Abstract
The utilization of biologically produced cells to treat diseases is a revolutionary invention in modern medicine after chemically synthesized small molecule drugs and biochemically made protein drugs. Cells are basic units of life with diverse functions in mature and developing organs, which biological properties could be utilized as a promising therapeutic approach for currently intractable and incurable diseases. Xenogeneic cell therapy utilizing animal cells other than human for medicinal purpose has been studied as a new way of treating diseases. Xenogeneic cell therapy is considered as a potential regenerative approach to fulfill current unmet medical needs because xenogeneic cells could be isolated from different animal organs and expanded ex vivo as well as maintain the characteristics of original organs, providing a versatile and plenty cell source for cell-based therapeutics beside autologous and allogeneic sources. The swine species is considered the most suitable source because of the similarity with humans in size and physiology of many organs in addition to the economic and ethical reasons plus the possibility of genetic modification. This review discusses the old proposed uses of xenogeneic cells such as xenogeneic pancreatic islet cells, hepatocytes and neuronal cells as a living drug for the treatment of degenerative and organ failure diseases. Novel applications of xenogeneic mesenchymal stroma cells and urothelial cells are also discussed. There are formidable immunological barriers toward successful cellular xenotransplantation in clinic despite major progress in the development of novel immunosuppression regimens and genetically multimodified donor pigs. However, immunological barriers could be turn into immune boosters by using xenogeneic cells of specific tissue types as a novel immunotherapeutic agent to elicit bystander antitumor immunity due to rejection immune responses. Xenogeneic cells have the potential to become a safe and efficacious option for intractable diseases and hard-to-treat cancers, adding a new class of cellular medicine in our drug armamentarium.
Keywords: rejection, xenoantigen, immunosuppression, neoantigen, antitumor immunity
Introduction
Cell therapy is a therapeutic approach that uses a biological product with therapeutic effect, derived from living cells of autologous, allogeneic or xenogeneic sources with the aim of preventing, treating or mitigating a disease1. The U.S. Food and Drug Administration (FDA) also defines somatic-cell therapy as the administration to humans of autologous, allogeneic, or xenogeneic living somatic cells that have been manipulated or processed to change their biologic characteristics2. Cellular therapies involving autologous or allogeneic use of cells have been applied in clinics as approve therapies. Autologous chimeric antigen receptor (CAR) T cell therapy has been validated and effective in treating relapsed and refractory hematological malignancies3,4. and allogeneic (or donor derived) expanded adipose-derived mesenchymal stem/stromal cells (eASCs) are clinically used for the treatment of complex perianal fistulas in adult Crohn’s disease patients5. There are many types of cell therapies in the experimental and clinical stages, paving the medicine revolution of cellular drugs after small molecule drugs and protein drugs. The development of human autologous or allogeneic cell therapy into more clinical applications is limited by the amount and availability of suitable functional and specialized cells to meet the general clinical needs since autologous cells can be only used in one individual and there are limited donated allogeneic cells. In addition, the use of allogeneic stem cell-derived cell is still challenged with the ability to precisely direct stem cells into functional specialized cells. However, xenogeneic cells could be isolated and expanded from primary cultures of tissues, and thus the cell fate is committed and specialized. Hence, xenogeneic cell therapy provides a promising new option by using the cells from different tissues of animals as a source for treating human diseases.
Xenogeneic cell therapy is a form of xenotransplantation, which, according to the definition of FDA, is considered as any procedure that involves the transplantation, implantation or infusion into a human recipient of either (a) live cells, tissues, or organs from a nonhuman animal source, or (b) human body fluids, cells, tissues, or organs that have had ex vivo contact with live nonhuman animal cells, tissues or organs6. Furthermore, based on FDA’s interpretation of Section 506(g) of the Food, Drug, and Cosmetic Act (as added by Section 3033 of the 21st Century Cures Act), xenogeneic cell products may also meet the definition of a regenerative medicine therapy and can be eligible for Expedited Programs for Regenerative Medicine Therapies for Serious Conditions (RMAT) designation7. European Medicines Agency (EMA) also classifies xenogeneic living cells as Advanced Therapy Medicinal Products (ATMPs)8. Organ xenotransplantation has been viewed as a potential solution to the existing shortage of human organs for transplantation, but challenged with immunological barriers cross species for successful clinical application9,10. Xenogeneic cell therapy uses animal cells not solid organs for therapeutic purpose could be a more diverse and feasible medical intervention to treat diseases such as diabetes and some degenerative disorders, as demonstrated in pre-clinical animal models and clinical trials (reviewed in Meier et al.9 and Ekser et al.10). That is because that xenogeneic cells face less rejection responses due to avasculature nature and the time period during cell product manufacturing, allowing for safety check the microbiological status11. In addition, due to the bounty supply of edible animal sources such as pigs, cattle, and sheep for xenogeneic cell production, it is highly feasible clinically to use xenogeneic cells as a therapeutic agent, a drug that could be administered repeatedly and metabolized by the body for the diagnosis, cure, mitigation, treatment, or prevention of diseases as small molecule drugs and protein drugs. And as small molecule drugs and protein drugs, in order to be used in clinics, xenogeneic cells must go through a rigorous evaluation of safety, quality, and effectiveness before they can be utilized to treat diseases.
Porcine Tissues Provide an Ideal Animal Source for Xenogeneic Cell Therapy
From an ethical and societal perspective, swine species is considered as the most potential as the donor species animal source of cells, tissues and organ for safety, effectiveness, ethical, and economic reasons12,13. Their favorable breeding characteristics (large litters and rapid maturation) and feasibility for genetic manipulation to insert or delete genes also help the development of clinical xenotransplantation. The porcine organs and biological systems as well as their functions are very close to those in humans in term of anatomical and physiological similarities. Porcine proteins and tissues have already been applied in clinics, such as prosthetic porcine heart valves, heparin for anti-coagulation, factor VIII to treat hemophilia, and porcine insulin to treat type 1 diabetes14–17. Xenogeneic cell therapy using porcine cells as a donor source has several distinct advantages: First, from a well characterized and identified animal source, xenogeneic cells with specialized characteristics and functions could be isolated and ex vivo expanded from different specific tissues without the need to directed differentiation that is required by allogeneic human stem cells. And second, xenogeneic cells from porcine donors could be well manufactured by raising animals under strict quality controlled conditions and streamlining cell production process to provide safe tissues and consistent, safe cell preparations. The risk of pathogen transmission could be mitigated through raising animals under designated pathogen-free (DPF) conditions and producing cells with good manufacturing practices (GMP) system18,19. Furthermore, precise and effective genetic engineering to genetically modify animals or porcine cells could be achieved by the development of mRNA-based expression and CRISPR-Cas9–based gene editing methodologies20,21.
Old Proposed Uses of Xenogeneic Cells for Treating Diseases
With the advantages of xenogeneic porcine cell therapy, various xenogeneic cell types isolated from different tissues have been tested in preclinical animal experiments and several therapies have advanced from pre-clinical safety and efficacy studies to human clinical trial.
