Abstract
Objective
To evaluate the long-term efficacy of once-daily baricitinib 4 mg in patients with active RA who were either naïve to DMARDs or who had inadequate response (IR) to MTX.
Methods
Analyses of data from two completed 52-week, phase III studies, RA-BEGIN (DMARD-naïve) and RA-BEAM (MTX-IR), and one ongoing long-term extension (LTE) study (RA-BEYOND) were performed (148 total weeks). At week 52, DMARD-naïve patients treated with MTX monotherapy or baricitinib 4 mg+MTX in RA-BEGIN were switched to open-label baricitinib 4 mg monotherapy; MTX-IR patients treated with adalimumab (+MTX) in RA-BEAM were switched to open-label baricitinib 4 mg (+MTX) in the LTE. Patients who received placebo (+MTX) were switched to baricitinib 4 mg (+MTX) at week 24. Low disease activity (LDA) [Simple Disease Activity Index (SDAI) ≤11], clinical remission (SDAI ≤ 3.3), and physical functioning [Health Assessment Questionnaire Disability Index (HAQ-DI) ≤ 0.5] were assessed. Data were assessed using a non-responder imputation.
Results
At week 148, SDAI LDA was achieved in up to 61% of DMARD-naïve patients and 59% of MTX-IR patients initially treated with baricitinib, and SDAI remission was achieved in up to 34% of DMARD-naïve patients and 24% of MTX-IR patients; HAQ-DI ≤ 0.5 was reached in up to 48% of DMARD-naïve patients and 38% of MTX-IR patients initially treated with baricitinib. Over 148 weeks, 3.6% and 10.7% of MTX-IR patients discontinued across treatment groups due to lack of efficacy or due to adverse events, respectively; discontinuation rates were similar in the DMARD-naïve population.
Conclusion
Treatment with baricitinib 4 mg demonstrated efficacy for up to 3 years and was well tolerated.
Keywords: baricitinib, clinical remission, long-term efficacy, low disease activity, rheumatoid arthritis
Rheumatology key messages
Baricitinib demonstrated efficacy and safety in rheumatoid arthritis, but research on long-term efficacy is needed.
At week 148, 61% of patients treated with baricitinib achieved low disease activity.
Baricitinib 4 mg may be considered for long-term treatment of early and refractory rheumatoid arthritis.
Introduction
RA is a chronic autoimmune disease characterized by progressive joint damage and multiple comorbidities that may lead to impaired physical function, diminished overall quality of life, and mortality. Long-term treatments that are safe and efficacious are needed to reduce disease symptomology, to prevent irreversible joint damage, and to reduce the burden of disease from comorbidities [1].
Baricitinib is an oral, selective and reversible Janus kinase (JAK) 1 and 2 inhibitor [2] approved for the treatment of adults with active RA. Baricitinib has demonstrated efficacy and safety in populations that span the clinical disease continuum, including patients who are naïve to DMARDs (RA-BEGIN [3]) and those with an inadequate response (IR) to MTX (RA-BEAM [4]), conventional synthetic DMARDs (RA-BUILD [5]) or biological DMARDs (RA-BEACON [6]).
As with other DMARDs, the long-term safety and efficacy profiles of baricitinib in the treatment of RA are and will continue to be salient factors in clinical decision making. Towards this end, an updated integrated analysis of safety using data from nine completed baricitinib RA clinical trials and an ongoing long-term extension (LTE) study, provided data from 3770 patients who received at least one dose of baricitinib with a total of about 10 000 PY of exposure [7].
Achievement and maintenance of low disease activity (LDA) or clinical remission with the use of DMARDs are foremost among treatment goals outlined in RA disease management recommendations [8, 9]. The objectives of this study were to evaluate the achievement and maintenance of LDA, remission and a normative state of physical functioning in patients who were treated with baricitinib 4 mg for up to 3 years who were either naïve to DMARDs or who had an inadequate response to MTX.
Methods
Patients and study design
RA-BEGIN (NCT01711359) and RA-BEAM (NCT01710358) are phase III clinical studies that evaluated the efficacy and safety of baricitinib over 52 weeks in adults (≥18 years of age) with moderate-to-severely active RA who were either naïve to DMARDs or who had an inadequate response to MTX. RA-BEYOND (NCT01885078) is an ongoing, phase III LTE study to assess the efficacy and safety of baricitinib over 7 years in patients who completed the phase III trials. Patients were not eligible for participation in RA-BEYOND if they demonstrated laboratory abnormalities or significant uncontrolled medical conditions that, in the opinion of the investigators, posed a risk to the administration of baricitinib.
Patients who enrolled in RA-BEGIN (DMARD-naïve) were initially randomized to once-daily baricitinib 4 mg monotherapy, baricitinib 4 mg + MTX, or MTX monotherapy. At week 24, patients considered to be non-responders [<20% improvement in tender joint count (TJC) and swollen joint count (SJC) from baseline] from any treatment group were given open-label baricitinib 4 mg + MTX as rescue therapy; this treatment continued until the start of the LTE in which patients were switched to baricitinib monotherapy.
Patients who enrolled in RA-BEAM (MTX-IR) were originally randomized to once-daily baricitinib 4 mg, adalimumab 40 mg subcutaneous once every 2 weeks, or placebo. All patients continued treatment with MTX as concomitant therapy throughout the study [background therapy is noted hereafter as (+MTX) for RA-BEAM]. Patients who received placebo (+MTX) were switched to baricitinib 4 mg (+MTX) at week 24 per the protocol. At week 16, patients considered to be non-responders (<20% improvement in TJC and SJC from baseline) from any treatment group were given open-label baricitinib 4 mg (+MTX) as rescue therapy; this treatment continued into the LTE study.
