Abstract
In the present study, we created a nanoscale platform that can deliver nutrients to pancreatic islets in a controlled manner. Our platform consists of a mesoporous silica nanoparticle (MSNP), which can be loaded with glutamine (G: an essential amino acid required for islet survival and function). To control the release of G, MSNPs were coated with a polydopamine (PD) layer. With the optimal parameters (0.5 mg/mL and 0.5 h), MSNPs were coated with a layer of PD, which resulted in a delay of G release from MSNPs over 14 d (57.4 ± 4.7% release). Following syngeneic renal subcapsule islet transplantation in diabetic mice, PDG-MSNPs improved the engraftment of islets (i.e., enhanced revascularization and reduced inflammation) as well as their function, resulting in re-establishment of glycemic control. Collectively, our data show that PDG-MSNPs can support transplanted islets by providing them with a controlled and sustained supply of nutrients.
Keywords: Mesoporous silica nanoparticles, Glutamine, Polydopamine, Islet transplantation, Diabetes, Nutrient delivery
Graphical Abstract
INTRODUCTION
Islet transplantation is a promising clinical therapy for patients with type 1 diabetes (T1D); however, it has yet to reach its full clinical potential. Indeed, 60% of islets are lost within the first two to three weeks following transplantation, mainly from them having an underdeveloped vascular supply, which, in turn, results in them suffering from hypoxia and nutrient deprivation.1,2 Reasons for this include islets being devascularized during their isolation procedure and then no surgical vascular anastomosis being created for transplanted islets, like most other organ transplants. Hence, for islets to survive over the long term, they need to build and secure a dedicated blood supply, which takes two to three weeks.3,4 In the short term, islets have to survive by relying on the diffusion of oxygen and nutrients from their microenvironment at the site where they are transplanted;5 this is not ideal, given their high metabolic requirements.6
One of the main nutrients required by pancreatic islets are amino acids, including glutamine,7–11 alanine,7–10 cysteine,9 tryptophan,9,12 leucine,9,10 methionine,9 isoleucine,13 arginine,13,14 lysine,10 proline,10 and homocysteine10 (Supporting Information, Table 1). These amino acids have been shown to be essential for islet function given their role as precursors of protein synthesis and their ability to regulate gene expression, provide energy, and stimulate insulin secretion.7,15 In the present study, we focused on glutamine (C5H10N2O3) given that (i) it is one of the most abundant amino acids in plasma;16 (ii) islets have a high demand and consumption rate for this amino acid;17 and (iii) it enhances islet function through the tricarboxylic acid (TCA) cycle, where it controls insulin secretion.7 Given that islets need a continual supply of glutamine, over two weeks (i.e., the time it takes for islets to build a dedicated blood supply), we designed a mesoporous silica nanoparticle (MSNP) platform that can be loaded with glutamine using a simple, scalable, cost-effective, and controllable procedure.18 To avoid this burst release from MSNPs, surface coating strategies are therefore required. One approach is to apply a layer of polydopamine,19 given that studies have shown that the rate at which polydopamine degrades can be modulated by controlling the thickness of its layer. Hence, by coating MSNPs with polydopamine, and determining the correct coating concentration and time to enable it to degrade over a defined time frame, we can now control the release of glutamine loaded into MSNPs, thereby preventing its immediate burst release.20
Several types of nanoparticles have been used in conjunction with islet transplantation, either for in vivo imaging of islets such as poly(lactic-co-glycolic acid) nanoparticles containing iron oxide,21 and exendin-4 conjugated iron oxide,22 or for controlled immunosuppressant delivery such as poly(lactide-co-glycolide)-poly(ethylene glycol) nanoparticles,23 and amphiphilic poly(D,L-lactide-co-glycolide)-block-poly(ethylene glycol) (PLGA-b-PEG-COOH) copolymer nanoparticles. However, to our knowledge, nanoplatforms have yet to be used for controlled nutrient delivery to islets. Hence, in the present study we synthesized a nanoparticle platform based on MSNPs that can be loaded with glutamine (G) and then coated with polydopamine (PD) to create PDG-MSNPs (Figure 1A). Next we optimized the coating parameters to ensure a sustained release of our cargo (i.e., G) from PDG-MSNPs over two weeks (i.e., the required time for islets to get revascularized following their transplantation24) and then tested this platform on islets in vitro as well as in vivo in a diabetic animal model.
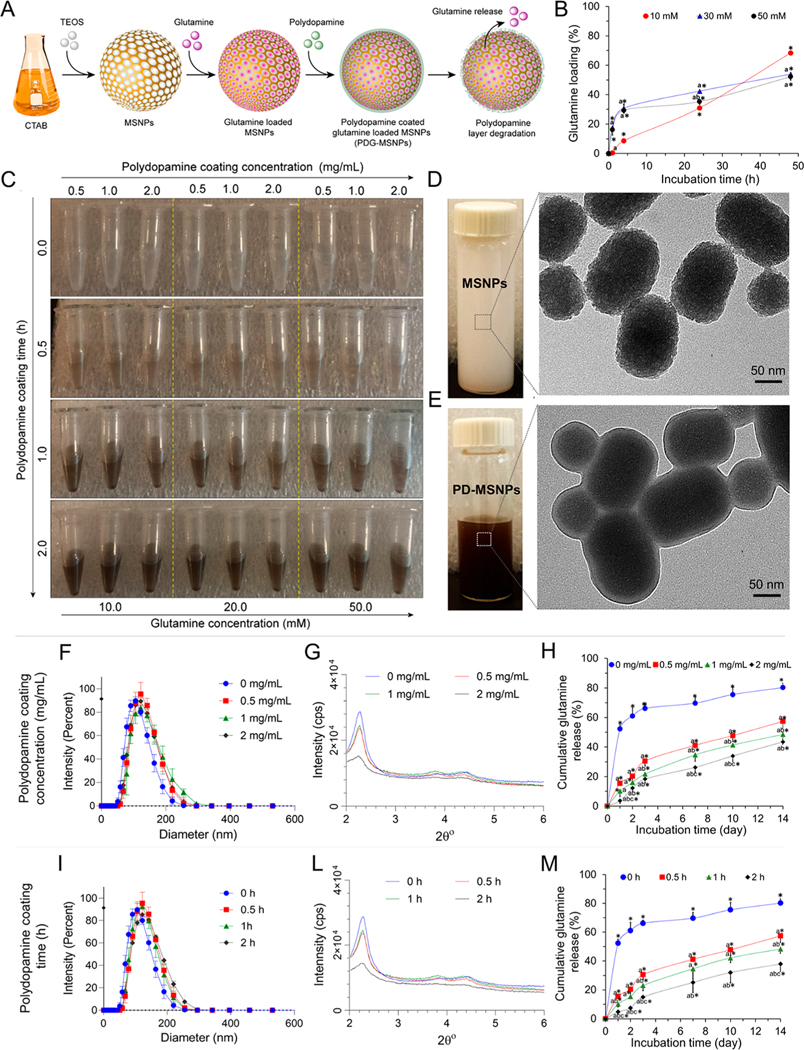
Figure 1.
