Abstract
Introduction
The ketogenic diet (KD) is a high-fat, low-carbohydrate, and moderate-protein diet that has shown benefit as a treatment in neurologic disorders and may serve as a therapeutic option in individuals with psychiatric disorders.
Methods
A search was conducted using EBSCOhost and PubMed databases for studies relating to ketogenic or low-carbohydrate diets and psychiatric disorders.
Results
A total of 32 experimental or observational studies were identified by initial search strategies, 14 of which met the criteria to be included in this analysis. Although specific diet formulations varied somewhat between studies, they all generally examined low-carbohydrate dietary intake with the goal of producing a ketotic state. The studies included in this review indicated the KD was beneficial in reducing symptoms associated with various psychiatric disorders.
Discussion
This review summarizes the available evidence regarding the efficacy of the ketogenic diet in psychiatric disease states. Data from the studies analyzed demonstrated a positive response in individuals who were able to remain on the diet, regardless of the disease state. However, there is a need for more data to clearly define the specific benefits the KD may provide.
Keywords: ketogenic diet, low carbohydrate diet, psychiatric disorder
Introduction
The 2015-2020 Dietary Guidelines for Americans recommends, based on percentage of calories, a diet that consists of 20% to 25% fat, 45% to 65% carbohydrates, and 10% to 35% protein.1 In contrast, the ketogenic diet (KD) is a high-fat, low-carbohydrate diet, with the protein component remaining the same. By restricting carbohydrates, lipolysis is activated, creating ketones to use as the body's primary energy source rather than glucose. The original KD consists of approximately 80% fat from long-chain fatty acids, which are found in animals or plants (eg, dairy products, avocados, vegetable oils, nuts, fatty fish), 15% protein, and 5% carbohydrates. To date, several variations of the original KD have been introduced, such as lowering the caloric intake from fat to 60% to 70%, switching the fat to medium-chain triglycerides, and increasing the carbohydrates to 10% to 20% while placing no restrictions on the amount of fat or protein.2,3 Specific examples include the modified Atkins diet (MAD; 15% carbohydrates with unlimited amounts of protein and fat) and the “John Radcliffe Diet” (71% of calories coming from a combination of medium-chain, long-chain, and saturated fats, 19% from carbohydrates, and 10% from protein).4 Regardless of the diet plan, the intent is to switch the body's fuel source from glucose to fat via the production of ketones, a process known as ketosis. As a result, the energy balance in the brain is significantly altered. It is hypothesized that ketone bodies, such as β-hydroxybutyrate (BHB), are more efficient in producing energy per gram of oxygen compared with glucose.5
Several theories for using the KD to treat psychiatric medical conditions have been proposed. It is believed that the KD alters the γ-aminobutyric acid (GABA) to glutamate ratio in the brain, favoring GABA. Hypothetically, this increase in GABA may help compensate for the imbalanced GABA levels of a person with schizophrenia, consequently alleviating symptoms such as hallucinations and delusions.6 Ketogenic diets are also thought to decrease reactive oxygen species and increase adenosine triphosphate and phosphocreatine levels, thus boosting metabolic efficiency, which may provide benefit for individuals taking medications with a higher risk of weight gain. This may reduce inflammation in the brain, which may lead to symptomatic relief in multiple disease states, including Alzheimer disease.7 Another theory involves ketosis limiting apoptosis and neuronal excitability, which may explain the theory behind successful reports of the KD in individuals with epilepsy.8,9 The metabolic changes associated with seizure reduction can also be applied to potential efficacy in patients with autism spectrum disorder (ASD) because of positive changes in the gut microbiome.10 In bipolar disorder, acidic plasma is thought to stabilize mood by reducing intracellular sodium and calcium.11 By inducing relative hypoglycemia in patients with narcolepsy, the KD increases activation of orexin-containing neurons to improve daytime sleepiness.12 The purpose of this systematic review is to examine the clinical impact of the KD on various psychiatric disease states. The reviewed studies provide supporting evidence for the potential benefit of using the KD in the treatment of Alzheimer disease, anorexia nervosa, ASD, bipolar disorder, MDD, narcolepsy, and schizophrenia.
