Abstract
Aims
Despite well‐established benefits of sacubitril/valsartan for cardiac reverse remodelling and the prognosis of patients with heart failure with reduced ejection fraction (HFrEF), there are some patients with limited therapeutic response, even with optimal therapy. We assessed the treatment response to sacubitril/valsartan in patients with HFrEF, focusing on the association between reverse remodelling and the prognosis.
Methods and results
Using a retrospective cohort of consecutive patients with HFrEF treated with sacubitril/valsartan, we compared the time trajectory of cardiac function in 415 patients (1258 echocardiograms), according to the occurrence of cardiovascular death and hospitalization for HF during a median follow‐up of 19.1 (interquartile range, 10.9–27.6) months. A higher sacubitril/valsartan dose was associated with a better prognosis, whereas advanced age, diabetes, left ventricular (LV) hypertrophy, left atrial enlargement, and pulmonary hypertension were associated with a worse prognosis. Patients without an event (n = 337; 81.2%) showed LV reverse remodelling (LV ejection fraction ≥45% or LV end‐systolic volume reduction by 15% from baseline), which was typically observed within 6 months of sacubitril/valsartan treatment. Reverse remodelling achievement was significantly associated with a better prognosis. However, patients without reverse remodelling had a worse prognosis, as poor as that in patients with HFrEF not treated with sacubitril/valsartan.
Conclusions
In patients with HFrEF treated with sacubitril/valsartan, LV reverse remodelling reflects the treatment response and predicts the prognosis, whereas a lack of reverse remodelling indicates the lack of treatment benefits. Prediction and assessment of reverse remodelling may facilitate the selection of patients with greater benefits by sacubitril/valsartan.
Keywords: Sacubitril/valsartan, Angiotensin receptor‐neprilysin inhibitor, Heart failure with reduced ejection fraction, Reverse remodelling
Introduction
One of the most important advances in the management of heart failure (HF) in the last decade was the development of angiotensin receptor‐neprilysin inhibitor (ARNI). The use of LCZ696 (sacubitril/valsartan) significantly improves the prognosis of patients with HF with reduced ejection fraction (HFrEF). 1 , 2 The prognostic benefits of ARNI treatment are associated with a reduction in N‐terminal pro B‐type natriuretic peptide (NT‐proBNP) level and cardiac reverse remodelling, which includes an improvement in the left ventricular ejection fraction (LV‐EF) and a reduction in LV volume. 3
Although the overall prognosis of patients with HFrEF has been further improved with the introduction of ARNI, we still encounter patients with HFrEF who have a poor prognosis, even with optimal therapy including beta blockers (BB), mineralocorticoid antagonists (MRA), and ARNI. 1 This finding indicates that there are still patients with a limited response to ARNI. However, most previous studies have focused on the beneficial effects of ARNI on cardiac reverse remodelling, neglecting the clinical course of such ‘non‐responders’. 1 , 2 , 3 , 4 Furthermore, the association between reverse remodelling and the prognosis has not been fully investigated, especially in non‐responders.
We hypothesized that the prognosis in patients with HFrEF treated with ARNI would be different according to the time trajectory of cardiac function, and that the achievement of cardiac reverse remodelling by ARNI treatment may predict the overall prognosis in these patients.
Methods
Patients
From March 2017 to May 2020, we retrospectively identified 700 patients with HFrEF who were prescribed ARNI at Seoul National University Bundang Hospital (Figure 1 ). In Korea, the prescription of ARNI is indicated, and can be reimbursed by the National Insurance, in patients with HFrEF who meet the following two criteria: (1) LV‐EF ≤ 40% with New York Heart Association functional class II to IV, and (2) under optimal HF therapy using angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers, with BB and MRA at stable doses for at least 4 weeks. All patients identified from electronic medical records met these criteria. Patients without echocardiograms, with only 1 echocardiogram, or with all echocardiograms performed exclusively before or exclusively after the initial ARNI prescription were excluded. Finally, 415 patients were identified. Comorbidities of patients were determined from electronic medical records.
Figure 1.
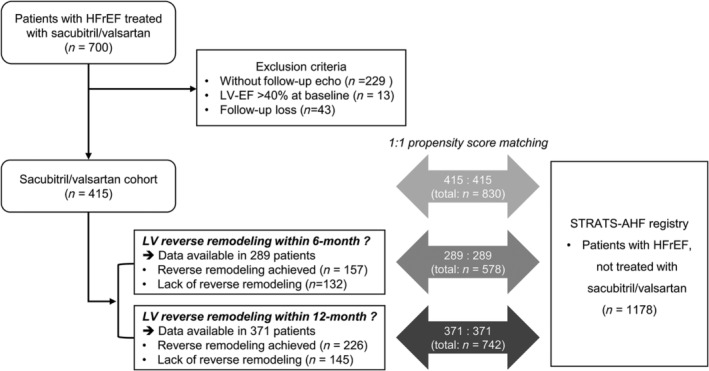
Study population flow chart. The study population, comprised 415 patients with HFrEF treated with ARNI (sacubitril/valsartan cohort), were matched to patients in a separate HF registry (STRATS‐AHF registry). 5 , 6 Patients with follow‐up echocardiography within 6 months (n = 289) and 12 months (n = 371) of ARNI treatment initiation were also matched. In the STRATS‐AHF registry, 1178 patients with HFrEF without sacubitril/valsartan treatment were identified for 1:1 propensity score matching. EF, ejection fraction; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; LV, left ventricular.
This study conformed with the principles outlined in the Declaration of Helsinki and was approved by the ethics committee of the Clinical Research Institute of Seoul National University Bundang Hospital. The requirement for informed consent was waived because of the retrospective nature of the study and minimal expected risk to the subjects.
Echocardiography
All images were obtained using a standard ultrasound device with a 2.5‐MHz probe. Standard techniques were used to obtain M‐mode, 2‐dimensional, and Doppler measurements in accordance with the guidelines of the American Society of Echocardiography (ASE). 7 , 8 LV diameter was measured using M‐mode imaging. LV end‐systolic and end‐diastolic volumes and LV‐EF were calculated using the biplane Simpson method from apical four‐ and two‐chamber views. LV volumes were indexed to body‐surface area (LV end‐diastolic volume index [LV‐EDVi] and LV end‐systolic volume index [LV‐ESVi]. The mitral E and A velocities, E/A ratio, deceleration time, and tissue Doppler analysis of septal mitral annular E′ velocity, and mitral E/e′ ratio were measured, and the continuous‐wave Doppler–derived pulmonary artery systolic pressure (PASP) was calculated from the peak tricuspid regurgitation (TR) velocity jet. Left atrial (LA) diameter was measured from the parasternal long‐axis view, and the LA maximal volume was measured at end systole using the area‐length method from apical 4‐ and 2‐chamber views, presented as the LA volume index (LAVI) after adjusting the body surface area. The severity of mitral regurgitation (MR) was evaluated in multiple aspects such as colour flow jet area, mitral E velocity, effective regurgitant orifice area, and regurgitant volume according to the guidelines of ASE, 9 and converted into scores (none or trivial, 0; mild, 1; moderate, 2; severe, 3).
