Key Points
Question
Do outcomes differ between non-Hispanic African American and non-Hispanic White women with triple-negative breast cancer (TNBC)?
Findings
In this population-based cohort study of 23 213 patients with TNBC, the risk of breast cancer mortality was significantly higher in African American women compared with White women, which is partially explained by disparities in receipt of surgery and chemotherapy.
Meaning
Improving treatment adherence and efficacy in African American women with TNBC is crucial in reducing TNBC disparities.
Abstract
Importance
To our knowledge, there is no consensus regarding differences in treatment and mortality between non-Hispanic African American and non-Hispanic White women with triple-negative breast cancer (TNBC). Little is known about whether racial disparities vary by sociodemographic, clinical, and neighborhood factors.
Objective
To examine the differences in clinical treatment and outcomes between African American and White women in a nationally representative cohort of patients with TNBC and further examine the contributions of sociodemographic, clinical, and neighborhood factors to TNBC outcome disparities.
Design, Setting, and Participants
This population-based, retrospective cohort study included 23 123 women who received a diagnosis of nonmetastatic TNBC between January 1, 2010, and December 31, 2015, followed up through December 31, 2016, and identified from the Surveillance, Epidemiology, and End Results data set. The study was conducted from July 2019 to November 2020. The analyses were performed from July 2019 to June 2020.
Exposures
Race and ethnicity, including non-Hispanic African American and non-Hispanic White race.
Main Outcomes and Measures
Using logistic regression analysis and competing risk regression analysis, we estimated odds ratios (ORs) of receipt of treatment and hazard ratios (HRs) of breast cancer mortality in African American patients compared with White patients.
Results
Of 23 213 participants, 5881 (25.3%) were African American women and 17 332 (74.7%) were White women. Compared with White patients, African American patients had lower odds of receiving surgery (OR, 0.69; 95% CI, 0.60-0.79) and chemotherapy (OR, 0.89; 95% CI, 0.81-0.99) after adjustment for sociodemographic, clinicopathologic, and county-level factors. During a 43-month follow-up, 3276 patients (14.2%) died of breast cancer. The HR of breast cancer mortality was 1.28 (95% CI, 1.18-1.38) for African American individuals after adjustment for sociodemographic and county-level factors. Further adjustment for clinicopathological and treatment factors reduced the HR to 1.16 (95% CI, 1.06-1.25). This association was observed in patients living in socioeconomically less deprived counties (HR, 1.26; 95% CI, 1.14-1.39), urban patients (HR, 1.21; 95% CI, 1.11-1.32), patients having stage II (HR, 1.19; 95% CI, 1.02-1.39) or III (HR, 1.15; 95% CI, 1.01-1.31) tumors that were treated with chemotherapy, and patients younger than 65 years (HR, 1.24; 95% CI, 1.12-1.37).
Conclusions and Relevance
In this retrospective cohort study, African American women with nonmetastatic TNBC had a significantly higher risk of breast cancer mortality compared with their White counterparts, which was partially explained by their disparities in receipt of surgery and chemotherapy.
This cohort study examines differences in clinical treatment and contributions of sociodemographic, clinical, and neighborhood factors to outcome disparaties between African American and White women with triple-negative breast cancer.
Introduction
Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer that is characterized by lacking protein expression of estrogen receptor (ER) and progesterone receptor (PR) and amplification of ERBB2 (formerly HER2). Thus, patients with TNBC do not benefit from hormone or ERBB2-targeted therapies, and chemotherapy is the main systemic option. Compared with hormone receptor–positive or ERBB2-positive subtypes, TNBC has a worse prognosis, with higher risks of recurrence, distant metastasis, and mortality, particularly within the first 5 years after diagnosis.1,2
Triple-negative breast cancer accounts for 10% to 12% of all invasive breast cancers in the US.3,4 While the overall incidence of breast cancer is lower in non-Hispanic African American women than in non-Hispanic White women, the incidence of TNBC in African American women is doubled.3 Differences in treatment and clinical outcomes between African American and White women with breast cancer are well documented. Compared with White women, African American women are less likely to receive guideline-recommended treatment regimens5,6 and more likely to die of the disease.3 However, studies of racial differences in TNBC outcomes have reported inconsistent results. While some studies have failed to detect survival differences between African American and White women with TNBC,7,8,9,10,11,12,13,14 others have demonstrated that African American women had higher risks of recurrence and breast cancer mortality.15,16,17,18 Most of these studies were limited by including patients generally from a single hospital or particular geographic region9,10,11,12,14,15,16,17,18 and a small sample of African American patients (n < 100).8,15,16 These limitations obscure whether TNBC outcomes differ by race and ethnicity.
Therefore, we comprehensively examined the differences in clinical treatment and outcomes between African American and White women in a nationally representative cohort of patients with TNBC, accounting for demographic, health insurance, clinicopathological, treatment, and neighborhood factors. Furthermore, we examined the magnitude of survival disparities within the strata of sociodemographic, clinical, and neighborhood factors to understand how these factors contributed to TNBC outcome disparities.
