Abstract
Objectives
The overall death toll from COVID-19 in Africa is reported to be low but there is little individual-level evidence on the severity of the disease. This study examined the clinical spectrum and outcome of patients monitored in COVID-19 care centres (CCCs) in two West-African countries.
Methods
Burkina Faso and Guinea set up referral CCCs to hospitalise all symptomatic SARS-CoV-2 carriers, regardless of the severity of their symptoms. Data collected from hospitalised patients by November 2020 are presented.
Result
A total of 1,805 patients (64% men, median age 41 years) were admitted with COVID-19. Symptoms lasted for a median of 7 days (IQR 4–11). During hospitalisation, 443 (25%) had a SpO2 < 94% at least once, 237 (13%) received oxygen and 266 (15%) took corticosteroids. Mortality was 5% overall, and 1%, 5% and 14% in patients aged <40, 40–59 and ≥60 years, respectively. In multivariable analysis, the risk of death was higher in men (aOR 2.0, 95% CI 1.1; 3.6), people aged ≥60 years (aOR 2.9, 95% CI 1.7; 4.8) and those with chronic hypertension (aOR 2.1, 95% CI 1.2; 3.4).
Conclusion
COVID-19 is as severe in Africa as elsewhere, and there must be more vigilance for common risk factors such as older age and hypertension.
Keywords: SARS-Cov-2, COVID-19, Sub-Saharan Africa, Mortality, Comorbidities
Introduction
As of 05 May 2021, it was estimated that 153 million people had been infected with SARS-CoV-2 and that COVID-19 had killed 3.2 million people worldwide. Africa accounted for 3% of deaths from COVID-19, although 17% of the world’s population lives there. Europe (10% of world population, 34% of deaths from COVID-19) and North America (4% of world population, 19% of deaths from COVID-19) have been impacted comparatively much higher (WHO Coronavirus (COVID-19) Dashboard, n.d.). Therefore, the death toll from COVID-19 seemed lower in Africa than in Europe and North America, although some predictions indicated the opposite (Martinez-Alvarez et al., 2020). This may have been due to the heterogeneity of systems for reporting cases and causes of death, the level of preparedness in a continent that has faced threatening epidemics in the recent past, or differences in environmental characteristics, circulating strains or population susceptibility (Galloway et al., 2020, Nagai et al., 2020, Price-Haywood et al., 2020, Williamson et al., 2020). To study this latter point, it would be useful to verify whether the clinical presentation, mortality and factors associated with COVID-19 are similar in Africa to those described in other continents. There have been a few retrospective population or hospital-based cohorts reporting mortality rates from COVID-19 in Africa so far, but no cohorts that provide individual-level prospective evidence on the severity of the disease (Abdela et al., 2020, Boulle et al., 2020, El Aidaoui et al., 2020, Elimian et al., 2020, Kirenga et al., 2020, Mekolo et al., 2021, Nachega et al., 2020). This study reports prospectively collected clinical data on patients hospitalised in the COVID-19 referral care centres of two West African countries between March and November 2020.
Methods
Participants, settings and follow-up
Burkina Faso and Guinea, two West African countries, decided at the start of the COVID-19 pandemic to accommodate everyone detected as carriers of SARS-CoV-2 in specific centres, whether asymptomatic or symptomatic, and in the latter case regardless of the intensity of their symptoms. The Ministries of Health in the two countries set up the COVID-19 referral care centres (CCCs) in collaboration with the non-governmental association ALIMA (The Alliance for International Medical Action). In Guinea, the CCC was opened at the Donka hospital in Conakry. In Burkina Faso, two CCCs were opened at the Tengandogo and Clinique Princesse Sarah hospitals, in Ouagadougou. This study describes the data collected from all people hospitalised with symptomatic COVID-19 and a positive RT-PCR SARS-CoV-2 test in each of these three centres. Those placed in isolation at the centres and asymptomatic were excluded from this study. The study period was between 01 April and 04 November 2020 in Guinea; and between 01 March and 12 November 2020 in Burkina Faso.
Care and follow-up
All people received basic care for COVID-19, as defined in the recommendations of the ministries of health of the two countries. No additional medical action or treatment was introduced for participants in this study. Basic care was similar at the three centres, including: (i) anti-infective treatment considered to have an anti-SARS-CoV-2 effect and recommended by the national authorities; (ii) oxygen therapy via nasal probe or mask, in the event of an SpO2 of <90%; and (iii) the possibility of corticosteroid therapy (methylprednisolone 120 mg/d or dexamethasone 6 mg/d for 3 days then decreased over a total of 10 days), anticoagulant treatment (enoxaparin 100 IU/kg twice a day) and broad-spectrum antibiotic therapy, of variable indication depending on the period and treating physician’s decision.
