Abstract
Objective
To assess non-inferiority of s.c. to i.v. CT-P13 in RA.
Methods
Patients with active RA and inadequate response to MTX participated in this phase I/III double-blind study at 76 sites. Patients received CT-P13 i.v. 3 mg/kg [week (W) 0 and W2] before randomization (1:1) at W6 to CT-P13 s.c. via pre-filled syringe (PFS) 120 mg biweekly until W28, or CT-P13 i.v. 3 mg/kg every 8 weeks until W22. Randomization was stratified by country, W2 serum CRP and W6 body weight. From W30, all patients received CT-P13 s.c. In a usability sub-study, patients received CT-P13 s.c. via auto-injector (W46–54) then PFS (W56–64). The primary endpoint was change (decrease) from baseline in disease activity score in 28 joints (DAS28)-CRP at W22 (non-inferiority margin: −0.6).
Results
Of 357 patients enrolled, 343 were randomized to CT-P13 s.c. (n = 167) or CT-P13 i.v. (n = 176) at W6. The least-squares mean change (decrease) from baseline (standard error) in DAS28-CRP at W22 was 2.21 (0.22) for CT-P13 s.c. (n = 162) and 1.94 (0.21) for CT-P13 i.v. [n = 168; difference 0.27 (95% CI: 0.02, 0.52)], establishing non-inferiority. Efficacy findings were similar between arms at W54. Safety was similar between arms throughout: 92 (54.8%; CT-P13 s.c.) and 117 (66.9%; CT-P13 i.v.) patients experienced treatment-emergent adverse events (from W6). There were no treatment-related deaths or new safety findings. Usability was similar for CT-P13 s.c. via auto-injector or PFS.
Conclusion
CT-P13 s.c. was non-inferior to CT-P13 i.v. in active RA. The convenience of s.c. administration could benefit patients.
Trial registration
ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03147248.
Keywords: biosimilar, non-inferiority, CT-P13, subcutaneous, rheumatoid arthritis, infliximab, switching, pharmacokinetics, immunogenicity
Rheumatology key messages
CT-P13 s.c. was non-inferior in terms of efficacy to CT-P13 i.v., without altering safety signals.
CT-P13 s.c. safety and efficacy were also well maintained after switching from CT-P13 i.v.
Usability was similar and high for CT-P13 s.c. self-administration via auto-injector or pre-filled syringe.
Introduction
CT-P13, an infliximab biosimilar, is a chimeric monoclonal antibody against TNF, with the same pharmaceutical form, strength, composition and i.v. administration route as reference infliximab [1]. CT-P13 i.v. has demonstrated comparable efficacy, pharmacokinetics (PK), immunogenicity and safety to reference infliximab in AS and RA [2, 3], and is approved for the same indications [4–7]. As for other biosimilars, CT-P13 availability contributes to reduced treatment costs and improved patient access to biologics [8, 9].
An s.c. formulation of CT-P13 could offer potential benefits for RA patients (particularly those who self-administer therapy) [10] in terms of convenience, medical resource optimization and health-care system costs [10–12]. Preliminary data with an s.c.-administered experimental infliximab formulation in patients with RA refractory to MTX suggest regular s.c. dosing could also provide more stable serum drug concentrations vs i.v. dosing, where flares may be experienced before the next dose [13, 14]. However, small sample size, heterogeneity of the patient population and lack of a placebo control arm limit the conclusions that can be drawn from that study [13].
This randomized phase I/III study evaluated the s.c. and i.v. formulations of CT-P13, in combination with MTX, in patients with active RA with inadequate response to MTX. Part 1 of the study [15, 16] was conducted to find the optimal CT-P13 s.c. dose and Part 2 to demonstrate the non-inferiority of CT-P13 s.c. to CT-P13 i.v. in terms of efficacy at week (W) 22. During Part 1, 48 patients were randomized (1:1:1:1) to receive CT-P13 i.v. [3 mg/kg every 8 weeks (q8w)] or CT-P13 s.c. [90, 120 or 180 mg every 2 weeks (q2w)] after initial CT-P13 i.v. dose-loading at W0 and W2; mean serum concentrations consistently exceeded the target therapeutic concentration (1 µg/ml) in all s.c. cohorts [15, 16]. The CT-P13 s.c. dosing regimen for Part 2 was selected based on Part 1 data [15, 16], population PK and PK-pharmacodynamic (PD) modelling, and simulation results comparing CT-P13 s.c. and CT-P13 IV i.v. dose regimens in RA patients. Part 2, reported here, evaluated the efficacy, PK, PD and safety of CT-P13 s.c. and CT-P13 i.v. in patients with active RA. In addition, the usability of CT-P13 s.c. administration via pre-filled syringe (PFS) or auto-injector (AI) was evaluated in a subset of patients.
Methods
Study design
This was a multicentre, randomized, double-blind, parallel-group, non-inferiority, phase I/III study (NCT03147248) initiated at 76 centres in multiple countries (Supplementary Table S1, available at Rheumatology online). The study was performed according to the principles of the Declaration of Helsinki and International Conference on Harmonisation Good Clinical Practice guidelines. Institutional review boards or ethics committees at each centre approved the study protocol. All patients provided written informed consent.
Patient and public involvement in research
This research was initiated without prior patient involvement. Patients did not contribute to the study design, were not involved in the interpretation of results and were not invited to contribute to the writing/editing of this document.
Patients
Full inclusion and exclusion criteria are provided in the Supplementary Material, section ‘Methods’, available at Rheumatology online. Patients were aged 18–75 years with active RA for ≥6 months prior to first study drug administration (day 0). RA was diagnosed according to 2010 ACR/EULAR criteria [17], and considered active if patients had ≥6 swollen joints (28-joint count), ≥6 tender joints (28-joint count) and a serum CRP concentration of >0.6 mg/dl. Eligible patients had an inadequate response to ≥3 months of MTX therapy and had received a stable MTX dose [12.5–25 mg/week (10–25 mg/week in Republic of Korea)] for 4 weeks prior to day 0.
