Abstract
One of the sequelae of chronic alcohol abuse is malnutrition. Importantly, a deficiency in thiamine (vitamin B1) can result in the acute, potentially reversible neurological disorder Wernicke encephalopathy (WE). When WE is recognized, thiamine treatment can elicit a rapid clinical recovery. If WE is left untreated, however, patients can develop Korsakoff syndrome (KS), a severe neurological disorder characterized by anterograde amnesia. Alcohol-related brain damage (ARBD) describes the effects of chronic alcohol consumption on human brain structure and function in the absence of more discrete and well-characterized neurological concomitants of alcoholism such as WE and KS. Through knowledge of both the well-described changes in brain structure and function that are evident in alcohol-related disorders such as WE and KS and the clinical outcomes associated with these changes, researchers have begun to gain a better understanding of ARBD. This Review examines ARBD from the perspective of WE and KS, exploring the clinical presentations, postmortem brain pathology, in vivo MRI findings and potential molecular mechanisms associated with these conditions. An awareness of the consequences of chronic alcohol consumption on human behavior and brain structure can enable clinicians to improve detection and treatment of ARBD.
Introduction
Alcoholism is an addictive disorder with multi faceted biological underpinnings (Box 1). Frequent concomitants of alcoholism are liver disease (steatosis, hepatitis and cirrhosis),1 cardiovascular disease2 and mal nutrition.3 The neurological consequences associated with this addictive disorder include hepatic encephalopathy, Wernicke encephalopathy (WE), Korsakoff syndrome (KS), Marchiafava–Bignami disease (MBD) and central pontine myelinolysis (CPM). Each of these relatively well-characterized alcohol-related CNS disorders is associated with a unique clinical presentation and a discrete neuropathological and neuroradiological signature (Figure 1).4 The structural changes to the brain and functional consequences that occur with chronic alcohol consumption in the absence of diagnosable neuro logical concomitants of alcoholism (that is, in cases of uncomplicated alcoholism) are grouped under the term ‘alcohol-related brain damage’ (ARBD).
Box 1 |. Prelude to alcoholism.
Alcoholism is the product of multiple interacting factors including complex genetics, the environment, predisposing personality characteristics, and psychiatric comorbidities.139 Variation in genes that modify the metabolism of ethanol, such as those encoding alcohol dehydrogenase140 or aldehyde dehydrogenase,140 has been shown to influence the risk of this condition. Environmental factors that increase vulnerability to alcoholism include severe childhood trauma (for example, emotional, physical or sexual abuse), maternal depression, and lack of peer or family support.141 An example of a gene–environment interaction that may contribute to the development of alcoholism is the influence of the environment on monoamine oxidase A (MAO-A), an enzyme that is important for the normal functioning of the serotonergic system. Studies have shown that young boys who experience a traumatic event can develop low levels of MAO-A expression, and that this decrease in MAO-A levels correlates with an increase in antisocial behavior, an antecedent of alcohol addiction.142,143
The offspring of individuals with alcoholism can display mild dysfunction of the frontal cortex,144 expressed as personality traits such as impulsivity, aggressiveness and perseveration, that increase their risk for alcoholism.145,146 Similarly, the mesolimbic dopaminergic system, which subserves reward-dependent behaviors, may be dysfunctional (that is, it confers an attenuated response to natural reward in certain individuals), thereby increasing the risk of severe alcoholism.147 Common psychiatric illnesses associated with alcoholism include schizophrenia,148,149 bipolar disorder,150 major depression,151 antisocial personality disorder,152,153 and general anxiety disorder.154
Figure 1 |.
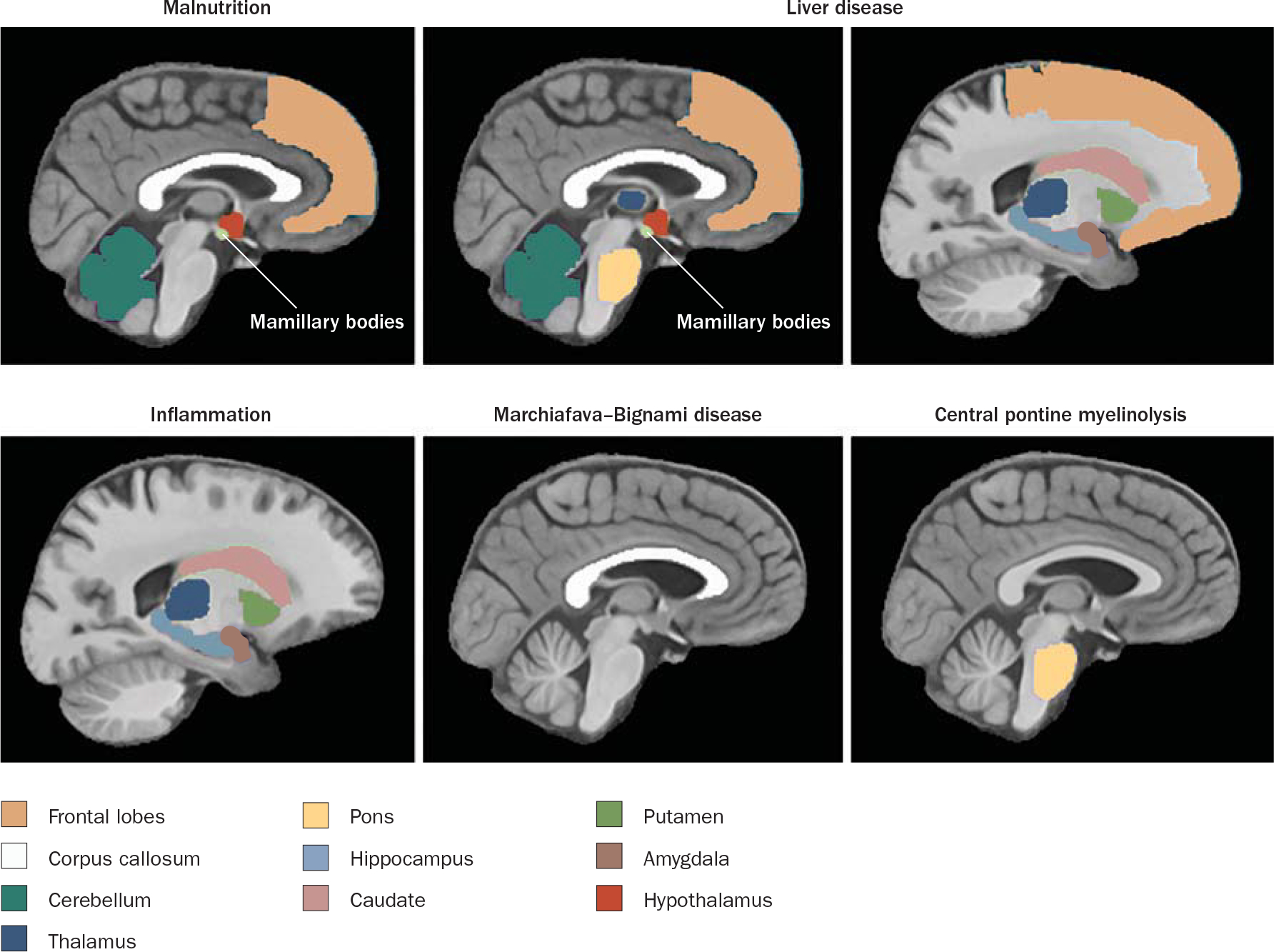
Brain regions targeted by alcohol-related disease. Figure courtesy of A. Pfefferbaum, SRI International, CA, USA and E. V. Sullivan, Stanford University, CA, USA.
Whether ARBD represents one end of a continuum of neurological deficits, with disorders such as KS and MBD at the other end,5 or one outcome in a range of dis continuous, graded deficits occurring with chronic alcohol exposure and, for example, aging6 remains unclear. In addition, whether people with certain genotypes (for example, individuals who are genetically susceptible to mal nutrition or liver compromise) are at a greater risk of particular neuro logical conditions and, consequently, are more likely to express specific alcoholism-related neuropsychological compromise than are individuals with a different genetic make-up remains to be determined. Our objective here is to describe a potential continuum between ARBD, WE and KS with respect to changes in human behavior and brain structure. Note that while this Review is extensive, it is not intended to be exhaustive.
The Wernicke–Korsakoff syndrome
WE is an acute, potentially reversible neurological disorder caused by a deficiency in or severe depletion of thiamine (vitamin B1) that can result from chronic alcoholism, poor nutrition, long-term parenteral feeding, hyper emesis gravi darum or bariatric surgery.7,8 Incidence rates of WE in the general population—on the basis of autopsy findings in Western countries—range from 0.1–2.8%, but can be as high as 12.5% in patients with alcoholism.9,10 Such individuals are at a high risk of thiamine deficiency because of the poor diet associated with their lifestyle, and the fact that chronic alcoholism compromises thiamine absorption from the gastro intestinal tract, impairs thiamine storage, and may reduce the phosphorylation of thiamine to its biologically active form, thiamine pyrophosphate (TPP; Figure 2).11–15
Figure 2 |.
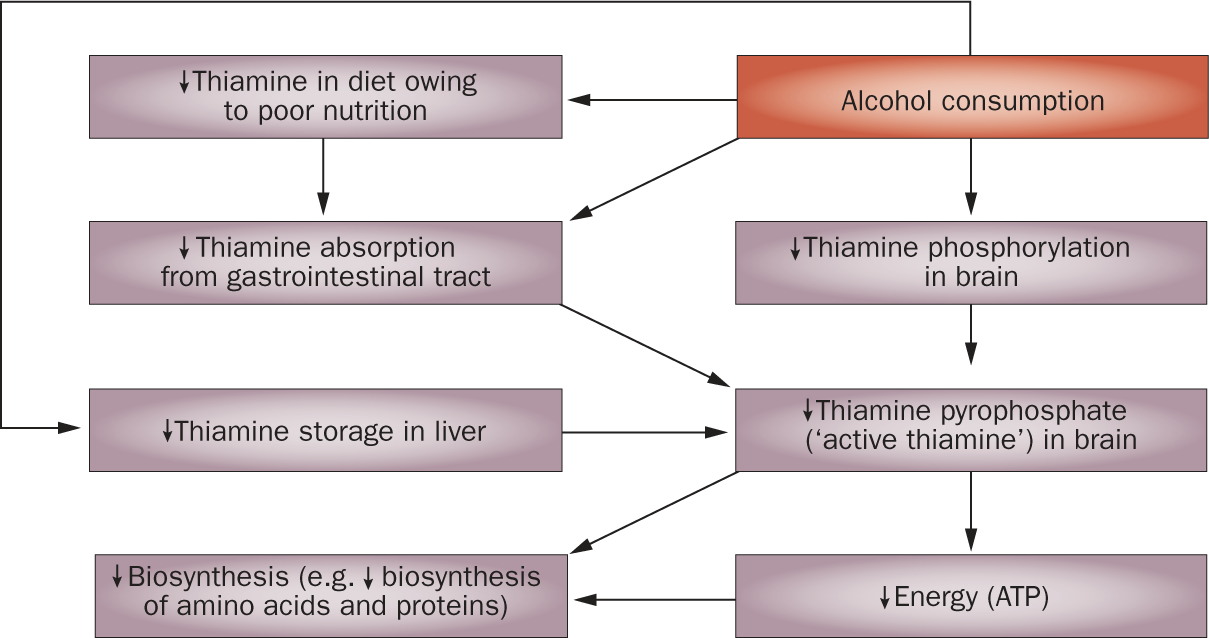
Interactions between alcohol consumption and thiamine deficiency.
