Abstract
Aims
Establishing a diagnosis of inflammatory cardiomyopathy (iCMP) by non‐invasive means remains challenging despite advances in cardiac magnetic resonance imaging. Previous studies suggested the involvement of microRNAs in the pathogenesis of iCMP. We examined the association of a predefined set of circulatory microRNAs with clinical characteristics of iCMP and evaluated their diagnostic performance in suspected iCMP.
Methods and results
Eighty‐nine patients with clinical suspicion of iCMP were included in the analysis. All patients underwent cardiac catheterization with left ventricular endomyocardial biopsy, echocardiography, and cardiac magnetic resonance imaging applying the Lake Louise criteria (LLC). Plasma levels of miR‐21, miR‐126, miR‐133a, miR‐146b, miR‐155, and miR‐206 were determined using real‐time polymerase chain reaction. Based on immunohistological findings on endomyocardial biopsy, iCMP was diagnosed in 67% of study participants (n = 60). Plasma levels of miR‐155 and miR‐206 were significantly increased in patients with iCMP as compared with patients with dilated cardiomyopathy (P = 0.008 and P = 0.009, respectively). In receiver operating characteristic curve analysis, miR‐155 and miR‐206 demonstrated superior diagnostic performance for iCMP (0.68 and 0.67, respectively) compared with LLC [area under the curve (AUC) 0.60], Troponin T (AUC 0.51), and N‐terminal pro‐brain natriuretic peptide (AUC 0.51). While baseline miR‐155 and miR‐206 plasma levels were predictive for biopsy‐proven iCMP (odds ratio = 2.61, 95% confidence interval = 1.28–5.31, P = 0.008 and odds ratio = 2.65, 95% confidence interval = 1.27–5.52, P = 0.009) on univariate logistic regression analysis, the presence of positive LLC, high baseline C‐reactive protein, or presence of clinical symptoms and signs of viral infection failed to predict iCMP (P > 0.05, respectively).
Conclusions
The present data suggest that plasma levels of miR‐206 and miR‐155 are potential novel biomarkers for confirming the diagnosis of iCMP.
Keywords: Inflammatory cardiomyopathy, Dilated cardiomyopathy, miRNA, Endomyocardial biopsy, Cardiovascular magnetic resonance imaging, Lake Louise criteria
Introduction
Inflammatory cardiomyopathy (iCMP) represents an important differential diagnosis in patients with new‐onset heart failure being associated with substantial long‐term functional disability. 1 , 2 Distinguishing iCMP from other forms of dilated cardiomyopathy (DCM) remains challenging because of its variable clinical presentation. 3 Immunohistological analysis of endomyocardial biopsies (EMB) is still regarded as the diagnostic reference standard and provides insights into possible underlying aetiologies and pathogenic mechanisms of iCMP. 4 However, routine use of EMBs in these patients is hampered by the invasiveness and potential complications of the procedure. 5 Cardiovascular magnetic resonance (CMR) imaging techniques yield a diagnostic accuracy for myocardial inflammation that varies between 60% and 80% depending on the type of symptoms and CMR protocol. 6 , 7 , 8 Yet CMR has still limited availability and is costly and imaging analysis techniques vary between different centres.
Evaluation of circulating biomarkers has recently been suggested as a non‐invasive alternative to improve diagnostic decision making in iCMP. MicroRNAs [miRNAs (miRs)] are a class of small non‐coding, single‐stranded RNAs acting as post‐transcriptional modulators of gene expression. Accumulating data indicate that miRNAs play a crucial role in a variety of biological processes and diseases including heart muscle development and homeostasis (miR‐133a and miR‐206), inflammation and immunity (miR‐155 and miR‐126), endothelial activation (miR‐146b), and fibrosis (miR‐21). Previous studies also indicated that dysregulation of miRNA expression contributes to the pathogenesis of iCMP. 9 , 10
The aim of the present study was to examine the association of plasma concentrations of selected miRNAs with immunohistological findings on EMB, CMR findings, and echocardiographic and laboratory characteristics in patients with iCMP and to evaluate the diagnostic utility of these miRNAs to distinguish patients with iCMP from patients with DCM.
Methods
Study design
For the purpose of this prospective single‐centre analysis within the MyoRacer Trial (Comprehensive Cardiac Magnetic Resonance Imaging in Patients With Suspected Myocarditis), we used plasma samples from 89 patients admitted to the Heart Center Leipzig between August 2012 and May 2015 fulfilling all of the following criteria: (i) new onset or persistent symptoms suggestive of myocarditis (shortness of breath, exercise intolerance, fatigue, palpitation, or chest pain); (ii) evidence of recent or ongoing myocardial damage [electrocardiogram (ECG) abnormalities, elevated enzymes of myocardial injury, and left ventricular (LV) dysfunction]; (iii) history of symptoms and signs typical for systemic viral infection; (iv) exclusion of relevant coronary artery disease on angiography (defined as stenosis >50% of vessel diameter); and (v) LV ejection fraction <55%. All patients included in the present study underwent LV EMB, CMR imaging, and regular clinical work‐up, containing evaluation of clinical history, echocardiography, ECG, and standard laboratory analysis. According to immunohistological findings on EMB, patients were divided into two groups, that is, patients with and patients without immunohistological evidence of myocardial inflammation. Myocardial inflammation was defined as the detection of ≥14 infiltrating immune cells/mm2 (CD3+ T lymphocytes and/or CD68+ macrophages) in addition to enhanced human leucocyte antigen class II expression in antigen‐presenting immune cells according to the classification criteria of the World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of Cardiomyopathies. 1 The patients not fulfilling these immunohistological criteria were assigned to the DCM group. 1
All patients provided written informed consent before participation. The study was conducted according to the Declaration of Helsinki and was approved by the ethics committee of the Medical Faculty of the University of Leipzig.
