Introduction
Osteoarthritis (OA) is a painful degenerative joint disease which in later stages can become severe to the point where the patient is a candidate for joint replacement, a costly and invasive procedure[41]. OA pain is managed by a wide variety of approaches that include lifestyle changes, topical creams and patches, systemic non-steroidal anti-inflammatory drugs (NSAIDs), opioids, intraarticular treatments and surgery[14–16; 41]. Nonetheless, pain from OA frequently remains an intractable problem in a majority of patients, with only 19% reporting that they are satisfied with their current treatment, in part because of unmanaged pain[1; 41].
Selective targeting of nociceptive primary afferents provides profound pain relief similar to what can be achieved with local anesthetics. Specific targeting of nociceptive primary afferents using agonists of the transient receptor potential cation channel V1 (TRPV1) such as resiniferatoxin (RTX) has been shown to block most clinically relevant pain while sparing protective pain sensations such as pinch and compression, as well as other somatic sensations[3; 4; 10; 11; 31; 49]. The molecular mechanism of RTX has been examined in detail[31; 32; 49], and cancer pain studies in canine and human patients demonstrate strong efficacy from intrathecal RTX[9; 10; 31; 49]. The application of RTX can be tailored to the pain problem[26]. In rodents, local injections reversibly lesion TRPV1+ nociceptive primary afferent nerve endings and show strong preclinical efficacy for burn[47], surgical incisional[45], and arthritic pain models[33; 40]. However, the translational potential of rodent models is subject of growing concern[37; 58]. The present study evaluates the efficacy of RTX in naturally-occurring canine OA and the explores the underlying molecular biology of canine dorsal root ganglia using deep RNA sequencing (RNA-Seq). We provide an improved toolset for quantification of nearly all expressed genes to create a comprehensive molecular fingerprint for canine sensory ganglia[36]. Intra-articular administration extends the range of RTX beyond cancer pain control and the transcriptomic profiling of the canine DRG provides the fundamental knowledge necessary to reach beyond reliance on rodent models.
Delineation of the full transcriptome of well characterized organisms such as mouse and human has revealed every molecule made by the dorsal root and trigeminal ganglia. The call for transitional animal models for pain studies, and the shortfall in drug development has exposed a gap at the molecular level[37]. Genomic annotations in several species including the dog are lacking, preventing full utilization of many of the powerful genetic tools available for mouse studies. We present a high-quality alignment of the dog DRG, allowing for molecular characterization of the first neurons in the canine pain circuit. Our results show preclinical evidence for the safety and efficacy of RTX for OA pain, and contribute to a broader foundation for an informed alternatives to rodent pain models. We propose that the overall strategy of transcriptomic assessment and treatments based thereon can serve to enhance predictability and translatability of preclinical animal studies.
Materials and Methods
Sample Population:
Control dogs and dogs with clinically significant osteoarthritis were recruited through a protocol approved by the University of Pennsylvania Institutional Animal Care and Use Committee. Owners received a written description of the protocol and provided written informed consent before their dogs were included for evaluation in the study. Inclusion criteria were animals weighing >10 kg and having a medical history, clinical signs, and physical examination and radiographic findings consistent with osteoarthritis (Figure 1). Arthritis in more than one joint was not criteria for exclusion, however one index joint needed to be identifiable (i.e., the most severely affected joint based on history and physical exam) for injection. All dogs had to score >2.0 on the pain severity domain of the Canine Brief Pain Inventory at the time of screening. Dogs were excluded if they had impairment due to neurologic disease, orthopedic disease (other than osteoarthritis), or any chronic disease for which the dog was receiving daily medication; or had clinically relevant abnormalities detected by complete blood count, and serology. The cohort used for transcriptomic analyses has been described previously[49] and we performed RNA-Seq on lumbar dorsal root ganglia (N=4, L5/L6) from healthy control animals from this study; demographics for these dogs are in Supplementary Table 1.
Figure 1: Lateral radiograph of the arthritic left elbow of a mixed breed male (Dog 7) and right knee of a mixed breed female (Dog 6).
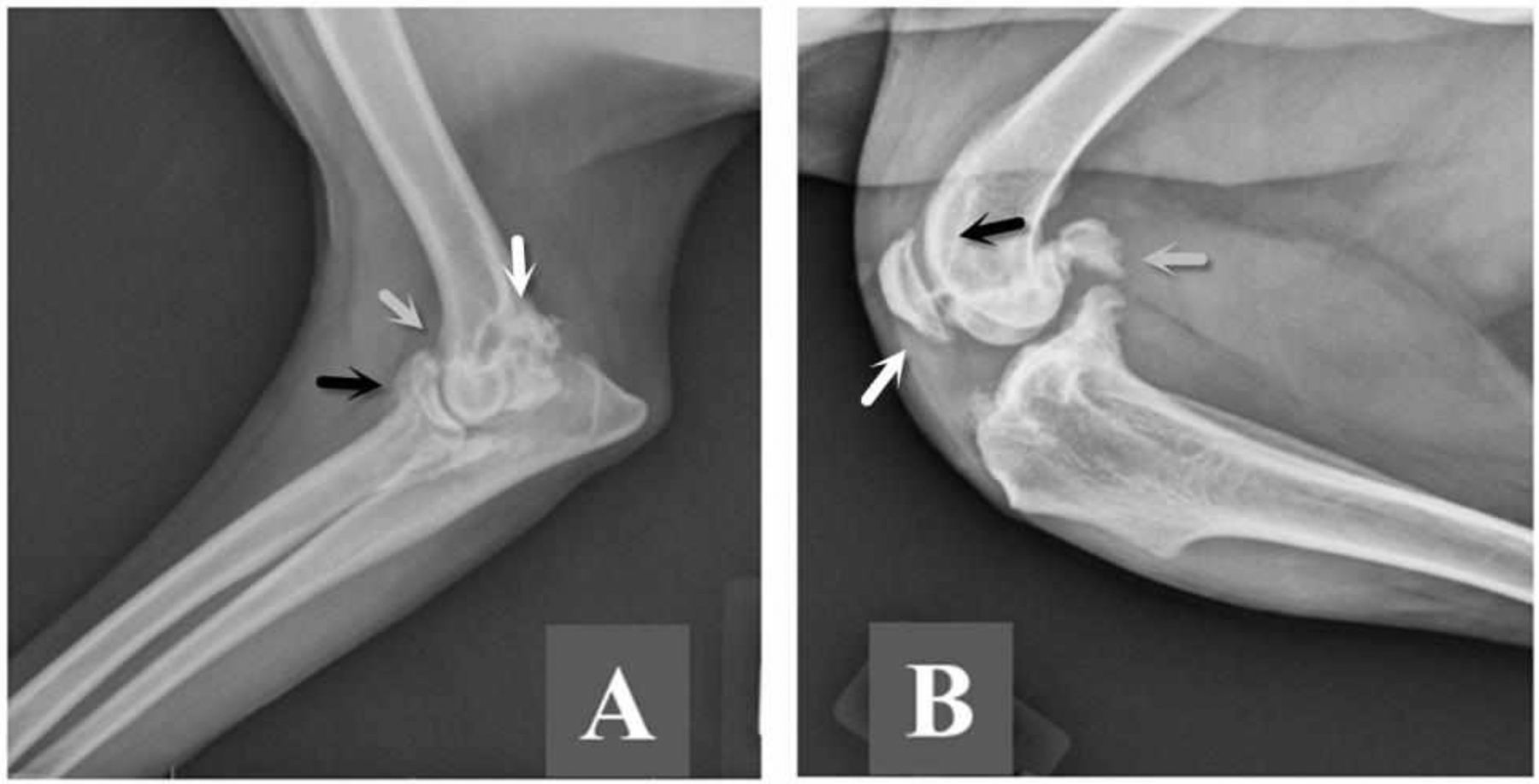
A. There is osteoptlytosis on the proximal aspect of the anconeal process (white arrow), the proximal aspect of the radial head (black arrow), and on the cranial aspect of the humeral condyle (grey arrow). Lateral radiograph of the arthritic right knee. B. There is marked peri-articular osteophytosis of the knee joint including osteophytes on the patella (white arrow), fabella (grey arrow), and trochlear ridge (black arrow).
