Abstract
The dentate gyrus (DG) is a unique brain structure in that neurons can be generated postnatally and integrated within existing circuitry throughout life. The maturation process of these newly generated neurons (granule cells) is modulated by nicotinic acetylcholine receptors (nAChRs) through a variety of mechanisms such as neural stem pool proliferation, cell survival, signal modulation, and dendritic integration. Disrupted nAChR signaling has been implicated in neuropsychiatric and neurodegenerative disorders, potentially via alterations in DG neurogenesis. GABAergic interneurons are known to express nAChRs, predominantly the α7 subtype, and have been shown to shape development, integration, and circuit reorganization of DG granule cells. Therefore, we examined histological and behavioral effects of knocking out α7 nAChRs in GABAergic neurons. Deletion of α7 nAChRs resulted in a reduction of radial glia-like cells within the subgranular zone of the DG and a concomitant trend towards decreased immature neurons, specifically in male mice, as well as sex-dependent changes in several behaviors, including social recognition and spatial learning. Overall, these findings suggest α7 nAChRs expressed in GABAergic neurons play an important role in regulating the adult neural stem cell pool and behavior in a sex-dependent manner. This provides important insight into the mechanisms by which cholinergic dysfunction contributes to the cognitive and behavioral changes associated with neurodevelopmental and neurodegenerative disorders.
Introduction
α7 nicotinic acetylcholine receptors (nAChRs) are highly expressed within excitatory and GABAergic interneurons in the hippocampus where they play an important role in supporting normal cognitive function via a variety of proposed mechanisms (Gotti et al. 2006; Haam and Yakel 2017). Aberrant α7 nAChR signaling has been implicated in neuropsychiatric and neurodegenerative disorders, such as schizophrenia and Alzheimer’s disease. Single nucleotide polymorphisms resulting in overexpression of a dominant negative version of the CHRNA7 gene (CHRNA7A) as well as reduced expression of α7 nAChRs have been found in the brains of schizophrenic patients (Stevens et al. 2014; Kunii et al. 2015). Furthermore, positive allosteric modulators of α7 nAChRs have been investigated as a potential treatment for a variety of disorders, including Alzheimer’s disease in which the cholinergic system is characteristically diminished (Bertrand et al. 2015; Dineley et al. 2015; Ma and Qian 2019). Thus, understanding the processes of α7 nAChR signaling in the hippocampus is critical to understanding normal brain function and developing treatments for neuropsychiatric and neurodegenerative disorders.
Neural stem cells are multipotent cells, which can undergo self-renewal symmetrically producing daughter cells or differentiate asymmetrically producing astrocytes or intermediate progenitors (Furutachi et al. 2015; Gonçalves et al. 2016). Neural stem cells within the subgranular zone (SGZ) of the dentate gyrus (DG) also generate new neurons that form the granule cell layer during postnatal development and continue to generate new neurons throughout life as part of the adult neurogenic niche. Among the adult neurogenic niche, radial glia-like astrocytes at the base of the DG granule cell layer are one such neural stem cell capable of generating newborn neurons and also provide a scaffold for the growth and migration of the immature neurons into the granule cell layer (Shapiro et al. 2005). Alterations to these radial glia-like astrocytes has also been shown to be associated with altered neurogenesis (Foresti et al. 2011; Song et al. 2012a). Thus, evaluating this population of astrocytes is an important component of evaluating the effects of experimental manipulations on the adult neurogenic niche.
Neurogenesis is known to be regulated by cholinergic signaling as shown by selective lesioning and pharmacological studies (Asrican et al. 2016). The targets of acetylcholine are muscarinic and nicotinic acetylcholine receptors found in various cell types throughout the hippocampus (Bertrand et al. 2015). α7 nAChRs in particular play a critical role in modulation of hippocampal neurogenesis, as the loss of α7 nAChRs results in reduced dendritic length and branch points of newly formed neurons (Campbell et al. 2010). Additionally, sex differences should be considered as neurogenesis within the DG has been reported to be influenced by gonadal hormones (Galea 2008). These findings may help explain common, but not invariable, sex differences in spatial-based task performance (Jonasson 2005; Linn and Petersen 2016; Yagi and Galea 2019). Prior findings in our lab found that the knockout of α7 nAChRs in nestin-positive neural stem cells increased the presence of newly generated neurons in both sexes, increased intermediate progenitors in male mice, diminished neural stem pools in male mice, and worsened spatial discrimination in male mice only (Otto and Yakel 2019). While α7 nAChRs clearly impact neurogenesis, there is much to delineate in regard to specific cell type and sex differences mediating these effects.
GABAergic interneurons play a vital role in distinct stages of DG neurogenesis, regulating neural stem cell quiescence, maturation, and apoptosis (Fernando et al. 2011; Gao et al. 2014; Catavero et al. 2018). α7 nAChRs presynaptically expressed on GABAergic interneurons are likely to play an important role in mediating these effects due to their high calcium permeability and impact on second messenger pathways (Haam and Yakel 2017). α7 nAChR-driven signaling has been proposed to directly modulate the level of GABA available to be released via PKA-dependent modulation of GAD67 enzymatic activity (Martin DL 2000; Dajas-Bailador et al. 2002; Wei et al. 2004; Thomsen et al. 2009; Adams et al. 2012; Bates et al. 2014; Cheng and Yakel 2015). In addition, cholinergic signaling is highly responsive to cognitive demand, increasing during encoding of novel information and playing an important role in regulating learning-related synaptic plasticity (Gu and Yakel 2011; Haam and Yakel 2017; Pelkey et al. 2017). Therefore, α7 nAChR signaling in interneurons is well positioned to drive learning-induced changes in neurogenesis that occur as a result of exposure to enriched environments and hippocampus-dependent learning (Kempermann et al. 1997; Lemaire et al. 1999; Dalla et al. 2007; Waddell et al. 2011).
The goal of the present study was to assess the structural and functional impact of loss of α7 nAChR expression in GABAergic neurons utilizing Cre-dependent disruption of the Chrna7 gene in a GAD2-IRES-Cre mouse line (referred to here as conditional knockout (cKO) mice). We assessed structural alterations within the hippocampal neurogenic niche by crossing the cKO line with a glial fibrillary acidic protein (GFAP) reporter line as well as staining for immature neurons using DCX and dividing cells using Ki-67. To address the functional impact, we conducted a battery of behavioral assays designed to test a broad range of functional domains. Postnatal hippocampal neurogenesis plays an important, though hotly debated, role in learning and memory processes (Shors et al. 2002; Nakashiba et al. 2012; Vicidomini et al. 2020). Disruptions of neurogenesis have been shown to impair performance in a wide array of behavioral assays, yet deficits are often subtle or require specific task parameters to be observed (Shors et al. 2002; Snyder et al. 2005). We hypothesized that loss of α7 nAChRs in GABAergic neurons would lead to reductions in neurogenic potential and spatial memory tasks without creating deficits in motor or sensory function.
Methods
Animals.
For histological assessment of the neurogenic niche, male and female hGFAP-GFP mice were purchased from Jackson Laboratory (JAX:003257). These mice were selected as radial glia-like cells and astrocytes within the DG express GFAP (Casper and McCarthy 2006). hGFAP-GFP mice were then crossed with either WT α7nAChRflox controls (JAX:026965) engineered with loxP sites flanking exon 4 of the Chrnα7 gene (Hernandez et al. 2014) or cKO GAD2-IRES-Cre mice (JAX:010802) crossed with α7nAChRflox mice to disrupt Chrnα7 expression within cells that express GAD2. Work from our laboratory has verified the functional loss of α7 nAChR expression in hippocampal GABAergic interneurons (Gu et al. 2020). Offspring used for immunostaining (7 male and 10 female WT and 8 male and 6 female cKO) were group housed (3–4 per cage) in reverse light/dark housing on a 12 hr cycle with food and water supplied ad libitum until sacrifice at 8 weeks of age. All procedures were approved and performed in compliance with the NIEHS/NIH Humane Care and Use of Animals Protocols.
For the behavioral battery, the UNC Mouse Behavioral Phenotyping Laboratory utilized 14 male and 13 female WT controls and 10 male and 10 female cKO mice. Mice were 6–8 weeks in age at the start of behavioral testing. For a detailed timeline see Supplemental Table 1 A. All animal care and procedures were conducted in strict compliance with the animal welfare policies set by the National Institutes of Health and by the University of North Carolina at Chapel Hill (UNC), and were approved by the UNC Institutional Animal Care and Use Committee. The NIEHS Neurobehavioral Core utilized 8 male and 8 female WT mice and 8 male and 8 female cKO mice for additional behavioral tests (trace fear conditioning, shock reactivity, spontaneous alternation, and spatial and novel object recognition - for a detailed timeline see Supplemental Table 1 B). Mice were 8–10 weeks in age at the start of behavioral testing. These procedures were conducted in strict compliance with the animal welfare policies set by the National Institutes of Health and approved by NIEHS Animal Care and Use Committee. During behavioral assays, animals were acclimated to handling for a minimum of one week prior to testing and acclimated to the experimental room for > 30 min prior to initiating testing. All tasks were separated by at least one week.
