Abstract
Aim
To compare the impact of two long‐term weight‐maintenance diets, a high protein (HP) and low glycaemic index (GI) diet versus a moderate protein (MP) and moderate GI diet, combined with either high intensity (HI) or moderate intensity physical activity (PA), on the incidence of type 2 diabetes (T2D) after rapid weight loss.
Materials and Methods
A 3‐year multicentre randomized trial in eight countries using a 2 x 2 diet‐by‐PA factorial design was conducted. Eight‐week weight reduction was followed by a 3‐year randomized weight‐maintenance phase. In total, 2326 adults (age 25‐70 years, body mass index ≥ 25 kg/m2) with prediabetes were enrolled. The primary endpoint was 3‐year incidence of T2D analysed by diet treatment. Secondary outcomes included glucose, insulin, HbA1c and body weight.
Results
The total number of T2D cases was 62 and the cumulative incidence rate was 3.1%, with no significant differences between the two diets, PA or their combination. T2D incidence was similar across intervention centres, irrespective of attrition. Significantly fewer participants achieved normoglycaemia in the HP compared with the MP group (P < .0001). At 3 years, normoglycaemia was lowest in HP‐HI (11.9%) compared with the other three groups (20.0%‐21.0%, P < .05). There were no group differences in body weight change (−11% after 8‐week weight reduction; −5% after 3‐year weight maintenance) or in other secondary outcomes.
Conclusions
Three‐year incidence of T2D was much lower than predicted and did not differ between diets, PA or their combination. Maintaining the target intakes of protein and GI over 3 years was difficult, but the overall protocol combining weight loss, healthy eating and PA was successful in markedly reducing the risk of T2D. This is an important clinically relevant outcome.
Keywords: behaviour change, carbohydrate, dietary intervention, exercise intervention, glycaemic control, obesity
1. INTRODUCTION
The rate of type 2 diabetes (T2D) continues to increase on a global level with serious consequences for the individual and the community. 1 Weight gain resulting in overweight and obesity is the main risk factor for developing T2D, whereas weight loss appears to be a key determinant of prevention of T2D in predisposed individuals. 2 , 3 Weight maintenance is a critical component of solving both the obesity and T2D epidemic and tools to effectively implement weight maintenance after significant weight loss are therefore needed. The DiRECT study showed that after a 12‐month intensive weight management programme in participants with T2D, the remission rate was highest in participants who achieved the greatest weight loss (46% in the intervention group compared with only 4% in the control group). 4 After 24 months, remission was still sustained in 36% versus 3% in the intervention versus control group. 5
Previous diabetes prevention studies have found that adherence to a healthy lifestyle (decreased energy intake, high fibre, high carbohydrate diets, increased physical activity [PA]) reduced the incidence of T2D by 28%‐58% in predisposed individuals compared with routine care. 6 , 7 , 8 , 9 The multicentre Diet, Obesity and Genes (DiOGenes) trial found that in overweight, healthy individuals a combined ad libitum high protein (HP), moderate carbohydrate, low glycaemic index (GI) diet was superior to four other diets of varying macronutrient and GI composition in preventing 6‐month weight regain after an initial 8‐week, low energy diet (LED). 10 A recent meta‐analysis of prospective cohort studies found that diets with lower GI and glycaemic load (GL) were associated with a reduced risk ratio for T2D. 11 Also, a Mediterranean‐based dietary pattern and a high carbohydrate quality index (in the PREDIMED trials) were proven to be beneficial concerning adiposity and risk factors for T2D and cardiovascular disease. 12 , 13
Despite the shown effect on weight maintenance after weight loss, the combined effect of an HP, low GI diet for the prevention of T2D has not yet been investigated. The main objective of the PREVIEW intervention study (PREVention of diabetes through lifestyle intervention and population studies In Europe and around the World) 14 was, therefore, to determine whether an ad libitum HP, low GI diet was superior to a conventional moderate protein, moderate GI diet for weight‐loss maintenance and thereby for prevention of T2D in adults with prediabetes. The second aim was to determine whether high intensity PA had additional positive effects on outcomes compared with moderate intensity PA. In order to study the effect on weight maintenance per se, an initial 8‐week weight‐loss phase using an LED was conducted adopting a similar design as the DiOGenes study. 10
2. METHODS
PREVIEW was a multicentre randomized trial including adults and children. The details of the study protocols have been described previously 14 , 15 (clinicaltrials.gov NCT01777893). This paper concerns the adult participants.
PREVIEW for adults was conducted at eight intervention centres in eight countries: University of Copenhagen (Denmark), University of Helsinki (Finland), University of Maastricht (the Netherlands), University of Nottingham (UK), University of Navarra (Spain), Medical University of Sofia (Bulgaria), University of Sydney (Australia) and University of Auckland (New Zealand). The study protocol and amendments were reviewed and approved by the local Human Ethics Committee at each of the eight intervention centres. The work of PREVIEW was carried out in full compliance with the relevant requirements of the latest version of the Declaration of Helsinki (59th WMA General Assembly, Seoul, Korea, October 2008) and The International Conference on Harmonisation for Good Clinical Practice, to the extent possible and relevant. All participants provided written informed consent prior any screening procedures. All information obtained during the trial was handled according to the local regulations and the European Directive 95/46/CE (directive on protection of individuals with regard to the processing of personal data and on the free movement of such data).
The 3‐year randomized intervention trial consisted of an 8‐week weight‐loss phase followed by a 148‐week weight‐maintenance phase. The intervention had a 2 x 2 factorial design with two diets and two PA programmes.
2.1. Participants
From June 2013 to April 2015, 2326 men and women with prediabetes (age 25‐70 years, body mass index [BMI] ≥ 25 kg/m2) were enrolled. The last participant visit was in March 2018. Prescreening was undertaken using the Finnish Diabetes Risk Score, 16 specific inclusion and exclusion criteria, 14 and prediabetes according to the recommended criteria of the American Diabetes Association (ADA). 17 A total of seven clinical investigation days (CIDs) were performed for each participant in the morning fasted state (at baseline, 8, 26, 52, 78, 104 and 156 weeks), including anthropometry, blood sampling and completion of questionnaires. Furthermore, at baseline, 26, 52, 104 and 156 weeks, 4‐day dietary records, 7‐day accelerometer data and 24‐hour urine samples were collected. Participants also attended 17 group counselling visits (8‐10 participants) with trained instructors to support the changes in habitual lifestyles.
2.2. Interventions
The Cambridge Weight Plan (Northants, UK) was used for the weight‐loss phase. 18 Participants who achieved a loss of initial body weight of 8% or higher could continue in the study. The intervention diets targeted different macronutrient compositions: HP 25 energy‐% (E%) protein, 30 E% fat, 45 E% carbohydrates, low GI (<50); and moderate protein (MP) 15 E% protein, 30 E% fat, 55 E% carbohydrates, moderate GI (>56). 14 Both intervention diets emphasized healthy food choices. The PA groups were: high intensity (HI) PA for 75 minutes per week; moderate intensity (MI) PA for 150 minutes per week as recommended. 19 More specifically, the HI group participated in 75 minutes of PA per week at six or more metabolic equivalents of task (METs) (450 MET minutes per week) and the MI group 150 minutes at 3‐5.9 METs (450 MET minutes per week). Both PA groups were therefore guided to expend the same amount of energy during PA.
The counselling visits (8‐12 participants) consisted of specific behavioural modification techniques designed to educate about and support adoption of the new diet and PA strategies (PREMIT). 20 The frequency of the visits decreased during weight maintenance. An instructors' network ensured consistency between centres.
Before trial start, all staff were trained in the procedures at joint training seminars and via standard operating procedures (SOPs). Instruction material for lifestyle changes, measures and questionnaires were also developed for the participants.
2.3. Primary outcome
The primary endpoint was incidence of T2D according to diet using an oral glucose tolerance test (OGTT) with 75 g glucose. T2D was diagnosed based on the World Health Organization criteria 21 of either (a) OGTT with fasting plasma glucose of more than 7.0 mmol/L and/or 2‐hour postprandial plasma glucose of 11.1 mmol/L or higher, or (b) T2D diagnosed by a medical doctor between the CIDs using random plasma glucose of 11.1 mmol/L or higher in the presence of symptoms of diabetes, an OGTT or HbA1c.
2.4. Secondary outcomes
Secondary endpoints included incidence of T2D according to PA and the four combinations, changes in fasting resting glucose, 2‐hour glucose, proportion of participants with normoglycaemia, HbA1c, insulin, C‐peptide, homeostatic model assessment of insulin resistance (HOMA‐IR), 22 Matsuda Index (subgroup, n = 328), 23 body weight, BMI, fat mass, fat‐free mass, waist, hip and thigh circumference, and proportion of participants maintaining ≥0%, ≥5% or ≥10% weight loss after 156 weeks.