Throughout the study, the anti-SARS-CoV-2 treatment recommended in the two countries consisted of dual therapy combining 5 days of azithromycin (500 mg at day 1 and then 250 mg/day) with 10 days of hydroxychloroquine (200 mg x tds). In Guinea, physicians had the choice of alternately using either hydroxychloroquine as monotherapy, lopinavir/ritonavir (200/50 mg bd for 10 days) as monotherapy or lopinavir/ritonavir in combination with hydroxychloroquine and azithromycin.
Each of the three centres had an intensive care unit, allowing patients on oxygen to be closely monitored and non-invasive or mechanical ventilation to be used if required.
Data
Data were recorded using the WHO COVID-19 rapid core case report form (CRF) (Global COVID-19 clinical platform: rapid core case report form (CRF), n.d.). In the context of the three urgently-established CCCs, the data were first collected on hard copy CRF and subsequently entered into an electronic database. The variables considered essential for the analysis were then monitored and the source data verified. Only these variables are presented in this article. The variables were: patient verification (exhaustivity of the patient list); sex; age; comorbidities; date of first symptoms; signs, symptoms, measurement of pulse oximetry (SpO2) at baseline and during follow-up; oxygen therapy, anti-infectious treatments, corticosteroid therapy; and vital status at the end of follow-up. Radiological data, and biological data other than the result of the initial SARS-CoV-2 test were not monitored.
Statistical analysis
The follow-up time was the time between admission to hospital and death or discharge alive from hospital. There was no post-discharge follow-up. The primary endpoint was in-hospital death. The secondary endpoint was COVID-19 clinical worsening, defined as a combination of an SpO2 < 94% at least once during follow-up (including at baseline), use of oxygen therapy at any time during follow-up, or death. The analysis describes the baseline characteristics, care received during follow-up, outcomes, and factors associated with COVID-19 clinical worsening or death.
The Kaplan Meier method was used to estimate the probability of occurrence of the primary and secondary endpoints over time, overall and by sex, age category, and presence or absence of the main comorbidities. Univariable then multivariable logistic regression were used to analyse the association between the primary and secondary endpoints and the following characteristics: sex, age (<60 vs. ≥60 years), time between first symptom and hospitalisation (≤7 vs. >7 days), chronic hypertension declared by the patient (yes vs. no), diabetes declared by the patient (yes vs. no), and initial severity (in three grades, mild, moderate, severe, according to the May 2020 WHO definitions). Variables associated with the endpoint with a p < 0.20 in univariable analysis were included in the multivariable model. The analyses were carried out using the software R, version 4.0.3
Ethical considerations
The study was approved in Burkina Faso by the Comité d’Ethique pour la Recherche en Santé (ID number 2020-6-116) and in Guinea by the Comité National d’Ethique pour la Recherche en Santé (ID number 069/CNERS/20). The committees of the two countries authorised the use of medical data collection in accordance with WHO methodology to describe the course of the COVID-19 disease during routine care, without requesting signatures for specific consent.
Role of the funding source
The funding sources took no part in designing the study, collecting, analysing and interpreting data, writing the report or making the decision to submit the article for publication. MJ, BS and SJ had access to the raw data. The corresponding author had full access to all data and the final responsibility for submitting it for publication.
Results
During the study period, 1,938 people (Guinea: 1,593; Burkina Faso: 345) were admitted to one of the three CCCs with a positive SARS-CoV-2 test on nasopharyngeal swab. Of these, 133 were completely asymptomatic and not included in the analysis. The remaining 1,805 were included in the analysis (Guinea: 1,593; Burkina Faso: 212). Their baseline characteristics are detailed in Table 1 ; 64% were men and 36% women, and the median age was 41 years (IQR 30–57, range 1–99 years). The two most common comorbidities were chronic hypertension (21%) and diabetes (12%). The median weight was 73 kg (IQR 60–85). Height, and therefore body mass index (BMI), could only be measured in 645 people. In this subgroup, the median BMI was 25 kg/m2 (IQR 22–29), and 119 (18%) had a BMI of ≥30 kg/m2. At the time of admission, symptoms lasted for a median of 7 days (IQR 4–11). The clinical picture was dominated by signs of lower respiratory tract involvement, present in 1,110 (61%) patients. Digestive signs were present in 311 (17%) patients. A total of 177 (10%) patients had an initially measured SpO2 of 90%, and 177 (10%) were considered severe from the start on the basis of this SpO2 or other signs of respiratory severity.
Table 1.
