Abstract
Background
Idiopathic normal pressure hydrocephalus (iNPH) is a reversible CNS disease characterized by disturbed cerebrospinal fluid (CSF) dynamics. Changes in the extracellular matrix (ECM) composition might be involved in the pathophysiology of iNPH. The aim of this study was to explore possible differences between lumbar and ventricular CSF concentrations of the ECM markers brevican and neurocan, matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinase-1 (TIMP-1) and their relation to clinical symptoms in iNPH patients before and after shunt surgery.
Methods
Paired lumbar and ventricular CSF was collected from 31 iNPH patients, before and four months after shunt surgery. CSF was analysed for concentrations of tryptic peptides originating from brevican and neurocan using a mass spectrometry-based panel, and for MMP-1, -2, -9, -10 and TIMP-1 using fluorescent or electrochemiluminescent immunoassays.
Results
Brevican and neurocan peptide levels were not influenced by CSF origin, but MMP-1, -2, -10 and TIMP-1 were increased (p ≤ 0.0005), and MMP-9 decreased (p ≤ 0.0003) in lumbar CSF compared with ventricular CSF. There was a general trend of ECM proteins to increase following shunt surgery. Ventricular TIMP-1 was inversely correlated with overall symptoms (rho = − 0.62, p < 0.0001). CSF concentrations of the majority of brevican and neurocan peptides were increased in iNPH patients with a history of cardiovascular disease (p ≤ 0.001, AUC = 0.84–0.94) compared with those without.
Conclusion
Levels of the CNS-specific proteins brevican and neurocan did not differ between the lumbar and ventricular CSF, whereas the increase of several CNS-unspecific MMPs and TIMP-1 in lumbar CSF suggests contribution from peripheral tissues. The increase of ECM proteins in CSF following shunt surgery could indicate disturbed ECM dynamics in iNPH that are restored by restitution of CSF dynamics.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12987-021-00256-1.
Keywords: Brevican, Idiopathic normal pressure hydrocephalus, Immunoassay, Mass spectrometry, Matrix metalloproteinase, Neurocan, Shunt surgery, Tissue inhibitor of metalloproteinases
Background
Idiopathic normal pressure hydrocephalus (iNPH) is the dominating form of hydrocephalus in adults, characterized by disturbed cerebrospinal fluid (CSF) dynamics with enlargement of the entire ventricular system, but no macroscopic obstruction to CSF flow [1]. The clinical features of iNPH include gait abnormality, problems with balance, urinary urgency and incontinence, and cognitive impairment [1, 2].
Symptoms of iNPH can be reversed by diverting CSF, usually by a ventriculo-peritoneal or ventriculo-atrial shunt, where CSF is diverted from the cerebral ventricular system into the peritoneal cavity or the right atrium of the heart, respectively [2]. Shunt surgery in iNPH is considered to be a definite and cost-effective medical treatment [3] with significant improvement in up to 80% of the patients [4]. Nevertheless, many iNPH patients remain undiagnosed and undertreated [5, 6]. Although iNPH is characterized by changes in CSF dynamics, the underlying disease mechanisms remain largely unknown. CSF markers can serve as an ideal tool for diagnostic and predictive purposes in iNPH. Changes in concentrations of CSF biomarkers reflecting a variety of pathological changes in iNPH patients have been previously reported. These include elevated neurofilament light (NFL) levels indicating damage to large myelinated axons [7–9] and increased glial fibrillary acidic protein (GFAP) levels implying astrogliosis [8, 10], while decreased levels of products derived from amyloid precursor protein (APP) might be explained by the reduced brain metabolism in the periventricular zone [9, 11]. Also, a reduced flow in the interstitial fluid space and impaired glymphatic circulation have been suggested [12, 13]. In addition, the water content in the brain extracellular space is markedly increased in iNPH patients [14], possibly leading to reduced ECM flow. Shunt surgery has been shown to induce biochemical changes in CSF, such as elevation of APP-derived products (sAPPα, sAPPβ and amyloid-β peptides) [9], albumin, tau and some monoamine metabolites [15]. Several preoperatively investigated CSF markers did not show any differences between patients that improved or did not improve after surgery, although high preoperative CSF NFL levels correlated with more favourable outcome after surgery [7, 8, 11]. There is still a great need for reliable markers for iNPH outcome prediction following shunt surgery. Identifying CSF-based markers with such an ability would aid in the selection of patients who could benefit from this procedure.
The differences in biochemical composition and dynamics between the two origins of CSF (lumbar and ventricular) are still largely unexplored, mainly because of ethical considerations regarding collection of ventricular CSF. As CSF travels from the ventricles to the spinal channel, there is a general progressive increase in cell count and total protein concentration in lumbar CSF with increasing distance from the brain [16, 17]. In addition, CSF absorption occurs mostly along cerebral convexities, either from the subarachnoid space to the venous system (across the arachnoid villi) or from the cerebral ventricular walls into the brain parenchyma (at the site of periventricular white matter) [18]. However, considerable portions of CSF may be absorbed into the cervical lymph nodes, nasal lymphatics or parenchymal capillaries of spinal cord [18–20]. It is generally accepted that the total protein content of lumbar CSF is 60% higher than that of ventricular CSF [16]. However, some proteins, e.g., tau, were reported to be higher in ventricular CSF than in lumbar CSF [15].
The appropriate assembly of brain extracellular matrix (ECM) is essential for maintaining a suitable environment for cellular functions. The two CNS-specific ECM proteoglycans, brevican and neurocan, are uniquely expressed in brain and spinal cord, and are, together with tenascins and hyaluronic acid the main modulators of brain plasticity by regulating cell migration and axonal growth [21]. Proteoglycans are the major substrates for several matrix metalloproteinases (MMPs), belonging to a 23-member family of zinc-dependent endopeptidases that are responsible for both physiological and pathological tissue remodelling [22]. The MMPs are divided into several subgroups based on substrate specificity and structural similarities, including collagenases (MMP-1), gelatinases (MMP-2 and -9) or stromelysins (MMP-10). Tissue inhibitor of metalloproteinases-1 (TIMP-1) is capable to inhibit the activity of most of the MMPs, although the efficacy of this inhibition varies for each MMP [23]. MMPs and TIMP-1 are non-CNS specific and play crucial roles in immune responses and their dysregulation occurs in many inflammatory and autoimmune diseases [22, 24].
