Abstract
Aims
This study aimed to examine if the cardiac changes associated with uraemic cardiomyopathy are reversed by renal transplantation.
Methods and results
MEDLINE, Embase, OpenGrey, and the Cochrane Library databases were searched from 1950 to March 2020. The primary outcome measure was left ventricular mass index. Secondary outcome measures included left ventricular dimensions and measures of diastolic and systolic function. Studies were included if they used any imaging modality both before and after successful renal transplantation. Data were analysed through meta‐analysis approaches. Weight of evidence was assessed through the Grading of Recommendations Assessment, Development and Evaluation system. Twenty‐three studies used echocardiography, and three used cardiac magnetic resonance imaging as their imaging modality. The methodological quality of the evidence was generally poor. Four studies followed up control groups, two using cardiac magnetic resonance imaging and two using echocardiography. Meta‐analysis of these studies indicated that there was no difference in left ventricular mass index between groups following transplantation {standardized mean difference −0.07 [95% confidence interval (CI) −0.41 to 0.26]; P = 0.67}. There was also no difference observed in left ventricular ejection fraction [mean difference 0.39% (95% CI −4.09% to 4.87%); P = 0.86] or left ventricular end‐diastolic volume [standardized mean difference −0.24 (95% CI −0.94 to 0.45); P = 0.49]. Inconsistent reporting of changes in diastolic dysfunction did not allow for any meaningful analysis or interpretation.
Conclusions
The evidence does not support the notion that uraemic cardiomyopathy is reversible by renal transplantation. However, the evidence is limited by methodological weaknesses, which should be considered when interpreting these findings.
Keywords: Echocardiography, Magnetic resonance imaging, Heart failure, Meta‐analysis, Kidney transplantation, Cardiomyopathy
Introduction
Over half of deaths in end‐stage kidney disease (ESKD) are due to cardiovascular disease; the age‐corrected relative risks are extreme, reaching over 100‐fold in younger patients.1 The majority of these deaths are not due to myocardial infarction as a result of coronary atheroma but due to heart failure and sudden cardiac death.2, 3, 4 Consistent with this observation, treatments for traditional cardiovascular risk factors such as hypertension and elevated cholesterol are relatively ineffective in this population.4, 5, 6 These observations can be explained by the near‐universal syndrome of uraemic cardiomyopathy in patients with ESKD.7, 8 Left ventricular hypertrophy is the cardinal feature of uraemic cardiomyopathy, in addition to ventricular dilatation and both systolic and diastolic dysfunction. Histologically, myocytes are severely hypertrophied with myocardial disarray and diffuse interstitial fibrosis.9 As renal function declines, these features become more prevalent and are present in up to 90% of those requiring renal replacement.10 Such changes are strongly linked to cardiovascular outcomes with the presence of left ventricular hypertrophy associated with increased mortality in both transplant recipients and those requiring haemodialysis.11, 12 The gold standard for the treatment of ESKD is renal transplantation.10 The associated improvement in glomerular filtration rate reduces cardiovascular risk below that of those on waiting lists.13 However, cardiovascular risk still remains higher than healthy individuals of the same age and sex with transplant recipients displaying a three‐fold increased risk.14
The restoration of renal function associated with renal transplantation improves many factors thought to cause uraemic cardiomyopathy. As a result, it is generally assumed that kidney transplantation reduces left ventricular mass index (LVMI) and volumes and improves diastolic and systolic function.15, 16 This assertion is based on the reduction of LVMI reported in small echocardiographic studies.15, 16 However, echocardiography is not a reliable or reproducible method for the measurement of LVMI, especially when there are large changes in loading such as before and after haemodialysis.17 As a result, cardiac magnetic resonance imaging (CMR) is now accepted as the gold‐standard imaging modality for patients with ESKD.7 Despite this, review articles continue to state that uraemic cardiomyopathy is reversed by renal transplantation. These articles will not cite any references, cite small, uncontrolled studies using either echocardiography or radionucleotide ventriculography‐gated blood pool (multigated acquisition scan) scans, or refer to other review articles.8, 15, 18, 19, 20
The aim of this study was to perform the first systematic review and meta‐analysis to establish if the features of uraemic cardiomyopathy are reversible following successful renal transplantation.
Methods
A Preferred Reporting Items for Systematic Reviews and Meta‐Analyses‐compliant systematic review was conducted and was registered with the International Prospective Register of Systematic Reviews (PROSPERO; http://www.crd.york.ac.uk/prospero/, Reference CRD42018115359).21 Published and unpublished articles and conference proceedings registered on or before 20 March 2020 were searched. The electronic databases used to search the published literature were MEDLINE, Embase, OpenGrey, and the Cochrane Library (clinical trials database and database of systematic reviews). All searches were limited to adult human studies. Reference lists of all pertinent review papers and eligible studies were reviewed. The search terms used are presented for the MEDLINE search in Table 1. These were modified for the specific databases searched.
Table 1.
Search strategy for MEDLINE database
| 1. exp Adult/ |
| 2. chronic kidney disease.mp. or exp Renal Insufficiency, Chronic/ |
| 3. exp Kidney Failure, Chronic/ |
| 4. 1 or 2 or 3 |
| 5. exp Echocardiography/or echocardiogram*.mp |
| 6. Heart Ventricles/dg [Diagnostic Imaging] |
| 7. exp Myocardial Perfusion Imaging |
| 8. Magnetic resonance imaging.mp. or exp Magnetic Resonance Imaging/ |
| 9. 5 or 6 or 7 or 8 |
| 10. exp Kidney Transplantation |
| 11. exp Renal Replacement Therapy/ |
| 12. exp Renal dialysis/ |
| 13. 10 or 11 or 12 |
| 14. exp Hypertrophy, Left Ventricular/ |
| 15. cardiomyopathy.mp. or exp Cardiomyopathies/ |
| 16. uremic cardiomyopathy.mp. |
| 17. exp Ventricular Remodeling/ |
| 18. exp Ventricular Dysfunction, Left/ |
| 19. exp Heart Failure, Diastolic/ |
| 20. left ventricular mass.mp. |
| 21. 14 or 15 or 16 or 17 or 18 or 19 or 20 |
| 22. 4 and 9 and 13 and 21 |
| 23. remove duplicates from 22 |
Inclusion criteria
All full‐text English‐language articles assessing changes in LVMI, before and after successful renal transplant, using any form of imaging technique were included. Single‐subject case reports, comments, letters, editorials, guidelines, or review papers were excluded. Studies were also excluded if participants received more than one organ type.
Study selection
Two reviewers (L.C.P. and J.P.L.) independently reviewed all titles and abstracts generated from the search strategy. Following this initial screening process, the full texts of eligible articles were reviewed independently by each author against the predefined eligibility criteria.
Critical appraisal
All papers were critically appraised independently by two reviewers (L.C.P. and A.R.). This appraisal was conducted using the Newcastle–Ottawa Scale.22 A maximum score of 9 points can be awarded based on participant selection, comparability, and study outcome including follow‐up.
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system was adopted to evaluate the quality of the evidence across studies for pooled analyses.23
Outcome measures and data extraction
Two reviewers (L.C.P. and J.P.L.) extracted data into a pre‐constructed table. Information gathered included number of participants, age range, sex distribution, dialysis modality, immunosuppression regime, and time to follow‐up after transplantation. The primary outcome measure was LVMI. Secondary outcome measures were left ventricular dimensions, measures of diastolic and systolic function. Any disagreement regarding study eligibility, data extraction, methodological quality, and GRADE assessment between reviewers was resolved through discussion until consensus was reached.
