Abstract
Objectives
There are signs that antidepressants and anticonvulsants are being prescribed more often for OA patients, despite limited evidence. Our objectives were to examine prescription rates and time trends for antidepressants and anticonvulsants in OA patients, to assess the percentage of long-term prescriptions, and to determine patient characteristics associated with antidepressant or anticonvulsant prescription.
Methods
A population-based cohort study was conducted using the Integrated Primary Care Information database. First, episodic and prevalent prescription rates for antidepressants (amitriptyline, nortriptyline and duloxetine) and anticonvulsants (gabapentinoids) in OA patients were calculated for the period 2008–17. Logistic regression was used to assess which patient characteristics were associated with prescriptions.
Results
In total, 164 292 OA patients were included. The prescription rates of amitriptyline, gabapentin and pregabalin increased over time. The increase in prescription rates for pregabalin was most pronounced. Episodic prescription rate increased from 7.1 to 13.9 per 1000 person-years between 2008 and 2017. Amitriptyline was prescribed most (15.1 episodic prescriptions per 1000 person-years in 2017). Prescription rates of nortriptyline and duloxetine remained stable at 3.0 and 2.0 episodic prescriptions per 1000 person-years, respectively. For ≤3% of patients with incident OA, medication was prescribed long-term (≥3 months). In general, all medication was prescribed more frequently for older patients (except duloxetine), women, patients with OA in ≥2 joints, patients with spinal OA and patients with musculoskeletal disorders.
Conclusion
Prescription rates of amitriptyline, gabapentin and pregabalin increased over time. Since there is little evidence to support prescription in OA, caution is necessary when prescribing.
Keywords: osteoarthritis, pain, analgesics, antidepressants, anticonvulsants, gabapentinoids
Rheumatology key messages
An increase was found in prescription rates for amitriptyline, gabapentin and pregabalin in osteoarthritis patients.
The prescription rate for pregabalin has doubled between 2008 and 2017.
The prescription rates for nortriptyline and duloxetine have remained stable between 2008 and 2017.
Introduction
OA is a highly prevalent chronic pain condition of the musculoskeletal system. Approximately 15% of the population suffers from OA [1, 2]. Since the population is ageing and the number of persons with obesity is increasing, it is expected that the disease burden will increase [3]. Pain, joint stiffness and reduced function are important complaints among patients with OA.
Different treatment options are available for OA-related pain [4, 5]. An important strategy is the use of analgesics in a stepwise approach. Paracetamol leads to a small, not clinically relevant, reduction of pain compared with a placebo (3%) and concerns have been raised about its long-term use [6]. Another treatment option is topical NSAIDs, with mean effect sizes of 0.30 for pain relief [7]. Oral NSAIDs have a clinically relevant effect for OA pain, but have serious gastrointestinal and cardiovascular side effects [8, 9]. Opioids are prescribed more frequently than in the past [10–12], but they lack a clinically relevant effect and have serious side effects and a risk of addiction [13, 14].
Another option may be the use of antidepressants or anticonvulsants, which are hypothesized to have an effect on central pain processing [15]. Signs of pain sensitization are present in around 40% of patients with OA [16, 17]. Most extensively studied for OA pain is the antidepressant duloxetine [18], a serotonin and noradrenalin reuptake inhibitor (SNRI). The Osteoarthritis Research Society International guideline recommends using duloxetine in polyarticular OA [5], while the National Institute for Health and Care Excellence guideline [19] and Dutch General Practitioner guideline [20] on OA do not advise using duloxetine because of insufficient evidence. Tricyclic antidepressants (TCAs) are used in the treatment of neuropathic pain and other chronic pain conditions; they inhibit presynaptic uptake of serotonin and noradrenaline [21]. The efficacy of TCAs for OA-related pain is unknown [22]. A study on the efficacy of the TCA nortriptyline in patients with knee OA is currently being carried out [23]. Also, the anticonvulsants pregabalin and gabapentin can inhibit centralized pain [24]. Pregabalin has been investigated for OA pain, and may have a positive effect on pain in hand and knee OA [25, 26].
Although none of these medications are registered for OA pain in the Netherlands, it might be that general practitioners (GPs) do already prescribe these as off-label medications for OA-related pain. A recent retrospective cohort study in the UK found an almost 3-fold increase in first prescriptions of gabapentin and pregabalin in patients with OA between 2005 and 2015 [27]. Earlier studies in US insurance claims databases found 20–30% of the OA patients used an antidepressant, and around 15% used an anticonvulsant, which was higher than in patients without OA. [28–30]. In the Netherlands, the antidepressants amitriptyline, nortriptyline and duloxetine, and the anticonvulsants gabapentin and pregabalin can be prescribed for neuropathic pain conditions [31], but it is unknown whether these medications are prescribed for pain in OA patients. The aim of the current study is to examine incident and prevalent prescription rates and time trends for prescriptions of antidepressants and anticonvulsants in OA patients. In addition, baseline characteristics associated with prescriptions will be assessed.
Methods
Setting
This study was conducted using the Integrated Primary Care Information (IPCI) database. The IPCI database is a primary healthcare database that contains the electronic patient records of over 1.5 million patients in the Netherlands. In the Netherlands, the GP acts as a gatekeeper to secondary care. The electronic records contain all relevant medical information: the medical journal recorded by the GP, diagnoses according to the International Classification of Primary Care (ICPC) codes, laboratory results and referrals to secondary care. Furthermore, it contains all drug prescriptions, which are coded according to the Anatomical Therapeutic Chemical (ATC) classification code.
Study cohort
The study cohort consisted of all patients aged ≥30 years with a diagnosis of OA in the period between 1 January 2008 and 31 December 2017. Patients with newly diagnosed OA in this period (incident OA) as well as patients with a medical history of OA (prevalent OA) were included in the cohort. The diagnosis of OA was based on the ICPC codes L84 (spinal OA), L89 (hip OA), L90 (knee OA) and L91 (other peripheral joints affected by OA). Earlier research showed that the positive predictive value of these codes for having OA is ∼90% [32]. Patients who had a total knee or hip replacement during or before the study period were retained in the cohort. Patients had to have at least 12 months of valid database history prior to the study entry to assure complete medical records. Patients were followed until the GP practice stopped contributing data to the database, until the end of the study period (31 December 2017) or until 1 year before first diagnosis of a malignancy or until 1 year before death. Those patients were excluded because there is a high probability that antidepressants and anticonvulsants were prescribed for pain not related to OA. GPs can use different healthcare information systems to record medical information. Healthcare information systems needed to provide at least 6 years of data so that time trends could be examined. This resulted in the exclusion of one healthcare information system (Webhis), which provided 5 years of data.