Patients who completed RA-BEGIN or RA-BEAM were switched to open-label baricitinib 4 mg monotherapy (RA-BEGIN) or baricitinib 4 mg (+MTX; RA-BEAM) in the LTE study, if they were not already randomized to these treatments (or given them as rescue therapy) in the originating studies (Supplementary Fig. S1, available at Rheumatology online). Rescue therapy was available for patients originating from RA-BEAM with a Clinical Disease Activity Index (CDAI) score >10 at 3 months or later following enrolment into the LTE. Rescue therapy was available for patients from RA-BEGIN based on investigator’s discretion at any time during the LTE.
The addition or change in dose of analgesics or non-steroidal, anti-inflammatory drugs could occur for all patients in the LTE at any time point at the investigator’s discretion. Conventional synthetic DMARDs could be added or increased in dose if needed after rescue for patients from RA-BEAM and could be added (or increased in dose if added) at any time based upon the investigator’s discretion for patients from RA-BEGIN. No washout period of adalimumab occurred in patients who were switched to baricitinib 4 mg upon entry into RA-BEYOND or who were rescued to baricitinib 4 mg during RA-BEAM [10].
Patients who had received baricitinib 4 mg for at least 15 months (including the duration of RA-BEGIN and RA-BEAM) and who had achieved sustained LDA (CDAI ≤ 10; RA-BEAM) or remission (CDAI ≤ 2.8; RA-BEGIN) at two consecutive time points ≥3 months apart, were eligible to participate in the dose step-down sub-study of the LTE. Patients treated with baricitinib 4 mg were re-randomized to either remain on baricitinib 4 mg or step down to baricitinib 2 mg. After the step-down, if CDAI LDA or remission status could not be maintained, patients could be rescued back to open-label baricitinib 4 mg. Study design and results from this dose step-down sub-study were previously published [11].
All studies informing this analysis were conducted or are being conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines and approved by each centre’s institutional review board or ethics committee. Written, informed consent was provided by all patients.
Efficacy and physical function
Efficacy was assessed by the proportion of patients who were in a state of LDA or remission, as assessed by the Simplified Disease Activity Index (SDAI) ≤ 11 and ≤ 3.3, respectively, at each time point. Physical function was assessed by the proportion of patients who reported scores that met or exceeded the population normative value of ≤0.5 based on HAQ-Disability Index (HAQ-DI ≤ 0.5). In addition, rates of rescue, discontinuation (and reasons), and dose step-down were summarized. These analyses were performed according to the treatment groups assigned upon entry to the originating studies.
Statistical analyses
The analyses of efficacy and physical function were conducted based on the modified intention-to-treat (mITT) population that included all patients who were randomized and had received ≥1 dose of study drug after randomization in the RA-BEGIN and RA-BEAM studies, respectively.
For the categorical measures (SDAI ≤ 11, SDAI ≤ 3.3, and HAQ-DI ≤ 0.5), two sets of analyses were conducted: non-responder imputation (NRI) analysis, which considered discontinued patients as non-responders, and completer analysis, which was based on patients who had data available at the analysis time point (as observed), in line with the EULAR recommendations for reporting extension studies [12]. Data collected from baricitinib 4 mg-treated patients who received baricitinib 2 mg following randomization in the dose step-down sub-study of the LTE were imputed based on data from patients who participated in the sub-study but remained on baricitinib 4 mg.
Patients who were rescued or switched to baricitinib 4 mg during any of the three studies were analysed based on the treatment groups to which they were originally randomized; the data collected after rescue or switch were analysed as observed.
Data included in this study were collected up to 13 February 2018. All analyses were post hoc.
Results
Disposition
In RA-BEGIN, 584 patients were randomized to three treatment arms: baricitinib 4 mg monotherapy (n = 159), baricitinib 4 mg + MTX (n = 215), and MTX monotherapy (n = 210; Supplementary Fig. S2, available at Rheumatology online); 85.5% of patients treated with baricitinib 4 mg monotherapy completed treatment at week 52, along with 80.5% in the baricitinib 4 mg + MTX and 76.7% in the MTX groups. Through week 148 of the LTE, the overall discontinuation rate in patients originating from RA-BEGIN was 30.1% (Table 1). Across all groups, 3.8% discontinued due to lack of efficacy and 11.3% discontinued for safety reasons (Supplementary Fig. S2, available at Rheumatology online).
Table 1.
