Abstract
The treatment of schizophrenia includes the control of symptoms, the prevention of relapses, and amelioration of adaptive skills for patient re-integration into society. Antipsychotic drugs are the agents of choice for the treatment of schizophrenia, as they reduce the positive symptoms of psychosis. Lurasidone is a second-generation antipsychotic drug representing a novel and useful clinical tool for the management of schizophrenia. A board consisting of a panel of Italian expert psychiatrists was organized with the following aims: (a) defining the current modalities of use of lurasidone, highlighted through 17 specific questions; (b) defining and agreeing the main features of the drug and the principal reasons to suggest its administration.
We established that lurasidone is suggested at any age, with no gender difference, at all stages of the disease. The switch from previous treatments is done primarily because of lack of efficacy as well as poor adherence/tolerability. Lurasidone is among the best-tolerated antipsychotics, and its use is indicated in the presence of different comorbidities. A wide range of dosages is available, allowing safe titration in particular cases, with the highest dose (148 mg) generally used for the treatment of the acute phase. The discontinuation rate due to poor tolerability, low compliance, and interactions with other drugs is very low. Akathisia is the most reported adverse event, but it may be controlled by dose reduction. Lurasidone does not possess a marked sedative action but, in agitated patients, can be associated with sedative drugs, such as benzodiazepines. The most frequent reason for switching to other therapies is the need for long-acting formulations, as in patients at risk of very low adherence or suicide. Lurasidone does not strongly impact metabolism or the cardiovascular system (QT interval), and does not influence the metabolism of other drugs, showing good efficacy and tolerability.
Keywords: adherence, antipsychotic agents, lurasidone, phenotypes, schizophrenia
Introduction
Schizophrenia is a chronic and severe mental illness that affects approximately 20 million people worldwide1; it is characterized by distortions of thinking, perceptions, emotions, language, sense of self, and behavior. Common symptoms include hallucinations (acoustic or visual, with the perception of voices and/or non-existent objects) and fixed and untrue beliefs, or suspicions not shared by others, that persist even in the face of evidence to the contrary.2 Schizophrenic patients also exhibit anomalous behaviors such as aimless wandering, mumbling and disorganized speech; moreover, many have poor self-care and appear unkempt. Finally, the emotional component can express itself by marked apathy or incoherence between reported emotions and expressive manifestations.3
Pathogenesis
It is thought that schizophrenia symptoms represent the consequence of the abnormal function of different neurotransmitters, although the hyperactivity and responsiveness of the dopamine mesolimbic circuit appear to be central for the disease, particularly for the manifestation of positive symptoms.4,5 On the other hand, negative symptoms and cognitive deficits in schizophrenia are probably associated with reduced activity of the mesocortical dopamine system, though other systems may play a role.2,6 Accordingly, a role for serotonin, gamma-aminobutyric acid (GABA) and glutamate has also been put forward. With respect to serotonin, it is known that drugs, such as lysergic acid diethylamide (LSD), that increase brain serotonin levels, produce hallucinations.7 Moreover, blockade of the serotonin receptor, particularly 5HT2A, is a common feature of second-generation antipsychotic drugs (APD) that, also through this mechanism, may lead to an amelioration of negative symptoms and cognitive impairment of schizophrenic patients.7
Furthermore, an unbalance between GABA and glutamate has also been proposed in schizophrenia, which may result in an inadequate control of the glutamatergic projections to the midbrain.8 In this respect, phencyclidine and ketamine, two noncompetitive N-methyl-d-aspartate (NMDA)/glutamate antagonists, induce schizophrenia-like symptoms.9 Moreover, alterations in a number of glutamatergic and GABAergic markers have been identified in schizophrenic patients.6
It is also important to point out that neurotransmitter alterations represent only one aspect of the dysfunction contributing to schizophrenia, since anatomical abnormalities as well as changes in neuronal and synaptic plasticity have been observed in affected individuals.10–12
Treatment
The objectives of the treatment of schizophrenia include the control of symptoms, the prevention of relapses, and the amelioration of adaptive skills so that patients can be socially integrated.12
The first-line psychiatric treatment for schizophrenia is the use of APD, which can reduce positive symptoms. The first generation of antipsychotics (FGAs) became available in the 1960s and are characterized by a potent antagonism at dopamine D2 receptors. While such a mechanism is highly effective in improving positive symptoms, the use of these agents was accompanied by significant side effects, including Parkinsonism and hyperprolactinemia, due to the blockade of D2 receptors in the nigrostriatal and the tuberoinfundibular pathways, respectively.2,13
Second-generation (atypical) antipsychotics (SGAs) were developed in order to improve efficacy and reduce side effects, and, according to the American Psychiatric Association, became the agents of choice in the treatment of schizophrenia.14,15 Indeed, SGAs are preferred over FGAs due to the lower risk of extrapyramidal symptoms, but also for better efficacy on negative symptoms and cognitive deficits,3,12 leading to patients’ appreciation.16 SGAs are highly heterogeneous since most possess a multi-receptor profile, with the opportunity to regulate different neurotransmitter systems and circuits that may be affected in schizophrenia. On the other hand, the use of some SGAs is associated with major side effects at the metabolic level, such as weight gain (even conspicuous), hyperlipidemia and diabetes, which may contribute to the increased risk of cardiovascular death observed in schizophrenic patients.17
Lurasidone
Lurasidone [(3aR,4S,7R,7aS)-2-{(1R,2R)-2-[4-(1,2-benzothiazole-3-yl)piperazin-1-ylmethyl]cyclohexylmethyl}hexahydro-4,7-methano-2H-isoindole-1,3-dione hydrochloride] is a second-generation antipsychotic drug approved by the European Medicines Agency in March 2014 for the treatment of schizophrenia in adult patients (>18 years), and in 2020 for adolescents (>13 years).18 In the United States, lurasidone is approved for the treatment of schizophrenia in patients >13 years and for the treatment of bipolar depression in adults, as monotherapy and as adjunctive therapy with lithium or valproate.19 Indeed, the pharmacodynamics of this SGA may suggest some sort of efficacy on the depressive core of schizophrenia, providing a rationale for its use in bipolar depression.
