Abstract
Background
There is a common perception that smoking generally helps people to manage stress, and may be a form of 'self‐medication' in people with mental health conditions. However, there are biologically plausible reasons why smoking may worsen mental health through neuroadaptations arising from chronic smoking, leading to frequent nicotine withdrawal symptoms (e.g. anxiety, depression, irritability), in which case smoking cessation may help to improve rather than worsen mental health.
Objectives
To examine the association between tobacco smoking cessation and change in mental health.
Search methods
We searched the Cochrane Tobacco Addiction Group's Specialised Register, Cochrane Central Register of Controlled Trials, MEDLINE, Embase, PsycINFO, and the trial registries clinicaltrials.gov and the International Clinical Trials Registry Platform, from 14 April 2012 to 07 January 2020. These were updated searches of a previously‐conducted non‐Cochrane review where searches were conducted from database inception to 13 April 2012.
Selection criteria
We included controlled before‐after studies, including randomised controlled trials (RCTs) analysed by smoking status at follow‐up, and longitudinal cohort studies. In order to be eligible for inclusion studies had to recruit adults who smoked tobacco, and assess whether they quit or continued smoking during the study. They also had to measure a mental health outcome at baseline and at least six weeks later.
Data collection and analysis
We followed standard Cochrane methods for screening and data extraction. Our primary outcomes were change in depression symptoms, anxiety symptoms or mixed anxiety and depression symptoms between baseline and follow‐up. Secondary outcomes included change in symptoms of stress, psychological quality of life, positive affect, and social impact or social quality of life, as well as new incidence of depression, anxiety, or mixed anxiety and depression disorders.
We assessed the risk of bias for the primary outcomes using a modified ROBINS‐I tool. For change in mental health outcomes, we calculated the pooled standardised mean difference (SMD) and 95% confidence interval (95% CI) for the difference in change in mental health from baseline to follow‐up between those who had quit smoking and those who had continued to smoke. For the incidence of psychological disorders, we calculated odds ratios (ORs) and 95% CIs. For all meta‐analyses we used a generic inverse variance random‐effects model and quantified statistical heterogeneity using I2. We conducted subgroup analyses to investigate any differences in associations between sub‐populations, i.e. unselected people with mental illness, people with physical chronic diseases.
We assessed the certainty of evidence for our primary outcomes (depression, anxiety, and mixed depression and anxiety) and our secondary social impact outcome using the eight GRADE considerations relevant to non‐randomised studies (risk of bias, inconsistency, imprecision, indirectness, publication bias, magnitude of the effect, the influence of all plausible residual confounding, the presence of a dose‐response gradient).
Main results
We included 102 studies representing over 169,500 participants. Sixty‐two of these were identified in the updated search for this review and 40 were included in the original version of the review. Sixty‐three studies provided data on change in mental health, 10 were included in meta‐analyses of incidence of mental health disorders, and 31 were synthesised narratively.
For all primary outcomes, smoking cessation was associated with an improvement in mental health symptoms compared with continuing to smoke: anxiety symptoms (SMD −0.28, 95% CI −0.43 to −0.13; 15 studies, 3141 participants; I2 = 69%; low‐certainty evidence); depression symptoms: (SMD −0.30, 95% CI −0.39 to −0.21; 34 studies, 7156 participants; I2 = 69%' very low‐certainty evidence); mixed anxiety and depression symptoms (SMD −0.31, 95% CI −0.40 to −0.22; 8 studies, 2829 participants; I2 = 0%; moderate certainty evidence). These findings were robust to preplanned sensitivity analyses, and subgroup analysis generally did not produce evidence of differences in the effect size among subpopulations or based on methodological characteristics. All studies were deemed to be at serious risk of bias due to possible time‐varying confounding, and three studies measuring depression symptoms were judged to be at critical risk of bias overall. There was also some evidence of funnel plot asymmetry. For these reasons, we rated our certainty in the estimates for anxiety as low, for depression as very low, and for mixed anxiety and depression as moderate.
For the secondary outcomes, smoking cessation was associated with an improvement in symptoms of stress (SMD −0.19, 95% CI −0.34 to −0.04; 4 studies, 1792 participants; I2 = 50%), positive affect (SMD 0.22, 95% CI 0.11 to 0.33; 13 studies, 4880 participants; I2 = 75%), and psychological quality of life (SMD 0.11, 95% CI 0.06 to 0.16; 19 studies, 18,034 participants; I2 = 42%). There was also evidence that smoking cessation was not associated with a reduction in social quality of life, with the confidence interval incorporating the possibility of a small improvement (SMD 0.03, 95% CI 0.00 to 0.06; 9 studies, 14,673 participants; I2 = 0%). The incidence of new mixed anxiety and depression was lower in people who stopped smoking compared with those who continued (OR 0.76, 95% CI 0.66 to 0.86; 3 studies, 8685 participants; I2 = 57%), as was the incidence of anxiety disorder (OR 0.61, 95% CI 0.34 to 1.12; 2 studies, 2293 participants; I2 = 46%). We deemed it inappropriate to present a pooled estimate for the incidence of new cases of clinical depression, as there was high statistical heterogeneity (I2 = 87%).
Authors' conclusions
Taken together, these data provide evidence that mental health does not worsen as a result of quitting smoking, and very low‐ to moderate‐certainty evidence that smoking cessation is associated with small to moderate improvements in mental health. These improvements are seen in both unselected samples and in subpopulations, including people diagnosed with mental health conditions. Additional studies that use more advanced methods to overcome time‐varying confounding would strengthen the evidence in this area.
Keywords: Humans; Middle Aged; Affect; Anxiety; Anxiety/therapy; Confidence Intervals; Controlled Before-After Studies; Depression; Depression/therapy; Incidence; Mental Disorders; Mental Disorders/epidemiology; Mental Disorders/therapy; Mental Health; Quality of Life; Smoking; Smoking/adverse effects; Smoking/psychology; Smoking Cessation; Smoking Cessation/methods; Smoking Cessation/psychology; Social Interaction; Stress, Psychological; Stress, Psychological/therapy; Tobacco Use Cessation; Tobacco Use Cessation/methods; Tobacco Use Cessation/psychology
Plain language summary
Does stopping smoking improve mental health?
Smoking and mental health
Some health providers and people who smoke believe that smoking helps reduce stress and other mental health symptoms, like depression and anxiety. They worry that stopping smoking may make mental health symptoms worse. However, studies have shown that smoking may have a negative impact on people's mental health, and stopping smoking could reduce anxiety and depression.
Why we did this Cochrane Review
We wanted to find out how stopping smoking affects people's mental health. If stopping smoking improves mental health symptoms, rather than worsening them, then this may encourage more people to try to quit smoking and more health professionals to help their patients to quit. It may also discourage people from beginning to smoke tobacco in the first place.
What did we do?
We searched for studies that lasted for at least six weeks that included people who were smoking at the start of the studies. To be included, studies also had to measure whether people did or did not stop smoking and any changes in mental health during the study.
We were interested in how stopping smoking affected:
‐ symptoms of anxiety;
‐ symptoms of depression;
‐ symptoms of anxiety and depression together;
‐ symptoms of stress;
‐ overall well‐being;
‐ mental health problems;
‐ social well‐being, personal relationships, isolation and loneliness.
Search date: we included evidence published up to 7 January 2020.
What we found
We found 102 studies in more than 169,500 people: some studies did not clearly report how many people took part. The studies used a range of different assessment scales to measure people's mental health symptoms.
Most studies included people from the general population (53 studies); 23 studies included people with mental health conditions; other studies included people with physical or mental health conditions, or long‐lasting physical conditions, who had recently had surgery, or who were pregnant.
We combined and compared the results from 63 studies that measured changes in mental health symptoms, and from 10 studies that measured how many people developed a mental health disorder during the study.
What are the results of our review?
Compared with people who continued to smoke, people who stopped smoking showed greater reductions in:
‐ anxiety (evidence from 3141 people in 15 studies);
‐ depression (7156 people in 34 studies); and
‐ mixed anxiety and depression (2829 people in 8 studies).
Our confidence in our results was very low (for depression), low (for anxiety), and moderate (for mixed anxiety and depression). Our confidence was reduced because we found limitations in the ways the studies were designed and carried out.
Compared with people who continued to smoke, people who stopped smoking showed greater improvements in:
‐ symptoms of stress (evidence from 4 studies in 1792 people);
‐ positive feelings (13 studies in 4880 people); and
‐ mental well‐being (19 studies in 18,034 people).
There was also evidence that people who stopped smoking did not have a reduction in their social well‐being, and their social well‐being may have increased slightly (9 studies in 14,673 people).
In people who stopped smoking, new cases of mixed anxiety and depression were fewer than in those who continued to smoke (evidence from 3 studies in 8685 people). New cases of anxiety were also fewer (2 studies in 2293 people). We were unable to come to a decision about the numbers of new cases of depression, as the results from different studies were too variable.
Key messages
People who stop smoking are not likely to experience a worsening in their mood long‐term, whether they have a mental health condition or not. They may also experience improvements in their mental health, such as reductions in anxiety and depression symptoms.
Summary of findings
Summary of findings 1. Associations between quitting smoking and change in mental health symptoms.
| Associations between quitting smoking and change in mental health symptoms | ||||
| Patient or population: various, including general population, pregnant people, psychiatric populations (ADHD, alcohol use disorder, anxiety disorder, depression, psychosis, PTSD, various SMI) and populations with chronic health conditions (acute coronary syndrome, AIDS, AS, brain injury, cancer, CHD, COPD, HIV) Setting: Australia, Belgium, Canada, China, Japan, Netherlands, Portugal, South Korea, Spain, Turkey, UK, USA Intervention: Quitting tobacco smoking Comparison: Continuing to smoke tobacco | ||||
| Outcomes | Probable outcome with intervention | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
|
Change in anxiety
assessed with various anxiety symptom scales
follow‐up: range 6 weeks to 2 years Higher score indicates higher‐intensity anxiety symptoms |
The mean change in anxiety score was 0.28 SDs lower (95% CI: −0.43 to −0.13) in people who quit smoking compared to people who continued smoking | 3141 (15 observational studies) | ⊕⊕⊝⊝ LOWa,b,c | According to Cohen 1988's interpretation of effect size 0.2 represents a small effect, 0.5 represents a moderate effect, and 0.8 represents a large effect. According to this rule of thumb, the effects found here are small to moderate. However, they are similar to the effects of antidepressant medications on anxiety disorder, which are deemed to be clinically meaningful (NCCMH 2011). |
|
Change in depression
assessed with various depression symptom scales
follow‐up: range 6 weeks to 6 years Higher score indicates higher‐intensity depression symptoms |
The mean change in depression score was 0.3 SDs lower (95% CI: −0.39 to −0.21) in people who quit smoking compared to people who continued smoking | 7156 (34 observational studies) | ⊕⊝⊝⊝ VERY LOWd,e,f | |
|
Mixed anxiety and depression
assessed with various mixed anxiety and depression symptom scales
follow‐up: range 3 months to 6 years Higher scores indicates higher‐intensity mixed anxiety & depression symptoms |
The mean change in mixed anxiety and depression score was 0.31 SDs lower (95% CI: −0.40 to −0.22) in people who quit smoking compared to people who continued smoking | 2829 (8 observational studies) | ⊕⊕⊕⊝ MODERATEa | |
| ADHD: attention deficit hyperactivity disorder; AIDS: acquired immune deficiency syndrome; AS: ankylosing spondylitis; CHD: coronary heart disease; CI: Confidence interval; COPD: chronic obstructive pulmonary disease; HIV: human immunodeficiency virus; PTSD: post‐traumatic stress disorder; SD: standard deviation; SMI: serious mental illness | ||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||
aDowngraded one level due to risk of bias: all studies were deemed to be at serious risk of bias. bDowngraded one level due to inconsistency: there was substantial heterogeneity between study effects (I2 = 69%) unaccounted for by subgroup and sensitivity analyses. Whilst some studies appeared to show evidence of a positive association with quitting smoking, others showed no clear evidence of benefit or harm. cPublication bias: although the funnel plot shows some evidence of asymmetry, statistical tests suggest this is unlikely to affect the pooled result. We therefore did not downgrade on the basis of publication bias. dDowngraded one level due to risk of bias: all studies were rated at serious or critical risk of bias. Removal of the three studies at critical risk of bias did not change the interpretation of the result. eDowngraded one level due to inconsistency: there was substantial statistical heterogeneity between study effects (I2 = 69%) unaccounted for by subgroup and sensitivity analyses. Whilst some studies appeared to show evidence of a positive association with quitting, others showed no clear evidence of benefit or harm, with only one study providing evidence of an increase in depression associated with quitting smoking. fPublication bias suspected, as the funnel plot indicates asymmetry, with a lack of smaller studies indicating an association between quitting and increased symptoms of depression. Egger's test also indicated potential publication bias.
Background
Description of the condition
Smoking is the world's leading cause of preventable illness and death (WHO 2011). One in every two people who smoke will die of a smoking‐related disease, unless they quit (Doll 2004; Pirie 2013). Although the prevalence of smoking has decreased markedly from the 1970s in high‐income countries; for example, in the UK it has fallen from 46% to approximately 14.9% in 2018 (ONS 2018; West 2019), prevalence remains higher in low‐ and middle‐income countries (GBD 2015 Tobacco Collaborators 2017). In addition, smoking prevalence amongst people with mental illness has declined only slightly and is currently around 32% in the UK (Richardson 2019; Szatkowski 2015; Taylor 2019a). People with mental illness who smoke are more heavily addicted, suffer from worse withdrawal (Hitsman 2013; Leventhal 2013; Leventhal 2014; RCP/RC PSYCH 2013), and are less responsive to standard treatments (Hitsman 2013; Taylor 2019a), even though they are motivated to quit (Haukkala 2000; Siru 2009). These inequalities contribute to a reduction in life expectancy of up to 17.5 years compared to the unselected population (Chang 2011; Chesney 2014).
Description of the intervention
Some people who smoke and healthcare providers believe that smoking can reduce stress and other symptoms related to mental illness, or that quitting smoking can exacerbate mental illness, and these beliefs maintain a culture of smoking (Cookson 2014; Sheals 2016). However, our previously‐published review (Taylor 2014) found an association between smoking cessation and improvements in mental health that were of a similar size to the effects reported in a systematic review of antidepressants for anxiety disorder (NCCMH 2011). We argued that this may be causal, and that smoking cessation could lead to improved mental health.
How the intervention might work
Chronic tobacco smoking is associated with neuroadaptations in nicotinic pathways in the brain. Neuroadaptations in these pathways are associated with the occurrence of withdrawal symptoms, such as depressed mood, agitation and anxiety. Withdrawal symptoms are alleviated by smoking and remain alleviated shortly after smoking, but symptoms return when blood levels of nicotine decline at around 20 minutes after smoking (Benowitz 1990; Benowitz 2010; Mansvelder 2002); this is known as the withdrawal cycle and is marked by fluctuations in a person's psychological state throughout the day (Benowitz 2010; Parrott 2003). People therefore mistake the ability of tobacco to alleviate tobacco withdrawal for an ability to alleviate mental health‐related symptoms. This misunderstanding has negative consequences in treating tobacco addiction in mental health populations, as many healthcare providers believe that by helping their patients to quit smoking, they will be harming their patients' mental health (Cookson 2014; Sheals 2016). Recent observational studies have used methods that support strong causal inference to indicate that smoking is associated with an increased risk of depression and schizophrenia (Wootton 2020), and that smoking cessation is associated with improved mental health outcomes (Taylor 2019a).
Why it is important to do this review
There is little convincing evidence that the smoking epidemic amongst people with mental illness is subsiding to the same level as that observed in the general population ‐ the gap in prevalence between people who smoke with and without mental illness is not closing (Richardson 2019; Taylor 2019a). Given evidence of therapeutic nihilism amongst healthcare professionals (Sheals 2016), strengthening, communicating and updating the evidence exploring the association between smoking and mental health is critically important to populations with mental illness. It could also encourage people without mental illness who smoke to quit, and discourage others from beginning to smoke tobacco.
Objectives
To examine the association between tobacco smoking cessation and change in mental health.
Methods
Criteria for considering studies for this review
Types of studies
Controlled before‐after studies, including randomised controlled trials (RCTs) analysed by smoking status at follow‐up rather than comparing the randomised study arms, and longitudinal cohort studies.
Types of participants
We included studies of adults who smoked tobacco (using the definitions given in included studies and excluding people who exclusively used electronic cigarettes). There were no restrictions by population type or co‐morbid conditions.
Types of interventions
The 'intervention' was quitting smoking and the 'comparator' was continued smoking. We included any definition of quitting smoking, as defined by the included studies (e.g. self‐report, biovalidated, point prevalence, continuous). There was no minimum length of abstinence specified, but studies had to be at least six weeks long to be included in the review. Where more than one measure of successful quitting was used we used the most stringent definition to categorise participants into the 'intervention' and 'comparator' groups. We preferred Intention‐to‐treat categorisation over complete‐case categorisation, where people without a smoking status were assumed to be still smoking, as is common in the field (West 2005).
Types of outcome measures
Self‐report or clinician‐scored measures of mental health, as follows. We included continuous and new incidence (dichotomous) measures of mental health, or mental ill‐health.
Outcome categories in this review were developed by examining the questions for each scale, to determine what each scale measured. What the scales actually measure, as determined by examining the questions they ask, can differ from what the scale’s name indicates.For example, the assumption might be that the stress reaction subscale of the Multidimensional Personality Questionnaire (MPQ) measures stress, but the scale developers note that people who score highly on the scale describe themselves using a range of terms, such as, tense, nervous, sensitive, vulnerable, prone to worry and feeling anxious, and feeling miserable without reason (Tellegen 2008). These terms describe mixed anxiety and depression symptoms, and were grouped as such in this review.
Primary outcomes
Change in depression symptoms
Change in anxiety symptoms
Change in mixed anxiety and depression symptoms
Secondary outcomes
Change in symptoms of stress, psychological quality of life, and positive affect
Cumulative incidence of mental ill‐health, including dichotomous measures of depression, anxiety, stress, psychological quality of life, positive affect, mixed anxiety and depression occurring after study start
Social impact or social quality of life, including measures of social satisfaction, interpersonal relationships, isolation and loneliness. This outcome was included as we carried out patient and public involvement work to identify any outcomes of particular relevance to members of the public, which were not considered in the previous version of this review (Taylor 2014). Our work highlighted that people who smoke may be concerned that quitting could disrupt their social networks, and lead to feelings of loneliness. This may be of particular significance to people also experiencing mental ill‐health. Where studies reported more than one social impact outcome, we selected the outcome that most closely represented social impact on friendships. This is the element which was highlighted most notably in our patient and public involvement work.
Outcomes had to be measured at least six weeks following baseline in order for data to be included in the review. Where outcomes were measured at multiple time points within one study we took the measure with the longest follow‐up in each of the following categories (where possible):
between six weeks and six months follow‐up (inclusive of the upper and lower time point)
over six months follow‐up
Search methods for identification of studies
Electronic searches
We searched the Cochrane Tobacco Addiction Group's Specialised Register, the Cochrane Central Register of Controlled Trials, MEDLINE, Embase, and PsycINFO. The most recent searches of these databases for the previous non‐Cochrane version of this review were carried out on 13 April 2012 (Taylor 2014). The inclusion criteria specified in this Cochrane update of the review do not differ from those in the original review, so we included studies from the previous review in this update and conducted update searches from 14 April 2012 to 07 January 2020, to identify any new studies. See Appendix 1 for the MEDLINE search strategy.
Searching other resources
We searched the trial registries clinicaltrials.gov and the International Clinical Trials Registry Platform (who.int/clinical-trials-registry-platform).
Data collection and analysis
Selection of studies
Our aim was to maximise sensitivity by including studies in initial screens even if data directly relevant to our question were not presented in the abstract. The titles and abstracts of eligible titles were screened independently by two review authors (from GT, NL, AF, AL‐J, KS, RtWN) for inclusion. We resolved disagreements by discussion, and involved a third review author in discussions where necessary. We obtained the full‐texts of articles that were included at the title and abstract screening stage. Two review authors (from GT, NL, AF, AL‐J, KS, RtWN, AT, PA) independently screened each full‐text for inclusion, resolving disagreements by discussion and with a third review author where necessary. We recorded reasons for exclusion at the full‐text examination stage. We translated non‐English language studies and included them where appropriate.
Data extraction and management
Review authors piloted the data extraction form and made appropriate changes (GT, NL, AF, PA). One review author extracted 100% of study characteristics data for each study (from KS, CB, NK).These data were then checked by GT or CB.
Two review authors (from GT, NL, AF, AL‐J, KS, RtWN, AT, PA) independently extracted outcome and 'Risk of bias' data for each study. We compared data for each study and resolved any disagreements by discussion and by involving a third review author where necessary.
We extracted the following data from each study.
Study design
Analysis method
Outcome measure(s)
Length of follow‐up
N at baseline and follow‐up
Population type
Percentage (%) male
Mean age (standard deviation (SD))
Covariates adjusted for
Motivation to quit
Intervention(s) used (if relevant)
Risk of bias using ROBINS‐I (Sterne 2016)
Data to calculate standardised mean difference (SMD) in mental health outcomes: for each group ‐ mean at baseline and follow‐up, mean change from baseline to follow‐up, and difference in mean change from baseline to follow‐up, and variance
Data to calculate new incidence of mental ill‐health outcomes: for each group ‐ N participants in continued‐smoking group at follow‐up, N participants with outcome in continued‐smoking group at follow‐up, N participants in quitter group at follow‐up, N participants with outcome in quitter group at follow‐up. If effect estimates had been calculated and reported in study reports then we also extracted these
Sources of study funding and authors' declarations of interests
Assessment of risk of bias in included studies
We assessed the risks of bias for each study that reported a primary outcome (continuously‐measured change in depression, anxiety or mixed anxiety and depression) using ROBINS‐I, which assesses studies based on risk of bias in the following domains: bias due to confounding; bias in selection of participants into the study; bias in classification of interventions; bias due to deviations from intended interventions; bias in measurement of outcomes; and bias in selection of the reported result (Sterne 2016). We modified the ROBINS‐I to ensure that it was appropriate to assess risk of bias for the association in question. The modified tool, and modifications with justifications, are available in Supplemental file 1 and Supplemental file 2 respectively.
The following relevant confounding variables were prespecified:
Time‐varying recreational drug and alcohol use, used individually or in combination, during the study period
Time‐varying psychoactive treatment use during the study period
Time‐varying significant life events during the study period (i.e. social changes, moving, divorce, having children, etc.)
The following psychoactive treatments that could have been different between people who quit and people who continued smoking, that could have impacted on mental health outcomes, were prespecified:
Psychological treatments that can improve mental health, i.e. cognitive behavioural therapy (CBT) for mood, mood management techniques, counselling for mood. Where treatments were provided that could be used for either smoking cessation or mood management we referred to the study protocol to ensure we did not misclassify smoking cessation treatments as mood treatments, e.g. 'CBT for smoking cessation'.
Pharmaceutical treatments, such as anti‐depressants (SSRIs, MAOIs or otherwise) and smoking cessation medicines that are also antidepressants (i.e. bupropion, nortriptyline, or otherwise).
Measures of treatment effect
For each study, for any outcome measured using continuous data, we calculated the standardised mean difference (SMD) (95% CI) in change in mental health from baseline to follow‐up, between those who had quit smoking and those who had continued to smoke. We calculated the SMD (95% CI) by extracting any of the following data, in order of preference:
adjusted or unadjusted mean difference (MD; difference in change from baseline to follow‐up) and measure of variation between exposure groups, with a preference for adjusted estimates;
mean change in mental health scores from baseline to follow‐up and measure of variance, by exposure group;
mean mental health scores and measures of variance at baseline and final follow‐up, by exposure group.
Where type 3) data were collected we then calculated the mean change and its variance for each exposure group (Follmann 1992); for data types 2) and 3) we calculated the SMD, using standard formulae outlined within the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020). We also sought statistical support where appropriate.
For each study, for any outcome measured using dichotomous data (new incidence of mental ill‐health data), we calculated odds ratios (ORs) and the 95% CI. We calculated the ORs (95% CI) by extracting any of the following data, in order of preference:
adjusted or unadjusted ORs and a measure of variance;
other types of effect estimate that could be converted into ORs (i.e. risk ratios, hazard ratios);
we extracted data to calculate ORs for new incidence of mental ill‐health and its variance (N participants in continued‐smoking group at follow‐up, N participants with outcome in continued‐smoking group at follow‐up, N participants in quit group at follow‐up, N participants with outcome in quit group at follow‐up).
We then logged ORs using a standard formula before inputting them into meta‐analyses. We sought statistical support to help with the above calculations where appropriate.
Unit of analysis issues
None.
Dealing with missing data
We contacted corresponding authors of studies for additional data where it was evident that data had been analysed comparing our exposure groups of interest (people who quit smoking versus people who continued to smoke), but the data needed for analyses were not reported, for any of our outcomes. If we were unable to obtain the necessary data for meta‐analysis, we reported studies narratively.
Assessment of heterogeneity
We quantified statistical heterogeneity using I2, which describes the percentage (%) of between‐study variability due to variance between studies rather than chance. We considered an I2 value between 50% and 75% as substantial heterogeneity, and above 75% we assessed whether it was appropriate to report a pooled analysis.
Assessment of reporting biases
We examined funnel plots for evidence of asymmetry and conducted Egger tests for evidence of small‐study bias where there were 10 or more studies contributing to any meta‐analysis. We used the Duval and Tweedie 'trim and fill' method to account for outcomes showing evidence of publication bias (Duval 2000). 'Trim and fill' adjusts the meta‐analysis to incorporate theoretically missing studies, and then estimates the pooled SMD incorporating imputed studies’ data. Using 'trim and fill' methods we imputed missing studies’ data, and compared pooled effect estimates between imputed and non‐imputed models.
We also conducted a subgroup analysis in which we compared effect estimates between studies in which mental health was the primary outcome and those in which it was not, to assess if there was evidence of publication bias (i.e. if mental health was a secondary outcome, was it more likely to be reported if it resulted in a clearly positive or negative effect).
Data synthesis
For continuous outcomes, we pooled SMDs (95% CI) across individual studies using a generic inverse variance random‐effects model. An SMD of greater than zero indicated that quitting smoking was associated with worse mental health at follow‐up for the anxiety, depression, mixed anxiety and depression, and stress outcomes, whereas an SMD of less than zero indicated that quitting smoking was associated with worse mental health at follow‐up for the positive affect, psychological quality of life, and social impact outcomes.
For dichotomous outcomes, we pooled log OR (95% CI) across individual studies using a generic inverse variance random‐effects model. An OR of greater than one indicated that people who quit smoking experienced a greater risk of mental ill‐health at follow‐up.
We conducted meta‐analyses of the SMD and OR for each outcome separately (i.e. depression, anxiety, stress, etc.) using RevMan 2014.
Subgroup analysis and investigation of heterogeneity
We conducted the following subgroup analyses:
Adjustment for covariates: we compared effect estimates from studies that present adjusted and unadjusted estimates;
Motivation to quit: we classified studies according to whether they selected participants for inclusion based on motivation to quit. Participants in RCTs were classed as motivated to quit, assuming that participants enrolled in a trial to help them stop smoking, unless otherwise specified. They were compared with any other studies that followed a group of people who smoked, where most were unlikely to want to quit in the near future;
Study design: we compared estimates between secondary analyses of RCTs, cohort studies, and one study where participants were randomised to quit smoking or to continue smoking and paid to do so;
Population comparison: we examined whether there was evidence of a difference in effect size between studies in different clinical populations, e.g. unselected samples, pregnant women, or participants who were postoperative, had a chronic physical condition, a psychiatric condition, or chronic psychiatric or physical conditions;
Length of follow‐up: we examined whether there was evidence of a difference in effect estimate between studies that assessed change in mental health between six‐week and six‐month follow‐up or at more than six‐months follow‐up. Effects may differ because people who achieve a difficult life goal, such as quitting smoking, may have a temporary improvement in mental health because of their achievement. However, should the effect persist long‐term, this supports the hypothesis that smoking itself is harmful to mental health and it is ceasing smoking and not the celebration of that achievement that improves mood.
Sensitivity analysis
We conducted the following sensitivity analyses:
Loss to follow‐up: we removed studies in which different numbers of participants were analysed at baseline and follow‐up;
Ascertainment of smoking status: we removed studies that did not biochemically validate follow‐up smoking status;
Psychotherapeutic/psychoactive component within cessation intervention: we removed studies that offered an evidence‐based psychotherapeutic or psychoactive (i.e. psychotherapy or antidepressants) component within the smoking cessation intervention;
Risk of bias: We removed studies judged to be at critical risk of bias.
Summary of findings and assessment of the certainty of the evidence
We created a 'Summary of findings' table, using GRADEpro GDT software, reporting the pooled effect estimates for our primary outcomes (depression, anxiety, and mixed depression and anxiety) and our secondary social impact outcome. We assessed these outcomes according to the eight GRADE considerations relevant to non‐randomised studies (Schünemann 2013; i.e. risk of bias, inconsistency, imprecision, indirectness, publication bias, magnitude of the effect, the influence of all plausible residual confounding, the presence of a dose‐response gradient) to assess the certainty of the body of evidence for these outcomes, and to draw conclusions about the certainty of the evidence within the text of the review.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
In this update of the review our searches identified 10,818 records. After removal of duplicates, we screened the titles and abstracts of 9858, of which 9106 were irrelevant. We assessed 752 full‐text studies for inclusion, of which we dropped 677, with reasons.
In the 2014 version of the review (searches conducted from inception to 30 April 2012) we identified 13,050 records. We screened the titles and abstracts of these records, of which 12,831 were irrelevant. We assessed 219 full‐texts for inclusion, of which we dropped 177, with reasons. We re‐examined 11 full‐text records that we had excluded from the previous version of this review for presenting dichotomous outcomes, and assessed if they were eligible for the current review.
Examples of studies that were excluded, with reasons for exclusion, are available in Characteristics of excluded studies. See Figure 1 for a flow diagram.
1.
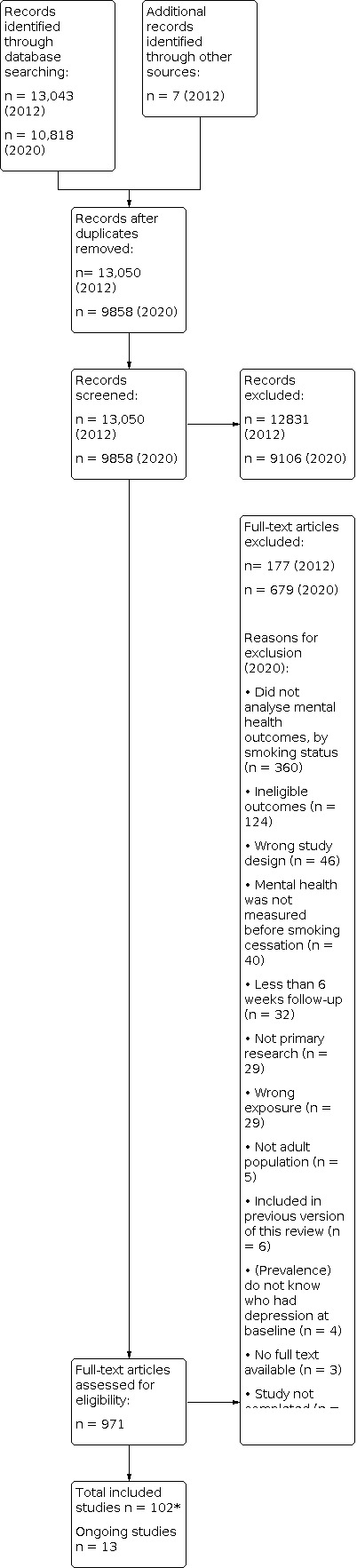
Study Flow Diagram 2020
(*40 of these studies were identified through the 2012 literature searches)
Included studies
As a result of both the 2014 review and the 2020 screening we include 102 studies in this review (40 that were included in the 2014 version, and 62 from the 2020 searches). We include 63 studies in our meta‐analyses of continuous measures of change in mental health, 10 were included in our meta‐analyses of incidence of clinical mental health disorders, and 31 were included in the narrative synthesis (two studies were included in the meta‐analysis and narrative review because they reported data suitable for meta‐analysis for one outcome, and data only suitable for narrative synthesis for another outcome: Mathew 2013; McFall 2006).
Forty‐five of the included studies were cohort studies, 56 were secondary analyses of randomised controlled trials analysed as observational cohorts, and one was a randomised trial in which participants were randomised to quit or to continue smoking (Dawkins 2009).
Participants
This review includes over 169,500 participants, with 42,000 included in meta‐analyses. It is not possible to give exact numbers, because some studies did not report the total number of participants that were included in their analyses.
The median age across studies was 45.2 years, and the median percentage of men was 51%. Participants smoked a median of 20.3 cigarettes a day at baseline and had a median score of 5.4 on the Fagerström test for nicotine dependence, indicating medium dependence levels.
Studies enrolled people from a range of populations: people with a chronic physical or psychiatric condition (2 studies) or both, people with a chronic physical condition (16 studies), post‐surgical patients (3 studies), pregnant women (4 studies), people with psychiatric conditions (23 studies), and the unselected population (57 studies). Three studies provided data on both the general population and on people with psychiatric conditions (Hammett 2019; Heffner 2019; Vermeulen 2019).
Intervention/exposure
The way abstinence was measured varied across studies. In 33 studies, abstinence was defined as a period of prolonged abstinence, typically defined from a point at or soon after the quit date. In all the other studies, apart from six where the definition was unclear (three studies included in meta‐analyses, and three summarised narratively), abstinence was defined as point prevalence (not smoking for a period, such as 24 hours or seven days, prior to the assessment, or at the time of the assessment only). In 66 studies, abstinence was biologically verified, usually through measuring participants' exhaled carbon monoxide or cotinine concentration.
In 33 studies, the length of participants' abstinence was unclear. In most cases this is where point prevalence abstinence was used as a definition, and so we could not tell when their time of abstinence began, i.e. participants were simply asked whether they were smoking or not at a follow‐up point. If people replied that they were not smoking at the follow‐up point it was not always clear when they had made their original quit attempt, and this has the potential to vary across participants within a study. However, the implications of this vary by study type. For RCTs, it would be reasonable to assume that, for most people, abstinence began on the study quit date, but for cohort studies the period of abstinence could have happened at any time from baseline to follow‐up. In the remaining studies length of abstinence was clearer: in 18 studies, with follow‐up at least six weeks after baseline, participants were assessed as abstinent for between one and three months of the follow‐up period; in 48 studies six‐ or 12‐month abstinence was assessed, and in three studies the maximum potential length of abstinence was over one year, with a maximum of 10 years (Sanchez‐Villegas 2008).
In 39 studies, participants were reported to be receiving a 'mood management' intervention (e.g. a psychological or medicinal treatment that could viably improve mental health), either externally to the study or as part of the study. Examples of these were antidepressants (Anthenelli 2013;Qi Zhang 2014; Sanchez‐Villegas 2008); behavioural mood‐management counselling (Blalock 2008; Krebs 2018; Qi Zhang 2014); or counselling on the emotional aspects of addiction (Segan 2011). In 73 studies, participants were motivated to quit, as defined by the study's selection criteria.
Outcomes
Forty‐two studies provided data for more than one of our prespecified outcomes. The median length of follow‐up was six months. Collectively the included studies reported on seven different measures of mental health: anxiety, depression, mixed anxiety and depression, positive affect, psychological quality of life, stress and social quality of life. Studies that reported social quality of life measured this using scales assessing the quality of social relationships. Where studies reported more than one social quality‐of‐life outcome, we selected the outcome which represented quality of friendships. We did this as the person involved in our patient and public involvement exercise was concerned about the impact of smoking cessation on their friendships. We chose between social quality‐of‐life scales in only one study (Leventhal 2014).
Full details of each included study can be found in Characteristics of included studies.
Ongoing studies
We identified 13 ongoing studies, which we will include in future updates. Full details of these studies can be found in Characteristics of ongoing studies.
Excluded studies
The primary reasons for excluding records were that the study
did not analyse mental health outcomes, by smoking status as an exposure;
did not collect mental health outcomes;
the outcomes were not eligible for this review (e.g. outcomes were cognitive in nature);
the study design was not eligible (e.g. qualitative study or cross‐sectional design); or
mental health was not measured before smoking cessation.
Other reasons are available in the PRISMA flow chart, and a list of sample excluded records is available in Excluded studies.
Risk of bias in included studies
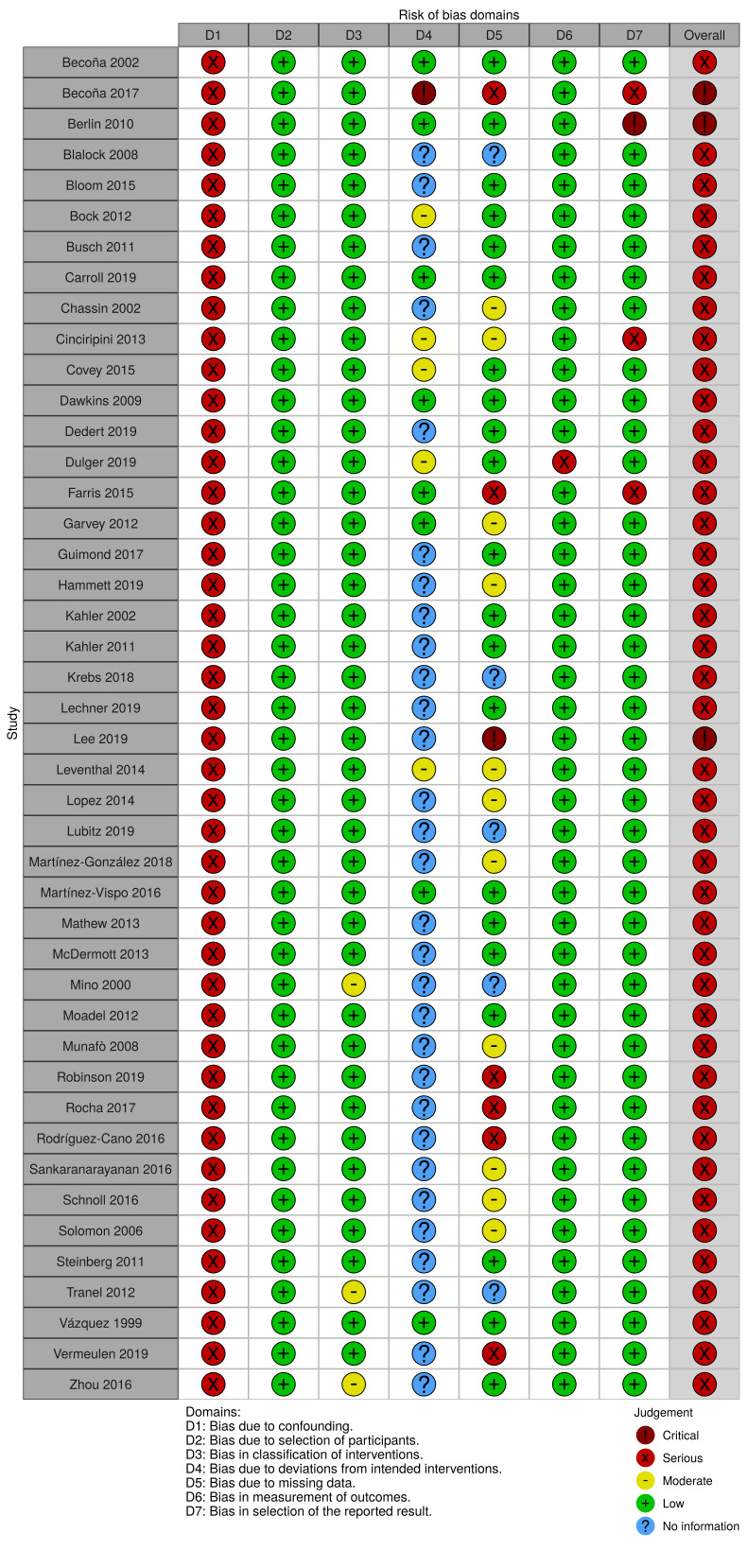
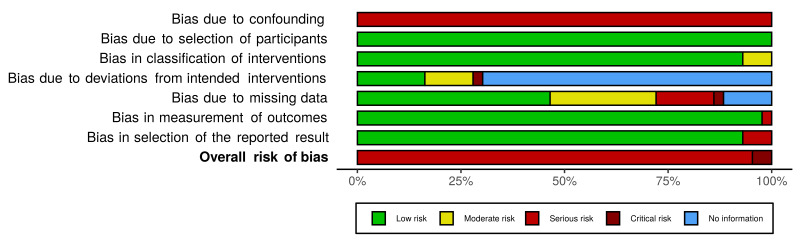
See Supplemental file 3; Supplemental file 4; Supplemental file 5; Figure 2; Figure 3 (figures generated using robvis; McGuiness 2020)
2.
Risk‐of‐bias: “traffic light” plot of the domain‐level judgements for each individual result according to the ROBINS‐I tool
3.
Risk‐of‐bias: Weighted bar plot of the distribution of risk‐of‐bias judgements within each bias domain according to the ROBINS‐I tool
We assessed risks of bias for each of the studies contributing to the meta‐analysis for the three primary outcomes (anxiety, depression, mixed anxiety and depression). We used a modified version of the ROBINS‐I tool to do so and assessed the domains highlighted below.
Bias due to confounding
We judged all studies across all three primary outcomes to be at serious risk of bias due to confounding. In all cases this was because the authors did not use methods that adequately controlled for all the potential time‐varying confounding, i.e. changes in recreational drug and alcohol use, change in psychoactive medication use and significant life events during the study period. This was because changes in each of these factors were not measured in the first instance, rather than that they were measured and then not adjusted for in analyses. An example of potential time‐varying confounding that was not accounted for in the studies is as follows: a person who experienced a stressful life event, such as divorce, during the course of the study would be both less likely to quit smoking (and therefore more likely to appear in the continuing‐smokers group), and more likely to experience worsened mental health. In this instance the difference in change between this person who continues smoking and someone else who quits would not be entirely explained by the change or lack of change in their smoking behaviour.
Bias in selection of participants into the study
We judged all studies across all three primary outcomes to be at low risk of bias for this domain. This is because the selection of participants into studies (or into the analyses) was not based on participant characteristics observed after participants made an attempt to quit smoking.
Bias in the classification of follow‐up smoking status
We rated all 15 studies contributing to the primary anxiety analysis at low risk of bias due to the classification of follow‐up smoking status. In all cases the differentiation between continued smoking and smoking cessation was clearly defined using a definition commonly used in the field.
We judged 32 of the studies that contributed to the primary depression analysis to be at low risk of bias for this domain; however we rated two studies (Tranel 2012; Zhou 2016) at moderate risk, as they did not clearly define abstinence.
Of the eight studies contributing to the primary mixed anxiety and depression outcome, we judged all but one study to be at low risk. Mino 2000 did not report the definition of cessation used and so was rated at moderate risk of bias for this domain.
Bias due to deviations from quitting smoking (i.e. relapsing) or through access to psychoactive treatments
For the anxiety outcome, we judged three of the studies contributing to the analysis to be at low risk of bias due to deviations from the 'intervention' (Becoña 2002; Dawkins 2009; Farris 2015). The ways that these studies were protected against this type of bias were by excluding potential participants who were receiving psychotherapy or psychoactive drugs, or had a psychiatric diagnosis; by not providing a psychoactive intervention as part of the study; or by adjusting for the imbalance in psychoactive treatment between the exposure and comparator groups in the analyses. We rated a further three studies at moderate risk of bias. In Bock 2012 there were some deviations from the intended intervention, but their impact on the outcome was expected to be slight. Participants were randomly allocated to either cognitive behavioural therapy for smoking cessation, Vinyasa yoga, or a general health and wellness programme. The authors reported that the yoga programme improved negative affect, and enhanced smoking cessation rates at eight‐week follow‐up, but that the effect on smoking cessation did not persist to the later follow‐up points. Covey 2015 did not use an analysis method that appropriately addressed the issue of relapsing, and in Dulger 2019 bupropion hydrochloride (an antidepressant and smoking cessation treatment) was provided to 42.6% of participants. The remaining nine studies did not provide enough information to answer the signalling questions in order to make an informed judgement for this domain, and so were categorised as 'No information'. In most cases this was because psychoactive treatments used by participants external to the study were not reported or were not reported split by exposure group.
Twenty‐five of the studies did not provide sufficient information to judge this domain for the depression outcome. We rated a further five studies at low risk of bias as they excluded people receiving psychotherapy or psychoactive drugs and did not provide a psychoactive intervention as part of the study, or they adjusted for any between‐group imbalance in psychoactive treatments through their analyses. We judged three studies to be at moderate risk of bias due to deviations from the 'intervention', as some of the participants received psychoactive interventions that were likely to be minimally associated with quitting smoking (Bock 2012; Dulger 2019), or the analysis did not appropriately account for relapse (Covey 2015). We rated one study at critical risk as some of the participants received behavioural activation therapy as part of the study, and this intervention increased quit rates (Becoña 2017).
For the mixed anxiety and depression outcome, five studies did not provide sufficient information to judge whether there was bias due to deviations from the 'intervention', and so we categorised them as 'No information'. We rated one study at low risk, as psychoactive treatments were not offered as part of the study and participants likely to need psychoactive treatment were excluded from the study (Carroll 2019). We deemed another two studies to be at moderate risk as some of the study participants received bupropion treatment and this was not adjusted for in the analyses (Cinciripini 2013; Leventhal 2014).
Bias due to missing data
For the anxiety outcome, we judged eight of the 15 studies to be at low risk of bias due to missing data, as outcome data was available for more than 70% of recruited participants, and participants were not excluded from the analysis due to missing smoking status or outcome data. We judged four of the studies to be at moderate risk of bias because more than 30% of participants were excluded from the analysis or participants were excluded from the analysis due to a lack of smoking cessation data, or both (Hammett 2019; Martínez‐González 2018; Schnoll 2016; Solomon 2006). We judged two studies to be at serious risk for this domain, as there was some evidence that the reasons for missing data may have differed between exposure groups (Farris 2015; Rocha 2017). The remaining study did not have enough information about missing data to make an informed judgement, and so was categorised as 'No information'.
For the depression outcome, we rated 17 studies to be at low risk, eight studies at moderate risk and four studies at serious risk for the same reasons as described above for the anxiety outcome. In addition, we deemed one study to be at critical risk of bias due to missing data, as less than 70% of the participants were included in the analysis of final follow‐up data and the proportions of missing data varied substantially by smoking status (Lee 2019). Another four studies did not provide enough information on smoking status to answer the signalling questions and assess bias due to missing data, and so were categorised as 'No information'.
For the mixed anxiety and depression outcome we rated two studies at low risk of bias, three studies at moderate risk, and three studies at serious risk for the reasons described for the anxiety outcome above. Two studies did not provide enough information to make a judgement, and so were categorised as 'No information' for this domain.
Bias in measurement of outcomes
We judged all studies across all three primary outcomes to be at low risk of bias for this domain. Bias is likely to be minimised as most studies did not set out to assess the impact of smoking cessation on mental health as a primary outcome, and participants and outcome assessors would not have known the hypothesis of this review. In addition, participants would have had to remember how they responded to questionnaires at baseline in order to provide a biased response to the follow‐up assessment, and the likelihood that there would be any systematic differences or errors in outcome assessment dependent on smoking status is unlikely.
Bias in selection of the reported result
We deemed most studies to be at low risk of bias across all of the primary outcomes for this domain. There was little evidence that the effect estimates were likely to be elected on the basis of results from multiple outcome measurements, multiple analyses of the association in question, or different subgroups. However, there were some exceptions to this. For the anxiety outcome we rated one of the included studies at serious risk of bias (Farris 2015), because although the Inventory of Depression and Anxiety Symptoms (IDAS) scale was used, which measures both anxiety and depression (subscales), the study only reported on anxious arousal at particular time points. For the depression outcome we judged a further two studies to be at serious risk of bias in selection of the reported result and one study was judged to be at critical risk. Becoña 2017 and Dulger 2019 both showed evidence of multiple tests of depression outcomes without reporting on all of these, and Berlin 2010 did the same, with further evidence that results appeared more likely to be reported if they showed a significant effect. Finally, we judged one of the studies measuring mixed anxiety and depression to be at serious risk of bias, as data from the Center for Epidemiologic Studies Depression scale (CES‐D) were not fully reported; results were reported for the unadjusted but not for the adjusted models (Cinciripini 2013).
Overall risk of bias assessment
As all studies were deemed to be at serious risk of bias due to confounding, we rated all included studies that contributed to the primary analyses at least at serious risk of bias overall. Although we rated most studies at serious risk, we judged a small number of studies to be at critical risk. All three studies contributed to the depression outcome (Becoña 2017; Berlin 2010; Lee 2019).
Effects of interventions
See: Table 1
Anxiety
Fifteen studies reported sufficient continuous data to calculate the pooled SMD for change in anxiety symptoms from baseline to follow‐up. Data from 798 people who quit smoking and 2343 people who continued smoking provided evidence that quitting smoking was associated with a decrease in anxiety symptoms from baseline to final follow‐up compared with continuing to smoke (SMD −0.28, 95% CI −0.43 to −0.13; 3141 participants; Analysis 1.1; Figure 4). However, we detected substantial statistical heterogeneity between studies (I2 = 69%).
1.1. Analysis.

Comparison 1: Change in anxiety, Outcome 1: Main continuous data analysis
4.

Funnel plot of comparison: 1 Main analysis: Difference in change (from baseline to longest follow‐up) between people who quit and people who continued smoking, outcome: 1.1 Primary outcome: Anxiety.
We carried out sensitivity analyses to test the robustness of this main effect. Removal of the three studies that did not biochemically confirm abstinence, the 10 studies that did not use a continuous measure of smoking abstinence, and the three studies where there was evidence that participants were receiving an evidence‐based psychoactive or psychotherapeutic component as part of an intervention did not account for the statistical heterogeneity or meaningfully change the estimate, such that it would change interpretation (Analysis 1.2; Analysis 1.3; Analysis 1.4). All studies included in this analysis (Analysis 1.1) analysed the same number of participants at baseline and at follow‐up, and all were judged to be at serious risk of bias; it was therefore not necessary to carry out our planned sensitivity analyses removing the studies at critical risk of bias and those that analysed a different number of participants at baseline compared to follow‐up.
1.2. Analysis.

Comparison 1: Change in anxiety, Outcome 2: Sensitivity analysis: no biochemical validation
1.3. Analysis.

Comparison 1: Change in anxiety, Outcome 3: Sensitivity analysis: point prevalence or no abstinence definition
1.4. Analysis.

Comparison 1: Change in anxiety, Outcome 4: Sensitivity analysis: psychoactive/psychological treatment used
We examined whether we were able to account for the statistical heterogeneity through subgroup analyses. Five studies that measured this outcome continuously enrolled people with a chronic physical condition, five studies enrolled people from the unselected population, one study enrolled post‐surgical participants, one study enrolled pregnant women and three studies enrolled people with psychiatric conditions. There was no evidence that the effect size differed across these different clinical populations (I2 = 0%; Analysis 1.5). Looking further at participant characteristics, 10 studies selected participants because they were motivated to quit smoking, and five studies did not select participants based on their motivation to quit. Again, there was no evidence of subgroup differences between these groups (I2 = 0%; Analysis 1.6). We also conducted subgroup analyses exploring methodological issues. Six studies presented adjusted effect estimates and 11 studies presented unadjusted effect estimates. However, there was no evidence that effect estimates when controlled for confounding differed from those calculated without adjustment (I2 = 0%; Analysis 1.7). One study provided both an adjusted and an unadjusted estimate (McDermott 2013), and they were very similar (Table 2). We also split studies according to whether they were longitudinal cohort or non‐randomised intervention studies, a randomised experiment comparing people allocated to quit or to continue smoking (only one study was categorised as the latter; Dawkins 2009), or a secondary observational analysis of an RCT. There was no evidence of subgroup differences between these differing study designs (I2 = 0%; Analysis 1.8). Eleven studies assessed anxiety at final follow‐up between six weeks and six months, and four studies measured final follow‐up at more than six months. There was no evidence for subgroup differences based on length of follow‐up (I2 = 0%; Analysis 1.9). Finally, nine studies reported that their original main aim was to report on the change in anxiety (classed as a primary outcome), and thus the decision to publish and the likelihood of successful publication may have been contingent on the strength or significance of this finding. The main aim of the other six studies was to report on other outcomes, meaning the changes in anxiety were secondary outcomes. There was no evidence for differences in the result of the studies based on the studies' aims (I2 = 0%; Analysis 1.10).
1.5. Analysis.

Comparison 1: Change in anxiety, Outcome 5: Subgroups: comparing clinical populations
1.6. Analysis.

Comparison 1: Change in anxiety, Outcome 6: Subgroups: motivation to quit
1.7. Analysis.

Comparison 1: Change in anxiety, Outcome 7: Subgroups: comparing adjusted & unadjusted estimates
1. Comparison between unadjusted and adjusted SMDs and 95% CIs for the association between smoking cessation compared to continued smoking and mental health from studies in which both were presented.
| Study | Outcome | Covariates adjusted for | Unadjusted SMD (95% CI) | Adjusted SMD (95% CI) |
| Becoña 2017 | Depression | Treatment group allocation, age, sex, baseline marital status, baseline education level, baseline working status, baseline FTND score, baseline number of years smoking | −1.61 (−2.34 to −0.88) |
−1.62 (−2.36 to −0.88) |
| Blalock 2008 | Depression | Baseline carbon monoxide expiration, baseline nicotine withdrawal score, treatment group allocation | −0.54 (−1.42 to 0.34) |
−0.58 (−1.01 to −0.15) |
| McDermott 2013 | Anxiety | Baseline STAI score, age, baseline nicotine dependence level, baseline cigarette consumption, and treatment group allocation | −0.62 (−0.88 to −0.36) |
−0.74 (−1.00 to −0.48) |
| Taylor 2015 | Psychological quality of life | Baseline FTND score, treatment group allocation, age started smoking, baseline report of calming effects from smoking, baseline report of unpleasant symptoms from smoking, baseline length of time to last cessation attempt, baseline experience from last cigarette, baseline longest period without smoking, baseline SF‐36 mental health score | 1.37 (0.41 to 2.33) |
1.13 (0.13 to 2.13) |
1.8. Analysis.

Comparison 1: Change in anxiety, Outcome 8: Subgroups: comparing study designs
1.9. Analysis.

Comparison 1: Change in anxiety, Outcome 9: Subgroups: length of longest follow‐up
1.10. Analysis.

Comparison 1: Change in anxiety, Outcome 10: Subgroups: primary versus secondary outcome
Two studies (Levy 2018; Shahab 2014) reported sufficient data to calculate the odds ratios for the association between abstinence and the cumulative incidence of anxiety at follow‐up. We pooled data from 165 people who quit, and 2128 people who continued smoking, which suggested that quitting smoking was associated with a reduced incidence of anxiety at final follow‐up compared with continuing to smoke (OR 0.61, 95% CI 0.34 to 1.12; 2293 participants; I2 = 46%; Analysis 1.11). However, there was imprecision, with CIs incorporating the potential for both a reduced and an increased incidence of anxiety.
1.11. Analysis.