Cumulative rates of rescue, discontinuation and dose step-down in RA-BEGIN patients
| Event | Week |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | 12 | 16 | 20 | 24 | 32 | 40 | 52 | LTE → | 56 | 64 | 76 | 88 | 100 | 112 | 124 | 136 | 148 | |
| Treatment at randomization: Bari 4 mg (N =159) | |||||||||||||||||||||
| Rescue | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 (3.1) | 7 (4.4) | 7 (4.4) | 7 (4.4) | Bari 4 mg Orig. group: Bari 4 mg | 14 (8.8) | 22 (13.8) | 24 (15.1) | 26 (16.4) | 28 (17.6) | 29 (18.2) | 29 (18.2) | 30 (18.9) | 31 (19.5) |
| Discontinuation | 1 (0.6) | 1 (0.6) | 1 (0.6) | 3 (1.9) | 3 (1.9) | 6 (3.8) | 9 (5.7) | 13 (8.2) | 18 (11.3) | 20 (12.6) | 23 (14.5) | 23 (14.5) | 25 (15.7) | 27 (17.0) | 32 (20.1) | 33 (20.8) | 34 (21.4) | 35 (22.0) | 36 (22.6) | 37 (23.3) | |
| Dose step-down to bari 2 mg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 (3.8) | 10 (6.3) | 13 (8.2) | 13 (8.2) | 14 (8.8) | 14 (8.8) | 15 (9.4) | 15 (9.4) | |
| Treatment at randomization: Bari 4 mg + MTX (N =215) | |||||||||||||||||||||
| Rescue | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (1.9) | 5 (2.3) | 6 (2.8) | 6 (2.8) | Bari 4 mg Orig. group: Bari 4 mg + MTX | 12 (5.6) | 17 (7.9) | 20 (9.3) | 20 (9.3) | 22 (10.2) | 26 (12.1) | 27 (12.6) | 28 (13.0) | 28 (13.0) |
| Discontinuation | 3 (1.4) | 7 (3.3) | 7 (3.3) | 12 (5.6) | 13 (6.0) | 18 (8.4) | 20 (9.3) | 22 (10.2) | 28 (13.0) | 35 (16.3) | 40 (18.6) | 44 (20.5) | 47 (21.9) | 51 (23.7) | 54 (25.1) | 55 (25.6) | 57 (26.5) | 62 (28.8) | 62 (28.8) | 65 (30.2) | |
| Dose step-down to bari 2 mg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 15 (7.0) | 24 (11.2) | 25 (11.6) | 31 (14.4) | 34 (15.8) | 37 (17.2) | 38 (17.7) | 38 (17.7) | |
| Treatment at randomization: MTX (N =210) | |||||||||||||||||||||
| Rescue | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 18 (8.6) | 24 (11.4) | 26 (12.4) | 26 (12.4) | Bari 4 mg Orig. group: MTX | 29 (13.8) | 33 (15.7) | 37 (17.6) | 37 (17.6) | 39 (18.6) | 39 (18.6) | 39 (18.6) | 41 (19.5) | 41 (19.5) |
| Discontinuation | 3 (1.4) | 5 (2.4) | 6 (2.9) | 13 (6.2) | 16 (7.6) | 20 (9.5) | 25 (11.9) | 27 (12.9) | 32 (15.2) | 38 (18.1) | 45 (21.4) | 49 (23.3) | 52 (24.8) | 57 (27.1) | 58 (27.6) | 61 (29.0) | 64 (30.5) | 66 (31.4) | 69 (32.9) | 74 (35.2) | |
| Dose step-down to bari 2 mg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 15 (7.1) | 21 (10.0) | 29 (13.8) | 31 (14.8) | |
Data are reported as n (%) and include up to 96 additional weeks in RA-BEYOND. Bari, baricitinib; LTE, long-term extension; orig., original.
In RA-BEAM, 1305 patients were randomized to three treatments arms: placebo (+MTX; n = 488), baricitinib 4 mg (+MTX; n = 487), and adalimumab (+MTX; n = 330; Supplementary Fig. S3, available at Rheumatology online). A total of 87.7% of patients treated with baricitinib 4 mg (+MTX) completed treatment at week 52, along with 83.4% of patients in the placebo (+MTX) group, who switched to baricitinib 4 mg treatment at week 24, and 86.7% in the adalimumab (+MTX) group. Through week 148 of the LTE, the overall discontinuation rate in patients originating from RA-BEAM was 24.8% (Table 2). Across all groups, 3.6% discontinued due to lack of efficacy and 10.7% discontinued for safety reasons (Supplementary Fig. S3, available at Rheumatology online).
Table 2.
Rates of rescue, discontinuation and dose step-down in RA-BEAM patients
| Event | Week |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | 12 | 16 | 20 | 24 | 32 | 40 | 52 | LTE → | 56 | 64 | 76 | 88 | 100 | 112 | 124 | 136 | 148 | |
| Treatment at randomization: PBO (+MTX) (N =488) | |||||||||||||||||||||
| Rescue | 0 | 0 | 0 | 0 | 0 | 65 (13.3) | 109 (22.3) | 128 (26.2) | 133 (27.3) | 133 (27.3) | 133 (27.3) | Bari 4 mg (+MTX) Orig. group: PBO (+MTX) | 133 (27.3) | 154 (31.6) | 172 (35.2) | 186 (38.1) | 188 (38.5) | 189 (38.7) | 189 (38.7) | 191 (39.1) | 192 (39.3) |
