Abstract
A partial skeleton of a hybodontiform shark-like chondrichthyan from the Upper Jurassic Kimmeridge Clay Formation of Dorset, England, is described and designated as a new genus and species, Durnonovariaodus maiseyi gen. et sp. nov. The holotype and only known specimen, which is represented by disarticulated splanchnocranial elements with associated teeth, a single dorsal fin spine, the pelvic girdle, as well as unidentifiable cartilage fragments, plus countless dermal denticles, exhibits a puzzling combination of dental and skeletal features, providing important new insights into the morphological and ecological diversity of hybodontiforms. Durnonovariaodus gen. nov. displays a unique set of dental characters, showing close morphological resemblance to Secarodus from the Middle Jurassic of England, which was erected for distinctive, strongly labio-lingually compressed multicuspid cutting teeth originally described as Hybodus polyprion. Skeletally, Durnonovariaodus gen. nov. resembles Hybodus and Egertonodus in having a palatoquadrate with a palatobasal process and an ethmoidal articular surface, combined with the possession of dorsal fin spines ornamented with costae. Therefore, and given the absence of any conclusive phylogenetic framework, Durnonovariaodus maiseyi gen. et sp. nov. is here tentatively referred to Hybodontidae until more complete material becomes available in order to enable a more reliable suprageneric identification. The holotype of Durnonovariaodus maiseyi gen. et sp. nov. contains two separate pelvic half-girdles, a feature previously considered as evolutionarily primitive among hybodontiforms. However, unfused pelvic half-girdles also occur in the supposedly closely related species Hybodus hauffianus and may in fact have been more widely distributed among hybodontiforms than previously thought, thus rendering the phylogenetic utility of separated pelvic half-girdles for inferring hybodontiform interrelationships difficult and unresolved.
Keywords: Chondrichthyes, Hybodontiformes, Taxonomy, Kimmeridge Clay Formation, Late Jurassic, Mesozoic, England
Introduction
Hybodontiformes, which forms a supposed extinct sister group to the elasmobranch crown comprising modern sharks, skates and rays (= Neoselachii sensu Compagno, 1973), represents a speciose clade of Palaeozoic to Mesozoic shark-like chondrichthyans characterized by distinct cranial and dental morphologies, and two dorsal fins supported by heavily ornamented spines exhibiting numerous retrorse denticles arranged along the posterior midline (Maisey, 1978, 1982; Maisey, Naylor & Ward, 2004; Ginter, Hampe & Duffin, 2010; Cappetta, 2012). In addition, a single or double pair of cephalic spines each with a trifid base carrying a prominent hook-shaped spine occurs in males on the skull posterior to the orbit (Maisey, 1982).
First appearing in the Late Devonian, hybodontiforms apparently reached their highest diversity during the Triassic and Jurassic where they flourished and expanded into various ecological niches, ranging from open marine to continental depositional environments (e.g., Rieppel, 1981; Duffin, 1997, 2010; Duffin & Thies, 1997; Rees & Underwood, 2006, 2008; Klug et al., 2010; Fischer et al., 2011; Leuzinger et al., 2015, 2017; Stumpf & Kriwet, 2019). By the Early Cretaceous, hybodontiforms had become almost entirely restricted to marginal marine and brackish water environments before they finally vanished at the end of the Cretaceous (Kriwet & Benton, 2004; Cuny, 2012).
As for elasmobranchs in general, hybodontiforms are characterized by a continuous, life-long tooth replacement resulting in a rich fossil record dominated by isolated teeth, which provide discrete combinations of morphological characters for use in species identification and establishing reliable diagnoses (e.g., Cuny et al., 2008; Cuny, Cavin & Suteethorn, 2009; Rees & Underwood, 2008; Underwood & Cumbaa, 2010; Koot et al., 2013, 2015; Rees et al., 2013; Leuzinger et al., 2017; Szabó & Főzy, 2020). Nevertheless, much uncertainty still surrounds the genus- and higher-level classification of many species, which resulted in the production of a series of different taxonomic and systematic schemes (e.g., Maisey, 1989; Rees, 2008; Cappetta, 2012). This is mainly because our knowledge of the taxonomy and systematics of hybodontiforms is strongly biased towards isolated teeth rather than those found associated with articulated or disarticulated skeletons, which otherwise remain extremely rare and limited to a few localities only, but commonly display important morphological features for inferring phylogenetic interrelationships (e.g., Maisey, 1982, 1983, 1986, 1987, 1989; Maisey, Naylor & Ward, 2004; Lane & Maisey, 2009, 2012; Stumpf et al., 2021). The incomplete nature of the hybodontiform skeletal fossil record consequently precludes deeper insights into their taxonomy and systematics in many cases, and therefore any new information about their skeletal morphology potentially increases our knowledge about their evolutionary history and ecological diversity.
Here, we describe a new hybodontidorm shark-like chondrichthyan, Durnonovariaodus maiseyi gen. et sp. nov., from the Upper Jurassic Kimmeridge Clay Formation of southern England based on a partial skeleton with teeth. This new taxon offers important insights into the morphological and taxonomic diversity, as well as the ecology of Mesozoic hybodontiforms and emphasizes the significance of the Jurassic as an important period in the evolutionary history of hybodontiforms before they witnessed a diversity decline and subsequent adaptation to brackish and freshwater environments from the Early Cretaceous onwards.
Material & Methods
Geological setting
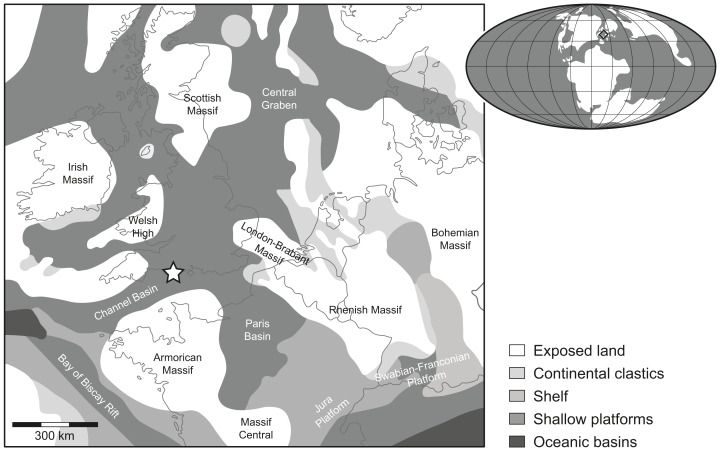
In England, Late Jurassic shallow epicontinental marine deposits referred to the Kimmeridge Clay Formation crop out at several localities aligned along a narrow, SW–NE trending strip connecting the coasts of Dorset and Yorkshire. In Dorset, the Kimmeridge Clay Formation consists of mudstones and organic-rich laminated shales with intercalated limestones spanning the Kimmeridgian and early Tithonian stages, and was deposited under calm environmental conditions with periods of anoxia (e.g., Hallam, 1987, 1992; Gallois, 2000). These beds are known to have produced a wide variety of fossil vertebrates, including bony and cartilaginous fishes (e.g., Dineley & Metcalf, 1999; Underwood, 2002; Cavin, Forey & Giersch, 2013; Underwood & Claeson, 2019), secondarily marine reptiles (e.g., Benson et al., 2013; Young, Steel & Middelton, 2014; Jacobs & Martill, 2020) and rare remains of pterosaurs and dinosaurs (Martill, Earland & Naish, 2006; Martill & Etches, 2013; O’Sullivan & Martill, 2015).
Material
The fossil chondrichthyan material described herein consists of an incomplete, disarticulated hybodontiform skeleton preserved on a slab of rock, which was collected by one of us (SE) from early Tithonian beds referred to the Pectinatites pectinatus ammonite zone accessible near Freshwater Steps, Encombe, Dorset (Fig. 1). The specimen is housed and curated in the Museum of Jurassic Marine Life (MJML) of Kimmeridge in Dorset, which was built to house the lifetime collection of one of us (SE) (see Noé, Gómez-Pérez & Nicholls, 2019), and was briefly described and referred to Planohybodus peterboroughensis Rees & Underwood, 2008 by Underwood (2020), together with additional specimens housed in the MJML, whose detailed descriptions will shed further light on the diversity of Kimmeridge Clay Formation hybodontiforms.
Figure 1. Location map.
Rough palaeogeographic reconstruction of the western Tethys during the early Tithonian (modified from Thierry et al., 2000) showing the type locality Durnonovariaodus maiseyi gen. et sp. nov. near Freshwater Steps, Encombe, Dorset, England (indicated by a star).
Methods
Photographs presented in the text were obtained by digital macro- and micro-photography using a Nikon D5300 DSLR camera with either an AF-S DX NIKKOR 18–140 mm f/3.5–5.6 G ED VR or an AF-S DX Micro NIKKOR 40 mm f/2.8G lens. All photographs were rendered utilizing the software package Adobe Photoshop CC 2018 and the accompanying figures were created using Adobe Illustrator CC 2018.
Descriptive terminologies used for the skeletal morphology correspond to those of Maisey (1982), and terminologies for the dental morphology largely follow that of Cappetta (2012).
Nomenclatural acts
The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Commission on Zoological Nomenclature (ICZN), and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is: urn:lsid:zoobank.org:pub:21199B53-9E93-44FF-987C-EDC115E8AA88. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central and CLOCKSS.
Results
Systematic palaeontology
Class CHONDRICHTHYES Huxley, 1880
Subclass ELASMOBRANCHII Bonaparte, 1838
Order HYBODONTIFORMES Maisey, 1975
Family HYBODONTIDAE Owen, 1846
DURNONOVARIAODUS gen. nov.
LSID: urn:lsid:zoobank.org:act:35DA49B9-B14E-4390-BEA3-9B99C9D9BAB0
Diagnosis: Hybodontiform shark-like chondrichthyan that is characterized by the following unique combination of morphological characters: palatoquadrate elongate with low and reduced palatobasal process and well-developed ethmoidal articular surface; Meckel’s cartilage elongate and rather deep with well-developed dental groove extending for approximately one-half its length of the Meckel’s cartilage; medial quadratomandibular joint on Meckel’s cartilage prominent and well-defined; articular cotylus on Meckel’s cartilage moderately well-developed and shallowly recessed; hyomandibular head formed into an anteriorly directed hook-like process; dorsal fin spines ornamented with strong, non-bifurcating costae; body covered by monodontode, thorn-like dermal denticles; dentition includes high-crowned multicuspid teeth that are symmetrical to slightly asymmetrical in labio-lingual view displaying disjunct monognathic heterodonty; tooth crown strongly labio-lingually flattened; main cusp high and fairly wide at its base without sigmoidal profile; main cusp flanked on each side by up to three pairs of low but well-developed lateral cusplets; cutting edges slightly labially displaced, continuous, and sharp without serrations; labial crown base is slightly incised above the crown-root junction and somewhat swollen; crown-root junction straight; lingual and labial crown faces ornamented with very short, inconspicuous vertical folds aligned along the base above the crown-root junction; tooth root prominent, about as high apico-basally as deep labio-lingually, and slightly lingually displaced beneath the tooth crown; basal root face flat with shallow depression extending along the labial edge; lingual and labial root face perforated by numerous small, densely arranged foramina and large, regularly arranged foramina that occur aligned along the bases; morphological variation passing posteriorly through the dentition encompasses distal inclination and reduction of principal cusp; anterior teeth symmetrical in labio-lingual view with moderately robust main cusp and divergent lateral cusplets; lateral teeth asymmetrical in labio-lingual aspect with wide, triangular-shaped and slightly distally inclined main cusp; posterior teeth asymmetrical and low in in labio-lingual view.
Etymology: The genus name is derived from Durnonovaria, the ancient name of the town of Dorchester from which the name Dorset derives, and the Greek noun odus (ὀδούς), meaning tooth.
Type species: Durnonovariaodus maiseyi sp. nov.
DURNONOVARIAODUS MAISEYI gen. et sp. nov.