All blood samples were drawn from the antecubital vein, stored at −80°C and subsequently analysed at the National Institute for Health and Welfare, Helsinki (T077, accredited by the Finnish Accreditation Service, fulfilling the requirements of standard SFS‐EN ISO/IEC 17025:2005). Laboratory measurements were performed on an Architect ci8200 integrated system (Abbott Laboratories, Abbott Park, IL, USA). Insulin for Matsuda Index was analysed by an immuno‐chemiluminescent method on a Siemens Immulite 2000 (Siemens Healthcare, Diagnostics Products, Gwynedd, UK). Measurements of body weight, body composition, height, waist, hip and thigh circumference, and blood pressure, were described previously. 18
Reported dietary intake was analysed from 4‐day weighed dietary records using national food composition software. Dietary compliance assessment by biomarkers was undertaken for protein intake (nitrogen or urea, 24‐hour urine samples) using the formula: 24‐hour urinary urea (mmol/day) x 0.22 + 12.5 g protein per day, and conversion factor urea x 0.4664 = nitrogen. 24 , 25 Nitrogen or urea were analysed locally at each intervention centre. Urine collection was assured by a SOP as well as instruction material and tools for the participants. A urine collection of less than 0.5 L/day was regarded as incomplete.
For assessment of PA, participants wore an ActiSleep+ (ActiGraph LLC, Pensacola, FL, USA) accelerometer as previously described. 14 , 26 In brief, the accelerometer was attached to an elastic waist belt worn over the right mid‐axillary line 24 hours a day for 7 consecutive days before each CID, only removing it for water‐based activities. After the removal of nocturnal sleep episodes, participants were included in the analyses if they wore the monitor for 10 hours or more on 4 or more days including 1 weekend day. Mean activity counts during valid wear time (counts per minute [CPM]) were used as an indicator of total PA volume. 27 Data were analysed centrally at Swansea University (UK). CPM was used to estimate PA.
Adverse events (AEs) were recorded at each CID.
2.5. Sample size
Sample size estimation of expected T2D incidence was based on the two diet intervention groups (HP and MP), hypothesizing that a risk reduction of 25.0% in MP would reduce T2D incidence to 15.8%. The estimated 25.0% risk reduction was based on outcomes from published diabetes prevention trials 7 , 8 and completer analyses. We further hypothesized that HP would achieve an overall 50.0% diabetes risk reduction from a baseline risk of 21.0% to a 3‐year risk of 10.5%. A conservative estimate of sample size to detect this difference (15.8% vs. 10.5%) was 649 per group or 1298 participants in total (two‐sided comparison, power = 80%, alpha = 0.05). We estimated an 30% drop‐out rate (similar to DiOGenes 10 ). Thus, 1854 subjects should start the weight‐maintenance phase. However, to allow for drop‐out after inclusion and for participants not losing ≥8% weight (estimate = 25%), a total of 2472 participants should be recruited. A secondary power calculation for HbA1c (using 3‐year results from the Diabetes Prevention Study) 8 anticipated a difference between the two diet groups of 0.2% points (SD = 0.6% points). Using an 80% power and alpha of 0.05, the estimated sample size for each group was 142. Allowing for 30% drop‐out, the sample size required was 205 per group.
2.6. Randomization and masking
Participants were randomly assigned to one of the four intervention groups, separately at each centre, stratified by age (25‐45, 46‐54 and 55‐70 years) and gender (men, women) by sequentially assigning participants within each stratum to the interventions. 28 The allocation order was concealed from staff and not disclosed to participants until the group meeting (week 8). Staff and study participants were not blinded because of the nature of the intervention. However, all staff involved in data handling and statistical analyses were blinded to the randomization until analyses were concluded.
2.7. Statistical analyses
Data are shown as mean ± SEM (± SD for baseline characteristics) unless otherwise stated. Three‐year incidence of T2D was compared between diets and PA groups by a semi‐parametric Cox proportional hazards regression model, including adjustments for age, BMI, gender, ethnicity, intervention centre, fasting glucose and 2‐hour glucose. Comparison of diets was adjusted for PA and vice versa. The proportional hazards model assumption was evaluated by means of residual plots based on Schoenfeld residuals as well as by means of the global Schoenfeld lack of fit test.
A post hoc analysis compared T2D incidence in intervention centres with higher (≥30%) versus lower (<30%) attrition rates.
Changes in continuous secondary outcomes from 8 to 156 weeks were analysed using an analysis of covariance (ANCOVA) linear mixed model, including three‐way interaction between diet, PA and time. The models were adjusted for baseline values (0 weeks), age and gender (fixed effects), and participant ID and intervention centre (random effects). For significant main effects and interactions, pairwise comparisons were reported. ANCOVA was used to investigate associations between outcomes. The binary outcome normoglycaemia (yes/no) was analysed using a logistic mixed effects model including the same fixed and random effects as the linear mixed models. A post hoc analysis was conducted for weight change after 3 years in participants with a protein intake of <0.8 and ≥0.8 g/kg per day (biomarker analyses).
Available‐case analyses were carried out assuming that missing data occurred at random. Sensitivity analysis was also conducted using the intention‐to‐treat population, defined as all individuals entering the weight‐loss phase. Imputation was conducted using baseline observation carried forward.
The statistical environment R was used for all analyses (version 3.6.3). 29 P less than .05 was considered statistically significant.
3. RESULTS
In total, 2326 individuals were enrolled and randomized into the trial (Figure 1; Figure S1 ). Baseline characteristics were similar in the four intervention groups (Table 1). Women represented 68% of participants. When 1857 participants had met the criteria for the weight‐maintenance phase (79.8% of all those enrolled), the statistical power for this timepoint had been reached and recruitment was consequently stopped. Between randomization and baseline, 102 individuals withdrew consent, mainly for personal reasons. One individual withdrew consent during the study and requested that all data be deleted. Therefore, 2223 individuals were included in the analyses. Overall, 1381 participants completed year 1 (74% of those eligible for the weight‐maintenance phase), 1093 year 2 (59%) and 962 year 3 (52%), corresponding to 74% of the goal for completers (n = 1298). Attrition rate during the 3 years was similar in the four groups.
FIGURE 1.
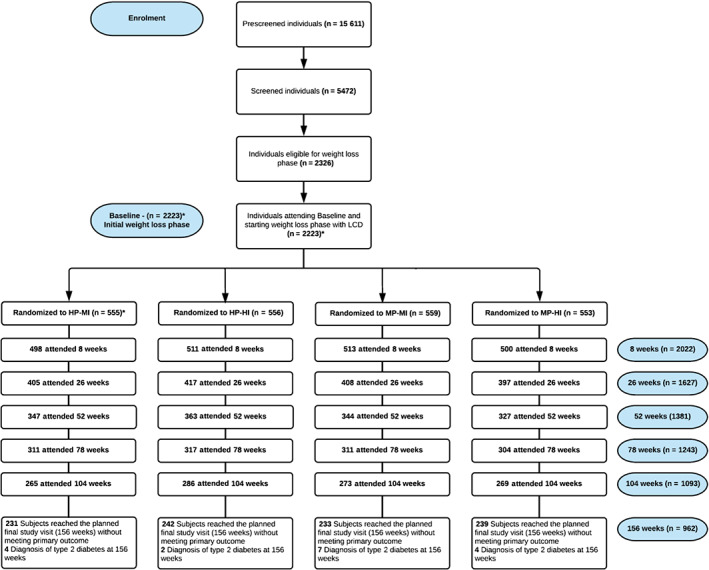
Participant flow during the trial (see Figure S1 for all details of exclusion). HP‐MI: high protein, moderate intensity. HP‐HI: high protein, high intensity. MP‐MI: moderate protein, moderate intensity. MP‐HI: moderate protein, high intensity. *n=2224 attended this visit, but 1 individual requested all data to be deleted and therefore, n= 2223
TABLE 1.
