Abstract
The purpose of this review is to explore how metabolomics can help uncover new biomarkers and mechanisms for cardiovascular ageing. Cardiovascular ageing refers to cardiovascular structural and functional alterations that occur with chronological ageing and that can lead to the development of cardiovascular disease. These alterations, which were previously only detectable on tissue histology or corroborated on blood samples, are now detectable with modern imaging techniques. Despite the emergence of powerful new imaging tools, clinical investigation into cardiovascular ageing is challenging because ageing is a life course phenomenon involving known and unknown risk factors that play out in a dynamic fashion. Metabolomic profiling measures large numbers of metabolites with diverse chemical properties. Metabolomics has the potential to capture changes in biochemistry brought about by pathophysiologic processes as well as by normal ageing. When combined with non‐invasive cardiovascular imaging tools, metabolomics can be used to understand pathological consequences of cardiovascular ageing. This review will summarize previous metabolomics and imaging studies in cardiovascular ageing. These methods may be a clinically relevant and novel approach to identify mechanisms of cardiovascular ageing and formulate or personalize treatment strategies.
Keywords: Ageing, Cardiovascular disease, Metabolomics, Cardiovascular imaging
Introduction
Metabolomics and cardiovascular imaging have been used as a strategy to study various disease states. However, combining metabolomics and imaging to better understand cardiovascular ageing is still in its infancy, and there are minimal published data. The purpose of this review is to consider the clinical manifestations and pathologic mechanisms for cardiovascular ageing and explore how metabolomics and cardiovascular imaging can help uncover new biomarkers and mechanisms for cardiovascular ageing.
Metabolomics
Metabolomic profiling is a systems biology tool that measures large numbers of metabolites with diverse chemical properties. The metabolome differs from the genome/transcriptome/proteome because its net output is influenced by genomic, transcriptomic, and proteomic variability. Metabolomics thus provides an integrated profile of an individual's biological status: ‘the genome defines what may happen, the metabolome defines what has happened’. 1 Metabolomics profiles are also influenced by environmental exposure. 2 , 3 , 4 , 5 , 6 , 7 , 8 There are two main analytical approaches used in metabolomics research: targeted and untargeted. Untargeted approaches involve comprehensive analysis of all the measurable analytes in a sample. The advantage to untargeted approaches is broad coverage of potentially important analytes and/or unbiased detection of biomarkers. The disadvantage to untargeted approaches includes a workflow that makes analysing large sample sets difficult, relative quantitation of compounds, a bias towards identifying compounds with high abundance, and frequent inability to identify peaks of interest. Targeted approaches involve measuring pre‐defined metabolites. Advantages to targeted approaches include use of internal standards that allows identification and absolute quantitation of analytes, including low abundance compounds as well as relatively fast workflow. The disadvantage of targeted metabolomics is that clinically important analytes can be overlooked. Metabolomics techniques have been applied to the study of human disease with novel findings that have helped our understanding of insulin resistance and diabetes, 9 , 10 chronic kidney disease, 11 , 12 depression, 13 , 14 and cardiovascular disease (CVD). 15 , 16 , 17 , 18 , 19 , 20 , 21
Cardiovascular ageing
Description
By the year 2030, approximately 20% of the world population will be aged 65 years or older. 22 CVD is a leading cause of death in older adults, 23 which underscores the importance of gaining a better understanding of the risk factors for CVD in older patients. The ageing process can lead to pathologic effects on the cardiovascular system, which can contribute to CVD. 23 Cardiovascular ageing refers to cardiovascular structural and functional alterations that may occur with chronological ageing and that can lead to the development of CVD. A prominent age‐related change is increased stiffness of central arteries referred to as vascular ageing. 24 This results from loss of elastic fibres and increase in collagen in the large elastic arteries. 25 Arterial stiffness exposes the endothelium to greater haemodynamic loading, thus promoting endothelial activation, inflammation, and damage. 26 Increased arterial stiffness can affect ventricular relaxation by altering afterload. 27 , 28 Altered relaxation is associated with cellular hyperplasia and fibrosis that can ultimately worsen left ventricular diastolic function. 29 Impairments in left ventricular relaxation cause the left atrium to compensate with increases in left atrial pressure to fill the left ventricle. 30 , 31 Elevated left atrial pressure results in left atrial enlargement secondary to pressure and volume overload. 31 With progressive worsening of diastolic function, left atrial volume increases, giving rise to complications including atrial fibrillation and stroke. 32 , 33 Large‐scale cohort studies have found increased incidences of chronic heart failure, atrial fibrillation, and left ventricular hypertrophy among ageing older adults. 33 , 34 , 35 , 36 , 37 , 38 , 39 Several mechanisms have been proposed to be involved in the pathogenesis of cardiovascular ageing including inflammation, redox stress, and endothelial dysfunction.
Inflammation
Inflammation is a well‐established driver of ageing and ageing‐related diseases. 40 , 41 The inflammatory process evolved as an immunologic defence system. 42 Acute inflammation acts as a response to noxious agents such as pathogens, allergens, and toxic substances. 42 The response includes activation of immune cells to eliminate pathogens and tissue remodelling processes to repair damage. However, when the acute inflammatory response fails to resolve, more components are activated to generate a continued immune activation that leads to longer‐term chronic inflammation. 43 , 44 , 45 Ageing is linked to dysregulation of the immune response with release of inflammatory mediators and cytokines, giving rise to a concept of age‐related chronic inflammation known as ‘senescent inflammation’. 46 , 47 , 48 This chronic low‐grade inflammatory state is characterized by increased levels of circulating cytokines such as interleukin‐1, interleukin‐6, and tumour necrosis factor. 47 , 48 In addition, age‐related changes in adipose tissue content and function enhance release of pro‐inflammatory cytokines. 49 , 50 Inflammation is associated with changes in metabolism pathways including fatty‐acid‐derived lipid signalling molecules. 51 , 52 Dietary and gut‐microbiome‐derived metabolites have also been implicated in human chronic inflammatory diseases. 53 These processes promote recruitment of macrophages and T cells into tissues like myocardium and vascular walls, altering vascular structure and function, leading to arterial stiffening, atherosclerosis, and hypertension. 54 Cardiac myofibroblasts respond to cytokines with resulting changes in cell proliferation, increased expression, and activity of extracellular matrix degrading metalloproteinases. 55 , 56 Over time, these lead to fibrotic cardiac remodelling and myocardial dysfunction. 57 Furthermore, cardiac fibrosis and modification contribute to increased vulnerability to cardiac arrhythmias. 58
Redox stress
The ageing heart shows changes in mitochondrial function, particularly in electron transport chain activity. 59 , 60 Mitochondrial oxidative stress is a molecular hallmark of cardiovascular ageing. Overproduction of reactive oxygen species (ROS) in the mitochondrial electron transport chain leads to formation of highly reactive products such as peroxide that promotes cellular damage including DNA mutations. Redox stress can cause inflammation and activation of cell death pathways and may eventually contribute to cellular senescence. 61 , 62 A rodent model of ageing has shown increased vascular endothelium redox stress in older animals. In this study, elevated levels of a major class of systemic bioactive lipids known as lysophosphatidylcholines (LPCs) contribute to the build‐up of redox stress. 63 LPCs stimulate oxidative stress through interaction with overactive 5‐lipoxygenase pathways in ageing endothelial cells. LPCs can also stimulate ROS production in human monocyte‐derived macrophages found in atherosclerotic arterial walls. 64 These ROS‐stimulated macrophages go on to activate expression of urokinase‐type plasminogen activator and its cell surface receptor. These observations at the cellular level may account for clinical findings. Levels of soluble urokinase‐type plasminogen activator receptor have been useful for predicting risks of myocardial dysfunction in aged adults. 65 LPC has also been shown to be predictive of ageing phenotypes such as cognitive impairment and gait speed in older adults. 66 , 67
Endothelial function
Ageing alters the endothelium and leads to reductions in vasodilatory and antithrombotic properties while also promoting atherogenesis and thrombosis. 68 , 69 The vascular changes that occur with ageing result in large artery thickening and stiffness in otherwise healthy older persons who are prone to increases in systolic and pulse pressures. These functional changes in vessel haemodynamics precede clinical disease and are associated with higher risks for developing atherosclerosis, hypertension, and stroke. 23 , 70 , 71 , 72 Brachial artery flow‐mediated dilation is a standard method for assessing endothelial function in a clinical research setting. Nitric oxide supplementation has been shown to improve brachial artery flow‐mediated dilation. 73 , 74 , 75 Among middle‐aged and older adults, a 10 week trial of sodium nitrite supplementation improved endothelial function and carotid artery elasticity. 73 Metabolites such as glycerophospholipids and fatty acyls predicted improved vascular function with nitrite. 76 Interestingly, improved endothelial function occurred independently of well‐known risk factors such as blood lipids, glucose, blood pressure, and body mass. These findings suggest that circulating metabolites may be helpful in gaining mechanistic insight into therapies that target endothelial function. 76 , 77 , 78
Dietary supplements have been shown to alter markers of redox stress, improve endothelial function, and reduce risks of chronic disease and premature ageing. 79 , 80 Circulating metabolites derived from dietary polyphenols (such as blueberries) have been linked to more robust vascular flow‐mediated dilatation, attenuated lipotoxicity‐induced endothelial dysfunction, and may complement therapies to reduce vascular complications. 81 , 82 All these suggest an important role for antioxidant and antioxidant defence for proper maintenance of endothelial cell function.