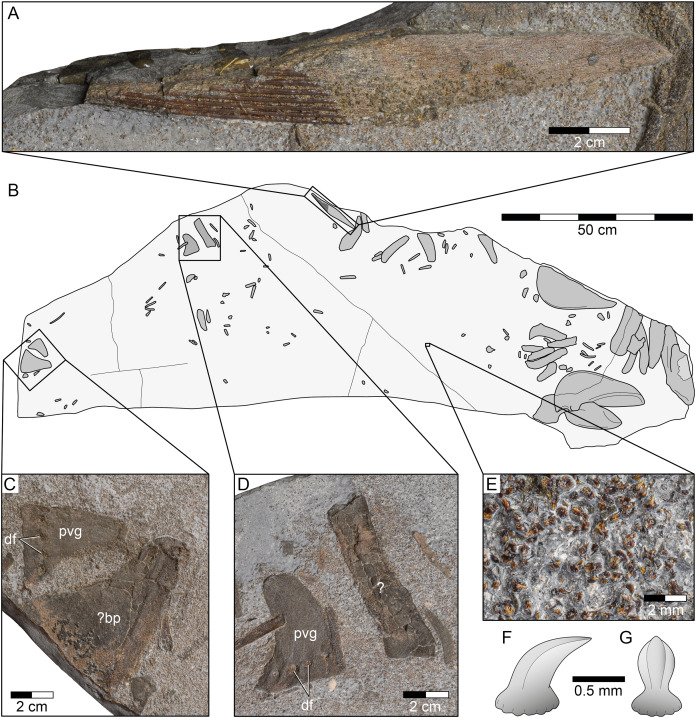
Figure 2. Durnonovariaodus maiseyi gen. et sp. nov., MJML K1624, holotype, from the Upper Jurassic Kimmeridge Clay Formation (early Tithonian) near Freshwater Steps, Encombe, Dorset, England.
(A) Slab containing specimen. (B) Interpretative line drawing (dashed box indicates splanchnocranial elements shown in Fig. 3).
Figure 6. Durnonovariaodus maiseyi gen. et sp. nov., MJML K1624, holotype, from the Upper Jurassic Kimmeridge Clay Formation (early Tithonian) near Freshwater Steps, Encombe, Dorset, England.
(A) Dorsal fin spine. (B) Interpretative line drawing of complete specimen. (C, D) Pelvic girdle. (E) Close-up view of dermal denticles. (F, G) Simplified sketch drawing of dermal denticle in (F) lateral and (G) anterior view. Anatomical abbreviations: bp, basal plate; df, diazonal nerve foramina; pvg, pelvic half-girdle.
2020 Planohybodus peterboroughensis Rees and Underwood; Underwood, text-fig. 2.3A.
LSID: urn:lsid:zoobank.org:act:2E9EAEC3-16FA-4304-A6FE-1123135A46BF
Diagnosis: As for genus (by monotypy).
Holotype: MJML K1624, a slab of rock preserving disarticulated elements of the splanchnocranium with associated teeth, numerous dermal denticles, a single fragmentary dorsal fin spine, and the pelvic girdle, plus abundant cartilage fragments of uncertain identity.
Type locality and horizon: Freshwater Steps, Encombe, Dorset, England; Upper Kimmeridge Clay Formation, Pectinatites pectinatus ammonite zone, early Tithonian, Late Jurassic.
Etymology: Species named in honour of John G. Maisey for his significant work on better understanding hybodontiform taxonomy and systematics and his contribution to the field of palaeoichthyology in general.
Description
The holotype and only specimen of Durnonovariaodus maiseyi gen. et sp. nov., MJML K1624, is preserved on a slab of rock of about 1,785 mm maximum length and 700 mm maximum width preserving disarticulated elements of the splanchnocranium with associated teeth, a single fragmentary dorsal fin spine, the pelvic girdle, as well as abundant cartilage fragments of uncertain identity, plus countless dermal denticles scattered all across the slab (Fig. 2). The endoskeletal remains are strongly compressed, but still show a certain degree of relief suitable for identifying morphological features. They are composed of well-mineralized, tessellated cartilage, which gives them a rough and scratchy surface texture. The scattered, but closely arranged skeletal elements support our interpretation that all belong to a single specimen.
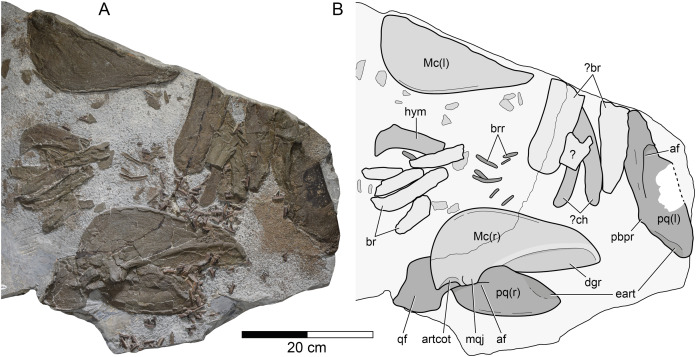
Splanchnocranium. The splanchnocranium is incomplete and highly disarticulated. It includes the mandibular arch as well as part of the hyoid arch and gill arches (Fig. 3).
Figure 3. Durnonovariaodus maiseyi gen. et sp. nov., MJML K1624, holotype, from the Upper Jurassic Kimmeridge Clay Formation (early Tithonian) near Freshwater Steps, Encombe, Dorset, England.
(A) Splanchnocranium; (B) Interpretative line drawing. Anatomical abbreviations: af, adductor fossa; artcot, articular cotylus; br, branchial element; brr, branchial rays; ch, ceratohyal; dgr, dental groove; eart, ethmoidal articulation; hym, hyomandibular; l, left (in parentheses); Mc, Meckel’s cartilage; mqj, medial quadratomandibular joint; pbpr, palatobasal process; pq, palatoquadrate; qf, quadrate flange; r, right (in parentheses).
The mandibular arch is disarticulated and includes the paired palatoquadrates and Meckel’s cartilages. The right palatoquadrate and the right Meckel’s cartilage are complete, while their left counterparts are incomplete.
The right palatoquadrate is visible in lateral aspect, measuring 257 mm in maximum length and 89 mm in height. The left palatoquadrate is less well-preserved and exposed in lateral view. It is incomplete in its most-posterior portion and along its ventral margin. The right Meckel’s cartilage is exposed in medial view, measuring 261 mm in maximum length and 125 mm in maximum height. Its left counterpart is visible in lateral aspect and lacks its dorsal portion.
The palatoquadrate of Durnonovariaodus maiseyi gen. et sp. nov. is elongate and rather massive. It can be roughly divided into an anterior palatine and a posterior quadrate portion. The latter is formed into a large, well-developed quadrate flange, which anteriorly gives rise to a prominent, well-defined ridge that bounds a deep adductor fossa dorsally. The dorsal margin of the palatoquadrate exhibits a low and reduced palatobasal process, which is located at about one-third the total palatoquadrate length from the anterior tip. The palatoquadrate is widely convex antero-dorsally and slopes slightly downwards towards its anterior tip. A large articulation surface for the ethmoid process of the neurocranium extends along the antero-dorsal margin of the palatoquadrate. The anterior tip of the palatoquadrate is bluntly pointed and the ventral margin of the anterior palatine portion of the palatoquadrate is widely convex and reinforced by a narrow, slightly elevated ridge.
The Meckel’s cartilage is elongate, rather deep posteriorly and tapers slightly towards its anterior tip, which is bluntly pointed rather than sharply tipped. Medially, there is a deep, well-developed dental groove, which extends approximately one-half the length of the Meckel’s cartilage. Ventrally, the dental groove is delimited by a prominent ridge. The dorsal margin of the Meckel’s cartilage is straight for the length of the accompanying dental groove until it forms a wide and low indentation that is delimited posteriorly by a large, well-defined medial quadratomandibular joint. The articular cotylus for the articulation with the palatoquadrate is moderately well-developed and shallowly recessed. A lateral quadratomandibular joint could not be observed. The postero-ventral margin of the Meckel’s cartilage is widely convex and merges smoothly into the ventral margin, which is straight for most of its length and reinforced by a narrow, slightly elevated ridge. Laterally, the Meckel’s cartilage bears a similarly developed ridge extending along its ventral margin. Labial cartilages could not be identified.
The hyoid arch and gill arches of Durnonovariaodus maiseyi gen. et sp. nov. are incomplete and very badly preserved. There is a single hyomandibular and two slender, slightly curved cartilages that are here tentatively identified as the ceratohyals, plus numerous well-calcified cartilage fragments of uncertain identity. The preserved hyomandibular is broken distally and could not be identified as either left or right. The proximal end of the hyomandibular, which articulates with the neurocranium, is formed into an anteriorly directed hook-like process.
Dentition. The holotype of Durnonovariaodus maiseyi gen. et sp. nov. comprises about 80 disarticulated teeth that are scattered on and around the right Meckel’s cartilage and right palatoquadrate (Fig. 4), suggesting that they derive from both the upper and lower dentition. Morphologically, the teeth can be differentiated into those coming from tooth files of anterior, lateral and posterior positions (see below), indicating a disjunct monognathic heterodonty. There is no indication for dignathic heterodonty in Durnonovariaodus maiseyi gen. et sp. nov., but this must be considered as tentative due to the incomplete and disarticulated nature of the holotype specimen, pending the discovery of more complete material.
Figure 4. Durnonovariaodus maiseyi gen. et sp. nov., MJML K1624, holotype, from the Upper Jurassic Kimmeridge Clay Formation (early Tithonian) near Freshwater Steps, Encombe, Dorset, England.
Overview of dentition (for anatomical abbreviations see caption to Fig. 3).
The dentition of Durnonovariaodus maiseyi gen. et sp. nov. encompasses relatively large, up to 18 mm wide and 12 mm high, symmetrical to slightly asymmetrical multicuspid teeth that are characterized by strongly labio-lingually flattened crowns displaying a high, fairly wide and pointed main cusp without a sigmoidal profile (Fig. 5). The main cusp is usually flanked by two to three pairs of low but well-developed lateral cusplets, which diminish in size away from the main cusp and reach up to one-third its height. The cutting edges are slightly labially displaced, sharp and continuous, extending from the principal cusp across all lateral cuplets. There are no serrations on the cutting edges. The labial crown base is slightly incised above the crown-root junction and somewhat swollen. The tooth crown ornamentation is reduced and comprises very short, inconspicuous vertical folds that occur on both the lingual and labial bases of the crown above the crown-root junction. The distribution of these vertical folds slightly differs on the lingual and labial faces, with those occurring on the lingual face occasionally being restricted to the bases below the lateral cusplets only, or may even be absent entirely. The crown-root junction is straight lingually and labially.
Figure 5. Durnonovariaodus maiseyi gen. et sp. nov., MJML K1624, holotype, from the Upper Jurassic Kimmeridge Clay Formation (early Tithonian) near Freshwater Steps, Encombe, Dorset, England.
Close-up view of teeth. (A–D) Anterior teeth in labial views. (E) Antero-lateral tooth in lingual view. (F) Lateral tooth in labial view. (G–I) Lateral teeth in lingual aspects (J) Postero-lateral teeth in labial views (K) Postero-lateral tooth in lingual view. (L, M) Extreme posterior teeth in (L) labial and (M) lingual aspect.
The tooth root is prominent, about as high apico-basally as deep labio-lingually, and slightly lingually displaced beneath the crown, forming a narrow, lingually sloping shelf. The basal root face is flat and bears a shallow depression that extends along the labial edge. The lingual and labial faces of the root are perforated by numerous tiny, densely arranged foramina, resulting in a somewhat trabecular appearance of the root. In addition, a series of larger, rather regularly arranged foramina occurs along both the lingual and labial base of the root.
