Abstract
Surgery to treat drug-resistant epilepsy can be quite effective but remains substantially underutilized. A pilot study was undertaken to test the feasibility of using a non-invasive, non-ablative, approach for producing focal neuronal loss to treat seizures in a rodent model of temporal lobe epilepsy. In this study, spontaneous, recurrent seizures were established in a mouse model of pilocarpine-induced status epilepticus (SE). After post-SE stabilization, baseline behavioral seizures were monitored for 30 days. Non-invasive opening of the Blood-Brain Barrier (BBB) targeting the hippocampus was then produced by using MRI-guided, low-intensity Focused Ultrasound (MRgFUS), through which a neurotoxin (Quinolinic Acid) administered intraperitoneally gained access to the brain parenchyma to produce focal neuronal loss. Behavioral seizures were then monitored for 30 days after this procedure, and brains were subsequently prepared for histological analysis of the sites of neuronal loss. The average frequency of behavioral seizures in all animals (n=11) was reduced by 21.2%. Histological analyses along the longitudinal axis of the hippocampus showed that most of the animals (n=8) exhibited neuronal loss located primarily in the intermediate aspect of the hippocampus, while sparing the septal aspect. Two other animals with damage to the intermediate hippocampus also exhibited prominent bilateral damage to the septal aspect of the hippocampus. A final animal had negligible neuronal loss overall. Notably, the site of neuronal loss along the longitudinal axis of the hippocampus influenced seizure outcomes. Animals that did not have bilateral damage to the septal hippocampus displayed a mean decrease in seizure frequency of 27.7%, while those with bilateral damage to septal hippocampus actually increased seizure frequency by 18.7%. The animal without neuronal loss exhibited an increase in seizure frequency of 19.6%. The findings indicate an overall decrease in seizure frequency in treated animals. And, the site of neuronal loss along the longitudinal axis of the hippocampus appears to play a key role in reducing seizure activity. These pilot data are promising, and they encourage additional and more comprehensive studies examining the effects of targeted, non-invasive, neuronal lesions for the treatment of epilepsy.
Keywords: epilepsy surgery, non-invasive, focused ultrasound, MR-guided, neuronal loss
Introduction
Epilepsy is a major neurological disorder that is primarily treated with one or more anti-epileptic drugs (AEDs). Unfortunately, one-third or more of epileptic patients are unresponsive to AEDs, and the inability to medically control seizures results in a poor quality of life and can be life-threatening. Neurosurgical interventions are quite effective in controlling seizures in appropriately-selected candidates with drug resistant epilepsy (DRE). Epilepsy surgery can reduce or eliminate seizures, and can greatly improve the quality of life in DRE patients (e.g. Wiebe et al. 2001; Engel et al. 2003; West et al. 2016). Temporal lobectomy is the most common type of surgery for patients with temporal lobe epilepsy. A portion of the anterior temporal lobe along with the amygdala and hippocampus are removed. A temporal lobectomy leads to a significant reduction or complete seizure control about 70% to 80% of the time (Sperling et al. 1996, Dupont et al. 2006). However, memory and language can be affected if this procedure is performed on the dominant hemisphere. With assistance of MRI, selective amygdalohippocampectomy (SAH) removes mesial structures, leaving lateral temporal cortex intact, resulting in less risk for language dysfunction and fewer neuropsychological sequelae (Spencer et al. 2012). However, despite extensive and growing evidence supporting of the benefits of epilepsy surgery, this treatment modality remains substantially underutilized (Haneef et al. 2010; Englot et al. 2012; Jette et al. 2012; Burneo et al. 2016). One reason for such underutilization is that well-established, resective surgeries have multiple contraindications and possible complications. They can be highly invasive, may require the removal of substantial amounts of brain tissue, can cause bleeding, infection, blood clots, strokes, seizures, swelling of the brain, and nerve damage, may require long recovery periods, and can be expensive (e.g. Rydenhag and Silander 2001; McClelland et al. 2011). Moreover, unintended functional deficits in memory, language comprehension, and visual processing can occur (e.g. Helmstaedter et al. 2004; Spencer and Huh 2008; Hader et al. 2013).
More recent advances using minimally-invasive and non-invasive procedures hold considerable promise for treating DRE, and can limit certain complications. For instance stereotactic laser ablation (SLA) is minimally invasive, and has recently been mentioned as a “technological advance (that) has revolutionized epilepsy management…” (Saipetch et al. 2016). However, SLA is not without its limitations, including bleeding, probe mal-positioning, and thermal effects on neighboring, non-target structures. SLA requires burr holes to be drilled in the skull and the insertion of one or more probes into the brain. The probes are then used to produce ablative, thermal lesions, which can risk injury to neighboring neural or ventricular structures, and/or fibers of passage (Gross et al. 2016). In addition, SLA cannot produce conformal areas of damage. Consequently, in order to treat an irregularly-shaped target, which is a common feature of seizure-genic tissue, multiple probe insertions can be necessary. Similar issues attend the use of radiofrequency thermocoagulation (e.g. Wellmer et al. 2014).
Other surgical options include radiosurgery (e.g. Quigg and Barbaro 2008) and High Intensity Focused Ultrasound (HIFU; e.g. Martin et al. 2009). A notable advantage of radiosurgery and HIFU is that they are both non-invasive procedures. However, with radiosurgery, there is a protracted delay to reductions in seizures, a need for ionizing radiation to the brain, and the risk of a postsurgical period of heightened seizure activity. HIFU is also non-invasive and has the advantage of being able to produce conformal targeting, allowing tailored treatment of irregularly-shaped targets. However, HIFU is also a thermal lesioning technique. Its ablation effect requires the deposition of a significant amount of energy in the brain tissue. The critical amount of energy deposition can be achieved only when the gain of the focused beam is high enough to overcome the dissipation effect of the skull. The resulting treatment envelope includes tissues located at greater distance from the skull, typically more central areas of the brain (e.g., thalamus), whereas superficial portions of the brain or structures located nearer to bone (e.g., hippocampus) are more challenging to treat. This was demonstrated by a previous study from our group with MR-guided HIFU treatment on the rat pups of different ages (from 9 to 43 days). The electric power was selected to always reach a target temperature of at least 50°C in the parenchyma. For the 30-day-old pups, with thin skull, the temperature in the brain tissue adjacent to the skull increased to 48.9°C; for rodents older than 33 days, with a thicker skull, reached 60°C or higher, which can produce undesired irreversible damage in this location (Zhang, et al. 2015).
The present study examined the feasibility of treating epilepsy using a newly-developed, non-invasive approach that produces focal neuronal loss in the brain. This approach utilizes MR-guided, low-intensity focused ultrasound (MRgFUS) together with intravenous microbubbles to transiently and focally open the BBB. The period of reversible BBB opening is then exploited to deliver a systemically-administered, BBB-impermeable neurotoxin to the targeted area of the brain parenchyma. Using this approach, previous evidence demonstrates that focal delivery to the brain of systemically-administered Quinolinic Acid (QA) produces neuronal loss in the area of BBB opening (Zhang et al. 2016; Zhang et al. 2019). This approach will be tested for its impact on seizures in a mouse model of temporal lobe epilepsy. In the interest of brevity, the procedure used to produce neuronal lesions using MRgFUS + microbubbles + Quinolinic Acid is termed PING (Precise Intracerebral Non-invasive Guided surgery).
Materials and Methods
Study Design
The animal protocol for this study was approved by the Stanford University Administrative Panel on Laboratory Animal Care (APLAC). All experiments were conducted in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. The basic time line for the study is shown in Figure 1.