Statistical analysis
Statistical analysis was conducted using Review Manager 5.0 for Apple (Nordic Cochrane Centre, Copenhagen, Cochrane Collaboration, 2008). Statistical heterogeneity was assessed by χ 2 and I 2. If χ 2 was greater than P = 0.10 and the I 2 statistic indicated that heterogeneity was present (>20%), a random‐effects statistical model was adopted to calculate mean difference or standardized mean difference (SMD) between groups. When χ 2and I 2 values demonstrated low heterogeneity, a fixed‐effects model was adopted.24 Where meta‐analysis was not possible because of insufficient data, a narrative approach was adopted.
Results
Search strategy results
The results of the search strategy are summarized in Figure 1. A total of 2547 potentially relevant citations were identified, with 26 being eligible for inclusion. The characteristics and outcomes of the 26 included studies are presented in Table 1.
Figure 1.
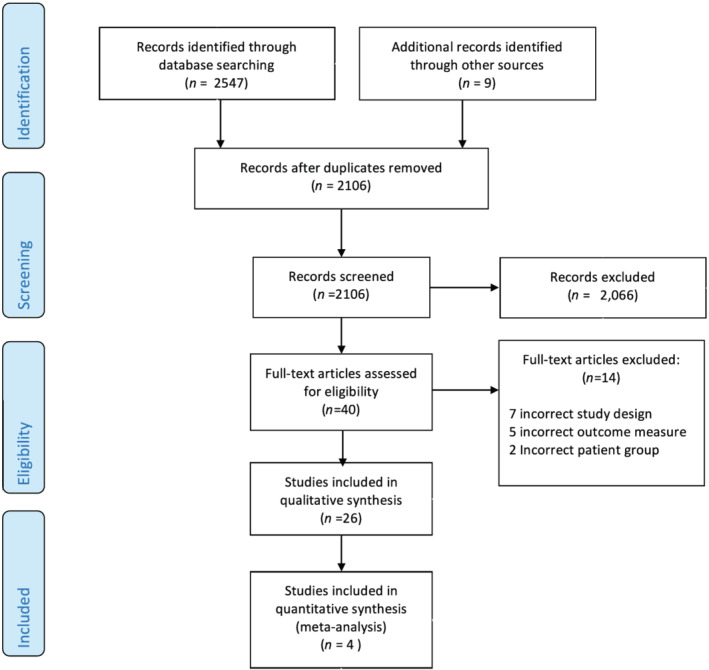
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flow diagram.
Methodological appraisal
There were 23 studies that used echocardiography.25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 Of these studies, eight were retrospective echocardiographic data collected as part of routine clinical practice.25, 31, 34, 37, 38, 45, 46, 47 Their methodological quality was largely poor (Table 1). One study was classified as fair,35 and none were classified as good. Two studies recruited control groups, which were followed up, both consisting of individuals receiving haemodialysis.27, 35 Assessor blinding was only employed in two studies,28, 30 and none used a sample size calculation.
Three studies employed CMR, two were classified as good48, 49 and one as fair.50 In each, recipients were recruited from local transplant waiting lists. No study performed a sample size calculation designed to detect change in LVMI. Prasad et al.,48 however, used a sample size calculation powered to detect changes in adiponectin levels. Assessor blinding was employed in all CMR studies.48, 49, 50 In one study, the indication for initial CMR was routine clinical practice,49 and in the remaining two, CMR was conducted for research purposes.48, 50
The length of follow‐up across all the studies varied from 1 week to 5 years, with the most common follow‐up time point being 12 months.
Study population
In total, 1998 renal transplant recipients were included of which 1229 were male. The pooled weighted mean age was 50 years (range 16–85 years). Fourteen studies reported type of transplant with a total of 840 live donor transplants and 377 deceased donor transplants.25, 27, 29, 31, 34, 35, 36, 38, 40, 42, 43, 44, 48, 50 A total of 1531 recipients were reported to be receiving renal replacement therapy in 24 studies.25, 26, 27, 29, 30, 31, 32, 33, 35, 36, 37, 38, 39, 40, 41, 43, 44, 45, 46, 47, 48, 49, 50 In total, 127 control patients were followed up in four studies.27, 35, 48, 49 Two CMR48, 49 studies recruited both recipients and controls from local transplant waiting lists. Comparisons between the groups at baseline showed that there was no difference in age, sex, systolic blood pressure, or history of ischaemic heart disease. Two echocardiographic studies also recruited controls. De Lima et al. 27 recruited 74 unselected ESKD patients on regular haemodialysis, 17 who were subsequently transplanted. There was no significant difference between those transplanted and those who remained on dialysis in terms of age, gender, race, or duration of haemodialysis. No data regarding blood pressure or prior cardiac disease were presented. Keven et al.35 also recruited both transplant recipients and randomly selected controls receiving haemodialysis. There were no significant differences reported between recipients and controls in terms of age, sex, and systolic blood pressure. There was also no recorded ischaemic heart disease in either group. Further two studies recruited controls who were studied at a single time point; in both cases, however, these were healthy controls.36, 50 Findings of all studies are summarized in Table 2.
Table 2.
Selected data for all studies
| Author | Subjects | Age (years) | Follow‐up |
RRT—modalitya Duration (months) |
Primary outcome measure findings | NOS | Comments |
|---|---|---|---|---|---|---|---|
|
Hayer et al.50 UK CMR |
Transplant group: 24 live donor recipients Control group: 18 healthy controls |
Transplant group: 46 ± 13 Control group: 49 ± 17 |
2 months |
HD 11 PD 3 Duration 13 (IQR 8–33) |
No significant reduction in LVMI (g/m2) from baseline 89 ± 38 to follow‐up 83 ± 23 | Fair |
Prospective Blinded Controlled |
|
Hamidi et al.30 Iran 2D Echob |
25 recipients on HD | 44.64 ± 13.91 | 1 month |
HD 25 Duration 56. ± 9.7 |
Significant reductions in LVMI (g/m2) −73.82 ± 11.6, P < 0.001, and relative wall thickness 0.056 ± 0.023, P = 0.021 | Poor |
Prospective Single blinded Non‐controlled |
|
Prasad et al.48 UK CMR |
Transplant group: 39 live donor recipients Control group: 43 on local waiting list |
Transplant group: 46.5 ± 12.4 Control group: 55.5 ± 11 |
12 months |
Transplant group: HD 27 PD 12 Control group: HD 31 PD 12 Duration NS |
No difference in LVMI (g/m2) change at 1 year between recipients −1.98 ± 5.5 and waiting list patients −0.36 ± 5.7 g/m2; P = 0.44 | Good |
Prospective Single blinded Controlled |
|
Hewing et al.31 Germany 2D Echob |
31 recipients | 44 year range: 19–85 | Median 19 months |
HD 23 Duration 33.5 (IQR 10.0–72.3) |
Significant reduction in LVMI (g/m2) 111. 2 (IQR 88.7–150.6) to 103.8 (IQR 78.4–113.8); P = 0.001. No change observed in LV diastolic function | Poor |
Retrospective Non‐blinded Non‐controlled |
|
An et al.25 Korea 2D Echob |
767 recipients | 45.0 ± 11.5 |
1 week 1 year 5 years |
HD 495 PD 108 Duration NS |
