Abstract
Background
Hepatocellular carcinoma (HCC) is one of the most common tumors. Transarterial chemoembolization (TACE) is the first choice of treatment for intermediate HCC and an important treatment option for advanced HCC. This retrospective study compared the prognosis between patients showing coagulative necrosis and patients showing liquefactive necrosis after the first TACE procedure.
Material/Methods
We divided 171 patients with Barcelona Clinic Liver Cancer (BCLC) Stage B or C HCC into 2 groups; a coagulative necrosis group (79 patients) and a liquefactive necrosis group (92 patients). The coagulative and liquefactive necroses were identified by computed tomography after the first TACE procedure. Kaplan-Meier analysis was used to identify the differences in the overall survival (OS) and progression-free survival (PFS) between the 2 groups, and the associated risk factors and safety of TACE were analyzed.
Results
The median OS durations were 23.27±1.40 months and 8.83±2.15 months (P=0.004) and the median PFS durations were 9.33±0.96 months and 3.70±0.44 months (P=0.002) in the coagulative necrosis and liquefactive necrosis groups, respectively. Intrahepatic in situ progression, new intrahepatic metastasis, and extrahepatic progression occurred significantly earlier in the liquefactive necrosis group (P<0.05). Univariate analysis and multivariate analyses showed liquefactive necrosis was the main risk factor for OS. There was no significant difference in the hepatic function impairment or post-embolism syndrome after TACE.
Conclusions
After the first TACE procedure, the patients with liquefactive necrosis experienced recurrence and metastasis earlier and had a worse prognosis. Therefore, these patients should be considered for earlier administration of targeted therapies or immunotherapies after TACE.
Keywords: Carcinoma, Hepatocellular; Chemoembolization, Therapeutic; Necrosis
Background
Hepatocellular carcinoma (HCC) is one of the most common tumors and globally is the 4th most common cause of cancer-related deaths [1]. The majority of patients with HCC are diagnosed at the intermediate or advanced stage of the disease, and only 30% to 40% of HCC patients can be diagnosed at an early stage and undergo radical procedures, including surgical resection, local ablation, and liver transplantation [2,3]. Despite these radical procedures, the recurrence rate within 5 years is as high as 70% [4]. Transarterial chemoembolization (TACE) is the first choice of treatment for patients with intermediate HCC, and an important treatment option for advanced HCC at the time of diagnosis [5,6].
TACE has been used for the clinical treatment of HCC for over 30 years [7]. According to the Barcelona Clinic Liver Cancer (BCLC) treatment recommendations, TACE is the first choice of treatment for BCLC Stage B, and several studies have confirmed that BCLC Stages B and C can benefit from TACE [8–10]. The TACE procedure is the injection of an iodized oil emulsion carrying chemotherapy drugs through the tumor’s blood-supply artery and the embolization of the blood vessels with granular embolic agents, including a gelatin sponge and microspheres. The strong cytotoxic effects of the chemotherapy drugs and severe ischemia kill the tumor cells [11]. Although the chosen embolic materials can vary, the majority of interventional physicians advocate the maximum degree of embolism to achieve maximum necrosis of the tumor. However, computed tomography (CT) imaging reveals that the manifestations of tumor necrosis after embolization are not uniform. Some lesions mainly manifest as coagulative necrosis, while others manifest as liquefactive necrosis. This study retrospectively evaluated the difference in the prognosis of the coagulative necrosis group and the liquefactive necrosis group after TACE in HCC patients receiving first-line treatment.
Material and Methods
Patients and Group Allocation
This was a retrospective study of patients diagnosed with HCC and their prognosis based on the type of necrosis after the first TACE treatment. From July 1, 2015 to July 1, 2018, 287 patients diagnosed with HCC (pathologically or clinically) were admitted to the Second Ward Area of the Interventional Oncology Department of Shandong Cancer Hospital. The inclusion criteria for this study were TACE as the initial local treatment, iodized oil as the main embolic agent, an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1, BCLC Stage B or Stage C, the patient requesting local treatment, Child-Pugh class A or class B, a maximum tumor diameter >5 cm, a clear boundary of the tumor and the presence of a measurable lesion, and significant necrosis after the first TACE procedure (>80% of the lesion). The exclusion criteria were a history of other local treatments or a combination of the first TACE procedure with other local treatments (ablation and radiotherapy), non-iodized oil as the main embolic agent, an ECOG performance status score of ≥2, BCLC Stage A or Stage D, Child-Pugh class C, the presence of a nonmeasurable lesion, and nonsignificant necrosis after the first TACE procedure. Based on these criteria, 116/287 patients were excluded. A total of 171 patients met the inclusion criteria, including 29 patients with BCLC Stage B and 142 patients with BCLC Stage C. The patients were followed up for at least 18 months, during which 149 patients died, 20 patients survived, and 2 patients were lost to follow-up (Figure 1).
Figure 1.