In addition, a subset of patients with OA were selected who did not have comorbidities for which antidepressants and anticonvulsants could be prescribed (OA without comorbidities). Comorbidities for which antidepressants and anticonvulsants could be prescribed were selected based on an earlier study using the IPCI database [33]. Frequently occurring indications for antidepressants were depression (P03 and P76), anxiety (P01 and P74), sleep disorders (P06), psychosis and schizophrenia (P71, P72 and P98) and neuropathic pain disorders (N94). Patients with epilepsy (N88) and fibromyalgia (L18.01) were often prescribed gabapentin and pregabalin. All patients with these comorbidities were excluded from 1 year prior to the first date of the diagnosis.
The presence of other musculoskeletal disorders during the cohort period was defined as a minimum of one ICPC diagnosis code for other complaints of the musculoskeletal system. This subset of codes includes non-specific diagnoses, for example shoulder or hip complaints. The group of back/neck symptoms also includes the ICPC codes for radiculopathy.
Outcomes
The prescriptions were identified by the ATC code. Prescriptions of amitriptyline (N06AA09), nortriptyline (N06AA10), duloxetine (N06AX21), pregabalin (N03AX16) and gabapentin (N03AX12) were evaluated. The first prescription rate was defined as the total number of patients with a first prescription of the medication divided by the total number of person-years per calendar year. Patients were excluded from the denominator for this subanalysis after receiving a first prescription. The episodic rate of these prescriptions was defined as the total number of new episodes of medication prescriptions divided by the total number of person-years per calendar year. A new episode was determined as occurring if there was no prescription in the preceding 6 months. The prevalent prescription rate was defined as the total number of patients who had at least one prescription of the specific drug divided by the number of person-years per calendar year. Furthermore, the percentage was determined of the patients with incident OA who were prescribed an antidepressant or anticonvulsant for a period longer than 3 months in a follow-up year (long-term prescription). In the IPCI database, the median prescription duration was 30 days for amitriptyline, 21 days for nortriptyline, 30 days for duloxetine, 30 days for gabapentin and 28 days for pregabalin. Therefore, four prescriptions of the medication per follow-up year was defined as long-term prescription.
Statistical analyses
Descriptive statistics were performed to assess baseline characteristics and to calculate first, incidence and prevalence rates (with their 95% CIs) of the medication prescribed. Possible time trends in medication prescriptions were assessed using joinpoint regression analysis. Permutation tests using Monte Carlo methods were used to determine whether a marked change in trend (e.g. joinpoint) occurred. Since trends were examined over 10 years (10 data points), only one joinpoint was allowed. The joinpoint analysis provides information on the trend over the complete 10 years, the average annual percentage change (AAPC). When a marked change in trend is present, the annual percentage change (APC) is additionally reported to describe the two different trends. Analyses were performed with the Joinpoint Regression Program (Version 4.8.0.1, released 22 April 2020, available at https://surveillance.cancer.gov/joinpoint/, National Cancer Institute.
To assess the baseline characteristics (age, sex, joints affected and presence of other musculoskeletal disorders) associated with antidepressant and anticonvulsant prescriptions, univariable and multivariable logistic regression analyses were performed and odds ratios (ORs) and their 95% CIs were calculated. Variables were included in the multivariable regression analyses if P <0.1 in the univariable analysis for that variable. Analyses were performed using SPSS Statistics version 24 (IBM Corp., Armonk, NY, USA).
Study approval
The study was approved by the Board of Directors of the IPCI database.
Results
Study population
In total, 164 292 patients with OA were included in the cohort, of whom 59 053 were newly diagnosed with OA. The average follow-up time per patient was 3 years and 5 months. Two-thirds of the patients were female, and the mean age was 66.6 years (s.d. 12.4) (Table 1). With regard to the joints affected, the biggest category was patients with knee OA (27.4%). Almost a quarter of patients had a diagnosis in two or more joint groups. It was common for there to be other musculoskeletal disorders during the cohort period (64.9%). The cohort subset of OA patients without comorbidities for which antidepressants and anticonvulsants could be prescribed, consisted of 99 099 patients. The baseline characteristics of these patients were very similar. However, these patients were slightly younger (mean age 66.4 as opposed to 66.6), and also had slightly fewer musculoskeletal (61.8% vs 64.9%) and cardiovascular disorders (54.4% vs 58.1%). Furthermore, patients with incident OA were younger (mean age 64.2 vs 67.9) and had fewer musculoskeletal and cardiovascular disorders than patients with prevalent OA (Supplementary Tables S1 and S2, available at Rheumatology online).
Table 1.