Endomyocardial biopsy
For EMB sampling, a myocardial biopsy forceps (Teleflex Medical Tuttlingen GmbH, Tuttlingen, Germany) was used. Five to six EMBs were taken from the left ventricle under fluoroscopic guidance. EMBs were taken from different locations within the ventricle. Histological and molecular pathological analyses confirming myocardial inflammation and viral infections were performed at the Department of Molecular Pathology, University Hospital Tübingen (Tübingen, Germany) as described previously. 11 , 12 Immunohistochemical staining was conducted using BenchMark ULTRA‐Ventana Medical Systems, as published. 12 Masson's trichrome staining and haematoxylin–eosin staining were used to determine the presence of myocyte necrosis, degeneration, and fibrosis. For the purpose of this study, myocyte degeneration was defined as the presence of morphological changes in the tissue structure and size fluctuations of myocytes on histological evaluation of EMB specimens. An independent experienced examiner, blinded to study objectives, evaluated EMB specimens (six LV biopsies with at least four tissue sections per staining), in accordance to published guidelines. 13 , 14 Electron microscopy and Congo red staining was performed for exclusion of hypertrophic cardiomyopathy and amyloidosis, respectively. Nested (reverse transcriptase) polymerase chain reaction (PCR) for detection of viral genomes [Epstein–Barr virus, parvovirus B19, enterovirus, human herpesvirus (HHV) 3, HHV 6, HHV 7, cytomegalovirus, adenovirus, influenza A and B] was performed, as described previously. 11 , 15
Cardiac magnetic resonance imaging
All study participants underwent CMR imaging on 1.5 and 3 T scanners. The CMR protocol included current standard Lake Louise criteria (LLC) for myocarditis as well as native T1, calculation of extracellular volume fraction, and T2 mapping (only on 1.5 T). 11 , 15
MicroRNA extraction
For determination of plasma miRNAs, blood samples obtained during EMB were centrifuged at 2000 g for 15 min. Plasma was aliquoted and immediately stored at −80°C. Total RNA was isolated from 200 μL plasma of each study participant using miRNeasyMini Kit according to the manufacturer's protocol (Qiagen, Hilden, Germany). cDNA preparation and real‐time PCR was performed using the miScript PCR system (Qiagen, Hilden, Germany) and a LightCycler 96 system (Roche, Mannheim, Germany) with primers detecting: Hs_miR‐21_2, Hs_miR‐126_1, Hs_miR‐133a_2, Hs_miR‐146b_1, Hs_miR155*_1, and Hs_miR‐206_1. Equal amounts of miR‐39_1 script (3.5 μL per 5.6 × 108 copies) were added to each evaluated plasma sample as an exogenous control for assessment of isolation efficiency, as well as for normalization of expression levels of analysed miRNAs. Samples were analysed in duplicates. The inter‐assay coefficient of variation was 12%.
Statistical analysis
Continuous variables of the patient population are presented as mean and standard deviation if normally distributed or as median and inter‐quartile range if non‐normally distributed. miRNA levels are presented as mean and standard error of the mean. Continuous variables were compared between groups using Student's t‐test, Mann–Whitney U test or Kruskal–Wallis one‐way analysis of variance, as appropriate. Categorical variables were compared using the χ 2 test. Analysis of miRNA levels in plasma samples using the Kolmogorov–Smirnov test revealed that the data were non‐normally distributed. The association between miRNA levels and histopathological findings in EMB was tested using Spearman correlation analysis. Myocyte necrosis, degeneration, and fibrosis were assessed as binary variables (either presence or absence). The diagnostic value of miRNAs was evaluated with receiver operating characteristic (ROC) curve analysis calculating optimal thresholds and areas under the curve (AUCs). The Youden index was used to depict optimal cut‐off values from the ROC and AUCs. According to the Youden index, sensitivity, specificity, and accuracy, defined as number of correct diagnostic assessments divided through number of all assessments, were calculated for each miRNA. Univariate logistic regression was used to test the utility of miRNA candidates from ROC analysis to diagnose iCMP by using EMB as the reference standard. Two‐tailed P‐values <0.05 were considered statistically significant for all statistical procedures. All statistical analyses were performed with the SPSS package Version 21.0 (SPSS, Inc., Chicago, IL).
Results
Study population
The baseline characteristics of the study population are presented in Table 1 . Based on immunohistological findings in EMB, iCMP was diagnosed in 67% of study participants (n = 60), whereas 29 patients did not show immunohistological signs of myocardial inflammation and were assigned to the DCM group. There were no statistically significant differences in age and gender between the two groups.
Table 1.
Baseline characteristics of patients with iCMP and DCM
| Clinical characteristic | All patients (n = 89) | iCMP (n = 60) | DCM (n = 29) | P |
|---|---|---|---|---|
| Age (years), mean ± SD | 45 ± 15 | 47 ± 15 | 43 ± 14 | 0.77 |
| Female (%) | 26% | 28% | 24% | 0.67 |
| BMI (kg/m2) | 31 | 32 | 31 | 0.42 |
| Clinical presentation | ||||
| Dyspnoea, n (%) | 61 (67%) | 41 (68%) | 20 (69%) | 0.96 |
| Fatigue, n (%) | 58 (64%) | 39 (65%) | 19 (65%) | 0.95 |
| Peripheral oedema, n (%) | 15 (16%) | 8 (13%) | 7 (24%) | 0.22 |
| Chest pain, n (%) | 44 (38%) | 29 (48%) | 15 (51%) | 0.95 |
| Recent viral infection, n (%) | 52 (58%) | 45 (75%) | 17 (60%) | 0.12 |
| CMR findings | ||||
| Lake Louise criteria (≥2) | 62% | 24% | 0.06 | |
| Number of positive Lake Louise criteria | ||||
| 1 | — | 14 (23%) | 8 (27%) | |
| 2 | — | 21 (41%) | 6 (21%) | |
| 3 | — | 10 (21%) | 1 (3%) | |
| Echocardiographic findings | ||||
| LVEF (%), mean ± SD | 36 ± 16 | 36 ± 15 | 35 ± 19 | 0.81 |
| LVEDD (mm), mean ± SD | 57 ± 10 | 56 ± 10 | 59 ± 11 | 0.33 |
| LVESD (mm), mean ± SD | 46 ± 12 | 46 ± 11 | 46 ± 14 | 0.92 |
| Atrioventricular conduction disturbance | 10 (11%) | 7 (11%) | 3 (10%) | 0.76 |
| ST‐segment elevation, n (%) | 14 (15%) | 10 (16%) | 6 (19%) | 0.44 |
| ST‐segment depression, n (%) | 37 (41%) | 27 (45%) | 9 (31%) | 0.14 |
| Troponin T (ng/L), mean ± SD | 202 ± 539 | 268 ± 643 | 64 ± 111 | 0.009 |
| CK‐MB (U/L), mean ± SD | 46 ± 324 | 29 ± 35 | 135 ± 564 | 0.01 |
| CRP (mg/L), mean ± SD | 26 ± 40 | 29 ± 43 | 18 ± 32 | 0.08 |
| Myoglobin (μg/L), mean ± SD | 217 ± 260 | 198 ± 271 | 269 ± 228 | 0.61 |
| ESR (mm/h), mean ± SD | 24 ± 23 | 28 ± 273 | 15 ± 12 | 0.01 |
| NT‐proBNP (pg/mL), mean ± SD | 2866 ± 5010 | 208 ± 5726 | 1994 ± 2255 | 0.09 |
| Immunohistochemistry (left ventricle) | ||||
| CD3+ T cells (cells/cm2 ± SD) | 11 ± 13 | 14 ± 14 | 3 ± 2 | 0.001 |
| CD68+ macrophages (cells/cm2 ± SD) | 25 ± 19 | 31 ± 20 | 11 ± 5 | 0.009 |
| Viral genome in EMB, n (%) | — | 20 (33%) | 6 (23%) | 0.20 |
| Enhanced MHC class II expression, n (%) | — | 60 (100) | 5 (18) | 0.001 |
| Cardiovascular risk factors, n (%) | 59 (65%) | 36 (60%) | 23 (82%) | 0.11 |
| Hypertension, n (%) | 49 (54%) | 31 (51%) | 18 (62%) | 0.49 |
| Diabetes, n (%) | 9 (10%) | 6 (10%) | 3 (10%) | 0.97 |
| Hyperlipoproteinaemia, n (%) | 29 (32%) | 19 (31%) | 10 (35%) | 0.91 |
BMI, body mass index; CK‐MB, creatine kinase myocardial band; CMR, cardiovascular magnetic resonance; CRP, C‐reactive protein; DCM, dilated cardiomyopathy; EMB, endomyocardial biopsy; ESR, erythrocyte sedimentation rate; iCMP, inflammatory cardiomyopathy; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; MHC, major histocompatibility complex; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; SD, standard deviation.
