Abstract
Over the last decade, great achievements have been made in the field of direct epigenetic reprogramming, which converts one type of adult somatic cells into another type of differentiated cells, such as direct reprograming of fibroblasts into cardiomyocytes, without passage through an undifferentiated pluripotent stage. Discovery of direct cardiac reprogramming offers a promising therapeutic strategy to prevent/attenuate cardiac fibrotic remodeling in a diseased heart. Furthermore, in vitro reprogramming of fibroblasts into cardiomyocyte-like cells provides new avenues to conduct basic mechanistic studies, to test drugs, and to model cardiac diseases in a dish. Here, we describe a detailed step-by-step protocol for in vitro production of induced cardiomyocyte-like cells (iCMs) from fibroblasts. The related procedures include high-quality fibroblast isolation of different origins (neonatal cardiac, tail-tip, and adult cardiac fibroblasts), retroviral preparation of reprogramming factors, and iCM generation by fibroblast reprogramming via retroviral transduction of Gata4, Mef2c and Tbx5. A detailed written protocol will help many other laboratories, inexperienced in this area, to use and further improve this technology in their studies of cardiac regenerative medicine.
Keywords: Cardiomyocytes, Fibroblasts, Epigenetic reprogramming, Transdifferentiation, Transcription factors, Heart regeneration, Cell therapy
1. Introduction
Cardiovascular diseases remain the leading cause of death worldwide [1]. Coronary artery disease is the most common form of cardiovascular diseases, resulting in the loss of cardiomyocytes (CMs) at the site of ischemic injury. To compensate for the loss of CMs, cardiac fibroblasts quickly respond to injury and initiate cardiac remodeling in an injured heart [2–4], which leads to dysfunction of the heart and eventually a heart failure. Heart transplantation remains the final solution for patients with an end-stage heart failure, but is limited by the shortage of donor organs. Cellular therapy offers more accessible options for a broader group of coronary heart patients and prevents a diseased heart from end-stage failure, and has been investigated with different strategies, including transplantation of autologous adult stem cells [5, 6] or CMs derived from embryonic stem cell (ESC) or induced pluripotent stem cell (iPSC) [7, 8], activation of endogenous progenitors [9–11], cell-cycle reentry of adult CMs [12–14], and direct epigenetic reprogramming [15–17].
Since lost CMs in an injured heart are replaced by cardiac fibroblasts it will be a promising therapy for cardiac regenerative medicine if the proliferated cardiac fibroblasts can be transdifferentiated into functional CMs. Transdifferentiation was initially reported in the early 1990s that MyoD alone converts fibroblasts and epithelial cells into skeletal muscle cells [18]. After decades of efforts, the discovery of iPSCs [19] demonstrated that, rather than a single transcription factor, a combination of several defined transcription factors might be required to directly convert a type of terminally-differentiated somatic cells into another type of cells. Indeed, in 2010, Ieda et al. [15] successfully identified a combination of three transcription factors (GMT: Gata4, Mef2c, and Tbx5) that can convert cardiac fibroblasts directly into induced cardiomyocyte-like cells (iCMs) without going through an intermediate pluripotency or progenitor state. Soon after the first discovery, in vitro mouse cardiac reprogramming by different combinations of defined factors [17, 20–22], in vivo mouse iCM-reprogramming [16, 17, 23], and in vitro human iCM-reprogramming [24–27] had been achieved in different laboratories around the world. Since then, many efforts have been invested to improve the efficiency and efficacy of reprogramming by manipulations of signaling pathways [28–30] epigenetic barriers [31], cell cycle regulation [32, 33], chemokine signaling [34], and inflammatory immune signaling [35]. Those progresses have been reviewed in recent publications [36, 37].
Despite the success of multiple groups, it remains challenging to achieve a high efficiency of in vitro reprogramming, which requires high quality of cultured fibroblasts and robust expression of all reprogramming factors in individual fibroblasts [38]. In vitro iCM reprogramming has significant advantages to study mechanisms of epigenetic reprogramming, to test chemicals for drug development, and to model cardiac diseases in a dish in the future; therefore, the detailed written protocol is important to help many research laboratories master it as well as to inspire further refinement of this technology.
This chapter provides step-by-step protocols for: 1) isolation and culture of high-quality starting cells for reprogramming (i.e. neonatal cardiac, neonatal tail tip, and adult cardiac fibroblasts); 2) preparation of high-titer retroviruses encoding the reprogramming factors; and 3) generation of iCMs from fibroblasts transduced with retroviruses encoding GMT.
2. Material
αMHC-GFP transgenic mice (Gladstone Institutes, Dr. Deepak Srivastava laboratory) (see Note 1).
Anti-Mouse CD90.1 (Thy-1.1) APC.
Blasticidin.
Fetal bovine serum (FBS) (Hyclone, Cat.# SH30910).
FuGENE® 6 Transfection Reagent.
FACS buffer: 2% FBS in PBS with 2 mM EDTA, store at 4 °C.
Fibroblast Explant Medium: Combine 395 mL of Iscove’s Modified Dulbecco’s Medium (IMDM), 100 mL FBS, and 5 mL Penicillin/Streptomycin. Store at 4 °C. Warm up before use.
Gelatin 0.1% (wt/vol) solution
iCM-Reprogramming Medium: combine 355 mL DMEM, 85 mL Medium 199 (M199), 50 mL FBS, 5 mL non-essential amino acids, and 5 mL Penicillin/Streptomycin. Store at 4 °C. Warm up before use.
Nalgene syringe filter, 0.45-μm pore-size, SFCA-membrane.
Opti-MEM I reduced-serum medium.
PBS without Ca2+ and Mg2+.