Outcome Measures:
Gait analysis was performed using a piezoelectric force plate, which is a gold standard method for quantifying lameness in dogs. Dogs were acclimated to the force plate before data collection. Five valid trials for each dog and each limb were recorded. A trial is considered valid when the ipsilateral thoracic and pelvic limbs only, fully contact the force platform and when velocity is controlled at 1.6–1.9 m/s with acceleration controlled at ± 0.5 m/s2 measured by 3 photocells. Ground reaction forces are sampled at 200Hz and recorded with associated software. All forces are normalized to body weight in kilograms. Data from the 5 valid trials for each limb are averaged to obtain a mean peak vertical force value in percent body weight at each time point. Only the results for the most severely affected limb, the one with the joint undergoing intra-articular injection of resiniferatoxin, were included in the analysis. Gait analysis was performed at Day 0 prior to intra-articular injection and Day 21 post injection.
Outcome assessment instruments such as the Canine Brief Pain Inventory, based on the Brief Pain Inventory (BPI) used in human clinical trials, have been developed and validated to capture pain severity and pain impact data in canine studies[5–8]. The Canine BPI is a publically available (www.CanineBPI.com) owner-completed questionnaire designed to quantify the severity and impact of chronic pain in companion dogs. The Canine BPI contains the same two domains as the human BPI: pain severity and pain’s interference with activities of daily living. All 4 questions in the severity domain are identical to those in the human BPI5,6,7. The responses to these are averaged to provide a pain severity score. There are 6 questions in the interference domain pertaining to how the pain interferes with the dog’s typical activities. Three of the questions are identical to the human BPI. The responses to these 6 questions are averaged to provide a pain interference score. Owners completed the Canine BPI at the screening appointment, Day 0 (just prior to intra-articular injection), and at days 2, 7, and 21 after injection.
Continued Follow-up:
Phone contact with the owner continued until owners documented a loss of analgesic efficacy following injection after which, additional standard-of-care analgesics and anti-inflammatories were prescribed as needed.
Intraarticular Injection:
Conscious sedation using 2.3ml/kg propofol IV provides adequate immobilization for intra-articular injection. With the dog in lateral recumbency, the anterior and lateral areas surrounding the joint are clipped free of hair and aseptically prepared with chlorhexidine solution. Two to three ml of a buffered lidocaine solution (9 parts 2% preservative-free lidocaine to 1 part 8.4% sodium bicarbonate making an 18mg/ml lidocaine solution) was used to block the joint 5 minutes prior to intra-articular injection of 10μg of resiniferatoxin in 100μl of vehicle (7.0%Tween 80, 0.05% ascorbic acid in phosphate buffered saline)[11; 49]. Dogs exhibited no signs of discomfort due to the vanilloid agonist activation at injection and were discharged to their owners approximately 30 minutes after injection.
Transcriptomic datasets:
Six RNA-Seq datasets were mined from existing publications[21; 28; 39; 48; 49; 52]. For the dog DRG dataset[49], the raw data was aligned to a novel CanFam3.1 genomic target using MAGIC[36] (available upon request). The quality of this alignment was examined by comparing alignment statistics to a dataset from high quality human DRG samples (Figure 3). While the polyA selection step in library preparation was apparently partial in some samples, the overall quality of the alignment was high.
Figure 3 – Alignment statistics for dog transcriptomic sequence alignment.
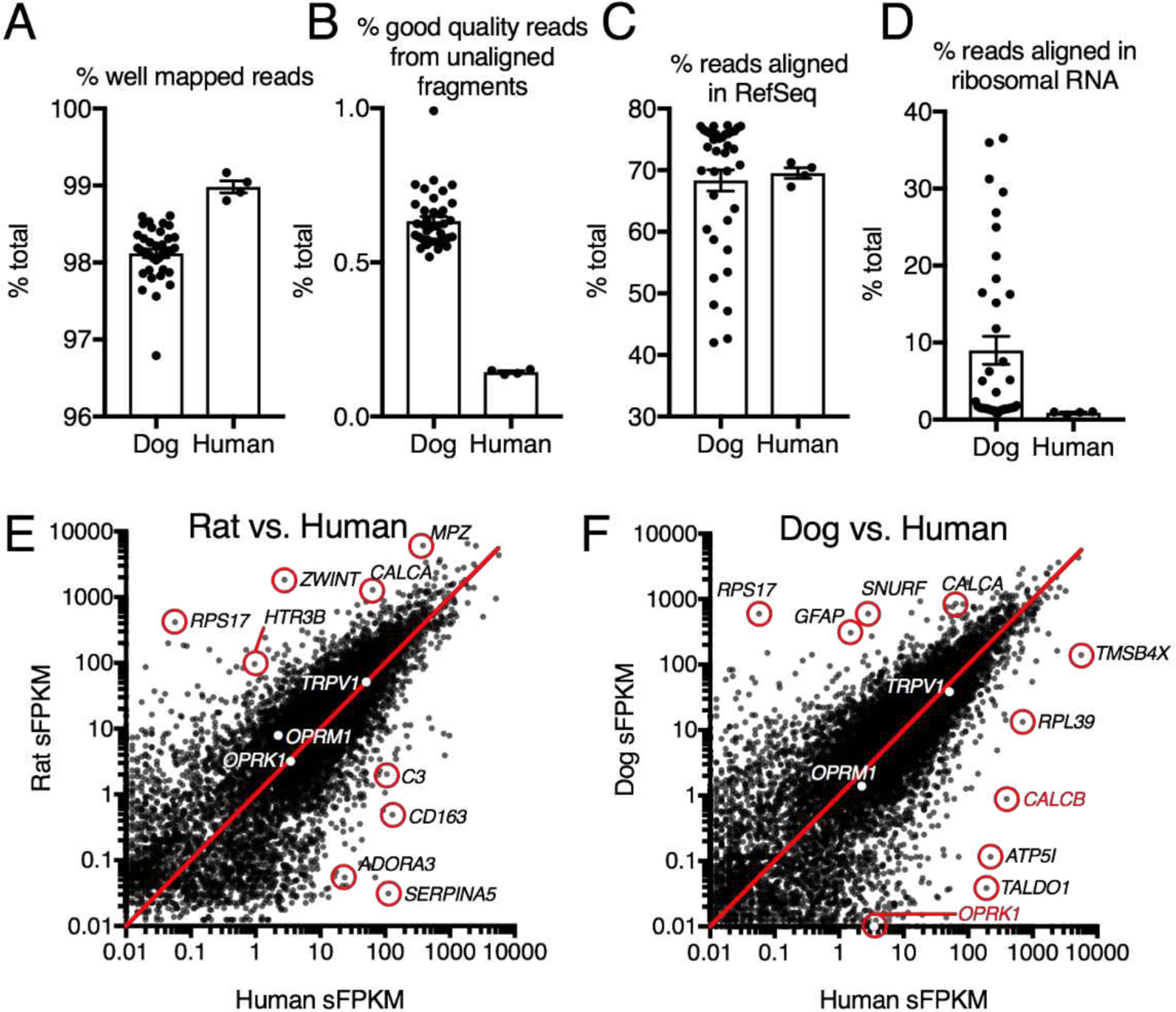
Several parameters were examined to evaluate quality of sequence alignment to the dog genome. Relatively few RNA-Seq experiments have been performed in the dog, leading to potential inaccuracies in the annotation. A. Compared to human DRG approximately the same number of reads were well mapped overall. B. In both dog and human, the majority of good quality reads aligned to the genomic targets used, accounting for 98%+ of high quality reads. C. In most samples, the percentage of reads aligned to RefSeq annotated genes was comparable to human, with some samples showing marked reduction in this parameter. D. Upon further examination, this was due to partial failure of the PolyA selection step in some samples, as indicated by the presence of ribosomal RNA in samples with low RefSeq alignment. E, F. Comparisons were made between the genes expressed in rat and human DRG as well as dog and human DRG. In both species genes such as OPRM1 and TRPV1 were expressed at comparable levels, while other genes such as the adenosine receptor ADORA3 was differential in the rat. In the dog, the kappa opioid receptor (OPRK1) and the protein precursors for the calcitonin gene-related peptide (CALCA and CALCB) were differential.