Synthesis, physical-chemical characterization, and release profile of PDG-MSNPs. (A) Schematic representation of PDG-MSNPs: MSNPs synthesis, glutamine loading (G-MSNPs), polydopamine coating on G-MSNPs (PDG-MSNPs), and glutamine release from PDG-MSNPs. (B) Cumulative loading profiles of glutamine into MSNPs showing that the glutamine loading efficiency is dose- and time- dependent. (C) Polydopamine coating shows a color change from light to dark brown confirming the polydopamine coating on G-MSNPs. The brown color becomes increasingly darker as the concentration and time of polydopamine coating increases. (D, E) Representative TEM images of uncoated- and PDG-MSNPs. Images show a layer of dopamine on PDG-MSNPs. (F, I) DLS analysis and (G, L) XRD analysis of uncoated- and PDG-MSNPs obtained using different polydopamine coating concentrations (0, 0.5, 1, 2 mg/mL) and times (0, 0.5, 1, 2 h), respectively. (H, M) Cumulative release profiles of glutamine from PDG-MSNPs in PBS (pH = 7.4) at 37 °C, showing polydopamine coating resulted in a delay in the release of glutamine, which, in turn, depended on the polydopamine coating concentration (H) and time (M). Results are expressed as average ± standard error of measure (n = 5). Statistical analysis (two-way analysis of variance posthoc Tukey test) is expressed as follows, with any differences considered statistically significant when p < 0.05: (B) *p < 0.05 incubation time 0 (baseline) vs all other time points, ap < 0.05 10 mM vs 30 and 50 mM; bp < 0.05 30 mM vs 50 mM; (F) see Table 1 in Supporting Information; (I) see Table 2 in Supporting Information; (H) *p < 0.05 incubation time 0 (baseline) vs all other time points, ap < 0.05 0 mg/mL vs 0.5, 1, and 2 mg/mL, bp < 0.05 0.5 mg/mL vs 1 and 2 mg/mL, cp < 0.05 1 mg/mL vs 2 mg/mL; (M) *p < 0.05 incubation time 0 (baseline) vs all other time points, ap < 0.05 0 h vs 0.5, 1, and 2 h, bp < 0.05 0.5 h vs 1 and 2 h, cp < 0.05 1 h vs 2 h.
RESULTS AND DISCUSSION
MSNPs loaded with glutamine showed a dose- and time-dependent cumulative loading profile. Incubation of MSNPs with 10 mM of glutamine, for 4 h, resulted in loading of 8.7 ± 0.8% of glutamine into nanoparticles. By increasing the glutamine concentration from 10 to 50 mM, there was a significant increase in the amount of glutamine loaded into MSNPs in 4 h (29.4 ± 2.0%; p < 0.05). Furthermore, by increasing the loading time from 4 to 48 h, the cumulative amount of glutamine loaded into MSNPs significantly increased at all concentrations (Figure 1B; p < 0.05).
The G-MSNPs were coated with polydopamine using different polydopamine coating concentrations and times. Photographs showed a color change from light to dark brown, which confirmed the polydopamine coating on G-MSNPs, since polydopamine coatings typically exhibit a brownish color. The brown color became increasingly darker as the concentration and time of polydopamine coating increased (Figure 1C). Transmission electron microscopy (TEM) images showed that uncoated and polydopamine-coated G-MSNPs were oval in shape with an average size of 120 nm (Figure 1D,E, Supporting Information Figures 3 and 4). Dynamic light scattering (DLS) measurements confirmed the average diameter of uncoated G-MSNPs at 113.9 ± 6.6 nm, a monodisperse population of particles (polydispersity index (PI) of 0.1), and a negative zeta potential (−36.57 ± 1.20). However, PDG-MSNPs obtained with different concentrations (0.5, 1, 2 mg/mL) and times (0.5, 1, 2 h) had a larger size (p < 0.05) and a more positive surface charge (p < 0.05) compared to uncoated G-MSNPs (Figure 1F,I, Supporting Information Figure 2 and Tables 3 and 4). The X-ray diffraction (XRD) analysis of the MSNPs showed diffraction peaks in low 2θ angles due to the MSNPs hexagonal structure. Three peaks at 2θ of 2.2°, 4.8°, and 23.2° corresponding to the 100, 110, and 200 planes, respectively, indicated the formation of a well-ordered structure. Following the polydopamine coating, the background XRD peaks increased due to the amorphous polymeric structure of polydopamine (Figure 1G,L and Supporting Information Figure 5).25–27
In addition, the cumulative release profiles showed that glutamine is released from PDG-MSNPs in a dose- and time-dependent manner. The polydopamine coating of G-MSNPs resulted in a delayed release of glutamine, which was dependent on both the polydopamine coating concentration and time (Figure 1H,M; p < 0.05). After 1 d of incubation, the amount of cumulative glutamine released significantly decreased from 52.3 ± 2.7% for uncoated G-MSNPs to 15.4 ± 0.7, 10.1 ± 1.9, and 4.8 ± 1.2% for PDG-MSNPs with polydopamine coating times of 0.5, 1, and 2 h, respectively (p < 0.05). Similar behavior was observed using different polydopamine coating concentrations (0.5, 1, 2 mg/mL). This effect was maintained over a period of 14 d (Figure 1H,M; p < 0.05).
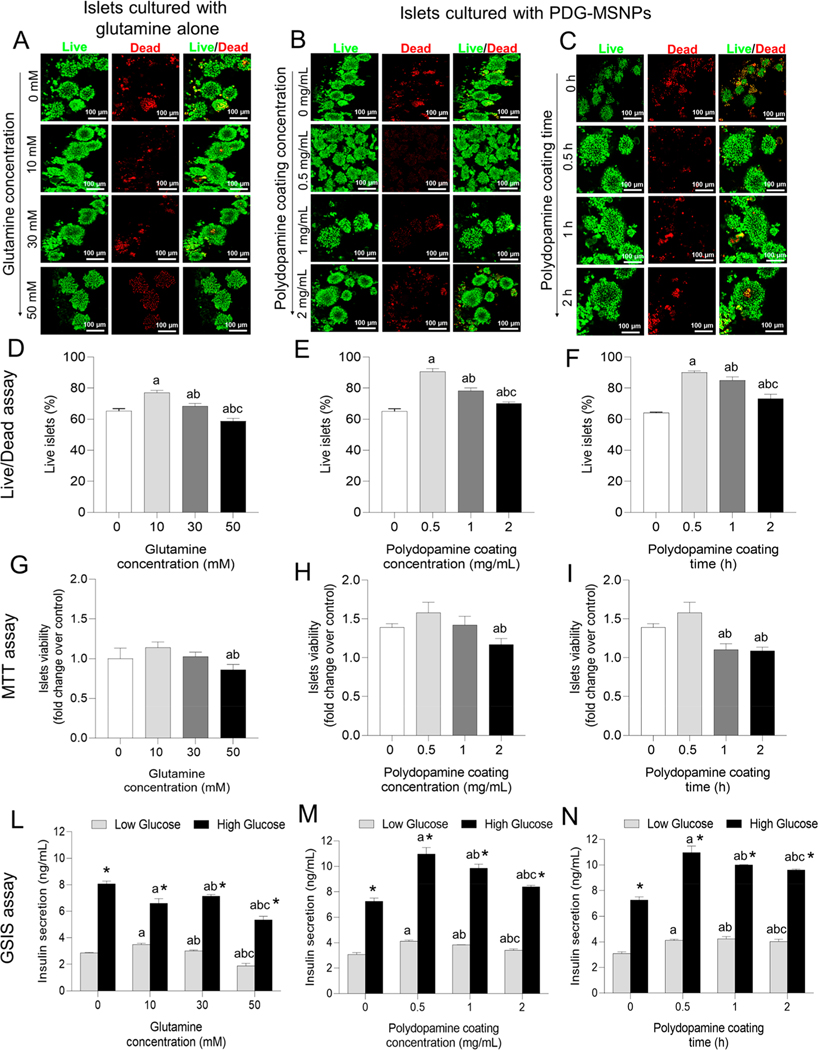
Confocal images showed islets are more viable (green) when seeded with glutamine (10, 30 mM) or PDG-MSNPs at different polydopamine coating concentrations (0.5, 1, 2 mg/mL) and times (0.5, 1, 2 h) compared to islets only. Specifically, a Live/Dead analysis showed that islets cultured with glutamine (10, 30 mM) demonstrated a significantly greater viability at day 7 compared to the control islets (i.e., islets cultured alone) (p < 0.05). Among the different concentrations of glutamine tested (i.e., 10–50 mM), 10 mM resulted in the highest islet viability compared to control islets (77.1 ± 2.6 vs 65.3 ± 2.5%, p < 0.05) (Figure 2A,D). Furthermore, when PDG-MSNPs were added to islets for 7 d, the viability increased significantly compared to the control islets (p < 0.05). Among the different tested polydopamine coating concentrations (0.5–2 mg/mL) and times (0.5–2 h), 0.5 mg/mL and 0.5 h resulted in the highest islet viability compared to islets only (Figure 2E,F; 90.7 ± 3.2 vs 65.3 ± 2.5%, p < 0.05). Similar results were also observed using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) viability assay (Figure 2G–I). The insulin secretory capacity of islets in response to the glucose challenges is reported in Figure 2L–N. The glucose stimulated insulin secretion (GSIS) data showed that, when the islets were cultured with 10 mM of glutamine alone, insulin secretion following stimulation with high glucose medium was significantly higher compared to the islets cultured without glutamine (Figure 2L; 8.1 ± 0.3 vs 6.6 ± 0.6 ng/mL, p < 0.05). Likewise, islets cultured with PDG-MSNPs, obtained using different polydopamine coating concentrations (0.5–2 mg/mL) and times (0.5–2 h), showed an elevated insulin secretory response compared to islets cultured without PDG-MSNPs (p < 0.05). Among the different polydopamine coating concentrations and times tested, when polydopamine was added using a concentration of 0.5 mg/mL over 0.5 h, the insulin secretion was significantly higher than control islets (Figure 2M,N; 10.9 ± 0.9 vs 7.3 ± 0.4 ng/mL, p < 0.05).