Plat-E Medium: combine 445 mL Dulbecco’s Modified Eagle Medium (DMEM) with L-Glutamine, 5 mL Non-essential amino acid solution (NEAA, 100X), and 50 mL FBS. Store at 4°C. Warm up before use.
Hexadimethrine bromide (Polybrene).
Propidium iodide.
Puromycin.
0.05% (wt/vol) Trypsin/EDTA.
Plasmids: pMX-Gata4, -Mef2c, -Tbx5 and -dsRed (Gladstone Institutes, Dr. Deepak Srivastava laboratory)
3. Method
To achieve a high efficiency of iCM-reprogramming, it requires freshly-purified fibroblasts with high quality and fresh retroviruses with high titers; therefore, all experiments should be coordinated and started as planned (Figure 1).
Figure 1.
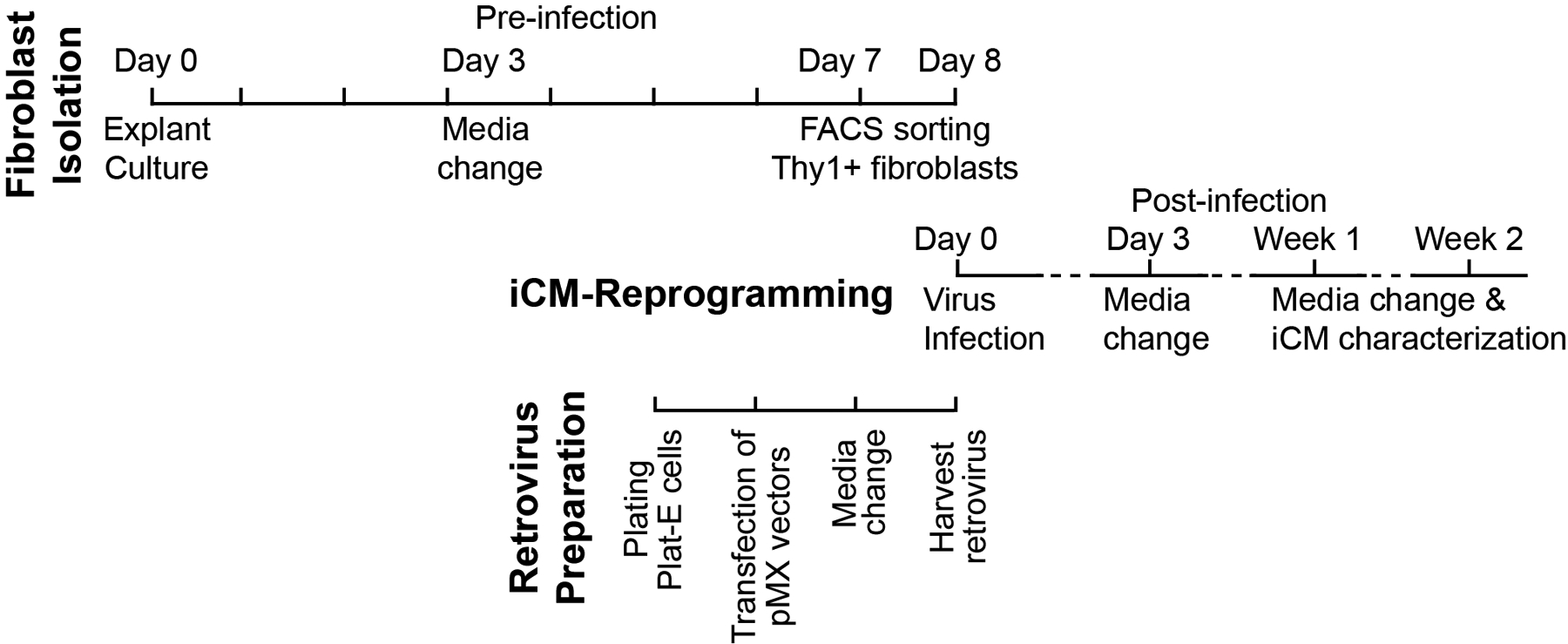
Scheme of producing cardiomyocytes by epigenetic reprogramming of fibroblasts, including fibroblast isolation, retrovirus preparation and reprogramming of induced cardiomyocytes (iCMs).
3.1. Fibroblast isolation by explant-culture method
Contamination could occur in primary cell culture from freshly-harvested tissues; therefore, all surgical tools (i.e., scissors and forceps) must be sterilized before experiments. Cell isolation should be carried out in a sterile biosafety level II tissue culture hood.
Coat cell-culture dishes with 0.1% gelatin solution at 37 °C for >1 hour (see Note 2).
Dissect hearts and/or collect tail-tips from neonatal pups (between postnatal 0.5 to 1.5 days) or adults (age: >8weeks) of the αMHC-GFP transgenic mice. Put the harvested hearts/tail tips in cold PBS and keep them on ice before fibroblast isolation (see Note 3).
Use a fluorescent dissecting microscope to examine for GFP fluorescence in the hearts and identify αMHC-GFP+ animals. After identification of the αMHC-GFP+ animals, use the tissues of heart or tail-tip from the αMHC-GFP+ mice for fibroblast isolation (see Note 4).
Right before cell isolation, use a curved forceps to transfer the tissues (i.e., hearts and/or tail-tips) of αMHC-GFP+ mice into a dish that contains 70% ethanol. After 3 to 5 seconds in the 70% ethanol, quickly transfer the tissues into another dish containing cold PBS to wash out the residual ethanol, and then transfer the tissues into a third dish containing cold PBS. These wash steps could reduce the chance of bacterial contamination of the cell culture.
Transfer hearts or tail tips into a 35-mm dish by a curved forceps and mince tissues into small pieces with a curved scissors (see Note 5).
