Abstract
Prepectoral prosthetic breast reconstruction has been widely reassessed in recent years and is taking on an increasingly important role in the field of immediate breast reconstruction. We report here a case series of 32 patients who underwent nipple-sparing mastectomy for breast carcinoma and prepectoral breast reconstruction involving an acellular dermal matrix (ADM) treated by means of a skin-graft mesher in our hospital from January 2015 to March 2016. The indications for this type of reconstruction were body mass index (BMI) less than 30 kg/m2; no history of radiotherapy; no active smokers; moderate grade breast; and good viability of mastectomy flap: normal skin colour, active bleeding at the fresh cut edges, and thicker than 1 cm mastectomy flaps; the viability of lower thicknesses was ascertained by the fluorescent dye indocyanine green xenon-based imaging technology (4 patients). The mean age of the patients was 56.4 years (range: 39-77 years). Their mean BMI was 27.4 kg/m2. Until the end of follow-up (mean: 17 months), major complications requiring reoperation occurred in 9% of patients and minor complications in 22% of patients. The mean of the 3 pain visual analogue scale scores taken in the first 24 hours after surgery was 1.8. Mean duration of hospital stay has been 2.2 days. Our complication rate was similar to those reported in other studies on prepectoral breast reconstruction featuring total ADM coverage of the implant.
Keywords: prepectoral breast reconstruction, acellular dermal matrix, meshed
Abstract
La reconstruction par prothèse mammaire prépectorale a été largement réévaluée ces dernières années et joue un rôle de plus en plus important dans le cadre des reconstructions mammaires immédiates. Les auteurs rendent compte d’une série de 32 patientes qui ont subi une mastectomie d’épargne cutanée à cause d’un carcinome du sein et d’une reconstruction mammaire prépectorale touchant la matrice du derme acellulaire traitée par une ampligreffe à l’hôpital entre janvier 2015 et mars 2016. Les indications pour ce type de reconstruction étaient un indice de masse corporelle inférieur à 30 kg/m2, aucun antécédent de radiothérapie, aucun tabagisme actif, des seins de dimension modérée, une bonne viabilité du lambeau de mastectomie, une couleur normale de la peau, un saignement actif aux bordures fraîchement coupées et des lambeaux de mastectomie de plus de 1 cm. La viabilité de l’épaisseur inférieure était évaluée par la technologie d’imagerie par fluorescence du vert d’indocyanine à base de xénon (chez quatre patientes). Les patientes avaient un âge moyen de 56,4 ans (moyenne de 39 à 77 ans) et avaient un indice de masse corporelle moyen de 27,4 kg/m2. Jusqu’à la fin du suivi (moyenne de 17 mois), 9 % des patientes ont souffert de complications majeures exigeant une réopération, et 22 % ont subi des complications mineures. La moyenne de trois scores de douleur sur l’échelle analogique visuelle calculés dans les 24 heures suivant l’opération s’élevait à 1,8. Le séjour hospitalier était d’une durée moyenne de 2,2 jours. Le taux de complication était semblable à celui déclaré dans d’autres études sur la reconstruction mammaire prépectorale touchant l’intégralité de la matrice dermique acellulaire de l’implant.
Introduction
Prepectoral prosthetic breast reconstruction has been widely reassessed in recent years and is taking on an increasingly important role in the field of immediate breast reconstruction.1 This trend reversal in a field in which submuscular mammary prostheses have long been considered safer is justified by the possibility of achieving valid aesthetic results with prepectoral reconstruction, even in cases of ptoticbreast, is partly due to the lesser surgical trauma involved.2 In addition, there have been great technological strides in terms of breast reconstruction materials.3 Indeed, although today’s breast prostheses are still made of silicone, their high degree of gel cohesiveness is sufficient to substantially reduce the rippling phenomenon. Furthermore, the introduction of acellular dermal matrix (ADM) and meshing to the procedure allows surgeons to create a layer of tissue between the mastectomy flap and implant that not only protects the latter from exposure but also keeps it in place, preventing lateral migration.
