Abstract
Background
Mechanical ventilation is a potentially painful and discomforting intervention that is widely used in neonatal intensive care. Newborn infants demonstrate increased sensitivity to pain, which may affect clinical and neurodevelopmental outcomes. The use of drugs that reduce pain might be important in improving survival and neurodevelopmental outcomes.
Objectives
To determine the benefits and harms of opioid analgesics for neonates (term or preterm) receiving mechanical ventilation compared to placebo or no drug, other opioids, or other analgesics or sedatives.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 9), in the Cochrane Library; MEDLINE via PubMed (1966 to 29 September 2020); Embase (1980 to 29 September 2020); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to 29 September 2020). We searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
We included randomised and quasi‐randomised controlled trials comparing opioids to placebo or no drug, to other opioids, or to other analgesics or sedatives in newborn infants on mechanical ventilation. We excluded cross‐over trials. We included term (≥ 37 weeks' gestational age) and preterm (< 37 weeks' gestational age) newborn infants on mechanical ventilation. We included any duration of drug treatment and any dosage given continuously or as bolus; we excluded studies that gave opioids to ventilated infants for procedures.
Data collection and analysis
For each of the included trials, we independently extracted data (e.g. number of participants, birth weight, gestational age, types of opioids) using Cochrane Effective Practice and Organisation of Care Group (EPOC) criteria and assessed the risk of bias (e.g. adequacy of randomisation, blinding, completeness of follow‐up). We evaluated treatment effects using a fixed‐effect model with risk ratio (RR) for categorical data and mean difference (MD) for continuous data. We used the GRADE approach to assess the certainty of evidence.
Main results
We included 23 studies (enrolling 2023 infants) published between 1992 and 2019. Fifteen studies (1632 infants) compared the use of morphine or fentanyl versus placebo or no intervention. Four studies included both term and preterm infants, and one study only term infants; all other studies included only preterm infants, with five studies including only very preterm infants. We are uncertain whether opioids have an effect on the Premature Infant Pain Profile (PIPP) Scale in the first 12 hours after infusion (MD ‐5.74, 95% confidence interval (CI) ‐6.88 to ‐4.59; 50 participants, 2 studies) and between 12 and 48 hours after infusion (MD ‐0.98, 95% CI ‐1.35 to ‐0.61; 963 participants, 3 studies) because of limitations in study design, high heterogeneity (inconsistency), and imprecision of estimates (very low‐certainty evidence ‐ GRADE). The use of morphine or fentanyl probably has little or no effect in reducing duration of mechanical ventilation (MD 0.23 days, 95% CI ‐0.38 to 0.83; 1259 participants, 7 studies; moderate‐certainty evidence because of unclear risk of bias in most studies) and neonatal mortality (RR 1.12, 95% CI 0.80 to 1.55; 1189 participants, 5 studies; moderate‐certainty evidence because of imprecision of estimates). We are uncertain whether opioids have an effect on neurodevelopmental outcomes at 18 to 24 months (RR 2.00, 95% CI 0.39 to 10.29; 78 participants, 1 study; very low‐certainty evidence because of serious imprecision of the estimates and indirectness). Limited data were available for the other comparisons (i.e. two studies (54 infants) on morphine versus midazolam, three (222 infants) on morphine versus fentanyl, and one each on morphine versus diamorphine (88 infants), morphine versus remifentanil (20 infants), fentanyl versus sufentanil (20 infants), and fentanyl versus remifentanil (24 infants)). For these comparisons, no meta‐analysis was conducted because outcomes were reported by one study.
Authors' conclusions
We are uncertain whether opioids have an effect on pain and neurodevelopmental outcomes at 18 to 24 months; the use of morphine or fentanyl probably has little or no effect in reducing the duration of mechanical ventilation and neonatal mortality. Data on the other comparisons planned in this review (opioids versus analgesics; opioids versus other opioids) are extremely limited and do not allow any conclusions. In the absence of firm evidence to support a routine policy, opioids should be used selectively ‐ based on clinical judgement and evaluation of pain indicators ‐ although pain measurement in newborns has limitations.
Plain language summary
Opioids for newborns receiving mechanical ventilation
Review question
Do drugs such as morphine and fentanyl (opioids) save lives, reduce pain, or improve the long‐term development of newborns needing breathing machines (mechanical ventilators)? Background
Breathing machines are widely used for newborn full‐term (≥ 37 weeks' gestational age) and preterm (< 37 weeks' gestational age) babies with breathing problems. Breathing machines may cause babies pain. Moreover, their use requires the presence and suctioning of a tube placed in the baby's trachea (which connects the larynx to the bronchi of the lungs), thus causing additional pain and distress. Since newborn babies are very sensitive to pain, which may have a bad effect on future development, pain reduction with drugs (including opioids such as morphine and fentanyl) might be very important. Pain in babies is assessed by adults by using different scales, which focus on the baby's appearance and behaviour, and on other parameters. Study characteristics We collected and analysed all relevant studies to answer the review question and found 23 studies enrolling 2023 babies. In most studies, babies were born before the due date (before 37 weeks' gestational age). Eight studies compared the use of morphine versus placebo (a substance with no therapeutic value) or no intervention, and seven versus fentanyl. We analysed the other studies separately because researchers compared the use of these two drugs with other opioids or other analgesics.
Key results
We are uncertain whether opioids have an effect on pain and neurodevelopmental outcomes at 18 to 24 months; use of morphine or fentanyl probably has little or no effect in reducing the duration of mechanical ventilation and neonatal mortality. Further research is needed.
Certainty of evidence
The certainty of evidence is very low to moderate because overall only a small number of studies have looked at this intervention, few babies were included in these studies, and some studies could have been better designed.
How up‐to‐date is this review?
We searched for studies that had been published up to 29 September 2020.
Summary of findings
Background
Description of the condition
Over the last few decades, important advances in perinatal care have led to higher survival rates among preterm newborns. The need for multiple invasive procedures, such as respiratory support ventilation, has increased, although non‐invasive ventilation techniques have become available. Prospectively collected registry data on infants at less than 1500 grams birth weight show that the need for ventilator support decreased significantly from 75% to 67% from 2000 to 2009 (Soll 2013). Similarly, one survey of 31 tertiary‐level neonatal intensive care units (NICUs) reported that the need for mechanical ventilation in infants before 30 weeks' gestational age (GA) decreased from 73% to 66% in the period 2006 to 2010, whereas use of non‐invasive ventilation increased from 77% to 85% (Vendettuoli 2014). In the EUROPAIN study, the need for mechanical ventilation in the NICU was 36% to 45% and 17% to 18% for neonates born at < 29 and at 30 to 36 weeks' GA, respectively (Anand 2017; Carbajal 2015; Lago 2017).
Mechanical ventilation (MV) is a potentially painful and uncomfortable intervention (Barker 1996; Hall 2007). In adults, MV may cause pain, anxiety, panic, and nightmares or distress (Fink 2015). Ventilated newborn infants are subjected to multiple painful procedures such as endotracheal suctioning, blood sampling, and central line insertion; in addition, both MV and any underlying disease may induce pain (Ancora 2019). Pain is a stressful experience that may have consequences for both the course of the acute illness and the development of the newborn. Pain and stress can interact negatively with MV, leading to unsynchronised breathing and sub‐optimal ventilation (Anand 1987). Moreover, pain can lead to clinical instability with changes in heart rate, respiratory rate, blood pressure, intracranial pressure, and oxygen saturation, as well as development of complications such as intraventricular haemorrhage (Anand 1998). Also, evidence suggests an endocrine stress response that leads to increased secretion of steroids, catecholamines, and glucagon, along with an increased rate of catabolism (Anand 1987). Metabolic and immune changes have been reported (Anand 1990). Neonates demonstrate heightened sensitivity to repetitive noxious stimuli (Fitzgerald 1989), which leads to chronic pain, discomfort, and possibly hyperalgesia (Taddio 2009). These responses may affect long‐term clinical and neurodevelopmental outcomes (Anand 1993). However, despite increasing knowledge about these consequences, consensus on how to control these complications in daily clinical care has not been reached.
Description of the intervention
Non‐pharmacological approaches are used in the management of neonatal pain. Non‐nutritive sucking, skin‐to‐skin contact, and swaddling have been shown to be effective and safe for treatment of pain associated with painful procedures (Pillai 2015). However, their applicability to repeated or chronic pain, such as that caused by MV, is not known (Nemergut 2013). Thus, pharmacological supports, such as opioids or other analgesics, are often needed to provide comfort during MV (Golianu 2007).
In 2017, a retrospective cohort study including more than 80,000 mechanically ventilated infants in 348 NICUs showed that administration of opioids increased from 5% of infant‐days in 1997 to 32% in 2012 (Zimmerman 2017). A similar increase was noted in usage of benzodiazepines, which increased from 5% to 24% in the same period.
Opioids are the main therapy used to treat severe pain. Opioids are μ (mu)‐receptor agonists that act on the central nervous system and provide both analgesia and sedation. Fentanyl and morphine are the most frequently used opioids for neonates (Zimmerman 2017). Other sedatives include benzodiazepine drugs that facilitate neurotransmitter gamma‐aminobutyric acid activity in neuronal inhibition in the central nervous system. They produce anxiolysis, sedation, amnesia, and muscle relaxation, but not analgesia. Analgesics include non‐steroidal anti‐inflammatory drugs (NSAIDs), which inhibit cyclo‐oxygenase enzymes, blocking prostaglandin‐induced inflammation. For ventilated infants, these drugs might help to reduce the doses of opioids and benzodiazepines usually required to treat pain (Aranda 2005).
Potential adverse effects of opioids include slowing of gastric and intestinal motility, feeding intolerance, dependence and tolerance (reduction of the normal response to a drug, requiring increased doses to achieve the desired effect), and adverse neurological effects (Taddio 2002). Concern also surrounds potential inhibition of the respiratory drive, leading to difficulties in weaning from MV (Darnall 2010).
How the intervention might work
Reduction of pain in mechanically ventilated newborns has been considered a critical part of supportive therapy (Anand 2001; Larsson 1999; Menon 1998), not only because it is important per se, but also because it is possibly associated with better outcomes. Sedation is routinely administered in intubated adults and children, but the approach to sedation of neonates shows considerable variability (Kahn 1998), specifically for the use of opioids, with up to a 100‐fold difference in doses (baseline, mean daily, and total) or in peak infusion rates (Anand 2013). Historically, the belief that newborns cannot feel pain may have accounted for the low usage of analgesics (Purcell‐Jones 1988).
Recommendations have been issued to promote a more rigorous approach to treatment and prevention of pain in the neonate (AAP 2016), but uncertainty remains about the long‐term effects of opioid use in neonates, and about which opioid is most effective and safe. Morphine and fentanyl are the most commonly used opioids, but other opioids are available for neonatal use. Benefits and risks of opioids in ventilated neonates have not yet been systematically reviewed.
Why it is important to do this review
This review addresses several questions involving newborns who are mechanically ventilated via an endotracheal tube. What evidence, from randomised and quasi‐randomised controlled trials, indicates that an opioid is better than a placebo, a sedative, or a non‐opioid analgesic for reducing pain? Does opioid treatment reduce the incidence of neonatal mortality and abnormal neurodevelopment? What evidence suggests that the goals of treatment can be accomplished without hampering cardiorespiratory functions, feeding, and weight gain?
Answering these questions is fundamental to synthesising current evidence on the use of sedation and analgesia in ventilated newborns.
This review will update the existing review, "Opioids for neonates receiving mechanical ventilation", which was published in the Cochrane Library in 2008 (Bellù 2008). Of note, the protocol for this updated review has been modified to include comparison of one opioid to another opioid (head‐to‐head comparison).
Objectives
To determine the benefits and harms of opioid analgesics for neonates (term or preterm) receiving mechanical ventilation compared to placebo or no drug, other opioids, or other analgesics or sedatives.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), cluster‐RCTs, and quasi‐RCTs. We excluded cross‐over trials.
Types of participants
We included term (≥ 37 weeks' GA) and preterm (< 37 weeks' GA) newborn infants on mechanical ventilation.
In this review, we will consider 'mechanical ventilation' as all forms of assisted ventilation, including volume‐, pressure‐ or flow‐limited ventilation; high‐frequency ventilation, patient‐triggered ventilation, and other models of ventilation requiring endotracheal intubation.
Types of interventions
We included the following comparisons.
Comparison 1: any opioid compared to control (placebo or no intervention).
Comparison 2: any opioid compared to another analgesic (e.g. paracetamol).
Comparison 3: any opioid compared to another sedative (e.g. midazolam, other benzodiazepines).
Comparison 4: any opioid compared to other opioids.
Opioids include morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, and codeine.
Non‐pharmacological interventions are permissible if they are administered to both groups in each comparison.
We included any duration of drug treatment and any dosage, given continuously or as bolus.
We excluded studies that gave opioids to ventilated infants for procedures.
Types of outcome measures
Primary outcomes
Pain assessed by validated methods during administration of selected drugs. The following scales, developed to assess pain, fulfil validity and reliability criteria for newborn infants (term and preterm on mechanical ventilation for any respiratory disease) when critically reviewed (Giordano 2019; Olsson 2021): Neonatal Facial Coding System (NFCS) (Grunau 1990); Neonatal Infant Pain Scale (NIPS) (Lawrence 1993); Premature Infant Pain Profile (PIPP) (Stevens 1996); COMFORTneo (van Dijk 2009); Astrid Lindgren and Lund Children's Hospital's Pain and Stress Assessment Scale for Preterm and Sick Newborn Infants (ALPS‐Neo) (Lundqvist 2014); CRIES (acronym of Crying, Requires oxygen, Increased vital signs, Expression, Sleeplessness) Scale (Krechel 1995); Échelle Douleur Inconfort Nouveau‐né (EDIN) Scale (Debillon 2001); and Neonatal Pain, Agitation and Sedation Scale (N‐PASS) (Hummel 2008)
Duration of mechanical ventilation (days)
Neonatal mortality (death within 28 days of birth) and mortality to discharge
Moderate to severe neurodevelopmental disability: cerebral palsy, developmental delay (Bayley Scales of Infant Development – Mental Development Index Edition II (BSID‐MDI‐II (Bayley 1993); Bayley Scales of Infant and Toddler Development – Edition III Cognitive Scale (BSITD‐III) (Bayley 2006); or Griffiths Mental Development Scale – General Cognitive Index (GCI) (Griffiths 1954; Griffiths 1970); assessment > 2 standard deviations (SDs) below the mean); intellectual impairment (intelligence quotient (IQ) > 2 SDs below the mean), blindness (vision < 6/60 in both eyes), or sensorineural deafness requiring amplification (Jacobs 2013). We separately assessed data on children aged 18 to 24 months and aged 3 to 5 years
Secondary outcomes
Growth parameters (weight, length, head circumference): at term or near term (36 to 40 weeks' postmenstrual age (PMA))
Days to reach full enteral feeding
Length of stay in hospital (days)
-
Bronchopulmonary dysplasia (BPD)/chronic lung disease (CLD), defined as:
respiratory support or oxygen, or both, at 28 days of life (NIH 1979)
treatment with oxygen > 21% for ≥ 28 days, with grade of severity scored at 36 weeks' PMA (Jobe 2001) or
physiological definition (measured at 36 weeks' PMA) (Walsh 2004)
Intraventricular haemorrhage (IVH; all (≥ grade 1) or severe (≥ grade 3) on cranial ultrasound, as per Papile classification (Papile 1978)
Periventricular leukomalacia (PVL) on the basis of ultrasound or magnetic resonance imaging (de Vries 1992)
Retinopathy of prematurity (all stages (≥ stage 1) and severe (defined as ≥ stage 3)) (ICCROP 2005)
Necrotising enterocolitis (NEC) (defined as ≥ Bell's stage II OR any grade; requiring surgery) (Bell 1978)
Focal gastrointestinal perforation
Hypotension requiring medical therapy (vasopressors or fluid boluses)
Pneumothorax (on chest X‐ray)
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy; neonatal.cochrane.org/resources-review-authors). We searched PubMed for errata or retractions for included studies published in full text (pubmed.ncbi.nlm.nih.gov/).
We collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review.
Electronic searches
We conducted a comprehensive search including the Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 9), in the Cochrane Library; MEDLINE via PubMed (1966 to 29 September 2020); Embase (1980 to 29 September 2020); and Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to 29 September 2020). We searched clinical trials databases, conference proceedings, and reference lists of retrieved articles for RCTs and quasi‐RCTs. We used Cochrane Neonatal's search strategy for neonates and RCTs (see Appendix 1 for full search strategies for each database). See Appendix 2 for the search method used for the last published version of this review (Bellù 2008). We applied no language restrictions.
We searched clinical trials registries (ClinicalTrials.gov (clinicaltrials.gov); World Health Organization International Trials Registry and Platform (www.who.int/ictrp/search/en/); and International Standard Randomized Controlled Trials Number (ISRCTN) Registry (www.isrctn.com/)) for recently completed and ongoing trials.
Searching other resources
We reviewed the reference lists of all identified articles for relevant articles not identified by the primary search.
Data collection and analysis
We collected information for each study regarding the method of randomisation, blinding, intervention, and stratification, and whether the trial was single‐centre or multi‐centre. We noted information regarding trial participants including birth weight, GA, number of participants, modality of administration, and dose of opioids. We analysed the clinical outcomes noted above under Types of outcome measures.
Selection of studies
For this update, two review authors (CN and MB) independently screened all titles and abstracts to determine which trials met the inclusion criteria. We retrieved full‐text copies of all papers that were potentially relevant. We resolved disagreements by discussion between review authors. We obtained additional data directly from study authors. We included RCTs and quasi‐RCTs fulfilling our inclusion criteria. We recorded reasons for exclusion in the Characteristics of excluded studies table. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009).
Data extraction and management
Two review authors (CN and MB) independently extracted data using a data extraction form integrated with a modified version of the Cochrane Effective Practice and Organisation of Care Group data collection checklist (Cochrane EPOC 2017). We piloted the form within the review team using a sample of included studies.
We extracted the following characteristics from each included study and completed a Characteristics of included studies table.
Administrative details: study author(s); published or unpublished; year of publication; year in which study was conducted; presence of vested interest; details of other relevant papers cited.
Study: study design; type, duration, and completeness of follow‐up (e.g. > 80%); country and locations of study; informed consent; ethics approval.
Participants: sex, birth weight, gestational age, number of participants.
Interventions: initiation, dose and duration of opioids.
Outcomes as mentioned above under Types of outcome measures.
We resolved disagreements by discussion. We described an ongoing study identified by our search by detailing the primary author, research question(s), methods, and outcome measures, together with an estimate of the reporting date, and we reported them in the Characteristics of ongoing studies table.
When any queries arose, or when additional data were required, we contacted study investigators/authors for clarification. Two review authors (CN and MB) used the Cochrane statistical tool for data entry (RevMan Web). We replaced any standard error of the mean (SEM) by the corresponding SD.
Assessment of risk of bias in included studies
Two review authors (CN and OR) independently assessed risk of bias (low, high, or unclear) of all included trials using the Cochrane 'Risk of bias' tool, for the following domains (Higgins 2011b).
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We resolved any disagreements by discussion or by consultation with a third review author. See Appendix 1 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We performed statistical analyses using RevMan Web. We summarised the data in a meta‐analysis when they were sufficiently homogeneous, both clinically and statistically.
Dichotomous data
For dichotomous data, we presented risk ratios (RRs) and risk differences (RDs) with 95% confidence intervals (CIs). We calculated the number needed to treat for an additional beneficial outcome (NNTB) or an additional harmful outcome (NNTH) with 95% CIs, if there was a statistically significant reduction (or increase) in RD.
Continuous data
For continuous data, we used mean difference (MD) when outcomes were measured in the same way between trials. We used standardised mean difference (SMD) to combine trials that measured the same outcome but used different methods. When trials reported continuous data as median and interquartile range (IQR) and data passed the test of skewness, we converted mean to median and estimated the SD as IQR/1.35.
If studies had used a variety of scales, we would have performed subgroup analysis to pool these measurements. Pain assessments six hours or longer after the start of continuous infusion or repeated bolus qualified for inclusion. For trials that measured pain after single administration of a drug, a measurement made within the period of the drug's duration of action qualified for inclusion (Taddio 2002).
Unit of analysis issues
The unit of analysis was the participating infant in individually randomised trials, and an infant was considered only once in the analysis. The participating neonatal unit or section of a neonatal unit or hospital would have been the unit of analysis in cluster‐randomised trials. We planned to analyse them using an estimate of the intra‐cluster correlation coefficient (ICC) derived from the trial (if possible) or from a similar trial, or from a study with a similar population, as described in Section 16.3.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). If we had used ICCs from a similar trial or from a study with a similar population, we would have reported this and conducted a sensitivity analysis to investigate the effect of variation in the ICC.
If we had identified both cluster‐randomised trials and individually randomised trials, we would have combined results from both only if there had been little heterogeneity between study designs, and if interaction between effects of the intervention and the choice of randomisation unit had been considered unlikely.
We would have acknowledged any possible heterogeneity in the randomisation unit and performed a sensitivity analysis to investigate possible effects of the randomisation unit.
Dealing with missing data
We planned to carry out analysis on an intention‐to‐treat basis for all outcomes. Whenever possible, we analysed all participants in the treatment group to which they were randomised, regardless of the actual treatment received. When we identified important missing data (in the outcomes) or unclear data, we requested them from the original investigators. We made explicit the assumptions of any methods used to deal with missing data. We planned to perform sensitivity analyses to assess how sensitive results were to reasonable changes in the undertaken assumptions. We planned to address the potential impact of missing data on findings of the review in the Discussion section.
Assessment of heterogeneity
We assessed clinical heterogeneity by comparing the distribution of important participant factors between trials and trial factors (randomisation concealment, blinding of outcome assessment, loss to follow‐up, treatment type, co‐interventions). We assessed statistical heterogeneity by examining the I² statistic (Higgins 2011a), a quantity that describes the proportion of variation in point estimates that is due to variability across studies rather than to sampling error.
We interpreted the I² statistic as follows, as described by Higgins 2003.
Less than 25%: no (none) heterogeneity.
25% to 49%: low heterogeneity.
50% to 74%: moderate heterogeneity.
75% or greater: high heterogeneity.