| Discontinuation | 6 (1.2) | 8 (1.6) | 14 (2.9) | 23 (4.7) | 31 (6.4) | 38 (7.8) | 45 (9.2) | 51 (10.5) | 61 (12.5) | 70 (14.3) | 80 (16.4) | 81 (16.6) | 84 (17.2) | 91 (18.6) | 103 (21.1) | 111 (22.7) | 114 (23.4) | 120 (24.6) | 125 (25.6) | 128 (26.2) | |
| Dose step-down to bari 2 mg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 64 (13.1) | 80 (16.4) | 82 (16.8) | 85 (17.4) | 87 (17.8) | 87 (17.8) | |
| Treatment at randomization: Bari 4 mg (+MTX) (N =487) | |||||||||||||||||||||
| Rescue | 0 | 0 | 0 | 0 | 0 | 20 (4.1) | 33 (6.8) | 35 (7.2) | 40 (8.2) | 43 (8.8) | 43 (8.8) | Bari 4 mg (+MTX) Orig. group: Bari 4 mg (+MTX) | 43 (8.8) | 73 (15.0) | 87 (17.9) | 93 (19.1) | 95 (19.5) | 98 (20.1) | 100 (20.5) | 100 (20.5) | 101 (20.7) |
| Discontinuation | 3 (0.6) | 3 (0.6) | 5 (1.0) | 9 (1.8) | 13 (2.7) | 16 (3.3) | 17 (3.5) | 25 (5.1) | 42 (8.6) | 49 (10.1) | 58 (11.9) | 62 (12.7) | 65 (13.3) | 72 (14.8) | 77 (15.8) | 84 (17.2) | 91 (18.7) | 101 (20.7) | 106 (21.8) | 116 (23.8) | |
| Dose step-down to bari 2 mg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.2) | 94 (19.3) | 131 (26.9) | 146 (30.0) | 150 (30.8) | 154 (31.6) | 155 (31.8) | 155 (31.8) | 156 (32.0) | |
| Treatment at randomization: ADA (+MTX) (N =330) | |||||||||||||||||||||
| Rescue | 0 | 0 | 0 | 0 | 0 | 17 (5.2) | 35 (10.6) | 40 (12.1) | 47 (14.2) | 51 (15.5) | 51 (15.5) | Bari 4 mg (+MTX) Orig. group: ADA (+MTX) | 51 (15.5) | 67 (20.3) | 78 (23.6) | 90 (27.3) | 98 (29.7) | 103 (31.2) | 106 (32.1) | 108 (32.7) | 108 (32.7) |
| Discontinuation | 0 | 3 (0.9) | 6 (1.8) | 11 (3.3) | 17 (5.2) | 17 (5.2) | 20 (6.1) | 23 (7.0) | 35 (10.6) | 38 (11.5) | 42 (12.7) | 46 (13.9) | 49 (14.8) | 53 (16.1) | 55 (16.7) | 59 (17.9) | 63 (19.1) | 68 (20.6) | 75 (22.7) | 79 (23.9) | |
| Dose step-down to bari 2 mg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 56 (17.0) | 70 (21.2) | 72 (21.8) | 74 (22.4) | |
Data are reported as n (%) and include up to 96 additional weeks in RA-BEYOND. ADA, adalimumab; bari, baricitinib; LTE, long-term extension; orig., original; PBO, placebo.
Per the study protocols, patients with symptomatic herpes zoster were mandated to drop out. Of all patients who discontinued due to adverse events over the 3-year study period, namely 11.3% for RA-BEGIN (DMARD-naïve) and 10.7% for RA-BEAM (MTX-IR), 21% and 26% discontinued due to herpes zoster across all treatment arms of the RA-BEGIN (through RA-BEYOND) and RA-BEAM (through RA-BEYOND) studies, respectively. The remaining reasons for discontinuations were reported by few patients [5 or less (5% or less of patients who discontinued due to AE)] and no adverse event type predominated within this group.
Efficacy
SDAI LDA
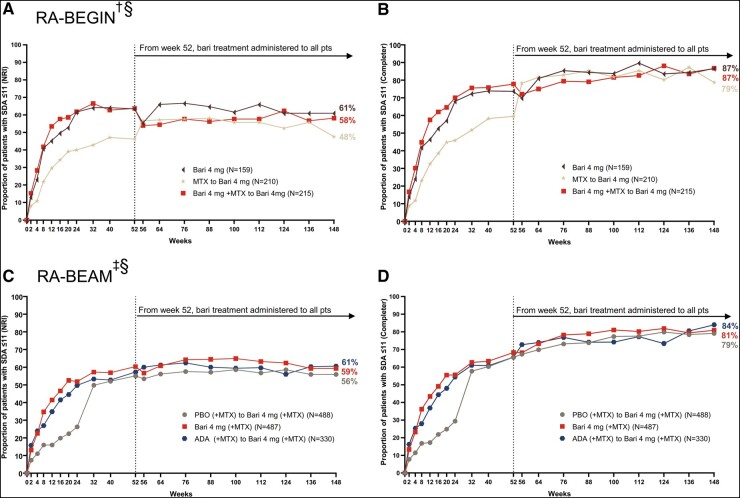
Greater proportions of DMARD-naïve patients from both baricitinib treatment groups of RA-BEGIN achieved LDA, as measured by SDAI ≤ 11, during the originating study than patients treated with MTX monotherapy (Fig. 1A and B). At week 148, 61%, 58%, and 48% of patients initially treated with baricitinib 4 mg monotherapy, baricitinib 4 mg + MTX, and MTX monotherapy, respectively, were in SDAI LDA based on the NRI method (Fig. 1A). The responses in patients initially treated with baricitinib were maintained from week 24 through week 148. An increased response was observed shortly after switching from MTX monotherapy to baricitinib 4 mg monotherapy upon entry into the LTE, which reflected the benefit of baricitinib 4 mg monotherapy over MTX monotherapy. The improvement achieved after switching was maintained through week 148.
Fig. 1.
Patients who achieved Simplified Disease Activity Index (SDAI) ≤11 in RA-BEGIN and RA-BEAM trials
(A) and (B) illustrate efficacy over time based on the non-responder imputation (NRI) and completer analyses in RA-BEGIN and RA-BEYOND. (C) and (D) illustrate efficacy over time based on the NRI and completer analysis in RA-BEAM and RA-BEYOND. †In RA-BEGIN, rescue was offered at week 24.