Tissue preparation.
Mice were deeply anesthetized using 0.2 ml phenobarbital (FatalPlus), transcardially perfused with ice cold 0.1 M phosphate buffer saline (PBS) at pH 7.4 with 0.1% heparin followed by ice cold 4% paraformaldehyde (PFA). Brains were post-fixed overnight at 4°C in 4% PFA, washed in 0.1 M PBS, and cryoprotected in 30% sucrose in 0.1 M PBS at 4°C. Brains were then frozen in Tissue Freezing Medium (Triangle Biomedical Sciences, Durham, NC) and cryosectioned into 50 μm free-floating coronal sections using a cryostat (Leica CM305 S; Leica Biosystems, Buffalo Grove, IL). After a random start, every sixth section of hippocampi were processed for immunolabeling. Sections were placed in blocking buffer (0.1 M PBS with 5% goat serum and 0.1% triton x-100) for 2 hr at room temperature or stored at 4°C until immunofluorescence. 50 μm free-floating sections were incubated with primary antibodies overnight at 4° C. Primary antibodies used include guinea pig anti-doublecortin (DCX; 1:500; cat# AB2253, Millipore, Billerica, MA), rabbit anti-Antigen KI-67 (Ki-67; 1:100; cat# AB15580 Abcam, Cambridge, MA), chicken anti-green fluorescent protein (GFP; 1:10,000; cat# AB13970, Abcam). Negative controls lacking primary were used to confirm specificity of staining. Tissue was triple washed in PBS and incubated in secondary antibody for 2 hr at room temperature or overnight at 4°C. Secondary antibodies were Alexa Fluor 647 goat anti-rabbit (cat# AB150087, Abcam), Alexa Fluor 568 goat anti-guinea pig (cat# AB175714, Abcam), Alexa Fluor 488 goat anti-chicken (cat# AB150169, Abcam). All secondary antibodies were used at 1:500. Tissue was triple washed in PBS with 4′,6-diamidino-2-phenylindole (DAPI; 2 μg/ml; cat# 508741, Millipore) in the final wash to stain cell nuclei. Tissue was mounted onto SuperFrost Plus slides (Fisher Scientific, Waltham, MA) using Prolong Diamond Anti-Fade Mounting Media (Molecular Probes, Eugene, OR).
Imaging.
Tile scan, Z-stack images of 50 μm thick brain sections were collected on a confocal microscope (LSM 710, Carl Zeiss Inc, Oberkochen, Germany) using a 40X objective. For the far-red channel, a 633 nm laser line was used for excitation of an Alexa 647 secondary antibody using a band pass filter of 640–717. For the red channel, a 561 nm dpss laser was used for excitation of Alexa 568 while a 561–639 filter collected the emission. For the green channel, a 488 nm ArKr laser line was used for excitation of the Alexa 488 secondary while a bandpass 503–552 filter was used for collection of the emission signal. For the blue channel, a 405 nm diode laser line was used for exshapi of DAPI while a bandpass 415–503 filter was used for the emission. Images were taken with the pinhole of the longest emission wavelength set to 1 airy unit, a zoom setting of 1, and line averaging set to 2.
Image analysis.
All images captured in Zen Black (2012, Carl Zeiss Inc, Oberkochen, Germany) were stitched and imported into an IMARIS (Bitplane, Oxford Industries, Atlanta, GA) analysis arena (Supplementary Video 1). 3D images were analyzed using a shape function to limit analysis to the SGZ of the DG within each section. Cells of interest were counted using the spots function. Fluorescent markers were included only when colocalized with DAPI. GFAP-positive cells located in the SGZ that were unipolar or bipolar in nature (which could be easily determined via three dimensional manipulation) were counted to quantify radial glia-like cells. Where possible, cell counts were automated using the batch feature within IMARIS. We determined the batch feature was adequate for counting DCX-positive and Ki-67-positive cells, but not GFAP-positive radial glia-like cells. Therefore, this population was entirely manually counted. Background was not subtracted as fluorescence intensity was not quantified and cell bodies were easily distinguishable. Data was recorded in Excel spreadsheets for further analysis. For presentation, images were cropped using IMARIS software, and captured via a snapshot feature. Images were modified solely after analysis by adjusting brightness and contrast to optimize the full dynamic range of the fluorescent signal. For image analysis, raw densities (cell count per total volume of SGZ as determined by the shape function) were computed from the averages of four dorsal DG sections per mouse (all within 2.5 to 1.75 mm from Bregma). Following image analysis by IMARIS, Excel (Microsoft Corporation, Redmond, WA), SPSS (SPSS Statistics, Chicago, IL), and/or GraphPad Prism (8.4.3, GraphPad Software, La Jolla, CA) was used for statistical analysis and graphical illustration. Higher throughput batch cell counting via 3D image analysis software allows for faster gathering of data yet still adds a degree of systematic error. To verify systematic errors inherent in batch counting were not affecting results, cell counts were verified by a separate, blinded count. A student’s t-test was used to analyze two independent samples where opposing hippocampi from a singular mouse provided an internal control (data not shown). A two-way ANOVA (genotype by sex) was used to analyze main effects and any interactions present. Post hoc analysis included Šídák’s multiple comparison to compare means within relevant rows. Data are presented at mean ± SEM. In all cases a value of p < 0.05 was considered statistically significant.
Open field.
Exploratory activity in a novel environment was assessed by a one-hour trial in an open field chamber (41 cm x 41 cm x 30 cm) crossed by a grid of photobeams (VersaMax system, AccuScan Instruments Inc., Columbus, OH). Counts were taken of the number of photobeams broken during the trial in five-min intervals, with measures taken of locomotion (total distance traveled), rearing movements, and time spent in the center region of the open field, an index of anxiety-like behavior.
Three chamber social approach.
The procedure consisted of three 10-min phases: a habituation period, a test for sociability, and a test for social novelty preference. For the sociability assay, mice were given a choice between being in the proximity of an unfamiliar conspecific (“Stranger Mouse”) versus a non-social novel object (“Empty Cage”). In the social novelty phase, mice were given a choice between the already investigated Stranger Mouse (“Familiar Mouse”), versus a new unfamiliar mouse (“Novel Mouse”). The social testing apparatus was a rectangular, three chambered box fabricated from clear Plexiglas. Dividing walls had doorways allowing access into each chamber. An automated image tracking system (Ethovision 15, Noldus, Leesburg, VA) provided measures of time spent in 5 cm proximity to each cage and numbers of entries into each side of the social test box.
At the start of the test, the mouse was placed in the middle chamber and allowed to explore for 10 min, with the doorways into the two side chambers open. After the habituation period, the test mouse was enclosed in the center compartment of the social test box, and an unfamiliar male C57BL/6J adult (Stanger Mouse) was placed in one of the side chambers. The Stranger Mouse was enclosed in a small Plexiglas cage drilled with holes. An identical empty Plexiglas cage was placed in the opposite side of the chamber. Following placement of the stranger and the empty cage, the doors were re-opened, and the subject was allowed to explore the entire social test box for a 10-min session. At the end of the sociability phase, Novel Mouse was placed in the empty Plexiglas container, and the test mouse was given an additional 10 min to explore the social test box. The social test box was 41.5 cm wide, 60 cm in length, and 20 cm in height. The box was divided into three chambers by two interior walls; each of the two side chambers was 41.5 cm wide and 21 cm in length.
Morris water maze.
The water maze was utilized to assess spatial and reversal learning, swimming ability, and vision. The water maze consisted of a large circular pool partially filled with water (122 cm wide, 45 cm deep, 12 cm diameter escape platform, 24–26° C), located in a room with numerous visual cues. The procedure involved three different phases: a visible platform test, acquisition in the hidden platform task, and a test for reversal learning (an index of cognitive flexibility).
Visible platform in the Morris water maze.
Each mouse was given four trials per day, across two days, to swim to an escape platform cued by a patterned cylinder extending above the surface of the water. For each trial, the mouse was placed in the pool at one of four possible locations (randomly ordered), and then given 60 sec to find the visible platform. If the mouse found the platform, the trial ended, and the animal was allowed to remain 10 sec on the platform before the next trial began. If the platform was not found, the mouse was placed on the platform for 10 sec, and then given the next trial. Measures were taken of latency to find the platform and swimming speed via an automated image tracking system (Ethovision 15).
