Abstract
Objectives
The classification of seronegative arthritides can be challenging. Our aim was to examine the incidence of SpA diagnosis among patients initially diagnosed as seronegative RA.
Methods
Using nationwide Finnish registers from social insurance institutions, we identified all adult patients who were diagnosed with incident seronegative RA [International Classification of Diseases (ICD)-10 code M06] from 1 January 2000 to 31 December 2014. The patients whose diagnoses subsequently changed to the ICD-10 codes of SpA (M07, M45, M46, K50 and K51) were identified in the national care register, until 31 December 2016.
Results
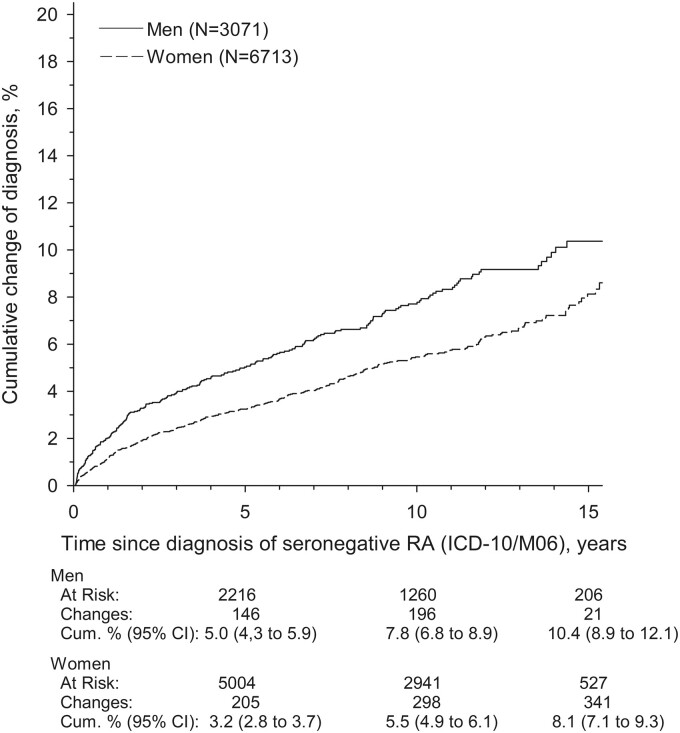
A total of 9784 adult seronegative RA patients were identified. Of these, 564 patients had their diagnosis subsequently changed to SpA: 275 (48.7%) patients with PsA, 245 (43.4%) patients with axial SpA and 44 (7.8%) patients with diagnoses related to IBD. The cumulative incidence of SpA diagnoses in 15 years was 10.4% (95% CI 8.9, 12.1) and 8.1% (95% CI 7.1, 9.3) in men and women, respectively.
Conclusion
This study calls for vigilance in seronegative RA patients, especially those with more atypical presentations, since the diagnosis could change. The possibility of SpA diagnosis should be considered and specifically looked for, as this could impact on management and response to treatment.
Keywords: seronegative rheumatoid arthritis, outcomes, spondyloarthropathy
Rheumatology key messages
Seronegative arthritis represents a heterogeneous group of various disease entities and can pose diagnostic challenges.
Long-term follow-up and observation is necessary, looking for any change in phenotypic and other characteristics.
Seronegative arthritis can evolve into different inflammatory arthritis categories over time, including SpA.
Introduction
Seronegative RA has long been recognized as a phenotype of RA without the presence of RF, and more recently ACPAs. However, seropositive and seronegative RA seem to ‘behave’ differently during the course of illness and in various ways [1].
Published studies support disparate disease mechanisms in seropositive vs seronegative RA, with different genetic and environmental risk factors [2–4]. Some studies implicate that seronegative patients may present with more severe clinical manifestations at baseline than seropositive patients, and in fact the current classification criteria require high disease activity in seronegative cases [5, 6]. On the other hand, in studies involving both early RA and undifferentiated arthritis (UA) patients, seronegative patients seem to have better prognosis in spite of higher disease activity at disease onset [5, 7]. Concerning the risk of radiographic progression, several studies report it to be lower in seronegative patients, including a long-term follow-up study of RA patients over 15–20 years [7–9]. The findings concerning the treatment outcomes between seronegative and seropositive patients vary across the studies reviewed [6, 10, 11]. Despite the aetiopathological and clinical differences between seronegative and seropositive RA patients, both clinical RA trials and real-life cohort studies have included up to a third seronegative patients [9, 12].
