Abstract
There is a range of proteomics methods to spot and analyze bacterial protein contents such as liquid chromatography-mass spectrometry (LC-MS), two-dimensional gel electrophoresis, and matrix-assisted laser desorption/ionization mass spectrometry (MALDI-TOF MS), which give comprehensive information about the microorganisms that may be helpful within the diagnosis and coverings of infections. Microorganism identification by mass spectrometry is predicted on identifying a characteristic spectrum of every species so matched with an outsized database within the instrument. MALDI-TOF MS is one of the diagnostic methods, which is a straightforward, quick, and precise technique, and is employed in microbial diagnostic laboratories these days and may replace other diagnostic methods. This method identifies various microorganisms such as bacteria, fungi, parasites, and viruses, which supply comprehensive information. One of the MALDI-TOF MS's crucial applications is bacteriology, which helps identify bacterial species, identify toxins, and study bacterial antibiotic resistance. By knowing these cases, we will act more effectively against bacterial infections.
1. Introduction
Bacterial pathogens are among the factors that threaten global health and can grow in humans [1], animals [2], and plants [3] and cause disease in their hosts under certain conditions, including the acquisition of virulence factors [4, 5]. These microorganisms have different macromolecules in their structure, including genome [6], proteins [7, 8], polysaccharides [9], and phospholipids [10], which various techniques and methods can study.
To study bacterial macromolecules, there are various methods, including genomics and proteomics. The genomics methods give us broad information about the bacteria's genome, and proteomics methods help us identify multiple bacterial proteins [11, 12]. The proteomics methods are liquid chromatography-mass spectrometry (LC-MS) [13–15], two-dimensional gel electrophoresis [16–19], and matrix-assisted laser desorption/ionization mass spectrometry (MALDI-TOF MS) [20–22], which are used for studying bacterial protein contents and give comprehensive information about the cell and can be helpful in the identification of the bacteria, and so treatment of the infections is performed well timed by creating effective drugs and vaccines [23].
MALDI-TOF MS is one of the most potent proteomics methods that can detect various microorganisms such as bacteria [24], viruses [25–27], fungi [28–30], and parasites [31–33] and also can provide us precise, quick, easy, and inexpensive comprehensive information about microorganisms [34]. This study aimed to comprehensively investigate the applications of MALDI-TOF in bacteriological studies and the advantages of this method over other methods of laboratory diagnosis of infections.
2. The General Applications of MALDI-TOF MS
There are various methods for identifying different cells; the study of cell proteins is one of those methods, which has been used in diagnostic laboratories for many years [35–37]. MALDI-TOF MS technique is a successful method of detecting microorganisms that have been widely used in microbiological laboratories in recent years [38–40].
MALDI-TOF MS is a device that generates ions from a sample and then separates the generated ions based on the mass-to-charge ratio in the gas phase [41]. Today, mass spectrometry is a susceptible method for the structural study of biomolecules [42]. At present, each mass spectrometer includes an ion source to generate ions from the sample, one or more mass analyzers to separate ions based on mass-to-charge ratio, a detector to record the number of ions removed from the analyzer, and a computer to process data and create spectrum and control the device through the feedback [43], which can use these data to get comprehensive information about the microorganisms (Figure 1).
Figure 1.
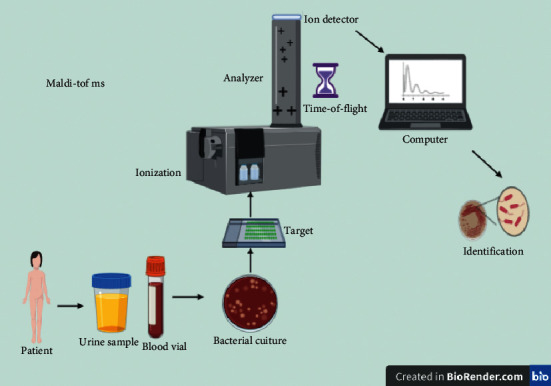
MALDI-TOF MS that includes an ion source to generate ions from the sample, one or more mass analyzers to separate ions based on mass-to-charge ratio, a detector to record the number of ions removed from the analyzer, and a computer to process data and create spectrum and control the device through the feedback, which can use these data to get comprehensive information about the microorganisms.
An essential feature of MALDI-TOF MS is that in addition to detecting bacteria, it can be used to detect microbial toxins [44], study antibiotic resistance [45–47], detect viruses [48, 49], parasites [50, 51], fungi [52, 53], and study of human [54–56] and plant cells [57, 58], which makes us more familiar with the creatures in our environment.
Essential applications of MALDI-TOF MS in virology include identifying various mutations in viruses and identifying different microorganisms' strains, which might help the rapid and accurate diagnosis of viruses [49]. For example, in the rapid detection of respiratory viruses including influenza A, influenza B, respiratory syncytial virus (RSV), parainfluenza viruses, adenovirus, and rhinovirus, the MALDI-TOF MS method is used, which has increased the speed and accuracy in the diagnosis of these viruses by detection of their protein structure such as nucleoprotein (NP), haemagglutinin (HA), neuraminidase (NA), matrix protein (M1), and a nonstructural protein (NS1) in the influenza A, which is one of the advantages of this method compared to other traditional diagnostic procedures, such as serology, cell culture, and real-time reverse transcription-polymerase chain reaction (rRT-PCR) [59], which by the rapid diagnosis of infection can help in the immediate initiation of treatment and surviving the patients.
Another application of MALDI-TOF MS is in mycology; it effectively identifies different fungal species, identifies subspecies, compares differences between other fungi, and identifies fungal resistance antifungal drugs [60]. For example, in the study by Antonietta Vella et al. [61], they used MALDI-TOF MS to evaluate the antifungal resistance of Candida glabrata against anidulafungin and fluconazole, and this technique shows the antifungal resistance of these fungi rapidly.
