Abstract
Obstructive sleep apnea (OSA) is underdiagnosed in women compared with men. Women have a tendency to underreport or present with atypical symptoms such as behavior changes, insomnia, fatigue, and depression. Nocturia, waking up from sleep 2 times or more to void, has been shown to be associated with OSA, but it is not an included symptom in commonly used screening questionnaires in primary provider offices. About 50% of patients with OSA have nocturia, and treatment of OSA improves it. Recognition of nocturia as a relevant symptom of OSA is important for primary providers to provide timely referral for the diagnosis of OSA.
Keywords: nocturia, nocturnal enuresis, obstructive sleep apnea, women, OSA screening
‘Interestingly, nocturia and enuresis are listed as common symptoms in the clinical guideline for managing chronic insomnia in adults . . .’
Introduction
Nocturia is defined as waking up from sleep 2 times or more to void and is a common symptom in patients with obstructive sleep apnea (OSA). Nocturnal enuresis defined as involuntary urination while asleep has also been associated with OSA.1,2 Increased urine production at night in patients with OSA has been well described in previous studies. However, it is also commonly associated with benign prostatic hypertrophy or overactive bladder, and therefore, primary care providers do not consider OSA when patients present with this symptom. Interestingly, nocturia and enuresis are listed as common symptoms in the clinical guideline for managing chronic insomnia in adults,3 but they are not included in the most recent American Academy of Sleep Medicine clinical practice guideline for the evaluation of OSA in adults.4 In contrast, snoring is traditionally recognized as a primary symptom of OSA. It is present in all or if not most of the screening algorithms and questionnaires for OSA. Romero et al5 demonstrated in a retrospective chart review of 1007 adult patients that nocturia is comparable to snoring in screening for OSA, with high sensitivity (>80%) and positive predictive value (>80%). These findings illustrate the utility of inquiring about OSA when patients present with nocturia or enuresis and substantiate the need for further research of this symptom in OSA.
The purpose of this review is to raise awareness of nocturia and nocturnal enuresis as being potential symptoms of OSA, particularly in women. If these symptoms are present in an at-risk patient, evaluation of OSA should be considered, given the known cardiovascular and other sequelae of untreated OSA.6
OSA Symptoms in Women
Women present with typical symptoms of OSA, such as loud snoring and daytime sleepiness, but the frequency of symptoms may vary compared with men. Women may underreport symptoms, present with symptoms at an older age, or have their symptoms disregarded and attributed to depression or insomnia, leading to an underestimation of the prevalence of OSA.7-9 For example, although snoring in general is more prevalent in men, the prevalence of snoring in women increases significantly in women after the age of 50 years, and the overall decline in prevalence after age 59 years compared with men is less steep.10 The age of 50 years corresponds to the start of menopause, which has been associated with increased risk of OSA and will be discussed later.11
In contrast, the prevalence of nocturia is higher in women compared with men. In a retrospective chart review study of 138 patients, it was noted that the prevalence of pathological nocturia, defined as having 2 or more urination episodes per night, was greater among women compared with men (60% and 40.9%, respectively).12 The prevalence of nocturia also increases with age in women. Kim et al13 divided 118 women among 3 age groups (under 40 years, 40 through 59 years, and 60 years and above) and determined the frequency of nocturia between the groups. The prevalence of nocturia was the highest in women >60 years old. Furthermore, a systematic review of 43 articles dating from 1990 to 2009 demonstrated that in younger men and women between the ages of 20 and 40 years, approximately 20% had nocturia. In older men and women, up to two-thirds of the population had nocturia.13,14
The increase in prevalence of nocturia in older adults is concerning because of its association with underlying sleep disorder. In a cohort study examining 1610 men and 2535 women who completed baseline and follow-up questionnaires of the Boston Area Community Health Survey, women had a higher prevalence of incident lower-urinary-tract symptoms (12.6% vs 7.1%) compared with men. The odds of lower-urinary-tract symptoms, including nocturia, were higher in those who reported sleep-related problems, such as poor sleep quality and sleep restriction. Body mass index (BMI) was shown to be a potential mediator.15 In a study by Oztura et al,16 1970 recruited individuals completed a questionnaire inquiring about nocturia. Frequency of nocturnal urination correlated with age, BMI, hypertension, respiratory disturbance index, apnea-hypopnea index (AHI), respiratory effort index, and nadir oxygen saturation. The prevalence of nocturia was 57.2% in mild sleep-disordered breathing (SDB), 64.3% in moderate SDB, and 76.9% in severe SDB: the prevalence of nocturia increases with the severity of SDB.16
Menopause and OSA