Study outcomes
The primary outcome was the composite of cardiovascular death and hospitalization for HF (HHF). Patients who received cardiac resynchronization therapy were censored at the time of device implantation. Vital status data with cause of death were collected from Statistics Korea which is a government organization responsible for the mortality census and causes of death of entire Korean population, and HHF data were identified from electronic medical records of the institution.
According to the occurrence of the study outcome, we analysed the serial echocardiographic measurements of each patient starting from the time of ARNI treatment initiation to the time of the last clinical follow‐up. The changes in the severity of MR from baseline (before ARNI treatment) to most recent echocardiography (after ARNI treatment) were analysed according to the occurrence of study outcomes. The achievement of LV reverse remodelling within 6 or 12 months of ARNI treatment initiation was defined using the following criteria, based on the results of our analyses and previous relevant studies 10 , 11 , 12 : an improvement in the LV‐EF to ≥45% or a reduction in LV end‐systolic volume (LV‐ESV) by 15% from baseline. Serial measurements of the NT‐proBNP level were also assessed.
Statistical analysis
Continuous variables are presented as medians with interquartile range (IQR) and categorical variables are presented as frequencies. Differences between groups were evaluated using the Student's t‐test for continuous variables and the χ 2 test for categorical variables. The changes in the severity of MR before and after ARNI treatment were analysed using paired t‐test. The time trajectories of echocardiographic parameters were assessed using penalized smoothing spline methods to show the overall trend over time, until the occurrence of the study outcome or the end of follow‐up. Survival was analysed using the Kaplan–Meier method and Cox‐proportional hazard analysis was used to determine the independent predictors of the study outcome in terms of the time to first adverse clinical event. Multivariable Cox proportional hazard model analysis was used to evaluate the significance of markers with a P‐value of <0.2 on univariable analysis, using the stepwise backward elimination method. We also used multivariable Cox proportional hazard model analysis to determine the independent predictors of LV reverse remodelling after ARNI treatment (within 6 or 12 months), using markers with a P‐value of <0.2 on univariable analysis.
In order to assess the temporal changes in cardiac function and the prognostic impact of LV reverse remodelling, the total study population (n = 415) and those for whom the achievement of LV reverse remodelling could be determined (within 6 months: n = 289; within 12 months: n = 371) were matched with patients with HFrEF included in the STrain for Risk Assessment and Therapeutic Strategies in patients with Acute Heart Failure (STRATS‐AHF) registry (NCT03513653) (Figure 1 ). 5 , 6 In the STRATS‐AHF registry, we identified 1178 patients with HFrEF (LV‐EF < 40%) who were not treated with ARNI because the drug had not been covered by the National Insurance during the follow‐up period. Patients were matched using propensity score matching, with a 1:1 ratio, for age, sex, body mass index, systolic blood pressure (SBP), heart rate, hypertension, diabetes, atrial fibrillation (AF), ischaemic HFrEF aetiology, glomerular filtration rate (GFR), BB use, MRA use, LV‐EF, and LV end‐diastolic volume (LV‐EDV) (Supporting Information, Tables S1 , S2 , and S3 ).
Data were analysed using SPSS version 22 (IBM, Chicago, IL, USA), and R version 4.0.2 (The R Foundation for Statistical Computing, Vienna, Austria). A two‐sided P‐value of <0.05 was considered statistically significant.
Results
Baseline characteristics
Among the 415 patients with HFrEF who were prescribed ARNI, the median age was 66.3 years (IQR, 56.6–75.7) and 73.3% were male (Table 1 ). The prevalence of hypertension, diabetes, dyslipidaemia, chronic kidney disease (CKD), AF, and stroke was 31.8%, 33.7%, 20.2%, 31.3%, 29.4%, and 14.0%, respectively. The aetiology of HFrEF was ischaemic in 135 patients (32.5%), and the proportion of HFrEF by ischaemic aetiology was higher in patients with an outcome event than in those without an outcome event. During a median 19.1 (IQR, 10.9–27.6) months of follow‐up, the composite event occurred in 78 patients (18.8%, cardiovascular death in 15 patients and HHF in 71 patients). Compared with those without an outcome event, patients with an outcome event had a higher prevalence of hypertension, diabetes, CKD, AF, stroke and prior cardiac resynchronization therapy (CRT) implantation. Also, patients with an outcome event had larger LV size and impaired diastolic function parameters, but similar LV‐EF, compared with those without an event.
Table 1.
Baseline characteristics
| Total (n = 415) | Without event (n = 337) | With event (n = 78) | P‐value | |
|---|---|---|---|---|
| Age (years) | 66 (57–76) | 65 (55–74) | 72 (63–79) | 0.005 |
| Male sex | 304 (73.3%) | 247 (73.3%) | 57 (73.1%) | 0.969 |
| BMI (kg/m2) | 24.1 (22.1–26.0) | 24.0 (22.0–26.2) | 24.2 (22.2–25.8) | 0.589 |
| Systolic blood pressure (mmHg) | 118 (106–131) | 119 (107–131) | 113 (102–126) | 0.162 |
| Diastolic blood pressure (mmHg) | 70 (63–79) | 70 (63–79) | 68 (60–76) | 0.384 |
| Heart rate (beat/min) | 84 (70–102) | 85 (71–101) | 79 (63–102) | 0.114 |
| Co‐morbidities | ||||
| Hypertension | 132 (31.8%) | 99 (29.4%) | 33 (42.3%) | 0.021 |
| Diabetes | 140 (33.7%) | 103 (30.6%) | 37 (47.4%) | 0.007 |
| Dyslipidaemia | 84 (20.2%) | 64 (19.0%) | 20 (25.6%) | 0.165 |
| Chronic kidney disease | 130 (31.3%) | 92 (27.3%) | 38 (48.7%) | <0.001 |