Xenogeneic islet cells for type 1 diabetes (T1D)
It was estimated that more than 463 million people worldwide suffer from diabetes, rising to 10.2% (578 million) by 2030 and 10.9% (700 million) and by 2045 global health expenditure on diabetes was estimated to be USD 727 billion22. T1D caused by destruction of insulin-producing pancreatic islet beta-cells due to irreversible immune-mediated attacks occurs predominantly in children and they require lifelong insulin injection therapy, but tight glucose control remains difficult. Even with optimal insulin therapy, some patient’s unstable insulin levels contribute to end stage micro- and macro-vascular injury and subsequently contribute to end-stage organ failure such as neuropathy, nephropathy, retinopathy and peripheral vascular disease as well as severe hypoglycemic events, causing substantial morbidity and mortality23,24. Islet cell transplantation is a valuable alternative to whole pancreas transplantation to treat diabetes and reduce the life-threatening hypoglycemia unawareness in patients with T1D23,24. Therefore, there is highly unmedical need for T1D patients with frequent hypoglycemic episodes and extreme glycemic lability, so-called “brittle disease.” Cell-based islet cell transplantation therapy with the Edmonton protocol and proper immunosuppression regimens has been demonstrated to long-term insulin independence and decreased risk of life-threatening hypoglycemia for many patients23,24. Although allogeneic islet cell transplantation has been clinically proved a safe and effective treatment for patients with labile T1D, but the shortage of suitable human donor islet cells severely limits the availability of this treatment. Alternative allogeneic stem cell-derived islet cell replacement therapy has been tested in in human clinical trial with inconsistent results25. Paucity of human organ donors has led to the consideration of potential alternative sources of islet cells, with porcine islet cells being attractive candidates for cell therapy. Since porcine insulin has long been used and functions in humans to treat patients before recombinant human insulin became available due to high similarity in porcine insulin protein sequence with only one amino acid residue difference to human insulin, xenogeneic porcine islet cell therapy has long been proposed to treat T1D26–28. Porcine islet cell xenotransplantation has been investigated as a potential bridge or the ultimate treatment for T1D in decades with substantial progresses in both pre-clinical and clinical studies. Xenogeneic islet cell therapy could become a clinically applicable treatment with proper clinically applicable immunosuppression regimens or efficient biocompatible matrix encapsulation devices that permit diffusion of oxygen and nutrients and prevent transplanted islet cells from the host immune attacks26,27. Preclinical pig-to-nonhuman primate transplantation models have been studied and the first long-term graft survival (>6 month) of pig islet cells in non-human primates (NHPs) has been demonstrated29–31. Subsequent study further confirmed reproducible curative potential in NHP model that pig islet grafts survived and maintained normoglycemia for >6 months with the longest up to 603 days32. The clinical studies with xenogeneic porcine islet cells demonstrated the cell survival and function in the human body with no noted serious adverse events, but these clinical islet cell xenotransplantation trials have not shown to durably prevent severe hypoglycemia while maintaining tight glycemic control in T1D patients28,33.
Xenogeneic hepatocytes for liver failures
Acute liver failure and end-stage liver diseases cause the loss of liver functions in carbohydrate, protein, and lipid metabolism as well as the synthesis of coagulation factors, complements, and acute phase proteins. Thus, severe liver failure results in impaired coagulation, hepatic encephalopathy and extrahepatic organ failures with high mortality rate34. Orthotopic liver transplantation (OLT) is recommended with proven clinical benefits and long-lasting effects as a standard of care for acute liver failure, end-stage liver disease, or metabolic defects of the liver -based metabolic disorders. However, because of the donor liver shortage, there is a significant waiting list for patients to get benefit from OLT. A potential solution to dress the shortage of organs for liver transplantation is allogeneic hepatocyte transplantation. Clinical hepatocyte transplantation studies have proven the safety and short-term efficacy of allogeneic hepatocytes in which transplanted cells provide the missing/impaired hepatic function once engrafted into the recipient’s liver, but still its clinical applications require stable supply of allogeneic hepatocytes, high cell engraftment, and long-lasting effects35–37. Xenotransplantation of hepatocytes has numerous potential advantages including only moderate invasiveness of the transplant procedure, low morbidity and high safety, a potentially unlimited supply of cells, the option to protect the cells by encapsulation, the use of genetically modified cells, and the possibility of cryopreserving hepatocytes. The xenogeneic hepatocyte therapy would provide temporary liver support to stabilize metabolic functions while the patient waits for an allotransplant or the regeneration of their own liver. Xenogeneic hepatocyte therapy has been tested in NHP model by transplanting porcine hepatocytes into the spleens of cynomolgus monkeys using conventional immunosuppression to control rejection and xenogeneic hepatocytes functioned for more than 80 days and, following re-transplantation, for more than 253 days based on the detection of porcine albumin in the recipients’ circulation38.
Xenogeneic nerve cells for Parkinson’s disease
Parkinson’s disease (PD), the second most common age-related neurodegenerative disease after Alzheimer’s disease, is a movement disorder due to the death of dopaminergic neurons in the substantia nigra because of misfolding of proteins and dysfunction of the ubiquitin-proteasome pathway or mitochondrial dysfunction and oxidative stress39. Current PD medications only provide symptomatic relief, but have no effects in stopping or even reversing dopaminergic neuron degeneration to improve outcomes40. Fetal/embryonic mesencephalic tissue containing dopamine cells were used to treat patients with the motor disability of advanced Parkinson’s disease by implanting tissues into the caudate and putamen and neurological improvement to some patients with advanced PD was observed41. Induced pluripotent stem cells (iPSCs) are a promising cell source to derive dopaminergic neurons to treat PD patients, which have been tested in a primate PD model, indicating that human iPSC-derived dopaminergic progenitors are clinically applicable for the treatment of patients with PD42. A single patient study using autologous, iPSC-derived dopaminergic progenitor cells for the treatment of progressive idiopathic PD showed cell survival and possible motor function benefit43. Although these exploratory studies using human fetal tissue allografts or stem cell derived dopaminergic neurons suggest that cell therapies have the potential to become an effective treatment for PD patients. However, because of the limited availability of human fetal tissue and ethical concern and the difficulties in controlling the differentiation of stem cells, the use of xenogeneic donor tissue was considered and tested in human clinical trials. The unilateral striatal transplantation of porcine fetal neural cells into PD patients has shown to be safe and clinically improve patient outcomes44. Long-term (over 7 months) graft survival was found with the presence of pig dopaminergic neurons and other pig neural and glial cells in a PD patient with unilateral transplantation of fetal pig neural cells into the caudate-putamen brain region through a post-mortem histological analysis45. Encapsulated Porcine choroid plexus cells from neonatal porcine choroid plexus (CP), an organ that secretes cerebrospinal fluid containing various types of neurotrophic and neuroprotective factors, was first demonstrated to improve neurological functions without requiring immunosuppression in PD disease NHP model46. However, a randomized, double-blind, placebo-controlled human trial on intra-striatal transplantation of encapsulated porcine choroid plexus cells for PD patients showed no evidence that the treatment brought significant clinical benefit in patients with moderately advanced Parkinson’s disease21,47.