Procedures
The treatment period comprised an i.v. dose-loading phase (CT-P13 i.v. 3 mg/kg, administered by a 2-h i.v. infusion at W0 and W2) for all patients followed by a maintenance phase from W6 to W54 (W64 for usability assessment) (Supplementary Fig. S1, available at Rheumatology online). At W6, patients who had received two full doses and displayed no safety concerns (investigator’s opinion) were randomized (1:1) to receive an s.c. injection of CT-P13 s.c. 120 mg q2w (administered via PFS in 1 ml) or a 2-h i.v. infusion of CT-P13 i.v. 3 mg/kg q8w (maintenance phase). Randomization was stratified by country, W2 serum CRP concentration (≤0.6 vs >0.6 mg/dl) and W6 body weight (≤100 vs >100 kg).
CT-P13 i.v. was manufactured by Celltrion, Inc. (Incheon, Republic of Korea). The PFS device/material and AI material were manufactured by Vetter Pharma-Fertigung GmbH & Co. (Ravensburg, Germany). The AI device was manufactured by SHL Pharma LLC (Deerfield Beach, FL, USA). CT-P13 s.c. (or placebo s.c.) was injected by a health-care professional at each study visit; patients could self-inject at other treatment weeks after appropriate training. Double-dummy matching placebos were administered to maintain blinding until W30, after which patients on CT-P13 i.v. were switched to CT-P13 s.c. 120 mg q2w via PFS until W54. Patients in Bulgaria, Poland and Russian Federation received CT-P13 s.c. q2w via AI (W46–54) followed by CT-P13 s.c. q2w via PFS (W56–64) to assess usability. All patients received MTX [12.5–25 mg/week (10–25 mg/week in Republic of Korea), oral or parenteral dose] and folic acid (≥5 mg/week, oral dose) throughout.
Outcome measures
Full details of efficacy, PK, PD, usability, safety and immunogenicity assessments are provided in the Supplementary Material, available at Rheumatology online.
The primary objective was to demonstrate the non-inferiority of CT-P13 s.c. to CT-P13 i.v. in terms of clinical response according to change (decrease) from baseline in DAS28-CRP at W22. CRP was assessed at a central laboratory. Secondary efficacy endpoints included mean change from baseline in DAS28 individual component, DAS28-CRP, DAS28-ESR, ACR response (individual component, ACR20, ACR50, ACR70 and hybrid ACR score), EULAR response rate, Clinical Disease Activity Index (CDAI) and Simplified Disease Activity Index (SDAI). Patient-reported outcomes included the HAQ and 36-Item Short Form Health Survey (SF-36). Steady-state PK sampling time points (W22–30) are shown in Supplementary Table S2, available at Rheumatology online. Secondary PK endpoints included model-predicted AUCτ, AUCW22–30, Cmax, and Ctrough. In addition, observed Ctrough was evaluated up to W54. Secondary PD endpoints included RF, anti-CCP, CRP and ESR. Usability endpoints included assessment of self-injection using PRE- and POST-Self-Injection Assessment Questionnaires (SIAQs), Successful Self-injection and the Potential Hazards Checklist. Safety outcomes included treatment-emergent adverse events (TEAEs), serious AEs (SAEs), AEs of special interest (see Supplementary Material, available at Rheumatology online), complement (C3, C4), total haemolytic complement, signs and symptoms of tuberculosis, local site pain and immunogenicity.
Statistical analysis
A sample size of 174 patients (87 per arm) was needed to achieve 80% statistical power for demonstrating non-inferiority of the primary endpoint between arms, based on a 97.5% one-sided CI for the difference in mean change (decrease) from baseline of DAS28-CRP at W22, assuming a non-inferiority margin of −0.6, one-sided alpha level of 2.5% and s.d. of 1.4. Anticipating a 20% dropout rate, the sample size was estimated to be 218 patients (109 per arm). The primary efficacy analysis was conducted on the all-randomized population and efficacy population using analysis of covariance; treatment was considered a fixed effect. Country, W2 serum CRP concentration (≤0.6 vs >0.6 mg/dl) and W6 body weight (≤100 vs >100 kg) were covariates. The primary efficacy endpoint, non-inferiority of CT-P13 s.c. to CT-P13 i.v., was concluded if the lower limit of the two-sided 95% CI for the difference in change (decrease) from baseline in DAS28-CRP at W22 between arms was more than −0.6. Sensitivity analysis with missing data imputation for primary efficacy endpoint and statistical analyses for secondary efficacy, PK, PD, safety and usability endpoints are described in the Supplementary Material, available at Rheumatology online. The all-randomized and intention-to-treat populations were analysed by randomized treatment at W6. Other populations were analysed by actual treatment received: patients were included in the CT-P13 s.c. arm if they received at least one CT-P13 s.c. dose before W30. Statistical analysis was performed using SAS software v9.4 (SAS Institute, Inc., Cary, NC, USA). Population PK model was employed to estimate the individual patient PK parameters by a non-linear mixed-effect PK model using NONMEM Version 7.2. An independent data safety monitoring board monitored the study.