Baseline characteristics
| OA patients (n = 164 292) | OA patients without comorbidities1 (n = 99 099) | |
|---|---|---|
| Age, mean (s.d.), years | 66.6 (12.4) | 66.4 (12.3) |
| Age category, n (%) | ||
| 30–39 | 2637 (1.6) | 1730 (1.7) |
| 40–49 | 12007 (7.3) | 7246 (7.3) |
| 50–59 | 32 786 (20.0) | 19 276 (19.5) |
| 60–69 | 48 756 (29.7) | 30 156 (30.4) |
| 70–79 | 40 749 (24.8) | 25 195 (25.4) |
| 80–89 | 23 639 (14.4) | 13 543 (13.7) |
| ≥90 | 3718 (2.3) | 1953 (2.0) |
| Female, n (%) | 107 438 (65.2) | 60 720 (61.3) |
| Joints affected, n (%) | ||
| Spine | 17 243 (10.5) | 10 180 (10.3) |
| Hip | 27 762 (16.9) | 18 075 (18.2) |
| Knee | 45 064 (27.4) | 28 610 (28.9) |
| Other joints | 37 753 (23.0) | 22 189 (22.4) |
| 2 or more joints | 36 470 (22.2) | 20 045 (20.2) |
| Comorbidities | ||
| Diabetes, n (%) | 25 175 (15.3) | 13 608 (13.7) |
| Hypertension, n (%) | 66 866 (40.7) | 38 005 (38.4) |
| Hyperlipidaemia, n (%) | 29 224 (17.8) | 15 497 (15.6) |
| MI/AP, n (%) | 18 868 (11.5) | 9674 (9.8) |
| Stroke/TIA, n (%) | 6352 (3.9) | 3114 (3.1) |
| PAD, n (%) | 6365 (3.9) | 3096 (3.1) |
| UGI/ulcer, n (%) | 5723 (3.5) | 2753 (2.8) |
| Heart failure, n (%) | 6989 (4.3) | 3374 (3.4) |
| Inflammatory arthritis, n (%) | 7949 (4.8) | 4122 (4.2) |
| Fibromyalgia, n (%) | 2492 (1.5) | NA |
| Neuropathic pain disorder, n (%) | 19 202 (11.7) | NA |
| Depression, n (%) | 28 714 (17.5) | NA |
| Anxiety, n (%) | 27 397 (16.7) | NA |
| Psychosis, n (%) | 4084 (2.5) | NA |
| Sleeping disorder, n (%) | 32 278 (19.6) | NA |
| Epilepsy, n (%) | 2404 (1.5) | NA |
| Other MSD during cohort time, n (%) | ||
| Upper extremity | 39 061 (23.8) | 21 702 (21.9) |
| Lower extremity | 61 076 (37.2) | 34 485 (34.8) |
| Back/neck | 51 260 (31.2) | 28 105 (28.4) |
| Other musculoskeletal | 50 559 (30.8) | 27 486 (27.7) |
| None | 57 609 (35.1) | 37 903 (38.2) |
| Renal function, n (%) | ||
| eGFR>60 ml/min | 103 952 (63.3) | 59 613 (60.2) |
| eGFR 30–60 ml/min | 19 382 (11.8) | 10 794 (10.9) |
| eGFR<30 ml/min | 1614 (1.0) | 896 (0.9) |
| Missing | 39 344 (23.9) | 27 796 (28.0) |
MI: myocardial infarction; AP: angina pectoris; TIA: transient ischemic attack; PAD: peripheral arterial disease; UGI: upper gastro-intestinal; MSD: musculoskeletal disorder; eGFR: estimated glomerular filtration rate.
Time trends for first prescriptions
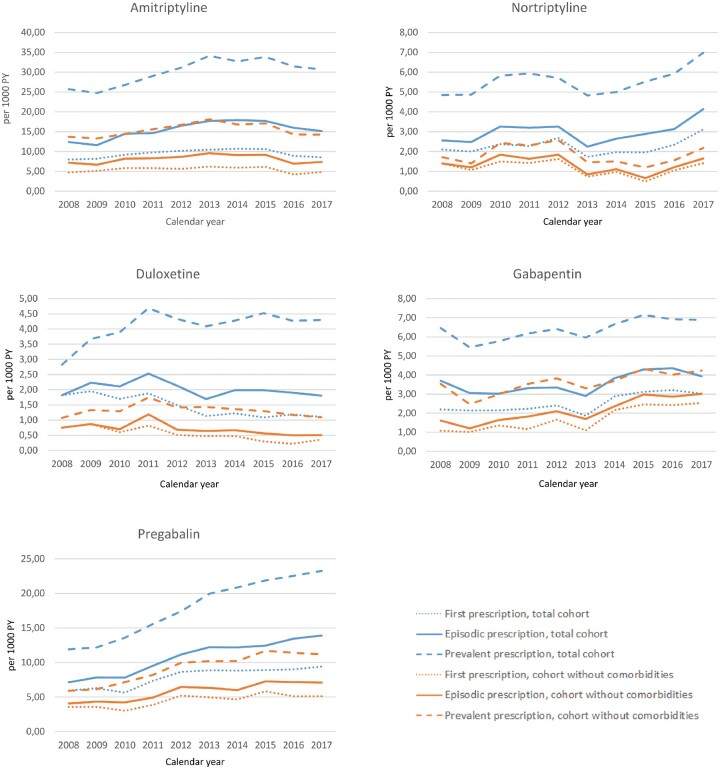
In the study period, the first prescription rates of gabapentin and pregabalin increased from 2.2 to 3.0 prescriptions per 1000 person-years [AAPC 12.0% (95% CI: 6.6, 17.8)] and from 6.0 to 9.4 prescriptions per 1000 person-years [AAPC 4.8% (95% CI: 0.9, 8.8)], respectively (Fig. 1 and Table 2). The first prescription rates of amitriptyline and nortriptyline remained stable. The first prescription rate of duloxetine decreased. The trends were similar for the subset of the cohort without comorbidities. The absolute number of prescriptions was lower. The prescription rates were similar for patients with incident and prevalent OA (see Supplementary Fig. S1 for prescription rates in incident and prevalent OA and Supplementary Tables S3 and S4 for the absolute numbers, available at Rheumatology online.)
Fig. 1.
Prescription rates of antidepressant and anticonvulsant prescriptions in OA patients
PY: person-years.
Table 2.