Guidelines for the diagnosis, treatment and prevention of WE have been released by the European Federation of Neurological Societies (EFNS), and are based on three decades of research into this condition (Box 2).16 If WE is recognized, treatment with thiamine can result in rapid clinical improvement.10 Indeed, the prevalence of WE has been reduced in a number of countries (including the US, the UK and Australia) that have instituted nationwide thiamine supplementation in staple foods such as bread.17
Box 2 |. EFNS guidelines for diagnosis, therapy and prevention of WE.
The clinical diagnosis of WE should take into account the different presentations of clinical signs between individuals with and without alcoholism; although the prevalence of WE is higher in the former than the latter group, WE should be suspected in all clinical conditions that could lead to thiamine deficiency
The clinical diagnosis of WE in patients with alcoholism requires the presence of two of the following four signs: dietary deficiencies, eye signs, cerebellar dysfunction, and either an altered mental state or mild memory impairment
Total thiamine levels in a blood sample should be measured immediately before thiamine administration
MRI should be used to support the diagnosis of acute WE in patients both with and without alcoholism
Thiamine is indicated for the treatment of suspected or manifest WE, and should be administered before any carbohydrate at a dose of 200 mg three times daily, preferably intravenously
The overall safety of thiamine is very good
After bariatric surgery, thiamine status should be monitored for at least 6 months and be accompanied by parenteral thiamine supplementation
Parenteral thiamine should be given to all at-risk individuals admitted to an emergency room
Patients who die from symptoms suggesting WE should have an autopsy
Derived from Galvin, R. et al. (2010).16 Abbreviations: EFNS, European Federation of Neurological Societies; WE, Wernicke encephalopathy.
When WE is left undiagnosed and untreated, ≈80% of patients with this condition develop KS, a severe, typically permanent neurological disorder characterized by anterograde amnesia.18 The term Wernicke–Korsakoff syndrome (WKS) is used to denote the range of brain and behavioral impairments associated with thiamine deficiency.19,20
Clinical and psychological features
Clinicians are often taught to diagnose WE on the basis of the presence of the classic clinical triad of ocular motor abnormalities, cerebellar dysfunction, and altered mental state. Ocular motor abnormalities occur in ≈30% of patients with WE and may include nystagmus or ophthalmo plegia, while cerebellar dysfunction can be found in ≈25% of patients with this disorder and may manifest as loss of equilibrium, incoordination of gait, trunk ataxia, dysdiadochokinesia and, occasionally, limb ataxia or dysarthria. Approximately 80% of patients with WE exhibit an altered mental state, which may comprise mental sluggishness, apathy, impaired awareness of an immediate situation, an inability to concentrate, confusion or agitation, hallucinations, behavioral disturbances mimicking an acute psychotic disorder, or coma.3,10,21 A retrospective analysis of the clinical signs and symptoms of patients diagnosed at autopsy as having WE revealed that only 20% of patients with this disorder presented with the full triad of clinical features and ≈30% of such indivi duals exhibited only cognitive impairment.21 Thus, through the requirement of the full triad for a positive diagnosis, WE is missed by routine clinical examination in 75–80% of cases, even in teaching hospitals. By contrast, the presence of just two of four signs (dietary deficiency, ocular motor abnormality, cerebellar dysfunction, and either altered mental state or mild memory impairment), which was first suggested by Caine and colleagues22 and is now recommended by the EFNS,16 can significantly improve the diagnostic accuracy for WE.
The most salient characteristic of KS is global amnesia.18 Neuropsychological assessments of multi ple functional domains targeting executive functions, declarative and procedural memory, visuospatial abilities and postural stability have revealed that individuals with KS have severe deficits in memory for new material and in gait and balance, despite sparing of general intelligence, short-term memory and visuoperceptual implicit learning.23–26 Patients with KS may also exhibit prefrontal neurobehavioral dysfunction, expressed as deficits on tests of problem solving, working memory, cognitive flexibility, perseverative responding, and self-regulation.27–29
Over 80% of individuals with uncomplicated alcoholism (that is, ARBD) are estimated to show cognitive deficits in executive functions,30,31 although such deficits are mild in comparison with those observed in patients with KS.32 Individuals with uncomplicated alcoholism also demonstrate deficits in explicit memory, visuospatial processes and motor control (for example, speed, gait and balance; Figure 3).33,34
Figure 3 |.
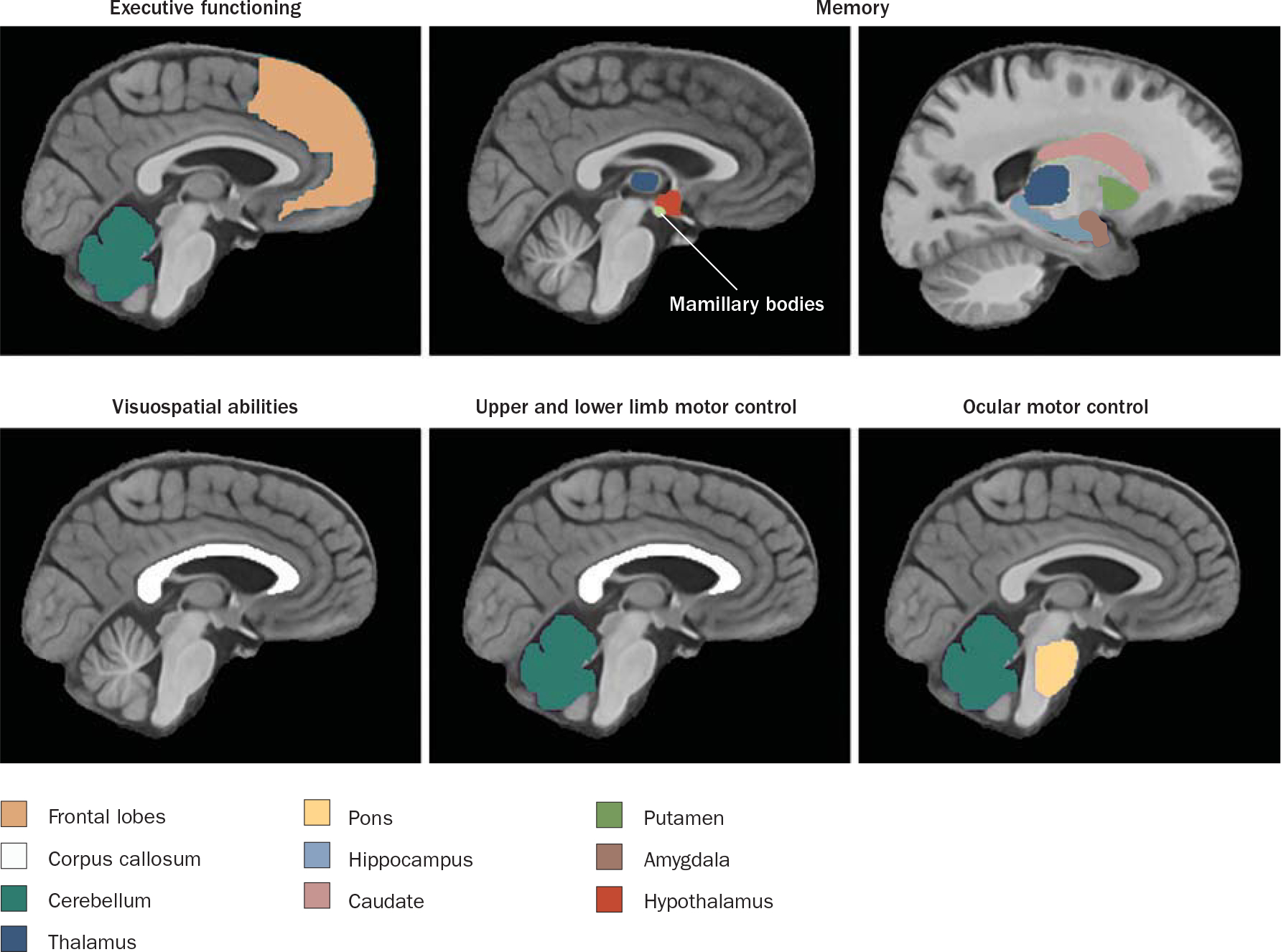
Functions and associated brain regions targeted by alcohol abuse and alcoholism. Figure courtesy of A. Pfefferbaum, SRI International, CA, USA and E. V. Sullivan, Stanford University, CA, USA.
