Abstract
Aims
Mechanical circulatory support (MCS) results in substantial improvement of prognosis and functional capacity. Currently, duration of MCS as a bridge to transplantation (BTT) is often prolonged due to shortage of donor hearts. Because long‐term results of exercise capacity after MCS are largely unknown, we studied serial cardiopulmonary exercise tests (CPETs) during the first year after MCS implantation.
Methods and results
Cardiopulmonary exercise tests at 6 and 12 months after MCS implantation in BTT patients were retrospectively analysed, including clinical factors related to exercise capacity. A total of 105 MCS patients (67% male, 50 ± 12 years) underwent serial CPET at 6 and 12 months after implantation. Power (105 ± 35 to 114 ± 40 W; P ≤ 0.001) and peak VO2 per kilogram (pVO2/kg) improved significantly (16.5 ± 5.0 to 17.2 ± 5.5 mL/kg/min (P = 0.008)). Improvement in pVO2 between 6 and 12 months after LVAD implantation was not related to heart failure aetiology or haemodynamic severity prior to MCS. We identified maximal heart rate at exercise as an important factor for pVO2. Younger age and lower BMI were related to further improvement. At 12 months, 25 (24%) patients had a normal exercise capacity (Weber classification A, pVO2 > 20 mL/kg/min).
Conclusions
Exercise capacity (power and pVO2) increased significantly between 6 and 12 months after MCS independent of Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profile or heart failure aetiology. Heart rate at exercise importantly relates to exercise capacity. This long‐term improvement in exercise capacity is important information for the growing group of long‐term MCS patients as this is critical for the quality of life of patients.
Keywords: Mechanical circulatory support, Quality of life, Functional capacity, Cardiopulmonary exercise test. VO2
Introduction
Mechanical circulatory support (MCS) by a continuous‐flow left ventricular assist device (cf‐LVAD) has shown to improve survival and quality of life in selected patients with end‐stage heart failure. 1 , 2 , 3 , 4 The present generation of cf‐LVADs consists of axial or centrifugal flow pumps operating at a fixed pump speed (RPM), thereby creating a totally different physiology than the native heart. Because of the shortage of donor hearts, resulting in longer waiting time and the use of LVAD's as destination therapy, long‐term MCS has increased substantially. In this respect, functional capacity after implantation is getting more and more important for a better quality of life and the opportunity to reintegrate into society.
Previous small studies reported improved exercise capacity after MCS compared with the situation prior to implantation, as assessed by cardiopulmonary exercise test (CPET). 5 , 6 , 7 , 8 , 9 However, information about long‐term exercise capacity in MCS patients is currently sparse. Previously, lower age, aerobic training, and LVAD settings, such as pump speed, pump flow, and power, have been associated with improved exercise capacity after MCS. 8 , 9 , 10 , 11 Aetiology of the cardiomyopathy was not associated with CPET results. 8 Markers for cardiac function, such as left ventricular ejection fraction (LVEF), peak heart rate, right ventricular function, and aortic valve opening, have not consistently been associated with better CPET results. 8 , 9 , 10
At our centre, MCS patients perform a CPET, together with an echocardiogram at rest, laboratory test, and full examination, on a routine basis at 6 and 12 months after implantation. As a result, many data were collected over the years.
The aim of this study was to investigate whether exercise capacity during the first year of MCS treatment showed a long‐term improvement, measured by CPET at 6 and 12 months after implantation, in a large cohort of MCS patients. Also, factors associated with exercise capacity were analysed, including aetiology of heart failure, echocardiographic parameters, markers for left ventricular unloading, heart failure medication, chronotropic (in)competence, and pre‐operative Inter‐agency Registry for Mechanically Assisted Circulatory Support (INTERMACS) classification. 12
Methods
Study population
Between 2006 and 2017, 229 adult patients received MCS [HeartMate‐II (HM‐II, Abbott), HVAD (Heartware, Medtronic), or HeartMate3 (HM3, Abbott)] at the University Medical Center Utrecht, the Netherlands, all implanted as a bridge‐to‐transplantation (BTT). After MCS, patients underwent cardiac rehabilitation two times a week for 1 h per session under the supervision of a second‐line physiotherapist. Outpatient rehabilitation started after discharge for a total duration of 6 weeks up to 3 months, conforming individual needs. During the training, patients improved their exercise tolerance on a treadmill/hometrainer and trained the large muscles for strength and endurance. Afterwards, patients are supported to improve or at least maintain the exercise performance achieved at the end of the rehabilitation programme. This study was approved by our institutional ethics committee.
Eighty‐three patients did not perform a CPET at all in the first year following MCS implantation, because of physical impairment in 53 patients (23%), early heart transplantation in three patients (1%), and death in 27 patients (12%). At 6 months, 146 patients performed a CPET. Thereafter, 13 patients underwent a heart transplantation, three patients died, and 25 were physically unable to perform a CPET at 12 months, mainly due to extra‐cardiac morbidity (Figure 1 ). Thus, 105 patients underwent sequential CPET both at 6 and 12 months after MCS implantation.
Figure 1.
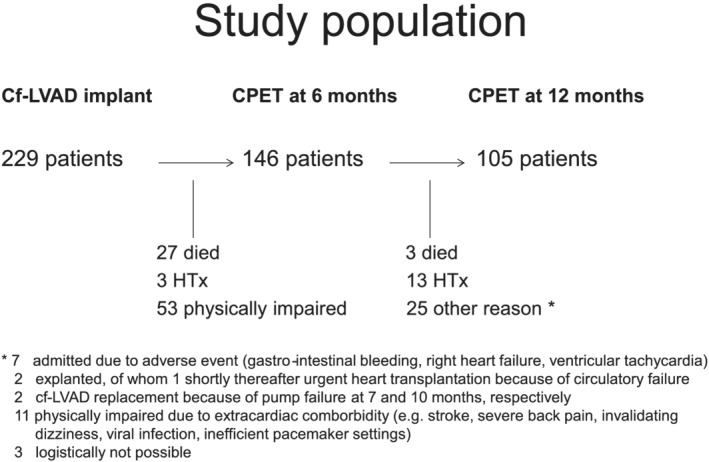
Study population.
