Abstract
Objective:
To determine which NH resident characteristics were most important to clinicians’ decision to prescribe antibiotics for a suspected urinary tract infection (UTI), including both evidence-based and non-evidence-based characteristics.
Design:
Web-based discrete choice experiment with 19 clinical scenarios. For each scenario, clinicians were asked whether they would prescribe an antibiotic for a suspected UTI.
Setting:
On-line survey
Participants:
Convenience sample of 876 NH physicians and advanced practice providers, who practiced primary care for NH residents in the United States.
Methods:
Each scenario varied information about 10 resident characteristics regarding urinalysis results, resident temperature, lower urinary tract symptoms, physical examination, antibiotic request, mental status, UTI risk, functional status, goals of care, and resident type. We derived importance scores for the characteristics and odds ratios (ORs) for specific information related to each characteristic from a multinomial logistic regression.
Results:
Approximately half of the participants were male (56%) with a mean age of 49 years. Resident characteristics differed in their importance (i.e., part-worth utility) when deciding whether to prescribe for a suspected UTI: urinalysis results (32%), body temperature (17%), lower urinary tract symptoms (17%), physical examination (15%), antibiotic request (7%), mental status (4%), urinary tract infection risk (4%), functional status (3%), goals of care (2%), and resident type (1%). Information about “positive leukocyte esterase, positive nitrates” was associated with highest odds of prescribing (OR 19.6, 95% CI 16.9, 22.7), followed by “positive leukocyte esterase, negative nitrates” (OR 6.7, 95% CI 5.8, 7.6), and “painful or difficult urination” (OR 4.8, 95% CI 4.2, 5.5).
Conclusions and Implications:
Although guidelines focus on lower urinary tract symptoms, body temperature, and physical examination for diagnosing a UTI requiring antibiotics, these characteristics were considered less important than urinalysis results, which have inconsistent clinical utility in NH residents. Point-of-care clinical decision support offers an evidence-based prescribing process.
Keywords: discrete choice experiment, urinary tract infection, nursing home, decision making
Brief Summary:
A discrete choice experiment of antibiotic prescribing for suspected urinary tract infections found urinalysis results were the most important resident characteristic.
Introduction
Antibiotic resistance globally endangers human health, with the World Health Organization recommending immediate and drastic changes to prescribing practices to avert catastrophic levels of untreatable infections.1,2 Although antibiotic stewardship programs can successfully reduce overuse in hospitals, nursing home (NH) antibiotic stewardship programs have had mixed effects. For example, although targeted programs for catheter-associated urinary tract infections (UTIs) successfully reduced antibiotic prescribing,3 and global stewardship efforts decreased prescribing without an increase in mortality or morbidity,4–6 their effect sizes were modest.7 Therefore, it is important to pursue new strategies to effect change. Targeting clinician decision-making may represent another route to improving antibiotic stewardship, particularly if decision-making is biased, misinformed, or hurried.8 Supporting the rationale for this tactic, NH clinicians often appear to deviate from the evidence when making decisions related to antibiotic prescribing9–12
Antibiotic prescribing for UTIs is particularly important because suspected UTIs account for the majority of antibiotics prescribed in NHs.6,13 In addition to evidence-based practice guidelines, they also consider non-evidence based information such as functional status changes that may be present in UTIs but are more likely in non-UTI conditions.9–11 Also, time pressure, a common issue in the healthcare setting, increases prescribing in primary care and may occur in NHs,14,15 suggesting that more deliberation may improve prescribing.
Understanding clinician decision-making when diagnosing UTIs may represent an important step to reduce inappropriate prescribing. Discrete choice experiment (DCE) methodology is widely used to understand medical decision-making because it allows a controlled examination of the influence of multiple types of information on a specific decision, and may predict real-world clinician choices.16,17 For example, a DCE examining patient preferences for topical acne antibiotics found that the results aligned with their actual choice of medication.18 Another DCE of physician decisions for antibiotic duration for uncomplicated pyelonephritis found that desires to limit treatment failure and avoid side effects most influenced the choice of antibiotic duration.19 Thus, a DCE can provide a quantitative valuation of the relative importance of NH resident characteristics to the antibiotic prescribing decision. To the best of our knowledge, no such experiment has been conducted to examine clinicians’ prescribing behavior in NH residents, which is the focus of this study.
Methods
Study Design
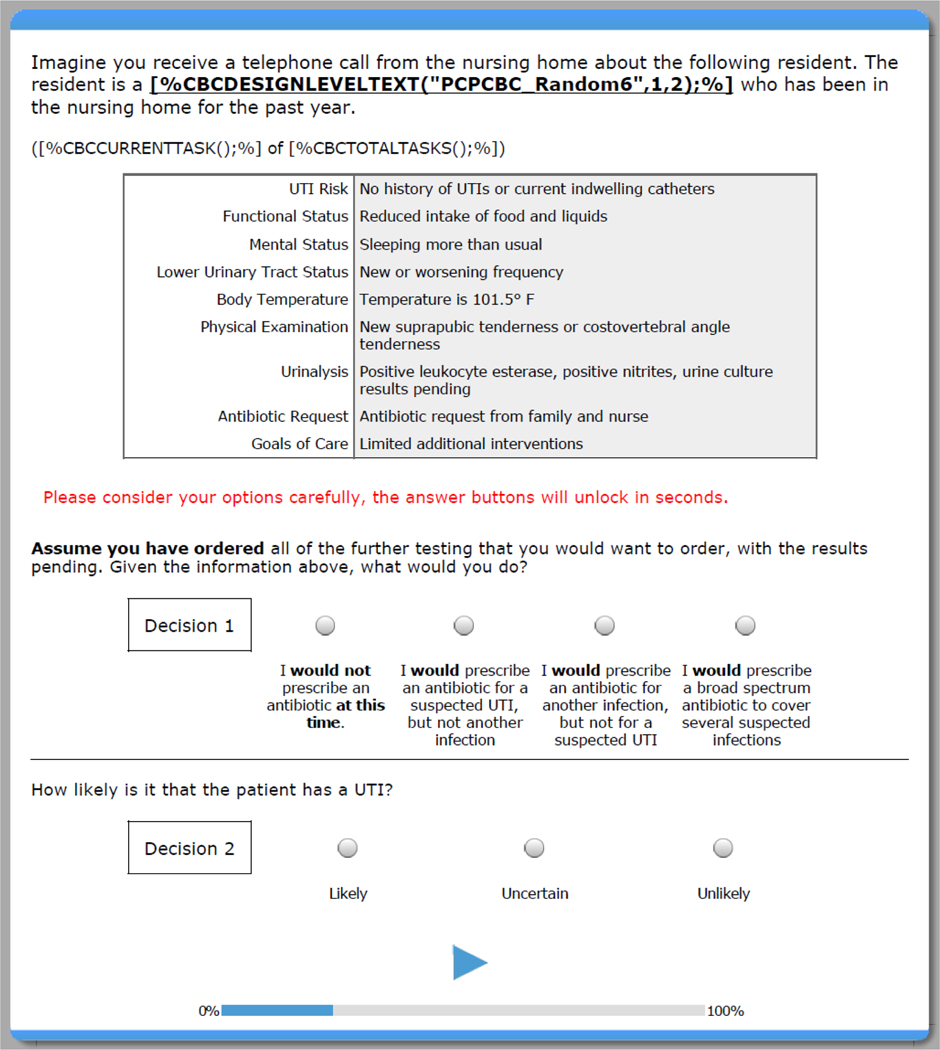