Methods
Study Population
We used the Surveillance, Epidemiology, and End Results (SEER) database (released in April 2019) to identify eligible cases. This study included women who received a diagnosis of stage I to III TNBC (ER-/PR-/ERBB2-) as a first primary malignancy between 2010 and 2015 (n = 29 274) because the SEER did not collect ERBB2 information until 2010. We excluded women younger than 18 years (n = 1), self-reported Hispanic individuals (n = 3723), and those of other races than self-reported African American or White (n = 2337). The analytic sample included 23 213 women (5881 African American [25.3%]). The deidentified data were determined exempt from informed consent by the institutional review board of Washington University School of Medicine in St. Louis, which approved the study.
Covariables
Demographic factors included age at diagnosis (18-39, 40-44, 45-49, 50-54, 55-59, 60-64, 65-69, 70-74, or ≥75 years) and registries. Pathological features included cancer stage (I, II, or III, based on the 7th edition of the American Joint Committee on Cancer Staging Atlas), tumor size (≤2 cm, >2-5 cm, >5 cm, or unknown), lymph node status (negative, 1-3 positive, >3 positive, or unknown), and grade (well differentiated, moderately differentiated, poorly differentiated/undifferentiated, or unknown). Cancer-directed treatment included surgery (no surgical treatment, breast-conserving surgery, mastectomy, unspecified type of surgery, or unknown), chemotherapy (yes or no), and radiation therapy (yes or no). Neighborhood socioeconomic deprivation and rurality were assessed based on the state county of the patient’s residence at diagnosis. The composite socioeconomic deprivation index was calculated using 21 variables from the 2008 to 2012 American Community Survey, as described elsewhere.19,20,21 Deprivation index scores were categorized based on quartiles of county-level deprivation index scores across the country, with a higher quartile representing greater socioeconomic deprivation. Rural counties were defined as nonmetropolitan areas and determined using the rural-urban continuum codes (4-9, 88, and 99) from the US Department of Agriculture.
Outcome
The SEER data set contains vital status data that are obtained through links to state vital records, state motor vehicle records, the National Death Index, and contact with treating physicians.22 We used the SEER cause-specific death classification variable to define deaths that were attributed to breast cancer. Follow-up months were calculated by the SEER data set from the date of diagnosis to the date of death, the date of last contact, or December 31, 2016, whichever occurred first.
Statistical Analysis
Logistic regression analyses were used to estimate odds ratios (ORs) of receiving surgery, chemotherapy, and radiation therapy in African American compared with White patients, adjusting for sociodemographic, clinicopathological, and neighborhood covariables. We estimated cumulative survival rates for White and African American patients using the Kaplan-Meier method and compared their survival rates using the log-rank test. We also performed a competing risk regression analysis, using the Fine and Gray method,23 to assess the association between race and risk of death of breast cancer. Deaths of other causes than breast cancer were considered competing events. Subdistribution hazard ratios (HRs) and 95% CIs were reported. The analysis was first adjusted for sociodemographic and neighborhood covariables and further adjusted for clinicopathological and treatment factors. Missing values in covariables were included in the models as a missing indicator category.24 The proportionality of hazards for race and covariables was tested by assessing the significance of interaction terms between race, covariables, and log-transformed follow-up time. Because the assumption of proportional hazards was violated for age, the models were stratified by age to allow varying baseline hazards. Stratified analyses were performed to examine the differences in the association between race and breast cancer mortality by demographic, health insurance, treatment, and neighborhood factors. Given that most of the patients 65 years or older were covered through Medicare, inclusion of health insurance in the analyses for this age group was uninformative, and the analysis by insurance was restricted to patients younger than 65 years. The interaction between race and other prognostic factors was assessed by including a cross-product term in the multivariable-adjusted models. The statistical significance of an interaction term was evaluated by the likelihood ratio test. All statistical analyses were performed using SAS (version 9.4; SAS Institute). Statistical significance was assessed as a 2-sided P < .05.
Results
The eTable in the Supplement shows the characteristics of TNBC cases, including 5881 African American women (25.3%) and 17 332 White women (74.7%). Compared with White women, African American women were younger at diagnosis (56.3 vs 59.7 years) and more likely to be insured through Medicaid (20.6% vs 8.8%) and live in the most deprived (highest quartile) counties (14.7% vs 7.1%) and urban counties (92.1% vs 86.2%). The TNBCs of African American women were pathologically more aggressive, including more stage III tumors (20.3% vs 15.2%), larger tumor size (>5 cm: 14.3% vs 9.6%), positive lymph nodes (39.0% vs 31.6%), and poor differentiation/undifferentiation (81.5% vs 76.0%).