Several bioinformatics approaches were used to examine TRPV1+ neuronal gene signatures. Heatmaps of expression data were constructed as described previously[12; 49]. Briefly, expression levels were normalized per gene across datasets to examine enrichment in each case/control experiment, as well as enrichment in single cell mouse subpopulations of DRG neurons. These datasets were also compared to human and dog DRG sequencing to explore expression levels in the dog and human. Co-expression plots were created based on a previously published deep-sequencing database of single DRG mouse neurons[39]. Single cells in this database were visualized using the Barnes-Hut implementation of the t-Distributed Stochastic Neighbor Embedding (t-SNE) technique according to their gene expression profiles[53]. Cells with similar gene expression profiles are plotted near each other in the 2-dimensional embedding. Cells were colored based on the expression of select genes (shown in the legend to the right of each panel). Plots were generated using the Rtsne package in R version 3.3.1. Coexpression was further explored in the same dataset using correlograms constructed from cells with > 5 FPKM Trpv1 expression. Percent of cells co-expressing several genes important for clinical pain management was examined. Alignments were constructed using MEGA7[35] by ClustalW methodology. The evolutionary history was inferred by using the Maximum Likelihood method based on the JTT matrix-based model[29].
Statistical Analysis:
Normally distributed continuous variables (pain scores) were evaluated as mean and standard deviation whereas the non-normally distributed continuous variable (peak vertical force) was evaluated as median values and ranges. One-way analysis of variance was used to compare the pain scores over time and the Bonferroni test was used to adjust for multiple comparisons. The Wilcoxon sign-rank test was used to compare Day 0 to Day 21 peak vertical force in the injected leg. Two-tailed assessments were used and p values ≤ 0.05 were considered significant. All statistical analyses in Figure 2 were performed using Stata 11.
Figure 2 -. Metrics of analgesic efficacy in 7 dogs with osteoarthritis treated with a single intra-articular injection of 10mcg of resiniferatoxin (RTX).
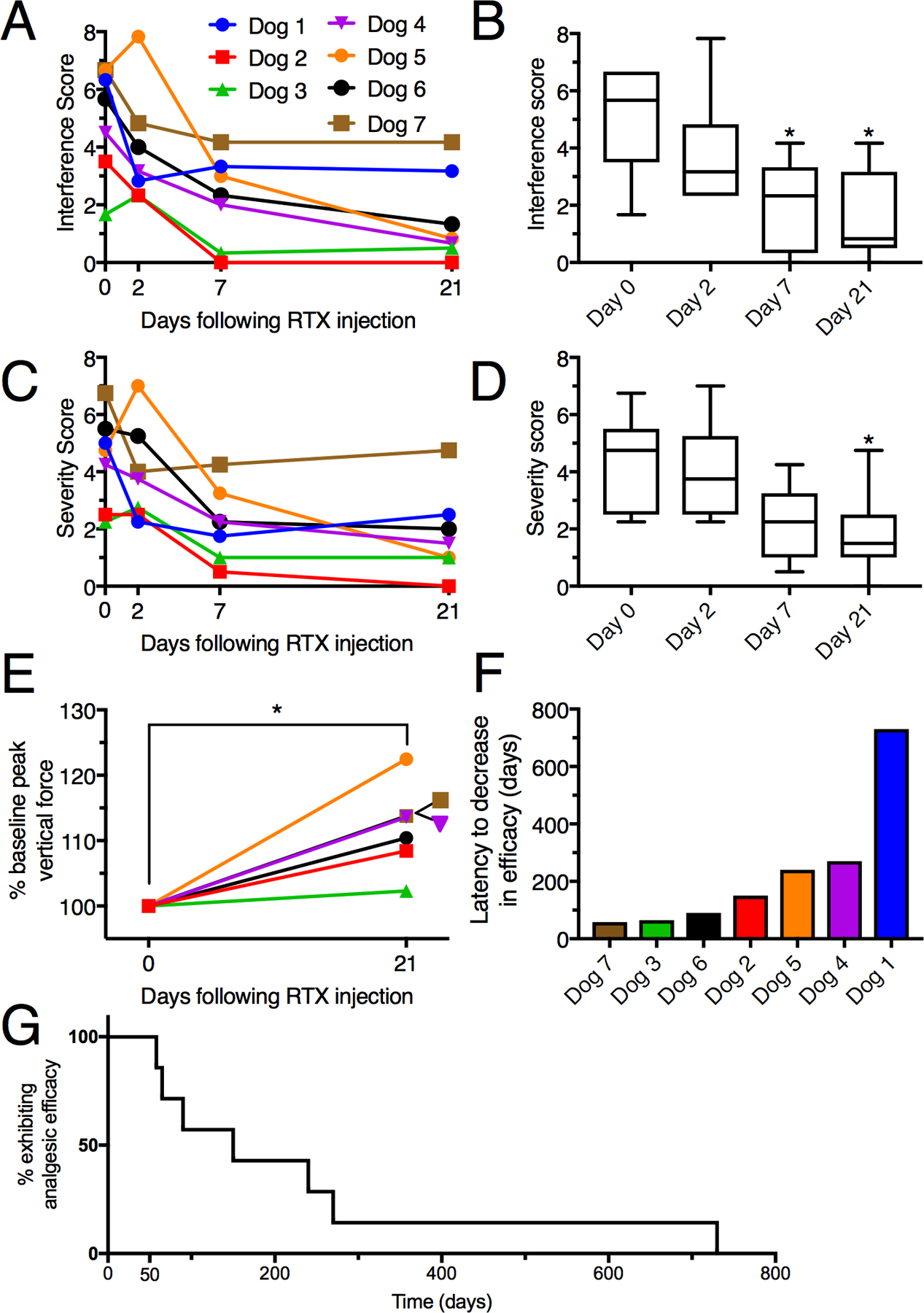
A. Pain interference scores are plotted for each invidivual dog treated with RTX. B. Aggregate data shows a significant decrease in interference scores at day 7 and day 21. C, D. Data are plotted similarly for the Canine Brief Pain Inventory pain severity scores, with a significant reduction observed at day 21 post-treatment relative to pre-treatment values. B, D. Bars show the range of individual values; *, p ≤ 0.05. E. Peak vertical forces were collected from force plate gait analysis in six of the seven dogs with osteoarthritis (5 knees and 1 elbow). There was significant improvement in lameness based on an increase in median peak vertical force in the treated leg from 46.0 (range 33.5 to 90.3) to 51.0 (range 38.0 to 102.7), three weeks following injection (p=0.027, Wilcoxon signed rank test). Statistics were performed on raw values for force measurements. Normalized values are plotted to correct for the wide range of initial force measurements, where each animal’s Day 0 measurements are taken as the 100% value. All six dogs show an improvement, with a median force at Day 21 equal to 112% of that at Day 0. A Mann Whitney test was also significant for normalized values (p=0.0022). F. The median time to owner-reported decrease in analgesic efficacy was 150 days (range 58 to 730 days).
