Abstract
Curcuma species (family: Zingiberaceae) are widely utilized in traditional medicine to treat diverse immune-related disorders. There have been many scientific studies on their immunomodulating effects to support their ethnopharmacological uses. In this review, the efficacy of six Curcuma species, namely, C. longa L., C. zanthorrhiza Roxb., C. mangga Valeton & Zijp, C. aeruginosa Roxb. C. zedoaria (Christm.) Roscoe, and C. amada Roxb., and their bioactive metabolites to modulate the immune system, their mechanistic effects, and their potential to be developed into effective and safe immunomodulatory agents are highlighted. Literature search has been carried out extensively to gather significant findings on immunomodulating activities of these plants. The immunomodulatory effects of Curcuma species were critically analyzed, and future research strategies and appropriate perspectives on the plants as source of new immunomodulators were discussed. Most of the pharmacological investigations to evaluate their immunomodulatory effects were in vivo and in vitro experiments on the crude extracts of the plants. The extracts were not chemically characterized or standardized. Of all the Curcuma species investigated, the immunomodulatory effects of C. longa were the most studied. Most of the bioactive metabolites responsible for the immunomodulating activities were not determined, and mechanistic studies to understand the underlying mechanisms were scanty. There are limited clinical studies to confirm their efficacy in human. Of all the bioactive metabolites, only curcumin is undergoing extensive clinical trials based on its anti-inflammatory properties and main use as an adjuvant for the treatment of cancer. More in-depth studies to understand the underlying mechanisms using experimental in vivo animal models of immune-related disorders and elaborate bioavailability, preclinical pharmacokinetics, and toxicity studies are required before clinical trials can be pursued for development into immunomodulatory agents.
Keywords: curcuma species, ethnopharmacology, phytochemicals, immunomodulation, immune system
Introduction
The human body has a remarkably sophisticated immune system consisting of white blood cells and specialized immune molecules that protect the body against invading pathogens (Tan and Vanitha, 2004). The immune system is made up of innate and adaptive immune immunity. Innate immunity provides first protection against pathogens, and then it will stimulate adaptive immunity to enhance the protection. Innate immunity is the most rapidly acting immunity. It mostly depends on neutrophils, macrophages, dendritic cells, and monocytes, while T and B cells are involved in adaptive immunity (Beutler, 2004; Saroj et al., 2012). In response to pathogens, leukocytes perform a number of phagocytic activities, including chemotaxis, leukocytes adhesion to vascular endothelial cells, and pathogen engulfment, followed by intracellular killing to eliminate the pathogens (Beutler, 2004; Kobayashi et al., 2005). Phagocytes migrate toward the chemoattractants such as complement (C3a and C3b) and formyl methionyl-leucyl-phenylalanine (fMLP) (a bacterial product) (Luster, 2001). Chemoattractants utilize a similar signal transduction system, namely, G protein–coupled receptor, that is, platelet-activating factor receptor (PAFR), formyl-methionyl-leucyl-phenylalanine receptor (fMLPR), and complement C5a receptor (C5aR). The interaction of chemotactic factor and its receptor stimulates cytoskeletal reorganization, calcium mobilization, and degranulation in heterologous cell types (Firtel and Chung, 2000). The adhesion of leukocytes to vascular endothelial cells is initiated by selection interaction, followed by the interaction of leukocyte integrin of the CD18 complex on the surface of phagocytes with adhesion molecule on endothelial cells (Beutler, 2004). Phagocytosis of microorganism triggers superoxide radical (O2 -) generation and release of reactive oxygen species (ROS) such as hydroxyl radical, hypochlorous acid (HOCl), and chloramines through the activity of myeloperoxidase (MPO). Besides, macrophages are involved in the release of nitric oxide (NO.) by inducible nitric oxide synthase (iNOS) (Bogdan, 2001).
Macrophages also modulate adaptive immunity by presenting antigen to CD4 T cells through major histocompatibility complex (MHC) class II antigen. CD4 T cells perform their functions by four subpopulations, which include Th-1, Th-2, Th-17, and CD4 T regulatory (Treg) cells (Chapel et al., 2006). Th cells help B cells develop into plasma cells which can produce antibody and also activate T cells to become activated cytotoxic T cells (Beutler, 2004; Luckheeram et al., 2011). Several cytokines also play essential roles in immune response, which consist of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin 1 (IL-1), IL-6, IL-11, IL-8, and anti-inflammatory cytokines or cytokines inhibitor such as IL-4, IL-10, and IL-13. Cytokines as intercellular messenger molecules have several functions, and these include stimulating phagocyte migration and coordinating early responses of monocytes, macrophages, dendritic cells, and lymphocytes during inflammatory states (Shaikh, 2011). The release of pro-inflammatory cytokines is regulated by nuclear factor-kappa B (NF-ĸB) and mitogen-activated protein kinase (MAPK) pathways (Beyaert et al., 2013). Defects or malfunctions in the immune system can cause disorders of the immune system. Inappropriate reaction to self-antigen is known as autoimmunity such as myasthenia gravis, type 1 diabetes (T1D), systemic lupus erythematosus, Graves’ disease, celiac disease, pernicious anemia, rheumatoid arthritis, and multiple sclerosis. Overactive immune response is known as hypersensitivity reactions, while ineffective immune response is known as immunodeficiency (Zhernakova et al., 2009; Warrington et al., 2011; Beyaert et al., 2013). The diseases which cause the body's immune system to attack the small intestine has affected 1 in 133 people in the United States (Rattue, 2012). A review on incidence and prevalence of Crohn’s disease in several countries reported a gradual increase in incidence and prevalence of this disease. In Malaysia, a study during 2001–2003 showed an increase of prevalence especially among Indians, compared to Chinese and Malay populations. Meanwhile, in Singapore, a study showed that majority patients were Chinese, and there was a trend of increased of prevalence (Economou et al., 2009). Therefore, modulation of the immune response is required in the management and treatment of diseases due to immune system dysfunction (Geetha et al., 2005).
The treatment of inflammatory and immune-related diseases due to defects or disorders of the immune system necessitates modulation of the immune response. Immunomodulation is the process of modifying an immune response by administration of a drug or compound, while immunomodulators are substances which are used to modulate the components of the immune system (Patil et al., 2012). There are several chemical immunomodulators available in the market, that is, prednisone, hydrocortisone, and dexamethasone, which have been used to treat numerous inflammatory diseases. Recombinant proteins have emerged as one important drug to treat cancer, immunodeficiency, and infectious diseases. Cyclosporin A, a microbial peptide, is the most widely used immunosuppressant in transplant rejection treatment (Elgert, 2009). Unfortunately, most of these commercial drugs have side effects. Gastric and intestinal mucosal damage are the commonest adverse effects of NSAIDS. Corticosteroids, an immunosuppressive drug, show various side effects, such as reduced bone marrow and increased skin fragility. Cyclosporin A exhibited toxicities and side effects including nephrotoxic activity and gingival hyperthrophy. Therefore, safer and more effective drugs are required as alternatives. Natural products remain one of the important sources of new and safe anti-inflammatory agents (Elgert, 2009).
In an effort to investigate for safer drugs, ethnopharmacological information can be used to provide preliminary data in the search for new drugs. It can be an indicator of pharmacological activity of natural products that could be further investigated for their mechanisms of action in cellular, animal, and human studies (Flores, 2017). Among them, some therapeutic activities of plant extracts or compounds have been proposed to be due to their effects on the immune system. Many plants of the genus Curcuma, especially C. longa, C. zanthorrhiza, C. amada, C. mangga, C. aeruginosa, and C. zedoaria, were reported to modulate the immune functions and possessed a variety of immunomodulatory effects. The strong immunomodulatory activity of these plants was due to their bioactive compounds as their main constituents. Curcuminoids, particularly curcumin, have been reported as the major components of plants in Curcuma species. Besides, other compounds, such as xanthorrhizol, have been reported to be present in other Curcuma species. A number of reviews on the phytochemistry, and biological and pharmacological activities of the genus Curcuma have been published recently (Rajkumari and Sanatombi, 2017; Sun et al., 2017; Dosoki and Setzer, 2018; Chanda and Ramachandra, 2019; Kaliyadasa and Samarasinghe, 2019; Kavitha and Mahadevi, 2020; USDA, 2021). However, there is either no or little and unconcise reports on the immunomodulatory effects of genus Curcuma and their bioactive molecules in these articles. In this review, we elaborated the ability of C. longa L., C. zanthorrhiza Roxb., C. mangga Valeton & Zijp, C. aeruginosa Roxb., C. zedoaria (Christm.) Roscoe, and C. amada Roxb. and their bioactive metabolites to modulate the immune response in different lineages of the immune system.
Methods
This comprehensive review was based on updated scientific databases on six major Curcuma species, namely, C. longa, C. zanthorrhiza, C. manga, C. aeruginosa, C. zedoaria, and C. amada. Databases were scanned from January 2000 until December 2020 for animal, in vitro, and clinical studies. A systematic search of databases with the use of the keywords “curcuma AND immune system,” “curcumin AND immune system,” and each species of Curcuma genus, such as “Curcuma mangga AND immune system,” “Curcuma longa AND immune system,” was carried out. Only published data were included in this study; meanwhile, references without title in English were excluded. Literature search has been carried out extensively to gather data, involving use of published scientific reports in Frontiers, the Science Direct, Scopus, Google Scholar, the Institute for Scientific Information (ISI)-Web of Science, Pub Med, Wiley Online Library, Elsevier, Springer, Taylor and Francis, ACS Publications Today, and other references over the past two decades. The gathered data on the immunomodulating effects of the Curcuma species were critically analyzed, and future strategies and appropriate perspectives for the plants as a source of new natural immunomodulators were discussed.
Taxonomy and Distribution
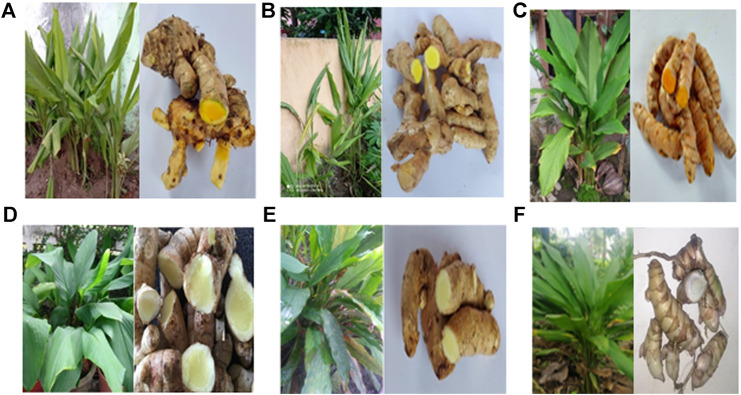
Curcuma L. is one of the largest genera in the family of Zingiberaceae, and there are approximately 100 accepted Curcuma species. It is found throughout tropical Asia from India to South China, Southeast Asia, Papua New Guinea, and northern Australia (Dosoky and Setzer, 2018). The word “curcuma” is derived from the Arabic word “kurkum,” which means yellow color (Kaliyadasa and Samarasinghe, 2019). Curcuma species are originated from the Indo-Malayan region and widespread in Asia, Africa, and Australia (Sasikumar, 2005). Figure 1 shows the Curcuma species: C. longa, C. zanthorrhiza, C. amada, C. mangga, C. aeruginosa, and C. zedoaria that are discussed in this review. The rhizomes of these plants are widely utilized in traditional medicine and as spices, food flavors, colorants, cosmetics, and perfumery. C. longa Linn (syn. Curcuma domestica Val.) is native to tropical South Asia, but it has been found throughout tropical areas (Li et al., 2011), such as Cambodia, China, India, Nepal, Indonesia, Madagascar, Malaysia, the Philippines, and Vietnam (Yadav and Tarun, 2017). It is commonly called as turmeric (Li et al., 2011; HMPC, 2017; Rajkumari and Sanatombi, 2017) and the Golden Spice of India (Yadav and Tarun, 2017). C. longa has been associated to the Indian culture for nearly 4000 years and probably reached China by 700 AD, East Africa by 800 AD, and West Africa by 1200 AD (Yadav and Tarun, 2017). C. longa has a specific name in some regions, namely, Haridra (Sanskrit, Ayurvedic), Jianghuang (Chinese), Kyoo or Ukon (Japanese) (HMPC, 2017), kurkum (Arabic), and haldi (Hindi and Urdu) (Dosoky and Setzer, 2018). C. longa has yellow-white flowers, 10–15 cm of stalk length, the seeds are brown ovoid, the plant grows upright, and part used for spices and medicine is the rhizome (Tung et al., 2019). C. zanthorrhiza is native to Indonesia (Rajkumari and Sanatombi, 2017), and it has been established by the Food and Drug Supervisory Agency (BADAN POM) as one of the leading medicinal plants (Ervintari et al., 2019). It is known as temu lawak (Dewi et al., 2012) and Java turmeric (Kim M-B et al., 2014; Astana et al., 2018), and distributed in Southeast Asia. It has been grown in Thailand, the Philippines, Sri Lanka, and Malaysia (Oon et al., 2015). It is grown simply to produce rhizomes which are commonly used in folk medicine (Wahono et al., 2017b). It is an ethnomedicinal plant from Indonesia and Malaysia (Kim M-B et al., 2014). It has 2-m tall erect pseudostems (Rajkumari and Sanatombi, 2017) and is generally cultivated in village home gardens. The rhizomes smell balmy and taste bitter (Ilene et al., 2020). C. zanthorrhiza has been used as an active ingredient in cosmetic and hygienic products in Germany and the Netherlands (Drugbank, 2021).
FIGURE 1.
Plants and rhizomes of Curcuma species. (A) Cucuma zanthorrhiza, (B) Curcuma mangga, (C) Curcuma longa, (D) Curcuma amada (Artfire, 2016; Snapdeal, 2020), (E) Curcuma zedoaria, and (F) Curcuma aeruginosa.
C. amada is widely distributed in Myanmar, and in southern and eastern India. Apart from Myanmar, C. amada is also distributed in the tropics of Asia to Africa and Australia. It is widely cultivated in West Bengal, Gujarat, Tamil Nadu, and the northeastern states of India (Sasikumar, 2005). It has the resemblance with ginger (Zingiber officinale) but imparts a raw mango (Mangifera indica) flavor (Policegoudra et al., 2011). Thereby, it is usually known as mango ginger due to its mango flavor. The flavor has been attributed to the presence of cis-ocimene and car-3-ene (Ayodele et al., 2018). C. amada rhizomes are fleshy, buff colored, 5–10 cm long, and 2–5 cm in diameter (Artfire, 2016; Policegoudra et al., 2011; Snapdeal, 2020). C. aeruginosa is an endemic species in Myanmar, but it is also distributed in West Bengal and Kerala (Rajkumari and Sanatombi, 2017). C. aeruginosa is also an ethnomedicinal plant in Indonesia, Malaysia, Thailand, Northern Australia, and Papua New Guinea (Sulfianti et al., 2019). It is commonly known as Kali Haldi (in India) and has a deep-blue or bluish-black colored cortex with pungent odor. In Indonesia, C. aeruginosa is known as Temu Ireng (Choudhury et al., 2013; George and Britto, 2015), and in English, it is known as pink and blue ginger (Sulfianti et al., 2019), temu hitam in Malaysia, and waan-maha-mek in Thailand. It is a perennial herb derived from Burma and spread to tropical countries in Malaysia, Thailand, India, and Indonesia (Dosoky and Setzer, 2018). C. zedoaria, known as white turmeric, is a perennial herb with perpendicular pseudostem and fleshy roots. It is a native plant from Bangladesh, India, and Sri Lanka (Lobo et al., 2009), but it is a critically threatened species in Bangladesh and India (Anisuzzaman et al., 2008). It is known by several names in India, and the most common are Krachura (Sanskrit), Gandamatsi (Hindi), and Sutha (Bengali) (Lobo et al., 2009). In China, it is generally called Ezhu (Lee et al., 2019). C. zedoaria is widely cultivated in subtropical regions (Southeast Asia, Thailand, Indonesia, Japan, and China). From outside, C. zedoaria looks like ginger, but inside, it looks like turmeric (Dosoky and Setzer, 2018). C. zedoaria rhizome has dark orange-fleshed tubers (Rahayu et al., 2020). C. mangga rhizome is commonly known as mango turmeric as it has the mango-like smell as in C. amada. It is a perennial herb with 30–110 cm of stem height. It is native from Java (Rajkumari and Sanatombi, 2017). It is distributed in most tropical countries such as Indonesia, Thailand, and Malaysia (Hong et al., 2016).
Ethnopharmacological Uses
C. longa is traditionally used as an antioxidant, anti-inflammatory, antidiabetic, hepatoprotective, and anticarcinogenic agent (Alsahli et al., 2018). It is well known as ethnomedicinal plant and used in different traditional systems in the world. In traditional medicine in Nigeria, C. longa is also used as an wound-healing agent (Adeshina et al., 2017). In Nepal, C. longa is applied as an anthelmintic, a tonic and blood purifier as well as for the treatment of Jaundice and liver disorder. In Peru, C. longa juice commonly known as Shapi natiyu is applied for the treatment of bronchitis and malaria. In Colombia, it is used for circulatory stimulant, healing wounds, liver cleaning, immune system booster, thrombosis, indigestion, diabetes, high cholesterol, and kidney infection (Ayati et al., 2019). The Ayurvedic Pharmacopoeia of India documented that C. longa is used as tonic, stomachic, and carminative. In Chinese Pharmacopoeia, C. longa has a potential for eliminating blood stasis, stimulating menstruation discharge, and relieving pain (Yue et al., 2010). In Pakistan traditional medicine, C. longa is used as a wound-healing agent and for the treatment of pimples. In Butanese folk medicine, it is known as Yung-ba and applied as tonic, antidote, antiseptic, anti-inflammatory, and as a good preservative (Ayati et al., 2019). C. zanthorrhiza is traditionally used for wound healing, as anti-inflammatory and anticarcinogecic agent, and for lowering of serum cholesterol levels (Kim et al., 2007) and booster immunity by Javanese (Setyati et al., 2019). In Malaysia, it is traditionally used to treat skin inflammation, rheumatism, stomach and liver disorders, and hepatitis (Kim M-B et al., 2014). In Ayurveda, C. amada is usually used for inflammation, asthma, bronchitis, biliousness, and skin disease (Policegoudra et al., 2006). The rhizomes are usually used for anorexia, dyspepsia, chronic ulcers, pruritus, gout, and inflammations (Thokchom and Phucho, 2015). Traditionally, C. amada is used for inflammation, stomach and skin diseases, cough, and rheumatism in Myanmar (Win et al., 2017). C. aeruginosa is used to booster immunity by Javanese (Setyati et al., 2019). It is used traditionally in Indonesia for gastrointestinal disease, and as antimicrobial and anti-inflammatory agents (Sulfianti et al., 2019). C. zedoaria is commonly known as white turmeric, and it is widely used as a traditional medicine in Indonesia (Putri, 2014; Aristyani et al., 2018), China and Japan (Kim et al., 2001), and India (Nan et al., 2014). C. zedoaria is traditionally used for treating cancer (Dutta, 2015) and also used as a traditional remedy to promote blood circulation in Korea and Japan (Kim et al., 2001). C. zedoaria is used to treat flatulent colic, hepatocirrhosis, and cancer in traditional Chinese medicine. It is also used to treat blood stagnation syndromes and for promoting menstruation (Carvalho et al., 2010). C. mangga is highly valued in Indonesian folk medicine for its healing properties in the treatment of stomach disorders, fever, and cancer-related diseases (Malek et al., 2011).