Methods
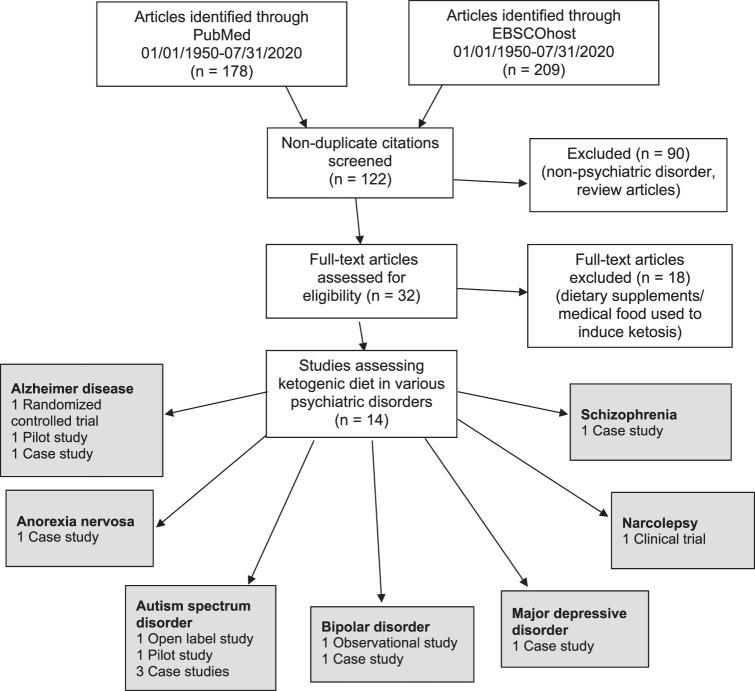
A search was conducted using EBSCOhost and PubMed databases for studies published through July 2020, with the following keywords: ketogenic diet, low carbohydrate diet, and psychiatric disorder. Initial search results were limited to studies with abstracts published in the English language that were conducted in human participants. Duplicate citations were removed during article screening, and references of pertinent articles were reviewed for the purpose of gathering additional references. Studies without abstracts, literature reviews, and letters to the editor describing case reports with limited details were excluded. Studies describing interventions in nonpsychiatric disorders (eg, epilepsy) were excluded. Studies that did not discuss dietary intake consisting of a low-carbohydrate or ketogenic diet (generally defined as at least 60%-80% of total daily calories from dietary fat with reduced consumption of carbohydrates), and studies that solely used dietary supplements or medical food interventions to produce ketosis were excluded. The Figure highlights the literature review process. The authors summarized the studies by the formulation of the KD listed (if provided) and the efficacy associated with a KD intervention.
FIGURE.
Systematic review process
Results
The initial search produced 178 citations in PubMed and 209 in EBSCOhost. Duplicates were removed, and 90 articles were excluded. After reviewing the abstracts, 32 full-text publications were retrieved. Studies that did not describe a low-carbohydrate diet or KD intervention in psychiatric disorders were excluded, which resulted in 14 experimental or observational studies. These studies examined the KD in psychiatric disorders, specifically Alzheimer disease, anorexia nervosa, ASD, bipolar disorder, MDD, narcolepsy, and schizophrenia. A summary of the results is presented in the Table.
TABLE.
Impact of the ketogenic diet (KD) in the treatment of various psychiatric disorders
|
Source |
Study Characteristics |
Intervention |
Outcomes |
|
Alzheimer Disease | |||
| Morrill and Gibas13 (2019) | Study design, duration: case report, 10 wk Setting: OP N = 1 | Low-carb, high-fat diet to produce sustained plasma ketones between 0.5 and 2 mg/dL | MoCA score increased from 21 to 28 Biometric changes: HOMA-IR decreased by 75% from 13.9 to 3.48 TG decreased by 50% from 170 to 85 mg/dL VLDL decreased by 50% from 34 to 17 mg/dL HbA1c decreased from 5.7% to 4.9% |
| Taylor et al14 (2018) | Study design, duration: pilot study, 31 mo Setting: OP N = 15 | KD, targeted macronutrient composition (70% fat), including MCT (10% total fat), 20% protein, and restriction of carbs (<10%); diet ratio of 1:1 (lipid to nonlipid) | 60% Reported some ketosis Serum β-hydroxybutyrate levels (mmol/L): Baseline: 0.11 1 mo: 0.52 2 mo: 0.34 3 mo: 0.31 (P < .001) Returned to 0.12 in 1-mo washout period MMSE (P < .05) and ADAS-Cog (P < .02) scores improved |
| Ota et al15 (2019) | Study design, duration: RCT, 12 wk Setting: OP N = 20 | MCT-ketogenic formula | No immediate benefit, but significant benefits in patients at 12 wk in WMS-R and WAIS-III (P < .05) |
|
Anorexia Nervosa | |||
| Scolnick et al16 (2020) | Study design, duration: case report, 6 mo Setting: OP N = 1 | KD, 2:1 to 1:1 ratio of fats to carbohydrates | Restrictive eating obsessions and compulsions stopped Continual improvement observed for >6 mo |
|
Autism Spectrum Disorder | |||
| Lee et al10 (2018) | Study design, duration: open-label clinical trial, 6 mo Setting: OP N = 15 | Modified ketogenic gluten-free diet regimen with supplemental MCT | ADOS-2 improvement at 3 mo: >7 units: 6 participants >3 units: 2 participants Little/none: 7 participants ADOS-2 improvement at 6 mo: Sustained improvement: 10 participants |