These mechanistic and interventional studies have contributed to our understanding of the pathogenesis of cardiovascular ageing. Circulating metabolites have been figured prominently in many of these studies, highlighting the potential role for metabolomics in elucidating the underlying mechanisms through which ageing exerts pathological changes in the cardiovascular system.
Metabolism, ageing, and the heart
Mitochondrial function is altered in the ageing heart. 59 , 60 Changes in mitochondrial function have a knock‐on effect on central carbon and related pathways. Fatty acid oxidation and ketone use declines with ageing in mouse hearts. 83 , 84 Reprogramming of cardiac metabolism away from fatty acid oxidation is a prominent feature of heart failure 85 , 86 and may partly explain changes in heart function with ageing. The sphingolipids are a major class of lipids that play an important role in tissue signalling and are active players in diseases such as insulin resistance 87 and CVD. 88 Sphingolipid physiology changes with ageing 89 and may contribute to ageing‐related decline in heart function. Declining mitochondrial function with ageing has been linked to redox state, nicotinamide adenine dinucleotide levels, and the activity of nicotinamide adenine dinucleotide‐dependent deacylases known as the sirtuins. 90 The sirtuins can be activated by caloric restriction, a well‐described and potent stimulus for longevity in animal models, 91 as well as resveratrol, a component of red wine. 92 Sirtuins can modulate tissue metabolic activity 93 and have been linked directly to CVD. 94 Alpha‐ketoglutarate is an important central carbon metabolite and intermediate in the tricarboxylic acid cycle. Alpha‐ketoglutarate has been shown to extend lifespan in worms, 95 flies, 96 and mice 97 likely through interactions with mammalian target of rapamycin and adenosine monophosphate‐activated protein kinase pathways. The related tricarboxylic acid cycle intermediate succinate has been shown to accumulate during ischaemia and contributes to ROS production and damage during reperfusions. 98
Metabolomics and cardiovascular ageing
Challenges
Many biomarkers are in use for detecting risk of CVD as well as for monitoring response to therapy. In contrast, few biomarkers have been identified for evaluating cardiovascular ageing. Because metabolomics provides an integrated profile of an individual's biological status, it may serve as a useful tool to help us understand the complexities that surround the mechanisms of pathologic ageing. The challenge going forward will be to design methods and studies to help identify new biomarkers that can predict risk of disease and/or follow disease progression.
Metabolomics and environmental exposure
Because ageing is a life course phenomenon, environmental exposure is an important consideration. Dynamic lifestyle factors such as physical activity, 99 alcohol use, and food intake can alter the course of physical ageing. 100 , 101 Dietary assessment in research studies is challenging. Dietary exposures are hard to measure, and established methods such as dietary recall and food frequency questionnaires have their limitations. Similar challenges exist for quantifying other important environmental exposures including lifetime exercise patterns, bouts of acute and chronic illness, medication and other drug use, and exposure to toxins from air pollution. Given that these dynamic factors all converge upon the human metabolome, metabolomics might provide a comprehensive and integrated picture of these lifelong environmental exposures. An example of how metabolomics can detect differences in environmental exposure comes from studies of host–gut microbiome interactions. The INTERMAP study performed nuclear magnetic resonance‐based urinary metabolome analyses among African–American compared with non‐Hispanic white Americans, where they found higher blood pressure levels among African–American men and women. 102 They found significant differences in metabolites between the two groups that were related to differing food intakes between the groups, as well as differences in gut microbiota. Another series of studies identified trimethylamine N‐oxide, a diet‐derived gut microbial metabolite, as an environment‐linked factor associated with increased cardiovascular and mortality risk. 103 , 104 , 105 Another lifestyle exposure such as exercise may also alter metabolomics profiles. 106 , 107 Exercise training may produce widespread changes in energy metabolism, owing to increases in lipolysis, fatty acid oxidation, or ketogenesis. 106 Differences in exercise intensity may also result in different profiles. Under moderate‐intensity exercise, for example, medium chain acylcarnitines appear to dominate, to support fat oxidation. 108
These studies highlight the importance of considering environmental exposure such as diet on cardiovascular health and strengthen the evidence base for applying metabolomics profiling onto life course exposures such as ageing.
Metabolomics and cardiovascular disease in the elderly
While there are community‐based studies that have looked at the association between metabolomics signatures, CVD, and cardiovascular function, 109 , 110 few have studied the elderly. A study by Rizza et al. 111 looked at a high‐risk cohort of elderly subjects in which over half of the participants had documented coronary artery disease or stroke. Rizza et al. found a distinct signature comprising medium‐chain and long‐chain acylcarnitines that predicted major adverse cardiac events in this high‐risk elderly population. Because these acylcarnitines are derived from fatty acid oxidation, 112 this finding suggests that mitochondrial beta‐oxidation pathways are linked to increased cardiovascular risk. In an interventional study that investigated the effect of Mediterranean diet on incident CVD, participants with higher baseline concentrations of short‐chain, medium‐chain, and long‐chain acylcarnitines had higher risk of CVD and stroke. 113 In both of these studies, metabolomics has been able to uncover new associations between disease and altered fuel metabolism pathways. The studies also show how metabolomics can be complementary to other omics technologies that have helped to unravel disease mechanisms in complex phenotypes such as heart failure, yielding biomarkers for diagnosis, prognosis, or identifying new therapeutic targets. 114
Table 1 contains a summary of human studies (selected for older age groups) that have used metabolomics to study CVD among older adults.
Table 1.