Immune-Mediated Barriers for Successful Clinical Xenogeneic Cell Therapy
The successful clinical application of xenogeneic cell therapy faces less immunological obstacles than organ xenotransplantation due to their avasculature nature (the vasculature of implanted xenogeneic cells is formed by the recipient vessels), but there are still formidable xenogeneic immune responses toward xenogeneic cells, first by innate and later by adaptive immunity, stopping cell engraftment, functions and survival48. Immune cell infiltration of tissue and solid organ xenografts starts with neutrophils as an early responding cell population, followed by macrophages and T cells48. The innate immune system, which is composed mainly of phagocytic cells (monocytes, macrophages and neutrophils), natural killer (NK) cells, as well as inflammatory mediator releasing granulocytes (basophils, eosinophils and mast cells), recognizes non-selfness of xenogeneic cells, like pathogen-associated molecular patterns (PAMPs), leading to the activation of innate immune cells49. Neutrophils are directly activated by xenogeneic cells and indirectly through xenoreactive natural antibodies (XNA) and complement to boost immune responses to eradicate xenogeneic cells50. Macrophages express signal-regulatory protein-a (SIRPa), an inhibitory receptor, which recognizes a ubiquitously expressed cell surface protein CD47 “don’t eat me for autologous cells” and controls phagocytic activity of macrophages preventing phagocytosis of autologous cells. The porcine CD47-human SIRPa interaction is not effective to inhibiting macrophage phagocytosis. Therefore, macrophage-mediated rejection leads to destruction of xenogeneic cells51. NK cells are activated by xenoreactive antibodies and the absence of human leukocyte antigen (HLA) for the inhibitory receptor CD94/NKG2A mechanisms to reject xenogeneic cells and human HLA-E/hubeta2 m expression on xenogeneic cells could protect cells from NK cell-mediated cytotoxicity and inhibit the secretion of interferon (IFN)-gamma by NK cells52,53. Humoral graft rejection is triggered by antibodies directed against the xenogeneic cell. These antibodies may be performed or produced de novo by B cells after recognition of xenoepitopes51. T-cell-mediated immunity play a critical role in rejecting xenogeneic cells, which is activated by innate immune cells or direct binding to porcine swine leukocyte antigen (SLA) class 1 and class 2 molecules, or indirectly by recipient antigen-presenting cells (APCs) expressing MHCs with processed xenoantigens48. Indirect T-cell activation triggered by xenogeneic cells is suggested to be more immunogenic than by allogeneic cells, because the large number of xenoantigen peptides are presented by human antigen presenting cells due to interspecies incompatibility of CD4754. T cell-mediated cytotoxicity toward xenogeneic cells is mediated predominantly by xenoreactive CD4+ T cells through the Fas/FasL pathway of apoptosis or other effector mechanisms55. The expression of LEA29Y, a human CTLA4-Ig derivative that binds human B7.1/CD80 and B7.2/CD86 with high affinity and is thus a potent inhibitor of T cell co-stimulation, on xenogeneic cells protects xenogeneic cells from human T cells56. Incidentally, the anti-CTLA4 antibody with the opposite action of CTLA4-Ig derivative is currently used in cancer immunotherapy and has demonstrated substantial and durable effects in patients with advanced melanoma57. This implies that xenogeneic cell could activate the immune responses suppressed by CTLA4 and this function could be used as a way to enhance the priming of immune active T cells.
In addition to cellular rejection, transplanted xenogeneic cells are also the targets of host humoral immune responses as in the case of transplanted porcine islet cells that faces instant blood-mediated inflammatory reaction with the activation of platelets, the coagulation and the complement systems, resulting in islet cell loss58,59. Furthermore, after cell administration, hypoxia due to slow or poor neo-vascularization for implanted cells also causes the loss of cells26. However, if utilizing xenogeneic cells as a drug, despite these barriers, the cells can be administered repeatedly to replenish the loss of cells and maintain the therapeutic effects.
Novel Uses of Xenogeneic Cells
The major uses of cell therapy have been focus on cell replacement to restore the function of failure tissues with the anticipated cell functions. However, the multiple properties of cells beyond anticipated cell functions could be further explored as mechanisms of action for working as therapeutic cell drugs for all autologous, allogeneic and xenogeneic cell therapies. The immunologic barriers have prevented the successful clinical application as proved therapies since the inception of xenotransplantation. However, if the immunological barriers incurred by xenogeneic cells could be tempered, discounted and even taken advantage of, xenogeneic cells could be repeatedly administered to maintain the effectiveness as a drug before the previous cells are completely eliminated due to rejection. More novel uses of xenogeneic cells with multiple cell properties would be possible and make them a new class of drugs to improve patients’ outcomes.
Xenogeneic mesenchymal stroma cell for inflammatory diseases
Allogeneic mesenchymal stem/stromal cells or medicinal signaling cells (MSCs) contain regenerative, anti-inflammatory, and immune modulatory properties have demonstrated promising therapeutic potential in clinical trials as a novel and safe cell therapy to treat a variety of diseases60,61. In a randomized, double-blind, parallel-group, placebo-controlled study, allogeneic, expanded, adipose-derived MSCs for patients with Crohn’s disease and treatment-refractory, draining complex perianal fistulas showed that MSCs appeared to promote healing of perianal fistulas62. Allogeneic MSCs infusion also was shown to be an effective therapy to improve survival for patients with steroid-refractory acute graft-versus-host disease following hematopoietic cell transplantation63,64. Several studies also have demonstrated that systemic administration of allogeneic MSCs was safe with favorable outcome in the treatment for acute respiratory distress syndrome (ARDS)65. Furthermore, a pilot study in China demonstrated that the intravenous infusions of donor allogeneic mesenchymal stem cells in seven severe Coronavirus disease 2019 (COVID-19) patients who had fever, shortness of breath, cough, and oxygen saturation at rest <95% improved functional outcomes of patients as well as changes of inflammatory and immune function levels and the majority of patients showed negative results for the SARS-CoV-2 nucleic acid test over a week or two after MSC infusion66,67.
As for xenotransplantation point of view, there are a lot of animal studies that use human MSCs to treat animals in different disease models without immunosuppression, suggesting that xenogeneic MSCs could still function, regenerate and differentiate as well as be engrafted into the recipients across the species barrier68,69. Xenogeneic MSCs can immuno-modulate human T cells through induction of apoptosis or anergy, or cause T cell phenotype switching with induction of regulatory T cells and show no more immunogenic than allogeneic MSC68,70. Xenogeneic mesenchymal stem cells (MSC) derived from porcine source was shown to suppress human peripheral blood lymphocyte (hPBLs) proliferation through FasL and TGF-beta1 mediated pathways in vitro71. Xenogeneic transplantation of porcine MSCs protected against renal injury and reduced inflammation in lupus nephritis prone mice72. Transplantation of coencapsulated pig islet cells with porcine adipose or bone marrow MSCs was shown to improve oxygenation and neoangiogenesis of encapsulated islet cells in subcutaneous tissue in a primate preclinical model69. Thus If xenogeneic MSCs could be demonstrated to have immunomodulatory effects for inflammatory diseases comparable to that of human MSCs, they have the potential to become a therapeutic agent.
