Abstract
Objective
The efficacy of the novel interleukin (IL)-23p19 inhibitor guselkumab for psoriatic arthritis (PsA) has recently been demonstrated in two phase 3 trials (DISCOVER-1 & -2) but has not been evaluated vs other targeted therapies for PsA. The objective was to compare guselkumab to targeted therapies for PsA for safety and joint and skin efficacy through network meta-analysis (NMA).
Methods
A systematic literature review was conducted in January 2020 to identify randomized controlled trials. Bayesian NMAs were performed to compare treatments on American College of Rheumatology (ACR) 20/50/70 response, mean change from baseline in van der Heijde-Sharp (vdH-S) score, Psoriasis Area Severity Index (PASI) 75/90/100 response, adverse events (AEs) and serious adverse events (SAEs).
Results
Twenty-six phase 3 studies evaluating 13 targeted therapies for PsA were included. For ACR 20 response, guselkumab 100 mg every 8 weeks (Q8W) was comparable to IL-17A inhibitors and subcutaneous tumor necrosis factor (TNF) inhibitors. Similar findings were observed for ACR 50 and 70. For vdH-S score, guselkumab Q8W was comparable to other agents except intravenous TNF therapies. Results for PASI 75 and PASI 90 response suggested guselkumab Q8W was better than most other agents. For PASI 100, guselkumab Q8W was comparable to other active agents. For AEs and SAEs, guselkumab Q8W ranked highly but comparative conclusions were uncertain. Similar results were observed for all outcomes for guselkumab 100 mg every four weeks.
Conclusions
In this NMA, guselkumab demonstrated favorable arthritis efficacy comparable to IL-17A and subcutaneous TNF inhibitors while offering better PASI response relative to many other treatments.
Keywords: guselkumab, psoriatic arthritis, interleukin, TNF, biologics, NMA, SLR, ACR, PASI
Rheumatology key messages
Guselkumab provides better PASI responses than many other agents available in PsA.
Guselkumab offers joint efficacy comparable to IL-17A and subcutaneous TNF inhibitors available in PsA.
Introduction
Psoriatic arthritis (PsA) is a clinically heterogeneous, progressive and chronic inflammatory condition that can cause irreversible joint damage and impact patient quality of life [1–4]. Treatment guideline recommendations for patients with active PsA depend on a variety of factors, including the PsA domain(s) involved (e.g. peripheral arthritis, axial disease, enthesitis, dactylitis, skin psoriasis, nail psoriasis), disease severity and line of therapy [5–7]. Current treatment options for PsA include non-biologic disease-modifying antirheumatic drugs (DMARDs; i.e. methotrexate, sulfasalazine, ciclosporin and leflunomide), biologic therapies (i.e. infliximab, golimumab, adalimumab, etanercept, certolizumab pegol, abatacept, ustekinumab, secukinumab and ixekizumab) and targeted synthetic DMARDs (i.e. apremilast and tofacitinib). These biologic and targeted therapies are generally indicated for use alongside optional concomitant DMARD treatment.
Guselkumab is a monoclonal antibody currently approved for the treatment of psoriasis and also for psoriatic arthritis in some regions [8]. Guselkumab offers a novel mechanism of action. It binds selectively to the p19 subunit of interleukin (IL)-23 with high specificity and affinity. Interleukin-23, a regulatory cytokine, affects the differentiation, expansion, and survival of T-cell subsets and innate immune cell subsets, which represent sources of effector cytokines that drive inflammatory disease [9, 10]. Guselkumab targets and inhibits the p19 subunit of IL-23, resulting in the disruption of IL-23-mediated signalling, activation and cytokine cascades, leading to clinical improvement in symptoms of psoriasis and PsA [11–14]. The efficacy and safety of guselkumab 100 mg every 8 weeks (Q8W) and 100 mg every 4 weeks (Q4W) was demonstrated in the placebo-controlled DISCOVER-1 and DISCOVER-2 phase 3 trials, the first to evaluate the efficacy of a selective IL-23p19 inhibitor in PsA [13, 14].
Although there are previous studies comparing IL-23 inhibitors in similar disease areas, such as the ECLIPSE trial evaluating guselkumab in psoriasis, [11] few head-to-head studies comparing biologic and targeted interventions have been conducted in PsA [15, 16]. Therefore, indirect comparisons are needed to inform the comparative efficacy and safety of guselkumab vs other targeted therapies. Network meta-analysis (NMA) is a widely used approach for comparing treatment effectiveness that synthesizes both direct and indirect evidence [17–19]. Several NMAs have compared the efficacy of treatments available for PsA, but none of these analyses have included phase 3 data for selective IL-23 inhibitors (i.e. guselkumab) [20–23]. Therefore, the objective of this study was to determine the relative skin and joint efficacy and safety of guselkumab compared with other targeted therapies available for PsA at the end of the induction period (i.e. 12–24 weeks) through NMA.
Materials and methods
The methods and reporting used in this review adhere to rigorous guidance documents designed to ensure the robustness of analyses and reproducibility of findings. The protocol for the SLR and NMA was drafted a priori, submitted to PROSPERO in September 2019, and was published in April 2020 (CRD42020152614). Both the methods and results of this study have been described as outlined by the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [24] and the corresponding extension statement for NMA [25].
Systematic review
A rigorous electronic search of the literature was designed in collaboration with an experienced information specialist (Supplementary Data S1, available at Rheumatology online). The strategy was peer reviewed by a second independent information specialist using the Peer Review of Electronic Search Strategies (PRESS) framework [26] prior to execution. The search covered multiple databases including EMBASE, MEDLINE® and Cochrane Central on the OVID platform. The original search was conducted in October 2018 and subsequently updated in January 2020 to expand the comparator scope.
Study selection
Predefined study eligibility criteria were used to screen all identified citations (Supplementary Table S1, available at Rheumatology online). Two reviewers independently screened the abstracts, with disagreements settled by discussion or involvement of a third reviewer, if needed. The same process was followed for review of full-text articles to establish final study selection.