Results
Patients
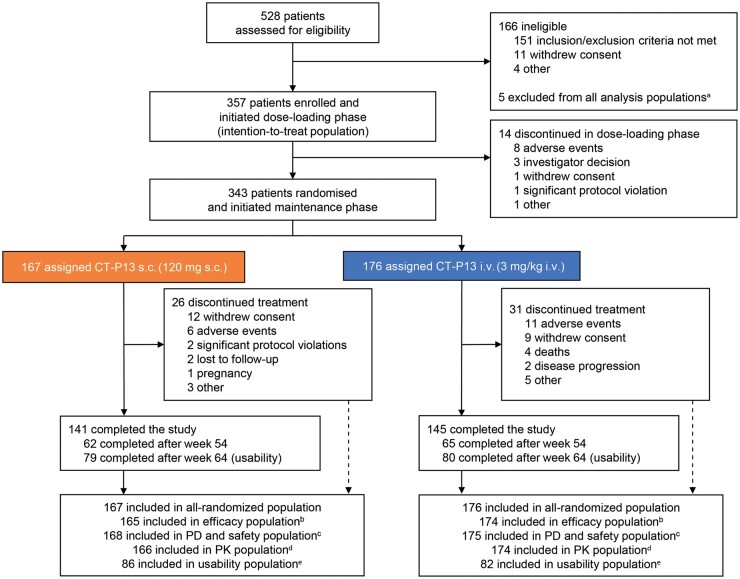
Patients were recruited to Part 2 of the study between 30 October and 18 December 2017 (last visit of last patient: 15 April 2019). Overall, 528 patients were screened, 357 were treated in the dose-loading phase and 343 were randomized to CT-P13 s.c. (n = 167) or CT-P13 i.v. (n = 176) at W6 (Fig. 1). One site in the Russian Federation displayed significant Good Clinical Practice non-compliance; 5 patients from this site were excluded from all analysis populations. At W30, 160 patients remaining in the CT-P13 i.v. arm switched to CT-P13 s.c. treatment. In the CT-P13 s.c. and i.v. arms, 141 and 145 patients, respectively, completed the study.
Fig. 1.
Patient disposition
aFive patients were excluded from all analysis populations because of significant Good Clinical Practice non-compliance of the study centre.
bThree patients (1 in the CT-P13 s.c. arm and 2 in the i.v. arm) with at least one major protocol deviation and 1 patient (in the CT-P13 s.c. arm) with no efficacy assessment after week 6 were excluded from the efficacy population.
cOne patient randomized to the CT-P13 i.v. arm received CT-P13 s.c. treatment instead of placebo s.c. treatment at week 14, thus receiving both CT-P13 i.v. 3 mg/kg and CT-P13 s.c. 120 mg at week 14. This patient was included in the CT-P13 s.c. arm for the PD and safety populations and excluded from the efficacy and PK populations.
dOne patient with a major protocol deviation (CT-P13 i.v. arm) and 2 patients without PK concentration data (1 in each of the CT-P13 s.c. and i.v. arms) were excluded from the PK population.
eAll 168 patients eligible for usability assessment (patients in Bulgaria, Poland and Russian Federation continuing the study at week 46) were included in the usability population.
PD, pharmacodynamics; PK, pharmacokinetics.
Overall, patient demographics and disease characteristics were similar between arms (Table 1).
Table 1.
Baseline demographics and disease characteristics and stratification criteria (all-randomized populationa)
| Variable b |
CT-P13 s.c.
(n = 167) |
CT-P13 i.v.
(n = 176) |
|---|---|---|
| Age, years | 50.9 (12.17) | 51.9 (12.42) |
| Sex, n (%) | ||
| Female | 130 (77.8) | 139 (79.0) |
| Race, n (%) | ||
| Asian/Oriental | 1 (0.6) | 2 (1.1) |
| White/Caucasian | 145 (86.8) | 151 (85.8) |
| Other | 21 (12.6) | 23 (13.1) |
| Screening height, cm | 164.73 (9.20) | 164.33 (9.31) |
| Screening weight, kg | 73.01 (15.13) | 72.75 (14.40) |
| Screening BMI, kg/m2 | 26.79 (4.42) | 26.82 (4.13) |
| Time since RA diagnosis, years | 6.82 (7.15) | 6.41 (6.39) |
| DAS28-CRPc | 6.01 (0.75) | 5.86 (0.81) |
| DAS28-ESRc | 6.70 (0.79) | 6.56 (0.78) |
| Tender joint count (DAS28 assessment)c | 16.1 (5.33) | 14.8 (5.55) |
| Swollen joint count (DAS28 assessment)c | 12.4 (4.42) | 11.0 (4.32) |
| HAQ estimate of physical abilityc | 1.58 (0.53) | 1.58 (0.60) |
| CDAIc | 42.53 (10.09) | 39.59 (10.08) |
| SDAIc | 44.36 (10.65) | 41.86 (11.12) |
| Patient’s assessment of pain (VAS)c | 69.09 (17.43) | 68.57 (17.85) |
| Patient’s global assessment of disease activity (VAS)c | 70.36 (15.80) | 69.16 (17.40) |
| Physician’s global assessment of disease activity (VAS)c | 70.22 (13.95) | 68.80 (15.26) |
| ESR, mm/hc | 41.8 (19.26) | 44.5 (23.61) |
| CRP, mg/dlc | 1.84 (2.39) | 2.24 (3.53) |
| MTX dose at first administration (mg/week)d | 17.01 (3.99) | 17.40 (3.98) |
| MTX dose at first administration of maintenance phase (mg/week)d | 16.98 (3.98) | 17.40 (3.98) |
| Stratification factors | ||
| Weight (W6), n (%) | ||
| >100 kg | 7 (4.2) | 10 (5.7) |
| ≤100 kg | 160 (95.8) | 166 (94.3) |
| Serum CRP (W2), n (%) | ||
| >0.6 mg/dl | 34 (20.4) | 47 (26.7) |
| ≤0.6 mg/dl | 133 (79.6) | 129 (73.3) |
Analysed according to randomized treatment at W6. bExcept where indicated otherwise, values are mean (s.d.). cEfficacy population; CT-P13 s.c. (n = 165) and CT-P13 i.v. (n = 174). dSafety population; CT-P13 s.c. (n = 168), CT-P13 i.v. (n = 175).
CDAI, Clinical Disease Activity Index; DAS28, disease activity score in 28 joints; SDAI, Simplified Disease Activity Index; VAS, visual analogue scale; W, week.
