Abstract
Purpose
To describe the relationship between comorbidities and survival following admission to the intensive care unit.
Methods
Retrospective observational study using several linked routinely collected databases from 16 general intensive care units between 2002 and 2011. Comorbidities identified from hospitalisation in the five years prior to intensive care unit admission. Odds ratios for survival in intensive care unit, hospital and at 30 days, 180 days and 12 months after intensive care unit admission derived from multiple logistic regression models.
Results
There were 41,230 admissions to intensive care units between 2002 and 2011. Forty-one percent had at least one comorbidity – 24% had one, 17% had more than one. Patients with comorbidities were significantly older, had higher Acute Physiology and Chronic Health Evaluation II scores and were more likely to have received elective rather than emergency surgery compared with those without comorbidities. After excluding elective hospitalisations, intensive care unit and hospital mortality for the cohort were 24% and 29%, respectively. Asthma (odds ratio 0.79, 95% confidence interval 0.63–0.99) and solid tumours (odds ratio 0.74, 0.67–0.83) were associated with lower odds of intensive care unit mortality than no comorbidity. Intensive care unit mortality was raised for liver disease (odds ratio 2.98, 2.43–3.65), cirrhosis (odds ratio 2.61, 1.9–3.61), haematological malignancy (odds ratio 2.29, 1.85–2.83), chronic ischaemic heart disease (odds ratio 1.53, 1.19–1.98), heart failure (odds ratio 1.79, 1.35–2.39) and rheumatological disease (odds ratio 1.53, 1.18–1.98).
Conclusions
Comorbidities affect two-fifths of intensive care unit admission and have highly variable effects on subsequent outcomes. Information on the differential effects of comorbidities will be helpful in making better decisions about intensive care unit support and understanding outcomes beyond surviving intensive care unit.
Keywords: Comorbidities, mortality, liver disease, haematological malignancy, solid malignancy
Introduction
Patients admitted to intensive care units (ICUs) often have other conditions (comorbidities) in addition to their principal acute illness. Comorbidities have been shown to have significant impact on the clinical course, complications and outcomes in the ICU.1 Consequently, they have a significant influence on decisions to admit patients to ICU. While severity of illness scoring systems such as Acute Physiology and Chronic Health Evaluation (APACHE) and simplified acute physiology score (SAPS) recognise the influence of comorbidities on outcomes, specific guidelines for admitting patients with comorbidities are outdated because the outcomes associated with some conditions, such as HIV infection and haematological malignancies, have changed a great deal.2
Among all comorbidities, patients with metastatic solid tumours and haematological cancers were previously considered to be particularly poor candidates for ICU admission. Attitudes may be changing in response to evidence showing them to have better outcomes than previously perceived.3 In addition, several studies have shown liver cirrhosis and chronic renal failure to be associated with increased short term mortality compared with patients without such comorbidities.1,4,5 However, no large-scale studies drawing direct comparisons of the outcomes of different comorbidities in the short and long term are available.6
The aim of our study was to evaluate the impact of different comorbidities in patients admitted to the ICU on both short- and long-term outcomes. We used data from all ICUs within a large geographic area in the UK.
Methodology
We performed a population-based study using routinely available data. Data were gathered for all admissions between January 2002 and December 2011 to all 16 general ICUs in the West of Scotland area of the UK (population 2.4 million). This comprises over 100 critical care beds in a range of hospitals from large teaching hospitals to smaller district generals. Patients under 18 were excluded. For patients who were readmitted to the ICU during the study period, only the first admission was included.
We used four routine databases for our study that were linked as described elsewhere.7 The primary source was WardWatcher (Critical Care Audit Ltd, Ilkley, Yorkshire), which collects clinical information for all ICU admissions. This information includes APACHE II score at admission and delivery of organ support during the course of the ICU stay. Each patient’s WardWatcher data were then linked to Scottish Morbidity Records (SMR). We used SMR06 (also known as the Scottish Cancer Registry, which records all incident cancers in the country) and SMR01 (which records clinical details of discharges from all National Health Service acute hospitals in Scotland). The fourth database was the National Records for Scotland deaths records.
Organ support was defined as receipt of invasive mechanical ventilation, vasoactive drugs or renal replacement therapy during the ICU stay.
Socio-economic deprivation was derived from the Scottish Index of Multiple Deprivation (SIMD), an area based index that use seven domains (income, employment, education, health, access to services, crime and housing) within a small area of about 760 people (datazone) to rank it from least deprived to most deprived. These have been divided into quintiles, where 1 is the most deprived and 5, the least deprived.