Figure 2.
In vitro interactions of PDG-MSNPs with pancreatic islets. (A) Representative Live/Dead confocal images of islets cultured with glutamine alone at different concentrations (0, 10, 30, and 50 mM) or (B, C) islets cultured with PDG-MSNPs with glutamine loading at 50 mM and different polydopamine coating concentrations (0, 0.5, 1, 2 mg/mL) (B) and times (0, 0.5, 1, and 2 h) (C) for 7 d after islet isolation. Red: Dead cells stained with PI. Green: Live cells stained with fluorescein diacetate (FDA). (D-I) Viability of islets performed with a Live/Dead assay (D-F) and an MTT assay (G-I) of islets cultured with glutamine alone at different concentrations (0, 10, 30, and 50 mM) (D, G) or PDG-MSNPs with different polydopamine coating concentrations (0, 0.5, 1, and 2 mg/mL) (E, H) and times (0, 0.5, 1, and 2 h) (F, I) after 7 d. (L-N) Functionality of islets evaluated with a GSIS assay after 7 d of incubation of islets in different conditions: glutamine alone at different concentrations (0, 10, 30, and 50 mM) (L), PDG-MSNPs with different polydopamine coating concentrations (0, 0.5, 1, and 2 mg/mL) (M) and times (0, 0.5, 1, and 2 h) (N). Functionality has been reported as the amount of insulin secretion (ng/mL) in low (2.8 mM of glucose) and high glucose (16.7 mM of glucose) conditions. Results are expressed as average ± standard error of measure (n = 5). Statistical analysis (two-way analysis of variance posthoc Tukey test) is expressed as follows, with any differences considered statistically significant when p < 0.05: (D, G) *p < 0.05 incubation time 0 (baseline) vs all other time points, ap < 0.05 10 mM vs 30 and 50 mM; bp < 0.05 30 mM vs 50 mM; (E, H) *p < 0.05 incubation time 0 (baseline) vs all other time points, ap < 0.05 0 mg/mL vs 0.5 mg/mL, 1 and 2 mg/mL, bp < 0.05 0.5 mg/mL vs 1 and 2 mg/mL, cp < 0.05 1 mg/mL vs 2 mg/mL; (F, I) *p < 0.05 incubation time 0 (baseline) vs all other time points, ap < 0.05 0 h vs 0.5, 1, and 2 h, bp < 0.05 0.5 h vs 1 and 2 h, cp < 0.05 1 h vs 2 h; (L) *p < 0.05 Low glucose vs High glucose, ap < 0.05 10 mM vs 30 and 50 mM; bp < 0.05 30 mM vs 50 mM; (M) *p < 0.05 Low glucose vs High glucose, ap < 0.05 0 mg/mL vs 0.5, 1, and 2 mg/mL, bp < 0.05 0.5 mg/mL vs 1 and 2 mg/mL, cp < 0.05 1 mg/mL vs 2 mg/mL; (N) *p < 0.05 Low glucose vs High glucose, ap < 0.05 0 h vs 0.5, 1, and 2 h, bp < 0.05 0.5 h vs 1 and 2 h, cp < 0.05 1 h vs 2 h.
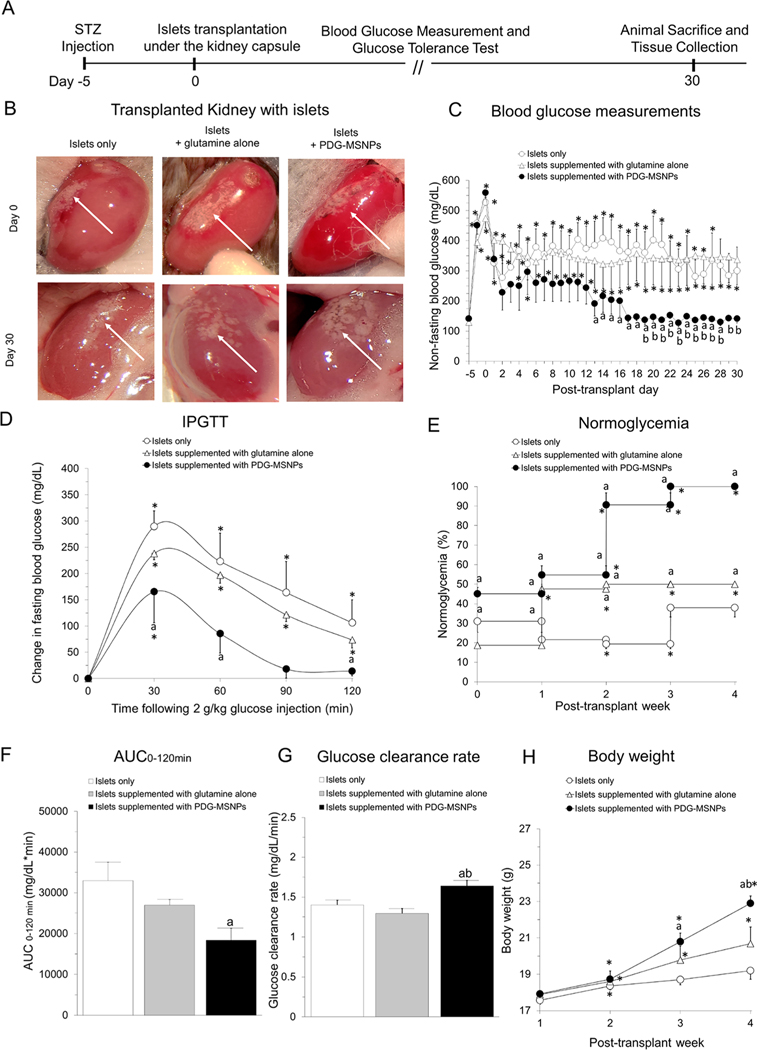
The experimental details of our in vivo transplantation experiments are outlined in Figure 3A,B. Following islet transplantation, all experimental groups showed a significant decrease in their blood glucose results (BGLs) within the first 48 h. After 48 h, the BGLs began to rise in animals transplanted with islets only or islets supplemented with glutamine alone, and this persisted for the duration of the experimental protocol (day 30: 300 ± 79 or 337 ± 84 mg/dL; respectively). In contrast, animals transplanted with islets supplemented with PDG-MSNPs sustained their reduction in nonfasting BGLs such that, from day 17 until the end of the experimental protocol, all animals were normoglycemic and had thus re-established glycemic control (day 30: 141 ± 15 mg/dL; Figure 3C). Indeed, 45 ± 3% animals transplanted with islets supplemented with PDG-MSNPs became normoglycemic in the first week post-transplantation, and this increased to 100 ± 0% in the fourth week post-transplantation (Figure 3E). Moreover, mice transplanted with islets and PDG-MSNPs progressively increased their body weight from 17.9 ± 0.1 g (week 1) to 22.9 ± 0.5 g (week 4) (p < 0.05), which was significantly higher compared to animals transplanted with islets only and islets supplemented with glutamine alone (19.2 ± 0.3 g and 20.7 ± 0.9 g at week 4 (p < 0.05), respectively) (Figure 3H). As shown in Figure 3D, BGLs significantly increased in all experimental groups after intraperitoneal glucose administration, with a peak value seen at 30 min (p < 0.05). However, in animals that received PDG-MSNPs, the peak incremental value following glucose injection was significantly lower (165 ± 58 vs 290 ± 30 or 238 ± 12 mg/dL for mice transplanted with islets alone or islets supplemented with glutamine alone, respectively; p < 0.05, Figure 3D), and these animals also restored their glucose values back to baseline levels by 120 min, unlike those animals that received transplantation of islets only or islets supplemented with glutamine alone. Accordingly, the area under the curve (AUC) of AUC0–120 min (18 326 ± 2932 vs 32 945 ± 4481 or 26935 ± 1347 mg/dL × min; p < 0.05, Figure 3F) was significantly lower, and the glucose clearance rate (1.6 ± 0.1 vs 1.4 ± 0.1 or 1.3 ± 0.1 mg/dL/min; p < 0.05, Figure 3G) was significantly faster in animals that had received islets supplemented with PDG-MSNPs compared to those animals that received an islet transplantation alone or islets supplemented with glutamine alone.