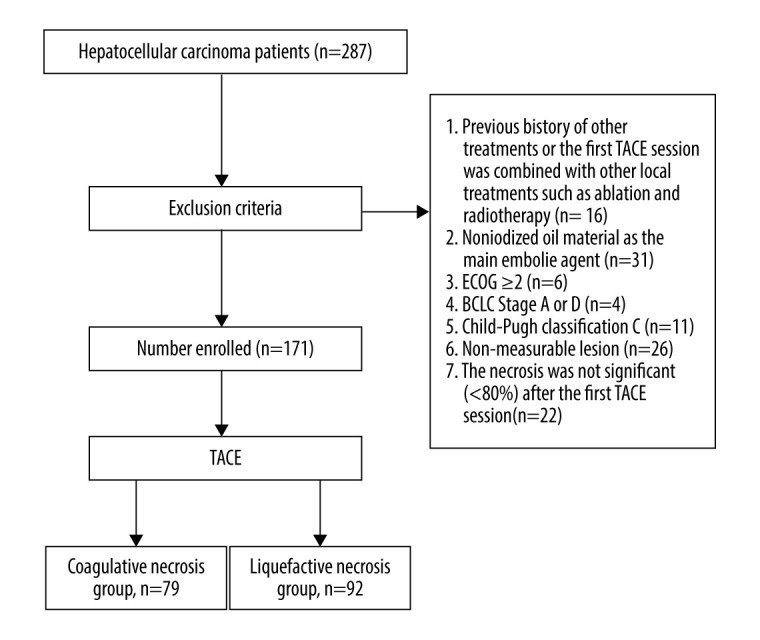
Flowchart for patient selection.
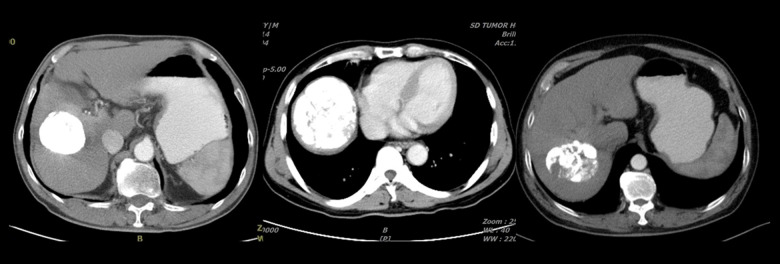
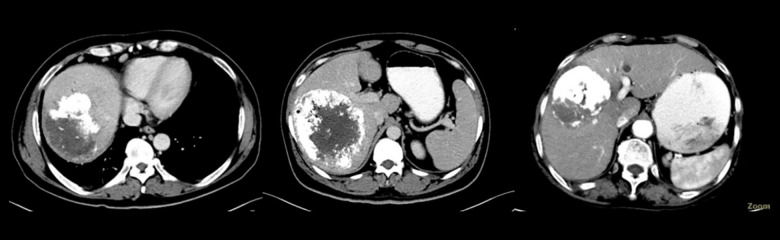
This study was based on the evaluation of the characteristics of lesion necrosis using computed tomography (CT) at 4 weeks to 6 weeks after the first TACE procedure. The patients were divided into 2 groups: a coagulative necrosis group (CN) and a liquefactive necrosis group (LN). For patients with multiple tumors, the outcome of the largest lesion was used for assigning the patients into the CN or LN group. The difference in the prognosis between the 2 groups was evaluated. Patients were assigned to the CN group if the CT of the plane of the tumor with the largest diameter showed a compact deposition of iodized oil without significant liquefaction or enhancement in each phase of the contrast-enhanced scan. CN was defined when the necrotic area showing the above features accounted for >70% of the total necrotic area in this plane [12]. Patients were assigned to the LN group if the CT of the plane of the tumor with the largest diameter showed no significant accumulation of the iodized oil, the lesions with LN had low density (<20 HU), were round or irregular, and seen without enhancement in each phase of the contrast-enhanced scan. If identification of the necrotic type was difficult, a comparison was made between the preoperative CT scans. LN was defined when the necrotic area showing the above-mentioned features accounted for >30% of the total necrotic area in this plane (Figures 2, 3) [13].
Figure 2.
Coagulative necrosis: a compact deposition of the iodized oil is seen; coagulative necrosis is seen in >70% of the total necrotic area in the plane of the largest tumor.
Figure 3.
Liquefactive necrosis: liquefactive necrosis is seen in >30% of the total necrotic area in the plane of the largest tumor.