Routine cardiopulmonary exercise test, echocardiography, and laboratory testing
Cardiopulmonary exercise test was planned prospectively at 6 and 12 months after MCS, together with an echocardiogram at rest, blood test, and full examination. CPET was performed on a bicycle ergometer using a symptom‐limited ramp protocol with an increase of workload by 10 Watt every minute. Respiratory gas analysis was analysed continuously (Ergostik, Geratherm Respiratory, Bad Kissingen, Germany).
Peak VO2 (pVO2), peak VO2 per kilogram (pVO2/kg), percentage of predicted peak VO2 (per kilogram) levels according to Jones, 13 the anaerobic threshold (AT), the respiratory exchange ratio (RER), EqCO2, maximal heart rate, and maximal workload were reported. The anaerobic threshold was defined as the oxygen uptake before the increase in the ventilatory equivalent for oxygen (VE/VO2), without an increase in the ventilatory equivalent for carbon dioxide (VE/VCO2), and using the V‐slope method. The ventilatory response to exercise was defined as VE/VCO2 (EqCO2) at peak exercise.
Delta pVO2 and delta pVO2/kg were defined as the absolute difference between pVO2(/kg) at 6 and 12 months. Results of CPET were reported by an independent cardiologist.
Echocardiography at rest was used to measure two‐dimensional left ventricular dimensions. Furthermore, assessment of the right ventricular function by the tricuspid annular plane systolic excursion (TAPSE) and tissue Doppler imaging (TDI‐RV) and (intermittent) opening of the aortic valve were performed.
Laboratory tests included haemoglobin (g/dL), bilirubin (mg/dL), B‐type natriuretic peptide (BNP) (pg/mL), and the kidney function, where the latter was divided into normal (estimated glomerular filtration rate (eGFR) > 60 mL/min/1.73 m2) and moderately impaired (eGFR 30–60 mL/min/1.73 m2).
Statistical analysis
For this retrospective analysis, the results of the CPET were collected in a central database, together with baseline characteristics, results of echocardiogram at rest, laboratory results, and medication at 6 and 12 months. Data were extracted to IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, N.Y., USA) for statistical analysis.
Comparison of continuous variables between two groups was performed by a t‐test or non‐parametric test if not normally distributed. Dichotomous variables were compared by χ 2 or Fisher's exact test in unrelated variables and by McNemar in related variables. Correlation between continuous variables was tested using a Pearson or Spearman rank test. Analysis of factors related to differences between the two test moments was analysed by univariable and multivariable linear regression.
Results
Patient characteristics
A total of 105 patients (67% male, mean age 50 ± 12 years at MCS implantation) performed serial CPET in the first year following MCS implantation after a mean of 5.4 (±1.4) months and 12 (±1) months. Prior to MCS implantation 45% of patients were in INTERMACS profile 2 (‘sliding on inotropes’) and 29% were stable on inotropes (INTERMACS 3). Because of severe cardiogenic shock (INTERMACS 1), temporary mechanical support (Centrimag, intra‐aortic balloon pump or extracorporeal membrane oxygenator) as bridge to implantation was necessary in 19% of the patients. Most patients (n = 75; 71%) received an HM‐II, followed by HVAD in 22 patients (21%) and HM3 in eight patients (8%). The underlying aetiology of heart failure was a dilated cardiomyopathy (DCM) in 55 (52%) patients, and 35 (33%) patients suffered from ischaemic heart disease (IHD). Other aetiologies included myocarditis and toxic (chemotherapy‐induced) cardiomyopathy (Table 1 ).
Table 1.
Baseline characteristics of patients who performed serial CPET at 6 and 12 months after MCS (n = 105)
| Baseline characteristics | |
|---|---|
| Gender, male (%) | 70 (67%) |
| Age at MCS implantation (years, mean ± SD) II | 50 ± 12 |
| Device type | |
| HM‐II (%) | 75 (71.4%) |
| HVAD (%) | 22 (21%) |
| HM3 (%) | 8 (7.6%) |
| Aetiology | |
| Dilated | 55 (52%) |
| Ischaemic | 35 (33%) |
| Myocarditis | 1 (1%) |
| Hypertrophic | 1 (1%) |
| Peripartum | 1 (1%) |
| Congenital | 1 (1%) |
| Toxic | 5 (5%) |
| Other | 6 (6%) |
| INTERMACS profile | |
| 1 with temporary support | 20 (19%) |
| 1 without temporary support | 1 (1%) |
| 2 | 47 (45%) |
| 3 | 30 (29%) |
| 4 | 6 (6%) |
| 6 | 1 (1%) |
| Hospital duration, days (mean ± SD) | 48 ± 22 |
HM‐II, HeartMate‐II; HM3, HeartMate 3; HVAD, HeartWare; INTERMACS, Inter‐agency Registry for Mechanically Assisted Circulatory Support; II MCS, mechanical circulatory support.
Cardiopulmonary exercise test
Patients achieved a maximal workload of 105 ± 35 Watt at 6 months, which increased to 114 ± 40 Watt (P ≤ 0.001) at 12 months. The pVO2 and pVO2/kg at 6 months postoperatively were 1.26 ± 0.42 L/min (53 ± 12% of predicted) and 16.5 ± 5.0 mL/kg/min (52 ± 12% of predicted). Peak VO2 further improved at 12 months after MCS both uncorrected (1.35 ± 0.46 L/min; P ≤ 0.001) and corrected for body weight (17.2 ± 5.5 mL/kg/min; P = 0.008), corresponding to an absolute increase in pVO2 of 7% (L/min). The percentage of the predicted pVO2 increased to 57 ± 12% (P ≤ 0.001) and to 55 ± 13% (P ≤ 0.001) for pVO2/kg. The AT increased from 10.8 ± 3.1 to 12.1 ± 3.7 mL/kg/min (P ≤ 0.001). Both maximal heart rate (141 ± 26 bpm vs. 144 ± 26 bpm; P = 0.178) and EqCO2 (36.1 ± 6.0 vs. 36.7 ± 5.8; P = 0.162) did not change between 6 and 12 months. Interestingly, RER was significantly lower during the CPET at 12 months (1.21 ± 0.11 resp. 1.18 ± 0.11; P = 0.021), indicating lower anaerobic metabolism and/or effort (Table 2 ). 14
Table 2.