| Stroke | 58 (14.0%) | 41 (12.2%) | 17 (21.8%) | 0.023 |
| Chronic obstructive pulmonary disease | 38 (9.2%) | 30 (8.9%) | 8 (10.3%) | 0.678 |
| AF | 122 (29.4%) | 92 (27.3%) | 30 (38.5%) | 0.041 |
| Prior ICD | 36 (8.7%) | 28 (8.3%) | 8 (10.3%) | 0.582 |
| Prior CRT | 24 (5.8%) | 15 (4.5%) | 9 (11.5%) | 0.027 |
| Ischaemic aetiology | 135 (32.5%) | 101 (30.0%) | 34 (43.6%) | 0.016 |
| Laboratory tests | ||||
| Total cholesterol (mg/dL) | 153.0 (125.0–179.0) | 157.0 (129.0–181.8) | 131.5 (107.0–167.0) | <0.001 |
| Haemoglobin (g/dL) | 13.4 (12.0–14.9) | 13.6 (12.1–15.0) | 12.8 (11.6–14.0) | 0.028 |
| Blood urea nitrogen (mg/dL) | 19.0 (15.0–25.0) | 18.0 (14.0–23.0) | 22.0 (16.3–30.8) | <0.001 |
| Creatinine (mg/dL) | 1.0 (0.8–1.3) | 1.0 (0.8–1.2) | 1.2 (0.9–1.6) | 0.059 |
| GFR (mL/min/1.73 m2) | 74.4 (54.0–91.1) | 78.5 (56.9–93.9) | 60.4 (40.9–78.9) | <0.001 |
| NT‐proBNP (pg/mL) | 1384.0 (449.7–4524.7) | 1472.2 (439.7–4597.5) | 1316.2 (535.6–3868.3) | 0.561 |
| Echocardiographic parameters | ||||
| LV‐EDD (mm) | 60.0 (55.0–65.0) | 60.0 (55.0–65.0) | 62.0 (56.0–67.0) | 0.083 |
| LV‐ESD (mm) | 50.0 (45.0–56.7) | 50.0 (44.6–56.0) | 51.5 (46.0–59.0) | 0.041 |
| LV‐EDV (mL) | 156.0 (123.0–196.0) | 154.0 (120.0–196.0) | 170.5 (130.0–200.8) | 0.136 |
| LV‐EDV index (mL/m2) | 90.9 (72.0–112.3) | 89.8 (70.4–110.5) | 100.6 (76.4–121.4) | 0.016 |
| LV‐ESV (ml) | 113.0 (85.0–146.0) | 110.0 (84.0–145.2) | 122.5 (93.5–154.5) | 0.125 |
| LV‐ESV index (mL/m2) | 65.2 (50.5–83.9) | 63.2 (48.6–81.6) | 74.5 (54.0–89.4) | 0.019 |
| LV‐EF (%) | 27.8 (22.9–33.1) | 28.1 (23.1–33.3) | 26.2 (21.7–32.6) | 0.156 |
| LV‐MI (g/m2) | 136.6 (113.6–163.7) | 134.3 (111.9–160.3) | 141.6 (123.3–176.4) | 0.026 |
| Mitral inflow E velocity (m/s) | 0.78 (0.56–1.02) | 0.74 (0.53–1.00) | 0.91 (0.70–1.06) | 0.002 |
| Septal mitral annulus s′ velocity (cm/s) | 4.4 (3.6–5.3) | 4.6 (3.6–5.4) | 4.1 (3.5–5.0) | 0.029 |
| Septal mitral annulus e′ velocity (cm/s) | 4.5 (3.5–5.7) | 4.6 (3.6–5.8) | 4.1 (3.2–5.4) | 0.002 |
| E/e′ ratio | 15.9 (11.3–24.1) | 15.2 (11.0–22.7) | 19.8 (13.4–29.3) | <0.001 |
| LA dimension (mm) | 44.0 (38.0–49.0) | 43.0 (38.0–48.0) | 46.0 (41.0–51.8) | 0.014 |
| LAVI (mL/m2) | 54.4 (43.0–71.6) | 52.7 (41.5–69.3) | 64.7 (51.4–82.2) | 0.002 |
| TR Vmax (m/s) | 2.6 (2.3–3.1) | 2.5 (2.2–3.0) | 3.0 (2.7–3.6) | <0.001 |
| PASP (mmHg) | 32.0 (26.2–43.7) | 30.0 (24.4–41.1) | 44.8 (34.2–60.3) | <0.001 |
| Medication | ||||
| Use of BB | 401 (96.6%) | 331 (97.9%) | 70 (90.9%) | 0.213 |
| Use of MRA | 247 (59.5%) | 196 (58.0%) | 51 (66.2%) | 0.183 |
| Use of ivabradine | 160 (38.6%) | 131 (38.8%) | 29 (37.7%) | 0.859 |
| Use of diuretics | 330 (79.5%) | 266 (78.9%) | 64 (82.1%) | 0.538 |
| Events | ||||
| Cardiovascular death | 15 (3.6%) | 0 (0.0%) | 15 (19.2%) | N/A |
| HHF | 71 (17.1%) | 0 (0.0%) | 71 (91.0%) | N/A |
| Follow‐up duration (months) | 19.1 (10.9–27.6) | 21.6 (14.8–29.2) | 5.1 (1.5–12.1) | <0.001 |
Values are given as the median with interquartile range or as a number (percentage).
AF, atrial fibrillation; BB, beta‐blockers; BMI, body‐mass index; CRT, cardiac resynchronization therapy; EDD, end‐diastolic dimension; EDV, end‐diastolic volume; EF, ejection fraction; ESD, end‐systolic dimension; ESV, end‐systolic volume; GFR, glomerular filtration rate; HHF, hospitalization for heart failure; ICD, implantable cardioverter defibrillator; LA, left atrium; LAVI, left atrial volume index; LV, left ventricular; MI, mass index; MRA, mineralocorticoid antagonists; N/A, not applicable; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; PASP, pulmonary artery systolic pressure; TR, tricuspid regurgitation.
Cardiac function trajectory according to the occurrence of event
In the total study population, 1258 echocardiographic examinations (median, 3; IQR, 2–4) and 975 NT‐proBNP measurements (median, 2; IQR, 0–4) were performed during the follow‐up period. The echocardiographic examinations and NT‐proBNP measurements were also obtained from the 415 patients with HFrEF not treated with ARNI, matched from the STRATS‐AHF registry: 1829 echocardiographic examinations (median 4; IQR, 2–6) and 340 NT‐proBNP measurements (median 1; IQR, 0–2). The serial echocardiographic measurements are plotted according to the use of ARNI and the occurrence of the composite outcome in Figure 2 . In patients without an outcome event, the echocardiographic parameters of cardiac function, including LV‐EF, LV‐EDVi, LV‐ESVi, LAVI, mitral E/e′ ratio, and PASP, showed prominent improvements in the early period of ARNI treatment, mostly within 6 months of the initial prescription for ARNI. In contrast, these parameters were not improved in the early period of ARNI treatment among those with an outcome event. The matched control group showed modest improvement in cardiac function, which was greater than the patients treated with ARNI and had an outcome event, but lesser than those treated with ARNI without an outcome event.
Figure 2.
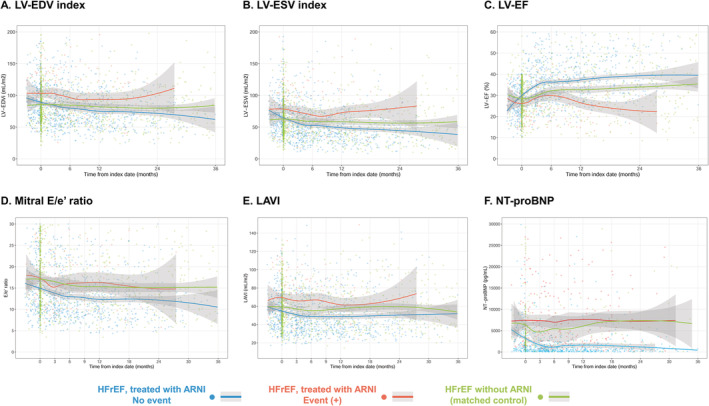
Trajectories of cardiac function and NT‐proBNP level in patients with HFrEF treated with sacubitril/valsartan. Serial measurements of (A) LV‐EDVi, (B) LV‐ESVi, (C) LV‐EF, (D) mitral E/e′ ratio, (E) LAVI, and (F) NT‐proBNP level are shown according to the occurrence of the study outcome (cardiovascular death or HHF). Measurements in patients without an outcome event are shown in blue dots with spline curves; those in patients with an outcome event are shown in red dots with spline curves; and those in the matched control group are shown in green dots with spline curves. EDVi, end‐diastolic volume index; EF, ejection fraction; ESVi, end‐systolic volume index; HFrEF, heart failure with reduced ejection fraction; LAVI, left atrial volume index; LV, left ventricular; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide.
