Abstract
Aims
Outflow graft obstruction is a poorly described complication following left ventricular assist device (LVAD) surgery. We sought to define the incidence of LVAD outflow graft obstruction and assess clinical outcomes with a percutaneous treatment strategy.
Methods and results
From January 2012 to October 2020, 322 patients with LVAD were managed at our institution. Patients with LVAD outflow graft obstruction were identified by cardiac computed tomography with angiography and invasive haemodynamic assessment and were subsequently treated with percutaneous intervention. Poisson regression was used to analyse time‐dependent differences in the incidence of LVAD outflow graft obstruction. Kaplan–Meier analysis was used to estimate survival. Twenty patients (6.2%) developed haemodynamically significant LVAD outflow graft obstruction at a rate of 0.03 events per patient‐year. Outflow graft obstruction presented a median of 33 (26–49) months after surgery. Patients presented with low estimated LVAD pump flow (95%), heart failure (90%), or both (85%), and 59% developed cardiogenic shock prior to intervention. The most common aetiology identified by cardiac computed tomography with angiography was external compression of the outflow graft (78%). On presentation, the median peak gradient in the outflow graft was 78 (64–100) mmHg. Outflow graft stenting was 100% successful with no in‐hospital mortality, and it reduced the peak outflow graft gradient to 10 (2–17) mmHg (P < 0.001). Outflow graft stenting was durable with two patients (10%) requiring a repeat procedure over a median follow‐up of 13 (7–20) months and did not impact survival.
Conclusions
Left ventricular assist device outflow graft obstruction is a relatively common and underappreciated cause of recurrent heart failure and LVAD dysfunction. Outflow graft stenting can be achieved with low morbidity and provides a long‐term solution to this complication.
Keywords: LVAD, Outflow graft, Stenosis, Stent
Introduction
Recurrent heart failure (HF) after left ventricular assist device (LVAD) implantation is a morbid complication most commonly caused by pump thrombosis or right ventricular dysfunction. 1 Obstruction of the LVAD outflow graft, the incidence of which is unknown, can also cause HF. Historically, surgical intervention has been the treatment of choice, but this carries substantial morbidity. Case reports suggest that percutaneous intervention is feasible although no published safety or long‐term outcomes data exist (Supporting Information, Tables S1 and S2 ). In this retrospective single‐centre analysis, we estimate the incidence, characterize the aetiology, and define clinical outcomes with percutaneous therapy in patients with LVAD outflow graft obstruction.
Methods
Left ventricular assist device parameters were obtained from log file data before LVAD dysfunction, at presentation, and after intervention (Supporting Information, Figure S1 ). Continuous variables are mean (± standard deviation) or median (interquartile range). Categorical variables are count (proportion). Between‐groups analyses were compared with Mann–Whitney or Kruskal–Wallis testing; when indicated, post hoc Dunn test was performed with Bonferroni correction for multiple hypothesis testing. Incidence was calculated as events per patient‐year (EPPY) relative to the entire cohort. Poisson regression was used to examine time‐dependent differences in incidence by LVAD type. Survival from time of LVAD implantation with and without outflow graft obstruction was determined by Kaplan–Meier analysis. The Emory University Institutional Review Board approved this study.
Results
Between 1 January 2012 and 31 October 2020, 309 patients underwent LVAD implantation at our centre. An additional 13 patients transferred their care to our centre, yielding a final cohort of 322 patients [median follow‐up of 17 (6–32) months; Supporting Information, Table S3 ]. Twenty patients (6.2%; 18 implanted at our centre and 2 implanted elsewhere) developed outflow graft obstruction, an incidence of 0.03 EPPY. The incidence was similar between HVAD (0.05 EPPY), HeartMate II (0.02 EPPY, P = 0.15 vs. HVAD), and HeartMate 3 (0.02 EPPY, P = 0.52 vs. HVAD). Two patients required restenting of their outflow graft at 15 and 43 months after the prior stent.