Hidden platform in the Morris water maze.
Following the visible platform task, mice were tested for their ability to find a submerged, hidden escape platform (12 cm diameter). Each animal was given four trials per day, with 1 min per trial, to swim to the hidden platform. Criterion for learning was an average group latency of 15 sec or less to locate the platform. Mice were tested until the group reached criterion at three days of testing. When criterion was reached, mice were given a one min probe trial in the pool with the platform removed. Selective quadrant search was evaluated by measuring number of crossings over the platform location, versus the corresponding location in the opposite quadrant.
Reversal learning in the Morris water maze.
Following the acquisition phase, mice were tested for reversal learning, using the same procedure as described above. In this phase, the hidden platform was re-located to the opposite quadrant in the pool. As before, measures were taken of latency to find the platform. On the fourth day of testing, when the criterion for learning was met, the platform was removed from the pool, and the group was given a one min probe trial to evaluate reversal learning.
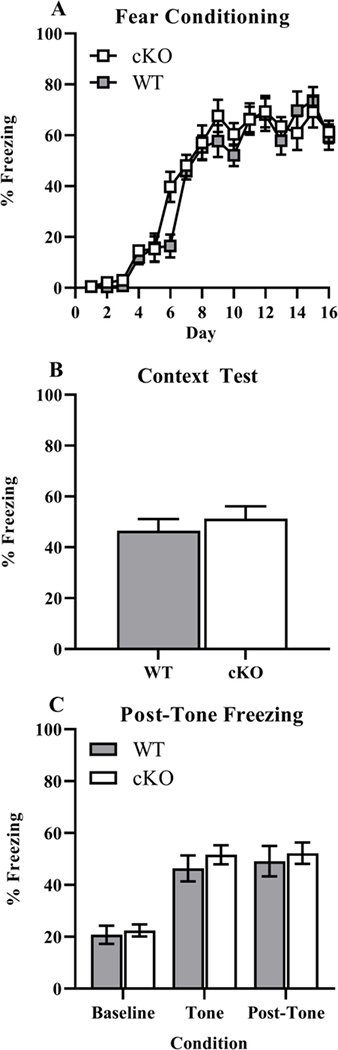
Fear conditioning.
Animals were held in an anteroom separated from the testing room to ensure that the animals did not hear testing of other animals for at least 30 min prior to training/testing. Training took place in four identical sound attenuating chambers (Context A; 28 cm wide, 21 cm deep, 21 cm tall; Med-Associates Inc., Fairfax, VT). The floor of each chamber consisted of a stainless-steel shock grid (1/2 in apart) wired to a shock generator and scrambler (Med-Associates Inc.) to deliver foot shocks. On the training day (Day 1) mice underwent trace fear conditioning as follows: mice were placed in Context A (mouse standard grid floor, fan in box on, three sprays of 75% isopropyl alcohol to sanitize per box, and five sprays of 50% Simple Green for scent in pan) and left to explore for 3 min prior to the first tone presentation (20 s, 75 dB, 2800 Hz). A 2 sec 0.5 mA shock was presented 20 sec later. This sequence was presented four more times for a total of five tone-shock pairings. After the last shock, animals were left for 2 min and then returned to their home cages. On Day 2 mice were returned to the condition chamber with identical configuration as during training for an 8 min context test. For the tone test on Day 3, the animals were placed in four novel and structurally distinct chambers (Context B: plastic white floor on top of standard grid, Black plastic A-frame, fan off, three sprays of 75% isopropyl alcohol to sanitize per box, and five sprays of Windex for scent in pan). The tone was delivered in the same way as in Context A during training, but with the shock omitted. This protocol provides two separate assessments of hippocampus-dependent fear learning (Chowdhury et al. 2005; Cushman and Fanselow 2010). The 20 sec “trace” interval between the tone and the shock requires intact hippocampal function in order to support associative learning, in addition to extra-hippocampal structures, such as the medial prefrontal cortex (Raybuck and Lattal 2014). Context fear, assessed on the context test on Day 2, also requires the hippocampus in order to generate a unified, multimodal contextual representation that becomes associated with the shock (Cushman and Fanselow 2010)
Social and novel object recognition.
Mice were first habituated to the experimental arena (45 cm by 45 cm) during Days 1 and 2 for 10 min over two daily sessions. The arena was placed inside a sound attenuating cubicle with an overhead camera to monitor behavior. The light was kept at 5–10 lux and four unique patterned walls were placed just outside the clear walls of the arena to provide distal visual information. On Day 3 the animals were presented with two identical objects (Supplemental Fig. 1) placed in opposite corners of the arena for 10 min. During the test phase 24 hours later, one object was moved to the opposite corner, with the side counterbalanced across animals. 24 hours later a novel object recognition assay was performed: the mice were placed back in the arena with two identical novel objects placed equidistant from the walls (6 cm) and from the center of the arena for 10 min. 10 min later one of the objects was replaced with a novel object and the mice were returned to the arena for 10 min (Supplemental Fig. 1). The novel versus familiar object was counterbalanced across mice. The amount of time spent exploring the objects was assessed using an automated image tracking system (Ethovision 15) based on parameters that were optimized to closely correlate with human scoring.
Behavioral analysis.
Measures were taken by observers blind to mouse genotype. Behavioral data were first analyzed using two-way or repeated measures ANOVA with genotype and sex as factors. If repeated measures ANOVAs indicated sex as a significant main effect, groups were then separated by sex and analyzed separately. Fisher’s protected least-significant difference (PLSD) tests were used for comparing group means only when a significant p value for interaction was determined in the ANOVA. For all comparisons, significance was set at p < 0.05. Please refer to supplementary methods for additional behavioral task descriptions.
Results.
Histological assessment of the hippocampal neurogenic niche.
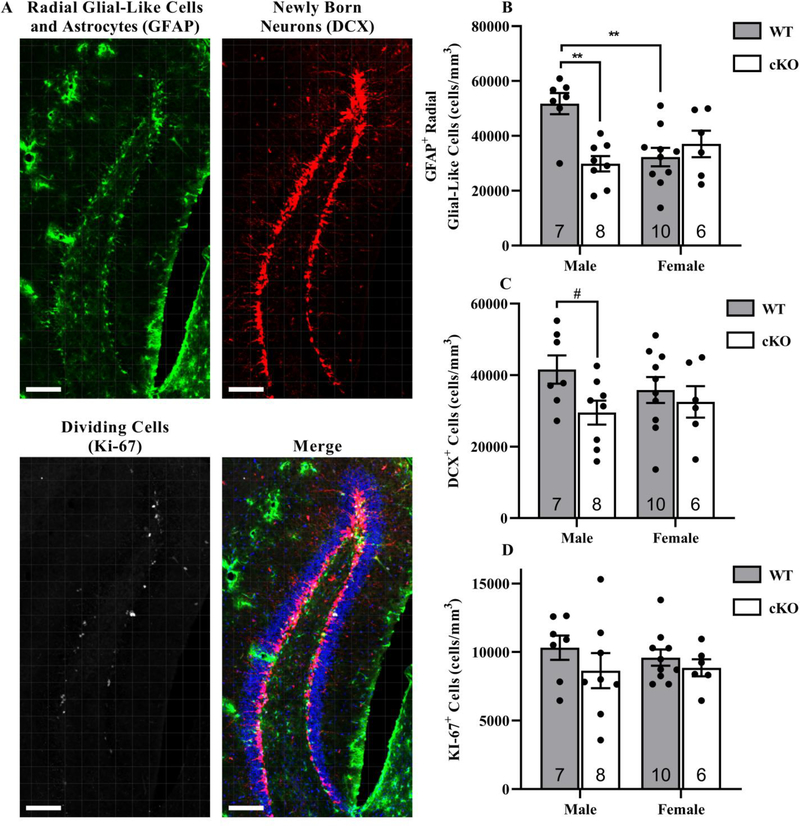
We found that the selective knockout of Chrna7 within GABAergic neurons (cKO mice) resulted in a sex by genotype interaction in radial glia-like cell density within the DG as marked by GFAP (Genotype: p = 0.029, Sex: p = 0.111, Interaction: p = 0.001, Fig. 1 B). Radial glia-like cell density was reduced in male cKO mice by 42 ± 10% compared to male WT mice, but no differences were detected between female genotypes. Within WT mice, we observed significantly fewer radial glia-like cells in females relative to males, but no sex difference was observed in the cKO mice (Fig. 1 B). Although there was no significant difference in the numbers of DCX-labeled cells or in actively dividing cells in either male or female mice, a trend was observed for decreased DCX-labeled cells in the cKO mice (DCX, Genotype: p = 0.058, Sex: p = 0.730, Interaction: p = 0.270, Fig. 1 C; Ki-67, Genotype: p = 0.196, Sex: p = 0.774, Interaction: p = 0.616, Fig. 1 D). The near significance in DCX-labeled cells was driven by a 29 ± 12% decrease in the cKO males compared to the WT males. A post hoc Student’s t-test comparing male genotypes only revealed a significant decrease in DCX-labeled cells in the cKO male mice compared to the WT male mice (p = 0.036).
