Abstract
This primer summarizes the diagnosis, treatment, complications, and prognosis of anti-N-methyl-d-aspartate receptor encephalitis for healthcare professionals, especially those in acute care specialities. Anti-N-methyl-d-aspartate receptor encephalitis is an immune-mediated encephalitis that is classically paraneoplastic and associated with ovarian teratomas in young women. Other less common neoplastic triggers include testicular cancers, Hodgkin lymphoma, lung and breast cancers. It may also be triggered by infection, occurring as a para-infectious phenomenon, seen most commonly after herpes simplex-1 encephalitis. Presentation varies but typically consists of behavioural and cognitive manifestations, seizures, dysautonomia, movement disorders, central hypoventilation, and coma, necessitating intensive care unit admission. Diagnosis of anti-N-methyl-d-aspartate receptor encephalitis requires high clinical suspicion plus ancillary testing, the most sensitive being cerebrospinal fluid analysis for anti-N-methyl-d-aspartate receptor antibodies. Imaging in search of an ovarian teratoma should be exhaustive and tumours need to be surgically treated. Treatment should be expeditious with pulsed steroids and either plasma exchange or intravenous immunoglobulin. Second-line treatments include intravenous rituximab, cyclophosphamide, azathioprine, and intrathecal methotrexate. Most patients recover to be functionally independent, but the in-hospital course can be months long followed by extensive rehabilitation. Given the lengthy course of illness, we explain why education and debriefing are important for staff, and where families can obtain additional help.
Keywords: Anti-N-methyl-d-aspartate receptor encephalitis, encephalitis, intensive care unit, ovarian teratoma
Introduction
Anti-N-methyl-d-aspartate receptor (NMDA-r) encephalitis is a serious, but treatable, cause of a profound disability. It commonly affects young women and can initially be misdiagnosed as a drug-related reaction or other disorder, which matters greatly because the quality of recovery is correlated with faster diagnosis and therapy.1–5 Accordingly, informed healthcare professionals (HCPs) play an important role, especially those in Critical Care (ICU), Emergency Medicine, Internal Medicine, Psychiatry, and Neurology. This primer outlines the essentials of NMDA-r encephalitis.
This immune-mediated encephalitis is often paraneoplastic and associated most commonly with ovarian teratoma.6 Other neoplasms associated with NMDA-r include Hodgkin lymphoma7 as well as testicular, lung, and breast cancers.3 However, many patients lack a clear trigger, and one case series of 44 patients found that the majority of cases were not associated with a neoplasm.8 Another identified risk factor is a history of herpes simplex virus-1 (HSV-1) encephalitis, where a study of 51 patients with HSV encephalitis found that 27% went on to develop autoimmune encephalitis, with 9 of the 14 patients developing autoimmune encephalitis having NMDA-r antibodies.9–11 Patients characteristically start with a constellation of cognitive and behavioural symptoms such as delusions, hallucinations, and/or catatonia, and therefore, may present to psychiatry.5,6 This usually follows a subacute course, evolving into movement disorders such as chorea, dystonia, and stereotypies,12 in addition to altered mental status, and ultimately involving the neurology service.5 Intensive care unit staff usually become involved with the subsequent development of seizures, status epilepticus, and profound dysautonomia, resulting in cardiac arrhythmias, hyperthermia, hypoventilation, and coma. However, patients may present early with seizures and in status epilepticus, warranting earlier ICU involvement.