Patients in both study groups presented with reduced LV ejection fraction (36 ± 15% in iCMP vs. 35 ± 19% in DCM; P = 0.81) and dilatation of the LV (left ventricular end‐diastolic diameter: 56 ± 10 mm in iCMP vs. 59 ± 11 mm in DCM; P = 0.33). ECG abnormalities (atrioventricular conduction disturbances and ST‐segment depression or elevation) were equally present in both groups. Troponin T levels, CK‐MB levels, and erythrocyte sedimentation rate were statistically higher in iCMP patients (P = 0.009 and P = 0.01, respectively). Enhanced expression of major histocompatibility complex class II was observed in all patients with iCMP and 18% of patients with DCM (P = 0.001). There were no differences in the distribution of cardiovascular risk factors between patients with iCMP and DCM (Table 1 ).
Lake Louise criteria on cardiac MRI were positive in 62% of patients in the iCMP group and in 24% of patients in the DCM group (P = 0.06), yielding an overall diagnostic accuracy of 60% for confirming iCMP (Table 1 ).
Plasma microRNA profile in patients with inflammatory cardiomyopathy and dilated cardiomyopathy
Patients with CMP demonstrated significantly higher plasma levels of miR‐155 and miR‐206 as compared with patients with DCM (P = 0.008 and P = 0.009, respectively). This difference was irrespective of enhanced major histocompatibility complex class II expression in patients with DCM (data not shown). Also, numerically higher levels of miR‐126 and miR‐146b were observed in patients with iCMP. However, this difference did not reach statistical significance (Figure 1 ). Plasma miR‐126 was the most abundant miRNA in plasma of evaluated subjects in the present analysis.
Figure 1.
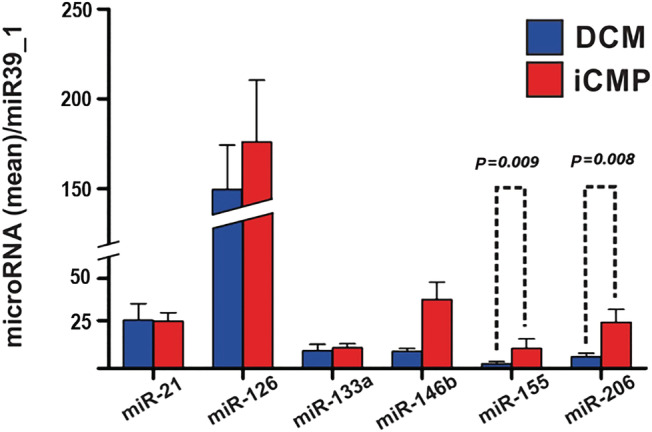
Differences in microRNA plasma concentration between patients with inflammatory cardiomyopathy (iCMP, n = 60, red bars) and dilated cardiomyopathy (DCM, n = 29, blue bars). Results are presented as mean ± standard error of the mean. Data were normalized to plasma concentration of miR39_1 in each patient.
Association of plasma microRNAs with histological and polymerase chain reaction findings in endomyocardial biopsy
Inflammatory cardiomyopathy patients without histological signs of fibrosis in EMB (n = 9) presented higher plasma concentrations of miR‐126 compared with those with evidence of fibrosis in EMB (n = 48, P = 0.01, Figure 2 ). We did not observe a significant difference in plasma miR‐21 levels between patients with and without myocardial fibrosis on EMB. Levels of evaluated miRNAs were not linked to the absence or presence of myocyte degeneration (n = 15 vs. n = 32, Figure 3 ) and necrosis (n = 8 vs. n = 43, Figure 4 ) on EMB in iCMP patients. There were no statistically significant differences in miRNA plasma level between iCMP patients with and without presence of viral genome in EMB specimens (n = 20 vs. n = 37, Figure 5 ).
Figure 2.
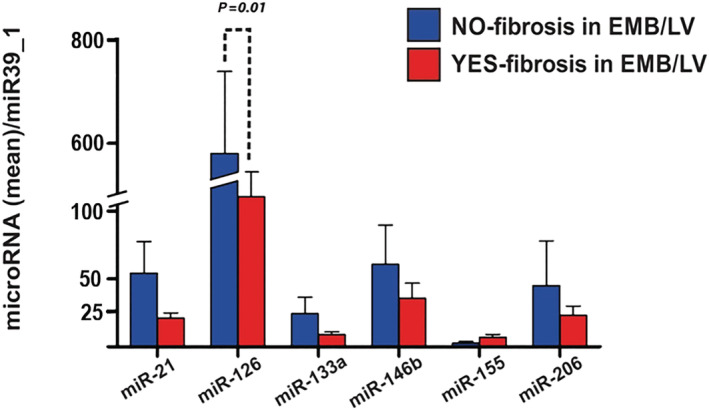
Differences in microRNA plasma concentration between inflammatory cardiomyopathy patients with (red bars, n = 48) and without (blue bars, n = 9) signs of fibrosis on histological analysis of left ventricular endomyocardial biopsy (EMB/LV). Results are presented as mean ± standard error of the mean. Data were normalized to plasma concentration of miR39_1 in each patient.
Figure 3.
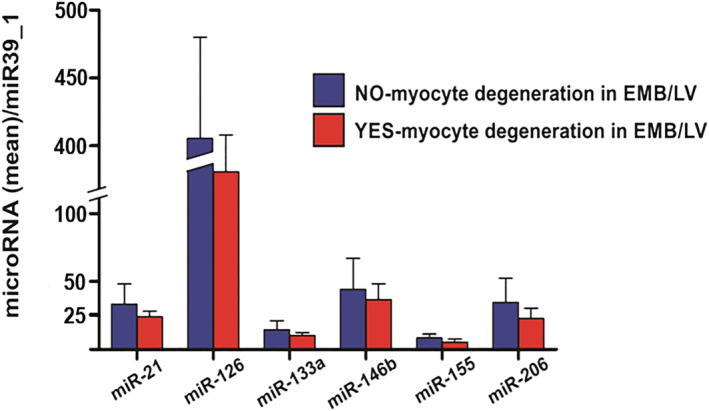
Difference in microRNA plasma concentration between inflammatory cardiomyopathy patients with (red bars, n = 32) and without (blue bars, n = 15) signs of myocyte degeneration on histological analysis of left ventricular endomyocardial biopsy (EMB/LV). Results are presented as mean ± standard error of the mean. Data were normalized to plasma concentration of miR39_1 in each patient.