| Evangeliou et al17 (2003) | Study design, duration: pilot study, 6 mo Setting: IP N = 30 | John Radcliffe diet | Improvement in CARS score ranged from minor to significant in 60% of patients |
| Herbert and Buckley18 (2013) | Study design, duration: case report, 8 y Setting: OP N = 1 | GFCF KD | CARS score decreased from 49 to 17 Intelligence quotient increased by 70 points |
| Żarnowska et al19 (2018) | Study design, duration: case report, 6 mo on diet, 10 mo observation Setting: OP N = 1 | Low-carbohydrate, moderate protein, high-fat, KD | Intellect on WISC-R improved from baseline in the 16 mo following KD implementation |
| El-Rashidy et al20 (2017) | Study design, duration: case-control study, 6 mo Setting: IP N = 45 | MAD (n = 15) and GFCF diet (n = 15) vs control (n = 15) | CARS score mean decrease: MAD 41.7 → 33.7 (P = .0001) GFCF 39.17 → 34.27 (P = .001) Control no change ATEC score mean decrease: MAD 58 → 44 (P = .003) GFCF 64.13-42.13 (P = .0001) Control 62.82 → 61.60 |
|
Bipolar Disorder | |||
| Campbell et al21 (2019) | Study design, duration: observational analytic study Setting: OP N = 141 | KD, omega-3 enriched, or vegetarian | Reports of significant mood stabilization or remission of symptoms were substantially higher for the KD than for other diets |
| Phelps et al11 (2013) | Study design, duration: case series, 7 mo Setting: OP N = 2 | KD, 70% fat, 22% protein, 8% carbs | Mood stabilization exceeded the improvement achieved with medication |
|
MDD | |||
| Cox et al22 (2019) | Study design, duration: case report, 12 wk Setting: OP N = 1 | KD, 65% fat, 25% protein, 10% carbs | PHQ-9 score decreased Biometric changes: HbA1c dropped from 8% to 5.4% Average daily glucose measurements declined from 216 to 96 mg/dL HOMA-IR and TG/HDL ratios improved by 75% |
|
Narcolepsy | |||
| Husain et al12 (2004) | Study design, duration: clinical trial, 8 wk Setting: OP N = 9 | KD, consisting of <20% carbs/d | NSSQ total score decreased by 18% (P < .0019) |
|
Schizophrenia | |||
| Kraft and Westman23 (2009) | Study design, duration: case report, 12 wk Setting: IP N = 1 | KD, <20 g carbs/d | Auditory and visual hallucinations stopped Continual improvement observed for 12 mo |
ADAS-cog = Alzheimer's Disease Assessment Scale-Cognitive Subscale; ADOS-2 = Autism Diagnostic Observation Schedule Second Edition; ATEC = Autism Treatment Evaluation Checklist; CARS = Childhood Autism Rating Scale; GFCF = gluten-free–casein-free; HOMA-IR = Homeostatic Model Assessment of Insulin Resistance; IP = inpatient; MAD = modified Atkins diet; MCT = medium-chain triglycerides; MMSE = Mini-Mental State Examination; MoCA = Montreal Cognitive Assessment; NSSQ = Narcolepsy Symptom Status Questionnaire; OP = outpatient; PHQ-9 = Patient Health Questionnaire; RCT = randomized control trial; TG = triglycerides; T2DM = type 2 diabetes mellitus; WAIS-III = Wechsler Adult Intelligence Scale-3rd edition; WISC-R = Wechsler Intelligence Scale for Children-Revised; WMS-R = Weschsler Memory Scale-revised.
Alzheimer Disease
Three studies assessing the effects of the KD on Alzheimer disease were included. A case study by Morrill and Gibas13 described alleviating mild Alzheimer disease symptoms of forgetfulness, delayed word recall, and frequent misplacement of objects in a 71-year-old female patient by using the KD. This low-carbohydrate, high-fat diet produced sustained plasma ketones between 0.5 and 2 mg/dL, indicating moderate-level, nutritional ketosis. The PEAK Brain Training Mobile Application for cognitive training in addition to guided low-impact exercises were used. The Montreal Cognitive Assessment (MoCA) score was used to measure change in cognition. By the end of the study, the patient's MoCA score increased from 21 to 28, showing a positive correlation between the diet and an improvement in symptoms.