Add proper amount of the fibroblast explant medium to the minced tissues (see Note 6), and then dissociate the tissue pieces by pipetting up and down gently.
Aspirate the coating gelatin solution from the dishes, and then plate the suspended minced tissues evenly on the coated dishes (see Note 7).
Carefully transfer the dishes into a cell-culture incubator (37 °C, 5% CO2) and culture for 2–4 hours to allow the minced tissue to settle (see Note 8).
Carefully take out the dishes from the incubator, attach the pipette on the wall of the dish and add 9 mL of the fresh fibroblast explant medium VERY GENTLY into a 100-mm dish, or 3 mL media for a 60-mm dish (see Note 9).
Carefully and slowly transfer dishes into the incubator and avoid any turbulence. Culture the tissues for 3 days WITHOUT any disturbance.
Afterward, take out the dishes and move dishes back and forth gently to suspend the unattached tissues (see Note 10). Aspirate media along with those unattached tissues. Attach the pipette on the wall of the dish and gently add fresh fibroblast explant medium (10 mL per 100-mm dish or 3 mL per 60-mm dish).
Culture for 3 to 4 days to allow more fibroblasts to migrate out from the attached tissues (see Note 11). Figure 2 shows representative images of fibroblasts from the neonatal hearts, neonatal tail-tip tissues, and adult hearts 7 days after explanation.
Figure 2.
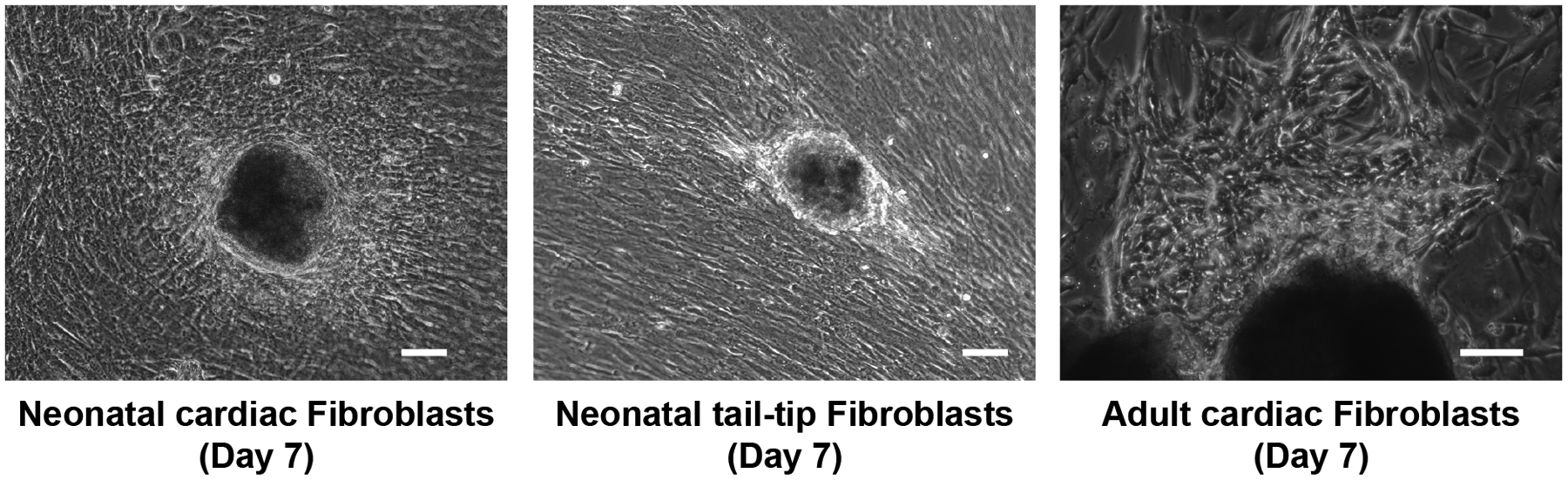
Representative pictures of cultured neonatal cardiac fibroblasts and tail-tip fibroblasts, and adult cardiac fibroblasts after 7 days of explant culture. Bars indicate 50 μm.
3.2. Fibroblast purification by fluorescence-activated cell sorting (FACS)
The health and freshness of the fibroblasts are critical to achieve a high efficiency of iCM reprogramming; therefore, explant-cultured fibroblasts are generally purified by FACS at Day 7 and could be also performed at Day 6 if there are plenty of fibroblasts from cultured tissues. Fibroblasts from the explanted tissue dishes (P0 fibroblasts) should be used for reprogramming (see Note 12).
Aspirate culture media, and add 5 mL PBS into one 100-mm dish of cultured cells to wash out the residual culture media. Aspirate PBS and repeat PBS wash twice.
Aspirate PBS, and add 2 mL Trypsin (0.05%) into the 100-mm dish. Digest for 5 minutes at 37 °C. Take out the dishes from the incubator, and gently pat the wall of the dishes. Examine the cells under a microscope to check if over 80% of the cells become round in shape and are detached from or loosely attached to the dishes.
Quench the digestion with 8 mL of fibroblast explant medium, and pipette up and down to break up clumps into single cells.
Harvest the digested cells, and pass the cells through a 40-μm cell strainer to remove undigested cell clumps and collect the filtered single cells into a 15-ml tube. Centrifuge the fibroblasts for 3 minutes at 300× g.
Aspirate off the media, and resuspend the cell pellet in 5 mL PBS. Centrifuge the cells for 3 minutes at 300× g.
Resuspend the cell pellet in 1 mL FACS buffer (see Note 13). Take an aliquot of the resuspended cells (25 μL or 50 μL). Add this aliquot into 0.5 mL FACS solution and keep it on ice without staining of Thy1, which will be used as the negative control in FACS assay.