Summary selection of human studies on metabolomics and cardiovascular disease endpoints among older adults
| a Human studies, publication year | Study population | Results | Inferences | Details/limitations |
|---|---|---|---|---|
| Rizza et al., 2014 111 |
N = 67 Mean age 85 ± 3 years High rate of prior CVD (85%) |
Medium‐chain and long‐chain acylcarnitines were associated with major adverse cardiac events (MACE) |
Ageing mitochondrial dysfunction associated with MACE |
Small sample size; high‐risk cohort |
| Ganna et al., 2014 115 |
N = 1028 Average age 70 years |
Lipid‐related metabolites lysophosphatidylcholine, monoglyceride, and sphingomyelin were associated with incident coronary heart disease over 3.9 to 10 years of median follow‐up |
Potential causal role in coronary heart disease development |
Population‐based, longitudinal cohorts; integrated genetic and metabolomic analyses |
| Cheng et al., 2015 116 |
N = 515 Average age 55 to 64 years across groups |
Metabolite panel consisting of methylarginine/arginine ratio, butyrylcarnitine, spermidine, total essential amino acids, and prognosticated endpoints of death or heart failure‐related hospitalization over 6 and 12 months |
Metabolite panel provided better prognostic value over B‐type natriuretic peptide | Targeted metabolomics; participants were in heart failure stages A, B, and C |
| Zordoky et al., 2015 19 |
Total N = 82 Heart failure with preserved ejection fraction (N = 24) Heart failure with reduced ejection fraction (N = 20) Age‐matched controls (N = 38) Average age 61 to 67 years across groups |
Short‐chain acylcarnitines were higher in both HFpEF and HFrEF than in controls Medium‐chain and long‐chain acylcarnitines were higher in HFpEF than both HFrEF and controls |
Metabolomics fingerprint of HFpEF is distinct from that of HFrEF and controls | Small sample size; 181 metabolites; other heterogeneous factors involved such as background coronary artery disease and medication usage |
| Hunter et al., 2016 16 |
CATHGEN study of sequential patients who underwent cardiac catheterization Comparison between HFpEF cases (N = 282) and HFrEF (N = 279) and controls (N = 191) Average age 55 to 66 years across groups |
Long‐chain acylcarnitines were higher in HFrEF than HFpEF, increasing linearly with declining ejection fraction |
Possible shared mechanism in HF regardless of ejection fraction | Replication cohort data unavailable; cardiac catheterization cohort could have over‐represented ischaemic phenotypes; clinically obtained data; targeted metabolite profiling |
| Ahmad et al., 2016 15 |
N = 453 chronic systolic heart failure patients (HF‐ACTION cohort) Median age 59 years |
Long‐chain acylcarnitines were associated with increased risk of all‐cause mortality, all‐cause hospitalization, cardiovascular death, and cardiovascular hospitalization |
Greater circulating levels of long‐chain acylcarnitines predicted functional status and mortality in patients with chronic systolic HF |
Subset study from HF‐ACTION cohort |
| Bedi Jr et al., 2016 117 |
N = 15 patients with chronic dilated nonischaemic cardiomyopathy N = 20 controls Transmural sampling of the left ventricular myocardium obtained during left ventricular assist device implantation or heart transplantation |
Increased abundance of ketogenic β‐hydroxybutyryl‐CoA, decreased levels of myocardial β‐OH‐butyrate, increased circulating levels of ketones | Increased ketone utilization in the end‐stage failing heart | End‐stage heart failure; male gender predominance in the failing heart group |
| Wang et al., 2017 118 |
PREDIMED trial N = 230 incident CVD cases N = 787 random participants Patients were randomized to Mediterranean diets or control diet Average age 67 to 69 years across groups |
Plasma ceramide concentrations associated with elevated risk of composite CVD outcome (acute myocardial infarction, stroke, and cardiovascular death) | Mediterranean diet may have the potential to mitigate detrimental effect associated with elevated baseline plasma ceramide concentrations on CVD risk | Participants were European Caucasians, limits generalizability to other populations; high CVD risk profiles |
| Menni et al., 2018 119 |
N = 617 middle‐aged women Average age 61 years TwinsUK cohort |
Pulse wave velocity correlated negatively with gut microbiome alpha diversity, adjusted for levels of gut‐derived metabolites (indolepropionate, trimethylamine oxide, and phenylacetylglutamine) | Gut microbiome diversity is inversely associated with arterial stiffness in women, only minimally mediated by metabolic syndrome |
Analyses limited to middle‐aged white female twins; faecal sampling not necessarily taken at time of arterial stiffness assessment |
CVD, cardiovascular disease; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Studies selected based on human studies, older age groups, and cardiovascular endpoints/surrogate endpoints.
Imaging as a tool to detect and manage cardiovascular ageing
Modern cardiovascular imaging has evolved to detect underlying disease that was previously only detectable on tissue histology or corroborated on blood samples. Disease processes commonly involved in ageing, such as inflammation, may be characterized by cardiovascular imaging (Figure 1 ). For example, in the setting of acute myocardial infarction, imaging of the myocardium via absolute native T1 values on magnetic resonance or fluorodeoxyglucose positron emission tomography correctly determined level of myocardial injury as well as systemic inflammation response. 120 Mitochondrial dysfunction has been implicated as a mechanism involved in cardiovascular ageing and can be assessed with imaging tools such as hyperpolarized carbon‐13 magnetic resonance spectroscopy in animals 121 , 122 and phosphorus‐31 spectroscopy in humans. 123 Endothelial dysfunction has traditionally been characterized by reactive hyperaemia‐peripheral arterial tonometry. 124 , 125 In recent times, coronary endothelial dysfunction, which leads to coronary microvascular dysfunction, has been quantitatively measured in humans by positron emission tomography. 126 , 127 , 128 Effects of environmental exposures such as smoking and infective agents on the cardiovascular system have also been imaged by cardiovascular imaging techniques. 129 , 130 , 131
Figure 1.
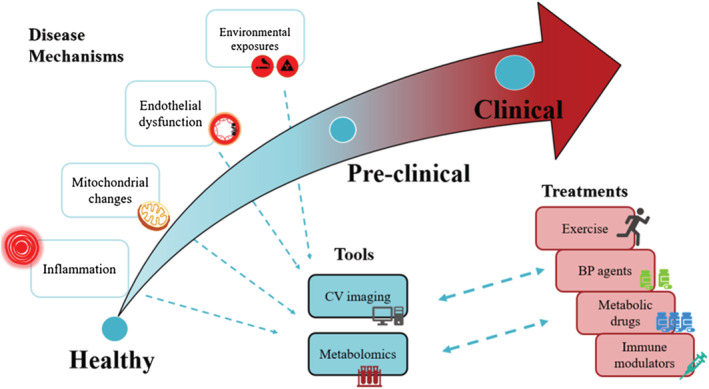
Cardiovascular ageing: metabolomics and associated approaches. The curved arrow depicts the course of cardiovascular (CV) ageing starting with normal health. Proposed underlying disease mechanisms are highlighted (left). As cardiovascular ageing progresses through the stages, tools such as CV imaging and metabolomics may be useful for detecting CV changes and disease mechanisms (unidirectional dashed arrows). As cardiovascular ageing becomes clinically apparent, insights from these changes and mechanisms may continue to assist in formulating and personalizing treatment strategies (bidirectional arrows). BP, blood pressure.
Quantifying subtle functional cardiovascular changes through imaging is important in the setting of preclinical disease and may be relevant in ageing. In a large community study from MESA (Multi‐Ethnic Study of Atherosclerosis), older age was associated with presence of myocardial fibrosis measured by T1 mapping on cardiac magnetic resonance imaging. 132 Cardiovascular imaging has also refined clinical phenotyping. Most cohorts define the presence of clinical disease solely by development of cardiovascular events such as acute myocardial infarction. This clinical event‐driven approach may underestimate actual disease among apparently healthy controls who may have substantial asymptomatic coronary atherosclerosis. 133 The use of non‐invasive imaging techniques to identify and better classify preclinical disease will enhance potential of novel omics technologies to discover early biomarkers.
Another key concept is that changes should occur as a result of ageing and be viewed distinctly from concomitant risk factors that frequently accompany ageing. Pre‐specified cohorts that study cardiovascular ageing independently of traditional risk factors might be necessary. Analysis of community and disease cohorts that include samples acquired prior to disease development may help to uncover novel biomarkers and pathways associated with ageing‐related diseases, CVD.
When used in conjunction with metabolomics techniques, non‐invasive cardiovascular imaging represents a way to understand pathological consequences of ageing‐related cardiovascular changes in preclinical cohorts. 134 , 135 In a community‐based study of older adults without clinical CVD, left atrial function assessed by cardiac magnetic resonance was used as a marker of cardiovascular ageing. 135 Left atrial function as represented by left atrial reservoir and conduit phases is known to decrease with age. 136 This study highlighted the impact of age on left atrial function, independent of traditional risk factors. Importantly, these specific left atrial functions were associated with a metabolic signature comprising medium‐chain and long‐chain dicarboxyl carnitines, serine, citrulline, and valine molecules, 135 highlighting the potential role of mitochondrial fuel metabolism on pathogenesis of atrial ageing. In another similar analysis, an imaging marker of arterial stiffness, known as pulse wave velocity assessed by applanation tonometry, was independently associated with a similar signature of medium‐chain and long‐chain dicarboxyl carnitines, independent of blood pressure. 137 These results demonstrate how preclinical imaging using established and new imaging markers, when used in conjunction with metabolomics, may be a clinically relevant and novel approach to help identify mechanisms of cardiovascular ageing. In a study that integrated whole‐genome sequencing, comprehensive metabolomics, and advanced human body imaging (by echocardiography, electrocardiography, computed tomography, and magnetic resonance imaging), genomics and metabolomics association analysis identified over 5% of heterozygotes with phenotypic manifestations affecting serum metabolite levels. 138 In the near future, precision medicine strategies may require integrated methodologies to identify and predict risk of disease prior to disease manifestation.