Figure 3.
In vivo interactions of PDG-MSNPs with pancreatic islets. (A) Experimental detail for in vivo experiments. (B) Photograph of the transplanted kidney using islets only, islets supplemented with glutamine alone, and islets supplemented with PDG-MSNPs at day 0 (i.e., at the time of transplantation) and day 30 (i.e., at the time of sacrifice) post-transplantation (white arrow = islets transplanted in kidney). (C) Nonfasting blood glucose measurements over 30 d post-transplantation. (D) Fasting Intraperitoneal Glucose Tolerance Test (IPGTT). (E) Normoglycemia percentage. (F) Area under the IPGTT curve (AUC0–120 min). (G) Blood glucose clearance rates calculated from the slope of the IPGTT curves from 30 to 90 min. (H) Weekly monitoring of the body weight of transplanted mice. Results are expressed as the average ± standard error of measure (n = 6). Statistical analysis (two-way analysis of variance posthoc Tukey test) is expressed as follows, with any differences considered statistically significant when p < 0.05: *p < 0.05 incubation time 0 (baseline) vs all other time points, ap < 0.05 islets only vs islets supplemented with glutamine alone and islets supplemented with PDG-MSNPs; bp < 0.05 islets supplemented with glutamine alone vs islets supplemented with PDG-MSNPs.
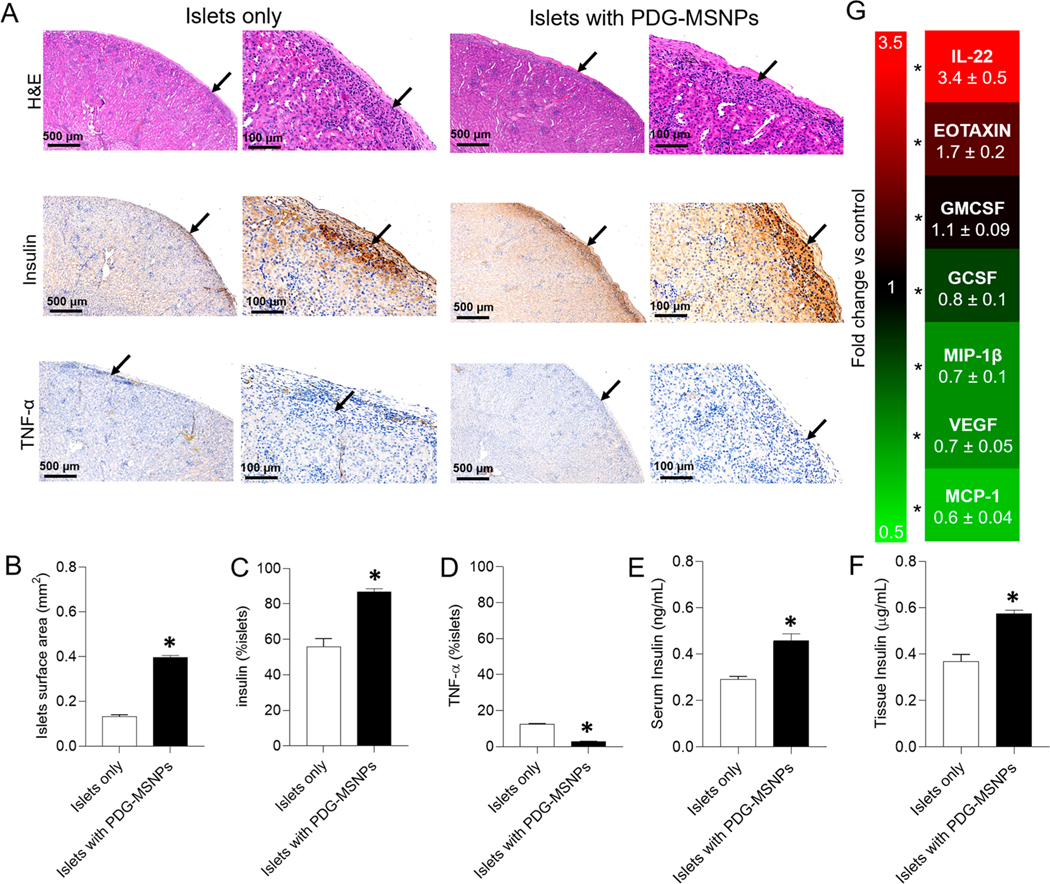
Histological analysis performed on tissue (i.e., kidneys) explanted 30 d post-transplantation showed that islets transplanted with PDG-MSNPs retained their native size, spherical morphology, and intrinsic architecture, findings that were not consistently seen in islets transplanted alone (Figure 4A). Moreover, animals transplanted with islets and PDG-MSNPs had a significantly greater number of viable transplanted islets compared to animals that did not receive any nanoparticles (i.e., islets only). Results were confirmed by quantifying the total islet area, which was higher for mice transplanted with islets supplemented with PDG-MSNPs compared to animals transplanted with islets only (total islet area: 0.4 ± 0.02 vs 0.1 ± 0.02 mm2, respectively; p < 0.05; Figure 4B). When transplanted islets are healthier, they are normally intact and have a spherical structure.28–30 However, when islets start to die, they lose their shape due to a loss in plasma membrane integrity and cell death.31 Here, our results support the cytoprotective effect provided by PDG-MSNP, given that islet survival and function was improved when the PDG-MSNPs were transplanted with islets; accordingly, islets had a more spherical and organized structure when compared to transplanted islets alone. There was also a greater number of β cells (insulin staining: 86.9 ± 4.5 vs 55.7 ± 12.4%; p < 0.05; Figure 4C) in addition to a reduction in the surrounding inflammation (TNF-α staining: 2.9 ± 0.4 vs 12.5 ± 0.7%; p < 0.05; Figure 4D) in mice transplanted with PDG-MSNPs compared to those transplanted with islets only. Furthermore, the analysis of serum and explanted kidneys demonstrated a significantly higher amount of insulin in animals transplanted with islets and PDG-MSNPs compared to those transplanted with islets only (for serum: 0.5 ± 0.08 vs 0.3 ± 0.03 ng/mL; for kidney tissue: 0.6 ± 0.04 vs 0.4 ± 0.08 μg/mL, respectively, p < 0.05; Figure 4E,F). Cytokine quantification was also performed in explanted kidneys to further investigate the immunological and inflammatory responses to transplanted islets alone versus transplanted islets supplemented with PDG-MSNPs. Our results showed an upregulation of monocyte chemoattractant protein-1 (MCP-1: (0.6 ± 0.04)-fold increase), vascular endothelial growth factor (VEGF: 0.7 ± 0.05), macrophage inflammatory protein 1-beta (MIP-1β: (0.7 ± 0.1)-fold increase), granulocyte colony-stimulating factor (GCSF: (0.8 ± 0.1)-fold increase), granulocyte-macrophage colony-stimulating factor (GMCSF: (1.1 ± 0.1)-fold increase), EOTAXIN ((1.7 ± 0.2)-fold increase), and Interleukin-22 (IL-22: (3.4 ± 0.5)-fold increase) in the kidneys that contained transplanted islets supplemented with PDG-MSNPs when compared to those containing transplanted islets only (Figure 4G; p < 0.05).