Menopause plays an important role in affecting sleep, the frequency of nocturia, and risk of OSA. Insomnia is more common in postmenopausal women compared with premenopausal women,17 but sleep quality did not differ according to studies in the Wisconsin sleep cohort examining sleep quality over time with polysomnography. In a group of 589 premenopausal, perimenopausal, and postmenopausal women, perimenopausal and postmenopausal women did not have worse sleep quality compared with premenopausal women. However, being postmenopausal increased the risk of having SDB.18,19 The mechanism of menopause increasing the risk of OSA is not clearly understood. Postmenopausal women tend to gain weight after menopause, which leads to increased BMI, central obesity, and larger neck circumference.20,21 The accumulation of adipose tissue around the upper airway makes it more prone to collapse during sleep. In a study evaluating 230 obese male and female individuals, postmenopausal women had higher BMI, neck circumference, waist-to-hip ratio, and respiratory disturbance index compared with premenopausal women. Additionally, the prevalence gap of OSA between men and women is narrowed after the age of 55 years.22 In contrast, a recent study of 219 women aged 38 to 62 years from the Wisconsin Sleep Cohort Study examined the relationship between menopause and SDB severity. The authors found that SDB severity increases with progression through menopause, but it is independent of aging and change in body habitus.23
The anatomical changes are associated with a decrease in or lack of sex hormones during menopause. Additionally, progesterone and estrogen have other properties that affect respiration and sleep. Progesterone is a respiratory stimulant. When progesterone is high, as during pregnancy, the occurrence of apnea during sleep is low even though weight gain is present.24 Therefore, absence of progesterone during menopause may decrease respiratory drive and increase apnea episodes. The lack of progesterone may also decrease pharyngeal dilator muscle activity, which leads to repetitive collapse of the upper airway during sleep.25 Estrogen improves sleep quality by decreasing arousals and increasing total, slow wave, and REM duration.26,27 It is also involved with temperature regulation. Therefore, low estrogen during menopause leads to hot flashes, which may disrupt sleep, and poor sleep quality. Women diagnosed with OSA are mostly postmenopausal.9,28 In the Wisconsin Sleep Cohort Study, the authors demonstrated that postmenopausal women were 2.6 times more likely to have an AHI of 5 or more and 3.5 times more likely to have OSA defined by an AHI of 15 or more compared with premenopausal women.16 To further help describe the relationship between sex hormones and OSA, in the Nurses’ Health Study and Nurses’ Health Study II, 50 473 and 53 827 postmenopausal women, respectively, were studied to examine the type of menopause and age at menopause in relation to OSA. The type of menopause is characterized as being natural or surgical (hysterectomy/oophorectomy). The risk of having OSA with surgical menopause was 1.27 times higher compared with that for natural menopause. The association did not change even after accounting for age at menopause. These findings suggest that the abrupt termination of sex hormones is associated with higher risk of OSA, and sex hormones play a role in OSA.29
As women increase in age, the prevalence of nocturia increases, but the number of voiding episodes per night is not related to parity.1,30 Factors associated with increase in urine production include advanced age, prior history of hysterectomy, hot flashes in the past month, worse depressive symptoms, vaginal estrogen cream use, and lack of physical activity.31 Of interest, nocturia and nocturnal enuresis are associated with OSA in menopause.2,32
In light of the association of menopause with OSA, hormone therapy (HT) has been studied to treat OSA in postmenopausal women. Several studies have demonstrated that HT reduces the prevalence and severity of OSA in postmenopausal women.11,33,34 However, there are no studies quantifying the effect of HT on nocturia in patients with OSA.
Although previous observational studies showed benefit of HT in decreasing the risk of OSA, the use of HT for treatment has been controversial. Two issues arose when HT was studied longitudinally in postmenopausal women. First, HT was thought to have cardiovascular benefits in postmenopausal women. A large study in the Women’s Health Initiative (WHI) showed no long-term benefit with HT in reducing cardiovascular risk. Participants in the WHI were followed for 5.2 years. There was a modest increase in coronary heart disease, breast cancer, and stroke.35 Second, previous observational studies showing benefit of HT in reducing OSA risk may be biased by healthy user effect. In the Wisconsin Sleep Cohort Study, 228 women were recruited. Overnight polysomnography was completed every 6 months from 1997 to 2006. AHI decreased prior to July 2002, but the effect waned after July 2002, resulting in similar AHI between users and nonusers of HT.36 Overall, the risks and benefits of HT will need to be weighed and individualized prior to initiating therapy.
Pathophysiology of Nocturia and Nocturnal Enuresis in OSA
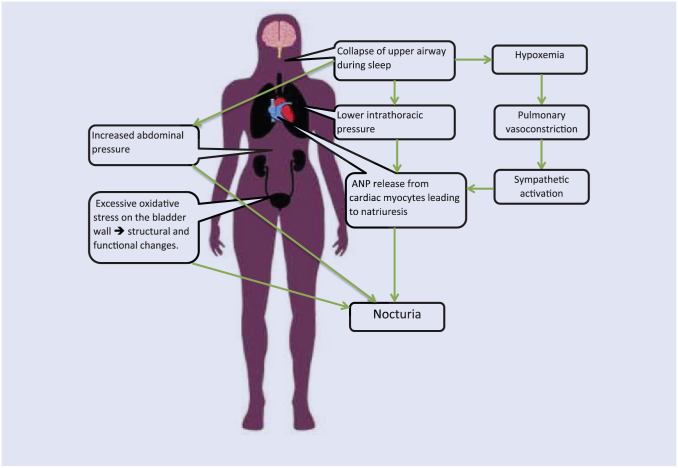
The pathophysiology explaining the relationship between OSA and nocturia remains poorly understood. Several possible mechanisms, however, have been suggested by previous studies, including hypoxemia, changes in intrathoracic pressure, and changes in renin-angiotensin-aldosterone axis. In one study, rats were exposed to intermittent hypoxia during sleep, simulating an OSA-like environment. These mice were found to have structural and functional changes in their bladder, resulting in nocturia. Additionally, hypoxia led to oxidative stress and increased protein and lipid oxidation at the microscopic level, leading to bladder overactivity.37 Whether or not this pathway is responsible for nocturia in human beings has not been studied.