Data extraction and study quality assessment
Data extraction was performed by one reviewer and validated by a second reviewer. Data were collected from the included studies using a structured form designed in Microsoft Excel (Microsoft Corporation, Seattle, WA, USA). The data collected consisted of information regarding publication characteristics, study populations, interventions and comparators studied, outcomes reported (namely summary measures such as the number of events and sample size for dichotomous outcomes) and study design. The National Institute for Health and Care Excellence (NICE) clinical effectiveness quality assessment checklist was used to appraise the validity of included studies [27].
Network meta-analysis
All NMAs were performed using a Bayesian framework [28–30]. Network diagrams were drawn to visualize the evidence base for each analysis. Placebo was used as the reference treatment throughout. Different doses of the same pharmaceutical were treated as separate interventions (e.g. guselkumab Q8W and Q4W). Outcomes of interest included American College of Rheumatology (ACR) 20/50/70 response, mean change from baseline in van der Heijde-Sharp (vdH-S) score, Psoriasis Area Severity Index (PASI) 75/90/100 response, as well as adverse events (AEs) and serious adverse events (SAEs). For ACR and PASI responses, analyses used data from the primary timepoint of assessment for each study, which varied from 12 to 24 weeks. For vdH-S score, analyses used data at 24 weeks as it was the only timepoint feasible for analyses during the placebo-controlled period. For safety outcomes, the latest placebo-controlled timepoint was used. An NMA model for dichotomous outcomes was used to compare interventions for ACR, PASI, AEs and SAEs, while an NMA model for continuous outcomes was used to derive comparisons between interventions for vdH-S score. Models appropriately accounted for multi-arm trials. Treatment effects for dichotomous outcomes were modeled on the log-odds ratio scale and transformed to relative risks (RR) using the unweighted average of trial placebo responses. For continuous outcomes, treatment effects were modeled and reported on the mean difference (MD) scale. Treatments populated entirely by zero events were dropped from networks of evidence. Convergence was monitored quantitatively using the latest implementation Gelman-Rubin diagnostic (Rhat) based on four chains [31] (Supplementary Data S2, available at Rheumatology online). Models were fit using four chains and used vague or weakly informative priors (Supplementary Data S3, available at Rheumatology online). All NMAs were performed using R (R Core Team, Vienna, Austria) and JAGS, based on the code adapted from the NICE Evidence Synthesis Decision Support Unit (DSU) Technical Support Document (TSD) Series [32–34]. An unrelated mean effects model was used to test for the presence of inconsistency.
Adjustment for heterogeneity
Given differences in patient characteristics and study designs (e.g. inclusion of bio-naïve and bio-experienced patients) and evidence of clinical heterogeneity highlighted by previous PsA publications and studies in similar theraueptic areas (i.e. psoriasis), heterogeneity was expected within networks [22, 35–39]. For this reason, random effects models were conducted by default, with fixed effect models considered when evidence networks were constructed entirely of connections with no more than two studies. In addition, adjustment for variation in placebo response through meta-regression on baseline risk was considered and applied where appropriate to further account for heterogeneity. Variation in placebo response represents an important proxy in clinical heterogeneity for both measured and unmeasured confounders [40]. Models that adjusted for placebo response were based on code reported in the NICE DSU TSD 3 [29]. Briefly, a single interaction effect that represents the relative treatment effect comparisons between treatments and placebo was used in all meta-regressions.
Approach to model selection
Assessment of model fit was performed as outlined in the NICE DSU TSD series [32–34]. In addition, the best-fitting model used was kept consistent across outcomes that are derived from the same clinical assessment (i.e. ACR 20/50/70 and PASI 75/90/100), so long as the model provided a reduction in between-trial heterogeneity. For example, if a baseline risk-adjusted model was best (as assessed by model fit diagnostics) for both PASI 75 and PASI 90, then a baseline risk-adjusted model was chosen for PASI 100 if there was a reduction in between-trial heterogeneity between the unadjusted and adjusted models, even if the 95% credible interval (CrI) for the regression coefficient included zero. This model selection approach was motivated by the clinical rationale that the heterogeneity observed in, for example, PASI 75 and PASI 90, should be similar to that observed for PASI 100, as they all involve the assessment of the same clinical characteristics and only vary according to the responder threshold. The lack of a meaningful regression coefficient for rarer outcomes (i.e. PASI 100) is instead likely due to the paucity of data and the inherent uncertainty associated with rare, dichotomous outcomes in NMAs, rather than a lack of meaningful treatment-effect relationship.
Results
Search results and study selection
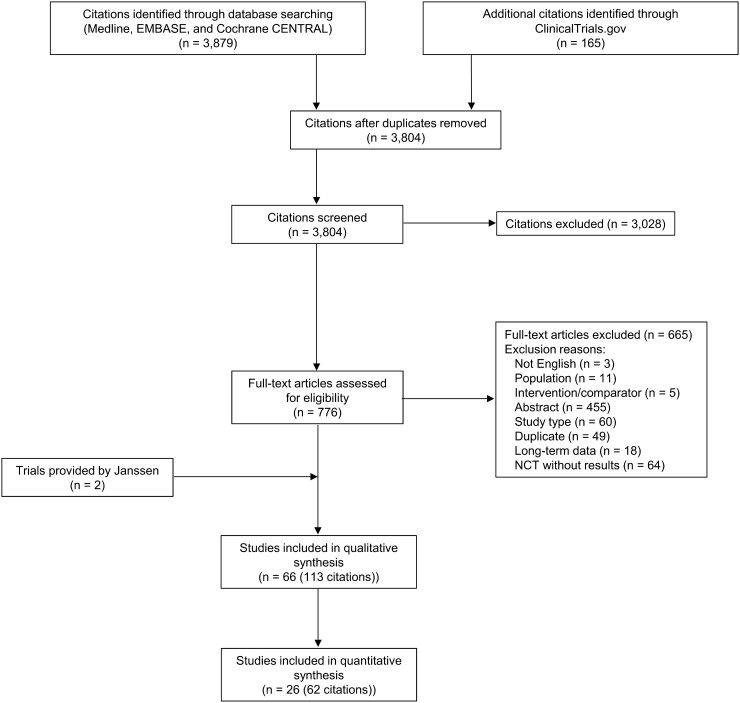
The literature search identified 3,804 unique citations, of which 113 citations reporting on 66 trials were included in the qualitative review. Two relevant clinical trials of guselkumab in PsA were provided directly by the manufacturer as they had not been published at the time of the original search. Of the 66 trials, 26 (62 citations) were included in the quantitative synthesis (i.e. NMA) [41–65]. These phase 3 trials included adults with active PsA that evaluated targeted therapies approved by the European Medicines Agency or the United States Food and Drug Administration. The PRISMA flow diagram for the selection of these studies is presented in Fig. 1.