Transarterial Chemoembolization Procedure and Treatment Strategy
The patients were positioned in a supine position. After routine sterilization and the spreading of sterile drapes, a local anesthetic was administered. According to the Seldinger technique, a vascular channel was established via a femoral artery puncture on 1 side. Under digital subtraction angiography, a 5-Fr catheter (Optitorque, Terumo, Tokyo, Japan or Angiographic Catheters, Cook, Bloomington, USA) was inserted to perform hepatic arteriography to identify the tumor’s blood-supply artery. A 2.7-Fr microcatheter (Progreat, Terumo, Tokyo, Japan) was inserted into the blood-supply artery, and an angiography confirmed the accurate position of the microcatheter. An emulsion containing epirubicin (Epirubicin Hydrochloride for Injection, Pfizer Pharmaceutical Co., Ltd., New York, USA), mitomycin (Mitomycin for Injection, Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, China), lobaplatin (Lobaplatin for Injection, Hainan Changan International Pharmaceutical Co., Ltd, Haikou, China), and iodized oil (Ethiodized Poppyseed Oil Injection, Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, China or Lipiodol, Guerbet, Paris, France) was injected. In addition, particulate embolic agents, including gelatin sponge particles (Gelatin Sponge Particle Embolic Agent, Hangzhou Alicon Pharmaceutical Technology Co., Ltd., Hangzhou, China) were injected until the blood flow slowed down or stagnated, observed as the absence of tumor staining on repeated imaging and considered the endpoint of the embolization. If there was a significant arteriovenous fistula, the embolization was adjusted. In addition to an iodized oil embolization, large-diameter gelatin particles or spring coils (Embolization Coil, Cook, Bloomington, USA) were used to embolize the fistula. The contrast embolization image and preoperative CT image were compared to ensure the embolization of all the tumor lesions in the liver. If the embolization was incomplete, the extrahepatic blood-supply vessels were searched and embolized.
TACE can be repeated after 1 month to 3 months based on the findings of the imaging, alpha-fetoprotein levels, and the patient’s status. Patients with stable reexamination results were continuously followed up until tumor progression. Other treatments, including targeted therapies and radiotherapy, were added to subsequent treatments according to the disease condition changes.
Follow-up
The enhanced CT of the upper abdomen, chest CT, alpha-fetoprotein levels, and hepatic function were reexamined 4 weeks to 6 weeks after the first TACE procedure. Other examinations included a brain CT every 3 months and a whole-body bone scan every 6 months to evaluate metastasis. The LightSpeed 16-slice spiral CT (GE Healthcare Life Sciences, Waukesha, WI, USA) was used and a high-quality scanning mode was applied. The parameters set were matrix 512×512, scanning cycle 0.8 seconds, layer thickness 5 mm, layer spacing 1.5 mm, tube voltage 120 kV, and tube current 150 mA. The target tumor lesions and all other tumor lesions were evaluated 1 month after the procedure, and the modified Response Evaluation Criteria in Solid Tumors were used as the evaluation criteria. A complete response was the disappearance of all the target lesions on the arterial-phase contrast-enhanced imaging; a partial response (PR) was ≥30% reduction in the total diameter of the target lesions on the arterial-phase contrast-enhanced imaging; stable disease was the reduction in diameter not reaching the level required to define a PR or an increase in diameter not reaching the level required to define progressive disease (PD); and PD was an increase in the total diameter of target lesions by ≥20% or the appearance of new lesions on the arterial-phase contrast-enhanced imaging.
Pain, nausea, vomiting, and fever were recorded after the first TACE procedure, and hepatic function was rechecked from day 3 to day 7 after the TACE treatment. Treatment-related toxic adverse effects were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) (version 4.0). The evaluated CTCAE ≥grade 2 (moderate or above) adverse events were alanine transaminase (ALT) elevation (>3 times to 5 times the upper limit of the normal level), aspartate transaminase (AST) elevation (>3 times to 5 times the upper limit of the normal level), total bilirubin elevation (>1.5 times to 3 times the upper limit of the normal level), nausea/vomiting (nausea: reduced food intake, dehydration, malnutrition or the requirement of an intravenous fluid infusion within 24 hours; vomiting: 3 episodes to 5 episodes within 24 hours), moderate abdominal pain, and fever (temperature >39°C). The doses of chemotherapeutic agents were reduced if CTCAE ≥grade 3 treatment-related adverse events occurred.
Study Endpoints
The time of the first TACE procedure was considered the start time and the death of the patient was considered to be the endpoint to record the overall survival (OS). Progression-free survival (PFS) was recorded until the time of detection of in situ intrahepatic progression, new intrahepatic metastasis, and extrahepatic progression, or the time of death or censoring; of these, the shortest time was recorded as the total PFS. Each patient was followed up for at least 18 months after the first TACE procedure. OS was considered the primary study endpoint, and PFS was considered the secondary study endpoint.
Statistical Analysis
For the analysis of baseline characteristics, categorical variables are presented as frequencies and percentages (n [%]), and continuous variables are presented as means and standard deviations. The chi-square test was used to compare categorical variables. Survival was estimated by the Kaplan-Meier method, and any differences in survival were evaluated using a stratified log rank test. Cox univariate analysis was performed to screen for possible independent variables (P<0.1). Linear regression was used to detect multicollinearity. After excluding the variables with significant collinearity, the independent variables were included in the Cox multivariate analysis. Logistic regression analysis was performed for factors affecting the types of necrosis. P values <0.05 were considered statistically significant. All statistical analyses were performed with SPSS v23.0 for Windows (IBM, Chicago, USA).
This study was approved by the Ethics Committee of Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences. All the participating patients signed informed consent before TACE treatment.