Risk stratification for cardiovascular ageing
For metabolomics approaches to be clinically relevant for future applications in cardiovascular ageing, we must move beyond mere cross‐sectional type analyses. Examples of how to proceed can be found in the literature. In the area of cardiovascular risk stratification, metabolomics has complemented well‐established risk scoring systems to provide finer cardiovascular risk stratification, 139 efficiently predicting CVD event risk in CVD cohorts, such as patients with coronary artery disease. 88 , 140 In clinical trials that target established risk factors such as hypertension and dyslipidaemia, metabolomics has been used before and after intervention to study the effects of salt‐lowering 141 and novel statin therapies. 142 Lifestyle factors such as diet factors have also used metabolomics to quantify effect of diet interventions on CVD incidence. 143 , 144 Physical activity, a key lifestyle factor that influences cardiovascular health, has also been shown to be associated with wide spectrum of acylcarnitines and amino acids. 145 These studies point to the role that metabolomics can play in a range of clinical settings, from observational studies, to risk stratification, to clinical trial interventions, to lifestyle‐type evaluations. All of these settings are pertinent to the entire life course phenomenon of ageing. What remains to be done is for more similar type study designs to be applied onto ageing cohorts, deeply focused on measuring age as a key exposure of interest, and intervening on specific mechanisms of cardiovascular ageing.
In summary, as cardiovascular ageing progresses, tools such as cardiovascular imaging and biological tools such as metabolomics may be useful for detecting early changes and also study mechanisms of progression. As cardiovascular ageing becomes clinically apparent, insights from these mechanisms may assist in formulating and/or personalizing treatment strategies (Figure 1 ).
Conclusions and implications
Cardiovascular ageing is a pathologic process that likely involves inflammation, redox stress, and endothelial dysfunction as well as other undefined mechanisms. Metabolomics has the potential to be a powerful tool to help unravel mechanisms of cardiovascular ageing and to identify new biomarkers that predict risk and/or monitor disease progression. Reaching these milestones will require more large‐scale studies with robust cross‐validation across cohorts.
Conflict of interest
None declared.
Funding
A.K. received grant support from the National Medical Research Council of Singapore (NMRC/TA/0031/2015, MOH‐000153), Hong Leong Foundation, Duke‐NUS Medical School, Estate of Tan Sri Khoo Teck Puat, and SingHealth Foundation.
Author contributions
A.K. and J.P.K.: conceptualization and methodology. A.K. and J.P.K.: writing—original draft preparation and writing—reviewing and editing.
Impact statement
Cardiovascular ageing is a life course phenomenon that involves known and unknown risk factors that play out in a dynamic fashion. As metabolomics provides an integrated profile of biological ageing that sums up complexities of cardiovascular ageing, metabolomics may represent a novel way to detect early changes as detected by cardiovascular imaging. A combined approach involving both metabolomics and cardiovascular imaging would expand mechanistic understanding about progressive cardiovascular ageing and provide targeted treatment strategies.
Acknowledgement
We thank Dr Yen How Tan for his artistic contributions.
Koh, A. S. , and Kovalik, J.‐P. (2021) Metabolomics and cardiovascular imaging: a combined approach for cardiovascular ageing. ESC Heart Failure, 8: 1738–1750. 10.1002/ehf2.13274
References
- 1. Dunn WB, Goodacre R, Neyses L, Mamas M. Integration of metabolomics in heart disease and diabetes research: current achievements and future outlook. Bioanalysis 2011; 3: 2205–2222. [DOI] [PubMed] [Google Scholar]
- 2. Cheng S, Shah SH, Corwin EJ, Fiehn O, Fitzgerald RL, Gerszten RE, Illig T, Rhee EP, Srinivas PR, Wang TJ, Jain M. Potential impact and study considerations of metabolomics in cardiovascular health and disease: a scientific statement from the American Heart Association. Circ Cardiovasc Genet 2017; 10: e000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rebholz CM, Surapaneni A, Levey AS, Sarnak MJ, Inker LA, Appel LJ, Coresh J, Grams ME. The serum metabolome identifies biomarkers of dietary acid load in 2 studies of adults with chronic kidney disease. J Nutr 2019; 149: 578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Everson TM, Marsit CJ. Integrating‐omics approaches into human population‐based studies of prenatal and early‐life exposures. Curr Environ Health Rep 2018; 5: 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang Y, Hui Q, Walker DI, Uppal K, Goldberg J, Jones DP, Vaccarino V, Sun YV. Untargeted metabolomics reveals multiple metabolites influencing smoking‐related DNA methylation. Epigenomics 2018; 10: 379–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ward‐Caviness CK, Breitner S, Wolf K, Cyrys J, Kastenmüller G, Wang‐Sattler R, Schneider A, Peters A. Short‐term NO2 exposure is associated with long‐chain fatty acids in prospective cohorts from Augsburg, Germany: results from an analysis of 138 metabolites and three exposures. Int J Epidemiol 2016; 45: 1528–1538. [DOI] [PubMed] [Google Scholar]
- 7. Townsend MK, Aschard H, De Vivo I, Michels KB, Kraft P. Genomics, telomere length, epigenetics, and metabolomics in the nurses' health studies. Am J Public Health 2016; 106: 1663–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cruickshank‐Quinn CI, Mahaffey S, Justice MJ, Hughes G, Armstrong M, Bowler RP, Reisdorph R, Petrache I, Reisdorph N. Transient and persistent metabolomic changes in plasma following chronic cigarette smoke exposure in a mouse model. PLoS One 2014; 9: e101855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched‐chain amino acid‐related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009; 9: 311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O'Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med 2011; 17: 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharma K, Karl B, Mathew AV, Gangoiti JA, Wassel CL, Saito R, Pu M, Sharma S, You YH, Wang L, Diamond‐Stanic M, Lindenmeyer MT, Forsblom C, Wu W, Ix JH, Ideker T, Kopp JB, Nigam SK, Cohen CD, Groop PH, Barshop BA, Natarajan L, Nyhan WL, Naviaux RK. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol 2013; 24: 1901–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu JJ, Ghosh S, Kovalik JP, Ching J, Choi HW, Tavintharan S, Ong CN, Sum CF, Summers SA, Tai ES, Lim SC. Profiling of plasma metabolites suggests altered mitochondrial fuel usage and remodeling of sphingolipid metabolism in individuals with type 2 diabetes and kidney disease. Kidney Int Rep 2016; 2: 470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shih PB. Metabolomics biomarkers for precision psychiatry. Adv Exp Med Biol 2019; 1161: 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hashimoto K. Metabolomics of major depressive disorder and bipolar disorder: overview and future perspective. Adv Clin Chem 2018; 84: 81–99 Epub;2018 Feb 1.:81‐99. [DOI] [PubMed] [Google Scholar]
- 15. Ahmad T, Kelly JP, McGarrah RW, Hellkamp AS, Fiuzat M, Testani JM, Wang TS, Verma A, Samsky MD, Donahue MP, Ilkayeva OR. Prognostic implications of long‐chain acylcarnitines in heart failure and reversibility with mechanical circulatory support. J Am Coll Cardiol 2016; 67: 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hunter WG, Kelly JP, McGarrah RW III, Khouri MG, Craig D, Haynes C, Ilkayeva O, Stevens RD, Bain JR, Muehlbauer MJ, Newgard CB. Metabolomic profiling identifies novel circulating biomarkers of mitochondrial dysfunction differentially elevated in heart failure with preserved versus reduced ejection fraction: evidence for shared metabolic impairments in clinical heart failure. J Am Heart Assoc 2016; 5: e003190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shah SH, Sun JL, Stevens RD, Bain JR, Muehlbauer MJ, Pieper KS, Haynes C, Hauser ER, Kraus WE, Granger CB, Newgard CB, Califf RM, Newby LK. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am Heart J 2012; 163: 844–850. [DOI] [PubMed] [Google Scholar]