Fig. 1.
PRISMA flow diagram of study selection for systematic literature review
n: number; NCT: National Clinical Trial.
Study and patient characteristics
The included RCTs evaluated the efficacy and safety of the following targeted therapies: IL-17A inhibitors (ixekizumab, secukinumab), IL-12/23 inhibitors (ustekinumab), tumor necrosis factor alpha (TNF) inhibitors (adalimumab, certolizumab pegol, etanercept, golimumab, infliximab), IL-23 inhibitors (guselkumab), cytotoxic T-lymphocyte-associated antigen 4 inhibitors (abatacept), small molecules (apremilast, tofacitinib), as well as placebo. The studies were published between 2004 and 2019. Baseline and additional study characteristics are summarized in Supplementary Table S2, available at Rheumatology online. Thirteen studies were conducted in biologic-naïve patients, two studies were conducted in biologic-experienced patients, and 11 studies included a mixed population. The timepoint of primary end point assessment varied across studies: week 12 certolizumab pegol, and tofacitinib; week 14 for golimumab; week 14 or 16 for infliximab; week 12 or 24 for adalimumab; week 16 for apremilast; week 24 for abatacept, etanercept, guselkumab, ixekizumab and ustekinumab; week 16 or 24 for secukinumab. Risk of bias assessments for each of the included studies are presented in detail in Supplementary Table S3, available at Rheumatology online. Overall, these assessments found the clinical trials included in NMAs to be of low risk of bias. The allocation concealment, blinding of personnel, and outcome assessment had unclear risk. A high risk of bias was rarely detected in any of the categories for any of the RCTs included in the NMAs.
Network meta-analysis results
In total, 21 distinct interventions were identified from the searches and subsequently included in analyses. No inconsistency was observed across networks (Supplementary Data S4, Supplementary Tables S7 to S15 and Supplementary Figs S1 to S9, available at Rheumatology online). Treatment rankings calculated from each NMA are reported and represent the rank-order of each treatment’s point estimates vs placebo. Rankings do not denote relative treatment effects between active agents, and do not reflect confidence regarding true difference between treatments in pairwise comparisons. To characterize pairwise comparison conclusions from the NMA, key results are presented in-text with forest plots displaying pairwise comparisons of guselkumab Q8W vs other treatments according to median RRs or MDs and 95% CrIs. A 95% CrI represents the interval in which there is a 95% probability that the true treatment effect lies within said interval. Conclusions are summarized by describing treatments as ‘better’ or ‘worse’ than guselkumab if the pairwise 95% CrI excludes no difference (0 for MDs and 1 for RRs), wherein there is a >95% probability that the two treatments are different, and as ‘comparable’ otherwise. The probability of guselkumab being better than a comparator is also shown for additional granularity in pairwise estimates. Supplementary Figs S18 to S26 (available at Rheumatology online) present full league tables and absolute probabilities/scores are presented in Supplementary Table S16, available at Rheumatology online.
Joint efficacy
Acr response
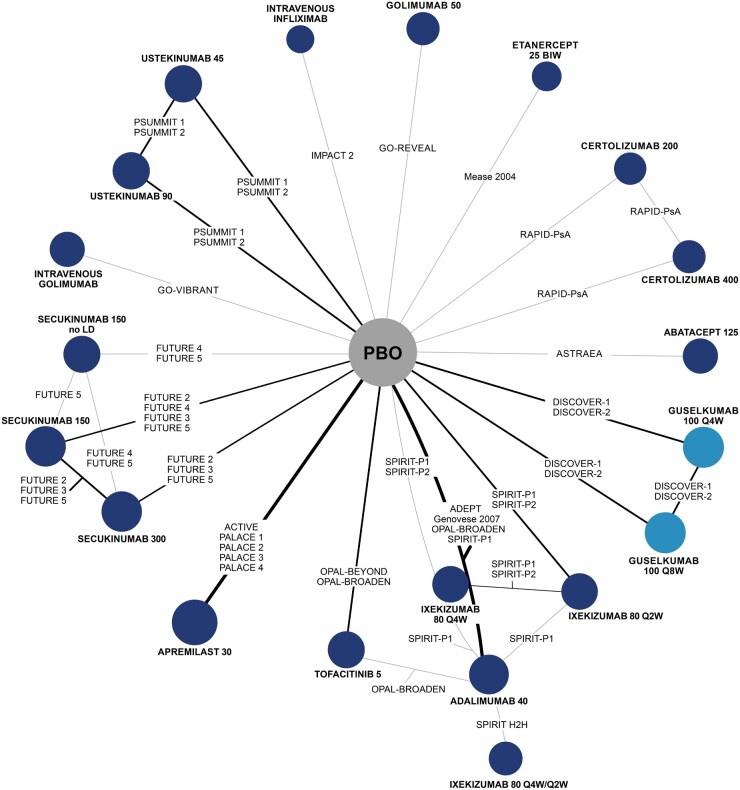
The network diagram of the evidence identified from the literature search and included in the NMA for ACR 20 response is shown in Fig. 2. Network diagrams for other outcomes are shown in Supplementary Figs S10 to S17, available at Rheumatology online. All studies reported ACR 20 and ACR 50 response, and all but one study reported ACR 70 response. Across all ACR outcomes, the baseline risk-adjusted model was a better fit for the data and was therefore used to inform results (Supplementary Table S6, available at Rheumatology online).
Fig. 2.
Evidence network for ACR 20
Treatment nodes are sized to reflect the proportionate number of patients randomized to each treatment in the network. Thickness of lines between nodes corresponds to the number of RCTs connecting treatments. BIW: biweekly; LD: loading dose; PBO: placebo; Q2W: every 2 weeks; Q4W: every 4 weeks; Q8W: every 8 weeks.