Figure 4.
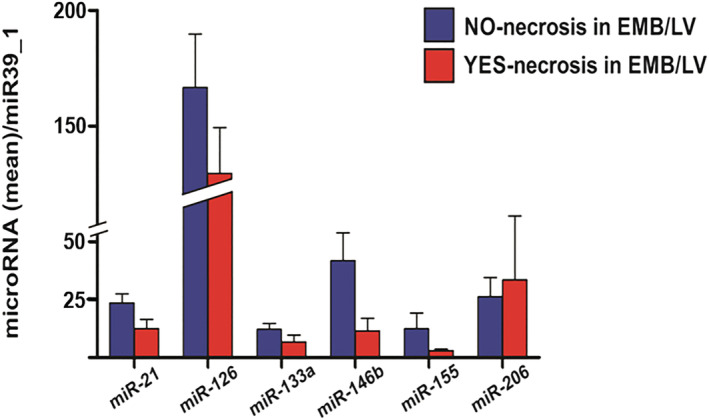
Difference in microRNA plasma concentration between inflammatory cardiomyopathy patients with (red bars, n = 8) and without (blue bars, n = 43) signs of necrosis on histological analysis of left ventricular endomyocardial biopsy (EMB/LV). Results are presented as mean ± standard error of the mean. Data were normalized to plasma concentration of miR39_1 in each patient.
Figure 5.
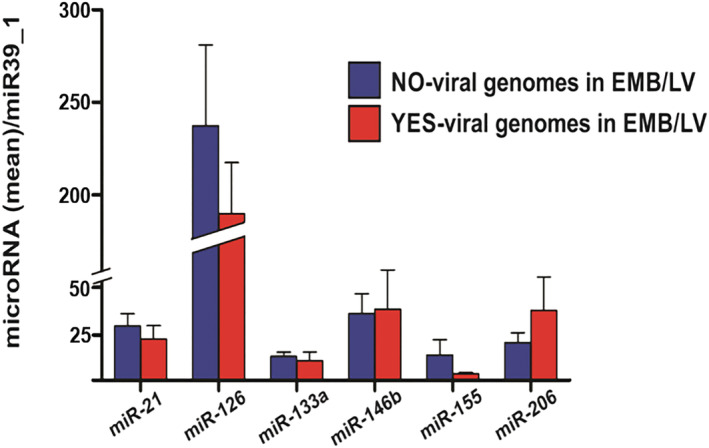
Difference in microRNA plasma concentration between inflammatory cardiomyopathy patients with (red bars, n = 20) and without (blue bars, n = 37) presence of viral genome in left ventricular endomyocardial biopsy (EMB/LV). Results are presented as mean ± standard error of the mean. Data were normalized to plasma concentration of miR39_1 in each patient.
In patients with iCMP, the most frequently detected virus on PCR analysis of EMB was parvovirus B19 (n = 13), followed by HHV 6 (n = 4), HHV 7 (n = 1), Epstein–Barr virus (n = 1), and enterovirus (n = 1). When patients with presence of the parvovirus B19 viral genome in EMB were compared with patients with detection of viral genomes other than parvovirus B19 or patients without viral genomes in EMB, no significant difference in plasma concentration of any evaluated miRNA was observed.
Association of plasma microRNAs with immunohistochemical and echocardiographic findings in inflammatory cardiomyopathy
Plasma miR‐126 and miR‐21 levels positively correlated with CD68+ macrophages in EMB specimens (r = 0.45 and r = 0.55, respectively, P < 0.05). Plasma miR‐133a, miR‐146b, miR‐155, and miR‐206 did not show a significant correlation with CD3+ T lymphocytes and CD68+ macrophages in myocardial tissue of iCMP patients (Table 2 ). Echocardiographic data were available for 82 patients (56 patients in the iCMP group and 26 patients in the DCM group). No relevant correlation between functional (LV ejection fraction) and morphological (end‐diastolic and end‐systolic diameter and end‐diastolic volume) parameters of LV function and plasma miRNA levels was observed (Table 2 ).
Table 2.
Correlation between plasma levels of evaluated miRNAs and degree of left ventricular inflammatory response, echocardiographic parameters of left ventricular function, and plasma concentration of markers of myocardial injury in patients with iCMP (n = 60)
| r | CD3+ | CD68+ | LVEF | LVEDD | LVESD | Troponin T | CK‐MB | CRP | ESR | NT‐proBNP |
|---|---|---|---|---|---|---|---|---|---|---|
| miR‐21 | 0.26 | 0.45* | −0.07 | −0.02 | −0.03 | 0.01 | −0.10 | 0.50* | 0.27 | 0.22 |
| miR‐126 | 0.38 | 0.55* | 0.06 | −0.03 | −0.03 | −0.04 | 0.12 | 0.18 | 0.18 | 0.07 |
| miR‐133a | −0.21 | −0.05 | −0.13 | −0.06 | −0.09 | 0.03 | 0.04 | 0.30 | 0.29 | 0.18 |
| miR‐146b | −0.01 | 0.07 | −0.11 | 0.12 | 0.16 | −0.04 | −0.07 | 0.31 | 0.37 | 0.22 |
| miR‐155 | −0.15 | −0.22 | −0.11 | 0.17 | 0.15 | −0.05 | 0.03 | 0.01 | −0.07 | −0.05 |
| miR‐206 | −0.04 | 0.03 | 0.13 | −0.14 | −0.09 | −0.06 | 0.33 | 0.03 | −0.13 | −0.06 |
CD3+, number of CD3‐positive T lymphocytes in left ventricular endomyocardial biopsy specimen; CD68+, number of CD68‐positive macrophages in left ventricular EMB specimen; CK‐MB, creatine kinase myocardial band; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; iCMP, inflammatory cardiomyopathy; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; miRNAs, microRNAs; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; r, Spearman correlation coefficient after Benjamini–Hochberg adjustment for multiple correlation testing.
Statistically significant correlation (P < 0.05).
Association of microRNAs with biomarkers of cardiac injury and inflammation
We did not find any clinically relevant correlation between plasma levels of analysed miRNAs and circulating markers of myocardial injury (Troponin T and CK‐MB). C‐reactive protein (CRP) exhibited a moderate correlation with miR‐21 (r = 0.50, P < 0.05), whereas miR‐126, miR‐133, miR‐146b, miR‐155, and miR‐206 did not show any association with CRP (for all r < 0.1, P > 0.05, Table 2 ).