Results
Cohort Characteristics:
Seven dogs comprised the clinical portion of this study (Table 1). Six had osteoarthritis of the knee and one had osteoarthritis of the elbow. Representative radiographs are presented in Figure 1. RNA-Seq transcriptome analysis on lumbar (L5 and L6) DRGs was performed on N=4 control dogs. C8 cervical or T1 thoracic ganglia from N=2 non-arthritic control dogs were also examined[49]. Raw reads and normalized expression values in significant fragments per kilobase per million aligned reads (sFPKM) are presented in the data appendix.
Table 1:
Cohort characteristics of 7 dogs with osteoarthritis treated with intra-articular resiniferatoxin.
| Dog | Breed | Sex | Weight (kg) | Age (years) | Joint Injected | Duration of Signs |
|---|---|---|---|---|---|---|
| 1 | Boxer | Female | 35 | 9 | Knee | 3 years |
| 2 | Mix Breed | Male | 27 | 6 | Knee | 4 years |
| 3 | Mastiff | Female | 46 | 6 | Knee | 2 years |
| 4 | Doberman | Male | 36 | 8 | Knee | 6 months |
| 5 | Rottweiler | Male | 67 | 7 | Knee | 2 years |
| 6 | Mix Breed | Female | 33 | 8 | Knee | 5 years |
| 7 | Mix Breed | Male | 22 | 11 | Elbow | 4 years |
Significant Improvement in Canine Brief Pain Inventory Pain Scores:
Pain scores were normally distributed. Correcting for multiple comparisons, the severity score also was significantly improved 21 days after injection (p=0.024); and the interference score was significantly improved at 7 (p=0.033) and 21 (p=0.006) days after injection compared to day 0. Without correcting for multiple comparisons, pain interference (f=5.85, p<0.004) and pain severity scores (f=4.88, p<0.009) from the Canine Brief Pain Inventory[5–8] were significantly improved following the intra-articular injection of 10μg RTX at 7 and 21 days (Figure 2A–D).
Significant Improvement in Force Plate Gait Analysis:
Peak vertical force data was not normally distributed. Due to the degree of lameness, baseline gait analysis data could not be collected on Dog 1. Force values for the remaining six dogs, were normalized such that baseline values were considered at 100%. The median force at Day 21 was 112%, and all six dogs tested showed an improvement (p=0.027; Two-tailed Mann-Whitney U-test; range 102–122% of baseline values, Figure 2E). There was also a significant (p=0.027) increase in peak vertical force in the treated leg for the raw values before normalization. The effect of a single injection of RTX was long-lived and assessed by the interval between treatment and the owner’s request for retreatment. Owners began to see a decreased analgesic effect in their dogs a median of 150 days after resiniferatoxin injection (range 58 to 730 days; Figure 2F). In addition to this quantitative evidence, several of the owners sent recordings of their animals at various intervals showing qualitative evidence of improvement. Supplementary Video 1 shows 3 clips of Dog 1 obtained pre-treatment and at 3.5 and 12 months following a single injection of RTX. Increased activity is evident at both post-injection recording times. The setting, the high motivational state of the animal and its interaction with the other animal are noteworthy elements of the companion dog model and, while not typical of a laboratory setting, this type of behavior is part of the full experiential context which the owners draw from in making the Canine BPI assessments.
Transcriptomic analyses:
Transcriptional analysis was also performed for the rat, dog and human DRG to examine levels of expression of common analgesic drug targets between species (Table 2). Transcripts encoding several analgesic target proteins and were robustly expressed in all three species. These include the ion channels TRPV1, TRPA1 and NaV1.7, 1.8 and 1.9), the two calcium channel subunits, and the NGF receptor-tyrosine kinase TrkA. The absolute expression level of the GPCRs was comparatively lower, which is a commonly observed feature. Species differences were observed among the prostaglandin receptors. Specifically, the prostaglandin receptor 3 is the most highly expressed prostaglandin receptor in the human, whereas prostaglandin receptor 4 is the most highly expressed in the dog, and all four prostaglandin receptors are expressed at comparable levels in the rat. The kappa opioid receptor (OPRK1) was detected at very low levels in dog DRG. This likely was not due to an alignment error because using the same alignment technique we were able to robustly detect OPRK1 transcripts in dog cortical brain samples.
Table 2. Transcriptomic analyses of DRG expression levels of analgesic target genes in dog and human subjects.
Expression levels for several common analgesic targets are shown for the rat, dog and human DRG.
| Human Gene | Protein name | Analgesic drugs | Rat sFPKM | Dog sFPKM | Human sFPKM |
|---|---|---|---|---|---|
| OPRM1 | Mu opiate receptor | Opiates | 7.94 | 1.40 | 2.23 |
| OPRK1 | Kappa opiate receptor | Opiates | 3.19 | †0.01 | 3.48 |
| OPRD1 | Delta opiate receptor | Opiates | 1.48 | 3.84 | 0.86 |
| TRPV1 | Transient receptor potential vanilloid 1 | Vanilloids | 51.42 | 38.38 | 51.10 |
| TRPA1 | Transient receptor potential ankyrin 1 | TRPA1 antagonists | 23.04 | 24.64 | 15.78 |
| PTGER1 | Prostaglandin E receptor 1 | NSAIDS | 3.55 | 0.60 | 0.10 |
| PTGER2 | Prostaglandin E receptor 2 | NSAIDS | 2.40 | 0.45 | 3.32 |
| PTGER3 | Prostaglandin E receptor 3 | NSAIDS | 4.26 | 0.55 | 29.08 |
| PTGER4 | Prostaglandin E receptor 4 | NSAIDS | 4.35 | 9.66 | 7.62 |
| NTRK1 | TrkA Neurotrophic receptor tyrosine kinase 1 | NGF antibodies | 80.13 | 82.52 | 49.40 |
| CACNA2D1 | CaVα2δ1 Voltage-gated Ca2+ channel subunit | Gabapentinoids | 37.08 | 86.34 | 36.88 |
| CACNA1B | CaV2.2 N-type voltage-dependent Ca2+ channel | Ziconotide | 51.13 | 31.87 | 18.39 |
| SCN9A | NaV1.7 Voltage-gated Na+ channel | Nav1.7 inhibitors | 143.36 | 77.79 | 127.79 |
| SCN10A | NaV1.8 Voltage-gated Na+ channel | Nav1.8 inhibitors | 171.29 | 61.97 | 47.75 |
| SCN11A | NaV1.9 Voltage-gated Na+ channel | Nav1.9 inhibitors | 121.51 | 59.51 | 192.20 |
OPRK1 quantification shows very low expression of this gene in the dog. To ensure proper quantification in our pipeline, samples of dog cortex were analyzed showing an average of 7.9 sFPKM, and indicating that the pipeline is capable of detecting expression of this gene. The dog paralog of OPRK1 is similar to rat and human by protein sequence homology.
