Abstract
The dental follicle is part of the tooth germ, and isolated stem cells from this tissue (dental follicle cells; DFCs) are considered, for example, for regenerative medicine and immunotherapies. However somatic stem cells can also improve pharmaceutical research. Cell proliferation is limited by the induction of senescence, which, while reducing the therapeutic potential of DFCs for cell therapy, can also be used to study aging processes at the cellular level that can be used to test anti-aging pharmaceuticals. Unfortunately, very little is known about cellular senescence in DFCs. This review presents current knowledge about cellular senescence in DFCs.
Keywords: Dental follicle, Dental stem cells, Senescence, Osteogenic differentiation
Introduction
Various types of tooth stem cells have been isolated from human mesodermal tooth tissue in the past 2 decades [1, 2, 3, 4]. Dental stem cells were isolated from the dental pulp and the periodontal ligament from permanent teeth, wisdom teeth, and deciduous teeth [5, 6, 7]. However, some human dental stem cells can only be isolated from impacted wisdom teeth such as dental follicle cells (DFCs), which are actually tooth germ cells [1, 8]. Somatic stem cells such as dental stem cells are discussed for a number of applications in regenerative medicine, for example, for biological implants in dentistry or for bone defects of critical size [9, 10]. Unfortunately, actual dental stem cell treatments are rare because induction of senescence is a major problem for cell proliferation in order to achieve a sufficient cell count for treatments. While this problem needs to be addressed in advance for stem cell-based therapies, the induction of senescence in somatic stem cells could also be an ideal cell model for in vitro aging studies to further investigate the molecular effects of pharmacological products. The induction of senescence in dental stem cells is, therefore, an opportunity for a new test method and of great importance for research. This article focuses on our current knowledge of senescence in a particular type of dental stem cell that can be isolated from the dental follicle.
Isolation of Undifferentiated Cells from the DFCs
The dental follicle can be easily separated from extracted wisdom teeth [8, 11]. This tooth germ tissue mainly consists of a collagen-like connective tissue-like stroma, which can be made visible by trichrome staining [12, 13, 14]. This connective tissue contains small vessels and around these vessels, some cells express Nestin or Notch-1, which are typical markers for cells of the neural crest or of neural precursor [8, 13, 15, 16]. A single-cell suspension was yielded from the dental follicle after mechanical treatment and protease treatment, and undifferentiated DFCs can be obtained from single cells as colony-forming unit fibroblasts (CFU-F). A colony contains at least 50 cells with a fibroblast-like morphology, and DFCs not only express Nestin and Notch-1 but also other typical mesenchymal stem cell markers such as CD105 [8, 17]. DFCs can be propagated in cell culture for at least 6 passages and differentiated into functional tissue cells such as fibroblasts, osteo-/cementoblasts, and neural cells (including glial cells) [8, 18, 19].
The multipotency of DFCs make them suitable for use in regenerative medicine, and they can be considered for a number of regenerative therapies of oral tissues, including hard and soft tooth tissues such as dentin, cementum, alveolar bone, gingiva, and the periodontium. The origin of the DFCs in cells of the neural crest also makes them interesting for the regeneration of nerve cells (tissue cells), including Schwann cells. In addition, it is also possible that DFCs could be used for immunotherapy in the near future [20].
Before dental stem cells can be applied, for example, in regenerative dentistry, however, extensive work in basic research is required. In addition, to some general questions regarding the assessment of differentiation potentials, the elucidation of differentiation mechanisms, and a thorough test of suitability in immunotherapy, an optimal protocol for the cultivation and multiplication of DFCs in cell culture is required [20, 21, 22]. Another important issue is how senescence can be induced in undifferentiated DFCs.
