Editor—We read with interest the recent meta-analysis by Weber and colleagues1 discussing strategies to reduce the dose of neuromuscular blocking agent (NMBA) with adjuncts. The prolonged use of muscle paralysis in patients with moderate-to-severe COVID-19 acute respiratory distress syndrome (ARDS), massively admitted during the first French wave of the COVID-19 outbreak,2 led to a shortage of NMBA supplies, especially cisatracurium. Conservation strategies were proposed, including use of neuromuscular block monitors measuring the train-of-four (TOF) count or ratio. ICU guidelines for sustained neuromuscular block recommend use of objective (quantitative, TOF ratio) monitoring, combined with clinical assessment, to ensure satisfactory recovery of neuromuscular function.3 , 4 However, no national recommendation for monitor use in COVID-19 ARDS patients as a strategy for reducing the dose of NMBA used was available. We evaluated whether use of a TOF-count monitor in adult COVID-19 patients receiving mechanical ventilation reduced daily and weight-adjusted NMBA consumption during a period of supply shortage. The study was registered with ClinicalTrials.gov (NCT04459533).
We retrospectively analysed records of adult patients requiring mechanical ventilation for COVID-19 ARDS and having received cisatracurium for at least 48 h in nine ICUs of five academic hospitals during the first epidemic peak in France from February 27 to April 21, 2020. At that time use of qualitative (TOF count) monitoring (TOFscan®; Draeger, Lübeck, Germany; applied to the adductor pollicis muscle) to adjust cisatracurium infusion rate was standardised (Supplementary Fig. S1) and applied per physician discretion. The primary endpoint was cisatracurium consumption in mg kg−1 day−1. Baseline characteristics, severity of patients using the Simplified Acute Physiology Score (SAPS) II and Sequential Organ Failure Assessment (SOFA scores, mechanical ventilation, arterial blood gas parameters at three time points (intubation, 48 h after intubation, 7 days after intubation), number of prone positionings, mechanical ventilation time, length of stay in ICU, and 90-day mortality were collected. Multiple logistic regression was used to identify independent risk factors for higher cisatracurium consumption (≥4 mg kg−1 day−1). A post hoc power analysis based on the primary endpoint was calculated and yielded 72.7% accuracy for 5% alpha error. All calculations were performed using R software version 3.4.4 (R Core Team 2017, Vienna, Austria), and significance level was set at P<0.05. The study was approved by the local ethics committee; no written consent was required.
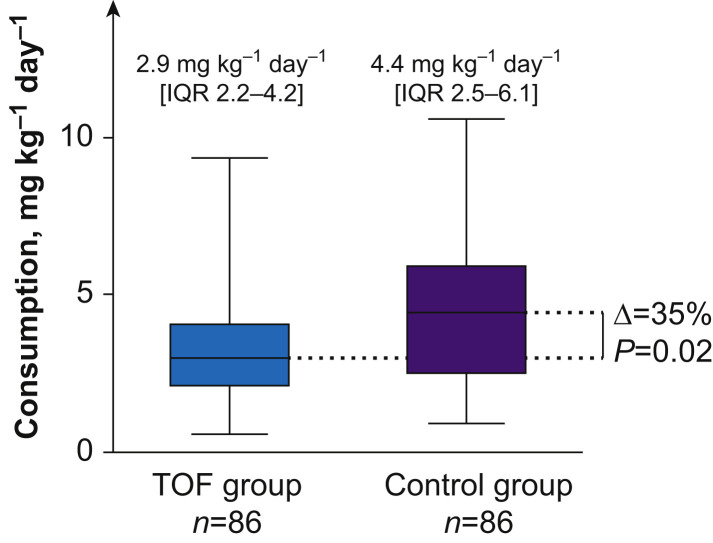
Out of 121 patients included, 86 were allocated to the TOF group (at least one qualitative TOF-count measurement reported in the electronic health record); otherwise they were allocated to the control group (n=35). Patient characteristics were comparable (Supplementary Table S1). No adjuncts (magnesium or alpha-adrenergic agonists) were used to reduce the dose of neuromuscular blocking drug administered.1 Cisatracurium consumption was significantly lower in the TOF group (2.9 mg kg−1 day−1 [inter-quartile range, IQR=2.2–4.2] vs 4.4 mg kg−1 day−1 [IQR=2.5–6.1]; P=0.02) (Fig. 1 ). The number of patients with higher cisatracurium consumption (>4 mg kg−1 day−1) in the TOF group was 26 (30.2%), and was 18 (51.4%) in the control group (P=0.02). The absence of monitoring was independently associated with higher cisatracurium consumption (odds ratio [OR]=2.77; 95% confidence interval [CI], 1.18–6.66; P=0.02). The number of prone positionings (OR=1.46; 95% CI, 1.11–1.46; P=0.0007) was also independently associated with greater cisatracurium consumption. No clinically significant difference in ventilation-related parameters was found in the first 7 days of mechanical ventilation or duration of cisatracurium administration (Supplementary Table S2). In the TOF group, duration of mechanical ventilation and length of hospital stay were significantly longer, but there was no difference in 90-day mortality (Table 1 ).
Fig 1.