In 2017, Taylor and colleagues14 assessed the feasibility and efficacy of the KD in participants with Alzheimer disease. This was a 3-month-long study, in which 10 of 15 total participants completed. After the study intervention period, there was a 1-month washout period where participants resumed their regular diet. Ketosis was evaluated using serum BHB levels, as well as a urine ketone test. The Mini-Mental State Examination (MMSE) and Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-cog) were performed at baseline and throughout the 3-month study period to determine the effect of the KD. Ten participants' ADAS-cog scores changed significantly from baseline, with a mean improvement of 4.1 points (25.5 vs 21.4, P < .02). MMSE scores in those who completed the study significantly improved (25.2 vs 26.3, P < .05). Following the 1-month washout period, participants were readministered the ADAS-cog and MMSE exams, which resulted in a return to mean baseline scores of 25.3 (ADAS-cog) and 25.1 (MMSE) for 9 participants. The participants continued cholinesterase inhibitors during the study. Diarrhea was the most common side effect reported. No serious adverse events occurred, and laboratory and electrocardiogram results were within normal limits.
A randomized, controlled trial to assess the impact of the KD on Alzheimer disease symptoms was published by Ota and colleagues.15 Twenty Japanese patients with mild to moderate Alzheimer disease were either started on a ketogenic diet formula containing medium-chain triglycerides (MCTs) or an isocaloric placebo formula containing emulsified long-chain triglycerides as a substitute for MCTs. Changes in symptom severity were measured by the Weschsler Memory Scale-revised (WMS-R), the Wechsler Adult Intelligence Scale-3rd edition (WAIS-III), the Stroop test, and the Trail Making Test (TMT). In a post-hoc analysis, there were significant improvements in the WMS-R in the digit-symbol coding category (P < .05), as well as in the logical memory (both immediate and delayed) in the WAIS-III test (P < .05). The study participants were allowed to continue their antidementia drugs (eg, cholinesterase inhibitors and N-methyl-d-aspartate receptor antagonists) at the same dose during the study. Diarrhea was the only reported side effect.
Anorexia Nervosa
Scolnick and colleagues16 published a case report detailing the effects of the KD on a 29-year-old female with a 15-year history of anorexia nervosa. A KD of 2:1 to 1:1 ratio of fats to carbohydrates was used, and a KD nutritionist provided dietary education. After 3 months on the diet, the patient reported improvement but was still experiencing compulsions associated with anorexia. Ketamine IV infusions were initiated after 3 months of the KD intervention, with a total of 4 infusions given during 14 days. The Patient Health Questionnaire (PHQ-9) score, a 9-item depression screening tool, decreased from 13 to 6 after the initial infusion and decreased to 2 after the third infusion. Six months after ketamine infusions the patient continued to sustain a KD diet. The patient's weight remained stable, and she claimed to be free of any anorexia obsessive thoughts and behaviors by the end of the study; however, eating disorder rating scales were not used to monitor progress. The patient did not experience any side effects with the KD.
Autism Spectrum Disorder
Five studies assessing the effects of the KD on ASD were included. Lee and colleagues10 studied the efficacy of a modified ketogenic, gluten-free diet regimen with supplemental MCTs in 15 children ages 2 to 17 years with ASD. At 3 months, substantial improvement, defined as a decrease of greater than 7 units in the Autism Diagnostic Observation Schedule, second edition (ADOS-2) total score, was observed in 6 participants. Moderate improvement occurred (>3 units) in 2 participants, and minor or no improvement (≤3 units) was observed in 7 participants. A total of 8 of 15 participants who responded to the KD improved their overall score by at least 4 points. An improvement in Childhood Autism Rating Scale (CARS) scores was also reported at 3 months. At 6 months, 10 participants achieved a sustained decrease in ADOS-2 total scores. Medications for comorbidities were continued during the study. Common side effects occurred in the first 2 to 4 weeks of diet initiation and included diarrhea (18.8%), vomiting (18.8%), fatigue (18.8%), constipation (12.5%), dehydration (12.5%), weight loss (12.5%), acidosis (6.3%), and hypoglycemia defined as blood glucose <50 mg/dL (6.3%).
A pilot study performed by Evangeliou and colleagues17 included 30 children ages 4 to 10 years with autistic behavior on the high-fat (71%), low-carb (19%) John Radcliffe diet. The diet was applied for 6 months, with 4-week periods of continuous administration followed by 2-week diet-free intervals. Patients were evaluated via CARS scores after 6 months. Significant improvement (>12 unit reduction in CARS score) was recorded in 2 patients, average improvement (>8 to 12 unit reduction) occurred in 8 patients, and minor improvement (2 to 8 unit reduction) was observed in 8 patients. Twelve patients did not demonstrate a decrease in CARS scores. Haloperidol was given at least 6 months before the initiation of a KD and was continued after the diet was completed, but no increased dosages were needed. Behavioral treatments were not given. No adverse effects were reported.
Herbert and colleagues18 described a 4-year-old female patient with severe regressive autism and a history of epilepsy for whom drug therapy was deemed ineffective. After being placed on a gluten-free, casein-free (GFCF) KD, she showed a reduction in ASD symptoms. During a follow-up period lasting more than 8 years, the patient achieved a decrease in CARS score from 49 to 17, representing a change from severely autistic to nonautistic. The patient also had an increase in her intelligence quotient (IQ) of 70 points. The patient remained on anticonvulsant therapy; however, doses were able to be reduced without an increase in seizure frequency.