Diagnostic potential of miR‐155 and miR‐206 in inflammatory cardiomyopathy
On ROC analysis, circulating biomarkers of cardiac injury provided limited diagnostic accuracy to identify iCMP (Table 3 ). CRP demonstrated numerically higher AUC compared with erythrocyte sedimentation rate on ROC analysis [AUC 0.62, 95% confidence interval (CI) 0.46–0.77 vs. AUC 0.58, 95% CI 0.41–0.72, Table 3 ]. In comparison with other circulating biomarkers of inflammation and cardiac injury, miR‐155 and miR‐206 demonstrated superior diagnostic performance in CMP (AUC 0.68, 95% CI 0.55–0.81 and AUC 0.67, 95% CI 0.55–0.79, respectively).
Table 3.
Diagnostic performance of circulating biomarkers and plasma levels of miR‐155 and miR‐206 in iCMP
| Circulating biomarker | Youden index | AUC | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|---|---|
| CRP | 6.30 mg/L | 0.62 (0.46–0.77) | 72% | 38% | 0.66 | 0.44 | 0.59 |
| ESR | 18.50 mm | 0.58 (0.44–0.72) | 73% | 40% | 0.56 | 0.57 | 0.56 |
| CK‐MB | 22.5 U/L | 0.51 (0.34–0.67) | 72% | 38% | 0.54 | 0.57 | 0.55 |
| Myoglobin | 251 μg/L | 0.39 (0.23–0.54) | 69% | 24% | 0.36 | 0.55 | 0.41 |
| Troponin T | 34.50 ng/L | 0.51 (0.36–0.65) | 73% | 38% | 0.55 | 0.57 | 0.43 |
| NT‐proBNP | 1076 g/mL | 0.51 (0.34–0.68) | 73% | 29% | 0.49 | 0.55 | 0.51 |
| Lake Louis criteria | — | 0.62 (0.47–0.77) | 62% | 63% | 0.81 | 0.38 | 0.60 |
| miR‐21 | 8.1 | 0.56 (0.43–0.69) | 68% | 31% | 0.71 | 0.39 | 0.57 |
| miR‐126 | 39.6 | 0.48 (0.34–0.61) | 68% | 35% | 0.62 | 0.33 | 0.57 |
| miR‐133a | 2.8 | 0.57 (0.45–0.71) | 68% | 35% | 0.63 | 0.35 | 0.58 |
| miR‐146b | 4.6 | 0.61 (0.49–0.73) | 68% | 38% | 0.61 | 0.37 | 0.59 |
| miR‐155 | 0.87 | 0.68 (0.55–0.80) | 75% | 42% | 0.65 | 0.53 | 0.61 |
| miR‐206 | 2.27 | 0.67 (0.55–0.79) | 77% | 48% | 0.70 | 0.57 | 0.66 |
CK‐MB, creatine kinase myocardial band; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
Receiver operating characteristic curve analysis was applied. Area under the curve (AUC), sensitivity, specificity, positive predictive value (PPV), negative predicative value (NPV), and diagnostic accuracy for diagnostic confirmation of inflammatory cardiomyopathy (iCMP) were calculated.
On univariate logistic regression analysis, higher baseline levels of miR‐155 and miR‐206 predicted the presence of iCMP on immunohistological analysis of EMB. LLC, CRP levels, and the presence of symptoms and signs typical for viral infection within the last 2 weeks prior to hospital presentation were not found to be predictive. Table 4 presents odds ratios associated with each predictor, their confidential intervals, and associated significances.
Table 4.
Results of univariate logistic regression analysis including five independent variables and its association with iCMP
| Variable | Odds ratio | Confidence interval (95%) | P‐value | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Lake Louis criteria | 2.79 | 0.93 | 8.34 | 0.06 |
| Symptoms of viral infection (<2 weeks) | 0.56 | 0.21 | 1.44 | 0.22 |
| CRP# | 1.02 | 0.99 | 1.02 | 0.27 |
| miR‐21 (logmiR‐21/miR‐39_1) | 1.30 | 0.57 | 3.01 | 0.52 |
| miR‐126 (logmiR‐126/miR‐39_1) | 1.01 | 0.44 | 2.35 | 0.97 |
| miR‐133a (logmiR‐133a/miR‐39_1) | 1.65 | 0.77 | 3.53 | 0.20 |
| miR‐146b (logmiR‐146b/miR‐39_1) | 2.06 | 0.90 | 4.53 | 0.09 |
| miR‐155 (logmiR‐155/miR‐39_1) | 2.61 | 1.28 | 5.31 | 0.008 |
| miR‐206 (log miR‐206/miR‐39_1) | 2.65 | 1.27 | 5.52 | 0.009 |
CRP, C‐reactive protein; iCMP, inflammatory cardiomyopathy.
Discussion
In the present study, we observed higher plasma levels of miR‐155 and miR‐206 in patients with iCMP as compared with patients with DCM. Furthermore, study participants with elevated baseline miR‐155 and miR‐206 demonstrated significantly higher probability for the presence of biopsy‐proven iCMP than those with positive LLC on CMR imaging. On ROC analysis, miR‐155 and miR‐206 provided higher accuracy for iCMP when compared either to standard circulating biomarkers of myocardial injury and inflammation, or CMR‐derived LLC.
Despite recent advances in comprehensive CMR imaging protocols for non‐invasive diagnosis of myocarditis, the distinction between iCMP and DCM still represents a significant challenge in clinical practice. 8 , 16 , 17 , 18 Several biomarkers are currently evaluated to improve diagnostic decision making and estimate prognosis by non‐invasive means in patients with iCMP. miRNAs have been implicated in a variety of cellular processes underlying cardiovascular disease. 19 The question about a potential diagnostic or therapeutic benefit of miRNAs in iCMP was proposed soon after recognizing the importance of miRNAs in myocardial homeostasis and disease. Interestingly, none of the miRNAs evaluated in the present study correlated with circulating cardiac troponin T levels, supporting the concept that miRNAs reflect pathophysiological processes beyond myocardial injury and may provide a diagnostic value superior to established laboratory markers used in current clinical practice.
