Abstract
Background
Acute invasive fungal rhinosinusitis (AIFRS) is aggressive morbidity affecting immunocompromised patients. Coronavirus disease 2019 (COVID-19) may allow secondary fungal disease through a propensity to cause respiratory infection by affecting the immune system leading to dysregulation and reduced numbers of T lymphocytes, CD4+T, and CD8+T cells, altering the innate immunity. The aim of this study is to evaluate the incidence of acute invasive fungal rhinosinusitis (AIFRS) in COVID-19 patients.
Methodology
Data for acute invasive rhinosinusitis was obtained from the Otorhinolaryngology departments at our tertiary hospital at the period from January 2017 to December 2020. Then the risk factors of comorbid diseases and fungal types between post-COVID-19 and non-COVID-19 groups regarding the incidence of AIFRS are compared.
Results
Consequently, the incidence of AIFRS showed a more significant difference (P < 0.05) in post-COVID-19 patients than in non-COVID-19 especially in immunocompromised patients, diabetic, renal, and liver dysfunction patients as well as patients with risk factors of AIFRS. The most common organisms affecting patients with AIFRS are Rhizopus oryzae, Aspergillus fumigatus, and Absidia mucor.
Conclusions
The incidence of AIFRS is markedly prominent in post-COVID-19 patients than in those of non-COVID-19, especially in immunocompromised, diabetic, renal, and liver dysfunction patients and patients with risk factors for rhinosinusitis.
Keywords: Coronavirus, Fungal, Immunocompromised, Rhinosinusitis
1. Introduction
Fungal sinusitis can be categorized into non-invasive and invasive groups. While non-invasive fungal sinusitis does not exhibit the penetration of mucosa by hyphae; in invasive fungal sinusitis hyphae do invade the mucosa [1].
Acute invasive fungal sinusitis is considered the most aggressive form of sinusitis. It can be more commonly found in immunocompromised patients and notably can lead to serious morbidity and mortality. Immunosuppression in these patients can be a result of widespread sources including hematologic malignancies, diabetes mellitus, solid organ or bone marrow transplantation, chemotherapy-induced neutropenia, and advanced AIDS. Furthermore, it is as a rule with sudden critical advancement of nasal congestion, facial pain, epistaxis and fever. Expansion into the sinus or intracranial compartments can lead to neurological impairments [2,3].
Fungal spores are copious in the atmosphere. Therefore, it is ready to cause morbidity in the nose and paranasal sinuses. These spores can lead to a pathological affection if the environment is suitable for their growth and active invasion of tissues. Normally, inhaled fungi form a part of the normal sinonasal flora, but they are significantly destroyed by the immunological system. However, in conditions such as prolonged antibiotics use, poor ventilation and moist environment as well as immunocompromised patients, these immunological pathways may be disrupted, making fungal invasion more likely to make a morbid affection of tissues [4].
Infection is often thought to arise in the nasal cavity (commonly the middle turbinate) and progress to the paranasal sinuses [5]. This suggests the involvement of multiple fungal agents, such as Aspergillus species (commonly in neutropenic patients), Zygomycetes (commonly in diabetic patients), Rhizopus species, Absidia species, Mucor species, and Rhizomucor species [1].
COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a primary acute respiratory disease which can lead to severe acute respiratory distress syndrome (ARDS), multiple organ dysfunction, and even death. Therefore, identifying risk and protective factors for COVID-19 is critical to developing efficient intervention and prevention strategies [6].
Particular pathophysiologic features of COVID-19 that may allow secondary fungal disease, a propensity to cause respiratory infection, may upgrade the risk of invasive fungal rhinosinusitis. Moreover, the immune dysregulation associated with COVID-19, with reduced numbers of T lymphocytes, CD4+T, and CD8+T cells, may alter innate immunity [7].
COVID-19 is associated with a significant incidence of secondary infections, both bacterial and fungal probably due to immune dysregulation. Additionally, the widespread use of steroids/monoclonal antibodies/broad-spectrum antibiotics as part of the armamentarium against COVID-19 may lead to the development/exacerbation of preexisting fungal diseases [8].
Acute invasive fungal rhinosinusitis is a rare, albeit highly comorbid, infection affecting immunosuppressed individuals. It may appear in patients with high risk of complications morbidity in the setting of COVID-19-related acute respiratory distress syndrome [9]. Therefore, the aim of this study is to evaluate the incidence of acute invasive fungal rhinosinusitis in COVID-19 patients.