At baseline, the severity of MR was greater in patients with an outcome event, compared with those without (P < 0.001) (Figure 3 ). Of note, the severity of MR was significantly decreased in patients without an event (P < 0.001), and the prevalence of moderate and severe MR were decreased by 10.4% and 2.1%, respectively. However, in patients with an outcome event, the overall severity of MR did not change (P = 0.744), while the prevalence of severe MR was increased from 12.8% to 20.5%.
Figure 3.
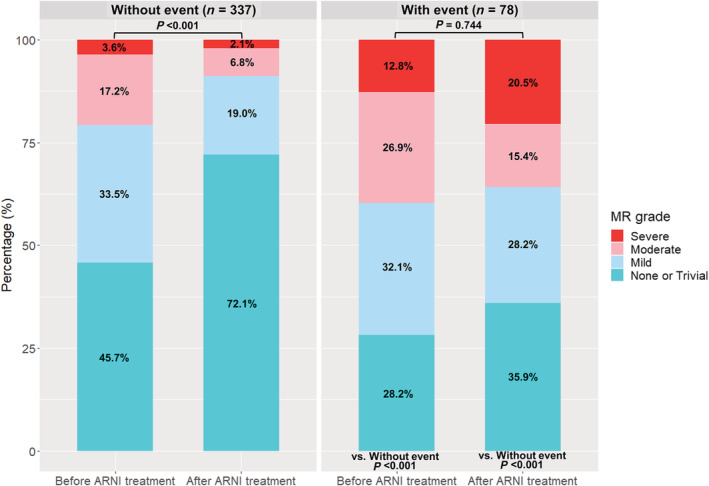
Changes in the severity of MR. The severity of MR was assessed at baseline (before ARNI treatment) and at last echocardiography (after ARNI treatment), according to the occurrence of outcome events. In patients without an outcome event, the severity of MR was decreased after ARNI treatment, whereas the patients with an outcome event did not show reduction in MR amount. Notably, the prevalence of severe MR was further increased in patients who experienced an outcome event. MR, mitral regurgitation.
Changes in N‐terminal pro B‐type natriuretic peptide levels in response to sacubitril/valsartan treatment
At baseline, NT‐proBNP levels did not differ between patients without an outcome event (median, 1472.2 pg/mL; IQR, 439.7–4597.5) and those with an outcome event (median, 1316.2; IQR, 535.6–3868.3) (Table 1 ). As shown in Figure 2 F , the NT‐proBNP levels were significantly decreased after ARNI treatment initiation in patients without an outcome event, but remained elevated throughout the follow‐up period in patients who had an outcome event.
Left ventricular reverse remodelling and its predictors
Among the 289 patients with available follow‐up echocardiographic measurements within 6 months of ARNI treatment initiation, 157 (54.3%) patients achieved LV reverse remodelling (Supporting Information, Table S4 ). Within 12 months of ARNI treatment initiation, 371 patients had follow‐up echocardiography; among these, 226 (60.9%) achieved LV reverse remodelling. The proportion of patients with LV reverse remodelling was significantly lower in those who experienced an outcome event than in patients without an outcome event.
The event‐free survival curves are plotted according to the achievement of LV reverse remodelling at 6 and 12 months of ARNI treatment in Figure 4 . The survival probability according to the achievement of LV reverse remodelling among patients treated with ARNI was further compared with that in patients with HFrEF without ARNI treatment, matched from a separate registry database (STRATS‐AHF registry). A lower risk of the composite event was prominent in those who achieved LV reverse remodelling (adjusted hazard ratio (HR), 0.409; 95% confidence interval (CI), 0.217–0.769; P = 0.006 for LV reverse remodelling within 6 months; adjusted HR, 0.313; 95% CI, 0.181–0.542; P < 0.001 for LV reverse remodelling within 12 months), compared with that in patients who did not achieve LV reverse remodelling. The survival probability of patients with HFrEF who did not achieve LV reverse remodelling after ARNI treatment was as poor as that in the matched HFrEF patients without ARNI treatment. A lower risk of the composite event in patients with LV reverse remodelling than in patients without LV reverse remodelling and matched patients with HFrEF who were not treated with ARNI was consistent across various subgroups based on clinical variables and LV‐EF (Figure 5 and Supporting Information, Figure S1 ).
Figure 4.
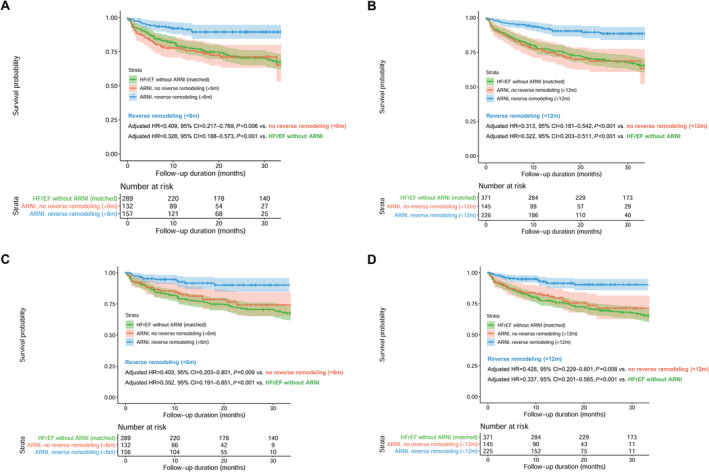
Survival curves according to the achievement of LV reverse remodelling. Event‐free survival curves according to the occurrence of LV reverse remodelling (A) within 6 months and (B) 12 months of sacubitril/valsartan treatment initiation are shown. Landmark analyses counting the outcome events after the determination of LV reverse remodelling (C) within 6 months and (D) within 12 months of treatment initiation are also shown. Survival curves of patients who achieved LV reverse remodelling are shown in blue, and those without LV reverse remodelling are shown in red. The overall survival in the study population was compared to that for propensity‐score matched patients with HFrEF who were not treated with sacubitril/valsartan (green colour), identified from a separate registry (STRATS‐AHF registry). 5 , 6 CI, confidence interval; HR, hazard ratio; LV, left ventricular.
Figure 5.