Xenogeneic urothelial cells for cystitis
Cystitis, the inflammation of bladder, is the results of urothelial injury due to physical and chemical stress or microbial infection73. Hemorrhagic cystitis, resulting in bleeding from the bladder mucosa, mainly caused by anticancer chemotherapy or radiotherapy for the treatment of pelvic malignancies74. Cyclophosphamide (CPP, a chemotherapeutic drug)-induced hemorrhagic cystitis occurs due to the toxic metabolite (acrolein) of cyclophosphamide concentrated in the bladder, which is a reactive, unsaturated aldehyde that induces the production of reactive oxygen species and nitric oxide to damage urothelium75,76. CPP-induced hemorrhagic cystitis is treated prophylactically by measures such as hyperhydration, and bladder irrigation, or detoxification with 2-mercaptoethane sodium sulphonate or treated with oral aminocaproic acid, estrogens, sodium pentosane polysulphate, endoscopic laser coagulation, intramural orgotein (free radical scavenger). Intravesical regimens of alum and formalin, hyperbaric oxygen, urinary diversion and even cystectomy are used, but their efficacy is variable73,77,78. A xenogeneic urothelial cell-based cytotherapy strategy was proposed to treat this disease. In a mouse CPP-induced cystitis model, the intravesical instillation of xenogeneic urothelial cells for porcine urothelium into bladders was shown to reduce the detrimental injury caused by CPP injection, with less injury-induced cell proliferation, cell apoptosis and the maintenance of urothelial integrity79. The host immunity is triggered to reject xenogeneic cells, but transplanted cells are able to protect urothelium from the toxic chemical attack, not only in decreasing the damages caused by CPP, but also preventing cell death that could be induced by CPP. Furthermore, if xenogeneic urothelial cells could be applied as a drug, repeated administration could be performed to maintain the therapeutic effect. There are several intravesical agents used to treat various degree of hemorrhagic cystitis such as alum (aluminum ammonium sulfate or aluminum potassium sulfate), hyaluronic acid and even formalin to contain hemorrhage in the mucosa and submucosa80–82. Intravesical instillation of xenogeneic urothelial cells represents a novel safe and effective therapeutic potential option for urothelial injury based cystitis.
Xenogeneic tissue specific cells as an immunostimulatory agent for cancer immunotherapy
Rejection of transplanted xenogeneic cells first starts cell cytolysis mediated by granulocytes and monocytes, which is activated by surface non-self xenoantigens from innate immunity. In addition to the innate immune response, xenogeneic cells are also rejected by the adaptive immune system involving xenoreactive antibody producing B cells and xenoreactive CD4+ or CD8+ T-cells. Xenogeneic cells are able to stimulate both innate and adaptive immune responses. In the other hand, although tumor cells express non-self neoantigens that activate multiple cell types including innate immune cells and adaptive immune cells to eradicate tumor cells, clinically detected tumor cells could escape anti-tumor immunity and grow progressively by creating an immunosuppressive tumor microenvironment through multiple mechanisms83–85. Multiple immunosuppression regimes are intensively studied to overcome immunological barriers of xenogeneic cell therapy with immunosuppressive agents such CTLA4-Ig to induce T cell co-inhibitory pathway and anti-IL-2 R to block T cell proliferation and activation86. Their opposite agents: anti-CTLA4 antibody to block T cell co-inhibitory pathway and IL-2 to induce T cell proliferation and activation are currently standard care of cancer immunotherapy to boost the immune system to fight cancers. Therefore, the use of xenogeneic cells as an immunostimulatory agent for cancer immunotherapy was proposed as a new class of anti-cancer agents87. General xenogeneic immune responses have been tested as a way to boost antitumor immunity, making non-self neoantigens more foreign. Whole cell vaccines with α (1,3) galactosyltransferase gene therapy for the synthesis of the α-galactosyl (αGal) xenoepitope on the surface of cell vaccine to induce hyperacute xenogeneic immune responses have been investigated to treat human cancers88. Xenogeneic polyantigenic vaccine prepared from murine melanoma B16 and carcinoma LLC cells used in 60 stage IV colorectal cancer patients was demonstrated to be safe and benefit patients associated with vaccine-induced, proinflammatory immune responses89. Xenogeneic immunization with homologous antigens derived from a different species (xenoantigens) to overcome immune ignorance and tolerance induced by tumor cells effectively boost antitumor immunity90–92. Xenogeneic DNA immunization with xenogeneic human TRP-2 (hTRP2) DNA immunization was shown to prevent local recurrence and the development of metastases in a mouse model of minimal residual melanoma by a mechanism requiring CD4(+) and CD8(+) T cells93. Xenogeneic human tyrosinase plasmid DNA vaccination of dogs with advanced malignant melanoma showed potentially therapeutic activity94. In human clinical trials on malignant melanoma patients, DNA vaccines encoding xenogeneic melanosomal antigens (tyrosinase, gp100) induced CD8(+) T-cell responses95, and epitope spreading of CD8+ T cell response was observed96. However, the clinical benefit for this xenogeneic vaccine approach has not been confirmed yet.
We have hypothesized a novel therapeutic xenogeneic cell cancer immunotherapy approach using xenogeneic tissue specific cells of the same tumor origin, which have homologous protein expression as tumor cells to induce cross-reactive antitumor T cells87. This treatment modality combines both cell therapy and immunotherapy, not only providing functional cells for tissue regeneration and repair, but also activating innate and adaptive rejection immunity to remove non-self xenogeneic tissue specific cells. The xenogeneic rejection immune responses share similar features for antitumor immunity through either tumor cell intrinsic or extrinsic immunomodulatory mechanisms to promote cancer immunity cycle97. We have tested this novel cancer immunotherapy approach on bladder cancer in pre-clinical mouse models98. First bladder cancer immunotherapy, intravesical bacillus Calmette-Guérin (BCG, a live attenuated form of Mycobacterium bovis) immunotherapy is the standard of care for patients with high-grade (HG), intermediate and high risk non-muscle invasive bladder cancer (NMIBC) after complete transurethral resection of the tumor99. BCG is the most effective agent in the treatment of NMIBC, but however, the exact mechanism of action of BCG in relation to its antitumor effect is still mainly unclear since its introduction in 1976 by Morales et al100. Despite that the exact antitumor mechanism of intravesical instillation of BCG is unknown, a set of local immune responses such as the induction of CD4 T cell infiltration, which may persist for a number of months, correlate with tumor suppression effect101,102, suggesting that anti-bacteria immune responses induce bystander antitumor immune responses. This bystander antitumor immune response could be exploited using xenogeneic immune responses as an another way to activate the host immune system in order to find more effective and durable immunotherapy. In mouse bladder cancer models, we showed that intravesical administration of xenogeneic urothelial cells isolated and expanded from porcine urothelium inhibits tumor growth and progression as well as extends survival and the combination with standard care chemotherapy is more efficacious than either single therapy through activation of host antitumor immune responses98. This novel xenogeneic cell immunotherapy strategy has the potential to open a new class of cancer immunotherapeutic agents for cancers of different tissue types using xenogeneic tissue specific cells of the same tissue types because of the advantages of xenogeneic cells as a form of cell medicine.