Significant reductions in LVMI (g/m2) at 1 and 5 years compared with pre‐transplant and 1 week; P < 0.001. Baseline 129.1 (IQR 103.0–161.6), 1 week 130.4 (IQR 103.7–161.6), 1 year 119.9 (IQR 96.5–150.4), and 5 years 110.0 (IQR 90.4–137.2); P < 0.001 | Poor |
Retrospective Non‐blinded Non‐controlled |
|
Hawwa et al.47 USA 2D Echob |
232 recipients | 54 ± 12 | 422 days (median) |
HD 163 PD 31 Duration 26 (IQR 8–24) |
Significant reduction in LVMI (g/m2) pre‐transplant 132 ± 46 and post‐transplant 125 ± 42; P = 0.32 | Poor |
Retrospective Non‐blinded Non‐controlled |
|
Deng et al.28 USA 2D Echob |
48 recipients with no history of MI, cardiomyopathy, CHF, arrhythmias, or OSA | Range (36–67) | 6 months |
NS NS |
Significant reduction in LVMI (g/m2) from 104.00 ± 16.47 to 95.50 ± 21.44; P = 0.043 | Poor |
Prospective Single blinded Non‐controlled |
|
Salerno et al.45 Italy 2D Echob |
104 recipients, two alternative immunosuppression strategies; CNI + EVE (28) or CNI + MMF (76) |
CNI + EVE: 47.5 ± 13.1 CNI + MMF: 47.8 ± 12.1 |
36 months |
CNI + EVE: RRT 28 Duration 48 ± 37.2 CNI + MMF: RRT 76 Duration 39.6 ± 37.2 |
No significant difference between immunosuppression groups. Both showed significant reductions in LVMI (g/m2) at 3 years in everolimus group 126.5 ± 46.4 to 121.9 ± 39.4 and in the mycophenolate group 116.6 ± 38.3 to 113 ± 28.9; P < 0.05 | Poor |
Retrospective Non‐blinded Non‐controlled |
|
Vaidya et al.46 USA 2D Echob |
105 recipients with ≥1 year of CKD prior to Tx | 53.8 ± 12.3 | Mean 2.2 years |
RRT 87 Duration 36 ± 36 |
57 participants had significant LVMI (g/m2) decrease, mean difference −37.2 ± 31.3, and 48 had no regression mean difference 15.7 ± 17.1. The extent of the LVMI before transplant was the only predictor of LVMI regression odds ratio 1.50 (95% CI 1.26–1.80) | Poor |
Retrospective Non‐blinded Non‐controlled |
|
Souza et al.42 Brazil 2D Echob |
40 live donor recipients | 31.6 ± 12.7 |
1 month 3 months 6 months |
NS Duration NS |
Significant reduction in LVMI (g/m2) from baseline 131.48 ± 38.93, to 1 month 126.41 ± 29.45, P < 0.05, to 3 months 128.81 ± 30.71 and 6 months 113.03 ± 29.99 (P = 0.02 comparison between 6 months and baseline. No significant difference between other follow‐up times and baseline) | Poor |
Prospective Non‐blinded Non‐controlled |
|
Namazi et al.39 Iran Echo modality NS |
47 recipients with no history of cardiovascular disease | Range 23–56 | 4 months |
HD 16 PD 4 NS |
Significant reduction in LVMI (g/m2) from baseline 120 to 110 (SD not given); P = 0.002 | Poor |
Prospective Non‐blinded Non‐controlled |
|
Patel et al.49 UK CMR |
Transplant group: 25 transplant recipients Control group: 25 patients transplant waiting list |
Transplant group: 45.9 ± 14.4 Control group: 52.7 ± 10.4 |
Mean 1.8 (±0.9) |
Transplant group: HD 10 Duration 36 ± 36 Control group: HD 12 Duration 28 ± 31 |
No difference in LVMI change (%/year) between recipients and those who remained on the waiting list, 2.75 ± 9.1 vs. 3.6 ± 16.7; P = 0.10 | Good |
Prospective Single blinded Controlled |
|
Keven et al.35 Turkey 2D Echob |
Transplant group: 28 recipients on HD Control group: 23 controls on HD |
34 ± 9 | 12 months |
Transplant group: HD 23 Duration 40 ± 35 Control group: HD 23 Duration 52 ± 20 |
No change in LVMI (g/m2) between transplant 132 ± 38 and HD 145 ± 38; P < 0.05 | Fair |
Prospective Non‐blinded Controlled |
|
Iqbal et al.34 Bangladesh 2D Echob |
Poor |
Retrospective Non‐blinded Non‐controlled |
|||||
| ‐ Group 1 | 22 recipients | 31 ± 9 | 3 months |
NS Duration 5 ± 1.2 |
LVMI (g/m2) reduced at 3 months from 379 ± 114 to 248 ± 58 g/m2 (P < 0.001) | ||
| ‐ Group 2 | 30 recipients | 31 ± 8 |
3 months 6 months 12 months |
NS Duration 7 ± 3 |
LVMI (g/m2) reduced significantly from baseline 275 ± 91, at 3 months 191 ± 38, 6 months 173 ± 39, and 12 months 159 ± 26; P < 0.001 | ||
|
Hernández et al.43 Spain 2D Echob |
60 divided based on the presence of LVH at baseline |
LVH: 52 ± 12 No LVH: 48 ± 12 |
19 months |
HD 43 PD 17 Duration 12 (IQR 6–24) |
52% (23) of participants with no LVH at baseline developed LVH or >20% increase in LVMI at follow‐up; 22% (8) participants with LVH at baseline showed regression to normal at follow‐up | Poor |
Prospective Non‐blinded Non‐controlled |
|
Montanaro et al.38 USA Echo modality NS |
23 recipients without diabetes | 43 ± 10 | 24 months |
HD 17 PD 7 Duration 33 ± 12 |
LVMI (g/m2) reduced at 24 months from 161.4 ± 48.2 to 122.1 ± 27.7 (P < 0.007) | Poor |
Retrospective Non‐blinded Non‐controlled |
|
Ferreira et al.29 Brazil 2D Echob |
24 recipients on RRT | 33.5 ± 10.0 |
3 months 6 months 12 months |
HD 21 PD 3 Duration 23 (range 9–119) |
LVMI (g/m2) reduced at 12 months from 164.6 ± 47.0 to 130.5 ± 39.8 (P = 0.004). The incidence of LVH decreased from 75% to 52.1% 12 months after transplant | Poor |
Prospective Non‐blinded Non‐controlled |
|
Sahagun‐Sanchez et al.41 Mexico 2D Echob |
13 recipients on HD | 33.64 ± 10.13 |
3 months 4 months |
HD 13 Duration 35.5 (SD NS) |
Reduction in LVMI (g/m2) from baseline 102.8 ± 27.7 to 3 months 83.5 ± 18.1 and 4 months 71.5 ± 16.2; P = 0.001 | Poor |
Prospective Non‐blinded Non‐controlled |
|
McGregor et al.37 UK 2D Echob |
67 recipients on RRT | 38.3 (18.7–64.5) | 4 months |
RRT 67 Duration NS |
No significant change in LVMI (g/m2) from baseline 143 (range 61–48) to 4 months 145 (range 62–37) (P = 0.71) | Poor |
Retrospective Non‐blinded Non‐controlled |
|
Hernandez et al.44 Spain 2D Echob |
38 on RRT, stratified according genotype DD or ID + II of intron 16 of the ACE gene |
DD: 46.2 ± 4.1 ID + II: 45.2 ± 2.9 |
6 months 12 months |
RRT 38 Duration DD: 32.3 ± 10.7 ID + II: 26.4 ± 7.3 |
LVMI increased at 12 months in those with DD genotype from 166.6 ± 10.4 to 201.5 ± 21.6; P < 0.05. There was no change in LVMI in the ID + II groups 181.3 ± 9.1 to 176.9 ± 9.4; P > 0.05 | Poor |
Prospective Non‐blinded Non‐controlled |
|
Palfrey et al.40 Canada 2D Echob |