Several prepectoral prosthetic breast reconstruction techniques involving ADM or other matrices have been described in the literature. Some involve anterior and posterior coverage of the implant,4 whereas others rely on a “sling” to cover the anterior part of the prosthesis.5 Other techniques involving the use of a combination of 2 ADM to cover the anterior surface of the prosthesis have also been proposed. We, on the other hand, report here a case series of patients who underwent prepectoral breast reconstruction involving an ADM support treated by means of a skin graft mesher.
Materials and Methods
Thirty-two patients underwent nipple-sparing mastectomy for breast carcinoma and immediate prepectoral breast reconstruction with meshed ADM in our hospital from January 2015 to March 2016. The indications for this type of reconstruction were body mass index (BMI) less than 30 kg/m2, no history of radiotherapy, no active smokers, moderate grade breast ptosis (patients with a high grade of breast ptosis were given skin-reducing mastectomy and were therefore ineligible for inclusion in this study), and viable mastectomy flap. Good viability was defined as normal skin colour, active bleeding at the fresh cut edges, and mastectomy flaps thicker than 1 cm; the viability of lower thicknesses was ascertained by the fluorescent dye indocyanine green (ICG) xenon-based imaging technology (Image 1 S, KARL STORZ SE & Co).
Post-operative pain scores were measured on a visual analogue scale (VAS) 3 times over the first 24 hours (2, 12, and 24 hours post-operatively) and used to calculate an average pain score. Hospital stay duration was recorded, and any complications occurring after admission were described. Patients were instructed to wear a sports bra 24 hours per day throughout the first month after surgery and during the day only for the second month. Participants were given outpatient medications and, upon healing, attended follow-up appointments for a minimum of 12 months. Any complications arising during this period were recorded; in particular, minor complications considered were partial nipple–areolar complex (NAC) necrosis, delayed wound healing, seroma, and medically treated infection. Major complications considered were any reconstruction-related condition that required reoperation (symptomatic haematoma, mastectomy flap necrosis and implant exposure, infection), capsular contracture, and chronic pain.
Reconstruction outcomes were objectively assessed by 2 external plastic surgeons, who were asked to provide a consensus opinion for each parameter. Parameters assessed by clinical examination were degree of capsule contracture on the Baker scale; the presence or absence of contouring defects, rippling, or implant visibility; and symmetry which was defined as excellent, good, acceptable, or not acceptable. This study was approved by the institutional review board of Santa Maria della Misericordia Hospital, Udine.
Surgical Technique
All patients underwent nipple-sparing mastectomy under general anaesthesia. After one-step nucleic acid amplification to check for sentinel lymph nodes, extemporaneous histological examination of the retroalveolar tissue was performed to ensure safe preservation of the NAC. Mastectomy flaps were clinically assessed and defined as suitable for subcutaneous implant placement with meshed ADM if the flap showed active bleeding at the edges, normal coloration, and 1-cm thickness. In cases in which the flap appeared clinically viable but was less than 1 cm thick, perfusion was assessed using the fluorescent indocyanine test.
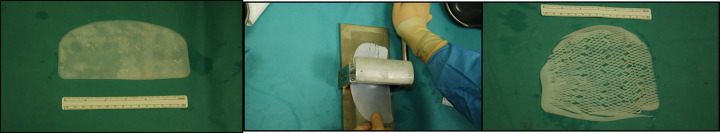
After washing and hydrating the Surgimend PRS 8 cm × 16 cm (Integra LifeSciences Corporation) ADM with sterile physiological solution, this was meshed to amplify the surface 3-fold and obtain a mesh of average size 16 cm × 18 cm (Figure 1). The mesh was sutured at the level of the submammary fold using Vicryl 2/0, and the patient was placed in a semi-seated position (Figure 2). A sizer was inserted and the ADM was sutured at the level of the anterior axillary line to the pectoral muscle surface at the medial end of the pocket and, without exerting tension, to the pectoral muscle proximal to the implant. Intra-operative symmetry verification was carried out with the patient in a sitting position, and the permanent prosthesis and an aspiration drain (2 in cases of axillary dissection) were positioned. The mastectomy wound was then sutured closed.
Figure 1.
Expansion of the acellular dermal matrix with a skin graft 3× mesher.