The neuropsychological expression of ARBD is marked by heterogeneity in the extent (severity) and type (component) of deficit, and not all individuals with uncomplicated alcoholism exhibit impairments in all the functions described above.33 Similarly, not all patients with KS have permanent amnesia.35 This heterogeneity suggests that the functions affected by chronic alcohol consumption are dissociable and supported by different neural systems.36 One study examined the component processes of episodic memory (memory of auto biographical events) and working memory (the ability to hold information ‘online’ while doing complex tasks) in individuals with uncomplicated alcoholism and patients with alcoholism and KS. The former could generally be differentiated from the latter via performance on tests of episodic memory (that is, the two groups showed graded impairment in episodic memory), despite significant overlap in performance on working-memory tasks between the two groups (that is, continuous impairment was seen between the two groups).37 On the basis of these findings, the study’s researchers suggested that impairment of episodic memory was the result of the untoward effects of alcohol on the Papez (limbic) circuit and was exacerbated by thiamine deficiency, while the observed impairment in working memory, which was not specific to KS, may have reflected the effects of chronic alcohol consumption on frontocerebellar circuitry.38
In an attempt to explain the cognitive heterogeneity commonly seen in patients with alcoholism, a recent prospective study applied the operational criteria of Caine and colleagues to a group of individuals with uncomplicated alcoholism, so as to determine whether the presence of any of the four signs, determined by history or current examination, could be used to predict performance on a battery of neuropsychological tests. Among the 56 patients with uncomplicated alcoholism who were assessed, 16% displayed two or more signs, 57% showed only one sign, and 27% met no criteria. In this sample of sober, community-dwelling individuals, self-reported dietary deficiency (n = 29) and cerebellar dysfunction (that is, ataxia; n = 20) were frequently described, while oculomotor abnormalities (n = 2) and mental impairment (n = 0) were rarely observed.39 This study revealed a graded effect in cognitive and motor performance among patient subgroups: individuals with alcoholism who did not meet any criteria performed at levels equivalent to healthy controls, whereas patients with one sign showed mild-to-moderate neuro psychological deficits, and patients with two or more signs showed the most severe deficits on each neuro psychological domain evaluated. This graded effect suggests that the heterogeneity in the severity of cognitive and motor deficits seen in patients with uncomplicated alcoholism can be accounted for, in part, by the number of WE signs present.
Postmortem pathological features
The neuropathological changes observed in WKS, as described by Victor and Adams in the early 1970s, include lesions in periventricular regions around the third and fourth ventricles, and atrophy of the mamillary bodies.40 By contrast, traditional clinical pathological methods have only been able to demonstrate mild cerebral atrophy and lower mean brain weight in cases of uncomplicated alcoholism.41–43 Thus, quantitative studies are required to characterize the relatively subtle structural abnormalities in the brain that are caused by the direct effects of alcohol (Box 3).
Box 3 |. The New South Wales Tissue Resource Centre.
For the past 25 years, the New South Wales Tissue Resource Centre ‘brain bank’155 has provided much of the postmortem tissue (fresh-frozen and formalin-fixed) used by research groups throughout the world to explore the effects of alcohol on the brain.156–158 The validity of research using brain bank material largely depends on careful clinical and pathological characterization of each case, precise matching to control cases, and appropriate storage. Brain bank tissue has been used for structural and molecular studies and to test hypotheses developed from animal models and in vivo studies. To ensure the long-term success of the brain bank, their premortem, ‘in-life’ donor program carefully details the lifestyle and medical histories of individuals who have committed to donating their tissue on their death.159,160 An important advantage of this tissue is that it is not restricted to the small sample of patients with alcoholism who are in treatment (estimated to be 25% of the total population of individuals with alcoholism in the USA), a limitation of in vivo studies, which typically rely on treatment-seeking patients.161
In one quantitative study, brain volume with respect to intracranial cavity volume was determined and the mean pericerebral space was shown to rise from 8.3% of the total intracranial cavity volume in healthy controls to 11.3% in patients with ARBD and 14.7% in patients with WKS.44 Stereometric studies have suggested that this reduction in brain volume is largely accounted for by the shrinkage of white matter.45–47 Cerebellar white matter volume (especially in the vermis) is reduced48 and the corpus callo sum area is significantly thinned in individuals with alcoholism,49,50 especially those with nutritional deficiencies,51 compared with healthy controls. This finding may represent a dose effect of alcohol rather than an effect of thiamine deficiency, as white matter volume was negatively correlated with maximum daily alcohol consumption.47 The nature of the white matter loss remains unknown; however, this phenomenon probably involves changes in both myelination and axonal integrity.52
In addition to atrophy of the mamillary bodies, WKS reveals neuronal loss in the anterior principal and medio-dorsal nuclei of the thalamus53 and in the basal forebrain.54 In patients with alcoholism and signs of WE, a reduction in Purkinje cell density and molecular layer atrophy are noted in the cerebellum, suggesting that this brain region is selectively vulnerable to thiamine deficiency.55 In patients with uncomplicated alcoholism, microscopic studies have revealed an ≈25% loss of pyramidal neurons in the superior frontal and frontal association (dorsolateral portion) cortices.56,57 Little evidence exists for neuronal loss in the primary motor cortex in ARBD. However, a silver impregnation technique has shown that pyramidal neurons in both the superior frontal and motor cortices have dendritic arbor shrinkage,58 indicating compromise in interneuronal communication. Dendritic shrinkage has been shown to be reversible in a rodent model of alcoholism following a prolonged period of abstinence.59 Subcortical regions of brains from patients with uncomplicated alcoholism exhibit neuronal loss in the supraoptic and para ventricular nuclei of the hypothalamus that shows a positive correlation with maximum daily alcohol consumption.60 With respect to the cerebellum, pathological studies do not consistently show a decrease in the number of neurons in cases of ARBD compared with normal controls, suggesting that chronic alcohol consumption per se does not necessarily cause neuronal death in this region of the brain. No changes have been documented in the number of neurons in the basal ganglia,61 hippocampus57,62 or serotonergic raphe nuclei63 in ARBD.
In vivo MRI features
MRI has shown that patients with KS have an increase in cerebrospinal fluid volume and widespread gray matter volume deficits.5,64 Moreover, group analysis has revealed substantial volume changes in the mamillary bodies of individuals with KS,65,66 although mamillary body shrinkage is not a necessary concomitant of this condition.3,67 MRI has also demonstrated volume losses in the orbito-frontal cortices and other hypothalamic nuclei in KS.5 The volume losses that best differentiate KS from uncomplicated alcoholism, however, involve the thalamus.68 In acute WE, MRI can be used to detect symmetrical, bi lateral hyperintense foci (clearly visible on T2-weighted and fluid-attenuated inversion recovery images) in peri-aqueductal gray matter, the mamillary bodies, and the tissue surrounding the third ventricle.5,69
In patients with uncomplicated alcoholism, MRI studies have generally confirmed postmortem studies by demonstrating that such patients have regional cortical volume deficits,64,70 especially in the frontal lobes.71,72 Among individuals with alcoholism, cerebral shrinkage is more pronounced in older patients than in younger patients, suggesting that the aging brain is especially susceptible to ARBD.70,73 Structural MRI has also demonstrated that individuals with alcoholism have significant volume deficits in the corpus callosum74,75 and cerebellar white matter.48,76 In contrast to postmortem findings, MRI has provided in vivo evidence for volume deficits in the an terior hippocampus of patients with chronic alcoholism.77,78 These deficits are potentially accounted for by a loss of non-neuronal cells; that is, glia. Altogether, use of MRI to characterize WKS structural brain changes in the context of the neuropathology of uncomplicated alcoholism has revealed a graded pattern of volume deficits (from mild deficits in ARBD to moderate or severe deficits in WKS) in the mamillary bodies, hippocampus, thalamus, cerebellum and pons (Figure 4).5 As brain regions outside those traditionally associated with thiamine depletion (for example, the frontal cortices, hippocampus and pons) are affected in both uncomplicated alcoholism and KS, alcohol ism alone or in combination with nutritional deficiencies may have roles in the mechanisms under lying these brain abnormalities. Indeed, multiple subclinical episodes of thiamine deficiency or other nutritional deficiencies may contribute to the graded nature of brain regional volume deficits and to the heterogeneity in presenting signs and neuro radiological profiles in patients with alcoholism.79
Figure 4 |.
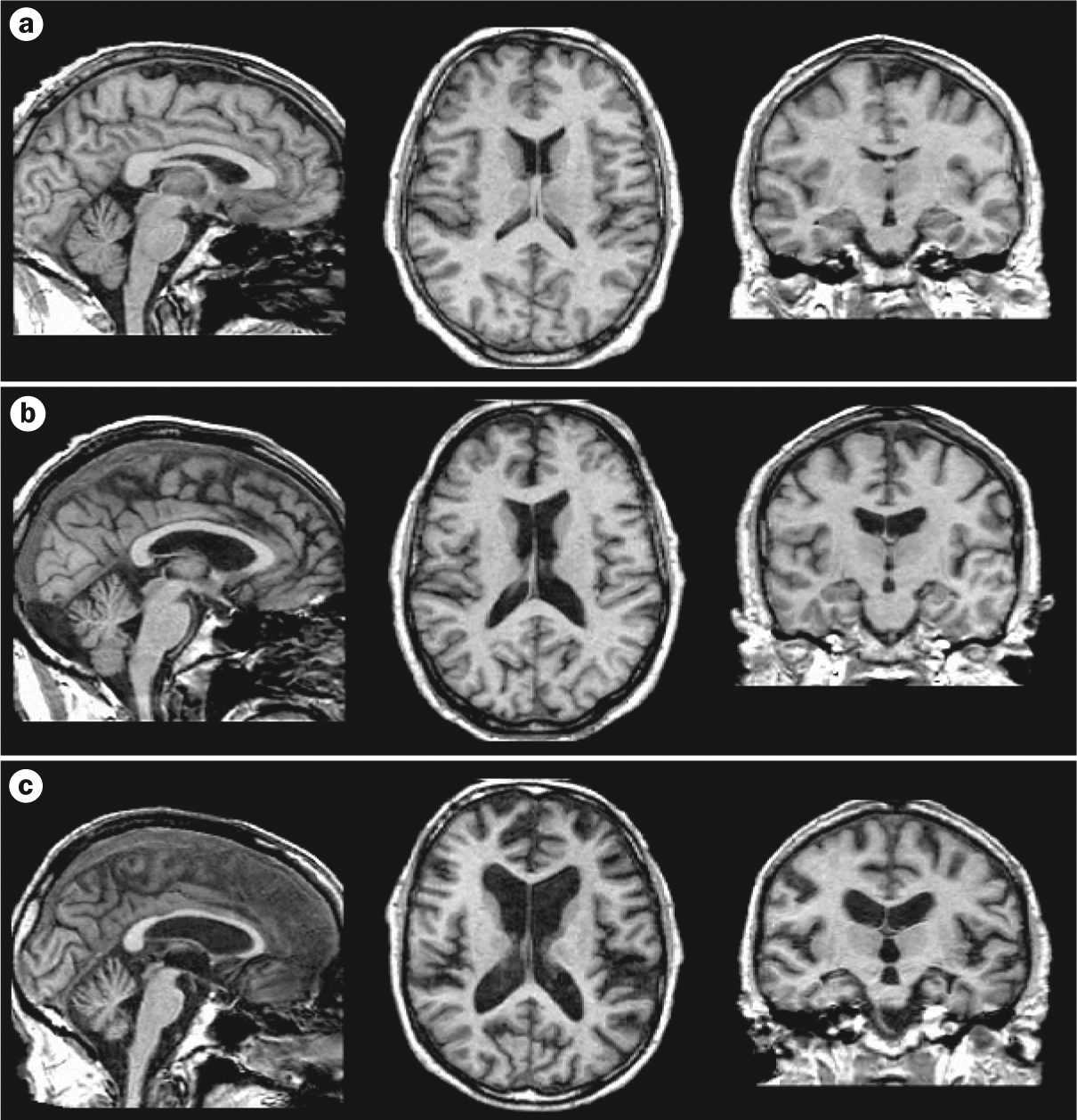
Graded brain-volume deficits in alcoholism and its sequelae. T1-weighted MRI scans from a | a 63-year-old healthy control male, b | a 59-year-old man with alcoholism, and c | a 63-year-old man with WKS. Graded enlargement of the ventricles (indicating shrinkage of the surrounding tissue) can be observed from the healthy control to the individual with WKS. Sagittal (left column), axial (middle column) and coronal (right column) brain images are shown. Abbreviation: WKS, Wernicke–Korsakoff syndrome. Figure courtesy of A. Pfefferbaum, SRI International, CA, USA.