Survival analysis was performed for patients that had an unanticipated ICU admission after exclusion of elective post-operative patients from the cohort. Hospital mortality was only available after 2005, and was complete for 99% of patients.
Calculation of comorbidities
We classified comorbidities using hospitalisation records in the five years prior to ICU admission. The SMR01 records up to six diagnoses for each hospital discharge using the International Classification of Diseases and Related Health Problems, revision 10 (ICD-10). We selected diagnoses from only the first three fields to reduce the likelihood of including conditions that were relatively minor or incidental. Table 1 gives the ICD-10 codes that we used to classify comorbidities. We made reference to the published literature but developed our own grouping as no existing method appeared to reflect current intensive care practice.8,9 We also created a group for patients with a single pre-existing comorbidity unrelated to their reason for ICU admission (‘Comorbidity 1’), and for patients who had two or more (‘Comorbidity 2+’). Patients with a single condition only were then further analysed.
Table 1.
International Classification of Disease, 10th revision (ICD-10) codes used to define comorbidities.
| Comorbidity | ICD-10 codes |
|---|---|
| Asthma | J45 |
| Chronic obstructive pulmonary disease (COPD) | J41-44 |
| Liver disease | K70.0-K70.2, K70.9, K71.0-K71.6, L71.8-71.9, K73, K74.0-K74.2, K76.0, K76.2-K76.4 |
| Cirrhosis and failure | K70.3, 70.4, K71.7, K72.1, K74.3-74.6, K76.5, K76.6, I85, I86.4, I98.2 |
| Renal disease | N18.0-N18.4, I12.9, N03, |
| Dialysis dependent | N18.5, N19, Z49, I12.0, I13.1 |
| Chronic ischaemic heart disease (CIHD) | I25 |
| Myocardial infarction (MI) | I21 I22 |
| Heart failure | I50 |
| Solid malignancy | C00-C76 |
| Haematological | C81-96 |
| Insulin-dependent diabetes mellitus | E10 |
| Non-insulin dependent diabetes mellitus | E11 |
| Rheumatological disease | M05, M06, M31.1, M32, M33, M34, M35.1, M35.3, M36 |
Ethics approval
Formal ethics committee approval was obtained from the West of Scotland Research Ethics Committee (reference 12/WS/0075).
Statistical analysis
Pearson’s chi-square test of association was used for categorical variables and two-sided Student’s t-test was performed for continuous variables. A conventional significance level of 5% was applied. Binary logistic regression was used to explore determinants of survival, to establish the individual impact of case-mix factors. All variables significant at p < 0.01 on univariable analysis were included in the multivariate model to adjust for potential confounding between variables. These were reported as odds ratios (ORs) along with 95 per cent confidence intervals (CIs) and p values. Analysis was performed using Stata 11.
Results
We identified 41,230 admissions to ICUs between 2002 and 2011. The mean age was 59 years, 55% were male and a third (36%) were from the most deprived quintile – Table 2. The mean APACHE II score overall was 15.6. Two-fifths of patients (43%) were admitted following surgery, with 17% of patients receiving elective and 26% urgent or emergency surgery. A fifth (22%) of patients did not require organ support. Where organ support was required, a third (31%) received single organ support only.
Table 2.
Number of admissions for the whole cohort and details of patients who had a single comorbidity only. χ2 for categorical variables and two-sided t-tests for continuous variables, no comorbidities (no Charlson) as the reference group.
