Abstract
Background
Vitamin D is a secosteroid hormone that is important for its role in calcium homeostasis to maintain skeletal health. Linear growth faltering and stunting remain pervasive indicators of poor nutrition status among infants and children under five years of age around the world, and low vitamin D status has been linked to poor growth. However, existing evidence on the effects of vitamin D supplementation on linear growth and other health outcomes among infants and children under five years of age has not been systematically reviewed.
Objectives
To assess effects of oral vitamin D supplementation on linear growth and other health outcomes among infants and children under five years of age.
Search methods
In December 2019, we searched CENTRAL, PubMed, Embase, 14 other electronic databases, and two trials registries. We also searched the reference lists of relevant publications for any relevant trials, and we contacted key organisations and authors to obtain information on relevant ongoing and unpublished trials.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs assessing the effects of oral vitamin D supplementation, with or without other micronutrients, compared to no intervention, placebo, a lower dose of vitamin D, or the same micronutrients alone (and not vitamin D) in infants and children under five years of age who lived in any country.
Data collection and analysis
We used standard Cochrane methodological procedures.
Main results
Out of 75 studies (187 reports; 12,122 participants) included in the qualitative analysis, 64 studies (169 reports; 10,854 participants) contributed data on our outcomes of interest for meta‐analysis. A majority of included studies were conducted in India, USA, and Canada. Two studies reported for‐profit funding, two were categorised as receiving mixed funding (non‐profit and for‐profit), five reported that they received no funding, 26 did not disclose funding sources, and the remaining studies were funded by non‐profit funding. Certainty of evidence varied between high and very low across outcomes (all measured at endpoint) for each comparison.
Vitamin D supplementation versus placebo or no intervention (31 studies)
Compared to placebo or no intervention, vitamin D supplementation (at doses 200 to 2000 IU daily; or up to 300,000 IU bolus at enrolment) may make little to no difference in linear growth (measured length/height in cm) among children under five years of age (mean difference (MD) 0.66, 95% confidence interval (CI) ‐0.37 to 1.68; 3 studies, 240 participants; low‐certainty evidence); probably improves length/height‐for‐age z‐score (L/HAZ) (MD 0.11, 95% CI 0.001 to 0.22; 1 study, 1258 participants; moderate‐certainty evidence); and probably makes little to no difference in stunting (risk ratio (RR) 0.90, 95% CI 0.80 to 1.01; 1 study, 1247 participants; moderate‐certainty evidence).
In terms of adverse events, vitamin D supplementation probably makes little to no difference in developing hypercalciuria compared to placebo (RR 2.03, 95% CI 0.28 to 14.67; 2 studies, 68 participants; moderate‐certainty evidence). It is uncertain whether vitamin D supplementation impacts the development of hypercalcaemia as the certainty of evidence was very low (RR 0.82, 95% CI 0.35 to 1.90; 2 studies, 367 participants).
Vitamin D supplementation (higher dose) versus vitamin D (lower dose) (34 studies)
Compared to a lower dose of vitamin D (100 to 1000 IU daily; or up to 300,000 IU bolus at enrolment), higher‐dose vitamin D supplementation (200 to 6000 IU daily; or up to 600,000 IU bolus at enrolment) may have little to no effect on linear growth, but we are uncertain about this result (MD 1.00, 95% CI ‐2.22 to 0.21; 5 studies, 283 participants), and it may make little to no difference in L/HAZ (MD 0.40, 95% CI ‐0.06 to 0.86; 2 studies, 105 participants; low‐certainty evidence). No studies evaluated stunting.
As regards adverse events, higher‐dose vitamin D supplementation may make little to no difference in developing hypercalciuria (RR 1.16, 95% CI 1.00 to 1.35; 6 studies, 554 participants; low‐certainty evidence) or in hypercalcaemia (RR 1.39, 95% CI 0.89 to 2.18; 5 studies, 986 participants; low‐certainty evidence) compared to lower‐dose vitamin D supplementation.
Vitamin D supplementation (higher dose) + micronutrient(s) versus vitamin D (lower dose) + micronutrient(s) (9 studies)
Supplementation with a higher dose of vitamin D (400 to 2000 IU daily, or up to 300,000 IU bolus at enrolment) plus micronutrients, compared to a lower dose (200 to 2000 IU daily, or up to 90,000 IU bolus at enrolment) of vitamin D with the same micronutrients, may make little to no difference in linear growth (MD 0.60, 95% CI −3.33 to 4.53; 1 study, 25 participants; low‐certainty evidence). No studies evaluated L/HAZ or stunting.
In terms of adverse events, higher‐dose vitamin D supplementation with micronutrients, compared to lower‐dose vitamin D with the same micronutrients, may make little to no difference in developing hypercalciuria (RR 1.00, 95% CI 0.06 to 15.48; 1 study, 86 participants; low‐certainty evidence) and probably makes little to no difference in developing hypercalcaemia (RR 1.00, 95% CI 0.90, 1.11; 2 studies, 126 participants; moderate‐certainty evidence).
Four studies measured hyperphosphataemia and three studies measured kidney stones, but they reported no occurrences and therefore were not included in the comparison for these outcomes.
Authors' conclusions
Evidence suggests that oral vitamin D supplementation may result in little to no difference in linear growth, stunting, hypercalciuria, or hypercalcaemia, compared to placebo or no intervention, but may result in a slight increase in length/height‐for‐age z‐score (L/HAZ). Additionally, evidence suggests that compared to lower doses of vitamin D, with or without micronutrients, vitamin D supplementation may result in little to no difference in linear growth, L/HAZ, stunting, hypercalciuria, or hypercalcaemia. Small sample sizes, substantial heterogeneity in terms of population and intervention parameters, and high risk of bias across many of the included studies limit our ability to confirm with any certainty the effects of vitamin D on our outcomes. Larger, well‐designed studies of long duration (several months to years) are recommended to confirm whether or not oral vitamin D supplementation may impact linear growth in children under five years of age, among both those who are healthy and those with underlying infectious or non‐communicable health conditions.
Plain language summary
Effects of vitamin D on linear growth and other health outcomes among children under 5 years of age
Background
Vitamin D is an essential nutrient that plays a major role in skeletal health. Deficiency in vitamin D has also been linked to non‐skeletal health outcomes such as growth. Stunting and poor growth among children under five years of age remain a major global challenge. Previous literature has shown that blood vitamin D level is associated with stunting and poor growth. We examined the evidence regarding vitamin D supplements and their potential effects on linear growth. We also explored other outcomes related to vitamin D status, including adverse effects.
Study characteristics
We included 187 reports representing 75 studies (12,122 participants), conducted most frequently in India, USA, and Canada, among children under five years of age. In addition, 33 studies were classified as currently being conducted (ongoing) and 21 studies as 'awaiting classification' because they did not provide enough information to be categorised as included, ongoing, or excluded. Comparisons included oral vitamin D supplementation versus placebo (dummy pill) or no intervention; higher‐dose vitamin D versus lower‐dose vitamin D; vitamin D plus micronutrients (vitamins or minerals or both) compared to the same micronutrients alone; and higher‐dose vitamin D plus micronutrients (vitamins or minerals or both) compared to lower‐dose vitamin D plus the same micronutrients. Two studies reported for‐profit funding, two were categorised as mixed funding (non‐profit and for‐profit), five reported that they had received no funding, 26 did not disclose funding sources, and the remaining studies were supported by non‐profit funding.
Key findings
Supplementation with vitamin D in comparison with placebo or no intervention probably makes little to no difference in developing hypercalciuria, probably improves length or height compared to the child's age, probably makes little to no difference in stunting, and may make little to no difference in child length or height. It is uncertain whether vitamin D in comparison with placebo or no intervention impacts the development of hypercalcaemia.
Supplementation with a higher dose of vitamin D compared to a lower dose of vitamin D may make little to no difference in length or height compared to the child's age and developing hypercalciuria, or hypercalcaemia; and we are uncertain about the effects of higher‐dose vitamin D on linear growth.
Supplementation with a higher dose of vitamin D along with micronutrients (vitamins or minerals, or both) compared to a lower dose of vitamin D and the same micronutrients may make little to no difference in linear growth in children under five years of age and developing hypercalciuria, and probably makes little to no difference in developing hypercalcaemia.
Conclusions
Current evidence suggests that vitamin D probably slightly improves length/height‐for‐age z‐score compared to placebo; however, because of the quality of the evidence, we are uncertain about the true effects of vitamin D on linear growth or adverse effects among children under five years of age compared to placebo, no intervention, or lower doses of vitamin D, with or without micronutrients.
Summary of findings
Background
Description of the condition
Linear growth faltering and stunting
Suboptimal health among children under five years of age remains a major global challenge (UNICEF, WHO, World Bank 2020; WHO 2016). Most of the 5.9 million deaths among children under five years of age in 2015 could be attributed to preventable causes with available treatment options, such as malnutrition (UNICEF, WHO, World Bank 2020).
Linear growth faltering, or failure to reach one’s linear growth potential compared to normative standards (Leroy 2019; Perumal 2018), is associated with negative short‐ and long‐term outcomes among children under five years of age. Linear growth faltering is a marker for poor health, reduced earnings, and lower cognitive capacity, as well as a direct factor in the causal pathway to biological states such as foetal growth restriction and shorter maternal height (Leroy 2019; Perumal 2018). A subset of children suffering from linear growth faltering may become stunted, which is defined as more than two standard deviations (SDs) below the World Health Organization (WHO) reference standard (length‐ or height‐for‐age z‐score) (WHO 2006). The prevalence of stunting in a community offers a useful marker of well‐being at the population level (Perumal 2018); however, it is not without limitations. Recent studies have suggested that the classical definition of stunting is based on an arbitrary cutoff and may fail to accurately represent the true proportion of children facing inadequate growth (Leroy 2019). Therefore, this review will use both linear growth faltering and stunting to better evaluate interventions.
Linear growth faltering and stunting have multiple causes, including cumulative poor nutrition in utero and postnatally (Dewey 2011). In addition, repeated infections, environmental enteropathy, and inadequate care have all been suggested as contributory to inadequate growth (Leroy 2019; Perumal 2018). A recent review of child stunting pinpointed growth faltering during childhood as both a causal mechanism for some poor outcomes and a non‐causal indicator of other consequences (Leroy 2019). Linear growth faltering can lead to (1) cephalopelvic distortion leading to difficult birth, morbidity, and mortality; and (2) maternal short stature leading to smaller infants, who are more likely to die or not grow to optimal height (Ramakrishnan 1999). Linear growth faltering has additionally been shown to be associated with reduced earnings, lower school achievement and work capacity, reduced physical strength, chronic diseases, or poor cognition in adulthood (Black 2008; Dewey 2011; Haas 1996; Leroy 2019). Women stunted in childhood tend to bear stunted offspring, creating an intergenerational cycle of adverse physical, mental, and economic outcomes (Martorell 2012). A seminal study by Hoddinott et al followed a cohort of Guatemalan adults and, using instrumental variables, found that stunting played a causal role in adult economic productivity independent of childhood malnutrition and socioeconomic status. The mechanism behind this remains unknown, but it may be attributable to discrimination in schooling or when seeking employment. Although it is not generalisable to other populations, the analysis performed in this study remains important to support interventions to directly address inadequate childhood growth to improve economic disparities.
One risk factor for linear growth faltering of infants is maternal undernutrition; the intergenerational cycle of malnutrition is perpetuated by intrauterine growth restriction and restricted blood flow to the uterus, placenta, and foetus (Dewey 2011). Intrauterine growth restriction may lead to the infant being born premature (gestational age less than 32 weeks) and/or with low birth weight (birth weight less than 2.5 kg), both of which are risk factors for stunting (Danaei 2016). Another risk factor is recurrent infection (Caulfield 2006); as children age, their exposure to the environment increases, along with their risk of infection (Caulfield 2006). Stunting remains the most prevalent form of undernutrition among children under five years of age; 149 million suffer from stunting globally (WHO 2019). Global stunting decreased from 32.5% in 2000 to 21.9% in 2018 among children under five years of age (WHO 2019), but it remains a critical challenge in numerous geographical regions (De Onis 2012; De Onis 2013; Prendergast 2014). In India, for instance, 46 million children (nearly 40%) under five years of age are stunted, accounting for more than a third of the stunted children in the developing world (MoHFW 2019). The World Health Assembly aims to reduce stunting in children under five years of age by 40% between 2010 and 2025 (WHO 2012; WHO 2014a). Therefore, it is crucial to delineate modifiable causes of, and effective interventions against, stunting and linear growth faltering, including micronutrient supplementation.
Given the widely recognised burden of disease associated with childhood stunting in diverse populations (Black 2008; Black 2013; De Onis 2012; Prendergast 2014), many global research and policy efforts have sought to reduce growth faltering (Victora 2010; WHO 2014a). It has been estimated that improved understanding and scaling up of effective, evidence‐informed, safe, and effective interventions can prevent stunting among 33.5 million children (Bhutta 2013; Huey 2016; WHO 2014a). In particular, investigators have explored vitamin D supplementation as an intervention to prevent and mitigate childhood stunting (Kumar 2011). Optimal vitamin D status, which is often assessed by measuring serum concentrations of calcifediol (i.e. 25(OH)D), allows calcium absorption and growth to support active vitamin D (i.e. calcitriol (1,25{OH}₂D₃)) (Holick 2010). Prolonged inadequate vitamin D status impairs transcriptional regulation of skeletal homeostasis and linear growth, which could result in stunting (Holick 2010).
Prior observational studies have provided evidence that stunting is associated with suboptimal vitamin D status among children (Walli 2017). Therefore, vitamin D supplementation as a potentially modifiable risk factor that can have an effect on linear growth requires further evaluation.
Description of the intervention
Vitamin D status
One billion people have suboptimal vitamin D status, according to global estimates (Holick 2010). Even in countries with sun exposure all year round, low vitamin D status is a global problem among all age groups (Palacios 2014). Consequences of low vitamin D include poor skeletal and extraskeletal health outcomes (Holick 2008a; Holick 2008b; Holick 2010).
Low circulating 25(OH)D serum concentration is widely regarded as the biomarker for vitamin D status (Heaney 2009), although cut‐off values indicating deficiency and insufficiency are debated (Holick 2011; Ross 2011). Between 30% and 50% of children in numerous countries in Africa, Asia, Europe, and North America (Holick 2010), including geographical areas with ample sunlight and heterogeneous economic resources, have 25(OH)D less than 50 nmol/L. In the context of vitamin D deficiency, infants and young children are considered a high‐risk population, given that vitamin D intake is low during exclusive breastfeeding (Leroy 2014; Shrimpton 2001), and early life represents a critical period for linear growth and development of the immune system (Adkins 2004; Levy 2007). As further detailed in the next section, pleiotropic actions of vitamin D can impact skeletal, muscular, and immunological functions, all of which are related to optimal growth.
Vitamin D sources
Vitamin D can be acquired through consumption of a diet containing naturally vitamin D‐rich and fortified foods, or vitamin D supplements, or through endogenous production via skin exposure to ultraviolet irradiation (Holick 2010). In this review, we focus on vitamin D supplementation, given that it overcomes the challenges of inadequate sunlight at some geographical latitudes, as well as minimal sun exposure based on individual lifestyle decisions and limited consumption of naturally vitamin D‐rich or fortified foods (Holick 2010). Vitamin D supplements are available in two chemical forms (ergocalciferol (D2) and cholecalciferol (D3)), which differ in their side‐chain structure (Holick 2010). Vitamins D2 and D3 have been observed to increase serum 25‐hydroxyvitamin D (serum 25(OH)D), although at higher doses (50,000 IU), vitamin D2 appears less potent than equivalent doses of D3 in maintaining serum 25(OH)D levels (Holick 2010).
Vitamin D requirements
According to the WHO and the Food and Agriculture Organization (FAO), 200 international units (IU) of vitamin D is the daily recommended nutrient intake (RNI) among children under five years of age (WHO, FAO 2004). In the USA, the Institute of Medicine recommends that children between one and five years of age should consume a recommended dietary allowance of 600 IU per day, and have an estimated average requirement (EAR) of 400 IU per day (Institute of Medicine 2011). From birth to 12 months, it is recommended that children in the USA consume adequate intake (AI) of 400 IU per day (Institute of Medicine 2011).
No adverse effects occur at vitamin D intakes recommended by WHO and by FAO (WHO, FAO 2004). In the USA, the recommended upper limits of vitamin D consumption are based on age: 1000 IU from birth to six months, 1500 IU from six to 12 months, 2500 IU from one to three years, and 3000 IU from four to five years (Institute of Medicine 2011). Vitamin D toxicity has been observed in a few rare cases with long‐term consumption of extreme pharmaceutical dosages (Barrueto 2005; Blank 1995; Holick 2011; Klontz 2007; Vieth 1999); it is caused primarily by excessive intestinal calcium or phosphate absorption and bone resorption (Holick 2010). Excess vitamin D may contribute to hypercalciuria, hypercalcaemia, hyperphosphataemia, and kidney stones (nephrolithiasis) (Holick 2010). Hypercalciuria, or high levels of calcium in the urine, is linked to the role of vitamin D in increasing intestinal calcium reabsorption and is defined differently across different age groups (Leslie 2020). In children over two years of age, hypercalciuria is defined as daily urinary excretion of more than 4 mg calcium per kg of body weight, or a 24‐hour urinary calcium concentration less than 200 mg calcium per litre of urine (Leslie 2020). For children under two years, a random or spot urinary calcium‐to‐creatine ratio less than 0.2 mg calcium per mg creatine is considered normal (Leslie 2020). Hypercalcaemia is mainly caused by excess parathyroid hormone (PTH), which can be induced by high vitamin D intake, and is defined as high levels of calcium in blood; it can be classified as mild (10.5 to 11.9 mg/dL), moderate (12.0 to 13.9 mg/dL), or a hypercalcaemic crisis (14.0 to 16.0 mg/dL) (Sadiq 2020). Hyperphosphataemia indicates plasma phosphate greater than 7 mg/dL in children and can be induced by the role of vitamin D in increasing intestinal phosphate absorption (Goyal 2020). Kidney stones, detected via ultrasound, are calcium crystal concretions (composed primarily of calcium oxalate or calcium phosphate) travelling from the kidney through the genitourinary system. Kidney stones can occur in the setting of hypercalciuria (Nojaba 2020).
Metabolism of vitamin D
Evidence from mechanistic and dose‐response studies suggests that increasing intake of vitamin D (via consumption (supplementation, dietary intake) or cutaneous synthesis) improves serum 25(OH)D concentration (Holick 2010; Holick 2011). After it enters the body, vitamin D is stored in fat or is metabolised by the liver (Holick 2010; Holick 2011). A 25‐hydroxylase (CYP27B1) in the liver converts vitamin D to 25(OH)D, which is the major circulating form (Holick 2010; Holick 2011).
Available data from dose‐response studies show that vitamin D supplementation increases serum 25(OH)D concentration, regardless of age (Heaney 2003; Holick 2008b; Holick 2010; Institute of Medicine 2011). A non‐linear response of 25(OH)D to vitamin D has been observed in murine and human models (Institute of Medicine 2011). Dosages greater than or equal to 1000 IU daily have resulted in more gradual responses (e.g. 0.95 nmol/L to 1.4 nmol/L for every 100 IU; Smith 2009), and dosages below 1000 IU daily have achieved steeper responses (e.g. approximately 2.0 nmol/L for every 40 IU; Cashman 2008; Cashman 2009; Institute of Medicine 2011). Moreover, studies including young children with stunting have confirmed that vitamin D supplementation increases 25(OH)D (Kumar 2011). Widely ranging vitamin D supplementation dosages across studies have included daily physiological doses (200 IU to 400 IU; Alizadeh Taheri 2014; Fort 2016), as well as pharmacological doses (50,000 IU at birth; Moodley 2015), and even a single dose of 100,000 IU (Gupta 2016). In summary, preliminary data highlight the need for assessment of potential beneficial effects of vitamin D supplementation on stunting among children.
How the intervention might work
Cells of kidney, immune system, bone, and epithelium, and of other tissues in the body, use 1‐OHase (CYP27R1) to metabolise 25(OH)D to the biologically active steroid hormone 1,25(OH)₂D (Bikle 2014; Christakos 2016; Holick 2010). In its hormonally active form, vitamin D plays pleiotropic roles in the human body, promoting skeletal health, muscle development and growth, and immune function.
1,25(OH)₂D functions through genomic and non‐genomic mechanisms (Bikle 2014; Christakos 2016; Holick 2010). First, genomic effects occur through binding of 1,25(OH)₂D to vitamin D receptor and retinoid X receptor, which results in a heterodimer complex that regulates gene activity (Bikle 2014; Christakos 2016; Holick 2010). At least 100 to 1250 target genes of vitamin D are known (Adams 2010; Holick 2007; Hossein‐Nezhad 2013; Ramagopalan 2010; Tarroni 2012). These are directly targeted by vitamin D (via a vitamin D response element; e.g. 1,25(OH)₂D has been shown to bind to vitamin D response element in the calcium‐sensing receptor gene and subsequently to modulate calcium‐sensing receptor expression (Bikle 2014; Canaff 2002; Christakos 2016; Holick 2010)). Second, 'rapid' or non‐genomic responses occur extracellularly via interaction with plasma membrane vitamin D receptor (VDR) (Bikle 2014; Christakos 2016; Holick 2010). Examples of these include stimulation of intestinal calcium absorption and inhibition of apoptosis in osteoblasts (Bikle 2014; Christakos 2016; Holick 2010). This nuclear receptor has been identified in nearly all human tissues and cells assessed (Bikle 2014; Christakos 2016; Holick 2010).
Skeletal homeostasis and linear growth
Vitamin D has well‐established effects on skeletal health, including bone mineralization and maintenance (Holick 2010). Active vitamin D (1,25(OH)₂D) functions in conjunction with two other hormones (parathyroid hormone and calcitonin) to maintain endocrine control of calcium and phosphorus concentrations (Holick 2010). This tight regulation of calcium and phosphorus flux (extracellular (bones, blood), intracellular) is critical for development and maintenance of bones (Holick 2010), which impacts linear growth. Specific roles of active vitamin D include increasing intestinal calcium absorption (Christakos 2012), renal calcium reabsorption, and skeletal calcium resorption (in conjunction with parathyroid hormone) (Holick 2010).
Previous studies have demonstrated that vitamin D deficiency is associated with stunting (Holick 2010), including stunting among children (Holick 2006; Wacker 2013). Maternal vitamin D deficiency has been associated with greater risk of stunting among neonates and children (Finkelstein 2012; Toko 2016).
Possible negative effects on linear growth in children have been noted with higher‐dose vitamin D supplementation. An early case series of nine infants consuming over 1500 units of vitamin D daily from cod liver oil sources were found to have lowered growth rates after six months of age compared to infants consuming 300 to 600 units of vitamin D daily (Jeans 1938). These findings have been raised as a matter to concern by the Dietary Reference Intakes Committees in their review of vitamin D in both 1997 and 2010 (Institute of Medicine 1997; Institute of Medicine 2011). However, a population‐based cohort study conducted in 2011 (n = 10,060 singletons) found that supplementation with 2000 IU vitamin D per day during infancy was not associated with height at age 14 or 31 years, and was not associated with reduced height at any age studied (Hyppönen 2011).
Muscle development and growth
Vitamin D may influence muscle mass and function, as well as related indicators (weight‐for‐height (WFH) and ‐age (WFA)). Observational studies have corroborated the link between severe vitamin D deficiency (≤ 8 ng/mL) and poor muscle health among individuals age 10 to 65 years (Plotnikoff 2003). As an example, among infants with HIV exposure and no infection, low 25(OH)D concentration (< 10 ng/mL or ~ 25 nmol/L) was associated with a higher incidence of wasting (hazard ratio 1.71, 95% confidence interval (CI) 1.20 to 2.43; Sudfeld 2015).
Previous studies have identified mechanisms that link vitamin D with myopathy (Bischoff‐Ferrari 2012). In vitro studies have assessed human muscle tissues and isolated VDR (Bischoff‐Ferrari 2004; Bischoff‐Ferrari 2012; Ceglia 2010; Simpson 1985), which facilitate genomic and non‐genomic effects (Haussler 1998; McDonnell 1987; Norman 2004; Vazquez 1998). Furthermore, murine models have demonstrated that deletion of VDR (via gene knockout) resulted in impaired skeletal muscle growth and muscle‐related gene expression (Bouillon 2008; Endo 2003). Mice without VDR had smaller muscle fibres in all striated muscles (Endo 2003).
Why it is important to do this review
Linear growth retardation (including stunting) continues to affect many children worldwide (WHO 2018), and global stunting remains a critical and complex challenge in numerous geographical regions (De Onis 2013; Prendergast 2014; UNICEF, WHO, World Bank 2020). This is reflected in the World Health Assembly nutrition target to reduce stunting by 40% among children under five years of age by 2025, and Sustainable Development Goal 2.2 to reduce the prevalence of stunting and wasting in children under five years of age by 2025, highlighting the global importance of addressing this issue (United Nations 2015; WHO 2012; WHO 2014a). Although stunting among children under five years of age has decreased from 39.7% (in 1990) to 21.3% (in 2019) (De Onis 2012; Dewey 2020), the World Health Assembly nutrition target will not be achieved at this current trajectory (De Onis 2013).
Linear growth is considered an important overall indicator of child development (De Onis 2016). Critically, children with stunting often show minimal (if any) catch‐up growth in later life (Martorell 1994). However, nutritional interventions have been seen to allow catch‐up growth among children (Martorell 1994), especially during key developmental windows (including between birth and five years) (Prentice 2013). Vitamin D is already a known beneficial intervention for prevention of rickets in the same early, crucial childhood years, and despite conclusive evidence, the drive to reduce growth retardation is an important one with a plethora of potential beneficial effects.
The systematic method of our review is intended to achieve comprehensive assessment of current evidence on effects of vitamin D supplementation on growth faltering and other health outcomes among children. This approach facilitates consideration of other modulating factors, particularly in subgroup analyses. Given the multi‐factorial origin of stunting, which needs further elucidation (Stewart 2013), accounting for other factors is important. Aside from nutritional factors that affect stunting, potential influences include repeated infections, poor sanitation, household environmental contamination, mycotoxin exposure, the gut, and associated enteropathy (Casanovas 2013; Owino 2016; Semba 2016; Stewart 2013; Waterlow 1994).
Separately, an estimated one billion people have suboptimal vitamin D status (Holick 2007), which is linked to numerous skeletal and extraskeletal outcomes (Holick 2010). Given the relative ease of administration, widespread availability, and ongoing acceptability, the benefits of supplementation for growth in the first five years of life should be explored. Despite the multitude of studies that have focused on vitamin D supplementation and clinical health indicators (Ferguson 2014; Jagannath 2010), particularly among adults (Avenell 2014; Bjelakovic 2014a; Palacios 2019; Straube 2015), evidence regarding growth and stunting among children under five years of age remains unclear. Thus, it is necessary to draw overall conclusions from currently available evidence regarding how vitamin D supplementation impacts the growth of children under five years of age.
Objectives
To assess effects of oral vitamin D supplementation on linear growth and other health outcomes among infants and children under five years of age.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs. Quasi‐RCTs included studies that did not involve a treatment regimen assignment with simple randomisation but systematically utilised another aspect of the study design (e.g. alternating assignments based on sequential study enrolment, medical record number). Cluster‐randomised and cross‐over trials were also eligible for inclusion.
Types of participants
Infants and children under five years of age who lived in any country, healthy and apparently non‐vitamin D‐deficient, as well as with diagnosed vitamin D deficiency, rickets, or other underlying health conditions (as defined by trialists). We included studies of children under five years of age and study participants who were both under and over five years of age (e.g. birth to 10 years) if study authors reported stratified outcomes; this review reports extracted results among children under five years of age. We included studies of vitamin D supplementation directly among infants and children under five years of age only. We excluded studies that provided vitamin D supplementation to mothers only and not to their offspring.
Types of interventions
Studies assessing effects of oral vitamin D supplementation, with or without micronutrients, compared to no intervention, placebo, a lower dose of vitamin D, or micronutrients alone in children under five years of age. Comparisons between intervention and comparator groups are described below (and in Table 4).
1. Intervention and comparator groups.
| Comparison | ||
| Name of comparison | Intervention group | Comparator group |
| 1. Vitamin D supplementation vs placebo or no intervention | Oral vitamin D (cholecalciferol D₃, ergocalciferol D₂, calcitriol) supplementationa | No intervention |
| Placebo | ||
| 2. Vitamin D supplementation (high dose) vs vitamin D (low dose) | Oral vitamin D (cholecalciferol D₃, ergocalciferol D₂, calcitriol) supplementation,a at a higher dose | Oral vitamin D (cholecalciferol D₃, ergocalciferol D₂, calcitriol) supplementation,a at a lower dose |
| 3. Vitamin D supplementation + micronutrient(s) vs micronutrient(s) alone | Other micronutrient(s),b including oral vitamin D (cholecalciferol D₃, ergocalciferol D₂, calcitriol) supplementationa | Other micronutrient(s),b not including vitamin D |
| 4. Vitamin D supplementation (high dose) + micronutrient(s) vs vitamin D (low dose) + micronutrient(s) | Other micronutrient(s),b including oral vitamin D (cholecalciferol D₃, ergocalciferol D₂, calcitriol) supplementation at a higher dosea | Other micronutrient(s),b including vitamin D at a lower dose |
aAny formulation, including capsules, tablets, soft gels, liquids, sprays/mists, or powders. bComparisons will include intervention and comparator groups with the same combination and content of vitamin(s) and/or mineral(s) to isolate the effects of vitamin D.
Interventions
Oral vitamin D (cholecalciferol D₃, ergocalciferol D₂, calcitriol) supplementation (Table 4). We included any form of oral consumption of vitamin D (such as capsules, tablets, soft gels, liquids, sprays/mists, and powders) and excluded alternative administration of vitamin D (e.g. intravenous injection, food fortification, dietary intake of vitamin D‐rich foods). We documented key differences across interventions (including treatment dosage, duration, and frequency) during data extraction. For studies assessing effects of higher versus lower doses of vitamin D, we considered the higher dose as the intervention arm (see Differences between protocol and review). Studies with micronutrient supplementation plus vitamin D as the intervention were included if the comparator arm involved the same micronutrients without vitamin D, or provided a lower dose of vitamin D as the reference group.
Comparators
Study participants who received placebo, no intervention, or a lower dose of vitamin D (Table 4). Additionally, for studies with micronutrient supplementation plus vitamin D as the intervention, we included comparisons that involved the same micronutrients without vitamin D or with a lower dose of vitamin D as the reference group.
Types of outcome measures
Primary outcomes
Linear growth (reported continuously in centimetres)
Length/height‐for‐age (L/HAZ; reported continuously as WHO z‐score; WHO 2006)
Stunting (reported as a categorical outcome; defined as L/HAZ more than 2 SDs below the reference WHO standard; WHO 2006)
-
Adverse effects relevant to excessive vitamin D (reported as categorical outcomes)
Hypercalciuria (high urinary calcium levels, defined by trialists)
Hypercalcaemia (high serum calcium levels, defined by trialists)
Hyperphosphataemia (high plasma phosphate levels, defined by trialists)
Kidney stones (nephrolithiasis, defined by trialists)
Secondary outcomes
Gain in linear growth (reported continuously in centimetres)
Weight‐for‐age (WAZ; reported continuously as WHO z‐score; WHO 2006)
Underweight (reported as a categorical outcome; defined as WAZ more than 2 SDs below the reference WHO standard; WHO 2006)
Weight‐for‐length/height (WL/HZ; reported continuously as WHO z‐score; WHO 2006)
Wasting (reported as a categorical outcome; defined as WHZ (or WLZ) more than 2 SDs below the reference WHO standard; WHO 2006)
Vitamin D status (based on serum 25(OH)D concentration (nmol/L); reported as continuous outcomes, including change in vitamin D status, and categorical outcomes, according to current recommended cut‐offs from the Institute of Medicine and the Endocrine Society (in the USA) (Holick 2011)). Usage of a wide spectrum of vitamin D assay instruments, including immunoassays (e.g. radioimmunoassays) and chromatographic methods (e.g. liquid chromatography‐tandem mass spectrometry)
Rickets (defined by trialists)
Search methods for identification of studies
Electronic searches
In March 2018, we searched the international and regional electronic databases and trial registers listed below. We updated the search in December 2019. We made some adjustments to our electronic search strategy post publication of our protocol (Yu 2017). Please see Differences between protocol and review.
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 12), in the Cochrane Library, which includes the Cochrane Developmental, Psychosocial and Learning Problems Specialised Register (searched 11 December 2019).
PubMed National Library of Medicine (www.ncbi.nlm.nih.gov/pubmed; searched 11 December 2019).
Embase Ovid (1980 to 11 December 2019).
CINAHL EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1982 to 11 December 2019).
CABI (Centre for Agriculture and Biosciences International): CAB Abstracts and Global Health Web of Science (1973 to 11 December 2019).
Web of Science Core Collection Clarivate (searched 11 December 2019).
Cochrane Database of Systematic Reviews (CDSR; 2019, Issue 12), part of the Cochrane Library (searched 11 December 2019).
DARE (Database of Abstracts of Reviews of Effects, Centre for Reviews and Dissemination; www.crd.york.ac.uk/CRDWeb; searched 11 December 2019).
IBECS (ibecs.isciii.es; searched 11 December 2019).
LILACS (Latin American and Caribbean Health Sciences Literature; lilacs.bvsalud.org/en; searched 11 December 2019).
PAHO (Pan American Health Library; iris.paho.org; searched 11 December 2019).
WHOLIS (WHO Library; dosei.who.int; searched 11 December 2019).
SciELO (Scientific Electronic Library Online; www.scielo.br; searched 11 December 2019).
WPRIM (Western Pacific Region Index Medicus; www.wprim.org; searched 11 December 2019).
IndMED (Indian Medical Journals; indmed.nic.in; searched 14 March 2018; IndMED was not available at this URL after 2018, and the database could not be located).
WHO ICTRP (World Health Organization International Clinical Trials Registry Platform; apps.who.int/trialsearch; searched 14 March 2018).
Epistemonikos (www.epistemonikos.org; searched 11 December 2019).
Scopus Elsevier (searched 11 December 2019).
EUCTR (European Union Clinical Trials registry; www.clinicaltrialsregister.eu/ctr-search/search; searched 11 December 2019).
The search strategies for each database are provided in Appendix 1. We did not limit the searches by publication year, language, country, or region.
Searching other resources
We searched the reference lists of relevant publications (including trials, reviews, meta‐analyses, reports) identified through our electronic searches, and we considered any potentially eligible trials included in these reference lists. Additionally, we attempted to obtain information on relevant ongoing and unpublished trials by contacting other entities such as the WHO Nutrition Section (www.who.int/nutrition/en), the United Nations Children’s Fund (UNICEF; www.unicef.org), Nutrition International (formerly Micronutrient Initiative; www.nutritionintl.org), the International Micronutrient Malnutrition Prevention and Control Programme (IMMPaCt; www.immpact.org) from the US Centers for Disease Control and Prevention (CDC), and the Vitamin D Workshop Group (vitamindworkshop.org).
Data collection and analysis
We performed this review in accordance with the recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020a). When possible, we used the methods described in our published protocol (Yu 2017). Unused methods may be found in Table 5.
2. Unused methods.
| Data analysis | Unused method | Reason for non‐use |
| Unit of analysis issues |
Cluster‐randomised trials Had we included cluster‐randomised trials, we would have accounted for randomisation of study participant groups by conducting analyses at the cluster level. We would have calculated effect estimates (with respective standard errors (SEs)) by using the generic inverse variance method presented in Review Manager 5 (RevMan 5) (Higgins 2020b; Review Manager 2014). Depending on analyses of included studies, we would have conducted approximately correct analyses, when possible (Higgins 2020b) |
No cluster‐randomised trials included in review |
|
Cross‐over trials We planned to assess data from a 2‐period, 2‐intervention cross‐over trial by using a paired t‐test to evaluate the difference between 2 measurements (subtracting the control measurement from the experimental measurement) for each study participant (Higgins 2020b). For studies with potential carry‐over effects, we planned to consider only the first period of trial intervention follow‐up (Higgins 2020b) |
No cross‐over trials included in quantitative analysis | |
| Subgroup analysis and investigation of heterogeneity | If at least 4 studies measuring a primary outcome had reported on age at time of intervention (birth to 6 months of age vs 7 to 12 months of age, 13 to 36 months of age, 37 to 59 months of age), frequency of supplementation (daily vs intermittent vs other), serum 25(OH)D at baseline (current cutoff levels recommended by the Institute of Medicine and the Endocrine Society (Holick 2011; Institute of Medicine 2011)), geographical latitude (between Tropics of Cancer and Capricorn, compared with north of Tropic of Cancer and south of Tropic of Capricorn), season at start of study (spring, summer, fall, winter), or baseline height/length‐for‐age z‐score, we would have performed subgroup analyses (see the protocol Yu 2017 for details). Subgroup analyses would have been undertaken in RevMan 5 (Review Manager 2014), using methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2020) | Not enough studies available (≤ 3) |
| Sensitivity analysis | If at least 10 studies measuring a primary outcome had been available to compare in terms of being published or unpublished, high risk of bias, longer intervention durations or greater sample sizes, influence of methods, and use of filters such as imputation, language of publication, source of funding, and country, we would have performed statistical tests, including Egger's test to assess asymmetry of funnel plots and as indicators of bias (Egger 1997) (see the protocol Yu 2017 for details). Sensitivity analyses would have been undertaken in RevMan 5 (Review Manager 2014), using methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2020) | Not enough studies available (≤ 10) |
| Publication bias | We searched 17 electronic databases and 2 trial registries to be as comprehensive as possible in examining all available evidence. However, we were not able to assess for publication bias using funnel plots due to lack of studies for comparison, thereby preventing us from drawing conclusions on publication bias of the included studies | Not enough studies available (≤ 10) |
Selection of studies
We modified the data extraction form on Covidence for use during screening of studies for this review. Using Covidence systematic review software (Covidence 2020), five review authors (SLH, AS, NA, RA, EAY) independently screened studies identified by the searches. Initially, they considered the title and abstract of each record to decide whether they met inclusion and exclusion criteria of this review (Criteria for considering studies for this review), and they selected 'No' for those that were irrelevant. For records that were not excluded, SLH, AS, and NA reviewed the full‐text reports for eligibility. We contacted study authors if clarifications were necessary, or if full‐text reports were not available (Dealing with missing data). SLH, AS, NA, EAY, and RA resolved discrepancies through discussion and, if necessary, through consultation with a sixth review author (SM).
We present the selection process in a PRISMA diagram (Moher 2009).
Data extraction and management
Three review authors (SLH, NA, AS) independently extracted data from eligible full‐text studies using customised forms in Covidence that were piloted on a sample of studies and modified accordingly before full data extraction was undertaken (Covidence 2020). If any data were unclear, or if data included children over five years of age, we attempted to contact the study authors to ask them to provide further details or to share age‐stratified data. SLH and NA extracted the data and entered them into Covidence; they then imported the data into Review Manager 5 (RevMan 5) (Review Manager 2014). SLH checked the data for accuracy.
SLH, NA, and AS resolved disagreements through discussion or through consultation with a fourth review author (SM). For this review, we aggregated study design details and findings from any duplicate or companion documents, as well as from multiple publications on a single study.
During data extraction, we recorded information regarding study design, setting, objectives and primary outcomes of the study, years the study was conducted, participants (inclusion and exclusion criteria), study methods (method of ascertaining vitamin D concentration and trial design), assessment of risk of bias, intervention information, and outcomes (see list in 'Study information' below). We recorded additional details beyond what we previously specified in our protocol (Yu 2017) (see Differences between protocol and review).
Study information
-
Identification
Sponsorship
Country
Setting
Study authors’ contact details
Study objectives
Primary outcomes measured
Year(s) of trial
-
Trial methods
Trial design (RCT or quasi‐RCT)
Vitamin D concentration quantification method
-
Participants
Inclusion criteria
Exclusion criteria
Group differences
Baseline characteristics
-
Intervention
Vitamin D content in IU
Formulation
Vitamin D type
Frequency of dosage
Duration of administration
Other micronutrient content
N (number) per group (in analysis)
Vitamin D brand/company
-
Comparator
None, placebo, other micronutrients, dosage of vitamin D
-
Outcomes
Primary and secondary outcomes (as outlined under Types of outcome measures)
Assessment of risk of bias in included studies
SLH, AS, and NA independently assessed the risk of bias in each included study using the certainty assessment form in Covidence (Covidence 2020), which follows Cochrane's domain‐based evaluation as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). These domains are sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessors; incomplete outcome data; selective reporting bias; and other sources of bias, which we measured as whether or not the sample size was calculated, and if calculated, met at randomisation and at endpoint of the study. We categorised each domain as low, high, or unclear risk of bias, depending on the sufficiency of information to characterise the risk of bias. Disagreements were resolved by discussion. Specific assessments by domain can be found in Appendix 2.
We detail our findings in the 'Risk of bias' tables and present a narrative summary of our findings in the Risk of bias in included studies section. We also present the findings graphically.
Measures of treatment effect
Continuous outcomes
When possible, we extracted means and standard deviations (SDs) for outcome data. When studies reported means and standard errors (SEs) or means and 95% confidence intervals (CIs), we extracted these values and used the calculator in RevMan 5 (Review Manager 2014) to back‐calculate the SD using methods from the Cochrane Handbook for Sytematic Reviews of Interventions (Li 2020). This step was not included in our original protocol (Yu 2017) (see Differences between protocol and review). Some studies reported medians and interquartile ranges (IQRs) or medians and ranges, or means without variance estimates such as SDs, SEs, or 95% CIs for specific outcomes. When studies reported medians and IQRs, and the sample size per group was large (n ≥ 30), we entered the reported median as the mean in RevMan 5 (Review Manager 2014), and we treated the IQR as approximately 1.35 × SD. If the sample size was < 30, we omitted these data from the analysis. When studies reported ranges as a measure of variance, we omitted these data from the analysis per guidelines provided in the Cochrane Handbook for Systematic Reviews of Intervention (Li 2020). When a study reported only the means and no variance estimates, we omitted these data from the analysis.
We reported continuous outcomes as mean differences (MDs) with corresponding 95% CIs (Deeks 2020). Specifically, these included primary (linear growth, HAZ, or LAZ) and secondary (WAZ, WHZ, serum 25(OH)D concentration) outcomes. If trials used different scales to measure the same continuous outcome across studies, we used standardised mean differences (SMDs) with 95% CIs, when possible (Deeks 2020).
Categorical outcomes
For categorical outcomes, when possible, we presented data as measures of association (risk, rate, odds ratio with corresponding 95% CI; Deeks 2020). These included primary (stunting, adverse effects (hypercalciuria, hypercalcaemia, hyperphosphataemia)) and secondary (vitamin D status, rickets) outcomes. For dichotomous outcomes, we calculated risk ratios (RRs) for the probability of an event happening. In studies where each arm had zero events for a particular outcome that was rare (e.g. rickets), we used risk differences (RDs) to perform the meta‐analysis (Higgins 2020b). To analyse dichotomous rickets outcomes, we summarised each study’s number of participants who experienced at least one event (i.e. signs of rickets, which may have included multiple signs per participant; participants were not counted twice) as events, as a proportion of the total number of participants per group (Li 2020) (see Differences between protocol and review). For categorical vitamin D outcomes (severe serum vitamin D deficiency defined by trialists as <25 to <30 nmol/L, serum vitamin D deficiency defined as <50 nmol/L (Holick 2011), and serum vitamin D insufficiency defined as <75 nmol/L (Holick 2011), we present these outcomes as the proportion of participants achieving above these cut‐offs, specifically ≥25 to ≥30 nmol/L, ≥50 nmol/L, or ≥75 nmol/L. For these outcomes, we combined both studies which presented participants developing severe deficiency, deficiency, or insufficiency, and those achieving vitamin D status above these cut‐offs, by converting these outcomes in the former to the proportion of participants above the cut‐offs to include them in analysis.
Unit of analysis issues
For each study included in this review, we documented the unit of randomisation during data extraction. The unit of randomisation included individual participants. We also considered whether individuals had undergone more than one intervention, as in a cross‐over trial, and whether a trial reported multiple observations for the same outcome(s), including repeated measurements or recurring events.
We included two cross‐over trials, Rodd 2011 and Lava 2011, neither of which assessed any outcomes within the scope of this review. We did not identify any cluster‐randomised trials. For methods to deal with cluster‐randomised trials should we find any in future updates of this review, please see Table 5.
Studies with more than two treatment groups
For multi‐arm studies, we included only the directly relevant arms (e.g. for one particular study, we excluded arms with only intramuscular injection of vitamin D but included arms administering oral vitamin D and oral placebo or control).
When studies included more than two intervention groups, we combined groups to perform a single pair‐wise comparison. Specifically, we combined all relevant experimental groups into one group, and all relevant control intervention groups into a second group. Thus, for studies that compared dichotomous outcomes among multiple vitamin D arms and one placebo or no intervention arm, we combined the vitamin D arms into one vitamin D group by summing each arm’s number of participants and number of events into one vitamin D group, which we then compared against the original placebo group. For studies that compared dichotomous outcomes among at least three varying dosages of vitamin D, we compared the lowest dose (control) of vitamin D to the combined higher‐dosage arms of vitamin D, again by summing each arm’s number of participants and number of events into one 'higher‐dosage vitamin D' group (Higgins 2020b). For studies that compared continuous outcomes among multiple vitamin D arms and one placebo or no intervention arm, we combined the vitamin D arms into one group using formulae for combining groups available in RevMan 5 (Higgins 2020b; Review Manager 2014). For studies that compared continuous outcomes among at least three varying dosages of vitamin D, we compared the lowest dose (control) of vitamin D arm to the combined higher‐dosage arms of vitamin D. We based our approach to meta‐analysis on information provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020b).
These methods were not described in our protocol (Yu 2017), but we have added them based on the studies identified and examined; see Differences between protocol and review for more details.
Dealing with missing data
As necessary, we contacted study authors via email to ask them to share further information. If no response was received after one week, we emailed again; if again no response was received, we did not contact the authors again.
We did not impute any missing data, except we calculated SDs from IQRs when the sample size was greater than 30 per group (see Measures of treatment effect > Continuous data), and we used the calculator in RevMan 5 to convert means with 95% CIs and means with SEs into means with SDs (Review Manager 2014).
From each included study, we documented the missingness of key data and study participant information (including loss to follow‐up) in 'Risk of bias' tables. Examples of unreported data include means and SDs of study participant subgroups. We recorded attrition as part of the 'Risk of bias' assessment. Loss to follow‐up data included additional information regarding attrition and treatment adherence, or data on study participants who did not complete the trial or follow the protocol.
We considered all outcomes based on the intention‐to‐treat approach, when possible. In summarising across studies, for every outcome, the denominator represented the total number of study participants randomised to a treatment regimen (minus any participants with missing outcomes).
Assessment of heterogeneity
We quantified statistical heterogeneity across studies by using forest plots, Chi² (significance of α (alpha) = 0.10) testing, I² (≥ 75%) statistics, and Tau² values (Deeks 2020). We also considered critical differences between study designs (including study population characteristics) and risk of bias. In the event that we observed substantial heterogeneity, we considered performing prespecified subgroup analyses to gain a better understanding of the differences (Subgroup analysis and investigation of heterogeneity). For outcomes with substantial heterogeneity (according to our assessments), we did not report a pooled estimate.
Assessment of reporting biases
For each study, we checked for existence of study protocols or trial registrations published before or after reports of the study were published. We also checked that outcomes described in the methods or protocols, when available, were reported in published studies. In addition, we visually examined funnel plots for our primary outcomes to assess for bias due to missing results. We summarised these findings per each study in the Risk of bias in included studies section.
Data synthesis
Among comparable studies in this review (including similar outcomes and populations), we conducted a meta‐analysis to estimate summary measures across studies. Specifically, these included studies with outcomes reported on the same scale (or as values that could be converted or standardised). For each outcome of interest, we considered reporting both continuous and categorical values across studies; we converted data to either continuous or categorical values to facilitate comparability, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2020).
We conducted meta‐analysis via RevMan 5 (Review Manager 2014), and we utilised the inverse variance method. Per our protocol (Yu 2017), we conducted random‐effects meta‐analyses for outcomes with two or more studies to account for differences across study designs (including intervention dosages, durations, and frequencies, as well as study populations) (Deeks 2020). We also anticipated heterogeneity of reported time points (by reporting endpoint data, change from baseline data, etc.). For analyses including only one study, we used a fixed‐effect model, as there is no inter‐study heterogeneity (see Differences between protocol and review). In the event that we identified too few studies or study data could not be pooled, we provided a narrative description of trial results.
Summary of findings
For each primary outcome, two review authors (SLH and NA) used the GRADE approach to rate the certainty of evidence as high, moderate, low, or very low, according to the presence of the following factors: within‐study risk of bias and limitations due to study design; directness of evidence; assessment of heterogeneity between studies; precision of effect estimates; and risk of publication bias (GRADEpro GDT 2020; Guyatt 2011). We assigned a grade of high certainty to evidence from RCTs and decreased this grade by one level for each factor present, up to a maximum of three levels. In the event of disagreement, we consulted an additional review author (SM or JPP, or both), who facilitated consensus through discussion. We present the grades of evidence for primary outcomes in a GRADE 'Summary of findings' table per each comparison.
We created 'Summary of findings' tables using GRADEpro GDT 2020 and Review Manager 2014 for our main comparisons when data were available: vitamin D versus placebo or no intervention (Table 1); vitamin D (higher dose) versus vitamin D (lower dose) (Table 2); and higher‐dose vitamin D plus micronutrient(s) versus micronutrient(s) with lower‐dose vitamin D (Table 3). We reported the following outcomes in each table, assessed at the end of the supplementation period, irrespective of whether or not there were data: linear growth; height‐for‐age z‐score; stunting; hypercalciuria; hypercalcaemia; hyperphosphataemia; and kidney stones. For each primary outcome, we provide the anticipated absolute or relative effect and an evidence certainty rating assessed through the GRADE approach (Guyatt 2011); a rationale for the GRADE certainty rating is provided in the table footnotes. The tables also provide information on study population, setting, outcome measurements, and timing of measurement, as well as the numbers of studies and participants included.
Summary of findings 1. Vitamin D versus placebo or no intervention.
| Vitamin D versus placebo or no intervention | ||||||
| Patient or population: children under 5 years of age Setting: any country Intervention: oral vitamin D (doses: 200 to 2000 IU daily; or up to 300,000 IU bolus at enrolment) Comparison: placebo or no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) |
№. of participants (studies) |
Certainty of evidence (GRADE) | Comments | |
| Risk with placebo or no intervention | Risk with vitamin D | |||||
|
Linear growth (length/height) Unit: cm Time frame: 6.3 months (mean) |
Mean length in control group was 62.7 cm | Mean length in intervention group was 0.66 cm longer (0.37 shorter to 1.68 longer). | ‐ | 240 (3 RCTs) |
⊕⊕⊝⊝ Lowa | Two studies showed an increase in linear growth, and 1 study found a decrease in linear growth. However, no difference was found overall |
|
Length/height‐for‐age z‐score (L/HAZ) Time frame: 6 months |
Mean height‐for‐age z‐score in control group was ‐1.95 | Mean height‐for‐age z‐score in intervention group was 0.11 units higher (0.001 to 0.22 higher). | ‐ | 1258 (1 RCT) |
⊕⊕⊕⊝ Moderateb | HAZ was higher among those receiving vitamin D |
|
Stunting Definition: L/HAZ < ‐2 Time frame: 6 months |
Study population | RR 0.90 (0.80 to 1.01) | 1247 (1 RCT) |
⊕⊕⊕⊝ Moderateb | ||
| 490 per 1000 | 441 per 1000 (392 to 495) | |||||
|
Adverse effect: hypercalciuria As defined by trialists Time frame: 6.5 months (mean) |
Study population | RR 2.03 (0.28 to 14.67) | 68 (2 RCTs) |
⊕⊕⊕⊝ Moderatec | There was no greater risk of increased calcium secretion in urine in groups receiving vitamin D | |
| 29 per 1000 | 60 per 1000 (1 to 238) | |||||
|
Adverse effect: hypercalcaemia As defined by trialists Time frame: 7.5 months (mean) |
Study population | RR 0.82 (0.35 to 1.90) | 367 (2 RCTs) |
⊕⊝⊝⊝ Very lowd | There was no greater risk of increased calcium concentration in blood in groups receiving vitamin D | |
| 124 per 1000 | 101 per 1000 (43 to 235) | |||||
| Adverse effect: hyperphosphataemiae | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Adverse effect: kidney stonese | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to serious risk of bias. Evidence was downgraded an additional level due to inconsistency (as indicated by an I² value of 49%; P = 0.14), suggesting moderate heterogeneity. bDowngraded one level due to indirectness as only one study conducted in India was included, restricting the population analysed. cDowngraded one level due to imprecision, as the confidence interval was wide around the effect size which included 1.0, the null value. dDowngraded one level due to serious risk of bias. Evidence was downgraded an additional level due to imprecision, as the confidence interval around the effect size included 1.0, the null value. Evidence was downgraded an additional level due to inconsistency (as indicated by an I² value of 48%; P = 0.64), suggesting moderate heterogeneity.
eNo data were available for this outcome.
Summary of findings 2. Vitamin D (higher dose) versus vitamin D (lower dose).
| Vitamin D (higher dose) versus vitamin D (lower dose) | ||||||
| Patient or population: children under 5 years of age Setting: any country Intervention: oral vitamin D (higher dose: 200 to 6000 IU daily; or up to 600,000 IU bolus at enrolment) Comparison: oral vitamin D (lower dose: 100 to 1000 IU daily; or up to 300,000 IU bolus at enrolment) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) |
№. of participants (studies) |
Certainty of evidence (GRADE) | Comments | |
| Risk with lower‐dose vitamin D | Risk with higher‐dose vitamin D | |||||
|
Linear growth (length/height) Unit: cm Time frame: 4.2 months (mean) |
Mean length in control group was 57.8 cm. | Mean length in intervention group was 1.00 cm shorter (2.22 shorter to 0.21 longer). | ‐ | 283 (5 RCTs) |
⊕⊝⊝⊝ Very lowa | Two studies showed an increase in linear growth, and 3 studies found a decrease in linear growth. However, no difference was found overall |
|
Length/height‐for‐age z‐score (L/HAZ) Unitless Time frame: 7 months (mean) |
Mean height‐for‐age z‐score in control group was ‐0.35. | Mean height‐for‐age z‐score in intervention group was0.40 units higher (0.06 units lower to 0.86 units higher). | ‐ | 105 (2 RCTs) |
⊕⊕⊝⊝ Lowb | No difference in HAZ was found between groups |
| Stuntingc | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
|
Adverse effect: hypercalciuria As defined by trialists Time frame: 3.9 months (mean) |
Study population | RR 1.16 (1.00 to 1.35) | 554 (6 RCTs) |
⊕⊕⊝⊝ Lowb | There was no greater risk of increased calcium secretion in urine in groups receiving vitamin D | |
| 276 per 1000 | 320 per 1000 (276 to 372) | |||||
|
Adverse effect: hypercalcaemia As defined by trialists Time frame: 8.6 months (mean) |
Study population | RR 1.39 (0.89 to 2.18) | 986 (5 RCTs) |
⊕⊕⊝⊝ Lowb | There was no greater risk of increased calcium concentrations in blood in groups receiving vitamin D | |
| 64 per 1000 | 88 per 1000 (57 to 139) | |||||
| Adverse effect: hyperphosphataemiac | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Adverse effect: kidney stonesc | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to serious risk of bias. Evidence was downgraded an additional level due to imprecision, as the confidence interval around the effect size included 0, the null value. Evidence was downgraded an additional level due to inconsistency between studies, indicated by an I² value of 71%, suggesting substantial heterogeneity. bDowngraded one level due to serious risk of bias. Evidence was downgraded an additional level due to imprecision, as the confidence interval around the effect size included 0 or 1.0, the null value. cNo data were available for this outcome.
Summary of findings 3. Vitamin D (higher dose) + micronutrient(s) versus vitamin D (lower dose) + micronutrient(s).
| Vitamin D (higher dose) + micronutrient(s) versus vitamin D (lower dose) + micronutrient(s) | ||||||
| Patient or population: children under 5 years of age Setting: any country Intervention: oral vitamin D (higher dose: 400 to 2000 IU daily, or up to 300,000 IU bolus at enrolment) + micronutrient(s), including minerals such as calcium phosphate, multi‐vitamin, or both Comparison: oral vitamin D (lower dose: 200 to 2000 IU daily, or up to 90,000 IU bolus at enrolment) + micronutrient(s), including minerals such as calcium phosphate, multi‐vitamin, or both | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) |
№. of participants (studies) |
Certainty of evidence (GRADE) | Comments | |
| Risk with lower‐dose vitamin D + micronutrient(s) | Risk with higher‐dose vitamin D + micronutrient(s) | |||||
|
Linear growth (length/height) Unit: cm Time frame: 3 months |
Mean length in control group was 49.2 cm | Mean length in intervention group was 0.6 cm longer (3.33 shorter to 4.53 longer) | ‐ | 25 (1 RCT) |
⊕⊕⊝⊝ Lowa | No difference in linear growth was found between groups |
| Length/height‐for‐age z‐score (L/HAZ)b | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Stuntingb | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
|
Adverse effect: hypercalciuria As defined by trialists Time frame: 3 months |
Study population | RR 1.00 (0.06 to 15.48) | 86 (1 RCT) |
⊕⊕⊝⊝ Lowc | There was no greater risk of increased calcium secretion in urine in groups receiving vitamin D | |
| 23 per 1000 | 23 per 1000 (1 to 360) | |||||
|
Adverse effect: hypercalcaemia As defined by trialists Time frame: 2.2 months (mean) |
Study population | RR 1.00 (0.90 to 1.11) | 126 (2 RCTs) |
⊕⊕⊕⊝ Moderated | There was no greater risk of increased calcium concentrations in blood in groups receiving vitamin D | |
| 145 per 1000 | 298 per 1000 (268 to 331) | |||||
| Adverse effect: hyperphosphataemiab | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Adverse effect: kidney stonesb | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to risk of bias and imprecision, as the 95% CI for the effect measure included the null value of 0. Evidence was downgraded an additional level due to indirectness as only one study conducted in Finland was included, restricting the population analysed. bNo data were available for this outcome. cDowngraded one level due to risk of bias and imprecision, as the 95% CI for the effect measure included the null value of 1.0. Evidence was downgraded an additional level due to indirectness as only one study conducted in India was included, restricting the population analysed. dDowngraded one level due to risk of bias and imprecision, as the 95% CI for the effect measure included the null value of 1.0.
Subgroup analysis and investigation of heterogeneity
We did not conduct our preplanned subgroup analyses because we did not find enough studies meeting the required number (more than three) for comparison by outcome (Yu 2017).
Sensitivity analysis
We did not conduct our preplanned sensitivity analyses because we did not find enough studies meeting the required number (more than 10) for comparison by outcome (Yu 2017).
Results
Description of studies
Please see Characteristics of included studies, Characteristics of excluded studies, Characteristics of ongoing studies, and Characteristics of studies awaiting classification tables.
Results of the search
We found a total of 17,044 records (16,986 from electronic searches and 58 from other sources). After removing 5790 duplicates, we screened the remaining 11,254 unique records by title and abstract. We deemed 10,910 records to be irrelevant during screening and retrieved the full texts of the remaining 344 records for assessing eligibility.
We categorised 37 studies (80 reports) as 'Excluded'.
We identified 40 studies that included children within our age range but grouped their results with the results of children who were older than we had specified. We contacted the authors of each of these studies to request that they share age‐stratified data. The authors of five studies shared age‐stratified data; therefore we included these studies in the review (Rianthavorn 2013Sánchez‐Armendáriz 2018; Tang 2019; Thacher 2014; Trilok‐Kumar 2011).
In total, 75 studies (187 total reports) met our inclusion criteria (Criteria for considering studies for this review). Of these, 64 studies (169 reports) reported on our prespecified outcomes and were included in meta‐analyses. The remaining 11 studies did not report on any of our prespecified outcomes and therefore were not included in quantitative meta‐analysis (Alam 2011; Aly 2019; Choudhary 2012; Kislal 2008; Lava 2011; Manaseki Holland 2010; Pehlivan 2003; Rodd 2011; Saad 2015; Sarhan 2019; Singh 2019).
We categorised 33 additional studies (55 reports) as 'Ongoing' because their trial registration status indicated that recruitment was currently ongoing, or because trial recruitment was complete and study author(s) indicated that a manuscript(s) from the trial would be published in the coming months.
We categorised an additional 21 studies (22 reports) as 'Awaiting classification' because the trial registration indicated that the trial recruitment status was complete but no current or upcoming manuscript or meeting abstract could be found, or because the status of the trial was unknown. We also categorised studies that did not provide enough information to assess eligibility as 'Awaiting classification', specifically if the age group was not specified (Bantz 2015; Behnamfar 2011), or if the study design was unclear and the full‐text report could not be obtained (Hagag 2020; Özkan 2000). When we could identify contact information, we contacted the authors of all studies awaiting classification to request more information, and we kept the study categorised as 'Awaiting classification' if these attempts were unsuccessful.
We present the study selection procedure in a PRISMA diagram (Figure 1).
1.
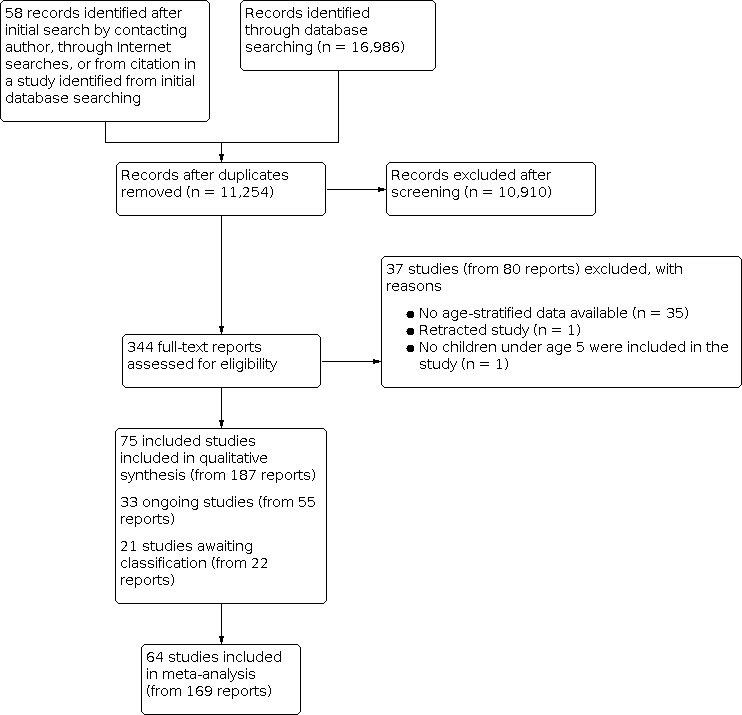
Study flow diagram.
Included studies
In total, we included in this review 75 studies (from 187 reports) with 12,122 participants. We summarise the key characteristics of these studies below. The Characteristics of included studies tables provide detailed information about the included trials in relation to the criteria prespecified in our protocol (Yu 2017). The earliest study was published in 1959 (Willi 1959), and the latest study was published in 2019 (Sarhan 2019).
Study design
Most included studies (70 studies) were parallel‐group, randomised controlled trials (RCTs). Four additional studies were quasi‐randomised controlled trials (Ala‐Houhala 1985; Holst‐Gemeiner 1978; Lagomarsino 1996; Willi 1959).
Two studies used a cross‐over design (Lava 2011; Rodd 2011), neither of which assessed any outcomes within the scope of this review. We did not find any cluster‐randomised trials.
Location/Setting
Most studies were conducted in India (14 studies), followed by the USA (10 studies), Canada (seven studies), and Finland (five studies). Four studies each took place in Egypt, Iran, and Turkey; three studies each were included from China and Germany; and two studies each were included from Afghanistan, Australia, Italy, Mexico, and Switzerland. The remaining studies reported on populations in Algeria, Austria, Bangladesh, Chile, Japan, Libya, London, Nigeria, Pakistan, Spain, and Thailand. Only six studies reported on children living at latitudes between the Tropics of Cancer (Northern Tropic) and Capricorn (Southern Tropic), and 67 studies reported on children living in latitudes outside the Tropic of Cancer or Capricorn. Two studies had multiple study sites falling both between and outside of the Northern and Southern Tropics.
A majority of studies (65 studies) were conducted in hospitals, primary care practices, or clinics, or had a point of contact in a hospital; four were run out of institutional settings (Ducharme 2019; Jensen 2016; Rao 2016; Ziegler 2014), and three reported catchment areas in cities or in areas around a hospital (Feliciano 1994; Manaseki‐Holland 2012; Specker 1992). Three studies did not report the exact setting (Rianthavorn 2013; Shajari 2009; Tomimoto 2018).
Participants
Collectively, participants at birth and up to five years of age were included. Eleven studies were conducted among both infants and children under five years of age, and nine additional studies were conducted among children older than one year. A majority of studies (55 studies) were conducted in infants younger than one year old. Four of the 55 infant studies followed up on the same participants after an extended follow‐up period without vitamin D supplementation in a subsequent report.
Baseline health status included being healthy; being preterm or (very) low birth weight, or both; having rickets; having severe acute malnutrition; having infectious diseases such as acute or recurrent otitis media, acute diarrhoea, bronchiolitis, pneumonia, or upper or lower respiratory tract infection; having non‐communicable diseases or disorders including asthma, chronic kidney disease, or chronic heart failure; or having autoimmune diseases such as juvenile idiopathic arthritis or atopic dermatitis.
Participant characteristics organised across the included studies are found in Table 6.
3. Participant characteristics.
Interventions
Study interventions involved oral vitamin D supplementation in the form of vitamin D₃ (53 studies) or vitamin D₂ (seven studies), or did not specify the type of vitamin D involved (12 studies). Two studies involved both vitamin D₃ and vitamin D₂ (Gallo 2013a; Gordon 2008), and one study involved D₂ and calcitriol (1,25(OH)₂D₃) (Chan 1978). We grouped studies by intervention into four comparisons: (1) those that compared vitamin D to placebo or no intervention; (2) those that compared a higher dose of vitamin D to a lower dose of vitamin D; (3) those that compared a micronutrient intervention plus vitamin D to the same micronutrient intervention without vitamin D; and (4) those that compared a micronutrient intervention plus a higher dose of vitamin D to the same micronutrient intervention with a lower dose of vitamin D) (Table 4). Please see Differences between protocol and review regarding our rationale for grouping the analysis by each of the following four comparisons.
Comparison 1: vitamin D versus placebo or no intervention
Thirty‐one studies compared vitamin D to placebo or no intervention, with a total of 7327 participants. Daily dosages of vitamin D ranged from 200 IU in Ponnapakkam 2010 to 2000 IU in Tang 2019. Bolus or pharmacological doses ranged from 40,000 IU in Rianthavorn 2013 to 300,000 IU in Singh 2019, which was usually given once, at enrolment ‐ Jensen 2016; Manaseki Holland 2010; Moodley 2015; Somnath 2017 ‐ or every few weeks ‐ Rianthavorn 2013; Saleem 2018 ‐ or months ‐ Manaseki‐Holland 2012; Singh 2019. The duration of follow‐up ranged from 60 hours in Chan 1978 to 20 months in Singh 2019.
Comparison 2: vitamin D (higher dose) versus vitamin D (lower dose)
Thirty‐four studies compared regimens of higher versus lower doses of vitamin D, with a total of 4027 participants. Daily dosages of the higher dose of vitamin D ranged from 200 IU in Specker 1992 to 6000 IU in Willi 1959, compared to lower doses of vitamin D of 100 IU in Specker 1992 up to 1000 IU in Morawa 1963. Nine studies investigated the effects of administering bolus or pharmacological doses, ranging from 50,000 IU in Huynh 2017; Shajari 2009; and Shakiba 2010 to 600,000 IU in Harnot 2017; Lagomarsino 1996; and Mittal 2014, compared to a daily lower‐dose vitamin D supplementation in Holst‐Gemeiner 1978; Huynh 2017; Mittal 2014; and Zeghoud 1994 or smaller bolus doses in Harnot 2017; Mittal 2014; and Zeghoud 1994. One study administered two bolus doses of 600,000 IU at months 1 and 5 of follow‐up (Lagomarsino 1996). Duration of administration ranged from 5 to 10 minutes in an acceptability study ‐ Lava 2011 ‐ to 24 months in Rosendahl 2018. One study did not report the duration of follow‐up (Pehlivan 2003). Finally, one study examining four vitamin D intervention groups with higher or lower doses of vitamin D included micronutrient supplementation (minerals, calcium, and phosphorus) in two of the four groups; therefore data from the two arms not containing calcium and phosphorus were included in this comparison (Backström 1999b).
Comparison 3: vitamin D + micronutrient(s) versus micronutrient(s) alone
One study was included in this comparison (Thacher 2014). This study investigated effects of 50,000 IU vitamin D₂ plus calcium against a placebo and calcium, given every month, for six months, among 53 participants.
Comparison 4: vitamin D (higher dose) + micronutrient(s) versus vitamin D (lower dose) + micronutrient(s)
Nine studies, with a total of 649 participants, investigated micronutrient(s) content plus vitamin D versus the same micronutrient(s) content with or without a lower dose of vitamin D (Alizadeh 2006; Alizadeh Taheri 2014; Backström 1999b; Evans 1989; Gordon 2008; Mathur 2016; Mittal 2018; Rao 2016; Tergestina 2016). Six studies compared daily vitamin D supplementation, ranging between 400 IU in Alizadeh Taheri 2014 and 2000 IU in Evans 1989 to a daily lower dose of vitamin D, ranging between 200 IU in Alizadeh Taheri 2014 and 2000 IU in Gordon 2008. Daily supplementation of 4000 IU in Rao 2016 or 2000 IU in Gordon 2008 was compared to weekly supplementation of 30,000 IU in Rao 2016 or 50,00 IU in Gordon 2008, respectively. A bolus dose (300,000 IU) was compared to 90,000 IU, both given once, at enrolment (Mittal 2018). Duration of follow‐up ranged from 51 days in Tergestina 2016 to 9 months in Rao 2016. Finally, one study examining four vitamin D groups with higher or lower doses of vitamin D included calcium and phosphorus supplementation in two of the four arms; therefore data from the two groups containing calcium and phosphorus supplementation were included in this comparison (Backström 1999b). Micronutrients administered in these studies mainly included minerals such as calcium (all studies), phosphorus (Alizadeh 2006; Alizadeh Taheri 2014; Backström 1999b; Mathur 2016; Tergestina 2016), and/or a multi‐vitamin (Mathur 2016; Tergestina 2016).
Outcomes
Primary outcomes
Thirty‐one studies included a primary outcome. Of these, 14 evaluated linear growth (Anderson‐Berry 2017; Backström 1999a; Backström 1999b; Chandy 2016; Gallo 2013b; Greer 1981; Greer 1989; Holmlund‐Suila 2012; Huynh 2017; Lagomarsino 1996; Natarajan 2014; Siafarikas 2011; Singh 2018a; Trilok‐Kumar 2011). Three reported on height‐for‐age z‐scores (HAZ) (Gallo 2013a; Gallo 2013b; Trilok‐Kumar 2011), and one reported on stunting (Trilok‐Kumar 2011). We found 29 studies reporting adverse effects (hypercalciuria, hypercalcaemia, hyperphosphataemia, and/or kidney stones).
Linear growth
Linear growth (length) was measured using infantometers or infant length boards (Greer 1981; Greer 1989; Chandy 2016; Singh 2018a), wall‐mounted stadiometer (Thacher 2014), clinical charts (Backström 1999a; Backström 1999b), or standardised calibrated equipment (Siafarikas 2011), in centimetres; several studies did not specify what type of equipment was used to measure length (Anderson‐Berry 2017; Holmlund‐Suila 2012; Huynh 2017; Lagomarsino 1996; Natarajan 2014).
Length/height‐for‐age and stunting
Height‐for‐age was measured using the WHO Child Growth Standards, with stunting defined as HAZ less than 2 standard deviations (SDs) below the WHO reference standard (WHO 2006). Linear growth for calculation of HAZ was measured using infantometers or infant length boards (Gallo 2013b; Trilok‐Kumar 2011), or it was not described (Gallo 2013a).
Adverse effects
Hypercalciuria
Hypercalciuria was measured using the urinary calcium‐to‐creatinine ratio. Urinary calcium was assayed by Beckman Coulter assay (Bozkurt 2017; Gallo 2013b; Natarajan 2014), photometric assay (Holmlund‐Suila 2012), or DiasSorin Auto Analyzer (Mittal 2018); colorimetrically using o‐cresol phthalein complexone (Pointe Scientific) (Shajari 2009), chemiluminescence (VitrosEci) (Singh 2018a), complexometric method with ethylenediaminetetraacetic acid (calcium), and the kinetic Jaffé reaction (creatinine) (Evans 1989); or by clinical chemistry analyser (Harnot 2017), urine spot (Tergestina 2016), or standard assays without further detail (Siafarikas 2011; Zeghoud 1994). Two studies did not specify which assay was used (Ducharme 2019; Gallo 2013a). Studies defined hypercalciuria as urinary calcium‐to‐creatinine ratio greater than 2.2 mmol/mmol (Holmlund‐Suila 2012); greater than 1.25 mmol/mmol (for one‐to‐two‐year‐olds) or > 1 mmol/mmol (for two‐to‐five‐year‐olds) (Ducharme 2019; Jensen 2016; Mittal 2018); > 0.8 mg/mg (Natarajan 2014); > 1.35 mg/mg (Tergestina 2016); > 0.21 mmol/mmol (Shajari 2009); or > 0.86, 0.6, and 0.4 for children < 7 months old, 7 to 18 months old, and 19 months to 6 years old, respectively (Harnot 2017); or did not define hypercalciuria (Bozkurt 2017; Gallo 2013a; Gallo 2013b; Siafarikas 2011; Singh 2018a).
Hypercalcaemia
Hypercalcaemia was measured using total serum calcium. Total serum calcium was assayed by the Beckman Coulter assay (Anderson‐Berry 2017; Hanson 2011; Huynh 2017; Natarajan 2014), randox (Chandy 2016), atomic absorption spectroscopy (Chan 1978), a multi‐channel analyser (Roche Diagnostics) (Gordon 2008), Dimension RxL Max clinical chemistry analyser (Harnot 2017), photometric assays (Holmlund‐Suila 2012), DiasSorin Auto Analyzer (Mittal 2018), flex gas analysers (Rosendahl 2018), spectrophotometric methods (Tergestina 2016), 'standard methods' without further detail (Siafarikas 2011; Zeghoud 1994), or ethylene glycol tetra‐acetic acid titration (Robinson 1981), or colorimetrically using o‐cresol phthalein complexone (Pointe Scientific) (Ziegler 2014). Six studies did not report the assay or method used (Aglipay 2017; Ducharme 2019; Gallo 2013a; Hibbs 2018; Mittal 2014; Shakiba 2010). Studies defined hypercalcaemia as total serum calcium > 10.5 mg/dL (Chan 1978; Chandy 2016), > 10.7 mg/dL (Hibbs 2018), > 10.8 mg/dL (Gupta 2016; Mittal 2014; Mittal 2018; Tergestina 2016), or > 11.2 mg/dL (Zeghoud 1994), or did not define hypercalcaemia (Aglipay 2017; Anderson‐Berry 2017; Gallo 2013b; Gordon 2008; Hanson 2011; Holmlund‐Suila 2012; Huynh 2017; Natarajan 2014; Robinson 1981; Rosendahl 2018; Siafarikas 2011; Shakiba 2010; Ziegler 2014).
Hyperphosphataemia
Hyperphosphataemia was measured using serum phosphorus. Methods for hyperphosphataemia were done using "standard assays" (Siafarikas 2011), or it was indicated that they were carried out at the study's clinical chemistry laboratory and otherwise not detailed (Aglipay 2017; Hibbs 2018). Only one study defined hyperphosphataemia as serum phosphorus > 9.5 mg/dL (3.07 mmol/L) (Hibbs 2018).
Kidney stones
Kidney stones were assessed using renal ultrasonography (Abdel‐Hady 2019; Singh 2018a), or methods were not reported (Natarajan 2014).
Secondary outcomes
Gain in length
Gain in length was reported by three studies (Feliciano 1994; Mathur 2016; Ziegler 2014). Length was assessed using an infantometer (Mathur 2016), or standardised methods were used (Ziegler 2014). In one study, the method of measurement was not described (Feliciano 1994).
Weight‐for‐age and weight‐for‐height/length
Four studies reported on weight‐for‐age and weight‐for‐height/length (Gallo 2013a; Gallo 2013b; Saleem 2018; Trilok‐Kumar 2011). Weight‐for‐age z‐score (WAZ) and weight‐for‐height/length z‐scores (WHZs) were measured using the WHO Child Growth standards (WHO 2006). Weight was measured using infant weighing scales (Gallo 2013b; Saleem 2018; Trilok‐Kumar 2011); in one study, the method of measurement was not reported (Gallo 2013a). Height/length was measured using a wall‐mounted stadiometer or infant length board (Gallo 2013b; Saleem 2018; Trilok‐Kumar 2011). Recumbent length was measured among participants under two years of age, and standing height was measured when the child was over two years of age. One study reported on both underweight and wasting (Trilok‐Kumar 2011).
Serum 25(OH)D concentration
Fifty‐nine studies reported on vitamin D status (continuously or categorically in terms of deficiency or insufficiency versus sufficiency). Vitamin D status was measured using chemiluminescence protein‐binding assay via the Cobase analyser kit with Elecsys Vitamin D Total Assay (Roche Diagnostics Ltd.) or automated immunoassay (IDS‐iSYS, Immunodiagnostic System Ltd.) (CLPBA) (Gallo 2013a; Holmlund‐Suila 2012; Manaseki‐Holland 2012; Mittal 2018; Natarajan 2014; Ponnapakkam 2010; Rosendahl 2018; Rueter 2019); competitive protein‐binding assay (CPBA) (Aglipay 2017; Ala‐Houhala 1985; Greer 1981; Mathur 2016; Robinson 1981; Specker 1992; Stögmann 1985; Zeghoud 1994); electro‐chemiluminescent assay (EIA) (Alizadeh Taheri 2014; Fort 2016; Gallo 2013b; Harnot 2017; Sánchez‐Armendáriz 2018; Somnath 2017); liquid chromatography with tandem mass spectrometry (LC‐MS/MS) (Anderson‐Berry 2017; Bozkurt 2017; Ducharme 2019; Gallo 2013a; Gallo 2013b; Huynh 2017; Jensen 2016; Moodley 2015; Saleem 2018; Thacher 2014); high‐performance liquid chromatography (HPLC) (Atas 2013; Backström 1999a; Backström 1999b; Greer 1989; Tang 2019); enzyme‐linked immunoabsorbent assay (ELISA) (Abdel‐Hady 2019); immunoassay (Hibbs 2018); radioimmunoassay (RIA) (Chan 1978; Chandy 2016; Evans 1989; Gallo 2013b; Gupta 2016; Hanson 2011; Holst‐Gemeiner 1978; Mittal 2014; Shedeed 2012; Siafarikas 2011; Trilok‐Kumar 2011; Ziegler 2014); and chemiluminescent assay (CLIA) (Gordon 2008; Marchisio 2013; Principi 2013; Rao 2016; Rianthavorn 2013; Shakiba 2010; Singh 2018a; Tergestina 2016). As shown, Gallo 2013a evaluated vitamin D concentration using two assays (CLPBA and LC‐MS/MS), and Gallo 2013b evaluated vitamin D concentration using three different assays (EIA, RIA, and LC‐MS/MS). One study did not report the vitamin D assay method used (Tomimoto 2018).
Rickets
Fourteen studies reported on rickets (Ala‐Houhala 1985; Alizadeh 2006; Chandy 2016; Greer 1981; Huynh 2017; Mittal 2014; Mittal 2018; Morawa 1963; Ponnapakkam 2010; Robinson 1981; Siafarikas 2011; Specker 1992; Thacher 2014; Willi 1959).
We observed variation across 15 studies in the trial definitions of 'rickets', which was one of our secondary outcomes. Definitions of rickets as a dichotomous outcome across these studies included biochemical concentrations (measured by serum calcium, phosphorus, magnesium, and alkaline phosphatase; thresholds unspecified) (Ala‐Houhala 1985); wide fontanelles, not defined (Huynh 2017), or defined as > 3 × 3 cm (Alizadeh 2006); craniotabes score, using a size‐based scale (Morawa 1963), or the rate of craniotabes, undefined (Huynh 2017); X‐ray changes, defined as fractures in the left‐hand radiograph (Alizadeh 2006), or presentation of florid changes (Morawa 1963); clinical signs, defined as a combination of rachitic rosary, craniotabes, or widened wrists (Greer 1981); radiological scores > 0 (Mittal 2014; Mittal 2018); widened epiphyses or limb deformities, undefined (Huynh 2017); combinations of signs, such as elevated alkaline phosphatase and evidence of X‐ray changes (Ponnapakkam 2010), or concavity and fraying of bone, widening of epiphyses (Specker 1992); radiological evidence, not defined (Robinson 1981); and clinical signs, including appearing translucent, pale, flushed, or showing failure (translated from German; Willi 1959). Two studies also reported symptoms of rickets as a continuous outcome, including mean radiographic score (Thacher 2014), median radiographic score (Evans 1989), and median anterior fontanelle size (Chandy 2016); these studies did not share the same control group (Chandy 2016 used placebo, and Evans 1989 and Thacher 2014 used a lower dose of vitamin D).
Missing data
We contacted study authors for additional information on included studies, as needed; most requests involved author sharing of age‐stratified data to include only children under five years of age in the results. We also asked study authors to send us a full‐text publication citation, if existing, of any meeting abstracts that we found, or to share unpublished data that could be incorporated into our analysis, if relevant.
In summary, we obtained a positive response (i.e. study authors shared specific information, published or unpublished data, or results) for nine studies (Aglipay 2017; Ponnapakkam 2010; Rianthavorn 2013; Rueter 2019; Sánchez‐Armendáriz 2018; Tang 2019; Thacher 2014; Trilok‐Kumar 2011; Ziegler 2014).
Funding sources
Studies were funded by a variety of sources, namely, non‐profit funding. Two studies reported provision of the drug by the manufacturer, along with non‐profit funding (Gallo 2013a; Huynh 2017). Two studies reported for‐profit funding (Rodd 2011; Tomimoto 2018). Two studies were categorised as mixed funding (non‐profit and for‐profit funding) (Chan 1978; Greer 1981). Five studies specifically reported no funding (Bozkurt 2017; Choudhary 2012; Lava 2011; Mittal 2018; Sarhan 2019), and 26 studies did not disclose funding sources. The remaining studies were funded by non‐profit sources. Information on specific funding sources may be found in the Characteristics of included studies tables.
Excluded studies
We excluded 37 studies (80 reports) for the following reasons: for 35 studies, no stratified data were available for population age group (which included children over five years of age), after contact with the study author; one study was retracted (Saad 2018); and one study's author indicated that no children under age five years were included in the study (Swangtrakul 2020). We considered conducting a sensitivity analysis including the studies from which we were unable to obtain age‐stratified data using a threshold of children under the age of five years constituting ≥ 80% of the study population, based on descriptive statistics presented for the whole population; however, no study appeared to meet this criterion, or studies did not present variance estimates, limiting our inference. As such, these studies have not been included in the review meta‐analyses. Further details may be found in the Characteristics of excluded studies tables.
Reasons for negative responses from study authors included not enough time to re‐analyse the data; most children were ineligible (over five years of age); data were unavailable; or no response was received to our follow‐up email after an initial positive response (see Characteristics of excluded studies).
Ongoing studies
We identified 33 ongoing studies (from 55 reports). These studies were registrations for trials for which no full‐text publication was identified, recruitment was currently ongoing, or trial recruitment was complete and study author(s) indicated that a manuscript(s) from a trial would be published in the coming months. We present a brief overview of these studies below. Further details may be found in the Characteristics of ongoing studies.
Study design
Ongoing studies included 32 parallel‐group RCTs and one cross‐over RCT (RBR‐4r6p5v).
Location/Setting
The studies are being conducted in India (nine studies), Canada (six studies), USA (three studies), Chile (two studies), and Poland (two studies), and one study a piece is being conducted in Australia, Brazil, China, France, Indonesia, Iran, Israel, Japan, Saudi Arabia, and Spain. An additional study is being conducted across three countries: Austria, Canada, and Chile.
Settings include hospitals (10 studies), intensive care units (two studies), clinics (one study), and a university medical centre (one study), or the setting has not been reported (19 studies).
Participants
Studies included or aimed to include infants and children. Studies among infants (12 studies) included healthy (5 studies), small‐for‐gestational‐age (1 study), preterm (5 studies), and low birth weight (2 studies) populations (with some overlap present). Studies among children (12 studies) included populations that were healthy (5 studies), or had asthma (1 study), atopic dermatitis (1 study), epilepsy (1 study), chronic kidney disease (1 study), Crohn's disease (1 study), or vitamin D deficiency plus low energy fracture (1 study). Several studies among children included only children over five age years of age (seven studies). Studies among both infants and children (nine studies) included populations that were healthy (two studies), or had rickets (two studies), cyanotic congenital heart disease (one study), vitamin D deficiency (two studies), lower respiratory tract infection (one study), or chronic heart disease requiring surgery (one study). Of these, five studies included children over the age of five years.
Interventions
Please see Differences between protocol and review regarding our rationale for grouping the analysis by each of the following four comparisons.
Comparison 1: vitamin D versus placebo or no intervention
Sixteen studies examined vitamin D versus placebo or no intervention. Doses ranged from 400 IU in ACTRN12614000334606/NCT02112734; CTRI/2013/04/003566; CTRI/2015/08/006132; UMIN000034864; and NCT01363167 to 100,000 IU in NCT03365687. Duration ranged from six weeks in NCT01996423 to one year in ACTRN12616000659404; and Galdo 2018, with one study not reporting the duration of follow‐up (CTRI/2017/12/010827).
Comparison 2: vitamin D (high dose) versus vitamin D (low dose)
An additional 16 studies examined a high dose of vitamin D versus a lower dose of vitamin D. As a note, one study examined two different dosages of vitamin D versus placebo; therefore it is applicable to both comparison 1 and comparison 2 (NCT02046577). Doses ranged from 400 IU in NCT02563015 to 150,000 IU in CTRI/2018/12/016760 in the higher‐dose group, and from 400 IU in NCT02563015 to 4000 IU in CTRI/2018/12/016760 in the lower‐dose group. Duration ranged from three weeks in CTRI/2018/04/013300 to three years in NCT02563015.
As a note, one additional study examined two interventions: 5600 IU vitamin D₃ versus 11,200 IU vitamin D₃, compared to placebo; as such, in a future version of this review, we may include this study in both comparison 1 and comparison 2 and analyse the study arms accordingly (comparison 1: 5600 IU D₃ versus placebo and 11,200 IU D₃ versus placebo; comparison 2: 5600 IU D₃ versus 11,200 IU D₃) (NCT02046577).
Comparison 3: vitamin D + micronutrient(s) versus micronutrient(s) alone
No studies are assessing this comparison.
Comparison 4: vitamin D (high dose) + micronutrient(s) versus vitamin D (low dose) + micronutrient(s)
One study was included in this comparison (IRCT20171030037093N4). This study investigated the effects of 300 IU vitamin D and an additional 400 IU vitamin D plus vitamin A, against 300 IU vitamin D and vitamin A, until 40 weeks' postmenstrual age.
Outcomes
Primary outcomes
Five studies listed "growth" (linear growth) in their protocol as an outcome (CTRI/2013/04/003566; CTRI/2015/08/006132; Galdo 2018; NCT03742310; NCT01363167). Eleven studies listed adverse effects (hypercalcaemia, hypercalciuria, and/or kidney stones) as outcomes of interest (CTRI/2017/11/010385; CTRI/2017/12/010827; CTRI/2018/12/016760; Galdo 2018; NCT03365687; NCT03536845; NCT03087149; NCT02452762; NCT01838447; NCT03742505; NCT01363167).
Secondary outcomes
Twenty‐five studies listed examining mean 25(OH)D concentrations, or changes in and/or achieving sufficiency. Although nine ongoing studies included serum calcium, urinary calcium, serum phosphorus, the urinary calcium‐to‐creatinine ratio, or adverse events/effects, they did not specifically list measuring hypercalciuria, hypercalcaemia, hyperphosphataemia, or kidney stones specifically as adverse effects.
Missing data
We contacted the authors of the trial registrations for additional information, including asking the authors to confirm a full‐text publication of any meeting abstracts found, or to share unpublished data that we could cite (see Characteristics of ongoing studies tables for details).
Funding sources
Fourteen studies were funded by non‐profit entities; one study was funded by a non‐profit organisation plus the company provided the drug; one study received no funding; and one study was funded by a for‐profit entity. The remaining 16 studies did not disclose funding sources.
Studies awaiting classification
We categorised an additional 21 studies (22 reports) as 'Awaiting classification' if the trial registration indicated that the trial recruitment status was complete but no current or upcoming manuscript or meeting abstract could be found, or if the status of the trial was unknown. We also categorised studies that did not provide enough information to assess eligibility as 'Awaiting classification', specifically, if the age group was not specified (Bantz 2015; Behnamfar 2011), or if the study design was unclear and the full‐text report could not be obtained (Hagag 2020; Özkan 2000). When we could identify contact information, we contacted the authors of all studies awaiting classification to ask for more information, and we kept the study categorised as 'Awaiting classification' if these attempts were unsuccessful. See Characteristics of studies awaiting classification for more information.
Risk of bias in included studies
Below, we summarise the results of our 'Risk of bias' assessment. Further details can be found in the 'Risk of bias' tables, beneath the Characteristics of included studies tables. Figure 2 and Figure 3 provide graphical summaries of the 'Risk of bias' assessment.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Included studies were individually randomised or block‐randomised controlled trials.
Sequence generation
We determined that 37 studies had adequate sequence generation and subsequently rated them at low risk of bias. Methods included computer‐based random number generators, such as random number tables, or Statiscal Analysis Software (SAS) procedures (31 studies); websites such as www.randomization.com and www.randomizer.org (four studies: Gallo 2013a; Moodley 2015; Rodd 2011; Rueter 2019); and the low‐tech technique of coin flips (two studies: Evans 1989; Thacher 2014). Seven studies reported inadequate methods of sequence generation due to employing alternating randomisation and therefore were rated at high risk of bias (Holst‐Gemeiner 1978; Lagomarsino 1996; Morawa 1963; Pehlivan 2003; Siafarikas 2011; Singh 2019; Willi 1959). The remaining 31 studies reported that groups were randomly allocated but did not provide details on how the randomisation sequence was generated and therefore were rated at unclear risk of bias.
Allocation concealment
We judged 37 studies to have adequate allocation concealment and thus low risk of bias, 14 studies to have inadequate methods of allocation concealment and therefore high risk of bias, and 24 studies to have unclear methods of allocation concealment and unclear risk of bias. The 14 studies with inadequate allocation concealment included studies in which allocation concealment was not described and the varying dosages/frequencies would indicate the allocation given (Atas 2013; Feliciano 1994; Gordon 2008; Holst‐Gemeiner 1978; Lagomarsino 1996; Ponnapakkam 2010; Rianthavorn 2013; Rodd 2011; Singh 2019; Tang 2019; Willi 1959); one study in which the types of interventions would indicate allocation, which included two study arms that were administered intramuscular vitamin D (Morawa 1963); one study in which parents were directly told the allocation (Chan 1978); and one study that used odd‐ and even‐numbered envelopes to allocate the intervention (Siafarikas 2011). The 24 studies with unclear risk of bias generally did not describe their allocation concealment procedures in enough detail to allow a judgement on their risk of selection bias.
Blinding
Twenty‐four studies were described as 'double‐blind' or appeared so, three studies were described as 'single‐blind' (Lava 2011; Principi 2013; Rao 2016), and 12 studies were specifically not blinded (i.e. 'open label') (Alonso 2011; Huynh 2017; Mittal 2014; Singh 2018a; Singh 2019; Somnath 2017; Stögmann 1985; Tang 2019; Thacher 2014; Tomimoto 2018; Willi 1959; Zeghoud 1994). Thirty‐seven studies had partial or non‐described blinding. One study was triple‐blind (Ducharme 2019).
Blinding of participants and staff (performance bias)
Many studies did not describe blinding, or were blinded only to staff and not parents, leading us to judge 39 studies as having high risk of performance bias. We judged 35 studies to be at low risk of bias as they either were double‐blind or were blinded to staff with likely blinding to parents of participants (even if not stated explicitly). We considered one study, Gallo 2013a, to have unclear risk of performance bias due to lack of description of blinding, but because of adequate allocation concealment, participants and staff were likely blinded.
Blinding of outcome assessors (detection bias)
We judged 33 studies to be at high risk of detection bias due to lack of description and the subjective nature of outcomes. We rated 37 studies at low risk of detection bias due to explicit mention of blinding to outcome assessors or mention of double‐blinding. We judged five studies to be at unclear risk of detection bias due to lack of a specific description but likely blinded due to the mention of a "double‐blind" study design (Evans 1989; Greer 1981; Rosendahl 2018; Saad 2015; Sarhan 2019).
Incomplete outcome data
We judged 16 studies to be at high risk of attrition bias, 34 at low risk of attrition bias, and 25 at unclear risk of attrition bias. Reasons for high risk of attrition bias included lack of reporting on the number of participants at randomisation compared to endpoint (Alizadeh 2006); high loss to follow‐up (Chandy 2016; Greer 1989; Mittal 2014); participants lost to follow‐up not examined for differences from those who were included (Alonso 2011; Feliciano 1994; Ponnapakkam 2010); reasons for loss to follow‐up not given, not compared by arm, or both (Alizadeh 2006; Atas 2013; Feliciano 1994; Greer 1989; Kislal 2008; Mittal 2018; Moodley 2015; Ponnapakkam 2010); outcomes reported at an intermediate study time point but not at the end of full follow‐up (Abdel‐Hady 2019); or use of complete case or per‐protocol analysis instead of intent‐to‐treat analysis (Backström 1999a; Chandy 2016; Greer 1989; Kislal 2008; Mittal 2018; Moodley 2015; Ponnapakkam 2010; Rao 2016; Shakiba 2010; Specker 1992). Reasons for low risk of bias included indistinguishable interventions/comparators; all randomised participants completing follow‐up or no missing data; reasons for missing data not related to the outcome (e.g. moving away); missing data balanced across groups and similar reasons; small proportion of missing data; and intention‐to‐treat analysis conducted, including all participants randomised. We assigned a judgement of unclear risk of bias when insufficient information was available to reach a judgement of high or low risk of bias.
Selective reporting
We considered most studies (50 studies) to be at unclear risk of reporting bias, as no study protocols or trial registration identifiers were reported (see Risk of bias in included studies), or a trial registration was found online but appeared to have been published after the study was completed. We judged two studies to be at high risk of bias because the methods sections mentioned measuring growth (Alonso 2011), or referred to specific biochemical parameters (Backström 1999a), but these measures were not reported in the results section; in addition, neither study had a published protocol or trial registration.
We judged 23 studies to be at low risk of bias because they had a protocol pre‐registered on a trial registry, or because they cited a published study protocol that proposed measuring the outcomes presented in the published study (Aglipay 2017; Rosendahl 2018).
Also, for each comparison, we visually inspected funnel plots to assess for bias due to missing results in our primary outcomes; we did not observe bias due to missing results.
Other potential sources of bias
We did not observe any other potential sources of bias in these studies and therefore rated all studies at low risk of bias on this domain.
Effects of interventions
See: Table 1; Table 2; Table 3
Please see Differences between protocol and review regarding our rationale for grouping the analysis by each of the following four comparisons.
Please see Table 7 for the results of sensitivity analyses conducted with fixed‐effect models for outcomes including at least two studies in the comparison.
4. Sensitivity analyses: results of analyses using fixed‐effect models with ≥ 2 studies.
| Results of sensitivity analysis with fixed‐effect model | |||||
| Comparison 1: vitamin D vs placebo or no intervention | Number of studies | Mean difference (95% CI) | Chi² | P value for overall effect | I²(%) |
| Linear growth (Analysis 1.1) | 3 | 0.73 (0.01 to 1.45) | 3.96 | 0.05 | 49 |
| Adverse effect: hypercalciuria (Analysis 1.4) | 2 | 2.03 (0.28 to 14.67) | 0.63 | 0.48 | 0 |
| Adverse effect: hypercalcaemia (Analysis 1.5) | 2 | 0.79 (0.43 to 1.44) | 1.93 | 0.44 | 48 |
| Weight‐for‐height (z‐score) (Analysis 1.8) | 2 | 0.06 (‐0.06 to 0.19) | 13.61 | 0.33 | 93 |
| Serum 25(OH)D (Analysis 1.10) | 21 | 25.04 (23.10 to 26.98) | 369.62 | < 0.001 | 95 |
| Change in 25(OH)D (Analysis 1.11) | 3 | 34.09 (28.90 to 39.28) | 17.35 | < 0.001 | 88 |
| Vitamin D sufficiency (≥ 50 nmol/L) (Analysis 1.12) | 6 | 1.88 (1.66 to 2.14) | 6.25 | < 0.001 | 20 |
| Vitamin D sufficiency (≥ 75 nmol/L) (Analysis 1.13) | 2 | 2.47 (1.50 to 4.06) | 11.30 | 0.0004 | 91 |
| Vitamin D severe deficiency (Analysis 1.14) | 3 | 0.26 (0.19 to 0.36) | 1.68 | < 0.001 | 0 |
| Comparison 2: vitamin D (higher dose) vs vitamin D (lower dose) | Number of studies | Mean difference (95% CI) | Chi² | P value for overall effect | I²(%) |
| Linear growth (Analysis 2.1) | 5 | ‐0.75 (‐1.33 to ‐0.17) | 13.64 | 0.01 | 71 |
| Length/height‐for‐age (z‐score) (Analysis 2.2) | 2 | 0.40 (‐0.06 to 0.86) | 0.04 | 0.09 | 0 |
| Adverse effect: hypercalciuria (Analysis 2.3) | 6 | 1.16 (1.00 to 1.35) | 1.88 | 0.06 | 0 |
| Adverse effect: hypercalcaemia (Analysis 2.4) | 5 | 1.39 (0.89 to 2.18) | 2.16 | 0.15 | 0 |
| Linear growth: gain in length (Analysis 2.5) | 3 | ‐0.01 (‐0.02 to 0.00) | 0.68 | 0.06 | 0 |
| Weight‐for‐age (z‐score) (Analysis 2.6) | 2 | 0.07 (‐0.44 to 0.58) | 0.01 | 0.78 | 0 |
| Serum 25(OH)D (Analysis 2.8) | 20 | 14.73 (13.24 to 16.22) | 493.04 | < 0.001 | 96 |
| Change in 25(OH)D (Analysis 2.9) | 3 | 1.68 (‐1.08 to 4.43) | 3.67 | 0.23 | 46 |
| Vitamin D sufficiency (≥ 50 nmol/L) (Analysis 2.10) | 12 | 1.02 (1.00 to 1.03) | 17.24 | 0.008 | 42 |
| Vitamin D sufficiency (≥ 75 nmol/L) (Analysis 2.11) | 6 | 1.25 (1.18 to 1.31) | 8.05 | < 0.001 | 38 |
| Rickets (Analysis 2.13) | 4 | 0.64 (0.46 to 0.90) | 1.24 | 0.009 | 0 |
| Comparison 4: vitamin D (higher dose) + micronutrient(s) vs vitamin D (lower dose) + micronutrient(s) | Number of studies | Mean difference (95% CI) | Chi² | P value for overall effect | I²(%) |
| Adverse effect: hypercalcaemia (Analysis 4.3) | 2 | 1.00 (0.90 to 1.11) | 0 | 1.00 | 0 |
| Serum 25(OH)D (Analysis 4.5) | 5 | 25.91 (20.50 to 31.32) | 112.69 | < 0.001 | 96 |
| Vitamin D sufficiency (≥ 75 nmol/L) (Analysis 4.7) | 3 | 1.13 (0.97 to 1.31) | 25.65 | 0.12 | 92 |
| Rickets (Analysis 4.8) | 2 | 1.23 (0.24 to 6.30) | 0.43 | 0.80 | 0 |
CI: confidence interval. Serum 25(OH)D: serum 25‐hydroxyvitamin D.
Comparison 1: vitamin D versus placebo or no intervention
Primary outcomes
Please see Table 1. All outcomes were measured at the end of the intervention, with average time frames ranging from 6 to 7.5 months.
Linear growth
There is little to no difference between vitamin D and placebo or no intervention in linear growth (mean difference (MD) 0.66 cm, 95% confidence interval (CI) ‐0.37 to 1.68; 3 studies, 240 participants; I² = 49%; tau² = 0.41; random‐effects model; Analysis 1.1; low‐certainty evidence). The results were similar with a fixed‐effect model (Table 7).
1.1. Analysis.