Like other SGA drugs, lurasidone is a potent antagonist of dopaminergic D2 and serotonergic 5HT2A receptors, but differs in its action on other receptors. In fact, it shows a high affinity for the serotoninergic 5HT7 receptors, an intermediate affinity at 5HT1a receptors and for α2C-adrenergic receptors, but weak affinity for α1 adrenergic and serotonergic 5HT2C receptors. Moreover, lurasidone has no affinity for histaminergic H1 or muscarinic M1 receptors, two receptors that have been associated with important side effects.20–22 Specifically concerning lurasidone role on 5HT7 receptors, there is strong evidence that this drug has the highest affinity for such receptors.20 5HT7 receptors are expressed mainly in the thalamus, hypothalamus, hippocampus, amygdala, and cortex areas, involved in the regulation of sleep, stress, memory and learning, motivation, and cognition.23,24 A drug that acts specifically on a subset of receptors represents a tool for the enhancement of those peculiar cognitive functions in patients by schizophrenia.25
On these bases, in comparison with other atypical antipsychotics, a lower incidence of cardiometabolic side effects, such as weight gain and QTc prolongation, has been observed with lurasidone.26
Lurasidone’s approval as a treatment for schizophrenia was based on the results of a clinical development program that included five similarly designed, 6-week-long, fixed-dose, placebo-controlled trials,27–31 and several long-term maintenance studies.31–34 Lurasidone has been shown to be more effective than placebo in the short term in the treatment of acute episodes in schizophrenic patients, with a significantly rapid time of onset of action (day 3 to day 7).27–29 The drug was found to be superior to quetiapine and placebo on cognitive function in a 6-week acute study along with a 6-month blinded extension.35
In long-term, double-blind comparison studies, lurasidone has been shown to be non-inferior to risperidone and to quetiapine.34,36,37 Open-label extension studies have demonstrated the safety of lurasidone and its capacity to continue to reduce the Positive and Negative Syndrome Scale (PANSS) over time.32,34,36 Some authors have also reported a reduction in depressive symptoms.29 Lurasidone has a good safety profile. It is distinguished from other second-generation antipsychotics by a favorable metabolic and cardiovascular safety profile.38 However, there appears to be a significant, albeit moderate, association with the onset of akathisia, extrapyramidal symptoms, and hyperprolactinemia at the start of treatment.38–40
Lurasidone is used in Italy in both acute emergencies (psychotic crisis) and in chronic, maintenance therapy for schizophrenic patients.
A project with a panel of expert psychiatrists was organized to identify the current modalities of prescription of lurasidone in Italy. The choice of lurasidone was done in dependence of the increasing use of the drug and the relative fewer publications available on its use. The final aim of the project was to identify patients with the most suitable characteristics for the clinical use of lurasidone.
Methods
The project had two aims: (a) to define the current modalities of use of lurasidone; and (b) to reach an agreement on the main features of the drug and the principal settings for its recommendation. The board included the presence of four scientific coordinators and a facilitator. Two groups of specialists (six from Northern Italy and seven from Southern Italy, with four scientific coordinators) joined two separate meetings. During each meeting, the participants were asked a series of 17 questions concerning their typical “lurasidone patient” and the situations of its clinical use. The questions were developed by the group of four scientific coordinators who took part in the meetings. The questions concerned patients’ sociodemographic characteristics (gender, age), disease stage (onset, acute exacerbation, maintenance), previous treatments and compliance/adherence to the therapies, and possible comorbidities, in particular, metabolic. Other questions explored the most frequent dosages of lurasidone adopted and the possible reasons for treatment withdrawal and contraindications. Each question was associated with 3–5 answers from which the participants had to choose. The results were expressed as percentages.
The results were analyzed and discussed, under the supervision of the scientific coordinators. The outcome of the survey allowed the definition of lurasidone use in the real world.
In the second part of the meeting, the Nominal Group Technique (NGT), a structured method for group brainstorming that encourages contributions from everyone and facilitates quick agreement on the relative importance of issues, problems, or solutions, was applied.41 This meeting was held with the participation of the four scientific coordinators and also included a neuropharmacologist, to enable a deeper look into the pharmacological characteristics of lurasidone.
Each participant was asked to list, individually and anonymously, the main factors that, in her/his opinion, better characterize lurasidone and encourage its recommendation to schizophrenic patients. After listing all identified factors, each participant was asked to give a relevance score of each factor anonymously from 1 (not at all relevant) to 9 (of maximum relevance). The resulting rating allowed the definition of a hierarchy of factors driving therapeutic choice. The outcome was discussed by the scientific coordinators, together with the participants, to reach a definitive and shared version of the final document.
The aim of this part of the meeting was to define what most characterizes lurasidone in the physician’s vision and to identify the major indications for its use.
The results of the two workshops were then presented and discussed during a third meeting, at the end of which it was possible to reach a shared result.
Results
Part I: identification of patients
The first set of questions investigated the demographic characteristics of patients; setting of the disease; comorbidities; dosages; and tolerability, compliance, and interactions (Figures 1–6).
Figure 1.
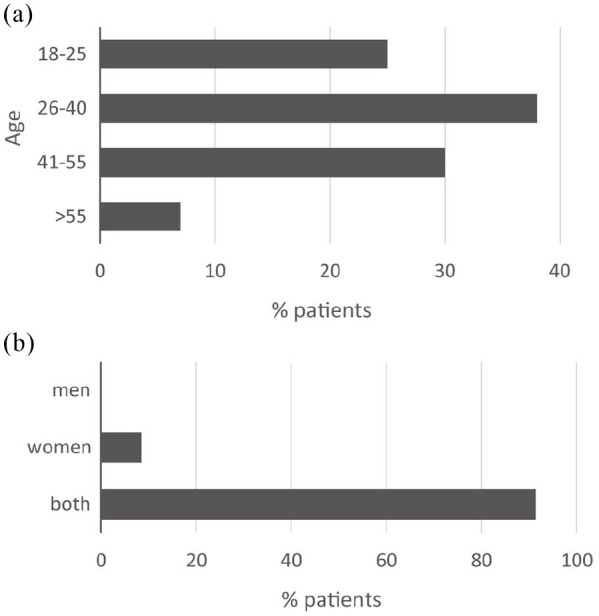
Demographic characteristics of patients prescribed with lurasidone. (a) Age distribution. (b) Gender.