Six RNA-Seq datasets were analyzed to present a comprehensive analysis of TRPV1+ sensory neurons affected by RTX treatment, and plotted as a heatmap (Supplementary Figure 1). Within each of the first three datasets (column pairs 1–3), the expression in each left column is plotted as a ratio of the expression of that in the right column pair, according to the flame scale. Rat trigeminal ganglia were treated in vitro with either RTX to kill TRPV1+ neurons, or DMSO (column pair 1)[28]. The genes most highly decreased by RTX treatment are shown, sorted by level of decrease. As sensory ganglia contain non-neural cells, DRG was compared to sciatic nerve (mainly Schwann cells) to reveal the neural subset of genes[48] (column pair 2) and only those genes enriched in DRG vs. sciatic nerve are shown. TRPV1+ lineage neurons were selectively sorted from mouse ganglia, sequenced, and compared to mice in which all of the TRPV1 lineage neurons were genetically ablated[21] (column pair 3). Only the TRPV1 lineage genes are shown. Single cell sequencing data showing fraction of positive cells in each DRG subclass are plotted[52] (column pair 4), with the TRPV1+ populations highlighted. The non-peptidergic 1 population does not express TRPV1 but also shows a similar gene expression pattern[49; 52]. Expression values (sFPKM) for the whole dog DRG and whole human DRG are shown to the right.
Phylogenetic tree analysis:
Expression of transcripts encoding Calcitonin Related Polypeptide Beta (CALCB) were highly divergent between dog and human, with dog transcripts being expressed at much lower levels. Peptide sequence within the protein precursor was aligned between several species, revealing many amino acid differences between dog and human CALCB in the region containing the beta-CGRP peptide. Examination of the evolutionary phylogenetic tree of the full protein precursor was examined, revealing a likely evolutionary divergence in this gene in several species including cats and dogs (Figure 4A and B). A similar analysis was performed for TRPV1 which is much more conserved between mammals and we include avian (chicken) TRPV1 which does not contain the capsaicin binding site for comparison (Figure 4C).
Figure 4. Expression and conservation analysis of selected nociceptive genes between human, dog, rodents, and other species.
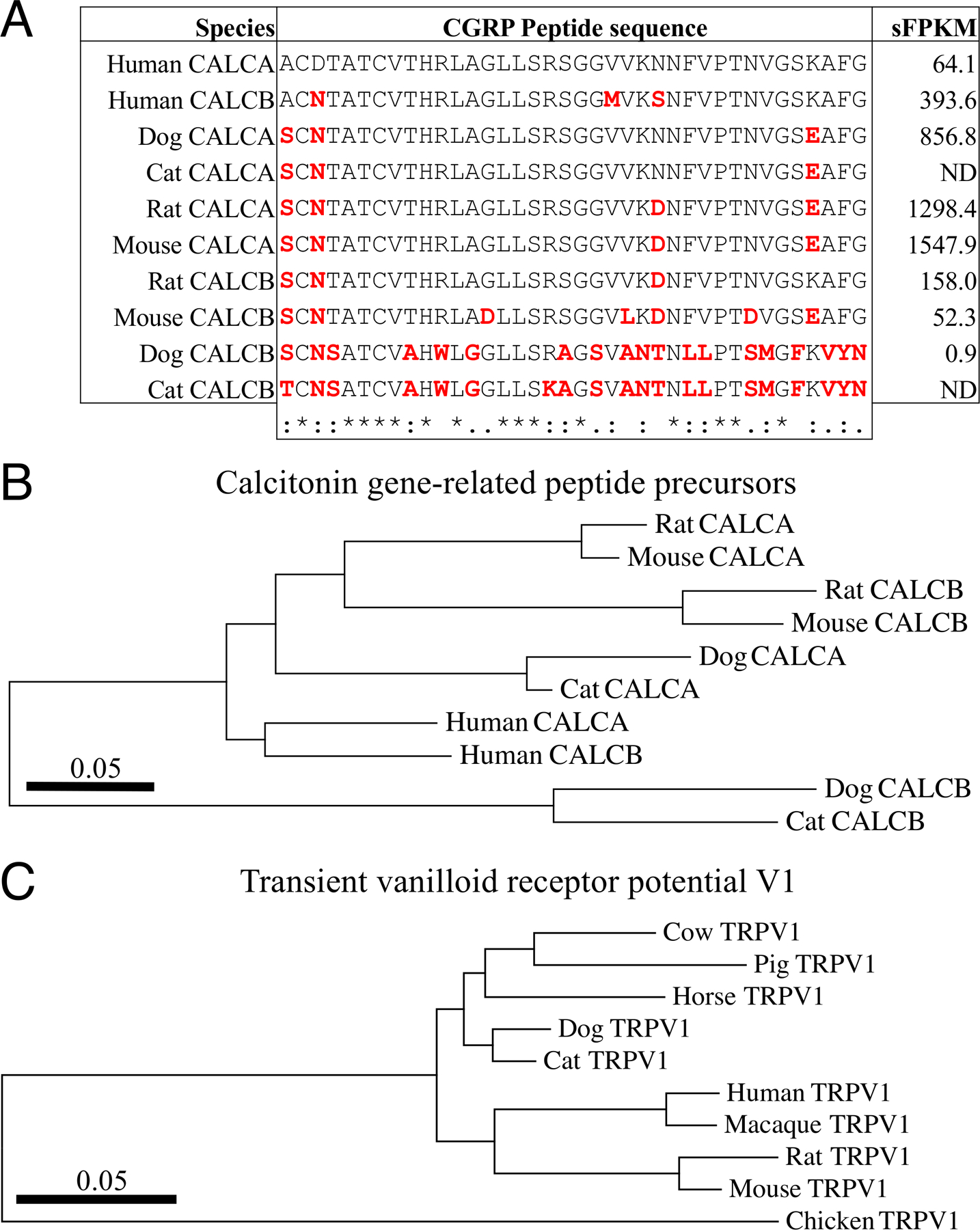
To identify critical species differences in nociceptive transcripts, an initial screen was performed to identify highly divergent transcript expression between human and dog DRG, identifying the CALCB transcript as divergent from human (see Figure 3). A. Human, dog, cat, rat, and mouse CGRP peptides from CALCA and CALCB precursor proteins were aligned. Amino acids that are divergent from Human alpha-CGRP (from CALCA) are highlighted in red. Alignments are clustered based on similarity computed in MEGA7[35]. The expression level (sFPKM, computed in MAGIC[36]) is shown for human, dog, rat and mouse DRG. Notably, the dog DRG expressed very low levels (0.9 sFPKM) of CALCB, while other species express this gene robustly (393.6 in human). B. The evolutionary history was inferred by using the Maximum Likelihood method based on the JTT matrix-based model[29] using the full protein sequence of CALCA and CALCB proteins. The tree with the highest log likelihood (−1115.56) is shown. Evolutionary analyses were conducted in MEGA7[35]. Dog and cat CALCB isoforms are highly divergent from other species with respect to CALCB sequence.