miR‐155 was among the first miRNAs linked to cardiac inflammation. 20 , 21 Data by Yan et al. suggested that miRNA‐155 is highly elevated in heart tissue and CD4+ T cells during experimental autoimmune myocarditis. 22 Corsten et al. found that miR‐155 is strongly up‐regulated during acute myocarditis in both humans and susceptible mice and that inhibition of miR‐155 attenuates cardiac infiltration of macrophages and reduces myocardial damage during acute myocarditis in mice. 23 Interestingly, a recent transcriptome analysis of EMB from patients with lymphocytic myocarditis suggested that a molecular signature of ultimately 13 genes provides an accuracy of 100% in confirming myocardial inflammation. Out of these genes, seven candidates (TLR1, TLR2, TLR7, SIGLEC1, ITGB2, FCER1G, and CD14) are involved in essential cascades of cellular and humoural immunity. Notably, expression of miR‐155 is greatly enhanced by toll‐like receptor agonist stimulation of macrophages and dendritic cells. 24 SIGLEC1 and ITGB2 encode cellular surface proteins on antigen‐presenting cells and promote initiation of innate immune system through NF‐κB‐dependent pathway, a process that is associated with higher expression and synthesis of miR‐155. 25 Increased plasma levels of miR‐155 in patients with iCMP in comparison with patients with DCM, as observed in the present study, are in line with these findings and additionally underline an involvement of miR‐155 in myocardial inflammation.
miR‐206 mediates multiple essential physiological functions, including tissue growth, differentiation, and angiogenesis. 26 A study by Westendorp et al. indicated a 10‐fold higher expression of miR‐206 in transgenic mice with E2F6 overexpression, suggesting that certain pathological conditions may induce cardiomyocyte‐specific miR‐206 synthesis. 27 Nevertheless, comprehensive insights into the role of miR‐206 in cardiomyocyte homeostasis are still lacking. Higher levels of miR‐206 in patients with iCMP observed in our study are concordant with previous findings, showing that myocardial injury due to inflammation may promote miR‐206 plasma release. 28 It is important to note that an increase of miR‐206 plasma levels may be of extracardiac origin, for example, resulting from generalized myositis in the setting of systemic viral infection, which to certain extent limits generalizability of our findings.
Based on serological studies, adenoviruses and enteroviruses have long been considered the most common cardiotropic viruses resulting in myocarditis, 29 but studies using PCR for viral diagnosis in cardiac biopsies pointed towards parvovirus B19 and HHV 6 as the most frequent pathogens in patients with acute myocarditis or inflammatory cardiomyopathy. The viral genome analysis of EMB in the present study is in line with these observations. However, the clinical relevance of parvovirus B19 presence and persistence of its DNA in myocardial tissue remains unclear. 30 , 31 It was previously shown that parvovirus B19 genomes persist in various human tissues, in both healthy individuals and patients, without clinical relevance or disease inducing potential. This phenomenon is commonly referred to as ‘bioportfolio’. 32 Interestingly, plasma miRNA distribution in the present study was similar when patients with parvovirus B19 viral genome detection at low copy numbers were compared with patients with other virus types or iCMP patients without viral genome detection in EMB. Profiling of humoural and T cellular immune response of iCMP patients against parvovirus B19 could help to differentiate latent from active forms of infection and, thereby, identify those patients at risk, who can profit from intensified diagnostic and management. 33 , 34 , 35 Only a limited number of studies further evaluated the link between histological features of specific myocardial pathologies and miRNA expression patterns in humans. 36 , 37 , 38 Most of the analysed plasma miRNAs in our study did not show any relevant association with signs of inflammation or fibrosis on histology. Plasma levels of miR‐126, a known regulator of myocardial apoptosis and angiogenesis, 39 were significantly lower in iCMP patients with histological signs of myocardial fibrosis in EMB in the present study. The question whether reduced myocardial synthesis and extracellular release of miR‐126 in patients with iCMP have beneficial effects on cardiac fibrosis and myocardial recovery needs to be addressed in further studies.
In recent years, CMR imaging has emerged as a promising additional diagnostic tool in patients with suspected myocarditis. 40 Diagnostic accuracy of CMR depends on the type of clinical presentation of myocarditis, as well as on the imaging sequences applied (i.e. T1, T2, early gadolinium enhancement, and late gadolinium enhancement), and varies between different studies. 6 Lurz et al. suggested that comprehensive CMR mapping using T1 and T2 sequences enhances the diagnostic performance of CMR in acute or chronic iCMP. 8 Implementation of LLC, as comprehensive and validated protocols for CMR imaging of myocardial inflammation, significantly improves the assessment of patients with suspected myocarditis. However, LLC present better diagnostic performance in ‘infarct‐like’ presentation of iCMP (sensitivity 80%) when compared with ‘heart failure‐like’ presentation of iCMP (sensitivity 50%), which demands further improvement of non‐invasive diagnostic protocols with higher accuracy across all clinical subentities of iCMP. 41 , 42 The results of the present study demonstrate that miR‐155 and miR‐206 could be suitable for these purposes. In direct comparison with LLC, baseline levels of miR‐155 and miR‐206 yield better diagnostic performance in iCMP when compared against EMB as the reference standard.
Several limitations have to be considered when interpreting the results of the present study. First, the observational character of the data is accompanied with a potential selection bias. Secondly, there is an ongoing controversy as to which method of normalization should be used to quantify miRNA levels in plasma. Exogenously added miR39_1 proved to be reliable and applicable in very different research settings, is rather unaffected by isolation processes, and allows for absolute quantification of miRNAs. However, we cannot exclude that other normalization methods would have been superior in the present study set‐up. Thirdly, considering that miRNA expression profiles are highly time and space dependent, and influenced by many confounding factors, serial sampling of miRNAs in time intervals during the course of iCMP may offer more detailed insights into the role of miRNAs in the complex pathophysiology of myocardial inflammation. In addition, despite taking several endomyocardial biopsies from different regions of the left ventricle, we cannot exclude the possibility of sampling error when the diagnosis of iCMP or DCM was established in the present cohort. Fourthly, there are several known clinical conditions (asthma, multiple sclerosis, etc.), which are associated with higher circulatory miR‐155 and/or miR‐206 levels and were not the primary focus of our investigation. Hence, a confounding effect of these coexisting clinical conditions cannot be excluded and underlines the need for cross‐validation of our results across a broader range of clinical scenarios. Lastly, because of the limited number of patients, our study results need further confirmation in larger cohorts of iCMP patients.
Conclusions
Together, the present data suggest that patients with iCMP have significantly higher plasma concentrations of miR‐155 and miR‐206 in comparison with patients with DCM, and these miRNAs may serve as novel biomarkers for the diagnosis of iCMP. We believe that further studies are warranted to further explore the diagnostic potential of miR‐155 and miR‐206 in larger cohorts of patients with suspected iCMP and to better understand pathophysiological implications of these miRNAs in myocardial inflammation.
Conflict of interest
None declared.
Funding
Costs for miRNA analysis were covered by a research grant provided by the Heart Center Leipzig.
Author contributions
Both last authors contributed equally to our study.
Acknowledgement
We would like to thank Martin Petzold for his support and effort in study conduction.
