Abstract
Objectives
To analyze the mechanical properties in different regions of the brain in healthy adults in a wide age range: 26 to 76 years old.
Methods
We used a multifrequency magnetic resonance elastography (MRE) protocol to analyze the effect of age on frequency-dependent (storage and loss moduli, G’ and G”, respectively) and frequency-independent parameters (μ1, μ2 and η, as determined by a standard linear solid model) of the cerebral parenchyma, cortical gray matter (GM), white matter (WM) and subcortical GM structures of 46 healthy male and female subjects. The multifrequency behavior of the brain and frequency-independent parameters were analyzed across different age groups.
Results
The annual change rate ranged from −0.32% to −0.36% for G’ and −0.43% to −0.55% for G” for the cerebral parenchyma, cortical GM and WM. For the subcortical GM, changes in G’ ranged from −0.18% to −0.23%, and G” changed −0.43%. Interestingly, males exhibited decreased elasticity, while females exhibited decreased viscosity with respect to age in some regions of subcortical GM. Significantly decreased values were also found in subjects over 60 years old.
Conclusion
Values of G’ and G” at 60 Hz and the frequency-independent μ2 of the caudate, putamen and thalamus may serve as parameters that characterize the aging effect on the brain. The decrease in brain stiffness accelerates in elderly subjects.
Keywords: brain, elasticity, magnetic resonance elastography, neuroimaging
Introduction
Protected by the skull, the human brain is an organ that is not accessible via palpation. Conventional neuroimaging techniques, including computed tomography (CT) and magnetic resonance imaging (MRI), can accurately characterize the morphologic features of the brain but do not typically provide information on the mechanical properties of the brain.
Elastography is an emerging technique to noninvasively measure the mechanical properties of soft tissues located deep in the human body [1]. Elastography together with phase-contrast MRI, known as magnetic resonance elastography (MRE) [2,3], can noninvasively measure the mechanical properties of brain tissue [4–7]. A number of studies have reported the storage and loss moduli (G’ and G”, respectively), which are typical measurements of the mechanical properties of the brain, in healthy subjects and in patient groups [4,7–11]. In some studies, the results were reported as the shear stiffness and damping ratio, which are also calculated based on values of G’ and G” [12–14]. Typically, these research studies used a frequency of vibration at 60 Hz; however, some used different frequencies, ranging from 25 Hz to 62.5 Hz [15]. The reported results are heterogeneous. It is extremely difficult to use existing literature data from different studies to evaluate age dependence because the results are dependent on the particular measurement method, reconstruction algorithm and symmetry assumptions of each study. The frequency-dependent features of the storage and loss moduli also cause heterogeneity in the results [4,16].
One approach that has been proposed to overcome this limitation is to use a multifrequency MRE protocol, apply a viscoelastic model and extract frequency-independent parameters to describe the mechanical properties of the brain tissue [15,17–20]. By using such a model, the resulting parameters describing viscoelastic properties are frequency-independent, facilitating direct comparisons across different research groups and adding to our understanding of the mechanical properties of the brain.
Variations in the viscoelastic properties of the brain have been observed in different age categories and in different anatomical regions of the brain by previous MRE studies. The stiffness of the brain and its lobes decreased continuously with age [9,11,15,21]. The evolution of the viscoelastic properties of the brain varies with age for different anatomical regions. For instance, subcortical gray matter, such as the caudate and putamen, is significantly stiffer in adolescents than in adults, while the hippocampus and amygdala are softer [12–14]. These differences may relate to different developmental states of brain structures [22,23]. The viscoelastic properties of the subcortical gray matter remain a hot topic, which was not specifically focused on previously or analyzed only with single-frequency MRE protocols but without frequency-independent parameters.
In this study, we used a multifrequency MRE protocol [24–26] to analyze the mechanical properties in different regions of the brain in healthy adults for a wide range of ages.
Subjects and Methods
Subjects
This study was approved by our local ethics committee. Written informed consent was obtained from all subjects prior to enrollment. A sample size calculation determined that at least 37 subjects were required in the study to have 80% power, assuming a Pearson correlation coefficient (r) of 0.4, and testing with a one-sided 95% confidence interval (CI). The calculation was based on the “pwr” package (version 1.2–2) in R Software (version 3.6.1, Stata Corp).
A total of 46 healthy subjects (22 males, 24 females) ranging from 26 to 76 years old were enrolled and included in this study. Subjects with a history of neurological or psychiatric disorders, head trauma, stroke, brain tumor, or claustrophobia were excluded.
Data acquisition
MRI data were acquired using a published and fully applied multifrequency MRE protocol [20,24,25] on a 3T scanner (General Electric 750, GE Healthcare) with a 32-channel phased array coil. Subjects were scanned in a supine position. The following sequences were acquired: (1) high-resolution structural images using the 3D-BRAVO sequence: 196 slices, field of view=24×24 cm, TR/TE=9.3/3.7 ms, TI=400 ms; matrix=256×256, and flip angle=13°, and (2) MRE data using a two-dimensional echo planar imaging (EPI) pulse sequence [27]: 48 transverse slices, slice thickness=3 mm, field of view=24×24 cm, TR/TE= 2000 ms/min full (e.g., frequencies of vibration/TE=40 Hz/74.5 ms, 60 Hz/62.0 ms, 80 Hz/51.6 ms, and 90 Hz/59.2 ms)(TE should change by changing the MRE frequency so it should not be the same for all actuation frequencies), matrix 128×128, and motion encoding gradient (MEG) strength=35 mT/m. Shear waves were generated by an activation pillow developed and provided by the Mayo Clinic (Mayo Clinic) and excited by a remote acoustic active driver. The activation pillow was placed underneath the subject’s head and induced skull vibrations, thus transmitting shear waves inside the brain. According to the protocol, vibration frequencies for the activation pillow were set as 40 Hz, 60 Hz, 80 Hz and 90 Hz [20,24,25]. We performed algebraic direct inversion without phase unwrapping by following the methods presented in previous studies. The total scan time was approximately 25 minutes per subject.