Baseline characteristics of the participants in the four intervention groups
| HP‐MI | HP‐HI | MP‐MI | MP‐HI | |
|---|---|---|---|---|
| No. (women: Men) | 555 a (371: 184) | 556 (379: 177) | 559 (379: 180) | 553 (374: 179) |
| Age, years | 51.6 ± 11.5 | 51.8 ± 11.7 | 51.4 ± 11.2 | 51.4 ± 11.8 |
| Weight, kg | 99.3 ± 20.8 | 100.6 ± 21.1 | 101.6 ± 22.6 | 98.7 ± 20.9 |
| Height, m | 1.68 ± 0.09 | 1.68 ± 0.09 | 1.68 ± 0.09 | 1.68 ± 0.10 |
| BMI, kg/m2 | 35.1 ± 6.5 | 35.7 ± 6.7 | 35.7 ± 6.6 | 35.0 ± 6.4139 |
| HOMA‐IR b | 3.8 ± 2.5 | 3.9 ± 2.6 | 3.7 ± 2.4 | 3.7 ± 2.2 |
| Fasting glucose, mmol/L | 6.2 ± 0.8 | 6.2 ± 0.7 | 6.2 ± 0.7 | 6.2 ± 0.8 |
| 2‐hour glucose, mmol/L | 7.8 ± 2.3 | 7.7 ± 2.3 | 7.5 ± 2.2 | 7.7 ± 2.1 |
| HbA1c, mmol/Mol | 36.6 ± 3.8 | 36.8 ± 3.9 | 36.7 ± 4.5 | 36.8 ± 4.0 |
| Insulin, pmol/L | 13.6 ± 7.9 | 14.0 ± 8.7 | 13.3 ± 7.9 | 13.1 ± 7.2 |
| C‐peptide, pmol/L | 918 ± 353 | 940 ± 359 | 916 ± 337 | 921 ± 348 |
| ALT, U/L | 27.9 ± 16.3 | 27.1 ± 16.1 | 29.3 ± 17.7 | 27.3 ± 15.1 |
| AST, U/L | 27.4 ± 9.6 | 27.4 ± 11.1 | 28.5 ± 11.4 | 27.5 ± 10.5 |
| Waist circumference, cm | 109.7 ± 14.4 | 111.2 ± 14.5 | 111.1 ± 15.4 | 109.7 ± 14.5 |
| Hip circumference, cm | 117.8 ± 13.7 | 119.0 ± 14.0 | 119.3 ± 13.9 | 117.9 ± 13.8 |
| Thigh circumference, cm | 60.2 ± 7.6 | 60.4 ± 7.3 | 60.7 ± 7.4 | 60.1 ± 7.0 |
| Fat‐free mass, kg | 56.4 ± 11.7 | 56.6 ± 12.0 | 57.2 ± 12.7 | 55.9 ± 11.6 |
| Fat mass, kg | 42.4 ± 13.5 | 43.3 ± 13.5 | 44.1 ± 14.3 | 42.3 ± 13.4 |
| Fat mass, % | 43.0 ± 7.5 | 43.5 ± 7.6 | 43.6 ± 7.5 | 43.1 ± 7.9 |
| SBP, mmHg | 128.5 ± 15.6 | 129.7 ± 16.2 | 128.7 ± 16.3 | 129.3 ± 15.5 |
| DBP, mmHg | 78.2 ± 11.0 | 77.6 ± 11.2 | 78.4 ± 11.5 | 78.3 ± 10.7 |
| Heart rate, bpm | 71.2 ± 10.4 | 71.3 ± 11.1 | 70.8 ± 10.4 | 71.7 ± 10.4 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DBP, diastolic blood pressure; HOMA‐IR, homeostasis model of assessment insulin resistance; HP‐HI, high protein diet, high intensity physical activity; HP‐MI, high protein diet, moderate intensity physical activity; MP‐HI, moderate protein diet, high intensity physical activity; MP‐MI, moderate protein diet, moderate intensity physical activity; SBP, systolic blood pressure; SD, standard deviation. Data are shown as means ± SD.
This number is one less than previously reported (14) as one participant withdrew consent during the intervention and asked to have all data removed.
The formula to calculate the HOMA‐IR was: fasting insulin (mU/L) x fasting glucose (mmol/L) / 22.5.22
3.1. Primary outcome
T2D incidence was 3.0% for the HP and 3.2% for the MP diet (ns) (Figure 2, top). Sixty‐two participants developed T2D, 30 on HP and 32 on MP (adjusted hazard ratio [aHR] for MP vs. HP: 1.22 (95% confidence interval: 0.73 to 2.05), P = .45; Figure 2, top). Incidence in both groups was less than one third of predicted incidence (HP = 10.5%, MP = 15.8%). Post hoc analyses showed that T2D incidence was 3.0% in intervention centres with higher (≥30%) and 3.4% in centres with lower (<30%) attrition rates (P = .24).
FIGURE 2.
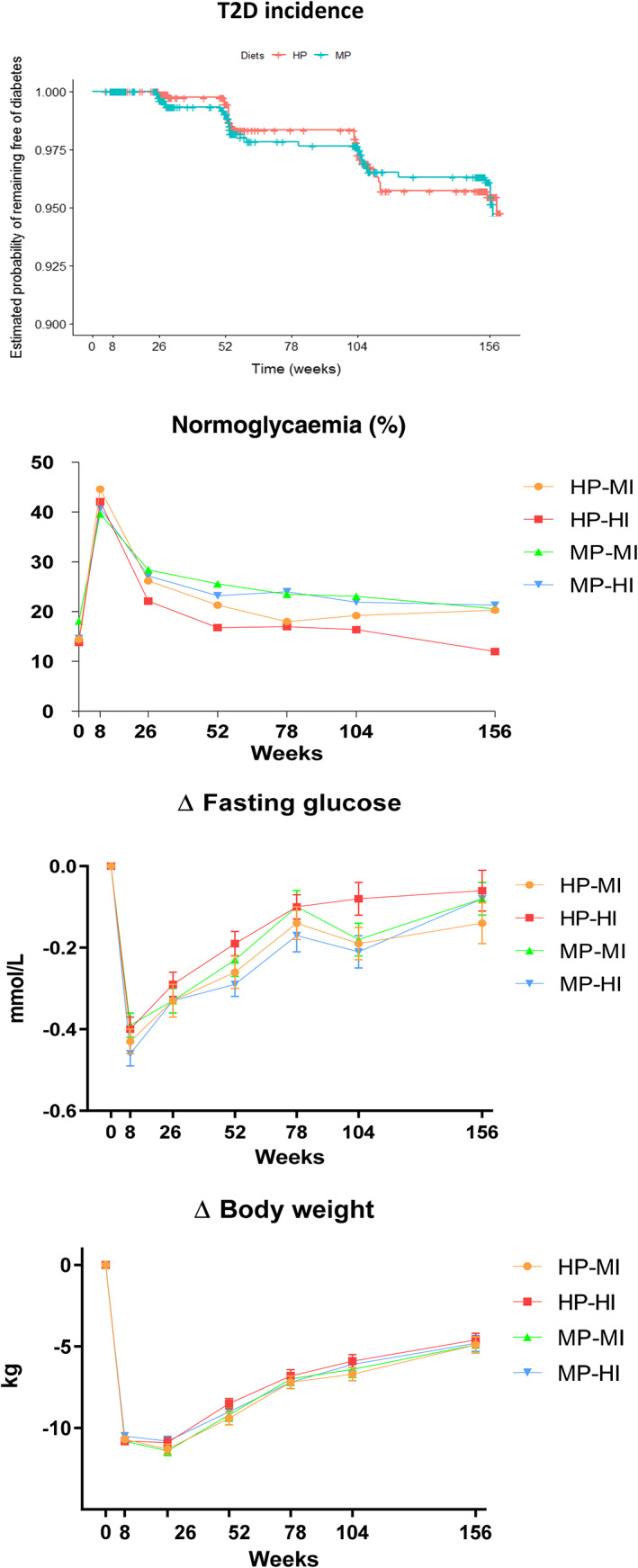
Top: T2D incidence per diet group. Estimated probability of remaining free of diabetes (Kaplan–Meier plot). HP: high protein, low GI diet. MP: moderate protein, moderate GI diet. HR for MP versus HP: 1.22 (95% confidence interval: 0.73‐2.05, P = .45). Normoglycaemia: percentage of participants with normoglycaemia based on fasting (only week 8) or fasting and 2‐hour glucose values by oral glucose tolerance test. Normoglycaemia (binary outcome: yes/no) was analysed using a logistic mixed effects model including fixed effects (outcome at 8 weeks, age, gender, PA or diet intervention) and participant ID and intervention centres (random effects). P‐values: diet effect: .000074 (fewer participants with normoglycaemia in HP than MP); PA effect: .09; time effect <.00001; combined (4) groups effect: .00028 (lower numbers in HP‐HI compared with MP‐HI, P = .006 and MP‐MI, P = .0005, but not HP‐MI, P = .08). ∆ fasting glucose: change in fasting glucose from week 0 presented as means ± SEM. ANCOVA linear mixed model analysis including three‐way interaction between diet, PA and time. Model was adjusted for baseline values (0 weeks), age and gender (fixed effects), and participant ID and intervention centre (random effects). P‐values: diet x PA x time effect: .33; diet x PA effect: .046 (post hoc pairwise analyses: ns); diet effect: .12; PA effect: .59; time effect: <.00001. ∆ body weight: change in body weight from week 0 presented as means ± SEM. ANCOVA linear mixed model analysis including three‐way interaction between diet, PA and time adjusted for baseline values (0 weeks), age and gender (fixed effects), and participant ID and intervention centre (random effects). Time effect: P < .00001. No other significant effects. HP‐MI: high protein, moderate intensity PA. HP‐HI: high protein, high intensity PA. MP‐MI: moderate protein, moderate intensity PA. MP‐HI: moderate protein, high intensity PA. N = week 0: 2223; week 8: 2022; week 26: 1627; week 52: 1381; week 78: 1243; week 104: 1093; week 156: 962
3.2. Secondary outcomes
T2D incidence was 2.5% (n = 27) for MI and 3.5% (n = 35) for HI PA. Adjusted HR was 1.35 (0.80‐2.27) for HI versus MI (P = .27). Incidence of T2D was 11 in HP‐MI, 19 in HP‐HI, 16 in MP‐MI and 16 in MP‐HI. Relative to HP‐HI, the aHR for MP‐MI was 0.92 (0.46‐1.83, P = .81), for MP‐HI 1.10 (0.56‐2.17, P = .79) and for HP‐MI 0.65 (0.30‐1.40, P = .27).