Diagnostic results at serial follow‐up after MCS implantation (n = 105)
| 6 months after implant | 12 months after implant | P‐value | |
|---|---|---|---|
| BMI (kg/m2, mean ± SD) | 24.5 ± 3.5 | 25.7 ± 5.0 | <0.001 |
| CPET results | |||
| Max workload (Watt, mean ± SD) | 105 ± 35 | 114 ± 40 | <0.001 |
| VO2 (L/min, mean ± SD) | 1.26 ± 0.42 | 1.35 ± 0.46 | <0.001 |
| VO2% predicted (mean ± SD) | 53 ± 12 | 57 ± 12 | <0.001 |
| VO2/kg (mL/kg/min, mean ± SD) | 16.5 ± 5.0 | 17.2 ± 5.5 | 0.008 |
| VO2/kg % predicted (mean ± SD) | 52 ± 12 | 55 ± 13 | <0.001 |
| Anaerobic threshold (mean ± SD) | 10.8 ± 3.1 | 12.1 ± 3.7 | <0.001 |
| Respiratory exchange ratio (mean ± SD) | 1.21 ± 0.11 | 1.18 ± 0.11 | 0.021 |
| EqCO2 (mean ± SD) | 36.0 ± 6.0 | 36.7 ± 5.8 | 0.162 |
| Max heart rate (bpm, mean ± SD) | 141 ± 26 | 144 ± 26 | 0.094 |
| Percentage of max predicted heart rate (mean ± SD) | 83 ± 14 | 85 ± 14 | 0.055 |
| Echocardiography | |||
| LVEDD (mean ± SD) | 59 ± 12 | 58 ± 12 | 0.073 |
| TAPSE (mean ± SD) | 12 ± 3 | 13 ± 3 | 0.637 |
| TDI‐RV (mean ± SD) | 6.1 ± 1.6 | 6.4 ± 2.1 | 0.640 |
| Aortic valve opening (%) | 18/68 (17%) | 20/60 (19%) | 0.302 |
| Laboratory results | |||
| Haemoglobin level (g/dL, mean ± SD) | 0.78 ± 0.35 | 0.88 ± 0.35 | 0.165 |
| Bilirubin (mg/dL, mean ± SD) | 12.9 ± 1.5 | 13.4 ± 1.6 | <0.001 |
| BNP level (pg/mL, mean ± SD) | 151 ± 113 | 190 ± 143 | 0.398 |
| Kidney function, eGFR >60 mL/min/1.73 m2 (%) | 85/104 (81%) | 80/104 (76%) | 0.227 |
| Kidney function, eGFR 30–60 mL/min/1.73 m2 (%) | 19/104 (18%) | 24/104 (23%) | 0.344 |
| Heart failure medication II | |||
| ACE inhibitor/ARB (%) | 54/97 (56%) | 56/94 (60%) | 0.289 |
| Beta‐blocker (%) | 11/97 (11%) | 17/94 (18%) | 0.063 |
| Antihypertensive (%) | 6/97 (6%) | 7/94 (7%) | 1.000 |
| Anti‐arrhythmics (%) | 44/97 (45%) | 47/94 (50%) | 0.727 |
| Sildenafil (%) | 24/97 (25%) | 23/94 (24%) | 1.000 |
| Diuretic (%) | 59/96 (62%) | 52/93 (56%) | 0.092 |
ARB, angiotensin receptor blocker; BMI, body mass index; BNP, B‐type natriuretic peptide; CPET, cardiopulmonary exercise test; eGFR, estimated glomerular filtration rate; EqCO2, ventilatory equivalent for carbon dioxide; II ACE inhibitor, angiotensin converting enzyme inhibitor; LVEDD, two‐dimensional left ventricular end‐diastolic diameter, measured in parasternal long axis (mm); percentage of max predicted heart rate: predicted maximal heart rate, 220‐age; TAPSE, tricuspid annular plane systolic excursion (mm); TDI‐RV, peak systolic tissue Doppler value of the right ventricle (cm/s); VO2(/kg) % predicted, percentage of predicted value according to Jones (mL/kg/min).
At 12 months, 25 (24%) patients had a normal exercise capacity, according to the Weber classification (pVO2 > 20 mL/kg/min, Weber A). 15 This subgroup of patients (mean age 40 ± 13 years, 80% male) had a maximal work load of 158 ± 41 Watt, a pVO2/kg of 24.6 ± 5.6 mL/kg/min, a RER of 1.18 ± 0.1, and a peak heart rate of 164 ± 19 bpm (91 ± 8% of maximal predicted heart rate).
Factors related to exercise capacity
Haemoglobin levels at 6 and 12 months after implantation were included in the analysis as anaemia is associated with worse CPET results due to lower oxygen‐carrying capacity. 14 Mean haemoglobin level at the first CPET was 12.9 ± 1.5 g/dL and increased to 13.4 ± 1.6 g/dL at the second CPET (P ≤ 0.001), but no correlation was found between the increase in haemoglobin levels and the increase (delta) in pVO2/kg (r = 0.183, P = 0.068). BNP levels (151 ± 113 pg/mL vs. 190 ± 143 pg/mL; P = 0.398) and left ventricular end‐diastolic diameter did not change between 6 and 12 months after MCS suggesting a similar amount of LV unloading over time (Table 2 ). Right ventricular function, assessed by TAPSE and TDI‐RV, did not change between 6 and 12 months after MCS. Furthermore, kidney function as a marker of organ perfusion, bilirubin level as a marker for right ventricular failure, heart failure medication affecting the afterload (i.e. ACE inhibitors), and heart rate (beta‐blocker) were analysed as these factors might influence the CPET results. However, no difference in kidney function, bilirubin level, and heart failure medication was noted (Table 2 ).