‡In RA-BEAM, rescue was offered at week 16. At week 24, all PBO (+MTX) patients were switched to baricitinib 4 mg (+MTX).
§Upon entering RA-BEYOND at week 52, patients who received MTX and Bari + MTX in RA-BEGIN were switched to Bari 4 mg monotherapy; patients who received ADA + (MTX) in RA-BEAM were switched to Bari 4 mg (+MTX). Data points are listed in Supplementary Tables S1 and S2, available at Rheumatology online.
ADA, adalimumab; Bari, baricitinib; NRI, non-responder imputation; PBO, placebo; pts, patients; SDAI, Simplified Disease Activity Index.
Similar results were seen in MTX-IR patients from RA-BEAM. Greater proportions of patients from both baricitinib 4 mg (+MTX) and adalimumab (+MTX) treatment groups achieved SDAI LDA during the originating study than patients treated with placebo (+MTX, switched to baricitinib 4 mg at week 24; Fig. 1C and D). At week 148, 59%, 61%, and 56% of patients initially treated with baricitinib 4 mg (+MTX), adalimumab (+MTX), and placebo (+MTX), respectively, were in SDAI LDA based on the NRI method (Fig. 1C). The response in patients initially treated with baricitinib (+MTX) was maintained from week 24 through week 148. The SDAI LDA response achieved with adalimumab (+MTX) treatment through week 52 was maintained through week 148 after patients switched to baricitinib 4 mg (+MTX). The SDAI LDA response increased from week 24 to week 52 following the switch from placebo (+MTX) to baricitinib 4 mg (+MTX) and was then maintained through week 148.
In both studies, the response trends were similar between the NRI and completer analyses; however, response rates were consistently higher based on the completer analysis. This was expected given that patients who discontinued from the study were defined as non-responders in NRI analysis but excluded from the completer analysis.
SDAI remission
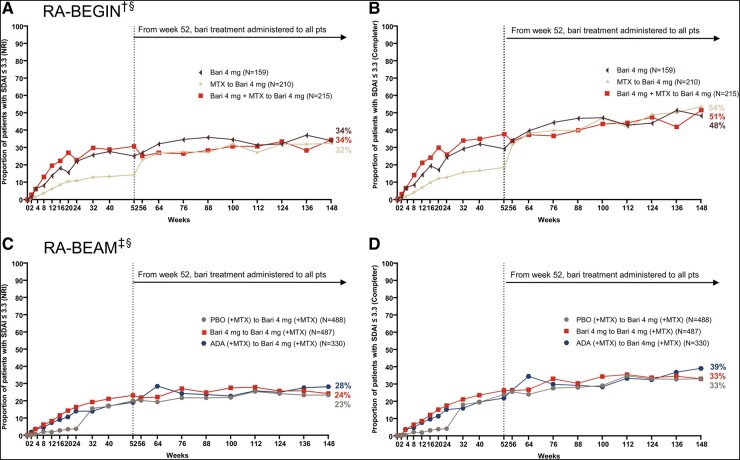
Greater proportions of patients from both baricitinib treatment groups in RA-BEGIN achieved remission, as measured by SDAI ≤ 3.3, during the originating study than patients who were treated with MTX monotherapy (Fig. 2A). At week 148, 34%, 34%, and 32% of patients initially treated with baricitinib 4 mg monotherapy, baricitinib 4 mg + MTX, and MTX monotherapy, respectively, were in SDAI remission based on the NRI method (Fig. 2). Responses in patients initially treated with baricitinib were maintained from week 24 through week 148. Similar to the trend observed with SDAI LDA, the remission response in the MTX monotherapy group increased shortly after switching from MTX monotherapy to baricitinib 4 mg monotherapy upon entry into the LTE. This clinical improvement demonstrated the benefit of treatment with baricitinib 4 mg monotherapy over MTX monotherapy. The improvement achieved after switching was maintained through week 148.
Fig. 2.
Patients who achieved SDAI ≤ 3.3 in RA-BEGIN and RA-BEAM trials
(A) and (B) illustrate efficacy over time based on the NRI and completer analyses in RA-BEGIN and RA-BEYOND. (C) and (D) illustrate efficacy over time based on the NRI and completer analyses in RA-BEAM and RA-BEYOND. †In RA-BEGIN, rescue was offered at week 24.
‡In RA-BEAM, rescue was offered at week 16. At week 24, all PBO (+MTX) patients were switched to baricitinib 4 mg (+MTX).
§Upon entering RA-BEYOND at week 52, patients who received MTX and Bari + MTX in RA-BEGIN were switched to Bari 4 mg monotherapy; patients who received ADA + (MTX) in RA-BEAM were switched to Bari 4 mg (+MTX). Data points are listed in Supplementary Tables S3 and S4, available at Rheumatology online.
ADA, adalimumab; Bari, baricitinib; NRI, non-responder imputation; PBO, placebo; pts, patients; SDAI, Simplified Disease Activity Index.
In RA-BEAM, greater proportions of patients from both baricitinib 4 mg (+MTX) and adalimumab (+MTX) treatment groups achieved SDAI ≤ 3.3 during the originating study than patients treated with placebo (+MTX, switched to baricitinib 4 mg at week 24; Fig. 2B). At week 148, 24%, 28%, and 23% of patients initially treated with baricitinib 4 mg (+MTX), adalimumab (+MTX), and placebo (+MTX), respectively, were in SDAI remission based on the NRI method. The response in patients initially treated with baricitinib (+MTX) was maintained from week 24 through week 148. SDAI remission response with adalimumab (+MTX) treatment through week 52 was maintained after patients switched to baricitinib 4 mg (+MTX) through week 148. Remission response rates increased from week 24 to week 52 following the switch from placebo (+MTX) to baricitinib 4 mg (+MTX) and were then maintained through week 148.