Phytochemistry
Plants from the genus Curcuma L. have been intensively studied for their phytochemical contents and bioactivity due to their tremendous ethnopharmacological and therapeutic potentials. There are recent reviews on the phytochemistry, and biological and pharmacological activities of Curcuma species (Rajkumari and Sanatombi, 2017; Dosoki and Setzer, 2018; Chanda and Ramachandra, 2019; Kavitha and Mahadevi, 2020; USDA, 2021). Phytochemical analysis has revealed that Curcuma species are made up mainly of terpenoids, flavonoids, phenolic compounds, organic acids, anthocyanin, tannins, and inorganic compounds. Until now, phytochemical studies on 32 Curcuma species have isolated and identified a total of 719 compounds, which include 529 terpenoids, 15 flavonoids, 102 diphenylalkanoids, 19 phenylpropene derivatives, 3 alkaloids, 7 steroids, and 44 other types of compounds (Sun et al., 2017). The phytochemical content of C. longa has been extensively investigated, and more than 235 compounds have been identified in the rhizome, which are mainly polyphenols and terpenoids. The major group of polyphenols is curcuminoids, which may contain up to 80% of curcumin, and other two are demethoxycurcumin and bisdemethoxycurcumin. In total, there are 109 sesquiterpenes, 68 monoterpenes, 22 diarylheptanoids and diarylpentanoids, eight phenylpropene and other phenolic compounds, five diterpenes, four sterols, three triterpenoids, two alkaloids, and 14 other compounds (Li et al., 2011). The essential oils of flowers and leaves are mainly made up of monoterpenes, while the root and rhizome oils are dominated by sesquiterpenes. A recent study reported that the average essential oil content in the rhizome was 3.97%, and the major components identified by gas chromatography were ar-turmerone (40%), α-turmerone (10%), and curlone (23%) (Guimarães et al., 2020). Xanthorrhizol, a bisabolane-type sesquiterpenoid compound, is the major compound of C. zanthorrhiza. Curcumin, demethoxycurcumin, and bisdemethoxycurcumin are also present in appreciable amounts. Sesquiterpenes of the bisabolene-type and their oxygenated derivatives were reported to comprise more than 92% of the rhizome oil of C. zanthorrhiza. Xanthorrhizol (32%) was the most abundant sesquiterpene phenol. ß-Curcumene (17.1%), zingiberene (13.2%), ß-bisabolol (3.5%), and ar-curcumene (2.6%) were the other major components of the oil (Jantan et al., 2012).
Several valuable sesquiterpenoids such as zedoarondiol zedoalactone A, zedoalactone B, curcumenol, isocurcumenol, zedoarol, isofuranodiene, and furanodiene have been isolated from C. aeruginosa rhizome. The rhizome oil of this plant was made up mainly of 1, 8-cineol, ß-pinene, camphor, curzerenone, furanodienone, furangermenone, curcumenol, zedoarol, isocurcumenol, and ß-elemene (Jose and Thomas, 2014). C. zedoaria rhizome is rich in sesquiterpenoids which are represented by furanodienone, furanodiene, curzerenone, zedorone, germacrone, curzeone, 13-hydroxy germacrone, curcumenol, curcumenone, dihydrocurdione, zedoaronediol, dihydrocurdione, zedoarol, 13-hydroxygermacrone, curzeone curcumenone, curcumanolide-A, curcumanolide-B, a-turmerone, ß-turmerone, epicurzerenone, and curzerene. GC and GC-MS analyses of the rhizome oil revealed the presence of curzerenone (22.3%) as the major component, together with 1,8-cineole, germacrone, cymene, a-phellandrene, and ß-eudesmol (Lobo et al., 2009). Based on percent yield, myrcene (88.6%), ocimene (47.2%), and ar-turmerone (29.12%) were reported to be the major chemical constituents of C. amada. Other compounds that were present in appreciable amounts were (Z)-β-farnesene, guaia-6,9-diene, cis ß-ocimene, cis-hydroocimene, trans-hydroocimene, a-longipinene, a-guaiene, linalool, ß-curcumene, and turmerone (Jatoi et al., 2007).
The presence of these diverse bioactive compounds in the plants contributes to the diverse pharmacological activities. Curcumin, one of the main active ingredients in Curcuma species, has been widely reported for its strong immunomodulating, antioxidant, anti-inflammatory, and antitumor activities. Structure–activity relationship studies have revealed that the presence of different functional entities on the diarylheptanoid structure which include methoxy, phenoxy, and carbon–carbon double bonds was found to be responsible for the antioxidant property. However, the remarkable anti-inflammatory property was associated with the symmetry of the structure and position of substituents along with the number of methoxy groups. In addition, electron-withdrawing substituents and the α,β-unsaturated carbonyl group were indicated imperative for reactivity (Arshad et al., 2017). Besides the curcuminoids (curcumin, demethoxycurcumin, bisdemethoxycurcumin, and dihydrocurcumin), other compounds from Curcuma spp. with significant activity on the immune system include xanthorrhizol, turmeronol, curdione, curcuzedoalide, curcumenol, and germacrone.
Immunomodulating Properties of Curcuma Species
Curcuma species and their bioactive compounds have been much investigated for their various biological and pharmacological activities, including antioxidant, anti-inflammatory, anticancer, hepato-protective, antifungal, antihypertensive, neuroprotective, and immunomodulatory effects through in vitro and in vivo studies. The six Curcuma species and their bioactive compounds discussed in this article have been documented to exhibit various pharmacological activities, particularly via modulation of the immune system. There are in-depth mechanistic studies on the immunomodulating effects of some of these species available in the literature. The immunomodulatory effects of the plant samples on the immune system are critically analyzed, and their underlying mechanisms of action are summarized in Table 1.
TABLE 1.
Immunomodulatory activity of some Curcuma species.
| Species | Subjects | Study design | Preparation | Immunomodulatory activities | Modulation | Parameters/mediators affected | References |
|---|---|---|---|---|---|---|---|
| Curcuma amada Roxb. | Rat PMNs | in vitro | Ethanol, petroleum ether, chloroform, and acetone extracts | Phagocytosis activity | ↑ | Phagocytosis | Karchuli and Pradhan (2011) |
| Sheep RBC-induced albino Wistar rats | in vivo | Ethanol extract | Cellular immunity | ↑ | Delayed-type hypersensitivity response | Karchuli and Pradhan (2011) | |
| Sheep RBC induced-albino Wistar rats | in vivo | Ethanol extract | Humoral immunity | ↑ | Antibody titer | Karchuli and Pradhan (2011) | |
| Curcuma aeruginosa Roxb. | Zymosan-stimulated human PMNs | in vitro | Methanol extract | ROS generation | ↓ | ROS | Jantan et al. (2011) |
| Zymosan-stimulated macrophages of BALB/c mice | in vitro | Methanol extract | ROS generation | ↓ | ROS | Jantan et al. (2011) | |
| Human PMNs | in vitro | Methanol extract | PMN chemotaxis | ↓ | Chemotaxis | Jantan et al. (2011) | |
| Human whole blood | in vitro | Methanol extract | CD18/11a expression | ↓ | CD18/11a | Harun et al. (2015) | |
| Human whole blood | in vitro | Methanol extract | Phagocytosis activity | ↓ | Phagocytosis | Harun et al. (2015) | |
| Lymphocytes of BALB/c mice | in vitro | Extract by steam distillation | Counts of CD4+ and CD8+ cells | ↑ | CD4+ and CD8+ cells | Anggriani et al. (2019) | |
| DMBA-induced Wistar rats | in vivo | Ethanol extract | Cytokine release | ↑ | TNF-α, IFN-γ, IL-2, and IL-12 | Sulfianti et al. (2019) | |
| Epinephelus fuscoguttatus | in vivo | C. aeruginosa, Piper retrofractum, and C. zanthorrhiza water extracts | Leukocyte number | ↑ | Total leukocyte count | Setyati et al. (2019) | |
| Epinephelus fuscoguttatus | in vivo | C. aeruginosa, Piper retrofractum, and C. zanthorrhiza water extracts | Phagocytosis activity | ↑ | Phagocytic index | Setyati et al. (2019) | |
| Curcuma longa Linn | CMS-induced Sprague–Dawley rats | in vivo | Ethanol extract | Cytokine release | ↓ | IL-6 and TNF-α | Xia et al. (2006) |
| Male Sprague–Dawley rats | in vivo | Ethanol extract | Splenic NK cell activity | ↑ | NK cell | Xia et al. (2006) | |
| Mice | in vivo | Methanol extract | Adaptive immune response | ↑ | Leukocytes number, antibody titer, spleen index, and delayed-type hypersensitivity response | Kumolosasi et al. (2018) | |
| Human peripheral blood mononuclear cells (PBMCs) | in vitro | Polar fraction of hot water extract | Proliferation response | ↑ | PBMC viability | Yue et al. (2010) | |
| Human peripheral blood mononuclear cells (PBMCs) | in vitro | Polysaccharide-enriched fraction at 200 μg/ml | Cytokine gene expression | ↑ | GM-CSF, IL-1, IL-5, IL-8, IL-10, and IL-13 | Yue et al. (2010) | |
| Human peripheral blood mononuclear cells (PBMCs) | in vitro | Polysaccharide-enriched fraction at 400 and 800 μg/ml | Cytokine release | ↑ | TNF-α and IL-6 | Yue et al. (2010) | |
| Human peripheral blood mononuclear cells (PBMCs) | in vitro | Polysaccharide-enriched fraction at 800 μg/ml | Cytokine release | ↑ | TGF-β | Yue et al. (2010) | |
| Human peripheral blood mononuclear cells (PBMCs) | in vitro | Polysaccharide-enriched fraction at 800 μg/ml | Lymphocyte population | ↑ | CD14+ | Yue et al. (2010) | |
| Unstimulated mouse splenocytes and mouse macrophage (RAW264.7) cells | in vitro | Water extract | Cytokine release | ↑ | NO, IL-2, IL-6, IL-10, IL-12, IFN-γ, TNF-α, and MCP-1 | Chinampudur et al. (2013) | |
| LPS stimulated mouse splenocytes | in vitro | Water extract | Cytokine release | ↓ | NO, IL-12, IL-6, and PGE2 | Chinampudur et al. (2013) | |
| Con-A–induced splenocytes | in vitro | Water extract | Cytokine release | ↑ | IL-2 and IFN-γ | Chinampudur et al. (2013) | |
| Con-A–induced splenocytes | in vitro | Water extract | Cytokine release | ↓ | IL-10 | Chinampudur et al. (2013) | |
| LPS-unstimulated and stimulated mouse splenocytes | in vitro | Polysaccharide fraction | Lymphocyte proliferation | ↑ | Splenocytes number | Chinampudur et al. (2013) | |
| LPS-stimulated mouse splenocytes | in vitro | Polysaccharide fraction | Cytokine release | ↑ | IL-10 | Chinampudur et al. (2013) | |
| LPS-stimulated mouse splenocytes | in vitro | Polysaccharide fraction | Cytokine release | ↓ | IL-12 and PGE2 | Chinampudur et al. (2013) | |
| RAW264.7 macrophages | in vitro | Water extract | Nitric oxide (NO) production | ↑ | NO levels | Pan et al. (2017) | |
| Diabetic infected rats | in vivo | Ethanol extract | Total IgE | ↓ | IgE levels | Shabana et al. (2020) | |
| Diabetic infected rats | in vivo | Ethanol extract | Leukocyte number | ↓ | Total leukocyte count (TLC) | Shabana et al. (2020) | |
| Diabetic infected rats | in vivo | Ethanol extract | NO production | ↓ | NO | Shabana et al., 2020 | |
| Diabetic infected rats | in vivo | Ethanol extract | Cytokine release | ↓ | IL-6, TNF-a, and IL-1β | Shabana et al., 2020 | |
| LP-BM5 MuLV-induced mice | in vivo | Alcohol extract | Proliferation | ↓ | T-cell, B-cell, and NK-cell | Kim O. K. et al. (2014) | |
| LP-BM5 MuLV-induced mice | in vivo | Alcohol extract | Cytokine imbalance | Prevented | Th1 (IL-2 and IFN-γ)/Th2) (IL-4 and IL-10) | Kim O. K. et al. (2014) | |
| C57BL/6 mice | in vivo | C. longa, Mulberry leaves, and purple sweet potato extracts | Proliferation | ↓ | T cell and B cell | Yoo et al. (2013) | |
| C57BL/6 mice | in vivo | C. longa, Mulberry leaves, and purple sweet potato extracts | Cytokine secretion | ↓ | Th 1 cytokines (IL-2 and IFN-γ), Th2 cytokines (TNF-α and IL-10) | Yoo et al. (2013) | |
| LP-BM5 MuLV-infected mice | in vivo | C. longa and sweet potato mixture | Messenger RNA (mRNA) expression | ↑ | MHC I and MHC II | Park et al. (2018) | |
| LP-BM5 MuLV-infected mice | in vivo | C. longa and sweet potato mixture | Population of CD4 (+)/CD8 (+) T cells | ↓ | CD4 (+)/CD8 (+) T cells | Park et al. (2018) | |
| LP-BM5 MuLV-infected mice | in vivo | C. longa powder and sweet potato mixture | Ig levels | ↓ | IgA, IgE, and IgG | Park et al. (2018) | |
| Human umbilical vein endothelial cells (HUVECs) | in vitro | Extract | mRNA levels | ↓ | NF-κB p65, IL-6, and TNF-α | Morales et al. (2012) | |
| C57BL mice | in vivo | Hot water extract | Cytokines release | ↓ | TNF-α, IL-6, and IL-6 m-RNA proteins | Uchio et al. (2017) | |
| Fusarium root | in vivo | Aqueous extract | mRNA of the defense-related genes | ↑ | Defensin and chitinase | Alsahli et al. (2018) | |
| Clarias gariepinus | in vivo | Powder | IgM level | ↑ | IgM | Adeshina et al. (2017) | |
| Clarias gariepinus | in vivo | Powder | Enzyme activity | ↑ | Lysozyme activity | Adeshina et al. (2017) | |
| Cyprinus carpio | in vivo | Powder | Leukocyte number | ↑ | Neutrophils, lymphocytes, monocyctes, eosinophils, and basophils | Arunkumar et al. (2016) | |
| Fish green terror (Andinocara rivulatus) | in vivo | Powder | White blood cell number | ↑ | White blood cells | Mooraki et al. (2019) | |
| Nile tilapia (Oreochromis niloticus) | in vivo | Powder | Leukocrit levels | ↑ | Leukocrit number | Hassan et al. (2018) | |
| M. rosenbergii | in vivo | Powder | Gene expression | ↑ | Crustin and lysozyme | Alambra et al. (2012) | |
| Chicks | in vivo | Powder | Lymphocyte percentage | ↑ | Lymphocytes | Naderi et al. (2014) | |
| Curcuma zedoaria Rosc. | LPS-stimulated RAW264.7 cells | in vitro | Methanol extract | NO production | ↓ | NO | Lee et al. (2019) |
| LPS-stimulated RAW264.7 cells | in vitro | Methanol extract | Pro-inflammatory protein expression | ↓ | iNOS and COX-2 | Lee et al. (2019) | |
| RBL-2H3 cells | in vitro | Aqueous acetone extract | Beta-hexosaminidase release | ↓ | Beta-hexosaminidase | Lobo et al. (2009) | |
| C57Bl/6J mice | in vivo | Ethanol extract | Total leukocytes count | ↑ | Leukocytes | Carvalho et al. (2010) | |
| L. monocytogenes and S. aureus–stimulated RAW264.7 cells | in vitro | Essential oil | Cytokine release | ↓ | TNF-α | Huang et al. (2019) | |
| PMA-stimulated RAW264.7 cells | in vitro | Polysaccharide fraction | Cytokine release | ↑ | TNF-α | Kim et al. (2001) | |
| PMA-stimulated RAW264.7 cells | in vitro | Polysaccharide fraction | NO production | ↑ | NO | Kim et al. (2001) | |
| Curcuma zanthorrhiza Roxb. | Zymosan-stimulated human whole blood | in vitro | Methanol extract | ROS generation | ↓ | ROS | Jantan et al. (2011) |
| Zymosan-stimulated PMNs | in vitro | Methanol extract | ROS generation | ↓ | ROS | Jantan et al. (2011) | |
| Zymosan-stimulated macrophages of BALB/c mice | in vitro | Methanol extract | ROS generation | ↓ | ROS | Jantan et al. (2011) | |
| Human PMNs | in vitro | Methanol extract | PMN chemotaxis | ↓ | Chemotaxis | Jantan et al. (2011) | |
| Human whole blood | in vitro | Methanol extract | Expression of CD18/11a | ↓ | CD18/11a | Harun et al. (2015) | |
| Human whole blood | in vitro | Methanol extract | Phagocytosis activity | ↑ | Phagocytosis | Harun et al. (2015) | |
| Hypercholesterolemic male Sprague–Dawley rats | in vivo | Curcuminoid cider | IL1β, TNFα, and chemokine gene expression | ↓ | IL1β, TNFα, and chemokine | Hardiwati et al. (2019) | |
| High cholesterol diet male Sprague–Dawley rats | in vivo | Curcuminoid cider | CD44, ICAM-1, iNOS, and LOX-1 gene expression | ↓ | CD44, ICAM-1, iNOS, and LOX-1 | Mauren et al. (2016) | |
| Human lymphocytes | in vitro | Volatile oil | Lymphocytes proliferation | ↑ | Lymphocytes | Miksusanti (2012) | |
| Alcohol-induced mice | in vivo | Ethanol extract | Lymphocytes proliferation | ↓ | Lymphocytes | Ilene et al. (2020) | |
| High-fat diet-induced C57BL/6 mice | in vivo | Ethanol extract | Cytokine genes expression | ↓ | TNF-α, IL-6, IL-1β, and C-reactive protein (CRP) | Kim M-B et al. (2014) | |
| RAW 264.7 cells | in vitro | Crude polysaccharide extract | Chemical mediators release | ↑ | TNF-α and PGE2 | Kim et al. (2007) | |
| RAW 264.7 cells | in vitro | Crude polysaccharide extract | Oxidative burst | ↑ | NO and H2O2 | Kim et al. (2007) | |
| RAW 264.7 cells | in vitro | Crude polysaccharide extract | Phosphorylation | ↑ | IκBα | Kim et al. (2007) | |
| LPS-stimulated human gingival fibroblast-1 cells | in vitro | Crude polysaccharide extract | mRNA levels | ↓ | IL-1β, NF-κB p65, MMP-2, and MMP-8 | Kim et al. (2018) | |
| HIV/AIDS patients | Clinical study | C. zanthorrhiza in combination with C. mangga and Phyllanthus niruri | Lymphocytes proliferation | Maintained | CD4+ value | Astana et al. (2018) | |
| Systemic lupus erythematosus (SLE) patients | Clinical study | C. zanthorrhiza supplementation with vitamin D3 | Cytokine release | No significant difference reduction | IL-6 | Wahono et al. (2017a) | |
| Systemic lupus erythematosus (SLE) patients | Clinical study | C. zanthorrhiza supplementation with vitamin D3 | Cytokine release | No significant difference reduction | IL-17 | Wahono et al. (2017b) | |
| Systemic lupus erythematosus (SLE) patients | Clinical study | Powder | Cytokine release | ↓ | TNF-α | Setiawati et al. (2017) | |
| Curcuma mangga Val. | Swiss albino mice | in vivo | Ethanol extract and its fraction (hexane, chloroform, ethyl acetate, and aqueous fractions) | Paw and ear edema | ↓ | Paw and ear volume | Ruangsang et al. (2010) |
| LPS and IFNγ–induced RAW264.7 macrophage cells | in vitro | Methanol extract | NO production | ↓ | NO | Abas et al. (2006) | |
| LPS-stimulated RAW264.7 macrophage cells | in vitro | Ethanol extract and chloroform, hexane, and ethyl acetate fractions | NO production | ↓ | NO | Kaewkroek et al. (2009) | |
| Zymosan-stimulated human whole blood | in vitro | Methanol extract | ROS inhibitory activity | ↓ | ROS | Jantan et al. (2011) | |
| Zymosan-stimulated human PMNs | in vitro | Methanol extract | ROS inhibitory activity | ↓ | ROS | Jantan et al. (2011) | |
| Zymosan-stimulated macrophages of BALB/c mice | in vitro | Methanol extract | ROS inhibitory activity | ↓ | ROS | Jantan et al. (2011) | |
| Human PMNs | in vitro | Methanol extract | PMN chemotaxis | ↓ | Chemotaxis | Jantan et al. (2011) | |
| Human whole blood | in vitro | Methanol extract | Expression of CD18/11a | ↓ | CD18/11a | Harun et al. (2015) | |
| Human whole blood | in vitro | Methanol extract | Phagocytosis activity | ↑ | Phagocytosis | Harun et al. (2015) | |
| Mice | in vivo | n-Hexane, ethyl acetate, and ethanol extracts | Phagocytosis activity | ↑ | Phagocytosis | Yuandani and Suwarso (2017b); Yuandani et al. (2019) | |
| Bovine RBC-stimulated mice | in vivo | Ethanol extract | Humoral immunity | ↑ | Antibody titer | Yuandani et al. (2018) | |
| Bovine RBC-stimulated mice | in vivo | Ethanol extract | Cellular immunity | ↑ | Delayed-type hypersensitivity response | Yuandani et al. (2018) | |
| Doxorubicin-induced immunosuppressive rats | in vivo | Ethanol extract | Humoral immunity | ↑ | Antibody titer | Yuandani et al. (2020) | |
| Doxorubicin-induced immunosuppressive rats | in vivo | Ethanol extract | Cellular immunity | ↑ | Delayed-type hypersensitivity response | Yuandani et al. (2020) |
↑, increase.