Comparison 1: Vitamin D versus placebo or no intervention, Outcome 1: Linear growth
Length/height‐for‐age (L/HAZ)
Compared to placebo or no intervention, vitamin D may improve length/height‐for‐age z‐score (L/HAZ) scores (MD 0.11, 95% CI 0.001 to 0.22; 1 study, 1258 participants; fixed‐effect model; Analysis 1.2; moderate‐certainty evidence).
1.2. Analysis.

Comparison 1: Vitamin D versus placebo or no intervention, Outcome 2: Length/height‐for‐age
Stunting
Some evidence suggests that, compared to placebo or no intervention, vitamin D has little to no effect on stunting (risk ratio (RR) 0.90, 95% CI 0.80 to 1.01; 1 study, 1247 participants; fixed‐effect model; Analysis 1.3; moderate‐certainty evidence).
1.3. Analysis.

Comparison 1: Vitamin D versus placebo or no intervention, Outcome 3: Stunting
Adverse effects
Hypercalciuria
Insufficient evidence suggests that, compared to placebo or no intervention, vitamin D has an effect on the incidence of hypercalciuria (RR 2.03, 95% CI 0.28 to 14.67; 2 studies, 68 participants; I² = 0%; tau² = 0.0; random‐effects model; Analysis 1.4; moderate‐certainty evidence). The results were similar with a fixed‐effect model (Table 7).
1.4. Analysis.

Comparison 1: Vitamin D versus placebo or no intervention, Outcome 4: Adverse effect: hypercalciuria
Hypercalcaemia
Compared to placebo or no intervention, we are uncertain whether vitamin D supplementation has an effect on the incidence of hypercalcaemia, as the certainty of the evidence was very low (RR 0.82, 95% CI 0.35 to 1.90; 2 studies, 367 participants; I² = 48%; tau² = 0.18; random‐effects model; Analysis 1.5). The results were similar with a fixed‐effect model (Table 7).
1.5. Analysis.

Comparison 1: Vitamin D versus placebo or no intervention, Outcome 5: Adverse effect: hypercalcaemia
No study included in this comparison measured the following primary outcomes: hyperphosphataemia and kidney stones.
Secondary outcomes
Weight‐for‐age (WAZ)
Insufficient evidence suggests that, compared to placebo or no intervention, vitamin D has an effect on mean WAZ scores (MD 0.09, 95% CI −0.02 to 0.20; 1 study, 1273 participants; fixed‐effect model; Analysis 1.6).
1.6. Analysis.

Comparison 1: Vitamin D versus placebo or no intervention, Outcome 6: Weight‐for‐age
Underweight
Insufficient evidence suggests that, compared to placebo or no intervention, vitamin D has an effect on differences in the proportion of underweight children between groups (RR 0.94, 95% CI 0.80 to 1.11; 1 study, 1282 participants; fixed‐effect model; Analysis 1.7).
1.7. Analysis.

Comparison 1: Vitamin D versus placebo or no intervention, Outcome 7: Underweight
Weight‐for‐length/height (WL/HZ)
Insufficient evidence suggests that, compared to placebo or no intervention, vitamin D has an effect on WL/HZ score between intervention arms (MD 0.65, 95% CI −0.67 to 1.97; 2 studies, 1442 participants; I² = 93%; tau² = 0.84; random‐effects model; Analysis 1.8). The results were similar with a fixed‐effect model (Table 7).
1.8. Analysis.

Comparison 1: Vitamin D versus placebo or no intervention, Outcome 8: Weight‐for‐length/height
Wasting
Insufficient evidence suggests that, compared to placebo or no intervention, vitamin D has an effect on differences in the proportion of wasted children between groups (RR 1.25, 95% CI 0.82 to 1.91; 1 study, 1282 participants; fixed‐effect model; Analysis 1.9)
1.9. Analysis.

Comparison 1: Vitamin D versus placebo or no intervention, Outcome 9: Wasting
Vitamin D status
Continuous 25(OH)D concentration (nmol/L)
Across 21 studies, children receiving vitamin D had higher serum 25(OH)D concentrations than children receiving placebo or no intervention (MD 30.91 nmol/L, 95% CI 21.82 to 40.00; 21 studies, 2202 participants; I² = 95%; tau² = 385.1; random‐effects model; Analysis 1.10). The results were similar with a fixed‐effect model (Table 7). We explored possible reasons for the high heterogeneity observed across studies, including analysis of studies examining physiological doses of vitamin D only; infants only; and children only (Table 8). We found that limiting the included studies to physiological doses of vitamin D and studies done in infants did not decrease inter‐study heterogeneity (I² = 95% for both analyses), but analysing only children over one year of age decreased inter‐study heterogeneity to I² = 87%.
1.10. Analysis.

Comparison 1: Vitamin D versus placebo or no intervention, Outcome 10: Serum 25‐hydroxyvitamin D
5. Sensitivity analysis: outcome 1.10.
| Serum 25(OH)D (nmol/L) (Analysis 1.10) | ||||||
| Category | Number of studies | Mean difference (95% CI) | Tau² | Chi² | P value | I²(%) |
| All studies | 20 | 30.91 (21.82 to 40.00) | 385.01 | 369.62 | < 0.001 | 95 |
| Physiological doses only | 15 | 31.00 (20.31 to 41.68) | 388.92 | 306.64 | < 0.001 | 95 |
| Infants only | 14 | 27.95 (17.36 to 38.54) | 357.03 | 240.76 | < 0.001 | 95 |
| Children only (> 1 year) | 5 | 42.50 (20.85 to 64.15) | 460.98 | 31.74 | < 0.001 | 87 |
CI: confidence interval.
Change in 25(OH)D concentration
Compared to placebo or no intervention, vitamin D resulted in a larger change in vitamin D concentration (MD 28.36 nmol/L, 95% CI 10.41 to 46.32; 3 studies, 495 participants; I² = 88%; tau² = 0.01; random‐effects model; Analysis 1.11). The results were similar with a fixed‐effect model (Table 7).
1.11. Analysis.

Comparison 1: Vitamin D versus placebo or no intervention, Outcome 11: Change in 25(OH)D levels (nmol/L)
25(OH)D ≥ 50 nmol/L
Groups receiving vitamin D were 88% more likely to have vitamin D status ≥ 50 nmol/L (RR 1.88, 95% CI 1.63 to 2.17; 6 studies, 982 participants; I² = 20%; tau² = 0.01; random‐effects model; Analysis 1.12) than groups receiving placebo or no intervention. The results were similar with a fixed‐effect model (Table 7).
1.12. Analysis.

Comparison 1: Vitamin D versus placebo or no intervention, Outcome 12: Vitamin D sufficiency (≥ 50 nmol/L)
25(OH)D ≥ 75 nmol/L
Insufficient evidence suggests that, compared to placebo or no intervention, vitamin D has an effect on achieving vitamin D status above 75 nmol/L (RR 5.75, 95% CI 0.49 to 67.59; 2 studies, 138 participants; I² = 91%; tau² = 2.90; random‐effects model; Analysis 1.13). With a fixed‐effect model, vitamin D had an effect on achieving vitamin D status above 75 nmol/L (Table 7).
1.13. Analysis.

Comparison 1: Vitamin D versus placebo or no intervention, Outcome 13: Vitamin D sufficiency (≥ 75 nmol/L)
25(OH)D < 25 to 30 nmol/L
In three studies, children in the vitamin D groups had 74% lower risk of severe vitamin D deficiency than those given placebo or no intervention (RR 0.26, 95% CI 0.19 to 0.36; 3 studies, 836 participants; I² = 0%; tau² = 0; random‐effects model; Analysis 1.14). The results were similar with a fixed‐effect model (Table 7).
1.14. Analysis.

Comparison 1: Vitamin D versus placebo or no intervention, Outcome 14: Vitamin D severe deficiency (< 25 to 30 nmol/L)
Rickets
Insufficient evidence suggests that, compared to placebo or no intervention, vitamin D has an effect on anterior fontanelle maximum diameter (MD ‐0.20 cm, 95% CI ‐0.61 to 0.21; 1 study, 101 participants; fixed‐effect model; Analysis 1.15).
1.15. Analysis.

Comparison 1: Vitamin D versus placebo or no intervention, Outcome 15: Rickets (continuous)
No study included in this comparison assessed the secondary outcome of gain in linear growth.
Comparison 2: vitamin D (higher dose) versus vitamin D (lower dose)
Primary outcomes
Please see Table 2. All outcomes were measured at completion of the intervention, with average time frames ranging from 3.9 to 8.6 months.
Linear growth
Data show little to no difference between higher doses of vitamin D and lower doses of vitamin D on linear growth, although we are uncertain about the result (MD ‐1.00 cm, 95% CI ‐2.22 to 0.21; 5 studies, 283 participants; I² = 71%; tau² = 1.22; random‐effects model; Analysis 2.1). With a fixed‐effect model, higher doses of vitamin D resulted in less linear growth than lower doses of vitamin D, although we are uncertain about the result (Table 7).
2.1. Analysis.

Comparison 2: Vitamin D (higher dose) versus vitamin D (lower dose), Outcome 1: Linear growth
Length/height‐for‐age (L/HAZ)
Insufficient evidence suggests that, compared to a lower dose of vitamin D, a higher dose of vitamin D has an effect on L/HAZ (MD 0.40 z‐score, 95% CI ‐0.06 to 0.86; 2 studies, 105 participants; I² = 0%; tau² = 0; random‐effects model; Analysis 2.2; low‐certainty evidence). The results were similar with a fixed‐effect model (Table 7).
2.2. Analysis.

Comparison 2: Vitamin D (higher dose) versus vitamin D (lower dose), Outcome 2: Length/height‐for‐age
Adverse effects
Hypercalciuria
Insufficient evidence suggests that, compared to a lower dose of vitamin D, a higher dose of vitamin D has an effect on the incidence of hypercalciuria (RR 1.16, 95% CI 1.00 to 1.35; 6 studies, 554 participants; I² = 0%; tau² = 0; random‐effects model; Analysis 2.3; low‐certainty evidence). The results were similar with a fixed‐effect model (Table 7).
2.3. Analysis.

Comparison 2: Vitamin D (higher dose) versus vitamin D (lower dose), Outcome 3: Adverse effect: hypercalciuria
Hypercalcaemia
Insufficient evidence suggests that, compared to a lower dose of vitamin D, a higher dose of vitamin D has an effect on the incidence of hypercalcaemia (RR 1.39, 95% CI 0.89 to 2.18; 5 studies, 986 participants; I² = 0%; tau² = 0; random‐effects model; Analysis 2.4; low‐certainty evidence). The results were similar with a fixed‐effect model (Table 7).
2.4. Analysis.

Comparison 2: Vitamin D (higher dose) versus vitamin D (lower dose), Outcome 4: Adverse effect: hypercalcaemia
No studies included in this comparison evaluated the primary outcome of stunting or had quantifiable data for the primary outcome of kidney stones or phosphataemia.
Secondary outcomes
Gain in linear growth
Insufficient evidence suggests that, compared to a lower dose of vitamin D, a higher dose of vitamin D has an effect on change in linear growth (MD ‐0.01 cm, 95% CI ‐0.02 to 0.00; 3 studies, 378 participants; I² = 0%; tau² = 0; random‐effects model; Analysis 2.5). The results were similar with a fixed‐effect model (Table 7).
2.5. Analysis.

Comparison 2: Vitamin D (higher dose) versus vitamin D (lower dose), Outcome 5: Linear growth: gain in length
Weight‐for‐age (WAZ)
Insufficient evidence suggests that, compared to a lower dose of vitamin D, a higher dose of vitamin D has an effect on WAZ scores (MD 0.07, 95% CI ‐0.44 to 0.58; 2 studies, 103 participants; I² = 0%; tau² = 0; random‐effects model; Analysis 2.6). The results were similar with a fixed‐effect model (Table 7).
2.6. Analysis.

Comparison 2: Vitamin D (higher dose) versus vitamin D (lower dose), Outcome 6: Weight‐for‐age
Weight‐for‐length/height (WL/HZ)
Insufficient evidence suggests that, compared to a lower dose of vitamin D, a higher dose of vitamin D has an effect on WL/HZ scores (MD ‐0.18, 95% CI ‐0.74 to 0.37; 1 study, 53 participants; fixed‐effect model; Analysis 2.7).
2.7. Analysis.

Comparison 2: Vitamin D (higher dose) versus vitamin D (lower dose), Outcome 7: Weight‐for‐length/height
Vitamin D status
Continuous 25(OH)D concentration (nmol/L)
Overall, compared to a lower dose of vitamin D, a higher dose of vitamin D increased vitamin D status (MD 16.13 nmol/L, 95% CI 7.11 to 25.15; 20 studies, 2765 participants; I² = 96%; tau² = 333.1; random‐effects model; Analysis 2.8). The results were similar with a fixed‐effect model (Table 7). We explored possible reasons for the high heterogeneity observed across studies, including analysis of studies examining physiological doses of vitamin D only; infants only; and preterm infants only (Table 9). We found that only the sensitivity analysis including preterm infants only decreased inter‐study heterogeneity to I² = 89%.
2.8. Analysis.

Comparison 2: Vitamin D (higher dose) versus vitamin D (lower dose), Outcome 8: Serum 25‐hydroxyvitamin D
6. Sensitivity analysis: outcome 2.8.
| Serum 25(OH)D (nmol/L) (Analysis 2.8) | ||||||
| Category | Number of studies | Mean difference (95% CI) | Tau² | Chi² | P value | I²(%) |
| All studies | 20 | 16.13 (7.11 to 25.15) | 333.01 | 493.04 | < 0.001 | 96 |
| Physiological doses only | 14 | 18.62 (8.86 to 28.39) | 268.61 | 243.46 | < 0.001 | 95 |
| Infants only | 18 | 16.02 (6.16 to 25.87) | 352.80 | 461.94 | < 0.001 | 96 |
| Preterm only | 9 | 12.96 (2.23 to 23.68) | 183.61 | 72.17 | < 0.001 | 89 |
CI: confidence interval.
Change in 25(OH)D concentration
Insufficient evidence suggests that, compared to a lower dose of vitamin D, a higher dose of vitamin D has an effect on change in vitamin D status (MD 4.12 nmol/L, 95% CI ‐5.82 to 14.07; 3 studies, 142 participants; I² = 46%; tau² = 37.3; random‐effects model; Analysis 2.9). The results were similar with a fixed‐effect model (Table 7).
2.9. Analysis.

Comparison 2: Vitamin D (higher dose) versus vitamin D (lower dose), Outcome 9: Change in 25(OH)D (nmol/L)
25(OH)D ≥ 50 nmol/L
Twelve studies comparing higher‐dose vitamin D to lower‐dose vitamin D found no association between higher‐dose vitamin D and attaining serum 25(OH)D concentrations ≥ 50 nmol/L (RR 1.04, 95% CI 1.00 to 1.08; 12 studies, 1735 participants; I² = 42%; tau² = 0; random‐effects model; Analysis 2.10). The results were similar with a fixed‐effect model (Table 7).
2.10. Analysis.

Comparison 2: Vitamin D (higher dose) versus vitamin D (lower dose), Outcome 10: Vitamin D sufficiency (≥ 50 nmol/L)
25(OH)D ≥ 75 nmol/L
Compared to the lower‐dose vitamin D group, those in the higher‐dose vitamin D group had 31% increased probability of reaching vitamin D sufficiency (RR 1.31, 95% CI 1.19 to 1.45; 6 studies, 1172 participants; I² = 38%; tau² = 0.01; random‐effects model; Analysis 2.11). The results were similar with a fixed‐effect model (Table 7).
2.11. Analysis.

Comparison 2: Vitamin D (higher dose) versus vitamin D (lower dose), Outcome 11: Vitamin D sufficiency (≥ 75 nmol/L)
25(OH)D < 25 to 30 nmol/L
Insufficient evidence suggests that, compared to a lower dose of vitamin D, a higher dose of vitamin D has an effect on the risk of severe vitamin D deficiency (RR 0.14, 95% CI 0.02 to 1.35; 1 study, 142 participants; fixed‐effect model; Analysis 2.12).
2.12. Analysis.

Comparison 2: Vitamin D (higher dose) versus vitamin D (lower dose), Outcome 12: Vitamin D severe deficiency (< 25 to 30 nmol/L)
Rickets
Compared to the lower‐dose vitamin D group, those in the higher‐dose vitamin D group had 36% lower risk of signs of rickets (RR 0.64, 95% CI 0.46 to 0.90; 4 studies, 212 participants; I² = 0%; tau² = 0; random‐effects model; Analysis 2.13). The results were similar with a fixed‐effect model (Table 7).
2.13. Analysis.

Comparison 2: Vitamin D (higher dose) versus vitamin D (lower dose), Outcome 13: Rickets (dichotomous)
Comparison 3: vitamin D + micronutrient(s) versus micronutrient(s) alone
Primary outcomes
The study included in this comparison, Thacher 2014, did not assess any primary outcomes (linear growth, length/height‐for‐age, stunting, adverse effects (hypercalciuria, hypercalcaemia, hyperphosphataemia, kidney stones)).
Secondary outcomes
Vitamin D status
Continuous 25(OH)D concentration (nmol/L)
Some evidence suggests that, compared to micronutrients alone, vitamin D + micronutrients increase vitamin D concentrations (MD 18.90 nmol/L, 95% CI 8.53 to 29.27; 1 study, 50 participants; fixed‐effect model; Analysis 3.1).
3.1. Analysis.

Comparison 3: Vitamin D + micronutrient(s) versus micronutrient(s) alone, Outcome 1: Serum 25‐hydroxyvitamin D
Rickets
Insufficient evidence suggests that, compared to micronutrients alone, vitamin D + micronutrients has an effect on mean radiographic scores (MD ‐0.94 radiographic score, 95% CI ‐2.10 to 0.22; 1 study, 53 participants; fixed‐effect model; Analysis 3.2).
3.2. Analysis.

Comparison 3: Vitamin D + micronutrient(s) versus micronutrient(s) alone, Outcome 2: Rickets (continuous)
Thacher 2014 did not assess any other secondary outcomes in this comparison (gain in linear growth, weight‐for‐age, underweight, weight‐for‐length/height, wasting).
Comparison 4: vitamin D (higher dose) + micronutrient(s) versus vitamin D (lower dose) + micronutrient(s)
Primary outcomes
Please see Table 3. All outcomes were measured at completion of the intervention, with average time frames ranging from 2.2 to 3 months.
Linear growth
Insufficient evidence suggests that compared to lower‐dose vitamin D + micronutrients, higher‐dose vitamin D + micronutrients may result in little to no difference on linear growth (MD 0.60 cm, 95% CI ‐3.33 to 4.53; 1 study, 25 participants; fixed‐effect model; Analysis 4.1; low‐certainty evidence).
4.1. Analysis.

Comparison 4: Vitamin D (higher dose) + micronutrient(s) versus vitamin D (lower dose) + micronutrient(s), Outcome 1: Linear growth
Adverse effects
Hypercalciuria
Insufficient evidence suggests that compared to lower‐dose vitamin D + micronutrients, higher‐dose vitamin D + micronutrients may result in little to no difference in the incidence of hypercalciuria (RR 1.00, 95% CI 0.06 to 15.48; 1 study, 86 participants; fixed‐effect model; Analysis 4.2; low‐certainty evidence).
4.2. Analysis.

Comparison 4: Vitamin D (higher dose) + micronutrient(s) versus vitamin D (lower dose) + micronutrient(s), Outcome 2: Adverse effect: hypercalciuria
Hypercalcaemia
Compared to lower‐dose vitamin D + micronutrients, higher‐dose vitamin D + micronutrients probably results in little to no effect on the incidence of hypercalcaemia (RR 1.00, 95% CI 0.90 to 1.11; 2 studies, 126 participants; I² = 0%; tau² = 0; random‐effects model; Analysis 4.3; moderate‐certainty evidence). The results were similar with a fixed‐effect model (Table 7).
4.3. Analysis.

Comparison 4: Vitamin D (higher dose) + micronutrient(s) versus vitamin D (lower dose) + micronutrient(s), Outcome 3: Adverse effect: hypercalcaemia
No study included in this comparison assessed the following primary outcomes: length/height‐for‐age; stunting; hyperphosphataemia; kidney stones.
Secondary outcomes
Gain in linear growth
Some evidence suggests that, compared to lower‐dose vitamin D + micronutrients, higher‐dose vitamin D + micronutrients is associated with greater gain in linear growth (MD 0.73 cm, 95% CI 0.12 to 1.34; 1 study, 50 participants; fixed‐effect model; Analysis 4.4).
4.4. Analysis.

Comparison 4: Vitamin D (higher dose) + micronutrient(s) versus vitamin D (lower dose) + micronutrient(s), Outcome 4: Linear growth: gain in length
Vitamin D status
Continuous 25(OH)D concentration (nmol/L)
Insufficient evidence suggests that, compared to lower‐dose vitamin D + micronutrients, higher‐dose vitamin D + micronutrients has an effect on vitamin D status (MD 27.94 nmol/L, 95% CI ‐2.75 to 58.63; 5 studies, 325 participants; I² = 96%; tau² = 1163.79; random‐effects model; Analysis 4.5. However, with a fixed‐effect model, children receiving higher‐dose vitamin D + micronutrients had higher serum 25(OH)D concentrations than children receiving lower‐dose vitamin D + micronutrients (Table 7).
4.5. Analysis.

Comparison 4: Vitamin D (higher dose) + micronutrient(s) versus vitamin D (lower dose) + micronutrient(s), Outcome 5: Serum 25‐hydroxyvitamin D
Change in 25(OH)D concentration
Some evidence suggests that, compared to lower‐dose vitamin D + micronutrients, higher‐dose vitamin D + micronutrients is associated with greater change in vitamin D concentration (MD 7.19 nmol/L, 95% CI 2.97 to 11.41; 1 study, 30 participants; fixed‐effect model; Analysis 4.6).
4.6. Analysis.

Comparison 4: Vitamin D (higher dose) + micronutrient(s) versus vitamin D (lower dose) + micronutrient(s), Outcome 6: Change in 25(OH)D (nmol/L)
25(OH)D ≥ 50 nmol/L
Insufficient evidence suggests that, compared to lower‐dose vitamin D + micronutrients, higher‐dose vitamin D + micronutrients has an effect on achieving vitamin D sufficiency (RR 1.34, 95% CI 0.76 to 2.35; 3 studies, 225 participants; I² = 92%; tau² = 0.23; random‐effects model; Analysis 4.7). The results were similar with a fixed‐effect model.
4.7. Analysis.

Comparison 4: Vitamin D (higher dose) + micronutrient(s) versus vitamin D (lower dose) + micronutrient(s), Outcome 7: Vitamin D sufficiency (≥ 50 nmol/L)
Rickets
Insufficient evidence suggests that, compared to lower‐dose vitamin D + micronutrients, higher‐dose vitamin D + micronutrients has an effect on signs of rickets (RR 1.23, 95% CI 0.24 to 6.30; 2 studies, 153 participants; I² = 0%; tau² = 0; random‐effects model; Analysis 4.8). The results were similar with a fixed‐effect model (Table 7).
4.8. Analysis.