In both studies, the response trends were similar between NRI and completer analyses with the expected consistently higher rates observed in the completer analysis.
Physical function (HAQ-DI)
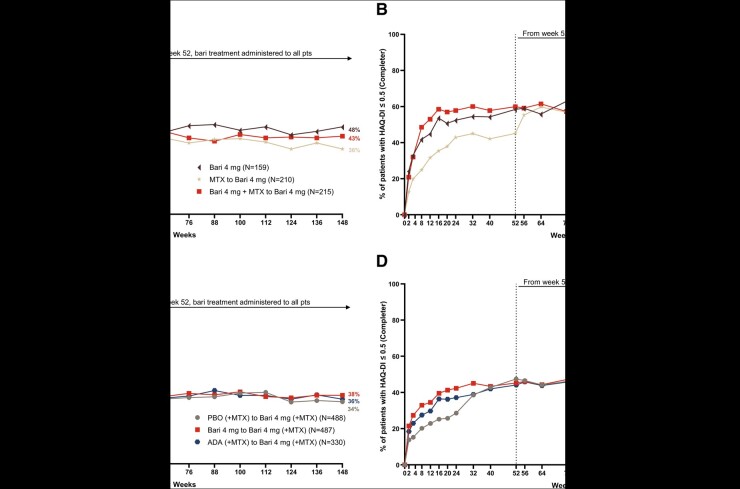
In RA-BEGIN, greater proportions of patients from both baricitinib treatment groups achieved HAQ-DI ≤ 0.5 during the originating study than patients treated with MTX monotherapy (Fig. 3A and B). At week 148, 48%, 43%, and 36% of patients initially treated with baricitinib 4 mg monotherapy, baricitinib 4 mg + MTX, and MTX monotherapy, respectively, had HAQ-DI ≤ 0.5 based on the NRI method. The HAQ-DI ≤ 0.5 responses at week 148 in patients initially treated with baricitinib were maintained from week 12. An increased response in the MTX monotherapy group was observed shortly after switching from MTX monotherapy to baricitinib 4 mg monotherapy upon entry into the LTE, which demonstrated the benefit of treatment with baricitinib 4 mg monotherapy over MTX monotherapy. The improvement achieved after switching was maintained through week 148.
Fig. 3.
Patients who achieved HAQ-Disability Index (HAQ-DI) ≤0.5 in RA-BEGIN and RA-BEAM trials
(A) and (B) illustrate the percentage of patients who achieved HAQ-DI ≤ 0.5 over time based on the NRI and completer analyses in RA-BEGIN and RA-BEYOND. (C) and (D) illustrate the percentage of patients who achieved HAQ-DI ≤ 0.5 over time based on the NRI and completer analyses in RA-BEAM and RA-BEYOND. †In RA-BEGIN, rescue was offered at week 24.
‡In RA-BEAM, rescue was offered at week 16. At week 24, all PBO (+MTX) patients were switched to baricitinib 4 mg (+MTX).
§Upon entering RA-BEYOND at week 52, patients who received MTX and Bari + MTX in RA-BEGIN were switched to Bari 4 mg monotherapy; patients who received ADA + (MTX) in RA-BEAM were switched to Bari 4 mg (+MTX). Data points are listed in Supplementary Tables S5 and S6, available at Rheumatology online.
ADA, adalimumab; Bari, baricitinib; HAQ-DI, HAQ-Disability Index; NRI, non-responder imputation; PBO, placebo; pts, patients.
In RA-BEAM, greater proportions of patients from both baricitinib 4 mg (+MTX) and adalimumab (+MTX) treatment groups achieved HAQ-DI ≤ 0.5 during the originating study than patients treated with placebo (+MTX, switched to baricitinib 4 mg at week 24; Fig. 3C and D). At week 148, 38%, 36%, and 34% of patients initially treated with baricitinib 4 mg (+MTX), adalimumab (+MTX), and placebo (+MTX), respectively, had HAQ-DI ≤ 0.5 based on the NRI method. The response in patients initially treated with baricitinib (+MTX) was maintained from week 12 through week 148. When patients switched from placebo (+MTX) to baricitinib 4 mg (+MTX) at week 24, an increased HAQ-DI ≤ 0.5 response was observed, and this improvement continued through week 148 (Fig. 3B).
In both studies, the response trends were similar between NRI and completer analyses with consistently higher rates observed in the completer analysis.
Safety
An updated assessment of baricitinib safety in patients with RA through a median of 3.1 years of treatment (maximum 7 years) using pooled data from 3770 patients for a total of 10 127 PY of exposure was recently reported [7]. Incidence rates per 100 PY for serious infections (2.8), herpes zoster (3.3), major adverse cardiovascular events (MACE; 0.5), deep vein thrombosis (DVT; 0.3), pulmonary embolism (PE; 0.2), DVT and/or PE (0.5), and malignancy (excluding non-melanoma skin cancer, 0.8) were similar to those previously reported [13].