Data processing
Anatomical reconstruction and segmentation
We applied an automated, region-specific parameter extraction tool (Simpleware, Synopsys https://www.synopsys.com/simpleware.html) to register the brain of each subject to a widely used atlas. Briefly, we utilized the SPM 12 software package and registered high-resolution structural images of each subject to the Montreal Neurological Institute (MNI152) template. The MRE results of the same subject were also registered to his or her corresponding reference structural images. Gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) areas and subcortical GM structures (including the bilateral amygdala, hippocampus, caudate, putamen, pallidum and thalamus) were automatically segmented using the automated anatomical labeling (AAL) atlas [29]. The frequency-dependent and frequency-independent MRE parameters were calculated and recorded only for the voxels in either gray or white matter or in both. Voxels containing any CSF were removed.
Frequency-dependent parameters: storage and loss moduli
A two-dimensional direct inversion technique was applied to calculate the elastograms from the MRE data [30]. Frequency-dependent parameters included storage and loss moduli (G’ and G”, respectively; the formulas for G’ and G” are listed below) [4]. G’ is the real component of the shear modulus. It is a measure of the restoration of mechanical energy and describes the elastic behavior of the brain. G” is the imaginary component of the shear modulus. It describes the viscous behavior of the material by measuring the energy dissipation in tissue as a result of the mechanical friction inherent to the material [4]. Both G’ and G” values for each cerebral subcortical GM region were recorded for all four activation frequencies.
Frequency-independent parameters: μ1, μ2 and η
We applied a standard linear solid model to calculate frequency-independent material properties [20,24]. Previous studies demonstrated that among different linear viscoelastic material models, the standard linear solid model represented the brain responses in the frequency ranges included in this study the best, along with the Maxwell model. This study also checked optimization bias by considering a 3-parameter model and confirmed that the Bayesian information criterion was still the lowest for the standard linear solid model. Briefly, the standard linear solid model consists of a spring with an elastic shear stiffness μ1, as well as a parallel arranged spring and damper with a viscoelastic shear stiffness μ2 and viscosity η, respectively. This model yields the following complex shear modulus G in the frequency domain:
In our study, the four activation frequencies, ω, were set to 40, 60, 80 and 90 Hz. The trends of the storage and loss moduli at extreme frequency values are such that the storage modulus is G’ = μ1 for frequencies ω → 0 and G’ = μ1 + μ2 for frequencies ω → ∞ [31]. The loss modulus G” is the highest with the frequency ω associated with the inverse relaxation time μ2/η. Based on this model, we calculated μ1, μ2 and η for the different brain regions of interest. Quality controls were ensured as in previous studies [20,24,25].In brief, we have checked confidence maps provided by the MRE sequence and used a 80% threshold in each case. Consequently, we have excluded regions and voxels that fall outside of this confidence range.
Statistical analysis
The demographic data of the enrolled subjects were recorded and summarized. Brain volume changes were measured as a function of age. Frequency-dependent (storage moduli G’ and loss moduli G”) and frequency-independent (μ1, μ2 and η) parameters were calculated for each activation frequency. We calculated the values in the ① cerebral parenchyma (including all of the GM and WM, excluding CSF), ② cortical GM, ③ WM and ④ subcortical GM as a whole. We then recorded the value of each region of interest (ROI) in the subcortical GM (⑤ hippocampus, ⑥ amygdala, ⑦ caudate, ⑧ putamen, ⑨ pallidum and ⑩ thalamus). We averaged the values on the right and left sides together, since they were not significantly different. The voxels used for consideration in the ROIs for the various structures were only those that contained gray or white matter; voxels that contained CSF were excluded. Since the ratio of G”/G’ is quantitatively related to the fluid-to-solid fractional composition, we calculated this ratio in the ROIs mentioned above. We also developed mixed linear models to estimate multifrequency data, including confounding variables such as age and sex. Mixed linear models were performed using R Software (version 3.6.1, Stata Corp).
Correlation and linear regression analyses were conducted to determine the changes in frequency-dependent and frequency-independent MRE parameters as a function of age for the ten ROIs. Since viscoelastic mechanical properties may be affected by brain volume, corresponding brain volumes were used as covariates. All calculations were performed using SPSS 16.0 software (SPSS, Inc.). The level of significance was set as P<0.05. The R2 values for the linear regression models for subcortical GM ROIs were reported. For the linear regression, the standard deviations were used as a weighting parameter. Furthermore, we compared the differences in these representative values among age groups (20–39, 40–59, over 60 years old) by using one-way analysis of variance (ANOVA) with a least significant difference (LSD) post hoc test. Thresholds were set at P<0.05.
Results
Subject population
Forty-six healthy adult volunteers ranging from 26 to 76 years old were recruited and enrolled in this study (mean age 47.0 years, standard deviation (SD) 16.1 years; 22 males, age range 26 to 76 years, 46.4±17.0 years; 24 females, age range 26 to 72 years, 47.6±15.5 years). Details about the subjects are listed in Table 1.
Table 1.
Characterization of enrolled subjects.
| Age | Total number | Gender (male/female) | Years of age (all) | Years of age (male) | Years of age (female) |
|---|---|---|---|---|---|
| 20–39 | 20 | 11/9 | 31.5±4.2 | 31.5±3.9 | 31.4±4.7 |
| 40–59 | 13 | 5/8 | 50.3±6.8 | 52.0±6.3 | 49.3±7.2 |
| 60+ | 13 | 6/7 | 67.6±4.8 | 68.8±5.5 | 66.6±4.3 |
Changes in brain volume with respect to age
Table 2 shows the expected decrease in whole cerebral parenchyma, cortical GM, WM and subcortical GM volumes with age. We observed a loss of 2.59 cm3/year of whole cerebral parenchyma tissue (R2=0.17, P=0.004) and a loss of 2.03 cm3/year of cortical GM tissue (R2=0.30, P<0.001). Volume changes in the WM and the whole subcortical GM regions were not statistically significant.
Table 2.