The ECM composition is tightly regulated under physiological conditions. Disruption of the balance between the production of ECM proteins as well as their proteolytic processing (by MMPs) and processing inhibition (by TIMPs) may result in pathological conditions associated with uncontrolled ECM turnover [25]. The iNPH pathophysiology seems to affect the ECM composition in CSF [26, 27], possibly reflecting astroglial activation in the iNPH patients [8, 10], although additional research is highly warranted. In addition, increased water content in the brain extracellular space might be a main cause of reduced ECM flow in iNPH patients [14]. Thus, in iNPH, ECM metabolism is likely to be modified and its dynamics, including the ECM flow, might be improved after shunt surgery.
The primary aim of this study was to explore the biochemical ECM changes in iNPH at baseline and after shunt surgery in both lumbar and ventricular CSF, and in relation to clinical symptoms. In addition, lumbar and ventricular CSF were compared in the context of ECM protein concentrations. To our knowledge, this is the first study on iNPH patients evaluating ECM components in both lumbar and ventricular CSF, in relation to shunt surgery.
Methods
Patient characteristics
The study cohort consisted of 31 patients [22 male and 9 female, 74 ± 7 years (mean ± SD)] diagnosed with iNPH according to international criteria [1] at the Hydrocephalus Research Unit, Sahlgrenska University Hospital, Gothenburg, Sweden (see Table 1 for the patients’ clinical characteristics). A pathologic ventricular enlargement was estimated using Evans’ index value of > 0.3, defined as the ratio between the maximal width of the frontal horns and the maximal internal diameter of the skull [28]. Patients were examined clinically pre- and four months postoperatively by a neurologist, a physiotherapist and a neuropsychologist. Severity of symptoms was scored on the iNPH scale introduced by Hellström et al. [29], composed of items assessing four domains—gait, balance, cognition and continence—yielding a score of 0–100 in each domain, 0 referring to maximal symptoms and 100 to no symptoms, and a total score of 0–100 of the combined domain scores. Outcome was defined as the difference between the post- and the preoperative iNPH scale score, i.e., a positive value indicating improvement, the higher the positive value, the better the improvement. Patients were classified as either improved if the postoperative change in iNPH scale was ≥ 5 points, or unimproved (change of < 5 points) [29].
Table 1.
Clinical characteristics of 31 iNPH patients at baseline, and pre- and postoperative iNPH scale scores
| Clinical characteristics | Value |
|---|---|
| Age (mean ± SD) | 74 ± 7 |
| Gender (male/female) | 22/9 |
| Symptom duration in months (mean ± SD) | 44 ± 40 |
| Diabetes (%) | 42 |
| Hypertension (%) | 61 |
| Cardiovascular disease (%) | 29 |
| MMSE (median, IQ interval) | 27 (24–28) |
| Daily need of sleep in hours (mean ± SD) | 9 ± 3 |
| iNPH scale score | |
| Baseline (median, IQ interval) | 56 (50–74) |
| Postoperative (median, IQ interval) | 70 (53–81) |
MMSE mini mental state examination
Cardiovascular disease: atrial fibrillation, ischemic heart disease, congestive heart disease
All patients received a ventriculo-peritoneal shunt with a programmable valve (PS Medical Strata, Medtronic Inc., Goleta, CA, USA) with an anti-siphon device and a Rickham reservoir. Three patients were excluded from the postoperative clinical outcome assessment due to development of symptoms caused by another progressive disorder (multiple sclerosis, amyotrophic lateral sclerosis or disseminated malignant disease). All patients had working shunts and none had complications at the time for follow-up evaluation.
Preoperative lumbar CSF (12 mL) was sampled in the morning at the routine preoperative examination in the L3/L4 or L4/L5 interspace with the patient in a recumbent position. Preoperative ventricular CSF was sampled through the catheter introduced in the right lateral ventricle at the time for shunt surgery: the first 2 mL of CSF were discarded, and the next 8 mL were collected. Postoperative ventricular CSF (8 mL) was sampled at the four months’ postoperative re-examination via a puncture of the Rickham reservoir followed by sampling of postoperative lumbar CSF as described above. No infection or other complication occurred due to the post-operative Rickham reservoir puncture. All CSF samples were centrifuged, aliquoted and kept at -80 °C until analysed.
Longitudinal samples from the same patient were placed next to each other on the same plates to reduce the effect of possible inter-plate variation. Study samples were run in singlicate, but analytical variation was monitored using CSF pool quality samples.
Brevican/neurocan analysis
The brevican/neurocan panel has previously been described in detail [30]. Briefly, in total 20 isotope-labelled tryptic peptides (nine for brevican and eleven for neurocan), labelled with 13C and 15 N at the C-terminal arginine or lysine, were used as internal standard (IS) peptides (JPT Peptide Technologies, Berlin, Germany) (Additional file 1: Table S1). Twenty-five μL of the IS mixture was spiked into 100 μL CSF, followed by reduction, alkylation, trypsin digestion and subsequent sample clean-up.
Next, the dried samples were reconstituted in 100 µL 50 mM ammonium bicarbonate. Samples were then analysed by high-performance liquid chromatography (HPLC)—mass spectrometry (MS)-based method with a parallel reaction monitoring (PRM) approach, using a Vanquish UHPLC coupled to a high resolution Q Exactive hybrid quadrupole-orbitrap mass spectrometer, with electrospray ionization (Thermo Fisher Scientific), operated as previously described [30, 31]. Each sample (50 µL) was loaded onto a Hypersil Gold reversed phase HPLC C18 column (Thermo Fisher Scientific) operated at a flow rate of 300 µL/min. Mobile phases were A: 0.1% formic acid in deionized water (v/v) and B: 0.1% formic acid/84% acetonitrile in deionized water (v/v/v). The applied gradient was 0 to 40% B over 20 min.