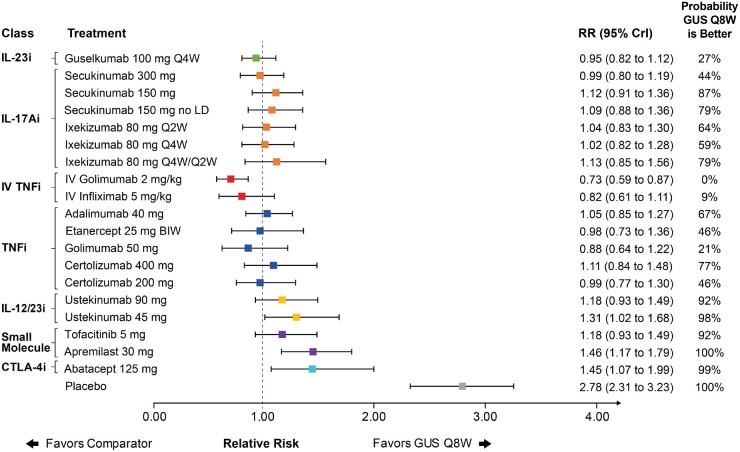
Guselkumab Q8W was ranked eighth in the network and had a comparable ACR 20 response to IL-17A inhibitors and subcutaneous TNF inhibitors as demonstrated by overlap in 95% CrI (Fig. 3). Similar results were observed for guselkumab Q4W in the full league table of results (Supplementary Fig. S18, available at Rheumatology online). Of note, guselkumab Q8W had a better ACR 20 response than ustekinumab 45 mg, abatacept and apremilast, as demonstrated by guselkumab ranking higher and lack of overlap in 95% CrI. Intravenous (IV) golimumab had a better ACR 20 response than guselkumab Q8W, as demonstrated by guselkumab ranking lower and lack of overlap in 95% CrI. Similar results were observed for ACR 50 and 70 response, although additional uncertainty in comparative effect estimates and variable treatment rankings was observed due to the lower baseline event rates associated with ACR 50 and 70 (Supplementary Figs S19 and S20, available at Rheumatology online).
Fig. 3.
Forest plot with pairwise comparisons of guselkumab Q8W vs all comparators for ACR 20
Comparisons are shown in terms of RRs and 95% CrIs. Treatments are grouped by therapeutic class. The vertical dotted line represents a RR of 1.00. The probability that guselkumab Q8W is better is also shown for each comparator. For the full league table of results, please consult the supplementary appendix, available at Rheumatology online. ACR: American College of Rheumatology; BIW: biweekly; CrI: credible interval; CTLA-4i: cytotoxic T-lymphocyte-associated protein 4; GUS: guselkumab; IL-17Ai: interleukin-17A inhibitor; IL-12/23i: interleukin-12/23 inhibitor; IL-23i: interleukin-23 inhibitor; IV: intravenous; LD: loading dose; Q2W: every 2 weeks; Q4W: every 4 weeks; Q8W: every 8 weeks; RR: relative risk; TNFi: tumor necrosis factor inhibitor.
Vdh-S score
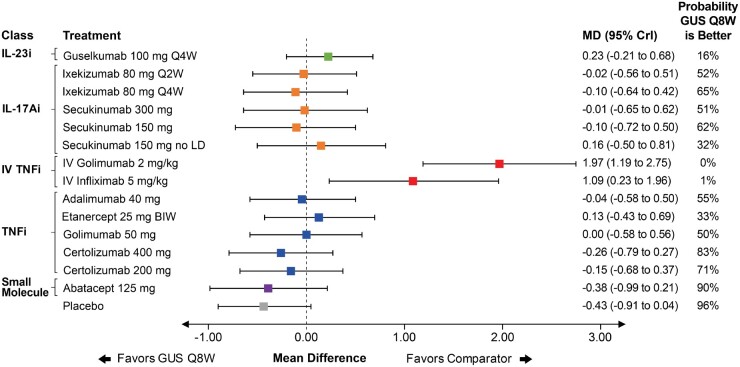
Only nine studies reported a mean change from baseline in vdH-S score. An unadjusted FE model was used to inform results because the evidence network was composed entirely of single-study connections, making random effects models inappropriate and adjustment for baseline risk impossible (Supplementary Table S6, available at Rheumatology online). Guselkumab Q8W ranked ninth in the network and had a comparable change in vdH-S score relative to most other agents as demonstrated by overlap in 95% CrI. Guselkumab Q8W was worse than IV TNF therapies (i.e. golimumab and infliximab) as demonstrated by lower ranking and lack in overlap in 95% CrI (Fig. 4). Results were similar for guselkumab Q4W (Supplementary Fig. S21, available at Rheumatology online).
Fig. 4.
Forest plot with pairwise comparisons of guselkumab Q8W vs all comparators for vdH-S score
Comparisons are shown in terms of MDs and 95% CrIs. Treatments are grouped by therapeutic class. The vertical dotted line represents a MD of 0.00. The probability that guselkumab Q8W is better is also shown for each comparator. For the full league table of results, please consult the supplementary appendix, available at Rheumatology online. BIW: biweekly; CrI: credible interval; CTLA-4i: cytotoxic T-lymphocyte-associated protein 4; GUS: guselkumab; IL-17Ai: interleukin-17A inhibitor; IL-12/23i: interleukin-12/23 inhibitor; IL-23i: interleukin-23 inhibitor; IV: intravenous; LD: loading dose; MD: mean difference; Q2W: every 2 weeks; Q4W: every 4 weeks; Q8W: every 8 weeks; TNFi: tumor necrosis factor inhibitor; vdH-S: van der Heijde-Sharp.