2. Material and methods
2.1. Ethical considerations
The bioethical approval was obtained from Al-Azhar University, Damietta faculty of medicine for this study (IRB 00012367 / 20- 11- 002). This retrospective study was conducted for patients with AIFRS at the period from Jan 2017 to Dec 2020 at Otorhinolaryngology departments of Al-Azhar University in both Cairo and Damietta branch, Egypt.
2.2. The inclusion criteria
Patients of any age and sex. AIFRS diagnosis based on clinical, endoscopic diagnosis (Fig. 1 ) and positive findings for acute sinusitis on sinus computed tomography (CT) scan (Fig. 2 ) and MRI (Fig. 3 ) in addition to fungal determination by histopathological examination of specimen of affected tissues for detection of the type of fungi. Positive PCR was confirmed from the patient's files at the isolation hospitals for COVID 19 group. Participants who did not meet inclusion criteria were excluded. Such as Patients who had acute rhinosinusitis (ARS) other than fungal type such as odontogenic rhinosinusitis and bacterial rhinosinusitis.
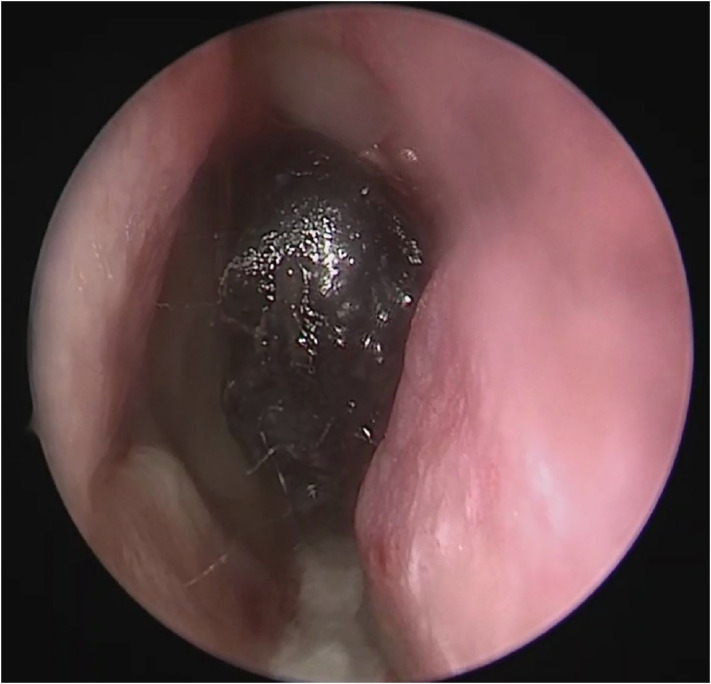
Fig. 1.
A photo of an endoscopic examination with ischemic changes in the middle turbinate suggesting AIFRS.
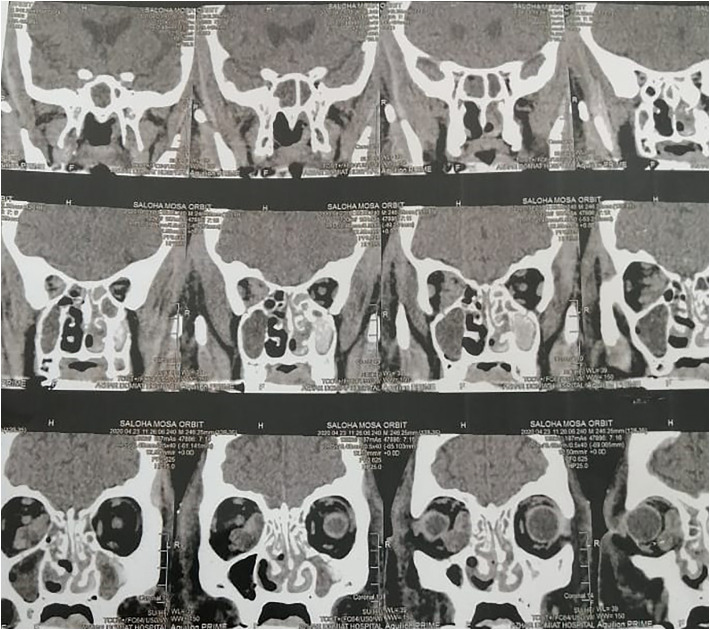
Fig. 2.
A CT picture of nose and paranasal sinuses of AIFRS with orbit invasion.