We considered statistical heterogeneity to be substantial when the I² statistic was 50% or greater. In addition, we employed the Chi² test of homogeneity to determine the strength of evidence that heterogeneity was genuine. We explored clinical variation across studies by comparing the distribution of important participant factors among trials and trial factors (randomisation concealment, blinding of outcome assessment, loss to follow‐up, treatment types, and co‐interventions). We considered a threshold of P value less than 0.1 as an indicator of whether heterogeneity (genuine variation in effect sizes) was present.
Assessment of reporting biases
We conducted a comprehensive search for eligible studies and were alert for duplication of data. We planned to investigate publication bias by using funnel plots if we included 10 or more clinical trials in the meta‐analysis. We planned to conduct a sensitivity analysis to determine the effects of including and excluding these studies in the analysis, if we should have uncovered reporting bias that could, in the opinion of the review authors, have introduced serious bias.
Data synthesis
As we identified multiple studies that we considered to be sufficiently similar, we performed meta‐analysis using RevMan Web. For categorical outcomes, we calculated typical estimates of RR and RD, each with its 95% CI; for continuous outcomes, we calculated MD or SMD, each with its 95% CI. We used a fixed‐effect model to combine data when it was reasonable to assume that studies were estimating the same underlying treatment effect. If we had judged meta‐analysis to be inappropriate, we would have analysed and interpreted individual trials separately. When we found evidence of clinical heterogeneity, we tried to explain this based on the different study characteristics and subgroup analyses.
Subgroup analysis and investigation of heterogeneity
We explored high statistical heterogeneity in outcomes by visually inspecting forest plots and removing outlying studies from the sensitivity analysis (Higgins 2011a). When statistical heterogeneity was significant, we interpreted results of the meta‐analyses accordingly; we downgraded the certainty of evidence in the 'Summary of findings' tables, according to GRADE recommendations.
We considered the following groups for subgroup analysis when data were available.
GA: term; moderately preterm (32 to 36 weeks' GA); very preterm (< 32 weeks' GA).
Whether infants receiving mechanical ventilation were unselected or selected for complications of mechanical ventilation including extrapulmonary air leaks, pneumothorax, CLD, or BPD.
Dosing schedule (continuous drug administration, or 'as needed', based on signs of pain, discomfort, or stress).
We restricted these analyses to primary outcomes.
Sensitivity analysis
Had we identified substantial heterogeneity, we would have conducted sensitivity analysis to determine if findings were affected by inclusion of only those trials considered to have used adequate methods with low risk of bias (selection and performance bias). We planned to report results of sensitivity analyses for primary outcomes only.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess certainty of evidence for the following (clinically relevant) outcomes.
Pain assessed by validated methods during administration of selected drugs.
Duration of mechanical ventilation.
Neonatal mortality (death within 28 days of birth).
Mortality to discharge.
Neurodevelopmental outcome at short term (one year).
Neurodevelopmental outcome and quality of life at medium term (one to three years).
Neurodevelopmental outcome and quality of life (measured by validated scales) at long‐term (longer than three years).
Two review authors (CN and OR) independently assessed the certainty of evidence for each of the outcomes above. We considered evidence from RCTs as high certainty, downgrading evidence by one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates, and presence of publication bias. We used GRADEpro GDT to create a ‘Summary of findings' table to report the certainty of evidence (GRADEpro GDT).
The GRADE approach results in an assessment of the certainty of a body of evidence by one of the following four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
We have provided results of the search for this review update in the study flow diagram (Figure 1). See Characteristics of included studies; Table 1; Table 2; Table 3; Table 4; Table 5; Table 6 and Table 7 for details.
1.
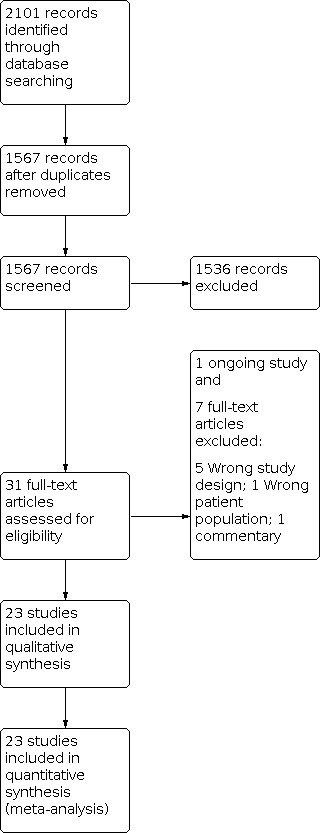
Study flow diagram: review update.
Summary of findings 1. Opioids compared to placebo or no treatment for neonates receiving mechanical ventilation.
| Opioids compared to placebo or no treatment for neonates receiving mechanical ventilation | ||||||
| Patient or population: neonates receiving mechanical ventilation Setting: neonatal intensive care units in USA, UK, Germany, Sweden, Australia, China, Netherlands, Italy, Brazil Intervention: opioids Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with opioids | |||||
| Pain (PIPP) ‐ time window: ≥ 12 and < 48 hours after infusion | Range 8.5 to 12.7 | MD 0.98 points lower (1.35 lower to 0.61 lower) | ‐ | 963 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW | Downgraded by 1 level for limitations in study design (unclear selective reporting in the 3 studies), by 1 level for high heterogeneity (difference between small and large beneficial effects), and by 2 level for imprecision of estimates (small sample size and wide confidence interval) |
| Duration of mechanical ventilation (days) | Range 4.4 to 19 | MD 0.23 days higher (0.38 lower to 0.83 higher) | ‐ | 1259 (7 RCTs) | ⊕⊕⊕⊝ MODERATE | Downgraded by 1 level for limitations in study design (unclear selective reporting in the 7 studies; unclear risk of bias in most domains in some studies) |
| Neonatal mortality | Study population | RR 1.12 (0.80 to 1.55) | 1189 (5 RCTs) | ⊕⊕⊕⊝ MODERATE | Downgraded by 1 level for imprecision of estimates (wide confidence interval) | |
| 101 per 1000 | 113 per 1000 (81 to 156) | |||||
| Mortality to discharge | Study population | RR 0.99 (0.52 to 1.88) | 178 (4 RCTs) | ⊕⊕⊝⊝ LOW | Downgraded by 1 level for limitations in study design (high or unclear risk of bias in most domains) and by 1 level for imprecision of estimates (wide confidence interval) | |
| 156 per 1000 | 154 per 1000 (81 to 292) | |||||
| Neurodevelopmental outcomes (18 to 24 months) | Study population | RR 2.00 (0.39 to 10.29) | 78 (1 RCT) | ⊕⊝⊝⊝ VERY LOW | Downgraded by 2 levels for serious imprecision of estimates (1 small study) and indirectness (characteristics of this study) | |
| 51 per 1000 | 103 per 1000 (20 to 528) | |||||
| Neurodevelopmental outcomes (3 to 5 years) | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| Neurodevelopmental outcome (5 to 6 years) | Study population | RR 1.60 (0.56 to 4.56) | 95 (1 RCT) | ⊕⊝⊝⊝ VERY LOW | Downgraded by 2 levels for serious imprecision of estimates (1 small study) and indirectness (characteristics of this study) | |
| 121 per 1000 | 194 per 1000 (68 to 553) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; PIPP: Premature Infant Pain Profile; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
Summary of findings 2. Opioids compared to other analgesics and sedatives for neonates receiving mechanical ventilation.
| Opioids compared to other analgesics and sedatives for neonates receiving mechanical ventilation | ||||||
| Patient or population: neonates receiving mechanical ventilation Setting: neonatal intensive care unit (Anand 1999 multi‐centre study in USA, UK, Germany, Sweden) Intervention: morphine Comparison: midazolam | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with other analgesics and sedatives | Risk with opioids | |||||
| Pain (PIPP) | Mean pain (PIPP) was 8.9 points | MD 1 points lower (2.66 lower to 0.66 higher) | Mean 8.9 | 46 (1 RCT) | ⊕⊝⊝⊝ VERY LOW | Serious imprecision of the estimatesa and indirectness |
| Duration of mechanical ventilation (days) | Mean duration of mechanical ventilation (days) was 14.2 days | MD 6.7 days lower (12.4 lower to 1 lower) | Mean 14.2 | 46 (1 RCT) | ⊕⊝⊝⊝ VERY LOW | Serious imprecision of the estimatesa and indirectness |
| Neonatal mortality | Study population | RR 0.31 (0.01 to 7.16) | 46 (1 RCT) | ⊕⊝⊝⊝ VERY LOW | Serious imprecision of the estimatesa and indirectness | |
| 45 per 1000 | 14 per 1000 (0 to 325) | |||||
| Mortality before discharge | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| Neurodevelopmental outcomes (18 to 24 months) | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| Neurodevelopmental outcomes (3 to 5 years) | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| Neurodevelopmental outcomes (5 to 6 years) | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PIPP: Premature Infant Pain Profile; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aFor "serious imprecision", downgraded by two levels.
Summary of findings 3. Morphine compared to fentanyl for neonates receiving mechanical ventilation.
| Morphine compared to fentanyl for neonates receiving mechanical ventilation | ||||||
| Patient or population: neonates receiving mechanical ventilation Setting: neonatal intensive care unit in Finland (Saarenmaa 1999) Intervention: morphine Comparison: fentanyl | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with fentanyl | Risk with morphine | |||||
| Pain (PIPP) | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| Duration of mechanical ventilation (days) | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| Neonatal mortality | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| Mortality to discharge | Study population | RR 1.21 (0.43 to 3.45) | 163 (1 RCT) | ⊕⊝⊝⊝ VERY LOW | Serious imprecision of the estimatesa and indirectness | |
| 72 per 1000 | 87 per 1000 (31 to 249) | |||||
| Neurodevelopmental outcomes (18 to 24 months) | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| Neurodevelopmental outcomes (3 to 5 years) | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| Neurodevelopmental outcomes (5 to 6 years) | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PIPP: Premature Infant Pain Profile; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aFor "serious imprecision", downgraded by two levels.
Summary of findings 4. Morphine compared to diamorphine for neonates receiving mechanical ventilation.
| Morphine compared to diamorphine for neonates receiving mechanical ventilation | ||||||
| Patient or population: neonates receiving mechanical ventilation Setting: neonatal intensive care unit in the UK (Wood 1998) Intervention: morphine Comparison: diamorphine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with diamorphine | Risk with morphine | |||||
| Pain (PIPP) | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| Duration of mechanical ventilation (days) | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| Neonatal mortality | Study population | RR 1.17 (0.43 to 3.19) | 88 (1 RCT) | ⊕⊝⊝⊝ VERY LOW | Serious imprecision of the estimatesa and indirectness | |
| 136 per 1000 | 160 per 1000 (59 to 435) | |||||
| Mortality before discharge | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| Neurodevelopmental outcomes (18 to 24 months) | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| Neurodevelopmental outcomes (3 to 5 years) | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| Neurodevelopmental outcomes (5 to 6 years) | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PIPP: Premature Infant Pain Profile; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aFor "serious imprecision", downgraded by two levels.
Summary of findings 5. Fentanyl compared to sufentanil for neonates receiving mechanical ventilation.
| Fentanyl compared to sufentanil for neonates receiving mechanical ventilation | |||||||
| Patient or population: neonates receiving mechanical ventilation Setting: neonatal intensive care unit in Germany (Schmidt 2010) Intervention: fentanyl Comparison: sufentanil | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with fentanyl | |||||||
| Pain (PIPP) | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome | ||
| Duration of mechanical ventilation (days) | MD 9 days higher (6.8 lower to 24.8 higher) | Mean 33 | 20 (1 RCT) | ⊕⊝⊝⊝ VERY LOW | Serious imprecision of the estimatesa and indirectness | ||
| Neonatal mortality | See comment | Not estimable | Not reported | ||||
| Mortality before discharge | See comment | See comment | See comment | Not estimable | Not reported | ||
| Neurodevelopmental outcomes (18 to 24 months) | See comment | See comment | See comment | Not estimable | Not reported | ||
| Neurodevelopmental outcomes (3 to 5 years) | See comment | See comment | See comment | Not estimable | Not reported | ||
| Neurodevelopmental outcomes (5 to 6 years) | See comment | See comment | See comment | Not estimable | Not reported | ||
| Not estimable | *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; PIPP: Premature Infant Pain Profile; RCT: randomised controlled trial. |
||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
aFor "serious imprecision", downgraded by two levels.
Summary of findings 6. Fentanyl compared to remifentanil for neonates receiving mechanical ventilation.
| Fentanyl compared to remifentanil for neonates receiving mechanical ventilation | ||||||
| Patient or population: neonates receiving mechanical ventilation Setting: one study conducted in Germany (Welzing 2012); none of the outcomes of this review were reported Intervention: fentanyl Comparison: remifentanil | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with remifentanil | Risk with fentanyl | |||||
| Pain (PIPP) | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| Duration of mechanical ventilation (days) | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| Neonatal mortality | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| Mortality before discharge | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| Neurodevelopmental outcomes (18 to 24 months) | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| Neurodevelopmental outcomes (3 to 5 years) | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| Neurodevelopmental outcomes (5 to 6 years) | See comment | See comment | Not estimable | Not reported | Not estimable | None of the studies reported on this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PIPP: Premature Infant Pain Profile. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1. Overview of included studies.
| Study ID | Country | Sample size | GA (weeks) | Intervention | Comparison | Administration | Open‐label boluses |
| Comparison 1: opioids vs placebo or no intervention | |||||||
| Anand 1999* | USA, UK, Germany, Sweden | 67a | 24 to 32 | Morphine | Dextrose 10% | LD + CIadj | Morphine |
| Anand 2004 | USA, UK, France, Sweden | 898 | 23 to 32 | Morphine | Placebo | LD + CIadj | Morphine |
| Dyke 1995 | Australia | 26 | 29 to 36 | Morphine | Dextrose 5% | LD + CI | No |
| Jiang 2012 | China | 46 | 33 to 39 | Morphine | Glucose 5% | LD + CI | No |
| Quinn 1992 | UK | 57 | < 34 | Morphine | No intervention | CI | Morphine |
| Quinn 1993 | UK | 41 | < 34 | Morphine | Dextrose 5% | LD + CI | No |
| Simons 2003 | Netherlands | 150 | 27 to 32 (interquartile) | Morphine | Glucose 5% | LD + CI | Morphine |
| Siwiec 1999 | Not reported | 20 | 26 to 35 | Morphine | No intervention | LD + CI | No |
| Ancora 2013 | Italy | 131 | 22 to 32 | Fentanyl | Placebo | LD + CI | Fentanyl |
| Chen 2015 | China | 30 | 28 to 39 | Fentanyl | No intervention | LD + CIadj | No |
| Guinsburg 1998 | Brazil | 22 | ≤ 32 | Fentanyl | Saline | Single dose | No |
| Lago 1998 | Italy | 55 | 26 to 34 | Fentanyl | No intervention | CIadj | No |
| Lago 1999 | Italy | 31 | 28 to 37 | Fentanyl | Placebo | CI | No |
| Orsini 1996 | USA | 20 | 26 to 36 | Fentanyl | Placebo | LD + CI | No |
| Qiu 2019 | China | 60 | < 32 | Fentanyl | Glucose 5% | LD + CI | Mo |
| Comparison 2: opioids vs other analgesics and sedatives | |||||||
| Anand 1999* | USA, UK, Germany, Sweden | 67a | 24 to 32 | Morphine | Midazolam | LD + CIadj | Morphine |
| Liem 1999 | Not reported | 8 | 30 to 32 | Morphine | Midazolam | LD + CI | No |
| Comparison 3: opioids vs other opioids | |||||||
| Ionides 1994 | USA | 27 | 30 to 38 | Morphine | Fentanyl | Single dose | No |
| Naderi 2017 | Iran | 32 | 26 to 38 | Morphine | Fentanyl | LD + CI | No |
| Saarenmaa 1999 | Finland | 163 | 29 to 36 (interquartile) | Morphine | Fentanyl | LD + CI | Drug not specified |
| Wood 1998 | UK | 88 | < 35 | Morphine | Diamorphine | LD + CI | No |
| e Silva 2008 | Brazil | 20 | 28 to 34 | Morphine | Remifentanil | CI | No |
| Schmidt 2010 | Germany | 20 | ≥ 37 | Fentanyl | Sufentanil | LD +CIadj | Midazolam |
| Welzing 2012 | Germany | 24 | ≤ 36 | Fentanyl | Remifentanil | CIadj | Midazolam, thiopental |
CI: continuous infusion; CIadj: continuous infusion and infusion rate adjustment based on pain score/clinical evaluation; GA: gestational age; LD: loading dose.
aThis study is described in both Comparison 1 and Comparison 2 because it comprised three arms: morphine sulphate, midazolam hydrochloride, and placebo.
Results of the search
The literature search run in September 2020 yielded 1567 references after duplicates were removed (Figure 1). After screening, we included 23 RCTs (2023 infants). We identified one ongoing trial (IRCT2017082417413N26).
Included studies
The 23 included studies were described in 46 separate reports: Anand 1999 (three reports), Anand 2004 (eight reports), Ancora 2013 (five reports), Simons 2003 (eight reports), and Welzing 2012 (three reports) (see Included studies). Moreover, late outcomes in the Quinn 1992 and Quinn 1993 studies were described in the follow‐up report of MacGregor 1998: for the purpose of this systematic review, all randomised infants in that report are referred to as Quinn 1992 and Quinn 1993.
There were differences in methods, participants, and interventions among the 23 included studies. Four studies included both term and preterm infants (Chen 2015; Ionides 1994; Jiang 2012; Naderi 2017), and one study included only term infants (Schmidt 2010). All other studies included only preterm infants, with five studies including only very preterm infants (< 32 weeks' gestation) (Anand 1999; Anand 2004; Ancora 2013; Guinsburg 1998; Qiu 2019).
Several different analgesia and sedation scores were used in the included studies, and some studies used modified scores. Each score is provided in the individual study description. A variety of opiates were used as interventions. Use of morphine was compared with placebo or no intervention in eight studies (Anand 1999; Anand 2004; Dyke 1995; Jiang 2012; Quinn 1992; Quinn 1993; Simons 2003; Siwiec 1999), with fentanyl in three studies (Ionides 1994; Naderi 2017; Saarenmaa 1999), with midazolam in two studies (Anand 1999; Liem 1999), with diamorphine in one study (Wood 1998), and with remifentanil in one study (e Silva 2008). In addition to the three studies on morphine mentioned above (Ionides 1994; Naderi 2017; Saarenmaa 1999), use of fentanyl was compared with placebo or no intervention in seven studies (Ancora 2013; Chen 2015; Guinsburg 1998; Lago 1998; Lago 1999; Orsini 1996; Qiu 2019).
Additional open‐label opioid boluses were permitted in five studies (Anand 1999; Anand 2004; Ancora 2013; Quinn 1992; Simons 2003). Six studies adjusted the infusion rate based on pain scale scores or clinical evaluation (Anand 1999; Anand 2004; Chen 2015; Lago 1998; Schmidt 2010; Welzing 2012).
Studies in which no dose adjustments or open‐label boluses were provided are shown as subgroup analyses (Dyke 1995; Guinsburg 1998; Ionides 1994; Jiang 2012; Lago 1999; Liem 1999; Naderi 2017; Orsini 1996; Qiu 2019; Quinn 1993; Siwiec 1999; Wood 1998; e Silva 2008) (see Data collection and analysis). Sixteen studies used a loading dose of opioid followed by continuous infusion (Anand 1999; Anand 2004; Ancora 2013; Chen 2015; Dyke 1995; Jiang 2012; Liem 1999; Naderi 2017; Orsini 1996; Qiu 2019; Quinn 1993; Saarenmaa 1999; Simons 2003; Siwiec 1999; Schmidt 2010; Wood 1998), five used only continuous infusion (e Silva 2008; Lago 1998; Lago 1999; Quinn 1992; Welzing 2012), and two used only one dose of opioid (Guinsburg 1998; Ionides 1994).
The loading dose (first dose) of morphine in different studies was 50 (Liem 1999), 100 (Anand 1999; Anand 2004; Dyke 1995; Jiang 2012; Naderi 2017; Simons 2003; Siwiec 1999), 140 (Saarenmaa 1999), or 200 microg/kg (Quinn 1993; Wood 1998). Continuous infusion of morphine was started after the loading dose at a dose of 1 microg/kg/hour (Liem 1999), 10 microg/kg/hour (Dyke 1995; e Silva 2008; Jiang 2012; Simons 2003), 12 microg/kg/hour (Naderi 2017), 20 microg/kg/hour (Saarenmaa 1999; Siwiec 1999), 25 microg/kg/hour (Quinn 1993; Wood 1998), 10 to 30 microg/kg/hour, depending on gestational age (Anand 1999; Anand 2004), or up to 50 to 100 microg/kg/hour (Quinn 1992).
The loading dose of fentanyl varied between 1 microg/kg in Ancora 2013 and Qiu 2019 and 10.5 microg/kg in Saarenmaa 1999, with a following continuous infusion of fentanyl of 3 microg/kg/hour or less (Chen 2015; Lago 1999; Naderi 2017; Orsini 1996; Qiu 2019; Saarenmaa 1999; Schmidt 2010; Welzing 2012). Lago 1998 used a continuous dose of fentanyl that was adjusted to render the baby sedated but arousable.
For additional data and information on the other opioids used, see Table 7 and Characteristics of included studies.
As specified in the protocol of this review, the following comparisons were planned.
-
Any opioid compared to control: Anand 1999; Anand 2004; Ancora 2013; Chen 2015; Dyke 1995; Guinsburg 1998; Jiang 2012; Lago 1998; Lago 1999; Orsini 1996; Qiu 2019; Quinn 1992; Quinn 1993; Simons 2003; Siwiec 1999 (see Comparison 1 and Table 1).
Morphine was used in eight studies (Anand 1999; Anand 2004; Dyke 1995; Jiang 2012; Quinn 1992; Quinn 1993; Simons 2003; Siwiec 1999).
Fentanyl was used in seven studies (Ancora 2013; Chen 2015; Guinsburg 1998; Lago 1998; Lago 1999; Orsini 1996; Qiu 2019).
Any opioid compared to other analgesics or sedatives: Anand 1999; Liem 1999 (see Comparison 2 and Table 2). Both studies compared morphine to midazolam.
-
Opioids compared to other opioids: e Silva 2008Ionides 1994; Naderi 2017; Saarenmaa 1999; Schmidt 2010; Welzing 2012; Wood 1998; that is:
three studies compared morphine to fentanyl: Ionides 1994; Naderi 2017; Saarenmaa 1999 (see Comparison 3 and Table 3);
one study compared morphine to diamorphine: Wood 1998 (see Comparison 4 and Table 4);
one study compared fentanyl to sufentanil: Schmidt 2010 (see Comparison 5 and Table 5);
one study compared fentanyl to remifentanil: Welzing 2012 (see Comparison 6 and Table 6); and
one study compared morphine to remifentanil: e Silva 2008 (no comparison was created in Data and analyses because none of the outcomes of this review were reported).
Anand 1999 is described in both Comparison 1 and Comparison 2 because this study comprised three arms: morphine sulphate, midazolam hydrochloride, and placebo. One ongoing study comparing fentanyl with midazolam was identified (IRCT2017082417413N26; see Characteristics of ongoing studies).
Excluded studies
We excluded seven studies because of the characteristics of the study design (Anand 2008; Bell 1987; Bergqvist 2007; Hwang 1999; Palmer 2005), because of the age of children in the population (Akkermans 2015), or because the article was a commentary (Perlman 2005).
Risk of bias in included studies
The overall quality of the studies was fair to good (Figure 2). Sixteen included studies had no high risk of bias for any of the items in the Cochrane 'Risk of bias' tool (Figure 3) (Anand 1999; Anand 2004; Ancora 2013; Dyke 1995; e Silva 2008; Guinsburg 1998; Jiang 2012; Lago 1999; Liem 1999; Naderi 2017; Orsini 1996; Qiu 2019; Quinn 1993; Saarenmaa 1999; Schmidt 2010; Simons 2003). Details of the methodological quality of each study are described under Characteristics of included studies.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation and allocation concealment were judged to be adequate in eight studies (Anand 1999; Anand 2004; Ancora 2013; Dyke 1995; Jiang 2012; Quinn 1993; Simons 2003; Welzing 2012). Nine studies stated that randomisation was performed and allocated treatment was obtained by drawing sealed envelopes (Chen 2015; Guinsburg 1998; Lago 1998; Liem 1999; Naderi 2017; Quinn 1992; Saarenmaa 1999; Schmidt 2010; Wood 1998). No details were given about how the randomisation list was generated, or how allocation concealment was ensured. In Orsini 1996, randomisation was performed in the pharmacy by a non‐specified random number generation. In Qiu 2019 and e Silva 2008, randomisation was done by using a random numbers table; however allocation concealment was not specified. In Ionides 1994, newborns were assigned by medical record number. Siwiec 1999 and Lago 1999 are abstract papers and do not provide details on randomisation or allocation concealment.
Blinding
Blinding of caregivers to the intervention was stated for all studies except Chen 2015, Lago 1999, Quinn 1992, and Siwiec 1999. A note of caution is warranted, as in most studies, caregivers could be more aware of the effects of pain control than was stated. Blinding of assessors to the intervention was stated for all studies except Lago 1999, Quinn 1992, and Siwiec 1999. In the latter study, blinding was not described (personal communication).
Incomplete outcome data
Follow‐up was almost complete for all studies. High risk of bias was identified in Lago 1998 and Wood 1998. In the large NEOPAIN study (Anand 2004), 30 of 391 surviving infants at 28 days in the morphine group and 43 of 402 in the placebo group did not undergo cranial ultrasonography at 4 to 7 days, nor at 28 to 35 days. MacGregor 1998 reported that with long‐term follow‐up, 8.4% of surviving patients were lost at 5 to 6 years.
Selective reporting
Only one study was at low risk of bias (Naderi 2017). In Welzing 2012, there are relevant differences between outcomes reported in the protocol and in the final article.
Other potential sources of bias
In Ionides 1994, the randomisation process between morphine and fentanyl groups was applied after the attending physician decided whether to use pancuronium, opiates, or both.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Comparison 1. Any opioid compared to control (placebo or no intervention)
Fifteen studies compared opioids to placebo or no intervention (Table 1): morphine was used in eight studies (Anand 1999; Anand 2004; Dyke 1995; Jiang 2012; Quinn 1992; Quinn 1993; Simons 2003; Siwiec 1999), and fentanyl was used in seven (Ancora 2013; Chen 2015; Guinsburg 1998; Lago 1998; Lago 1999; Orsini 1996; Qiu 2019). Infants randomised to midazolam in Anand 1999 are included in Comparison 2, not in Comparison 1.
Primary outcomes
Pain ‐ Premature Infant Pain Profile (PIPP) (Outcome 1.1)
PIPP ‐ less than 12 hours after infusion (Outcome 1.1.1)
Two trials reported on this outcome (MD ‐5.74, 95% CI ‐6.88 to ‐4.59; 50 participants, 2 studies; I² = 96%; subgroup analysis 1.1.1; Figure 4) (Chen 2015; Siwiec 1999).
4.