Efficacy
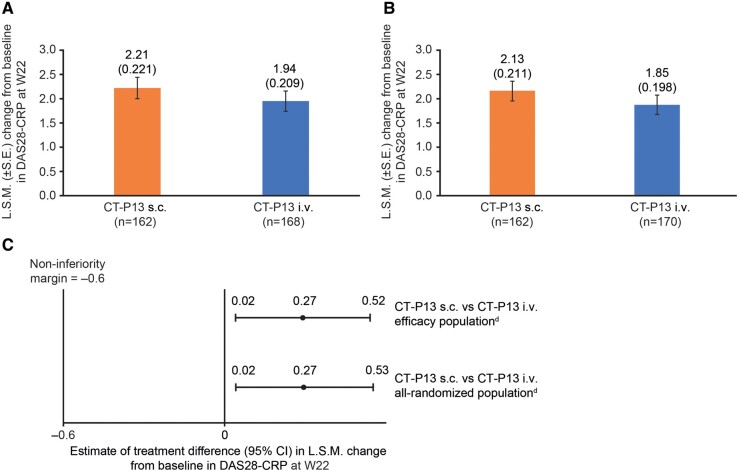
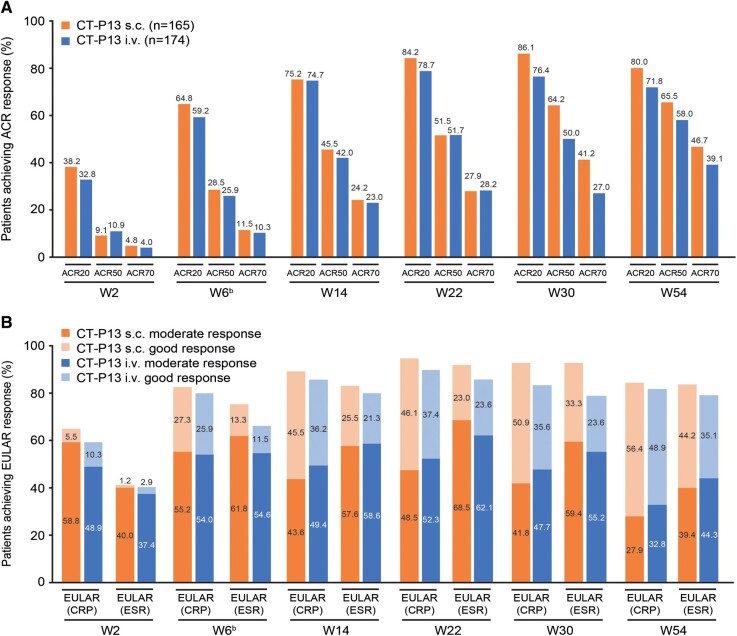
The primary outcome, the least-squares mean (SE) change (decrease) from baseline at W22 in DAS28-CRP for the efficacy population, was 2.21 (0.22) in the CT-P13 s.c. arm (n = 162) and 1.94 (0.21) in the CT-P13 i.v. arm (n = 168; Fig. 2A). Results for the all-randomized population supported those for the efficacy population (Fig. 2B). The difference between arms was 0.27 (95% CI: 0.02, 0.52; efficacy population; Fig. 2C). The lower bound of the CI was above the pre-specified non-inferiority margin (−0.6), indicating non-inferiority of CT-P13 s.c. to CT-P13 i.v. Sensitivity analysis provided similar results to the primary analysis (Supplementary Fig. S2, available at Rheumatology online). CT-P13 s.c. appeared to have similar improvement in efficacy up to W22 and slightly improved efficacy at W30 vs CT-P13 i.v. with respect to hybrid ACR score, ACR and EULAR responses, DAS28-CRP, DAS28-ESR, CDAI and SDAI (Fig. 3; Supplementary Tables S3 and S4, available at Rheumatology online). After switching from CT-P13 i.v. to CT-P13 s.c. at W30, substantial improvements in efficacy measures were seen in the CT-P13 i.v. arm, with similar efficacy to the CT-P13 s.c. arm observed at W54 (Supplementary Tables S3 and S4, available at Rheumatology online). Mean scores for HAQ estimate of physical ability generally decreased up to W54, and mean change from baseline was similar between arms (Supplementary Table S4, available at Rheumatology online). There were no notable differences between arms in mean change from baseline in SF-36 Physical and Mental Component summary scores (Supplementary Fig. S3, available at Rheumatology online).
Fig. 2.
ANCOVA analysis of change (decrease) from baseline of DAS28-CRP at W22a
(A) L.S.M. (s.e.) change from baseline in DAS28-CRP at W22 (efficacy populationb). (B) L.S.M. (s.e.) change from baseline in DAS28-CRP at W22 (all-randomized populationc). (C) Estimate of treatment difference (95% CI) in L.S.M. change from baseline in DAS28-CRP at W22 for efficacyb and all-randomizedc populations.
aChange (decrease) from baseline for this primary analysis was defined as decrease from baseline and calculated as (DAS28-CRP at baseline − DAS28-CRP at W22).
bAnalysed according to actual treatment received.
cAnalysed according to randomized treatment at W6.
dCriteria for non-inferiority met.
ANCOVA, analysis of covariance; DAS28, disease activity score in 28 joints; L.S.M., least-squares mean; W, week.
Fig. 3.
Response (efficacy populationa)
(A) Proportion of patients achieving clinical response according to ACR20, ACR50 and ACR70 criteria. (B) Proportion of patients with good/moderate response according to EULAR (CRP) and EULAR (ESR).
aFrom W30 to W54, all patients received CT-P13 s.c. and are analysed according to treatment received prior to W30.
bAt W6, patients were randomized to treatment; efficacy results up to W6 represent the efficacy of CT-P13 i.v. loading dose, regardless of randomized arm.
ACR20, 20% improvement in ACR criteria; ACR50, 50% improvement in ACR criteria; ACR70, 70% improvement in ACR criteria; W, week.