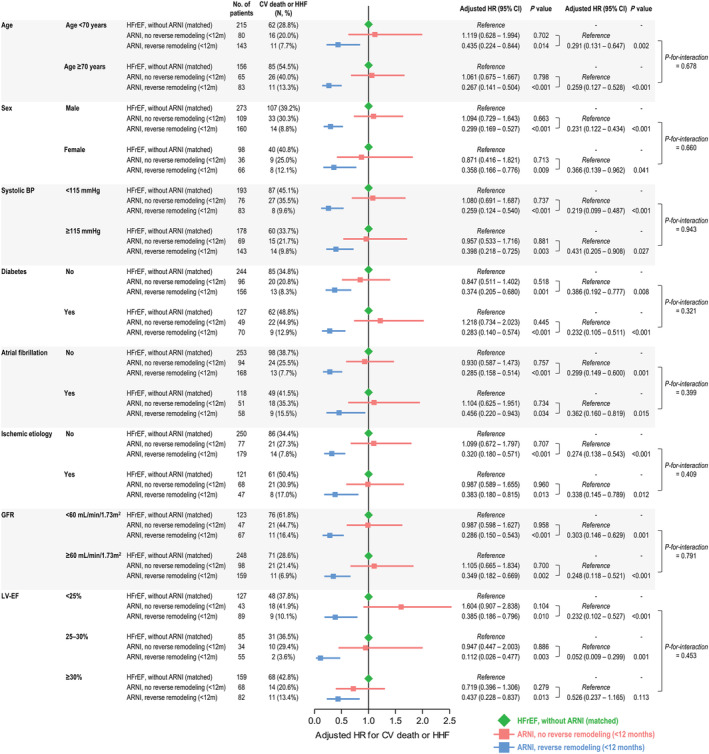
Subgroup analyses for LV reverse remodelling within 12 months of treatment initiation. The adjusted HRs are shown for the composite of CV death or HHF in subgroups based on clinical features and LV‐EF. Using propensity‐score matched patients with HFrEF without ARNI treatment as a reference (green), the HRs of patients treated with ARNI who did (blue) and did not (red) achieve reverse remodelling within 12 months are summarized. Comparisons between patients with and without reverse remodelling, and P‐values for the interaction, are also shown in the rightmost column of the table. BP, blood pressure; CI, confidence interval; CV, cardiovascular; GFR, glomerular filtration rate; HHF, hospitalization for heart failure; HR, hazard ratio; LV‐EF, left ventricular ejection fraction.
We evaluated the associations between baseline clinical variables and echocardiographic parameters with LV reverse remodelling (Table 2 and Supporting Information, Table S5 ). SBP > 110 mmHg (adjusted HR, 1.821; 95% CI, 1.228–2.700; P = 0.003), LV‐EF < 25% (adjusted HR, 1.872; 95% CI 1.313–2.669; P = 0.001), and PASP <40 mmHg (adjusted HR, 1.536; 95% CI, 1.048–2.253; P = 0.028) were significantly associated with LV reverse remodelling within 6 months of ARNI treatment (Table 2 ). Similarly, SBP > 120 mmHg (adjusted HR, 1.355; 95% CI 1.010–1.818; P = 0.043) and LV‐EF < 30% (adjusted HR, 1.536; 95% CI 1.135–2.080; P = 0.005) showed significant associations with LV reverse remodelling within 12 months of ARNI treatment, and of note, non‐ischaemic aetiology of HFrEF showed a significant association with LV reverse remodelling (adjusted HR, 1.661; 95% CI 1.172–2.353; P = 0.004).
Table 2.
Predictors of the achievement of LV reverse remodelling
| Reverse remodelling within 6 months a | Reverse remodelling within 12 months b | |||||
|---|---|---|---|---|---|---|
| Adjusted HR | 95% CI | P‐value | Adjusted HR | 95% CI | P‐value | |
| SBP >110 mmHg | 1.821 | 1.228–2.700 | 0.003 | ‐ | ‐ | ‐ |
| SBP >120 mmHg | ‐ | ‐ | ‐ | 1.355 | 1.010–1.818 | 0.043 |
| Non‐ischaemic aetiology | ‐ | ‐ | ‐ | 1.661 | 1.172–2.353 | 0.004 |
| LV‐EF <25% | 1.872 | 1.313–2.669 | 0.001 | ‐ | ‐ | ‐ |
| LV‐EF <30% | ‐ | ‐ | ‐ | 1.536 | 1.135–2.080 | 0.005 |
| PASP <40 mmHg | 1.536 | 1.048–2.253 | 0.028 | ‐ | ‐ | ‐ |
Univariable factors with P‐values <0.200 were entered into the multivariable Cox proportional hazard regression analysis, using the stepwise backward elimination method.
LV reverse remodelling within 6 months of sacubitril/valsartan treatment initiation was determined in 289 patients for whom follow‐up echocardiograms within 6 months were available. Multivariable Cox regression analysis was performed with these 289 patients.
LV reverse remodelling within 12 months of sacubitril/valsartan treatment initiation was determined in 371 patients for whom follow‐up echocardiograms within 6 months were available. Multivariable Cox regression analysis was performed with these 371 patients.
CI, confidence interval; LV‐EF, left ventricular ejection fraction; HR, hazard ratio; PASP, pulmonary artery systolic pressure; SBP, systolic blood pressure.
Predictors of cardiovascular death and hospitalization for heart failure
On univariable analysis, advanced age, hypertension, diabetes, CKD, stroke, ischaemic aetiology, prior CRT implantation, lower GFR, larger LV size, larger LV mass index, larger LAVI, higher mitral E/e′ ratio, and higher PASP were significantly associated with the occurrence of the composite outcome. In contrast, more BB prescriptions and a higher daily dose of ARNI were associated with a lower risk of cardiovascular death or HHF (Supporting Information, Table S6 ). On multivariable analysis using the backward selection method, advanced age (age >70 years; adjusted HR, 1.810; 95% CI, 1.080–3.031; P = 0.024), presence of diabetes (adjusted HR, 1.725; 95% CI, 1.058–2.813; P = 0.029), larger LV‐EDVi (LV‐EDVi ≥91 mL/m2; adjusted HR, 1.754; 95% CI, 1.031–2.983; P = 0.038), and PASP >50 mmHg (adjusted HR, 2.657; 95% CI, 1.603–4.405; P < 0.001) were associated with a higher risk of the composite outcome (Table 3 ). In contrast, more BB prescriptions (adjusted HR, 0.426; 95% CI, 0.190–0.958; P = 0.039) and a higher daily dose of ARNI (≥200 mg/day; adjusted HR, 0.399; 95% CI, 0.239–0.665; P = 0.001) was associated with a lower risk of the composite outcome. Similar results were observed in the analyses among the patients for whom the achievement of LV reverse remodelling could be determined (within 6 months: n = 289; within 12 months: n = 371), while the achievement of LV reverse remodelling was significantly associated with a lower risk of study outcome.
Table 3.