↓, decrease.
Curcuma longa L.
In Vitro Immunomodulating Effect of C. longa
Of all the Curcuma species investigated, the immunomodulatory effects of C. longa were the most studied. Interestingly, most experimental studies on the extracts of C. longa were carried out using in vivo animal models, and there were few in vitro studies. The followings are reports on the few in vitro studies that have been carried out to evaluate the immunomodulating effects of C. longa. C longa fermented by Aspergillus oryzae (FCL) exhibited immunomodulatory effects in RAW 264.7 cells. The different extracts of FLC on phagocytic activity, TNF-α, NO production, NK cell activity, and mRNA expression of LP-BM5 eco displayed the following results: hot water and 20% ethanol extracts increased the phagocytic activity, but there was no significant change in the production of NO relative to the control. There was also suppression of mRNA expression of LP-BM5 eco in FCL extracts and a four-fold increase in NK cell cytotoxity relative to the control group, especially in the 20% ethanol extract treatment group. However, TNF-α was significantly increased by the addition of FCL extracts (Yoo et al., 2014). Curcuminoid extract from C. longa has been reported to modulate TNF-⍺ and IL-6 at protein and gene levels in adipocytes in vitro (Hardiwati et al., 2019). C. longa decreased mRNA levels of NF-κB p65, IL-6, and TNF-α at 2.5–5 mg/L in LPS-induced human umbilical vein endothelial cells (HUVEC) (Morales et al., 2012). The polysaccharide extract isolated from C. longa was reported to possess immunostimulatory activities. Investigation of the polar fractions of C. longa hot water extract displayed that the extract stimulated PBMC proliferation using the [methyl-3H]-thymidine incorporation assay. Furthermore, its polysaccharide-enriched fraction at 200 μg/ml enhanced the cytokine expression (IL-1, IL-5, IL-8, IL-10, IL-13, and GM-CSF) detected by semiquantitatively using the antibody-based RayBio human cytokine array. However, the fraction at 200 μg/ml did not significantly enhance TNF-α, IFN-γ, TGF-β, and IL-6 productions. The production of IL-6 and TNF-α was only enhanced after treatment with the fraction at the higher doses of 400 and 800 μg/ml, respectively. The polysaccharide fraction at 800 μg/ml stimulated TGF-β release and CD14+ lymphocyte and population. However, the CD4+/CD8+ ratio was not altered after administration with polysaccharide fraction (Yue et al., 2010). In a related study, the immunostimulant and anti-inflammatory effects of C. longa aqueous extract and its polysaccharide fractions in the presence and absence of mitogens were determined. The extract enhanced splenocyte proliferation in unstimulated and LPS or concanavalin A-stimulated cells. The extract increased the levels of IL-2, IL-10, NO, IL-6, IL-12, TNF-α, IFN-γ, and MCP-1 in the absence of mitogen. Interestingly, C. longa extract decreased the levels of IL-12, IL-6, NO, and PGE-2 in LPS-stimulated cells, while TNF-α, IL-10, and MCP-1 levels were not altered. In contrast, the extract stimulated IL-2 and IFN-γ production but decreased IL-10 production from Con-A–induced splenocytes. Furthermore, its polysaccharide fraction showed stimulatory activity on lymphocyte proliferation in the absence or presence of LPS. The levels of IL-10 were increased, but the levels of IL-12 and PGE-2 were decreased after treatment with C. longa in LPS-stimulated cells (Chinampudur et al., 2013). In another study, a C. longa root aqueous extract standardized to a minimum of 20% of polysaccharides ukonan A, B, C, and D was shown to stimulate NO production in RAW264.7 macrophages (Pan et al., 2017).
In Vivo Immunomodulating Effect of C. longa
Most immunomodulating studies were carried out using aqueous and alcoholic extracts. The ethanol extract of C. longa was reported to suppress immune function, and behavioral and neuroendocrine alterations in a rat chronic mild stress (CMS) model. The enhancement of cytokine level (TNF-α and IL-6) activity and NK cell activity inhibition in the CMS-induced rat in splenocytes were reversed by administration of 35 mg/kg of C. longa ethanol extract and 7 mg/kg of fluoxetine as a control. The putative antidepressant properties of the extract were due to suppressive effects on cytokine biosynthesis. However, the extract increased the IL-6 level in the nonstress group, but there was no significant difference as compared with those of the normal group and caused a slight but no significant decrease in TNF-α levels. Although the extract enhanced splenic NK cell activity in CMS-treated rats, the NK cell activity of nonstressed rat did not change after treatment with C. longa (Xia et al., 2006). In another study, treatment with C. longa methanol extract with a single dose of 200 mg/kg for 14 days in mice stimulated innate and adaptive immunity. The effect of the extract on adaptive immunity was investigated by immunizing and challenging the mice with sheep red blood cells (sRBCs) on days 7 and 14, respectively. C. longa enhanced the adaptive immunity by increasing leukocyte number, antibody titer, spleen index, and delayed-type hypersensitivity response (Kumolosasi et al., 2018). However, the results of this study are preliminary as different doses of the extract need to be used to determine a dose–response relationship and the optimal dose for efficacy.
A previous study reported that treatment with C. longa in diabetic rats infected with Staphylococcus aureus resulted in a decrease of IgE, total leukocyte number (TLC), NO, and cytokine production (IL-6, IL-1β, and TNF-α). The results indicated that there was improvement of immune function by reducing levels of pro-inflammatory cytokines in the diabetic rats (Shabana et al., 2020). It was reported that 20% C. longa alcohol extract suppressed the increase of liver weights, lymph node, and spleen, and reduction of proliferation of T and B cells and NK cell activity stimulated by murine leukemia viruses–induced murine acquired immunodeficiency syndrome (AIDS) infection. Moreover, the extract suppressed Th1/Th2 (IL-2, IFN-γ/IL-4, and IL-10) cytokine imbalance and pro-inflammatory cytokine production (Kim O-K et al., 2014). This is in agreement with another study which showed that a diet consisted of C. longa; mulberry leaves and purple sweet potato extracts have the ability to prevent splenomegaly and lymphadenopathy induced by retrovirus, decrement of B- and T-cell proliferation, as well as reduction of Th 1 cytokine (IFN-γ and IL-2) release. It also reduced Th2 cytokine (TNF-α and IL-10) release (Yoo et al., 2013). Moreover, C. longa alone and in combination with purple sweet potato inhibited LP-BM5 murine leukemia virus (MuLV)-induced lymphadenopathy. The mixture of C. longa and purple sweet potato at the doses of 2 and 5 g/kg body weight increased the mRNA expression of MHC I and II as compared to those of the infected control group. The mixture at 5 g/kg body weight decreased the population of CD4+ T cells as compared to the infected control group, and also, the population of CD8+ T cells was lower than that of the normal group. Moreover, the extracts also affected T- and B-cell proliferation. The levels of Th1-type cytokines (IL-12 and IL-15) were enhanced after treatment by the mixture; meanwhile, Th2-type cytokine (IL-4, IL-10, IL-6, and TNF-α) production was significantly decreased as compared to the infected control group. In addition, the mixture at the doses of 2 and 5 g/kg decreased the levels of IgA, IgE, and IgG. Besides, C. longa alone or in mixture enhanced the phagocytosis activity of LP-BM5 MuLV-infected mice (Park et al., 2018). C. longa hot water extract protected the C57BL mice liver from acute injury induced by ethanol at 3 g/kg. The hepatic injury caused an increase in TNF-α, IL-6, and IL-6 m-RNA proteins. However, an increase in these proteins was not found in mice treated with hot water extract of C. longa 30 min before induction (Uchio et al., 2017). C. longa aqueous extract has been evaluated for its immunotherapeutic and hepatoprotective activities in CCl4 intoxicated Swiss albino mice. The aqueous extract reduced the levels of bilirubin and transaminase enzymes (SGOT and SGPT) in mice. Treatment with CCl4 resulted in liver damage and reduced nonspecific host–response parameters such as NO and MPO release, phagocytosis, intracellular killing capacity of peritoneal macrophages, and morphological alteration. Treatment with the extract also significantly protected the adverse effects of CCl4 on the nonspecific host response in the peritoneal macrophages of the mice (Sengupta et al., 2011).
Interestingly, there are several studies on the ability of C. longa to modulate the immune response of fish, chick, and prawn. C. longa increased plant defense by enhancing the defense-related genes such as defensin and chitinase of treated sunflower seedlings (Alsahli et al., 2018). The enhancement of host defense in fish has also been reported. C. longa leaf–enriched diet was fed to the fish to satiation twice daily for 12 weeks. Then, the fish was challenged with Aeromonas hydrophila. The highest stimulation on immunoglobulin M (IgM) level and lysozyme activity was observed in fish fed with 2.5% C. longa–fortified diets (Adeshina et al., 2017). A study reported that Mesocyclops thermocyclopoides enriched with C. longa enhanced the differential leukocyte number in fish (Cyprinus carpio), including enhancement of neutrophils, lymphocytes, monocytes, eosinophils, and basophils (Arunkumar et al., 2016). This result was supported by a previous study which reported the ability of 0.3% turmeric powder–enriched fish diet to enhance the white blood cell number significantly as compared to those of the control group (Mooraki et al., 2019). Turmeric in combination with rosemary (Rosmarinus officinalis) and thyme (Thymus vulgaris) increased the leukocrit levels in fish (Hassan et al., 2018).
C. longa was also able to enhance the immune response of prawns (Macrobrachium rosenbergii) after being infected by Vibrio alginolyticus. Identification using RT-PCR revealed that C. longa–enhanced feeds increased the gene expression of crustin and lysozyme in M. rosenbergii, indicating a remarkable increase in the expression of AMPs (antimicrobial peptides). Production of AMPs is a first-line host defense mechanism of innate immunity, and they are thought to be essential for organisms lacking adaptive immunity (Alambra et al., 2012). The ability of C. longa to modulate the immune response in chicks was also reported. C. longa powder constituted 2.5 and 7.5 g/kg of the diet, which significantly enhanced lymphocyte percentage in chicks. Supplementation of the diet with the powder at 2.5 g/kg of the diet resulted in a significant increase in anti-infectious bronchitis virus (IBV) titer compared to the control group (Naderi et al., 2014). In another study, 2.5% of C. longa–enriched diet protected chicken from Salmonella pullorum infection (Purwanti et al., 2018). Moreover, the cellular immunity of broiler chicken to phytohemagglutinin-P (PHA-P) was significantly higher in groups fed with higher amount of C. longa. The primary antibody titer to sRBCs was also stimulated (Sethy et al., 2017). These studies revealed that C. longa mostly enhanced the cellular and humoral responses of fish, chick, and prawns. Thus, this plant can be used as animal feed to enhance the immune defense of the animals.
Extensive cellular and animal studies have been performed to evaluate the immunomodulatory effects of C. longa by using various immune cells such as macrophages, monocytes, neutrophils, lymphocytes (T and B cells), and NK cells. There is a need to explore the immune effect of the plant with other immune cells, particularly the antigen-presenting cells such as dendritic cells. The existing reports should be supported by exploring the effects of the plant samples on various animal disease models of immune-related and chronic inflammatory disorders. All the extracts of Curcuma species used in the in vitro and in vivo immunomodulating studies were not analyzed for their chemical constituents or standardized to marker compounds. C. longa samples were mostly in the form of crude aqueous and alcoholic extracts. Some of the samples were curcuminoids or polysaccharide-rich extracts, but the chemical composition of the extracts were not determined. It has been suggested that the curcuminoids and polysaccharides might be the main contributors for immunomodulatory activity of the plant. The extracts used should be determined qualitatively and quantitatively by using validated analytical methods such as reversed-phase HPLC methods. Some of the bioactive compounds—especially the curcuminoids—have been isolated from the extracts, and their mechanistic effects in modulating the immune system have been determined.
Curcuma zanthorrhiza Roxb.
In Vitro Immunomodulating Effect of C. zanthorrhiza
C. zanthorrhiza methanol extract has been reported to inhibit ROS generation in a luminol and lucigenin-enhanced chemiluminescence (CL) assay. C. zanthorrhiza rhizomes reduced ROS production from whole blood of human by in vitro study. Moreover, the extract significantly inhibited the release of ROS from zymosan-induced PMNs and macrophages. C. zanthorrhiza also showed strong inhibition on PMN migration, with an IC50 value of 2.5 μg/ml (Jantan et al., 2011). A previous study reported that the methanol extract of C. zanthorrhiza rhizomes showed strong inhibition on the expression of CD18/11a; meanwhile, the extract has low effect on leukocyte phagocytosis (Harun et al., 2015). The mRNA levels of IL-1β, NF-κB p65, MMP-2, and MMP-8 on LPS-induced human gingival fibroblast-1 cells were reduced after treatment with crude polysaccharide extract of C. zanthorrhiza. The extract of C. zanthorrhiza inhibited MAPK/activator protein-1 (AP-1) signaling pathways. C. zanthorrhiza has been documented to exhibit anti-inflammatory activities in LPS-induced RAW264.7 monocytes and H2O2-treated HT22 hippocampal cells (Kim et al., 2018).
In Vivo Immunomodulating Effect of C. zanthorrhiza
Curcuminoid cider, a traditional fermented product made by the addition of Acetobacter xylinum to curcuminoid fraction isolated from C. zanthorrhiza, reduced the gene expression of IL1β, TNFα, and chemokine in hypercholesterolemic rats (Hardiwati et al., 2019). The data were in accordance with a previous study which demonstrated the inhibitory activity of curcuminoid cider and curcuminoid fraction from C. zanthorrhiza on the gene expression of CD44, ICAM-1, iNOS, and LOX-1 in high-cholesterol diet rats (Mauren et al., 2016). Volatile oil from C. zanthorrhiza enhanced the lymphocyte proliferation from human male B blood type (Miksusanti, 2012). C. zanthorrhiza extract administration was able to reduce inflammatory lymphocytes in alcohol-induced hepatitis in mice (Ilene et al., 2020). C. zanthorrhiza ethanol extracts strongly reduced cytokine gene expression, which include TNF-α, IL-6, IL-1β, and C-reactive protein (CRP) in the liver, adipose tissue, and muscle of high-fat diet-induced obese mice (Kim M-B et al., 2014). The crude polysaccharide extract of C. zanthorrhiza consisted of glucose, galactose, arabinose, xylose, mannose, and rhamnose, and was also reported to significantly enhance the phagocytosis of macrophages and the production of NO, H2O2, TNF-α, and PGE2. In addition, it clearly enhanced phosphorylation of IκBα, suggesting a role as a NF-ĸB activator (Kim et al., 2007; Huang et al., 2010). C. zanthorrhiza–inhibited pro-inflammatory cytokine production in mice induced high-fat diet. C. zanthorrhiza extract at 100 mg/kg body weight/day decreased IL-1β gene expression by 89.9% compared to the control group (Ilene et al., 2020). C. zanthorrhiza was also reported to stimulate total and differential leukocytes in African catfish (Clarias gariepinus) (Lestari et al., 2019). C. zanthorrhiza rhizome in combination with Zingiber officinale rhizome, Vitex trifolia leaves, Echinacea purpurea, and citrus fruit in a herbal formula increased the number of macrophages phagocytizing Candida albicans as compared to those of E. purpurea–only group in mice. In addition, the herbal formula also displayed immunostimulatory activities on lymphocyte proliferation and the level of IgG actively phagocytizing C. albicans (Ikawati et al., 2019).
Clinical Studies of C. zanthorrhiza on the Immune System
An unsystematic clinical study of C. zanthorrhiza reported that C. zanthorrhiza extract reduced the population of B lymphocytes (Dewi et al., 2012). A previous study reported that C. zanthorrhiza in combination with C. mangga and Phyllanthus niruri maintained the levels of CD4+ in HIV/AIDS patients (Astana et al., 2018). C. zanthorrhiza in combination with Vitex trifolia did not cause liver and kidney damage after 14 days, 3 times a day treatment in women (Baroroh et al., 2011). Supplementation of C. zanthorrhiza with vitamin D3 was not able to decrease IL-6 level and elevate TGF-β1 systemic lupus erythematosus (SLE) in patients with hypovitaminosis D (Wahono et al., 2017b). These data were supported by a double-blind randomized controlled study on active SLE patients with hypovitaminosis D, which reported that addition of C. zanthorrhiza in vitamin D3 did not reduce IL-17 level as compared to those of singular vitamin D administration (Wahono et al., 2017a). Furthermore, a placebo-controlled double-blind clinical study showed that TNF-α release was reduced after treatment with the extract of C. zanthorrhiza for 4 weeks in SLE patients (Setiawati et al., 2017).
C. zanthorrhiza Roxb. is the second most popular plant among the genus Curcuma that has been investigated for its immunomodulating properties. Similar to C. longa, the crude extracts of C. zanthorrhiza were used in experimental studies to evaluate its in vivo immunomodulating effect using various animal models. There were a few in vitro studies, and the chemical constituents of the extracts were mostly not determined or the extracts were not standardized. Some clinical trials have been conducted on C. zanthorrhiza extracts, but they were unsystematic and not well designed. Despite the regulatory requirements for clinical studies and sufficient data not being generated on preclinical testing of C. zanthorrhiza, there were already reports on a few unsystematic case studies to evaluate the immunomodulating properties of C. zanthorrhiza in human. For clinical studies, sufficient preclinical testing should be generated using standardized extracts, which include bioavailability, and pharmacokinetic and toxicological studies, before they can be subjected to clinical studies.
Curcuma aeruginosa Roxb.