Time trends in prescription rates of antidepressants and anticonvulsants
| Overall trend [AAPC (95% CI)] | Joinpoint (95% CI) | Trend 1 | Trend 2 | ||
|---|---|---|---|---|---|
| APC (95% CI) | APC (95% CI) | ||||
| TOTAL COHORT | |||||
| Amitriptyline | First | 0.4 (−1.5, 2.3) | 2014 (2012, 2015) | 5.2 (2.3, 8.2) | −8.6 (−13.1, −3.9) |
| Episodic | 2.6 (0.6, 4.6) | 2014 (2012, 2015) | 7.6 (4.5, 10.8) | −6.7(−11.2, −2.0) | |
| Prevalent | 3.1 (1.2, 4.9) | 2013 (2012, 2015) | 7.5 (3.8, 11.4) | −2.2 (−5.0, 0.6) | |
| Nortriptyline | First | 5.0 (−1.3, 11.6) | |||
| Episodic | 3.7 (−1.2, 8.9) | ||||
| Prevalent | 2.8 (−0.3, 6.0) | ||||
| Duloxetine | First | −7.0 (−9.7, −4.1) | |||
| Episodic | −2.4 (−5.1, 0.3) | ||||
| Prevalent | 3.8 (0.1, 7.7) | 2011 (2010, 2012) | − | −0.6 (−2.7, 1.6) | |
| Gabapentin | First | 5.9 (2.3, 9.7) | |||
| Episodic | 4.1 (1.0, 7.4) | ||||
| Prevalent | 2.4 (0.9, 3.8) | ||||
| Pregabalin | First | 6.6 (2.9, 10.4) | 2012 (2010, 2015) | 12.6 (2.4, 23.8) | 1.9 (−1.5, 5.5) |
| Episodic | 8.2 (5.9, 10.5) | 2013 (2011, 2014) | 12.0 (7.2, 17.0) | 3.5 (0.3, 6.8) | |
| Prevalent | 8.8 (7.7, 9.9) | 2013 (2012, 2015) | 12.7(10.4, 15.0) | 4.1 (2.7, 5.6) | |
| WITHOUT COMORBIDITIES | |||||
| Amitriptyline | First | −1.8 (−5.5, 2.1) | |||
| Episodic | 0.8 (−3.8, 5.6) | 2013 (2011, 2015) | 7.5 (−1.6, 17.5) | −7.1 (−14.2, 0.7) | |
| Prevalent | 1.2 (−1.6, 4.0) | 2013 (2012, 2015) | 7.3 (1.8, 13.0) | −5.9 (−10.3, −1.3) | |
| Nortriptyline | First | −2.6 (−11.1, 6.6) | |||
| Episodic | −2.2 (−10.5, 6.8) | ||||
| Prevalent | −2.4 (−9.6, 5.4) | ||||
| Duloxetine | First | −12.3 (−17.5, −6.8) | |||
| Episodic | −7.9 (−13.2,−2.4) | ||||
| Prevalent | 0.2 (−3.5, 4.0) | 2011 (2010, 2012) | 14.4 (−0.4, 31.3) | −6.3 (−8.7, −3.8) | |
| Gabapentin | First | 12.0 (6.6, 17.8) | |||
| Episodic | 9.9 (6.2, 13.8) | ||||
| Prevalent | 4.1 (1.5, 6.9) | ||||
| Pregabalin | First | 4.8 (0.9, 8.8) | |||
| Episodic | 6.3 (3.4, 9.2) | ||||
| Prevalent | 8.9 (5.1, 12.9) | 2012 (2011, 2015) | 16.5 (5.8, 28.3) | 3.2 (−0.2, 6.8) | |
Time trends for episodic prescriptions
In the period 2008–17, amitriptyline was the drug most often prescribed in patients with OA (Fig. 1). The incident prescription rate was 15.1 per 1000 person-years in 2017. The episodic prescription rates for amitriptyline, gabapentin and pregabalin increased over that time period. The increase in prescription rates for pregabalin was most pronounced, from 7.1 per 1000 person-years in 2008 to 13.8 per 1000 person-years in 2017. This was an average annual percentage change (AAPC) of 8.3% (95% CI: 5.9, 10.5) (Table 2). The prescription rates for nortriptyline and duloxetine remained stable over this decade. Absolute prescription rates were lower in the subset of patients without comorbidities than in the total cohort (Fig. 1). The prescription rate for pregabalin almost doubled in this subset as well, from 4.1 to 7.1 prescriptions per 1000 person-years. The prescription rates for amitriptyline remained relatively stable in this subset, at around 8.0 prescriptions per 1000 person-years, while there was an increase in the total cohort. The increase in prescription rates of gabapentin in this subset was from 1.6 to 3.0 [AAPC 9.9% (95% CI: 6.2, 13.8)] and was more pronounced than in the total cohort.
Time trends for prevalent prescriptions
The time trends for the prevalent prescription rates showed similar patterns to the episodic prescription rates (Fig. 1). In the total population, prescription rates increased for amitriptyline, gabapentinand and pregabalin (Table 2). In addition, the prescription rate of duloxetine increased [AAPC 3.8% (95% CI: 0.1, 7.7)]. The prescription rate for pregabalin almost doubled from 11.9 prescriptions per 1000 person-years in 2008 to 23.3 prescriptions per 1000 person-years in 2017. Prescription rates were lower in the subset of patients without comorbidities. The prescription rate for gabapentin and pregabalin increased in this subset, while the prescription rates for the other medications remained stable.
Rates for long-term prescription
Long-term prescription was defined as the prescription of antidepressants or anticonvulsants for more than 3 months in a follow-up year. In patients with incident OA, ∼40% of the patients who were prescribed an antidepressant had a long-term prescription for that antidepressant (Table 3). In the patients who were prescribed an anticonvulsant, this figure was 30%.
Table 3.
Long-term prescription of antidepressants and anticonvulsants in patients with incident OA
| Age | Amitriptyline | Nortriptyline | Duloxetine | Gabapentin | Pregabalin | |
|---|---|---|---|---|---|---|
| N (% users/% population) | N (% users/% population) | N (% users/% population) | N (% users/% population) | N (% users/% population) | ||
| Female | 30–49 | 98 (44.1/2.9) | 10 (41.7/0.3) | 20 (64.5/1.9) | 11 (33.3/0.3) | 41 (38.0/1.2) |
| 50–69 | 392 (41.7/2.5) | 61 (46.2/0.4) | 66 (53.7/0.3) | 72 (34.0/0.5) | 233 (37.4/1.5) | |
| 70+ | 246 (40.7/2.4) | 67 (49.6/0.7) | 24 (36.9/0.4) | 52 (34.2/0.5) | 142 (36.1/1.4) | |
| Male | 30–49 | 29 (37.6/1.4) | 8 (47.1/0.4) | 6 (46.2/2.3) | 3 (11.1/0.1) | 17 (27.4/0.8) |
| 50–69 | 140 (40.2/1.5) | 25 (38.5/0.3) | 19 (45.2/0.5) | 31 (28.4/0.3) | 111 (40.1/1.2) | |
| 70+ | 71 (42.3/1.5) | 12 (32.4/0.2) | 4 (20.0/0.4) | 10 (21.3/0.2) | 59 (37.6/1.2) |
Characteristics associated with antidepressant and anticonvulsant prescriptions
A higher age at baseline was associated with higher episodic prescription rates of amitriptyline, nortriptyline, gabapentin (except for age ≥ 90) and pregabalin (Table 4, multivariable analyses; see Supplementary Table S5, available at Rheumatology online, for univariable analyses). Nortriptyline in particular was prescribed in elderly patients [age ≥ 90: OR 2.23 (1.18–4.20)]. Prescription rates for duloxetine declined with increasing age. Men were less likely to be prescribed antidepressants and anticonvulsants than women. Furthermore, patients with ≥2 joint groups affected were more likely to be prescribed duloxetine, gabapentin and pregabalin than patients with spinal OA, while patients with knee, hip or other peripheral OA were less likely to be prescribed amitriptyline, nortriptyline and pregabalin than patients with spinal OA (Table 4 and Supplementary Fig. S2 and Table S6, available at Rheumatology online). Finally, patients who visited their GP regarding other musculoskeletal disorders during the cohort period were more likely to be prescribed antidepressants and anticonvulsants; this was especially marked for patients with ≥2 musculoskeletal disorders.