Induction of Senescence in DFCs
During cell culture, more and more DFCs acquire senescence, which is noticed, for example, in cell culture due to a reduced possibility of cell proliferation [23]. Senescence is generally induced by a number of factors in cell culture, for example, DNA damage, telomere erosion, or oxidative stress [24, 25, 26]. Senescent stem cells are of limited value for regenerative medicine because they can, for example, secrete senescence-associated secretory phenotype (SASP), which is an important mediator of the pathophysiological functions of senescent cells (fibrosis, wound healing, etc.) [27, 28].
Previous observations have shown that DFCs can easily be cultivated for more than 6 passages [8]. Here, the cell morphology and the population doubling time did not change significantly. After passage 10 and in later stages of cell culture, however, cell proliferation decreased significantly and finally stopped [23, 29]. The appearance of longer population doubling times is associated with the induction of β-galactosidase activity in senescent cells. This is an important and reliable marker of cellular senescence, and the enzyme catalyzes the hydrolysis of β-galactosides [27]. DFCs exhibit a high β-galactosidase activity at later stages of cell culture [23]. In earlier times of DFC cultures − under 10 passages − almost none of the cells examined showed any visible β-galactosidase activity. The observed induction of β-galactosidase activity between passages 10 and 16 indicates the most susceptible point in time for the induction of cellular senescence in DFCs [29, 30]. Another feature for the upcoming cellular senescence is the increasing cell size. An increased size of DFCs is visible, at least after passage 14 in cell culture, but the heterogeneity of the cell size is very high [23]. Interestingly, we have shown in preliminary data that the expression of dental stem cell markers such as CD105 did not change significantly after induction of cellular senescence. Only minor changes for the markers CD146 and Nestin were found between cell passages 7 and 18. We do not know why the expression of stem cell markers did not decrease, but − as I will discuss later − the differentiation potential in senescent DFCs is decreasing [23].
Telomere Length and the Induction of Cellular Senescence in DFCs
Since the publication of the Hayflick limit on the relation of the limited ability of cell division and the shortening of telomeres in the 1960s, a lot of publications have shown that the limit ability of cell division (senescence) depends highly on the telomere length [31, 32]. In DFCs, the shortening of the telomeres is small and probably only plays a subordinate role for the induction of senescence, since it hardly or only slightly decreases [23]. However, cellular senescence induction is still associated with telomere length. Recent works with dental pulp stem cells have shown that significant heterogeneity in stem cell expansion and regeneration capacity is associated with different telomere lengths and an associated susceptibility to replicative senescence [33, 34]. However, the original telomeric length of DFCs could be critical for induction of cellular senescence. A recent study compared telomeric length of 6 different isolations of DFCs [29]. Telomeric lengths of 5 DFC cell lines were almost similar, but 1 cell line had shorter telomeres. This cell line (DFC_F) with short telomeres was compared with another cell line with longer “standard” telomeres (DFC_S) [29]. Although we expect cells with shorter telomeres to have higher β-galactosidase activity than cells with longer telomeres, the opposite was observed in this study. The DFC_Fs with shorter telomeres had lower β-galactosidase activity than the DFC_S with longer telomeres in an early phase of cell culture. Contrary to the relationship between DFC_F and DFC_S at high passages, here the relationship was reversed, indicating a higher susceptibility to senescence in cells with short telomeres. Previous studies on telomere length in dental pulp stem cells and induction of cellular senescence have shown similar results [33]. Interestingly, the telomeric erosion did not occur differently in the 2 cell lines during cell culture since almost no erosion was found for DFC_F and DFC_S in senescent DFCs [29]. Genomically higher rates of DNA damage and chromosome reorganization or aberration in DFC_F was the reason for the accelerated induction of cellular senescence. DFC_F became aneuploid after long-term cell cultures, while the number of aneuploid cells in DFC_S was almost negligible. The same applies if the expression of genes that are associated with DNA damage and cellular senescence was compared [29]. In conclusion, telomeric erosion probably plays a minor role in the induction of cellular senescence, but short telomeres favor induction.