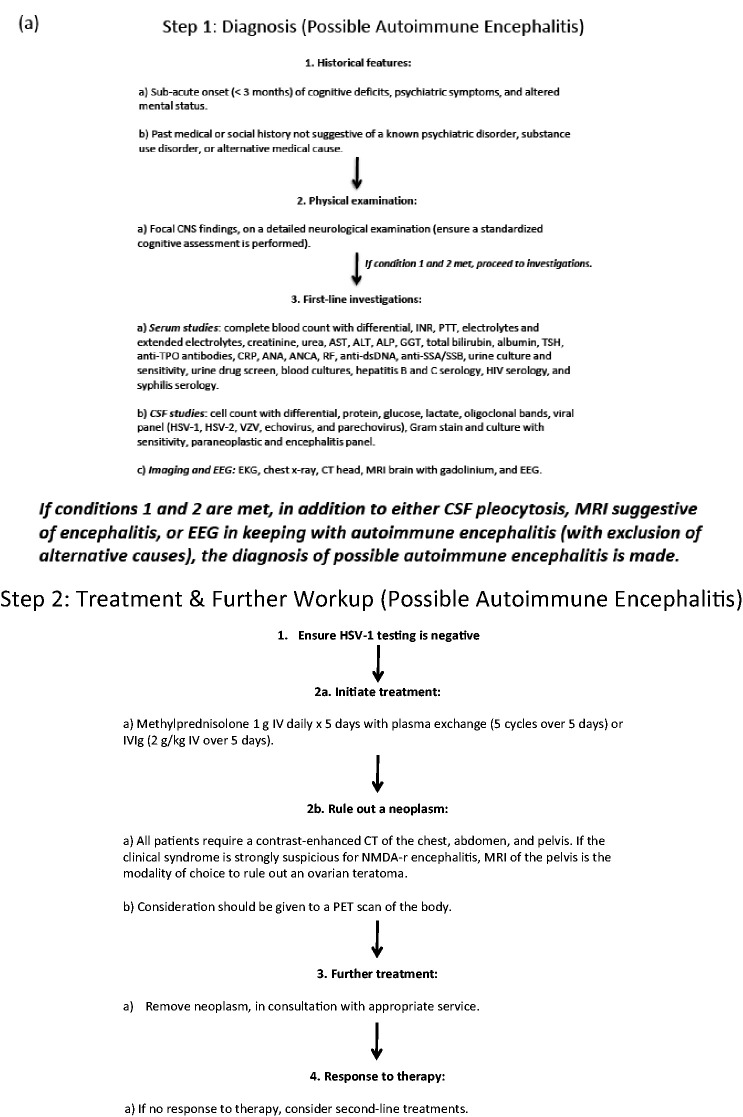
In addition to highlighting key points about NMDA-r encephalitis (Figure 1), this review summarizes the steps required to secure an early diagnosis and spur expeditious and aggressive management (Figure 2(a) and (b)). We also outline unique complications, and what to do when first-line treatments fail. Importantly, a diagnosis of NMDA-r encephalitis can cause considerable emotional toll on families and HCPs, given its protracted course and dramatic symptoms. It is important to emphasize that it has a generally favourable long-term outcome with high-quality survival (Figure 3). Accordingly, ongoing ICU effort and resources are justified, and patients, HCPs, and families may benefit from support and counselling (Figure 4).
Figure 1.
Ten summary points for ICU staff related to NMDA-r encephalitis.
CSF: cerebrospinal fluid; EEG: electroencephalogram; HSV: herpes simplex virus; ICU: intensive care unit; IVIg: intravenous immunoglobulin; NMDA-r: anti-N-methyl-d-aspartate receptor.
Figure 2.
Algorithmic approach to NMDA-R encephalitis. ALP: alkaline phosphatase; ALT: alanine transaminase; ANA: antinuclear antibody; ANCA: anti-neutrophil cytoplasmic antibodies; AST: aspartate aminotransferase; CNS: central nervous system; CRP: c-reative protein; CSF: cerebrospinal fluid; CT: computed tomography; EEG: electroencephalogram; EKG: electrocardiogram; GGT: gamma-glutamyl transferase; HSV-1: herpes simplex virus-1; INR: international normalized ratio; IVIg: intravenous immunoglobulin; MRI: magnetic resonance imaging; NMDA-r: anti-N-methyl-d-aspartate receptor; PET: positron emission tomography; PTT: partial thromboplastin time; RF: rheumatoid factor; SSA: anti-Sjogren's syndrome type a; SSB: anti-Sjogren's syndrome type b; TPO: thyroid peroxidase; TSH: thyroid stimulating hormone; VZV: varicella zoster virus.
Figure 3.
The mRS.
A 0–6 scale for quantifying disability or dependence following a neurological illness (especially stroke).34
mRS: modified Rankin scale.
Note: In contrast, the Glasgow Outcome Score (GOS) is a 1–5 scale used to assess neurologic recovery, usually cerebral trauma. A low GOS equates with worse outcome (i.e. score of 1 equates death and 5 equates low disability).
Figure 4.
Resources for families following the diagnosis of NMDA-r encephalitis.