Brain volume changes with age.
| Average volume (cm3) | Annual changes (cm3) | 95% CI of Annual Changes | Annual changes% | R2 | P | Linear regression | ||
|---|---|---|---|---|---|---|---|---|
| Cerebral parenchyma | all | 1020.1±100.7 | −2.59 | −4.324 to −0.858 | −0.24 | 0.17 | 0.004* | Y = −2.59*X + 1142.0 |
| male | 1086.2±83.2 | −3.01 | −4.810 to −1.205 | −0.26 | 0.38 | 0.002* | Y = −3.01*X + 1226.0 | |
| female | 959.6±74.1 | −1.81 | −3.765 to −0.139 | −0.18 | 0.14 | 0.07 | Y = −1.81*X + 1046.0 | |
| Cortical GM | all | 568.6±59.1 | −2.03 | −2.962 to −1.095 | −0.32 | 0.30 | <0.001* | Y = −2.03*X + 663.5 |
| male | 603.4±52.1 | −2.27 | −3.229 to −1.311 | −0.34 | 0.55 | <0.001* | Y = −2.27*X + 708.6 | |
| female | 535.7±45.7 | −1.59 | −2.687 to −0.502 | −0.27 | 0.29 | 0.006* | Y = −1.59*X + 611.6 | |
| WM | all | 409.3±42.9 | −0.50 | −1.305 to 0.288 | −0.12 | 0.04 | 0.205 | Y = −0.50*X + 433.2 |
| male | 437.8±33.6 | −0.67 | −1.539 to 0.193 | −0.15 | 0.12 | 0.12 | Y = −0.67*X + 469.0 | |
| female | 383.3±33.0 | −0.19 | −1.124 to 0.749 | −0.05 | 0.01 | 0.68 | Y = −0.19*X + 392.2 | |
| Subcortical GM | all | 42.7±3.6 | −0.05 | −0.121 to 0.012 | −0.11 | 0.06 | 0.107 | Y = −0.05*X + 45.3 |
| male | 45.1±3.5 | −0.06 | −0.156 to 0.027 | −0.13 | 0.10 | 0.16 | Y = −0.06*X + 48.1 | |
| female | 40.6±2.1 | −0.05 | −0.105 to 0.107 | −0.13 | 0.14 | 0.07 | Y = −0.05*X + 38.2 |
Data are presented as mean ± SD. The level of significance was set as P<0.05.
Changes in brain mechanical properties with respect to age
Supplementary Table 1 lists the brain mechanical properties of the different brain regions in the group of all subjects, males and females. In general, the values of G’, G”, μ1 and μ2 were higher in females than in males. However, there was no statistical significance among the sexes. The results were further analyzed by linear regression to assess the relationship between the mechanical properties of the brain and age. Brain volume was normalized for calculation. Changes in the mechanical properties of the brain with respect to age were analyzed in cerebral parenchyma, cortical GM, WM and subcortical GM regions (Supplementary Table 2). All of the frequency-dependent and independent parameters of the cerebral parenchyma, cortical GM and WM showed strong negative correlations with age. The changes in frequency-dependent parameters showed a stronger correlation with age (R2 up to 0.66 in all subject groups); changes in frequency-independent parameters showed a weaker correlation with age (R2 up to 0.50). However, for the subcortical GM, only the storage modulus at 60 Hz and 80 Hz, the loss modulus at 60 Hz, μ2 and η were significantly correlated with age. The R2 values ranged from 0.09 to 0.21 for results with statistical significance. The R2 values were higher in most cases when divided by sex, indicating that changes in the mechanical properties were different in males and females.
Absolute annual changes in G’ and G” values as well as the G”/G’ ratio were higher when the activation vibration frequency was higher (Figure 1a), but the annual change rates were relatively similar for all frequencies. The annual percentage change rates ranged from −0.32% to −0.36% for G’ and from −0.43% to −0.55% for G” for the cerebral parenchyma, cortical GM and WM. The annual percentage change rates for subcortical GM ranged from −0.18% to −0.23% for G’ and from −0.21% to −0.43% for G”. Figure 1b roughly shows the effect of age on the values of G’ and G”. The average annual percentage change rate of the G”/G’ ratio was −0.18% for cortical GM, −0.16% for subcortical GM and −0.13% for WM. The results of mixed linear model analysis showed that different frequencies give significantly different results (Supplementary Table 4).
Figure 1. The effects of vibration frequency and age on G’ (a) and G” (b).