Multivariable predictors of cardiovascular mortality and HHF
| Variables | Total study population (n = 415) | Patients with follow‐up echocardiography within 6 months (n = 289) b | Patients with follow‐up echocardiography within 12 months (n = 371) c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Adjusted HR | 95% CI | P‐value | Adjusted HR | 95% CI | P‐value | Adjusted HR | 95% CI | P‐value | |
| Age >70 years | 1.810 | 1.080–3.031 | 0.024 | 2.460 | 1.363–4.440 | 0.003 | ‐ | ‐ | ‐ |
| Diabetes mellitus | 1.725 | 1.058–2.813 | 0.029 | 2.460 | 1.363–4.440 | 0.003 | 2.234 | 1.295–3.854 | 0.004 |
| LV hypertrophy a | 2.093 | 0.928–4.721 | 0.075 | 6.065 | 1.845–19.938 | 0.003 | 2.815 | 1.251–6.335 | 0.012 |
| LV‐EDV index ≥91 mL/m2 (median) | 1.754 | 1.031–2.983 | 0.038 | ||||||
| PASP >50 mmHg | 2.657 | 1.603–4.405 | <0.001 | 2.115 | 1.120–3.992 | 0.021 | 1.815 | 1.009–3.267 | 0.047 |
| Use of BB | 0.370 | 0.165–0.833 | 0.016 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Dosage of sacubitril/valsartan at last follow‐up ≥200 mg/day | 0.407 | 0.243–0.680 | 0.001 | 0.494 | 0.260–0.938 | 0.031 | 0.362 | 0.207–0.633 | <0.001 |
| Reverse remodelling within 6 months b | ‐ | ‐ | ‐ | 0.409 | 0.217–0.769 | 0.006 | ‐ | ‐ | ‐ |
| Reverse remodelling within 12 months c | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 0.313 | 0.181–0.542 | <0.001 |
Univariable factors with P values <0.200 were entered into the multivariable Cox proportional hazard regression analysis, using the stepwise backward elimination method. Variables with a significant association with the composite endpoint of cardiovascular death and HHF are shown.
LV hypertrophy was defined according to the American Society of Echocardiography's guidelines 7 : LV‐MI > 95 g/m2 in women and >115 g/m2 in men.
LV reverse remodelling within 6 months of sacubitril/valsartan treatment initiation was determined in 289 patients for whom follow‐up echocardiograms within 6 months were available. Multivariable Cox proportional hazard regression analysis was performed with these 289 patients.
LV reverse remodelling within 12 months of sacubitril/valsartan treatment initiation was determined in 371 patients for whom follow‐up echocardiograms within 6 months were available. Multivariable Cox proportional hazard regression analysis was performed with these 371 patients.
BB, beta‐blockers; CI, confidence interval; HHF, hospitalization for heart failure; HR, hazards ratio; LV, left ventricular; MI, mass index; PASP, pulmonary artery systolic pressure.
Discussion
In the present study, we investigated the occurrence of cardiovascular death and HHF according to the trajectory of cardiac function in patients with HFrEF treated with ARNI. LV reverse remodelling was observed in patients without these events, typically in the early period of ARNI treatment. The occurrence of LV reverse remodelling was significantly associated with a lower risk of cardiovascular mortality and HHF. However, in patients with HFrEF treated with ARNI who did not show LV reverse remodelling, the overall survival was similar to that in patients with HFrEF not treated with ARNI. These findings suggest that 1 improvement in cardiac function assessed by echocardiography could be used as an indicator of treatment response and a predictor of a better prognosis, and 2 the prediction and assessment of LV reverse remodelling may facilitate the selection of patients with HFrEF who will have greater clinical benefits with ARNI treatment.
Benefits of angiotensin receptor‐neprilysin inhibitor treatment in patients with heart failure with reduced ejection fraction
The development of sacubitril/valsartan is considered as one of the most important advances in the management of HFrEF, and its role is rapidly expanding to first‐line treatment in symptomatic patients with HFrEF. 13 , 14 According to the Prospective Comparison of ARNI with ACEI [Angiotensin‐Converting–Enzyme Inhibitor] to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM‐HF) trial, the use of ARNI reduced cardiovascular death by 20% and the risk of HHF by 21% compared with that with the use of enalapril. 1
The prognostic benefits of ARNI were further elaborated in a series of studies that reported changes in echocardiographic parameters; the Prospective Study of Biomarkers, Symptom Improvement, and Ventricular Remodelling During Sacubitril/Valsartan Therapy for Heart Failure (PROVE‐HF) showed that the reduction in NT‐proBNP level is correlated with improvement in LV function parameters in patients with HFrEF treated with ARNI. 3 In the Effect of Sacubitril‐Valsartan versus Enalapril on Aortic Stiffness in Patients with Heart Failure and Reduced Ejection Fraction (EVALUATE‐HF) trial, the patients treated with ARNI showed a significant reduction in LV size, LAVI, and mitral E/e′ ratio, compared with those treated with enalapril. 15 LV reverse remodelling by ARNI treatment was also demonstrated in patients with functional MR secondary to LV dysfunction. 16 According to the Pharmacological Reduction of Functional, Ischemic Mitral Regurgitation (PRIME) trial, the 12‐month treatment with ARNI significantly reduced the severity of MR and LV‐EDVi, compared with those treated with valsartan. Several retrospective studies reported similar results for LV reverse remodelling, which was observed in the early phase after ARNI treatment initiation. 17 , 18 , 19
In the present study, we observed significant improvements in LV function parameters in patients with HFrEF treated with ARNI, which is consistent with previous reports. Relevant echocardiographic parameters, including LV‐EF, indexed LV volume, LAVI, mitral E/e′ ratio, PASP, and the severity of MR demonstrated concordant trends towards improvement. These findings suggest that the use of ARNI can provide comprehensive benefits for cardiac geometry and both systolic and diastolic function, and that. LV reverse remodelling provides a mechanistic explanation for the prognostic benefits of ARNI in patients with HFrEF.
Existence of patients with a poor prognosis despite optimal treatment
Although the benefits of ARNI are robust, there are still some patients who experience cardiovascular death or HHF that cannot be prevented with the current optimal treatment protocol. According to the PARADIGM‐HF trial, the overall event rate (cardiovascular death or HHF) in patients with HFrEF treated with optimal medical therapy, including ARNI, was 21.8% during a median of 27 months (annual event rate, 9.7%). While most of the published studies have focused on the positive results of ARNI, including its prognostic benefits and associated LV reverse remodelling, little attention has been paid to this small, but still significant, proportion of poor responders. In this regards, in addition to the robust findings on the benefits of ARNI for LV reverse remodelling in patients with HFrEF, we further demonstrated that there are certain patients who do not show LV reverse remodelling despite ARNI treatment (i.e. non‐responders), and that these patients have a worse prognosis compared with that in ARNI‐responders.
Clinical implications of early reverse remodelling
In the present study, the difference in the time trajectory of LV function between ARNI‐responders and non‐responders became obvious in the early phase of ARNI treatment, within 6 months after treatment initiation. Furthermore, it was noted that the worse prognosis in non‐responders was similar to that in propensity‐score matched patients with HFrEF who were not treated with ARNI. The non‐responders, as well as the matched patients from the STRATS‐AHF registry, were on optimal medical therapy, including renin‐angiotensin system blockers (100%), BB (97%), and MRA (~60%), with the exception of ARNI.