Table 4.
Multivariable regression analyses characteristics associated with antidepressant or anticonvulsant prescription
| Amitriptyline |
Nortriptyline |
Duloxetine |
Gabapentin |
Pregabalin |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | OR (95% CI) | n (%) | OR (95% CI) | n (%) | OR (95% CI) | n (%) | OR (95% CI) | n (%) | OR (95% CI) | |
| Age category | ||||||||||
| 30–39 | 86 (3.3) | 1 | 13 (0.5) | 1 | 26 (1.0) | 1 | 15 (0.6) | 1 | 67 (2.5) | 1 |
| 40–49 | 685 (5.7) | 1.69 (1.34, 2.12) | 107 (0.9) | 1.73 (0.97, 3.07) | 126 (1.0) | 1.00 (0.65, 1.52) | 125 (1.0) | 1.70 (0.99, 2.91) | 429 (3.6) | 1.34 (1.03, 1.74) |
| 50–59 | 1863 (5.7) | 1.62 (1.30, 2.03) | 275 (0.8 | 1.59(0.91, 2.78) | 301 (0.9) | 0.83 (0.55, 1.24) | 402 (1.2) | 1.90 (1.13, 3.19) | 1234 (3.8) | 1.37 (1.06, 1.76) |
| 60–69 | 2494 (5.1) | 1.50 (1.20, 1.87) | 462 (0.9) | 1.86 (1.07, 3.24) | 300 (0.6) | 0.56 (0.37, 0.84) | 675 (1.4) | 2.10 (1.26, 3.51) | 1905 (3.9) | 1.46 (1.14, 1.86) |
| 70–79 | 2361 (5.8) | 1.63 (1.31, 2.03) | 549 (1.3) | 2.56 (1.47, 4.46) | 270 (0.7) | 0.57 (0.37, 0.85) | 590 (1.4) | 2.06 (1.23, 3.46) | 1794 (4.4) | 1.59 (1.24, 2.04) |
| 80–89 | 1248 (5.3) | 1.49 (1.19, 1.87) | 365 (1.5) | 2.98 (1.71, 5.21) | 133 (0.6) | 0.49 (0.32, 0.74) | 295 (1.2) | 1.79 (1.06, 3.02) | 868 (3.7) | 1.36 (1.05, 1.75) |
| > 90 | 126 (3.6) | 1.05 (0.80, 1.40) | 39 (1.0) | 2.23 (1.18, 4.20) | 9 (0.2) | 0.72 (0.11, 0.50) | 20 (0.5) | 0.86 (0.78, 0.95) | 85 (2.3) | 0.98 (0.71, 1.36) |
| Gender | ||||||||||
| Female | 7237 (6.3) | 1 | 1371 (1.3) | 1 | 862 (0.8) | 1 | 1495 (1.4) | 1 | 4570 (4.3) | 1 |
| Male | 2202 (3.6) | 0.62 (0.59, 0.65) | 439 (0.8) | 0.69 (0.63, 0.76) | 303 (0.5) | 0.72 (0.63, 0.82) | 627 (1.1) | 0.86 (0.78, 0.95) | 1812 (3.2) | 0.84 (0.79, 0.89) |
| Joint affected | ||||||||||
| Spine | 1138 (6.2) | 1 | 207 (1.2) | 1 | 131 (0.8) | 1 | 196 (1.1) | 1 | 739 (4.3) | 1 |
| Hip | 1256 (4.2) | 0.70 (0.64, 0.76) | 277 (1.0) | 0.82 (0.78, 0.98) | 166 (0.6) | 0.91 (0.73, 1.15) | 348 (1.3) | 1.14 (0.96, 1.37) | 868 (3.1) | 0.76 (0.69, 0.84) |
| Knee | 2043 (4.3) | 0.70 (0.65, 0.74) | 387 (0.9) | 0.72 (0.61, 0.85) | 230 (0.5) | 0.75 (0.60, 0.93) | 543 (1.2) | 1.09 (0.93, 1.29) | 1363 (3.0) | 0.73 (0.67, 0.80) |
| Other peripheral joints | 1957 (4.8) | 0.73 (0.67, 0.79) | 362 (1.0) | 0.81 (0.69, 0.97) | 250 (0.7) | 0.84 (0.68, 1.04) | 305 (0.8) | 0.71 (0.60, 0.84) | 1238 (3.3) | 0.76 (0.69, 0.83) |
| Two or more joints | 3045 (7.9) | 1.04 (0.96, 1.11) | 577 (1.6) | 1.00 (0.85, 1.18) | 388 (1.1) | 1.34 (1.09, 1.64) | 730 (2.0) | 1.48 (1.26, 1.74) | 2174 (6.0) | 1.14 (1.04, 1.24) |
| MSD | ||||||||||
| No MSD | 1373 (2.3) | 1 | 322 (0.6) | 1 | 198 (0.3) | 1 | 352 (0.6) | 1 | 802 (1.4) | 1 |
| Upper extremity | 240 (3.0) | 1.28 (1.11, 1.48) | 60 (0.8) | 1.44 (1.09, 1.90) | 32 (0.4) | 1.17 (0.80, 1.70) | 66 (0.9) | 1.43 (1.10, 1.86) | 190 (2.5) | 1.79 (1.53, 2.10) |
| Lower extremity | 718 (4.0) | 1.78 (1.62, 1.95) | 135 (0.8) | 1.42 (1.16, 1.74) | 73 (0.4) | 1.23 (0.94, 1.61) | 200 (1.2) | 1.87 (1.57, 2.22) | 456 (2.7) | 1.93 (1.72, 2.17) |
| Neck/back | 690 (5.5) | 2.43 (2.21, 2.68) | 157 (1.3) | 2.39 (1.98, 2.90) | 90 (0.8) | 2.12 (1.65, 2.72) | 154 (1.3) | 2.12 (1.75, 2.57) | 519 (4.5) | 3.19 (2.85, 3.65) |
| Other MSD | 485 (4.2) | 1.76 (1.58, 1.96) | 105 (1.0) | 1.72 (1.38, 2.15) | 49 (0.5) | 1.23 (0.90, 1.69) | 110 (1.0) | 1.63 (1.31, 2.02) | 278 (2.6) | 1.81 (1.57, 2.07) |
| ≥2 MSD | 5933 (9.1) | 3.89 (3.65, 4.14) | 1031 (1.7) | 2.97 (2.62, 3.38) | 723 (1.2) | 3.07 (2.61, 3.60) | 1240 (2.1) | 3.15 (2.79, 3.56) | 4137 (6.9) | 4.82 (4.46, 5.21) |
Values in bold are statistically significant (P< 0.05). The multivariable regression analyses were adjusted for age, gender, joint affected and musculoskeletal disorders. MSD: musculoskeletal disorders.