- 18. Shah SH, Kraus WE, Newgard CB. Metabolomic profiling for the identification of novel biomarkers and mechanisms related to common cardiovascular diseases: form and function. Circulation 2012; 126: 1110–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zordoky BN, Sung MM, Ezekowitz J, Mandal R, Han B, Bjorndahl TC, Bouatra S, Anderson T, Oudit GY, Wishart DS, Dyck JRB, Alberta HEART . Metabolomic fingerprint of heart failure with preserved ejection fraction. PLoS One 2015; 10: e0124844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tang WW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013; 368: 1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ussher JR, Elmariah S, Gerszten RE, Dyck JR. The emerging role of metabolomics in the diagnosis and prognosis of cardiovascular disease. J Am Coll Cardiol 2016; 68: 2850–2870. [DOI] [PubMed] [Google Scholar]
- 22. Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd‐Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ, American Heart Association Advocacy Coordinating Committee , Stroke Council , Council on Cardiovascular Radiology and Intervention , Council on Clinical Cardiology , Council on Epidemiology and Prevention , Council on Arteriosclerosis , Thrombosis and Vascular Biology , Council on Cardiopulmonary , Critical Care , Perioperative and Resuscitation , Council on Cardiovascular Nursing , Council on the Kidney in Cardiovascular Disease , Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research . Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 2011; 123: 933–944. [DOI] [PubMed] [Google Scholar]
- 23. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation 2003; 107: 139–146. [DOI] [PubMed] [Google Scholar]
- 24. Meyer ML, Tanaka H, Palta P, Cheng S, Gouskova N, Aguilar D, Heiss G. Correlates of segmental pulse wave velocity in older adults: the Atherosclerosis Risk in Communities (ARIC) study. Am J Hypertens 2016; 29: 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fritze O, Romero B, Schleicher M, Jacob MP, Oh DY, Starcher B, Schenke‐Layland K, Bujan J, Stock UA. Age‐related changes in the elastic tissue of the human aorta. J Vasc Res 2012; 49: 77–86. [DOI] [PubMed] [Google Scholar]
- 26. Walker AE, Henson GD, Reihl KD, Morgan RG, Dobson PS, Nielson EI, Ling J, Mecham RP, Li DY, Lesniewski LA, Donato AJ. Greater impairments in cerebral artery compared with skeletal muscle feed artery endothelial function in a mouse model of increased large artery stiffness. J Physiol 2015; 593: 1931–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Choong CY, Abascal VM, Thomas JD, Guerrero JL, McGlew S, Weyman AE. Combined influence of ventricular loading and relaxation on the transmitral flow velocity profile in dogs measured by Doppler echocardiography. Circulation 1988; 78: 672–683. [DOI] [PubMed] [Google Scholar]
- 28. Urthaler F, Walker AA, James TN. The effect of aging on ventricular contractile performance. Am Heart J 1978; 96: 481–485. [DOI] [PubMed] [Google Scholar]
- 29. Anversa P, Palackal T, Sonnenblick EH, Olivetti G, Meggs LG, Capasso JM. Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circ Res 1990; 67: 871–885. [DOI] [PubMed] [Google Scholar]
- 30. Triposkiadis F, Tentolouris K, Androulakis A, Trikas A, Toutouzas K, Kyriakidis M, Gialafos J, Toutouzas P. Left atrial mechanical function in the healthy elderly: new insights from a combined assessment of changes in atrial volume and transmitral flow velocity. J Am Soc Echocardiogr 1995; 8: 801–809. [DOI] [PubMed] [Google Scholar]
- 31. Leung DY, Boyd A, Ng AA, Chi C, Thomas L. Echocardiographic evaluation of left atrial size and function: current understanding, pathophysiologic correlates, and prognostic implications. Am Heart J 2008; 156: 1056–1064. [DOI] [PubMed] [Google Scholar]
- 32. Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol 2002; 90: 1284–1289. [DOI] [PubMed] [Google Scholar]
- 33. Morley JE, Reese SS. Clinical implications of the aging heart. Am J Med 1989; 86: 77–86. [DOI] [PubMed] [Google Scholar]
- 34. Strait JB, Lakatta EG. Aging‐associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin 2012; 8: 143–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lakatta EG. Changes in cardiovascular function with aging. Eur Heart J 1990: C:22–9–22–9. [DOI] [PubMed] [Google Scholar]
- 36. Lakatta EG. Age‐associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail Rev 2002; 7: 29–49. [DOI] [PubMed] [Google Scholar]
- 37. Harvey A, Montezano AC, Touyz RM. Vascular biology of ageing—implications in hypertension. J Mol Cell Cardiol 2015; 83: 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chantler PD, Lakatta EG. Arterial‐ventricular coupling with aging and disease. Front Physiol 2012; 3: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet 2014; 383: 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss‐Coray T, Sierra F. Geroscience: linking aging to chronic disease. Cell 2014; 159: 709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton‐Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, Pahor M. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation 2003; 108: 2317–2322. [DOI] [PubMed] [Google Scholar]
- 42. Freire MO, van Dyke TE. Natural resolution of inflammation. Periodontol 2000 2013; 63: 149–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kwon HJ, Sung BK, Kim JW, Lee JH, Kim ND, Yoo MA, Kang HS, Baek HS, Bae SJ, Choi JS, Takahashi R, Goto S, Chung HY. The effect of lipopolysaccharide on enhanced inflammatory process with age: modulation of NF‐κB. J Am Aging Assoc 2001; 24: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jorgensen T, Pedersen BK. Predicting death from tumour necrosis factor‐alpha and interleukin‐6 in 80‐year‐old people. Clin Exp Immunol 2003; 132: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gordon CJ, Rowsey PJ, Bishop BL, Ward WO, Macphail RC. Serum biomarkers of aging in the Brown Norway rat. Exp Gerontol 2011; 46: 953–957. [DOI] [PubMed] [Google Scholar]
- 46. Chung HY, Kim DH, Lee EK, Chung KW, Chung S, Lee B, Seo AY, Chung JH, Jung YS, Im E, Lee J, Kim ND, Choi YJ, Im DS, Yu BP. Redefining chronic inflammation in aging and age‐related diseases: proposal of the senoinflammation concept. Aging Dis 2019; 10: 367–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Cesari M, Nourhashemi F. Proinflammatory cytokines, aging, and age‐related diseases. J Am Med Dir Assoc 2013; 14: 877–882. [DOI] [PubMed] [Google Scholar]
- 48. Alvarez‐Rodriguez L, Lopez‐Hoyos M, Munoz‐Cacho P, Martinez‐Taboada VM. Aging is associated with circulating cytokine dysregulation. Cell Immunol 2012; 273: 124–132. [DOI] [PubMed] [Google Scholar]
- 49. Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL. Fat tissue, aging, and cellular senescence. Aging Cell 2010; 9: 667–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nosalski R, Guzik TJ. Perivascular adipose tissue inflammation in vascular disease. Br J Pharmacol 2017; 174: 3496–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro‐resolving superfamily of mediators. J Clin Invest 2018; 128: 2657–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ishihara T, Yoshida M, Arita M. Omega‐3 fatty acid‐derived mediators that control inflammation and tissue homeostasis. Int Immunol 2019; 31: 559–567. [DOI] [PubMed] [Google Scholar]
- 53. Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “western‐lifestyle” inflammatory diseases. Immunity 2014; 40: 833–842. [DOI] [PubMed] [Google Scholar]
- 54. Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol 2009; 78: 539–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther 2009; 123: 255–278. [DOI] [PubMed] [Google Scholar]
- 56. Zhang J, Zhao X, Vatner DE, McNulty T, Bishop S, Sun Z, Shen YT, Chen L, Meininger GA, Vatner SF. Extracellular matrix disarray as a mechanism for greater abdominal versus thoracic aortic stiffness with aging in primates. Arterioscler Thromb Vasc Biol 2016; 36: 700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gullestad L, Ueland T, Vinge LE, Finsen A, Yndestad A, Aukrust P. Inflammatory cytokines in heart failure: mediators and markers. Cardiology 2012; 122: 23–35. [DOI] [PubMed] [Google Scholar]