Figure 4.
Histological and molecular analyses of kidney tissue and serum after mice sacrifice. (A) Representative histological (haematoxylin & eosin staining) and immunohistochemical images (insulin and TNF-α staining) of islets transplanted under the kidney capsule (black arrow = islets). (B) Quantification of islet surface area, positive (C) insulin and (D) TNF-α staining. (E) The level of insulin within blood serum and (F) kidney tissue. (G) Fold change in cytokine expression within kidney tissue that contained transplanted islets supplemented with PDG-MSNPs relative to those samples of transplanted islets only (i.e., control). Results were analyzed with at least 15–20 islets from five different sections through the kidney of each animal. Results are expressed as average ± standard error of measure (n = 6). Statistical analysis (unpaired Student’s t test) is expressed as follows, with any differences considered statistically significant when p < 0.05: *p < 0.05: islets only vs islets with PDG-MSNPs.
MSNPs have been used for different biomedical applications including the delivery of proteins,32 chemotherapeutic drugs,33 and cytokines,34 as well as cellular imaging.35 To control the rate of release of these therapeutic agents to achieve effective local concentrations, great efforts have been made to test various parameters that can be modulated/controlled (i.e., temperature,36 redox,37 pH,38 light,39 and enzymes40) in order to create a responsive MSNP-based system. In addition, MSNPs can be coated with various polymers including poly(ethylene glycol) (PEG),41 PEGylated lipid bilayer,42 poly(N-isopropylacrylamide) (PNIPAAm) coating,43 poly(L-lysine)44 alginate,45 and poly(acrylic acid) (PAA).46 Although promising, these approaches require a multistep, complex, and often time-consuming chemistry with the use of toxic solvents to synthesize a coating layer on the surface of MSNPs. An external (e.g., light39) or internal (e.g., pH38) stimulation is also then required to degrade the formed coating layer for the release of any encapsulated therapeutic agents. We used MSNPs for nutrient (glutamine) delivery to pancreatic islets; however, to prevent the immediate unloading of glutamine and sustain its release over a period of 14 d (i.e., the approximate time it takes for islets to establish their own microcirculation following transplantation),24 we used a simple (one-step synthesis), quick (<2 h), inexpensive (<$10 per 106 MSNPs), and eco-friendly (no toxic solvents) method using polydopamine to coat our glutamine-loaded MSNPs (G-MSNPs). Our results showed that changing the polydopamine coating concentration (0.5–2 mg/mL) and time (0.5–2 h) allowed us to control the release of glutamine from MSNPs over 1–14 d. PDG-MSNPs made using polydopamine at 0.5 mg/mL over 0.5 h demonstrated significant increase in islet viability and function compared to islets supplemented with either glutamine alone (at the same concentration) or uncoated G-MSNPs. We attributed this to the sustained and controlled release of glutamine from MSNPs over time, instead of it being provided in a “burst phenomenon” to islets. Our in silico and in vitro results demonstrate that, when islets are cultured with glutamine alone, their survival and function improves compared to when islets are cultured alone without glutamine; this is in keeping with previous work examining the role of glutamine on islet survival.9 These results can be due to the fact that glutamine protects islets from nutrient deprivation by sustaining mitochondria oxidation via anaplerosis9 and/or activating protein synthesis via mammalian target of rapamycin (mTOR), which is normally inhibited under nutrient-limiting conditions.47
When the islets were transplanted with PDG-MSNPs, diabetic animals were able to re-establish glycemic control for 30 d post-transplantation, with mice demonstrating a faster dynamic response to glucose challenges. Histological examination of the islet graft 30 d post-transplantation also confirmed that islets transplanted with PDG-MSNPs had better morphology and reduced inflammation (decrease in tumor necrosis factor (TNF)-α staining) compared to islets transplanted alone. In support of the ability of islets supplemented with PDG-MSNPs to facilitate islet revascularization, we also measured cytokine expression in the islet graft and found an upregulation of pro-angiogenic factors including VEGF. Indeed, VEGF has been shown to be key for islet revascularization following transplantation,48 and β-cells themselves have been shown to secrete large amounts of VEGF.49 This is important, given that a delay or insufficient revascularization in transplanted islets will affect their overall survival and function.50 We also found that transplanted islets supplemented with PDG-MSNPs had a significantly higher expression of the anti-inflammatory cytokine IL-22,51 compared to transplanted islets alone. These results confirm the ability of glutamine in being able to reduce inflammation within the microenvironment of transplanted islets. However, a previous study did show that, when glutamine alone is supplemented to stem-cell-derived β cells, although there was an improvement early after transplantation, this protective effect was not persistent.9 Similarly, our in vitro (GSIS assay) and in vivo (metabolic analysis) data show that islets supplemented with glutamine alone were not as responsive compared to when islets were supplemented with PDG-MSNPs. One possible explanation for this could be due to the gradual and sustained delivery of glutamine to islets provided by PDG-MSNPs, compared to a one-time supplementation when glutamine alone is added to islets. Indeed, with high levels of glutamine provided in a burst supply, β cells may get overworked, which over time may adversely affect both their survival and function.
The anatomical site that is currently being explored for islets to be transplanted with novel platforms such as bioscaffolds or nanoparticles is the omentum given that it has the ability to accommodate novel three-dimensional (3D) platforms (i.e., nanoparticles and bioscaffolds52–54). Indeed, human clinical trials are already underway examining the feasibility of the omentum as a site for poly(dimethylsiloxane) (PDMS)-based bioscaffolds for islet transplantation.55 In small animals, the epididymal fat pad (EFP) is often used as a surrogate site for the omentum in humans.55–57 Hence, in our biocompatibility study we examined both the EFP and the subcutaneous space with our PDG-MSNPs, given that these sites both have the potential to be widely adopted in human patients. Since our current data support that PDG-MSNPs can augment islet survival and function with excellent biocompatibility, future research will now aim to examine islet transplantation with PDG-MSNPs, over the short and long terms, at these other sites either alone or in combination with 3D bioscaffolds. In the latter case, bioscaffolds can be easily scaled to 100 cm3 to accommodate the required number of islets for human islet transplantation (i.e., a typical islet transplant requires 5000 islets per kg, which, on average, translates to ∼350 000–450 000 islets58). Given that islets housed in these bioscaffolds will require nutrients until they are revascularized, combining our PDG-MSNPs with bioscaffolds can address this issue to ensure islets have the optimal microenvironment following their transplantation. Finally, our PDG-MSNP is composed of two components: MSNP and PD. In general, silica is recognized as “safe” by the Food & Drug Administration (FDA),59 and it is often used in tablet form in drug formulations as an excipient and frequently also taken as a dietary supplement for nourishing skin and nails.59 Recently, silica nanoparticles in the form of Cornell dots (“C-dots”)60 were FDA-approved for a phase I human clinical trial.61 While we,62,63 and others,64–66 have shown PD is a biocompatible material, to our knowledge there are currently no clinical trials that have used PD. Taken together, PDG-MSNPs should therefore be well-placed to be assessed in human clinical trials following their optimization for use in islet transplantation.