In Vitro Immunomodulating of C. aeruginosa
The methanol extract of C. aeruginosa at 100 and 6.25 μg/ml showed moderate inhibition on CD18/11a expression on the surface of phagocytes, which was determined using a flow cytometry method. The extract at the same concentrations also demonstrated low inhibition on phagocytosis of leukocytes (Harun et al., 2015). Investigation on the effect of C. aeruginosa methanol extract on ROS generation from polymorphonuclear cells (PMNs) and peritoneal macrophages in human whole blood revealed that the extract possessed ROS inhibitory activity for luminol-stimulated chemiluminescence (CL). C. aeruginosa rhizomes inhibited oxidative burst of PMNs and macrophages, with IC50 values of 1.8 and 4.6 μg/ml, respectively. Interestingly, C. aeruginosa extract also possessed significant ROS inhibitory activity for lucigenin-enhanced CL. However, C. aeruginosa revealed low inhibition on PMN chemotaxis toward the chemoattractant, N-formyl-methionyl-leucyl-phenylalanine (fMLP), with percentage inhibition of 49.9% (Jantan et al., 2011).
In Vivo Immunomodulating of C. aeruginosa
C. aeruginosa extract, obtained by steam distillation, has been reported to increase the percentage of CD4+ and CD8+ cells (Anggriani et al., 2019). A previous study reported that C. aeruginosa ethanol extract was able to increase IFN-γ, TNF-α, IL-2, and IL-12 levels in 7,12-dimethylbenz [a]anthracene (DMBA)-induced Wistar rats. The highest stimulation on cytokines release was shown after treatment with the ethanol extract of C. aeruginosa at a dose of 80 mg/200 g body weight (Sulfianti et al., 2019). The aqueous extract of C. aeruginosa in combination with Piper retrofractum and Curcuma zanthorrhiza supplemented in a fish fed at the concentrations of 0.5, 1, and 1.5%, respectively, enhanced nonspecific immunity of Epinephelus fuscoguttatus. The addition of C. aeruginosa extract induced significant difference in the total leukocyte count of Epinephelus fuscoguttatus after being infected by Vibrio alginolyticus and V. parahaemolyticus during 15 days of observation. C. aeruginosa treatment increased the total leukocyte count on day 4 and day 8. Moreover, C. aeruginosa at concentration of 1% showed the strongest stimulation on phagocytosis activity, which was determined on day 8 (Setyati et al., 2019).
The in vitro and in vivo immunomodulating studies on C. aeruginosa were carried out on their crude aqueous and ethanol extracts. The bioactive metabolites contributing to the modulating effects were not identified. It is important to chemically characterize the extract to determine the bioactive compounds contributing to the immunomodulatory properties and mechanistic investigation to conclude the plant potency and effects on the immune-related disorders.
Curcuma zedoaria (Christm.) Roscoe
In Vitro Immunomodulating Effect of C. zedoaria
C. zedoaria (Christm.) Roscoe rhizome extract has been reported to inhibit NO production from LPS-stimulated RAW264.7 cells. It has also been found to reduce iNOS and COX-2 expressions (Lee et al., 2019). In another study, C. zedoaria prevented ß-hexosaminidase release in RBL-2H3 cells and showed passive cutaneous anaphylaxis reaction in mice. ß-Hexosaminidase is a marker of antigen-IgE–mediated degranulation (Lobo et al., 2009). Essential oil from C. zedoaria was reported to reduce TNF-α release from L. monocytogenes and S. aureus–stimulated RAW264.7 cells (Huang et al., 2019). Polysaccharide fraction of C. zedoaria rhizome was found to enhance phagocytosis activity and splenocyte proliferation. It also stimulated the primary and secondary titers as well as delayed-type hypersensitivity response (Faradilla and Iwo, 2014). This work was supported by a previous study which showed that polysaccharide fraction of C. zedoaria enhanced phagocytosis of FITC-labeled Gram-negative bacteria (E. coli) or Gram-positive bacteria (S. aureus) by peritoneal macrophages. It also stimulated two microbicidal routes, oxygen-dependent and oxygen-independent mechanisms. Lysosomal activity increased after treatment with polysaccharide fraction as well as in vivo and in vitro respiratory burst. It was reported that PMA-induced respiratory burst of peritoneal macrophage was higher than those of RAW 264 cells identified using luminol-chemiluminescence–based assay. The production of H2O2, NO, and TNF-α was also enhanced at the doses of 10, 50, and 100 μg/ml, dose dependently (Kim et al., 2001).
In Vivo Immunomodulating Effect of C. zedoaria
The effect of C. zedoaria extract on tumor progression and peripheral blood cells in C57Bl/6J mice injected with B16F10 murine melanoma cells was determined using different routes of administration. A decrease in peritoneal cell number and a significant increase in total red and white blood cell counts were observed. Oral administration of the extract revealed a noteworthy increase only in the total leukocyte count (Carvalho et al., 2010). C. zedoaria has also been reported to stimulate immune response in fish. Supplemented diets with C. zedoaria increased the phagocytic rate and lysosome activity in Epinephelus coioedes fish. C. zedoaria was able to increase reactive oxygen production, identified using two different methods, NBT test and chemiluminescent assay (Nan et al., 2014).
Similar to the other Curcuma species already discussed, the metabolite profiles of C. zedoaria extracts were not determined. It is necessary to analyze the chemical constituents of the extracts or use standardized extracts in the studies as the phytochemical constituents of the plant may vary with variation in genetic adaptation of the plant population growing at different altitudes, its geographical distribution due to the changes in soil composition, and other environmental factors. Thus, using standardized extracts will ensure the dynamic change of varying amounts of phytochemical constituents in the plant is taken into consideration.
Curcuma mangga Valeton & Zijp
In Vitro Immunomodulating Effect of C. mangga
A previous study reported in vitro NO inhibition activity of C. mangga which might contribute to its anti-inflammatory effect (Abas et al., 2006; Kaewkroek et al., 2009; Liu and Nair, 2011). Furthermore, C. mangga rhizome extract and its chloroform, hexane, and ethyl acetate fractions reduced NO production from LPS-induced RAW 264.7 cells. Among the fractions, the chloroform fraction showed the highest NO inhibition, followed by hexane, and then ethyl acetate fractions (Kaewkroek et al., 2009). A previous study of the methanol extract of C. mangga rhizomes on whole blood showed that the extract exhibited strong inhibitory activity upon activation by zymosan. C. mangga rhizome extract possessed high ROS inhibitory activity in PMNs and peritoneal macrophages as investigated in a luminol-enhanced CL assay. The extract also inhibited the release of ROS from PMNs and macrophages in a lucigenin-enhanced CL assay, with IC50 values of 0.9 and 6.6 μg/ml, respectively (Jantan et al., 2011). C. mangga methanol extract has also been found to significantly suppress the cell surface expression of CD18/11a as compared to the negative control. However, the extract of C. mangga rhizome at the concentration of 100 and 6.25 μg/ml showed immunostimulatory activity on phagocytosis of leukocytes (Harun et al., 2015).
In Vivo Immunomodulating Effect of C. mangga
C. mangga Valeton & Zijp rhizome ethanol extract, its different organic fractions (hexane, chloroform, and ethyl acetate), and aqueous fraction have showed appreciable anti-inflammatory and analgesic activities in mice and inflammatory models using croton oil-induced mouse ear edema and carrageenan-induced rat paw edema. The plant extract and its fractions at 200 mg/kg demonstrated analgesic activity by reducing the number of writhing and also produced antinociception using hot plate and formalin test. At 200 mg/kg, the hexane and chloroform fractions significantly prolonged the latency time, but ethyl acetate and aqueous fractions were not active. In addition, the ethanol extract of C. mangga rhizome and its fractions displayed significant reduction of paw and ear edema in rat (Ruangsang et al., 2010). Our previous study reported that the n-hexane, ethyl acetate, and ethanol extracts of C. mangga rhizomes at the doses of 100, 200, and 400 mg/kg increased the carbon clearance rate, indicating the enhancement of carbon engulfment by cells in the reticuloendothelial system of mice, thus stimulating the phagocytosis activity in mice (Yuandani and Suwarso, 2017a; Yuandani et al., 2019). In addition, the C. mangga rhizome ethanol extract exhibited stimulation of antibody titer against bovine red blood cells in a dose-dependent way by using the hemagglutination method. The cellular immunity was also enhanced after treatment with C. mangga ethanol extract by increasing the bovine red blood cell–induced mice paw volume (Yuandani et al., 2018). Moreover, the ethanol extract of C. mangga rhizome stimulated the immune response in doxorubicin-induced immunosuppressive rats, which was indicated by the elevation of antibody titer and delayed hypersensitivity (DTH) response (Yuandani et al., 2020).
As with other Curcuma species already discussed, the effects of C. mangga on the immune cells and experimental animals may vary considerably, depending on the experimental conditions used, including the solvent of extraction, extraction method, cell line, animal model, treatment scheme, and different disease animal models. Dosage and concentration of raw extracts of the plant are crucial in order to achieve the desired benefit. Thus, to ensure the results are reproducible when the study is replicated, the same methodology has to be used by other researchers.
Curcuma amada Roxb.
The ethanol, petroleum ether, chloroform, and acetone extracts of C. amada enhanced the phagocytosis activity of PMNs. The ethanol extract at a concentration 3 mg/ml showed the highest stimulation on percentage of phagocytosis. Further study on delayed hypersensitivity response against sRBCs showed that the ethanol extract of C. amada increased the paw volume. Moreover, the ethanol extract at the doses of 100, 200, and 400 mg/kg enhanced the antibody titer dose-dependently (Karchuli and Pradhan, 2011). Supercritical carbon dioxide (CO2) extract prepared from C. amada rhizomes has potential to be used for the treatment of immune disorder such as autoimmune diseases. Specifically, the extract can be used to treat or prevent hypersensitivity diseases, in particular IgE-mediated allergic reactions as well as autoimmune disorders (Weidner et al., 2001). C. amada in combination with Tinospora cordifolia, Piper longum, and Albizia lebbeck in a herbal preparation can be used to treat allergy (Palpu et al., 2008). The chemical constituents responsible for eliciting the activity were not determined, although a few potent activities have been reported on C. amada extract. There is a need to proceed to study in detail the underlying mechanisms on relevant signaling events followed by in vivo studies to explore the potential of this plant as a natural immunomodulating agent.
Immunomodulatory Effects of Bioactive Compounds of Curcuma Species
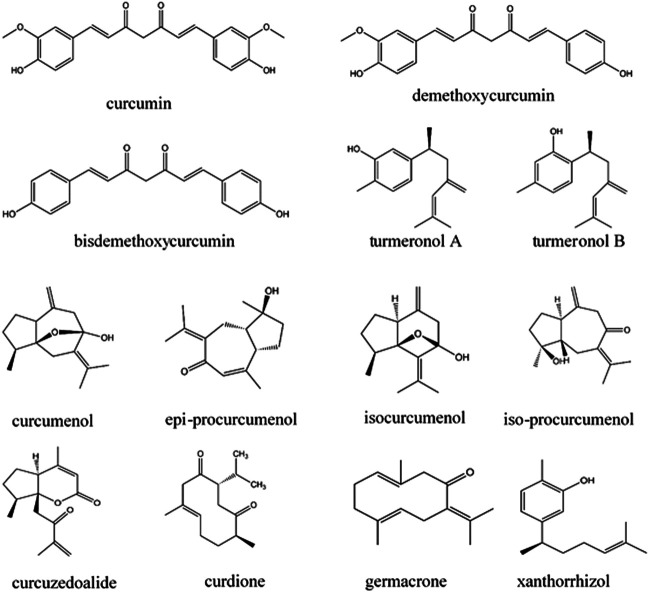
Plants in the genus Curcuma contain many compounds which contribute to the immunomodulatory activity of the plants, as shown in Table 2. Among the compounds from Curcuma species, curcumin and xanthorrhizol have been discussed in detail in this review as they have been widely investigated for their immunomodulating effects on the innate and adaptive immune system. Other compounds including turmeronols, curdione, curcuzedoalide, demethoxycurcumin, bisdemethoxycurcumin, dihydrocurcumin, curcumenol, epi-procurcumenol, isocurcumenol, and iso-procurcumenol germacrone are also included in this review, but their data are limited as they have not been well investigated for their immunomodulating effects. The chemical structures of these compounds are included in Figure 2.
TABLE 2.
Bioactive compounds of Curcuma species with immunomodulating activity and their mechanisms of action.
| Main compound | Species | Subjects | Study design | Immunomodulatory activities | Modulation | Parameters/mediators affected | References |
|---|---|---|---|---|---|---|---|
| Curcumin | Curcuma species | High glucose-cultured monocytes | in vitro | Cytokine production | ↓ | IL6, IL8, TNFα, and MCP1 | Jain et al. (2009) |
| Streptozotocin-induced rats | in vivo | Cytokine production | ↓ | IL6, TNFα, and MCP1 | Jain et al. (2009) | ||
| Mice pancreatic | in vivo | Leukocyte infiltration | ↓ | Leukocytes | Castro et al. (2014) | ||
| M-stimulated BDC2.5-splenocytes | in vitro | T-cell proliferation | ↓ | CD4+, T cells, and IFN-γ | Castro et al. (2014) | ||
| BDC2.5 mice T lymphocite | in vitro | T-cell proliferation | ↓ | T lymphocyte | Castro et al. (2014) | ||
| PMN leukocytes | in vitro | DHA synthesis | ↑ | DHA | Pisani et al. (2009), Wu et al. (2015) | ||
| PMN leukocytes | in vitro | ROS production | ↓ | ROS | Pisani et al. (2009), Wu et al. (2015) | ||
| LPS-induced mice mastitis | in vivo | Myeloperoxidase activity | ↓ | MPO | Fu et al. (2014) | ||
| LPS-induced mice mastitis | in vivo | Cytokine production | ↓ | TNF-α, IL-6, IL-1β, and TLR4 | Fu et al. (2014) | ||
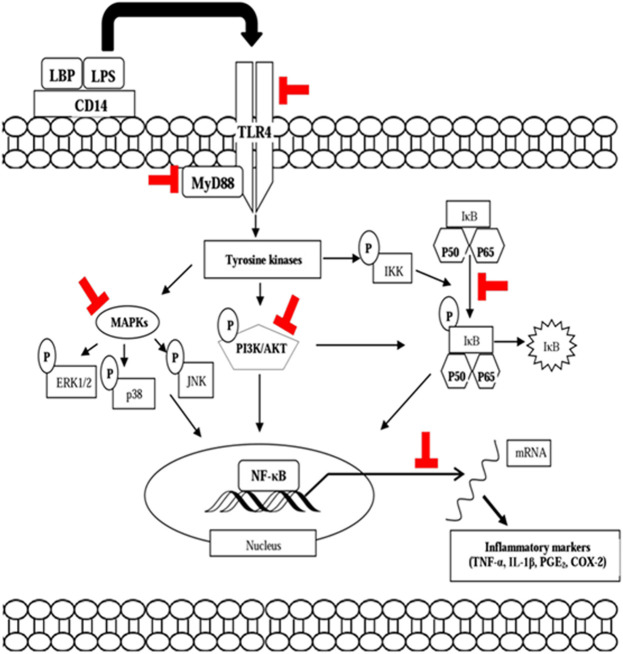
| LPS-induced mice mastitis | in vivo | Phosphorylation | ↓ | IκB-α and NF-κB p65 | Fu et al. (2014) | ||
| Microglial cells | in vitro | NO production | ↓ | NO | Cianciulli et al. (2016) | ||
| Microglial cells | in vitro | Phosphorylation | ↓ | IL-1β, IL-6, TNF-α, and PI3K/Akt | Cianciulli et al. (2016) | ||
| Microglial cells | in vitro | NF-κB and iNOS expression | ↓ | NF-κB and iNOS | Cianciulli et al. (2016) | ||
| Microglial cells | in vitro | Cytokine production | ↓ | NO, PGE2, TNF-α, iNOS, and COX-2 | Yu et al. (2018) | ||
| C. longa | Healthy albino mice | in vivo | White blood cells production and weight lymphoid | ↑ | Lymphoid organs and white blood cells | Afolayan et al. (2018) | |
| Dendritic cells | in vitro | Surface molecule expression | ↓ | CD80, CD86, MHC class II, and IL-1 | Kim et al. (2005) | ||
| Dendritic cells | in vitro | Cytokine production | ↓ | IL-6, IL-12, and TNF- α | Kim et al. (2005) | ||
| Dendritic cells | in vitro | NF-κB p65 translocation | ↓ | NF-κB p65 | Kim et al., 2005 | ||
| Bronchoalveolar of Balb/c mice | in vivo | Allergic response | ↓ | Eosinophils | Ravikumar and Kavitha (2020) | ||
| Bronchoalveolar of Balb/c mice | in vivo | Cytokine production | ↓ | IL-4 | Ravikumar and Kavitha (2020) | ||
| PBMCs | in vitro | T-cell proliferation | ↓ | Lymphocyte | Yadav et al. (2005) | ||
| PBMCs | in vitro | Cytokine production | ↓ | IL-2 and TNF-α | Yadav et al. (2005) | ||
| PBMCs | in vitro | NF-κB | ↓ | NF-κB | Yadav et al. (2005) | ||
| Erythroleukemic cell line K562 | in vitro | Cytotoxicity | ↑ | NK cell | Yadav et al. (2005) | ||
| Lupus BALB/c mice | in vivo | Adaptive immune response | ↓ | Th1, Th2, and Th17 | Kalim et al. (2017) | ||
| Lupus BALB/c mice | in vivo | ANA levels | ↓ | ANA | Kalim et al. (2017) | ||
| Monocytes and liver macrophages | in vivo | ROS production | ↓ | ROS | Inzaugarat et al. (2017) | ||
| Monocytes | in vivo | TNF-α and IFN- γ production | ↑ | TNF-α and IFN- γ | Inzaugarat et al. (2017) | ||
| C. longa | Fish | in vivo | Immune response | ↑ | Immune | Alambra et al. (2012) | |
| C. zedoaria | RBL-2H3 cells | in vitro | beta-Hexosaminidase production | ↓ | Beta-hexosaminidase | Matsuda et al. (2004) | |
| RBL-2H3 cells | in vitro | Cytokine production | ↑ | TNF–α and IL–4 | Matsuda et al. (2004) | ||
| Turmeronol | C. longa | RAW264.7 cells | in vitro | PGE2 and NO production | ↓ | PGE2 and NO | Okuda-Hanafusa et al. (2019) |
| RAW264.7 cells | in vitro | Cytokine production | ↓ | IL-1β and IL-6 | Okuda-Hanafusa et al. (2019) | ||
| Cytoplasm into the nucleus | in vitro | NF-κB translocation | ↓ | NF-κB | Okuda-Hanafusa et al. (2019) | ||
| Curdione | C. aeruginosa | CD95 protein | in silico | Docking score | ↓ | Curdione to CD95 | Anggriani et al. (2019) |
| 1,8-cineol | CD95 protein | in silico | Docking score | ↑ | 1,8-cineol to CD95 | Anggriani et al. (2019) | |
| Isocurcumenol | Chicken embryo fibroblast | in vitro | Toxicity | - | Fibroblast cells and lymphocytes | Lakshmi et al. (2011) | |
| Isoprocurcumenol | RAW264.7 cells | in vitro | NO activity | ↓ | NO | Lee et al. (2019) | |
| Germacrone | C. zedoaria | RAW264.7 cells | in vitro | NO activity | ↓ | NO | Lee et al. (2019) |
| Curzerenone | RAW264.7 cells | in vitro | NO activity | ↓ | NO | Lee et al., 2019 | |
| Curcumenol | RAW264.7 cells | in vitro | NO activity | ↓ | NO | Lee et al. (2019) | |
| Curcuzedoalide | RAW264.7 cells | in vitro | NO activity | ↓ | NO | Lee et al. (2019) | |
| RAW264.7 cells | in vitro | iNOS and COX-2 response | ↓ | iNOS and COX-2 | Lee et al. (2019) | ||
| Dihydrocurcumin | RBL-2H3 cells | in vitro | beta-Hexosaminidase production | ↓ | beta-Hexosaminidase | ||
| RBL-2H3 cells | in vitro | Cytokine production | ↑ | TNF-α and IL-4 | Matsuda et al. (2004) | ||
| Tetrahydrodemethoxycurcumin | RBL-2H3 cells | in vitro | beta-Hexosaminidase production | ↓ | beta-Hexosaminidase | Matsuda et al. (2004) | |
| RBL-2H3 cells | in vitro | Cytokine production | ↑ | TNF-α and IL-4 | Matsuda et al. (2004) | ||
| Tetrahydrobisdemethoxycurcumin | RBL-2H3 cells | in vitro | Hexosaminidase production | ↓ | beta-Hexosaminidase | Matsuda et al. (2004) | |
| RBL-2H3 cells | in vitro | Cytokine production | ↑ | TNF-α and IL-4 | Matsuda et al. (2004) | ||
| 1,7-bis(4-hydroxyphenyl)-1,4,6-heptatrien-3-one | Lipopolysaccharide (LPS)-activated macrophages | in vitro | TNF-α production | ↓ | TNF-α | Jang et al. (2001) | |
| Macrophages | in vitro | NO production and iNOS expression | ↓ | NO and iNOS | Jang et al. (2004) | ||
| Procurcumenol | lipopolysaccharide (LPS)-activated macrophages | in vitro | TNF-α production | ↓ | TNF-α | Jang et al. (2001) | |
| Xanthorrhizol | C. zanthorrhiza | Human gingival fibroblast-1 cells | in vitro | mRNA levels | ↓ | IL-1β, NF-κB p65, MMP-2, and MMP-8 | Kim et al. (2018) |
| RAW 264.7 cell line | in vitro | MAPK and AP-1 response | ↓ | MAPK and AP-1 | Kim et al. (2018) | ||
| Demethoxycurcumin | C. mangga | RAW 264.7 cell line | in vitro | NO production | ↓ | NO | Kaewkroek et al. (2009) |
| RAW 264.7 cell line | in vitro | NO and PGE2 production | ↓ | NO and PGE2 | Kaewkroek et al. (2010) | ||
| RAW 264.7 cell line | in vitro | mRNA expressions | ↓ | iNOS and COX-2 | Kaewkroek et al. (2010) | ||
| Bisdemethoxycurcumin | RAW 264.7 cell line | in vitro | NO production | ↓ | NO | Kaewkroek et al. (2009) | |
| RAW 264.7 cell line | in vitro | NO and PGE2 production | ↓ | NO and PGE2 | Kaewkroek et al. (2010) | ||
| RAW 264.7 cell line | in vitro | mRNA expressions | ↓ | iNOS and COX-2 | Kaewkroek et al. (2010) | ||
| 4-[(1R, 4aR, 8aR)-decahydro-5, 5, 8a-trimethyl-2-methylene-1-naphthalenyl]-, (3E)-rel | RAW 264.7 cell line | in vitro | NO and PGE2 production | ↓ | NO and PGE2 | Kaewkroek et al. (2009) | |
| RAW 264.7 cell line | in vitro | mRNA expressions | ↓ | iNOS and COX-2 | Kaewkroek et al. (2009) | ||
| 15,16 bisnorlabda-8(17), 11-dien-13-one | RAW 264.7 cell line | in vitro | NO and PGE2 production | ↓ | NO and PGE2 | Kaewkroek et al. (2010) | |
| RAW 264.7 cell line | in vitro | mRNA expressions | ↓ | iNOS and COX-2 | Kaewkroek et al. (2010) | ||
| (E)-15,15-diethoxylabda-8 (17),12-dien-16-al | RAW 264.7 cell line | in vitro | NO and PGE2 production | ↓ | NO and PGE2 | Kaewkroek et al. (2010) | |
| RAW 264.7 cell line | in vitro | mRNA expressions | ↓ | iNOS and COX-2 | Kaewkroek et al. (2010) |
↑, increase.