Discussion
Given the chronic and progressive nature of RA, treatment options must offer long-term solutions for patients and health-care practitioners. Results from this study demonstrated the long-term maintenance of clinically relevant treatment goals achieved with baricitinib 4 mg, which include LDA, remission and normative physical function. Important to clinically relevant questions about efficacy following treatment switching, outcomes in patients initially assigned to established first- (MTX) and second-line (adalimumab; anti-TNF biologic DMARD) RA treatments were evaluated for an additional 2 years following a switch to baricitinib 4 mg. At week 148, as many as 61%, 34%, and 48% of DMARD-naïve patients initially treated with baricitinib were in SDAI LDA, SDAI remission, and a state of physical function that met or exceeded the population normative value, respectively (NRI analysis method); 59%, 24%, and 38% of MTX-IR patients initially treated with baricitinib were in SDAI LDA, SDAI remission, and a state of normative physical function, respectively (NRI). Increased response rates across all measures were observed following the switch from MTX monotherapy to baricitinib 4 mg monotherapy with maintenance of achieved outcomes with baricitinib for an additional 2 years. It is noteworthy that in the population of patients who had failed MTX and potentially other csDMARDs, essentially 1 of 4 patients who started baricitinib 4 mg were able to achieve and maintain stringent SDAI remission, and more than half of the patients achieved and maintained LDA in the long term, which was the treatment goal in this patient population [14]. Furthermore, patients who were in a state of LDA (SDAI ≤ 11) were close to remission with a mean SDAI of ∼3.4 (DMARD-naïve) and 4.6 (MTX-IR). Also, outcomes achieved following initial adalimumab treatment for 1 year were maintained for an additional 2 years following the switch to baricitinib 4 mg.
Overall treatment discontinuation and the reasons attributed to the discontinuations are able to augment understanding of treatment response maintenance over time. Discontinuation due to loss of efficacy or for reasons related to safety are foremost among clinically relevant considerations that may challenge patients staying on treatment over the long term. After 3 years of treatment, 10.7% of MTX-IR patients discontinued due to reasons related to safety across all groups. A pragmatic approach to ‘background’ therapy (csDMARDs, corticosteroids, NSAIDs, and analgesics) adjustment was permitted in these studies. Based on this approach, 3.6% of MTX-IR patients discontinued due to lack of efficacy. Rates were similar in the DMARD-naïve population. While an analysis of predictors for specific reasons for discontinuation would be of interest, the number of patients discontinuing for the individual reasons is too small to perform such analyses. These will have to come from larger trials of JAK inhibitors.
The efficacy and safety of baricitinib, an oral, selective and reversible JAK1 and JAK2 inhibitor, were previously demonstrated in clinical trials for up to 1 year in patients with active RA [3–6]. LTEs can demonstrate the maintenance of achieved outcomes beyond what studies can offer given their shorter duration. However, the overall study design may differ between originating and LTE studies, which can complicate data interpretation. Furthermore, sponsors may also choose to pool data across clinically relevant populations rather than focus on particular patient groups. The current study, however, followed patients who were randomized to treatment in originator trials and continues to assess response rates in an LTE using both NRI and completer (observed) analyses. The tofacitinib LTE, ORAL Sequel, was previously the only study with data reported from a period of at least 3 years from the JAK inhibitor class [15]. Due to trial design, most patients enrolled into ORAL Sequel from phase III originator trials received 10 mg twice daily, a dose that is not approved to treat RA in major geographies—the recommended dose for RA is 5 mg administered twice daily in both the European Union and the United States [16, 17]. Further, data were pooled from clinically diverse populations and only observed data analysed from the start of the LTE, not from the time of initial randomization in the originator trials, in contrast to how the current study was designed.
The advantage of well-designed LTE studies in RA clinical research programmes is to utilize systematic reporting and data monitoring to identify trends over time from cumulative exposure. In accordance with the EULAR recommendations for the reporting of LTE studies [12], the analysis plan for this LTE was carefully considered to align with this guidance. All mITT patients randomized at the originating study entry were included in the analyses in this LTE to avoid bias from only reporting data from selected patient subgroups. Further, frequencies and number of patients discontinued from study, rescued or stepped down at each time point during the analysis period were reported, which provides clarity on the efficacy response over time.
The effectiveness of TNF inhibitors and other biologics might be lost over time despite a good initial response (secondary treatment inefficacy). The development of anti-drug antibodies is a notable reason for secondary failure [18], and loss of efficacy represents the foremost reason for TNF inhibitor treatment discontinuation [19]. Secondary loss of efficacy has not been documented with small molecule JAK inhibitors and the present study provides evidence that loss of treatment response may not be a challenge with JAK inhibitors, including baricitinib.
The comprehensive evaluation of a drug’s safety over time is also necessary to characterize the risk–benefit. While safety was not the focus of the present study, an evaluation of the safety profile of baricitinib in >3700 patients with RA treated for up to 7 years was recently reported [7]. Overall, in baricitinib-treated patients with active RA, a consistent safety profile has been observed over long-term exposure.
The study had potential limitations, which should be noted. As in all clinical research, patients were ineligible for the trial if significant comorbidities existed, which may have influenced patient profiles, although our findings were consistent with previous research [3, 4]. The analyses detailed in this paper were descriptive summaries. No comparisons of long-term data between groups were provided due to insufficient statistical power for this post hoc analysis. Further, not all patients had data available from their originally randomized treatment during the entire analysis period. This was due to either study discontinuation or to a protocol-defined treatment switch, such as when patients stepped down from baricitinib 4 mg to baricitinib 2 mg therapy. In these instances, data imputation was required in the analysis.
In conclusion, these results demonstrated the long-term efficacy of baricitinib 4 mg for up to 3 years. Low discontinuation rates indicated that baricitinib 4 mg treatment was both efficacious and well tolerated over the long term.