102 recipients | 37 ± 12 | 12 months |
HD 72 PD 27 Duration 15 ± 15 |
LVMI (g/m2) reduced from baseline 158 ± 39 to 1 year 132 ± 39; P < 0.001 | Poor |
Prospective Non‐blinded Non‐controlled |
|
De Lima et al.27 Brazil 2D Echob |
Transplant group: 17 live donor recipients Control group: 36 on HD |
Transplant group: 44 ± 13 Control group: 40.5 ± 10 |
15 months |
HD 74 Duration minimum 12 months |
No change in LVMI (g/m2) in recipients 156.7 ± 51.3 vs. 132.9 ± 31.0, P > 0.05, or controls 170.6 ± 50.8 vs. 155.6 ± 43.1, P > 0.05 | Poor |
Prospective Non‐blinded Controlled |
|
De Castro et al.26 Italy 2D Echob |
23 non‐diabetic recipients on HD | 39.1 ± 13.7 | 1 year |
HD 23 Duration 15 ± 14.3 |
LVMI (g/m2) decreased from 157.78 ± 53.5 to 108.1 ± 19.5 (P‐value not stated) | Poor |
Prospective Non‐blinded Non‐controlled |
|
Huting32 Germany 2D Echob |
24 recipients on HD | 47 ± 12 | Mean 41 ± 30 months |
HD 24 Duration 50 ± 29 |
No change in LVMI (g/m2) from baseline 175 ± 48 to follow‐up 171 ± 49; P = 0.05 | Poor |
Prospective Non‐blinded Non‐controlled |
|
Larsson et al.36 Sweden M‐modec |
Transplant group: 27 recipients with juvenile onset diabetes. Control group: 27 healthy men |
Transplant group: 33 range (27–45) Control group: 26 ± 2 |
Transplant group: 6 months 13 months 44 months Control group: single echo |
HD 6 Duration NS |
LVMI (g/m2) decreased from baseline 176 ± 51, to 6 months 143 ± 44, 13 months 133 ± 44, and 44 months 111 ± 22; P < 0.01 | Poor |
Prospective Non‐blinded Controlled |
|
Ikaheimo et al.33 Finland M‐modec |
13 recipients on HD | 31 (20–50) | 9 months |
13 Duration NS |
LVMI (g/m2) decreased from baseline pre‐HD session 197.7 ± 44.8 and post‐HD session 143.5 ± 47.3 to 143.5 ± 47.3, P = 0.001, after transplant | Poor |
Prospective Non‐blinded Non‐controlled |
ACE, angiotensin‐converting enzyme; CHF, congestive heart failure; CI, confidence interval; CKD, chronic kidney disease; CMR, cardiac magnetic resonance imaging; CNI, calcineurin inhibitor; D, deletion; EVE, everolimus; HD, haemodialysis; I, insertion; IQR, inter‐quartile range; LV, left ventricular; LVH, left ventricular hypertrophy; LVMI, left ventricular mass index; NOS, Newcastle–Ottawa score; NS, not stated; OSA, obstructive sleep apnoea; PD, peritoneal dialysis; RRT, renal replacement therapy; SD, standard deviation; Tx, transplantation.
2D Echo: acquisition of two‐dimensional images of cardiac structures.
M‐mode: acquisition of monodimensional view of cardiac structures along a single ultrasound line.
RRT indicates number receiving dialysis where a specific modality is not specified.
Aetiology of ESKD was reported in 12 studies,25, 27, 29, 30, 31, 33, 35, 39, 41, 47, 48, 50 with glomerulonephritis being the most commonly reported aetiology. Five studies excluded patients with ischaemic heart disease or congestive cardiac failure,25, 28, 30, 39, 50 and a further two32, 41 only included patients who were asymptomatic from cardiovascular disease. Prasad et al.48 reported that 10% of transplanted patients had undergone coronary revascularization. Vaidya et al.46 reported that 43% of their cohort had a diagnosis of coronary artery disease, and McGregor et al.37 indicated that 84% of participants had a dilated cardiomyopathy at baseline. The cohort reported by Hawwa et al.47 included 26% with coronary artery disease and 31% with a prior diagnosis of heart failure.
Left ventricular mass index
Nineteen echocardiographic studies25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 47 reported the mean changes in LVMI following transplantation for their entire cohort with 16 reporting significant reductions in LVMI at follow‐up.25, 26, 28, 29, 30, 31, 33, 34, 35, 36, 38, 39, 40, 41, 42, 47 The magnitude of change observed varied greatly between studies. Iqbal et al.,34 in a cohort of 22 participants, reported the largest reduction in LVMI of 131 g/m2 three months after transplantation. Three studies found no significant change in LVMI.27, 32, 37
Four echocardiography studies only presented changes in LVMI based on predetermined subgroups.43, 44, 45, 46 These two studies examined the effect of baseline LVMI on subsequent changes.43, 46 In their cohort, Vaidya et al.46 reported that pre‐transplantation LVMI was the only predictor of subsequent LVMI regression following transplantation [odds ratio 1.50, 95% confidence interval (CI) 1.26–1.80]. Hernandez et al.43 studied 60 patients, with initial LVMI shown to be an independent predictor of subsequent change in LVMI. Salerno et al.45 examined changes in LVMI in 104 patients treated with either everolimus or mycophenolate mofetil. While a significant reduction in LVMI from baseline to follow‐up was seen in both groups (everolimus 126.5 ± 46.4 to 121.9 ± 39.4 g/m2, P < 0.05; mycophenolate 116.6 ± 38.3 to 113 ± 28.9 g/m2, P < 0.05), there was no significant difference between the groups. Hernandez et al.44 studied the effect of angiotensin‐converting enzyme polymorphisms. Those with an unfavourable genotype (highest angiotensin‐converting enzyme activity) had a significant increase in LVMI after transplantation (23.3 ± 7.9%; P < 0.05), whereas in those with a more favourable genotype, no change was observed (−0.08 ± 4.9%; P > 0.05).
All three CMR studies reported no significant overall change in LVMI.48, 49, 50 However, the trends in mean change were conflicting. Patel et al.49 observed an increase in LVMI in transplant recipients and a decrease in the control group of haemodialysis patients (−3.6 ± 16.7%/year vs. 2.75 ± 9.1%/year). Prasad et al.48 reported a reduction in LVMI in both recipients and controls who remained on the waiting list (recipients −1.98 ± 5.5 g/m2 and controls −0.36 ± 5.7 g/m2; P = 0.44). The third CMR study by Hayer et al.50 also reported no significant change in LVMI, from baseline (89 ± 38 to 83 ± 23 g/m2; P ≥ 0.05).