After the LED, 40.2% of those achieving ≥8% weight loss no longer had prediabetes according to ADA criteria (based on fasting glucose; Figure 2). During the weight‐maintenance phase, a significant effect of diet group was observed, with fewer participants having normoglycaemia in HP than MP (P < .0001). A significant main effect of the four groups was also observed (P = .0003), with lower numbers in HP‐HI compared with MP‐HI (P = .006) and MP‐MI (P = .0005), but not HP‐MI (P = .08) (Figure 2). After 3 years, 18.2% of participants (175 of 962) had normoglycaemia, but significantly fewer in HP‐HI (11.9%) than in HP‐MI (20.0%, P = .02), MP‐HI (21.0%, P = .0096) and MP‐MI (20.0%, P = .02; Figure 2).
Fasting glucose showed a significant diet*PA interaction (P = .046; Figure 2), but post hoc pairwise comparisons only showed a tendency (HP‐HI vs. MP‐HI, P = .06). Two‐hour glucose, fasting insulin and C‐peptide tended to differ (diet*PA*time interaction, P = .05‐.1), but not HOMA‐IR, HbA1c (Table 2) or Matsuda Index (Table S1 ). Body weight decreased by 11% during the LED phase (P < .0001) and 3‐year weight loss averaged 4.6‐4.9 kg (n = 962) or ~5% of initial body weight (completers, ns between groups). Imputation or sensitivity analyses did not change this (Figure S3). At 3 years, 47% of completers still had a body weight loss of ≥5% (Table S2). Time effect was significant for all outcomes (P < .0001), and 2 hour‐glucose, fasting insulin and HOMA‐IR remained significantly lower after 3 years compared with baseline (P < .05; Table 2). Three‐year changes in fasting glucose, 2‐hour glucose, insulin, HbA1c, waist and hip circumference were significantly correlated with changes in body weight (P < .001).
TABLE 2.
Changes in anthropometrics and blood variables during the intervention
| Outcome | Group | 8 weeks (n = 2022) | 26 weeks (n = 1627) | 52 weeks (n = 1381) | 78 weeks (n = 1243) | 104 weeks (n = 1093) | 156 weeks (n = 962) | P‐value for diet x PA x time three‐way interaction | P‐value for diet x PA interaction | P‐value for diet main effect | P‐value for PA main effect | P‐value for time main effect |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ∆HbA1c, mmol/mol | HP‐MI | −2.09 ± 0.11 | −1.85 ± 0.12 | −1.43 ± 0.14 | −0.72 ± 0.15 | −0.61 ± 0.15 | −0.33 ± 0.15 | .81682 | .41235 | .66929 | .42009 | <.00001 |
| HP‐HI | −2.12 ± 0.10 | −1.66 ± 0.12 | −1.33 ± 0.13 | −0.64 ± 0.15 | −0.42 ± 0.16 | −0.25 ± 0.16 | ||||||
| MP‐MI | −2.01 ± 0.10 | −1.94 ± 0.11 | −1.41 ± 0.13 | −0.73 ± 0.16 | −0.76 ± 0.16 | −0.25 ± 0.16 | ||||||
| MP‐HI | −2.17 ± 0.10 | −1.91 ± 0.12 | −1.25 ± 0.15 | −0.83 ± 0.16 | −0.77 ± 0.14 | −0.34 ± 0.15 | ||||||
| ∆ 120 min glucose, mmol/L | HP‐MI | −1.06 ± 0.10 | −1.18 ± 0.12 | −0.78 ± 0.13 | −0.61 ± 0.14 | .06657 | .79322 | .34678 | .11778 | <.00001 | ||
| HP‐HI | −0.96 ± 0.10 | −1.10 ± 0.10 | −0.51 ± 0.13 | −0.49 ± 0.13 | ||||||||
| MP‐MI | −1.15 ± 0.10 | −1.06 ± 0.11 | −0.57 ± 0.13 | −0.35 ± 0.14 | ||||||||
| MP‐HI | −0.98 ± 0.10 | −1.15 ± 0.11 | −0.65 ± 0.14 | −0.69 ± 0.14 | ||||||||
| ∆ insulin, pmol/L | HP‐MI | −4.72 ± 0.30 | −4.51 ± 0.31 | −4.12 ± 0.36 | −3.13 ± 0.36 | −3.39 ± 0.40 | −2.88 ± 0.43 | .06023 | .45269 | .16519 | .27689 | <.00001 |
| HP‐HI | −4.87 ± 0.30 | −4.54 ± 0.33 | −3.47 ± 0.38 | −2.58 ± 0.40 | −2.94 ± 0.43 | −2.18 ± 0.51 | ||||||
| MP‐MI | −4.42 ± 0.26 | −4.39 ± 0.27 | −3.31 ± 0.32 | −1.76 ± 0.33 | −2.67 ± 0.31 | −1.83 ± 0.41 | ||||||
| MP‐HI | −3.80 ± 0.26 | −3.89 ± 0.27 | −3.19 ± 0.30 | −2.13 ± 0.29 | −2.40 ± 0.31 | −1.65 ± 0.33 | ||||||
| HOMA‐IR a | HP‐MI | −1.47 ± 0.10 | −1.39 ± 0.10 | −1.28 ± 0.12 | −0.95 ± 0.13 | −1.05 ± 0.14 | −0.89 ± 0.16 | .10755 | .25167 | .29449 | .33987 | <.00001 |
| HP‐HI | −1.50 ± 0.10 | −1.39 ± 0.10 | −1.07 ± 0.12 | −0.79 ± 0.13 | −0.86 ± 0.14 | −0.63 ± 0.17 | ||||||
| MP‐MI | −1.38 ± 0.08 | −1.34 ± 0.09 | −1.01 ± 0.10 | −0.53 ± 0.11 | −0.80 ± 0.10 | −0.52 ± 0.13 | ||||||
| MP‐HI | −1.23 ± 0.08 | −1.23 ± 0.09 | −1.01 ± 0.09 | −0.67 ± 0.09 | −0.73 ± 0.09 | −0.47 ± 0.10 | ||||||
| ∆ C‐peptide, pmol/L | HP‐MI | −215.3 ± 12.9 | −237.4 ± 11.9 | −210.5 ± 13.8 | −173.0 ± 14.6 | −162.8 ± 17.5 | −137.9 ± 18.0 | .09727 | .0532 | .28546 | .36015 | <.00001 |
| HP‐HI | −209.5 ± 10.7 | −235.7 ± 13.1 | −186.9 ± 14.8 | −138.6 ± 15.1 | −130.3 ± 16.8 | −91.7 ± 20.5 | ||||||
| MP‐MI | −201.3 ± 10.5 | −220.2 ± 13.3 | −185.4 ± 13.2 | −108.2 ± 15.4 | −128.6 ± 15.3 | −85.2 ± 21.3 | ||||||