Conclusion
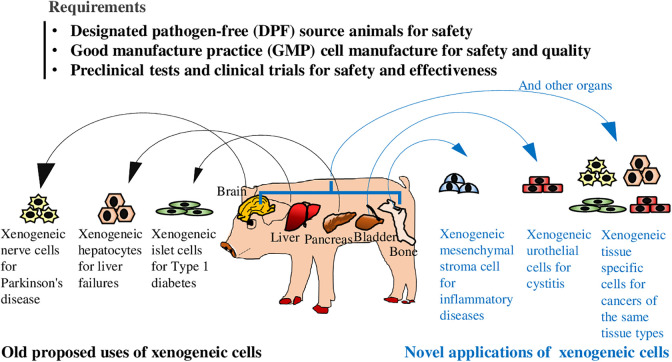
Remarkable pre-clinical and clinical research progress have been made with demonstration of efficacy and safety in the development of utilizing xenogeneic cells as a therapeutic drug to treat a varieties of diseases using different types of xenogeneic tissue-specific cells for old proposed and novel applications (Fig. 1). Immunological barriers of xenogeneic cell therapy still require the development of suitable immunosuppression regimes or induced tolerance and multiple genetic engineering. However, if barriers could be accepted such as the applications with xenogeneic MSCs and urothelial cells or even more taken advantage of, turning into immunological booster like xenogeneic cell cancer immunotherapy, more potential uses of xenogeneic cells could be explored. However, there are still more formidable hurdles to be removed before meaningful xenogeneic cell clinical applications, regarding isolation, expansion and well characterization of xenogeneic cells from safe donor animals, improvement of cell engraftment, and most importantly, demonstration of significant and durable efficacy and long-term safety. We believe that xenogeneic cells provide a potential therapy to manage the body damages caused by diseases with mechanisms of action much different to small molecule drugs and protein drugs.
Fig 1.
The old uses and novel applications of xenogeneic cells as a therapeutic agent in treating a variety of diseases including diabetes, liver failure, neurodegenerative, inflammatory diseases, cystitis, and cancers. Various tissue types of xenogeneic cells could be isolated from safe organs of designated pathogen free (DPF) pigs and expanded under good manufacturing practices (GMP) standards to ensure the safety and quality of cell proudcts. After a series of preclinal tests and clinical trials to determine that xenogeneic cells are safe when used to treat a disease and provides a real health benefit, they can be used as a therapeutic agent to treat diseases.
Acknowledgments
This work was supported by China Medical University Hospital grants (DMR-104-038 and DMR-104-045).
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Chih-Rong Shyr  https://orcid.org/0000-0003-4762-8877
https://orcid.org/0000-0003-4762-8877
References
- 1. Arrighi N. 3 - Stem Cells at the Core of Cell Therapy. In: Arrighi N, editor. Stem Cells: Elsevier; 2018. 73–100. [Google Scholar]
- 2. Guidance for Industry: Human Somatic Cell Therapy and Gene Therapy. I U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research. March, 1998. [Google Scholar]
- 3. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, Liedtke M, Rosenblatt J, Maus MV, Turka A, Lam LP, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380(18):1726–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Panes J, Garcia-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L, Grimaud JC, et al. Long-term efficacy and safety of stem cell therapy (Cx601) for complex perianal fistulas in patients with crohn’s disease. Gastroenterology. 2018;154(5):1334–1342 e4. [DOI] [PubMed] [Google Scholar]
- 6. Source Animal, Product, Preclinical, and Clinical Issues Concerning the Use of Xenotransplantation Products in Humans; Guidance for Industry. U.S. Department of Health and Human Services. Food and Drug Administration, Center for Biologics Evaluation and Research. December, 2016. [Google Scholar]
- 7. Expedited Programs for Regenerative Medicine Therapies for Serious Conditions. Services. U.S. Department of Health and Human Services. Food and Drug Administration, Center for Biologics Evaluation and Research. February, 2019. [Google Scholar]
- 8. Guideline on xenogeneic cell-based medicinal products. European Medicines Agency. 2009. [Google Scholar]
- 9. Meier RPH, Muller YD, Balaphas A, Morel P, Pascual M, Seebach JD, Buhler LH. Xenotransplantation: back to the future? Transpl Int. 2018;31(5):465–477. [DOI] [PubMed] [Google Scholar]
- 10. Ekser B, Li P, Cooper DKC. Xenotransplantation: past, present, and future. Curr Opin Organ Transplant. 2017;22(6):513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hawthorne WJ, Cowan PJ, Buhler LH, Yi S, Bottino R, Pierson RN, Ahn C, 3rd, Azimzadeh A, Cozzi E, Gianello P, Lakey JRT, et al. Third WHO global consultation on regulatory requirements for xenotransplantation clinical trials, Changsha, Hunan, China December 12-14, 2018: “The 2018 Changsha Communique” the 10-year anniversary of the international consultation on xenotransplantation. Xenotransplantation. 2019;26(2):e12513. [DOI] [PubMed] [Google Scholar]
- 12. Cooper DKC, Gaston R, Eckhoff D, Ladowski J, Yamamoto T, Wang L, Iwase H, Hara H, Tector M, Tector AJ. Xenotransplantation—the current status and prospects. British Medical Bulletin. 2017;125(1):5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sykes M, Sachs DH. Transplanting organs from pigs to humans. Sci Immunol. 2019;4(41):eaau6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manji RA, Menkis AH, Ekser B, Cooper DK. Porcine bioprosthetic heart valves: the next generation. Am Heart J. 2012;164(2):177–185. [DOI] [PubMed] [Google Scholar]
- 15. Mulloy B, Gray E, Barrowcliffe TW. Characterization of unfractionated heparin: comparison of materials from the last 50 years. Thromb Haemost. 2000;84(6):1052–1056. [PubMed] [Google Scholar]
- 16. Morrison AE, Ludlam CA, Kessler C. Use of porcine factor VIII in the treatment of patients with acquired hemophilia. Blood. 1993;81(6):1513–1520. [PubMed] [Google Scholar]
- 17. Mann NP, Johnston DI, Reeves WG, Murphy MA. Human insulin and porcine insulin in the treatment of diabetic children: comparison of metabolic control and insulin antibody production. Brit Med J (Clinical research ed.). 1983;287(6405):1580–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spizzo T, Denner J, Gazda L, Martin M, Nathu D, Scobie L, Takeuchi Y. First update of the international xenotransplantation association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes--chapter 2a: source pigs--preventing xenozoonoses. Xenotransplantation. 2016;23(1):25–31. [DOI] [PubMed] [Google Scholar]