Results
Baseline Characteristics
In this study, 171/287 patients met the inclusion criteria and were analyzed retrospectively. Of these, 149 patients died and 22 patients were censored (20 alive and 2 lost to follow-up). Seventy-nine (46.2%) patients showed CN and 92 (53.8%) patients showed LN after the first TACE procedure. The patient characteristics and therapies for both groups are presented in Table 1. There were no significant differences in the gender, age, alpha-fetoprotein level, hepatitis B virus or hepatitis C virus, liver cirrhosis, Child-Pugh class, BCLC Stages, extrahepatic vessel invasion, other treatments, and survival status between the 2 groups (Table 1). In total, 25.7% of tumors were located in the left lobe (CN 24.1% vs LN 27.2%), 74.3% of tumors were located in the right lobe (CN 75.9% vs LN 72.8%), and 50.9% of tumors were massive HCCs with a diameter of >10 cm (CN 43.0% vs LN 57.6%). The patients with LN had a slightly higher incidence of vascular fistulae (CN 55.7% vs LN 69.6%) and portal vein tumor thrombosis (CN 17.7% vs LN 23.9%) than those with CN. There was no significant difference between the 2 groups for location, maximum diameter, pseudocapsule, portal vein tumor, thrombus, and arteriovenous fistulae (P>0.05) (Table 2).
Table 1.
Baseline characteristics of the study population.
| Coagulative necrosis | Liquefactive necrosis | P value | |
|---|---|---|---|
| No. of patients | 79 (46.2%) | 92 (53.8%) | |
| Gender | |||
| Men | 73 (92.4%) | 79 (85.9%) | 0.225 |
| Women | 6 (7.6%) | 13 (14.1%) | |
| Age | |||
| >60 years | 32 (40.5%) | 27 (29.3%) | 0.148 |
| <60 years | 47 (59.5%) | 65 (70.7%) | |
| Alpha-fetoprotein | |||
| >400 ng/ml | 47 (59.5%) | 50 (54.3%) | 0.538 |
| <400 ng/ml | 32 (40.5%) | 42 (45.7%) | |
| HBV or HCV | 69 (87.3%) | 75 (81.5%) | 0.401 |
| Liver cirrhosis | 38 (48.1%) | 45 (48.9%) | 1.00 |
| Child-Pugh | 5.76±0.91 | 5.72±0.94 | 0.767 |
| Class B (7–9) | 13 (16.5%) | 17 (18.5%) | 0.841 |
| Class A (5–6) | 66 (83.5%) | 75 (81.5%) | |
| Total bilirubin (umol/L) | 22.63±24.08 | 23.06±27.73 | 0.913 |
| Albumin (g/L) | 36.71±4.63 | 36.13±4.02 | 0.382 |
| Prothrombin time (s) | 11.62±1.37 | 11.42±1.31 | 0.342 |
| Ascites | 0.13±0.34 | 0.10±0.30 | 0.554 |
| Hepatic encephalopathy | 0 | 0 | - |
| Barcelona Clinic Liver Cancer | |||
| Stage B | 18 (22.8%) | 11 (12.0%) | 0.068 |
| Stage C | 61 (77.2%) | 81 (88.0%) | |
| Extrahepatic metastasis | 14 (17.7%) | 26 (28.3%) | 0.147 |
| Intrahepatic vessel invasion | 53 (67.1%) | 73 (79.3%) | 0.082 |
| TACE frequency | 8.68±3.97 | 5.63±3.59 | 0.000 |
| Other treatments | |||
| Systemic therapy | 8 (10.1%) | 13 (14.1%) | 0.513 |
| Local therapy | 9 (11.4%) | 8 (8.7%) | |
| Survival status | |||
| Dead | 70 (88.6%) | 79 (85.9%) | 0.652 |
| Censored | 9 (11.4%) | 13 (14.1%) | |
HBV – hepatitis B virus; HCV – hepatitis C virus; TACE – transarterial chemoembolization. P<0.05 was considered a statistically significant difference.
Table 2.
Baseline characteristics of the target lesions.
| Coagulative necrosis (n=79) | Liquefactive necrosis (n=92) | P value | |
|---|---|---|---|
| Location | |||
| Left lobe | 19 (24.1%) | 25 (27.2%) | 0.727 |
| Right lobe | 60 (75.9%) | 67 (72.8%) | |
| Maximum diameter | |||
| >10 cm | 34 (43.0%) | 53 (57.6%) | 0.067 |
| <10 cm | 45 (57.0%) | 39 (42.4%) | |
| Pseudocapsule | 54 (68.4%) | 60 (65.2%) | 0.745 |
| Portal vein tumor thrombus | 44 (55.7%) | 64 (69.6%) | 0.08 |
| Arteriovenous fistula | 14 (17.7%) | 22 (23.9%) | 0.352 |
P<0.05 was considered a statistically significant difference.