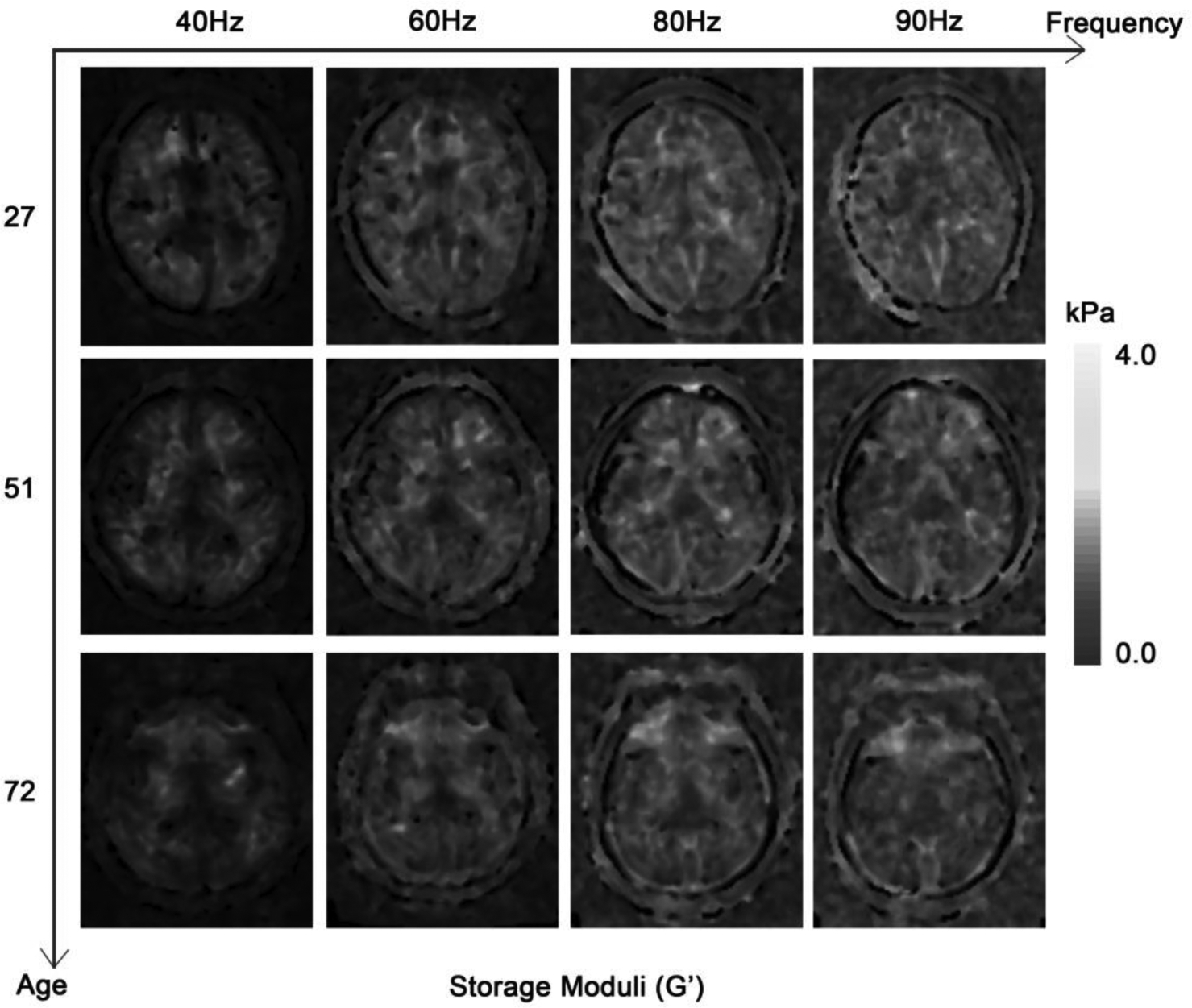
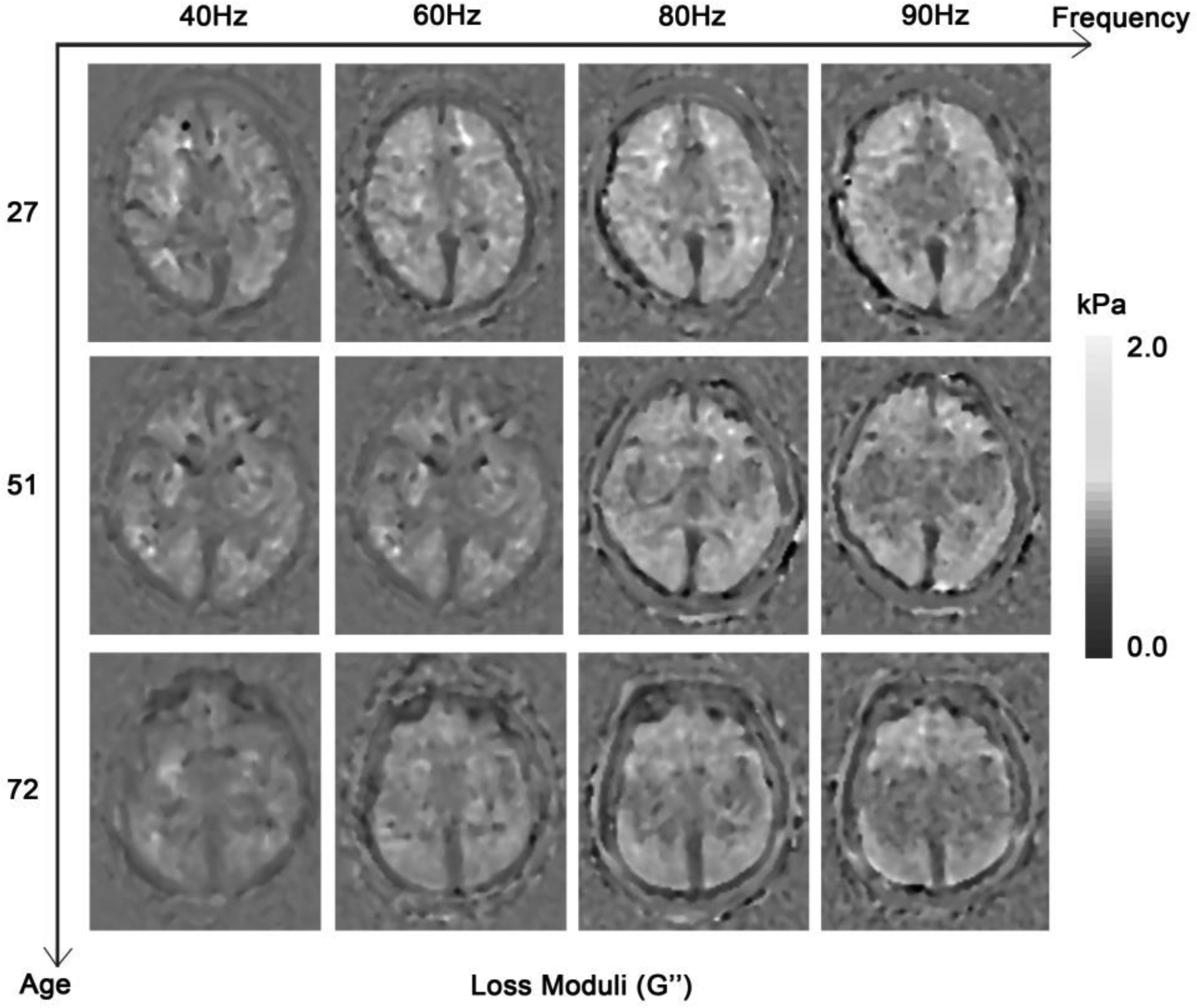
On the grayscale figures, the brighter the voxels, the higher the value of the results. It can be roughly discerned that the values of G’ and G” were higher when the activation vibration frequency was higher. The ages of the patients were 27, 51 and 72 years old, respectively. The values of G’ and G” also decreased with age.