Fig. 1. Reduced radial glia-like cells within the dorsal DG of male cKO mice.
Images are representative samples from the dorsal DG at 40x from a hGFAP-GFP x α7nAChRflox x GAD2-IRES-Cre mouse A. Expression of GFP was amplified using ck-antiGFP (Green; 1:10,000). Mice were also probed for immature neuron expression using gp-antiDCX (Red; 1:500) and dividing cells using rb-antiKi-67 (White; 1:400). Cell nuclei were marked using DAPI (Blue; 1:500). Scale bar = 100 μm. GFAP-positive with radial glia-like morphology, DCX-positive, and Ki-67-positive cell densities were averaged from four sections per mouse and plotted against sex and genotype B-D. **p < 0.01. # post-hoc Student’s t-test, p = 0.0362. Data expressed as mean ± SEM.
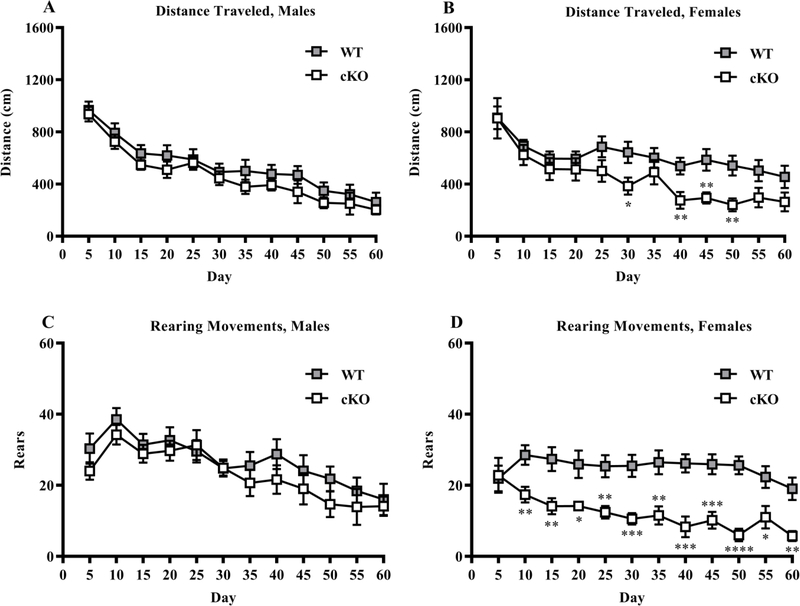
Open field.
As shown in Fig. 2, no differences were observed between the male WT and cKO mice for distance traveled (Genotype: p = 0.254, Time: p < 0.001, Interaction: p = 0.996) or rearing (Genotype: p = 0.308, Time: p < 0.001, Interaction: p = 0.899). Within females, the cKO mice had decreased locomotion, measured by distance traveled, 30 to 50 min into the test (Genotype: p = 0.037, Time: p < 0.001, Interaction: p = 0.191). Further, the female cKO group had a large reduction in rearing responses during all but the initial 5 min (Genotype: p < 0.001, Time: p < 0.001, Interaction: p = 0.004). No genotype effects were found for time spent in the center region within either sex (summary data in Supplemental Table 2).
Fig 2. Reduced distance traveled and rearing movements in cKO female mice during the open field test.
Distanced traveled and number of rearing movements during 60 min in an open field task A-D. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data expressed as mean ± SEM.
Three chamber social approach.
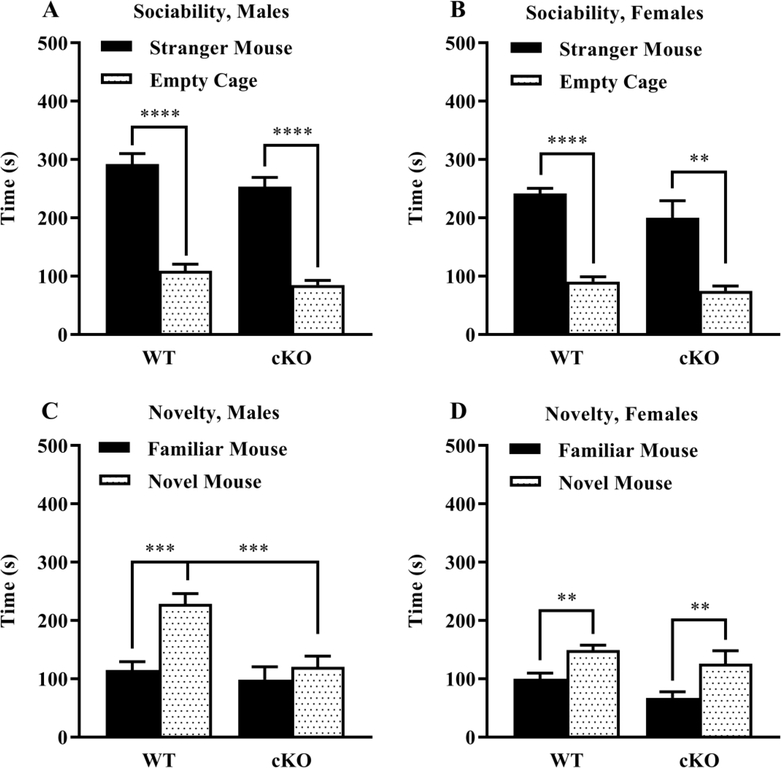
A repeated measures ANOVA on time in proximity to each cage indicated a significant main effect of sex (Sex: p < 0.001); therefore, data from males and females were separately analyzed. As shown in Fig. 3 A,B, both WT and cKO mice had robust preference for spending more time in proximity to the stranger mouse versus the empty cage (Side within males: p < 0.0001; Side within females: p < 0.0001). During the test for social novelty, male cKO mice failed to exhibit significant preference for the newly introduced novel mouse (Genotype: p = 0.002; Interaction: p = 0.021, Fig. 3 C). Data were removed for one outlier female cKO mouse that remained in proximity to the second stranger mouse for 466 sec. No significant effects of genotype were observed in the female groups during the social novelty test (Genotype: p = 0.459; Interaction: p = 0.262, Fig. 3 D). As shown in Supplemental Fig. 2, the WT and cKO groups had similar numbers of entries during the social approach test, indicating the significant differences in the male groups were not due to altered activity or exploration in the cKO mice.
Fig. 3. Reduced social novelty preference in cKO male mice during the three chamber social approach test.
Measures were taken of time spent in proximity to each cage during the test for sociability A,B and preference for social novelty C,D. Data were lost from one male cKO mouse due to experimenter error. Data were removed for one outlier female cKO mouse that remained in proximity to novel mouse for 466 sec. **p < 0.01, ***p < 0.001, ****p < 0.0001. Data expressed as mean ± SEM.
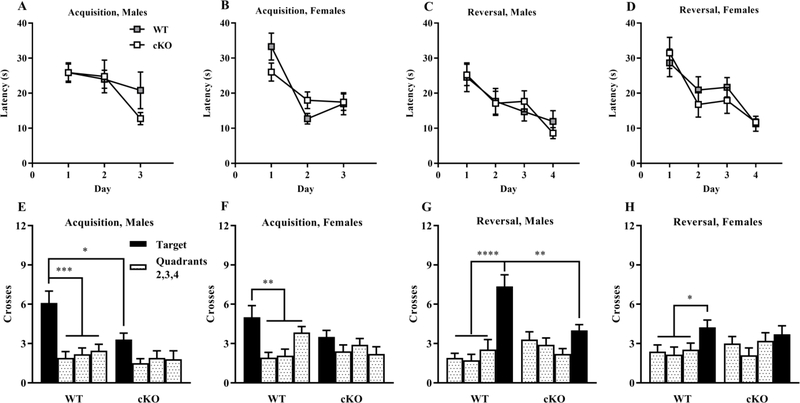
Acquisition phase in the Morris water maze.
The WT and cKO mice showed high proficiency in the visible platform phase of the water maze test. All experimental groups had mean latencies of less than 10 sec by the second day of visible platform testing. The WT and cKO mice demonstrated similar rates of learning, measured by latency to reach the escape platform, during acquisition of the hidden platform task (Genotype: p = 0.538, Sex: p = 0.499, Interaction: p = 0.687, Fig. 4 A,B) and the reversal phase (Genotype: p = 0.761, Sex: p = 0.162, Interaction: p = 0.7842, Fig. 4 C,D). However, a significant main effect of genotype was found for swimming speed on the first day of testing for each phase. Post hoc analysis indicated the female cKO mice had lower swimming speeds than the female WT at each time point (Supplemental Table 3).