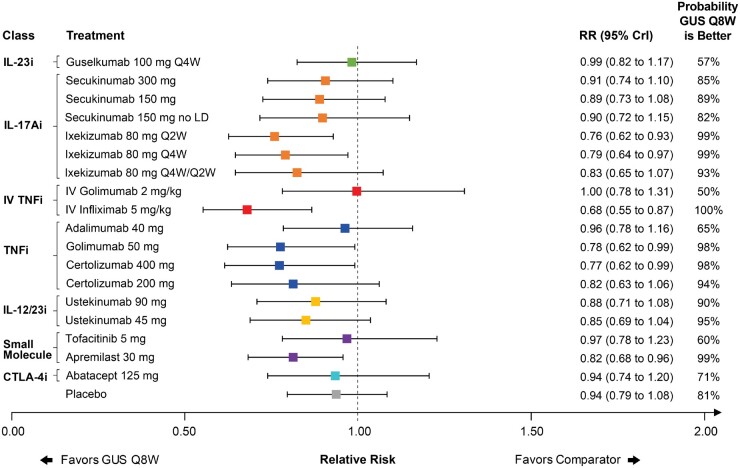
All but two included studies reported PASI 75 response, most reported PASI 90 response, and few reported PASI 100 response. The baseline risk-adjusted model was used for all PASI outcomes (Supplementary Table S6, available at Rheumatology online). Guselkumab Q8W ranked second in the network and had a better PASI 90 response than many other treatments, including TNFs and lower doses of secukinumab, as demonstrated by guselkumab ranking higher and lack of overlap in 95% CrI (Fig. 5). Similar results were observed for PASI 75 response (Supplementary Fig. S22, available at Rheumatology online). For PASI 100, low baseline event rates led to uncertainty in pairwise estimates, with guselkumab Q8W ranking fourth and being comparable to other active treatments, as demonstrated by overlap in 95% CrI (Supplementary Fig. S24, available at Rheumatology online). Comparisons vs guselkumab Q4W were similar to those vs Q8W for all PASI responses (Supplementary Figs S22 to S24, available at Rheumatology online).
Fig. 5.
Forest plot with pairwise comparisons of guselkumab Q8W vs all comparators for PASI 90
Comparisons are shown in terms of RRs and 95% CrIs. Treatments are grouped by therapeutic class. The vertical dotted line represents a RR of 1.00. The probability that guselkumab Q8W is better is also shown for each comparator. For the full league table of results, please consult the supplementary appendix, available at Rheumatology online. BIW: biweekly; CrI: credible interval; CTLA-4i: cytotoxic T-lymphocyte-associated protein 4; GUS: guselkumab; IL-17Ai: interleukin-17A inhibitor; IL-12/23i: interleukin-12/23 inhibitor; IL-23i: interleukin-23 inhibitor; IV: intravenous; PASI: Psoriasis Area Severity Index; LD: loading dose; Q2W: every 2 weeks; Q4W: every 4 weeks; Q8W: every 8 weeks; RR: relative risk; TNFi: tumor necrosis factor inhibitor.
Adverse event outcomes
All but two studies reported AEs while all but one reported SAEs. The baseline risk-adjusted model provided the best fit for both safety outcomes (Supplementary Table S6, available at Rheumatology online). For AEs, guselkumab Q8W ranked highly in the network (Supplementary Fig. S25, available at Rheumatology online) but significant uncertainty in pairwise estimates was observed as demonstrated by overlap in 95% CrI vs most other agents (Fig. 6). Results were similar in analyses of SAEs, where once again guselkumab Q8W ranked highly in the network, but low baseline event rates caused significant uncertainty in pairwise point estimates as demonstrated by overlap in 95% CrI vs most other agents (Supplementary Fig. S26, available at Rheumatology online). Similar results were observed for guselkumab Q4W for both AEs and SAEs (Supplementary Figs S25 and S26, available at Rheumatology online).
Fig. 6.
Forest plot with pairwise comparisons of guselkumab Q8W vs all comparators for AEs
Comparisons are shown in terms of RRs and 95% CrIs. Treatments are grouped by therapeutic class. The vertical dotted line represents a RR of 1.00. The probability that guselkumab Q8W is better is also shown for each comparator. For the full league table of results, please consult the supplementary appendix, available at Rheumatology online. AEs: adverse events; CrI: credible interval; CTLA-4i: cytotoxic T-lymphocyte-associated protein 4; GUS: guselkumab; IL-17Ai: interleukin-17A inhibitor; IL-12/23i: interleukin-12/23 inhibitor; IL-23i: interleukin-23 inhibitor; IV: intravenous; LD: loading dose; Q2W: every 2 weeks; Q4W: every 4 weeks: Q8W: every 8 weeks; RR: relative risk; TNFi: tumor necrosis factor inhibitor.
Discussion
Given the number of treatment options available in PsA, combined with the clinical complexity of the disease (e.g. involvement of both skin and joints), healthcare decision makers face a challenge to identify the most appropriate treatment option available for patients. Evaluating the comparative safety and efficacy of the treatments available for PsA through NMAs can help inform medical decision-making in the absence of direct evidence from head-to-head RCTs. Within the current NMAs, data from the placebo-controlled period of 26 RCTs were used to derive comparisons of guselkumab Q8W and Q4W with other targeted therapies for the treatment of active PsA.
This is the first NMA in PsA to evaluate the comparative efficacy and safety of the novel IL-23p19 inhibitor, guselkumab, using data from the phase 3 DISCOVER-1 and DISCOVER-2 trials. The results of the NMAs demonstrated that guselkumab Q8W and Q4W were associated with comparable efficacy to IL-17A and subcutaneous TNF inhibitors for both ACR responses and vdH-S score. Guselkumab also had better PASI responses relative to many other treatments, a finding observed in a previous NMA evaluating treatments in psoriasis [34] as well as the head-to-head ECLIPSE study vs secukinumab in psoriasis [11]. Of note, this is one of the first NMAs in PsA to include comparative assessment of structural damage and progression. In addition, guselkumab Q8W and Q4W had the highest PASI 90 responses while also offering ACR 20 responses comparable to IL-17A and subcutaneous TNF inhibitors (see Supplementary Fig. S27, available at Rheumatology online). Lastly, both guselkumab Q8W and Q4W ranked highly in the network for AEs and SAEs but significant uncertainty in pairwise estimates was observed, as demonstrated by overlap in 95% CrI vs most other agents.
A previous review by Lu et al. [23] incorporated phase 2 data for guselkumab in a frequentist NMA. However, the authors did not account for differences in baseline risk across trials, which, according to our review, represents an important source of clinical heterogeneity in PsA. Lu et al. concluded that infliximab, golimumab, guselkumab, adalimumab, secukinumab and ustekinumab might be the safest and most efficacious targeted treatments available for PsA. Our study adds additional granularity to these findings, suggesting that IV TNF therapies offer the highest joint responses and guselkumab offers the highest skin responses.