Fig 1.
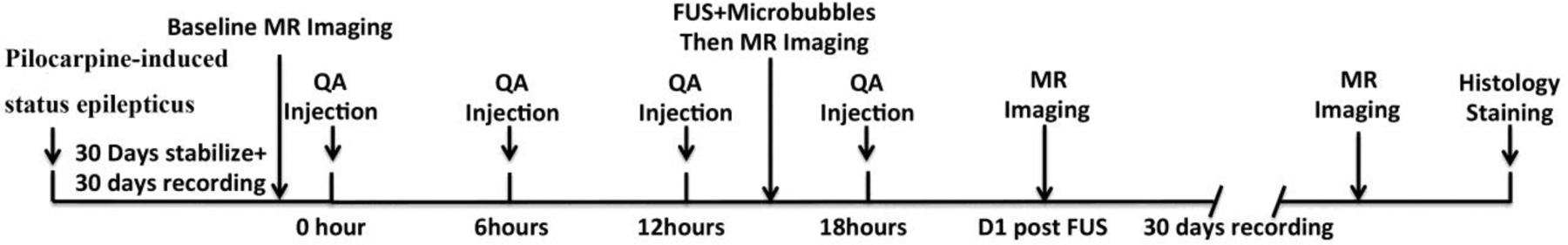
Timeline of the study. Day 0 was defined as the day of pilocarpine administration and status epilepticus. Animals were allowed a 30-day period of post-status stabilization and seizure development. Behavioral seizures were monitored on Days 31 to 60, and Days 62 to 91. Magnetic Resonance Imaging (MRI), Quinolinic Acid injection (QA), and Focused Ultrasound + microbubbles (FUS) were performed at the indicated time points. Animals were euthanized on Day 92, and brains were prepared for histological assessment of sites of neuronal loss (Histo).
Induction of Status Epilepticus (SE)
Rodents that survive status epilepticus after systemic treatment with kainic acid or pilocarpine are among the most widely used models in epilepsy research (Cavalheiro et al., 2006) since its first description a quarter of a century ago (Turski et al., 1983a,b). Mice are increasingly popular because of the abundant availability of transgenic and knockout animals. Kainate treatment in mice can be problematic, because some commonly used strains are resistant to kainate’s excitotoxic (and epileptogenic) effects (Schauwecker and Steward, 1997), which is not the case for pilocarpine (Shibley and Smith, 2002; Schauwecker, 2012). Consequently, pilocarpine-treated mice have become an important model of temporal lobe epilepsy. In its most common application, the model involves intraperitoneally administering a peripherally acting muscarinic acetylcholine receptor antagonist, such as methyl scopolamine or atropine methyl bromide, minutes before a single high-dose of pilocarpine (Turski et al., 1984). Pilocarpine’s actions include activation of M1 receptors, which evokes status epilepticus (Maslanksi et al., 1994; Hamilton et al., 1997). After surviving status epilepticus, mice begin displaying spontaneous seizures days later, and their epileptic condition is permanent (Turski et al., 1989; Cavalheiro et al., 1996).
An established model of temporal lobe epilepsy involving pilocarpine-induced status epilepticus followed by spontaneous, recurrent seizures was utilized for this study (Buckmaster and Haney 2012). Sixty male FVB/N mice (Charles River Laboratories, Wilmington, MA, USA), 7 to 8 weeks of age, were administered pilocarpine hydrochloride (Sigma) dissolved in bacteriostatic 0.9% NaCl to a final concentration of 200 mg/ml. 250 mg/kg of the pilocarpine solution was injected intraperitoneally. Twenty minutes prior to pilocarpine treatment, 5mg/kg of scopolamine methyl bromide (Sigma, non-pharmaceutical grade) was administered subcutaneously, in order to antagonize peripheral side effects of pilocarpine. Status Epileptics (SE) was gauged by the presence of continuous or repetitive motor convulsions. Twenty-three animals died during or soon after pilocarpine treatment. After 2 hours of SE, seizures were suppressed with diazepam administered intraperitoneally at 5 mg/kg. Diazepam treatment was repeated every 2–3 hours for up to 6 hours, in order to maintain seizure suppression. After SE, subcutaneous lactated Ringers solution was also administered to maintain hydration, and mice were kept warm with a heating pad under their cage. Twenty-six animals exhibiting SE did not develop spontaneous, recurrent seizures, and were removed from the study. Eleven animals exhibiting SE did develop spontaneous, recurrent seizures, and were used for the study.
Monitoring of Behavioral Seizures
A post-SE period of 30 days was allowed for stabilizing the mice and to allow the development of spontaneous, recurrent seizures. After this period, mice were video-recorded daily for 30 days to detect and quantify behavioral seizures. A 30-day recording period for the seizure rate baseline was selected because previous evidence from this model has shown that seizure rates are either stable or increase slightly after this post-SE time frame (Arida et al. 1999). Recordings were begun at approximately 8:00 a.m. each day, and were continued for 10–11 hours per day. During recordings, mice were transferred from their home cage to an aquarium with physical divisions. This allowed each mouse to be monitored individually and separately from other mice. Video recordings were reviewed in a fast-forward playback setting for identifying behavioral seizures of grade 3 (forelimb clonus) or greater (Racine 1972). Seizure frequency was measured by an investigator who was blinded to the histological outcomes for the animals. The same video monitoring procedure was repeated for an additional 30 days post-PING.
Magnetic Resonance Imaging and PING
At the end of the initial 30 days of video monitoring (Day 60), baseline MRI scans were performed. T2-weighted fast spin echo (FSE) images (TR/TE=4800/85 ms, 1 average, field of view=25 mm, matrix size=240×256, slice thickness 1.2 mm) were obtained for structural assessments.
Administration of QA was then initiated with a total of 4 injections given at 6-hour intervals. Quinolinic acid (Santa Cruz Biotechnology, Dallas, TX, USA) was dissolved in saline (10 mg/mL) and injected intraperitoneally at 0.012 mmol per injection. This injection protocol was designed to achieve a relatively stable plasma concentration of QA during the period of BBB opening. Two hours after the third QA injection, mice were anesthetized with isoflurane (4% induction and 2% maintenance), and microbubbles were administered. Definity® Microbubbles (300 uL/kg, mean diameter range: 1.1–3.3 μm, 1:20 diluted to a concentration of 5.0–8.0 × 108 bubbles per ml; Lantheus Medical Imaging, MA, USA) were injected through the tail vein.
Magnetic Resonance-guided Focused Ultrasound was then delivered at an acoustic power of 0.5 MPa, in order to open the BBB. Sonication parameters were 1.5 MHz, pulse duration 20-ms, duty cycle of 2%, 1-Hz pulse repetition frequency, 120-s duration per sonication. Multiple sonications were administered in the vicinity of the targeted area of the hippocampus by moving the sonication zones slightly rostro-caudally and medio-laterally. After sonicating a target on one side of the brain, the system was re-directed to sonicate same the target on the contralateral side of the brain. The MRgFUS system (Image Guided Therapy, Pessac, France) was configured as previously described (Zhang et al. 2015; Zhang et al. 2016). The system includes an MR-compatible, pre-focused, eight-element annular array, 1.5-MHz transducer (spherical radius = 20 +/− 2 mm, active diameter=25 mm [focal ratio =0.8]; Imasonic, Voray sur l’Ognon, France), which was connected to a phased array generator and radiofrequency power amplifier. An MR-compatible motorized positioning stage was used to move the transducer in the rostral-caudal and medial-lateral directions. The membrane in front of the transducer was filled with degassed water and inflated to ensure good ultrasonic coupling between the membrane and the head of the animal. For sonication, the animals were placed in a prone position and maintained in that position using a bite bar and ear bars. The scalp hair was shaved and removed with depilatory cream. Acoustic gel was applied between the transducer and skin. The experimental apparatus of this study is shown in Figure 2.