NMDA-r: anti-N-methyl-d-aspartate receptor.
Diagnosing NMDA-r encephalitis: Chance favours a prepared mind
Diagnosis starts with a thorough history and physical exam, followed by laboratory confirmation. ‘Probable NMDA-r encephalitis’ incorporates three criteria: (1) a rapid onset (over less than three months) of at least four of the following symptoms: abnormal behaviour or cognitive dysfunction, speech dysfunction, seizures, movement disorder, decreased level of consciousness, autonomic dysfunction or central hypoventilation; (2) abnormal electroencephalogram (EEG) (i.e. extreme delta brush) or abnormal cerebrospinal fluid (CSF) with pleocytosis or oligoclonal bands; and (3) reasonable exclusion of other disorders, most notably primary psychiatric disorders, exposure to drugs or toxins, CNS infections, alternate immune mediated conditions, or metabolic disorders.13 ‘Definite NMDA-r encephalitis’ is made following the detection of CSF antibodies to the GluN1 subunit of the NMDA receptor.13
In five years, our institution has had 27 patients with definite NMDA-r encephalitis. Just over half (14/27) required ICU involvement before definitive diagnosis. Thus, acute care HCPs play an important collaborative role in caring for these patients and their families. This is emphasized by the association between worse outcome and delays in diagnosis and treatment.1,4–6,14 Intensive care unit HCPs should also understand that there are many forms of encephalitis (i.e. autoimmune, paraneoplastic, and infectious), and the approach to diagnosis, treatment, and outcomes differs.
Following multidisciplinary input (neurology, psychiatry, infectious diseases, and intensive care), along with established criteria,13 we developed an algorithmic protocol (Figure 2). This is intended to summarize and expedite diagnosis and treatment. It is informed by local experience and worldwide literature. Diagnostic tests include (i) serum studies, (ii) CSF analysis, (iii) EEG, and (iv) imaging studies. Serum studies serve to rule out systemic inflammatory or infectious conditions. CSF analysis is employed to rule out infectious causes of encephalitis (especially HSV-1), to identify a lymphocytic pleocytosis or CSF-specific oligoclonal bands, and to identify GluN1 autoantibodies. Notably, CSF is more sensitive than serum for the NMDA-r antibody,15 and therefore, should be tested even in the absence of serum antibodies. Either flow cytometry or cell-based assays can be used to detect NMDA-r antibodies with cell-based assays appearing to have higher sensitivity without compromising specificity.16
Magnetic resonance imaging (MRI) of the brain with and without gadolinium is often unremarkable in NMDA-r encephalitis patients.17,18 However, MRI is still recommended to rule out other mimics including inflammatory, infectious, or neoplastic lesions of the CNS. EEG can be useful, as it is rarely normal in patients with NMDA-r encephalitis; the pattern of extreme delta brush is highly specific.19 Moreover, EEG abnormalities correlate with the need for ICU admission and prolonged recovery time.20
Treatment: Multipronged, aggressive, and expeditious
Treatment should start without delay once HSV-1 testing (either by polymerase chain reaction or serological analysis) is confirmed negative in CSF. If there is low suspicion of HSV encephalitis and features suggestive of NMDA-r encephalitis (see above), treatment should be initiated promptly. First-line treatment includes intravenous methylprednisolone (1 g daily for five days), alongside either plasma exchange (five cycles over five days) or intravenous immunoglobulin (IVIg) (2 g/kg IV over five days) (Figure 2). Our local preference is to use plasma exchange rather than IVIg providing the patient is cooperative. For psychotic or agitated patients, plasma exchange includes the risk of patients pulling out their central lines. In these cases, we either judiciously sedate or use IVIg instead.