Comparison 1: Change in anxiety, Outcome 11: New incidence of anxiety
We were unable to include eight studies reporting change in anxiety symptoms in our meta‐analysis and summarised them narratively (see Supplemental file 6). These studies included a total of 7613 participants. Four studies reported an improvement in anxiety symptoms in people who quit that was greater than any change which occurred in the people who continued smoking. In four studies it was not possible to discern the direction of effect.
Depression
Thirty‐four studies reported sufficient data to calculate the pooled SMD for change in depression symptoms from baseline to follow‐up. We pooled data from 1863 people who quit, and 5293 people who continued smoking, which resulted in evidence that quitting smoking was associated with a decrease in depression symptoms from baseline to final follow‐up when compared to continuing to smoke (SMD −0.30, 95% CI −0.39 to −0.21; 7156 participants; Analysis 2.1; Figure 5). There was substantial statistical heterogeneity detected between studies (I2 = 69%).
2.1. Analysis.

Comparison 2: Change in depression, Outcome 1: Main continuous data analysis
5.

Funnel plot of comparison: 2 Change in depression, outcome: 2.1 Main continuous data analysis.
Our sensitivity analyses removing the two studies judged to be at critical risk of bias (as opposed to serious risk), removing the eight studies that did not biochemically confirm abstinence, removing the 22 studies that did not measure smoking cessation using continuous abstinence measures, removing the 13 studies that included participants who were in receipt of an evidence‐based psychoactive or psychotherapeutic treatment, and removing the five studies that reported mean depression scores at baseline on more people than were present at follow‐up did not result in any meaningful change in the pooled effect estimates nor diminish heterogeneity substantially (Analysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.5; Analysis 2.6).
2.2. Analysis.

Comparison 2: Change in depression, Outcome 2: Sensitivity analysis: risk of bias
2.3. Analysis.

Comparison 2: Change in depression, Outcome 3: Sensitivity analysis: no biochemical validation
2.4. Analysis.

Comparison 2: Change in depression, Outcome 4: Sensitivity analysis: point prevalence or no abstinence definition
2.5. Analysis.

Comparison 2: Change in depression, Outcome 5: Sensitivity analysis: psychoactive/psychological treatment used
2.6. Analysis.

Comparison 2: Change in depression, Outcome 6: Sensitivity analysis: differing Ns analysed
We assessed whether there was evidence that the strength of association differed based on clinical context (people with a chronic physical condition; post‐surgical participants; pregnant women; people with psychiatric conditions; the unselected population). There was weak evidence that the effect size differed across these different clinical populations (I2 = 58.5%). However, in all cases, point estimates suggested that quitters experienced a decrease in depression symptoms. For people with psychiatric conditions, pregnant women and post‐surgical participants the estimates were smaller, and for the latter two groups these were also imprecise, suggesting the possibility of either a decrease, no change, or an increase in depression symptoms associated with successfully quitting smoking (Analysis 2.7). We also investigated whether effect estimates differed by the motivation of participants to quit smoking. Twenty‐five studies selected participants because they were motivated to quit smoking, whereas nine studies did not select participants based on motivation to quit. There was no clear evidence of meaningful subgroup differences (I2 = 27.9%; Analysis 2.8).
2.7. Analysis.

Comparison 2: Change in depression, Outcome 7: Subgroups: comparing clinical populations
2.8. Analysis.

Comparison 2: Change in depression, Outcome 8: Subgroups: motivation to quit
Ten studies presented adjusted effect estimates and 24 studies presented unadjusted effect estimates. A subgroup analysis provided no clear evidence that effect estimates differed between studies that had and had not adjusted for confounders (I2 = 0%; Analysis 2.9). Two of these studies (Becoña 2017; Blalock 2008) provided both adjusted and unadjusted estimates of the effects of smoking cessation on depression, and the within‐study comparisons of effects also indicated that adjustment did not result in any meaningful difference in the results (Table 2).
2.9. Analysis.

Comparison 2: Change in depression, Outcome 9: Subgroups: comparing adjusted & unadjusted estimates
We also found no evidence that effect estimates varied based on study design (longitudinal cohort, non‐randomised intervention study, randomised experimental study, or secondary analyses of RCTs; I2 = 5.4%; Analysis 2.10), length of final follow‐up (between six weeks and six months versus greater than six months; I2 = 0%; Analysis 2.11), or the main aim of the study (to report on change in depression versus to report on other outcomes with depression as a secondary outcome; I2 = 0%; Analysis 2.12).
2.10. Analysis.

Comparison 2: Change in depression, Outcome 10: Subgroups: comparing study designs
2.11. Analysis.

Comparison 2: Change in depression, Outcome 11: Subgroups: length of longest follow‐up
2.12. Analysis.

Comparison 2: Change in depression, Outcome 12: Subgroups: primary versus secondary outcome
Seven additional included studies reported data on the incidence of depression between baseline and follow‐up in sufficient detail to calculate the pooled odds ratio. We analysed data from 19,521 people who quit, and 89,221 people who continued smoking. The result was subject to substantial unexplained statistical heterogeneity (I2 = 87%; Analysis 2.13), and we therefore deemed it inappropriate to present the pooled result. Two studies favoured a beneficial effect of smoking cessation on incidence of depression, two a harmful effect, and three were close to the null.
2.13. Analysis.

Comparison 2: Change in depression, Outcome 13: New incidence of depression
We were unable to include 16 studies with 15,285 participants reporting change in depression symptoms in our meta‐analysis, so we summarised them narratively (see Supplemental file 7). Six studies reported a larger improvement in depression symptoms in people who quit compared to people who continued to smoke, in four studies the association was equivocal, in one study there was an improvement in people who continued to smoke compared with people who quit, and in the remaining five studies it was not possible to discern the direction of effect.
Mixed anxiety and depression
Eight studies reported sufficient data to calculate the pooled SMD for the change in mixed anxiety and depression measures between baseline and follow‐up. We analysed data from 793 people who quit and 2036 people who continued smoking. Results suggested that quitting smoking was associated with a decrease in mixed anxiety and depression symptoms compared with continued smoking (SMD −0.31, 95% CI −0.40 to −0.22; 2829 participants; I2 = 0%; Analysis 3.1). None of our sensitivity analyses investigating the effects of removing studies that did not biochemically validate abstinence, that measured point prevalence abstinence only, that included participants who were receiving psychoactive or psychological treatment, or that analysed different numbers of participants at baseline and follow‐up, found any meaningful difference in the effect estimates (Analysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.5)
3.1. Analysis.

Comparison 3: Change in mixed anxiety and depression, Outcome 1: Main continuous data analysis
3.2. Analysis.

Comparison 3: Change in mixed anxiety and depression, Outcome 2: Sensitivity analysis: no biochemical validation
3.3. Analysis.

Comparison 3: Change in mixed anxiety and depression, Outcome 3: Sensitivity analysis: point prevalence or no abstinence definition
3.4. Analysis.

Comparison 3: Change in mixed anxiety and depression, Outcome 4: Sensitivity analysis: psychoactive/psychological treatment used
3.5. Analysis.

Comparison 3: Change in mixed anxiety and depression, Outcome 5: Sensitivity analysis: differing Ns analysed
We also carried out subgroup analyses to examine whether prespecified factors contributed to the variation in effects across studies. There was no evidence of subgroup differences by population type (Analysis 3.6), or between people motivated to quit smoking versus participants not selected on motivation (Analysis 3.7). A further three subgroup analyses investigated methodological factors; there was no evidence of subgroup differences when studies were split based on study type (Analysis 3.8), maximum length of follow‐up (Analysis 3.9), or whether mixed anxiety and depression was investigated as a primary outcome or a secondary outcome (Analysis 3.10).
3.6. Analysis.

Comparison 3: Change in mixed anxiety and depression, Outcome 6: Subgroups: comparing clinical populations
3.7. Analysis.

Comparison 3: Change in mixed anxiety and depression, Outcome 7: Subgroups: motivation to quit
3.8. Analysis.

Comparison 3: Change in mixed anxiety and depression, Outcome 8: Subgroups: comparing study designs
3.9. Analysis.

Comparison 3: Change in mixed anxiety and depression, Outcome 9: Subgroups: length of longest follow‐up
3.10. Analysis.

Comparison 3: Change in mixed anxiety and depression, Outcome 10: Subgroups: primary versus secondary outcome
Three additional studies (Cavazos‐Rehg 2014; Chen 2015; Giordano 2011) reported sufficient data to calculate the odds ratio for the association between smoking cessation and incidence of mixed anxiety and depression at follow‐up. These studies included data from 1752 people who quit and 6933 people who continued smoking. There was evidence that quitting smoking was associated with a reduced incidence of mixed anxiety and depression at final follow‐up when compared to continuing to smoke (OR 0.76, 95% CI 0.66 to 0.86; 8685 participants; Analysis 3.11); there was moderate statistical heterogeneity between studies (I2 = 57%).
3.11. Analysis.

Comparison 3: Change in mixed anxiety and depression, Outcome 11: New incidence of mixed anxiety and depression
We were unable to include two studies with 130 participants reporting change in mixed anxiety and depression symptoms in our meta‐analysis, so we summarised these narratively (see Supplemental file 8). For both studies, the association was equivocal, with no evidence of a difference in mixed anxiety and depression symptoms between quitters and continuing smokers.
Stress
Four studies reported sufficient data to calculate the SMD for change in stress from baseline to follow‐up, pooling data from 651 people who quit, and 1141 people who continued smoking. Evidence suggests that quitting smoking was associated with decreased stress symptoms from baseline to final follow‐up when compared with continuing to smoke (SMD −0.19, 95% CI −0.34 to −0.04; 1792 participants; Analysis 4.1). Moderate statistical heterogeneity was detected (I2 = 50%).
4.1. Analysis.

Comparison 4: Change in stress, Outcome 1: Main continuous data analysis
We carried out the following sensitivity analyses to assess the robustness of the result: removal of two studies that did not biochemically confirm abstinence (Analysis 4.2); removal of two studies that did not measure abstinence using a continuous measure (Analysis 4.3), removal of one study that based baseline analyses on larger numbers than follow‐up analyses (Analysis 4.4). None of these analyses provided evidence for any meaningful change in the effect estimates. No studies recruited participants that were clearly receiving psychoactive or psychotherapeutic treatment. One study included in this analysis (Taylor 2015) provided both an adjusted and unadjusted estimate of the effect of smoking cessation on stress. Adjustment did not meaningfully change the effect estimate (Table 2).
4.2. Analysis.

Comparison 4: Change in stress, Outcome 2: Sensitivity analysis: no biochemical validation
4.3. Analysis.

Comparison 4: Change in stress, Outcome 3: Sensitivity analysis: point prevalence or no abstinence definition
4.4. Analysis.

Comparison 4: Change in stress, Outcome 4: Sensitivity analysis: differing Ns analysed
Subgroup analyses examined whether prespecified factors contributed to the variation in effects across studies. There was no evidence of subgroup differences between the association in people with a chronic physical condition and the general population (Analysis 4.5), or between people motivated to quit smoking and studies that did not select participants based on their motivation (Analysis 4.6). A further two subgroup analyses investigated methodological factors; there was no evidence of subgroup differences when studies were split based on study type (longitudinal cohort studies or non‐randomised intervention studies versus secondary analyses of RCTs; Analysis 4.7) or based on the maximum length of follow‐up (between six weeks and six months follow‐up versus more than six months follow‐up; Analysis 4.8). The primary aim of all four studies that measured stress was to report on the change in stress symptoms, so the prespecified subgroup analysis comparing stress as a primary versus secondary outcome was not appropriate.
4.5. Analysis.

Comparison 4: Change in stress, Outcome 5: Subgroups: comparing clinical populations
4.6. Analysis.

Comparison 4: Change in stress, Outcome 6: Subgroups: motivation to quit
4.7. Analysis.

Comparison 4: Change in stress, Outcome 7: Subgroups: comparing study designs
4.8. Analysis.

Comparison 4: Change in stress, Outcome 8: Subgroups: length of longest follow‐up
We were unable to include four studies with 1554 participants, reporting change in stress in our meta‐analysis, so we summarise them narratively (see Supplemental file 9). Three studies reported a larger improvement in stress in people who quit compared with people who continued smoking, and in the remaining study it was not possible to discern the direction of effect.
Positive affect
Thirteen studies reported sufficient data to calculate the SMD change in positive affect from baseline to follow‐up. We pooled data from 1965 people who quit, and 2915 people who continued smoking. Quitting smoking was associated with an increase in positive affect from baseline to final follow‐up when compared with continuing to smoke (SMD 0.22, 95% CI 0.11 to 0.33; 4880 participants; Analysis 5.1; Figure 6). There was substantial statistical heterogeneity (I2 = 75%), but all point estimates favoured quitting smoking, apart from one, which was only slightly in favour of continuing to smoke.
5.1. Analysis.

Comparison 5: Change in positive affect, Outcome 1: Main continuous data analysis
6.

Funnel plot of comparison: 5 Change in positive affect, outcome: 5.1 Main continuous data analysis.
Where possible we carried out our prespecified sensitivity and subgroup analyses to investigate any potential explanations for this variation (Analysis 5.2; Analysis 5.3; Analysis 5.4; Analysis 5.5; Analysis 5.6; Analysis 5.7; Analysis 5.8; Analysis 5.9; Analysis 5.10; Analysis 5.11). Two of the subgroup analyses provided evidence of variance across subgroups; one subgroup examined whether the effect differed by subgroups based on comorbidities, and the other based on the timing of their longest follow‐up. The effect was much larger in people with physical or psychological diseases than in the unselected samples (Analysis 5.6). The effect on positive affect was larger in studies where the follow‐up was six months or less than where it was longer than six months (Analysis 5.10).
5.2. Analysis.

Comparison 5: Change in positive affect, Outcome 2: Sensitivity analysis: no biochemical validation
5.3. Analysis.

Comparison 5: Change in positive affect, Outcome 3: Sensitivity analysis: point prevalence or no abstinence definition
5.4. Analysis.

Comparison 5: Change in positive affect, Outcome 4: Sensitivity analysis: psychoactive/psychological treatment used
5.5. Analysis.

Comparison 5: Change in positive affect, Outcome 5: Sensitivity analysis: differing Ns analysed
5.6. Analysis.

Comparison 5: Change in positive affect, Outcome 6: Subgroups: comparing clinical populations
5.7. Analysis.

Comparison 5: Change in positive affect, Outcome 7: Subgroups: motivation to quit
5.8. Analysis.

Comparison 5: Change in positive affect, Outcome 8: Subgroups: comparing adjusted & unadjusted estimates
5.9. Analysis.

Comparison 5: Change in positive affect, Outcome 9: Subgroups: comparing study designs
5.10. Analysis.

Comparison 5: Change in positive affect, Outcome 10: Subgroups: length of longest follow‐up
5.11. Analysis.

Comparison 5: Change in positive affect, Outcome 11: Subgroups: primary versus secondary outcome
We were unable to include five studies with 3155 participants reporting change in positive affect in our meta‐analysis, so we summarised these narratively (see Supplemental file 10). One study reported a larger improvement in positive affect in people who quit compared with people who continued smoking, in two studies the association was equivocal, and in the remaining two studies it was not possible to discern the direction of effect.
Psychological quality of life
Nineteen studies reported sufficient data to calculate the SMD for change in psychological quality of life from baseline to follow‐up. We pooled data from 5234 people who quit and 12,800 people who continued smoking. We found evidence that quitting smoking was associated with increased psychological quality of life when compared with continuing to smoke (SMD 0.11, 95% CI 0.06 to 0.16; 18,034 participants; I2 = 42%; Analysis 6.1; Figure 7).
6.1. Analysis.

Comparison 6: Change psychological quality of life, Outcome 1: Main continuous data analysis
7.

Funnel plot of comparison: 6 Change psychological quality of life, outcome: 6.1 Main continuous data analysis.
We carried out sensitivity analyses removing the following study types: those that did not biochemically validate abstinence (Analysis 6.2), those that did not use a continuous measure of smoking cessation (Analysis 6.3), those where there was clear evidence that the participants were in receipt of psychoactive or psychotherapeutic treatment (Analysis 6.4), and those where a larger number of participants contributed to the analysis at baseline than at follow‐up (Analysis 6.5). None of these sensitivity analyses resulted in meaningful changes in the point estimate, but in the first, second and third analyses the increased imprecision meant that the lower boundary of the 95% CI fell below zero.
6.2. Analysis.

Comparison 6: Change psychological quality of life, Outcome 2: Sensitivity analysis: no biochemical validation
6.3. Analysis.

Comparison 6: Change psychological quality of life, Outcome 3: Sensitivity analysis: point prevalence or no abstinence definition
6.4. Analysis.

Comparison 6: Change psychological quality of life, Outcome 4: Sensitivity analysis: psychoactive/psychological treatment used
6.5. Analysis.

Comparison 6: Change psychological quality of life, Outcome 5: Sensitivity analysis: differing Ns analysed
None of the subgroup analyses showed convincing evidence that there were differences between subgroups in the effect of stopping smoking on psychological quality of life (Analysis 6.6; Analysis 6.7; Analysis 6.8; Analysis 6.9; Analysis 6.10; Analysis 6.11).
6.6. Analysis.

Comparison 6: Change psychological quality of life, Outcome 6: Subgroups: comparing clinical populations
6.7. Analysis.

Comparison 6: Change psychological quality of life, Outcome 7: Subgroups: motivation to quit
6.8. Analysis.

Comparison 6: Change psychological quality of life, Outcome 8: Subgroups: comparing adjusted & unadjusted estimates
6.9. Analysis.

Comparison 6: Change psychological quality of life, Outcome 9: Subgroups: comparing study designs
6.10. Analysis.

Comparison 6: Change psychological quality of life, Outcome 10: Subgroups: length of longest follow‐up
6.11. Analysis.

Comparison 6: Change psychological quality of life, Outcome 11: Subgroups: primary versus secondary outcome
We were unable to include nine studies with 4301 participants reporting change in psychological quality of life in our meta‐analysis, so we summarise them narratively (see Supplemental file 11). One study reported a larger improvement in psychological quality of life in people who quit compared to people who continued smoking, in four studies the association was equivocal, and in the remaining four studies it was not possible to discern the direction of effect.
Social impact or social quality of life
Nine studies reported sufficient data to calculate the SMD for change in social impact or social quality of life from baseline to follow‐up. We pooled data from 3876 people who quit, and 10,797 people who continued to smoke. Quitting smoking appeared to be associated with a small increase in social quality of life when compared to continuing to smoke (SMD 0.03, 95% CI 0.00 to 0.06; 14,67 participants; I2 = 0%; Analysis 7.1), although the lower boundary of the confidence interval suggests that there is the possibility of no association between quitting smoking and social quality of life. None of our sensitivity analyses (investigating biovalidation, abstinence definition, use of psychoactive/psychological treatment, differing participant numbers at measurement points) resulted in meaningful differences in the effect estimates generated (Analysis 7.2; Analysis 7.3; Analysis 7.4; Analysis 7.5).
7.1. Analysis.

Comparison 7: Change in social quality of life, Outcome 1: Main continuous data analysis
7.2. Analysis.

Comparison 7: Change in social quality of life, Outcome 2: Sensitivity analysis: no biochemical validation
7.3. Analysis.

Comparison 7: Change in social quality of life, Outcome 3: Sensitivity analysis: point prevalence or no abstinence definition
7.4. Analysis.

Comparison 7: Change in social quality of life, Outcome 4: Sensitivity analysis: psychoactive/psychological treatment used
7.5. Analysis.

Comparison 7: Change in social quality of life, Outcome 5: Sensitivity analysis: differing Ns analysed
We used subgroup analyses to examine whether prespecified factors contributed to the variation in effects across studies. There was no evidence of subgroup differences by population type (Analysis 7.6), or between people motivated to quit smoking versus participants not selected on motivation (Analysis 7.7). A further three subgroup analyses investigated methodological factors; there was no evidence of subgroup differences when studies were split based on whether they contributed adjusted or unadjusted effect estimates to the meta‐analysis (Analysis 7.8), study type (Analysis 7.9), or maximum length of follow‐up (Analysis 7.10).
7.6. Analysis.

Comparison 7: Change in social quality of life, Outcome 6: Subgroups: comparing clinical populations
7.7. Analysis.

Comparison 7: Change in social quality of life, Outcome 7: Subgroups: motivation to quit
7.8. Analysis.

Comparison 7: Change in social quality of life, Outcome 8: Subgroups: comparing adjusted & unadjusted estimates
7.9. Analysis.

Comparison 7: Change in social quality of life, Outcome 9: Subgroups: comparing study designs
7.10. Analysis.