Baseline characteristics (n = 1,805).
| Country, n (%) | ||
|---|---|---|
| Burkina Faso | 212 | 12% |
| Guinea | 1,593 | 88% |
| Sex, n (%)a | ||
| Male | 1,151 | 64% |
| Female | 651 | 36% |
| Age, years, n (%)a | ||
| <20 | 132 | 7% |
| 20–39 | 691 | 39% |
| 40–59 | 579 | 32% |
| ≥60 | 388 | 22% |
| Comorbidities, n (%) | ||
| Diabetes | 219 | 12% |
| Chronic hypertension | 386 | 21% |
| Any otherb | 147 | 8% |
| Time from first symptom to admission (days), median [IQR]a | 7 | [4; 11] |
| Signs and symptoms, n (%) | ||
| Fever ≥38.0 °C | 297 | 16% |
| Fatigue | 748 | 41% |
| Loss of smell or taste | 490 | 27% |
| Headache | 673 | 37% |
| Arthralgia or myalgia | 294 | 16% |
| Higher respiratory tract symptomsc | 425 | 24% |
| Any lower respiratory tract symptom | 1,110 | 61% |
| Cough | 833 | 46% |
| Dyspnoea | 505 | 28% |
| Chest pain | 294 | 16% |
| Any digestive tract symptom | 311 | 17% |
| Vomiting | 156 | 9% |
| Diarrhoea | 95 | 5% |
| Abdominal pain | 125 | 7% |
| Glasgow <15 | 21 | 1% |
| Other signs or symptomsd | 36 | 2% |
| SpO2 under ambient air | ||
| ≥94% | 1,545 | 86% |
| 90–93% | 83 | 5% |
| <90% | 177 | 10% |
| Severity, n (%)e | ||
| Mild | 867 | 48% |
| Moderate | 761 | 42% |
| Severe | 177 | 10% |
N, number; %, percentage; IQR, interquartile range.
• Mild: Symptomatic patients meeting the case definition for COVID-19 without evidence of viral pneumonia or hypoxia.
• Moderate:
- Adolescent or adult with clinical signs of pneumonia (fever, cough, dyspnoea, fast breathing) but no signs of severe pneumonia, including SpO2 ≥ 90% on room air.
- Child with clinical signs of non-severe pneumonia (cough or difficulty breathing + fast breathing and/or chest indrawing) and no signs of severe pneumonia.
• Severe:
- Adolescent or adult with clinical signs of pneumonia (fever, cough, dyspnoea, fast breathing) plus one of the following: respiratory rate >30 breaths/min; severe respiratory distress; or SpO2 < 90% on room air.
- Child with clinical signs of pneumonia (cough or difficulty in breathing) + at least one of the following: Central cyanosis or SpO2 < 90%; severe respiratory distress (e.g., fast breathing, grunting, very severe chest indrawing); general danger sign: inability to breastfeed or drink, lethargy or unconsciousness, or convulsions (55, 56). Fast breathing (breaths/min): <2 months: ≥60; 2–11 months: ≥50; 1–5 years: ≥40.
Missing values: sex, n = 3; age, n = 15; time since first symptoms, n = 517.
Chronic cardiac disease (n = 25, 1%), current smoking (n = 12, 1%), chronic pulmonary disease (n = 17, 1%), active or previous tuberculosis (n = 23, 1%), asthma (n = 39, 2%), asplenia (n = 0, 0%), chronic kidney disease (n = 13, 1%), malignant neoplasm (n = 4, 0%), chronic hepatic disease (n = 19, 1%), chronic neurological disorder (n = 8, 0%), HIV infection (n = 5, 0%).
Runny nose, nasal congestion, sneezing.
Other signs or symptoms: confusion (n = 18, 1%), seizure (n = 6, 0%), conjunctivitis (n = 7, 0%), skin rash (n = 3, 0%), skin ulcer (n = 1, 0%), lymphadenopathy (n = 3, 0%), bleeding (n = 1, 0%).
Adapted from WHO May 2020 interim guidance (WHO, 2020a).
The follow-up characteristics are detailed in Table 2 . During hospitalization, 1,697 people (95%) received at least one specific treatment recommended by national authorities, and 97 (5%) received none. The most common treatment was the hydroxychloroquine plus azithromycin combination, received by 1,244 (73%) participants. Between admission and the end of follow-up, 231 people (13%) had a measured SpO2 of <90% at least once, 237 (13%) received oxygen and 266 (15%) received corticosteroids.
Table 2.
Follow-up characteristics (n = 1,805).
| Vital signs during follow-up, n (%) | ||
|---|---|---|
| Heart rate >110 beats/min at least once | 338 | 19% |
| Glasgow <15 at least once | 37 | 2% |
| SpO2 under ambient air, worse value | ||
| <90% | 231 | 13% |
| 90–93% | 212 | 12% |
| ≥94% | 1,362 | 75% |
| Care received, n (%) | ||
| Specific drugs:a | 1,697 | 95% |
| Hydroxychloroquine + azithromycin | 1,244 | 73% |
| Hydroxychloroquine | 227 | 13% |
| Hydroxychloroquine + azithromycin + lopinavir/ritonavir | 212 | 12% |
| Hydroxychloroquine + lopinavir/ritonavir | 7 | 0.4% |
| Lopinavir/ritonavir | 7 | 0.4% |
| No specific drugs | 97 | 5% |
| Other drugs | ||
| Other antibiotics | 1,491 | 83% |
| Corticosteroids | 266 | 15% |
| Systemic anticoagulation | 315 | 17% |
| Received oxygen therapy | 237 | 13% |
| WHO ordinal scale (higher grade)b | ||
| Stage 4: Oxygen by mask or nasal prongs | 151 | 64% |
| Stage 5: Non-invasive ventilation or high-flow oxygen | 83 | 35% |
| Stage 6: Intubation and mechanical ventilation | 3 | 1% |
| Admitted to intensive care unit | 275 | 15% |
| Final vital status | ||
| Death, n (%) | 86 | 5% |
| Time from admission to death (days), median [IQR] | 5 | [2; 10] |
| Alive, n (%) | 1,719 | 95% |
| Time from admission to discharge (days), median [IQR]a | 11 | [8; 16] |
| Combined secondary outcome | ||
| Death or oxygen therapy or oxygen saturation <94%, n (%) | 552 | 31% |
N, number; %, percentage; IQR, interquartile range.