Pharmacokinetics
Mean CT-P13 serum levels were well maintained in both arms up to W54 (Supplementary Fig. S4; Supplementary Table S5, available at Rheumatology online). During the PK monitoring period (W22–30), mean serum concentration of CT-P13 s.c. gradually increased and then decreased before the next administration. For CT-P13 i.v., mean serum concentration peaked at the end of the infusion at W22, then rapidly decreased towards the pre-infusion level until W30. Mean predicted AUCW22–30 for CT-P13 s.c. (20 926.6 h·µg/ml) was greater than the predicted AUCτ at W22 for CT-P13 i.v. (14 156.9 h·µg/ml; Table 2). Model-predicted mean Ctrough from W22 to W28 was consistently greater for CT-P13 s.c. than CT-P13 i.v., but the model-predicted mean Cmax was lower for CT-P13 s.c. than CT-P13 i.v. (Table 2). Observed Ctrough values (Supplementary Table S6, available at Rheumatology online) were consistent with predicted Ctrough results from W22 to W28. Mean observed Ctrough gradually increased in the CT-P13 i.v. arm after switching to CT-P13 s.c. at W30 and was similar between arms from W44 to W52. In the CT-P13 s.c. arm, mean serum concentration exceeded the target therapeutic concentration (1 µg/ml) throughout the treatment period (Supplementary Fig. S4, available at Rheumatology online). Observed Ctrough values exceeded this target for a greater proportion of patients in the CT-P13 s.c. arm vs the CT-P13 i.v. arm. At W28, 81.6% of patients in the CT-P13 s.c. arm achieved observed Ctrough >1 µg/ml; of those, 91.1% achieved ACR20 response at W30 (Supplementary Table S7, available at Rheumatology online).
Table 2.
|
Parameter
Week |
CT-P13 s.c.
(n = 166) |
CT-P13 i.v.
(n = 174) |
||
|---|---|---|---|---|
| n | Mean (CV%) | n | Mean (CV%) | |
| AUCτ, h·µg/ml | ||||
| 22 | 162 | 5311.5 (45.6) | 165 | 14,156.9 (46.3) |
| 24 | 160 | 5187.9 (45.3) | N/A | N/A |
| 26 | 161 | 5273.1 (47.3) | N/A | N/A |
| 28 | 160 | 5157.2 (46.6) | N/A | N/A |
| AUCW22–30, h·µg/ml | ||||
| 22 | 162 | 20,926.6 (45.4) | N/A | N/A |
| Cmax, µg/ml | ||||
| 22 | 162 | 17.74 (40.87) | 165 | 71.60 (16.89) |
| 24 | 160 | 17.62 (40.73) | N/A | N/A |
| 26 | 161 | 17.63 (41.10) | N/A | N/A |
| 28 | 160 | 17.54 (40.63) | N/A | N/A |
| Ctrough, µg/ml | ||||
| 22 | 162 | 12.19 (54.25) | 165 | 1.49 (168.41) |
| 24 | 160 | 12.30 (53.96) | N/A | N/A |
| 26 | 161 | 12.18 (53.42) | N/A | N/A |
| 28 | 160 | 12.17 (54.58) | N/A | N/A |
PK parameters (AUCτ, Cmax and Ctrough) were estimated from population PK modelling, and AUCW22–30 for the CT-P13 s.c. arm was estimated due to the different dosing interval of s.c. and i.v. administration [every 2 weeks (W6–28) and every 8 weeks (W6, W14 and W22), respectively]. bAnalysed according to actual treatment received. AUCτ, model-predicted area under the concentration–time curve at steady-state between W22 and W30; AUCW22–30, model-predicted area under the concentration–time curve due to different dosing interval of s.c. and i.v. administration using population PK model; Cmax, model-predicted maximum serum concentration after study drug administration; Ctrough, model-predicted trough serum concentration; CV%, per cent coefficient of variation; N/A, not applicable; PK, pharmacokinetic; W, week.
Pharmacodynamics
In general, mean concentrations of PD parameters decreased from baseline to W54 in both arms (Supplementary Table S8, available at Rheumatology online). In the CT-P13 i.v. arm, change from baseline in CRP and ESR remained relatively consistent until W30; after patients switched to CT-P13 s.c., CRP and ESR generally decreased (W38–54).
Usability
Usability of CT-P13 s.c. via AI and PFS was high and similar to each other: mean scores exceeded 7 for almost all PRE-/POST-SIAQ domains from W46 to W64. Among the three domains that have both PRE- and POST-SIAQ scores, SIAQ scores of self-confidence were increased after injection for both AI and PFS (Supplementary Table S9, available at Rheumatology online). Almost all patients successfully self-administered CT-P13 s.c. via AI and PFS, and completed all instructions from the AI and PFS Self-Injection Assessment Checklist at W46, W54, W56 and W64 (Supplementary Table S10, available at Rheumatology online). The proportion of patients with hazard-free self-injection was consistently high.
Safety
Incidence of TEAEs was higher with CT-P13 i.v. [117 (66.9%)] than CT-P13 s.c. [92 (54.8%)] during the maintenance phase (Table 3). Most TEAEs were grade 1 or 2 in intensity (Table 3; Supplementary Table S11, available at Rheumatology online) and there were no notable differences in safety profiles between arms after switching from CT-P13 i.v. to CT-P13 s.c. (Supplementary Table S12, available at Rheumatology online). Incidence of TEAEs classified as infection during the maintenance phase was higher with CT-P13 i.v. [60 (34.3%) patients] than CT-P13 s.c. [49 (29.2%)] patients; Table 3).
Table 3.
Summary of adverse events in the maintenance phase (safety populationa)
|
CT-P13 s.c.
(n = 168) |
CT-P13 i.v.