The Cell Cycle Regulator Protein P16 Supports Induction of Senescence
In a very simplified model, 2 signaling pathways are involved in the induction of cellular senescence in mammalian cells [35, 36, 37, 38]. These 2 pathways control the progression of cell cycle in the G1 phase by inhibition of cyclin-dependent kinases (CDKs) [37, 39, 40, 41]. Each of these 2 pathways can be represented by proteins that are unique to that pathway. One pathway, for example, uses protein p21, which is activated by protein p53 (TP53), and the other pathway uses protein p16. Both pathways are indirectly or directly induced by oxidative stress, telomerase inhibition, oncogenes, and enzymes that are involved in DNA repair [42, 43, 44]. Both pathways are also involved in additional biological processes such as autophagy and apoptosis, which can alternatively be induced [39]. However, it must be elucidated individually which of the pathways is responsible for the induction of cellular senescence.
A previous study, therefore, examined both pathways by investigating the expression of genes and proteins, which are involved in DNA damage and by the inhibition of telomerase activity and cellular senescence [41]. DFCs were selected at different stages of cell culture: before the induction of senescence (passage 7), at an early stage of senescence induction (passage 12), at a later stage of senescence induction (passage 16), and from a cell culture with almost only senescent DFCs (passage 21). PCR array analyses containing gene expression data of the genes from p21, p53, and p16 showed that only the gene of the p16 protein (CDKN2A) was induced in senescent DFCs [41]. In contrast, p21 and p53 proteins were downregulated or constitutively expressed in senescent DFCs [41, 45]. During cellular senescence, p21 gene expression resembles genes associated with cell cycle progression and senescence inhibition, such as CDK2 or E2F1 [41]. Western blot analyses confirmed that p16 protein expression was induced after induction of cellular senescence, while p21 protein expression was downregulated. The protein expression of p21 was again comparable to the expression of CDKs [41]. The relationship between p16 expression and induction of cellular senescence confirmed that inhibition of p16 significantly inhibited induction of senescence. These experiments supported the main assumption that p16 induced senescence in DFCs.
It is already known that typical stem cell characteristics such as the osteogenic differentiation in dental stem cells are affected after the induction of cellular senescence [33, 34, 44, 46, 47]. In senescent dental stem cells, for example, the expression of osteogenic markers is repressed and the biomineralization is greatly reduced, but the molecular mechanisms for this phenomenon are unknown. Induction of p16 in senescent DFCs does not inhibit osteogenic differentiation because downregulation of p16 inhibited the expression of osteogenic differentiation markers and apparently had no influence on the alkaline phosphatase activity of DFCs, which is an important marker for osteogenic differentiation [41]. These data provide some evidence that p16 supports but cannot inhibit osteogenic differentiation in senescent DFCs. The molecular mechanism that inhibits differentiation in senescent DFCs remained unclear after this study.
Role of WNT5A in Senescent DFCs
While p16 plays an important role in the induction of senescence, its expression does not cause a reduced osteogenic differentiation potential of senescent cells. Good opportunities to find key factors for the reduced osteogenic differentiation potential are investigations on the role of typical proteins, which are generally involved in the induction of cellular senescence and/or the osteogenic differentiation of stem/progenitor cells. One protein is WNT5A, which is involved in the non-canonical WNT pathway [30]. Previous studies have shown that WNT5A is involved in both osteogenic differentiation and induction of cellular senescence [48, 49, 50, 51]. Studies with DFCs from rats, for example, showed that WNT5A supports bone morphogenetic protein (BMP)2-induced differentiation, WNT5A specifically inducing JNK [49]. Interestingly, WNT5A is also associated with an alternative activation of β-catenin [52]. A published study by Nemoto and co-workers suggests a feedback mechanism between the canonical and the non-canonical WNT signaling pathways that could regulate the specific activation of β-catenin in DFCs during osteogenic differentiation [53]. The studies by Nemoto and co-workers [53, 54] and by Ling and co-workers [49] on WNT5A and osteogenic differentiation were made with murine DFCs, however, and these 2 groups showed very different effects of WNT5a on osteogenic differentiation. In contrast to the study by Ling and co-workers, WNT5A inhibited the alkaline phosphatase activity in the study by Nemoto and colleagues [53]. Studies with human DFCs showed that both the canonical WNT signaling pathway and BMP signaling pathway are involved in the osteogenic differentiation of DFCs [55, 56]. However, the roles that some members of the canonical WNT pathways such as β-catenin or APCDD1, a known inhibitor of the WNT pathway, play are not clear [57].