Subgroup analyses
Dilated cardiomyopathy versus ischaemic heart disease
Previous studies did not show differences in exercise capacity between patients with IHD and a non‐ischaemic cardiomyopathy, probably related to small sample sizes. 16 , 17 In our analysis, patients with a DCM (n = 55) had significant better CPET results both at 6 and 12 months after implantation compared with patients with IHD (n = 35). At 6 months, pVO2 was 1.35 ± 0.46 L/min in DCM patients and 1.11 ± 0.26 L/min in patients with IHD (P = 0.011). This increased to 1.46 ± 0.45 L/min (DCM) versus 1.18 ± 0.36 L/min (IHD) at 12 months (P = 0.003). Maximal workload was also substantially higher in DCM compared with IHD patients both at 6 months (110 ± 36 Watt vs. 90 ± 25 Watt; P = 0.014) and 12 months (120 ± 39 Watt vs. 99 ± 33 Watt; P = 0.015) after implantation. Importantly, the improvement in pVO2 (delta pVO2) and pVO2/kg (delta pVO2/kg) from 6 to 12 months was similar in both groups (P = 0.27 and P = 0.23), as shown in Table 3 . Age, gender, heart failure medication, BNP, and haemoglobin levels did not differ between DCM and IHD patients.
Table 3.
Diagnostic results in dilated and ischaemic cardiomyopathy (n = 90)
| Dilated cardiomyopathy | Ischaemic cardiomyopathy | P value | |
|---|---|---|---|
| Baseline | |||
| Number of patients (%) | 55 (61%) | 35 (39%) | |
| Age at implant | 48.2 ± 14 | 53.1 ± 8.1 | 0.181 |
| Male (%) | 38 (69%) | 23 (66%) | 0.738 |
| INTERMACS 1 with temporary support | 7 (13%) | 12 (34%) | 0.015 |
| INTERMACS 1 without temporary support | 0 (0%) | 1 (3%) | 0.389 |
| INTERMACS 2 | 25 (46%) | 15 (43%) | 0.809 |
| INTERMACS 3 | 18 (33%) | 5 (14%) | 0.051 |
| INTERMACS 4 | 4 (7%) | 2 (6%) | 1.000 |
| INTERMACS 6 | 1 (2%) | 0 (0%) | 1.000 |
| HM‐II | 42 (76.4%) | 22 (63%) | 0.168 |
| HVAD II | 10 (18.2%) | 10 (26%) | 0.248 |
| HM3 | 3 (5.5%) | 3 (9%) | 0.674 |
| Diagnostics: 6 months | |||
| Body mass index (kg/m2, mean ± SD) | 24.4 ± 3.7 | 24.7 ± 3.1 | 0.524 |
| Max load (Watt, mean ± SD) | 110 ± 36 | 90 ± 25 | 0.014 |
| VO2 (L/min, mean ± SD) | 1.35 ± 0.46 | 1.11 ± 0.27 | 0.011 |
| VO2/kg (mL/kg/min, mean ± SD) | 17.4 ± 5.5 | 15.1 ± 3.7 | 0.057 |
| Anaerobic threshold (mean ± SD) | 11.3 ± 3.4 | 10.3 ± 2.6 | 0.250 |
| Respiratory exchange ratio (mean ± SD) | 1.20 ± 0.11 | 1.22 ± 0.10 | 0.312 |
| Max heart rate (bpm, mean ± SD) | 142 ± 26 | 133 ± 26 | 0.127 |
| EqCO2 (mean ± SD) | 35.3 ± 6.2 | 38.1 ± 4.6 | 0.021 |
| Haemoglobin (g/dL, mean ± SD) | 13 ± 1.7 | 12.6 ± 1.3 | 0.444 |
| BNP (pg/mL, mean ± SD) | 186 ± 162 | 211 ± 100 | 0.061 |
| Bilirubin (mg/dL, mean ± SD) | 0.86 ± 0.44 | 0.71 ± 0.22 | 0.242 |
| Use of beta‐blocker (%) | 5/50 (10%) | 4/33 (12%) | 1.000 |
| Use of ACE inhibitor/ARB (%) | 30/50 (60%) | 16/33 (49%) | 0.302 |
| Use of antihypertensive (%) | 5/50 (10%) | 1/33 (3%) | 0.395 |
| Diagnostics: 12 months | |||
| Body mass index (kg/m2, mean ± SD) | 25.3 ± 3.8 | 26.5 ± 6.5 | 0.413 |
| Max load (Watt, mean ± SD) | 120 ± 39 | 99 ± 33 | 0.015 |
| VO2 (L/min, mean ± SD) | 1.46 ± 0.45 | 1.18 ± 0.36 | 0.003 |
| VO2/kg (mL/kg/min, mean ± SD) | 18.3 ± 5.3 | 15.3 ± 4.5 | 0.002 |
| Anaerobic threshold (mean ± SD) | 12.2 ± 3.6 | 11.5 ± 3.3 | 0.477 |
| Respiratory exchange ratio (mean ± SD) | 1.18 ± 0.09 | 1.17 ± 0.11 | 0.659 |
| Max heart rate (bpm, mean ± SD) | 147 ± 26 | 137 ± 26 | 0.166 |
| EqCO2 (mean ± SD) | 35.6 ± 5.0 | 38.9 ± 5.8 | 0.008 |
| Haemoglobin (g/dL, mean ± SD) | 13.6 ± 1.7 | 13 ± 1.6 | 0.137 |
| BNP (pg/mL, mean ± SD) | 115 ± 88 | 190 ± 126 | 0.390 |
| Bilirubin (mg/dL, mean ± SD) | 0.88 ± 0.4 | 0.8 ± 0.3 | 0.092 |
| Use of beta‐blocker (%) | 6/51 (11%) | 6/30 (17%) | 0.345 |
| Use of ACE inhibitor/ARB (%) | 32/51 (63%) | 16/30 (53%) | 0.405 |
| Use of antihypertensive (%) | 5/51 (9%) | 2/30 (6%) | 1.000 |
| Delta VO2 (L/min, mean ± SD) | 0.1 ± 2.3 | 0.1 ± 3.5 | 0.27 |
| Delta VO2/kg (mL/kg/min, mean ± SD) | 0.9 ± 2.8 | 0.2 ± 2.2 | 0.23 |
BNP, B‐type natriuretic peptide; EqCO2, ventilatory equivalent for carbon dioxide; HM‐II, HeartMate‐II; HM3, HeartMate 3; II HVAD, HeartWare; INTERMACS, Inter‐agency Registry for Mechanically Assisted Circulatory Support.