Changes in mechanical properties with respect to age differed in different subregions of the subcortical GM (Supplementary Table 5). For all subjects, mechanical parameters in the hippocampus, amygdala and globus pallidum were relatively stable with age. In contrast, several mechanical parameters varied with age in the caudate nucleus, putamen and thalamus. Mainly, the viscoelastic parameters were G’ at 60 Hz, 80 Hz, 90 Hz, and G” at 60 Hz, 80 Hz, μ2 and η. The corresponding complex moduli linearly decreased with age from −0.22% to −0.73% per year, with R2 ranging from 0.09 to 0.34 (P<0.05). Differences were also identified in males and females. (Supplementary Tables 2, 3, and 5). The average annual percentage change rates in females were lower than those observed in males in most of the analyzed brain regions. Interestingly, significant results were identified in G” at 60 Hz in the hippocampus and amygdala and at 80 Hz in the caudate nucleus, putamen, and globus pallidum in females but not in males. There were no significant changes in the G”/G’ ratio in subregions of the subcortical GM.
The results with the most prominent R2 in the subcortical GM were the G’ of the putamen at 60 Hz, the G” of the caudate and thalamus at 60 Hz, and the μ2 of the caudate and thalamus. Significant differences in these five mechanical properties were also found among age groups (Figure 2). Specifically, decreased mechanical properties in subjects over 60 years old were statistically significant. In contrast, there were no significant differences between subjects under 59 years of age.
Figure 2. Linear regressions of the annual changes and differences among age groups of the subcortical GM mechanical properties.
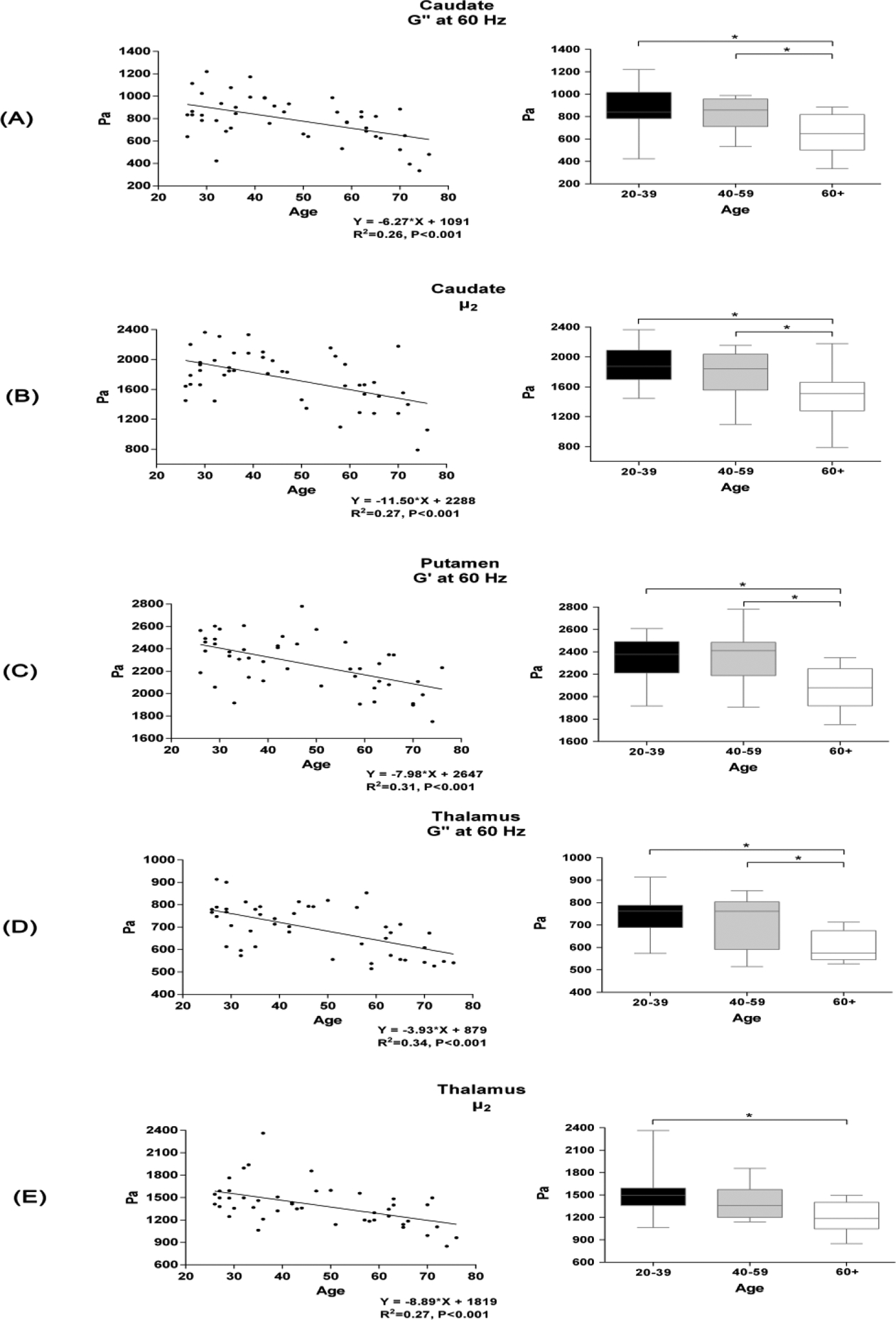
The age-related decrease in brain viscoelasticity was most prominent in the caudate, putamen and thalamus, mostly revealed by values of G’ and G” at 60 Hz as well as μ2. Differences in the mechanical properties of these brain regions among age groups are also shown on the right side (ANOVA and post hoc, P<0.05). A: G” at 60 Hz in the caudate. B: Frequency-independent μ2 in the caudate. C: G’ at 60 Hz in the putamen. D: G” at 60 Hz in the thalamus. E: μ2 in the thalamus. G’, storage modulus; G”, loss modulus.