In contrast to postmortem studies, in vivo magnetic resonance (MR) modalities constitute safe, noninvasive methods for longitudinal examination of the condition of the brain in patients with alcoholism during the natural course of chronic alcohol consumption, detoxification, abstinence or relapse. Such MR studies have demonstrated that some structural brain changes are reversible with prolonged abstinence from alcohol.72,80–82 Indeed, ARBD may have two components, one of which is transient and the other being permanent.83 If brain volume loss is due to neuronal loss, brain volume recovery will be incomplete with abstinence. For example, some MR spectroscopy studies have shown that in spite of prolonged abstinence, individuals who have chronically consumed alcohol demonstrate persistent N-acetylaspartate (a putative marker of neuronal integrity) decreases in the frontal lobes,84–86 the thalamus86 and the cerebellum.86,87 Other studies, however, have found improvements in the levels of N-acetylaspartate and choline—another metabolite that may indicate remyelination—with abstinence.88–90 Structural repair of myelin could explain the increase in white matter volume that has been shown to occur after periods of abstinence from alcohol.91–93
Another advantage of in vivo MR tools is the facility to conduct behavioral experiments concurrently with imaging, so as to determine brain structure–function relationships. Combined neuropsychological and neuroimaging studies suggest that the amnesia observed in KS may be caused by interruption of a complex diencephalic–hippocampal circuitry that includes thalamic nuclei and mamillary bodies, rather than through an insult to a single node in the circuit such as the hippo campus.94 This hypothesis has received support from a study using a novel ‘resting state’ functional MRI analysis, which demonstrated that improvement in memory function in patients recovering from WE parallels the level of mammillothalamic ‘functional connectivity’.95 Diffusion tensor imaging (DTI), which is particularly useful in the characterization of the integrity of white matter microstructure, supports a positive correlation between disruption of the microstructural integrity of the corpus callosum and deficits in visuospatial performance, gait and balance.81,96–98 In addition to confirming the contribution of cerebellar white matter volume loss (especially in the vermis) to ataxia in patients with chronic alcoholism, combined brain imaging and neuropsychological methods have demonstrated the importance of frontocerebellar connections99 to cognitive and sensory functioning,100–102 including perceptual motor tasks, executive functions, and learning and memory.99,103,104 Improvements with abstinence in brain structure and biochemical status have been demonstrated105 that correspond with improvement or reversal of functional deficits in working memory, postural stability and visuospatial ability.106,107
Molecular features
A number of incompletely understood, mutually inclusive mechanisms have been proposed to explain how ethanol causes brain damage (Box 4). These mechanisms include neurotoxicity of the ethanol molecule itself, and the consequences of nutritional deficiencies or liver dysfunction, each of which can lead to the intriguing possibility of alcohol-induced neuroinflammation.
Box 4 |. Select pathophysiological mechanisms underlying ARBD.
Ethanol-specific effects
Toxic metabolites of ethanol such as acetaldehyde or fatty acid ethyl esters can accumulate and lead to adduct formation, which can disorder lipids, interrupt mitochondrial function, and induce neuronal damage162,163
Ethanol increases the generation of reactive oxygen species (such as nitric oxide and lipid peroxidation products), which can accumulate and cause DNA damage, inhibition of gene expression, and neuronal death164–167
Ethanol lowers brain-derived neurotrophic factor levels and, hence, may impair intracellular signaling pathways involved in cell survival, growth and differentiation, thereby enhancing natural cell death168
Removal of ethanol causes brain disinhibition, dysregulation of glutamate release and uptake, and stimulation of NMDA receptors that mediate excitotoxicity169
Thiamine deficiency
Thiamine deficiency leads to low levels of thiamine pyrophosphate and, hence, impairment of several biochemical pathways in the brain, including carbohydrate metabolism (for energy production), lipid metabolism (for production and maintenance of myelin), and amino acid metabolism (for production of glucose-derived neurotransmitters; for example, glutamic acid and γ-aminobutyric acid)10
Liver dysfunction
A liver directly damaged by the toxic effects of ethanol is unable to remove neurotoxic substances such as ammonia and manganese from blood;170 accumulation of ammonia affects cerebral blood flow, metabolism and astrocytic function,171,172 while manganese at high levels can affect the dopaminergic system, enhance oxidative stress, and induce neurotoxicity173,174
Synergistic effects
Acetaldehyde protein adduct formation175 or oxidative stress176 may induce inflammatory liver damage,177 potentially resulting in a generalized immune response178 and, consequently, central inflammation110,179,180 and neuronal degeneration181
Liver dysfunction182 or thiamine deficiency113 can contribute to astrocytic pathology, which may compromise glutamate homeostasis and enhance NMDA-receptor-mediated excitoxicity
Abbreviations: ARBD, alcohol-related brain damage; NMDA, N-methyl-d-aspartate.
Neuroinflammation is considered to be involved in the pathogenesis and progression of many neurodegenerative disorders,108,109 and a mechanistic role for this process in ARBD has recently been proposed.110 The brain cells that might mediate neuroinflammation are microglia, which can exist in multiple states, with the activation of these cells resulting in either anti-inflammatory or pro inflammatory responses.111 A recent study investigated several markers of brain inflammation in ‘brain bank’ tissue from patients with alcoholism and individuals who had moderate levels of alcohol consumption. Various patterns of positive signs of neuroinflammation were identified in the ventral tegmental area, substantia nigra, hippocampus and amygdala in the individuals with alcoholism,110 providing some support for a mechanistic role for inflammation in al cohol-related alterations to the brain reward system.
As described above, individuals with alcoholism have a high risk of thiamine deficiency because of poor nutrition, impaired absorption of thiamine from the gastrointestinal tract, and reduced liver stores.112 Moreover, alcohol interferes with the conversion of thiamine to its metabolically active form, namely TPP.113 A reduction in TPP levels disrupts the following processes: carbohydrate metabolism, thereby interrupting energy production through the Krebs cycle and pentose phosphate pathway; lipid metabolism, thereby interrupting the production and maintenance of myelin; and amino acid metabolism, thereby interrupting the production of glucose-derived neurotransmitters.10 These metabolic deficits can contribute to neuronal and white matter damage.
Bouts of thiamine deficiency may occur in upwards of 80% of patients with alcoholism;114,115 however, only ≈13% of such individuals develop WKS,116 raising the possibility that a genetic predisposition to WKS may exist in some individuals.15 Some studies have shown that transketolase binds TPP less effectively in patients with WKS than in healthy controls.117,118 No consistent correlation, however, has been found between transketolase variants and thiamine deficiency.119 Other genetic loci or variants associated with WKS susceptibility include the X-linked transketolase-like 1 gene,120 the high-affinity thiamine transporter protein gene SLC19A2,121 the γ-aminobutyric acid A receptor subunit gene cluster on chromosome 5q33,122 and the aldehyde dehydrogenase-2 ADH21 allele.123 One possibility is that several genetic variants and environmental factors must be present to generate a WKS phenotype, which only becomes clinically relevant when an individual’s diet is deficient in thiamine.10
In recent years, high-throughput genomic and proteomic approaches have been used extensively to provide clues about the molecular mechanisms underlying ARBD. Oligonucleotide and complementary DNA microarray studies of samples of human frontal cortex from individuals with alcoholism have identified alcohol-responsive genes relating to several broad categories, namely myelination, synaptic structure, mitochondria, signal transduction, intracellular metabolism, protein trafficking, apoptosis and transcriptional regulation.124–127 Moreover, samples of human temporal cortex from patients with alcoholism have exhibited changes in the expression of genes encoding proteins related to mitochondria, the ubiquitin system or signal transduction.128 Alcohol-responsive genes expressed in the nucleus accumbens and the ventral tegmental area are primarily associated with changes in neurotransmission and signal transduction, suggesting neuroplastic changes that may contribute to changes in reward response. The results of such genomic approaches suggest that multiple pathways may be involved in causing altered neuronal function and structural changes in ARBD, although changes in myelin-related genes seem particularly important. Indeed, altered expression levels of proteolipid protein and myelin basic protein, both of which are involved in stabilization and compaction of the myelin sheath,129,130 could explain the structural and functional changes in white matter in patients with alcoholism.131
Protein expression studies have been conducted in various brain regions including the occipital cortex,127 hippo campus132 and cerebellum.133 Again, while such studies suggest that several pathways may be associated with ARBD, relevant protein expression studies in patients with uncomplicated alcoholism show dysregulation of key energy-regulating and metabolic proteins, notably those involved in thiamine-dependent cascades in prefrontal gray and white matter,134,135 cerebellar vermis133 and corpus callosum.136,137 These findings lend weight to the continuum hypothesis of ARBD, WE and KS.
