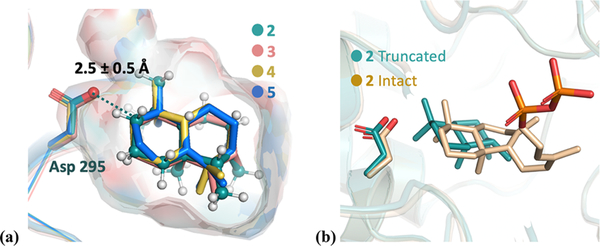
Figure 3.
(a) Overlay of lowest-RMSD structures for intermediates 2–5 (colored green, salmon, yellow, and blue, respectively). Asp295 is highlighted as sticks. The protons of intermediate 2 are colored white. The protonated carbon is highlighted as a dark blue sphere, which is a constraint to the Asp295 oxygen during the docking simulation. The surface indicates the active-site cavity. (b) Overlay of the lowest-energy docking result of intact 2 (beige) and truncated 2 (green, same structure as 2 in panel a).