↓, decrease.
-, no changes.
FIGURE 2.
Chemical structures of potential immunomodulators from Curcuma species.
Curcumin
It is a major compound of C. longa and can also be found in other Curcuma species. This natural diarylheptanoid compound has been mainly isolated from the rhizomes of C. longa and studied extensively for various pharmacological activities, including antioxidant, anti-inflammatory, immunomodulatory, antiangiogenic, anticancer, antiproliferative, and proapoptotic. It has been one of the most intensively investigated compounds for its immunomodulatory properties. Many preclinical investigations which include in vitro cell assays and in vivo studies in animal models have been carried out on curcumin to evaluate its modulatory effects in the immune system. It is also undergoing extensive clinical trials based on its anti-inflammatory properties for the treatment of cancer.
In Vitro Immunomodulating Effect of Curcumin
The immunomodulating activity of curcumin has been demonstrated by many in vitro studies using several immune cells. Curcumin has been shown to inhibit inflammatory responses by suppressing COX-2 and NO, NF-ĸB, iNOS, and lipoxygenase in IFN-γ or TNF-α–activated macrophages and NK cells (Surh et al., 2001). A study by Jain et al. (2009) revealed that curcumin significantly reduced the production of IL-6, IL-8, TNF-α, and MCP-1 from high glucose-cultured monocytes. Low concentration of curcumin reduced NOS activity and NO production from macrophages. In another study, curcumin inhibited the immunostimulatory function of dendritic cells, leading to the reduction of CD80, CD86, and MHC class II expression, but not MHC class I expression as well as IL-12 expression and cytokine release (IL-1, IL-6, and TNF- α). Curcumin also inhibited LPS-induced MAPK activation and the translocation of NF-B p65 as well as impaired induction of Th1 responses (Kim et al., 2005). Gao et al. (2004) demonstrated that curcumin inhibited pro-inflammatory cytokine (TNF-α, IL-1, IL-12, and IL-6) expression in PMA or LPS-activated macrophages, dendritic cells, monocytes, and splenic lymphocytes. Curcumin has also been found to suppress PHA-stimulated lymphocyte proliferation and IL-2 release as well as transcription factor NF-KB and TNF-α production from LPS-stimulated PBMC to enhance NK cell cytotoxicity (Yadav et al., 2005). Curcumin has been shown to be able to increase ω-3 polyunsaturated fatty acid (PUFA) synthesis in the brain. An in vitro study has showed that PUFAs have beneficial effects on stimulating immune response by stimulating neutrophil phagocytosis activity, while decreasing the release of ROS in goat neutrophils (Pisani et al., 2009; Wu et al., 2015). Curcumin also increased ROS production from linoleic acid–stimulated monocytes and liver macrophages as well as TNF-α production from leptin-induced monocytes and IFN-γ release from CD4+ T cells (Inzaugarat et al., 2017). Pretreatment with curcumin significantly reduced the production of NO, the expression and release of TNF-α, IL-1β, IL-6, PI3K/Akt phosphorylation, NF-κB activation, and iNOS expression in LPS-stimulated microglial cells (Cianciulli et al., 2016). In addition, NO, PGE2, and TNF-α production as well as iNOS and COX-2 expression in LTA-activated microglial cells were reduced by curcumin (Yu et al., 2018). In an in silico study, curcumin has been shown to bind to viral S1 protein, which is important for SARS-CoV-2 entry; hence, it may prevent cytokine storm in a severe form of COVID-19 (Pawitan, 2020). The pathways and inflammatory mediators involved in the immunomodulating property of curcumin are illustrated in Figure 3.
FIGURE 3.
Modulatory effects of curcumin on the NF-κB, MAPK, and Akt signaling pathways. The thick red block sign indicates the possible point of modulation of the signal transduction pathways. NF-κB, nuclear factor kappa β; MAPK, mitogen-activated protein kinase; PI3K/Akt, phosphatidylinositol 3-kinase and protein kinase B; P, phosphoryl group.
In Vivo Immunomodulating Effect of Curcumin
In vivo studies to determine the immunomodulatory effects of curcumin were carried out using several animal models. Curcumin reduced the levels of IL6, TNFα, and MCP1 in streptozotocin-induced type 1 diabetes rats. Moreover, curcumin prevented pancreatic leukocytes infiltration that might initiate ß-cell destruction. In addition, curcumin reduced CD4+ T cell proliferation and IFN-γ release from M-stimulated BDC2·5-splenocytes as well as reduced LPS/IFN-γ–induced dendritic cell maturation. Antigen-specific T-lymphocyte proliferation has also been reduced by curcumin action on both T cells and antigen-presenting cells (APCs) (Castro et al., 2014). Administration of curcumin to lactating mice prevented mice mastitis by reducing the MPO activity; expression of TNF-α, IL-6, IL-1β, TLR4; and phosphorylation of IκB-α and NF-κB p65 after being induced by LPS (Fu et al., 2014). Nanoparticulate curcumin demonstrated stronger activity on cellular and humoral immunity, and increased lymphoid organs and white blood cell production than those of control (Afolayan et al., 2018). A previous study reported that curcumin displayed immunomodulatory effects in comorbid diabetic asthma mice by reducing eosinophil number and Il-4 level with a high IFN-γ to IL-4 ratio in the blood and bronchoalveolar after ovalbumin injection (Ravikumar and Kavitha, 2020). Administration of curcumin to pristane-induced lupus mice decreased Th1, Th2, and Th17 and slightly increased Treg percentages. In addition, ANA levels were also decreased after curcumin treatment (Kalim et al., 2017). Curcumin was able to enhance immune response in Macrobrachium rosenbergii after being challenged with Vibrio alginolyticus (Alambra et al., 2012).
Clinical Studies of Curcumin on Immune System
Presently, curcumin is under extensive clinical investigation where there are 116 ongoing clinical trials on curcumin, the status of which can be found on http://www.clinicaltrials.gov/. Among the clinical trials on curcumin, 99 of them were based on its anti-inflammatory properties. Cancer (e.g., breast, pancreatic, lung, colorectal, and prostate), inflammatory bowel diseases (IBD; Crohn’s disease and ulcerative colitis) and rheumatoid arthritis were the major diseases for which trials had been conducted, reflecting the pleiotropic actions of curcumin. In these trials, curcumin often acted as a dietary supplement or an adjunct treatment to the standard therapy. Studies on the efficacy and safety of curcumin as adjuvant in the treatment of cancer and cognitive damage will continue to dominate in future clinical trials (Jurenka, 2009). In their review on the clinical effects of curcumin in ulcerative colitis, Kumar et al. (2012) suggested that curcumin may be a safe and effective therapy for the maintenance of remission when given as adjunct therapy in quiescent ulcerative colitis. However, the results were preliminary due to the low number of enrolled patients participated in the clinical trials, as suggested by Fürst and Zündorf (2014). They suggested more thorough controlled randomized trials are required to be pursued to determine the safety level and efficacy of the compound for human use. The success of curcumin as a potent anti-inflammatory agent in future depends on the findings of high-quality and big cohort studies. Moreover, curcumin has poor bioavailability, and many studies have been carried out to address this issue via chemical and technological methods. Preparation of more stable curcumin derivatives and use of nanotechnology for curcumin delivery are actively being pursued to improve the bioavailability of curcumin (Anand et al., 2007). Curcumin still has potential to be used clinically for the treatment of the abovementioned indications as it is nontoxic with good safety profile and well tolerated.
Xanthorrhizol
Xanthorrhizol is a bisabolane-type sesquiterpenoid, isolated from C. zanthorrhiza Roxb. It is known to possess diverse pharmacological activities, including antioxidant, anti-inflammatory, antimicrobial, anticancer, hepatoprotective, nephroprotective, antihypertensive, antihyperglycemic, antiestrogenic, and antiplatelet effects. Xanthorrhizol reduced mRNA levels of MMP-2, MMP-8, NF-κB p65, and IL-1β in LPS-induced human gingival fibroblast-1 cells. The compound also has anti-inflammatory activities in H2O2-treated HT22 hippocampal cells and inhibited MAPK/activator protein-1 (AP-1) signaling pathways (Kim et al., 2018). In a study to evaluate the effects of standardized C. zanthorrhiza extract and its marker compound, xanthorrhizol, on hyperglycemia and inflammatory markers in high-fat diet–induced obese mice, xanthorrhizol was found to significantly inhibit inflammatory cytokine release, such as IL-1β, IL-6, TNF-α, and C-reactive protein (CRP), in the liver, muscle, and adipose tissue (Kim M-B et al., 2014).
Other Compounds
In addition to curcuminoids, sesquiterpenes like turmeronols have potential to suppress the immune response. Turmeronol A and turmeronol B from C. longa were tested on mouse macrophages (RAW 264,7 cells). Both turmeronols showed inhibitory of PGE2 and NO production as well as IL-6 and IL-1β at the mRNA and protein levels in LPS-induced cells. Turmeronols also inhibited the translocation of NF-κB from the cytoplasm into the nucleus (Okuda-Hanafusa et al., 2019). Curdione, a major compound of C. aeruginosa, has been proposed as a potential immunomodulatory agent. Molecular docking analysis revealed that there is a high probability of interaction between curdione and CD95 protein, as the replacement of native ligand. The docking score of curdione to the protein was lower than that of the native ligand. In addition, 1,8-cineol from C. aeruginosa also has a high docking score to CD95 protein, but not significant as compared to those of native ligand of CD95 (Anggriani et al., 2019). Isocurcumenol isolated from C. zedoaria did not demonstrate any significant toxicity on normal chicken embryo lymphocytes and fibroblast cells (Lakshmi et al., 2011). A recent study showed that five sesquiterpenoids (isoprocurcumenol, germacrone, curzerenone, curcumenol, and curcuzedoalide) from C. zedoaria demonstrated inhibitory activity on NO synthesis. Among the compounds, curcuzedoalide showed the highest inhibition. Further study showed that curcuzedoalide inhibited the expression of pro-inflammatory mediators (iNOS and COX-2) (Lee et al., 2019). Curcumin, dihydrocurcumin, tetrahydrodemethoxycurcumin, and tetrahydrobisdemethoxycurcumin in C. zedoaria enhanced the release of IL-4 and TNF–α, and inhibited the production of ß-hexosaminidase. ß-Hexosaminidase is a marker of antigen-IgE–mediated degranulation (Putri, 2014).
Isolated compounds from C. zedoaria, that is, epiprocurcumenol, procurcumenol, and 1,7-bis(4-hydroxyphenyl)-1,4,6-heptatrien-3-one, inhibited the production of TNF-α from LPS-activated macrophages (Jang, et al., 2001). These compounds, especially 1,7-bis(4-hydroxyphenyl)-1,4,6-heptatrien-3-one, were also found to exhibit strong inhibition against production of NO and expression of iNOS in activated macrophages (Jang et al., 2004). Demethoxycurcumin, bisdemethoxycurcumin, and 3-buten-2-one, 4-[(1R, 4aR, 8aR)-decahydro-5, 5, 8a-trimethyl-2-methylene-1-naphthalenyl]- (3E)-rel isolated from C. manga reduced the production of NO from LPS-stimulated RAW 264.7 cells. Among the compounds tested, demethoxycurcumin showed the highest NO inhibition (Kaewkroek et al., 2009). In an effort to elaborate the anti-inflammatory mechanism of compounds from C. mangga rhizomes, Kaewkroek et al. (2010) evaluated the anti-inflammatory effects of several compounds against production of PGE2 and NO from RAW 264.7 cells. These include demethoxycurcumin, bisdemethoxycurcumin, 15,16 bisnorlabda-8 (17),11-dien-13-one, and (E)-15,15-diethoxylabda-8 (17),12-dien-16-al. Of all the compounds tested, 11-dien-13-one (E)-15,15-diethoxylabda-8 (17),12-dien-16-al demonstrated the strongest NO inhibitory activity, while demethoxycurcumin displayed the strongest activity on PGE2 release. Moreover, investigation of mechanism at the transcriptional level showed that all the compounds reduced the mRNA expressions of COX-2 and iNOS, except 15,16 bisnorlabda-8 (17), 11-dien-13-one, which only downregulated the mRNA of iNOS.
Most of the studies on the bioactive secondary metabolites of Curcuma species were carried out at cellular and molecular levels on various immune cells to explore their effects on the release and expression of pro-inflammatory mediators via various signaling pathways, such as NF-κB, MAPKs, and other events. More in-depth studies to understand the underlying mechanisms using experimental in vivo animal models of immune-related disorders and elaborate bioavailability, preclinical pharmacokinetics, and toxicity studies are required before clinical trials can be pursued for development into immunomodulatory agents.
Toxicological Studies
Systematic safety evaluations and toxicological investigations on C. longa and curcumin have indicated that they are nontoxic for human consumption, especially by oral administration. It is considered non-genotoxic, non-mutagenic, and generally recognized as safe. Several studies have indicated that oral administration of C. longa and curcumin in animals was safe without reproductive toxicity at certain doses. Clinical trials have indicated that the safe dose for human consumption was at an oral dose of 6 g/day for 4–7 weeks. In rare cases, minor side effects like gastrointestinal upsets may happen (Soleimani et al., 2018). A cheminformatics approach was used to predict toxicity, which includes human hepatotoxicity, rodent carcinogenicity, and bacterial mutagenicity of 200 chemical compounds found in C. longa. Of the compounds studied, 136 compounds were predicted as mutagenic, 184 were toxigenic, 64 were hepatotoxic, and 153 were carcinogenic. Interestingly, a dose-dependent hepatotoxicity may occur with curcumin and its derivatives. The study also predicted that few other constituents of C. longa are noncarcinogenic, non-mutagenic, non-hepatotoxic, and devoid of any side effects (Balaji and Chemakam, 2010).
Liju et al. (2013) reported the acute and sub-chronic toxicity studies of the essential oil of C. longa (EOCL). For the acute toxicity test, up to 5 g of EOCL per kg body weight was administered in a single dose to Wistar rats, while for the subacute toxicity study, the rats were administered with a daily oral administration at doses of 0.1, 0.25, and 0.5 g/kg for 13 weeks. The results indicated that the EOCL was nontoxic as there were no changes in body weight, and no mortality or adverse clinical signs during both acute and sub-chronic toxicity studies. The hepatic function was normal, and the biomarkers, alanine amino transferase (ALT), alkaline phosphatase (ALP), and aspartate aminotransferase (AST) remained unchanged in treated animals. There was no subacute toxicity as triglycerides, total cholesterol, serum electrolyte parameters, histopathology of tissues, and markers of renal function remained unchanged after 13 weeks of treatment with curcumin. There was no mutagenicity to Salmonella typhimurium up to 3 mg/plate. Oral administration of 1 g/kg body weight EOCL for 14 days did not produce any genotoxicity as there was no DNA damage and chromosome aberration or micronuclei in rat bone marrow cells (Mary et al., 2012).
The acute toxicity study of C. zanthorrhiza ethanol extract at 5000 mg/kg revealed that the extract did not show any toxicity signs such as salivation, sleeping, diarrhea, or lethargy in mice (Devaraj et al., 2010). The result was in accordance with a previous study which showed no toxicity sign was observed in rats after administration of C. zanthorrhiza ethanol extract at 2000 and 5000 mg/kg. During 14 days of observation, rats showed no clinical toxic signs, such as hypoactivity, hyperactivity, lethargy, dermatitis, anorexia, depression, and jaundice as well as no abnormalities in the kidney and liver (Rahim et al., 2014). Listyawati (2006) reported the chronic toxicity study of ethanol extract of C. zanthorrhiza. The extract at 50 mg/kg/day did not induce significant effects on spermatogenic and hematological changes (Listyawati, 2006). C. zanthorrhiza supplement at 2000 mg/kg showed no significant abnormalities on the lung, heart, liver, kidney, and stomach. The LD50 of C. zanthorrhiza supplement as hepatoprotective was greater than 5000 mg/kg bw (Arifin et al., 2020). Based on a clinical study on 30 healthy subjects, the administration of C. zanthorrhiza in combination with Vitex trifolia at doses of 1,500 and 4,500 mg/day for 14 days did not alter the liver and kidney function, while at a dose of 9000 mg/day, the administration altered the AST and serum creatinine values, indicating the extract affected the liver and kidney functions (Baroroh et al., 2011). An aqueous extract of C. zanthorrhiza at 2000 and 5000 mg/kg body weights also showed no toxicity in mice or rats. Xanthorhizol, the active constituent of C. zanthorrhiza, at a dose of 500 mg/kg did not cause mortality in mice (HMPC, 2014).