WNT5A is likely to play a role in human DFCs during senescence induction. It is gradually downregulated after the induction of cellular senescence; similar to osteogenic differentiation markers [30]. After induction of cellular senescence, inhibition of WNT5A affects osteogenic differentiation. However, WNT5A had no significant influence on the expression of osteogenic differentiation markers in DFC before induction of senescence. The effect of WNT5A is, therefore, not directly related to pathways or genes that are directly involved in the expression of osteogenic differentiation markers. Since WNT5A strongly affects cell proliferation, induction of senescence, and the number of apoptotic and viable cells, it is important for the viability of DFCs after induction of osteogenic differentiation [30]. Interestingly, also another factor related to cell viability and cellular senescence is involved in a reduced osteogenic differentiation potential of senescent DFCs. The TP53 transcription factors are almost always involved in cell division and cell viability, but also in other biological processes such as ostoegenic differentiation [35, 40, 58, 59, 60, 61, 62]. A recent study showed that TP53 inhibits the osteogenic differentiation in senescent DFCs, but is not involved in senescence induction [45].
Conclusion
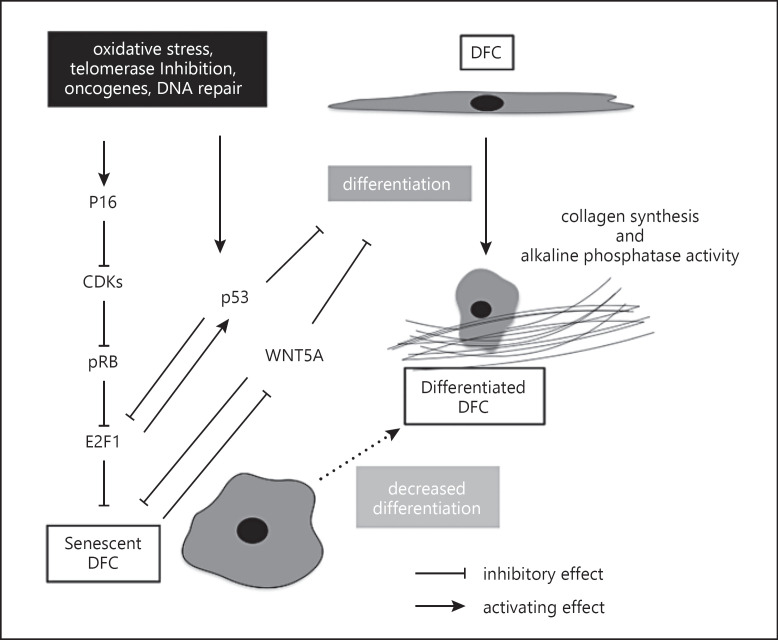
Cellular senescence affects the use of DFCs in stem cell therapies and needs to be regulated. Basic research on cellular senescence is aimed at this problem, and its current progress can be summarized by the following points (Fig. 1):
Fig. 1.
Putative mechanisms of cellular senescence in DFCs. This figure also contains proteins CDK and pRB, which are generally involved in this biological process. ALP, alkaline phosphatase; DFCs, dental follicle cells; CDK, cyclin-dependent kinase; pRB, retinoblastoma protein.
The shortening of the telomeres is not strongly related to the senescence induction of DFCs.
Shorter telomere length of DFCs accelerates senescence induction and favor DNA damage (aneuploidy).
The p16 signaling pathways drives senescence induction.
Senescence inhibits osteogenic differentiation of DFCs.