INTERMACS profile
As patients with INTERMACS profile 1 at time of implantation are in a considerably worse condition, we compared the CPET results of these patients with the results of patients in INTERMACS profile 2 and 3. At both test moments, CPET results were comparable between INTERMACS 1 (n = 21) and INTERMACS 2 + 3 (n = 77), both uncorrected and corrected for body weight. At 6 months, pVO2 was 1.31 ± 0.52 and 1.25 ± 0.40 L/min (P = 0.806) and pVO2/kg 17.4 ± 6.1 and 16.2 ± 4.8 mL/kg/min (P = 0.556) for INTERMACS 1 and INTERMACS 2 + 3, respectively. At 12 months, pVO2 was 1.35 ± 0.56 and 1.37 ± 0.44 (P = 0.792) and pVO2/kg 17.4 ± 7.1 and 17.2 ± 5.2 mL/kg/min (P = 0.710), respectively. Delta pVO2 (P = 0.216) and pVO2/kg (P = 0.188) were also similar between INTERMACS 1 and 2 + 3.
Chronotropic (in)competence
Previous studies evaluating the chronotropic response after MCS showed a significantly lower maximal heart rate during CPET compared with healthy individuals. 18 , 19 Therefore, we compared exercise capacity in patients with chronotropic incompetence (CI) versus patients without CI, defined as a peak heart rate <80% of the predicted maximal heart rate for age (220‐age). At 6 months, 67 patients (46%) met the criteria for CI, and 79 (54%) patients reached a maximal heart rate above 80% of predicted (not CI). Compared with patients without CI, patients with CI had a significantly lower pVO2 (1.07 ± 0.24 L/min vs. 1.37 ± 0.45 L/min; P < 0.001) and pVO2/kg (14.1 ± 2.8 mL/kg/min vs. 18.0 ± 5.4 mL/kg/min; P < 0.001). Importantly, RER did not differ between the groups (1.21 ± 0.11, P = 0.972). The importance of heart rate was further illustrated by the presence of a significant correlation between the percentage of predicted maximal heart rate and pVO2/kg at both 6 months (r = 0.299; P = 0.002) and 12 months (r = 0.310; P = 0.001) after implantation as a continuous variable.
Patients with one cardiopulmonary exercise test (6 months only)
Selection bias may be introduced as 53 patients were physically not able to perform any CPET in the first year after MCS implantation and 41 only performed one CPET at 6 months (physically impaired, died, or received heart transplantation). To gain more insight into the reasons for physical impairment, the 1 year incidence of adverse events was registered. In patients who were not able to perform any CPET, the incidence of stroke (haemorrhagic or ischaemic), VAD‐related infection, sepsis, and major bleeding were higher compared with patients who performed a CPET at 6 and 12 months (0.23, 0.25, 0.54, and 0.90 events per patient year vs. 0.12, 0.13, 0.16, and 0.62).
Importantly, no differences were observed in patient characteristics including age, gender, aetiology, INTERMACS profile, and duration of hospitalization in patients who performed no or only one CPET compared with patients who performed a CPET at both 6 and 12 months. Furthermore, pVO2(/kg), echocardiographic, and laboratory results did not differ between patients who performed one CPET and patients who performed two CPETs during the first year after implantation.
Predictors for pVO2/kg over time
Using linear regression analysis, factors contributing to the difference between pVO2/kg at 6 and 12 months (delta pVO2/kg, Appendix A) were identified. Multivariable linear regression analysis including CPET results at 6 months, age, and gender, resulted in a significant model; F(7, 89) = 4.879, P < 0.001, explaining 22% of the variance in delta VO2/kg (adjusted R 2 = 0.22). Significant predictors for a higher delta pVO2/kg were a younger age, a lower body mass index, lower maximal work load, and anaerobic threshold during the CPET at 6 months.
An improvement of peak oxygen uptake >6% on repeated CPET, which is defined clinically important in heart failure patients, was observed in 46 (44%) of the patients. 20
Discussion
This single centre study is the first study to examine exercise capacity in a large cohort of MCS patients during 1 year of follow‐up. It demonstrated an increase of maximal workload by 9% and pVO2 by 7% between 6 and 12 months postoperatively. Although this increase seems only modest, a recent position paper on the role of CPET in heart failure patients showed that an increase of >6% in maximal exercise capacity is considered clinically relevant and related to improved prognosis. 20 This was observed in almost half of the patients. Furthermore, approximately a quarter of patients had a normal maximal exercise capacity after 1 year, defined as a pVO2/kg > 20 mL/kg/min according to Weber. 15
The improvement in exercise capacity could not be explained by increasing effort or motivation, as the respiratory exchange ratio was lower at 12 months while pVO2 values increased. Although a considerable amount of patients were not able to perform sequential exercise testing at 6 and 12 months, selection bias seems unlikely. No differences were observed in pVO2 at 6 months between patients who were physically able to perform two CPETs and those who performed a CPET at 6 months only. Patient characteristics, echocardiographic, and laboratory results were also similar, and the most important reason for drop‐out was extra‐cardiac morbidity.
The explanation for the further improvement of exercise performance over the first year after LVAD implantation is probably multifactorial. 21 , 22 , 23 As a consequence of the continuous flow, haemodynamics change dramatically with MCS. Device output is mainly determined by the pump speed and the preload and afterload of the left ventricle. During exercise, total output might be further enhanced by residual left ventricular output through the aortic valve. As mentioned earlier, in cf‐LVADs, the pump speed (RPM) presently is fixed, independent of the physical activity of the patient. 23 In our study, LVAD settings during the exercise test were not changed. Earlier studies evaluating the effect of increasing pump speed also showed an increase in pVO2. 17 , 24 Although performed in a small number of patients, this might advocate the need for an algorithm to adapt speed settings during exercise in cf‐LVADs.