Conclusions
We have described a potential continuum between ARBD, WE and KS with respect to changes in human behavior and brain structure. The clinical diagnosis of ARBD and WE remains difficult; however, an awareness of current research findings and a high index of suspicion can aid in the detection of these conditions. An intimate relationship seems to exist between alcohol use and thiamine deficiency, and we hypothesize that both ARBD and WE may develop as a result of repeated episodes of subclinical thiamine deficiency.138 Neuroradiological examination (with MRI) is a valuable tool in the diagnosis of acute WE and enables in vivo tracking of the progression of brain pathology from ARBD to KS. An awareness of the facts presented herein by clinicians and other health workers could help minimize the overall burden of ARBD. Moreover, public education programs should be promoted so that individuals using alcohol become aware of the associated risks and gain an understanding that components of the structural and functional changes linked to alcohol use are potentially reversible with abstinence.
Key points.
Alcohol can cause a spectrum of untoward structural and functional changes in the brain
The spectrum of disruption includes alcohol-related brain damage at one end and complications such as hepatic encephalopathy, Wernicke encephalopathy, Korsakoff syndrome, Marchiafava–Bignami disease and central pontine myelinolysis at the other
The clinical diagnoses of alcohol-related brain damage and even Wernicke encephalopathy can be difficult to make, and many cases of these conditions are missed
Changes to the brain associated with alcohol intake are regionally specific and can affect both gray and white matter; some of these changes are reversible with abstinence
Pathogenic mechanisms associated with alcoholism are under investigation, with neuroinflammation currently receiving particular attention
Review criteria.
Articles were selected on the basis of their contribution to the field of alcohol-related brain damage research, with a particular focus on neuropathological and neuroimaging studies in humans. Referenced articles were mostly identified from MEDLINE using access search engines PubMed and NLM Gateway. Articles were retrieved using keywords such as “alcohol”, “ethanol”, “alcoholism”, “brain damage”, “white matter loss”, “atrophy”, “neuropathology”, “Wernicke encephalopathy”, “Korsakoff psychosis”, “Wernicke–Korsakoff sydrome”, “thiamine deficiency”, “pathogenesis”, “neuroimaging”, “genomics” and “proteomics”. All articles cited were published in English and most represent peer-reviewed original research articles. This Review aimed to include the most recent pathological and radiological data; however, many articles date back to the 1970s and 1980s when much of the original neuropathology research was performed. Some review articles were also cited and their reference lists scrutinized. Related citation lists generated by PubMed searches were also a useful source of references.
Acknowledgments
The authors would like to thank E. V. Sullivan for her invaluable support and advice in preparing this Review. The authors would also like to thank A. Pfefferbaum for contributing the illustrations.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Lieber CS Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 34, 9–19 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Hillbom M, Juvela S & Karttunen V Mechanisms of alcohol-related strokes. Novartis Found. Symp 216, 193–204 (1998). [DOI] [PubMed] [Google Scholar]
- 3.Victor M, Adams RD & Collins GH The Wernicke–Korsakoff Syndrome and Related Neurologic Disorders due to Alcoholism and Malnutrition, 2nd edn (Davis FA, Philadelphia, 1989). [Google Scholar]
- 4.Zuccoli G et al. Neuroimaging findings in alcohol-related encephalopathies. AJR Am. J. Roentgenol 195, 1378–1384 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Sullivan EV & Pfefferbaum A Neuroimaging of the Wernicke–Korsakoff syndrome. Alcohol Alcohol. 44, 155–165 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oscar-Berman M & Marinkovic K Alcoholism and the brain: an overview. Alcohol Res. Health 27, 125–133 (2003). [PMC free article] [PubMed] [Google Scholar]
- 7.Harper C Thiamine (vitamin B1) deficiency and associated brain damage is still common throughout the world and prevention is simple and safe! Eur. J. Neurol 13, 1078–1082 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Burns EM et al. Introduction of laparoscopic bariatric surgery in England: observational population cohort study. BMJ 341, c4296 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Thomson AD, Cook CC, Touquet R & Henry JA The Royal College of Physicians report on alcohol: guidelines for managing Wernicke’s encephalopathy in the accident and emergency department. Alcohol Alcohol. 37, 513–521 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Sechi G & Serra A Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 6, 442–455 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Thomson AD, Jeyasingham MD, Pratt OE & Shaw GK Nutrition and alcoholic encephalopathies. Acta Med. Scand 717 (Suppl.), 55–65 (1987). [DOI] [PubMed] [Google Scholar]
- 12.Todd KG & Butterworth RF Early microglial response in experimental thiamine deficiency: an immunohistochemical analysis. Glia 25, 190–198 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Lieber CS Relationships between nutrition, alcohol use, and liver disease. Alcohol Res. Health 27, 220–231 (2003). [PMC free article] [PubMed] [Google Scholar]
- 14.Thomson AD Mechanisms of vitamin deficiency in chronic alcohol misusers and the development of the Wernicke–Korsakoff syndrome. Alcohol Alcohol. 35 (Suppl.), 2–7 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Martin PR, Singleton CK & Hiller-Sturmhofel S The role of thiamine deficiency in alcoholic brain disease. Alcohol Res. Health 27, 134–142 (2003). [PMC free article] [PubMed] [Google Scholar]
- 16.Galvin R et al. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur. J. Neurol 17, 1408–1418 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Harper C The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain? J. Neuropathol. Exp. Neurol 57, 101–110 (1998). [DOI] [PubMed] [Google Scholar]
- 18.Butters N The Wernicke–Korsakoff syndrome: a review of psychological, neuropathological and etiological factors. Curr. Alcohol 8, 205–232 (1981). [PubMed] [Google Scholar]
- 19.Feinberg I, Fein G, Price LJ, Jernigan TL & Floyd TC in Aging in the 1980s: Psychological Issues (ed. Poon LW) 71–77 (American Psychological Association, Washington D. C., 1980). [Google Scholar]
- 20.Butters N & Brandt J in Recent Developments in Alcoholism Vol. 3 (ed. Galanter M) 207–226 (Plenum Publishing, New York, 1985). [DOI] [PubMed] [Google Scholar]
- 21.Harper CG, Giles M & Finlay-Jones R Clinical signs in the Wernicke–Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J. Neurol. Neurosurg. Psychiatry 49, 341–345 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caine D, Halliday GM, Kril JJ & Harper CG Operational criteria for the classification of chronic alcoholics: identification of Wernicke’s encephalopathy. J. Neurol. Neurosurg. Psychiatry 62, 51–60 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fama R, Marsh L & Sullivan EV Dissociation of remote and anterograde memory impairment and neural correlates in alcoholic Korsakoff syndrome. J. Int. Neuropsychol. Soc 10, 427–441 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Fama R, Pfefferbaum A & Sullivan EV Visuoperceptual priming in alcoholic Korsakoff syndrome. Alcohol. Clin. Exp. Res 30, 680–687 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Sullivan EV, Deshmukh A, Desmond JE, Lim KO & Pfefferbaum A Cerebellar volume decline in normal aging, alcoholism, and Korsakoff’s syndrome: relation to ataxia. Neuropsychology 14, 341–352 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Kopelman MD, Thomson AD, Guerrini I & Marshall EJ The Korsakoff syndrome: clinical aspects, psychology and treatment. Alcohol Alcohol. 44, 148–154 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Oscar-Berman M & Ellis RJ Cognitive deficits related to memory impairments in alcoholism. Recent Dev. Alcohol 5, 59–80 (1987). [DOI] [PubMed] [Google Scholar]
- 28.Dirksen CL, Howard JA, Cronin-Golomb A & Oscar-Berman M Patterns of prefrontal dysfunction in alcoholics with and without Korsakoff’s syndrome, patients with Parkinson’s disease, and patients with rupture and repair of the anterior communicating artery. Neuropsychiatr. Dis. Treat 2, 327–339 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oscar-Berman M in Review of NIAAA’s Neuroscience and Behavioral Research Portfolio, NIAAA Research Monograph No. 34 (eds Noronha, Eckardt M & Warren K) 437–472 (NIH, Bethesda, 2000). [Google Scholar]
- 30.Giancola PR & Moss HB Executive cognitive functioning in alcohol use disorders. Recent Dev. Alcohol. 14, 227–251 (1998). [DOI] [PubMed] [Google Scholar]
- 31.Bates ME, Bowden SC & Barry D Neurocognitive impairment associated with alcohol use disorders: implications for treatment. Exp. Clin. Psychopharmacol 10, 193–212 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Oscar-Berman M & Marinkovic K Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol. Rev 17, 239–257 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan EV, Harris RA & Pfefferbaum A Alcohol’s effects on brain and behavior. Alco. Res. Health 33, 127–143 (2010). [PMC free article] [PubMed] [Google Scholar]