Survival Analysis and Prognostic Factors for Survival
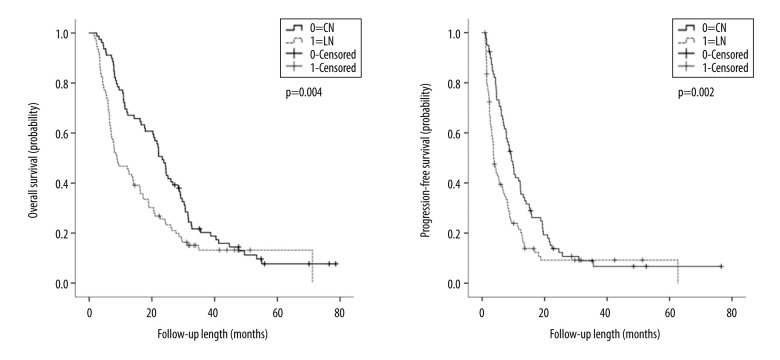
Of the 171 patients enrolled in the study, 70 (88.6%) patients from the CN group and 79 (85.9%) patients from the LN group died. One patient from each group was lost to follow-up. Overall, the median OS and PFS were 16.27±2.29 months and 6.73±0.86 months, respectively. The median OS (23.27±1.40 months vs 8.83±2.15 months, P=0.004) and PFS (9.33±0.96 months vs 3.70±0.44 months, P=0.002) were significantly longer in the CN group than in the LN group. The 1-year and 2-year OS rates in the CN and LN groups were 69.6% vs 46.7% and 48.1% vs 25.7%, respectively. The 1-year and 2-year PFS rates were 40.8% vs 21.4% and 13.8% vs 9.2% in the CN and LN groups, respectively (Figure 4).
Figure 4.
Kaplan-Meier estimates of overall survival (OS) and progression-free survival (PFS).
In both groups, the intrahepatic primary tumor site was the earliest to show disease progression. Intrahepatic in situ primary tumor progression, new intrahepatic metastasis, and extrahepatic progression occurred significantly earlier in the LN group than in the CN group (P<0.05) and extrahepatic metastasis occurred earlier than intrahepatic metastasis in the LN group (Table 3).
Table 3.
Comparison of the prognosis for the coagulative necrosis and liquefactive necrosis groups.
| Coagulative necrosis group (months) (median±SD) | Liquefactive necrosis group (months) (median±SD) | P value | |
|---|---|---|---|
| Overall survival | 23.27±1.40 | 8.83±2.15 | 0.004 |
| Progression-free survival | 9.33±0.96 | 3.70±0.44 | 0.002 |
| Intrahepatic in situ progression | 12.23±1.74 | 5.53±1.29 | 0.008 |
| New intrahepatic metastasis | 14.33±1.32 | 7.20±0.66 | 0.009 |
| Extrahepatic progression | 15.27±3.24 | 6.67±1.29 | 0.011 |
SD – standard deviation. P<0.05 was considered a statistically significant difference.
Prognostic Factor Analysis
Table 4 shows the results of the univariate analysis. LN (hazard ratio [HR] 0.623, 95% confidence interval [CI] 0.450–0.863; P=0.004), liver cirrhosis (HR 0.607, 95% CI 0.439–0.840; P=0.002), portal vein tumor thrombosis (HR 0.700, 95% CI 0.501–0.978; P=0.036), Child-Pugh class B (HR 0.194, 95% CI 0.126–0.300; P=0.000), and arteriovenous fistula(e) (HR 0.624, 95% CI, 0.421–0.925; P=0.018) were risk factors that affected the OS. Multivariate analysis showed that LN (HR 0.596, 95% CI, 0.426–0.834; P=0.003), liver cirrhosis (HR 0.664, 95% CI 0.471–0.936; P=0.019), Child-Pugh class B (HR 0.210, 95% CI 0.134–0.329; P=0.000), and the presence of a tumor(s) in the left lobe (HR 0.675, 95% CI 0.461–0.989; P=0.044) were independent risk factors that affected OS.
Table 4.
Univariate and multivariate analyses of the factors affecting overall survival.
| Parameter | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | |
| Necrosis type (liquefactive/coagulative) | 0.623 | 0.450–0.863 | 0.004 | 0.596 | 0.426–0.834 | 0.003 |
| Sex (Male/Female) | 1.354 | 0.823–2.226 | 0.231 | |||
| Age (>60 years/<60 years) | 1.232 | 0.879–1.727 | 0.226 | |||
| Hepatitis (present/absent) | 0.944 | 0.604–1.474 | 0.799 | |||
| Liver cirrhosis (present/absent) | 0.607 | 0.439–0.840 | 0.002 | 0.664 | 0.471–0.936 | 0.019 |
| Child-Pugh classification (B/A) | 0.194 | 0.126–0.300 | 0.000 | 0.210 | 0.134–0.329 | 0.000 |
| Barcelona Clinic Liver Cancer (stage C/stage B) | 0.780 | 0.510–1.193 | 0.250 | |||
| Maximum diameter (>10 cm/<10 cm) | 0.942 | 0.683–1.299 | 0.715 | |||
| Pseudocapsule (present/absent) | 0.994 | 0.702–1.408 | 0.972 | |||
| Alpha-fetoprotein (>400 ng/ml/<400 ng/ml) | 0.793 | 0.571–1.099 | 0.163 | |||
| Location (left lobe/right lobe) | 0.698 | 0.486–1.005 | 0.052 | 0.675 | 0.461–0.989 | 0.044 |
| Portal vein tumor thrombus (present/absent) | 0.700 | 0.501–0.978 | 0.036 | 0.910 | 0.629–1.315 | 0.615 |
| Arteriovenous fistula (present/absent) | 0.624 | 0.421–0.925 | 0.018 | 0.729 | 0.481–1.104 | 0.136 |
| Other treatments (present/absent) | 0.757 | 0.512–1.121 | 0.163 | |||
CI – confidence interval. P<0.05 was considered a statistically significant difference.