TIMP-1 analysis
TIMP-1 concentrations in CSF were measured using a singleplex electrochemiluminescent immunoassay (K151JFC, MSD Multi-Array, Meso Scale Discovery, Rockville, MD, USA), following the manufacturer’s instructions.
MMP panel analysis
MMPs (MMP-1, -2, -9 and -10) concentrations in CSF were measured using a Milliplex MAP Human MMP magnetic bead panel (HMMP2MAG-55 K, EMD Millipore Corp., Billerica, MA, USA), following the manufacturer’s instructions.
Statistical analysis
Apart from MMP-2, none of the analytes were normally distributed based on the Kolmogorov–Smirnov test. Changes between the pre- and postoperative measurements in CSF of two different origins (lumbar and ventricular) were analysed using Friedman test with Dunn’s multiple comparisons. Mann–Whitney test or Kruskal–Wallis test with Dunn’s multiple comparisons were applied to investigate the differences between two or more independent groups of various clinical characteristics (clinical outcome after shunt surgery, cardiovascular disease, diabetes and/or hypertension), respectively. To evaluate if the patients were age- and gender-matched, Mann–Whitney test or Chi-square test were applied, respectively. Area under the curve (AUC) from receiver operating characteristic analysis was used as a measure of the effect size. Spearman correlation coefficients were calculated in correlation analyses. A p-value of less or equal to 0.002, after adjustment using Bonferroni correction for multiple testing (n = 23), was considered significant. Statistical analyses were performed using GraphPad Prism, version 7.03 (GraphPad Software, Inc., San Diego, CA, USA) and SPSS software, version 26 (IBM Corp., Armonk, NY, USA).
Validation
For the brevican/neurocan panel, four replicates each of two different CSF pool quality samples were evenly spread out throughout the 96-well plate. For the MMP panel and TIMP-1 assay, the two different CSF pool quality samples were placed at the beginning and end of the 96-well plate. The intra- and inter-assay variabilities were determined by calculating the coefficient of variation (CV) for these replicates. A more extensive validation of the in-house brevican/neurocan panel was performed previously [30]. Briefly, the majority of brevican and neurocan peptides in CSF showed analytical stability for up to five freeze–thaw cycles and storage stability under different conditions: −80 °C for one month, − 20 °C for one month, 5–8 °C for 24 h, 5–8 °C for 7 days, and room temperature for 24 h. In addition, the relative error of the back-calculated concentrations were below 20% for the majority of calibrators, except for N1195.
Data availability
The data supporting the findings in this study are available from the corresponding author, upon reasonable request.
Results
Lumbar vs. ventricular CSF
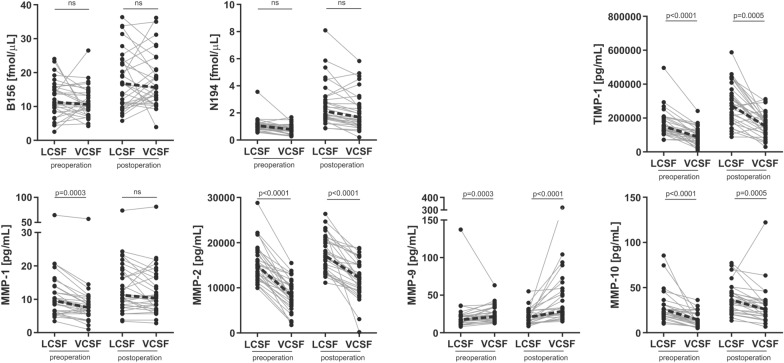
The brevican and neurocan peptide concentrations did not differ between the lumbar and ventricular CSF, neither pre- nor post- operatively (p = 0.003–0.99) (Fig. 1 and Additional file 1: Fig. S1). The concentrations of MMP-1, -2 and -10 were higher in lumbar CSF compared with ventricular CSF before shunt surgery (all three MMPs) and after (MMP-2 and -3) (p ≤ 0.0005), whereas MMP-9 showed the opposite pattern—its levels were elevated in ventricular CSF both pre- and postoperatively (p ≤ 0.0003) (Fig. 1). The lumbar CSF concentrations of TIMP-1 were higher compared with ventricular CSF in iNPH patients before and after shunt surgery (p ≤ 0.0005) (Fig. 1). The majority of CSF brevican and neurocan peptides levels correlated with each other, although this correlation was much stronger in ventricular CSF compared with lumbar CSF (Additional file 1: Fig. S2).
Fig. 1.
CSF concentrations of ECM proteins between lumbar and ventricular CSF before and after shunt surgery. The CNS-specific brevican and neurocan peptide concentrations did not differ between the lumbar and ventricular CSF (both pre- and post-operation), while the majority of MMPs and TIMP-1 concentrations were increased in lumbar CSF compared with ventricular CSF. MMP-9 showed opposite dynamics—its concentrations were higher in ventricular CSF compared with lumbar CSF. Friedman test with Dunn’s multiple comparisons and Bonferroni correction for multiple testing were applied to investigate the differences between the lumbar and ventricular CSF. A representative peptide of brevican (B156) and of neurocan (N194) has been displayed (see Additional file 1: Fig. S1 for all peptides). Dotted line represents change in median. LCSF lumbar CSF, VCSF ventricular CSF
Pre- vs. postoperative differences
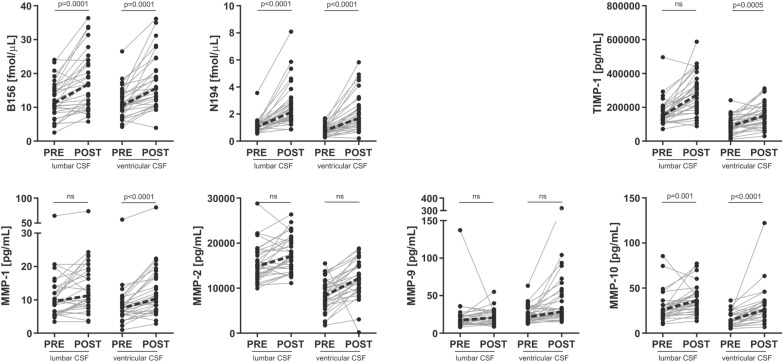
The tryptic peptide levels of brevican and neurocan increased in both lumbar and ventricular CSF following shunt surgery compared with preoperative levels (p ≤ 0.002) (Fig. 2 and Additional file 1: Fig. S3). However, based on the correlation matrices, there was no clear change in brevican/neurocan fragment pattern after shunt surgery (Additional file 1: Fig. S2). Ventricular CSF MMPs and TIMP-1 showed a postoperative increase, either significant or at trend level (p < 0.0001–0.04), whereas the postoperative changes in lumbar CSF were less clear (p = 0.001–0.57) (Fig. 2).