- 34.Green A et al. The effect of moderate to heavy alcohol consumption on neuropsychological performance as measured by the repeatable battery for the assessment of neuropsychological status. Alcohol. Clin. Exp. Res 34, 443–450 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Bowden SC Separating cognitive impairment in neurologically asymptomatic alcoholism from Wernicke–Korsakoff Syndrome: is the neuropsychological distinction justified? Psychol. Bull 107, 355–366 (1990). [DOI] [PubMed] [Google Scholar]
- 36.Squire L & Butters N (eds) Neuropsychology of Memory 2nd edn (Guilford Press, New York, 1992). [Google Scholar]
- 37.Pitel AL et al. Episodic and working memory deficits in alcoholic Korsakoff patients: the continuity theory revisited. Alcohol. Clin. Exp. Res 32, 1229–1241 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Sullivan EV et al. Disruption of frontocerebellar circuitry and function in alcoholism. Alcohol. Clin. Exp. Res 27, 301–309 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Pitel AL et al. Signs of preclinical Wernicke’s encephalopathy and thiamine levels as predictors of neuropsychological deficits in alcoholism without Korsakoff’s syndrome. Neuropsychopharmacology 36, 580–588 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Victor M, Adams RD & Collins GH The Wernicke–Korsakoff Syndrome (Davis FA, Philadelphia, 1971). [PubMed] [Google Scholar]
- 41.Harper CG & Blumbergs PC Brain weights in alcoholics. J. Neurol. Neurosurg. Psychiatry 45, 838–840 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skullerud K Variations in the size of the human brain. Influence of age, sex, body length, body mass index, alcoholism, Alzheimer changes, and cerebral atherosclerosis. Acta Neurol. Scand 102, 1–94 (1985). [PubMed] [Google Scholar]
- 43.Harper CG & Kril JJ in Alcohol Induced Brain Damage: NIAAA Research Monograph No. 22 (eds Hunt WA & Nixon SJ) 39–69 (NIH, Rockville, 1993). [Google Scholar]
- 44.Harper CG, Kril JJ & Holloway RL Brain shrinkage in chronic alcoholics: a pathological study. Br. Med. J 290, 501–504 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De la Monte SM Disproportionate atrophy of cerebral white matter in chronic alcoholics. Arch. Neurol 45, 990–992 (1988). [DOI] [PubMed] [Google Scholar]
- 46.Harper C & Kril JJ Brain atrophy in chronic alcoholic patients: a quantitative pathological study. J. Neurol. Neurosurg. Psychiatry 48, 211–217 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kril JJ & Butterworth RF Diencephalic and cerebellar pathology in alcoholic and nonalcoholic patients with end-stage liver disease. Hepatology 26, 837–841 (1997). [DOI] [PubMed] [Google Scholar]
- 48.Phillips SC, Harper CG & Kril J A quantitative histological study of the cerebellar vermis in alcoholic patients. Brain 110, 301–314 (1987). [DOI] [PubMed] [Google Scholar]
- 49.Harper CG & Kril JJ Corpus callosal thickness in alcoholics. Br. J. Addict 83, 577–580 (1988). [DOI] [PubMed] [Google Scholar]
- 50.Tarnowska-Dziduszko E, Bertrand E & Szpak G Morphological changes in the corpus callosum in chronic alcoholism. Folia Neuropathol. 33, 25–29 (1995). [PubMed] [Google Scholar]
- 51.Lee ST, Jung YM, Na DL, Park SH & Kim M Corpus callosum atrophy in Wernicke’s encephalopathy. J. Neuroimaging 15, 367–372 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Harper C et al. The pathophysiology of ‘brain shrinkage’ in alcoholics structural and molecular changes and clinical implications. Alcohol. Clin. Exp. Res 29, 1106–1115 (2005). [Google Scholar]
- 53.Harding A, Halliday G, Caine D & Kril J Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain 123, 141–154 (2000). [DOI] [PubMed] [Google Scholar]
- 54.Cullen KM, Halliday GM, Caine D & Kril JJ The nucleus basalis (Ch4) in the alcoholic Wernicke–Korsakoff syndrome: reduced cell number in both amnesic and nonamnesic patients. J. Neurol. Neurosurg. Psychiatry 63, 315–320 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baker K, Harding A, Halliday G, Kril J & Harper C Neuronal loss in functional zones of the cerebellum of chronic alcoholics with and without Wernicke’s encephalopathy. Neuroscience 91, 429–438 (1999). [DOI] [PubMed] [Google Scholar]
- 56.Harper C & Kril J An introduction to alcohol-induced brain damage and its causes. Alcohol Alcohol. 2 (Suppl.), 237–243 (1994). [PubMed] [Google Scholar]
- 57.Kril JJ, Halliday GM, Svoboda MD & Cartwright H The cerebral cortex is damaged in chronic alcoholics. Neuroscience 79, 983–998 (1997). [DOI] [PubMed] [Google Scholar]
- 58.Harper C & Corbett D Changes in the basal dendrites of cortical pyramidal cells from alcoholic patients—a quantitative Golgi study. J. Neurol. Neurosurg. Psychiatry 53, 856–861 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McMullen PA, Saint-Cyr JA & Carlen PL Morphological alterations in the rat CA1 hippocampal pyramidal cell dendrites resulting from chronic ethanol consumption and withdrawal. J. Comp. Neurol 225, 111–118 (1984). [DOI] [PubMed] [Google Scholar]
- 60.Harding AJ, Halliday GM, Ng JL, Harper CG & Kril JJ Loss of vasopressin-immunoreactive neurons in alcoholics is dose-related and time-dependent. Neuroscience 72, 699–708 (1996). [DOI] [PubMed] [Google Scholar]
- 61.Harper C, Dixon G, Sheedy D & Garrick T Neuropathological alterations in alcoholic brains. Studies arising from the New South Wales Tissue Resource Centre. Prog. Neuropsychopharmacol. Biol. Psychiatry 27, 951–961 (2003). [DOI] [PubMed] [Google Scholar]
- 62.Harding AJ, Wong A, Svoboda M, Kril JJ & Halliday GM Chronic alcohol consumption does not cause hippocampal neuron loss in humans. Hippocampus 7, 78–87 (1997). [DOI] [PubMed] [Google Scholar]
- 63.Baker KG, Halliday GM, Kril JJ & Harper CG Chronic alcoholics without Wernicke–Korsakoff syndrome or cirrhosis do not lose serotonergic neurons in the dorsal raphe nucleus. Alcohol. Clin. Exp. Res 20, 61–66 (1996). [DOI] [PubMed] [Google Scholar]
- 64.Jernigan TL et al. Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcohol. Clin. Exp. Res 15, 418–427 (1991). [DOI] [PubMed] [Google Scholar]
- 65.Sullivan EV et al. In vivo mammillary body volume deficits in amnesic and nonamnesic alcoholics. Alcohol. Clin. Exp. Res 23, 1629–1636 (1999). [PubMed] [Google Scholar]
- 66.Sheedy D, Lara A, Garrick T & Harper C Size of mamillary bodies in health and disease: useful measurements in neuroradiological diagnosis of Wernicke’s encephalopathy. Alcohol. Clin. Exp. Res 23, 1624–1628 (1999). [PMC free article] [PubMed] [Google Scholar]
- 67.Shear PK, Sullivan EV, Lane B & Pfefferbaum A Mammillary body and cerebellar shrinkage in chronic alcoholics with and without amnesia. Alcohol. Clin. Exp. Res 20, 1489–1495 (1996). [DOI] [PubMed] [Google Scholar]
- 68.Jernigan TL, Schafer K, Butters N & Cermak LS Magnetic resonance imaging of alcoholic Korsakoff patients. Neuropsychopharmacology 4, 175–186 (1991). [PubMed] [Google Scholar]
- 69.Lenz V et al. Value of MRI findings in Gayet–Wernicke encephalopathy [French]. J. Neuroradiol 29, 153–160 (2002). [PubMed] [Google Scholar]
- 70.Pfefferbaum A et al. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol. Clin. Exp. Res 16, 1078–1089 (1992). [DOI] [PubMed] [Google Scholar]
- 71.Pfefferbaum A, Sullivan EV, Mathalon DH & Lim KO Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol. Clin. Exp. Res 21, 521–529 (1997). [DOI] [PubMed] [Google Scholar]
- 72.Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC & Meyerhoff DJ Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage 34, 879–887 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pfefferbaum A et al. Increase in brain cerebrospinal fluid volume is greater in older than in younger alcoholic patients: a replication study and CT/MRI comparison. Psychiatry Res 50, 257–274 (1993). [DOI] [PubMed] [Google Scholar]
- 74.Pfefferbaum A, Lim KO, Desmond JE & Sullivan EV Thinning of the corpus callosum in older alcoholic men: a magnetic resonance imaging study. Alcohol. Clin. Exp. Res 20, 752–757 (1996). [DOI] [PubMed] [Google Scholar]
- 75.Estruch R et al. Atrophy of the corpus callosum in chronic alcoholism. J. Neurol. Sci 146, 145–151 (1997). [DOI] [PubMed] [Google Scholar]
- 76.Sullivan EV et al. Cerebellar volume deficits and neuropsychological function in alcoholics [abstract]. Alcohol. Clin. Exp. Res 22, 63A (1998). [Google Scholar]
- 77.Agartz I, Momenan R, Rawlings RR, Kerich MJ & Hommer DW Hippocampal volume in patients with alcohol dependence. Arch. Gen. Psychiatry 56, 356–363 (1999). [DOI] [PubMed] [Google Scholar]
- 78.Sullivan EV, Marsh L, Mathalon DH, Lim KO & Pfefferbaum A Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol. Clin. Exp. Res 19, 110–122 (1995). [DOI] [PubMed] [Google Scholar]
- 79.Blansjaar B, Vielvoye G, van Dijk J & Rijnders R Similar brain lesions in alcoholics and Korsakoff patients: MRI, psychometric and clinical findings. Clin. Neurol. Neurosurg 93, 197–203 (1992). [DOI] [PubMed] [Google Scholar]
- 80.O’Neill J, Cardenas VA & Meyerhoff DJ Effects of abstinence on the brain: quantitative magnetic resonance imaging and magnetic resonance spectroscopic imaging in chronic alcohol abuse. Alcohol. Clin. Exp. Res 25, 1673–1682 (2001). [PubMed] [Google Scholar]
- 81.Pfefferbaum A, Adalsteinsson E & Sullivan EV Dysmorphology and microstructural degradation of the corpus callosum: interaction of age and alcoholism. Neurobiol. Aging 27, 994–1009 (2006). [DOI] [PubMed] [Google Scholar]
- 82.Schroth G, Naegele T, Klose U, Mann K & Petersen D Reversible brain shrinkage in abstinent alcoholics, measured by MRI. Neuroradiology 30, 385–389 (1988). [DOI] [PubMed] [Google Scholar]