Reduced expression of WNT5A in senescent DFCs decreases cell viability and osteogenic differentiation of DFCs.
P53 is unlikely to be involved in senescence induction but affects osteogenic differentiation of DFCs.
Future studies will need to evaluate new cell culture protocols that reduce senescence induction in DFC and/or improve osteogenic differentiation in senescent cells. It will be of great importance to manipulate certain targets such as WNT5A to directly obtain DFCs that are suitable for therapies. The type of manipulation is still unclear. However, the summarized studies in this article show that a DFC-based cell culture model can already be used for in vitro tests, for example, to investigate the molecular and cellular consequences of drugs in aging cells.
Statement of Ethics
The author has no ethical conflicts to disclose.
Conflict of Interest Statement
The author has no conflicts of interest.
Funding Sources
This paper was not funded.
Author Contributions
Christian Morsczeck had the idea and is the only author of this article.
verified
References
- 1.Luan X, Ito Y, Dangaria S, Diekwisch TG. Dental follicle progenitor cell heterogeneity in the developing mouse periodontium. Stem Cells Dev. 2006;15((4)):595–608. doi: 10.1089/scd.2006.15.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morsczeck C, Schmalz G, Reichert TE, Völlner F, Galler K, Driemel O. Somatic stem cells for regenerative dentistry. Clin Oral Investig. 2008;12((2)):113–8. doi: 10.1007/s00784-007-0170-8. [DOI] [PubMed] [Google Scholar]
- 3.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88((9)):792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morsczeck C, Huang GTJ, Shi S. Morsczeck, christian. Regensburg, Germany: Univ Hosp Regensburg, Dept Cranial and Maxillofacial Surg; 2013. Stem and progenitor cells of dental and gingival tissue origin. [Google Scholar]
- 5.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97((25)):13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100((10)):5807–12. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364((9429)):149–55. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 8.Morsczeck C, Götz W, Schierholz J, Zeilhofer F, Kühn U, Möhl C, et al. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24((2)):155–65. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seo BM, Sonoyama W, Yamaza T, Coppe C, Kikuiri T, Akiyama K, et al. SHED repair critical-size calvarial defects in mice. Oral Dis. 2008;14((5)):428–34. doi: 10.1111/j.1601-0825.2007.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kémoun P, Laurencin-Dalicieux S, Rue J, Farges JC, Gennero I, Conte-Auriol F, et al. Human dental follicle cells acquire cementoblast features under stimulation by BMP-2/-7 and enamel matrix derivatives (EMD) in vitro. Cell Tissue Res. 2007;329((2)):283–94.. doi: 10.1007/s00441-007-0397-3. [DOI] [PubMed] [Google Scholar]
- 12.Diekwisch TG. The developmental biology of cementum. Int J Dev Biol. 2001;45((5–6)):695–706. [PubMed] [Google Scholar]
- 13.Morsczeck C, Moehl C, Götz W, Heredia A, Schäffer TE, Eckstein N, et al. In vitro differentiation of human dental follicle cells with dexamethasone and insulin. Cell Biol Int. 2005;29((7)):567–75. doi: 10.1016/j.cellbi.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Kémoun P, Laurencin-Dalicieux S, Rue J, Vaysse F, Roméas A, Arzate H, et al. Localization of STRO-1, BMP-2/-3/-7, BMP receptors and phosphorylated Smad-1 during the formation of mouse periodontium. Tissue Cell. 2007;39((4)):257–66. doi: 10.1016/j.tice.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Hermann A, Gastl R, Liebau S, Popa MO, Fiedler J, Boehm BO, et al. Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. J Cell Sci. 2004;117((Pt 19)):4411–22. doi: 10.1242/jcs.01307. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida S, Shimmura S, Nagoshi N, Fukuda K, Matsuzaki Y, Okano H, et al. Isolation of multipotent neural crest-derived stem cells from the adult mouse cornea. Stem Cells. 2006;24((12)):2714–22. doi: 10.1634/stemcells.2006-0156. [DOI] [PubMed] [Google Scholar]