We conducted an online DCE study with U.S. clinicians who provide primary care for NH residents. Clinicians were recruited via an email invitation describing the study (i.e., duration, focus, incentives), including an electronic link to eligibility questions, an informed consent page, and then the survey. Once participants provided online consent, they began the survey, starting with an introduction about the resident characteristics under consideration in the study and an explanation of the discrete choice scenarios. They were told that for each scenario, they would receive a “telephone call from the NH” about a hypothetical resident (see Figure 1). Each scenario included 10 types of clinical information that varied over each scenario. After providing their responses for 19 scenarios, they completed demographic and clinical background measures. We used Sawtooth Software© to design and conduct the DCE. This research was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill (IRB # 16–0207).
Figure 1. General Layout of the Discrete Choice Scenarios*.
*[%CBCDESIGNLEVELTEXT(“PCPCBC_Random6”, 1,2);%] populates with resident’s age and gender based on the individual scenario. [CBCCURRENTTASK();%] OF [%CBCTOTALTASKS();%]) populates with the number of the current scenario out of the 19 scenarios to be completed.
To understand whether encouraging deliberation influenced the decision-making process, we randomized all participants to either a self-paced (n=421, 48.6%) or deliberative (n=446, 51.4%) time condition for the DCE scenarios. In the self-paced group, participants answered questions at their own pace (as quickly or slowly as they chose), whereas in the deliberative group, participants were required to wait at least 30 seconds before an answer could be selected. Self-paced participants completed the 19 scenarios 9 minutes faster (p = .003) than the deliberative participants (M = 18.6, SD = 39.4 vs, M = 27.7, SD = 48.9).
Participants
All participants were recruited from the research panel of Medefield®, an Accredited Gold Seal Member of the Marketing Research and Intelligence Agency, located in the United States. The primary care medical research panel is verified through lists from licensure databases such as the American Board of Medical Specialties. Panel members are actively screened and must offer 100% validation of medical professionals with regulatory bodies. All panelists are double opt-in and managed on Confirmit, the world’s leading software for Market Research (MR). Their internal processes are audited semi-annually by a third party. Eligibility criteria to participate in our study included being (1) a licensed physician (allopathic or osteopathic medicine) or advance practice provider (nurse practitioner or physician’s assistant), (2) having English language proficiency, and (3) currently practicing primary care in a NH in the United States. We specifically requested at least one-third of the sample be APPs, given the rising presence of APPs in NHs.20
Survey Development and Pretesting
The survey was developed in three steps. First, using clinical experience and literature, the research team developed a list of resident characteristics, each with a range of information typically used in the decision to diagnose a UTI.5,9,21–26 A survey prototype was developed with 19 DCE scenarios and demographic information (described below). From December 2016 to February 2017, we conducted in-depth cognitive interviews with 28 clinicians from the research panel. Interviews were conducted over the phone using a structured interview guide via shared screens; they gauged participants’ reactions to the experimental design format, individual items, scenarios, response options, and use of the web interface. Interviews lasted approximately 30 to 50 minutes; interviewees received $175 for participating.
We then conducted a pilot test of the survey in September 2017 in a sample of 82 clinicians from the research panel to confirm the feasibility of administering the survey and to explore illogical response patterns to the DCE scenarios. Participants received an honorarium of $75 via Medefield upon completion of the survey. After minor modifications to wording, the final version of the survey containing a total of 94 items was administered in April 2018 and then again in July 2018 to boost the sample as a result of detected outliers (see below). Participants who were randomized into the self-paced condition received an honorarium of $40 and participants in the deliberative condition received $75 upon completion of the survey.
Measures
The primary component of the study was the 19 DCE scenarios related to NH residents with suspected UTIs. Information in the scenarios was systematically varied on 10 distinct resident characteristics: urinalysis results, resident temperature, lower urinary tract symptoms, physical examination, antibiotic request, mental status, UTI risk, functional status, goals of care, and resident type (Table 1). We included characteristics and information that are indications to prescribe and also not to prescribe. Each scenario contained 1 type of information related to each characteristic, with each characteristic containing 3 to 6 possible types of information. At the end of the scenario, clinicians were asked “What would you do?” given the information available (with additional work-up pending; see Figure 1).
Table 1.