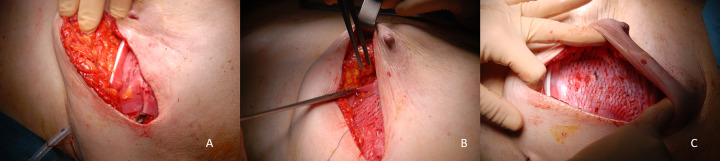
Figure 2.
Securing of the meshed ADM to the inframammary fold and to the anterior axillary pillar. After positioning (A), the meshed ADM is secured via separate stitches to the inframammary fold (B and C).
All patients were fitted with elastic compression stockings and administered low-molecular-weight heparin, both to prevent thrombosis, and post-operative antibiotics from the evening of the surgery.
Results
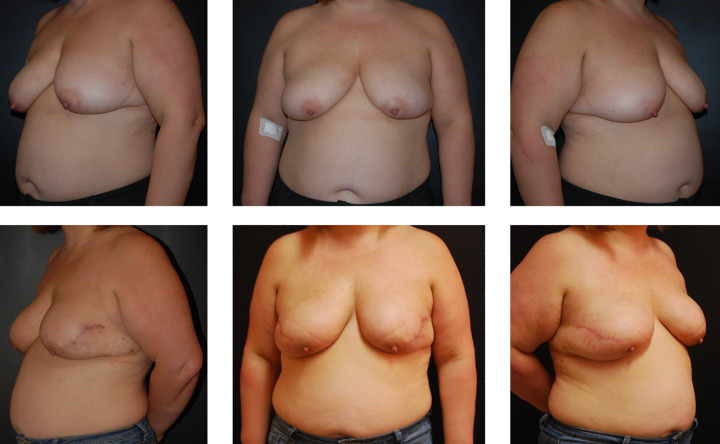
A total of 32 patients underwent prepectoral breast reconstruction with meshed ADM. One patient had a bilateral mastectomy and a bilateral reconstruction with meshed ADM (Figure 3). The mean age of the patients was 56.4 years (range: 39 to 77 years). Their mean BMI was 27.4 kg/m2, and comorbidities recorded were arterial hypertension (2 patients), dyslipidaemia (1), hypothyroidism (1), osteoporosis (2), and type 2 diabetes (1) (Table 1). A radial, lateral mastectomy incision was chosen in all cases and was performed within a few millimetres from the areolar margin toward the axilla. The mean mastectomy weight was 374 g, and the mean implant volume was 330 mL. The ADM used in all cases was Surgimend PRS 8 cm × 16 cm (Integra LifeSciences Corporation). The mastectomy flap was greater than 1-cm thick in 28 of 32 patients. In the remaining 4 patients, flap viability was confirmed by fluorescent ICG test.
Figure 3.
Bilateral breast reconstruction with meshed acellular dermal matrix. Preoperative (above) and 5-month postoperative (below) view. The patient received adjuvant chemotherapy.
Table 1.
Demographic Features.a
| Demographic | |
|---|---|
| Mean age | 56.4 years (39-77) |
| Body mass index | 27.4 kg/m2 |
| Comorbidities | No. of patients |
| Arterial hypertension | 2 |
| Dyslipidaemia | 1 |
| Hypothyroidism | 1 |
| Osteoporosis | 2 |
| Diabetes mellitus II | 1 |
a Main demographic information and comorbidities describing our series.
The mean of the 3 pain VAS scores taken in the first 24 hours after surgery, before administration of analgesics, was 1.8. The mean duration of stay was 2.2 days. Drains were removed when the recorded output was less than 40 mL/24 hours; in all cases, the drains were removed within 10 days of surgery, as per our department’s protocols.
One patient required further surgery in the first 36 hours due to symptomatic haematoma. In this case, the matrix was detached laterally from the pectoral muscle and the implant removed. After clot removal, repeated lavage, and careful haemostasis, the implant was repositioned as in the first surgical sitting. One case (3%) of seroma, requiring repeated external drainages in the weeks following the surgery, was recorded, but this resolved within a month.
One case (3%) of partial NAC necrosis (but no cases of total NAC necrosis) occurred, and in 2 cases (6%), there was a delay in healing with superficial marginal necrosis of the mastectomy flap which required prolonged dressing. In 1 case, cellulitis developed, but this was treated successfully with outpatient antibiotic therapy. In 1 case (3%), the infection and implant exposure necessitated re-surgery to remove the implant and position a submuscular expander, as well as prolonged antibiotics. No cases of red breast syndrome were encountered.