Previous reports did not show a clear correlation between right ventricular function and exercise capacity in MCS recipients. 10 , 16 , 17 More recently, no correlation was found between right ventricular ejection fraction (measured by four‐dimensional cardiac computed tomography) and pVO2. 25 In line with these data, our study observed an identical right ventricular function (TAPSE and TDI‐RV) at 6 and 12 months after MCS, while maximal exercise performance increased, further negating the importance of RV function for exercise capacity in MCS patients.
On the other hand, maximal heart rate seems to play an important role in exercise capacity after MCS. 26 , 27 Approximately half of the MCS recipients in our study were chronotropic incompetent, as was also observed in previous studies. 18 , 19 , 26
Furthermore, we demonstrated a significant correlation between maximal heart rate and pVO2/kg, and patients who achieved a normal exercise capacity had a peak heart rate >90% of predicted during exercise. Therefore, an impaired chronotropic response might largely explain the relatively limited maximal exercise performance in the majority of MCS patients.
Nevertheless, the improvement of maximal exercise capacity between 6 and 12 months in our study did not go hand in hand with a significant additional increase in maximal heart rate. So other factors must also contribute to the improvement of pVO2 beyond 6 months postoperatively.
Despite the significantly higher haemoglobin levels at 12 months, it is not obvious that this is an important factor because the (small) increase in haemoglobin did not correlate with delta pVO2/kg. Unfortunately, we were not informed about the iron status (and possible iron deficiency) in these patients, as it is known that iron deficiency is associated with reduced exercise capacity in heart failure patients, improving by intravenous iron suppletion. 28
Additionally, increased muscle strength may also contribute to improved exercise capacity over time, which is probably enhanced by the cardiac rehabilitation programme after MCS. 29
We identified that the magnitude of improvement of exercise capacity was more pronounced in younger patients and in those with a lower BMI. The fact that a lower maximal workload and AT at 6 months were predictive of further improvement at 12 months illustrates that cf‐LVAD patients might benefit from prolonged cardiac rehabilitation, extending even beyond 6 months after implantation. Cardiac rehabilitation has already demonstrated to affect maximal exercise capacity in MCS patients positively, together with an increased muscle strength. 29 However, the effect of long‐term cardiac rehabilitation on maximal exercise capacity in chronic MCS has not been studied yet.
Subgroups
In this study, patients with a DCM showed a better exercise capacity than patients with IHD at both test moments, which could not be explained by differences in age and/or gender. Hypothetically, a lower maximal exercise performance in IHD might relate to the presence of generalized atherosclerosis and a subsequent decrease in organ and muscle perfusion compared with DCM patients, in which primarily the heart is affected.
Another important finding was that maximal exercise capacity in INTERMACS 1 patients was identical to that of INTERMACS 2 + 3 patients. Although survival differs substantially, improvement in exercise capacity in patients surviving the first year is similar between INTERMACS 1 and 2 + 3. These findings illustrate that functional ‘recovery’ after receiving MCS is substantial, even for haemodynamically severely compromised patients.
This does not correspond to the findings of a recent study by Gustafsson et al., which identified an INTERMACS profile 1 or 2 before HM 3 implantation to be predictive of a lower exercise capacity (i.e. 6 min walk distance below 300 m) at 6 months postoperatively. However, because of the relatively low number of INTERMACS 1 and 2 patients included in that study, the results may be less reliable and cannot be fully compared with our group of LVAD patients, consisting 20% INTERMACS 1 and 45% INTERMACS 2. 30
The detrimental effect of an impaired chronotropic response on maximal exercise capacity in MCS patients advocates the need for studies evaluating the mechanisms of chronotropic incompetence in these patients and analysis of interventions to increase maximal heart rate, for example, a more aggressive rate response in patients with pacing devices. 26
Limitations
The study sample consisted of patients with MCS implanted as a bridge to transplantation. Results of CPET might not be extrapolated to implantations as destination therapy, as these patients generally are older and have a worse outcome than patients who received MCS as a bridge to transplantation. Although echocardiograms during the actual CPET were not made, resting echocardiograms showed no difference in aortic valve opening at 6 and 12 months, suggesting comparable intrinsic contractile contribution. The effect of adaptive pump speed changes during exercise was not investigated in the current study but may lead to further improvement in exercise capacity based on the study by Jung et al. 17 This may also account for optimization of pacemaker settings, for example, rate response, as a substantial number of patients on MCS has a cardiac resynchronization device and/or implantable cardioverter defibrillator in situ. 31 The improvement between 6 and 12 months was more pronounced in pVO2 (7%) than pVO2/kg (4%). This is likely related to the increase in body weight over time.
Conclusions
This study demonstrates a significant increase in exercise capacity between 6 and 12 months after MCS in end‐stage heart failure patients. Maximal heart rate at exercise plays an important role in this improved exercise capacity. RV function seems to be less important. DCM patients generally have a better exercise capacity after MCS than patients with IHD, irrespective of age, but improvement over time was equal in both groups. Another important finding was that patients in INTERMACS 1 at the time of LVAD implantation, meaning severe cardiogenic shock, are able to achieve the same exercise capacity after MCS as less sick patients (INTERMACS 2 and 3). The improvement of exercise capacity over time after MCS correlates with younger age and lower BMI at the time of LVAD implantation and highlights the importance of prolonged cardiac rehabilitation.
Our results are important information for the growing group of patients on long‐term MCS, as improvement in exercise capacity is critical for the quality of life of patients.
Conflict of interest
None declared.
Acknowledgement
F. Ramjankhan is a proctor of Abbott. W. Suyker reports grants from Abbott, outside the submitted work. Folkert W. Asselbergs is supported by UCL Hospitals NIHR Biomedical Research Centre.