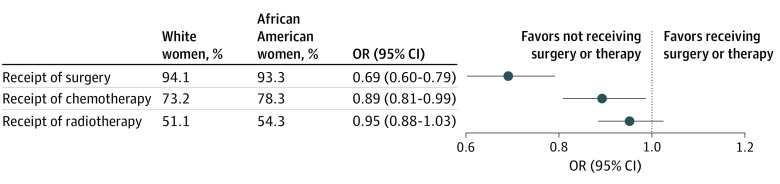
Most patients with TNBC received surgical treatments (94.7%), chemotherapy (74.5%), and/or radiation therapy (51.9%). After adjustment for sociodemographic, clinicopathological, and neighborhood covariables, African American patients had lower odds of receiving surgery (OR, 0.69; 95% CI, 0.60-0.79) and chemotherapy (OR, 0.89; 95% CI, 0.81-0.99) compared with White patients (Figure 1). There was no significant difference in use of radiation therapy.
Figure 1. Odds Ratios of Treatment in Non-Hispanic African American vs Non-Hispanic White Women With Triple-Negative Breast Cancer (TNBC).
The analyses were adjusted for age, health insurance, county-level socioeconomic deprivation, rural residency, cancer stage, tumor grade, tumor size, and the number of positive lymph nodes. OR indicates odds ratio.
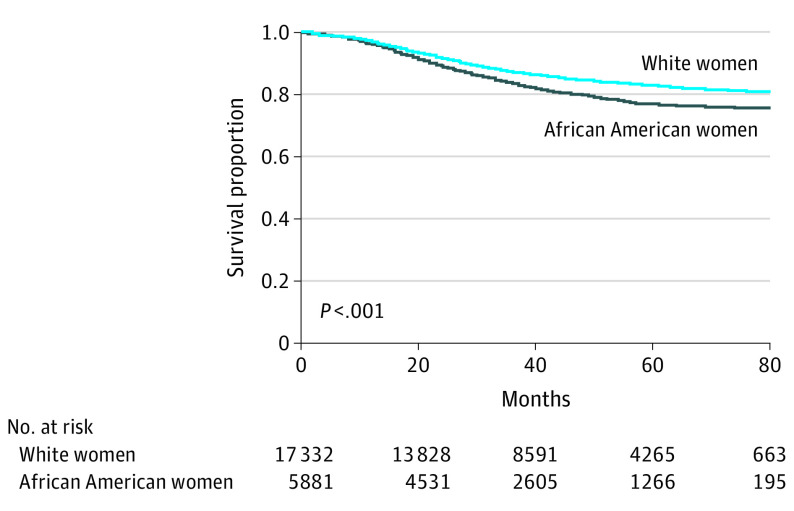
During a median follow-up of 43 months (interquartile range, 22-59 months), 987 African American patients (16.8%) and 2289 White patients (13.2%) died of breast cancer, and 263 African American patients (4.5%) and 875 White patients (5.0%) died of other causes. Cumulative breast cancer-specific survival was significantly lower in African American women than White women (P < .001; Figure 2); the 5-year survival rate was 76.9% in African American women and 82.9% in White women.
Figure 2. Cumulative Breast Cancer-Specific Survival in Non-Hispanic African American and Non-Hispanic White Women With Triple-Negative Breast Cancer.
The HR of breast cancer mortality in African American patients was 1.28 (95% CI, 1.18-1.38) compared with White patients after adjustment for age, insurance status, county-level socioeconomic deprivation, and rural residency. Further adjustment for clinicopathological and treatment factors reduced the HR to 1.16 (95% CI, 1.06-1.25) (Table). African American patients also had a higher risk of overall mortality (HR, 1.13; 95% CI, 1.06-1.21) after adjustment for all aforementioned covariables. We further analyzed the race-associated risk of breast cancer mortality according to sociodemographic, neighborhood, and treatment factors (Table). The association between race and breast cancer mortality significantly varied by age at diagnosis, county-level socioeconomic deprivation, and rural/urban residency. The increased risk in African American patients was observed in women younger than 65 years (HR, 1.24; 95% CI, 1.12-1.37) but not in older women, those living in socioeconomically less deprived counties (HR, 1.26; 95% CI, 1.14-1.39) but not in more deprived counties, and those from urban areas (HR, 1.21; 95% CI, 1.11-1.32). African American women living in rural areas had lower risk of death from breast cancer than White women who lived in rural areas (HR, 0.72; 95% CI, 0.53-0.96).
Table. Risk of Death From Breast Cancer in Non-Hispanic African American Women With Triple-Negative Breast Cancer Compared With Non-Hispanic White Counterparts.