The morphological variation that passes posteriorly through the dentition of Durnonovariaodus maiseyi gen. et sp. nov. mainly involves a distal inclination and reduction of the principal cusp. Teeth from anterior positions are symmetrical and display a moderately robust and erect principal cusp that is flanked by two pairs of low, slightly divergent lateral cusplets (Figs. 5A–5C). These are, as measured from the crown-root junction, up to one-half the height of the crown. In addition, a third pair of very small to incipient lateral cusplets may be developed (Fig. 5D).
Lateral teeth exhibit a fairly wide, triangular-shaped and slightly distally inclined main cusp, which has a more or less straight mesial but a slightly concave distal cutting edge (Figs. 5E–5I). The main cusp is usually flanked on each side by three pairs of lateral cusplets. These are up to one-half the height of the crown as in teeth of anterior positions.
Posterior teeth have a wide and very low profile. The main cusp is wide, particularly low and distally inclined and has a long, slightly undulating mesial cutting edge (Figs. 5J–5M). It is flanked on each side by two to three pairs of low, commonly reduced lateral cusplets.
Dorsal fin spine. The holotype of Durnonovariaodus maiseyi gen. et sp. nov. includes a single dorsal fin spine only (Figs. 6A, 6B). The fin spine is incomplete and exposed in left lateral view, lacking its distal portion. It is ornamented with strong, non-bifurcating costae. The unornamented fin spine base, which includes the deeply inserted posterior slot that received the cartilaginous basal plate of the dorsal fin, appears to have been rather long. No further information can be retrieved due to the poor state of preservation of the dorsal fin spine. There is a cartilage fragment of roughly triangular shape, which may represent a dorsal basal fin plate (Figs. 6B, 6C).
Pelvic girdle. The pelvic girdle of Durnonovariaodus maiseyi gen. et sp. nov. is represented by two separate pelvic half-girdles, both displaying a series of diazonal nerve foramina aligned along the distal margin (Figs. 6B–6D). There is an elongate, broken cartilage preserved in close proximity to one of the pelvic girdle halves (Figs. 6B, 6D), whose precise identity remains unknown due to preservation.
Dermal denticles. The holotype of Durnonovariaodus maiseyi gen. et sp. nov. encompasses countless very small, densely packed dermal denticles that occur all across the bedding plane (Fig. 6E). Morphologically, the dermal denticles correspond to the ‘non-growing’ (monodontode) type. They all have a thorn-like appearance, measuring less than 1 mm in maximum height, with a circular to oval base and an upright, slightly recurved cusp displaying a few strong vertical folds that extend from the apex to the base of the cusp (Figs. 6F, 6G). These folds usually merge apically to form a keel-like leading edge extending along the anterior face of the cusp.
Discussion
Comparison
In the following, detailed comparisons between Durnonovariaodus maiseyi gen. et sp. nov. and other hybodontiforms is drawn. While the first section focuses on comparing and contrasting the dentition of Durnonovariaodus maiseyi gen. et sp. nov. with that of other hybodontiforms, particularly those that are known to have developed teeth of similar morphologies, the second section addresses similarities and differences in skeletal anatomy between the new taxon and better known hybodontiforms. Note that the genus Asteracanthus Agassiz, 1837 is here considered distinct from Strophodus Agassiz, 1838, following Stumpf et al. (2021). In addition, the systematic position of Durnonovariaodus maiseyi gen. et sp. nov. within Hybodontiformes is discussed in the light of currently available hypotheses of their interrelationships.
Specimen MJML K1624, which is here designated as new genus and species, Durnonovariaodus maiseyi gen. et sp. nov., was briefly described by Underwood (2020), who referred it to Planohybodus peterboroughensis Rees & Underwood, 2008, which is known from rare dental and fragmentary skeletal material from the Callovian–Oxfordian of England. Teeth of P. peterboroughensis, although morphologically similar, are readily distinguished from those of Durnonovariaodus maiseyi gen. et sp. nov., by possessing fairly symmetrical, more strongly ornamented crowns with a higher and more slender central cusp, which is flanked by two or three pairs of lateral cusplets that are up to one-quarter the height of the of the central cusp. Furthermore, unlike in Durnonovariaodus maiseyi gen. et sp. nov., the dentition of P. peterboroughensis appears to have been characterized by a gradual rather than disjunct monognathic heterodonty, as expressed by variations in height and width of the main cusp and by a weak dignathic heterodonty, with teeth of the lower jaw being narrower and less heavily ornamented, exhibiting a rather gracile main cusp, which may be slightly mesio-distally expanded at mid-height, plus smaller, less well-developed lateral cusplets (Rees & Underwood, 2008).
The remaining species currently placed in Planohybodus are known from isolated teeth only and include P. grossiconus (Agassiz, 1833–1844) from the Bathonian of England, Scotland and France (Woodward, 1889; Rees & Underwood, 2006, 2008) and P. ensis (Woodward, 1916) from the Berriasian–Barremian of England and Spain (Patterson, 1966; Underwood & Rees, 2002; Bermúdez-Rochas, 2009; Duffin & Sweetman, 2011; Turmine-Juhel et al., 2019). Additionally, poorly preserved teeth from the Berriasian of Bornholm, Denmark, may present an as yet undescribed species of Planohybodus (Rees, 2001; Rees & Underwood, 2008).
The species P. marki Pinheiro et al., 2013 from the pre-Aptian Early Cretaceous of Brazil, which is represented by a few fragmentary tooth crowns, is here regarded as nomen dubium due to the poor state of preservation and the absence of any dental features that would unambiguously support its inclusion in the genus Planohybodus.
Teeth of P. grossiconus and P. ensis are very similar to those of P. peterboroughensis, which makes species identification of isolated tooth crowns difficult, particularly because dental characters for use in differentiation between these three species mainly relate to differences in main cusp proportions and the number of lateral cusplets, besides minor variations in ornamentation (Rees & Underwood, 2008). In addition, faint serrations may be developed on the cutting edges in larger teeth of P. ensis, unlike in P. peterboroughensis and P. grossiconus (Underwood & Rees, 2002; Bermúdez-Rochas, 2009) as well as Durnonovariaodus maiseyi gen. et sp. nov.
High-crowned, labio-lingually flattened dental morphologies with partly serrated cutting edges also characterize teeth of Secarodus, which was erected by Rees & Underwood (2008) for distinctive teeth from the Bathonian of England that were originally described as Hybodus polyprion by Agassiz (1843). However, teeth of Secarodus differ from those of Planohybodus ensis (and Planohybodus in general) in exhibiting lower crowns with a fairly wide, triangular-shaped main cusp and in possessing cutting edges characterized by more strongly developed serrations. In addition, the dentition of Secarodus is characterized by a disjunct monognathic heterodonty, with anterior teeth being almost symmetrical in profile, a condition clearly separating them from teeth of lateral and posterior positions. A quite similar heterodonty pattern characterizes the dentition of Durnonovariaodus gen. nov., although lateral teeth of the latter are less asymmetrical in profile as compared to those of Secarodus. The ornamentation is reduced in teeth of Secarodus and consists of short vertical folds along the base of the crown, resembling the condition in Durnonovariaodus gen. nov., which otherwise lacks a small, knob-like protuberance at the base of the labial crown face, a feature that has been found to occasionally occur in teeth of Secarodus. The main character separating teeth of Secarodus from those Durnonovariaodus gen. nov. is the presence of weak to moderately well-developed serrations occurring on the lower mesial cutting edges of the main cusp and cusplets.
The presence of high-crowned, strongly labio-lingually compressed multicuspid teeth with fully serrated cutting edges characterizes teeth of Priohybodus arambourgi d’Erasmo, 1960 from the Kimmeridgian–Hauterivian/Barremian of Africa, Yemen and Uruguay (Tabaste, 1963; Goodwin et al., 1999; Duffin, 2001; Cuny et al., 2004; Soto, Perea & Toriño, 2012). However, unlike in Secarodus, the dentition of Priohybodus is rather homodont to include close to symmetrical teeth with a prominent principal cusp and up to five pairs of strongly divergent lateral cusplets, suggesting a closer phylogenetic relationship with Planohybodus than with Secarodus and other hybodontiforms (Rees & Underwood, 2008; Soto, Perea & Toriño, 2012).
The remaining hybodontiforms that have developed teeth with fully serrated cutting edges are Pororhiza molimbaensis Casier, 1969 from the Albian of Congo, Thaiodus ruchae Cappetta, Buffetaut & Suteethorn, 1990 from the Aptian–Albian of Thailand, Tibet and China (Cappetta et al., 2006; Cuny et al., 2008; Mo et al., 2016), and Mukdahanodus trisivakulii Cuny, Cavin & Suteethorn, 2009 from the pre-Aptian Early Cretaceous of Thailand. In addition, Mukdahanodus is also represented by a possible second species from the Barremian–Aptian of Malaysia (Teng et al., 2019). All of these species are characterized by quite uniquely shaped teeth with distinctively low crowns. A main cusp is either absent in these species or it is very low and blunt.
Durnonovariaodus gen. nov. has costate dorsal fin spines, a condition shared with most other hybodontiforms (Maisey, 1978), except for Asteracanthus and Strophodus, which currently are the only known hybodontiforms for which dorsal fin spines with an ornamentation consisting of small to moderately well-developed, more or less regularly arranged tubercles can be unambiguously be confirmed (Stumpf et al., 2021), although this feature certainly was more widely distributed among hybodontiforms (cf. e.g., Werner, 1989; Case & Cappetta, 2004; Underwood & Cumbaa, 2010; Cicimurri, Ciampalgio & Runyon, 2014). Planohybodus, which shares with Durnonovariaodus gen. nov. the presence of costate dorsal fin spines, seems to be differentiated from the latter in having fin spines with proximally bifurcating costae, as inferred from fin spine material referred to the Planohybodus type species, P. peterboroughensis (Rees & Underwood, 2008). However, the phylogenetic significance of this difference in fin spine ornamentation needs to be tested.
The countless small thorn-like dermal denticles present in the holotype of Durnonovariaodus maiseyi gen. et sp. nov., which would certainly have covered the body in life, closely resemble those covering the body of Hamiltonichthys mapesi Maisey, 1989 and those occurring on top of the head of Egertonodus basanus (Egerton, 1845) and Hybodus delabechei Charlesworth, 1839 (Reif, 1978; Maisey, 1983, 1989; note that the generic identity of H. delabechei remains unresolved, see Rees, 1998; Maisch & Matzke, 2016). Dermal denticles of quite similar morphology cover the body of Hybodus fraasi Brown, 1900, a species tentatively referred to Egertonodus by Maisey (1987). Dermal denticles of this species, however, differ from those of the aforementioned taxa in having a larger base that is ovoid in outline carrying a rather low, more strongly compressed cusp that is formed into a blade-like keel (Maisey, 1986; Thies & Leidner, 2011). The shagreen covering the body of Tribodus limae Brito & Ferreira, 1989 is composed of similarly developed denticles, but also includes smaller denticles of different morphology, which co-occur intercalated between the larger ones, resulting in a unique two-size squamation pattern otherwise unknown in hybodontiforms (Maisey & Denton, 2016). Dermal denticles of Asteracanthus ornatissimus have a circular base carrying an upright, cone-like cusp that exhibits numerous vertical folds that radiate from the apex to the base of the cusp (Stumpf et al., 2021), resembling those covering the head of Planohybodus peterboroughensis (Rees & Underwood, 2008). In addition, similarly developed denticles have been observed in the snout region of Hamiltonichthys (Maisey, 1989). In ‘Hybodus’ delabechei, cone-like dermal denticles are present on both the lower jaws and on the roof of the mouth cavity, with the latter co-occurring with dermal denticles that correspond to the ‘growing’ (polydontode) type (Reif, 1978). Despite being absent in the holotype specimen of Durnonovariaodus maiseyi gen. et sp. nov., growing dermal denticles actually may have been present in life, because this type of dermal denticles is likely to have been restricted to the oropharyngeal region in at least some, if not all, hybodontiforms (Maisey & Denton, 2016).