(n = 175) |
|
|---|---|---|
| Total TEAEs, n | 309 | 313 |
| Patients with ≥1 TEAE | 92 (54.8) | 117 (66.9) |
| Treatment-related TEAE | 73 (43.5) | 72 (41.1) |
| TEAE grade ≥3 | 13 (7.7) | 8 (4.6) |
| Total TESAEs, n | 8 | 15 |
| Patients with ≥1 TESAE | 6 (3.6) | 13 (7.4) |
| Treatment-related TESAE | 3 (1.8) | 4 (2.3) |
| Patients with ≥1 TEAE leading to discontinuation of study drug | 6 (3.6) | 14 (8.0) |
| Patients with ≥1 TEAE classified as localized ISR | 30 (17.9)e | 22 (12.6)e |
| Patients with ≥1 TEAE classified IRRb | 0 | 7 (4.0)f |
| Patients with ≥1 TEAE classified as SIRb | 2 (1.2)g | 3 (1.7)g |
| Patients with ≥1 TEAE classified as delayed hypersensitivityc | 4 (2.4)h | 0 |
| Patients with ≥1 TEAE classified as infections | 49 (29.2) | 60 (34.3) |
| Most common infections and infestationsd | ||
| Viral upper respiratory tract infection | 10 (6.0) | 14 (8.0) |
| Upper respiratory tract infection | 8 (4.8) | 13 (7.4) |
| Latent tuberculosis | 8 (4.8) | 10 (5.7) |
| Urinary tract infection | 9 (5.4) | 7 (4.0) |
| Bronchitis | 5 (3.0) | 4 (2.3) |
| Patients with ≥1 TEAE classified as malignancy | 1 (0.6) | 0 |
Data are n (%) unless stated otherwise. aAll patients received CT-P13 s.c. from W30 to W54 (or W64 for patients participating in the usability assessment) and were analysed according to actual treatment received prior to W30. bIRRs occurred between start of administration and 24 h after the i.v. infusion of CT-P13 or placebo. SIRs occurred between start of administration and 24 h after the s.c. injection of CT-P13 or placebo. cTEAEs classified as delayed hypersensitivity were defined as IRRs or SIRs that occurred after 24 h from study drug or placebo administration. dReported by ≥3% of patients in either treatment arm. eAll localized ISRs were grade 1 or 2 and considered by the investigator to be related to study drug, except for one treatment-related grade 3 localized ISR in each treatment arm, and one grade 2 localized ISR considered unrelated to study treatment in the CT-P13 s.c. arm. fOne grade 3 IRR; all other IRRs were grade 1 or 2. gAll SIRs were grade 1 or 2. hThree grade 2 delayed hypersensitivity reactions and one grade 3 reaction. IRR, infusion-related reaction; ISR, injection site reaction; SIR, systemic injection reaction; TEAE, treatment-emergent adverse event; TESAE, treatment-emergent serious adverse event; W, week.
Localized injection site reactions (ISRs) were reported by 30 (17.9%; CT-P13 s.c.) and 22 (12.6%; CT-P13 i.v.) patients (Table 3); 7 (4.0%) patients in the CT-P13 i.v. arm reported an infusion-related reaction; and systemic injection reactions occurred in 2 (1.2%; CT-P13 s.c.) and 3 (1.7%; CT-P13 i.v.) patients. The majority of these events were grade 1 or 2. Four (2.4%) patients in the CT-P13 s.c. arm experienced a TEAE classified as delayed hypersensitivity during the maintenance phase. One of these patients underwent additional testing 11 days after the reaction: complement (C3; C4) and the majority of clinical laboratory tests were normal and anti-drug antibodies (ADAs) tested negative. ADA results prior to delayed hypersensitivity were also negative for the other 3 patients. A lower proportion of patients in the CT-P13 s.c. vs i.v. arm experienced TEAEs leading to discontinuation of study drug during the maintenance phase [6 (3.6%) vs 14 (8.0%)].
Overall, 6 (3.6%; CT-P13 s.c.) and 13 (7.4%; CT-P13 i.v.) patients experienced at least one treatment-emergent SAE in the maintenance phase (Supplementary Table S13, available at Rheumatology online). Five patients died during the maintenance phase: one (0.6%) in the CT-P13 s.c. arm, as a complication of hereditary haemochromatosis; four (2.3%) in the CT-P13 i.v. arm, as a result of myocardial infarction (n = 2), sudden death (n = 1) and cardiac arrest (n = 1). All deaths were considered unrelated to study drug. One patient (0.6%; CT-P13 s.c.) reported a malignancy (ovarian) during the maintenance phase (Table 3).
Local site pain was high following the first administration of CT-P13 s.c. injection (CT-P13 s.c. arm: W6; CT-P13 i.v.: W30; Supplementary Table S14, available at Rheumatology online). Local site pain generally decreased with repeated use of either administration method and was similar between arms, although a slight increase was reported in both arms at W54.
Immunogenicity
Immunogenicity was similar between arms: 114 (67.9%; CT-P13 s.c.) and 129 (73.7%; CT-P13 i.v.) patients had at least one positive post-treatment ADA result. In both arms, the proportion of ADA-positive patients increased after the dose-loading phase (Supplementary Fig. S5, available at Rheumatology online) and remained similar between arms throughout the study.
Discussion
The primary endpoint of Part 2 of this phase I/III study was met by demonstrating that the mean change (decrease) from baseline in DAS28-CRP at W22 for patients treated with CT-P13 s.c. was non-inferior to CT-P13 i.v. Interestingly, the 95% CI for the treatment difference not only lay within the pre-specified non-inferiority margin, but was also above zero (Fig. 2C), providing evidence of superiority of CT-P13 s.c. over CT-P13 i.v. at the 5% statistical significance level. Overall, secondary efficacy endpoints suggested slightly improved efficacy of CT-P13 s.c. vs i.v. at W30, with similar efficacy observed between arms at W54 after switching from CT-P13 i.v. to CT-P13 s.c. Findings were similar for PD endpoints. CT-P13 s.c. was well tolerated throughout the study. Except for localized ISR, there were no clinically meaningful differences in the safety profile of CT-P13 s.c. and CT-P13 i.v. Usability of CT-P13 s.c. via AI and PFS was high and did not differ between administration methods.