Alongside expeditious treatment, it is important to thoroughly investigate for a neoplasm with computed tomography (CT) of the chest and abdomen. We order pelvic MRI for an ovarian teratoma because pelvic ultrasound can miss a small teratoma. Moreover, there should be a low suspicion for diagnostic laparoscopy, if all imaging modalities are normal. If a neoplasm is found it should be surgically removed. If no neoplasm is found, we perform whole body positron emission tomography (PET). Still if a neoplasm is not detected, PET body or CT of the chest, abdomen, and pelvis and MRI of the pelvis should be repeated at regular intervals. This is because of case reports of ovarian teratomas detected years after initial presentation of NMDA-r encephalitis. Dalmau and colleagues recommend screening for ovarian teratomas for at least two years after initial presentation regardless of recovery. Although not currently standard practice, there are case reports of exploratory laparotomies where occult ovarian teratomas were discovered and removed, with subsequent improvement in the patient’s clinical status.21,22
When primary treatments fail (do not give up)
Treatment failure is defined as little to no clinical response after four weeks of treatment. Currently, there is no standardized approach to second-line treatment, if the first-line combination of pulsed steroids, plasma exchange, and/or IVIg fails. Justifiable second-line options include rituximab,5,22 cyclophosphamide,5 azathioprine,24 and intrathecal methotrexate.25 High-quality evidence for these therapeutic agents is lacking in the setting of NMDA-r encephalitis, and their rationale comes from their effectiveness in other autoimmune conditions.
Aside from the aforementioned second-line agents, bortezomib has shown benefit in small case series of refractory NMDA-r encephalitis. This proteasome inhibitor is approved for use in multiple myeloma and functions by depleting plasma cells.26 In one case series of five patients, bortezomib was associated with clinical improvement and a concomitant decrease in NMDA receptor antibody titres in all but one patient.27 Separately, in a report of two cases, both patients benefited from bortezomib, one of whom received the therapy following relapse.28 In a third case series of five patients with refractory disease, some of the patients showed some clinical improvement and a reduction in antibody titres, but did not improve their modified Rankin score (mRS score; see Figure 3 for a description of this useful functional scoring system).29 Locally, we have used bortezomib in five refractory NMDA-r encephalitis cases and witnessed subjective improvement in all five. Furthermore, the safety profile of bortezomib appears to be favourable.27,28 Accordingly, and while more study data are required, this agent has been added to the arsenal of immune treatments for refractory NMDA-r encephalitis.
Complications for patients with NMDA-r encephalitis
In the acute phase of the illness, complications include seizures, dysautonomia, central hypoventilation, movement disorders, agitation, and catatonia. Dysautonomia most often presents with bradycardia and tachycardia, hypotension and hypertension, hyperthermia, and hypersalivation.6 Bradycardia can be significant enough to necessitate cardiac pacing.6 Central hypoventilation can disrupt the patient’s synchronization of breathing. This can prolong the need for mechanical ventilation (necessitating tracheostomy and a percutaneous feeding tube) and cause ventilator dyssynchrony (delaying ventilatory weaning and ward transfer).
Movement disorders associated with NMDA-r encephalitis are a highly disabling patient complication. Movement disorders are also extremely emotionally distressing for families and HCPs. They often present later and include choreoathetosis, dystonia, stereotypies, ballism, catatonia, myoclonus, and opisthotonos.5,12 Oro-lingual-facial dyskinesias are common and can cause mouth trauma such as tongue laceration and broken teeth.6 Bite blocks may mitigate such injuries. Currently there is no accepted approach to managing NMDA-r encephalitis associated movement disorders. Dopamine depleting agents such as tetrabenazine, deutetrabenazine, and valbenazine have been described in the literature as potential options for treating hyperkinetic movement disorders.30 We have employed botulinum toxin, injected into the masseter muscles, to mitigate the complications of the oro-lingual-facial dyskinesias. Agitation and catatonia are also common. Involvement of the psychiatry service may help. High doses of benzodiazepines can control agitation, insomnia, and catatonia.31 Electroconvulsive therapy may alleviate catatonia.31
Outcomes
Friends and family may assume that any patient with severe and long-lasting neurologic symptoms will almost certainly suffer significant permanent brain damage. Therefore, it is important to emphasize that the majority of NMDA-r encephalitis patients eventually do well. In a large case series (n = 100) at a median follow-up of 17 months, 75% either fully recovered or had only mild residual deficits with a mRS of 0 and/or returned to work.6 However, recovery can take many months and usually requires prolonged physical and cognitive rehabilitation. Importantly, it should be noted 25% do not recover as well, and between 5 and 10% are dead within a year of diagnosis.6 Recovery in that study was defined as having an mRS of 0, mild deficits, and/or if patients were able to return to their activities of daily living.6 Cognitive dysfunction is also a common consequence of NMDA-r encephalitis, with one paper reporting eight of nine patients having persistent cognitive impairment up to 2–3 years after the illness.14
Given the patient’s typical young age and long inpatient battle, this disease can be especially distressing for both staff and families. Moreover, HCPs may not be accustomed to persisting with treatment in patients with severe neurological disease for as long as may be required in this disease. As such, we strongly encourage staff education about the prognosis coupled with an opportunity to debrief about their own feelings. We also recommend maximizing physiotherapy for patients and in-hospital family support. Families may also benefit from contact with support groups. Figure 4 summarizes five resources which may be of benefit to both families and HCPs seeking basic knowledge and support.