Morphologically, the palatoquadrate of Durnonovariaodus gen. nov. closely resembles that of Egertonodus and Hybodus, particularly in having a distinct, antero-dorsally positioned articulation surface for the ethmoid process of the neurocranium and forming a palatobasal process that projects dorsally from the palatine moiety (Maisey, 1982, 1989). The jaw suspension of Durnonovariaodus gen. nov. is therefore likely to have been hyostylic (sensu Lane & Maisey, 2012). This type of jaw suspension may have also been present in Asteracanthus, whose palatoquadrates otherwise lack a palatobasal process and instead display a deeply recessed dorso-medial articulation facet, presumably for the articulation with the ectethmoid process of the neurocranium (Stumpf et al., 2021). Tribodus is unique among hybodontiforms in having a jaw suspension reminiscent of the euhyostylic condition present in modern batomorphs (Maisey & de Carvalho, 1997; Lane & Maisey, 2012). Its palatoquadrates are short, transversally oriented and connected symphyseally but not fused, lacking any direct articulation with the neurocranium, although there may have been ligamentous connections between the palatoquadrates and the neurocranium, probably homologous to the palatobasal articulation present in most other hybodontiforms (Lane & Maisey, 2012).
The Meckel’s cartilage of Durnonovariaodus gen. nov. is reminiscent of that of Hybodus, Egertonodus and Asteracanthus in displaying an elongate and comparably low profile (Maisey, 1982, 1983, 1987; Stumpf et al., 2021). This distinguishes it from other hybodontiforms like Palaeobates Meyer, 1849, Acrodus Agassiz, 1837 and Crassodus Maisch & Matzke, 2016, which have relatively massive and deep Meckel’s cartilages (Maisey, 1982; Romano & Brinkmann, 2010; Lane & Maisey, 2012; Maisch & Matzke, 2016). The antero-posterior dimension displayed by the dental groove, which extends about one-half the length of the Meckel’s cartilage, is similar to that observed in other hybodontiforms (Fraas, 1896; Maisey, 1983, 1987; Romano & Brinkmann, 2010; Lane & Maisey, 2012) and many other chondrichthyan outgroup taxa basal to hybodontiforms (e.g., Hotton, 1952; Coates & Sequeira, 2001; Long et al., 2015).
Durnonovariaodus gen. nov. shares with Hybodus and Egertonodus a hyomandibular with an elongate, slightly anteriorly directed proximal end (Maisey, 1982, 1983). This contrasts with Tribodus, in which the hyomandibular is rather short and more rod-like, lacking an enlarged proximal end (Lane & Maisey, 2012).
The holotype specimen of Durnonovariaodus gen. nov. preserves two separate pelvic half-girdles, a condition present in Tristychius Agassiz, 1837 (Dick, 1978) and more basal chondrichthyans (e.g., Zangerl & Case, 1976; Dick, 1981; Lund, 1985). In elasmobranchs, the paired halves of the pelvic girdle are fused to form a continuous puboischiadic bar, a feature considered by Compagno (1973, 1977) to be a synapomorphy separating them from more basal chondrichthyan outgroups. This view, however, was refuted by Maisey (1989) based on the presence of a continuous puboischiadic bar occurring in males of the apparently primitive hybodontiform Hamiltonichthys. A puboischiadic bar is otherwise absent in females of the same genus, which retain the plesiomorphic condition of separate pelvic half-girdles. Maisey (1989) regarded this intraspecific variation in pelvic girdle morphology as an evolutionary primitive character state separating Hamiltonichthys from more derived hybodontiforms, which he suggested to have developed independently of their sex a continuous puboischiadic bar. However, direct fossil support for this hypothesis is still lacking due to the limited number of sufficiently and well-preserved fossil material. In fact, currently available information on the pelvic girdle morphology of hybodontiforms other than Hamiltonichthys is limited to males of Lissodus and Hybodus only, more precisely to males of Lissodus cassangensis (Teixeira, 1956) from the Lower Triassic of Angola and Hybodus hauffianus Fraas, 1895 from the Lower Jurassic of Germany, which are traditionally accepted to have developed a puboischiadic bar (Brown, 1900; Maisey, 1982; Antunes et al., 1990). However, a re-investigation of the specimen on which the presence of a puboischiadic bar in males of Hybodus hauffianus was initially claimed (Fig. 7A), actually revealed the possession of two separate pelvic half-girdles, which are in part covered by an amorphous, light-brown phosphatic mass that presents preserved part of the stomach contents (see Fig. 7B; cf. Brown, 1900: pl. 16, fig. 1.; Maisey, 1982: text-fig. 13B). This feature is also shared by female individuals of H. hauffianus (Figs. 7C, 7D; see also Hauff et al., 2014: fig. 190), and may in fact have been more widely distributed among supposedly more advanced hybodontiforms than previously thought, as also suggested by a large indeterminate hybodontiform from the Lower Jurassic of Lyme Regis, England, preserving an incomplete clasper complex associated with a single half-girdle (Fig. 7E). In addition, according to Maisey (1982), males of Lissodus cassangensis possess a puboischiadic bar, although the presence of this feature cannot unambiguously be attested for either males or females of this species due to the poor preservation of the available material (Maisey, 1982; Antunes et al., 1990). In fact, as judged by the interpretative drawing of the pelvic fin and clasper complex of L. cassangensis provided by Maisey (1982: text-fig. 13A), males of this species actually appear to have developed two separate pelvic half-girdles, which Maisey (1982: 25) interpreted as a preservation artefact due to “superposition of one fin on the other”, but more complete material is needed to confirm or refute Maisey’s interpretation.
Figure 7. Separate pelvic half-girdles (pvg) in hybodontiforms.
(A, B) Male specimen of Hybodus hauffianus Fraas, 1895, SMNS 10060, from the Lower Jurassic Posidonienschiefer Formation of Holzmaden, Baden-Württemberg, Germany, in (A) total view and (B) close-up view of pelvic girdle. (C, D) Female specimen of H. hauffianus, SMNS 15150, from the Posidonienschiefer Formation of Holzmaden in (C) total view and (D) close-up view of pelvic girdle. (E) Hybodontiformes gen. et sp. indet., NHMUK PV P 339, from the Lower Jurassic of Lyme Regis, Dorset, England, showing pelvic girdle and associated clasper complex.
In summary, males of Hamiltonichthys are as yet the only known hybodontiforms for which the presence of a puboischiadic bar can unambiguously be confirmed. This contrasts with females of Hamiltonichthys, which together with both males and females of Hybodus retain the evolutionary primitive condition of two separate pelvic half-girdles otherwise present in chondrichthyan outgroups basal to Hybodontiformes. Whether the possession of two separate pelvic half-girdles is present in individuals of both sexes of Durnonovariaodus gen. nov. or related to sex-specific variation in pelvic girdle morphology as in Hamiltonichthys nevertheless remains impossible to determine without having more complete material.
Systematic affinities
Durnonovariaodus maiseyi gen. et sp. nov. is characterized by a unique combination of dental characters, indicating close architectural similarities to Secarodus polyprion, whose familial affinities still remain ambiguous and unresolved. When initially described, Secarodus was placed together with Planohybodus in Hybodontinae by Rees & Underwood (2008) based on the presence of high-crowned multicuspid teeth, following Maisey (1989) who included this subfamily together with Acrodontinae in the family Hybodontidae. The grouping proposed by Maisey (1989) is mainly based on the presence of an osteodont tooth histotype, which he considered as a derived feature among hybodontiforms. On the contrary, genera with low-crowned teeth possessing the orthodont tooth histotype were regarded by Maisey (1989) to form an assemblage of phylogenetically plesiomorphic hybodontiforms, except for the supposed durophagous genus Palaeobates, which he tentatively referred to Hybodontidae, particularly due to the presence of cephalic spines with a T-shaped basal plate as well as some cranial features shared with species included in Hybodontinae and Acrodontinae. Although generally accepted, this classification scheme still remains open to question, mainly because tooth histology patterns in hybodontiforms are more heterogenous and diverse than previously thought (e.g., Rees, 2001; Blażekowski, 2004; Stumpf et al., 2021), which makes a conclusive assessment of hybodontiform interrelationships as inferred from tooth histologies impossible based on the current data available, pending further research.
Rees (2008) proposed a phylogenetic hypothesis that placed Secarodus together with Priohybodus and Planohybodus in an unnamed hybodontid subfamily (informally referred to as “priohybodontines” by Soto, Perea & Toriño, 2012) based on the shared presence of strongly labio-lingually compressed, high-crowned multicuspid teeth with serrated cutting edges to form the sister group to Hybodontinae comprising Hybodus and Egertonodus, which both share high-crowned multicuspid grasping teeth with a slender main cusp that is close to circular in cross-section. However, the monophyletic grouping of Secarodus, Priohybodus and Planohybodus proposed by Rees (2008) may not be a natural one, because teeth of Planohybodus are in fact rather more similar to those of Egertonodus and Hybodus than to those of Secarodus and Priohybodus (Bermúdez-Rochas, 2009; Duffin & Sweetman, 2011; Turmine-Juhel et al., 2019), except for the presence of serrated cutting edges, a feature that is otherwise known to occur only rarely in teeth of Planohybodus. Further differences that may argue against the phylogenetic ties proposed by Rees (2008) relate to differences in heterodonty. In fact, the high degree of overlap in both dental morphology and heterodonty between Secarodus and Durnonovariaodus gen. nov. may suggest that both genera have formed a discrete monophyletic group of Mesozoic hybodontiforms characterized by uniquely shaped high-crowned multicuspid teeth. In consequence, one could argue that the dental traits shared by Secarodus and Durnonovariaodus gen. nov. are sufficient enough to justify the introduction of a new hybodontid subfamily for these two genera. However, dental morphology alone may not necessarily mirror evolutionary relationships of hybodontiforms, because some of them may have convergently evolved similar dentitions, as inferred from puzzling skeletal characteristics displayed by Durnonovariaodus gen. nov. and other apparently closely related hybodontiforms. For instance, Durnonovariaodus gen. nov. shares with Hybodus and Egertonodus a palatoquadrate with a palatobasal process and an ethmoidal articular surface, contrasting with Asteracanthus, which lacks a palatobasal process, but otherwise has teeth reminiscent of Hybodus and Egertonodus (Stumpf et al., 2021). Dorsal fin spines of Asteracanthus are ornamented with tubercles as opposed to costae present in fin spines of Durnonovariaodus gen. nov., Hybodus, Egertonodus and Planohybodus. In addition, cephalic spines of Asteracanthus have a uniquely shaped basal plate forming a robust posterior lobe and short lateral lobes. This differs to Hybodus, Egertonodus, and Planohybodus, which possess cephalic spines with a less robust, somewhat T-shaped basal plate (Maisey, 1983, 1987; Duffin, 1997; Rees & Underwood, 2008). On the other hand, Egertonodus has a single pair of cephalic spines (Maisey, 1983), while Hybodus, Planohybodus and Asteracanthus have a double pair of cephalic spines (Maisey, 1987; Rees & Underwood, 2008; Stumpf et al., 2021). The phylogenetic importance of all these differences, however, still remains unclear and needs to be tested. Likewise, much uncertainty still surrounds the variation in pelvic girdle morphology among hybodontiforms, pending the discovery of more complete skeletal material.
Consequently, given all these inconsistencies, we tentatively recommend referring Durnonovariaodus gen. nov. to Hybodontidae, particularly due to the close skeletal similarities shared with Hybodus and Egertonodus. However, further research is needed, pending a detailed re-evaluation of hybodontiform tooth mineralization patterns, combined with a subsequent phylogenetic analysis utilizing robust cladistic principles. However, resolving these issues is beyond the scope of the present study and will be published elsewhere.