Missing values: Specific drugs, n = 11; time from admission to discharge, n = 1.
See reference (WHO, 2020b).
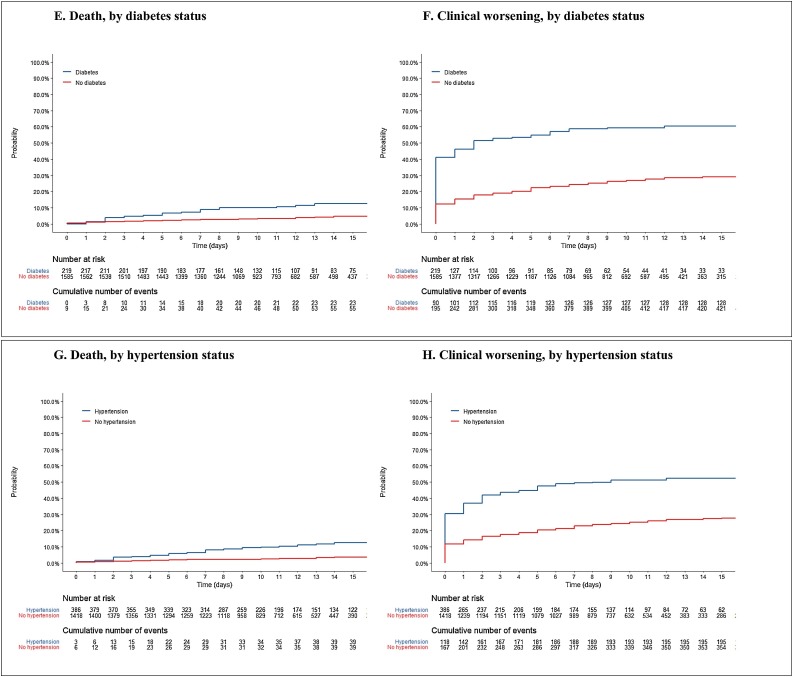
The rate of clinical worsening was 31% overall; 24% in women and 34% in men; 23%, in patients aged <60 years and 57% in those ≥60 years; 59% in diabetics and 27% in non-diabetics; 51% in patients with self-declared chronic hypertension and 25% in those without hypertension.
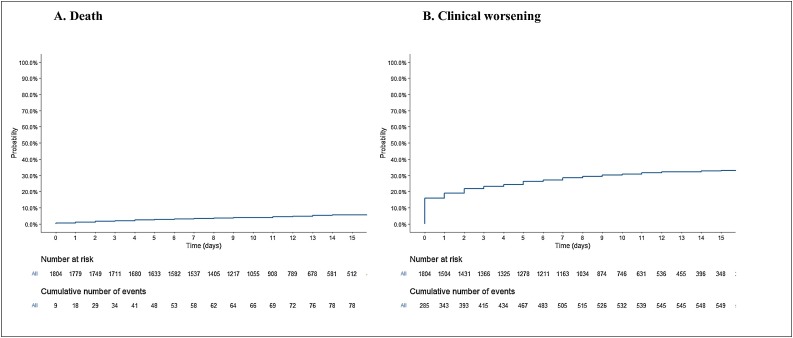
Overall, 86 (5%) patients died within a median of 5 days (IQR 2–10) of hospital admission. The final percentage of deaths by group was: 3% in women and 6% in men; 0%, 1%, 5% and 14% in patients aged <20, 20–39 years, 40–59 years and ≥60, years, respectively; 13% in diabetics and 4% in non-diabetics; 12% in patients with chronic hypertension and 3% in those without hypertension; and 26%, 3% and 2% in patients initially considered to be at a severe, moderate and mild stage, respectively. Patients aged <60 years at admission accounted for 38% of death (33/86). For these patients, median age was 50 years (IQR 41–56), 60% were at a severe stage at admission and 32% and 36% had diabetes and chronic hypertension, respectively. In the primary analysis, the risk of death was significantly higher in men, those aged ≥60 years, those with chronic hypertension, and those initially considered to be at a severe stage. The duration of symptoms prior to admission was not significantly associated with the risk of death (Table 3A , Figure 1, Figure 2 ). In the secondary analysis, male patients, those aged ≥60 years, those with chronic hypertension, and those with diabetes had a higher risk of clinical worsening (Table 3B , Figure 1, Figure 2). Patients who recovered were discharged after a median of 11 days (IQR 8–16).