Treatment-related Toxicity
A liver injury after TACE was primarily evaluated based on the elevated levels of transaminases and bilirubin. There was no significant difference in the liver injuries between the CN and LN groups. The CTCAE ≥grade 2 elevations in liver injury markers observed were ALT elevation (CN 36.7% vs LN 29.3%), AST elevation (CN 35.4% vs LN 40.2%), and total bilirubin elevation (CN 20.3% vs LN 23.9%). Nausea, vomiting, abdominal pain, fever, and other symptoms of post-embolism syndrome were common after TACE. The frequency of post-embolism symptoms for CTCAE ≥grade 2 were nausea and vomiting (CN 13.9% vs LN 8.7%), abdominal pain (CN 24.1% vs LN 18.5%), and fever (CN 5.1% vs LN 12.0%). However, there were no significant differences between the 2 groups (Table 5). This suggests that there were no significant differences in the liver injuries and post-embolism syndrome between the 2 types of necrosis.
Table 5.
Treatment-related toxicities.
| Coagulative necrosis group (n=79) | Liquefactive necrosis group (n=92) | P value | |
|---|---|---|---|
| ALT elevation | 29 (36.7%) | 27 (29.3%) | 0.330 |
| AST elevation | 28 (35.4%) | 37 (40.2%) | 0.532 |
| TB elevation | 16 (20.3%) | 22 (23.9%) | 0.586 |
| Nausea/vomiting | 11 (13.9%) | 8 (8.7%) | 0.333 |
| Abdominal pain | 19 (24.1%) | 17 (18.5%) | 0.452 |
| Fever | 4 (5.1%) | 11 (12.0%) | 0.174 |
ALT – alanine transaminase; AST – aspartate transaminase; TB – total bilirubin. P value <0.05 was considered a statistically significant difference.
Analysis of Factors Affecting Necrosis Types
Logistic regression analysis was conducted for factors (tumor location, tumor diameter, pseudocapsule, portal vein tumor thrombus, liver cirrhosis, arteriovenous fistula, hepatitis, alpha-fetoprotein level, gender, age, and BCLC stages B and C) that influenced the type of necrosis (Table 6). Nonsignificant correlations were found between the factors and the necrosis types (P>0.05); furthermore, in this study, there was no significant correlation between each factor and the type of necrosis.
Table 6.
Logistic regression analysis for factors affecting the type of necrosis.
| r | OR | OR 95% CI | P value | |
|---|---|---|---|---|
| Tumor location | −0.137 | 0.872 | 0.418–1.819 | 0.715 |
| Tumor diameter | −0.653 | 0.521 | 0.268–1.010 | 0.54 |
| Pseudocapsule | 0.155 | 1.167 | 0.570–2.392 | 0.672 |
| Portal vein tumor thrombus | −0.622 | 0.141 | 0.235–1.228 | 0.141 |
| Liver cirrhosis | −0.006 | 0.994 | 0.513–1.927 | 0.987 |
| Arteriovenous fistula | −0.140 | 0.870 | 0.378–1.999 | 0.742 |
| Hepatitis | 0.305 | 1.357 | 0.518–3.554 | 0.534 |
| Alpha-fetoprotein | 0.436 | 1.547 | 0.784–3.053 | 0.209 |
| Gender | 1.069 | 2.913 | 0.939–9.029 | 0.064 |
| Age | 0.523 | 1.687 | 0.846–3.367 | 0.138 |
| Barcelona Clinic Liver Cancer staging | −0.171 | 0.842 | 0.296–2.396 | 0.748 |
CI – confidence interval; OR – odds ratio; r – correlation coefficient. P<0.05 was considered a statistically significant difference.
Discussion
HCC is a common tumor, with 841 000 new cases diagnosed every year; 46.71% of these involve Chinese patients [1]. The majority of patients with HCC present with intermediate or advanced stage disease at diagnosis, and TACE is typically the first choice of treatment [5,6]. TACE is also the first choice of treatment for BCLC Stage B and experts believe that patients with BCLC Stage C can benefit from TACE; therefore, it is widely used in clinical practice [14]. TACE aims for maximum tumor necrosis to reduce the tumor load and control tumor growth.
Necrosis is categorized into CN, LN, caseous necrosis, fat necrosis, and gangrenous necrosis. Tumor tissue necrosis mainly manifests as CN and LN. The necrosis seen in HCC tissue after TACE is primarily CN; however, LN is the main manifestation seen in some HCC patients [15,16]. Hsu et al reported 11 resection cases of HCC after transarterial embolization (TAE). Their study suggested that after TAE, CN occurred first and then LN. The formation of a large tumor and a long duration after embolization were more likely to form LN [17]. However, their study reported no single factor that affected the type of tumor necrosis.