Add 20 μL anti-mouse Thy1-APC (1:50 dilution) in the resuspended cells, and mix gently by finger tapping. Protect the tube from light, and incubate for 30 minutes at room temperature.
Add 9 mL PBS, and centrifuge the cells for 3 minutes at 300× g. Repeat PBS wash twice.
Resuspend the cells in 1 mL FACS buffer, and add propidium iodide (50 μg/mL) into the Thy1-stained fibroblasts and the aliquoted unstained cells. Keep the stained cells on ice and protect them from light until FACS sorting. In general, more than 70% of the cells should be stained as Thy1+/αMHC-GFP− fibroblasts (Figure 3), which will be sorted and collected into a tube with 0.5 mL of the fibroblast explant medium.
Centrifuge the purified Thy1+/αMHC-GFP− fibroblasts for 3 minutes at 300× g, and then resuspend the cells with the fibroblast explant media; plate the fibroblasts in a gelatin-coated 6-well plate at a density of 104/cm2, at around 1–1.2×105/per well of 6-well plate in 2 mL media. Those sorted fibroblasts will be used for iCM reprogramming the next day by retroviral transduction of GMT.
Figure 3.
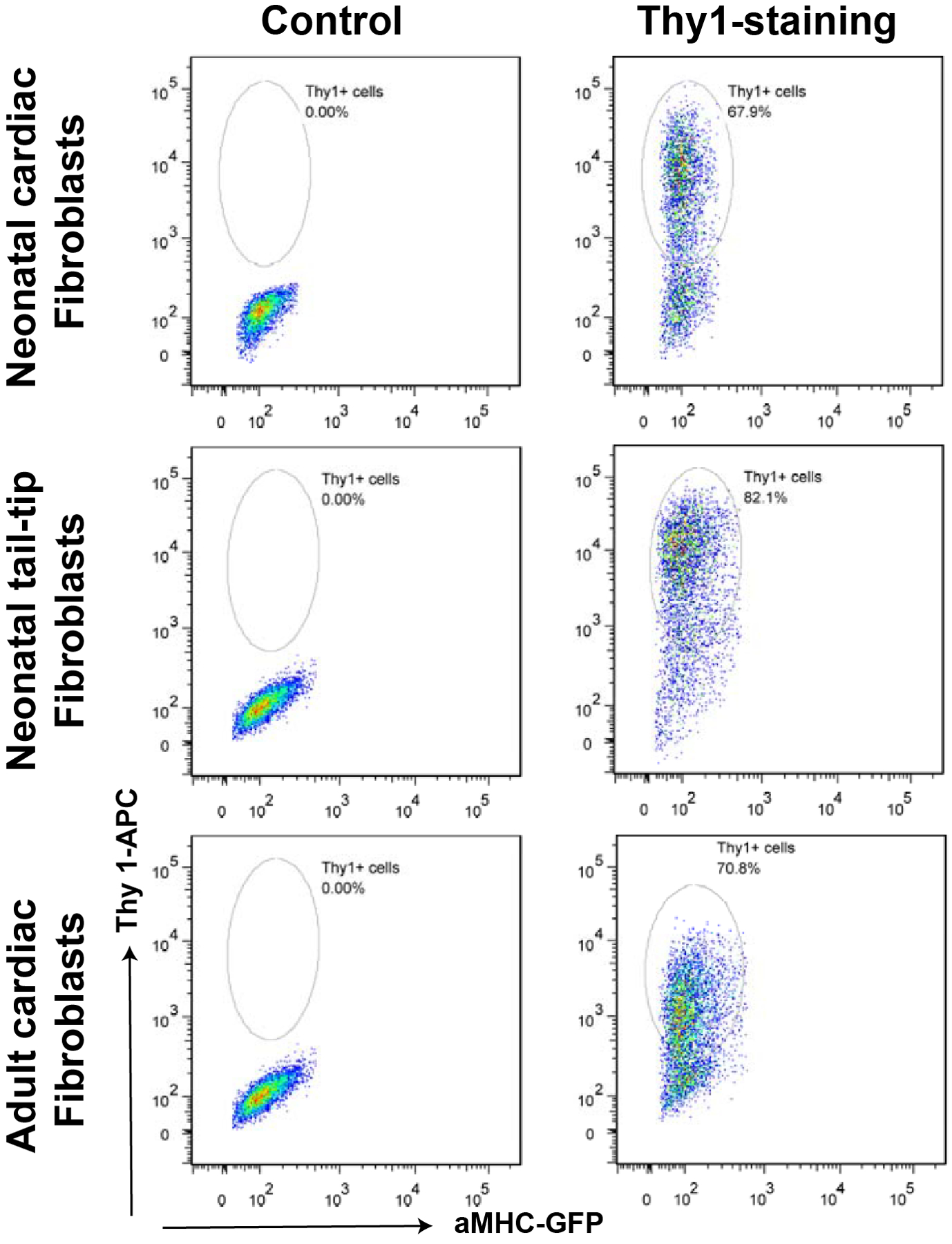
Representative plots of FACS assays after Thy1-APC staining to purify fibroblasts.
3.3. Retrovirus preparation
The platinum-E (Plat-E) cells, a derivative of the HEK293T cell line, was established by using the packaging constructs with the EF1α promoter, which ensures stable and robust expression of the retroviral structure proteins (gag, pol, and ecotropic env). In order to maintain robust expression of those retroviral structure proteins, the Plat-E cells are cultured with the Plat-E cell medium in the presence of puromycin (1 μg/mL) and blasticidin (10 μg/mL), which will selectively kill cells that have lost the expression of the packaging constructs. The Plat-E cell line is designed for rapid and transient production of high-titer ecotropic retroviruses (see Note 14).