Obradovic, D. , Rommel, K.‐P. , Blazek, S. , Klingel, K. , Gutberlet, M. , Lücke, C. , Büttner, P. , Thiele, H. , Adams, V. , Lurz, P. , Emrich, F. , and Besler, C. (2021) The potential role of plasma miR‐155 and miR‐206 as circulatory biomarkers in inflammatory cardiomyopathy. ESC Heart Failure, 8: 1850–1860. 10.1002/ehf2.13304
References
- 1. Caforio ALP, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggeweis H, Tavazzi L, Thiene G, Yilmaz A, Charren A, Eliot MP. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013; 34: 2636–2648. [DOI] [PubMed] [Google Scholar]
- 2. Kindermann I, Barth C, Mahfoud F, Ukena C, Lenski M, Yilmaz A, Klingel K, Kandolf R, Sechtem U, Cooper LT, Böhm M. Update on myocarditis. J Am Coll Cardiol 2012; 59: 779–792. [DOI] [PubMed] [Google Scholar]
- 3. Biesbroek PS, Beek AM, Germans T, Niessen HW, van Rossum AC. Diagnosis of myocarditis: current state and future perspectives. Int J Cardiol 2015; 191: 211–219. [DOI] [PubMed] [Google Scholar]
- 4. Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, Levine GN, Narula J, Starling RC, Towbin J, Virmani R. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation 2007; 116: 2216–2233. [DOI] [PubMed] [Google Scholar]
- 5. Shanes JG, Ghali J, Billingham ME, Ferrans VJ, Fenoglio JJ, Edwards WD, Tsai CC, Saffitz JE, Isner J, Furner S. Interobserver variability in the pathologic interpretation of endomyocardial biopsy results. Circulation 1987; 75: 401–405. [DOI] [PubMed] [Google Scholar]
- 6. Friedrich MG, Sechtem U, Schulz‐Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel‐Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P, International Consensus Group on CMR in Myocarditis . Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol 2009; 53: 1475–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lurz P, Eitel I, Adam J, Steiner J, Grothoff M, Desch S, Fuernau G, de Waha S, Sareban M, Luecke C, Klingel K, Kandolf R, Schuler G, Gutberlet M, Thiele H. Diagnostic performance of CMR imaging compared with EMB in patients with suspected myocarditis. J Am Coll Cardiol Img 2012; 5: 513–524. [DOI] [PubMed] [Google Scholar]
- 8. Lurz P, Luecke C, Eitel I, Föhrenbach F, Frank C, Grothoff M, de Waha S, Rommel KP, Lurz JA, Klingel K, Kandolf R, Schuler G, Thiele H, Gutberlet M. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis: the MyoRacer‐Trial. J Am Coll Cardiol 2016; 67: 1800–1811. [DOI] [PubMed] [Google Scholar]
- 9. Bao JL, Lin L. MiR‐155 and miR‐148a reduce cardiac injury by inhibiting NF‐κB pathway during acute viral myocarditis. Eur Rev Med Pharmacol Sci 2014; 18: 2349–2356. [PubMed] [Google Scholar]
- 10. van den Hoogen P, van den Akker F, Deddens JC, Sluijter JPG. Heart failure in chronic myocarditis: a role for microRNAs? Curr Genomics 2015; 16: 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT, Klingel K, Kandolf R, Sechtem U. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 2006; 114: 1581–1590. [DOI] [PubMed] [Google Scholar]
- 12. Baccouche H, Mahrholdt H, Meinhardt G, Merher R, Voehringer M, Hill S, Klingel K, Kandolf R, Sechtem U, Yilmaz A. Diagnostic synergy of non‐invasive cardiovascular magnetic resonance and invasive endomyocardial biopsy in troponin‐positive patients without coronary artery disease. Eur Heart J 2009; 30: 2869–2879. [DOI] [PubMed] [Google Scholar]
- 13. Calabrese F, Angelini A, Carturan E, Thiene G. Myocarditis and inflammatory cardiomyopathy: histomorphological diagnosis. Ernst Schering Res Found Workshop 2006; 55: 305–321. [DOI] [PubMed] [Google Scholar]
- 14. Leone O, Veinot JP, Angelini A, Baandrup UT, Basso C, Berry G, Bruneval P, Burke M, Butany J, Calabrese F, d'Amati G, Edwards WD, Fallon JT, Fishbein MC, Gallagher PJ, Halushka MK, McManus B, Pucci A, Rodriguez ER, Saffitz JE, Sheppard MN, Steenbergen C, Stone JR, Tan C, Thiene G, van der Wal AC, Winters GL. 2011 consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc Pathol 2012; 21: 245–274. [DOI] [PubMed] [Google Scholar]
- 15. Yilmaz A, Kindermann I, Kindermann M, Mahfoud F, Ukena C, Athanasiadis A, Hill S, Mahrholdt H, Voehringer M, Schieber M, Klingel K, Kandolf R, Böhm M, Sechtem U. Comparative evaluation of left and right ventricular endomyocardial biopsy: differences in complication rate and diagnostic performance. Circulation 2010; 122: 900–909. [DOI] [PubMed] [Google Scholar]
- 16. Gutberlet M, Spors B, Thoma T, Bertram H, Denecke T, Felix R, Noutsias M, Schultheiss HP, Kühl U. Suspected chronic myocarditis at cardiac MR: diagnostic accuracy and association with immunohistologically detected inflammation and viral persistence. Radiology 2008; 246: 401–409. [DOI] [PubMed] [Google Scholar]
- 17. Tschöpe C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB, Hare JM, Heidecker B, Heymans S, Hübner N, Kelle S, Klingel K, Maatz H, Parwani AS, Spillmann F, Starling RC, Tsutsui H, Seferovic P, van Linthout S. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol 2020: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weintraub RG, Semsarian C, Macdonald P. Dilated cardiomyopathy. Lancet 2017; 390: 400–414. [DOI] [PubMed] [Google Scholar]
- 19. De Rosa S, Curcio A, Indolfi C. Emerging role of microRNAs in cardiovascular diseases. Circ J 2014; 78: 567–575. [DOI] [PubMed] [Google Scholar]
- 20. Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, Vetrie D, Okkenhaug K, Enright AJ, Dougan G, Turner M, Bradley A. Requirement of bic/microRNA‐155 for normal immune function. Science 2007; 316: 608–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vigorito E, Kohlhaas S, Lu D, Leyland R. miR‐155: an ancient regulator of the immune system. Immunol Rev 2013; 253: 146–157. [DOI] [PubMed] [Google Scholar]
- 22. Yan L, Hu F, Yan X, Wei Y, Ma W, Wang Y, Lu S, Wang Z. Inhibition of microRNA‐155 ameliorates experimental autoimmune myocarditis by modulating Th17/Treg immune response. J Mol Med (Berl) 2016; 94: 1063–1079. [DOI] [PubMed] [Google Scholar]