| Group | Total (%) | No. of admissions with single comorbidity | % single comorbidity | Age | Sex (% men) | APACHE II (mean) | SIMD1 (most deprived) | SIMD5 (least deprived) | Elective surgery | Emergency surgery | Organ support |
Nights in ICU | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||||||||||||
| Whole cohort | 41230 | 41230 | 59.3 | 55% | 15.6 | 36% | 9% | 17% | 26% | 22% | 31% | 26% | 8% | 4.7 | |
| No comorbidities | 24222 (58.7) | 24222 | 56.1 | 55% | 15.0 | 36% | 10% | 12% | 28% | 21% | 32% | 25% | 8% | 4.9 | |
| 1 Comorbidity | 9862 (23.9) | 9862 | 62.7** | 54% | 15.5** | 35%** | 10% | 27%** | 22%** | 24%** | 29%** | 25% | 7%* | 4.7* | |
| 2+ Comorbidities | 7146 (17.3) | 7146 | 65.2** | 58%** | 17.8** | 38% | 8%** | 20%** | 25%** | 22%** | 29%** | 28%** | 9%** | 4.2** | |
| Asthma | 1874 (4.5) | 760 | 41% | 46.7** | 38%** | 15.0 | 44%** | 7%* | 8%* | 16%** | 24%* | 36%* | 21%* | 7% | 4.3* |
| COPD | 2960 (7.2) | 959 | 32% | 66.5** | 46%** | 18.2** | 47%** | 6%** | 7%** | 23%* | 19% | 29% | 30%** | 7% | 5.4 |
| Liver disease | 1293 (3.1) | 436 | 34% | 50.7** | 57% | 19.0** | 54%** | 7% | 3%** | 19%** | 11%** | 30% | 37%** | 11%* | 4.7 |
| Cirrhosis and failure | 814 (2.0) | 166 | 20% | 55.2 | 63% | 20.3** | 43% | 8% | 2%** | 8% | 10%** | 30% | 35%** | 13%* | 4.6 |
| Renal disease | 993 (2.4) | 149 | 15% | 62.7** | 54% | 21.7** | 29% | 9% | 11% | 26% | 15% | 28% | 23% | 23%** | 3.8 |
| Dialysis dependent | 802 (1.9) | 91 | 11% | 61.1** | 55% | 19.7** | 45% | 8% | 14% | 22% | 14% | 35% | 20% | 18%** | 4.8 |
| CIHD | 4395 (10.7) | 801 | 18% | 70.1** | 57% | 15.1 | 31%** | 9% | 20%** | 32%* | 26%** | 24%** | 26% | 7% | 4.2* |
| MI | 2294 (5.6) | 123 | 5% | 68.5** | 67%* | 14.7 | 31% | 7% | 20%** | 22% | 22% | 24% | 21% | 8% | 4.6 |
| Heart failure | 1766 (4.3) | 223 | 13% | 70.2** | 51% | 18.5** | 36% | 9% | 9% | 28% | 15%* | 27% | 32%* | 8% | 4.2 |
| Solid malignancy | 6797 (16.5) | 4968 | 73% | 66.1** | 56% | 12.3** | 28%** | 13%** | 50%** | 26% | 32%** | 28%** | 19%** | 4%** | 4.2** |
| Haematological malignancy | 515 (1.2) | 272 | 53% | 58.6** | 58% | 21.6** | 24%** | 22%** | 6% | 18%** | 15% | 25%* | 37%** | 9% | 5.7 |
| Rheumatological disease | 746 (1.8) | 310 | 42% | 63.4** | 26%* | 17.2** | 31%* | 12% | 11% | 29% | 19% | 25%** | 32%* | 9% | 5.9 |
| T1 Diabetes | 936 (2.3) | 253 | 27% | 45.6** | 55% | 17.3** | 37% | 4%* | 5%* | 13%** | 29%** | 26%* | 18%* | 9% | 5.0 |
| T2 Diabetes | 2523 (6.1) | 606 | 24% | 65.5** | 54% | 17.6** | 35% | 7% | 17% | 25% | 20% | 27%** | 28% | 15%** | 5.4 |
APACHE: Acute Physiology and Chronic Health Evaluation; CIHD: chronic ischaemic heart disease; COPD: chronic obstructive pulmonary disease; MI: myocardial infarction.
P < 0.05. **p < 0.01.
The majority of patients (59%) had no prior documented comorbidity – Table 2. Among those with a comorbidity, 24% had one and 17% had more than one. Patients with comorbidities were significantly older, had higher APACHE II scores and were more likely to have received elective rather than emergency surgery compared with those without comorbidities. The most frequent comorbidities were solid malignancy (17%), chronic ischaemic heart disease (CIHD) (11%) and chronic obstructive pulmonary disease (7%).
Patients with a solid malignancy were significantly older, had lower mean APACHE II scores, were from less deprived areas and were more likely to have had elective surgery compared with those without comorbidities. Patients with liver disease, cirrhosis and liver failure were younger, had higher APACHE II scores, came from more deprived areas and were more likely to require multiple organ support.
Outcomes
After exclusion of patients who had been admitted to ICU following elective surgery, there were 32,344 patients who had an unanticipated admission to ICU. Survival is described for this cohort in Table 3 differentiated by presence of comorbidity.