Comparison 7: Change in social quality of life, Outcome 10: Subgroups: length of longest follow‐up
We were unable to include one study reporting change in social impact or social quality of life in our meta‐analysis, which we summarised narratively (see Supplemental file 12). This study included 943 participants and reported equivocal evidence on the association between change in social quality of life and quitting smoking.
Discussion
Summary of main results
We compared the change in mental health‐related outcomes in people who stopped smoking with changes occurring in people who continued to smoke. Across six separate outcomes, there was no evidence to suggest that quitting smoking resulted in worsening mental health relative to continuing to smoke. In addition, there was evidence to suggest that negative mental‐health symptoms (depression, anxiety, mixed depression and anxiety, and stress) decreased, while positive symptoms (psychological quality of life and quality of social interaction) increased in people who stopped relative to those continuing to smoke. Sensitivity analyses removing studies at the highest risk of bias left the results largely unchanged. Subgroup analyses examined whether the effect differed by groups defined by disease status or condition, and, most crucially, people with current psychological disorders. Taken across these analyses, there was very little evidence that the effect differed for this group or any other subgroup defined by disease status or condition, such as pregnancy or chronic physical illness. Where there was some evidence of this for the positive affect outcome, effect estimates differed in magnitude rather than the direction of effect. The pooled effect sizes we found for our anxiety, depression, and mixed anxiety and depression outcomes were similar at around 0.3. Although this could be deemed to be a small‐to‐moderate effect (Cohen 1988), it is similar in size to that observed in a meta‐analysis of antidepressants for anxiety disorder (NCCMH 2011), which is generally considered to be clinically significant.
There were far fewer data available to assess the impact of smoking cessation on the incidence of psychological disorders in people who stopped compared with those who continued smoking. The data we identified were equivocal, with marked unexplained heterogeneity for the depression outcome, while studies on the incidence of anxiety and mixed anxiety and depression showed a somewhat lower incidence in people who stopped smoking, but with imprecision.
Overall completeness and applicability of evidence
We believe, and our public contributor believes, that all relevant outcomes have been studied in this review. We added the impact of stopping smoking on social relationships after consulting with a member of the public who smoked, as one fear they had was of losing friends through not smoking and no longer being part of a group of people who smoked.
The evidence in this review is directly relevant to the question of whether stopping smoking influences mood. In conducting the review, we summarised 102 observational studies, giving 112 comparisons of the association between change in smoking status and change in mood. In addition, a much smaller number (12 studies) gave data on the incidence of psychological disorders by change in smoking status for those who did not have such disorders at baseline. While directly relevant to estimating the effect size that stopping smoking may have on mood, such data are naturally subject to greater biases than randomised trials would be. Randomised trials with long‐term follow‐up are not ethical in this area, because they require investigators to randomise participants to stop smoking or continue smoking in the longer term. However, we included one such study in which participants were randomised to quit or to continue smoking, with a short‐term assessment of outcome, which produced an effect estimate similar to that seen in the observational data (Dawkins 2009). Differential dropout from the arm assigned to achieve abstinence (because of relapse) somewhat clouds this study.
It is important to assess the degree to which these findings are applicable worldwide to everyone who smokes. As with nearly all medical research, the great majority of studies were conducted in high‐income countries with a western cultural outlook, where the public typically view smoking as harmful to health and a minority smoke. As such, it is possible that people who stop smoking feel relieved and mood improves, an effect not achieved in cultures where there is not widespread concern about smoking. However, it seems implausible that relief from no longer smoking would explain the improvement in mood seen in people who have stopped smoking many months or years ago, which is what we see in many of the included studies. Instead, the effect of stopping smoking on mood could be explained by neuroadaptations to chronic nicotine inhalation that reverse on cessation (Taylor 2020). If so, these results are likely to apply worldwide to anyone who finds stopping smoking difficult.
Quality of the evidence
All of the included studies were observational and there is therefore potential for bias. One bias may be thought to arise because people with current or past psychological disorders are less likely to achieve abstinence, and therefore are likely to be over‐represented in the group who continue smoking compared with those who stop (Hitsman 2013). However, we primarily compared change in mental health over time between people who stopped and people who did not. If worsened mental health predicted failure to stop smoking at baseline it is likely that by follow‐up it would have improved through regression to the mean, creating a bias that should favour the continuing‐smoking group. This bias therefore could not explain the apparent benefit of abstinence on improving mental health. Moreover, it is unlikely to be quantitatively serious. The differences between people who achieved abstinence and those who did not emerged because those achieving abstinence mostly improved, while those continuing to smoke showed minimal change in mean scores. The within‐person design we used for our main analyses was intended to control for most confounding. Any factors that differed between those who stopped smoking and those who did not, which were likely also to influence mental health scores, would have influenced baseline and follow‐up scores equally. For example, variables such as age, gender, social class and ethnicity are likely to be causally related to mental health, but their influence would have been manifest both at baseline and at follow‐up, and thus the change in mental health should not be subject to confounding by these individual fixed factors. In support of this contention, when we compared unadjusted and adjusted estimates both within study and through subgroup analyses, there was little evidence that estimates differed meaningfully.
We also considered a range of other biases, particularly access to psychotherapeutic interventions that may have supported both smoking abstinence and mood. Sensitivity analyses removing studies that provided evidence that the likelihood of psychotherapeutic intervention may have differed between the exposure and comparator groups made no meaningful difference to our estimates. Similarly, where smoking abstinence had not lasted a long time, it is plausible that people who were abstinent would be experiencing withdrawal symptoms, which generally include mood disturbances. However, such an effect would bias the estimate against seeing a benefit from cessation, which would not explain our findings.
Time‐varying factors that both influence the likelihood of stopping smoking and mood are of concern, but only one study adjusted for these (Weinhold 2017). Examples of such factors are problematic alcohol or illegal drug use, intercurrent illness or stressful life events. Problematic substance use and stressful life events are likely to make stopping smoking less likely and may depress mood, while intercurrent illness may depress mood but increase the likelihood of quitting. These factors are likely to confound to some extent, and so we judged all studies contributing to our primary outcomes to be at least at serious risk of bias during our ROBINS‐I assessments, due to lack of adjustment for these variables. However, we think it unlikely that this confounding would completely reverse the associations found in this study because such exposures are likely to be uncommon, and so we are confident that quitting smoking is unlikely to lead to a worsening of mental health symptoms.
Our 'Summary of findings' table relates to changes in the symptoms of the most common psychological disorders: anxiety, depression, and mixed anxiety and depression, where we graded the certainty in our estimates of effect as low, very low, and moderate respectively (Table 1). The downgrading was partly due to the difficulties in controlling for the more complex time‐varying confounding discussed above, and also due to inconsistencies in study estimates for anxiety and for depression. However, the unexplained statistical heterogeneity detected related to differences in the size of the association across studies and not its direction, which almost without exception favoured positive mood in those who stopped smoking over those who continued. This contributes to our confidence in the evidence that suggests quitting smoking is unlikely to lead to a worsening of mental health symptoms. For the depression outcome we also downgraded the evidence due to the likelihood that publication bias may be influencing the result, which is discussed further below. We did not downgrade any of our primary outcomes due to imprecision as they were all measured by a reasonable number of studies, with a substantial number of participants, resulting in reasonably precise estimates of the association between stopping smoking and change in mood.
Less evidence was available from the 12 studies that investigated the incidence of new anxiety, depression, and mixed anxiety and depression. Furthermore, there were marked differences in the direction of effect between studies and hence future studies would help to clarify these findings. The most extreme example related to Glassman 2001, which showed a marked harm, by showing an increase in the incidence of depression in people who stopped smoking compared with those who continued. However, this study had marked differential follow‐up, with 95% of people who stopped smoking supplying data on depression, compared with 61% of people who continued smoking. If depression incidence was underestimated in those who continued smoking, as seems likely, this could partly explain this effect.
As well as the clinical significance of the effects observed and the marked consistency in the direction of our findings across studies and outcomes, there is a plausible causal mechanism by which smoking could improve mood. Chronic nicotine inhalation leads to neuroadaptations in brain pathways (Benowitz 2010). The constant fluctuation in withdrawal‐induced psychological symptoms experienced by people who smoke could worsen mental health over time, and the associated biological effects could increase the risk of mental illness (Parrott 2003; Wootton 2020). These adaptations revert after ceasing smoking (Mamede 2007). This is consistent with reports that withdrawal symptoms abate a few weeks after quitting smoking (Hughes 2007). These arguments support the contention that the associations observed could be causal.
Potential biases in the review process
We deployed extensive searching developed with an information specialist to maximise the sensitivity of our search. This was coupled with citation searching as well as extensive full‐text review to identify estimates. However, in many cases, included studies provided evidence on the association between change in smoking status and mental health that was not the main focus of the paper. As such, it is possible that we missed some studies with relevant data. Some studies where our outcomes were not the focus of the paper did include evidence that they had examined the association between change in smoking status and mental health, but not in sufficient detail to allow us to extract numerical estimates for the meta‐analyses. Where it was possible to discern the direction of effect, this was generally consonant with the numerical analyses, suggesting bias from incompleteness is unlikely to be differential. Five studies showed potential evidence of multiple testing and selectively reporting significant results (Becoña 2017; Berlin 2010; Cinciripini 2013; Dulger 2019; Farris 2015), but we found no other evidence of this in our primary outcomes. There were sufficient studies to create funnel plots for anxiety, depression, positive affect and psychological quality of life. The plots were asymmetrical for anxiety (Figure 4) and depression (Figure 5), but symmetrical for positive affect (Figure 6) and psychological quality of life (Figure 7). Egger's tests indicated that small studies measuring the depression and positive affect outcomes provided larger effect estimates than studies with larger samples (Table 3), so we used trim and fill to examine the possible biasing effect of missing studies on the outcome. For depression, the trim‐and‐fill method was not compatible with the data due to high heterogeneity. For positive affect, trim and fill estimated that there were five potential missing studies. The original pooled SMD and 95% CI for the association between smoking cessation and change in positive affect was 0.22 (0.11 to 0.21), and, after using trim and fill to impute the plausibly‐missing data, the SMD and 95% CI was 0.12 (0.01 to 0.23); I2 = 75.5%. Thus, while it is likely that some data, either unpublished or published, are missing from the analyses, there is no strong evidence that missing data would nullify the apparent effect of smoking cessation on mental health.
2. Egger test for small study bias: Egger's test regression coefficients and 95% CI for outcomes 1.1 to 1.7.
| Outcome | N studies | Egger's test regression coefficient | Standard error | CI lower bound | CI upper bound | P‐value |
| Anxiety | 15 | −0.97 | 0.61 | −2.28 | 0.34 | 0.13 |
| Depression | 34 | −1.23 | 0.33 | −1.91 | −0.55 | < 0.001 |
| Mixed anxiety and depression | 8 | −0.28 | 0.58 | −1.7 | 1.13 | 0.64 |
| Stress | 4 | −3.08 | 1.69 | −10.37 | 4.21 | 0.21 |
| Positive affect | 13 | 1.98 | 0.31 | 1.3 | 2.65 | < 0.001 |
| Psychological quality of life | 19 | 0.72 | 0.4 | −0.11 | 1.56 | 0.09 |
| Social | 9 | 0.47 | 0.41 | −0.51 | 1.44 | 0.29 |
Agreements and disagreements with other studies or reviews
An earlier version of this review was published outside of the Cochrane Library by many of the same team (Taylor 2014). We now include more than twice as many studies, but the effect estimates are very similar. The findings in these reviews suggest that smoking cessation does not worsen mood, and may improve it. A further Mendelian randomisation study (Wootton 2020) provides evidence that the effect may be causal. Mendelian randomisation effectively randomises exposure (here continuing or stopping smoking) via meiosis, and thereby equalises confounders in each exposure state, allowing assessment of an association that is free from confounding and reverse causation, providing certain assumptions are met. In the case of smoking, genetic factors are important in explaining smoking behaviour, but inheritance of these risk genes is not associated with inheritance of other genetic characteristics that influence mood. Wootton 2020 reported evidence that genetic propensity to smoke was associated with depression in adulthood, thereby providing strong support that smoking causes depression and, by implication, that stopping smoking improves mood. Another study used the same statistical approach, instrumental variable analysis, to examine whether stopping smoking improved mental health (Taylor 2019a). The instrument in this case related to varenicline use, as this medication is more effective than alternative smoking cessation treatments. Varenicline use was associated with decreased odds of receiving mental health diagnoses and prescriptions, and the instrumental variable analysis suggests this was causally associated with stopping smoking, and not by uncontrolled confounding.
Authors' conclusions
Implications for practice.
People who smoke can be reassured that stopping smoking will not worsen and may improve their mood, by reducing anxiety, depression, and stress, and boosting positive mood. It is also unlikely to worsen their social relationships.
Clinicians treating people who smoke should be reassured that encouraging and supporting smoking cessation in their patients will not worsen and may improve mood. In particular, there is no reason to fear that people with psychological disorders will have their condition worsened by smoking cessation.
Implications for research.
Further studies that examine the impact of smoking cessation on the incidence of psychological disorders would greatly strengthen the evidence on this important outcome.
Studies using designs that overcome confounding, such as instrumental variable analysis, would strengthen the evidence in this area.
Investigators should report all outcomes investigating the relationship between smoking cessation and mental health, regardless of the result and the status of the outcome.
History
Protocol first published: Issue 1, 2020 Review first published: Issue 3, 2021
Acknowledgements
We would like to thank Mr Alan Girling (University of Birmingham) for his contributions to a previous version of this review (Taylor 2014). We would like to thank Professor Ann McNeill for her contributions to the previous version of this review (Taylor 2014), and to the protocol for the current review (Taylor 2020). We would like to thank Dr Jonathan Livingstone‐Banks for conducting the searches for the review and for providing editorial advice throughout the project. We would also like to thank Dr Thomas Fanshawe for statistical support, and for generating calculators so that we could convert extracted data ready for input into the meta‐analysis. We gratefully acknowledge the review of Dr Jamie Hartmann‐Boyce, University of Oxford as Cochrane Editor; Dr Leonie Brose, Institute of Psychiatry, Psychology and Neuroscience, King’s College London and Dr Tim Bradshaw, Division of Nursing, Midwifery and Social Work, School of Health Sciences, The University of Manchester as peer reviewers, Christopher‐James Harvey as consumer reviewer, and Carolyn Hughes for her assistance drafting the Plain Language Summary. We would also like to thank all the study authors who provided additional information for this review.
This project was partially supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Tobacco Addiction Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health and Social Care.
Appendices
Appendix 1. MEDLINE search strategy
1. exp "Tobacco Use Cessation"/ or exp Smoking Cessation/ 2. smoking cessation.mp. 3. ((reduc* or modif*) adj3 (smok* or cigar* or tobacco)).mp. 4. ((quit* or stop* or give* or cease) adj3 (smok* or cigar* or tobacco)).mp. 5. Harm Reduction/ or harm reduction.mp. 6. Smoking Reduction/ 7. tobacco consumption.mp. 8. cold turkey.mp. 9. Smoking Cessation Agents/ 10. "Tobacco Use Cessation Devices"/ 11. Electronic Nicotine Delivery Systems/ 12. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 13. Mental Health/ or mental health.mp. 14. Stress, Psychological/ 15. psychological health.mp. 16. Resilience, Psychological/ or psychological resilience.mp. 17. Anxiety/ or anxiety.mp. or Anxiety Disorders/ 18. anxious.mp. 19. Depression/ or depression.mp. 20. Depressive Disorders/ or depressive.mp. 21. Emotions/ or emotion*.mp. 22. psychological process$.mp. 23. mental hygiene.mp. 24. "Quality of Life"/ or quality of life.mp. 25. (well being or well?being).mp. 26. Affect/ or affect.mp. or Affective Symptoms/ 27. Adaptation, Psychological/ 28. 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 29. 12 and 28 30. limit 29 to yr="2011 ‐Current"
Data and analyses
Comparison 1. Change in anxiety.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Main continuous data analysis | 15 | 3141 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.43, ‐0.13] |
| 1.2 Sensitivity analysis: no biochemical validation | 12 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.44, ‐0.09] | |
| 1.3 Sensitivity analysis: point prevalence or no abstinence definition | 5 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.74, ‐0.06] | |
| 1.4 Sensitivity analysis: psychoactive/psychological treatment used | 12 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.49, ‐0.21] | |
| 1.5 Subgroups: comparing clinical populations | 15 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.45, ‐0.14] | |
| 1.5.1 Chronic physical condition | 5 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.61, ‐0.07] | |
| 1.5.2 General (unselected) population | 5 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.62, 0.11] | |
| 1.5.3 Post‐surgical patients | 1 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.64, 0.10] | |
| 1.5.4 Pregnant | 1 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.41, 0.29] | |
| 1.5.5 Psychiatric condition | 3 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.66, ‐0.01] | |
| 1.6 Subgroups: motivation to quit | 15 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.43, ‐0.13] | |
| 1.6.1 Participants were selected by being motivated to quit | 10 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.45, ‐0.09] | |
| 1.6.2 Participants were not selected by being motivated to quit | 5 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.57, ‐0.02] | |
| 1.7 Subgroups: comparing adjusted & unadjusted estimates | 15 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.43, ‐0.13] | |
| 1.7.1 Adjusted estimates | 6 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.62, ‐0.10] | |
| 1.7.2 Unadjusted estimates | 9 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.38, ‐0.04] | |
| 1.8 Subgroups: comparing study designs | 15 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.43, ‐0.13] | |
| 1.8.1 Longitudinal cohort study/Non‐randomised intervention study | 5 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.54, 0.03] | |
| 1.8.2 Randomised experiment comparing people allocated to quit/continue to smoke | 1 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.68, 0.30] | |
| 1.8.3 Secondary analysis of RCT | 9 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.49, ‐0.09] | |
| 1.9 Subgroups: length of longest follow‐up | 15 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.43, ‐0.13] | |
| 1.9.1 6 weeks to ≤6 months follow‐up | 11 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.42, ‐0.06] | |
| 1.9.2 >6 months follow‐up | 4 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.62, ‐0.15] | |
| 1.10 Subgroups: primary versus secondary outcome | 15 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.43, ‐0.13] | |
| 1.10.1 Primary outcome | 9 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.46, ‐0.10] | |
| 1.10.2 Secondary outcome | 6 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.54, 0.03] | |
| 1.11 New incidence of anxiety | 2 | Odds Ratio (IV, Random, 95% CI) | 0.61 [0.34, 1.12] |
Comparison 2. Change in depression.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Main continuous data analysis | 34 | 7156 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.39, ‐0.21] |
| 2.2 Sensitivity analysis: risk of bias | 32 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.36, ‐0.19] | |
| 2.3 Sensitivity analysis: no biochemical validation | 26 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.55, ‐0.27] | |
| 2.4 Sensitivity analysis: point prevalence or no abstinence definition | 12 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.42, ‐0.19] | |
| 2.5 Sensitivity analysis: psychoactive/psychological treatment used | 21 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.42, ‐0.19] | |
| 2.6 Sensitivity analysis: differing Ns analysed | 29 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.40, ‐0.20] | |
| 2.7 Subgroups: comparing clinical populations | 34 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.41, ‐0.22] | |
| 2.7.1 Chronic physical condition | 7 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.53, ‐0.25] | |
| 2.7.2 General (unselected) population | 11 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.79, ‐0.31] | |
| 2.7.3 Post‐surgical patients | 2 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.73, 0.31] | |
| 2.7.4 Pregnant population | 3 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.41, 0.09] | |
| 2.7.5 Psychiatric condition | 11 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.32, ‐0.04] | |
| 2.8 Subgroups: motivation to quit | 34 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.39, ‐0.21] | |
| 2.8.1 Participants were selected by being motivated to quit | 25 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.45, ‐0.23] | |
| 2.8.2 Participants were not selected by being motivated to quit | 9 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.39, ‐0.06] | |
| 2.9 Subgroups: comparing adjusted & unadjusted estimates | 34 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.38, ‐0.21] | |
| 2.9.1 Adjusted estimates | 10 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.49, ‐0.19] | |
| 2.9.2 Unadjusted estimates | 24 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.39, ‐0.16] | |
| 2.10 Subgroups: comparing study designs | 34 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.39, ‐0.21] | |
| 2.10.1 Longitudinal cohort study/Non‐randomised intervention study | 11 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.36, ‐0.10] | |
| 2.10.2 Randomised experiment comparing people allocated to quit/continue to smoke | 1 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.87, 0.11] | |
| 2.10.3 Secondary analysis of RCT | 22 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.48, ‐0.24] | |
| 2.11 Subgroups: length of longest follow‐up | 34 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.38, ‐0.21] | |
| 2.11.1 6 weeks to ≤6 months follow‐up | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.40, ‐0.14] | |
| 2.11.2 >6 months follow‐up | 14 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.52, ‐0.20] | |
| 2.12 Subgroups: primary versus secondary outcome | 34 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.39, ‐0.21] | |
| 2.12.1 Primary outcome | 21 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.39, ‐0.17] | |
| 2.12.2 Secondary outcome | 13 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.58, ‐0.18] | |
| 2.13 New incidence of depression | 7 | Odds Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 3. Change in mixed anxiety and depression.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Main continuous data analysis | 8 | 2829 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.40, ‐0.22] |
| 3.2 Sensitivity analysis: no biochemical validation | 5 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.40, ‐0.21] | |
| 3.3 Sensitivity analysis: point prevalence or no abstinence definition | 3 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.46, ‐0.08] | |
| 3.4 Sensitivity analysis: psychoactive/psychological treatment used | 4 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.49, ‐0.24] | |
| 3.5 Sensitivity analysis: differing Ns analysed | 5 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.46, ‐0.12] | |
| 3.6 Subgroups: comparing clinical populations | 8 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.40, ‐0.22] | |
| 3.6.1 Chronic physical and/or psychiatric condition | 1 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.56, ‐0.02] | |
| 3.6.2 Chronic physical condition | 1 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.97, ‐0.03] | |
| 3.6.3 General (unselected) population | 5 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.40, ‐0.21] | |
| 3.6.4 Psychiatric condition | 1 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐1.07, 0.65] | |
| 3.7 Subgroups: motivation to quit | 8 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.40, ‐0.22] | |
| 3.7.1 Participants were selected by being motivated to quit | 6 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.41, ‐0.22] | |
| 3.7.2 Participants were not selected by being motivated to quit | 2 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.50, ‐0.04] | |
| 3.8 Subgroups: comparing study designs | 8 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.40, ‐0.22] | |
| 3.8.1 Longitudinal cohort study/Non‐randomised intervention study | 3 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.45, ‐0.10] | |
| 3.8.2 Secondary analysis of RCT | 5 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.42, ‐0.22] | |
| 3.9 Subgroups: length of longest follow‐up | 8 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.40, ‐0.22] | |
| 3.9.1 6 weeks to ≤6 months follow‐up | 3 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.52, ‐0.15] | |
| 3.9.2 >6 months follow‐up | 5 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.40, ‐0.20] | |
| 3.10 Subgroups: primary versus secondary outcome | 8 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.40, ‐0.22] | |
| 3.10.1 Primary outcome | 5 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.46, ‐0.14] | |
| 3.10.2 Secondary outcome | 3 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.42, ‐0.21] | |
| 3.11 New incidence of mixed anxiety and depression | 3 | 8685 | Odds Ratio (IV, Fixed, 95% CI) | 0.76 [0.66, 0.86] |
Comparison 4. Change in stress.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Main continuous data analysis | 4 | 1792 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.34, ‐0.04] |
| 4.2 Sensitivity analysis: no biochemical validation | 2 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.38, ‐0.07] | |
| 4.3 Sensitivity analysis: point prevalence or no abstinence definition | 2 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.41, ‐0.12] | |
| 4.4 Sensitivity analysis: differing Ns analysed | 3 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.36, ‐0.00] | |
| 4.5 Subgroups: comparing clinical populations | 4 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.34, ‐0.04] | |
| 4.5.1 Chronic physical condition | 1 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.40, ‐0.04] | |
| 4.5.2 General (unselected) population | 3 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.41, 0.03] | |
| 4.6 Subgroups: motivation to quit | 4 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.34, ‐0.04] | |
| 4.6.1 Participants were selected by being motivated to quit | 2 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.38, ‐0.07] | |
| 4.6.2 Participants were not selected by being motivated to quit | 2 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.49, 0.13] | |
| 4.7 Subgroups: comparing study designs | 4 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.34, ‐0.04] | |
| 4.7.1 Longitudinal cohort study/Non‐randomised intervention study | 2 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.49, 0.13] | |
| 4.7.2 Secondary analysis of RCT | 2 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.38, ‐0.07] | |
| 4.8 Subgroups: length of longest follow‐up | 4 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.34, ‐0.04] | |
| 4.8.1 6 weeks to ≤6 months follow‐up | 1 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.58, 0.08] | |
| 4.8.2 >6 months follow‐up | 3 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.36, ‐0.00] |
Comparison 5. Change in positive affect.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Main continuous data analysis | 13 | 4880 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [0.11, 0.33] |
| 5.2 Sensitivity analysis: no biochemical validation | 7 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [0.15, 0.51] | |
| 5.3 Sensitivity analysis: point prevalence or no abstinence definition | 3 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [0.06, 0.87] | |
| 5.4 Sensitivity analysis: psychoactive/psychological treatment used | 9 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [0.04, 0.22] | |
| 5.5 Sensitivity analysis: differing Ns analysed | 11 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [0.12, 0.39] | |
| 5.6 Subgroups: comparing clinical populations | 13 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [0.11, 0.33] | |
| 5.6.1 Chronic physical and/or psychiatric condition | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.04, 0.90] | |
| 5.6.2 Chronic physical condition | 3 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [0.17, 1.00] | |
| 5.6.3 Post‐surgical patients | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.05, 0.43] | |
| 5.6.4 General (unselected) population | 6 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [0.01, 0.14] | |
| 5.6.5 Psychiatric condition | 2 | Std. Mean Difference (IV, Random, 95% CI) | 0.69 [0.33, 1.06] | |
| 5.7 Subgroups: motivation to quit | 13 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [0.11, 0.33] | |
| 5.7.1 Participants were selected by being motivated to quit | 9 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [0.09, 0.31] | |
| 5.7.2 Participants were not selected by being motivated to quit | 4 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [0.02, 0.67] | |
| 5.8 Subgroups: comparing adjusted & unadjusted estimates | 13 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [0.11, 0.33] | |
| 5.8.1 Adjusted estimates | 5 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [0.01, 0.24] | |
| 5.8.2 Undjusted estimates | 8 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [0.12, 0.48] | |
| 5.9 Subgroups: comparing study designs | 13 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [0.11, 0.33] | |
| 5.9.1 Longitudinal cohort study/Non‐randomised intervention study | 6 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [0.01, 0.42] | |
| 5.9.2 Seconary analysis of RCT | 7 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [0.11, 0.37] | |
| 5.10 Subgroups: length of longest follow‐up | 13 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [0.11, 0.33] | |
| 5.10.1 6 weeks to ≤6 months follow‐up | 4 | Std. Mean Difference (IV, Random, 95% CI) | 0.60 [0.27, 0.93] | |
| 5.10.2 >6 months follow‐up | 9 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [0.04, 0.22] | |
| 5.11 Subgroups: primary versus secondary outcome | 13 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [0.11, 0.33] | |
| 5.11.1 Primary outcome | 3 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.13, 0.97] | |
| 5.11.2 Secondary outcome | 10 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [0.10, 0.32] |
Comparison 6. Change psychological quality of life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Main continuous data analysis | 19 | 18034 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [0.06, 0.16] |
| 6.2 Sensitivity analysis: no biochemical validation | 9 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.02, 0.24] | |
| 6.3 Sensitivity analysis: point prevalence or no abstinence definition | 3 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.03, 0.63] | |
| 6.4 Sensitivity analysis: psychoactive/psychological treatment used | 15 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [0.06, 0.18] | |
| 6.5 Sensitivity analysis: differing Ns analysed | 18 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [0.06, 0.17] | |
| 6.6 Subgroups: comparing clinical populations | 19 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [0.06, 0.16] | |
| 6.6.1 Chronic physical and/or psychiatric condition | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.60 [0.17, 1.03] | |
| 6.6.2 Chronic physical condition | 5 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.03, 0.29] | |
| 6.6.3 General (unselected) population | 9 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [0.03, 0.14] | |
| 6.6.4 Post‐surgical patients | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.17, 0.31] | |
| 6.6.5 Psychiatric condition | 3 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.12, 0.31] | |
| 6.7 Subgroups: motivation to quit | 19 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [0.06, 0.16] | |
| 6.7.1 Participants were selected by being motivated to quit | 10 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [0.05, 0.15] | |
| 6.7.2 Participants were not selected by being motivated to quit | 9 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [0.03, 0.24] | |
| 6.8 Subgroups: comparing adjusted & unadjusted estimates | 19 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [0.06, 0.16] | |
| 6.8.1 Adjusted estimates | 8 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [0.06, 0.20] | |
| 6.8.2 Unadjusted estimates | 11 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.01, 0.18] | |
| 6.9 Subgroups: comparing study designs | 19 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [0.06, 0.16] | |
| 6.9.1 Longitudinal cohort study/Non‐randomised intervention study | 12 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [0.04, 0.20] | |
| 6.9.2 Secondary analysis of RCT | 7 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [0.03, 0.18] | |
| 6.10 Subgroups: length of longest follow‐up | 19 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [0.06, 0.16] | |
| 6.10.1 6 weeks to ≤6 months follow‐up | 6 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [0.05, 0.19] | |
| 6.10.2 >6 months follow‐up | 13 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [0.03, 0.18] | |
| 6.11 Subgroups: primary versus secondary outcome | 19 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [0.06, 0.16] | |
| 6.11.1 Primary outcome | 5 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [0.04, 0.24] | |
| 6.11.2 Secondary outcome | 14 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [0.02, 0.18] |
Comparison 7. Change in social quality of life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Main continuous data analysis | 9 | 14673 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [0.00, 0.06] |
| 7.2 Sensitivity analysis: no biochemical validation | 5 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.07, 0.15] | |
| 7.3 Sensitivity analysis: point prevalence or no abstinence definition | 2 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.10, 0.36] | |
| 7.4 Sensitivity analysis: psychoactive/psychological treatment used | 5 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.03, 0.16] | |
| 7.5 Sensitivity analysis: differing Ns analysed | 7 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [0.00, 0.07] | |
| 7.6 Subgroups: comparing clinical populations | 9 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [0.00, 0.06] | |
| 7.6.1 Chronic physical condition | 3 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.09, 0.34] | |
| 7.6.2 General (unselected) population | 4 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.04, 0.10] | |
| 7.6.3 Post‐surgical patients | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.18, 0.30] | |
| 7.6.4 Psychiatric condition | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.01, 0.11] | |
| 7.7 Subgroups: motivation to quit | 9 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [0.00, 0.06] | |
| 7.7.1 Participants were selected by being motivated to quit | 6 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [0.00, 0.11] | |
| 7.7.2 Participants were not selected by being motivated to quit | 3 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.02, 0.06] | |
| 7.8 Subgroups: comparing adjusted & unadjusted estimates | 9 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [0.00, 0.06] | |
| 7.8.1 Adjusted estimates | 3 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.01, 0.10] | |
| 7.8.2 Unadjusted estimates | 6 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.02, 0.10] | |
| 7.9 Subgroups: comparing study designs | 9 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [0.00, 0.06] | |
| 7.9.1 Secondary analysis of RCT | 4 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.00, 0.10] | |
| 7.9.2 Longitudinal cohort study/Non‐randomised intervention study | 5 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.01, 0.06] | |
| 7.10 Subgroups: length of longest follow‐up | 9 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [0.00, 0.06] | |
| 7.10.1 6 weeks to ≤6 months follow‐up | 3 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.01, 0.10] | |
| 7.10.2 >6 months follow‐up | 6 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.02, 0.10] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anthenelli 2013.
| Study characteristics | ||
| Methods |
Study design: RCT Country: Bosnia and Herzegovina, Croatia, Germany, Hungary, Romania, Russian Federation, Spain, United States Data collection period: March 2010 – June 2012 Registry ID: ClinicalTrials.gov: NCT01078298 |
|
| Participants |
Number of participants: N = 525 Sample characteristics (at baseline): Age (mean): 45.4 years (SD 10.9) varenicline group, 47.1 years (SD 10.8) placebo group; Sex (% male): 37.3% (196/525) Population category: psychiatric population; Specific population: persons with stably‐treated current or past major depressive disorder (MDD) Nicotine dependence: FTND 5.9 (SD 1.9) varenicline group, 5.9 (SD 2.0) placebo group; Baseline cigarettes per day: 22 cigarettes daily in the past month (varenicline group 21.9 (SD 7.5), placebo group 21.5 (SD 8.7)); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: manual‐guided smoking cessation counselling developed by a study author in accordance with Agency for Healthcare Research and Quality guidelines – provided at each clinic and telephone visit from baseline through to week 52 Pharmacological support for smoking cessation: varenicline or placebo Psychotherapeutic or psychoactive support for mental health or mood: received mood management ‐ most participants (varenicline 70.7%, placebo 73.2%) were receiving antidepressant medications at study entry; selective serotonin reuptake inhibitors and serotonin–norepinephrine reuptake inhibitors the predominant choice (varenicline, 61.3%; placebo, 67.7%), other most common psychotropic treatment was alprazolam (varenicline, 8.6%; placebo, 13.4%) |
|
| Outcomes |
Definition of cessation used: continuous abstinence rates week 9 – 12; self‐reported 7‐day point‐prevalence of abstinence at weeks 12, 24 and 52 Cessation definition used for outcome(s) in this analysis: continuous abstinence Measure of biovalidation: exhaled CO levels (≤ 10 ppm) Definition of people who continued smoking used: ‘non‐quitters’ (exact definition not reported), carbon monoxide levels > 10 ppm Time point(s) at which follow‐up was conducted: baseline, every week during treatment phase and weeks 13, 14, 16, 20, 24, 28, 43, 36, 40, 44, 48 and 52 Outcome category: Depression Outcome measure(s): Montgomery‐Asberg Depression Rating Scale (MADRS) |
|
| Funding source | Study was funded by Pfizer. Dr. Anthenelli’s writing of this manuscript was funded, in part, by a Department of Veterans Affairs Merit Review award (NEUA‐003‐08S) and by a National Institute on Alcohol Abuse and Alcoholism grant (AA019720). Dr. Morris was supported, in part, by grants from the University of California, San Francisco, Smoking Cessation Leadership Center and Colorado Department of Public Health and Environment. Drs. Ramey, Tsilkos, Russ, and Yunis and Ms. Dubrava are employees of Pfizer. Editorial support was provided by Abegale Templar, PhD, of Engage Scientific and funded by Pfizer | |
| Author conflicts of interest | Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNumM13‐0777 | |
| Notes | Outcome data source: additional data requested, but this was not received by the deadline and we were unable to include it in this version of the review | |
Aversa 2013.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated Registry ID: VA Cooperative study #519 |
|
| Participants |
Number of participants: N = 943 Sample characteristics (at baseline): Age (mean): 54.1 years (SD 8.7); Sex (% male): 94% (886/943) Population category: psychiatric population; Specific population: veterans with PTSD Nicotine dependence: not measured; Baseline cigarettes per day: 21.7 (SD 10.5); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: integrated cognitive behavioural smoking cessation intervention into participant’s individual PTSD psychotherapy, or treatment as usual (TAU) where participants received separate standardised smoking cessation and individual PTSD treatment Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: received mood management ‐ PTSD psychotherapy ‐ type and amount of PTSD treatment received (i.e. evidenced‐based) were not reported and monitored for as part of the paper |
|
| Outcomes |
Definition of cessation used: prolonged abstinence (PA) ‐ defined as 1 year of self‐reported (via average cigarettes per day over past 3 months) and bioverified abstinence without relapse between the 6‐month to 18‐month follow‐up visits Cessation definition used for outcome(s) in this analysis: prolonged abstinence Measure of biovalidation: expired CO of < 8 ppm and urine cotinine level of < 100 ng/ml Definition of people who continued smoking used: not having prolonged abstinence (PA) Time point(s) at which follow‐up was conducted: pre‐treatment, post‐treatment (18‐months post‐enrolment) and every 3 months in between Outcome category: Positive Affect, Psychological Quality of Life (QoL), Social Outcome Outcome measure(s): Veterans Short‐Form (VR – 36; vitality, mental health, social functioning) |
|
| Funding source | Funding was provided by the Cooperative Studies Program of the Clinical Science Research and Development Service, U.S. Department of Veterans Affairs (DVA; CSP #519, NCT00118534) and the Tobacco‐Related Disease Research Program 19DT‐0003. Dr. Baker receives research support from the Department of Defense (Navy BUMED and CDMRP PTO 090738) and the DVA (HSR&D SDR09‐128) and is supported in part by the VA Center of Excellence for Stress and Mental Health. Dr. McFall receives research support from VA Merit #821 and DVA. Dr. Saxon receives research support from NIAAA (1 P20 AA017839‐01), NIDA (5 U10 DA013714‐08), and from VA HSR&D (1 IO1 HX000616‐01) | |
| Author conflicts of interest | The authors report no financial relationships with commercial interests | |
| Notes | Outcome data source: Published data | |
Avery 2014.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 230 Sample characteristics (at baseline): Age (mean): 55.6 years (SD 6.7); Sex (% male): 0% (0/230) Population category: general population; Specific population: postmenopausal women Nicotine dependence: not reported; Baseline cigarettes per day: 18.3 (SD 9.9); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: therapeutic sessions with motivational and cognitive behavioural techniques (NRT study); supervised relaxation programme (REST study) Pharmacological support for smoking cessation: assigned to use 21 mg nicotine or placebo patch for 3 months (NRT study); varenicline treatment for 12 weeks (REST study) Psychotherapeutic or psychoactive support for mental health or mood: received mood management – participants in the REST study undertook a supervised relaxation programme |
|
| Outcomes |
Definition of cessation used: self‐reported and biovalidated 7‐day point‐prevalence abstinence Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: exhaled CO level of ≤ 8 ppm; participants with discrepancies were coded as people who smoke Definition of people who continued smoking used: people who smoke – not reported abstinence in last 7 days; missed visits coded as people who smoke Time point(s) at which follow‐up was conducted: baseline, 6 and 12 weeks Outcome category: Depression Outcome measure(s): Center for Epidemiologic Studies Depression Scale (CESD; 10‐item) |
|
| Funding source | The Patrick and Catherine Weldon Donaghue Foundation, The University of Connecticut Center on Aging, and NIH grants R01 DA13334, R01DA024872, and M01 RR06192 (University of Connecticut General Clinical Research Center) and P50AA15632. GlaxoSmithKline Pharmaceuticals donated nicotine and placebo patches | |
| Author conflicts of interest | Authors declared no competing financial interests exist | |
| Notes | Outcome data source: Published data | |
Becoña 2002.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: Spain Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 214; Number included in meta‐analysis: N = 200 Sample characteristics (at baseline): Age (mean): 37.3 years (SD 10.3); Sex (% male): 53% (106/200) Population category: general population; Specific population: people seeking treatment for smoking who do not use NRT, other drugs, or receive psychotherapy Nicotine dependence: FTND 5.4 (SD 2.4); Baseline cigarettes per day: 26.4 (SD 10.4); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: multicomponent behavioural programme delivered in 6 weekly behavioural support sessions, including nicotine fading, withdrawal symptom avoidance and relapse prevention strategies Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: self‐reported point‐prevalence abstinence – immediately after programme (not smoking in previous 24 hours) and at the 3‐, 6‐ and 12‐month follow‐ups (not smoking previous 7 days; self‐reported continuous abstinence – at the 3‐, 6‐ and 12‐month follow‐ups (i.e. not smoking since initial quitting) Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: expired CO (< 9 ppm) Definition of people who continued smoking used: not stated Time point(s) at which follow‐up was conducted: immediately after programme (no smoking in previous 24 hours) and at 3‐, 6‐ and 12‐month follow‐ups (no smoking in previous 7 days) Outcome category: Anxiety Outcome measure(s): State Trait Anxiety Inventory (STAI) |
|
| Funding source | None specified | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Becoña 2017.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: Spain Data collection period: January 2016 – April 2018 Registry ID: ClinicalTrials.gov: NCT02844595 |
|
| Participants |
Number of participants: N = 275; Number included in meta‐analysis: N = 127 Sample characteristics (at baseline): Age (mean): SCBSCT‐BA 45.2 years (SD 11.2), SCBSCT 44.6 years (SD 10.7), WL 47.1 years (SD 10.6); Sex (% male): SCBSCT‐BA 39.1% (43/110), SCBSCT 36.7% (40/109), WL 41.1% (23/56) Population category: general population; Specific population: adults who smoke Nicotine dependence: Fagerström Test for Cigarette Dependence SCBSCT‐BA 4.6 (SD 2.1), SCBSCT 5.0 (SD 2.2), WL 4.9 (SD 2.1); Baseline cigarettes per day: SCBSCT‐BA 18.9 (SD 7.3), SCBSCT 19.3 (SD 7.4), WL 19.0 (SD 7.2); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: cognitive behavioural smoking cessation treatment with components of behavioural activation (SCBSCT‐BA), standard cognitive‐behavioral treatment for smoking cessation (SCBSCT) and wait‐list control group (WL) Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: received mood management ‐ SCBSCT‐BA included analysis of the relationship between behaviour and mood, identification of situations and behaviours that worsen mood, identification of avoidance behaviours and rumination, self‐report of pleasant daily activities, and pleasant activity scheduling to increase engagement in non‐smoking‐related rewarding activities |
|
| Outcomes |
Definition of cessation used: 7‐day point‐prevalence abstinence Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: expired CO reading of ≤ 9 ppm Definition of people who continued to smoke used: self‐reported smoking; self‐reported abstinence but carbon monoxide ≥ 10 ppm Time point(s) at which follow‐up was conducted: end of treatment, 3‐, 6‐ and 12‐month follow‐ups Outcome category: Depression Outcome measure(s): Beck Depression Inventory II (BDI‐II) |
|
| Funding source | Supported by the Spanish Ministry of Economy and Competiveness (Project reference: PSI2015‐66755‐R) and co‐financed by FEDER (European Regional Development Fund; pluri‐annual plan 2014‐2020) | |
| Author conflicts of interest | Authors declared that no competing interests exist | |
| Notes | Outcome data source: Published and unpublished data | |
Berlin 2010.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: October 1993 – January 1997 Registry ID: not reported |
|
| Participants |
Number of participants: N = 134; Number included in meta‐analysis: 133 Sample characteristics (at baseline): Age (mean): 44.5 years (SD 10.7); Sex (% male): 37% (49/133) Population category: psychiatric population; Specific population: people with depression (DSM‐III‐R) who had entered remission > 6 m prior to start of study Nicotine dependence: FTQ, people who quit 6.6 (SD 2.8), people who continued to smoke 6.7 (SD 2.4); Baseline cigarettes per day: people who quit 23.3 (SD 8.6), people who continued to smoke 26 (SD 10.6); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: behavioural support and counselling, incorporating standard smoking cessation techniques (i.e. benefits of cessation, avoiding relapse) Pharmacological support for smoking cessation: either sertraline or placebo (in combination with behavioural support) Psychotherapeutic or psychoactive support for mental health or mood: received mood management ‐ mood management counselling (supportive approach to help people who smoke to recognise, express and manage negative affects related to quit effort); some participants received sertraline |
|
| Outcomes |
Definition of cessation used: point‐prevalence abstinence ‐ defined as abstinence from smoking during the previous 7 days; continuous abstinence ‐ defined as complete 7 days’ abstinence from quit day until end of study (biochemically verified, from week 4 – week 11) Cessation definition used for outcome(s) in this analysis: continuous abstinence Measure of biovalidation: serum cotinine concentration ≤ 15 ng/ml Definition of people who continued to smoke used: participants whose diary was not available were considered to still be smoking; did not report abstinence in last 7 days Time point(s) at which follow‐up was conducted: every week (point‐prevalence abstinence); week 11 (end of study; continuous abstinence), data included in this study is from week 11 Outcome category: Depression Outcome measure(s): Hamilton Depression Rating Scale |
|
| Funding source | Secondary analysis partially funded by investigator‐initiated research agreement awarded by Pfizer Ltd to the Research Foundation for Mental Hygiene (Principle Investigator: Lirio Covey) | |
| Author conflicts of interest | I. Berlin reports having received occasional honoraria for participating in advisory panels of Sanofi‐Aventis and Pfizer Ltd during the last 3 years. Lirio S. Covey received research support from Pfizer, Inc. None of the authors have any connection with tobacco, alcohol or gaming industries | |
| Notes | Outcome data source: Published data | |
Blalock 2008.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated Registry ID: NCI‐2012‐02097 |
|
| Participants |
Number of participants: N = 21; Number included in meta‐analysis: N = 21 Sample characteristics (at baseline): Age (mean): 48.0 years (SD 12.1); Sex (% male): 19% (4/21) Population category: psychiatric population; Specific population: people with depressive disorders (DSM‐IV/CES‐D/psychotropic medication) Nicotine dependence: FTND people who quit 3.9 (SD 1.6), people who continued smoking 5.6 (SD 2.4); Baseline cigarettes per day: people who quit 23.1 (SD 7.0), people who continued smoking 24.0 (SD 5.3); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: behavioural support or mood management counselling Pharmacological support for smoking cessation: participants in both groups were provided with 6‐weeks supply of the 21‐mg nicotine patch, starting on the quit date, followed by 2 weeks each of the 14‐mg and 7‐mg nicotine patches Psychotherapeutic or psychoactive support for mental health or mood: received mood management ‐ behavioural counselling (based on relapse prevention) or mood management counselling (based on cognitive behavioural analysis system of psychotherapy ‐ CBASP) |
|
| Outcomes |
Definition of cessation used: continuous abstinence ‐ defined as self‐reported sustained abstinence beginning 2 weeks after quit date, through the 3‐month follow‐up assessment Cessation definition used for outcome(s) in this analysis: continuous abstinence Measure of biovalidation: expired CO level of ≤ 10 parts per million at each treatment visit following a 2‐week grace period and at the 3‐month follow‐up Definition of people who continued to smoke used: non‐abstainers (i.e. participants not meeting criteria for abstinence) Time point(s) at which follow‐up was conducted: baseline (before first treatment visit), each weekly treatment session and 3 months after baseline Outcome category: Depression, Mixed Anxiety and Depression, Positive Affect Outcome measure(s): Beck Depression Inventory (BDI), Positive and Negative Affect Scale (PANAS) |
|
| Funding source | University of Texas M. D. Anderson Cancer Center Institutional Research Grant | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published and unpublished data | |
Bloom 2015.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 134; Number included in meta‐analysis: N = 134 Sample characteristics (at baseline): Age (mean): 58.6 years (SD 11.2); Sex (% male): 56.7% (76/134) Population category: postoperative; Specific population: cancer patients scheduled for surgery Nicotine dependence: FTND 5.8 (SD 2.2); Baseline cigarettes per day: 24.2 (SD 11.7); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: participants who self‐reported 7‐day abstinence at all 4 follow‐ups classified as abstinent ‐ any other pattern not classified as abstinent Cessation definition used for outcome(s) in this analysis: continuous abstinence Measure of biovalidation: self‐reports confirmed via exhaled carbon monoxide from a subsample who reported abstinence and were seen at a hospital visit Definition of people who continued to smoke used: did not report 7‐day abstinence at one or more follow‐ups Time point(s) at which follow‐up was conducted: telephone follow‐up assessments occurred 2‐, 4‐, 6‐ and 12‐months post‐surgery; head and neck (HN) patients completed baseline at pre‐operative appointment (usually 1 week prior to surgery); thoracic (TH) patients completed baseline in their hospital room (generally 2 ‐ 3 days post‐surgery) Outcome category: Depression Outcome measure(s): Center for Epidemiologic Studies Depression Scale (CES‐D) |
|
| Funding source | Supported by the National Cancer Institute R03 CA 126409 and in part by the Biostatistics Core Facility at the H. Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center (P30‐CA76292) | |
| Author conflicts of interest | There are no financial disclosures for any of the authors | |
| Notes | Outcome data source: Published data | |
Bloom 2017.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 61; Number included in meta‐analysis: N = not clear (somewhere between 48 and 51) Sample characteristics (at baseline): Age (mean): aerobic exercise (AE) group 47.1 years (SD 8.5), health education (HEC) group 47.5 years (SD 10.7); Sex (% male): AE 36.7% (11/30), HEC 32.3% (10/31) Population category: general population; Specific population: adults who smoke and not recently engaged in aerobic exercise Nicotine dependence: FTND AE 5.9 (SD 2.1), HEC 5.6 (SD 1.6); Baseline cigarettes per day: AE 20.3 (SD 9.9), HEC 19.4 (SD 8.1); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: all participants (AE and HEC) received 8 sessions of telephone counselling for smoking cessation Pharmacological support for smoking cessation: all participants received 8 weeks of transdermal nicotine patch (TNP) Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: self‐reported 7‐day point prevalence abstinence at follow‐ups Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: expired carbon monoxide (CO; < 10 ppm cut‐off); in 1 instance, by the report of a family member of the participant because the participant was unable to provide CO Definition of people who continued to smoke used: exact definition not reported; ‘continued smoking’, carbon monoxide > 10 ppm Time point(s) at which follow‐up was conducted: 3 months (end of treatment), 6 months, and 12‐month follow‐up after baseline Outcome category: Positive Affect; Psychological Quality of Life (QoL) Outcome measure(s): QoL Enjoyment and Satisfaction Questionnaire – Short Form (Q‐LES‐Q‐SF; well‐being and satisfaction domains) |
|
| Funding source | Supported by the National Institute on Drug Abuse (grant number K23 DA019950) awarded to Ana M. Abrantes, PhD | |
| Author conflicts of interest | No potential conflict of interest was reported by the authors | |
| Notes | Outcome data source: Published data | |
Bock 2012.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 55; Number included in meta‐analysis: N = 55 Sample characteristics (at baseline): Age (mean): 45.6 years (SD 8.3); Sex (% male): 0% (0/55) Population category: general population; Specific population: women who smoke Nicotine dependence: FTQ 5.0 (SD 1.4); Baseline cigarettes per day: 16 (SD 7.3); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: all participants were given a weekly 1‐hour group‐based CBT for smoking cessation session Pharmacological support for smoking cessation: participants were not provided with nicotine replacement therapy or other smoking cessation medications but were allowed to use them in conjunction with the programme if advised to do so by primary care physician Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: primary smoking outcome variable was 7‐day point‐prevalence abstinence (7PPA) Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: 7PPA verified by saliva cotinine level less than 57 nmol/L (15 ng/mL) at the end of treatment (week 8) and all follow‐up assessments Definition of people who continued to smoke used: ‘smoking’ ‐ no 7PPA Time point(s) at which follow‐up was conducted: baseline, end of treatment (week 8) and 3‐ and 6‐month follow‐up Outcome category: Depression, Psychological Quality of Life (QoL) and Anxiety Outcome measure(s): Center for Epidemiological Studies Depression Scale (CES‐D‐10); Short‐Form Health Survey (SF‐36); State Trait Anxiety Inventory – Trait (STAI‐T) |
|
| Funding source | This study was funded by a grant from the National Center for Complementary and Alternative Medicine (NCCAM) to Dr. Bock (AT003669) | |
| Author conflicts of interest | No competing financial interests exist for any of the authors | |
| Notes | Outcome data source: Published data | |
Buchanan 2015.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: USA Data collection period: January 2003 – June 2004, June 2005 – December 2008 Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 4003; Number included in meta‐analysis: N = 1484 Sample characteristics (at baseline): Age (mean): never smoked 62.8 years (SD 13.3), people who previously smoked 64.1 years (SD 10.7), people who recently quit 54.6 years (SD 9.8), people who continued to smoke 54.6 years (SD 9.8); Sex (% male): never smoked 55.4% (634 /1145), people who previously smoked 74.9% (1029/1374), people who recently quit 71.2% (486/683), people who continued smoking 66.7% (534/801) Population category: chronic physical condition; Specific population: people hospitalised with acute myocardial infarction (AMI) Nicotine dependence: not measured; Baseline cigarettes per day: not measured; Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: at 1‐year those who responded they had quit in the past year were designated as 'recent quitters' Cessation definition used for outcome(s) in this analysis: unclear point‐prevalence Measure of biovalidation: not bioverified Definition of people who continued to smoke used: reported not quit in the past year i.e. ‘persistent smokers’ Time point(s) at which follow‐up was conducted: 1, 6 and 12 months after AMI Outcome category: Psychological Quality of Life (QoL) Outcome measure(s): Medical Outcomes Study 12‐item Short Form (SF‐12; Mental Component Summary) |
|
| Funding source | Funding support was received for the Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER) Registry from CV Therapeutics, Inc and for the Translational Research Investigating Underlying disparities in acute Myocardial infarction Patient’s Health status (TRIUMPH) Registry from the National Heart Lung and Blood Institute. S. Cresci’s effort is supported in part by the National Institutes of Health | |
| Author conflicts of interest | (Disclosures) J.A. Spertus owns the copyright for the Seattle Angina Questionnaire | |
| Notes | Outcome data source: Published data | |
Busch 2011.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 212; Number included in meta‐analysis: N = 131 Sample characteristics (at baseline): Age (mean): 32.9 years (SD 8.6); Sex (% male): 10.4% (22/212) Population category: general population; Specific population: caregivers who smoke and have children with asthma Nicotine dependence: FTND 4.6 (SD 1.4); Baseline cigarettes per day: 15.1 (SD 8.6); Motivation to quit: not selected by motivation to quit (“drawn from a cessation induction trial i.e. included smokers who were not motivated to quit”) |
|
| Interventions |
Behavioural support for smoking cessation: nurse‐delivered smoking cessation intervention, embedded within in‐home asthma education programme: a motivational enhancement treatment based on Precaution Adoption Model or a treatment based on the Behavioural Action Model Pharmacological support for smoking cessation: participants who reported readiness to quit were provided NRT (patches) Psychotherapeutic or psychoactive support for mental health or mood: some participants received standard support, and some received mood management |
|
| Outcomes |
Definition of cessation used: point‐prevalence abstinence of 7 or more days (self‐report) Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: expired air carbon monoxide test (abstinent if ≤ 10 ppm) Definition of people who continued to smoke used: people who smoked (either abstinence for < 7 d, or abstinence reported but CO measurement > 10 ppm) Time point(s) at which follow‐up was conducted: end of treatment (EOT); EOT + 2 m, EOT + 6 m, EOT + 12 m Outcome category: Depression Outcome measure(s): Center for Epidemiologic Studies Depression Scale (CES‐D) |
|
| Funding source | NIH grant R01 HL‐062165 to Belinda Borrelli National Institute of Health | |
| Author conflicts of interest | All authors declared no conflicts of interest | |
| Notes | Outcome data source: Published data | |
Carey 1993.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: USA Data collection period: not stated Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 308 Sample characteristics (at baseline): Age (mean): 42.0 years (SD 12.7); Sex (% male): 46% Population category: general population; Specific population: general population Nicotine dependence: Fagerström Tolerance Questionnaire (FTQ), confirmed quitters 5.5 (SD 2.1); non‐quitters 6.5 (SD 1.7); Baseline cigarettes per day: 27 (SD 11.5); Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: self‐reported 7‐day point‐prevalence abstinence (7 days prior to assessments) Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: saliva cotinine verified Definition of people who continued to smoke used: relapser – currently smoking but had been abstinent for at least 24 hours; person who smokes – currently smoking with no periods of abstinence exceeding 24 hours; people lost to follow‐up were assumed to be smoking Time point(s) at which follow‐up was conducted: 1, 6 and 12 months after baseline Outcome category: Stress Outcome measure(s): Perceived Stress Scale: Short Form (PSS‐4) |