Aspirate culture media and add 5 mL PBS into the cultured Plat-E cells to wash out the residual culture media; aspirate PBS and repeat PBS wash once again.
Aspirate PBS and add 2 mL Trypsin (0.05%) into the 100-mm dish; digest the Plat-E cells for 5 minutes at 37 °C. Quench the digestion with 8 mL of the Plat-E cell medium, and pipette up and down to break up clumps into single cells.
Collect the digested Plat-E cells into a 15-ml tube, and centrifuge for 3 minutes at 300× g.
Resuspend the Plate-E cells in the Plat-E cell medium, and count cell numbers.
Plate 8×106 cells in one gelatin-coated 100-mm dish in the Plat-E cell medium without any antibiotics (see Note 15).
- Transfect Plat-E cells in the next day (see Note 16) by using FuGENE® 6 system (see Note 17). Here is an example for transfection of a 100-mm dish, which produces 10 mL of retroviral supernatants of one reprogramming factor (see Note 18).
- For each retroviral vector of GMT factors, add 300 μL of Opti-MEM I into a 1.5-ml Eppendorf tube; add 27 μL Fugene® 6 in Opti-Mem, and mix gently by finger tapping. Incubate for 5 minutes at room temperature.
- Add 9 μg of one retroviral vector DNA (i.e., pMXs-Gata4, -Mef2c, -Tbx5 or -dsRed), and mix thoroughly by finger tapping. Incubate for 15 minutes at room temperature.
- Add the DNA-FuGENE complex into the culture media dropwise, and move the dishes back and forth gently to mix the DNA-FuGENE complex with the media in the dishes. Culture the transfected cells overnight.
In the morning of the next day after transfection, remove the spent medium (see Note 19), and add 10 mL of fresh pre-warmed Plat-E cell medium. Culture the transfected Plat-E cells for additional 24 hours to produce high-titer viruses.
Harvest and filter retroviral supernatants through a Nalgene syringe filter (0.45-μm pore-size, SFCA-membrane) using a 10-mL sterile disposable syringe to remove the cell debris.
Add polybrene (final concentration 5 μg/mL) into the filtered virus-containing supernatant, and mix gently by pipetting up and down. To make a retroviral reprogramming cocktail, add equal amount of retroviral supernatant of each factor (i.e. Gata4, Mef2c, and Tbx5 in our study), and mix.
3.4. Production of iCMs from mouse fibroblasts by retroviral transduction of GMT
Aspirate the medium from the cultured FACS-purified fibroblasts from Step 10 of Section 3.2. Add 0.5 mL of the fresh iCM reprogramming medium with polybrene (5 μg/mL) into each well of fibroblasts.
Add 1.5 mL of the retroviral cocktail of the three factors into each well of fibroblasts, i.e., 0.5 mL retrovirus of each factor (Gata4, Mef2c, and Tbx5) (see Note 20).
Culture the cells overnight. After 24 hours, remove the transduction medium (see Note 19), and add 2 mL fresh iCM reprogramming medium into one well of a 6-well plate.
Change medium every 2–3 days. Examine the progress of iCM reprogramming by checking for αMHC-GFP+ cells under a fluorescence microscope (Figure 4A) (see Note 21).
Determine the reprogramming efficiency by FACS assay to quantify the number of αMHC-GFP+ cells, or by quantification of cells positive for cardiac troponin-T (cTnT) via immunostaining (Figure 4B).
Evaluate the reprogramming by examining the expression of the cardiac enriched genes (e.g., α-actinin, cTnT) and the fibroblast-enriched genes at different time points after retroviral transduction. For examples, αMHC-GFP+ and GFP− cells can be purified by FACS sorting and used to profile gene expression of the reprogrammed iCMs by RNA-microarray or RNA-sequencing; activation of the cardiac genes and inactivation of the fibroblast-enriched genes (see Note 22) can be validated by qRT-PCR.
Conduct functional characterization of iCMs, such as Ca2+ transients, action potential and cell contraction, at 2 weeks to 8 weeks after retroviral transduction (see Note 23).
Figure 4.