Fig. 2.
Lumbar and ventricular CSF concentrations of ECM proteins pre- and postoperatively in 31 iNPH patients. There was a general trend of ECM proteins to increase following shunt surgery, in both lumbar and ventricular CSF. Friedman test with Dunn’s multiple comparisons and Bonferroni correction for multiple testing were applied to investigate the differences before (``PRE``) and after (``POST``) shunt surgery. Dotted line represents change in median. A representative peptide of brevican (B156) and of neurocan (N194) has been displayed (see Additional file 1: Fig. S3 for all peptides)
Associations between ECM protein concentrations and clinical symptoms and postoperative outcome
At baseline, TIMP-1 was negatively correlated to total iNPH scale score (rho = − 0.62, p < 0.0001), otherwise there were no or weak correlations between the CSF ECM protein concentrations and the total iNPH scale score or the gait, balance, cognition or continence domain scores (Table 2). In addition, there was no association between the CSF ECM protein concentrations and a measure of ventriculomegaly (Evans’ index) (Table 2). None of the ECM proteins in preoperative lumbar or ventricular CSF showed any differences between patients that improved (n = 20) after surgery and those who remained unchanged (n = 8) (Fig. 3). Patients who suffered from cardiovascular comorbidity (atrial fibrillation, ischemic heart disease, congestive heart disease) had increased ventricular, but not lumbar, preoperative CSF concentrations of the majority of brevican and neurocan peptides compared with patients without (p ≤ 0.001, AUC = 0.84–0.94) (Fig. 4 and Additional file 1: Fig. S4).
Table 2.
Correlations of ECM protein levels with various clinical variables in preoperative lumbar and ventricular CSF
| B156 | N194 | MMP-1 | MMP-2 | MMP-9 | MMP-10 | TIMP-1 | |
|---|---|---|---|---|---|---|---|
| Lumbar | |||||||
| Age | − 0.15 | 0.37 | 0.41 | 0.48 | 0.15 | -0.27 | 0.45 |
| Symptom duration | − 0.09 | 0.07 | − 0.04 | 0.25 | − 0.22 | 0.27 | 0.31 |
| Gait domain | 0.26 | 0.09 | 0.01 | − 0.38 | 0.01 | − 0.25 | − 0.37 |
| Balance domain | 0.18 | 0.04 | 0.21 | − 0.25 | − 0.15 | − 0.08 | − 0.41 |
| Cognition domain | 0.62* | 0.41 | − 0.18 | − 0.41 | 0.15 | − 0.28 | − 0.39 |
| Continence domain | − 0.06 | 0.13 | 0.42 | 0.45 | 0.28 | − 0.16 | 0.31 |
| Total inph scale | 0.31 | 0.12 | 0.03 | − 0.39 | 0.22 | − 0.26 | − 0.43 |
| Daily need of sleep in hours | 0.08 | 0.07 | − 0.23 | − 0.16 | − 0.14 | − 0.29 | 0.06 |
| ΔiNPHscale score | − 0.05 | − 0.25 | − 0.32 | − 0.09 | 0.00 | 0.11 | 0.02 |
| Evans’ index | 0.03 | 0.08 | − 0.06 | 0.18 | 0.05 | − 0.12 | 0.29 |
| Ventricular | |||||||
| Age | − 0.05 | 0.15 | 0.31 | 0.11 | 0.00 | − 0.30 | 0.57* |
| Symptom duration | 0.37 | 0.52 | 0.02 | 0.45 | − 0.21 | 0.46 | 0.44 |
| Gait domain | 0.26 | 0.19 | 0.06 | − 0.16 | 0.14 | 0.06 | − 0.27 |
| Balance domain | 0.20 | 0.00 | 0.22 | − 0.11 | − 0.04 | 0.05 | − 0.15 |
| Cognition domain | 0.12 | 0.02 | − 0.36 | − 0.33 | 0.18 | 0.01 | − 0.54* |
| Continence domain | 0.13 | 0.12 | 0.40 | 0.14 | 0.18 | − 0.18 | 0.26 |
| Total inph scale | 0.06 | − 0.15 | − 0.32 | − 0.44 | 0.37 | − 0.11 | − 0.62* |
| Daily need of sleep in hours | 0.18 | 0.16 | − 0.09 | 0.16 | − 0.30 | 0.00 | 0.17 |
| ΔiNPHscale score | − 0.17 | − 0.29 | − 0.46 | − 0.07 | 0.05 | 0.08 | − 0.11 |
| Evans’ index | 0.37 | 0.50 | 0.03 | 0.39 | − 0.11 | 0.14 | 0.21 |
Spearman correlation coefficients were calculated in correlation analyses and rho values are displayed. Results with p < 0.002 are marked with an asterisk (*). A representative peptide of brevican (B156) and of neurocan (N194) has been included. The other brevican/neurocan peptides showed correlations very similar to the representative peptides. Gait, balance, cognition and continence domain refers to iNPH scale domains
ΔiNPH scale score refers to the postoperative change in total iNPH scale score
Fig. 3.