In the post-operative period, 18 patients underwent chemotherapy and 5 radiotherapy. One-year follow-up assessment, carried out by 2 external plastic surgeons, resulted in a consensus on all cases. Specifically, the dimensional and volumetric symmetry was judged as excellent in 24 cases (75%), good in 7 cases (22%), and acceptable in 1 patient (3%), who in any case was satisfied with the outcome and refused corrective surgery. Three patients showed a contouring defect with implant visibility, and a lipofilling was performed to correct the defect. Of these 3 patients, 2 had displayed a flap thickness of <1 cm after the initial mastectomy.
Our mean follow-up was 17 months (range: 12-24 months), at which 1 case of grade II capsular contracture was recorded, as well as 1 case of grade III. No cases of grade IV capsular contracture were noted, and the patient with the grade III capsular contracture had undergone post-operative radiotherapy.
Discussion
The implantation of subcutaneous breast prostheses after mastectomy was introduced 40 years ago6 but has since been superseded by submuscular reconstruction due to the high rate of mastectomy flap necrosis, capsular contracture, and poor implant positioning associated with the former technique. However, in addition to demolition techniques that were less focussed on reconstruction, and more primitive implants, these complications were ascribable to the inability of the mastectomy flap, even if well vascularised, to support and protect mammary prostheses, especially large ones.
Subcutaneous reconstruction does enable recreation of natural-looking mammary ptosis, which is difficult to achieve by means of single-plane submuscular techniques. The results have been enhanced by the introduction of ADM, which, sutured like a sling to the inferior margin of the pectoral muscle and the submammary fold, can increase the pocket height, reduce the weight of the prosthesis on the mastectomy flap, and enable partial safe subcutaneous placement. It acts as a protective layer between the mastectomy flap and the implant before the capsule is formed.
The introduction of ADM has not overcome all of the limitations of sub-pectoral reconstruction. In particular, the detachment of the pectoral muscle and, if necessary, the serratus anterior prolongs operating times, increases blood loss, and delays the patient’s return to normal daily activities.2 Furthermore, pocket creation inevitably requires the exposure of nervous structures that may be injured or stretched during surgery, thereby predisposing the patient to chronic post-operative pain.7,8 Pectoral muscle detachment also causes a certain degree of loss of force in arm abduction, irrespective of the amount of muscle fibres dissected.9 Moderate grade pectoral animation deformity is reported by a high number of submuscular breast reconstruction patients10,11 and is particularly evident upon muscle contraction, which causes lateral displacement of the implant. Hence, many authors propose subcutaneous repositioning of the implant, with or without ADM.10,12 In other cases of sub-pectoral reconstruction, the implants may appear flattened and the submuscular portion constrained beneath the muscle itself, a defect that is not improved to any great extent by wearing a bra.
These limitations make a muscle-sparing reconstruction appealing. Outcomes need to be aesthetically valid and symmetrical, with low functional impact, and the operation should be as rapid as possible. In order to achieve these aims, several authors have re-proposed subcutaneous reconstruction with ADM for total coverage of the implant. For example, Berna et al13 and Vidya et al4 used an envelope of ADM, sutured to the pectoral muscle, to cover both the anterior and posterior surface of the implant. Reitsamer and Peintinger,14 on the other hand, made a pouch to fully the cover the implant by suturing 2 ADMs together. We, on the other hand, rely on only one matrix, which considerably reduces costs.
According to a recent review on the use of various ADMs in prepectoral reconstruction, the pooled complication rate was low, with the most common being partial NAC necrosis (4.5%), followed by implant explantation (4.1%), minor infection (2.3%), seroma (2.9%), hematoma (2.3%), and wound healing problems (2.3%).5 The complications recorded in our case series were comparable with those reported by other surgical teams (Table 2).
Table 2.