Figure 2.
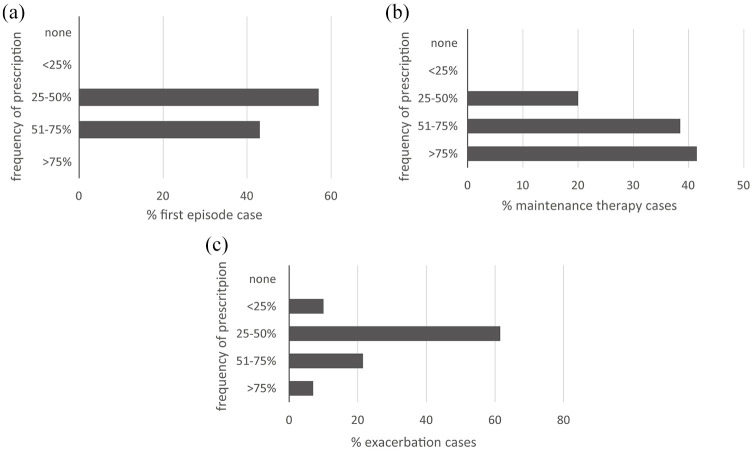
Indications for lurasidone prescription. (a) First episode*. (b) Maintenance therapy. (c) Exacerbation.
*Results available only from the Northern Italy session.
Figure 3.
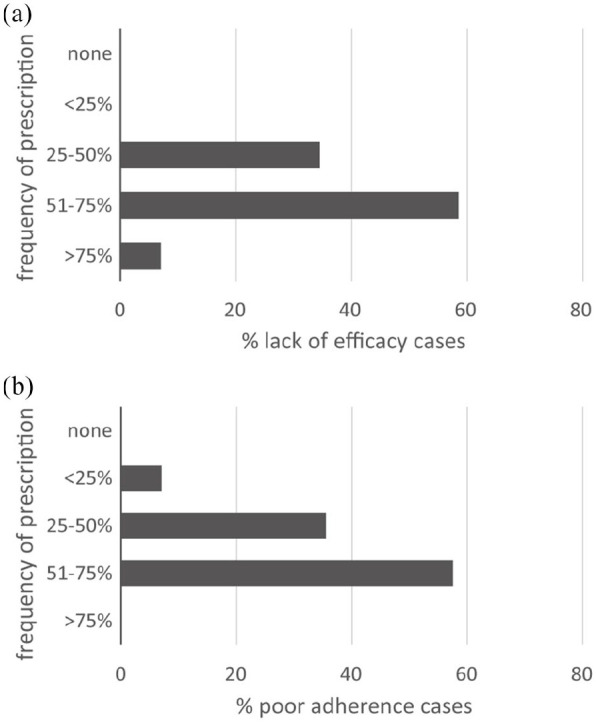
Reasons for switching to lurasidone from other therapies. (a) Lack of efficacy. (b) Poor adherence.
Figure 4.
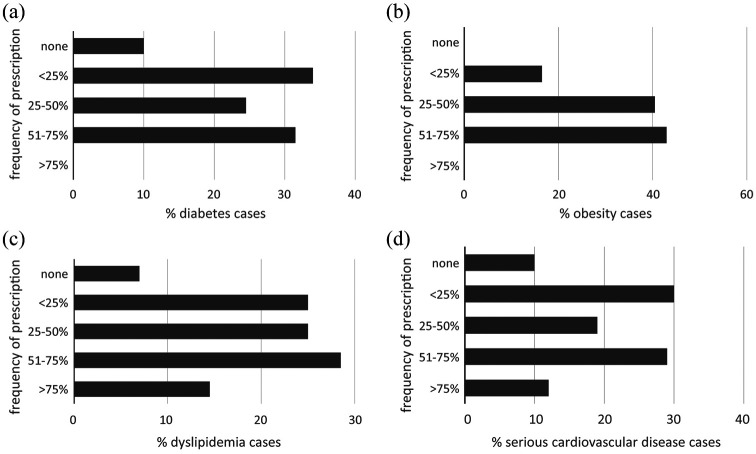
Percentage of lurasidone prescriptions according to comorbidities. (a) Diabetes. (b) Obesity. (c) Dyslipidemias. (d) Serious cardiovascular disease.
Figure 5.
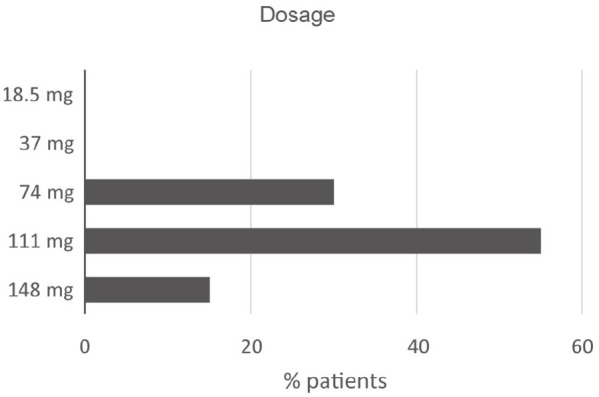
Most frequent lurasidone dosages.
Figure 6.
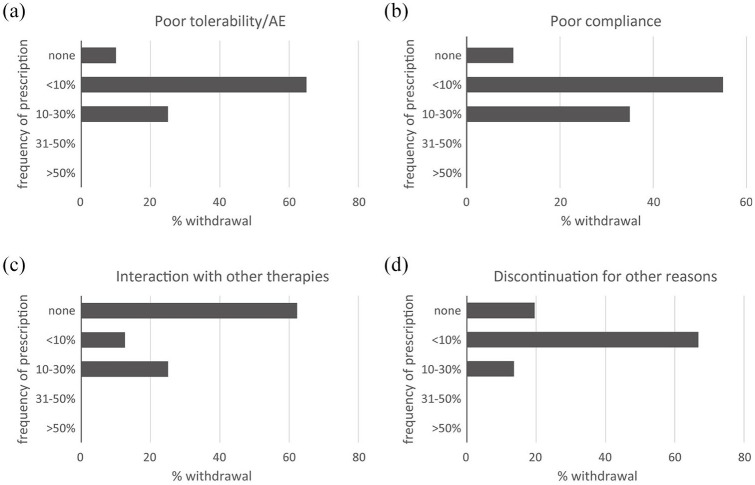
Reasons for discontinuation of lurasidone. (a) Poor tolerability, adverse events. (b) Poor compliance. (c) Interactions with other therapies. (d) Other.