Table 3A.
Factors associated with the risk of death.
| Univariable |
Multivariable |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n/N | % | cOR | 95% CI | p | n/N | % | aOR | 95% CI | p | ||
| Sex | Female | 18/651 | 3% | – | – | – | 18/643 | 3% | – | – | – |
| Male | 68/1,151 | 6% | 2.2 | [1.3; 3.8] | 0.002 | 68/1,144 | 6% | 2.0 | [1.1; 3.6] | 0.019 | |
| Age | <60 years | 33/1,402 | 2% | – | – | – | 33/1,400 | 2% | – | – | – |
| ≥60 years | 53/388 | 14% | 6.5 | [4.2; 10.4] | <0.001 | 53/387 | 14% | 2.9 | [1.7; 4.8] | <0.001 | |
| Diabetes | No | 58/1,586 | 4% | – | – | – | 58/1,570 | 4% | – | – | – |
| Yes | 28/219 | 13% | 3.9 | [2.4; 6.2] | <0.001 | 28/217 | 13% | 1.3 | [0.8; 2.3] | 0.32 | |
| Chronic hypertension | No | 41/1,419 | 3% | – | – | – | 41/1,403 | 3% | – | – | – |
| Yes | 45/386 | 12% | 4.4 | [2.9; 6.9] | <0.001 | 45/384 | 12% | 2.1 | [1.2; 3.4] | 0.006 | |
| Initial severity gradea | Mild | 18/867 | 2% | – | – | – | 18/857 | 2% | – | – | – |
| Moderate | 22/761 | 3% | 1.4 | [0.7; 2.7] | 0.30 | 22/753 | 3% | 1.3 | [0.7; 2.6] | 0.37 | |
| Severe | 46/177 | 26% | 16.4 | [9.4; 30.0] | <0.001 | 46/177 | 26% | 9.0 | [5.0; 16.8] | <0.001 | |
| Time since first symptom | ≤7 days | 31/778 | 4% | – | – | – | – | – | – | – | – |
| >7 days | 27/510 | 5% | 1.4 | [0.8; 2.3] | 0.28 | – | – | – | – | – | |
n = total number of patients in the category; n = number of patients in the category who died; CI: confidence interval; cOR: crude odds ratio; aOR: adjusted odds ratio: the OR for each variable shown in the table was adjusted on the other variables shown in the table.
See reference (WHO, 2020b).
Figure 1.
Kaplan Meier estimate of the probability of clinical worsening and death in the overall population.
Figure 2.
Kaplan Meier estimate of the probability of clinical worsening and death, according to sex, age, and comorbidities.
Table 3B.
Factors associated with the risk of clinical worsening.
| Univariable |
Multivariable |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n/N | % | cOR | 95% CI | p | n/N | % | aOR | 95% CI | p | ||
| Sex | Female | 157/651 | 24% | – | – | – | 157/643 | 24% | – | – | – |
| Male | 394/1,151 | 34% | 1.6 | [1.3; 2.0] | <0.001 | 392/1,144 | 34% | 1.6 | [1.3; 2.1] | <0.001 | |
| Age | <60 years | 327/1,402 | 23% | – | – | – | 326/1,400 | 23% | – | – | – |
| ≥60 years | 223/388 | 57% | 4.4 | [3.5; 5.6] | <0.001 | 223/387 | 58% | 3.1 | [2.4; 4.0] | <0.001 | |
| Diabetes | No | 423/1,586 | 27% | – | – | – | 420/1,570 | 27% | – | – | – |
| Yes | 129/219 | 59% | 4.0 | [2.9; 5.3] | <0.001 | 129/217 | 59% | 2.3 | [1.6; 3.1] | <0.001 | |
| Chronic hypertension | No | 356/1,419 | 25% | – | – | – | 354/1,403 | 25% | – | – | – |
| Yes | 196/386 | 51% | 3.1 | [2.4; 3.9] | <0.001 | 195/384 | 51% | 1.7 | [1.3; 2.2] | <0.001 | |
| Time since first symptom | ≤7 days | 223/778 | 29% | – | – | – | – | – | – | – | – |
| >7 days | 188/510 | 37% | 1.5 | [1.1; 1.8] | 0.002 | – | – | – | – | – | |
n = total number of patients in the category; n = number of patients in the category who had an SpO2 < 94%, received oxygen, or died; CI: confidence interval; cOR: crude odds ratio; aOR: adjusted odds ratio: the OR for each variable shown in the table was adjusted on the other variables shown in the table.