Discussion
In this study, we examined the prescription rates and time trends in prescription rates for antidepressants and anticonvulsants in patients with OA. We found an increase in episodic and prevalent prescription rates for amitriptyline, gabapentin and pregabalin and an increase in first prescription rates for gabapentin and pregabalin between 2008 and 2017. The increase was most pronounced for pregabalin, for which the episodic prescription rate increased from 7.1 to 13.8 per 1000 person-years. The prescription rates for nortriptyline and duloxetine remained stable over time. Amitriptyline was the most prescribed drug in patients with OA. Prescriptions were positively associated with a higher age at baseline (except for duloxetine), the diagnosis of other musculoskeletal disorders during the cohort period, and the diagnosis of OA in two or more joint groups when compared with a diagnosis of spinal OA. The diagnosis of OA in hip, knee or other peripheral joints was associated with lower prescription rates for amitriptyline, nortriptyline and pregabalin compared with spinal OA.
The prevalent prescription rates we found were lower than the rates in other studies. Two US insurance claim studies of OA patients found that ∼30% of the population was prescribed an antidepressant in a year [28, 29]. Another US insurance claim study, which included patients with at least one opioid prescription in a 2-year period, found that 10% of the patients were prescribed an antidepressant and 7% an anticonvulsant, which could also be prescribed for pain [30]. These studies were cross-sectional and examined the prescriptions of all antidepressants and anticonvulsants [28–30, 34], whereas a specific selection was made in our study. Moreover, earlier studies of antidepressant prescription rates found that these rates were lower in the Netherlands than in other countries [35]. Furthermore, we included all patients with an incident or prevalent diagnosis of OA in the cohort (including people with total hip or total knee replacements), while other studies included patients with a medication claim or opioid use, which could also be a reason for the lower numbers in our study.
Time trends for prescription rates for antidepressants and anticonvulsants have been examined in the general population in various countries. The prescription rates of TCAs have remained stable or even decreased in the past decade [33, 35], and TCAs are prescribed more frequently for indications other than depression and anxiety, e.g. for neuropathic pain disorders, sleeping disorders and other off-label indications [33]. One study evaluating the prescription rates of antidepressants by British GPs found a stable prescription rate for TCAs, but an increase in prescriptions of low-dose amitriptyline, indicating prescribing for indications other than depression and anxiety [36]. We have found an increase in prescription rates of amitriptyline and nortriptyline in the total cohort of OA patients, in contrast to the stable prescription rate other studies have found. This reflects the increase in the prescription for indications other than depression and anxiety.
The prescription rate of pregabalin almost doubled in our population between 2008 and 2017, and the prescription rate of gabapentin also increased. The rapid increase in prescriptions of gabapentin and pregabalin is also found in the general population [37, 38]: up to a tripling of prescription rates in the past decade has been reported in the UK, with incident prescription rates of 6.8 per 1000 persons per year for gabapentin and 3.8 per 1000 persons per year for pregabalin [37]. For OA, a retrospective cohort study in the UK found an almost tripled rate of first prescriptions of gabapentinoids between 2005 and 2015, with a prescription rate of 27.6 per 1000 person-years in 2015 [27]. In this study, we found a less steep increase and lower absolute numbers. In the Netherlands, data from pharmacies show a tripling of prescription rates for pregabalin and relatively stable rates of gabapentin prescriptions [39]. Pregabalin was registered earlier than gabapentin for neuropathic pain disorders in the Netherlands [40], which may have influenced prescription rates. Concerns about this increase have been raised [41, 42]. The use is associated with side effects, especially in older patients [43], and gabapentin and pregabalin are often prescribed off-label without enough clinical evidence to support prescription. Only a few studies on anticonvulsants for OA pain have been carried out. In addition, there is some evidence for the misuse and abuse of gabapentin and pregabalin [44, 45]. In the UK, gabapentin and pregabalin became controlled class C drugs in April 2019 since the number of deaths related to these medications had increased [46].
We found that increasing age, being female, the presence of other musculoskeletal disorders and the location of OA (spinal OA, and OA in ≥2 joint groups) were positively associated with prescriptions of antidepressants and anticonvulsants. Increasing age and being female are also found to be associated with higher prescription rates in other studies [34, 36, 47]. To our knowledge, the effect of the presence of other musculoskeletal disorders and the type of joints involved in OA has not previously been investigated. It might be that GPs see more reason to prescribe these medicines to patients with back complaints and to patients with more generalized pain.
We calculated the time trends of prescription rates for all patients and for patients without comorbidities. The absolute numbers of prescriptions were lower in the OA group without comorbidities. Also, in this subgroup an increase in prescription rates was found for gabapentin and pregabalin but not for the antidepressants. This subgroup is more likely to consist of patients for whom these medications are prescribed for OA-related pain, but even in patients without comorbidities for which these medications are usually prescribed, antidepressants and anticonvulsants may have been prescribed for indications other than OA-related pain. Anxiety, depression and sleep disorders are associated with pain and functional impairment and are more common in OA patients [48–50]. A GP might decide that antidepressants or anticonvulsants could benefit patients with these comorbidities since multiple problems exist.