Patient demographics
The demographic characteristics of patients prescribed with lurasidone are shown in Figure 1a,b.
From the answers provided, it is evident that lurasidone is recommended to any age group, even in young patients where it has proven to be effective, especially on cognitive aspects, and where the prospect of a long duration of therapy makes its tolerability of particular importance.42 The drug is indicated in all intervals of age because, as recently underlined, the tolerability and efficacy seem similar in young and older patients.43,44 The reported data reflect the epidemiological distribution of patients referred to the public healthcare centers the specialists belong to. In these centers, younger patients are less represented compared with those spanning the range 26–40 and 41–55 years. These patients, together with the >55-year age group, are most likely already subjected to a therapy (FGAs and SGAs) and lurasidone can be given as a switch from previous therapy.
The age group around 40 years is the one most affected by negative and cognitive symptoms and by anhedonia: these are the cases in which lurasidone may show larger benefits due to its high affinity for 5-HT7 receptors, whose modulation is associated with significant antidepressant and pro-cognitive effects.45,46
The recommendation of lurasidone to younger patients may be limited by the lack of a long-acting injectable (LAI) lurasidone formulation; often patients in their first episode have poor insight, are non-adherent to oral formulations, and may need a LAI antipsychotic.
Gender appears not to influence the clinical use at all. However, the lack of effect on body weight makes lurasidone particularly indicated and appreciated by women.
Disease setting
The indications for the use of lurasidone in first-episode schizophrenia, as maintenance therapy, and in exacerbations are shown in Figure 2a–c.
These results indicate that lurasidone is suggested at any stage of the disease. In particular, based on the low propensity to induce sedation, lurasidone can be given at the onset of the disease. When a sedative effect is required during an exacerbation of schizophrenia, benzodiazepines may be added to lurasidone. It may be better to use an antipsychotic without a sedative effect because in the following stages of illness, during maintenance phases, add-on treatments may be withdrawn. Choosing an antipsychotic with a sedative effect may instead be associated with persistent side effects (because of a higher H1 affinity), which may hamper adherence over the long term. When necessary, rapid dose titration of lurasidone up to 148 mg/day may be used to reach a relief of positive symptoms and agitation. The dosage and the route of administration of the other drugs however, vary according to whether the patient is hospitalized in a psychiatric ward (rapid transition to high doses, intramuscular/intravenous drug administration) or is an outpatient (slower titration, oral drug administration). Overall, all of the participants consider the immediate start of lurasidone therapy to be crucial, thanks to its good tolerability. The first subjective experience of the patient will likely influence the overall adherence to future therapy.
After the first usage, lurasidone therapy is easily maintained. Cases of a switch to another therapy are rare and depend mainly on the onset of adverse events. Moreover, lurasidone is also suggested for a possible exacerbation phase when there is an inadequate response or tolerability to other drugs.
Switching from previous treatments
Figure 3a,b shows the reasons for switching to lurasidone from other therapies because of lack of efficacy (a) or poor adherence (b).
Despite being a drug used widely in treatment-naïve patients, lurasidone is also often used after discontinuation of other drugs due to various reasons, including lack of efficacy as well as reduced adherence to previous treatments. The lack of adherence to treatment is an important motivation for the switch to lurasidone: this may be ascribed largely to poor tolerability and onset of adverse events with other drugs.
Comorbidities
Figure 4a–d shows the distribution of prescribing for lurasidone according to the presence of comorbidities.
Among antipsychotic agents, lurasidone has the lowest metabolic impact. For this reason, it is the drug of choice in patients with metabolic syndrome, diabetes, dyslipidemia, and obesity, as well as in patients with a history of cardiovascular disease. This group of answers confirms this statement as the drug is frequently used in patients with these comorbidities resulting not suggested only to 10% of the patients with diabetes or cardiovascular disease, to 7% of dyslipidemic patients, while it was always prescribed to obese individuals. The latter case is of particular interest as lurasidone was shown to induce a loss of weight in obese patients, as opposed to the outcome with olanzapine or quetiapine.47,48
Dosage
The most frequent lurasidone dosage levels are shown in Figure 5.
There is a good agreement on the dosage used: it is evident that low dosages (18.5–37 mg) are unusual. However, the dose of 37 mg can be useful in patients with affective-negative symptoms, without any occurrence of productive symptoms. There is evidence of a good outcome by the off-label use of low dosages in bipolar syndrome.
Conversely, all the range of intermediate dosages (74–111 mg, with a marked action on dopaminergic and 5-HT7 serotoninergic receptors) is widely suggested, with an optimal efficacy based on clinical conditions, such as stage of disease, symptom pattern, and previous treatments.
The highest dosage (148 mg) has a prevalent action on the dopaminergic system and is administered less frequently, generally for the acute phases and in the case of severe symptoms. Also, this high dosage is very well tolerated.
Overall, lurasidone is used at low dosage at the beginning of therapy, with a gradual increase after the first days of use. In case of an inappropriate response, the dose increment is achieved in 5–6 days. A higher dosage can be required to reach a therapeutic effect in case of switching from other drugs like haloperidol. The broad range of dosages is very useful in case of polytherapy, as it happens with the association with benzodiazepines, that is, when the patient experiences a rebound following discontinuation of a previous therapy.
Tolerability, compliance, and interactions
Figure 6a–d shows the reasons why patients discontinued lurasidone.
All participants consider the rate of discontinuation of lurasidone therapy very low, and the three reasons listed in the questions (poor tolerability, low compliance, and interactions) very rare. Among adverse events, akathisia can occur but it can be easily controlled. Lurasidone does not have a marked sedative action, but, in particularly agitated patients, the association with more sedative drugs, such as benzodiazepines, can be used. The most frequent reason for switching to other therapy is the need for long-acting formulations, for example, in patients at risk for very low adherence or suicide.