Discussion
It is believed that this is the first African study describing the clinical presentation and outcomes of COVID-19 among symptomatic patients prospectively monitored in specialist hospital care centres. This study has strengths in that it provides partial answers to some of the questions surrounding COVID-19 in Africa, and weaknesses in that it leaves other questions unanswered.
In two West African countries where the policy was to hospitalise everyone with a positive SARS-Cov-2 RT-PCR test, regardless of the severity of the clinical picture, 5% of symptomatic people who were hospitalised died. Mortality was 14% in patients aged ≥60 years and 26% in people with severe respiratory signs on admission. Although no comparisons can be made between hospitals in different countries with different hospitalisation practices and levels of care, these figures are consistent with those described elsewhere (Lv et al., 2020, Olumade and Uzairue, 2021, Singer et al., 2020) and strongly suggest that COVID-19 is at least as serious in Africa as it is in other continents (Boulle et al., 2020, Elimian et al., 2020, Kirenga et al., 2020, Nachega et al., 2020). If the reported death toll from COVID-19 is lower in Africa than in Europe or the USA (Chilimuri et al., 2020, Galloway et al., 2020, Rossi et al., 2020, Zhou et al., 2020), it is probably not because the disease is less lethal in Africa. This reasoning is also compatible with the 2.8%, 9.2% and 13.2% mortality rates reported from retrospective population or hospital-based cohorts in South Africa, Nigeria and the Democratic Republic of the Congo (Boulle et al., 2020, Elimian et al., 2020, Nachega et al., 2020) as well as the 5.6% of an African meta-analysis (Olumade and Uzairue, 2021).
In the present study, the predominant clinical presentation was respiratory. Digestive signs and general signs, headaches, myalgia and asthenia were nevertheless quite frequent, as was a lack of taste and smell. Mortality was twice as high in people with hypertension, 2 times higher in men, and 3 times higher in people aged ≥60 years. These clinical spectrum and factors associated with mortality are also consistent with what has been described elsewhere (Boulle et al., 2020, El Aidaoui et al., 2020, Elimian et al., 2020, Guan et al., 2020, Huang et al., 2020, Kirenga et al., 2020, Nachega et al., 2020, Singer et al., 2020, Zhou et al., 2020). They emphasise the particular severity of the disease in groups at risk, in Africa as elsewhere, and the need to set up appropriate care to try to reduce the high mortality rate in these groups.
The first type of appropriate care is oxygen therapy. In the present study, participants received oxygen therapy when their SpO2 dropped below 90%, but few people with an SpO2 of between 90% and 93% did. Very few patients also benefited from non-invasive or invasive ventilation. It can be hypothesised that expanding the indications for oxygen therapy and increasing options for ventilation could have reduced mortality (Baker et al., 2020).
Regarding other ways to improve care, the data is of very limited value. The anti-infective treatments recommended in both countries and widely prescribed to patients in this study have not been proven effective so far (Kaptein et al., 2020, Maisonnasse et al., 2020, RECOVERY Collaborative Group et al., 2020, Skipper et al., 2020). The data do not provide any new insights in this regard. In the present study, a large percentage of patients received corticosteroids; although this percentage is consistent with what could be expected based on SpO2 figures, there are no more details on its duration or precise indications. In addition, the data do not allow comment on anything concerning elaborate management of the complications of the disease in its inflammatory phase, but since substantial resuscitation capacities are limited, there is little hope for improving survival this way. Hopes for therapy lean more toward earlier intervention. Treatments of this nature are still experimental, and it is important for African centres to participate in trials of new curative treatments.
Finally, this study was also limited as follows. Because it was carried out at the height of an emergency, the monitoring of collected data was limited to a small number of key variables to ensure the analysis provided solid descriptive elements. Since the number of variables was limited, the analysis of factors associated with outcomes probably suffered from confusion bias. Nevertheless, the analyses were consistent with what was known elsewhere, and this external consistency has the advantage of increasing confidence that the data are robust. It is therefore believed that the main results, which are the overall and group mortality rates, are credible.
In conclusion, COVID-19 is a serious disease in Africa, as it is elsewhere. Men are more likely to experience complications than women, and certain common risk factors such as age, diabetes and hypertension should make us particularly vigilant in the event of COVID-19 in these countries. Further studies should now focus on finding ways to reduce mortality from COVID-19 in Africa, overall and in these at-risk groups.
Data sharing statement
The anonymised individual data and the data dictionary of the study will be made available to other researchers by Professor Denis Malvy (denis.malvy@chu-bordeaux.fr) after approval of a methodologically sound proposal and the signature of a data access agreement.