To date, there is insufficient data to conclude which factors are associated with neurological and overall functional outcomes after NMDA-r encephalitis. Generally, prognosis is worse if patients present with a low Glasgow Coma Scale score, if the diagnosis is delayed, if treatment is ineffective, if ICU care is prolonged, or if NMDA-r encephalitis is associated with other diseases.1,4,32,33 Balu and colleagues examined the probability of a poor functional outcome using a cutoff mRS score of 3 or more at one-year. Specifically, they identified the following independent predictors of poor outcome: the need for ICU admission, treatment delay greater than four weeks, lack of clinical improvement within four weeks, elevated CSF white blood cell count (>20 cells/µl), and an abnormal MRI. They assigned each of these variables one point to create the anti-NMDAR encephalitis One-Year Functional Status (NEOS) score, where a higher NEOS score was associated with greater probability of poor one-year functional outcome.4
Another multicentre study of patients with autoimmune encephalitis necessitating ICU admission found that the need for mechanical ventilation, tracheostomy, the presence of a tumour, sepsis, or autonomic dysfunction were associated with poor neurological outcome.33 Another study found that receiving immunotherapy early and a low CSF white blood cell count were associated with a good neurological outcome, defined as an mRS score of 0–2 at six months after ICU admission.1
Importantly, even after recovery, patients need long term follow-up. This is because 20–25% of patients relapse from this condition.6 After such an investment of time, resources, and empathy, it would be especially tragic to lose patients to follow-up. In summary, with a combination of knowledge, coordination, persistence, and attention, HCPs can significantly help patients and families suffering with NMDA-r encephalitis, and the likelihood of meaningful recovery.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.de Montmollin E, Demeret S, Brule N, et al. Anti-N-methyl-D-aspartate receptor encephalitis in adult patients requiring intensive care. Am J Respir Crit Care Med 2017; 195: 491–499. [DOI] [PubMed] [Google Scholar]
- 2.Mittal MK, Rabinstein AA, Hocker SE, et al. Autoimmune encephalitis in the ICU: analysis of phenotypes, serologic findings, and outcomes. Neurocrit Care 2016; 24: 240–250. [DOI] [PubMed] [Google Scholar]
- 3.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013; 12: 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balu R, McCracken L, Lancaster E, et al. A score that predicts 1-year functional status in patients with anti-NMDA receptor encephalitis. Neurology 2019; 92: e244–e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalmau J, Lancaster E, Martinez-Hernandez E, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011; 10: 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008; 7: 1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zandi MS, Irani SR, Follows G, et al. Limbic encephalitis associated with antibodies to the NMDA receptor in Hodgkin lymphoma. Neurology 2009; 73: 2039–2040. [DOI] [PubMed] [Google Scholar]
- 8.Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 2010; 133: 1655–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armangue T, Leypoldt F, Malaga I, et al. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Annals Neurol 2014; 75: 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armangue T, Moris G, Cantarin-Extremera V, et al. Autoimmune post-herpes simplex encephalitis of adults and teenagers. Neurology 2015; 85: 1736–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armangue T, Spatola M, Vlagea A, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol 2018; 17: 760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varley JA, Webb AJS, Balint B, et al. The Movement disorder associated with NMDAR antibody-encephalitis is complex and characteristic: an expert video-rating study. J Neurol Neurosurg Psychiatry 2019; 90: 724–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016; 15: 391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finke C, Kopp UA, Pruss H, et al. Cognitive deficits following anti-NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry 2012; 83: 195–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang R, Guan HZ, Ren HT, et al. CSF findings in patients with anti-N-methyl-D-aspartate receptor-encephalitis. Seizure 2015; 29: 137–142. [DOI] [PubMed] [Google Scholar]