Clinical Information Incorporated into the Discrete Choice Scenarios by Resident Characteristic
| Resident Characteristic | Type of Information | Evidence-based Informationa |
|
|---|---|---|---|
| Indication to Prescribe | Indication to Not Prescribe | ||
| Urinalysis | • Negative leukocyte esterase, negative nitrates, urine results pendingb | No | Yes1,2 |
| • Positive leukocyte esterase, positive nitrates, urine results pending | Yes3 | No | |
| • Positive leukocyte esterase, negative nitrates, urine results pending | Equivocal3 | No | |
| • Unavailable/Not performed | No | No | |
| Body Temperature | • Temperature is 97.5°F | No | Yes4,5 |
| • Temperature is 101.5°F | Yes3,6–8 | No | |
| • Temperature is 99.5°F | Yes3,8,9 | No | |
| • Temperature is 96.5°F | No | No | |
| Lower Urinary Tract Status | • No lower urinary tract signs or symptoms | No | Yes4,5 |
| • Painful or difficult urination | Yes3,6–8,10 | No | |
| • Obvious blood in urine | Yes3,11 | Yes8 | |
| • Change in urine clarity or odor | No | Yes11 | |
| • New or worsening frequency | Yes6,7,10,11 | No | |
| Physical Examination | • Normal physical exam | No | Yes5,12 |
| • New suprapubic tenderness or costovertebral angle tenderness | Yes6–8,10,11 | No | |
| • New or increased area of redness and warmth on left lower leg | No | Yes4 | |
| • New or increased cough and work of breathing | No | Yes4 | |
| Antibiotic Requestc13–19 | • No antibiotic request from either resident, family, or nurse | No | No |
| • Antibiotic request from family and nurse | No | No | |
| • Antibiotic request from resident and nurse | No | No | |
| • Antibiotic request from resident | No | No | |
| • Antibiotic request from family | No | No | |
| • Antibiotic request from nurse but not resident or family | No | No | |
| Mental Status | • Usual state of health | No | Yes4,5 |
| • New or worsening confusion | Yes6,7,10 | Yes20/Equivocal21,22 | |
| • New or worsening agitation | Yes6,7,10 | Yes8 | |
| • Sleeping more than usual | No | Yes8 | |
| UTI Risk | • No history of UTIs or current indwelling catheters | No | No |
| • Current indwelling catheter and history of three UTIs over the past year | Equivocal23,24 | No | |
| • History of three UTIs over past year but no current indwelling catheter | No | No | |
| • Current indwelling catheter but no history of prior UTIs over past year | Equivocal23,24 | No | |
| Functional Status | • Usual state of health | No | Yes4,5 |
| • New or worsening difficulties with ambulation or transfers | No | Yes8 | |
| • New or increased falls | No | Yes8 | |
| • Reduced intake of food and liquids | No | Yes8 | |
| • New or increased resistance to care | No | Yes | |
| Goals of Care | • Comfort care measures | No | Yes3 |
| • Full scope of treatment | No | No | |
| • Limited additional interventions | No | No | |
| Resident Type | • 84 year-old cognitively-intact man | No | No |
| • 84 year-old man with dementia | Equivocal24,25 | Equivocal26 | |
| • 84 year-old cognitively-intact woman | Equivocal24 | No | |
| • 84 year-old woman with dementia | Equivocal24,25 | Equivocal26 | |
Evidence-based Information could either be in favor or against prescribing antibiotics, a “yes” response means evidence favors that column, “equivocal” response means evidence is mixed, and a “no” means there is no evidence for that type of information being related to that column.
Italicized items represent DCE reference category.
Although antibiotic request is neither an indication for antibiotic prescribing or not prescribing, it is known to influence prescribing and so was included for that reason.
Demographic and clinical data included items for gender, age, race, ethnicity, and response to 2 personality scales, the Ten-Item Personality Inventory (TIPI).27 TIPI items are coupled into pairs assessing the five factors of personality, i.e., extraversion, agreeableness, conscientiousness, emotional stability, and openness to experiences.28 They are scored using reverse scoring for 1 item in each factor, serving the dual purpose as a personality measure (not included in these analyses) and an attention check.27 We expect that a participant, who answers one item in a certain direction, would answer its companion item in a similar way. If a participant was inattentive, he or she would have discrepant scores on the paired TIPI items due to reverse coding. Clinical data included characteristics of the NH where they practice (e.g., use of electronic health records), work and resident load in the NH (e.g., days per week at NH, percent of residents in NH), certified Medical Director status, geriatrics subspecialty, and education (e.g., degree type, degree year).
Data Analysis
We used recently published methodologies to calculate the sample size of our multi-attribute DCE.29 Given an α= 0.05, we needed 878 clinicians to have 80% power to detect an effect size of 0.5. Although 1,070 individuals participated in the survey, a proportion (n=135, 12.6%) were excluded because they failed the eligibility questions or did not complete the DCE or demographic questions; an additional proportion (n=68, 6.4%) were excluded as outliers if they met any of 4 pre-specified criteria: (1) total survey completion time > 720 minutes, (2) total survey completion time < [(median survey minutes) − 2.5 × (median absolute deviation)], (3) complete avoidance of a UTI choice option, and (4), total TIPI item pair difference score > [(median score) + 5.0 × (median absolute deviation)]. Bivariate comparisons on those designated as outliers (n=68) found that these excluded participants did not demonstrate any demographic or clinical differences from included participants aside from having a greater likelihood for working more days/week in the NH (+1.9 days/week, p=.007). Non-qualifier and non-completer participants did not complete enough items to merit meaningful bivariate comparisons. After removing non-qualifiers, non-completers, and outliers, 867 (81.0%) of participants remained for analysis. Statistical significance was defined throughout as p<.05 (two-tailed). Part-worth utilities were transformed into odds ratio (OR) estimates with corresponding 95% confidence interval (CI) estimates for ease of interpretation.
We estimated the importance of each characteristic and type of information using unconditional multinomial logistic regression appropriate for the categorical responses of the DCE scenarios. The part-worth utilities of each characteristic (i.e., “attribute”) and type of information (i.e., level) were estimated using a multinomial logistic regression at the sample level. Because all utilities are uniformly scaled, the utilities within characteristics can be compared across clinicians and used to determine the relative importance of each type of information compared to all other types of information. Each part-worth utility is therefore a numerical representation of the relative importance of each type of information in the clinician’s decision to prescribe an antibiotic. Reference groups were chosen to reflect the type of information least likely to encourage or indicate antibiotic prescribing. To examine the potential role of deliberation, the sample-level utilities were (1) first compared between the groups (self-paced versus deliberative) to compare the scales of the utilities via a Swait-Louviere test that decomposed the traditional Chow test into sub-tests for differences in preferences versus differences in scales appropriate for ordinal responses, and then were (2) used to compute importance scores.30 Additionally, we compared the distribution of individual-level clinicians’ importance scores by the clinical characteristics in Table 2 to determine if any differences in what type of information is important to clinicians is due to underlying characteristics. We also sought to see if any of these differences in prescribing decisions might be associated with what they considered to be the most important resident characteristic.