Single-cell transcriptomics:
Using data mined from a publicly available database[39] coexpression of Trpv1 and other key genes in nociceptive neurons were assessed. All of these genes have some degree of coexpression with Trpv1 expressing neurons, indicating multiple subsets of Trpv1+ neurons that express the targets of major analgesic drugs (Supplementary Figure 2). The highest degree of colocalization is observed with the broadly expressed genes Scn9a and Calca, indicating that these genes are highly expressed , robustly detected by single-cell sequencing, and present in many neurons. High expression makes some genes more readily detectable in the single cell format which has limited sequencing depth. Substantial coexpression is also observed in cells expressing the gene encoding the Mu opiate receptor (Oprm1) and the gene encoding the calcium channel putatively targeted by gabapentin (Cacna2d1). Plots were generated using the Rtsne package in R version 3.3.1. Trpv1+ DRG cells from the single-cell RNA-Seq dataset[39] were examined further using correlation plots to look for degree of coexpression (Supplementary Figure 3). Cells with expression >5 FKPM for Trpv1 are shown in red indicating the high level of coexpression of other common analgesic target molecules with Trpv1. Specifically, 35% of Trpv1+ neurons express Oprm1, 54% express Cacna2d1, 86% express Ntrk1, and 98% express Scn9a. These analgesic drug targets are also correlated with each other, indicating that all of these TRPV1+ subgroups can be impacted by RTX. As a technical note, in general, not all genes are detected in every single cell. Higher expressed genes such as Ntrk1 and Scn9a are highly correlated (83.8%) most likely because they are not only expressed but also detected in a large percentage of the single cells.
Discussion
The present study examines one of the most potent and long-lasting known analgesic treatments in a heterogeneous naturally-occurring disease in a veterinary setting. This is in sharp contrast to experimental models which are generally induced acutely, either mechanically or chemically, in a laboratory setting[9; 58]. A single IA injection of RTX elicited marked, long-lasting decreases in pain scores and improvement in limb use (Figure 2). Intraplantar RTX injection in rats leads to calcium cytotoxicity of TRPV1-containing primary afferent terminals within ~10 minutes[43; 56] with recovery over the next ~14 days. Based on this, we had predicted rapid development of analgesia with gradual return of pain as the nociceptive nerve terminals regenerate. However, the analgesia far outlasted our 2–3 week projection (Figure 2F,G). One explanation for the progressive improvement in pain scores over several weeks, as well as the owners’ perception that there was sustained efficacy for many months, could be that, in addition to the direct analgesic action elicited by eliminating the TRPV1-containing primary afferent terminals, RTX treatment blocks local neurogenic inflammation mediated by these afferents[44; 49]. The long duration suggests that RTX treatment may have disease modifying action(s) as well as blocking nociceptive neural activity emanating from the joint. Chronic inflammatory states are partially maintained by neurogenic inflammation, which is triggered by release of neuropeptides from the primary afferent terminals thereby causing plasma extravasation and edema. This process can exacerbate and maintain joint inflammation[38; 42; 54]. It is also important to note that an analgesic effect was documented with intra-articular injection of just one joint (Table 1), despite the fact that all dogs had osteoarthritis in more than one joint. For example, dogs undergoing total hip replacement for advanced coxofemoral OA typically have severe disease in both hips, but only require joint replacement in one to have a significant improvement in outcome[18–20; 55], although this improvement would likely be better if all affected joints were treated, which is not always possible. However, interventional injection of RTX is comparatively less invasive, and is likely a more tractable approach to treating multiple joints than surgical replacement.
Given the number of compounds that fail clinical development due to toxicity or other types of side effects, it is important to note the lack of adverse events documented in these dogs after injection. This is consistent with observations in several previous studies of intrathecal RTX[4; 10; 25; 31; 49]. All dogs recovered uneventfully from the procedure, returning to their owners within 30 min of RTX injection, which contrasts with canine joint replacements which take 3–6 months on average for recovery. In the pain scores, it can be seen that 2 of 7 dogs had higher pain scores two days after injection (Figure 2). We attribute this to two factors: manipulation of the joint during the injection procedure and residual effects of gait assessment, which can cause joint stress. In these moderately to severely affected dogs, dozens of passes over the force plate are usually required to obtain the 10 valid passes needed for analysis. TRPV1 is expressed at low levels in the articular cartilage, but this low level of expression and their overall morphology makes them unlikely to be susceptible to RTX[32] (Supplementary Figure 4).
All of the dogs in this study are now two to three years post IA RTX injection and none have shown signs of accelerated OA progression. Such a long-term adverse event is a major concern with IA corticosteroids and the recently evaluated anti-nerve growth factor agents[22; 50; 51]. In a rat model of OA, chronic pathological joint changes such as bone erosion, trabecular damage, and decrease in bone volume were actually reduced in animals pre-treated with the TRPV1 agonist capsaicin compared to controls[30]. Similar attenuation of arthritic joint swelling and hyperalgesia has been observed in Trpv1 knockout mice[2]. As the presence of inflammatory neuropeptides is associated with increased severity of OA in mice, it was hypothesized that the functional loss of the primary sensory neurons caused by capsaicin leads to a decrease in the neurogenic component of inflammation in the joint and thus protects against the progression of OA[30]. The present study provides additional support for the long-term effects of TRPV1+ nerve terminal ablation.
In order to perform informed preclinical studies in animals, availability of a modern molecular genetic toolset is useful[21; 36; 46; 48; 49; 52]. A high-quality transcriptome of the relevant target tissue of the model organism in comparison to human can be informative for planning and interpretation of these studies. As RTX ablates Trpv1+ nerve terminals, it preempts the need for other peripherally acting analgesics that might have acted on these peripheral nociceptors (Table 2). These targets are highly conserved between dog and human, indicating similar regulatory pathways for modulation of nociception. We present a summary of genes which are highly and selectively expressed by the TRPV1+ neuronal populations targeted by RTX (Supplementary Figure 1), and suggest that these marker genes cluster with, and are co-expressed on the same neurons (Supplementary Figure 2, 3). Additionally, many other analgesic targets are also expressed within the populations of TRPV1+ DRG neurons. These data argue that this population of neurons is the clinically relevant population[4; 10; 26; 27; 31; 49]. The network of genes expressed by these key populations of sensory neurons defines a repertoire of druggable targets for pain control[21; 48; 49; 57], most of which are comparable between dog and human (Table 2).
With the inclusion of the dog DRG data, a transcriptomically informed approach to analgesic mechanisms and drug development is becoming realistic. Assuming that the molecular target of a compound is known, the existence of the drug target within the model animal can be verified and quantitated by RNA-Seq. The presence and conservation of paralogs and other related transcripts that may be relevant to its function are always ascertained because of the comprehensive nature of the technique[36]. Gene similarities to human orthologs should also be examined, with particular attention to the conservation of the drug binding site, if this is known. For example, the capsaicin binding site in TRPV1 is completely absent in chickens, rendering them insensitive to agonist stimulation by capsaicin and RTX. These are important considerations for establishing the validity of the organism as a predictor for the human. The high-quality alignment of the dog DRG transcriptome (Figure 3A–D) can be used as a tool for analgesic drug development in this non-rodent species and provides a dataset to establish inclusion and exclusion criteria. For example, in examining several relevant analgesic genes, we report the lack of conservation in the expression level of CALCB and OPRK1 in the dog DRG (Figure 3F), indicating a substantial species difference. While gene quantification across species is subject to several sources of technical variation, high magnitude changes are generally indicative of biologically meaningful species differences. As single-cell sequencing resources such as the Sequence Read Archive become more widespread, additional examinations can be made into whether the expression of targets of interest is localized to specific cells within the pain circuit, and whether this expression pattern is conserved[13]. These fundamental molecular insights across species are important for interpretation of animal studies in relationship to the human and emphasize that no species in isolation is an ideal model for human physiology.