Our previous study reported the acute toxicity evaluation of ethanol extract of C. mangga rhizomes. Mice were administered with the extract at 500, 1000, 2000, and 5000 mg/kg body weights as a single dose, followed by 14 days of observation. Signs of toxicity were revealed as lethargy was observed after treatment with C. mangga extract at doses of 2000 and 5000 mg/kg body weight. Meanwhile, other signs of toxicity such as diarrhea, coma, and salivation were not recorded. In addition, the extract did not cause deleterious effect on mice body weight. Macroscopic examination of two main organs (liver and kidney) showed that the texture and color of both organs were comparable to those of normal group. C. mangga extract at the dose of 5000 mg/kg caused sinusoidal dilation in the liver and glomerular lesion in the right kidney; however, there was no lesion in the left kidney. The extract at the highest dose did not cause mortality; hence, it can be considered that the LD50 of C. mangga extract was estimated to be more than 5000 mg/kg body weight (Yuandani and Suwarso, 2017a).
The cytotoxicity evaluation of C. aeruginosa rhizomes on fibroblast test has been conducted by Yuliawati and Hestianah (2010). The results revealed that the extract at the concentrations ranging from 1 to 25 ppm was not toxic, as indicated by the percentage of cell viability ranging from 81.60 to 90.57% (Yuliawati and Hestinah, 2010). Sub-chronic toxicity evaluation of C. aeruginosa starch was conducted in Wistar rats. C. aeruginosa starch was administered daily for 90 days. The observation was performed for 90 days and followed until 120 days for satellite group to evaluate the reversible or irreversible effect. The hematological parameters were observed, these include leukocytes, hemoglobin, red blood cell (RBC), hematocrit, mean corpuscular hemoglobin concentration (MCHC), mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), and platelet levels. The results indicated that there was no toxic effect on hematological parameters (Kusumarin et al., 2020). C. aeruginosa in combination with Allium sativum, Terminalia bellirica, and Amomum compactum has been evaluated for their safety. Acute toxicity evaluation has been performed according to the fixed-dose method of OECD guideline 420. The herbal formulation did not cause any toxic signs and symptoms. The were no abnormalities found on body weight gain, macroscopic and microscopic examinations as well as the relative organ weight after treatment with the herbal preparation at the doses of 300 and 2000 mg/kg body weight. Further study on sub-chronic toxicity showed that the extracts did not induce any physical toxic symptoms as well as abnormal weight gain and hematological parameters. Moreover, the herbal formulation at a dose of 4,032 mg/kg did not cause any toxic effects on the liver and kidney, which was indicated by the normal values of urea, creatinine, total protein, albumin, globulin, aspartate aminotransferase (AST) or glutamic oxaloacetic transaminase (GOT), and alanine aminotransferase (ALT). In addition, macroscopic and microscopic examinations showed that there were no toxic effect on all organs tested (Sholikhah et al., 2020).
The evaluation of acute toxicity study of the purified fraction of C. zedoaria revealed that the fraction at the dose of 41.6 and 35.7 mg/kg did not significantly alter the liver and kidney enzyme levels. The LD50 was 500 mg/kg bw (Lakshmi et al., 2011). Furthermore, C. zedoaria ethanol extract at 150 mg/kg/day revealed a significant reduction of RBC, Hb level, and spermatozoa quality in chronic toxicity evaluation (Listyawati, 2006). The essential oil of C. zedoaria at 100 or 200 mg/kg revealed weight loss, and abnormal hematological and biochemical changes on dams and embryos in GD17 pregnant rats. The toxicity mechanism may be related to placental calcification in angiogenesis (Zhou et al., 2013). Sudeepthi et al. (2014) reported the safety evaluation of C. amada rhizomes in short-term treatment. The acetone extract of C. amada (500–2000 mg/kg) was administered to the test animals. The results indicated that the highest dose tested did not cause mortality (Sudeepthi et al., 2014).
Conclusion and Future Directions
In the last 20 years, many plants of the genus Curcuma especially C. longa, C. zanthorrhiza, C. amada, C. mangga, C. aeruginosa, and C. zedoaria and some of their bioactive compounds have been investigated for their immunomodulating effects on the immune system. Most of the studies were in vitro and in vivo and only a few of the preclinical studies have progressed into clinical studies. The up-to-date literature gathered indicated that the immunological investigations on the plant extracts were mainly preliminary with little mechanistic studies. Most of the studies were on the crude extracts of the rhizomes. The extracts were not appropriately characterized chemically or standardized to the bioactive marker compounds which were responsible for the activity. The contributions of the chemical constituents of the plant to the bioactivities were not clearly correlated and identified. It is necessary for the immunomodulatory activity studies of the plant extracts to be accompanied with analyses of their bioactive compounds and identification of the chemical markers for standardization purposes. The extracts used in these studies should be quantitative and qualitative analyzed by using validated analytical methods. Some of the bioactive compounds especially the curcuminoids (curcumin, demethoxycurcumin and bisdemethoxycurcumin) and some sesquiterpenoids have been isolated from the extracts and their mechanistic effects in modulating the immune system have been determined. However, more mechanistic studies should be carried out for in depth understanding of the modulating effects of the plant samples on the innate and adaptive immune system. Of all the Curcuma species investigated, the immunomodulatory effects of C. longa and its major compound, curcumin, were the most studied. Their modulatory effects on various signaling pathways at molecular level have been reported. However, extensive molecular work on the other Curcuma species need to be carried out. Despite the regulatory requirements for clinical studies and sufficient data have not been generated on preclinical testing, there were already reports on a few unsystematic case studies to evaluate the immunomodulating properties of Curcuma species in human. Some clinical trials have been conducted on C. zanthorrhiza but they were unsystematic and not well designed. There was also lack of sufficient preclinical data and the extracts were not appropriately standardized. For clinical studies, sufficient preclinical testing should be generated using standardized extracts, which include bioavailability, pharmacokinetic and toxicological studies, before they can be subjected to clinical studies. Of all the bioactive metabolites of Curcuma species, only curcumin is undergoing extensive clinical trials for its anti-inflammatory properties and potential use as an adjuvant in the treatment of cancer. Curcumin has showed significant ability to modulate the immune response in experimental and clinical studies. However, more systematic and operationally thorough controlled randomized trials are needed to prove its safety and efficacy for human use. Other compounds from Curcuma species such as xanthorrhizol, curdione, curcuzedoalide, isoprocurcumenol and turmeronols have also been reported to modulate various lineages of immune response. More in depth studies including elaborate bioavailability, preclinical pharmacokinetics and toxicity studies are required to understand the underlying mechanisms and safety level before clinical trials can be pursued for development into potent and safe immunomodulatory agents.
Author Contributions
All authors participated in the concept and preparation of draft, revised the manuscript, and approved the final version for submission to this journal.
Funding
This work was funded by the Ministry of Education and Culture Republic of Indonesia under the World Class University Program Year 2020. The grant number is 1879/UN5.1.R/SK/PPM/2020.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
- ADMET
absorption, distribution, metabolism, elimination, and toxicity
- ALP
alkaline phosphatase
- ALT
alanine amino transferase
- AP-1
activator protein-1
- AMPs
antimicrobial peptides
- APC
antigen-presenting cells
- AST
aspartate aminotransferase
- C5aR
complement C5a receptor
- CL
chemiluminescence
- CMS
chronic mild stress
- CO2
carbon dioxide
- CRP
C-reactive protein
- DTH
delayed hypersensitivity
- EOCL
essential oil of C. longa
- fMLP
formyl methionyl-leucyl-phenylalanine;
- fMLPR
formyl-methionyl-leucyl-phenylalanine receptor
- GOT
glutamic oxaloacetic transaminase
- HFD-
high-fat diet-
- HOCl
hypochlorous acid
- HUVEC
human umbilical vein endothelial cells
- IBV
infectious bronchitis virus
- IBD
inflammatory bowel diseases
- IgM
immunoglobulin M
- IL-12
Interleukin 12
- IL-6
interleukin-6
- IL-1β
interleukin-1β
- iNOS
inducible nitric oxide synthase
- ISI
Institute for Scientific Information
- IκB-α
I kappa B alpha
- LPS
lipopolysaccharide
- MAPKs
mitogen-activated protein kinases
- MCHC
mean corpuscular hemoglobin concentration
- MCH
mean corpuscular hemoglobin
- MCP1
monocyte chemoattractant protein-1
- MCV
mean corpuscular volume
- MIP1α
macrophage inflammatory protein-1α
- MHC
major histocompatibility complex
- MPO
myeloperoxidase
- NO
nitric oxide
- NF-ĸB
nuclear factor-kappa B
- PAFR
platelet-activating factor receptor
- PGE2
prostaglandin E2
- PHA-P
phytohemagglutinin-P
- PMNs
polymorphonuclear cells
- PMA
phorbol 12-myristate 13-acetate
- PUFAs
polyunsaturated fatty acids
- ROS
reactive oxygen species
- SLE
systemic lupus erythematosus
- sRBCs
sheep red blood cells
- T1D
type 1 diabetes
- TLC
total leukocytes number
- TNF-α
tumor necrosis factor-alpha.
References
- Abas F., Lajis N. H., Israf D. A., Khozirah S., Umi Kalsom Y. (2006). Antioxidant and Nitric Oxide Inhibition Activities of Selected Malay Traditional Vegetables. Food Chem. 95, 566–573. 10.1016/j.foodchem.2005.01.034 [DOI] [Google Scholar]
- Adeshina I., Aewale Y. A., Tiamiyu L. O. (2017). Growth Performance and Innate Immune Response of Clarias gariepinus Infected with Aeromonas hydrophila Fed Diets Fortified with Curcuma longa Leaf. West. Afr. J. Appl. Ecol. 25, 87–99. [Google Scholar]
- Afolayan F. I. D., Erinwusi B., Oyeyemi O. T. (2018). Immunomodulatory Activity of Curcumin-Entrapped poly d,l -lactic- Co -glycolic Acid Nanoparticles in Mice. Integr. Med. Res. 7, 168–175. 10.1016/j.imr.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alambra J. R., Alenton R. R. R., Gulpeo P. C. R., Mecenas C. L., Miranda A. P., Thomas R. C., et al. (2012). Immunomodulatory Effects of Turmeric, Curcuma longa (Magnoliophyta, Zingiberaceae) on Macrobrachium rosenbergii (Crustacea, Palaemonidae) against Vibrio alginolyticus (Proteobacteria, Vibrionaceae). Aquac. Aquar. Conserv Legis. 5, 13–17. [Google Scholar]
- Alsahli A. A., Alaraidh I. A., Rashad Y. M., Abdel razik E. S. (2018). Extract from Curcuma longa L. Triggers the Sunflower Immune System and Induces Defence-Related Genes against Fusarium Root Rot. Phytopathol. Mediterr. 57, 26–36. 10.14601/Phytopathol_Mediterr-21176 [DOI] [Google Scholar]
- Anand P., Kunnumakkara A. B., Newman R. A., Aggarwal B. B. (2007). Bioavailability of Curcumin: Problems and Promises. Mol. Pharmaceutics 4, 807–818. 10.1021/mp700113r [DOI] [PubMed] [Google Scholar]
- Anggriani L., Yasmin A., Wulandari A. R., Leksono G. M., Ikawati M., Meiyanto E. (2019). Extract of Temu Ireng (Curcuma aeruginosa Roxb.) Rhizome Reduces Doxorubicin-Induced Immunosuppressive Effects. AIP Conf. Proc. 2099, 020001. 10.1063/1.5098406 [DOI] [Google Scholar]
- Anisuzzama M., Sharmin S. A., Mondal S. C., Sultana R., Khalekuzza M., Alam I., et al. (2008). In vitro Microrhizome Induction in Curcuma zedoaria (Christm.) Roscoe-A Conservation Prioritized Medicinal Plant. J. Biol. Sci. 8, 1216–1220. 10.3923/jbs.2008.1216.1220 [DOI] [Google Scholar]
- Arifin P. F., Setiawan I. M., Purwantiningsih, Astuti R. B., Azima F., Susilowidodo R. A., et al. (2020). Acute Toxicity Evaluation of Temulawak (Curcuma xanthorrhiza Roxb.) Hepatoprotective Supplement. Res. J. Pharm. 11 (2), 13–18. 10.33887/rjpbcs/2020.11.2.3 [DOI] [Google Scholar]
- Aristyani S., Widyarti S., Sumitro S. B. (2018). Network Analysis of Indigenous Indonesia Medical Plants for Treating Tuberculosis. Pharmacogn. J. 10, 1159–1164. 10.5530/pj.2018.6.198 [DOI] [Google Scholar]
- Arshad L., Md. Areeful Haque Md. A., Bukhari S. N. A., Jantan I. (2017). An Overview of Structure-Activity Relationship Studies of Curcumin Analogs as Antioxidant and Anti-Inflammatory Agents. Future Med. Chem. 9, 605–626. 10.4155/fmc-2016-0223 [DOI] [PubMed] [Google Scholar]
- Artfire (2016). Rare Curcuma amada. Available at: https://www.artfire.com/(Accessed December 1, 2020).
- Arunkumar P., Ramasubramanian V., Munirasu S. (2016). Effect of Curcuma longa Enriched Mesocylops Thermocyclopoides on Fresh Water Fish, Cyprinus carpio . Int. J. Res. Dev. Pharm. L. Sci. 6, 2848–2492. [Google Scholar]
- Astana P. R. W., Ardiyanto D., Mana T. A. (2018). Change in quality of life and CD4+ value on HIV/AIDS patients with immuostimulant Jamu Formula in Sragen regency. Indonesian J. Clin. Pharm. 7, 227-235. 10.15416/ijcp.2018.7.4.227 [DOI] [Google Scholar]
- Ayati Z., Ramezani M., Amiri M. S., Moghadam A. T., Rahimi H., Abdollahzade A., et al. (2019). Ethnobotany, Phytochemistry and Traditional Uses of Curcuma spp. and Pharmacological Profile of Two Important Species (C. longa and C. zedoaria): A Review. Cpd 25, 871–935. 10.2174/1381612825666190402163940 [DOI] [PubMed] [Google Scholar]
- Ayodele V. O., Olowe O. M., Afolabi C. G., Kehinde I. A. (2018). Identification, Assessment of Diseases and Agronomic Parameters of Curcuma amada Roxb (Mango ginger). Curr. Plant Biol. 15, 51–57. 10.1016/j.cpb.2018.10.001 [DOI] [Google Scholar]
- Balaji S., Chempakam B. (2010). Toxicity Prediction of Compounds from Turmeric (Curcuma longa L). Food Chem. Toxicol. 48 (10), 2951–2959. 10.1016/j.fct.2010.07.032 [DOI] [PubMed] [Google Scholar]
- Baroroh H. N., Ikawati Z., Sudarman K. (2011). A Safety Study of Extract Combination of Legundi (Vitex trifolia L.) Leaves and Temulawak (Curcuma xanthorrhiza R.) Rhizome as Anti-allergy in Healthy Volunteers. Int. J. Pharm. Pract. 2, 165–170. [Google Scholar]
- Beutler B. (2004). Innate Immunity: An Overview. Mol. Immunol. 40, 845–859. 10.1016/j.molimm.2003.10.005 [DOI] [PubMed] [Google Scholar]
- Beyaert R., Beaugerie L., Van Assche G., BrochezRenauld L. J. C., Renauld J.-C., Viguier M., et al. (2013). Cancer Risk in Immune-Mediated Inflammatory Diseases (IMID). Mol. Cancer 12, 98. 10.1186/1476-4598-12-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C. (2001). Nitric Oxide and the Immune Response. Nat. Immunol. 2, 907–916. 10.1038/ni1001-907 [DOI] [PubMed] [Google Scholar]
- Carvalho F., Vassão R., Nicoletti M., Maria D. (2010). Effect of Curcuma zedoaria Crude Extract against Tumor Progression and Immunomodulation. J. Venom. Anim. Toxins Incl. Trop. Dis. 16, 324–341. 10.1590/s1678-91992010000200013 [DOI] [Google Scholar]
- Castro C. N., Barcala Tabarrozzi A. E., Winnewisser J., Gimeno M. L., Antunica Noguerol M., Liberman A. C., et al. (2014). Curcumin Ameliorates Autoimmune Diabetes. Evidence in Accelerated Murine Models of Type 1 Diabetes. Clin. Exp. Immunol. 177, 149–160. 10.1111/cei.12322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda S., Ramachandra T. V. (2019). Phytochemical and Pharmacological Importance of Turmeric (Curcuma longa): A Review. Res. Rev. J. Pharm. 9, 16–23. [Google Scholar]
- Chandrasekaran C. V., Sundarajan K., Kannan J. R., Gururaja G. M., Mundkinajeddu D., Agarwal A. (2013). Immune-Stimulatory and Anti-inflammatory Activities of Curcuma longa Extract and its Polysaccharide Fraction. Pharmacognosy Res. 5, 71–79. 10.4103/0974-8490.110527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapel H., Haeney M., Misbah S., Snowden N. (2006). Clinical Immunology. 5th Edn. Massachusetts: Blackwell Publishing. [Google Scholar]
- Choudhury D., Ghosal M., Das A. P., Mandal P. (2013). Development of Single Node Cutting Propagation Techniques and Evaluation of Antioxidant Activity of Curcuma aeruginosa Roxnurgh Rhizome. Int. J. Pharm.Sci. Res. 5, 227–234. [Google Scholar]
- Cianciulli A., Calvello R., Porro C., Trotta T., Salvatore R., Panaro M. A. (2016). PI3k/Akt Signalling Pathway Plays a Crucial Role in the Anti-inflammatory Effects of Curcumin in LPS-Activated Microglia. Int. Immunopharmacol. 36, 282–290. 10.1016/j.intimp.2016.05.007 [DOI] [PubMed] [Google Scholar]
- Devaraj S., Esfahani A. S., Ismail S., Ramanathan S., Yam M. F. (2010). Evaluation of the Antinociceptive Activity and Acute Oral Toxicity of Standardized Ethanolic Extract of the Rhizome of Curcuma xanthorrhiza Roxb. Molecules 15 (4), 2925–2934. 10.3390/molecules15042925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewi M., Aries M. H., Dwiriani C. M., Januwati N. (2012). Knowledge on Health Benefit of Curcuma and the Clinical Trial of Its Effect on Humoral Immune System in Obese Adults. Indones. J. Agric. 17, 166–171. [Google Scholar]
- Dosoky N. S., Setzer W. N. (2018). Chemical Composition and Abiological Activities of Essential Oils of Curcuma Species. Nutrients 10, 1196. 10.3390/nu10091196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drugbank (2021). Curcuma xanthorrhiza oil. Available at: https://go.drugbank.com/drugs/DB11265 (Accessed January 26, 2021).