| Characteristic | Person-years | Deaths | HR (95% CI)a |
|---|---|---|---|
| Overall | |||
| White | 59 488 | 2289 | 1 [Reference] |
| African American | 19 048 | 987 | 1.16 (1.06-1.25) |
| Age, <65 y | |||
| White | 39 046 | 1300 | 1 [Reference] |
| African American | 14 617 | 735 | 1.24 (1.12-1.37) |
| Age ≥65 | |||
| White | 20 442 | 989 | 1 [Reference] |
| African American | 4430 | 252 | 0.99 (0.86-1.15) |
| P value for interaction | NA | NA | .01 |
| Privately insuredb | |||
| White | 33 879 | 1013 | 1 [Reference] |
| African American | 10 631 | 455 | 1.27 (1.13-1.43) |
| Uninsured or Medicaid-insuredb | |||
| White | 4639 | 266 | 1 [Reference] |
| African American | 3805 | 271 | 1.18 (0.97-1.42) |
| P value for interaction | NA | NA | .27 |
| Socioeconomically less deprived countiesc | |||
| White | 48 268 | 1762 | 1 [Reference] |
| African American | 12 427 | 670 | 1.26 (1.14-1.39) |
| Socioeconomically more deprived countiesc | |||
| White | 11 220 | 527 | 1 [Reference] |
| African American | 6621 | 317 | 0.93 (0.80-1.08) |
| P value for interaction | NA | NA | <.01 |
| Urban counties | |||
| White | 51 369 | 1935 | 1 [Reference] |
| African American | 17 555 | 923 | 1.21 (1.11-1.32) |
| Rural counties | |||
| White | 8118 | 354 | 1 [Reference] |
| African American | 1493 | 64 | 0.72 (0.53-0.96) |
| P value for interaction | NA | NA | <.01 |
| No surgery | |||
| White | 1785 | 262 | 1 [Reference] |
| African American | 796 | 161 | 1.23 (0.99-1.51) |
| Surgical treatment | |||
| White | 57 601 | 2016 | 1 [Reference] |
| African American | 18 232 | 820 | 1.12 (1.02-1.22) |
| P value for interaction | NA | NA | .44 |
| No radiation therapy | |||
| White | 27 835 | 1256 | 1 [Reference] |
| African American | 8342 | 530 | 1.17 (1.04-1.30) |
| Radiation therapy | |||
| White | 31 653 | 1033 | 1 [Reference] |
| African American | 10 705 | 457 | 1.13 (1.00-1.28) |
| P value for interaction | NA | NA | .59 |
Abbreviations: HR, hazard ratio; NA, not applicable.
Models were adjusted for age, type of health insurance, county-level socioeconomic deprivation, rural residency, cancer stage, tumor grade, surgery, radiation therapy, and chemotherapy.
The analysis was restricted to patients younger than 65 years.
County-level socioeconomic deprivation was dichotomized using the median.
Considering a strong association between cancer stage and chemotherapy in this study (62.2% of stage I, 80.2% of stage II, and 85.1% of stage III; P < .001), the analysis of outcome disparities was stratified by cancer stage and chemotherapy (Figure 3). Within stage I tumors, race-associated increased risk did not significantly vary by chemotherapy. Among patients with stage II tumors, an excess risk of death of breast cancer in African American women was observed in those who received chemotherapy (HR, 1.19; 95% CI, 1.02-1.39; P for interaction = .04), but not in those who did not (HR, 0.90; 95% CI, 0.69-1.17). Women with stage III tumors had similar association patterns as women with stage II tumors, although the interaction was not statistically significant (P for interaction = .23); the HR was 1.15 (95% CI, 1.01-1.31) in the group that received chemotherapy and 0.97 (95% CI, 0.73-1.27) in the group that did not receive chemotherapy.
Figure 3. Stage and Chemotherapy-Stratified Risk of Breast Cancer Mortality in Non-Hispanic African American vs White Patients With Triple-Negative Breast Cancer.
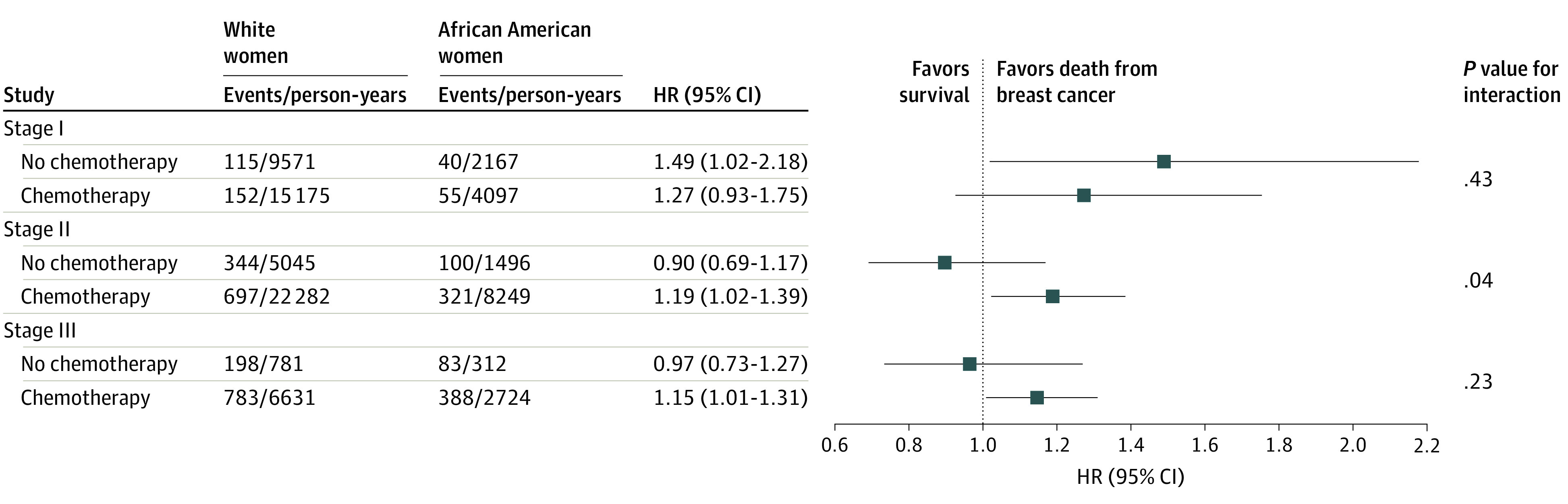
The models were adjusted for age, type of health insurance, county-level socioeconomic deprivation, rural residency, tumor grade, surgery, and radiation therapy. HR indicates hazard ratio.