Two echocardiographic studies and two CMR studies recruited suitable control groups.27, 35 Keven et al.35 studied 28 transplant recipients and 23 haemodialysis patients with follow‐up at 1 year. There was a significant reduction of LVMI in transplant recipients from baseline to follow‐up. However, the magnitude of change was not significantly different from that observed in the control group (recipients −10 ± 24 g/m2 vs. controls −5.6 ± 22 g/m2; P > 0.05). De Lima et al.27 studied 36 haemodialysis patients and 17 transplant recipients at mean follow‐up of 30 ± 8 months. In both groups, no significant change was observed in LVMI from baseline to follow‐up [LVMI (g/m2) recipients 156.7 ± 51.3 to 132.9 ± 31.0, P > 0.05; controls 170.6 ± 50.8 to 155.6 ± 43.1, P > 0.05]. The two CMR studies reported no significant overall change in LVMI following transplantation compared with the control group.48, 49
A meta‐analysis was conducted of the four studies reporting change in LVMI in transplant recipient and controls (Figure 2). A total of 236 participants were included in this analysis; the overall SMD was −0.07 (95% CI −0.41 to 0.26), P = 0.67, suggesting no difference between transplant and control groups. However, heterogeneity was moderate (I 2 = 38%). This was regarded as low‐quality evidence using the GRADE approach due to low participant numbers and heterogeneity between the studies included. Subgroup analysis is also presented based on imaging modality. The two echocardiographic studies27, 35 [SMD −0.20 (95% CI −0.60 to 0.20); P = 0.33] and the two CMR studies48, 49 [SMD 0.07(95% CI −0.67 to 0.80)] showed no mean change in LVMI. There was no significant difference between the findings of the two imaging modalities (P = 0.53). However, heterogeneity in the echocardiographic subanalysis was low (I 2 = 0%) but substantial (I 2 = 77%) in the CMR subanalysis.
Figure 2.
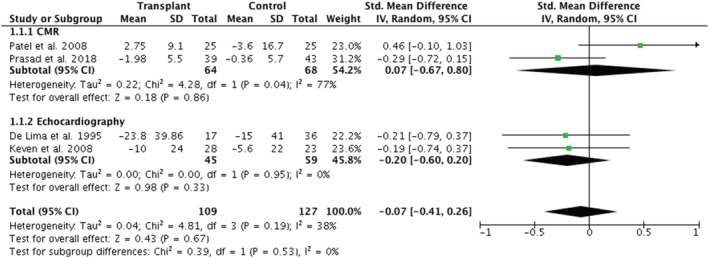
Meta‐analysis of changes in left ventricular mass index following renal transplantation. Subgroup analysis presented based on imaging modality. CI, confidence interval; CMR, cardiac magnetic resonance imaging; IV, inverse variance; SD, standard deviation.
Systolic function
Fifteen studies reported changes in left ventricular ejection fraction (LVEF) across their whole cohort: 11 using echocardiography25, 28, 29, 30, 31, 32, 36, 39, 41, 42, 47 and three using CMR.48, 49, 50 None of the studies using echocardiography included a control group. Eight studies25, 28, 30, 32, 36, 39, 42, 47 reported significant increases in LVEF following transplantation, six of which had recruited individuals with normal mean LVEF.25, 30, 32, 36, 39, 42 Deng et al.28 recruited 48 participants with a mean LVEF of 40 ± 11%, which increased to 60 ± 14% (P < 0.05). Hawwa et al.47 also reported that in participants with reduced LVEF, significant improvements were observed following transplant (ejection fraction 41 ± 10% to 50 ± 12%; P < 0.0001).
One CMR study by Hayer et al.50 reported a significant improvement in LVEF from baseline to follow‐up (ejection fraction 68 ± 9% to 73 ± 9%; P < 0.05). However, when comparing changes to control participants with ESKD, both Patel et al.49 and Prasad et al.48 reported no statistically significant change in LVEF. Meta‐analysis of these two studies, consisting of 64 transplant recipients and 68 control participants receiving regular dialysis, showed no overall change in LVEF in transplant recipients compared with controls [mean difference 0.39% (95% CI −4.09% to 4.87%); P = 0.86] with high heterogeneity (I 2 = 62%) (Figure 3). The quality of evidence (GRADE) was rated as very low quality due inconsistency between the results, the low numbers of trials included, and overall participant numbers.
Figure 3.
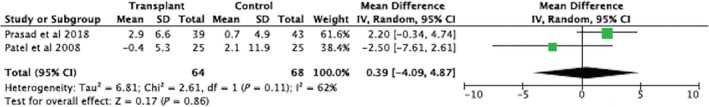
Meta‐analysis of cardiac magnetic resonance imaging studies representing change in left ventricular ejection fraction after renal transplant. CI, confidence interval; IV, inverse variance; SD, standard deviation.
Left ventricular dimensions
The most reported measure was left ventricular internal diameter in diastole in 13 non‐controlled echocardiographic studies with all but three reporting a significant reduction.25, 29, 31, 32, 33, 34, 36, 37, 40, 41, 42, 43, 47 All three CMR studies reported left ventricular end‐diastolic volume (LVEDV) with Hayer et al.50 reporting significant reduction from baseline (79 ± 24 to 63 ± 20 mL/m2; P < 0.05). Conflicting results however were observed in the other two studies with follow‐up of control groups. Prasad et al.48 reported a reduction in LVEDV compared with controls (recipients −4.9 ± 8.5 mL/m2 vs. controls 0.3 ± 9.2 mL/m2; P = 0.02), whereas Patel et al.49 reported no significant difference in mean percentage change (controls −3.4 ± 31.5% vs. recipients 0.1 ± 19.5%; P = 0.64). Meta‐analysis of these CMR studies also highlighted that there were high levels of heterogeneity (I 2 = 74%) and that there was no overall significant change in LVEDV following transplantation [SMD −0.24 (95% CI −0.94 to 0.45); P = 0.49] (Figure 4). The quality of evidence (GRADE) was rated as very low quality; this was again due to inconsistency between the results of the two included trials and low participant numbers.
Figure 4.
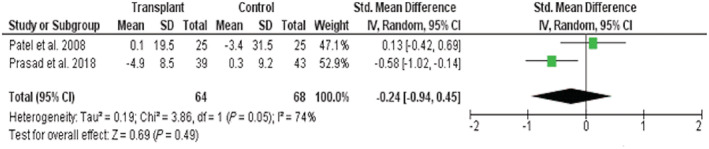
Meta‐analysis of cardiac magnetic resonance imaging studies representing change in end‐diastolic volume after renal transplant. CI, confidence interval; IV, inverse variance; SD, standard deviation.
Diastolic dysfunction
The most reported parameter of diastolic dysfunction was E/A ratio with three studies reporting statistically significant changes following transplantation.25, 27, 28 One controlled study by De Lima et al.27 reported a small reduction in E/A ratio (1.42 ± 0.6 to 1.10 ± 0.4; P < 0.05) at 1 year follow‐up, whereas Deng et al.28 reported a small increase (1.04 ± 0.57 to 1.21 ± 0.52; P = 0.001). An et al.25 reported that recipients with moderate diastolic dysfunction (Grade 2) before transplantation showed a significant reduction in E/A ratio at 12 months (baseline 1.13 vs. 0.98; P < 0.05), whereas those with mild dysfunction (Grade 1) only exhibited a significant change at the 5 year follow‐up (baseline 0.72 vs. 0.81 at 5 years; P < 0.05).34 Mitral valve deceleration time was also reported in four studies,28, 29, 31, 42 with one study reporting a small significant increase42 and one a small significant decrease.28 Neither of these changes represented a change in the grade of diastolic function observed.
Discussion
Reversing uraemic cardiomyopathy is potentially the key to reducing cardiovascular morbidity and mortality in ESKD. Although no targeted therapy has been shown to achieve this, it is generally assumed that restoration of kidney function by kidney transplantation reverses the changes observed. At present, however, the evidence does not support this.