| MP‐HI | −200.6 ± 12.9 | −217.1 ± 12.2 | −204.5 ± 13.0 | −131.7 ± 13.4 | −127.5 ± 13.5 | −90.4 ± 15.0 | ||||||
| ∆ BMI, kg/m2 | HP‐MI | −3.8 ± 0.1 | −3.9 ± 0.1 | −3.3 ± 0.1 | −2.5 ± 0.1 | −2.3 ± 0.1 | −1.7 ± 0.2 | .48217 | .54623 | .80283 | .4793 | <.00001 |
| HP‐HI | −3.8 ± 0.1 | −3.9 ± 0.1 | −3.0 ± 0.1 | −2.4 ± 0.1 | −2.0 ± 0.1 | −1.6 ± 0.2 | ||||||
| MP‐MI | −3.8 ± 0.1 | −4.0 ± 0.1 | −3.2 ± 0.1 | −2.5 ± 0.2 | −2.2 ± 0.2 | −1.7 ± 0.2 | ||||||
| MP‐HI | −3.7 ± 0.1 | −3.8 ± 0.1 | −3.2 ± 0.1 | −2.5 ± 0.1 | −2.1 ± 0.2 | −1.7 ± 0.2 | ||||||
| ∆ fat mass, kg | HP‐MI | −7.8 ± 0.2 | −9.4 ± 0.3 | −7.8 ± 0.4 | −5.1 ± 0.4 | −3.5 ± 0.4 | .74052 | .40815 | .76205 | .5155 | <.00001 | |
| HP‐HI | −8.0 ± 0.2 | −9.1 ± 0.3 | −7.1 ± 0.3 | −4.4 ± 0.4 | −3.0 ± 0.4 | |||||||
| MP‐MI | −7.9 ± 0.2 | −9.3 ± 0.3 | −7.5 ± 0.4 | −4.9 ± 0.5 | −3.0 ± 0.5 | |||||||
| MP‐HI | −7.9 ± 0.2 | −9.0 ± 0.2 | −7.4 ± 0.3 | −4.6 ± 0.5 | −2.9 ± 0.4 | |||||||
| ∆ fat‐free mass, kg | HP‐MI | −2.7 ± 0.1 | −1.9 ± 0.2 | −1.5 ± 0.2 | −1.6 ± 0.2 | −1.4 ± 0.2 | .56413 | .76296 | .85176 | .2078 | <.00001 | |
| HP‐HI | −2.7 ± 0.1 | −1.6 ± 0.1 | −1.4 ± 0.2 | −0.9 ± 0.2 | −1.5 ± 0.2 | |||||||
| MP‐MI | −2.7 ± 0.1 | −1.9 ± 0.1 | −1.6 ± 0.2 | −1.4 ± 0.2 | −1.4 ± 0.2 | |||||||
| MP‐HI | −2.5 ± 0.1 | −1.5 ± 0.1 | −1.4 ± 0.1 | −1.1 ± 0.2 | −1.5 ± 0.3 | |||||||
| ∆ thigh circumference, cm | HP‐MI | −3.6 ± 0.2 | −4.0 ± 0.2 | −3.6 ± 0.3 | −2.8 ± 0.2 | −2.6 ± 0.3 | −2.4 ± 0.4 | .2101 | .05872 | .23572 | .93376 | <.00001 |
| HP‐HI | −3.7 ± 0.2 | −3.6 ± 0.2 | −2.8 ± 0.2 | −2.8 ± 0.2 | −2.6 ± 0.3 | −2.6 ± 0.3 | ||||||
| MP‐MI | −3.9 ± 0.2 | −4.0 ± 0.2 | −3.3 ± 0.2 | −3.1 ± 0.3 | −2.8 ± 0.3 | −1.7 ± 0.5 | ||||||
| MP‐HI | −3.8 ± 0.2 | −3.9 ± 0.2 | −3.3 ± 0.3 | −3.3 ± 0.2 | −2.7 ± 0.2 | −2.6 ± 0.3 | ||||||
| ∆ waist circumference, cm | HP‐MI | −9.7 ± 0.2 | −10.5 ± 0.4 | −8.2 ± 0.4 | −6.7 ± 0.4 | −6.0 ± 0.4 | −4.1 ± 0.5 | .25851 | .69829 | .89252 | .19469 | <.00001 |
| HP‐HI | −9.9 ± 0.2 | −9.9 ± 0.4 | −7.5 ± 0.4 | −6.6 ± 0.4 | −4.9 ± 0.4 | −3.7 ± 0.5 | ||||||
| MP‐MI | −9.7 ± 0.2 | −10.1 ± 0.4 | −8.5 ± 0.5 | −6.7 ± 0.4 | −5.3 ± 0.5 | −3.5 ± 0.5 | ||||||
| MP‐HI | −9.2 ± 0.2 | −9.8 ± 0.3 | −8.3 ± 0.5 | −6.4 ± 0.5 | −5.3 ± 0.5 | −2.8 ± 0.6 | ||||||
| ∆ hip circumference, cm | HP‐MI | −7.2 ± 0.2 | −7.6 ± 0.3 | −6.0 ± 0.3 | −4.2 ± 0.4 | −3.8 ± 0.4 | −2.2 ± 0.4 | .22499 | .65382 | .81448 | .43981 | <.00001 |
| HP‐HI | −7.2 ± 0.2 | −7.6 ± 0.3 | −5.1 ± 0.3 | −4.2 ± 0.4 | −3.4 ± 0.4 | −2.5 ± 0.4 | ||||||
| MP‐MI | −7.2 ± 0.2 | −7.8 ± 0.3 | −5.9 ± 0.4 | −4.6 ± 0.4 | −3.7 ± 0.4 | −2.5 ± 0.4 | ||||||
| MP‐HI | −7.1 ± 0.2 | −7.2 ± 0.3 | −5.7 ± 0.4 | −4.7 ± 0.4 | −3.6 ± 0.4 | −2.3 ± 0.4 |
Abbreviations: BMI, body mass Index; HOMA‐IR, homeostasis model of assessment insulin resistance; HP‐HI, high protein diet and high intensity physical activity; HP‐MI, high protein diet and moderate intensity physical activity; MP‐MI, moderate protein diet and moderate intensity physical activity; MP‐HI, moderate protein diet and high intensity physical activity. Unadjusted mean changes from baseline (0 weeks) ± SE. Changes from 8 to 156 weeks were analysed using analysis of covariance (ANCOVA) linear mixed model, including three‐way interaction between diet, PA and time adjusted for baseline values (0 weeks), age and gender (fixed effects), and participant ID and intervention centre (random effects). Comparison of diets was adjusted for PA and vice versa. Available case analyses were carried out under the assumption that missing data appear randomly.
The formula to calculate the HOMA‐IR was: fasting insulin (mU/L) x fasting glucose (mmol/L) / 22.5. 22
Recorded dietary intake was similar at baseline (Table 3). During weight maintenance, significantly lower GI, carbohydrate intake and GL, and significantly higher protein, fat and energy intake, were observed in HP compared with MP. Compliance by biomarker analyses showed higher protein intake in HP than MP at 26 and 52 weeks, but not at 104 or 156 weeks (interaction diet*time, P < .0001). Still, mean protein intake in MP appeared to remain above 0.8 g/kg daily from week 26 to 156 (Figure S2, top). A post hoc analysis showed that participants consuming ≥0.8 g/kg protein daily (n = 639) regained 1.5% points less weight than participants consuming less than 0.8 g/kg (n = 261) during weight maintenance (P = .0054, data not shown). Dietary intake in the four intervention groups is given in Table S3. Overall, significant effects were only related to diet and time.
TABLE 3.