- 19. Schuurman H-J. Regulatory aspects of clinical xenotransplantation. Int J Surg. 2015;23(Pt B):312–321. [DOI] [PubMed] [Google Scholar]
- 20. Barrangou R, Doudna JA. Applications of CRISPR technologies in research and beyond. Nat Biotechnol. 2016;34(9):933–941. [DOI] [PubMed] [Google Scholar]
- 21. Mulroy E, Snow B, Bok A, Simpson M, Smith A, Taylor KM, Lockhart M, Lam BBJ, Frampton C, Finucane G, Schweder P, et al. A long-term follow-up of safety and clinical efficacy of NTCELL® [Immunoprotected (Alginate-encapsulated) porcine choroid plexus cells for xenotransplantation] in patients with Parkinson’s disease. Parkinsonism Relat Disord. 2020;82:128–132. [DOI] [PubMed] [Google Scholar]
- 22. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- 23. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. [DOI] [PubMed] [Google Scholar]
- 24. Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hunsicker LG, Kaufman DB, et al. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2016;39(7):1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pullen LC. Stem Cell-derived pancreatic progenitor cells have now been transplanted into patients: report from IPITA 2018. Am J Transplant. 2018;18(7):1581–1582. [DOI] [PubMed] [Google Scholar]
- 26. Park CG, Bottino R, Hawthorne WJ. Current status of islet xenotransplantation. Int J Surg. 2015;23(Pt B):261–266. [DOI] [PubMed] [Google Scholar]
- 27. Coe TM, Markmann JF, Rickert CG. Current status of porcine islet xenotransplantation. Curr Opin Organ Transplant. 2020;25(5):449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsumoto S, Abalovich A, Wechsler C, Wynyard S, Elliott RB. Clinical benefit of islet xenotransplantation for the treatment of type 1 diabetes. EBioMedicine. 2016;12:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dufrane D, Goebbels RM, Saliez A, Guiot Y, Gianello P. Six-month survival of microencapsulated pig islets and alginate biocompatibility in primates: proof of concept. Transplantation. 2006;81(9):1345–1353. [DOI] [PubMed] [Google Scholar]
- 30. Hering BJ, Wijkstrom M, Graham ML, Hardstedt M, Aasheim TC, Jie T, Ansite JD, Nakano M, Cheng J, Li W, Moran K, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12(3):301–303. [DOI] [PubMed] [Google Scholar]
- 31. Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, Bello-Laborn H, Hacquoil B, Strobert E, Gangappa S, Weber CJ, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12(3):304–306. [DOI] [PubMed] [Google Scholar]
- 32. Shin JS, Kim JM, Kim JS, Min BH, Kim YH, Kim HJ, Jang JY, Yoon IH, Kang HJ, Kim J, Hwang ES, et al. Long-term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. Am J Transplant. 2015;15(11):2837–2850. [DOI] [PubMed] [Google Scholar]
- 33. Wang W, Mo Z, Ye B, Hu Pa, Liu S, Yi S. A clinical trial of xenotransplantation of neonatal pig islets for diabetic patients. Zhong nan da xue xue bao. Yi xue ban = journal of central south university. Med Sci. 2011;36(12):1134–1140. [DOI] [PubMed] [Google Scholar]
- 34. Arroyo V, Moreau R, Jalan R. Acute-on-chronic liver failure. N Engl J Med. 2020;382(22):2137–2145. [DOI] [PubMed] [Google Scholar]
- 35. Iansante V, Mitry RR, Filippi C, Fitzpatrick E, Dhawan A. Human hepatocyte transplantation for liver disease: current status and future perspectives. Pediatr Res. 2018;83(1-2):232–240. [DOI] [PubMed] [Google Scholar]
- 36. Dhawan A, Strom SC, Sokal E, Fox IJ. Human hepatocyte transplantation. Methods Mol Biol. 2010;640:525–534. [DOI] [PubMed] [Google Scholar]
- 37. Fisher RA, Strom SC. Human hepatocyte transplantation: worldwide results. Transplantation. 2006;82(4):441–449. [DOI] [PubMed] [Google Scholar]
- 38. Nagata H, Nishitai R, Shirota C, Zhang JL, Koch CA, Cai J, Awwad M, Schuurman HJ, Christians U, Abe M, Baranowska-Kortylewicz J, et al. Prolonged survival of porcine hepatocytes in cynomolgus monkeys. Gastroenterology. 2007;132(1):321–329. [DOI] [PubMed] [Google Scholar]
- 39. Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. [DOI] [PubMed] [Google Scholar]
- 40. McFarthing K, Buff S, Rafaloff G, Dominey T, Wyse RK, Stott SRW. Parkinson’s disease drug therapies in the clinical trial pipeline: 2020. J Parkinson’s Dis. 2020;10(3):757–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Freed CR, Breeze RE, Rosenberg NL, Schneck SA, Kriek E, Qi JX, Lone T, Zhang YB, Snyder JA, Wells TH, et al. Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson’s disease. N Engl J Med. 1992;327(22):1549–1555. [DOI] [PubMed] [Google Scholar]
- 42. Kikuchi T, Morizane A, Doi D, Magotani H, Onoe H, Hayashi T, Mizuma H, Takara S, Takahashi R, Inoue H, Morita S, et al. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature. 2017;548(7669):592–596. [DOI] [PubMed] [Google Scholar]
- 43. Schweitzer JS, Song B, Herrington TM, Park TY, Lee N, Ko S, Jeon J, Cha Y, Kim K, Li Q, Henchcliffe C, et al. Personalized iPSC-derived dopamine progenitor cells for Parkinson’s disease. N Engl J Med. 2020;382(20):1926–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fink JS, Schumacher JM, Ellias SL, Palmer EP, Saint-Hilaire M, Shannon K, Penn R, Starr P, VanHorne C, Kott HS, Dempsey PK, et al. Porcine xenografts in Parkinson’s disease and Huntington’s disease patients: preliminary results. Cell Transplant. 2000;9(2):273–278. [DOI] [PubMed] [Google Scholar]
- 45. Deacon T, Schumacher J, Dinsmore J, Thomas C, Palmer P, Kott S, Edge A, Penney D, Kassissieh S, Dempsey P, Isacson O. Histological evidence of fetal pig neural cell survival after transplantation into a patient with Parkinson’s disease. Nat Med. 1997;3(3):350–353. [DOI] [PubMed] [Google Scholar]
- 46. Luo XM, Lin H, Wang W, Geaney MS, Law L, Wynyard S, Shaikh SB, Waldvogel H, Faull RL, Elliott RB, Skinner SJ, et al. Recovery of neurological functions in non-human primate model of Parkinson’s disease by transplantation of encapsulated neonatal porcine choroid plexus cells. J Parkinsons Dis. 2013;3(3):275–291. [DOI] [PubMed] [Google Scholar]