Comparison 1 (Any opioid compared to control). Outcome 1.1: PIPP = Premature Infant Pain Profile scale
PIPP ‐ greater than or equal to 12 and less than 48 hours after infusion (Outcome 1.1.2)
Three trials reported on this outcome (MD ‐0.98, 95% CI ‐1.35 to ‐0.61; 963 participants, 3 studies; I² = 90%; subgroup analysis 1.1.2; Figure 4) (Anand 1999; Anand 2004; Siwiec 1999).
Scores for this pain scale at any other time points were not reported.
Pain ‐ Neonatal Facial Coding System (NFCS) (Outcome 1.2)
One study reported on this outcome at less than 12 hours after infusion (MD 0.19, 95% CI ‐1.15 to 1.53; 22 participants, 1 study; Analysis 1.2) (Guinsburg 1998). The test for heterogeneity was not applicable.
1.2. Analysis.

Comparison 1: Any opioid (e.g. morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, codeine) compared to control (placebo or no intervention), with or without other non‐pharmacological measures, Outcome 2: Pain (NFCS)
Scores for this pain scale at any other time points were not reported.
Pain ‐ Neonatal Infant Pain Scale (NIPS) (Outcome 1.3)
Simons 2003 reported on this outcome by multiple measurements during endotracheal suctioning (MD 0.19, 95% CI ‐0.72 to 0.34; 150 participants, 1 study; Analysis 1.3). The test for heterogeneity was not applicable.
1.3. Analysis.

Comparison 1: Any opioid (e.g. morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, codeine) compared to control (placebo or no intervention), with or without other non‐pharmacological measures, Outcome 3: Pain (NIPS)
Scores for this pain scale at any other time points were not reported.
Pain ‐ COMFORTneo (Outcome 1.4)
Two trials reported on this outcome at the time point greater than or equal to 12 and less than 48 hours after infusion (MD ‐3.33, 95% CI ‐4.98 to ‐1.68; 65 participants, 2 studies; I² = 0%; Analysis 1.4) (Anand 1999; Siwiec 1999).
1.4. Analysis.

Comparison 1: Any opioid (e.g. morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, codeine) compared to control (placebo or no intervention), with or without other non‐pharmacological measures, Outcome 4: Pain (COMFORTneo)
Scores for this pain scale at any other time points were not reported.
Duration of ventilation (days) (Outcome 1.5)
Seven trials reported on this outcome (MD 0.23, 95% CI ‐0.38 to 0.83; 1259 participants, 7 studies; I² = 30%; Analysis 1.5; Figure 5) (Anand 1999; Anand 2004; Lago 1998; Lago 1999; Qiu 2019; Simons 2003; Siwiec 1999).
1.5. Analysis.

Comparison 1: Any opioid (e.g. morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, codeine) compared to control (placebo or no intervention), with or without other non‐pharmacological measures, Outcome 5: Duration of ventilation (days)
5.

Comparison 1 (Any opioid compared to control). Outcome 1.5: Duration of mechanical ventilation (respiratory support with an endotracheal tube)
Only Anand 2004, the largest study reporting on this outcome, found a significant difference in time spent on the ventilator between opioid and control groups. Data on this outcome were presented in a figure with a P value (P = 0.0338). Data used for meta‐analysis were obtained via personal communication.
Results on duration of ventilation could not be combined by meta‐analysis for the studies of Dyke and Quinn (Dyke 1995; Quinn 1992; Quinn 1993), because they presented this outcome as a median and a range. Orsini 1996 described in his article that no significant differences were found in duration of ventilator use between the two groups, but no actual data were available for analysis.
Meta‐analysis of results of the three studies with no dose adjustments or open‐label boluses did not show any statistically significant effect (MD 0.03, 95% CI ‐0.62 to 0.67; 111 participants, 3 studies; I² = 0%; subgroup 1.5.2; Figure 5) (Lago 1999; Qiu 2019; Siwiec 1999).
Meta‐analysis of results of the two studies that considered only very preterm infants did not show any statistically significant effect (MD 1.95, 95% CI ‐0.50 to 4.39; 943 participants, 2 studies; I² = 80%; subgroup 1.5.3; Figure 5) (Anand 1999; Anand 2004). Because of its high heterogeneity, this meta‐analysis (outcome 1.5.3) should be interpreted with caution.
Neonatal mortality (Outcome 1.6)
Five trials reported on this outcome (RR 1.12, 95% CI 0.80 to 1.55; 1189 participants, 5 studies; I² = 0%; Analysis 1.6; Figure 6) (Anand 1999; Anand 2004; Lago 1998; Quinn 1992; Simons 2003).
1.6. Analysis.

Comparison 1: Any opioid (e.g. morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, codeine) compared to control (placebo or no intervention), with or without other non‐pharmacological measures, Outcome 6: Neonatal mortality
6.

Comparison 1 (Any opioid compared to control). Outcome 1.6: Neonatal mortality is defined as mortality during the first 28 days of life
The only study with no dose adjustments or open‐label boluses reporting on this outcome did not show any statistically significant effect (RR 1.27, 95% CI 0.32 to 4.98; 41 participants, 1 study; subgroup 1.6.2; Figure 6) (Quinn 1993). The test for heterogeneity was not applicable.
Meta‐analysis of results of the two studies that considered only very preterm infants did not show any statistically significant effect (RR 1.18, 95% CI 0.82 to 1.68; 943 participants, 2 studies; I² for RR = 38%, for RD = 60%; subgroup 1.6.3; Figure 6) (Anand 1999; Anand 2004).
Mortality to discharge (Outcome 1.7)
Four trials reported on this outcome (RR 1.09, 95% CI 0.52 to 1.88; 178 participants, 4 studies; I² for RR and RD = 0%; Analysis 1.7) (Dyke 1995; Lago 1998; Quinn 1992; Quinn 1993).
1.7. Analysis.

Comparison 1: Any opioid (e.g. morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, codeine) compared to control (placebo or no intervention), with or without other non‐pharmacological measures, Outcome 7: Mortality to discharge
Two studies with no dose adjustments and no open‐label boluses reported on this outcome (Dyke 1995; Quinn 1993). No events occurred in Dyke 1995. Quinn 1993 did not show any statistically significant effect (RR 1.43, 95% CI 0.47 to 4.32; 41 participants, 1 study; I² for RR = not applicable, for RD = 0%; subgroup 1.7.2).
No studies considering only very preterm infants reported on this outcome (subgroup 1.7.3).
Neurodevelopmental outcome at 18 to 24 months (moderate to severe disability) (Outcome 1.8)
Ancora 2013 reported on this outcome (RR 2.00, 95% CI 0.39 to 10.29; 78 participants, 1 study; Analysis 1.8). The test for heterogeneity was not applicable. This study included very preterm infants; open‐label boluses of fentanyl were allowed.
1.8. Analysis.

Comparison 1: Any opioid (e.g. morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, codeine) compared to control (placebo or no intervention), with or without other non‐pharmacological measures, Outcome 8: Neurodevelopmental outcome at 18 to 24 months (moderate to severe disability)
Neurodevelopmental outcome at 5 to 6 years (moderate to severe disability) (Outcome 1.9)
Quinn 1993 reported on this outcome (RR 1.60, 95% CI 0.56 to 4.56; 95 participants, 1 study; Analysis 1.9). The test for heterogeneity was not applicable. This study included only very preterm infants; open‐label boluses of morphine were allowed. This time point (5 to 6 years) was added post hoc.;
1.9. Analysis.

Comparison 1: Any opioid (e.g. morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, codeine) compared to control (placebo or no intervention), with or without other non‐pharmacological measures, Outcome 9: Neurodevelopmental outcome at 5 to 6 years (disability)
Secondary outcomes
Weight gain at hospital discharge (Outcome 1.10)
Anand 1999 reported on this outcome (MD ‐0.18, 95% CI ‐0.64 to 0.28; 45 participants, 1 study; Analysis 1.10). The test for heterogeneity was not applicable. This study included very preterm infants; open‐label boluses of morphine were allowed.
1.10. Analysis.

Comparison 1: Any opioid (e.g. morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, codeine) compared to control (placebo or no intervention), with or without other non‐pharmacological measures, Outcome 10: Weight gain at discharge (g/kg per day)
Days to reach full enteral feeding (Outcome 1.11)
Three trials reported on this outcome (MD 1.68, 95% CI 0.12 to 3.25; 974 participants, 3 studies; I² = 0%; Analysis 1.11) (Anand 1999; Anand 2004; Lago 1999).
1.11. Analysis.

Comparison 1: Any opioid (e.g. morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, codeine) compared to control (placebo or no intervention), with or without other non‐pharmacological measures, Outcome 11: Days to reach full enteral feeding
The only study with no dose adjustments or open‐label boluses reporting on this outcome did not show any statistically significant effect (MD 0.00, 95% CI ‐3.52 to 3.52; 31 participants, 1 study; subgroup 1.11.2) (Lago 1999). The test for heterogeneity was not applicable.
Meta‐analysis of results of the two studies that considered only very preterm infants showed longer time to reach full enteral feeding (MD 2.10, 95% CI 0.35 to 3.85; 943 participants, 2 studies; I² = 0%; subgroup 1.11.3) (Anand 1999; Anand 2004). Data obtained via personal communication were used.
Length of stay in hospital (days) (Outcome 1.12)
Three trials reported on this outcome (MD 1.68, 95% CI 0.12 to 3.25; 131 participants, 3 studies; I² = 0%; Analysis 1.12) (Anand 1999; Lago 1998; Lago 1999).
1.12. Analysis.

Comparison 1: Any opioid (e.g. morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, codeine) compared to control (placebo or no intervention), with or without other non‐pharmacological measures, Outcome 12: Length of stay in hospital (days)
The only study with no dose adjustments and no open‐label boluses reporting on this outcome did not show any statistically significant effect (MD ‐2.00, 95% CI ‐26.74 to 22.74; 31 participants, 1 study; subgroup 1.12.2) (Lago 1999). The test for heterogeneity was not applicable.
The only study considering only very preterm infants did not show any statistically significant effect (MD ‐1.40, 95% CI ‐18.48 to 15.68; 45 participants, 1 study; subgroup 1.12.3) (Anand 1999). The test for heterogeneity was not applicable.
Results from Dyke 1995 and Anand 2004 (no statistically significant differences between opioid and control groups) could not be combined in the meta‐analysis because researchers presented this outcome as median and range.
Bronchopulmonary dysplasia (BPD; oxygen need at 28 days of life) (Outcome 1.13)
Four trials reported on this outcome (RR 1.17, 95% CI 0.75 to 1.82; 262 participants, 4 studies; I² for RR and RD = 0%; Analysis 1.13) (Dyke 1995; Lago 1998; Lago 1999; Simons 2003).
1.13. Analysis.

Comparison 1: Any opioid (e.g. morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, codeine) compared to control (placebo or no intervention), with or without other non‐pharmacological measures, Outcome 13: Oxygen at 28 days of life (BPD any grade)
Two studies with no dose adjustments and no open‐label boluses reported on this outcome (RR 1.10, 95% CI 0.36 to 3.37; 57 participants, 2 studies; I² for RR and RD = 0%; subgroup 1.13.2) (Dyke 1995; Lago 1999).
No studies considering only very preterm infants reported on this outcome (subgroup 1.13.3).
Chronic lung disease (oxygen need at 36 weeks' postmenstrual age) (Outcome 1.14)
Four trials reported on this outcome (RR 0.97, 95% CI 0.83 to 1.15; 964 participants, 4 studies; I² for RR = 38%, for RD = 62%; Analysis 1.14) (Anand 2004; Ancora 2013; Orsini 1996; Siwiec 1999).
1.14. Analysis.

Comparison 1: Any opioid (e.g. morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, codeine) compared to control (placebo or no intervention), with or without other non‐pharmacological measures, Outcome 14: Oxygen at 36 weeks' postmenstrual age (BPD moderate or severe)
Two studies with no dose adjustments and no open‐label boluses reported on this outcome (RR 0.18, 95% CI 0.03 to 0.92; 40 participants, 2 studies; I² for RR and RD = 0%; subgroup 1.14.2) (Orsini 1996; Siwiec 1999).
One study considering only very preterm infants reported on this outcome (RR 1.02, 95% CI 0.78 to 1.32; 175 participants, 2 studies; subgroup 1.14.3) (Anand 1999). The test for heterogeneity was not applicable.
Necrotising enterocolitis (NEC) (Outcome 1.15)
Five trials reported on this outcome (RR 1.03, 95% CI 0.61 to 1.76; 1278 participants, 5 studies; I² for RR and RD = 0%; Analysis 1.15) (Anand 2004; Ancora 2013; Jiang 2012; Lago 1998; Simons 2003).
1.15. Analysis.

Comparison 1: Any opioid (e.g. morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, codeine) compared to control (placebo or no intervention), with or without other non‐pharmacological measures, Outcome 15: Necrotising enterocolitis
One study with no dose adjustments and no open‐label boluses reported on this outcome (RR 1.09, 95% CI 0.07 to 16.41; 46 participants, 1 study; subgroup 1.15.2) (Jiang 2012). The test for heterogeneity was not applicable.
No studies considering only very preterm infants reported on this outcome (subgroup 1.15.3).
Any intraventricular haemorrhage (IVH any Papile grade) (Outcome 1.16)
Eight trials reported on this outcome (RR 0.84, 95% CI 0.67 to 1.05; 515 participants, 8 studies; I² for RR = 28%, for RD = 34%; Analysis 1.16) (Anand 1999; Ancora 2013; Dyke 1995; Jiang 2012; Orsini 1996; Quinn 1992; Quinn 1993; Simons 2003).
1.16. Analysis.

Comparison 1: Any opioid (e.g. morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, codeine) compared to control (placebo or no intervention), with or without other non‐pharmacological measures, Outcome 16: Any intraventricular haemorrhage (IVH)
Four studies with no dose adjustments and no open‐label boluses reported on this outcome (RR 0.87, 95% CI 0.51 to 1.49; 133 participants, 4 studies; I² for RR = 0%, for RD = 22%; subgroup 1.16.2) (Dyke 1995; Jiang 2012; Orsini 1996; Quinn 1993).
One study considering only very preterm infants reported on this outcome (RR 0.66, 95% CI 0.17 to 2.60; 45 participants, 1 study; subgroup 1.16.3) (Anand 1999). The test for heterogeneity was not applicable.
Severe intraventricular haemorrhage (IVH Papile grade 3/4) (Outcome 1.17)
Six trials reported on this outcome (RR 1.03, 95% CI 0.81 to 1.30; 1299 participants, 6 studies; I² for RR = 15%, for RD = 33%; Analysis 1.17) (Anand 1999; Anand 2004; Ancora 2013; Lago 1998; Simons 2003; Siwiec 1999).
1.17. Analysis.

Comparison 1: Any opioid (e.g. morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, codeine) compared to control (placebo or no intervention), with or without other non‐pharmacological measures, Outcome 17: Severe intraventricular haemorrhage (Papile grade 3/4)
One study with no dose adjustments and no open‐label boluses reported on this outcome (RR 0.33, 95% CI 0.02 to 7.32; 20 participants, 1 study; subgroup 1.17.2) (Siwiec 1999). The test for heterogeneity was not applicable.
Two studies considering only very preterm infants reported on this outcome (RR 1.12, 95% CI 0.78 to 1.60; 943 participants, 2 studies; I² for RR = 57%, for RD = 73%; subgroup 1.17.2) (Anand 1999; Anand 2004).
Periventricular leukomalacia (PVL) (Outcome 1.18)
Seven trials reported on this outcome (RR 0.83, 95% CI 0.57 to 1.21; 1345 participants, 7 studies; I² for RR and RD = 0%; Analysis 1.18) (Anand 1999; Anand 2004; Ancora 2013; Jiang 2012; Lago 1998; Simons 2003; Siwiec 1999).
1.18. Analysis.

Comparison 1: Any opioid (e.g. morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, codeine) compared to control (placebo or no intervention), with or without other non‐pharmacological measures, Outcome 18: Periventricular leukomalacia (PVL)
Two studies with no dose adjustments and no open‐label boluses reported on this outcome (RR 1.07, 95% CI 0.48 to 2.42; 66 participants, 2 studies; I² for RR = 42%, for RD = 65%; subgroup 1.18.2) (Jiang 2012; Siwiec 1999).
Two studies considering only very preterm infants reported on this outcome (RR 0.80, 95% CI 0.49 to 1.29; 943 participants, 2 studies; I² for RR and RD = 0%; subgroup 1.18.3) (Anand 1999; Anand 2004).
Hypotension requiring medical treatment (Outcome 1.19)
Three studies reported on this outcome (RR 1.48, 95% CI 1.26 to 1.74; 1083 participants, 3 studies; I² for RR = 71%, for RD = 31%; Analysis 1.19) (Anand 2004; Quinn 1993; Simons 2003).
1.19. Analysis.

Comparison 1: Any opioid (e.g. morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, codeine) compared to control (placebo or no intervention), with or without other non‐pharmacological measures, Outcome 19: Hypotension requiring medical treatment
One study with no dose adjustments and no open‐label boluses reported on this outcome (RR 1.27, 95% CI 0.32 to 4.98; 41 participants, 1 study; subgroup 1.19.2) (Quinn 1993). The test for heterogeneity was not applicable.
One study considering only very preterm infants reported on this outcome (RR 1.63, 95% CI 1.25 to 2.11; 898 participants, 1 study; subgroup 1.19.3) (Anand 2004). The test for heterogeneity was not applicable.
Within Comparison 1, no studies reported on ALPS‐Neo, CRIES, EDIN, N‐PASS, neurodevelopmental outcome at 18 to 24 months, retinopathy of prematurity, and focal gastrointestinal perforation.
Comparison 2. Morphine compared to midazolam
Two studies compared morphine to midazolam (Anand 1999; Liem 1999; Table 2). Only Anand 1999 reported on outcomes specified in this review. The test for heterogeneity was not applicable. This study included only very preterm infants; open‐label boluses of morphine were allowed.
Infants randomised to dextrose 10% in Anand 1999 are included in Comparison 1, not in Comparison 2.
Primary outcomes
Pain ‐ Premature Infant Pain Profile (PIPP) (Outcome 2.1)
Anand 1999 reported on this outcome (MD ‐1.00, 95% CI ‐2.66 to 0.66; 46 participants, 1 study; Analysis 2.1).
2.1. Analysis.

Comparison 2: Morphine compared to midazolam, Outcome 1: Pain (PIPP)
Pain ‐ COMFORTneo (Outcome 2.2)
Anand 1999 reported on this outcome (MD ‐0.20, 95% CI ‐2.51 to 2.11; 46 participants, 1 study; Analysis 2.2).
2.2. Analysis.

Comparison 2: Morphine compared to midazolam, Outcome 2: Pain (COMFORTneo)
Duration of ventilation (days) (Outcome 2.3)
Anand 1999 reported on this outcome (MD ‐6.70, 95% CI ‐12.40 to ‐1.00; 46 participants, 1 study; Analysis 2.3).
2.3. Analysis.

Comparison 2: Morphine compared to midazolam, Outcome 3: Duration of ventilation (days)
Neonatal mortality (Outcome 2.4)
Anand 1999 reported on this outcome (RR 0.31, 95% CI 0.01 to 7.16; 46 participants, 1 study; Analysis 2.4).
2.4. Analysis.