|
| Funding source | American Cancer Society, National Institute on Drug Abuse | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Carroll 2019.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: May 2013 – June 2017 Registry ID: ClinicalTrials.gov: NCT01756885 (primary RCT) |
|
| Participants |
Number of participants: N = 119; Number included in meta‐analysis: not clear for PANAS‐N (somewhere between 82 and 132), N = 117 for PANAS‐P Sample characteristics (at baseline): Age (mean): non‐adherent 58.6 years (SD 9.0), adherent 59.4 years (SD 8.4); Sex (% male): non‐adherent 48% (11/23), adherent 51% (49/96) Population category: chronic physical condition; Specific population: diagnosed with cancer within 5 years Nicotine dependence: FTCD non‐adherent 4.7 (SD 2.5), adherent 4.4 (SD 2.1); baseline cigarettes per day: not presented; Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: smoking cessation counselling (based on PHS guidelines) Pharmacological support for smoking cessation: varenicline following standard dosing (0.5 mg once per day days 1 – 3, 0.5 mg twice per day days 4 – 7, increasing to 1.0 mg twice per day for remainder of treatment) Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: 24‐hour point‐prevalence abstinence (i.e. not a cigarette, even a puff in past 24 hours) Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: not bioverified Definition of people who continue to smoke used: smoking a cigarette in previous 24 hours Time point(s) at which follow‐up was conducted: week 1 (target quit day), week 4, week 8 and week 12 after pre‐quit baseline (week 0) Outcome category: Mixed Depression and Anxiety, Positive Affect Outcome measure(s): Positive and Negative Affect Schedule (PANAS; negative and positive affect subscales) |
|
| Funding source | This research was supported by grants from the National Cancer Institute (R01 CA165001; R01 CA184211) and the National Institute on Drug Abuse (K24 DA045244). NCI and NIDA had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication | |
| Author conflicts of interest | Dr. Schnoll receives medication and placebo free from Pfizer; Dr. Schnoll has provided consultation to Pfizer and GlaxoSmithKline, and consults with Curaleaf; all other authors declare that they have no conflicts of interest | |
| Notes | Outcome data source: Published and unpublished data | |
Cather 2017.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: New Zealand Data collection period: March 2008 – May 2013 Registry ID: ClinicalTrials.gov: NCT00621777 |
|
| Participants |
Number of participants: N = 179 Sample characteristics (at baseline): Age (mean): 47.9 years (SD 10.4) (≥ 2 weeks abstinence at week 12), 46.9 years (SD 10.0) (< 2 weeks abstinence at week 12), 47.2 years (SD 10.0) (early discontinuation); Sex (% male): 67.6% (50/74) (≥ 2 weeks), 64.7% (22/34) (< 2 weeks), 54.9% (39/71) (ED) Population category: psychiatric population; Specific population: people with schizophrenia or schizoaffective disorder, depressed type (SSD) Nicotine dependence: FTND 5.9 (SD 2.0) (≥ 2 weeks), 6.4 (SD 1.8) (< 2 weeks), 6.3 (SD 1.7) (ED); Baseline cigarettes per day: 20.6 (SD 12.7) (≥ 2 weeks), 24.1 (SD 10.8) (< 2 weeks), 25.2 (SD 11.9) (ED); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: manualised group CBT for smoking cessation tailored for people who smoke with severe mental illness Pharmacological support for smoking cessation: varenicline 0.5 mg daily for 3 days, 0.5 mg twice daily for 4 days and 1 mg twice daily for 11 weeks Psychotherapeutic or psychoactive support for mental health or mood: at enrolment nearly 60% were taking an antidepressant, 32% were taking an anxiolytic and all were taking antipsychotic medication i.e. received mood management |
|
| Outcomes |
Definition of cessation used: point‐prevalence abstinence ‐ defined as at least 2 weeks of continuous abstinence at week 12 (operationalised as self‐report of smoking no cigarettes in previous week) Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: expired CO < 9 ppm (week 11 and 12), semi‐qualitative urinary cotinine analysis (week 12) Definition of people who continue to smoke used: ‘non‐abstinent’ defined as < 2 weeks continuous abstinence at week 12; carbon monoxide > 9ppm Time point(s) at which follow‐up was conducted: weekly during treatment and 12 weeks after baseline Outcome category: Depression Outcome measure(s): Calgary Depression Scale for Schizophrenia (CDSS) |
|
| Funding source | This work was funded by the National Institute of Health ‐ R01 DA021245 (Evins) and K24 DA030443 (Evins) | |
| Author conflicts of interest | Dr. Evins, within the past 5 years has received research study support to her institution from Pfizer, Forum Pharmaceuticals, GlaxoSmithKline (GSK), National Institute of Mental Health (NIMH), National Institute on Drug Abuse (NIDA), and Patient‐Centered Outcomes Research Institute (PCORI) and has provided consulting and/or advisory board services to Pfizer and Reckitt Benckiser. Dr. Achtyes has received research grant support from AssurEx, Janssen, Michigan State University, Pine Rest Foundation, Priority Health, University of Chicago, American Recovery and Reinvestment Act (ARRA), Avanir, Centers for Medicare and Medicaid Services (CMMS), Dartmouth College, Janssen, National Institute on Alcohol Abuse and Alcoholism (NIAAA), NIDA, NIMH, North Shore Long Island Jewish Health System, Otsuka, Pfizer, Vanguard Research Group, University of Texas Southwestern. He has served on advisory boards to Roche and Vanguard Research Group. No other authors have conflicts of interest to declare | |
| Notes | Outcome data source: Published data | |
Cavazos‐Rehg 2014.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: USA Data collection period: 2001 – 2002 (Wave I) and 3‐year follow‐up (Wave II) Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 4853; Number included in meta‐analysis: N = 2873 Sample characteristics (at baseline): Age (mean): mean not presented, 18 – 29 years (24.2%), 30 – 44 years (34.4%), 45 ‐ 64 years (33.6%), ≥ 65 years (7.9%); Sex (% male): 52.2% (2223/4853) Population category: general population; Specific population: general population (adults 18 years +) Nicotine dependence: not measured; Baseline cigarettes per day: 18.9 (95% CI 18.5 – 19.3); Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: people who smoked daily at Wave 1 were classified into 10 ‐ 49% reduction, 50 ‐ 99% reduction or 100% reduction in cigarettes smoked at Wave 2 Cessation definition used for outcome(s) in this analysis: unclear point‐prevalence Measure of biovalidation: not bioverified Definition of people who continue to smoke used: not defined Time point(s) at which follow‐up was conducted: 3 years after baseline (Wave I) Outcome category: Mixed Anxiety and Depression Outcome measure(s): DSM‐IV defined mood or anxiety disorders |
|
| Funding source | This publication was made possible by grants from the National Center for Research Resources (NCRR; grants no. UL1 RR024992 and KL2 RR024994), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research and by grant no. K02DA021237 from the NIH. Other support includes an American Cancer Study (ACS) grant no. IRG‐58‐010‐54, NIH Career Development Award (NIDA, no. K01DA025733) and an additional NIDA (no. R01 DA032843 awarded to P.A.C.‐R.), and NIH Midcareer Investigator Awards to L.J.B. (K02 DA021237 awarded to L.J.B and no. R01 DA031288 awarded to R.A.G.) | |
| Author conflicts of interest | L.J.B. is listed as an inventor on issued US Patent no. 8,080,371, ‘Markers for Addiction’ covering the use of certain single nucleotide polymorphisms (SNPs) in determining the diagnosis, prognosis and treatment of addiction. D.H. received a grant from Nabi Biopharmaceuticals to test a nicotine immunotherapy | |
| Notes | Outcome data source: Published data | |
Chassin 2002.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: USA Data collection period: recruitment 1980 ‐1983; follow‐up in 1987, 1993 and 1999 (only 1993 and 1999 included in this study) Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 5395; Number included in meta‐analysis: N = 308 Sample characteristics (at baseline): Age: median 27 years; Sex (% male): 47.7% (2573/5395) Population category: general population; Specific population: consenting 6th – 12th graders at country schools in Midwest Nicotine dependence: not measured; Baseline cigarettes per day: people who quit median 10 – 14, people who smoke median 10 ‐ 14; Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support provided Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: continuous abstinence for at least 1 year (self‐report) Cessation definition used for outcome(s) in this analysis: continuous abstinence Measure of biovalidation: not bio‐verified Definition of people who continued to smoke used: those participants who reported having formerly smoked in 1993, but who smoked monthly or more in 1999; or those who reported at least monthly smoking in both 1993 and 1999 Time point(s) at which follow‐up was conducted: measured at time 1 (1993) and time 2 (1999) Outcome category: Mixed Anxiety and Depression, Stress Outcome measure(s): Mental Health Inventory (MHI; negative affect scale), Stress (non‐standardised composite) |
|
| Funding source | National Institute of Child Health and Human Development Grant HD13449; National Institute on Drug Abuse (NIDA) Grant DA13555, and NIDA Research Scientist Award K05 DA00492 to Steven J. Sherman. | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published and unpublished data | |
Chen 2015.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: Taiwan Data collection period: January – September 2007 and follow‐ups (July 2007 – September 2008) Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 3514; Number included in meta‐analysis: N = 3207 Sample characteristics (at baseline): Age (mean): people who quit long‐term: < 30 y 8.8%, 30 – 44 y 37.8%, 45 – 59 y 28.6%, ≥ 60 y 24.8% / people who quit short‐term: < 30 y 13.1%, 30 – 44 y 31.1%, 45 – 59 y 29.2%, ≥ 60 y 26.6% / people who relapsed to smoking: < 30 y 8.9%, 30 – 44 y 17.8%, 45 – 59 y 44.4%, ≥ 60y 28.9% / people who smoked: < 30 y 14.1%, 30 – 44 y 38.5%, 45 – 59 y 31.0%, ≥ 60y 16.5%; Sex (% male): people who quit long‐term 87.8% (230/262) / people who quit short‐term 79.9% (306/383)/people who relapsed to smoking 84.4% (38/45) / people who smoke 85.7% (2421/2824) Population category: general population; Specific population: people who smoked who participated in outpatient smoking cessation programme Nicotine dependence: not measured; Baseline cigarettes per day: not measured; Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: outpatient smoking cessation service (OSCS) – smoking cessation counselling provided Pharmacological support for smoking cessation: OSCS – smoking cessation pharmacotherapy provided Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: long‐term quitters ‐ participants who had quit tobacco use for 1 year; short‐term quitters ‐ participants who had been smoking for at least 6 months and then quit tobacco for 6 months (180 days) after participating in the programme (i.e. OSCS) Cessation definition used for outcome(s) in this analysis: continuous abstinence Measure of biovalidation: not bioverified Definition of people who continued to smoke used: people who relapsed to smoking ‐ participants who relapsed into tobacco use after ceasing tobacco use for 6 months; and people who smoked ‐ participants who failed to quit smoking for at least 1 year, despite participating in the programme Time point(s) at which follow‐up was conducted: 6 months and 1 year after finishing the smoking cessation programme Outcome category: Mixed Anxiety and Depression Outcome measure(s): EuroQol‐5D‐3L (EQ‐5D‐3L; depression and anxiety subscale) |
|
| Funding source | This study was supported by the Health Promotion Administration, Ministry of Health and Welfare, Taiwan (No. 95039‐1) | |
| Author conflicts of interest | None declared | |
| Notes | Outcome data source: Published data | |
Cinciripini 2013.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: 31 August 2006 – 27 October 2010 Registry ID: ClinicalTrials.gov: NCT00507728 |
|
| Participants |
Number of participants: N = 294; Number included in meta‐analysis: N = 180 Sample characteristics (at baseline): Age (mean): 44.3 years (SD 10.4); Sex (% male): 61.2% (180/294) Population category: general population; Specific population: adults who smoke Nicotine dependence: FTND 4.5 (SD 2.2); Baseline cigarettes per day: 19.7 (SD 9.4); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: all people who smoke who received individual behavioural smoking cessation counselling which included active effort to prepare for quitting, self‐monitoring, identification of high‐risk situations and coping skill development Pharmacological support for smoking cessation: varenicline tartrate or placebo and bupropion hydrochloride sustained release (SR) or placebo Psychotherapeutic or psychoactive support for mental health or mood: received mood management; behavioural counselling included stress management and relaxation visualisation; some participants received bupropion (anti‐depressant) |
|
| Outcomes |
Definition of cessation used: prolonged abstinence (primary smoking outcome), whereby relapse was defined as 7 or more consecutive days of smoking or smoking at least 1 cigarette over 2 consecutive weeks from the end of the grace period to a selected future time point (e.g. EOT, 3 and 6 months post‐quit date); 7‐day point‐prevalence abstinence defined as self‐report of no smoking (not even a puff), in 7 days prior to time point of interest; continuous abstinence defined as self‐report of no smoking (not even a puff) from 2‐weeks post‐quit date to future time point or last 4 weeks of treatment or week 8 of medication Cessation definition used for outcome(s) in this analysis: continuous abstinence Measure of biovalidation: expired CO < 10 ppm; salivary cotinine values < 15 ng/mL Definition of people who continued smoking used: ‘non‐abstinent’; carbon monoxide > 10 ppm; participants unavailable for assessment considered non‐abstinent Time point(s) at which follow‐up was conducted: 3‐, 4‐ and 6‐month post‐quit follow‐up visits Outcome category: Mixed Anxiety and Depression Outcome measure(s): Positive and Negative Affect Schedule (PANAS; negative affect scale) |
|
| Funding source | Support for this research was provided by grant DA017073 from the National Institute on Drug Abuse (Dr Cinciripini) and by Cancer Center Support Grant P50CA70907 from the National Cancer Institute. Varenicline was provided by Pfizer | |
| Author conflicts of interest | Dr Cinciripini served on the scientific advisory board of Pfizer, conducted educational talks sponsored by Pfizer on smoking cessation (2006‐2008), and has received grant support from Pfizer | |
| Notes | Outcome data source: Unpublished data | |
Cohen 1990.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: USA Data collection period: not stated Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 260 Sample characteristics (at baseline): Age (mean): 40 years; Sex (% male): 29% Population category: general population; Specific population: general population Nicotine dependence: mean time before first cigarette in morning 28.3 minutes, mean longest period without cigarette in last year 12.1 days; Baseline cigarettes per day: 27.4; Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: 24‐hour quitters – participants reported at 1‐month they had stopped smoking at some point since pre‐quit interview for at least 1 x 24‐hour period; point‐prevalent abstinent – participants self‐reported not currently smoking and not smoking ‘even a puff’ during the last week; 6‐month continuous abstinence – participants self‐reported point‐prevalent abstinence at all follow‐up interviews and had not smoked > 3 days since quitting Cessation definition used for outcome(s) in this analysis: multiple (point‐prevalence and continuous abstinence) Measure of biovalidation: self‐reported abstinence was verified at 6 months by carbon monoxide (CO) and saliva cotinine – not presented in analysis Definition of people who continued smoking used: people who continued smoking (smoking‐smoking) Time point(s) at which follow‐up was conducted: 1, 3 and 6 months following participant expected quit date Outcome category: Stress Outcome measure(s): Perceived Stress Scale: Short Form (PSS‐4) |
|
| Funding source | Preparation of the article was supported by National Institute of Mental Health Research Scientist Development Award K02 MH00721 to Sheldon Cohen; research in this article was supported by National Cancer Institute Grant CA38243 to Sheldon Cohen and Edward Lichtenstein | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published | |
Cooper 2016.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: Canada, UK, USA and Australia Data collection period: waves 5 ‐ 8 (2006/2007 – 2010/2011) Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: waves 5 – 6 (N = 4676), waves 6 – 7 (N = 4717), waves 7 – 8 (N = 3710); Number included in meta‐analysis: N = 4676 Sample characteristics (at baseline): Age (mean): waves 5 – 6 18 ‐ 39 y (29.0%), 40 ‐ 55 y (42.4%). 55+ (28.6%) / waves 6 – 7 18 ‐ 39 y (23.5%), 40 – 55 y (43.4%), 55+ (30.5%) / waves 7 – 8 18 ‐ 39 y (26.1%), 40 – 55 y (43.8%), 55+ (32.7%); Sex (% male): waves 5 – 6 42.4% (1983/4676), waves 6 – 7 42.7% (2014/4717), waves 7 – 8 43.7% (1621/3710) Population category: general population; Specific population: adults who smoked Nicotine dependence: heaviness of smoking index (HSI) means not reported; Baseline cigarettes per day: measured but not reported; Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: quitting status at T2 defined as those who reported making an attempt to quit since T1 and whether they were still stopped smoking (i.e. 'quit and still abstinent') Cessation definition used for outcome(s) in this analysis: unclear point‐prevalence Measure of biovalidation: not bioverified Definition of people who continued to smoke used: made ‘no quit attempt’ Time point(s) at which follow‐up was conducted: approximately 12 months after T1 survey Outcome category: Depression Outcome measure(s): Primary Care Evaluation of Mental Disorders Procedure questionnaire (2 questions assessing depressed mood) |
|
| Funding source | The ITC Four‐Country Survey is supported by multiple grants, including R01 CA 100362 and P50 CA111236 (Roswell Park Transdisciplinary Tobacco Use Research Center) and also in part from grant P01 CA138389 (Roswell Park Cancer Institute, Buffalo, New York), all funded by the National Cancer Institute of the United States, Robert Wood Johnson Foundation (045734), Canadian Institutes of Health Research (57897, 79551), National Health and Medical Research Council of Australia (265903, 450110, APP1005922), Cancer Research UK (C312/A3726), Canadian Tobacco Control Research Initiative (014578); and the Centre for Behavioural Research and Program Evaluation, National Cancer Institute of Canada/Canadian Cancer Society | |
| Author conflicts of interest | Authors declare that they have no competing interests | |
| Notes | Outcome data source: Published data | |
Covey 2015.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: December 2005 – January 2008 Registry ID: ClinicalTrials.gov: NCT00253747 |
|
| Participants |
Number of participants: N = 255; Number included in meta‐analysis: N = 225 Sample characteristics (at baseline): Age (mean): 37.8 years (SD 9.9); Sex (% male): 57% (145/255) Population category: psychiatric population; Specific population: people with ADHD Nicotine dependence: not measured; Baseline cigarettes per day: not measured; Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: smoking cessation counselling standardised using manual‐based counselling following principles of CBT (individual, 10‐minute sessions, weeks 1 ‐ 11) Pharmacological support for smoking cessation: osmotic release oral system methylphenidate (OROS‐MPH) or placebo and 21 mg/24 h nicotine patches daily Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management ‐ no psychological or psychoactive support for mental health (methylyphenidate is psychoactive but no certainty in evidence for impact on depression/anxiety symptoms) |
|
| Outcomes |
Definition of cessation used: point‐prevalence weekly abstinence i.e. ‘abstainer’, at week 1 and week 6 after target quit date (TQD) Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: exhaled carbon monoxide < 8 ppm Definition of people who continued to smoke used: ‘non‐abstainer’, carbon monoxide > 8 ppm Time point(s) at which follow‐up was conducted: weeks 1 and 6 after TQD Outcome category: Anxiety, Depression Outcome measure(s): Beck Anxiety Inventory, Beck Depression Inventory II (BDI‐II) |
|
| Funding source | Supported by: NIDAK24 DA022412 (Nunes), NIDAU10 DA013035 (Nunes & Rotrosen), U10‐DA013732 (Winhusen) | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Croghan 2005.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: USA Data collection period: participants using services from 1st September 1996 – 31st May 1998 and 1‐year follow‐up (31st May 1999) Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 206; Number included in meta‐analysis: N = 206 Sample characteristics (at baseline): Age (mean): people who quit 52.8 years (SD 11.2), people who continued smoking 54.6 years (SD 15.6); Sex (% male): people who quit 52% (76/146), people who continued smoking 50% (30/60) Population category: general population; Specific population: participants using clinical services of the Nicotine Dependence Centre (NDC) Nicotine dependence: not measured; Baseline cigarettes per day: median 20 ‐ 39; Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: behavioural support, relapse prevention or pharmacological support or both Pharmacological support for smoking cessation: behavioural support, relapse prevention or pharmacological support or both Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: 7‐day point‐prevalence abstinence (self‐report); 1‐year continuous abstinence (self‐report) Cessation definition used for outcome(s) in this analysis: continuous abstinence Measure of biovalidation: not bioverified Definition of people who continued smoking used: longest period of smoking abstinence ≤ 6 days (during the prior year) Time point(s) at which follow‐up was conducted: 1 year after baseline Outcome category: Positive Affect; Psychological Quality of Life (QoL), Social Outcome Outcome measure(s): Short Form Health Survey – 36 (SF‐36; energy, vitality and social functioning subscales and mental health composite) |
|
| Funding source | The Mayo Foundation | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Cui 2019.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: Japan Data collection period: recruitment January 2011 – March 2014 Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 80,872; Number included in meta‐analysis: N = 14,047 Sample characteristics (at baseline): Age (mean): total 31.3 years (SE 0.02); quit before pregnancy 32.0 years (SE 0.03); quit after becoming pregnant 29.5 years (SE 0.05); smoked during pregnancy 30.1 years (SE 0.1); Sex (% male): 0% (0/80872) Population category: pregnant population; Specific population: pregnant women Nicotine dependence: not measured; Baseline cigarettes per day: not measured; Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: self‐reported smoking cessation (quit before pregnancy, quit after becoming pregnant) Cessation definition used for outcome(s) in this analysis: unclear point‐prevalence Measure of biovalidation: not bioverified Definition of continuing smoker used: self‐reported smoking (smoked during pregnancy) Time point(s) at which follow‐up was conducted: second/third trimester and postpartum period (1 month after childbirth) Outcome category: Depression Outcome measure(s): Edinburgh Postnatal Depression Scale (EPDS) |
|
| Funding source | The Japan Environment and Children's Study was funded by the Ministry of the Environment, Japan | |
| Author conflicts of interest | Authors declare no competing interests exist | |
| Notes | Outcome data source: Published data | |
Dawkins 2009.
| Study characteristics | ||
| Methods |
Study design: randomised experiment Country: UK Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 145; Number included in meta‐analysis: N = 64 Sample characteristics (at baseline): Age (mean): 34.5 years; Sex (% male): 47% (30/64) Population category: general population; Specific population: recruited through advertisement in South‐East London Nicotine dependence: FTND, people who quit 4.6 (SD 1.7), people who continued smoking 5.3 (SD 1.8); Baseline cigarettes per day: people who quit 17.8 (SD 5.9), people who continued smoking 18.5 (SD 6.3); Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support but participants received financial incentive for maintaining smoking or abstinence status as allocated Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: continuous abstinence (maintained cotinine‐verified abstinence at all follow‐ups i.e. ‘successful quitters’) Cessation definition used for outcome(s) in this analysis: continuous abstinence Measure of biovalidation: breath CO levels < 11 ppm; salivary cotinine level of more than 20 ng/ml were considered non‐abstinent Definition of people who continued smoking used: participants from 'quit' group who reported more than 1 lapse since the last session, or with a salivary cotinine level > 20 ng/ml were classified as relapsers Time point(s) at which follow‐up was conducted: 7 days, 1 month and 3 months Outcome category: Anxiety, Depression Outcome measure(s): Hospital Anxiety and Depression Scale (HADS) |
|
| Funding source | National Institute of Drug Abuse (NIDA); grant code 3527427 | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published and unpublished data | |
Dedert 2019.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: December 2013 – July 2017 Registry ID: ClinicalTrials.gov: NCT01901848 |
|
| Participants |
Number of participants: N = 40; Number included in meta‐analysis: N = 31 Sample characteristics (at baseline): Age (mean): CPTS 43.1 years (SD 10.8), PS 53.3 years (SD 10.8); Sex (% male): CPTS 93% (14/15), PS 76% (19/25) Population category: psychiatric population; Specific population: outpatients with PTSD randomised to receive combined Cognitive Processing Therapy and smoking cessation (CPTS) or present‐focused smoking cessation (PS) Nicotine dependence: FTND CPTS 4.8 (SD 1.9), PS 5.0 (SD 2.2);Baseline cigarettes per day: CPTS 13.9 (SD 4.8), PS 5.0 (SD 2.2); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: randomised to A) CPTS – 12 sessions, twice per week (where possible), beginning with 60 minutes of CPT (manualised psychotherapy for PTSD) followed by 25 minutes of smoking cessation based on the Integrated Care for Smoking Cessation (ICSC) treatment manual; or B) PS – 12 sessions, 25 minutes of standard cognitive behavioural smoking cessation treatment based on treatment manual for ICSC only, provided by same therapists as CPTS condition; participants in both conditions contacted by phone monthly after end of treatment for brief relapse prevention call and all participants encouraged to sign up for free supportive text messaging service (SmokeFreeVET) which provided automated texts from group of messages specifically for veterans; the PS smoking cessation was identical to smoking cessation component of CPTS group except the PS arm did not use formal cognitive restructuring to address smoking triggers Pharmacological support for smoking cessation: all participants encouraged to use standard cessation pharmacotherapy prescribed by study physician including 6 weeks of nicotine patch, 1 nicotine rescue method (gum/lozenge) through follow‐up period; participants with no contraindications encouraged to use bupropion to reduce withdrawal/cravings Psychotherapeutic or psychoactive support for mental health or mood: received mood management – 45% of total sample were prescribed bupropion; 15 participants randomised to CPTS which included cognitive processing of extreme cognitions related to traumatic event |
|
| Outcomes |
Definition of cessation used: bioverified 7‐day point‐prevalence smoking abstinence at end of treatment and 6‐month follow‐up Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: expired CO < 4 ppm Definition of people who continued smoking used: exact definition not reported; non‐abstinence ‐ missing data assumed to indicate smoking Time point(s) at which follow‐up was conducted: post‐treatment and 6‐month follow‐up Outcome category: Depression Outcome measure(s): Beck Depression Inventory II (BDI‐II) |
|
| Funding source | This work was primarily supported by award number 1IK2CX000718 to Dr. Dedert from the CSR&D Service of the VA Office of Research and Development | |
| Author conflicts of interest | The authors declare no conflicts of interest | |
| Notes | Outcome data source: Unpublished data | |
Dulger 2019.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: Turkey Data collection period: not stated Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 92; Number included in meta‐analysis: N = 54 Sample characteristics (at baseline): Age (mean): 38.7 years (SD 9.3); Sex (% male): 74% (68/92) Population category: chronic physical condition; Specific population: participants diagnosed with ankylosing spondylitis (AS) Nicotine dependence: FTND median 6 (1 – 10 min‐max); Baseline cigarettes per day: median 20 (2 – 60; min‐max); Motivation to quit: not selected by motivation to quit (some participants were motivated to quit, some were not motivated to quit) |
|
| Interventions |
Behavioural support for smoking cessation: participants who agreed to quit smoking were treated with cognitive behavioral therapy (duration: 6 months); first approach in smoking cessation programme conducted with all people who smoked (5A and 5R principles) i.e. brief cessation advice and repeated for those who did not agree to quit smoking Pharmacological support for smoking cessation: participants who agreed to quit smoking were treated with monotherapy (varenicline tartrate, nicotine replacement therapy, or bupropion hydrochloride) – duration 3 months Psychotherapeutic or psychoactive support for mental health or mood: received mood management – of those who smoked, 23 participants (42.6%) received bupropion hydrochloride (i.e. anti‐depressant); split by quit status, 30.4% (n = 7/17) who quit received bupropion, 69.6% who did not quit (n = 16) received bupropion (n = 16/37) |
|
| Outcomes |
Definition of cessation used: at end of 6th month the people who smoked were separated into subgroups of ‘quit smoking’ (exact definition not provided) versus ‘not quit smoking’ Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: exhaled CO (cut‐off level 6 ppm) Definition of people who continued to smoke: not quit smoking, carbon monoxide level > 6 ppm Time point(s) at which follow‐up was conducted: 6‐month follow‐up Outcome category: Anxiety, Depression, Positive Affect, Psychological Quality of Life (QoL), Social Outcome Outcome measure(s): Beck Anxiety Index (BAI), Beck Depression Index (BDI), Short‐Form Health Survey (SF‐36; emotional health, power/live/vitality, social functioning subscales) |
|
| Funding source | None specified | |
| Author conflicts of interest | Authors declare no conflict of interest | |
| Notes | Outcome data source: Published data | |
Farris 2015.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: January 2008 (study start date) – August 2013 (actual primary completion date) Registry ID: ClinicalTrials.gov; NCT01753141 |
|
| Participants |
Number of participants: N = 185; Number included in meta‐analysis: N = 185 Sample characteristics (at baseline): Age (mean): non‐quitters 38.6 years (SD 14.1), quitters 40.0 years (SD 13.6); Sex (% male): non‐quitters 50% (40/80), quitters 42.9% (60/105) Population category: psychiatric population; Specific population: people diagnosed with anxiety disorder Nicotine dependence: non‐quitters 5.3 (SD 2.2), quitters 5.0 (SD 2.3); Baseline cigarettes per day: eligibility criteria for the parent study included smoking ≥ 8 cigarettes per day for at least the past year; Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: smoking cessation treatment programme consisted of either (1) standard smoking cessation programme; or (2) anxiety‐focused smoking cessation treatment Pharmacological support for smoking cessation: nicotine replacement therapy via the transdermal nicotine patch, which was initiated at treatment session 4 (quit day) Psychotherapeutic or psychoactive support for mental health or mood: received mood management‐ anxiety‐focused smoking cessation treatment |
|
| Outcomes |
Definition of cessation used: continuous abstinence ‐ ‘successful quitters’ based on consistent biochemical verification at week 1, week 2 and month 1 post‐intervention (i.e. quit‐day) Cessation definition used for outcome(s) in this analysis: continuous abstinence Measure of biovalidation: CO breath samples at each time point were used as an indicator of abstinence (expired CO ≤ 4 ppm, as abstinent) Definition of people who continued to smoke: ‘non‐quitters’ defined as no continuous abstinence, carbon monoxide > 4 ppm Time point(s) at which follow‐up was conducted: week 1, week 2, month 1, month 3 and month 6, year 1 and year 2 follow‐up (NCT record) Outcome category: Anxiety Outcome measure(s): Inventory of Depression and Anxiety Symptoms (IDAS; anxiety subscale) |
|
| Funding source | Funded by a National Institute of Mental Health grant awarded to Drs. Michael J. Zvolensky and Norman B. Schmidt (R01‐MH076629‐01A1). Ms. Farris is supported by a pre‐doctoral National Research Service Award from the National Institute of Drug Abuse (F31‐DA035564) | |
| Author conflicts of interest | No authors have any conflicts of interests or financial disclosures to report | |
| Notes | Outcome data source: Published data | |
Fujita 2019.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: Japan Data collection period: fiscal years 2010 to 2014 Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 87,255; Number included in meta‐analysis: N = 87,255 Sample characteristics (at baseline): Age (mean): mean not presented, 30 – 39 y (21.4%), 40 – 49 y (35.8%), 50 – 59 y (30.8%), 60 – 69 y (12.0%); Sex (% male): 82.6% (72,114/87,255) Population category: general population; Specific population: employees who underwent health checks in fiscal years 2010 and 2011 Nicotine dependence: not measured; Baseline cigarettes per day: not measured; Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: none reported but states in the introduction that only varenicline and nicotine patches are permitted to be prescribed under the insurance coverage for smoking cessation in Japan; overall, 1.3% reported using varenicline and 0.1% nicotine patches ‐ in the smoking cessation group this was 7.4% and 0.2% respectively Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: those who reported smoking in the fiscal year 2010 and reported "no" to smoking within the previous month in fiscal year 2011 were labelled the smoking cessation group Cessation definition used for outcome(s) in this analysis: unclear point‐prevalence Measure of biovalidation: not bioverified Definition of people who continued to smoke used: resumption of smoking if in the smoking cessation group and continued smoking if in the smoking group Time point(s) at which follow‐up was conducted: 4 years Outcome category: Depression Outcome measure(s): Depressive disorder was defined as receiving any medical care for depressive episodes, classified as ICD‐10 code F32 in the medical administrative claim data |
|
| Funding source | The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not‐for‐profit sectors | |
| Author conflicts of interest | None declared | |
| Notes | Outcome data source: Published data | |
Garvey 2012.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 278; Number included in meta‐analysis: N = 106 Sample characteristics (at baseline): Age (mean): 46.9 years (SD 11.5); Sex (% male): 47.1% (131/278) Population category: general population; Specific population: recruited people who smoked via using advertisements placed in Boston area newspapers, excluded medical and psychiatric co‐morbidities Nicotine dependence: FTND 4.9 (SD 2.3); Baseline cigarettes per day: 17.9 (SD 7.9); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: front‐loaded (FL) counselling condition or weekly counselling (WC) condition. Both conditions received 2 pre‐quit (approximately 45 minutes each) and 12 post‐quit individual face‐to‐face counselling sessions (average 20 – 30 mins per session). FL condition received 6 of the 2 post‐quit counselling sessions in the first 2 weeks following quit days, WC condition received 2 of the 12 sessions in the first 2 post‐quit weeks. Counsellors were trained to deliver a manual‐based cognitive–behavioural smoking cessation therapy developed for the study ‐ included development of an individualised coping plan for preventing smoking in high‐risk situations and provision of counselling support (discussions covered guarding against rationalisations, the abstinence violation effect, proactive versus reactive coping, and developing the identity of someone who does not smoke) Pharmacological support for smoking cessation: both treatment groups received nicotine patches (7 ‐ 21 mg doses); those with CPD ≥ 10 at baseline were given the 21 mg patch for 8 weeks and then were reduced to 14 mg for 2 weeks and 7 mg for 2 weeks; those CPD < 10 at baseline were provided the 14 mg patch for 8 weeks and then reduced to 7 mg for 4 weeks Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: 'continuous abstinence’ ‐ defined as not even a puff smoked at any point during 1 year follow‐up; The National Heart, Lung, and Blood Institute (NHLBI) definition ‐ defined as never smoking for 7 or more consecutive days nor for 7 or more consecutive episodes; ‘point prevalence’ abstinence ‐ defined as no smoking in 7 days prior to 1 year assessment (bioverified) Cessation definition used for outcome(s) in this analysis: continuous abstinence Measure of biovalidation: expired CO levels assessed in post‐quit period (abstinence cut‐off level of < 8 ppm) Definition of people who continued to smoke used: day of relapse was defined as the day post‐cessation that began the regular pattern of smoking Time point(s) at which follow‐up was conducted: 1 year (3 months unpublished data) Outcome category: Depression Outcome measure(s): Center for Epidemiological Studies Depression Scale (CES‐D) |
|
| Funding source | Study was supported by grant 5 R01 DA016739 from the National Institute on Drug Abuse of the National Institutes of Health | |
| Author conflicts of interest | No competing financial interests exist | |
| Notes | Outcome data source: Unpublished data | |
Giordano 2011.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: UK Data collection period: data used for this longitudinal study come from British Household Panel Survey (BHPS) individual‐level responses in years 2003 (Wave 13) and 2005 (Wave 15) Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 10,512; Number included in meta‐analysis: N = 2605 Sample characteristics (at baseline): Age (mean): mean not presented, age categories 16 ‐ 34 y (29.5%), 35 ‐ 44 y (20.5%), 45 ‐ 54 y (16.8%), 55 ‐ 64 y (14.9%) 65+ (18.3%) Sex (% male): 45.4% (4777/10,512) Population category: general population; Specific population: participants in the BHPS 2003 ‐ 2005 Nicotine dependence: not measured; Baseline cigarettes per day: not measured; Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: in 2003 and 2005, the same individuals were asked: ‘Do you smoke cigarettes?’ to which the replies were either ‘Yes’ or ‘No’. From this created 4 potential outcomes for assessing smoking over time: (i) still a smoker; (ii) now a non‐smoker; (iii) now a smoker; and (iv) still a non‐smoker. Cessation group ‐ ‘now a non‐smoker’. Cessation definition used for outcome(s) in this analysis: unclear point‐prevalence Measure of biovalidation: not bioverified Definition of people who continued to smoke used: exact definition unclear; concerned with change in smoking status (cessation and initiation) Time point(s) at which follow‐up was conducted: 2 years Outcome category: Mixed Anxiety and Depression Outcome measure(s): General Health Questionnaire‐12 (GHQ‐12) |
|
| Funding source | Study was supported by the Swedish Research Council (Vetenskapsrädet) (grant number K2008‐70X01‐3), the Swedish Research Council Linnaeus Centre for Economic Demography (grant number VR 79) and the Research Funds of Malmo University Hospital | |
| Author conflicts of interest | None declared | |
| Notes | Outcome data source: Unpublished data | |
Glassman 2001.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 100; Number included in meta‐analysis: N = 76 Sample characteristics (at baseline): Age (mean): people who quit 44.4 years (SD 11.3), people who continued to smoke 42.8 (SD 11.3); Sex (% male): people who quit 36%, people who continued to smoke 35% Population category: psychiatric population; Specific population: people with history of major depression Nicotine dependence: Fagerström score, people who quit 6.3 (SD 2.6). people who continued to smoke 6.4 (2.4); Baseline cigarettes per day: 27.4 (SD 9.2); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: participants randomly assigned sertraline or identical placebo Psychotherapeutic or psychoactive support for mental health or mood: sertraline or placebo i.e. did receive mood management |
|
| Outcomes |
Definition of cessation used: self‐reported smoking abstinence 9 weeks after the start of the study (end of treatment), and 6 months after the end of treatment Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: self‐reported smoking status verified by serum‐sample cotinine concentrations at end of treatment and 6‐month follow‐up Definition of people who continued smoking used: continued to smoke at end of treatment Time point(s) at which follow‐up was conducted: 3 and 6 months after treatment ended Outcome category: Depression Outcome measure(s): structured clinical interview for the diagnostic and statistical manual of mental disorders that are used to assess mood disorders (SCID I) |
|
| Funding source | This study was supported by Pfizer, Suzanne C Murphy Foundation, and Perry and Martin Granoff Family Foundations | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Grabovac 2017.
| Study characteristics | ||
| Methods |
Study design: non‐randomised intervention study Country: Germany and Austria Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 447; Number included in meta‐analysis: N = 224 Sample characteristics (at baseline): Age (mean): 45.5 years (range 21 ‐ 82); Sex (% male): 86.4% (386/447) Population category: chronic physical condition; Specific population: people living with HIV Nicotine dependence: not measured; Baseline cigarettes per day: up to 10 (24.0%), 11 – 20 (37.1%), 21 – 30 (22.2%), 31+ (13.1%); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: 2 smoking cessation interventions delivered by trained physicians were offered to all people who smoked; a short intervention (5‐minute structured motivational intervention focused on increasing knowledge about benefits of smoking cessation) and a longer programme (full smoking cessation programme) with 5 sessions (approximately 30 minutes, focused on strengthening problem‐solving skills and developing social skills to improve patient social support) – people who smoked were invited to participate in full programme after short intervention, time‐frame between sessions was not pre‐determined to facilitate flexibility Pharmacological support for smoking cessation: pharmacological support (nicotine replacement products, bupropion, varenicline) was offered for participants to use if they wished; 4.0% participants received nicotine replacement products, 2.7% participants received varenicline and 1.8% bupropion in the recommended dosages Psychotherapeutic or psychoactive support for mental health or mood: some of the sample received mood‐management intervention (1.8% of participants; n = 4) received bupropion and 16% of total sample (14% of people who smoked) were receiving ‘antidepressant therapy’ not otherwise specified |
|
| Outcomes |
Definition of cessation used: smoking status assessed with question ‘have you smoked within the last 7 days’ i.e. point‐prevalence abstinence Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: smoking status at baseline confirmed with CO level of > 6 ppm ; smoking status measured at study end using same protocol (i.e. CO measured at study end) but does not explicitly state it was used to confirm abstinence Definition of people who continued to smoke used: ‘participants who continued to smoke’ i.e. reported smoking in the previous 7‐days Time point(s) at which follow‐up was conducted: 8 months Outcome category: Psychological Quality of Life (QoL), Social Outcome Outcome measure(s): World Health Organisation Quality of Life – HIV‐Bref (WHOQOL HIV‐Bref) |
|
| Funding source | Study was funded by ViiV healthcare Germany and GSK Austria | |
| Author conflicts of interest | The authors report no conflict of interest | |
| Notes | Outcome data source: Published data | |
Guimond 2017.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: Canada Data collection period: not stated Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 110; Number included in meta‐analysis: N = 110 Sample characteristics (at baseline): Age (mean): quitters 53.1 years (SD 10), non‐quitters 55.4 years (SD 10.3); Sex (% male): quitters 36.4% (20/55), non‐quitters: 67.3% (37/55) Population category: postoperative; Specific population: people with confirmed diagnosis of non‐metastatic cancer scheduled to receive surgery with curative intent Nicotine dependence: not measured; Baseline cigarettes per day: quitters: 16.8 (SD 7.9), non‐quitters: 16.5 (SD 10.0); Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: smoking cessation was defined as a participant who reported an occasional or a daily usage at one time point and a non‐usage at the following time point Cessation definition used for outcome(s) in this analysis: unclear point‐prevalence Measure of biovalidation: not bioverified Definition of people who continued to smoke used: smoking relapse was defined as a participant who reported smoking cessation at a specific time point and resumed tobacco usage on an occasional or daily basis 4 months later Time point(s) at which follow‐up was conducted: 4 months Outcome category: Anxiety, Depression Outcome measure(s): Hospital Anxiety and Depression Scale (HADS; anxiety and depression sub‐scales) |
|
| Funding source | Study was supported by master and doctoral research training scholarships from the Fonds pour la recherche en santédu Québec and a scholarship from the Psychosocial Oncology ResearchTraining program of the Canadian Institutes of Health Research and a grant from the CanadianInstitutes of Health Research (MOP‐69073) | |
| Author conflicts of interest | The authors declare that they have no conflict of interest | |
| Notes | Outcome data source: Unpublished data | |
Hajek 2010.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: UK Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 469; Number included in meta‐analysis: N = 469 Sample characteristics (at baseline): Age (mean): 56 years (SD 9.9); Sex (% male): 77.4% (363/469) Population category: chronic physical conditions; Specific population: cardiology patients recovering from myocardial infarction or admission for coronary artery bypass surgery Nicotine dependence: 75.3% of cohort smoked within 30 minutes of waking; Baseline cigarettes per day: 20.7 (SD 12.6); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: single brief support (bedside intervention by cardiac rehabilitation nurses to prevent smoking relapse) Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: continuous abstinence at 1 year defined as self‐report of having not smoked a single puff in the past week and having smoked no more than 5 cigarettes (or rollups, cigars or pipes) since recruitment Cessation definition used for outcome(s) in this analysis: continuous abstinence Measure of biovalidation: salivary cotinine level (< 20 ng/ml) and expired CO level (< 10 ppm) Definition of people who continued to smoke used: people who continued to smoke Time point(s) at which follow‐up was conducted: 1 year after baseline Outcome category: Stress Outcome measure(s): non‐standardised measurement of perceived stress (self‐reported on scale of 1 = no stress – 10 = extreme stress) |
|
| Funding source | Grant received from NHS R&D Programme on Cardiovascular Disease and Stroke | |
| Author conflicts of interest | Drs Hajek and McRobbie have undertaken research and consultancy for, and received honoraria for speaking at meetings for, the manufacturers of smoking cessation medications | |
| Notes | Outcome data source: Published data | |
Hammett 2019.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated ‐ secondary analysis of data from the 'Proactive Smoking Cessation Treatment for Minnesota Priority Populations' (OPTIN) study Registry ID: ClinicalTrials.gov: NCT01123967 |
|
| Participants |
Number of participants: N = 2321; Number included in meta‐analysis: ranges from N = 527 – N = 879 dependent on SMI category and outcome Sample characteristics (at baseline): Age (mean): mean not reported, 18 – 24 years (SMI 15.2%, non‐SMI 22.3%), 25 – 34 years (SMI 32.7%, non‐SMI, 35.9%), 35 – 64 years (SMI 52.1%, non‐SMI 41.8%); Sex (% male): SMI 26.6% (250/939), non‐SMI 41.6% (436/1382) Population category: general population and psychiatric population; Specific population: people who smoked, enrolled in Minnesota Health Care Programs (MHCP), people with serious mental illness (SMI, n = 939; categorised using ICD 9 codes) and remaining people who smoked categorised as non‐SMI (n = 1382) Nicotine dependence: minutes until fist cigarette ≤ 5 (SMI 32.2%, non‐SMI 22.%); Baseline cigarettes per day: SMI: 14.7 (SD 9.6), non‐SMI: 13.0 (SD 8.8); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: the proactive care intervention systematically offers low‐income people who smoke free and easy access to evidence‐based treatments and has 2 primary components: (1) proactive outreach to people who currently smoke in the form of mailed invitation materials and telephone calls containing targeted health messages, and (2) facilitated access to free, comprehensive, evidence‐based tobacco cessation treatments in the form of NRT and intensive, telephone‐based behavioural counselling. The proactive tobacco treatment intervention is based on social cognitive theory, the stages of change model and the biopsychosocial model of perceived discrimination. Comparison group received usual care which included similar smoking cessation treatment components (including pharmacotherapy and telephone counselling) ‐ participants must self‐initiate access to treatment Pharmacological support for smoking cessation: NRT available for both the intervention and usual‐care group in the original trial Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: a self‐report measure was used to assess 6‐month prolonged smoking abstinence at 12‐month follow‐up Cessation definition used for outcome(s) in this analysis: prolonged abstinence Measure of biovalidation: not bioverified Definition of people who continued to smoke used: participants were asked whether they had smoked 1) at least once on 7 consecutive days, or 2) at least once on 2 consecutive weekends in the 6‐month period prior to the follow‐up survey – those who responded yes to either question were considered to be people continuing to smoke Time‐point(s) at which follow‐up was conducted: 12 months Outcome category: Anxiety, Depression Outcome measure(s): Patient‐Reported Outcomes Measurement Information System (PROMIS); Patient Health Questionnaire 2‐item (PHQ‐2) |
|
| Funding source | Primary funding provided by NCI Grant R01CA141527‐01. Additional support provided by NCI Grant 2T32CA163184 and the Center for Chronic Disease Outcomes Research, VA, Minneapolis, MN | |
| Author conflicts of interest | No conflict declared | |
| Notes | Outcome data source: Published and unpublished data | |
Hays 2012.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: June 2003 – March 2005 Registry ID: ClinicalTrials.gov: NCT00143364 and NCT00141206 |
|
| Participants |
Number of participants: N = 2052 Sample characteristics (at baseline): Age (mean): varenicline: 43.5 years (SD 11.3), bupropion SR 42.5 years (SD 11.8), placebo 42.5 years (SD 11.7); Sex (% male): varenicline 52.6% (366/696), bupropion SR 59.3% (398/671), placebo 56.1% (384/685) Population category: general population; Specific population: generally healthy people between 18 and 75 years of age who smoked ≥ 10 cigarettes per day Nicotine dependence: Fagerström varenicline 5.3 (SD 2.2), bupropion SR 5.3 (SD 2.1), placebo 5.3 (SD 2.1); Baseline cigarettes per day: varenicline 21.8 (range 10 ‐ 70), bupropion SR 21.4 (range 10 ‐ 65), placebo 21.5 (range 10 ‐ 80); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: brief office counselling (≤ 10 min) in accord with recommended clinical practice guidelines provided Weeks 1 – 12 during double‐blind treatment phase and Weeks 13, 24, 36, 44 and 52 during non‐treatment follow‐up Pharmacological support for smoking cessation: all participants randomly assigned for 12 weeks to 1 of a) varenicline 1 mg twice a day; b) bupropion SR 150 mg twice a day; or c) placebo Psychotherapeutic or psychoactive support for mental health or mood: N = 671 received bupropion SR (anti‐depressant) i.e. received mood management |
|
| Outcomes |
Definition of cessation used: continuous abstinence from smoking was assessed at each clinic visit by self‐report and confirmed by measurement of expired carbon monoxide (CO) levels ≤ 10 ppm Cessation definition used for outcome(s) in this analysis: continuous abstinence Measure of biovalidation: exhaled CO levels ≤ 10 ppm Definition of people continuing to smoke used: exact definition not reported Time point(s) at which follow‐up was conducted: week 12, 24 and 52 Outcome category: Anxiety, Positive Affect, Psychological Quality of Life (QoL) Outcome measure(s): Smoking Cessation Quality of Life (SCQoL) questionnaire, a validated instrument composed of the SF‐ 36 (Version 1) as the generic core and 5 additional smoking‐related domains |
|
| Funding source | This work was supported by Pfizer Inc which was the sponsor and funding source for both clinical trials reported here (NCT Identifier: NCT00143364 and NCT00141206. | |
| Author conflicts of interest | J.C.C., C.L.B. and A.G.B. are employees and share‐holders of Pfizer | |
| Notes | Outcome data source: Published data | |
Heffner 2019.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: Argentina, Australia, Brazil, Canada, Chile, Mexico, New Zealand, Russia, South Africa, USA Data collection period: November 2011 – January 2015 Registry ID: ClinicalTrials.gov: NCT01456936 |
|
| Participants |
Number of participants: N = 3079 Sample characteristics (at baseline): Age (mean): BD sub‐cohort 45.2 years (11.9), NPC cohort 45.7 years (13.2); Sex (% male): BD sub‐cohort 41.8% (119/285), NPC cohort 49.5% (1382/2794) Population category: general population and psychiatric population; Specific population: stably‐treated bipolar disorder (BD) without current substance use disorders (DSM‐IV‐TR criteria for either BD I or II as a primary diagnosis or co‐morbidity) ‐ comparison data available also for non‐psychiatric cohort (NPC) Nicotine dependence: BD sub‐cohort FTCD 6.6 (1.7), NPC cohort FTCD 5.4 (2.0); Baseline cigarettes per day: BD sub‐cohort 20.4 (8.2), NPC cohort 20.1 (8.2); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: weekly brief (≤ 10 min) behavioural counselling (included setting a quit date, managing withdrawal and cravings – did not include counselling for mental health) provided Pharmacological support for smoking cessation: placebo ‐ triple dummy placebo for each treatment arm 12 weeks; Varenicline tartrate ‐ participants titrated to the full dose during the first week (0.5 mg (tablet form) once a day for 3 days, 0.5 mg twice a day for 4 days, then 1 mg twice a day for 11 weeks); Bupropion hydrochloride – participants received 150 mg (tablet form) once a day for 3 days and then took 150 mg twice a day for the remainder of the treatment period (11 weeks and 4 days); NRT patch – participants started active dosing the morning of the Week 1 visit and received a 21 mg transdermal patch per day x 7 weeks, followed by a 14 mg transdermal patch per day x 2 weeks, and then a 7 mg transdermal patch x 2 weeks for a total of 11 weeks of treatment Psychotherapeutic or psychoactive support for mental health or mood: 86/285 of BD sub‐cohort and 692/2794 of NPC cohort received bupropion SR (150 mg twice daily) i.e. received mood management |
|
| Outcomes |
Definition of cessation used: continuous abstinence from smoking was assessed at each week by self‐report and bioverified; CO‐confirmed complete abstinence during final 4 weeks was the primary outcome with CO‐confirmed complete abstinence for Weeks 9 – 24 and 7‐day point prevalence abstinence at Weeks 12 and 24 as secondary outcome Cessation definition used for outcome(s) in this analysis: continuous abstinence Measure of biovalidation: expired CO (abstinence cut‐off < 10 ppm) Definition of people continuing to smoke used: exact definition not reported – assessed via self‐report questionnaire and CO level > 10 ppm considered indicative of smoking; participants lost to follow‐up or missing at Week 12 visit were considered still smoking Time point(s) at which follow‐up was conducted: 24 weeks (12 week follow‐up after the end of the intervention) Outcome category: Clinically‐significant neuropsychiatric AEs (NPSAEs) Outcome measure(s): trial adverse events (not a validated scale) ‐ the occurrence of ≥ 1 moderate‐to‐severe NPSAE fitting 1 of the prespecified 16 categories of events: anxiety, depression, feeling abnormal, hostility, agitation, aggression, delusions, hallucinations, homicidal ideation, mania, panic, paranoia, psychosis, suicidal behaviour, suicidal ideation, or completed suicide |
|
| Funding source | Study was funded by Pfizer and GlaxoSmithKline | |
| Author conflicts of interest | CR, DL, LSA, and TM are employees and stockholders of Pfizer. AK is a PAREXEL employee working on behalf of GlaxoSmithKline. AEE reports research grants to her institution from Forum Pharmaceuticals and Pfizer and personal fees for advisory board services from Pfizer and Reckitt Benckiser. RW is a consultant to Pfizer, Johnson & Johnson, and GlaxoSmithKline and has received research funding from Pfizer and Johnson & Johnson; RW's salary is funded by Cancer Research UK. RMA reports his university receiving grants from Alkermes and Pfizer and providing consulting and/or advisory board services to Arena Pharmaceuticals, Cerecor, Pfizer, and US WorldMeds | |
| Notes |
Outcome data source: Published data Other: EAGLES trial included countries from Europe but these were excluded from this secondary analysis as regulatory authorities contra‐indicated use of bupropion in BD |
|
Hughes 1991.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 315 Characteristics (at baseline): Age (mean): nicotine gum 37.4 years (SD 9.7), placebo 36.3 years (SD 10.3); Sex (% male): nicotine gum 45%, placebo 41% Population category: psychiatric population; Specific population: people with addiction disorders Nicotine dependence: Fagerström score, nicotine gum 5.8 (SD 1.5), placebo 5.7 (SD 1.5); Baseline cigarettes per day: nicotine gum 29.8 (SD 10.7), placebo 29.2 (SD 12.0); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: participants counselled by the study nurse and their physician for 10 minutes each Pharmacological support for smoking cessation: participants randomly assigned nicotine gum or placebo gum Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: exact definitions unclear, reference to ‘complete abstinence’ and ‘slippers’ (defined as reporting smoking very few cigarettes) Cessation definition used for outcome(s) in this analysis: unclear point‐prevalence Measure of biovalidation: at 12‐month follow‐up participants self‐reporting abstinence were paid to provide a breath sample for carbon monoxide and saliva sample for cotinine and thiocyanate Definition of people who continued to smoke used: self‐reported return to smoking i.e. ‘smokers who did not quit’; non‐responders to follow‐up considered still smoking Time point(s) at which follow‐up was conducted: 1, 6 and 12 months after participant quit date Outcome category: Anxiety Outcome measure(s): observer and participant withdrawal rating scales ‐ the scales asked participants or observers to rate the participant's symptoms, with 0 indicating not present; 1, mild; 2, moderate; and 3, severe. Ratings were based on the last 24 hours. Observers were asked to ignore self‐report as much as possible and base their rating on observed behaviour. Observers were not asked to rate several symptoms because pilot work indicated they could not do so reliably. Observers were also asked to indicate whether they thought each day's rating was reliable |
|
| Funding source | Funded by grants DA‐04066,DA‐03728, and DA‐0298, Research Scientist Development Award DA‐00109 (Dr Hughes) from the National Institute on Drug Abuse, and grant HL‐39220 from the National Heart, Lung, and Blood Institute, Bethesda, Md | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Kahler 2002.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 179; Number included in meta‐analysis: N = 164 Sample characteristics (at baseline): Age (mean): 45.1 years (SD 9.3); Sex (% male): 40.2% (72/179) Population category: psychiatric population; Specific population: people with history of major depressive disorder (MDD) Nicotine dependence: FTND 6.4 (SD 1.8); Baseline cigarettes per day: 27.3 (SD 11.3); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: some participants received standard smoking cessation treatment (ST) only, including self‐management, nicotine fading and relapse prevention Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: received mood management‐some participants received smoking cessation treatment plus cognitive behavioural therapy for depression (CBT‐D) |
|
| Outcomes |
Definition of cessation used: self‐reported point‐prevalence abstinence in 2 weeks following quit date; continuous abstinence during each week of 52 weeks leading up to 1‐year follow‐up Cessation definition used for outcome(s) in this analysis: continuous abstinence Measure of biovalidation: CO level < 10 ppm and cotinine level < 46 ng/ml, in cases where biochemical verification could not be obtained (6.5%) verification was by interview with a significant other Definition of people who continued to smoke used: minimal smoking (average of less than 1 cig per day during the week), moderate smoking (average of 1 ‐ 9 cigs per day), relapsed (smoking an average 10 or more cigs per day) Time point(s) at which follow‐up was conducted: each treatment session from quit date to end of treatment (session 5 – 4 weeks after session 1); 1‐, 6‐ and 12‐month follow‐ups (from quit date ‐ unclear) Outcome category: Depression Outcome measure(s): Beck Depression Inventory (BDI) |
|
| Funding source | Partially supported by Grant DA08511 from the National Institute on Drug Abuse to Richard A. Brown | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Kahler 2011.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated Registry ID: ClinicalTrials.gov: NCT00107575 |
|
| Participants |
Number of participants: N = 236; Number included in meta‐analysis: N = 113 Sample characteristics (at baseline): Age (mean): 41.5 years (SD 2.6); Sex (% male): 55% (130/236) Population category: psychiatric population; Specific population: people who were drinking heavily and smoking Nicotine dependence: FTND 5.0 (SD 2.2); Baseline cigarettes per day: 21.3 (SD 9.4); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: either counselling only or counselling plus brief alcohol intervention (ST‐BI), no mood management Pharmacological support for smoking cessation: all received NRT (transdermal nicotine patch) Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: self‐reported 7‐day point‐prevalence abstinence Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: expired CO level (≤ 10 ppm), saliva sample for cotinine level (≤ 15 ng/ml) – if bioverification was not possible abstinence was confirmed by a significant other Definition of people who were continuing to smoke used: non‐abstainers if no self‐reported abstinence or self‐reported abstinence missing data Time point(s) at which follow‐up was conducted: at 2, 8, 16 and 26 weeks after each participant quit date Outcome category: Depression Outcome measure(s): Center for Epidemiologic Studies Depression Scale (CES‐D) |
|
| Funding source | National Institute on Drug Abuse, grants R01 DA15534 to CK, K08 DA029094 to NS, and K08 DA025041 to AL | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published and unpublished data | |
Kahler 2014.
| Study characteristics | ||
| Methods |
Study design: non‐randomised intervention study Country: USA Data collection period: February – November 2011 Registry ID: not reported |
|
| Participants |
Number of participants: N = 19 Sample characteristics (at baseline): Age (mean): 45 years (SD 9.9); Sex (% male): 68.4% Population category: general population; Specific population: participants who wanted help to quit smoking and reported low positive affect (average item score of ≤ 2.5 on the positive affect subscale of the CES‐D) Nicotine dependence: FTND 5.7 (SD 1.7); Baseline cigarettes per day: 18.3 (SD 5.2); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: standard behavioural treatment based on recent clinical practice guidelines for smoking cessation (Fiore, 2008). The focus of the first 2 sessions was on identifying reasons for quitting, seeking social support for quitting, and problem‐solving in high‐risk situations for smoking relapse. Participants were also instructed on the proper use of nicotine patch. Identifying and planning for high‐risk situations remained the focus of the treatment after quit date with counsellors providing support, reinforcing success, and bolstering self‐efficacy, as well as managing slips in the event that participants smoked after quit date Pharmacological support for smoking cessation: participants smoking more than 10 CPD at baseline received treatment with transdermal nicotine patch with the initial dose starting on quit date at 21 mg for 4 weeks, followed by 2 weeks of 14 mg patch, and then 2 weeks of 7 mg patch; participants smoking 5 – 10 CPD started with 14 mg for 6 weeks, followed by 2 weeks of 7 mg patch Psychotherapeutic or psychoactive support for mental health or mood: received mood management ‐ the Positive Psychotherapy for Smoking Cessation (PPT‐S) intervention integrated standard smoking cessation counselling with nicotine patch and a package of positive psychology interventions. During the course of this feasibility study the positive psychology intervention components were developed further but started with 6 exercises used previously in trials of mild‐to‐moderate depression: (1) using Signature Strengths in a new way, (2) Three Good Things, (3) Gratitude Visit, (4) Savoring, (5) Active/constructing Responding, and (6) Positive Service. A Savoring Kindness exercise was later added in place of Positive Service, while the Signature Strengths exercise was modified to focus solely on using one's strengths to aid smoking cessation |