We compared i.v. dosing, using a body weight-based dose administered q8w, with an s.c. regimen, using a fixed dose (120 mg) administered q2w. Overall, the mean serum concentration of CT-P13 was well maintained with s.c. and i.v. dosing. As a result of sparse PK sampling time points, a non-linear mixed-effect PK model estimated certain PK parameters for individual patients. The predicted mean Ctrough was higher and the predicted mean Cmax was lower with CT-P13 s.c. vs CT-P13 i.v., reflecting the more frequent administration of lower doses with the s.c. regimen and delayed drug absorption via the s.c. route [18, 19], leading to more constant exposure over time vs i.v. dosing. After switching to CT-P13 s.c. at W30, the PK profile in the CT-P13 i.v. arm became similar to the CT-P13 s.c. arm. Previous studies of i.v.-administered infliximab in RA patients have suggested an association between Ctrough serum concentrations ≥1 µg/ml and clinical response [20–23]. In the CT-P13 s.c. arm, observed mean Ctrough levels remained higher than this target throughout the maintenance phase, suggesting that the s.c. dosing regimen was appropriate. In addition, observed Ctrough values exceeded this target for a greater proportion of patients in the CT-P13 s.c. vs i.v. arm. Dose-loading with CT-P13 i.v. was conducted prior to randomization at W6 to ensure that steady-state serum concentrations exceeding this therapeutic target could be rapidly achieved.
While the proportion of ADA-positive patients increased after the dose-loading phase, CT-P13 s.c. and i.v. immunogenicity was similar throughout the study. As expected, more localized ISRs were reported with CT-P13 s.c., but were rarely serious or severe in intensity. No new safety findings were observed with CT-P13 s.c., consistent with other s.c.- or i.v.-administered biologics [24–26].
Clinical responses in our study are consistent with previous reports. In the PLANETRA study, 73.4% of RA patients treated with CT-P13 i.v. achieved ACR20 response at W30 [3]. This compared with 86.1% (CT-P13 s.c.) and 76.4% (CT-P13 i.v.) of patients in our study; W54 findings were also similar to PLANETRA results at W54 [27]. Similarly, during a phase I study of an experimental s.c. infliximab formulation, ∼80–87% of RA patients achieved ACR20 response 2 weeks post-treatment [13], consistent with our W22 results. Taken together, these data suggest that s.c. administration of infliximab can achieve good clinical response rates in RA patients.
We report a robust randomized study using well-established outcome measures, conducted across 12 countries, which suggests findings should be applicable to the wider RA patient population. Nevertheless, the study had some limitations. Patients with a BMI ≥ 35 were excluded, and follow-up was limited (≤56–66 weeks, depending on country). Future studies should investigate longer-term efficacy and safety of CT-P13 s.c. and effects in patients with high body weight/BMI.
In conclusion, CT-P13 s.c. was non-inferior in efficacy to CT-P13 i.v. in patients with active RA. Efficacy and safety of CT-P13 treatment were well maintained following switching from CT-P13 i.v. to s.c. CT-P13 s.c. could represent a beneficial treatment option because of the alternative, convenient administration method.
Supplementary Material
Acknowledgements
We thank all study investigators, staff and patients who contributed to this study. Medical writing support (including development of a draft outline and subsequent drafts in consultation with the authors, assembling tables and figures, collating author comments, copyediting, fact checking and referencing) was provided by Emma Evans, PhD CMPP and Beatrice Tyrrell, DPhil at Aspire Scientific Limited (Bollington, UK), and was funded by Celltrion, Inc. Selected data up to week 30 from the CT-P13 3.5 Part 2 study were presented on 15 June 2019 in a poster at EULAR 2019 (Westhovens R, Wiland P, Zawadzki M et al. A novel formulation of CT-P13 for subcutaneous administration: 30 week results from a Part 2 of phase I/III randomized controlled trial in patients with rheumatoid arthritis (abstract SAT0170). Ann Rheum Dis 2019;78(Suppl 2):A1158), and selected data up to week 54 were presented on 10 November 2019 in a poster at the 2019 ACR/ARP Annual Meeting (Westhovens R, Wiland P, Zawadzki M et al. Efficacy and safety of a novel subcutaneous formulation of CT-P13 over the 1-year treatment period and after switching from intravenous CT-P13 in patients with active rheumatoid arthritis: results from Part 2 of phase I/III randomized controlled trial. Arthritis Rheumatol 2019:71(Suppl 10):Abstract 548). R.W., S.J.L., J.H.S. and D.H.Y. contributed to the conception and design of the study, interpretation of the data and review of study results. P.W., M.Z., D.I., A.B.K., E.C.E-K., E.B., S.S., L.E., M.S., R.Y., P.H., J.J., V.Z., J.T. and P.S. contributed to the acquisition of data and review of study results. S.H.K. and N.R.H. contributed to the interpretation of data and review of study results. S.G.L. contributed to the acquirement, management and analysis of data. All authors contributed to manuscript development, had full access to all study data, approved the final manuscript and have accountability for all aspects of the work. The study protocol is available as part of the online Supplementary Material, available at Rheumatology online. All data relevant to the study are included in the article or uploaded as supplementary information.
Funding: This work was supported by Celltrion, Inc. (Incheon, Republic of Korea).