Author contribution statement
MJ, SJ, BS, RK, ED, AP, MSS, Eudoxie Koumbem (EK), Halidou Tinto (HT), Adama Sanou (ASa), Apoline Sondo (ASo), Billy Sivahera (BSh), Caroline Martin (CM), Moumouni Kinda (MK), Joseph Donamou (JD), Jean-Paul-Yassa Guilavogui (JPYG), Fode Bangaly Sako (FBS), Fode Amara Traore (FAT), Flavien Kabore (FK), Brice Bicaba (BB), Hans-Joerg Lang (HJL), Sani Sayadi (SS) and Augustin Augier (AA) set up the study in Burkina Faso and Guinea, enrolled and followed the patients and recorded clinical data.
MJ, SJ, MSS, ED and BS had access to the raw data.
MJ, SJ, BS and XA performed the analysis. MJ, SJ, BS, ED, MSS, RK, Olivier Marcy (OM), XA and DM drafted the manuscript.
All authors revised the manuscript critically for important intellectual content and approved the final version before submission.
Conflict of interest
All authors declare not conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Ethic approval
The study was approved in Burkina Faso by the Comité d’Ethique pour la Recherche en Santé (ID number 2020-6-116) and in Guinea by the Comité National d’Ethique pour la Recherche en Santé (ID number 069/CNERS/20).
The committees of the two countries authorised the use of medical data collection in accordance with WHO methodology to describe the course of the COVID-19 disease during routine care, without requesting signatures for specific consent.
Acknowledgments
We thank all the patients involved in the current study, as well as their caregivers and the investigators and research staff at the participating care centres. We thank the World Health Organization for technical advice.
The COVISTA STUDY GROUP is constituted as follows: Xavier Anglaret, Augustin Augier, Fatoumata Bah, Hadjiratou Bah, Ibrahima Bah, Ibrahima Balde, Edouard Florent Bangoura, Moumié Barry, Aguibou Barry, Souleymane Barry, Mamadou Kolon Barry, Thierno Amadou Bailo Barry, Thierno Amadou Bella Barry, Eric Barte de Sainte Fare, Jean Thona Beavogui, Brice Wilfried Bicaba, Joachim Bongono, Erica Bonnet-Laverge, Marion Bererd Camara, Amadou Souleymane Camara, Saidou Cherif Camara, Gnékéré Camara, Cheick Oumar Camara, Sekou Ditin Cisse, Aurore Claudia Bidossesse Deguenonga, Fatoumata Abdoulaye Diallo, Adama Hawa Diallo, Mamadou Lamarana Diallo, Mamadou Oury Safiatou Diallo, Fatoumata Lamarana Diallo, Aboubacar Diallo, Mamadou Sarafou Diallo, Thierno Tahirou Diallo, Daouda Diawara, Eric Dienderé, Joseph Donamou, Lancinet Doumbouya, Mohamed Lamine Fofana, Aly 2 Fofana, Joseph Fokam, Theolinde Gentil, Drissa Gouba, Jean-Paul-Yassa Guilavogui, Victoire Hubert, Marie Jaspard, Sylvain Juchet, Ibrahima Kaba, Abdoulaye Kaba, Flavien Kaboré, Saa Pascal Kamano, Issa Malam Kanta, Judith Katoudi, Kaba Keita, Sakoba Keita, Moumouni Kinda, Richard Kojan, Justin Kolié, Eudoxie Koumbem, Jules Aly Koundouno, Samagbè Kourouma, Hans-Joerg Lang, Réné Lolamou, Catherine Loua, Denis Malvy, Olivier Marcy, Caroline Martin, Camille Montfort, Nicolas Mouly, Dally Muamba, Felicité Nana, Armel Poda, Mamadou Aliou Samoura, Alpha Yaya Sampil, Freddy Sangala, Salif Sankara, Adama Sanou, Sani Sayadi, Beatrice Serra, Ahmadou Sidibe, Billy Sivahera, Apoline Sondo, Mohamed Soumah, Mamadou Saliou Sow, Mamadou Binta Sylla, Nathalie Theuillon, Halidou Tinto, Tamba Kallas Tonguino, Mohamed Toure, Abdoulaye Toure, Fanny Velardo, Eric Kabre Wendmanegda, Bounna Yattasaye.