We have shown that the majority of uncontrolled echocardiographic studies reported significant reductions in LVMI. However, making conclusions based on these data is problematic. Echocardiography is unreliable when measuring LVMI due to inaccuracy where large volume fluctuations occur.17 CMR is more accurate and reproducible and is accepted as the gold‐standard imaging modality for patients with ESKD.7 None of the three CMR studies included in our review found a significant change in LVMI. Furthermore, in a meta‐analysis of the four available studies with control groups, renal transplantation was not associated with any reduction in LVMI, and subgroup analysis indicated that this finding was not affected by imaging modality. This analysis also clearly highlights that none of the controlled studies, regardless of imaging modality, reported significant changes in LVMI following transplantation.
A similar pattern was also observed in left ventricular function, with the majority of echocardiographic non‐controlled longitudinal studies reporting significant improvements in LVEF following transplant. This finding is also supported by the work of Wali et al.51 where 102 transplant recipients with left ventricular dysfunction showed significant improvement at 1 year when assessed with radionuclide ventriculography. However, this study was not included in the systematic review as LVMI was not considered. Among the three CMR studies, the patterns of change observed were conflicting. Hayer et al.50 report a significant change in recipients from baseline to follow‐up, whereas both Patel et al. and Prasad et al. did not. In addition, there was also no convincing evidence that successful renal transplantation improves diastolic left ventricular function. It would, therefore, appear that the assumption that the features of uraemic cardiomyopathy are reversed by successful renal transplantation is not supported by the current published literature.
Before concluding that uraemic cardiomyopathy is irreversible, it is important to examine the quality of the evidence available. Studies were generally classified as poor with only two rated as good and two as fair using the Newcastle–Ottawa scoring system. In addition, the assessment of the evidence across studies for each comparison (GRADE) ranged from ‘very low quality’ to ‘low quality’. The majority were opportunistic and unblinded, with little attempt to reduced risk of systematic bias. Only four studies, comprising a total of 109 transplant recipients, recruited a suitable control group, which was followed up.27, 35, 48, 49 A further limitation was the lack of sample size justification with no studies powered to detect a change in LVMI. Previous work, however, has indicated that to detect a change in left ventricular mass of 10 g with 90% power using 2D and M‐mode echocardiography, 78 and 162 participants would be required, respectively. As a result, only five echocardiographic studies included in the review can be considered to have sufficient power to reliably detect clinically significant changes in left ventricular mass.52 The number required to detect the same change using CMR is much smaller, with only 13 participants required indicating that all three CMR studies recruited adequate numbers of participants.52 The fact that only three CMR studies have been conducted, with a total of 88 transplant recipients included, is a major weakness of the current evidence base. The meta‐analyses also demonstrated high heterogeneity, suggesting that the currently available studies do not reliably answer the question of whether uraemic cardiomyopathy is reversible. Some of this may be explained by many studies appearing to have an opportunistic design that is examining patients that happened to have a heart scan performed before and after transplantation with consequential bias, rather than being prospectively designed.
While there are weaknesses in the evidence base, it may also be true that uraemic cardiomyopathy is not reversible. Indeed, the presented meta‐analysis looking at controlled studies, including those using the gold‐standard technique of CMR, suggests that this might well be the case. Following renal transplantation, many traditional risk factors for cardiovascular disease persist and in some cases may develop de novo.53 Hypertension, dyslipidaemia, and diabetes are all recognized complications of both steroids and calcineurin inhibitors, which are routinely administered following transplant. In addition, there is also persistence of non‐traditional risk factors including uraemia, proteinuria, and chronic inflammation.53 Transplantation cannot fully reverse these factors, which may explain the persistence of uraemic cardiomyopathy.
Our study has several strengths in that it included data from both echocardiography and CMR studies, which enabled all relevant data pertaining to the subject to be incorporated. The number of studies identified ensured that there was a significant pooled sample size on which conclusion could be based, although with 26 studies identified, the number of participants was only 1998, highlighting the fact that many studies were very small. There were, however, significant limitations. While there were an appropriate number of studies included in the systematic review, the number suitable for meta‐analysis was small with only four studies eligible. There were also moderate levels of heterogeneity noted among the studies when meta‐analysis was undertaken. Subsequent sensitivity analysis suggested that this was being driven by the conflicting findings of the CMR studies. Such heterogeneity can make the interpretation of any findings difficult. However, we took the view that demonstrating this variability between studies highlights the need for further work to be conducted in this area. Another limitation of the studies included is the short length of follow‐up. It therefore cannot be concluded that uraemic cardiomyopathy might be reversible in the longer term with no study having more than 12 month follow‐up. Furthermore, because we used the assessment of LVMI as the primary selection criteria, studies looking at other important features such as longitudinal strain and right heart changes were not systematically examined.
Conclusions
Reversing uraemic cardiomyopathy is a potential target for reducing the cardiovascular morbidity and mortality associated with chronic kidney disease. This syndrome has generally been assumed to be reversible by renal transplantation. Our review has highlighted that at present, it is unclear if this is true.
This review also highlights the need for adequately powered and controlled studies to answer this fundamental question and provides further insights into other potential strategies to reverse uraemic cardiomyopathy and improve the increased cardiovascular risk associated with ESKD.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
Funding
This work was supported by the British Heart Foundation Clinical Research Training Fellowships (FS/18/29/33554 to L.C.P., FS/16/73/32314 to A.M.P., and FS/19/16/34169 to J.P.L.).
Author contributions
C.J.F. and L.C.P were responsible for the concept and design of the review. L.C.P. and J.P.L. performed the literature search and data extraction. L.C.P. and A.R. performed the methodological quality analysis using the Newcastle–Ottawa score and the GRADE system. L.C.P. performed the data analysis and was the primary author of the manuscript. All authors were involved in the preparation and editing of the final manuscript.