Fig. 4. Normal acquisition learning in a hidden platform task but lack of quadrant preference in cKO mice during Morris water maze probe trials.
Criterion for learning was a group average of 15 sec to find the escape platform A-D. Mice were given a one-min probe trial without the platform following an initial 3-day acquisition of the hidden platform task E,F and a subsequent 4-day reversal phase G,H. ‘Target’ indicates the quadrant where the platform was located in each phase. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data expressed as mean ± SEM.
Probe trials in the Morris water maze.
At the end of the acquisition phase, mice were given one min probe trials with the platform removed. An ANOVA on number of platform-location crossings during the hidden platform probe trial revealed significant effects of genotype (Genotype by Quadrant Interaction: p = 0.017). As shown in Fig. 4 E,F, cKO mice in both sexes failed to exhibit a higher number of crossings across the target quadrant. In male groups, the cKO mice had significantly fewer crossings than WT across the target (post hoc test following Genotype by Quadrant interaction in male groups, p = 0.007). A similar pattern was observed in the probe test following the reversal phase wherein the cKO mice again failed to show quadrant selectivity (Genotype by Quadrant Interaction: p = 0.004, Fig. 4 G,H). In the male groups, the cKO mice had decreased numbers of crossings over the new location for the platform (post hoc test following Genotype by Quadrant interaction in male groups, p = 0.001).
Fear conditioning.
Freezing increased across the session during initial acquisition training but did not differ by genotype (Genotype: p = 0.101, Time: p < 0.001, Interaction: p = 0.981, Fig. 5 A). No differences in shock reactivity were observed (Genotype: p = 0.611, Sex: p = 0.281, Interaction: p = 0.110), which was calculated as the averaged movement index during the 2 sec shock period across all five shocks normalized by the average movement index prior to the first shock. No genotype or sex differences were detected during context testing (Genotype: p = 0.484, Sex: p = 0.343, Interaction: p = 0.778, Fig. 5 B). No differences in baseline (Genotype: p = 0.704, Sex: p = 0.0.634, Interaction: p = 0.0.995), tone (Genotype: p = 0.403, Sex: p = 0.235, Interaction: p = 0.307), or post-tone freezing (Genotype: p = 0.676, Sex: p = 0.527, Interaction: p = 0.647) were detected (Fig. 5 C).
Fig. 5. Lack of differences in fear conditioning and context tests.
Measures were taken of total freezing during sixteen trials across training A. Total percentage freezing during context test was recorded B with baseline, tone, and post-tone freezing C. Data expressed as mean ± SEM.
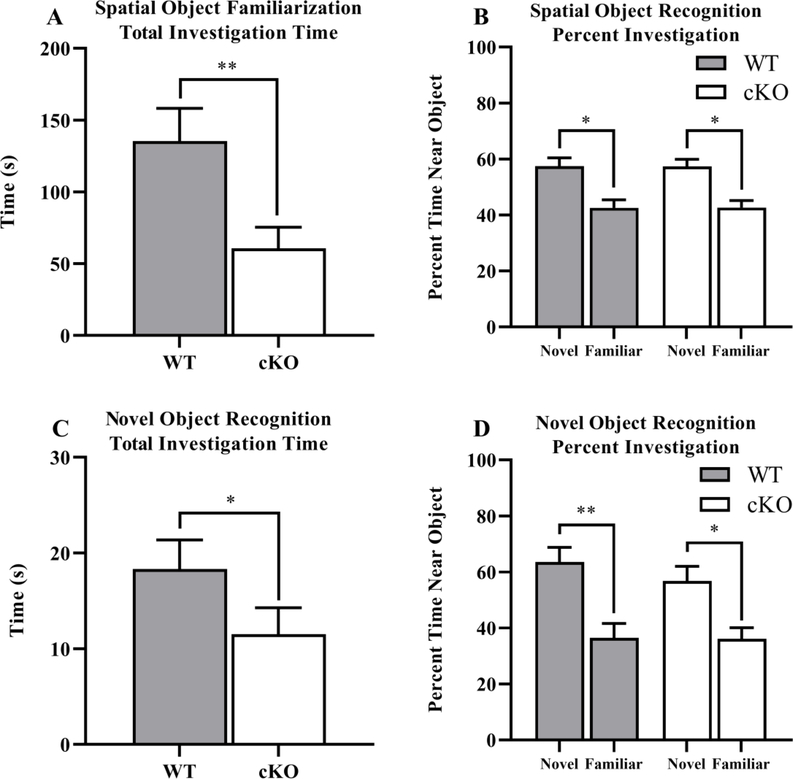
Spatial and novel object recognition.
On Day 3 during object familiarization, cKO mice spent significantly less total time investigating the novel objects (Genotype: p = 0.008, Sex: p = 0.096, Interaction: p = 0.363, Fig. 6 A). Despite this overall reduction in investigation, the cKO mice demonstrated a similar preference for the displaced object on the test day (Day 4) when calculated as percent investigation (displaced/total investigation versus stationary/total investigation) which normalizes for the total amount of investigation time (Object: p = 0.001, Object within WT mice: p = 0.029, Object within cKO mice: p = 0.015, Object by Sex Interaction: p = 0.724, Three-Way Interaction: p = 0.990, Fig. 6 B). cKO mice spent less time investigating during the familiarization phase of the novel object recognition test as well (Genotype: p = 0.045, Sex: p = 0.010, Interaction p = 0.553, Fig. 6 C) and a similar preference for the novel object as measured by percent investigation (Object: p = 0.001, Object within WT: p = 0.022; Object within cKO: p = 0.005, Object by Sex Interaction: p = 0.152, Three-Way Interaction: p = 0.291). See Supplemental Fig. 3 for more details on spatial and novel object recognition and break down by sex where appropriate.
Fig. 6. Decreases in object investigation in cKO mice during spatial and novel object recognition tests.
Measures were taken of total object investigation familiarization time during spatial object recognition as an average of time and percentage of time near novel versus familiar objects A,B. Total investigation time during novel object recognition was recorded and plotted as an average of time and percentage of time near novel versus familiar objects C,D. *p < 0.05, **p < 0.01. Data expressed as mean ± SEM.
Additional Findings.
The cKO mice had increased body mass in comparison to the WT mice at most time points during the behavioral study (Supplemental Fig. 4). No differences were found in the elevated plus maze (Supplemental Table 2), marble-bury (Supplemental Table 2), rotarod (Supplemental Fig. 5), acoustic startle (Supplemental Fig. 6 and 7), buried food (Supplemental Table 2), wire hang, or spontaneous alternation tests (Supplemental Fig. 8).
Discussion
Our findings indicate that α7 nAChRs expressed in GABAergic neurons play an important, sex-dependent role in the regulation of the hippocampal neurogenic niche and in several behavior domains. Male α7 cKO mice showed a reduction in GFAP-positive radial glia-like cells within the SGZ, indicating a diminished neural stem pool and potentially reduced topological scaffolding for newly born neurons, whereas female cKO mice were unchanged relative to female WT mice. We also observed fewer radial glia-like cells in female WT mice relative to WT males, suggesting an underlying sex difference, consistent with prior findings (Juraska et al. 1985; Falconer and Galea 2003; Brydges et al. 2018; Yagi and Galea 2019; Otto and Yakel 2019). While analysis of main effects on DCX-labeled and Ki-67-labeled cell numbers revealed no significant differences, a significant post hoc statistic indicated male cKO mice had a reduction in DCX-labeled cells compared to male WT mice. In a battery of behavioral tests, the most notable deficits were a male-specific deficiency in social recognition and spatial learning.