Comparison 2: Morphine compared to midazolam, Outcome 4: Neonatal mortality
Secondary outcomes
Weight gain at hospital discharge (Outcome 2.5)
Anand 1999 reported on this outcome (MD ‐0.19, 95% CI ‐0.66 to 0.28; 46 participants, 1 study; Analysis 2.5).
2.5. Analysis.

Comparison 2: Morphine compared to midazolam, Outcome 5: Weight gain at discharge (g/kg per day)
Days to reach full enteral feeding (Outcome 2.6)
Anand 1999 reported on this outcome (MD 1.70, 95% CI ‐7.09 to 10.49; 46 participants, 1 study; Analysis 2.6).
2.6. Analysis.

Comparison 2: Morphine compared to midazolam, Outcome 6: Days to reach full enteral feeding
Length of stay in hospital (days) (Outcome 2.7)
Anand 1999 reported on this outcome (MD ‐21.90, 95% CI ‐43.56 to ‐0.24; 46 participants, 1 study; Analysis 2.7).
2.7. Analysis.

Comparison 2: Morphine compared to midazolam, Outcome 7: Length of stay in hospital (days)
Any intraventricular haemorrhage (IVH any Papile grade) (Outcome 2.8)
Anand 1999 reported on this outcome (RR 0.28, 95% CI 0.09 to 0.87; 46 participants, 1 study; Analysis 2.8).
2.8. Analysis.

Comparison 2: Morphine compared to midazolam, Outcome 8: Any intraventricular haemorrhage (IVH)
Severe intraventricular haemorrhage (IVH Papile grade 3/4) (Outcome 2.9)
Anand 1999 reported on this outcome (RR 0.08, 95% CI 0.00 to 1.43; 46 participants, 1 study; Analysis 2.9).
2.9. Analysis.

Comparison 2: Morphine compared to midazolam, Outcome 9: Severe intraventricular haemorrhage (Papile grade 3/4)
Periventricular leukomalacia (PVL) (Outcome 2.10)
Anand 1999 reported on this outcome (RR 0.23, 95% CI 0.03 to 1.90; 46 participants, 1 study; Analysis 2.10).
Within Comparison 2, no studies reported on NFCS, NIPS, ALPS‐Neo, CRIES, EDIN, N‐PASS, mortality to discharge, neurodevelopmental outcome, BPD, retinopathy of prematurity (ROP), NEC, focal gastrointestinal perforation, and hypotension.
Comparison 3. Morphine compared to fentanyl
Three studies compared morphine to fentanyl (see Table 3) (Ionides 1994; Naderi 2017; Saarenmaa 1999). Only Saarenmaa 1999 reported on outcomes specified in this review. The test for heterogeneity was not applicable. This study included both term and preterm infants; open‐label boluses of morphine or fentanyl were allowed.
Primary outcomes
Mortality to discharge (Outcome 3.1)
Saarenmaa 1999 reported on this outcome (RR 1.21, 95% CI 0.43 to 3.45; 163 participants, 1 study; Analysis 3.1).
3.1. Analysis.

Comparison 3: Morphine vs fentanyl, Outcome 1: Mortality to discharge
Secondary outcomes
Severe intraventricular haemorrhage (IVH Papile grade 3/4) (Outcome 3.2)
Saarenmaa 1999 reported on this outcome (RR 0.59, 95% CI 0.18 to 1.95; 163 participants, 1 study; Analysis 3.3).
3.3. Analysis.

Comparison 3: Morphine vs fentanyl, Outcome 3: Necrotising enterocolitis
Necrotising enterocolitis (NEC) (Outcome 3.3)
Saarenmaa 1999 reported on this outcome (RR 0.83, 95% CI 0.35 to 2.00; 163 participants, 1 study; Analysis 3.2).
3.2. Analysis.

Comparison 3: Morphine vs fentanyl, Outcome 2: Severe intraventricular haemorrhage (Papile grade 3/4)
Hypotension requiring medical treatment (Outcome 3.4)
Saarenmaa 1999 reported on this outcome (RR 1.10, 95% CI 0.94 to 1.29; 163 participants, 1 study; Analysis 3.4).
3.4. Analysis.

Comparison 3: Morphine vs fentanyl, Outcome 4: Hypotension requiring medical treatment
Within Comparison 3, no studies reported on pain scales, duration of mechanical ventilation (MV), neonatal mortality, neurodevelopmental outcome, growth parameters, time to full enteral feeding, length of hospital stay, BPD, IVH any grade, PVL, ROP, and focal gastrointestinal perforation.
Comparison 4. Morphine compared to diamorphine
One study compared morphine to diamorphine (see Table 4) (Wood 1998), and these researchers reported on three of the outcomes specified in this review. The test for heterogeneity was not applicable. This study included preterm and very preterm infants (< 35 weeks); open‐label boluses of opioids were not allowed.
Primary outcome
Neonatal mortality (Outcome 4.1)
Wood 1998 reported on this outcome (RR 1.17, 95% CI 0.43 to 3.19; 88 participants, 1 study; Analysis 4.1).
4.1. Analysis.

Comparison 4: Morphine vs diamorphine, Outcome 1: Neonatal mortality
Secondary outcomes
Any intraventricular haemorrhage (IVH any Papile grade) (Outcome 4.2)
Wood 1998 reported on this outcome (RR 0.65, 95% CI 0.40 to 1.07; 88 participants, 1 study; Analysis 4.2).
4.2. Analysis.

Comparison 4: Morphine vs diamorphine, Outcome 2: Any intraventricular haemorrhage (IVH)
Bronchopulmonary dysplasia (BPD; oxygen need at 28 days of life) (Outcome 4.3)
Wood 1998 reported on this outcome (RR 1.07, 95% CI 0.60 to 1.88; 88 participants, 1 study; Analysis 4.3).
4.3. Analysis.

Comparison 4: Morphine vs diamorphine, Outcome 3: Bronchopulmonary dysplasia (BPD) defined as oxygen required at 28 days
Within Comparison 4, no studies reported on pain scales, duration of MV, mortality to discharge, neurodevelopmental outcome, growth parameters, time to full enteral feeding, length of hospital stay, BPD at 36 weeks' postconceptional age, severe IVH, PVL, NEC, ROP, focal gastrointestinal perforation, and hypotension.
Comparison 5. Fentanyl compared to sufentanil
Schmidt 2010 compared fentanyl to sufentanil (see Table 5); these researchers reported on one of the outcomes specified in this review. The test for heterogeneity was not applicable. This study included only term infants (< 35 weeks); open‐label boluses of opioids were not allowed.
Primary outcome
Duration of ventilation (days) (Outcome 5.1)
Schmidt 2010 reported on this outcome (MD 9.00, 95% CI ‐6.80 to 24.80; 20 participants, 1 study; Analysis 5.1).
5.1. Analysis.

Comparison 5: Fentanyl vs sufentanil, Outcome 1: Duration of mechanical ventilation
Within Comparison 5, no studies reported on pain scales, mortality, neurodevelopmental outcome, growth parameters, time to full enteral feeding, length of hospital stay, BPD, IVH, PVL, NEC, ROP, focal gastrointestinal perforation, and hypotension.
Comparison 6. Fentanyl compared to remifentanil
Welzing 2012 compared fentanyl to remifentanil (see Table 6); these investigators reported on one of the outcomes specified in this review. The test for heterogeneity was not applicable. This study included very preterm and preterm infants (< 37 weeks); open‐label boluses of opioids were allowed.
Secondary outcome
Hypotension requiring medical treatment (Outcome 6.1)
Welzing 2012 reported on this outcome (RR 1.20, 95% CI 0.50 to 2.88; 24 participants, 1 study; Analysis 6.1).
6.1. Analysis.

Comparison 6: Fentanyl vs remifentanil, Outcome 1: Hypotension requiring medical treatment
Within Comparison 6, no studies reported on pain scales, duration of MV, mortality, neurodevelopmental outcome, growth parameters, time to full enteral feeding, length of hospital stay, BPD, IVH, PVL, NEC, ROP, and focal gastrointestinal perforation.
Discussion
Summary of main results
We evaluated the benefits and harms of opioids for infants receiving mechanical ventilation for any respiratory disease compared to placebo or no drug, other opioids, or other analgesics or sedatives. The primary comparison (i.e. use of opioids versus placebo or no intervention) is the most relevant to address the main research question of this review. Furthermore, it included 15 studies (corresponding to 1632 infants) of the 23 included studies (2023 infants): morphine versus placebo or no intervention in eight studies, and fentanyl versus placebo or no intervention in seven studies. We are uncertain whether morphine or fentanyl has an effect on pain because of limitations in study design, high heterogeneity, and imprecision of estimates (see Quality of the evidence; Table 1). The use of morphine or fentanyl probably has little or no effect in reducing the duration of mechanical ventilation and neonatal mortality. We are uncertain whether opioids have an effect on neurodevelopmental outcomes at 18 to 24 months because of serious imprecision of the estimates and indirectness. Limited data were available for the other comparisons (i.e. two studies (54 infants) on morphine versus midazolam, three (222 infants) on morphine versus fentanyl, and one each on morphine versus diamorphine (88 infants), morphine versus remifentanil (20 infants), fentanyl versus sufentanil (20 infants), and fentanyl versus remifentanil (24 infants)). For these comparisons, no meta‐analyses were conducted because outcomes were reported by one study. Study authors reported extremely limited data on outcomes such as long‐term neurodevelopmental assessment and ratings of pain. Anand 2004 has raised concerns about higher hypotension incidence among the most vulnerable population of very preterm infants.
Data on the other comparisons planned in this review are scarce. Two studies (54 infants) compared morphine to midazolam, three (222 infants) morphine to fentanyl, and one each morphine to diamorphine (88 infants), morphine to remifentanil (20 infants), fentanyl to sufentanil (20 infants), and fentanyl to remifentanil (24 infants). The limited information size within these comparisons does not allow assessment of the benefits and harms of these interventions. When morphine was compared to midazolam, one small study showed similar pain scores between the two groups but fewer adverse effects with morphine.
Analysis of the effects of opioids in studies with no dose adjustments or open‐label boluses was limited by very small information size and therefore could not elucidate this aspect. Different dosage regimens were used across studies, but no clear relationship to effect could be found. We identified one ongoing trial comparing fentanyl with midazolam.
Overall completeness and applicability of evidence
Most studies evaluated the effect of an opioid started at the beginning of mechanical ventilation, assuming that mechanical ventilation is a painful or distressful procedure. The overall effect of opioids in this setting was found to be small and quite inconsistent. Given the pharmacological characteristics of opioids, possible explanations for this finding are numerous: pain could have been measured incorrectly, or newborns on mechanical ventilation may not always feel pain. Effects of opioids given 'on demand' in studies that allowed for extra protocol opioid administration could have altered measurements of pain. Non‐pharmacological interventions that were not mentioned in these papers could reduce pain and stress among newborns on the ventilator, thus reducing the need for drug control of pain. Indeed, Simons 2003 found very low pain scores for a situation in which lack of effect of opioids would be expected.
One major problem regarding pain scale scores involves differences in measuring pain across studies. Pain was assessed with well‐validated and less well‐validated scales (Giordano 2019; Olsson 2021). For validated pain scales (e.g. Premature Infant Pain Profile (PIPP) score), the mean difference of 0.98 points at the most relevant time window (i.e. 12 to 48 hours after opioid infusion) was statistically significant in favour of the opioid group, but this difference is not considered clinically significant (Ballantyne 1999). At the earlier time point (i.e. the first 12 hours after opioid infusion), the mean difference was 5.74 points; however information size was limited to two small studies (50 infants in total) and certainty of the evidence was very low (limitations in study design, heterogeneity, and imprecision of estimates ‐ GRADE). Marked statistical heterogeneity was found in both analyses (Figure 4). Heterogeneity can be caused by the small sample sizes of groups or by differences between small and large beneficial effects. Siwiec 1999, when evaluating PIPP score without any stimulus, found a PIPP score below 6 in the morphine group, which is considered to represent minimal pain (Ballantyne 1999). The study measuring PIPP during suctioning did not find any reduction in PIPP score below 7.9 points (i.e. incomplete control of pain) (Simons 2003).
One of the objectives of the review ‐ to test effects of opioids according to administration of drugs on an 'as needed' basis based on signs of pain ‐ was addressed by Lago 1998. This study showed a significant reduction in pain when fentanyl was titrated (administered in proportion to need) according to assessment of pain in the newborn. However, the limitations (a limited number of patients evaluated with a scale that was not well validated, i.e. behavioural sedation score) of this trial do not allow conclusions to be drawn.
We could not perform an appropriate a priori subgroup analysis to detect differential effects because of the paucity of outcome data amongst the included trials.
We were able to summarise available evidence in a comprehensive way, as we obtained additional information about study design and outcome data from study authors.
Quality of the evidence
We rated the certainty of evidence for clinically relevant outcomes as very low to moderate (see Table 1; Table 2; Table 3; Table 4; Table 5; Table 6). We downgraded the overall certainty of evidence for critical outcomes because of limitations in study design (i.e. unclear and high risk of bias in most domains) and imprecision of results (small information size and wide confidence intervals). We downgraded the certainty of evidence for pain scales by three levels because of limitations in study design (unclear selective reporting), high heterogeneity (differences between small and large beneficial effects), and imprecision of estimates (small sample size and wide confidence interval). We downgraded certainty of evidence for duration of mechanical ventilation by one level because of unclear risk of bias in most studies, and neonatal mortality by one level because of imprecision of estimates. We downgraded the certainty of evidence for neurodevelopmental outcomes by three levels because of serious imprecision of the estimates and indirectness. When secondary outcomes were considered, the certainty of evidence was low (limitations in study design and imprecision of estimates) for bronchopulmonary dysplasia, intraventricular haemorrhage, and time to reach full enteral feeding (limitations in study design and imprecision of estimates), and it was very low for weight gain (limitations in study design and serious imprecision of estimates) and length of hospital stay (indirectness and serious imprecision of estimates).
Heterogeneity was significantly high in the PIPP analyses. Causes of heterogeneity were not formally analysed in a post‐hoc analysis because of the paucity of studies within each subgroup. However, possible explanations include inconsistency among opioids used, differences in dosage of opioids used, differences in outcome measures, and differences in statistical reporting of results (see Table 7). In addition, high heterogeneity was identified in the analysis of three studies reporting duration of mechanical ventilation among very preterm infants, and in three studies reporting hypotension requiring medical treatment. The 'extra protocol' use of opioids in more recent studies may dilute the effect of the intervention being studied. On the other hand, this issue confirms the fact that opioids are now widely accepted for use in the neonatal intensive care unit, and that it is considered unethical to not give newborns the analgesic relief they need, if clinically justified. We could not assess the presence of publication bias by creating funnel plots because of the paucity of studies included in the meta‐analyses.
Potential biases in the review process
We used standard methods of Cochrane Neonatal in conducting this systematic review. It is unlikely that the literature search applied to this review may have missed relevant trials; thus we are confident that this systematic review summarises all presently available randomised trial evidence on opioids in ventilated infants. We applied no language restrictions and succeeded in having two trials translated from Mandarin to English (Chen 2015; Jiang 2012). We excluded seven trials because of characteristics of the study design (Anand 2008; Bell 1987; Bergqvist 2007; Hwang 1999; Palmer 2005), because of the age of children in the population (Akkermans 2015), or because the article was a commentary (Perlman 2005).
We succeeded in obtaining additional information from study authors.
Agreements and disagreements with other studies or reviews
In 2005 Aranda and colleagues reviewed the use of opioids and other drugs (midazolam, ibuprofen, indomethacin, and acetaminophen) in neonates receiving mechanical ventilation (Aranda 2005). Similar to our review, their meta‐analysis reported that morphine and fentanyl can reduce pain and agitation but may prolong the duration of mechanical ventilation. Use of drugs other than opioids for sedation and analgesia in ventilated infants has been addressed in other Cochrane Reviews (e.g. on midazolam (Ng 2017), on clonidine (Romantsik 2017a)). However, comparisons to opioids were not included. A systematic review on opioids and alpha‐2‐agonists for analgesia and sedation in newborn infants, including ventilated infants, is in preparation (Kinoshita 2020). Finally, a Cochrane overview of systematic reviews on pharmacological pain and sedation interventions for prevention of intraventricular haemorrhage in ventilated preterm infants is under preparation (Romantsik 2017b).
Previous versions of this review compared the use of opioids with no intervention, placebo, or other analgesics (Bellù 2005; Bellù 2008). For this update, we added the head‐to‐head comparison to the analyses, thus identifying seven additional studies: three on morphine versus fentanyl (Ionides 1994; Naderi 2017; Saarenmaa 1999), and one each on morphine versus diamorphine (Wood 1998), fentanyl versus sufentanil (Schmidt 2010), fentanyl versus remifentanil (Welzing 2012), and morphine versus remifentanil (e Silva 2008). However, limited data were available for most of these comparisons, and the effects of different opioids could not be determined.
For this update, we could add data from follow‐up studies of patients enrolled in trials conducted in the previous decade.
Authors' conclusions
Implications for practice.
We are uncertain whether opioids have an effect on pain and neurodevelopmental outcomes at 18 to 24 months; the use of morphine or fentanyl probably has little or no effect in reducing the duration of mechanical ventilation and neonatal mortality.
Data on the other comparisons planned for this review (opioids versus analgesics; opioids versus other opioids) are extremely limited and do not allow any conclusions.
In the absence of firm evidence to support a routine policy, opioids should be used selectively ‐ based on clinical judgement and evaluation of pain indicators ‐ although pain measurement in newborns is subject to limitations.
Implications for research.
There is a need to investigate this issue fully in large, well‐conducted studies. Future studies should enrol only newborns who express indicators of pain (based on validated pain scores) when on mechanical ventilation. Data are specifically needed for very preterm infants. Medium‐term (one to three years) and long‐term (longer than three years) neurodevelopmental consequences of opioid treatment have not been adequately addressed to date, so if additional data could be obtained from follow‐up studies of patients enrolled in recent trials, they would be very valuable. More research is needed to clarify the clinical importance of hypotension in very preterm infants and the underlying mechanisms leading to hypotension.
History
Protocol first published: Issue 9, 2020 Review first published: Issue 3, 2021
| Date | Event | Description |
|---|---|---|
| 2 January 2013 | Amended | Edit made to Anand 1999, 'Characteristics of included studies' table Interventions: Loading dose corrected to read "100 mcg/kg for all gestational ages" Placebo group (n = 21) corrected to read "dextrose 10%" |
| 10 June 2008 | Amended | Converted to new review format |
| 31 August 2007 | New search has been performed | This updates the review, "Opioids for neonates receiving mechanical ventilation", published in the Cochrane Library, Issue 1, 2005 (Bellù 2005) Updated strategy for search done in June 2007 found no new studies. Secondary reports from previous studies were identified. Data from these reports are now included in the review. Hypotension requiring medical treatment is now included among the secondary outcomes |
| 31 August 2007 | New citation required and conclusions have changed | Substantive amendments made |
Acknowledgements
The Methods section of this review is based on a standard template used by Cochrane Neonatal.
We would like to thank Cochrane Neonatal: Colleen Ovelman, Managing Editor; Jane Cracknell, Assistant Managing Editor; Roger Soll, Co‐coordinating Editor; and Bill McGuire, Co‐coordinating Editor, who provided editorial and administrative support. Carol Friesen, Information Specialist, designed and ran the literature searches.
As a Cochrane Neonatal Associate Editor, Thangaraj Abiramalatha has peer‐reviewed and offered feedback on this review.
We thank Iris PL Wong (Tian, Xiuying, Department of Neonatology, Tianjin Central Hospital of Gynecology and Obstetrics, Tianjin, China) and Yu‐Tian Xiao (Department of Urology, Shanghai Changhai Hospital, Second Military Medical University, Shanghai, China) for translation of two of the included studies from Mandarin to English.
We thank Matthias Bank (Library and ICT Services, Lund University) for defining and running the search strategy for this review.
For previous versions of this review, we thank Prof PG Duca, Department of Statistics, University of Milan, for statistical advice, and Mrs V Castagna for help in document retrieval. Thanks to all trial authors who were contacted and kindly answered.
Appendices
Appendix 1. 'Risk of bias' tool
We will use the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality of the trials. For each trial, we will seek information regarding the method of randomisation, blinding, and reporting of all outcomes for all infants enrolled in the trial. We will assess each criterion as having low, high, or unclear risk of bias. Two review authors will separately assess each study. We will resolve any disagreements by discussion. We will add this information to the 'Characteristics of included studies' table. We will evaluate the following issues and will enter the findings into the 'Risk of bias' table.
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we will categorise the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we will categorise the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we will categorise the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding will be assessed separately for different outcomes or class of outcomes. We will categorise the methods as:
low risk, high risk, or unclear risk for participants; and
low risk, high risk, or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we will categorise the methods used to blind outcome assessment. Blinding will be assessed separately for different outcomes or classes of outcomes. We will categorise the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we will describe the completeness of data including attrition and exclusions from analysis. We will note whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion when reported, and whether missing data were balanced across groups or were related to outcomes. When sufficient information is reported or supplied by the trial authors, we will re‐include missing data in the analyses. We will categorise the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Were reports of the study free of suggestion of selective outcome reporting?
For each included study, we will describe how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we will compare pre‐specified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we will contact study authors to gain access to the study protocol. We will assess the methods as:
low risk (when it is clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review were reported);
high risk (when not all of the study's pre‐specified outcomes were reported; one or more reported primary outcomes were not pre‐specified outcomes of interest and were reported incompletely and so cannot be used; study failed to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at high risk of bias?
For each included study, we will describe any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design, whether the trial was stopped early due to some data‐dependent process). We will assess whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If needed, we will explore the impact of the level of bias using sensitivity analyses.
Appendix 2. Search methods for previous publication
This review will update the existing review, "Opioids for neonates receiving mechanical ventilation", which was published in the Cochrane Library in 2008 (Bellù 2008). For the previous publications, trials were searched for using Cochrane Neonatal's search strategy. Randomised and quasi‐randomised controlled trials of opioid use in neonates were identified by electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL, 2007, Issue 2), in the Cochrane Library; MEDLINE (from 1966 to June 2007); Embase (from 1974 to June 2007), and CINAHL (from 1982 to 2007). Expert informants in the areas of anaesthesia, paediatric surgery, and neonatology were contacted, and previous reviews and lists of relevant articles cross‐referenced in the search for relevant trials. Abstracts from the Society for Pediatric Research and the European Society for Pediatric Research from 1995 until 2006 were handsearched.
Because of the nature of the questions, the search strategy was focused on patients and interventions and involved the following text words or MeSH subject headings (or both): infan*, neonat*, newborn*, morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, codeine, methadone, narcotics, sedation, analgesia.
No language restriction was used. No attempts were made to identify unpublished studies.
Appendix 3. Search strategy
The RCT filters have been created using Cochrane's highly sensitive search strategies for identifying randomised trials (Higgins 2011a). The neonatal filters were created and tested by the Cochrane Neonatal Information Specialist
CENTRAL
#1 MeSH Descriptor: [Infant, newborn] EXPLODE ALL
#2 (infan* or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm* or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU):ti,ab,kw
#3 (morphine OR diamorphine OR fentanyl OR alfentanil OR sufentanil OR pethidine OR meperidine OR codeine OR methadone OR remifentanil):ti,ab,kw
#4 (articifial respiration OR respiration OR mechanical ventilation OR ventilat* OR intubat* OR intubation): ti,ab,kw
#5 #1 OR #2
#6 #5 AND #3 AND #4
Cochrane Library
#1 MeSH Descriptor: [Infant, newborn] EXPLODE ALL
#2 (infan* or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm* or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU):ti,ab,kw
#3 (morphine OR diamorphine OR fentanyl OR alfentanil OR sufentanil OR pethidine OR meperidine OR codeine OR methadone OR remifentanil):ti,ab,kw
#4 (articifial respiration OR respiration OR mechanical ventilation OR ventilat* OR intubat* OR intubation): ti,ab,kw
#5 #1 OR #2
#6 #5 AND #3 AND #4 5
PubMed
#1 (((infant, newborn[MeSH] OR newborn*[TIAB] OR "new born"[TIAB] OR "new borns"[TIAB] OR "newly born"[TIAB] OR baby*[TIAB] OR babies*[TIAB] OR premature[TIAB] OR prematurity[TIAB] OR preterm[TIAB] OR "pre term"[TIAB] OR “low birth weight”[TIAB] OR "low birthweight"[TIAB] OR VLBW[TIAB] OR LBW[TIAB] OR infan*[TIAB] OR neonat*[TIAB])))
#2 (((((morphine OR diamorphine OR fentanyl OR alfentanil OR sufentanil OR pethidine OR meperidine OR codeine OR methadone))) OR ("Narcotics"[Majr] OR "Analgesia"[Majr] OR sedation[Title/Abstract] OR opioid*[Title/Abstract] OR remifentanil)) OR (((((((("Morphine"[Mesh]) OR "Heroin"[Mesh]) OR "Fentanyl"[Mesh]) OR "Alfentanil"[Mesh]) OR "Sufentanil"[Mesh]) OR "Meperidine"[Mesh]) OR "Codeine"[Mesh]) OR "Methadone"[Mesh] OR “Remifentanil”[Mesh]))
#3 (("respiration, artificial"[Mesh]) OR respiration OR mechanical ventilation OR ventilat* OR intubat* OR intubation)
#4 ((((randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab])) NOT (animals[MH] NOT humans[MH])))
#5 #1 AND #2 AND #3 AND #4
Embase
#1. 'narcotic analgesic agent'/exp/mj OR 'analgesia'/exp/mj OR sedation:ti,ab
#2. 'morphine':ab,ti OR 'diamorphine':ab,ti OR 'fentanyl':ab,ti OR 'alfenatil':ab,ti OR 'sufentanil':ab,ti OR 'pethidine':ab,ti OR 'meperidine':ab,ti OR 'codeine':ab,ti OR 'methadone':ab,ti OR 'morphine'/exp OR 'diamorphine'/exp OR 'fentanyl'/exp OR 'alfentanil'/exp OR 'sufentanil'/exp OR 'pethidine'/exp OR 'methadone'/exp OR 'remifentanil':ab,ti OR 'remifentanil'/exp OR opioid*:ti,ab
#3. #1 OR #2
#4. 'prematurity'/exp OR 'infant'/exp
#5. newborn*:ti,ab OR 'new born':ti,ab OR 'new borns':ti,ab OR 'newly born':ti,ab OR baby*:ti,ab OR babies:ti,ab OR premature:ti,ab OR prematurity:ti,ab OR preterm:ti,ab OR 'pre term':ti,ab OR 'low birth weight':ti,ab OR 'low birthweight':ti,ab OR vlbw:ti,ab OR lbw:ti,ab OR infant:ti,ab OR infants:ti,ab OR infantile:ti,ab OR infancy:ti,ab OR neonat*:ti,ab
#6. #4 OR #5
#7. 'randomized controlled trial'/exp OR 'randomized controlled trial' OR 'controlled clinical trial'/exp OR 'controlled clinical trial' OR 'randomized':ab,ti OR 'placebo':ab,ti OR 'randomly':ab,ti OR 'trial':ab,ti OR 'clinical trial'/exp OR 'clinical trial'
#8 'artificial ventilation'/exp OR ventilation OR 'ventilat*':ab,ti OR 'intuba*':ab,ti OR respiration:ab,ti
#9 #3 AND #6 AND #7 AND #8
CINAHL
#1 (infant or infants or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW)
#2 (morphine OR diamorphine OR fentanyl OR alfentanil OR sufentanil OR pethidine OR meperidine OR codeine OR methadone OR MH morphine OR MH diamorphine OR MH fentanyl OR MH alfentanil OR MH sufentanil OR MH pethidine OR MH meperidine OR MH codeine OR MH methadone OR MH remifentanil OR MJ narcotics OR MJ sedation OR MJ analgesia OR TI opioid* OR AB opioid*)
#3 ((respiration, artificial MH) OR respiration OR mechanical ventilation OR ventilat* OR intubat* OR intubation)
#4 (TX "randomized controlled trial" OR TX "controlled trial" OR TX randomized OR TX placebo OR TX "clinical trials as topic" OR TX randomly OR TX trial OR PT clinical trial)
#5 #1 AND #2 AND #3 AND #4
Clinicaltrials.gov
infant, newborn, opioid
WHO ICTRP
Search term: opioid
Search filter: Clinical trials in children
Data and analyses
Comparison 1. Any opioid (e.g. morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, codeine) compared to control (placebo or no intervention), with or without other non‐pharmacological measures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Pain (PIPP) | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 < 12 hours after infusion | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐5.74 [‐6.88, ‐4.59] |
| 1.1.2 ≥ 12 and < 48 hours after infusion | 3 | 963 | Mean Difference (IV, Fixed, 95% CI) | ‐0.98 [‐1.35, ‐0.61] |
| 1.1.3 Multiple time points (e.g. during endotracheal suctioning) | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.73, 0.93] |
| 1.2 Pain (NFCS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 < 12 hours after infusion | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐1.15, 1.53] |
| 1.3 Pain (NIPS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 Multiple time points (e.g. during endotracheal suctioning) | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.72, 0.34] |
| 1.4 Pain (COMFORTneo) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.4.1 ≥ 12 and < 48 hours after infusion | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐3.33 [‐4.98, ‐1.68] |
| 1.5 Duration of ventilation (days) | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 All studies | 7 | 1259 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.38, 0.83] |
| 1.5.2 With no dose adjustments or open‐label boluses | 3 | 111 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.62, 0.67] |
| 1.5.3 Very preterm infants only | 3 | 1003 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.47, 0.80] |
| 1.6 Neonatal mortality | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.6.1 All studies | 5 | 1189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.80, 1.55] |
| 1.6.2 With no dose adjustments or open‐label boluses | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.32, 4.98] |
| 1.6.3 Very preterm infants only | 2 | 943 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.82, 1.68] |
| 1.7 Mortality to discharge | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 All studies | 4 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.52, 1.88] |
| 1.7.2 With no dose adjustments or open‐label boluses | 2 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.47, 4.32] |
| 1.8 Neurodevelopmental outcome at 18 to 24 months (moderate to severe disability) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.8.1 All studies | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.39, 10.29] |
| 1.8.2 Very preterm infants only | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.39, 10.29] |
| 1.9 Neurodevelopmental outcome at 5 to 6 years (disability) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.9.1 All studies | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.56, 4.56] |
| 1.10 Weight gain at discharge (g/kg per day) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.10.1 All studies | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.64, 0.28] |
| 1.10.2 Very preterm infants only | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.64, 0.28] |
| 1.11 Days to reach full enteral feeding | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.11.1 All studies | 3 | 974 | Mean Difference (IV, Fixed, 95% CI) | 1.68 [0.12, 3.25] |
| 1.11.2 With no dose adjustments or open‐label boluses | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐3.52, 3.52] |
| 1.11.3 Very preterm infants only | 2 | 943 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [0.35, 3.85] |
| 1.12 Length of stay in hospital (days) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.12.1 All studies | 3 | 131 | Mean Difference (IV, Fixed, 95% CI) | 1.85 [‐6.88, 10.57] |
| 1.12.2 With no dose adjustments or open‐label boluses | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐2.00 [‐26.74, 22.74] |
| 1.12.3 Very preterm infants only | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐18.48, 15.68] |
| 1.13 Oxygen at 28 days of life (BPD any grade) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.13.1 All studies | 4 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.73, 1.92] |
| 1.13.2 With no dose adjustments or open‐label boluses | 2 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.36, 3.37] |
| 1.14 Oxygen at 36 weeks' postmenstrual age (BPD moderate or severe) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.14.1 All studies | 4 | 964 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.23] |
| 1.14.2 With no dose adjustments or open‐label boluses | 2 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.03, 0.92] |
| 1.14.3 Very preterm infants only | 2 | 924 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.85, 1.31] |
| 1.15 Necrotising enterocolitis | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.15.1 All studies | 5 | 1278 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.61, 1.76] |
| 1.15.2 With no dose adjustments or open‐label boluses | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.07, 16.41] |
| 1.15.3 Very preterm infants only | 2 | 1029 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.56, 2.10] |
| 1.16 Any intraventricular haemorrhage (IVH) | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.16.1 All studies | 8 | 515 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.65, 1.09] |
| 1.16.2 With no dose adjustments or open‐label boluses | 4 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.51, 1.49] |
| 1.16.3 Very preterm infants only | 2 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.53, 1.33] |
| 1.17 Severe intraventricular haemorrhage (Papile grade 3/4) | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.17.1 All studies | 6 | 1299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.71, 1.34] |
| 1.17.2 With no dose adjustments or open‐label boluses | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 1.17.3 Very preterm infants only | 3 | 1074 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.78, 1.52] |
| 1.18 Periventricular leukomalacia (PVL) | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.18.1 All studies | 7 | 1345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.57, 1.21] |
| 1.18.2 With no dose adjustments or open‐label boluses | 2 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.48, 2.42] |
| 1.18.3 Very preterm infants only | 3 | 1074 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.47, 1.19] |
| 1.19 Hypotension requiring medical treatment | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.19.1 All studies | 3 | 1083 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.13, 1.72] |
| 1.19.2 With no dose adjustments or open‐label boluses | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.32, 4.98] |
| 1.19.3 Very preterm infants only | 1 | 898 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.25, 2.11] |
1.1. Analysis.