Comparison 4: Vitamin D (higher dose) + micronutrient(s) versus vitamin D (lower dose) + micronutrient(s), Outcome 8: Rickets (dichotomous)
No study included in this comparison assessed the following secondary outcomes: weight‐for‐age, underweight, weight‐for‐length/height, wasting.
Discussion
This systematic review evaluated the effects of oral vitamin D supplementation on linear growth, anthropometric z‐scores, stunting, adverse effects, vitamin D status, and rickets.
Summary of main results
In total, we included 75 studies (from 187 reports), 31 of which discussed at least one of our primary outcomes in this review.
For linear growth, vitamin D compared to placebo (3 randomised controlled trials (RCTs), 240 participants; low‐certainty evidence); higher‐dose vitamin D compared to lower‐dose vitamin D (5 RCTs, 283 participants; very low‐certainty evidence); and vitamin D (higher dose) plus micronutrients compared to vitamin D (lower dose) plus micronutrients (1 RCT, 25 participants; moderate‐certainty evidence) were not associated with any differences in mean length/height (cm) between groups.
Mean length/height‐for‐age z‐scores were slightly higher in groups receiving vitamin D compared to those given placebo (1 RCT, 1258 participants; moderate‐certainty evidence) but were not different between groups in the higher‐dose versus lower‐dose vitamin D comparison (2 RCTs, 105 participants; low‐certainty evidence).
Prevalence of stunting was not different in the vitamin D versus placebo groups (1 RCT, 1247 participants; moderate‐certainty evidence). However, in the original study, Trilok‐Kumar 2011 reported an adjusted risk ratio (RR), which showed that children in the vitamin D group had a 27% lower risk of stunting (95% confidence interval (CI) 5% to 43%) compared to children in the placebo group. The adjusted RR accounted for all characteristics associated with missing data, including sex, quintiles of socioeconomic status, quintiles of exposure to sunlight, season, socioeconomic status, housing materials, material possessions, and breastfeeding. Stunting was not reported by studies included in the other comparisons.
Adverse effects of oral vitamin D reported by studies included hypercalciuria, hypercalcaemia, hyperphosphataemia, and kidney stones. We found no evidence of differences in the risk of hypercalciuria or hypercalcaemia across the four comparisons. All trials measuring hyperphosphataemia or kidney stones, or both, reported no occurrences.
Overall completeness and applicability of evidence
In this review, we sought to determine the effects of oral vitamin D supplementation on our primary outcomes of linear growth, length/height‐for‐age, stunting, and adverse effects in children from birth to five years of age, as determined by randomised and quasi‐randomised controlled trials. We aimed to systematically review the evidence that already exists for oral vitamin D supplementation and linear growth, and to compare oral vitamin D supplementation against placebo, no intervention, and a lower dose of vitamin D intervention, with or without micronutrients.
A major limitation that we encountered while conducting this review is that we were able to synthesise very few studies for the primary outcomes of interest per each comparison. For example, in total, we identified 14 studies that evaluated linear growth, three that evaluated length/height‐for‐age (L/HAZ), and one that evaluated stunting. Studies measuring linear growth were analysed across Comparison 1 (three studies), Comparison 2 (five studies), and Comparison 4 (one study), showing the limited number of studies available for inclusion in a meta‐analysis. In contrast, Comparison 1 and Comparison 2 each included more than 20 studies that analysed vitamin D status. These findings highlight the need to study in future trials the primary outcomes of interest ‐ linear growth, L/HAZ, and stunting.
All of these studies measured linear growth at the end of the supplementation period in infants (either preterm or term). The only studies (three studies) evaluating linear growth, L/HAZ, or stunting in children between one and five years of age were performed after a longer follow‐up period among infants who were previously supplemented but did not receive vitamin D supplementation during that time (Gallo 2013b; Greer 1981; Trilok‐Kumar 2011). This represents a major gap in evidence for the effects of oral vitamin D supplementation on linear growth at the end of the supplementation period in children specifically between one and five years of age. In comparisons including more than one study, evidence was rated as low or very low certainty. No comparisons were judged to have high‐certainty evidence, demonstrating the need for further research. Measurable effects on linear growth, L/HAZ, and stunting may be observed only after a long period of supplementation and follow‐up and among large cohorts. Twenty‐eight studies reported on adverse effects of vitamin D supplementation, including hypercalciuria, hypercalcaemia, hyperphosphataemia, and kidney stones, and overall found no greater risk of incidence in the vitamin D groups. Studies in these comparisons involved mainly infants, with two studies reporting on children only, and three studies reporting on both infants and children, with a range of health issues at baseline (preterm, very low birth weight, asthma or upper respiratory tract infection, rickets).
Heterogeneity of studies across outcomes was an issue in the current evidence base. Across the 75 studies included in this review, participants ranged in baseline health status, which included being healthy; being vitamin D deficient; being preterm or of low birth weight, or both; having rickets; having infectious diseases such as acute or recurrent otitis media, acute diarrhoea, bronchiolitis, pneumonia, or upper or lower respiratory tract infection; or having non‐communicable diseases or disorders, including asthma, dermatitis, chronic kidney disease, juvenile idiopathic arthritis, or chronic heart failure. The included studies were conducted as early as 1959, which presented challenges in 'Risk of bias' assessments and data extraction due to reporting standards changing over time, such as lack of study design or randomisation sequence generation details or reasons for loss‐to‐follow‐up. Oral vitamin D supplementation doses were variable in range, quantity, frequency, and duration across studies. Often studies did not meet their target sample size, if calculated, raising the likelihood of low power to detect an effect in individual studies. There were not enough studies per any one comparison or primary outcome to investigate potential subgroup differences in terms of participant characteristics or intervention administration. The heterogeneity in intervention doses and durations as well as population characteristics, coupled with small sample sizes that were often underpowered at the analysis stage, and lack of reporting of full measurements of outcomes (i.e. not including variance estimates) for estimates of effect in many studies limited our ability to conduct a full meta‐analysis of all available evidence identified by the literature search. Further, trials that may have been included but were not eligible due to lack of age‐stratified data represent a gap in the evidence that could not be analysed in this review (see Characteristics of excluded studies tables).
A majority of studies were performed outside of the Tropic of Cancer and the Tropic of Capricorn, where populations are considered to be at higher susceptibility to vitamin D deficiency. However, among the studies conducted completely or partially between these latitudes (i.e. thought to be at lower risk for vitamin D deficiency due to more abundant sunshine), most studies reported baseline deficiency in vitamin D, either < 50 nmol/L (Rianthavorn 2013; Somnath 2017; Singh 2019; Thacher 2014), or < 75 nmol/L (Tergestina 2016), or they did not report baseline vitamin D status (Feliciano 1994; Specker 1992), showing the need for further investigation in these areas.
Quality of the evidence
In this review, we included 75 studies, 64 of which reported quantifiable data on our primary or secondary outcomes, or both. Our primary outcomes were measured by studies in three of our four comparisons, and secondary outcomes were measured by all studies across all four comparisons. We made efforts to contact study authors to request additional data. The certainty of evidence varied between high and very low across outcomes in each comparison.
Our primary outcomes included linear growth, length/height‐for‐age z‐score (L/HAZ), stunting, and adverse effects (hypercalciuria, hypercalcaemia, hyperphosphataemia, and kidney stones). Among all studies measuring at least one primary outcome across all comparisons (31 studies), 53% lacked caregiver or investigator blinding, and 35% lacked (or lacked a description of) blinding of outcome assessors, and 29% were open‐label or included no description of blinding. In studies with no intervention as the comparator (one study), blinding was not possible. Lack of blinding is unlikely to have impacted the results, all of which were measured objectively by study personnel; however, lack of blinding of caregivers could potentially have raised the risk for differential attrition. Over 50% of studies were considered to have unclear or high risk of attrition bias due to high loss to follow‐up, differential by study arm, or overall, in particular when reasons for loss to follow‐up were not detailed and intention‐to‐treat analysis was not carried out. Most studies had low risk of selection bias regarding sequence generation and allocation concealment.
We evaluated the certainty of evidence using the GRADE method (GRADEpro GDT 2020); our findings are shown in the 'Summary of findings' tables (Table 1; Table 2; Table 3) for our primary outcomes linear growth, L/HAZ, stunting, hypercalciuria, and hypercalcaemia. We planned to conduct a GRADE assessment for hyperphosphataemia and kidney stones, but no data were available for analysis of those outcomes; in three studies reporting on hyperphosphataemia, and in one study reporting on kidney stones, the outcome did not occur.
The certainty of evidence across Comparisons 1, 2, and 4, respectively, as assessed by GRADE, was low, very low, and low for linear growth; moderate and low for L/HAZ (Comparisons 1 and 2 only); moderate for stunting (Comparison 1 only); moderate, low, and low for hypercalciuria; and very low, low, and moderate for hypercalcaemia. Overall, the majority of reasons for downgrading the evidence included moderate to high heterogeneity, imprecision about the estimate, and serious risk of bias.
Potential biases in the review process
We believe that potential biases were minimal in the creation of this review. We conducted a systematic assessment of studies by having at least two reviewers evaluate each potential study at every stage (literature searches, screening of titles and abstracts, screening of full‐text reports, extraction of data, and performance of 'Risk of bias' assessments, and GRADE assessments). We searched 17 electronic databases and two trial registries to be as comprehensive as possible in examining all available evidence. However, we were not able to assess for publication bias using funnel plots due to lack of studies for comparison, thereby preventing us from drawing conclusions on publication bias of the included studies (Table 5; Differences between protocol and review).
Agreements and disagreements with other studies or reviews
Below, we compare the results of previous reviews assessing effects of oral vitamin D supplementation on health outcomes in children.
A previous umbrella (i.e. overview) review of systematic reviews and meta‐analyses examined observational associations between circulating vitamin D concentrations and clinical outcomes, and randomised controlled trials (RCTs) assessing vitamin D supplementation and health outcomes. This umbrella review analysed some outcomes similar to those discussed in this review among neonates, infants, and children, including (birth) length and bone mineral density (Theodoratou 2014). No conclusion was reached regarding effects of vitamin D on neonatal and infant growth (i.e. birth length) and bone mineral density (in lumbar spine) in children, and a substantial effect was unlikely for bone mineral density in general, specifically in the forearm, or in the hip in children. However, it seems that these results are pooled from both reviews of observational studies and RCTs, limiting comparability with our study, which analysed only RCTs.
A previous Cochrane Review assessed effects of vitamin D supplementation for improving bone mineral density in children and adolescents age 1 month up to 20 years (Winzenberg 2011). This review graded available evidence between moderate and high certainty and reported no improvements in total body, hip bone, lumbar spine, and forearm bone mineral density from baseline after one to two years of follow‐up. This is similar to the findings of our review, which analysed six studies reporting on bone mineral density (total, forearm shaft, tibia, distal forearm, lumbar spine) as a secondary outcome and found no differences between any comparisons at the end of the supplementation period or at long‐term follow‐up. As a note, this review found an effect of vitamin D supplementation on bone mineral density among children who were deficient in vitamin D, but not among children with replete vitamin D levels; however, given that there are no deficiency cutoff recommendations for vitamin D for linear growth, we did not examine effects by deficiency status.
Another Cochrane Review analysed effects of vitamin D supplementation on asthma among both children and adults (Martineau 2016). This review found that vitamin D supplementation had a positive effect on asthma outcomes, such as reduced risk of asthma exacerbation (high‐certainty evidence), but we did not find any effect of vitamin D supplementation on asthma. However, it is difficult to compare our findings, as only three studies in our review analysed asthma in association with vitamin D, one of which was terminated early and included only children. Another non‐Cochrane review assessing higher‐dose vitamin D supplementation among children and adolescents age 5 to 18 years for asthma found a reduction in asthma exacerbation with vitamin D ≥ 500 IU per day compared to control (Pojsupap 2015).
A previous Cochrane Review analysed effects of vitamin D supplementation for prevention of nutritional rickets in children born at full term (Lerch 2007). Based on data from four studies, specifically among term‐born children, review authors concluded that it was reasonable to offer vitamin D as a preventive measure to groups at high risk, such as infants and toddlers, and those from settings such as Africa, Asia, or the Middle East. In our review, vitamin D compared to placebo or no intervention did not result in any differences in signs of rickets at endpoint, but higher‐dose vitamin D compared to lower‐dose vitamin D showed reduced risk of rickets signs at endpoint; these studies were conducted in Finland, Germany, India, Australia, London, and Switzerland, and most participants were infants. Our results are consistent with the findings of the 2007 review and provide some support for potentially updating this review with trials published since 2007.
Finally, a systematic review analysed the response of serum 25[OH]D concentration to vitamin D supplementation among children and adolescents (age 3 to 17 years) and adults and found that, overall, vitamin D intervention groups obtained a higher serum vitamin D concentration than controls, with an obvious dose‐response effect among low‐, moderate‐, and higher‐dose groups (Mo 2019). These findings are consistent with our results, which showed higher vitamin D in intervention groups across all three comparisons (vitamin D versus placebo or no intervention, higher‐dose vitamin D versus lower‐dose vitamin D, and vitamin D plus multiple micronutrients versus micronutrients only), although the populations studied were slightly non‐overlapping in terms of age group.
A previous Cochrane Review analysed effects of vitamin D among children under five years of age but on outcomes not covered in this review. That review examined the effects of oral vitamin D on preventing infection and, overall, found no evidence of effects of vitamin D supplementation on death, incidence of pneumonia, or diarrhoea, among a limited number of studies with low‐certainty evidence (Yakoob 2016).
Authors' conclusions
Implications for practice.
The studies included in this review were performed in populations that were healthy or had preexisting conditions. Evidence suggests that oral vitamin D supplementation may result in little to no difference in linear growth, stunting, hypercalciuria, or hypercalcaemia. However, vitamin D supplementation probably leads to a slight increase in length‐for‐age z‐score compared to placebo, based on one study in low birth weight infants between birth and six months of age, which found a 0.11 unit increase in length/height‐for‐age z‐score (L/HAZ). For context, this will be equivalent to 0.22 cm and 0.27 cm for males and females, respectively, based on a standard deviation (SD) of 2.04 cm for males and 2.42 cm for females for the reference population (for six months of age) for World Health Organization (WHO) Growth Standards (WHO 2006). For linear growth, there are no recommendations for the dose of vitamin D supplementation. To determine if any dose is efficacious in impacting linear growth, a majority of trials in this review examined a range of physiological doses, while some involved pharmacological doses. Current evidence does not support the recommendation of vitamin D supplementation for linear growth.
Implications for research.
This review highlights the need for randomised controlled trials (RCTs) to evaluate effects of oral vitamin D supplementation on linear growth among children under five years of age, given the few studies available for data synthesis. Larger, well‐designed, rigorous RCTs of longer durations, carried out in populations stratified by age, and in cohorts of varying health status, with complete, high‐certainty reporting regarding all methodological aspects, are highly recommended. Further, future research should consider dose‐response trials that address infant‐ and child‐specific serum vitamin D concentrations, and should be appropriately powered to address all clinical outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 18 March 2021 | Amended | The GRADE judgement for the outcome Adverse events: Hypercalciuria in the comparison vitamin D versus placebo or no treatment has been ammended from high to moderate. |
History
Protocol first published: Issue 11, 2017 Review first published: Issue 12, 2020
Acknowledgements
We would like to thank the study authors who contributed additional data for this review. We would also like to thank Dr Zulfiqar A Bhutta for his contributions during the protocol stage (Yu 2017), and all staff at the Cochrane Developmental, Psychosocial and Learning Problems (CDPLP) editorial office for their support in preparation of this review.
We are grateful to the following reviewers for their time and comments on this review: Sina Gallo, Associate Professor, University of Georgia, USA; Rehana A Salam, Aga Khan University, Pakistan; and Yohanes Aditya Adhi Satria, Indonesia. We also thank Professor Pradeep Deshmukh for his comments on the protocol.
We gratefully acknowledge the following individuals for their contributions: Ms Sarah Young (for her expertise and assistance in developing the initial search strategy) and Ms Kate Ghezzi‐Kopel (for her expertise and guidance in translating the search strategies across our databases and in providing additional support in searching databases such as Embase).
The protocol for this review was developed during the WHO/Cochrane/Cornell University Summer Institute for Systematic Reviews in Nutrition for Global Policy Making, hosted at the Division of Nutritional Sciences, Cornell University, Ithaca, New York, USA, from 27 July to 7 August 2015.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library
Searched 14 March 2018 (2169 records) Searched 11 December 2019 (8 records)
IDSearch #1(("Vitamin D" OR "Vitamin D deficiency" OR "Vitamin D*" OR ergocalciferol* OR cholecalciferol* OR calcifediol* OR calcitriol* OR dihydrotachysterol* OR "hydroxyvitamin D*" OR alfacalcidol* OR "alpha‐ calcidol*" OR colecalciferol*)):ti,ab,kw (Word variations have been searched) #2("Dietary supplements" OR supplement* OR capsul* OR gel OR liquid OR powder* OR tablet* OR syrup OR drop* OR spray* OR mist* OR pill*) #3#1 AND #2 #4(infant OR child) #5(Infant* OR baby OR babies OR newborn* OR neonat* OR toddler* OR child* OR preschool* OR schoolchild* OR boy* OR girl* OR pre‐school*) #6#4 OR #5 #7#3 AND #6
PubMed (MEDLINE)
Searched 14 March 2018 (1564 records) Searched 11 December 2019 (146 records)
SearchAdd to builderQueryItems #10AddSearch (#8 NOT #9) #9AddSearch (Animals [mh] NOT humans [mh]) #8AddSearch (#3 AND #6 AND #7) #7AddSearch (Randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR randomised [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) #6AddSearch (#4 OR #5) #5AddSearch (Infant*[tiab] OR baby[tiab] OR babies[tiab] OR newborn*[tiab] OR neonat*[tiab] OR toddler*[tiab] OR child*[tiab] OR preschool*[tiab] OR schoolchild*[tiab] OR boy*[tiab] OR girl*[tiab] OR pre‐school*[tiab]) #4AddSearch (Infant[mh] OR child[mh]) #3AddSearch (#1 AND #2) #2AddSearch (Dietary supplements[mh] OR supplement*[tiab] OR capsul*[tiab] OR gel[tiab] OR liquid[tiab] OR powder* [tiab] OR tablet*[tiab] OR syrup[tiab] OR drop*[tiab] OR spray*[tiab] OR mist*[tiab] OR pill*[tiab]) #1AddSearch (Vitamin D[mh] OR Vitamin D deficiency [mh] OR Vitamin D*[tiab] OR ergocalciferol*[tiab] OR cholecalciferol*[tiab] OR calcifediol*[tiab] OR calcitriol*[tiab] OR dihydrotachysterol*[tiab] OR hydroxyvitamin D*[tiab] OR alfacalcidol*[tiab] OR alpha‐ calcidol*[tiab] OR colecalciferol*[tiab])
Embase (OVID)
Searched 14 March 2018 (1632 records) Searched 11 December 2019 (102 records)
1 Vitamin D/ or Vitamin D deficiency/ or Vitamin D*.mp. or ergocalciferol*.mp. or cholecalciferol*.mp. or calcifediol*.mp. or calcitriol*.mp. or dihydrotachysterol*.mp. or hydroxyvitamin D*.mp. or alfacalcidol*.mp. or alpha‐ calcidol*.mp. or colecalciferol*.mp. 2 Dietary supplements/ or supplement*.mp. or capsul*.mp. or gel.mp. or liquid.mp. or powder*.mp. or tablet*.mp. or syrup.mp. or drop*.mp. or spray*.mp. or mist*.mp. or pill*.mp. 3 1 and 2 4 infant/ or child/ 5 (Infant* or baby or babies or newborn* or neonat* or toddler* or child* or preschool* or schoolchild* or boy* or girl* or pre‐school*).mp. 6 4 or 5 7 crossover‐procedure/ or double‐blind procedure/ or randomized controlled trial/ or single‐blind procedure/ or (random* or factorial* or crossover* or cross over* or placebo* or (doubl* adj blind*) or (singl* adj blind*) or assign* or allocat* or volunteer*).mp. 8 3 and 6 and 7 9 animals/ not humans/ 10 8 not 9
Notes: Line 7 contains the search terms suggested inLefebvre 2020for the identification of RCTs in Embase.
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO)
Searched 14 March 2018 (146 records) Searched 11 December 2019 (509 records)
S11(MH "animals") NOT (MH "humans") S10S3 AND S6 AND S9 S9S7 OR S8 S8"Infant*" OR "baby" OR "babies" OR "newborn*" OR "neonat*" OR "toddler*" OR "child*" OR "preschool*" OR "schoolchild*" OR "boy*" OR "girl*" OR "pre‐school*" S7(MH "infant") OR (MH "child")Limiters ‐ Published Date: ‐20191231 S6S4 OR S5Limiters ‐ Published Date: ‐20191231 S5"supplement*" OR "capsul*" OR "gel" OR "liquid" OR "powder*" OR "tablet*" OR "syrup" OR "drop*" OR "spray*" OR "mist*" OR "pill*"Limiters ‐ Published Date: ‐20191231 S4MH "Dietary supplements"Limiters ‐ Published Date: ‐20191231 S3S1 OR S2Limiters ‐ Published Date: ‐20191231 S2"Vitamin D*" OR "ergocalciferol*" OR "cholecalciferol*" OR "calcifediol*" OR "calcitriol*" OR "dihydrotachysterol*" OR "hydroxyvitamin D*" OR "alfacalcidol*" OR "alpha‐ calcidol*" OR "colecalciferol*"Limiters ‐ Published Date: ‐20191231 S1(MH "Vitamin D") OR (MH "Vitamin D deficiency")
Centre for Agriculture and Biosciences (CAB) Abstracts & Web of Science Core Collection databases
Web of Science CAB Abstracts
Searched 14 March 2018 (1371 records) Searched 11 December 2019 (229 records)
# 10#8 NOT #9 Indexes=CAB Abstracts # 9TS=(animals NOT humans) Indexes=CAB Abstracts # 8#3 AND #6 AND #7 Indexes=CAB Abstracts # 7TS=("randomised controlled trial" OR "controlled clinical trial" OR randomized OR randomised OR placebo OR "drug therapy" OR randomly OR trial OR groups) Indexes=CAB Abstracts # 6#4 OR #5 Indexes=CAB Abstracts # 5TS=(Infant* OR baby OR babies OR newborn* OR neonat* OR toddler* OR child* OR preschool* OR schoolchild* OR boy* OR girl* OR pre‐school*) Indexes=CAB Abstracts # 4TS=(infant OR child) Indexes=CAB Abstracts # #1 AND #2 Indexes=CAB Abstracts # 2TS=("Dietary supplements" OR supplement* OR capsul* OR gel OR liquid OR powder* OR tablet* OR syrup OR drop* OR spray* OR mist* OR pill*) Indexes=CAB Abstracts # 1TS=("Vitamin D" OR "Vitamin D deficiency" OR "Vitamin D*" OR ergocalciferol* OR cholecalciferol* OR calcifediol* OR calcitriol* OR dihydrotachysterol* OR "hydroxyvitamin D*" OR alfacalcidol* OR alpha‐ calcidol* OR colecalciferol*) Indexes=CAB Abstracts
Web of Science Core Collection
Searched 14 March 2018 (1850 records) Searched 11 December 2019 (512 records)
# 10#8 NOT #9 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED # 9TS=(animals NOT humans) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED # 8#7 AND #6 AND #3 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED # 7TS=("randomised controlled trial" OR "controlled clinical trial" OR randomized OR randomised OR placebo OR "drug therapy" OR randomly OR trial OR groups) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED # 6#5 OR #4 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED # 5TS=(Infant* OR baby OR babies OR newborn* OR neonat* OR toddler* OR child* OR preschool* OR schoolchild* OR boy* OR girl* OR pre‐school*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED # 4TS=(infant OR child) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED # 3#2 AND #1 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED # 2TS=("Dietary supplements" OR supplement* OR capsul* OR gel OR liquid OR powder* OR tablet* OR syrup OR drop* OR spray* OR mist* OR pill*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED # 1TS=("Vitamin D" OR "Vitamin D deficiency" OR "Vitamin D*" OR ergocalciferol* OR cholecalciferol* OR calcifediol* OR calcitriol* OR dihydrotachysterol* OR "hydroxyvitamin D*" OR alfacalcidol* OR alpha‐ calcidol* OR colecalciferol*) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED
Notes: Web of Science Core Collection includes: Science Citation Index Expanded (SCI‐EXPANDED, 1900‐11 December 2019), Social Sciences Citation Index (SSCI, 1900‐11 December 2019), Arts & Humanities Citation Index (A&HCI, 1975‐11 December 2019), Conference Proceedings Citation Index‐ Science (CPCI‐S, 1990‐11 December 2019), Conference Proceedings Citation Index‐ Social Science & Humanities (CPCI‐SSH, 1990‐11 December 2019), Book Citation Index‐Science (BKCI‐S, 2005‐11 December 2019), Book Citation Index‐Social Sciences & Humanities (BKCI‐SSH, 2005‐11 December 2019), Emerging Sources Citation Index (ESCI, 2015‐11 December 2019), Current Chemical Reactions (CCR‐Expanded, 1985‐11 December 2019), Index Chemicus (IC, 1993‐11 December 2019).
Cochrane Database of Systematic Reviews (CDSR), in the Cochrane Library
Searched 14 March 2018 (2169 records) Searched 11 December 2019 (8 records)
IDSearch #1(("Vitamin D" OR "Vitamin D deficiency" OR "Vitamin D*" OR ergocalciferol* OR cholecalciferol* OR calcifediol* OR calcitriol* OR dihydrotachysterol* OR "hydroxyvitamin D*" OR alfacalcidol* OR "alpha‐ calcidol*" OR colecalciferol*)):ti,ab,kw (Word variations have been searched) with Cochrane Library publication date to Dec #2("Dietary supplements" OR supplement* OR capsul* OR gel OR liquid OR powder* OR tablet* OR syrup OR drop* OR spray* OR mist* OR pill*) with Cochrane Library publication date to Dec 2019 (Word variations have been searched) #3#1 AND #2 with Cochrane Library publication date to Dec 2019 (Word variations have been searched) #4(infant OR child) with Cochrane Library publication date to Jan 2019 (Word variations have been searched) #5(Infant* OR baby OR babies OR newborn* OR neonat* OR toddler* OR child* OR preschool* OR schoolchild* OR boy* OR girl* OR pre‐school*) with Cochrane Library publication date to Dec 2019 (Word variations have been searched) #6#4 OR #5 with Cochrane Library publication date to Dec 2019 (Word variations have been searched) #7#3 AND #6 with Cochrane Library publication date to Dec 2019 (Word variations have been searched) #8(animals NOT humans) with Cochrane Library publication date to Dec 2019 (Word variations have been searched) #9#7 NOT #8 with Cochrane Library publication date to Dec 2019 (Word variations have been searched)
Database of Abstracts of Reviews of Effects (DARE) (www.crd.york.ac.uk/CRDWeb)
Searched 14 March 2018 (7 records)
#11 5 AND 8 AND 10 #10 8 NOT 9 #9 (Animals) NOT (humans) #8 6 OR 7 #7 (Infant* OR baby OR babies OR newborn* OR neonat* OR toddler* OR child* OR preschool* OR schoolchild* OR boy* OR girl* OR (pre‐school*)):TI #6 (MeSH DESCRIPTOR Infant) OR (MeSH DESCRIPTOR Child) #5 4 AND 3 #4 ((Dietary supplements) OR supplement* OR capsul* OR gel OR liquid OR powder* OR tablet* OR syrup OR drop* OR spray* OR mist* OR pill*):TI #3 1 OR 2 #2 (MeSH DESCRIPTOR Vitamin D) OR (MeSH DESCRIPTOR Vitamin D deficiency) #1 ((Vitamin D*) OR ergocalciferol* OR cholecalciferol* OR calcifediol* OR calcitriol* OR dihydrotachysterol* OR (hydroxyvitamin D) OR alfacalcidol* OR alphacalcidol* OR colecalciferol*):TI
Notes: The DARE database was not updated after 14 March 2018 and therefore omitted from the 11 December 2019 search.
Spanish Bibliographic Index of the Health Sciences (IBECS)/Latin American & Caribbean Health Sciences Literature (LILACS)/Pan American Health Organization (PAHO)/WHO Library Database (WHOLIS)
Searched 11 December 2019 (all years; 547 records total)
Search strategy was identical for these 4 databases:
Search on :((Vitamin D$) OR (Vitamin D deficiency) OR ergocalciferol$ OR cholecalciferol$ OR calcifediol$ OR calcitriol$ OR dihydrotachysterol$ OR (hydroxyvitamin D) OR alfacalcidol$ OR alphacalcidol$ OR colecalciferol$) [Words] and ((Dietary supplements) OR supplement$ OR capsul$ OR gel OR liquid OR powder$ OR tablet$ OR syrup OR drop$ OR spray$ OR mist$ OR pill$) [Words] and (Infant$ OR child OR baby OR babies OR newborn$ OR neonat$ OR toddler$ OR child$ OR preschool$ OR schoolchild$ OR boy$ OR girl$ OR (pre‐school$)) [Words]
Database: PAHO References found:16
Database: LILACS References found:366
Database: WHOLIS References found:22
Database: IBECS References found:143
SciELO (Scientific Electronic Library Online)
Searched 14 March 2018 (231 records) Searched 11 December 2019 (4 records)
#4Expression: (#1 AND #2 AND #3) Filters:4Add item to search field Edit search expression Remove from list #3Expression: (ti: (Infant* OR baby OR babies OR newborn* OR neonat* OR toddler* OR child* OR preschool* OR schoolchild* OR boy* OR girl* OR (pre‐school*))) OR (ab: (Infant* OR baby OR babies OR newborn* OR neonat* OR toddler* OR child* OR preschool* OR schoolchild* OR boy* OR girl* OR (pre‐school*))) Filters:302Add item to search field Edit search expression Remove from list #2Expression: (ti: ((Dietary supplements) OR supplement* OR capsul* OR gel OR liquid OR powder* OR tablet* OR syrup OR drop* OR spray* OR mist* OR pill*)) OR (ab:((Dietary supplements) OR supplement* OR capsul* OR gel OR liquid OR powder* OR tablet* OR syrup OR drop* OR spray* OR mist* OR pill*)) Filters:43.982Add item to search field Edit search expression Remove from list #1Expression: (ti: ((Vitamin D*) OR (Vitamin D Deficiency) OR ergocalciferol* OR cholecalciferol* OR calcifediol* OR calcitriol* OR dihydrotachysterol* OR (hydroxyvitamin D) OR alfacalcidol* OR alphacalcidol* OR colecalciferol*)) OR (ab: ((Vitamin D*) OR (Vitamin D Deficiency) OR ergocalciferol* OR cholecalciferol* OR calcifediol* OR calcitriol* OR dihydrotachysterol* OR (hydroxyvitamin D) OR alfacalcidol* OR alphacalcidol* OR colecalciferol*))
Western Pacific Region Index Medicus (WPRIM)
Searched 14 March 2018 (1731 records) Searched 11 December 2019 (25 records)
#1. Search All:Vitamin D OR All:Vitamin D Deficiency OR All:ergocalciferol OR All:cholecalciferol OR All:calcifediol OR All:calcitriol OR All:dihydrotachysterol OR All:hydroxyvitamin D OR All:alfacalcidol or All:alphacalcidol
IndMED (Indian Medical Journals)
Searched 14 March 2018 (360 records)
#4 1 OR 2 AND 3 #3 Infant OR baby OR babies OR newborn OR neonatal OR toddler OR child OR preschool OR schoolchild OR boy OR girl OR pre‐school #2 Supplement OR capsule OR gel OR liquid OR powder OR tablet OR syrup OR drop OR spray OR mist OR pill #1 Vitamin D OR Vitamin D deficiency OR ergocalciferol OR cholecalciferol OR calcifediol OR calcitriol OR dihydrotachysterol OR hydroxyvitamin D OR alfacalcidol OR alphacalcidol OR colecalciferol
Note: Database was no longer available at time of 11 December 2019 search
WHO International Clinical Trials Registry Platform (ICTRP)
Searched 14 March 2018 (91 records)
Intervention AND Condition
Condition: Vitamin D OR Vitamin D deficiency OR Vitamin D* OR ergocalciferol* OR cholecalciferol* OR calcifediol* OR calcitriol* OR dihydrotachysterol* OR hydroxyvitamin D OR alfacalcidol* OR alphacalcidol* OR colecalciferol*
Intervention: Dietary supplements OR supplement* OR capsul* OR gel OR liquid OR powder* OR tablet* OR syrup OR drop* OR spray* OR mist* OR pill*
Notes: Selected "search for clinical trials in children" and "recruitment status ALL.” ICTRP records are now added to CENTRAL, so a separate search of the WHO website was not performed
Epistemonikos
Searched 14 March 2018 (92 records) Searched 11 December 2019 (61 records)
Full query:(title:((title:(Animals NOT humans) OR abstract:(Animals NOT humans))) OR abstract:((title:(Animals NOT humans) OR abstract:(Animals NOT humans)))) 1)(title:((title:(Animals NOT humans) OR abstract:(Animals NOT humans))) OR abstract:((title:(Animals NOT humans) OR abstract:(Animals NOT humans))))
Scopus
Searched 14 March 2018 (4891 records) Searched 11 December 2019 (226 records)
5 ( ( TITLE‐ABS‐KEY ( "Vitamin D*" OR ergocalciferol* OR cholecalciferol* OR calcifediol* OR calcitriol* OR dihydrotachysterol* OR "hydroxyvitamin D" OR alfacalcidol* OR alphacalcidol* OR colecalciferol* ) ) AND ( TITLE‐ABS‐KEY ( "Dietary supplements" OR supplement*or AND capsul* OR gel OR liquid OR powder* OR tablet* OR syrup OR drop* OR spray* OR mist* OR pill* ) ) ) AND ( ( infant OR child ) OR ( TITLE‐ABS‐KEY ( infant* OR baby OR babies OR newborn* OR neonat* OR toddler* OR child* OR preschool* OR schoolchild* OR boy* OR girl* OR "pre‐school*" ) ) ) AND ( ( ( INDEXTERMS ( "clinical trials" OR "clinical trials as a topic" OR "randomized controlled trial" OR "Randomized Controlled Trials as Topic" OR "controlled clinical trial" OR "Controlled Clinical Trials" OR "random allocation" OR "Double‐Blind Method" OR "Single‐Blind Method" OR "Cross‐Over Studies" OR "Placebos" OR "multicenter study" OR "double blind procedure" OR "single blind procedure" OR "crossover procedure" OR "clinical trial" OR "controlled study" OR "randomization" OR "placebo" ) ) OR ( TITLE‐ABS‐KEY ( ( "clinical trials" OR "clinical trials as a topic" OR "randomized controlled trial" OR "Randomized Controlled Trials as Topic" OR "controlled clinical trial" OR "Controlled Clinical Trials as Topic" OR "random allocation" OR "randomly allocated" OR "allocated randomly" OR "Double‐Blind Method" OR "Single‐Blind Method" OR "Cross‐Over Studies" OR "Placebos" OR "cross‐over trial" OR "single blind" OR "double blind" OR "factorial design" OR "factorial trial" ) ) ) OR ( TITLE‐ABS ( clinical AND trial* OR trial* OR rct* OR random* OR blind* ) ) ) AND NOT ( KEY ( animals AND NOT humans ) ) )
4 ( ( INDEXTERMS ( "clinical trials" OR "clinical trials as a topic" OR "randomized controlled trial" OR "Randomized Controlled Trials as Topic" OR "controlled clinical trial" OR "Controlled Clinical Trials" OR "random allocation" OR "Double‐Blind Method" OR "Single‐Blind Method" OR "Cross‐Over Studies" OR "Placebos" OR "multicenter study" OR "double blind procedure" OR "single blind procedure" OR "crossover procedure" OR "clinical trial" OR "controlled study" OR "randomization" OR "placebo" ) ) OR ( TITLE‐ABS‐KEY ( ( "clinical trials" OR "clinical trials as a topic" OR "randomized controlled trial" OR "Randomized Controlled Trials as Topic" OR "controlled clinical trial" OR "Controlled Clinical Trials as Topic" OR "random allocation" OR "randomly allocated" OR "allocated randomly" OR "Double‐Blind Method" OR "Single‐Blind Method" OR "Cross‐Over Studies" OR "Placebos" OR "cross‐over trial" OR "single blind" OR "double blind" OR "factorial design" OR "factorial trial" ) ) ) OR ( TITLE‐ABS ( clinical AND trial* OR trial* OR rct* OR random* OR blind* ) ) ) AND NOT ( KEY ( animals AND NOT humans ) )
3 KEY ( animals AND NOT humans )
2 ( INDEXTERMS ( "clinical trials" OR "clinical trials as a topic" OR "randomized controlled trial" OR "Randomized Controlled Trials as Topic" OR "controlled clinical trial" OR "Controlled Clinical Trials" OR "random allocation" OR "Double‐Blind Method" OR "Single‐Blind Method" OR "Cross‐Over Studies" OR "Placebos" OR "multicenter study" OR "double blind procedure" OR "single blind procedure" OR "crossover procedure" OR "clinical trial" OR "controlled study" OR "randomization" OR "placebo" ) ) OR ( TITLE‐ABS‐KEY ( ( "clinical trials" OR "clinical trials as a topic" OR "randomized controlled trial" OR "Randomized Controlled Trials as Topic" OR "controlled clinical trial" OR "Controlled Clinical Trials as Topic" OR "random allocation" OR "randomly allocated" OR "allocated randomly" OR "Double‐Blind Method" OR "Single‐Blind Method" OR "Cross‐Over Studies" OR "Placebos" OR "cross‐over trial" OR "single blind" OR "double blind" OR "factorial design" OR "factorial trial" ) ) ) OR ( TITLE‐ABS ( clinical AND trial* OR trial* OR rct* OR random* OR blind* ) )
1 ( infant OR child ) OR ( TITLE‐ABS‐KEY ( infant* OR baby OR babies OR newborn* OR neonat* OR toddler* OR child* OR preschool* OR schoolchild* OR boy* OR girl* OR "pre‐school*" ) ) AND ( EXCLUDE ( PUBYEAR, 2020 ) )
European Union Clinical Trials Register (EUCTR)
Searched 11 December 2019 (6 records; all years)
((Vitamin D OR Vitamin D deficiency OR Vitamin D OR ergocalciferol OR cholecalciferol OR calcifediol OR calcitriol OR dihydrotachysterol OR hydroxyvitamin D OR alfacalcidol OR alpha‐calcidol OR colecalciferol) AND (Dietary supplements OR supplement OR capsule OR capsules OR gel OR liquid OR powder OR tablet OR tablets OR syrup OR drop OR drops OR spray OR mist OR pill OR pills)) AND ((Infant OR child) OR (Infant OR baby OR babies OR newborn OR newborns OR neonate OR neonates OR toddler OR toddlers OR child OR children OR preschool OR schoolchild OR schoolchildren OR boy OR girl OR pre‐school)) NOT (Animals NOT humans)
Appendix 2. Criteria for assessing risk of bias
Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence. We assessed the method as follows.
Low risk of bias: any truly random process (e.g. random number table, computer random number generator, stratified or block randomisation, low‐tech methods (coin toss, shuffling cards or envelopes, throwing dice, drawing lots)).
High risk of bias: any non‐random process (e.g. sequence based on date of birth, week of month, even or odd days, case record number, date of presentation, alternate allocation, non‐random or choice of clinician or participant, based on test results or availability).
Unclear risk of bias: insufficient information to facilitate a judgement of low or high risk of bias; or study authors state that they randomly allocated participants but do not describe how they generated the randomisation sequence.
Allocation concealment (checking for possible selection bias)
For each included study, we described the method used to conceal the allocation sequence (when applicable) and assessed whether the intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We assessed the method as follows.
Low risk of bias: concealed allocation using, for example, central allocation done by a third party or by use of consecutively numbered, sealed, opaque envelopes or drug containers (or equivalent).
High risk of bias: allocation based on, for example, open random allocation, unsealed or non‐opaque envelopes; or if the random sequence is known to staff in advance; or if sequence generation was considered at high risk of bias.
Unclear risk of bias: insufficient information (no description of how interventions were indistinguishable) to facilitate a judgement of low or high risk of bias.
Blinding of participants and personnel (checking for possible performance bias)
For each included study, we described the methods used to blind performance. We described the methods used, if any, for blinding study participants and personnel from knowledge of the allocated intervention during the study.
Low risk of bias: both participants and personnel are blinded and the outcome is unlikely to have been influenced; or no blinding or incomplete blinding but outcome is unlikely to have been influenced.
High risk of bias: no, incomplete, or broken blinding and outcome is likely to be have been influenced.
Unclear risk of bias: insufficient information (blinding of participants or personnel, or both, is not described) to facilitate a judgement of low or high risk of bias.
Blinding of outcome assessors (checking for possible detection bias)
For each included study, we described methods used to blind outcome assessors. We described the methods used, if any, for blinding outcome assessors from knowledge of the allocated intervention during the study.
Low risk of bias: blinded and unlikely that blinding was broken; or not blinded but measurement is unlikely to have been influenced.
High risk of bias: no, incomplete, or broken blinding and outcome is likely to have been influenced.
Unclear risk of bias: insufficient information (blinding of outcome assessors is not described) to facilitate a judgement of low or high risk of bias.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
For each included study, we described completeness of data, including attrition and exclusions from analysis, and noted if attrition levels were higher for one prespecified outcome or group of outcomes. We also noted whether missing data were imbalanced across groups, reasons for attrition or exclusions when reported, or whether data were imputed (and, if so, the methods used). We assessed the methods as follows.
Low risk of bias: all randomised participants completed follow‐up or there were no missing data; reasons for missing data were not related to the outcome (e.g. moving away); missing data were balanced across groups and reasons were similar; the proportion of missing data was small; intention‐to‐treat analysis, including all participants randomised, was conducted.
High risk of bias: reasons for loss to follow‐up (LTFU) not described or reasons for LTFU related to the outcome (e.g. recovered, adverse effects, refusal, withdrawal) and imbalanced across groups in numbers; missing data were imputed or a complete case analysis was done (omitting the missing data); no attempts were made to check if excluded participants were different than those included; intention‐to‐treat or per‐protocol analysis was performed when non‐compliers were excluded from the analysis.
Unclear risk of bias: insufficient information to facilitate a judgement of low or high risk of bias.
Selective reporting bias
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as follows.
Low risk of bias: available protocol’s prespecified outcomes of interest are reported in the study in a prespecified way (this includes a published study protocol or a ClinicalTrials.gov ID that was registered before enrolment began).
High risk of bias: outcomes are not reported as prespecified or expected such as due to missing data, adding participants or groups, looking at subsets, or unexpected measurements or methods.
Unclear risk of bias: insufficient information (study protocol does not exist to compare prespecified outcomes to reported outcomes; there is no trial registration code or prepublished protocol referenced) to facilitate a judgement of low or high risk of bias.
Other sources of bias
For each included study, we described any important concerns that we had about other possible sources of bias (particularly reporting of a calculated sample size target and whether or not this target was met at randomisation and for analysis) and assessed them as follows.
Low risk of bias: sample size calculation reported and met at randomisation and at analysis.
High risk of bias: sample size calculation not reported; sample size calculation reported but number randomised or in the final analysis does not meet the sample size (so study is underpowered to analyse outcome).
Unclear risk of bias: insufficient information to facilitate a judgement of low or high risk of bias.
Data and analyses
Comparison 1. Vitamin D versus placebo or no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Linear growth | 3 | 240 | Mean Difference (IV, Random, 95% CI) | 0.66 [‐0.37, 1.68] |
| 1.2 Length/height‐for‐age | 1 | 1258 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.00, 0.22] |
| 1.3 Stunting | 1 | 1247 | Risk Ratio (IV, Fixed, 95% CI) | 0.90 [0.80, 1.01] |
| 1.4 Adverse effect: hypercalciuria | 2 | 68 | Risk Ratio (IV, Random, 95% CI) | 2.03 [0.28, 14.67] |
| 1.5 Adverse effect: hypercalcaemia | 2 | 367 | Risk Ratio (IV, Random, 95% CI) | 0.82 [0.35, 1.90] |
| 1.6 Weight‐for‐age | 1 | 1273 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.02, 0.20] |
| 1.7 Underweight | 1 | 1282 | Risk Ratio (IV, Fixed, 95% CI) | 0.94 [0.80, 1.11] |
| 1.8 Weight‐for‐length/height | 2 | 1442 | Mean Difference (IV, Random, 95% CI) | 0.65 [‐0.67, 1.97] |
| 1.9 Wasting | 1 | 1282 | Risk Ratio (IV, Fixed, 95% CI) | 1.25 [0.82, 1.91] |
| 1.10 Serum 25‐hydroxyvitamin D | 21 | 2202 | Mean Difference (IV, Random, 95% CI) | 30.91 [21.82, 40.00] |
| 1.11 Change in 25(OH)D levels (nmol/L) | 3 | 495 | Mean Difference (IV, Random, 95% CI) | 28.36 [10.41, 46.32] |
| 1.12 Vitamin D sufficiency (≥ 50 nmol/L) | 6 | 982 | Risk Ratio (IV, Random, 95% CI) | 1.88 [1.63, 2.17] |
| 1.13 Vitamin D sufficiency (≥ 75 nmol/L) | 2 | 138 | Risk Ratio (IV, Random, 95% CI) | 5.75 [0.49, 67.59] |
| 1.14 Vitamin D severe deficiency (< 25 to 30 nmol/L) | 3 | 836 | Risk Ratio (IV, Random, 95% CI) | 0.26 [0.19, 0.36] |
| 1.15 Rickets (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. Vitamin D (higher dose) versus vitamin D (lower dose).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Linear growth | 5 | 283 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐2.22, 0.21] |
| 2.2 Length/height‐for‐age | 2 | 105 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.06, 0.86] |
| 2.3 Adverse effect: hypercalciuria | 6 | 554 | Risk Ratio (IV, Random, 95% CI) | 1.16 [1.00, 1.35] |
| 2.4 Adverse effect: hypercalcaemia | 5 | 986 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.89, 2.18] |
| 2.5 Linear growth: gain in length | 3 | 378 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.02, 0.00] |
| 2.6 Weight‐for‐age | 2 | 103 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.44, 0.58] |
| 2.7 Weight‐for‐length/height | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.74, 0.37] |
| 2.8 Serum 25‐hydroxyvitamin D | 20 | 2765 | Mean Difference (IV, Random, 95% CI) | 16.13 [7.11, 25.15] |
| 2.9 Change in 25(OH)D (nmol/L) | 3 | 142 | Mean Difference (IV, Random, 95% CI) | 4.12 [‐5.82, 14.07] |
| 2.10 Vitamin D sufficiency (≥ 50 nmol/L) | 12 | 1735 | Risk Ratio (IV, Random, 95% CI) | 1.04 [1.00, 1.08] |
| 2.11 Vitamin D sufficiency (≥ 75 nmol/L) | 6 | 1172 | Risk Ratio (IV, Random, 95% CI) | 1.31 [1.19, 1.45] |
| 2.12 Vitamin D severe deficiency (< 25 to 30 nmol/L) | 1 | 142 | Risk Ratio (IV, Fixed, 95% CI) | 0.14 [0.02, 1.35] |
| 2.13 Rickets (dichotomous) | 4 | 212 | Risk Ratio (IV, Random, 95% CI) | 0.64 [0.46, 0.90] |
Comparison 3. Vitamin D + micronutrient(s) versus micronutrient(s) alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Serum 25‐hydroxyvitamin D | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 18.90 [8.53, 29.27] |
| 3.2 Rickets (continuous) | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐2.10, 0.22] |
Comparison 4. Vitamin D (higher dose) + micronutrient(s) versus vitamin D (lower dose) + micronutrient(s).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Linear growth | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐3.33, 4.53] |
| 4.2 Adverse effect: hypercalciuria | 1 | 86 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.06, 15.48] |
| 4.3 Adverse effect: hypercalcaemia | 2 | 126 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.90, 1.11] |
| 4.4 Linear growth: gain in length | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.73 [0.12, 1.34] |
| 4.5 Serum 25‐hydroxyvitamin D | 5 | 325 | Mean Difference (IV, Random, 95% CI) | 27.94 [‐2.75, 58.63] |
| 4.6 Change in 25(OH)D (nmol/L) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 7.19 [2.97, 11.41] |
| 4.7 Vitamin D sufficiency (≥ 50 nmol/L) | 3 | 225 | Risk Ratio (IV, Random, 95% CI) | 1.34 [0.76, 2.35] |
| 4.8 Rickets (dichotomous) | 2 | 153 | Risk Ratio (IV, Random, 95% CI) | 1.23 [0.24, 6.30] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdel‐Hady 2019.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: Egypt Study period: September 2014 to August 2016 |
|
| Participants |
Included criteria: prematurity with gestational age 28 weeks and
< 37 weeks, postnatal age > 72 hours, and presence of clinical and
haematological signs suggestive of late‐onset sepsis, ascertained by a
scoring system containing 11 clinical and haematological domains including
skin colour, capillary refill, tone, feeding intolerance, hepatomegaly,
apnoea, bradycardia, metabolic acidosis, thrombocytopenia, leukocytosis, and
shift to left. Total score is 25 points. Infants with a score < 5 were
considered normal, with a score of 5 to 10 were suspected to have sepsis,
and score > 10 were considered clinically septic Excluded criteria: major congenital anomalies, chromosomal anomalies, known inborn errors of metabolism, immunodeficiency disorders Group differences: average total vitamin D daily intake (feeding along with supplementation) was significantly greater in the 800 IU group (Table Supplement, Supplemental Digital Content; links.lww.com/MPG/B551) Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 400 IU D₃
800 IU D₃
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: baseline, 1 week, discharge from neonatal intensive care unit (40 weeks' PMA) |
|
| Notes | Sample size calculated as n = 50, but this was met only at randomisation and not during analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "infants with [late onset sepsis] were randomized using
computer‐generated stratified randomization codes" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "the allocation sequence was concealed by using sealed opaque
envelopes that contained the serial number and the group to which a subject
would be enrolled" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "double blind... Clinicians and primary caregivers were masked to
the intervention... Antibiotic therapy and supportive care were continued
according to managing physician who was not aware of the group assignment...
After each parental consent, an envelope would be opened by the principle
[sic] investigator and group assignment would be established" Judgement comment: caregivers were blinded; although indicated to be double‐blind, the principal investigator was aware of group allocation and may have been biased toward a particular outcome, which could increase the risk of performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "clinicians and primary caregivers were masked to the
intervention... Antibiotic therapy and supportive care were continued
according to managing physician, who was not aware of the group
assignment" Judgement comment: outcome assessors blinded; outcome measurements not subjective and unlikely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: low loss to follow‐up (reasons given: mortality, discontinued intervention; flow diagram in Figure Supplement) similar in both arms; intent‐to‐treat analysis not performed |
| Selective reporting (reporting bias) | Low risk | Judgement comment: study was registered at ClinicalTrials.gov (ID: NCT02273843) prospectively; reported in text; prespecified outcomes and reported outcomes consistent |
| Other bias | Low risk | Judgement comment: no other risks observed |
Aglipay 2017.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Canadian Institutes of Health Research Institute of Human Development, Child and Youth Health and Nutrition, Metabolism and Diabetes, and the Thrasher Research Fund Country: Canada Study period: winter months between 13 September 2011 and 30 June 2015 |
|
| Participants |
Included criteria: healthy children age 1 to 5 years Excluded criteria: gestational age under 32 weeks, chronic illness (other than asthma) Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 400 IU D₃
2000 IU D₃
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: enrolment, 4 to 8 months |
|
| Notes | Sample size calculated and met | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the randomization sequence was generated using a computer‐based
random‐number generator by the SickKids research pharmacy" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "the research pharmacy prepared the vitamin D formulations in
sealed, serially numbered bottles identical in appearance and weight to
maintain allocation concealment" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "study personnel, parents, attending physicians, laboratory
personnel, investigators, and data analysts were all blinded to group
allocation throughout the study period" Judgement comment: all personnel and participants blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "study personnel, parents, attending physicians, laboratory
personnel, investigators, and data analysts were all blinded to group
allocation throughout the study period" Judgement comment: all personnel and participants blinded; outcome measurements not subjective and unlikely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "complete case analysis was performed for all primary and secondary
outcomes in which only cases with available data were analyzed... all
analyses were conducted using the intention‐to‐treat principle" Judgement comment: complete case analysis violates the intention‐to‐treat principle and leads to bias unless data were missing at random, but study authors do not examine missingness. Few participants were lost to follow‐up (reasons not described), while 31 and 24 participants discontinued the intervention per arm, possibly increasing the risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | Quote: "the primary outcome was the number of all‐cause
laboratory‐confirmed viral upper respiratory tract infections per child.
Secondary outcomes included time to first laboratory‐confirmed, total
parent‐reported, laboratory‐confirmed influenza, and [non‐influenza] upper
respiratory tract infections and serum 25‐hydroxyvitamin D levels. Other
secondary outcomes not presented in this article included asthma
exacerbations among children with asthma, physician‐diagnosed otitis media
and pneumonia, emergency department visits, and hospitalizations. Trial
procedures have been described in detail elsewhere (see Supplement 1)" Quote (from 2011 protocol): "the primary analysis will be a comparison of laboratory‐confirmed upper respiratory tract infection rate (per child) between study groups using a Poisson regression model. Secondary analyses will include a comparison of vitamin D serum levels, asthma exascerbations [sic] and the frequency of respiratory syncitial [sic] virus, adenovirus and influenza viruses between arms. Furthermore, a cost effectiveness analysis on the effect of wintertime vitamin D supplementation of preschoolers will be undertaken using the net benefit regression approach" Judgement comment: prespecified protocol in supplemental content; describes outcomes measured and reported on; study registered prospectively at ClinicalTrials.gov (ID: NCT01419262) and reported in text |
| Other bias | Low risk | Judgement comment: no other risks observed |
Ala‐Houhala 1985.
| Study characteristics | ||
| Methods |
Study design: quasi‐randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Piltti grant from the Foundation of Pediatric Research, the National Board of Health, and the Academy of Finland Country: Finland Study period: winter 1981 |
|
| Participants |
Included criteria: healthy, term, breastfed infants and their
mothers Excluded criteria: mother‐infant pairs that failed to complete breastfeeding Maternal pretreatment: "during pregnancy the mothers had vitamin D supplementation of 0‐500 IU/day: one‐half of the mothers had no supplementation during pregnancy; one‐fourth of the mothers received 500 IU/day vitamin D during middle pregnancy; and one‐fourth of mothers, 500 IU/day vitamin D during one entire pregnancy" (quote). However, study authors do not specify which group each of the infants' mothers fell into (winter or summer, Group 2 or 3) Baseline vitamin D status (mean ± standard error, nmol/L)
|
|
| Interventions |
Intervention characteristics 400 IU D₂ (winter)
400 IU D₂ (summer)
1000 IU D₂ (winter)
1000 IU D₂ (summer)
|
|
| Outcomes |
Secondary
Measurement
Time points: birth, 8 weeks of age, 20 weeks of age |
|
| Notes | No sample size calculation; study may be underpowered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "they were randomly allocated to three groups with different
supplementation protocols of vitamin D" Judgement comment: study authors state that they randomly allocated the interventions but did not describe the random sequence generation method |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. Not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, while investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. However, outcomes measured are not subjective in nature and are less likely to be influenced by knowledge of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or protocol identified. Outcomes in methods presented in results |
| Other bias | Low risk | Judgement comment: no other risks observed |
Alam 2011.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: Bangladesh Study period: not reported |
|
| Participants |
Included criteria: children age 6 to 36 months with acute diarrhoea
attending the International Centre for Diarrhoeal Disease Research,
Bangladesh Hospital Excluded criteria: not specified Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics 1000 IU D₃ + milk suji
Milk suji
1000 IU D₃ + L‐isoleucine + milk suji
L‐isoleucine + milk suji
|
|
| Outcomes | None within scope of this review | |
| Notes | Meeting abstracts available only; milk suji: mixture of milk and rice powder (70 kcal/100 mL) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not described (meeting abstract) |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not described (meeting abstract) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind" Judgement comment: double‐blind but not further detailed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind" Judgement comment: double‐blind but not further detailed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or protocol identified; outcomes in methods presented in results |
| Other bias | Low risk | Judgement comment: no other risks observed |
Alizadeh 2006.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: Iran Study period: May 2001 to May 2002 |
|
| Participants |
Included criteria: gestational age < 38 weeks, birth weight <
2000 g Excluded criteria: use of specific medications interacting with vitamin D metabolism (e.g. anticonvulsants, diuretics, corticosteroids) in mother, diabetes mellitus in mother, previous intrauterine growth restriction or small for gestational age, long‐term use of furosemide in infant, having nothing by mouth for longer than 2 weeks Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics 400 IU
1000 IU
|
|
| Outcomes |
Secondary
Measurement
Time points: birth, 9 weeks |
|
| Notes | Sample size calculated and met | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "within 2 weeks of birth, eligible infants randomly divided in two
groups by block randomization of two, to receive a vitamin D 400 IU/d (group
A) and 1000 IU/d (group B)" Judgement comment: random sequence generation method not described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding of participants and/or personnel not described. Not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, while investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "physical examination and x‐ray analysis were made blind by expert
pediatricians" Judgement comment: blinding of X‐ray analysis was done; other outcomes not specified to have been assessed by blinded personnel, but outcome measurements not subjective and unlikely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: the number of participants randomised and completing follow‐up not specified. No analysis or reasons given for loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial protocol or clinical trial registration identified. Outcomes in methods presented in results |
| Other bias | Low risk | Judgement comment: no other risks observed |
Alizadeh Taheri 2014.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: Iran Study period: May 2010 to May 2012 |
|
| Participants |
Included criteria: gestational age 37 weeks, birth weight 2000 g Excluded criteria: taking specific medications interacting with vitamin D metabolism (e.g. anticonvulsants, diuretics, corticosteroids) in mother, diabetes mellitus in mother, previous intrauterine growth restricted or small‐for‐gestational‐age baby, long‐term use of furosemide in infant, NPO (non per oral) > 2 weeks Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 200 IU
400 IU
|
|
| Outcomes |
Secondary
Measurement
Time points: birth, 6 to 8 weeks of life |
|
| Notes | No sample size calculation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the newborns were randomly divided into two groups by block
randomization of two, to receive a 200 IU/d vitamin D (Group 1) and 400 IU/d
vitamin D (Group 2) since they tolerated full enteral nutrition" Judgement comment: study authors state that they randomly allocated interventions by block randomisation but did not describe the random sequence generation method used |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. Not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, while investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "physical examination and X‐ray evaluation were made by blinded
expert neonatalogists [sic]" Judgement comment: outcome assessors were blinded, which is appropriate for subjective outcomes from physical examination and X‐ray evaluation; other outcomes were not specified to have been assessed by blinded personnel, but outcome measurements are not subjective and are unlikely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: no discussion of loss to follow‐up or evidence of no loss to follow‐up; unclear |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or protocol identified; outcomes in methods presented in results |
| Other bias | Low risk | Judgement comment: no other risks observed |
Alonso 2011.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Supported in part by grant from the Instituto de Salud Carlos III and by the Fundación Nutrición y Crecimiento Country: Spain Study period: February 2007 to February 2008 |
|
| Participants |
Included criteria: healthy term infants who were seen for a routine
health visit in the first 15 days of life at 11 participating primary
healthcare centres in a community of northern Spain Excluded criteria: chronic disease; use of medications known to affect vitamin D metabolism; refusal of parents to participate; prematurity; dark skin pigmentation; sunlight exclusion for cultural, religious, or other reasons; breastfeeding by vegetarian mothers. Thus, no child with risk factors for vitamin D deficiency was included in the study. During follow‐up, additional exclusion criteria were long hospitalisation, refusal of parents, loss to follow‐up, and non‐compliance with prophylaxis or study visits Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics 402 IU D₃
Nothing
|
|
| Outcomes |
Secondary
Measurement
Time points: 3, 6, 12 months of age |
|
| Notes | No sample size calculation and no indication of how many were screened for enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the principal investigator (AA) made the assignment by phone using
a computer software, Epi Dat 3.1 (Xunta de Galicia, La Coruña. Spain, and
Pan‐American Health Organization, Washington, DC)" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the study was not blinded to parents and investigators" Judgement comment: not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, while investigators who know the interventions' allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: blinding not done; outcomes measured not subjective in nature and less likely to be influenced by knowledge of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: somewhat unbalanced loss to follow‐up: 11 patients lost to follow up in group 1, 4 lost to follow‐up in group 2. No analysis or reasons given for loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Judgement comment: no trial registration or protocol identified; outcomes in methods presented in results |
| Other bias | Low risk | Judgement comment: no other risks observed |
Aly 2019.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: Egypt Study period: January 2017 to December 2017 |
|
| Participants |
Included criteria: gestational age between 28 0/7 and 33 6/7 weeks,
postnatal age 14 days at the time of enrolment, receiving enteral feed ≥ 100
mL/kg/d Excluded criteria: congenital and chromosomal anomalies, diagnosed with necrotising enterocolitis (NEC) Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 400 IU D₃
800 IU D₃
|
|
| Outcomes | None within scope of review | |
| Notes | Sample size calculated and met | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was done using a computerized program ((Statistical
Package for the Social Sciences) SPSS)" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "the allocation sequence was concealed by using sealed opaque
envelopes that contained the serial number and the group to which a subject
would be enrolled" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "all care providers and laboratory personnel were blinded to the
study group allocation" Judgement comment: all laboratory personnel and participants blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "laboratory personnel were blinded to the study group
allocation" Judgement comment: all laboratory personnel and participants blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: no loss to follow‐up reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: study registered retrospectively at ClinicalTrials.gov (ID: NCT03793309), as reported in text. No prepublished protocol identified |
| Other bias | Low risk | Judgement comment: no other risks observed |
Anderson‐Berry 2017.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Supported in part by the Edna Ittner Pediatric Research Support Fund through the University of Nebraska Medical Center, and in part by a grant from the National Institute of Standards and Technology Country: USA Study period: not reported |
|
| Participants |
Included criteria: parents age 19 or over, patients at 32 weeks'
gestational age Excluded criteria: congenital anomalies, disorders of calcium metabolism, inborn error of metabolism, kidney disease, liver disease, use of steroids Baseline vitamin D status (mean (interquartile range); nmol/L)
|
|
| Interventions |
Intervention characteristics 400 IU D₃
800 IU D₃
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: birth, 4 weeks, 8 weeks |
|
| Notes | Sample size calculated and met at final analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the study statistician generated a randomization sequence
stratified by race (white and non‐white) using SAS software and the study
pharmacist randomized each infant" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "infants were randomized to receive either 400 IU or 800 IU of
vitamin D3 enterally with the initiation of enteral feedings in addition to
parenteral multivitamin injection while on parenteral nutrition and enteral
vitamin D from breast milk and human milk fortifier or preterm formula. The
study vitamin D was delivered in a brown oral syringe (to protect the
product from light) and the product was identical in color, volume and smell
regardless of dose... Formulations were prepared and dispensed by a research
pharmacist who was independent of the study" Judgement comment: appropriate allocation concealment by a third party, although serial labelling was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blinded... Investigators and neonatal intensive care unit
staff were blinded to subject group assignment" Judgement comment: although indicated to be double‐blind, participants were not specifically blinded. However, intervention was administered enterally, limiting the likelihood of caregivers distinguishing the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "investigators and neonatal intensive care unit staff were blinded
to subject group assignment" Judgement comment: outcome assessors blinded; outcome measurements not subjective and unlikely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "thirty‐two infants were enrolled in the study (16 per group) and
were included in the final analysis" Judgement comment: although the diagram shows no loss to follow‐up, when patients were discharged from the neonatal intensive care unit, they were discontinued in the study. N=32 infants were randomised equally to the 2 arms and no loss to follow‐up occurred according to Figure 1. Intention‐to‐treat analysis was used |
| Selective reporting (reporting bias) | Low risk | Judgement comment: study registered prospectively at ClinicalTrials.gov (ID: NCT01469650), as reported in text, and protocol published (Maguire 2014). Prespecified outcomes consistent with those reported |
| Other bias | Low risk | Judgement comment: no other risks observed |
Atas 2013.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: Turkey Study period: June 2006 to May 2007 |
|
| Participants |
Included criteria: none Excluded criteria: prematurity, any natal or postnatal complications, metabolic disorders, dysmorphic features, formula feeding Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics 200 IU D₃
400 IU D₃
|
|
| Outcomes |
Secondary
Measurement
Time points: ~ 15 days of age; 4 months of age |
|
| Notes | Sample size calculation not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "one hundred and sixty‐nine participants were randomly assigned with
simple randomization procedures (computerized random numbers) to groups" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | High risk | Judgement comment: allocation concealment not described; given that both groups were given different amounts of the same intervention, allocation concealment is unlikely |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. Not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, while investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. However, outcomes measured are not subjective in nature and are less likely to be influenced by knowledge of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "82% ... thirty infants who did not complete the study were excluded
with following reasons: (1) loss of follow‐up, (2) formula‐feeding, (3)
improper vitamin D supplementation" Judgement comment: reasons for attrition include (1) loss to follow‐up, (2) formula feeding, (3) improper vitamin D supplementation, but not given by arm; impossible to know if reasons were balanced. No attempt described to check if excluded participants differed in some way from included participants. Attrition led Group 1 to have 11 more participants than Group 2, which may increase the risk for attrition bias |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial protocol cited or found; outcomes in methods presented in results |
| Other bias | Low risk | Judgement comment: no other risks observed |
Backström 1999a.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: Finland Study period: May 1994 to January 1996 |
|
| Participants |
Included criteria: gestational age less than 33 weeks, appropriate
weight for gestational age Excluded criteria: major congenital malformation, failure to supplement vitamin D Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 200‐400 IU D₃
960 IU D₃
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: birth; 6 weeks', 12 weeks', 3 and 6 months' corrected age |
|
| Notes | Sample size calculations were reported, but no outcomes contain the full sample size, suggesting loss to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: random sequence generation method not described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "at hospital discharge all parents received written instructions on
how to lower vitamin D dose according to the amount of formula used in order
to maintain the constancy of the dose" Judgement comment: very likely parents were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the weight and length of the infants were obtained from clinical
charts... The randomisation was concealed from those performing bone
densitometry and determination of serum vitamin D metabolites" Judgement comment: outcome assessors blinded; outcome measurements not subjective and unlikely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: no discussion of loss to follow‐up; does not specify if analysis was intention‐to‐treat or how missing data were handled. Few reported outcomes were based on entire sample |
| Selective reporting (reporting bias) | High risk | Judgement comment: no trial registration or protocol identified; outcomes in methods presented in results |
| Other bias | Low risk | Judgement comment: no other risks observed |
Backström 1999b.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: Finland Study period: August 1985 to May 1987 |
|
| Participants |
Included criteria: preterm infants with birth weight < 2000 g and
gestational age < 37 weeks Excluded criteria: major congenital malformation Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics 500 IU, CaP+
1000 IU, CaP+
500 IU, CaP‐
1000 IU, CaP‐
|
|
| Outcomes |
Primary
Secondary
Measurement
Time point: 3 months of age |
|
| Notes | Sample size calculation described; possibly done retrospectively CaP+ included calcium and phosphorus; CaP‐ did not include calcium and phosphorus |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned to four groups" Judgement comment: randomisation sequence generation method not described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. Not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, while investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the randomization was concealed from those performing measurements
by bone densitometry and assaying the serum vitamin D metabolites. All scans
and analyses were made by the same experienced laboratory technician in a
blinded fashion" Judgement comment: outcome assessors blinded; outcome measurements not subjective and unlikely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "the original study group sizes were: 12 (500 IU, CaP+), 13 (1000
IU, CaP+), 22 (500 IU, CaP‐), and 23 (1000 IU, CaP‐)" Judgement comment: no loss to follow‐up at the 3‐month time point |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no published trial protocol or trial registration identified; outcomes in methods presented in results |
| Other bias | Low risk | Judgement comment: no other risks observed |
Bozkurt 2017.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: no funding Country: Turkey Study period: January 2014 to March 2016 |
|
| Participants |
Included criteria: preterm infants with gestational age 24 to 32
weeks, admitted to neonatal intensive care unit, achieved ≥ 75% of total
nutrition by enteral feedings at postnatal 2 weeks Excluded criteria: infants with perinatal asphyxia, major congenital or chromosomal anomalies, twin‐twin transfusion syndrome, requirement of dopamine ≥ 15 μg/kg/min or > 1 inotrope, no expectation of survival in first 2 weeks, total parenteral nutrition not ceased by first 2 weeks Group differences: frequency of multiple births was higher in 400 IU group Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 400 IU D₃
800 IU D₃
1000 IU D₃
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: birth, 36 weeks' postmenstrual age |
|
| Notes | Sample size calculation met | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization cards were generated using computer generated random
number list and concealed in opaque, sequentially numbered, sealed
envelopes" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "after parental consent, infants were randomly allocated to either
of the 3 groups designating oral Vitamin D 3 dose of: 1) 400 IU/day; 2) 800
IU/ day; 3) 1000 IU/day by sealed opaque envelopes... The envelopes were
opened and each infant was randomised just after achieving 75% of total
nutrition as enteral feeding" Judgement comment: appropriate allocation concealment, although not specified if envelopes were sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. Not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, while investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. However, outcomes measured are not subjective in nature and are less likely to be influenced by knowledge of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "138 infants with gestational age of 24–32 completed weeks were
randomized to one of the 3 vitamin D supplementation dose. After
intervention 17 infants were excluded for the declared reasons in consort
diagram, eventually 121 infants completed the study and a total of 40
infants in the 400 IU, 41 infants in the 800 IU, 40 infants in the 1000 IU