Canine studies provide an informative transitional bridge to analgesic drug evaluation in humans[23]. We document a significant and prolonged analgesic effect of a single intra-articular injection of 10μg of RTX in naturally-occurring canine OA, which is physiologically, and symptomatically analogous to human OA. These results are also consistent with earlier examinations of the utility of RTX in rodent intra-articular carrageenan model of OA[34]. Our earlier studies in naturally-occurring canine osteosarcoma provided evidence to bring the compound to human clinical trial (ClinicalTrials.gov Identifier: NCT00804154). Dose estimations, and other critical information from previous canine studies translated directly to the human cancer pain trial[24; 49]. This pilot study provides proof-of-concept data for translation to a human OA trial. A lower cost, non-surgical approach to OA pain, such as RTX, may reduce barriers for patient populations that do not extensively seek arthroplasty as a solution[17]. Lastly, intraarticular RTX offers a clinical alternative that uses a relatively simple administration protocol, making it transportable to areas with minimal medical resources.
Supplementary Material
Acknowledgments:
For their assistance with this project, the authors would like to thank Michael DiGregorio CVT, Managing Director; Molly Love RN, Clinical Trials Coordinator; of the Veterinary Clinical Investigations Center, University of Pennsylvania School of Veterinary Medicine, Philadelphia, PA USA, and Jacklyn Gross of the DPM.
Funding:
This study was funded by a generous gift from Woodmere Foundation and by the Department of Perioperative Medicine, Clinical Center, Intramural Research Program, NIH and by a research award to MJI from the NIH Center for Complementary and Integrative Health (1ZIAAT000017-03).
Competing interests:
The authors declare no competing interests at the time of study design, funding, implementation, and analysis. MJI is an inventor on US patent US 8338457 B2, “Selective ablation of pain-sensing neurons by administration of a vanilloid receptor agonist,” issued to the NIH, and is on the scientific advisory board of Ark Animal Health.
References
- [1].National Institutes of Health Consensus Development Conference Statement, NIH Consensus Development Conference on Total Knee Replacement. 2003.
- [2].Barton NJ, McQueen DS, Thomson D, Gauldie SD, Wilson AW, Salter DM, Chessell IP. Attenuation of experimental arthritis in TRPV1R knockout mice. Exp Mol Pathol 2006;81(2):166–170. [DOI] [PubMed] [Google Scholar]
- [3].Bates BD, Mitchell K, Keller JM, Chan CC, Swaim WD, Yaskovich R, Mannes AJ, Iadarola MJ. Prolonged analgesic response of cornea to topical resiniferatoxin, a potent TRPV1 agonist. Pain 2010;149(3):522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brown DC, Agnello K, Iadarola MJ. Intrathecal resiniferatoxin in a dog model: efficacy in bone cancer pain. Pain 2015;156(6):1018–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brown DC, Bell M, Rhodes L. Power of treatment success definitions when the Canine Brief Pain Inventory is used to evaluate carprofen treatment for the control of pain and inflammation in dogs with osteoarthritis. Am J Vet Res 2013;74(12):1467–1473. [DOI] [PubMed] [Google Scholar]
- [6].Brown DC, Boston R, Coyne JC, Farrar JT. A novel approach to the use of animals in studies of pain: validation of the canine brief pain inventory in canine bone cancer. Pain medicine 2009;10(1):133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brown DC, Boston RC, Coyne JC, Farrar JT. Development and psychometric testing of an instrument designed to measure chronic pain in dogs with osteoarthritis. Am J Vet Res 2007;68(6):631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brown DC, Boston RC, Coyne JC, Farrar JT. Ability of the canine brief pain inventory to detect response to treatment in dogs with osteoarthritis. J Am Vet Med Assoc 2008;233(8):1278–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Brown DC, Iadarola MJ. TRPV1 Agonist Cytotoxicity for Chronic Pain Relief: From Mechanistic Understanding to Clinical Application. TRP Channels as Therapeutic Targets: From Basic Science to Clinical Use, 2015. pp. 99–118. [Google Scholar]
- [10].Brown DC, Iadarola MJ, Perkowski SZ, Erin H, Shofer F, Laszlo KJ, Olah Z, Mannes AJ. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology 2005;103(5):1052–1059. [DOI] [PubMed] [Google Scholar]
- [11].Brown JD, Saeed M, Do L, Braz J, Basbaum AI, Iadarola MJ, Wilson DM, Dillon WP. CT-guided injection of a TRPV1 agonist around dorsal root ganglia decreases pain transmission in swine. Sci Transl Med 2015;7(305):305ra145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Burbelo PD, Iadarola MJ, Alevizos I, Sapio MR. Transcriptomic Segregation of Human Autoantigens Useful for the Diagnosis of Autoimmune Diseases. Mol Diagn Ther 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cheng OT, Souzdalnitski D, Vrooman B, Cheng J. Evidence-based knee injections for the management of arthritis. Pain medicine 2012;13(6):740–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chevalier X Intraarticular treatments for osteoarthritis: new perspectives. Curr Drug Targets 2010;11(5):546–560. [DOI] [PubMed] [Google Scholar]
- [16].da Costa BR, Nuesch E, Kasteler R, Husni E, Welch V, Rutjes AW, Juni P. Oral or transdermal opioids for osteoarthritis of the knee or hip. The Cochrane database of systematic reviews 2014(9):CD003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dominick KL, Baker TA. Racial and ethnic differences in osteoarthritis: prevalence, outcomes, and medical care. Ethn Dis 2004;14(4):558–566. [PubMed] [Google Scholar]
- [18].Druen S, Boddeker J, Meyer-Lindenberg A, Fehr M, Nolte I, Wefstaedt P. Computer-based gait analysis of dogs: evaluation of kinetic and kinematic parameters after cemented and cementless total hip replacement. Vet Comp Orthop Traumatol 2012;25(5):375–384. [DOI] [PubMed] [Google Scholar]
- [19].Forster KE, Wills A, Torrington AM, Moores AP, Thomson D, Arthurs G, Brown G, Denny HR, Scott HW, MacQueen I, Dunne J, Onyett J, Walker JD, Prior J, Owen MR, Burton N, Whitelock R, Girling S, Morrison S, Gilbert S, Langley-Hobbs SJ, Gemmill TJ, Innes JF. Complications and owner assessment of canine total hip replacement: a multicenter internet based survey. Vet Surg 2012;41(5):545–550. [DOI] [PubMed] [Google Scholar]
- [20].Gemmill TJ, Pink J, Renwick A, Oxley B, Downes C, Roch S, McKee WM. Hybrid cemented/cementless total hip replacement in dogs: seventy-eight consecutive joint replacements. Vet Surg 2011;40(5):621–630. [DOI] [PubMed] [Google Scholar]
- [21].Goswami SC, Mishra SK, Maric D, Kaszas K, Gonnella GL, Clokie SJ, Kominsky HD, Gross JR, Keller JM, Mannes AJ, Hoon MA, Iadarola MJ. Molecular signatures of mouse TRPV1-lineage neurons revealed by RNA-Seq transcriptome analysis. The journal of pain : official journal of the American Pain Society 2014;15(12):1338–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Habib GS, Saliba W, Nashashibi M. Local effects of intra-articular corticosteroids. Clin Rheumatol 2010;29(4):347–356. [DOI] [PubMed] [Google Scholar]
- [23].Hayashida K Substance P-saporin for bone cancer pain in dogs: can man’s best friend solve the lost in translation problem in analgesic development? Anesthesiology 2013;119(5):999–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Heiss J, Iadarola M, Cantor F, Oughourli A, Smith R, Mannes A. A Phase I study of the intrathecal administration of resiniferatoxin for treating severe refractory pain associated with advanced cancer. Journal of Pain 2014;15(4):S67–S67. [Google Scholar]