Comparison 1: Any opioid (e.g. morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, codeine) compared to control (placebo or no intervention), with or without other non‐pharmacological measures, Outcome 1: Pain (PIPP)
Comparison 2. Morphine compared to midazolam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Pain (PIPP) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.2 Pain (COMFORTneo) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.3 Duration of ventilation (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.4 Neonatal mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.5 Weight gain at discharge (g/kg per day) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.6 Days to reach full enteral feeding | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.7 Length of stay in hospital (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.8 Any intraventricular haemorrhage (IVH) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.9 Severe intraventricular haemorrhage (Papile grade 3/4) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.10 Periventricular leukomalacia (PVL) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
2.10. Analysis.

Comparison 2: Morphine compared to midazolam, Outcome 10: Periventricular leukomalacia (PVL)
Comparison 3. Morphine vs fentanyl.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Mortality to discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.2 Severe intraventricular haemorrhage (Papile grade 3/4) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.3 Necrotising enterocolitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.4 Hypotension requiring medical treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 4. Morphine vs diamorphine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Neonatal mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.2 Any intraventricular haemorrhage (IVH) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.3 Bronchopulmonary dysplasia (BPD) defined as oxygen required at 28 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 5. Fentanyl vs sufentanil.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Duration of mechanical ventilation | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 6. Fentanyl vs remifentanil.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Hypotension requiring medical treatment | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.50, 2.88] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anand 1999.
| Study characteristics | ||
| Methods | Multi‐centre randomised placebo‐controlled trial | |
| Participants | 67 preterm infants (24 to 32 weeks) on a ventilator by < 8 hours were eligible for inclusion Exclusion criteria: postnatal age > 72 hours, positive‐pressure ventilation ≥ 8 hours, major congenital anomalies, severe intrapartum asphyxia (Apgar score ≤ 3 at 5 min), participation in other studies interfering with NOPAIN trial procedures criteria | |
| Interventions | Morphine group (n = 24): loading dose 100 mcg/kg for all gestational ages Midazolam group (n = 22): loading dose 200 mcg/kg followed by infusion of 20, 40, or 60 mcg/kg/hour for infants of gestational ages 24 to 26, 27 to 29, and 30 to 32 weeks, respectively Placebo group (n = 21): dextrose 10% Treatment continued as long as necessary (written protocol for stopping drugs), max 14 days. Additional analgesia with morphine bolus doses was allowed. Amount and frequency of additional morphine were recorded as an outcome measure | |
| Outcomes | Primary outcome: incidence of adverse neurological events (neonatal death, grade III/IV IVH, PVL) Secondary outcomes: level of sedation (measured by COMFORT score); pain response to tracheal suctioning (assessed by PIPP) ‐ all scores assessed before start of treatment, after 24 hours of infusion, and at 10 to 12 hours after treatment was discontinued; incidence of pneumothorax; days of ventilatory support; continuous positive airway pressure and oxygen; length of intensive care unit and hospital stay; days needed to reach full enteral feeding; weight gain at hospital discharge; neurodevelopmental outcome (measured by Neurobehavioral Assessment of NAPI cluster scores at 36 weeks' corrected for gestational age) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by an automated procedure in blocks, stratified by centre |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was ensured by an automated telephone response system |
| Blinding of participants and personnel (performance bias) | Low risk | Caregivers were blinded to treatment |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded to treatment |
| Incomplete outcome data (attrition bias) | Low risk | Outcomes were reported for all patients enrolled |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered; protocol not available |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Anand 2004.
| Study characteristics | ||
| Methods | Multi‐centre randomised placebo‐controlled trial | |
| Participants | 898 preterm (23 to 32 weeks) babies intubated within 72 hours of birth and ventilated for < 8 hours at enrolment Exclusion criteria: major congenital anomalies, asphyxia, intrauterine growth retardation, maternal opioid addiction, participation in other clinical trials | |
| Interventions | Morphine group (n = 449): loading dose 100 mcg/kg followed by infusion of 10, 20, or 30 mcg/kg/hour for infants of gestational ages 23 to 26, 27 to 29, and 30 to 32 weeks, respectively Placebo group (n = 449): additional analgesia with morphine bolus doses was allowed | |
| Outcomes | Primary outcomes: a composite of neonatal death, grade III/IV IVH, and PVL Secondary outcomes: pain response to tracheal suctioning (assessed by PIPP) ‐ scores were assessed before start of treatment, after 24 and 72 hours of infusion, and at 12 hours after treatment was discontinued; days of ventilatory support; days of oxygen supplementation; days to full volume feeding | |
| Notes | Data on PIPP scores, duration of ventilation, days to reach full enteral feeding were obtained by personal communication with the study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by an automated procedure, stratified by centre and groups of gestational age at birth |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was ensured by an automated telephone response system |
| Blinding of participants and personnel (performance bias) | Low risk | Caregivers were blinded to treatment |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment |
| Incomplete outcome data (attrition bias) | Low risk | Outcomes were reported for all patients enrolled |
| Selective reporting (reporting bias) | Unclear risk | Trail not registered; protocol not available |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Ancora 2013.
| Study characteristics | ||
| Methods | Multi‐centre randomised double‐blind placebo‐controlled study | |
| Participants | 131 preterm infants (22 to 32 weeks) mechanically ventilated during the first 72 hours of life were eligible for inclusion Exclusion criteria: known genetic or chromosomal disorders; severe IVH (grade III and intraparenchymal haemorrhage); cystic PVL; need for postoperative analgesic therapy during the first week of life; participation in another clinical trial; probable rapid extubation | |
| Interventions | Fentanyl group (n = 64): intravenous loading dose of 1 microg/kg in 30 minutes, followed by continuous intravenous infusion of 1 mg/kg/hour Placebo group (n = 67): continuous infusion of placebo Masked treatment was started within 24 hours from initiation of MV and had to be continued until the end of MV (if interrupted before 7 days of life). After the seventh day of life, newborns still receiving MV were treated for pain according to local protocols. Infants could receive open‐label boluses of fentanyl as required In both groups, infants could receive open‐label boluses of fentanyl as required | |
| Outcomes | Primary outcome: acute pain response to daily heel prick (assessed by PIPP Scale); prolonged pain measured 3 times daily on the EDIN validated algometric scale Secondary outcomes: infant’s clinical status; duration of hospitalisation; duration of MV; chronic lung disease (need for supplemental oxygen at 36 weeks' PMA); use of MV at 1 week of age; duration of the first cycle of MV (hours); age at full enteral feeding (i.e. milk volume of 150 mL/kg/d); age at first meconium passage (hours); IVH, cystic PVL, or death within 28 days of life; chest wall rigidity; bladder size during first week of life. Diuresis, blood pressure, and use of vasopressors were recorded as well At 24 months' corrected age, developmental assessment was performed with the revised Griffiths Mental Development Scales, providing a general developmental quotient (DQ) of infants’ abilities and 5 subscale quotients (locomotor, personal and social, hearing and language, eye and hand co‐ordination, performance) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation code was developed with a computerised random number generator |
| Allocation concealment (selection bias) | Low risk | Allocation was made by a request sent to the local pharmacy, which had a specific randomisation list, stratified by GA and balanced in blocks of 6 at 1:1, developed by the pharmacy at the co‐ordinating centre |
| Blinding of participants and personnel (performance bias) | Low risk | The local pharmacy prepared study medication (fentanyl or placebo) and packed it in a similar way |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "double‐blind design" |
| Incomplete outcome data (attrition bias) | Low risk | All infants accounted for For follow‐up study (Ancora 2017), outcomes were missing for more than 10% of participants, although dropout did not differ between treatment groups (23.5% and 25.0% lost at follow‐up in fentanyl and placebo groups, respectively) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Chen 2015.
| Study characteristics | ||
| Methods | Single‐centre randomised controlled trial | |
| Participants | 30 newborns (28 to 39 weeks) mechanically ventilated longer than 24 hours, with disease including neonatal pneumonia, neonatal respiratory distress syndrome, neonatal aspiration pneumonia, and neonatal wet lung Exclusion criteria: not stated | |
| Interventions | Fentanyl group (n = 15): 2 mcg/kg by intravenous bolus injection (bolus time > 10 minutes), followed by continuous intravenous infusion (2 mcg/kg/hour; dose was adjusted according to degree of pain in the patient) Control group (n = 15): no intervention | |
| Outcomes | Heart rate; respiratory rate; blood pressure; percutaneous oxygen saturation; PIPP score recorded before treatment and at 30 minutes, 2 hours, and 4 hours after drug administration Mental development index (MDI) and psychomotor development index (PDI) were measured using the CDCC intellectual development scale for infants, during follow‐up at 3, 6, 9, and 12 months after discharge | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation is not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment is not specified |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessment is not specified |
| Incomplete outcome data (attrition bias) | Low risk | All infants are accounted for |
| Selective reporting (reporting bias) | Unclear risk | We could not ascertain if there were deviations from the original protocol in the final publication (trial not registered, protocol not available) |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Dyke 1995.
| Study characteristics | ||
| Methods | Single‐centre randomised double‐blind placebo‐controlled trial | |
| Participants | 26 preterm infants (29 to 36 weeks) requiring mechanical ventilation for hyaline membrane disease Exclusion criteria: major congenital malformations |
|
| Interventions | Morphine group (n = 12): loading dose 100 mcg/kg over 30 minutes followed by continuous infusion 10 mcg/kg/hour Placebo group (n = 14): dextrose 5% Infusion continued until weaning from intermittent mandatory ventilation, or for maximum of 48 hours' therapy. Pancuronium allowed for infants not stabilised by ventilatory adjustment and markedly asynchronous with the ventilator (2/12 infants in morphine group, 3/14 in the placebo group) | |
| Outcomes | Primary outcomes: heart rate, blood pressure, respiratory rate hourly; severity of respiratory distress; interaction of the infant with positive‐pressure ventilation Secondary outcomes: duration of oxygen therapy; ventilator therapy and hospitalisation; incidence of bronchopulmonary dysplasia, periventricular haemorrhage, and pneumothorax | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by a computer‐generated list in the pharmacy |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was adequate |
| Blinding of participants and personnel (performance bias) | Low risk | Caregivers were blinded to treatment |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment |
| Incomplete outcome data (attrition bias) | Low risk | There was no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered; protocol not available |
| Other bias | Low risk | Study appears to be free of other sources of bias |
e Silva 2008.
| Study characteristics | ||
| Methods | Single‐centre double‐blind randomised controlled trial | |
| Participants | 20 neonates were enrolled according to the inclusion criteria: preterm neonates (28 to 34 weeks) with clinical and radiological features compatible with RDS, requiring elective tracheal intubation and surfactant therapy Exclusion criteria: major congenital malformations; birth weight < 1000 g; previous or concurrent use of opioid for any reason; haemodynamic instability before indication of tracheal intubation; volume expansion need | |
| Interventions | Remifentanil group (n = 10): continuous infusion of 0,5 mcg/kg/min Morphine group (n = 10): continuous infusion of 10 mcg/kg/hour Both infusions began 15 minutes after successful intubation. If extubation was possible (gas results were normal, and there was no other concomitant event to contraindicate extubation), continuous drug infusion was stopped | |
| Outcomes | Primary outcome: time to awaken and time to extubate after interruption of opioid administration. The presence of open eyes, limb movements, and individual respiratory incursions defined awake Secondary outcomes: level of pain (NIPS Scale, COMFORT Scale); HR; BP; temperature; SpO₂; continuous positive airway pressure (CPAP) therapy duration | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number table was used to randomise premature neonates |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Carers were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered; protocol not available |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Guinsburg 1998.
| Study characteristics | ||
| Methods | Single‐centre randomised double‐blind placebo‐controlled trial | |
| Participants | 22 preterm infants (≤ 32 weeks) mechanically ventilated since birth, with postnatal age 12 to 48 hours, with an indwelling arterial umbilical line Exclusion criteria: maternal opioid use or abuse during pregnancy, labour, or delivery; administration of muscle relaxants, analgesics, or sedatives before or during the study period; grade III to IV intraventricular haemorrhage; central nervous system malformations; gross neurological abnormalities; intubation or re‐intubation within 4 hours before patient observation | |
| Interventions | Intervention group (n = 11): single dose of fentanyl (3 mcg/kg) injected over 2 minutes Control group (n = 11): 0.2 mL normal saline injected over 2 minutes | |
| Outcomes | Serum cortisol and growth hormone; blood glucose and lactate; vital signs. Behavioural pain scales: modified postoperative COMFORT Scale and NFCS for 10 minutes. All outcomes measured before treatment, at 30 minutes and at 60 minutes after treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not stated |
| Allocation concealment (selection bias) | Unclear risk | The use of sealed envelopes was stated, but it is not clear how allocation concealment was dealt with |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "the drug randomization code was broken and statistical analysis was performed after all data had been recorded" |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were stated to be blinded to treatment |
| Incomplete outcome data (attrition bias) | Low risk | Outcomes were reported for all patients enrolled |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered; protocol not available |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Ionides 1994.
| Study characteristics | ||
| Methods | Single‐centre randomised controlled trial | |
| Participants | Infants mechanically ventilated who required the use of pancuronium and/or morphine or fentanyl, according to the attending physician's judgement, were included Exclusion criteria: asphyxia (Apgar score ≤ 3 at 5 minutes); foetal drug exposure; sepsis; major congenital anomalies Among the 27 neonates enrolled, 2 were excluded because of inadequate blood sampling, 3 received only pancuronium, and the remaining 22 were randomly assigned to fentanyl or morphine | |
| Interventions | Fentanyl group (n = 10): 3 mcg/kg as a single intravenous dose Morphine group (n = 12): 0.1 mg/kg as a single intravenous dose 4 newborns in the fentanyl group and 2 in the morphine group received both pancuronium and opioid; pancuronium was always administered first, followed by opiate in 1 hour | |
| Outcomes | β‐Endorphin concentration before and 1 hour after administration of each medicine; HR; systolic and diastolic BP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients were assigned by medical record number |
| Allocation concealment (selection bias) | High risk | The chosen sequence did not allow for allocation concealment |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding was not clearly ensured |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding was not clearly ensured |
| Incomplete outcome data (attrition bias) | Low risk | All infants accounted for 2 infants subsequently excluded because of inadequate blood sampling |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered; protocol not available |
| Other bias | Unclear risk | The randomisation process between morphine and fentanyl groups was started after the attending physician decided whether to use pancuronium, opiates, or both |
Jiang 2012.
| Study characteristics | ||
| Methods | Single‐centre randomised controlled double‐blind trial | |
| Participants | 46 full‐term or premature neonates who required MV were recruited Exclusion criteria: history of severe asphyxia and hypoxia (5‐minute Apgar score < 4, umbilical blood pH < 7.0); severe intraventricular haemorrhage; severe congenital malformation (e.g. congenital heart disease, diaphragmatic hernia, cleft palate); paralysis; history of use of neuromuscular blocker agents | |
| Interventions | Morphine group (n = 22): i.v. infusion (> 1 hour) of 5% glucose‐saline 10 mL + morphine 100 mcg/kg, followed by continuous i.v. infusion of 5% glucose‐saline 24 mL + morphine 10 mcg/kg/hour for several days until criteria for withdrawal were met Control group (n = 24): i.v. infusion (> 1 hour) of 5% glucose‐saline 10 mL, followed by continuous i.v. infusion of 5% glucose‐saline 24 mL for several days until criteria for withdrawal were met | |
| Outcomes | Respiratory rate (RR); peak inspiratory pressure (PIP); positive end‐expiratory pressure (PEEP); fraction of inspired oxygen (FiO₂); mean arterial pressure (MAP); heart rate (HR). Pain assessment by N‐PASS score and COMFORT score was measured at 30 minutes, 2 hours, 6 hours, 12 hours, 24 hours, and 48 hours after administration; brain ultrasound was done at Day 1 (before administration), 5, 7, and 14. Total ventilation time, total time from withdrawal to extubation, total oxygen time after ventilation, and adverse events were evaluated |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done with 15 sets of opaque envelopes containing 4 cards reporting A or B (A = intervention; B = control). A member of the research group took out a random card from a random set for each newborn included |
| Allocation concealment (selection bias) | Low risk | Envelopes were opaque |
| Blinding of participants and personnel (performance bias) | Low risk | The nurse knew only the meaning of "A" and "B" as intervention or control and labelled drugs so they were not different in volume, appearance, and odour |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment |
| Incomplete outcome data (attrition bias) | Low risk | All infants were accounted for |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered; protocol not available Not reported in results: total ventilation time, total time from withdrawal to extubation, total oxygen time after ventilation, brain ultrasound |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Lago 1998.
| Study characteristics | ||
| Methods | Single‐centre randomised double‐blind controlled trial | |
| Participants | 55 preterm infants (26 to 34 weeks) requiring mechanical ventilation for hyaline membrane disease, with indwelling catheters
Exclusion criteria: asphyxia (Apgar < 5 at 5 minutes); foetal drug exposure; sepsis; major congenital anomalies 2 infants died (1 in fentanyl group and 1 in control group) and were excluded from analysis |
|
| Interventions | Fentanyl group (n = 26): continuous infusion at 0.5 to 2 mcg/kg/hour adjusted to render the neonate sedated but arousable (according to the behavioural sedation score) Control group (n = 27): no intervention | |
| Outcomes | Urine metanephrine:normetanephrine molar ratio concentration; behavioural sedation score (assessed every 2 hours during the study period); severity of hyaline membrane disease; need for surfactant replacement; evidence of clinically significant patent ductus arteriosus; days of ventilatory support and oxygen treatment; air leak; IVH; PVL; BPD; days to exclusive enteral feeding; days to reach birth weight; length of hospital stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not stated |
| Allocation concealment (selection bias) | Low risk | The use of sealed envelopes was reported (study author's communication) |
| Blinding of participants and personnel (performance bias) | High risk | Caregivers were not blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Assessors were stated to be blinded to treatment, but blinding was unlikely |