Paleoecology
Durnonovariaodus gen. nov. was certainly among the larger hybodontiforms, probably reaching a maximum length of up two meters, as inferred from size comparisons with taxa for which more complete skeletal material is available (cf. Fig. 7A; see also Koken, 1907; Urlichs, Wild & Ziegler, 1994; Stumpf et al., 2021), thus making it one of the largest chondrichthyans to have ever roamed the Jurassic seas.
The Jurassic represents an important period in the evolutionary history of chondrichthyans, because it was the time when crown group elasmobranchs comprising sharks, skates and rays underwent their first major radiations, resulting in profound faunal turnover events among chondrichthyan communities (Underwood, 2006; Kriwet, Kiesling & Klug, 2009; Guinot & Cavin, 2016, 2020; Stumpf & Kriwet, 2019). By the Late Jurassic, crown group elasmobranchs had become taxonomically diverse and geographically widespread forming the most dominant chondrichthyan group (e.g., Underwood, 2002; Kriwet & Klug, 2004, 2008; Szabó, 2020), suggesting an increasing risk of niche overlap with hybodontiforms. However, despite the apparently high competition potential with their more advanced chondrichthyan counterparts, Late Jurassic hybodontiforms may have avoided direct competition by exploiting different food resources, as suggested by differences in body size. While sharks, rays and skates rarely reached a body size of two meters in maximum length (Kriwet & Klug, 2004, 2015; see also Pimiento et al., 2019), some hybodontiforms easily exceeded their phylogenetically more derived relatives reaching an estimated maximum body size length of up to three meters, in particular those that are known to have predominantly inhabited open marine environments, such as Asteracanthus, Planohybodus and Strophodus (e.g., Underwood, 2002; Leuzinger et al., 2015, 2017; Citton et al., 2019; Szabó & Főzy, 2020; Stumpf et al., 2021). By contrast, the rather limited facies distribution of small-bodied hybodontiforms suggests that they predominantly inhabited marginal marine environments with reduced or fluctuating salinities (e.g., Duffin & Thies, 1997; Kriwet, 2004; Vullo et al., 2014).
Unlike most similarly sized hybodontiforms, Durnonovariaodus maiseyi gen. et sp. nov. appears to have predominantly inhabited deeper-water environments, possibly along the outer continental shelves and upper continental slopes, as also suggested for the rare, dentally similar species Secarodus polyprion from the Bathonian of England (Rees & Underwood, 2008). Both species may have occasionally moved to more shallow water environments for feeding, similar to modern hexanchiform sharks, which are generally bound to deep-water environments, but sometimes also occur in inshore continental waters (Ebert, Fowler & Compagno, 2013; see also Priede & Froese, 2013).
Hybodontiforms reported from the Late Jurassic Kimmeridge Clay Formation, aside from the holotype of Durnonovariaodus maiseyi gen. et sp. nov., are represented by teeth, isolated cephalic and dorsal fin spines, as well as partial skeletons attributable to different, predominantly large-bodied taxa (Woodward, 1889; Underwood, 2002, 2020). The precise systematic and taxonomic classification of these hybodontiforms, however, still remain problematic and unresolved in many cases, particularly given recently published efforts to better understand the diversity of Mesozoic hybodontiforms (e.g., Rees et al., 2013; Leuzinger et al., 2017; Szabó & Főzy, 2020; Stumpf et al., 2021), pending further research. According to current available data (Underwood, 2002, 2020; Stumpf et al., 2021; this study), hybodontiforms from the Kimmeridge Clay Formation include five large-bodied genera, comprising Durnonovariaodus gen. nov., Planohybodus, Asteracanthus, Strophodus, and Meristodonoides. The latter is represented by new, as yet unnamed species (Underwood, 2020) that extends the stratigraphic range of Meristodonoides, which was initially recognized in the Cretaceous (Underwood & Cumbaa, 2010), back to the Late Jurassic (see also Leuzinger et al., 2017). In addition, there is also a small-bodied hybodontiform, which is assigned by Underwood (2020) to Hybodus lusitanicus Kriwet, 2004, a species otherwise considered consistent with referral to the genus Parvodus Rees & Underwood, 2002 (Rees et al., 2013).
The genus Planohybodus, which formed a common and widely distributed constituent of Mesozoic marine ecosystems, apparently ranging from the Middle Jurassic to the Late Cretaceous (e.g., Rees & Underwood, 2008; Bermúdez-Rochas, 2009; Bourdon et al., 2011; Duffin & Sweetman, 2011; Alvarado-Ortega et al., 2014), was certainly among the most common hybodontiforms encountered in the Kimmeridge Clay Formation, as inferred from abundant but in many cases poorly preserved tooth crowns, which commonly co-occur with crowns of Meristodonoides (Underwood, 2020; SS pers. obs.). Although incomplete, these teeth can readily be distinguished from those of Durnonovariaodus gen. nov. by differences in tooth cusp morphology and ornamentation (Underwood & Cumbaa, 2010). Although tooth morphologies alone do not necessarily mirror feeding behaviours in chondrichthyans, those displayed by Planohybodus and Meristodonoides suggests that both taxa were adapted towards clutching and tearing rather than cutting prey. The disjunct monognathic heterodonty displayed by Durnonovariaodus gen. nov. suggests that the symmetrical, more gracile anterior teeth were probably used for capturing and handling prey, while the asymmetrical teeth from lateral and posterior positions more likely performed a cutting function. Therefore, based on the current available data, it seems likely that within the European Late Jurassic marine communities Durnonovariaodus gen. nov. has occupied an ecological niche distinct from that of other morphologically and presumably ecologically similar hybodontiforms, probably in order to avoid risk of direct competition.
Conclusions
Hybodontiforms have an extensive fossil record elucidating a speciose clade of Palaeozoic to Mesozoic shark-like chondrichthyans that have developed diverse dental adaptations in relation to prey and feeding. However, even after almost two centuries of research, the taxonomy and systematics of hybodontiforms still remain poorly understood. This is mainly due to the scarcity of well-preserved skeletal material, which commonly provide important morphological features for inferring phylogenetic interrelationships.
The Etches Collection, which is now housed and curated in the Museum of Jurassic Marine Life of Kimmeridge, England, contains well-preserved but largely unstudied hybodontiform skeletal material from the Upper Jurassic Kimmeridge Clay Formation of southern England, including a partial skeleton of a comparably large-bodied hybodontiform, which is here described and designated as a new genus and species, Durnonovariaodus maiseyi gen. et sp. nov., and which significantly adds to our limited understanding of the diversity, ecology and distribution of Late Jurassic hybodontiforms.
The holotype and only known specimen of Durnonovariaodus maiseyi gen. et sp. nov., which could not be assigned to a particular gender given its incomplete preservation, shows a puzzling combination of dental and skeletal characters. Although skeletally similar to the better-known genera Hybodus and Egertonodus, which are traditionally referred to the family Hybodontidae due to close dental and skeletal similarities, teeth of Durnonovariaodus gen. nov. exhibit a unique combination of morphological characters reminiscent of Secarodus, which was erected to include distinctive, strongly labio-lingually compressed multicuspid cutting teeth from the Bathonian of England originally described as Hybodus polyprion. Skeletally, Durnonovariaodus gen. nov. resembles Hybodus and Egertonodus in possessing a palatoquadrate with a palatobasal process and an ethmoidal articular surface and in having costate dorsal fin spines. These features, however, contrast with Asteracanthus, whose teeth are otherwise rather more similar to those of Hybodus and Egertonodus than to those of Durnonovariaodus gen. nov. and Secarodus, rendering the perception of currently available phylogenetic hypotheses of hybodontiforms difficult and unresolved, which consequently led us to tentatively refer Durnonovariaodus gen. nov. to Hybodontidae.
The holotype of Durnonovariaodus maiseyi gen. et sp. nov. preserves two unfused pelvic half-girdles, a feature that has previously been considered as evolutionary primitive among hybodontiforms. However, unlike previously described, separate pelvic half-girdles also occur in the supposedly closely related species Hybodus hauffianus and may in fact have been more widely distributed among hybodontiforms than previously thought, thus rendering the phylogenetic utility of unfused pelvic half-girdles for inferring hybodontiform interrelationships difficult and unresolved.
All these discrepancies can only be countered by conducting more comprehensive comparative studies focusing on hybodontiform species that are presented by dental and skeletal material, combined with a subsequent phylogenetic analysis utilizing robust cladistic principles. These future studies will focus not only on new, yet largely unstudied hybodontiform skeletons from the Late Jurassic Kimmeridge Clay Formation, but also on historically collected skeletons from the Early Jurassic Posidonienschiefer Formation of Germany such as those referred to Hybodus hauffianus, which are in urgent need of re-investigation.
Acknowledgments
We are grateful to Emma Bernard (NHMUK) and Erin Maxwell (SMNS) for granting access to the collections under their care. We thank Christopher Duffin and Gilles Cuny for helpful comments that improved the manuscript.
Institutional abbreviations
- MJML
Museum of Jurassic Marine Life, Kimmeridge, Dorset, England
- NHMUK
Natural History Museum, London, England
- SMNS
Staatliches Museum für Naturkunde Stuttgart, Germany
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Sebastian Stumpf conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Steve Etches analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Charlie J. Underwood analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Jürgen Kriwet analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
Specimen shown in Figs. 2–6 is housed in the Museum of Jurassic Marine Life, England, and catalogued under MJML K1624.
Specimens shown in Figs. 7A–7D are stored in the Staatliches Museum für Naturkunde Stuttgart, Germany, and catalogued under SMNS 10060 and SMNS 15150, respectively.
Specimen shown in Fig. 7E is housed in the Natural History Museum, London, England, and catalogued under NHMUK PV P 339.
New Species Registration
The following information was supplied regarding the registration of a newly described species:
Publication LSID: urn:lsid:zoobank.org:pub:21199B53-9E93-44FF-987C-EDC115E8AA88.
Durnonovariaodus gen. nov. LSID: urn:lsid:zoobank.org:act:35DA49B9-B14E-4390-BEA3-9B99C9D9BAB0.
Durnonovariaodus maiseyi gen. et sp. nov. LSID: urn:lsid:zoobank.org:act:2E9EAEC3-16FA-4304-A6FE-1123135A46BF.