Appendix A. Linear regression analysis on delta VO2/kg
Methods
To assess the association between the results of the CPET at 6 months and the delta VO2/kg, which was normally distributed, linear regression analysis was performed.
First, univariable analysis was performed, including the results of the CPET at 6 months and the demographic parameters, age and gender, using Pearson correlation.
Then, multivariable linear regression was performed, including the variables of the CPET at 6 months as well as age and gender, in relation to the delta VO2/kg.
Results
The assumptions for linear regression were met. Multivariable linear regression built a significant model, F(7, 89) = 4.879, P < 0.001, explaining 22% of the variance in delta VO2/kg (adjusted R 2 = 0.22). Significant predictors for a higher delta VO2/kg were a younger age and a lower body mass index, maximal work load, and anaerobic threshold during the CPET at 6 months (Table A1 ).
Table A1.
Results of the multivariable linear regression analysis on the absolute difference between pVO2/kg from 6 to 12 months
| Standardized beta coefficient | 95% CI lower | Upper | P‐value | |
|---|---|---|---|---|
| Age at implant | −0.265 | −0.111 | −0.004 | 0.034 |
| Gender | −0.105 | −1.783 | 0.599 | 0.326 |
| BMI | −0.318 | −0.389 | −0.089 | 0.002 |
| Max workload | −0.367 | 0.005 | 0.051 | 0.017 |
| Anaerobic threshold | −0.524 | −0.655 | −0.239 | <0.001 |
| EqCO2 | 0.128 | −0.042 | 0.157 | 0.255 |
| Percentage max HR | −0.033 | −0.046 | 0.033 | 0.753 |
Felix, S. E. A. , Oerlemans, M. I. F. , Ramjankhan, F. Z. , Muller, S. A. , Kirkels, H. H. , van Laake, L. W. , Suyker, W. J. L. , Asselbergs, F. W. , and de Jonge, N. (2021) One year improvement of exercise capacity in patients with mechanical circulatory support as bridge to transplantation. ESC Heart Failure, 8: 1796–1805. 10.1002/ehf2.13234
References
- 1. Kormos RL, Cowger J, Pagani FD, Teuteberg JJ, Goldstein DJ, Jacobs JP, Higgins RS, Stevenson LW, Stehlik J, Atluri P, Grady KL, Kirklin JK. The Society of Thoracic Surgeons Intermacs database annual report: evolving indications, outcomes, and scientific partnerships. J Heart Lung Transplant 2019; 38: 114–126. [DOI] [PubMed] [Google Scholar]
- 2. Slaughter MS, Meyer AL, Birks EJ. Destination therapy with left ventricular assist devices: patient selection and outcomes. Curr Opin Cardiol 2011; 26: 232–236. [DOI] [PubMed] [Google Scholar]
- 3. Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH, HeartMate II Investigators . Advanced heart failure treated with continuous‐flow left ventricular assist device. N Engl J Med 2009; 361: 2241–2251. [DOI] [PubMed] [Google Scholar]
- 4. Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, Walsh MN, Milano CA, Patel CB, Hutchins SW, Ransom J, Ewald GA, Itoh A, Raval NY, Silvestry SC, Cogswell R, John R, Bhimaraj A, Bruckner BA, Lowes BD, Um JY, Jeevanandam V, Sayer G, Mangi AA, Molina EJ, Sheikh F, Aaronson K, Pagani FD, Cotts WG, Tatooles AJ, Babu A, Chomsky D, Katz JN, Tessmann PB, Dean D, Krishnamoorthy A, Chuang J, Topuria I, Sood P, Goldstein DJ, MOMENTUM 3 Investigators . A fully magnetically levitated left ventricular assist device—final report. N Engl J Med 2019; 380: 1618–1627. [DOI] [PubMed] [Google Scholar]
- 5. Kugler C, Malehsa D, Tegtbur U, Guetzlaff E, Meyer AL, Bara C, Haverich A, Strueber M. Health‐related quality of life and exercise tolerance in recipients of heart transplants and left ventricular assist devices: a prospective, comparative study. J Heart Lung Transplant 2011; 30: 204–210. [DOI] [PubMed] [Google Scholar]
- 6. Jakovljevic DG, McDiarmid A, Hallsworth K, Seferovic PM, Ninkovic VM, Parry G, Schueler S, Trenell MI, MacGowan GA. Effect of left ventricular assist device implantation and heart transplantation on habitual physical activity and quality of life. Am J Cardiol 2014; 114: 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pruijsten RV, Lok SI, Kirkels HH, Klöpping C, Lahpor JR, de Jonge N. Functional and haemodynamic recovery after implantation of continuous flow left ventricular assist devices in comparison with pulsatile left ventricular assist devices in patients with end‐stage heart failure. Eur J Heart Fail 2012; 14: 319–325. [DOI] [PubMed] [Google Scholar]
- 8. Loyaga‐Rendon RY, Plaisance EP, Arena R, Shah K. Exercise physiology, testing, and training in patients supported by a left ventricular assist device. J Heart Lung Transplant 2015; 34: 1005–1016. [DOI] [PubMed] [Google Scholar]
- 9. Jung MH, Gustafsson F. Exercise in heart failure patients supported with a left ventricular assist device. J Heart Lung Transplant 2015; 34: 489–496. [DOI] [PubMed] [Google Scholar]
- 10. Rosenbaum AN, Dunlay SM, Pereira NL, Allison TG, Maltais S, Stulak JM, Joyce LD, Kushwaha SS. Determinants of improvement in cardiopulmonary exercise testing after left ventricular assist device implantation. ASAIO J 2018; 64: 610–615. [DOI] [PubMed] [Google Scholar]
- 11. Jung MH, Houston B, Russell SD, Gustafsson F. Pump speed modulations and sub‐maximal exercise tolerance in left ventricular assist device recipients: a double‐blind, randomized trial. J Heart Lung Transplant 2017; 36: 36–41. [DOI] [PubMed] [Google Scholar]