Due to the heterogeneous nature of seronegative arthritides it is impossible predict the prognosis of these patients at disease onset. Some patients may require intense and long-standing therapy with DMARDs, but many do not develop chronic or destructive inflammatory disease in the long run. Since there is no differential diagnostic test available for this heterogeneous group of arthritides, it is imperative that these patients are followed up closely, observing disease course and evolution to enable stricter classification.
Our previous study indicated that during a 10-year follow-up, a majority of the patients in a clinical cohort who were originally diagnosed as seronegative RA could be classified with a more specific rheumatic disease, including a quarter of patients who were reclassified as SpA cases [1]. In the current study we aimed to evaluate the nationwide incidence of new SpA diagnoses among patients initially diagnosed as seronegative RA in Finland by using national registry data.
Methods
Setting
According to national guidelines, patients with RA are diagnosed and cared for in rheumatology outpatient clinics in Finland. Finland has a statutory national social insurance system, organized by the Social Insurance Institution (SII) of Finland, which provides social security coverage for all Finnish residents. This social security provides various benefits, including reimbursement of medical expenses. Patients with long-term inflammatory rheumatic disease such as RA, SpA and PsA can be granted a special reimbursement (40–65% of the total cost) for DMARDs (conventional and biologic) after submitting a medical certificate provided by a rheumatologist to the SII. This special reimbursement for conventional DMARDs is granted for the rest of one’s life. If a patient’s diagnosis is later changed, for example from seronegative RA to SpA diagnosis, there is no need to apply for a new reimbursement. Only if a patient requires treatment with biologics a new medical certificate is needed for SII, and then it should be done with the possible updated diagnosis. The SII maintains a register on the reimbursements, including patient’s age, sex, International Classification of Diseases (ICD)-10 code of the illness and date of entitlement.
In addition to the SII register, many public registries in Finland provide nationwide, reliable data covering all residents [13]. The Care Registers for Social Welfare and Health Care (HILMO) is a nationwide register in Finland maintained by The National Institute for Health and Welfare (THL). The HILMO register is systematically quality controlled and the completeness of the register has been found to be very good [14]. The HILMO register includes a variety of data on outpatient visits in primary and secondary care, including all the diagnoses made in outpatient Finnish rheumatology clinics. Furthermore, possible changed diagnoses are also registered into the HILMO register. Each Finnish resident has a unique identification code that is used in all registers and allows linking of the data between various registers.
From the SII national register data, we identified all patients (aged ≥18 years) granted the first special reimbursement related to medications prescribed for a diagnosis of seronegative RA [classified according to the ICD-10 code as seronegative RA (M06)] from 1 January 2000 to 31 December 2014. The date of the first reimbursement decision was defined as the index date in this study. Among these seronegative RA patients we identified all patients with new SpA diagnoses registered in the HILMO register after the index date until 31 December 2016, including PsA, non-radiographic axial SpA (nr-axSpA), AS and IBD, such as ulcerative colitis and Crohn’s disease. We included only diagnoses made by specialists in rheumatology clinics. The patients with SpA diagnoses were classified according to the ICD code into three groups: PsA (M07), axSpA including AS and nr-axSpA (M45-M46) and IBD including Crohn’s disease and ulcerative colitis (K50–K51). Reactive arthritis could not be reliably analysed from the register data due to the natural, short-term duration of the disease and thus the lack of follow-up in secondary care.
Permission to use the databases was obtained from the SII and National Institute for Health and Welfare. No additional ethical approval was necessary as this was a register-based study without any direct patient contact.
Statistical methods
Time-to-event analysis was based on the Kaplan–Meier failure function. Kaplan–Meier curves were also adjusted for sex using inverse probability weighting. Hazard ratios and 95% CIs were estimated using Cox proportional hazards regression models. The appropriateness of the Cox proportional hazards assumption was further visualized using log–log survival plots, that is, plotting log {–log[S(t)] against log(t)}. A possible non-linear (effect modification) relationship between gender and age was assessed by using a restricted cubic spline Cox model (with four knots, placed according to Harrell’s recommended percentiles). The knots were located at the 5th, 35th, 65th and 95th percentiles. All analyses were performed using STATA 11 (StataCorp LP, College Station, TX, USA).