References
- Agassiz (1833–1844).Agassiz LJR. Recherches sur les poissons fossiles, 5 vols. Imprimerie de Neuchâtel: Petitpierre; 1833–1844. p. 1420. [Google Scholar]
- Alvarado-Ortega et al. (2014).Alvarado-Ortega J, Barrientos-Lara JI, Espinosa-Arrubarrena L, Melgarejo-Damián MDP. Late Jurassic marine vertebrates from Tlaxiaco, Oaxaca State, southern Mexico. Paleontologica Electronica. 2014;17:1–25. doi: 10.26879/454. [DOI] [Google Scholar]
- Antunes et al. (1990).Antunes MT, Maisey JG, Marques MM, Schaeffer B, Thomson KS. Triassic fishes from the Cassange depression (RP. de Angola) Ciências da Terra (UNL) 1990;1:1–16. [Google Scholar]
- Benson et al. (2013).Benson RBJ, Evans M, Smith AS, Sassoon J, Moore-Faye S, Ketchum HF, Forrest R. A giant pliosaurid skull from the Late Jurassic of England. PLOS ONE. 2013;8(5):e65989. doi: 10.1371/journal.pone.0065989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermúdez-Rochas (2009).Bermúdez-Rochas DD. New hybodont shark assemblage from the Early Cretaceous of the Basque-Cantabrian Basin. Geobios. 2009;42(6):675–686. doi: 10.1016/j.geobios.2009.06.004. [DOI] [Google Scholar]
- Blażekowski (2004).Blażekowski B. Shark teeth from the Lower Triassic of Spitsbergen and their histology. Polish Polar Research. 2004;25(2):153–167. [Google Scholar]
- Bonaparte (1838).Bonaparte CL. Synopsis vertebratorum systematis. Nuovi Annali delle Scienze Naturali Bologna. 1838;2:105–133. [Google Scholar]
- Bourdon et al. (2011).Bourdon J, Wright K, Lucas SG, Spielmann JA, Pence R. Selachians from the Upper Cretaceous (Santonian) Hosta Tongue of the Point Lookout Sandstone, central New Mexico. New Mexico Museum of Natural History and Science Bulletin. 2011;52:1–54. [Google Scholar]
- Brito & Ferreira (1989).Brito PM, Ferreira PLN. The first hybodont shark, Tribodus limae n.g., n.sp., from the Lower Cretaceous of Chapada do Araripe (North-East Brazil) Anais da Academia Brasileira de Ciências. 1989;61(1):53–57. [Google Scholar]
- Brown (1900).Brown C. Ueber das Genus Hybodus und seine systematische Stellung. Palaeontographica. 1900;46:149–174. [Google Scholar]
- Cappetta (2012).Cappetta H. Chondrichthyes. Mesozoic and Cenozoic Elasmobranchii: teeth. Handbook of Paleoichthyology 3E; Munich: Verlag Dr. Friedrich Pfeil; 2012. p. 512. [Google Scholar]
- Cappetta, Buffetaut & Suteethorn (1990).Cappetta H, Buffetaut E, Suteethorn V. A new hybodont from the Lower Cretaceous of Thailand. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte. 1990;1990(11):659–666. doi: 10.1127/njgpm/1990/1990/659. [DOI] [Google Scholar]
- Cappetta et al. (2006).Cappetta H, Buffetaut E, Cuny G, Suteethorn V. A new elasmobranch assemblage from the Lower Cretaceous of Thailand. Palaeontology. 2006;49(3):547–555. doi: 10.1111/j.1475-4983.2006.00555.x. [DOI] [Google Scholar]
- Case & Cappetta (2004).Case GR, Cappetta H. Additions to the elasmobranch fauna from the late Cretaceous of New Jersey (lower Navesink Formation, early Maastrichtian) Palaeovertebrata. 2004;33:1–16. [Google Scholar]
- Casier (1969).Casier E. Addenda aux connaissances sur la faune ichthyologique de la série de Bokungu (Congo) Annales du Musée Royal de l’Afrique Centrale, Sciences Géologiques. 1969;62:1–22. [Google Scholar]
- Cavin, Forey & Giersch (2013).Cavin L, Forey PL, Giersch S. Osteology of Eubiodectes libanicus (Pictet & Humbert, 1866) and some other ichthyodectiformes (Teleostei): phylogenetic implications. Journal of Systematic Palaeontology. 2013;11(2):115–177. doi: 10.1080/14772019.2012.691559. [DOI] [Google Scholar]
- Charlesworth (1839).Charlesworth E. Illustrated zoological notes. On the remains of a species of Hybodus from Lyme Regis. Magazine of Natural History, New Series. 1839;3:242–248. [Google Scholar]
- Cicimurri, Ciampalgio & Runyon (2014).Cicimurri DJ, Ciampalgio CN, Runyon KE. Late Cretaceous elasmobranchs from the Eutaw Formation at Luxapalila Creek, Lowndes County, Mississippi. PalArch’s Journal of Vertebrate Palaeontology. 2014;11(2):1–36. [Google Scholar]
- Citton et al. (2019).Citton P, Fabbi S, Cipriani A, Jansen M, Romano M. Hybodont dentition from the Upper Jurassic of Monte Nerone Pelagic Carbonate Platform (Umbria-Marche Apennine, Italy) and its ecological implications. Geological Journal. 2019;54(1):278–290. doi: 10.1002/gj.3174. [DOI] [Google Scholar]
- Coates & Sequeira (2001).Coates MI, Sequeira SEK. A new stethacanthid chondrichthyan from the Lower Carboniferous of Bearsden, Scotland. Journal of Vertebrate Paleontology. 2001;21(3):438–459. doi: 10.1671/0272-4634(2001)021. [DOI] [Google Scholar]
- Compagno (1973).Compagno LJV. Interrelationships of living elasmobranchs. Zoological Journal of the Linnean Society. 1973;53:15–61. [Google Scholar]
- Compagno (1977).Compagno LJV. Phyletic relationships of living sharks and rays. American Zoologist. 1977;17:303–322. [Google Scholar]
- Cuny (2012).Cuny G. Freshwater hybodont sharks in Early Cretaceous ecosystems: a review. In: Godefroit P, editor. Bernissart Dinosaurs and Early Cretaceous Terrestrial Ecosystems. Bloomington: Indiana University Press; 2012. pp. 518–529. [Google Scholar]
- Cuny et al. (2004).Cuny G, Ouaja M, Srarfi D, Schmitz L, Buffetaut E, Benton MJ. Fossil sharks from the Early Cretaceous of Tunisia. Revue de Paléobiologie. 2004;9:127–142. [Google Scholar]
- Cuny et al. (2008).Cuny G, Suteethorn V, Kamha S, Buffetaut E. Hybodont sharks from the Lower Cretaceous Khok Kruat Formation of Thailand, and hybodont diversity during the Early Cretaceous. In: Cavin L, Longbottom A, Richter M, editors. Fishes and the Break-up of Pangaea. Vol. 295. London: The Geological Society, Special Publications; 2008. pp. 93–107. [Google Scholar]
- Cuny, Cavin & Suteethorn (2009).Cuny G, Cavin L, Suteethorn V. A new hybodont with a cutting dentition from the Lower Cretaceous of Thailand. Cretaceous Research. 2009;30(3):515–520. doi: 10.1016/j.cretres.2008.09.003. [DOI] [Google Scholar]
- Dick (1978).Dick JRF. On the Carboniferous shark Tristychius arcuatus Agassiz from Scotland. Transactions of the Royal Society of Edinburgh. 1978;70(4):63–109. doi: 10.1017/S0080456800012898. [DOI] [Google Scholar]
- Dick (1981).Dick JRF. Diplodoselache woodi gen. et sp. nov., an early Carboniferous shark from the Midland Valley of Scotland. Transactions of the Royal Society of Edinburgh. 1981;72(2):99–113. doi: 10.1017/S0263593300009937. [DOI] [Google Scholar]
- Dineley & Metcalf (1999).Dineley DL, Metcalf SJ. Fossil fishes of Great Britain. London: Joint Nature Conservation Committee; 1999. p. 675. [Google Scholar]
- Duffin (1997).Duffin CJ. The dentition of Hybodus hauffianus Fraas, 1895 (Toarcian, Early Jurassic) Stuttgarter Beiträge zur Naturkunde, Serie B. 1997;256:1–20. [Google Scholar]
- Duffin (2001).Duffin CJ. The hybodont shark, Priohybodus d’Erasmo, 1960 (Early Cretaceous, northern Africa) Zoological Journal of the Linnean Society. 2001;133(3):303–308. doi: 10.1006/zjls.2000.0274. [DOI] [Google Scholar]
- Duffin (2010).Duffin CJ. Fishes. In: Lord AR, Davis PG, editors. Fossils from the Lower Lias of the Dorset Coast. Vol. 13. London: Palaeontological Association, Field Guide to Fossils 13; 2010. pp. 317–340. [Google Scholar]
- Duffin & Thies (1997).Duffin CJ, Thies D. Hybodont shark teeth from the Kimmeridgian (Late Jurassic) of northwest Germany. Geologica et Palaeontologica. 1997;31:235–256. [Google Scholar]
- Duffin & Sweetman (2011).Duffin CJ, Sweetman SC. 17. Sharks. In: Batten DJ, editor. Field Guide to Fossils. Vol. 14. London: Palaeontological Association; 2011. pp. 205–224. [Google Scholar]
- d’Erasmo (1960).d’Erasmo G. Nuovi avanci ittiolitici della ‘‘Serie di Lugh’’ in Somalia conservatori nel Museo Geologico di Firenze. Palaeontographica Italica. 1960;55:1–23. [Google Scholar]
- Ebert, Fowler & Compagno (2013).Ebert DA, Fowler S, Compagno L. Sharks of the world: a fully illustrated guide. Plymouth: Wild Nature Press; 2013. p. 528. [Google Scholar]
- Egerton (1845).Egerton PMG. Description of the Mouth of a Hybodus found by Mr. Boscawen Ibbetson in the Isle of Wight. Quarterly Journal of the Geological Society. 1845;1(1):197–199. doi: 10.1144/GSL.JGS.1845.001.01.51. [DOI] [Google Scholar]
- Fischer et al. (2011).Fischer J, Voigt S, Schneider JW, Buchwitz M, Voigt S. A selachian freshwater fauna from the Triassic of Kyrgyzstan and its implications for Mesozoic shark nurseries. Journal of Vertebrate Paleontology. 2011;31(5):937–953. doi: 10.1080/02724634.2011.601729. [DOI] [Google Scholar]
- Fraas (1895).Fraas E. Ein Fund von Skeletresten von Hybodus (Hybodus Hauffianus E. Fraas) Jahresbericht und Mitteilungen des Oberrheinischen Geologischen Vereins. 1895;28:24–26. [Google Scholar]
- Fraas (1896).Fraas E. Neue Selachier-Reste aus dem oberen Lias von Holzmaden in Württemberg. Jahreshefte des Vereins für vaterländische Naturkunde in Württemberg. 1896;52:1–25. [Google Scholar]
- Gallois (2000).Gallois RW. The stratigraphy of the Kimmeridge Clay Formation (Upper Jurassic) in the RGGE boreholes at Swanworth Quarry and Metherhills, south Dorset. Proceedings of the Geologists’ Association. 2000;111(3):265–280. doi: 10.1016/S0016-7878(00)80019-X. [DOI] [Google Scholar]
- Ginter, Hampe & Duffin (2010).Ginter M, Hampe O, Duffin CJ. Paleozoic Elasmobranchii: Teeth. Handbook of Paleoichthyology 3D. Munich: Verlag Dr. Friedrich Pfeil; 2010. Chondrichthyes; p. 168. [Google Scholar]
- Goodwin et al. (1999).Goodwin MB, Clemens WA, Hutchison HJ, Wood CB, Zavada MS, Kemp A, Duffin CJ, Schaff CR. First Mesozoic terrestrial vertebrates with associated palynostratigraphic dates from the northwestern Ethiopian Plateau. Journal of Vertebrate Paleontology. 1999;19(4):728–741. doi: 10.1080/02724634.1999.10011185. [DOI] [Google Scholar]
- Guinot & Cavin (2016).Guinot G, Cavin L. ‘Fish’ (Actinopterygii and Elasmobranchii) diversification patterns through deep time. Biological Reviews. 2016;91(4):950–981. doi: 10.1111/brv.12203. [DOI] [PubMed] [Google Scholar]
- Guinot & Cavin (2020).Guinot G, Cavin L. Distinct responses of elasmobranchs and ray-finned fishes to long-term global change. Frontiers in Ecology and Evolution. 2020;7:513. doi: 10.3389/fevo.2019.00513. [DOI] [Google Scholar]