- Dutta B. (2015). Study of Secondary Metabolite Constituents and Curcumin Contents of Six Different Species of Genus Curcuma . J. Med. Plants Stud. 3, 116–119. [Google Scholar]
- Economou M., Zambeli E., Michopoulos S. (2009). Incidence and Prevalence of Crohn’s Disease and its Etiological Influences. Ann. Gastroenterol. 22, 158–167. [Google Scholar]
- Elgert K. D. (2009). Immunology Understanding the Immune System. 2nd Edn. Virginia: A John Wiley & Sons, Inc., Publication. [Google Scholar]
- Ervintari F., Puspitawati R., Utami S. (2019). Effect of Curcuma Xanthorrhiza Roxb. Ethanol Extract on the Viability of Streptococcus Mutans and Streptococcus Sanguinis Dual-Species Biofilms. Int. J. App. Pharm. 11, 75–78. 10.22159/ijap.2019.v11s1.181 [DOI] [Google Scholar]
- Faradilla M., Iwo M. I. (2014). Immunomodulatory Effect of Polysaccharide from White Turmeric [Curcuma zedoria (Christm.) Roscoe)]. Indonesian J. Pharm. 12, 73–278. [Google Scholar]
- Firtel R. A., Chung C. Y. (2000). The Molecular Genetics of Chemotaxis: Sensing and Responding to Chemoattractant Gradients. BioEssays 22, 603–615. [DOI] [PubMed] [Google Scholar]
- Flores G. (2017). Curcuma longa L. Extract Improves the Cortical Neural Connectivity during the Aging Process. Neural Regen. Res. 12, 875–880. 10.4103/1673-5374.208542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Gao R., Cao Y., Guo M., Wei Z., Zhou E., et al. (2014). Curcumin Attenuates Inflammatory Responses by Suppressing TLR4-Mediated NF-κB Signaling Pathway in Lipopolysaccharide-Induced Mastitis in Mice. Int. Immunopharmacol. 20, 54–58. 10.1016/j.intimp.2014.01.024 [DOI] [PubMed] [Google Scholar]
- Fürst R., Zündorf I. (2014). Plant-Derived Anti-Inflammatory Compounds: Hopes and Disappointments Regarding the Translation of Preclinical Knowledge into Clinical Progress. Mediators Inflamm. 2014, 146832. 10.1155/2014/146832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Kuo J., Jiang H., Deeb D., Liu Y., Divine G., et al. (2004). Immunomodulatory Activity of Curcumin: Suppression of Lymphocyte Proliferation, Development of Cell-Mediated Cytotoxicity, and Cytokine Production in vitro . Biochem. Pharmacol. 68, 51–61. 10.1016/j.bcp.2004.03.015 [DOI] [PubMed] [Google Scholar]
- Geetha S., Singh V., Ram M. S., Ilavazhagan G., Banerjee P. K., Sawhney R. C. (2005). Immunomodulatory Effects of Seabuckthorn (Hippophae rhamnoides L.) against Chromium (VI) Induced Immunosuppression. Mol. Cel. Biochem. 278, 101–109. 10.1007/s11010-005-7095-9 [DOI] [PubMed] [Google Scholar]
- George M., Britto S. J. (2015). Phytochemicaland Antioxidant Studies on the Essential Oil of the Rhizome of Curcuma Aeruginosa Roxb. Int. Res. J. Pharm. 6, 573–579. 10.7897/2230-8407.068113 [DOI] [Google Scholar]
- Guimarães A. F., Vinhas A. C. A., Gomes A. F., Souza L. H., Krepskya P. B. (2020). Essential Oil of Curcuma longa L. Rhizomes Chemical Composition, Yield Variation and Stability. Quim. Nova 43, 909–913. 10.21577/0100-4042.20170547 [DOI] [Google Scholar]
- Hardiwati K. T., Seninha M., Lay B. W., Yanti (2019). Curcuminoid Cider Fermented from Curcuma xanthorrhiza Curcuminoids Attenuates Gene Expression Related to Obesity-Induced Inflammation in Hypercholesterolaemic Rats. Int. Food Res. J. 26, 859–867. [Google Scholar]
- Harun N. H., Septama A. W., Jantan I. (2015). Immunomodulatory Effects of Selected Malaysian Plants on the CD18/11a Expression and Phagocytosis Activities of Leukocytes. Asian Pac. J. Trop. Biomed. 5, 48–53. 10.1016/s2221-1691(15)30170-2 [DOI] [Google Scholar]
- Hassan A. A. M., Yacout M. H., Khalel M. S., Hafsa S. H. A., Ibrahim M. A. R., Mocuta D. N., et al. (2018). Effects of Some Herbal Plant Supplements on Growth Performance and the Immune Response in Nile Tilapia (Oreochromis Niloticus). Sciendo 1, 134–141. 10.2478/alife-2018-0020 [DOI] [Google Scholar]
- Hong G. W., Hong S. L., Lee G. S., Yaacob H., Malek S. N. A. (2016). Non-Aqueous Extracts of Curcuma mangga Rhizomes Induced Cell Death in Human Colorectal Adenocarcinoma Cell Line (HT29) via Induction of Apoptosis and Cell Cycle Arrest at G0/G1 Phase. Asian Pac. J. Trop. Med. 9, 8–18. 10.1016/j.apjtm.2015.12.003 [DOI] [PubMed] [Google Scholar]
- HMPC (2017). Assessment report on Curcuma longa L. (C. domestica Valeton) rhizoma. London: European Medicines Agency, 1–5. [Google Scholar]
- HMPC (2014). Assessment report on Curcuma xanthorrhiza Roxb. (C. xanthorrhiza D. Dietrich), rhizoma. London: European Medicines Agency, 15. [Google Scholar]
- Huang J. K., Kim A. J., Sohn J. H., Han K. L., Choo J. H. (2010). Immunostimulating Polysaccharide Isolated from Curcuma xanthorrhiza and Manufacturing Method Thereof. Patent Appl. Publ., 1–11. [Google Scholar]
- Huang Y., Xue C., He W., Zhao X. (2019). Inhibition Effect of Zedoary Turmeric Oil on Listeria Monocytogenes and Staphylococcus aureus Growth and Exotoxin Proteins Production. J. Med. Microbiol. 68, 657–666. 10.1099/jmm.0.000949 [DOI] [PubMed] [Google Scholar]
- Ikawati Z., Hertiani T., Izzati F. (2019). Immunomodulatory Activity of an Indonesian Herbal Formulation for Respiratory Disorder. Phcog Mag. 15, 130–134. 10.4103/pm.pm_314_18 [DOI] [Google Scholar]
- Ilene A., Sandhika W., Hasanatuludhhiyah N. (2020). Effect of Javanese Turmeric (Curcuma xanthorrhiza) Extract on Hepatitis Model of Alcohol-Induced Mice. Jurnal Kedokteran Brawijaya 1, 39–44. 10.21776/ub.jkb.2020.031.01.8 [DOI] [Google Scholar]
- Inzaugarat M. E., Matteo E. D., Baz P., Lucero D., Garcia C. C., Ballerga E. G., et al. (2017). New Evidence for the Therapeutic Potential of Curcumin to Treat Nonalcoholic Faty Liver Disease in Humans. Plos One 12 (3), e0172900. 10.1371/journal.pone.0172900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S. K., Rains J., Croad J., Larson B., Jones K. (2009). Curcumin Supplementation Lowers TNF-α, IL-6, IL-8, and MCP-1 Secretion in High Glucose-Treated Cultured Monocytes and Blood Levels of TNF-α, IL-6, MCP-1, Glucose, and Glycosylated Hemoglobin in Diabetic Rats. Antioxid. Redox Signaling 11, 241–249. 10.1089/ars.2008.2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M. K., Lee H. J., Kim J. S., Ryu J.-H. (2004). A Curcuminoid and Two Sesquiterpenoids from Curcuma Zedoaria as Inhibitors of Nitric Oxide Synthesis in Activated Macrophages. Arch. Pharm. Res. 27, 1220–1225. 10.1007/bf02975885 [DOI] [PubMed] [Google Scholar]
- Jang M., Sohn D., Ryu J.-H. (2001). A Curcuminoid and Sesquiterpenes as Inhibitors of Macrophage TNF-α Release from Curcuma Zedoaria. Planta Med. 67, 550–552. 10.1055/s-2001-16482 [DOI] [PubMed] [Google Scholar]
- Jantan I., Saputri F. C., Qaisar M. N., Buang F. (2012). Correlation between Chemical Composition of Curcuma domestica and Curcuma xanthorrhiza and Their Antioxidant Effect on Human Low-Density Lipoprotein Oxidation. Evid. Based Complement. Alternat. Med. 2012, 438356. 10.1155/2012/438356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantan I., Harun N. H., Septama A. W., Murad S., Mesaik M. A. (2011). Inhibition of Chemiluminescence and Chemotactic Activity of Phagocytes in vitro by the Extracts of Selected Medicinal Plants. J. Nat. Med. 65, 400–405. 10.1007/s11418-010-0492-8 [DOI] [PubMed] [Google Scholar]
- Jatoi S. A., Kikuchi A., Gilani S. A., Watanabe K. N. (2007). Phytochemical, Pharmacological and Ethnobotanical Studies in Mango Ginger (Curcuma amada Roxb.; Zingiberaceae). Phytother. Res. 21, 507–516. 10.1002/ptr.2137 [DOI] [PubMed] [Google Scholar]
- Jose S., Thomas T. D. (2014). Comparative Phytochemical and Anti-Bacterial Studies of Two Indigenous Medicinal Plants Curcuma caesia Roxb. and Curcuma aeruginosa Roxb. Int. J. Green. Pharm. 8, 65–71. 10.4103/0973-8258.126828 [DOI] [Google Scholar]
- Jurenka J. S. (2009). Anti-Inflammatory Properties of Curcumin, a Major Constituent of Curcuma longa: A Review of Preclinical and Clinical Research. Altern. Med. Rev. 14, 141–153. [PubMed] [Google Scholar]
- Kaewkroek K., Wattanapiromsakul C., Tewtrakul S. (2010). Anti-Inflammatory Mechanisms of Compounds from Curcuma mangga Rhizomes using RAW264.7 Macrophage Cells. Nat. Prod. Commun. 5, 1547–1550. 10.1177/1934578x1000501006 [DOI] [PubMed] [Google Scholar]
- Kaewkroek K., Wattanapiromsakul C., Tewtrakul S. (2009). Nitric Oxide Inhibitory Substances from Curcuma mangga Rhizomes. Songklanakarin J. Sci. Technol. 31, 293–297. [Google Scholar]
- Kalim H., Handono K., Khalasha T., Pratama M., Dantara T. I., Wulandari A., et al. (2017). Immune Modulation Effects of Curcumin in Pristane-Induced Lupus Mice. Indian J. Rheumatol. 12, 86–93. 10.4103/injr.injr_95_16 [DOI] [Google Scholar]
- Kaliyadasa E., Samarasinghe B. A. (2019). A Review on Golden Species of Zingiberaceae Family Around the World: Genus Curcuma . Afr. J. Agric. Res. 14, 519–531. 10.5897/AJAR2018.13755 [DOI] [Google Scholar]
- Karchuli M. S., Pradhan D. (2011). Curcuma amada Roxb. Rhizome Extract Modulates Cellular and Humoral Immune System. Pharmcologyonline 3, 947–952. [Google Scholar]
- Kavitha R., Mahadevi R. (2020). Phytochemical and Pharmacological Properties of Curcuma amada: A Review. Int. J.Pharm. Sci. Res. 11, 3546–3555. 10.26452/ijrps.v11i3.2510 [DOI] [Google Scholar]
- Kim A.-J., Kim Y.-O., Shim J.-S., Hwang J.-K. (2007). Immunostimulating Activity of Crude Polysaccharide Extract Isolated from Curcuma xanthorrhiza Roxb. Biosci. Biotechnol. Biochem. 71, 1428–1438. 10.1271/bbb.60241 [DOI] [PubMed] [Google Scholar]
- Kim G.-Y., Kim K.-H., Lee S.-H., Yoon M.-S., Lee H.-J., Moon D.-O., et al. (2005). Curcumin Inhibits Immunostimulatory Function of Dendritic Cells: MAPKs and Translocation of NF-κB as Potential Targets. J. Immunol. 174, 8116–8124. 10.4049/jimmunol.174.12.8116 [DOI] [PubMed] [Google Scholar]
- Kim K. I., Shin K. S., Jun W. J., Hong B. S., Shin D. H., Cho H. Y., et al. (2001). Effects of Polysaccharides from Rhizomes of Curcuma Zedoaria on Macrophage Functions. Biosci. Biotechnol. Biochem. 65, 2369–2377. 10.1271/bbb.65.2369 [DOI] [PubMed] [Google Scholar]
- Kim M.-B., Kim C., Song Y., Hwang J.-K. (2014). Antihyperglycemic and Anti-Inflammatory Effects of Standardized Curcuma xanthorrhiza Roxb. Extract and Its Active Compound Xanthorrhizol in High-Fat Diet-Induced Obese Mice. Evid. Based. Complement. Alternat. Med. 2014, 205915. 10.1155/2014/205915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim O. K., Yoo S. A., Nam D.-E., Kim Y., Kim E., Jun W., et al. (2014). Immunomodulatory Effects of Curcuma longa L. Extract in LP-BM5 Murine Leukemia Viruses-Induced Murine Acquired Immune Deficiency Syndrome. J. Korean Soc. Food Sci. Nutr. 43, 1317–1324. 10.3746/jkfn.2014.43.9.1317 [DOI] [Google Scholar]
- Kim S., Kook K. E., Kim C., Hwang J.-K. (2018). Inhibitory Effects of Curcuma xanthorrhiza Supercritical Extract and Xanthorrhizol on LPS-Induced Inflammation in HGF-1 Cells and RANKL-Induced Osteoclastogenesis in RAW264.7 Cells. J. Microbiol. Biotechnol. 28, 1270–1281. 10.4014/jmb.1803.03045 [DOI] [PubMed] [Google Scholar]
- Kobayashi S. D., Voyich J. M., Burlak C., DeLeo F. R. (2005). Neutrophils in the Innate Immune Response. Arch. Immunol. Ther. Exp. 53, 505–517. [PubMed] [Google Scholar]
- Kumar S., Ahuja V., Sankar M. J., Kumar A., Moss A. C. (2012). Curcumin for Maintenance of Remission in Ulcerative Colitis. Cochrane Database Syst. Rev. 10, CD008424. 10.1002/14651858.CD008424.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumolosasi E., Ibrahim S. N. A., Ahmad Shukri S. M., Ahmad W. (2018). Immunostimulant Activity of Standardised Extracts of Mangifera Indica Leaf and Curcuma Domestica Rhizome in Mice. Trop. J. Pharm. Res. 17, 77–84. 10.4314/tjpr.v17i1.12 [DOI] [Google Scholar]
- Kusumarin N., Lianah, Hafsha M. (2020). Evaluation of the Potential of Ireng Gathering Starch (Curcuma aeruginosa Roxb.) as Alternative Food Ingredients and the Processed Organoleptic Aspects. Int. J. Agric. Biol. Sci. 2, 71–76. 10.5281/zenodo.3710771 [DOI] [Google Scholar]
- Lakshmi S., Padmaja G., Remani P. (2011). Antitumour Effects of Isocurcumenol Isolated from Curcuma zedoaria Rhizomes on Human and Murine Cancer Cells. Int. J. Med. Chem. 2011, 253962. 10.1155/2011/253962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. K., Trinh T. A., Lee S. R., Kim S., So H. M., Moon E., et al. (2019). Bioactivity-Based Analysis and Chemical Characterization of Anti-Inflammatory Compounds from Curcuma zedoaria Rhizomes using LPS-Stimulated RAW264.7 Cells. Bioorg. Chem. 82, 26–32. 10.1016/j.bioorg.2018.09.027 [DOI] [PubMed] [Google Scholar]
- Lestari M. D., Arief M., Satyantini W. H. (2019). Addition of Curcuma (Curcuma xanthorrhiza) as an Antioxidant on African Catfish (Clarias gariepinus) Commercial Fish Feeding. Int. J. Civ. Eng. 10, 380–385. [Google Scholar]
- Li S., Yuan W., Deng G., Wang P., Yang P., Aggarwal B. B. (2011). Chemical Composition and Product Quality Control of Turmeric (Curcuma longa L.). Topharmcj 5, 28–54. 10.2174/2210290601102010028 [DOI] [Google Scholar]
- Liju V. B., Jeena K., Kuttan R. (2013). Acute and Subchronic Toxicity as Well as Mutagenic Evaluation of Essential Oil from Turmeric (Curcuma longa L). Food Chem. Toxicol. 53, 52–61. 10.1016/j.fct.2012.11.027 [DOI] [PubMed] [Google Scholar]
- Listyawati S. (2006). Toxicity Studies of the Rhizome Curcuma xanthorrhiza Roxb. and Curcuma zedoaria Roscoe on Hematological and Male Reproduction System of Mice (Mus musculus L.). J. Nat. Prod. Biochem. 4 (1), 10–13. 10.13057/biofar/f040103 [DOI] [Google Scholar]
- Liu Y., Nair M. G. (2011). Labdane Diterpenes in Curcuma mangga Rhizomes Inhibit Lipid Peroxidation, Cyclooxygenase Enzymes and Human Tumour Cell Proliferation. Food Chem. 124, 527–532. 10.1016/j.foodchem.2010.06.064 [DOI] [Google Scholar]
- Lobo R., Prabhu K. S., Shirwaikar A., Shirwaikar A. (2009). Curcuma zedoaria Rosc. (white turmeric): A Review of Its Chemical, Pharmacological and Ethnomedicinal Properties. J. Pharm. Pharmacol. 61, 13–21. 10.1211/jpp/61.01.0003 [DOI] [PubMed] [Google Scholar]
- Luckheeram R. V., Zhou R., Verma A. D., Xia B. (2012). CD4+T Cells: Differentiation and Functions. Clin. Develop. Immunol. 2012, 1. 10.1155/2012/925135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster A. D. (2001). “Chemotaxis: Role in Immune Response,” in Encyclopedia of Life Sciences. Massachusetts: Nature Publishing Grup, 1–12. [Google Scholar]
- Malek S. N. A., Lee G. S., Hong S. L., Yaacob H., Wahab N. A., Faizal Weber J.-F., et al. (2011). Phytochemical and Cytotoxic Investigations of Curcuma mangga Rhizomes. Molecules 16, 4539–4548. 10.3390/molecules16064539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mary H. P., Susheela G. K., Jayasree S., Nizzy A., Rajagopal B., Jeeva S. (2012). Phytochemical Characterization and Antimicrobial Activity of Curcuma xanthorrhiza Roxb. Asian Pac. J. Trop. Biomed. 2, S637–S640. 10.1016/s2221-1691(12)60288-3 [DOI] [Google Scholar]
- Matsuda H., Tewtrakul S., Morikawa T., Nakamura A., Yoshikawa M. (2004). Anti-allergic Principles from Thai Zedoary: Structural Requirements of Curcuminoids for Inhibition of Degranulation and Effect on the Release of TNF-α and IL-4 in RBL-2H3 Cells. Bioorg. Med. Chem. 12, 5891–5898. 10.1016/j.bmc.2004.08.027 [DOI] [PubMed] [Google Scholar]
- Mauren F. M., Yanti, Lay B. W. (2016). Efficacy of Oral Curcuminoid Fraction from Curcuma xanthorrhiza and Curcuminoid Cider in High-Cholesterol Fed Rats. Pharmacogn. Res. 8, 153–159. 10.4103/0974-8490.181468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miksusanti (2012). Lymphocyte Proliferation by Temu Lawak (Curcuma xanthorrhiza ROXB) Essential Oil. Int. Conf. Bioinform. Biomed. Eng. 29, 205–209. [Google Scholar]
- Mooraki N., Batmany Y., Zoriehzahra S. J., Kakoolaki S. (2019). Evaluating the Effect of using Turmeric (Curcuma longa) of Growth Performance and Hematological Parameters of the Ornamental Fish, Green Terror (Andinocara rivulatus). J. Surv. Fish. Sci. 5, 37–47. 10.18331/sfs2019.5.2.5 [DOI] [Google Scholar]