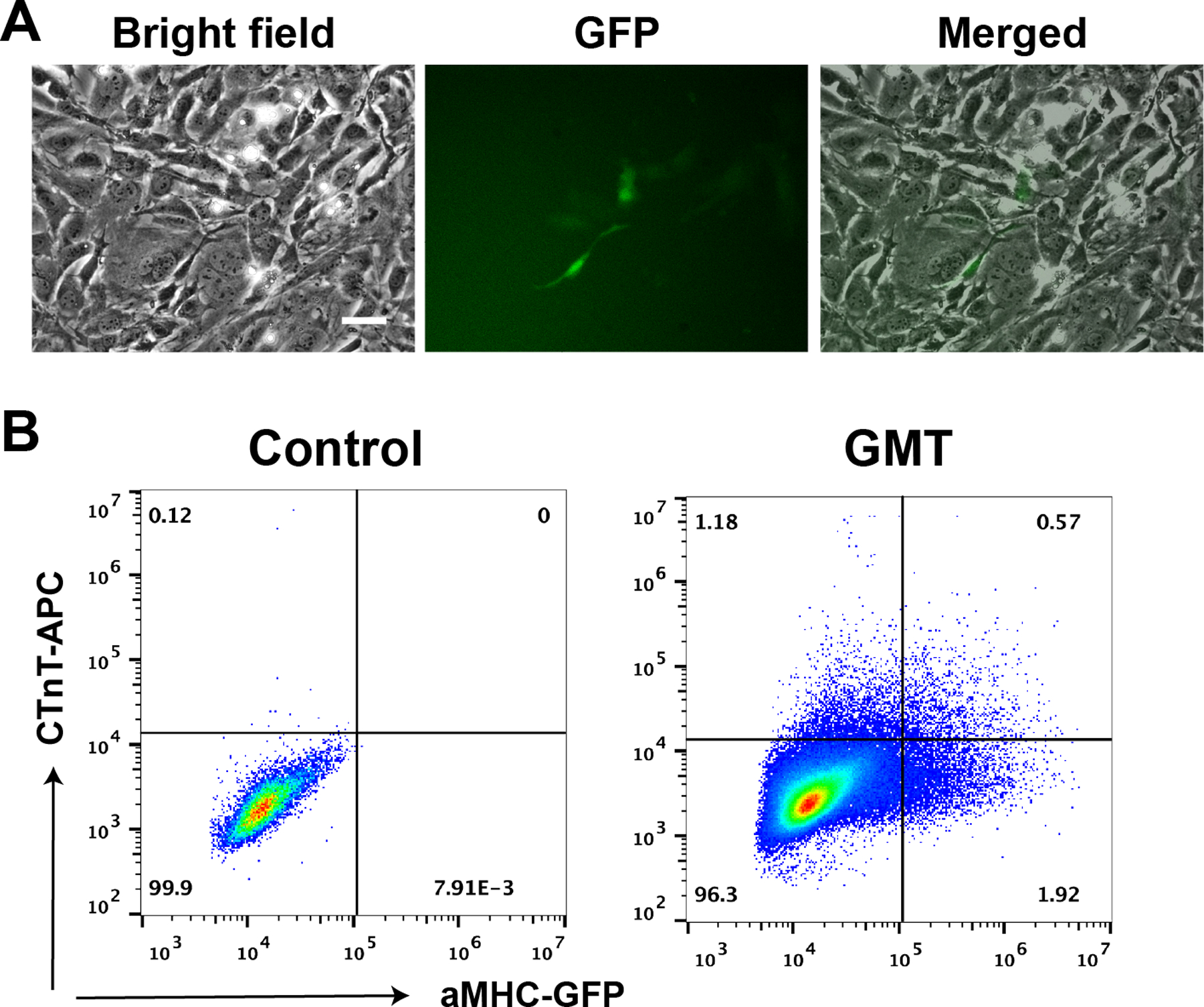
A) Representative pictures of reprogrammed αMHC-GFP+ iCMs from neonatal cardiac fibroblasts three days after retrovirus infection of Gata4, Meft2c and Tbx5 (GMT). Bar indicates 50 μm. B) FACS assay of cardiac troponin T (cTnT) and αMHC-GFP staining of reprogrammed neonatal cardiac fibroblasts three days after GMT retrovirus infection.
4. Notes
Cardiac epigenetic reprogramming could be studied by using other transgenic mouse of cardiac lineage reporter, such as αMHC-mCherry transgenic mice (Stock No: 021577) from The Jackson laboratory.
In our experience, tissues from one heart of a neonatal mouse should be plated into one 60-mm dish and three hearts into one 100-mm dish; two tail-tip tissues, harvested at 0.5–0.75 cm in length, should be plated in one 100-mm dish; tissues from one ventricle of an adult mouse should be plated in two or three 100-mm dishes.
Keep tissues cold to minimize cell death or senescence. The harvested tissues should be used as soon as possible, but can be stored on ice for 2 hours without obvious negative effect on fibroblast isolation.
Place the heart in one well of 24-well plate and the tail tip from the same animal into the next well of the 24-well plate, so that αMHC-GFP-harboring tail tips can be determined based on GFP expression of the heart from the same animal and will be used for fibroblast isolation.
It is critical to mince the tissues into small pieces so that the tissues can attach to cell culture vessels. Attachment is required for fibroblasts to emigrate from the isolated tissues. It is more efficient to mince tissues without addition of media.
The amount of media depends on the number of collected αMHC-GFP+ tissues that are used for cell culture. For examples, we use 0.5 mL media for tissues of one neonatal heart that will be plated into one 60-mm dish, and 1.5 mL media for tissues of three neonatal hearts that will be plated into one 100-mm dish.
It is important to use a proper amount of media so that the media barely cover the surface of dish. Too much medium prevents attachment of the minced tissues onto the bottom of the culture dishes.
Tissue attachment requires a minimum of 2 hours. A period of four hours of attachment is recommended.
It is very important to avoid disturbing the attached tissues. Don’t add media directly upon tissue.
It is a good sign if the media become yellow after 3 days of culture, suggesting that many fibroblasts have emigrated out from those attached tissues. Under a microscope, emigrated fibroblasts can be observed around those attached tissues.
Adult cardiac fibroblasts can be cultured for another extra 3 days if needed.
It is not recommended to use frozen or passaged fibroblasts for iCM reprogramming.
To save the amount of antibody used in Thy1-staining of Step 7, it is fine to resuspend cells in 0.5 ml FACS solution if there are not huge number of cells.
The ecotropic retroviruses, prepared from Plat-E cells, infect mouse cells only, and do not infect human cells.
It is important that the media of retrovirus preparation shouldn’t include any residual puromycin and blasticidin, which will kill fibroblasts when they are infected by the retroviral supernatants.
After overnight culture (~16 hours), the culture of Plat-E cells should become >80% confluent for transfection. To improve the virus titer, it is recommended to refresh the Plat-E Cell Medium 1 hour before transfection.
Other transfection reagents might be used for transfection. In our experience, FuGENE® 6 gives us the best yield of retrovirus.
For each batch of retrovirus preparation, dsRed virus should be included as a control of red fluorescence to monitor the efficiency of transfection and virus preparation. For example, to determine the transfection efficiency, dsRed fluorescence should be observed in most of the Plat-E cells (>80%) in the pMX-dsRed control. dsRed retroviruses could be used for virus titration as needed. To save transfection reagents, retrovirus of pMX-dsRed can be prepared in a 60-mm dish.