Table 3.
Showing outcomes at several time points when elective surgical patients were excluded. Chi-square test comparing patients to those with no comorbidities. ‘Missing’ is missing data for hospital mortality, after 2005.
| Mortality |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No ‘pure’ admissions | ICU |
Hospital |
30 Days |
180 Days |
12 Months |
|||||||
| Group | % | P value | % | P value | Missing | % | P value | % | P value | % | P value | |
| Whole cohort | 32,344 | 24% | 29% | 1% | 31% | 38% | 41% | |||||
| No comorbidity | 21,419 | 21% | 25% | 1% | 27% | 32% | 34% | |||||
| 1 Comorbidity | 7215 | 27% | <0.01 | 33% | <0.01 | 1% | 35% | <0.01 | 42% | <0.01 | 46% | <0.01 |
| 2+ Comorbidities | 5710 | 31% | <0.01 | 39% | <0.01 | 1% | 42% | <0.01 | 52% | <0.01 | 58% | <0.01 |
| Asthma | 696 | 14% | <0.01 | 15% | <0.01 | 1% | 17% | <0.01 | 21% | <0.01 | 23% | <0.01 |
| COPD | 890 | 30% | <0.01 | 36% | <0.01 | 1% | 38% | <0.01 | 46% | <0.01 | 51% | <0.01 |
| Liver disease | 422 | 41% | <0.01 | 47% | <0.01 | 0% | 51% | <0.01 | 57% | <0.01 | 62% | <0.01 |
| Cirrhosis and failure | 162 | 41% | <0.01 | 53% | <0.01 | 0% | 54% | <0.01 | 63% | <0.01 | 64% | <0.01 |
| Renal disease | 133 | 34% | 0.01 | 35% | 0.01 | 2% | 47% | <0.01 | 52% | <0.01 | 56% | <0.01 |
| Dialysis dependent | 78 | 32% | 0.01 | 36% | 0.04 | 0% | 37% | 0.04 | 46% | 0.01 | 55% | <0.01 |
| CIHD | 640 | 26% | <0.01 | 31% | <0.01 | 1% | 33% | <0.01 | 39% | <0.01 | 41% | <0.01 |
| MI | 98 | 33% | 0.01 | 34% | 0.07 | 2% | 36% | 0.05 | 46% | <0.01 | 49% | <0.01 |
| Heart failure | 204 | 42% | <0.01 | 46% | <0.01 | 0% | 50% | <0.01 | 57% | <0.01 | 60% | <0.01 |
| Solid malignancy | 2476 | 21% | 0.56 | 30% | <0.01 | 1% | 31% | <0.01 | 45% | <0.01 | 52% | <0.01 |
| Haematological malignancy | 391 | 40% | <0.01 | 49% | <0.01 | 1% | 51% | <0.01 | 63% | <0.01 | 68% | <0.01 |
| Rheumatological disease | 277 | 34% | <0.01 | 39% | <0.01 | 1% | 44% | <0.01 | 47% | <0.01 | 52% | <0.01 |
| T1 Diabetes mellitus | 240 | 17% | 0.15 | 16% | 0.01 | 1% | 21% | 0.04 | 26% | 0.10 | 31% | 0.61 |
| T2 Diabetes mellitus | 505 | 27% | <0.01 | 33% | <0.01 | 1% | 34% | <0.01 | 41% | <0.01 | 43% | <0.01 |
CIHD: chronic ischaemic heart disease; COPD: chronic obstructive pulmonary disease; MI: myocardial infarction.
ICU and hospital mortality
ICU and hospital mortality were 24% and 29%, respectively, for the whole group and 21% and 25%, respectively, for patients with no comorbidities. Short-term mortality increased as the number of comorbidities present increased, with hospital mortality of 33% in those with one comorbidity (p < 0.01) when compared with patients without comorbidity), and 39% in those with two or more comorbidities (p < 0.01).
There was a large variation in mortality by different comorbidities. Mortality was highest among patients with liver cirrhosis/failure (53% hospital mortality, p < 0.01 when compared with patients without comorbidity), those with haematological malignancies (49% hospital mortality, p < 0.01) and in patients with heart failure (hospital mortality 46%, p < 0.01). In contrast, mortality was significantly lower for patients with asthma (hospital mortality 15%, p < 0.01) and type 1 diabetes mellitus (hospital mortality 16%, p value 0.01).