Żarnowska and colleagues19 studied a 6-year-old female patient with high-functioning autism who responded poorly to several behavioral and pharmacologic interventions during a period of 16 months. The patient was placed on a low-carbohydrate, moderate-protein, high-fat KD. One month following initiation of the diet, marked improvements were reported in behavior and intellect (including hyperactivity, attention span, abnormal reactions to visual and auditory stimuli, use of objects, adaptability to changes, communication skills, fear, anxiety, and emotional reactions). Intellect was measured via WISC-R scores: Full Scale IQ increased from 82 to 99, Verbal Scale IQ increased from 102 to 113, and Performance Scale IQ increased from 62 to 83 during the 16 months following initiation of the KD. Improvements continued until the end of the observation period. Pharmacologic treatment was not needed during the study period, and there were no side effects observed.
El-Rashidy and colleagues20 randomized 45 children ages 3 to 8 years diagnosed with ASD into 3 groups. A total of 15 children received a ketogenic MAD, 15 children received a GFCF diet, and 15 received balanced nutrition as the control group. All patients were assessed via neurologic examination, anthropometric measures, CARS score, and Autism Treatment Evaluation Test before and after 6 months following initiation of the diets. Both intervention groups showed significant improvement in Autism Treatment Evaluation Test and CARS scores at 6 months (P < .0001, P < .003), and the ketogenic MAD group showed better results in cognitive awareness (P = .0001) and sociability (P < .034) when compared to the GFCF group. Five patients in the MAD group were not included in the data analysis because of poor compliance.
Bipolar Disorder
Two articles evaluated the effects of the KD in bipolar disorder. Campbell and Campbell21 completed an analytic observational study that used online bipolar disorder forums to retrieve data from patients. A total of 85% of the 141 study participants' self-reported comments demonstrated positive effects on mood while following a ketogenic diet. Reports of mood stabilization or relief of symptoms were significantly higher (P < .0001) for patients on the KD compared with other diets, such as omega-3 enriched or vegetarian. Additionally, study participants reported a decrease in depression, improved clarity of thought and speech, weight loss, and increased energy overall.
Phelps and colleagues11 described the effects of the KD on bipolar disorder in 2 patients who experienced a decrease in quality of life despite taking medication for mood stabilization. A 69-year-old female experienced confusion due to lamotrigine, and a 30-year-old female wanted to discontinue lamotrigine because of concerns about becoming pregnant while taking this medication. The KD formulation consisted of 70% fat, 22% protein, and 8% carbohydrates. Both patients maintained ketosis for 2 to 3 years, successfully discontinued their mood stabilizers, and demonstrated significant subjective improvement. There were no adverse effects of the KD reported in either case.
Major Depressive Disorder
Cox and colleagues22 reported the effects of the KD in a 65-year-old female with a 26-year history of both type 2 diabetes and MDD. The intervention consisted of a KD formulation with 65% fat, 25% protein, and 10% carbohydrates during 12 weeks. The PHQ-9 was used. The patient's scores decreased substantially from a baseline score of 17 (moderately severe) to 0 (no symptoms reported). The patient's quality of life dramatically improved with the use of the KD. Additionally, the patient's HbA1C decreased from 8% to 5.4%, and her average daily glucose readings were lowered from 216 to 96 mg/dL. Although specific details were not provided, the patient was able to reduce her medications by 75% by the end of the study, which included a SSRI.
Narcolepsy
An experimental study by Husain and colleagues12 evaluated the effects of a low-carbohydrate KD on sleepiness and narcolepsy symptoms. Nine participants with a diagnosis of narcolepsy were asked to adhere to the Atkins diet plan, and their symptoms were assessed during an 8-week period. At the end of the 8 weeks, the combined Narcolepsy Symptom Status Questionnaire score of all participants showed a statistically significant decrease of 18% (161.9 to 133.5, P = .0019) from baseline. Patients reported less daytime sleepiness. The participants were taking optimal doses of stimulant medications. There were no serious adverse events reported, but 4 patients experienced mild and self-limiting side effects (eg, headache, leg cramps, irritability, and difficulty with concentration). No adverse changes were noted in vitals or laboratory tests.
Schizophrenia
Kraft and Westman23 described the effects of the KD on a 70-year-old female with a 17-year history of schizophrenia. Her medication regimen at the time of the study was risperidone 4 mg by mouth at bedtime. The goal of the case report was to evaluate if the KD would lessen the patient's schizophrenia symptoms (paranoia, disorganized speech, and hallucinations). This particular KD consisted of a restriction of less than 20 g of carbohydrates per day. The patient reported diminished auditory and visual hallucinations approximately 8 days after initiation of the diet. She had no recurrences during the course of the 12-month intervention period. The patient experienced weight loss (approximately 10 kg) and noted increased energy while following the KD. Two additional case reports about the KD in patients with schizophrenia have been published as letters, but these were excluded from this review because of insufficient details.