- 47. Snow B, Mulroy E, Bok A, Simpson M, Smith A, Taylor K, Lockhart M, Lam BJ, Frampton C, Schweder P, Chen B, et al. A phase IIb, randomised, double-blind, placebo-controlled, dose-ranging investigation of the safety and efficacy of NTCELL((R)) [immunoprotected (alginate-encapsulated) porcine choroid plexus cells for xenotransplantation] in patients with Parkinson’s disease. Parkinsonism Relat Disord. 2019;61:88–93. [DOI] [PubMed] [Google Scholar]
- 48. Vadori M, Cozzi E. The immunological barriers to xenotransplantation. Tissue Antigens. 2015;86(4):239–253. [DOI] [PubMed] [Google Scholar]
- 49. Carvalho-Oliveira M, Valdivia E, Blasczyk R, Figueiredo C. Immunogenetics of xenotransplantation. Int J Immunogenet. 2021;48(2):120–134. [DOI] [PubMed] [Google Scholar]
- 50. Al-Mohanna F, Saleh S, Parhar RS, Khabar K, Collison K. Human neutrophil gene expression profiling following xenogeneic encounter with porcine aortic endothelial cells: the occult role of neutrophils in xenograft rejection revealed. J Leukoc Biol. 2005;78(1):51–61. [DOI] [PubMed] [Google Scholar]
- 51. Griesemer A, Yamada K, Sykes M. Xenotransplantation: immunological hurdles and progress toward tolerance. Immunol Rev. 2014;258(1):241–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Karlsson-Parra A, Ridderstad A, Wallgren AC, Möller E, Ljunggren HG, Korsgren O. Xenograft rejection of porcine islet-like cell clusters in normal and natural killer cell-depleted mice. Transplantation. 1996;61(9):1313–1320. [DOI] [PubMed] [Google Scholar]
- 53. Weiss EH, Lilienfeld BG, Muller S, Muller E, Herbach N, Kessler B, Wanke R, Schwinzer R, Seebach JD, Wolf E, Brem G. HLA-E/human beta2-microglobulin transgenic pigs: protection against xenogeneic human anti-pig natural killer cell cytotoxicity. Transplantation. 2009;87(1):35–43. [DOI] [PubMed] [Google Scholar]
- 54. Tahara H, Ide K, Basnet N, Tanaka Y, Ohdan H. Determination of the precursor frequency and the reaction intensity of xenoreactive human t lymphocytes. Xenotransplantation. 2010;17(3):188–196. [DOI] [PubMed] [Google Scholar]
- 55. Yi S, Feng X, Wang Y, Kay TW, Wang Y, O’Connell PJ. CD4+ cells play a major role in xenogeneic human anti-pig cytotoxicity through the Fas/Fas ligand lytic pathway. Transplantation. 1999;67(3):435–443. [DOI] [PubMed] [Google Scholar]
- 56. Bähr A, Käser T, Kemter E, Gerner W, Kurome M, Baars W, Herbach N, Witter K, Wünsch A, Talker SC, Kessler B, et al. Ubiquitous LEA29Y expression blocks t cell co-stimulation but permits sexual reproduction in genetically modified Pigs. PLOS One. 2016;11(5): e0155676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kirchhof N, Shibata S, Wijkstrom M, Kulick DM, Salerno CT, Clemmings SM, Heremans Y, Galili U, Sutherland DE, Dalmasso AP, Hering BJ. Reversal of diabetes in non-immunosuppressed rhesus macaques by intraportal porcine islet xenografts precedes acute cellular rejection. Xenotransplantation. 2004;11(5):396–407. [DOI] [PubMed] [Google Scholar]
- 59. Nilsson B. The instant blood-mediated inflammatory reaction in xenogeneic islet transplantation. Xenotransplantation. 2008;15(2):96–98. [DOI] [PubMed] [Google Scholar]
- 60. Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cells Transl Med. 2017;6(6):1445–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Thompson M, Mei SHJ, Wolfe D, Champagne J, Fergusson D, Stewart DJ, Sullivan KJ, Doxtator E, Lalu M, English SW, Granton J, et al. Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: An updated systematic review and meta-analysis. EClinicalMedicine. 2020;19:100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L, Grimaud JC, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388(10051):1281–1290. [DOI] [PubMed] [Google Scholar]
- 63. Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–1586. [DOI] [PubMed] [Google Scholar]
- 64. Kurtzberg J, Abdel-Azim H, Carpenter P, Chaudhury S, Horn B, Mahadeo K, Nemecek E, Neudorf S, Prasad V, Prockop S, Quigg T, et al. A phase 3, single-arm, prospective study of remestemcel-l, ex vivo culture-expanded adult human mesenchymal stromal cells for the treatment of pediatric patients who failed to respond to steroid treatment for acute graft-versus-host disease. Biol Blood Marrow Transplant. 2020;26(5):845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yip HK, Fang WF, Li YC, Lee FY, Lee CH, Pei SN, Ma MC, Chen KH, Sung PH, Lee MS. Human umbilical cord-derived mesenchymal stem cells for acute respiratory distress syndrome. Crit Care Med. 2020;48(5):e391–e399. [DOI] [PubMed] [Google Scholar]
- 66. Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge M, Deng K, Zhang L, Zou B, Cheng B, Xu J. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res. 2014;15:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, et al. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li J, Ezzelarab MB, Cooper DK. Do mesenchymal stem cells function across species barriers? relevance for xenotransplantation. Xenotransplantation. 2012;19(5):273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262(5):509–525. [DOI] [PubMed] [Google Scholar]
- 70. Ezzelarab M, Ayares D, Cooper DK. The potential of genetically-modified pig mesenchymal stromal cells in xenotransplantation. Xenotransplantation. 2010;17(1):3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu J, Lu XF, Wan L, Li YP, Li SF, Zeng LY, Zeng YZ, Cheng LH, Lu YR, Cheng JQ. Suppression of human peripheral blood lymphocyte proliferation by immortalized mesenchymal stem cells derived from bone marrow of Banna Minipig inbred-line. Transplant Proc. 2004;36(10):3272–3275. [DOI] [PubMed] [Google Scholar]
- 72. Liu J, Lu X, Lou Y, Cai Y, Cui W, Wang J, Nie P, Chen L, Li B, Luo P. Xenogeneic transplantation of human placenta-derived mesenchymal stem cells alleviates renal injury and reduces inflammation in a mouse model of lupus nephritis. Biomed Res Int. 2019;2019:9370919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lee G, Romih R, Zupancic D. Cystitis: from urothelial cell biology to clinical applications. Biomed Res Int. 2014;2014:473536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bon K, Lichtensteiger CA, Wilson SG, Mogil J. Characterization of cyclophosphamide cystitis, a model of visceral and referred pain, in the mouse: species and strain differences. J Urol. 2003;170(3):1008–1012. [DOI] [PubMed] [Google Scholar]