Patients with solid malignancies experienced no significant difference in ICU mortality from the no comorbidity group (21%, p value 0.56), however, the hospital mortality for this group was higher (30%, p value < 0.01).
Thirty- and 180-day mortality
Patients with no comorbidities had 30- and 180-day mortality rates of 27% and 34%, respectively. Short- and medium-term mortality were generally closely correlated. Thus, patients with liver disease, cirrhosis, haematological malignancy and heart failure had some of the highest 30- and 180-day mortalities.
One-year mortality
A year after admission to ICU, 41% of patients had died. There was a significantly higher mortality among patients with single (46%, p < 0.01) and multiple (58%, p < 0.01) comorbidities when compared to those without comorbidity (34%). Liver disease/cirrhosis, heart failure and haematological malignancies had the highest one-year mortality. Patients with asthma had significantly lower one-year mortality compared to those with no comorbidity (23% vs. 34%, respectively, p < 0.01).
Mortality rate
Mortality is greatest within the first few days of admission to ICU but the additional risk declines thereafter – Figure 1. Some difference in risk over time is evident between comorbidity groups. Patients with no comorbidity had the most favourable survival at all time points and this effect became more pronounced with time.
Figure 1.
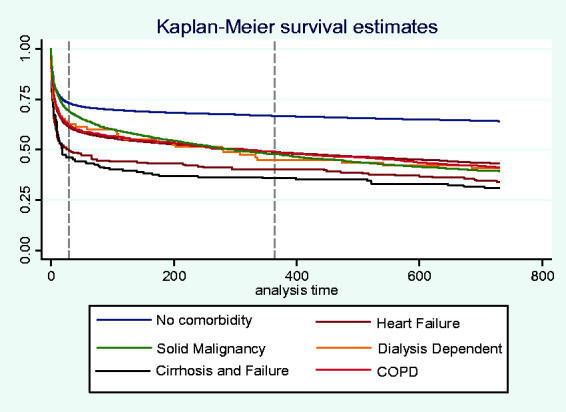
Kaplan–Meier survival curve comparing no comorbidities, heart failure, solid malignancy, COPD, cirrhosis and dialysis dependent patients. Dotted line at 30 days and 12 months. Log-rank test for equivalence, p value < 0.01. COPD: chronic obstructive pulmonary disease.
Multivariable analysis of comorbidity groups
The odds of mortality in ICU, at 30 days and 12 months were compared between patients with a range of comorbidities and none – Table 4. Odds were adjusted for APACHE II score, age, Scottish Index of Multiple Deprivation and ICU year, which were demonstrated to be significantly associated with mortality. The presence and number of comorbidities were associated with mortality at all time points with odds of death at ICU discharge, 30 days and 12 months of OR 1.19 (95% CI 1.12–1.27), OR 1.20 (1.13–1.28) and OR 1.36 (1.29–1.44) respectively for patients with one comorbidity, and OR 1.35 (1.26–1.44), OR 1.53 (1.44–1.63) and OR 2.0 (1.87–2.12) respectively for patients with multiple comorbidities.
Table 4.
Odds of death in intensive care unit (ICU), 30 days and 12 months, adjusted for age, SIMD, year of admission and Acute Physiology and Chronic Health Evaluation II (APACHE II) score, with elective surgical patients removed. The comparison group is those with no comorbidity.