Discussion
In this study, non-Hispanic African American women experienced a disproportional burden of poor breast cancer outcomes than non-Hispanic White women, which is associated with a higher incidence of TNBC and more advanced stages at diagnosis in African American women.7,25 However, it remained uncertain if African American women have inferior outcomes than White women within the TNBC subtype. To our knowledge, this study represents the most comprehensive analysis of the differences in TNBC treatment and outcomes between African American and White patients in a nationally representative cohort of women with TNBC. We observed that African American women were less likely to receive chemotherapy and surgery than White women. However, the risk of death of breast cancer remained significantly higher in African American women compared with White women after adjustment for demographic, health insurance, neighborhood, clinicopathological, and treatment factors.
Studies of the association between race and survival outcomes in women with TNBC have yielded inconsistent results. Comparable TNBC outcomes between African American and White women were reported in hospital-based studies in which treatment patterns were uniformly distributed between these 2 racial groups.9,11,13 For example, Dawood et al11 examined racial differences in clinical outcomes among patients with TNBC (including 100 African American and 371 White patients) who were treated with standard neoadjuvant therapy at the MD Anderson Cancer Center (Houston, Texas) and found no difference in survival or likelihood of attaining a pathological complete response between African American and White patients. However, a population-based study using the 2005 to 2012 California Cancer Registry data (including 1896 African American and 8589 White patients) showed that African American women with TNBC still had a 21% (HR, 1.21; 95% CI, 1.06-1.37) higher risk of breast cancer mortality than their White counterparts after adjustment for sociodemographic, neighborhood, clinicopathological, and treatment factors.17 Our finding of a 16% higher risk of breast cancer mortality in African American women than White women with TNBC using the 2010 to 2015 SEER data demonstrated racial disparities in TNBC outcomes in a much larger patient population (including 5881 African American and 17 332 White patients) from much wider geographic areas. The drivers of TNBC outcome disparities are not clear. An analysis of 55 642 African American and White women age 18 to 64 years with hormone receptor–negative breast cancer (unknown ERBB2 status) showed that differences in health insurance and clinicopathology explained approximately three-fifths of the excess breast cancer mortality in African American women, and differences in comorbidities and treatment accounted for less than one-tenth of the excess risk.26 Collectively, these data suggest a possible role of as yet undefined tumor microenvironment or tumor biological features in the racial differences observed in TNBC clinical outcomes.
We identified a higher risk of breast cancer mortality in African American patients than White patients, even though both groups were treated with chemotherapy. Killelea et al27 reported that African American patients with TNBC had a lower likelihood of a pathological complete response to neoadjuvant chemotherapy compared with White patients with TNBC, which is associated with reduced survival.28 These findings suggest that the TNBC of African American women may be more resistant to chemotherapy than the TNBC of White women. The development of TNBC chemoresistance is considered as a result of interactions of the tumor microenvironment, drug efflux, cancer stem cells, and tumor cells through multiple signaling pathways.29 Triple-negative breast cancer comprises distinct molecular subtypes with significant variations in their genomic landscape and cellular composition of their microenvironment, leading to differences in the response to chemotherapy and prognostic outcomes.30 The luminal androgen receptor–positive and mesenchymal subtypes of TNBC tumors appear to be more resistant to neoadjuvant chemotherapy than the basal-like subtype.31 Future studies that examine racial differences in TNBC molecular subtypes and other molecular factors/pathways that regulate TNBC chemoresistance may be warranted.
An interesting observation in this study is the racial disparity in TNBC outcomes among patients younger than 65 years, which was independent of health insurance status at diagnosis, clinicopathological features, and treatment. African American women have significantly higher rates of TNBC than White women after age 35 years.32 Triple-negative breast cancer tumors diagnosed before age 50 years are more aggressive pathologically and molecularly than those diagnosed after age 65 years, including higher grade, larger tumor size, higher number of positive lymph nodes, higher expression of proliferation-associated genes, and more baselike subtypes.33 To our knowledge, it remains unknown if tumor biological differences between the TNBC of African American patients and the TNBC of White patients are more pronounced in women younger than 65 years.