- 17.Press T, Viale-Bouroncle S, Felthaus O, Gosau M, Morsczeck C. EGR1 supports the osteogenic differentiation of dental stem cells. Int Endod J. 2015;48((2)):185–92. doi: 10.1111/iej.12299. [DOI] [PubMed] [Google Scholar]
- 18.Völlner F, Ernst W, Driemel O, Morsczeck C. A two-step strategy for neuronal differentiation in vitro of human dental follicle cells. Differentiation. 2009;77((5)):433–41. doi: 10.1016/j.diff.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Felthaus O, Ernst W, Driemel O, Reichert TE, Schmalz G, Morsczeck C. TGF-beta stimulates glial-like differentiation in murine dental follicle precursor cells (mDFPCs) Neurosci Lett. 2010;471((3)):179–84. doi: 10.1016/j.neulet.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 20.Morsczeck C, Reichert TE. Dental stem cells in tooth regeneration and repair in the future. Expert Opin Biol Ther. 2018;18:1–196. doi: 10.1080/14712598.2018.1402004. [DOI] [PubMed] [Google Scholar]
- 21.Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006;24((2)):462–71. doi: 10.1634/stemcells.2004-0331. [DOI] [PubMed] [Google Scholar]
- 22.Locatelli F, Algeri M, Trevisan V, Bertaina A. Remestemcel-L for the treatment of graft versus host disease. Expert Rev Clin Immunol. 2017;13((1)):43–56. doi: 10.1080/1744666X.2016.1208086. [DOI] [PubMed] [Google Scholar]
- 23.Morsczeck C, Gresser J, Ettl T. The induction of cellular senescence in dental follicle cells inhibits the osteogenic differentiation. Mol Cell Biochem. 2016;417((1–2)):1–339. doi: 10.1007/s11010-016-2708-z. [DOI] [PubMed] [Google Scholar]
- 24.Sherr CJ, DePinho RA. Cellular senescence: mitotic clock or culture shock? Cell. 2000;102((4)):407–10. doi: 10.1016/s0092-8674(00)00046-5. [DOI] [PubMed] [Google Scholar]
- 25.Shay JW, Wright WE. Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis. 2005;26((5)):867–74. doi: 10.1093/carcin/bgh296. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Wu Q, Wang Y, Li L, Bu H, Bao J. Senescence of mesenchymal stem cells (Review) Int J Mol Med. 2017;39((4)):775. doi: 10.3892/ijmm.2017.2912. [DOI] [PubMed] [Google Scholar]
- 27.Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8((9)):729–40. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 28.Malaquin N, Martinez A, Rodier F. Keeping the senescence secretome under control: molecular reins on the senescence-associated secretory phenotype. Exp Gerontol. 2016;82:39–49. doi: 10.1016/j.exger.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Morsczeck C, Reck A, Reichert TE. Short telomeres correlate with a strong induction of cellular senescence in human dental follicle cells. Bmc Mol Cell Biol. 2019;20((1)):5. doi: 10.1186/s12860-019-0185-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morsczeck C, Reck A, Reichert TE. WNT5A supports viability of senescent human dental follicle cells. Mol Cell Biochem. 2019 May;455((1–2)):21–8. doi: 10.1007/s11010-018-3467-9. [DOI] [PubMed] [Google Scholar]
- 31.Hayflick L, Perkins F, Stevenson RE. Human diploid cell strains. Science. 1965;143((3609)):976–636. doi: 10.1126/science.143.3609.976. [DOI] [PubMed] [Google Scholar]
- 32.Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000;1((1)):72–6. doi: 10.1038/35036093. [DOI] [PubMed] [Google Scholar]