Increase in the serum level of atrial natriuretic peptide (ANP) in patients with OSA has also been cited as a potential explanation of increased urine production at night. ANP is primarily synthesized in mammalian cardiac cells, and the stimuli for its release include stretch, volume overload, high salt diet, and stress on cardiac myocytes.38 The mechanism of ANP secretion is depicted in Figure 1. Negative intrathoracic pressure increases venous return on the right side of the heart, which causes the myocardium to stretch and release ANP. Increased levels of ANP lead to natriuresis and urine production. Its release has been noted in hypoxemia and increased intravascular volume and has many effects, including systemic and renal vasodilation, antidiuretic hormone inhibition, and activation of the rennin-angiotensin-aldosterone system.39 Hypoxemia could also lead to pulmonary vasoconstriction and sympathetic activation. Mackay et al40 explored the association of negative intrathoracic pressure generated by airway closure during sleep increasing circulating ANP and causing nocturia. Their study showed an increase in the level of ANP in OSA patients. However, it failed to show any significant improvement in the symptoms of nocturia with oxygen supplementation and correction of hypoxia. Another study reported a statistically significant decline in circulating ANP and improvement in nocturia episodes with the use of positive airway pressure.41
Figure 1.
Pathophysiology of nocturia in obstructive sleep apnea.
In addition to increased urine production caused by ANP, the sensation of needing to urinate at night and having nocturia or nocturnal enuresis could also occur mechanically from increased abdominal pressure during apneic episodes or loss of neurological control during an arousal episode.41 If nocturia occurs solely from intra-abdominal pressure changes, complete resolution of nocturia is possible by treating OSA.
Less is known about nocturnal enuresis. Nocturnal enuresis is uncommon in adult patients with OSA. It is mainly reported in pediatric cases of OSA. The presence of this symptom has been described in small case studies. Kramer et al42 found that enuresis was associated with sleep apnea symptoms, and treatment of OSA with positive airway pressure prevented further episodes of enuresis. A recent study by Koo et al2 found an association between OSA risk factors and nocturnal enuresis in postmenopausal women. The mechanism of nocturnal enuresis was considered to be similar to that for nocturia. In postmenopausal women, nocturnal enuresis is more common because of higher prevalence of bladder dysfunction or anatomical changes from hormonal imbalance.2
Role of Nocturia in Screening for OSA
With OSA becoming more common in the general population and the known negative health effects it has if left untreated, recognition of OSA by primary care providers has become increasingly more important. There have been many studies that aimed to determine the best screening tool in the primary care setting. Hoffstein and Szalai43 examined the predictive value of clinical features in diagnosing OSA. Helpful clinical predictors include BMI, sex, age, pharyngeal anatomy, snoring, nocturnal choking, excessive daytime sleepiness, impotence, and bed partner’s observations of apneic episodes. Based on the clinician’s history and physical exam, specifically focusing on pharyngeal abnormalities, the sensitivity and specificity of diagnosing OSA were 60% and 63%, respectively.43 The use of the history and physician exam to adequately identify OSA was about 50%. This indicates that 50% of patients with OSA go undetected by their primary care physicians.
Currently available screening questionnaires only include BMI, snoring, other respiratory-related symptoms, and presence of hypertension (Table 1). Nocturia has not been included even though it is a commonly reported symptom for patients with OSA and has a relevant pathophysiological association with OSA. A recent study that examined a large population of sleep patients from a tertiary care center provided greater insight on the association of nocturia with OSA. A total of 1084 patients who completed a sleep questionnaire, Berlin questionnaire, and Epworth sleepiness scare and diagnostic polysomnography were included in the study. Commonly reported OSA symptoms, including snoring, apneas, frequent awakenings, excessive daytime sleepiness, and nocturia, and atypical OSA symptoms such as diaphoresis, nightmares, and morning headaches were recorded. Of the 1084 patients, 788 had the diagnosis of OSA. About 40% of those who had OSA were female. With regard to nocturia, overall 40% of those who had OSA reported the symptom. Of the 40%, 100 (32.9%) were female, and 143 (29.5%) were male. Nocturia was also reported in 69 (23.3%) patients who did not have OSA. The sensitivity of nocturia for OSA was low (31%), and the specificity was high (77%). The positive likelihood ratio (+LR) was 1.35. Snoring, a symptom that has been included in many questionnaires had high sensitivity (91%) but very low specificity (31%). The +LR for snoring (+LR = 1.32) was similar to that for nocturia. As with previous studies, the authors did not find any association between gender and nocturia in those with OSA.44
Table 1.