groups were analyzed (Fig. 1)" Judgement comment: CONSORT diagram shows exact reasons for loss to follow‐up per arm, and no infants were excluded from analysis; it appears it was intent‐to‐treat, although this was not specified |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: study registered retrospectively at ClinicalTrials.gov (ID: NCT02941185), as reported in text. No protocol identified |
| Other bias | Low risk | Judgement comment: no other risks observed |
Chan 1978.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: non‐profit and for‐profit funding: grant from Ross Laboratories, Columbus, OH, USA; Maternal and Child Health Training Project No.174 and grant AM 14881 Country: USA Study period: 24 November 1975 to 23 October 1976 |
|
| Participants |
Included criteria: gestation ≤ 37 weeks, appropriate for gestational
age Excluded criteria: uncertain date of last menstrual period, 2 weeks or more was apparent between calculated and clinical measurements, family history of diabetes Baseline vitamin D status: unclear |
|
| Interventions |
Intervention characteristics Placebo
400 IU D₂
20 IU 1,25‐dihydroxyvitamin D₃ (1,25(OH)₂D₃)
400 IU 1,25(OH)₂D₃
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: 12 and 72 hours of age |
|
| Notes | Sample size was not calculated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the 32 infants were divided randomly and equally into one of four
groups" Judgement comment: random sequence generation method not described |
| Allocation concealment (selection bias) | High risk | Quote: "parents of infants given placebo were told that a placebo was
used" Judgement comment: allocation not concealed from parents |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "parents of infants given placebo were told that a placebo was
used" Judgement comment: parents of infants receiving placebo may be less likely to adhere to the allocated placebo and may increase vitamin D from other sources. Investigator blinding was not described; investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. However, outcomes measured are not subjective in nature and are less likely to be influenced by knowledge of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or protocol identified; outcomes in methods presented in results |
| Other bias | Low risk | Judgement comment: no other risks observed |
Chandy 2016.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Grants to study author from Department of Biotechnology, Government of India, and Indian Society for Bone and Mineral Research Country: India Study period: September 2012 to June 2014 |
|
| Participants |
Included criteria: all mothers giving birth in 2 maternity units of
the institution who intended to continue exclusive breastfeeding through
first 6 months and come to hospital of birth for immunisation Excluded criteria: birth weight ≤ 2 kg, sick neonate admitted to intensive care unit, mother or infant on treatment with anticonvulsants or antitubercular drugs, mothers who had received any vitamin D other than the 10 μg present in calcium tablets Pretreatment: all mothers instructed to give infants 15 minutes of traditional baby massage once per day, under the sun between 9 am and 4 pm Baseline vitamin D status (median (interquartile range); nmol/L)
|
|
| Interventions |
Intervention characteristics 400 IU D₃
Placebo
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: birth, 3.5 and 9 months of age |
|
| Notes | This study also included a maternal supplementation group, which we did not include here, because supplement was not given directly to infants. Sample size was calculated but was not met in the group receiving 400 IU/d | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "after maternal blood sample was collected for serum 25(OH)D 2–4 day
after delivery, mother–infant pairs were randomly assigned at birth to one
of three treatment regimens described below, to be followed for 9 months.
Numbers were computer‐generated and allocation was done by one research
staff who supervised medication distribution. This staff member was not
involved in data collection" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "allocation was done by one research staff who supervised medication
distribution. This staff member was not involved in data collection" Judgement comment: allocation was done by a third party who was not involved in data collection, minimising risk of bias of knowing allocation sequence; however, further details (serial numbering, etc.) were not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. Not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, while investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. Outcomes such as measuring anterior fontanelle may be subjective, increasing the risk of detection bias; however, outcomes measured are not subjective in nature and are less likely to be influenced by knowledge of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "of 230 recruited mother–infant pairs, 152 came for the 3.5 month
visit (66% response rate). Those who came for follow‐up did not differ from
those who were absent, in maternal (socio‐economic score, and body mass
index and 25(OH)D 2–4 d after delivery) and infant (birth length, weight,
head circumference, chest circumference and maximum anterior fontanelle
diameter) characteristics (online Supplementary Table S1)... All analyses
were done as per protocol" Judgement comment: reasons for high loss to follow‐up (Figure 1) are not described; further analyses were done on those lost to follow‐up, who were found to not be significantly different from those who completed follow‐up. Per‐protocol analyses suggest no intent‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Judgement comment: study registered prospectively with Clinical Trial Registry of India (CTRI, ctri.nic.in) (ID: CTRI/2012/09/002958). All prespecified outcomes reported; no prepublished protocol identified |
| Other bias | Low risk | Judgement comment: no other risks observed |
Choudhary 2012.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: no funding Country: India Study period: not reported |
|
| Participants |
Included criteria: age 2 to 60 months; clinical diagnosis of severe
pneumonia; presenting to paediatric emergency department; severe pneumonia
diagnosed as fever, cough, tachypnoea, crepitations; tachypnoea defined as
respiratory rate ≥ 50/min in children 2 to 12 months and ≥ 40/min in
children 1 to 5 years; pneumonia and chest indrawing or ≥ danger sign
(inability to feed, lethargy, cyanosis) diagnosed as severe pneumonia Excluded criteria: severe wasting (weight for height < 3 standard deviations), chronic illness, previous history of vitamin D intake over last 4 weeks, known asthma Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics 1000 IU D₃
Placebo
|
|
| Outcomes | None within scope of review | |
| Notes | Sample size calculated and met | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was done according to computer generated random
number table" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "both looked alike in terms of appearance, taste and color. The code
key was opened only after the intervention, data collection, follow up and
tabulation were completed" Quote: "allocation concealment was done by sealed envelope technique" Judgement comment: appropriate allocation concealment; however, envelopes not specified as sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind" Judgement comment: double‐blind but not further detailed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" Judgement comment: double‐blind but not further detailed; however, this study did not analyse any outcomes within the scope of this review |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "overall 173 (86.5%) children improved (vitamin D: 87; placebo: 86)
and 23 (11.5%) remained in the same condition. Worsening occurred in 4 (2%)
children only. Two children died, 1 each in vitamin D and placebo group. A
total of 7 children could not complete the study as parents left against
medical advice (Fig. 1). There was no difference between the two groups in
the proportion of children who improved. A total of 191 children received
all five doses of the drug" Judgement comment: all loss to follow‐up reasons documented and equivalent across both arms of the trial; intent‐to‐treat analysis performed |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or protocol identified; outcomes in methods presented in results |
| Other bias | Low risk | Judgement comment: no other risks observed |
Ducharme 2019.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Two research bridge‐funding grants (# 313322 and 142741) of the Canadian Institutes of Health Research (CIHR) Country: Canada Study period: September 2014 to July 2016 |
|
| Participants |
Included criteria: age 1 to 5 years, physician‐diagnosed asthma
based on clinical signs of airflow obstruction and reversibility, upper
respiratory tract infection (URTI) reported by parents as the main asthma
trigger; ≥ 4 URTIs in the preceding year, ≥1 exacerbation requiring rescue
oral corticosteroids (OCS) in the preceding 6 months (or ≥ 2 in the past 12
months) confirmed by pharmacy or medical records or both Excluded criteria: intake of or intention to use > 400 IU/d of vitamin D supplement, extreme prematurity (< 28 weeks’ gestation), high risk of vitamin D deficiency (e.g. vegan diet), condition(s) (e.g. rickets) or drug(s) altering calcium or vitamin D absorption or metabolism (e.g. antiepileptic, diuretic, antacid, antifungal), anticipated difficult follow‐up Group differences: most baseline characteristics were similar between groups but some appeared slightly imbalanced, with a greater proportion of male participants, environmental tobacco exposure, use of combination therapy, more school days missed, fewer Caucasians, and lower vitamin D dietary intake in the intervention group compared to the placebo group (not statistically tested) Baseline vitamin D status (n (%) < 75 nmol/L)
|
|
| Interventions |
Intervention characteristics 100,000 IU D₃
Placebo
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: enrolment; 3. 5, and 7 months |
|
| Notes | Sample size not calculated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated random numbers with variable permuted
blocks" Judgement comment: sequence generation method adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "the Central Pharmacy (SJUHC) held the allocation codes, prepared
the study supplements in sequentially coded syringes, and dispensed as per
randomisation 2 mL of vitamin D 3 (100,000 IU of cholecalciferol) or
identical placebo, administered by the nurse at baseline and 3.5 months" Judgement comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "triple‐blind... At the end of follow up, parents, nurse, and
physician independently guessed the child’s group assignment" Judgement comment: data related to this statement were not reported; however, triple‐blind indicates that participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "triple‐blind... At the end of follow up, parents, nurse, and
physician independently guessed the child’s group assignment" Judgement comment: data related to this statement were not reported; however, triple‐blind implies that outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "an intention‐to‐treat (ITT) analysis was carried out whereby all
randomised children were included in the analysis, wherever possible" Judgement comment: minimal loss to follow‐up; intention‐to‐treat analysis done |
| Selective reporting (reporting bias) | Unclear risk | Quote: "after premature trial cessation due partial funding enabling only a
2‐year single‐centre pilot trial, rather than an adequately powered
multicentre study of 865 children, the primary outcome was modified post hoc
to the overall change (Δ) from baseline in total serum 25OHD and at 3.5 and
7 months, similar to our previous pilot study [21]" Judgement comment: study was registered prospectively at ClinicalTrials.gov (ID: NCT02197702), as reported in text. Outcomes were changed following the start of the study; however this change was due to lack of funding ‐ not to intervention or outcome |
| Other bias | Low risk | Judgement comment: no other risks observed |
Evans 1989.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Medical Research of Canada Grant to Dr David Cole Country: Canada Study period: not reported |
|
| Participants |
Included criteria: infants born at Grace Maternity Hospital
(Halifax, Nova Scotia, Canada) who weighed < 1500 g at birth and survived
to 72 hours of postnatal age Excluded criteria: major congenital anomaly, congenital infection, inherited metabolic disease Pretreatment: infants were entered into the study after informed consent was obtained, and were stratified according to size for gestational age at birth and requirement for mechanical ventilation. Three control infants received high doses of vitamin D at the discretion of the attending physician after 4‐week wrist radiographs obtained for clinical indications were interpreted as showing moderate bone disease (scores of 4, 4, and 6, respectively). For these 3 infants, only data obtained up to the day of the switch were used in subsequent analyses. Control and experimental groups were well matched for known possible confounding variables. No significant difference was noted between the 2 groups with respect to gestational age; birth weight; mean daily weight gain; intake of calories, calcium, or phosphorus; number who were small for gestational age at birth, who required mechanical ventilation, or who had more than 30% of their total enteral intake from human milk or commercial soy formula (Isomil); or time (in days) to establishment of enteral feedings Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics 400 IU D₂
2000 IU D₂
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: 72 hours' postnatal age, 6 weeks of age |
|
| Notes | Sample size stated but calculation not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the first of each pair of infants entered into a stratum was
randomly assigned by a coin flip to receive a daily oral supplement of
either 2000 IU vitamin D₂, begun by 72 hours of postnatal age (experimental
group), or 400 IU vitamin D₂, begun once oral feedings were established
according to standard nursery policy (control group). The second of each
pair of infants entered into that stratum received the alternate treatment.
Infants were removed from the study if they did not survive to 6 weeks of
postnatal age or if they developed prolonged obstructive jaundice" Quote: "stratified according to size for gestational age at birth and requirement for mechanical ventilation" Judgement comment: infants were stratified, using low‐tech coin flip ‐ an appropriate randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. Not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, while investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "assessments of each radiograph, done on three different occasions
by the pediatric radiologist, who was unaware of study group assignments,
previous radiographic assessment, and biochemical data, were used to assign
the grade" Judgement comment: outcome assessors were blinded for radiograph assessment ‐ a subjective outcome; other outcome measurements were not subjective and were unlikely to be at risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "of the remaining 89 eligible infants, 87 were enrolled after
consent was obtained. One infant was inadvertently missed, and consent could
not be obtained for one infant. Six enrolled infants did not complete the
study, four because they died before 6 weeks of postnatal age (two in each
study group) and two because of the development of severe obstructive
jaundice (both in the experimental group). Of the 81 infants who completed
the study, 40 were randomly assigned to the control group and 41 to the
experimental group" Judgement comment: n = 3 control infants were excluded from final follow‐up due to presence of bone disease. Because this outcome is related to the intervention, this loss to follow‐up may introduce some bias (effect may have been underestimated), but only 3 infants were excluded, and therefore this is unlikely to impact the effect estimate |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or protocol identified; prespecified outcomes consistent with those reported |
| Other bias | Low risk | Judgement comment: no other risks observed |
Feliciano 1994.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Funded in part by the Thrasher Research Fund, Salt Lake City, UT, USA; and the Perinatal Research Institute, Cincinnati, OH, USA Country: China Study period: fall (September and October 1986) and spring (March and April 1987) |
|
| Participants |
Included criteria: gestational age 37 weeks or over, absence of
gastrointestinal disease and congenital anomaly Excluded criteria: none specified Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics 100 IU, North China, Spring born
200 IU, North China, Spring born
400 IU, North China, Spring born
100 IU, North China, Fall born
200 IU, North China, Fall born
400 IU, North China, Fall born
100 IU, South China, Spring born
200 IU, South China, Spring born
400 IU, South China, Spring born
100 IU, South China, Fall born
200 IU, South China, Fall born
400 IU, South China, Fall born
|
|
| Outcomes |
Secondary
Measurement
Time points: birth, 6 months of age |
|
| Notes | Sample size not calculated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "at age 3‐5 days, the infants were randomly assigned to receive
either 100, 200, or 400 IU of vitamin D a day" Judgement comment: random sequence generation method not described |
| Allocation concealment (selection bias) | High risk | Judgement comment: allocation concealment is not described; given that both groups were given different amounts of the same intervention, allocation concealment is unlikely |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. Not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, while investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. However, outcomes measured are not subjective in nature and are less likely to be influenced by knowledge of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "eighty‐two per‐cent of the infants enrolled at birth completed the
study (209/255)" Judgement comment: overall loss to follow‐up (18%) is noted but not by group, and no reasons are described. Whether analysis was intent‐to‐treat, or if infants failing to complete the study were different from completers, is not addressed |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or protocol identified; outcomes reported in methods and in results |
| Other bias | Low risk | Judgement comment: no other risks observed |
Fort 2016.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. National Institutes of Health (Kaul Pediatric Research Institute Senior Investigator Award U10 HD34216) Country: USA Study period: June 2012 to October 2014 |
|
| Participants |
Included criteria: inborn infants with gestational age between 23
and 27 completed weeks admitted to neonatal intensive care unit at
University of Alabama Hospital Excluded criteria: major congenital or chromosomal anomalies, moribund infant with low likelihood of survival as out‐born infant Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics Placebo
200 IU D₃
800 IU D₃
|
|
| Outcomes |
Secondary
Measurement
Time points: birth, postnatal day 28; followed up at 2 years of age in associated report (Fort 2016; see Salas 2018) |
|
| Notes | Sample size was calculated and met except for 800 IU group; study is powered to determine a difference of 50% in vitamin D concentrations on postnatal day 28 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "after consent, infants were randomly allocated by the research
pharmacy staff using computer‐generated stratified randomization codes to
one of three groups" Judgement comment: appropriate sequence generation method, by third party |
| Allocation concealment (selection bias) | Low risk | Quote: "the medication was dispensed by a research pharmacist in an amber
syringe to mask the caregivers" Judgement comment: appropriate allocation concealment; no description of sequentially labelled containers or envelopes; however, intervention administered by blinded study staff ‐ not caregivers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind... The medication was dispensed by a research
pharmacist in an amber syringe to mask the caregivers" Judgement comment: participants were blinded, and personnel appear to have been blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind... Clinical data were collected by a trained research
coordinator" Judgement comment: outcome assessors appear to be blinded; outcome measurements are not subjective and are unlikely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "between June 2012 and October 2014, 100 infants with birth weights
ranging from 360 g to 1290 g (mean 770 ± 215 g) were randomized to three
vitamin D daily intake groups: 36 infants to the placebo group, 34 to the
200 IU/day, and 30 to the 800 IU/day. Of the 100 infants, 37 did not
complete the study: 15 due to death, 12 developed necrotizing enterocolitis
or spontaneous intestinal perforation, and 12 were not fed for more than 24
hours (Figure 1)" Judgement comment: from Figure 1, 37% of infants did not complete the study; reasons for discontinuing included those related to the outcome (death (n = 15), necrotising enterocolitis/consuming nothing by mouth/spontaneous intestinal perforation (n = 12)) or not (lack of feeding for longer than 24 hours (n = 12)). Numbers of those discontinuing the intervention were much higher (n = 17) in the 200 IU group and n = 9 in the 800 IU group, compared to n = 5 in the placebo group. Intention‐to‐treat analysis was performed. Children who did not complete the study were not compared with those who completed follow‐up |
| Selective reporting (reporting bias) | Low risk | Judgement comment: study was registered prospectively at ClinicalTrials.gov (ID: NCT01600430), as reported in text; prespecified outcomes are consistent with those reported. No prepublished protocol was identified |
| Other bias | Low risk | Judgement comment: no other risks observed |
Gallo 2013a.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: non‐profit + provision of drug. Grant from the Canadian Foundation for Dietetic Research and the D Drops Company provided vitamin D supplements in kind; Canadian Foundation for Innovation Country: Canada Study period: May 2010 to September 2011 |
|
| Participants |
Included criteria: healthy, singleton, term infants born at
appropriate size for gestational age as assessed according to the World
Health Organization (WHO) Child Growth Charts (between 5th and 95th
percentiles) to healthy, breastfeeding women (consuming > 80% of total
feeds from breast milk) Excluded criteria: infants of mothers with history of gestational diabetes or hypertension in pregnancy, malabsorption syndromes (coeliac and Crohn's diseases), or taking medications that interfere with vitamin D metabolism (anticonvulsants and corticosteroids), and mothers taking ≥ 50 µg/d of vitamin D through supplementation Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 400 IU D₂
400 IU D₃
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: 1 and 4 months of age |
|
| Notes | Calculated sample size was attained at randomisation but not at primary analysis, and primary analysis does not include all who were randomised. Did not meet sample size in D₂ group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "infants were randomly assigned to receive a 10‐mg/d oral dose of
either D₂ or D₃ in a 1:1 ratio stratified by sex" Judgement comment: study authors state that they randomly allocated interventions but do not describe the random sequence generation method used |
| Allocation concealment (selection bias) | Low risk | Quote: "there were no differences in appearance and both products were
tasteless and odorless. These products are oil based (coconut and palm) and
dosages were delivered in 1‐drop volumes (0.03 mL) using a standardized
Eurodropper" Judgement comment: appropriate allocation concealment; no description of sequentially labelled envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: given concealed allocation, participants were likely blinded. If blinding was done, this was not described. Not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, while investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. However, outcomes measured are not subjective in nature and are less likely to be influenced by knowledge of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "the intent‐to‐treat principle was applied for all outcomes" Judgement comment: Figure 1 gives those lost to follow‐up but not reasons for loss to follow‐up. Intention‐to‐treat analysis is done but does not include those lost to follow‐up. However, loss to follow‐up is minimal (Figure 1) |
| Selective reporting (reporting bias) | Low risk | Judgement comment: study was registered retrospectively at ClinicalTrials.gov (ID: NCT01190137), as reported in text; prespecified outcomes are consistent with those reported. No prepublished protocol was identified |
| Other bias | Low risk | Judgement comment: no other risks observed |
Gallo 2013b.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Canadian Institutes for Health Research, Nutricia Research Foundation, and Canadian Foundation for Innovation, and in‐kind support from Euro‐Pharm International Canada Inc. for provision of the supplements. Fonds de la Recherche en Santé du Québec provided personal funding for the doctoral student (Ms Gallo), and Canada Research Chairs provided a salary award to Dr Weiler Country: Canada Study period: May 2007 to August 2010 |
|
| Participants |
Included criteria: healthy, term, singleton, appropriate size for
gestational age, breastfeeding (consuming 80% of total milk volume) Excluded criteria: infants of mothers with gestational diabetes, hypertension in pregnancy, chronic alcohol use, or malabsorption syndrome Group differences: maternal and infant baseline characteristics were similar among groups except for mother’s race (P = 0.03); thus, race was included as a covariate in all analyses. There were no differences in attrition rates, referring centres, or reported adherence across treatment groups Baseline vitamin D status (mean (95% confidence interval); nmol/L)
|
|
| Interventions |
Intervention characteristics 400 IU D₃
800 IU D₃
1200 D₃
1600 IU D₃
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: 1, 3, 6, 9, and 12 months of age; followed up at 3 years of age |
|
| Notes | Sample size calculated and met at randomisation for groups randomised to 400 IU, 800 IU, and 1200 IU vitamin D. Group randomised to 1600 IU vitamin D per day did not meet the target sample size at randomisation. Participants in this group received 1600 IU vitamin D per day until age 12 months (n = 6), until age 6 months (n = 4), or until age 9 months (n = 6) before investigators re‐assigned these participants to the 400 IU per day group, thereafter receiving 400 IU per day. Children in the 1600 IU group were not included in statistical models owing to discontinuation of the intervention (see Gallo 2013b for details) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "following enrollment into the study and baseline measurements, the
infants were randomly assigned to 1 of the 4 groups in a 1:1:1:1 allocation
ratio. Randomization was stratified by sex in equal blocks of 4. The
randomization list was generated using http://www.randomization.com and
blinded supplement codes. The codes were revealed only after the statistical
analysis was complete" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "supplements containing 400, 800, 1200, or 1600 IU of vitamin D 3
were formulated by Europharm International Canada Inc and administered in
2‐mL/day volume using a standardized dropper; all had similar taste, smell,
and appearance. Supplements were provided in precoded bottles of 60‐mL
volume" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "parents and researchers were blinded to treatment dosage" Judgement comment: participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the codes were revealed only after the statistical analysis was
complete" Judgement comment: outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "careful comparisons of participants with missing and fully observed
data were consistent with data missing at random. The mixed‐model analysis
of variance estimates the effect size based on available data (Figure 1 and
Figure 2), and participants with missing data are not dropped, mitigating
the need for imputation" Judgement comment: similar proportions of loss to follow‐up across study groups; reasons for dropouts not given explicitly, only "lost to follow‐up", and "insufficient blood". Analysis done by intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | Judgement comment: study was registered prospectively at ClinicalTrials.gov (ID: NCT00381914), as reported in text; prespecified outcomes are consistent with those reported. No study protocol was identified |
| Other bias | Low risk | Judgement comment: no other risks observed |
Gordon 2008.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Grants from Allen Foundation and McCarthy Family Foundation; National Institutes of Health Grant MO1‐RR‐2172 to Children’s Hospital Boston General Clinical Research Center; and Project T71 MC00009 Maternal and Child Health Bureau, Human Resources and Services Administration Country: USA Study period: October 2005 to June 2007 |
|
| Participants |
Included criteria: age 8 to 24 months, enrolled from Children's
Hospital Boston Primary Care Center, vitamin D deficient 50 nmol/L Excluded criteria: chronic disease (e.g. asthma, seizure disorder, sickle cell disease), use of oral glucocorticoid over previous 3 months, other therapy known to affect vitamin D metabolism Baseline vitamin D status (mean; nmol/L)
|
|
| Interventions |
Intervention characteristics 2000 IU D₂
50,000 IU D₂
2000 IU D₃
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: enrolment, 6 weeks |
|
| Notes | Sample size calculated but not met | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients identified to have hypovitaminosis D were randomly
assigned to one treatment protocol. The randomization list was stratified by
age at screening (9 or 18 months) and blocked in randomly permuted sequences
of 3 or 6, ensuring that no treatment would be disproportionately
represented in any season or age group" Judgement comment: random sequence generation method not described |
| Allocation concealment (selection bias) | High risk | Quote: "the vitamin D₂ preparation (200 IU per drop or 0.025 ml) was
manufactured by Sanofi‐Synthelabo Inc. (Bridgewater, NJ), and doses were
provided as 10 drops or 0.25 ml daily for the 2,000 IU dose and 6.25 ml
weekly for the 50,000 IU dose; for each vitamin D 2 dose, the suspension was
administered via a provided dropper onto the tongue" Judgement comment: given that both groups were given different amounts of the same intervention, allocation concealment is unlikely |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. Not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, while investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. However, outcomes measured are not subjective in nature and are less likely to be influenced by knowledge of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "we conducted an intention‐to‐treat analysis, attributing the
assigned treatment to all randomized subjects regardless of compliance" Judgement comment: low loss‐to‐follow‐up; intention‐to‐treat analysis performed |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or protocol identified; outcomes in methods presented in results |
| Other bias | Low risk | Judgement comment: no other risks observed |
Greer 1981.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: for‐profit and non‐profit: Grant from Ross Laboratories and National Institute of Child Health and Human Development Country: USA Study period: summer 1979 and November 1979 |
|
| Participants |
Included criteria: healthy, term, exclusively breastfed infants Excluded criteria: none specified Pretreatment: no differences in baseline infant bone mineral content, biochemical measurements, maternal intake, and breast milk minerals Baseline vitamin D status (mean ± standard error; nmol/L)
|
|
| Interventions |
Intervention characteristics Placebo
400 IU D₂
|
|
| Outcomes |
Secondary
Measurement
Time points: birth, 12 weeks of age; followed up at 1 year of age |
|
| Notes | Sample size not calculated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "eighteen healthy, term, exclusively breast‐fed infants were divided
randomly into two groups" Judgement comment: random sequence generation method not described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "neither mothers nor investigators knew whether vitamin D or placebo was given to the infants... After 12 weeks, the study was unblinded to the investigators. At six months, the study was unblinded to the mothers of the study infants, at which time all infants were allowed solid foods and the placebo group was given a daily vitamin D supplement of 400 IU" Judgement comment: mothers were blinded until age 6 months (reported in Greer 1982); investigators were unblinded at 12 weeks (end of study) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind fashion; after 12 weeks, the study was unblinded to
the investigators" Judgement comment: outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "at 9 months, six of 13 (46%) infants remaining in the study from the placebo and supplemented groups were still breast‐feeding. At one year, three infants were still breast‐feeding, and ten infants were receiving cow milk. An additional 12 term, healthy, exclusively formula‐fed infants from the same private practice served as a comparison group for bone mineral content only" Judgement comment: no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or protocol identified; all outcomes in methods reported in results |
| Other bias | Low risk | Judgement comment: no other risks observed |
Greer 1989.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. US Department of Agriculture grant Country: USA Study period: October 1985 to January 1987 |
|
| Participants |
Included criteria: breastfed, term; mothers must plan to exclusively
breastfeed until 6 months Excluded criteria: none specified Group differences: birth length was significantly lower in the formula group compared with the human milk groups; gestational age of formula group was lower than that of the group receiving no vitamin D Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 400 IU D₂
Placebo
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: birth, 6 months of age |
|
| Notes | Sample size calculated and met | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "forty‐six term, breast‐fed infants were divided randomly into two
groups and studied in a double‐blind fashion" Judgement comment: random sequence generation method not described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "studied in a double‐blind fashion" Judgement comment: 'double‐blind' implies that participants and personnel were blinded to assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: 'double‐blind' implies that outcome assessors were blinded; outcome measurements are not subjective and are unlikely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "fifty‐eight patients completed the initial 1 1/2 months of the
study, 12 in the formula group, 22 in the group fed human milk supplemented
with vitamin D₂, and 24 in the group fed human milk and given placebo. All
of the 12 formula‐fed infants completed 6 months of the study. By 3 months,
one infant in each of the human milk‐fed groups was eliminated for
noncompliance. An additional seven infants dropped out after 3 months
because breast‐feeding was discontinued. Ultimately, 19 infants in each of
the groups fed human milk completed 6 months of the study" Judgement comment: moderate loss to follow‐up (17%); no reasons given; no investigations of lost patients; appears to show complete case analysis |
| Selective reporting (reporting bias) | Unclear risk | Quote: "we measured bone mineral content, growth, and serum concentrations
of 25(OH)D₃, 25(OH)D₂, 1,25‐(OH)₂D, and parathyroid hormone as indicators of
vitamin D deficiency or sufficiency" Judgement comment: trial not registered and no protocol available; outcomes in methods presented in results |
| Other bias | Low risk | Judgement comment: no other risks observed |
Gupta 2016.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Indian Council of Medical Research Country: India Study period: 25 August 2012 to 27 January 2015 |
|
| Participants |
Included criteria: children age 6 months to 5 years with clinical
diagnosis of severe pneumonia (defined as presence of lower chest indrawing
in children presenting with cough or difficult breathing); family staying
within 10‐km radius of the hospital Excluded criteria: children with history or clinical features suggestive of rickets (presence of wide wrists, delayed closure of anterior fontanelle, presence of rachitic rosary, bow legs or knock knee), severe acute malnutrition, asthma, hypertension, complicated pneumonia (lung abscess, pleural effusion, empyema) or illness severe enough to require ventilation, chronic respiratory disease, heart disease, renal or hepatic insufficiency, neurological illness resulting in abnormalities of muscle tone/power, and known immunodeficiency. Children having received vitamin D or calcium supplements within 4 weeks before enrolment, those diagnosed with hypercalcaemia or allergy to vitamin D, and those immunised with pneumococcal/flu vaccine were also excluded Baseline vitamin D status (n (%) < 75 nmol/L)
|
|
| Interventions |
Intervention characteristics 100,000 IU D₃
Placebo
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: baseline, 2 weeks, 3 months |
|
| Notes | Sample size calculated and met at randomisation and analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "eligible children were randomized using computer‐generated block
randomization to receive 100,000 IU of vitamin D (cholecalciferol) or
placebo orally. Eight, ten, and twelve blocks consisting of 10, 10, and 12
subjects, respectively were created" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "both drug and placebo were identical in appearance, color, odor,
amount, and taste. Five sachets of the drug were weighed and repackaged into
three airtight zip pouch containing 100,000 IU of cholecalciferol each with
the help of electronic weighing scale (0.001 g calibration). Placebo was
also processed in similar manner. Only 15 doses were prepared at a time.
Both drug and placebo were stored in a cool, dry, and dark place till
dispensed. The next lot was prepared afresh when 4 doses were left. The
allocation was further concealed by using sealed opaque envelopes.
Randomization, repackaging, sequencing, and allocation concealment were done
independently by a biostatistician and an office secretary who were not
members of the investigating team. ... None of the investigators, study
staff, and participants was aware of the drug or placebo being dispensed.
The codes were revealed only at the time of final data analysis" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "none of the investigators, study staff, and participants [were]
aware of the drug or placebo being dispensed. The codes were revealed only
at the time of final data analysis" Judgement comment: participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "none of the investigators, study staff, and participants [were]
aware of the drug or placebo being dispensed. The codes were revealed only
at the time of final data analysis." Judgement comment: outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "the effect of vitamin D supplementation on outcome variables was
analyzed on an intention‐to‐treat basis" Judgement comment: reasons for low loss to follow‐up are described (migration, address not traceable, left against medical advice, death); seem equal among groups. Intention‐to‐treat analysis was performed but on final available numbers (not on original randomised numbers) |
| Selective reporting (reporting bias) | Low risk | Quote: "the primary outcome variables were (a) the time to resolution of
severe pneumonia (the duration from the enrolment till the chest indrawing
was no longer present, and continued to be absent for next 24 hours); and
(b) the proportion of children having a recurrence of pneumonia in next six
months" Judgement comment: study was registered prospectively with Clinical Trial Registry of India (CTRI, ctri.nic.in) (ID: CTRI/2013/01/003317). All prespecified outcomes were reported. No protocol was identified |
| Other bias | Low risk | Judgement comment: no other risks observed |
Hanson 2011.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Grant from Nebraska Medical Center and University of Nebraska Medical Center, and Department of Pediatrics, University of Nebraska Medical Center, Omaha, NE, USA Country: USA Study period: August 2009 to June 2010 |
|
| Participants |
Included criteria: 32 weeks' gestational age, birth weight 1500 g,
mother indicated intention to formula‐feed her infant Excluded criteria: infants exclusively receiving maternal breast milk; those with congenital abnormalities; gastrointestinal, liver, or kidney disease; inborn errors of metabolism; parathyroid disease; disorders of calcium metabolism; infants receiving seizure medication or steroids Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics Placebo
400 IU D₃
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: birth; 7, 14, and 21 days of life |
|
| Notes | Calculated sample size met at randomisation but not at analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: random sequence generation method not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "matching placebo" Judgement comment: suggests placebo was matched to appearance of intervention but does not describe concealment processes. Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "investigators and neonatal intensive care unit staff were blinded
to subject group assignment" Judgement comment: personnel were blinded; no mention of participant blinding. Caregivers were likely blinded because they did not administer the supplement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "investigators and neonatal intensive care unit staff were blinded
to subject group assignment" Judgement comment: investigators blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "fifty‐six infants were enrolled in the study; the primary reason
for exclusion from the study was the mother’s intention to provide maternal
breast milk for her infant. Fifty‐two infants were included in the analysis;
four were excluded from the analysis for the following reasons:
phenobarbital was initiated in two infants, one infant was discharged, and
one infant was transferred to another institution" Judgement comment: reasons for low loss to follow‐up are given, but not by arm (reasons are unlikely to be related to outcome). Intention‐to‐treat analysis was performed |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: study was registered prospectively at ClinicalTrials.gov (ID: NCT01042561), which we identified through additional searching; prespecified outcomes are consistent with those reported. No prepublished protocol was identified |
| Other bias | Low risk | Judgement comment: no other risks observed |
Harnot 2017.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Indian Council of Medical Research (ICMR) (Grant no 3/2/2012/PG‐thesis‐HRD) Country: India Study period: July 2012 to June 2013 |
|
| Participants |
Included criteria: age 3 months to 3 years, attending paediatric
outpatient department with evidence of vitamin D deficiency based on
clinical (hypocalcaemic seizure or features of rickets like bowing legs or
rachitic rosary) or radiological (frying of radius ulna or costochondral
beading) features, those found to have vitamin D < 15 ng/mL Excluded criteria: chronic liver or kidney disease; congenital malformation; taking anticonvulsants, diuretics, or steroids longer than 1 month within past 6 months; known hypersensitivity to vitamin D Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions | 600,000 IU D₃
300,000 IU D₃
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: days 7 to 10 |
|
| Notes | Sample size calculated and met | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random allocation sequence was generated by computer using block
randomization of variable block size; by an independent physician, not
involved in patient management" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "allocation concealment was ensured by using serially numbered,
tamper proof, opaque and sealed envelopes" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "patient and clinician administering the drug were blinded from the
study details" Judgement comment: all personnel and participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind randomized" Judgement comment: implies that outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "from the initial cohort of 60 patients, 55 completed the study
(Fig. 1). Five patients, two from the 600,000 IU group and three from
300,000 IU group were lost to follow‐up (Fig. 1). The reason for lack of
follow up could not be ascertained as the caregivers did not come for even a
single follow up visit" Judgement comment: reasons for loss to follow‐up not ascertained but minimal attrition. Intent‐to‐treat analysis done only as a sensitivity analysis with no change in study results |
| Selective reporting (reporting bias) | Low risk | Judgement comment: study was prospectively registered with Clinical Trial Registry of India (CTRI, ctri.nic.in) (ID: CTRI/2012/05/002621), as reported in text. All prespecified outcomes were reported |
| Other bias | Low risk | Judgement comment: no other risks observed |
Hibbs 2018.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. National Heart, Lung, and Blood Institute (NHLBI) and Office of Dietary Supplements (ODS) (grant R01HL109293) Country: USA Study period: January 2013 to March 2017 |
|
| Participants |
Included criteria: infants at 28 to 36 weeks’ gestational age (GA)
at birth, child identified by family as black or African American; received
28 days or less of supplemental oxygen; admitted to a participating nursery
as a neonate; 40 weeks’ adjusted GA or younger at enrolment; lived within
predefined geographical area at each site Excluded criteria: diagnosed as having bronchopulmonary dysplasia; preexisting diagnosis of moderate to severe osteopenia of prematurity or alkaline phosphatase level > 700 U/L (to convert to μkat/L, multiply by 0.0167), or both; history of fracture; history of gastrointestinal surgery, including for necrotising enterocolitis, known gastrointestinal malabsorption, major congenital anomaly, congenital pulmonary or airway disorder, documented wheezing, or stridor before enrolment; previous vitamin D supplementation > 400 IU/d; family planned to move out of the region. Infants were also ineligible if their serum phosphorus concentration was outside the range of 4.0 to 9.5 mg/dL (to convert to mmol/L, multiply by 0.323) or serum calcium was outside the range of 8.5 to 10.7 mg/dL (to convert to mmol/L, multiply by 0.25). A serum 25‐hydroxyvitamin D concentration < 10 ng/mL or > 80 ng/mL also made infants ineligible (to convert to nmol/L, multiply by 2.496) Baseline vitamin D status (mean ± (IQR); nmol/L)
|
|
| Interventions |
Intervention characteristics 400 IU D₃ (sustained)
Placebo (diet limited)
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: 3, 6, and 12 months' adjusted age |
|
| Notes | Sample size calculated and met | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "infants were randomized with randomly permuted blocks, sizes 2 to
6, using computer‐generated random numbers" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "they received masked study drug (liquid cholecalciferol or a
placebo, dispensed in an amber bottle)" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "families, clinical caregivers, and study staff were blinded to
assignment and block size" Judgement comment: all personnel and participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "families, clinical caregivers, and study staff were blinded to
assignment and block size" Judgement comment: outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "of the 300 infants enrolled in the study, 18 withdrew from the
study and 1 died while co‐sleeping (Figure 1). Follow‐up rates of surviving
non withdrawn infants at the 3‐, 6‐, 9‐, and 12‐month visits were 97.9%,
96.5%, 95.0%, and 94.0%, respectively. Due to missing 12‐month visits in
infants who had not yet met criteria for recurrent wheezing, we were unable
to determine recurrent wheezing status for 8 children, and these cases were
considered as missing data in the primary analysis" Judgement comment: loss to follow‐up was well described and missing data were examined. Modified intent‐to‐treat approach was used |
| Selective reporting (reporting bias) | Low risk | Judgement comment: study was registered at ClinicalTrials.gov (ID: NCT01601847), as reported in text; study protocol is available. Outcomes proposed match reported outcomes |
| Other bias | Low risk | Judgement comment: no other risks observed |
Holmlund‐Suila 2012.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit: Finnish Foundation for Pediatric Research, Academy of Finland (no 277843), Sigrid Jusélius Foundation, Finska Läkaresällskapet, Biomedicum Helsinki Foundation, Folkhälsan Research Foundation, and a grant from the special governmental subsidy for health sciences research, Helsinki, Finland Country: Finland Study period: September 2010 to February 2011 |
|
| Participants |
Included criteria: born at term, with birth weight appropriate for
gestational age Excluded criteria: none specified Pretreatment: maternal vitamin D supplementation: 88% overall Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 400 IU D₃
1200 IU D₃
1600 IU D₃
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: 2 weeks of age, 3 months of age |
|
| Notes | Sample size calculated and met at randomisation and endpoint when compliance not considered, but not met when compliance considered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "each infant was randomized to receive 10, 30, or 40 g vitamin D3
supplementation daily for 10 wk" Quote: "infants were randomized into three groups stratified by gender and received vitamin D3 10 g (400 IU), 30 g (1200 IU), or 40 g (1600 IU) daily from age 2 weeks to 3 months in a double‐blinded fashion" Judgement comment: random sequence generation method not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "the Helsinki University Central Hospital Pharmacy prepared the
appropriate concentrations (10, 30, and 40 g/ml) and carried out
randomization after stratification by gender" Judgement comment: appropriate allocation concealment by a third party |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the study was double‐blinded; personnel responsible for the
subjects’ assessments remained blinded to the child’s intervention group
throughout the study" Judgement comment: personnel were blinded, but blinding of participants was not specified, although double‐blinding would indicate that participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the study was double‐blinded; personnel responsible for the
subjects’ assessments remained blinded to the child’s intervention group
throughout the study" Judgement comment: outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "this study included 113 children; in 93 subjects (82%), compliance
with the study vitamin D3 preparation" Quote: "we conducted an intention‐to‐treat analysis, regardless of compliance" Judgement comment: low loss to follow‐up; intention‐to‐treat analysis performed |
| Selective reporting (reporting bias) | Low risk | Quote: "our aim was to evaluate the effect of a higher than currently
recommended dose of vitamin D supplementation to determine a daily dose
ensuring S‐25‐OHD concentration at or above 80 nmol/liter in infants,
without ensuing signs of vitamin D excess" Quote: "study protocol was approved by the Finnish Medicines Agency (EudraCT 2009‐015940‐40) and Children’s Hospital, Helsinki University Central Hospital" Judgement comment: study was registered at European Union Clinical Trial Registry (ID: EUDRA2009‐015940‐40), as well as at ClinicalTrials.gov (ID: NCT01723852), as reported in text. Prespecified outcomes reported |
| Other bias | Low risk | Judgement comment: no other risks observed |
Holst‐Gemeiner 1978.
| Study characteristics | ||
| Methods |
Study design: quasi‐randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: Germany Study period: January to March 1976 |
|
| Participants |
Included criteria: newborns born at Gottfriend von Preyer Children's
Hospital in January, February, and March of 1976 Excluded criteria: none specified Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics 1200 IU D₃
200,000 IU D₃
|
|
| Outcomes |
Secondary
Measurement
Time points: 2nd to 10th week of life, 4th to 6th week of life |
|
| Notes | This study was translated from German; no sample size calculation; may be underpowered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "10 of the children received a daily pro from the 2nd to the 10th
day of life phylaxis of 1200 1. E. = 0.03 mg of a gly neutral alcoholic
solution of vitamin D3 per os, the remaining 11 got one 200,000 I. E. = 5 mg
of vitamin D3 per tablet" Quote: "the studies were performed in 21 consecutive newborns" Judgement comment: sequence appears to be based on date of presentation; possibly convenient or alternating randomisation; considered at high risk of selection bias |
| Allocation concealment (selection bias) | High risk | Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. Not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, while investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. However, outcomes measured are not subjective in nature and are less likely to be influenced by knowledge of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: no loss to follow‐up described |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registry or protocol available; outcomes in methods presented in results |
| Other bias | Low risk | Judgement comment: no other risks observed |
Huynh 2017.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: non‐profit + provision of drug. Women's and Children’s Division Sunshine Hospital, St Albans, Australia, for provision of trial medications and pharmacy costs. Australian Institute for MusculoSkeletal Science, Sunshine Hospital, St Albans, Australia, funded the publication and conference costs related to this study. Bayer Health donated Infant‐Pentavite in kind Country: Australia Study period: August 2013 to May 2014 |
|
| Participants |
Included criteria: born at 37 to 42 weeks' gestation, singleton
pregnancy, birth weight appropriate for gestational age according to
standardised Centers for Disease Control growth charts Excluded criteria: illicit drug use during pregnancy; infants requiring resuscitation for more than 10 minutes at birth; preexisting maternal conditions such as type 1 and type 2 diabetes mellitus, parathyroid disease, uncontrolled thyroid disease, and systemic glucocorticoid/anti‐inflammatory or cytotoxicity; major congenital anomalies and subcutaneous fat necrosis in the newborn Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 400 IU D₃
50,000 IU D₃
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: birth; 1 and 2 weeks of age |
|
| Notes | Study was underpowered at 3‐ to 4‐month follow‐up. Adherence was 31%; therefore this sample size calculation was not performed for a large enough sample size | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation (in random blocks of 2, 4 and 6) was undertaken in a
blinded manner. Babies of eligible mothers were randomised at birth using a
computer‐generated schedule" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "the allocated treatment arm was kept inside opaque, sealed
envelopes, which were numbered sequentially and opened, in numerical order
by the study recruiters" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "we conducted a single centre, open‐label randomised clinical
trial" Judgement comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "statistical analyses were undertaken by the trial statistician who
was blinded to treatment allocation" Judgement comment: not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "all predetermined analyses were performed according to intention to
treat principle" Judgement comment: moderate loss to follow‐up at 3 to 4 months, with loss to follow‐up reasons given, including patient transport difficulties, did not attend, switched treatment, declined blood tests, loss to follow‐up (not contactable/untraceable), foetal arrhythmia, decision to formula‐feed (not related to outcome) etc. All analysis was done by intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | Quote: "the full trial protocol can be accessed from the Western Health
Centre for Research and Education, Sunshine Hospital, St Albans,
Australia" Judgement comment: study was registered at Australian and New Zealand Clinical Trial Registry (ID: ACTRN12613001234707), as reported in text; full protocol may be accessed |
| Other bias | Low risk | Judgement comment: no other risks observed |
Jensen 2016.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. MEJ is supported by Canadian Institute of Health Research/Canadian LungAssociation/GlaxoSmithKline Post‐doctoral Fellowship (XCL‐120981). Funding for the trial was provided by a Thrasher Research Fund Early Career Award. The Sainte‐Justine Research Centre is supported by Fond de recherche Santé Québec Country: Canada Study period: November 2013 to February 2014 |
|
| Participants |
Included criteria: children age 1 to 5 years with (1)
physician‐diagnosed asthma, based on clinical signs of airflow obstruction
and reversibility; (2) upper respiratory tract infection (URTI) as the main
exacerbation trigger, as reported by parents; (3) ≥ 4 parent‐reported URTIs
in the past 12 months; and (4) ≥ 1 exacerbation requiring oral
corticosteroids in the past 6 months or ≥ 2 in the past 12 months Excluded criteria: extreme prematurity (28 weeks’ gestation); high risk of vitamin D deficiency; other chronic respiratory disease; disordered calcium or vitamin D metabolism; oral medications interfering with vitamin D metabolism; vitamin D supplementation > 1000 IU/d in the past 3 months Baseline vitamin D status (mean (interquartile range); nmol/L)
|
|
| Interventions |
Intervention characteristics 100,000 + 400 IU D₃
Placebo + 400 IU D₃
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: enrolment, 10 days, 3 months, 6 months |
|
| Notes | Sample size was calculated but was not met due to trial termination before sample size target was reached. Study was underpowered for the primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using computer‐generated randomisation with variable block sizes of
2–4, participants were randomised 1:1 to the intervention or control group.
Group assignment, recorded on a sequentially numbered list, was allocated by
the Sainte‐Justine Hospital Research Pharmacy, which held the randomisation
code" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "or identical placebo" Quote: "group assignment, recorded on a sequentially numbered list, was allocated by the Sainte‐Justine Hospital Research Pharmacy, which held the randomisation code. To maintain blinding, the intervention and placebo dose were identical in colour, appearance, volume, taste, and packaging" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "all research personnel, physicians, nurses, participants and their
parents were blinded to group allocation" Judgement comment: all personnel and participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: all personnel and participants were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "twenty‐two children were randomised to the intervention (N = 11) or
control group (N = 11) (Additional file 2). The trial was terminated before
reaching the target sample size, as funding was received to commence the
larger definitive trial. Retention in the intervention versus control group
was 91% vs. 100% at 3 months, and 73% vs. 91% at 6 months" Judgement comment: from supplementary data, each arm has similar numbers of patients lost to follow‐up; no reasons given for loss to follow‐up, no explanation of characteristics of patients lost to follow‐up. Trial was terminated early due to receipt of more funding for larger study |
| Selective reporting (reporting bias) | Low risk | Judgement comment: study was prospectively registered at ClinicalTrials.gov (ID: NCT01999907), as reported in text. No protocol was identified |
| Other bias | Low risk | Judgement comment: no other risks observed |
Kislal 2008.
| Study characteristics | ||
| Methods |
Study design: quasi‐randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: Turkey Study period: not reported |
|
| Participants |
Included criteria: gestational age 33 weeks, appropriate weight for
gestational age Excluded criteria: congenital malformations, failure to supplement vitamin D according to protocol Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics 200 IU
400 IU
800 IU
|
|
| Outcomes | None within scope of review | |
| Notes | No sample size calculation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "preterm infants were randomly selected either to receive a vitamin
D supplement of 200 IU/kg (group 1, 11 infants) or 400 IU/kg (group 2, 15
infants) or 800 IU/kg" Judgement comment: random sequence generation method not described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. Not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, while investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described; however, outcomes measured are not subjective in nature and are less likely to be influenced by knowledge of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "forty‐eight preterm infants were enrolled in the study.
...Thirty‐seven infants completed the study" Judgement comment: no reasons given for loss to follow‐up and no intention‐to‐treat analysis performed |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registry or protocol published; all outcomes measured and specified in methods and results |
| Other bias | Low risk | Judgement comment: no other risks observed |
Lagomarsino 1996.
| Study characteristics | ||
| Methods |
Study design: quasi‐randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Direccion de Investigaciones Universidad Catolica (DIUC 09/84) Country: Chile Study period: not reported |
|
| Participants |
Included criteria: birth in ambulatory paediatric unit at Diagnostic
Centre of the Pontificia Universidad Catolica, in Santiago de Chile
(CEDIUC); born at term, with weight appropriate for gestational age; without
neonatal conditions; receiving breast milk or formula of known composition
and quantity; not taking vitamins other than vitamin D given by the study
team Excluded criteria: none specified Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics 600,000 IU D₃
400 IU D₃
|
|
| Outcomes |
Primary
Measurement
Time points: various throughout 6‐month follow‐up |
|
| Notes | This study was translated from Spanish to English. No sample size calculation was performed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "the participants were assigned by order of admission to alternating
programs: one group 1 of 35 children, received 600 000 IU of Vitamin D, at 1
and 6 months of age; group 2 of 43 children, received 400 IU of Vitamin D
per day (20 drops) from the 15th day until 6 months of life" Judgement comment: alternating randomisation based on order of admission |
| Allocation concealment (selection bias) | High risk | Quote: "in the children from group 1, the solution of Vitamin D was for
oral usage packaged in 1 mL ampoules by the Laboratorio Chile, contained 15
mg of vit D, or cholecalciferol, equivalent to 600 000 IU. Since there were
no commercially available drops that contained exclusively Vitamin D, the
aforementioned laboratory made specially for this study a preparation that
in 20 drops contained 400 IU of vitamin D3 for the children in group 2" Judgement comment: sequence generation was at high risk of bias. Allocation concealment was not described, but given different regimens for each group, concealment seems unlikely |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. Not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, while investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. However, outcomes measured are not subjective in nature and are less likely to be influenced by knowledge of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: children lost to follow‐up (reasons, etc.) not discussed; appears to show a complete case analysis |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registry or protocol available; outcomes in methods reported in results |
| Other bias | Low risk | Judgement comment: no other risks observed |
Lava 2011.
| Study characteristics | ||
| Methods |
Study design: cross‐over trial Study grouping: parallel group Funding: no funding Country: Switzerland Study period: 1 March to 30 April 2010 |
|
| Participants |
Included criteria: Swiss singleton, newborn infants with gestational
age of 36 weeks or more and neonatal body weight of 2 kg or more; not
previously exposed to vitamin D Excluded criteria: not specified Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics Vi‐De₃
Vitamin D₃ Wild
|
|
| Outcomes | None within scope of review | |
| Notes | Target sample size described and met but not calculated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "an independent statistician had generated a randomization list to
balance the order of presentation of the preparations so that each
preparation was tasted first an equal number of times" Judgement comment: random sequence generation method not described |
| Allocation concealment (selection bias) | Low risk | Quote: "42 sequentially numbered, opaque sealed envelopes containing the
assignment. The envelopes were opened in sequence after accompanying the
infant with the mother to the test area" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "single‐blind" Judgement comment: only participants were blinded; if investigators know the intervention allocations, may be biased toward a particular outcome, increasing the risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: outcome assessors were not blinded; however, no outcomes were within the scope of this review |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol or trial registration found; outcomes in methods presented in results |
| Other bias | Low risk | Judgement comment: no other risks observed&& |
Manaseki Holland 2010.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. New Zealand Aid Cooperation Country: Afghanistan Study period: February to May 2007 |
|
| Participants |
Included criteria: all children between 1 week and 3 years of age in
the socio‐economically deprived population of Kabul, diagnosed clinically
with pneumonia, defined as (1) age‐specific tachypnoea (> 60 ⁄ min if
< 2 months; > 50 ⁄ min if 2 to 11 months; > 40 if 12 to 24 months)
and (2) absence of wheeze (with or without fever) Excluded criteria: clinical signs of rickets, known to have received high‐dose vitamin D treatment in the past 3 months, severe vomiting, pronounced wheeze, very severe pneumonias, other severe illness (meningitis, heart or renal disorder, measles, severe malnutrition, suspected tuberculosis), likely to migrate out of study area within 3 months Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics 100,000 IU D₃
Placebo
|
|
| Outcomes | None within scope of review | |
| Notes | Sample size calculated and met | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the children were individually randomised into intervention or
placebo groups using a random number sequence generated in an Excel
spreadsheet with no restrictions" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "vitamin D was contained in 1 ml of olive oil and individually
packaged into sealed 2‐mL plastic syringes at Aga Khan University and
labelled with unique ID number (only office was aware of the randomization
codes). Placebo (olive oil alone) and vitamin D syringes looked the same and
tasted the same" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind... On random questioning of parents, there were no
indications at any stage that families or doctors knew which child may have
received placebo or vitamin" Judgement comment: all personnel and participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind... On random questioning of parents, there were no
indications at any stage that families or doctors knew which child may have
received placebo or vitamin" Judgement comment: outcome assessors were blinded; however, no outcomes were within the scope of this review |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "all children randomized were included in the analysis on an
Intention‐to‐treat analysis" Judgement comment: minimal loss to follow‐up, due for the most part to recovery from pneumonia within the 24‐hour period after enrolment; intent‐to‐treat analysis performed |
| Selective reporting (reporting bias) | Low risk | Judgement comment: study was registered prospectively at ClinicalTrials.gov (ID: NCT00548379), which we identified through further searching; prespecified outcomes are consistent with those reported. Study protocol was not identified |
| Other bias | Low risk | Judgement comment: no other risks observed |
Manaseki‐Holland 2012.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Wellcome Trust and British Council Country: Afghanistan Study period: 4 November 2008 to August 2009 |
|
| Participants |
Included criteria: infants age 1 to 11 months and living in the
study region (catchment area of Maiwand Teaching Hospital, inner city
Kabul) Excluded criteria: families expecting to move to another town within 18 months, diagnosis of rickets or treatment with vitamin D in previous 3 months, clinical diagnosis of Kwashiorkor or Marasmus Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics 100,000 IU D₃
Placebo
|
|
| Outcomes |
Secondary
Measurement
Time points: various across 18‐month follow‐up |
|
| Notes | Sample size calculated and met | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "an independent statistician (Shabbar Jaff ar, London School of
Hygiene and Tropical Medicine, London, UK) randomised unique identification
numbers individually in fixed blocks of 20 to the vitamin D₃ or placebo
group by use of a random number generator with the SAS routine" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "by use of the randomisation list, a pharmacist in the Department of
Pharmacy, Aga Khan University Hospital, Karachi prepared 100 000 IU (2.5 mg)
of vitamin D₃ (cholecalciferol) in olive oil (Sinochem Ningbo Laboratory,
China) or placebo (olive oil) in sealed 2 mL plastic syringes labelled with
the unique identification numbers. The vitamin D 3 and the placebo were the
same colour (pale yellow), taste, and quantity (0.5 mL) and therefore the
study staff and the families did not know to which group the children were
assigned. Fieldworkers allocated children to randomisation groups during
recruitment and gave vitamin D or placebo" Judgement comment: appropriate allocation concealment by a third party |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the study staff and the families did not know to which group the
children were assigned. Fieldworkers allocated children to randomisation
groups during recruitment and gave vitamin D or placebo" Judgement comment: participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "many children lost at one time because of travel rejoined the study
later with rates being similar between the two groups (figure 1). There was
no statistically significant difference in any of the baseline
characteristics between the groups (table 1), including reported sun
exposure" Quote: "by the end of our trial 2616 of the 3046 recruited children were present in our study and 17 had died" Judgement comment: reasons for low loss to follow‐up were not given (other than death); children who dropped out rejoined the study in some cases. Intention‐to‐treat analysis was performed and was compared with per‐protocol analysis |
| Selective reporting (reporting bias) | Low risk | Judgement comment: trial was registered prospectively on ClinicalTrials.gov (ID: NCT00548379), as reported in text. Prespecified outcomes are consistent with those reported |
| Other bias | Low risk | Judgement comment: no other risks observed |
Marchisio 2013.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Grant (Ricerca Corrente 2012 850/02) from Italian Ministry of Health to Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca’ Granda Ospedale Maggiore Policlinico Country: Italy Study period: 1 November 2011 to 31 May 2012 |
|
| Participants |
Included criteria: children age 1 to 5 years with history of
recurrent acute otitis media (AOM) (defined as ≥ 3 episodes in preceding 6
months or ≥ 4 episodes in preceding 12 months, with most recent episode in
the previous 2 to 8 weeks), who were regularly followed by the outpatient
section of Pediatric Clinic 1, Fondazione IRCCS Ca’ Granda Ospedale Maggiore
Policlinico, University of Milan, Italy. The minimum number of episodes of
AOM for inclusion of patients in the otitis‐prone group had to be diagnosed
by pneumatic otoscopy in the outpatient section of Pediatric Clinic 1 by
trained investigators included among the authors of the study and documented
by medical records, with ≥ 2 episodes supported also by tympanometric
findings. At the time of enrolment, children had to be free of AOM but could
be affected by otitis media with effusion Excluded criteria: factors that can favour development of AOM, including severe atopy, acquired or congenital immunodeficiency, cleft palate, chronically ruptured eardrum, craniofacial abnormalities or obstructive adenoids, sleep apnoea syndrome, or placement of tympanostomy tubes Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 1000 IU D₃
Placebo
|
|
| Outcomes |
Secondary
Measurement
Time point: 4 months |
|
| Notes | No sample size calculated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a random number generator was then used to randomize the enrolled
children to receive oral vitamin D 1000 IU/d (10 drops of Pédiatre, Vitamin
D 3, Pediatrica, Livorno, Italy) or placebo for 4 months" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "similarly, the physicians involved in clinical monitoring were
blinded to the treatment assignment. The parents were given 4 numbered
bottles, each of which contained a number of drops needed for 1 month’s
treatment" Quote: "the study was blinded by labeling the identical bottles of VD and placebo drops and only revealing the randomization codes to the staff at the data monitoring center, who had no contact with the patients; similarly, the physicians involved in clinical monitoring were blinded to the treatment assignment" Judgement comment: appropriate allocation concealment by a third party |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "this prospective, randomised, double‐blind and placebo‐controlled
study" Judgement comment: double‐blind implies that participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "physicians involved in clinical monitoring were blinded to the
treatment assignment" Judgement comment: double‐blind implies that outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "the study involved 116 children (64 males, 55.2%; mean age 33.7 ±
11.7 months) with a history of (recurrent acute otitis media): 58 received
placebo and 58 VD" Judgement comment: loss to follow‐up not described; appears no loss to follow‐up occurred |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registry or protocol identified; all outcomes in methods described in results |
| Other bias | Low risk | Judgement comment: no other risks observed |
Mathur 2016.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: India Study period: April to December 2013 |
|
| Participants |
Included criteria: very low birth weight (1500 g) neonates who were
born preterm (37 weeks) Excluded criteria: major congenital malformation, not tolerating at least 100 mL/kg/d enteral feeds by day 10 of life Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 400 IU
1000 IU
|
|
| Outcomes |
Secondary
Measurement
Time point: 6 weeks |
|
| Notes | Sample size calculated and met | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "eligible neonates were randomized using a computer‐generated random
number sequence" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "concealed using sealed opaque envelopes on the day 100 ml/kg
enteral feeds were tolerated" Judgement comment: appropriate allocation concealment; sequential numbering not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "randomized, double‐blinded controlled trial in a teaching
hospital" Judgement comment: participants were likely blinded due to sealed envelopes; however personnel blinding was not specified although implied |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the radiologist and biochemist were blinded to the group allocation
and intervention given" Judgement comment: radiologist and biochemist were blinded; outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: Figure 1 implies that there was no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Quote: "Clinical Trial Registry of India (No. 2013/04/004953)" Judgement comment: study was registered retrospectively with Clinical Trial Registry of India (CTRI, ctri.nic.in) (ID: CTRI/2018/02/012058), which we found through additional searching. A different ID number was referenced in the text; however the quoted ID was not found when the CTRI database was searched |
| Other bias | Low risk | Judgement comment: no other risks observed |
Mittal 2014.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Indian Council of Medical Research, New Delhi, India; University College of Medical College, Delhi, India Country: India Study period: November 2010 to April 2012 |
|
| Participants |
Included criteria: age 6 months to 5 years presenting to paediatric
outpatient or emergency department with combination of clinical evidence of
rickets (wide wrists, bow legs, frontal bossing, rachitic rosary, etc.) and
radiological findings (fraying, splaying, and cupping at epiphyseal ends of
long bones in wrist/knee) consistent with diagnosis of nutritional