Our finding of variations in the race-associated breast cancer mortality by county-level socioeconomic deprivation and rural contexts suggests a role of neighborhood contexts in TNBC outcome disparities. In general, living in socioeconomically disadvantaged neighborhoods and rural (vs large metropolitan) areas is associated with increased risk of cancer mortality20,34,35,36 because of the association with health behaviors and access to cancer care. The analysis of racial disparities in cancer outcomes stratified by neighborhood socioeconomic status and rurality would minimize variability in access to care and other health behaviors. We observed excess breast cancer mortality in African American patients compared with White patients in socioeconomically less deprived, but not more deprived, counties and in urban, but not rural, areas. This is consistent with an analysis of the California Cancer Registry data, in which socioeconomically advantaged, rather than socioeconomically disadvantaged, census blocks showed a higher risk of death of breast cancer in African American patients than White patients.37 This California Cancer Registry-based study did not distinguish patients by hormone receptor status. In addition, a lower likelihood of stage-appropriate chemotherapy use in African American vs White patients with breast cancer was observed in large urban and suburban areas,38,39 but not seen in a study of a rural region in Georgia.40 However, this study’s data showed that the excess breast cancer mortality in African American women who live in urban areas vs White women who live in urban areas remained persistent after adjustment for treatment. Together, these findings reflected that African American women with TNBC in neighborhoods with higher socioeconomic levels and urban areas did not achieve the same gains in cancer outcomes as their White counterparts. The reason for a lower risk of mortality in African American women living in rural areas vs White women living in rural areas is unclear. Future studies of treatment details, comorbidities, and lifestyles in explaining the association between race and TNBC outcomes in rural and urban areas are warranted.
Chemotherapy and surgery are the primary treatment options for TNBC. However, we observed that African American race was independently associated with disparities in chemotherapy and surgical resection for TNBC, even when controlling for the type of health insurance, clinicopathological features, neighborhood socioeconomic deprivation, and rural residency. Zhang et al41 comprehensively examined the racial difference in the use of chemotherapy for TNBC among the patients identified from 10 cancer registries of the National Program of Cancer Registry. They reported that African American patients were more likely to receive delayed chemotherapy, even though there was no significant difference in chemotherapy use between African American and White women. Maintaining the schedule and dosage is of central concern in delivering chemotherapy, because delayed initiation and a low dose intensity are associated with worse survival of patients with TNBC.42,43 Because of the lack of treatment details, we were unable to assess the quality of chemotherapy, including timely initiation, appropriate dosage, and completion, in African American women with TNBC and its association with TNBC outcome disparities. Although lack of health insurance is an important barrier to cancer treatment, underuse of chemotherapy and surgical treatment in African American women with TNBC, even when controlling for the type of health insurance, reflected a critical role of other factors in adherence to recommended treatment in this underserved patient population. Among women with breast cancer (regardless of hormone receptor status), African American patients were more likely to receive treatment in underresourced public and safety net hospitals, have unsatisfying communication with their health care clinicians, and experience discriminatory practices in the health care system, all of which contributed to treatment-related disparities.44 In addition, travel burden45 and cancer-related financial hardship46 might disproportionately affect breast cancer treatment for African American patients.
Limitations
This study had limitations. Some confounding factors, such as comorbidities, obesity, alcohol use, and physical activity, were not accounted for because of the unavailability in the SEER data set. We did not consider individual-level socioeconomic status, such as household income, debt, educational attainment, and changes in insurance coverage, which could directly affect the use of cancer treatment,47 although health insurance at cancer diagnosis and county-level socioeconomic deprivation status were accounted for. Women in this study were followed for 43 months on average. Future studies could investigate long-term survival differences between African American and White women with TNBC.
Conclusions
This study provides evidence that suggests that African American women with nonmetastatic TNBC were less likely to receive surgery and chemotherapy and were more likely to die of the disease compared with their White counterparts. Worse TNBC outcomes of African American women were observed in socioeconomically less deprived counties and in urban areas, suggesting that the place of residence should be considered in addressing racial disparities in TNBC outcomes. Future studies that focus on tumor microenvironment, tumor biology, treatment efficacy, and access to care in African American women with TNBC could advance understanding of the drivers of TNBC outcome disparities and complement a deeper understanding of social factors.