Table 2.
Clinician Characteristics (NClinicians = 867)
| Characteristic | N (%) or M (SD) |
|---|---|
| Demographic | |
| Male | 485 (55.9) |
| Age | 49.0 (11.0) |
| Race | |
| White | 665 (75.6) |
| Asian-American | 139 (16.0) |
| Black | 34 (3.9) |
| Other | 39 (4.5) |
| Hispanic/Latino | 40 (4.6) |
| Ten-Item Personality Inventory (1–7) | |
| Extraversion | 4.2 (1.5) |
| Agreeableness | 5.7 (1.1) |
| Conscientiousness | 6.2 (0.9) |
| Emotional Stability | 5.6 (1.2) |
| Openness to Experiences | 5.2 (1.1) |
| Clinical | |
| NH uses electronic health records | 775 (89.4) |
| NH has wireless network | 627 (72.3) |
| Days per week in NH | 1.8 (1.5) |
| Hours per month in NH | 32.3 (42.4) |
| % of patients in NH | 25.9 (26.4) |
| Work in a practice that serves long-term care only a | 44 (23.3) |
| Certified Medical Director a | 39 (20.6) |
| Specialty | |
| General Practice | 71 (8.2) |
| Family Medicine | 477 (55.0) |
| Internal Medicine | 301 (34.7) |
| Other | 18 (2.1) |
| Has a sub-specialty | 189 (21.8) |
| Geriatrics | 84 (44.4) |
| Hospice and palliative medicine | 30 (15.9) |
| Other | 95 (50.3) |
| Degree | |
| MD, DO | 515 (59.4) |
| Nurse Practitioner | 209 (24.1) |
| Physician Assistant | 143 (16.5) |
| Years since obtained degree | 20.2 (11.4) |
| Obtained degree in United States | 780 (90.0) |
| Prescribing Decision a | |
| “I would not prescribe an antibiotic.” | 5,084 (30.9) |
| “I would prescribe for a UTI.” | 5,562 (33.8) |
| “I would prescribe for another infection.” | 2,502 (15.2) |
| “I would prescribe a broad-spectrum antibiotic.” | 3,325 (20.2) |
Note.
NObservations = 16,473 (867 × 19).
Results
Sample Characteristics
Of the 867 participants, 56% were male, 76% White, with a mean age of 49 years (Table 2). Participants reported 1.8 days/week and 32.3 hours/month in the NH on average. Almost one-quarter (23%) worked in a long-term care only practice. Over one-fifth (22%) had a sub-specialty with 44% of those noting their sub-specialty was geriatrics. Most participants (59%) had a medical degree (doctor of medicine or doctor of osteopathy); 24% were nurse practitioners and 17% physician assistants.
Resident Characteristics
For the decision to prescribe an antibiotic for a UTI, urinalysis results had the highest importance score (32%) (Table 3). The rest of the categories had the following importance: body temperature (17%), lower urinary tract symptoms (17%), physical examination (15%), antibiotic request (7%), mental status (4%), UTI risk (4%), functional status (3%), goals of care (2%), and resident type (1%). The most important characteristic to clinicians’ choice to prescribe for another infection or a broad-spectrum antibiotic, was physical examination (45% and 24%, respectively); body temperature was the second most important characteristic (26% and 21% respectively) for those decisions, and urinalysis was also important to the decision for a broad-spectrum antibiotic (21%). Also, for these choices, the importance scores for characteristics related to mental status, UTI risk, functional status, goals of care, and resident type were consistently low (≤ 4.5%).
Table 3.
Resident Characteristics and Types of Information with Associated Importance Scores and Utility-Derived Odds Ratios by Response Option (NClinicians = 867)
| Resident Characteristics and Types of Information | Importance Scores (%) and Utilities (OR, [95% CI]) |
||
|---|---|---|---|
| “I would prescribe for a UTI.” | “I would prescribe for another infection.” | “I would prescribe a broad-spectrum” | |
| Urinalysis | 31.7% | 3.7% | 21.4% |
| Negative leukocyte esterase, negative nitrates | 0.00 | 0.00 | 0.00 |
| Positive leukocyte esterase, positive nitrates | 19.54 (16.92, 22.57) a | 0.77 (0.64, 0.93) a | 8.77 (7.50, 10.27) a |
| Positive leukocyte esterase, negative nitrates | 6.65 (5.83, 7.58) a | 0.87 (0.75, 1.01) | 3.29 (2.84, 3.81) a |
| Unavailable/Not performed | 2.53 (2.22, 2.87) a | 0.82 (0.72, 0.94) a | 1.77 (1.53, 2.04) a |
| Body Temperature | 16.9% | 25.8% | 21.3% |
| Temperature is 97.5°F | 0.00 | 0.00 | 0.00 |
| Temperature is 101.5°F | 4.65 (4.05, 5.33) a | 6.17 (5.24, 7.27) a | 8.68 (7.44, 10.11) a |
| Temperature is 99.5°F | 1.89 (1.68, 2.14) a | 1.83 (1.57, 2.13) a | 2.02 (1.75, 2.33) a |
| Temperature is 96.5°F | 0.95 (0.84, 1.07) | 1.12 (0.97, 1.30) | 1.02 (0.89, 1.18) |
| Lower Urinary Tract Status | 16.7% | 4.2% | 13.2% |
| No lower urinary tract signs/symptoms | 0.00 | 0.00 | 0.00 |
| Painful or difficult urination | 4.80 (4.15, 5.54) a | 1.15 (0.97, 1.37) | 3.80 (3.22, 4.47) a |
| Obvious blood in urine | 3.14 (2.72, 3.61) a | 0.85 (0.72, 1.01) | 2.80 (2.38, 3.28) a |