- 83.Carlen PL, Wilkinson DA, Wortzman G & Holgate R Partially reversible cerebral atrophy and functional improvement in recently abstinent alcoholics. Can. J. Neurol. Sci 11, 441–446 (1984). [DOI] [PubMed] [Google Scholar]
- 84.Schweinsburg BC et al. Chemical pathology in brain white matter of recently detoxified alcoholics: a 1H magnetic resonance spectroscopy investigation of alcohol-associated frontal lobe injury. Alcohol. Clin. Exp. Res 25, 924–934 (2001). [PubMed] [Google Scholar]
- 85.Fein G, Meyerhoff DJ & Weiner MW Magnetic resonance spectroscopy of the brain in alcohol abuse. Alcohol Health Res. World 19, 3056–3314 (1995). [PMC free article] [PubMed] [Google Scholar]
- 86.Jagannathan NR, Desai NG & Raghunathan P Brain metabolite changes in alcoholism: An in vivo proton magnetic resonance spectroscopy (MRS) study. Magn. Reson. Imaging 14, 553–557 (1996). [DOI] [PubMed] [Google Scholar]
- 87.Seitz D et al. Localized proton magnetic resonance spectroscopy of the cerebellum in detoxifying alcoholics. Alcohol. Clin. Exp. Res 23, 158–163 (1999). [PubMed] [Google Scholar]
- 88.Durazzo TC, Gazdzinski S, Rothlind JC, Banys P & Meyerhoff DJ Brain metabolite concentrations and neurocognition during short-term recovery from alcohol dependence: preliminary evidence of the effects of concurrent chronic cigarette smoking. Alcohol. Clin. Exp. Res 30, 539–551 (2006). [DOI] [PubMed] [Google Scholar]
- 89.Martin PR et al. Brain proton magnetic resonance spectroscopy studies in recently abstinent alcoholics. Alcohol. Clin. Exp. Res 19, 1078–1082 (1995). [DOI] [PubMed] [Google Scholar]
- 90.Bartsch AJ et al. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain 130, 36–47 (2007). [DOI] [PubMed] [Google Scholar]
- 91.Shear PK, Jernigan TL & Butters N Volumetric magnetic resonance imaging quantification of longitudinal brain changes in abstinent alcoholics. Alcohol. Clin. Exp. Res 18, 172–176 (1994). [DOI] [PubMed] [Google Scholar]
- 92.Pfefferbaum A et al. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol. Clin. Exp. Res 19, 1177–1191 (1995). [DOI] [PubMed] [Google Scholar]
- 93.Gazdzinski S, Durazzo TC & Meyerhoff DJ Temporal dynamics and determinants of whole brain tissue volume changes during recovery from alcohol dependence. Drug Alcohol Depend. 78, 263–273 (2005). [DOI] [PubMed] [Google Scholar]
- 94.Sullivan EV & Marsh L Hippocampal volume deficits in alcoholic Korsakoff’s syndrome. Neurology 61, 1716–1719 (2003). [DOI] [PubMed] [Google Scholar]
- 95.Kim E et al. Mammillothalamic functional connectivity and memory function in Wernicke’s encephalopathy. Brain 132, 369–376 (2009). [DOI] [PubMed] [Google Scholar]
- 96.Schulte T, Sullivan EV, Muller-Oehring EM, Adalsteinsson E & Pfefferbaum A Corpus callosal microstructural integrity influences interhemispheric processing: a diffusion tensor imaging study. Cereb. Cortex 15, 1384–1392 (2005). [DOI] [PubMed] [Google Scholar]
- 97.Pfefferbaum A, Adalsteinsson E & Sullivan EV Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biol. Psychiatry 59, 364–372 (2006). [DOI] [PubMed] [Google Scholar]
- 98.Pfefferbaum A, Adalsteinsson E & Sullivan EV Dysmorphology and microstructural degradation of the corpus callosum: interaction of age and alcoholism. Neurobiol. Aging 27, 994–1009 (2006). [DOI] [PubMed] [Google Scholar]
- 99.Sullivan EV Compromised pontocerebellar and cerebellothalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcohol. Clin. Exp. Res 27, 1409–1419 (2003). [DOI] [PubMed] [Google Scholar]
- 100.Leiner HC, Leiner AL & Dow RS Cognitive and language functions of the human cerebellum. Trends Neurosci 16, 444–447 (1993). [DOI] [PubMed] [Google Scholar]
- 101.Chanraud S et al. Diffusion tensor tractography in mesencephalic bundles: relation to mental flexibility in detoxified alcohol-dependent subjects. Neuropsychopharmacology 34, 1223–1232 (2009). [DOI] [PubMed] [Google Scholar]
- 102.Zahr NM, Pitel AL, Chanraud S & Sullivan EV Contributions of studies on alcohol use disorders to undrestanding cerebellar function. Neuropsychol. Rev 20, 280–289 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Parks MH et al. Longitudinal brain metabolic characterization of chronic alcoholics with proton magnetic resonance spectroscopy. Alcohol. Clin. Exp. Res 26, 1368–1380 (2002). [DOI] [PubMed] [Google Scholar]
- 104.Schmahmann J & Sherman J The cerebellar cognitive affective syndrome. Brain 121, 561–579 (1998). [DOI] [PubMed] [Google Scholar]
- 105.Meyerhoff DJ Brain spectroscopic imaging, morphometry, and cognition in social drinkers and recovering alcoholics. Alcohol. Clin. Exp. Res 29, 153–154 (2005). [Google Scholar]
- 106.Sullivan EV, Rosenbloom MJ, Lim KO & Pfefferbaum A Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: relationships to changes in brain structure. Neuropsychology 14, 178–188 (2000). [PubMed] [Google Scholar]
- 107.Rosenbloom MJ, Pfefferbaum A & Sullivan EV Recovery of short-term memory and psychomotor speed but not postural stability with long-term sobriety in alcoholic women. Neuropsychology 18, 589–597 (2004). [DOI] [PubMed] [Google Scholar]
- 108.Shatz CJ MHC class I: an unexpected role in neuronal plasticity. Neuron 64, 40–45 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luna-Medina R et al. NP031112, a thiadiazolidinone compound, prevents inflammation and neurodegeneration under excitotoxic conditions: potential therapeutic role in brain disorders. J. Neurosci 27, 5766–5776 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.He J & Crews FT Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp. Neurol 210, 349–358 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Barres BA The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60, 430–440 (2008). [DOI] [PubMed] [Google Scholar]
- 112.Cook CC, Hallwood PM & Thomson AD B vitamin deficiency and neuropsychiatric syndromes in alcohol misuse. Alcohol Alcohol. 33, 317–336 (1998). [DOI] [PubMed] [Google Scholar]
- 113.Butterworth RF, Kril JJ & Harper CG Thiamine-dependent enzyme changes in the brains of alcoholics—relationship to the Wernicke–Korsakoff syndrome. Alcohol. Clin. Exp. Res 17, 1084–1088 (1993). [DOI] [PubMed] [Google Scholar]
- 114.Morgan MY Alcohol and nutrition. Br. Med. Bull 38, 21–29 (1982). [DOI] [PubMed] [Google Scholar]
- 115.Tallaksen CM, Bøhmer T & Bell H Blood and serum thiamin and thiamin phosphate esters concentrations in patients with alcohol dependence syndrome before and after thiamin treatment. Alcohol. Clin. Exp. Res 16, 320–325 (1992). [DOI] [PubMed] [Google Scholar]
- 116.Harper C, Rodriguez M, Gold J & Perdices M The Wernicke–Korsakoff syndrome in Sydney—a prospective necropsy study. Med. J. Aust 149, 718 (1988). [DOI] [PubMed] [Google Scholar]
- 117.Blass JP & Gibson GE Abnormality of a thiamine-requiring enzyme in patients with Wernicke–Korsakoff syndrome. N. Engl. J. Med 297, 1367–1370 (1977). [DOI] [PubMed] [Google Scholar]
- 118.Mukherjee AB et al. Transketolase abnormality in cultured fibroblasts from familial chronic alcoholic men and their male offspring. J. Clin. Invest 79, 1039–1043 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nixon PF, Kaczmarek MJ, Tate J, Kerr RA & Price J An erythrocyte transketolase isoenzyme pattern associated with the Wernicke–Korsakoff syndrome. Eur. J. Clin. Invest 14, 278–281 (1984). [DOI] [PubMed] [Google Scholar]
- 120.Coy JF et al. Molecular cloning of tissue-specific transcripts of a transketolase-related gene: implications for the evolution of new vertebrate genes. Genomics 32, 309–316 (1996). [DOI] [PubMed] [Google Scholar]
- 121.Guerrini I et al. Direct genomic PCR sequencing of the high affinity thiamine transporter (SLC19A2) gene identifies three genetic variants in Wernicke Korsakoff syndrome (WKS). Am. J. Med. Genet. B Neuropsychiatr. Genet 137B, 17–19 (2005). [DOI] [PubMed] [Google Scholar]
- 122.Loh EW et al. Association between variants at the GABAAβ2, GABAAα6 and GABAAγ2 gene cluster and alcohol dependence in a Scottish population. Mol. Psychiatry 4, 539–544 (1999). [DOI] [PubMed] [Google Scholar]
- 123.Singleton CK & Martin PR Molecular mechanisms of thiamine utilization. Curr. Mol. Med 1, 197–207 (2001). [DOI] [PubMed] [Google Scholar]
- 124.Liu J et al. Patterns of gene expression in the frontal cortex discriminate alcoholic from nonalcoholic individuals. Neuropsychopharmacology 31, 1574–1582 (2006). [DOI] [PubMed] [Google Scholar]
- 125.Mayfield RD et al. Patterns of gene expression are altered in the frontal and motor cortices of human alcoholics. J. Neurochem 81, 802–813 (2002). [DOI] [PubMed] [Google Scholar]
- 126.Iwamoto K et al. Decreased expression of NEFH and PCP4/PEP19 in the prefrontal cortex of alcoholics. Neurosci. Res 49, 379–385 (2004). [DOI] [PubMed] [Google Scholar]
- 127.Etheridge N, Lewohl JM, Mayfield RD, Harris RA & Dodd PR Synaptic proteome changes in the superior frontal gyrus and occipital cortex of the alcoholic brain. Proteomics Clin. Appl 3, 730–742 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sokolov BP, Jiang L, Trivedi NS & Aston C Transcription profiling reveals mitochondrial, ubiquitin and signaling systems abnormalities in postmortem brains from subjects with a history of alcohol abuse or dependence. J. Neurosci. Res 72, 756–767 (2003). [DOI] [PubMed] [Google Scholar]
- 129.Weimbs T & Stoffel W Proteolipid protein (PLP) of CNS myelin: positions of free, disulfide-bonded, and fatty acid thioester-linked cysteine residues and implications for the membrane topology of PLP. Biochemistry 31, 12289–12296 (1992). [DOI] [PubMed] [Google Scholar]