Part II: characterization of phenotypes
From the descriptions of the patients treated by the clinicians participating in this project, it appears that most schizophrenic patients can benefit from treatment with lurasidone. However, the panel has identified certain clinical characteristics in which the drug appears particularly useful and indicated.
In schizophrenia, cognitive symptoms are common and consist of disorganized thinking, difficulty in understanding and concentration, and poor memory. In these patients, all the participating physicians agreed that lurasidone is particularly effective, as confirmed by their clinical practice.
In the experience of the participating physicians, the percentage of patients with metabolic diseases is very high: diabetes, dyslipidemia and obesity are common conditions in schizophrenic patients, and, in recent years, cardiovascular mortality has overtaken suicide as the most common cause of premature death in patients with schizophrenia.49 When compared with other antipsychotic drugs, lurasidone has the lowest impact on metabolic dysfunction, and it does not impact the QT interval.50 For this reason, it is the drug of choice in patients with metabolic disorders and cardiovascular risk. Lurasidone does not cause weight gain, and patients with obesity caused by previous therapies can sometimes experience a weight loss when shifted to lurasidone.
The wide range of available dosages makes lurasidone very versatile. Schizophrenic patients are often treated with many drugs, and therefore require treatments, such as lurasidone, that do not present significant drug–drug interactions. In this context, the use of lurasidone can be suggested due to the broad range of dosages available, which can be modified easily in order to target the specific needs of each patient.
The drug is used both in early and in maintenance therapy, thanks to its excellent tolerability profile, which allows it to be taken over the long term.
Overall, the participants agreed that schizophrenic patients show multiple and heterogeneous manifestations of the different domains of the disease, and, within the same patient, clinical conditions can be different. With respect to this, the study’s participants agreed that lurasidone is a drug that can be used in multiple situations due to its effectiveness on a wide range of symptoms, particularly on cognitive dysfunction, as well as for its tolerability, in particular the extreme safety on metabolic and cardiovascular complications.
In patients with a prevalence of negative symptoms, other drugs may be the first choice, although lurasidone can be used in association.
Very agitated patients can be treated with lurasidone in combination with sedative drugs, such as benzodiazepines, which can be removed once the acute situation has been cleared. Lurasidone is therefore suitable for use in polytherapy in specific conditions, with the possibility of going back to monotherapy once the emergency situation has been solved.
Discussion
With this project, a group of psychiatrists expert in schizophrenia had the opportunity to discuss the features of patients who may benefit from the prescription of lurasidone. Indeed, in the lack of specific real-world data concerning the use of lurasidone, the opinion of a panel of experts daily facing the necessity to make decisions regarding patient treatment could be of practical help to clinicians.
Based on such discussion, a range of important and useful information on the use of the drug has emerged. Indeed, the administration of lurasidone is suggested for a wide range of patients, including young subjects,44 with no gender differences. Lurasidone has been shown to improve a wide range of symptoms, including cognitive, affective, and negative symptoms, and it may also ameliorate anhedonia.34 The pharmacological and clinical features of the drug allow its use from the first onset of symptoms to maintenance therapy. Although there are no current real-world data supporting the specific use of this drug both in terms of dosage range and the peculiar clinical cases, a recent Expert Opinion underlines the broad spectrum of symptoms that are addressed by lurasidone, which may facilitate its adoption as monotherapy for long-term maintenance.38
In addition, lurasidone may represent the drug of choice for patients who need a switch from a previous treatment. Indeed, lurasidone shows an excellent response for patients presenting low emotionality with the use of other drugs. The switch to lurasidone has been reported to produce a significant improvement of such critical domain, as described by patients. The low discontinuation rate, together with a good overall outcome in psychopathology (including improvements in both positive and negative symptoms) were shown by a meta-analysis on eight randomized controlled trials on the use of lurasidone in patients with acute schizophrenia.51 This analysis showed that, besides the positive results in the treatment, one of the side effects was weight gain. Actually, the increment in weight was not significant compared with placebo, and the trials considered in the analysis were all short-term studies. The effect on weight gain needs to be considered in a wider range of patients, with a longer follow-up time.
In specific clinical cases, such as obese patients, the switch from quetiapine or olanzapine to lurasidone can greatly improve the weight gain and dyslipidemia that can develop as a consequence of the treatment with such drugs.30,31,37 Moreover, the prolactin increase with lurasidone is mild and significantly lower when compared with that observed following treatment with haloperidol and risperidone.52 The overall good safety profile of lurasidone in comparison with other antipsychotics is also highlighted by a large network meta-analysis analyzing not only efficacy but a great variety of side-effects and outcomes, such as depression, quality of life, and social functioning.53 A recently published extension study confirmed the good tolerability profile of lurasidone, particularly on weight gain and metabolic dysfunction as well as on prolactin elevation. The same study highlighted the good outcome obtained when considering the same parameters (weight, metabolism, and prolactin) after the switch from 12-month use of risperidone,36 pointing to a solid rationale for the switch to lurasidone or to its use as a first treatment in patients with comorbidities. The aforementioned network meta-analysis confirmed the moderate incidence on weight gain resulting from the use of lurasidone, accounted as one of the best three drugs for metabolic outcomes.53 Of note, lurasidone was the antipsychotic with the safest cardiovascular profile, as shown by the lowest effect on QTc prolongation.53 Concerning the cardio-metabolic effects (QTc prolongation and weight gain) and social functioning outcomes, the profile of lurasidone was even better than that of the third-generation antipsychotic aripiprazole. In several other areas (positive and negative symptoms, depression) these two drugs’ effect are almost overlapping. Giving the similar outcome on overall efficacy, the results in cardio-metabolic areas and social functioning are considered pivotal to therapy decisions, as they converge to affect improvements in a patient’s quality of life.
Overall, based on its efficacy and safety profiles, the use of lurasidone as a first-line treatment for schizophrenic patients will eventually require less need to switch to another drug. It is worth mentioning the lack of a long-acting formulation as a possible downside for the use of lurasidone, which may be particularly useful in specific subgroups of patients.