Results
Over the years 2000–2014 there were altogether 18 163 (66.9% female) patients with a diagnosis of seropositive RA and a total of 9784 patients (68.6% female) with a diagnosis of seronegative RA who were entitled to the use of reimbursed DMARDs in the SII register. Among the 9784 seronegative RA patients a total of 564 patients (61% female) had a subsequent new diagnosis of SpA (PsA, nr-axSpA, AS, IBD) in the HILMO register, providing a period prevalence of 5.5%. The mean age (s.d.) of these patients at the index date was 45 (14) years, while the mean (s.d.) age of the patients whose diagnosis remained as seronegative RA was 56 (16) years. The 564 patients who emerged with SpA diagnoses included 275 (48.8%) PsA patients, 245 (43.4%) nr-axSpA, AS patients and 44 (7.8%) IBD patients.
The cumulative incidence of the SpA diagnoses was 3.8% (95% CI 3.4, 4.2) in 5 years, 6.2% (95% CI 5.6, 6.7) in 10 years and 8.8% (95% CI 8.8, 9.8) in 15 years. The cumulative incidence of the SpA diagnoses was higher among men compared with women (Fig. 1).
Fig. 1.
The cumulative change of diagnosis
The cumulative incidence of new SpA diagnosis (PsA, axial SpA and IBD) between men and women in patients initially diagnosed as seronegative RA during years 2000 and 2014.
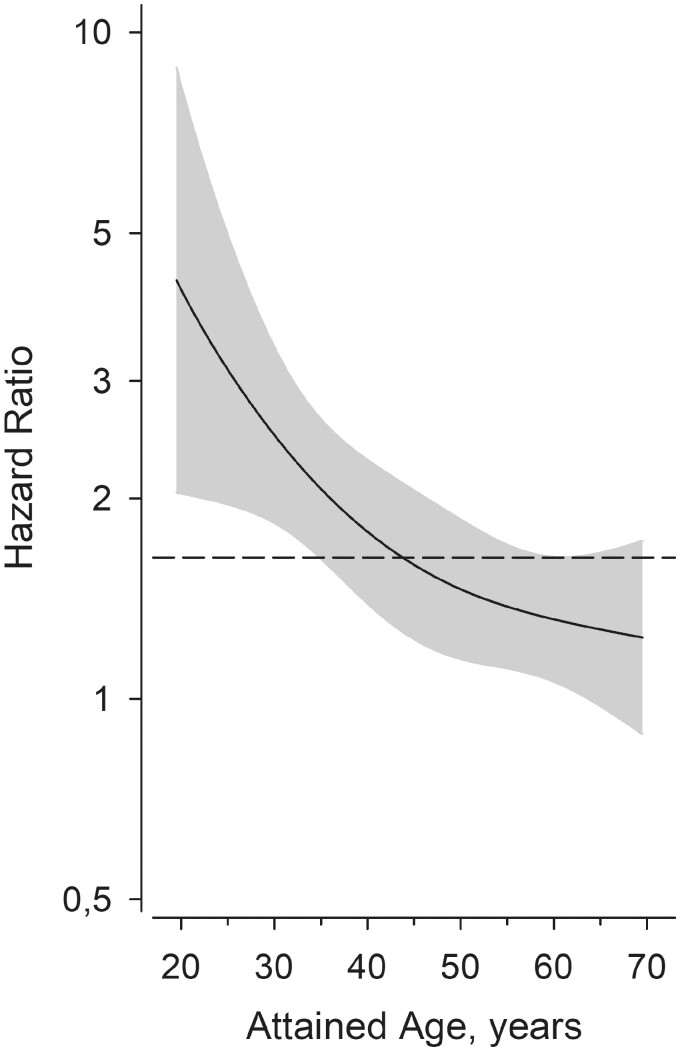
Male gender and younger age at the time of diagnosis in males was associated with greater probability for the change of diagnosis from seronegative RA to a SpA diagnosis (Fig. 2).
Fig. 2.
The attained age and the risk of change of diagnosis
The age at diagnosis of seronegative RA and its effect on the risk of a new SpA diagnosis (PsA, axial SpA and IBD), adjusted to the age and sex. Grey area shows the 95% CIs.