Preoperative ventricular CSF concentrations of ECM proteins in relation to clinical outcome after shunt surgery. None of the ECM proteins showed any differences between patients that improved after surgery and those who remained unchanged. Mann–Whitney test was applied to investigate the differences between the patients who did (improved; n = 20) or did not (non-improved; n = 8) improve following shunt surgery. INPH patients without any co-morbidities are displayed in the graphs as squares. The two groups were age- and gender-matched. A representative peptide of brevican (B156) and of neurocan (N194) has been displayed
Fig. 4.
Ventricular, preoperative CSF concentrations of ECM proteins in relation to cardiovascular disease comorbidity as a single risk factor. Brevican and neurocan peptide levels were increased in patients who suffered from CVD comorbidity. Mann–Whitney test was applied to investigate the differences between the patients who did (CVD + ; n = 9) and did not (CVD-; n = 22) suffer from cardiovascular disease (CVD). The two groups were age- and gender-matched. A representative peptide of brevican (B156) and of neurocan (N194) has been displayed (see Additional file 1: Fig. S3 for all peptides)
A history of diabetes or hypertension did not have any significant effect on the ECM protein concentrations in CSF (Supplementary figs. 5 and 6), but there was a gradual trend of brevican/neurocan levels to increase with the number of vascular risk factors present, although the change did not reach significant level (p = 0.06–0.25) (Additional file 1: Fig. S7). The two independent patients groups, e.g., improved vs unimproved patients, did not differ in age or gender.
Precision
The intra- and inter- assay variabilities of brevican and neurocan peptides, MMP-1, -2, -9 and TIMP-1 measurements in two different CSF pool quality samples were below or equal to 20%. Although the intra-assay variability of MMP-10 measurements were 3–4% in both CSF pool quality samples, the inter-assay variation was higher (27–28%). As the paired samples from the same individual were placed in the same plate, the high inter-assay variability does not affect within individual comparisons. The B834 and N1242 peptides were excluded from the analysis due to a large degree of both intra- and inter-assay variability (CV > 40%).
Discussion
To our knowledge, this is the first study on iNPH patients evaluating ECM components in both lumbar and ventricular CSF, in relation to shunt surgery. The sparse associations between CSF ECM protein levels and measures of the patients’ clinical symptomathology and the lack of associations with outcome following shunt surgery suggest that the concentration patterns of these ECM proteins are not primarily related to core pathophysiological mechanisms or reversibility in iNPH, and therefore not reliable markers for outcome prediction, but probably rather associated with more general or secondary processes. The negative correlations found between TIMP-1 and measures of cognitive and overall performance are interesting but require further exploration in larger sample sets or need to be repeated. The lack of associations between the CSF ECM proteins and degree of ventriculomegaly is consistent with the result from a previous study [32] where several other CSF biomarkers also did not correlate with the ventricular CSF volume.
This paper presents an interesting discrepancy between the concentrations of CNS-specific and -unspecific components of ECM in two different sources of CSF: lumbar and ventricular. The CNS-specific brevican and neurocan were not influenced by CSF origin, which indicates that their levels can be evaluated using the less invasive lumbar sampling for future research. However, the majority of MMPs as well as TIMP-1, that are widely expressed in a multiple range of tissues, were higher in lumbar CSF compared with ventricular CSF. The higher protein levels in lumbar CSF vs. ventricular CSF was previously observed for two other blood-derived proteins, IgG and albumin, and was explained either by their local synthesis in the subarachnoid space or increased endothelial cell permeability [16, 33, 34]. The elevated lumbar CSF levels of MMP-1, -2, -10 and TIMP-1 might also be caused by their potential release to the subarachnoid space from periphery along the spine. Interestingly, MMP-9 showed different, and difficult to explain, dynamics compared with the other MMPs. Its CSF levels were decreased in lumbar CSF compared with ventricular CSF. The CSF MMP-9 levels did not correlate with any of the other studied ECM proteins indicating that MMP-9 might measure different pathological changes in iNPH. In a previous study [27] we reported that MMP-9 did not strongly correlate neither to other MMPs nor to brain injury markers. In addition, MMP-9 seems to have different substrate preferences compared with other MMPs. It did not show any potential to degrade brevican, while MMP-1, -2 and -10 did [35, 36]. Neurocan also cannot be degraded by MMP-9, while MMP-2 showed this potential [35].
There is a general trend of ECM proteins to be elevated in CSF following shunt surgery. The comparatively high preoperative levels of brevican and neurocan in CSF was previously explained to possibly reflect chronic stages of iNPH, e.g., astroglial activation [26]. Interestingly, shunt surgery has also been shown to lead to an increase in the CSF levels of markers for other pathologies such as NFL, APP-derived products, p-tau and albumin [9]. Shunt surgery improves the clinical symptoms in the vast majority of patients with iNPH [4] and thus, the observed overall postoperative increase of ECM proteins in CSF reported here might be a result of improved or normalized CSF flow and reversed iNPH pathophysiology. Speculatively, ECM proteins might be more easily released from the brain tissue to the ventricles as a result of improved CSF dynamics with reduction of ICP pulsations [37], decompression of the brain, improved periventricular white matter extracellular flow and better clearance of protein fragments into the CSF [38–41]. Interestingly, the non-CNS-specific part of ECM (MMPs and TIMP-1) showed an increase following operation mostly in ventricular CSF, while these changes were only partially reflected in lumbar CSF. The peripheral origin of MMPs and TIMP-1 might contribute to the lack of a clear increase in lumbar CSF following the shunt surgery. However, their increased levels in ventricular CSF following shunt surgery might be a result of improved CSF dynamics, as these ECM proteins are also expressed in the brain, in addition to other tissues.