| ICU |
30 Days |
12 Months |
||||
|---|---|---|---|---|---|---|
| Comorbidity | Odds ratio (CI) | P value | Odds ratio (CI) | P value | Odds ratio (CI) | P value |
| 1 Comorbidity | 1.19 (1.12–1.27) | <0.01 | 1.20 (1.13–1.28) | <0.01 | 1.36 (1.29–1.44) | <0.01 |
| 2+ Comorbidity | 1.35 (1.26–1.44) | <0.01 | 1.53 (1.44–1.63) | <0.01 | 2.00 (1.87–2.12) | <0.01 |
| Asthma | 0.79 (0.63–0.99) | 0.04 | 0.72 (0.57–0.89) | <0.01 | 0.81 (0.68–0.99) | 0.03 |
| COPD | 1.14 (0.98–1.33) | 0.84 | 1.13 (0.98–1.31) | 0.08 | 1.37 (1.19–1.58) | <0.01 |
| Liver disease | 2.98 (2.43–3.65) | <0.01 | 3.34 (2.73–4.09) | <0.01 | 4.01 (3.26–4.93) | <0.01 |
| Cirrhosis and failure | 2.61 (1.90–3.61) | <0.01 | 3.19 (2.32–4.39) | <0.01 | 3.82 (2.74–5.33) | <0.01 |
| Renal disease | 1.66 (1.15–2.41) | <0.01 | 2.05 (1.43–2.92) | <0.01 | 2.18 (1.52–3.14) | <0.01 |
| Dialysis dependent | 1.48 (0.90–2.41) | 0.11 | 1.31 (0.81–2.10) | 0.27 | 2.05 (1.28–3.29) | <0.01 |
| CIHD | 1.53 (1.19–1.98) | <0.01 | 0.83 (0.70–0.99) | 0.03 | 0.80 (0.68–0.95) | 0.01 |
| MI | 1.27 (0.83–1.94) | 0.28 | 0.98 (0.64–1.49) | 0.92 | 1.16 (0.77–1.74) | 0.48 |
| Heart failure | 1.79 (1.35–2.39) | <0.01 | 1.73 (1.30–2.29) | <0.01 | 1.74 (1.30–2.32) | <0.01 |
| Solid malignancy | 0.74 (0.67–0.83) | <0.01 | 0.84 (0.77–0.93) | <0.01 | 1.46 (1.33–1.59) | <0.01 |
| Haematological | 2.29 (1.85–2.83) | <0.01 | 2.64 (2.15–3.26) | <0.01 | 4.19 (3.34–5.26) | <0.01 |
| T1 diabetes | 1.01 (0.71–1.43) | 0.96 | 0.96 (0.69–1.33) | <0.81 | 1.32 (0.99–1.77) | 0.06 |
| T2 diabetes | 1.05 (0.86–1.29) | 0.61 | 1.00 (0.83–1.22) | 0.95 | 1.04 (0.86–1.25) | 0.38 |
| Rheumatological disease | 1.53 (1.18–1.98) | <0.01 | 1.63 (1.27–2.08) | <0.01 | 1.66 (1.29–2.10) | <0.01 |
CI: confidence interval; MI: myocardial infarction; CIHD: chronic ischaemic heart disease; COPD: chronic obstructive pulmonary disease.
Asthma (OR 0.79, 0.63–0.99) and solid tumour (OR 0.74, 0.67–0.83) were associated with lower odds of ICU mortality than no comorbidity. ICU mortality was raised for liver disease (OR 2.98, 2.43–3. 65), cirrhosis (OR 2.61, 1.90–3.65), haematological malignancy (OR 2.29, 1.85–2.83), heart failure (OR 1.79, 1.35–2.39), renal disease (OR 1.66, 1.15–2.41), CIHD (OR 1.53, 1.19–1.98) and rheumatological disease (OR 1.53, 1.18–1.98).
At one year most of these conditions had a continued increase in mortality; liver disease (OR 4.01, 3.26–4.93), cirrhosis (OR 3.28, 2.74–5.33), haematological malignancy (OR 4.19, 3.34–5.26), heart failure (OR 1.74, 1.3–2.32), renal disease (OR 2.18, 1.52–3.14) and rheumatological disease (OR 1.66, 1.29–2.1) with the exception of CIHD which had lower odds of one-year mortality (OR 0.8, 0.68–0.95). Patients with asthma continued to have lower odds of death at one year (OR 0.81, 0.68–0.99).
ICU mortality was similar among patients with one (OR 1.10, 1.02–1.18) and multiple (OR 1.09, 1.01–1.17) comorbidities. However, a difference emerged over time, such that patients with two or more comorbidities had considerably increased odds of mortality by 12 months.
Discussion
In a large unselected cohort of admissions to general ICUs, we found that two-fifths of patients had at least one comorbidity, of which a solid malignancy, chronic obstructive pulmonary disease (COPD), asthma and CIHD were the most frequent. Mortality within ICU was greatest among patients with liver disease, heart failure and haematological malignancies but lower for those with type 1 diabetes and asthma. We found a higher rate of solid cancer patient admissions, but a lower proportion of haematological patients than reported in the literature.10,11 The appreciation of the poor outcomes for haematological malignancy patients may explain the low admission rate in this cohort. We found that haematological malignancy patients had amongst the highest crude mortality rates of any condition and on multivariate adjusted analysis. In comparison, patients with solid malignancy had a lower adjusted ICU mortality with OR 0.74 (0.67–0.83) when compared to patients without any comorbidity. This may reflect the case mix with a larger proportion of surgical admissions in the solid tumour population who would be expected to have superior survival. The mortality benefit seen in solid tumours had reversed by one year with a higher odds of death at this time point.