|
| Outcomes |
Definition of cessation used: 7‐day point‐prevalence abstinence which was bioverified Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: Expired CO (≤ 10 ppm); saliva cotinine (≤ 15 ng/ml) Definition of people who continued to smoke used: exact definition not reported; not biochemically confirmed as smoking abstinent Time point(s) at which follow‐up was conducted: follow‐ups were conducted 8, 16, and 26 weeks after quit date Outcome category: Depression, Positive Affect Outcome measure(s): Center for Epidemiologic Studies Depression Scale (CES‐D), Positive and Negative Affect Schedule (PANAS‐P; positive affect subscale) |
|
| Funding source | This research was supported by the National Cancer Institute grant R01CA156241 to CWK | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Kohata 2016.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: Japan Data collection period: May 2011 – November 2013 Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 191; Number included in meta‐analysis: N = 191 Sample characteristics (at baseline): Age (mean): Success 47.0 years (SD 13.9); Failure 46.4 years (SD 12.4); Sex (% male): Success 58.2% (82/141), Failure 64% (32/50) Population category: general population; Specific population: participants who visited smoking cessation clinics of their own volition, and were treated with varenicline Nicotine dependence: not measured; Baseline cigarettes per day: Success 20.4 (SD 9.7), Failure 23.4 (SD 16.7); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: none reported but participants were attending smoking cessation clinics Pharmacological support for smoking cessation: participants initiated varenicline treatment 1 week prior to their intended smoking cessation date ‐ taken once or twice daily after eating according to the following treatment regimen: 0.5 mg once daily from day 1 to day 3, 0.5 mg twice daily from day 4 to day 7, and 1 mg twice daily from day 8 to the end of the treatment (participants took varenicline for a total of 12 weeks) Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: participants who had undergone smoking cessation therapy for 12 weeks received a survey through the mail 1 year after the treatment and asked whether smoking cessation was achieved, participants were subsequently divided into ‘success’ or ‘failure’ groups Cessation definition used for outcome(s) in this analysis: unclear point‐prevalence Measure of biovalidation: not bioverified Definition of people who continued to smoke used: exact definition not reported; "patients who did not succeed at smoking cessation at 1 year after the treatment" Time point(s) at which follow‐up was conducted: 1 year Outcome category: Positive Affect, Psychological Quality of Life (QoL) Outcome measure(s): SF‐8 vitality subscale, SF‐8 mental composite score |
|
| Funding source | The authors have no support or funding to report | |
| Author conflicts of interest | Prof. Arakawa received lecture fees from Otsuka and Eisai and research grants from Otsuka, Eisai, Astellas, Abbott Japan,Takeda, Dainippon Sumitomo, and Daiichi Sankyo. The remaining authors have no conflicts to disclose | |
| Notes | Outcome data source: Unpublished data | |
Krebs 2018.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 577; Number included in meta‐analysis: N = 577 Sample characteristics (at baseline): Age (mean): VA counselling 53.8 years (SD 12.2), Quitline 53.6 years (SD 11.7); Sex (% male): VA counselling 91.8% (248/270), Quitline 92.5% (284/307) Population category: psychiatric population; Specific population: veterans with mental health conditions Nicotine dependence: time to first cigarette ≤ 5 min – VA counselling 36.6%, Quitline 30.8%; Baseline cigarettes per day: VA counselling 17.5 (SD 12.1), Quitline 14.9 (SD 10.5); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: all enrolled participants received mailed self‐help materials (provided brief advice on preparing to quit smoking, setting a quit date, preventing relapse and corrective information on common myths about smoking among persons with mental health diagnosis) and smoking cessation medications, unless they declined or contraindications noted. Booklet’s imagery was tailored for veteran mental health using military personnel images. Participants randomly assigned to A) VA counselling based on a structured counselling protocol created specifically for the study, the content was based on Motivational Interviewing and Problem Solving Therapy and protocol allowed for up to 10 calls, comprising either planning (pre‐quit) and follow‐up (post‐quit) sessions at 0, 1, 3, 7, 14, 21, and 30 days after their quit date ‐ addressed both behavioural and cognitive issues, including motivation, self‐efficacy, difficult situations, comorbid mental health symptoms, coping strategies, medication usage, and relapse prevention; or B) Quitline counselling where a research assistant initiated a “warm transfer” of the participant to their Quitline via a 3‐way call to start the counselling process after which study personnel were not involved in any aspect of the Quitline counselling ‐ Quitlines followed their regular service protocols, which depending on the Veteran’s state, ranged from 1 ‐ 6 sessions Pharmacological support for smoking cessation: all enrolled participants received smoking cessation medications, unless they declined or had contraindications noted by their referring provider Psychotherapeutic or psychoactive support for mental health or mood: received mood management – the content of VA counselling “addressed both behavioural and cognitive issues including…comorbid mental health symptoms”; booklet also provided corrective information on 5 common myths about smoking among persons with a mental health diagnosis |
|
| Outcomes |
Definition of cessation used: self‐reported abstinence (smoked even a puff) in the past 30 days assessed at 2 and 6 months from enrolment Cessation definition used for outcome(s) in this analysis: prolonged abstinence Measure of biovalidation: not bioverified Definition of people who continued to smoke used: exact definition not reported; self‐reported non‐abstinence Time point(s) at which follow‐up was conducted: 2 and 6 months from enrolment Outcome category: Depression, Psychological Quality of Life (QoL), Social Outcome Outcome measure(s): Behaviour and Symptom Identification Scale 24‐item (BASIS‐24; depression/function, emotional lability and interpersonal relationships subscales) |
|
| Funding source | The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This material is based upon work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, and Health Services Research and Development, grant #SDP 07‐034 | |
| Author conflicts of interest | The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article | |
| Notes | Outcome data source: Published data | |
Lechner 2019.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 150; Number included in meta‐analysis: N = 37 Sample characteristics (at baseline): Age (mean): 42.1 years (SD 12.7); Sex (% male): 58.7% (88/150) Population category: general population; Specific population: people who were drinking heavily and smoking (at least once per month on average (≥ 4 drinks per occasion for women; ≥ 5 drinks for men)) recruited from the community Nicotine dependence: FTCD 5.3 (SD 2.3); Baseline cigarettes per day: 17.9 (SD 9.5); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: participants received 6‐sessions of counselling that addressed heavy drinking and smoking. The Counseling and Medication Management (CMM) intervention provided (i) smoking cessation treatment consistent with clinical practice guidelines, (ii) counselling on alcohol and its impact on smoking cessation, and (iii) monitoring of oral study medication use and safety following guidelines in medical management – in addition to standard smoking cessation modules, session 1 included normative feedback on drinking and discussion of goals related to short‐ and long‐term changes in drinking. subsequent sessions focused on study medication use, side effects, progress in quitting smoking, provision of support, review of current drinking, efforts to modify drinking, and problem‐solving for high‐risk situations for smoking relapse; a quit date was set in the second week of the 10‐week treatment Pharmacological support for smoking cessation: participants received 6 weeks of transdermal nicotine patches; participants were drawn from a parent study in which they were randomised to receive 10 weeks of either i) 50 mg naltrexone (n = 75), or ii) placebo (n = 75) daily Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: 7‐day point‐prevalence smoking abstinence was assessed at 2, 8, 16, and 26 weeks after quit date and was bioverified Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: expired CO ( ≤ 4 ppm) Definition of people who continued to smoke used: exact definition not reported; ‘no significant change in use relevant to baseline’ Time point(s) at which follow‐up was conducted: 2, 8, 16, and 26 weeks after quit date Outcome category: Depression Outcome measure(s): Center for Epidemiologic Studies Depression Scale (CES‐D) |
|
| Funding source | This study was supported by NIHNIAAA R01‐ AA01718 (Kahler) | |
| Author conflicts of interest | No conflict declared | |
| Notes | Outcome data source: Published data | |
Lee 2019.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: South Korea Data collection period: September 2013 – February 2019 Registry ID: ClinicalTrials.gov: NCT02916628 |
|
| Participants |
Number of participants: N = 163; Number included in meta‐analysis: N = 54 Sample characteristics (at baseline): Age (mean): people who quit 26.9 years (SD 5.2), people who continued smoking 27.2 years (SD 5.5); Sex (% male): people who quit 100% (15/15), people who continued to smoke 95.3% (141/148) Population category: general population; Specific population: participants in a RCT of smoking cessation Nicotine dependence: not measured; Baseline cigarettes per day: people who quit 9.7 (SD 4.9), people who continued to smoke 13.5 (SD 6.6); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: the smoking cessation intervention consisted of 6 sessions of motivational interviewing, positive group psychotherapy, and auricular acupressure ‐ total intervention time was 6 hours (1 hour per week for 6 weeks) Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: participants received positive group psychotherapy, but there is no clear evidence that this is effective for treatment of low mood or other mood‐related symptoms |
|
| Outcomes |
Definition of cessation used: ‘abstainers’, exact definition not reported in paper; NCT record states self‐reported 7‐day point‐prevalence abstinence (where abstinence reported) would be bioverified Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: not reported in paper; NCT record states urine cotinine and serum cotinine ( ≤ 10 ng/ml) Definition of people who continued to smoke used: ‘smokers’, exact definition not reported; ‘failed to maintain smoking cessation’ Time point(s) at which follow‐up was conducted: 6 weeks, 6 months, 1 year, and 2 years after the smoking cessation intervention Outcome category: Depression Outcome measure(s): Patient Health Questionnaire (PHQ‐9) |
|
| Funding source | None specified | |
| Author conflicts of interest | Dr. Lee has received research grants from the National Research Foundation of Korea (NRF) (2014002505) | |
| Notes | Outcome data source: Published data | |
Leventhal 2014.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: January 2005 – January 2007 Registry ID: ClinicalTrials.gov: NCT00332644 |
|
| Participants |
Number of participants: N = 1469; Number included in meta‐analysis: N = ranges from 964 (Social Outcome); 966 (PANAS) Sample characteristics (at baseline): Age (mean): 44.7 years (SD 11.1); Sex (% male): 41.6% (611/1469) Population category: general population; Specific population: participants in a RCT of smoking cessation Nicotine dependence: FTND 5.4 (2.1); Baseline cigarettes per day: 21.4 (SD 8.9); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: all participants received individual cessation counselling (manualised, lasting 10 – 20 minutes each and provided social support as well as problem‐solving training at the pre‐quit visit, quit day, and every subsequent study visit up to 8 weeks post‐quit day; counsellors were bachelors‐level case managers supervised by a licensed clinical psychologist Pharmacological support for smoking cessation: randomisation into 1 of 6 treatment conditions (bupropion SR (n = 264; 150 mg twice per day for 1 week pre‐quit and 8 weeks post‐quit); nicotine lozenge (n = 260; 2 or 4 mg lozenges, dose based on package instructions for 12 weeks post‐quit); nicotine patch (n = 262; 24‐hour patch of 21, 14, and 7 mg titrated over 8 weeks post‐quit); nicotine patch + nicotine lozenge (n = 267); bupropion SR + nicotine lozenge (n = 262); or placebo (n = 189; 5 conditions that matched the 5 active conditions)) Psychotherapeutic or psychoactive support for mental health or mood: received mood management ‐ 526/1504 participants received bupropion SR as pharmacological support |
|
| Outcomes |
Definition of cessation used: 7‐day point‐prevalence abstinence (“Have you smoked at all, even a puff, in the last seven days?” yes/no) was assessed at 8 weeks and 6 months following the target quit day and self‐reports were bioverified; participants who did not provide outcome data were coded as non‐abstinent Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: expired CO ( < 10 ppm) Definition of people who continued to smoke used: exact definition not reported in paper; no CO‐confirmed 7‐day point‐prevalence abstinence Time point(s) at which follow‐up was conducted: study visits on participant quit day and at 1, 2, 4, 8, and 52 weeks post‐quit; follow‐up also at 3 years Outcome category: Mixed Anxiety + Depression, Positive Affect, Social Outcome Outcome measure(s): Positive and Negative Affect Scale (PANAS); Quality of Life Inventory (QOLI; friendships subscale) |
|
| Funding source | This research was supported by grants P50DA019706, K08DA021311, K08DA02504, and 1K05CA139871 from National Institutes of Health (NIH), 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources (NCRR), and an Institutional Clinical and Translational Science Award (UW‐Madison; KL2 Grant # 1KL2RR025012‐01). GlaxoSmithKline provided medication to patients at no cost. Additional funding source in Piper 2012/2013: grant no. M01 RR03186 from the General Clinical Research Centers Program of the National Center for Research Resources, NIH | |
| Author conflicts of interest | Not reported in Leventhal 2014 but in Piper 2013: Megan E. Piper, Matthew Rodock, Jessica W. Cook, Tanya R. Schlam and Timothy B. Baker have no potential conflicts of interest to disclose. Over the last 3 years, Michael C. Fiore served as an investigator on research studies at the University of Wisconsin that were funded in part by NabiBiopharmaceuticals. From 1998 to 2010, Dr. Fiore held a University of Wisconsin named Chair, made possible by a gift from GlaxoWellcome | |
| Notes | Outcome data source: Unpublished data | |
Levy 2018.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: December 2012 – July 2014 Registry ID: ClinicalTrials.gov: NCT01714323 |
|
| Participants |
Number of participants: N = 1357; Number included in meta‐analysis: N = 1357 Sample characteristics (at baseline): Age (mean): 49.7 years (SD 12.6); Sex (% male): 50.8% (689/1357) Population category: general population; Specific population: people who smoked recruited during hospitalisation for RCT of standard vs sustained cessation interventions Nicotine dependence: not measured; Baseline cigarettes per day: not measured; Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: all participants received a brief in‐hospital smoking intervention before randomisation at discharge to either A) Standard Care – information handout about how to contact state quitline for additional cessation support and to use cessation medication as recommended by hospital smoking counsellor; or B) Extended Care – 3‐month programme consisting of 1) free medication and 2) Interactive Voice Response (IVR) triage to telephone counselling from national quitline provider which aims to identify people who smoke needing post‐discharge support with immediate transfer to live telephone counsellor (aims to encourage medication adherence and enhance counselling efficiency) Pharmacological support for smoking cessation: free medication ‐ a 30‐day supply of FDA‐approved medication (nicotine replacement, bupropion, or varenicline) given at hospital discharge and refillable for a total of 90 days to encourage medication use and adherence Psychotherapeutic or psychoactive support for mental health or mood: received mood management – some participants received bupropion |
|
| Outcomes |
Definition of cessation used: biochemically confirmed 7‐day point‐prevalence abstinence at 6 months, was the primary outcome for the trial, assessed at 1, 3, and 6 months post‐discharge; also time‐varying measure of continuous abstinence using 7‐PPA from any tobacco (including e‐cigarettes), considered ‘continuously abstinent’ if no indication of smoking from measurement time point back to baseline (hospital discharge) Cessation definition used for outcome(s) in this analysis: multiple (point‐prevalence and continuous abstinence) Measure of biovalidation: saliva cotinine (≤ 10 ng/mL) or expired CO (< 9 ppm) for those using NRT Definition of people who continued to smoke used: exact definition not reported; ‘smokers’ or ‘non‐abstinent’ – study participants not reporting smoking status at follow‐up were considered to still be smoking at that time point Time point(s) at which follow‐up was conducted: 1, 3, and 6 months post‐hospital discharge Outcome category: Depression Outcome measure(s): Patient Health Questionnaire 4‐item (PHQ‐4) |
|
| Funding source | This work was supported by the National Heart Lung and Blood Institute (R01‐HL111821) | |
| Author conflicts of interest | Dr. Rigotti receives royalties from UpToDate. Dr. Rigotti has been an unpaid consultant for Pfizer, Inc., and Optum, Inc., regarding smoking cessation. She has received travel expenses from Pfizer to attend a consultant meeting for which she received no honorarium. Dr. Levy has been a paid consultant to CVS, Inc., to provide expertise on tobacco policy. Dr. Singer has been a paid consultant for Pfizer, Inc., but on matters separate from smoking cessation | |
| Notes | Outcome data source: Published data | |
Longmore 2007.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: USA Data collection period: the PREMIER registry began on January 1st 2003 and ended on June 28th 2004 Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 1144; Number included in meta‐analysis: N = 640 Sample characteristics (at baseline): Age (mean): participants were 18+ years; Sex (% male): not reported Population category: chronic physical conditions; Specific population: people with myocardial infarction (MI) Nicotine dependence: not reported; Baseline cigarettes per day: not reported; Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: quitters defined as those who were smoking at baseline but not 6‐ and 12‐months post‐MI Cessation definition used for outcome(s) in this analysis: unclear point‐prevalence Measure of biovalidation: not bioverified Definition of people who continued to smoke used: not reported Time point(s) at which follow‐up was conducted: baseline (time of MI), 6‐ and 12‐months post‐MI Outcome category: Psychological Quality of Life (QoL) Outcome measure(s): Short Form‐12 (SF‐12; mental component scale (MCS)) |
|
| Funding source | This study was principally supported by CV Therapeutics, Inc, Palo Alto, CA, and R‐01 HS11282‐01 from the Agency for Healthcare Research and Quality, Rockville, MD, This study was also supported by a Veterans Affairs Health Services Research Advanced Research Career Development Award (ARCD‐98‐341‐2) (Dr Rumsfeld), Washington, DC | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data (conference abstract) | |
Lopez 2015.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: 2001 ‐ 2013 Registry ID: not reported ‐ data from 4 controlled clinical trials |
|
| Participants |
Number of participants: N = 289; Number included in meta‐analysis: N = 234 Sample characteristics (at baseline): Age (mean): Contigent 24.3 years (SD 5.2), Non‐contingent 23.8 years (SD 4.9); Sex (% male): 0% (0/289) Population category: pregnant population; Specific population: pregnant women who smoked enrolled in 1 of 4 controlled clinical trials of financial incentives for smoking cessation recruited from the same area Nicotine dependence: not measured; Baseline cigarettes per day: Contigent 9.0 (SD 6.6), Non‐contingent 9.5 (SD 6.6); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: all participants were assigned to A) abstinence‐contingent incentives (earned vouchers exchangeable for retail items contingent on biochemically‐verified abstinence from recent smoking; beginning at relatively low value and escalating per consecutive negative specimen to maximum value) or B) non‐contingent incentives control conditions (vouchers of comparable monetary value delivered independent of smoking status); incentive interventions were in place from study initiation to 12‐weeks postpartum and earnings did not differ significantly between condition (averaged approximately USD 450 (range USD 0 ‐ USD 1,180) per woman); women in both conditions received using smoking cessation care via obstetric clinics Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: 7‐day point‐prevalence abstinence (self‐report via questionnaire and biochemically verified at each time point) Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: urine cotinine Definition of people who continued to smoke used: exact definition not reported Time point(s) at which follow‐up was conducted: 1 month after the study intake assessment (mid‐pregnancy), at a late‐pregnancy assessment (≥ 28‐weeks gestation), and at 2, 4, 8, 12, and 24 weeks postpartum Outcome category: Depression Outcome measure(s): Beck Depression Inventory (BDI) |
|
| Funding source | This research was supported by National Institute of Health (NIH) Research Awards R01DA014028 and R01HD075669, Institutional Training Grant T32DA007242, Tobacco Centers of Regulatory Science Award P50DA036114, and Centers of Biomedical Research Excellence Center Award P20GM103644 | |
| Author conflicts of interest | None declared | |
| Notes | Outcome data source: Unpublished data | |
Lubitz 2019.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: October 2012 ‐ June 2018 Registry ID: ClinicalTrials.gov: NCT01710137 |
|
| Participants |
Number of participants: N = 179; Number included in meta‐analysis: N = 179 Sample characteristics (at baseline): Age (mean): 48.6 years (SD 9.9); Sex (% male): 68.2% (122/179) Population category: chronic physical condition; Specific population: people living with HIV/AIDS (PLWHA) recruited through Penn’s health system, media ads, and through a community‐based HIV clinic Nicotine dependence: FTND 4.7 (SD 2.1); Baseline cigarettes per day: 11.5 (SD 7.9); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: all participants were offered 6 standardised, Public Health Service guideline‐based smoking cessation counselling sessions at Weeks 0, 1, 3, 5, 7, and 9, in‐person one‐to‐one or by telephone; sessions were delivered by trained counsellors supervised by a clinical psychologist; sessions were designed to help participants understand the risks associated with smoking, prepare for a target quit‐date at Week 1 and develop skills to manage nicotine withdrawal and avoid relapse Pharmacological support for smoking cessation: randomly allocated to placebo (n = 90) or varenicline (n = 89) was provided at Week 0 based on U.S. Food and Drug Administration labelling: Day 1 ‐ Day 3 (0.5 mg once daily); Days 4 – 7 (0.5 mg twice daily); and Days 8 ‐ Day 84 (1.0 mg twice daily); placebo was identical in appearance and dosing regimen Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: primary smoking cessation outcome was 7‐day point‐prevalence abstinence at Week 12 (EOT), based on no self‐reported tobacco use (not even a puff) during the 7 days preceding the assessment and a CO ≤ 10 ppm Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: saliva cotinine (measured at baseline) or CO (≤ 10 ppm) Definition of people who continued to smoke used: exact definition not reported; participants lost to follow‐up or failed to provide CO measure considered ‘non‐abstinent’ Time point(s) at which follow‐up was conducted: week 0 (pre‐quit session) and week 12 (end of treatment) Outcome category: Anxiety Outcome measure(s): Hospital and Depression Anxiety Scale (HADS); HIV/AIDS‐Targeted Quality of Life Scale (life‐satisfaction subscale) |
|
| Funding source | This work was supported by National Institutes of Health [grant number K24 DA045244 and R01 DA033681] and University of Pennsylvania Center for AIDS Research [P30AI045008] and Penn Mental Health AIDS Research Center [P30 MH097488]. Pfizer provided the medication and placebo supply. National Institute of Allergy and Infectious Diseases; National Institute of Mental Health; National Institute on Drug Abuse | |
| Author conflicts of interest | Dr. Schnoll received medication and placebo free from Pfizer and has provided consultation to Pfizer. Dr. Schnoll has provided consultation to GlaxoSmithKline and CuraLeaf. Dr.Gross serves on a DSMB for a Pfizer medication unrelated to smoking or HIV. No other conflicts are declared | |
| Notes | Outcome data source: Published data | |
Manning 2005.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 300; Number included in meta‐analysis: N = 249 Sample characteristics (at baseline): Age (mean): 44.4 years (SD 11.3); Sex (% male): 30.7% (92/300) Population category: general population; Specific population: recruited from community health centre and using advertisements Nicotine dependence: not measured; Baseline cigarettes per day: 17.1 (SD 8.5); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: 8 weekly sessions of motivational interviewing and a culturally‐sensitive smoking cessation guide Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: self‐reported 7‐day point‐prevalence abstinence Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: expired CO level (≤ 10 ppm); saliva cotinine level (abstinence < 20 ng/ml) was used to resolve any discrepancies between self‐report and expired CO assessments Definition of people who continued to smoke used: people who did not quit smoking Time point(s) at which follow‐up was conducted: baseline (7 days prior to target quit day), end of treatment (6 weeks post‐target quit‐day) and follow‐up (6 months post‐target quit‐day) Outcome category: Stress Outcome measure(s): Perceived Stress Scale – 14 (PSS‐14) |
|
| Funding source | Grants RO1CA77856 and K07 CA87714 from the National Cancer Institute; Glaxo‐Wellcome, Inc. provided study medication but played no role in the design, conduct of study or interpretation of data analysis | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Marqueta 2010.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort (smoking cessation service data) Country: Spain Data collection period: 2005 ‐ 2008 Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 569 Sample characteristics (at baseline): Age (mean): 43.3 years (SD 9.9); Sex (% male): 50.6% (288/569) Population category: general population; Specific population: general population Nicotine dependence: Fagerström score, 6 (SD 2.3); Baseline cigarettes per day: 23.1 (SD 9.9); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: multicomponent treatment, including 9 sessions of psychological group therapy where the therapeutic relationship emphasised the active role of the participant Pharmacological support for smoking cessation: 1 of the 3 drugs recommended by clinical practice guidelines and based on participant preference: NRT in form of transdermal patches or chewing gum, bupropion or varenicline Psychotherapeutic or psychoactive support for mental health or mood: some participants in the cohort were using psychotropic drugs (13%) and 30.3% of total sample were prescribed bupropion i.e. received mood management |
|
| Outcomes |
Definition of cessation used: continued abstinence – self‐reported no smoking since first day Cessation definition used for outcome(s) in this analysis: continuous abstinence Measure of biovalidation: self‐reported smoking status verified by expired carbon monoxide (CO ≤ 10 ppm) Definition of people who continued to smoke used: did not self‐report continued abstinence; participants for which no data available were assumed to still be smoking Time point(s) at which follow‐up was conducted: day of clinical assessment, day before quitting (Pre‐D), day after quitting (Post‐D), 1st week, 4th week (1‐month) and 12th week (3‐months) Outcome category: Anxiety Outcome measure(s): State Trait Anxiety Inventory (STAI) |
|
| Funding source | None specified | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Martínez‐González 2018.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: Spain Data collection period: Baseline from January 2010 and 2‐year appointment at time of analysis (February 2016) Registry ID: ClinicalTrials.gov: NCT01122758 |
|
| Participants |
Number of participants: N = 1081; Number included in meta‐analysis: N = 188 Sample characteristics (at baseline): Age (mean): 65.2 years (SD 8.9); Sex (% male): 80.8% (873/1081) Population category: chronic physical condition; Specific population: data from CHAIN (COPD History Assessment in Spain), a Spanish multi‐centre study carried out at pulmonary clinics including people who actively or previously smoked with COPD Nicotine dependence: not measured; Baseline cigarettes per day: CPD per day not reported, pack years cohort 55.3 (SD 28.2); Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: yearly minimal smoking cessation counselling offered during follow‐up, no other specific tobacco intervention was carried out to quit tobacco Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: 'those who stopped smoking at time of second visit and persisted without smoking until third visit’; smoking status was evaluated from the clinical history and it was confirmed by co‐oximetry Cessation definition used for outcome(s) in this analysis: continuous abstinence Measure of biovalidation: expired CO Definition of people who continued to smoke used: exact definition not reported; ‘those who continued smoking at all 3 visits’ Time point(s) at which follow‐up was conducted: 2 years Outcome category: Anxiety, Depression Outcome measure(s): Hospital Anxiety and Depression Scale (HADS) |
|
| Funding source | This study has been funded by AstraZeneca | |
| Author conflicts of interest | C. C. has received speaker fees from Novartis, Menarini, Boehringer Ingelheim, AstraZeneca, GlaxoSmithKline, and Teva, and consulting fees from AstraZeneca, Esteve, GlaxoSmithKline, and Novartis. B. G. C. has received speaker fees from AstraZeneca,Novartis, Menarini, and Chiesi. I. S. has received company training fees from Novartis and speaker fees from Esteve. J. L. L.‐C. has received honoraria for lecturing, scientific advice, participation in clinical studies or writing for publications for: Almirall, AstraZeneca, Bayer,Boehringer Ingelheim, Cantabria Pharma, Chiesi, Esteve, Faes, Ferrer, Gebro, GlaxoSmithKline, Grifols, Menarini, MSD, Novartis, Pfizer, Rovi, Teva and Takeda. None declared for the remaining authors | |
| Notes | Outcome data source: Published data | |
Martínez‐Vispo 2016.
| Study characteristics | ||
| Methods |
Study design: non‐randomised intervention study Country: Spain Data collection period: January – October 2015 Registry ID: not reported |
|
| Participants |
Number of participants: N = 92; Number included in meta‐analysis: N = 92 Sample characteristics (at baseline): Age (mean): 44.5 years (SD 11.9); Sex (% male): 38% (35/92) Population category: general population; Specific population: people who smoked and who wanted to quit recruited via local media and healthcare systems Nicotine dependence: FTND 4.8 (SD 2.2); Baseline cigarettes per day: 19.2 (SD 8.0); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: cognitive‐behavioral psychological intervention Program to Quit Smoking was delivered for 8 sessions, once a week. The intervention consisted of therapeutic contract, self‐registration and graphic representation of cigarette consumption, general information on tobacco and smoking, fading (gradual reduction of nicotine and tar intake), stimulus control, strategies for not having withdrawal syndrome of nicotine, physiological feedback of cigarette consumption by measuring carbon monoxide (CO) in exhaled air in each session and relapse prevention strategies Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: a participant was considered abstinent at the end of the intervention if there was no smoking in the 24 hours prior to the last session and CO level was less than 10 ppm Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: expired CO < 10 ppm Definition of people who continued to smoke used: exact definition not reported; participants not available for bioverification at follow‐up considered a ‘smoker’ Time point(s) at which follow‐up was conducted: end of the intervention (8 weeks) Outcome category: Depression Outcome measure(s): Beck Depression Inventory II (BDI‐II) |
|
| Funding source | None specified | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Unpublished data | |
Mathew 2013.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 49; Number included in meta‐analysis: N = 49 Sample characteristics (at baseline): Age (mean): 41.9 years (SD 11.5); Sex (% male): 61.2% (30/49) Population category: psychiatric population; Specific population: participants meeting criteria for chronic form of depressive disorder and experiencing, or be in partial remission of, a major depressive episode at baseline (PHQ‐9 and DSM‐IV) Nicotine dependence: FTND 5.3 (SD 2.0); Baseline cigarettes per day: not reported; Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: Intervention group ‐ Cognitive Behavioral Analysis System of Psychotherapy in combination with standard smoking cessation treatment (CBASP/ST); Comparison group ‐ Health Education plus standard smoking cessation treatment (HE/ST); for both treatment groups, 12 treatment sessions were provided by clinical psychologists and participants were instructed to set a quit date following Week 6 of treatment Pharmacological support for smoking cessation: nicotine replacement therapy (NRT) was provided to participants in both groups. Participants were provided with a total of 8 weeks of NRT, beginning on the scheduled quit date and tapering from patches with 21 mg nicotine dosages to 14 and 7 mg patches Psychotherapeutic or psychoactive support for mental health or mood: received mood management ‐ intervention group comprised a Cognitive Behavioral Analysis System of Psychotherapy (referenced: McCullough 2000. Treatment for chronic depression: Cognitive behavioral analysis system of psychotherapy (CBASP). New York: Guilford Press.) |
|
| Outcomes |
Definition of cessation used: ‘prolonged abstinence’ –defined as sustained abstinence from end of treatment (EOT; visit 12) to 3‐month follow‐up, biochemically verified by expired CO (< 10 ppm) at the 3‐month follow‐up assessment; 7‐day point‐prevalence abstinence defined as self‐report of no smoking, not even a puff, in the 7 days prior to the selected time point of interest (visits 8 – 12) plus a corresponding CO < 10 ppm Cessation definition used for outcome(s) in this analysis: prolonged abstinence Measure of biovalidation: expired CO < 10 ppm Definition of people who continued to smoke used: prolonged abstinence at 3‐month follow‐up used to assign participants to ‘prolonged abstainers’ or ‘non‐abstainers’; participants considered ‘non‐abstainers’ if reported smoking on 7 or more consecutive days or smoking at least once each week over 2 consecutive weeks Time point(s) at which follow‐up was conducted: data were collected from participants at the 12 weekly treatment sessions and follow‐up sessions at 3 and 6 months Outcome category: Depression, Mixed Anxiety + Depression, Positive Affect Outcome measure(s): Beck Depression Inventory II (BDI‐II); Positive and Negative Affect Scale (PANAS) |
|
| Funding source | This work was supported the National Institute of Mental Health (R01 MH076776‐03 to JAB), National Cancer Institute (R25T CA057730 to ARM; S. Chang, Principal Investigator), and National Institutes of Health (P30 CA016672 to the University of Texas MD Anderson Cancer Center) | |
| Author conflicts of interest | None declared | |
| Notes | Outcome data source: Published data | |
McDermott 2013.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: UK Data collection period: June 2007 – September 2009 Registry ID: ISRCTN 14352545 |
|
| Participants |
Number of participants: N = 491; Number included in meta‐analysis: N = 491 Sample characteristics (at baseline): Age (mean): people who quit 51.5 years, people who smoked 47.7 years; Sex (% male): 45% (221/491) Population category: general population; Specific population: people who smoked recruited from primary care Nicotine dependence: FTND people who quit 4.8 (SD 2.0), people who smoked 5.7 (SD 2.2); Baseline cigarettes per day: people who quit 18.7 (SD 7.8), people who smoked 21.0 (SD 8.9); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: 7 weekly behavioural support sessions Pharmacological support for smoking cessation: all prescribed NRT (nicotine patch) based on heaviness of smoking; also randomised to receive additional oral NRT (6 mg or 12 mg) based on genotype or nicotine dependence (phenotype) Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: self‐reported continuous abstinence at 6 months (with initial 2‐week grace period); participants considered to be continuously abstinent if they reported they had smoked no more than 5 cigarettes since start of abstinence period Cessation definition used for outcome(s) in this analysis: prolonged abstinence Measure of biovalidation: salivary cotinine level (< 15 ng/ml) Definition of people who continued to smoke used: relapsers; non‐responders or invalidated self‐reports counted as smoking Time point(s) at which follow‐up was conducted: assessed at 6 months Outcome category: Anxiety Outcome measure(s): State Trait Anxiety Inventory: State Scale Short Form (STAI‐6) |
|
| Funding source | This study was funded as part of a grant from the Medical Research Council, UK: G0500274 | |
| Author conflicts of interest | Paul Aveyard has done consultancy and research on smoking cessation for pharmaceutical companies | |
| Notes | Outcome data source: Published data | |
McFall 2006.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 107; Number included in meta‐analysis: N = 107 Sample characteristics (at baseline): Age (mean): 52.1 years (SD 6.8); Sex (% male): 91.6% (98/107) Population category: psychiatric population; Specific population: Veterans with Post Traumatic Stress Disorder (PTSD) Nicotine dependence: FTND 6.2 (SD 2.2); Baseline cigarettes per day: 26.3 (SD 14.5); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: behavioural support and mood management – smoking cessation behavioural counselling rolled into regularly scheduled mental health visits i.e. integrated care (IC) Pharmacological support for smoking cessation: pharmacological support received (bupropion or NRT, or both) Psychotherapeutic or psychoactive support for mental health or mood: received mood management ‐ psychotropic medications for PTSD, supportive psychotherapy and some participants received bupropion |
|
| Outcomes |
Definition of cessation used: 7‐day point‐prevalence abstinence (self‐report) Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: expired CO (CO ≤ 10 ppm) Definition of people who continued to smoke used: people who continued to smoke – failed to achieve 7‐day point‐prevalence abstinence criterion Time‐point(s) at which follow‐up was conducted: 2‐, 4‐, 6‐ and 9‐months post‐enrolment Outcome category: Psychological Quality of Life (QoL) Outcome measure(s): Short Form Health Survey – 36 (SF‐36; mental health subscale) |
|
| Funding source | The Northwest Network Mental Illness Research, Education and Clinical Center, the Center for Excellence in Substance Abuse Treatment and Education, and a grant from the University of Washington Alcohol and Drug Abuse Institute | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
McMahon 1998.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 844 Sample characteristics (at baseline): Age (Mean): 38 years; Sex (% male): 37% Population category: general population; Specific population: employees from worksites Nicotine dependence: not measured; Baseline cigarettes per day: 21; Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: a) self‐help manuals only which included strategies to learn about pattern (e.g. triggers, tracking habits), changing the pattern (e.g. making plan of action) and reinforcing quitting (e.g. anticipating smoking urges); b) self‐help manuals and incentives (USD 1 each day abstinent); or c) self‐help manuals, incentives and group support (cognitive behavioural techniques and social support) Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: received mood management ‐ group intervention were taught relaxation techniques as aid for coping with stress and quitting |
|
| Outcomes |
Definition of cessation used: point‐prevalence – self‐reported cessation (people who quit) Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: self‐reported abstinence verified using exhaled carbon monoxide (cut‐off point of 9 ppm); cotinine samples collected at end of 6‐month intervention Definition of people who continued to smoke used: not reported point‐prevalence abstinence at follow‐up Time point(s) at which follow‐up was conducted: pre‐test (prior to any intervention), post‐test (immediately following 3‐week intervention), 6‐months (immediately following 2nd phase of intervention) and 12, 18 and 24 months post‐test Outcome category: Stress Outcome measure(s): Perceived Stress Scale: Short Form (PSS‐4) |
|
| Funding source | None specified | |
| Author conflicts of interest | None specified | |
| Notes |
Outcome data source: Published data Other: description of interventions and biovalidation measures detailed in the 1994 paper (McMahon, Jason & Salina, 1994) |
|
Mino 2000.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: Japan Data collection period: not stated Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 191; Number included in meta‐analysis: N = 162 Sample characteristics (at baseline): Age (mean): people who quit 33.3 years (SD 9.2), people who continued smoking 36.1 years (SD 8.8); Sex (% male): 100% (191/191) Population category: general population; Specific population: factory workers Nicotine dependence: not measured; Baseline cigarettes per day: people who quit 15.7 (SD 10.1), people who continued smoking 20.0 (SD 8.4); Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: definition unclear Cessation definition used for outcome(s) in this analysis: unclear definition Measure of biovalidation: not bioverified Definition of people who continued smoking used: people who continued smoking (‘smoking group’) Time point(s) at which follow‐up was conducted: 6 months and 1 year post‐enrolment Outcome category: Mixed Anxiety and Depression Outcome measure(s): General Health Questionnaire‐30 (GHQ‐30) |
|
| Funding source | None specified | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Mitra 2004.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: USA Data collection period: phase 1 (1996 – 1997) and phase 3 (1999 – 2000) Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 656; Number included in meta‐analysis: N = 110 Sample characteristics (at baseline): Age (mean): 43.8 years (SD 13.3); Sex (% male): 41.3% (271/656) Population category: chronic physical and/or psychiatric condition; Specific population: people living independently with disabilities Nicotine dependence: not measured; Baseline cigarettes per day: not reported; Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: people who quit reported smoking in phase I but not in phase III Cessation definition used for outcome(s) in this analysis: unclear point prevalence Measure of biovalidation: not bioverified Definition of people who continued to smoke used: people who are currently smoking – respondents who reported smoking in past month in both study periods Time point(s) at which follow‐up was conducted: in phase 3 of study (1999 ‐ 2000), 2 years after first measurement Outcome category: Positive Affect, Psychological Quality of Life (QoL) Outcome measure(s): Short Form Health Survey‐36 (SF‐36; energy vitality and mental health subscales) |
|
| Funding source | None specified | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Moadel 2012.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 145; Number included in meta‐analysis: ranges from N = 124 (Depression) to 138 (Anxiety) Sample characteristics (at baseline): Age (mean): Intervention: 49.2 years (SD 7.4), Control: 47.9 years (SD 6.6); Sex (% male): Intervention: 49.3% (36/73), Control: 48.6% (35/72) Population category: chronic physical condition; Specific population: RCT of Positively Smoke Free (PSF), an intensive group‐therapy intervention targeting people with HIV who smoke, compared to standard care Nicotine dependence: Intervention Modified Fagerström Tolerance Questionnaire 4.8 (SD 2.1), Control 5.2 (SD 2.0); Baseline cigarettes per day: Intervention 12.8 (SD 10.1), Control 11.1 (SD 7.3); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: A) Standard Care ‐ participants were given a quit‐smoking brochure, brief advice to quit (i.e. < 5 minutes), and an offer of a 3‐month supply of NRT; or B) Intervention ‐ Positively Smoke Free (PSF; an intensive 8‐session group‐therapy intervention targeting people with HIV who smoke based on Social Cognitive Theory principles), choice of day or evening programme with 6 – 8 participants per group; PSF programme emphasised the use of a buddy (supportive friend/family member) to provide encouragement through quit attempt and were welcome to attend sessions; travel fare provided to each PSF attendee (and buddies); PSF content was crafted to address concerns particularly relevant to PLWH who smoke (e.g. specific risks of smoking to PLWH, co‐morbid psychiatric illness and substance use, social isolation, stress reduction, etc.); each session led by 2 co‐facilitators (a “professional” and a peer) and encourages the recounting of personal experiences as well as a group interaction dynamic; sessions are 90 minutes long and occur weekly, except for the session after Quit Day (Session 5) which is scheduled 3 days after the quit; at the first session, Quit Day is defined as the day that all tobacco use will cease and is planned for the entire group 4 weeks thereafter Pharmacological support for smoking cessation: standard care ‐ 3‐month supply of NRT Psychotherapeutic or psychoactive support for mental health or mood: received mood management – PSF content was crafted to address concerns particularly relevant to PLWH who smoke (e.g. specific risks of smoking to PLWH, co‐morbid psychiatric illness and substance use, social isolation, stress reduction, etc.) |
|
| Outcomes |
Definition of cessation used: primary endpoint was 7‐day self‐reported, point‐prevalence abstinence at the final study visit (approximately 3 months post‐quit date); participants reporting abstinence with discrepant ECO levels of ≥ 10 parts per million (ppm) were coded as non‐abstinent Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of bio‐validation: expired CO (< 10 ppm) Definition of people who continued smoking used: exact definition not reported; ‘non‐abstinent’ Time point(s) at which follow‐up was conducted: participants completed self‐report questionnaires on days 0, 42, and 132, as well as expired CO Outcome category: Anxiety, Depression Outcome measure(s): State‐Trait Anxiety Inventory (STAI), Center for Epidemiologic Studies Depression Scale (CES‐D) |
|
| Funding source | This work was supported by grant R21 DA023362 from the National Institutes of Health/National Institute on Drug Abuse. It was also supported by the Clinical Core of the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center funded by the National Institutes of Health (NIH AI‐51519) | |
| Author conflicts of interest | Dr. Shuter has received grant support from the American Legacy Foundation and the Abbott Laboratories Investigator Initiated Research Program | |
| Notes | Outcome data source: Unpublished data | |
Munafò 2008.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: UK Data collection period: 1991 ‐ 1995 Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 7089; Number included in meta‐analysis: N = 991 Sample characteristics (at baseline): Age (mean): at birth 29 years (IQR 26 – 32); Sex (% male): 0% (0/7089) Population category: pregnant population; Specific population: pregnant women Nicotine dependence: not reported; Baseline cigarettes per day: not reported; Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: point prevalence (exact definitions not provided) Cessation definition used for outcome(s) in this analysis: unclear point prevalence Measure of biovalidation: not bioverified Definition of people who continued smoking used: people who temporarily quit or who continued to smoke (exact definitions not provided) Time point(s) at which follow‐up was conducted: assessments of current smoking asked at 6 intervals – 18 weeks and 32 weeks antenatal; 8 weeks, 8, 21 and 33 months postnatal Outcome category: Depression Outcome measure(s): Edinburgh Postnatal Depression Scale (EPDS) |
|
| Funding source | The UK Medical Research Council, the Wellcome Trust, and the University of Bristol provided core support for this cohort study | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Pacheco 2017.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: Spain Data collection period: March 2011 ‐ October 2012 Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 678 Sample characteristics (at baseline): Age (mean): 49.9 years (SD 11); Sex (% male): 57.5% (390/678) Population category: general population; Specific population: people attending the smoking cessation clinic at Seville’s Hospital Universitario Virgen Macarena Nicotine dependence: Fagerström test 6 (SD 2.4); Baseline cigarettes per day: CPD not reported, pack years 37.4 (SD 22.2); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: a combined programme of pharmacological and individualised cognitive‐behavioural psychological treatment was carried out at 5 time points: baseline, 15 days, first, second and third month; in other follow‐up visits possible problems connected with the medication were assessed and abstinence was monitored; participants who did not complete the scheduled visits were telephoned to check for possible adverse effects, especially those of a neuropsychiatric nature Pharmacological support for smoking cessation: at first visit, the pneumologist decided on the pharmacological treatment‐based participant characteristics (comorbidity, concomitant medication, cost of treatment and preferences agreed with the participant): Bupropion (150 or 300 mg) or varenicline (0.5 mg/day for 3 days; 0.5 mg/12 hours from the 4th to 7th day and 1 mg/12 hours from the 8th day on), with a duration of 2 months in both cases; rescue nicotine gum was allowed at a decreasing rate over the first few days after complete cessation Psychotherapeutic or psychoactive support for mental health or mood: received mood management – 56% of final population received bupropion; also received individualised cognitive‐behavioural psychological treatment but no further details |
|
| Outcomes |
Definition of cessation used: abstinence rate established by measuring exhaled CO – no further details Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: expired CO Definition of people who continued to smoke used: exact definition not reported, ‘smoker’ at 3‐month follow‐up Time point(s) at which follow‐up was conducted: 3 months Outcome category: Anxiety, Depression Outcome measure(s): Hospital and Anxiety Depression Scale (HADS) |
|
| Funding source | This research project was financed by Fundación Neumosur 7/2008 | |
| Author conflicts of interest | The authors declare no conflict of interest | |
| Notes | Outcome data source: Published data | |
Pawlina 2015.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: Brazil Data collection period: May 2012 – August 2012 Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 142 Sample characteristics (at baseline): Age (mean): mean not reported, 64% between 40 ‐ 59 years; Sex (% male): 29.6% (42/142) Population category: general population; Specific population: participants spontaneously sought out the smoking cessation programme at 1 of 4 healthcare facilities in the city of Cuiabá, Brazil Nicotine dependence: 98/142 (69%) had a FTND score ≥ 6 (indicating high or very high nicotine dependence); Baseline cigarettes per day: 56 (39.4%) smoked 11 ‐ 20 cigarettes/day; Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: all participants were treated with the same protocol ‐ nicotine replacement therapy, bupropion and CBT; at the initial session participants were submitted to an initial psychological evaluation by a psychologist, who counselled them in relation to the proposed treatment plan, and scheduled the first CBT session; participants were invited to attend 4 weekly 90‐minute group CBT sessions (10 ‐ 15 participants each); participants were also offered the option to attend 5 follow‐up sessions, at 15, 30, 60, 90, and 180 days after the initial 4‐week treatment period Pharmacological support for smoking cessation: during the initial interview, the participants were evaluated by the physician responsible for the programme and subsequently received the appropriate medication; states participants received nicotine replacement therapy and bupropion – no further details Psychotherapeutic or psychoactive support for mental health or mood: received mood management – states all participants received bupropion |
|
| Outcomes |
Definition of cessation used: exact definition not reported; ‘able to quit smoking’ Cessation definition used for outcome(s) in this analysis: unclear definition Measure of biovalidation: not bioverified Definition of people who continued to smoke used: exact definition not reported; ‘those who continued to smoke’ Time point(s) at which follow‐up was conducted: 45 days, 6 months Outcome category: Anxiety, Depression, Stress Outcome measure(s): Beck Anxiety Inventory (BAI); Beck Depression Inventory (BDI); Lipp Inventory of Stress Symptoms for Adults |
|
| Funding source | None | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Peltzer 2015.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: Thailand Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 620 Sample characteristics (at baseline): Age (mean): 33.6 years (SD 11.6); Sex (% male): 97.7% (606/620) Population category: general population; Specific population: hospital outpatients in Nakhon Pathom province (Thailand) who screened positive for moderate risk for tobacco and alcohol use and involved in a RCT comparing smoking cessation treatment that integrated a brief alcohol intervention and smoking cessation or alcohol intervention alone Nicotine dependence: not measured; Baseline cigarettes per day: not measured; Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: treatment consisted of 3‐weekly individual counselling sessions, with a quit date occurring at the second session in the integrated smoking and alcohol intervention, and the smoking cessation‐only intervention; in the brief alcohol‐only intervention, no quit date for smoking occurred Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: timeline follow‐back (TLFB) interview was used to assess alcohol and cigarette use. Participants report the number of cigarettes smoked and number of alcoholic drinks consumed on each day in the past 7 days; 7‐day point‐prevalence smoking abstinence was assessed at 3 and 6 months after counselling completion Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: not bioverified Definition of people who continued smoking used: exact definition not reported; ‘no abstinence’ Time point(s) at which follow‐up was conducted: 3 and 6 months after counselling completion Outcome category: Anxiety, Depression Outcome measure(s): Hospital and Anxiety Depression Scale (HADS) |
|
| Funding source | Thai Health Promotion Foundation | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Pertschuk 1979.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: USA Data collection period: not stated Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 28 Sample characteristics (at baseline): Age (mean): 40.0 years (SD 12.5); Sex (% male): 37.5% Population category: general population; Specific population: participants in a smoking cessation programme Nicotine dependence: not measured; Baseline cigarettes per day: 34.7 (SD 11.6); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: behavioural support for smoking cessation (weekly group sessions and follow‐up meetings), including identifying patterns of tobacco use, stimulus control and contingency management techniques Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: some participants reported use of psychotropic medication i.e. mood management |
|
| Outcomes |
Definition of cessation used: self‐reported (exact definition of abstinence not reported) Cessation definition used for outcome(s) in this analysis: unclear definition Measure of biovalidation: not bioverified Definition of people who continued to smoke used: participants who did not successfully stop smoking in the treatment programme Time point(s) at which follow‐up was conducted: from smoking records throughout treatment and 2‐month follow‐up after completion of treatment programme Outcome category: Psychological Quality of Life (QoL) Outcome measure(s): participants completed a questionnaire reporting any overall change in anxiety, depression, anger, or irritability experienced during the previous 4 months; participants were also asked to indicate the occurrence of a range of symptoms over the 4‐month period, including appetite loss, insomnia, nightmares, hopelessness, tension, apathy, difficulty in concentration, and temper outbursts |
|
| Funding source | None specified | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Prochaska 2008.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: April 2000 – June 2003 Registry ID: not reported |
|
| Participants |
Number of participants: N = 322 Sample characteristics (at baseline): Age (mean): 42 years (SD 13); Sex (% male): 30% (97/322) Population category: psychiatric population; Specific population: people with unipolar depression Nicotine dependence: FTND 4.0 (SD 2.5); Baseline cigarettes per day: 15.5 (SD 10.1); Motivation to quit: not selected by motivation to quit, “recruitment material stated that participants did not have to want to quit smoking to participate” |
|
| Interventions |
Behavioural support for smoking cessation: a computerised system that provided individualised feedback to motivate people who smoked to quit. Followed by 6 sessions of individual cognitive–behavioural counselling (motivational counselling and cessation treatment). The control group received brief cessation advice only Pharmacological support for smoking cessation: nicotine patches and possible use of bupropion (not for control group) Psychotherapeutic or psychoactive support for mental health or mood: received mood management ‐ individual cognitive‐behavioural counselling, which included mood management |
|
| Outcomes |
Definition of cessation used: self‐reported 7‐day point‐prevalence abstinence Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: expired air carbon monoxide (≤ 10 ppm) Definition of people who continued to smoke used: people who smoked and had not reported 7‐day point‐prevalence abstinence and did not score CO ≤ 10 ppm Time point(s) at which follow‐up was conducted: baseline (before randomisation into treatment group), 3, 6, 12 and 18 months after baseline Outcome category: Depression and Psychological Quality of Life (QoL) Outcome measure(s): Beck's Depression Inventory‐II (BDI‐II); The Mental Component Summary of the Medical Outcomes Study Short Form (SF–36), days with emotional problems |
|
| Funding source | National Institute on Drug Abuse (grants K23 DA018691, K05 DA016752, and P50 DA09253) and the, State of California Tobacco‐ Related Disease Research Program (grant 13KT–0152) | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Qi Zhang 2014.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: multiple ‐ most sites in USA and Canada, also sites in India, Iran, Pakistan and Tunisia Data collection period: December 2005 ‐ June 2010 Registry ID: ClinicalTrials.gov: NCT00689611 |
|
| Participants |
Number of participants: N = 225 Sample characteristics (at baseline): Age (mean): 54 years (SD 10); Sex (% male): 84.4% (190/225) Population category: chronic physical condition; Specific population: people who actively smoked (average ≥ 10 CPD in past year) who suffered a MI with ≥ 24 hours hospitalisation were recruited in the ZESCA (9 weeks bupropion versus placebo) Nicotine dependence: FTND bupropion 5.4 (SD 2.2), placebo 5.5 (SD 2.0);Baseline cigarettes per day: not measured; Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: prior to initiation of study medication, all participants received a standardised non‐pharmacologic smoking cessation intervention Pharmacological support for smoking cessation: 9 weeks of bupropion (150 mg once daily for 3 days, then 150 mg twice daily) or placebo Psychotherapeutic or psychoactive support for mental health or mood: received mood management – 109/225 participants included in analysis were in the bupropion group |
|
| Outcomes |
Definition of cessation used: abstinence was biochemically validated by an exhaled carbon monoxide level of ≤ 10 ppm during all clinic visits; secondary endpoint of ZESCA trial was point‐prevalence smoking abstinence at 12 months; ‘persistent quitters’ defined as abstinence at all follow‐ups Cessation definition used for outcome(s) in this analysis: continuous abstinence Measure of biovalidation: expired CO (≤ 10 ppm) Definition of people who continued to smoke used: ‘smokers’ defined as reported smoking on at least 1 occasion Time point(s) at which follow‐up was conducted: 6 and 12 months Outcome category: Depression, Psychological Quality of Life (QoL) Outcome measure(s): Beck Depression Inventory II (BDI‐II) used in circulation abstract; EuroQol‐5D (EQ‐5D) used in main paper |
|
| Funding source | This ZESCA trial was funded by the Canadian Institutes of Health Research (Grant NCT64989) and the Heart and Stroke Foundation of Quebec | |
| Author conflicts of interest | Dr. Eisenberg received funding from Pfizer Canada Inc., to conduct the Evaluation of Varenicline (Champix) in Smoking Cessation for Patients Post‐Acute Coronary Syndrome(EVITA) Trial (NCT00794573); all other authors have no potential conflicts to disclose | |
| Notes | Outcome data source: Published data | |
Quist‐Paulsen 2006.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: Norway Data collection period: February 1999 – September 2001 Registry ID: not reported |
|
| Participants |
Number of participants: N = 240; Number included in meta‐analysis: N = 218 Sample characteristics (at baseline): Age (mean): 56.5 years; Sex (% male): 79.3% (173/218) Population category: chronic physical conditions; Specific population: people with myocardial infarction/unstable angina/recent coronary bypass Nicotine dependence: not measured; Baseline cigarettes per day: people who quit 14 (SD 6), people who continued to smoke 16 (SD 7); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: self‐help booklet with regular health practitioner contact (intervention) or very brief advice (usual care) Pharmacological support for smoking cessation: those with strong withdrawal urges were encouraged to use nicotine replacements (gum or patch) Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: self‐reported point‐prevalence smoking cessation (i.e. people who smoked who claimed they had quit) Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: nicotine metabolite concentration in urine < 2.0 mmol/mol creatine Definition of people who continued to smoke used: people who sustained smoking – if stated they were still smoking or claimed to have quit but urinary nicotine metabolite concentration above cut‐off Time‐point(s) at which follow‐up was conducted: 12‐month follow‐up Outcome category: Psychological Quality of Life (QoL), Social outcome Outcome measure(s): Cardiac Arrhythmia Suppression Trial (CAST) inventory (mental health and social function subscales) |
|
| Funding source | Vest‐Agder Council for Public Health, Sørlandet Hospital HF, Charity "Sykehuset i vaare hender" | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Robinson 2019.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: December 2010 ‐ September 2014 Registry ID: ClinicalTrials.gov: NCT01314001 |
|
| Participants |
Number of participants: N = 1246; Number included in meta‐analysis: N = 823 Sample characteristics (at baseline): Age (mean): 45.7 years; Sex (% male): 56.4% Population category: general population; Specific population: people who smoked, who were seeking treatment and recruited from the community by 4 North‐American academic medical centres Nicotine dependence: FTCD 5.3 (SD2.3); Baseline cigarettes per day: 18.3; Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: all participants received the National Cancer Institute's 'Clearing the Air' smoking cessation self‐help manual and counselling, starting with a 1‐hour in‐person pre‐quit session; a target quit date was set for week 1, at which time a second counselling session occurred; subsequent brief (15‐minute) counselling sessions were conducted over the telephone at weeks 4 and 8 post‐TQD; the counselling and manual both took a cognitive‐behavioural approach to smoking cessation, including coping with triggers and withdrawal‐related symptoms Pharmacological support for smoking cessation: participants randomised to receive NRT, varenicline or placebo (stratified by nicotine metabolite ratio or ‘NMR’); subsequently attended a pre‐quit session for counselling and received study medication; A) varenicline (or placebo pills) were taken for 12 weeks, starting the day after the pre‐quit visit; B) NRT (or placebo patches) was taken for 11 weeks, starting on the morning of the TQD; participants in the varenicline group (n = 420) received active medication, with dose titration at days 1 – 3 (0.5 mg once daily), days 4 – 7 (0.5 mg twice daily), and days 8 – 84 (1.0 mg twice daily), and placebo patches; participants in the NRT group (n = 418) received placebo pills and active NRT, with dose titration at weeks 1 ‐ 6 (21 mg), weeks 7 ‐ 8 (14 mg) and weeks 9 ‐ 11 (7 mg); participants in the placebo group (n = 408) received placebo pills and patches Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: 7‐day point‐prevalence abstinence at weeks 24 and week 52 post‐quit Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: expired CO (≤ 8 ppm) measured at each clinic visit Definition of people who continued to smoke used: not fulfilling 7PPA at 24‐ or 52‐week follow‐up Time point(s) at which follow‐up was conducted: weeks 1, 4, 8, 11, 24, and 52 post‐TQD Outcome category: Mixed Anxiety + Depression, Positive Affect Outcome measure(s): Positive and Negative Affect Scale (PANAS) |