Discussion
MR elastography results are influenced by the activation vibration frequency. Indeed, almost all biological tissues display frequency-dependent viscoelastic behavior. The activation frequency can be set at a wide range from almost 20 Hz to 100 Hz. In previous studies, the activation frequency for MRE of the brain was often set at 50 or 60 Hz [9,11–15]. It has been demonstrated that the shear modulus increases when the vibration frequency increases [15,17,18,20,25]. In addition, different measurement methods, reconstruction algorithms and symmetry assumptions of different research groups make cross-study comparisons challenging. Recently, multifrequency MRE protocols have been reported [20,24–26] to extract frequency-independent parameters to characterize the mechanical properties of the brain. In this study, we used one such multifrequency MRE protocol [20]. Using this protocol, we present a detailed characterization of the viscoelastic mechanical properties (frequency-dependent parameters, G’ and G”; frequency-independent parameters, μ1, μ2 and η) of different regions in a group of healthy adults with a wide range of ages. The multifrequency behavior of the brain as well as the directly comparable frequency-independent values can be analyzed using this protocol. Importantly, the frequency-independent parameters may serve as standardized baseline values that facilitate direct comparison across results from different research groups. These parameters might also be used for specific clinical applications, i.e., acquiring data from patients with brain tumors to avoid an overlap of frequency-dependent parameters between different tumor types [16].
In this study, we observed similar changes in the mechanical properties of the brain with respect to age compared to previously published articles [9,11,15,21]. We observed similar annual changes and a similar ratio of G’ and G” in the whole brain at a vibration frequency of 60 Hz, as well as similar changes in frequency-independent parameters when applying the same viscoelastic model [15]. Interestingly, the G”/G’ ratio changes were similar in both cortical and subcortical GM (−0.18%, −0.16%, respectively) but different in WM (−0.13%), indicating different patterns of fluid-to-solid fractional composition alteration over age. Specifically, our research focused on the effect of aging on the viscoelastic mechanical properties of the subcortical GM and different subregions of the subcortical GM (hippocampus, amygdala, caudate, putamen, pallidum, thalamus) in vivo. The application of an automatic, region-specific parameter extraction tool ensured the quality of the study results.
We found the brain volume decreased by 0.24% per year, which is consistent with previous studies [21]. The results of viscoelastic mechanical properties may be affected by the decreased brain volume. To exclude this confounding factor, we added the brain volume as a covariate in the linear regression models. After adjusting for brain volume loss with age, we found that the absolute changes in G’ and G” of the cerebral parenchyma, cortical GM, WM and subcortical GM regions increased with increasing vibration frequency. However, interestingly, the annual percentage change rates were relatively similar for all frequencies. The viscoelastic properties of the brain varied depending on the anatomical region, including the different regions of the subcortical GM, which was the original focus of our study. Interestingly, the annual change rates (%) were relatively similar for all frequencies. This indicates that the decrease in mechanical properties may be a stable value that can reflect changes in the brain more objectively.
Overall, the subregions of the subcortical GM showed decreasing frequency-dependent or frequency-independent values with age, which was also observed in a recently published study [11]. The values of the amygdala, hippocampus and pallidum mainly varied with age, while parameters of the caudate, putamen and thalamus showed a stronger correlation with age. Recently, it was reported that the amygdala and hippocampus exhibited separate stiffness and damping ratio patterns among subcortical GM subregions in an adolescent group [12]. Relatively higher standard deviations of stiffness and damping ratio values for the amygdala, hippocampus and pallidum were also observed in adults [12,13], which may additionally decrease the correlation and regression significance. The subregions of the subcortical GM for which changes in mechanical properties were the most correlated with age were the caudate, putamen and thalamus. The parameters that showed the highest correlation were G’ and G” at 60 Hz, as well as frequency-independent μ2. These values and their changes in the caudate, putamen and thalamus may serve as objective parameters that can be compared between healthy subjects and patient groups. Additionally, the results indicated that changes in the mechanical properties were different in males and females. Interestingly, for different regions of subcortical GM, the results with significance were identified in one sex but not another. This also indicated that males are characterized by decreased elasticity, while females exhibit decreased viscosity corresponding with age in some regions of subcortical GM. In addition, we found that the average changes in the annual rates were lower in females than in males in most of the analyzed brain regions.
Most of these mechanical properties were significantly lower between subjects over 60 years old and subjects between 40–59 years old. In contrast, there were no significant differences between subjects under 59, as shown in Figure 2. This may indicate that the decrease in brain stiffness is accelerated in elderly subjects.
A good understanding of the natural evolution of the viscoelastic properties of the brain is necessary to identify and interpret changes observed in pathological conditions, i.e., to differentiate a change that is expected in the patient’s age group versus a pathological change [32–38]. For instance, Streitberger et al. found that the elasticity of the white matter and of the thalamic regions in patients with neuromyelitis optica was significantly reduced [35]. Decreased viscosity in the hippocampus was observed in an animal model of Alzheimer’s disease [32]. This may be due to different patterns of demyelination, cytoskeletal crosslinking and axonal organization in normal aging and pathological changes [7,39]. This understanding may increase our comprehension of the frequency-dependent response of the human brain [40,41].
There are several limitations. First, the imaging quality of the recently developed 3D MRE is supposed to be superior to that of the 2D MRE used in this study. It would be valuable to assess signal-to-noise or phase-to-noise ratios to ensure imaging quality.
A frequency of 90 Hz was one of the vibration options. Waves at 90 Hz may not penetrate deep enough to the center of the brain. Frequency-independent parameters may be more vulnerable to noise since they require two sets of calculations. It is necessary to measure their repeatability in subsequent studies.
The linear regression results may not fully characterize the features of change over age. Theoretically, the influence of aging should be studied longitudinally with the same subjects. Subjects younger than 26 years old are needed in further studies since the influence of age on the mechanical properties of the brain is particularly important in teenagers [42].