Discussion
The differential diagnosis possibilities of seronegative arthritis are extensive. The group of SpA comprises a number of closely related rheumatic diseases, including PsA, nr-axSpA, AS, IBD-related arthritis, reactive arthritis and undifferentiated SpA. The clinical presentation of SpA conditions is heterogeneous and can include inflammatory back pain, oligoarthritis predominantly of the lower limbs, dactylitis and enthesitis, but extra-articular manifestations, such as uveitis, psoriasis and IBD, may also be present [15]. The group of SpA conditions is one of the differential diagnoses for early seronegative RA [1]. Our present study supports this view. These results also emphasize the importance of long-term follow-up of patients, which can help reveal the true nature of their disease.
We previously published a 10-year follow-up study of 435 early seronegative RA patients from a rheumatology outpatient clinic in Finland [1]. We found substantial heterogeneity in diagnoses during follow-up, including over a dozen diagnostic groups. SpA conditions represented the largest group with 24% of the patients, including PsA, peripheral SpA, nr-axSpA, AS, IBD and reactive arthritis. Another group in Finland followed 64 seronegative UA patients over 20 years. The majority of these patients [40/64 (63%)] evolved in terms of their diagnosis into mild spondyloarthritides [16].
The proportion of SpA patients in the present register-based study is considerably smaller compared with the earlier findings from ordinary rheumatology clinics. In fact, it is possible that the proportion of diagnoses changing from seronegative RA to SpA is higher in real life than recorded in our present register-based study. For example, patients who receive reimbursed DMARD treatment on the grounds of their arthritis do not necessary always develop extra-articular manifestations (such as colitis or psoriasis) due to the medication masking them, and therefore the correct diagnosis cannot be made. In particular, if the clinical disease is mild and responsive to DMARD treatment these patients do not necessarily develop SpA-related extra-articular manifestations over time. Further, if the patient outcomes are mild with no need for continuing visits to specialist rheumatology clinics, the possible SpA-related symptoms and signs can remain unnoticed, and therefore possible updates to the diagnosis missed.
National registers provide a great source of information, allowing for instance the analysis of the incidence of various rheumatic diseases and secular trends. For example, in Finland a declining trend in the incidence of seronegative RA has been reported recently, whereas the incidence of seropositive RA has remained stable and the incidence of UA increased [17]. The shifting profiles in incidences of seronegative RA and UA are probably at least partly a reflection of the greater application of the present 2010 ACR/EULAR RA classification criteria, which emphasizes the positive serology [18]. Even though patients are mainly diagnosed on clinical grounds, the classification criteria of individual rheumatic diseases can guide the rheumatologists towards formulating an earlier and more accurate diagnosis. Considering the changes in classification criteria of rheumatic diseases and the evolving diagnostic procedures, it is likely that patients’ initial diagnoses will also continue to change over time.
The role of imaging in general, and in the diagnosis, management and follow-up of patients with inflammatory arthritis, has dramatically increased [19]. The accuracy of diagnostics is improved with the use of imaging. For example, the use of US has become a promising and user-friendly diagnostic means to detect synovitis for rheumatologists [20]. With the help of imaging modalities, such as radiographs, US and especially with MRI, rheumatologists are now in a position to do more precise differential diagnostics at an earlier phase of the disease [21]. In fact, the combination of an expert interpretation of MRI and US findings along with the reasoning of the clinical findings of an experienced rheumatologist greatly enhances the detection of peripheral manifestations (such as enthesitis), and thus the diagnostics of spondyloarthritides [22, 23].
In the current study, PsA was the largest group within the new SpA diagnoses. The diagnosis of PsA can be challenging in the early phase. PsA can have various sub-types and joint involvement can be highly variable. It may appear as oligoarthritis, but it can also involve the small joints of hands and feet and behave in a similar way to RA (RA-like) [21]. Furthermore, it is estimated that arthritis precedes the skin disease in up to 15% of PsA patients [24]. If an initial manifestation of PsA is polyarthritis in small joints, it can be falsely diagnosed as seronegative RA. However, one distinguishing feature between RA-like PsA and true RA, apart from the skin involvement, is enthesitis. Enthesitis is not a common clinical finding in RA, whereas it is common in PsA, with a reported prevalence up to 35% [25, 26].