Our findings provide several implications regarding the use of ARNI in patients with HFrEF. First, serial echocardiographic assessments can be used as indicators of treatment response. The achievement of early LV reverse remodelling indicates an appropriate response to ARNI, leading to greater hemodynamic improvements and a reduced risk of cardiovascular mortality and morbidity. Second, the failure to achieve LV reverse remodelling by ARNI may indicate a lack of treatment benefits, not only in terms of echocardiographic recovery, but also in terms of additive survival gain. Therefore, it can be postulated that serial echocardiographic assessments can differentiate patients who will benefit from the current treatment with ARNI and those who will have a limited response. Patients with limited response to ARNI treatment may require additional treatment options, such as a cardiac implantable electronic device, ventricular assist device, or cardiac transplantation. Third, there might be predictors of early reverse remodelling after ARNI treatment initiation.. In the present study, patients with higher SBP, at baseline can be considered as having a better response to ARNI treatment. Patients with higher SBP are more likely to be prescribed with a higher dose of ARNI, as reported in a substudy of the TITRATION trial. 20 , 21 The higher SBP at baseline, as well as the maintenance of target dose of ARNI, are good prognostic factors, according to the sub‐analyses of PARADIGM‐HF trial. 22 , 23 In line with these findings, we observed that the higher SBP at baseline is associated with the early LV reverse remodelling, and that the higher dose of ARNI at last follow‐up is associated with better outcome.
Regarding the LV reverse remodelling, the duration of HFrEF is considered as an important predictor. In a prospective study of 1160 patients, the long‐term trajectories of LV‐EF showed a more prominent improvement in patients with new‐onset HF compared with those with longer HF duration. 24 This tendency was also reported in a small retrospective study of patients with HFrEF treated with ARNI: the patients with LV reverse remodelling tended to have a shorter duration of HFrEF. 25 In the present study, we could not determine the duration of HFrEF due to the retrospective study design, but we observed that non‐ischaemic HFrEF aetiology was associated with early reverse remodelling. Considering the potential association between the duration of HFrEF and LV reverse remodelling, it could be assumed that the patients with ischaemic aetiology of HFrEF in our study might have a longer duration of HFrEF. Meanwhile, the aetiology of HFrEF was not associated with prognosis, which is concordant with a substudy of PARADIM‐HF trial by Balmforth et al.; the benefits of ARNI were similar between patients with ischaemic aetiology and those with non‐ischaemic aetiology. 26 Furthermore, as shown in the Figure 5 , the prognostic benefits of ARNI were concordant across various subgroups, which are in line with previous landmark trials. 1 , 27 Thus, although we could not confirm the cause‐effect relationship, our findings suggest that the identification of potential predictors for early reverse remodelling after ARNI treatment can facilitate the selection of candidates who may have greater prognostic benefits with ARNI treatment among patients with HFrEF. These findings need to be further investigated in large real‐world studies or prospective trials.
Limitations
The present study has several limitations. First, study population was identified from a retrospective database. However, the entire study population was prescribed ARNI under the same indication criteria, defined by the National Insurance. Therefore, the study utilized a homogenous study population while reflecting real‐world practice. Second, echocardiography was performed according to the attending physician's discretion, rather than by a pre‐specified schedule. However, the distinct difference in the trajectory of cardiac function between patients with and without an outcome event suggests that the findings of our study are relevant. Still, a prospective study with a pre‐defined echocardiographic follow‐up schedule could further clarify the temporal changes in cardiac function. Third, data regarding changes in symptomatic status and quality of life were not available due to the retrospective design of the study. Given the significant correlation between changes in symptoms and Kansas City Cardiomyopathy Questionnaire‐23 scores with changes in NT‐proBNP levels and echocardiographic parameters observed in the PROVE‐HF study, 3 , 28 the lack of this information does not affect the interpretation of the present results. Fourth, we focused on the changes in echocardiographic parameters and NT‐proBNP, but could not investigated the strain measurements (i.e. LV global longitudinal strain or LA reservoir strain), or cardiac magnetic resonance parameters (i.e. native T1 or extracellular volume). Future studies are warranted to investigate the comprehensive changes in cardiac function and structures by ARNI treatment.
Conclusions
In patients with HFrEF treated with ARNI, LV reverse remodelling occurs in the early period of ARNI treatment and is associated with a lower risk of cardiovascular mortality and HHF. However, a lack of LV reverse remodelling by ARNI treatment sometimes occurs and may indicate a lack of additive benefit by ARNI treatment. Thus, LV reverse remodelling by ARNI can be an indicator of the treatment response and a predictor of the prognosis in patients with HFrEF.
Conflict of interest
None declared.
Supporting information
Figure S1. Subgroup analysis for LV reverse remodelling within 6‐month of treatment.
Table S1. Propensity score matching for total study population.
Table S2. Propensity score matching for patients with follow‐up echocardiography within 6‐month.
Table S3. Propensity score matching for patients with follow‐up echocardiography within 12‐month.
Table S4. Achievement of LV reverse remodelling after sacubitril/valsartan treatment initiation.
Table S5. Univariable predictors for achievement of LV reverse remodelling.
Table S6. Univariable predictors of cardiovascular mortality and HHF.
Acknowledgement
We thank Lia Ju, a registered diagnostic cardiac sonographer (RDCS), for her dedication and support on this study.