In conclusion, we used a multifrequency MRE protocol to assess the changes in the mechanical properties of the brain with age. We found different types of changes in different subregions of the subcortical GM in males and females. Values of G’, G” at 60 Hz and frequency-independent μ2 of the caudate, putamen and thalamus may serve as parameters to characterize the aging effects on the brain. The decrease in brain stiffness accelerates in elderly subjects. The frequency-independent parameters may serve as standardized baseline values that facilitate direct comparison across results from different research groups. Our research contributes to the understanding of the mechanical properties of the brain.
Supplementary Material
Key points.
We used a multifrequency MRE protocol to assess changes in the mechanical properties of the brain with age.
Frequency-dependent (storage moduli G’ and loss moduli G”) and frequency-independent (μ1, μ2 and η) parameters can be quantitatively measured by our protocol.
The decreased value of viscoelastic properties due to aging varies in different regions of subcortical GM in males and females.
The decrease in brain stiffness is accelerated in elderly subjects over 60 years old.
Acknowledgements
We thank Richard L. Ehman from the Mayo Clinic Rochester for providing the MRE activation device. We acknowledge Karla Epperson, Kevin Epperson, and Anne M. Sawyer from the Richard M. Lucas Center, Stanford University for their support during the experiments.
Funding information
This work was supported by Grant No.61801311 from the National Natural Science Foundation of China, Grant No.7182044 from Beijing Natural Science Foundation, No. PX2018001 from Beijing Hospitals Authority, QML20180103 from Beijing Hospitals Authority Youth Programme, No. YYZZ2017B01 from Beijing Friendship Hospital, Capital Medical University and No. 2019M660717 from China Postdoctoral Science Foundation.
Abbreviations
- AAL
automated anatomical labeling
- ANOVA
analysis of variance
- CSF
cerebrospinal fluid
- CT
computed tomography
- G’
storage moduli
- G”
loss moduli
- GE
General Electric
- GM
gray matter
- LSD
least significant difference
- MEG
motion encoding gradient
- MNI
Montreal Neurological Institute
- MRE
magnetic resonance elastography
- MRI
magnetic resonance imaging
- ROI
region of interest
- SD
standard deviation
- SEM
standard error of the mean
- WM
white matter
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Guarantor:
The scientific guarantor of this publication is Max Wintermark.
Conflict of Interest:
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and Biometry:
One of the authors has significant statistical expertise.
Informed Consent:
Written informed consent was obtained from all subjects (patients) in this study.
Ethical Approval:
Institutional Review Board approval was obtained.
- retrospective
- cross sectional study
- performed at one institution
References
- 1.Ophir J, Alam SK, Garra B et al. (1999) Elastography: ultrasonic estimation and imaging of the elastic properties of tissues. Proc Inst Mech Eng H 213:203–233. [DOI] [PubMed] [Google Scholar]
- 2.Muthupillai R, Lomas DJ, Rossman PJ et al. (1995) Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. SCIENCE 269:1854–1857. [DOI] [PubMed] [Google Scholar]
- 3.Muthupillai R, Ehman RL (1996) Magnetic resonance elastography. NATURE MEDICINE 2:601–603. [DOI] [PubMed] [Google Scholar]
- 4.Hiscox LV, Johnson CL, Barnhill E et al. (2016) Magnetic resonance elastography (MRE) of the human brain: technique, findings and clinical applications. PHYSICS IN MEDICINE AND BIOLOGY 61:R401–R437. [DOI] [PubMed] [Google Scholar]
- 5.Glaser KJ, Manduca A, Ehman RL (2012) Review of MR elastography applications and recent developments. JOURNAL OF MAGNETIC RESONANCE IMAGING 36:757–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Ieva A, Grizzi F, Rognone E et al. (2010) Magnetic resonance elastography: a general overview of its current and future applications in brain imaging. NEUROSURGICAL REVIEW 33:137–145, 145. [DOI] [PubMed] [Google Scholar]
- 7.Murphy MC, Huston J. Rd, Ehman RL (2019) MR elastography of the brain and its application in neurological diseases. NEUROIMAGE 187:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin Z Romano AJ Manduca A Ehman RL Huston J. Rd (2018) Stiffness and Beyond: What MR Elastography Can Tell Us About Brain Structure and Function Under Physiologic and Pathologic Conditions. Top Magn Reson Imaging 27:305–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arani A, Murphy MC, Glaser KJ et al. (2015) Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults. NEUROIMAGE 111:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sack Ingolf Jöhrens Korinna Würfel Jens Braun Jürgen (2013) Structure-sensitive elastography: on the viscoelastic powerlaw behavior of in vivo human tissue in health and disease. Soft Matter 9:5672–5680. [Google Scholar]
- 11.Takamura T, Motosugi U, Sasaki Y et al. (2020) Influence of Age on Global and Regional Brain Stiffness in Young and Middle-Aged Adults. JOURNAL OF MAGNETIC RESONANCE IMAGING 51:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McIlvain G Schwarb H Cohen NJ Telzer EH Johnson CL (2018) Mechanical properties of the in vivo adolescent human brain. Dev Cogn Neurosci 34:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiscox LV, Johnson CL, McGarry MDJ et al. (2018) High-resolution magnetic resonance elastography reveals differences in subcortical gray matter viscoelasticity between young and healthy older adults. NEUROBIOLOGY OF AGING 65:158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson CL, Schwarb H, D J. McGarry M et al. (2016) Viscoelasticity of subcortical gray matter structures. HUMAN BRAIN MAPPING 37:4221–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sack I, Beierbach B, Wuerfel J et al. (2009) The impact of aging and gender on brain viscoelasticity. NEUROIMAGE 46:652–657. [DOI] [PubMed] [Google Scholar]