| Incomplete outcome data (attrition bias) | High risk | 2 patients died after enrolment and were excluded from statistical analysis |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered; protocol not available |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Lago 1999.
| Study characteristics | ||
| Methods | Single‐centre randomised double‐blind placebo‐controlled trial | |
| Participants | 31 preterm infants (> 28 weeks to < 37 weeks) ventilated for hyaline membrane disease Exclusion criteria: not stated | |
| Interventions | Fentanyl group (n = 15): continuous infusion of 1.5 mcg/kg/hour scaled down by 0.5 mcg/kg/hour every 24 hours, for a total of 72 hours Placebo group (n = 16): 5% glucose | |
| Outcomes | Ventilator setting; sedation score (not described) every 4 hours; severity of respiratory disorder; radiological score; duration of ventilation; need for surfactant therapy; duration of oxygen dependence; electromyographic activity of intercostal muscles | |
| Notes | Data, methods, and details were obtained by personal communication with the study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not stated |
| Allocation concealment (selection bias) | Low risk | The use of sealed envelopes was reported (study author's communication) |
| Blinding of participants and personnel (performance bias) | Low risk | Caregivers were unaware of treatment |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were stated to be blinded to treatment |
| Incomplete outcome data (attrition bias) | Unclear risk | All infants were accounted for. However, as the study is available as an abstract only, risk for attrition bias is unclear |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered; protocol not available |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Liem 1999.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 8 preterm infants (29.9 to 32.1 weeks) who needed MV Exclusion criteria: no information | |
| Interventions | Midazolam group (n = 4): loading dose 0.2 mg/kg, maintenance 0.2 mg/kg/hour Morphine group (n = 4): loading dose 0.05 mg/kg, maintenance 0.001 mg/kg/hour | |
| Outcomes | Concentration changes of oxyhaemoglobin, deoxyhaemoglobin, and total haemoglobin in the brain were continuously measured with near‐infrared spectrophotometry for 1 hour Changes in cerebral blood flow velocity (pulsed Doppler US) in internal carotid artery (in %) were determined before and at 15, 30, and 60 minutes after sedation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding was not specified |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding was not specified |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of infants lost to follow‐up or analysed for each outcome was not specified |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered; protocol not available |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Naderi 2017.
| Study characteristics | ||
| Methods | Single‐centre randomised double‐blinded clinical trial | |
| Participants | 32 newborns with gestational age between 26 and 38 weeks undergoing intubation and mechanical ventilation (for at least 24 hours) were included Exclusion criteria: asphyxia (possible hepatic or renal damage); Apgar scores below 5 at fifth minute after birth; direct hyperbilirubinaemia; neonatal hepatitis; biliary system anomalies; cardiovascular anomalies; gastrointestinal obstructions | |
| Interventions | Morphine group (n = 16): intravenous loading dose of 100 mcg/kg in the first hour, with maintenance dose of 12 mcg/kg/hour for the next 24 hours Fentanyl group (n = 16): intravenous loading dose of 2 mcg, with maintenance dose of 0.25 mcg/kg/hour | |
| Outcomes | Ultrasonographic measurements of gallbladder dimensions (length, width, depth, and volume), as well as occurrence of hydrops of gallbladder, were evaluated on the third and sixth days after drug administration | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not stated |
| Allocation concealment (selection bias) | Unclear risk | The use of sealed envelopes was stated, but it is not clear how allocation concealment was dealt with |
| Blinding of participants and personnel (performance bias) | Low risk | Carers were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | All infants were accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Orsini 1996.
| Study characteristics | ||
| Methods | Single‐centre randomised double‐blind placebo‐controlled trial | |
| Participants | 20 preterm infants (26 to 36 weeks), > 1000 grams birth weight, undergoing mechanical ventilation for respiratory distress syndrome, with indwelling arterial catheter Exclusion criteria: any analgesic, sedating agent or muscle relaxant given before informed consent could be obtained | |
| Interventions | Fentanyl group (n = 11): loading dose 5 mcg/kg over 20 minutes followed by continuous infusion of 2 mcg/kg/hour for 72 hours, 1 mcg/kg/hour for the next 24 hours, and 0.5 mcg/kg/hour for the final 24 hours (total: 5 days) Placebo group (n = 9): dextrose 5% in water | |
| Outcomes | Behavioral score; vital signs every 2 hours; cortisol and 11‐deoxycortisol levels at baseline and daily 3‐methyl histidine/urinary creatinine ratio and urea excretion (as indicators of catabolism); incidence of intraventricular haemorrhage; patent ductus arteriosus; bronchopulmonary dysplasia; sepsis | |
| Notes | Data on pain and on incidence of CLD and IVH were obtained by personal communication with the study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was performed in the pharmacy by random number generation (not otherwise specified) |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was adequate |
| Blinding of participants and personnel (performance bias) | Low risk | Caregivers were blinded to treatment |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded to treatment |
| Incomplete outcome data (attrition bias) | Low risk | There was no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered; protocol not available |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Qiu 2019.
| Study characteristics | ||
| Methods | Single‐centre double‐blind randomised placebo‐controlled trial | |
| Participants | 60 premature newborns (< 32 weeks' GA) mechanically ventilated in the first 72 hours after birth Exclusion criteria: serious birth injury; serious malformation; significant parenchymal brain injury (grade IV IVH or PVL); treatment with analgesics or sedatives | |
| Interventions | Fentanyl group (n = 30): intravenous loading dose of 1 mcg/kg in 30 minutes, followed by continuous intravenous infusion of 1 mcg/kg/hour immediately after MV Placebo group (n = 30): 5% glucose at the same rate as fentanyl Dose of fentanyl/glucose was decreased to 0.5 mcg/kg/hour when default parameters for MV were as follows: FiO₂ < 25%, RR < 25 bpm, and MAP < 7 cmH₂O. 30 minutes later, MV was stopped after discontinuation of infusion | |
| Outcomes | PIPP; cerebral blood flow velocity (by transcranial Doppler at 1, 3, and 7 days after MV); neuron‐specific enolase (NSE) concentrations in plasma samples (immediately before and after MV); cerebral function monitoring (CFM) recordings (obtained at 37 weeks' GA) Also, pH, PaO₂, PaCO₂, MAP, heart rate, cardiac output, PIP, PEEP, mean airway pressure, and FiO₂ were recorded immediately before administration and at 1, 12, 24, 48, and 72 hours after the start of the infusion | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done with a random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not specified |
| Blinding of participants and personnel (performance bias) | Low risk | Carers were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | 3 neonates in the fentanyl group and 4 neonates in the control group were excluded after randomisation because they were discharged within 2 weeks; they were not included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered; protocol not available |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Quinn 1992.
| Study characteristics | ||
| Methods | Single‐centre randomised double‐blind controlled trial | |
| Participants | 95 preterm newborns (57, excluding those receiving only morphine) Entry criteria: newborns with hyaline membrane disease who were fighting against the ventilator (on clinical impression); postnatal age > 4 hours and < 48 hours; no prior treatment with narcotic analgesic or neuromuscular blocking agent | |
| Interventions | Morphine group (n = 29): 50 mcg/kg/hour by continuous infusion, increased to 100 mcg/kg/hour if the newborn was still struggling after 2 hours. Babies in this group were allowed to receive pancuronium if still fighting the ventilator after 4 hours (n = 7) Pancuronium group (n = 28): 100 mcg/kg per dose given as required to inhibit breathing. Babies in this group were given morphine for painful procedures (n = 4) Morphine + pancuronium group (n = 38): continuous morphine at 50 mcg/kg/hour + intermittent pancuronium at 100 mcg/kg as required; morphine not increased above 50 mcg/kg/hour We extracted data from the morphine + pancuronium group and the pancuronium group (i.e. pancuronium was a co‐intervention); we did not include the group receiving only morphine Drug therapy continued until FiO₂ < 0.45. 1 baby stopped treatment within 24 hours because FiO₂ fell below 0.45 | |
| Outcomes | Catecholamines; plasma levels; blood pressure; heart rate; peak inspiratory pressure; FiO₂ on entry to the study and after 6 hours' treatment; days on ventilator; air leaks; intraventricular haemorrhage; PDA; death 5 to 6 years' follow‐up outcomes: IQ (by Weschler Preschool and Primary Scale of Intelligence); motor impairment (by Movement Assessment Battery for Children); behaviour problems (by Child Behaviour Checklist completed by a parent); blood thyroid‐stimulating hormone level | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study authors stated that randomisation was performed by drawing a sealed envelope. These authors did not state how the list of randomisation was generated |
| Allocation concealment (selection bias) | High risk | Allocation concealment was inadequate |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of interventions and outcomes was not clearly ensured |
| Blinding of outcome assessment (detection bias) | High risk | Blinding of interventions and outcomes was not clearly ensured |
| Incomplete outcome data (attrition bias) | Unclear risk | Clinical outcomes were reported for 28 of the 38 randomised infants to morphine and for 28 of 28 in the control group. Unclear if due to a typo in the manuscript or attrition bias. A power calculation was made for differences in catecholamine levels. Analysis of clinical data was performed on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered; protocol not available |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Quinn 1993.
| Study characteristics | ||
| Methods | Single‐centre randomised double‐blind placebo‐controlled trial | |
| Participants | 41 preterm (< 34 weeks) infants who required mechanical ventilation and received surfactant (Curosurf) for hyaline membrane disease Exclusion criteria: babies who did not have an arterial line in situ | |
| Interventions | Morphine group (n = 21): loading dose 100 mcg/kg/hour for 2 hours followed by 25 mcg/kg/hour as a continuous infusion. Treatment was continued until the baby was on a ventilator Placebo group (n = 20): dextrose 5% | |
| Outcomes | Catecholamine; plasma levels; blood pressure; heart rate and ventilator setting (peak inspiratory pressure and oxygen concentration) at study entry and after 6 hours' treatment; arterial/alveolar oxygen ratio at 0 hours and at 24 hours; pain score (based on level of consciousness, crying, posture, and facial expression); days on ventilator; air leaks; intraventricular haemorrhage; PDA; death during first 6 months of life 5 to 6 years' follow‐up outcomes: IQ (by Weschler Preschool and Primary Scale of Intelligence); motor impairment (by Movement Assessment Battery for Children); behaviour problems (by Child Behaviour Checklist completed by a parent); blood thyroid‐stimulating hormone level | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by using a stratified table |
| Allocation concealment (selection bias) | Low risk | Pharmacy allowed for adequate allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | Caregivers were stated to be blinded, except for the consultant physician responsible for the baby |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were stated to be blinded |
| Incomplete outcome data (attrition bias) | Low risk | There was no loss to follow‐up for clinical outcomes. A power calculation was made for differences in catecholamines levels. Analysis of clinical data was performed on an intention‐to‐treat basis. The trial was terminated after an interim analysis showed differences in adrenaline concentrations |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered; protocol not available |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Saarenmaa 1999.
| Study characteristics | ||
| Methods | Single‐centre double‐blind randomised controlled trial | |
| Participants | 163 babies mechanically ventilated during the first day of life fulfilled study entry criteria: 24 weeks' or longer GA, estimated duration of MV ≥ 1 day, with indwelling arterial line Exclusion criteria: chromosomal aberrations; major anomalies | |
| Interventions | Fentanyl group (n = 83): loading dose of 10.5 mcg/kg in 1 hour, followed by maintenance rate of 1.5 mcg/kg/hour for ≥ 24 hours Morphine group (n = 80): loading dose of 140 mcg/kg in 1 hour, followed by maintenance dose of 20 mcg/kg/hour for ≥ 24 hours Additional analgesia with opioid boluses was allowed. The bolus, equal to a 1‐hour maintenance infusion dose, could be given 4 times a day at the most. Muscle relaxation could be used if the infant was struggling against the ventilator despite analgesia | |
| Outcomes | Primary outcome (used for sample size calculation): gastrointestinal motility and urinary retention Secondary outcomes: duration of MV; beginning of enteral feeding (days); duration of opioid infusion (hours); administration of opioid boluses; pancuronium relaxation; surfactant treatment; RDS; infection; PPHN; NEC; IVH (grades 3 + 4); mortality; severity of pain assessed with (i) physiological parameters (heart rate, arterial blood pressure, and oxygen saturation); (ii) behavioural pain responses before, during, and after tracheal suction assessed with a pain score adapted from the Neonatal Infant Pain Scale of the Children´s Hospital of East Ontario Pain Scale; (iii) stress hormone concentrations (noradrenaline, adrenaline, and beta‐endorphin) before and 2 and 24 hours after the start of treatment | |
| Notes | Power calculation was made on urinary retention and decreased gastrointestinal motility | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Infants were randomised in 5 blocks with closed envelopes. Sequence generation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not specified |
| Blinding of participants and personnel (performance bias) | Low risk | Carers were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | 2 infants in the fentanyl group did not receive the infusion; their data are included in an ITT analysis. Data on plasma catecholamine and endorphin levels were not available for all infants; however loss to follow‐up seems similar in the 2 groups |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered; protocol not available |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Schmidt 2010.
| Study characteristics | ||
| Methods | Single‐centre randomised double‐blind trial | |
| Participants | 20 term neonates (≥ 37 weeks) mechanically ventilated longer than 24 hours were included Exclusion criteria: known allergy to fentanyl or sufentanil; renal or hepatic dysfunction (serum creatinine > 1.5 mg/dL, urinary production < 0.5 mL/(kg × hour) after 48 hours; glutamate‐oxaloacetate‐transferase (GOT) > 150 U/L, Quick < 25%); maternal history of prenatal opioid abuse; chromosomal disorders; major anomalies | |
| Interventions | Fentanyl group (n = 10): intravenous loading dose of 2 to 3 μg/kg in 15 minutes, followed by maintenance rate of 1 μg/(kg × hour) Sufentanil group (n = 10): intravenous loading dose of 0.28 to 0.42 μg/kg in 15 minutes, followed by maintenance rate of 0.14 μg/(kg × hour) In case of inadequate analgesia (estimated by the Hartwig Behavioural Pain Scale score), opioid dose was adjusted in steps of 0.5 to 1 μg/(kg × hour) fentanyl or 0.07 to 0.14 μg/(kg × hour) sufentanil according to flow rate. Administration of an additional bolus equal to a 2‐hour maintenance infusion dose was possible. As sedative, co‐medication midazolam was allowed | |
| Outcomes | Primary outcome: duration of weaning, defined as time to extubation after opioid discontinuation Secondary outcomes: SNAP score (Score for Neonatal Acute Physiology) within the first 24 hours; TISS (Therapeutic Intervention Scoring System) daily; behavioural analgesic and sedation scores (Hartwig score) every 4 hours; HR; ABP; oxygen saturation; diuresis; urinary cortisol levels; Quick value, GOT, and creatinine (daily); co‐medications; enteral/oral feeding; withdrawal symptoms (Finnegan score); duration of MV also measured | |
| Notes | Power calculation was made on the duration of weaning | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation by concealed envelops in blocks of 4 patients. Sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not specified |
| Blinding of participants and personnel (performance bias) | Low risk | Doctors as well as nurses were blinded with respect to medication |
| Blinding of outcome assessment (detection bias) | Low risk | Doctors as well as nurses were blinded with respect to medication |
| Incomplete outcome data (attrition bias) | Unclear risk | Tables 1 and 2 report 10 patients for each group, whereas in the Methods, it is reported (quote): "comparison of weaning time at the interim analysis (intention‐to‐treat population n = 13, fentanyl n = 6, and sufentanil group = 7)" |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported; protocol not available |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Simons 2003.
| Study characteristics | ||
| Methods | Randomised double‐blind placebo‐controlled trial in 2 NICUs | |
| Participants | 150 ventilated neonates Inclusion criteria: all neonates admitted to NICU who required mechanical ventilation; postnatal age < 3 days; ventilation < 8 hours Exclusion criteria: severe asphyxia; severe IVH; major congenital malformations; facial malformations; neurological disorders; continuous or intermittent treatment with neuromuscular blockers | |
| Interventions | Morphine group (n = 73): loading dose 100 mcg/kg followed by 10 mcg/kg/hour continuous infusion Placebo group (n = 77): sodium chloride in 5% glucose Masked treatment was continued for 7 days or less (as for clinical conditions); after 7 days, study medication was weaned or stopped, or was replaced by open‐label morphine infusion During study period, additional morphine was allowed if patients from either group were judged to be in pain or distress based on decisions of the attending physician | |
| Outcomes | Primary outcomes: pain response: PIPP, NIPS, and visual analogue scale at standardised time points
Secondary outcomes: incidence of all grades of IVH and poor neurological outcome (severe IVH, PVL, or death); duration of MV; length of NICU stay; incidence of comorbidity (chronic lung disease; sepsis; necrotising enterocolitis; PDA); number of painful procedures; MAP; administration of volume expanders and vasopressor drugs; number of infants with hypotensive MAP measurements as compared with "normal" values of BP; concentrations of adrenaline and noradrenaline in arterial blood plasma
Primary and secondary outcomes were measured during hospital stay and were reported by Simons 2003 and Simons 2005 Follow‐up outcomes:
|
|
| Notes | Data on duration of ventilator days were presented for the first period (NICU stay) and as total duration of ventilator days during admission. Data from the first period were used for the meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation: computer‐generated randomisation list to select 10 random permuted blocks stratified into 5 groups of gestational age ranges |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was ensured |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of caregivers was attained |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of assessors was attained |
| Incomplete outcome data (attrition bias) | Low risk | There was no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered; protocol not available |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Siwiec 1999.
| Study characteristics | ||
| Methods | Randomised double‐blind controlled trial | |
| Participants | 20 preterm infants (26 to 35 weeks), birth weight 810 to 2750 grams, receiving mechanical ventilation Exclusion criteria: not stated | |
| Interventions | Morphine group (n = 10): loading dose 100 mcg/kg over 30 minutes followed by continuous infusion of 20 mcg/kg/hour for 1 to 5 days Control group (n = 10): no intervention | |
| Outcomes | PIPP and COMFORT scores; ventilatory setting; mean airways pressure, ventilatory rate, FiO₂; pneumothorax; grade IV intraventricular haemorrhage; periventricular leukomalacia; BPD | |
| Notes | Data on pain scores were obtained by personal communication with the study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not stated |
| Allocation concealment (selection bias) | Low risk | The use of sealed envelopes was reported (study author's communication) |
| Blinding of participants and personnel (performance bias) | High risk | Caregivers were stated to be not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Assessors were stated to be not blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up not reported; number of patients evaluated for each outcome not reported |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered; protocol not available |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Welzing 2012.
| Study characteristics | ||
| Methods | Single‐centre double‐blind randomised controlled trial | |
| Participants | 24 mechanically ventilated infants were recruited Inclusion criteria: gestational age ≥ 36 weeks with postnatal age no greater than 60 days; intubation within the last 12 hours; expected analgesia and sedation requirement due to mechanical ventilation for a further 12 to 96 hours Exclusion criteria: CNS insult (e.g. asphyxia); structural brain disorder affecting ability to assess the level of sedation | |