- 23. Corsten MF, Papageorgiou A, Verhesen W, Carai P, Lindow M, Obad S, Summer G, Coort SLM, Hazebroek M, van Leeuwen R, Gijbels MJJ, Wijnands E, Biessen ELA, de Winther MPJ, Stassen FRM, Carmeliet P, Kauppinen S, Schroen B, Heymans S. MicroRNA profiling identifies microRNA‐155 as an adverse mediator of cardiac injury and dysfunction during acute viral myocarditis. Circ Res 2012; 111: 415–425. [DOI] [PubMed] [Google Scholar]
- 24. Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, Fabbri M, Alder H, Liu CG, Calin GA, Croce CM. Modulation of miR‐155 and miR‐125b levels following lipopolysaccharide/TNF‐α stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol 2007; 179: 5082–5089. [DOI] [PubMed] [Google Scholar]
- 25. Takeda K, Akira S. Toll‐like receptors in innate immunity. Int Immunol 2005; 17: 1–14. [DOI] [PubMed] [Google Scholar]
- 26. Lin CY, Lee HC, Fu CY, Ding YY, Chen JS, Lee MH, Huang WJ, Tsai HJ. MiR‐1 and miR‐206 target different genes to have opposing roles during angiogenesis in zebrafish embryos. Nat Commun 2013; 4: 2829. [DOI] [PubMed] [Google Scholar]
- 27. Westendorp B, Major JL, Nader M, Salih M, Leenen FH, Tuana BS. The E2F6 repressor activates gene expression in myocardium resulting in dilated cardiomyopathy. FASEB J 2012; 26: 2569–2579. [DOI] [PubMed] [Google Scholar]
- 28. Shan ZX, Lin QX, Fu YH, Deng CY, Zhou ZL, Zhu JN, Liu XY, Zhang YY, Li Y, Lin SG, Yu XY. Upregulated expression of miR‐1/miR‐206 in a rat model of myocardial infarction. Biochem Biophys Res Commun 2009; 381: 597–601. [DOI] [PubMed] [Google Scholar]
- 29. Bowles NE, Ni J, Kearney DL, Pauschinger M, Schultheiss HP, McCarthy R, Hare J, Bricker JT, Bowles KR, Towbin JA. Detection of viruses in myocardial tissues by polymerase chain reaction: evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol 2003; 42: 466–472. [DOI] [PubMed] [Google Scholar]
- 30. Kuethe F, Sigusch HH, Hilbig K, Tresselt C, Glück B, Egerer R, Figulla HR. Detection of viral genome in the myocardium: lack of prognostic and functional relevance in patients with acute dilated cardiomyopathy. Am Heart J 2007; 153: 850–858. [DOI] [PubMed] [Google Scholar]
- 31. Rigopoulos AG, Klutt B, Matiakis M, Apostolou A, Mavrogeni S, Noutsias M. Systematic review of PCR proof of parvovirus B19 genomes in endomyocardial biopsies of patients presenting with myocarditis or dilated cardiomyopathy. Viruses 2019; 11: 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Norja P, Hokynar K, Aaltonen LM, Chen R, Ranki A, Partio EK, Kiviluoto O, Davidkin I, Leivo T, Eis‐Hübinger AM, Schneider B, Fischer HP, Tolba R, Vapalahti O, Vaheri A, Söderlund‐Venermo M, Hedman K. Bioportfolio: lifelong persistence of variant and prototypic erythrovirus DNA genomes in human tissue. Proc Natl Acad Sci U S A 2006; 103: 7450–7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Verdonschot J, Hazebroek M, Merken J, Debing Y, Dennert R, Brunner‐La Rocca HP, Heymans S. Relevance of cardiac parvovirus B19 in myocarditis and dilated cardiomyopathy: review of the literature. Eur J Heart Fail 2016; 18: 1430–1441. [DOI] [PubMed] [Google Scholar]
- 34. Lindner J, Noutsias M, Lassner D, Wenzel J, Schultheiss HP, Kuehl U, Modrow S. Adaptive immune responses against parvovirus B19 in patients with myocardial disease. J Clin Virol 2009; 44: 27–32. [DOI] [PubMed] [Google Scholar]
- 35. Streitz M, Noutsias M, Volkmer R, Rohde M, Brestrich G, Block A, Klippert K, Kotsch K, Ay B, Hummel M, Kühl U, Lassner D, Schultheiss HP, Volk HD, Kern F. NS1 specific CD8+ T‐cells with effector function and TRBV11 dominance in a patient with parvovirus B19 associated inflammatory cardiomyopathy. PLoS One 2008; 3: e2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Besler C, Urban D, Watzka S, Lang D, Rommel KP, Kandolf R, Klingel K, Thiele H, Linke A, Schuler G, Adams V, Lurz P. Endomyocardial miR‐133a levels correlate with myocardial inflammation, improved left ventricular function, and clinical outcome in patients with inflammatory cardiomyopathy. Eur J Heart Fail 2016; 18: 1442–1451. [DOI] [PubMed] [Google Scholar]
- 37. Kuehl U, Lassner D, Gast M, Stroux A, Rohde M, Siegismund C, Wang X, Escher F, Gross M, Skurk C, Tschoepe C, Loebel M, Scheibenbogen C, Schultheiss HP, Poller W. Differential cardiac microRNA expression predicts the clinical course in human enterovirus cardiomyopathy. Circ Heart Fail 2015; 8: 605–618. [DOI] [PubMed] [Google Scholar]
- 38. Satoh M, Minami Y, Takahashi Y, Tabuchi T, Nakamura M. Expression of microRNA‐208 is associated with adverse clinical outcomes in human dilated cardiomyopathy. J Card Fail 2010; 16: 404–410. [DOI] [PubMed] [Google Scholar]
- 39. Jakob P, Doerries C, Briand S, Mocharla P, Kränkel N, Besler C, Mueller M, Manes C, Templin C, Baltes C, Rudin M, Adams H, Wolfrum M, Noll G, Ruschitzka F, Lüscher TF, Landmesser U. Loss of angiomiR‐126 and 130a in angiogenic early outgrowth cells from patients with chronic heart failure: role for impaired in vivo neovascularization and cardiac repair capacity. Circulation 2012; 126: 2962–2975. [DOI] [PubMed] [Google Scholar]
- 40. Skouri HN, Dec GW, Friedrich MG, Cooper LT. Noninvasive imaging in myocarditis. J Am Coll Cardiol 2006; 48: 2085–2093. [DOI] [PubMed] [Google Scholar]
- 41. Lagan J, Schmitt M, Miller CA. Clinical applications of multi‐parametric CMR in myocarditis and systemic inflammatory diseases. Int J Cardiovasc Imaging 2018; 34: 35–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ammirati E, Frigerio M, Adler ED, Basso C, Birnie DH, Brambatti M, Friedrich MG, Klingel K, Lehtonen J, Moslehi JJ, Pedrotti P, Rimoldi OE, Schultheiss HP, Tschöpe C, Cooper LT Jr, Camici PG. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail 2020; 13: e007405. [DOI] [PMC free article] [PubMed] [Google Scholar]