The selection of ECM proteins was based on previous results, where CSF brevican and neurocan concentrations were indicated to be involved in the pathological processes of iNPH [26]. Moreover, CSF MMP-1, -2, -9 and -10 exhibited different dynamics in iNPH [27] compared with patients following traumatic brain injury. To our knowledge, CSF TIMP-1 levels have not been reported before in regard to iNPH. The reason for analysis of various brevican and neurocan fragments, not total proteins, was to evaluate if their fragmentation patterns and consequently the concentration of proteolytic break-down products are affected by shunt surgery. The previous study [42] showed that various brevican fragments might reflect different pathological changes in the brain, e.g., the two peptides, B741 and B834, but not others, showed different dynamics and were significantly correlated to S100B. Thus, they might serve as potential indicators for astroglial pathophysiology. Although all the brevican and neurocan peptides correlated with each other in the iNPH group in the previous study [42], it would still be interesting to evaluate if the dynamics of individual peptides differ in longitudinal data of pre/post surgery. However, no discrepancy between the peptides was observed in the current study. Correlations between the brevican and neurocan peptides indicate that there is no evident dysregulated proteolytic processing of these proteins along their protein core in iNPH, and it is not affected by shunt surgery. Stronger correlations in ventricular CSF than in lumbar CSF might be explained by the CNS-specific origin of both proteoglycans and that ventricular CSF reflects more pure CNS pathophysiology. MMPs and TIMP-1 are abundantly present in various non-CNS tissues, which can explain that there is no clear difference in their correlations with each other between the two CSF origins.
We recently reported that CSF brevican and neurocan peptide concentrations were decreased in vascular dementia compared with healthy controls [30], and thus it was of interest to explore their levels in iNPH patients who showed vascular-based pathology. This study shows that ventricular CSF concentrations of brevican and neurocan peptides show a potential to distinguish iNPH patients who suffer from cardiovascular disease, and thus, might hypothetically serve as novel biomarker candidates to reflect cardiovascular disease related brain abnormalities in iNPH. We suggest that future studies explore this notion further and include associations between CSF brevican and neurocan peptides and measures of white matter hyperintensities in patients with iNPH. Moreover, since CSF brevican and neurocan peptide levels were decreased in vascular dementia compared with healthy controls [30], and thus might serve as potential diagnostic biomarkers, the even lower levels of these peptides in iNPH without cardiovascular comorbidities, might reflect further, different pathological processes specific to iNPH. However, as the two ECM proteins in iNPH have not been studied in comparison with healthy controls, it is difficult to evaluate the specificity of brevican/neurocan to diagnose iNPH when compared with its diagnostic ability for vascular dementia. Further studies should explore the association of the markers to radiological biomarkers of subcortical damage and/or white matter damage.
The strengths of this study include a well characterized patient group with detailed clinical assessment of symptoms and the collection of paired lumbar and ventricular CSF before and after shunt surgery for each patient.
However, there are some limitations to the study that should be acknowledged, including lack of healthy individuals in the cohort. Without a control group, it is not possible to fully evaluate the ECM changes involved in pathological processes specific for iNPH. In addition, the samples were not normalized to the total protein content. However, such normalization is not common practice for most immunoassays and not for targeted MS assays with internal standards. Another limitation is the low number of cases in the separate clinical groups as well as in the combined groups, which introduces a risk that associations or differences between groups remain undetected.
Conclusions
In conclusion, CSF ECM protein levels are not reliable markers for outcome prediction after shunt surgery in iNPH. However, this study demonstrated prominent biochemical changes in ECM between lumbar and ventricular CSF of iNPH patients induced by shunt surgery. While brevican and neurocan concentrations are not affected by CSF origin, the majority of MMPs and TIMP-1 are elevated in lumbar CSF compared with ventricular CSF, suggesting contribution from peripheral tissues to the lumbar CSF levels of these proteins.
Supplementary Information
Additional file 1. Additional figures and table.
Acknowledgements
Not applicable.
Abbreviations
- APP
Amyloid precursor protein
- AUC
Area under the curve
- CVD
Cardiovascular disease
- CSF
Cerebrospinal fluid
- CV
Coefficient of variation
- EVD
External ventricular drain
- ECM
Extracellular matrix
- iNPH
Idiopathic normal pressure hydrocephalus
- IS
Internal standard
- MMP
Matrix metalloproteinase
- MS
Mass spectrometry
- MMSE
Mini-Mental State Examination
- NFL
Neurofilament light
- PRM
Parallel reaction monitoring
- TIMP
Tissue inhibitor of metalloproteinases
Authors' contributions
KM, GB, UA, AJ, MT, HZ and KB created the concept of the study. AJ and MT recruited subjects and acquired data. KM carried out the experiments, statistical analysis and drafted the manuscript. GB, UA, EP, HZ, KB, AJ and MT contributed to the interpretation of the results and provided critical feedback of the manuscript. All authors read and approved the final manuscript.
Funding
Open access funding provided by University of Gothenburg. KM acknowledges fundings from Stiftelsen för Gamla Tjänarinnor, Herbert och Karin Jacobssons Stiftelse and Gun och Bertil Stohnes Stiftelse. HZ is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018-02532), the European Research Council (#681712), Swedish State Support for Clinical Research (#ALFGBG-720931), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer's Association (#ADSF-21–831376-C, #ADSF-21–831381-C and #ADSF-21–831377-C), the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2019-0228), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), and the UK Dementia Research Institute at UCL. KB is supported by the Swedish Research Council (#2017–00915), the Alzheimer Drug Discovery Foundation (ADDF), USA (#RDAPB-201809–2016615), the Swedish Alzheimer Foundation (#AF-742881), Hjärnfonden, Sweden (#FO2017-0243), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (#ALFGBG-715986), and European Union Joint Program for Neurodegenerative Disorders (JPND2019-466–236). MT acknowledges support from the ALF agreement (#ALFGBG 720121 and 872441), the Edit Jacobson foundation and the Rune and Ulla Amlöv foundation.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Helsinki Declaration and approved by the University of Gothenburg Ethics Committee (Dnr: 154-05 and 328-14). Eligible patients received oral and written information about the study and gave written informed consent of acceptance.
Consent for publication
Not applicable.