| Interventions | Fentanyl group (n = 12): 3 mcg/kg/hour combined with midazolam 50 μg/kg/hour. Subsequently, opioid was adjusted in steps of 1 μg/kg/hour (maximum 10 mcg/kg/hour) to achieve and maintain a Hartwig score between 9 and 13 Remifentanil group (n = 12): 9 μg/kg/hour combined with midazolam 50 μg/kg/hour. Subsequently, opioid was adjusted in steps of 3 μg/kg/hour (maximum 30 mcg/kg/hour) to achieve and maintain a Hartwig score between 9 and 13 Pre‐medication for endotracheal intubation was given and boluses as needed to keep the infant sedated and pain‐free until the start of study medication, which had to be started at the latest 12 hours after intubation. Increased dosage of midazolam and boli of thiopental were allowed. If analgesia and sedation were insufficient despite the maximum allowed opioid and midazolam dosage, the infant was excluded from the study | |
| Outcomes | Primary outcome: extubation time (time from cessation of opioid infusion until extubation) Secondary outcomes: efficacy in analgesia and sedation (Hartwig score); haemodynamic stability (MBP, HR); adverse effects; opioid tolerance (number of doses, number of dose adjustments); opioid withdrawal (Finnegan score); opioid‐induced hyperalgesia (CHIPPS Scale score and Flexion Withdrawal Reflex threshold) in the first 48 hours after extubation | |
| Notes | Of 24 infants randomised, 1 was withdrawn from the fentanyl group because of insufficient analgesia despite the maximum allowed dosage, and 2 were withdrawn from the remifentanil group because of protocol deviation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was ensured by the central pharmacy of the hospital |
| Blinding of participants and personnel (performance bias) | Low risk | Interventions were masked; the trial was stated to be double‐blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Interventions were masked; the trial was stated to be double‐blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | If analgesia and sedation were insufficient despite the maximum allowed opioid and midazolam dosage, the infant was excluded from the study (not considered for the ITT analysis) |
| Selective reporting (reporting bias) | High risk | Outcomes reported in the article seemed much more than those established in the protocol. Moreover, not all outcomes in the protocol were reported in the article (discharge time from the PICU) |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Wood 1998.
| Study characteristics | ||
| Methods | Single‐centre double‐blind randomised controlled trial | |
| Participants | 88 babies were eligible Inclusion criteria: neonates less than 35 weeks' PMA at birth, greater than 2 hours but less than 48 hours old at trial entry; who required IPPV (intermittent positive‐pressure ventilation) and therefore sedation. An indwelling intra‐arterial access was needed as an inclusion criterion Exclusion criteria: early neonatal surgery; severe congenital malformation; previous administration of opiates | |
| Interventions | Morphine group (n = 44): 200 mcg/kg over 2 hours, followed by maintenance infusion of 25 mcg/kg/hour Diamorphine group (n = 44): 120 mcg/kg over 2 hours, then 15 mcg/kg/hour | |
| Outcomes | Mean arterial blood pressure (MABP); coefficient of variation (CV) of 10 successive systolic blood pressure values; sedation level (by a 4‐parameter sedation score and a qualitative judgement) recorded at 0, 2, 6, and 24 hours into the infusion; plasma concentrations of adrenaline and noradrenaline evaluated at 0 and 24 hours Duration of infusion (hours); days of MV; IVH (all grades); parenchymal brain lesions; air leak; PDA; oxygen required at 28 days; death (up to 28 days) also recorded | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not stated |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Clinicians, nurses, and parents were blinded to randomisation. Infusion solutions were made up by the hospital pharmacy in identically presented syringes |
| Blinding of outcome assessment (detection bias) | Low risk | Trial was stated to be double‐blinded: probably done (clinicians and nurses were blinded) |
| Incomplete outcome data (attrition bias) | High risk | For many outcomes, data for almost 50% of infants were missing |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered; protocol not available |
| Other bias | Low risk | Study appears to be free of other sources of bias |
ABP: arterial blood pressure; BP: blood pressure; BPD: bronchopulmonary dysplasia; bpm: beats per minute; CBCL: Child Behavior Checklist; CDCC: Child Development Center of China; CFM: cerebral function monitoring; CHIPPS: Children and Infants Postoperative Pain Scale; CLD: chronic lung disease; CNS: central nervous system; CPAP:continuous positive airway pressure; CV: coefficient of variation; DQ: developmental quotient; EDIN: Échelle de Douleur et d'Inconfort du Nouveau‐né; FiO₂: fraction of inspired oxygen: GA: gestational age; GOT: glutamate‐oxaloacetate‐transferase; HR: heart rate; IPPV: intermittent positive‐pressure ventilation; IQ: intelligence quotient; ITT: intention‐to‐treat; i.v.: intravenous; IVH: intraventricular haemorrhage; MABP: mean arterial blood pressure; MAP: mean arterial pressure; MBP: mean arterial blood pressure; min: minutes; MDI: mental development index; MV: mechanical ventilation; N‐PASS: Neonatal Pain, Agitation and Sedation Scale; NAPI: Neurobehavioral Assessment of the Preterm Infant; NEC: necrotising enterocolitis; NFCS: Neonatal Facial Coding System; NICU: neonatal intensive care unit; NIPS: Neonatal‐Infant Pain Scale; NOPAIN: Neonatal Outcome and Prolonged Analgesia in Neonates; NSE: neuron‐specific enolase; PDA: patent ductus arteriosus; PDI: psychomotor development index; PEEP: positive end‐expiratory pressure; PICU: paediatric intensive care unit; PIP: peak inspiratory pressure; PIPP: premature infant pain profile; PMA: post‐menstrual age; PPHN: persistent pulmonary hypertension of the newborn; PVL: periventricular leukomalacia; RDS: respiratory distress syndrome; RR: respiratory rate; SNAP score: Score for Neonatal Acute Physiology; TISS: Therapeutic Intervention Scoring System.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akkermans 2015 | Wrong patient population |
| Anand 2008 | Wrong study design |
| Bell 1987 | Wrong study design |
| Bergqvist 2007 | Wrong study design |
| Hwang 1999 | Wrong study design |
| Palmer 2005 | Wrong study design |
| Perlman 2005 | Commentary |
Characteristics of ongoing studies [ordered by study ID]
IRCT2017082417413N26.
| Study name | Comparing the effect of fentanyl and midazolam on the sedation of infants under mechanical ventilation: a randomized clinical trial |
| Methods | Unblinded mono‐centre randomised controlled trial Sample size: 60 newborns Setting: Najmiyeh Hospital, Tehran, Iran |
| Participants | Inclusion criteria: ventilated infants weighing between 2.5 and 4 kg Exclusion criteria: congenital heart disease; brain anomalies; metabolic or chromosomal syndromes |
| Interventions | Fentanyl 0.5 microg/kg Midazolam 0.1 mg/kg |
| Outcomes | Primary outcomes: duration of hospital stay; duration of mechanical ventilation Secondary outcome: pneumothorax |
| Starting date | Expected recruitment start date: 1 July 2016 |
| Contact information | Mohammad Hossein Khosravi; dr.mhkhosravi@gmail.com |
| Notes |
Differences between protocol and review
We added a subgroup analysis for studies in which no dose adjustments or open‐label boluses were made
-
We created 4 time windows for each pain scale:
Less than 12 hours after infusion
Greater than or equal to 12 and less than 48 hours after infusion
Greater than or equal to 48 hours after infusion
Multiple time points (e.g. during endotracheal suctioning).
We modified post hoc time points for the outcome neurodevelopmental outcome (Types of outcome measures; Analysis 1.8; Analysis 1.9)
Contributions of authors
R Bellu Development and writing of the protocol Literature search and identification of trials for inclusion Evaluation of methodological quality of included trials Abstraction of data independently of co‐reviewer Entry of data into RevMan Writing of the description of studies section Writing of the results section Writing of the discussion section
K.A. de Waal Literature search and identification of trials for inclusion Evaluation of methodological quality of included trials Abstraction of data independently of co‐reviewer Entry of data into RevMan Writing of the description of studies section Writing of the results section Writing of the discussion section
R. Zanini Development of the protocol Writing of the discussion section Revision of the final review
O. Romantsik, only for 2019 update Evaluation of methodological quality of included trials Data extraction Writing of the discussion section
C. Nava, only for 2019 update Update of the background Identification of trials for inclusion Evaluation of methodological quality of included trials Abstraction of data independently of co‐reviewer Entry of data into RevMan Writing of the description of studies section Writing of the results section
M. Bruschettini, only for 2019 update Revision of the methods section Identification of trials for inclusion Abstraction of data independently of co‐reviewer Entry of data into RevMan Writing of the description of studies section Writing of the results section Writing of the discussion section
Sources of support
Internal sources
-
Institute for Clinical Sciences, Lund University, Lund, Sweden
to OR and MB
-
National Institute for Health Research, UK
Editorial support for Cochrane Neonatal has been funded with funds from a UK National Institute of Health Research (NIHR) Cochrane Programme Grant (16/114/03). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the NIHR, or the UK Department of Health.
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
Region Skåne, Skåne University Hospital, Lund University and Region Västra Götaland, Sweden, Sweden, Sweden
Cochrane Sweden is supported from Region Skåne, Skåne University Hospital Lund University and Region Västra Götaland.
Declarations of interest
RB has no interest to declare.
KAdW has no interest to declare.
RZ has no interest to declare.
OR has no interest to declare.
CN has no interest to declare.
MB has received research funding from an ALF grant (non‐profit ‐ Lund University) and from the Crafoord Foundation (non‐profit) for research projects not related to Cochrane.
New
References
References to studies included in this review
Anand 1999 {published data only}
- Anand KJ, Barton BA, McIntosh N, Lagercrantz H, Pelausa E, Young TE, et al. Analgesia and sedation in preterm neonates who require ventilatory support: results from the NOPAIN trial. Neonatal Outcome and Prolonged Analgesia in Neonates. Archives of Pediatrics & Adolescent Medicine 1999;153(4):331-8. [10.1001/archpedi.153.4.331] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Anand KJ, McIntosh N, Lagercrantz H, Gauthier T, Pelausa E, Young T, et al. The Pilot NOPAIN Trial: morphine and midazolam infusions decrease pain/stress and may alter clinical outcomes in ventilated preterm neonates. Pediatric Research 1997;41(4 Pt 2):136A. [PMID: 10.1203/00006450-199704001-00819] [DOI] [Google Scholar]
- Anand KJS, McIntosh N, Lagercrantz H, Young TE, Vasa R, Barton BA. Erratum: Analgesia and sedation in preterm neonates who require ventilatory support: result from the NOPAIN trial (Archives of Pediatrics and Adolescent Medicine (April 1999) 153 (331-338)). Archives of Pediatrics and Adolescent Medicine 1999;153(8):895. [DOI] [PubMed] [Google Scholar]
Anand 2004 {published data only}
- Anand KJ, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE, et al, NEOPAIN Trial Investigators Group. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet 2004;363(9422):1673-82. [PMID: 10.1016/S0140-6736(04)16251-X] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Bhandari V, Bergqvist LL, Kronsberg SS, Barton BA, Anand KJ, NEOPAIN Trial Investigators Group. Morphine administration and short-term pulmonary outcomes among ventilated preterm infants. Pediatrics 2005;116(2):352-9. [DOI: 10.1542/peds.2004-2123] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Boyle EM, Wong CM, Freer Y, Ahmadian M, Maxwell A, McIntosh N, et al. Mean heart rate and heart rate variability in preterm ventilated infants receiving morphine analgesia. Pediatric Research 2003;54:569. [Google Scholar]
- Ferguson SA, Ward WL, Paule MG, Hall RW, Anand KJ. A pilot study of preemptive morphine analgesia in preterm neonates: effects on head circumference, social behavior, and response latencies in early childhood. Neurotoxicology and Teratology 2012;34(1):47-55. [PMID: 10.1016/j.ntt.2011.10.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hall RW, Kronsberg SS, Barton BA, Kaiser JR, Anand KJ. Morphine, hypotension and adverse outcomes among preterm neonates: who's to blame? Secondary results from the NEOPAIN trial; NEOPAIN Trial Investigators Group. Pediatrics 2005;115(5):1351-9. [DOI: 10.1542/peds.2004-1398] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Menon G, Boyle EM, Bergqvist LL, McIntosh N, Barton BA, Anand KJ. Morphine analgesia and gastrointestinal morbidity in preterm infants: secondary results from the NEOPAIN trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(5):F362-7. [DOI: 10.1136/adc.2007.119297] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NEOPAIN Multicenter Group. Effects of morphine therapy on neurological outcomes in ventilated preterm neonates: primary outcomes from the NEOPAIN (NEurologic Ouctomes & Pre-Emptive Analgesia In Neonates) Multicenter trial. Pediatric Research 2002;51:361A. [Google Scholar]
- Ward-Begnoche WL, Ferguson S, Hall RW, Anand KS. Long-term effects of morphine analgesia in ventilated preterm neonates. In: Pediatric Academic Societies (PAS) Annual Meeting. 25 May 2009.
Ancora 2013 {published data only}
- Ancora G, Lago P, Garetti E, Pirelli A, Merazzi D, Mastrocola M, et al. Efficacy and safety of continuous infusion of fentanyl for pain control in preterm newborns on mechanical ventilation. Journal of Pediatrics 2013;163(3):645-51.e1. [DOI: 10.1016/j.jpeds.2013.02.039] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ancora G, Lago P, Garetti E, Pirelli A, Merazzi D, Mastrocola M, et al. Efficacy and safety of continuous infusion of fentanyl for pain control in preterm newborns on mechanical ventilation (MV). In: Pediatric Academic Societies (PAS) Annual Meeting. 2011. [DOI] [PubMed]
- Ancora G, Lago P, Garetti E, Pirelli A, Merazzi D, Pierantoni L, et al. Follow-up at the corrected age of 24 months of preterm newborns receiving continuous infusion of fentanyl for pain control during mechanical ventilation. Pain 2017;158(5):840-5. [DOI: 10.1097/j.pain.0000000000000839] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ancora G, Pierantoni L, Grandi S, Maranella E. Continuous infusion of fentanyl in preterm on mechanical ventilation. In: 50th Annual Meeting of the European Society for Paediatric Research. 2009.
- NCT00571636. Continuous infusion of fentanyl in preterm on MV. clinicaltrials.gov/ct2/show/study/NCT00571636 (first received 9 May 2006).
Chen 2015 {published data only}
- Chen SS, Liu L, Hu P, Shi BZ, Fu YK, Luo R, et al. [Analgesic effect of fentanyl in neonates during mechanical ventilation]. Zhongguo dang dai er ke za zhi = Chinese Journal of Contemporary Pediatrics 2015;17(10):1045-50. [PMID: ] [PubMed] [Google Scholar]
Dyke 1995 {published data only}
- Dyke MP, Kohan R, Evans S. Morphine increases synchronous ventilation in preterm infants. Journal of Paediatrics and Child Health 1995;31(3):176-9. [DOI: 10.1111/j.1440-1754.1995.tb00780.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
e Silva 2008 {published data only}
- e Silva YP, Gomez RS, Marcatto Jde O, Maximo TA, Barbosa RF, e Silva AC. Early awakening and extubation with remifentanil in ventilated premature neonates. Paediatric Anaesthesia 2008;18(2):176-83. [DOI: 10.1111/j.1460-9592.2007.02378.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Guinsburg 1998 {published data only}
- Guinsburg R, Kopelman BI, Anand KJ, Almeida MF, Peres Cde A, Miyoshi MH. Physiological, hormonal, and behavioral responses to a single fentanyl dose in intubated and ventilated preterm neonates. Journal of Pediatrics 1998;132(6):954-9. [DOI: 10.1016/s0022-3476(98)70390-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ionides 1994 {published data only}
- Ionides SP, Weiss MG, Angelopoulos M, Myers TF, Handa RJ. Plasma beta-endorphin concentrations and analgesia-muscle relaxation in the newborn infant supported by mechanical ventilation. Journal of Pediatrics 1994;125(1):113-6. [DOI: 10.1016/s0022-3476(94)70136-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jiang 2012 {published data only}
- Jiang HH, Cheng R, Kan Q, Shen X, Li F, Fu CH, et al. [Clinical evaluation of the effects of morphine in mechanical ventilation of neonates]. Zhonghua er ke za zhi = Chinese Journal of Pediatrics 2012;50(5):350-5. [PMID: ] [PubMed] [Google Scholar]
Lago 1998 {published data only}
- Lago P, Benini F, Agosto C, Zacchello F. Randomised controlled trial of low dose fentanyl infusion in preterm infants with hyaline membrane disease. Archives of Disease in Childhood. Fetal and Neonatal Edition 1998;79(3):F194-7. [DOI: 10.1136/fn.79.3.f194] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lago 1999 {published data only}
- Lago P, Benini F, Salvadori S, Bettiol T, Agosto C, Zacchello F. Effect of administering low-dose fentanyl infusion on respiratory dynamics in the premature ventilated for respiratory distress syndrome - a randomized double-blind trial. Pediatric Research 1999;45(7):308. [DOI: 10.1203/00006450-199904020-01835] [DOI] [Google Scholar]
Liem 1999 {published data only}
- Liem KD, Der Velden AA, Klaessens JH, De Bor M. Changes of cerebral oxygenation and hemodynamics in ventilated preterm infants after sedation with midazolam and morphine: preliminary report. Pediatric Research 1999;45(6):906. [DOI: 10.1203/00006450-199906000-00134] [DOI] [Google Scholar]
Naderi 2017 {published data only}
- Naderi S, Goodarzi R, Naziri GRP, Mohammad AM, Kheiltash A, Shafaeizadeh A. Effect of fentanyl and morphine on gallbladder dimensions in newborns admitted to the neonatal intensive care unit: a randomized double-blinded clinical trial. Iranian Journal of Pediatrics 2017;27(1):e5726. [DOI: 10.5812/ijp.5726] [DOI] [Google Scholar]
Orsini 1996 {published data only}
- Orsini AJ, Leef KH, Costarino A, Dettorre MD, Stefano JL. Routine use of fentanyl infusions for pain and stress reduction in infants with respiratory distress syndrome. Journal of Pediatrics 1996;129(1):140-5. [DOI: 10.1016/s0022-3476(96)70201-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Qiu 2019 {published data only}
- Qiu J, Zhao L, Yang Y, Zhang JH, Feng Y, Cheng R. Effects of fentanyl for pain control and neuroprotection in very preterm newborns on mechanical ventilation. Journal of Maternal-Fetal & Neonatal Medicine 2019;32(22):3734-40. [DOI: 10.1080/14767058.2018.1471593] [PMID: ] [DOI] [PubMed] [Google Scholar]
Quinn 1992 {published data only}
- MacGregor R, Evans D, Sugden D, Gaussen T, Levene M. Outcome at 5-6 years of prematurely born children who received morphine as neonates. Archives of Disease in Childhood. Fetal and Neonatal Edition 1998;79(1):F40-3. [DOI: 10.1136/fn.79.1.f40] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn MW, Otoo F, Rushforth JA, Dean HG, Puntis JW, Wild J, et al. Effect of morphine and pancuronium on the stress response in ventilated preterm infants. Early Human Development 1992;30(3):241-8. [DOI: 10.1016/0378-3782(92)90073-p] [PMID: ] [DOI] [PubMed] [Google Scholar]
Quinn 1993 {published data only}
- MacGregor R, Evans D, Sugden D, Gaussen T, Levene M. Outcome at 5-6 years of prematurely born children who received morphine as neonates. Archives of Disease in Childhood. Fetal and Neonatal Edition 1998;79(1):F40-3. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn MW, Wild J, Dean HG, Hartley R, Rushforth JA, Puntis JW, et al. Randomised double-blind controlled trial of effect of morphine on catecholamine concentrations in ventilated pre-term babies. Lancet 1993;342(8867):324-7. [DOI: 10.1016/0140-6736(93)91472-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Saarenmaa 1999 {published data only}
- Saarenmaa E, Huttunen P, Leppaluoto J, Meretoja O, Fellman V. Advantages of fentanyl over morphine in analgesia for ventilated newborn infants after birth: a randomized trial. Journal of Pediatrics 1999;134(2):144-50. [DOI: 10.1016/s0022-3476(99)70407-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schmidt 2010 {published data only}
- Schmidt B, Adelmann C, Stutzer H, Welzing L, Hunseler C, Kribs A, et al. Comparison of sufentanil versus fentanyl in ventilated term neonates. Klinische Padiatrie 2010;222(2):62-6. [DOI: 10.1055/s-0029-1225348] [PMID: ] [DOI] [PubMed] [Google Scholar]
Simons 2003 {published data only}
- Graaf J, den Akker EL, Lingen RA, Groot Jebbink LJ, Jong FH, Grunau RE, et al. Five-year follow-up of effects of neonatal intensive care and morphine infusion during mechanical ventilation on diurnal cortisol rhythm. Journal of Pediatrics 2014;165(3):459-63.e2. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Graaf J, Lingen RA, Simons SH, Anand KJ, Duivenvoorden HJ, Weisglas-Kuperus N, et al. Long-term effects of routine morphine infusion in mechanically ventilated neonates on children's functioning: five-year follow-up of a randomized controlled trial. Pain 2011;152(6):1391-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Graaf J, Lingen RA, Valkenburg AJ, Weisglas-Kuperus N, Groot Jebbink L, Wijnberg-Williams B, et al. Does neonatal morphine use affect neuropsychological outcomes at 8 to 9 years of age? Pain 2013;154(3):449-58. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Simons SH, Roofthooft DW, Dijk M, Lingen RA, Duivenvoorden HJ, den Anker JN, et al. Morphine in ventilated neonates: its effects on arterial blood pressure. Archives of Disease in Childhood. Fetal and Neonatal Edition 2006;91(1):F46-51. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons SH, Dijk M, Lingen RA, Roofthooft D, Boomsma F, den Anker JN, et al. Randomised controlled trial evaluating effects of morphine on plasma adrenaline/noradrenaline concentrations in newborns. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90(1):F36-40. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons SH, Dijk M, Lingen RA, Roofthooft D, Duivenvoorden HJ, Jongeneel N, et al. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. JAMA 2003;290(18):2419-27. [DOI: 10.1001/jama.290.18.2419] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Simons SHP, Dijk M, Lingen RA, Roofthooft D, den Anker JN, Tibboel D. The effect of continuous morphine infusion in ventilated newborns: a blinded randomized placebo controlled trial. Pediatric Research 2003;53:35. [Google Scholar]