Fig 2.
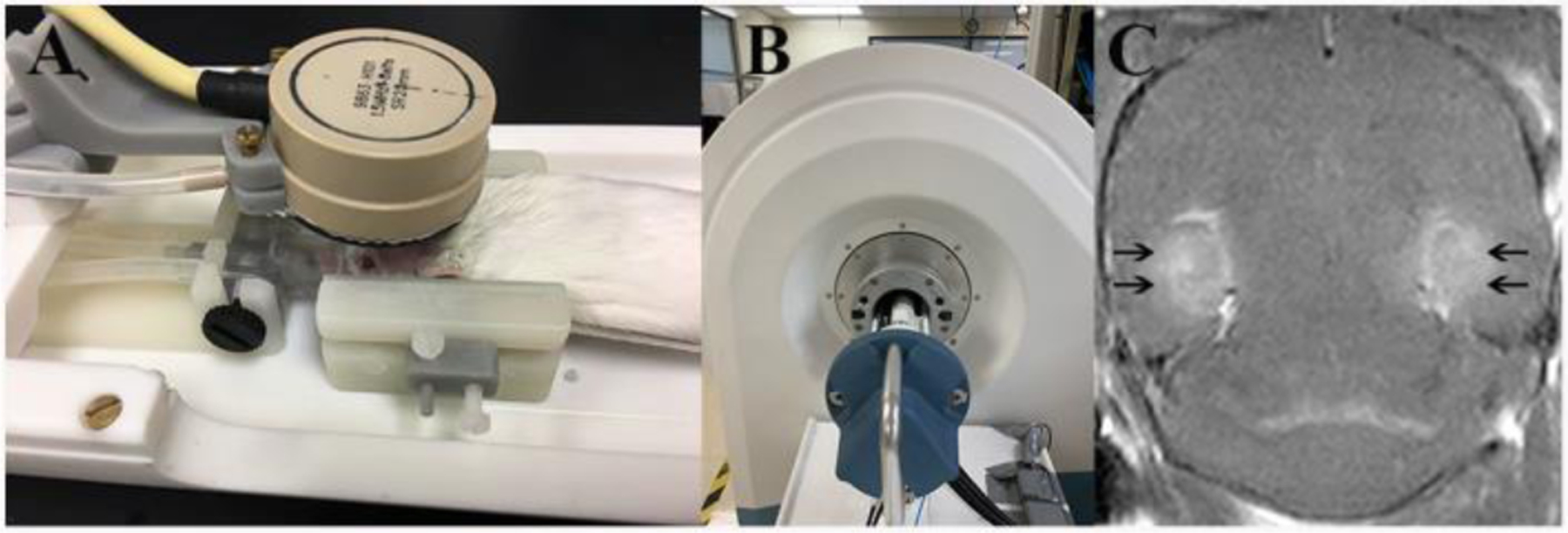
Experimental apparatus and post contrast T1-weighted image immediately after sonication. A: FUS system, a 1.5-MHz transducer in brown rests upon the top of the rodent head and can move in X–Y planes and be focused in the Z axis. B: A 3T MRI scanner was used to detect the BBB-opening after sonication. C: Post contrast T1-weighted image immediately post sonication; enhancement of the bilateral ventral hippocampus area (black arrows) indicated BBB opening.
Opening of the BBB was confirmed immediately after sonication utilizing post-contrast (gadobenate dimeglumine; Multihance, Bracco Diagnostics Inc., Monroe Township, NJ 08831, USA) T1-weighted imaging (TR/TE=720/11 milliseconds, 4 averages, field of view=25 mm, matrix size=248×12, slice thickness=0.6 mm). T2-FSE images were acquired immediately, one day, and 31 days after sonication, in order to assess resulting lesions. In addition, T2*-weighted gradient echo images (repetition time/echo time [TR/TE]=391/20 ms, flip angle 20°, 3 averages, field of view 25 mm, matrix size=256×256, slice thickness 0.8 mm) were obtained at the same time points, in order to identify possible hemorrhagic complications. Images were reviewed and analyzed using the DICOM viewer Horos. Animals were allowed to recover for one day post-sonication, after which the second 30-day period of video monitoring was initiated.
Tissue Preparation and Analysis
Mice were euthanized on Day 92 post-SE with pentobarbital (>100 mg/kg, i.p.) and perfused through the left ventricle at 15 mL/min for 1 min with 0.9% NaCl and then for 30 min with 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). Brains were post-fixed overnight at 4°C and then transferred into 30% (w/v) sucrose in PB. After equilibrating in the 30% sucrose solution, the brains were sectioned coronally (40 μm) with a sliding microtome. Semi-serial sections were collected in 30% ethylene glycol and 25% glycerol in 50 mM PB and stored at −20°C until use. Series of adjacent sections were processed for Nissl staining. The sites of lesions were identified in Nissl-stained sections by an investigator blinded to the seizure frequency outcomes. The hippocampus proper was present in approximately 40 sections in each animal. Four areas were examined (CA1, CA3, dentate gyrus), and each area was assessed in three aspects of the longitudinal axis of the hippocampus (septal, intermediate, and temporal). To measure the neuronal loss, Nissl-stained sections was evaluated with a 10X objective and a Neurolucida system (MBF Bioscience, Williston, VT) to draw contours around the hippocampus and lines along the pyramidal cell layer, granule cell layer, and gaps in both. Cell layer length and hippocampal volume were measured. Cell layer length correlated with hippocampal volume (correlation coefficient = 0.922, p < 0.001, ANOVA). Gaps in the cell layer were used as the primary metric of neuronal loss (Figure 3).
Fig 3.