- Morales G. A., Noratto G., Talcott S. U. M. (2012). Standardized Curcuminoid Extract (Curcuma longa l.) Decreases Gene Expression Related to Inflammation and Interacts with Associated microRNAs in Human Umbilical Vein Endothelial Cells (HUVEC). Food Funct. 3, 1286–1293. 10.1039/c2fo30023k [DOI] [PubMed] [Google Scholar]
- Naderi M., Akbari M. R., Asadi K. E., Khaksar K., Khajali F. (2014). Effects of Dietary Inclusion of Turmeric (Curcuma longa) and Cinnamon (Cinnamomum verum) Powders on Performance, Organs Relative Weight and Some Immune System Parameters in Broiler Chickens. Poult. Sci. J. 2, 153–163. 10.22069/PSJ.2014.1963 [DOI] [Google Scholar]
- Nan F. H., Agus P. A. S., Margie, Bargir B., Lee M. C. (2014). The Effects of Curcuma zedoaria and Zingiber zerumbet on Non-Specific Immune Responses of Grouper Epinephelus coioides . Iran. J. Fish. Sci. 14, 598–611. [Google Scholar]
- Okamoto A., Noble E. E., Tyagi E., Ying Z., Zhuang Y., Gomez-Pinilla F. (2015). Curcumin Boosts DHA in the Brain: Implications for the Prevention of Anxiety Disorders. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 1852, 951–961. 10.1016/j.bbadis.2014.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda-Hanafusa C., Uchio R., Fuwa A., Kawasaki K., Muroyama K., Yamamoto Y., et al. (2019). Turmeronol A and Turmeronol B from Curcuma longa Prevent Inflammatory Mediator Production by Lipopolysaccharide-Stimulated RAW264.7 Macrophages, Partially via Reduced NF-κB Signaling. Food Funct. 10, 5779–5788. 10.1039/c9fo00336c [DOI] [PubMed] [Google Scholar]
- Oon S. F., Nallappan M., Tee T. T., Shohaimi S., Kassim N. K., Sa’ariwijaya M. S. F., et al. (2015). Xanthorrhizol: A Review of its Pharmacological Activities and Anticancer Properties. Cancer Cel. Int. 15, 1–15. 10.1186/s12935-015-0255-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palpu P., Rao C. V., Rawat A. K. S., Pjha S. K., Reddy G. D. (2008). Anti-Allergic Herbal Formulation. U.S. Patent No 7344739 B2. Washington, DC: U.S. Patent and Trademark Office. [Google Scholar]
- Pan M. H., Wu J. C., Ho C. T., Badmaev V. (2017). Effect of Water Extract of Curcuma longa (L.) Roots on Immunity and Telomerase Function. J. Complement. Integr. Med. 14, 1–6. 10.1515/jcim-2015-0107 [DOI] [PubMed] [Google Scholar]
- Park S.-J., Lee D., Lee M., Kwon H.-O., Kim H., Park J., et al. (2018). The Effects of Curcuma longa L., Purple Sweet Potato, and Mixtures of the Two on Immunomodulation in C57BL/6J Mice Infected with LP-BM5 Murine Leukemia Retrovirus. J. Med. Food 21, 689–700. 10.1089/jmf.2017.4093 [DOI] [PubMed] [Google Scholar]
- Patil U. S., Jaydeokar A. V., Bandawane D. D. (2012). Immunomodulators: A Pharmacological Review. Int. J. Pharm. Sci. 4, 30–36. [Google Scholar]
- Pawitan J. A. (2020). Curcumin as Adjuvant Therapy in COVID-19: Friend or Foe?. J. Int. Dent. Med. Res. 13, 824–829. [Google Scholar]
- Pisani L. F., Lecchi C., Invernizzi G., Sartorelli P., Savoini G., Ceciliani F. (2009). In vitro Modulatory Effect of ω-3 Polyunsaturated Fatty Acid (EPA and DHA) on Phagocytosis and ROS Production of Goat Neutrophils. Vet. Immunol. Immunopathol. 131, 79–85. 10.1016/j.vetimm.2009.03.018 [DOI] [PubMed] [Google Scholar]
- Policegoudra R. S., Divakar S., Aradhya S. M. (2006). Identification of Difurocumenonol, a New Antimicrobial Compound from Mango Ginger (Curcuma amada Roxb.) Rhizome. J. Appl. Microbiol. 102, 1594–1602. 10.1111/j.1365-2672.2006.03186.x [DOI] [PubMed] [Google Scholar]
- Policegoudra R. S., Aradhya S. M., Singh L. (2011). Mango Ginger (Curcuma amada Roxb.) - A Promising Spice for Phytochemicals and Biological Activities. J. Biosci. 36, 739–748. 10.1007/s12038-011-9106-1 [DOI] [PubMed] [Google Scholar]
- Purwanti S., Agustina L., Syamsu J. A., Adriyansyah A., Latief M. F. (2018). Turmeric (Curcuma domestica) and Garlic (Allium sativum) towards Broiler Immune System Infected by Salmonella pullorum Bacteria as a Feed Additive. IOP Conf. Ser. Earth Environ. Sci. 247, 1–6. 10.1088/1755-1315/247/1/012063 [DOI] [Google Scholar]
- Putri M. S. (2014). White Turmeric (Curcuma zedoaria): Its Chemical Substance and the Pharmacological Benefit. J. Majority 3, 88–93. [Google Scholar]
- Rahayu D. U. C., Setyani D. A., Dianhar H., Sugita P. (2020). Phenolic Compounds from Indonesian White Turmeric (Curcuma zedoaria) Rhizomes. Asian J. Pharm. Clin. Res. 13, 194–198. 10.22159/ajpcr.2020.v13i7.38249 [DOI] [Google Scholar]
- Rahim N. A., Hassandarvish P., Golbabapour S., Ismail S., Tayyab S., Abdulla M. A. (2014). Gastroprotective Effect of Ethanolic Extract of Curcuma xanthorrhiza Leaf against Ethanol-Induced Gastric Mucosal Lesions in Sprague-Dawley Rats. Biomed. Res. Int. 2014, 416409. 10.1155/2014/416409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumari S., Sanatombi K. (2017). Nutritional Value, Phytochemical Composition, and Biological Activities of Edible Curcuma Species: A Review. Int. J. Food Prop. 20, S2668–S2687. 10.1080/10942912.2017.1387556 [DOI] [Google Scholar]
- Rattue G. (2012). Autoimmune Disease Rates Increasing. Medical News Today. Available at: http://www.medicalnewstoday.com/articles/246960.php (Accessed February 15, 2015). [Google Scholar]
- Ravikumar N., Naga Kavitha C. (2020). Therapeutic Potential of Curcumin on Immune Dysregulation in Comorbid Diabetic Asthma in Mice. Biomed. Pharmacol. J. 13, 821–831. 10.13005/bpj/1948 [DOI] [Google Scholar]
- Ruangsang P., Tewtrakul S., Reanmongkol W. (2010). Evaluation of the Analgesic and Anti-Inflammatory Activities of Curcuma mangga Val and Zijp Rhizomes. J. Nat. Med. 64, 36–41. 10.1007/s11418-009-0365-1 [DOI] [PubMed] [Google Scholar]
- Saroj P., Verma M., Jha K. K., Pal M. (2012). An Overview on Immunomodulation. J. Adv. Sci. Res. 3, 7–12. [Google Scholar]
- Sasikumar B. (2005). Genetic Resources of Curcuma: Diversity, Characterization and Utilization. Plant Genet. Resour. 3, 230–251. 10.1079/pgr200574 [DOI] [Google Scholar]
- Sengupta M., Sharma G. D., Chakraborty B. (2011). Hepatoprotective and Immunomodulatory Properties of Aqueous Extract of Curcuma longa in Carbon Tetra Chloride Intoxicated Swiss Albino Mice. Asian Pac. J. Trop. Biomed. 1, 193–199. 10.1016/s2221-1691(11)60026-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethy K., Swain P., Behera K., Sahoo N., Agrawalla J., Khandanga S., et al. (2017). Effect of Turmeric (Curcuma longa) Supplementation on Antioxidants and Immunity of Broiler Birds. Livest. Sci. 8, 103–106. 10.4081/ijas.2015.3870 [DOI] [Google Scholar]
- Setiawati M. C., Ikawati Z., Kertia I. N. (2017). Antiinflamatory and Antidepressive Activities of Extract Curcuma xanthorrhiza Roxb in Systemic Lupus Erythematosus. Indonesian J. Pharm. 28, 185–190. 10.14499/indonesianjpharm28iss3pp185 [DOI] [Google Scholar]
- Setyati W., Subagiyo S., Pramesti R., Pramesti D. (2019). Effectiveness of Herbal Extract (Piper retrofractum, Curcuma aeruginosa, and Curcuma zanthorrhiza) as Immunomodulator in Non-Specific Immunity System of Tiger Grouper (Epinephelus fuscoguttatus) against Infection from Vibrio Alginolyticus and Vibrio Parah. Sci. Technol. Indones. 4, 94–100. 10.26554/sti.2019.4.4.94-100 [DOI] [Google Scholar]
- Shabana M. H., Shahy E. M., Taha M. M., Mahdy G. M., Mahmoud M. H. (2020). Phytoconstituents from Curcuma longa L. Aqueous Ethanol Extract and Its Immunomodulatory Effect on Diabetic Infected Rats. Egypt. Pharmaceut J. 14, 36–43. 10.4103/1687-4315.154713 [DOI] [Google Scholar]
- Shaikh P. Z. (2011). Cytokines & Their Physiologic and Pharmacologic Functions in Inflammation: A Review. Int. J. Pharm. Life Sci. 2, 1247–1263. [Google Scholar]
- Sholikhah E. N., Mustofa M., Nugrahaningsih D. A. A., Yuliani F. S., Purwono S., Sugiyono S., et al. (2020). Acute and Subchronic Oral Toxicity Study of Polyherbal Formulation Containing Allium sativum L., Terminalia bellirica (Gaertn.) Roxb., Curcuma aeruginosa Roxb., and Amomum compactum Sol. ex. Maton in Rats. Biomed. Res. Int. 2020, 8609364. 10.1155/2020/8609364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapdeal (2020). Curcuma Amada. Available at: https://www.snapdeal.com/(Accessed December 1, 2020).
- Soleimani V., Sahebkar A., Hosseinzadeh H. (2018). Turmeric (Curcuma longa) and its Major Constituent (Curcumin) as Nontoxic and Safe Substances: Review. Phytotherapy Res. 32, 985–995. 10.1002/ptr.6054 [DOI] [PubMed] [Google Scholar]
- Sudeepthi N. L., Kumar K. E., Kola P. K. (2014). Effect of Curcuma amada (mango ginger) Roxb. n Scopolamine Induced Memory Deficit in Rats. Int. J. Res. Pharm. Chem. 4, 1127–1134. [Google Scholar]
- Sulfianti A., Ningsih S., Agustini K. (2019). Chemoprevention Effect of Curcuma aeruginosa in DMBA-Induced Cytokines Production. Int. Res. J. Pharm. 10, 54–59. 10.7897/2230-8407.100378 [DOI] [Google Scholar]
- Sun W., Wang S., Zhao W., Wu C., Guo S., Gao H., et al. (2017). Chemical Constituents and Biological Research on Plants in the Genus Curcuma . Crit. Rev. Food Sci. Nutr. 57, 1451–1523. 10.1080/10408398.2016.1176554 [DOI] [PubMed] [Google Scholar]
- Surh Y.-J., Chun K.-S., Cha H.-H., Han S. S., Keum Y.-S., Park K.-K., et al. (2001). Molecular Mechanisms Underlying Chemopreventive Activities of Anti-Inflammatory Phytochemicals: Down-Regulation of COX-2 and iNOS through Suppression of NF-κB Activation. Mutat. Res./Fundam. Mol. Mech. Mutagen. 480–481, 243–268. 10.1016/S0027-5107(01)00183-X [DOI] [PubMed] [Google Scholar]
- Tan B., Vanitha J. (2004). Immunomodulatory and Antimicrobial Effects of Some Traditional Chinese Medicinal Herbs: A Review. Cmc 11, 1423–1430. 10.2174/0929867043365161 [DOI] [PubMed] [Google Scholar]
- Thokchom S. S., Phucho I. T. (2015). Elemental Analysis, Ditermination of Alkaloid, Saponin and Flavonoid of Three Selected Species of Zingiberaceae Family. Int. J. Pharm. Sci. Res. 6, 3044–3048. 10.13040/IJPSR.0975-8232.6(7).3044-48 [DOI] [Google Scholar]
- Tung B. T., Nham D. T., Hai N. T., Thu D. K. (2019). Curcuma longa, the Polyphenolic Curcumin Compound and Pharmacological Effects on Liver. Dietary Interventions Liver Dis. 2019, 125–134. 10.1016/b978-0-12-814466-4.00010-0 [DOI] [Google Scholar]
- Uchio R., Higashi Y., Kohama Y., Kawasaki K., Hirao T., Muroyama K., et al. (2017). A Hot Water Extract of Turmeric (Curcuma longa) Suppresses Acute Ethanol-Induced Liver Injury in Mice by Inhibiting Hepatic Oxidative Stress and Inflammatory Cytokine Production. J. Nutr. Sci. 6, 1–9. 10.1017/jns.2016.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA (2021). Natural Resources Conservation Services. Plants Database. Available at: https://plants.usda.gov/java/ClassificationServlet?source=display&classid=CURCU (Accessed January 25, 2021).
- Wahono C. S., Setyorini C. D., Kalim H., Nurdiana, Handono K. (2017a). Effect of Curcuma xanthorrhiza Supplementation on Systemic Lupus Erythematosus Patients with Hypovitamin D which were Given Vitamin D3 towards Disease Activity (SLEDAI), IL-6, and TGF-β1 Serum. Int. J. Rheumatol. 2017, 7687053. 10.1155/2017/7687053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahono C. S., Wahyuni Z. D., Kalim H. (2017b). Effect of Curcuma xanthorrhiza Suplementation in Vitamin D3 Administration towards Proteinuria, Serum Anti-dsDNA and IL-17 Levels on Systemic Lupus Erythematosus (sle) Patients with Hypovitamin. D Int. J. Clin. Rheumatol. 12, 121–129. [Google Scholar]
- Warrington R., Watson W., Kim H. L., Antonetti F. R. (2011). An Introduction to Immunology and Immunopathology. All Asth Clin. Immun. 7, S1. 10.1186/1710-1492-7-s1-s1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner M. S., Peterson M. J., Jacobsen N. (2001). Certain Diterpenes and Extracts or Concentrates of Curcuma amada Containing Them for Use as Medicaments. U.S. Patent No 6235287 B1. Washington, DC: U.S. Patent and Trademark Office. [Google Scholar]
- Win N. N., Ito T., Ngwe H., Win Y. Y., Prema, Okamoto Y., et al. (2017). Labdane Diterpenoids from Curcuma amada Rhizomes Collected in Myanmar and their Antiproliferative Activities. Fitoterapia 122, 34–39. 10.1016/j.fitote.2017.08.006 [DOI] [PubMed] [Google Scholar]
- Wu A., Noble E. E., Tyagi E., Ying Z., Zhuang Y., Pinilla F. G., et al. (2015). Curcumin boosts DHA in the brain: implications for the prevention of anxiety disorders. Biochim. Biophys. Acta 1852, 951–961. 10.1016/j.bbadis.2014.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X., Pan Y., Zhang W.-Y., Cheng G., Kong L.-D. (2006). Ethanolic Extracts from Curcuma longa Attenuates Behavioral, Immune, and Neuroendocrine Alterations in a Rat Chronic Mild Stress Model. Biol. Pharm. Bull. 29, 938–944. 10.1248/bpb.29.938 [DOI] [PubMed] [Google Scholar]
- Yadav R. P., Tarun G. (2017). Versality of Turmeric: a Review the Golden Spice of Life. J. Pharmacogn. Phytochem. 6, 41–46. [Google Scholar]
- Yadav V. S., Mishra K. P., Singh D. P., Mehrotra S., Singh V. K. (2005). Immunomodulatory Effects of Curcumin. Immunopharmacol. Immunotoxicol. 27, 485–497. 10.1080/08923970500242244 [DOI] [PubMed] [Google Scholar]
- Yoo S. A., Kim O. K., Nam D.-E., Kim Y., Baek H., Jun W., et al. (2014). Immunomodulatory Effects of Fermented Curcuma longa L. Extracts on RAW 264.7 Cells. J. Korean Soc. Food Sci. Nutr. 43, 216–223. 10.3746/jkfn.2014.43.2.216 [DOI] [Google Scholar]
- Yoo S. A., Kim O. K., Nam D. E., Park S. J., Han D. K., Kwon H. O., et al. (2013). Immunomodulatory Effects of Curcuma longa L., Mulberry Leaves, and Purple Sweet Potato Extracts: Modulation of Immune Functions during Murine Leukemia Virus Infection. Nutrition 27, 1079. 10.1096/fasebj.27.1_supplement.1079.18 [DOI] [Google Scholar]
- Yu Y., Shen Q., Lai Y., Park S. Y., Qu X., Lin D., et al. (2018). Anti-Inflammatory Effect of Curcumin in Microglial Cells. Front. Pharmacol. 9, 1–10. 10.3389/fphar.2018.00386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuandani, Nugraha S. E., Laila L., Silaban S. D., Ramadhani F. (2020). Stimulatory Effect of Curcuma mangga on Immune Response against Staphylococcus aureus . Nusantara Biosci. 12, 109–113. 10.13057/nusbiosci/n120204 [DOI] [Google Scholar]
- Yuandani Y., Suwarso E. (2017a). Acute Toxicity of Ethanol Extract of Curcuma mangga Rhizome. Asian J. Pharm. Clin. Res. 10, 383–385. 10.22159/ajpcr.2017.v10i9.18398 [DOI] [Google Scholar]
- Yuandani Y., Suwarso E. (2017b). Immunomodulatory Effects of Ethanol Extract of Curcuma mangga Rhizomes in Mice. Asian J. Pharm. Clin. Res. 10, 383–385. 10.22159/ajpcr.2017.v10i9.18398 [DOI] [Google Scholar]
- Yuandani, Yuliasmi S., Satria D, F., Dongoran R, S., Sinaga M. H. A., Marpaung N. (2019). Correlation between the Phytochemical Constituents of Curcuma mangga and Its Immunomodulatory Effect. Rasayan J. Chem. 12, 1–6. 10.31788/rjc.2019.1215050 [DOI] [Google Scholar]
- Yuandani, Yuliasmi S., Yuliasmi S., Satria D. (2018). Analysis of Compounds and Immunostimulatory Properties of Curcuma mangga Rhizomes on Male Mice. Rasayan J. Chem. 11, 844–849. 10.31788/rjc.2018.1122097 [DOI] [Google Scholar]
- Yue G. L., Chan B. C. L., Hon P. M., Kennelly E. J., Yeung S. K., et al. (2010). Immunostimulatory Activities of Polysaccharide Extract Isolated from Curcuma longa . Int. J. Biol. Macrol. 47, 42–347. 10.1016/j.ijbiomac.2010.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuliawati T. H., Hestinah E. P. (2010). Cytotoxicity Effect of Curcuma aeruginosa Extract on Fibroblast with MTT Assay Method. Folia Med. Indonesiana. 46, 120–124. [Google Scholar]
- Zhernakova A., van Diemen C. C., Wijmenga C. (2009). Detecting Shared Pathogenesis from the Shared Genetics of Immune-Related Diseases. Nat. Rev. Genet. 10, 43–55. 10.1038/nrg2489 [DOI] [PubMed] [Google Scholar]
- Zhou L., Zhang K., Li J., Cui X., Wang A., Huang S., et al. (2013). Inhibition of Vascular Endothelial Growth Factor-Mediated Angiogenesis Involved in Reproductive Toxicity Induced by Sesquiterpenoids of Curcuma zedoaria in Rats. Reprod. Toxicol. 37, 62–69. 10.1016/j.reprotox.2013.02.001 [DOI] [PubMed] [Google Scholar]