- Hallam (1987).Hallam A. Mesozoic marine organic-rich shales. Geological Society, London, Special Publications. 1987;26(1):251–261. doi: 10.1144/GSL.SP.1987.026.01.17. [DOI] [Google Scholar]
- Hallam (1992).Hallam A. Jurassic. In: Curry D, Duff PMcLD, Smith AJ, editors. Geology of England and Wales. London: Geological Society; 1992. pp. 325–354. [Google Scholar]
- Hauff et al. (2014).Hauff RB, Hochsprung U, Ilger J-M, Joger U, Klopschar M, Kosma R, Krüger FJ, Thies D, Zellmer H. Jurameer. Niedersachsen versunkene Urwelt. Munich: Verlag Dr. Friedrich Pfeil; 2014. p. 96. [Google Scholar]
- Hotton (1952).Hotton N. Jaws and teeth of American xenacanth sharks. Journal of Paleontology. 1952;26(3):489–500. [Google Scholar]
- Huxley (1880).Huxley TH. On the application of the laws of evolution to the arrangement of the Vertebrata and more particularly of the Mammalia. Proceedings of the Zoological Society of London. 1880;43:649–662. [Google Scholar]
- Jacobs & Martill (2020).Jacobs ML, Martill DM. A new ophthalmosaurid ichthyosaur from the Upper Jurassic (Early Tithonian) Kimmeridge Clay of Dorset, UK, with implications for Late Jurassic ichthyosaur diversity. PLOS ONE. 2020;15(12):e0241700. doi: 10.1371/journal.pone.0241700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug et al. (2010).Klug S, Tütken T, Wings O, Pfretzschner H-U, Martin T. A Late Jurassic freshwater shark assemblage (Chondrichthyes, Hybodontiformes) from the southern Junggar Basin, Xinjiang, Northwest China. Palaeobiodiversity & Palaeoenvironments. 2010;90(3):241–257. doi: 10.1007/s12549-010-0032-2. [DOI] [Google Scholar]
- Koken (1907).Koken E. Ueber Hybodus. Geologische und Paläontologische Abhandlungen. 1907;5:261–276. [Google Scholar]
- Koot et al. (2013).Koot MB, Cuny G, Tintori A, Twitchett RJ. A new diverse shark fauna from the Wordian (Middle Permian) Khuff Formation in the interior Haushi-Huqf area, Sultanate of Oman. Palaeontology. 2013;56(2):303–343. doi: 10.1111/j.1475-4983.2012.01199.x. [DOI] [Google Scholar]
- Koot et al. (2015).Koot MB, Cuny G, Orchard MJ, Richoz S, Hart MB, Twitchett RJ. New hybodontiform and neoselachian sharks from the Lower Triassic of Oman. Journal of Systematic Palaeontology. 2015;13(10):891–917. doi: 10.1080/14772019.2014.963179. [DOI] [Google Scholar]
- Kriwet (2004).Kriwet J. Late Jurassic selachians (Chondrichthyes: Hybodontiformes, Neoselachii) from Central Portugal. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte. 2004;2004(4):233–256. doi: 10.1127/njgpm/2004/2004/233. [DOI] [Google Scholar]
- Kriwet & Benton (2004).Kriwet J, Benton MJ. Neoselachian (Chondrichthyes, Elasmobranchii) diversity across the Cretaceous-Tertiary boundary. Palaeogeography, Palaeoclimatology, Palaeoecology. 2004;214(3):181–194. doi: 10.1016/j.palaeo.2004.02.049. [DOI] [Google Scholar]
- Kriwet & Klug (2004).Kriwet J, Klug S. Late Jurassic selachians (Chondrichthyes, Elasmobranchii) from southern Germany: re-evaluation on taxonomy and diversity. Zitteliana, Reihe A. 2004;44:67–95. [Google Scholar]
- Kriwet & Klug (2008).Kriwet J, Klug S. Diversity and biogeography patterns of Late Jurassic neoselachians (Chondrichthyes, Elasmobranchii) In: Cavin L, Longbottom A, Richter M, editors. Fishes and the Break-up of Pangaea. Vol. 295. London: Geological Society, Special Publications; 2008. pp. 55–69. [Google Scholar]
- Kriwet & Klug (2015).Kriwet J, Klug S. Knorpelfische (Chondrichthyes) In: Arratia G, Schultze HP, Tischlinger H, Viohl G, editors. Solnhofen. Ein Fenster in die Jurazeit. Munich: Verlag Dr. Friedrich Pfeil; 2015. pp. 334–359. [Google Scholar]
- Kriwet, Kiesling & Klug (2009).Kriwet J, Kiesling W, Klug S. Diversification trajectories and evolutionary life-history traits in early sharks and batoids. Proceedings of the Royal Society B. 2009;276(1658):945–951. doi: 10.1098/rspb.2008.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane & Maisey (2009).Lane JA, Maisey JG. Pectoral Anatomy of Tribodus limae (Elasmobranchii: Hybodontiformes) from the Lower Cretaceous of Northeastern Brazil. Journal of Vertebrate Paleontology. 2009;29(1):25–38. doi: 10.1080/02724634.2009.10010359. [DOI] [Google Scholar]
- Lane & Maisey (2012).Lane JA, Maisey JG. The visceral skeleton and jaw suspension in the durophagous hybodontid shark Tribodus limae from the Lower Cretaceous of Brazil. Journal of Palaeontology. 2012;86(5):886–905. doi: 10.1666/11-139.1. [DOI] [Google Scholar]
- Leuzinger et al. (2015).Leuzinger L, Kocsis L, Billon-Bruyat J-P, Spezzaferri S, Vennemann T. Stable isotope study of a new chondrichthyan fauna (Kimmeridgian, Porrentruy, Swiss Jura): an unusual freshwater-influenced isotopic composition for the hybodont shark Asteracanthus. Biogeosciences. 2015;12(23):6945–6954. doi: 10.5194/bg-12-6945-2015. [DOI] [Google Scholar]
- Leuzinger et al. (2017).Leuzinger L, Cuny G, Popov E, Billon-Bruyat J-P. A new chondrichthyan fauna from the Late Jurassic of the Swiss Jura (Kimmeridgian) dominated by hybodonts, chimaeroids and guitarfishes. Papers in Palaeontology. 2017;3(4):471–511. doi: 10.1002/spp2.1085. [DOI] [Google Scholar]
- Long et al. (2015).Long JA, Burrow CW, Ginter M, Maisey JG, Trinajstic KM, Coates MI, Young GC, Senden TJ. First shark from the Late Devonian (Frasnian) Gogo Formation, Western Australia sheds new light on the development of tessellated calcified cartilage. PLOS ONE. 2015;10(5):e0126066. doi: 10.1371/journal.pone.0126066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund (1985).Lund R. The morphology of Falcatus falcatus (St. John and Worthen), a Mississippian stethacanthid chondrichthyan from the Bear Gulch Limestone of Montana. Journal of Vertebrate Paleontology. 1985;5(1):1–19. doi: 10.1080/02724634.1985.10011842. [DOI] [Google Scholar]
- Maisch & Matzke (2016).Maisch MW, Matzke AT. A new hybodontid shark (Chondrichthyes, Hybodontiformes) from the Lower Jurassic Posidonienschiefer Formation of Dotternhausen, SW Germany. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen. 2016;280(3):241–257. doi: 10.1127/njgpa/2016/0577. [DOI] [Google Scholar]
- Maisey (1975).Maisey JG. The interrelationships of phalacanthous selachians. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte. 1975;1975(9):553–567. [Google Scholar]
- Maisey (1978).Maisey JG. Growth and form of finspines in hybodont sharks. Palaeontology. 1978;21(3):657–666. [Google Scholar]
- Maisey (1982).Maisey JG. The anatomy and interrelationships of Mesozoic hybodont sharks. American Museum Novitates. 1982;2724:1–48. [Google Scholar]
- Maisey (1983).Maisey JG. Cranial antomy of Hybodus basanus Egerton from the Lower Cretaceous of England. American Museum Novitates. 1983;2758:1–26. [Google Scholar]
- Maisey (1986).Maisey JG. Anatomical revision of the fossil shark Hybodus fraasi (Chondrichthyes: Elasmobranchii) American Museum Novitates. 1986;2857:1–16. [Google Scholar]
- Maisey (1987).Maisey JG. Cranial anatomy of the Lower Jurassic shark Hybodus reticulatus (Chondrichthyes: Elasmobranchii), with comments on hybodontid systematics. American Museum Novitates. 1987;2878:1–39. [Google Scholar]
- Maisey (1989).Maisey JG. Hamiltonichthys mapesi, g. & sp. nov. (Chondrichthyes; Elasmobranchii), from the Upper Pennsylvanian of Kansas. American Museum Novitates. 1989;2931:1–42. [Google Scholar]
- Maisey & de Carvalho (1997).Maisey JG, de Carvalho MR. New look at old sharks. Nature. 1997;385(6619):779–780. doi: 10.1038/385779a0. [DOI] [Google Scholar]
- Maisey & Denton (2016).Maisey JG, Denton JSS. Dermal denticle patterning in the Cretaceous hybodont shark Tribodus limae (Euselachii, Hybodontiformes), and its implications for the evolution of patterning in the chondrichthyan dermal skeleton. Journal of Vertebrate Paleontology. 2016;36(5):e1179200. doi: 10.1080/02724634.2016.1179200. [DOI] [Google Scholar]
- Maisey, Naylor & Ward (2004).Maisey JG, Naylor GJP, Ward DJ. Mesozoic elasmobranchs, neoselachian phylogeny and the rise of modern elasmobranch diversity. In: Arratia G, Tintori A, editors. Mesozoic fishes 3: Systematics, paleoenvironments and biodiversity. Munich: Verlag Dr. Friedrich Pfeil; 2004. pp. 17–56. [Google Scholar]
- Martill & Etches (2013).Martill DM, Etches S. A new monofenestratan pterosaur from the Kimmeridge Clay Formation (Upper Jurassic, Kimmeridgian) of Dorset, England. Acta Palaeontologica Polonica. 2013;58(2):285–294. doi: 10.4202/app.2011.0071. [DOI] [Google Scholar]
- Martill, Earland & Naish (2006).Martill DM, Earland S, Naish D. Dinosaurs in marine strata: evidence from the British Jurassic, including a review of the allochthonous vertebrate assemblage from the marine Kimmeridge Clay Formation (Upper Jurassic) of Great Britain. Colectivo Arqueológico y Paleontológico Salense, ed Actas de las III Jornadas Intrernacionales sobre Paleontología de Dinosaurios y su Entorno, 16–17 Sep. 2004; Burgos: Salas de los Infantes; 2006. pp. 47–84. [Google Scholar]
- Meyer (1849).Meyer HV. Fossile Fische aus dem Muschelkalk von Jena, Querfurt und Esperstädt. Palaeontographica. 1849;1:195–208. [Google Scholar]
- Mo et al. (2016).Mo J, Buffetaut E, Tong H, Amiot R, Cavin L, Cuny G, Suteethorn V, Suteethorn S, Jiang S. Early Cretaceous vertebrates from the Xinlong Formation of Guangxi (southern China): a review. Geological Magazine. 2016;153(1):143–159. doi: 10.1017/S0016756815000394. [DOI] [Google Scholar]
- Noé, Gómez-Pérez & Nicholls (2019).Noé LF, Gómez-Pérez M, Nicholls R. Mary Anning, Alfred Nicholson Leeds and Steve Etches. Comparing the three most important UK ‘amateur’ fossil collectors and their collections. Proceedings of the Geologists’ Association. 2019;130(3–4):366–389. doi: 10.1016/j.pgeola.2018.09.001. [DOI] [Google Scholar]
- O’Sullivan & Martill (2015).O’Sullivan M, Martill DM. Evidence for the presence of Rhamphorhynchus (Pterosauria: Rhamphorhynchinae) in the Kimmeridge Clay of the UK. Proceedings of the Geologists’ Association. 2015;126(3):390–401. doi: 10.1016/j.pgeola.2015.03.003. [DOI] [Google Scholar]
- Owen (1846).Owen R. Lectures on the comparative anatomy and physiology of the vertebrate animals, delivered at the Royal College of Surgeons of England in 1844 and 1846. Part 1. Fishes. London: Longman; 1846. p. 308. [Google Scholar]