CONCLUSION
In summary, our results show the potential of PDG-MSNPs to be used as a translatable platform for the controlled and sustained delivery of nutrients in the short term (i.e., over 14 d), which is especially important in the context of islet transplantation. The present in vitro and in vivo data show that PDG-MSNPs not only protect islets but also promote their survival and function.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by research grants from the NIDDK (R01DK119293 and P30DK116074), the Stanford Nano Shared Facilities grant (1161726–146-DAARZ), as part of the grant supported by the National Science Foundation grant (ECCS-1542152) and Stanford Neuroscience Microscopy Service grant (NIH NS069375) as well as the Akiko Yamazaki and Jerry Yang Faculty Scholar Fund in Pediatric Translational Medicine, and the Stanford Maternal and Child Health Research Institute. The authors thank Research Professor Y. J. Zhou, PhD. Director of Morrison Microscopy Core Research Facility, Center for Biotechnology, University of Nebraska-Lincoln for the technical support related to the TEM data. The authors also acknowledge A. Thomas for her help on preparing the TOC graphic.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.nanolett.0c02576.
Experimental details including the synthesis and characterization, determination of glutamine loading and release, computational modeling of glutamine delivery to pancreatic islets, in vitro and in vivo analyses, long-term biocompatibility, in vivo tracking, and statistical analysis. Results of characterizations, computational modeling, islet protein expression, in vivo tracking, and histological analysis, and long-term biocompatibility (PDF)
The authors declare no competing financial interest.
Contributor Information
Mehdi Razavi, Interventional Regenerative Medicine and Imaging Laboratory, Stanford University School of Medicine, Palo Alto, California 94304, United States; Biionix (Bionic Materials, Implants & Interfaces) Cluster, Department of Internal Medicine, College of Medicine and Department of Materials Science & Engineering, University of Central Florida, Orlando, Florida 32827, United States.
Rosita Primavera, Interventional Regenerative Medicine and Imaging Laboratory, Stanford University School of Medicine, Palo Alto, California 94304, United States.
Bhavesh D Kevadiya, Interventional Regenerative Medicine and Imaging Laboratory, Stanford University School of Medicine, Palo Alto, California 94304, United States.
Jing Wang, Interventional Regenerative Medicine and Imaging Laboratory, Stanford University School of Medicine, Palo Alto, California 94304, United States.
Mujib Ullah, Interventional Regenerative Medicine and Imaging Laboratory, Stanford University School of Medicine, Palo Alto, California 94304, United States.
Peter Buchwald, Diabetes Research Institute, Miller School of Medicine, University of Miami, Miami, Florida 33136, United States.
Avnesh S Thakor, Interventional Regenerative Medicine and Imaging Laboratory, Stanford University School of Medicine, Palo Alto, California 94304, United States.
REFERENCES
- (1).Biarnés M; Montolio M; Nacher V; Raurell M; Soler J; Montanya E. Β-Cell Death and Mass in Syngeneically Transplanted Islets Exposed To Short- and Long-Term Hyperglycemia. Diabetes 2002, 51 (1), 66–72. [DOI] [PubMed] [Google Scholar]
- (2).Nilsson B; Ekdahl KN; Korsgren O. Control of Instant Blood-Mediated Inflammatory Reaction to Improve Islets of Langerhans Engraftment. Current Opinion in Organ Transplantation 2011, 620–626, DOI: 10.1097/MOT.0b013e32834c2393. [DOI] [PubMed] [Google Scholar]
- (3).Pepper AR; Gala-Lopez B; Ziff O; Shapiro AMJ Revascularization of Transplanted Pancreatic Islets and Role of the Transplantation Site. Clin. Dev. Immunol. 2013, 2013, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Carlsson PO; Palm F; Andersson A; Liss P. Markedly Decreased Oxygen Tension in Transplanted Rat Pancreatic Islets Irrespective of the Implantation Site. Diabetes 2001, 50 (3), 489–495. [DOI] [PubMed] [Google Scholar]
- (5).Komatsu H; Cook C; Wang CH; Medrano L; Lin H; Kandeel F; Tai YC; Mullen Y. Oxygen Environment and Islet Size Are the Primary Limiting Factors of Isolated Pancreatic Islet Survival. PLoS One 2017, 12 (8), e0183780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Komatsu H; Kang D; Medrano L; Barriga A; Mendez D; Rawson J; Omori K; Ferreri K; Tai YC; Kandeel F; et al. Isolated Human Islets Require Hyperoxia to Maintain Islet Mass, Metabolism, and Function. Biochem. Biophys. Res. Commun. 2016, 470 (3), 534–538. [DOI] [PubMed] [Google Scholar]
- (7).Dixon G; Nolan J; McClenaghan N; Flatt PR; Newsholme P. A Comparative Study of Amino Acid Consumption by Rat Islet Cells and the Clonal Beta-Cell Line BRIN-BD11 - The Functional Significance of L-Alanine. J. Endocrinol. 2003, 179 (3), 447–454. [DOI] [PubMed] [Google Scholar]
- (8).Newsholme P; Brennan L; Bender K. Amino Acid Metabolism, β-Cell Function, and Diabetes. Diabetes 2006, 55, S39. [Google Scholar]
- (9).Faleo G; Russ HA; Wisel S; Parent AV; Nguyen V; Nair GG; Freise JE; Villanueva KE; Szot GL; Hebrok M; et al. Mitigating Ischemic Injury of Stem Cell-Derived Insulin-Producing Cells after Transplant. Stem Cell Rep. 2017, 9 (3), 807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Liu Z; Jeppesen PB; Gregersen S; Chen X; Hermansen K. Dose- and Glucose-Dependent Effects of Amino Acids on Insulin Secretion from Isolated Mouse Islets and Clonal INS-1E Beta-Cells. Rev. Diabet. Stud. 2008, 5 (4), 232–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Jang HJ; Kwak JH; Cho EY; We YM; Lee YH; Kim SC; Han DJ Glutamine Induces Heat-Shock Protein-70 and Glutathione Expression and Attenuates Ischemic Damage in Rat Islets. Transplant. Proc. 2008, 40 (8), 2581–2584. [DOI] [PubMed] [Google Scholar]
- (12).Lindström, P.; Sehlin, J. Aromatic Amino Acids and Pancreatic Islet Function: A Comparison of l-Tryptophan and l-5-Hydroxytryptophan. Mol. Cell. Endocrinol. 1986, 48 (2–3), 121–126. [DOI] [PubMed] [Google Scholar]
- (13).Neuman JC; Truchan NA; Joseph JW; Kimple ME A Method for Mouse Pancreatic Islet Isolation and Intracellular CAMP Determination. J. Visualized Exp. 2014, No. 88, No. e50374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Mullooly N; Vernon W; Smith DM; Newsholme P. Elevated Levels of Branched-Chain Amino Acids Have Little Effect on Pancreatic Islet Cells, but l-Arginine Impairs Function through Activation of the Endoplasmic Reticulum Stress Response. Exp. Physiol. 2014, 99, 538. [DOI] [PubMed] [Google Scholar]