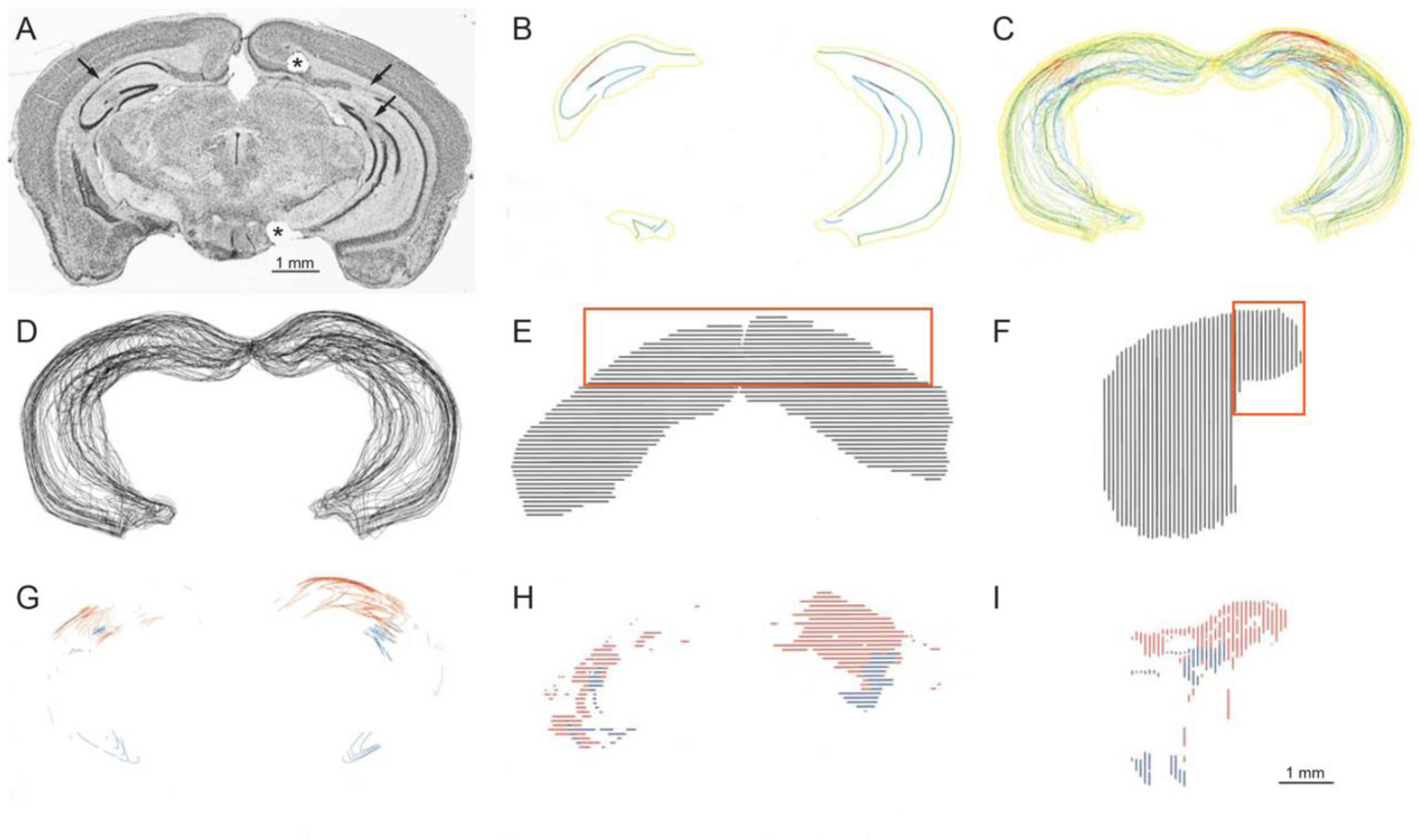
Histological analysis of hippocampi. (A) Nissl-stained coronal section of mouse brain. Asterisk indicates fiduciary marker identifying the right side. Some gaps or lareas of extreme thinning are indicated by arrows. (B) Contours drawn from the section shown in panel A. Hippocampus, yellow; pyramidal cell layer, green; granule cell layer, cyan; pyramidal cell layer gap, red; granule cell layer gap, blue. (C) Colored- and (D) black-line coronal views of contours of entire hippocampi. (E) Dorsal and lateral (F) views of hippocampi. The septal hippocampus is indicated by the orange rectangles. (G) Coronal, (H) dorsal, and (I) lateral views of gaps in the pyramidal cell layer (red) and granule cell layer (blue). The scale bar pertains to all reconstructions in panels B–I.
Results
Pilocarpine-induced Status Epilepticus and Spontaneous Seizures
Status epilepticus (SE) was observed in all 11 animals included in the study, and SE was terminated by treatment with diazepam two hours after injection of pilocarpine. Video recordings initiated 30 days after SE showed spontaneous, recurrent seizures in each of the 11 animals. The seizure clusters were evident in all the mice. The total number of spontaneous, recurrent seizures recorded for 30 days after pilocarpine treatment are between 42–105 times with an average seizure frequency of 0.221 +/− 0.019 seizures per hour (mean +/− SEM). Figure 4 shows the seizure numbers (daily and total) of each individual animal.
Fig 4.
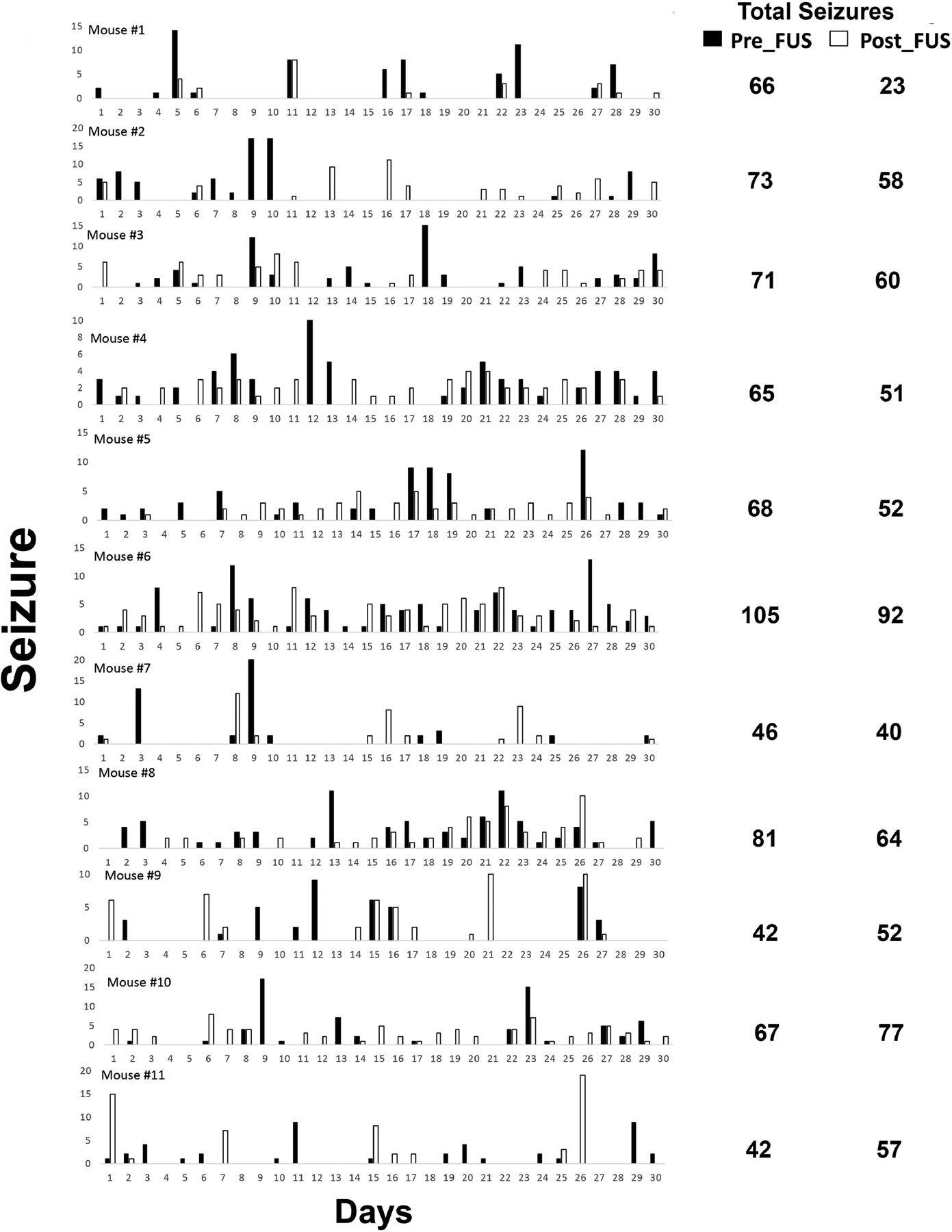
Frequency of spontaneous, behavioral seizures in epileptic pilocarpine-treated mice. (a) Example of data from 11 mice. Mice were video-recorded 10–11 hrs/day, every day for 1 month. Seizures of grade 3 or greater on the Racine (1972) scale were counted. (b) Histogram showing the distribution of seizure frequency for all the mice in this study.