| Change in urine clarity or odor | 2.31 (2.01, 2.66) a | 1.02 (0.87, 1.19) | 1.78 (1.52, 2.10) a |
| New/Worsening frequency | 1.96 (1.68, 2.28) a | 1.10 (0.92, 1.31) | 1.87 (1.57, 2.23) a |
| Physical Examination | 14.7% | 44.8% | 24.4% |
| Normal physical exam | 0.00 | 0.00 | 0.00 |
| Suprapubic/costovertebral angle tenderness | 2.43 (2.18, 2.72) a | 1.38 (1.08, 1.77) a | 2.00 (1.66, 2.40) a |
| Redness and warmth on left lower leg | 0.72 (0.63, 0.83) a | 23.69 (19.54, 28.72) a | 11.89 (10.10, 14.00) a |
| Cough and work of breathing | 0.61 (0.54, 0.70) a | 10.71 (8.84, 12.98) a | 7.82 (6.67, 9.16) a |
| Antibiotic Request | 6.8% | 8.8% | 6.7% |
| No antibiotic request | 0.00 | 0.00 | 0.00 |
| Antibiotic request from family + nurse | 1.88 (1.59, 2.23) a | 1.87 (1.51, 2.30)aa | 1.97 (1.61, 2.40) a |
| Antibiotic request from resident + nurse | 1.81 (1.53, 2.14) a | 1.65 (1.34, 2.02) a | 1.61 (1.33, 1.95) a |
| Antibiotic request from resident | 1.70 (1.44, 2.02) a | 1.42 (1.16, 1.75) a | 1.71 (1.41, 2.07) a |
| Antibiotic request from family | 1.65 (1.40, 1.95) a | 1.58 (1.28, 1.95) a | 1.80 (1.48, 2.19)a |
| Antibiotic request from nurse | 1.59 (1.37, 1.85) a | 1.38 (1.15, 1.66)a | 1.63 (1.37, 1.93) a |
| Mental Status | 4.1% | 3.6% | 3.7% |
| Usual state of health | 0.00 | 0.00 | 0.00 |
| New/Worsening confusion | 1.48 (1.30, 1.67) a | 1.29 (1.11, 1.50) a | 1.45 (1.26, 1.67) a |
| New/Worsening agitation | 1.36 (1.20, 1.54) a | 1.29 (1.11, 1.51) a | 1.41 (1.23, 1.63) a |
| Sleeping more than usual | 1.22 (1.08, 1.38) a | 1.18 (1.02, 1.37) a | 1.14 (0.99, 1.32) |
| UTI Risk | 3.5% | 1.3% | 2.8% |
| No history of UTIs or current indwelling catheters | 0.00 | 0.00 | 0.00 |
| Current indwelling catheter + history of three UTIs | 1.39 (1.22, 1.59) a | 1.10 (0.93, 1.29) | 1.33 (1.14, 1.55) a |
| History of three UTIs over past year | 1.37 (1.21, 1.56) a | 1.01 (0.86, 1.19) | 1.26 (1.09, 1.45) a |
| Current indwelling catheter | 1.08 (0.94, 1.23) | 1.01 (0.87, 1.18) | 1.02 (0.88, 1.19) |
| Functional Status | 2.5% | 1.7% | 1.3% |
| Usual state of health | 0.00 | 0.00 | 0.00 |
| New/Worsening difficulties with ambulation/transfers | 1.26 (1.10, 1.45) a | 1.12 (0.94, 1.32) | 1.14 (0.98, 1.34) |
| New/Increased falls | 1.24 (1.08, 1.42) a | 1.06 (0.89, 1.25) | 1.12 (0.95, 1.31) |
| Reduced intake of food and liquids | 1.20 (1.05, 1.38) a | 1.13 (0.96, 1.34) | 1.06 (0.90, 1.24) |
| New/Increased resistance to care | 1.17 (1.02, 1.34) a | 1.08 (0.91, 1.28) | 1.06 (0.91, 1.25) |
| Goals of Care | 2.2% | 4.5% | 3.8% |
| Comfort care measures | 0.00 | 0.00 | 0.00 |
| Full scope of treatment | 1.23 (1.11, 1.37) a | 1.37 (1.20, 1.56) a | 1.47 (1.30, 1.66) a |
| Limited additional interventions | 1.13 (1.01, 1.26) a | 1.24 (1.09, 1.41) a | 1.24 (1.09, 1.40) a |
| Resident Type | 0.8% | 1.5% | 1.4% |
| 84 year-old cognitively-intact man | 0.00 | 0.00 | 0.00 |
| 84 year-old man with dementia | 1.06 (0.89, 1.26) | 0.96 (0.78, 1.19) | 0.96 (0.78, 1.17) |
| 84 year-old cognitively-intact woman | 1.02 (0.90, 1.16) | 0.96 (0.82, 1.12) | 0.96 (0.83, 1.10) |
| 84 year-old woman with dementia | 0.98 (0.82, 1.17) | 0.90 (0.72, 1.11) | 0.87 (0.71, 1.06) |
Note. Evidence-based information are bolded. Reference response not displayed (“I would not prescribe an antibiotic). OR = Odds ratio, CI = Confidence interval.
p < .05; two-side
Types of Clinical Information
The ORs of the utilities for the 33 types of clinical information (i.e., the levels of all resident characteristics) are found in Table 3. Across all 33 types of information, 28 (85%) were significantly associated with prescribing an antibiotic for UTI. Information about “positive leukocyte esterase, positive nitrates” was associated with highest odds of prescribing (OR 19.6,95% CI 16.9, 22.6), followed by “positive leukocyte esterase, negative nitrates” (OR 6.7, 95% CI 5.8, 7.6), and “painful or difficult urination” (OR 4.8, 95% CI 4.2, 5.5). The least important clinical information was related to the presence or absence of dementia by gender, where none of this type of information was more important than the reference category of “an 84-year-old cognitively-intact man.” A low body temperature “Temperature is 96.5°F” and a “current indwelling catheter” were also not more important than “Temperature is 97.5°F” and a “No history of UTIs or current indwelling catheter”. Only the physical examination information of “new or increased area of redness and warmth on the left lower leg” and “new or increased cough and work of breathing” were significantly and negatively associated with prescribing for a UTI [OR 0.72 (95% CI 0.63, 0.83) and OR 0.61 (95% CI 0.54, 0.70), respectively].