- 58. Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol 2015; 12: 230–243. [DOI] [PubMed] [Google Scholar]
- 59. Lesnefsky EJ, Chen Q, Hoppel CL. Mitochondrial metabolism in aging heart. Circ Res 2016; 118: 1593–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tatarkova Z, Kuka S, Racay P, Lehotský J, Dobrota D, Mistuna D, Kaplán P. Effects of aging on activities of mitochondrial electron transport chain complexes and oxidative damage in rat heart. Physiol Res 2011; 60: 281–289. [DOI] [PubMed] [Google Scholar]
- 61. Camici GG, Savarese G, Akhmedov A, Luscher TF. Molecular mechanism of endothelial and vascular aging: implications for cardiovascular disease. Eur Heart J 2015; 36: 3392–3403. [DOI] [PubMed] [Google Scholar]
- 62. Kornfeld OS, Hwang S, Disatnik MH, Chen CH, Qvit N, Mochly‐Rosen D. Mitochondrial reactive oxygen species at the heart of the matter: new therapeutic approaches for cardiovascular diseases. Circ Res 2015; 116: 1783–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zou Y, Kim DH, Jung KJ, Heo HS, Kim CH, Baik HS, Yu BP, Yokozawa T, Chung HY. Lysophosphatidylcholine enhances oxidative stress via the 5‐lipoxygenase pathway in rat aorta during aging. Rejuvenation Res 2009; 12: 15–24. [DOI] [PubMed] [Google Scholar]
- 64. Oka H, Kugiyama K, Doi H, Matsumura T, Shibata H, Miles LA, Sugiyama S, Yasue H. Lysophosphatidylcholine induces urokinase‐type plasminogen activator and its receptor in human macrophages partly through redox‐sensitive pathway. Arterioscler Thromb Vasc Biol 2000; 20: 244–250. [DOI] [PubMed] [Google Scholar]
- 65. Koh AS, Velmurugan B, Gao F, Tan RS, Wong JI, Teo LLY, Keng BMH, Chua SJM, Yuan JM, Koh WP, Cheung C. Value of soluble Urokinase plasminogen activator receptor over age as a biomarker of impaired myocardial relaxation. BMC Geriatr 2017; 17: 275–0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mapstone M, Cheema AK, Fiandaca MS, Zhong X, Mhyre TR, MacArthur LH, Hall WJ, Fisher SG, Peterson DR, Haley JM, Nazar MD, Rich SA, Berlau DJ, Peltz CB, Tan MT, Kawas CH, Federoff HJ. Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med 2014; 20: 415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gonzalez‐Freire M, de Cabo R, Bernier M, Sollott SJ, Fabbri E, Navas P, Ferrucci L. Reconsidering the role of mitochondria in aging. J Gerontol A Biol Sci Med Sci 2015; 70: 1334–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lakatta EG, Wang M, Najjar SS. Arterial aging and subclinical arterial disease are fundamentally intertwined at macroscopic and molecular levels. Med Clin North Am 2009; 93: 583–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium‐dependent dilation and upregulation of nuclear factor‐κB. Circ Res 2007; 100: 1659–1666. [DOI] [PubMed] [Google Scholar]
- 70. Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, Levy D. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation 2001; 103: 1245–1249. [DOI] [PubMed] [Google Scholar]
- 71. Sagie A, Larson MG, Levy D. The natural history of borderline isolated systolic hypertension. N Engl J Med 1993; 329: 1912–1917. [DOI] [PubMed] [Google Scholar]
- 72. Sesso HD, Stampfer MJ, Rosner B, Hennekens CH, Gaziano JM, Manson JAE, Glynn RJ. Systolic and diastolic blood pressure, pulse pressure, and mean arterial pressure as predictors of cardiovascular disease risk in men. Hypertension 2000; 36: 801–807. [DOI] [PubMed] [Google Scholar]
- 73. DeVan AE, Johnson LC, Brooks FA, Evans TD, Justice JN, Cruickshank‐Quinn C, Reisdorph N, Bryan NS, McQueen MB, Santos‐Parker JR, Chonchol MB. Effects of sodium nitrite supplementation on vascular function and related small metabolite signatures in middle‐aged and older adults. J Appl Physiol (1985) 2016; 120: 416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double‐blind, placebo‐controlled study. Hypertension 2015; 65: 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rammos C, Hendgen‐Cotta UB, Sobierajski J, Bernard A, Kelm M, Rassaf T. Dietary nitrate reverses vascular dysfunction in older adults with moderately increased cardiovascular risk. J Am Coll Cardiol 2014; 63: 1584–1585. [DOI] [PubMed] [Google Scholar]
- 76. Basak T, Varshney S, Akhtar S, Sengupta S. Understanding different facets of cardiovascular diseases based on model systems to human studies: a proteomic and metabolomic perspective. J Proteomics 2015; 127: 50–60. [DOI] [PubMed] [Google Scholar]
- 77. Krauss RM, Zhu H, Kaddurah‐Daouk R. Pharmacometabolomics of statin response. Clin Pharmacol Ther 2013; 94: 562–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Patel S, Ahmed S. Emerging field of metabolomics: big promise for cancer biomarker identification and drug discovery. J Pharm Biomed Anal 2015; 107: 63–74. [DOI] [PubMed] [Google Scholar]
- 79. Alehagen U, Johansson P, Aaseth J, Alexander J, Surowiec I, Lundstedt‐Enkel K, Lundstedt T. Significant changes in metabolic profiles after intervention with selenium and coenzyme Q10 in an elderly population. Biomolecules 2019; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ames BN. Prolonging healthy aging: longevity vitamins and proteins. Proc Natl Acad Sci U S A 2018; 115: 10836–10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bharat D, Cavalcanti RRM, Petersen C, Begaye N, Cutler BR, Costa MMA, Ramos RKLG, Ferreira MR, Li Y, Bharath LP, Toolson E, Sebahar P, Looper RE, Jalili T, Rajasekaran NS, Jia Z, Symons JD, Anandh Babu PV. Blueberry metabolites attenuate lipotoxicity‐induced endothelial dysfunction. Mol Nutr Food Res 2018; 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. de Bruyne T, Steenput B, Roth L, de Meyer GR, Santos CND, Valentová K, Dambrova M, Hermans N. Dietary polyphenols targeting arterial stiffness: interplay of contributing mechanisms and gut microbiome‐related metabolism. Nutrients 2019; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Abu‐Erreish GM, Neely JR, Whitmer JT, Whitman V, Sanadi DR. Fatty acid oxidation by isolated perfused working hearts of aged rats. Am J Physiol 1977; 232: E258–E262. [DOI] [PubMed] [Google Scholar]
- 84. Hyyti OM, Ledee D, Ning XH, Ge M, Portman MA. Aging impairs myocardial fatty acid and ketone oxidation and modifies cardiac functional and metabolic responses to insulin in mice. Am J Physiol Heart Circ Physiol 2010; 299: H868–H875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lai L, Leone TC, Keller MP, Martin OJ, Broman AT, Nigro J, Kapoor K, Koves TR, Stevens R, Ilkayeva OR, Vega RB, Attie AD, Muoio DM, Kelly DP. Energy metabolic reprogramming in the hypertrophied and early stage failing heart: a multisystems approach. Circ Heart Fail 2014; 7: 1022–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Peterzan MA, Lygate CA, Neubauer S, Rider OJ. Metabolic remodeling in hypertrophied and failing myocardium: a review. Am J Physiol Heart Circ Physiol 2017; 313: H597–H616. [DOI] [PubMed] [Google Scholar]
- 87. Meikle PJ, Summers SA. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat Rev Endocrinol 2017; 13: 79–91. [DOI] [PubMed] [Google Scholar]
- 88. de Carvalho LP, Tan SH, Ow GS, Tang Z, Ching J, Kovalik JP, Poh SC, Chin CT, Richards AM, Martinez EC, Troughton RW, Fong AYY, Yan BP, Seneviratna A, Sorokin V, Summers SA, Kuznetsov VA, Chan MY. Plasma ceramides as prognostic biomarkers and their arterial and myocardial tissue correlates in acute myocardial infarction. JACC Basic Transl Sci 2018; 3: 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Trayssac M, Hannun YA, Obeid LM. Role of sphingolipids in senescence: implication in aging and age‐related diseases. J Clin Invest 2018; 128: 2702–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. van de Ven RAH, Santos D, Haigis MC. Mitochondrial sirtuins and molecular mechanisms of aging. Trends Mol Med 2017; 23: 320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009; 325: 201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003; 425: 191–196. [DOI] [PubMed] [Google Scholar]