The median OS was significantly different between the CN patients and the LN patients in the present study (23.27 months vs 8.83 months). A meta-analysis by Lencioni et al analyzed 101 articles from 1980 to 2013 with data from 10 108 patients treated with iodized oil TACE. They reported the median OS after TACE treatment in HCC patients to be 19.4 months [18]. In the present study, the OS was longer than 19.4 months in the CN group (23.27 months), while the OS in the LN group (8.83 months) was shorter, which further confirmed that the survival duration after the first TACE procedure was longer in patients with CN than in patients with LN. According to our study, in situ recurrence as well as intrahepatic and extrahepatic metastases occurred significantly earlier in the LN group than in the CN group. There are numerous possible mechanisms underlying this difference.
Acute ischemia and hypoxia, tumor cell necrosis, and neutrophil-dominated inflammatory cell infiltration occur in HCC tissues after TACE [19]. The common outcome after the necrosis of cells and tissues is dissolution and absorption. Lysosomal enzymes released by the necrotic cells and hydrolytic enzymes released by the infiltrating neutrophils liquefy and dissolve necrotic tissues into macromolecules, which are transported away from the local area via veins or lymphatic vessels. The unabsorbable fragments are phagocytized and removed by the macrophages, which enter the venous system. Necrotic tissues show coagulative changes that retain the general structure of the tumor. However, when there is an imbalance between the liquefaction and absorption, an excessive amount of neutrophil aggregate, releasing a large amount of hydrolase, and the tumor tissue is completely liquefied. Meanwhile, the edematous tissue compresses drainage veins and lymphatic vessels in the vascular region, resulting in inefficient drainage of the liquefied tissue and aggregation; this results in the formation of liquefactive lesions, which manifest as LN. A large amount of necrotic fluid is formed, accompanied by drainage obstruction. This in turn increases the pressure on the lumen, squeezing the surviving tumor cells around the tumor lesions and forcing them to shift and spread. Therefore, the prognosis is worse in the LN group. It is speculated that the reduction of neutrophils and severe edema can reduce the occurrence of liquefaction necrosis. However, this aspect requires further study.
TACE can cause some complex changes in tumors at a molecular level, including a dramatic increase in HIF-1α expression, PD-L1 overexpression, and hypersecretion of the macrophage colony-stimulating factor. The changes in cytokine levels promote the tumor-associated macrophages to present the M2 phenotype, promote the differentiation of immature T cells to Th17 cells, and increase the counts of T regulatory cells and neutrophils; this promotes the liquefaction of necrotic tumors, tumor recurrence, and metastasis [18–28].
Univariate and multivariate analyses in the present study indicated that LN and liver cirrhosis were risk factors affecting the OS. In addition, the multivariate analysis indicated that the presence of a tumor(s) in the left lobe was a risk factor affecting the OS; this could be related to the inadequate enhancement of the tumor(s) in the left lobe(s), resulting in reduced efficacy of the TACE [29,30].
In the present study, there was no significant difference in the hepatic function impairment or post-embolism syndrome between the 2 groups after TACE. The factors affecting the type of necrosis were not identified in this study. Therefore, further investigation is needed for information about screening patients, ensuring the occurrence of coagulative necrosis, and avoiding LN.
This study had some limitations. First, this was a single-center study, and the sample size was relatively small. Second, some cases were clinically diagnosed by CT without a pathological confirmation.
Conclusions
After the first TACE procedure, the prognosis of the patients can be predicted based on the type of tumor necrosis observed. This helps in the provision of well-informed treatment. Compared with the CN group, patients with LN experience recurrence and metastasis earlier and show a worse prognosis. Therefore, TACE should be combined at an earlier stage with other treatments, including targeted therapies and immunotherapies for these patients.
Footnotes
Department and Institution Where Work Was Done
Interventional Oncology Department of Shandong Cancer Hospital, Jinan, Shandong, China.
Conflict of Interest
None.