- (15).Cunningham GA; Mcclenaghan NH; Flatt PR; Newsholme P. L-Alanine Induces Changes in Metabolic and Signal Transduction Gene Expression in a Clonal Rat Pancreatic β-Cell Line and Protects from pro-Inflammatory Cytokine-Induced Apoptosis. Clin. Sci. 2005, 109 (5), 447–455. [DOI] [PubMed] [Google Scholar]
- (16).Curi R; Lagranha CJ; Doi SQ; Sellitti DF; Procopio J; Pithon-Curi TC; Corless M; Newsholme P. Molecular Mechanisms of Glutamine Action. J. Cell. Physiol. 2005, 204, 392–401. [DOI] [PubMed] [Google Scholar]
- (17).Smith RJ; Wilmore DW Glutamine Nutrition and Requirements. JPEN, J. Parenter. Enteral Nutr. 1990, 14, 94S–99S. [DOI] [PubMed] [Google Scholar]
- (18).Moreno-Villaécija MÁ; Sedó-Vegara J; Guisasola E; Baeza A; Regí MV; Nador F; Ruiz-Molina D. Polydopamine-like Coatings as Payload Gatekeepers for Mesoporous Silica Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10 (9), 7661–7669. [DOI] [PubMed] [Google Scholar]
- (19).Baeza A; Colilla M; Vallet-Regí M. Advances in Mesoporous Silica Nanoparticles for Targeted Stimuli-Responsive Drug Delivery. Expert Opin. Drug Delivery 2015, 12 (2), 319–337. [DOI] [PubMed] [Google Scholar]
- (20).Liu R; Guo Y; Odusote G; Qu F; Priestley RD Core-Shell Fe3O4 Polydopamine Nanoparticles Serve Multipurpose as Drug Carrier, Catalyst Support and Carbon Adsorbent. ACS Appl. Mater. Interfaces 2013, 5 (18), 9167–9171. [DOI] [PubMed] [Google Scholar]
- (21).Hwang JH; Noh YW; Choi JH; Noh JR; Kim YH; Gang GT; Kim KS; Park HS; Lim YT; Moon H; et al. In Vivo Imaging of Islet Transplantation Using PLGA Nanoparticles Containing Iron Oxide and Indocyanine Green. Magn. Reson. Med. 2014, 71 (3), 1054–1063. [DOI] [PubMed] [Google Scholar]
- (22).Wang P; Yoo B; Yang J; Zhang X; Ross A; Pantazopoulos P; Dai G; Moore A. GLP-1R-Targeting Magnetic Nanoparticles for Pancreatic Islet Imaging. Diabetes 2014, 63 (5), 1465–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Pham TT; Nguyen TT; Pathak S; Regmi S; Nguyen HT; Tran TH; Yong CS; Kim JO; Park PH; Park MH; et al. Tissue Adhesive FK506-Loaded Polymeric Nanoparticles for Multi-Layered Nano-Shielding of Pancreatic Islets to Enhance Xenograft Survival in a Diabetic Mouse Model. Biomaterials 2018, 154, 182–196. [DOI] [PubMed] [Google Scholar]
- (24).Morini S; Brown ML; Cicalese L; Elias G; Carotti S; Gaudio E; Rastellini C. Revascularization and Remodelling of Pancreatic Islets Grafted under the Kidney Capsule. J. Anat. 2007, 210 (5), 565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Ferreira Soares DC; de Sousa Andrada A; Andrade Ramaldes G. Silica Nanoparticles Containing Gadolinium Complex as Potential Alternative to Anticancer Radiotherapy. Part. Sci. Technol. 2015, 33 (4), 331–338. [Google Scholar]
- (26).Yin NQ; Wu P; Yang TH; Wang M. Preparation and Study of a Mesoporous Silica-Coated Fe3O4 Photothermal Nanoprobe. RSC Adv. 2017, 7 (15), 9123–9129. [Google Scholar]
- (27).Santha Moorthy M; Subramanian B; Panchanathan M; Mondal S; Kim H; Lee KD; Oh J. Fucoidan-Coated Core-Shell Magnetic Mesoporous Silica Nanoparticles for Chemotherapy and Magnetic Hyperthermia-Based Thermal Therapy Applications. New J. Chem. 2017, 41 (24), 15334–15346. [Google Scholar]
- (28).Ionescu-Tirgoviste C; Gagniuc PA; Gubceac E; Mardare L; Popescu I; Dima S; Militaru MA 3D Map of the Islet Routes throughout the Healthy Human Pancreas. Sci. Rep. 2015, 5. DOI: 10.1038/srep14634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Huang HH; Harrington S; Stehno-Bittel L. The Flaws and Future of Islet Volume Measurements. Cell Transplantation 2018, 27, 1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Rackham CL; Jones PM; King AJF Maintenance of Islet Morphology Is Beneficial for Transplantation Outcome in Diabetic Mice . PLoS One 2013, 8 (2), e57844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Kaviani M; Keshtkar S; Azarpira N; Aghdaei MH; Geramizadeh B; Karimi MH; Shamsaeefar A; Motazedian N; Nikeghbalian S; Al-Abdullah IH Cytoprotective Effects of Olesoxime on Isolated Human Pancreatic Islets in Order to Attenuate Apoptotic Pathway. Biomed. Pharmacother. 2019, 112, 108674. [DOI] [PubMed] [Google Scholar]
- (32).Liu B; Ejaz W; Gong S; Kurbanov M; Canakci M; Anson F; Thayumanavan S. Engineered Interactions with Mesoporous Silica Facilitate Intracellular Delivery of Proteins and Gene Editing. Nano Lett. 2020, 20 (5), 4014–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Llopis-Lorente A; Garciá-Fernández A; Murillo-Cremaes N; Hortelaõ AC; Patinõ T; Villalonga R; Sancenón F; Martínez-Máñez R; Sánchez S. Enzyme-Powered Gated Mesoporous Silica Nanomotors for on-Command Intracellular Payload Delivery. ACS Nano 2019, 13 (10), 12171–12183. [DOI] [PubMed] [Google Scholar]
- (34).Kwon D; Cha BG; Cho Y; Min J; Park EB; Kang SJ; Kim J. Extra-Large Pore Mesoporous Silica Nanoparticles for Directing in Vivo M2Macrophage Polarization by Delivering IL-4. Nano Lett. 2017, 17 (5), 2747–2756. [DOI] [PubMed] [Google Scholar]
- (35).Sun Q; You Q; Wang J; Liu L; Wang Y; Song Y; Cheng Y; Wang S; Tan F; Li N. Theranostic Nanoplatform: Triple-Modal Imaging-Guided Synergistic Cancer Therapy Based on Liposome-Conjugated Mesoporous Silica Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10 (2), 1963–1975. [DOI] [PubMed] [Google Scholar]
- (36).You YZ; Kalebaila KK; Brock SL; Oupický D. Temperature-Controlled Uptake and Release in PNIPAM-Modified Porous Silica Nanoparticles. Chem. Mater. 2008, 20 (10), 3354–3359. [Google Scholar]
- (37).Sauer AM; Schlossbauer A; Ruthardt N; Cauda V; Bein T; Bräuchle C. Role of Endosomal Escape for Disulfide-Based Drug Delivery from Colloidal Mesoporous Silica Evaluated by Live-Cell Imaging. Nano Lett. 2010, 10 (9), 3684–3691. [DOI] [PubMed] [Google Scholar]
- (38).Park C; Oh K; Lee SC; Kim C. Controlled Release of Guest Molecules from Mesoporous Silica Particles Based on a PH-Responsive Polypseudorotaxane Motif. Angew. Chem., Int. Ed. 2007, 46 (9), 1455–1457. [DOI] [PubMed] [Google Scholar]
- (39).Lu J; Choi E; Tamanoi F; Zink JI Light-Activated Nanoimpeller-Controlled Drug Release in Cancer Cells. Small 2008, 4 (4), 421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Schlossbauer A; Kecht J; Bein T. Biotin-Avidin as a Protease-Responsive Cap System for Controlled Guest Release from Colloidal Mesoporous Silica. Angew. Chem., Int. Ed. 2009, 48 (17), 3092–3095. [DOI] [PubMed] [Google Scholar]