Pickup, L. C., Law, J. P., Radhakrishnan, A., Price, A. M., Loutradis, C., Smith, T. O., Edwards, N. C., Steeds, R. P., Townend, J. N., and Ferro, C. J. (2021) Changes in left ventricular structure and function associated with renal transplantation: a systematic review and meta‐analysis. ESC Heart Failure, 8: 2045–2057. 10.1002/ehf2.13283
References
- 1.Kochanek KD, Murphy SL, Xu J, Tejada‐Vera B. Deaths: final data for 2014. Natl Vital Stat Rep 2016; 65: 1–122. [PubMed] [Google Scholar]
- 2.Saran R, Robinson B, Abbott KC, Agodoa LY, Albertus P, Ayanian J, Balkrishnan R, Bragg‐Gresham J, Cao J, Chen JL, Cope E, Dharmarajan S, Dietrich X, Eckard A, Eggers PW, Gaber C, Gillen D, Gipson D, Gu H, Hailpern SM, Hall YN, Han Y, He K, Hebert H, Helmuth M, Herman W, Heung M, Hutton D, Jacobsen SJ, Ji N, Jin Y, Kalantar‐Zadeh K, Kapke A, Katz R, Kovesdy CP, Kurtz V, Lavalee D, Li Y, Lu Y, McCullough K, Molnar MZ, Montez‐Rath M, Morgenstern H, Mu Q, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O'Hare AM, Obi Y, Pearson J, Pisoni R, Plattner B, Port FK, Potukuchi P, Rao P, Ratkowiak K, Ravel V, Ray D, Rhee CM, Schaubel DE, Selewski DT, Shaw S, Shi J, Shieu M, Sim JJ, Song P, Soohoo M, Steffick D, Streja E, Tamura MK, Tentori F, Tilea A, Tong L, Turf M, Wang D, Wang M, Woodside K, Wyncott A, Xin X, Zang W, Zepel L, Zhang S, Zho H, Hirth RA, Shahinian V. US renal data system 2016 annual data report: epidemiology of kidney disease in the United States. Am J kidney Diseases: off j Nat Kidney Foundation 2017; 69: S465–S480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Methven S, Steenkamp R, Fraser S. UK renal registry 19th annual report: Chapter 5 survival and causes of death in UK adult patients on renal replacement therapy in 2015: national and centre‐specific analyses. Nephron 2017; 137: 117–150. [DOI] [PubMed] [Google Scholar]
- 4.Ferro CJ, Mark PB, Kanbay M, Sarafidis P, Heine GH, Rossignol P, Massy ZA, Mallamaci F, Valdivielso JM, Malyszko J, Verhaar MC, Ekart R, Vanholder R, London G, Ortiz A, Zoccali C. Lipid management in patients with chronic kidney disease. Nat Rev Nephrol 2018; 14: 727–749. [DOI] [PubMed] [Google Scholar]
- 5.Cholesterol Treatment Trialists Collaboration , Herrington WG, Emberson J, Mihaylova B, Blackwell L, Reith C, Solbu MD, Mark PB, Fellstrom B, Jardine AG, Wanner C, Holdaas H, Fulcher J, Haynes R, Landray MJ, Keech A, Simes J, Collins R, Baigent C. Impact of renal function on the effects of LDL cholesterol lowering with statin‐based regimens: a meta‐analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol 2016; 4: 829–839. [DOI] [PubMed] [Google Scholar]
- 6.Kramer HJ, Townsend RR, Griffin K, Flynn JT, Weiner DE, Rocco MV, Choi MJ, Weir MR, Chang TI, Agarwal R, Beddhu S. KDOQI US commentary on the 2017 ACC/AHA hypertension guideline. Am J Kidney Dis 2019; 73: 437–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards NC, Moody WE, Chue CD, Ferro CJ, Townend JN, Steeds RP. Defining the natural history of uremic cardiomyopathy in chronic kidney disease: the role of cardiovascular magnetic resonance. JACC Cardiovasc Imaging 2014; 7: 703–714. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Shapiro JI. Evolving concepts in the pathogenesis of uraemic cardiomyopathy. Nat Rev Nephrol 2019; 15: 159–175. [DOI] [PubMed] [Google Scholar]
- 9.Aoki J, Ikari Y, Nakajima H, Mori M, Sugimoto T, Hatori M, Tanimoto S, Amiya E, Hara K. Clinical and pathologic characteristics of dilated cardiomyopathy in hemodialysis patients. Kidney Int 2005; 67: 333–340. [DOI] [PubMed] [Google Scholar]
- 10.Al Rahbi F, Al SI. Commercial kidney transplantation: attitude, knowledge, perception, and experience of recipients. Kidney Int Rep 2017; 2: 626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stack AG, Saran R. Clinical correlates and mortality impact of left ventricular hypertrophy among new esrd patients in the United States. Am J Kidney Dis 2002; 40: 1202–1210. [DOI] [PubMed] [Google Scholar]
- 12.Paoletti E, Bellino D, Signori A, Pieracci L, Marsano L, Russo R, Massarino F, Ravera M, Fontana I, Carta A, Cassottana P, Garibotto G. Regression of asymptomatic cardiomyopathy and clinical outcome of renal transplant recipients: a long‐term prospective cohort study. Nephrol Dial Transplant 2016; 31: 1168–1174. [DOI] [PubMed] [Google Scholar]
- 13.Neale J, Smith AC. Cardiovascular risk factors following renal transplant. World J Transplant 2015; 5: 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devine PA, Courtney AE, Maxwell AP. Cardiovascular risk in renal transplant recipients. J Nephrol 2019; 3232: 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alhaj E, Alhaj N, Rahman I, Niazi TO, Berkowitz R, Klapholz M. Uremic cardiomyopathy: an underdiagnosed disease. Congest Heart Fail 2013; 19: E40–E45. [DOI] [PubMed] [Google Scholar]
- 16.Patel H, Madanieh R, Kosmas CE, Vatti SK, Vittorio TJ. Reversible cardiomyopathies. Clin Med Insights Cardiol 2015; 9: 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart GA, Foster J, Cowan M, Rooney E, McDonagh T, Dargie HJ, Rodger RS, Jardine AG. Echocardiography overestimates left ventricular mass in hemodialysis patients relative to magnetic resonance imaging. Kidney Int 1999; 56: 2248–2253. [DOI] [PubMed] [Google Scholar]
- 18.Kaesler N, Babler A, Floege J, Kramann R. Cardiac remodeling in chronic kidney disease. Toxins (Basel) 2020; 12: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawwa N, Schreiber MJ Jr, Tang WH. Pharmacologic management of chronic reno‐cardiac syndrome. Curr Heart Fail Rep 2013; 10: 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lekawanvijit S, Adrahtas A, Kelly DJ, Kompa AR, Wang BH, Krum H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur Heart J 2010; 31: 1771–1779. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–269 W264. [DOI] [PubMed] [Google Scholar]
- 22.Wells GA SB, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses [cited 3/3/2019].
- 23.Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O'Connell D, Oxman AD, Phillips B, Schunemann HJ, Edejer T, Varonen H, Vist GE, Williams JW Jr, Zaza S, Group GW . Grading quality of evidence and strength of recommendations. BMJ 2004; 328: 1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sage W, Pickup L, Smith TO, Denton ER, Toms AP. The clinical and functional outcomes of ultrasound‐guided vs landmark‐guided injections for adults with shoulder pathology—a systematic review and meta‐analysis. Rheumatology (Oxford) 2013; 52: 743–751. [DOI] [PubMed] [Google Scholar]
- 25.An JN, Kim YH, Park JB, Hwang JH, Yoo KD, Park JY, Kim CT, Kim HL, Kim YJ, Han DJ, Lim CS, Kim YS, Lee JP. The reciprocal interaction between LV remodelling and allograft outcomes in kidney transplant recipients. Heart 2015; 101: 1826–1833. [DOI] [PubMed] [Google Scholar]
- 26.De Castro S, Migliau G, Giannantoni P, Maurin F, Poli L, Pretagostini R, Di Nicuolo N, Berloco P, Alfani D, Cortesini R. Persistence of abnormal left ventricular filling following renal transplantation. Transplant Proc 1993; 25: 2603–2604. [PubMed] [Google Scholar]