Inquiry of Symptoms and Risk Factors by Questionnaires.
| Epworth Sleepiness Scale | STOP | STOP-BANG | Berlin | |
|---|---|---|---|---|
| Age | Not used in interpretation | Not used in interpretation | Yes | Not used in interpretation |
| Gender | Not used in interpretation | Not used in interpretation | Yes | Not used in interpretation |
| Neck size | No | No | Yes | No |
| Obesity | No | No | Yes | Yes |
| Snoring | No | Yes | Yes | Yes |
| Daytime sleepiness | Yes | Yes | Yes | Yes |
| Poor sleep quality | No | No | No | No |
| Frequent awakenings | No | No | No | No |
| Apnea | No | Yes | Yes | Yes |
| Nocturia | No | No | No | No |
| Hypertension | No | Yes | Yes | Yes |
Based on the lower prevalence of nocturia as compared with snoring and its high specificity, nocturia may be underreported. Many studies demonstrated a significant association between nocturia and hypertension.45-47 Hypertension also has a strong association with OSA, which is also associated with nocturia.48 Per Dr Feldstein, the presence of nocturia is seen in many comorbid conditions, but it is frequently overlooked and underreported.49 Although nocturia is considered to be a common symptom and the expectation that the bother should prompt patients to report the symptom, the actual reporting of the symptom is low. Urinary symptoms, interactions with health care providers, treatment practices and expectations, and comorbid conditions were examined in 1046 women via survey. Most of the women rate urinary symptoms such as nocturia as moderately to extremely bothersome. However, more than half of the women did not seek medical help. More than a third of the women would like their health care provider to inquire about urinary symptoms.50 The barriers that lead to underreporting include embarrassment, thinking that nocturia is normal with aging, minimizing the seriousness of the condition, and believing that there is no treatment for nocturia.14,51 Given the implications, it would be prudent for physicians to inquire about nocturia in patients who are at risk for OSA.
Changes in Nocturia With OSA Treatment
The use of positive airway pressure to treat OSA has been well established. Although positive airway pressure is the most common and more efficacious treatment, other treatment options for patients, especially those in the mild to moderate OSA groups, are available. They include oral devices, positional therapy, and even surgery in some cases. Each treatment strategy has its risk and benefits. The efficacy of these treatment options depends on the severity of OSA and compliance. The effectiveness of each treatment modality in reducing nocturia is presented in Table 2.
Table 2.
| Treatment | n | Pretreatment | Posttreatment | Reference |
|---|---|---|---|---|
| Mandibular airway device | ||||
| Attali et al, 2016 | 279 | 48% Of the population reported nocturia | 13% Of the population reported nocturia. The incident increased to 57% after 6 months | 54 |
| Positional therapy | No data | No data | ||
| Uvulopalatopharyngoplasty | ||||
| Park et al, 2016 | 66 | Mean nocturia episodes per night: 1.8 ± 1.1 | Mean nocturia episodes per night: 0.8 ± 1.2 | 60 |
| Continuous positive airway pressure | ||||
| Miyauchi et al, 2015 | 51 | Mean nocturia episodes per night: 1.6 ± 1.3 | Mean nocturia episodes per night: 1.1 ± 0.9 | 68 |
| Guilleminault et al, 2004 | 31 | Mean nocturia episodes per night: 3.8 ± 0.4 | Mean nocturia episodes per night: 0.7 ± 0.3 | 71 |
| Margel et al, 2006 | 97 | Mean nocturia episodes per night: 2.4 ± 2.4 | Mean nocturia episodes per night: 0.7 ± 0.6 | 72 |
| McMillan et al, 2014 | 113 | Mean nocturia episodes per night: 1.9 ± 1.3 | Mean nocturia episodes per night: 1.6 ± 1.4 | 73 |
| Liu and Liu, 2001 | 15 | Mean nocturia episodes per night: 2.9 ± 1.5 | Mean nocturia episodes per night: 1.3 ± 0.8 | 74 |
The use of mandibular airway devices (MADs) is a common treatment modality in OSA. These devices mechanically protrude the mandible, which improves the patency of the upper airway and reduces the collapsibility of the upper airway during sleep. The American Academy of Sleep Medicine recommends their use in patients with mild to moderate OSA or in those who cannot tolerate positive airway pressure treatment. Although they are not used as first-line therapy, they are becoming more popular. Because of its ease of use and less intrusive nature, the compliance of using MAD is better compared with positive airway pressure therapy.52,53 In a study that followed 176 patients who wore MAD for nearly 1000 days, there was a significant short-term reduction in nocturia. At the beginning of the study, 48% of those recruited reported nocturia. At the short-term follow-up period (3-6 months after initiation of MAD), 13% reported nocturia.54 Over time, relapse of nocturia occurred likely as a result of worsening OSA severity55 or device wear from long-term use.52
Positional therapy by which patients avoid sleeping in the supine position and/or sleep with neck extension could be considered in patients with mild to moderate OSA and predominant airway obstruction while sleeping in the supine position. It has been shown to normalize AHI and reduce AHI by greater than 50%. Its effectiveness is comparable to positive airway pressure therapy in some studies.56,57 However, a meta-analysis of 3 crossover trials from 1999 to 2010 comparing continuous positive airway pressure (CPAP) with positional therapy demonstrated that CPAP is superior to positional therapy in reducing OSA severity and improving oxygenation in those with positional OSA.58 There are no studies evaluating the effectiveness of positional therapy on reducing or eliminating nocturia.