MR imaging
MR images obtained prior to the PING procedure were unremarkable, providing no evidence of lesions, edema, or hemorrhage. Immediately after FUS, post-contrast T1 imaging showed evidence of BBB opening in all animals. Opening of the BBB in the intermediate aspect of the hippocampus was observed in 10 of 11 animals. In two animals, the septal hippocampus also displayed BBB opening. T2 images demonstrated edema in 5 animals, and no evidence of bleeding was observed on the gradient echo images.
Effect of PING on Seizure Frequency
Recordings over a 30-day post-PING period demonstrated an average seizure frequency of 0.174 +/− 0.017 seizures per hour (mean +/− SEM), and total seizure numbers ranging 42–105. This represented a decrease of 21.2% in seizure frequency as compared to the pre-PING baseline, and this effect achieved statistical significance (T=2.748, p=0.021, DF=10). Notably, 8 of the 11 mice exhibited decreases in seizure frequency, while three animals exhibited increases.
Location of PING-induced Neuronal Loss
The location of neuronal loss in each animal was identified by analyzing semi-serial, Nissl-stained sections. Subfields CA1, CA3, and dentate gyrus were examined along the longitudinal axis of the hippocampus at septal, intermediate, and temporal sites (Fig. 5). Examples of the patterns of neuronal loss observed across the longitudinal axis of the hippocampus are shown in Figure 5. Most of the animals (n=8) exhibited neuronal loss located primarily in the intermediate hippocampus in CA1 and CA3, and to a lesser extent in the dentate gyrus. However, in these animals the septal aspect of the hippocampus was mostly spared from damage. Two other animals also displayed neuronal loss in the intermediate aspect of the hippocampus, but also exhibited prominent bilateral damage to the septal aspect of the hippocampus. Damage to the temporal hippocampus was sporadic and limited in all animals. A final animal had negligible neuronal loss overall.
Fig 5.
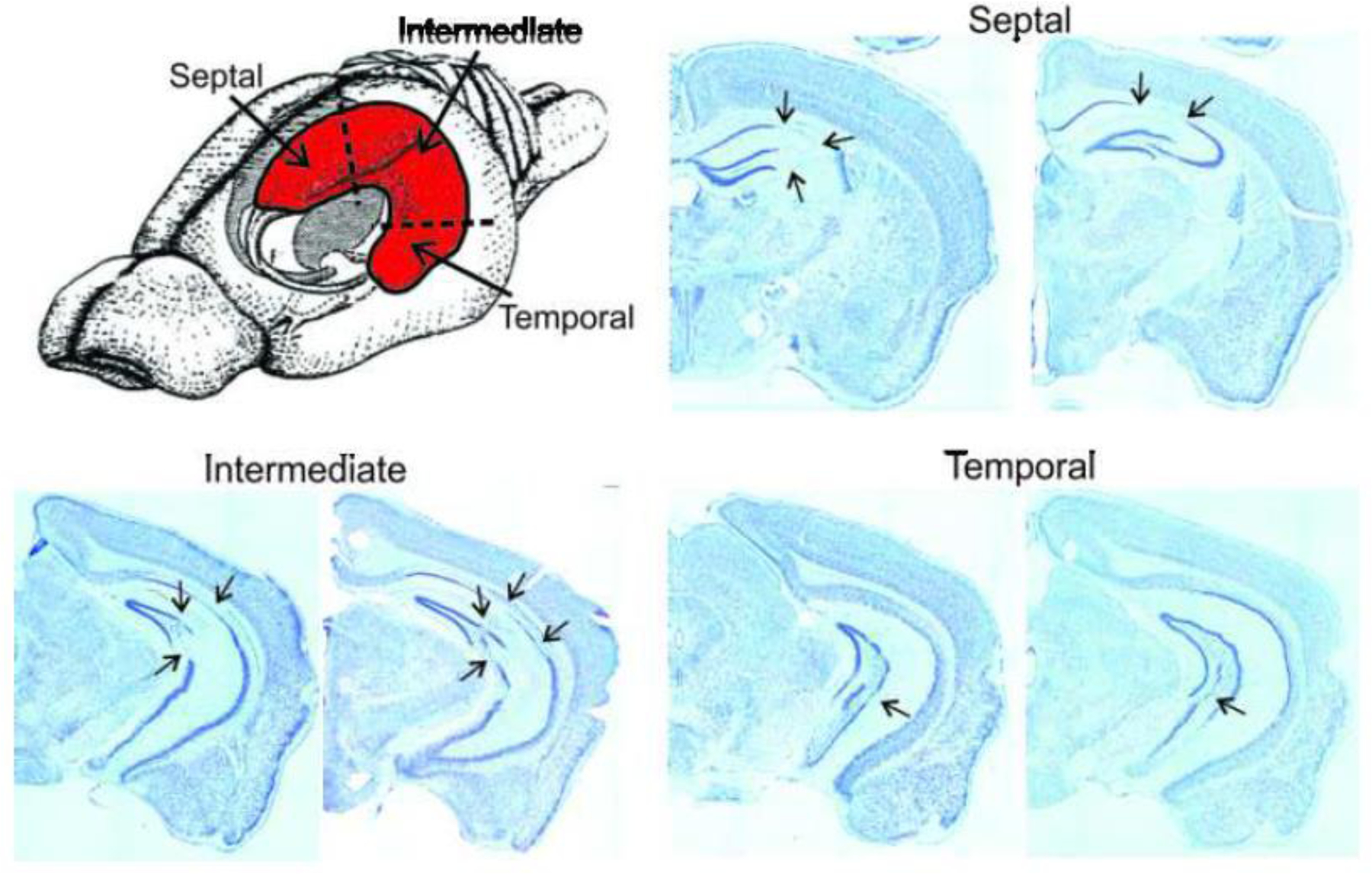
Location of PING-induced neuronal loss. A drawing of the rodent brain with the hippocampus (in red) shows the divisions of the hippocampus that were assessed for neuronal loss. Examples of the types of neuronal loss that occurred are shown in Nissl-stained, coronal sections taken along the longitudinal axis of the hippocampus. Septal: The left frame in the septal hippocampus shows neuronal loss in CA1 and CA3 (arrows), and the right frame illustrates loss primarily in CA1. Intermediate: The left frame in the intermediate hippocampus shows neuronal loss in CA1, CA3, and dentate gyrus (arrows), and the right shows a similar pattern of loss. Temporal: The left frame in the temporal hippocampus shows neuronal loss in the dentate gyrus (arrows), and the right frame illustrates damage in CA3.
Impact of the Location of Neuronal Loss on Seizure Frequency
A comparison between changes in seizure frequency and the sites of neuronal loss was undertaken to determine whether the location of neuronal loss influenced the impact of PING on seizures (Fig. 6). Both of the animals with bilateral neuronal loss in the septal hippocampus exhibited increases in seizure frequency (18.7%) after PING. The single animal with negligible neuronal loss also showed an increase in seizure frequency (19.6%). In contrast, all of the animals with neuronal loss in the intermediate hippocampus, but not in the septal hippocampal hippocampus (n=8), exhibited decreases in seizure frequency (27.7%) after PING (P=0.002, Fig 6b).
Fig 6.