References
- Abdela S.G., Abegaz S.H., Demsiss W., Tamirat K.S., van Henten S., van Griensven J. Clinical profile and treatment of COVID-19 patients: experiences from an Ethiopian treatment center. Am J Trop Med Hyg. 2020;104(2):532–536. doi: 10.4269/ajtmh.20-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T., Schell C.O., Petersen D.B., Sawe H., Khalid K., Mndolo S. Essential care of critical illness must not be forgotten in the COVID-19 pandemic. Lancet Lond Engl. 2020;395:1253–1254. doi: 10.1016/S0140-6736(20)30793-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulle A., Davies M.-A., Hussey H., Ismail M., Morden E., Vundle Z. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020 doi: 10.1093/cid/ciaa1198. online ahead of print. [DOI] [Google Scholar]
- Chilimuri S., Sun H., Alemam A., Mantri N., Shehi E., Tejada J. Predictors of mortality in adults admitted with COVID-19: retrospective cohort study from New York city. West J Emerg Med. 2020;21:779–784. doi: 10.5811/westjem.2020.6.47919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Aidaoui K., Haoudar A., Khalis M., Kantri A., Ziati J., El Ghanmi A. Predictors of severity in Covid-19 patients in Casablanca, Morocco. Cureus. 2020;12 doi: 10.7759/cureus.10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elimian K.O., Ochu C.L., Ebhodaghe B., Myles P., Crawford E.E., Igumbor E. Patient characteristics associated with COVID-19 positivity and fatality in Nigeria: retrospective cohort study. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-044079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway J.B., Norton S., Barker R.D., Brookes A., Carey I., Clarke B.D. A clinical risk score to identify patients with COVID-19 at high risk of critical care admission or death: an observational cohort study. J Infect. 2020;81:282–288. doi: 10.1016/j.jinf.2020.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global COVID-19 clinical platform: rapid core case report form (CRF). n.d. https://apps.who.int/iris/handle/10665/333229. [Accessed 30 December 2020].
- Guan W., Ni Z., Yu Hu, Liang W., Ou C., He J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptein S.J.F., Jacobs S., Langendries L., Seldeslachts L., Ter Horst S., Liesenborghs L. Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity. Proc Natl Acad Sci U S A. 2020;117(43):26955–26965. doi: 10.1073/pnas.2014441117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirenga B., Muttamba W., Kayongo A., Nsereko C., Siddharthan T., Lusiba J. Characteristics and outcomes of admitted patients infected with SARS-CoV-2 in Uganda. BMJ Open Respir Res. 2020;7 doi: 10.1136/bmjresp-2020-000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Z., Cheng S., Le J., Huang J., Feng L., Zhang B. Clinical characteristics and co-infections of 354 hospitalized patients with COVID-19 in Wuhan, China: a retrospective cohort study. Microbes Infect. 2020;22:195–199. doi: 10.1016/j.micinf.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonnasse P., Guedj J., Contreras V., Behillil S., Solas C., Marlin R. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature. 2020;585:584–587. doi: 10.1038/s41586-020-2558-4. [DOI] [PubMed] [Google Scholar]
- Martinez-Alvarez M., Jarde A., Usuf E., Brotherton H., Bittaye M., Samateh A.L. COVID-19 pandemic in west Africa. Lancet Glob Health. 2020;8:e631–2. doi: 10.1016/S2214-109X(20)30123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekolo D., Bokalli F.A., Chi F.M., Fonkou S.B., Takere M.M., Ekukole C.M. Clinical and epidemiological characteristics and outcomes of patients hospitalized for COVID-19 in Douala, Cameroon. Pan Afr Med J. 2021;38 doi: 10.11604/pamj.2021.38.246.28169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachega J.B., Ishoso D.K., Otokoye J.O., Hermans M.P., Machekano R.N., Sam-Agudu N.A. Clinical characteristics and outcomes of patients hospitalized for COVID-19 in Africa: early Insights from the Democratic Republic of the Congo. Am J Trop Med Hyg. 2020;103:2419–2428. doi: 10.4269/ajtmh.20-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M., Oikawa M., Tamura T., Egami Y., Fujita N. Can we apply lessons learned from Ebola experience in West Africa for COVID-19 in lower income countries? Glob Health Med. 2020;2:140–141. doi: 10.35772/ghm.2020.01028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olumade T.J., Uzairue L.I. Clinical characteristics of 4499 COVID-19 patients in Africa: a meta-analysis. J Med Virol. 2021;93:3055–3061. doi: 10.1002/jmv.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price-Haywood E.G., Burton J., Fort D., Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382:2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECOVERY Collaborative Group, Horby P., Mafham M., Linsell L., Bell J.L., Staplin N. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi P.G., Marino M., Formisano D., Venturelli F., Vicentini M., Grilli R. Characteristics and outcomes of a cohort of COVID-19 patients in the Province of Reggio Emilia, Italy. PLoS One. 2020;15 doi: 10.1371/journal.pone.0238281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer A.J., Morley E.J., Meyers K., Fernandes R., Rowe A.L., Viccellio P. Cohort of four thousand four hundred four persons under investigation for COVID-19 in a New York hospital and predictors of ICU care and ventilation. Ann Emerg Med. 2020;76:394–404. doi: 10.1016/j.annemergmed.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper C.P., Pastick K.A., Engen N.W., Bangdiwala A.S., Abassi M., Lofgren S.M. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med. 2020;173:623–631. doi: 10.7326/M20-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Clinical management of COVID-19 -intermin guidance - WHO. [Google Scholar]
- WHO . 2020. COVID-19 Therapeutic Trial Synopsis - WHO R&D Blueprint. [Google Scholar]
- WHO Coronavirus (COVID-19) Dashboard. n.d. https://covid19.who.int. [Accessed 5 May 2021].
- Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet Lond Engl. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]