There are several surgical procedures that could be performed to treat OSA, specifically if there is an anatomical abnormality that is causing airway obstruction during sleep. Uvulopalatopharyngoplasty removes or repositions excess tissue from the soft palate and/or uvula. Surgical correction is offered on a patient-by-patient basis and should be considered as primary treatment in patients with mild OSA and severe obstructing anatomy.59 A recent study examined nocturia after surgical correction in 66 patients with OSA. Of the 66 patients who had the surgery, 37 responded to the follow-up telephone interview. Using the International Prostatic Symptom Score item 7, which inquires about the frequency of nocturia, the authors found that the number of episodes decreased from 1.8 times to 0.8 times per night. Nocturia was abolished in 41% of the patients. Lower-urinary-tract symptoms and quality of life also improved after surgical treatment.60
Positive Airway pressure is the most common and effective modality to treat OSA. It works by pneumatically splinting open the upper airway to prevent upper airway collapse.61 It reduces snoring, gasping or choking, nocturnal awakenings, daytime sleepiness, and nocturia and improves quality of life.62-67 It has been found to reduce not only nocturia but also volume of urine.68 In a meta-analysis of 5 publications performed by Wang et al,66 the authors found that CPAP reduced the standardized mean difference of nocturia incidents by 2.28 and that of nighttime urine volume by 183.12 mL. The 5 studies reported significant reductions in nocturia incidents after treatment with CPAP (Table 2).66 One of the pathophysiological explanations is the significantly reduced ANP secretion with positive airway pressure.69 Krieger et al70 demonstrated that positive airway pressure significantly decreased urinary sodium excretion secondary to sodium resorption. They also showed that with reduction in apnea episodes per night, there was an associated decrease in fractional urinary flow, sodium and potassium excretion, and osmolal clearance.70
Conclusion
Nocturia is an important symptom of OSA that can help primary care providers in screening for this disease, particularly in women, because they generally present with atypical symptoms compared with men with OSA. Most of the screening tools we have today do not include nocturia as one of the symptoms. Treatment of OSA with mandibular advancement devices, positive airway pressure, or surgery improves nocturia. Therefore, further research is needed to investigate whether routinely questioning about nocturia in women would improve efficacy in screening for OSA.
Footnotes
Authors’ Note: Megan Doyle-McClam and Muhammad H. Shahid contributed equally to the work.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: Not applicable, because this article does not contain any studies with human or animal subjects.
Informed Consent: Not applicable, because this article does not contain any studies with human or animal subjects.
Trial Registration: Not applicable, because this article does not contain any clinical trials.
ORCID iD: Patrick Koo  https://orcid.org/0000-0002-8482-1353.
https://orcid.org/0000-0002-8482-1353.