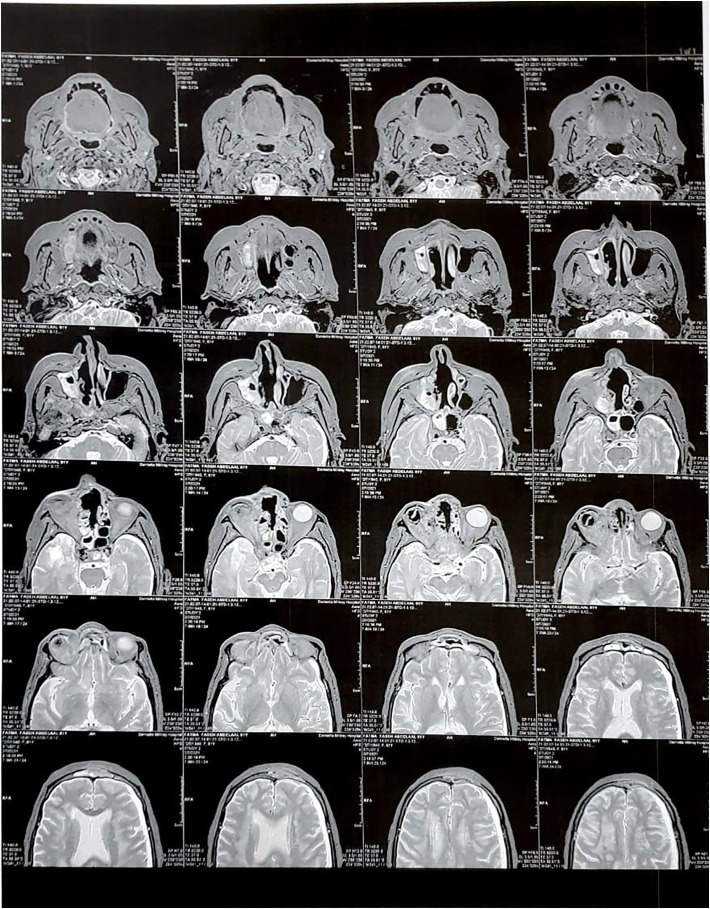
Fig. 3.
MRI picture of AIFRS with orbit extension.
2.3. Sample collection
Our samples were collected each year separately (from 2017 to 2020). Each year was recorded as demographic data of the patients, onset and duration of the disease, etiology of RS, risk factors or complications. All collected data and laboratory findings were recorded and statistically analyzed then tabulated for comparison purposes.
2.4. Statistical analysis
Statistical analyses were performed using SPSS v23 statistical software (SPSS, Inc., Chicago, Illinois). Descriptive statistics (means, standard deviations, frequencies, and correlation coefficients) were calculated for all measures. To compare the two groups, a paired t-test was carried out to determine P values using the Pearson's correlation test and a χ2 test and a one-sample t-test and Wilcoxon test performed when appropriate. The level of significance calculated as P < 0.05 was considered statistically significant, while P > 0.05 was considered statistically non-significant.
3. Results
The study included 56 patients with acute invasive fungal rhinosinusitis: 30 males and 26 females. They were collected from the year 2017 to 2020. Nine patients in 2017, 8 p in 2018, 10 in 2019 and 29 patients in 2020 as shown in Table 1 .
Table 1.
Incidence of acute invasive fungal rhinosinusitis per year (AIFRS).
| Year of study | Males |
Females |
Total |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| 2017 | 3 | 33.3 | 6 | 66.7 | 9 | 16.07 |
| 2018 | 4 | 50.0 | 4 | 50.0 | 8 | 14.29 |
| 2019 | 4 | 40.0 | 6 | 60.0 | 10 | 17.86 |
| 2020 | 19 | 65.5 | 10 | 34.5 | 29 | 51.78 |
| Total | 30 | 53.57 | 26 | 46.43 | 56 | 100 |
Diabetes mellitus, liver and renal dysfunction, immunosuppressive drug, and leukemia were the common risk factors of AIFRS patients as shown in Table 2 . In comparison of these common risk factors between Post-COVID-19 and Non-COVID-19 AIFRS patients Table 3 showed a statistically significant difference (P < 0.01).
Table 2.