Disclosure statement: R.W. reports advisory board and speaker fees from Celltrion related to the submitted work and advisory board and speaker fees from Galapagos and Gilead outside of the submitted work. P.W. reports personal fees from AbbVie, Celltrion, Inc., Gedeon Richter, Lilly, Novartis, Pfizer, Roche and Sandoz during the conduct of the study. M.Z., E.C.E-K., S.S. and L.E. report grants from Celltrion, Inc. during the conduct of the study. A.B.K. reports grants from Celltrion, Inc. and personal fees from Pfizer during the conduct of the study. P.H. reports grants and personal fees from Celltrion Healthcare related to CT-P13 study conduct. J.J. reports personal fees from Celltrion related to and outside of the submitted work. S.J.L., S.H.K., J.H.S., S.G.L. and N.R.H. are employees of Celltrion, Inc. D.H.Y. reports personal fees for advisory board and speakers’ bureau from Celltrion, Inc. and has received research grants from Celltrion, Inc. outside of the submitted work. The other authors have declared no conflict of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1.European Medicines Agency Committee for Medicinal Products for Human Use (CHMP). Assessment report: Remsima (Infliximab) [cited 01 October 2018]. https://www.ema.europa.eu/documents/assessment-report/remsima-epar-public-assessment-report_en.pdf.
- 2. Park W, Hrycaj P, Jeka S. et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis 2013;72:1605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yoo DH, Hrycaj P, Miranda P. et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis 2013;72:1613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Becciolini A, Raimondo MG, Crotti C. et al. A review of the literature analyzing benefits and concerns of infliximab biosimilar CT-P13 for the treatment of rheumatologic diseases: focus on interchangeability. Drug Des Devel Ther 2017;11:1969–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Medicines Agency. Remsima summary of product characteristics [cited 22 January 2019]. https://www.ema.europa.eu/documents/product-information/remsima-epar-product-information_en.pdf.
- 6.European Medicines Agency. Press Release 28 June 2013: European Medicines Agency recommends approval of first two monoclonal antibody biosimilars. https://www.ema.europa.eu/en/news/european-medicines-agency-recommends-approval-first-two-monoclonal-antibody-biosimilars.
- 7.European Medicines Agency. Remsima [cited 01 October 2018]. https://www.ema.europa.eu/medicines/human/EPAR/remsima.
- 8. Gabbani T, Deiana S, Annese V.. CT-P13: design, development, and place in therapy. Drug Des Devel Ther 2017;11:1653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoo DH. Comparative effectiveness of the biosimilar CT-P13. J Comp Eff Res 2017;6:693–712. [DOI] [PubMed] [Google Scholar]
- 10. Jackisch C, Muller V, Maintz C, Hell S, Ataseven B.. Subcutaneous administration of monoclonal antibodies in oncology. Geburtshilfe Frauenheilkd 2014;74:343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chilton F, Collett RA.. Treatment choices, preferences and decision-making by patients with rheumatoid arthritis. Musculoskeletal Care 2008;6:1–14. [DOI] [PubMed] [Google Scholar]
- 12. Viola M, Sequeira J, Seica R. et al. Subcutaneous delivery of monoclonal antibodies: how do we get there? J Control Release 2018;286:301–14. [DOI] [PubMed] [Google Scholar]
- 13. Westhovens R, Houssiau F, Joly J. et al. A phase I study assessing the safety, clinical response, and pharmacokinetics of an experimental infliximab formulation for subcutaneous or intramuscular administration in patients with rheumatoid arthritis. J Rheumatol 2006;33:847–53. [PubMed] [Google Scholar]
- 14. Zhu YW, Pendley C, Sisco D. et al. Pharmacokinetics and pharmacodynamics of infliximab, an anti-tumor necrosis factor-alpha monoclonal antibody, following single subcutaneous administrations in rheumatoid arthritis patients. Clin Pharmacol Ther 2005;77:P43. [Google Scholar]
- 15. Yoo DH, Jaworski J, Matyska-Piekarska E. et al. A novel formulation of CT-P13 (infliximab biosimilar) for subcutaneous administration: 1-year results from a part 1 of phase I/III randomized controlled trial in patients with active rheumatoid arthritis. Ann Rheum Dis 2019;78(Suppl 2):A733. [Google Scholar]
- 16. Westhovens R, Yoo DH, Jaworski J. et al. Novel formulation of CT-P13 for subcutaneous administration in patients with rheumatoid arthritis: initial results from a phase I/III randomised controlled trial (abstract THU0191). Ann Rheum Dis 2018;77(Suppl 2):315.28476879 [Google Scholar]
- 17. Aletaha D, Neogi T, Silman AJ. et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–8. [DOI] [PubMed] [Google Scholar]
- 18. Richter WF, Jacobsen B.. Subcutaneous absorption of biotherapeutics: knowns and unknowns. Drug Metab Dispos 2014;42:1881–9. [DOI] [PubMed] [Google Scholar]
- 19. Ryman JT, Meibohm B.. Pharmacokinetics of monoclonal antibodies. CPT Pharmacometrics Syst Pharmacol 2017;6:576–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mori S. A relationship between pharmacokinetics (PK) and the efficacy of infliximab for patients with rheumatoid arthritis: characterization of infliximab-resistant cases and PK-based modified therapy. Mod Rheumatol 2007;17:83–91. [DOI] [PubMed] [Google Scholar]
- 21. Takeuchi T, Miyasaka N, Inoue K, Abe T, Koike T.. Impact of trough serum level on radiographic and clinical response to infliximab plus methotrexate in patients with rheumatoid arthritis: results from the RISING study. Mod Rheumatol 2009;19:478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. St Clair EW, Wagner CL, Fasanmade AA. et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2002;46:1451–9. [DOI] [PubMed] [Google Scholar]
- 23. Wolbink GJ, Voskuyl AE, Lems WF. et al. Relationship between serum trough infliximab levels, pretreatment C reactive protein levels, and clinical response to infliximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis 2005;64:704–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davies A, Berge C, Boehnke A. et al. Subcutaneous rituximab for the treatment of B-cell hematologic malignancies: a review of the scientific rationale and clinical development. Adv Ther 2017;34:2210–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matucci A, Vultaggio A, Danesi R.. The use of intravenous versus subcutaneous monoclonal antibodies in the treatment of severe asthma: a review. Respir Res 2018;19:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scott LJ. Tocilizumab: a review in rheumatoid arthritis. Drugs 2017;77:1865–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoo DH, Racewicz A, Brzezicki J. et al. A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther 2016;18:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.