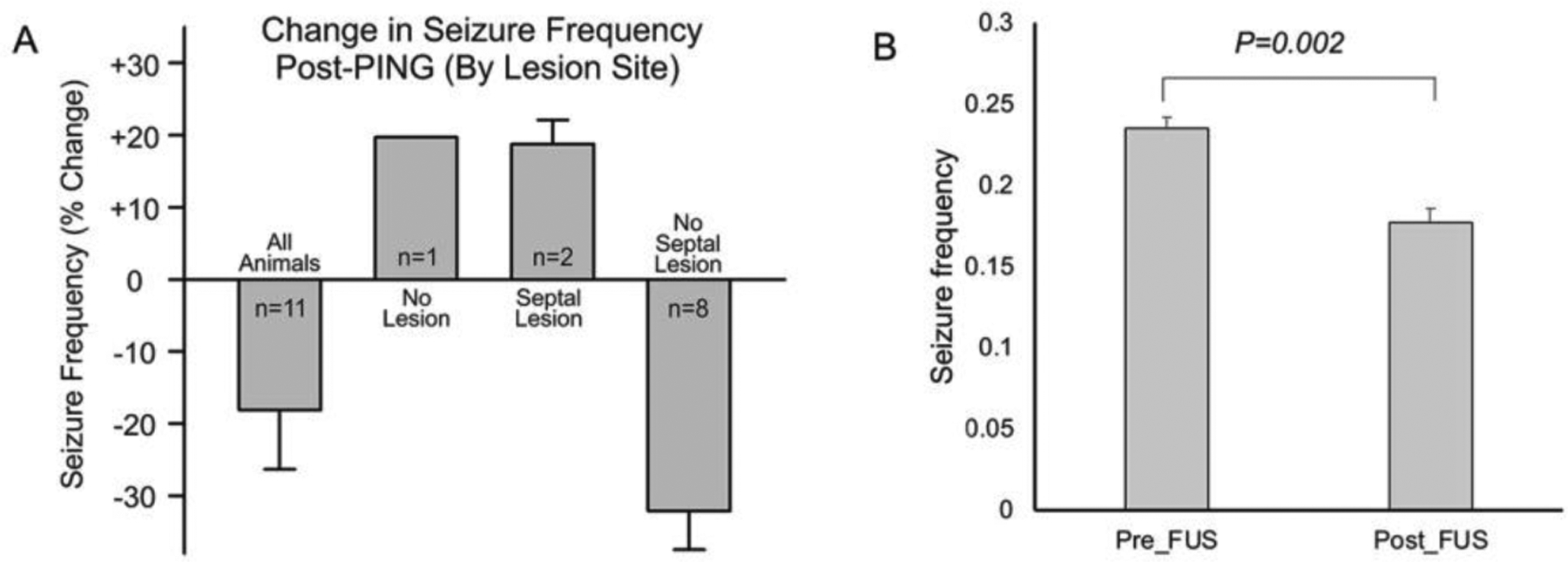
Effect of location of neuronal loss on change in seizure frequency. The average seizure frequency for all animals was reduced post-PING. Animals with bilateral neuronal loss in the septal hippocampus (Septal Lesion) exhibited increases in seizure frequency. Animals with intermediate hippocampal neuronal loss but no septal damage (No Septal Lesion) displayed reduced seizure frequency. A single animal with negligible neuronal loss (No Lesion) exhibited an increase in seizure frequency (Fig 6a). For the 8 animals with no septal lesion, the seizure frequency decreased significantly (p=0.002, Fig 6b). Values shown are means +/− SEMs.
Correlation between the seizure frequency and cell loss level
The 11 mice showed different extent of neuronal loss in both the pyramidal cell layer and in the granular cell layer. The mice with seizure reduction did not show neuronal loss in the septal granule cell layer (Table 1)
Table 1.
Histology evidence of neuronal loss
| Location | Measurement on histology | Seizure Monitor | ||
|---|---|---|---|---|
| Worsening | Improvement | p value | ||
| Full | PCL gap length (um) | 22321.2 | 10328.7 | 0.303 |
| GCL gap length (um) | 5247.3 | 4489.9 | 0.88 | |
| PCL gap % | 11.20% | 4.10% | 0.235 | |
| GCL gap % | 5.00% | 4.00% | 0.836 | |
| Septal | PCL gap length (um) | 14208.9 | 3232 | 0.179 |
| GCL gap length (um) | 663.5 | 0 | 0.033* | |
| PCL gap % | 24.80% | 4.20% | 0.169 | |
| GCL gap % | 1.40% | 0.00% | 0.035* | |
| Difference | PCL gap length (um) | 8112.3 | 7096.7 | 0.84 |
| GCL gap length (um) | 4583.8 | 4489.9 | 0.985 | |
| PCL gap % | 6.00% | 4.30% | 0.612 | |
| GCL gap % | 7.40% | 7.20% | 0.978 | |
PCL: Pyramidal cell layer; GCL: Granule cell layer; Difference=Full-Septal
p < 0.05
Discussion
This study provides the first feasibility test of a non-invasive, non-ablative surgical approach for treating seizures in a model of epilepsy. Focal neuronal loss was produced by MRgFUS-facilitated delivery of a systemically-administered neurotoxin to a targeted region of the brain parenchyma. Notably, this procedure does not produce pannecrotic lesions, but rather spares other non-neuronal cell types in the vicinity of neuronal loss. Our findings demonstrate that this approach, termed PING, reduced the frequency of spontaneous, recurrent seizures in a mouse model of temporal lobe epilepsy.
In our study, 8 animals with seizure reduction of 27.7% (p=0.002) after PING exhibited neuronal loss located primarily in the intermediate hippocampus in CA1 and CA3, and to a lesser extent in the dentate gyrus; the septal aspect of the hippocampus was mostly spared from damage in these animals. Two other animals with seizure frequency increase of 19.6% also displayed neuronal loss in the intermediate aspect of the hippocampus, but also exhibited prominent bilateral damage to the septal aspect of the hippocampus. These results support the concept that the site of damage along the longitudinal axis of the hippocampus influences the impact on seizure frequency. Substantial evidence derived from anatomical, electrophysiological, behavioral, genomic, and pathophysiological studies indicates that the hippocampus is functionally differentiated across its longitudinal axis (for reviews, see Moser and Moser 1998; Fanselow and Dong 2010; Strange et al. 2014). Moreover, regional characteristics of neurons and circuits appear to predispose more temporal aspects of the hippocampus to heightened excitability, as compared with the septal aspect of the hippocampus. (Lee et al. 1983; Bragdon et al. 1986; Derchansky et al. 2004; Toyoda et al. 2013; Kouvaros and Papatheodoropoulos 2017). Our observation that PING-induced neuronal loss in the intermediate and temporal hippocampus reduces seizures is consistent with these previous findings. Moreover, the results are parsimonious with observations in human temporal lobe epilepsy patients, in which seizures originate preferentially in the anterior (temporal) aspect of the hippocampus, and resection or ablation of the anterior hippocampus is effective for reducing seizures. Our findings also raise the possibility that the septal aspect of the hippocampus may actually serve to repress seizure activity. This finding, albeit preliminary in nature, suggests that different sites along the longitudinal axis of the hippocampus play opposing roles in seizure generation and/or maintenance.