|
| Funding source | This project was supported by a grant from the National Institutes of Health (U01DA20830) and by MD Anderson's Cancer Center Support Grant (P30CA016672); Pfizer provided varenicline and placebo pills at no cost | |
| Author conflicts of interest | Caryn Lerman received study medication and support for medication packaging from Pfizer; she has also consulted to Gilead, and has been a paid expert witness in litigation against tobacco companies. Rachel Tyndale has acted as a consultant to Apotex and to Quinn Emmanuel. Paul Cinciripini served on the scientific advisory board of Pfizer Pharmaceuticals, did educational talks sponsored by Pfizer on smoking cessation from 2006 to 2008, and has received grant and medication support from Pfizer. Dr. Schnoll receives medication and placebo free of charge from Pfizer for other clinical trials and has provided consultation to Pfizer and GlaxoSmithKline. Dr. Benowitz has been a consultant to pharmaceutical companies that market smoking cessation medications, including Pfizer, and has served as an expert witness in litigation against tobacco companies. Dr. Hawk receives medication and placebo free of charge from Pfizer for an ongoing clinical trial. The remaining authors declare no competing interests | |
| Notes | Outcome data source: Unpublished data | |
Rocha 2017.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: Portugal Data collection period: November 2013 – July 2015 Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 65; Number included in meta‐analysis: N = 65 Sample characteristics (at baseline): Age (mean): 55.7 years (SD 10.8); Sex (% male): 91% (59/65) Population category: chronic physical condition; Specific population: participants who smoked and suffered an acute coronary syndrome, followed from hospital discharge Nicotine dependence: not measured; Baseline cigarettes per day: not measured; Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: primary outcome was 7‐day point‐prevalence tobacco abstinence 6 and 12 months after clinical discharge; participants responded either ‘yes’ or ‘no’ to the question: ‘Have you smoked a cigarette, even a puff, in the past seven days?’ Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: not bioverified Definition of people who continued to smoke used: exact definition not reported; ‘smokers’ Time point(s) at which follow‐up was conducted: 6 and 12 months after clinical discharge Outcome category: Anxiety, Depression Outcome measure(s): Hospital and Anxiety Depression Scale (HADS) |
|
| Funding source | None specified | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Unpublished data | |
Rodríguez‐Cano 2016.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: Spain Data collection period: 2009 ‐ 2014 Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 242; Number included in meta‐analysis: N = 242 Sample characteristics (at baseline): Age (mean): 41.7 years (SD 10.1); Sex (% male): 37.6% (91/242) Population category: general population; Specific population: people who smoked and wished to take part in a smoking cessation programme Nicotine dependence: FTND 5.1 (SD 2.1); Baseline cigarettes per day: 20.6 (SD 7.1); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: the Smoking Cessation Programme based on CBT was delivered in groups of 6 ‐ 8; it is a standardised and manualised treatment consisting of 6 sessions (1 per week) with the following elements ‐ treatment contract, self‐report and graphic representation of cigarette consumption, information about tobacco, stimulus control, activities for the avoidance of withdrawal syndrome, physiological feedback (CO in exhaled air) on cigarette consumption, nicotine fading (change of cigarette brands each week progressively decreasing the intake of nicotine and tar), and relapse‐prevention strategies Pharmacological support for smoking cessation: participants did not use pharmacological smoking‐cessation treatment during the Smoking Cessation Programme or during follow‐up Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: criteria used for point‐prevalence abstinence at the end of treatment was not smoking in the past 24 hours; at 1 and 3 months follow‐up point‐prevalence abstinence was defined as not smoking in the past 7 days and at 6‐ and 12‐months follow‐up, not smoking in the past 30 days. A participant presenting continuous abstinence at the 12‐months follow‐up (not having smoked, not even a puff since the end of treatment) was considered to belong to the abstainer group Cessation definition used for outcome(s) in this analysis: multiple (prolonged and continuous abstinence) Measure of biovalidation: expired CO (≤ 10 ppm) Definition of people who continued to smoke used: those who did not quit smoking at the end of treatment and continued smoking during the follow‐ups were considered ‘smokers’; those who stopped smoking at the end of the treatment but were smoking during follow‐up were considered ‘relapsers’ Time point(s) at which follow‐up was conducted: 1, 3, 6 and 12 months follow‐up Outcome category: Depression Outcome measure(s): Beck Depression Inventory II (BDI‐II) |
|
| Funding source | This research was supported by the Spanish Ministerio de Ciencia y Competi‐tividad (Project reference: PSI2012‐31196) and by FEDER (European Regional Development Fund) | |
| Author conflicts of interest | "There are none" | |
| Notes | Outcome data source: Published data | |
Saiz Martinez 2016.
| Study characteristics | ||
| Methods |
Study design: non‐randomised intervention study Country: Spain Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 81 Sample characteristics (at baseline): Age (mean): 43.4 years (SD 8.8); Sex (% male): 72.8% Population category: psychiatric population; Specific population: People with schizophrenia allocated to continue smoking or take varenicline or use NCT patches Nicotine dependence: not measured; Baseline cigarettes per day: 28 (SD 12.3); Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: Varenicline or NCT patches, no further details. Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: not reported Cessation definition used for outcome(s) in this analysis: unclear definition Measure of biovalidation: not reported Definition of people who continued to smoke used: not reported Time point(s) at which follow‐up was conducted: 3 and 6 months Outcome category: Depression, Mixed Anxiety + Depression, Positive Affect Outcome measure(s): Hamilton Depression Rating Scale (HDRS); Positive and Negative Syndrome Scale (PANSS) |
|
| Funding source | None specified | |
| Author conflicts of interest | Authors have not supplied their declaration of competing interest | |
| Notes | Outcome data source: Published data | |
Sales 2009.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: Brazil Data collection period: August 2006 – August 2007 Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 60 Sample characteristics (at baseline): Age (mean): 54 years (SD 10); Sex (% male): 52% (31/60) Population category: chronic physical conditions; Specific population: people with cardiothoracic disease Nicotine dependence: FTND, quitters 5.6, non‐quitters 6.6; Baseline cigarettes per day: 39.2 (SD 19.6); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: weekly and bi‐weekly group counselling sessions based on behaviour modification counselling, followed by monthly phone contact after end of group treatment Pharmacological support for smoking cessation: combination of NRT and bupropion recommended Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: ‘self‐reported cessation’; reference to 'remaining abstinent' and 'continuously abstinent' Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: expired air carbon monoxide (≤ 10 ppm) Definition of people who continued to smoke used: ‘non‐quitters’ (not reported cessation and exhaled CO > 10 ppm) Time point(s) at which follow‐up was conducted: 12 months after baseline Outcome category: Positive Affect; Psychological Quality of Life (QoL) Outcome measure(s): Medical Outcomes Study 36‐item Short‐Form Health Survey (SF‐36) domains: vitality, mental health and mental component summary |
|
| Funding source | None specified | |
| Author conflicts of interest | No declarations specified; however, Maria Penha Uchoa Sales listed as Co‐ordinator of the Smoking Control Program at the Dr Carlos Alberto Studhart Gomes Hospital de Messejana, Fortaleza, Brazil | |
| Notes | Outcome data source: Published data | |
Sanchez‐Villegas 2008.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: Spain Data collection period: the SUN project started December 1999 with participants followed up every 2 years Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 8556 Sample characteristics (at baseline): Age (mean): information not available; Sex (% male): information not available Population category: general population; Specific population: general population Nicotine dependence: information not available; Baseline cigarettes per day: information not available; Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: some participants were using antidepressants at follow‐ups, i.e. received mood management |
|
| Outcomes |
Definition of cessation used: continuous abstinence ‐ ‘ex‐smokers who had quit smoking more than 10 years ago’ Cessation definition used for outcome(s) in this analysis: continuous abstinence Measure of biovalidation: not bioverified Definition of people who continued to smoke used: ‘smokers’ Time point(s) at which follow‐up was conducted: baseline and at 2‐, 4‐, 6‐year follow‐ups after baseline; mean follow‐up period was 47.4 months Outcome category: Depression Outcome measure(s): presence of self‐reported physician diagnosis or use of antidepressant medication, or both |
|
| Funding source | The SUN Project has received funding from the Spanish Government (current Grants PI10/02658, PI10/02293, PI13/00615, PI14/01798, RD06/0045, G03/140 and 87/2010), the Navarra Regional Government (45/2011, 27/2011) and the University of Navarra | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Sankaranarayanan 2016.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: Australia Data collection period: 4th August 2009 – 30th April 2014 Registry ID: Australian New Zealand Clinical Trials Registry: ACTRN12609001039279 |
|
| Participants |
Number of participants: N = 235; Number included in meta‐analysis: N = 121 Sample characteristics (at baseline): Age (mean): 42 years (SD 11); Sex (% male): 59% (138/235) Population category: psychiatric population; Specific population: people who smoked with a psychotic disorder were recruited across 3 sites (in Newcastle, Sydney and Melbourne, Australia) Nicotine dependence: not measured; Baseline cigarettes per day: 28.2 (SD 14.6); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: participants received either (a) a face‐to‐face multicomponent ‘Healthy Lifestyle’ intervention for smoking cessation and cardiovascular (CVD) risk reduction (NRT plus a total of 17 sessions consisting of the initial 90‐minute common session then 7 further 1‐hour weekly sessions, plus 3 fortnightly hour‐long sessions and monthly ‘booster’ sessions of 1 hour duration for 6 months base on CBT and using motivational interviewing and contingency management); or (b) a predominantly telephone‐based, less intensive intervention focusing mainly on monitoring use of NRT and CVD risk behaviours, and delivered at the same intervals as the Healthy Lifestyles intervention condition; telephone‐based sessions were scheduled to be approximately 10 mins duration and at weeks 4, 8, and 15; participants attended 30‐min face‐to‐face sessions where NRT was dispensed Pharmacological support for smoking cessation: for the first 6 months of the study, all participants had access to NRT Psychotherapeutic or psychoactive support for mental health or mood: received mood management ‐ 92.7% of overall sample were taking antipsychotics (N = 205); 61.5% (N = 136) were taking antidepressant and mood stabilisers; 14.9% (N = 33) were taking an anxiolytic |
|
| Outcomes |
Definition of cessation used: (point prevalence) abstinent vs. non‐abstinent (at 12 months) Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: expired CO (reported in NCT record) Definition of people who continued to smoke used: exact definition not reported, 'non‐abstinent' Time point(s) at which follow‐up was conducted: 3.5 and 12 months Outcome category: Depression Outcome measure(s): Beck Depression Inventory II (BDI‐II) |
|
| Funding source | Funding for this work was received from the Australian National Health and Medical Research Council (NHMRC project Grantnumber: 569210) and the Commonwealth Department of Health and Ageing. The Priority Research Centre for Brain and Mental Health Research, University of Newcastle, Australia provided financial assistance for statistical analysis. Nicotine Replacement Therapy (NRT) was provided free to the study by GlaxoSmithKline (GSK) | |
| Author conflicts of interest | The authors report no financial partnership or speaking engagements with GSK, and GSK was not involved in any other aspect of the study design or analysis. None of the authors have any conflicts of interest to declare | |
| Notes | Outcome data source: Unpublished data | |
Sarna 2008.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: USA Data collection period: 1992/1993, 1996/1997, 2000/2001 Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 158,734; Number included in meta‐analysis: N = 11,809 Sample characteristics (at baseline): Age (mean): range 29 – 71 years; Sex (% male): 0% (0/158,734) Population category: general population; Specific population: female nurses Nicotine dependence: not measured; Baseline cigarettes per day: 16.8 (SD 10.3); Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: self‐reported point prevalence (exact definition of abstinence not reported) Cessation definition used for outcome(s) in this analysis: unclear point prevalence Measure of biovalidation: not bioverified Definition of people who continued smoking used: people who currently smoked (self‐reported) Time point(s) at which follow‐up was conducted: baseline, 4 years and 8 years after baseline Outcome category: Psychological Quality of Life (QoL), Social outcome Outcome measure(s): Short Form Health Survey – 36 (SF‐36; mental health composite and social functioning subscale) |
|
| Funding source | Supported by grants CA87979, CA50385, and K07 CA92696‐02 (Cooley) from the National Institutes of Health, and a grant from the Robert Wood Johnson Foundation #55769 (Sarna) | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Schnoll 2016.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: June 2009 – April 2014 Registry ID: ClinicalTrials.gov: NCT01047527 |
|
| Participants |
Number of participants: N = 180; Number included in meta‐analysis: N = 180 Sample characteristics (at baseline): Age (mean): 45.9 years (SD 12.3); Sex (% male): 50% (90/180) Population category: general population; Specific population: people who smoked taking part in a RCT of standard (8 weeks), extended (24 weeks), or maintenance (52 weeks) transdermal nicotine therapy Nicotine dependence: FTND: 5.3 (SD 1.9); Baseline cigarettes per day: 16.9 (SD 8.4); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: all participants received brief counselling (8 sessions) based on established treatment guidelines and focused on preparing for cessation, managing urges and triggers to smoking, and developing strategies to avoid relapse Pharmacological support for smoking cessation: participants randomised to standard (8 weeks), extended (24 weeks), or maintenance (52 weeks) transdermal nicotine therapy (21 mg); for the current study, only participants randomised to 8 weeks of treatment were included in order to standardise nicotine patch treatment duration Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management. |
|
| Outcomes |
Definition of cessation used: point‐prevalence abstinence at 6 months ‐ self‐reported abstinence from smoking for 7 days prior to the assessment and biochemically confirmed with breath carbon monoxide Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: expired CO (≤ 10 ppm) Definition of people who continued to smoke used: exact definition not reported; participants who withdrew from the study, failed to provide a CO sample, or had a CO greater than 10 ppm were considered continuing to smoke Time point(s) at which follow‐up was conducted: week 8 and 6 months Outcome category: Anxiety, Depression Outcome measure(s): Beck Anxiety Inventory (BAI); Inventory of Depressive Symptomology (IDS) |
|
| Funding source | This research was supported by grants from the National Institute on Drug Abuse (R01DA025078 and R01DA033681) and from the National Cancer Institute (R01CA165001 and P50CA143187) | |
| Author conflicts of interest | Dr.Schnoll and Dr. Hitsman receive medication and placebo free of charge from Pfizer and have provided consultation to Pfizer; Dr.Schnoll has also consulted with GlaxoSmithKline; these companies had no involvement in this study | |
| Notes | Outcome data source: Published data | |
Schwartz 1968a.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 158 Sample characteristics (at baseline): Age (mean): not reported, age range 25 – 44 years; Sex (% male): 100% (158/158) Population category: general population; Specific population: general population Nicotine dependence: not measured; Baseline cigarettes per day: average not reported; Motivation to quit; selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: individual counselling or group methods Pharmacological support for smoking cessation: 1 group received medication (pill), but were not included in this analysis Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: point‐prevalence ‐ self‐reported at least 85% reduction in smoking from the pretreatment level Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: not bioverified Definition of people who continued to smoke used: did not have at least 85% reduction in smoking from the pretreatment level Time point(s) at which follow‐up was conducted: end of treatment (8 weeks after baseline) Outcome category: Anxiety Outcome measure(s): The Reaction Index |
|
| Funding source | Public Health Service grant 05‐15‐D67 (a Cancer demonstration grant) from the National Center for Chronic Disease Control | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Secades‐Villa 2015.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: Spain Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 147 Sample characteristics (at baseline): Age (mean): CBT 43.8 years (SD 12.8), CBT+CM 43.5 years (SD 13.5); Sex (% male): CBT 44.6% (33/74), CBT+CM 32.9% (24/73) Population category: general population; Specific population: people who smoked and were seeking treatment Nicotine dependence: CBT FTND 5.4 (SD 1.8), CBT+CM 5.4 (SD 1.9); Baseline cigarettes per day: CBT 20.7 (SD 8), CBT+CM 20.2 (SD 8.4); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: randomised to CBT or CBT + contingency management (CM); CBT protocol was implemented in group‐based sessions of 5 or 6 participants, for 1 hour per week for 6 weeks with quit date occurring at the 5th session; the main component was nicotine fading where from the first to the fourth week, participants were asked to gradually reduce their nicotine intake based on a weekly reduction of 30%; other components included information about tobacco, a behavioural contract, self‐monitoring and graphical representation of cigarette smoking, stimulus control, strategies for controlling nicotine withdrawal symptoms, physiological feedback (measured by CO and cotinine), training in alternative behaviours, social reinforcement of objective completion and abstinence, and relapse prevention strategies; CO and cotinine specimens were collected twice a week and participants were informed of the outcome; CM for smoking abstinence included a voucher programme in which nicotine abstinence was reinforced on an escalating schedule of reinforcement with a reset contingency ‐ points were earned for specimens testing negative for cotinine (80 ng/ml); CM for shaping abstinence included a voucher programme in which progressive reductions in cotinine (with abstinence also as the final target) were reinforced according to an individualised percentile schedule; maximum amount that participants could earn in both conditions was EUR 300 (USD 339) + points were exchangeable for vouchers with a variety of uses Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: self‐reported and bioverified 7‐day point‐prevalence abstinence at end of treatment, 1‐month and 6‐month follow‐up Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: expired CO (< 4 ppm), urine cotinine levels (< 80 ng/ml) Definition of people who continued to smoke used: exact definition not reported; missing participants at assessment period were considered non‐abstinent Time point(s) at which follow‐up was conducted: EOT, 1 month and 6 months Outcome category: Depression Outcome measure(s): Beck Depression Inventory II (BDI‐II) |
|
| Funding source | This research was supported by the Spanish Ministry of Science and Innovation Grants (PSI2011‐22804 and PSI2011‐23395) and by the Pre‐doctoral Grants BP12‐037, from the Foundation for the Promotion of Applied Scientific Research and Technology in Asturias and BES‐2012‐053988, from the Spanish Ministry of Economy and Competitiveness | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Secades‐Villa 2019.
| Study characteristics | ||
| Methods |
Study design: RCT Country: Spain Data collection period: January 2015 – July 2018 Registry ID: ClinicalTrials.gov: NCT03163056 |
|
| Participants |
Number of participants: N = 120 Sample characteristics (at baseline): Age (mean): CBT+BA 50.6 years (SD 10.2), CBT+BA+CM 52.7 years (SD 8.9); Sex (% male): CBT+BA 35% (21/60), CBT+BA+CM 23.3% (14/60) Population category: general population; Specific population: people who smoked and were seeking treatment recruited from the community via flyers, radio, television, web‐based, and newspaper advertisements Nicotine dependence: CBT+BA FTND 6.9 (SD 1.8), CBT+BA+CM 6.2 (SD 1.8); Baseline cigarettes per day: CBT+BA 23 (SD 8.2), CBT+BA+CM 20.7 (SD 6.7); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: participants randomised to A) CBT plus behavioural activation (BA) – empirically‐supported psychological intervention for quitting smoking; main components were psychoeducation on cigarette use, nicotine fading (i.e. a 30% weekly reduction of nicotine consumption from the 1st to the 4th week and abstinence from 48 hours prior to the 5th session onwards), self‐monitoring and graphical representations of nicotine intake reductions, stimulus control, relaxation, role‐playing in alternative behaviours, development of a preventive relapse plan through training in coping skills, and enhancement of social support (BA element described below); or B) CBT plus BA plus contingency management (CM) which includes interventions previously described + CM protocol for reinforcing abstinence ‐ participants were given vouchers redeemable for a variety of uses contingent upon negative CO and cotinine samples, incentives followed an escalating schedule of reinforcement; if participants had CO or cotinine readings indicating smoking status (i.e. CO ≥ 5 ppm and cotinine levels ≥ 81 ng/ml), they did not receive the voucher and the next abstinent sample was reset to the initial value; after the reset occurred, if participants provided 3 consecutive abstinent samples, the voucher value that they received was the same as the one given before the reset ‐ total amount that participants could earn during the 8‐week treatment phase was EUR 175 (USD 200) Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: received mood management ‐ participants in both conditions took part in BA module which was delivered from the 1st session, its main goal being to increase the number of pleasant activities participants engage in ‐ activation assignment was based on participants’ activity hierarchy of level of difficulty and interest; therapists worked with participants in a collaborative way to identify life values and objectives; through sessions 2 – 8, therapists and participants agreed on 2 – 3 activation goals aimed to enhance activation and decrease avoidance patterns of behaviour; other treatment components included ‐ discussion of the treatment rationale, psychoeducation on the relationship between smoking and depression (through functional analysis of behaviour and written materials developed by therapists), weekly activity and mood monitoring, and social support through social contracts that consisted of identifying which people in the participants’ lives they would like to get support from when engaging in new positive activities |
|
| Outcomes |
Definition of cessation used: 2 measures of smoking were assessed, 1) point prevalence (i.e. the percentage of participants being abstinent for a minimum of 24 hours at the post‐treatment and 7 days prior to the visit at 1‐, 2‐, 3‐ and 6‐month follow‐ups) and 2) continuous abstinence, defined as number of days without smoking, not even a puff, since quit day in each of the time frames Cessation definition used for outcome(s) in this analysis: continuous abstinence Measure of biovalidation: expired CO; urine cotinine levels Definition of people who continued to smoke used: exact definition not reported; ‘smoker’; instances in which participants did not submit urine/CO samples were rendered as positive Time point(s) at which follow‐up was conducted: 1‐, 2‐, 3‐ and 6‐month follow‐up Outcome category: Depression Outcome measure(s): Beck Depression Inventory II (BDI‐II) |
|
| Funding source | This research was supported by the National Agency of Research of the Spanish Ministry of Science, Innovation and Universities and the European Regional Development Fund MINECO/FEDER(PSI2015‐64371‐P)and by two predoctoral grants from the National Agency of Research of the Spanish Ministry of Science, Innovation and Universities (BES‐2016‐076663/FPU15/04327) | |
| Author conflicts of interest | No conflict declared | |
| Notes | Outcome data source: Unpublished data | |
Segan 2011.
| Study characteristics | ||
| Methods |
Study design: ‘uncontrolled before‐and‐after study’ Country: Australia Data collection period: recruitment May 2007 – July 2008 and 6‐month follow‐up Registry ID: not reported |
|
| Participants |
Number of participants: N = 227 Sample characteristics (at baseline): Age (mean): 45.2 years (SD 13.6); Sex (% male): 34.8% (79/227) Population category: psychiatric population; Specific population: people with depression Nicotine dependence: not measured; Baseline cigarettes per day: 23.0 (SD 11.3); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: behavioural support and mood management provided via Quitline Pharmacological support for smoking cessation: 64.4% of total sample (n = 227) used cessation medication including NRT, varenicline or bupropion Psychotherapeutic or psychoactive support for mental health or mood: co‐management with doctor – tailored call‐back counselling was provided during which advisers focused on the emotional aspect of addiction and relationship between smoking and mood; strategies provided to monitor mood during quitting and to help quit smoking and regulate mood; 68.8% of total sample were taking prescription medication for well‐being, i.e. received mood management |
|
| Outcomes |
Definition of cessation used: self‐reported point‐prevalence abstinence – quit for at least 24 hours between baseline and 2‐month follow‐up and at 6‐month follow‐up; sustained abstinence ‐ period prevalence (quit for 4 months) at 6‐month follow‐up Cessation definition used for outcome(s) in this analysis: prolonged abstinence Measure of biovalidation: not bioverified Definition of people who continued to smoke used: not making a quit attempt or failed quit attempt – had not tried/failed to quit for at least 24 hours between baseline and 2‐month follow‐up; had not quit for at least 4 months at 6‐month follow‐up Time point(s) at which follow‐up was conducted: baseline (after initial Quitline call), 2 months (end of treatment) and 6 months (after initial Quitline call) Outcome category: Depression Outcome measure(s): Patient Health Questionnaire 9 (PHQ‐9) |
|
| Funding source | Primarily funded by the Victorian Centre of Excellence in Depression and Related Disorders an (initiative between beyond‐blue and the State Government of Victoria, with contribution from Quit Victoria | |
| Author conflicts of interest | Ainslie Hannan and Ian Ferretter are employed by Quit Victoria. Together with Catherine Segan, Kay Wilhelm and Sunil Bhar, they developed and implemented Quit Victoria’s tailored service for depression‐history smokers evaluated in this study. Ron Borland is employed by The Cancer Council Victoria, which houses Victoria’s Quitline. He has conducted research on the Quitline and has an ongoing interest in helping them improve the quality of their services | |
| Notes | Outcome data source: Published data | |
Shahab 2014.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: Multiple ‐ Canada, France, UK, USA. Data collection period: phase 2 of the ATTEMPT cohort set up in 2004 with 12‐month follow‐up Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 1640; Number included in meta‐analysis: ranges from N = 936 (Anxiety) ‐ N = 1027 (Depression) Sample characteristics (at baseline): Age (mean): 46.3 years (SD 7.5); Sex (% male): 54.8% (899/1640) Population category: general population; Specific population: participants in the ATTEMPT cohort ‐ those who smoked at least 5 cigarettes per day and intended to quit within the following 3 months Nicotine dependence: FTND 4.7 (SD 2.4); Baseline cigarettes per day: not reported; Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: point‐prevalence abstinence – self‐reported not having a cigarette (even a puff) in last 3 months (‘ex‐smokers ≥ 3 months’), or last 30 days or 7 days (‘ex‐smokers < 3 months’) Cessation definition used for outcome(s) in this analysis: prolonged abstinence Measure of biovalidation: not bioverified Definition of people who continued to smoke used: people who currently smoked – self‐reported currently smoking or smoking a cigarette on day of 12‐month follow‐up, or both Time point(s) at which follow‐up was conducted: 12 months Outcome category: Anxiety, Depression Outcome measure(s): Bespoke scale (symptoms of anxiety + depression yes/no in last 30 days or 3 months or doctor diagnosed in last 3 months ‐ yes to any of these 3 questions) |
|
| Funding source | Funded by Sanofi‐Aventis Recherche et Développement (SAR&D) and the report write‐up by Cancer Research UK (C1417/A14135) | |
| Author conflicts of interest | L.S. has received an honorarium for a talk and travel expenses from a pharmaceutical company making smoking cessation products. R.W. undertakes research and consultancy for developers and manufacturers of smoking cessation treatments such as nicotine replacement products | |
| Notes | Outcome data source: Published data | |
Slopen 2013.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: USA Data collection period: MIDUS I (1995/1996) to MIDUS II (2004/2005) Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 4938; Number included in meta‐analysis: N = 766 Sample characteristics (at baseline): Age (mean): mean not reported, < 35 years 19.7% (975), 35 – 44 years 26.8% (1325) , 45 – 54 years 25.4% (1251), 46 ‐ 55 years 28.1% (1385); Sex (% male): 46.6 % (2302/4938) Population category: general population; Specific population: participants contributing to both MIDUS I and II (MIDUS is a nationally‐representative cohort of adults (25 – 74 years) to examine the influence of behavioural, psychological, and social factors on mental and physical health in midlife) Nicotine dependence: not measured; baseline cigarettes per day: not measured; Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: at baseline and follow‐up participants were asked “Do you smoke cigarettes regularly now?” and participants who responded ‘‘yes’’ were categorised as currently smoking; based on combined responses at both time points, participants were sorted into 1 of 4 categories: (1) not smoking (at baseline and follow‐up); (2) continued smoking (i.e. smoking regularly at baseline and follow‐up); (3) people who previously smoked (i.e. smoked at baseline, not smoking at follow‐up); and (4)relapse smoking (i.e. reported previous smoking at baseline, smoking at follow‐up); individuals who reported regular smoking at baseline were also asked if they had ‘‘tried to quit smoking’’ during the time since the last interview (yes/no) – using this information (in combination with smoking status in 2004/2005), people smoking at baseline who attempted to quit smoking between baseline and follow‐up were categorised as ‘‘successful’’ or ‘‘unsuccessful’’ Cessation definition used for outcome(s) in this analysis: unclear point‐prevalence Measure of biovalidation: not bioverified Definition of people who continued to smoke used: ‘persistent smokers’ (those who reported regular smoking at baseline and follow‐up), and ‘relapsed smokers’ (those who reported previously smoking at baseline and smoking at follow‐up); ‘unsuccessful’ quit attempt between baseline and follow‐up Time point(s) at which follow‐up was conducted: 9 ‐ 10 years Outcome category: Stress Outcome measure(s): Cumulative Stress Score (non‐standardised measure, encompassing 8 domains of stressors: relationship, financial, work, work‐family spill‐over, perceived inequality, neighbourhood, discrimination and recent problems in the family) |
|
| Funding source | The MIDUS study was supported by a grant from the National Institute on Aging (P01‐AG020166) to conduct a longitudinal follow‐up of the MIDUS (Midlife in the U.S.) investigation; the original study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development; this research was also supported by a grant from National Cancer Institute (P50‐CA148596) to the Harvard Lung Cancer Disparities Center; the first author is supported by a post‐doctoral fellowship at the Center on the Developing Child sponsored by the Robert Wood Johnson Foundation | |
| Author conflicts of interest | The authors have no financial disclosures or conflicts of interest to report | |
| Notes | Outcome data source: Unpublished data | |
Solomon 2006.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: USA Data collection period: not stated Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 151; Number included in meta‐analysis: N = 129 Sample characteristics (at baseline): Age (mean): 24.3 years; Sex (% male): 0% (0/129) Population category: pregnant population; Specific population: pregnant women Nicotine dependence: not measured; Baseline cigarettes per day: people who quit 9.7 (SD 6.0), people who continued smoking 22.4 (SD 9.6); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: abstinence monitoring schedule plus TAU, no mood management – participants allocated to receive a financial incentive after successful bioverification or independent of smoking status Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: self‐reported 7‐day point‐prevalence abstinence (7 days prior to assessments) Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of bio‐validation: urinary cotinine level (≤ 80 ng/ml) Definition of people who continued smoking used: never‐quitters if continued smoking throughout; later quitters if stopped smoking after second assessment Time point(s) at which follow‐up was conducted: baseline, 4 ‐ 8 weeks after baseline and 25 weeks gestation Outcome category: Depression Outcome measure(s): Brief Symptom Inventory (BSI; depression subscale) |
|
| Funding source | This study was supported by research grant no. R01DA14028 from the National Institute on Drug Abuse | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Steinberg 2011.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: USA Data collection period: July 2006 – December 2008 Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 723; Number included in meta‐analysis: N = 220 Sample characteristics (at baseline): Age (mean): 45 years (range 16 – 78); Sex (% male): 47% (338/723) Population category: chronic physical or psychiatric conditions, or both; Specific population: attendees at chronic tobacco‐dependence clinic treating co‐occurring conditions Nicotine dependence: time to first cigarette > 30 minutes (15%), wake up at night to smoke (yes 46%); Baseline cigarettes per day: 19; Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: behavioural support based on the US Public Health Service Guidelines Pharmacological support for smoking cessation: single medications (NRT, bupropion or varenicline alone) or combinations (patch and/or bupropion plus spray/inhaler/gum/lozenge) Psychotherapeutic or psychoactive support for mental health or mood: received mood management (bupropion) |
|
| Outcomes |
Definition of cessation used: self‐reported 7‐day point‐prevalence abstinence (7 days prior to assessments) Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: exhaled CO level (< 10 ppm) Definition of people who continued smoking used: not abstinent; persons not contactable at 6‐month follow‐up assumed to be smoking Time point(s) at which follow‐up was conducted: 4 weeks and 6 months post‐pre‐agreed quit date Outcome category: Mixed Anxiety and Depression Outcome measure(s): Kessler Psychological Distress Scale (K‐6) |
|
| Funding source | There was no financial support for this study and there was no support from any pharmaceutical company for any aspect of this research. none for study. the UMDNJ‐Tobacco Dependence Clinic is funded through a grant from the New Jersey Department of Health and Senior Services – Comprehensive Tobacco Control Program | |
| Author conflicts of interest | All authors indicated current/previous status as consultant or contractor for companies involved in cessation pharmacotherapy (MS has conducted research funded by Pfizer; JF has previously worked for Pfizer, Novartis, GSK, Celtic Pharma; JW has conducted research supported by Pfizer and is consultant for Novartis and BeBetter Inc.) | |
| Notes | Outcome data source: Published and unpublished data | |
Stewart 1995.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 350; Number included in meta‐analysis: N = 323 Sample characteristics (at baseline): Age (mean): 40.6 years (SD 10.4); Sex (% male): 52% (182/350) Population category: general population; Specific population: people who currently smoked contacted randomly by phone Nicotine dependence: measured but not reported; Baseline cigarettes per day: 20; Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: self‐help booklet with no mood management described; financial inventive Pharmacological support for smoking cessation: NRT (gum) Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: self‐reported 7‐day point‐prevalence abstinence (7 days prior to assessments) at both 3 and 6 months Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: saliva cotinine level (< 20 ng/l); for initial eligibility assessment expired CO level (< 9 ppm) i.e. quit for 24 hours prior to enrolment Definition of people who continued to smoke used: people who smoked; participants who refused to come to the centre for cotinine test of saliva were assumed to be smoking Time point(s) at which follow‐up was conducted: baseline and 6‐month follow‐up Outcome category: Psychological Quality of Life (QoL), Social outcome Outcome measure(s): Short Form Health Survey (SF‐36; mental health and social functioning subscale) |
|
| Funding source | Grant from State of California Tobacco‐Related Research Program, and grant from National Heart, Lung, and Blood Institute | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Taylor 2015.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: Europe, USA and Australia Data collection period: 1997 ‐ 2003 Registry ID: secondary analysis of 5 RCTs, individual registry numbers not reported |
|
| Participants |
Number of participants: N = 627; Number included in meta‐analysis: N = 627 Sample characteristics (at baseline): Age (mean): people who continued smoking 45.6 years (SD 10.6), people who quit 46.2 years (SD 10.2); Sex (% male): people who continued smoking 48% (258/560), people who quit 52% (35/67) Population category: general population; Specific population: all participants were adults who had smoked for at least 3 years and were selected because they wanted to reduce but not stop smoking Nicotine dependence: FTND people who continued smoking 6.2 (SD 1.9), people who quit 5.3 (SD 2.5); Baseline cigarettes per day: not reported; Motivation to quit: not selected by motivation to quit (participants were selected by motivated to reduce) |
|
| Interventions |
Behavioural support for smoking cessation: participants followed up 8 – 10 times over 2 years and encouraged at each occasion to reduce smoking by using NRT or placebo and given behavioural strategies to assist Pharmacological support for smoking cessation: data from 5 placebo‐controlled trials of NRT Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: classified a person as having achieved prolonged cessation if they were abstinent at both 6 and 12 months and were biologically verified to be so on both occasions by having a CO reading of < 10 ppm Cessation definition used for outcome(s) in this analysis: prolonged abstinence Measure of biovalidation: CO reading of < 10 ppm Definition of people who continued to smoke used: classified a person as smoking continuously if they reported smoking at both times and had a CO reading of ≥ 10 ppm Time point(s) at which follow‐up was conducted: 6 months and 12 months Outcome category: Psychological Quality of Life (QoL) Outcome measure(s): Short Form Survey 36‐item (SF‐36) |
|
| Funding source | GT was funded by a National Coordinating Centre for Research Capacity Development scholarship during the conduct of the study. AG was funded by The National Institute for Health Research (NIHR) Collaborations for Leadership in Applied Health Research and Care for West Midlands(CLAHRC WM) during the conduct of the study. AM receives funding from the UK Centre for Tobacco and Alcohol Studies. GT, AM and PA are part of the UK Centre for Tobacco and Alcohol Studies, a UKCRC Public Health Research: Centre of Excellence. Funding from British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council, and the National Institute for Health Research, under the auspices of the UK Clinical Research Collaboration | |
| Author conflicts of interest | All authors have completed an ICMJE form for disclosure of potential conflicts of interest; PA reports personal fees from Pfizer, grants and personal fees from McNeil, outside the submitted work | |
| Notes | Outcome data source: Published data | |
Tian 2016.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: Australia Data collection period: baseline 2004 ‐ 2006/follow‐up 2009 ‐ 2011 Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 3868; Number included in meta‐analysis: N = 356 Sample characteristics (at baseline): Age (mean): 32 (range 26 ‐ 37); Sex (% male): 41.1% (1590/3868) Population category: general population; Specific population: participants in the Childhood Determinants of Adult Health Study (CDAH) Nicotine dependence: not measured; Baseline cigarettes per day: not measured; Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: smoking status was defined via 3 questions: 1) ‘Over your lifetime, have you smoked at least 100 cigarettes, or a similar amount of tobacco?’ – participants answered ‘yes’ = ‘ever smokers’; ‘ever smokers’ subdivided into ‘current’ or ‘former smokers’ based on second question; 2) ‘How often do you now smoke cigarettes, cigars, pipes or any other tobacco products?’ –‘daily’ / ‘at least once per week’ classified as ‘current smokers’, participants who answered ‘not at all’ asked a third question; 3) ‘In the past have you ever been a daily smoker?’ – participants answered ‘yes’ classified as ‘former smokers’; for the longitudinal analyses, exposure variable was change in smoking status from baseline to follow‐up, categorised as ‐ 'stable never smokers' (had never smoker at either time point), 'stable former smokers' (had previously smoked at both time points), 'continuing smokers' (reported current smoking at both time points), 'quitters' (reported current smoking at baseline and previous smoking at follow‐up) and 'resumed smokers' (reported former smoking at baseline and current smoking at follow‐up) Cessation definition used for outcome(s) in this analysis: unclear point prevalence Measure of biovalidation: not bioverified Definition of people who continued to smoke used: people currently smoking at both time points; people who resumed smoking defined as people who had previously smoked at baseline and were smoking at follow‐up) Time point(s) at which follow‐up was conducted: 5 years Outcome category: Psychological Quality of Life (QoL) Outcome measure(s): Short Form Health Survey 12‐item (SF‐12; mental component summary) |
|
| Funding source | This study was supported by grants from the National Heart Foundation (Grant GOOH0578 and FellowshipPH11H6047 to S.L.G.); the National Health and Medical Research Council (Grants 211316 and 544923 and fellowship APP1008299 to A.J.V.); the Tasmania Graduate Research Scholarship (to J.T.); the Tasmanian Community Fund (Grant D0013808); and Veolia Environmental Services (Sydney, New South Wales, Australia). The study was sponsored by Sanitarium Health and Wellbeing Australia (Melbourne, Victoria, Australia), ASICS Ltd. (Kobe, Japan) and Target Australia Pty. Ltd. (North Geelong, Victoria, Australia) | |
| Author conflicts of interest | The authors declare that they have no conflict of interest | |
| Notes | Outcome data source: Published data | |
Tomioka 2014.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: Japan Data collection period: September 2007 ‐ August 2013 Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 277; Number included in meta‐analysis: N = 277 Sample characteristics (at baseline): Age (mean): 60.9 years (SD 12.2); Sex (% male): 65% (180/277) Population category: general population; Specific population: smoking cessation clinic attendees Nicotine dependence: Tobacco Dependence Screener (TDS) 7.7 (SD 1.6); Baseline cigarettes per day: 22.9 (SD 12.4); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: an educational seminar introduced the programme and explained the following topics ‐ harmful effects of smoking, possible benefits of quitting smoking, how to handle withdrawal symptoms and how to prevent relapses; a leaflet was used to reinforce the information; the programme consisted of 5 sessions ‐ participants returned 2, 4, 8 and 12 weeks after their baseline visit date for follow‐up; at each visit, CO concentration was measured and the attending physician and a nurse with experience in smoking cessation confirmed whether smoking cessation had continued; brief counselling (≤ 15 mins) was provided and the staff praised those who continued with cessation or expressed appreciation of the efforts of those who had continued to smoke and recommended a rechallenge; no psychosocial support was provided Pharmacological support for smoking cessation: all participants received either transdermal nicotine patches or varenicline following discussion with the attending physician; a pharmacist explained the effect of each drug, its usage and side effects using a leaflet to support the information; a target quit date was set on the day that nicotine patches were applied or on the 8th day after the first dose of varenicline ‐ initial prescriptions were transdermal nicotine patches in 160 participants and varenicline in 117 (6 participants in each group were switched to another medication because of adverse events or participant demand) Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: participants were considered to be quitters even if they smoked at 8 weeks but had quit completely between the 8‐week and 12‐week visits ‐ reports of abstinence were subjected to verification by an exhaled CO level of ≤ 10 ppm Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: expired CO (≤ 10 ppm) Definition of people who continued to smoke used: those who smoked between the 8‐week and 12‐week visits Time point(s) at which follow‐up was conducted: 12 weeks Outcome category: Psychological Quality of Life (QoL) Outcome measure(s): St George’s Respiratory Questionnaire (SGRQ; mental subscale) |
|
| Funding source | This research received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors | |
| Author conflicts of interest | None | |
| Notes | Outcome data source: Unpublished data | |
Tranel 2012.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: USA Data collection period: participants studied in 2007, no further details Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 70; Number included in meta‐analysis: N = 70 Sample characteristics (at baseline): Age (mean): quitters 47.4 years (SD 14.1), non‐quitters 44.8 years (SD 11.7); Sex (% male): quitters 62.5% (20/32), non‐quitters 55.3% (21/38) Population category: chronic physical condition; Specific population: people who smoked and were deemed to be nicotine‐dependent (> 5 CPD for > 2 years at point of brain injury) drawn from the Iowa Patient Registry maintained in the Department of Neurology at the University of Iowa College of Medicine; to enrol in the Registry patients had to be free of any premorbid history of primary psychiatric disease, alcohol or substance abuse (other than nicotine), learning disability, and dementia Nicotine dependence: not measured; Baseline cigarettes per day: 27.0; Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: participants were categorised as quitters who had stopped smoking after their brain lesions Cessation definition used for outcome(s) in this analysis: unclear definition Measure of biovalidation: not bioverified Definition of people who continued to smoke used: participants were categorised as non‐quitters who had kept smoking after their brain lesions Time point(s) at which follow‐up was conducted: acute epoch (within 2 weeks of brain lesion) and chronic epoch (3 months or more after brain lesion onset) Outcome category: Depression Outcome measure(s): Depression rating chronic epoch (the medical record data were used to formulate a variable of Depression Rating) ‐ participants were classified as having depression on a scale of 0 (none), 1 (mild), or 2 (moderate/severe); Beck Depression Inventory‐II (BDI‐II) |
|
| Funding source | Study was supported by NIDA R01 DA16708 and NINDS P50 NS19632, and partially by NIDA R01 DA023051, NIDA R01 DA022549, and NCI R01 CA152062 | |
| Author conflicts of interest | The authors declare no conflicts of interest | |
| Notes | Outcome data source: Published data | |
Tsoh 2000.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 304; Number included in meta‐analysis: N = 304 Sample characteristics (at baseline): Age (mean): 41.0 years (SD 9.7); Sex (% male): 43.3% (132/304) Population category: general population; Specific population: general population Nicotine dependence: Fagerström Tolerance Questionnaire 5.8 (SD 2.1); Baseline cigarettes per day: 23.3 (SD 10.4); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: cognitive behavioural mood management; or health education Pharmacological support for smoking cessation: nicotine gum (Trial 1); nortriptyline or placebo (Trial 2) Psychotherapeutic or psychoactive support for mental health or mood: received mood management ‐ 10 sessions of cognitive behavioural mood management intervention or 5 sessions of health education |
|
| Outcomes |
Definition of cessation used: self‐reported abstinence during the last 7 days of the assessment (point‐prevalence abstinence) Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: expired‐air carbon monoxide of ≤ 10 ppm (both trials); urine cotinine level < 60 ng/ml (Trial 2 only) Definition of people who continued to smoke used: self‐report did not abstain during last 7 days of assessment Time point(s) at which follow‐up was conducted: end of treatment (week 12) and at 26 and 52 weeks after baseline (Trial 1); and at 24, 38 and 64 weeks after baseline (Trial 2) Outcome category: Depression Outcome measure(s): major depressive episode (MDE) diagnosed according to DSM‐III‐R criteria using ‘Inventory to Diagnose Depression’ |
|
| Funding source | Supported by grants DA‐02538, DA‐23625, DA‐09253, and DA07250 from the National Institute on Drug Abuse | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Vázquez 1999.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: September 1994 – September 1996 Registry ID: not reported |
|
| Participants |
Number of participants: N = 160; Number included in meta‐analysis: N = 160 Sample characteristics (at baseline): Age (mean): 34.9 years (SD 9.4); Sex (% male): 35% (56/160) Population category: general population; Specific population: recruited from community health centre and using advertisements Nicotine dependence: FTND 4.8 (SD 2.3); Baseline cigarettes per day: 24.6 (SD 8.9); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: multicomponent behavioural treatment, including self‐monitoring, nicotine fading and relapse prevention strategies; no mood management Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: at end of treatment – self‐reported 24‐hour point‐prevalence abstinence; at 3‐ and 6‐month follow‐up – self‐reported 7‐day point‐prevalence abstinence; at 12‐month follow‐up – self‐reported continuous abstinence during previous 30 days Cessation definition used for outcome(s) in this analysis: continuous abstinence Measure of biovalidation: expired CO level (< 9 ppm) Definition of people who continued to smoke used: 'smokers' Time point(s) at which follow‐up was conducted: baseline, 3‐, 6‐ and 12‐month follow‐up Outcome category: Depression Outcome measure(s): Beck Depression Inventory (BDI) |
|
| Funding source | None specified | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Vermeulen 2019.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: Netherlands and Belgium Data collection period: January 2004 ‐ March 2014 Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 2720; Number included in meta‐analysis: ranges from N = 427 (Psych QoL) to N = 719 (Depression; combined groups) Sample characteristics (at baseline): Age (mean): (people who smoked) NAP 26.8 years (SD 6.9), US 27.5 years (SD 8.1), HC 26.9 years (SD 9.9); Sex (% male): (people who smoked) NAP 83% (603/729), US 49% (198/401), HC 50% (73/145) Population category: psychiatric population (Psych QoL and Depression) and general population (Depression); Specific population: mixed population ‐ recruited participants with a non‐affective psychosis (NAP; n = 1094), unaffected siblings (US; n = 1047), and healthy controls (HC; n = 579) Nicotine dependence: not measured; Baseline cigarettes per day: NAP 17.5 (SD 8.8), US 12.6 (SD 8.1), HC 11.9 (SD 7.8); Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: 95% of smoking participants reported antipsychotic drug use, i.e. received mood management |
|
| Outcomes |
Definition of cessation used: exact definition not reported; the Composite International Diagnostic Interview (CIDI) was used to assess tobacco use during past year; ‘changes in smoking status’ used to explore differences between those who ‘quit smoking’ versus ‘no change in smoking behaviour’ versus ‘started to smoke’ Cessation definition used for outcome(s) in this analysis: unclear point prevalence Measure of biovalidation: not bioverified Definition of people who continued to smoke used: people who smoked daily during 1 month or more during the past 12 months; data were also collected about the number of cigarettes per day in the period of most severe smoking in the past 12 months; smoking data collected at all 3 time points (baseline, 3 and 6 years) – no further details Time point(s) at which follow‐up was conducted: 3 and 6 years Outcome category: Depression, Psychological Quality of Life (QoL) Outcome measure(s): Community Assessment of Psychic Experience (CAPE; depression subscale); WHO Quality of Life BREF (WHOQOL‐BREF; psychological subscale) |
|
| Funding source | The infrastructure for the GROUP study is funded through the Geestkracht programme of the Dutch Health Research Council (ZonMw, grant number 10‐000‐1001) and matching funds from participating pharmaceutical companies (Lundbeck, AstraZeneca, Eli Lilly, Janssen Cilag) and universities and mental health‐care organisations (Academic Psychiatric Center of Amsterdam UMC, Meibergdreef, and the mental health institutions GGZ inGeest, Arkin, Dijk en Duin, GGZ Rivierduinen, Erasmus Medical Center, GGZ Noord Holland Noord; University Medical Center Groningen, and the mental health institutions Lentis, GGZ Friesland, GGZ Drenthe, Dimence, Mediant, GGNet Warnsveld, Yulius Dordrecht, and Parnassia psycho‐medical center, The Hague; Maastricht University Medical Center and the mental health institutions GGzE, GGZ Breburg, GGZ Oost‐Brabant, Vincent van Gogh voor Geestelijke Gezondheid, Mondriaan, Virenze riagg, Zuyderland GGZ, MET ggz, Universitair Centrum Sint‐Jozef Kortenberg, CAPRI University of Antwerp, PC Ziekeren Sint‐Truiden, PZ Sancta Maria Sint‐Truiden, GGZ Overpelt, OPZ Rekem; University Medical Center Utrecht and the mental health institutions Altrecht, GGZ Centraal, and Delta) | |
| Author conflicts of interest | WvdB received speakers’ fees from Lundbeck, Indivior, Eli Lilly, and Pfizer and he is a consultant to Indivior, Mundipharma, Novartis, Bioproject, D&A Pharmaceuticals, and Opiant Pharmaceuticals. All other authors declare no competing interests | |
| Notes | Outcome data source: Unpublished data | |
Ward 2005.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 296 Sample characteristics (at baseline): Age (mean): 66.4 years (SD 5.3); Sex (% male): 53.7% Population category: general population; Specific population: older adults Nicotine dependence: information not available; Baseline cigarettes per day: information not available; Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: randomised to receive 1 of 3 smoking cessation interventions consisting of a) usual care (MD advice plus 1 behavioral counselling session); b) standard behavioural counselling (4 sessions) + nicotine patch; or c) 4 sessions of behavioural counselling targeted to older adults + nicotine patch Pharmacological support for smoking cessation: 2 of 3 interventions included provision of nicotine patch Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: biochemically‐confirmed point‐prevalence abstinence Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: expired carbon monoxide (< 10 ppm) and saliva cotinine Definition of people who continued to smoke used: non‐confirmed quitters defined as non‐quitters Time point(s) at which follow‐up was conducted: at 7 weeks, 6‐ and 12‐month follow‐up Outcome category: Psychological Quality of Life (QoL) Outcome measure(s): The Short Form Health Status Questionnaire (SF‐36) |
|
| Funding source | Supported by PHS grants HL56626 and HL68569 | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Weinberger 2009.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: USA Data collection period: 1998 and 2007 Registry ID: NCT00593099 and NCT00124683 |
|
| Participants |
Number of participants: N = 135 Sample characteristics (at baseline): Age (mean): 41.1 years (SD 12.4); Sex (% male): 57.8% (78/135) Population category: psychiatric population; Specific population: nicotine‐dependent adults who smoked with schizophrenia or schizoaffective disorder Nicotine dependence: FTND 6.9 (SD 1.6); Baseline cigarettes per day: 25.9 (SD 12.4); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: manualised group behavioural counselling including motivational enhancement, psychoeducation and relapse prevention Pharmacological support for smoking cessation: NRT (transdermal nicotine patch) and/or sustained‐release bupropion or placebo Psychotherapeutic or psychoactive support for mental health or mood: received mood management ‐ manualised group behavioural counselling included motivational enhancement therapy, stratified treatment by antipsychotic medication class |
|
| Outcomes |
Definition of cessation used: smoking abstinence during the last week of trials (end of trial, EOT) i.e. 7‐day point prevalence abstinence; continuous abstinence (CA) over the last 4 weeks of the trials Cessation definition used for outcome(s) in this analysis: multiple (point‐prevalence and continuous abstinence) Measure of biovalidation: biochemically‐verified by expired breath carbon monoxide (CO) levels < 10 ppm Definition of people who continued to smoke used: people who continued to smoke, including those lost during trials or to follow‐up Time point(s) at which follow‐up was conducted: trial end point (EOT) and last 4 weeks of trials (CA) Outcome category: Depression Outcome measure(s): Beck Depression Inventory‐II (BDI‐II) |
|
| Funding source | Supported by grants DA‐02538, DA‐23625, DA‐09253, and DA07250 from the National Institute on Drug Abuse, the National Cancer Institute, the Transdisciplinary Tobacco Use Research Center at Brown and the National Alliance for Research in Schizophrenia and Depression | |
| Author conflicts of interest | Dr. Weinberger reports that she has received grant support from Sepracor. Dr. George reports that he has received grant support from Targacept, Inc., Sepracor, Pfizer and Sanofi~Aventis, and has served as a consultant or received honoraria from Pfizer, Evotec, Prepharm, Janssen‐Ortho International and Memory Pharmaceuticals | |
| Notes | Outcome data source: Published data | |
Weinhold 2017.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: Netherlands Data collection period: 2008 ‐ 2012 Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 5227; Number included in meta‐analysis: N = 1782 Sample characteristics (at baseline): Age (mean): new quit 48 years (SD 15.3), smoke now 48 years (SD 12.8); Sex (% male): new quit 51.3%, smoke now 54.9% Population category: general population; Specific population: data from the Longitudinal Internet Studies for the Social Sciences (LISS) Nicotine dependence: not measured; Baseline cigarettes per day: not measured; Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: the Health module of the LISS asks respondents ‘Have you ever smoked?’ and ‘Do you smoke now?’; ‘smokers who quit during survey’ are respondents who changed status from smoking in the first observation to not smoking in the last observation Cessation definition used for outcome(s) in this analysis: unclear point prevalence Measure of biovalidation: not bioverified Definition of people who continued to smoke used: those who reported smoking in the first and last observation Time point(s) at which follow‐up was conducted: 5 years Outcome category: Positive Affect Outcome measure(s): Subjective Wellbeing – SWB – 1; measures “happiness” in “the respondent’s personal life” |
|
| Funding source | None specified | |
| Author conflicts of interest | None declared | |
| Notes | Outcome data source: Published and unpublished data | |
Wiggers 2006.
| Study characteristics | ||
| Methods |
Study design: secondary analysis of RCT Country: Netherlands Data collection period: not stated Registry ID: not reported |
|
| Participants |
Number of participants: N = 344 Sample characteristics (at baseline): Age (mean): 59 years (SD 12); Sex (% male): 63.4% (218/344) Population category: chronic physical condition; Specific population: people recruited from vascular surgery, cardiology and vascular medicine outpatient departments Nicotine dependence: FTND low‐dependency (≤ 5) 59.6% and high‐dependency (≥ 6) 40.4%; Baseline cigarettes per day: not measured; Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: minimal Intervention Strategy for Cardiology Patients (C‐MIS), a brief form of behavioural counselling to promote smoking cessation – provided to intervention group only Pharmacological support for smoking cessation: NRT in both control and intervention group Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: self‐reported 7‐day point‐prevalence abstinence at all 4 measurement occasions (baseline, 2, 6 and 12 months post‐intervention) Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: not bioverified Definition of people who continued smoking used: reported smoking in the last 7 days i.e. ‘failed quitters’ Time point(s) at which follow‐up was conducted: at 2, 6 and 12 months (T2, T3, T4 respectively) Outcome category: Psychological Quality of Life (QoL) Outcome measure(s): Medical Outcomes Study Short Form Health Status Questionnaire (SF‐36): generic mental QoL |
|
| Funding source | Financial support for the present study was provided entirely by a grant from the Netherlands Heart Foundation (2000/B216) | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Xue 2017.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: China Data collection period: May 2011 ‐ November 2013 Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 649; Number included in meta‐analysis: N = 298 Sample characteristics (at baseline): Age (mean): people who continued to smoke 60.3 years (SD 9.7), people who never smoked 65.1 years (SD 9.3), people who quit 60.2 years (SD 9.1); Sex (% male): people who continued to smoke 90.1% (155/172), people who never smoked 60.1% (211/351), people who quit: 93.7% (118/126) Population category: postoperative; Specific population: coronary artery disease patients treated with drug‐eluting stents Nicotine dependence: not measured; Baseline cigarettes per day: not measured; Motivation to quit: not selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: no behavioural support Pharmacological support for smoking cessation: no pharmacological support Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: participants who smoked during the year before baseline interview but stopped smoking during the follow‐up period were considered quitters Cessation definition used for outcome(s) in this analysis: unclear point prevalence Measure of biovalidation: not bioverified Definition of people who continued to smoke used: participants who smoked before baseline interview and continued smoking during the follow‐up period Time point(s) at which follow‐up was conducted: 6 months and 12 months Outcome category: Psychological Quality of Life (QoL) Outcome measure(s): Short Form Health Survey (SF‐36; mental component summary) |