Risk factors of the studied AIFRS patients.
| Risk factors |
Males |
Females |
Total |
|||
|---|---|---|---|---|---|---|
| 2017 | No. | % | No. | % | N = 9 | % |
| Diabetes mellitus | 3 | 50.0 | 3 | 50.0 | 6 | 66.7 |
| Immunosuppressive drugs | 0 | 0.00 | 2 | 100 | 2 | 22.2 |
| Liver cell failure | 0 | 0.00 | 1 | 100 | 1 | 11.1 |
| Risk factors |
Males |
Females |
Total |
|||
|---|---|---|---|---|---|---|
| 2018 | No. | % | No. | % | N = 8 | % |
| Diabetes mellitus | 4 | 50.0 | 4 | 50.0 | 8 | 100 |
| Liver cell failure | 2 | 100 | 0 | 0.00 | 2 | 25.0 |
| Renal failure | 1 | 100 | 0 | 0.00 | 1 | 12.5 |
| Risk factors |
Males |
Females |
Total |
|||
|---|---|---|---|---|---|---|
| 2019 | No. | % | No. | % | N = 10 | % |
| Diabetes mellitus | 2 | 28.57 | 5 | 71.43 | 7 | 70.0 |
| Immunosuppressive drugs | 0 | 0.00 | 1 | 100 | 1 | 10.0 |
| Liver cell failure | 2 | 66.7 | 1 | 33.3 | 3 | 30.0 |
| Leukemia | 1 | 100 | 0 | 0.00 | 1 | 10.0 |
| Risk factors |
Males |
Females |
Total |
|||
|---|---|---|---|---|---|---|
| 2020 | No. | % | No. | % | N = 29 | % |
| Diabetes mellitus | 5 | 62.5 | 3 | 37.5 | 8 | 27.6 |
| Renal failure | 1 | 100 | 0 | 0.00 | 1 | 3.45 |
| Liver cell failure | 1 | 100 | 0 | 0.00 | 1 | 3.45 |
| Immunosuppressive drugs | 0 | 0.00 | 1 | 100 | 1 | 3.45 |
| Post-COVID-19 | 12 | 66.67 | 6 | 33.33 | 18 | 62.1 |
Table 3.
Incidence of covid-19 in AIFRS patients as regard risk factors.
| Risk factors | No. | COVID-19 |
Non-COVID-19 |
Significance |
|||
|---|---|---|---|---|---|---|---|
| N = 18 | % | N = 38 | % | χ2 | P | ||
| Diabetes mellitus | 19 | 8 | 44.4 | 11 | 28.9 | 6.227 | 0.003⁎ |
| Immunosuppressive drugs | 4 | 3 | 16.7 | 1 | 2.63 | 5.878 | 0.004⁎ |
| Liver cell failure | 7 | 5 | 27.8 | 2 | 5.26 | 9.256 | 0.001⁎ |
| Chronic kidney disease | 2 | 1 | 5.56 | 1 | 2.63 | 3.582 | 0.014⁎ |
| Leukemia | 1 | 0 | 0.00 | 1 | 2.63 | 3.142 | 0.037⁎ |
| Cardiac diseases | 8 | 5 | 27.8 | 3 | 7.69 | 8.941 | 0.001⁎ |
| Bronchial asthma | 6 | 5 | 27.8 | 1 | 2.63 | 10.31 | 0.001⁎ |
| Overweight | 24 | 13 | 72.2 | 11 | 28.9 | 17.36 | 0.000⁎ |
χ2 = Chi square test.
P < 0.05 = significant.
The older age was statistically significant in Post-COVID-19 group compared with non-COVID-19 AIFRS patients (P = 0.001). The study showed comorbidities of AIFRS patients Table 4 and showed a statistically significant difference (P < 0.05) in Post-COVID-19 compared with Non-COVID-19 AIFRS patients.
Table 4.
Age and associated comorbidity of the studied patients.