rickets Excluded criteria: critically ill children; those with coexisting fat malabsorption, liver or renal insufficiency, and hypercalcaemia; those with history of having received vitamin D, calcium supplements, or other medications affecting vitamin D metabolism (e.g. anticonvulsants, steroids, cancer chemotherapy) in previous 6 months Baseline vitamin D status (mean (95% confidence interval); nmol/L)
|
|
| Interventions |
Intervention characteristics 300,000 IU D₃
600,000 IU D₃
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: enrolment, 12 weeks |
|
| Notes | Sample size calculated, met at randomisation but not at final follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomization was done by block randomization (18 blocks of 4 each
and 2 blocks of 2 participants each) to 300,000 IU or 600,000 IU of oral
vitamin D3 in a single day" Judgement comment: random sequence generation method not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "allocation concealment was done by sealed envelope technique" Judgement comment: appropriate allocation concealment (sealed envelopes) but unclear if sequentially numbered or how they were opened |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "design: randomized, open‐labeled, controlled trial" Judgement comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "of the 76 children enrolled..." Judgement comment: high loss to follow‐up in Group 2 particularly, reasons reported (Figure 1). At randomisation, n = 28/600,000 IU arm and n = 32/300,000 IU arm. Reasons for loss to follow‐up before 4 weeks were mostly equal across groups, including (1) did not come in for follow‐up and could not be contacted; (2) systemic illness; (3) discontinuation of treatment. Reasons for loss to follow‐up before 12 weeks were noted only in the 600,000 IU arm and were (1) and (3); blood samples were not analysable ‐ relation to outcome is possible. Analysis was intention‐to‐treat but appears to analyse those who adhered to study protocol only |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or protocol identified; outcomes in methods presented in results |
| Other bias | Low risk | Judgement comment: no other risks observed |
Mittal 2018.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: no funding Country: India Study period: 19 December 2014 to unknown end date |
|
| Participants |
Included criteria: children age 6 months to 5 years with
radiological rickets (Thacher score > 1.5) Excluded criteria: any participant already diagnosed with any disease affecting absorption, or taking oral steroids, antitubercular, or antiepileptic drugs; patients who had taken calcium or vitamin D supplementation in last 6 months Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 90,000 IU
300,000 IU
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: enrolment; 1, 4, 12 weeks |
|
| Notes | Sample size calculated and appropriate at randomisation but not met by first follow‐up visit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was done on the basis of 1:1 subjects in both the
groups (random table generated from www.randomization.com)" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were allocated to one of two treatment arms according to
web‐generated sequence using block randomization (block sizes of 10, 8, and
4). The sequence was transcribed to sequentially numbered opaque sealed
envelopes by a person not directly involved in the study" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. Participants likely unblinded, as 1 group required half tablet and the other required full tablet. Not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, while investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: outcome assessors were blinded; radiological changes were scored by the same radiologist who was blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: equal loss to follow‐up across groups (22%); reasons for loss to follow‐up not given; intention‐to‐treat analysis not specified |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or protocol identified; outcomes in methods and presented in results |
| Other bias | Low risk | Judgement comment: no other risks observed |
Moodley 2015.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. National Institutes of Health grants R21AI084573 and R01NS077874, Early Career Award from Thrasher Research Foundation Country: Mexico Study period: February 2011 to July 2012 |
|
| Participants |
Included criteria: healthy infants born to women age 18 years at
Tijuana General Hospital, Mexico, were enrolled within 24 hours after birth
and before routine tuberculosis vaccine administration Excluded criteria: preterm (37 weeks' gestation), low birth weight (2500 g), had received vitamin D supplementation Baseline vitamin D status (mean (95% confidence interval); nmol/L)
|
|
| Interventions |
Intervention characteristics 50,000 IU D₃
Placebo
|
|
| Outcomes |
Secondary
Measurement
Time points: birth, 2 and 6 months of age |
|
| Notes | No sample size calculation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "infants were then randomized to receive oral vitamin D₃ or placebo.
None of the infants vomited or regurgitated the liquid in the 15 min after
administration. A randomization list was generated in blocks of 10 using
http://www.randomizer.org" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "a clear, tasteless liquid containing 2000 IU of vitamin D₃
(cholecalciferol) per drop was used (Carlson Laboratories Inc., Arlington
Heights, IL). The study dose of 50 000 IU was dispensed in 0.7 ml of liquid
vitamin D₃ solution. The placebo was a tasteless, colorless liquid that
contained 0.7 ml of medium chain triglycerides. Vitamin D₃ and placebo were
administered in prefilled and precoded syringes that were
indistinguishable" Judgement comment: appropriate allocation concealment; unclear if treatments were packaged by a third party, of if they were sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "a single‐center, double‐blind, placebo controlled trial was
conducted in 51 mother–infant pairs" Judgement comment: double‐blind implies that both participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "a single‐center, double‐blind, placebo controlled trial was
conducted in 51 mother–infant pairs" Judgement comment: double‐blind implies that study staff (i.e. outcome assessors) were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: Tables 3 and 4 indicate that there was loss to follow‐up; reasons were not given; intention‐to‐treat analysis was not specified |
| Selective reporting (reporting bias) | Low risk | Judgement comment: trial registered prospectively on ClinicalTrials.gov (ID: NCT01288950), which we identified through separate searching. Outcomes specified in methods were presented in results and are consistent with trial registration |
| Other bias | Low risk | Judgement comment: no other risks observed |
Morawa 1963.
| Study characteristics | ||
| Methods |
Study design: quasi‐randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: Germany Study period: October 1961 to July 1962 |
|
| Participants |
Included criteria: preterm, birth weight between 1500 and 2000 g Excluded criteria: none specified Pretreatment: all mothers had taken Vigantol menge (vitamin D supplement) in the first trimester Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics 720,000 IU D₃
720,000 IU D₃, CaP+
1000 IU D₃
750 to 1000 IU D₃
|
|
| Outcomes |
Secondary
Measurement
Time point: 3 months of age |
|
| Notes | We have not included the first 2 larger‐dose groups listed (720,000 IU)
because the dose was given intramuscularly or with calcium and therefore was
not eligible for analysis. This study was translated from German. No sample
size was calculated; appears to be a convenient sample CaP+: includes calcium and phosphorus |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "the various methods of the Vit.D prophylaxis, which are compared in
4 different groups with each other, were performed strictly alternating in
the above‐mentioned observation time" Judgement comment: alternating randomisation |
| Allocation concealment (selection bias) | High risk | Quote: "b) Groups of rickets for the treatment of rickets 1st Group: 15
children On day 3 of life, they received 3 mg Vigantol aquat D3 IM. In
addition, the children were given an oral shock of 5 mg D3 at the age of 4
weeks, an additional dose of 5 mg D3 at the age of 6 weeks and 5 mg D3
orally at the age of 10 weeks. 2nd group: 16 children The same procedure as
in group 1. In addition to the daily intake of food, 0.5 g
Calciumphosphoricum bibasicum was added to the diet daily until the 6th
week, when mixed milk (half milk, half pelargon) was administered. From this
point on there was no further mineral addition. 3rd group: 16 children The
children were given 1 Vigantolette (1 tablet contains 1,000 I.U. Vit.D3)
daily for 3 months from the 8th day of life. 4th group: 17 children The
children were given limevigantol tablets starting from the 8th day of life,
ie 1/2 tablet / kg weight (1 tabl. = 500 I.U. D3 + 0.5 g calcium
phosphoricum bibasicum), ie 750 ‐ 1000 I.U. daily, as long as 50% of the
women's milk could be replaced by Pelargon, this was the case in the 6th
week of life. Then transfer to 1 Vigantolette (1000 I.U. D3), daily until
the 3rd month of life. The children of groups 1 ‐ 2 received a total of
720,000 I.E. D3 in the form of small bumps within the first 3 months of
life. The children of groups 3 and 4 received 70‐80,000 I, E. D in
protracted daily dose" Judgement comment: allocation concealment unlikely, as each group was dosed at different times. No description of how interventions were indistinguishable |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding of participants and personnel is unlikely because doses were given at different times. Because rickets symptoms were the main symptoms (soft fontanelles), they could have been assessed by parents who would give kids a higher dose if suspected that the dose was low. Outcomes were more subjective based on scoring craniotabes and X‐rays, so were likely to be biased if personnel knew groupings |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: blinding of outcome assessors is not described ‐ introduces detection bias. Outcomes were more subjective based on scoring craniotabes and X‐rays, so likely to be biased if personnel knew groupings |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: no loss to follow‐up indicated, as per Tables 6 and 7 |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or protocol identified. Primary outcomes were serum alkaline phosphatase, cases of craniotabes, number of rachitic X‐ray changes; serum alkaline phosphatase could not be evaluated and reason is not clear; data on craniotabes results and rachitic X‐ray changes were reported |
| Other bias | Low risk | Judgement comment: no other risks observed |
Natarajan 2014.
| Study characteristics | ||
| Methods |
Study design: randomised‐controlled trial Study grouping: parallel group Funding: 100% non‐profit. Study drug was procured through Indian Council of Medical Research grant 5/7/305/08‐RHN Country: India Study period: August 2011 to March 2012 |
|
| Participants |
Included criteria: preterm infants born between 28 and 34 weeks’
gestational age and receiving ≥ 100 mL/kg/d of enteral feedings by 2 weeks’
postnatal age Excluded criteria: infants with major malformations, those who received parenteral nutrition for ≥ 2 weeks, those born to mothers receiving phenytoin therapy or with HIV infection Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 800 IU D₃
400 IU D₃
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: enrolment, 40 weeks' postmenstrual age, 3 months' corrected age |
|
| Notes | Sample size calculated and met at randomisation and analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "infants in both strata were randomly assigned to receive oral
vitamin D3 at a dose of 800 or 400 IU/day. We used computer‐generated random
numbers to allocate infants to 1 of the study groups with a fixed block size
of 4" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "random allocation was concealed by assigning sequential numbers to
identical‐appearing bottles containing 2 different amber‐colored, identical
appearing drug suspensions" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "amber‐colored bottles containing identical‐appearing drug
suspensions ensured blinding of investigator and parents" Judgement comment: participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: double‐blind implies blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "we enrolled 96 infants in the study (Fig 1). Clinical and baseline
characteristics of the study population were comparable between the 2 groups
(Table 1). Study intervention was initiated in 94 infants because consent
was withdrawn by 2parentssoonafter randomization.Of these 94 infants, 3
infants died before follow‐up at 40 weeks (1 due to stage 3 necrotizing
enterocolitis and 2 due to probable milk aspiration); another 4 infants were
lost to follow‐up. Thus, a total of 87..." Quote: "analysis was performed by intention to treat" Judgement comment: low loss to follow‐up overall; reasons documented. Reasons for loss to follow‐up included death and loss to follow‐up not described. Balanced among groups. Intention‐to‐treat analysis but seems to include only those finishing follow‐up; possible attrition bias |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: study was registered retrospectively with Clinical Trial Registry of India (CTRI, ctri.nic.in) (ID: CTRI/2012/02/002459), as reported in text. All prespecified outcomes were reported |
| Other bias | Low risk | Judgement comment: no other risks observed |
Pehlivan 2003.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: Turkey Study period: December 2012 to February 2013 |
|
| Participants |
Included criteria: healthy pregnant women, infants of normal birth
weight (> 2.5 kg) Excluded criteria: pregnant women with chronic disease or who were taking medication or had obstetrical problems (gestational diabetes, hypertension, preeclampsia, eclampsia, or premature delivery), twin pregnancy Baseline vitamin D status: unclear |
|
| Interventions |
Intervention characteristics 800 IU
400 IU
|
|
| Outcomes | None within scope of this review | |
| Notes | Data from this study were not clearly written, and numbers per intervention group were not given; therefore, data could not be included in any analyses in this review. It appears that 78 pregnant women and 65 infants were followed up. It is unclear if these 65 infants were a separate population, or if they were born to these moms, as study authors state that 65 infants were given vitamin D but then mention 65 infants again and label them as "controls". Further, study authors then state that 40 infants who were breastfed and received recommended doses of vitamin D on a regular basis were randomly assigned to either 400 IU per day or 800 IU per day of vitamin D, but do not specify sample sizes per group. One statement regarding results listed vitamin D concentration for the whole population of 83.7 ± 53.7 nmol/L, and indicated that 24.6% of infants were vitamin D deficient (measured as < 40 nmol/L), but it is unclear whether this occurred at baseline or after supplementation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement comment: randomisation of infants is not clear (which population was randomised) and random sequence generation method is not described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. Not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, while investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. However, outcomes measured are not subjective in nature and are less likely to be influenced by knowledge of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: loss to follow‐up is not described. Duration of administration is not specified |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial protocol or registration was identified |
| Other bias | Low risk | Judgement comment: no other risks were observed |
Ponnapakkam 2010.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Grant from The Gerber Foundation, a private, independent foundation promoting research in paediatric nutrition and health Country: USA Study period: August 2007 to December 2009 |
|
| Participants |
Included criteria: term babies with no known bone disorders, those
whose parents indicated that they intended to breastfeed (> 50% of total
intake) for at least the first 3 months of life Excluded criteria: none specified Baseline vitamin D status (mean ± standard error; nmol/L)
|
|
| Interventions |
Intervention characteristics 200 IU D₃ (at birth)
200 IU D₃ (at 2 months)
Placebo
|
|
| Outcomes |
Secondary
Measurement
Time points: birth; 2, 4, and 6 months of age |
|
| Notes | Sample size not calculated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the study participants were randomized into 1 of the 3 study
groups" Quote: "prior to randomization, the study population was stratified (as low risk and high risk) based on the presence or absence of additional risk factors for rickets (dark skin color or full‐body clothing/draping) to reduce the influence of this potentially confounding factor on the overall results" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | High risk | Quote: "commercial preparations of Vitamin D drops (D3) and placebo were
purchased from Patio drugs (Metairie, LA). The preparation consisted of 200
IU of vitamin D per 0.5 mL, for daily dosing. Patients were given a new
container of medication every 2 months and were encouraged to throw away any
leftover medicine. Approximate numbers of missed doses were noted on the
questionnaires" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not indicated; because parents were giving intervention and age at start of intervention was part of the randomisation group, performance bias may be increased. If investigators know the intervention allocations may be biased toward a particular outcome, this increases the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: blinding not indicated; outcomes assessed by parents as well as study personnel who were not blinded to allocation, which could introduce detection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "we recruited 80 patients into the study; 25 of these patients (30%)
completed the study (Table 1). Of the patients completing the study, 18 were
low risk, and 7 were high risk. There were 2 adverse events: 1 urinary tract
infection in group 1 (Vitamin D starting at birth, unclear if study related)
and 1 sudden infant death syndrome in group 3 (placebo, not study
related)" Judgement comment: study authors noted if loss to follow‐up (30% attrition) reasons were study related but did not compare dropouts to those who adhered to the study protocol. Study authors did not state number randomised to each arm and number of dropouts per arm. Reasons not given for loss to follow‐up except for adverse events. Analysis was not intention‐to‐treat |
| Selective reporting (reporting bias) | Unclear risk | Quote: "height and weight were documented, and data were collected from the
parents through questionnaires at the 0‐, 2‐, 4‐, and 6‐month pediatric
visits regarding nutrition" Judgement comment: no trial registration or protocol identified; outcomes in methods presented in results |
| Other bias | Low risk | Judgement comment: no other risks observed |
Principi 2013.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Grant from Italian Ministry of Health (Bando Giovani Ricercatori 2007) Country: Italy Study period: 1 October 2011 and 30 April 2012 |
|
| Participants |
Included criteria: children age 2 to 5 years with history of
recurrent acute otitis media (defined as ≥ 3 episodes in preceding 6 months,
or ≥ 4 episodes in preceding 12 months, with most recent episode in previous
2 to 8 weeks) who had not been previously vaccinated against influenza Excluded criteria: free of clinically evident febrile infectious disease, severe atopy, acquired or congenital immunodeficiency, recent administration of blood products, presence of anatomical abnormalities capable of favouring development of acute otitis media, long‐term treatment with drugs capable of interfering with absorption or metabolism of vitamin D, such as barbiturates, corticosteroids, and cholestyramine Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 1000 IU D₃
Placebo
|
|
| Outcomes |
Secondary
Measurement
Time points: enrolment, 6 months |
|
| Notes | Calculated sample size not given but ~ 55/group stated. This was met by treatment end | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the enrolled children were randomly divided into two groups and
assigned to receive daily vitamin D 1,000 IU (four drops of Dibase, Vitamin
D3, Abiogen Pharma S.p.A.) or placebo orally for four months" Judgement comment: random sequence generation method not described |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the study was single blinded because investigators knew whether the
children were receiving vitamin D or placebo, but parents were not
aware" Judgement comment: caregivers were blinded, while investigators were not blinded; investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: outcome assessors were not blinded; however, outcomes measured are not subjective in nature and are less likely to be influenced by knowledge of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "the study involved 116 children (61 males, 52.6%; mean age 3.0 ±
1.0 y), none of whom had been previously vaccinated against influenza: 59
(50.9%; mean age 3.3 ± 1.1 y) were administered vitamin D and 57 (49.1%;
mean age 2.9 ± 0.9 y) received placebo" Judgement comment: no discussion of loss to follow‐up; no examination of missing data; intention‐to‐treat analysis not done |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or protocol identified |
| Other bias | Low risk | Judgement comment: no other risks observed |
Rao 2016.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: India Study period: not reported |
|
| Participants |
Included criteria: age 2 to 5 years, vitamin D deficiency (20
ng/mL); parents had given informed written consent Excluded criteria: children with chronic illness, children taking steroid, other factor influencing vitamin D in children, acute illness for 2 weeks Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 4000 IU D₃
30,000 IU D₃
|
|
| Outcomes |
Secondary
Measurement
Time points: baseline, 3 and 12 months |
|
| Notes | Study authors describe calculation but do not explicitly state the actual sample size calculated. Assumed that sample size calculation was n = 19. Possibly retrospectively justified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects eligible for the study were divided in 2 strata based on
gender (male or female). Stratified randomization with a block size of 3 was
used to assign patients to groups 1, 2, and 3. The randomization lists were
computer‐generated prior to the start of the study and kept
confidential" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Unclear risk | Quote: "group 1 received 4,000 IU/day of vitamin D3 for 12 weeks along with
calcium carbonate (50‐mg elemental calcium/kg/day), group 2 received 30,000
IU/wk of vitamin D3 for 12 weeks along with calcium carbonate (50‐mg
elemental calcium/kg/day), and group 3 received 3,00,000 IU of vitamin D3
once intramuscular along with calcium carbonate (50‐mg elemental calcium/kg/
day)" Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "it was a single blind study and patients involved in the study were
unaware of assignment to treatment groups" Judgement comment: caregivers were blinded, while investigators were not. Investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: unblinding of study personnel could lead to detection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "a total of 19 subjects each was included in groups 1, 2, and 3,
respectively. In group 1, 3 subjects migrated and were lost to follow up and
one subject opted out of the study. In group 2, 4 subjects were lost to
follow up. In group 3, 4 subjects opted out of the study" Judgement comment: loss to follow‐up; reasons specified. After loss‐to‐follow‐up, n = 15/group, and only those completing the study protocol were analysed. 15% to 21% attrition may be causing bias. No Intention‐to‐treat analysis. Similar loss to follow‐up in each group; no discussion of the characteristics of lost participants. 80% completion rate is borderline. No mention of how study authors dealt with missing data |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or protocol identified |
| Other bias | Low risk | Judgement comment: no other risks observed |
Rianthavorn 2013.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: Thailand Study period: not reported |
|
| Participants |
Included criteria: patients age 18 years with chronic kidney
disease, Stage 5 or 5D, and 25‐hydroxyvitamin D (serum 25(OH)D, nmol/L)
levels of 30 ng/mL; haemoglobin levels of 10.0 to 12.5 g/dL, serum
phosphorus levels of 6.5 mg/dL, corrected serum calcium levels of 10.5
mg/dL, and calcium–phosphorus product of 65 mg/dL for ≥ 1 month before
recruitment Excluded criteria: thalassemia, chronic liver disease, gastrointestinal malabsorption, significant blood loss, serum parathyroid hormone levels > 800 pg/mL, proteinuria > 2 mg/mg of urine creatinine, blood transfusion, long‐term anticonvulsant therapy, prior ergocalciferol supplementation, kidney transplantation Pretreatment: degree of vitamin D insufficiency in participants was classified into 3 categories based on serum 25(OH)D levels (5, 5 to 15, 16 to 30 ng/mL). In patients with severe 25(OH)D deficiency (serum 25(OH)D level 5 ng/mL), 40,000 IU of ergocalciferol was given weekly for 4 weeks followed by 40,000 IU biweekly for 8 weeks (total 320,000 IU of ergocalciferol). For mild 25D deficiency (25D level 5 to 15 ng/mL), 40,000 IU of ergocalciferol was given biweekly for 12 weeks (total 240,000 IU of ergocalciferol). For 25D insufficiency (25D level 16 to 30 ng/mL), 40,000 IU of ergocalciferol was given every 4 weeks for 12 weeks (total 120,000 IU of ergocalciferol) Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 40,000 IU D₂
No intervention
|
|
| Outcomes |
Secondary
Measurement
Time points: baseline, 12 weeks |
|
| Notes | Post‐hoc power calculation showed this study had 50% power to detect a 30% change in effect estimate. Age‐stratified data were shared by study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "twenty patients were divided into two groups by simple
randomization" Judgement comment: random sequence generation method not described |
| Allocation concealment (selection bias) | High risk | Quote: "ten patients received oral ergocalciferol supplementation
(treatment), whereas the other group did not (control)" Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: not possible to blind participants due to control group receiving no intervention; investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: blinding of outcome assessors is not described; however, outcomes measured are not subjective in nature and are less likely to be influenced by knowledge of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "all patients completed the 12‐week study without any major adverse
effects from ergocalciferol" Judgement comment: no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no registration of trial nor cited study protocol |
| Other bias | Low risk | Judgement comment: no other risks observed |
Robinson 1981.
| Study characteristics | ||
| Methods |
Study design: quasi‐randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: London Study period: July 1977 to February 1978 |
|
| Participants |
Included criteria: preterm babies, patients of Professor Scopes Excluded criteria: none specified Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 400 IU D₃
1000 IU D₃
|
|
| Outcomes |
Primary
Secondary
Measurements
Time points: 14th day of life, 36 and 39 weeks' postmenstrual age |
|
| Notes | No sample size calculated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "18 babies (13 white and 5 West Indian) were randomly allocated to
two groups (1 or 2) between July 1977 and February 1978" Judgement comment: random sequence generation method not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "those in group 1 received 400 and those in group 2 1000 IU of
vitamin D3 daily by mouth from day 15" Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. Not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, while investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described; however, outcomes measured are not subjective in nature and are less likely to be influenced by knowledge of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: no loss to follow‐up described |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or protocol identified |
| Other bias | Low risk | Judgement comment: no other risks observed |
Rodd 2011.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: cross‐over Funding: 100% for profit. BioEnvelop, a division of Paladin Labs Inc., Laval, Quebec, Canada Country: Canada Study period: March to July 2009 |
|
| Participants |
Included criteria: healthy, term, singleton newborns of any racial
background and any feeding method Excluded criteria: infants unable to accept the supplement, congenital malformations, and (or) parents not sufficiently fluent in English or French to provide informed consent Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics 400 IU D₃ (syrup)
400 IU D₃ (filmstrip)
|
|
| Outcomes | None within scope of review | |
| Notes | Sample size not calculated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed using the Web site www.randomization.
com; selecting the first generator function randomized participants to
treatment groups by using the method of randomly permuted blocks" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | High risk | Judgement comment: allocation concealment not possible |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding, not possible with intervention/comparator formulations. Not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, while investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: no blinding; not possible with intervention/comparator formulations. However, this study did not analyse any outcomes within the scope of this review |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: minimal loss to follow‐up (n = 1 from liquid to filmstrip arm; n = 2 from filmstrip to liquid arm) |
| Selective reporting (reporting bias) | Low risk | Judgement comment: trial registered prospectively on ClinicalTrials.gov (ID: NCT00846677), as reported in text. Prespecified outcomes are consistent with those reported |
| Other bias | Low risk | Judgement comment: no other risks observed |
Rosendahl 2018.
| Study characteristics | ||
| Methods |
flex Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit Country: Finland Study period: 14 January 2013 to 30 May 2016 |
|
| Participants |
Included criteria: Northern European, term, birth weight within 2
standard deviations of the mean for gestational age Excluded criteria: infants requiring intravenous glucose, antibiotics, nasal continuous positive airway pressure treatment longer than 1 day, phototherapy longer than 3 days, or nasogastric tube feeding longer than 1 day; infants with seizures Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 400 IU D₃
1200 IU D₃
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: enrolment, 24 months |
|
| Notes | Sample size calculated at n ~ 300/group, which was met by analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "infants were randomized (1:1) to receive 400 IU or 1200 IU of
vitamin D₃ daily from age 2 weeks to 24 months. To ensure fair distribution
across the year, a pharmacist at Helsinki University Hospital with no
relation to the study performed randomization in blocks of 50" Judgement comment: random sequence generation method not described |
| Allocation concealment (selection bias) | Low risk | Quote: "both study preparations, manufactured by Orion Pharmaceuticals,
contained vitamin D₃ dissolved in medium‐chain triglyceride oil and were
identical in appearance. Participants and investigators were masked to group
assignment, and no changes to the methods were made after trial
commencement" Judgement comment: appropriate allocation concealment; however, no mention of sequentially labelled envelopes or containers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "participants and investigators were masked to group assignment, and
no changes to the methods were made after trial commencement" Judgement comment: all personnel and participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: possible that outcome assessors were not blinded and grading was done by staff; subjective and possibly increasing risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "we performed pQCT bone scans of the left tibia in 783 of the 823
children (95.1%) attending the age 24‐month follow‐up. Owing to motion
artifacts, 79 (10.1%) of the scans failed and were excluded. A total of 704
scans (89.9%) were included in the analyses. Of these, scan quality was
assessed as good in 165 (48.1%) of the 400‐IU group and 193 (53.5%) of the
1200‐IU group participants, moderate in 124 (36.2%) of the 400‐IU group and
133 (36.8%) of the 1200‐IU group, and poor in 54 (15.7%) of the 400‐IU group
and 35 (9.7%) of the 1200‐IU group" Quote: "in the analyses, we applied the intention‐to‐treat principle. Per‐protocol analyses included participants with treatment adherence of at least 80%" Judgement comment: loss to follow‐up was balanced across groups and reasons were documented. Out of 975 children, 783 (80%) had bone scans. Uncertain if children whose scans had motion artifacts are different from children whose scans did not. Grading of scans was subjective. Possible intention‐to‐treat analysis in 83.5% of the cohort |
| Selective reporting (reporting bias) | Low risk | Quote: "the project protocol is provided in Supplement 1 and has been
described in a previously published article" Judgement comment: registered on ClinicalTrials.gov (ID: NCT01723852); previously published protocol's prespecified outcomes are reported in study results |
| Other bias | Low risk | Judgement comment: no other risks observed |
Rueter 2019.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. DJP is supported by a Career Development Fellowship funded from the Medical Research Future Fund Next Generation Clinical Researchers Program. This study was supported by grants from Telethon–New Children’s Hospital Research Fund, Australia; Asthma Foundation of Western Australia, Australia; and Princess Margaret Hospital Foundation, Australia Country: Australia Study period: 9 October 2012 to 4 July 2017 |
|
| Participants |
Included criteria: healthy, term, singleton, before 28 days of age;
first‐degree relative (mother, father, or sibling) with history of allergic
disease (asthma, eczema, and allergic rhinitis) Excluded criteria: infants whose mothers had smoked during pregnancy or had an underlying immunodeficiency/autoimmune disease; those with maternal 25‐hydroxyvitamin D (25(OH)D) level serum concentrations < 50 nmol/L or > 100 nmol/L between 36 and 40 weeks’ gestation, which was intended to reduce risk of vitamin D deficiency or toxicity in infant participants Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics 400 IU D₃
Placebo
|
|
| Outcomes |
Secondary
Measurement
Time points: birth, 3 and 6 months of age |
|
| Notes | Sample size not calculated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was conducted by the Princess Margaret Hospital for
Children Clinical Trials Pharmacy and stratified according to a history of
maternal allergic disease and the participant’s sex. The pharmacy created a
randomization plan from an online source (www.randomization.com)" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "both the intervention (vitamin D) and control (placebo) oils were
packaged to appear identical and to maintain the blind" Judgement comment: appropriate allocation concealment, by a third party |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "all research staff remained blind to the allocations until analyses
were completed" Quote: "both the intervention (vitamin D) and control (placebo) oils were packaged to appear identical and to maintain the blind. Pharmacy staff had no contact with participants, and all research staff remained blind to the allocations until analyses were completed" Judgement comment: personnel were blinded; double‐blind implies that participants were blinded as well |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "both the intervention (vitamin D) and control (placebo) oils were
packaged to appear identical and to maintain the blind. Pharmacy staff had
no contact with participants, and all research staff remained blind to the
allocations until analyses were completed" Judgement comment: outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "analyses were performed according to the intention‐to‐treat
principle" Quote: "a total of 195 infants were randomized into the trial, 97 to the intervention vitamin D group and 98 to the placebo group. Fig 1 shows the participant flow diagram. Baseline characteristics of the 2 groups are described in Table I. Allocations in the vitamin D group compared with those in the placebo group were not different across seasons. Data collection was completed on July 4, 2017. Ninety‐two percent (180/195) of infant participants attended their appointment at 3 months of age, and 89% (173/195) of infants attended their appointment at 6 months of age. Nine (n = 6 from the vitamin D group) parents withdrew consent to participate during the intervention period" Judgement comment: low loss to follow‐up was balanced across groups; reasons were described. Intention‐to‐treat analysis was performed; appears that a subsample of infants gave blood, but how the sample was selected is not described |
| Selective reporting (reporting bias) | Low risk | Judgement comment: trial registered retrospectively on Australian New Zealand Clinical Trials Registry (ID: ACTRN12606000281594), as reported in text. Outcomes on trial registration are consistent with those reported |
| Other bias | Low risk | Judgement comment: no other risks observed |
Saad 2015.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: Egypt Study period: not reported |
|
| Participants |
Included criteria: infants with bronchiolitis; bronchiolitis
diagnosed by 2 senior paediatricians and defined as acute‐onset lower
respiratory tract symptoms for < 2 weeks with (a) evidence of a viral
infection (rhinorrhoea, coryza, cough, or fever); (b) abnormal auscultatory
findings (wheeze or crackles, or both); and (c) increased respiratory effort
(tachypnoea and intercostal retractions), who presented to the emergency
room within 7 days of onset of symptoms Excluded criteria: severe respiratory distress, admitted to intensive care unit, evidence of bacterial pneumonia (diagnosis of pneumonia was based on cough, chest wall in‐drawing and/or difficult breathing and tachypnoea, fever, and lobar, or bronchopneumonic, infiltration demonstrated by X‐ray), atopic disorders (asthma, known chronic cardiopulmonary disease, immunodeficiency, chronic medical condition including anaemia, severe malnutrition, meningitis, neurological disease, metabolic disease, gastrointestinal disease associated with malabsorption), any micronutrient supplementation or vitamin D therapy within the 4 weeks before enrolment Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 100 IU D₃/kg
Placebo
|
|
| Outcomes | None within scope of review | |
| Notes | Sample size not calculated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computerized random number generator" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "...drop solutions with identical outer covers and size of bottles.
The randomization and allocation process was done by a physician blinded to
the study" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind... Throughout the study, the parents of children who
administered the medications to their children were blind to
assignments" Judgement comment: participants were likely blinded due to adequate allocation concealment; double‐blind implies investigator blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double blind" Judgement comment: 'double‐blind' implies that outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "all side effects were mild and transient and all patients continued
with the study" Judgement comment: no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial protocol or registration identified |
| Other bias | Low risk | Judgement comment: no other risks observed |
Saleem 2018.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Supported by a grant from the Higher Education Commission of Pakistan under its International Research Support Initiative Program; reference no. 1‐8/HEC/HRD/2016/6029 Country: Pakistan Study period: not reported |
|
| Participants |
Included criteria: infants age 6 to 59 months at enrolment, whose
parents gave consent for them to participate, provided they had severe acute
malnutrition without complications, as defined by WHO (i.e. children with
mid‐upper arm circumference < 115 mm, weight‐for‐height z‐score < ‐3,
or grade 1 or 2 bilateral oedema who were clinically well and alert with
good appetite) Excluded criteria: ingestion of a dose of vitamin D > 200,000 IU (5 mg)/mo in the last 3 months (confirmed by medical records, or by maternal recall when these were unavailable), presence of complications of severe malnutrition (severe dehydration, severe anaemia, severe pitting oedema, anorexia, hypothermia, hyperpyrexia, acute lower respiratory tract infection, or hypoglycaemia) Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics 200,000 IU D₃
Placebo
|
|
| Outcomes |
Secondary
Measurement
Time points: enrolment, 8 weeks |
|
| Notes | Sample size calculated and met | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the random allocation sequence was generated on a Microsoft Excel
spreadsheet by a statistician who was independent of the study (Mr. Arslan
Chughtai, Rashid Latif Medical Collage, Lahore); a copy was held by the
principal investigator (JS), but she did not consult this during the trial.
Consecutive numbers from 001 to 200 were assigned to active and placebo
groups in a 1:1 ratio. No restrictions (e.g. stratification, block size)
were applied" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "double‐blind; the active and placebo medications were presented
identically (syringes of oily solution for oral administration) and had the
same appearance and taste" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the parents or guardians of all the study participants were blinded
to the allocations, as were the health workers, the research nurse, and the
pediatrician who enrolled participants and/or performed study
assessments" Judgement comment: all personnel and participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind; the parents or guardians of all the study
participants were blinded to the allocations, as were the health workers,
the research nurse, and the pediatrician who enrolled participants and/or
performed study assessments" Judgement comment: outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "one child allocated to the vitamin D 3 group died before taking the
first dose of study medication (cause of death: dehydration secondary to
gastroenteritis), and a further 8 children (3 allocated to vitamin D 3, 5
allocated to placebo) moved away from the study site prior to administration
of the first dose of study medication. The remaining 185 participants (93
allocated to vitamin D 3, 92 allocated to placebo) all took both doses of
study medication, completed the follow‐up and were included in the
analysis" Judgement comment: minimal loss to follow‐up (5%), reasons described |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: trial registered retrospectively on ClinicalTrials.gov (ID: NCT03170479), as reported in text; primary outcomes listed on registration but not secondary outcomes |
| Other bias | Low risk | Judgement comment: no other risks observed |
Sánchez‐Armendáriz 2018.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: Mexico Study period: April 2013 to March 2014 |
|
| Participants |
Included criteria: diagnosed with moderate to severe atopic
dermatitis according to Scoring of Atopic Dermatitis index Excluded criteria: some primary immunodeficiency, renal tubular acidosis, pregnancy, those who took other supplements, lack of follow‐up at 12 weeks Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 5000 IU D₃
Placebo
|
|
| Outcomes |
Secondary
Measurement
Time points: enrolment, 6 weeks, 12 weeks |
|
| Notes | Stratified data sent by study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "simple randomization was performed using the Epidat V3.1
software" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "the patients were divided into two groups; the first group received
water‐soluble capsules of 5000 IU/day of vitamin D3 (n = 33), and the second
group received cellulose capsules (n = 32). The placebo capsules were the
same size and color as the vitamin D3 capsules" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "both patients and doctors were blind to the study; in this way, a
pharmacist who did not participate in the taking of blood samples or in the
analysis of results was the only one who knew which patient belonged to each
group and gave them the capsules" Judgement comment: all personnel and participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" Judgement comment: all personnel and participants were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "sixty‐five patients diagnosed with AD were included; seven (10.8%)
were excluded because they dropped out of the study for reasons other than
this (lack of follow‐up)" Judgement comment: lack of detail on reasons for loss to follow‐up. Methods to deal with missing data not described. Intention‐to‐treat analysis not done |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or protocol identified |
| Other bias | Low risk | Judgement comment: no other risks observed |
Sarhan 2019.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: no funding Country: Egypt Study period: October 2016 to March 2017 |
|
| Participants |
Included criteria: age 1 to 24 months; diagnosed clinically as
suffering from acute bronchiolitis; presenting with any of the following:
persistent resting oxygen saturation < 92% in room air; marked tachypnoea
(> 70/min) or intercostal retractions indicating respiratory distress, or
both; difficulty of oral intake; inability of caregivers to care for the
child at home. Diagnosis of acute bronchiolitis was defined as a first
episode of respiratory distress with wheezing or crackles, or both, preceded
by infection of the upper airways (rhinorrhoea, coryza, cough, fever).
Disease severity was evaluated using the modified Tal score Excluded criteria: history of prematurity (< 37 weeks), chronic cardiopulmonary disease, immunodeficiency, neuromuscular disease, any other chronic medical condition; receiving vitamin D for 4 weeks before the study period; infants with recurrent wheezing or a physician’s diagnosis of asthma; patients with acute bronchiolitis having a very severe clinical score Baseline vitamin D status (n (%) <75 nmol/L)
|
|
| Interventions |
Intervention characteristics 100 IU D₃/kg
Placebo
|
|
| Outcomes | None within scope of review | |
| Notes | Sample size calculated and met | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the randomization and allocation process was carried out by a
higher nursing staff blinded to the study" Judgement comment: random sequence generation method not described |
| Allocation concealment (selection bias) | Low risk | Quote: "the assignments were kept in sealed envelopes until data
analysis" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the medical staff and parents were blind to assignments during the
study period" Judgement comment: all personnel and participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "the assignments were kept in sealed envelopes until data
analysis" Judgement comment: suggests outcome assessors were blinded, but possibly not those analysing the data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: no loss to follow‐up; all outcomes described in the methods reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: trial registered retrospectively on ClinicalTrials.gov (ID: NCT03799406), which we identified through separate searching; outcomes on trial registration consistent with those reported |
| Other bias | Low risk | Judgement comment: no other risks observed |
Shajari 2009.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: Iran Study period: not reported |
|
| Participants |
Included criteria: 15 days old, healthy, exclusively breastfeeding,
weighing 2500 to 4100 g Excluded criteria: kidney disease, malnutrition, prematurity Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics 200 IU D₃
400 IU D₃
50,000 IU D₃
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: 0 to 15 days of age, 3 months of age |
|
| Notes | No sample size calculation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "subjects divided randomly into three groups" Judgement comment: random sequence generation method not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "supplemented daily with 200 IU/daily vitamin D3 (Group I), 400
IU/daily vitamin D 3 (Group II) and the third group received 50,000 IU
vitamin D3 twice in fifteenth and sixtieth day after birth (Group III)" Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. Participants could learn which group they were allocated to by the dosing schedule. Not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, and investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described; however, outcomes measured are not subjective in nature and are less likely to be influenced by knowledge of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: analysis using Intention‐to‐treat analysis not noted; no discussion of loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or protocol identified |
| Other bias | Low risk | Judgement comment: no other risks observed |
Shakiba 2010.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: Iran Study period: January to September 2007 |
|
| Participants |
Included criteria: healthy breastfed infants weighing 2500 to 4000 g
from 3 primary care clinics in urban areas of Yazd City; infants' mothers
healthy and not under medication Excluded criteria: none specified Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics 200 IU D₃
400 IU D₃
50,000 IU D₃
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: enrolment, 6 months |
|
| Notes | Sample size calculated and met at randomisation but not at analysis for group 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the infants were randomised based on a computer‐generated
randomisation list (restricted randomisation), with a randomisation ratio
3:1, so that for each infant in the bolus group, three infants in the daily
group were selected, which distributed them equally within the 200 IU and
400 IU daily dosage groups" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "the paediatrician responsible for the infant allocated the next
available number on entry into the trial, and each parent collected the
vitamin drop and complete instructions directly from the pharmacy. The code
was revealed to the researchers at the end of the analysis of the
results" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the paediatrician responsible for the infant allocated the next
available number on entry into the trial, and each parent collected the
vitamin drop and complete instructions directly from the pharmacy. The code
was revealed to the researchers at the end of the analysis of the
results" Judgement comment: blinding not described; personnel and participants were unlikely to have been blinded due to different dosing regimens |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the code was revealed to researchers at the end of the analysis of
results" Judgement comment: blinding not described; quote implies that outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "the parents of three infants expressed an unwillingness to provide
a blood sample from their infants, and these patients were subsequently
eliminated from the study" Quote: "they were eliminated from the study if they failed to give their infants more than 15% of the daily doses (> 4 days in a month [36 infants]), or if they did not remember the number of missed days, or in the case of Group I, if more than 200 IU of vitamin D was consumed per day (6 infants)" Judgement comment: high attrition due to non‐compliance or inability to remember the number of missed days; possible attrition bias. Quote and Table 2 suggest that those lost to follow‐up were excluded from analysis; complete case analysis was done. Characteristics of those lost to follow‐up were not evaluated |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or protocol identified |
| Other bias | Low risk | Judgement comment: no other risks observed |
Shedeed 2012.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: Libya Study period: April 2008 to July 2010 |
|
| Participants |
Included criteria: admitted with congestive heart failure due to
dilated cardiomyopathy or congenital heart disease, with systemic left
ventricular systolic dysfunction (dilated left ventricle > 2 standard
deviations for age and sex together with an ejection fraction > 40%) Excluded criteria: any infant with hypercalcaemia, hypocalcaemia, serum creatinine concentration (> 1.5 mg/dl), and nephrolithiasis; actual intake of supplements containing vitamin D and calcium Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 1000 D₃
Placebo
|
|
| Outcomes |
Secondary
Measurement
Time points: enrolment, 12 weeks |
|
| Notes | Sample size not calculated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "subjects randomly allocated by systematic random sampling into two
groups: group I included 42 patients who received a daily supplement of 25
µg (1,000 IU) cholecalciferol (D‐Vi‐Sol Infant Drops; Mead Johnson
Nutritionals), and group II included the other 38 subjects who received the
placebo oral drops" Judgement comment: random sequence generation method not described |
| Allocation concealment (selection bias) | Low risk | Quote: "group I included 42 patients who received a daily supplement of 25
lg (1,000 IU) cholecalciferol (D‐Vi‐Sol Infant Drops; Mead Johnson
Nutritionals), and group II included the other 38 subjects who received the
placebo oral drops (vitamin D‐free distilled water). Both groups and the
investigators were unaware with the nature of the oral drops bottles (the
vitamin D and placebo bottles were identical in shape)" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "both groups and the investigators were unaware with the nature of
the oral drops bottles (the vitamin D and placebo bottles were identical in
shape)" Judgement comment: blinding of participants and investigators not specifically indicated but implied due to allocation concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judgement comment: blinding of outcome assessors not specifically indicated; outcomes objective in nature |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: no discussion of loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or protocol cited |
| Other bias | Low risk | Judgement comment: no other risks observed |
Siafarikas 2011.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Northern German Society of Paediatric and Adolescent Medicine Country: Germany Study period: autumn/winter (October to March) and spring/summer (April to September) (year not reported) |
|
| Participants |
Included criteria: delivery at Hospital Berlin‐Lichtenberg, Germany;
breastfeeding Excluded criteria: vitamin D supplementation during pregnancy, drug abuse, premature delivery, highly pigmented skin Baseline vitamin D status (mean (95% confidence interval); nmol/L)
|
|
| Interventions |
Intervention characteristics 250 IU D₃
500 IU D₃
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: birth, 6 weeks |
|
| Notes | Sample size calculated but not met | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "using odd and even numbers taken from opaque envelopes,
participants were randomised into two subgroups (n=20) on either 250 or 500
units of vitamin D3 as a daily supplement" Judgement comment: even/odd (non‐sequentially numbered) envelopes are considered at high risk of selection bias |
| Allocation concealment (selection bias) | High risk | Quote: "families received detailed instructions on how to dissolve either
one (500 IU) or half a tablet (250 IU) in a spoon and administer the tablet
to their child" Quote: "using odd and even numbers taken from opaque envelopes, participants were randomised into two subgroups (n=20) on either 250 or 500 units of vitamin D3 as a daily supplement (figure 1)" Judgement comment: opaque envelope but not sequentially numbered; the 2 interventions varied by protocol. Therefore allocation could not have been concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not described; as allocations varied by protocol, participants may not have been blinded, leading to parental compensation of vitamin D in the control group or impact on nutrition diaries/conduct. Nature of intervention (half tablet and whole tablet) would not facilitate blinding. If investigators knew the intervention allocations, they may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: blinding of outcome assessors is not described. Some outcomes are subjective (clinical signs of rickets) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: a significant number of subjects ended up being excluded due to insufficient blood sample; no discussion of how this many have impacted outcomes. No loss to follow‐up; however. Intention‐to‐treat analysis was not done |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: trial registered retrospectively on Australian New Zealand Clinical Trials Registry (ID: ACTRN12609000919213) and World Health Organization (ID: U1111–1112‐2443), as described in text. Outcomes on registrations are presented in results |
| Other bias | Low risk | Judgement comment: no other risks observed |
Singh 2018a.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: India Study period: January 2013 to February 2014 |
|
| Participants |
Included criteria: consecutively born, full‐term, healthy neonates
born to mothers who were residents of Delhi and chose to exclusively
breastfeed their infant and consented for participation in this follow‐up
study Excluded criteria: babies with life‐threatening congenital malformations, those born to HIV‐positive mothers Group differences: total alkaline phosphatase was higher in the vitamin D group; serum 25‐hydroxyvitamin D (serum 25(OH)D, nmol/L) was higher in the control group; parathyroid hormone was higher in the control group Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 400 IU D₃
No intervention
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: enrolment, 6 months of age |
|
| Notes | Sample size calculated and met for analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "series of computer‐generated random number sequence was prepared by
Inclen Trust, New Delhi, using Stata 9.0 software. Block randomisation was
done using alternate block sizes of 4 and 6" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "allocation concealment was achieved using sequentially numbered
opaque sealed envelopes; safely secured with a person not involved in the
study until subject enrolment" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "was an open‐label (unblinded), parallel, superiority randomised
controlled trial with 1:1 allocation ratio, conducted in post‐natal ward
setting" Judgement comment: unblinded; as no placebo was used, the nature of the intervention would have made blinding impossible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "was an open‐label (unblinded), parallel, superiority randomised
controlled trial with 1:1 allocation ratio, conducted in post‐natal ward
setting" Judgement comment: unblinded; no mention of outcome assessor blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "a total of 100 babies meeting inclusion criteria were enrolled and
randomised to respective groups within 24 hours of birth. Two were lost to
follow‐up (shifted out of Delhi) and one withdrew consent within initial one
week of the study [Table/Fig‐2]" Judgement comment: Figure 2 shows minimal loss to follow‐up, with balanced, documented reasons (2 were lost to follow‐up (shifted out of Delhi) and 1 withdrew consent within first week of the study (Table/Fig 2)). Intention‐to‐treat analysis analysis was done |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no citation of trial registration or protocol |
| Other bias | Low risk | Judgement comment: no other risks observed |
Singh 2019.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: India Study period: January 2013 to September 2014 |
|
| Participants |
Included criteria: age 0 to 5 years, diagnosed with recurrent
pneumonia according to World Health Organization criteria Excluded criteria: diagnosed with rickets, vitamin D deficiency, congenital heart disease, wheezing associated with lower respiratory tract infection, neurological illness, congenital anomaly (kyphosis, scoliosis, cleft lip and palate), measles, whooping cough, tuberculosis, HIV infection, previous vitamin D supplementation, not residing in the given locality for > 1 year Baseline vitamin D status (n (%) < 75 nmol/L)
|
|
| Interventions |
Intervention characteristics 300,000 IU D₃
Placebo
|
|
| Outcomes | None within scope of review | |
| Notes | Sample size not calculated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgement comment: random sequence generation method not described |
| Allocation concealment (selection bias) | High risk | Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: blinding not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: 9 children (9%) were excluded from analysis but only reason appears to be haemolysis of blood samples. Other reasons for loss to follow‐up are not described. Intention‐to‐treat analysis was not done |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or protocol identified |
| Other bias | Low risk | Judgement comment: no other risks observed |
Somnath 2017.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Jawaharlal Institute of Post‐graduate Medical Education & Research (JIPMER) Intramural Grant Country: India Study period: March 2013 to April 2014 |
|
| Participants |
Included criteria: age 2 months to 5 years, lower respiratory
infection (LRI) Excluded criteria: chronic chest condition presenting as acute LRI such as tuberculosis, bronchial asthma, congenital lung malformation, immunodeficiency state (both congenital and acquired); conditions that interfered with absorption and metabolism of vitamin D (malabsorption syndrome, chronic diarrhoea, liver disease, kidney disease); any known contraindications for vitamin D administration (e.g. nephrocalcinosis, urolithiasis) Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 100,000 IU D₃
Nothing
|
|
| Outcomes |
Secondary
Measurement
Time points: enrolment, 72 hours |
|
| Notes | Sample size calculated n = 70/group. This was met at analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was done by using computer generated random number
tables by a resident not involved in the study" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "opaque sealed and serially numbered envelopes were used for
allocation concealment" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open labelled" Judgement comment: not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, while investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the outcome assessors were blinded to the intervention" Judgement comment: outcome assessors were blinded; outcome measurements are not subjective and are unlikely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "there were a total of 154 children in the age range 2 months 5
years" Judgement comment: no loss to follow‐up, but 2 participants had tuberculosis at the end and were excluded. Intention‐to‐treat analysis was not done |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: study was registered retrospectively with Clinical Trial Registry of India (CTRI, ctri.nic.in) (ID: CTRI/2014/09/005032), as reported in text |
| Other bias | Low risk | Judgement comment: no other risks observed |
Specker 1992.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Thrasher Research Fund, Salt Lake City, UT, USA; and Perinatal Research Institute, Cincinnati, OH, USA Country: China Study period: fall (September to October 1986) and spring (March and April 1987) |
|
| Participants |
Included criteria: gestational age ≥ 37 weeks Excluded criteria: major congenital abnormality, gastrointestinal disease Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics 100 IU, North China
200 IU, North China
400 IU, North China
100 IU, South China
200 IU, South China
400 IU, South China
|
|
| Outcomes |
Secondary
Measurement
Time points: 3 to 5 days of age, 6 months of age |
|
| Notes | Sample size calculated; met for analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "at 3 to 5 days of age each infant was randomly assigned to one of
three groups to receive vitamin D supplements of 100, 200, or 400
IU/day" Judgement comment: random sequence generation method not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "vitamin D was prepared in propylene glycol (Kremers‐Urban Co.,
Milwaukee, Wis.) and mothers were instructed to give the vitamin preparation
to their infants daily. The vitamin D supplements were distributed monthly
to mothers... Both mothers and investigators were unaware of assigned
dosage" Judgement comment: appropriate allocation concealment; lacking detail |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "both mothers and investigators were unaware of assigned dosage" Judgement comment: all personnel and participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all radiographs were interpreted; ossification centers, as well as
signs of rickets (concavity and fraying of bone and widening of epiphysis),
were recorded by a pediatric radiologist at Cincinnati Children's Hospital
Medical Center who was unaware of the dosage group" Judgement comment: more subjective measures were obtained by a blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "ninety percent of the infants enrolled (280/312) completed the
study; cord and 6‐month blood samples were available for 256 (82%) of the
infants" Judgement comment: loss to follow‐up (18%); reasons not given. Appears to be a complete case analysis |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or protocol identified |
| Other bias | Low risk | Judgement comment: no other risks observed |
Stögmann 1985.
| Study characteristics | ||
| Methods |
Study design: quasi‐randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: Austria Study period: not reported |
|
| Participants |
Included criteria: clinical, biochemical, and radiological signs of
vitamin D deficiency Excluded criteria: none specified Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics 200,00 IU D₃
9600 IU D₃
|
|
| Outcomes |
Secondary
Measurement
Time points: enrolment; days 3, 7, 14, and 21 |
|
| Notes | Translated from German to English. Sample size not calculated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "for this reason, 5 children with vitamin D deficiency echinitis
were randomized to receive vitamin D bump therapy or continuous therapy, and
the changes in calcium, phosphorus, alkaline phosphatase,
25‐OH‐cholecalciferol, parathyroid hormone, and calcitonin serum
assessed" Judgement comment: random sequence generation method not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "children received a vitamin D beating therapy (5 mg each = 200,000
IU of vitamin D3 on day 1 and day 3, referred to below as group I), 5
children a continuous therapy (9600 IU of vitamin D3 (2 x 12 drops)
glycollarcolic cholecalciferol solution by 18 days, hereinafter called group
II)" Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no description of blinding; could have led to parental compensation; unlikely parents were blinded, as dosing schedules were different |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: no description of blinding; unlikely outcome assessors were blinded, which may lead to bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or protocol identified |
| Other bias | Low risk | Judgement comment: no other risks observed |
Tang 2019.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Children's Hospital of Chongqing Medical University and Chongqing City Health and Family Planning Committee (grant no 2016MSXM033) Country: China Study period: 20 October 2016 to 15 February 2018 |
|
| Participants |
Included criteria: met 2001 International League of Associations for
Rheumatology classification criteria, children with newly diagnosed juvenile
idiopathic arthritis (JIA), signed informed consent Excluded criteria: history of kidney stones, hypercalciuria, intestinal malabsorption, primary cardiovascular disease, lung disease, blood disease, liver disease; history of using drugs that inhibit bone resorption; history of allergy to vitamin D; refusal to participate in the study; treatment with methylprednisolone, biological agents, or cyclophosphamide Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 2000 IU D₃
No intervention
|
|
| Outcomes |
Secondary
Measurement
Time points: enrolment, 12 weeks, 24 weeks |
|
| Notes | Stratified data sent by study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were selected using a table of random numbers" Judgement comment: random sequence generation method unclear |
| Allocation concealment (selection bias) | High risk | Judgement comment: control group received nothing; allocation was not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label" Judgement comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label" Judgement comment: not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "the remaining patients were assigned randomly to the CG (n=22) or
the EG (n=20). A total of six patients withdrew for personal reasons or were
lost to follow‐up. Finally, 36 subjects completed the trial and were
included in the analysis (n=18 per group)" Judgement comment: some loss to follow‐up; intent‐to‐treat analysis not performed |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: trial registered prospectively on Chinese Clinical Trial Registry (ID: ChiCTR‐INR‐16009235), as described in text. Outcomes listed in trial registration are cytokines but not 25(OH)D, JADAS‐27, or BMD z‐score |
| Other bias | Low risk | Judgement comment: no other risks observed |
Tergestina 2016.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Institutional Fluid Research Grant Country: India Study period: January to November 2013 |
|
| Participants |
Included criteria: gestational age of 27 to 34 weeks Excluded criteria: major congenital anomaly, maternal condition or medication likely to influence vitamin D or calcium metabolism, neonates not attaining 100 mL/kg feeds by 14 days of life Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 400 IU D₃
1000 IU D₃
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: enrolment, 40 weeks' corrected gestational age |
|
| Notes | Sample size calculated n = 50/arm. This was met at randomisation but not for group 1 at analysis. Study was underpowered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "block randomization with sizes of 2, 4 and 6 with 25, 25 and 50%
allocation were used" Judgement comment: random sequence generation method not described |
| Allocation concealment (selection bias) | Low risk | Quote: "serially numbered opaque sealed envelopes were used to conceal the
allocation. The drugs were identical in appearance, color and taste and were
contained in amber‐colored bottles labeled with serial numbers corresponding
to the envelopes. The randomization code was known only to the pharmacy and
the statistician" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind... blinding the investigators and the family... the
randomization code was known only to the pharmacy and the statistician,
blinding the investigators and the family" Judgement comment: all personnel and participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind... blinding the investigators and the family... the
randomization code was known only to the pharmacy and the statistician,
blinding the investigators and the family" Judgement comment: outcome assessors were blinded, although this quote implies that statisticians were not blinded for analysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "120 babies were enrolled in the trial, with 60 babies randomized
into each arm. Twenty one babies were lost to follow‐up—12 babies in the 400
IU arm and 9 babies in the 1000 IU arm. Post discharge, three sets of twins
were not exclusively administered 400 or 1000 IU of vitamin D by their
caregivers. This constituted a breach of study protocol, was recorded as
such and analysis was on an intention to treat basis" Judgement comment: loss to follow‐up (18%); reasons were not given; relatively balanced by arm; intention‐to‐treat analysis done. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: study was registered retrospectively with Clinical Trial Registry of India (CTRI, ctri.nic.in) (ID: CTRI/2012/11/003154), as reported in text. All prespecified outcomes were reported |
| Other bias | Low risk | Judgement comment: no other risks observed |
Thacher 2014.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Grant Number 1 UL1 RR024150, from the National Center for Research Resources Country: Nigeria Study period: February 2004 and November 2006 |
|
| Participants |
Included criteria: children with active rickets, identified using
radiographs of the wrists and knees from among children who presented with
leg deformities. Children were eligible for enrolment if they had a
radiographic score ≥ 2.5 on a validated 10‐point scoring method that
assessed the severity of rickets in growth plates of the distal radius and
ulna and around the knee Excluded criteria: none specified Pretreatment: Nigerian children with active rickets treated with calcium carbonate as limestone (approximately 938 mg elemental calcium twice daily) were randomised to receive either oral vitamin D 250,000 IU (calcium and vitamin D, n = 44) or placebo (calcium, n = 28) monthly for 24 weeks. All children were treated with calcium carbonate as powdered limestone. Powdered limestone was locally available at a much lower cost than calcium tablets. The content of elemental calcium in 1.0 g of limestone was 268 mg (courtesy of Michael Gruzak, USA Department of Agriculture/Agriculture Research Service (USDA/ARS), Children’s Nutrition Research Center, Houston, TX, USA). Samples of limestone had no toxic concentrations of heavy metals. One level teaspoon of powdered limestone (approximately 3.5 g = 938 mg of elemental calcium) was mixed with the child’s food or porridge twice daily Baseline vitamin D status (mean (95% confidence interval); nmol/L)
|
|
| Interventions |
Intervention characteristics 50,000 IU D₂, Ca+
Placebo, Ca+
|
|
| Outcomes |
Secondary
Measurement
Time points: enrolment, 24 weeks |
|
| Notes | Received age‐stratified information from study author (n = 53 under 5 years
of age, out of 68). Due to the coin toss method, the calcium‐only group was
underpowered. Calculations were done but were not met, target being n =
40/group; this was not met at randomisation nor at analysis. Power to detect
was only 46%. However, study authors state that lack of power does not
affect conclusions related to findings that were statistically
significant Ca+: included calcium |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "enrolled children were randomised by coin toss (performed by TDT)
to receive under direct observation either oral vitamin D 2 as 50 000 IU
(ergocalciferol; Pliva, Inc., East Hanover, New Jersey) once every 4 weeks
(Ca+D group) or placebo, which was a single vitamin B complex tablet, once
every 4 weeks (Ca group) for 24 weeks" Judgement comment: coin toss, a low‐tech appropriate randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Quote: "to receive under direct observation either oral vitamin D2 as
50,000 IU (ergocalciferol; Pliva, Inc., East Hanover, New Jersey) once every
4 weeks (calcium and vitamin D group) or placebo, which was a single vitamin
B complex tablet, once every 4 weeks (Calcium group) for 24 weeks" Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no blinding described; it is possible that caregivers were blinded as intervention was given under direct observation of study personnel; however, differences in interventions would make blinding impossible. Not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, while investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: no blinding is described; outcomes such as symptoms of rickets and diet are subjective and therefore are prone to possible bias if not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "total of 254 children presented with leg deformities, and 72
subjects with radiographically active rickets were enrolled between February
2004 and November 2006 (figure 1)" Judgement comment: loss to follow‐up: 1 and 3 per arm ‐ a low proportion. Reasons for loss to follow‐up not given. Intention‐to‐treat analysis not done |
| Selective reporting (reporting bias) | Unclear risk | Quote: "trial registration number ClinicalTrials.gov NCT00949832" Judgement comment: study was carried out between 2004 and 2006, but study was registered in 2009 so was not prespecified. Prespecified and reported outcomes match |
| Other bias | Low risk | Judgement comment: no other risks observed |
Tomimoto 2018.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% for profit. Morishita Jintann Co., Osaka, Japan Country: Japan Study period: 19 January 2016 to unknown end date |
|
| Participants |
Included criteria: 3 to 4 months of age with vitamin D deficiency
(< 50 nmol/L) Excluded criteria: underlying disease, mixed feeding (artificial milk > 50 mL/d), fever, anorexia, vitamin D supplementation for mother or child, vitamin D deficiency with clinical manifestations of rickets Baseline vitamin D status (n (%) < 50 nmol/L)
|
|
| Interventions |
Intervention characteristics 160 IU
400 IU
|
|
| Outcomes |
Secondary
Measurement
Time points: enrolment, 4 weeks |
|
| Notes | Sample size not described (abstract of full‐text paper only) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: random sequence generation method not described (abstract only) |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not described (abstract only) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open label" (trial registration) Judgement comment: unblinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open label" (trial registration) Judgement comment: unblinded study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: not described |