The results of this pilot study encourage future investigations undertaking more comprehensive assessments of the utility of PING in the treatment of epilepsy. In this context, it is important to highlight the strengths and limitations of the current study. The study provides the first evidence that non-invasive, focal neuronal lesions produced by PING are capable of reducing spontaneous, recurrent seizures in an established model of temporal lobe epilepsy. We have previously shown that when saline is substituted for QA in the PING paradigm that neuronal loss is not observed (Zhang et al. 2016). This indicates that low-intensity FUS, by itself, is insufficient to produce the neuronal loss observed in the current study. It will be valuable for future studies to confirm these findings, and expand the evaluation of how neuronal loss at different sites along the longitudinal axis of the hippocampus influences seizure activity. It is also important to note that the current study evaluated seizures during a post-SE time course over which baseline seizure rates have been shown not to decrease (Arida et al. 1999). Consequently, the post-PING reduction in seizures would not appear to be the result of a decline in the baseline seizure rate in this model of epilepsy. Future studies should also incorporate additional comparator groups, in particular a group receiving QA but not FUS. One could posit that systemically-administered QA by itself (i.e. in the absence of FUS) might be capable of producing neuronal lesions or influencing seizure frequency. However, QA does not cross the BBB, and regions of the brain not receiving FUS do not display neuronal loss. It is also conceivable that transient opening of the BBB by seizures themselves is sufficient to provide access of QA to the brain, and thus produce neuronal loss. However, recurrent seizures in the pilocarpine model generalize to the whole hippocampus and other brain areas. If seizure-induced opening of the BBB were responsible for providing QA access to the brain parenchyma, then neuronal loss would be more widespread, and not restricted to the area of FUS-targeted BBB opening. A final issue concerns whether some areas of neuronal loss observed in this study reflect damage produced by SE and/or recurrent seizures, rather than PING-induced damage. The extent of seizure-induced damage can vary considerably in the pilocarpine model as a function of the time post-SE and the region under study (Lopim et al. 2016). Consequently, it will be important for future studies to assess the sites and size of such loss in a parametric and comprehensive manner.
We acknowledge the following limitations to our study: (i) the small sample size: we only induced stable seizures in 11 mice. We could not allocate animals into more different groups to test the effect of PING through targeting temporal hippocampus, septal hippocampus, and the intermediate aspect of hippocampus. (ii) We only included male mice in this study. In Buckmaster’s study in 2017, male mice showed mean seizure frequency of 0.132/h, and female mice showed 0.111/h. In the future, animals of both genders need to be included. (iii) Future studies also need to incorporate an additional group to test the effect of QA alone (without focused ultrasound) on seizure frequency.
A broader issue pertaining to this study is whether there is actually a need for another new surgical strategy for treating drug resistant epilepsy. With the advent of minimally-invasive (e.g. SLA) and non-invasive (e.g. radiosurgery and HIFU) procedures, would another new procedure contribute substantively to improving surgical treatment of epilepsy? In this regard, the PING strategy could provide multiple advantages over existing procedures. It is non-invasive which, as described above, avoids a range of potential complications. Unlike resective or ablative procedures, it produces damage specifically to neurons. This avoids off-target damage, such as injury to fibers of passage, vessels of passage, ventricular structures, and neighboring eloquent parenchymal tissue. BBB opening can be produced in a conformal manner, allowing for more precise treatment of irregularly-shaped targets with PING. This is an important consideration because surgical targets with complex contours are a common challenge for the treatment of seizure-genic cortical dysplasias. It is also important to consider how PING might influence the endemic underutilization of surgery for the treatment of epilepsy. Surgical treatment of epilepsy remains one of the few interventions for treating any neurological disorder that is actually highly effective, but is only used for a small minority of patients who stand to benefit. There are a variety of reasons reported for this underutilization (de Flon et al. 2010; Haneef et al. 2010; Englot et al. 2012; Jette et al. 2012; Burneo et al. 2016). From the patient’s perspective, these include reluctance to undergo an invasive procedure, fear of complications and pain, concern about long recovery periods, high costs and, in the case of radiosurgery, a fear of radiation treatment. From a societal perspective, the utilization of epilepsy surgery is impacted by socioeconomic status, race, and access to health insurance. From a medical perspective, poor referral rates persist despite guidelines from major professional societies supporting increased early referrals to comprehensive epilepsy clinics with the capacity for surgical treatment. Obviously, the PING strategy, if translated into the clinic, would not be a panacea for these major impediments. Nonetheless, the availability of a new, precise, non-invasive, procedure could encourage the use of epilepsy surgery in patients who are reluctant to undergo more complex, invasive procedures.
Conclusions
A new surgical strategy for non-invasive, non-ablative treatment of seizures was shown to be effective in an initial feasibility study using a mouse model of temporal lobe epilepsy. This approach may offer multiple advantages over existing surgical procedures. The potential clinical applications for this strategy could extend beyond the treatment of epilepsy to include other neurological disorders (e.g. movement disorders) in which aberrant neuronal activity plays a role.
Acknowledgements
The authors thank Matthew J. Anzivino for his assistance in preparation of the manuscript.
This work was supported by National Institutes of Health Grants R01 CA217953-01 and R01 NS102194.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no conflict of interest.
References
- Arida RM, Scorza FA, Peres CA, Cavalheiro EA. The course of untreated seizures in the pilocarpine model of epilepsy. Epilepsy Res 1999;34:99–107. [DOI] [PubMed] [Google Scholar]
- Bragdon AC, Taylor DM, Wilson WA. Potassium-induced epileptiform activity in area CA3 varies markedly along the septotemporal axis of the rat hippocampus. Brain Res 1986;378:169–173. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Haney MM. Factors affecting outcomes of pilocarpine treatment in a mouse model of temporal lobe epilepsy. Epilepsy Res 2012;102:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burneo JG, Shariff SZ, Liu K, Leonard S, Saposnik G, Garg AX. Disparities in surgery among patients with intractable epilepsy in a universal health system. Neurology 2016;86:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalheiro EA, Santos NF, Priel MR. The pilocarpine model of epilepsy in mice. Epilepsia. 1996;37:1015–1019. [DOI] [PubMed] [Google Scholar]
- Cavalheiro EA; Naffah-Mazzacoratti MG; Mello LE; Leite JP The pilocarpine model of seizures. In: Pitkänen A; Schwartzkroin PA; Moshé SL, editors. Models of Seizures and Epilepsy. New York: Elsevier; 2006. p. 433–448. [Google Scholar]
- de Flon P, Kumlien E, Reuterwall C, Mattsson P. Empirical evidence of underutilization of referrals for epilepsy surgery evaluation. Eur J Neurol 2010;17:619–625. [DOI] [PubMed] [Google Scholar]
- Derchansky M, Shahar E, Wennberg RA, Samoilova M, Jahromi SS, Abdelmalik PA, Zhang L, Carlen PL. Model of frequent, recurrent, and spontaneous seizures in the intact mouse hippocampus. Hippocampus 2004;14:935–947. [DOI] [PubMed] [Google Scholar]