Changes in dietary intake during the intervention a
| Outcome | Group | 0 weeks (n = 1822) | 26 weeks (n = 1292) | 52 weeks (n = 1080) | 104 weeks (n = 889) | 156 weeks (n = 833) | P‐value for diet x time interaction | P‐value for diet main effect | P‐value for time main effect |
|---|---|---|---|---|---|---|---|---|---|
| Glycaemic index | HP | 56.3 ± 0.2 a | 51.2 ± 0.3 b | 51.0 ± 0.4 b | 51.7 ± 0.4 b | 51.6 ± 0.5 b | <.00001 | <.00001 | <.00001 |
| MP | 56.5 ± 0.2 a | 55.9 ± 0.3 ac | 54.8 ± 0.4 d | 55.2 ± 0.4 cd | 54.9 ± 0.4 d | ||||
| Glycaemic load | HP | 119.3 ± 1.5 a | 82.5 ± 1.3 b | 82.8 ± 1.4 b | 81.4 ± 1.5 b | 79.7 ± 1.5 b | <.00001 | <.00001 | <.00001 |
| MP | 119.0 ± 1.5 a | 98.7 ± 1.5 c | 95.5 ± 1.6 cd | 94.3 ± 1.8 cd | 93.3 ± 1.9 d | ||||
| Protein, E% | HP | 17.9 ± 0.1 a | 22.4 ± 0.2 b | 22.0 ± 0.2 bd | 21.6 ± 0.2 de | 21.2 ± 0.2 e | <.00001 | <.00001 | <.00001 |
| MP | 17.7 ± 0.1 a | 18.9 ± 0.1 c | 18.6 ± 0.2 c | 18.8 ± 0.2 c | 18.9 ± 0.2 c | ||||
| Carbohydrate, E% | HP | 40.1 ± 0.2 a | 37.7 ± 0.3 b | 37.8 ± 0.3 b | 37.7 ± 0.4 b | 37.8 ± 0.4 b | <.00001 | <.00001 | .00158 |
| MP | 40.1 ± 0.2 a | 42.9 ± 0.3 c | 42.9 ± 0.4 c | 42.2 ± 0.4 cd | 41.4 ± 0.4 d | ||||
| Fat, E% | HP | 36.6 ± 0.2 ab | 34.0 ± 0.3 cd | 34.8 ± 0.3 cf | 35.0 ± 0.3 f | 35.0 ± 0.3 bf | <.00001 | <.00001 | <.00001 |
| MP | 37.3 ± 0.2 a | 32.7 ± 0.3 e | 33.1 ± 0.3 eg | 33.7 ± 0.3 dg | 34.3 ± 0.3 cd | ||||
| Protein, g | HP | 93 ± 1 a | 93 ± 1 a | 92 ± 1 a | 88 ± 1 c | 85 ± 1 c | <.00001 | <.00001 | <.00001 |
| MP | 91 ± 1 a | 76 ± 1 b | 74 ± 1 b | 74 ± 1 b | 76 ± 1 b | ||||
| Carbohydrate, g | HP | 211 ± 2 a | 159 ± 2 bc | 161 ± 2 be | 155 ± 2 bc | 153 ± 2 c | <.00001 | <.00001 | <.00001 |
| MP | 210 ± 2 a | 175 ± 2 d | 174 ± 3 df | 169 ± 3 def | 168 ± 3 bef | ||||
| Fat, g | HP | 87 ± 1 a | 64 ± 1 bcd | 66 ± 1 b | 65 ± 1 bc | 64 ± 1 bcd | <.00001 | <.00001 | <.00001 |
| MP | 87 ± 1 a | 60 ± 1 e | 60 ± 1 e | 61 ± 1 de | 63 ± 1 cde | ||||
| Energy, kJ | HP | 8811 ± 90 a | 7052 ± 76 bc | 7126 ± 95 c | 6919 ± 99 bcd | 6788 ± 89 bd | .00506 | .00433 | <.00001 |
| MP | 8751 ± 91 a | 6829 ± 80 bd | 6771 ± 88 d | 6711 ± 91 bd | 6810 ± 105 bd | ||||
| Fibre, g | HP | 22.4 ± 0.3 ab | 22.5 ± 0.3 ab | 23.3 ± 0.4 ab | 21.8 ± 0.4 cd | 21.7 ± 0.4 d | .0056 | .05723 | <.00001 |
| MP | 21.8 ± 0.3 ab | 22.8 ± 0.3 a | 22.7 ± 0.3 ab | 22.1 ± 0.4 abc | 22.2 ± 0.4 bcd |
Abbreviations: HP, high protein diet; MP, moderate protein diet.
From four‐day dietary records. Unadjusted means ± SE. Changes in continuous secondary outcomes from 8 to 156 weeks analysed using analysis of covariance (ANCOVA) linear mixed model, including two‐way interaction between diet and time adjusted for baseline values (0 weeks), age and gender (fixed effects), and participant ID and intervention centre (random effects). For significant main effects and interactions, pairwise comparison are presented with superscript letters a‐g, where means with differing superscript letters are significantly different (P < .05, single step‐adjusted).
Regarding PA, there were no significant differences in total CPM (Figure S2, bottom), moderate‐to‐high PA, high PA or sedentary time (data not shown).
A significant positive association was found between % attendance at group counselling visits and % weight‐loss maintenance (r = 0.17, P < .0001).
The overall numbers of serious AEs (178 in total) were similar by diet (P = .27) and PA (P = .91) groups (Table S4).
Participants completing the 3‐year intervention were slightly older, had lower BMI and an overall healthier metabolic profile at baseline compared with those who withdrew (P < .0001; Table S5).
4. DISCUSSION
To our knowledge, PREVIEW is the first study to compare 3‐year T2D incidence on an ad libitum HP and low GI (lower GL) versus a MP and moderate GI (higher GL) weight‐maintenance diet in adults with overweight and prediabetes. The 3‐year incidence of T2D was very low (3.1%) and did not differ between the two diets or the four diet and PA combinations. However, fewer participants achieved normoglycaemia in HP than in MP during the weight‐maintenance phase.
The incidence of T2D was not different between groups, but was substantially lower than predicted from previous diabetes prevention studies (10.5%‐15.8%). 7 , 8 This may be because of the large and fast initial weight loss, which was still partially present after 3 years. Other possible reasons for the low incidence of T2D include selection bias in the recruitment process, increasing attrition, higher cases among the drop‐outs, or actually a true null effect.
For the recruitment process, we aimed to use the best possible methods, that is, the Finnish Diabetes Risk Score (FINDRISK) for prescreening and an OGTT for screening. It is possible that especially health‐interested volunteers contacted us, and we therefore missed a more vulnerable part of the population, which would then have resulted in selection bias. However, this is also the case for most other dietary or lifestyle intervention studies that recruit volunteers from the general public.
The attrition rate was higher than estimated. We needed 1298 participants to complete the 3‐year intervention and we achieved 962 (i.e. 74% of the target). Still, the incidence of T2D was similar in the centres with higher (≥30%) versus lower attrition rates (<30%), which suggests that the low incidence was indeed representative.
Undiagnosed cases among the drop‐outs can be an issue. We were, however, unable to approach drop‐outs in all intervention centres, despite continued efforts, and therefore these data are not available.
There was no beneficial effect of the HP, low GI diet, as we had hypothesized. Indeed, the number of participants with normoglycaemia was lower in this group. Therefore, it can be speculated that an HP, low carbohydrate (even with low GI) combination or an HP diet per se, may not be favourable in a population at risk of T2D. The mechanisms are unclear. Some epidemiological studies support that an HP diet may increase the risk of T2D 30 , 31 , while other studies have observed a reduced risk of T2D. 32 Adjusting for BMI or waist circumference often removed or reduced the associations. Also, the way protein was expressed (e.g. g per day or g per kg of body weight daily) greatly influenced the results. 32 Because of the tight interplay of adiposity, insulin sensitivity and an individual's response to protein, it is difficult to disentangle these components. However, an HP diet may increase insulin demand, and this may raise the burden on insulin secretion in vulnerable individuals, such as those with prediabetes. 33
The recorded dietary intake showed significant differences in the main targets, GI and protein content. However, differences were smaller than planned. This corresponds to previous findings (e.g. the DiOGenes study), 10 and indicates that it is difficult to adhere to diets with specific protein and GI targets. Compliance for protein intake, assessed by urinary biomarkers, showed differences up to 52 weeks, but MP had a mean protein intake of greater than 0.8 g/kg daily, which may be sufficiently high to promote body weight maintenance. 34 , 35 , 36 , 37 , 38 Post hoc analyses showed a difference in 3‐year weight‐loss maintenance between participants with a protein intake of <0.8 versus ≥0.8 g/kg daily, which confirmed a role of protein in weight‐loss maintenance. Adverse effects of a higher protein intake were not observed or reported, although fewer participants achieved normoglycaemia in HP than in MP at 3 years. Likewise, there were no adverse effects on kidney function as assessed after 1 year in an elderly group 26 or in colon cancer risk markers assessed in a subgroup. 39
As for GI, the differences were also smaller than planned (4.7 to 3.3 units from week 26 to 156 compared with a goal of ≥6). But it is more relevant to consider GL, which also takes total carbohydrate intake into account. The differences between HP and MP that we observed (16 to 13 units from week 26 to 156) apparently did not affect our outcomes.
We did not see any differences in PA between the HI and MI groups. The finding that fewer participants had normoglycaemia after 3 years in the HP‐HI group compared with the other three groups may, therefore, be solely by chance. Conversely, the differences in normoglycaemia between HP and MP seemed robust throughout the weight‐maintenance phase.
The use of a specific behaviour modification tool (PREMIT) for PREVIEW can be considered a strength. 20 Thus, a higher visit attendance was associated with greater weight‐loss maintenance after 3 years. Further, PREVIEW used an LED for the first 8 weeks, which resulted in a large, rapid weight loss (−11%). This degree of weight loss appeared to be highly motivating for the participants as reflected in higher intentions for healthy eating, PA and self‐efficacy, and a more positive perception about expected outcomes in those who had achieved target weight loss compared with those who had not. 40
The higher than expected attrition rate during the weight‐maintenance phase must be considered a weakness because it decreased the statistical power for our primary outcome. The statistical power also decreased because of the very low number of T2D cases, which (as stated above) was probably caused by the large initial weight loss. The partial reversal of most improvements from 8 weeks to the end is a concern, and it is possible that at 5 years there would be no benefit concerning a reduction in T2D incidence. Unfortunately, 5‐year data do not exist, but at 3 years the interventions were successful compared with the predicted incidence.