A strength of the current study is that it was conducted using a large database containing a representative sample of the Dutch population. There are some limitations to the current study. Patients were only included in the cohort when diagnosed with OA. Since GPs may have been too rigorous or not rigorous enough in deciding when to use these ICPC codes, this may have led to an underestimation or overestimation of the number of patients with OA. UK primary healthcare database research found that OA is probably under-recorded in patients having total hip or knee replacements [51]. This would mean that underestimation of the total number of patients with OA is more likely. Furthermore, as mentioned earlier, the indication for the prescriptions of antidepressants and anticonvulsants is not always clear in the medical records, and prescriptions may relate to another indication than OA-related pain.
In conclusion, prescription rates of amitriptyline, gabapentin and pregabalin increased in the past decade in patients with OA. Prescription rates of duloxetine and nortriptyline remained stable. This rise is concerning since these prescriptions are for off-label indications and there is little evidence to support the prescription of these antidepressants and anticonvulsants in patients with OA. Since these medicines have side effects, and concerns about the misuse of gabapentin and pregabalin have been raised, these medications should be prescribed with caution for OA-related pain.
Supplementary Material
Acknowledgements
J.J.v.d.D., D.S., M.d.W., P.J.E.B., J.v.d.L. and S.M.A.B.-Z. participated in the design of the study. J.J.v.d.D. and M.d.W. conducted statistical analysis. J.J.v.d.D., D.S., M.d.W., P.J.E.B., J.v.d.L. and S.M.A.B.-Z. were involved in the writing, editing and approval of the final manuscript.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: S.M.A.B.-Z. reports grants from The Netherlands Organisation for Health Research and Development, the Dutch Arthritis Association, the European Commission 7th Framework Programme, the European Institute of Innovation and Technology Health and the Foundation for Research in Rheumatology, personal fees from Infirst healthcare and personal fees from Osteoarthritis Research Society International, outside the submitted work. J.v.d.L. reports grants from The Netherlands Organisation for Health Research and Development, the European Commission Framework Programme and a number of pharmaceutical companies, outside the submitted work. The other authors have declared no conflicts of interest.
Data availability
The aggregated data are available from the corresponding author upon reasonable request.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Johnson VL, Hunter DJ.. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol 2014;28:5–15. [DOI] [PubMed] [Google Scholar]
- 2. Hunter DJ, Bierma-Zeinstra S.. Osteoarthritis. Lancet 2019;393:1745–59. [DOI] [PubMed] [Google Scholar]
- 3. Cross M, Smith E, Hoy D. et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;73:1323–30. [DOI] [PubMed] [Google Scholar]
- 4. Zhang W, Doherty M, Arden N. et al. EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2005;64:669–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McAlindon TE, Bannuru RR, Sullivan MC. et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014;22:363–88. [DOI] [PubMed] [Google Scholar]
- 6. Leopoldino AO, Machado GC, Ferreira PH. et al. Paracetamol versus placebo for knee and hip osteoarthritis. Cochrane Database Syst Rev 2019;2:CD013273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeng C, Wei J, Persson MSM. et al. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: a systematic review and network meta-analysis of randomised controlled trials and observational studies. Br J Sports Med 2018;52:642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. da Costa BR, Reichenbach S, Keller N. et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet 2017;390:e21–e33. [DOI] [PubMed] [Google Scholar]
- 9. Schmidt M, Sorensen HT, Pedersen L.. Diclofenac use and cardiovascular risks: series of nationwide cohort studies. BMJ 2018;362:k3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ackerman IN, Zomer E, Gilmartin-Thomas JF, Liew D.. Forecasting the future burden of opioids for osteoarthritis. Osteoarthritis Cartilage 2018;26:350–5. [DOI] [PubMed] [Google Scholar]
- 11. Thorlund JB, Turkiewicz A, Prieto-Alhambra D, Englund M.. Opioid use in knee or hip osteoarthritis: a region-wide population-based cohort study. Osteoarthritis Cartilage 2019;27:P871–7. [DOI] [PubMed] [Google Scholar]
- 12. van den Driest JJ, Schiphof D, de Wilde M. et al. Opioid prescriptions in patients with osteoarthritis: a population-based cohort study. Rheumatology 2020; Advance Access published 20 January 2020, doi: 10.1093/rheumatology/kez646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Noble M, Treadwell JR, Tregear SJ. et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev 2010;2010:CD006605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheatle MD. Prescription opioid misuse, abuse, morbidity, and mortality: balancing effective pain management and safety. Pain Med 2015;16:S3–8. [DOI] [PubMed] [Google Scholar]
- 15. Schaible HG. Mechanisms of chronic pain in osteoarthritis. Curr Rheumatol Rep 2012;14:549–56. [DOI] [PubMed] [Google Scholar]
- 16. Hochman JR, Davis AM, Elkayam J, Gagliese L, Hawker GA.. Neuropathic pain symptoms on the modified painDETECT correlate with signs of central sensitization in knee osteoarthritis. Osteoarthritis Cartilage 2013;21:1236–42. [DOI] [PubMed] [Google Scholar]
- 17. French HP, Smart KM, Doyle F.. Prevalence of neuropathic pain in knee or hip osteoarthritis: a systematic review and meta-analysis. Semin Arthritis Rheum 2017;47:1–8. [DOI] [PubMed] [Google Scholar]
- 18. Chen L, Gong M, Liu G. et al. Efficacy and tolerability of duloxetine in patients with knee osteoarthritis: a meta-analysis of randomized controlled trials. Intern Med J 2019;49:1514–23. [DOI] [PubMed] [Google Scholar]
- 19.National Institute for Health and Care Excellence (NICE). Osteoarthritis: care and management. 2014. https://www.nice.org.uk/guidance/cg177 (1 December 2019, date last accessed).
- 20.NHG. NHG Standaard Niet-traumatische knieklachten. 2016. https://www.nhg.org/standaarden/volledig/nhg-standaard-niet-traumatische-knieklachten#Richtlijnendiagnostiek (15 December 2019, date last accessed).