An evaluation of the dose-response for the diverse antipsychotics, including lurasidone, is reported in a recent meta-analysis.54 This study identified 147 mg/day as the 95% effective dose for the drug, as deduced by the reduction of both positive and negative symptoms expressed by the Positive and Negative Syndrome Scale. Other reports account for the wide range of available dosages for lurasidone, allowing its versatile use in clinical practice, favoring the treatment of a variety of different situations with good efficacy. According to real-world data, published in 2018 by Osborne, the highest therapy discontinuation for lack of efficacy occurred with low dosages.55 While, in clinical practice, the intermediate dose appears to be the most useful, the possibility to use a wide range of doses allows the adjustment of the treatment in the different phases of the disease, using a higher dose at the beginning (exacerbation phase), followed by a step-by-step reduction during the maintenance phase. On the other hand, the lower dose (37 mg) may be particularly useful in patients with a predominant emotional component.
The use of the NGT allowed experts to share and agree on clinical situations observed in schizophrenic patients. By analyzing their different experiences, the expert panel examined the possible use of lurasidone in subgroups of patients with peculiar underlying conditions.
Lurasidone appears to be particularly effective on a group of symptoms, such as disorganized thinking, difficulty in understanding and concentration, poor memory, as witnessed both by data obtained in clinical trials and in the real-world setting.30,35,55 This effect appears to be related primarily to potent antagonism at serotonin 5-HT7 as well as to the agonism at 5-HT1A receptors, which are known to exert a beneficial effect on cognitive function.56
Although the available evidence regarding interactions with other drugs is limited, this possibility cannot be ruled out completely. This necessity demands more studies, since the pattern of lurasidone is suggested to be similar to that of quetiapine.57 Attention should be paid to the concomitant administration of strong cytochrome P450 3A4 inhibitors or inducers,58 as reported in the related SmPC,59 and in a recent review that showed the effect of concomitant administration of some anti-infective agents on lurasidone metabolism, underlining the supposed similarity with quetiapine and cariprazine.60 Lastly, akathisia is a possible side effect described for the drug, reported in some cases either by naïve patients or following the switch from another treatment. This condition can be solved by the adoption of concomitant treatment with benzodiazepines or β-blockers.
Conclusions
Different schizophrenia phenotypes have been proposed, as defined by symptoms as well as psychological, electrophysiological, biochemical, or simply clinical features. The definition and characterization of these phenotypes into biologically and pharmacologically significant groups is underway. The overall importance of this task lies in the definition of molecular targets of the disease to facilitate rational pharmacological development as well as better use of available drugs. The ability to target specific pathophysiological domains of schizophrenia may ultimately lead to a significant improvement of disease outcomes. From this point of view, lurasidone appears to be a drug with excellent efficacy and tolerability, which allows its use in a wide range of patients.
Acknowledgments
We thank Ray Hill, an independent medical writer, who provided English-language editing and journal styling prior to submission on behalf of Health Publishing & Services Srl. This assistance was funded by Angelini S.p.A., Italy.
Footnotes
Conflict of interest statement: Marco Andrea Riva has received compensation as speaker/consultant from Angelini, Lundbeck, Otzuka, Recordati and Sumitomo Dainippon Pharma, and he has received research grants from Sumitomo Dainippon Pharma and Sunovion. Umberto Albert is/has been a consultant and/or a speaker for Angelini, Neuraxpharm, Janssen-Cilag, Lundbeck, InnovaPharma. Sergio de Filippis declares no conflict of interests. Antonio Vita, in the last 2 years, has received, directly or indirectly, support for clinical studies or trials, conferences, consultancies, Congress presentations, advisory boards from: Angelini, Boehringer Ingelheim, Fidia, Innovapharma, Janssen- Cilag, Lundbeck, Otsuka, Pfizer, Recordati, Roche, Takeda. Domenico De Berardis received in the last years, directly or indirectly, support for clinical studies or trials, conferences, consultancies, congress presentations, advisory boards from: Eli-Lilly, FB Health, Janssen-Cilag, Lundbeck, Otsuka, Pfizer, Italfarmaco, Angelini, Abbot, Recordati, Laborest, Neuraxpharm, IQVIA
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Domenico De Berardis  https://orcid.org/0000-0003-4415-5058
https://orcid.org/0000-0003-4415-5058
Contributor Information
Marco Andrea Riva, Department of Pharmacological and Biomolecular Sciences, University of Milan, Milano, Italy; Biological Psychiatry Laboratory, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy.
Umberto Albert, Department of Medicine, Surgery and Health Sciences, University of Trieste, Trieste, Friuli-Venezia Giulia, Italy; Azienda Sanitaria Universitaria Giuliano-Isontina - ASUGI, Clinica Psichiatrica, Trieste, Italy.
Sergio de Filippis, Villa Von Siebenthal Neuropsychiatric Clinic, Genzano, Roma, Italy.
Antonio Vita, Department of Clinical and Experimental Sciences, University of Brescia, Brescia, Italy; Department of Mental Health and Addiction Services, Spedali Civili Hospital, Brescia, Italy.
Domenico De Berardis, NHS, Department of Mental Health, Hospital “G. Mazzini”, ASL 4, Teramo, 64100, Italy.