Baseline characteristics are presented in Table 1 . Laboratory evaluation was unrevealing. Notably, lactate dehydrogenase levels were within normal limits in 13 patients (65%), and none exceeded 1.5× the upper limit of normal, excluding significant haemolysis, which is frequently caused by LVAD pump thrombosis. Patients presented with low estimated LVAD flow (95%) or HF (90%), with most (85%) having both (Figure 1 A,B ). A majority of patients presented or developed cardiogenic shock requiring pre‐procedural inotropic or intra‐aortic balloon pump support (59%). On presentation, pump power had decreased by 19% (7–28%) from baseline (0.9 ± 0.7 W; P = 0.01); none had elevated power. Estimated LVAD flow declined by 46% (39–55%) from baseline (2.1 ± 0.9 L/min, P < 0.001).
Table 1.
Characteristics at presentation with outflow graft obstruction
| Clinical parameters | N = 20 |
|---|---|
| Age, years | 49 (43–60) |
| Male | 13 (65) |
| Body mass index, kg/m2 | 31.4 ± 6.3 |
| Hypertension | 13 (65) |
| Diabetes mellitus | 10 (50) |
| Atrial fibrillation | 8 (40) |
| Ischaemic cardiomyopathy | 5 (25) |
| Medications | |
| Aspirin | 18 (90) |
| Warfarin | 20 (100) |
| INR | 3.2 ± 1.3 |
| Beta‐blocker | 13 (65) |
| ACEi/ARB | 11 (55) |
| Aldosterone antagonist | 11 (55) |
| Loop diuretic | 18 (90) |
| LVAD details | |
| Implantation strategy | |
| Destination therapy | 14 (70) |
| Bridge‐to‐transplantation | 6 (30) |
| Device type | |
| HeartMate II | 3 (15) |
| HeartWare | 14 (70) |
| HeartMate 3 | 3 (15) |
| Clinical presentation | |
| Heart failure | 18 (90) |
| Low LVAD flow | 19 (95) |
| Presenting labs | |
| LDH, whole cohort (unit/L) | 263 ± 74 |
| LDH, HMII | 296 ± 55 |
| LDH, HVAD | 245 ± 68 |
| LDH, HM3 | 312 ± 75 |
| Sodium (mmol/L) | 137 ± 3 |
| Blood urea nitrogen (mg/dL) | 23 ± 14 |
| Creatinine (mg/dL) | 1.46 ± 0.41 |
| GFR (mL/min/1.73 m2) | 63 ± 22 |
| Haemoglobin (g/dL) | 10.9 ± 1.5 |
| Platelet count (103/μL) | 203 ± 68 |
| Total bilirubin (mg/dL) | 1.0 ± 0.6 |
| Aspartate aminotransferase (unit/L) | 31 ± 19 |
| Lesion details | (N = 18) a |
| Lesion type | |
| External compression of graft | 14 (78) |
| Stenosis of the aortic anastomosis | 7 (39) |
| Kink | 7 (39) |
| Multiple lesions | 10 (56) |
ACEi/ARB, angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; GFR, glomerular filtration rate; HM3, HeartMate 3; HMII, HeartMate II; INR, international normalized ratio; LDH, lactate dehydrogenase; LVAD, left ventricular assist device.
Data are expressed as n (%), mean ± standard deviation, or median (interquartile range).
Not identified in two patients due to severe kidney disease.
Figure 1.
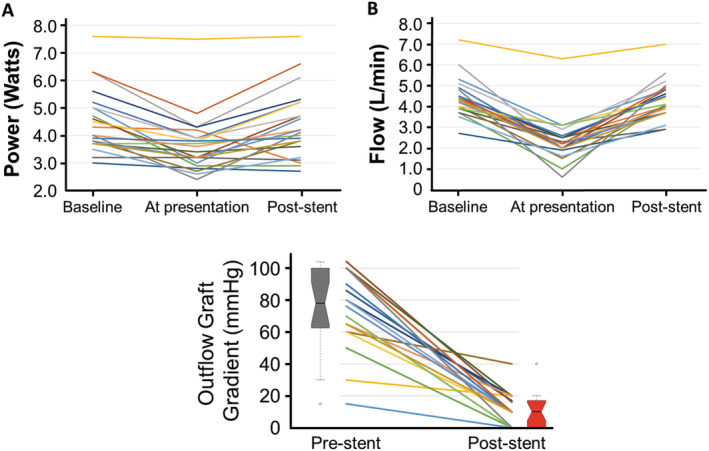
Changes in left ventricular assist device (A) power and (B) estimated flow at baseline, on presentation with outflow graft obstruction, and after percutaneous intervention. (C) Change in left ventricular assist device outflow graft pressure gradient pre‐stent and post‐stent placement. Boxes demonstrate median and interquartile range; notches represent the 95% confidence interval around the median; whiskers identify pressures up to 1.5× interquartile range; and circles represent extreme outliers.