| Selective reporting (reporting bias) | Low risk | Judgement comment: trial registration number available (JMA‐IIA00243) and outcomes specified on registration reported, quantitatively and qualitatively, in identified abstract |
| Other bias | Low risk | Judgement comment: no other risks observed |
Trilok‐Kumar 2011.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Department of Biotechnology, Ministry of Science and Technology, Government of India; Nutrition Third World; Sight and Life Country: India Study period: March 2007 to July 2010 |
|
| Participants |
Included criteria: singleton, ≥ 37 weeks' gestation, birth weight
1.8 to 2.5 kg, 48 hours of age, living within a 15‐km radius of Safdarjung
Hospital, parental informed consent Excluded criteria: severe congenital abnormality, morbidity severe enough to result in death before age 7 days, intention to live outside catchment area before the infant reaches 6 months of age, lack of consent Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics 1400 IU D₃
Placebo
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: enrolment, 6 months, follow‐up at 3 to 5 years |
|
| Notes | Calculated sample size met at randomisation but not met for analysis of anthropometry or morbidity | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a simple randomisation list without blocking was computer generated
and held by the data safety and monitoring board only" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "at enrolment the infants were randomised to receive each week,
starting at 7 days of age and continuing to 6 months (maximum of 25 doses),
either 35 µg (1400 IU) granulated vitamin D 3 (cholecalciferol), which is
the Food and Agriculture Organization/World Health Organization recommended
nutrient intake of 5 µg (200 IU) per day, 14 or an identical appearing and
tasting placebo (both prepared by Cadilla Pharmaceuticals, Gujarat, India).
The ethics committee did not permit use of a larger vitamin D dose. The data
safety and monitoring board individually labelled the sachets containing
vitamin D or placebo crystals with the participant identification
number" Judgement comment: appropriate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the study team remained blinded to treatment allocation until the
primary and growth outcomes had been analysed" Judgement comment: double‐blind implies that both participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the study team remained blinded to treatment allocation until the
primary and growth outcomes had been analysed" Judgement comment: double‐blind implies that outcome assessors, as well as statisticians, were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "the study’s main limitation was the large loss to follow‐up, but
controlling for factors associated with missing data did not alter the
results. In addition, although the death rate was similar to that used in
the calculations of sample size, inpatient admissions were much lower so
power was reduced and the confidence interval was wide and included the
original estimate of a 25% reduction in the primary outcome. We estimate
that, with the observed rate for the primary outcome in the placebo group
and the observed loss to follow‐up, we would have needed to recruit 1500
infants per group to detect the planned 25% reduction in mortality plus
admission to hospital" Judgement comment: large loss to follow‐up (Figure 1), with reasons documented unrelated to study interventions; missingness was examined. Intention‐to‐treat was performed |
| Selective reporting (reporting bias) | Low risk | Judgement comment: trial registered prospectively on ClinicalTrials.gov (ID: NCT00415402), as reported in text. Prespecified outcomes are consistent with those reported |
| Other bias | Low risk | Judgement comment: no other risks observed |
Willi 1959.
| Study characteristics | ||
| Methods |
Study design: quasi‐randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: Switzerland Study period: 1954 to 1959 |
|
| Participants |
Included criteria: preterm infants with birth weight 1500 g Excluded criteria: none specified Baseline vitamin D status: not reported |
|
| Interventions |
Intervention characteristics 4000 to 6000 IU D₂
1000 to 2000 IU D₂
500 to 1000 IU D₂
500 to 800 IU D₂
|
|
| Outcomes |
Secondary
Measurements
Time point: 4 weeks of age |
|
| Notes | This study was translated from German. Sample size and power were not calculated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "the experiments were mainly carried out alternately" Judgement comment: alternating randomisation methods indicate high risk of bias |
| Allocation concealment (selection bias) | High risk | Quote: "preparation (Oldevit Gewo) containing 20 000 IU per ml and a
powdered vitamin D 2 preparation containing, in 1 g, 750 IU of vitamin D and
600 mg of calcium phosphoricum bibasicum (decalcit Gewo)" Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. Not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, while investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: if blinding was done, this was not described. However, outcomes measured are not subjective in nature and are less likely to be influenced by knowledge of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: loss to follow‐up not described |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or reference protocol found |
| Other bias | Low risk | Judgement comment: no other risks observed |
Zeghoud 1994.
| Study characteristics | ||
| Methods |
Study design: quasi‐randomised controlled trial Study grouping: parallel group Funding: undisclosed Country: Algeria Study period: 1991 to 1992 |
|
| Participants |
Included criteria: born during September or October 1991, normal
pregnancy Excluded criteria: vitamin D supplementation during pregnancy Pretreatment: Group 1 was given 1 oral dose (at birth) of 5 mg cholecalciferol; Group 2 was given 3 trimestral oral doses (at birth, 3 months, and 6 months of age) of 2.5 mg cholecalciferol Baseline vitamin D status (mean, nmol/L)
|
|
| Interventions |
Intervention characteristics 200,000 IU D₃
100,000 IU D₃
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: enrolment; 0.5, 3, 6, 6.5, and 9 months of age |
|
| Notes | No sample size calculation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "30 neonates born during September and October were randomly
assigned to one of two groups: group" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "group 1 was given one oral dose of 5 mg cholecalciferol at birth
(Uvedose; Cninex laboratories, Montrouge, France); group 2 was given 2.5 mg
cholecalciferol at birth and 3 and 6 mo of age (Uvedose)" Judgement comment: blinding not described but not possible: Group 1 was given a single dose, and Group 2 was given 3 separate doses across 6 months. Not blinding participants may lead caregivers in control arm to compensate by administering additional vitamin D doses to children, while investigators who know the intervention allocations may be biased toward a particular outcome; both increase the risk for performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: blinding not described but unlikely: Group 2 (2.5 mg) was assessed at more time points than Group 1 (5 mg); therefore it is clear to both participants and personnel who was included in each group and what dose they were receiving, and that Group 2 was being assessed more often |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: from Table 2, only the 2.5 mg cholecalciferol group has data reported at all time points. Intent‐to‐treat analysis was not done. Loss to follow‐up was not described. Attrition is not reported |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no trial registration or protocol identified |
| Other bias | Low risk | Judgement comment: no other risks observed |
Ziegler 2014.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. National Institutes of Health, grant HD048870 Country: USA Study period: September 2006 to October 2010 |
|
| Participants |
Included criteria: born between June and November, term infants
(gestational age ≥ 37 weeks), either gender, birth weight > 2500 g;
considered normal by parents, physicians, and investigators; exclusively
breastfed at the time of enrolment Excluded criteria: none Baseline vitamin D status (mean ± standard deviation; nmol/L)
|
|
| Interventions |
Intervention characteristics 200 IU D₃
400 IU D₃
600 IU D₃
800 IU D₃
|
|
| Outcomes |
Primary
Secondary
Measurement
Time points: enrolment (1 month of age); 2, 4, 5.5, 7.5, 9, and 12 months of age |
|
| Notes | Sample size re‐calculation: n = 32/group. Dose of 800 IU/d was added after study was under way. This was met up until the 4‐month time point | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "under the modified design, the new dose (800 IU/day) received three
codes and the original doses each received two new codes. In this way, it
could be expected that at the conclusion of the trial approximately equal
numbers of infants would have received each of the four doses. New random
sequences of nine codes (G–O) were generated" Quote: "randomization and blinding: Under the initial design, two letter codes were used for each of the three doses. Random sequences of the six codes (A–F) were generated using SAS proc plan. Randomization was stratified for gender and birth weight (2,500–3,350 g vs. >3,350 g). At enrollment, infants were assigned to the next letter code on the list. Infants continued to receive the assigned supplement until 9 mo of age. All study personnel and parents were blinded to the identity of the supplements" Judgement comment: appropriate sequence generation method |
| Allocation concealment (selection bias) | Low risk | Quote: "the study vD supplements were prepared by UnitDrugCo in Centennial,
CO, who also kept the code until the intervention was completed. Supplements
were supplied in opaque bottles containing 50 ml each" Judgement comment: appropriate allocation concealment by a third party |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind... Under the modified design, the new dose (800
IU/day) received three codes and the original doses each received two new
codes. In this way, it could be expected that at the conclusion of the trial
approximately equal numbers of infants would have received each of the four
doses. New random sequences of nine codes (G–O) were generated. The identity
of all codes was kept by the manufacturer of the supplements and was broken
only after all study data had been gathered" Judgement comment: double‐blind implies that participants and study personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind... The identity of all codes was kept by the
manufacturer of the supplements and was broken only after all study data had
been gathered" Judgement comment: double‐blind implies that outcomes assessors were blinded, and code was kept by a third party |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "the data were analyzed on an intention‐to‐treat basis" Judgement comment: intention‐to‐treat analysis analysis; moderate loss to follow‐up throughout study period due to parental feeding choices; characteristics of infants who withdrew from the study did not differ from those of infants who completed the study to 9 or 12 months |
| Selective reporting (reporting bias) | Low risk | Quote: "the trial was registered with ClinicalTrials.gov under
NCT00494104" Quote: "at the time the study was initiated, the recommended dose of supplemental vD was 200 IU/day (11). In its original design, the study was to test 200, 400, and 600 IU/day. The addition of a dose of 800 IU/day was deemed necessary when a number of infants showed 25(OH)D levels <50 nmol/l in spite of receiving [vitamin D] supplements. The primary endpoint was plasma 25(OH)D concentration. Secondary outcomes were illness incidence and growth. Bone mineral content and measures of bone turnover were determined, but the findings are to be reported separately" Judgement comment: trial registered retrospectively on ClinicalTrials.gov (ID: NCT00494104), which we identified through separate searching; outcomes in registration are presented in results |
| Other bias | Low risk | Judgement comment: no other risks observed |
AOM: acute otitis media. CI: confidence interval. HPLC: high‐performance liquid chromatography. L/HAZ: length/height‐for‐age z‐score. NEC: necrotising enterocolitis. NICU: neonatal intensive care unit. PMA: postmenstrual age. RIA: radioimmunoassay.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abrams 2013 | No stratified data available for study population; age group 4 to 8 years (study author contacted via email but no response received) |
| Atkinson 2017 | No stratified data available for study population; age group 1 to 21 years (study author contacted via email but no response received) |
| Camargo 2014 | No stratified data available for study population; age group 2 to 17 years (study author contacted via email and responded: no time to re analyse data) |
| Dehbroki 2019 | No stratified data available for study population; age group 2 to 18 years (study author contacted via email but no response received) |
| Galli 2015 | No stratified data available for study population; age group 6 to 195 months (study author contacted via email but no response received) |
| Gottschlich 2017 | No stratified data available for study population; age group 0.7 to 18.4 years (study author contacted via email but no response received) |
| Hamidieh 2016 | No stratified data available for study population; age group 1 to 15 years (study author contacted via email but no response received) |
| Homola 2011 | No stratified data available for study population; age group not explicitly stated (study author contacted via email and responded: no time to share raw data) |
| Kakalia 2011 | Age group above 5 years (study author contacted via email and responded that stratified data were available for study age group of 3 to 18 years, but only 3 participants were under 5 years of age; they did not provide the data in response to follow‐up emails) |
| Kashif 2014 | No stratified data available for study population; age group 1 to 12 years (study author contacted via email and responded, but follow‐up attempts were unsuccessful) |
| Kazemi 2010 | No stratified data available for study population; age group over 3 years; upper age range not stated (study author contacted via email but no response received) |
| Kerley 2017 | No stratified data available for age group under 18 years (study author contacted via email and responded: data are not retrievable) |
| Lal 2018 | No stratified data available for study population; age group 1.5 to 18 years (study author contacted via email but no response received) |
| Lara‐Corrales 2019 | No stratified data available for study population; age group 0 to 18 years (study author contacted via email but no response received) |
| Lee 2018 | No stratified data for study population; age group 3 to 20 years (study author contacted via email but no response received) |
| Loeb 2019 | No stratified data for study population; data on age group 3 to 17 years were available via study authors (study authors contacted and responded: provided information via email) |
| Mazahery 2019 | No stratified data for study population; age group 2.5 to 8 years (study author contacted via email but no response received) |
| Merrikhi 2018 | No stratified data available for study population; age group 2 to 12 years (study author contacted via email but no response received) |
| Morcos 1998 | No stratified data available for study population; age group 1.5 to 13 years (study author contacted via email but no response received) |
| Mortensen 2016 | No stratified data available for study population; age group 4 to 8 years (study author contacted via email but no response received) |
| Muske 2018 | No stratified data available for study population; age group 1 to 18 years (study author contacted via email but no response received) |
| Nwosu 2014 | No stratified data available for study population; age group 3 to 18 years (study author contacted via email but no response received) |
| Rahmati 2018 | No stratified data available for study population; age group 3 to 14 years (study author contacted via email but no response received) |
| Saad 2018 | Retracted |
| Sharma 2017 | No stratified data available for study population; age group 1 to 18 years (study author contacted via email but no response received) |
| Shroff 2012 | No stratified data available for study population; age group under 18 years (study author contacted via email and responded: requested blank spreadsheet to provide stratified data, which was sent; no further response received) |
| Siafarikas 2009 | Meeting abstract. No stratified data available for study population; age group ≤ 16 years (study author contacted via email but no response received) |
| Sidbury 2008 | No stratified data available for study population; age group 1 to 18 years (study author contacted via email and responded: most study participants were older than 5 years; could not share stratified data) |
| Simon 2016 | No stratified data available for study population; age group 1 to 18 years (study author contacted via email but no response received) |
| Singh 2018b | No stratified data available for study population; age group 1 to 18 years (study author contacted but no response received) Note: study cites CTRI registration number as "CTRI/2015/10/009984" with the title, "Comparison of the efficacy of two dosing regimens of Vitamin D for bone protection in children with difficult nephrotic syndrome"; however, the correct CTRI number with this title is "CTRI/2016/10/007405," as referenced in this review |
| Suryanto 2018 | No stratified data available for study population; age group 1 to 18 years (study author contacted via email but no response received) |
| Swangtrakul 2020 | No children younger than 5 years included in study (study author contacted via email and responded with confirmation) |
| Talaat 2016 | No stratified data available for study population; age group 2 to 16 years (study author contacted via email but no response received) |
| Tannous 2018 | No stratified data available for study population; age group 1 to 18 years (study author contacted via email but no response received) |
| Udompataikul 2015 | No stratified data available for study population; age group 1 to 18 years (study author contacted via email but no response received) |
| Wadia 2018 | No stratified data available for study population; age group 5.5 months to 16 years (study author contacted via email but no response received) |
| Zulkarnain 2019 | No stratified data available for study population; age group 2 to 12 years (study author contacted via email but no response received) |
Characteristics of studies awaiting classification [ordered by study ID]
Bantz 2015.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: USA |
| Participants |
Included criteria: atopic children with inadequate vitamin D
levels Excluded criteria: not specified |
| Interventions |
Intervention characteristics 1000 IU, for children with serum 25‐hydroxyvitamin D (25(OH)D) concentration 20 to 30 ng/mL
2000 IU, for children with serum 25(OH)D concentration < 20 ng/mL
400 IU
*Meeting abstract indicates n = 47 total were randomised |
| Outcomes |
Secondary
Measurement
Time point: 3 months |
| Notes | Notes: age group not specified in meeting abstract; not enough information to assess eligibility. Study author contacted but no response received |
Behnamfar 2011.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: Iran |
| Participants |
Included criteria: young children in day care centres Excluded criteria: not specified |
| Interventions |
Intervention characteristics Treatment
Base of vitamin D/placebo
*Meeting abstract indicated a total of n = 50 children were randomised. |
| Outcomes |
Secondary
Measurement
Time point: 3 months |
| Notes | Notes: age group not specified in meeting abstract; not enough information to assess eligibility. Study author contacted via email but no response received |
CTRI/2014/04/004574.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: no funding Country: India |
| Participants |
Included criteria: age 1 to 12 years with iron deficiency
anaemia Excluded criteria: history of fever within last 4 weeks, acute or chronic medical disorder, haemolytic anaemia, haemoglobin 6 gm%, receiving iron/vitamin/mineral supplements (including herbal drugs), blood transfusion within 8 weeks, malignancy, congestive cardiac failure, congenital heart disease/rheumatic heart disease/cardiomyopathy, features of rickets |
| Interventions |
Intervention characteristics 400 IU D₃ + Iron
Iron only
|
| Outcomes | None within scope of this review |
| Notes | Notes: completed recruitment (26 December 2012). Study author contacted to inquire about additional outcomes but no response received |
CTRI/2015/08/006084.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: none Country: India |
| Participants |
Included criteria: age 1 to 13 years, apparently healthy Excluded criteria: vitamin D/mineral supplements (including herbal drugs) within 8 weeks from the day of screening for the study; chronic medical disorder (e.g. chronic diarrhoea, chronic liver disease, chronic renal disease); children taking anticonvulsant medications, glucocorticoids, antifungals such as ketoconazole, and antiretroviral drugs; blood transfusion within 3 months from the day of screening for the study; obese children; malignancy; features of rickets; history of recurrent fractures; refusal of parents/guardians to consent |
| Interventions |
Intervention characteristics 1000 IU D₃
600 IU D₃
*Trial registration indicates total enrolment of n = 45 participants |
| Outcomes |
Secondary
Measurement
Time point: 90 days |
| Notes | Notes: completed recruitment (3 August 2015). Study author contacted but no response received |
CTRI/2019/02/017374.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. All India Institute of Medical Sciences (AIIMS), Jodhpur, India‐342005 Country: India |
| Participants |
Included criteria: inborn healthy, term, singleton, appropriate for
gestational age (as defined by Fenton’s growth charts) infants Excluded criteria: birth weight < 2.5 kg, multiple pregnancy, families from far off places not willing for follow‐up, mother or infant on anticonvulsant or antitubercular treatment, major congenital malformations, severe birth asphyxia, need for neonatal intensive care unit stay > 48 hours |
| Interventions |
Intervention characteristics 800 IU D₃
400 IU D₃
|
| Outcomes |
Secondary
Measurement
Time point: 14 weeks' postnatal age |
| Notes | Notes: completed recruitment (31 December 2019). Study author contacted but no response received |
Hagag 2020.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: Egypt |
| Participants |
Included criteria: neonates with sepsis Excluded criteria: none noted |
| Interventions |
Intervention characteristics Vitamin D + antibiotics
Antibiotics only
|
| Outcomes |
Secondary
Measurement
Time points: not specified |
| Notes | Notes: study design unclear from abstract; full‐text study not obtainable. Study author contacted but no response received |
IRCT20111206008307N.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Tabriz University of Medical Science, Tabriz, Iran Country: Iran |
| Participants |
Included criteria: age 14 to 28 days, very low birth weight (<
1500 g), born in Alzahra Teaching Hospital, consent provided by parents,
tolerating 120 cc/kg/d breast milk or breast milk with formula (full
feed) Excluded criteria: infants with major malformation, congenital cardiac disease, familial history of bone disease, receiving corticosteroids, born from mothers with renal failure |
| Interventions |
Intervention characteristics Calcitriol
400 IU D₃
|
| Outcomes |
Secondary
Measurement
Time points: 3 weeks, 6 weeks |
| Notes | Notes: completed recruitment (16 July 2018). Study author contacted but no response received. Duplicate registration found under the registration number IRCT20111206008307N28 in the World Health Organization International Clinical Trials Registry |
IRCT20131013014994N5.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Kermanshah University of Medical Sciences, Kermanshah, Iran Country: Iran |
| Participants |
Included criteria: desire for parental collaboration, age range 3 to
13 years, diagnosis of autism disorder based on DSM‐5 criteria Excluded criteria: reluctance to continue co‐operation; children with significant hearing loss and vision loss; other neurological disorders such as cerebral palsy, phenylketonuria, seizure disorders; history of head trauma; genetic abnormalities; premature children; children with nutritional and malnutrition problems; children with digestive problems; immune disorders; children with endocrine, cardiovascular, pulmonary, kidney, or liver disease; children 2 months before study given supplements or the following medications: vitamin A, vitamin D, fish liver oil, steroids, cimetidine, heparin, diuretics, digoxin, diltiazem, and verapamil; children with serum vitamin D level > 80 ng/mL |
| Interventions |
Intervention characteristics 6000 IU
Placebo
|
| Outcomes |
Secondary
Measurement
Time point: 3 months |
| Notes | Notes: completed recruitment (18 April 2018). Study author contacted but no response received |
IRCT2014053117843N3.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Ardabil University of Medical Sciences, Ardabil, Iran Country: Iran |
| Participants |
Included criteria: age 4 to 18 years; has asthma; Bouali Hospital
admission Excluded criteria: pneumonia; rickets; use of higher‐dose vitamin D in last 3 months; severe malnutrition; diseases such as cystic fibrosis |
| Interventions |
Intervention characteristics 100,000 IU D₃
Placebo
|
| Outcomes | None within scope of this review |
| Notes | Notes: completed recruitment (04 August 2014). Study author contact information was inaccurate or out of date for successful communication |
NCT01229189.
| Methods |
Study design: interventional randomised clinical trial Study grouping: parallel group Funding: not reported Country: Pakistan |
| Participants |
Included criteria: pregnant women from 20 to 22 weeks' gestation and
their infants Excluded criteria: pregnant women with preexisting type 1 or 2 diabetes, women with multiple foetuses/babies |
| Interventions |
Intervention characteristics 400 IU
Placebo
|
| Outcomes |
Primary
Secondary
Measurement
Time point: 6 months |
| Notes | Notes: completed (August 2011). Study author contacted but no response received |
NCT01419821.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: Israel |
| Participants |
Included criteria: infants 9 to 12 months old in Tipat Chalav and
Kupat Holim Clalit in Beitar Illit undergoing a blood draw for CBC at 1 year
of age Excluded criteria: parents who refuse to participate in this study, infants with any diagnosed chronic disease, preterm infants at less than 34 weeks |
| Interventions |
Intervention characteristics 800 IU (serum vitamin D < 15 ng/mL)
Placebo (serum vitamin D < 15 ng/mL)
No intervention (serum vitamin D > 15 ng/mL)
*Trial registration lists both 1 year and 2 years |
| Outcomes |
Primary
Measurement
Time point: 3 years of age |
| Notes | Notes: unknown recruitment status (estimated study completion September 2016). Study authors contacted but no response received |
NCT01656070.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: Italy |
| Participants |
Included criteria: vertically acquired HIV infection, age < 30
years, serum 25(OH)D concentration < 30 ng/mL, signed written informed
consent Excluded criteria: hyperparathyroidism, as detected by an intact serum parathyroid hormone (PTH) ≥ 65 pg/mL; black ethnic group; any supplementation with vitamin D in previous 12 months; use of any treatment known to alter vitamin D status in previous 6 months (excluding antiretroviral treatment); any concomitant severe illness |
| Interventions |
Intervention characteristics 100,000 IU D₃
Placebo
|
| Outcomes |
Secondary
Measurement
Time point: 12 months |
| Notes | Notes: completed (July 2012). Study author contacted but no response received |
NCT01724190.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: USA |
| Participants |
Included criteria: age 4 to 18 years with newly diagnosed type 1
diabetes Excluded criteria: under 4 years of age, pregnant female, previous or known history of vitamin D deficiency or insufficiency, current daily use of vitamin D supplementation or multi‐vitamin containing > 800 IU, concurrent development or history (or both) of other significant systemic illness or non‐endocrine autoimmune disorder |
| Interventions |
Intervention characteristics 3000 IU D₃
Placebo
|
| Outcomes |
Secondary
Measurement
Time point: 9 months |
| Notes | Notes: completed (June 2014). Study author contacted but no response received |
NCT02054182.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: South Africa |
| Participants | Included criteria: 1 month to 5 years of age, admitted to paediatric unit of Dr George Mukhari Hospital with acute lower respiratory tract infection (i.e. bronchiolitis or pneumonia (or both)) Excluded criteria: children whose caregivers decline participation in the study, those with comorbid chronic respiratory condition(s), those who have received vitamin D supplementation in the past 30 days |
| Interventions |
Intervention characteristics 500 IU D₃
Placebo
|
| Outcomes | None within scope of this review |
| Notes | Notes: unknown recruitment status (estimated study completion January 2015). Study authors contacted but no response received |
NCT02185196.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: Bangladesh |
| Participants |
Included criteria: 3 to 59 months of age, clinical diagnosis of
severe pneumonia with or without diarrhoea Excluded criteria: known case of hypercalcaemia or allergy to vitamin D, as determined by history or previous medical records; congenital heart disease, as evidenced by clinical exam or past medical records; renal or hepatic insufficiency, as evidenced by clinical exams or past medical records; known case of tuberculosis, as evidenced by medical records; known case of asthma, as evidenced by history and clinical exam findings; critically ill children requiring ICU care, such as those with septic shock or cardiac arrest or apnoea; those who have received vitamin D or calcium supplementation within the last 4 weeks before current admission, as evidenced by history or medical prescription; any children diagnosed with hypernatraemia during the main phase of the study |
| Interventions |
Intervention characteristics Vitamin D
Placebo
*Trial registration indicates a total of 197 participants **Trial registration indicates same dosages of IU in placebo arm but this is likely reported in error |
| Outcomes | None within scope of this review |
| Notes | Notes: completed (31 December 2017). Study authors contacted but no response received |
NCT02186028.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: India |
| Participants |
Included criteria: age up to 48 hours, gestation > 37 weeks,
birth weight > 2.5 kg, informed consent of 1 of the parents, place of
residence < 10 km Excluded criteria: presence of gross congenital malformation, need for resuscitation at birth, need for admission to neonatal intensive care unit, refusal of consent |
| Interventions |
Intervention characteristics 400 IU
200 IU
|
| Outcomes |
Secondary
Measurement
Time point: 6 months |
| Notes | Notes: completed (September 2016). Study authors contacted but no response received |
NCT02936895.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: Iran |
| Participants |
Included criteria: 2 months to 6 years of age, definitive diagnosis
of pneumonia Excluded criteria: immunocompromised patients, airway hypersensitivity or asthma, allergies, nasal polyps, use of inhaled medications to 1 month before the study, receiving high doses of vitamin D, avoiding signing of informed consent form |
| Interventions |
Intervention characteristics 50,000 IU D₃
Placebo
|
| Outcomes | None within scope of this review |
| Notes | Notes: completed (July 2015). Study authors contacted but no response received |
NCT03176849.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: USA |
| Participants |
Included criteria: preterm infants born at between 24 and 32 weeks
of gestation (estimated by ultrasound), in‐born or admitted to the unit
within 48 hours from birth, randomisation within 7 days from birth, mothers
willing to return for follow‐up visits Excluded criteria: preterm delivery (at least 33 weeks of gestation or term delivery, estimated by ultrasound), major congenital abnormality, participation in another trial, severe illness at birth deemed incompatible with survival, congenital human immunodeficiency virus infection, total parenteral nutrition > 14 days, cholestasis |
| Interventions |
Intervention characteristics Higher‐dose
Standard
|
| Outcomes |
Primary
Secondary
Measurement
Time point: 100 days |
| Notes | Notes: enrolling by invitation (estimated completion: 1 September 2019). Study authors contacted but no response received |
NCT03544671.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: Mexico |
| Participants |
Included criteria: 12 to 30 months of age; attend day care centres;
children whose parents accepted their child to participate and signed
informed consent Excluded criteria: children receiving multiple micronutrient supplementation or other vitamin D supplement; children whose parents did not accept to participate; children with capillary haemoglobin concentration < 90 g/L at baseline |
| Interventions |
Intervention characteristics 1000 IU D₃
800 IU D₃
400 IU D₂
Placebo
*Actual total sample size enrolled: n = 220 |
| Outcomes |
Secondary
Measurement
Time point: 16 weeks |
| Notes | Notes: completed (27 December 2017). Study authors contacted but no response received |
NTR477.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: The Netherlands |
| Participants |
Included criteria: age 4 to 18 with acute lymphoblastic leukaemia,
without physical handicap Excluded criteria: age < 3 years |
| Interventions |
Intervention characteristics Trial registration is unclear as to what are the intervention and comparator groups, which appear to contain physical activities, vitamin D, and calcium |
| Outcomes | None within the scope of this review |
| Notes | Notes: no longer recruiting; completed 27 January 2006. Study authors contacted but no response received |
Özkan 2000.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: Turkey |
| Participants |
Included criteria: age 4 to 19 months with nutritional rickets Excluded criteria: not specific |
| Interventions |
Intervention characteristics 300,000 IU D₃
600,000 IU D₃
300,000 IU D₃ (intramuscular)
|
| Outcomes |
Primary
Secondary
Measurement
Time point: 25 to 30 days |
| Notes | Notes: an additional third intervention group was randomly assigned to receive 300,000 IU intramuscular vitamin D, once at enrolment. Study design unclear as control group was included with age‐ and sex‐matched children who may not have been randomised. Study authors contacted but no response received |
ICU: intensive care unit. PTH: parathyroid hormone.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12614000334606/NCT02112734.
| Study name |
Public trial: Can vitamin D supplementation in infants prevent food
allergy in the first year of life? The VITALITY trial Scientific title: A placebo‐controlled, randomised trial of vitamin D supplementation for infants in their first year of life, to prevent the development of food allergy by age 12 months. The VITALITY trial |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Isabel & John Gilbertson Charitable Trust and Murdoch Children’s Research Institute. KJA, MP, JJK, SCD, A‐LP, LCG, MW all receive fellowship funding from National Health and Medical Research Council (NHMRC) of Australia. NC receives funding from University of Melbourne, McKenzie Postdoctoral Fellowship Country: Australia |
| Participants |
Included criteria: healthy, term, 6– to 8‐week‐old breastfed infants
whose mothers intend to continue to predominantly breastfeed until 6
months Excluded criteria: already receiving vitamin D supplementation, born premature (< 37 weeks) or at low birth weight (< 2500 g), multiple births, poor health due to current or past significant disease state or congenital abnormality or taking medication that interferes with vitamin D metabolism |
| Interventions |
Intervention characteristics 400 IU D₃
Placebo
|
| Outcomes |
Secondary
Measurement
Time point: endpoint (12 months of age) |
| Starting date | December 2014 |
| Contact information | Michael Field, Murdoch Children's Research Institute; email:
vitality@mcri.edu.au Jennifer Koplin, Murdoch Children's Research Institute; email: jennifer.koplin@mcri.edu.au |
| Notes | Notes: recruiting (estimated study completion: December 2022) |
ACTRN12616000659404.
| Study name |
Public title: PREVARID ‐ PREVention of Acute Respiratory Infections
with Vitamin D Scientific title: Does vitamin D supplementation prevent acute respiratory infection health care visits among children under 2 years old? A randomised controlled trial |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Cure Kids, Auckland, New Zealand Country: New Zealand |
| Participants |
Included criteria: New Zealand residents, < 2 years old at the
time of acute lower respiratory infection hospital admission, reside in
Auckland District Health Board catchment area Excluded criteria: receiving vitamin D supplements, have a complex chronic condition known to be associated with recurrent hospital admission (e.g. cystic fibrosis, tracheostomy) |
| Interventions |
Intervention characteristics 5000 IU D₃
Placebo
|
| Outcomes |
Secondary
Measurement
Time points: enrolment, 6 months and 12 months after enrolment |
| Starting date | July 2016 |
| Contact information | Cameron Grant, University of Auckland, Principal Investigator; email: cc.grant@auckland.ac.nz |
| Notes | Notes: not yet recruiting (estimated recruitment completion 30 November 2017) |
CTRI/2013/04/003566.
| Study name |
Public title: Vitamin D supplementation and responses to vaccines in
infants Scientific title: Vitamin D supplementation to improve immune responses to vaccines administered in early infancy ‐ the Nutrivac‐D trial |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Government funding agency Country: India |
| Participants |
Included criteria: Inclusion criteria during pregnancy and labour are: pregnant at any gestation when screened antenatally; Pregnancy full term (> 37 and < 41 completed weeks) when screened again at labour (consent will only be taken in the antenatal period and not during labour); will stay in study area for a period of at least 6 months after delivery; delivery by vaginal route or by elective Cesarean section. Inclusion criteria at birth and within 24 hours: single term newborn (gestational age > 37 and < 41 weeks at birth) < 24 hours old; born by normal vaginal route or elective caesarean section; will stay in study area for a period of at least 6 months after delivery; breastfeeding established Excluded criteria: Exclusion criteria during pregnancy and intrapartum period: a mother with a history of > 5 pregnancies; multiple gestation; presence of any documented major maternal medical or surgical illness e.g. HIV, Hepatitis B, Tuberculosis, TORCH infections, syphilis, malignancy or immunodeficiency, etc; presence of fetal (major) congenital anomalies diagnosed in utero; any infection during pregnancy that required hospitalisation; blood transfusion during pregnancy; history of maternal eclampsia / preeclampsia / hypertension with significant proteinuria (> 3+) during pregnancy. Exclusion criteria at birth and within 24 hrs: one minute Apgar of < 7/10; birth weight < 1.8 kg; multiple gestation; major congenital anomalies diagnosed prior to birth or during a clinical examination by a paediatrician performed within the first 24 hours; newborn required admission to neonatal intensive care prior to randomisation; informed written consent not provided by parents |
| Interventions |
Intervention characteristics 400 IU
Placebo
|
| Outcomes |
Primary
Measurements
Time point: 6 months of age |
| Starting date | December 2012 |
| Contact information | Uma Chandra Mouli Natchu, Translational Health Science and Technology Institute, Principal Investigator; email: unatchu@thsti.res.in |
| Notes | Notes: open to recruitment (first recruitment 21 December 2012) |
CTRI/2015/08/006132.
| Study name |
Public title: A clinical trial to evaluate the need for routine
vitamin D supplementation till six months age in full term babies who are
being exclusively breastfed Scientific title: Vitamin D oral supplementation evaluation in full‐term, exclusively breastfed infants ‐ a randomised controlled study |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: India |
| Participants |
Included criteria: born at 37 weeks' completed gestation or
thereafter, birth weight ≥ 2500 g, exclusively breastfed, parents provided
written consent for infant to participate in the study after detailed
information was disseminated to them Excluded criteria: infants born before 37 completed weeks of gestation; low birth weight (i.e. birth weight < 2500 g); sick neonate, including birth asphyxia; neonate not exclusively breastfed from birth for any reason even though may afterward be on exclusive breastfeeds; neonate with major congenital anomaly; neonate whose parents decline consent to participate in the study (presence of any 1 criterion will result in exclusion) |
| Interventions |
Intervention characteristics 400 IU
No intervention
|
| Outcomes |
Primary
Secondary
Measurements
Time points: birth (cord blood), 1 month of age, 6 months of age |
| Starting date | July 2015 |
| Contact information | Shankar Narayan, Indian Naval Hospital Ship (INHS) Asvini, Principal Investigator; email: drshankarnarayan@gmail.com |
| Notes | Notes: open to recruitment (first enrolment 1 July 2015) |
CTRI/2016/12/007519.
| Study name |
Public title: Vitamin D levels in preterm babies Scientific title: Vitamin D levels of the term small‐for‐date newborns at birth and at 3 month of age after vitamin D supplementation with 2 different doses 400 IU v/s 800 IU ‐ a randomised controlled trial |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Sri Devaraj Urs Academy of Higher Education and Research Country: India |
| Participants |
Included criteria: healthy, small‐for‐gestational‐age infants born
with normal delivery, gestational period ≥ 37 weeks, birth weight < 2500
g, exclusively breastfed for 3 months Excluded criteria: preterm babies with gestational age < 36 weeks; birth weight < 2500 g; liver, renal, intestinal, and other problems that can affect vitamin D metabolism; on medications such as anticonvulsants, glucocorticoids, antifungal medications; mother with systematic disease that can alter vitamin D metabolism (renal, hepatic); malignancy; features of rickets; repeated fractures |
| Interventions |
Intervention characteristics 1400 IU
2800 IU
Total target sample size: n = 35 |
| Outcomes |
Secondary
Measurement
Time points: birth, 3 months of age |
| Starting date | February 2016 |
| Contact information | Syed Manazir Ali, Jawaharlal Nehru Medical College, Aligarh Muslim University, Principal Investigator; email: manazir1958@yahoo.com |
| Notes | Notes: open to recruitment (first enrolment 2 February 2016) |
CTRI/2017/10/010274.
| Study name |
Public title: Role of vitamin D3 intake and decrease in respiratory
infections Scientific title: Randomised trial of two different doses of vitamin D supplementation and risk of acute respiratory infection in children in rural Kolar, Karnataka |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Jawaharlal Nehru Medical College Aligarh Muslim University Aligarh Country: India |
| Participants |
Included criteria: healthy, age 3 to 6 years, attends RL
Jallappaschool in Kolar Excluded criteria: chronic illness (except asthma), clinical rickets, child on vitamin supplementation |
| Interventions |
Intervention characteristics 3000 IU
600 IU
|
| Outcomes |
Secondary
Measurement
Time points: 3 and 12 months |
| Starting date | January 2018 |
| Contact information | Kanak N Venkateshwara Prasad, Sri Devraj Urs Medical College, Principal Investigator; email: drknvp@gmail.com |
| Notes | Notes: not yet recruiting (record last modified 28 October 2017) |
CTRI/2017/11/010385.
| Study name |
Public title: Dose of vitamin D in children with chronic kidney
disease Scientific title: Optimal dose of cholecalciferol supplementation in Indian children with chronic kidney disease ‐ a randomised controlled trial |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Navajbai Ratan Tata Trust, Bombay House, 24 Homi Mody Street, Mumbai ‐ 400 001, Maharashtra, India (reference no: Health‐CKCC‐20141118). This being an “Investigator initiated trial” will receive only partial support from the Trust towards supporting expenses of clinical tests and vitamin D supplements Country: India |
| Participants |
Included criteria: 1 to 18 years of age, chronic kidney disease
stages 2 to 4 (estimated glomerular filtration rate 15 to 90 mL/min/1.73
m²), serum 25‐hydroxyvitamin D level < 30 ng/mL Excluded criteria: therapy with cholecalciferol, including over‐the‐counter multi‐vitamin or intramuscular serum 25‐hydroxyvitamin D, in preceding 3 months; known nephrocalcinosis; refusal to give consent; known poor adherence to medications; inability to attend a follow‐up visit |
| Interventions |
Intervention characteristics 3000 IU D₃
25,000 IU D₃
100,000 IU D₃
*After 3 months, children with serum 25‐hydroxyvitamin D ≥ 30 ng/mL will receive maintenance 1000 IU D₃ orally daily for 9 months. Children with serum 25‐hydroxyvitamin D < 30 ng/mL will be given a second course of intensive treatment, using same dosage schedule as per allocation at randomisation. Those who fail to achieve serum 25‐hydroxyvitamin D ≥ 30 ng/mL will receive a third course of intensive replacement therapy |
| Outcomes |
Primary
Secondary
Measurement
Time points: end of an intensive phase |
| Starting date | January 2016 |
| Contact information | Arpana Aprameya Iyengar, Government Medical College; email: drarpanaiyengar@gmail.com |
| Notes |
Notes: trial completed (ended: 20 November 2019), trial protocol
publication and meeting abstract with preliminary data available. Study
author contacted and indicated manuscript is forthcoming Note: trial protocol lists Clinical Trials Registry of India registration number as "CTRI/2015/11/010180"; however; this was not found in the CTRI database. The CTRI registration referenced in this review, "CTRI/2017/11/010385", appears to be the correct number |
CTRI/2017/12/010827.
| Study name |
Public title: Effect of vitamin D supplementation on postoperative
surgical outcomes in children with cyanotic congenital heart disease
undergoing open heart surgery: a randomised controlled trial Scientific title: Effect of vitamin D supplementation on outcome in children undergoing open heart surgery: a randomised controlled trial |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Institutional research fund, All India Institute of Medical Science (AIMS), New Delhi, India Country: India |
| Participants |
Included criteria: 1 month to 12 years of age, transposition of
great arteries, total anomalous pulmonary venous connection, tetralogy of
Fallot, tricuspid atresia, univentricular physiology, shunts with reversal
of flow, left‐to‐right shunt like atrial septal defect, ventricular septal
defect, undergoing open heart surgery electively under cardiopulmonary
bypass Excluded criteria: urgent/emergency surgery, syndromic child, closed heart surgery, preoperative infection/antibiotic administration and ventilation |
| Interventions |
Intervention characteristics 400,000 IU
Placebo
|
| Outcomes |
Primary
Measurement
Time points: postoperative course in intensive care unit |
| Starting date | January 2018 |
| Contact information | Manoj Kumar Sahu, AIMS, Principal Investigator; email: drmanojsahu@gmail.com |
| Notes | Notes: not yet recruiting (record last modified 26 November 2019) |
CTRI/2018/04/013300.
| Study name |
Public title: A clinical trial to compare three different regimes
for treatment of nutritional rickets in children Scientific title: To evaluate the efficacy of daily vitamin D therapy versus Stoss therapy in nutritional rickets in Indian children: a randomised controlled trial |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Maulana Azad Medical College and Lok Nayak Hospital Country: India |
| Participants |
Included criteria: age 6 months to 12 years; diagnosis of
nutritional rickets defined by clinical, biochemical, and radiological
parameters; residence within 50 km of hospital; willing for follow‐up
visits Excluded criteria: patients with confirmed or suspected diagnosis of malabsorption or chronic kidney or hepatic disease, severe systemic illness compromising oral intake (tachycardia, tachypnoea, shock, weak peripheral pulses, increased capillary refill time); have taken calcium supplements or vitamin D preparation in last 6 months |
| Interventions |
Intervention characteristics 60,000 IU
2000 IU
|
| Outcomes |
Secondary
Measurement
Time point: 12 weeks |
| Starting date | April 2018 |
| Contact information | Aashima Dabas, Maulana Azad Medical College, Principal Investigator; email: dr.aashimagupta@gmail.com |
| Notes | Notes: not yet recruiting (record last modified 13 December 2018) |
CTRI/2018/12/016760.
| Study name |
Public title: Daily versus bolus oral vitamin D3 for treatment of
rickets In children Scientific title: Daily versus depot oral vitamin D3 for treating nutritional rickets |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. University College of Medical Sciences and GTB Hospital, Dilshad Garden, New Delhi, India 110095 Country: India |
| Participants |
Included criteria: 3‐month‐old to 5‐year‐old children with
nutritional rickets presenting in outpatient department, wards, emergency of
paediatrics department based on history, examination, and biochemical (serum
calcium ‐ normal/low, serum phosphorus ‐ normal/low and serum alkaline
phosphatase ‐ high) and radiological features (Thacher score ≥ 1.5) Excluded criteria: previous treatment of rickets, child admitted to paediatric intensive care unit, secondary cause of rickets such as medication, vitamin D disorder of metabolism, fat malabsorption syndrome |
| Interventions |
Intervention characteristics 60,000 IU to 150,000 IU
2000 IU to 4000 IU
|
| Outcomes |
Primary
Secondary
Measurement
Time points: 4 weeks, 12 weeks |
| Starting date | January 2019 |
| Contact information | Ravneet T Kaur Saluja, University College of Medical Sciences and Guru Teg Bahadur Hospital, Principal Investigator |
| Notes | Notes: closed to recruitment (last enrolment November 2019) |
Galdo 2018.
| Study name |
Public title: Effect of supplementation with vitamin D on acute
bronchitis prevention during the first year of life Scientific title: Effect of supplementation with vitamin D on acute bronchitis prevention during the first year of life |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Grant from Spanish Ministry of Health (EC11‐476) Country: Spain |
| Participants |
Included criteria: healthy, on‐term newborns of appropriate size for
gestational age during first 2 weeks of age (range 5 days), on exclusive
breastfeeding or exclusive formula feeding Excluded criteria: infant with gestational age < 37 weeks; low birth weight for gestational age (birth weight < 2500 g); mixed feeding at baseline; newborn with major congenital anomaly; infant with chronic gastrointestinal, hepatic, renal, respiratory, cardiac, neurological, or metabolic disorder; any disease that is accompanied by hypercalcaemia and hypercalciuria, calcium lithiasis, hypersensitivity to vitamin D, hypervitaminosis D, renal osteodystrophy with hyperphosphataemia |
| Interventions |
Intervention characteristics 2000 IU D₃
Placebo
|
| Outcomes |
Primary
Secondary
Measurement
Time point: 12 months |
| Starting date | February 2013 |
| Contact information | Antonio Moreno Galdó, University Hospital Vall d'Hebron, Principal Investigator; email: amoreno@vhebron.net |
| Notes | Notes: completed recruitment (ended 20 August 2018). Meeting abstract (preliminary results) available; study author contacted and indicated publication is forthcoming |
IRCT20171030037093N4.
| Study name |
Public title: Effect of vitamin D supplement on the level of serum
vitamin D in preterm neonates Scientific title: Comparing the effect of different doses of vitamin D supplement on the level of serum 25(OH) vitamin D and bone metabolism related factors in preterm neonates |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Shahrekord University of Medical Sciences, Shahrekord, Iran Country: Iran |
| Participants |
Included criteria: gestational age between 28 and 34 weeks; absence
of major disorders and malformations; absence of systemic disease, such as
asphyxia or cholestasis Excluded criteria: supportive nutrition longer than 2 weeks, use of anticonvulsant and anti‐HIV drugs by infant's mother, use of injectable vitamin D during the study, formula feeding, nephrocalcinosis in the infant |
| Interventions |
Intervention characteristics 300 IU
300 IU
|
| Outcomes |
Secondary
Measurements
Time point: modified age 40 weeks after last menstrual period |
| Starting date | July 2018 |
| Contact information | Roya Choopani, Shahrekord University of Medical Sciences, Principal Investigator; email: choopani.r@skums.ac.ir |
| Notes | Notes: recruitment completed (ended 25 July 2019); study author contacted to clarify interventions but no response received |
Kishore 2019.
| Study name | Study of daily vitamin D supplementation in preterm infants: a randomised trial |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: India |
| Participants |
Included criteria: preterm neonates Excluded criteria: not reported |
| Interventions |
Intervention characteristics 400 IU
800 IU
|
| Outcomes |
Secondary
Measurement
Time point: 40 weeks' postmenstrual age |
| Starting date | Not reported |
| Contact information | Sai Sunil Kishore, Maharajah Institute of Medical Sciences; email: mssk81@gmail.com |
| Notes | Note: preliminary results included in meeting abstract; study author contacted but no response received |
NCT01050387.
| Study name |
Public title: A randomised trial of vitamin D supplementation in
healthy inner‐city children Official title: A randomised, controlled trial of vitamin D supplementation in infants and children: effects of vitamin D dose and genotype of the binding protein |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), Thrasher Research Fund Country: USA |
| Participants |
Included criteria: 6 months to 6 years of age, healthy or free from
any disease or condition that may affect nutritional status or bone
metabolism, willingness of family to participate in a 6‐month study of
vitamin D supplementation. *In preliminary data (meeting abstract), children
2 to 10 years of age of predominantly Hispanic background were included Excluded criteria: chronic disease, prematurity < 32 weeks' gestational age, liver disease such as hepatitis or renal/urologic disease (e.g. recurrent urinary tract infection), use of pharmacological or prescription‐level dosage of vitamin D or its metabolites. Also excluded are users of any systemic glucocorticoid preparation and users of inhaled steroids that are considered greater than medium dose for age 4 years. Specifically, this would exclude users of more than 1 mg/d of budesonide, and more than 352 mcg/d of fluticasone. Current or recent (within 1 month) use of anticonvulsants or other medications known to affect bone and mineral homeostasis or alkaline phosphatase levels also excluded |
| Interventions |
Intervention characteristics 1000 IU
400 IU
*Trial registration indicated total enrolment of 193 participants Intervention characteristics (preliminary data from meeting abstract) 7000 IU D₃
2800 IU D₃
|
| Outcomes |
Secondary
Measurement
Time point: 6 months |
| Starting date | January 2010 |
| Contact information | Thomas Carpenter, Yale University, Principal Investigator; email: thomas.carpenter@yale.edu |
| Notes | Notes: recruitment completed (ended February 2013); study authors contacted, who indicated that data are currently being analysed (meeting abstract is available) and reflect trial registration NCT01050387; publication is forthcoming |
NCT01363167.
| Study name |
Public title: Identifying vitamin D deficiency in very low birth
weight (VLBW) infants part 2 Official title: Identifying vit D deficiency in VLBW infants part 2 |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Country: USA |
| Participants |
Included criteria: any infant born at Medical University of South
Carolina at < 34 weeks' gestation, < 1500 g at birth, adequate
gestational age, must be African American or Caucasian. Each infant born of
twin or triplet pregnancy also eligible Excluded criteria: major congenital anomaly or haemolytic disease requiring exchange transfusion, infant born small‐for‐gestational‐age or large‐for‐gestational‐age, maternal uncontrolled thyroid disease, maternal parathyroid disease, other race (non‐African American or Caucasian) |
| Interventions |
Intervention characteristics 400 IU D₃
Placebo
|
| Outcomes |
Primary
Secondary
Measurement
Time point: 2 to 4 months |
| Starting date | October 2011 |
| Contact information | Sarah N Taylor, MD, Medical University of South Carolina, Principal Investigator; email: taylorse@musc.edu |
| Notes | Notes: recruitment completed (ended October 2013) |
NCT01698840.
| Study name |
Public title: Effect of vitamin D in diets of preterm infants Official title: An evaluation of the effects of two levels of vitamin D in infants fed preterm or transitional formula on serum 25‐hydroxyvitamin D and bone status in preterm infants: a double‐blind, randomised controlled trial |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: USA |
| Participants |
Included criteria: born at 28 0/7 to 34 6/7 weeks' postmenstrual age
(PMA) and 1000 to 2250 g birth weight. Currently, 34 0/7 to 38 6/7 weeks'
PMA at time of consent. Born at Texas Children's (including Pavilion for
Women) or Methodist campus hospital or transferred within 48 hours of birth.
Care expected to be provided at one of these institutions until discharge to
home. Any initial feeding will be permitted but expected to transition to
primarily (80% of feeds or up to 2 breast milk feeds per day) infant formula
by 38 6/7 weeks' PMA or hospital discharge, whichever comes first. Able to
tolerate 22 kcal/oz transitional formula and to receive a volume of ≥ 130
mL/kg/d total feeding volume. No longer receiving any form of mechanical
ventilation or diuretics. Low‐flow nasal cannula will be permitted if it is
anticipated, and this will be discontinued before hospital discharge Excluded criteria: bronchopulmonary dysplasia requiring daily use of diuretics beyond 38 6/7 weeks; PMA (or hospital discharge, whichever comes first) and > 22 kcal/oz concentration formula beyond 38 6/7 weeks' PMA; major congenital anomaly; history of proven stage 2 or above necrotising enterocolitis or severe feeding intolerance; caloric density > 22 kcal/oz; higher‐order multiples ‐ however, twins are acceptable and will be randomised together but only data from 1 twin picked at random will be used in the final analyses |
| Interventions |
Intervention characteristics Vitamin D
Placebo
*Trial registration reports total sample size of n = 39 participants |
| Outcomes |
Secondary
Measurement
Time points: last 7 days of hospitalisation, 52 weeks' postmenstrual age |
| Starting date | January 2013 |
| Contact information | Amy Hair, Baylor College of Medicine, Principal Investigator |
| Notes | Notes: active, not recruiting (estimated trial completion December 2021) |
NCT01838447.
| Study name |
Public title: Prevention of vitamin D deficiency following pediatric
chronic heart disease surgery: a phase II dose evaluation randomised
controlled trial comparing usual care with a high dose preoperative
supplementation regimen based on the Institute of Medicine Daily Upper
Tolerable Intake Level (HICCUPS 2) Official title: Prevention of post‐cardiac surgery vitamin D deficiency in children with congenital heart disease: a pilot dose evaluation randomised controlled trial |
| Methods |
Study design: randomised double‐blind controlled trial Study grouping: parallel group Funding: not reported Country: Canada |
| Participants |
Included criteria: newborn (corrected gestational age between 36
weeks and 18 years) with chronic heart disease that will require surgery
within the next 12 months, chronic heart failure requiring surgical
intervention with cardiopulmonary bypass Excluded criteria: born at less than 32 weeks' gestational age, corrected gestational age < 36 weeks, cardiac or gastrointestinal disease preventing enteral feeds or drug administration before surgery, confirmed or suspected Williams syndrome, proposed surgery to take place at another centre (outside of Children's Hospital of Eastern Ontario) |
| Interventions |
Intervention characteristics 400 IU (0 to 1 year); 600 IU (1 to 17 years); placebo (formula‐fed, 0 to 1 year)
1200 to 1600 IU (0 to 1 year); 2400 IU (1 to 17 years); 1200 IU (formula‐fed, 0 to 1 year)
|
| Outcomes |
Primary
Secondary
Measurement
Time points: days 1, 3, 5, and 10 |
| Starting date | July 2013 |
| Contact information | James Dayre McNally, Children's Hosptial of Eastern Ontario, Principal Investigator; email: dmcnally@cheo.on.ca |
| Notes | Notes: recruitment completed (ended December 2015); study author contacted via email, who shared unpublished meeting abstract |
NCT01996423.
| Study name |
Public title: Impact of vitamin D supplementation on severity of
pediatric atopic dermatitis (VIDATOPIC) Official title: Impact of vitamin D supplementation on clinical severity and immunologic tolerance of pediatric atopic dermatitis |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. National Fund for Scientific and Technological Development (FONDECYT) Country: Chile |
| Participants |
Included criteria: atopic dermatitis diagnosed according to Hanifin
and Rajka criteria, age 2 to 17 years, Scoring of Atopic Dermatitis (SCORAD)
score of 10 to 103 Excluded criteria: active skin infection; history of underlying illness causing immunosuppression within past 2 years; immunosuppressor taken within past month; parathyroid disease; sarcoidosis; acute or chronic renal disease; hypercalcaemia or hypocalcaemia; thyroid disease; osteomalacia or Paget's disease of bone malabsorption; use of vitamin D supplements (> 400 IU daily) or fish oil supplements in past month; treatment for known VD deficiency in last 6 months; treatment with moderate‐ or high‐potency topical corticosteroids, oral or topical antibiotics, oral antivirals, immune enhancers, or topical calcineurin inhibitors in past 7 days; phototherapy in past month; autoimmune disease or immunodeficiency; planned trip to sunny climate during 6‐week study. |
| Interventions |
Intervention characteristics 8000 IU D₃
Placebo
*Trial registration indicated n = 101 participants enrolled |
| Outcomes | None within the scope of this review |
| Starting date | April 2014 |
| Contact information | Arturo Borzutzky, MD, Pontifica Universidad Catolica de Chile, Principal Investigator; email: arturobor@med.puc.cl |
| Notes | Notes: trial completed (ended December 2014); study author contacted via email and indicated manuscript is in progress |
NCT02046577.
| Study name |
Publict title: Study of vitamin D for the prevention of acute
respiratory infections in children Official title: A randomised, double‐blind, controlled trial of vitamin D for the prevention of acute respiratory infections in children age 18 to 36 months in Santiago, Coyhaique, and Punta Arenas, Chile |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Award Fonis SA13I20173, Fondo Nacional de Investigación y Desarrollo en Salud Country: Chile |
| Participants |
Included criteria: age 18 to 36 months, attending day care in
Santiago, Coyhaique, or Punta Arenas, Chile Excluded criteria: history of chronic illness requiring immunosuppression; history of metabolic bone disease; use of vitamin D supplementation > 400 IU daily, by milk formula or by vitamin supplements, in last 3 months; use of fish oil supplements in last 3 months; immunodeficiency; planned trip to sunny climate during study period |
| Interventions |
Intervention characteristics 5600 IU D₃
11,200 IU D₃
Placebo
*Trial registration indicates n = 276 participants enrolled; meeting abstract describes n = 303 participants included in analysis |
| Outcomes |
Secondary
Measurement
Time point: 6 months |
| Starting date | January 2010 |
| Contact information | María L Reyes, Pontificia Universidad Catolica de Chile, Principal Investigator |
| Notes | Notes: trial completed (ended May 2016); meeting abstract with preliminary data available; study author contacted via email, specified that primary outcomes were not measured (e.g. growth) |
NCT02404623.
| Study name |
Public title: The effect of vitamin D administration to premature
infants on vitamin D status and respiratory morbidity Official title: The effect of vitamin D administration to premature infants on vitamin D status and respiratory morbidity during the first year of life |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: Israel |
| Participants |
Included criteria: preterm infant born at 32 + 6 to 36 + 6 weeks of
gestational age, born at Saroka University Medical Center, with signed
informed consent Excluded criteria: chromosomal abnormality; neurological or muscular congenital anomaly; congenital cardiac defect; congenital respiratory anomaly; congenital gastrointestinal, liver, or renal anomaly that affects absorption or metabolism (or both) of vitamin D or other substances (or both); admission after birth to neonatal intensive care unit persisting longer than 5 days |
| Interventions |
Intervention characteristics 400 IU D₃
800 IU D₃
|
| Outcomes |
Secondary
Measurement
Time points: enrolment, 12 months of age |
| Starting date | April 2015 |
| Contact information | Inbal Golan‐Tripto, Soroka University Medical Center, Principal Investigator; email: inbalgt@clalit.org.il |
| Notes | Notes: recruitment completed (ended 12 February 2018). Meeting abstract and conference poster shared by study author |
NCT02452762.
| Study name |
Public title: Rapid normalization of vitamin D in critically ill
children: a phase II dose evaluation randomised controlled trial
(VITdAL‐PICU) Official title: Rapid normalization of vitamin D in critically ill children: a phase II dose evaluation randomised controlled trial |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: Canadian Institutes of Health Research through the Project Scheme Grant and the Academic Health Sciences Centre Alternative Funding Plan Innovation Fund 2014–2015 at Children’s Hospital of Eastern Ontario; Euro‐Pharm International Canada Inc. provided study drug in kind; Qualigen Inc. provided FastPak (R) Vitamin D immunoassay kits in kind Country: Canada, Austria, Chile |
| Participants |
Included criteria: admitted to intensive care unit (ICU), corrected
gestational age > 37 weeks to age < 18 years, expected ICU admission
in excess of 48 hours and likely to have access for blood work at 7 days of
hospital stay (determined by medical team), 25‐hydroxyvitamin D level <
50 nmol/L Excluded criteria: significant gastrointestinal disorder preventing enteral drug administration; hypercalcaemia, excluding transient abnormality and related to parenteral calcium administration for hypocalcaemia; confirmed or suspected Williams syndrome; patient known to have nephrolithiasis or nephrocalcinosis; imminent plan for withdrawal of care or transfer to another ICU; physician refusal; previous enrolment in VITdAL‐PICU pilot study; patient known to have granulomatous disease (tuberculosis or sarcoidosis); severe liver dysfunction or failure; patient known to have hypersensitivity or allergy to vitamin D or any of the non‐medicinal ingredients of the formulation; patient on thiazide diuretics and also receiving regular ongoing calcium supplementation above daily recommended intake for reasons other than hypocalcaemia; adolescent female of childbearing age with positive pregnancy serum test; patient on digoxin therapy |
| Interventions |
Intervention characteristics 10,000 IU D₃
Placebo
*Sites in Austria and Chile will use vitamin D and placebo from Fresenius Kabi (Oleovit D3) and Laboratorios Andromaco SA (D’Vidamax 50,000 IU Oral Solution), respectively |
| Outcomes |
Primary
Secondary
Measurement
Time points: days 1, 2, 3, 7, 30, 60, and 90; at hospital discharge |
| Starting date | January 2016 |
| Contact information | James Dayre McNally, Children's Hosptial of Eastern Ontario, Principal Investigator; email: dmcnally@cheo.on.ca |
| Notes | Notes: trial completed (ended January 2018); study author contacted and indicated that data are currently being analysed |
NCT02563015.
| Study name |
Public title: Can correction of low vitamin D status in infancy
program for a leaner body composition? Official title: Novel functional outcomes of vitamin D in infancy; can correction of low vitamin D status program for a leaner body composition phenotype? |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: Canada |
| Participants |
Included criteria: term, healthy, appropriate weight for gestational
age; infants born to mothers with otherwise healthy pregnancy and free of
medications that impact vitamin D metabolism (except vitamin or mineral
supplements) or faetal growth; intent to breastfeed to at least 3 months;
age up to 1 week Excluded criteria: preterm, small‐for‐gestational‐age, maternal smoking in pregnancy, diabetes, preeclampsia, celiac disease, inflammatory bowel disease, medications that impact vitamin D or mineral metabolism |
| Interventions |
Intervention characteristics 400 IU D₃
1000 IU D₃
400 IU D₃ "reference"
*Trial registration reports a total of N = 132 participants enrolled |
| Outcomes |
Secondary
Measurement
Time point: 3 years |
| Starting date | March 2016 |
| Contact information | Hope Weiler, McGill University, Principal Investigator; email: catherine.vanstone@mcgill.ca |
| Notes | Notes: trial terminated (stopped 20 September 2020) due to the coronavirus disease 2019 (COVID‐19) pandemic, stopping recruitment |
NCT02975492.
| Study name |
Public title: Assessing the impact of a mode of vitamin D
supplementation (sequential dose vs daily dose) on the incidence of
hypercalciuria in children age from 2 to 6 years (DonneDVit) Official title: Evaluation de l'impact d'un mode de supplémentation en vitamine D (dose séquentielle vs dose quotidienne) sur l'incidence de l'hypercalciurie chez des enfants des départements du gard et de l'hérault agés de 2 à 6 ans. Etude contrôlée randomisée en 2 groupes parallèles |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: France |
| Participants |
Included criteria: age 2 to 6 years included, obtaining signed
informed consent of parents Excluded criteria: none reported |
| Interventions |
Intervention characteristics 100,000 IU D₃
1000 IU D₃
*Trial registration indicates estimated enrolment of N = 280 participants |
| Outcomes | None within the scope of this review |
| Starting date | December 2017 |
| Contact information | Denis Morin, MD, University Hospital, Montpelier, Principal Investigator; email: d‐morin@chu‐montpellier.fr |
| Notes | Notes: recruiting (estimated recruitment completion November 2023) |
NCT03087149.
| Study name |
Public title: Monitored vs standard supplementation of vitamin D in
preterm infants (MOSVID) Official title: Supplementation of vitamin D in preterm infants ‐ monitored therapy vs standard therapy. A randomised controlled trial |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: no funding Country: Poland |
| Participants |
Included criteria: preterm infants born between 24 and 32 weeks of
gestation (estimated by ultrasound), born or admitted to the unit within 48
hours from birth, randomisation within 7 days from birth, mothers willing to
return for follow‐up visits Excluded criteria: preterm delivery (at least 33 weeks of gestation or term delivery, estimated by ultrasound), major congenital abnormalities, participation in another trial, severe illness at birth deemed incompatible with survival, congenital HIV infection, total parenteral nutrition > 14 days, cholestasis |
| Interventions |
Intervention characteristics Monitored
Standard
|
| Outcomes |
Primary
Secondary
Measurement
Time points: 35, 40, and 52 ± 2 weeks' postmenstrual age |
| Starting date | May 2017 |
| Contact information | Alicja Kołodziejczyk, Medical University of Warsaw, Warsaw, Poland; email: alicja.kolodziejczyk@uwr.edu.pl |
| Notes | Notes: unknown recruitment status (estimated recruitment completion May 2020) |
NCT03365687.
| Study name |
Public title: Vitamin D In the prevention of viral‐induced asthma in
preschoolers Official title: Vitamin D In the prevention of viral‐induced asthma in preschoolers: a randomised controlled multicenter trial (DIVA) |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: 100% non‐profit. Canadian Institutes of Health Research, grant no. 153252 Country: Canada |
| Participants |
Included criteria: age 1 to 5 years, with a physicians’ diagnosis of
asthma based on clinical signs of airflow obstruction and reversibility
according to Canadian guidelines, a recent history of asthma exacerbation(s)
requiring oral corticosteroids (OCSs) (≥ 1 in the past 6 months or ≥ 2 in
the past year, as documented in pharmacy or medical records, or both),
frequent upper respiratory tract infections (URTIs) (≥ 4 in the past year),
and URTIs identified by parents as the main asthma trigger Excluded criteria: current intake or intention to use > 400 IU/d of vitamin D supplement, or combined dietary and supplemental vitamin D intake that would exceed the recommended daily upper limit (i.e. 2500 IU for children age 1 to 3 years and 3000 IU for children age 4 to 8 years) if combined with the intervention dose; extreme prematurity (< 28 weeks' gestation); no vitamin D supplementation if exclusively breastfed in the past 6 months; vitamin D restrictive diet; undernourished (body mass index (BMI)‐for‐age in children ≥ 2 years of age, or either weight‐ or length‐for‐age in those < 2 years less than the third percentile); recent (< 1 year) refugees and immigrants from regions at high risk of rickets; other chronic respiratory disease; diagnosed condition(s) or use of medication(s) that alter calcium or vitamin D absorption/metabolism, and anticipated follow‐up difficulties |
| Interventions |
Intervention characteristics 100,000 IU D₃
Placebo
|
| Outcomes |
Primary
Secondary
Measurements
Time points: 3.5 ± 0.5 months, 7 ± 0.5 months |
| Starting date | October 2018 |
| Contact information | Connie Yang, British Columbia Children's Hospital, Principal Investigator; email: connie.yang@cw.bc.ca |
| Notes | Notes: recruiting (estimated recruitment completion December 2023) |
NCT03536845.
| Study name |
Public title: Vitamin D supplementation to prevent vitamin D
deficiency for children with epilepsy Official title: Vitamin D supplementation to prevent vitamin D deficiency for children with epilepsy: a randomised controlled clinical trial |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: non‐profit and for‐profit. Dallah Healthcare, Kingdom of Saudi Arabia. Grant number (CMRC‐DHG‐1/006) Country: Saudi Arabia |
| Participants |
Included criteria: age 2 to 16 years, being treated with
antiepileptic drugs Excluded criteria: preexisting vitamin D metabolism problems such as vitamin D‐dependent rickets, malabsorption syndrome, kidney disease, or liver disease. In addition to hypercalcaemia at baseline, total corrected calcium > 2.5 mg/dL, serum 25‐hydroxyvitamin D (25(OH)D) level > 250 nmol/L, or urine calcium‐to‐creatinine ration > 1.2 mol/mol or > 0.41 g/g Notes: children with baseline serum 25‐hydroxyvitamin D < 75 nmol/L will be given a treatment course of 5000 IU vitamin D₃ daily for 8 weeks + 30 to 75 mg/kg/d of elemental calcium in 3 divided doses for 4 weeks, and given the option of taking 35,000 IU weekly during the treatment phase according to patient preference. Upon normalisation of serum vitamin D level, patients will be randomised. Children with serum vitamin D > 75 nmol/L will be randomised immediately to the maintenance intervention |
| Interventions |
Intervention characteristics 400 IU D₃
1000 IU D₃
|
| Outcomes |
Primary
Secondary
Measurement
Time points: 3 months, 6 months |
| Starting date | January 2018 |
| Contact information | Reem Al Khalifah, MBBS, FRCPs Msc, King Saud University, Principal Investigator; email: ralkahlifah@ksu.edu.sa |
| Notes | Notes: recruiting (estimated recruitment completion January 2021) |
NCT03742310.
| Study name |
Public title: The relationship between VDR gene polymorphism and
children's physical and intellectual development (RVDRGPCPID) Official title: Multi‐center clinical study on the relationship between vitamin D receptor gene polymorphism and children's physical and intellectual development |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: China |
| Participants |
Included criteria: age 0 to 3 years, healthy, no history of specific
diseases Excluded criteria: current or past serious lung infection, nervous system disease, kidney disease, malignant tumour; bone metabolic disease or other genetic metabolic disease; taking drugs that affect bone metabolism |
| Interventions |
Intervention characteristics Low‐risk vitamin D receptor (VDR) genotype 400 IU D₃
Middle‐risk VDR genotype 600 IU D₃
High‐risk VDR genotype 800 IU D₃
General 400 IU D₃
|
| Outcomes |
Primary
Measurement
Time point: 3 years of age |
| Starting date | January 2019 |
| Contact information | Hui Li, PhD, First Affiliated Hospital of Xi'an Jiaotong University, Principal Investigator; email: huili@mail.xjtu.edu.cn |
| Notes | Notes: not yet recruiting (estimated start 1 March 2021) |
NCT03742505.
| Study name |
Public title: Rapid normalization of vitamin D deficiency in PICU
(VITdALIZE‐KIDS) Official title: Rapid normalization of vitamin D deficiency in PICU: a multi‐centre phase III double‐blind randomised controlled trial |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: Canada |
| Participants |
Included criteria: anticipated paediatric intensive care unit stay ≥
48 hours; corrected gestational age 37 weeks to age 18 years; expected to
require clinically indicated blood work > 48 hours following study
enrolment (range 2 to 7 days); vitamin D deficiency, defined by blood