References
- 1. GBD 2017. Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet 2018; 392: 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marder SR, Cannon TD. Schizophrenia. N Engl J Med 2019; 381: 1753–1761. [DOI] [PubMed] [Google Scholar]
- 3. Patel KR, Cherian J, Gohil K, et al. Schizophrenia: overview and treatment options. P T 2014; 39: 638–645. [PMC free article] [PubMed] [Google Scholar]
- 4. Schwartz JH. Neurotransmitters. In: Kandel E, Jessell T, Siegelbaum S, et al. (eds) Principles of neural science. New York, NY: McGraw-Hill, 2013, pp.289–305. [Google Scholar]
- 5. Sthal SM. (ed.). Psychosis and schizophrenia. In: Essential psychopharmacology. Cambridge: Cambridge University Press, 2000, pp.365–399. [Google Scholar]
- 6. Marsman A, Mandl RC, Klomp DW, et al. GABA and glutamate in schizophrenia: a 7 T 1 H-MRS study. Neuroimage Clin 2014; 6: 398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lavretsky H. History of schizophrenia as a psychiatric disorder. In: Mueser K, Jeste D. (eds) Clinical handbook of schizophrenia. New York, NY: Guilford Press, 2008, pp.3–12. [Google Scholar]
- 8. McCutcheon RA, Krystal JH, Howes OD. Dopamine and glutamate in schizophrenia: biology, symptoms and treatment. World Psychiatry 2020; 19: 15–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 1999; 20: 201–225. [DOI] [PubMed] [Google Scholar]
- 10. Ren W, Lui S, Deng W, et al. Anatomical and functional brain abnormalities in drug-naive first-episode schizophrenia. Am J Psychiatry 2013; 170: 1308–1316. [DOI] [PubMed] [Google Scholar]
- 11. Crabtree GW, Gogos JA. Synaptic plasticity, neural circuits, and the emerging role of altered short-term information processing in schizophrenia. Front Synaptic Neurosci 2014; 6: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crismon L, Argo TR, Buckley PF. Schizophrenia. In: Dipiro JT, Talbert RL, Yee GC, et al. (eds) Pharmacotherapy: a pathophysiologic approach. New York, NY: McGraw-Hill Medical, 2014, pp.1147–1172. [Google Scholar]
- 13. Weinstein JJ, Chohan MO, Slifstein M, et al. Pathway-specific dopamine abnormalities in schizophrenia. Biol Psychiatry 2017; 81: 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry 2004; 161: 1–56. [PubMed] [Google Scholar]
- 15. Moore TA, Buchanan RW, Buckley PF, et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. J Clin Psychiatry 2007; 68: 1751–1762. [DOI] [PubMed] [Google Scholar]
- 16. Awad AG, Voruganti LN. The impact of newer atypical antipsychotics on patient-reported outcomes in schizophrenia. CNS Drugs 2013; 27: 625–636. [DOI] [PubMed] [Google Scholar]
- 17. Raedler TJ. Cardiovascular aspects of antipsychotics. Curr Opin Psychiatry 2010; 23: 574–581. [DOI] [PubMed] [Google Scholar]
- 18. European Medicines Agency. Latuda (lurasidone hydrochloride) 18.5 mg film-coated tablets: summary of product characteristics, https://www.ema.europa.eu/2020 (2020, accessed 12 December 2020).
- 19. U.S. Food and Drug Administration. LATUDA (lurasidone hydrochloride) tablets, for oral use. Highlights of prescribing information, https://www.acessdata.fda.gov2010 (2010, accessed 12 December 2020).
- 20. Ishibashi T, Horisawa T, Tokuda K, et al. Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J Pharmacol Exp Ther 2010; 334: 171–181. [DOI] [PubMed] [Google Scholar]
- 21. Samalin L, Garnier M, Llorca PM. Clinical potential of lurasidone in the management of schizophrenia. Ther Clin Risk Manag 2011; 7: 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tarazi FI, Riva MA. The preclinical profile of lurasidone: clinical relevance for the treatment of schizophrenia. Expert Opin Drug Discov 2013; 8: 1297–1307. [DOI] [PubMed] [Google Scholar]
- 23. Thomas DR, Atkinson PJ, Hastie PG, et al. [3H]-SB-269970 radiolabels 5-HT7 receptors in rodent, pig and primate brain tissues. Neuropharmacology 2002; 42: 74–81. [DOI] [PubMed] [Google Scholar]
- 24. Varnas K, Thomas DR, Tupala E, et al. Distribution of 5-HT7 receptors in the human brain: a preliminary autoradiographic study using [3H]SB-269970. Neurosci Lett 2004; 367: 313–316. [DOI] [PubMed] [Google Scholar]
- 25. Terry AV, Jr, Buccafusco JJ, Wilson C. Cognitive dysfunction in neuropsychiatric disorders: selected serotonin receptor subtypes as therapeutic targets. Behav Brain Res 2008; 195: 30–38. [DOI] [PubMed] [Google Scholar]
- 26. Leucht S, Leucht C, Huhn M, et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: systematic review, bayesian meta-analysis, and meta-regression of efficacy predictors. Am J Psychiatry 2017; 174: 927–942. [DOI] [PubMed] [Google Scholar]
- 27. Ogasa M, Kimura T, Nakamura M, et al. Lurasidone in the treatment of schizophrenia: a 6-week, placebo-controlled study. Psychopharmacology (Berl) 2013; 225: 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakamura M, Ogasa M, Guarino J, et al. Lurasidone in the treatment of acute schizophrenia: a double-blind, placebo-controlled trial. J Clin Psychiatry 2009; 70: 829–836. [DOI] [PubMed] [Google Scholar]
- 29. Nasrallah HA, Silva R, Phillips D, et al. Lurasidone for the treatment of acutely psychotic patients with schizophrenia: a 6-week, randomized, placebo-controlled study. J Psychiatr Res 2013; 47: 670–677. [DOI] [PubMed] [Google Scholar]
- 30. Meltzer HY, Cucchiaro J, Silva R, et al. Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study. Am J Psychiatry 2011; 168: 957–967. [DOI] [PubMed] [Google Scholar]
- 31. Loebel A, Cucchiaro J, Xu J, et al. Effectiveness of lurasidone vs. quetiapine XR for relapse prevention in schizophrenia: a 12-month, double-blind, noninferiority study. Schizophr Res 2013; 147: 95–102. [DOI] [PubMed] [Google Scholar]
- 32. Stahl SM, Cucchiaro J, Simonelli D, et al. Effectiveness of lurasidone for patients with schizophrenia following 6 weeks of acute treatment with lurasidone, olanzapine, or placebo: a 6-month, open-label, extension study. J Clin Psychiatry 2013; 74: 507–515. [DOI] [PubMed] [Google Scholar]