- Patterson (1966).Patterson C. British Wealden sharks. Bulletin of the British Museum (Natural History) Geology. 1966;11:283–350. [Google Scholar]
- Pimiento et al. (2019).Pimiento C, Cantalpiedra JL, Shimada K, Field DJ, Smaers JB. Evolutionary pathways toward gigantism in sharks and rays. Evolution. 2019;73(3):588–599. doi: 10.1111/evo.13680. [DOI] [PubMed] [Google Scholar]
- Pinheiro et al. (2013).Pinheiro FL, de Figueiredo AEQ, Dentzien-Dias PC, Fortier DC, Schultz CL, Viana MSS. Planohybodus marki sp. nov., a new fresh-water hybodontid shark from the Early Cretaceous of northeastern Brazil. Cretaceous Research. 2013;41:210–216. doi: 10.1016/j.cretres.2012.12.005. [DOI] [Google Scholar]
- Priede & Froese (2013).Priede IG, Froese R. Colonization of the deep sea by fishes. Journal of Fish Biology. 2013;83(6):1528–1550. doi: 10.1111/jfb.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees (1998).Rees J. Early Jurassic selachians from the Hasle Formation on Bornholm. Denmark Acta Palaeontologica Polonica. 1998;43(3):439–452. [Google Scholar]
- Rees (2001).Rees J. Jurassic and Early Cretaceous selachians—focus on southern Scandinavia. Lund Publications in Geology. 2001;153:1–19. [Google Scholar]
- Rees (2008).Rees J. Interrelationships of Mesozoic hybodont sharks as indicated by dental morphology-preliminary results. Acta Geologica Polonica. 2008;58:217–221. [Google Scholar]
- Rees & Underwood (2002).Rees J, Underwood CJ. The status of the shark genus Lissodus, and the position of nominal Lissodus species within the Hybodontoidea. Journal of Vertebrate Paleontology. 2002;22(3):471–479. doi: 10.1671/0272-4634(2002)022. [DOI] [Google Scholar]
- Rees & Underwood (2006).Rees J, Underwood CJ. Hybodont sharks from the Middle Jurassic of the Inner Hebrides, Scotland. Transactions of the Royal Society of Edinburgh: Earth Sciences. 2006;96(4):351–363. doi: 10.1017/S0263593300001346. [DOI] [Google Scholar]
- Rees & Underwood (2008).Rees J, Underwood CJ. Hybodont sharks of the English Bathonian and Callovian (Middle Jurassic) Palaeontology. 2008;51(1):117–147. doi: 10.1111/j.1475-4983.2007.00737.x. [DOI] [Google Scholar]
- Rees et al. (2013).Rees J, Cuny G, Pouech J, Mazin J-M. Non-marine selachians from the basal Cretaceous of Charente, SW France. Cretaceous Research. 2013;44:122–131. doi: 10.1016/j.cretres.2013.04.002. [DOI] [Google Scholar]
- Reif (1978).Reif W-E. Types of morphogenesis of the dermal skeleton in fossil sharks. Paläontologische Zeitschrift. 1978;52(1–2):110–128. doi: 10.1007/BF03006733. [DOI] [Google Scholar]
- Rieppel (1981).Rieppel O. The hybodontiform sharks from the Middle Triassic of Mte. San Giorgio, Switzerland. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen. 1981;16:324–353. [Google Scholar]
- Romano & Brinkmann (2010).Romano C, Brinkmann W. A new specimen of the hybodont shark Palaeobates polaris with three-dimensionally preserved Meckel’s cartilage from the Smithian (Early Triassic) of Spitsbergen. Journal of Vertebrate Paleontology. 2010;30(6):1673–1683. doi: 10.1080/02724634.2010.521962. [DOI] [Google Scholar]
- Soto, Perea & Toriño (2012).Soto M, Perea D, Toriño P. New remains of Priohybodus arambourgi (Hybodontiformes: Hybodontidae) from Late Jurassice–?earliest Cretaceous deposits in Uruguay. Cretaceous Research. 2012;35(11):118–123. doi: 10.1016/j.cretres.2011.12.001. [DOI] [Google Scholar]
- Stumpf & Kriwet (2019).Stumpf S, Kriwet J. A new Pliensbachian elasmobranch (Vertebrata, Chondrichthyes) assemblage from Europe, and its contribution to the understanding of late Early Jurassic elasmobranch diversity and distributional patterns. PalZ. 2019;93(4):637–658. doi: 10.1007/s12542-019-00451-4. [DOI] [Google Scholar]
- Stumpf et al. (2021).Stumpf S, López-Romero FA, Kindlimann R, Lacombat F, Pohl B, Kriwet J. A unique hybodontiform skeleton provides novel insights into Mesozoic chondrichthyan life. Papers in Palaeontology. 2021;61:53. doi: 10.1002/spp2.1350. [DOI] [Google Scholar]
- Szabó (2020).Szabó M. A Late Jurassic (Kimmeridgian-early Tithonian) fish fauna of the Eperkés-hegy (Olaszfalu, Bakony Mts., Hungary): the oldest record of Notidanodon Cappetta, 1975 and a short revision of Mesozoic Hexanchidae. Palaeobiodiversity and Palaeoenvironments. 2020;100(1):151–170. doi: 10.1007/s12549-018-00368-x. [DOI] [Google Scholar]
- Szabó & Főzy (2020).Szabó M, Főzy I. Asteracanthus (Hybodontiformes: Acrodontidae) remains from the Jurassic of Hungary, with the description of a new species and with remarks on the taxonomy and paleobiology of the genus. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen. 2020;297(3):295–309. doi: 10.1127/njgpa/2020/0926. [DOI] [Google Scholar]
- Tabaste (1963).Tabaste N. Étude des restes de Poissons du Crétacé Saharien. Memoires de l’Institut Français d’Afrique Noire. 1963;68:437–486. [Google Scholar]
- Teixeira (1956).Teixeira C. Sur un Hybodonitdé du Karroo de l’Angola. Revista da Faculdade de Ciências de Lisboa, 2a, série C, Ciências Naturais. 1956;5:135–136. [Google Scholar]
- Teng et al. (2019).Teng YH, Sone M, Hirayama R, Yoshida M, Komatsu T, Khamha S, Cuny G. First Cretaceous fish fauna from Malaysia. Journal of Vertebrate Paleontology. 2019;39(1):e1573735. doi: 10.1080/02724634.2019.1573735. [DOI] [Google Scholar]
- Thierry et al. (2000).Thierry J, Barrier E, Abbate E, Alekseev AS, Ait-Ouali R, Ait-Salem H, Bouaziz S, Canerot J, Georgiev G, Guiraud R, Hirsch F, Ivanik M, Le Metour J, Le Nindre YM, Medina F, Mouty M, Nazarevich B, Nikishin AM, Page K, Panov DL, Pique E, Poisson A, Sandulescu M, Sapunov IG, Seghedi A, Soussi M, Tchoumatchenko PV, Vaslet D, Vishnevskaya V, Volozh YA, Voznezenski A, Walley CO, Wong TE, Ziegler M, Ait-Abrahim L, Bergerat F, Bracene R, Brunet MF, Cadet JP, Guezou JC, Jabaloy A, Lepvrier C, Rimmele G. Map 11: Early Tithonian (141-139 Ma) In: Dercourt J, Gaetani M, Vrielynck B, Barrier E, Biju-Duval B, Brunet MF, Cadet JP, Crasquin S, Sadulescu M, editors. Peri-Tethys Palaeogeographical Atlas. Paris: CCGM/CGMW; 2000. [Google Scholar]
- Thies & Leidner (2011).Thies D, Leidner A. Sharks and guitarfishes (Elasmobranchii) from the Late Jurassic of Europe. Palaeodiversity. 2011;4:63–184. [Google Scholar]
- Turmine-Juhel et al. (2019).Turmine-Juhel P, Wilks R, Brockhurst D, Austen PA, Duffin CJ, Benton MJ. Microvertebrates from the Wadhurst Clay Formation (Lower Cretaceous) of Ashdown Brickworks, East Sussex, UK. Proceedings of the Geologists’ Association. 2019;130(6):752–769. doi: 10.1016/j.pgeola.2019.08.003. [DOI] [Google Scholar]
- Underwood (2002).Underwood CJ. Sharks, rays and a chimaeroid from the Kimmeridgian (Late Jurassic) of Ringstead, southern England. Palaeontology. 2002;45(2):297–325. doi: 10.1111/1475-4983.00238. [DOI] [Google Scholar]
- Underwood (2006).Underwood CJ. Diversification of the Neoselachii (Chondrichthyes) during the Jurassic and Cretaceous. Paleobiology. 2006;32(2):215–235. doi: 10.1666/04069.1. [DOI] [Google Scholar]
- Underwood (2020).Underwood CJ. 2. Sharks and rays. In: Martill DM, Etches S, editors. Fossils of the Kimmeridge Clay Formation. Vol. 2. London: Palaeontological Association, Field Guide to Fossils 16; 2020. pp. 14–32. [Google Scholar]
- Underwood & Rees (2002).Underwood CJ, Rees J. Selachian faunas from the earliest Cretaceous Purbeck Group of Dorset, southern England. Special Papers in Palaeontology. 2002;68:107–119. [Google Scholar]
- Underwood & Cumbaa (2010).Underwood CJ, Cumbaa SL. Chondrichthyans from a Cenomanian (Late Cretaceous) bonebed, Saskatchewan, Canada. Palaeontology. 2010;53(4):903–944. doi: 10.1111/j.1475-4983.2010.00969.x. [DOI] [Google Scholar]
- Underwood & Claeson (2019).Underwood CJ, Claeson KM. The Late Jurassic ray Kimmerobatis etchesi gen. et sp. nov. and the Jurassic radiation of the Batoidea. Proceedings of the Geologists’ Association. 2019;130(3–4):345–354. doi: 10.1016/j.pgeola.2017.06.009. [DOI] [Google Scholar]
- Urlichs, Wild & Ziegler (1994).Urlichs M, Wild R, Ziegler B. Der Posidonien-Schiefer und seine Fossilien. Stuttgarter Beiträge zur Naturkunde C. 1994;36:1–95. [Google Scholar]
- Vullo et al. (2014).Vullo R, Abit D, Ballèvre M, Billon-Bruyat J-P, Bourgeais R, Buffetaut E, Daviero-Gomez V, Garcia G, Gomez B, Mazin J-M, Morel S, Néraudeau D, Pouech J, Rage J-C, Schnyder J, Tong H. Palaeontology of the Purbeck-type (Tithonian, Late Jurassic) bonebeds of Chassiron (Oléron Island, western France) Comptes Rendus Palevol. 2014;13(5):421–441. doi: 10.1016/j.crpv.2014.03.003. [DOI] [Google Scholar]
- Werner (1989).Werner C. Die Elasmobranchier-Fauna des Gebel Dist Member der Bahariya Formation (Obercenoman) der Oase Bahariya, Ägypten. Palaeo Ichthyologica. 1989;5:1–112. [Google Scholar]
- Woodward (1889).Woodward AS. Catalogue of the Fossil Fishes in the British Museum (Natural History). Part I. London: British Museum (Natural History); 1889. p. 474. [Google Scholar]
- Woodward (1916).Woodward AS. The fossil fishes of the English Wealden and Purbeck formations. Monograph of the Palaeontographical Society London. 1916;69:1–48. doi: 10.1017/CBO9781139680851. [DOI] [Google Scholar]
- Young, Steel & Middelton (2014).Young MT, Steel L, Middelton H. Evidence of the metriorhynchid crocodylomorph genus Geosaurus in the Lower Kimmeridge Clay Formation (Late Jurassic) of England. Historical Biology. 2014;26(5):551–555. doi: 10.1080/08912963.2013.801468. [DOI] [Google Scholar]
- Zangerl & Case (1976).Zangerl R, Case GR. Cobelodus aculeatus (Cope), an anacanthous shark from Pennsylvanian black shales of North America. Palaeontographica, Abteilung A. 1976;154:107–157. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
Specimen shown in Figs. 2–6 is housed in the Museum of Jurassic Marine Life, England, and catalogued under MJML K1624.
Specimens shown in Figs. 7A–7D are stored in the Staatliches Museum für Naturkunde Stuttgart, Germany, and catalogued under SMNS 10060 and SMNS 15150, respectively.
Specimen shown in Fig. 7E is housed in the Natural History Museum, London, England, and catalogued under NHMUK PV P 339.