| Age |
Post-COVID-19 |
Non-COVID-19 |
t-Test |
P |
||
|---|---|---|---|---|---|---|
| Mean ± SD (years) |
58.38 ± 12.2 |
38.64 ± 7.78 |
0.968 |
0.001⁎ |
||
| Comorbidity | N = 18 | % | N = 38 | % | χ2 | P |
| Hypertension | 10 | 55.56 | 7 | 18.42 | 0.999 | 0.000⁎ |
| Obesity | 8 | 44.44 | 14 | 36.84 | 0.396 | 0.009⁎ |
| Smoking | 12 | 66.67 | 17 | 44.74 | 0.898 | 0.001⁎ |
| Allergic rhinitis | 14 | 77.78 | 20 | 52.63 | 0.947 | 0.000⁎ |
| Asthma | 4 | 22.22 | 5 | 13.16 | 0.418 | 0.008⁎ |
| COBD & ARDS | 3 | 16.67 | 0 | 0.00 | 0.789 | 0.001⁎ |
| Cardiac diseases | 5 | 27.78 | 0 | 0.00 | 0.986 | 0.000⁎ |
| Otitis media | 6 | 33.33 | 12 | 31.58 | 0.089 | 0.0967 |
| Renal dysfunction | 3 | 16.67 | 0 | 0.00 | 0.789 | 0.001⁎ |
| Liver dysfunction | 2 | 11.11 | 2 | 5.26 | 0.321 | 0.011⁎ |
| Thrombocytopenia & leukopenia | 4 | 22.22 | 0 | 0.00 | 0.902 | 0.000⁎ |
| Immunosuppressive drugs | 3 | 16.67 | 1 | 2.63 | 0.622 | 0.002⁎ |
| Antibiotic or antiviral therapy | 12 | 66.67 | 17 | 44.74 | 0.898 | 0.001⁎ |
| Prolonged steroid use | 7 | 38.89 | 6 | 15.79 | 0.914 | 0.000⁎ |
| Chemotherapy | 1 | 5.556 | 0 | 0.00 | 0.309 | 0.013⁎ |
COBD: chronic obstructive pulmonary disease, ARDS: Acute respiratory distress syndrome.
t-Test: paired t-test, χ2 = Chi square test.
P < 0.05 = significant.
The most common fungal species represented in our study was Rhizopus oryzae, Aspergillus fumigatus, and Absidia mucor. They were more prominent in Post-COVID-19 compared than in Non-COVID-19 AIFRS patients and showed a significant difference in comparison between the two groups (P < 0.05) as shown in Table 5 .
Table 5.
Type of fungal species found in the studied patients.
| Fungal species | Post-COVID-19 |
Non-COVID-19 |
Significance |
|||
|---|---|---|---|---|---|---|
| N = 18 | % | N = 38 | % | χ2 | P | |
| Rhizopus oryzae | 8 | 44.44 | 7 | 18.42 | 9.972 | 0.001⁎ |
| Aspergillus fumigatus | 6 | 33.33 | 10 | 26.32 | 5.982 | 0.003⁎ |
| Absidia mucor | 2 | 11.11 | 1 | 2.63 | 6.454 | 0.002⁎ |
| Others | 2 | 11.11 | 20 | 52.63 | −14.25 | 0.000⁎ |
χ2 = Chi square test.
P < 0.05 = significant.
4. Discussion
Although AIFR is rare, it is the most aggressive form of fungal infection [1,10]. It is most commonly encountered in immuno-compromise patients. These have two categories and each of these has commonly associated pathogens with them. The first is diabetic patients (roughly 50%), particularly if poorly controlled, and is frequently associated with diabetic ketoacidosis. Second is the defect in immune system such as hematological malignancies and chronic renal insufficiency [4,11].
Pathology examination is the corner stone in diagnosing several challenging diseases including the fungal sinusitis, where is the microscopic examination of fungal ball on low power might be confused with the eosinophilic mucin seen in allergic rhinosinusitis, since both have a “layered” appearance, but this confusion is usually lost on high power revealing abundant fungal organisms [[12], [13], [14], [15]].
Our patients were diabetics in 44.4% of post-COVID-19 AIFRS and in 28.9% of AIFRS patients with non-COVID-19. Bakhshaee et al. [1] found that the most common underlying disorder in AIFRS patients was diabetes mellitus (50%), then leukemia (44.4%). In studies of Kursun et al. [16], Mohammadi et al. [17], Kermani et al. [18], the most common underlying disorders affecting the immune system were the diabetes mellitus and, to a lesser extent, hematological malignancies and chronic renal insufficiency. This may be due to the fungal affinity for acidotic environments with high glucose concentrations [19].
The second subset of patients are those who are immunosuppressed such as those with neutropenia, HIV/AIDS, hematological malignancies and patients receiving chemotherapy [4]. Patients who receive immuno-suppressive drugs in the present study represent 16.7% in post-COVID-19 patients AIFRS and 2.63% in patients with non-COVID-19. Although neutropenia is strongly associated with AIFR- (22.2%) in post-COVID-19, the vast majority of these patients have a hematological depletion.