Source of support: Departmental sources
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Zhang G, Wu J, et al. Adjuvant therapy for hepatocellular carcinoma: current situation and prospect. Drug Discov Ther. 2013;7(4):137–43. [PubMed] [Google Scholar]
- 4.Shiraha H, Yamamoto K, Namba M. Human hepatocyte carcinogenesis (Review) Int J Oncol. 2013;42(4):1133–38. doi: 10.3892/ijo.2013.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin WH, Yeh SH, Yeh KH, et al. Hypoxia-activated cytotoxic agent tirapazamine enhances hepatic artery ligation-induced killing of liver tumor in HBx transgenic mice. Proc Natl Acad Sci USA. 2016;113(42):11937–42. doi: 10.1073/pnas.1613466113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raoul JL, Forner A, Bolondi L, et al. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28–36. doi: 10.1016/j.ctrv.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Bonnemain B, Guerbet M. [The history of Lipiodol (1901–1994) or How a medication may evolve with the times]. Rev Hist Pharm (Paris) 1995;42(305):159–70. [in French] [PubMed] [Google Scholar]
- 8.Chen RX, Gan YH, Ge NL, et al. A new prediction model for prognosis of patients with intermediate-stage HCC after conventional transarterial chemoembolization: An internally validated study. J Cancer. 2019;10(26):6535–42. doi: 10.7150/jca.34064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yen YH, Cheng YF, Wang JH, et al. Real world clinical practice in treating advanced hepatocellular carcinoma: When East meets West. PLoS One. 2020;15(3):e0230005. doi: 10.1371/journal.pone.0230005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golfieri R, Bargellini I, Spreafico C, et al. Patients with Barcelona Clinic Liver Cancer stages B and C hepatocellular carcinoma: Time for a subclassification. Liver Cancer. 2019;8(2):78–91. doi: 10.1159/000489791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52(2):762–73. doi: 10.1002/hep.23725. [DOI] [PubMed] [Google Scholar]
- 12.Boatta E, Corona M, Cannavale A, et al. Endovascular treatment of hepatocellular carcinoma with drug eluting microparticles (DC-Beads): CT evaluation of response to the treatment. Indian J Radiol Imaging. 2013;23(2):126–33. doi: 10.4103/0971-3026.116564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venook AP, Stagg RJ, Lewis BJ, et al. Chemoembolization for hepatocellular carcinoma. J Clin Oncol. 1990;8(6):1108–14. doi: 10.1200/JCO.1990.8.6.1108. [DOI] [PubMed] [Google Scholar]
- 14.Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE Study. Liver Int. 2015;35(9):2155–66. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adigun R, Basit H, Murray J. StatPearls. Treasure Island (FL): StatPearls Publishing; 2020. Necrosis, cell (liquefactive, coagulative, caseous, fat, fibrinoid, and gangrenous) [Google Scholar]
- 16.Higuchi T, Kikuchi M, Okazaki M. Hepatocellular carcinoma after transcatheter hepatic arterial embolization. A histopathologic study of 84 resected cases. Cancer. 1994;73(9):2259–67. doi: 10.1002/1097-0142(19940501)73:9<2259::aid-cncr2820730905>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 17.Hsu HC, Wei TC, Tsang YM, et al. Histologic assessment of resected hepatocellular carcinoma after transcatheter hepatic arterial embolization. Cancer. 1986;57(6):1184–91. doi: 10.1002/1097-0142(19860315)57:6<1184::aid-cncr2820570620>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Lencioni R, de Baere T, Soulen MC, et al. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology. 2016;64(1):106–16. doi: 10.1002/hep.28453. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida T, Kanai T, Monden M, et al. [Immunological and histological analyses of transarterial immuno-embolization therapy (TIE) in operable patients with hepatocellular carcinoma]. Gan To Kagaku Ryoho. 1994;21(13):2111–14. [in Japanese] [PubMed] [Google Scholar]
- 20.Nishida N, Kudo M. Immunological microenvironment of hepatocellular carcinoma and its clinical implication. Oncology. 2017;92(Suppl 1):40–49. doi: 10.1159/000451015. [DOI] [PubMed] [Google Scholar]
- 21.Xu W, Kwon JH, Moon YH, et al. Influence of preoperative transcatheter arterial chemoembolization on gene expression in the HIF-1α pathway in patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2014;140(9):1507–15. doi: 10.1007/s00432-014-1713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu K, Zhang X, Xu W, et al. Targeting the vasculature in hepatocellular carcinoma treatment: Starving versus normalizing blood supply. Clin Transl Gastroenterol. 2017;8(6):e98. doi: 10.1038/ctg.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budhu A, Forgues M, Ye QH, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10(2):99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Komiyama K. [Changes on hosts’ immunological response in HCC patients treated with transcatheter arterial embolization (TAE)]. Nihon Shokakibyo Gakkai Zasshi. 1992;89(10):2594–603. [in Japanese] [PubMed] [Google Scholar]
- 25.Zhou ZJ, Xin HY, Li J, et al. Intratumoral plasmacytoid dendritic cells as a poor prognostic factor for hepatocellular carcinoma following curative resection. Cancer Immunol Immunother. 2019;68(8):1223–33. doi: 10.1007/s00262-019-02355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim MJ, Jang JW, Oh BS, et al. Change in inflammatory cytokine profiles after transarterial chemotherapy in patients with hepatocellular carcinoma. Cytokine. 2013;64(2):516–22. doi: 10.1016/j.cyto.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 27.He M, Peng A, Huang XZ, et al. Peritumoral stromal neutrophils are essential for c-Met-elicited metastasis in human hepatocellular carcinoma. Oncoimmunology. 2016;5(10):e1219828. doi: 10.1080/2162402X.2016.1219828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cescon M, Bertuzzo VR, Ercolani G, et al. Liver transplantation for hepatocellular carcinoma: Role of inflammatory and immunological state on recurrence and prognosis. World J Gastroenterol. 2013;19(48):9174–82. doi: 10.3748/wjg.v19.i48.9174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miki I, Murata S, Uchiyama F, et al. Evaluation of the relationship between hepatocellular carcinoma location and transarterial chemoembolization efficacy. World J Gastroenterol. 2017;23(35):6437–47. doi: 10.3748/wjg.v23.i35.6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okubo H, Mogami M, Ozaki Y, et al. Liver function test by gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging with consideration of intrahepatic regional differences. Hepatogastroenterology. 2013;60(127):1547–51. doi: 10.5754/hge13291. [DOI] [PubMed] [Google Scholar]