Sex differences of hippocampal neurogenesis have been widely reported (Yagi and Galea 2019) but remain an area of considerable complexity due to the interactions of neurogenesis and behavior (De Vries 2004) as well as interspecies effects (Amrein et al. 2004). Many studies report larger hippocampal formations in males that can usually be explained by total brain volume (Tan et al. 2016). Cell proliferation rates reported between sexes are also highly variable with studies indicating an impact from sex hormones and some report no influence (Galea 2008b). The sex differences present in our model are intriguing as the greater radial glia-like cell density in WT males compared to WT females is not explicitly paired with differences in DCX-labeled or Ki-67-labeled cells. However, the significant post hoc test indicating cKO males had reduced DCX-labeled cells compared to WT males implies our study was likely underpowered to detect main effects of this size and should be considered an important, but inconclusive, finding. Accumulating evidence suggests radial glia-like cell populations are heterogenous and can influence multiple facets of neurogenesis such as self-replication, production of neurons, astrocytes or type β cells, scaffolding for newly born neurons, and can even act as an extension of the neurogenic niche (Gebara et al. 2016). The reduction of radial glia-like cells in male cKO mice is supported by evidence that indicates neural stem cells are quiescent when bathed in GABA (Song et al. 2012b) of which release probability is affected by densely expressed α7 nAChRs in the SGZ on GABAergic interneuron terminals (Jones and Yakel 1997; Radcliffe et al. 1999; John et al. 2015). Of note, while the SGZ is also innervated by GABAergic projections from the medial septum, there is no evidence for presynaptic α7 nAChR expression. The radial glia-like cell reduction in males alone may indicate females were either protected from the cKO or do not receive an added benefit initially from α7 nAChRs’ modulation of GABA release probability. While we have provided evidence for a role of α7 nAChR-mediated radial glia-like cell production and/or survival in male mice, progenitor fate and functional circuitry changes remain unknown. Radial glia-like cell processes are important for supporting outgrowth of daughter neurons in a one-to-one relationship (Shapiro et al. 2005). Thus, the reduction of radial glia-like cells in combination with the near significant reduction in immature neurons suggests there is a high chance for dysfunctional neurogenesis. This conclusion is supported by previous results from our lab where stage 1 progenitor cells are also reduced with a global α7 nAChR knockout. However, without whole hippocampal stereology quantifying mature granule cells and astrocytes, the current data remain limited.
The behavioral changes in α7 cKO mice were highly selective indicating specific functional roles for Chrna7 expressed in GABAergic neurons. One caveat to these behavioral results is that each animal was subjected to a series of behavioral test (see Supplemental Table 1 A,B for summary). Although this is an established approach in the field (Paylor et al. 2006), we cannot rule out that prior testing could have influenced subsequent performance on other tasks, and this may have interacted with loss of the α7 nAChRs, potentially in a neurogenesis-dependent manner. Disruption of Chrna7 did not lead to changes in anxiety-like behavior in the elevated plus maze or marble burying tests. Female cKO mice showed reduced distance traveled and rearing in the 1 hr open field test, slower swimming speeds in the Morris water maze, and reduced object investigation in comparison to WT females. Although these changes suggest a possible motor impairment, both male and female cKO mice were proficient in the rotarod and wire hang tests for motor coordination indicating the differences are likely due to anxiety or motivation. The selective deletion of Chrnα7 did not lead to alterations in prepulse inhibition of acoustic startle responses, indicating the cKO mice did not model the deficits in sensorimotor gating associated with schizophrenia, which have previously been observed in animal models and patients alike (Ludewig et al. 2003; Shoji and Miyakawa 2018). Increased sensitivity to thermal stimuli was present in both male and female cKOs, consistent with previously reported findings of α7 nAChRs mediating nociception by reducing pain evoked by heat (Rowley et al. 2008). The augmentation in weight for cKOs warrants further study as α7 nAChRs have been shown to be important in metabolic function, including cancer progression and oxidative stress (Bertrand et al. 2015). Oxidative stress accumulation in adipose tissue has been associated with insulin resistance, a biomarker for weight gain or obesity (Aroor and DeMarco 2014), which could potentially involve α7 nAChR signaling.
In a 3-chamber test for sociability, both WT and cKO mice had similar preference for proximity to a stranger mouse, versus a non-social novel object. However, in a test for social novelty preference, the male cKO mice failed to demonstrate the typical shift in attention towards a newly-introduced stranger mouse, indicating a specific deficit in social recognition. This male-specific behavioral difference mirrors the male-specific deficit in radial glia-like cells and immature neurons, which could indicate a link between the two. Decreases in social approach have been correlated with decreases in DG volume, reported in both humans and in mouse models (Kalman and Keay 2017), and deletion of the α4 GABA receptor subunit has been shown to impair social recognition (Fan et al. 2020). Furthermore, studies have shown male-specific social and behavioral deficits following exposure to the pesticide chlorpyrifos (Lan et al. 2019), which is known to target the α7 nAChR (Slotkin et al. 2004). Given the greater preponderance of autism in males, these findings suggest GABAergic and cholinergic signaling may contribute to social deficits by impacting the neurogenic niche in a sex-dependent manner. Evident sex differences with regards to social housing and chronic stress have been correlated to levels of neurogenesis, where male rats experience a reduction and females experience an increase in proliferating cell counts within granule cell layer (Westenbroek et al. 2004). Future research will be required to determine if the male-specific social recognition deficits in α7 cKO mice are indeed driven by hippocampal neurogenesis-related mechanisms or by α7 nAChR elsewhere in the brain.
In the Morris water maze task, α7 cKO mice showed impaired preference for the platform location during the probe trial indicating a deficit in spatial learning and memory. This deficit is strongest in the male cKO, although female cKO also failed to show a spatial bias for the target quadrant. Female controls showed a less pronounced spatial bias relative to control males, consistent with some prior evidence of relative spatial deficits in females (Yagi and Galea 2019). This reduced bias likely created a floor effect such that the target quadrant preference in the female cKO mice was not significantly reduced relative to female controls. The overall sex-dependent pattern in spatial performance mirrors the differences that we observed in alterations within the hippocampal neurogenic niche: a relative reduction in control females relative to control males and a specific reduction in male cKO relative to male controls. These findings mirror our prior results which showed full body α7 KO produced sex dependent alterations reduction in hippocampal neurogenesis and a male-specific deficit in spatial pattern separation (Otto and Yakel 2019). Together these findings suggest a possible relationship between the neurogenic niche and overall spatial performance. Postnatal hippocampal neurogenesis has been shown to have little role in short term spatial memory in the water maze (Snyder et al. 2005), however it does impact spatial search strategies (Yu et al. 2019). The spatial impairment in the cKO mice could also be due to more direct effects of aberrant cholinergic signaling on encoding, retrieval and task performance. Prior studies have shown that hilar interneurons play a critical role in water maze performance, which argues that impaired cholinergic modulation of interneurons could mediate the observed spatial deficit in α7 cKO mice (Andrews-Zwilling et al. 2012).
In contrast to the spatial learning deficit, α7 cKO mice showed normal hippocampus-dependent performance in a number of other behavioral assays indicating that α7-dependent cholinergic modulation of interneurons plays a very specific role in hippocampus-dependent learning and memory. Spontaneous alternation, considered a measure of spatial working memory, was normal, indicating the deficit may be more specific to long term retention of the spatial location of the escape platform. In contrast to this, however, object location performance, which is a hippocampus-dependent form of object learning (see Cohen and Stackman 2015 for review), was intact, suggesting that some forms of long-term spatial learning and memory are unaffected in the α7 cKO mice. This task was complicated however, by a decrease in the overall time the α7 cKO mice spent investigating the objects. Despite this reduction, they showed a similar preference for the displaced object when normalizing by the total investigation time. The exact mechanisms underlying this reduced object investigation are unclear, however prior studies have linked general reactivity to novelty to hippocampal neurogenesis (Lemaire et al. 1999) and to differences in investigation of novel objects, in particular (Denny et al. 2012). The exact role of the hippocampus in object recognition tasks and the involvement of extra-hippocampal regions is a the subject of ongoing debate and research (Piterkin et al. 2008; Cohen and Stackman 2015). The present findings argue that α7 nAChRs expressed on GABAergic interneurons may contribute primarily to the overall level of object investigation without impacting the encoding or retrieval of object identity, spatial location or contextual information.
No differences were found in trace or contextual fear conditioning, both of which are hippocampus-dependent (Chowdhury et al. 2005), again confirming, the specificity of the spatial deficits. This is surprising given the well-established role for cholinergic and GABAergic signaling in hippocampus-dependent forms of fear conditioning (Moore et al. 2010; Cushman et al. 2014; Hersman et al. 2017). The increased thermal sensitivity suggests that pain sensitivity to the shock may have been altered in the α7 cKO mice, however the magnitude of the activity burst response to the shock did not differ. One possibility for the lack of an effect is that nicotinic manipulations have been shown to have opposing roles in the dorsal versus ventral hippocampus (Kenney et al. 2012). The effects of losing α7-mediated cholinergic modulation of GABAergic neurons in the whole hippocampus might therefore be counteracted by differential dorsal versus ventral effects.
Overall, we have shown that α7 nAChRs on GABAergic neurons play a sex dependent role in regulating radial glia-like cell quantity, social recognition, and spatial learning and memory. Loss of α7 nAChRs in GABAergic neurons appears to impact highly specific domains of hippocampal function as some hippocampus-dependent measures were unaffected. As our cKO was not localized to GABAergic neurons within the dorsal DG, connections between structure and behavior is limited. Additional research examining brain wide effects should be completed. The finding of a male-specific role for α7 nAChRs in the modulation of both neurogenesis-related mechanisms and behavior may provide a potential explanation for why there are sex differences in cholinergic related neuropsychiatric and neurodegenerative disorders.