In a study by Turner et al. [20] they found that 47.8% of their patients were diabetics, 39.0% having hematologic malignancies, 27.6% with corticosteroid use, 6.6% renal or liver failure, 6.3% organ transplantation, 2.3% AIDS, and 1.2%autoimmune disease.
These patients often have Rhizopus oryzae (44.4%) and Aspergillus fumigatus (33.3%) species isolated in post-COVID-19 patients and 18.4% & 26.3% in non-COVID-19 patients, respectively. There is an additional small subset of patients with a propensity to develop AIFR. It is those who are iron overloaded or in renal failure and receive deferoxamine for iron chelation [4]. Some fungi (Rhizopus) can bind to deferoxamine that supply fungus with extra iron which aids its growth [21].
There is a role that suggests that diabetic patient's survival is better than patients with immunosuppression [19]. This has been attributed to the more easily optimized disease state. However, there is no evidence to suggest that the specific pathogen isolated can help inform prognosis. Other factors which appear to be related to a poorer prognosis include: older patients, aplastic anemia, delayed diagnosis, concurrent hepatorenal failure, intracranial complications and neutropenia [20,22].
Heard et al. [23] suggested COVID-19 fungal research investigate invasive Candida species as potential pathogens, the environmental factors such as rapid changes to ICU capacity and the infra-structure might increase the risk of COVID-19-associated respiratory aspergillosis, and the potential hazards of un-treating invasive aspergillosis.
The consolidation of a key demonstrative calculation is pivotal for recognizing obtrusive contagious illness in patients with COVID-19 who require critical-care and ought to be done as often as possible all through the period of serious respiratory trouble [24].
In our study we found that prolonged use of antibiotics, steroids, immunosuppressive drugs that may be used in treatment of COVID-19 exacerbate acute invasive fungal infection.
Mehta and Pandey [8] concluded that COVID-19 is associated secondary infections; both bacterial and fungal due to immune dysregulation. Additionally, the widespread use of steroids/monoclonal antibodies/broad-spectrum antibiotics as part of the armamentarium against COVID-19 may lead to the development/exacerbation of preexisting fungal diseases.
Doctors ought to be mindful of the plausibility of invasive secondary fungal infections in patients with COVID-19 disease particularly in patients with preexisting risk factors and ought to empower early diagnosis and treatment with the ensuing diminishment of mortality and morbidity. The use of medications should to be checked to attain a perfect dose in the shortest duration. The use of broad-spectrum anti-microbials should be re-evaluated.
Patients hospitalized in intensive care units (ICU) for COVID-19 share risk factors and underlying diseases reported for invasive fungal disease, corticosteroid therapy, intubation or mechanical ventilation, and cytokinic storm were blamed [7].
Recent publications reported at least 10% of co-infection during COVID-19 in patients hospitalized in ICU for ARDS, among them Aspergillus infections [25]. Besides, the incidence of invasive pulmonary aspergillosis (IPA) in ICU patients admitted for severe influenza A and B is high reached 19% versus 5% in patients with severe pneumonia other than flu [26].
In addition, the pathophysiology of COVID-19 had co-morbidity with invasive fungal infection (IFI); first, the high aggressive feature of SARS-CoV-2 virus to the respiratory system made the IFI occurrence very likely, specifically those with airborne route of infection such as pneumocystosis and mucormycosis [27], second, absolute number of T lymphocytes, CD4+T and CD8+T cells are markedly lower in severe COVID-19 cases than in moderate cases, associated with markedly higher levels of IL-2R, IL-6, IL-10, TNF-alpha and some other inflammatory markers [28,29]. The complement system, while a first-line immune defense against invading pathogens, has off-target effects that lead to increases in inflammation, tissue damage, and thrombosis; these are common, life-threatening complications seen in patients with COVID-19 [30].
Turbin et al. [31] studied CT images for COVID-19 in sinusitis patients and found 2 cases characterized by dense and T1 hyperintense sinus substance, who accepted to speak to inspissated secretions and deposition of calcium salts or metals such as Mg, Mn and Fe, and typically seen with chronic invasive or allergic fungal sinusitis. However, they demonstrated features associated with acute invasive disease. Another case was characterized by hypodense, fluid signal contents, unlike the increased density seen with chronic invasive fungal sinusitis. Moreover, there was subtle involvement of the peri-antral fat, a finding relatively specific for invasive fungal sinusitis.