Discussion
This review summarizes the available literature on the effects of the KD on various psychiatric disorders. Symptom improvement included but is not limited to mood, cognition, communication skills, energy, anxiety, and auditory and visual hallucinations. Other benefits reported included positive biometric changes, such as improved lipid profile, reduced weight, positive change in blood glucose, and decreased HbA1c. These ancillary benefits may facilitate management of comorbid conditions and enhance overall health and well-being.
Niepoetter and Gopalan24 published a literature review in 2019 on the effect of the KD in autism, depression, anxiety, and schizophrenia in humans and animal models. The authors noted the use of the KD in psychiatric diseases may be a feasible method of treatment and incorporating the KD may allow for a reduction in pharmacologic treatment. Although there appears to be a correlation between the KD and symptom improvement, the evidence is limited. Because there are no well-controlled, large trials investigating the efficacy and safety of the KD for treatment of these psychiatric disorders, more research is needed before such a dramatic change in diet can be considered a primary treatment modality.
There are known challenges and risks associated with this diet intervention. A KD typically consists of approximately 75% fat, 15% protein, and 5% carbohydrates, and strict adherence to the very low daily intake of carbohydrates is required to produce a sustained state of ketosis. It may be challenging to generalize this type of diet for each patient, and long-term sustainability may not be feasible. Side effects of the KD reported in the literature include constipation, nausea, vomiting, diarrhea, fatigue, and hunger and were generally mild and self-limiting. However, this diet has been associated with negative health outcomes, including increased risk of heart disease, hyperlipidemia, and kidney stones.25
One of the most important limitations found in this review is the variation in the KD components among studies. Although several experimental studies confirmed a state of ketosis via ketone body testing, most did not. There were numerous versions of the KD used, each with different macronutrient allotments. For example, some KD plans included daily carbohydrate intake that ranged from 5% to 20% or higher. Some studies added a dietary supplement, such as MCT powders, to the KD to assist in producing ketosis rather than using dietary adjustments alone. Furthermore, the detail in which the studies reported the composition of intervention and control of diets was wide-ranging, and in some cases was lacking. Outcome measures were also variable among the studies. Some used validated objective measures, but others employed more subjective measures, which produces bias. Subjectivity of the results and the different types of tests used make it difficult to directly compare the data between the studies.
Although not included in this review because the study population did not follow a KD, Gardener and colleagues26 assessed the association between carbohydrate intake and cognitive performance in 666 cognitively normal patients who were genotyped for the apolipoprotein E (APOE) 4 allele. Nearly one third of these patients were determined to be at high risk for developing Alzheimer disease because of carrying the APOE 4 allele. Upon completion of this study, a negative association was discovered between cognitive function and a higher amount of daily carbohydrates consumed in patients with the APOE 4 allele. This may suggest benefits of following a lower carbohydrate diet that is not necessarily ketogenic; however, further investigation is warranted to determine if there is an optimal amount of carbohydrates or level of ketosis required to provide benefit in the treatment of psychiatric conditions.
Another study27 that did not meet inclusion criteria but is worth noting examined the effects of the KD in 10 female patients ages 19 to 63 years with schizophrenia. The study participants continued their medications and electroconvulsive therapy. The evaluation methods described did not include the specific symptoms listed in the nursing ward checks, the Minimal Social Behavior scale, or the Beckomberga rating scale; however, the authors reported a clinically observed decrease in symptoms after 2 weeks of treatment. Overall, 7 of the 10 patients studied showed improved symptoms while maintaining the KD.
Conclusion
This review presents the available evidence for the use of the KD in the treatment of several psychiatric disorders. The results are positive, and all studies that met the inclusion criteria showed some form of benefit in those who were able to remain on the diet, regardless of the disease state. However, the small number of study participants is a limitation that prevents generalization. The benefits and risks of the KD should be considered for each patient before implementing dietary changes. Because the evidence is limited, there is a need for more appropriately controlled, larger trials to clearly define the benefits of incorporating the KD in the psychiatric treatment regimen. Medical food studies were not included in the scope of this article but may be a future area of interest in the treatment of psychiatric diseases. Clinical pharmacists and providers may consider the KD as a potential adjunctive treatment option for patients living with Alzheimer disease, anorexia nervosa, autism, bipolar disorder, MDD, narcolepsy, and schizophrenia. The KD may allow for a reduction in pharmacotherapy and potentially reduce unwanted medication side effects.