- [25].Hockman TM, Cisternas AF, Jones B, Butt MT, Osborn KG, Steinauer JJ, Malkmus SA, Yaksh TL. Target engagement and histopathology of neuraxial resiniferatoxin in dog. Vet Anaesth Analg 2018;45(2):212–226. [DOI] [PubMed] [Google Scholar]
- [26].Iadarola MJ, Gonnella GL. Resiniferatoxin for Pain Treatment: An Interventional Approach to Personalized Pain Medicine. Open Pain J 2013;6:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Iadarola MJ, Mannes AJ. The vanilloid agonist resiniferatoxin for interventional-based pain control. Current topics in medicinal chemistry 2011;11(17):2171–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Isensee J, Wenzel C, Buschow R, Weissmann R, Kuss AW, Hucho T. Subgroup-elimination transcriptomics identifies signaling proteins that define subclasses of TRPV1-positive neurons and a novel paracrine circuit. PloS one 2014;9(12):e115731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci 1992;8(3):275–282. [DOI] [PubMed] [Google Scholar]
- [30].Kalff KM, El Mouedden M, van Egmond J, Veening J, Joosten L, Scheffer GJ, Meert T, Vissers K. Pre-treatment with capsaicin in a rat osteoarthritis model reduces the symptoms of pain and bone damage induced by monosodium iodoacetate. European journal of pharmacology 2010;641(2–3):108–113. [DOI] [PubMed] [Google Scholar]
- [31].Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, Wellisch OM, Neubert JK, Olah Z, Iadarola MJ. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. The Journal of clinical investigation 2004;113(9):1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Karai LJ, Russell JT, Iadarola MJ, Olah Z. Vanilloid receptor 1 regulates multiple calcium compartments and contributes to Ca2+-induced Ca2+ release in sensory neurons. The Journal of biological chemistry 2004;279(16):16377–16387. [DOI] [PubMed] [Google Scholar]
- [33].Kim Y, Kim EH, Lee KS, Lee K, Park SH, Na SH, Ko C, Kim J, Yooon YW. The effects of intra-articular resiniferatoxin on monosodium iodoacetate-induced osteoarthritic pain in rats. Korean J Physiol Pha 2016;20(1):129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kissin EY, Freitas CF, Kissin I. The effects of intraarticular resiniferatoxin in experimental knee-joint arthritis. Anesthesia and analgesia 2005;101(5):1433–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol 2016;33(7):1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].LaPaglia DM, Sapio MR, Burbelo PD, Thierry-Mieg J, Thierry-Mieg D, Raithel SJ, Ramsden CE, Iadarola MJ, Mannes AJ. RNA-Seq investigations of human post-mortem trigeminal ganglia. Cephalalgia 2017:333102417720216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lascelles BDX, Brown DC, Maixner W, Mogil JS. Spontaneous painful disease in companion animals can facilitate the development of chronic pain therapies for humans. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 2018;26(2):175–183. [DOI] [PubMed] [Google Scholar]
- [38].Levine JD, Khasar SG, Green PG. Neurogenic inflammation and arthritis. Ann N Y Acad Sci 2006;1069:155–167. [DOI] [PubMed] [Google Scholar]
- [39].Li CL, Li KC, Wu D, Chen Y, Luo H, Zhao JR, Wang SS, Sun MM, Lu YJ, Zhong YQ, Hu XY, Hou R, Zhou BB, Bao L, Xiao HS, Zhang X. Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res 2016;26(8):967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mapp PI, Kerslake S, Brain SD, Blake DR, Cambridge H. The effect of intra-articular capsaicin on nerve fibres within the synovium of the rat knee joint. J Chem Neuroanat 1996;10(1):11–18. [DOI] [PubMed] [Google Scholar]
- [41].Matthews GL, Hunter DJ. Emerging drugs for osteoarthritis. Expert Opin Emerg Drugs 2011;16(3):479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].McDougall JJ. Arthritis and pain. Neurogenic origin of joint pain. Arthritis research & therapy 2006;8(6):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Neubert JK, Karai L, Jun JH, Kim HS, Olah Z, Iadarola MJ. Peripherally induced resiniferatoxin analgesia. Pain 2003;104(1–2):219–228. [DOI] [PubMed] [Google Scholar]
- [44].Neubert JK, Mannes AJ, Karai LJ, Jenkins AC, Zawatski L, Abu-Asab M, Iadarola MJ. Perineural resiniferatoxin selectively inhibits inflammatory hyperalgesia. Molecular pain 2008;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Raithel SJ, Sapio MR, LaPaglia DM, Iadarola MJ, Mannes AJ. Transcriptional Changes in Dorsal Spinal Cord Persist after Surgical Incision Despite Preemptive Analgesia with Peripheral Resiniferatoxin. Anesthesiology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ray P, Torck A, Quigley L, Wangzhou A, Neiman M, Rao C, Lam T, Kim JY, Kim TH, Zhang MQ, Dussor G, Price TJ. Title: Comparative transcriptome profiling of the human and mouse dorsal root ganglia: an RNA-seq-based resource for pain and sensory neuroscience research. Pain 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Salas MM, Clifford JL, Hayden JR, Iadarola MJ, Averitt DL. Local Resiniferatoxin Induces Long-Lasting Analgesia in a Rat Model of Full Thickness Thermal Injury. Pain medicine 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sapio MR, Goswami SC, Gross JR, Mannes AJ, Iadarola MJ. Transcriptomic analyses of genes and tissues in inherited sensory neuropathies. Experimental neurology 2016;283(Pt A):375–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sapio MR, Neubert JK, LaPaglia DM, Maric D, Keller JM, Raithel SJ, Rohrs EL, Anderson EM, Butman JA, Caudle RM, Brown DC, Heiss JD, Mannes AJ, Iadarola MJ. Pain control through selective chemo-axotomy of centrally projecting TRPV1+ sensory neurons. The Journal of clinical investigation 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Seidel MF, Lane NE. Control of arthritis pain with anti-nerve-growth factor: risk and benefit. Curr Rheumatol Rep 2012;14(6):583–588. [DOI] [PubMed] [Google Scholar]
- [51].Syed HM, Green L, Bianski B, Jobe CM, Wongworawat MD. Bupivacaine and triamcinolone may be toxic to human chondrocytes: a pilot study. Clin Orthop Relat Res 2011;469(10):2941–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nature neuroscience 2015;18(1):145–153. [DOI] [PubMed] [Google Scholar]
- [53].van der Maaten L Accelerating t-SNE using Tree-Based Algorithms. J Mach Learn Res 2014;15:3221–3245. [Google Scholar]
- [54].Vilensky JA, Cook JA. Neurogenic acceleration of osteoarthritis. Curr Opin Rheumatol 1998;10(3):251–255. [DOI] [PubMed] [Google Scholar]
- [55].Warnock JJ, Dyce J, Pooya H, Schulz KS. Retrospective analysis of canine miniature total hip prostheses. Vet Surg 2003;32(3):285–291. [DOI] [PubMed] [Google Scholar]
- [56].Yang HY, Mitchell K, Keller JM, Iadarola MJ. Peripheral inflammation increases Scya2 expression in sensory ganglia and cytokine and endothelial related gene expression in inflamed tissue. Journal of neurochemistry 2007;103(4):1628–1643. [DOI] [PubMed] [Google Scholar]
- [57].Yekkirala AS, Roberson DP, Bean BP, Woolf CJ. Breaking barriers to novel analgesic drug development. Nature reviews Drug discovery 2017;16(8):545–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Yezierski RP, Hansson P. Inflammatory and Neuropathic Pain From Bench to Bedside: What Went Wrong? The journal of pain : official journal of the American Pain Society 2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