Complications Recorded.a
| Complication | No. of reconstructions (%) |
|---|---|
| Major complications | |
| Early | |
| Symptomatic haematoma (within 36 hours) | 1 (3%) |
| Infection requiring re-operation | 1 (3%) |
| Mastectomy flap necrosis with implant exposure | 0 (0%) |
| Late | |
| Chronic pain | 0 (0%) |
| Capsular contracture | 2 (6%) |
| Total | 4 (12%) |
| Minor complications | |
| Early | |
| Partial nipple–areolar complex necrosis | 1 (3%) |
| Delayed wound healing | 2 (6%) |
| Seroma | 1 (3%) |
| Minor infections | 1 (3%) |
| Late | |
| Red breast syndrome | 0 (0%) |
| Late seroma | 0 (0%) |
| Contouring defect | 2 (6%) |
| Total | 7 (22%) |
a Summary of complications recorded, subdivided by severity and time.
The meshing technique enables the drainage of any fluids accumulating between the matrix and mastectomy flap, which could otherwise delay or prevent integration (as with the meshed skin graft in acute burn treatment). Furthermore, meshing enables the ADM to conform to the shape of the implant with no gaps or folds, as recommended for their routine use, and without compromising their resistance, which is sufficient to support medium–large implants (330 cc) by means of cardinal sutures. In fact, we were in a position to verify the resistance of the matrix in the one case of haematoma that necessitated surgical intervention that we encountered. During the second operation, we saw that the matrix was intact and appeared to have expanded even further due to the pressure of the haematoma.
The technique presented here does present some limitations. In particular, some criteria that can contraindicate the technique can only be determined while the surgery is underway and, in some cases, rely on the ICG, not available in all centres. Although clinical assessment via the pinch test for mastectomy flap thickness with a cut-off of 1 cm appears to be reliable in terms of determining flap suitability for the procedure, and is used by several authors,15 it may not be best practice. Indeed, although we found good sensitivity for the pinch test in our case series (no necrosis in flaps greater than 1 cm thick), it seemed to have low specificity, which would reduce the number of candidates eligible for muscle-sparing reconstruction in centres unable to make use of fluorescent ICG test. In fact, all of the 4 patients who had a flap thickness of less than 1 cm on the pinch test were shown to have good perfusion by indocyanine angiography. None of these patients displayed any complications linked to mastectomy flap perfusion, although 2 did show a rippling defect between the medial breast quadrants that required correction via lipofilling.
However, a lack of muscle coverage, especially in patients with thin mastectomy flap, predisposes them to implant visibility, which may require corrective intervention. As tissue coverage is responsible for such contouring defects, when prepectoral reconstruction is performed using mastectomy flaps <1-cm thick, the likelihood of needing a corrective procedure should be taken into account. Many corrective procedures for contouring defects have been proposed, but authors seem to prefer lipofilling in such cases, as it is rapid, low invasive, and well tolerated by patients.
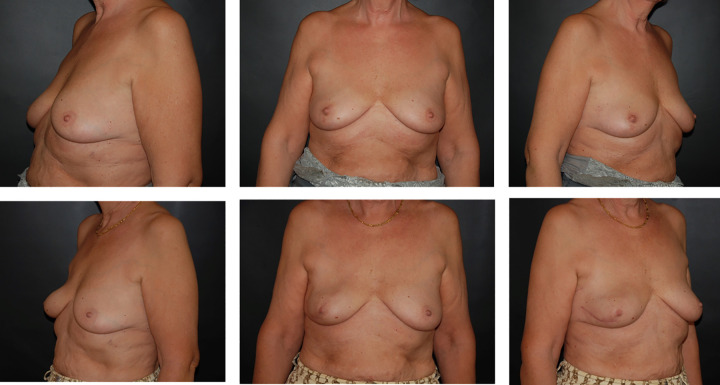
Another limitation of this study is that the technique was only introduced 2 years ago in our department, meaning that we can only report on short-term follow-up in a low number of cases at this time. That is presumably why we saw none of the rarer complications, namely red breast syndrome or delayed seroma.16 Meanwhile, the overall outcomes of one-step prepectoral breast reconstruction with meshed ADM were highly satisfactory (Figure 4), with good–excellent symmetry judged by consensus in a great majority of our, admittedly small, number of cases. Our complication rate was similar to those reported in other studies on prepectoral breast reconstruction featuring total ADM coverage of the implant.
Figure 4.