Four-week mortality of patients with severe heart failure reported by Zannad et al. was considerably lower than in this study probably due to reporting on mainly specialist coronary care units which treat less severely ill patients than UK general ICUs.12 In a study of general hospital admissions chronic heart failure patients were shown to have worse survival than cancer patients; in this paper heart failure patients have much higher crude mortality than solid malignancy patients at all time points so the assertion that heart failure is ‘more malignant’ than cancer could perhaps also be applied to an ICU population.13
The ICU, hospital and 180-day mortality outcomes were broadly similar to the COPD and asthma outcome study (CAOS) study for COPD patients.14 This paper provides a fairly crude method for distinguishing COPD and asthma, possibly introducing classification bias. However, patients with a previous admission for asthma have considerably lower mortality rate at all-time points than COPD patients and on multivariate analysis asthma had an OD less than 1 in comparison to those with no comorbidity, though the mechanism for this is unclear and may reflect the more promptly reversible nature of asthma or the younger population more commonly affected. Consistent with the literature, liver cirrhosis patients have extremely poor short-term survival, and long-term survival is inevitably poor with nearly two-thirds dying within one year. There is little to distinguish the outcome of those with pre-cirrhotic liver disease and those with established cirrhosis, only doing slightly better in the long term. Therefore Welch’s study which groups all stages of alcoholic liver disease (ALD) together seems logical; though of course this paper considered many aetiologies of liver disease, not just alcohol.15
Strengths and weaknesses
Our study has a number of strengths, such as use of high-quality linked hospitalisation records to classify comorbidities. Also, the cancer patients were established using Scottish Cancer Registry data, the gold standard for identifying incident cancer cases. Our study had a large cohort size, being drawn from several centres which helps to account for case-mix bias and centre bias, such as differences in referral practices, and allows direct comparisons to be drawn in terms of the effect of so many different conditions. We considered the potential confounding effects of socio-economic deprivation on outcomes, which few other studies have done.
Weaknesses of our study include potential selection or misclassification bias because of under recognition of some conditions that do not result in a hospital admission. However, a patient with a previous admission for a condition is likely to represent those with more established disease. There is no consistent evidence of under reporting in this paper with percentage of admissions being comparable to that seen in the literature. A potential confounder which was not addressed was reason for admission as described; many studies simply considered patients who had been admitted for reasons relating to the condition but this paper considered any reason for admission. However, the impact of a comorbidity may be different when it has precipitated an admission to the ICU, although because these were all serious conditions they will probably have been a significant factor in any admission. A further confounder is the chronic health component of the APACHE II score, which contributes to the score for patients admitted with evidence of liver cirrhosis, heart failure New York Heart Association (NYHA) class IV, severe respiratory disease, chronic dialysis and immunocompromise (e.g. chemotherapy or haematological malignancy).16 The impact of this on multivariate analysis is unlikely to have affected the direction of the results but may have reduced the ODs. A difficulty was developing appropriate groups of comorbidities. The well-known Charlson Comorbidity Index for example makes no distinction between asthma and COPD, and describes alcoholic cirrhosis of the liver as mild liver disease and thus we felt was not fit for purpose but one might suggest this introduces bias. Ideally we would also have had data detailing severity of comorbidity such as Child Pugh scores for liver disease but this was not available due to the retrospective nature of the study. It would also have been beneficial to have data on factors such as nutrition and functional status but these are not routinely recorded. This paper considers comorbidities individually and they may interact in was beyond its scope to address how these comorbidities interact, and as shown by Barnett et al. is not simply linear.17
Implications for practice
The impact of single comorbidities on survival after admission to ICU is significant and does not necessarily reflect possible biases within the ICU community. While this information should not dictate admission policies it should be used to facilitate discussions with patients and families about realistic outcomes. Clinicians should be aware that outcomes in patients with liver disease, cirrhosis, haematological malignancy, heart failure and rheumatological disease are significantly poorer than that seen in patients with other comorbidities. In the presence of other negative prognostic factors this may raise questions about whether these patients are receiving appropriate end-of-life care by being admitted to ICU.
Further research
Future papers could improve identification of patients with comorbidities by linking to further disease registers, such as the UK Renal Registry. Also the APACHE II score might be disaggregated to remove the chronic health component, and further work on the multivariate model taking into account time dependent differentials would give more accurate results.