eTable. Characteristics of non-Hispanic African American women and non-Hispanic European American women with triple-negative breast cancer in the Surveillance, Epidemiology, and End Results, 2010-2015 (n = 23,213)
References
- 1.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429-4434. doi: 10.1158/1078-0432.CCR-06-3045 [DOI] [PubMed] [Google Scholar]
- 2.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275-1281. doi: 10.1200/JCO.2007.14.4147 [DOI] [PubMed] [Google Scholar]
- 3.DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438-451. doi: 10.3322/caac.21583 [DOI] [PubMed] [Google Scholar]
- 4.DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66(1):31-42. doi: 10.3322/caac.21320 [DOI] [PubMed] [Google Scholar]
- 5.Freedman RA, Virgo KS, He Y, et al. The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer. 2011;117(1):180-189. doi: 10.1002/cncr.25542 [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Li CI. Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer Epidemiol Biomarkers Prev. 2015;24(11):1666-1672. doi: 10.1158/1055-9965.EPI-15-0293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warner ET, Tamimi RM, Hughes ME, et al. Racial and ethnic differences in breast cancer survival: mediating effect of tumor characteristics and sociodemographic and treatment factors. J Clin Oncol. 2015;33(20):2254-2261. doi: 10.1200/JCO.2014.57.1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ademuyiwa FO, Tao Y, Luo J, Weilbaecher K, Ma CX. Differences in the mutational landscape of triple-negative breast cancer in African Americans and Caucasians. Breast Cancer Res Treat. 2017;161(3):491-499. doi: 10.1007/s10549-016-4062-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacheco JM, Gao F, Bumb C, Ellis MJ, Ma CX. Racial differences in outcomes of triple-negative breast cancer. Breast Cancer Res Treat. 2013;138(1):281-289. doi: 10.1007/s10549-012-2397-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma H, Lu Y, Malone KE, et al. Mortality risk of black women and white women with invasive breast cancer by hormone receptors, HER2, and p53 status. BMC Cancer. 2013;13:225. doi: 10.1186/1471-2407-13-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawood S, Broglio K, Kau SW, et al. Triple receptor-negative breast cancer: the effect of race on response to primary systemic treatment and survival outcomes. J Clin Oncol. 2009;27(2):220-226. doi: 10.1200/JCO.2008.17.9952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prasad S, Efird JT, James SE, Walker PR, Zagar TM, Biswas T. Failure patterns and survival outcomes in triple negative breast cancer (TNBC): a 15 year comparison of 448 non-Hispanic black and white women. Springerplus. 2016;5(1):756. doi: 10.1186/s40064-016-2444-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider BP, Shen F, Jiang G, et al. Impact of genetic ancestry on outcomes in ECOG-ACRIN-E5103. JCO Precis Oncol. 2017;2017. doi: 10.1200/po.17.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collin LJ, Jiang R, Ward KC, et al. Racial disparities in breast cancer outcomes in the metropolitan Atlanta area: new insights and approaches for health equity. JNCI Cancer Spectr. 2019;3(3):pkz053. doi: 10.1093/jncics/pkz053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lund MJ, Trivers KF, Porter PL, et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat. 2009;113(2):357-370. doi: 10.1007/s10549-008-9926-3 [DOI] [PubMed] [Google Scholar]
- 16.Sachdev JC, Ahmed S, Mirza MM, Farooq A, Kronish L, Jahanzeb M. Does race affect outcomes in triple negative breast cancer? Breast Cancer (Auckl). 2010;4:23-33. doi: 10.1177/117822341000400003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tao L, Gomez SL, Keegan TH, Kurian AW, Clarke CA. Breast cancer mortality in African-American and non-Hispanic White women by molecular subtype and stage at diagnosis: a population-based study. Cancer Epidemiol Biomarkers Prev. 2015;24(7):1039-1045. doi: 10.1158/1055-9965.EPI-15-0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez CA, Zumsteg ZS, Gupta G, et al. Black race as a prognostic factor in triple-negative breast cancer patients treated with breast-conserving therapy: a large, single-institution retrospective analysis. Breast Cancer Res Treat. 2013;139(2):497-506. doi: 10.1007/s10549-013-2550-x [DOI] [PubMed] [Google Scholar]
- 19.Lian M, Struthers J, Liu Y. Statistical assessment of neighborhood socioeconomic deprivation environment in spatial epidemiologic studies. Open J Stat. 2016;6(3):436-442. doi: 10.4236/ojs.2016.63039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lian M, Pérez M, Liu Y, et al. Neighborhood socioeconomic deprivation, tumor subtypes, and causes of death after non-metastatic invasive breast cancer diagnosis: a multilevel competing-risk analysis. Breast Cancer Res Treat. 2014;147(3):661-670. doi: 10.1007/s10549-014-3135-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S, Liu Y, Yun S, Lian M, Komaie G, Colditz GA. Impacts of neighborhood characteristics on treatment and outcomes in women with ductal carcinoma in situ of the breast. Cancer Epidemiol Biomarkers Prev. 2018;27(11):1298-1306. doi: 10.1158/1055-9965.EPI-17-1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lund JL, Harlan LC, Yabroff KR, Warren JL. Should cause of death from the death certificate be used to examine cancer-specific survival? a study of patients with distant stage disease. Cancer Invest. 2010;28(7):758-764. doi: 10.3109/07357901003630959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fine JP, Gray RJ. A proportional hazards model for the Subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 24.Song M, Pazaris M, Spiegelman D. The missing covariate indicator method is nearly valid almost always. Paper presented at 2016 Epidemiology Congress of the Americas; June 21, 2016; Miami, Florida. [Google Scholar]