|
| Funding source | Supported by the Scientific and Technological Foundation Projects of Shanghai Jiao Tong University, School of Medicine (No.13XJ10050); the Shanghai Ninth People’s Hospital Foundation (No.2013A10) | |
| Author conflicts of interest | The authors declare that they have no competing interests | |
| Notes | Outcome data source: Published data | |
Zhou 2016.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: China Data collection period: March 2014 ‐ September 2014 Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 690; Number included in meta‐analysis: N = 690 Sample characteristics (at baseline): Age (mean): 45.5 years (SD 12.1); Sex (% male): 83.2% Population category: chronic physical condition; Specific population: people (Beijing Anzhen Hospital) with coronary heart disease (CHD) Nicotine dependence: not measured; Baseline cigarettes per day: not measured; Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: 541 participants attended a smoking cessation clinic but no further detail on support provided Pharmacological support for smoking cessation: 306 participants received a smoking medication ‐ no further detail Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: exact definition not reported; participants self‐reported cessation Cessation definition used for outcome(s) in this analysis: unclear definition Measure of biovalidation: not bioverified Definition of people who continued smoking used: people who smoked defined according to the WHO 1997 definition (continuously or cumulatively smoking for 6 months or more during their life), but no explanation of whether and how smoking cessation was verified Time point(s) at which follow‐up was conducted: 6 months Outcome category: Depression Outcome measure(s): Hamilton Anxiety and Depression Scale (HAMD; depression scale) |
|
| Funding source | National Key Clinical Specialty Construction Project; Beijing Hospital Administration Bureau of Clinical Medicine Funding | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
Zillich 2002.
| Study characteristics | ||
| Methods |
Study design: longitudinal cohort Country: USA Data collection period: July 2000 – December 2001 Registry ID: N/A (cohort) |
|
| Participants |
Number of participants: N = 31 Sample characteristics (at baseline): Age (mean): 41.2 years (SD 10.5); Sex (% male): 35% Population category: general population; Specific population: general population (recruited using advertisements) Nicotine dependence: Fagerström score 6.1 (SD 1.9); Baseline cigarettes per day: 25.5 (SD 10.4); Motivation to quit: selected by motivation to quit |
|
| Interventions |
Behavioural support for smoking cessation: weekly, pharmacist‐led 1‐hour group meetings including participant description of cessation attempt during the previous week and techniques for behaviour modification Pharmacological support for smoking cessation: NRT (nicotine gum 4 mg pieces or nicotine patch 14 mg or 21 mg dependent on starting number of packs smoked) Psychotherapeutic or psychoactive support for mental health or mood: did not receive mood management |
|
| Outcomes |
Definition of cessation used: self‐reported abstinence during previous 7 days recorded at each weekly meeting – point prevalence abstinence Cessation definition used for outcome(s) in this analysis: point‐prevalence abstinence Measure of biovalidation: exhaled carbon monoxide levels (< 10 ppm) assessed at end of treatment (3 months) and at 6‐month follow‐up Definition of people who continued to smoke used: people who smoked; participants who missed sessions and did not perform CO test were considered smoking at that time point Time point(s) at which follow‐up was conducted: baseline, 2 weeks and 1, 2, 3 and 6 months; abstinence self‐reported weekly at group meetings and validated biochemically at 3 and 6 months Outcome category: Psychological Quality of Life (QoL) Outcome measure(s): Smoking Cessation Quality of Life Questionnaire (SCQOL): Vitality and Mental Health domains |
|
| Funding source | Start‐up funds provided by University of Kentucky Medical Center Human Resources Department. An unrestricted educational grant and exhaled carbon monoxide equipment provided by GlaxoSmithKline | |
| Author conflicts of interest | None specified | |
| Notes | Outcome data source: Published data | |
FTND: Fagerström Test for Nicotine Dependence
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abrams 1987 | Mental health was not measured before smoking cessation |
| Abrantes 2018 | Did not analyse mental health outcomes, by smoking status |
| Acri 1992 | Mental health outcome was measured during the withdrawal period. |
| Almeida 2011 | Mental health outcome was measured during the withdrawal period. |
| Auer 2014 | Ineligible outcomes |
| Bakhshaie 2015 | Wrong study design |
| Balduyck 2011 | Mental health was not measured before smoking cessation |
| Balfour 2017 | Did not analyse mental health outcomes, by smoking status |
| Bekele 2017 | Wrong study design |
| Billert 2006 | Mental health outcome was measured during the withdrawal period. |
| Bock 2014 | Did not analyse mental health outcomes, by smoking status |
| Bolliger 2002 | Ineligible outcomes |
| Breslau 1998 | Wrong exposure |
| Brody 2017 | Wrong study design |
| Brook 2012 | Did not analyse mental health outcomes, by smoking status |
| Brown 2014 | Did not analyse mental health outcomes, by smoking status |
| Brunette 2018 | Did not analyse mental health outcomes, by smoking status |
| Burris 2014 | Did not analyse mental health outcomes, by smoking status |
| Campbell 2018 | Wrong study design |
| Caponnetto 2013 | Ineligible outcomes |
| Carter 2014 | Mental health was not measured before smoking cessation |
| Chen 2012 | Ineligible outcomes |
| Chengappa 2014 | Ineligible outcomes |
| Dalack 1999 | Mental health outcome was measured during the withdrawal period. |
| Dickson‐Spillmann 2012 | Did not analyse mental health outcomes, by smoking status |
| Duffy 2012 | Did not analyse mental health outcomes, by smoking status |
| El Hajj 2015 | Ineligible outcomes |
| Etter 2006 | Mental health outcome was measured during the withdrawal period. |
| Faseru 2013 | Ineligible outcomes |
| Flensborg‐Madsen 2011 | Wrong study design |
| Fond 2013 | Did not analyse mental health outcomes, by smoking status |
| Frederick 1998 | Mental health outcome was measured during the withdrawal period. |
| Garces 2004 | Ineligible outcomes |
| George 2002 | Did not analyse mental health outcomes, by smoking status |
| Gilbert 1998 | Mental health outcome was measured during the withdrawal period. |
| Gilbert 1999 | Mental health outcome was measured during the withdrawal period. |
| Gilbert 2002 | Mental health outcome was measured during the withdrawal period. |
| Gilbert 2003 | Mental health outcome was measured during the withdrawal period. |
| Guiterrez‐Bedmar 2009 | No psychological outcome data presented at baseline. |
| Haibach 2016 | Ineligible outcomes |
| Hall 1990 | Mental health outcome was measured during the withdrawal period |
| Heffner 2012 | Mental health was measured during the withdrawal period |
| Hendricks 2014 | Did not analyse mental health outcomes, by smoking status |
| Hirdes 1994 | Ineligible outcomes |
| Hsu 2015 | Did not analyse mental health outcomes, by smoking status |
| Hughes 1992 | Mental health was measured during the withdrawal period |
| Hurt 1997 | Randomised controlled trial. Analysis by treatment group. |
| Hwang 2012 | Ineligible outcomes |
| IRCT20100127003210N16 2019 | Did not analyse mental health outcomes, by smoking status |
| Jang 2010 | No mental health outcome |
| John 2004 | Smoking status as the outcome. Binary outcome. |
| Kaetsu 2002 | Ineligible outcomes |
| Kahler 2004 | Mental health was measured during the withdrawal period. |
| Kahler 2009 | Ineligible outcomes |
| Kerkvliet 2015 | Did not analyse mental health outcomes, by smoking status |
| Khaled 2009 | Does not present data of change in mental health. |
| Khaled 2012 | Mental health was not measured before smoking cessation |
| Kim 2019 | (Prevalence) do not know who had depression at baseline |
| Kinnunen 2006 | Mental health was not measured before smoking cessation |
| Kocak 2015 | Ineligible outcomes |
| Lam 2012 | Mental health was measured during the withdrawal period. |
| Lappan 2018 | Mental health was not measured before smoking cessation |
| Lerman 2004 | Randomised controlled trial. Analyses data by treatment group. |
| Lewis 2011 | (Prevalence) do not know who had depression at baseline |
| McClure 2009 | Randomised controlled trial. Analyses data by treatment group. |
| Miguez 2019 | Mental health was not measured before smoking cessation |
| Moreno‐Coutino 2007 | Continuing smoking group included recent relapsers. |
| Morris 2011 | Randomised controlled trial. Analyses data by treatment group. |
| Myneni 2018 | No full text available |
| NCT01789125 2013 | Did not analyse mental health outcomes, by smoking status |
| NCT02845687 | Study not completed |
| Nieva 2017 | Ineligible outcomes |
| O'Toole 2018 | (Prevalence) do not know who had depression at baseline |
| Ong 2016 | Mental health was not measured before smoking cessation |
| Park 2009 | Ineligible outcomes |
| Piper 2012 | Ineligible outcomes |
| Roson 2017 | Did not analyse mental health outcomes, by smoking status |
| Schlyter 2016 | Ineligible outcomes |
| Schwartz 1968b | No figures presented in paper |
| Seghatoleslam 2014 | No full text available |
| Shaw 2001 | Mental health outcome was measured during the withdrawal period |
| Shimadu 2016 | Ineligible outcomes |
| Siahpush 2007 | Ineligible outcomes |
| Solak 2018 | Ineligible outcomes |
| Stepankova 2016 | No full text available |
| Strong 2012 | Did not analyse mental health outcomes, by smoking status |
| Thorsteinsson 2001 | Mental health was measured during the withdrawal period |
| Vidrine 2007 | Change in mental health measured during the withdrawal period |
| Vidrine 2015 | Did not analyse mental health outcomes, by smoking status |
| Weaver 2015 | Did not analyse mental health outcomes, by smoking status |
| Weinberger 2012 | Ineligible outcomes |
| Welch 2015 | Did not analyse mental health outcomes, by smoking status |
| Zvolensky 2014 | Mental health was not measured before smoking cessation |
Characteristics of ongoing studies [ordered by study ID]
Allen 2017.
| Study name | Reduced nicotine cigarettes in smokers with mood and anxiety disorders |
| Methods | A 2‐site, 2‐arm, 34‐week, double‐blind, parallel‐group, randomised controlled trial of reduced nicotine cigarettes |
| Participants | Psychiatric population: adults who smoke and meet lifetime diagnostic criteria for mood or anxiety disorders |
| Interventions | (a) Usual Nicotine Content (11.6 mg); or (b) Reduced Nicotine Content: the nicotine content per cigarette is progressively reduced from approximately 11.6 mg to 0.2 mg in 5 steps over 18 weeks. Both arms offered brief smoking cessation counselling after week 10 |
| Outcomes | Primary outcome measure is blood cotinine Key secondary outcomes are: exhaled carbon monoxide; urinary total NNAL‐ 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanol and 1‐hydroxypyrene; oxidative stress biomarkers including 8‐isoprostanes; measures of psychiatric symptoms (e.g., depression, anxiety); smoking behaviour and dependence (e.g., cigarette consumption, quit attempts); and health effects (e.g., blood pressure, respiratory symptoms) |
| Starting date | Recruitment of participants started in September 2015 |
| Contact information | Name: Sophia Allen; Email: sallen@phs.psu.edu |
| Notes | Registry ID: Clinicaltrials.gov NCT01928758 Funding source: National Institute on Drug Abuse of the National Institutes of Health under Award Number P50DA036107 (JM & JF PIs for parent grant; JF and AEE PIs for this study) and the Center for Tobacco Products of the U.S. Food and Drug Administration. NIH NIDA (P50DA036107), NCATS (UL1 TR000127), FDA Declaration of interests: JF has done paid consulting for pharmaceutical companies involved in producing smoking cessation medications, including GSK, Pfizer, Novartis, J&J, and Cypress Bioscience, and received a research grant from Pfizer Inc. (not related to reduced nicotine cigarettes). There are no competing interests to declare for other authors |
Bernstein 2016.
| Study name | A multicomponent intervention including texting to promote tobacco abstinence in emergency department smokers: a pilot study |
| Methods | A prospective, 2‐arm, parallel group, randomised controlled trial of a multicomponent intervention (brochure + text‐based smoking cessation intervention + NRT + quitline referral) compared to standard care (brochure only). Outcome assessments blinded, recruiting research staff not blinded to group assignment |
| Participants | General population: adults visiting a hospital emergency department |
| Interventions | Multicomponent intervention consisting of: a) brochure containing information on health benefits of cessation and local quitline number; b) 4‐week supply of NRT; c) referral to local quitline; d) enrolment by research team in supported texting programme for tobacco dependence. Texting programme used was ‘SmokefreeTXT’ (smokefree.gov/smokefreetxt) with message content based on principles of CBT and some tips/advice for remaining abstinent. Some texts also employed momentary assessment of mood, cravings, tobacco use and physician contact; texting programme lasted for 28 days Control arm/standard care: brochure only |
| Outcomes | Main objective: To assess feasibility of multicomponent ED‐initiated programme of tobacco dependence treatment using a publicly‐available text messaging programme and compare with control condition
Primary outcome(s)/Primary end point(s): self‐reported 7‐day tobacco abstinence at 1‐ and 3‐months telephone follow‐up assessed using (“Have you smoked, even a puff, in the last 7 days?”). Time point(s) of evaluation of endpoint: 1‐month and 3‐month post‐enrolment; both 1 and 3 months considered primary in this study; biochemical verification not performed Secondary outcome(s)/Secondary end point(s): associations between responses/adherence to text‐delivered ecological momentary assessments (EMA) and tobacco abstinence. EMAs assessed mood and craving on 3‐point ordinal scale (mood: good, OK, bad; craving: low, medium, high). EMAs used were taken from SmokefreeTXT library of messages and participants received either 0 or 1 EMA message per day. Time point(s) of evaluation of endpoint: frequency of response to EMAs was measured throughout intervention, i.e. 28 days Secondary objectives: to obtain a measure of effect size for follow‐up trial and assess willingness of eligible participants to participate and undergo randomisation, to assess study procedures, follow‐up rates and adherence to intervention, i.e. proportion of participants engaging in quitline services to which they were referred |
| Starting date | Participants were enrolled in May 2014 |
| Contact information | Name: Steven L. Bernstein; E‐mail: Steven.bernstein@yale.edu |
| Notes | Registry ID: NCT02081144 Funding source: Supported in part by grant R01CA141479 from the National Cancer Institute, National Institutes of Health Declaration of interests: Authors had no potential conflicts to disclose |
Bunker 2012.
| Study name | A cluster‐randomised trial to examine the impact of early intervention by practice nurse‐general practitioner (GP) teams on quality of life, health status and lung function of Chronic Obstructive Pulmonary Disease (COPD) patients |
| Methods | A pragmatic, parallel‐group, cluster‐randomised controlled trial of early intervention by a practice nurse‐GP partnership vs usual care for newly‐diagnosed COPD. Participating GPs, practice nurses and participants not blinded. Research staff collecting outcome measures and statistician blinded to group allocation |
| Participants | General population: patients at risk of COPD aged 40 ‐ 85 years with documented smoking history who have attended a GP practice at least twice, who are newly diagnosed with COPD through case‐finding visits |
| Interventions | Practice nurses will identify people who are at risk of COPD and undertake case finding. People newly diagnosed with COPD who attend the 'intervention practices' will be offered a management involving the GP and practice nurse working in partnership. The people who attend the 'control practices' will receive usual care from their GPs |
| Outcomes | Primary outcome [1]: The St George Respiratory Questionnaire (SGRQ) will be used for 2 purposes: 1) to collect participants' COPD‐related quality of data, and 2) participants' overall health status data (from the preamble question of (SGRQ): Timepoint [1] 1) at the first visit by project officer soon after the case‐finding visit by practice nurse; 2) 6 months from the first visit; 3) 12 months from the first visit. Secondary outcome [1]: Lung function ‐ forced expiratory volume in 1 second (FEV1) assessed by spirometry performed according to the standard methods. Timepoint [1] 1) at the first visit by project officer soon after the case‐finding visit by practice nurse; 2) 12 months from the first visit Secondary outcome [2]: Medication audit ‐ Participant report of medication use. These data will be collected by project officer at all 3 visits, except that at 6 months data will only be collected about respiratory medications. Timepoint [2]: 1) at the first visit by project officer soon after the case‐finding visit by practice nurse; 2) 6 months from the first visit; 3) 12 months from the first visit Secondary outcome [3]: Inhaler technique ‐ Assessment of inhaler technique assessed using published inhaler technique checklists. Timepoint [3]: 1) at first visit by project officer soon after the case‐finding visit by practice nurse; 2) 12 months from the first visit Secondary outcome [4]: Participant report of participation in a pulmonary rehabilitation programme Timepoint [4]: 1) at the first visit by project officer soon after the case‐finding visit by practice nurse; 2) 6 months from the first visit; 3) 12 months from the first visit Secondary outcome [5]: Patient knowledge ‐ Participant knowledge of COPD will be assessed using a previously‐published measure Timepoint [5]: 1) at the first visit by project officer soon after the case‐finding visit by practice nurse; 2) 12 months from the first visit Secondary outcome [6]: Health service use ‐ Participant report of hospital admissions, emergency department attendances, or unscheduled visits to a doctor in the previous 6 months Timepoint [6]: 1) at the first visit by project officer soon after the case‐finding visit by practice nurse; 2) 6 months from the first visit; 3) 12 months from the first visit Secondary outcome [7]: Smoking status ‐ Attendance at a smoking cessation programme and quit rates will be recorded (participant report). Validation of participant report of smoking status using Smokerlyser analysis Timepoint [7]: 1) at the first visit by project officer soon after the case‐finding visit by practice nurse; 2) 12 months from the first visit Secondary outcome [8]: Immunisation status ‐ Immunisation status (influenza and pneumococcal) by participant report Timepoint [8]: 1) at the first visit by project officer soon after the case‐finding visit by practice nurse; 2) 12 months from the first visit Secondary outcome [9]: Validation of COPD Diagnostic Questionnaire (CDQ) in Australian context Timepoint [9]: At the case‐finding visit by practice nurse. Secondary outcome [10]: COPD Assessment Test (CAT) ‐ a tool to quantify the impact of COPD on participants' health status Timepoint [10]: 1) at the first visit by project officer soon after the case‐finding visit by practice nurse; 2) 6 months from the first visit; 3) 12 months from the first visit |
| Starting date | Recruitment of participants started in 6th April 2011 |
| Contact information | Name: Prof Nicholas Zwar (School of Public Health & Community Medicine, The University of New South Wales Sydney, NSW 2052 Country Australia, Phone +61 2 93852515); Email: n.zwar@unsw.edu.au |
| Notes | Registry ID: ACTRN12610000592044 Funding source: The study was funded by the National Health and Medical Research Council, Project Grant No. 630421 Declaration of interests: HR has participated on COPD advisory committees for Novartis, has spoken about COPD guidelines at symposia funded by AstraZeneca and BoehringerIngelheim, has received travel support from AstraZeneca, GlaxoSmithKlineand Novartis, and has received independent research funding fromGlaxoSmithKline for an investigator‐initiated COPD study. GBM is on an advisory board for Novartis and his institution has received funds fromAstraZeneca for consultancies. He has spoken at education symposia sponsored by AstraZeneca and GlaxoSmithKline. NZ has provided expert advice on smoking cessation education programs to Pfizer Pty Ltd andGlaxoSmithKline Australia Pty Ltd and has received support to attend smoking cessation conferences. Other authors have no competing interests |
CTRI/2012/12/003262 2012.
| Study name | A controlled field trial to assess the effect of a community‐based tobacco cessation intervention package on tobacco use and expenditure on tobacco in women self‐help group members in rural Gadchiroli, Maharashtra, India: The Women’s Empowerment through Liberation From Tobacco (WE LiFT) project |
| Methods | A group‐randomised, parallel‐group, multiple‐arm field trial to assessed the effectiveness of a community‐based tobacco cessation intervention on tobacco use and expenditure on tobacco among women micro‐finance self‐help group (SHG) members. Blinding/Masking (N/A), randomisation performed at cluster level via computer‐generated randomisation |
| Participants | General population: women who are members of the MAVIM SHG in villages from selected clusters of Mahila Arthik Vikas Mahamandal (MAVIM) and their husbands, aged 18 ‐ 90 years old |
| Interventions | A) community‐based tobacco‐cessation intervention package from women SHG members (group health education, individual counselling of users, distribution of earthen saving pots to save money spent on tobacco, oral evaluation by dentists to assess oral lesions due to tobacco and distribution of iron and folic acid (ferrous sulphate 300 mg and folic acid 0.5 mg ‐ 1 tablet once a week by mouth for 7 months), B‐complex vitamin tablets (1 tablet twice daily by mouth for 7 days) and calcium tablets (calcium lactate 300 mg, 1 tablet twice daily by mouth for 7 days) as a health promotion measure and bisacodyl tablets (5 mg tablet, 1 tablet at bedtime for 7 days) for those reporting using tobacco to prevent constipation); B) community‐based tobacco cessation intervention package for women SHG members and their husbands (SHG members and their husbands receive tobacco cessation intervention package described above); C) no intervention (comparison cluster in which community‐based intervention package is not provided) |
| Outcomes | Primary outcome: decrease in % of SHG members using tobacco (7‐months); Secondary outcome(s): decrease in average daily expenditure on tobacco (7 months), amount of money saved in earthen money‐saving pots by families of tobacco users in the intervention areas (7 months), decrease in % of family members of the SHG members using tobacco (7 months), increase in % of SHG members and husbands with a sense of well‐being (7 months), decrease in % of SHG members and their husbands with oral discomfort (7 months) |
| Starting date | Date of first enrolment (India): 19 October 2012 |
| Contact information | Name: Dr Abhay Bang; Site address: Gadchiroli, Search Shodhgram Gadchiroli, MAHARASHTRA; Tel: 07138255407; Email: search@sify.com |
| Notes | Registry ID: CTRI/2012/12/003262 Funding source: Indira foundation, Greenwich, CT, USA ; Mahila Arthik Vikas Mahamandal (Women Economic Development Corporation), Gadchiroli, Maharashtra, India Declaration of interests: None specified |
DRKS00014920 2018.
| Study name | Reducing stress, alcohol and tobacco use in pregnant women to improve the children’s later mental health (MINDFUL/PMI) |
| Methods | A 2‐arm, parallel‐group, randomised controlled trial of a 15‐week mindfulness‐oriented app‐based programme compared to an education app‐based programme. Assessor and data analyst are blinded. Control group is active control (i.e. effective treatment of control group) |
| Participants | Pregnant population: pregnant women aged 18 ‐ 50 years, between 8+0 to 14+0 weeks of pregnancy |
| Interventions | A) Intervention group: pregnancy mindfulness ‐ 15‐week mindfulness‐oriented app‐based programme B) Control group: pregnancy education ‐ 15‐week pregnancy education app‐based programme |
| Outcomes | Primary outcome(s): second‐to‐fourth finger length ratio (2D:4D) in the 11‐ to 12‐month‐old child. Secondary outcome(s): A) Child (birth and 11‐ to 12‐month of age): Index “Orientation and Regulation“ of the Infant Behavior Questionnaire Revised (IBQ‐R); subscale scores of the German Parent Questionnaire of Child Development supplement to the Pediatric Screenings (EEE U6); subscale scores cognition, language, and motor function of the Bayley Scales of Infant and Toddler Development (BSID); sum and subscale scores of the Child Behavior Checklist (CBCL); infant anogenital distance; strength of the transient‐evoked oto‐acoustic emissions (TEOAEs); ethylglucuronide (EtG) in meconium; B) Mother (longitudinal): sum and subscale scores of the Perceived Stress Scale (PSS10); sum scale score of the Pregnancy‐Related Anxiety Questionnaire (PRAQ‐R2); sum and subscale scores of the Mindful Attention and Awareness Scale (MAAS) and the Five Facet‐Mindfulness Questionnaire (FFMQ‐D); consumption quantified by the modified Alcohol Use Disorder Identification Test (AUDIT‐C); consumption quantified by the modified smoking questionnaire Robert Koch‐Institut; cotinine in maternal hair; ethylglucuronide (EtG) in maternal hair; cortisol in maternal hair |
| Starting date | Date of first enrolment: 12 November 2018 |
| Contact information | Name: Johannes Kornhuber, Address: Schwabachanlage 6 91054 Erlangen Germany, Telephone: +49 9131 85 34166, Email: johannes.kornhuber@uk‐erlangen.de, Affiliation: Universitätsklinikum Erlangen, Psychiatrische und Psychotherapeutische Klinik |
| Notes | Registry ID: DRKS00014920 Funding source: With the public funded research project IMAC‐Mind: Improving Mental Health and Reducing Addiction in Childhood and Adolescence through Mindfulness: Mechanisms, Prevention and Treatment (2018–2022, 01GL1745C), the Federal Ministry of Education and Research contributes to improving the prevention and treatment of children and adolescents with substance use disorders and associated mental disorders. The project coordination is realised by the German Center of Addiction Research in Childhood and Adolescence at the University Medical Center Hamburg‐Eppendorf. For more information please visit our homepage www.IMAC‐Mind.de Declaration of interests: The authors quote some of their own publications on which this work and the idea of the study are based |
EUCTR2012‐002731‐28‐PL 2012.
| Study name | Randomised placebo‐controlled trial assessing the efficacy and safety of BP1.4979 in smoking cessation |
| Methods | A multicentre, randomised, double‐blind, placebo‐controlled, phase IIb trial with parallel groups to assess safety and efficacy of BP1.4979 in people who smoke |
| Participants | General population: adults (18 ‐ 65 years) who smoke who have made at least 2 attempts to stop |
| Interventions | Product Code: BP1.4979; Pharmaceutical Form: Tablet; CAS Number; 1000036‐77‐0; Drug BP1.4979 at 3 mg, 10 mg, 15 mg or placebo during 3 months; Placebo form: tablet (oral use) |
| Outcomes | Primary outcome(s)/Main objective: To assess the efficacy (abstinence measured using diaries and confirmed by exhaled CO (abstinent = 10 ppm) and safety of BP1.4979 for smoking cessation, in people who smoke heavily and are willing to quit smoking Primary end point(s): 4‐week prolonged abstinence from smoking cigarettes at the end of a 12‐week double‐blind treatment phase (i.e. from V3 to V4); continuous abstinence measured by participant diary and verified by exhaled CO (abstinent < 10 ppm) will define response to the treatment. Secondary objective: No secondary objectives were established. Time point(s) of evaluation of this end point: During treatment phase and up to 3 months after treatment. Secondary outcome(s)/Secondary end point(s): 7‐day tobacco point prevalence abstinence (PPA) assessed at each visit by analysis of cigarettes consumption using self‐report diaries and measurement of exhaled CO; daily cigarettes consumption (mean consumption variation to baseline to determine % of participants having reduced cigarettes consumption between baseline and last week of treatment); assessment of craving and urge to smoke performed at each visit except screening (V0) by QSU‐Brief Questionnaire (Measure: variation from V1 to V2, V3, V4, V5 and V‐TQD), on (7‐days PPA) responders; continuous abstinence from V3 (W8) to V6 (W16) and from V3 (W8) to V8 (W24); nicotine withdrawal syndrome assessed by Minnesota Nicotine Withdrawal Scale (MNWS) (assessing urge to smoke, depressed mood, irritability, anxiety, poor concentration, restlessness, increased appetite and insomnia)(measure: variation from V1 to V2, V3, V4, V5 and V‐TQD ), on (7‐days PPA) responders; smoking satisfaction measured by the modified Cigarette Evaluation Questionnaire (mCEQ) (recording measures of smoking satisfaction, psychological reward, enjoyment of respiratory tract sensations, craving reduction and aversion) if the participant continues to smoke (measure: variation from V1 to V2, V3, V4, V5 and V‐TQD), in participants still smoking; body weight variation in treatment responders; BDI mean depressive score variation in responders; abstinence in a subgroup of participants having TQD up until treatment Day 8; safety will be assessed by evaluation of adverse events, various questionnaires and vital signs (measurement of heart rate, blood pressure, and body weight) at each study visit, by physical examinations (V0 and V4), ECG and laboratory tests (blood chemistry, haematology, urinanalysis tests, prolactin dosage) at screening (V0), after 4‐week treatment (V2) and at V4 (time points of evaluation of this end point: throughout the whole study duration) |
| Starting date | Date of first enrolment: 01 March 2013 |
| Contact information | Name: Bioprojet Clinical Department, Address: 9 rue Rameau 75002 PARIS France, Telephone: +33(0)1 47 03 66 33, Affiliation: Bioprojet |
| Notes | Registry ID: EUCTR2012‐002731‐28‐CZ/NCT01785147 Funding source: Bioprojet Declaration of interests: none specified |
EUCTR2017‐004715‐40‐ES 2018.
| Study name | An effectiveness and safety study of varenicline for smoking cessation in hospitalised patients with psychiatric disorders |
| Methods | A 2‐arm, parallel group, randomised controlled trial of varenicline (12‐weeks) relative to nicotine patch (active comparator) for smoking cessation in hospitalised patients with psychiatric disorders. Blinding/Masking: None (Open‐Label) |
| Participants | Psychiatric population: aged 18 ‐ 65 years with at least 1 psychiatric disorder, hospitalised at 1 of 3 acute psychiatric facilities |
| Interventions | Trade Name: CHANTIX (varenicline) tablets, for oral use, Pharmaceutical form: film‐coated tablet, INN or proposed INN: VARENICLINE, CAS Number: 375815‐87‐5; Dose: 3 days of 0.5 mg, followed by 4 days of 1 mg, followed by 2 mg of varenicline until week 12. Trade Name: NICOTINELL TTS, Pharmaceutical Form: Cutaneous patch, INN or Proposed INN: NICOTINE, CAS Number: 54‐11‐5. Dose: 8 weeks of 21 mg patch, followed by 2 weeks of 14 mg patch and 2 weeks of 7 mg patch |
| Outcomes | Primary outcome(s). Main objective: This study will compare varenicline to nicotine patch initiated in‐hospital on smoking abstinence rates post‐discharge. In addition, safety will be assessed by comparing the incidence of severe neuropsychiatric adverse events in participants with varenicline or nicotine patch Primary end point(s): EFFICACY, 1. To compare smoking abstinence rates of varenicline relative to nicotine patch measured by CO‐confirmed continuous abstinence rate (CAR) between week 9 and week 12; 2. To compare smoking abstinence rates of varenicline relative to nicotine patch measured by CO‐confirmed continuous abstinence rate (CAR) between week 12 and week 16; SAFETY 1. The primary safety endpoint is the occurrence of at least 1 treatment‐emergent 'severe' neuropsychiatric event during or after hospitalisation. In case of a pre‐existing 'severe' neuropsychiatric event, only a worsening of this event will be reported. Time point(s) of evaluation of this end point: Efficacy: week 9 ‐ 12 and week 12 ‐ 16. Safety: number of severe neuropsychiatric events from the screening to the end of study Secondary objective: N/A. Secondary outcome(s)/Secondary end point(s): EFFICACY 1. To compare smoking abstinence rates of varenicline relative to nicotine patch measured by 7‐day CO‐confirmed abstinence at week 16. 2. To compare smoking withdrawal symptoms of varenicline relative to nicotine patch during and after hospitalisation; SAFETY It will be individual subscales scores on the following questionnaires: ‐ Hospital Anxiety and Depression Scale (HADS), ‐ Columbia Suicide Severity Rating Scale (C‐SSRS), ‐ Clinical Global Impression of Improvement (CGI‐I). Timepoint(s) of evaluation of this end point: 0, week 1, week 2, week 3, week 4, week 5, week 7, week 9, week 12, week 16 |
| Starting date | Date of first enrolment: 04 July 2018 |
| Contact information | Name: Psychiatry Department Vall d’Hebron, Address: Pg. de la Vall d’Hebron, 119‐129 08035 Barcelona Spain, Telephone: 3493489 42 95, Email: ebruguer@vhebron.net, Affiliation: Fundació Hospital Universitari Vall d’Hebron‐Institut de Recerca (VHIR) |
| Notes | Registry ID: EUCTR2017‐004715‐40‐ES Funding source: Pfizer Declaration of interests: none specified |
Garrison 2015.
| Study name | Smartphone application for smoking cessation. |
| Methods | A 2‐arm, parallel‐group, pragmatic randomised controlled trial comparing an app which trains behavioural strategies for smoking cessation to a standard smartphone application to support people who smoke working to become quit‐free. Blinding/masking: single (outcomes assessor at follow‐up will be blind to group allocation). |
| Participants | General population: people who smoke aged 18 ‐ 65 years who own a smartphone and are motivated to quit smoking |
| Interventions | Intervention: smartphone app (Craving to Quit (www.cravingtoquit.com) which delivers mindfulness training and experience sampling for 22 days and consists of 22 modules of 10 ‐ 15 minutes each, designed to teach behavioural strategies for smoking cessation using psycho‐education‐based audio and videos, animations to reinforce key concepts and in vivo exercises. Active comparator: 3‐week smartphone application for smoking cessation in which people who smoke self‐monitor their smoking habits, mood and experience to quit smoking, comprising 6 prompts per day asking individuals about their experience, behaviour and smoking habits for 22 days |
| Outcomes | Primary outcome: 1‐week point‐prevalence abstinence at 6 months, verified by CO monitoring, analysed using intention‐to‐treat approach Secondary outcome(s): smoking, craving, mood and mindfulness as measured by experience sampling using the Tracker |
| Starting date | Study start date: October 2014; actual study completion date: June 2016 |
| Contact information | Correspondence: kathleen.garrison@yale.edu 1 Department of Psychiatry, Yale School of Medicine, New Haven, CT, USA 4 1 Church Street, Room 730, New Haven CT 06510, USA |
| Notes | Registry ID: Clinicaltrials.gov NCT02134509 Funding source: This research is funded by a grant from the American Heart Association (to KAG: 14CRP18200010). Additional research support is provided by a grant from the National Institutes of Health, National Institute on Drug Abuse (to KAG and JAB: K12DA00167) Declaration of interests: Judson A. Brewer and Prasanta Pal own stock in Claritas Mindsciences, the company that developed the apps used in this study. All other authors declare that they have no competing interests |
NCT02002858 2013.
| Study name | Integrated smoking cessation treatment for emotional dysregulation |
| Methods | A 2‐arm, parallel‐group, randomised controlled trial comparing 2 group treatment approaches: (1): an educational‐supportive psychotherapy and standard smoking cessation treatment; and (2) an integrated smoking cessation and anxiety and depression management treatment programme (SDAT). Blinding/masking: single‐blind (outcome‐assessors will be blinded to group allocation) |
| Participants | Psychiatric population: aged 18 ‐ 65 years with elevated anxiety or depression who smoke an average of at least 6 cigarettes per day and report being motivated to quit smoking |
| Interventions | Comparing 2 group treatment approaches: A) cognitive behavioural treatment program which blends smoking cessation, anxiety and depression management/reduction treatment strategies (SDAT), with nicotine patch B) educational‐based psychotherapy and standard smoking cessation treatment programme (active comparator) with nicotine patch |
| Outcomes | Primary outcome: smoking status using the Timeline Follow‐Back Assessment (Time Frame: Change from baseline at 2, 4, 8, 10, 16, and 24 weeks post quit day) |
| Starting date | October 2014 (completed May 2017) |
| Contact information | Michael J Zvolensky, Ph.D. Anxiety and Health Research Laboratory and Substance Use Treatment Clinic, University of Houston Houston, Texas, United States, 77205 |
| Notes | Registry ID: NCT02002858. Smoking Cessation for Depression and Anxiety Treatment. 2013 Funding source: University of Houston, National Institute on Drug Abuse (NIDA) Declaration of interests: none specified |
Pavey 2015.
| Study name | Assessing the effectiveness of High Intensity Interval Training (HIIT) for smoking cessation in women: HIIT to quit study |
| Methods | A 2‐arm, parallel‐group, randomised controlled trial comparing the effects of 2 exercise interventions (HIIT and '10,000 steps' pedometer‐based intervention) combined with usual care smoking‐cessation support, on cessation rates in women who smoke and wish to quit. Blinding/masking: single (outcome assessor will be blinded to group allocations) Randomisation: allocation sequence generated by a computer programme and concealed within sealed opaque envelopes |
| Participants | General population: women aged 18 ‐ 55 years who smoke ≥ 5 cigarettes/day, and want to quit smoking |
| Interventions | Participants in both groups receive usual care smoking‐cessation support based on Smoking Cessation Guidelines for Australian General Practice (quit pack, signposting to other resources, directed to call Quitline). A) 10,000 steps: participants will receive a resource pack and pedometer, and will be asked to use the 10,000 steps log book to record steps and other physical activities. The aim will be to increase daily steps to 10,000 steps/day B) HIIT group: participants will complete 2 gym‐based supervised HIIT sessions/week and 1 home‐based HIIT session/week. At each training session participants will be asked to complete four 4‐min (4 × 4‐min) intervals at approximately 90% of maximum heart rate interspersed with 3‐min recovery periods |
| Outcomes | Primary outcome: % of participants who have ceased smoking using the Russell standard self‐reported abstinence (previous 2 weeks) and CO concentration < 10 ppm. Time points: 13 and 26 weeks after randomisation. Secondary outcome(s): number of cigarettes smoked on a daily basis (baseline, 13 and 26 weeks); withdrawal symptoms and cravings assessed using the Moods and Physical Symptoms Scale (MPSS; weeks 3, 6, 9 and 12); smoking dependency (FTND; baseline, weeks 6, 13 and 26); subjective stress (PSS; weeks 3, 6, 9 and 12); well‐being (SF36; baseline, 13 and 26 weeks); motivation assessed using the Behavioural Regulation in Exercise Questionnaire (BREQ‐2; baseline, 13 and 26 weeks); Cardiorespiratory fitness (VO2max) will be assessed using a graded exercise test to exhaustion; lung function will be assessed by forced vital capacity [FVC], forced expiratory volume in 1 sec [FEV1] and FEV1/FVC ratio, using a Vitalograph (2150, Ennis, Ireland); body composition will be assessed by researcher‐measured body mass index (height, weight), and waist circumference; physical activity and sedentary time will be measured using a wrist‐worn GENEActiv accelerometer, which will be worn for 1 week at each measurement time |
| Starting date | Date of first enrolment 10 December 2014, last enrolment 30 March 2016, date of last data collection 3 October 2016 |
| Contact information | Dr Toby Pavey. Address: School of Human Movement Studies, Blair Drive, The University of Queensland, St. Lucia Campus, Brisbane, Australia, QLD 4072 Country Australia. Phone: +61 7 3346 9898. Email: t.pavey@uq.edu.au |
| Notes | Registry ID: ACTRN12614001255673 Funding source: This study is supported by a Heart Foundation of Australia Vanguard Grant (#100587). TP is supported by a National Health and Medical Research Council program grant (#569940) at The University of Queensland, School of Human Movement and Nutrition Sciences Declaration of interests: The authors declare that they have no competing interests |
Smits 2012.
| Study name | Smoking termination enhancement project (STEP) |
| Methods | A 2‐arm, parallel‐group, randomised controlled trial to compare the effectiveness of 2 smoking cessation programmes that integrate counselling and nicotine replacement with either a wellness programme or exercise. Blinding/masking: none (open‐label) |
| Participants | Psychiatric population: people who smoke (aged 18 ‐ 65 years) who report elevated anxiety sensitivity (AS) and report smoking an average of at least 10 cigarettes per day |
| Interventions | All participants receive standard treatment (ST) for smoking cessation based on the most recent clinical practice guideline from the US Department of Health and Human Services which broadly includes CBT for relapse prevention along with NRT. A) ST + EX: a 15‐week programme involving 3 x 45‐min exercise sessions each week B) ST + CTRL: a 15‐week programme involving 3 x 45‐min wellness sessions All participants asked to set a quit date for 6 weeks after the baseline visit |
| Outcomes | Primary outcome: % of participants who achieved point‐prevalence abstinence from smoking at 10‐ and 30‐weeks post‐quit‐day (EOT i.e. 10‐weeks post‐quit and 30‐week follow‐up). Main outcome analysis based on 7‐day point‐prevalence abstinence. Self‐reported abstinence verified by saliva cotinine (< 10 ng/mL) at 16 and 24‐week follow‐ups, or expired CO (< 8 ppm). Measures of putative mediators: Minnesota Withdrawal Scale (MWS); Anxiety Sedherence and patient adherence, adverse events and concurrent treatment |
| Starting date | Study start date: September 2009; Study completion date: August 2013 |
| Contact information | Name: Dr Jasper Smits (PI) ‐ University of Texas at Austin, Email: jsmits@smu.edu |
| Notes | Registry ID: ClinicalTrials.gov, NCT01065506 Funding source: The study is funded by the National Institute of Drug Abuse (NIDA; R01DA027533) Declaration of interests: Drs Smits and Otto receive royalties from Oxford University Press for books on exercise for mood and anxiety disorders. All other authors declare that they have no competing interests |
Taylor 2019b.
| Study name | IntEgrating Smoking Cessation treatment As part of usual Psychological care for dEpression and anxiety (ESCAPE) |
| Methods | A 2‐arm, parallel‐group, multicentre randomised and controlled feasibility trial to compare the effectiveness of offering smoking cessation treatment alongside usual psychological care relative to usual psychological care plus referral to smoking cessation services. Blinding/masking: single (outcome assessments will be conducted by a researcher blinded to treatment allocation). Randomisation requested via RedCap |
| Participants | Psychiatric population: people who smoke (18+ years) with a current diagnosis of depression (PHQ‐9) or anxiety (GAD‐7) or both, who report smoking daily for at least 1 year and are interested in receiving help to quit smoking |
| Interventions | Participants are randomly allocated to 1 of 2 treatments (usual care vs usual care + integrated smoking cessation treatment). Both treatments are very similar and involve behavioural, psychological support and medicine to help participants to quit. The difference between the treatments is that one will be delivered alongside psychology therapy, and the other treatment will involve being referred to the local stop‐smoking service at the end of the IAPT therapy. Participants assigned to receive the smoking treatment alongside their psychology therapy will talk to their Psychological Well‐being Practitioner about their smoking for up to 15 minutes during each therapy appointment. They will be guided through behavioural techniques to support them through the quit attempt. The Psychological Well‐being Practitioner will also talk about the psychology of quitting, and how quitting might improve mental health. In addition, participants receive a smoking cessation medication of their choice to help with withdrawal symptoms. Participants assigned to receive smoking treatment after their psychology therapy is finished will receive a referral to their local stop‐smoking service, who will offer a very similar treatment as described above based at their service. Treatment will last a maximum of 12 weeks over 7 appointments |
| Outcomes | Primary outcome measure: Retention in the smoking cessation treatment, measured at treatment appointments 1 to 6 Secondary outcome measures: Smoking‐related: 1. Biochemically‐verified 7‐day point prevalence smoking cessation at 3 and 6 months after baseline 2. Number of cigarettes smoked per day, measured at enrolment, treatment appointments 1 to 6, and 3 and 6 months after baseline 3. Heaviness of Smoking Index, measured at enrolment, treatment appointments 1 to 6, and 3 and 6 months after baseline. Mental health‐related: Symptoms of depression, measured using PHQ‐9. Service‐related: Number of 'Did Not Attends', number of planned and completed IAPT sessions, measured at treatment appointments 1 to 6. Acceptability and feasibility: Participant and clinician acceptability and satisfaction of intervention as assessed by questionnaires and qualitative interviews; interviews also explore acceptability and feasibility of data collection procedures, and impact of smoking cessation treatment on usual care and psychological recovery Assessed at 3 months after baseline. Implementation‐related: intervention delivery checklist, qualitative analysis of intervention delivery, measured at treatment appointments 1 to 6 |
| Starting date | January 2018 and estimated completion February 2021 |
| Contact information | Name: Dr Gemma Taylor. Email: g.m.j.taylor@bath.ac.uk. Address: Addiction and Mental Health Group (AIM), Department of Psychology, University of Bath, 10 West, Bath BA2 7AY, UK |
| Notes | Registry ID: World Health Organization's International Clinical Trials Registry Platform ID: ISRCTN99531779/ Protocol reference: Pilot and Feasibility Studies 2019, 5:16 Funding source: The study is funded by Cancer Research UK. (Dr. Gemma Taylor is funded by a Cancer Research UK Postdoctoral Fellowship (C56067/A21330). Dr. Ali Shaw is funded through NIHR Research Capability Funding. The MRC Integrative Epidemiology Unit at the University of Bristol is supported by the Medical Research Council and the University of Bristol [MC_UU_12013/6]. Dr. David Kessler is funded by The Centre for Primary Care at the University of Bristol. Professor Paul Aveyard is an NIHR senior investigator and is funded by NIHR Oxford Biomedical Research Centre and CLAHRC. Dr. Gemma Taylor,and Professors Marcus Munafò and Paul Aveyard are members of the UK Centre for Tobacco and Alcohol Studies, a UKCRC Public Health Research: Centre of Excellence. Funding from the British Heart Foundation, Cancer Research UK,Economic and Social Research Council, Medical Research Council, and the National Institute for Health Research, under the auspices of the UK Clinical Research Collaboration. Dr. Kate Bartlem is funded by a National Health and Medical Research Council Early Career Fellowship (#1142272). Professor Chris Metcalfe is funded by the Higher Education Funding Council for England) Declaration of interests: Drs. Ali Shaw, David Kessler and Kate Bartlem have no conflicts of interest. Professor Chris Metcalfe has no conflicts of interest. Dr Gemma Taylor and Professor Marcus Munafò have received funding from Pfizer, who manufacture smoking cessation products Professor Paul Aveyard led a trial funded by the NIHR and Glaxo SmithKline donated nicotine patches to the NHS in support of the trial |
Webb Hooper 2018.
| Study name | Addressing racial/ethnic tobacco health disparities via group intervention |
| Methods | A 2‐arm, dual‐site, parallel‐group, randomised controlled trial aimed at evaluating the efficacy of group CBT in eliminating racial/ethnic differences in smoking cessation and distress. Blinding/masking: none (open‐label); 2 (group intervention) x 3 (race/ethnicity) design. Participants not informed of study condition ‐ informed they will receive a supportive group‐based cessation intervention combined with NRT |
| Participants | General population: adults who smoke (18+ years) that self‐identify as African American/Black, Hispanic (any race), or non‐Hispanic White and report smoking at least 5 cigarettes per day |
| Interventions | Experimental: Group Cognitive Behavioural Therapy ‐ participants may receive 8 group cognitive behavioural therapy (CBT) sessions and 8 weeks of transdermal nicotine patches (TNP) (21 mg (4 weeks), 14 mg (2 weeks), and 7 mg (2 weeks)). Active Comparator: General Health Education: Participants may receive group general health education (GHE) sessions and 8 weeks of transdermal nicotine patches (TNP) (21 mg (4 weeks), 14 mg (2 weeks), and 7 mg (2 weeks)) |
| Outcomes | Primary outcome(s): biochemically‐confirmed 7‐day point‐prevalence abstinence (ppa), which will be assessed over a 12‐month follow‐up period, and change in perceived distress and depressive symptoms pre‐ to post‐intervention. The mediating role of physiological distress is considered exploratory. Other outcome measures: Change in salivary cortisol level (Time Frame: 12‐months) |
| Starting date | Study start date: August 2015; actual study completion date: October 23, 2019 |
| Contact information | Corresponding author at: Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, 10900 Euclid Ave, Cleveland, OH 44106‐7285, USA. E‐mail address: monica.hooper@case.edu (M. Webb Hooper) |
| Notes | Registry ID: Clinicaltrials.gov NCT02511236 Funding source: The Florida Department of Health, James and Esther King Biomedical Research Program funding study (5JK01) Declaration of interests: None specified |
Differences between protocol and review
In our protocol we planned to compare estimates derived from cohort studies that adopted causal designs (e.g. propensity‐score matching, or instrumental variable analyses) with estimates from non‐causal designs. However, there were insufficient studies available for this.
We used the Duval and Tweedie 'trim and fill' method to account for outcomes showing evidence of publication bias (Duval 2000). Trim and fill adjusts the meta‐analysis to incorporate theoretically missing studies, and then estimates the pooled SMD incorporating imputed studies’ data. Using trim‐and‐fill methods, we imputed missing study data, and compared pooled effect estimates between imputed and non‐imputed models.
For the meta‐analysis of incidence data we decided to pool ORs, rather than the RRs, given that most studies reported ORs (adjusted or unadjusted). After statistical advice (from Dr Thomas Fanshawe) we agreed that this was the most sensible decision to ensure that the meta‐analysis would be conducted on the same scale that was used for the modelling in most of the papers.
We renamed the outcome 'social impact' to 'social quality of life', to better represent the outcome measures that we pooled.
In our protocol we planned to carry out a GRADE assessment for the social quality‐of‐life outcome, but we were unable to do this as we did not assess risks of bias for this outcome.
Contributions of authors
GT and PA conceived the study. GT, NL, AF, AL‐J, KS, RtWN, AT, NK, CB, and PA were all involved in writing and editing the manuscript.
Sources of support
Internal sources
-
University of Oxford, UK
Employer or academic host of AT, NL, PA & RtWN
-
University of Birmingham, UK
Employer of AF
-
University of Bath, UK
Employer or acedemic host of CB, GT, KS & NK
-
New York University Abu Dhabi, UK
Employer of ALJ
External sources
-
NIHR Biomedical Research Centre, UK
PA is funded by the NIHR Oxford Biomedical Research Centre
-
NIHR Oxford Applied Research Collaboration, UK
PA is funded by NIHR Oxford Applied Research Collaboration
-
Cancer Research UK, UK
GT is funded by Cancer Research UK Population Researcher Postdoctoral Fellowship award (reference: C56067/A21330)
-
NIHR Cochrane Infrastructure Funding, UK
NIHR infrastructure funds the Cochrane Tobacco Addiction Group. NL is employed as Managing Editor of the group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health and Social Care.
-
NIHR senior investigator, UK
PA is an NIHR senior investigator
Declarations of interest
GT's salary and research activity is paid for by a Cancer Research UK Postdoctoral Fellowship (C56067/A21330) that has been paid to University of Bath.
NL is employed by the University of Oxford to work as Managing Editor for the Cochrane Tobacco Addiction Group (TAG). TAG's infrastructure is funded by the NIHR. Nicola has received payment for lectures on systematic review methodology, and has been an applicant on project funding to carry out priority setting and systematic reviews in the area of tobacco control (NIHR funded). None of this is deemed a conflict of interest.
AF is employed by the University of Birmingham, has been awarded grant funding from the CRUK, NIHR and Ethicon (Johnson and Johnson) researcher‐led funding.
AL‐J: none known
KS: none known
RtWN: none known
AT: none known
NK: none known
CB: none known
PA: none known.
New
References
References to studies included in this review
Anthenelli 2013 {published data only (unpublished sought but not used)}
- Anthenelli RM, Morris C, Ramey TS, Dubrava SJ, Tsilkos K, Russ C, et al. Effects of varenicline on smoking cessation in adults with stably treated current or past major depression: a randomized trial. Annals of Internal Medicine 2013;159(6):390-400. [DOI: 10.7326/0003-4819-159-6-201309170-00005] [DOI] [PubMed] [Google Scholar]
Aversa 2013 {published data only (unpublished sought but not used)}
- Aversa LH, Stoddard JA, Doran NM, Au S, Chow B, McFall M, et al. Longitudinal analysis of the relationship between PTSD symptom clusters, cigarette use, and physical health-related quality of life. Quality of Life Research 2013;22(6):1381-9. [DOI] [PubMed] [Google Scholar]
- Aversa LH, Stoddard JA, Doran NM, Au S, Chow B, McFall M, et al. PTSD and depression as predictors of physical health-related quality of life in tobacco-dependent veterans. Journal of Psychosomatic Research 2012;73(3):185-90. [DOI: 10.1016/j.jpsychores.2012.06.010] [DOI] [PubMed] [Google Scholar]
- Harder L. Health-related Quality of Life (HRQoL) in veterans with chronic Posttraumatic Stress Disorder (PTSD) and tobacco dependence. Dissertation Abstracts International: Section B: The Sciences and Engineering 2013;73(7-B(E)):No pagination specified. [Google Scholar]
Avery 2014 {published data only (unpublished sought but not used)}
- Avery N, Kenny AM, Kleppinger A, Brindisi J, Litt MD, Oncken CA. Effects of varenicline, nicotine or placebo on depressive symptoms in postmenopausal smokers. American Journal on Addictions 2014;23(5):459-65. [DOI: 10.1111/j.1521-0391.2014.12130.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Becoña 2002 {published data only}
- Becoña E, Vazquez FL, Miguez MD. Smoking cessation and anxiety in a clinical sample. Personality and Individual Differences 2002;32(3):489-94. [DOI: 10.1016/S0191-8869(01)00050-2] [DOI] [Google Scholar]
Becoña 2017 {published and unpublished data}
- Becoña E, Martinez-Vispo C, Senra C, Lopez-Duran A, Rodriguez-Cano R, Fernandez del Rio E. Cognitive-behavioural treatment with behavioural activation for smokers with depressive symptomatology: study protocol of a randomized controlled trial. BMC Psychiatry 2017;17(1):No pagination specified. [DOI: 10.1186/s12888-017-1301-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Vispo C, Rodriguez-Cano R, Lopez-Duran A, Senra C, Fernandez Del Rio E, Becoña E. Cognitive-behavioural treatment with behavioural activation for smoking cessation: randomized controlled trial. PLOS One 2019;14(4):e0214252. [DOI: 10.1371/journal.pone.0214252] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berlin 2010 {published data only}
- Berlin I, Chen H, Covey LS. Depressive mood, suicide ideation and anxiety in smokers who do and smokers who do not manage to stop smoking after a target quit day. Addiction 2010;105(12):2209-16. [DOI: 10.1111/j.1360-0443.2010.03109.x] [DOI] [PubMed] [Google Scholar]
Blalock 2008 {published and unpublished data}
- Blalock JA, Robinson JD, Wetter DW, Schreindorfer LS, Cinciripini PM. Nicotine withdrawal in smokers with current depressive disorders undergoing intensive smoking cessation treatment. Psychology of Addictive Behaviours 2008;22(1):122-8. [DOI: 10.1037/0893-164X.22.1.122] [DOI] [PubMed] [Google Scholar]
Bloom 2015 {published data only}
- Bloom EL, Oliver JA, Sutton SK, Brandon TH, Jacobsen PB, Simmons VN. Post-operative smoking status in lung and head and neck cancer patients: association with depressive symptomatology, pain, and fatigue. Psycho-Oncology 2015;24(9):1012-9. [DOI: 10.1002/pon.3682] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bloom 2017 {published data only (unpublished sought but not used)}
- Bloom EL, Minami H, Brown RA, Strong DR, Riebe D, Abrantes AM. Quality of life after quitting smoking and initiating aerobic exercise. Psychology, Health & Medicine 2017;22(9):1127-35. [DOI: 10.1080/13548506.2017.1282159] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom EL, Minami H, Brown RA, Zywiak W, Strong DR, Riebe D, et al. Quality of life after quitting smoking and increasing aerobic exercise (POS2-34). In: Society for Research on Nicotine & Tobacco 19th Annual Meeting. 2013.
Bock 2012 {published data only}
- Bock BC, Fava JL, Gaskins R, Morrow KM, Williams DM, Jennings E, et al. Yoga as a complementary treatment for smoking cessation in women. Journal of Women's Health 2012;21(2):240-8. [DOI: 10.1089/jwh.2011.2963] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchanan 2015 {published data only}
- Buchanan DM, Arnold SV, Gosch KL, Jones PG, Longmore LS, Spertus JA, et al. Association of smoking status with angina and health-related quality of life after acute myocardial infarction. Circulation: Cardiovascular Quality and Outcomes 2015;8(5):493-500. [DOI: 10.1161/CIRCOUTCOMES.114.001545] [DOI] [PMC free article] [PubMed] [Google Scholar]
Busch 2011 {published data only}
- Busch AM, Wagener TL, Gregor KL, Ring KT, Borrelli B. Utilizing reliable and clinically significant change criteria to assess for the development of depression during smoking cessation treatment: the importance of tracking idiographic change. Addictive Behaviors 2011;36(12):1228-32. [DOI: 10.1016/j.addbeh.2011.07.031] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carey 1993 {published data only (unpublished sought but not used)}
- Carey MP, Kalra DL, Carey KB, Halperin S, Richards CS. Stress and unaided smoking cessation: a prospective investigation. Journal of Consulting & Clinical Psychology 1993;61(5):831-8. [DOI: 10.1037/0022-006X.61.5.831] [DOI] [PubMed] [Google Scholar]
Carroll 2019 {published and unpublished data}
- Carroll AJ, Kim K, Miele A, Olonoff M, Leone FT, Schnoll RA, et al. Longitudinal associations between smoking and affect among cancer patients using varenicline to quit smoking. Addictive Behaviors 2019;95:206-10. [DOI: 10.1016/j.addbeh.2019.04.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price S, Hitsman B, Veluz-Wilkins A, Blazekovic S, Brubaker TR, Leone F, et al. The use of varenicline to treat nicotine dependence among patients with cancer. Psycho-Oncology 2017;26(10):1526-34. [DOI: 10.1002/pon.4166] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cather 2017 {published data only (unpublished sought but not used)}
- Cather C, Hoeppner S, Pachas G, Pratt S, Achtyes E, Cieslak KM, et al. Improved depressive symptoms in adults with schizophrenia during a smoking cessation attempt with varenicline and behavioral therapy. Journal of Dual Diagnosis 2017;13(3):168-78. [DOI: 10.1080/15504263.2017.1319585] [DOI] [PubMed] [Google Scholar]
- Pachas GN, Cather C, Pratt SA, Hoeppner B, Nino J, Carlini SV, et al. Varenicline for smoking cessation in schizophrenia: safety and effectiveness in a 12-week, open-label trial. Journal of Dual Diagnosis 2012;8(2):117-25. [DOI: 10.1080/15504263.2012.663675] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cavazos‐Rehg 2014 {published data only}
- Cavazos-Rehg PA, Breslau N, Hatsukami D, Krauss MJ, Spitznagel EL, Grucza RA, et al. Smoking cessation is associated with lower rates of mood/anxiety and alcohol use disorders. Psychological Medicine 2014;44(12):2523-35. [DOI: 10.1017/S0033291713003206] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chassin 2002 {published and unpublished data}
- Chassin L, Presson CC, Sherman SJ, Kim K. Long-term psychological sequelae of smoking cessation and relapse. Health Psychology 2002;21(5):438-43. [DOI: 10.1037//0278-6133.21.5.438] [DOI] [PubMed] [Google Scholar]
Chen 2015 {published data only}
- Chen PC, Kuo RNC, Lai CK, Tsai ST, Lee YC. The relationship between smoking status and health-related quality of life among smokers who participated in a 1-year smoking cessation programme in Taiwan: a cohort study using the EQ-5D. BMJ Open 2015;5(5):e007249. [DOI: 10.1136/bmjopen-2014-007249] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cinciripini 2013 {unpublished data only}
- Cinciripini PM, Robinson JD, Karam-Hage M, Minnix JA, Lam C, Versace F, et al. Effects of varenicline and bupropion sustained-release use plus intensive smoking cessation counselling on prolonged abstinence from smoking and on depression, negative affect, and other symptoms of nicotine withdrawal. JAMA Psychiatry 2013;70(5):522-33. [DOI: 10.1001/jamapsychiatry.2013.678] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1990 {published data only (unpublished sought but not used)}
- Cohen S, Lichtenstein E. Perceived stress, quitting smoking, and smoking relapse. Health Psychology 1990;9(4):466-78. [DOI: 10.1037//0278-6133.9.4.466] [DOI] [PubMed] [Google Scholar]
Cooper 2016 {published data only}
- Cooper J, Borland R, Yong HH, Fotuhi O. The impact of quitting smoking on depressive symptoms: findings from the International Tobacco Control Four-Country Survey. Addiction 2016;111(8):1448-56. [DOI: 10.1111/add.13367] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covey 2015 {published data only}
- Covey LS, Hu M-C, Winhusen T, Lima J, Berlin I, Nunes E. Anxiety and depressed mood decline following smoking abstinence in adult smokers with attention deficit hyperactivity disorder. Journal of Substance Abuse Treatment 2015;59:104-8. [DOI: 10.1016/j.jsat.2015.07.004] [DOI] [PMC free article] [PubMed] [Google Scholar]
Croghan 2005 {published data only}
- Croghan IT, Schroeder DR, Hays JT, Eberman KM, Patten CA, Berg EJ, et al. Nicotine dependence treatment: Perceived health status improvement with 1-year continuous smoking abstinence. European Journal of Public Health 2005;15(3):251-5. [DOI: 10.1093/eurpub/cki076] [DOI] [PubMed] [Google Scholar]
Cui 2019 {published data only}
- Cui M, Kimura T, Ikehara S, Dong JY, Ueda K, Kawanishi Y, et al. Prenatal tobacco smoking is associated with postpartum depression in Japanese pregnant women: the Japan Environment and Children's Study. Journal of Affective Disorders 2019;264:76-81. [DOI: 10.1016/j.jad.2019.11.145] [DOI] [PubMed] [Google Scholar]
Dawkins 2009 {published and unpublished data}
- Dawkins L, Powell JH, Pickering A, Powell J, West R. Patterns of change in withdrawal symptoms, desire to smoke, reward motivation and response inhibition across 3 months of smoking abstinence. Addiction 2009;104(5):850-8. [DOI: 10.1111/j.1360-0443.2009.02522.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dedert 2019 {unpublished data only}
- Dedert EA, Resick PA, Dennis PA, Wilson SM, Moore SD, Beckham JC. Pilot trial of a combined cognitive processing therapy and smoking cessation treatment. Journal of Addiction Medicine 2019;13(4):322-30. [DOI: 10.1097/ADM.0000000000000502] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedert EA, Resick PA, McFall ME, Dennis PA, Olsen M, Beckham JC. Pilot cases of combined cognitive processing therapy and smoking cessation for smokers with posttraumatic stress disorder. Behaviour Therapy 2016;47(1):54-65. [DOI: 10.1016/j.beth.2015.09.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dulger 2019 {published data only (unpublished sought but not used)}
- Dulger S, Aykurt Karlibel I, Kasapoglu Aksoy M, Altan L, Sengoren Dikis O, Yildiz T. How does smoking cessation affect disease activity, function loss, and quality of life in smokers with ankylosing spondylitis? JCR: Journal of Clinical Rheumatology 2019;25(7):288-96. [DOI: 10.1097/RHU.0000000000000851] [DOI] [PubMed] [Google Scholar]
Farris 2015 {published data only (unpublished sought but not used)}
- Farris SG, Allan NP, Morales PC, Schmidt NB, Zvolensky MJ. Does successful smoking cessation reduce anxious arousal among treatment-seeking smokers? Journal of Anxiety Disorders 2015;36:92-8. [DOI: 10.1016/j.janxdis.2015.07.009] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fujita 2019 {published data only}
- Fujita T, Babazono A, Harano Y, Jiang P. Risk of depressive disorders after tobacco smoking cessation: a retrospective cohort study in Fukuoka, Japan. BMJ Open 2019;9(3):e025124. [DOI: 10.1136/bmjopen-2018-025124] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garvey 2012 {unpublished data only}
- Garvey AJ, Kalman D, Hoskinson RA Jr, Kinnunen T, Wadler BM, Thomson CC, et al. Front-loaded versus weekly counselling for treatment of tobacco addiction. Nicotine & Tobacco Research 2012;14(5):578-85. [DOI: 10.1093/ntr/ntr256] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giordano 2011 {unpublished data only}
- Giordano GN, Lindstrom M. The impact of social capital on changes in smoking behaviour: a longitudinal cohort study. European Journal of Public Health 2011;21(3):347-54. [DOI: 10.1093/eurpub/ckq048] [DOI] [PubMed] [Google Scholar]
Glassman 2001 {published data only}
- Glassman AH, Covey LS, Stetner F, Rivelli S. Smoking cessation and the course of major depression: a follow-up study. Lancet 2001;357(9272):1929-32. [DOI: 10.1016/S0140-6736(00)05064-9] [DOI] [PubMed] [Google Scholar]
Grabovac 2017 {published data only}