By comparing the number of patients of AIFRS in the last 4 years it is found that the number of patients increased up to triple in 2020. The increased number of AIFRS in 2020 is attributed to the increased number of COVID-19 with subsequent use of high dose of corticosteroid and immunosuppressant drugs and also to the immunological disturbance associated with COVID 19 infection.
5. Conclusions
The incidence of AIFRS is markedly more prominent in post-COVID-19 patients than in non-COVID-19 especially in immuno-compromised patients, diabetic, renal and liver dysfunction patients and patients with risk factors for rhinosinusitis.
Funding
This research received no funding.
CRediT authorship contribution statement
Conceptualization: Ismaiel WF, Eldsoky I, Ibrahim AA, Alsobky ME
Methodology: Ismaiel WF, Eldsoky I, Alsobky ME>
Design: Ismaiel WF, Alsobky ME, Zafan E, Hasan A
Software: Eldsoky I, Zafan E, Hasan A
Data curation: Ismaiel WF, Eldsoky I, Ibrahim AA, Alsobky ME, Zafan E
Writing- Original draft preparation: Ismaiel WF, Eldsoky I, Ibrahim AA, Alsobky ME, Hasan A
Investigation: Ismaiel WF, Eldsoky I, Ibrahim AA, Alsobky ME
Writing- Reviewing and Editing: ALL
Declaration of competing interest
The authors declare no conflict of interest.
References
- 1.Bakhshaee M., Bojdi A., Allahyari A., et al. Acute invasive fungal rhinosinusitis: our experience with 18 cases. Eur Arch Otorhinolaryngol. 2016;273(12):4281–4287. doi: 10.1007/s00405-016-4109-z. [DOI] [PubMed] [Google Scholar]
- 2.Aribandi M., McCoy V.A., Bazan C. Imaging features of invasive and noninvasive fungal sinusitis: a review. RadioGraphics. 2007;27(5):1283–1296. doi: 10.1148/rg.275065189. [DOI] [PubMed] [Google Scholar]
- 3.Momeni A.K., Roberts C.C., Chew F.S. Imaging of chronic and exotic sinonasal disease: review. AJR Am J Roentgenol. 2007;189(6):S35–S45. doi: 10.2214/AJR.07.7031. [DOI] [PubMed] [Google Scholar]
- 4.Deutsch P.G., Whittaker J., Prasad S. Invasive and non-invasive fungal rhinosinusitis—a review and update of the evidence. Medicina (Kaunas) 2019;55(7):319. doi: 10.3390/medicina55070319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillespie M.B., O’Malley B.W., Francis H.W. An approach to fulminant invasive fungal rhinosinusitis in the immunocompromised host. Arch Otolaryngol Head Neck Surg. 1998;124(5):520–526. doi: 10.1001/archotol.124.5.520. [DOI] [PubMed] [Google Scholar]
- 6.Wang H., Song J., Pan L., et al. The characterization of chronic rhinosinusitis in hospitalized patients with COVID-19. J Allergy Clin Immunol Pract. 2020;8(10):3597–3599. doi: 10.1016/j.jaip.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gangneux J.P., Bougnoux M.E., Dannaoui E., et al. Invasive fungal diseases during COVID-19: we should be prepared. J Mycol Med. 2020;30(2):100971. doi: 10.1016/j.mycmed.2020.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta S., Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12(9) doi: 10.7759/cureus.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mekonnen Z.K., Ashraf D.C., Jankowski T., et al. Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthalmic Plast Reconstr Surg. 2020 Nov 19 doi: 10.1097/IOP.0000000000001889. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei H., Li Y., Han D., et al. The values of (1,3)-β-D-glucan and galactomannan in cases of invasive fungal rhinosinusitis. Am J Otolaryngol. 2021 Mar 1;42(2):102871. doi: 10.1016/j.amjoto.2020.102871. [DOI] [PubMed] [Google Scholar]
- 11.Cavada M.N., Wong E., Orgain C.A., et al. Fungal ball of the maxillary sinus and the risk of persistent sinus dysfunction after simple antrostomy. Am J Otolaryngol. 2020 Jul 1;41(4):102541. doi: 10.1016/j.amjoto.2020.102541. [DOI] [PubMed] [Google Scholar]