- 27.De Lima JJ, Abensur H, da Fonseca JA, Krieger EM, Pileggi F. Comparison of echocardiographic changes associated with hemodialysis and renal transplantation. Artif Organs 1995; 19: 245–250. [DOI] [PubMed] [Google Scholar]
- 28.Deng Y, Pandit A, Heilman RL, Chakkera HA, Mazur MJ, Mookadam F. Left ventricular torsion changes post kidney transplantation. J Cardiovasc Ultrasound 2013; 21: 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferreira SR, Moises VA, Tavares A, Pacheco‐Silva A. Cardiovascular effects of successful renal transplantation: a 1‐year sequential study of left ventricular morphology and function, and 24‐hour blood pressure profile. Transplantation 2002; 74: 1580–1587. [DOI] [PubMed] [Google Scholar]
- 30.Hamidi S, Kojuri J, Attar A, Roozbeh J, Moaref A, Nikoo MH. The effect of kidney transplantation on speckled tracking echocardiography findings in patients on hemodialysis. J Cardiovasc Thorac Res 2018; 10: 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hewing B, Dehn AM, Staeck O, Knebel F, Spethmann S, Stangl K, Baumann G, Dreger H, Budde K, Halleck F. Improved left ventricular structure and function after successful kidney transplantation. Kidney Blood Press Res 2016; 41: 701–709. [DOI] [PubMed] [Google Scholar]
- 32.Huting J. Course of left ventricular hypertrophy and function in end‐stage renal disease after renal transplantation. Am J Cardiol 1992; 70: 1481–1484. [DOI] [PubMed] [Google Scholar]
- 33.Ikaheimo M, Linnaluoto M, Huttunen K, Takkunen J. Effects of renal transplantation on left ventricular size and function. Br Heart J 1982; 47: 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iqbal MM, Rashid HU, Banerjee SK, Rahman MH, Mohsin M. Changes in cardiac parameters of renal allograft recipients: a compilation of clinical, laboratory, and echocardiographic observations. Transplant Proc 2008; 40: 2327–2329. [DOI] [PubMed] [Google Scholar]
- 35.Keven K, Calayoglu R, Sengul S, Dincer I, Kutlay S, Erturk S, Erbay B, Nergizoglu G. Comparative effects of renal transplantation and maintenance dialysis on arterial stiffness and left ventricular mass index. Clin Transplant 2008; 22: 360–365. [DOI] [PubMed] [Google Scholar]
- 36.Larsson O, Attman PO, Beckman‐Suurkula M, Wallentin I, Wikstrand J. Left ventricular function before and after kidney transplantation. A prospective study in patients with juvenile‐onset diabetes mellitus. Eur Heart J 1986; 7: 779–791. [DOI] [PubMed] [Google Scholar]
- 37.McGregor E, Stewart G, Rodger RS, Jardine AG. Early echocardiographic changes and survival following renal transplantation. Nephrol Dial Transplant 2000; 15: 93–98. [DOI] [PubMed] [Google Scholar]
- 38.Montanaro D, Gropuzzo M, Tulissi P, Vallone C, Boscutti G, Mioni R, Risaliti A, Baccarani U, Adani GL, Sainz M, Lorenzin D, Bresadola F, Mioni G. Effects of successful renal transplantation on left ventricular mass. Transplant Proc 2005; 37: 2485–2487. [DOI] [PubMed] [Google Scholar]
- 39.Namazi MH, Parsa SA, Hosseini B, Saadat H, Safi M, Motamedi MR, Vakili H. Changes of left ventricular mass index among end‐stage renal disease patients after renal transplantation. Urol J 2010; 7: 105–109. [PubMed] [Google Scholar]
- 40.Parfrey PS, Harnett JD, Foley RN, Kent GM, Murray DC, Barre PE, Guttmann RD. Impact of renal transplantation on uremic cardiomyopathy. Transplantation 1995; 60: 908–914. [PubMed] [Google Scholar]
- 41.Sahagun‐Sanchez G, Espinola‐Zavaleta N, Lafragua‐Contreras M, Chavez PY, Gomez‐Nunez N, Keirns C, Romero‐Cardenas A, Perez‐Grovas H, Acosta JH, Vargas‐Barron J. The effect of kidney transplant on cardiac function: an echocardiographic perspective. Echocardiography 2001; 18: 457–462. [DOI] [PubMed] [Google Scholar]
- 42.Souza FL, Bezerra KB, Sousa AR, Ferreira TC, Oliveira MI, Martins GP, Silva FA, Santos AM, Salgado FN. Study of echocardiographic alterations in the first six months after kidney transplantation. Arq Bras Cardiol 2012; 98: 505–513. [DOI] [PubMed] [Google Scholar]
- 43.Hernandez D, Gonzalez A, Rufino M, Laynez I, de la Rosa A, Porrini E, Lacalzada J, Barragan A, Lorenzo V, Torres A. Time‐dependent changes in cardiac growth after kidney transplantation: the impact of pre‐dialysis ventricular mass. Nephrol Dial Transplant: off publ Eur Dial Transplant Assoc‐Eur Renal Assoc 2007; 22: 2678–2685. [DOI] [PubMed] [Google Scholar]
- 44.Hernandez D, Lacalzada J, Rufino M, Torres A, Martin N, Barragan A, Barrios Y, Macia M, de Bonis E, Lorenzo V, Rodriguez A, Gonzalez‐Posada JM, Salido E. Prediction of left ventricular mass changes after renal transplantation by polymorphism of the angiotensin‐converting‐enzyme gene. Kidney Int 1997; 51: 1205–1211. [DOI] [PubMed] [Google Scholar]
- 45.Salerno MP, Rossi E, Favi E, Pedroso JA, Spagnoletti G, Romagnoli J, Citterio F. The reduction of left ventricular hypertrophy after renal transplantation is not influenced by the immunosuppressive regimen. Transplant Proc 2013; 45: 2660–2662. [DOI] [PubMed] [Google Scholar]
- 46.Vaidya OU, House JA, Coggins TR, Patil H, Vaidya A, Awad A, Main ML. Effect of renal transplantation for chronic renal disease on left ventricular mass. Am J Cardiol 2012; 110: 254–257. [DOI] [PubMed] [Google Scholar]
- 47.Hawwa N, Shrestha K, Hammadah M, Yeo PSD, Fatica R, Tang WHW. Reverse remodeling and prognosis following kidney transplantation in contemporary patients with cardiac dysfunction. J Am Coll Cardiol 2015; 66: 1779–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prasad GVR, Yan AT, Nash MM, Kim SJ, Wald R, Wald R, Lok C, Gunaratnam L, Karur GR, Kirpalani A, Connelly PW. Determinants of left ventricular characteristics assessed by cardiac magnetic resonance imaging and cardiovascular biomarkers related to kidney transplantation. Can J Kidney Health Dis 2018; 5 2054358118809974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel RK, Mark PB, Johnston N, McGregor E, Dargie HJ, Jardine AG. Renal transplantation is not associated with regression of left ventricular hypertrophy: a magnetic resonance study. Clin J Am Soc Nephrol: CJASN 2008; 3: 1807–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayer MK, Radhakrishnan A, Price AM, Baig S, Liu B, Ferro CJ, Captur G, Townend JN, Moon JC, Edwards NC, Steeds RP. Early effects of kidney transplantation on the heart—a cardiac magnetic resonance multi‐parametric study. Int J Cardiol 2019; 293: 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wali RK, Wang GS, Gottlieb SS, Bellumkonda L, Hansalia R, Ramos E, Drachenberg C, Papadimitriou J, Brisco MA, Blahut S, Fink JC, Fisher ML, Bartlett ST, Weir MR. Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end‐stage renal disease. J Am Coll Cardiol 2005; 45: 1051–1060. [DOI] [PubMed] [Google Scholar]
- 52.Myerson SG, Bellenger NG, Pennell DJ. Assessment of left ventricular mass by cardiovascular magnetic resonance. Hypertension 2002; 39: 750–755. [DOI] [PubMed] [Google Scholar]
- 53.Rangaswami J, Mathew RO, Parasuraman R, Tantisattamo E, Lubetzky M, Rao S, Yaqub MS, Birdwell KA, Bennett W, Dalal P, Kapoor R, Lerma EV, Lerman M, McCormick N, Bangalore S, McCullough PA, Dadhania DM. Cardiovascular disease in the kidney transplant recipient: epidemiology, diagnosis and management strategies. Nephrol Dial Transplant 2019; 34: 760–773. [DOI] [PubMed] [Google Scholar]