Supplementary Material
Acknowledgements:
We would like to acknowledge Patricia Lamb for creation and maintenance of mouse lines and Charles J. Tucker for assistance with confocal microscopy. This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Adams CE, Yonchek JC, Schulz KM, et al. (2012) Reduced Chrna7 expression in mice is associated with decreases in hippocampal markers of inhibitory function: Implications for neuropsychiatric diseases. Neuroscience 207:274–282. 10.1016/j.neuroscience.2012.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein I, Slomianka L, Poletaeva II, et al. (2004) Marked species and age-dependent differences in cell proliferation and neurogenesis in the hippocampus of wild-living rodents. Hippocampus 14:1000–1010. 10.1002/hipo.20018 [DOI] [PubMed] [Google Scholar]
- Andrews-Zwilling Y, Gillespie AK, Kravitz A V., et al. (2012) Hilar GABAergic Interneuron Activity Controls Spatial Learning and Memory Retrieval. PLoS One 7:e40555. 10.1371/journal.pone.0040555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroor AR, DeMarco VG (2014) Oxidative stress and obesity: The chicken or the egg? Diabetes 63:2216–2218. 10.2337/db14-0424 [DOI] [PubMed] [Google Scholar]
- Asrican B, Paez-Gonzalez P, Erb J, Kuo CT (2016) Cholinergic circuit control of postnatal neurogenesis. Neurogenesis 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates RC, Stith BJ, Stevens KE, Adams CE (2014) Reduced Chrna7 expression in C3H mice is associated with increases in hippocampal parvalbumin and glutamate decarboxylase-67 (GAD67) as well as altered levels of GABAA receptor subunits. Neuroscience 273:52–64. 10.1016/j.neuroscience.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D, Lee C-HL, Flood D, et al. (2015) Therapeutic Potential of a7 Nicotinic Acetylcholine Receptors. Pharmacol Rev Pharmacol Rev 67:1025–1073. 10.1124/pr.113.008581 [DOI] [PubMed] [Google Scholar]
- Brydges NM, Moon A, Rule L, et al. (2018) Sex specific effects of pre-pubertal stress on hippocampal neurogenesis and behaviour. Transl Psychiatry 8:. 10.1038/s41398-018-0322-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell NR, Fernandes CC, Halff AW, Berg DK (2010) Endogenous signaling through α7-containing nicotinic receptors promotes maturation and integration of adult-born neurons in the hippocampus. J Neurosci 30:8734–8744. 10.1523/JNEUROSCI.0931-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper KB, McCarthy KD (2006) GFAP-positive progenitor cells produce neurons and oligodendrocytes throughout the CNS. Mol Cell Neurosci 31:676–684. 10.1016/j.mcn.2005.12.006 [DOI] [PubMed] [Google Scholar]
- Catavero C, Bao H, Song J (2018) Neural mechanisms underlying GABAergic regulation of adult hippocampal neurogenesis. Cell Tissue Res. 371:33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Yakel JL (2015) Activation of α7 nicotinic acetylcholine receptors increases intracellular cAMP levels via activation of AC1 in hippocampal neurons. Neuropharmacology 95:405–414. 10.1016/j.neuropharm.2015.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury N, Quinn JJ, Fanselow MS (2005) Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behav Neurosci 119:1396–1402. 10.1037/0735-7044.119.5.1396 [DOI] [PubMed] [Google Scholar]
- Cohen SJ, Stackman RW (2015) Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res 285:105–117. 10.1016/j.bbr.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman JD, Fanselow MS (2010) Fear Conditioning. 1:524–531 [Google Scholar]
- Cushman JD, Moore MD, Olsen RW, Fanselow MS (2014) The role of the δ GABA(A) receptor in ovarian cycle-linked changes in hippocampus-dependent learning and memory. Neurochem Res 39:1140–1146. 10.1007/s11064-014-1282-6 [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador FA, Soliakov L, Wonnacott S (2002) Nicotine activates the extracellular signal-regulated kinase 1/2 via the α7 nicotinic acetylcholine receptor and protein kinase A, in SH-SY5Y cells and hippocampal neurones. J Neurochem 80:520–530. 10.1046/j.0022-3042.2001.00725.x [DOI] [PubMed] [Google Scholar]
- Dalla C, Bangasser DA, Edgecomb C, Shors TJ (2007) Neurogenesis and learning: Acquisition and asymptotic performance predict how many new cells survive in the hippocampus. Neurobiol Learn Mem 88:143–148. 10.1016/j.nlm.2007.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ (2004) Minireview: Sex Differences in Adult and Developing Brains: Compensation, Compensation, Compensation. Endocrinology 145:1063–1068. 10.1210/en.2003-1504 [DOI] [PubMed] [Google Scholar]
- Denny CA, Burghardt NS, Schachter DM, et al. (2012) 4- To 6-Week-Old Adult-Born Hippocampal Neurons Influence Novelty-Evoked Exploration and Contextual Fear Conditioning. Hippocampus 22:1188–1201. 10.1002/hipo.20964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineley KT, Pandya AA, Yakel JL (2015) Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol. Sci 36:96–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer EM, Galea LAM (2003) Sex differences in cell proliferation, cell death and defensive behavior following acute predator odor stress in adult rats. Brain Res 975:22–36. 10.1016/S0006-8993(03)02542-3 [DOI] [PubMed] [Google Scholar]
- Fan C, Gao Y, Liang G, et al. Transcriptomics of Gabra4 knockout mice reveals common NMDAR pathways underlying autism, memory, and epilepsy. 10.1186/s13229-020-0318-9 [DOI] [PMC free article] [PubMed]
- Fernando RN, Eleuteri B, Abdelhady S, et al. (2011) Cell cycle restriction by histone H2AX limits proliferation of adult neural stem cells. Proc Natl Acad Sci U S A 108:5837–5842. 10.1073/pnas.1014993108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresti ML, Arisi GM, Shapiro LA (2011) Role of glia in epilepsy-associated neuropathology, neuroinflammation and neurogenesis. Brain Res. Rev 66:115–122 [DOI] [PubMed] [Google Scholar]
- Furutachi S, Miya H, Watanabe T, et al. (2015) Slowly dividing neural progenitors are an embryonic origin of adult neural stem cells. Nat Neurosci 18:657–665. 10.1038/nn.3989 [DOI] [PubMed] [Google Scholar]
- Galea LAM (2008a) Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res. Rev 57:332–341 [DOI] [PubMed] [Google Scholar]
- Galea LAM (2008b) Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res. Rev 57:332–341 [DOI] [PubMed] [Google Scholar]
- Gao J, Wang H, Liu Y, et al. (2014) Glutamate and GABA imbalance promotes neuronal apoptosis in hippocampus after stress. Med. Sci. Monit 20:499–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebara E, Bonaguidi MA, Beckervordersandforth R, et al. (2016) Heterogeneity of Radial Glia-Like Cells in the Adult Hippocampus. Stem Cells 34:997–1010. 10.1002/stem.2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves JT, Schafer ST, Gage FH (2016) Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 167:897–914 [DOI] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F (2006) Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol. Sci 27:482–491 [DOI] [PubMed] [Google Scholar]
- Gu Z, Smith KG, Alexander GM, et al. (2020) Hippocampal Interneuronal α7 nAChRs Modulate Theta Oscillations in Freely Moving Mice. Natl Institutes Heal 31:. 10.1016/j.celrep.2020.107740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Yakel JL (2011) Timing-Dependent Septal Cholinergic Induction of Dynamic Hippocampal Synaptic Plasticity. Neuron 71:155–165. 10.1016/j.neuron.2011.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haam J, Yakel JL (2017) Cholinergic modulation of the hippocampal region and memory function. J. Neurochem 142:111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez CM, Cortez I, Gu Z, et al. (2014) Research tool: Validation of floxed α7 nicotinic acetylcholine receptor conditional knockout mice using in vitro and in vivo approaches. J Physiol 592:3201–3214. 10.1113/jphysiol.2014.272054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersman S, Cushman J, Lemelson N, et al. (2017) Optogenetic excitation of cholinergic inputs to hippocampus primes future contextual fear associations. Sci Rep 7:. 10.1038/s41598-017-02542-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John D, Shelukhina I, Yanagawa Y, et al. (2015) Functional alpha7 nicotinic receptors are expressed on immature granule cells of the postnatal dentate gyrus. Brain Res 1601:15–30. 10.1016/j.brainres.2014.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson Z (2005) Meta-analysis of sex differences in rodent models of learning and memory: A review of behavioral and biological data. Neurosci. Biobehav. Rev 28:811–825 [DOI] [PubMed] [Google Scholar]