Dishes, pipettes, tips and media, all of which possibly contain virus, must be treated by 10% bleach for >1 hour before discard. After overnight transfection, it is expected that Plat-E cells have produced retroviruses.
We routinely use 0.5 mL of viruses for each factor, but 0.3 mL of each viruses gave similar results in our experience. Our FACS assay showed that both 0.3 mL and 0.5 mL dsRed retroviruses could transduce >90% of fibroblasts without significant difference.
In our experience, αMHC-GFP+ cells can be observed at day 3 after transduction, suggesting a success of reprogramming; 1 week later, many αMHC-GFP+ cells should be observed. iCM reprogramming failed if there are very few or no αMHC-GFP+ cells 7 days after retroviral transduction.
Cardiac muscle genes include Actc1, Myh6, Ryr2, Myl7, Scn5a, Slc8a1, Myl2, Tnnt2, Pln, Kcna5, Kcnj2, Cacba1c, Gja1, Atp2a2; fibroblast-enriched genes include Col1a1, Col1a2, Col3a1, Vim, Posn, Fsp1, Fn.
Fibroblasts infected by dsRed-retroviruses should be included as a negative control in assays of αMHC-GFP+ iCM characterization.
Acknowledgement:
Research in Fu’s group is supported by the Start-up Fund from The Ohio State University (to J.D.F), American Heart Association (13SDG14580035, to J.D.F.), and NIH (1R01HL139006, to J.D.F.).
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, Vanwagner LB, Wilkins JT, Wong SS, Virani SS, American Heart Association Council On E, Prevention Statistics C, Stroke Statistics S (2019) Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 139:e56–e528 [DOI] [PubMed] [Google Scholar]
- 2.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG (2005) CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 96:881–889 [DOI] [PubMed] [Google Scholar]
- 3.Frangogiannis NG, Michael LH, Entman ML (2000) Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb). Cardiovasc Res 48:89–100 [DOI] [PubMed] [Google Scholar]
- 4.Ivey MJ, Kuwabara JT, Pai JT, Moore RE, Sun Z, Tallquist MD (2018) Resident fibroblast expansion during cardiac growth and remodeling. J Mol Cell Cardiol 114:161–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savi M, Bocchi L, Fiumana E, Karam JP, Frati C, Bonafe F, Cavalli S, Morselli PG, Guarnieri C, Caldarera CM, Muscari C, Montero-Menei CN, Stilli D, Quaini F, Musso E (2015) Enhanced engraftment and repairing ability of human adipose-derived stem cells, conveyed by pharmacologically active microcarriers continuously releasing HGF and IGF-1, in healing myocardial infarction in rats. J Biomed Mater Res A 103:3012–3025 [DOI] [PubMed] [Google Scholar]
- 6.Suzuki G, Iyer V, Lee TC, Canty JM Jr., (2011) Autologous mesenchymal stem cells mobilize cKit+ and CD133+ bone marrow progenitor cells and improve regional function in hibernating myocardium. Circ Res 109:1044–1054 [DOI] [PubMed] [Google Scholar]
- 7.Giacomelli E, Mummery CL, Bellin M (2017) Human heart disease: lessons from human pluripotent stem cell-derived cardiomyocytes. Cell Mol Life Sci 74:3711–3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandel Y, Weissman A, Schick R, Barad L, Novak A, Meiry G, Goldberg S, Lorber A, Rosen MR, Itskovitz-Eldor J, Binah O (2012) Human embryonic and induced pluripotent stem cell-derived cardiomyocytes exhibit beat rate variability and power-law behavior. Circulation 125:883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smart N, Bollini S, Dube KN, Vieira JM, Zhou B, Davidson S, Yellon D, Riegler J, Price AN, Lythgoe MF, Pu WT, Riley PR (2011) De novo cardiomyocytes from within the activated adult heart after injury. Nature 474:640–U117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, Anversa P (2005) Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res 97:663–673 [DOI] [PubMed] [Google Scholar]
- 11.Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Bohm M, Quaini F, Urbanek K, Leri A, Hintze TH, Kajstura J, Anversa P (2005) Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A 102:8966–8971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, Giacca M (2012) Functional screening identifies miRNAs inducing cardiac regeneration. Nature 492:376–381 [DOI] [PubMed] [Google Scholar]
- 13.Miyawaki A, Obana M, Mitsuhara Y, Orimoto A, Nakayasu Y, Yamashita T, Fukada SI, Maeda M, Nakayama H, Fujio Y (2017) Adult murine cardiomyocytes exhibit regenerative activity with cell cycle reentry through STAT3 in the healing process of myocarditis. Sci Rep 7:1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohamed TMA, Ang YS, Radzinsky E, Zhou P, Huang Y, Elfenbein A, Foley A, Magnitsky S, Srivastava D (2018) Regulation of Cell Cycle to Stimulate Adult Cardiomyocyte Proliferation and Cardiac Regeneration. Cell 173:104–116 e112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D (2010) Direct Reprogramming of Fibroblasts into Functional Cardiomyocytes by Defined Factors. Cell 142:375–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu J-DD, Srivastava D (2012) In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 485:593–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN (2012) Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature 485:599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi J, Costa ML, Mermelstein CS, Chagas C, Holtzer S, Holtzer H (1990) MyoD converts primary dermal fibroblasts, chondroblasts, smooth muscle, and retinal pigmented epithelial cells into striated mononucleated myoblasts and multinucleated myotubes. Proceedings of the National Academy of Sciences of the United States of America 87:7988–7992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676 [DOI] [PubMed] [Google Scholar]