- 33.Alraies A, Alaidaroos NY, Waddington RJ, Moseley R, Sloan AJ. Variation in human dental pulp stem cell ageing profiles reflect contrasting proliferative and regenerative capabilities. BMC Cell Biol. 2017;18((1)):12. doi: 10.1186/s12860-017-0128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morsczeck C. Cellular senescence in dental pulp stem cells. Arch Oral Biol. 2019;99:150–5. doi: 10.1016/j.archoralbio.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, et al. Crucial role of p53-dependent cellular senescence in suppression of pten-deficient tumorigenesis. Nature. 2005;436((7051)):725–30. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Courtois-Cox S, Jones SL, Cichowski K. Many roads lead to oncogene-induced senescence. Oncogene. 2008;27((20)):2801–9. doi: 10.1038/sj.onc.1210950. [DOI] [PubMed] [Google Scholar]
- 37.Feng X, Xing J, Feng G, Huang D, Lu X, Liu S, et al. p16(INK4A) mediates age-related changes in mesenchymal stem cells derived from human dental pulp through the DNA damage and stress response. Mech Ageing Dev. 2014;141–142:46–55. doi: 10.1016/j.mad.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530((7589)):184–9. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vicencio JM, Galluzzi L, Tajeddine N, Ortiz C, Criollo A, Tasdemir E, et al. Senescence, apoptosis or autophagy? When a damaged cell must decide its path: a mini-review. Gerontology. 2008;54((2)):92–9. doi: 10.1159/000129697. [DOI] [PubMed] [Google Scholar]
- 40.Laine A, Westermarck J. Molecular pathways: harnessing E2F1 regulation for prosenescence therapy in p53-defective cancer cells. Clin Cancer Res. 2014;20((14)):3644–50. doi: 10.1158/1078-0432.CCR-13-1942. [DOI] [PubMed] [Google Scholar]
- 41.Morsczeck C, Hullmann M, Reck A, Reichert TE. The cell cycle regulator protein P16 and the cellular senescence of dental follicle cells. Mol Cell Biochem. 2018;439:45–52. doi: 10.1007/s11010-017-3134-6. [DOI] [PubMed] [Google Scholar]
- 42.Lou Z, Chen J. Cellular senescence and DNA repair. Exp Cell Res. 2006;312((14)):2641–6. doi: 10.1016/j.yexcr.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Chandeck C, Mooi WJ. Oncogene-induced cellular senescence. Adv Anat Pathol. 2010;17((1)):42–8. doi: 10.1097/PAP.0b013e3181c66f4e. [DOI] [PubMed] [Google Scholar]
- 44.Mas-Bargues C, Viña-Almunia J, Inglés M, Sanz-Ros J, Gambini J, Ibáñez-Cabellos JS, et al. Role of p16. Redox Biol. 2017;12:690–8. doi: 10.1016/j.redox.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pieles OR A, Reichert TE, Morsczeck C. p53 inhibits the osteogenic differentiation but does not induce senescence in human dental follicle cells. Differentiation. 2020;114:20–6. doi: 10.1016/j.diff.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Choi YJ, Lee JY, Chung CP, Park YJ. Cell-penetrating superoxide dismutase attenuates oxidative stress-induced senescence by regulating the p53-p21(Cip1) pathway and restores osteoblastic differentiation in human dental pulp stem cells. Int J Nanomedicine. 2012;7:5091–106. doi: 10.2147/IJN.S31723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Despars G, Carbonneau CL, Bardeau P, Coutu DL, Beauséjour CM. Loss of the osteogenic differentiation potential during senescence is limited to bone progenitor cells and is dependent on p53. PloS One. 2013;8:e73206. doi: 10.1371/journal.pone.0073206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santos A, Bakker AD, de Blieck-Hogervorst JM, Klein-Nulend J. WNT5A induces osteogenic differentiation of human adipose stem cells via rho-associated kinase ROCK. Cytotherapy. 2010;12:924–32. doi: 10.3109/14653241003774011. [DOI] [PubMed] [Google Scholar]