References
- 1. Umlauf MG, Chasens ER. Sleep disordered breathing and nocturnal polyuria: nocturia and enuresis. Sleep Med Rev. 2003;7:403-411. [DOI] [PubMed] [Google Scholar]
- 2. Koo P, McCool FD, Hale L, Stone K, Eaton CB. Association of obstructive sleep apnea risk factors with nocturnal enuresis in postmenopausal women. Menopause. 2016;23:175-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504. [PMC free article] [PubMed] [Google Scholar]
- 4. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:479-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Romero E, Krakow B, Haynes P, Ulibarri V. Nocturia and snoring: predictive symptoms for obstructive sleep apnea. Sleep Breath. 2010;14:337-343. [DOI] [PubMed] [Google Scholar]
- 6. Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA: implications for comorbidities. Chest. 2015;147:266-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Golabek T, Skalski M, Przydacz M, et al. Lower urinary tract symptoms, nocturia and overactive bladder in patients with depression and anxiety [in Polish]. Psychiatr Pol. 2016;50:417-430. [DOI] [PubMed] [Google Scholar]
- 8. Shepertycky MR, Banno K, Kryger MH. Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome. Sleep. 2005;28:309-314. [PubMed] [Google Scholar]
- 9. Saaresranta T, Anttalainen U, Polo O. Sleep disordered breathing: is it different for females? ERJ Open Res. 2015;1:pii: 00063-2015. doi: 10.1183/23120541.00063-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chuang LP, Lin SW, Lee LA, et al. The gender difference of snore distribution and increased tendency to snore in women with menopausal syndrome: a general population study. Sleep Breath. 2017;21:543-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3, pt 1):608-613. [DOI] [PubMed] [Google Scholar]
- 12. Hajduk IA, Strollo PJ, Jr, Jasani RR, Atwood CW, Jr, Houck PR, Sanders MH. Prevalence and predictors of nocturia in obstructive sleep apnea-hypopnea syndrome—a retrospective study. Sleep. 2003;26:61-64. [PubMed] [Google Scholar]
- 13. Kim SO, Kim JS, Kim HS, et al. Age related change of nocturia in women. Int Neurourol J. 2010;14:245-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bosch JL, Weiss JP. The prevalence and causes of nocturia. J Urol. 2013;189(1, suppl):S86-S92. [DOI] [PubMed] [Google Scholar]
- 15. Araujo AB, Yaggi HK, Yang M, McVary KT, Fang SC, Bliwise DL. Sleep related problems and urological symptoms: testing the hypothesis of bidirectionality in a longitudinal, population based study. J Urol. 2014;191:100-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oztura I, Kaynak D, Kaynak HC. Nocturia in sleep-disordered breathing. Sleep Med. 2006;7:362-367. [DOI] [PubMed] [Google Scholar]
- 17. Kuh DL, Wadsworth M, Hardy R. Women’s health in midlife: the influence of the menopause, social factors and health in earlier life. Br J Obstet Gynaecol. 1997;104:923-933. [DOI] [PubMed] [Google Scholar]
- 18. Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167:1181-1185. [DOI] [PubMed] [Google Scholar]
- 19. Young T, Rabago D, Zgierska A, Austin D, Laurel F. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep. 2003;26:667-672. [DOI] [PubMed] [Google Scholar]
- 20. Dancey DR, Hanly PJ, Soong C, Lee B, Hoffstein V. Impact of menopause on the prevalence and severity of sleep apnea. Chest. 2001;120:151-155. [DOI] [PubMed] [Google Scholar]
- 21. Donato GB, Fuchs SC, Oppermann K, Bastos C, Spritzer PM. Association between menopause status and central adiposity measured at different cutoffs of waist circumference and waist-to-hip ratio. Menopause. 2006;13:280-285. [DOI] [PubMed] [Google Scholar]
- 22. Resta O, Caratozzolo G, Pannacciulli N, et al. Gender, age and menopause effects on the prevalence and the characteristics of obstructive sleep apnea in obesity. Eur J Clin Invest. 2003;33:1084-1089. [DOI] [PubMed] [Google Scholar]
- 23. Mirer AG, Young T, Palta M, Benca RM, Rasmuson A, Peppard PE. Sleep-disordered breathing and the menopausal transition among participants in the Sleep in Midlife Women Study. Menopause. 2017;24:157-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eichling PS, Sahni J. Menopause related sleep disorders. J Clin Sleep Med. 2005;1:291-300. [PubMed] [Google Scholar]
- 25. Popovic RM, White DP. Upper airway muscle activity in normal women: influence of hormonal status. J Appl Physiol (1985). 1998;84:1055-1062. [DOI] [PubMed] [Google Scholar]
- 26. Scharf MB, McDannold MD, Stover R, Zaretsky N, Berkowitz DV. Effects of estrogen replacement therapy on rates of cyclic alternating patterns and hot-flush events during sleep in postmenopausal women: a pilot study. Clin Ther. 1997;19:304-311. [DOI] [PubMed] [Google Scholar]
- 27. Antonijevic IA, Stalla GK, Steiger A. Modulation of the sleep electroencephalogram by estrogen replacement in postmenopausal women. Am J Obstet Gynecol. 2000;182:277-282. [DOI] [PubMed] [Google Scholar]