Footnotes
Disclosures: None
References
- 1.US Department of Health and Human Services [Internet] President's Council on Sports, Fitness & Nutrition. Dietary guidelines for Americans; 2012 [cited 2020 May 21]. Available from: https://www.hhs.gov/fitness/eat-healthy/dietary-guidelines-for-americans/index.html.
- 2.Rogovik AL, Goldman RD. Ketogenic diet for treatment of epilepsy. Can Fam Physician. 2010;56(6):540–2. PubMed PMID: 20547519. [PMC free article] [PubMed] [Google Scholar]
- 3.Seo JH, Lee YM, Lee JS, Kang HC, Kim HD. Efficacy and tolerability of the ketogenic diet according to lipid:nonlipid ratios--comparison of 3:1 with 4:1 diet. Epilepsia. 2007;48(4):801–5. doi: 10.1111/j.1528-1167.2007.01025.x. DOI: 10.1111/j.1528-1167.2007.01025.x PubMed PMID: 17386059. [DOI] [PubMed] [Google Scholar]
- 4.ScienceDirect Topics [Internet] Modified Atkins diet - an overview. 2020 [cited. May 21]. Available from: https://www.sciencedirect.com/topics/medicine-and-dentistry/modified-atkins-diet.
- 5.Brietzke E, Mansur RB, Subramaniapillai M, Balanzá-Martínez V, Vinberg M, González-Pinto A, et al. Ketogenic diet as a metabolic therapy for mood disorders: evidence and developments. Neurosci Biobehav Rev. 2018;94(1-2):11–16. doi: 10.1016/j.neubiorev.2018.07.020. DOI: 10.1016/j.neubiorev.2018.07.020 PubMed PMID: 30075165. [DOI] [PubMed] [Google Scholar]
- 6.Włodarczyk A, Wiglusz MS, Cubała WJ. Ketogenic diet for schizophrenia: nutritional approach to antipsychotic treatment. Med Hypotheses. 2018;118:74–7. doi: 10.1016/j.mehy.2018.06.022. DOI: 10.1016/j.mehy.2018.06.022 PubMed PMID: 30037619. [DOI] [PubMed] [Google Scholar]
- 7.Gasior M, Rogawski MA, Hartman AL. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav Pharmacol. 2006;17(5-6):431–9. doi: 10.1097/00008877-200609000-00009. DOI: 10.1097/00008877-200609000-00009 PubMed PMID: 16940764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59(2):293–315. doi: 10.1016/j.brainresrev.2008.09.002. DOI: 10.1016/j.brainresrev.2008.09.002 PubMed PMID: 18845187 PubMed Central PMCID: PMC2649682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ułamek-Kozioł M, Czuczwar SJ, Januszewski S, Pluta R. Ketogenic diet and epilepsy. Nutrients. 2019;11(10):2510. doi: 10.3390/nu11102510. DOI: 10.3390/nu11102510 PubMed PMID: 31635247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee RWY, Corley MJ, Pang A, Arakaki G, Abbott L, Nishimoto M, et al. A modified ketogenic gluten-free diet with MCT improves behavior in children with autism spectrum disorder. Physiol Behav. 2018;188(3):205–11. doi: 10.1016/j.physbeh.2018.02.006. DOI: 10.1016/j.physbeh.2018.02.006 PubMed PMID: 29421589 PubMed Central PMCID: PMC5863039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phelps JR, Siemers SV, El-Mallakh RS. The ketogenic diet for type II bipolar disorder. Neurocase. 2013;19(5):423–6. doi: 10.1080/13554794.2012.690421. DOI: 10.1080/13554794.2012.690421 PubMed PMID: 23030231. [DOI] [PubMed] [Google Scholar]
- 12.Husain AM, Yancy WS, Carwile ST, Miller PP, Westman EC. Diet therapy for narcolepsy. Neurology. 2004;62(12):2300–2. doi: 10.1212/wnl.62.12.2300. DOI: 10.1212/WNL.62.12.2300 PubMed PMID: 15210901. [DOI] [PubMed] [Google Scholar]
- 13.Morrill SJ, Gibas KJ. Ketogenic diet rescues cognition in ApoE4+ patient with mild Alzheimer's disease: a case study. Diabetes Metab Syndr. 2019;13(2):1187–91. doi: 10.1016/j.dsx.2019.01.035. DOI: 10.1016/j.dsx.2019.01.035 PubMed PMID: 31336463. [DOI] [PubMed] [Google Scholar]
- 14.Taylor MK, Sullivan DK, Mahnken JD, Burns JM, Swerdlow RH. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer's disease. Alzheimers Dement (N Y) 2018;4(1):28–36. doi: 10.1016/j.trci.2017.11.002. DOI: 10.1016/j.trci.2017.11.002 PubMed PMID: 29955649 PubMed Central PMCID: PMC6021549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ota M, Matsuo J, Ishida I, Takano H, Yokoi Y, Hori H, et al. Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer's disease. Neurosci Lett. 2019;690:232–6. doi: 10.1016/j.neulet.2018.10.048. DOI: 10.1016/j.neulet.2018.10.048 PubMed PMID: 30367958. [DOI] [PubMed] [Google Scholar]