- 12. Stevenson LW, Pagani FD, Young JB, Jessup M, Miller L, Kormos RL, Naftel DC, Ulisney K, Desvigne‐Nickens P, Kirklin JK. INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant 2009; 28: 535–541. [DOI] [PubMed] [Google Scholar]
- 13. Jones NL, Makrides L, Hitchcock C, Chypchar T, McCartney N. Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis 1985; 131: 700–708. [DOI] [PubMed] [Google Scholar]
- 14. Luks AM, Glenny RW, Robertson HT. Introduction to Cardiopulmonary Exercise Testing. New York: Springer; 2013. [Google Scholar]
- 15. Weber KT, Kinasewitz GT, Janicki JS, Fishman AP. Oxygen utilization and ventilation during exercise in patients with chronic heart failure. Circulation 1982; 65: 1213–1223. [DOI] [PubMed] [Google Scholar]
- 16. Dunlay SM, Allison TG, Pereira NL. Changes in cardiopulmonary exercise testing parameters following continuous flow left ventricular assist device implantation and heart transplantation. J Card Fail 2014; 20: 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jung MH, Hansen PB, Sander K, Olsen PS, Rossing K, Boesgaard S, Russell SD, Gustafsson F. Effect of increasing pump speed during exercise on peak oxygen uptake in heart failure patients supported with a continuous‐flow left ventricular assist device. A double‐blind randomized study. Eur J Heart Fail 2014; 16: 403–408. [DOI] [PubMed] [Google Scholar]
- 18. Dimopoulos S, Diakos N, Tseliou E, Tasoulis A, Mpouchla A, Manetos C, Katsaros L, Drakos S, Terrovitis J, Nanas S. Chronotropic incompetence and abnormal heart rate recovery early after left ventricular assist device implantation. Pacing Clin Electrophysiol 2011; 34: 1607–1614. [DOI] [PubMed] [Google Scholar]
- 19. Grosman‐Rimon L, McDonald MA, Pollock Bar‐Ziv S, Jacobs I, Tumiati LC, Cherney DZ, Rao V. Chronotropic incompetence, impaired exercise capacity, and inflammation in recipients of continuous‐flow left ventricular assist devices. J Heart Lung Transplant 2013; 32: 930–932. [DOI] [PubMed] [Google Scholar]
- 20. Corrà U, Agostoni PG, Anker SD, Coats AJS, Crespo Leiro MG, de Boer RA, Harjola VP, Hill L, Lainscak M, Lund LH, Metra M, Ponikowski P, Riley J, Seferović PM, Piepoli MF. Role of cardiopulmonary exercise testing in clinical stratification in heart failure. A position paper from the Committee on Exercise Physiology and Training of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018; 20: 3–15. [DOI] [PubMed] [Google Scholar]
- 21. Leibner ES, Cysyk J, Eleuteri K, El‐Banayosy A, Boehmer JP, Pae WE. Changes in the functional status measures of heart failure patients with mechanical assist devices. ASAIO J 2013; 59: 117–122. [DOI] [PubMed] [Google Scholar]
- 22. Martina J, de Jonge N, Rutten M, Kirkels JH, Klöpping C, Rodermans B, Sukkel E, Hulstein N, Mol B, Lahpor J. Exercise hemodynamics during extended continuous flow left ventricular assist device support: the response of systemic cardiovascular parameters and pump performance. Artif Organs 2013; 37: 754–762. [DOI] [PubMed] [Google Scholar]
- 23. Stainback RF, Estep JD, Agler DA, Birks EJ, Bremer M, Hung J, Kirkpatrick JN, Rogers JG, Shah NR, American Society of Echocardiography . Echocardiography in the management of patients with left ventricular assist devices: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2015; 28: 853–909. [DOI] [PubMed] [Google Scholar]
- 24. Schmidt T, Bjarnason‐Wehrens B, Schulte‐Eistrup S, Reiss N. Effects of pump speed changes on exercise capacity in patients supported with a left ventricular assist device‐an overview. J Thorac Dis 2018; 10: S1802–S1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mirza KK, Jung MH, Sigvardsen PE, Kofoed KF, Elming MB, Rossing K, Gustafsson F. Computed tomography estimated right ventricular function and exercise capacity in patients with left ventricular assist devices. ASAIO J 2020; 66: 8–16. [DOI] [PubMed] [Google Scholar]
- 26. Mirza KK, Cuomo K, Jung MH, Russell SD, Gustafsson F. Effect of heart rate reserve on exercise capacity in patients treated with a continuous left ventricular assist device. ASAIO J 2020; 66: 160–165. [DOI] [PubMed] [Google Scholar]
- 27. Hydren JR, Cornwell WK 3rd, Richardson RS, Drakos SG. Exercise capacity in mechanically supported advanced heart failure patients: it is all about the beat. ASAIO J 2020; 66: 339–342. [DOI] [PubMed] [Google Scholar]
- 28. von Haehling S, Ebner N, Evertz R, Ponikowski P, Anker SD. Iron deficiency in heart failure: an overview. JACC Heart Fail 2019; 7: 36–46. [DOI] [PubMed] [Google Scholar]
- 29. Marko C, Danzinger G, Käferbäck M, Lackner T, Müller R, Zimpfer D, Schima H, Moscato F. Safety and efficacy of cardiac rehabilitation for patients with continuous flow left ventricular assist devices. Eur J Prev Cardiol 2015; 22: 1378–1384. [DOI] [PubMed] [Google Scholar]
- 30. Gustafsson F, Mirza KK, Pya Y, Shaw S, Diegeler A, Netuka I, Lavee J, Garbade J, Morshuis M, Heatley J, Saeed D, Potapov E, Schmitto JD, Zimpfer D, ELEVATE investigators . Predictors of physical capacity 6 months after implantation of a full magnetically levitated left ventricular assist device: an analysis from the ELEVATE registry. J Card Fail 2020; 26: 580–587. [DOI] [PubMed] [Google Scholar]
- 31. Berg DD, Vaduganathan M, Upadhyay GA, Singh JP, Mehra MR, Stewart GC. Cardiac implantable electronic devices in patients with left ventricular assist systems. J Am Coll Cardiol 2018; 71: 1483–1493. [DOI] [PubMed] [Google Scholar]