Conclusion
This large observational study of all admissions to 16 general ICUs has established the individual effect of many chronic conditions on mortality for patients admitted to ICU. It demonstrates that liver disease, liver cirrhosis, heart failure, haematological malignancy and rheumatological disease are independently associated with increased odds of death in the ICU; and at 12 months COPD, renal disease and solid malignancy were also associated with increased odds of death. This paper has demonstrated the considerable and varied impact of chronic conditions on outcomes for acutely unwell patients. It is hoped that this paper will provide a valuable resource to clinicians managing patients with critical illness, as well as aiding conversations with patients and family.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from Cancer Research UK (C47114A / A16662).
ORCID iDs
Alasdair Simpson https://orcid.org/0000-0002-1578-3568
Philip McLoone https://orcid.org/0000-0003-0002-8700
References
- 1.Esper AM, Martin GS. The impact of comorbid [corrected] conditions on critical illness. Crit Care Med 2011; 39: 2728–2735. [DOI] [PubMed] [Google Scholar]
- 2.Guidelines for intensive care unit admission, discharge, and triage. Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine. Crit Care Med 1999; 27: 633–638. [PubMed] [Google Scholar]
- 3.Puxty K, McLoone P, Quasim T, et al. Survival in solid cancer patients following intensive care unit admission. Inten Care Med 2014; 40: 1409–1428. [DOI] [PubMed] [Google Scholar]
- 4.Hutchison CA, Crowe AV, Stevens PE, et al. Case mix, outcome and activity for patients admitted to intensive care units requiring chronic renal dialysis: a secondary analysis of the ICNARC Case Mix Programme Database. Crit Care 2007; 11: R50(2007). 10.1186/cc5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomson SJ, Moran C, Cowan ML, et al. Outcomes of critically ill patients with cirrhosis admitted to intensive care: an important perspective from the non-transplant setting. Aliment Pharmacol Ther 2010; 32: 233–243. [DOI] [PubMed] [Google Scholar]
- 6.Azoulay E, Soares M, Darmon M, et al. Intensive care of the cancer patient: recent achievements and remaining challenges. Ann Inten Care 2011; 1. : 5. DOI: 10.1186/2110-5820-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puxty K, Mcloone P, Quasim T, et al. Risk of critical illness among patients with solid cancers. JAMA Oncol 2015; 1: 1078. [DOI] [PubMed] [Google Scholar]
- 8.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic co-morbidity in longitudinal-studies – development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 9.Sundararajan V, Henderson T, Perry C, et al. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 2004; 57: 1288–1294. [DOI] [PubMed] [Google Scholar]
- 10.Taccone FS, Artigas AA, Sprung CL, et al. Characteristics and outcomes of cancer patients in European ICUs. Crit Care 2009; 13: R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bos MMEM, de Keizer NF, Meynaar IA, et al. Outcomes of cancer patients after unplanned admission to general intensive care units. Acta Oncol 2012; 51: 897–905. [DOI] [PubMed] [Google Scholar]
- 12.Zannad F, Mebazaa A, Juilliere Y, et al. Clinical profile, contemporary management and one-year mortality in patients with severe acute heart failure syndromes: the EFICA study. Eur J Heart Fail 2006; 8: 697–705. [DOI] [PubMed] [Google Scholar]
- 13.Stewart S, MacIntyre K, Hole DJ, et al. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail 2001; 3: 315–322. [DOI] [PubMed] [Google Scholar]
- 14.Wildman MJ, Sanderson C, Groves J, et al. Implications of prognostic pessimism in patients with chronic obstructive pulmonary disease (COPD) or asthma admitted to intensive care in the UK within the COPD and asthma outcome study (CAOS): multicentre observational cohort study. Br Med J 2007; 335: 1132–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welch C, Harrison D, Short A, et al. The increasing burden of alcoholic liver disease on United Kingdom critical care units: secondary analysis of a high quality clinical database. J Health Serv Res Policy 2008; 13: 40–44. [DOI] [PubMed] [Google Scholar]
- 16.Knaus WA, Draper EA, Wagner DP, et al. Apache-II – a severity of disease classification-system. Crit Care Med 1985; 13: 818–829. [PubMed] [Google Scholar]
- 17.Barnett K, Mercer S, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. The Lancet 2012; 380: 37–43. [DOI] [PubMed] [Google Scholar]