- 33. Tandon R, Cucchiaro J, Phillips D, et al. A double-blind, placebo-controlled, randomized withdrawal study of lurasidone for the maintenance of efficacy in patients with schizophrenia. J Psychopharmacol 2016; 30: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Citrome L, Cucchiaro J, Sarma K, et al. Long-term safety and tolerability of lurasidone in schizophrenia: a 12-month, double-blind, active-controlled study. Int Clin Psychopharmacol 2012; 27: 165–176. [DOI] [PubMed] [Google Scholar]
- 35. Harvey PD, Ogasa M, Cucchiaro J, et al. Performance and interview-based assessments of cognitive change in a randomized, double-blind comparison of lurasidone vs. ziprasidone. Schizophr Res 2011; 127: 188–194. [DOI] [PubMed] [Google Scholar]
- 36. Mattingly GW, Haddad PM, Tocco M, et al. Switching to Lurasidone following 12 months of treatment with risperidone: results of a 6-month, open-label study. BMC Psychiatry 2020; 20: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Loebel A, Cucchiaro J, Sarma K, et al. Efficacy and safety of lurasidone 80 mg/day and 160 mg/day in the treatment of schizophrenia: a randomized, double-blind, placebo- and active-controlled trial. Schizophr Res 2013; 145: 101–109. [DOI] [PubMed] [Google Scholar]
- 38. Javed A, Arthur H, Curtis L, et al. Practical guidance on the use of lurasidone for the treatment of adults with schizophrenia. Neurol Ther 2019; 8: 215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sacchetti E, Antonio V. Oral lurasidone for people with schizophrenia: clinical practice recommendations emerging from a review of the literature. Evid Based Psychiatric Care 2016; 2: 32–52. [Google Scholar]
- 40. Demyttenaere K, Detraux J, Racagni G, et al. Medication-induced akathisia with newly approved antipsychotics in patients with a severe mental illness: a systematic review and meta-analysis. CNS Drugs 2019; 33: 549–566. [DOI] [PubMed] [Google Scholar]
- 41. Manera K, Hanson CS, Gutman T, et al. Consensus methods: nominal group technique. In: Liamputtong P. (ed.) Handbook of research methods in health social sciences. Singapore: Springer, 2019, pp.737–750. [Google Scholar]
- 42. Goldman R, Loebel A, Cucchiaro J, et al. Efficacy and safety of lurasidone in adolescents with schizophrenia: a 6-week, randomized placebo-controlled study. J Child Adolesc Psychopharmacol 2017; 27: 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Potkin S, Michael T, Pikalov A, et al. Safety and tolerability of lurasidone in older adults with schizophrenia: analysis of 7 placebo-controlled studies. In: Proceedings of the 29th annual US psychiatric & mental health congress, San Antonio, TX, 21–24 October 2016. [Google Scholar]
- 44. Arango C, Ng-Mak D, Finn E, et al. Lurasidone compared to other atypical antipsychotic monotherapies for adolescent schizophrenia: a systematic literature review and network meta-analysis. Eur Child Adolesc Psychiatry 2020; 29: 1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Luoni A, Macchi F, Papp M, et al. Lurasidone exerts antidepressant properties in the chronic mild stress model through the regulation of synaptic and neuroplastic mechanisms in the rat prefrontal cortex. Int J Neuropsychopharmacol 2014; 18: pyu061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Calabrese F, Brivio P, Sbrini G, et al. Effect of lurasidone treatment on chronic mild stress-induced behavioural deficits in male rats: the potential role for glucocorticoid receptor signalling. J Psychopharmacol 2020; 34: 420–428. [DOI] [PubMed] [Google Scholar]
- 47. Meyer JM, Ng-Mak DS, Chuang CC, et al. Weight changes before and after lurasidone treatment: a real-world analysis using electronic health records. Ann Gen Psychiatry 2017; 16: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Meyer JM, Mao Y, Pikalov A, et al. Weight change during long-term treatment with lurasidone: pooled analysis of studies in patients with schizophrenia. Int Clin Psychopharmacol 2015; 30: 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J 2005; 150: 1115–1121. [DOI] [PubMed] [Google Scholar]
- 50. Kane JM. Lurasidone: a clinical overview. J Clin Psychiatry 2011; 72(Suppl. 1): 24–28. [DOI] [PubMed] [Google Scholar]
- 51. Zheng W, Cai DB, Yang XH, et al. Short-term efficacy and tolerability of lurasidone in the treatment of acute schizophrenia: a meta-analysis of randomized controlled trials. J Psychiatr Res 2018; 103: 244–251. [DOI] [PubMed] [Google Scholar]
- 52. Loebel A, Citrome L. Lurasidone: a novel antipsychotic agent for the treatment of schizophrenia and bipolar depression. BJPsych Bull 2015; 39: 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet 2019; 394: 939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leucht S, Crippa A, Siafis S, et al. Dose-response meta-analysis of antipsychotic drugs for acute schizophrenia. Am J Psychiatry 2020; 177: 342–353. [DOI] [PubMed] [Google Scholar]
- 55. Osborne IJ, Mace S, Taylor D. A prospective year-long follow-up of lurasidone use in clinical practice: factors predicting treatment persistence. Ther Adv Psychopharmacol 2018; 8: 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Caccia S, Pasina L, Nobili A. Critical appraisal of lurasidone in the management of schizophrenia. Neuropsychiatr Dis Treat 2012; 8: 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Spina E, Hiemke C, de Leon J. Assessing drug-drug interactions through therapeutic drug monitoring when administering oral second-generation antipsychotics. Expert Opin Drug Metab Toxicol 2016; 12: 407–422. [DOI] [PubMed] [Google Scholar]
- 58. Greenberg WM, Citrome L. Pharmacokinetics and pharmacodynamics of lurasidone hydrochloride, a second-generation antipsychotic: a systematic review of the published literature. Clin Pharmacokinet 2017; 56: 493–503. [DOI] [PubMed] [Google Scholar]
- 59. Chiu YY, Ereshefsky L, Preskorn SH, et al. Lurasidone drug-drug interaction studies: a comprehensive review. Drug Metabol Drug Interact 2014; 29: 191–202. [DOI] [PubMed] [Google Scholar]
- 60. Spina E, Barbieri MA, Cicala G, et al. Clinically relevant interactions between atypical antipsychotics and anti-infective agents. Pharmaceuticals (Basel) 2020; 13: 439. [DOI] [PMC free article] [PubMed] [Google Scholar]