Reconstruction of a ptotic breast with meshed acellular dermal matrix. Preoperative (above) and 3-month postoperative (below) view. The patient received adjuvant chemotherapy.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Alessandra Fin, MD  https://orcid.org/0000-0002-5745-720X
https://orcid.org/0000-0002-5745-720X
Level of Evidence: Level 4, Therapeutic
References
- 1. Highton L, Johnson R, Kirwan C, Murphy J. Prepectoral implant-based breast reconstruction. Plast Reconstr Surg Glob Open. 2017;5(9):e1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tasoulis MK, Iqbal FM, Cawthorn S, MacNeill F, Vidya R. Subcutaneous implant breast reconstruction: time to reconsider? Eur J Surg Oncol. 2017;43(9):1636–1646. [DOI] [PubMed] [Google Scholar]
- 3. Brown MH, Shenker R, Silver SA. Cohesive silicone gel breast implants in aesthetic and reconstructive breast surgery. Plast Reconstr Surg. 2005;116(3):768–779; discussion 780-781 [DOI] [PubMed] [Google Scholar]
- 4. Vidya R, Masià J, Cawthorn S, et al. Evaluation of the effectiveness of the prepectoral breast reconstruction with Braxon dermal matrix: first multicenter European report on 100 cases. Breast J. 2017;23(6):670–676. [DOI] [PubMed] [Google Scholar]
- 5. Salibian AA, Frey JD, Choi M, Karp NS. Subcutaneous implant-based breast reconstruction with acellular dermal matrix/mesh: a systematic review. Plast Reconstr Surg Glob Open. 2016;4(11):e1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Snyderman RK, Guthrie RH Reconstruction of the female breast following radical mastectomy. Plast Reconstr Surg. 1971;47(6):565–567. [DOI] [PubMed] [Google Scholar]
- 7. Wallace MS, Wallace AM, Lee J, Dobke MK. Pain after breast surgery: a survey of 282 women. Pain. 1996;66(2-3):195–205. [DOI] [PubMed] [Google Scholar]
- 8. Ducic I, Seiboth LA, Iorio ML Chronic postoperative breast pain: danger zones for nerve injuries. Plast Reconstr Surg. 2011;127(1):41–46. [DOI] [PubMed] [Google Scholar]
- 9. Roxo AC, Nahas FX, Pinheiro Rodrigues NC, et al. Functional and volumetric analysis of the pectoralis major muscle after submuscular breast augmentation. Aesthet Surg J. 2017;37(6):654–661. [DOI] [PubMed] [Google Scholar]
- 10. Becker H, Fregosi N. The impact of animation deformity on quality of life in post-mastectomy reconstruction patients. Aesthet Surg J. 2017;37(5):531–536. [DOI] [PubMed] [Google Scholar]
- 11. Nigro LC, Blanchet NP. Animation deformity in postmastectomy implant-based reconstruction. Plast Reconstr Surg Glob Open. 2017;5(7):e1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gabriel A, Sigalove S, Sigalove NM, et al. Prepectoral revision breast reconstruction for treatment of implant-associated animation deformity: a review of 102 reconstructions. Aesthet Surg J. 2018;38(5):519–526. [DOI] [PubMed] [Google Scholar]
- 13. Berna G, Cawthorn SJ, Papaccio G, Balestrieri N. Evaluation of a novel breast reconstruction technique using the Braxon® acellular dermal matrix: a new muscle-sparing breast reconstruction. ANZ J Surg. 2017;87(6):493–498. [DOI] [PubMed] [Google Scholar]
- 14. Reitsamer R, Peintinger F. Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: a new technique for direct-to-implant breast reconstruction after nipple-sparing mastectomy. J Plast Reconstr Aesthet Surg. 2015;68(2):162–167. [DOI] [PubMed] [Google Scholar]
- 15. Jordan D, Frey MD, Ara AS, et al. Mastectomy flap thickness and complications in nipple-sparing mastectomy: objective evaluation using magnetic resonance imaging. Plast Reconstr Surg Glob Open. 2017;5(8):e1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu PS, Winocour S, Jacobson SR. Red breast syndrome: a review of available literature. Aesthetic Plast Surg. 2015;39(2):227–230. [DOI] [PubMed] [Google Scholar]