Deliberation and Clinician Characteristics
As Table 4 shows, no differences existed by deliberation group on the distribution of importance scores for any of the three choices and only small differences across all categories. Swait-Louviere tests found that the deliberation groups did not have significant differences in their utility preferences after adjusting for scale differences (p = .75). The distribution of importance scores varied somewhat in bivariate analyses of a few purposively chosen clinical characteristics. We examined days per week spent working in the NH, practice location (long-term care only or other), training background (doctor of medicine, doctor of osteopathy, nurse practitioner, or physician assistant), the presence of specialty training (geriatrics or other), and duration of clinical practice (>20 years or ≤ 20 years). To examine how much differences in the distributions of importance scores were driven by the most important resident characteristic, we used a multivariable model to examine the association between these clinician characteristics and having urinalysis as the most important resident characteristic for prescribing antibiotics for a UTI. As Table 4 shows, we found that clinicians who spent >1 day a week in the NH had lower odds of having urinalysis as the most important resident characteristic as compared to clinicians who spent 1 day a week or less in the NH (OR 0.66, 95% CI 0.45, 0.96). No association was found between the other clinical characteristics (e.g., practice location, training background, specialty, or duration of clinical practice) and having urinalysis as the most important resident characteristic.
Table 4.
Importance Scores for the Five Most Important Resident Characteristics by Time Pressure and Clinician Characteristics (NClinicians = 867)
| Characteristic | Evidence Category |
||||||
|---|---|---|---|---|---|---|---|
| Urinalysis | Body Temperature | Lower Urinary Tract Status | Physical Examination | Antibiotic Request | All Others | p | |
| Total | 31.7% | 16.9% | 16.7% | 14.7% | 6.8% | 13.1% | |
| Time Pressure, (0,1) | 0.61 | ||||||
| Self-Paced | 32.4% | 17.1% | 16.5% | 14.5% | 6.9% | 12.6% | |
| Deliberative | 30.6% | 16.5% | 16.7% | 14.6% | 6.6% | 15.1% | |
| Clinician Characteristics, (0,1) | |||||||
| Days in NH | <.001 | ||||||
| 0–1 | 29.9% | 16.1% | 16.8% | 14.3% | 7.4% | 15.4% | |
| >1 | 32.5% | 17.3% | 16.4% | 14.8% | 6.5% | 12.6% | |
| Long-term care only | <.001 | ||||||
| Yes | 28.6% | 14.0% | 12.5% | 11.4% | 10.6% | 23.0% | |
| No | 27.5% | 16.3% | 14.9% | 15.5% | 8.8% | 17.0% | |
| Degree type | <.001 | ||||||
| MD, DO | 30.2% | 15.8% | 17.1% | 13.9% | 7.0% | 16.1% | |
| NP, PA | 31.7% | 17.5% | 14.9% | 14.7% | 7.8% | 13.4% | |
| Sub-specialty | .009 | ||||||
| Geriatric | 24.6% | 14.4% | 15.7% | 12.1% | 9.3% | 23.7% | |
| Other | 32.0% | 17.1% | 16.6% | 14.6% | 6.6% | 13.0% | |
| Years since degree | .12 | ||||||
| > Mean (20.2 years) | 30.3% | 15.6% | 17.9% | 13.7% | 7.6% | 14.9% | |
| ≤ Mean | 31.2% | 17.2% | 14.7% | 14.7% | 6.8% | 15.3% | |
Note. Importance scores for response “I would prescribe for a UTI.”. p-value tests structure of utilities of models (0, 1) against total model using Swait-Louviere likelihood ratio test; two-sided.
Discussion
This national study of NH clinicians represents the first to our knowledge to provide a quantitative valuation of the relative importance of NH resident characteristics to the decision to prescribe antibiotics. We found that urinalysis was the most important resident characteristic in deciding to prescribe an antibiotic for a presumed UTI. This is an alarming finding given that guidelines do not recommend the use of a urinalysis to diagnose a UTI in a NH resident. 5,26,31–33 The importance of resident characteristics did not vary by deliberation, although clinicians who practice >1 day/week in the NH had reduced odds of having urinalysis as the most important resident characteristic in their decision-making.
Guidelines by the Society for Healthcare Epidemiology of America and the Infectious Disease Society of America recommend that a decision to prescribe an antibiotic for a suspected UTI should be based on clinical signs/symptoms of the lower urinary tract such as dysuria, urgency, frequency, and incontinence and the presence of systemic signs or symptoms of infection.26,32 New Delphi-driven guidelines also recommend similar approaches.33,34 A key aspect of these guidelines is that none of them recommend using a urinalysis to make prescribing decisions. However, in our study, urinalysis was the most important resident characteristic when prescribing antibiotics. Moreover, urinalysis may simply be a proxy for urine culture results, a known gateway for overprescribing.35 Urine cultures are only useful for determining the right antibiotic choice after the decision to prescribe has been made.36,37 The fact that urinalysis results were the most important resident characteristic demonstrates that significant efforts are needed to reframe clinical understanding of UTIs in NH residents. This finding remained consistent across all clinicians except those in NHs >1 day/week which may give them a better understanding of asymptomatic bacteriuria than other groups.
Because inappropriate reliance on the urinalysis appears to be widespread, clinicians seem to need more than just support in knowing and understanding clinical guidelines; they also need support in avoiding reliance on generalizations and heuristics.38 One path forward could be through electronic clinical decision support systems at the point of care. To develop truly effective and feasible clinical decision support, developers will need to understand how decisions are actually made in the clinical setting.39–41 Dual Process theory posits that it is a natural tendency of people to make decisions through both rapid, intuitive means, and more deliberative, analytical processes (e.g., carefully weighing one’s options and their consequences).42,43 Grounding clinical decision support in dual process theory would give clinicians access to more deliberative cognitive processing, helping them avoid reliance on non-evidence-based information such as the urinalysis results.