- 25.Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J Clin Oncol. 2018;36(1):25-33. doi: 10.1200/JCO.2017.74.2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jemal A, Robbins AS, Lin CC, et al. Factors that contributed to Black-White disparities in survival among nonelderly women with breast cancer between 2004 and 2013. J Clin Oncol. 2018;36(1):14-24. doi: 10.1200/JCO.2017.73.7932 [DOI] [PubMed] [Google Scholar]
- 27.Killelea BK, Yang VQ, Wang SY, et al. Racial differences in the use and outcome of neoadjuvant chemotherapy for breast cancer: results from the National Cancer Data Base. J Clin Oncol. 2015;33(36):4267-4276. doi: 10.1200/JCO.2015.63.7801 [DOI] [PubMed] [Google Scholar]
- 28.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164-172. doi: 10.1016/S0140-6736(13)62422-8 [DOI] [PubMed] [Google Scholar]
- 29.Nedeljković M, Damjanović A. Mechanisms of chemotherapy resistance in triple-negative breast cancer—how we can rise to the challenge. Cells. 2019;8(9):E957. doi: 10.3390/cells8090957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masuda H, Baggerly KA, Wang Y, et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res. 2013;19(19):5533-5540. doi: 10.1158/1078-0432.CCR-13-0799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Echavarria I, López-Tarruella S, Picornell A, et al. Pathological response in a triple-negative breast cancer cohort treated with neoadjuvant carboplatin and docetaxel according to Lehmann’s Refined Classification. Clin Cancer Res. 2018;24(8):1845-1852. doi: 10.1158/1078-0432.CCR-17-1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarke CA, Keegan TH, Yang J, et al. Age-specific incidence of breast cancer subtypes: understanding the black-white crossover. J Natl Cancer Inst. 2012;104(14):1094-1101. doi: 10.1093/jnci/djs264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gulbahce HE, Bernard PS, Weltzien EK, et al. Differences in molecular features of triple-negative breast cancers based on the age at diagnosis. Cancer. 2018;124(24):4676-4684. doi: 10.1002/cncr.31776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kish JK, Yu M, Percy-Laurry A, Altekruse SF. Racial and ethnic disparities in cancer survival by neighborhood socioeconomic status in Surveillance, Epidemiology, and End Results (SEER) registries. J Natl Cancer Inst Monogr. 2014;2014(49):236-243. doi: 10.1093/jncimonographs/lgu020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: part I–all cancers and lung cancer and part II–colorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011:107497. doi: 10.1155/2011/107497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obeng-Gyasi S, Timsina L, Bhattacharyya O, Fisher CS, Haggstrom DA. Breast cancer presentation, surgical management and mortality across the rural-urban continuum in the National Cancer Database. Ann Surg Oncol. 2020;27(6):1805-1815. doi: 10.1245/s10434-020-08376-y [DOI] [PubMed] [Google Scholar]
- 37.Parise CA, Caggiano V. Disparities in race/ethnicity and socioeconomic status: risk of mortality of breast cancer patients in the California Cancer Registry, 2000-2010. BMC Cancer. 2013;13:449. doi: 10.1186/1471-2407-13-449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hershman D, McBride R, Jacobson JS, et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23(27):6639-6646. doi: 10.1200/JCO.2005.12.633 [DOI] [PubMed] [Google Scholar]
- 39.Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24(9):1357-1362. doi: 10.1200/JCO.2005.04.5799 [DOI] [PubMed] [Google Scholar]
- 40.Lipscomb J, Gillespie TW, Goodman M, et al. Black-white differences in receipt and completion of adjuvant chemotherapy among breast cancer patients in a rural region of the US. Breast Cancer Res Treat. 2012;133(1):285-296. doi: 10.1007/s10549-011-1916-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang L, King J, Wu XC, et al. Racial/ethnic differences in the utilization of chemotherapy among stage I-III breast cancer patients, stratified by subtype: Findings from ten National Program of Cancer Registries states. Cancer Epidemiol. 2019;58:1-7. doi: 10.1016/j.canep.2018.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, Yu Q, Wu XC, et al. Impact of chemotherapy relative dose intensity on cause-specific and overall survival for stage I-III breast cancer: ER+/PR+, HER2- vs. triple-negative. Breast Cancer Res Treat. 2018;169(1):175-187. doi: 10.1007/s10549-017-4646-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH. Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol. 2016;2(3):322-329. doi: 10.1001/jamaoncol.2015.3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in African American women: a review. JAMA Surg. 2017;152(5):485-493. doi: 10.1001/jamasurg.2017.0005 [DOI] [PubMed] [Google Scholar]
- 45.Ambroggi M, Biasini C, Del Giovane C, Fornari F, Cavanna L. Distance as a barrier to cancer diagnosis and treatment: review of the literature. Oncologist. 2015;20(12):1378-1385. doi: 10.1634/theoncologist.2015-0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wheeler SB, Spencer JC, Pinheiro LC, Carey LA, Olshan AF, Reeder-Hayes KE. Financial impact of breast cancer in Black versus White women. J Clin Oncol. 2018;36(17):1695-1701. doi: 10.1200/JCO.2017.77.6310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siddharth S, Sharma D. Racial disparity and triple-negative breast cancer in African-American women: a multifaceted affair between obesity, biology, and socioeconomic determinants. Cancers (Basel). 2018;10(12):E514. doi: 10.3390/cancers10120514 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Characteristics of non-Hispanic African American women and non-Hispanic European American women with triple-negative breast cancer in the Surveillance, Epidemiology, and End Results, 2010-2015 (n = 23,213)