- Jones S, Yakel JL (1997) Functional nicotinic ACh receptors on interneurones in the rat hippocampus. J Physiol 504:603–610. 10.1111/j.1469-7793.1997.603bd.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraska JM, Fitch JM, Henderson C, Rivers N (1985) Sex differences in the dendritic branching of dentate granule cells following differential experience. Brain Res 333:73–80. 10.1016/0006-8993(85)90125-8 [DOI] [PubMed] [Google Scholar]
- Kalman E, Keay KA (2017) Hippocampal volume, social interactions, and the expression of the normal repertoire of resident–intruder behavior. Brain Behav 7:. 10.1002/brb3.775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH (1997) More hippocampal neurons in adult mice living in an enriched environment. Nature 386:493–495. 10.1038/386493a0 [DOI] [PubMed] [Google Scholar]
- Kenney JW, Raybuck JD, Gould TJ (2012) Nicotinic receptors in the dorsal and ventral hippocampus differentially modulate contextual fear conditioning. Hippocampus 22:1681–1690. 10.1002/hipo.22003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunii Y, Zhang W, Xu Q, et al. (2015) CHRNA7 and CHRFAM7A mRNAs: Co-localized and their expression levels altered in the postmortem dorsolateral prefrontal cortex in major psychiatric disorders. Am J Psychiatry 172:1122–1130. 10.1176/appi.ajp.2015.14080978 [DOI] [PubMed] [Google Scholar]
- Lan A, Stein D, Portillo M, et al. (2019) Impaired innate and conditioned social behavior in adult C57Bl6/J mice prenatally exposed to chlorpyrifos. Behav Brain Funct 15:. 10.1186/s12993-019-0153-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V, Aurousseau C, Le Moal M, Abrous DN (1999) Behavioural trait of reactivity to novelty is related to hippocampal neurogenesis. Eur J Neurosci 11:4006–4014. 10.1046/j.1460-9568.1999.00833.x [DOI] [PubMed] [Google Scholar]
- Li R, Bilkei-Gorzo A, Sakkattu Rao M, et al. (2019) Increased Anxiety-Related Behavior, Impaired Cognitive Function and Cellular Alterations in the Brain of Cend1-deficient Mice. 10.3389/fncel.2018.00497 [DOI] [PMC free article] [PubMed]
- Linn MC, Petersen AC (2016) Emergence and Characterization of Sex Differences in Spatial Ability: A Meta-Analysis. 10.1111/j.1467-8624.1985.tb00213.x [DOI] [PubMed]
- Ludewig K, Geyer MA, Vollenweider FX (2003) Deficits in prepulse inhibition and habituation in never-medicated, first-episode schizophrenia. Biol Psychiatry 54:121–128. 10.1016/s0006-3223(02)01925-x [DOI] [PubMed] [Google Scholar]
- Ma KG, Qian YH (2019) Alpha 7 nicotinic acetylcholine receptor and its effects on Alzheimer’s disease. Neuropeptides 73:96–106 [DOI] [PubMed] [Google Scholar]
- Martin DLTAJ (2000) Mechanisms controlling GABA synthesis and degradation in the brain. In: GABA in the nervous system. pp 25–41 [Google Scholar]
- Moore MD, Cushman J, Chandra D, et al. (2010) Trace and contextual fear conditioning is enhanced in mice lacking the α4 subunit of the GABAA receptor. Neurobiol Learn Mem 93:383–387. 10.1016/j.nlm.2009.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, et al. (2012) Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell 149:188–201. 10.1016/j.cell.2012.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto SL, Yakel JL (2019) The α7 nicotinic acetylcholine receptors regulate hippocampal adult-neurogenesis in a sexually dimorphic fashion. Brain Struct Funct 224:829–846. 10.1007/s00429-018-1799-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paylor R, Spencer CM, Yuva-Paylor LA, Pieke-Dahl S (2006) The use of behavioral test batteries, II: Effect of test interval. Physiol Behav 87:95–102. 10.1016/j.physbeh.2005.09.002 [DOI] [PubMed] [Google Scholar]
- Pelkey KA, Chittajallu R, Craig MT, et al. (2017) Hippocampal gabaergic inhibitory interneurons. Physiol Rev 97:1619–1747. 10.1152/physrev.00007.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piterkin P, Cole E, Cossette M-P, et al. (2008) A limited role for the hippocampus in the modulation of novel-object preference by contextual cues. Learn Mem 15:785–791. 10.1101/lm.1035508 [DOI] [PubMed] [Google Scholar]
- Radcliffe KA, Fisher JL, Gray R, Dani JA (1999) Nicotinic modulation of glutamate and GABA synaptic transmission in hippocampal neurons. Ann N Y Acad Sci 868:591–610. 10.1111/j.1749-6632.1999.tb11332.x [DOI] [PubMed] [Google Scholar]
- Raybuck JD, Lattal KM (2014) Bridging the interval: Theory and neurobiology of trace conditioning. Behav Processes 101:103–111. 10.1016/j.beproc.2013.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley TJ, Payappilly J, Lu J, Flood P (2008) The Antinociceptive Response to Nicotinic Agonists in a Mouse Model of Postoperative Pain. Anesth Analg 107:1052–1057. 10.1213/ane.0b013e318165e0c0 [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Korn MJ, Shan Z, Ribak CE (2005) GFAP-expressing radial glia-like cell bodies are involved in a one-to-one relationship with doublecortin-immunolabeled newborn neurons in the adult dentate gyrus. Brain Res 1040:81–91. 10.1016/j.brainres.2005.01.098 [DOI] [PubMed] [Google Scholar]
- Shoji H, Miyakawa T (2018) Relationships between the acoustic startle response and prepulse inhibition in C57BL/6J mice: a large-scale meta-analytic study. Mol Brain 11:42. 10.1186/s13041-018-0382-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, et al. (2002) Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus 12:578–584. 10.1002/hipo.10103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Southard MC, Adam SJ, et al. (2004) α7 Nicotinic acetylcholine receptors targeted by cholinergic developmental neurotoxicants: Nicotine and chlorpyrifos. Brain Res Bull 64:227–235. 10.1016/j.brainresbull.2004.07.005 [DOI] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM (2005) A role for adult neurogenesis in spatial long-term memory. Neuroscience 130:843–852. 10.1016/j.neuroscience.2004.10.009 [DOI] [PubMed] [Google Scholar]
- Song J, Zhong C, Bonaguidi MA, et al. (2012a) Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 489:150–154. 10.1038/nature11306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Zhong C, Bonaguidi MA, et al. (2012b) Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 489:150–154. 10.1038/nature11306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens KE, Choo KS, Stitzel JA, et al. (2014) Long-term improvements in sensory inhibition with gestational choline supplementation linked to α7 nicotinic receptors through studies in Chrna7 null mutation mice. Brain Res 1552:26–33. 10.1016/j.brainres.2014.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan A, Ma W, Vira A, et al. (2016) The human hippocampus is not sexually-dimorphic: Meta-analysis of structural MRI volumes. Neuroimage 124:350–366. 10.1016/j.neuroimage.2015.08.050 [DOI] [PubMed] [Google Scholar]
- Thomsen M, Hansen H, Timmerman M, Mikkelsen J (2009) Cognitive Improvement by Activation of α7 Nicotinic Acetylcholine Receptors: From Animal Models to Human Pathophysiology. Curr Pharm Des 16:323–343. 10.2174/138161210790170094 [DOI] [PubMed] [Google Scholar]
- Vicidomini C, Guo N, Sahay A (2020) Communication, Cross Talk, and Signal Integration in the Adult Hippocampal Neurogenic Niche. Neuron 105:220–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Anderson ML, Shors TJ (2011) Changing the rate and hippocampal dependence of trace eyeblink conditioning: Slow learning enhances survival of new neurons. Neurobiol Learn Mem 95:159–165. 10.1016/j.nlm.2010.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Davis KM, Wu H, Wu JY (2004) Protein phosphorylation of human brain glutamic acid decarboxylase (GAD)65 and GAD67 and its physiological implications. Biochemistry 43:6182–6189. 10.1021/bi0496992 [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Den Boer JA, Veenhuis M, Ter Horst GJ (2004) Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res Bull 64:303–308. 10.1016/j.brainresbull.2004.08.006 [DOI] [PubMed] [Google Scholar]
- Yagi S, Galea LAM (2019) Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology 44:200–213. 10.1038/s41386-018-0208-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu RQ, Cooke M, Seib DR, et al. (2019) Adult neurogenesis promotes efficient, nonspecific search strategies in a spatial alternation water maze task. Behav Brain Res 376:. 10.1016/j.bbr.2019.112151 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.