25‐hydroxyvitamin D (25(OH)D) < 50 nmol/L at the time of screening Excluded criteria: treating physician refuses enteral drug administration due to gastrointestinal disorder; persistent hypercalcaemia (ionised calcium > 1.40 mmol/L (age ≥ 2 months), > 1.45 (age < 2 months)) excluding transient abnormalities and those related to parenteral calcium administration for hypocalcaemia; confirmed or suspected Williams syndrome; known nephrolithiasis or nephrocalcinosis; imminent plan for withdrawal of treatment or transfer to another intensive care unit not participating in the VITdALIZE‐KIDS trial; physician refusal; previous enrolment in this trial; granulomatous disease (tuberculosis or sarcoidosis); severe liver failure; hypersensitivity or allergy to vitamin D or any of the non‐medicinal ingredients of the formulation; taking thiazide diuretics while receiving regular ongoing calcium supplementation above daily recommended intake; adolescent female of childbearing age with positive pregnancy serum test; receiving digoxin therapy; treating physician intends to administer vitamin D doses above 1000 IU (e.g. patient presents with isolated clinical symptoms of severe VDD, severe burns) |
| Interventions |
Intervention characteristics 400,000 IU D₃
Placebo
|
| Outcomes |
Primary
Measurement
Time point: up to 90 days post randomisation |
| Starting date | June 2019 |
| Contact information | James Dayre McNally, Children's Hospital of Eastern Ontario, Principal Investigator; email: dmcnally@cheo.on.ca |
| Notes | Notes: recruiting (estimated recruitment completion 31 August 2023) |
NCT03871322.
| Study name |
Public title: The vitamin K₂ and D₃ intervention trial in children
and adolescents with low‐energy fractures Official title: Rationale and design of the vitamin K₂ and vitamin D₃ intervention trial in children and adolescents with low‐energy bone fractures |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: Poland |
| Participants |
Included criteria: age 3 to 18 years, presence of low‐energy
fracture, vitamin D serum level < 30 ng/mL Excluded criteria: age > 18 years, lack of low‐energy bone fracture, oral anticoagulant treatments that interfere with vitamin K cycle, current supplementation with vitamin K₂ or vitamin D₃, osteogenesis imperfecta or other bone disease, vitamin D concentration > 30 ng/mL |
| Interventions |
Intervention characteristics 2000 IU D₃
2000 IU D₃ + Vitamin K₂
Arm not to be included in analysis Placebo
|
| Outcomes |
Secondary
Measurement
Time point: 3 months |
| Starting date | July 2019 |
| Contact information | Michał Karpiński, Medical University of Bialystok, Principal Investigator; email: gufkarp@gmail.com |
| Notes | Notes: recruiting (estimated recruitment completion 20 January 2021) |
NCT03999580.
| Study name |
Public title: The vitamin D in pediatric Crohn's disease (ViDiPeC‐2)
(ViDiPeC‐2) Official title: A pragmatic randomised controlled trial on high dose vitamin D to prevent relapses of Crohn's disease in children |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: Canada |
| Participants |
Included criteria: age at randomisation between 4 and 17 years
inclusive; Pediatric Crohn's Disease (CD) Activity Index (PCDAI) ≤ 10 with
no clinical symptoms (abdominal pain or blood in the stool) at inclusion;
receiving a stable dose for at least 4 weeks of any of the following drugs:
thiopurines, methotrexate, or tumour necrosis factor‐α inhibitors
(infliximab/adalimumab); dosage of fecal calprotectin < 250 µg/g stool at
inclusion Excluded criteria: history of surgery resulting in a permanent colostomy or ileostomy (because of inability to calculate PCDAI at baseline), patients who have already been included in the pilot vitamin D trials, patients actively enrolled in other CD drug trials |
| Interventions |
Intervention characteristics 3000 to 4000 IU D₃
600 IU D₃
|
| Outcomes | None within scope of this review |
| Starting date | August 2019 |
| Contact information | Prevost Jantchou, MD, PhD, St Justine's Hospital, Principal Investigator; email: prevost.jantchou@umontreal.ca |
| Notes | Notes: not yet recruiting (estimated trial completion December 2024) |
RBR‐4r6p5v.
| Study name | Effect of physical exercise and nutritional programs on the health status of schoolchildren age 4 to 11 years old from Santo Antônio de Goiás |
| Methods |
Study design: randomised controlled trial Study grouping: cross‐over Funding: 100% non‐profit. Fundação Cargill ‐ Goiânia, GO, Brazil Country: Brazil |
| Participants |
Included criteria: age between 4 and 11 years; both genders;
residing in Santo Antônio de Goiás; enrolled in the city elementary
school Excluded criteria: cognitive or physical disabilities; pathologies such as respiratory, cardiologic, renal, or hepatic chronic disease, which prevent data collection, and vitamin D supplementation; using any medication that influences the serum concentration of lipoproteins; having used cholecalciferol supplement in the last 10 weeks; serum 25‐hydroxyvitamin D levels > 75 ng/dL |
| Interventions |
Intervention characteristics 1000 IU D₃
Placebo
|
| Outcomes | None within scope of this review |
| Starting date | September 2017 |
| Contact information | Ana Gabriella Pereira Alves, Universidade Federal de Goiás, Principal Investigator; email:anagabriela_alves@hotmail.com |
| Notes | Notes: recruitment completed (ended January 2020); study author contacted and indicated that few children were under 5 years of age and additional data are forthcoming |
UMIN000034864.
| Study name |
Public title: Prevention of allergic march by vitamin D
supplementation during infancy Scientific title: Prevention of allergic march by vitamin D supplementation during infancy |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: other Country: Japan |
| Participants |
Included criteria: age 1 to 5 years Excluded criteria: premature; surgical disease (oesophageal atresia, diaphragmatic hernia) requiring tube feeding or inability to take vitamin D; ineligible per judgement of research facility director or doctor |
| Interventions |
Intervention characteristics 400 IU
Placebo
|
| Outcomes |
Secondary
Measurement
Time points: 6 months of age, 1 year of age |
| Starting date | July 2018 |
| Contact information | Taiji Nakano, Chiba University, Department of Pediatrics, Principal Investigator; email: t‐nakano@chiba‐u.jp |
| Notes | Notes: recruiting (estimated recruitment completion July 2023) |
Yani 2018.
| Study name | Vitamin D supplementation and tuberculin skin test conversion among healthy under‐five children with tuberculosis contact |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Funding: not reported Country: Indonesia |
| Participants |
Included criteria: healthy children < 5 years of age with
tuberculosis contact with negative tuberculin tests Excluded criteria: none noted |
| Interventions |
Intervention characteristics 25,000 IU D₃
Placebo
|
| Outcomes |
Secondary
Measurement
Time point: 12 weeks |
| Starting date | March 2014 |
| Contact information | Finny Fitry Yani, MD, University Andalas, Principal Investigator; email: finny_fy@yahoo.com |
| Notes | Notes: trial completed (ended December 2015); study author contacted and indicated that manuscript is forthcoming |
BMI: body mass index. ICU: intensive care unit. PMA: postmenstrual age.
Differences between protocol and review
Title. We changed the title to "Oral vitamin D supplementation on linear growth and other health outcomes among children under five years of age" to focus our review on our primary and secondary outcomes related to linear growth, including adverse effects and rickets. We made this decision after reviewing the literature and noting that many of the trials investigating non‐communicable diseases, including atopy, allergy, metabolic disease, and bone health outcomes, had already been included in previous Cochrane Reviews.
Authorship. We added three new authors ‐ NA, AS, and RA ‐ for their substantial contributions to the review.
Description of the condition and Why it is important to do this review. We revised these sections to reflect updated estimates and statistics published since 2017.
Objectives. We edited our objectives to be in line with changes to the title and scope of the review (see #1 above).
Types of interventions. We had planned to conduct two comparisons: (1) vitamin D versus placebo or no intervention; and (2) vitamin D+micronutrient(s) versus micronutrient(s) alone. After conducting the search, we found that many studies compared higher‐dose vitamin D to lower‐dose vitamin D (across both arms, with or without micronutrient(s)). Upon discussion amongst all review authors, we chose to include such studies as a third and fourth comparison for the review, to more deeply describe, and to gain further clarity over, the literature base in this research area (Table 4).
-
We added 'gain in linear growth' as a relevant secondary outcome, as two studies included from our search results included this outcome.
We added 'change in vitamin D concentration' as a relevant secondary outcome, as several studies included from our search results included this outcome.
We added 'underweight' and 'wasting' as relevant secondary outcomes to include these dichotomised outcomes in parallel with including 'stunting' as a primary outcome.
We removed secondary outcomes #8 'Atopic diseases (i.e. asthma, including recurring wheeze, dermatitis, and/or rhinitis; as defined by trialists)' and #9, 'Other non‐communicable disease outcomes (i.e. bone health, number of fractures, bone mineral density, any type of cancer, type 1 and type 2 diabetes mellitus, insulin resistance, and other autoimmune disorders; congestive heart failure; as defined by trialists)', as they are covered by previous Cochrane Reviews (Winzenberg 2011; Martineau 2016), as well as by other reviews (Pojsupap 2015), and we sought to narrow our review scope to focus on linear growth and adverse effects (see #1 above).
-
Electronic searches. Our specific changes are detailed below.
-
PubMed.
We removed quotation marks to increase sensitivity.
We added wildcard to hydroxyvitamin D*
We corrected the spelling of 'randomised controlled trial [pt]' to 'randomised controlled trial [pt]'.
-
Scopus.
We did not limit to conference papers only, thereby conducting a broader search.
-
WPRO (WHO Western Pacific Regional Office).
We corrected the name WPRO to WPRIM (Western Pacific Region Index Medicus); WPRO is the office.
-
IMSEAR (Indian Medicus for the South East Asia Region).
This database was not available at the time of searching (14 March 2018 and 11 December 2019) and therefore was not included.
-
WHO ICTRP.
In December 2019, we did not search WHO ICTRP directly because the trials records were available in CENTRAL.
-
IndMED.
We tried to access IndMED in 2019 but the database was no longer available at the last known URL, and we could not find an alternative location.
-
Data extraction and management. After piloting our data extraction forms, we found additional information to capture beyond what we had originally proposed, which included only "intervention, participants, trial identification numbers if available, results, and adverse events". We recorded this and additional details in the aforementioned section because we considered these details to be relevant in making comparisons.
-
Measures of treatment effect.
After screening and extracting data, we found that many studies reported medians, ranges, interquartile ranges, and standard errors, rather than means and standard deviations, as described in our protocol (Yu 2017). Using methods in the Cochrane Handbook for Systematic Reviews of Interventions (Li 2020), we were able to include these data in comparisons by back‐calculating means and standard deviations, when appropriate.
To examine our secondary outcome 'rickets', we chose to include any study reporting on signs and symptoms of rickets as a dichotomous variable, and to combine these into one variable for meta‐analysis (see Included studies > Outcomes). We analysed rickets this way due to the heterogeneity in rickets' definitions across studies reporting this outcome.
We analysed two studies that reported rickets as an outcome using continuous measures, as reported in the original study (see Included studies > Outcomes). We analysed continuous data on rickets separately from categorical measures of rickets to include both types of data in our review.
-
Unit of analysis issues > Studies with more than two treatment groups.
After screening and extracting data, we found that some studies assessed effects of oral vitamin D compared to a control as well as compared to other forms of vitamin D administration, such as intramuscular injection. We did not anticipate this in our protocol (Yu 2017), but we have accounted for this in the Review by extracting data only from relevant trial arms.
Because we included another comparison to examine higher‐dose vitamin D compared to lower‐dose vitamin D (Table 4), if a study involved two or more comparison arms, we added our method to accommodate this, which was not described in our protocol (Yu 2017).
Assessment of reporting biases. To avoid repetition, we did not populate this section in our protocol (Yu 2017); we had explained how we would assess reporting bias under 'Selective reporting' in the 'Assessment of risk of bias' section. However, for clarity for the reader, we have explained how we assessed reporting bias in this section of the review.
Data synthesis. After screening and extracting data, we found that many outcomes included only one study in the analysis. For analyses including only one study, we used fixed‐effect models, as they are more appropriate than the random‐effects analyses originally proposed.
Potential biases in the review process. We searched 17 electronic databases and two trial registries to be as comprehensive as possible in examining all available evidence. However, we were not able to assess for publication bias using funnel plots due to lack of studies for comparison, thereby preventing us from drawing conclusions on publication bias of the included studies (Table 5).
Contributions of authors
Samantha Huey (SLH) and Nina Archarya (NA) drafted the review. Elaine Yu (EAY) wrote an earlier draft of this review. SLH, NA, EAY, Ashley Silver (AS), and Risha Sheni (RS) performed search strategy translation and screened records. SLH, NA, and AS extracted data and assessed 'Risk of bias' in included studies. SLH and NA performed the GRADE assessment. Juan Pablo Peña‐Rosas (JPP) and Saurabh Mehta (SM) revised and critically reviewed the protocol and the review, and arbitrated disagreements.
SM is the guarantor for the review.
Sources of support
Internal sources
-
Division of Nutritional Sciences, Cornell University, USA
SM is faculty, and SH and EY are doctoral candidates of the Division of Nutritional Sciences at Cornell University.
-
Department of Nutrition and Food Safety, World Health Organization (WHO), Switzerland
JPP is a full‐time member of staff of the Department of Nutrition and Food Safety at the WHO.
External sources
-
Bill & Melinda Gates Foundation, USA
WHO gratefully acknowledges financial support from the Bill & Melinda Gates Foundation. Donors do not fund specific guidelines and do not participate in any decision related to the guideline development process including the composition of policy questions, membership of the guideline groups, the conduct and interpretation of systematic reviews, or the formulation of recommendations.
Declarations of interest
Samantha L Huey: none known.
Nina Acharya: none known.
Ashley Silver: none known.
Risha Sheni: none known.
Elaine Yu: none known.
Juan Pablo Peña‐Rosas: the WHO receives partial financial support from the Bill & Melinda Gates Foundation to support commissioning of systematic reviews of interventions for health throughout the life course. Donors do not fund specific guidelines and do not participate in any decision related to the guideline development process, including the composition of policy questions, membership of the guideline groups, the conduct and interpretation of systematic reviews, or the formulation of recommendations.
Disclaimer: Juan Peña‐Rosas is a full‐time staff member at the World Health Organization. The review authors alone are responsible for the views expressed in this publication, which do not necessarily represent the official position, decisions, policy, or views of the WHO.
Saurabh Mehta (SM) is an unpaid board member with an equity stake/stocks/stock options in a diagnostic start‐up company, VitaScan, which is focused on developing assays for low‐cost and point‐of‐care measurement of certain nutrients from a drop of blood, using results from his research as a faculty member at Cornell University. SM is also the principal investigator on competitive research grants from HarvestPlus/International Food Policy Research Institute to conduct efficacy trials for crops biofortified with iron, zinc, and vitamin A among children in India, for which the outcomes include child growth and nutritional status. SM was paid a consulting fee as external reviewer for the nutrition programme at New York Academy of Sciences and was paid travel and accommodation expenses by Foundation Merieux for a conference presentation on precision nutrition and gut microbiome. SM received partial financial support for this work from the WHO.
Edited (no change to conclusions)
References
References to studies included in this review
Abdel‐Hady 2019 {published data only}
- Abdel-Hady H, Yahia S, Megahed A, Mosbah A, Seif B, Nageh E, et al. Mediators in preterm infants with late-onset sepsis: a randomized controlled trial. Journal of Pediatric Gastroenterology and Nutrition 2019;68(4):578-84. [DOI: 10.1097/MPG.0000000000002238] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT02273843. A trial on different dosages of vitamin D in preterm infants with late-onset sepsis [Effect of different dosages oral vitamin D on serum interleukin-6 in preterm infants with late-onset sepsis]. clinicaltrials.gov/ct2/show/NCT02273843 (first received 20 October 2014).
Aglipay 2017 {published and unpublished data}
- Aglipay M, Birken C, Dai D, Parkin P, Maguire J. High dose vitamin D for the prevention of wheezing in preschoolers: a secondary analysis of a randomized clinical trial. Paediatrics & Child Health 2019;24(Suppl 2):e27-8. [DOI: 10.1093/pch/pxz066.069] [Abstract #70] [DOI] [Google Scholar]
- Aglipay M, Birken CS, Parkin PC, Loeb MB, Thorpe K, Chen Y, et al. Effect of high-dose vs standard-dose wintertime vitamin D supplementation on viral upper respiratory tract infections in young healthy children. JAMA 2017;318(3):245-54. [DOI: 10.1001/jama.2017.8708] [PMC5817430] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aglipay M, Maguire JL. Vitamin D supplementation and upper respiratory tract infections in children—reply. JAMA 2017;318(21):2139-40. [DOI: 10.1001/jama.2017.15154] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Davidson BL, Alansari K. Vitamin D supplementation and upper respiratory tract infections in children—comment. JAMA 2017;318(21):2138-9. [DOI: 10.1001/jama.2017.15150] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gibson E, Aglipay M, Keown-Stoneman C, Birken C, Thorpe K, O'Connor D, et al. Effect of high vs standard dose wintertime vitamin D supplementation on adiposity in young healthy children: a secondary analysis of a pragmatic RCT. Paediatrics & Child Health 2019;24(Suppl 2):e23. [DOI: 10.1093/pch/pxz066.057] [Abstract #58] [DOI] [Google Scholar]
- Hueniken K, Aglipay M, Birken CS, Parkin PC, Loeb MB, Thorpe KE, et al. Effect of high-dose vitamin D supplementation on upper respiratory tract infection symptom severity in healthy children. Pediatric Infectious Disease Journal 2019;38(6):564-8. [DOI: 10.1097/INF.0000000000002225] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Maguire JL, Birken CS, Loeb MB, Mamdani M, Thorpe K, Hoch JS, et al. DO IT trial: vitamin D outcomes and interventions in toddlers—a TARGet Kids! randomized controlled trial. BMC Pediatrics 2014;14:37. [DOI: 10.1186/1471-2431-14-37] [PMC3942179] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01419262. DO IT trial: vitamin D Outcomes and Interventions in Toddlers (DO IT). clinicaltrials.gov/ct2/show/NCT01419262 (first received 16 August 2011).
Ala‐Houhala 1985 {published data only}
- Ala-Houhala M. 25-hydroxyvitamin D levels during breast-feeding with or without maternal or infantile supplementation of vitamin D. Journal of Pediatric Gastroenterology and Nutrition 1985;4(2):220-6. [DOI: 10.1097/00005176-198504000-00011] [PMID: ] [DOI] [PubMed] [Google Scholar]
Alam 2011 {published data only (unpublished sought but not used)}
- Alam NH, Ashraf H, Gyr NE, Meier RF. Efficacy of L-isoleucine supplemented food and vitamin D in the treatment of acute diarrhea in children. Gastroenterology 2011;140(5 Suppl 1):S571. [DOI: 10.1016/S0016-5085(11)62364-0] [Abstract #Mo1158] [DOI] [Google Scholar]
- Alam NH, Ashraf H. Evaluation of the efficacy of l-isoleucine supplemented food and vitamin D in the treatment of acute diarrhea in children. Archives of Disease in Childhood 2012;97(Suppl 2):A12. [DOI: 10.1136/archdischild-2012-302724.0043] [Abstract #43] [DOI] [Google Scholar]
Alizadeh 2006 {published data only}
- Alizadeh P, Naderi F, Sotoudeh K. A randomized clinical trial of prophylactic effects of vitamin D on different indices of osteopenia of prematurity. Iranian Journal of Public Health 2006;35(3):58-63. [ijph.tums.ac.ir/index.php/ijph/article/view/2165] [Google Scholar]
Alizadeh Taheri 2014 {published data only}
- Alizadeh Taheri P, Sajjadian N, Beyrami B, Shariat M. Prophylactic effect of low dose vitamin D in osteopenia of prematurity: a clinical trial study. Acta Medica Iranica 2014;52(9):671-4. [PMID: ] [PubMed] [Google Scholar]
Alonso 2011 {published data only}
- Alonso A, Rodríguez J, Carvajal I, Prieto MAL, Rodríguez RMA, Pérez AMA, et al. Prophylactic vitamin D in healthy infants: assessing the need. Metabolism: Clinical and Experimental 2011;60(12):1719-25. [DOI: 10.1016/j.metabol.2011.04.011] [PMID: ] [DOI] [PubMed] [Google Scholar]
Aly 2019 {published data only (unpublished sought but not used)}
- Aly H, Mohsen L, Bhattacharjee I, Malash A, Atyia A, Elanwary S, et al. Vitamin D supplementation and T cell regulation in preterm infants: a randomized controlled trial. Journal of Pediatric Gastroenterology and Nutrition 2019;69(5):607-10. [DOI: 10.1097/MPG.0000000000002448] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT03793309. Different doses of vitamin D and T regulatory cells in preterm infants [A randomized controlled trial on the effect of 400 vs 800 IU of vitamin D on T regulatory cells in preterm infants]. clinicaltrials.gov/ct2/show/NCT03793309 (first received 24 December 2018).
Anderson‐Berry 2017 {published data only}
- Anderson-Berry A, Jones G, Lyden E, Kaufmann M, Jones G, Hanson C. Dynamics of 3-epi-25-hydroxyvitamin D3 in premature infants during neonatal intensive care unit hospitalization. Clinical Chemistry 2014;60(10 Suppl 1):S198. [Google Scholar]
- Anderson-Berry A, Thoene M, Wagner J, Lyden E, Jones G, Kaufmann M, et al. Randomized trial of two doses of vitamin D3 in preterm infants < 32 weeks: dose impact on achieving desired serum 25(OH)D3 in a NICU population. PloS One 2017;12(10):e0185950. [DOI: 10.1371/journal.pone.0185950] [PMC5634602] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson C, Jones G, Lyden E, Kaufmann M, Armas L, Anderson-Berry A. Vitamin D metabolism in the premature newborn: a randomized trial. Clinical Nutrition 2016;35(4):835-41. [DOI: 10.1016/j.clnu.2015.07.023] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hanson C, Lyden E, Kaufman M, Jones G, Anderson-Berry A. Dynamics of 24,25-hydroxyvitamin D3 in premature infants during neonatal intensive care unit hospitalization. Clinical Nutrition 2014;33(Suppl 1):S9. [Abstract #OP021] [tinyurl.com/y5l2zgag] [Google Scholar]
- Hanson C, Lyden E, Nelson A, Thoene M, Wagner J, Wu A, et al. Response of vitamin D binding protein and free vitamin D concentrations to vitamin D supplementation in hospitalized premature infants. Journal of Pediatric Endocrinology & Metabolism 2015;28(9-10):1107-14. [DOI: 10.1515/jpem-2015-0089] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hanson C, Lyden E, Thoene M, Wagner J, Anderson-Berry A. Vitamin D supplementation and NICU outcomes in premature infants. In: Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2014 May 3-6; Vancouver (BC). 2014. [Abstract #436]
- NCT01469650. Serum 25(OH)D levels, supplemental vitamin D, and parathyroid hormone levels in premature infants. clinicaltrials.gov/ct2/show/NCT01469650 (first received 8 November 2011).
Atas 2013 {published data only (unpublished sought but not used)}
- Atas E, Karademır F, Ersen A, Meral C, Aydınoz S, Suleymanoglu S, et al. Comparison between daily supplementation doses of 200 versus 400 IU of vitamin D in infants. European Journal of Pediatrics 2013;172(8):1039-42. [DOI: 10.1007/s00431-013-1997-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Backström 1999a {published data only}
- Backström MC, Mäki R, Kuusela AL, Sievänen H, Koivisto AM, Ikonen RS, et al. Randomised controlled trial of vitamin D supplementation on bone density and biochemical indices in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 1999;80(3):F161-6. [DOI: 10.1136/fn.80.3.f161] [PMC1720926] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Backström 1999b {published data only}
- Backström MC, Mäki R, Kuusela AL, Sievänen H, Koivisto AM, Koskinen M, et al. The long-term effect of early mineral, vitamin D, and breast milk intake on bone mineral status in 9- to 11-year-old children born prematurely. Journal of Pediatric Gastroenterology and Nutrition 1999;29(5):575-82. [DOI: 10.1097/00005176-199911000-00019] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Zamora SA, Rizzoli R, Belli DC. Long-term effect of early vitamin D supplementation on bone mineral status in prematurely born infants—comment. Journal of Pediatric Gastroenterology and Nutrition 2000;31(1):94. [DOI: 10.1097/00005176-200007000-00025] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bozkurt 2017 {published data only}
- Bozkurt O, Uras N, Sari FN, Atay FY, Sahin S, Alkan AD, et al. Multi-dose vitamin d supplementation in stable very preterm infants: prospective randomized trial response to three different vitamin D supplementation doses. Early Human Development 2017;112:54-9. [DOI: 10.1016/j.earlhumdev.2017.07.016] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT02941185. Multi-dose vitamin D supplementation in preterm infants. clinicaltrials.gov/ct2/show/NCT02941185 (first received 18 October 2016).
Chan 1978 {published data only}
- Chan GM, Tsang RC, Chen IW, DeLuca HF, Steichen JJ. The effect of 1,25(OH)2 vitamin D3 supplementation in premature infants. Journal of Pediatrics 1978;93(1):91-6. [DOI: 10.1016/s0022-3476(78)80613-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chandy 2016 {published data only}
- Chandy DD, Kare J, Singh SN, Agarwal A, Das V, Singh U, et al. Effect of vitamin D supplementation, directly or via breast milk for term infants, on serum 25 hydroxyvitamin D and related biochemistry, and propensity to infection: a randomised placebo-controlled trial. British Journal of Nutrition 2016;116(1):52-8. [DOI: 10.1017/S0007114516001756] [PMID: ] [DOI] [PubMed] [Google Scholar]
- CTRI/2012/09/002958. A study of vitamin D supplementation in breastfeeding young children [A study of vitamin D supplementation regimens in exclusively breastfed term infants]. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=4632&EncHid=&modid=&compid=%27,%274632det%27 (first received 4 September 2012).
Choudhary 2012 {published data only}
- Choudhary N, Gupta P. Vitamin D supplementation for severe pneumonia--a randomized controlled trial. Indian Pediatrics 2012;49(6):449-54. [DOI: 10.1007/s13312-012-0073-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ducharme 2019 {published data only}
- Ducharme FM, Jensen M, Mailhot G, Alos N, White J, Rousseau E, et al. Impact of two oral doses of 100,000 IU of vitamin D3 in preschoolers with viral-induced asthma: a pilot randomised controlled trial. Trials 2019;20(1):138. [DOI: 10.1186/s13063-019-3184-z] [PMC6379931] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02197702. Vitamin D in preschoolers with viral-induced asthma (DIVA) [Vitamin D In the prevention of viral-induced asthma of preschoolers: a randomised controlled trial (RCT) - (DIVA)]. clinicaltrials.gov/ct2/show/NCT02197702 (first received 18 July 2014).
Evans 1989 {published data only}
- Evans JR, Allen AC, Stinson DA, Hamilton DC, St John Brown B, Vincer MJ, et al. Effect of high-dose vitamin D supplementation on radiographically detectable bone disease of very low birth weight infants. Journal of Pediatrics 1989;115(5 Pt 1):779-86. [DOI: 10.1016/s0022-3476(89)80662-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Feliciano 1994 {published data only}
- Feliciano ES, Ho ML, Specker BL, Falciglia G, Shui QM, Yin TA, et al. Seasonal and geographical variations in the growth rate of infants in China receiving increasing dosages of vitamin D supplements. Journal of Tropical Pediatrics 1994;40(3):162-5. [DOI: 10.1093/tropej/40.3.162] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fort 2016 {published data only}
- Craig C, Ambalavanan N, McNair T. Early vitamin d supplementation for prevention of respiratory morbidity in extremely preterm infants. Journal of Investigative Medicine 2014;62(2):492. [DOI: 10.231/JIM.0000000000000055] [Abstract #285] [DOI] [Google Scholar]
- Fort P, Salas AA, Ambalavanan N. Randomized clinical trial of vitamin D supplementation in extremely preterm infants. Journal of Investigative Medicine 2015;63(2):417. [DOI: 10.1097/JIM.0000000000000146] [Abstract #324] [DOI] [Google Scholar]
- Fort P, Salas AA, Nicola T, Craig CM, Carlo WA, Ambalavanan N. A comparison of 3 vitamin D dosing regimens in extremely preterm infants: a randomized controlled trial. Journal of Pediatrics 2016;174:132-8.e1. [DOI: 10.1016/j.jpeds.2016.03.028] [PMC4925243] [PMID: 27079965 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01600430. Vitamin D supplementation for extremely preterm infants [Early vitamin D supplementation for prevention of respiratory morbidity in extremely preterm infants: a randomized clinical trial]. clinicaltrials.gov/ct2/show/NCT01600430 (first received 14 May 2012).
- Salas AA, Woodfin T, Philips V, Paralta-Carcelen M, Carlo WA, Ambalavanan N. Dose-response relationship between early enteral vitamin D supplementation and neurodevelopmental outcomes at 2 years of age: follow-up data from a randomized controlled trial in extremely preterm infants. Journal of Investigative Medicine 2017;65(2):572. [DOI: 10.1136/jim-2016-000393.439] [Abstract #436] [DOI] [Google Scholar]
- Salas AA, Woodfin T, Phillips V, Peralta-Carcelen M, Carlo WA, Ambalavanan N. Dose-response effects of early vitamin D supplementation on neurodevelopmental and respiratory outcomes of extremely preterm infants at 2 years of age: a randomized trial. Neonatology 2018;113(3):256-62. [DOI: 10.1159/000484399] [PMC5860938] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallo 2013a {published data only}
- Gallo S, Phan A, Vanstone CA, Rodd C, Weiler HA. The change in plasma 25-hydroxyvitamin D did not differ between breast-fed infants that received a daily supplement of ergocalciferol or cholecalciferol for 3 months. Journal of Nutrition 2013;143(2):148-53. [DOI: 10.3945/jn.112.167858] [PMC3969107] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01190137. Study comparing two isoforms of vitamin D supplements for infants [Bio-equivalency study of the effects of vitamin D2 and vitamin D3 supplements on 25-hydroxyvitamin D levels in exclusively breast fed Canadian infants]. clinicaltrials.gov/ct2/show/NCT01190137 (first received 26 August 2010).
Gallo 2013b {published data only}
- Gallo S, Comeau K, Vanstone C, Agellon S, Sharma A, Jones G, et al. Effect of different dosages of oral vitamin D supplementation on vitamin D status in healthy, breastfed infants: a randomized trial. JAMA 2013;309(17):1785-92. [DOI: 10.1001/jama.2013.3404] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gallo S, Hazell T, Vanstone CA, Agellon S, Jones G, L'Abbé M, et al. Vitamin D supplementation in breastfed infants from Montréal, Canada: 25-hydroxyvitamin D and bone health effects from a follow-up study at 3 years of age. Osteoporosis International 2016;27(8):2459-66. [DOI: 10.1007/s00198-016-3549-z] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gallo S, Rodd C, Vanstone C, Agellon S, Comeau K, Jones G, et al. Vitamin D dose-response study in breast fed infants from Montreal, Canada: 400 IU/day is sufficient to meet the plasma 25-hydroxy vitamin D threshold of 50 nmol/L but not 75 nmol/L by 12 months of age. FASEB Journal 2012;26(Suppl 1):41.4. [DOI: 10.1096/fasebj.26.1_supplement.41.4] [DOI] [Google Scholar]
- Gallo S, Rodd C, Vanstone C, Agellon S, L'Abbe M, Khamessan A, et al. Lumbar spine bone mineral density is enhanced in breast fed infants receiving 800 or 1200 IU of vitamin d daily from 4 to 20 weeks of age. Journal of Bone and Mineral Research 2011;26(Suppl 1):S48. [DOI: 10.1002/jbmr.564] [Abstract #1140] [DOI] [Google Scholar]
- Gallo S, Rodd C, Weiler H. Vitamin D supplementation during first 12 months of life—reply. JAMA 2013;310(8):853-4. [DOI: 10.1001/jama.2013.124272] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gallo S, Vanstone CA, Comeau K, Agellon S, Rodd C, Sharma A, et al. Vitamin D dose response study in Canadian infants: 25-hydroxy-vitamin D status during a 20 week intervention. FASEB Journal 2010;24(Suppl 1):537.11. [DOI: 10.1096/fasebj.24.1_supplement.537.11] [DOI] [Google Scholar]
- Hazell TJ, Gallo S, Vanstone CA, Agellon S, Rodd C, Weiler HA. Vitamin D supplementation trial in infancy: body composition effects at 3 years of age in a prospective follow-up study from Montréal. Pediatric Obesity 2017;12(1):38-47. [DOI: 10.1111/ijpo.12105] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT00381914. Vitamin D dose-response study to establish dietary requirements in infants. clinicaltrials.gov/ct2/show/NCT00381914 (first received 27 September 2006).
- Sofiyan A, Nana RKH. Vitamin D supplementation during first 12 months of life—comment. JAMA 2013;310(8):853. [DOI: 10.1001/jama.2013.124263] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Wicklow B, Gallo S, Majnemer A, Vanstone C, Agellon S, Comeau K, et al. Impact of vitamin D supplementation on gross motor development: the result of a randomized dose-response trial in Canada. Journal of Bone and Mineral Research 2012;27(Suppl 1):S358. [DOI: 10.1002/jbmr.1852] [Abstract #MO0042] [DOI] [Google Scholar]
- Wicklow B, Gallo S, Majnemer A, Vanstone C, Comeau K, Jones G, et al. Impact of vitamin D supplementation on gross motor development of healthy term infants: a randomized dose-response trial. Physical & Occupational Therapy in Pediatrics 2016;36(3):330-42. [DOI: 10.3109/01942638.2015.1050150] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gordon 2008 {published data only}
- Gordon CM, Williams AL, Feldman HA, May J, Sinclair L, Vasquez A, et al. Treatment of hypovitaminosis D in infants and toddlers. Journal of Clinical Endocrinology & Metabolism 2008;93(7):2716-21. [DOI: 10.1210/jc.2007-2790] [PMC2729207] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Greer 1981 {published data only}
- Greer FR, Searcy JE, Levin RS, Steichen JJ, Asche PS, Tsang RC. Bone mineral content in breast-fed infants with and without supplemental vitamin D (D). Pediatric Research 1980;14(4, II):500. [Google Scholar]
- Greer FR, Searcy JE, Levin RS, Steichen JJ, Asch PS, Tsang RC. Bone mineral content and serum 25-hydroxyvitamin D concentration in breast-fed infants with and without supplemental vitamin D. Journal of Pediatrics 1981;98(5):696-701. [DOI: 10.1016/s0022-3476(81)80827-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Greer FR, Searcy JE, Levin RS, Steichen JJ, Steichen-Asche PS, Tsang RC. Bone mineral content and serum 25-hydroxyvitamin D concentrations in breast-fed infants with and without supplemental vitamin D: one-year follow-up. Journal of Pediatrics 1982;100(6):919-22. [DOI: 10.1016/s0022-3476(82)80514-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Greer 1989 {published data only}
- Greer FR, Marshall S. Bone mineral content, serum vitamin D metabolite concentrations, and ultraviolet B light exposure in infants fed human milk with and without vitamin D2 supplements. Journal of Pediatrics 1989;114(2):204-12. [DOI: 10.1016/s0022-3476(89)80784-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gupta 2016 {published data only}
- CTRI/2013/01/003317. Vitamin D supplementation for severe pneumonia in under-five children [Vitamin D supplementation for severe pneumonia in under-five children: a double blind, randomized placebo controlled trial]. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=4625&EncHid=&modid=&compid=%27,%274625det%27 (first received 23 January 2013).
- Gupta P, Dewan P, Shah D, Sharma N, Bedi N, Kaur IR, et al. Vitamin D supplementation for treatment and prevention of pneumonia in under-five children: a randomized double-blind placebo controlled trial. Indian Pediatrics 2016;53(11):967-76. [DOI: 10.1007/s13312-016-0970-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hanson 2011 {published data only}
- Hanson C, Armas L, Lyden E, Anderson-Berry A. Vitamin D status and associations in newborn formula-fed infants during initial hospitalization. Journal of the American Dietetic Association 2011;111(12):1836-43. [DOI: 10.1016/j.jada.2011.09.010] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT01042561. Vitamin D status and dose response in infants. clinicaltrials.gov/ct2/show/NCT01042561 (first received 31 December 2009).
Harnot 2017 {published data only (unpublished sought but not used)}
- CTRI/2012/05/002621. Appropriate dose of vitamin-D for treatment of vitamin-D deficiency [Appropriate dose of vitamin-D (3L IU or 6L IU) for treatment of vitamin-D deficiency in small children: a double blind randomised trial]. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=4048&EncHid=&modid=&compid=%27,%274048det%27 (first received 1 May 2012).
- Harnot J, Sanjay V, Singhi S, Sankhyan V, Sachdeva N, Bharti B. Comparison of 300,000 and 600,000 IU oral vitamin-D bolus for vitamin-D deficiency in young children. Indian Journal of Pediatrics 2017;84(2):111-6. [DOI: 10.1007/s12098-016-2233-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hibbs 2018 {published data only}
- Gao Y, Zhang D, Hou B. Vitamin D supplementation in young infants and recurrent wheezing—comment. JAMA 2018;320(16):1708. [DOI: 10.1001/jama.2018.11533] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Goldsobel A. Effect of vitamin D supplementation on recurrent wheezing in black infants who were born preterm: the D-Wheeze randomized clinical trial. Pediatrics 2018;142(Suppl 4):S250-1. [DOI: 10.1542/peds.2018-2420PPP] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs AM, Ross K, Kerns LA, Wagner C, Fuloria M, Groh-Wargo S, et al. Effect of vitamin D supplementation on recurrent wheezing in black infants who were born preterm: the D-Wheeze randomized clinical trial. JAMA 2018;319(20):2086-94; Erratum in: JAMA 2018; 320(6):605. DOI: 10.1001/jama.2018.10664. [DOI: 10.1001/jama.2018.5729] [PMC6583240] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs AM, Wagner CL, Tatsuoka C. Vitamin D supplementation in young infants and recurrent wheezing—reply. JAMA 2018;320(16):1708-9. [DOI: 10.1001/jama.2018.11537] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Lintonjua AA. Vitamin D reduces risk of recurrent wheezing in premature black infants—comment. Journal of Pediatrics 2018;202:330-3. [DOI: 10.1016/j.jpeds.2018.08.052] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT01601847. Wheezing in black preterm infants: impact of vitamin D supplementation strategy (D-Wheeze) [Wheezing in black preterm infants: impact of vitamin D supplementation strategy]. clinicaltrials.gov/ct2/show/NCT01601847 (first received 16 May 2012).
Holmlund‐Suila 2012 {published data only}
- Eudra-CT 2009-015940-40. VIDI-P [Vitamin-D intervention pilotti]. www.clinicaltrialsregister.eu/ctr-search/trial/2009-015940-40/FI (first received 5 October 2009).
- Holmlund-Suila E, Viljakainen H, Hytinantti T, Lamberg-Allardt C, Andersson S, Mäkitie O. High-dose vitamin D intervention in infants—effects on vitamin D status, calcium homeostasis, and bone strength. Journal of Clinical Endocrinology & Metabolism 2012;97(11):4139-47. [DOI: 10.1210/jc.2012-1575] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Holmlund-Suila E, Viljakainen H, Ljunggren Ö, Hytinantti T, Andersson S, Mäkitie O. Fibroblast growth factor 23 concentrations reflect sex differences in mineral metabolism and growth in early infancy. Hormone Research in Paediatrics 2016;85(4):232-41. [DOI: 10.1159/000443988] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Holmlund-Suila E, Viljakainen H, Ljunggren O, Carlsson E, Hytinantti T, Andersson S, et al. Gender, but not vitamin D supplementation, affects FGF23 concentration in healthy infants. In: Pediatric Academic Societies Annual Meeting; 2015 Apr 25-28; San Diego (CA). 2015. [Abstract #172] [Publication #1522.172] [mydigitalpublication.com/publication/?i=251676]
- Holmlund-Suila E. Vitamin D deficiency and supplementation: studies from infancy to young adulthood [Doctoral Thesis]. Helsinki: University of Helsinki, 2016. [Google Scholar]
- NCT01723852. Vitamin D intervention in infants (VIDI) [Vitamin D intervention in infants]. clinicaltrials.gov/ct2/show/NCT01723852 (first received 6 November 2012).
Holst‐Gemeiner 1978 {published data only}
- Holst-Gemeiner D, Gemeiner M, Pilz I, Swoboda W. Plasma 25-hydroxycholecalciferol after daily vitamin D administration in comparison with massive single-dose prophylaxis [Plasmaspiegel des 25-hydroxycholecalciferols nach tages- und "stossprophylaxe" mit vitamin]. Wiener kKlinische Wochenschrift 1978;90(14):509-12. [PMID: ] [PubMed] [Google Scholar]
Huynh 2017 {published data only}
- ACTRN12613001234707. A randomised controlled trial comparing daily (400 international units) and single bolus dosing (50,000 international units) of vitamin D in healthy breastfed infants of vitamin D deficient mothers [A randomised controlled trial evaluating the efficacy and safety of single bolus (50,000 international units) versus daily (400 international units) dosing of vitamin D in healthy breastfed infants of vitamin D deficient mothers]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364356 (first received 9 October 2013).
- Bandyopadhyay T, Saini I. Vitamin D in newborns. A randomised controlled trial comparing daily and single oral bolus vitamin D in infants—comment. Journal of Paediatrics and Child Health 2017;53(6):615. [DOI: 10.1111/jpc.13534] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Huynh J, Liew D, Rodda C. Neonatal vitamin D supplementation—reply. Journal of Paediatrics and Child Health 2017;53(7):725-6. [DOI: 10.1111/jpc.13582] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Huynh J, Lu T, Liew D, Doery J, Tudball R, Jona M, et al. The VINE study: vitamin D in newborns: a randomized controlled trial comparing daily and bolus supplementation in breastfed infants of vitamin D deficient mothers. In: Bone Abstracts. 7th International Conference on Children's Bone Health; 2015 Jun 27-30; Salzburg, Austria. Vol. 4. bioscientifica, 2015. [DOI: 10.1530/boneabs.4.OC19] [Abstract #OC19] [DOI]
- Huynh J, Lu T, Liew D, Doery JC, Tudball R, Jona M, et al. Vitamin D in newborns. A randomised controlled trial comparing daily and single oral bolus vitamin D in infants. Journal of Paediatrics and Child Health 2017;53(2):163-9. [DOI: 10.1111/jpc.13338] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jensen 2016 {published data only}
- Jensen M, Mailhot G, Alos N, Khamessan A, Rousseau E, White J, et al. A single vitamin D dose of 100,000 IU rapidly raises serum vitamin D levels > 75nmol/l, with no alteration in calcium metabolism biomarkers, in preschool-aged children with asthma. Respirology 2016;21(Suppl 2):181. [DOI: 10.1111/resp.12755] [Abstract #TP 213] [DOI] [Google Scholar]
- Jensen ME, Mailhot G, Alos N, Rousseau E, White JH, Khamessan A, et al. Vitamin D intervention in preschoolers with viral-induced asthma (DIVA): a pilot randomised controlled trial. Trials 2016;17(1):353. [DOI: 10.1186/s13063-016-1483-1] [PMC4960871] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen ME, Mailhot G, Alos N, White JH, Rousseau E, Khamessan A, et al. A vitamin D intervention in preschoolers with viral-induced asthma (DIVA): a pilot randomised controlled trial. American Journal of Respiratory and Critical Care Medicine 2015;191:A3360. [B53 new aspects of pediatric asthma, thematic poster session] [www-atsjournals-org.proxy.library.cornell.edu/doi/pdf/10.1164/ajrccm-conference.2015.191.1_MeetingAbstracts.A3360] [Google Scholar]
- NCT01999907. Vitamin D to reduce colds and asthma attacks in young children (DIVA-pilot) [Vitamin D vs placebo in the prevention of viral-induced exacerbations in preschoolers with asthma: a pilot RCT]. clinicaltrials.gov/ct2/show/NCT01999907 (first received 25 November 2013).
Kislal 2008 {published data only}
- Kislal FM, Dilmen U. Effect of different doses of vitamin D on osteocalcin and deoxypyridinoline in preterm infants. Pediatrics International 2008;50(2):204-7. [DOI: 10.1111/j.1442-200X.2008.02553.x] [DOI] [PubMed] [Google Scholar]
Lagomarsino 1996 {published data only}
- Lagomarsino FE, Arnaiz GP, Vial CP, Heusser RF, Cantwell AM, Johnson PMC, et al. Effects of two forms of vitamin D supplementation on bone metabolism and infant growth [Efecto de dos formas de suplementación de vitamina D en el crecimiento y metabolismo óseo del lactante]. Chilean Journal of Pediatrics [Revista Chilena de Pediatría] 1996;67(5):219-23. [DOI: 10.4067/S0370-41061996000500005 ] [Google Scholar]
Lava 2011 {published data only}
- Lava SAG, Caccia G, Osmetti-Gianini S, Simonetti GD, Milani GP, Falesi M, et al. Acceptance of two liquid vitamin D₃ formulations among mothers with newborn infants: a randomized, single-blind trial. European Journal of Pediatrics 2011;170(12):1559-62. [DOI: 10.1007/s00431-011-1477-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Manaseki Holland 2010 {published data only (unpublished sought but not used)}61245920
- ISRCTN61245920. The effect of vitamin D supplementation at diagnosis of pneumonia upon response to treatment. www.isrctn.com/ISRCTN61245920 (first received 27 February 2007). [DOI: 10.1186/ISRCTN61245920] [DOI]
- Manaseki-Holland S, Qader G, Isaq Masher M, Bruce J, Zulf Mughal M, Chandramohan D, et al. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Tropical Medicine & International Health 2010;15(10):1148-55. [DOI: 10.1111/j.1365-3156.2010.02578.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Maroof Z, Manaseki-Holland S, Masher MI, Bruce J. Effects of vitamin D supplementation to children admitted to hospital for pneumonia in Kabul: a randomised controlled trial. Tropical Medicine & International Health 2009;14(Suppl 2):145. [DOI: 10.1111/j.1365-3156.2009.02354_1.x] [Abstract #5.1-138] [DOI] [PubMed] [Google Scholar]
Manaseki‐Holland 2012 {published data only}
- Aluisio AR, Maroof Z, Chandramohan D, Bruce J, Masher MI, Manaseki-Holland S, et al. Risk factors associated with recurrent diarrheal illnesses among children in Kabul, Afghanistan: a prospective cohort study. Plos One 2015;10(2):e0116342. [DOI: 10.1371/journal.pone.0116342] [PMC4332656] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaseki-Holland S, Maroof Z, Bruce J, Mughal MZ, Masher MI, Bhutta ZA, et al. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. Lancet 2012;379(9824):1419-27. [DOI: 10.1016/S0140-6736(11)61650-4] [PMC3348565] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroof Z, Manaseki-Holland S, Chandramohan D, Bhutta Z, Mughal Z, Bruce J, et al. RCT methodology, population profile and pneumonia rates in socio-economically deprived infants of Kabul: from an RCT investigating the effects of vitamin D supplementation on the incidence of infant pneumonia. Tropical Medicine & International Health 2009;14(Suppl 2):145-6. [DOI: 10.1111/j.1365-3156.2009.02354_1.x] [Abstract #5.1-139] [DOI] [Google Scholar]
- Maroof Z. The Effects of Vitamin D Supplementation on the Incidence of Pneumonia in Infants and Young Children in Kabul, Afghanistan: A Double Blind Randomized Controlled Trial [PhD thesis]. London (UK): London School of Hygiene & Tropical Medicine, 2011. [Google Scholar]
- NCT00548379. Kabul vitamin D supplementation trial [The effect of vitamin D supplementation on the incidence of pneumonia in children in Afghanistan: a randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT00548379 (first received 23 October 2007).
- Zabihullah Z, Manaseki-Holland S, Chandramohan D, Bhutta Z, Mughal Z. RCT methodology, population profile and pneumonia rates in socio-economically deprived infants of Kabul: data from an RCT investigating the effects of vitamin D supplementation on the incidence of infant pneumonia. International Journal of Infectious Diseases 2010;14(1):e421-2. [DOI: 10.1016/j.ijid.2010.02.557] [Abstract #80.019] [DOI] [Google Scholar]
Marchisio 2013 {published data only}
- Marchisio P, Consonni D, Baggi E, Zampiero A, Bianchini S, Terranova L, et al. Vitamin D supplementation reduces the risk of acute otitis media in otitis-prone children. Pediatric Infectious Disease Journal 2013;32(10):1055-60. [DOI: 10.1097/INF.0b013e31829be0b0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mathur 2016 {published data only}
- CTRI/2018/02/012058. Evaluating sufficiency of oral vitamin D dose in babies with birth weight less than 1500 g born prematurely before 37 weeks of gestation [Assessment of adequacy of supplementation of vitamin D in very low birth weight pre term neonates]. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=6491&EncHid=&userName=mathur (first received 21 February 2018).
- Mathur NB, Saini A, Mishra TK. Assessment of adequacy of supplementation of vitamin D in very low birth weight preterm neonates: a randomized controlled trial. Journal of Tropical Pediatrics 2016;62(6):429-35. [DOI: 10.1093/tropej/fmv110] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mittal 2014 {published data only}
- Jacobs B. Is single oral dose of 300,000 IU vitamin D3 adequate for treatment of nutritional rickets? Indian Pediatrics 2014;51(4):260-1. [PMID: ] [PubMed] [Google Scholar]
- Mittal H, Rai S, Shah D, Madhu SV, Mehrotra G, Malhotra RK, et al. 300,000 IU or 600,000 IU of oral vitamin D3 for treatment of nutritional rickets: a randomized controlled trial. Indian Pediatrics 2014;51(4):265-72. [DOI: 10.1007/s13312-014-0399-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Pettifor JM. How should we manage vitamin D deficiency rickets? Indian Pediatrics 2014;51(4):259-60. [DOI: 10.1007/s13312-014-0388-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mittal 2018 {published data only}
- CTRI/2017/02/007791. A study on children suffering from nutritional rickets aged 6 months to 5 years comparing a lower dose of vitamin D given as a single oral dose for treatment (90,000 IU) with the standard dose (3,00,00 IU) [To compare the efficacy of 90,000 IU and 3,00,00 IU oral single dose vitamin D for treatment of nutritional rickets in children aged 6 months to 5 years—a randomized controlled trial]. ctri.nic.in/Clinicaltrials/showallp.php?mid1=10170&EncHid=&userName=007791 (first received 6 February 2017).
- Mittal M, Yadav V, Khadgawat R, Kumar M, Sherwani P. Efficacy and safety of 90,000 IU versus 300,000 IU single dose oral vitamin D in nutritional rickets: a randomized controlled trial. Indian Journal of Endocrinology and Metabolism 2018;22(6):760-5. [DOI: 10.4103/ijem.IJEM_84_18] [PMC6330863] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal M, Yadav V, Khadgawat R, Kumar M, Sherwani P. Single dose oral vitamin D3 90,000 IU is safe and as effective as 300,000 IU in treatment of nutritional rickets in children. EBSCO Abstracts 2016;21:51. [Google Scholar]
Moodley 2015 {published data only}
- Moodley A, Qin M, Spector S. Vitamin D supplementation at birth boosts immune response to Bacille Calmette Guerin (BCG) in infants. In: Pediatric Academic Societies Annual Meeting; 2013 May 4-7; Washington (DC). 2013.
- Moodley A, Qin M, Spector S. Vitamin D supplementation at birth corrects vitamin D deficiency in infants born in Tijuana, Mexico. In: Pediatric Academic Societies Annual Meeting; 2013 May 4-7; Washington (DC). 2013.
- Moodley A, Spector SA. Single high-dose vitamin D at birth corrects vitamin D deficiency in infants in Mexico. International Journal of Food Sciences and Nutrition 2015;66(3):336-41. [DOI: 10.3109/09637486.2014.992006] [PMC4648545] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodley A, Spector SA. Single high-dose vitamin D at birth corrects vitamin D deficiency in infants in Mexico. International Journal of Food Sciences and Nutrition 2015;66(3):336-41. [DOI: 10.3109/09637486.2014.992006] [PMC4648545] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01288950. Vitamin D supplementation enhances immune response to Bacille-Calmette-Guerin (BCG) vaccination in infants (BCG-25-D) [Vitamin D supplementation enhances immune response to BCG vaccination in infants]. clinicaltrials.gov/ct2/show/NCT01288950 (first received 1 February 2011).
Morawa 1963 {published data only}
- Morawa A. Prevention of Rickets in Premature Infants [Beitrag zur Rachitisprophylaxe bei Fruhgeborenen] [Doctoral Thesis]. Gelsenkirchen: Rheinische Friedrich-Wilhelms Universität zu Bonn, 1963. [Google Scholar]
Natarajan 2014 {published data only}
- CTRI/2012/02/002459. Efficacy of different regimens of vitamin D supplementation in preterm infants - a randomized double blind trial. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=3299&EncHid=&modid=&compid=%27,%273299det%27 (first received 27 February 2012).
- Kumar C, Sankar M, Agarwal R, Pratap O, Jain V, Gupta N. Daily vitamin D supplementation with 800 IU vs 400 IU in preterm infants: a randomized trial. In: Pediatric Academic Societies 2013 Annual Meeting; 2013 May 04-07; Washington (DC). 2013.
- Natarajan CK, Sankar MJ, Agarwal R, Pratap OT, Jain V, Gupta N, et al. Trial of daily vitamin D supplementation in preterm infants. Pediatrics 2014;133(3):e628-34. [DOI: 10.1542/peds.2012-3395] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pehlivan 2003 {published data only}
- Pehlivan I, Hatun Ş, Aydoǧan M, Babaoǧlu K, Gökalp AS. Maternal vitamin D deficiency and vitamin D supplementation in healthy infants. Turkish Journal of Pediatrics 2003;45(4):315-20. [PMID: ] [PubMed] [Google Scholar]
Ponnapakkam 2010 {published data only}
- Bradford EM, Gensure R, Ponnapakkam I. Vitamin D supplementation in the breastfed infants: preliminary results of a prospective trial. Journal of Investigative Medicine 2010;58(2):448. [DOI: 10.1002/jbmr.5650231305] [Abstract #327] [DOI] [Google Scholar]
- Ponnapakkam T, Bradford E, Gensure R. A treatment trial of vitamin D supplementation in breast-fed infants: universal supplementation is not necessary for rickets prevention in Southern Louisiana. Clinical Pediatrics 2010;49(11):1053-60. [DOI: 10.1177/0009922810376320] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ponnapakkam T, Bradford E, Gensure R. Vitamin D supplementation in breastfed infants: results of a prospective trial in the Southern United States. Journal of Bone and Mineral Research 2010;25(Suppl 1):S232. [DOI: 10.1002/jbmr.5650251304] [Abstract #SU0026] [DOI] [Google Scholar]
- Ponnapakkam T, Bradford E, Gensure R. Vitamin D supplementation in the breastfed infants: preliminary results of a prospective trial. Hormone Research 2009;72(Suppl 3):198. [DOI: 10.1159/000239668)] [Abstract #LB-PO1-007] [DOI] [Google Scholar]
- Ponnapakkam T, Bradford E, Gensure RC. Vitamin D supplementation in breast fed infants: a prospective trial-recruitment problem encountered along the way. Journal of Bone and Mineral Research 2008;23(Suppl 1):S371. [DOI: 10.1002/jbmr.5650231305] [Abstract #SU500] [DOI] [Google Scholar]
Principi 2013 {published data only}
- Principi N, Eposito S. Vitamin D and influenza vaccination—reply. Human Vaccines & Immunotherapeutics 2013;9(5):976. [DOI: 10.4161/hv.23848] [PMC3899165] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Principi N, Marchisio P, Terranova L, Zampiero A, Baggi E, Daleno C, et al. Impact of vitamin D administration on immunogenicity of trivalent inactivated influenza vaccine in previously unvaccinated children. Human Vaccines & Immunotherapeutics 2013;9(5):969-74. [DOI: 10.4161/hv.23540] [PMC3899163] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiwanitkit V. Vitamin D and influenza vaccination—comment. Human Vaccines & Immunotherapeutics 2013;9(5):975. [DOI: 10.4161/hv.23847] [PMC3899164] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rao 2016 {published data only}
- Rao YK, Midha T, Singh S, Bajpai A, Tilak A. Increment in vitamin D level and bone mineral accrual in children with vitamin D deficiency. Korean Journal of Pediatrics 2016;59(7):292-7. [DOI: 10.3345/kjp.2016.59.7.292] [PMC5007424] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rianthavorn 2013 {published and unpublished data}
- Rianthavorn P, Boonyapapong P. Ergocalciferol decreases erythropoietin resistance in children with chronic kidney disease stage 5. Pediatric Nephrology 2013;28(8):1261-6. [DOI: 10.1007/s00467-013-2431-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Robinson 1981 {published data only}
- Robinson MJ, Merrett AL, Tetlow VA, Compston JE. Plasma 25-hydroxyvitamin D concentrations in preterm infants receiving oral vitamin D supplements. Archives of Disease in Childhood 1981;56(2):144-5. [DOI: 10.1136/adc.56.2.144] [PMC1627110] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodd 2011 {published data only (unpublished sought but not used)}
- NCT00846677. Study comparing two vitamin D supplements for infants: liquid versus D-strips [A randomized, open-label, cross-over study comparing two vitamin D supplements for infants: liquid versus D-strips]. clinicaltrials.gov/ct2/show/study/NCT00846677 (first received 17 February 2009).
- Rodd C, Jean-Philippe S, Vanstone C, Weiler H. Comparison of 2 vitamin D supplementation modalities in newborns: adherence and preference. Applied Physiology, Nutrition, and Metabolism 2011;36(3):414-18. [DOI: 10.1139/h11-018] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Rodd C, Jean-Philippe S, Vanstone C, Weiler H. Comparison of parent-perceived acceptance of two vitamin D supplementation modalities in newborn infants. Pediatrics and Child Health 2010;15(Suppl A):68A-9A. [DOI: 10.1093/pch/15.suppl_A.68Ab] [Abstract #183] [DOI] [Google Scholar]
Rosendahl 2018 {published data only}
- Enlund-Cerullo M, Koljonen L, Holmlund-Suila E, Hauta-Alus H, Rosendahl J, Valkama S, et al. Genetic variation of the vitamin D binding protein affects vitamin D status and response to supplementation in infants. Journal of Clinical Endocrinology & Metabolism 2019;104(11):5483-98. [DOI: 10.1210/jc.2019-00630] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Enlund-Cerullo M, Valkama S, Holmlund-Suila E, Rosendahl J, Hauta-Alus H, Viljakainen H, et al. No severe hypercalcemia during a 12-month high-dose vitamin D intervention in infants. Hormone Research in Paediatrics 2016;86(Suppl 1):170. [DOI: 10.1159/000449142] [Abstract #P1-P117] [DOI] [PubMed] [Google Scholar]
- Helve O, Viljakainen H, Homlund-Suila E, Rosendahl J, Hauta-lus H, Enlund-Cerullo M, et al. Towards evidence-based vitamin D supplementation in infants: vitamin D intervention in infants (VIDI)—study design and methods of a randomised controlled double-blinded intervention study. BMC Pediatrics 2017;17(1):91. [DOI: 10.1186/s12887-017-0845-5] [PMC5372327] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Lin X, Yuan LQ. Vitamin D3 supplementation and bone health—comment. JAMA 2018;172(12):1202. [DOI: 10.1001/jamapediatrics.2018.3349] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT01723852. Vitamin D intervention in infants (VIDI) [Vitamin D intervention in infants]. clinicaltrials.gov/ct2/show/NCT01723852 (first received 6 November 2012).
- Rosendahl J, Pelkonen AS, Helve O, Hauta-Alus H, Holmlund-Suila E, Valkama S, et al. High-dose vitamin D supplementation does not prevent allergic sensitization of infants. Journal of Pediatrics 2019;209:139-45.e1. [DOI: 10.1016/j.jpeds.2019.02.021] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Rosendahl J, Valkama S, Andersson S. Vitamin D3 supplementation and bone health—reply. JAMA Pediatrics 2018;172(12):1202-3. [DOI: 10.1001/jamapediatrics.2018.3357] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Rosendahl J, Valkama S, Holmlund-Suila E, Enlund-Cerullo M, Hauta-alus H, Helve O, et al. Effect of higher vs standard dosage of vitamin D3 supplementation on bone strength and infection in healthy infants: a randomized clinical trial. JAMA Pediatrics 2018;172(7):646-54. [DOI: 10.1001/jamapediatrics.2018.0602] [PMC6137511] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkama S, Enlund-Cerullo M, Holmlund-Suila E, Rosendahl J, Hauta-alus H, Helve O, et al. No severe hypercalcemia during a high-dose vitamin D3 intervention in infants. In: 55th Annual Meeting of the European Society for Paediatric Endocrinology (ESPE); 2016 Sep 10-12; Paris (FR). Paris (FR): Hormone Research in Paediatrics, 2016.
- Valkama S, Holmlund-Suila E, Enlund-Cerullo M, Rosendahl J, Hauta-Alus H, Helve O, et al. No severe hypercalcemia with daily vitamin D3 supplementation of up to 30 µg during the first year of life. Hormone Research in Paediatrics 2017;88(2):147-54. [DOI: 10.1159/000477298] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rueter 2019 {published data only}
- ACTRN12606000281594. Infant fish oil study [The immunomodulatory effects of omega-3 polyunsaturated fatty acids: role in allergy prevention]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=1438 (first received 29 June 2006).
- Rueter K, Jones A, Siafarikas A, Bear N, Lim EM, Noakes P, et al. The effect of vitamin D and UV-light exposure on immune development in infancy. Allergy: European Journal of Allergy & Clinical Immunology 2019;74(Suppl 106):241. [DOI: 10.1111/all.13959] [Abstract #PD0430] [DOI] [PubMed] [Google Scholar]
- Rueter K, Jones A, Siafarikas A, Lim EM, Prescott SL, Palmer DJ. The influence of vitamin D and UV-light exposure on the developing immune phenotype in infancy. Internal Medicine Journal 2016;46(Suppl 4):21-2. [DOI: 10.1111/imj.55_13197] [Abstrcat #ASCIA-P55] [DOI] [Google Scholar]
- Rueter K, Jones AP, Siafarikas A, Lim EM, Bear N, Noakes PS, et al. Direct infant UV light exposure is associated with eczema and immune development. Journal of Allergy and Clinical Immunology 2019;143(3):1012-20.e2. [DOI: 10.1016/j.jaci.2018.08.037] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Rueter K, Jones AP, Siafarikas A, Lim EM, Clarke MW, Noakes PS, et al. The effect of vitamin D and sunlight exposure on immune development in the first 6 months of life. Internal Medicine Journal 2018;48(Suppl 6):29. [DOI: 10.1111/imj.8_14078] [Abstract #R8] [DOI] [Google Scholar]
Saad 2015 {published data only (unpublished sought but not used)}
- Saad K, Abd Aziz NHR, El-Houfey AA, El-Asheer O, Mohamed SAA, Ahmed AE, et al. Trial of vitamin D supplementation in infants with bronchiolitis: a randomized, double-blind, placebo-controlled study. Pediatric Allergy, Immunology, and Pulmonology 2015;28(2):102-6. [DOI: 10.1089/ped.2015.0492] [DOI] [Google Scholar]
Saleem 2018 {published data only}
- NCT03170479. Developmental screening and nutritional intervention of severe acute malnourished children in Southern Punjab, Pakistan. clinicaltrials.gov/ct2/show/NCT03170479 (first received 16 October 2016).
- Saleem J, Zakar R, Zakar MZ, Belay M, Rowe M, Timms PM, et al. High-dose vitamin D3 in the treatment of severe acute malnutrition: a multicenter double-blind randomized controlled trial. American Journal of Clinical Nutrition 2018;107(5):725-33. [DOI: 10.1093/ajcn/nqy027] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sánchez‐Armendáriz 2018 {published and unpublished data}
- Sánchez-Armendáriz K, García-Gil A, Romero CA, Contreras-Ruiz J, Karam-Orante M, Balcazar-Antonio D, et al. Oral vitamin D3 5000 IU/day as an adjuvant in the treatment of atopic dermatitis: a randomized control trial. International Journal of Dermatology 2018;57(12):1516-20. [DOI: 10.1111/ijd.14220] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sarhan 2019 {published data only (unpublished sought but not used)}
- NCT03799406. Vitamin D supplementation for acute bronchiolitis [Does oral vitamin D supplementation in Egyptian infants with acute bronchiolitis improve the outcome? A double blind randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT03799406 (first received 29 December 2018).
- Sarhan AA, Saeed NM, Mostafa AA, Osman AM. Vitamin D supplementation for acute bronchiolitis: a double-blind randomized controlled trial. Alexandria Journal of Pediatrics 2019;32(2):61-7. [DOI: 10.4103/AJOP.AJOP_28_19] [DOI] [Google Scholar]
Shajari 2009 {published data only}
- Shajari A, Shakiba M, Nourani F, Zaki M, Kheirandish M. Urinary calcium/creatinine ratio with different dosages of vitamin D3 prophylaxis in infants. Iran Journal of Pediatrics 2009;19(2):159-63. [www.bioline.org.br/pdf?pe09025] [Google Scholar]
Shakiba 2010 {published data only}
- Shakiba M, Sadr S, Nefei Z, Mozaffari-Khosravi H, Lotfi MH, Bemanian MH. Combination of bolus dose vitamin D with routine vaccination in infants: a randomised trial. Singapore Medical Journal 2010;51(5):440-5. [PMID: ] [PubMed] [Google Scholar]
Shedeed 2012 {published data only}
- Shedeed SA. Vitamin D supplementation in infants with chronic congestive heart failure. Pediatric Cardiology 2012;33(5):713-9. [DOI: 10.1007/s00246-012-0199-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Siafarikas 2011 {published data only}
- ACTRN12609000919213. Optimizing vitamin D supplementation and sun exposure for breastfed infants in Berlin, Germany [An assessment of the minimal amount of vitamin D supplementation and sun exposure required to provide a sufficient vitamin D status in breastfed infants during their first 6 weeks of life]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=320733 (first received 22 October 2009).
- Siafarikas A, Piazena H, Feister U, Bulsara MK, Meffert H, Hesse V. Randomised controlled trial analysing supplementation with 250 versus 500 units of vitamin D3, sun exposure and surrounding factors in breastfed infants. Archives of Disease in Childhood 2011;96(1):91-5. [DOI: 10.1136/adc.2009.178301] [PMID: ] [DOI] [PubMed] [Google Scholar]
Singh 2018a {published data only}
- Singh H, Maria A, Shukla A, Sharma N, Mani K, Das MK. Relation of vitamin D supplementation, up to six months of age with total and bone specific alkaline phosphatase: a randomised control trial. Journal of Clinical and Diagnostic Research 2018;12(10):SC01-5. [DOI: 10.7860/JCDR/2018/34751.12095] [DOI] [Google Scholar]
- Singh H, Shukla A, Maria A, Mani K. Daily supplementation with 400 IU vitamin D in term breast fed infants from 0-6 months and changes in total and bone specific alkaline phosphatase--an RCT. European Journal of Pediatrics 2017;176(11):1528. [DOI: 10.1007/s00431-017-2979-8] [Abstract #456] [DOI] [Google Scholar]
Singh 2019 {published data only}
- Singh N, Kamble D, Mahantshetti NS. Effect of vitamin D supplementation in the prevention of recurrent pneumonia in under-five children. Indian Journal of Pediatrics 2019;86(12):1105-11. [DOI: 10.1007/s12098-019-03025-z] [PMID: ] [DOI] [PubMed] [Google Scholar]
Somnath 2017 {published data only}
- CTRI/2014/09/005032. Vitamin D in pneumonia in under five children--a randomized controlled trial [Outcome of severe lower respiratory infection in under-five children with vitamin D supplementation]. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=9095&EncHid=&modid=&compid=%27,%279095det%27 (first received 18 September 2014).
- Somnath SH, Biswal N, Chandrasekaran V, Jagadisan B, Bobby Z. Therapeutic effect of vitamin D in acute lower respiratory infection: a randomized controlled trial. Clinical Nutrition ESPEN 2017;20:24-8. [DOI: 10.1016/j.clnesp.2017.02.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Specker 1992 {published data only}
- Specker BL, Ho ML, Oestreich A, Yin TA, Shui QM, Chen XC, et al. Prospective study of vitamin D supplementation and rickets in China. Journal of Pediatrics 1992;120(5):733-9. [DOI: 10.1016/s0022-3476(05)80236-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stögmann 1985 {published data only}
- Stögmann W, Sacher M, Blümel P, Woloszczuk W. Vitamin D deficiency rickets: single-dose therapy versus continuous therapy. Padiatrie und Padologie 1985;20(4):385-92. [PMID: ] [PubMed] [Google Scholar]
Tang 2019 {published and unpublished data}
- ChiCTR-INR-16009235. Effects of vitamin D in children with juvenile idiopathic arthritis (JIA) about the level of serum interleukin and tumor necrosis factor alpha [维生素D对幼年特发性关节炎(JIA)患儿血清白介素及肿瘤坏死因子α水平的影响]. www.chictr.org.cn/hvshowproject.aspx?id=10921 (first received 21 September 2016).
- Tang T, Zhang Y, Luo C, Liu M, Xu L, Tang X. Adjunctive vitamin D for the treatment of active juvenile idiopathic arthritis: an open-label, prospective, randomized controlled trial. Experimental and Therapeutic Medicine 2019;18(6):4921-6. [DOI: 10.3892/etm.2019.8133] [PMC6880388] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tergestina 2016 {published data only}
- CTRI/2012/11/003154. Comparison of the efficacy of two regimens of vitamin D supplementation in preventing vitamin D deficiency among preterm infants [A randomized double blind controlled trial comparing two regimens of vitamin D supplementation in preterm neonates]. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=5533&EncHid=&modid=&compid=%27,%275533det%27 (first received 29 November 2012).
- Tergestina M, Rebekah G, Job V, Simon A, Thomas N. A randomized double-blind controlled trial comparing two regimens of vitamin D supplementation in preterm neonates. Journal of Perinatology 2016;36(9):763-7. [DOI: 10.1038/jp.2016.70] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thacher 2014 {published and unpublished data}
- NCT00949832. Vitamin D and genetics in nutritional rickets. clinicaltrials.gov/ct2/show/NCT00949832 (first received 29 July 2009).
- Thacher TD, Fischer PR, Pettifor JM. Vitamin D treatment in calcium-deficiency rickets: a randomised controlled trial. Archives of Disease in Childhood 2014;99(9):807-11. [DOI: 10.1136/archdischild-2013-305275] [PMC4145444] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomimoto 2018 {published data only (unpublished sought but not used)}
- JMA-IIA00243. A study of vitamin D supplementation in breast fed infant with vitamin D deficiency. dbcentre3.jmacct.med.or.jp/JMACTR/App/JMACTRS06/JMACTRS06.aspx?seqno=5784 (first received 04 February 2016).
- Tomimoto K. A study on appropriate vitamin D supplementation in vitamin D-deficient breastfed infants. Journal of the Japan Pediatric Society 2018;122(11):1683-91. [www.jpeds.or.jp/journal/abstract/122-11e.html] [Google Scholar]
Trilok‐Kumar 2011 {published and unpublished data}
- Kumar GT, Sachdev HPS, Chellani H, Rehman AM, Singh V, Arora H, et al. Effect of weekly vitamin D supplements to Indian low birth weight term infants on mortality, morbidity, and growth in the first 6 months of life: a randomised controlled trial. In: Proceedings of the Nutrition Society. Vitamins in Early Development and Healthy Ageing: Impact on Infectious and Chronic Disease. Joint Irish Section and American Society for Nutrition Meeting, 70th Anniversary. 2011 Jun 15-17. Cork. Vol. 70. 2011:E98. [DOI: 10.1017/S0029665111001388] [DOI]
- NCT00415402. Effect of vitamin D supplementation on health of low birth weight infants (DIVIDS) [Randomised controlled trial to evaluate the preventive effect on mortality and serious morbidity/ Hospitalisations of daily vitamin D supplements in small for gestational age term infants]. clinicaltrials.gov/ct2/show/NCT00415402 (first received 21 December 2006).
- Trilok-Kumar G, Arora H, Rajput M, Chellani H, Singh V, Raynes J, et al. Effect of vitamin D supplementation of low birth weight term Indian infants from birth on cytokine production at 6 months. European Journal of Clinical Nutrition 2012;66(6):746-50. [DOI: 10.1038/ejcn.2012.33] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Trilok-Kumar G, Kaur M, Rehman AM, Arora H, Rajput MM, Chugh R, et al. Effects of vitamin D supplementation in infancy on growth, bone parameters, body composition and gross motor development at age 3-6 years: follow-up of a randomized controlled trial. International Journal of Epidemiology 2015;44(3):894-905. [DOI: 10.1093/ije/dyv116] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Trilok-Kumar G, Sachdev HS, Chellani H, Rehman AM, Singh V, Arora H, et al. Effect of weekly vitamin D supplements on mortality, morbidity, and growth of low birthweight term infants in India up to age 6 months: randomised controlled trial. BMJ 2011;342:d2975. [DOI: 10.1136/bmj.d2975] [PMC3104477] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Willi 1959 {published data only}
- Willi H. On the prophylaxis of rickets in premature infants [Zur Prophylaxe der Frühgeborenenrachitis]. Helvetica Paediatrica Acta 1959;14:351-71. [PMID: ] [PubMed] [Google Scholar]
Zeghoud 1994 {published data only}
- Zeghoud F, Ben-Mekhbi H, Djeghri N, Garabédian M. Vitamin D prophylaxis during infancy: comparison of the long-term effects of three intermittent doses (15, 5, or 2.5 mg) on 25-hydroxyvitamin D concentrations. American Journal of Clinical Nutrition 1994;60(3):393-6. [DOI: 10.1093/ajcn/60.3.393] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ziegler 2014 {published data only}
- NCT00494104. Prevention of vitamin D deficiency [Prevention of vitamin D deficiency in breastfed infants]. clinicaltrials.gov/ct2/show/NCT00494104 (first received 28 June 2007).
- Ziegler EE, Koo WW, Nelson SE, Jeter JM. Lack of effect of graded doses of vitamin D on bone metabolism of breastfed infants. Journal of Clinical Nutrition and Metabolism 2017;1(1):105. [PMC5744599] [PMID: ] [PMC free article] [PubMed] [Google Scholar]
- Ziegler EE, Nelson SE, Jeter JM. Vitamin D supplementation of breastfed infants: a randomized dose-response trial. Pediatric Research 2014;76(2):177-83. [DOI: 10.1038/pr.2014.76] [PMC4104134] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abrams 2013 {published data only (unpublished sought but not used)}
- Abrams S, Hawthorne K, Chen Z. Vitamin D3 supplementation leads to increased total calcium absorption in 4 to 8 yr old children. FASEB Journal 2012;26(Suppl 1):365.1. [DOI: 10.1096/fasebj.26.1_supplement.365.1] [DOI] [Google Scholar]
- Abrams SA, Hawthorne KM, Chen Z. Supplementation with 1000 IU vitamin D/d leads to parathyroid hormone suppression, but not increased fractional calcium absorption, in 4-8-y-old children: a double-blind randomized controlled trial. American Journal of Clinical Nutrition 2013;97(1):217-23. [DOI: 10.3945/ajcn.112.046102] [PMC3522137] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00868738. Evaluation of calcium, vitamin D, magnesium, and zinc absorption in children [Evaluation of calcium, vitamin D, magnesium, and zinc absorption in healthy children 4 to 8 years of age]. clinicaltrials.gov/ct2/show/NCT00868738 (first received 24 March 2009).
Atkinson 2017 {published data only (unpublished sought but not used)}
- Atkinson MA, Juraschek SP, Bertenthal MS, Detrick B, Furth SL, Miller ER 3rd. Pilot study of the effect of cholecalciferol supplementation on hepcidin in children with chronic kidney disease: results of the D-fense trial. Pediatric Nephrology 2017;32(5):859-68. [DOI: 10.1007/s00467-016-3563-6] [PMC5735842] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01532349. Vitamin D as a modifier of serum hepcidin in children with chronic kidney disease (D-fense) [Cholecalciferol as a modifier of serum hepcidin in children with chronic kidney disease]. clinicaltrials.gov/ct2/show/NCT01532349 (first received 8 February 2012).
Camargo 2014 {published data only (unpublished sought but not used)}
- Camargo CA Jr, Ganmaa D, Sidbury R, Erdenedelger Kh, Radnaakhand N, Khandsuren B. Randomized trial of vitamin D supplementation for winter-related atopic dermatitis in children. Journal of Allergy and Clinical Immunology 2014;134(4):831-5.e1. [DOI: 10.1016/j.jaci.2014.08.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT00879424. Vitamin D supplementation in childhood atopic dermatitis [Randomized trial of vitamin D supplementation in winter-related, childhood atopic dermatitis]. clinicaltrials.gov/ct2/show/NCT00879424 (first received 9 April 2009).
Dehbroki 2019 {published data only (unpublished sought but not used)}
- Dehbokri N, Noorazar G, Ghaffari A, Mehdizadeh G, Sarbakshsh P, Ghaffary S. Effect of vitamin D treatment in children with attention-deficit hyperactivity disorder. World Journal of Pediatrics 2019;15(1):78-84. [DOI: 10.1007/s12519-018-0209-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
- IRCT2017012826998N2. Effect of vitamin D deficiency treatment on children with attention deficit hyperactivity disorder (ADHD). en.irct.ir/trial/22244 (first received 1 March 2017).
Galli 2015 {published data only (unpublished sought but not used)}
- Galli E, Rocchi L, Carella R, Giampietro PG, Panei P, Meglio P. Serum vitamin D levels and vitamin D supplementation do not correlate with the severity of chronic eczema in children. European Annals of Allergy and Clinical Immunology 2015;47(2):41-7. [www.eurannallergyimm.com/cont/journals-articles/358/volume-serum-vitamin-levels-supplementation-correlate-951allasp1.pdf] [PMID: ] [PubMed] [Google Scholar]
Gottschlich 2017 {published data only (unpublished sought but not used)}
- Gottschlich MM, Mayes T, Khoury J, Kagan R. Differential effects of three vitamin D supplementation practices on clinical outcome postburn. Journal of Burn Care & Research 2011;32(Suppl 2):S73. [DOI: 10.1097/BCR.0b013e318211ec89] [DOI] [Google Scholar]
- Gottschlich MM, Mayes T, Khoury J, Kagan RJ. Clinical trial of vitamin D2 vs D3 supplementation in critically ill pediatric burn patients. JPEN. Journal of Parenteral and Enteral Nutrition 2017;41(3):412-21. [DOI: 10.1177/0148607115587948] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Mayes T, Gottschlich MM, Brunner C, Khoury J, Kagan, RJ. Long-term follow-up fracture incidence in children receiving vitamin D supplementation during the acute phase postburn. Journal of Burn Care & Research 2012;33(Suppl 1):S82. [DOI: 10.1097/BCR.0b013e3182508c98] [DOI] [Google Scholar]
- Mayes T, Gottschlich MM, Khoury J, Kagan RJ. Investigation of bone health subsequent to vitamin D supplementation in children following burn injury. Nutrition in Clinical Practice 2015;30(6):830-7. [DOI: 10.1177/0884533615587720] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT00536276. Evaluation of vitamin D status in children with acute burns (VitaminD) [Vitamin D deficiency in acutely injured pediatric burn patients: incidence, etiology, metabolic sequelae and prevention]. clinicaltrials.gov/ct2/show/NCT00536276 (first received 25 September 2007).