Outflow graft flow velocity estimated by echocardiography revealed an elevated peak gradient of 42 mmHg (21–69 mmHg) but could not be measured in 29%. Cardiac computed tomography with angiography identified the aetiology in all cases (Supporting Information, Figure S2 ): external graft compression (78%), stenosis of the aortic anastomosis (39%), and discrete kinking (39%); 56% presented with multiple lesions. Notably, we did not identify thrombus within the outflow graft. Otherwise, our data are similar to published reports (Supporting Information, Tables S1 and S2 ).
Cardiac catheterization with haemodynamic assessment and stenting of the outflow graft was performed in all patients (Supporting Information, Table S4 ). Median time from LVAD implant to stenting was 33 (26–49) months; 15 patients (75%) presented >2 years after LVAD implantation; only three (15%) presented within the first post‐operative year. These data are similar to published reports [26 (12–40) months; P = 0.11]. At catheterization, the median pre‐stent peak gradient was 78 mmHg (64–100 mmHg; Figure 1 C ), which was higher than that measured by echocardiography (P = 0.004). After stenting, the gradient decreased to 10 mmHg (2–17 mmHg; P < 0.001) and estimated LVAD flow increased by 1.9 ± 0.9 L/min (P < 0.001).
There was 0% in‐hospital mortality. Twenty‐three total procedures were performed in 20 patients. One individual underwent a staged procedure while two underwent repeat stenting of different parts of the outflow graft for recurrent HF and low flow. Four procedural complications occurred (17%; see Supplementary Methods): one embolic stroke and three instances of acute blood loss anaemia requiring transfusion (range 1–4 units). Notably, all four complications occurred during our first six procedures. Patients were discharged a median of 4 (2–10) days post‐stent. Over 15 ± 10 months of follow‐up, two patients (10%) developed recurrent outflow graft stenosis and underwent a second procedure, two patients (10%) were successfully bridged to heart transplantation, and nine patients (45%) died a median of 13 (7–17) months after stent placement. LVAD outflow graft obstruction was not associated with survival (Supporting Information, Figure S3 ).
Conclusions
These data are the first to estimate the incidence of LVAD outflow graft obstruction and demonstrate the durability of percutaneous intervention. Our data suggest that LVAD outflow graft obstruction is more common than previously appreciated and is highly morbid with over half of our population (59%) developing shock. Long‐term LVAD support is rising due to steadily improving survival, increasing use of a destination therapy implant strategy, and recent changes to the heart transplant listing criteria that prolong wait times for bridge‐to‐transplant patients in the USA. As this complication usually presents >2 years after implant, the incidence of outflow graft obstruction is likely to increase.
Identifying patients with outflow graft obstruction requires elimination of other causes of HF or low LVAD flow. We created a diagnostic algorithm to guide management (Figure 2 ). Outflow graft obstruction can be distinguished from VAD thrombosis by near‐normal lactate dehydrogenase levels with stable to decreased pump power. LVAD log files revealed that outflow graft obstruction more commonly presented with an insidious decline in flow over weeks to months. However, in 25% estimated flow decreased abruptly within days. Therefore, the rapidity with which flow drops does not distinguish between inflow and outflow cannula problems as proposed elsewhere. 2 Reports suggest that adequate echocardiographic images of the outflow graft can be obtained in >90%. 3 However, our echocardiographic success rate was only 71%, and the pressure gradient estimated was significantly lower than by catheterization. Thus, additional testing must be considered if the clinical suspicion remains high.
Figure 2.