- (41).Mu S; Liu Y; Wang T; Zhang J; Jiang D; Yu X; Zhang N. Unsaturated Nitrogen-Rich Polymer Poly(L-Histidine) Gated Reversibly Switchable Mesoporous Silica Nanoparticles Using “Graft to” Strategy for Drug Controlled Release. Acta Biomater. 2017, 63, 150–162. [DOI] [PubMed] [Google Scholar]
- (42).Lin J; Cai Q; Tang Y; Xu Y; Wang Q; Li T; Xu H; Wang S; Fan K; Liu Z; et al. PEGylated Lipid Bilayer Coated Mesoporous Silica Nanoparticles for Co-Delivery of Paclitaxel and Curcumin: Design, Characterization and Its Cytotoxic Effect. Int. J. Pharm. 2018, 536 (1), 272–282. [DOI] [PubMed] [Google Scholar]
- (43).Hegazy M; Zhou P; Wu G; Wang L; Rahoui N; Taloub N; Huang X; Huang Y. Construction of Polymer Coated Core-Shell Magnetic Mesoporous Silica Nanoparticles with Triple Responsive Drug Delivery. Polym. Chem. 2017, 8 (38), 5852–5864. [Google Scholar]
- (44).Du X; Zhang T; Ma G; Gu X; Wang G; Li J. Glucose-Responsive Mesoporous Silica Nanoparticles to Generation of Hydrogen Peroxide for Synergistic Cancer Starvation and Chemistry Therapy. Int. J. Nanomed. 2019, 14, 2233–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Yuan N. -n.; Li S.-j.; Li G.-q. Sodium Alginate Coated Mesoporous Silica for Dual Bio-Responsive Controlled Drug Delivery. J. Drug Delivery Sci. Technol. 2018, 46, 348–353. [Google Scholar]
- (46).Liu J; Liang H; Li M; Luo Z; Zhang J; Guo X; Cai K. Tumor Acidity Activating Multifunctional Nanoplatform for NIR-Mediated Multiple Enhanced Photodynamic and Photothermal Tumor Therapy. Biomaterials 2018, 157, 107–124. [DOI] [PubMed] [Google Scholar]
- (47).Yu L; McPhee CK; Zheng L; Mardones GA; Rong Y; Peng J; Mi N; Zhao Y; Liu Z; Wan F; et al. Termination of Autophagy and Reformation of Lysosomes Regulated by MTOR. Nature 2010, 465 (7300), 942–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Brissova M; Shostak A; Shiota M; Wiebe PO; Poffenberger G; Kantz J; Chen Z; Carr C; Jerome WG; Chen J; et al. Pancreatic Islet Production of Vascular Endothelial Growth Factor-A Is Essential for Islet Vascularization, Revascularization, and Function. Diabetes 2006, 55 (11), 2974–2985. [DOI] [PubMed] [Google Scholar]
- (49).Peiris H; Bonder CS; Coates PTH; Keating DJ; Jessup CF The β-Cell/EC Axis: How Do Islet Cells Talk to Each Other? Diabetes 2014, 63, 3–11. [DOI] [PubMed] [Google Scholar]
- (50).Del Toro-Arreola A; Robles-Murillo AK; Daneri-Navarro A; Rivas-Carrillo JD The Role of Endothelial Cells on Islet Function and Revascularization after Islet Transplantation. Organogenesis 2016, 12 (1), 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Parks OB; Pociask DA; Hodzic Z; Kolls JK; Good M. Interleukin-22 Signaling in the Regulation of Intestinal Health and Disease. Front. Cell Dev. Biol. 2016. DOI: 10.3389/fcell.2015.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Razavi M; Hu S; Thakor AS A Collagen Based Cryogel Bioscaffold Coated with Nanostructured Polydopamine as a Platform for Mesenchymal Stem Cell Therapy. J. Biomed. Mater. Res., Part A 2018, 106 (8), 2213–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Razavi M; Primavera R; Kevadiya BD; Wang J; Buchwald P; Thakor AS A Collagen Based Cryogel Bioscaffold That Generates Oxygen for Islet Transplantation. Adv. Funct. Mater. 2020, 30 (15), 1902463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Razavi M; Thakor AS An Oxygen Plasma Treated Poly(Dimethylsiloxane) Bioscaffold Coated with Polydopamine for Stem Cell Therapy. J. Mater. Sci.: Mater. Med. 2018, 29 (5). DOI: 10.1007/s10856-018-6077-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Schmidt C. Pancreatic Islets Find a New Transplant Home in the Omentum. Nat. Biotechnol. 2017, 35 (1), 8–8. [DOI] [PubMed] [Google Scholar]
- (56).Berman DM; Molano RD; Fotino C; Ulissi U; Gimeno J; Mendez AJ; Kenyon NM; Kenyon NS; Andrews DM; Ricordi C; et al. Bioengineering the Endocrine Pancreas: Intraomental Islet Transplantation within a Biologic Resorbable Scaffold. Diabetes 2016, 65 (5), 1350–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Gibly RF; Zhang X; Lowe WL; Shea LD Porous Scaffolds Support Extrahepatic Human Islet Transplantation, Engraftment, and Function in Mice. Cell Transplant. 2013, 22 (5), 811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).McCall M; James Shapiro AM Update on Islet Transplantation. Cold Spring Harbor Perspect. Med. 2012, 2 (7), a007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Rosenholm JM; Mamaeva V; Sahlgren C; Lindén M. Nanoparticles in Targeted Cancer Therapy: Mesoporous Silica Nanoparticles Entering Preclinical Development Stage. Nanomedicine 2012, 7, 111–120. [DOI] [PubMed] [Google Scholar]
- (60).Ow H; Larson DR; Srivastava M; Baird BA; Webb WW; Wiesner U. Bright and Stable Core-Shell Fluorescent Silica Nanoparticles. Nano Lett. 2005, 5 (1), 113–117. [DOI] [PubMed] [Google Scholar]
- (61).Benezra M; Penate-Medina O; Zanzonico PB; Schaer D; Ow H; Burns A; DeStanchina E; Longo V; Herz E; Iyer S; et al. Multimodal Silica Nanoparticles Are Effective Cancer-Targeted Probes in a Model of Human Melanoma. J. Clin. Invest. 2011, 121 (7), 2768–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Razavi M; Hu S; Thakor AS A Collagen Based Cryogel Bioscaffold Coated with Nanostructured Polydopamine as a Platform for Mesenchymal Stem Cell Therapy. J. Biomed. Mater. Res., Part A 2018, 106 (8), 2213–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Razavi M; Thakor AS An Oxygen Plasma Treated Poly(Dimethylsiloxane) Bioscaffold Coated with Polydopamine for Stem Cell Therapy. J. Mater. Sci.: Mater. Med. 2018, 29 (5). DOI: 10.1007/s10856-018-6077-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Liu X; Cao J; Li H; Li J; Jin Q; Ren K; Ji J. Mussel-Inspired Polydopamine: A Biocompatible and Ultrastable Coating for Nanoparticles in Vivo. ACS Nano 2013, 7 (10), 9384–9395. [DOI] [PubMed] [Google Scholar]
- (65).Lee DJ; Lee YT; Zou R; Daniel R; Ko CC Polydopamine-Laced Biomimetic Material Stimulation of Bone Marrow Derived Mesenchymal Stem Cells to Promote Osteogenic Effects. Sci. Rep. 2017, 7 (1), 12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Jun DR; Moon SK; Choi SW Uniform Polydimethylsiloxane Beads Coated with Polydopamine and Their Potential Biomedical Applications. Colloids Surf., B 2014, 121, 395–399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.