- 49.Xiang L, Chen M, He L, Cai B, Du Y, Zhang X, et al. Wnt5a regulates dental follicle stem/progenitor cells of the periodontium. Stem Cell Res Ther. 2014;5:135. doi: 10.1186/scrt525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Webster MR, Xu M, Kinzler KA, Kaur A, Appleton J, O'Connell MP, et al. Wnt5A promotes an adaptive, senescent-like stress response, while continuing to drive invasion in melanoma cells. Pigment Cell Melanoma Res. 2015;28:184–95. doi: 10.1111/pcmr.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hasegawa D, Wada N, Yoshida S, Mitarai H, Arima M, Tomokiyo A, et al. Wnt5a suppresses osteoblastic differentiation of human periodontal ligament stem cell-like cells via Ror2/JNK signaling. J Cell Physiol. 2018;233:1752–62. doi: 10.1002/jcp.26086. [DOI] [PubMed] [Google Scholar]
- 52.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakisaka Y, Tsuchiya M, Nakamura T, Tamura M, Shimauchi H, Nemoto E. Wnt5a attenuates Wnt3a-induced alkaline phosphatase expression in dental follicle cells. Exp Cell Res. 2015;336((1)):85–93. doi: 10.1016/j.yexcr.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 54.Nemoto E, Sakisaka Y, Tsuchiya M, Tamura M, Nakamura T, Kanaya S, et al. Wnt3a signaling induces murine dental follicle cells to differentiate into cementoblastic/osteoblastic cells via an osterix-dependent pathway. J Periodontal Res. 2016 Apr;51((2)):164–74. doi: 10.1111/jre.12294. [DOI] [PubMed] [Google Scholar]
- 55.Viale-Bouroncle S, Felthaus O, Schmalz G, Brockhoff G, Reichert TE, Morsczeck C. The transcription factor DLX3 regulates the osteogenic differentiation of human dental follicle precursor cells. Stem Cells Dev. 2012;21:1936–47. doi: 10.1089/scd.2011.0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Viale-Bouroncle S, Klingelhoffer C, Ettl T, Reichert TE, Morsczeck C. A protein kinase A (PKA)/beta-catenin pathway sustains the BMP2/DLX3-induced osteogenic differentiation in dental follicle cells (DFCs) Cell Signal. 2015;27:598–605. doi: 10.1016/j.cellsig.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 57.Viale-Bouroncle S, Klingelhöffer C, Ettl T, Morsczeck C. The WNT inhibitor APCDD1 sustains the expression of β-catenin during the osteogenic differentiation of human dental follicle cells. Biochem Biophys Res Commun. 2015 Feb 13;457((3)):314–7. doi: 10.1016/j.bbrc.2014.12.107. [DOI] [PubMed] [Google Scholar]
- 58.Yu J, Baron V, Mercola D, Mustelin T, Adamson ED. A network of p73, p53 and Egr1 is required for efficient apoptosis in tumor cells. Cell Death Differ. 2007;14:436–46. doi: 10.1038/sj.cdd.4402029. [DOI] [PubMed] [Google Scholar]
- 59.Berman SD, Yuan TL, Miller ES, Lee EY, Caron A, Lees JA. The retinoblastoma protein tumor suppressor is important for appropriate osteoblast differentiation and bone development. Mol Cancer Res. 2008;6:1440–51. doi: 10.1158/1541-7786.MCR-08-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vigneron A, Vousden KH. p53, ROS and senescence in the control of aging. Aging. 2010;2:471–4. doi: 10.18632/aging.100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Egbuniwe O, Idowu BD, Funes JM, Grant AD, Renton T, Di Silvio L. P16/p53 expression and telomerase activity in immortalized human dental pulp cells. Cell Cycle. 2011;10:3912–9. doi: 10.4161/cc.10.22.18093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laine A, Sihto H, Come C, Rosenfeldt MT, Zwolinska A, Niemela M, et al. Senescence sensitivity of breast cancer cells is defined by positive feedback loop between CIP2A and E2F1. Cancer Discov. 2013;3:182–97. doi: 10.1158/2159-8290.CD-12-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]