Daily cisatracurium consumption in COVID-19 ARDS patients with qualitative TOF monitoring (TOF group) or without TOF (control group). Data are expressed as median [IQR]. ARDS, acute respiratory distress syndrome; TOF, train-of-four; IQR, inter-quartile range. Wilcoxon–Mann–Whitney test comparison.
Table 1.
Clinical outcomes of COVID-19 patients requiring mechanical ventilation and cisatracurium infusion with TOF monitoring (TOF group) or without TOF (control group). Data are expressed as median [inter-quartile range] or number (proportion). TOF, train-of-four.
| TOF group (n=86) | Control group (n=35) | P | |
|---|---|---|---|
| Invasive ventilation, days | 19 [12–30] | 13 [8–22] | 0.006 |
| ICU stay, days | 25 [17–40] | 16 [10–30] | 0.011 |
| Mortality at day 90 | 42 (48.8%) | 17 (48.6%) | 0.999 |
Although underpowered, our study demonstrated a clinically relevant (35%) decrease in cisatracurium consumption in patients with neuromuscular block for more than 48 h. These findings may inform decision-making during situations with acute or expected NMBA shortage.
Use of TOF monitoring has been shown to reduce NMBA use hourly and cumulatively with no impairment in quality of neuromuscular block, ventilator and blood gas parameters, or occurrence of adverse events.5 Use of a bundle directed (sedation, clinical assessment, and qualitative TOF-count monitoring) protocol was also beneficial in terms of NMBA dose reduction.6 However, in the beginning of the COVID-19 pandemic, with limited time and material resources, little was known regarding the pathophysiology of severe acute respiratory syndrome coronavirus 2 (SARS-COV-2)-induced lung injury.7 , 8 Physicians may have used greater doses of NMBA because of the frequently observed high respiratory drive in COVID-19 ARDS patients.9
The major limitation of our study is the retrospective case-control design and the absence of sample size calculation. Patients were hospitalised in different ICUs, including temporary units created on demand to increase ICU bed capacity during the COVID-19 outbreak. TOF count may have been estimated but not recorded.
The NMBA-monitoring protocol implemented used a qualitative reading, only estimating TOF count combined with clinical judgement. Poor agreement regarding neuromuscular block quality between solely used clinical assessment and qualitative TOF (number of twitches) alone has been reported.10 However, combined use of qualitative TOF-count monitoring and clinical assessment together may help modify the infusion rate of NMBA in a considerable proportion of paralysed ICU patients, helping to reduce total NMBA dose administered. We did observe such saving in our study.
In conclusion, we observed a 35% reduction of weight-adjusted cisatracurium daily use in patients with TOF-count monitoring. No clinically relevant difference in ventilation related parameters were found in the first 7 days of mechanical ventilation, or in duration of neuromuscular block. Our study supports the use of qualitative TOF monitoring in COVID-19 ARDS patients as part of a strategy to reduce the dose of NMBA used in the context of limited supply. A larger RCT is warranted to study the remote outcomes of mechanical ventilation guided with neuromuscular block in critically ill adult patients.
Declarations of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
The study was supported by Hospices Civils de Lyon.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2021.04.028.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Weber V., Abbott T.E.F., Ackland G.L. Reducing the dose of neuromuscular blocking agents with adjuncts: a systematic review and meta-analysis. Br J Anaesth. 2021;126:608–621. doi: 10.1016/j.bja.2020.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Courcelle R., Gaudry S., Serck N. Neuromuscular blocking agents (NMBA) for COVID-19 acute respiratory distress syndrome: a multicenter observational study. Crit Care. 2020;24:446. doi: 10.1186/s13054-020-03164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welhengama C., Hall A., Hunter J.M. Neuromuscular blocking drugs in the critically ill. BJA Educ. 2021;21(7):P258–P263. doi: 10.1016/j.bjae.2021.02.002. S2058534921000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray M.J., DeBlock H., Erstad B. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. 2016;44:2079–2103. doi: 10.1097/CCM.0000000000002027. [DOI] [PubMed] [Google Scholar]
- 5.Hraiech S., Forel J.-M., Guervilly C. How to reduce cisatracurium consumption in ARDS patients: the TOF-ARDS study. Ann Intensive Care. 2017;7:79. doi: 10.1186/s13613-017-0305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hadique S., Badami V., Forte M. The implementation of protocol-based utilization of neuromuscular blocking agent using clinical variables in acute respiratory distress syndrome patients. Crit Care Explor. 2021;3 doi: 10.1097/CCE.0000000000000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gattinoni L., Chiumello D., Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24:154. doi: 10.1186/s13054-020-02880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schenck E.J., Hoffman K., Goyal P. Respiratory mechanics and gas exchange in COVID-19-associated respiratory failure. Ann Am Thorac Soc. 2020;17:1158–1161. doi: 10.1513/AnnalsATS.202005-427RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esnault P., Cardinale M., Hraiech S. High respiratory drive and excessive respiratory efforts predict relapse of respiratory failure in critically ill patients with COVID-19. Am J Respir Crit Care Med. 2020;202:1173–1178. doi: 10.1164/rccm.202005-1582LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouju P., Tadié J.-M., Barbarot N. Clinical assessment and train-of-four measurements in critically ill patients treated with recommended doses of cisatracurium or atracurium for neuromuscular blockade: a prospective descriptive study. Ann Intensive Care. 2017;7:10. doi: 10.1186/s13613-017-0234-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.