- 12.Hasan A., Deyab A., Monazea K., et al. Clinico-pathological assessment of surgically removed abdominal wall endometriomas following cesarean section. Ann Med Surg. 2021 Feb 1;62:219–224. doi: 10.1016/j.amsu.2021.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montone K.T. Pathology of fungal rhinosinusitis: a review. Head Neck Pathol. 2016 Mar 1;10(1):40–46. doi: 10.1007/s12105-016-0690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eldsoky I, Ismaiel WF, Hasan A, et al. The predictive value of nasolacrimal sac biopsy in endoscopic dacryocystorhinostomy. Ann Med Surg (Lond). 2021 Apr 16;65:102317. doi: 10.1016/j.amsu.2021.102317. PMID: 33981427; PMCID: PMC8085898. [DOI] [PMC free article] [PubMed]
- 15.Hasan A., Abozied H., Youssef A., et al. A rare case of collecting duct carcinoma with first presentation of respiratory symptoms. Urol Case Rep. 2020 Nov 1;33:101367. doi: 10.1016/j.eucr.2020.101367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kursun E., Turunc T., Demiroglu Y.Z., et al. Evaluation of 28 cases of mucormycosis. Mycoses. 2015;58(2):82–87. doi: 10.1111/Myc.12278. [DOI] [PubMed] [Google Scholar]
- 17.Mohammadi R., Meidani M., Mostafavizadeh K., et al. Case series of rhi-nocerebral mucormycosis occurring in diabetic patients. Caspian J Intern Med. 2015;6(4):243–246. 26644901 [PMC free article] [PubMed] [Google Scholar]
- 18.Kermani W., Bouttay R., Belcadhi M., et al. ENT mucormycosis. Report of 4 cases. Eur Ann Otorhinolaryngol Head Neck Dis. 2016;133(2):83–86. doi: 10.1016/j.anorl.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 19.Watkinson JC, Clarke RW. Scott-Brown's otorhinolaryngology and head and neck surgery (8th Edition): Volume 1: Basic sciences, endocrine surgery, rhinology. CRC Press; Boca Raton, FL, USA: 2018. ISBN 9781138094611.
- 20.Turner J.H., Soudry E., Nayak J.V., Hwang P.H. Survival outcomes in acute invasive fungal sinusitis: a systematic review and quantitative synthesis of published evidence. Laryngoscope. 2013;123(5):1112–1118. doi: 10.1002/lary.23912. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim A.S., Spellberg B., Edwards J. Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment. Curr Opin Infect Dis. 2008;21:620–625. doi: 10.1097/QCO.0b013e3283165fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craig J.R. Updates in management of acute invasive fungal rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2019;27(1):29–36. doi: 10.1097/moo.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 23.Heard K.L., Hughes S., Mughal N., Moor L.S.P. COVID-19 and fungal superinfection. Lancet Microbe. 2020;1(3) doi: 10.1016/S2666-5247(20)30065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White P.L., Dhillon R., Hughes H., et al. COVID-19 and fungal infection: the need for a strategic approach. Lancet Microbe. 2020;1(5) doi: 10.1016/S2666-5247(20)30127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schauwvlieghe A., Rijnders B.J.A., Philips N., et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6:782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 27.He F., Deng Y., Li W. Coronavirus disease 2019 (COVID-19): what we know? J Med Virol. 2020;92(7):719–725. doi: 10.1002/jmv.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen G, Wu D, Guo W, e. Clinical and immunologic features in severe and moderate Coronavirus disease 2019. J Clin Invest. 2020:137244. Doi: 10. 1172/JCI137244. [DOI] [PMC free article] [PubMed]
- 29.Mutiawati E, Fahriani M, Mamada SS, et al. Anosmia and dysgeusia in SARS-CoV-2 infection: incidence and effects on COVID-19 severity and mortality, and the possible pathobiology mechanisms-a systematic review and meta-analysis. F1000Research. 2021 Jan 21;10(40):40. DOI: 10.12688/f1000research.28393.1. [DOI] [PMC free article] [PubMed]
- 30.Rabaan A.A., Al-Ahmed S.H., Garout M.A, et al. Diverse Immunological Factors Influencing Pathogenesis in Patients with COVID-19: A Review on Viral Dissemination, Immunotherapeutic Options to Counter Cytokine Storm and Inflammatory Responses. Pathogens. 2021;10(5):565. doi: 10.3390/pathogens10050565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turbin R.E., Wawrzusin P.J., Sakla N.M., et al. Orbital cellulitis, sinusitis and intracranial abnormalities in two adolescents with COVID-19. Orbit. 2020;39(4):305–310. doi: 10.1080/01676830.2020.1768560. [DOI] [PubMed] [Google Scholar]