- Grabovac I, Brath H, Schalk H, Degen O, Dorner TE. Clinical setting-based smoking cessation programme and the quality of life in people living with HIV in Austria and Germany. Quality of Life Research 2017;26(9):2387-95. [DOI: 10.1007/s11136-017-1580-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guimond 2017 {unpublished data only}
- Guimond AJ, Croteau VA, Savard MH, Bernard P, Ivers H, Savard J. Predictors of smoking cessation and relapse in cancer patients and effect on psychological variables: an 18-month observational study. Annals of Behavioral Medicine 2017;51(1):117-27. [DOI: 10.1007/s12160-016-9834-4] [DOI] [PubMed] [Google Scholar]
Hajek 2010 {published data only}
- Hajek P, Taylor T, McRobbie H. The effect of stopping smoking on perceived stress levels. Addiction 2010;105(8):1466-71. [DOI: 10.1111/j.1360-0443.2010.02979.x] [DOI] [PubMed] [Google Scholar]
Hammett 2019 {published and unpublished data}
- Hammett PJ, Lando HA, Taylor BC, Widome R, Erickson DJ, Joseph AM, et al. The relationship between smoking cessation and binge drinking, depression, and anxiety symptoms among smokers with serious mental illness. Drug and Alcohol Dependence 2019;194:128-35. [DOI: 10.1016/j.drugalcdep.2018.08.043] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammett PJ. Elucidating the smoking cessation process among socioeconomically disadvantaged smokers with serious mental illness. Dissertation Abstracts International: Section B: The Sciences and Engineering 2018;79(9-B(E)):No pagination specified. [Google Scholar]
Hays 2012 {published data only (unpublished sought but not used)}
- Hays JT, Croghan IT, Baker CL, Cappelleri JC, Bushmakin AG. Changes in health-related quality of life with smoking cessation treatment. European Journal of Public Health 2012;22(2):224-9. [DOI: 10.1093/eurpub/ckq137] [DOI] [PubMed] [Google Scholar]
Heffner 2019 {published data only (unpublished sought but not used)}
- Heffner JL, Evins AE, Russ C, Lawrence D, Ayers CR, McRae T, et al. Safety and efficacy of first-line smoking cessation pharmacotherapies in bipolar disorders: subgroup analysis of a randomized clinical trial. Journal of Affective Disorders 2019;256:267-77. [DOI: 10.1016/j.jad.2019.06.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 1991 {published data only (unpublished sought but not used)}
- Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal: a replication and extension. Archives of General Psychiatry 1991;48(1):52-9. [DOI: 10.1001/archpsyc.1991.01810250054007] [DOI] [PubMed] [Google Scholar]
Kahler 2002 {published data only}
- Kahler CW, Brown RA, Ramsey SE, Niaura R, Abrams DB, Goldstein MG, et al. Negative mood, depressive symptoms, and major depression after smoking cessation treatment in smokers with a history of major depressive disorder. Journal of Abnormal Psychology 2002;111(4):670-5. [DOI: 10.1037//0021-843x.111.4.670] [DOI] [PubMed] [Google Scholar]
Kahler 2011 {published and unpublished data}
- Kahler CW, Spillane NS, Busch AM, Leventhal AM. Time-varying smoking abstinence predicts lower depressive symptoms following smoking cessation treatment. Nicotine & Tobacco Research 2011;13(2):146-50. [DOI: 10.1093/ntr/ntq213] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kahler 2014 {published data only (unpublished sought but not used)}
- Kahler CW, Spillane NS, Day A, Clerkin E, Parks A, Leventhal AM, et al. Positive psychotherapy for smoking cessation: treatment development, feasibility and preliminary results. Journal of Positive Psychology 2014;9(1):19-29. [DOI: 10.1080/17439760.2013.826716] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kohata 2016 {unpublished data only}
- Kohata Y, Fujiwara Y, Kobayashi M, Takemoto Y, Kamata N, Yamagami H, et al. Long-term benefits of smoking cessation on gastroesophageal reflux disease and health-related quality of life. PLOS One 2016;11(2):e0147860. [DOI: 10.1371/journal.pone.0147860] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohata Y, Fujiwara Y, Watanabe T, Kobayashi M, Takemoto Y, Kamata N, et al. Correction: long-term benefits of smoking cessation on gastroesophageal reflux disease and health-related quality of life. PLOS One 2016;11(3):e0150554. [DOI: 10.1371/journal.pone.0150554] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krebs 2018 {published data only}
- Krebs P, Rogers E, Smelson D, Fu S, Wang B, Sherman S. Relationship between tobacco cessation and mental health outcomes in a tobacco cessation trial. Journal of Health Psychology 2018;23(8):1119-28. [DOI: 10.1177/1359105316644974] [DOI] [PubMed] [Google Scholar]
Lechner 2019 {published data only (unpublished sought but not used)}
- Lechner WV, Sidhu NK, Cioe PA, Kahler CW. Effects of time-varying changes in tobacco and alcohol use on depressive symptoms following pharmaco-behavioral treatment for smoking and heavy drinking. Drug and Alcohol Dependence 2019;194:173-7. [DOI: 10.1016/j.drugalcdep.2018.09.030] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2019 {published data only}
- Lee EJ. Long-term effects of smoking cessation on depressive symptoms, resilience, coping skills, and serotonin. Psychiatric Quarterly 2019;91:263-71. [DOI: 10.1007/s11126-019-09689-2] [DOI] [PubMed] [Google Scholar]
Leventhal 2014 {unpublished data only}
- Leventhal AM, Piper ME, Japuntich SJ, Baker TB, Cook JW. Anhedonia, depressed mood, and smoking cessation outcome. Journal of Consulting and Clinical Psychology 2014;82(1):122-9. [DOI: 10.1037/a0035046] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Kenford S, Fiore MC, Baker TB. Smoking cessation and quality of life: changes in life satisfaction over 3 years following a quit attempt. Annals of Behavioral Medicine 2012;43(2):262-70. [DOI: 10.1007/s12160-011-9329-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Rodock M, Cook JW, Schlam TR, Fiore MC, Baker TB. Psychiatric diagnoses among quitters versus continuing smokers 3 years after their quit day. Drug and Alcohol Dependence 2013;128(1-2):148-54. [DOI: 10.1016/j.drugalcdep.2012.08.023] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlam TR, Piper ME, Cook JW, Fiore MC, Baker TB. Life 1 year after a quit attempt: real-time reports of quitters and continuing smokers. Annals of Behavioral Medicine 2012;44(3):309-19. [DOI: 10.1007/s12160-012-9399-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Levy 2018 {published data only}
- Levy DE, Chang Y, Regan S, Tindle HA, Singer DE, Rigotti NA. Improvements in health-related quality of life among smokers who quit after hospitalisation. Preventive Medicine 2018;110:38-46. [DOI: 10.1016/j.ypmed.2018.02.006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Longmore 2007 {published data only}
- Longmore LS, Buchanan DM, Xiao L, Jones PG, Spertus JA. Smoking cessation is associated with better mental health outcomes after myocardial infarction. Circulation 2007;115(21):E551-2. [Google Scholar]
Lopez 2015 {unpublished data only}
- Lopez AA, Skelly JM, Higgins ST. Financial incentives for smoking cessation among depression-prone pregnant and newly postpartum women: effects on smoking abstinence and depression ratings. Nicotine & Tobacco Research 2014;17(4):455-62. [DOI: 10.1093/ntr/ntu193] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lubitz 2019 {published data only}
- Lubitz SF, Flitter A, Ashare RL, Thompson M, Leone F, Gross R, et al. Improved clinical outcomes among persons with HIV who quit smoking. AIDS Care Psychological and Socio Medical Aspects of AIDS/HIV 2019;32(10):1217-23. [DOI: 10.1080/09540121.2019.1703891] [DOI] [PMC free article] [PubMed] [Google Scholar]
Manning 2005 {published data only}
- Manning BK, Catley D, Harris KJ, Mayo MS, Ahluwalia JS. Stress and quitting among African American smokers. Journal of Behavioural Medicine 2005;28(4):325-33. [DOI: 10.1007/s10865-005-9004-9] [DOI] [PubMed] [Google Scholar]
Marqueta 2010 {published data only (unpublished sought but not used)}
- Marqueta A, Jimenez-Muro A, Beamonte A, Gargallo P, Nerin I. Evolution of anxiety during the smoking cessation process at a smoking cessation Clinic. Adicciones 2010;22(4):317-24. [DOI: 10.20882/adicciones.173] [DOI] [PubMed] [Google Scholar]
Martínez‐González 2018 {published data only}
- Martínez-González C, Casanova C, de-Torres JP, Marin JM, Lucas P, Fuster A, et al. Changes and clinical consequences of smoking cessation in patients With COPD: a prospective analysis from the CHAIN cohort. Chest 2018;154(2):274-85. [DOI: 10.1016/j.chest.2018.02.007] [DOI] [PubMed] [Google Scholar]
Martínez‐Vispo 2016 {unpublished data only}
- Martínez-Vispo C, Rio EF, Lopez-Duran A, Becoña E. Influence of anxiety sensitivity in a psychological smoking cessation intervention. Revista de Psicopatologia y Psicologia Clinica 2016;21(1):11-9. [DOI: 10.5944/rppc.vol.21.num.1.2016.15977] [DOI] [Google Scholar]
Mathew 2013 {published data only}
- Mathew AR, Robinson JD, Norton PJ, Cinciripini PM, Brown RA, Blalock JA. Affective trajectories before and after a quit attempt among smokers with current depressive disorders. Nicotine & Tobacco Research 2013;15(11):1807-15. [DOI: 10.1093/ntr/ntt036] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew AR. Changes in affect following smoking cessation in depressed smokers. Dissertation Abstracts International: Section B: The Sciences and Engineering 2014;75(2-B(E)):No pagination specified. [Google Scholar]
McDermott 2013 {published data only}
- McDermott MS, Marteau TM, Hollands GJ, Hankins M, Aveyard P. Change in anxiety following successful and unsuccessful attempts at smoking cessation: cohort study. British Journal of Psychiatry 2013;202(1):62-7. [DOI: 10.1192/bjp.bp.112.114389] [DOI] [PubMed] [Google Scholar]
McFall 2006 {published data only}
- McFall M, Atkins DC, Yoshimoto D, Thompson CE, Kanter E, Malte CA, et al. Integrating tobacco cessation treatment into mental health care for patients with posttraumatic stress disorder. American Journal on Addictions 2006;15(5):336-44. [DOI: 10.1080/10550490600859892] [DOI] [PubMed] [Google Scholar]
McMahon 1998 {published data only (unpublished sought but not used)}
- McMahon SD, Jason LA, Salina D. Stress, coping, and appraisal in a smoking cessation intervention. Anxiety Stress and Coping 1994;7:161-71. [DOI: 10.1080/10615809408249342] [DOI] [Google Scholar]
- McMahon SD, Jason LA. Stress and coping in smoking cessation: a longitudinal examination. Anxiety Stress and Coping 1998;11:327-43. [DOI: 10.1080/10615809808248318] [DOI] [Google Scholar]
Mino 2000 {published data only}
- Mino Y, Shigemi J, Otsu T, Tsuda T, Babazono A. Does smoking cessation improve mental health? Psychiatry and Clinical Neurosciences 200;54(2):169-72. [DOI: 10.1046/j.1440-1819.2000.00654.x] [DOI] [PubMed] [Google Scholar]
Mitra 2004 {published data only}
- Mitra M, Chung MC, Wilber N, Klein WD. Smoking status and quality of life: a longitudinal study among adults with disabilities. American Journal of Preventive Medicine 2004;27(3):258-60. [DOI: 10.1016/j.amepre.2004.06.002] [DOI] [PubMed] [Google Scholar]
Moadel 2012 {unpublished data only}
- Moadel AB, Bernstein SL, Mermelstein RJ, Arnsten JH, Dolce EH, Shuter J. A randomized controlled trial of a tailored group smoking cessation intervention for HIV-infected smokers. Journal of Acquired Immune Deficiency Syndromes 2012;61(2):208-15. [DOI: 10.1097/QAI.0b013e3182645679] [DOI] [PMC free article] [PubMed] [Google Scholar]
Munafò 2008 {published data only}
- Munafò MR, Heron J, Araya R. Smoking patterns during pregnancy and postnatal period and depressive symptoms. Nicotine & Tobacco Research 2008;10(11):1609-20. [DOI: 10.1080/14622200802412895] [DOI] [PubMed] [Google Scholar]
Pacheco 2017 {published data only (unpublished sought but not used)}
- Pacheco VA, Fernandez AP, Morales AV, Crespo EL, Garcia SM, Rubio TM. Anxiety, depression and tobacco abstinence. Adicciones 2017;29(4):233-44. [DOI] [PubMed] [Google Scholar]
Pawlina 2015 {published data only (unpublished sought but not used)}
- Pawlina MM, Rondina Rde C, Espinosa MM, Botelho C. Depression, anxiety, stress, and motivation over the course of smoking cessation treatment. Jornal Brasileiro De Pneumologia 2015;41(5):433-9. [DOI: 10.1590/S1806-37132015000004527] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peltzer 2015 {published data only (unpublished sought but not used)}
- Peltzer K, Pengpid S. Anxiety and depression symptoms following smoking cessation and/or brief alcohol treatment among moderate risk smokers and drinkers. Journal of Psychology in Africa 2015;25(4):361-3. [DOI: 10.1080/14330237.2015.1078095] [DOI] [Google Scholar]
Pertschuk 1979 {published data only}
- Pertschuk MJ, Pomerleau OF, Adkins D, Hirsh C. Smoking cessation: the psychological costs. Addictive Behaviors 1979;4(4):345-8. [DOI: 10.1016/0306-4603(79)90005-4] [DOI] [PubMed] [Google Scholar]
Prochaska 2008 {published data only (unpublished sought but not used)}
- Prochaska JJ, Hall SM, Tsoh JY, Eisendrath S, Rossi JS, Redding CA, et al. Treating tobacco dependence in clinically depressed smokers: effect of smoking cessation on mental health functioning. American Journal of Public Health 2008;98(3):446-8. [DOI: 10.2105/AJPH.2006.101147] [DOI] [PMC free article] [PubMed] [Google Scholar]
Qi Zhang 2014 {published data only (unpublished sought but not used)}
- Filion KB, Grandi SM, Joseph L, O'Loughlin J, Paradis G, Pilote L, et al. The effect of bupropion on symptoms of depression among patients attempting to quit smoking post-myocardial infarction: the zesca trial. Circulation 2012;125(10 SUPPL. 1):No pagination. [CENTRAL: CN-01029170] [Google Scholar]
- Qi Zhang DD, Eisenberg MJ, Grandi SM, Joseph L, O'Loughlin J, Paradis G, et al. Bupropion, smoking cessation, and health-related quality of life following an acute myocardial infarction. Journal of Population Therapeutics and Clinical Pharmacology 2014;21(3):e346-56. [PMID: ] [PubMed] [Google Scholar]
Quist‐Paulsen 2006 {published data only}
- Quist-Paulsen P, Bakke PS, Gallefoss F. Does smoking cessation improve quality of life in patients with coronary heart disease? Scandinavian Cardiovascular Journal 2006;40(1):11-6. [DOI: 10.1080/14017430500384855] [DOI] [PubMed] [Google Scholar]
Robinson 2019 {unpublished data only}
- Robinson JD, Li L, Chen M, Lerman C, Tyndale RF, Schnoll RA, et al. Evaluating the temporal relationships between withdrawal symptoms and smoking relapse. Psychology of Addictive Behaviors 2019;33(2):105-16. [DOI: 10.1037/adb0000434] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rocha 2017 {unpublished data only}
- Rocha V, Guerra M P, Lemos M S, Maciel J, Williams GC. Smoking abstinence twelve months after an acute coronary syndrome. Spanish Journal of Psychology 2017;20:E63. [DOI: 10.1017/sjp.2017.61] [DOI] [PubMed] [Google Scholar]
Rodríguez‐Cano 2016 {published data only}
- Rodríguez-Cano R, Lopez-Duran A, Del Rio EF, Martinez-Vispo C, Martinez U, Becoña E. Smoking cessation and depressive symptoms at 1-, 3-, 6-, and 12-months follow-up. Journal of Affective Disorders 2016;191:94-9. [DOI: 10.1016/j.jad.2015.11.042] [DOI] [PubMed] [Google Scholar]
Saiz Martinez 2016 {published data only (unpublished sought but not used)}
- Saiz Martinez PA, Al-Halabi S, Fernandez-Artamendi S, Garcia-Alvarez L, Diaz-Mesa E, Martinez-Santamaria E, et al. Effects of nicotine abstinence on clinical symptoms. Study at 3 and 6-months follow-up of outpatients with schizophrenia. European Psychiatry 2016. [DOI: 10.1016/j.eurpsy.2016.01.669] [DOI]
Sales 2009 {published data only (unpublished sought but not used)}
- Sales MP, Oliveira MI, Mattos IM, Viana CM, Pereira ED. The impact of smoking cessation on patient quality of life. Jornal Brasileiro de Pneumologia 2009;35(5):436-41. [DOI: 10.1590/S1806-37132009000500008] [DOI] [PubMed] [Google Scholar]
Sanchez‐Villegas 2008 {published data only}
- Sanchez-Villegas A, Serrano-Martinez M, Alonso A, Irala J, Tortosa A, Martinez- Gonzalez MA. Role of the tobacco use on the depression incidence in the SUN cohort study. Medicina Clinica 2008;130(11):405-9. [DOI: 10.1157/13117850] [DOI] [PubMed] [Google Scholar]
Sankaranarayanan 2016 {unpublished data only}
- Sankaranarayanan A, Clark V, Baker A, Palazzi K, Lewin TJ, Richmond R, et al. Reducing smoking reduces suicidality among individuals with psychosis: complementary outcomes from a healthy lifestyles intervention study. Psychiatry Research 2016;243:407-12. [DOI: 10.1016/j.psychres.2016.07.006] [DOI] [PubMed] [Google Scholar]
Sarna 2008 {published data only}
- Sarna L, Bialous SA, Cooley ME, Jun HJ, Feskanich D. Impact of smoking and smoking cessation on health-related quality of life in women in the Nurses' Health Study. Quality of Life Research 2008;17(10):1217-27. [DOI: 10.1007/s11136-008-9404-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schnoll 2016 {published data only}
- Goelz PM, Audrain-McGovern JE, Hitsman B, Leone FT, Veluz-Wilkins A, Jepson C, et al. The association between changes in alternative reinforcers and short-term smoking cessation. Drug and Alcohol Dependence 2014;138:67-74. [DOI: 10.1016/j.drugalcdep.2014.02.007] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann A, Hitsman B, Goelz PM, Veluz-Wilkins A, Blazekovic S, Powers L, et al. Rate of nicotine metabolism and smoking cessation outcomes in a community-based sample of treatment-seeking smokers. Addictive Behaviors 2015;51:93-9. [DOI: 10.1016/j.addbeh.2015.07.019] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Hitsman B, Blazekovic S, Veluz-Wilkins A, Wileyto EP, Leone FT, et al. Longitudinal changes in smoking abstinence symptoms and alternative reinforcers predict long-term smoking cessation outcomes. Drug and Alcohol Dependence 2016;165:245-52. [DOI: 10.1016/j.drugalcdep.2016.06.017] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwartz 1968a {published data only}
- Schwartz JL, Dubitzky M. Changes in anxiety, mood, and self-esteem resulting from an attempt to stop smoking. American Journal of Psychiatry 1968;124(11):1580-4. [DOI: 10.1176/ajp.124.11.1580] [DOI] [PubMed] [Google Scholar]
Secades‐Villa 2015 {published data only (unpublished sought but not used)}
- Secades-Villa R, Vallejo-Seco G, Garcia-Rodriguez O, Lopez-Nunez C, Weidberg S, Gonzalez-Roz A. Contingency management for cigarette smokers with depressive symptoms. Experimental and Clinical Psychopharmacology 2015;23(5):351-60. [DOI: 10.1037/pha0000044] [DOI] [PubMed] [Google Scholar]
- Weidberg S, Garcia-Rodriguez O, Yoon JH, Secades-Villa R. Interaction of depressive symptoms and smoking abstinence on delay discounting rates. Psychology of Addictive Behaviors 2015;29(4):1041-7. [DOI: 10.1037/adb0000073] [DOI] [PubMed] [Google Scholar]
Secades‐Villa 2019 {unpublished data only}
- Secades-Villa R, Gonzalez-Roz A, Vallejo-Seco G, Weidberg S, Garcia-Perez A, Alonso-Perez F. Additive effectiveness of contingency management on cognitive behavioural treatment for smokers with depression: six-month abstinence and depression outcomes. Drug and Alcohol Dependence 2019;204:107495. [DOI: 10.1016/j.drugalcdep.2019.06.003] [DOI] [PubMed] [Google Scholar]
Segan 2011 {published data only (unpublished sought but not used)}
- Segan CJ, Borland R, Wilhelm KA, Bhar SS, Hannan AT, Dunt DR, et al. Helping smokers with depression to quit smoking: collaborative care with Quitline. Medical Journal of Australia 2011;195(3):7-11. [DOI: 10.5694/j.1326-5377.2011.tb03258.x] [DOI] [PubMed] [Google Scholar]
Shahab 2014 {published data only}
- Bolam B, West R, Gunnell D. Does smoking cessation cause depression and anxiety? Findings from the ATTEMPT cohort. Nicotine & Tobacco Research 2011;13(3):209-14. [DOI: 10.1093/ntr/ntq244] [DOI] [PubMed] [Google Scholar]
- Shahab L, Andrew S, West R. Changes in prevalence of depression and anxiety following smoking cessation: results from an international cohort study (ATTEMPT). Psychological Medicine 2014;44(1):127-41. [DOI: 10.1017/S0033291713000391] [DOI] [PubMed] [Google Scholar]
Slopen 2013 {unpublished data only}
- Slopen N, Kontos EZ, Ryff CD, Ayanian JZ, Albert MA, Williams DR. Psychosocial stress and cigarette smoking persistence, cessation, and relapse over 9-10 years: a prospective study of middle-aged adults in the United States. Cancer Causes and Control 2013;24(10):1849-63. [DOI: 10.1007/s10552-013-0262-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Solomon 2006 {published data only}
- Solomon LJ, Higgins ST, Heil SH, Badger GJ, Mongeon JA, Bernstein IM. Psychological symptoms following smoking cessation in pregnant smokers. Journal of Behavioral Medicine 2006;29(2):151-60. [DOI: 10.1007/s10865-005-9041-4] [DOI] [PubMed] [Google Scholar]
Steinberg 2011 {published and unpublished data}
- Steinberg MB, Bover MT, Richardson DL, Schmelzer AC, Williams JM, Foulds J. Abstinence and psychological distress in co-morbid smokers using various pharmacotherapies. Drug and Alcohol Dependence 2011;114(1):77-81. [DOI: 10.1016/j.drugalcdep.2010.06.022] [DOI] [PubMed] [Google Scholar]
Stewart 1995 {published data only}
- Stewart A, King A, Killen J, Ritter P. Does smoking cessation improve health-related quality-of-life? Annals of Behavioral Medicine 1995;17(4):331-8. [DOI: 10.1007/BF02888598] [DOI] [PubMed] [Google Scholar]
Taylor 2015 {published data only}
- Taylor G, Girling A, McNeill A, Aveyard P. Does smoking cessation result in improved mental health? A comparison of regression modelling and propensity score matching. BMJ Open 2015;5(10):e008774. [DOI: 10.1136/bmjopen-2015-008774] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G, McNeill A, Aveyard P. Does deterioration in mental health after smoking cessation predict relapse to smoking? BMC Public Health 2015;15:1150. [DOI: 10.1186/s12889-015-2473-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tian 2016 {published data only}
- Tian J, Venn AJ, Blizzard L, Patton GC, Dwyer T, Gall SL. Smoking status and health-related quality of life: a longitudinal study in young adults. Quality of Life Research 2016;25(3):669-85. [DOI: 10.1007/s11136-015-1112-6] [DOI] [PubMed] [Google Scholar]
Tomioka 2014 {unpublished data only}
- Tomioka H, Sekiya R, Nishio C, Ishimoto G. Impact of smoking cessation therapy on health-related quality of life. BMJ Open Respiratory Research 2014;1:e000047. [DOI: 10.1136/bmjresp-2014-000047] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tranel 2012 {published data only (unpublished sought but not used)}
- Tranel D, McNutt A, Bechara A. Smoking cessation after brain damage does not lead to increased depression: implications for understanding the psychiatric complications of varenicline. Cognitive and Behavioral Neurology 2012;25(1):16-24. [DOI: 10.1097/WNN.0b013e3182492a9c] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsoh 2000 {published data only}
- Tsoh JY, Humfleet GL, Munoz RF, Reus VI, Hartz DT, Hall SM. Development of major depression after treatment for smoking cessation. American Journal of Psychiatry 2000;157(3):368-74. [DOI: 10.1176/appi.ajp.157.3.368] [DOI] [PubMed] [Google Scholar]
Vázquez 1999 {published data only}
- Vázquez FL, Becoña E. Depression and smoking in a smoking cessation programme. Journal of Affective Disorders 1999;55(2-3):125-32. [DOI: 10.1016/s0165-0327(98)00215-8] [DOI] [PubMed] [Google Scholar]
Vermeulen 2019 {unpublished data only}
- Vermeulen J, Schirmbeck F, Blankers M, Van Tricht M, Van den Brink W, De Haan L, et al. Smoking, symptoms, and quality of life in patients with psychosis, siblings, and healthy controls: a prospective, longitudinal cohort study. Lancet Psychiatry 2019;6(1):25-34. [DOI: 10.1016/S2215-0366%2818%2930424-3] [DOI] [PubMed] [Google Scholar]
- Vermeulen J, Schirmbeck F, Blankers M, Van Tricht M, Van den Brink W, De Haan L. Smoking, symptoms, and quality of life in patients with psychosis, siblings, and healthy controls: a prospective, longitudinal cohort study": Correction. Lancet Psychiatry 2019;6(2):e5. [DOI: 10.1016/S2215-0366%2818%2930514-5] [DOI] [PubMed] [Google Scholar]
Ward 2005 {published data only (unpublished sought but not used)}
- Ward KD, Relyea G, Weg MW, Sherrill-Mittleman D, Klesges R, Debon M, et al. Changes in quality of life after smoking cessation among older adults. Nicotine & Tobacco Research 2005;7(4):700. [Google Scholar]
Weinberger 2009 {published data only (unpublished sought but not used)}
- Weinberger AH, Hitsman B, Papandonatos GD, Sacco KA, Vessicchio JC, George TP. Predictors of abstinence and changes in psychiatric symptoms in a pooled sample of smokers with schizophrenia receiving combination pharmacotherapy and behavioural therapy for smoking cessation. Journal of Clinical Psychopharmacology 2009;29(6):601-3. [DOI: 10.1097/JCP.0b013e3181bfd0b4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinhold 2017 {published and unpublished data}
- Weinhold D, Chaloupka FJ. Smoking status and subjective well-being. Tobacco Control 2017;26(2):195-201. [DOI: 10.1136/tobaccocontrol-2015-052601] [DOI] [PubMed] [Google Scholar]
Wiggers 2006 {published data only (unpublished sought but not used)}
- Wiggers LC, Oort FJ, Peters RJ, Legemate DA, De Haes HC, Smets EM. Smoking cessation may not improve quality of life in atherosclerotic patients. Nicotine & Tobacco Research 2006;8(4):581-9. [DOI: 10.1080/14622200600790005] [DOI] [PubMed] [Google Scholar]
Xue 2017 {published data only}
- Xue C, Bian L, Xie YS, Yin ZF, Xu ZJ, Chen QZ, et al. Impact of smoking on health-related quality of life after percutaneous coronary intervention treated with drug-eluting stents: a longitudinal observational study. Health and Quality of Life Outcomes 2017;15(1):1-10. [DOI: 10.1186/s12955-016-0578-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2016 {published data only}
- Zhou ZM, Xu XH, Liang J, Hu B, Cheng YJ, Shi C, et al. The impact of cigarette cessation intervention on mental state of patients with coronary heart disease. Chung-Hua Nei Ko Tsa Chih [Chinese Journal of Internal Medicine] 2016;55(11):854-8. [DOI: 10.3760/cma.j.issn.0578-1426.2016.11.008] [DOI] [PubMed] [Google Scholar]
Zillich 2002 {published data only (unpublished sought but not used)}
- Zillich AJ, Ryan M, Adams A, Yeager B, Farris K. Effectiveness of a pharmacist-based smoking-cessation program and its impact on quality of life. Pharmacotherapy 2002;22(6):759-65. [DOI: 10.1592/phco.22.9.759.34073] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abrams 1987 {published data only}
- Abrams DB, Monti PM, Pinto RP, Elder JP, Brown RA, Jacobus SI. Psychosocial stress and coping in smokers who relapse or quit. Health Psychology 1987;6(4):289-303. [DOI: 10.1037/0278-6133.6.4.289] [DOI] [PubMed] [Google Scholar]
Abrantes 2018 {published data only}
- Abrantes AM, Farris SG, Minami H, Strong DR, Riebe D, Brown RA. Acute effects of aerobic exercise on affect and smoking craving in the weeks before and after a cessation attempt. Nicotine & Tobacco Research 2018;20(5):575-82. [DOI: 10.1093/ntr/ntx104] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrantes AM, Zywiak W, Strong DR, Riebe D, Desaulniers J, Brown RA. Smoking status as a moderator of the acute effects of aerobic exercise among smokers in cessation treatment (POS1-155). In: Society for Research on Nicotine & Tobacco 19th Annual Meeting, March 13-16, Boston, MA. 2013.
Acri 1992 {published data only}
- Acri JB, Grunberg NE. A psychophysical task to quantify smoking cessation-induced irritability: the reactive irritability scale (RIS). Addictive Behaviors 1992;17(6):587-601. [DOI: 10.1016/0306-4603(92)90068-7] [DOI] [PubMed] [Google Scholar]
Almeida 2011 {published data only}
- Almeida OP, Garrido GJ, Alfonso H, Hulse G, Lautenschlager NT, Hankey GJ. 24-month effect of smoking cessation on cognitive function and brain structure in later life. Neuroimage 2011;55(4):1480-9. [DOI: 10.1016/j.neuroimage.2011.01.063] [DOI] [PubMed] [Google Scholar]
Auer 2014 {published data only}
- Auer R, Vittinghoff E, Kiefe C, Reis JP, Rodondi N, Khodneva YA, et al. Change in physical activity after smoking cessation: The Coronary Artery Risk Development in Young Adults Study (CARDIA) study. Addiction 2014;109(7):1172-83. [DOI: 10.1111/add.12561] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bakhshaie 2015 {published data only}
- Bakhshaie J, Zvolensky MJ, Goodwin RD. Cigarette smoking and the onset and persistence of depression among adults in the United States: 1994-2005. Comprehensive Psychiatry 2015;60:142-8. [DOI: 10.1016/j.comppsych.2014.10.012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Balduyck 2011 {published and unpublished data}
- Balduyck B, Sardari NP, Cogen A, Dockx Y, Lauwers P, Hendriks J, et al. The effect of smoking cessation on quality of life after lung cancer surgery. European Journal of Cardio-Thoracic Surgery 2011;40(6):1432-8. [DOI: 10.1016/j.ejcts.2011.03.004] [DOI] [PubMed] [Google Scholar]
Balfour 2017 {published data only}
- Balfour L, Wiebe SA, Cameron WD, Sandre D, Pipe A, Cooper C, et al. An HIV-tailored quit-smoking counselling pilot intervention targeting depressive symptoms plus Nicotine Replacement Therapy. AIDS Care 2017;29(1):24-31. [DOI: 10.1080/09540121.2016.1201195] [DOI] [PubMed] [Google Scholar]
Bekele 2017 {published data only}
- Bekele T, Rueda S, Gardner S, Raboud J, Smieja M, Kennedy R, et al. Trends and correlates of cigarette smoking and its impacts on health-related quality of life among people living with HIV: findings from the Ontario HIV treatment network cohort study, 2008-2014. AIDS Patient Care and STDs 2017;31(2):49-59. [DOI: 10.1089/apc.2016.0174] [DOI] [PubMed] [Google Scholar]
Billert 2006 {published data only}
- Billert H, Gaca M, Adamski D, Miluska J, Breborowicz G. Significance of smoking and cigarette abstinence regarding anxiety in gynecologic patients in a perioperative period. Przeglad Lekarski 2006;63(10):870-7. [PMID: ] [PubMed] [Google Scholar]
Bock 2014 {published data only}
- Bock BC, Papandonatos GD, De Dios MA, Abrams DB, Azam MM, Fagan M, et al. Tobacco cessation among low-income smokers: motivational enhancement and nicotine patch treatment. Nicotine & Tobacco Research 2014;16(4):413-22. [DOI: 10.1093/ntr/ntt166] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolliger 2002 {published data only (unpublished sought but not used)}
- Bolliger CT, Zellweger JP, Danielsson T, Van Biljon X, Robidou A, Westin A, et al. Influence of long-term smoking reduction on health risk markers and quality of life. Nicotine & Tobacco Research 2002;4(4):433-9. [DOI: 10.1080/1462220021000018380] [DOI] [PubMed] [Google Scholar]
Breslau 1998 {published data only}
- Breslau N, Peterson EL, Schultz LR, Chilcoat HD, Andreski P. Major depression and stages of smoking: a longitudinal investigation. Archives of General Psychiatry 1998;55(2):161-6. [DOI: 10.1001/archpsyc.55.2.161] [DOI] [PubMed] [Google Scholar]
Brody 2017 {published data only}
- Brody AL, Zorick T, Hubert R, Hellemann GS, Balali S, Kawasaki SS, et al. Combination extended smoking cessation treatment plus home visits for smokers with schizophrenia: a randomized controlled trial. Nicotine & Tobacco Research 2017;19(1):68-76. [DOI: 10.1093/ntr/ntw190] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brook 2012 {published data only}
- Brook DW, Rubenstone E, Zhang C, Brook JS. Trajectories of cigarette smoking in adulthood predict insomnia among women in late mid-life. Sleep Medicine 2012;13(9):1130-7. [DOI: 10.1016/j.sleep.2012.05.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2014 {published data only}
- Brown RA, Abrantes AM, Strong DR, Niaura R, Kahler CW, Miller IW, et al. Efficacy of sequential use of fluoxetine for smoking cessation in elevated depressive symptom smokers. Nicotine & Tobacco Research 2014;16(2):197-207. [DOI: 10.1093/ntr/ntt134] [DOI] [PubMed] [Google Scholar]
Brunette 2018 {published data only}
- Brunette MF, Pratt SI, Bartels SJ, Scherer EA, Sigmon SC, Ferron JC, et al. Randomized trial of interventions for smoking cessation among medicaid beneficiaries with mental illness. Psychiatric Services 2018;69(3):274-80. [DOI: 10.1176/appi.ps.201700245] [DOI] [PubMed] [Google Scholar]
Burris 2014 {published data only}
- Burris JL. Cigarette smoking among recently diagnosed head and neck cancer patients: a prospective study. Dissertation Abstracts International: Section B: The Sciences and Engineering 2014;75(5-B(E)):No pagination specified. [Google Scholar]
Campbell 2018 {published data only}
- Campbell B, Yip D, Le T, Gubner N, Guydish J. Relationship between tobacco use and health-related quality of life (hrqol) among clients in substance use disorders treatment. Journal of Psychoactive Drugs 2018;51(1):48-57. [DOI: 10.1080/02791072.2018.1555651] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caponnetto 2013 {published data only}
- Caponnetto P, Auditore R, Russo C, Cappello GC, Polosa R. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. International Journal of Environmental Research and Public Health 2013;10(2):446-61. [DOI: 10.3390/ijerph10020446] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carter 2014 {published data only}
- Carter KN, Van der Deen FS, Wilson N, Blakely T. Smoking uptake is associated with increased psychological distress: results of a national longitudinal study. Tobacco Control 2014;23(1):33-8. [DOI: 10.1136/tobaccocontrol-2012-050614] [DOI] [PubMed] [Google Scholar]
Chen 2012 {published data only}
- Chen J, Qi Y, Wampfler JA, Jatoi A, Garces YI, Busta AJ, et al. Effect of cigarette smoking on quality of life in small cell lung cancer patients. European Journal of Cancer 2012;48(11):1593-601. [DOI: 10.1016/j.ejca.2011.12.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chengappa 2014 {published data only}
- Chengappa KN, Perkins KA, Brar JS, Schlicht PJ, Turkin SR, Hetrick ML, et al. Varenicline for smoking cessation in bipolar disorder: a randomized, double-blind, placebo-controlled study. Journal of Clinical Psychiatry 2014;75(7):765-72. [DOI: 10.4088/JCP.13m08756] [DOI] [PubMed] [Google Scholar]
Dalack 1999 {published data only}
- Dalack GW, Becks L, Hill E, Pomerleau OF, Meador-Woodruff JH. Nicotine withdrawal and psychiatric symptoms in cigarette smokers with schizophrenia. Neuropsychopharmacology 1999;21(2):195-202. [DOI: 10.1016/S0893-133X(98)00121-3] [DOI] [PubMed] [Google Scholar]
Dickson‐Spillmann 2012 {published data only}
- Dickson-Spillmann M, Kraemer T, Rust K, Schaub M. Group hypnotherapy versus group relaxation for smoking cessation: an RCT study protocol. BMC Public Health 2012;12:271. [DOI: 10.1186/1471-2458-12-271] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duffy 2012 {published data only}
- Duffy SA, Ronis DL, Titler MG, Blow FC, Jordan N, Thomas PL, et al. Dissemination of the nurse-administered Tobacco Tactics intervention versus usual care in six Trinity community hospitals: study protocol for a comparative effectiveness trial. Trials 2012;13:125. [DOI: 10.1186/1745-6215-13-125] [DOI] [PMC free article] [PubMed] [Google Scholar]
El Hajj 2015 {published data only}
- El Hajj MS, Kheir N, Al Mulla AM, Al-Badriyeh D, Al Kaddour A, Mahfoud ZR, et al. Assessing the effectiveness of a pharmacist-delivered smoking cessation program in the State of Qatar: study protocol for a randomized controlled trial. Trials 2015;16:65. [DOI: 10.1186/s13063-015-0570-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Etter 2006 {published data only}
- Etter J-F, Hughes JR. A comparison of the psychometric properties of three cigarette withdrawal scales. Addiction 2006;101(3):362-72. [DOI: 10.1111/j.1360-0443.2005.01289.x] [DOI] [PubMed] [Google Scholar]
Faseru 2013 {published data only}
- Faseru B, Nollen NL, Mayo MS, Krebill R, Choi WS, Benowitz NL, et al. Predictors of cessation in African American light smokers enrolled in a bupropion clinical trial. Addictive Behaviors 2013;38(3):1796-803. [DOI: 10.1016/j.addbeh.2012.11.010] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flensborg‐Madsen 2011 {published data only}
- Flensborg-Madsen T, Von Scholten MB, Flachs EM, Mortensen EL, Prescott E, Tolstrup JS. Tobacco smoking as a risk factor for depression. A 26-year population-based follow-up study. Journal of Psychiatric Research 2011;45(2):143-9. [DOI: 10.1016/j.jpsychires.2010.06.006] [DOI] [PubMed] [Google Scholar]
Fond 2013 {published data only}
- Fond G, Guillaume S, Artero S, Bernard P, Ninot G, Courtet P, et al. Self-reported major depressive symptoms at baseline impact abstinence prognosis in smoking cessation program. A one-year prospective study. Journal of Affective Disorders 2013;149(1-3):418-21. [DOI: 10.1016/j.jad.2012.11.066] [DOI] [PubMed] [Google Scholar]
Frederick 1998 {published data only}
- Frederick S, Reus V, Ginsberg D, Hall S, Munoz R, Ellman G. Cortisol and response to dexamethasone as predictors of withdrawal distress and abstinence success. Biological Psychiatry 1998;43(7):525-30. [DOI: 10.1016/S0006-3223(97)00423-X] [DOI] [PubMed] [Google Scholar]
Garces 2004 {published data only}
- Garces YI, Yang P, Parkinson J, Zhao XH, Wampfler JA, Ebbert JO. The relationship between cigarette smoking and quality of life after lung cancer diagnosis. Chest 2004;126(6):1733-41. [DOI: 10.1378/chest.126.6.1733] [DOI] [PubMed] [Google Scholar]
George 2002 {published data only (unpublished sought but not used)}
- George TP, Vessicchio JC, Termine A, Sahady DM, Head CA, Pepper WT, et al. Effects of smoking abstinence on visuospatial working memory function in schizophrenia. Neuropsychopharmacology 2002;26(1):75-85. [DOI: 10.1016/S0893-133X(01)00296-2] [DOI] [PubMed] [Google Scholar]
Gilbert 1998 {published data only}
- Gilbert DG, McClernon FJ, Rabinovich NE, Plath LC, Jensen RA, Meliska CJ. Effects of smoking abstinence on mood and craving in men: influences of negative-affect-related personality traits, habitual nicotine intake and repeated measurements. Personality and Individual Differences 1998;25(3):399-423. [DOI: 10.1016/S0191-8869(98)00003-8] [DOI] [Google Scholar]
Gilbert 1999 {published data only}
- Gilbert DG, McClernon FJ, Rabinovich NE, Dibb WD, Plath LC, Hiyane S. EEG, physiology, and task-related mood fail to resolve across 31 days of smoking abstinence: relations to depressive traits, nicotine exposure, and dependence. Experimental and Clinical Psychopharmacology 1999;7(4):427-43. [DOI: 10.1037//1064-1297.7.4.427] [DOI] [PubMed] [Google Scholar]
Gilbert 2002 {published data only}
- Gilbert DG, McClernon FJ, Rabinovich NE, Plath LC, Masson CL, Anderson AE, et al. Mood disturbance fails to resolve across 31 days of cigarette abstinence in women. Journal of Consulting and Clinical Psychology 2002;70(1):142-52. [DOI: 10.1037//0022-006x.70.1.142] [DOI] [PubMed] [Google Scholar]
Gilbert 2003 {published data only}
- Gilbert HM, Warburton DM. Individual variation in psychological and psychomotor symptoms following smoking cessation: the implications for treatment. Psychology & Health 2003;18(5):613-24. [DOI: 10.1080/0887044032000069892] [DOI] [Google Scholar]
Guiterrez‐Bedmar 2009 {published data only}
- Guiterrez-Bedmar M, Segui-Gomez M, Gomez-Gracia E, Bes-Rastrollo M, Martinez- Gonzalez MA. Smoking status, changes in smoking status and health-related quality of life: findings from the SUN ("Seguimiento Universidad de Navarra") cohort.. International Journal of Environmental Research & Public Health 2009;6(1):310-20. [DOI: 10.3390/ijerph6010310] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haibach 2016 {published data only}
- Haibach JP, Homish GG, Collins RL, Ambrosone CB, Giovino GA. Fruit and vegetable intake as a moderator of the association between depressive symptoms and cigarette smoking. Substance Abuse 2016;37(4):571-8. [DOI: 10.1080/08897077.2016.1179703] [DOI] [PubMed] [Google Scholar]
Hall 1990 {published data only}
- Hall SM, Munoz R, Reus V. Smoking cessation, depression and dysphoria. NIDA Research Monograph 1990;105:312-3. [PubMed] [Google Scholar]
Heffner 2012 {published data only}
- Heffner JL, DelBello MP, Anthenelli RM, Fleck DE, Adler CM, Strakowski SM. Cigarette smoking and its relationship to mood disorder symptoms and co-occurring alcohol and cannabis use disorders following first hospitalization for bipolar disorder. Bipolar Disorders 2012;14(1):99-108. [DOI: 10.1111/j.1399-5618.2012.00985.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hendricks 2014 {published data only}
- Hendricks PS, Delucchi KL, Benowitz NL, Hall SM. Clinical significance of early smoking withdrawal effects and their relationships with nicotine metabolism: preliminary results from a pilot study. Nicotine & Tobacco Research 2014;16(5):615-20. [DOI: 10.1093/ntr/ntt204] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hirdes 1994 {published data only}
- Hirdes JP, Maxwell CJ. Smoking cessation and quality of life outcomes among older adults in the Campbell's survey on well-being. Canadian Journal of Public Health 1994;85(2):99-102. [PMID: ] [PubMed] [Google Scholar]
Hsu 2015 {published data only}
- Hsu H, Chang W. Reducing the risks of morbidity, disability, and mortality using successful aging strategies. Journal of the American Geriatrics Society 2015;63(11):2426-8. [DOI: 10.1111/jgs.13817] [DOI] [PubMed] [Google Scholar]
Hughes 1992 {published data only}
- Hughes JR. Tobacco withdrawal in self-quitters. Journal of Consulting and Clinical Psychology 1992;60(5):689-97. [DOI: 10.1037//0022-006x.60.5.689] [DOI] [PubMed] [Google Scholar]
Hurt 1997 {published data only}
- Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. New England Journal of Medicine 1997;337(17):1195-202. [DOI: 10.1056/NEJM199710233371703] [DOI] [PubMed] [Google Scholar]
Hwang 2012 {published data only}
- Hwang GS, Jung HS, Yi Y, Yoon C, Choi JW. Smoking cessation intervention using stepwise exercise incentives for male workers in the workplace. Asia-Pacific Journal of Public Health 2012;24(1):82-90. [DOI: 10.1177/1010539510370991] [DOI] [PubMed] [Google Scholar]
IRCT20100127003210N16 2019 {published data only}
- IRCT20100127003210N16. Cytisine versus nicotine for smoking cessation in hospitalized psychiatric patients. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20100127003210N16 (first received 14 January 2019).
Jang 2010 {published data only}
- Jang A-S, Park S-W, Kim D-J, Uh S, Kim YH, Whang HG. Effects of smoking cessation on airflow obstruction and quality of life in asthmatic smokers. Allergy, Asthma and Immunology Research 2010;2(4):254-9. [DOI: 10.4168/aair.2010.2.4.254] [DOI] [PMC free article] [PubMed] [Google Scholar]
John 2004 {published data only}
- John U, Meyer C, Rumpf HJ, Hapke U. Smoking, nicotine dependence and psychiatric comorbidity -a population-based study including smoking cessation after three years. Drug and Alcohol Dependence 2004;76(3):287-95. [DOI: 10.1016/j.drugalcdep.2004.06.004] [DOI] [PubMed] [Google Scholar]
Kaetsu 2002 {published data only}
- Kaetsu A, Fukushima T, Moriyama M, Shigematsu T. Change of the smoking behaviour and related lifestyle variables among physicians in Fukuoka, Japan: a longitudinal study. Journal of Epidemiology 2002;12(3):208-16. [DOI: 10.2188/jea.12.208] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kahler 2004 {published data only}
- Kahler CW, Strong DR, Niaura R, Brown RA. Hostility in smokers with past major depressive disorder: relation to smoking patterns, reasons for quitting, and cessation outcomes. Nicotine & Tobacco Research 2004;6(5):809-18. [DOI: 10.1080/1462220042000282546] [DOI] [PubMed] [Google Scholar]
Kahler 2009 {published data only}
- Kahler CW, Spillane NS, Leventhal AM, Strong DR, Brown RA, Monti PM. Hostility and smoking cessation treatment outcome in heavy social drinkers. Psychology of Addictive Behaviors 2009;23(1):67-76. [DOI: 10.1037/a0012655] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerkvliet 2015 {published data only}
- Kerkvliet JL, Wey H, Fahrenwald NL. Cessation among state quitline participants with a mental health condition. Nicotine & Tobacco Research 2015;17(6):735-41. [DOI: 10.1093/ntr/ntu239] [DOI] [PubMed] [Google Scholar]
Khaled 2009 {published data only}
- Khaled SM, Bulloch A, Exner DV, Patten SB. Cigarette smoking, stages of change, and major depression in the Canadian population. Canadian Journal of Psychiatry 2009;54(3):204-8. [DOI: 10.1177/070674370905400309] [DOI] [PubMed] [Google Scholar]
Khaled 2012 {published data only}
- Khaled SM, Bulloch AG, Williams JVA, Hill JC, Lavorato DH, Patten SB. Persistent heavy smoking as risk factor for major depression (MD) incidence: evidence from a longitudinal Canadian cohort of the National Population Health Survey. Journal of Psychiatric Research 2012;46(4):436-43. [DOI: 10.1016/j.jpsychires.2011.11.011] [DOI] [PubMed] [Google Scholar]
Kim 2019 {published data only}
- Kim SJ, Chae W, Park WH, Park MH, Park EC, Jang SI. The impact of smoking cessation attempts on stress levels. BMC Public Health 2019;19(1):267. [DOI: 10.1186/s12889-019-6592-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinnunen 2006 {published data only}
- Kinnunen T, Haukkala A, Korhonen T, Quiles ZN, Spiro A, Garvey AJ. Depression and smoking across 25 years of the normative aging study. International Journal of Psychiatry in Medicine 2006;36(4):413-26. [DOI: 10.2190/G652-T403-73H7-2X28] [DOI] [PubMed] [Google Scholar]
Kocak 2015 {published data only}
- Kocak ND, Eren A, Boga S, Akturk UA, Ozturk UA, Arinc S, et al. Relapse rate and factors related to relapse in a 1-year follow-up of subjects participating in a smoking cessation program. Respiratory Care 2015;60(12):1796-803. [DOI: 10.4187/respcare.03883] [DOI] [PubMed] [Google Scholar]
Lam 2012 {published data only}
- Lam CY, Robinson JD, Versace F, Minnix JA, Cui Y, Carter BL. Affective reactivity during smoking cessation of never-quitters as compared with that of abstainers, relapsers, and continuing smokers. Experimental and Clinical Psychopharmacology 2012;20(2):139-50. [DOI: 10.1037/a0026109] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lappan 2018 {published data only}
- Lappan S, Thorne CB, Long D, Hendricks PS. Longitudinal and reciprocal relationships between psychological well-being and smoking. Nicotine & Tobacco Research 2018;22(1):18-23. [DOI: 10.1093/ntr/nty185] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lerman 2004 {published data only}
- Lerman C, Niaura R, Collins BN, Wileyto P, Audrain-McGovern J, Pinto A. Effect of bupropion on depression symptoms in a smoking cessation clinical trial. Psychology of Addictive Behaviors 2004;18(4):362-6. [DOI: 10.1037/0893-164X.18.4.362] [DOI] [PubMed] [Google Scholar]
Lewis 2011 {published data only}
- Lewis SJ, Araya R, Smith GD, Freathy R, Gunnell D, Palmer T, et al. Smoking is associated with, but does not cause, depressed mood in pregnancy--a Mendelian randomization study. PLOS One 2011;6(7):e21689. [DOI: 10.1371/journal.pone.0021689] [DOI] [PMC free article] [PubMed] [Google Scholar]
McClure 2009 {published data only}
- McClure JB, Swan GE, Jack L, Catz SL, Zbikowski SM, McAfee TA. Mood, side-effects and smoking outcomes among persons with and without probable lifetime depression taking varenicline. Erratum.. Journal of General Internal Medicine 2009;24(10):1173. [PMID: 10.1007/s11606-009-0966-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miguez 2019 {published data only}
- Miguez MC, Pereira B, Pinto TM, Figueiredo B. Continued tobacco consumption during pregnancy and women's depression and anxiety symptoms. International Journal of Public Health 2019;64(9):1355-65. [DOI: 10.1007/s00038-019-01308-y] [DOI] [PubMed] [Google Scholar]
Moreno‐Coutino 2007 {published data only}
- Moreno-Coutino A, Calderon-Ezquerro C, Drucker-Colin R. Long-term changes in sleep and depressive symptoms of smokers in abstinence. Nicotine & Tobacco Research 2007;9(3):389-96. [DOI: 10.1080/14622200701188901] [DOI] [PubMed] [Google Scholar]
Morris 2011 {published data only}
- Morris CD, Waxmonsky JA, May MG, Tinkelman DG, Dickinson M, Giese AA. Smoking reduction for persons with mental illnesses: 6-month results from community-based interventions. Community Mental Health Journal 2011;47(6):694-702. [DOI: 10.1007/s10597-011-9411-z] [DOI] [PubMed] [Google Scholar]
Myneni 2018 {published data only}
- Myneni AA. Dietary factors, smoking cessation and lung cancer. Dissertation Abstracts International: Section B: The Sciences and Engineering 2018;79(8-B(E)):No pagination specified. [Google Scholar]
NCT01789125 2013 {published data only}
- NCT01789125. Smoking Termination / Anxiety Reduction Treatment (ST/ART). clinicaltrials.gov/show/NCT01789125 (first received 11 February 2013).
NCT02845687 {published data only}
- NCT02845687. Study on smoking cessation and cost outcomes in the Duke smoking cessation program. clinicaltrials.gov/ct2/show/NCT02845687 (first received 27 July 2016).
Nieva 2017 {published data only (unpublished sought but not used)}
- Nieva G, Comin M, Valero S, Bruguera E. Cigarette dependence and depressive symptoms as predictors of smoking status at five-year follow-up after a workplace smoking cessation program. Addictive Behaviors 2017;73:9-15. [DOI] [PubMed] [Google Scholar]
O'Toole 2018 {published data only}
- O'Toole BI, Kirk R, Bittoun R, Catts SV. Combat, posttraumatic stress disorder, and smoking trajectory in a cohort of male Australian Army Vietnam veterans. Nicotine & Tobacco Research 2018;20(10):1198-205. [DOI: 10.1093/ntr/ntx257] [DOI] [PubMed] [Google Scholar]
Ong 2016 {published data only}
- Ong J, Plueckhahn I, Cruickshank D, Churilov L, Mileshkin L. A smoking cessation programme for current and recent ex-smokers following diagnosis of a potentially curable cancer. Internal Medicine Journal 2016;46(9):1089-96. [DOI: 10.1111/imj.13172] [DOI] [PubMed] [Google Scholar]
Park 2009 {published data only}
- Park ER, Chang Y, Quinn V, Regan S, Cohen L, Viguera A. The association of depressive, anxiety, and stress symptoms and postpartum relapse to smoking: a longitudinal study. Nicotine & Tobacco Research 2009;11(6):707-14. [DOI: 10.1093/ntr/ntp053] [DOI] [PMC free article] [PubMed] [Google Scholar]
Piper 2012 {published data only}
- Piper ME, Kenford S, Fiore MC, Baker TB. Smoking cessation and quality of life: changes in life satisfaction over 3 years following a quit attempt. Annals of Behavioral Medicine 2012;43(2):262-70. [DOI: 10.1007/s12160-011-9329-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roson 2017 {published data only}
- Roson NF, Panadero-Paz C, Almadana-Pacheco V, Benito-Bernaldez C, Rodriguez-Martin PJ, Montemayor-Rubio T. Influence of psychiatric disorders in patients treated with varenicline. European Respiratory Journal 2017;50:PA4478. [DOI: 10.1183/1393003.congress-2017.PA4478] [DOI] [Google Scholar]
Schlyter 2016 {published data only}
- Schlyter M, Leosdottir M, Engström G, André-Petersson L, Tydén P, Östman M. Smoking cessation after acute myocardial infarction in relation to depression and personality factors. International Journal of Behavioral Medicine 2016;23(2):234-42. [DOI: 10.1007/s12529-015-9514-y] [DOI] [PubMed] [Google Scholar]
Schwartz 1968b {published data only}5651704
- Schwartz JL, Dubitzky M. One-year follow-up results of a smoking cessation program. Canadian Journal of Public Health 1968;59(4):161-5. [PMID: ] [PubMed] [Google Scholar]
Seghatoleslam 2014 {published data only}
- Seghatoleslam T, West R, Habil H, Zahiroddin A. A pilot study of managing depression and controlling smoking. International Medical Journal 2014;21(1):14-7. [Google Scholar]
Shaw 2001 {published data only}
- Shaw JW, Coons SJ, Foster SA, Leischow SJ, Hays RD. Responsiveness of the Smoking Cessation Quality of Life (SCQoL) questionnaire. Clinical Therapeutics 2001;23(6):957-69. [DOI: 10.1016/s0149-2918(01)80083-7] [DOI] [PubMed] [Google Scholar]
Shimadu 2016 {published data only (unpublished sought but not used)}27303107
- Shimadu S, Hamajima N, Okada Y, Oguri T, Murohara T, Ban N, et al. Factors influencing sustainable efficacy of smoking cessation treatment with varenicline beyond nine months. Nagoya Journal of Medical Science 2016;78(2):205-13. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Siahpush 2007 {published data only}
- Siahpush M, Spittal M, Singh GK. Association of smoking cessation with financial stress and material well-being: Results from a prospective study of a population-based national survey. American Journal of Public Health 2007;97(12):2281-7. [DOI: 10.2105/AJPH.2006.103580] [DOI] [PMC free article] [PubMed] [Google Scholar]
Solak 2018 {published data only (unpublished sought but not used)}
- Solak I, Yildirim DI, Gumus F, Eren I, Eryilmaz MA. Effects of depression and anxiety scores on smoking cessation treatment. Konuralp Tip Dergisi 2018;10(2):128-33. [DOI: 10.18521/ktd.351519] [DOI] [Google Scholar]
Stepankova 2016 {published data only (unpublished sought but not used)}
- Stepankova L, Kralikova E, Zvolska K, Pankova A, Felbrova V, Kulovana S. Improvement of depressive symptoms after a successful treatment of tobacco dependence. Ceska a Slovenska Psychiatrie 2016;112(5):221-5. [Google Scholar]
Strong 2012 {published and unpublished data}
- Strong DR, Taylor AN, Abrantes A, Kahler CW, Miller V, Brown RA, et al. Mood management phone counseling for smokers with recurrent depression (POS3-22). In: Society for Research on Nicotine and Tobacco 18th Annual Meeting March 13-16, 2012, Houston, Texas. 2012:98.
Thorsteinsson 2001 {published data only}
- Thorsteinsson HS, Gillin JC, Patten CA, Golshan S, Sutton LD, Drummond S, et al. The effects of transdermal nicotine therapy for smoking cessation on depressive symptoms in patients with major depression. Neuropsychopharmacology 2001;24(4):350-8. [DOI: 10.1016/S0893-133X(00)00217-7] [DOI] [PubMed] [Google Scholar]
Vidrine 2007 {published data only}
- Vidrine DJ, Arduino RC, Gritz ER. The effects of smoking abstinence on symptom burden and quality of life among persons living with HIV/AIDS. Aids Patient Care STDS 2007;21(9):659-66. [DOI: 10.1089/apc.2007.0022] [DOI] [PubMed] [Google Scholar]
Vidrine 2015 {published data only}
- Vidrine DJ, Kypriotakis G, Li L, Arduino RC, Fletcher FE, Tami-Maury I, et al. Mediators of a smoking cessation intervention for persons living with HIV/AIDS. Drug and Alcohol Dependence 2015;147:76-80. [DOI: 10.1016/j.drugalcdep.2014.12.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weaver 2015 {published data only}
- Weaver K, Kaplan S, Urbanic J, Case D, Zbikowski S, Dakhil CSR, et al. Incorporating evidence-based smoking cessation into community oncology practices: feasibility and preliminary efficacy of an enhanced quitline-based smoking cessation intervention for cancer survivors. Psycho-oncology 2015;24(Suppl. 2):32. [DOI: 10.1002/pon.3873] [DOI] [Google Scholar]
Weinberger 2012 {published data only}
- Weinberger AH, Pilver CE, Desai RA, Mazure CM, McKee SA. The relationship of major depressive disorder and gender to changes in smoking for current and former smokers: longitudinal evaluation in the US population. Addiction 2012;107(10):1847-56. [DOI: 10.1111/j.1360-0443.2012.03889.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Welch 2015 {published data only}
- Welch AE, Jasek JP, Caramanica K, Chiles MC, Johns M. Cigarette smoking and 9/11-related posttraumatic stress disorder among world trade center health registry enrollees, 2003-12. Preventive Medicine 2015;73:94-9. [DOI: 10.1016/j.ypmed.2015.01.023] [DOI] [PubMed] [Google Scholar]
Zvolensky 2014 {published data only}
- Zvolensky MJ, Bogiaizian D, Salazar PL, Farris SG, Bakhshaie J. An anxiety sensitivity reduction smoking-cessation program for Spanish-speaking smokers (Argentina). Cognitive and Behavioral Practice 2014;21(3):350-63. [DOI: 10.1016/j.cbpra.2013.10.005] [DOI] [Google Scholar]
References to ongoing studies
Allen 2017 {published data only (unpublished sought but not used)}
- Allen SI, Foulds J, Pachas GN, Veldheer S, Cather C, Azzouz N, et al. A two-site, two-arm, 34-week, double-blind, parallel-group, randomized controlled trial of reduced nicotine cigarettes in smokers with mood and/or anxiety disorders: trial design and protocol. BMC Public Health 2017;17(1):100. [DOI: 10.1186/s12889-016-3946-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernstein 2016 {published data only (unpublished sought but not used)}
- Bernstein SL, Rosner J, Toll B. A multicomponent intervention including texting to promote tobacco abstinence in emergency department smokers: a pilot study. Academic Emergency Medicine 2016;23(7):803-8. [DOI: 10.1111/acem.12990] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bunker 2012 {published data only}
- Bunker JM, Reddel HK, Dennis SM, Middleton S, Van Schayck C, Crockett AJ, et al. A pragmatic cluster randomized controlled trial of early intervention for chronic obstructive pulmonary disease by practice nurse-general practitioner teams: Study Protocol. Implementation Science 2012;7:83. [PMID: 10.1186/1748-5908-7-83] [DOI] [PMC free article] [PubMed] [Google Scholar]
CTRI/2012/12/003262 2012 {published data only}
- CTRI/2012/12/003262. Empowering women in self-help groups (SHGs) in Gadchiroli district, Maharashtra to reduce tobacco consumption and increase savings by a community-based tobacco cessation intervention package. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2012/12/003262 (first received 26 December 2012).
DRKS00014920 2018 {published data only}
- DRKS00014920. Reducing stress, alcohol and tobacco use in pregnant women to improve the children's later mental health. www.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00014920 (first received 8 October 2018).
EUCTR2012‐002731‐28‐PL 2012 {published data only}
- EUCTR2012-002731-28-PL. Smoking cessation. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2012-002731-28-PL 2012.
EUCTR2017‐004715‐40‐ES 2018 {published data only}
- EUCTR2017-004715-40-ES. We try to assess the effectiveness and safety of varenicline for smoking cessation in hospitalized patients with psychiatric disorders. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2017-004715-40-ES 2018.
Garrison 2015 {published data only}
- Garrison KA, Pal P, Rojiani R, Dallery J, O'Malley SS, Brewer JA. A randomized controlled trial of smartphone-based mindfulness training for smoking cessation: a study protocol. BMC Psychiatry 2015;15(83):1-7. [DOI: 10.1186/s12888-015-0468-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02002858 2013 {published data only}
- NCT02002858. Smoking cessation for depression and anxiety treatment. clinicaltrials.gov/show/NCT02002858 (first received 06 December 2013).
Pavey 2015 {published data only}
- Pavey TG, Gartner CE, Coombes JS, Brown WJ. Assessing the effectiveness of high intensity interval training (HIIT) for smoking cessation in women: HIIT to quit study protocol. BMC Public Health 2015;15:1309. [DOI: 10.1186/s12889-015-2631-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smits 2012 {published data only}
- Smits JA, Zvolensky MJ, Rosenfield D, Marcus BH, Church TS, Frierson GM, et al. The efficacy of vigorous-intensity exercise as an aid to smoking cessation in adults with elevated anxiety sensitivity: study protocol for a randomized controlled trial. Trials 2012;13:207. [DOI: 10.1186/1745-6215-13-207] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2019b {published data only}
- Taylor G, Aveyard P, Bartlem K, Shaw A, Player J, Metcalfe C, et al. IntEgrating Smoking Cessation treatment As part of usual Psychological care for dEpression and anxiety (ESCAPE): protocol for a randomised and controlled, multicentre, acceptability, feasibility and implementation trial. Pilot and Feasibility Studies 2019;5:16. [DOI: 10.1186/s40814-018-0385-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Webb Hooper 2018 {published data only}
- Webb Hooper M, Lee DJ, Simmons VN, Brandon KO, Antoni MH, Unrod M, et al. Reducing racial/ethnic tobacco cessation disparities via cognitive behavioral therapy: design of a dualsite randomized controlled trial. Contemporary Clinical Trials 2018;68:127-32. [DOI: 10.1016/j.cct.2018.03.017] [DOI] [PubMed] [Google Scholar]
Additional references
Benowitz 1990
- Benowitz NL, Porchet H, Jacob P, Wonnacott S, Russell MA, Stolerman IP. Nicotine Psychopharmacology: Molecular, Cellular, and Behavioural Aspects. Oxford (UK): Oxford University Press, 1990. [Google Scholar]
Benowitz 2010
- Benowitz NL. Nicotine addiction. New England Journal of Medicine 2010;362(24):2295-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2011
- Chang CK, Hayes RD, Perera G, Broadbent MT, Fernandes AC, Lee WE, et al. Life expectancy at birth for people with serious mental illness from a secondary mental health care case register in London, UK. American Journal of Epidemiology 2011;173(s311):e19590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chesney 2014
- Chesney E, Goodwin G, Fazel S. Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry 2014;13(2):153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York: Routledge Academic, 1988. [Google Scholar]
Cookson 2014
- Cookson C, Strang J, Ratschen E, Sutherland G, Finch E, McNeill A. Smoking and its treatment in addiction services: clients’ and staff behaviour and attitudes. BMC Health Services Research 2014;14(1):304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doll 2004
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years observations on male British doctors. BMJ 2004;328(7455):1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duval 2000
- Duval, S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56(2):455-463. [DOI: 10.1111/j.0006-341x.2000.00455.x] [DOI] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769-73. [DOI] [PubMed] [Google Scholar]
GBD 2015 Tobacco Collaborators 2017
- GBD 2015 Tobacco Collaborators. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet 2017;389(10082):1885-906. [DOI: 10.1016/S0140-6736(17)30819-X] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEproGDT. Version accessed 13 January 2020. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Haukkala 2000
- Haukkala A, Uutela A, Vartiainen E, Mcalister A, Knekt P. Depression and smoking cessation: the role of motivation and self-efficacy. Addictive Behaviors 2000;25(2):311-6. [DOI] [PubMed] [Google Scholar]
Higgins 2020
- Higgins JP, Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). The Cochrane ollaboration, 2020. Available from www.training.cochrane.org/handbook. [Available from www.handbook.cochrane.org]
Hitsman 2013
- Hitsman B, Papandonatos G, McChargue D, DeMott A, Herrera M, Spring B, et al. Past major depression and smoking cessation outcome: a systematic review and meta-analysis update. Addiction 2013;108(2):294-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 2007
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine & Tobacco Research 2007;9(3):315-27. [DOI] [PubMed] [Google Scholar]
Leventhal 2013
- Leventhal AM, Ameringer KJ, Osborn E, Zvolensky MJ. Anxiety and depressive symptoms and affective patterns of tobacco withdrawal. Drug and Alcohol Dependence 2013;133(2):324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mamede 2007
- Mamede M, Ishizu K, Ueda M, Mukai T, Lida Y, Kawashima H, et al. Temporal change in human nicotinic acetylcholine receptor after smoking cessation: 5IA SPECT study. Journal of Nuclear Medicine 2007;48(11):1829-35. [DOI] [PubMed] [Google Scholar]
Mansvelder 2002
- Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. Journal of Neurobiology 2002;53(4):606-17. [DOI] [PubMed] [Google Scholar]
McGuiness 2020
- McGuinness LA, Higgins JP. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Research Synthesis Methods 2020;12(1):55-61. [DOI: ] [DOI] [PubMed] [Google Scholar]
NCCMH 2011
- National Collaborating Centre for Mental Health. Generalised Anxiety Disorder in Adults: Management in Primary, Secondary and Community Care. British Psychological Society, 2011. [PubMed] [Google Scholar]
ONS 2018
- Office for National Statistics. Statistics on Smoking - England, 2018. digital.nhs.uk/data-and-information/publications/statistical/statistics-on-smoking/statistics-on-smoking-england-2018 2018.
Parrott 2003
- Parrott AC. Cigarette-derived nicotine is not a medicine. World Journal of Biological Psychiatry 2003;4(2):49-55. [DOI] [PubMed] [Google Scholar]
Pirie 2013
- Pirie K, Peto R, Reeves G, Green J, Beral V. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet 2013;381(9861):133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
RCP/RC PSYCH 2013
- Royal College of Physicians and Royal College of Psychiatrists. Smoking and Mental Health. London, UK: Royal College of Physicians, 2013. [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager (RevMan). Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richardson 2019
- Richardson S, McNeill A, Brose L. Smoking and quitting behaviours by mental health conditions in Great Britain (1993-2014). Addictive Behaviors 2019;90:14-10. [DOI: 10.1016/j.addbeh.2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sheals 2016
- Sheals K, Tombor I, McNeill A, Shahab L. A mixed-method systematic review and meta-analysis of mental health professionals' attitudes toward smoking and smoking cessation among people with mental illnesses. Addiction 2016;111(9):1536-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siru 2009
- Siru R, Hulse GK, Tait RJ. Assessing motivation to quit smoking in people with mental illness: a review. Addiction 2009;104(5):719-33. [DOI] [PubMed] [Google Scholar]
Sterne 2016
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Szatkowski 2015
- Szatkowski L, McNeill A. Diverging trends in smoking behaviors according to mental health status. Nicotine & Tobacco Research 2015;17(3):356–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2019a
- Taylor G, Davies N, Thomas K, Rai D, Jones T, Windmeijer F, et al. Prescribing prevalence, long-term effectiveness, and mental health safety of varenicline and nicotine replacement therapy in patients with mental disorders: a prospective cohort study of electronic medical records. Nicotine & Tobacco Research 2019;ntz072:1-10. [DOI: 10.1093/ntr/ntz072] [DOI] [Google Scholar]
Taylor 2020
- Taylor GM, McNeill A, Farley A, Lindson N, Aveyard P. Smoking cessation for improving mental health. Cochrane Database of Systematic Reviews 2020, Issue 1. Art. No: CD013522. [DOI: 10.1002/14651858.CD013522] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2021
- Taylor G, Lindson N, Farley A, Leinberger-Jabari A, Sawyer K, te Water Naudé R, et al. Supplementary materials for "Smoking cessation for improving mental health". Bath: University of Bath Research Data Archive 2021. [DOI: ] [DOI] [PMC free article] [PubMed]
Tellegen 2008
- Tellegen A, Waller NG. Exploring personality through test construction: Development of the Multidimensional Personality Questionnaire. In: The SAGE Handbook of Personality Theory and Assessment, Vol. 2. Personality Measurement and Testing. Sage Publications, Inc., 2008:261–92. [Google Scholar]
West 2005
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100(3):299-303. [DOI: 10.1111/j.1360-0443.2004.00995.x.] [DOI] [PubMed] [Google Scholar]
West 2019
- West R, Brown J. Latest trends on smoking in England from the Smoking Toolkit Study. www.smokinginengland.info/latest-statistics 2019.
WHO 2011
- World Health Organization. WHO report on the global tobacco epidemic: warning about the dangers of tobacco. www.who.int/tobacco/global_report/2011/en/ 2011.
Wootton 2020
- Wootton RE, Richmond RC, Stuijfzand BG, Lawn RB, Sallis HM, Taylor GM, et al. Evidence for causal effects of lifetime smoking on risk for depression and schizophrenia: a Mendelian randomisation study. Psychological Medicine 2020;50(14):2435-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Taylor 2014
- Taylor G, McNeill A, Girling A, Farley A, Lindson N, Aveyard P. Change in mental health after smoking cessation: systematic review and meta-analysis. BMJ 2014;348:g1151. [DOI] [PMC free article] [PubMed] [Google Scholar]