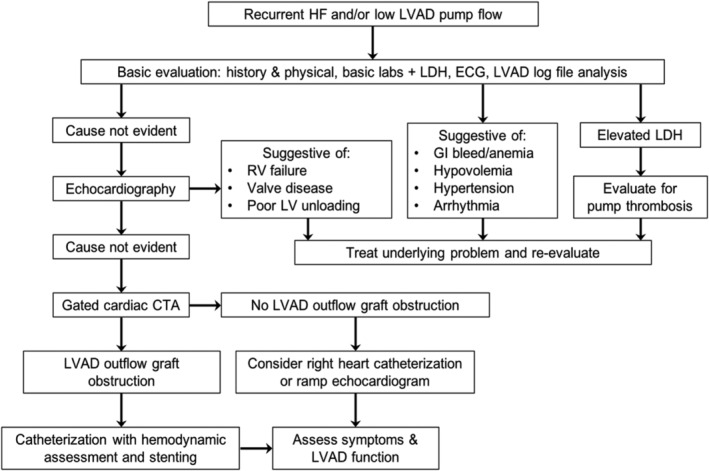
Algorithm for the evaluation of LVAD outflow graft obstruction. CTA, computed tomography with angiography; ECG, electrocardiogram; GI, gastrointestinal; HF, heart failure; LDH, lactate dehydrogenase; LV, left ventricular; LVAD, left ventricular assist device; RV, right ventricular.
Cardiac computed tomography with angiography is essential to define the cause of outflow graft obstruction. The most common aetiology in our series and other reports is external graft compression. At implant, the outflow graft is typically wrapped circumferentially in Gore‐Tex to protect it from direct trauma and adhesions on future sternal re‐entry. 4 External graft compression is due to build‐up of a largely acellular fibrinous material within this protective wrap. We and others now advocate leaving the wrap open posteriorly at implant, 5 although it is noted that compression is still possible in the circumferentially wrapped bend relief segment. Stent placement at the site of external compression typically caused migration of this material within the wrap leading to a shift in the area of maximal stenosis (akin to squeezing a tube of toothpaste). Consequently, our approach is to stent the entire length of the outflow graft. Two patients from our early experience who did not have the entire length of the outflow graft stented presented 15 and 43 months later, respectively, with low flow and HF, requiring restenting of the entire outflow graft.
This study has several key limitations. As a retrospective single‐centre study, our data are subject to bias and unmeasured confounding and, therefore, may not be generalizable. However, 4% of our patients, including two with outflow graft obstruction, were implanted elsewhere, and our literature review revealed a similar spectrum of outflow graft obstruction from centres around the world. Additionally, the small number of affected patients, lack of prospective testing, and our evolving approach with growing recognition of this problem also make our analysis of incidence susceptible to error.
In summary, these data critically extend our understanding of this common and morbid complication and mirror case reports from around the world. We also demonstrate that a percutaneous treatment strategy has a high likelihood of success and low operative morbidity and therapeutic durability, making it a safe alternative to surgical correction.
Conflict of interest
None of the authors except V.B. have any disclosures. Dr. Babaliaros is a consultant for Edwards Lifesciences and Abbott Vascular and has an equity interest in Transmural Systems.
Funding
No funding was received for the production of this manuscript.
Supporting information
Table S1. Published literature on LVAD outflow graft obstruction.
Table S2. Summary of published LVAD outflow graft obstruction case reports.
Table S3. Baseline Characteristics of Entire LVAD Cohort
Table S4. Procedural Details.
Figure S1. LVAD Log File Analysis: Sample log file data illustrating changes in LVAD speed, pump power and flow before and after outflow graft stent placement. LVAD outflow graft obstruction can present (A) slowly over months and even years, or (B) rapidly over days and even hours. RPM – revolutions per minute (VAD set speed); W – Watts, mW – milliWatts (VAD pump power); mL/min – milliliters per minute, L/m – liters/min (estimated VAD flow).
Figure S2. LVAD Outflow Graft Assessment: Example patient imaging: (A) 3D volume rendering CTA showing 2 regions of external compression (arrowheads) and stenosis of the aortic anastomosis (circle, inset). (B) Linear interpolation of the outflow graft showing the areas in question (arrows). (C) Direct angiography demonstrating overlapping placement of six stents (approximate location represented by colored lines). Invasive hemodynamics demonstrated a pre‐stent peak pressure gradient of 86 mmHg (D) with improvement to 17mmgHg (E) after stenting.