- 130.Boison D & Stoffel W Disruption of the compacted myelin sheath of axons of the central nervous system in proteolipid protein-deficient mice. Proc. Natl Acad. Sci. USA 91, 11709–11713 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lewohl JM, Dodd PR, Mayfield RD & Harris RA Application of DNA microarrays to study human alcoholism. J. Biomed. Sci 8, 28–36 (2001). [DOI] [PubMed] [Google Scholar]
- 132.Matsuda-Matsumoto H, Iwazaki T, Kashem MA, Harper C & Matsumoto I Differential protein expression profiles in the hippocampus of human alcoholics. Neurochem. Int 51, 370–376 (2007). [DOI] [PubMed] [Google Scholar]
- 133.Alexander-Kaufman K, Harper C, Wilce P & Matsumoto I Cerebellar vermis proteome of chronic alcoholic individuals. Alcohol. Clin. Exp. Res 31, 1286–1296 (2007). [DOI] [PubMed] [Google Scholar]
- 134.Alexander-Kaufman K, Dedova I, Harper C & Matsumoto I Proteome analysis of the dorsolateral prefrontal region from healthy individuals. Neurochem. Int 51, 433–439 (2007). [DOI] [PubMed] [Google Scholar]
- 135.Alexander-Kaufman K, James G, Sheedy D, Harper C & Matsumoto I Differential protein expression in the prefrontal white matter of human alcoholics: a proteomics study. Mol. Psychiatry 11, 56–65 (2006). [DOI] [PubMed] [Google Scholar]
- 136.Kashem MA, James G, Harper C, Wilce P & Matsumoto I Differential protein expression in the corpus callosum (splenium) of human alcoholics: a proteomics study. Neurochem. Int 50, 450–459 (2007). [DOI] [PubMed] [Google Scholar]
- 137.Kashem MA, Harper C & Matsumoto I Differential protein expression in the corpus callosum (genu) of human alcoholics. Neurochem. Int 53, 1–11 (2008). [DOI] [PubMed] [Google Scholar]
- 138.Lishman WA Cerebral disorder in alcoholism: syndromes of impairment. Brain 104, 1–20 (1981). [DOI] [PubMed] [Google Scholar]
- 139.Crabbe JC Alcohol and genetics: new models. Am. J. Med. Genet 114, 969–974 (2002). [DOI] [PubMed] [Google Scholar]
- 140.Chen YC et al. Pharmacokinetic and pharmacodynamic basis for overcoming acetaldehyde-induced adverse reaction in Asian alcoholics, heterozygous for the variant ALDH2*2 gene allele. Pharmacogenet. Genomics 19, 588–599 (2009). [DOI] [PubMed] [Google Scholar]
- 141.Enoch MA Genetic and environmental influences on the development of alcoholism: resilience vs. risk. Ann. NY Acad. Sci 1094, 193–201 (2006). [DOI] [PubMed] [Google Scholar]
- 142.Caspi A et al. Role of genotype in the cycle of violence in maltreated children. Science 297, 851–854 (2002). [DOI] [PubMed] [Google Scholar]
- 143.Foley DL et al. Childhood adversity, monoamine oxidase a genotype, and risk for conduct disorder. Arch. Gen. Psychiatry 61, 738–744 (2004). [DOI] [PubMed] [Google Scholar]
- 144.Rangaswamy M et al. A functional MRI study of visual oddball: evidence for frontoparietal dysfunction in subjects at risk for alcoholism. Neuroimage 21, 329–339 (2004). [DOI] [PubMed] [Google Scholar]
- 145.Wiers RW, Sergeant JA & Gunning WB Psychological mechanisms of enhanced risk of addiction in children of alcoholics: a dual pathway? Acta Paediatr. Suppl 404, 9–13 (1994). [DOI] [PubMed] [Google Scholar]
- 146.Whipple SC & Noble EP Personality characteristics of alcoholic fathers and their sons. J. Stud. Alcohol 52, 331–337 (1991). [DOI] [PubMed] [Google Scholar]
- 147.Blum K et al. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J. Psychoactive Drugs 32 (Suppl. i–iv), 1–112 (2000). [DOI] [PubMed] [Google Scholar]
- 148.Mathalon DH, Pfefferbaum A, Lim KO, Rosenbloom MJ & Sullivan EV Compounded brain volume deficits in schizophrenia–alcoholism comorbidity. Arch. Gen. Psychiatry 60, 245–252 (2003). [DOI] [PubMed] [Google Scholar]
- 149.Sullivan EV et al. Contribution of alcohol abuse to cerebellar volume deficits in men with schizophrenia. Arch. Gen. Psychiatry 57, 894–902 (2000). [DOI] [PubMed] [Google Scholar]
- 150.Krystal JH et al. The vulnerability to alcohol and substance abuse in individuals diagnosed with schizophrenia. Neurotox. Res 10, 235–252 (2006). [DOI] [PubMed] [Google Scholar]
- 151.Cargiulo T Understanding the health impact of alcohol dependence. Am. J. Health Syst. Pharm 64, S5–S11 (2007). [DOI] [PubMed] [Google Scholar]
- 152.Ceballos NA, Nixon SJ, Phillips JA & Tivis R Semantic processing in alcoholics with and without antisocial symptomatology. J. Stud. Alcohol 64, 286–291 (2003). [DOI] [PubMed] [Google Scholar]
- 153.Grant BF et al. Co-occurrence of 12-month alcohol and drug use disorders and personality disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch. Gen. Psychiatry 61, 361–368 (2004). [DOI] [PubMed] [Google Scholar]
- 154.Kessler RC, Chiu WT, Demler O, Merikangas KR & Walters EE Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry 62, 617–627 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.New South Wales Tissue Resource Centre. ‘Brain Bank’ [online], http://sydney.edu.au/medicine/pathology/trc/index.php (2010).
- 156.Harper C et al. How important are brain banks for alcohol research? Alcohol. Clin. Exp. Res 27, 310–323 (2003). [DOI] [PubMed] [Google Scholar]
- 157.Pfefferbaum A, Sullivan EV, Adalsteinsson E, Garrick T & Harper C Postmortem MR imaging of formalin-fixed human brain. Neuroimage 21, 1585–1595 (2004). [DOI] [PubMed] [Google Scholar]
- 158.Dedova I et al. The importance of brain banks for molecular neuropathological research: the New South Wales Tissue Resource Centre experience. Int. J. Mol. Sci 10, 366–384 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Garrick T, Azizi L, Merrick J & Harper C Brain donation for research, what do people say? Intern. Med. J 33, 475 (2003). [DOI] [PubMed] [Google Scholar]
- 160.Glaw XM et al. Brain donation: who and why? Cell Tissue Bank 10, 241–246 (2009). [DOI] [PubMed] [Google Scholar]
- 161.Fein G & Landman B Treated and treatment-naive alcoholics come from different populations. Alcohol 35, 19–26 (2005). [DOI] [PubMed] [Google Scholar]
- 162.Nakamura K et al. Acetaldehyde adducts in the brain of alcoholics. Arch. Toxicol 77, 591–593 (2003). [DOI] [PubMed] [Google Scholar]
- 163.Bora PS & Lange LG Molecular mechanism of ethanol metabolism by human brain to fatty acid ethyl esters. Alcohol. Clin. Exp. Res 17, 28–30 (1993). [DOI] [PubMed] [Google Scholar]
- 164.Coyle JT & Puttfarcken P Oxidative stress, glutamate, and neurodegenerative disorders. Science 262, 689–695 (1993). [DOI] [PubMed] [Google Scholar]
- 165.Tsai GE et al. Increased glutamatergic neurotransmission and oxidative stress after alcohol withdrawal. Am. J. Psychiatry 155, 726–732 (1998). [DOI] [PubMed] [Google Scholar]
- 166.Ikegami Y et al. Increased TUNEL positive cells in human alcoholic brains. Neurosci. Lett 349, 201–205 (2003). [DOI] [PubMed] [Google Scholar]
- 167.Brooks PJ Brain atrophy and neuronal loss in alcoholism: a role for DNA damage? Neurochem. Int 37, 403–412 (2000). [DOI] [PubMed] [Google Scholar]
- 168.Climent E, Pascual M, Renau-Piqueras J & Guerri C Ethanol exposure enhances cell death in the developing cerebral cortex: role of brain-derived neurotrophic factor and its signaling pathways. J. Neurosci. Res 68, 213–225 (2002). [DOI] [PubMed] [Google Scholar]
- 169.Fadda F & Rossetti ZL Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog. Neurobiol 56, 385–431 (1998). [DOI] [PubMed] [Google Scholar]
- 170.Butterworth RF Hepatic encephalopathy—a serious complication of alcoholic liver disease. Alcohol Res. Health 27, 143–145 (2003). [PMC free article] [PubMed] [Google Scholar]
- 171.Felipo V & Butterworth RF Neurobiology of ammonia. Prog. Neurobiol 67, 259–279 (2002). [DOI] [PubMed] [Google Scholar]
- 172.Zahr NM et al. Glutamate and glutamine changes induced by ethanol treatment in the rat brain detectable at 3T. In Proc. ISMRM 18th Annual Meeting 917 (Stockholm, Sweden, 2010). [Google Scholar]
- 173.Mousseau DD, Perney P, Layrargues GP & Butterworth RF Selective loss of pallidal dopamine D2 receptor density in hepatic encephalopathy. Neurosci. Lett 162, 192–196 (1993). [DOI] [PubMed] [Google Scholar]
- 174.Donaldson J, LaBella FS & Gesser D Enhanced autoxidation of dopamine as a possible basis of manganese neurotoxicity. Neurotoxicology 2, 53–64 (1981). [PubMed] [Google Scholar]
- 175.Niemela O et al. Antibodies against acetaldehyde-modified protein epitopes in human alcoholics. Hepatology 7, 1210–1214 (1987). [DOI] [PubMed] [Google Scholar]
- 176.Albano E Alcohol, oxidative stress and free radical damage. Proc. Nutr. Soc 65, 278–290 (2006). [DOI] [PubMed] [Google Scholar]
- 177.Yokoyama H et al. Experimental hepatitis induced by ethanol after immunization with acetaldehyde adducts. Hepatology 17, 14–19 (1993). [PubMed] [Google Scholar]
- 178.Horner M, Behrens UJ, Worner T & Lieber CS Humoral immune response to acetaldehyde adducts in alcoholic patients. Res. Comm. Chem. Pathol. Pharmacol 54, 3–12 (1996). [PubMed] [Google Scholar]
- 179.Qin L et al. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J. Neuroinflammation 5, 10 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Crews FT & Nixon K Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol 44, 115–127 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Phillips SC Cytoprotective value of lysine, penicillamine, and pyridoxal phosphate against the neurotoxicity of acetaldehyde. Toxicol. Appl. Pharmacol 98, 553–560 (1989). [DOI] [PubMed] [Google Scholar]
- 182.Butterworth RF Pathophysiology of alcoholic brain damage: synergistic effects of ethanol, thiamine deficiency and alcoholic liver disease. Metab. Brain Dis 10, 1–8 (1995). [DOI] [PubMed] [Google Scholar]