- 21. Sindrup SH, Otto M, Finnerup NB, Jensen TS.. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol 2005;96:399–409. [DOI] [PubMed] [Google Scholar]
- 22. van den Driest JJ, Bierma-Zeinstra SMA, Bindels PJE, Schiphof D.. Amitriptyline for musculoskeletal complaints: a systematic review. Fam Pract 2017;34:138–46. [DOI] [PubMed] [Google Scholar]
- 23. Hudson B, Williman JA, Stamp LK. et al. Nortriptyline in knee osteoarthritis (NortIKA Study): study protocol for a randomised controlled trial. Trials 2015;16:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kremer M, Salvat E, Muller A, Yalcin I, Barrot M.. Antidepressants and gabapentinoids in neuropathic pain: mechanistic insights. Neuroscience 2016;338:183–206. [DOI] [PubMed] [Google Scholar]
- 25. Ohtori S, Inoue G, Orita S. et al. Efficacy of combination of meloxicam and pregabalin for pain in knee osteoarthritis. Yonsei Med J 2013;54:1253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sofat N, Harrison A, Russell MD. et al. The effect of pregabalin or duloxetine on arthritis pain: a clinical and mechanistic study in people with hand osteoarthritis. J Pain Res 2017;10:2437–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Appleyard T, Ashworth J, Bedson J, Yu D, Peat G.. Trends in gabapentinoid prescribing in patients with osteoarthritis: a United Kingdom national cohort study in primary care. Osteoarthritis Cartilage 2019;27:1437–44. [DOI] [PubMed] [Google Scholar]
- 28. Gore M, Tai KS, Sadosky A, Leslie D, Stacey BR.. Clinical comorbidities, treatment patterns, and direct medical costs of patients with osteoarthritis in usual care: a retrospective claims database analysis. J Med Econ 2011;14:497–507. [DOI] [PubMed] [Google Scholar]
- 29. Gore M, Tai KS, Sadosky A, Leslie D, Stacey BR.. Use and costs of prescription medications and alternative treatments in patients with osteoarthritis and chronic low back pain in community-based settings. Pain Pract 2012;12:550–60. [DOI] [PubMed] [Google Scholar]
- 30. Kozma CM, Provenzano DA, Slaton TL, Patel AA, Benson CJ.. Complexity of pain management among patients with nociceptive or neuropathic neck, back, or osteoarthritis diagnoses. J Manag Care Spec Pharm 2014;20:455–66b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NHG. NHG Standaard Pijn (M106). 2018. https://richtlijnen.nhg.org/standaarden/pijn (15 December 2019, date last accessed).
- 32. Ferguson RJ, Prieto-Alhambra D, Walker C. et al. Validation of hip osteoarthritis diagnosis recording in the UK Clinical Practice Research Datalink. Pharmacoepidemiol Drug Saf 2019;28:187–93. [DOI] [PubMed] [Google Scholar]
- 33. Noordam R, Aarts N, Verhamme KM. et al. Prescription and indication trends of antidepressant drugs in the Netherlands between 1996 and 2012: a dynamic population-based study. Eur J Clin Pharmacol 2015;71:369–75. [DOI] [PubMed] [Google Scholar]
- 34. Gisev N, Nielsen S, Campbell G. et al. Antidepressant use among people prescribed opioids for chronic noncancer pain. Pain Med 2019;20:2450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abbing-Karahagopian V, Huerta C, Souverein PC. et al. Antidepressant prescribing in five European countries: application of common definitions to assess the prevalence, clinical observations, and methodological implications. Eur J Clin Pharmacol 2014;70:849–57. [DOI] [PubMed] [Google Scholar]
- 36. Lockhart P, Guthrie B.. Trends in primary care antidepressant prescribing 1995–2007: a longitudinal population database analysis. Br J Gen Pract 2011;61:e565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johansen ME. Gabapentinoid use in the United States 2002 through 2015. JAMA Intern Med 2018;178:292–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Montastruc F, Loo SY, Renoux C.. Trends in first gabapentin and pregabalin prescriptions in primary care in the United Kingdom, 1993–2017. Jama 2018;320:2149–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stichting Farmaceutische Kengetallen, 2019. Anti-epileptica niet alleen bij epilepsie. https://www.sfk.nl/publicaties/PW/2017/anti-epileptica-niet-alleen-bij-epilepsie (24 December 2019, date last accessed).
- 40.Zorginstituut Nederland, 2008 CFH-rapport 08/18 Anti-epileptica cluster 0N03AXB0. https://www.zorginstituutnederland.nl/publicaties/rapport/2008/07/28/anti-epileptica-cluster-0n03axb0 (24 December 2019, date last accessed).
- 41. Goodman CW, Brett AS.. Gabapentin and pregabalin for pain – is increased prescribing a cause for concern? N Engl J Med 2017;377:411–4. [DOI] [PubMed] [Google Scholar]
- 42. Wallach JD, Ross JS.. Gabapentin approvals, off-label use, and lessons for postmarketing evaluation efforts. Jama 2018;319:776–8. [DOI] [PubMed] [Google Scholar]
- 43. Enke O, New HA, New CH. et al. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ 2018;190:E786–E93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Driot D, Jouanjus E, Oustric S, Dupouy J, Lapeyre-Mestre M.. Patterns of gabapentin and pregabalin use and misuse: results of a population-based cohort study in France. Br J Clin Pharmacol 2019;85:1260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schifano F. Misuse and abuse of pregabalin and gabapentin: cause for concern? CNS Drugs 2014;28:491–6. [DOI] [PubMed] [Google Scholar]
- 46. Mayor S. Pregabalin and gabapentin become controlled drugs to cut deaths from misuse. BMJ 2018;363:k4364. [DOI] [PubMed] [Google Scholar]
- 47. Baftiu A, Johannessen Landmark C, Rusten IR. et al. Changes in utilisation of antiepileptic drugs in epilepsy and non-epilepsy disorders-a pharmacoepidemiological study and clinical implications. Eur J Clin Pharmacol 2016;72:1245–54. [DOI] [PubMed] [Google Scholar]
- 48. Pickering ME, Chapurlat R, Kocher L, Peter-Derex L.. Sleep disturbances and osteoarthritis. Pain Pract 2016;16:237–44. [DOI] [PubMed] [Google Scholar]
- 49. Rathbun AM, Stuart EA, Shardell M, Yau MS. et al. Dynamic effects of depressive symptoms on osteoarthritis knee pain. Arthritis Care Res 2018;70:80–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Scopaz KA, Piva SR, Wisniewski S, Fitzgerald GK.. Relationships of fear, anxiety, and depression with physical function in patients with knee osteoarthritis. Arch Phys Med Rehabil 2009;90:1866–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yu D, Jordan KP, Peat G.. Underrecording of osteoarthritis in United Kingdom primary care electronic health record data. Clin Epidemiol 2018;10:1195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The aggregated data are available from the corresponding author upon reasonable request.