In addition, our findings are generally well-aligned with previous NMAs in PsA evaluating targeted therapies in PsA. Ruyssen-Witrand et al. [20] recently evaluated the safety and efficacy of biologics in PsA at an earlier 12–16-week period using a baseline risk-adjusted model for ACR and PASI outcomes. The authors found TNF inhibitors offered the highest ACR responses, which agrees with our results. For PASI responses, the authors found that ixekizumab and intravenous infliximab offered the best responses, which also aligns with our observations without the consideration of guselkumab. The authors also found few differences between treatments in evaluations of safety endpoints. Consistency of the current NMA with previous analyses strengthens our conclusions despite the variations in the analytical approaches taken.
The comprehensive search and analyses used in this study have several strengths. All literature search and analytical methods used for this study adhere to various methodological guidelines required by NICE and similar HTAs [32–34]. The protocol for the SLR and NMA was drafted a priori and both the methods and results of this study have been described using the PRISMA statement [24] and the corresponding extension statement for NMA [25]. Analyses adjusted for variation in placebo response across trials, an important proxy for both measured and unmeasured clinical characteristics that may bias treatment effects as observed in a similar therapeutic area, psoriasis [35, 66]. We have also adopted a conservative approach to interpretation of analysis results, relying on overlap of pairwise 95% CrI with no difference to determine comparability or superiority of treatments.
There are some limitations with the current analyses that should be recognized. Although all outcomes were assessed within the context of the placebo-controlled induction period, the timepoint of assessment varied from 12 to 24 weeks for all outcomes except vdH-S score, which had an assessment timepoint of 24 weeks. Despite allowing for comparison of all therapies within the induction period, this variation may introduce some heterogeneity in results. Likewise, this analysis included patients regardless of previous biologic exposure, which may also introduce heterogeneity. Analyses controlling for such heterogeneity will be explored in subsequent studies. Adjustment for placebo response may mitigate some of this clinical heterogeneity by controlling for various effect modifying variables, including unmeasured variables such as practice changes over time, but there may be residual bias that remains. However, as in all clinical trials, patients in RCTs may differ from those treated in contemporary clinics, which may affect the generalizability of findings if the populations differ with respect to important effect modifiers. Further, data limitations and low baseline event rates rendered certain analyses (e.g. PASI 100 and SAEs) highly uncertain, where almost all treatments were considered comparable to one another, as demonstrated by overlap in 95% CrI.
Because PsA is a complex disease involving several clinical domains, the full efficacy profile of treatments, and the overall value to patients, may not be captured by only assessing ACR, vdH-S score, PASI, AEs and SAEs. Analyses of other outcomes or disease domains, such as patient-reported and additional clinical outcomes, will be explored in subsequent studies. Finally, because PsA is a chronic, life-long disease, long-term comparisons should be explored to evaluate the maintenance of treatment response, especially with respect to safety outcomes such as AEs and SAEs. However, long-term NMAs are currently unfeasible due to the lack of a common comparator beyond the placebo-controlled period, combined with a lack of active head-to-head trials in PsA. Therefore, alternative analytical methods may be required to assess relative long-term safety and efficacy of treatments in PsA in future studies.
In conclusion, analyses suggest that guselkumab has joint efficacy (i.e. ACR and vdH-S score) comparable to IL-17A and subcutneous TNF inhibitors while offering particularly robust efficacy on skin manifestations through the placebo-controlled trial period. Guselkumab ranked highly in analyses of AEs and SAEs, but rarity of events led to significant uncertainty in pairwise comparisons. Overall, guselkumab offers favorable outcomes for patients with PsA by improving both rheumatological and dermatological outcomes coupled with a favorable safety profile.
Supplementary Material
Acknowledgements
This work acknowledges the intellectual contributions made by the broader team at EVERSANA and Janssen Pharmaceuticals, including Alicia Pepper, Fareen Hassan, Cheryl Druchok, Raji Rajalingam, Meaghan Bartlett and Sheryl Fogarty.
Author contributions are as follows. Substantial contributions to the conception or design of the work: K.E., S.P., A.S., S.D.C. and C.S.K. Substantial contributions to the acquisition of the data: K.E., S.P. and A.S. Substantial contributions to the analysis of the data: K.E., S.P., A.S. and A.P. Substantial contributions to the interpretation of data for the work, drafting the work or revising it critically for important intellectual content, final approval of the version to be published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated: all authors.
Funding: This work was supported by Janssen Research and Development.
Disclosure statement: P.J.M. consultancies: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Galapagos, Gilead, GlaxoSmithKline, Janssen, Leo, Eli Lilly, Merck, Novartis, Pfizer, Sun Pharmaceutical Industries, INC pharma, UCB, Crescendo Bioscience; member of speakers’ bureAbbVie, Amgen, Bristol-Myers Squibb, Celgene, Genentech, Janssen, Leo, Merck, Novartis, Pfizer, UCB, Crescendo Bioscience; grants/research support: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Leo, Eli Lilly, Merck, Novartis, Pfizer, Sun Pharmaceutical Industries, INC pharma, UCB, Crescendo Bioscience. I.B.M. consultancies: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Eli Lilly, Novartis, Pfizer, UCB; honoraria: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Eli Lilly, Novartis, Pfizer, UCB; grants/research support: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Novartis, Pfizer, UCB. L.-S.T. consultancies: Janssen, Pfizer, Sanofi, AbbVie, Boehringer Ingelheim, Lilly; grant/research support: Amgen, Boehringer Ingelheim, Janssen, GSK, Novartis and Pfizer. K.E. employee of EVERSANA; consultant for Janssen Pharmaceuticals. S.P. employee of Janssen Pharmaceuticals and shareholder of Johnson & Johnson. A.S. employee of Janssen Pharmaceuticals and shareholder of Johnson & Johnson. S.D.C. employee of Janssen Scientific Affairs, LLC, and shareholder of Johnson & Johnson. A.P. employee of EVERSANA; consultant for Janssen Pharmaceuticals. C.S.K. employee of Janssen Pharmaceuticals and shareholder of Johnson & Johnson. S.N. employee of Janssen Pharmaceuticals. W.-H.B. support from AbbVie, Almirall, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis, Pfizer, UCB. C.R. consultancies: AbbVie, Amgen, Janssen, Eli Lilly, Novartis, Pfizer, UCB; grants/research support: AbbVie, Amgen, Janssen, UCB.