Moon, M.‐G. , Hwang, I.‐C. , Choi, W. , Cho, G.‐Y. , Yoon, Y. E. , Park, J.‐B. , Lee, S.‐P. , Kim, H.‐K. , and Kim, Y.‐J. (2021) Reverse remodelling by sacubitril/valsartan predicts the prognosis in heart failure with reduced ejection fraction. ESC Heart Failure, 8: 2058–2069. 10.1002/ehf2.13285
References
- 1. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, Investigators P‐H, Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 2. Solomon SD, Claggett B, McMurray JJ, Hernandez AF, Fonarow GC. Combined neprilysin and renin‐angiotensin system inhibition in heart failure with reduced ejection fraction: a meta‐analysis. Eur J Heart Fail 2016; 18: 1238–1243. [DOI] [PubMed] [Google Scholar]
- 3. Januzzi JL Jr, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Pina IL, Rocha RA, Shah AM, Williamson KM, Solomon SD, Investigators P‐H. Association of change in N‐terminal pro‐B‐type natriuretic peptide following initiation of sacubitril‐valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA 2019; 2: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Y, Zhou R, Lu C, Chen Q, Xu T, Li D. Effects of the angiotensin‐receptor neprilysin inhibitor on cardiac reverse remodeling: meta‐analysis. J Am Heart Assoc 2019; 8: e012272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park JJ, Park JB, Park JH, Cho GY. Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol 2018; 71: 1947–1957. [DOI] [PubMed] [Google Scholar]
- 6. Hwang IC, Cho GY, Choi HM, Yoon YE, Park JJ, Park JB, Park JH, Lee SP, Kim HK, Kim YJ, Sohn DW. Derivation and validation of a mortality risk prediction model using global longitudinal strain in patients with acute heart failure. Eur Heart J Cardiovasc Imaging 2019; 21: 1412–1420. [DOI] [PubMed] [Google Scholar]
- 7. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39.e14. [DOI] [PubMed] [Google Scholar]
- 8. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016; 29: 277–314. [DOI] [PubMed] [Google Scholar]
- 9. Zoghbi WA, Adams D, Bonow RO, Enriquez‐Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD, Weissman NJ. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017; 30: 303–371. [DOI] [PubMed] [Google Scholar]
- 10. Stellbrink C, Breithardt OA, Franke A, Sack S, Bakker P, Auricchio A, Pochet T, Salo R, Kramer A, Spinelli J, Investigators P‐C, Group CPIGCHFR . Impact of cardiac resynchronization therapy using hemodynamically optimized pacing on left ventricular remodeling in patients with congestive heart failure and ventricular conduction disturbances. J Am Coll Cardiol 2001; 38: 1957–1965. [DOI] [PubMed] [Google Scholar]
- 11. Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, Yu CM, Gorcsan J 3rd, St John Sutton M, De Sutter J, Murillo J. Results of the predictors of response to CRT (PROSPECT) trial. Circulation 2008; 117: 2608–2616. [DOI] [PubMed] [Google Scholar]
- 12. Mastenbroek MH, Van't Sant J, Versteeg H, Cramer MJ, Doevendans PA, Pedersen SS, Meine M. Relationship between reverse remodeling and cardiopulmonary exercise capacity in heart failure patients undergoing cardiac resynchronization therapy. J Card Fail 2016; 22: 385–394. [DOI] [PubMed] [Google Scholar]
- 13. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017; 70: 776–803. [DOI] [PubMed] [Google Scholar]
- 14. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Group ESCSD . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 15. Desai AS, Solomon SD, Shah AM, Claggett BL, Fang JC, Izzo J, McCague K, Abbas CA, Rocha R, Mitchell GF, Investigators E‐H. Effect of sacubitril‐valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2019; 2: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kang DH, Park SJ, Shin SH, Hong GR, Lee S, Kim MS, Yun SC, Song JM, Park SW, Kim JJ. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation 2019; 139: 1354–1365. [DOI] [PubMed] [Google Scholar]
- 17. Mazzetti S, Scifo C, Abete R, Margonato D, Chioffi M, Rossi J, Pisani M, Passafaro G, Grillo M, Poggio D, Mortara A. Short‐term echocardiographic evaluation by global longitudinal strain in patients with heart failure treated with sacubitril/valsartan. ESC Heart Fail 2020; 7: 964–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Romano G, Vitale G, Ajello L, Agnese V, Bellavia D, Caccamo G, Corrado E, Di Gesaro G, Falletta C, La Franca E, Mina C, Storniolo SA, Sarullo FM, Clemenza F. The Effects of sacubitril/valsartan on clinical, biochemical and echocardiographic parameters in patients with heart failure with reduced ejection fraction: the "hemodynamic recovery". J Clin Med 2019; 8: 2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang HY, Chen KC, Fong MC, Feng AN, Fu HN, Huang KC, Chong E, Yin WH. Recovery of left ventricular dysfunction after sacubitril/valsartan: predictors and management. J Cardiol 2020; 75: 233–241. [DOI] [PubMed] [Google Scholar]
- 20. Senni M, McMurray JJ, Wachter R, McIntyre HF, Reyes A, Majercak I, Andreka P, Shehova‐Yankova N, Anand I, Yilmaz MB, Gogia H, Martinez‐Selles M, Fischer S, Zilahi Z, Cosmi F, Gelev V, Galve E, Gomez‐Doblas JJ, Nociar J, Radomska M, Sokolova B, Volterrani M, Sarkar A, Reimund B, Chen F, Charney A. Initiating sacubitril/valsartan (LCZ696) in heart failure: results of TITRATION, a double‐blind, randomized comparison of two uptitration regimens. Eur J Heart Fail 2016; 18: 1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Senni M, McMurray JJV, Wachter R, McIntyre HF, Anand IS, Duino V, Sarkar A, Shi V, Charney A. Impact of systolic blood pressure on the safety and tolerability of initiating and up‐titrating sacubitril/valsartan in patients with heart failure and reduced ejection fraction: insights from the TITRATION study. Eur J Heart Fail 2018; 20: 491–500. [DOI] [PubMed] [Google Scholar]
- 22. Bohm M, Young R, Jhund PS, Solomon SD, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Swedberg K, Zile MR, Packer M, McMurray JJV. Systolic blood pressure, cardiovascular outcomes and efficacy and safety of sacubitril/valsartan (LCZ696) in patients with chronic heart failure and reduced ejection fraction: results from PARADIGM‐HF. Eur Heart J 2017; 38: 1132–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vardeny O, Claggett B, Packer M, Zile MR, Rouleau J, Swedberg K, Teerlink JR, Desai AS, Lefkowitz M, Shi V, McMurray JJ, Solomon SD, Prospective Comparison of AwAtDIoGM , Morbidity in Heart Failure I . Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM‐HF trial. Eur J Heart Fail 2016; 18: 1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lupon J, Gavidia‐Bovadilla G, Ferrer E, de Antonio M, Perera‐Lluna A, Lopez‐Ayerbe J, Domingo M, Nunez J, Zamora E, Moliner P, Diaz‐Ruata P, Santesmases J, Bayes‐Genis A. Dynamic trajectories of left ventricular ejection fraction in heart failure. J Am Coll Cardiol 2018; 72: 591–601. [DOI] [PubMed] [Google Scholar]
- 25. Castrichini M, Manca P, Nuzzi V, Barbati G, De Luca A, Korcova R, Stolfo D, Lenarda AD, Merlo M, Sinagra G. Sacubitril/valsartan induces global cardiac reverse remodeling in long‐lasting heart failure with reduced ejection fraction: standard and advanced echocardiographic evidences. J Clin Med 2020; 9: 906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balmforth C, Simpson J, Shen L, Jhund PS, Lefkowitz M, Rizkala AR, Rouleau JL, Shi V, Solomon SD, Swedberg K, Zile MR, Packer M, McMurray JJV. Outcomes and effect of treatment according to etiology in HFrEF: an analysis of PARADIGM‐HF. JACC Heart Fail 2019; 7: 457–465. [DOI] [PubMed] [Google Scholar]
- 27. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E, Investigators P‐H. Angiotensin‐neprilysin inhibition in acute decompensated heart failure. N Engl J Med 2019; 380: 539–548. [DOI] [PubMed] [Google Scholar]
- 28. Pina IL, Camacho A, Ibrahim NE, Felker GM, Butler J, Maisel AS, Prescott MF, Williamson KM, Claggett BL, Desai AS, Solomon SD, Januzzi JL, Investigators P‐H. Improvement of health status following initiation of sacubitril/valsartan in heart failure and reduced ejection fraction. JACC Heart Fail 2021; 9: 42–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Subgroup analysis for LV reverse remodelling within 6‐month of treatment.
Table S1. Propensity score matching for total study population.
Table S2. Propensity score matching for patients with follow‐up echocardiography within 6‐month.
Table S3. Propensity score matching for patients with follow‐up echocardiography within 12‐month.
Table S4. Achievement of LV reverse remodelling after sacubitril/valsartan treatment initiation.
Table S5. Univariable predictors for achievement of LV reverse remodelling.
Table S6. Univariable predictors of cardiovascular mortality and HHF.