In conclusion, 3‐year incidence of T2D was lower than predicted and did not differ between diets, PA or the four combined groups. Maintaining the target intakes of protein and GI over 3 years appeared difficult. However, the overall protocol was highly successful in reducing the risk of conversion to T2D compared with estimates from the literature, and the combination of rapid weight loss, healthy eating and PA seemed to be effective in achieving this. As far as clinically relevant outcomes are concerned, this is of great importance.
CONFLICT OF INTEREST
AR has received honorariums from Novo Nordisk A/S, the International Sweeteners Association, Nordic Sugar and Unilever. PSV has received travel grants from the Cambridge Weight Plan, UK. IAM is member of the UK Government Scientific Advisory Committee on Nutrition, Treasurer of the Federation of European Nutrition Societies, Treasurer of the World Obesity Federation, member of the Mars Scientific Advisory Council, member of the Mars Europe Nutrition Advisory Board and Scientific Adviser to the Waltham Centre for Pet Nutrition. He is also a member of the Nestle Research Scientific Advisory Board and of the Novozymes Scientific Advisory Board. JB‐M is President and Director of the Glycemic Index Foundation, oversees a glycaemic index testing service at the University of Sydney and is a co‐author of books about diet and diabetes. SP was the Fonterra Chair in Human Nutrition and Principle Investigator for NZ National Science Challenge High Value Nutrition during the PREVIEW intervention. TML is advisor for the ‘Sense’ diet programme. JAM is President of IUNS. All other authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
The protocol for the PREVIEW adult intervention study was written by MF, TML and AR. MW‐P, IAM, JAM, SP, WS, GS and SH were involved in developing the study design. All authors contributed to the implementation of the experimental trial and contributed to analysis and interpretation of the data. AR drafted the manuscript. PSV and CR were responsible for the statistical analyses. All authors contributed to critical revision of the manuscript for important intellectual content. All authors agreed that the accuracy and integrity of the work has been appropriately investigated and resolved, and all approved the final version of the manuscript. The corresponding author had full access to the data and had final responsibility for the decision to submit for publication. The corresponding author attests that all listed authors meet authorship criteria, and that no others meeting the criteria have been omitted. AR and MF are the guarantors.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14219.
Supporting information
Figure S1 Supporting information.
Table S1 Supporting information.
ACKNOWLEDGEMENTS
The PREVIEW consortium would like to thank all study participants at every intervention centre for their time and commitment and all scientists, advisors and students for their dedication and contributions to the study. Specifically, we would like to thank Louise Dye (chairman of the Scientific Advisory Board, SAB), University of Leeds, UK, Richard Atkinson (Ethical Officer of the SAB), Virginia Commonwealth University, USA, and medical expert and consultant Stephen Colagiuri (University of Sydney, Australia). The PREVIEW project was designed by AR, JB‐M, MW‐P, MF, WS and Edith Feskens (Wageningen University, NL). Meyers Madhus A/S is acknowledged for providing training and producing the two cooking books. We gratefully thank the research staff from each centre. From UCPH: Laura Pastor‐Sanz, Grith Møller, Lone Vestergaard Nielsen, Kasper Nowak, Arne Astrup, Finn Sandø‐Pedersen, Morten Bo Johansen, Ulla Skovbæch Pedersen, Maria Roed Andersen, Marianne Juhl Hansen, Jane Jørgensen, Sofie Skov Frost and Lene Stevner. From HEL: Heikki Tikkanen, Saara Kettunen, Tiia Kunnas, Sanna Ritola, Laura Korpipää, Heini Hyvärinen, Karoliina Himanen, Tiina Pellinen, Elina Malkamäki, Heidi Jokinen, Pauliina Kokkonen, Liisi Korhonen, Jaana Valkeapää, Heli Pikkarainen, Martta Nieminen, Tuulia Ingman, Pihla Mäkinen and Sonja Toijonen. From UNOTT: Clare Randall, Nicky Gilbert, Shelley Archer, Sally Maitland, Melanie Marshall, Cheryl Percival, Jakki Pritchard, Laura Helm and Peter Mansell. From UNAV: Blanca Martinez de Morentin, Maria Hernandez Ruiz de Eguilaz, Salome Perez Diez, Veronica Ciaurriz, Angels Batlle and Maria Jose Cobo. From MU: Georgi Bogdanov, Pavlina Gateva, Rossica Metodieva and Galia Dobrevska. From SU: Nils Swindell, Jeff Stephens, Gareth Dunseath, Steve Luzio and Masoumeh Minou. From THL: Merja Tukiainen, Ira Greinert, Laura Karjalainen and Jukka Lauronen. From UNSYD: Fiona Atkinson, Michele Whittle, Jessica Burke, Kylie Simpson, Kimberley Way, Sally McClintock, Radhika Seimon, Shelly Keating, Kirsten Bell, Tania Markovic, Cathy Corry, Evalyn Eldering and Ian Caterson. From UOA: Jim Mann (University of Otago), Boyd Swinburn, Lindsay Plank, Nicholas Gant, Jon Woodhead, Anne‐Thea McGill, Katya Volkova, Madhavi Bollineni, Clarence Vivar, Kelly Storey, Niamh Brennan and Audrey Tay.
The EU framework programme 7 (FP7/2007–2013) grant agreement # 312057. National Health and Medical Research Council ‐ EU Collaborative Grant, AUS 8, ID 1067711. The Glycemic Index Foundation Australia through royalties to the University of Sydney. The NZ Health Research Council (14/191) and University of Auckland Faculty Research Development Fund. The Cambridge Weight Plan donated all products for the 8‐week LED period. The Danish Agriculture & Food Council. The Danish Meat and Research Institute. National Institute for Health Research Biomedical Research Centre (NIHR BRC) (UK). Biotechnology and Biological Sciences Research Council (BBSRC) (UK). Engineering and Physical Sciences Research Council (EPSRC) (UK). Nutritics (Dublin) donated all dietary analyses software used by UNOTT. Juho Vainio Foundation (FIN), Academy of Finland (grant numbers: 272376, 314383, 266286, 314135), Finnish Medical Foundation, Gyllenberg Foundation, Novo Nordisk Foundation, Finnish Diabetes Research Foundation, University of Helsinki, Government Research Funds for Helsinki University Hospital (FIN), Jenny and Antti Wihuri Foundation (FIN), Emil Aaltonen Foundation (FIN).
The funders of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report.
Raben A, Vestentoft PS, Brand‐Miller J, et al. The PREVIEW intervention study: Results from a 3‐year randomized 2 x 2 factorial multinational trial investigating the role of protein, glycaemic index and physical activity for prevention of type 2 diabetes. Diabetes Obes Metab. 2021;23:324–337. 10.1111/dom.14219
Funding information The Cambridge Weight Plan ©, Ltd, Northants (UK); Emil Aaltosen Säätiö; Engineering and Physical Sciences Research Council (EPSRC) (UK); EU Framework programme 7, Grant/Award Number: 312057; Finnish Diabetes Research Foundation; Government Research Funds for Helsinki University Hospital; Jenny ja Antti Wihurin Rahasto; Juho Vainio Foundation (FIN), Academy of Finland, Grant/Award Numbers: 272376, 314383, 266286, 314135; National Health and Medical Research Council ‐ EU collaborative grant, AUS 8, ID 1067711; National Institute for Health Research Biomedical Research Centre (NIHR BRC) (UK); Novo Nordisk Fonden; Nutritics (Dublin, IRE); Signe ja Ane Gyllenbergin Säätiö; Suomen Lääketieteen Säätiö; The Danish Agriculture & Food Council; The Danish Meat and Research Institute; The Glycemic Index Foundation Australia; The NZ Health Research Council (14/191) and University of Auckland Faculty Research Development Fund
DATA AVAILABILITY STATEMENT
Protocol, technical appendix, statistical codes, and datasets are available from the corresponding author (ara@nexs.ku.dk).
REFERENCES
- 1. WHO . Global report on diabetes, 2016. http://www.who.int/diabetes/global-report/en/. Accessed November 27, 2018.