Our work adds to the evidence that clinicians use a variety of resident characteristics and types of information when deciding to prescribe antibiotics. Other work has examined the associations between resident characteristics and positive urine cultures post hoc, which is problematic given the rates of asymptomatic bacteriuria in the NH population.44 DCE allows us to quantify the importance of all characteristics in relationship to each other.16,17 Reassuringly, our findings demonstrate that while characteristics with less evidence (such as worsening mental or functional status) are important to prescribing, they are markedly less important than more evidence-based characteristics such as body temperature and lower urinary tract status. Additionally, the physical examination findings related to other types of infections were negatively associated with prescribing for a UTI.
Limitations and Future Directions
This study has several limitations. Although large and geographically diverse, participants were a convenience sample; also, we do not have information about those who declined participation. We did not ask participants about the number of NHs in which they practiced; instead, we asked whether they practiced in other settings as well. We recognize that factors other than time in a NH may explain differences in UTI prescribing. Additionally, we measured responses to hypothetical scenarios and not actual prescribing; however, this limitation is offset by our ability to disentangle the amount of influence each type of patient information had on decision-making, impossible to do in a clinical setting. Because of the complexities of decision-making, this work supports the CDC recommendation to use clinical decision support to improve antibiotic prescribing.45 That effort, combined with system efforts to control the use of urinalysis and urine cultures, promote local champions, and employ other educational efforts may be enough to bend the overprescribing rate.22,46
Conclusions and Implications
In a large U.S. survey of NH primary care clinicians, we found a reliance on urinalysis to guide antibiotic prescribing for suspected UTIs, a practice known to lead to overprescribing. Future efforts need to focus on creating clinical support systems that not only educate clinicians on UTI management but encourage clinicians to use evidence-based information when making prescribing decisions.
Supplementary Material
Acknowledgments:
The sponsor had no role in the design, methods, subject recruitment, data collections, analysis and preparation of paper. We would like to acknowledge Zarett Ramirez for her help in preparing this manuscript for submission.
Funding Source: This work was supported by the Agency for Healthcare Research and Quality (GRANT11921828, 1R01HS024519-01)
References
- 1.Marston HD, Dixon DM, Knisely JM, et al. Review of Antimicrobial Resistance. JAMA. 2016;316(11):1193–1204. [DOI] [PubMed] [Google Scholar]
- 2.Organization WH. Antimicrobial resistance: global report on surveillance. World Health Organization; 2014. [Google Scholar]
- 3.Mody L, Greene MT, Meddings J, et al. A National Implementation Project to Prevent Catheter-Associated Urinary Tract Infection in Nursing Home Residents. JAMA Internal Medicine. 2017;177(8):1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monette J, Miller MA, Monette M, et al. Effect of an educational intervention on optimizing antibiotic prescribing in long-term care facilities. J Am Geriatr Soc. 2007;55(8):1231–1235. [DOI] [PubMed] [Google Scholar]
- 5.Loeb M, Brazil K, Lohfeld L, et al. Effect of a multifaceted intervention on number of antimicrobial prescriptions for suspected urinary tract infections in residents of nursing homes: cluster randomised controlled trial. BMJ. 2005;331(7518):669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimmerman S, Sloane PD, Bertrand R, et al. Successfully reducing antibiotic prescribing in nursing homes. J Am Geriatr Soc. 2014;62(5):907–912. [DOI] [PubMed] [Google Scholar]
- 7.Fleming A, Browne J, Byrne S. The effect of interventions to reduce potentially inappropriate antibiotic prescribing in long-term care facilities: a systematic review of randomised controlled trials. Drugs Aging. 2013;30(6):401–408. [DOI] [PubMed] [Google Scholar]
- 8.Tamma PD, Miller MA, Cosgrove SE. Rethinking How Antibiotics Are Prescribed: Incorporating the 4 Moments of Antibiotic Decision Making Into Clinical PracticeRethinking How Antibiotics Are PrescribedRethinking How Antibiotics Are Prescribed. JAMA. 2019;321(2):139–140. [DOI] [PubMed] [Google Scholar]
- 9.Juthani-Mehta M, Drickamer MA, Towle V, et al. Nursing home practitioner survey of diagnostic criteria for urinary tract infections. J Am Geriatr Soc. 2005;53(11):1986–1990. [DOI] [PubMed] [Google Scholar]
- 10.Boscia JA, Kobasa WD, Abrutyn E, et al. Lack of association between bacteriuria and symptoms in the elderly. The American journal of medicine. 1986;81(6):979–982. [DOI] [PubMed] [Google Scholar]
- 11.Walker S, McGeer A, Simor AE, et al. Why are antibiotics prescribed for asymptomatic bacteriuria in institutionalized elderly people? A qualitative study of physicians’ and nurses’ perceptions. CMAJ. 2000;163(3):273–277. [PMC free article] [PubMed] [Google Scholar]
- 12.Kistler CE, Sloane PD, Platts-Mills TF, et al. Challenges of antibiotic prescribing for assisted living residents: perspectives of providers, staff, residents, and family members. J Am Geriatr Soc. 2013;61(4):565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicolle LE, Strausbaugh LJ, Garibaldi RA. Infections and antibiotic resistance in nursing homes. Clin Microbiol Rev. 1996;9(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson A, Childs S. The relationship between consultation length, process and outcomes in general practice: a systematic review. The British Journal of General Practice. 2002;52(485):1012–1020. [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell SM, Hann M, Hacker J, et al. Identifying predictors of high quality care in English general practice: observational study. Vol 3232001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Bekker-Grob EW, Hol L, Donkers B, et al. Labeled versus unlabeled discrete choice experiments in health economics: an application to colorectal cancer screening. Value Health. 2010;13(2):315–323. [DOI] [PubMed] [Google Scholar]
- 17.Bech-Larsen T, Nielsen NA. A comparison of five elicitation techniques for elicitation of attributes of low involvement products. Journal of Economic Psychology. 1999;20(3):315–341. [Google Scholar]