- 16.Ramberger M, Peschl P, Schanda K, et al. Comparison of diagnostic accuracy of microscopy and flow cytometry in evaluating N-methyl-D-aspartate receptor antibodies in serum using a live cell-based assay. PLoS One 2015; 10: e0122037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang R, Lai XH, Liu X, et al. Brain magnetic resonance-imaging findings of anti-N-methyl-D-aspartate receptor encephalitis: a cohort follow-up study in Chinese patients. J Neurol 2018; 265: 362–369. [DOI] [PubMed] [Google Scholar]
- 18.Zhang T, Duan Y, Ye J, et al. Brain MRI characteristics of patients with anti-N-methyl-D-aspartate receptor encephalitis and their associations with 2-year clinical outcome. AJNR Am J Neuroradiol 2018; 39: 824–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitt SE, Pargeon K, Frechette ES, et al. Extreme delta brush: a unique EEG pattern in adults with anti-NMDA receptor encephalitis. Neurology 2012; 79: 1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillinder L, Warren N, Hartel G, et al. EEG findings in NMDA encephalitis – a systematic review. Seizure 2019; 65: 20–24. [DOI] [PubMed] [Google Scholar]
- 21.Abdul-Rahman ZM, Panegyres PK, Roeck M, et al. Anti-N-methyl-D-aspartate receptor encephalitis with an imaging-invisible ovarian teratoma: a case report. J Med Case Rep 2016; 10: 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boeck AL, Logemann F, Krauss T, et al. Ovarectomy despite negative imaging in anti-NMDA receptor encephalitis: effective even late. Case Rep Neurol Med 2013; 2013: 843192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee WJ, Lee ST, Byun JI, et al. Rituximab treatment for autoimmune limbic encephalitis in an institutional cohort. Neurology 2016; 86: 1683–1691. [DOI] [PubMed] [Google Scholar]
- 24.Shin YW, Lee ST, Park KI, et al. Treatment strategies for autoimmune encephalitis. Ther Adv Neurol Disord 2018; 11: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang XZ, Zhu HD, Ren HT, et al. Utility and safety of intrathecal methotrexate treatment in severe anti-N-methyl-D-aspartate receptor encephalitis: a pilot study. Chin Med J 2018; 131: 156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hideshima T, Richardson PG, Anderson KC. Mechanism of action of proteasome inhibitors and deacetylase inhibitors and the biological basis of synergy in multiple myeloma. Mol Cancer Ther 2011; 10: 2034–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheibe F, Pruss H, Mengel AM, et al. Bortezomib for treatment of therapy-refractory anti-NMDA receptor encephalitis. Neurology 2017; 88: 366–370. [DOI] [PubMed] [Google Scholar]
- 28.Behrendt V, Krogias C, Reinacher-Schick A, et al. Bortezomib treatment for patients with anti-N-Methyl-D-aspartate receptor encephalitis. JAMA Neurol 2016; 73: 1251–1253. [DOI] [PubMed] [Google Scholar]
- 29.Shin YW, Lee ST, Kim TJ, et al. Bortezomib treatment for severe refractory anti-NMDA receptor encephalitis. Ann Clin Transl Neurol 2018; 5: 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baizabal-Carvallo JF, Jankovic J. Autoimmune and paraneoplastic movement disorders: an update. J Neurol Sci 2018; 385: 175–184. [DOI] [PubMed] [Google Scholar]
- 31.Kruse JL, Jeffrey JK, Davis MC, et al. Anti-N-methyl-D-aspartate receptor encephalitis: a targeted review of clinical presentation, diagnosis, and approaches to psychopharmacologic management. Ann Clin Psychiatry 2014; 26: 111–119. [PubMed] [Google Scholar]
- 32.Chi X, Wang W, Huang C, et al. Risk factors for mortality in patients with anti-NMDA receptor encephalitis. Acta Neurol Scand 2017; 136: 298–304. [DOI] [PubMed] [Google Scholar]
- 33.Schubert J, Bramer D, Huttner HB, et al. Management and prognostic markers in patients with autoimmune encephalitis requiring ICU treatment. Neurol Neuroimmunol Neuroinflamm 2019; 6: e514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farrell B, Godwin J, Richards S, Warlow C. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry 1991; 54: 1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]