- 2. Franz MJ, Boucher JL, Rutten‐Ramos S, VanWormer JJ. Lifestyle weight‐loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta‐analysis of randomized clinical trials. J Acad Nutr Diet. 2015;115:1447‐1463. [DOI] [PubMed] [Google Scholar]
- 3. Guess ND. Dietary interventions for the prevention of type 2 diabetes in high‐risk groups: current state of evidence and future research needs. Nutrients. 2018;10:1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lean MEJ, Leslie WS, Barnes AC, et al. Primary care‐led weight management for remission of type 2 diabetes (DiRECT): an open‐label, cluster‐randomised trial. Lancet. 2018;391:541‐551. [DOI] [PubMed] [Google Scholar]
- 5. Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care‐led weight‐management intervention for remission of type 2 diabetes: 2‐year results of the DiRECT open‐label, cluster‐randomised trial. Lancet Diabetes Endocrinol. 2019;7:344‐355. [DOI] [PubMed] [Google Scholar]
- 6. Walker KZ, O'Dea K, Gomez M, Girgis S, Colagiuri R. Diet and exercise in the prevention of diabetes. J Hum Nutr Diet. 2010;23:344‐352. [DOI] [PubMed] [Google Scholar]
- 7. Knowler WC, Barrett‐Connor E, Fowler SE, et al. Diabetes prevention program research group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tuomilehto J, Lindström J, Eriksson JG, et al. Finnish diabetes prevention study group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343‐1350. [DOI] [PubMed] [Google Scholar]
- 9. Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and diabetes study. Diabetes Care. 1997;20:537‐544. [DOI] [PubMed] [Google Scholar]
- 10. Larsen TM, Dalskov S‐M, van Baak M, et al. Diets with high or low protein content and glycemic index for weight‐loss maintenance. N Engl J Med. 2010;363:2102‐2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Livesey G, Taylor R, Livesey HF, et al. Dietary glycemic index and load and the risk of type 2 diabetes: a systematic review and updated meta‐analyses of prospective cohort studies. Nutrients. 2019;11:1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sayón‐Orea C, Razquin C, Bulló M, et al. Effect of a nutritional and behavioral intervention on energy‐reduced Mediterranean diet adherence among patients with metabolic syndrome: interim analysis of the PREDIMED‐plus randomized clinical trial. JAMA. 2019;322(15):1486‐1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martínez‐González MA, Fernandez‐Lazaro CI, Toledo E, et al. Carbohydrate quality changes and concurrent changes in cardiovascular risk factors: a longitudinal analysis in the PREDIMED‐plus randomized trial. J Clin Nutr. 2020;111(2):291‐306. 10.1093/ajcn/nqz298. [DOI] [PubMed] [Google Scholar]
- 14. Fogelholm M, Larsen T, Westerterp‐Plantenga M, et al. PREVIEW: prevention of diabetes through lifestyle intervention and population studies in Europe and around the world. Design, methods, and baseline participant description of an adult cohort enrolled into a three‐year randomised clinical trial. Nutrients. 2017;9:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dorenbos E, Drummen M, Rijks J, et al. PREVIEW: prevention of diabetes through lifestyle intervention in a multicentre study in Europe in children (10‐17 y). Design, methods, and baseline results. Diabetes Obes Metab. 2018;20:1096‐1101. [DOI] [PubMed] [Google Scholar]
- 16. Silventoinen K, Pankow J, Lindström J, Jousilahti P, Hu G, Tuomilehto J. The validity of the Finnish diabetes risk score for the prediction of the incidence of coronary heart disease and stroke, and total mortality. Eur J Cardiovasc Prev Rehabil. 2005;12:451‐458. [DOI] [PubMed] [Google Scholar]
- 17. American Diabetes Association . Standards of medical care in diabetes—2011. Diabetes Care. 2011;34:S11‐S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Christensen P, Larsen TM, Westerterp‐Plantenga M, et al. Men and women respond differently to rapid weight loss: metabolic outcomes of a multicentre intervention study after a low‐energy diet in 2500 overweight, individuals with pre‐diabetes (PREVIEW). Diabetes Obes Metab. 2018;20:2840‐2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. WHO . Global Recommendations on Physical Activity for Health. https://www.who.int/dietphysicalactivity/global-PA-recs-2010.pdf. Accessed May 28, 2019.
- 20. Kahlert D, Unyi‐Reicherz A, Stratton G, et al. PREVIEW behavior modification intervention toolbox (PREMIT): a study protocol for a psychological element of a multicenter project. Front Psychol. 2016;7:1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. WHO . Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia. http://www.who.int/diabetes/publications/diagnosis_diabetes2006/en/. Accessed July 19, 2017.
- 22. Mathews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412‐419. [DOI] [PubMed] [Google Scholar]
- 23. DeFronzo RA, Matsuda M. Reduced time points to calculate the composite index. Diabetes Care. 2010;33:e93. [DOI] [PubMed] [Google Scholar]
- 24. Bingham SA, Cummings JH. Urine nitrogen as an independent validatory measure of dietary intake: a study of nitrogen balance in individuals consuming their normal diet. Am J Clin Nutr. 1985;42:1276‐1289. [DOI] [PubMed] [Google Scholar]
- 25. Bingham SA. Urine nitrogen as a biomarker for the validation of dietary protein intake. J Nutr. 2003;133(Suppl 3(3)):921S‐924S. [DOI] [PubMed] [Google Scholar]
- 26. Møller G, Ritz C, Sluik D, et al. Higher protein intake is not associated with decreased kidney function in pre‐diabetic older adults following a one‐year intervention ‐ a PREVIEW sub‐study. Nutrients. 2018;10:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boyer WR, Wolff‐Hughes DL, Bassett DR, Churilla JR, Fitzhugh EC. Accelerometer‐derived Total activity counts, Bouted minutes of moderate to vigorous activity, and insulin resistance: NHANES 2003‐2006. Prev Chronic Dis. 2016;13:E146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsumoto M, Nishimura T. Mersenne Twister: a 623‐dimensionally equidistributed uniform pseudorandom number generator. ACM Trans Model Comput Simul. 1998;8(1):3‐30. [Google Scholar]
- 29. Core Team R. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. https://www.R-project.org/. [Google Scholar]
- 30. van Nielen M, Feskens EJ, Mensink M, et al. Dietary protein intake and incidence of type 2 diabetes in Europe: the EPIC‐InterAct case‐cohort study. Diabetes Care. 2014;37(7):1854‐1862. [DOI] [PubMed] [Google Scholar]
- 31. Malik VS, Li Y, Tobias DK, Pan A, Hu FB. Dietary protein intake and risk of type 2 diabetes in US men and women. Am J Epidemiol. 2016;183(8):715‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhao LG, Zhang QL, Liu XL, Qu H, Zheng JL, Xiang YB. Dietary protein intake and risk of type 2 diabetes: a dose‐response meta‐analysis of prospective studies. Eur J Nutr. 2019;58:1351‐1367. [DOI] [PubMed] [Google Scholar]
- 33. Sluik D, Berendsen A, Mikkilä V, et al. Protein intake and the incidence of pre‐diabetes and diabetes in four population‐based studies: the PREVIEW project. Am J Clin Nutr. 2019;109:1310‐1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Azzout‐Marniche D, Gaudichon C, Tome D. Dietary protein and blood glucose control. Curr Opin Clin Nutr Metab Care. 2014;17(4):349‐354. [DOI] [PubMed] [Google Scholar]
- 35. Soenen S, Bonomi AG, Lemmens SG, et al. Relatively high‐protein or 'low‐carb' energy‐restricted diets for body weight loss and body weight maintenance? Physiol Behav. 2012;107:374‐380. [DOI] [PubMed] [Google Scholar]
- 36. Soenen S, Martens EA, Hochstenbach‐Waelen A, Lemmens SG, Westerterp‐Plantenga MS. Normal protein intake is required for body weight loss and weight maintenance, and elevated protein intake for additional preservation of resting energy expenditure and fat free mass. J Nutr. 2013;143:591‐596. [DOI] [PubMed] [Google Scholar]
- 37. Drummen M, Tischmann L, Gatta‐Cherifi B, Adam T, Westerterp‐Plantenga M. Dietary protein and energy balance in relation to obesity and co‐morbidities. Front Endocrinol. 2018;9:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Westerterp‐Plantenga MS, Lejeune MP, Nijs I, van Ooijen M, Kovacs EM. High protein intake sustains weight maintenance after body weight loss in humans. Int J Obes Relat Metab Disord. 2004;28:57‐64. [DOI] [PubMed] [Google Scholar]
- 39. Møller G, Andersen JR, Jalo E, et al. The association of dietary animal and plant protein with putative risk markers of colorectal cancer in overweight pre‐diabetic individuals during a weight reducing programme: a PREVIEW sub‐study. Eur J Nutr. 2020;59(4):1517‐1527. [DOI] [PubMed] [Google Scholar]
- 40. Huttunen‐Lenz M, Hansen S, Christensen P, et al. PREVIEW study ‐ influence of a behavior modification intervention (PREMIT) in over 2,300 people with pre‐diabetes: intention, self‐efficacy and outcome expediencies during the early phase of a lifestyle intervention. Psychol Res Behav Manag. 2018;11:383‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Supporting information.
Table S1 Supporting information.
Data Availability Statement
Protocol, technical appendix, statistical codes, and datasets are available from the corresponding author (ara@nexs.ku.dk).