- 75. Korkmaz A, Oter S, Deveci S, Ozgurtas T, Topal T, Sadir S, Bilgic H. Involvement of nitric oxide and hyperbaric oxygen in the pathogenesis of cyclophosphamide induced hemorrhagic cystitis in rats. J Urol. 2003;170(6 Pt 1):2498–2502. [DOI] [PubMed] [Google Scholar]
- 76. Korkmaz A, Topal T, Oter S. Pathophysiological aspects of cyclophosphamide and ifosfamide induced hemorrhagic cystitis; implication of reactive oxygen and nitrogen species as well as PARP activation. Cell Biol Toxicol. 2007;23(5):303–312. [DOI] [PubMed] [Google Scholar]
- 77. Sandhu SS, Goldstraw M, Woodhouse CR. The management of haemorrhagic cystitis with sodium pentosan polysulphate. BJU Int. 2004;94(6):845–847. [DOI] [PubMed] [Google Scholar]
- 78. Chong KT, Hampson NB, Corman JM. Early hyperbaric oxygen therapy improves outcome for radiation-induced hemorrhagic cystitis. Urology. 2005;65(4):649–653. [DOI] [PubMed] [Google Scholar]
- 79. Huang CP, Chen CC, Tsai YT, Wu CC, Shyr CR. Intravesical administration of xenogeneic porcine urothelial cells attenuates cyclophosphamide-induced cystitis in mice. Cell Transplant. 2019;28(3):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Donahue LA, Frank IN. Intravesical formalin for hemorrhagic cystitis: analysis of therapy. J Urol. 1989;141(4):809–812. [DOI] [PubMed] [Google Scholar]
- 81. Westerman ME, Boorjian SA, Linder BJ. Safety and efficacy of intravesical alum for intractable hemorrhagic cystitis: a contemporary evaluation. Int Braz J Urol. 2016;42(6):1144–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Montgomery BD, Boorjian SA, Ziegelmann MJ, Joyce DD, Linder BJ. Intravesical silver nitrate for refractory hemorrhagic cystitis. Turk J Urol. 2016;42(3):197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Houghton AN. Cancer antigens: immune recognition of self and altered self. J Ex Med. 1994;180(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Murciano-Goroff YR, Warner AB, Wolchok JD. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res. 2020;30(6):507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Thompson P, Badell IR, Lowe M, Turner A, Cano J, Avila J, Azimzadeh A, Cheng X, Pierson RN, 3rd, Johnson B, Robertson J, et al. Alternative immunomodulatory strategies for xenotransplantation: CD40/154 pathway-sparing regimens promote xenograft survival. Am J Transplant. 2012;12(7):1765–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Huang CP, Chen CC, Shyr CR. Xenogeneic cell therapy provides a novel potential therapeutic option for cancers by restoring tissue function, repairing cancer wound and reviving anti-tumor immune responses. Cancer Cell Int. 2018;18:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. McCormick KA, Coveler AL, Rossi GR, Vahanian NN, Link C, Chiorean EG. Pancreatic cancer: update on immunotherapies and algenpantucel-L. Hum Vaccin Immunother. 2016;12(3):563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Seledtsova GV, Shishkov AA, Kaschenko EA, Seledtsov VI. Xenogeneic cell-based vaccine therapy for colorectal cancer: Safety, association of clinical effects with vaccine-induced immune responses. Biomed Pharmacother. 2016;83:1247–1252. [DOI] [PubMed] [Google Scholar]
- 90. Perales MA, Blachere NE, Engelhorn ME, Ferrone CR, Gold JS, Gregor PD, Noffz G, Wolchok JD, Houghton AN. Strategies to overcome immune ignorance and tolerance. Semin Cancer Biol. 2002;12(1):63–71. [DOI] [PubMed] [Google Scholar]
- 91. Strioga MM, Darinskas A, Pasukoniene V, Mlynska A, Ostapenko V, Schijns V. Xenogeneic therapeutic cancer vaccines as breakers of immune tolerance for clinical application: to use or not to use? Vaccine. 2014;32(32):4015–4024. [DOI] [PubMed] [Google Scholar]
- 92. Srinivasan R, Wolchok JD. Tumor antigens for cancer immunotherapy: therapeutic potential of xenogeneic DNA vaccines. J Transl Med. 2004;2(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hawkins WG, Gold JS, Blachere NE, Bowne WB, Hoos A, Lewis JJ, Houghton AN. Xenogeneic DNA immunization in melanoma models for minimal residual disease. J Surg Res. 2002;102(2):137–143. [DOI] [PubMed] [Google Scholar]
- 94. Bergman PJ, McKnight J, Novosad A, Charney S, Farrelly J, Craft D, Wulderk M, Jeffers Y, Sadelain M, Hohenhaus AE, Segal N, et al. Long-term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: a phase I trial. Clin Cancer Res. 2003;9(4):1284–1290. [PubMed] [Google Scholar]
- 95. Wolchok JD, Yuan J, Houghton AN, Gallardo HF, Rasalan TS, Wang J, Zhang Y, Ranganathan R, Chapman PB, Krown SE, Livingston PO, et al. Safety and immunogenicity of tyrosinase DNA vaccines in patients with melanoma. Mol Ther. 2007;15(11):2044–2050. [DOI] [PubMed] [Google Scholar]
- 96. Ginsberg BA, Gallardo HF, Rasalan TS, Adamow M, Mu Z, Tandon S, Bewkes BB, Roman RA, Chapman PB, Schwartz GK, Carvajal RD, et al. Immunologic response to xenogeneic gp100 DNA in melanoma patients: comparison of particle-mediated epidermal delivery with intramuscular injection. Clin Cancer Res. 2010;16(15):4057–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chen Daniel S, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. [DOI] [PubMed] [Google Scholar]
- 98. Huang CP, Wu CC, Shyr CR. Combination of novel intravesical xenogeneic urothelial cell immunotherapy and chemotherapy enhances anti-tumor efficacy in preclinical murine bladder tumor models. Cancer Immunol Immunother. 2021;70(5):1419–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Babjuk M, Bohle A, Burger M, Capoun O, Cohen D, Comperat EM, Hernandez V, Kaasinen E, Palou J, Roupret M, van Rhijn BW, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. 2017;71(3):447–461. [DOI] [PubMed] [Google Scholar]
- 100. Morales A, Eidinger D, Bruce AW. Intracavitary bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180–183. [DOI] [PubMed] [Google Scholar]
- 101. Alexandroff AB, Jackson AM, O’Donnell MA, James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet. 1999;353(9165):1689–1694. [DOI] [PubMed] [Google Scholar]
- 102. Bohle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003;169(1):90–95. [DOI] [PubMed] [Google Scholar]