- 93. Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese Jr RV, Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty‐acid oxidation by reversible enzyme deacetylation. Nature 2010; 464: 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Winnik S, Auwerx J, Sinclair DA, Matter CM. Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur Heart J 2015; 36: 3404–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chin RM, Fu X, Pai MY, Vergnes L, Hwang H, Deng G, Diep S, Lomenick B, Meli VS, Monsalve GC, Hu E, Whelan SA, Wang JX, Jung G, Solis GM, Fazlollahi F, Kaweeteerawat C, Quach A, Nili M, Krall AS, Godwin HA, Chang HR, Faull KF, Guo F, Jiang M, Trauger SA, Saghatelian A, Braas D, Christofk HR, Clarke CF, Teitell MA, Petrascheck M, Reue K, Jung ME, Frand AR, Huang J. The metabolite α‐ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature 2014; 510: 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Su Y, Wang T, Wu N, Li D, Fan X, Xu Z, Mishra SK, Yang M. Alpha‐ketoglutarate extends Drosophila lifespan by inhibiting mTOR and activating AMPK. Aging (Albany NY) 2019; 11: 4183–4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Shahmirzadi AA, Edgar D, Liao CY, Hsu YM, Lucanic M, Shahmirzadi AA, Wiley CD, Gan G, Kim DE, Kasler HG, Kuehnemann C. Alpha‐ketoglutarate, an endogenous metabolite, extends lifespan and compresses morbidity in aging mice. Cell Metab 2020; 32: 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cheng SM, Blume M, Vogel R, Hung C, Lee SG, Hung PP. Characterization of the simian adenovirus type 30 inverted terminal repeat. Gene 1989; 80: 381–384. [DOI] [PubMed] [Google Scholar]
- 99. Pang Y, Kartsonaki C, Du H, Millwood IY, Guo Y, Chen Y, Bian Z, Yang L, Walters R, Bragg F, Lv J. Physical activity, sedentary leisure time, circulating metabolic markers, and risk of major vascular diseases. Circ Genom Precis Med 2019; 12: 386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kurihara A, Okamura T, Sugiyama D, Higashiyama A, Watanabe M, Okuda N, Kadota A, Miyagawa N, Fujiyoshi A, Yoshita K, Ohkubo T, Okayama A, Miura K, Ueshima H, for the NIPPON DATA90 Research Group . Vegetable protein intake was inversely associated with cardiovascular mortality in a 15‐year follow‐up study of the general Japanese population. J Atheroscler Thromb 2019; 26: 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Holmes E, Loo RL, Stamler J, Bictash M, Yap IKS, Chan Q, Ebbels T, de Iorio M, Brown IJ, Veselkov KA, Daviglus ML, Kesteloot H, Ueshima H, Zhao L, Nicholson JK, Elliott P. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature 2008; 453: 396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Stamler J, Brown IJ, Yap IK, Chan Q, Wijeyesekera A, Garcia‐Perez I, Chadeau‐Hyam M, Ebbels TM, de Iorio M, Posma J, Daviglus ML, Carnethon M, Holmes E, Nicholson JK, Elliott P, INTERMAP Research Group . Dietary and urinary metabonomic factors possibly accounting for higher blood pressure of black compared with white Americans: results of International Collaborative Study on macro‐/micronutrients and blood pressure. Hypertension 2013; 62: 1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta‐analysis of prospective studies. J Am Heart Assoc 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, Trimarco B, Esposito G, Perrino C. Gut microbe‐generated metabolite trimethylamine‐N‐oxide as cardiovascular risk biomarker: a systematic review and dose‐response meta‐analysis. Eur Heart J 2017; 38: 2948–2956. [DOI] [PubMed] [Google Scholar]
- 105. Yu D, Shu XO, Rivera ES, Zhang X, Cai Q, Calcutt MW, Xiang YB, Li H, Gao YT, Wang TJ, Zheng W. Urinary levels of trimethylamine‐N‐oxide and incident coronary heart disease: a prospective investigation among urban Chinese adults. J Am Heart Assoc 2019; 8: e010606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Karl JP, Margolis LM, Murphy NE, Carrigan CT, Castellani JW, Madslien EH, Teien HK, Martini S, Montain SJ, Pasiakos SM. Military training elicits marked increases in plasma metabolomic signatures of energy metabolism, lipolysis, fatty acid oxidation, and ketogenesis. Physiol Rep 2017; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhang J, Light AR, Hoppel CL, Campbell C, Chandler CJ, Burnett DJ, Souza EC, Casazza GA, Hughen RW, Keim NL, Newman JW, Hunter GR, Fernandez JR, Garvey WT, Harper ME, Fiehn O, Adams SH. Acylcarnitines as markers of exercise‐associated fuel partitioning, xenometabolism, and potential signals to muscle afferent neurons. Exp Physiol 2017; 102: 48–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lehmann R, Zhao X, Weigert C, Simon P, Fehrenbach E, Fritsche J, Machann J, Schick F, Wang J, Hoene M, Schleicher ED, Häring HU, Xu G, Niess AM. Medium chain acylcarnitines dominate the metabolite pattern in humans under moderate intensity exercise and support lipid oxidation. PLoS One 2010; 5: e11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Havulinna AS, Sysi‐Aho M, Hilvo M, Kauhanen D, Hurme R, Ekroos K, Salomaa V, Laaksonen R. Circulating ceramides predict cardiovascular outcomes in the population‐based FINRISK 2002 cohort. Arterioscler Thromb Vasc Biol 2016; 36: 2424–2430. [DOI] [PubMed] [Google Scholar]
- 110. Zhang ZY, Marrachelli VG, Thijs L, Yang WY, Wei FF, Monleon D, Jacobs L, Nawrot T, Verhamme P, Voigt JU, Kuznetsova T, Redón J, Staessen JA. Diastolic left ventricular function in relation to circulating metabolic biomarkers in a general population. J Am Heart Assoc 2016; 5: e002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Rizza S, Copetti M, Rossi C, Cianfarani MA, Zucchelli M, Luzi A, Pecchioli C, Porzio O, di Cola G, Urbani A, Pellegrini F, Federici M. Metabolomics signature improves the prediction of cardiovascular events in elderly subjects. Atherosclerosis 2014; 232: 260–264. [DOI] [PubMed] [Google Scholar]
- 112. Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes 2013; 62: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Guasch‐Ferré M, Zheng Y, Ruiz‐Canela M, Hruby A, Martínez‐González MA, Clish CB, Corella D, Estruch R, Ros E, Fitó M, Dennis C. Plasma acylcarnitines and risk of cardiovascular disease: effect of Mediterranean diet interventions. Am J Clin Nutr 2016; 103: 1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bayes‐Genis A, Liu PP, Lanfear DE, de Boer RA, González A, Thum T, Emdin M, Januzzi JL. Omics phenotyping in heart failure: the next frontier. Eur Heart J 2020; 41: 3477–3484. [DOI] [PubMed] [Google Scholar]
- 115. Ganna A, Salihovic S, Sundström J, Broeckling CD, Hedman ÅK, Magnusson PK, Pedersen NL, Larsson A, Siegbahn A, Zilmer M, Prenni J. Large‐scale metabolomic profiling identifies novel biomarkers for incident coronary heart disease. PLoS Genet 2014; 10: e1004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cheng ML, Wang CH, Shiao MS, Liu MH, Huang YY, Huang CY, Mao CT, Lin JF, Ho HY, Yang NI. Metabolic disturbances identified in plasma are associated with outcomes in patients with heart failure: diagnostic and prognostic value of metabolomics. J Am Coll Cardiol 2015; 65: 1509–1520. [DOI] [PubMed] [Google Scholar]