Concerning axSpA, the axial manifestations are again not always present at baseline. It is estimated that peripheral manifestations, such as arthritis and enthesitis, are found in 30–50% of the SpA patients at presentation or even in the history [27]. By following patients with seronegative arthritis systematically and over time, and by being vigilant to evolving signs and symptoms, rheumatologists will be in a better position to detect possible signs and symptoms of PsA or other SpA-related diseases, and thus a potential change in the diagnosis and management.
A limitation of our study is that the two nationwide registers used in the study do not include any clinical data on the fulfilment of the classification criteria. Another limitation is that it is possible that not all SpA-related diagnoses are systematically registered in rheumatology clinics and further into the HILMO register. After the diagnosis of seronegative RA is made it is possible that clinicians as a rule are content with the existing diagnosis, and there is no interest in altering or, put to it better, changing the diagnosis. However, the ICD code data in the Finnish nationwide registers are based on registered clinical diagnoses made in rheumatology clinics by specialists, with reliable diagnoses expected. The strengths of the study are the large national data gathered into nationwide registers and the long observation period. Moreover, studies concerning long-term seronegative RA follow-up are limited. To our knowledge, this is the first study to specifically examine the incidence of new SpA diagnoses in patients initially diagnosed as seronegative RA. Our present study strengthens the knowledge of seronegative patients’ long-term outcome in a real-life setting.
In conclusion, we found that the 15-year cumulative incidence for changing diagnosis to SpA diagnoses in seronegative RA patients in Finland diagnosed between 2000 and 2015 was 8.8%. It is of note that male patients and those diagnosed with seronegative RA before the age of 50 years appear to be more prone to a change in their diagnosis to SpA over the years. Our study calls for vigilance in seronegative RA patients, since the SpA diagnosis can become apparent over time, necessitating longer-term follow-up and observation. Importantly, our findings remind of the need to consider SpA in the differential diagnosis of seronegative RA.
Acknowledgements
The authors would like to thank Hannu Kautiainen for his statistical assistance, as well as the medical staff at the Rheumatology department in Jyväskylä Central Hospital.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: T.S. has received consulting fees from BMS, Roche, Celgene, Medac, Merck, Novartis, UCB, Sandoz, Orion Pharma and Boehringer Ingelheim. The other authors have declared no conflicts of interest.
References
- 1. Paalanen K, Rannio K, Rannio T. et al. Does early seronegative arthritis develop into rheumatoid arthritis? A 10-year observational study. Clin Exp Rheumatol 2019;37:37–43. [PubMed] [Google Scholar]
- 2. Klareskog L, Alfredsson L, Rantapää-Dahlqvist S. et al. What precedes development of rheumatoid arthritis? Ann Rheum Dis 2004;63:ii28–ii31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huizinga TWJ, Amos CI, van der Helm-van Mil AHM. et al. Refining the complex rheumatoid arthritis phenotype based on specificity of the HLA-DRB1 shared epitope for antibodies to citrullinated proteins. Arthritis Rheum 2005;52:3433–8. [DOI] [PubMed] [Google Scholar]
- 4. Lundström E, Källberg H, Alfredsson L, Klareskog L, Padyukov L.. Gene-environment interaction between the DRB1 shared epitope and smoking in the risk of anti-citrullinated protein antibody-positive rheumatoid arthritis: all alleles are important. Arthritis Rheum 2009;60:1597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barra L, Pope JE, Orav JE. et al. ; CATCH Investigators. Prognosis of seronegative patients in a large prospective cohort of patients with early inflammatory arthritis. J Rheumatol 2014;41:2361–9. [DOI] [PubMed] [Google Scholar]
- 6. Nordberg LB, Lillegraven S, Lie E. et al. Patients with seronegative RA have more inflammatory activity compared with patients with seropositive RA in an inception cohort of DMARD-naïve patients classified according to the 2010 ACR/EULAR criteria. Ann Rheum Dis 2017;76:341–5. [DOI] [PubMed] [Google Scholar]