- Dupont S, Tanguy ML, Clemenceau S, Adam C, Hazemann P, Baulac M. Long-term prognosis and psychosocial outcomes after surgery for MTLE. Epilepsia. 2006;47:2115–2124. [DOI] [PubMed] [Google Scholar]
- Engel J Jr., Wiebe S, French J, Sperling M, Williamson P, Spencer D, Gumnit R, Zahn C, Westbrook E, Enos B; Quality Standards Subcommittee of the American Academy of Neurology; American Epilepsy Society; American Association of Neurological Surgeons. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology 2003;60:538–547. [DOI] [PubMed] [Google Scholar]
- Englot DJ, Ouyang D, Garcia PA, Barbaro NM, Chang EF. Epilepsy surgery trends in the United States, 1990–2008. Neurology 2012;78:1200–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 2010; 65:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross RE, Willie JT, Drane DL. The Role of Stereotactic Laser Amygdalohippocampotomy in Mesial Temporal Lobe Epilepsy. Neurosurg Clin N Am 2016;27:37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hader WJ, Tellez-Zenteno J, Metcalfe A, Hernandez-Ronquillo L, Wiebe S, Kwon CS, Jette N. Complications of epilepsy surgery: a systematic review of focal surgical resections and invasive EEG monitoring. Epilepsia 2013;54:840–847. [DOI] [PubMed] [Google Scholar]
- Hamilton SE, Loose MD, Qi M, Levey A, Hille B, McKnight GS, Idzerda RL, Nathanson NM. Disruption of the m1 receptor gene ablates muscarinic receptor-dependent M current regulation and seizure activity in mice. Proc Natl Acad Sci USA. 1997;94:13311–13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneef Z, Stern J, Dewar S, Engel J Jr. Referral pattern for epilepsy surgery after evidence-based recommendations: a retrospective study. Neurology 2010;75:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter C, Sonntag-Dillender M, Hoppe C, Elger CE. Depressed mood and memory impairment in temporal lobe epilepsy as a function of focus lateralization and localization. Epilepsy Behav 2004;5:696–701. [DOI] [PubMed] [Google Scholar]
- Jette N, Quan H, Tellez-Zenteno JF, Macrodimitris S, Hader WJ, Sherman EM, Hamiwka LD, Wirrell EC, Burneo JG, Metcalfe A, Faris PD, Hernandez-Ronquillo L, Kwon CS, Kirk A, Wiebe S; CASES Expert Panelists. Development of an online tool to determine appropriateness for an epilepsy surgery evaluation. Neurology 2012;79:1084–1093. [DOI] [PubMed] [Google Scholar]
- Kouvaros S, Papatheodoropoulos C. Prominent differences in sharp waves, ripples and complex spike bursts between the dorsal and the ventral rat hippocampus. Neuroscience 2017;352:131–143. [DOI] [PubMed] [Google Scholar]
- Lee KS, Reddington M, Schubert P, Kreutzberg G. Regulation of the strength of adenosine modulation in the hippocampus by a differential distribution of the density of A1 receptors. Brain Res 1983;260:156–15. [DOI] [PubMed] [Google Scholar]
- Lopim GM, Vannucci Campos D, Gomes Da Silva S, De Almeida AA, Lent R, Cavalheiro EA, Arida RM. Relationship between seizure frequency and number of neuronal and non-neuronal cells in the hippocampus throughout the life of rats with epilepsy. Brain Res 2016;1634:179–186. [DOI] [PubMed] [Google Scholar]
- Martin E, Jeanmonod D, Morel A, Zadicario E, Werner B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann Neurol 2009;66:858–861. [DOI] [PubMed] [Google Scholar]
- Maslanski JA, Powelt R, Deirmengiant C, Patelt J. Assessment of the muscarinic receptor subtypes involved in pilocarpine-induced seizures in mice. Neurosci Lett. 1994;168:225–228. [DOI] [PubMed] [Google Scholar]
- McClelland S 3rd, Guo H, Okuyemi KS. Population-based analysis of morbidity and mortality following surgery for intractable temporal lobe epilepsy in the United States. Arch Neurol 2011;68:725–729. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus 1998;8:608–619. [DOI] [PubMed] [Google Scholar]
- Quigg M, Barbaro NM. Stereotactic radiosurgery for treatment of epilepsy. Arch Neurol 2008;65:177–183. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 1972;32:281–294. [DOI] [PubMed] [Google Scholar]
- Rydenhag B, Silander HC. Complications of epilepsy surgery after 654 procedures in Sweden, September 1990–1995: a multicenter study based on the Swedish National Epilepsy Surgery Register. Neurosurgery 2001;49: 51–56; discussion 56–57. [DOI] [PubMed] [Google Scholar]
- Saipetch C, Sachs E, Haneef Z. Epilepsy: Five new things. Neurol Clin Pract 2016;6:444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauwecker PE. Strain differences in seizure-induced cell death following pilocarpine-induced status epilepticus. Neurobiol Dis. 2012;45:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauwecker PE, Steward O. Genetic determinants of susceptibility to excitotoxic cell death: implications for gene targeting approaches. Proc Natl Acad Sci USA. 1997;94:4103–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibley H, Smith BN. Pilocarpine-induced status epilepticus results in mossy fiber sprouting and spontaneous seizures in C57BL/6 and CD-1 mice. Epilepsy Res. 2002;49:109–120. [DOI] [PubMed] [Google Scholar]
- Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol 2008;7:525–537. [DOI] [PubMed] [Google Scholar]
- Spencer D and Burchiel K. Selective Amygdalohippocampectomy Epilepsy Res Treat. 2012; 2012: 382095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling MR, O’Connor MJ, Saykin AJ, Plummer C. Temporal lobectomy for refractory epilepsy. JAMA. 1996. August 14;276(6):470–5. [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci 2014;15:655–669. [DOI] [PubMed] [Google Scholar]
- Toyoda I, Bower MR, Leyva F, Buckmaster PS. Early activation of ventral hippocampus and subiculum during spontaneous seizures in a rat model of temporal lobe epilepsy. J Neurosci 2013;33:11100–11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SLJ, Kleinrok Z, Turski L Limbic seizures produced by pilocarpine I rats: behavioral, electroencephalographic and neuropathological study. Behav Brain Res. 1983;9:315–335. [DOI] [PubMed] [Google Scholar]
- Turski WA, Czuczwar SJ, Kleinrok Z, Turski L Cholinomimetics produce seizures and brain damage in rats. Experientia. 1983;39:1408–1411. [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Bortolotto ZA, Mello LM, Schwarz M, Turski L Seizures produced by pilocarpine in mice: a behavioral, electroencephalographic and morphological analysis. Brain Res. 1984;32:237–253. [DOI] [PubMed] [Google Scholar]
- Turski L, Ikonomidou C, Turski WA, Bortolotto ZA, Calalheiro EA. Review: cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine: a novel experimental model of intractable epilepsy. Synapse. 1989;3:154–171. [DOI] [PubMed] [Google Scholar]
- Wellmer J, Kopitzki K, Voges J. Lesion focused stereotactic thermo-coagulation of focal cortical dysplasia IIB: a new approach to epilepsy surgery? Seizure 2014;23:475–478. [DOI] [PubMed] [Google Scholar]
- West S, Nolan SJ, Newton R. Surgery for epilepsy: a systematic review of current evidence. Epileptic Disord 2016;18:113–121. [DOI] [PubMed] [Google Scholar]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M, Effectiveness, and Efficiency of Surgery for Temporal Lobe Epilepsy Study G. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 2001;345:311–318. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Aubry JF, Zhang J, Wang Y, Roy J, Mata JF, Miller W, Dumont E, Xie M, Lee K, Zuo Z, Wintermark M. Defining the optimal age for focal lesioning in a rat model of transcranial HIFU. Ultrasound Med Biol 2015;41:449–455. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liao C, Qu H, Huang S, Jiang H, Zhou H, Abrams E, Habte FG, Yuan L, Bertram EH, Lee KS, Pauly KB, Buckmaster PS, Wintermark M. Testing Different Combinations of Acoustic Pressure and Doses of Quinolinic Acid for Induction of Focal Neuron Loss in Mice Using Transcranial Low-Intensity Focused Ultrasound. Ultrasound Med Biol 2019; 45:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tan H, Bertram EH, Aubry JF, Lopes MB, Roy J, Dumont E, Xie M, Zuo Z, Klibanov AL, Lee KS, Wintermark M. Non-Invasive, Focal Disconnection of Brain Circuitry Using Magnetic Resonance-Guided Low-Intensity Focused Ultrasound to Deliver a Neurotoxin. Ultrasound Med Biol 2016;42:2261–2269. [DOI] [PubMed] [Google Scholar]