Competing interests
HZ has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics and CogRx, has given lectures in symposia sponsored by Fujirebio, Alzecure and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. KB has served as a consultant or at advisory boards for Abcam, Axon, Biogen, Lilly, MagQu, Novartis and Roche Diagnostics, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. KM, GB, EP, AJ, MT and UA declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PM. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57(3 Suppl):S4–16. (discussion ii-v). [DOI] [PubMed]
- 2.Ghosh S, Lippa C. Diagnosis and prognosis in idiopathic normal pressure hydrocephalus. Am J Alzheimers Dis Other Demen. 2014;29(7):583–589. doi: 10.1177/1533317514523485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tullberg M, Persson J, Petersen J, Hellstrom P, Wikkelso C, Lundgren-Nilsson A. Shunt surgery in idiopathic normal pressure hydrocephalus is cost-effective-a cost utility analysis. Acta Neurochir (Wien) 2018;160(3):509–518. doi: 10.1007/s00701-017-3394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klinge P, Hellstrom P, Tans J, Wikkelso C, European i NPHMSG. One-year outcome in the European multicentre study on iNPH. Acta Neurol Scand. 2012;126(3):145–53. [DOI] [PubMed]
- 5.Jaraj D, Rabiei K, Marlow T, Jensen C, Skoog I, Wikkelso C. Prevalence of idiopathic normal-pressure hydrocephalus. Neurology. 2014;82(16):1449–1454. doi: 10.1212/WNL.0000000000000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tisell M, Hoglund M, Wikkelso C. National and regional incidence of surgery for adult hydrocephalus in Sweden. Acta Neurol Scand. 2005;112(2):72–75. doi: 10.1111/j.1600-0404.2005.00451.x. [DOI] [PubMed] [Google Scholar]
- 7.Tullberg M, Blennow K, Mansson JE, Fredman P, Tisell M, Wikkelso C. Cerebrospinal fluid markers before and after shunting in patients with secondary and idiopathic normal pressure hydrocephalus. Cerebrospinal Fluid Res. 2008;5:9. doi: 10.1186/1743-8454-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tullberg M, Rosengren L, Blomsterwall E, Karlsson JE, Wikkelso C. CSF neurofilament and glial fibrillary acidic protein in normal pressure hydrocephalus. Neurology. 1998;50(4):1122–1127. doi: 10.1212/WNL.50.4.1122. [DOI] [PubMed] [Google Scholar]
- 9.Jeppsson A, Zetterberg H, Blennow K, Wikkelso C. Idiopathic normal-pressure hydrocephalus: pathophysiology and diagnosis by CSF biomarkers. Neurology. 2013;80(15):1385–1392. doi: 10.1212/WNL.0b013e31828c2fda. [DOI] [PubMed] [Google Scholar]
- 10.Albrechtsen M, Sorensen PS, Gjerris F, Bock E. High cerebrospinal fluid concentration of glial fibrillary acidic protein (GFAP) in patients with normal pressure hydrocephalus. J Neurol Sci. 1985;70(3):269–274. doi: 10.1016/0022-510X(85)90168-6. [DOI] [PubMed] [Google Scholar]
- 11.Jeppsson A, Holtta M, Zetterberg H, Blennow K, Wikkelso C, Tullberg M. Amyloid mis-metabolism in idiopathic normal pressure hydrocephalus. Fluids Barriers CNS. 2016;13(1):13. doi: 10.1186/s12987-016-0037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reeves BC, Karimy JK, Kundishora AJ, Mestre H, Cerci HM, Matouk C, et al. Glymphatic system impairment in Alzheimer's disease and idiopathic normal pressure hydrocephalus. Trends Mol Med. 2020;26(3):285–295. doi: 10.1016/j.molmed.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasan-Olive MM, Enger R, Hansson HA, Nagelhus EA, Eide PK. Loss of perivascular aquaporin-4 in idiopathic normal pressure hydrocephalus. Glia. 2019;67(1):91–100. doi: 10.1002/glia.23528. [DOI] [PubMed] [Google Scholar]
- 14.Aygok G, Marmarou A, Fatouros P, Young H. Brain tissue water content in patients with idiopathic normal pressure hydrocephalus. Acta Neurochir Suppl. 2006;96:348–351. doi: 10.1007/3-211-30714-1_72. [DOI] [PubMed] [Google Scholar]
- 15.Tullberg M, Blennow K, Mansson JE, Fredman P, Tisell M, Wikkelso C. Ventricular cerebrospinal fluid neurofilament protein levels decrease in parallel with white matter pathology after shunt surgery in normal pressure hydrocephalus. Eur J Neurol. 2007;14(3):248–254. doi: 10.1111/j.1468-1331.2006.01553.x. [DOI] [PubMed] [Google Scholar]
- 16.Rubalcava MA, Sotelo J. Differences between ventricular and lumbar cerebrospinal fluid in hydrocephalus secondary to cysticercosis. Neurosurgery. 1995;37(4):668–71 (discussion 71–2). [DOI] [PubMed]
- 17.Podkovik S, Kashyap S, Wiginton Jt, Kang C, Mo K, Goodrich M, et al. Comparison of Ventricular and Lumbar Cerebrospinal Fluid Composition. Cureus. 2020;12(7):e9315. [DOI] [PMC free article] [PubMed]
- 18.Miyajima M, Arai H. Evaluation of the production and absorption of cerebrospinal fluid. Neurol Med Chir (Tokyo) 2015;55(8):647–656. doi: 10.2176/nmc.ra.2015-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 2004;1(1):2. doi: 10.1186/1743-8454-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edsbagge M, Tisell M, Jacobsson L, Wikkelso C. Spinal CSF absorption in healthy individuals. Am J Physiol Regul Integr Comp Physiol. 2004;287(6):R1450–R1455. doi: 10.1152/ajpregu.00215.2004. [DOI] [PubMed] [Google Scholar]
- 21.Zimmermann DR, Dours-Zimmermann MT. Extracellular matrix of the central nervous system: from neglect to challenge. Histochem Cell Biol. 2008;130(4):635–653. doi: 10.1007/s00418-008-0485-9. [DOI] [PubMed] [Google Scholar]
- 22.Khokha R, Murthy A, Weiss A. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol. 2013;13(9):649–665. doi: 10.1038/nri3499. [DOI] [PubMed] [Google Scholar]