- 117. Bedi KC Jr, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, Rame JE. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation 2016; 133: 706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wang DD, Toledo E, Hruby A, Rosner BA, Willett WC, Sun Q, Razquin C, Zheng Y, Ruiz‐Canela M, Guasch‐Ferré M, Corella D. Plasma ceramides, Mediterranean diet, and incident cardiovascular disease in the PREDIMED trial. Circulation 2017. CIRCULATIONAHA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Menni C, Lin C, Cecelja M, Mangino M, Matey‐Hernandez ML, Keehn L, Mohney RP, Steves CJ, Spector TD, Kuo CF, Chowienczyk P, Valdes AM. Gut microbial diversity is associated with lower arterial stiffness in women. Eur Heart J 2018; 39: 2390–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kunze KP, Dirschinger RJ, Kossmann H, Hanus F, Ibrahim T, Laugwitz KL, Schwaiger M, Rischpler C, Nekolla SG. Quantitative cardiovascular magnetic resonance: extracellular volume, native T1 and 18F‐FDG PET/CMR imaging in patients after revascularized myocardial infarction and association with markers of myocardial damage and systemic inflammation. J Cardiovasc Magn Reson 2018; 20: 33–0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Chen W, Sharma G, Jiang W, Maptue NR, Malloy CR, Sherry AD, Khemtong C. Metabolism of hyperpolarized 13C‐acetoacetate to β‐hydroxybutyrate detects real‐time mitochondrial redox state and dysfunction in heart tissue. NMR Biomed 2019; 32: e4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Dodd MS, Atherton HJ, Carr CA, Stuckey DJ, West JA, Griffin JL, Radda GK, Clarke K, Heather LC, Tyler DJ. Impaired in vivo mitochondrial Krebs cycle activity after myocardial infarction assessed using hyperpolarized magnetic resonance spectroscopy. Circ Cardiovasc Imaging 2014; 7: 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Bates MG, Newman JH, Jakovljevic DG, Hollingsworth KG, Alston CL, Zalewski P, Klawe JJ, Blamire AM, MacGowan GA, Keavney BD, Bourke JP. Defining cardiac adaptations and safety of endurance training in patients with m.3243A>G‐related mitochondrial disease. Int J Cardiol 2013; 168: 3599–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Fujiyoshi K, Yamaoka‐Tojo M, Minami Y, Kutsuna T, Obara S, Kakizaki R, Nemoto T, Hashimoto T, Namba S, Shimohama T, Tojo T, Ako J. Endothelial dysfunction is associated with cognitive impairment of elderly cardiovascular disease patients. Int Heart J 2018; 59: 1034–1040. [DOI] [PubMed] [Google Scholar]
- 125. Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow‐mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 2011; 300: H2–H12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Yoshinaga K, Manabe O, Katoh C, Chen L, Klein R, Naya M, deKemp RA, Williams K, Beanlands RSB, Tamaki N. Quantitative analysis of coronary endothelial function with generator‐produced 82Rb PET: comparison with 15O‐labelled water PET. Eur J Nucl Med Mol Imaging 2010; 37: 2233–2241. [DOI] [PubMed] [Google Scholar]
- 127. Campisi R, Marengo FD. Coronary microvascular dysfunction in women with nonobstructive ischemic heart disease as assessed by positron emission tomography. Cardiovasc Diagn Ther 2017; 7: 196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bucerius J, Duivenvoorden R, Mani V, Moncrieff C, Rudd JH, Calcagno C, Machac J, Fuster V, Farkouh ME, Fayad ZA. Prevalence and risk factors of carotid vessel wall inflammation in coronary artery disease patients: FDG‐PET and CT imaging study. JACC Cardiovasc Imaging 2011; 4: 1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sollini M, Ciccarelli M, Cecconi M, Aghemo A, Morelli P, Gelardi F, Chiti A. Vasculitis changes in COVID‐19 survivors with persistent symptoms: an [18F]FDG‐PET/CT study. Eur J Nucl Med Mol Imaging 2020: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Naya M, Morita K, Yoshinaga K, Manabe O, Goto D, Hirata K, Katoh C, Tamaki N, Tsutsui H. Long‐term smoking causes more advanced coronary endothelial dysfunction in middle‐aged smokers compared to young smokers. Eur J Nucl Med Mol Imaging 2011; 38: 491–498. [DOI] [PubMed] [Google Scholar]
- 131. Guaraldi G, Milic J, Prandini N, Ligabue G, Esposito F, Ciusa G, Malagoli A, Scaglioni R, Besutti G, Beghetto B, Nardini G, Roncaglia E, Mussini C, Raggi P. 18Fluoride‐based molecular imaging of coronary atherosclerosis in HIV infected patients. Atherosclerosis 2020; 297: 127–135 Epub;2020 Feb 17.:127‐35. [DOI] [PubMed] [Google Scholar]
- 132. Liu CY, Liu YC, Wu C, Armstrong A, Volpe GJ, van der Geest RJ, Liu Y, Hundley WG, Gomes AS, Liu S, Nacif M, Bluemke DA, Lima JAC. Evaluation of age‐related interstitial myocardial fibrosis with cardiac magnetic resonance contrast‐enhanced T1 mapping: MESA (Multi‐Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2013; 62: 1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Fernández‐Friera L, Fuster V, López‐Melgar B, Oliva B, García‐Ruiz JM, Mendiguren J, Bueno H, Pocock S, Ibáñez B, Fernández‐Ortiz A, Sanz J. Normal LDL‐cholesterol levels are associated with subclinical atherosclerosis in the absence of risk factors. J Am Coll Cardiol 2017; 70: 2979–2991. [DOI] [PubMed] [Google Scholar]
- 134. Leng S, Zhao X, Koh AS, Zhao L, Allen JC, Tan RS, Ma X, Zhong L. Age‐related changes in four‐dimensional CMR‐derived atrioventricular junction velocities and displacements: implications for the identification of altered annular dynamics for ventricular function assessment. Int J Cardiol Heart Vasc 2019; 22: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Koh AS, Gao F, Leng S, Kovalik JP, Zhao X, Tan RS, Fridianto KT, Ching J, Chua SJM, Yuan JM, Koh WP, Zhong L. Dissecting clinical and metabolomics associations of left atrial phasic function by cardiac magnetic resonance feature tracking. Sci Rep 2018; 8: 8138–26456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Evin M, Redheuil A, Soulat G, Perdrix L, Ashrafpoor G, Giron A, Lamy J, Defrance C, Roux C, Hatem SN, Diebold B, Mousseaux E, Kachenoura N. Left atrial aging: a cardiac magnetic resonance feature‐tracking study. Am J Physiol Heart Circ Physiol 2016; 310: H542–H549. [DOI] [PubMed] [Google Scholar]
- 137. Koh AS, Gao F, Liu J, Fridianto KT, Ching J, Tan RS, Wong JI, Chua SJM, Leng S, Zhong L, Keng BMH, Huang FQ, Yuan JM, Koh WP, Kovalik JP. Metabolomic profile of arterial stiffness in aged adults. Diab Vasc Dis Res 2018; 15: 74–80. [DOI] [PubMed] [Google Scholar]
- 138. Hou YCC, Yu HC, Martin R, Cirulli ET, Schenker‐Ahmed NM, Hicks M, Cohen IV, Jönsson TJ, Heister R, Napier L, Swisher CL. Precision medicine integrating whole‐genome sequencing, comprehensive metabolomics, and advanced imaging. Proc Natl Acad Sci U S A 2020; 117: 3053–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Paynter NP, Balasubramanian R, Giulianini F, Wang DD, Tinker LF, Gopal S, Deik AA, Bullock K, Pierce KA, Scott J, Martínez‐González MA, Estruch R, Manson JAE, Cook NR, Albert CM, Clish CB, Rexrode KM. Metabolic predictors of incident coronary heart disease in women. Circulation 2018; 137: 841–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hilvo M, Meikle PJ, Pedersen ER, Tell GS, Dhar I, Brenner H, Schöttker B, Lääperi M, Kauhanen D, Koistinen KM, Jylhä A, Huynh K, Mellett NA, Tonkin AM, Sullivan DR, Simes J, Nestel P, Koenig W, Rothenbacher D, Nygård O, Laaksonen R. Development and validation of a ceramide‐ and phospholipid‐based cardiovascular risk estimation score for coronary artery disease patients. Eur Heart J 2020; 41: 371–380. [DOI] [PubMed] [Google Scholar]
- 141. Chen L, He FJ, Dong Y, Huang Y, Harshfield GA, Zhu H. Sodium reduction, metabolomic profiling, and cardiovascular disease risk in untreated black hypertensives. Hypertension 2019; 74: 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Sliz E, Kettunen J, Holmes MV, Williams CO, Boachie C, Wang Q, Männikkö M, Sebert S, Walters R, Lin K, Millwood IY, Clarke R, Li L, Rankin N, Welsh P, Delles C, Jukema JW, Trompet S, Ford I, Perola M, Salomaa V, Järvelin MR, Chen Z, Lawlor DA, Ala‐Korpela M, Danesh J, Davey Smith G, Sattar N, Butterworth A, Würtz P. Metabolomic consequences of genetic inhibition of PCSK9 compared with statin treatment. Circulation 2018; 138: 2499–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Loo RL, Zou X, Appel LJ, Nicholson JK, Holmes E. Characterization of metabolic responses to healthy diets and association with blood pressure: application to the Optimal Macronutrient Intake Trial for Heart Health (OmniHeart), a randomized controlled study. Am J Clin Nutr 2018; 107: 323–334. [DOI] [PubMed] [Google Scholar]
- 144. Wang DD, Toledo E, Hruby A, Rosner BA, Willett WC, Sun Q, Razquin C, Zheng Y, Ruiz‐Canela M, Guasch‐Ferré M, Corella D, Gómez‐Gracia E, Fiol M, Estruch R, Ros E, Lapetra J, Fito M, Aros F, Serra‐Majem L, Lee CH, Clish CB, Liang L, Salas‐Salvadó J, Martínez‐González MA, Hu FB. Plasma ceramides, Mediterranean diet, and incident cardiovascular disease in the PREDIMED trial (Prevencion con Dieta Mediterranea). Circulation 2017; 135: 2028–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Koh AS, Gao F, Tan RS, Zhong L, Leng S, Zhao X, Fridianto KT, Ching J, Lee SY, Keng BMH, Yeo TJ, Tan SY, Tan HC, Lim CT, Koh WP, Kovalik JP. Metabolomic correlates of aerobic capacity among elderly adults. Clin Cardiol 2018; 41: 1300–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]