- 28. Lose G, Alling-Møller L, Jennum P. Nocturia in women. Am J Obstet Gynecol. 2001;185:514-521. [DOI] [PubMed] [Google Scholar]
- 29. Huang T, Lin BM, Redline S, Curhan GC, Hu FB, Tworoger SS. Type of menopause, age at menopause, and risk of developing obstructive sleep apnea in postmenopausal women [published online January 22, 2018]. Am J Epidemiol. doi: 10.1093/aje/kwy011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Asplund R, Aberg HE. Nocturia and health in women aged 40-64 years. Maturitas. 2000;35:143-148. [DOI] [PubMed] [Google Scholar]
- 31. Ishman S, Carey RM, Meltel T, Gourin CG. Depression, sleepiness, and disease severity in patients with obstructive sleep apnea. Laryngoscope. 2010;120:2331-2335. [DOI] [PubMed] [Google Scholar]
- 32. Gopal M, Sammel D, Pien G, et al. Investigating the associations between nocturia and sleep disorders in perimenopausal women. J Urol. 2008;180:2063-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Keefe DL, Watson R, Naftolin F. Hormone replacement therapy may alleviate sleep apnea in menopausal women: a pilot study. Menopause. 1999;6:196-200. [DOI] [PubMed] [Google Scholar]
- 34. Shahar E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med. 2003;167:1186-1192. [DOI] [PubMed] [Google Scholar]
- 35. Rossouw JE, Anderson GL, Prentice RL, et al. ; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333. [DOI] [PubMed] [Google Scholar]
- 36. Mirer AG, Peppard PE, Palta M, Benca RM, Rasmuson A, Young T. Menopausal hormone therapy and sleep-disordered breathing: evidence for a healthy user bias. Ann Epidemiol. 2015;25:779-784.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Witthaus MW, Nipa F, Yang JH, Li Y, Lerner LB, Azadzoi KM. Bladder oxidative stress in sleep apnea contributes to detrusor instability and nocturia. J Urol. 2015;193:1692-1699. [DOI] [PubMed] [Google Scholar]
- 38. Ruskoaho H, Lang RE, Toth M, Ganten D, Unger T. Release and regulation of atrial natriuretic peptide (ANP). Eur Heart J. 1987;8(suppl B):99-109. [DOI] [PubMed] [Google Scholar]
- 39. Broaddus VC, Mason RJ, Ernst JD, et al. Murray and Nadel’s Textbook of Respiratory Medicine, 2-Volume Set. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016. [Google Scholar]
- 40. Mackay TW, Fitzpatrick MF, Freestone S, Lee MR, Douglas NJ. Atrial natriuretic peptide levels in the sleep apnoea/hypopnoea syndrome. Thorax. 1994;49:920-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Krieger J, Follenius M, Sforza E, Brandenberger G, Peter JD. Effects of treatment with nasal continuous positive airway pressure on atrial natriuretic peptide and arginine vasopressin release during sleep in patients with obstructive sleep apnoea. Clin Sci (Lond). 1991;80:443-449. [DOI] [PubMed] [Google Scholar]
- 42. Kramer NR, Bonitati AE, Millman RP. Enuresis and obstructive sleep apnea in adults. Chest. 1998;114:634-637. [DOI] [PubMed] [Google Scholar]
- 43. Hoffstein V, Szalai J. Predictive value of clinical features in diagnosing obstructive sleep apnea. Sleep. 1993;16:118-122. [PubMed] [Google Scholar]
- 44. Nigro CA, Dibur E, Borsini E, et al. The influence of gender on symptoms associated with obstructive sleep apnea [published online February 1, 2018]. Sleep Breath. doi: 10.1007/s11325-017-1612-4 [DOI] [PubMed] [Google Scholar]
- 45. Bing MH, Moller LA, Jennum P, Mortensen S, Skovgaard LT, Lose G. Prevalence and bother of nocturia, and causes of sleep interruption in a Danish population of men and women aged 60-80 years. BJU Int. 2006;98:599-604. [DOI] [PubMed] [Google Scholar]
- 46. Johnson TM, II, Sattin RW, Parmelee P, Fultz NH, Ouslander JG. Evaluating potentially modifiable risk factors for prevalent and incident nocturia in older adults. J Am Geriatr Soc. 2005;53:1011-1016. [DOI] [PubMed] [Google Scholar]
- 47. Yoshimura K, Terada N, Matsui Y, Terai A, Kinukawa N, Arai Y. Prevalence of and risk factors for nocturia: analysis of a health screening program. Int J Urol. 2004;11:282-287. [DOI] [PubMed] [Google Scholar]
- 48. Parthasarathy S, Fitzgerald M, Goodwin JL, Unruh M, Guerra S, Quan SF. Nocturia, sleep-disordered breathing, and cardiovascular morbidity in a community-based cohort. PLoS One. 2012;7:e30969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Feldstein CA. Nocturia in arterial hypertension: a prevalent, underreported, and sometimes underestimated association. J Am Soc Hypertens. 2013;7:75-84. [DOI] [PubMed] [Google Scholar]
- 50. MacDiarmid S, Rosenberg M. Overactive bladder in women: symptom impact and treatment expectations. Curr Med Res Opin. 2005;21:1413-1421. [DOI] [PubMed] [Google Scholar]
- 51. Weiss JP. Nocturia: focus on etiology and consequences. Rev Urol. 2012;14:48-55. [PMC free article] [PubMed] [Google Scholar]
- 52. Vecchierini MF, Attali V, Collet JM, et al. ; ORCADES Investigators. A custom-made mandibular repositioning device for obstructive sleep apnoea-hypopnoea syndrome: the ORCADES study. Sleep Med. 2016;19:131-140. [DOI] [PubMed] [Google Scholar]