- 16.Bunevicius A Schregel K Sinkus Golby A Patz S (2019) REVIEW: MR elastography of brain tumors. Neuroimage Clin 25:102109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dittmann F, Hirsch S, Tzschatzsch H et al. (2016) In vivo wideband multifrequency MR elastography of the human brain and liver. MAGNETIC RESONANCE IN MEDICINE 76:1116–1126. [DOI] [PubMed] [Google Scholar]
- 18.Feng Y Clayton EH Chang Y Okamoto RJ Bayly PV (2013) Viscoelastic properties of the ferret brain measured in vivo at multiple frequencies by magnetic resonance elastography. JOURNAL OF BIOMECHANICS 46:863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klatt D Hamhaber U Asbach P Braun J Sack I (2007) Noninvasive assessment of the rheological behavior of human organs using multifrequency MR elastography: a study of brain and liver viscoelasticity. PHYSICS IN MEDICINE AND BIOLOGY 52:7281–7294. [DOI] [PubMed] [Google Scholar]
- 20.Kurt M, Wu L, Laksari K et al. (2019) Optimization of a Multifrequency Magnetic Resonance Elastography Protocol for the Human Brain. JOURNAL OF NEUROIMAGING 29:440–446. [DOI] [PubMed] [Google Scholar]
- 21.Sack I Streitberger KJ Krefting D Paul F Braun J (2011) The influence of physiological aging and atrophy on brain viscoelastic properties in humans. PLoS One 6:e23451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Squarzoni P Duran FLS Busatto GF Alves TCTF (2018) Reduced Gray Matter Volume of the Thalamus and Hippocampal Region in Elderly Healthy Adults with no Impact of APOE varepsilon4: A Longitudinal Voxel-Based Morphometry Study. JOURNAL OF ALZHEIMERS DISEASE 62:757–771. [DOI] [PubMed] [Google Scholar]
- 23.Raz N, Lindenberger U, Rodrigue KM et al. (2005) Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. CEREBRAL CORTEX 15:1676–1689. [DOI] [PubMed] [Google Scholar]
- 24.Weickenmeier J, Kurt M, Ozkaya E et al. (2018) Magnetic resonance elastography of the brain: A comparison between pigs and humans. J Mech Behav Biomed Mater 77:702–710. [DOI] [PubMed] [Google Scholar]
- 25.Mehmet Kurt, Han Lv, Kaveh Laksari et al. (2016) In vivo multi-frequency magnetic resonance elastography of the human brain: Which frequencies matter? In. Biomedical Engineering Society Annual Meeting. Phoenix, AZ, USA [Google Scholar]
- 26.Chartrain AG, Kurt M, Yao A et al. (2019) Utility of preoperative meningioma consistency measurement with magnetic resonance elastography (MRE): a review. NEUROSURGICAL REVIEW 42:1–7. [DOI] [PubMed] [Google Scholar]
- 27.Murphy MC, Huston J. Rd, Jack CR Jr et al. (2011) Decreased brain stiffness in Alzheimer’s disease determined by magnetic resonance elastography. JOURNAL OF MAGNETIC RESONANCE IMAGING 34:494–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glaser K, Ehman R (2009) MR Elastography Inversions Without Phase Unwrapping. Proc. Intl. Soc. Mag. Reson. Med:4669. [Google Scholar]
- 29.Tzouriomazoyer N, Landeau B, Papathanassiou D et al. (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NEUROIMAGE 15:273. [DOI] [PubMed] [Google Scholar]
- 30.Oliphant TE Manduca A Ehman RL Greenleaf JF (2001) Complex-valued stiffness reconstruction for magnetic resonance elastography by algebraic inversion of the differential equation. MAGNETIC RESONANCE IN MEDICINE 45:299–310. [DOI] [PubMed] [Google Scholar]
- 31.Fung YC, Cowin SC (1994) Biomechanics: Mechanical Properties of Living Tissues, 2nd edn.. Springer Science+Business Media, Berlin [Google Scholar]
- 32.Munder T, Pfeffer A, Schreyer S et al. (2018) MR elastography detection of early viscoelastic response of the murine hippocampus to amyloid beta accumulation and neuronal cell loss due to Alzheimer’s disease. JOURNAL OF MAGNETIC RESONANCE IMAGING 47:105–114. [DOI] [PubMed] [Google Scholar]
- 33.Murphy MC, Jones DT, Jack CR Jr et al. (2016) Regional brain stiffness changes across the Alzheimer’s disease spectrum. Neuroimage Clin 10:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.ElSheikh M, Arani A, Perry A et al. (2017) MR Elastography Demonstrates Unique Regional Brain Stiffness Patterns in Dementias. AJR Am J Roentgenol 209:403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Streitberger KJ, Fehlner A, Pache F et al. (2017) Multifrequency magnetic resonance elastography of the brain reveals tissue degeneration in neuromyelitis optica spectrum disorder. EUROPEAN RADIOLOGY 27:2206–2215. [DOI] [PubMed] [Google Scholar]
- 36.Huston J. Rd, Murphy MC, Boeve BF et al. (2016) Magnetic resonance elastography of frontotemporal dementia. JOURNAL OF MAGNETIC RESONANCE IMAGING 43:474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riek K, Millward JM, Hamann I et al. (2012) Magnetic resonance elastography reveals altered brain viscoelasticity in experimental autoimmune encephalomyelitis. Neuroimage Clin 1:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weickenmeier J, Kurt M, Ozkaya E et al. (2018) Brain stiffens post mortem. J Mech Behav Biomed Mater 84:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo J, Bertalan G, Meierhofer D et al. (2019) Brain maturation is associated with increasing tissue stiffness and decreasing tissue fluidity. Acta Biomaterialia 99:433–442. [DOI] [PubMed] [Google Scholar]
- 40.Javid Abderezaei, Wei Zhao, Carissa Grijalva et al. (2019) Nonlinear dynamical behavior of the deep white matter during head impact. Physical Review Applied. DOI: 10.1103/PhysRevApplied.12.014058 [DOI] [Google Scholar]
- 41.Laksari K Kurt M Babaee H Kleiven S Camarillo D (2018) Mechanistic Insights into Human Brain Impact Dynamics through Modal Analysis. PHYSICAL REVIEW LETTERS 120:138101. [DOI] [PubMed] [Google Scholar]
- 42.Johnson CL, Telzer EH (2018) Magnetic resonance elastography for examining developmental changes in the mechanical properties of the brain. Dev Cogn Neurosci 33:176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