- 7. van der Helm-van Mil AHM, Verpoort KN, Breedveld FC, Toes REM, Huizinga TWJ.. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther 2005;7:R949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Asikainen J, Nikiphorou E, Kaarela K. et al. Is long-term radiographic joint damage different between men and women? Prospective longitudinal data analysis of four early RA cohorts with greater than 15 years of follow-up. Clin Exp Rheumatol 2016;34:641–5. [PubMed] [Google Scholar]
- 9. Goekoop-Ruiterman YPM, de Vries-Bouwstra JK, Allaart CF. et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2005;52:3381–90. [DOI] [PubMed] [Google Scholar]
- 10. Wevers-de Boer K, Visser K, Heimans L. et al. Remission induction therapy with methotrexate and prednisone in patients with early rheumatoid and undifferentiated arthritis (the IMPROVED study). Ann Rheum Dis 2012;71:1472–7. [DOI] [PubMed] [Google Scholar]
- 11. Van den Broek M, Dirven L, Klarenbeek NB. et al. The association of treatment response and joint damage with ACPA-status in recent-onset RA: a subanalysis of the 8-year follow-up of the BeSt study. Ann Rheum Dis 2012;71:245–8. [DOI] [PubMed] [Google Scholar]
- 12. Sokka T, Kautiainen H, Pincus T. et al. Disparities in rheumatoid arthritis disease activity according to gross domestic product in 25 countries in the QUEST–RA database. Ann Rheum Dis 2009;68:1666–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sokka T. National databases and rheumatology research I: longitudinal databases in Scandinavia. Rheum Dis Clin 2004;30:851–67. [DOI] [PubMed] [Google Scholar]
- 14. Sund R. Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health 2012;40:505–15. [DOI] [PubMed] [Google Scholar]
- 15. van Tubergen A, Weber U.. Diagnosis and classification in spondyloarthritis: identifying a chameleon. Nat Rev Rheumatol 2012;8:253–61. [DOI] [PubMed] [Google Scholar]
- 16. Jäntti JK, Kaarela K, Lehtinen KES.. Seronegative oligoarthritis: a 23-year follow-up study. Clin Rheumatol 2002;21:353–6. [DOI] [PubMed] [Google Scholar]
- 17. Muilu P, Rantalaiho V, Kautiainen H. et al. Increasing incidence and shifting profile of idiopathic inflammatory rheumatic diseases in adults during this millennium. Clin Rheumatol 2019;38:555–62. [DOI] [PubMed] [Google Scholar]
- 18. Aletaha D, Neogi T, Silman AJ. et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–8. [DOI] [PubMed] [Google Scholar]
- 19. Mandl P, Navarro-Compán V, Terslev L. et al. EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann Rheum Dis 2015;74:1327–39. [DOI] [PubMed] [Google Scholar]
- 20. Epis O, Paoletti F, d'Errico T. et al. Ultrasonography in the diagnosis and management of patients with inflammatory arthritides. Eur J Intern Med 2014;25:103–11. [DOI] [PubMed] [Google Scholar]
- 21. Felbo SK, Terslev L, Ostergaard M.. Imaging in peripheral and axial psoriatic arthritis: contributions to diagnosis, follow-up, prognosis and knowledge of pathogenesis. Clin Exp Rheumatol 2018;36 (Suppl 1):24–34. [PubMed] [Google Scholar]
- 22. Taniguchi Y, Kumon Y, Takata T. et al. Imaging assessment of enthesitis in spondyloarthritis. Ann Nucl Med 2013;27:105–11. [DOI] [PubMed] [Google Scholar]
- 23. Fournié B, Margarit-Coll N, Champetier de Ribes TL. et al. Extrasynovial ultrasound abnormalities in the psoriatic finger. Prospective comparative power-doppler study versus rheumatoid arthritis. Joint Bone Spine 2006;73:527–31. [DOI] [PubMed] [Google Scholar]
- 24. Ritchlin CT, Colbert RA, Gladman DD.. Psoriatic arthritis. N Engl J Med 2017;376:957–70. [DOI] [PubMed] [Google Scholar]
- 25. Veale DJ, Fearon U.. What makes psoriatic and rheumatoid arthritis so different? RMD Open 2015;1:e000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Polachek A, Li S, Chandran V, Gladman DD.. Clinical enthesitis in a prospective longitudinal psoriatic arthritis cohort: incidence, prevalence, characteristics, and outcome. Arthritis Care Res (Hoboken) 2017;69:1685–91. [DOI] [PubMed] [Google Scholar]
- 27. Sieper J, Poddubnyy D.. Axial spondyloarthritis. Lancet 2017;390:73–84. [DOI] [PubMed] [Google Scholar]