- Valkenburg AJ, den Bosch GE, Graaf J, Lingen RA, Weisglas-Kuperus N, Rosmalen J, et al. Long-term effects of neonatal morphine infusion on pain sensitivity: follow-up of a randomized controlled trial. Journal of Pain 2015;16(9):926-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Siwiec 1999 {published data only}
- Siwiec J, Porzucek I, Gadzinowski J, Bhat R, Vidyasagar D. Effect of short term morphine infusion on premature infant pain profile (PIPP) and hemodynamics. Pediatric Research 1999;45(7):69. [DOI: 10.1203/00006450-199904020-00416] [DOI] [Google Scholar]
Welzing 2012 {published data only}
- NCT00419601. RAPIP study: clinical trial on remifentanil for analgesia and sedation of ventilated neonates and infants. clinicaltrials.gov/ct2/show/NCT00419601 (first received 8 January 2007).
- Welzing L, Link F, Junghaenel S, Oberthuer A, Harnischmacher U, Stuetzer H, et al. Remifentanil-induced tolerance, withdrawal or hyperalgesia in infants: a randomized controlled trial. RAPIP trial: remifentanil-based analgesia and sedation of paediatric intensive care patients. Neonatology 2013;104(1):34-41. [DOI: 10.1159/000348790] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Welzing L, Oberthuer A, Junghaenel S, Harnischmacher U, Stutzer H, Roth B. Remifentanil/midazolam versus fentanyl/midazolam for analgesia and sedation of mechanically ventilated neonates and young infants: a randomized controlled trial. Intensive Care Medicine 2012;38(6):1017-24. [DOI: 10.1007/s00134-012-2532-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wood 1998 {published data only}
- Wood CM, Rushforth JA, Hartley R, Dean H, Wild J, Levene MI. Randomised double blind trial of morphine versus diamorphine for sedation of preterm neonates. Archives of Disease in Childhood. Fetal and Neonatal Edition 1998;79(1):F34-9. [DOI: 10.1136/fn.79.1.f34] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Akkermans 2015 {published data only}
Anand 2008 {published data only}
- Anand KJ, Anderson BJ, Holford NH, Hall RW, Young T, Shephard B, et al. Morphine pharmacokinetics and pharmacodynamics in preterm and term neonates: secondary results from the NEOPAIN trial. British Journal of Anaesthesia 2008;101(5):680-9. [DOI: 10.1093/bja/aen248] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bell 1987 {published data only}
- Bell SG, Ellis LJ. Use of fentanyl for sedation of mechanically ventilated neonates. Neonatal Network 1987;6(2):27-31. [PMID: ] [PubMed] [Google Scholar]
Bergqvist 2007 {published data only}
- Bergqvist L, Eriksson M, Kronsberg S, Schollin J, Barton B, Anand K. Seeing through the blind! Ability of hospital staff to differentiate morphine from placebo, in neonates at a placebo controlled trial. Acta Paediatrica 2007;96(7):1004-7. [DOI: 10.1111/j.1651-2227.2007.00270.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hwang 1999 {published data only}
- Hwang WK, Kim HM. Pain reduction effects of continuous fentanyl infusion to intensive care neonates. Journal of the Korean Pediatric Society 1999;42(5):657-65. [Google Scholar]
Palmer 2005 {published data only}
- Palmer KG, Kronsberg SS, Barton BA, Hobbs CA, Hall RW, Anand KJ. Effect of inborn versus outborn delivery on clinical outcomes in ventilated preterm neonates: secondary results from the NEOPAIN trial. Journal of Perinatology 2005;25(4):270-5. [DOI: 10.1038/sj.jp.7211239] [PMID: ] [DOI] [PubMed] [Google Scholar]
Perlman 2005 {published data only}
- Perlman JM. Morphine, hypotension, and intraventricular hemorrhage in the ventilated premature infant. Pediatrics 2005;115(5):1416-8. [DOI: 10.1542/peds.2005-0501] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
IRCT2017082417413N26 {published data only}
- IRCT2017082417413N26. Comparing the effect of fentanyl and midazolam on the sedation of infants under mechanical ventilation: a randomized clinical trial. en.irct.ir/trial/16036 (first received 2016 July 1).
Additional references
AAP 2016
- American Academy of Pediatrics Committee on Fetus and Newborn and Section on Anesthesiology and Pain Medicine. Prevention and management of procedural pain in the neonate: an update. Pediatrics 2016;137(2):e20154271. [DOI: 10.1542/peds.2015-4271] [PMID: ] [DOI] [PubMed] [Google Scholar]
Anand 1987
- Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. New England Journal of Medicine 1987;317(21):1321-9. [DOI: 10.1056/NEJM198711193172105] [PMID: ] [DOI] [PubMed] [Google Scholar]
Anand 1990
- Anand KJ. Hormonal-metabolic stress response in neonates undergoing cardiac surgery. Anesthesiology 1990;73(4):661-70. [DOI: 10.1097/00000542-199010000-00012] [PMID: ] [DOI] [PubMed] [Google Scholar]
Anand 1993
- Anand KJ. Relationships between stress responses and clinical outcome in newborns, infants, and children. Critical Care Medicine 1993;21(9 Suppl):S358-9. [DOI: 10.1097/00003246-199309001-00035] [PMID: ] [DOI] [PubMed] [Google Scholar]
Anand 1998
- Anand KJ. Clinical importance of pain and stress in preterm neonates. Biology of the Neonate 1998;73(1):1-9. [DOI: 10.1159/000013953] [PMID: ] [DOI] [PubMed] [Google Scholar]
Anand 2001
- Anand KJ, International Evidence-Based Group for Neonatal Pain. Consensus statement for the prevention and management of pain in the newborn. Archives of Pediatric and Adolescent Medicine 2001;155(2):173-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Anand 2013
- Anand KJ, Clark AE, Willson DF, Berger J, Meert KL, Zimmerman JJ, et al, Eunic Kennedy Shriver National Institute of Child Health, Human Development (NICHD) Collaborative Pediatric Critical Care Research Network (CPCCRN). Opioid analgesia in mechanically ventilated children: results from the multicenter Measuring Opioid Tolerance Induced by Fentanyl study. Pediatric Critical Care Medicine 2013;14(1):27-36. [DOI: 10.1097/PCC.0b013e318253c80e] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anand 2017
- Anand KJ, Eriksson M, Boyle EM, Avila-Alvarez A, Andersen RD, Sarafidis K, et al, EUROPAIN survey working group of the NeoOpioid Consortium. Assessment of continuous pain in newborns admitted to NICUs in 18 European countries. Acta Paediatrica 2017;106(8):1248-59. [DOI: 10.1111/apa.13810] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ancora 2017
- Ancora G, Lago P, Garetti E, Pirelli A, Merazzi D, Pierantoni L, et al. Follow-up at the corrected age of 24 months of preterm newborns receiving continuous infusion of fentanyl for pain control during mechanical ventilation. Pain 2017;158(5):840-5. [DOI: 10.1097/j.pain.0000000000000839] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ancora 2019
- Ancora G, Lago P, Garetti E, Merazzi D, Savant Levet P, Bellieni CV, et al. Evidence-based clinical guidelines on analgesia and sedation in newborn infants undergoing assisted ventilation and endotracheal intubation. Acta Paediatrica 2019;108(2):208-17. [DOI: 10.1111/apa.14606] [PMID: ] [DOI] [PubMed] [Google Scholar]
Aranda 2005
- Aranda JV, Carlo W, Hummel P, Thomas R, Lehr VT, Anand KJ. Analgesia and sedation during mechanical ventilation in neonates. Clinical Therapeutics 2005;27(6):877-99. [DOI: 10.1016/j.clinthera.2005.06.019] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ballantyne 1999
- Ballantyne M, Stevens B, McAllister M, Dionne K, Jack A. Validation of the premature infant pain profile in the clinical setting. Clinical Journal of Pain 1999;15:297-303. [DOI: 10.1097/00002508-199912000-00006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Barker 1996
- Barker DP, Rutter N. Stress, severity of illness, and outcome in ventilated preterm infants. Archives of Disease in Childhood Fetal and Neonatal Edition 1996;75(3):187-90. [DOI: 10.1136/fn.75.3.f187] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bayley 1993
- Bayley N. Bayley Scales of Infant Development. 2nd edition. San Antonio (TX): The Psychological Corporation, 1993. [Google Scholar]
Bayley 2006
- Bayley N. Bayley Scales of Infant and Toddler Development. San Antonio (TX): Harcourt Assessment, 2006. [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1-7. [DOI: 10.1097/00000658-197801000-00001] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carbajal 2015
- Carbajal R, Eriksson M, Courtois E, Boyle E, Avila-Alvarez A, Andersen DR, et al, EUROPAIN Survey Working Group. Sedation and analgesia practices in neonatal intensive care units (EUROPAIN): results from a prospective cohort study. The Lancet Respiratory Medicine 2015;3(10):796-812. [DOI: 10.1016/S2213-2600(15)00331-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cochrane EPOC 2017
- Cochrane Effective Practice and Organisation of Care (EPOC). EPOC resources for review authors, 2017. epoc.cochrane.org/epoc-resources-review-authors (accessed prior to 12 July 2019).
Darnall 2010
- Darnall RA. The role of CO(2) and central chemoreception in the control of breathing in the fetus and the neonate. Respiratory Physiology & Neurobiology 2010;173(3):201-12. [DOI: 10.1016/j.resp.2010.04.009] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Debillon 2001
- Debillon T, Zupan V, Ravault N, Magny JF, Dehan M. Development and initial validation of the EDIN scale, a new tool for assessing prolonged pain in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2001;85(1):F36-41. [DOI: 10.1136/fn.85.1.f36] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Graaf 2011
- Graaf J, Lingen RA, Simons SH, Anand KJ, Duivenvoorden HJ, Weisglas-Kuperus N, et al. Long-term effects of routine morphine infusion in mechanically ventilated neonates on children's functioning: five-year follow-up of a randomized controlled trial. Pain 2011;152(6):1391-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
de Graaf 2013
- Graaf J, Lingen RA, Valkenburg AJ, Weisglas-Kuperus N, Groot Jebbink L, Wijnberg-Williams B, et al. Does neonatal morphine use affect neuropsychological outcomes at 8 to 9 years of age? Pain 2013;154(3):449-58. [PMID: ] [DOI] [PubMed] [Google Scholar]
de Graaf 2014
- Graaf J, den Akker EL, Lingen RA, Groot Jebbink LJ, Jong FH, Grunau RE, et al. Five-year follow-up of effects of neonatal intensive care and morphine infusion during mechanical ventilation on diurnal cortisol rhythm. Journal of Pediatrics 2014;165(3):459-63.e2. [PMID: ] [DOI] [PubMed] [Google Scholar]
de Vries 1992
- Vries LS, Eken P, Dubowitz LM. The spectrum of leukomalacia using cranial ultrasound. Behavioural Brain Research 1992;49(1):1-6. [DOI: 10.1016/s0166-4328(05)80189-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fink 2015
- Fink RM, Makic MB, Poteet AW, Oman KS. The ventilated patient's experience. Dimensions of Critical Care Nursing 2015;34(5):301-8. [DOI: 10.1097/DCC.0000000000000128] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fitzgerald 1989
- Fitzgerald M, Millard C, McIntosh N. Cutaneous hypersensitivity following peripheral tissue damage in newborn infants and its reversal with topical anaesthesia. Pain 1989;39(1):31-6. [DOI: 10.1016/0304-3959(89)90172-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Giordano 2019
- Giordano V, Edobor J, Deindl P, Wildner B, Goeral K, Steinbauer P, et al. Pain and sedation scales for neonatal and pediatric patients in a preverbal stage of development: a systematic review. JAMA Pediatrics 2019;173(12):1186-97. [DOI: 173 10.1001/jamapediatrics.2019.3351] [PMID: ] [DOI] [PubMed] [Google Scholar]
Golianu 2007
- Golianu B, Krane E, Seybold J, Almgren C, Anand KJ. Non-pharmacological techniques for pain management in neonates. Seminars in Perinatology 2007;31(5):318-22. [DOI: 10.1053/j.semperi.2007.07.007] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 3 May 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Griffiths 1954
- Griffiths R. The Abilities of Babies: A Study in Mental Measurement. New York (NY): McGraw-Hill Book Co. Inc, 1954. [Google Scholar]
Griffiths 1970
- Griffiths R. The Abilities of Young Children: A Comprehensive System of Mental Measurement for the First Eight Years. London (UK): Child Development Research Centre, 1970. [Google Scholar]
Grunau 1990
- Grunau RV, Craig KD. Facial activity as a measure of neonatal pain expression. In: Tyler DC, Krane EJ, editors(s). Advances in Pain Research Therapy. Pediatric Pain. Vol. 15. New York (NY): Raven Press, 1990:147-54. [Google Scholar]
Hall 2007
- Hall RW, Boyle E, Young T. Do ventilated neonates require pain management? Seminars in Perinatology 2007;31(5):289-97. [DOI: 10.1053/j.semperi.2007.07.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI: 10.1136/bmj.327.7414.557] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Altman DG, Sterne JA, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8. Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hummel 2008
- Hummel P, Puchalski M, Creech SD, Weiss MG. Clinical reliability and validity of the N-PASS: neonatal pain, agitation and sedation scale with prolonged pain. Journal of Perinatology 2008;28(1):55-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
ICCROP 2005
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Archives of Ophthalmology 2005;123(7):991-9. [DOI: 10.1001/archopht.123.7.991] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jacobs 2013
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No: CD003311. [DOI: 10.1002/14651858.CD003311.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jobe 2001
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine 2001;163(7):1723-9. [DOI: 10.1164/ajrccm.163.7.2011060] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kahn 1998
- Kahn DJ, Richardson DK, Gray JE, Bednarek F, Rubin LP, Shah B, et al. Variation among neonatal intensive care units in narcotic administration. Archives of Pediatric and Adolescent Medicine 1998;152(9):844-51. [DOI: 10.1001/archpedi.152.9.844] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kinoshita 2020
- Kinoshita M, Stempel K, do Nascimento IJB, Vejayaram DN, Norman E, Bruschettini M. Opioids and alpha-2-agonists for analgesia and sedation in newborn infants: protocol of a systematic review. Systematic Reviews 2020;9(1):183. [DOI: 10.1186/s13643-020-01436-0] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krechel 1995
- Krechel SW, Bildner J. CRIES: a new neonatal postoperative pain measurement score. Initial testing of validity and reliability. Paediatric Anaesthesia 1995;5(1):53-61. [DOI: 10.1111/j.1460-9592.1995.tb00242.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lago 2017
- Lago P, Frigo AC, Baraldi E, Pozzato R, Courtois E, Rambaud J, et al. Sedation and analgesia practices at Italian neonatal intensive care units: results from the EUROPAIN study. Italian Journal of Pediatrics 2017;43(1):26. [DOI: 10.1186/s13052-017-0343-2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Larsson 1999
- Larsson BA. Pain management in neonates. Acta Paediatrica 1999;88(12):1301-10. [DOI: 10.1080/080352599750029952] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lawrence 1993
- Lawrence J, Alcock D, McGrath P, Kay J, MacMurray SB, Dulberg C. The development of a tool to assess neonatal pain. Neonatal Network 1993;12(6):59-66. [PMID: ] [PubMed] [Google Scholar]
Lundqvist 2014
- Lundqvist P, Kleberg A, Edberg AK, Larsson BA, Hellstrom-Westas L, Norman E. Development and psychometric properties of the Swedish ALPS-Neo pain and stress assessment scale for newborn infants. Acta Paediatrica 2014;103(8):833-9. [DOI: 10.1111/apa.12672] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
MacGregor 1998
- MacGregor R, Evans D, Sugden D, Gaussen T, Levene M. Outcome at 5-6 years of prematurely born children who received morphine as neonates. Archives of Disease in Childhood. Fetal and Neonatal Edition 1998;79(1):F40-3. [DOI: 10.1136/fn.79.1.f40] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Menon 1998
- Menon G, Ananad KJ, McIntosh N. Practical approach to analgesia and sedation in the neonatal intensive care unit. Seminars in Perinatology 1998;22(5):417-24. [DOI: 10.1016/s0146-0005(98)80057-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [DOI: 10.1016/j.jclinepi.2009.06.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nemergut 2013
- Nemergut ME, Yaster M, Colby CE. Sedation and analgesia to facilitate mechanical ventilation. Clinics in Perinatology 2013;40(3):539-58. [DOI: 10.1016/j.clp.2013.05.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ng 2017
- Ng E, Taddio A, Ohlsson A. Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit. Cochrane Database of Systematic Reviews 2017, Issue 1. Art. No: CD002052. [DOI: 10.1002/14651858.CD002052.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
NIH 1979
- National Institutes of Heart, Lung, and Blood Institute of Lung Diseases. In: Report of Workshop on Bronchopulmonary Dysplasia; Dec 4-8 1978. Bethesda (MD): U.S. Dept. of Health, Education, and Welfare, Public Health Service, National Institutes of Health, 1979.
Olsson 2021
- Olsson E, Ahl H, Bengtsson K, Vejayaram DN, Norman E, Bruschettini M, et al. The use and reporting of neonatal pain scales: a systematic review of randomized trials. Pain 2021;162(2):353-60. [DOI: 10.1097/j.pain.0000000000002046] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529-34. [DOI: 10.1016/s0022-3476(78)80282-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pillai 2015
- Pillai Riddell RR, Racine NM, Gennis HG, Turcotte K, Uman LS, Horton RE, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD006275. [DOI: 10.1002/14651858.CD006275.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Purcell‐Jones 1988
- Purcell-Jones G, Dormon F, Sumner E. Paediatric anaesthetists' perceptions of neonatal and infant pain. Pain 1988;33(2):181-7. [PMID: 10.1016/0304-3959(88)90089-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
RevMan Web [Computer program]
- The Cochrane Collaboration Review Manager Web (RevMan Web). The Cochrane Collaboration, 2019. Available at revman.cochrane.org.
Romantsik 2017a
- Romantsik O, Calevo MG, Norman E, Bruschettini M. Clonidine for sedation and analgesia for neonates receiving mechanical ventilation. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD012468. [DOI: 10.1002/14651858.CD012468.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Romantsik 2017b
- Romantsik O, Bruschettini M, Calevo MG, Banzi R, Ley D. Pharmacological pain and sedation interventions for the prevention of intraventricular haemorrhage in preterm infants on assisted ventilation - an overview of systematic reviews. Cochrane Database of Systematic Reviews 2017, Issue 6. Art. No: CD012706. [DOI: 10.1002/14651858.CD012706] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Simons 2005
- Simons SH, Dijk M, Lingen RA, Roofthooft D, Boomsma F, den Anker JN, et al. Randomised controlled trial evaluating effects of morphine on plasma adrenaline/noradrenaline concentrations in newborns. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90(1):F36-40. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soll 2013
- Soll RF, Edwards EM, Badger GJ, Kenny MJ, Morrow KA, Buzas JS, et al. Obstetric and neonatal care practices for infants 501 to 1500 g from 2000 to 2009. Pediatrics 2013;132(2):222-8. [PMID: 10.1542/peds.2013-0501] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stevens 1996
- Stevens B, Johnstone C, Petryshen P, Taddio A. Premature infant pain profile: development and initial validation. Clinical Journal of Pain 1996;12(1):13-22. [DOI: 10.1097/00002508-199603000-00004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Taddio 2002
- Taddio A. Opioid analgesia for infants in the neonatal intensive care unit. Clinics in Perinatology 2002;29(3):493-509. [DOI: 10.1016/s0095-5108(02)00017-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Taddio 2009
- Taddio A, Shah V, Atenafu E, Katz J. Influence of repeated painful procedures and sucrose analgesia on the development of hyperalgesia in newborn infants. Pain 2009;144(1-2):43-8. [DOI: 10.1016/j.pain.2009.02.012] [PMID: ] [DOI] [PubMed] [Google Scholar]
Valkenburg 2015
- Valkenburg AJ, den Bosch GE, Graaf J, Lingen RA, Weisglas-Kuperus N, Rosmalen J, et al. Long-term effects of neonatal morphine infusion on pain sensitivity: follow-up of a randomized controlled trial. Journal of Pain 2015;16(9):926-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
van Dijk 2009
- Dijk M, Roofthooft DW, Anand KJ, Guldemond F, Graaf J, Simons S, et al. Taking up the challenge of measuring prolonged pain in (premature) neonates: the COMFORTneo scale seems promising. Clinical Journal of Pain 2009;25(7):607-16. [DOI: 10.1097/AJP.0b013e3181a5b52a] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vendettuoli 2014
- Vendettuoli V, Bellu R, Zanini R, Mosca F, Gagliardi L. Changes in ventilator strategies and outcomes in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(4):F321-4. [DOI: 10.1136/archdischild-2013-305165] [PMID: ] [DOI] [PubMed] [Google Scholar]
Walsh 2004
- Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al, National Institute of Child Health and Human Development Neonatal Research Network. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004;114(5):1305-11. [DOI: 10.1542/peds.2004-0204 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zimmerman 2017
- Zimmerman KO, Smith PB, Benjamin DK, Laughon M, Clark R, Traube C, et al. Sedation, analgesia, and paralysis during mechanical ventilation of premature infants. Journal of Pediatrics 2017;180:99-104. [DOI: 10.1016/j.jpeds.2016.07.001] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Bellù 2005
- Bellù R, Waal KA, Zanini R. Opioids for neonates receiving mechanical ventilation. Cochrane Database of Systematic Reviews 2005, Issue 1. Art. No: CD004212. [DOI: 10.1002/14651858.CD004212.pub2] [DOI] [PubMed] [Google Scholar]
Bellù 2008
- Bellù R, Waal KA, Zanini R. Opioids for neonates receiving mechanical ventilation. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD004212. [DOI: 10.1002/14651858.CD004212.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