Supplementary Material
Acknowledgements
Medical writing support was provided by Katie Crosslin, PhD, of Evidera/PPD and was funded by Eli Lilly and Company (Indianapolis, IN, USA) in accordance with Good Publication Practice guidelines. The authors thank Yun-Fei Chen and Jorge Alfonso Ross Terres for providing statistical and medical reviews, respectively, during manuscript development. Dr Peter C. Taylor acknowledges support from the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC).
Funding: This work was supported by Eli Lilly and Company.
Disclosure statement: L.X., B.J., A.E., A.C., R.O. and C.W. are full-time employees of Eli Lilly and Company and they may own stock or stock options in the company. G.B. has worked as a paid consultant/paid speaker for AbbVie, Eli Lilly and Company, Gilead, and Pfizer. P.T. has received grant/research support from Celgene, Eli Lilly and Company, Galapagos, and Gilead; he also was a paid consultant for AbbVie, Biogen, Eli Lilly and Company, Fresenius, Galapagos, Gilead, GlaxoSmithKline, Janssen, Nordic Pharma, Pfizer, Roche, and UCB. M.D. received grant/research support and was a paid consultant for AbbVie, BMS, Eli Lilly and Company, Pfizer, Novartis, Merck, Roche, and UCB. J.S. received grant/research support from AbbVie, Eli Lilly and Company, Janssen, MSD, Novartis, Pfizer, and Roche; he was also a paid consultant for AbbVie, Amgen, AstraZeneca, Astro, BMS, Celgene, Celltrion, Chugai, Eli Lilly and Company, Gilead, ILTOO, Janssen, Medimmune, MSD, Novartis, Pfizer, Roche, Samsung, Sanofi, and UCB. Y.T. received grant/research support from Mitsubishi Tanabe, Chugai, AbbVie, Takeda, UCB, Daiichi Sankyo, Eisai; he was also a paid speaker for Daiichi Sankyo, Eli Lilly and Company, Novartis, YL Biologics, Bristol Myers, Eisai, Chugai, AbbVie, Astellas, Pfizer, Sanofi, Asahi Kasei, GlaxoSmithKline, Mitsubishi Tanabe, Gilead, Janssen.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Deb A, Dwibedi N, LeMasters T. et al. Burden of depression among working-age adults with rheumatoid arthritis. Arthritis 2018;2018:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fridman JS, Scherle PA, Collins R. et al. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol 2010;184:5298–307. [DOI] [PubMed] [Google Scholar]
- 3. Fleischmann R, Schiff M, van der Heijde D. et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol 2017;69:506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taylor PC, Keystone EC, van der Heijde D. et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med 2017;376:652–62. [DOI] [PubMed] [Google Scholar]
- 5. Dougados M, van der Heijde D, Chen YC. et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann Rheum Dis 2017;76:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Genovese MC, Kremer J, Zamani O. et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med 2016;374:1243–52. [DOI] [PubMed] [Google Scholar]
- 7. Genovese MS, Takeuchi T, Burmester G. et al. Safety profile of baricitinib for the treatment of rheumatoid arthritis up to 7 years: an updated integrated safety analysis. The Lancet Rheumatology 2020;2(6):e347–e357. [DOI] [PubMed]
- 8. Gaujoux-Viala C, Nam J, Ramiro S. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2014;73:510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singh JA, Saag KG, Bridges SL Jr. et al. 2015 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68:1–26. [DOI] [PubMed] [Google Scholar]
- 10. Tanaka Y, Emoto K, Cai Z. et al. Efficacy and safety of baricitinib in Japanese patients with active rheumatoid arthritis receiving background methotrexate therapy: a 12-week, double-blind, randomized placebo-controlled study. J Rheumatol 2016;43:504–11. [DOI] [PubMed] [Google Scholar]
- 11. Takeuchi T, Genovese MC, Haraoui B. et al. Dose reduction of baricitinib in patients with rheumatoid arthritis achieving sustained disease control: results of a prospective study. Ann Rheum Dis 2019;78:171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buch MH, Silva-Fernandez L, Carmona L. et al. Development of EULAR recommendations for the reporting of clinical trial extension studies in rheumatology. Ann Rheum Dis 2015;74:963–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smolen JS, Genovese MC, Takeuchi T. et al. Safety profile of baricitinib in patients with active rheumatoid arthritis with over 2 years median time in treatment. J Rheumatol 2019;46:7–18. [DOI] [PubMed] [Google Scholar]
- 14. Smolen JS, Breedveld FC, Burmester GR. et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 2016;75:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wollenhaupt J, Lee EB, Curtis JR. et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: final results of a global, open-label, long-term extension study. Arthritis Res Ther 2019;21:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Medicines Agency. Summary of tofacitinib characteristics. [cited on 2 Sept 2020]. Available from: https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf.
- 17.Food and Drug Administration. Product insert for XELJANZ® (tofacitinib) tablets. [updated 2020 July; cited 2 Sept 2020]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/203214s024,208246s010lbl.pdf.
- 18. Kalden JR, Schulze-Koops H.. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol 2017;13:707–18. [DOI] [PubMed] [Google Scholar]
- 19. Garcia-Lagunar MH, Gutierrez-Civicos MR, Garcia-Simon MS. et al. Reasons for discontinuation and adverse effects of TNFalpha inhibitors in a cohort of patients with rheumatoid arthritis and ankylosing spondylitis. Ann Pharmacother 2017;51:388–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.