Data availability statement
The data underlying this article are sourced from the public domain and are available in the articles cited throughout.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Gudu T, Gossec L.. Quality of life in psoriatic arthritis. Expert Rev Clin Immunol 2018;14:405–17. [DOI] [PubMed] [Google Scholar]
- 2. Giannelli A. A review for physician assistants and nurse practitioners on the considerations for diagnosing and treating psoriatic arthritis. Rheumatol Ther 2019;6:5–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Merola JF, Espinoza LR, Fleischmann R.. Distinguishing rheumatoid arthritis from psoriatic arthritis. RMD Open 2018;4:e000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McArdle A, Pennington S, FitzGerald O.. Clinical features of psoriatic arthritis: a comprehensive review of unmet clinical needs. Clin Rev Allergy Immunol 2018;55:271–94. [DOI] [PubMed] [Google Scholar]
- 5. Ritchlin CT, Colbert RA, Gladman DD.. Psoriatic arthritis. N Engl J Med 2017;376:957–70. [DOI] [PubMed] [Google Scholar]
- 6. Coates LC, Helliwell PS.. Psoriatic arthritis: state of the art review. Clin Med 2017;17:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shah K, Paris M, Mellars L, Changolkar A, Mease PJ.. Real-world burden of comorbidities in US patients with psoriatic arthritis. RMD Open 2017;3:e000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boehncke W-H, Brembilla NC, Nissen MJ.. Guselkumab: the first selective IL-23 inhibitor for active psoriatic arthritis in adults. Expert Rev Clin Immunol 2020;17:5–13. [DOI] [PubMed] [Google Scholar]
- 9. Suzuki E, Mellins ED, Gershwin ME, Nestle FO, Adamopoulos IE.. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev 2014;13:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Girolomoni G, Strohal R, Puig L. et al. The role of IL-23 and the IL-23/TH 17 immune axis in the pathogenesis and treatment of psoriasis. J Eur Acad Dermatol Venereol 2017;31:1616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reich K, Armstrong AW, Langley RG. et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet 2019;394:831–9. [DOI] [PubMed] [Google Scholar]
- 12. Deodhar A, Gottlieb AB, Boehncke WH. et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet 2018;391:2213–24. [DOI] [PubMed] [Google Scholar]
- 13. Deodhar A, Helliwell PS, Boehncke WH. et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFalpha inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2020;395:1115–25. [DOI] [PubMed] [Google Scholar]
- 14. Mease PJ, Rahman P, Gottlieb AB. et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2020;395:1126–36. [DOI] [PubMed] [Google Scholar]
- 15. McInnes IB, Behrens F, Mease PJ. et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3b trial. Lancet 2020;395:1496–505. [DOI] [PubMed] [Google Scholar]
- 16. Mease PJ, Smolen JS, Behrens F, Nash P. et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naive patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis 2020;79:123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salanti G, Higgins JP, Ades AE, Ioannidis JP.. Evaluation of networks of randomized trials. Stat Methods Med Res 2008;17:279–301. [DOI] [PubMed] [Google Scholar]
- 18. Catalá-López F, Tobías A, Cameron C, Moher D, Hutton B.. Network meta-analysis for comparing treatment effects of multiple interventions: an introduction. Rheumatol Int 2014;34:1489–96. [DOI] [PubMed] [Google Scholar]
- 19. Caldwell DM, Ades AE, Higgins JP.. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ruyssen-Witrand A, Perry R, Watkins C. et al. Efficacy and safety of biologics in psoriatic arthritis: a systematic literature review and network meta-analysis. RMD Open 2020;6:e001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kawalec P, Holko P, Mocko P, Pilc A.. Comparative effectiveness of abatacept, apremilast, secukinumab and ustekinumab treatment of psoriatic arthritis: a systematic review and network meta-analysis. Rheumatol Int 2018;38:189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McInnes IB, Nash P, Ritchlin C. et al. Secukinumab for psoriatic arthritis: comparative effectiveness versus licensed biologics/apremilast: a network meta-analysis. J Comp Eff Res 2018;7:1107–23. [DOI] [PubMed] [Google Scholar]
- 23. Lu C, Wallace BI, Waljee AK. et al. Comparative efficacy and safety of targeted DMARDs for active psoriatic arthritis during induction therapy: a systematic review and network meta-analysis. Semin Arthritis Rheum 2019;49:381–8. [DOI] [PubMed] [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P.. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hutton B, Salanti G, Caldwell DM. et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–84. [DOI] [PubMed] [Google Scholar]
- 26. McGowan J, Sampson M, Salzwedel DM. et al. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- 27.(NICE) NIfHaCE. Single technology appraisal: User guide for company evidence submission template (updated April 2017). Available from: https://www.nice.org.uk/process/pmg24/chapter/clinical-effectiveness#quality-assessment-of-the-relevant-clinical-effectiveness-evidence.
- 28. Dias S, Sutton AJ, Ades AE, Welton NJ.. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making 2013;33:607–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dias S, Sutton AJ, Welton NJ, Ades AE.. Evidence synthesis for decision making 3: heterogeneity–subgroups, meta-regression, bias, and bias-adjustment. Med Decis Making 2013;33:618–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dias S, Welton NJ, Sutton AJ. et al. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making 2013;33:641–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vehtari A, Gelman A, Simpson D, Carpenter B, Bürkner P-C. Rank-normalization, folding, and localization: An improved R for assessing convergence of MCMC. Bayesian Anal 2021; doi:10.1214/20-BA1221.