Figure S3. Kaplan–Meier analysis of survival in LVAD patients with or without outflow graft obstruction. A sensitivity analysis was performed by removal of patients with early post‐op mortality (n = 35; 29 (83%) died; 5 (14%) remain alive but are less than 30d out from implant by October 31, 2020; and 1 pt transferred their care to another facility <30d after implant).
Agrawal, A. , Alexy, T. , Kamioka, N. , Shafi, T. , Stowe, J. , Morris, A. A. , Vega, J. D. , Babaliaros, V. , and Burke, M. A. (2021) Outflow graft obstruction after left ventricular assist device implantation: a retrospective, single‐centre case series. ESC Heart Failure, 8: 2349–2353. 10.1002/ehf2.13333
References
- 1. Burke MA, Givertz MM. Assessment and management of heart failure after left ventricular assist device implantation. Circulation 2014; 129: 1161–1166. [DOI] [PubMed] [Google Scholar]
- 2. Scandroglio AM, Kaufmann F, Pieri M, Kretzschmar A, Muller M, Pergantis P, Dreysse S, Falk V, Krabatsch T, Potapov EV. Diagnosis and treatment algorithm for blood flow obstructions in patients with left ventricular assist device. J Am Coll Cardiol 2016; 67: 2758–2768. [DOI] [PubMed] [Google Scholar]
- 3. Grinstein J, Kruse E, Collins K, Sayer G, Fedson S, Kim GH, Sarswat N, Adatya S, Ota T, Jeevanandam V, Mor‐Avi V, Uriel N, Lang RM. Screening for outflow cannula malfunction of left ventricular assist devices (LVADs) with the use of Doppler echocardiography: new LVAD‐specific reference values for contemporary devices. J Card Fail 2016; 22: 808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leprince P, Rahmati M, Bonnet N, Bors V, Rama A, Leger P, Gandjbakhch I, Pavie A. Expanded polytetrafluoroethylene membranes to wrap surfaces of circulatory support devices in patients undergoing bridge to heart transplantation. Eur J Cardiothorac Surg 2001; 19: 302–306. [DOI] [PubMed] [Google Scholar]
- 5. Rajagopal K, Salas de Armas IA, Jumean MF, Gregoric ID. Surgical treatment of late left ventricular assist device outflow obstruction due to extrinsic compression. J Heart Lung Transplant 2019; 38: 472–474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Published literature on LVAD outflow graft obstruction.
Table S2. Summary of published LVAD outflow graft obstruction case reports.
Table S3. Baseline Characteristics of Entire LVAD Cohort
Table S4. Procedural Details.
Figure S1. LVAD Log File Analysis: Sample log file data illustrating changes in LVAD speed, pump power and flow before and after outflow graft stent placement. LVAD outflow graft obstruction can present (A) slowly over months and even years, or (B) rapidly over days and even hours. RPM – revolutions per minute (VAD set speed); W – Watts, mW – milliWatts (VAD pump power); mL/min – milliliters per minute, L/m – liters/min (estimated VAD flow).
Figure S2. LVAD Outflow Graft Assessment: Example patient imaging: (A) 3D volume rendering CTA showing 2 regions of external compression (arrowheads) and stenosis of the aortic anastomosis (circle, inset). (B) Linear interpolation of the outflow graft showing the areas in question (arrows). (C) Direct angiography demonstrating overlapping placement of six stents (approximate location represented by colored lines). Invasive hemodynamics demonstrated a pre‐stent peak pressure gradient of 86 mmHg (D) with improvement to 17mmgHg (E) after stenting.
Figure S3. Kaplan–Meier analysis of survival in LVAD patients with or without outflow graft obstruction. A sensitivity analysis was performed by removal of patients with early post‐op mortality (n = 35; 29 (83%) died; 5 (14%) remain alive but are less than 30d out from implant by October 31, 2020; and 1 pt transferred their care to another facility <30d after implant).