- 16.Scolnick B, Zupec-Kania B, Calabrese L, Aoki C, Hildebrandt T. Remissions from chronic anorexia nervosa with ketogenic diet and ketamine: case report. Front Psychiatry. 2020;11:763. doi: 10.3389/fpsyt.2020.00763. DOI: 10.3389/fpsyt.2020.00763 PubMed PMID: 32848935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evangeliou A, Vlachonikolis I, Mihailidou H, Spilioti M, Skarpalezou A, Makaronas N, et al. Application of a ketogenic diet in children with autistic behavior: pilot study. J Child Neurol. 2003;18(2):113–8. doi: 10.1177/08830738030180020501. DOI: 10.1177/08830738030180020501 PubMed PMID: 12693778. [DOI] [PubMed] [Google Scholar]
- 18.Herbert MR, Buckley JA. Autism and dietary therapy. J Child Neurol. 2013;28(8):975–82. doi: 10.1177/0883073813488668. DOI: 10.1177/0883073813488668 PubMed PMID: 23666039. [DOI] [PubMed] [Google Scholar]
- 19.Żarnowska I, Chrapko B, Gwizda G, Nocuń A, Mitosek-Szewczyk K, Gasior M. Therapeutic use of carbohydrate-restricted diets in an autistic child; a case report of clinical and 18FDG PET findings. Metab Brain Dis. 2018;33(4):1187–92. doi: 10.1007/s11011-018-0219-1. DOI: 10.1007/s11011-018-0219-1 PubMed PMID: 29644487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Rashidy O, El-Baz F, El-Gendy Y, Khalaf R, Reda D, Saad K. Ketogenic diet versus gluten free casein free diet in autistic children: a case-control study. Metab Brain Dis. 2017;32(6):1935–41. doi: 10.1007/s11011-017-0088-z. DOI: 10.1007/s11011-017-0088-z PubMed PMID: 28808808. [DOI] [PubMed] [Google Scholar]
- 21.Campbell IH, Campbell H. Ketosis and bipolar disorder: controlled analytic study of online reports. BJPsych Open. 2019;5(4):e58. doi: 10.1192/bjo.2019.49. DOI: 10.1192/bjo.2019.49 PubMed PMID: 31530294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox N, Gibas S, Salisbury M, Gomer J, Gibas K. Ketogenic diets potentially reverse type II diabetes and ameliorate clinical depression: a case study. Diabetes Metab Syndr. 2019;13(2):1475–9. doi: 10.1016/j.dsx.2019.01.055. DOI: 10.1016/j.dsx.2019.01.055 PubMed PMID: 31336509. [DOI] [PubMed] [Google Scholar]
- 23.Kraft BD, Westman EC. Schizophrenia, gluten, and low-carbohydrate, ketogenic diets: a case report and review of the literature. Nutr Metab (Lond) 2009;6(1):10. doi: 10.1186/1743-7075-6-10. DOI: 10.1186/1743-7075-6-10 PubMed PMID: 19245705 PubMed Central PMCID: PMC2652467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niepoetter P, Gopalan C. The effects of ketogenic diets on psychiatric disorders involving mitochondrial dysfunction: a literature review of the influence of dieting on autism, depression, anxiety, and schizophrenia. HAPS Ed. 2019;23(2):426–31. doi: 10.21692/haps.2019.002. [DOI] [Google Scholar]
- 25.Paoli A, Bianco A, Damiani E, Bosco G. Ketogenic diet in neuromuscular and neurodegenerative diseases. Biomed Res Int. 2014;2014:474296. doi: 10.1155/2014/474296. DOI: 10.1155/2014/474296 PubMed PMID: 25101284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardener SL, Rainey-Smith SR, Sohrabi HR, Weinborn M, Verdile G, Fernando WMADB, et al. AIBL Research Group. Increased carbohydrate intake is associated with poorer performance in verbal memory and attention in an APOE genotype-dependent manner. J Alzheimers Dis. 2017;58(1):193–201. doi: 10.3233/JAD-161158. DOI: 10.3233/JAD-161158 PubMed PMID: 28387666. [DOI] [PubMed] [Google Scholar]
- 27.Pacheco A, Easterling WS, Pryer MW. A pilot study of the ketogenic diet in schizophrenia. Am J Psychiatry. 1965;121(11):1110–1. doi: 10.1176/ajp.121.11.1110. DOI: 10.1176/ajp.121.11.1110 PubMed PMID: 14283310. [DOI] [PubMed] [Google Scholar]