- 20.Addis RC, Ifkovits JL, Pinto F, Kellam LD, Esteso P, Rentschler S, Christoforou N, Epstein JA, Gearhart JD (2013) Optimization of direct fibroblast reprogramming to cardiomyocytes using calcium activity as a functional measure of success. Journal of molecular and cellular cardiology 60:97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Protze S, Khattak S, Poulet C, Lindemann D, Tanaka EM, Ravens U (2012) A new approach to transcription factor screening for reprogramming of fibroblasts to cardiomyocyte-like cells. J Mol Cell Cardiol 53:323–332 [DOI] [PubMed] [Google Scholar]
- 22.Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, Pandya K, Zhang Z, Rosenberg P, Mirotsou M, Dzau VJ (2012) MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res 110:1465–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srivastava D, Ieda M, Fu J, Qian L (2012) Cardiac repair with thymosin β4 and cardiac reprogramming factors. Annals of the New York Academy of Sciences 1270:66–72 [DOI] [PubMed] [Google Scholar]
- 24.Fu J-DD, Stone NR, Liu L, Spencer CI, Qian L, Hayashi Y, Delgado-Olguin P, Ding S, Bruneau BG, Srivastava D (2013) Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem cell reports 1:235–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wada R, Muraoka N, Inagawa K, Yamakawa H, Miyamoto K, Sadahiro T, Umei T, Kaneda R, Suzuki T, Kamiya K, Tohyama S, Yuasa S, Kokaji K, Aeba R, Yozu R, Yamagishi H, Kitamura T, Fukuda K, Ieda M (2013) Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proceedings of the National Academy of Sciences of the United States of America 110:12667–12672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bektik E, Dennis A, Prasanna P, Madabhushi A, Fu J-DD (2017) Single cell qPCR reveals that additional HAND2 and microRNA-1 facilitate the early reprogramming progress of seven-factor-induced human myocytes. PloS one 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nam YJ, Song K, Luo X, Daniel E, Lambeth K, West K, Hill JA, Dimaio JM, Baker LA, Bassel-Duby R, Olson EN (2013) Reprogramming of human fibroblasts toward a cardiac fate. Proc Natl Acad Sci U S A 110:5588–5593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ifkovits JL, Addis RC, Epstein JA, Gearhart JD (2014) Inhibition of TGFbeta signaling increases direct conversion of fibroblasts to induced cardiomyocytes. PLoS One 9:e89678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y, Londono P, Cao Y, Sharpe EJ, Proenza C, O’rourke R, Jones KL, Jeong MY, Walker LA, Buttrick PM, Mckinsey TA, Song K (2015) High-efficiency reprogramming of fibroblasts into cardiomyocytes requires suppression of pro-fibrotic signalling. Nature communications 6:8243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamakawa H, Muraoka N, Miyamoto K, Sadahiro T, Isomi M, Haginiwa S, Kojima H, Umei T, Akiyama M, Kuishi Y, Kurokawa J, Furukawa T, Fukuda K, Ieda M (2015) Fibroblast Growth Factors and Vascular Endothelial Growth Factor Promote Cardiac Reprogramming under Defined Conditions. Stem cell reports 5:1128–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Y, Wang L, Vaseghi HR, Liu Z, Lu R, Alimohamadi S, Yin C, Fu J-DD, Wang GG, Liu J, Qian L (2016) Bmi1 Is a Key Epigenetic Barrier to Direct Cardiac Reprogramming. Cell stem cell 18:382–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bektik E, Dennis A, Pawlowski G, Zhou C, Maleski D, Takahashi S, Laurita KR, Deschênes I, Fu J-DD (2018) S-phase Synchronization Facilitates the Early Progression of Induced-Cardiomyocyte Reprogramming through Enhanced Cell-Cycle Exit. International journal of molecular sciences 19:pii: E1364. doi: 1310.3390/ijms19051364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Y, Wang L, Liu Z, Alimohamadi S, Yin C, Liu J, Qian L (2017) Comparative Gene Expression Analyses Reveal Distinct Molecular Signatures between Differentially Reprogrammed Cardiomyocytes. Cell Reports 20:3014–3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yijing G, Ienglam L, Shuo T, Wenbin G, Karatas H, Yangbing L, Shaomeng W, Liu L, Zhong W (2019) Enhancing Cardiac Reprogramming by Suppressing Specific C-C Chemokine Signaling Pathways. bioRxiv 522995
- 35.Muraoka N, Nara K, Tamura F, Kojima H, Yamakawa H, Sadahiro T, Miyamoto K, Isomi M, Haginiwa S, Tani H, Kurotsu S, Osakabe R, Torii S, Shimizu S, Okano H, Sugimoto Y, Fukuda K, Ieda M (2019) Role of cyclooxygenase-2-mediated prostaglandin E2-prostaglandin E receptor 4 signaling in cardiac reprogramming. Nat Commun 10:674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tani H, Sadahiro T, Ieda M (2018) Direct Cardiac Reprogramming: A Novel Approach for Heart Regeneration. International journal of molecular sciences 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bektik E, Fu JD (2019) Ameliorating the Fibrotic Remodeling of the Heart through Direct Cardiac Reprogramming. Cells 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stone NR, Gifford CA, Thomas R, Pratt KJB, Samse-Knapp K, Mohamed TMA, Radzinsky EM, Schricker A, Ye L, Yu P, Van Bemmel JG, Ivey KN, Pollard KS, Srivastava D (2019) Context-Specific Transcription Factor Functions Regulate Epigenomic and Transcriptional Dynamics during Cardiac Reprogramming. Cell Stem Cell 25:87–102 e109 [DOI] [PMC free article] [PubMed] [Google Scholar]