- 23.Arpino V, Brock M, Gill SE. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015;44–46:247–254. doi: 10.1016/j.matbio.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Gardner J, Ghorpade A. Tissue inhibitor of metalloproteinase (TIMP)-1: the TIMPed balance of matrix metalloproteinases in the central nervous system. J Neurosci Res. 2003;74(6):801–806. doi: 10.1002/jnr.10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803(1):55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minta K, Cullen NC, Nimer FA, Thelin EP, Piehl F, Clarin M, et al. Dynamics of extracellular matrix proteins in cerebrospinal fluid and serum and their relation to clinical outcome in human traumatic brain injury. Clin Chem Lab Med. 2019;57(10):1565–1573. doi: 10.1515/cclm-2019-0034. [DOI] [PubMed] [Google Scholar]
- 27.Minta K, Brinkmalm G, Al Nimer F, Thelin EP, Piehl F, Tullberg M, et al. Dynamics of cerebrospinal fluid levels of matrix metalloproteinases in human traumatic brain injury. Sci Rep. 2020;10(1):18075. doi: 10.1038/s41598-020-75233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Synek V, Reuben JR, Du Boulay GH. Comparing Evans' index and computerized axial tomography in assessing relationship of ventricular size to brain size. Neurology. 1976;26(3):231–233. doi: 10.1212/WNL.26.3.231. [DOI] [PubMed] [Google Scholar]
- 29.Hellstrom P, Klinge P, Tans J, Wikkelso C. A new scale for assessment of severity and outcome in iNPH. Acta Neurol Scand. 2012;126(4):229–237. doi: 10.1111/j.1600-0404.2012.01677.x. [DOI] [PubMed] [Google Scholar]
- 30.Minta K, Brinkmalm G, Portelius E, Johansson P, Svensson J, Kettunen P, et al. Brevican and neurocan peptides as potential cerebrospinal fluid biomarkers for differentiation between vascular dementia and Alzheimer's disease. J Alzheimers Dis. 2021 (in press). [DOI] [PubMed]
- 31.Brinkmalm G, Sjodin S, Simonsen AH, Hasselbalch SG, Zetterberg H, Brinkmalm A, et al. A parallel reaction monitoring mass spectrometric method for analysis of potential CSF biomarkers for Alzheimer's Disease. Proteomics Clin Appl. 2018;12:1. doi: 10.1002/prca.201700131. [DOI] [PubMed] [Google Scholar]
- 32.Edsbagge M, Andreasson U, Ambarki K, Wikkelso C, Eklund A, Blennow K, et al. Alzheimer's disease-associated cerebrospinal fluid (CSF) Biomarkers do not Correlate with CSF Volumes or CSF Production Rate. J Alzheimers Dis. 2017;58(3):821–828. doi: 10.3233/JAD-161257. [DOI] [PubMed] [Google Scholar]
- 33.Kaschka WP, Theilkaes L, Eickhoff K, Skvaril F. Disproportionate elevation of the immunoglobulin G1 concentration in cerebrospinal fluids of patients with multiple sclerosis. Infect Immun. 1979;26(3):933–941. doi: 10.1128/IAI.26.3.933-941.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandberg-Wollheim M, Zettervall O, Muller R. In vitro synthesis of IgG by cells from the cerebrospinal fluid in a patient with multiple sclerosis. Clin Exp Immunol. 1969;4(4):401–405. [PMC free article] [PubMed] [Google Scholar]
- 35.Muir EM, Adcock KH, Morgenstern DA, Clayton R, von Stillfried N, Rhodes K, et al. Matrix metalloproteases and their inhibitors are produced by overlapping populations of activated astrocytes. Brain Res Mol Brain Res. 2002;100(1–2):103–117. doi: 10.1016/S0169-328X(02)00132-8. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura H, Fujii Y, Inoki I, Sugimoto K, Tanzawa K, Matsuki H, et al. Brevican is degraded by matrix metalloproteinases and aggrecanase-1 (ADAMTS4) at different sites. J Biol Chem. 2000;275(49):38885–38890. doi: 10.1074/jbc.M003875200. [DOI] [PubMed] [Google Scholar]
- 37.Qvarlander S, Lundkvist B, Koskinen LO, Malm J, Eklund A. Pulsatility in CSF dynamics: pathophysiology of idiopathic normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 2013;84(7):735–741. doi: 10.1136/jnnp-2012-302924. [DOI] [PubMed] [Google Scholar]
- 38.Demura K, Mase M, Miyati T, Osawa T, Hattori M, Kasai H, et al. Changes of fractional anisotropy and apparent diffusion coefficient in patients with idiopathic normal pressure hydrocephalus. Acta Neurochir Suppl. 2012;113:29–32. doi: 10.1007/978-3-7091-0923-6_6. [DOI] [PubMed] [Google Scholar]
- 39.Tullberg M, Ziegelitz D, Ribbelin S, Ekholm S. White matter diffusion is higher in Binswanger disease than in idiopathic normal pressure hydrocephalus. Acta Neurol Scand. 2009;120(4):226–234. doi: 10.1111/j.1600-0404.2009.01165.x. [DOI] [PubMed] [Google Scholar]
- 40.Reiss-Zimmermann M, Scheel M, Dengl M, Preuss M, Fritzsch D, Hoffmann KT. The influence of lumbar spinal drainage on diffusion parameters in patients with suspected normal pressure hydrocephalus using 3T MRI. Acta Radiol. 2014;55(5):622–630. doi: 10.1177/0284185113502334. [DOI] [PubMed] [Google Scholar]
- 41.Graff-Radford NR. Alzheimer CSF biomarkers may be misleading in normal-pressure hydrocephalus. Neurology. 2014;83(17):1573–1575. doi: 10.1212/WNL.0000000000000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minta K, Brinkmalm G, Thelin EP, Al Nimer F, Piehl F, Tullberg M, et al. Cerebrospinal fluid brevican and neurocan fragment patterns in human traumatic brain injury. Clin Chim Acta. 2021;512:74–83. doi: 10.1016/j.cca.2020.11.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Additional figures and table.
Data Availability Statement
The data supporting the findings in this study are available from the corresponding author, upon reasonable request.
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.