- 18.Kellett N, West F, Finlay AY. Conjoint analysis: a novel, rigorous tool for determining patient preferences for topical antibiotic treatment for acne. A randomised controlled trial. Br J Dermatol. 2006;154(3):524–532. [DOI] [PubMed] [Google Scholar]
- 19.McGregor JC, Harris AD, Furuno JP, et al. Relative influence of antibiotic therapy attributes on physician choice in treating acute uncomplicated pyelonephritis. Medical decision making : an international journal of the Society for Medical Decision Making. 2007;27(4):387–394. [DOI] [PubMed] [Google Scholar]
- 20.Intrator O, Miller EA, Gadbois E, et al. Trends in Nurse Practitioner and Physician Assistant Practice in Nursing Homes, 2000–2010. Health Serv Res. 2015;50(6):1772–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kistler CE, Zimmerman S, Scales K, et al. The Antibiotic Prescribing Pathway for Presumed Urinary Tract Infections in Nursing Home Residents. J Am Geriatr Soc. 2017;65(8):1719–1725. [DOI] [PubMed] [Google Scholar]
- 22.Sloane PD, Huslage K, Kistler CE, Zimmerman S. Optimizing Antibiotic Use in Nursing Homes Through Antibiotic Stewardship. N C Med J. 2016;77(5):324–329. [DOI] [PubMed] [Google Scholar]
- 23.Midthun S, Paur R, Bruce AW, Midthun P. Urinary tract infections in the elderly: a survey of physicians and nurses. Geriatr Nurs. 2005;26(4):245–251. [DOI] [PubMed] [Google Scholar]
- 24.Mody L, Juthani-Mehta M. Urinary tract infections in older women: a clinical review. JAMA. 2014;311(8):844–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sloane PD, Kistler C, Mitchell CM, et al. Role of body temperature in diagnosing bacterial infection in nursing home residents. J Am Geriatr Soc. 2014;62(1):135–140. [DOI] [PubMed] [Google Scholar]
- 26.Stone ND, Ashraf MS, Calder J, et al. Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol. 2012;33(10):965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gosling SD, Rentfrow PJ, Swann WB. A very brief measure of the Big-Five personality domains. Journal of Research in Personality. 2003;37(6):504–528. [Google Scholar]
- 28.Costa PT, Mccrae RR. The 5-Factor Model of Personality and Its Relevance to Personality-Disorders. J Personal Disord. 1992;6(4):343–359. [Google Scholar]
- 29.de Bekker-Grob EW, Donkers B, Jonker MF, Stolk EA. Sample Size Requirements for Discrete-Choice Experiments in Healthcare: a Practical Guide. Patient. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swait J, Louviere J. The Role of the Scale Parameter in the Estimation and Comparison of Multinomial Logit Models. Journal of Marketing Research. 1993;30(3):305–314. [Google Scholar]
- 31.Loeb M, Bentley DW, Bradley S, et al. Development of minimum criteria for the initiation of antibiotics in residents of long-term-care facilities: results of a consensus conference. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2001;22(2):120–124. [DOI] [PubMed] [Google Scholar]
- 32.High KP, Bradley SF, Gravenstein S, et al. Clinical practice guideline for the evaluation of fever and infection in older adult residents of long-term care facilities: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(2):149–171. [DOI] [PubMed] [Google Scholar]
- 33.van Buul LW, Vreeken HL, Bradley SF, et al. The Development of a Decision Tool for the Empiric Treatment of Suspected Urinary Tract Infection in Frail Older Adults: A Delphi Consensus Procedure. J Am Med Dir Assoc. 2018;19(9):757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nace DA, Perera SK, Hanlon JT, et al. The Improving Outcomes of UTI Management in Long-Term Care Project (IOU) Consensus Guidelines for the Diagnosis of Uncomplicated Cystitis in Nursing Home Residents. Journal of the American Medical Directors Association. 2018;19(9):765–769.e763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sloane PD, Kistler CE, Reed D, et al. Urine Culture Testing in Community Nursing Homes: Gateway to Antibiotic Overprescribing. Infect Control Hosp Epidemiol. 2017;38(5):524–531. [DOI] [PubMed] [Google Scholar]
- 36.Genao L, Buhr GT. Urinary Tract Infections in Older Adults Residing in Long-Term Care Facilities. Ann Longterm Care. 2012;20(4):33–38. [PMC free article] [PubMed] [Google Scholar]
- 37.Daley P, Penney C, Wakeham S, et al. Urinary tract infection diagnosis and response to therapy in long-term care: A prospective observational study. The Canadian journal of infectious diseases & medical microbiology = Journal canadien des maladies infectieuses et de la microbiologie medicale. 2015;26(3):133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mamede S, Splinter TAW, van Gog T, et al. Exploring the role of salient distracting clinical features in the emergence of diagnostic errors and the mechanisms through which reflection counteracts mistakes. BMJ Qual Saf. 2012;21(4):295–300. [DOI] [PubMed] [Google Scholar]
- 39.Djulbegovic B, Hozo I, Beckstead J, et al. Dual processing model of medical decision-making. BMC Med Inform Decis Mak. 2012;12:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Croskerry P Clinical cognition and diagnostic error: applications of a dual process model of reasoning. Adv Health Sci Educ Theory Pract. 2009;14 Suppl 1:27–35. [DOI] [PubMed] [Google Scholar]
- 41.Stolper E, van Royen P, Dinant GJ. The ‘sense of alarm’ (‘gut feeling’) in clinical practice. A survey among European general practitioners on recognition and expression. Eur J Gen Pract. 2010;16(2):72–74. [DOI] [PubMed] [Google Scholar]
- 42.Kahneman D, Tversky A. Prospect Theory - Analysis of Decision under Risk. Econometrica. 1979;47(2):263–291. [Google Scholar]
- 43.Kahneman D,Thinking fast and slow. 1st ed. New York: Farrar, Straus and Giroux; 2011. [Google Scholar]
- 44.Gbinigie OA, Ordonez-Mena JM, Fanshawe TR, et al. Diagnostic value of symptoms and signs for identifying urinary tract infection in older adult outpatients: Systematic review and meta-analysis. J Infect. 2018;77(5):379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Centers for Disease C, Prevention. CDC’s campaign to prevent antimicrobial resistance in health-care settings. MMWR Morb Mortal Wkly Rep. 2002;51(15):343. [PubMed] [Google Scholar]
- 46.Jump RLP, Gaur S, Katz MJ, et al. Template for an Antibiotic Stewardship Policy for Post-Acute and Long-Term Care Settings. Journal of the American Medical Directors Association. 2017;18(11):913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.