- 32. Dias S, Welton NJ, Sutton AJ, Ades A. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. 2011; http://nicedsu.org.uk/wp-content/uploads/2016/03/A-general-linear-modelling-framework-for-pair-wise-and-network-meta-analysis-of-randomised-controlled-trials..pdf. [PubMed]
- 33. Dias S, Sutton AJ, Welton N, J, Ades A. NICE DSU Technical Support Document 3: Heterogeneity: subgroups, meta-regression, bias and bias-adjustment. 2011. [PubMed]
- 34. Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades A. NICE DSU Technical Support Document 4: Inconsistency in Networks of Evidence Based on Randomised Controlled Trials. 2011. [PubMed]
- 35. Cameron C, Druchok C, Hutton B. et al. Guselkumab for the treatment of moderate-to-severe plaque psoriasis during induction phase: a systematic review and network meta-analysis. J Psoriasis Psoriatric Arthritis 2019;4:81–92. [Google Scholar]
- 36. Nash P, McInnes IB, Mease PJ. et al. Secukinumab versus adalimumab for psoriatic arthritis: comparative effectiveness up to 48 weeks using a matching-adjusted indirect comparison. Rheumatol Ther 2018;5:99–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Strand V, Betts KA, Mittal M. et al. Comparative effectiveness of adalimumab versus secukinumab for the treatment of psoriatic arthritis: a matching-adjusted indirect comparison. Rheumatol Ther 2017;4:349–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Van SS, Diels J, Van LJ, Hemels M.. Network meta-analysis with baseline risk adjustment to assess the relative efficacy of ustekinumab in adult patients with active psoriatic arthritis. Value Health 2014;17:A373. [DOI] [PubMed] [Google Scholar]
- 39. Kirson NY, Rao S, Birnbaum HG. et al. Matching-adjusted indirect comparison of adalimumab vs etanercept and infliximab for the treatment of psoriatic arthritis. J Med Econ 2013;16:479–89. [DOI] [PubMed] [Google Scholar]
- 40. Cameron C, Hutton B, Druchok C. et al. Importance of assessing and adjusting for cross-study heterogeneity in network meta-analysis: a case study of psoriasis. J Comp Eff Res 2018;7:1037–51. [DOI] [PubMed] [Google Scholar]
- 41. Janssen A Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled Study Evaluating the Efficacy and Safety of Guselkumab Administered Subcutaneously in Subjects with Active Psoriatic Arthritis - DISCOVER 2. 2019; https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)302634/fulltext#:~:text=DISCOVER%2D2%20is%20a%20randomised,%5D%2C%20apremilast%2C%20or%20non%2D.
- 42. Janssen A Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled Study Evaluating the Efficacy and Safety of Guselkumab Administered Subcutaneously in Subjects with Active Psoriatic Arthritis including those Previously Treated with Biologic Anti-TNFα Agent(s) - DISCOVER-1. 2019; https://pubmed.ncbi.nlm.nih.gov/32178765/.
- 43. Nash P, Ohson K, Walsh J. et al. Early and sustained efficacy with apremilast monotherapy in biological-naive patients with psoriatic arthritis: a phase IIIB, randomised controlled trial (ACTIVE). Ann Rheum Dis 2018;77:690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mease PJ, Gladman DD, Ritchlin CT. et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2005;52:3279–89. [DOI] [PubMed] [Google Scholar]
- 45. McInnes IB, Mease PJ, Kirkham B. et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015;386:1137–46. [DOI] [PubMed] [Google Scholar]
- 46. Nash P, Mease PJ, McInnes IB. et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther 2018;20:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kivitz AJ, Nash P, Tahir H. et al. Efficacy and safety of subcutaneous secukinumab 150 mg with or without loading regimen in psoriatic arthritis: results from the FUTURE 4 study. Rheumatol Ther 2019;6:393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mease P, van der Heijde D, Landewe R. et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann Rheum Dis 2018;77:890–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kavanaugh A, McInnes I, Mease P. et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum 2009;60:976–86. [DOI] [PubMed] [Google Scholar]
- 50. Kavanaugh A, Husni ME, Harrison DD. et al. Safety and efficacy of intravenous golimumab in patients with active psoriatic arthritis: results through week twenty-four of the GO-VIBRANT study. Arthritis Rheum 2017;69:2151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Antoni C, Krueger GG, de Vlam K. et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis 2005;64:1150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Genovese MC, Mease PJ, Thomson GT. et al. Safety and efficacy of adalimumab in treatment of patients with psoriatic arthritis who had failed disease modifying antirheumatic drug therapy. J Rheumatol 2007;34:1040–50. [PubMed] [Google Scholar]
- 53. Gladman D, Rigby W, Azevedo VF. et al. Tofacitinib for Psoriatic Arthritis in Patients with an Inadequate Response to TNF Inhibitors. N Engl J Med 2017;377:1525–36. [DOI] [PubMed] [Google Scholar]
- 54. Mease P, Hall S, FitzGerald O. et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med 2017;377:1537–50. [DOI] [PubMed] [Google Scholar]
- 55. Kavanaugh A, Mease PJ, Gomez-Reino JJ. et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis 2014;73:1020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cutolo M, Myerson GE, Fleischmann RM. et al. A Phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol 2016;43:1724–34. [DOI] [PubMed] [Google Scholar]
- 57. Edwards CJ, Blanco FJ, Crowley J. et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis 2016;75:1065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wells AF, Edwards CJ, Kivitz AJ. et al. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: results of the randomized, placebocontrolled PALACE 4 trial. Rheumatology 2018;57:1253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McInnes IB, Kavanaugh A, Gottlieb AB. et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 2013;382:780–9. [DOI] [PubMed] [Google Scholar]
- 60. Ritchlin C, Rahman P, Kavanaugh A. et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis 2014;73:990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mease PJ, Fleischmann R, Deodhar AA. et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis 2014;73:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mease PJ, Van Der Heijde D, Ritchlin CT. et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebocontrolled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis 2017;76:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nash P, Kirkham B, Okada M. et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet 2017;389:2317–27. [DOI] [PubMed] [Google Scholar]
- 64. Mease PJ, Gottlieb AB, van der Heijde D. et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis 2017;76:1550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mease PJ, Kivitz AJ, Burch FX. et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum 2004;50:2264–72. [DOI] [PubMed] [Google Scholar]
- 66. Signorovitch JE, Betts KA, Yan YS. et al. Comparative efficacy of biological treatments for moderate-to-severe psoriasis: a network meta-analysis adjusting for cross-trial differences in reference arm response. Br J Dermatol 2015;172:504–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are sourced from the public domain and are available in the articles cited throughout.