Hamidieh 2016 {published data only (unpublished sought but not used)}
- Hamidieh AA, Sherafatmand M, Mansouri A, Hadjibabaie M, Ashouri A, Jahangard-Rafsanjani Z, et al. Calcitriol for oral mucositis prevention in patients with Fanconi anemia undergoing hematopoietic SCT: a double-blind, randomized, placebo-controlled trial. American Journal of Therapeutics 2016;23(6):e1700-8. [DOI: 10.1097/MJT.0000000000000269] [PMID: ] [DOI] [PubMed] [Google Scholar]
- IRCT201307141030N15. Calcitriol in prevention of mucositis in patients with Fanconi anemia undergoing hematopoietic stem cell transplantation [Efficacy of calcitriol in prevention of high dose chemotherapy induced mucositis in patients with Fanconi anemia undergoing hematopoietic stem cell transplantation]. en.irct.ir/trial/24 (first received 16 July 2013).
Homola 2011 {published data only (unpublished sought but not used)}
- Homola L, Holcikova A, Mikolasek P, Pavelka J. Vitamin D supplementation in children with cystic fibrosis: sunshine, cholecalciferol and ergocalciferol. Journal of Cystic Fibrosis 2011;10(Suppl 1):S76. [DOI: 10.1016/S1569-1993(11)60315-9] [Abstract #302] [DOI] [Google Scholar]
Kakalia 2011 {published data only (unpublished sought but not used)}
- Kakalia S, Sochett E, Assor E, Arneson C, Stephens D, Read S, et al. Vitamin D supplementation and CD4 count in HIV infected children. Canadian Journal of Infectious Diseases and Medical Microbiology 2010;21(3 Suppl B):53B. [Abstract #P152] [Article ID 923563] [Google Scholar]
- Kakalia S, Sochett EB, Stephens D, Assor E, Read SE, Bitnun A. Vitamin D supplementation and CD4 count in children infected with human immunodeficiency virus. Journal of Pediatrics 2011;159(6):951-7. [DOI: 10.1016/j.jpeds.2011.06.010] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT00911664. Vitamin D supplementation and CD4 count in HIV-infected children. clinicaltrials.gov/ct2/show/NCT00911664 (first received 29 May 2009).
Kashif 2014 {published data only (unpublished sought but not used)}
- Kashif M, Rab ZZ, Ahmad J, Hasan A, Mirza S. To compare the effect of iron therapy alone versus combined iron therapy and vitamin D supplementation on cardiovascular functions in children with iron deficiency anemia by echocardiography: a randomized controlled trial. Annals of Pediatric Cardiology 2014;7(Suppl 4):S93. [Abstract #PA 70] [www.annalspc.com/showBackIssue.asp?issn=0974-2069;year=2014;volume=7;issue=4;month=February;supp=Y] [Google Scholar]
Kazemi 2010 {published data only (unpublished sought but not used)}
- Kazemi SAN, Bordbar M, Amirmoghaddami HR, Mousavinasab N. The effect of high dose vitamin D3 on the level of serum alkaline phosphatase, calcium, phosphor and vitamin D3 in children older than 3 years old who were receiving phenobarbital. Journal of Advances in Medical and Biomedical Research 2010;18(72):62-70. [www.sid.ir/en/Journal/ViewPaper.aspx?ID=256376] [Google Scholar]
Kerley 2017 {published data only (unpublished sought but not used)}
- Kerley CP, Elnazir B, Greally P, Coghlan D. Blunted serum 25(OH)D response to vitamin D3 supplementation in children with autism. Nutritional Neuroscience 2020;23(7):537-42. [DOI: 10.1080/1028415X.2018.1529342] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Kerley CP, Power C, Gallagher L, Coghlan D. Lack of effect of vitamin D3 supplementation in autism: a 20-week, placebo-controlled RCT. Archives of Disease in Childhood 2017;102(11):1030-6. [DOI: 10.1136/archdischild-2017-312783] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT02508922. Trial of vitamin D3 supplementation in paediatric autism [Vitamin D3 for autism spectrum disorder: a randomized, double-blind, placebo-controlled trial]. clinicaltrials.gov/ct2/show/NCT02508922 (first received 24 July 2015).
Lal 2018 {published data only (unpublished sought but not used)}
- Lal BB, Alam S, Khanna R, Rawat D. Weekly regimen of vitamin D supplementation is more efficacious than Stoss regimen for treatment of vitamin D deficiency in children with chronic liver diseases. European Journal of Pediatrics 2018;177(6):827-34. [DOI: 10.1007/s00431-018-3123-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Lal BB, Alam S, Khanna R, Rawat D. Weekly regimen of vitamin D supplementation is more efficacious than Stoss regimen for treatment of vitamin D deficiency in children with non-cholestatic chronic liver diseases. Hepatology International 2018;12(Suppl 2):S615. [DOI: 10.1007/s12072-018-9852-3] [Abstract #LATEB-32] [DOI] [PubMed] [Google Scholar]
- Lal BB, Khanna R, Alam S, Rawat D. Weekly regimen of vitamin D supplementation is more effective than Stoss regimen for treatment of hypovitaminosis D in children with chronic liver disease. Journal of Clinical and Experimental Hepatology 2017;7(Suppl 2):S94-5. [DOI: 10.1016/j.jceh.2017.05.170] [DOI] [Google Scholar]
Lara‐Corrales 2019 {published data only (unpublished sought but not used)}
- Lara-Corrales I, Gomez GR, De Los Rios CP, Pope E. Vitamin D in patients with atopic dermatitis: a randomized, double-blinded, placebo-controlled study preliminary analysis. Pediatric Dermatology 2013;30(5):638. [DOI: 10.1111/pde.12241] [DOI] [Google Scholar]
- Lara-Corrales I, Huang CM, Parkin PC, Rubio-Gomez GA, Posso-De Los Rios, Maguire J, et al. Vitamin D level and supplementation in pediatric atopic dermatitis: a randomized controlled trial. Journal of Cutaneous Medicine and Surgery 2019;23(1):44-9. [DOI: 10.1177/1203475418805744] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lee 2018 {published data only (unpublished sought but not used)}
- Lee MT, Kattan M, Fennoy I, Apradi SM, Miller RL, Cremers S, et al. Randomized phase 2 trial of monthly vitamin D to prevent respiratory complications in children with sickle cell disease. Blood Advances 2018;2(9):969-78. [DOI: 10.1182/bloodadvances.2017013979] [PMC5941998] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01443728. Vitamin D for sickle-cell respiratory complications. clinicaltrials.gov/ct2/show/NCT01443728 (first received 27 September 2011).
Loeb 2019 {published data only (unpublished sought but not used)}
- Loeb M, Dang AD, Thiem VD, Thanablan V, Wang B, Nguyen NB, et al. Effect of vitamin D supplementation to reduce respiratory infections in children and adolescents in Vietnam: a randomized controlled trial. Influenza and Other Respiratory Viruses 2019;13(2):176-83. [DOI: 10.1111/irv.12615] [PMC6379634] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01705314. A randomized trial of vitamin D to reduce respiratory infection [Effect of vitamin D3 supplementation to reduce respiratory infections in children and adolescents in Vietnam: a blinded, placebo-controlled randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT01705314 (first received 9 October 2012).
Mazahery 2019 {published data only (unpublished sought but not used)}
- ACTRN12615000144516. Omega-3, vitamin D and autism in children [Effect of vitamin D and omega-3 fatty acid supplements on behavioural measures in children with autism spectrum disorder (ASD): a randomised, double-blind, placebo-controlled trial]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=366358 (first received 29 October 2014).
- Mazahery H, Conlon C, Beck KL, Kruger MC, Stonehouse W, Camargo CA Jr, et al. Vitamin D and omega-3 fatty acid supplements in children with autism spectrum disorder: a study protocol for a factorial randomised, double-blind, placebo-controlled trial. Trials 2016;17(1):295. [DOI: 10.1186/s13063-016-1428-8] [PMC4917935] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazahery H, Conlon CA, Beck KL, Mugridge O, Kruger MC, Stonehouse W, et al. A randomised-controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of core symptoms of autism spectrum disorder in children. Journal of Autism and Developmental Disorders 2019;49(5):1778-94. [DOI: 10.1007/s10803-018-3860-y] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Mazahery H, Conlon CA, Beck KL, Mugridge O, Kruger MC, Stonehouse W, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. Journal of Steroid Biochemistry and Molecular Biology 2019;187:9-16. [DOI: 10.1016/j.jsbmb.2018.10.017] [PMID: ] [DOI] [PubMed] [Google Scholar]
Merrikhi 2018 {published data only (unpublished sought but not used)}
- IRCT2017052221968N2. Effectiveness of vitamin D supplement in prevention of recurrent urinary tract infections in children [Effectiveness of vitamin D supplement in prevention of urinary tract infections in children with recurrent urinary tract infection]. en.irct.ir/trial/19083 (first received 10 August 2017).
- Merrikhi A, Ziaei E, Shahsanai A, Kelishadi R, Maghami-Mehr A. Is vitamin D supplementation effective in prevention of recurrent urinary tract infections in the pediatrics? A randomized triple-masked controlled trial. Advanced Biomedical Research 2018;7:150. [DOI: 10.4103/abr.abr_149_18] [PMC6289001] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morcos 1998 {published data only (unpublished sought but not used)}
- Morcos MM, Gabr AA, Samuel S, Kamel M, El Baz M, El Beshry M, et al. Vitamin D administration to tuberculous children and its value. Bollettino Chimico Farmaceutico 1998;137(5):157-64. [PMID: ] [PubMed] [Google Scholar]
Mortensen 2016 {published data only (unpublished sought but not used)}
- Hauger H, Mølgaard C, Mortensen C, Ritz C, Frøkiær H, Smith TJ, et al. Winter cholecalciferol supplementation at 55°N has no effect on markers of cardiometabolic risk in healthy children aged 4-8 years. Journal of Nutrition 2018;148(8):1261-8. [DOI: 10.1093/jn/nxy080] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hauger H, Ritz C, Mortensen C, Mølgaard C, Metzdorff SB, Frøkiær H, et al. Winter cholecalciferol supplementation at 55°N has little effect on markers of innate immune defense in healthy children aged 4–8 years: a secondary analysis from a randomized controlled trial. European Journal of Nutrition 2019;58(4):1453-62. [DOI: 10.1007/s00394-018-1671-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Mortensen C, Damsgaard CT, Hauger H, Ritz C, Lanham-New SA, Smith TJ, et al. Estimation of the dietary requirement for vitamin D in white children aged 4-8 y: a randomized, controlled, dose-response trial. American Journal of Clinical Nutrition 2016;104(5):1310-7. [DOI: 10.3945/ajcn.116.136697] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Mortensen C, Mølgaard C, Hauger H, Kristensen M, Damsgaard CT. Winter vitamin D3 supplementation does not increase muscle strength, but modulates the IGF-axis in young children. European Journal of Nutrition 2019;58(3):1183-92. [DOI: 10.1007/s00394-018-1637-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT02145195. The effect of vitamin D supplements on vitamin D status, cardiometabolic health and immune defense in Danish children (ODIN Junior) [Food-based solutions for optimal vitamin D nutrition and health through the life cycle (ODIN) Junior]. clinicaltrials.gov/ct2/show/NCT02145195 (first received 20 May 2014).
Muske 2018 {published data only (unpublished sought but not used)}
- CTRI/2015/04/005721. Comparison of two different vitamin D doses for bone strength protection in nephrotic syndrome [Comparison of two vitamin D dosing regimens for osteoprotection in children with new-onset and infrequently relapsing nephrotic syndrome treated with steroids: a randomized trial]. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=11326&EncHid=&modid=&compid=%27,%2711326det%27 (first received 27 April 2015).
- Muske S, Krishnamurthy S, Kamalanathan SK, Rajappa M, Harichandrakumar KT, Sivamurukan P. Effect of two prophylactic bolus vitamin D dosing regimens (1000 IU/day vs 400 IU/day) on bone mineral content in new-onset and infrequently-relapsing nephrotic syndrome: a randomised clinical trial. Paediatrics and International Child Health 2018;38(1):23-33. [DOI: 10.1080/20469047.2017.1319528] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nwosu 2014 {published data only (unpublished sought but not used)}
- Nwosu BU, Maranda L. The effects of vitamin D supplementation on hepatic dysfunction, vitamin D status, and glycemic control in children and adolescents with vitamin D deficiency and either type 1 or type 2 diabetes mellitus. PloS One 2014;9(6):e99646. [DOI: 10.1371/journal.pone.0099646] [PMC4053366] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahmati 2018 {published data only (unpublished sought but not used)}
- Rahmati MB, Nikbakht A, Ahmadi M. Evaluation of the effect of vitamin D on the length of hospital stay in children with gastroenteritis aged 3 months to 14 years admitted to the Pediatric Hospital of Bandar Abbas. Hormozgan Medical Journal 2018;22(3):e86323. [DOI: 10.5812/hmj.86323] [DOI] [Google Scholar]
Saad 2018 {published data only}
- Beale DJ. Letter to the Editor: unreported statistics lead to unverifiable results in study of vitamin D supplementation in children with autism spectrum disorder—comment regarding Saad K, et al (2016). Journal of Child Psychology and Psychiatry 2018;59(1):e1. [DOI: 10.1111/jcpp.12776] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Saad K, Abdel-Rahman A, Elserogy Y, Al-Atram A, El-Houfey A, Othman H, et al. Retraction: randomized controlled trial of vitamin D supplementation in children with autism spectrum disorder. Journal of Child Psychology and Psychiatry 2019;60(6):711. [DOI: 10.1111/jcpp.13076] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Saad K, Abdel-Rahman AA, Elserogy YM, Al-Atram AA, El-Houfey AA, Othman HA-K, et al. Randomized controlled trial of vitamin D supplementation in children with autism spectrum disorder. Journal of Child Psychology and Psychiatry 2018;59(1):20-9. Retraction in: Journal of Child Psychology and Psychiatry 2019;60(6):711. DOI: 10.1111/jcpp.13076; PubMed: 31087556. [DOI: 10.1111/jcpp.12652] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Saad K. Response to letters: randomized controlled trial of vitamin D supplementation in children with autism spectrum disorder—correction and additional information. Journal of Child Psychology and Psychiatry 2018;59(1):e3-e5. [DOI: 10.1111/jcpp.12788] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E. Letter to the author from Editor-in-Chief seeking clarifications. Journal of Child Psychology and Psychiatry 2018;59(1):e2-e3. [DOI: 10.1111/jcpp.12800] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Stevenson J. Letter to the Editor: unreported statistics lead to unverifiable results in study of vitamin D supplementation in children with autism spectrum disorder—Comment regarding Saad K, et al (2016). Journal of Child Psychology and Psychiatry 2018;59(1):e1-e2. [DOI: 10.1111/jcpp.12799] [PMID: ] [DOI] [PubMed] [Google Scholar]
- UMIN000020281. Randomized-controlled trial of vitamin D supplementation in children with autism spectrum disorder. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000023414 (first received 31 December 2015).
Sharma 2017 {published data only (unpublished sought but not used)}
- CTRI/2014/07/004739. Role of vitamin D as an added therapy for type 1 diabetes mellitus patients [Role of oral cholecalciferol as an adjunctive therapy in type 1 diabetes mellitus: a randomized controlled trial]. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=8907&EncHid=&modid=&compid=%27,%278907det%27 (first received 15 July 2014).
- Sharma S, Biswal N, Bethou A, Rajappa M, Kumar S, Vinayagam V. Does vitamin D supplementation improve glycaemic control in children with type 1 diabetes mellitus? A randomized controlled trial. Journal of Clinical and Diagnostic Research 2017;11(9):SC15-7. [DOI: 10.7860/JCDR/2017/27321.10645] [PMC5713820] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shroff 2012 {published data only (unpublished sought but not used)}
- Shroff R, Wan M, Gullett A, Ledermann S, Shute R, Knott C, et al. Ergocalciferol supplementation in children with CKD delays the onset of secondary hyperparathyroidism: a randomized trial. Clinical Journal of the American Society of Nephrology 2012;7(2):216-23. [DOI: 10.2215/CJN.04760511] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Shroff R, Wan M, Gullett A, Ledermann S, Shute R, Knott C, et al. Ergocalciferol supplementation in children with CKD delays the onset of secondary hyperparathyroidism--a randomised, double-blinded placebo controlled study. Pediatric Nephrology 2011;26(9):1634. [DOI: 10.1007/s00467-011-1958-y] [Abstract #PS1-THU-506] [DOI] [PubMed] [Google Scholar]
Siafarikas 2009 {published data only (unpublished sought but not used)}
- Siafarikas A, Pascoe E, Banfiel S, Cherian S, Geddes J, Nemba K, et al. An evidence based approach to vitamin D deficiency in the child and adolescent refugee population of Western Australia (WA). Bone 2009;44(Suppl 1):S89-S90. [DOI: 10.1016/j.bone.2009.01.200] [DOI] [Google Scholar]
Sidbury 2008 {published data only (unpublished sought but not used)}
- Sidbury R, Sullivan AF, Thadhani RI, Camargo CA Jr. Randomized controlled trial of vitamin D supplementation for winter-related atopic dermatitis in Boston: a pilot study. British Journal of Dermatology 2008;159(1):245-7. [DOI: 10.1111/j.1365-2133.2008.08601.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Simon 2016 {published data only}
- Pragathesh P. A Randomized Clinical Trial Comparing the Effects of Three Different Cholecalciferol Supplementation Protocols on Serum 25 Hydroxy Vitamin D Level in Asymptomatic Vitamin D Deficient Children [MD Pediatrics thesis]. Chennai (TN): Christian Medical College Vellore, 2013. [Google Scholar]
- Simon A, Pragathesh P, Priyambada L. An RCT comparing the effect of three different vitamin D supplementation regimens on Se 25 OH Vit D in asymptomatic vit D deficient children. Hormone Research in Paediatrics 2016;86(Suppl 1):176-7. [DOI: 10.1159/000449142] [Abstract #P1-P134] [DOI] [Google Scholar]
Singh 2018b {published data only (unpublished sought but not used)}
- CTRI/2016/10/007405. Comparison of the efficacy of two dosing regimens of vitamin D for bone protection in children with difficult nephrotic syndrome [Comparison of the efficacy of two dosing regimens of vitamin D supplementation for osteoprotection in children with difficult nephrotic syndrome: an open-label randomized clinical trial]. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=12607&EncHid=&modid=&compid=%27,%2712607det%27 (first received 25 October 2016).
- Singh DN, Krishnamurthy S, Kamalanathan SK, Harichandrakumar KT, Sivamurukan P. Three-monthly bolus vitamin D supplements (1000 vs 400 IU/day) for prevention of bone loss in children with difficult-to-treat nephrotic syndrome: a randomised clinical trial. Paediatrics and International Child Health 2018;38(4):251-60. [DOI: 10.1080/20469047.2018.1505589] [PMID: ] [DOI] [PubMed] [Google Scholar]
Suryanto 2018 {published data only (unpublished sought but not used)}
- Suryanto T, Tjahjono HA, Widjajanto E. Levels of 25(OH)D3, IL-2, and C-peptide in children with type 1 diabetes mellitus (T1DM) receiving vitamin D3 supplementation. Journal of Tropical Life Science 2018;8(1):28-36. [DOI: 10.11594/jtls.08.01.06] [DOI] [Google Scholar]
Swangtrakul 2020 {published data only (unpublished sought but not used)}
- Swangtrakul N, Manuyakorn W, Mahachoklertwattana P, Kiewngam P, Sasisakulporn C. Effect of vitamin D on lung function assessed by forced oscillation technique in asthmatic children with vitamin D deficiency: a randomized double-blind placebo-controlled trial. Asian Pacific Journal of Allergy and Immunology 2020 Dec 14 [Epub ahead of print]. [DOI: 10.12932/AP-010519-0553] [PMID: ] [DOI] [PubMed]
- TCTR20170422001. A randomized double-blind placebo controlled trial of vitamin D supplementation in asthmatic children. www.clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=2488 (first received 20 April 2017).
Talaat 2016 {published data only (unpublished sought but not used)}
- Talaat IM, Kamal NM, Alghamdi HA, Alharthi AA, Alsharani MA. A randomized clinical trial comparing 3 different replacement regimens of vitamin D in clinically asymptomatic pediatrics and adolescents with vitamin D insufficiency. Italian Journal of Pediatrics 2016;42(1):106. [DOI: 10.1186/s13052-016-0314-z] [PMC5142392 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tannous 2018 {published data only (unpublished sought but not used)}
- ACTRN12609000874213. This is a randomised study to assess the safety and efficacy of a high dose vitamin D supplement compared to the standard vitamin D therapy in children [A study to assess the safety and efficacy of Stoss therapy (high dose vitamin D supplement) compared to standard daily therapy in children diagnosed with simple vitamin D deficiency]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=320672 (first received 10 October 2009).
- Tannous P, Munns C. A randomized cohort study comparing the safety and effectiveness of high dose vitamin D to standard daily vitamin D therapy in children with vitamin D deficiency. Pediatrics & Therapeutics 2018;8(3):72. [10.4172/2161-0665-C3-058] [Google Scholar]
Udompataikul 2015 {published data only (unpublished sought but not used)}
- NCT02058186. Effect of oral vitamin D supplement on atopic dermatitis; a clinical trial with Staphylococcus aureus colonization determination. clinicaltrials.gov/ct2/show/NCT02058186 (first received 6 February 2014).
- Udompataikul M, Huajai S, Chalermchai T, Taweechotipatr M, Kamanamool N. The effects of oral vitamin D supplement on atopic dermatitis: a clinical trial with Staphylococcus aureus colonization determination. Journal of the Medical Association of Thailand 2015;98 Suppl 9:S23-30. [PMID: ] [PubMed] [Google Scholar]
Wadia 2018 {published data only (unpublished sought but not used)}
- ACTRN12611001177943. Comparison of depot versus daily vitamin D3 for supplementation in refugees in Western Australia [Randomized controlled trial comparing depot and daily vitamin D3 therapy in 0-16 year old refugees in Western Australia looking at the change in 25(OH)D levels as the primary outcome]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=347634 (first received 10 November 2011).
- Wadia U, Cherian S, Thambiran A, Burgner D, Siafarikas A. Randomised controlled trial comparing daily versus depot vitamin D3 for the treatment of vitamin D deficiency in 0-16 year old refugees in Western Australia. Bone 2011;48(Suppl 2):S247. [DOI: 10.1016/j.bone.2011.03.599] [Abstract #PP468-T] [DOI] [Google Scholar]
- Wadia U, Soon W, Chivers P, Thambiran A, Burgner D, Cherian S, et al. Randomised controlled trial comparing daily versus depot vitamin D3 therapy in 0-16-year-old newly settled refugees in western Australia over a period of 40 weeks. Nutrients 2018;10(3):348. [DOI: 10.3390/nu10030348] [PMC5872766] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zulkarnain 2019 {published data only (unpublished sought but not used)}
- Zulkarnain I, Rahmawati YW, Setyaningrum T, Citrashanty I, Aditama L, Avanti C. Vitamin D3 supplementation reduced Staphylococcus aureus colonization in the skin of pediatric patients with atopic dermatitis. European Journal of Pediatric Dermatology 2019;29(3):143-9. [DOI: 10.26326/2281-9649.29.3.2001] [DOI] [Google Scholar]
References to studies awaiting assessment
Bantz 2015 {published data only (unpublished sought but not used)}
- Bantz SK, Dy T, Herzog R. Vitamin D deficiency in a young, atopic pediatric population. Journal of Allergy and Clinical Immunology 2015;135(2 Suppl):AB148. [DOI: 10.1016/j.jaci.2014.12.1421] [Abstract #481] [DOI] [Google Scholar]
Behnamfar 2011 {published data only (unpublished sought but not used)}
- Behnamfar Z, Mehrdad S, Zahra B. Effect of maintenance dose (30000 unit per month) 25-hydroxyvitamin D on upper respiratory tract infection in children of day care center. European Journal of Medical Research 2011;16(Suppl 1):49. [DOI: 10.1186/2047-783X-16-S1-1] [Abstract #641] [DOI] [Google Scholar]
CTRI/2014/04/004574 {published data only (unpublished sought but not used)}
- CTRI/2014/04/004574. A clinical trial to compare the effects of iron therapy alone versus combined iron therapy and vitamin D supplementation on cardiovascular functions in children with iron deficiency anemia [Comparison of effect of iron therapy alone versus combined iron therapy and vitamin Dsupplementation on cardiovascular functions in children with iron deficiency anemia]. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2014/04/004574 (first received 28 April 2014).
CTRI/2015/08/006084 {published data only (unpublished sought but not used)}
- CTRI/2015/08/006084. Effect of two different regimens of vitamin D supplementation on serum vitamin D levels in children [A study to compare the effect of two different regimens of vitamin D supplementation on serum vitamin D levels in children: a randomized controlled trial]. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2015/08/006084 (first received 10 August 2015).
CTRI/2019/02/017374 {published data only (unpublished sought but not used)}
- CTRI/2019/02/017374. Comparison of the efficacy of two doses of vitamin D supplementation to prevent Vitamin D deficiency in healthy term breastfed infants [Efficacy of 800 IU versus 400 IU per day of oral vitamin D3 supplementation on vitamin D sufficiency in healthy, breastfed infants: a randomized controlled trial]. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2019/02/017374 (first received 1 February 2019).
Hagag 2020 {published data only (unpublished sought but not used)}
- Hagag AA, El Frargy MS, Houdeeb HA. Therapeutic value of vitamin D as an adjuvant therapy in neonates with sepsis. Infectious Disorders. Drug Targets 2020;20(4):440-7. [DOI: 10.2174/1871526519666190626141859] [PMID: ] [DOI] [PubMed] [Google Scholar]
IRCT20111206008307N {published data only (unpublished sought but not used)}
- IRCT20111206008307N. Evaluation of effect of different type of vitamin D on biochemical markers of osteopenia of prematurity [Comparison of calcitriol and cholecalciferol on biochemical markers of metabolic bone disease in very low birth weight infants]. en.irct.ir/trial/30580 (first received 10 June 2018).
IRCT20131013014994N5 {published data only (unpublished sought but not used)}
- IRCT20131013014994N5. Effect of vitamin D on autism spectrum disorders [Effect of vitamin D supplementation on core symptoms and serum biomarkers (serotonin and IL-6) in children with autism spectrum disorders]. en.irct.ir/trial/14421 (first received 18 April 2018).
IRCT2014053117843N3 {published data only (unpublished sought but not used)}
- IRCT2014053117843N3. Vitamin D supplementation and its effect on markers of oxidative stress and antioxidant in asthmatic children [Effect of oral vitamin D supplementation on serum markers of oxidative stress and antioxidant in asthmatic children and its comparison with control group]. en.irct.ir/trial/16324?revision=16324 (first received 4 August 2014).
NCT01229189 {published data only (unpublished sought but not used)}
- NCT01229189. Evaluation of the effectiveness of vitamin D supplementation to pregnant women and their infants in Pakistan. clinicaltrials.gov/ct2/show/NCT01229189 (first received 26 October 2010).
NCT01419821 {published data only (unpublished sought but not used)}
- NCT01419821. Vitamin D and its affect on growth rates and bone mineral density until age 5 (VitD) [Supplemental vitamin D administered to one year old vitamin D deficient infants until age 3 and its affect on growth rates and bone mineral density until age 5]. clinicaltrials.gov/ct2/show/NCT01419821 (first received 17 August 2011).
NCT01656070 {published data only (unpublished sought but not used)}
- NCT01656070. Vitamin D supplementation in HIV-infected youth [Vitamin D status and T cell phenotype in HIV-infected youth supplemented with cholecalciferol: a randomized clinical trial]. clinicaltrials.gov/ct2/show/NCT01656070 (first received 31 July 2012).
NCT01724190 {published data only (unpublished sought but not used)}
- NCT01724190. Effect of vitamin D on the honeymoon period in children and adolescents with type 1 diabetes [Effect of Vitamin D supplementation on rate of partial clinical remission in children and adolescents with type 1 diabetes]. clinicaltrials.gov/ct2/show/NCT01724190 (first received 7 November 2012).
NCT02054182 {published data only (unpublished sought but not used)}
- NCT02054182. Effect of vitamin D supplementation in young South African children hospitalized with acute lower respiratory infection [Effect of vitamin D supplementation in young children with acute lower respiratory tract infection at Dr George Mukhari Academic Hospital, Pretoria, South Africa]. clinicaltrials.gov/ct2/show/NCT02054182 (first received 18 January 2014).
NCT02185196 {published data only (unpublished sought but not used)}
- NCT02185196. Vitamin-D supplementation: impact on severe pneumonia among under-five children (Vitamin-D) [Vitamin-D supplementation: impact on severe pneumonia among under-five children]. clinicaltrials.gov/ct2/show/NCT02185196 (first received 22 June 2014).
NCT02186028 {published data only (unpublished sought but not used)}
- NCT02186028. Effect of oral daily supplementation with 400 IU Vs 200 IU of vitamin D in term healthy neonates [Effect of oral daily supplementation with 400 IU Vs 200 IU of vitamin D In term healthy newborns: a randomised control trial]. clinicaltrials.gov/ct2/show/NCT02186028 (first received 7 July 2014).
NCT02936895 {published data only (unpublished sought but not used)}
- NCT02936895. Vitamin D supplementation and respiratory index of severity in children (RISC) in pneumonia [The effects of vitamin D supplementation in Respiratory Index of Severity in Children (RISC) of hospitalized patients with community-acquired pneumonia]. clinicaltrials.gov/ct2/show/NCT02936895 (first received 12 October 2016).
NCT03176849 {published data only (unpublished sought but not used)}
- NCT03176849. A randomized phase IV control trial of single high dose oral vitamin D3 in pediatric patients undergoing HSCT [A randomized phase IV control trial of single high dose oral vitamin D3 (Stoss therapy) in pediatric patients undergoing HSCT to prevent vitamin D deficiency and insufficiency during transplant]. clinicaltrials.gov/ct2/show/NCT03176849 (first received 26 May 2017).
NCT03544671 {published data only (unpublished sought but not used)}
- NCT03544671. Effect of vitamin D3 supplementation in children from 12 to 30 months of age [Effect of vitamin D3 supplementation over 25-hydroxivitamin D (25-OH-D) status in children from 12-30 months of age: randomized clinical trial]. clinicaltrials.gov/ct2/show/NCT03544671 (first received 17 May 2018).
NTR477 {published data only (unpublished sought but not used)}
- ISRCTN23173889. Molecular determinants of bone mineral density (BMD) in children with acute lymphoblastic leukemia (ALL) and the role of intervention by physical activities and calcium and vitamin D supplements as a preventive strategy. www.isrctn.com/ISRCTN23173889 (first received 27 January 2006). [DOI: 10.1186/ISRCTN23173889] [DOI]
- NTR477. Molecular determinants of bone mineral density in children with acute lymphoblastic leukemia and the role of intervention by physical activities and calcium and vitamin D supplement as a preventive strategy [Molecular determinants of bone mineral density in children with acute lymphoblastic leukemia (ALL) and the role of intervention by physical activities and calcium and vitamin D supplement as a preventive strategy]. www.who.int/trialsearch/Trial2.aspx?TrialID=NTR477 (first received 14 September 2005).
Özkan 2000 {published data only (unpublished sought but not used)}
- Özkan B, Büyükavci M, Energin M, Dirican ME, Alp H, Akdağ R. Nutritional rickets: comparison of three different therapeutic approaches (300.000 U p.o., 300.000 U i.m. and 600.000 U p.o.) [Nütrisyonel riketsde farklı tedavi șekillerinin (300.000 U oral, 300.000 U intramusküler ve 600.000 U oral vitamin D) karșılaștırılması]. Çocuk Sağliğı ve Hastalıkları Dergisi 2000;43(1):30-5. [Record #20001412893] [www.cabdirect.org/cabdirect/abstract/20001412893] [Google Scholar]
References to ongoing studies
ACTRN12614000334606/NCT02112734 {published data only}
- Allen KJ, Panjari M, Koplin JJ, Ponsonby A-L, Vuillermin P, Gurrin LC, et al. VITALITY trial: protocol for a randomised controlled trial to establish the role of postnatal vitamin D supplementation in infant immune health. BMJ Open 2015;5(12):e009377. [DOI: 10.1136/bmjopen-2015-009377] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANZCTR12614000334606. Can vitamin D supplementation in infants prevent food allergy in the first year of life? The VITALITY trial [A placebo-controlled, randomised trial of vitamin D supplementation for infants in their first year of life, to prevent the development of food allergy by age 12 months. The VITALITY Trial]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12614000334606 (first received 20 March 2014).
- NCT02112734. Can vitamin D supplementation in infants prevent food allergy in the first year of life? The VITALITY trial (VITALITY) [Can vitamin D supplementation in infants prevent food allergy in the first year of life? The VITALITY trial]. clinicaltrials.gov/ct2/show/NCT02112734 (first received 10 April 2014).
ACTRN12616000659404 {published data only}
- ACTRN12616000659404. PREVARID—PREVention of Acute Respiratory Infections with Vitamin D [Does vitamin D supplementation prevent acute respiratory infection health care visits among children under 2 years old? A randomised controlled trial]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=369897 (first received 25 January 2016).
CTRI/2013/04/003566 {published data only}
- CTRI/2013/04/003566. Vitamin D supplementation and responses to vaccines in infants [Vitamin D supplementation to improve immune responses to vaccines administered in early infancy – the Nutrivac-D trial]. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=6170&EncHid=&modid=&compid=%27,%276170det%27 (first received 17 April 2013).
CTRI/2015/08/006132 {published data only}
- CTRI/2015/08/006132. A clinical trial to evaluate the need for routine vitamin D supplementation till six months age in full term babies who are being exclusively breastfed [Vitamin D oral supplementation evaluation in full-term, exclusively breastfed infants—a randomized controlled study]. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=10328&EncHid=&modid=&compid=%27,%2710328det%27 (first received 21 August 2015).
CTRI/2016/12/007519 {published data only}
- CTRI/2016/12/007519. Vitamin D levels in preterm babies [Vitamin D levels of the term small-for-date newborns at birth and at 3 month of age after vitamin D supplementation with 2 different doses 400 IU v/s 800 IU—a randomized control trial]. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2016/12/007519 (first received 1 December 2016).
CTRI/2017/10/010274 {published data only}
- CTRI/2017/10/010274. Role of vitamin D3 intake and decrease in respiratory infections [Randomised trial of two different doses of vitamin D supplementation and risk of acute respiratory infection in children in Rural Kolar, Karnataka]. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/10/010274 (first received 31 October 2017).
CTRI/2017/11/010385 {published data only (unpublished sought but not used)}
- CTRI/2017/11/010385. Dose of vitamin D in children with chronic kidney disease [Optimal dose of cholecalciferol supplementation in Indian children with chronic kidney disease—a randomised control trial]. ctri.nic.in/Clinicaltrials/showallp.php?mid1=13495&EncHid=&userName=CTRI/2017/11/010385 (first received 6 November 2017).
- Iyengar AA, Kamath N, Hamsa V, Uthup S, Sharma J, Singhal J, et al. Determining the optimal dose of cholecalciferol supplementation in children with chronic kidney disease (C3 trial): design of an open-label multicenter randomized controlled trial. Asian Journal of Pediatric Nephrology 2018;1(2):67-73. [DOI: 10.4103/ajpn.Ajpn_34_18] [DOI] [Google Scholar]
- Kamath N, Iyengar A, Reddy HV, Uthup S, Sharma J, Singhal J, et al. Determining the optimal dose of cholecalciferol supplementation in children with chronic kidney disease (C3 trial)—an open label multicentre randomized controlled trial. Kidney International Reports 2019;4(Suppl 7):S120-1. [DOI: 10.1016/j.ekir.2019.05.308] [Abstract #Sat-271] [DOI] [Google Scholar]
CTRI/2017/12/010827 {published data only}
- CTRI/2017/12/010827. Effect of vitamin D supplementation on post operative surgical outcomes in children with cyanotic congenital heart disease undergoing open heart surgery: a randomised controlled trial [Effect of vitamin D supplementation on outcome in children undergoing open heart surgery: a randomised controlled trial]. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/12/010827 (first received 11 December 2017).
CTRI/2018/04/013300 {published data only}
- CTRI/2018/04/013300. A clinical trial to compare three different regimes for treatment of nutritional rickets in children [To evaluate the efficacy of daily vitamin D therapy versus Stoss therapy in nutritional rickets inIndian children: a randomized controlled trial]. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/04/013300 (first received 16 April 2018).
CTRI/2018/12/016760 {published data only}
- CTRI/2018/12/016760. Daily versus bolus oral vitamin D3 for treatment of rickets in children [Daily versus depot oral vitamin D3 for treating nutritional rickets]. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/12/016760 (first received 21 December 2018).
Galdo 2018 {published data only (unpublished sought but not used)}
- EUCTR 2013-005326-38. Digitoxin to improve outcomes in patients with advanced systolic chronic heart failure [A multicenter, randomized, double-blind, placebo-controlled study to demonstrate that digitoxin reduces a composite of overall mortality and hospitalization for worsening heart failure in patients with chronic heart failure and reduced ejection fraction]. www.clinicaltrialsregister.eu/ctr-search/trial/2013-005326-38/DE (first received 18 November 2014).
- Galdo AM, De Aguileta AL, Mercader PT, Estela AC, Marcos MIM, Murua JK, et al. Effect of supplementation with vitamin D on acute bronchitis prevention during the first year of life. European Respiratory Journal 2018;52(Suppl 62):PA4593. [DOI: 10.1183/13993003.congress-2018.PA4593] [Abstract #PA4593] [DOI] [Google Scholar]
- NCT01875757. Effect of supplementation with vitamin D on the acute bronchitis prevention during the first year of life (VitDBR2012) [Effect of supplementation with vitamin D on the acute bronchitis prevention during the first year of life]. clinicaltrials.gov/ct2/show/NCT01875757 (first received 10 June 2013).
IRCT20171030037093N4 {published data only (unpublished sought but not used)}
- IRCT20171030037093N4. Effect of vitamin D supplement on the level of serum vitamin D in preterm neonates [Comparing the effect of different doses of vitamin D supplement on the level of serum 25(OH) vitamin D and bone metabolism related factors in preterm neonates]. en.irct.ir/trial/37624 (first received 3 March 2019).
Kishore 2019 {published data only (unpublished sought but not used)}
- Kishore SS, Gadiraju M. Study of daily vitamin D supplementation in preterm infants: a randomized trial. Journal of Pediatric Gastroenterology and Nutrition 2019;68(Suppl 1):1167. [Abstract #N-P-121] [Google Scholar]
NCT01050387 {published data only (unpublished sought but not used)}
- NCT01050387. Effects of vitamin D dose and genotype of the binding protein in infants and children (VitaD) [A randomized, controlled trial of vitamin D supplementation in infants and children: effects of vitamin D dose and genotype of the binding protein]. clinicaltrials.gov/ct2/show/NCT01050387 (first received 13 January 2010).
- Simpson C, Zhang J, Vanderschueren D, Fu L, Pennestri T, Bouillon R, et al. A randomized trial of vitamin D supplementation in healthy inner-city children. Journal of Bone and Mineral Research 2018;33:402. [DOI: 10.1002/jbmr.3621] [MON-0856] [DOI] [Google Scholar]
NCT01363167 {published data only (unpublished sought but not used)}
- NCT01363167. Identifying vitamin D deficiency in very low birth weight infant (VLBW) infants part 2 [Identifying vit D deficiency in VLBW infants part 2]. clinicaltrials.gov/ct2/show/NCT01363167 (first received 18 April 2011).
- Taylor S, Wagner C, Finch C, Ebeling M, Hollis B. Very low birth weight infant vitamin D supplementation: double-blind, randomized clinical efficacy trial. FASEB Journal 2014;28(Suppl 1):828.13. [DOI: 10.1096/fasebj.28.1_supplement.828.13] [DOI] [Google Scholar]
- Taylor SN, Wagner CL, Finch C, Ebeling M, Hollis HW. Very low birth weight infant mineral and bone health outcomes with vitamin D supplementation: double-blind, randomized clinical efficacy trial. In: Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2014 May 3-6; Vancouver (BC). 2014. [Abstract #432] [bluetoadpublishing.co.uk/publication/?i=203855&p=193&pp=1&view=issueViewer]
NCT01698840 {published data only}
- NCT01698840. Effect of vitamin D in diets of preterm infants [An evaluation of the effects of two levels of vitamin D in infants fed preterm or transitional formula on serum 25-hydroxyvitamin D and bone status in preterm infants: a double-blind, randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT01698840 (first received 24 September 2012).
NCT01838447 {published and unpublished data}
- McNally D, O'Hearn K, Lougheed J, Fergusson D, Maharajh G, Weiler H, et al. Prevention of post-cardiac surgery vitamin D deficiency in children with congenital heart disease: results of a pilot dose evaluation randomized controlled trial. Pediatric Critical Care Medicine 2018;19(Suppl 6):46. [DOI: 10.1097/01.pcc.0000537451.52429.d6] [Abstract #PD-049] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally JD, O'Hearn K, Lawson ML, Maharajh G, Geier P, Wiler H, et al. Prevention of vitamin D deficiency in children following cardiac surgery: study protocol for a randomized controlled trial. Trials 2015;16:402. [DOI: 10.1186/s13063-015-0922-8] [PMC4564959] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01838447. Prevention of vitamin D deficiency following pediatric CHD surgery: a phase II dose evaluation randomized controlled trial comparing usual care with a high dose pre-operative supplementation regimen based on the Institute of Medicine daily upper tolerable Intake level (HICCUPS 2) [Prevention of post-cardiac surgery vitamin D deficiency in children with congenital heart disease: a pilot dose evaluation randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT01838447 (first received 11 April 2013).
NCT01996423 {published data only (unpublished sought but not used)}
- Borzutzky A, Cifuentes L, Silva-Valenzuela S, Vera-Kellet C, Iturriaga C, Perez-Mateluna G, et al. Effect of vitamin D supplementation on the severity of atopic dermatitis in children: the VIDATOPIC randomized controlled trial. In: Takaoka R , editors(s). ISAD 2016: 9th Georg Rajka International Symposium on Atopic Dermatitis; 2016 May 19-21; São Paulo (BR). Sao Paulo (BR): ISAD, 2016:70-1. [Abstract #PT45] [www.isadsociety.org/wp-content/uploads/ISAD-2016_program.pdf?v=2]
- Cristi F, Perez-Mateluna G, Vera-Kellet C, Silva-Valenzuela S, Iturriaga C, Hoyos-Bachiloglu R, et al. Vitamin D modulates the allergic phenotype of dendritic cells in children with atopic dermatitis. Experimental Dermatology 2019;28(3):308-11. [DOI: 10.1111/exd.13873] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT01996423. Impact of vitamin D supplementation on severity of pediatric atopic dermatitis (VIDATOPIC) [Impact of vitamin D supplementation on clinical severity and immunologic tolerance of pediatric atopic dermatitis]. clinicaltrials.gov/ct2/show/study/NCT01996423 (first received 11 November 2013).
NCT02046577 {published data only (unpublished sought but not used)}
- Loureiro C, Brinkmann K, Campos L, Vizcaya C, Leroy C, Arancibia M, et al. Randomized study of vitamin D supplementation for the prevention of acute respiratory infections Aris of Chile in preschools. Hormone Research in Paediatrics 2018;90(Suppl 2):31-2. [DOI: 10.1177/1203475418805744] [DOI] [Google Scholar]
- NCT02046577. Study of vitamin D for the prevention of acute respiratory infections in children [A randomized, double-blind, controlled trial of vitamin D for the prevention of acute respiratory infections in children aged 18 to 36 months in Santiago, Coyhaique and Punta Arenas, Chile]. clinicaltrials.gov/show/NCT02046577 (first received 23 January 2014).
NCT02404623 {published and unpublished data}
- Golan Tripto I, Bistritzer J, Loewenthal N, Dizitzer Y, Goldbart A. The effect of vitamin D administration on vitamin D status and respiratory morbidity in premature infants. Pediatric Pulmonology 2019;54(Suppl 1):S81. [DOI: 10.1002/ppul.24373] [Abstract #A-22] [DOI] [PubMed] [Google Scholar]
- NCT02404623. The effect of vitamin D administration to premature infants on vitamin D status and respiratory morbidity [The effect of vitamin D administration to premature infants on vitamin D status and respiratory morbidity during the first year of life]. clinicaltrials.gov/ct2/show/NCT02404623 (first received 18 March 2015).
NCT02452762 {published data only (unpublished sought but not used)}
- EUCTR 2016-002459-38. Rapid normalization of vitamin D in critically ill children [Rapid normalization of vitamin D in critically ill children: a phase II dose evaluation randomized controlled trial (VITdAL-PICU pilot)]. www.clinicaltrialsregister.eu/ctr-search/trial/2016-002459-38/AT (first received 19 January 2017).
- McNally D, Amrein K, O'Hearn K, Fergusson D, Geier P, Henderson M, et al. Study protocol for a phase II dose evaluation randomized controlled trial of cholecalciferol in critically ill children with vitamin D deficiency (VITdAL-PICU study). Pilot Feasibility Studies 2017;3(70):1-13. [DOI: 10.1186/s40814-017-0214-z] [PMC5721544] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02452762. Rapid normalization of vitamin D in critically Ill children: a phase II dose evaluation randomized controlled trial (VITdAL-PICU) [Rapid normalization of vitamin D in critically Ill children: a phase II dose evaluation randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT02452762 (first received 15 May 2015).
NCT02563015 {published data only (unpublished sought but not used)}
- NCT02563015. Can correction of low vitamin D status in infancy program for a leaner body composition? [Novel functional outcomes of vitamin D in infancy; can correction of low vitamin D status program for a leaner body composition phenotype?]. clinicaltrials.gov/ct2/show/NCT02563015 (first received 28 September 2015).
NCT02975492 {published data only}
- EUCTR 2016-001807-22-FR. Modalities of vitamin D supplementation and the risk of hypercalcemia in children aged 2 to 6 years. www.clinicaltrialsregister.eu/ctr-search/trial/2016-001807-22/FR (first received 20 July 2016).
- NCT02975492. Assessing the impact of a mode of vitamin D supplementation (sequential dose vs daily dose) on the incidence of hypercalciuria in children aged from 2 to 6 years (DonneDVit) [Evaluation de l'Impact d'un mode de supplémentation en vitamine D (dose séquentielle vs dose quotidienne) sur l'Incidence de l'hypercalciurie chez des enfants des départements du gard et de l'hérault agés de 2 à 6 ans. Etude contrôlée randomisée en 2 groupes parallèles]. clinicaltrials.gov/ct2/show/NCT02975492 (first received 7 November 2016).
NCT03087149 {published data only (unpublished sought but not used)}
- Kołodziejczyk A, Borszewska-Kornacka MK, Seliga-Siwecka J. MOnitored supplementation of VItamin D in preterm infants (MOSVID trial): study protocol for a randomised controlled trial. Trials 2017;18(1):424. [DOI: 10.1186/s13063-017-2141-y] [PMC5594536] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03087149. Monitored vs standard supplementation of vitamin D in preterm infants (MOSVID) [Supplementation of vitamin D in preterm infants—monitored therapy vs standard therapy. A randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT03087149?id=NCT03087149 (first received 15 March 2017). [NCT03087149]
NCT03365687 {published data only}
- Jensen ME, Ducharme FM, Alos N, Mailhot G, Mâsse B, White JH, et al. Vitamin D in the prevention of exacerbations of asthma in preschoolers (DIVA): protocol for a multicentre randomised placebo-controlled triple-blind trial. BMJ Open 2019;9(12):e033075. [DOI: 10.1136/bmjopen-2019-033075] [PMC6955525] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03365687. Vitamin D In the prevention of viral-induced asthma in preschoolers (DIVA) [Vitamin D In the prevention of viral-induced asthma in preschoolers: a randomized controlled multicenter trial (DIVA)]. clinicaltrials.gov/ct2/show/NCT03365687 (first received 3 December 2017).
NCT03536845 {published data only}
- Khalifah RA, Hudairi A, Homyani DA, Hamad MH, Bashiri FA. Vitamin D supplementation to prevent vitamin D deficiency for children with epilepsy: randomized pragmatic trial protocol. Medicine 2018;97(40):e12734. [DOI: 10.1097/MD.0000000000012734] [PMC6200520] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03536845. Vitamin D supplementation to prevent vitamin D deficiency for children with epilepsy [Vitamin D supplementation to prevent vitamin D deficiency for children with epilepsy: a randomized controlled clinical trial]. clinicaltrials.gov/ct2/show/NCT03536845 (first received 14 May 2018).
NCT03742310 {published data only}
- NCT03742310. The relationship between VDR gene polymorphism and children's physical and intellectual development (RVDRGPCPID) [Multi-center clinical study on the relationship between vitamin D receptor gene polymorphism and children's physical and intellectual development]. clinicaltrials.gov/ct2/show/NCT03742310 (first received 12 November 2018).
NCT03742505 {published data only}
- NCT03742505. Rapid normalization of vitamin D deficiency in PICU (VITdALIZE-KIDS) [Rapid normalization of vitamin D deficiency in PICU: a multi-centre phase III double-blind randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT03742505 (first received 12 November 2018).
NCT03871322 {published data only}
- NCT03871322. The vitamin K2 and D3 intervention trial in children and adolescents with the low-energy fractures [Rationale and design of the vitamin K2 and vitamin D3 intervention trial in children and adolescents with the low-energy bone fractures]. clinicaltrials.gov/ct2/show/NCT03871322 (first received 7 March 2019).
NCT03999580 {published data only}
- NCT03999580. The vitamin D in pediatric Crohn's disease (ViDiPeC-2) (ViDiPeC-2) [A pragmatic randomized controlled trial on high dose vitamin D to prevent relapses of Crohn's disease in children]. clinicaltrials.gov/ct2/show/NCT03999580 (first received 24 June 2019).
RBR‐4r6p5v {published data only (unpublished sought but not used)}
- RBR-4r6p5v. Effect of physical exercise and nutritional programs on the health status of schoolchildren aged 4 to 11 years old from Santo Antônio de Goiás [Efeito de programas de exercício físico e nutricional sobre o estado de saúde deescolares de 4 a 11 anos de Santo Antôniode Goiás] [Metabolic syndrome: metabolic, oxidative and inflammatory responses of physical exercise and nutritional programs in schoolchildren aged 4 to 11 years old from Santo Antônio de Goiás [Síndrome metabólica: respostas metabólicas, oxidativas e inflamatórias de programas de exercício físico e nutricional em escolares de 4 a 11 anos de Santo Antônio de Goiás]]. www.ensaiosclinicos.gov.br/rg/RBR-4r6p5v/ (first received 23 May 2018). [UTN #U1111-1214-5845]
UMIN000034864 {published data only}
- UMIN000034864. Prevention of allergic march by vitamin D supplementation during infancy. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000037789 (first received 12 November 2018).
Yani 2018 {published data only (unpublished sought but not used)}
- Yani FF, Amelin F, Hamdi, Erisma R, Basir D, Lusita L, et al. Vitamin D level & tuberculin conversion among under five children with TB contact history after vitamin D supplementation. In: Asia Pacific Congress of Pediatrics 2018; 2018 Aug 27-29; Bali (ID). 2018. [Abstract #APCP406]
- Yani FF, Lipoeto NI, Supriyatno B, Darwin E. In: Vitamin D supplementation and tuberculin skin test conversion among healthy under-five children with tuberculosis contact. 17th International Congress of Pediatric Pulmonology; 2018 Jun 21-24; Toledo (ES). 2018. [Abstract #M125]
Additional references
Adams 2010
- Adams JS, Hewison M. Update in vitamin D. Journal of Clinical Endocrinology and Metabolism 2010;95(2):471-8. [DOI: 10.1210/jc.2009-1773] [PMC2840860] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adkins 2004
- Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nature Reviews Immunology 2004;4(7):553-64. [DOI: 10.1038/nri1394] [PMID: ] [DOI] [PubMed] [Google Scholar]
Avenell 2014
- Avenell A, Mak JCS, O'Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database of Systematic Reviews 2014, Issue 4. Art. No: CD000227. [DOI: 10.1002/14651858.CD000227.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barrueto 2005
- Barrueto F Jr, Wang-Flores HH, Howland MA, Hoffman RS, Nelson LS. Acute vitamin D intoxication in a child. Pediatrics 2005;116(3):e453-6. [DOI: 10.1542/peds.2004-2580] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bhutta 2013
- Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, et al. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet 2013;382(9890):452-77. [DOI: 10.1016/S0140-6736(13)60996-4] [DOI] [PubMed] [Google Scholar]
Bikle 2014
- Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chemistry and Biology 2014;21(3):319-29. [DOI: 10.1016/j.chembiol.2013.12.016] [PMC3968073] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bischoff‐Ferrari 2004
- Bischoff-Ferrari HA, Borchers M, Gudat F, Dürmüller U, Stähelin HB, Dick W. Vitamin D receptor expression in human muscle tissue decreases with age. Journal of Bone and Mineral Research 2004;19(2):265-9. [DOI: 10.1359/jbmr.2004.19.2.265] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bischoff‐Ferrari 2012
- Bischoff-Ferrari HA. Relevance of vitamin D in muscle health. Reviews in Endocrine and Metabolic Disorders 2012;13(1):71-7. [DOI: 10.1007/s11154-011-9200-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bjelakovic 2014a
- Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Krstic G, Wetterslev J, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database of Systematic Reviews 2014, Issue 6. Art. No: CD007469. [DOI: 10.1002/14651858.CD007469.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Black 2008
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, De Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371(9608):243-60. [DOI: 10.1016/S0140-6736(07)61690-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Black 2013
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, De Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013;382(9890):427-51. [DOI: 10.1016/S0140-6736(13)60937-X] [PMID: ] [DOI] [PubMed] [Google Scholar]
Blank 1995
- Blank S, Scanlon KS, Sinks TH, Lett S, Falk H. An outbreak of hypervitaminosis D associated with the overfortification of milk from a home-delivery dairy. American Journal of Public Health 1995;85(5):656-9. [PMC1615443] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bouillon 2008
- Bouillon R, Carmeliet G, Verlinden L, Van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocrine Reviews 2008;29(6):726-76. [DOI: 10.1210/er.2008-0004] [PMC2583388] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Canaff 2002
- Canaff L, Hendy GN. Human calcium-sensing receptor gene. Vitamin D response elements in promoters P1 and P2 confer transcriptional responsiveness to 1,25-dihydroxyvitamin D. Journal of Biological Chemistry 2002;277(33):30337-50. [DOI: 10.1074/jbc.M201804200] [PMID: ] [DOI] [PubMed] [Google Scholar]
Casanovas 2013
- Casanovas MC, Lutter CK, Mangasaryan N, Mwadime R, Hajeebhoy N, Aguilar AM, et al. Multi-sectoral interventions for healthy growth. Maternal and Child Nutrition 2013;9(Suppl 2):46-57. [DOI: 10.1111/mcn.12082] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cashman 2008
- Cashman KD, Hill TR, Lucey AJ, Taylor N, Seamans KM, Muldowney S, et al. Estimation of the dietary requirement for vitamin D in healthy adults. American Journal of Clinical Nutrition 2008;88(6):1535-42. [DOI: 10.3945/ajcn.2008.26594] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cashman 2009
- Cashman KD, Wallace JM, Horigan G, Hill TR, Barnes MS, Lucey AJ, et al. Estimation of the dietary requirement for vitamin D in free-living adults >=64 y of age. American Journal of Clinical Nutrition 2009;89(5):1366-74. [DOI: 10.3945/ajcn.2008.27334] [PMID: ] [DOI] [PubMed] [Google Scholar]
Caulfield 2006
- Caufield LE, Richard SA, Rivera JA, Musgrove P, Black RE. Stunting, wasting, and micronutrient deficiency disorders. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al, editors(s). Disease Control Priorities in Developing Countries. 2nd edition. Washington (DC); New York (NY): International Bank for Reconstruction and Development/World Bank; Oxford University Press, 2006:551-68. [Google Scholar]
Ceglia 2010
- Ceglia L, Da Silva Morais M, Park LK, Morris E, Harris SS, Bischoff-Ferrari HA, et al. Multi-step immunofluorescent analysis of vitamin D receptor loci and myosin heavy chain isoforms in human skeletal muscle. Journal of Molecular Histology 2010;41(2-3):137-42. [DOI: 10.1007/s10735-010-9270-x] [PMC2958104] [DOI] [PMC free article] [PubMed] [Google Scholar]
Christakos 2012
- Christakos S. Mechanism of action of 1,25-dihydroxyvitamin D3 on intestinal calcium absorption. Reviews in Endocrine and Metabolic Disorders 2012;13(1):39-44. [DOI: 10.1007/s11154-011-9197-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Christakos 2016
- Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiological Reviews 2016;96(1):365-408. [DOI: 10.1152/physrev.00014.2015] [PMC4839493] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence 2020 [Computer program]
- Veritas Health Innovation Covidence. Version accessed 7 December 2020. Melbourne, Australia: Veritas Health Innovation, 2020. Available at covidence.org.
Danaei 2016
- Danaei G, Andrews KG, Sudfeld CR, Fink G, McCoy DC, Peet E, et al. Risk factors for childhood stunting in 137 developing countries: a comparative risk assessment analysis at global, regional, and country levels. PLoS Medicine 2016;13(11):e1002164. [DOI: 10.1371/journal.pmed.1002164] [PMC5089547] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2020
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
De Onis 2012
- De Onis M, Blössner M, Borghi E. Prevalence and trends of stunting among pre-school children, 1990-2020. Public Health Nutrition 2012;15(1):142-8. [DOI: 10.1017/S1368980011001315] [PMID: ] [DOI] [PubMed] [Google Scholar]
De Onis 2013
- De Onis M, Dewey KG, Borghi E, Onyango AW, Blössner M, Daelmans B, et al. The World Health Organization's global target for reducing childhood stunting by 2025: rationale and proposed actions. Maternal and Child Nutrition 2013;9(Suppl 2):6-26. [DOI: 10.1111/mcn.12075] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Onis 2016
- De Onis M, Branca F. Childhood stunting: a global perspective. Maternal and Child Nutrition 2016;12(Suppl 1):12-26. [DOI: 10.1111/mcn.12231] [PMC5084763] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dewey 2011
- Dewey KG, Begum K. Long-term consequences of stunting in early life. Maternal and Child Nutrition 2011;7(Suppl 3):5-18. [DOI: 10.1111/j.1740-8709.2011.00349.x] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dewey 2020
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI: 10.1136/bmj.315.7109.629] [PMC2127453] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Endo 2003
- Endo I, Inoue D, Mitsui T, Umaki Y, Akaike M, Yoshizawa T, et al. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology 2003;144(12):5138-44. [DOI: 10.1210/en.2003-0502] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ferguson 2014
- Ferguson JH, Chang AB. Vitamin D supplementation for cystic fibrosis. Cochrane Database of Systematic Reviews 2014, Issue 5. Art. No: CD007298. [DOI: 10.1002/14651858.CD007298.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Finkelstein 2012
- Finkelstein JL, Mehta S, Duggan C, Manji KP, Mugusi FM, Aboud S, et al. Maternal vitamin D status and child morbidity, anemia, and growth in human immunodeficiency virus-exposed children in Tanzania. Pediatric Infectious Disease Journal 2012;31(2):171-5. [DOI: 10.1097/INF.0b013e318245636b] [PMC3813463] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goyal 2020
- Goyal R, Jialal I. Hyperphosphatemia. In: StatPearls [Internet]. [Updated 2020 June 25] edition. Treasure Island (FL): StatPearls Publishing, 2020 Jan. [https://www.ncbi.nlm.nih.gov/books/NBK551586/] [Google Scholar]
GRADEpro GDT 2020 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro Guideline Development Tool [Software]. GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction--GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI: 10.1016/j.jclinepi.2010.04.026] [PMID: ] [DOI] [PubMed] [Google Scholar]
Haas 1996
- Haas JD, Murdoch S, Rivera J, Martorell R. Early nutrition and later physical work capacity. Nutrition Reviews 1996;54(2 Pt 2):S41-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Haussler 1998
- Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. Journal of Bone and Mineral Research 1998;13(3):325-49. [DOI: 10.1359/jbmr.1998.13.3.325] [PMID: ] [DOI] [PubMed] [Google Scholar]
Heaney 2003
- Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. American Journal of Clinical Nutrition 2003;77(1):204-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Heaney 2009
- Heaney RP, Horst RL, Cullen DM, Armas LA. Vitamin D3 distribution and status in the body. Journal of the American College of Nutrition 2009;28(3):252-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC, editor(s), on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1.
Higgins 2020a
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Higgins 2020b
- Higgins JPT, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Holick 2006
- Holick MF. Resurrection of vitamin D deficiency and rickets. Journal of Clinical Investigation 2006;116(8):2062-72. [DOI: 10.1172/JCI29449] [PMC1523417] [DOI] [PMC free article] [PubMed] [Google Scholar]
Holick 2007
- Holick MF. Vitamin D deficiency. New England Journal of Medicine 2007;357(3):266-81. [DOI: 10.1056/NEJMra070553] [PMID: ] [DOI] [PubMed] [Google Scholar]
Holick 2008a
- Holick MF. Vitamin D: a D-lightful health perspective. Nutrition Reviews 2008;66(10 Suppl 2):S182-94. [DOI: 10.1111/j.1753-4887.2008.00104.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Holick 2008b
- Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. Journal of Clinical Endocrinology & Metabolism 2008;93(3):677-81. [DOI: 10.1210/jc.2007-2308] [PMC2266966] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Holick 2010
- Holick MF. Vitamin D: Physiology, Molecular Biology, and Clinical Applications. New York (NY): Springer Science & Business Media, 2010. [Google Scholar]
Holick 2011
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 2011;96(7):1911-30. [DOI: 10.1210/jc.2011-0385] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hossein‐Nezhad 2013
- Hossein-nezhad A, Spira A, Holick MF. Influence of vitamin D status and vitamin D3 supplementation on genome wide expression of white blood cells: a randomized double-blind clinical trial. PLoS One 2013;8(3):e58725. [DOI: 10.1371/journal.pone.0058725] [NCT01696409] [PMC3604145] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huey 2016
- Huey SL, Mehta S. Stunting: the need for application of advances in technology to understand a complex health problem. EBioMedicine 2016;6:26-7. [DOI: 10.1016/j.ebiom.2016.03.013] [PMC4856790] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hyppönen 2011
- Hyppönen E, Fararouei M, Sovio U, Hartikainen A-L, Pouta A, Robertson C, et al. High-dose vitamin D supplements are not associated with linear growth in a large Finnish cohort. Journal of Nutrition 2011;141(5):843-8. [DOI: 10.3945/jn.110.133009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Institute of Medicine 1997
- Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington (DC): National Academies Press, 1997. [PubMed] [Google Scholar]
Institute of Medicine 2011
- Institute of Medicine of the National Academies. Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): National Academies Press, 2011. [PubMed] [Google Scholar]
Jagannath 2010
- Jagannath VA, Fedorowicz Z, Asokan GV, Robak EW, Whamond L. Vitamin D for the management of multiple sclerosis. Cochrane Database of Systematic Reviews 2010, Issue 12. Art. No: CD008422. [DOI: 10.1002/14651858.CD008422.pub2] [DOI] [PubMed] [Google Scholar]
Jeans 1938
- Jeans PC, Stearns G. The effect of vitamin D on linear growth in infancy: II. The effect of intakes above 1,800 USP units daily. Journal of Pediatrics 1938;13(5):730-40. [DOI: 10.1016/S0022-3476(38)80162-1] [DOI] [Google Scholar]
Klontz 2007
- Klontz KC, Acheson DW. Dietary supplement-induced vitamin D intoxication. New England Journal of Medicine 2007;357(3):308-9. [DOI: 10.1056/NEJMc063341] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kumar 2011
- Kumar GT, Sachdev HS, Chellani H, Rehman AM, Singh V, Arora H, et al. Effect of weekly vitamin D supplements on mortality, morbidity, and growth of low birthweight term infants in India up to age 6 months: randomised controlled trial. British Medical Journal 2011;342:d2975. [DOI: 10.1136/bmj.d2975] [PMC3104477] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2020
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Lerch 2007
- Lerch C, Meissner T. Interventions for the prevention of nutritional rickets in term born children. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD006164. [DOI: 10.1002/14651858.CD006164.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leroy 2014
- Leroy JL, Ruel M, Habicht J-P, Frongillo EA. Linear growth deficit continues to accumulate beyond the first 1000 days in low- and middle-income countries: global evidence from 51 national surveys. Journal of Nutrition 2014;144(9):1460-6. [DOI: ] [DOI] [PubMed] [Google Scholar]
Leroy 2019
- Leroy JL, Frongillo EA. Perspective: what does stunting really mean? A critical review of the evidence. Advances in Nutrition 2019;10(2):196-204. [DOI: 10.1093/advances/nmy101] [PMC6416038] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leslie 2020
- Leslie SW, Sajjad H. Hypercalciuria; 2020 [updated 20 July 2020]. Available at www.ncbi.nlm.nih.gov/books/NBK448183/.
Levy 2007
- Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nature Reviews Immunology 2007;7(5):379-90. [DOI: 10.1038/nri2075] [PMID: ] [DOI] [PubMed] [Google Scholar]
Li 2020
- Li T, Higgins JPT, Deeks JJ (editors). Chapter 5: Collecting data. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Martineau 2016
- Martineau AR, Cates CJ, Urashima M, Jensen M, Griffiths AP, Nurmatov U, et al. Vitamin D for the management of asthma. Cochrane Database of Systematic Reviews 2016, Issue 9. Art. No: CD011511. [DOI: 10.1002/14651858.CD011511.pub2] [PMC6457769] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martorell 1994
- Martorell R, Khan LK, Schroeder DG. Reversibility of stunting: epidemiological findings in children from developing countries. European Journal of Clinical Nutrition 1994;48(Suppl 1):S45-57. [PMID: ] [PubMed] [Google Scholar]
Martorell 2012
- Martorell R, Zongrone A. Intergenerational influences on child growth and undernutrition. Paediatric and Perinatal Epidemiology 2012;26(Suppl 1):302-14. [DOI: 10.1111/j.1365-3016.2012.01298.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
McDonnell 1987
- McDonnell DP, Mangelsdorf DJ, Pike JW, Haussler MR, O'Malley BW. Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science 1987;235(4793):1214-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mo 2019
- Mo M, Wang S, Chen Z, Muyiduli X, Wang S, Shen Y, et al. A systematic review and meta-analysis of the response of serum 25-hydroxyvitamin D concentration to vitamin D supplementation from RCTs from around the globe. European Journal of Clinical Nutrition 2019;73(6):816-34. [DOI: 10.1038/s41430-019-0417-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine 2009;151(4):264-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
MoHFW 2019
- Ministry of Health and Family Welfare (MoHFW), Government of India, UNICEF, Population Council. Comprehensive National Nutrition Survey 2016-2018; 2019. www.popcouncil.org/uploads/pdfs/2019RH_CNNSreport.pdf.
Nojaba 2020
- Nojaba L, Guzman N. Nephrolithiasis; 2020 [updated 10 August 2020]. Available at www.ncbi.nlm.nih.gov/books/NBK559227.
Norman 2004
- Norman AW, Mizwicki MT, Norman DP. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nature Reviews Drug Discovery 2004;3(1):27-41. [DOI: 10.1038/nrd1283] [PMID: ] [DOI] [PubMed] [Google Scholar]
Owino 2016
- Owino V, Ahmed T, Freemark M, Kelly P, Loy A, Manary M, et al. Environmental enteric dysfunction and growth failure/stunting in global child health. Pediatrics 2016;138(6):e20160641. [DOI: 10.1542/peds.2016-0641] [PMID: ] [DOI] [PubMed] [Google Scholar]
Palacios 2014
- Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? Journal of Steroid Biochemistry and Molecular Biology 2014;144(Pt A):136-45. [DOI: 10.1016/j.jsbmb.2013.11.003] [PMC4018438] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palacios 2019
- Palacios C, Kostiuk LK, Peña‐Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2019, Issue 7. Art. No: CD008873. [DOI: 10.1002/14651858.CD008873.pub4] [PMC6659840 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perumal 2018
- Perumal N, Bassani DG, Roth DE. Use and misuse of stunting as a measure of child health. Journal of Nutrition 2018;148(3):311-5. [DOI: 10.1093/jn/nxx064] [PMID: ] [DOI] [PubMed] [Google Scholar]
Plotnikoff 2003
- Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clinic Proceedings 2003;78(12):1463-70. [DOI: 10.4065/78.12.1463] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pojsupap 2015
- Pojsupap S, Illiriani K, Sampaio TZAL, O'Hearn K, Kovesi T, Menon K, et al. Efficacy of high-dose vitamin D in pediatric asthma: a systematic review and meta-analysis. Journal of Asthma 2015;52(4):382-90. [DOI: 10.3109/02770903.2014.980509] [PMID: ] [DOI] [PubMed] [Google Scholar]
Prendergast 2014
- Prendergast AJ, Humphrey JH. The stunting syndrome in developing countries. Paediatrics and International Child Health 2014;34(4):250-65. [DOI: 10.1179/2046905514Y.0000000158] [PMC4232245] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prentice 2013
- Prentice AM, Ward KA, Goldberg GR, Jarjou LM, Moore SE, Fulford AJ, et al. Critical windows for nutritional interventions against stunting. American Journal of Clinical Nutrition 2013;97(5):911-8. [DOI: 10.3945/ajcn.112.052332] [PMC3628381] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramagopalan 2010
- Ramagopalan SV, Heger A, Berlanga AJ, Maugeri NJ, Lincoln MR, Burrell A, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Research 2010;20(10):1352-60. [DOI: 10.1101/gr.107920.110] [PMC2945184] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramakrishnan 1999
- Ramakrishnan U, Martorell R, Schroeder DG, Flores R. Role of intergenerational effects on linear growth. Journal of Nutrition 1999;129(2):544S-9S. [DOI: 10.1093/jn/129.2.544S] [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ross 2011
- Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. Journal of Clinical Endocrinology and Metabolism 2011;96(1):53-8. [DOI: 10.1210/jc.2010-2704] [PMC3046611] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sadiq 2020
- Sadiq NM, Naganathan S, Badireddy M. Hypercalcemia; 2020 [updated 10 Sep 2020]. Available at www.ncbi.nlm.nih.gov/books/NBK430714/.
Semba 2016
- Semba RD, Shardell M, Sakr Ashour FA, Moaddel R, Trehan I, Maleta KM, et al. Child stunting is associated with low circulating essential amino acids. EBioMedicine 2016;6:246-52. [DOI: 10.1016/j.ebiom.2016.02.030] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shrimpton 2001
- Shrimpton R, Victora CG, De Onis M, Lima RC, Blössner M, Clugston G. Worldwide timing of growth faltering: implications for nutritional interventions. Pediatrics 2001;107(5):e75. [DOI: 10.1542/peds.107.5.e75] [PMID: ] [DOI] [PubMed] [Google Scholar]
Simpson 1985
- Simpson RU, Thomas GA, Arnold AJ. Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. Journal of Biological Chemistry 1985;260(15):8882-91. [PMID: ] [PubMed] [Google Scholar]
Smith 2009
- Smith SM, Gardner KK, Locke J, Zwart SR. Vitamin D supplementation during Antarctic winter. American Journal of Clinical Nutrition 2009;89(4):1092-8. [DOI: 10.3945/ajcn.2008.27189] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stewart 2013
- Stewart CP, Iannotti L, Dewey KG, Michaelsen KF, Onyango AW. Contextualising complementary feeding in a broader framework for stunting prevention. Maternal and Child Nutrition 2013;9(Suppl 2):27-45. [DOI: 10.1111/mcn.12088] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Straube 2015
- Straube S, Derry S, Straube C, Moore RA. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database of Systematic Reviews 2015, Issue 5. Art. No: CD007771. [DOI: 10.1002/14651858.CD007771.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sudfeld 2015
- Sudfeld CR, Duggan C, Aboud S, Kupka R, Manji KP, Kisenge R, et al. Vitamin D status is associated with mortality, morbidity, and growth failure among a prospective cohort of HIV-infected and HIV-exposed Tanzanian infants. Journal of Nutrition 2015;145(1):121-7. [DOI: 10.3945/jn.114.201566] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tarroni 2012
- Tarroni P, Villa I, Mrak E, Zolezzi F, Mattioli M, Gattuso C, et al. Microarray analysis of 1,25(OH)2D3 regulated gene expression in human primary osteoblasts. Journal of Cellular Biochemistry 2012;113(2):640-9. [DOI: 10.1002/jcb.23392] [PMID: ] [DOI] [PubMed] [Google Scholar]
Theodoratou 2014
- Theodoratou E, Tzoulaki I, Zgaga L, Ionnidis JPA. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 2014;348:g2035. [DOI: ] [PMC3972415] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Toko 2016
- Toko NE, Sumba PO, Daud II, Ogolla S, Majiwa M, Krisher TJ, et al. Maternal vitamin D status and adverse birth outcomes in children from rural western Kenya. Nutrients 2016;8(12):794. [DOI: 10.3390/nu8120794] [PMC5188449] [DOI] [PMC free article] [PubMed] [Google Scholar]
UNICEF, WHO, World Bank 2020
- UNICEF, World Health Organization, World Bank Group. Levels and Trends in Child Malnutrition. UNICEF/WHO/World Bank Group Joint Child Malnutrition Estimates. Key Findings of the 2020 Edition; March 2020. Available at www.unicef.org/media/69816/file/Joint-malnutrition-estimates-2020.pdf.
United Nations 2015
- UN General Assembly. Transforming our World: The 2030 Agenda for Sustainable Development. A/RES/70/1; October 2015. Avalilabe at www.refworld.org/docid/57b6e3e44.html.
Vazquez 1998
- Vazquez G, Boland AR, Boland RL. 1α, 25-Dihydroxy-vitamin-D3-induced store-operated Ca2+ influx in skeletal muscle cells modulation by phospholipase C, protein kinase C, and tyrosine kinases. Journal of Biological Chemistry 1998;273(51):33954-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Victora 2010
- Victora CG, De Onis M, Hallal PC, Blössner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics 2010;125(3):e473-80. [DOI: 10.1542/peds.2009-1519] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vieth 1999
- Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. American Journal of Clinical Nutrition 1999;69(5):842-56. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wacker 2013
- Wacker M, Holick MF. Vitamin D--effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013;5(1):111-48. [DOI: 10.3390/nu5010111] [PMID: PMC3571641] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walli 2017
- Walli NZ, Munubhi EK, Aboud S, Manji KP. Vitamin D levels in malnourished children under 5 years in a tertiary care center at Muhimbili National Hospital, Dar es Salaam, Tanzania--a cross-sectional study. Journal of Tropical Pediatrics 2017;63(3):203-9. [DOI: 10.1093/tropej/fmw081] [PMID: ] [DOI] [PMC free article] [PubMed]
Waterlow 1994
- Waterlow JC. Introduction. Causes and mechanisms of linear growth retardation (stunting). European Journal of Clinical Nutrition 1994;48(Suppl 1):S1-4. [PMID: ] [PubMed] [Google Scholar]
WHO, FAO 2004
- World Health Organization, Food and Agriculture Organization of the United Nations. Vitamin and Mineral Requirements in Human Nutrition. Second Edition; 2004. Available at apps.who.int/iris/bitstream/10665/42716/1/9241546123.pdf.
WHO 2006
- World Health Organization Multicentre Growth Reference Study Group. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age. Methods and development. www.who.int/childgrowth/standards/Technical_report.pdf (accessed 15 August 2017).
WHO 2012
- World Health Organization (WHO). WHA65.6: comprehensive implementation plan on maternal, infant and young child nutrition. In: Resolutions and decisions annexes. Sixty-Fifth World Health Assembly; 2012 May 21-26; Geneva (CH). 2012.
WHO 2014a
- World Health Organization. WHA global nutrition targets 2025: stunting policy brief. www.who.int/nutrition/topics/globaltargets_stunting_policybrief.pdf (accessed 15 August 2017).
WHO 2016
- World Health Organization. Towards a grand convergence for child survival and health: a strategic review of options for the future building on lessons learnt from IMNCI. apps.who.int/iris/bitstream/10665/251855/1/WHO-MCA-16.04-eng.pdf (accessed 15 August 2017).
WHO 2018
- World Health Organization. Reducing Stunting in Children: Equity Considerations for Achieving the Global Nutrition Targets 2025; 2018. Available at apps.who.int/iris/bitstream/handle/10665/260202/9789241513647-eng.pdf?sequence=1.
WHO 2019
- WHO/NMH/NHD/1920. Levels and Trends in Child Malnutrition. UNICEF/WHO/World Bank Group Joint Vhild Malnutrition Estimates. Key Findings of the 2019 Edition; March 2019. Available at www.who.int/nutgrowthdb/jme-2019-key-findings.pdf?ua=1.
Winzenberg 2011
- Winzenberg T, Powel S, Shaw KA, Jones G. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ 2011;342:c7254. [DOI: 10.1136/bmj.c7254] [PMC3026600] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yakoob 2016
- Yakoob M, Salam R, Khan F, Bhutta Z. Vitamin D supplementation for preventing infections in children under five years of age. Cochrane Database of Systematic Reviews 2016, Issue 11. Art. No: CD008824. [DOI: 10.1002/14651858.CD008824.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Yu 2017
- Yu EA, Huey SL, Peña-Rosas JP, Mehta S. The effects of oral vitamin D supplementation on linear growth and non-communicable diseases among infants and children younger than five years of age. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD012875. [DOI: 10.1002/14651858.CD012875] [PMC6486017] [DOI] [PMC free article] [PubMed] [Google Scholar]