- 53. Schwartz M, Acosta L, Hung YL, Padilla M, Enciso R. Effects of CPAP and mandibular advancement device treatment in obstructive sleep apnea patients: a systematic review and meta-analysis [published online November 11, 2017]. Sleep Breath. doi: 10.1007/s11325-017-1590-6 [DOI] [PubMed] [Google Scholar]
- 54. Attali V, Chaumereuil C, Arnulf I, et al. Predictors of long-term effectiveness to mandibular repositioning device treatment in obstructive sleep apnea patients after 1000 days. Sleep Med. 2016;27-28:107-114. [DOI] [PubMed] [Google Scholar]
- 55. Marklund M. Long-term efficacy of an oral appliance in early treated patients with obstructive sleep apnea. Sleep Breath. 2016;20:689-694. [DOI] [PubMed] [Google Scholar]
- 56. Mador MJ, Kufel TJ, Magalang UJ, Rajesh SK, Watwe V, Grant BJ. Prevalence of positional sleep apnea in patients undergoing polysomnography. Chest. 2005;128:2130-2137. [DOI] [PubMed] [Google Scholar]
- 57. Permut I, Diaz-Abad M, Chatila W, et al. Comparison of positional therapy to CPAP in patients with positional obstructive sleep apnea. J Clin Sleep Med. 2010;6:238-243. [PMC free article] [PubMed] [Google Scholar]
- 58. Ha SC, Hirai HW, Tsoi KK. Comparison of positional therapy versus continuous positive airway pressure in patients with positional obstructive sleep apnea: a meta-analysis of randomized trials. Sleep Med Rev. 2014;18:19-24. [DOI] [PubMed] [Google Scholar]
- 59. Epstein LJ, Kristo D, Strollo PJ, Jr, et al. ; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276. [PMC free article] [PubMed] [Google Scholar]
- 60. Park HK, Paick SH, Kim HG, et al. Nocturia improvement with surgical correction of sleep apnea. Int Neurourol J. 2016;20:329-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Katsantonis GP, Schweitzer PK, Branham GH, Chambers G, Walsh JK. Management of obstructive sleep apnea: comparison of various treatment modalities. Laryngoscope. 1988;98:304-309. [DOI] [PubMed] [Google Scholar]
- 62. Berry RB, Block AJ. Positive nasal airway pressure eliminates snoring as well as obstructive sleep apnea. Chest. 1984;85:15-20. [DOI] [PubMed] [Google Scholar]
- 63. Antic NA, Catcheside P, Buchan C, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011;34:111-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cruz IA, Drummond M, Winck JC. Obstructive sleep apnea symptoms beyond sleepiness and snoring: effects of nasal APAP therapy. Sleep Breath. 2012;16:361-366. [DOI] [PubMed] [Google Scholar]
- 65. Cao MT, Sternback JM, Guilleminault C. Continuous positive airway pressure therapy in obstructive sleep apnea: benefits and alternatives. Expert Rev Respir Med. 2017;11:259-272. [DOI] [PubMed] [Google Scholar]
- 66. Wang T, Huang W, Zong H, Zhang Y. The efficacy of continuous positive airway pressure therapy on nocturia in patients with obstructive sleep apnea: a systematic review and meta-analysis. Int Neurourol J. 2015;19:178-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fitzgerald MP, Mulligan M, Parthasarathy S. Nocturic frequency is related to severity of obstructive sleep apnea, improves with continuous positive airways treatment. Am J Obstet Gynecol. 2006;194:1399-1403. [DOI] [PubMed] [Google Scholar]
- 68. Miyauchi Y, Okazoe H, Okujyo M, et al. Effect of the continuous positive airway pressure on the nocturnal urine volume or night-time frequency in patients with obstructive sleep apnea syndrome. Urology. 2015;85:333-336. [DOI] [PubMed] [Google Scholar]
- 69. Baruzzi A, Riva R, Cirignotta F, Zucconi M, Cappelli M, Lugaresi E. Atrial natriuretic peptide and catecholamines in obstructive sleep apnea syndrome. Sleep. 1991;14:83-86. [DOI] [PubMed] [Google Scholar]
- 70. Krieger J, Imbs JL, Schmidt M, Kurtz D. Renal function in patients with obstructive sleep apnea. Effects of nasal continuous positive airway pressure. Arch Intern Med. 1988;148:1337-1340. [PubMed] [Google Scholar]
- 71. Guilleminault C, Lin CM, Goncalves MA, Ramos E. A prospective study of nocturia and the quality of life of elderly patients with obstructive sleep apnea or sleep onset insomnia. J Psychosom Res. 2004;56:511-515. [DOI] [PubMed] [Google Scholar]
- 72. Margel D, Shochat T, Getzler O, Livne PM, Pillar G. Continuous positive airway pressure reduces nocturia in patients with obstructive sleep apnea. Urology. 2006;67:974-977. [DOI] [PubMed] [Google Scholar]
- 73. McMillan A, Bratton DJ, Faria R, et al. ; PREDICT Investigators. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med. 2014;2:804-812. [DOI] [PubMed] [Google Scholar]
- 74. Liu S, Liu L. Effect of treatment with continuous positive airway pressure on nocturnal polyuria in patients with obstructive sleep apnea syndrome [in Chinese]. Zhonghua Jie He He Hu Xi Za Zhi. 2001;24:158-160. [PubMed] [Google Scholar]