MALDI-TOF MS also is used in parasitology, where it is used to detect various parasites such as Leishmania [62], Cryptosporidium parvum [63], Giardia lamblia [64], and Entamoeba histolytica [65], which provides us with comprehensive information about them.
3. Identification of Bacteria
Until recently, only phenotypic and genotypic methods were used to detect bacteria in bacteriological laboratories [66], and by the advancement of science, other techniques were used [67], such as MALDI-TOF MS, which is based on the study of bacterial proteins, which is an easy, fast, and accurate method that can replace other diagnostic procedures [68]. This method makes mass spectral for each microorganism unique as a fingerprint, identifying different bacterial genus and subspecies [34].
Bacteria use protein for various structural and functional purposes. For example, they use protein to build many parts such as cell envelope [69], flagella [70], secretory systems [71], enzymes [72], and biofilm [73, 74], and MALDI-TOF MS helps detecting various bacteria by detecting these proteinaceous parts.
MALDI-TOF technique has been used in bacteriology to study different types of bacteria such as Gram-positive [75], Gram-negative [76], mycobacteria [77], and anaerobic [78], which give complete and comprehensive information about those microorganisms, useful in the fast diagnosis of bacterial infections. For example, in the study of Sun et al. [79], they used MALDI-TOF MS to identify species of Lactobacillus plantarum, a Gram-positive bacterium, and they identified 34 proteins as the biomarker proteins and used these proteins for identifying species of bacterium in the various cultures. In another study by Friedrichs et al. [80], MALDI-TOF MS was used to identify 99 species of clinical Streptococci, a Gram-positive bacterium; they identified 71 Streptococcus mitis, 23 Streptococcus anginosus, and five Streptococcus salivarius. Another study carried out on mycobacteria by Hettick et al. [81] used MALDI-TOF MS to identify various mycobacteria and acid-fast bacteria, such as Mycobacterium tuberculosis, Mycobacterium intracellulare, Mycobacterium avium, Mycobacterium bovis, Mycobacterium kansasii, and Mycobacterium fortuitum. Due to the development of tuberculosis by the M. tuberculosis, which is one of the most dangerous global infections and health problems, rapid and accurate diagnosis of this bacterium is very important, and the use of the MALDI-TOF MS technique can be helpful in this operation [82].
Another vital application of MALDI-TOF MS is identifying anaerobic bacteria that face many difficulties due to their hard growing on solid media, the ineffectiveness of routine biochemical tests for their identification, and the need for specific environmental conditions that develop the need for new and effective diagnostic procedures [83]. For example, the study of Eigner et al. [84] is on more than 1000 bacteria isolated from laboratories, since the detection of 95.2% of isolation was correct, and shows this method's effectiveness for detecting anaerobe bacteria.
4. Identification of Bacterial Toxin
Different microorganisms such as bacteria produce proteins called toxins to increase their virulence [85]. The toxin is a part of the bacterial structure called endotoxin [86] or secretes out of the cell called exotoxin [87]. Many bacteria form toxins that can be identified by different MALDI-TOF MS methods, which help in their detection [88]. For example, Staphylococcus aureus threatens people's health by causing dangerous infections by producing a toxin called Panton–Valentine leukocidin (PVL); fast and accurate detection of this toxin is crucial to save the life of people [89]. In the study of Bittar et al. [90], they used MALDI-TOF MS for detecting PVL for identification of 81 S. aureus isolated from patients of a hospital, and they were able to identify the isolates effective, fast, and efficiently by using this method.
Another study by Ranasinghe and Akhurst [91] was performed to study crystal toxin of Bacillus thuringiensis by MALDI-TOF MS to detect these bacteria and shows that this method can identify the toxin rapidly and sensitively that indicates the effectiveness of this method in rapid identification of microbial toxins for quick control of the infection.
5. Study of Antibiotic Resistance
Antibiotic resistance is the ability of the microorganisms to resist medications that are used to destroy them. This resistance can be caused by excessive consumption of antibiotics and a mutation in microbes that have caused this phenomenon to become a global health threat [92].
To resist antibiotics, the bacteria attempt to perform various actions, including constructing specific structures called efflux pumps, which drive the antibiotic out of the bacteria [89] and make enzymes that can destroy antibiotics [93]. For fast, accurate, and effective detection of antibiotic-resistant bacteria, new methods must identify the factors that make the bacteria resist antibiotics. MALDI-TOF MS is one of these methods that can locate efflux pumps and enzymes made up of protein [94].
Rapid identification of some bacteria is essential and plays a vital role in preventing transmission to other people, and delays in identifying them can lead to countless deaths, which indicate the need for rapid diagnostic methods such as MALDI-TOF MS. Among these bacteria, we can mention methicillin-resistant Staphylococcus aureus (MRSA), one of the main threats to public health. For example, in a study by Edwards-Jones et al. [95], they used MALDI-TOF MS to detect MRSA. Its fast identification is vital for appropriate therapeutic actions and timely interposition for controlling the infection.
6. Advantages and Disadvantages of MALDI-TOF MS
MALDI-TOF approaches effectively discover viruses in a wide range of biological specimens, and the concordance rate between MALDI-TOF and other standard-based methods is high [96]. PCR-based identification methods have some restrictions such as a lot longer turn-around time than MS, reagent and labor expenses, and some practical issues [97]. On the other hand, MALDI-TOF modern technology can likewise be applied to recognize infections in coinfected tests. These approaches can synchronize the discovery of several pathogens in a solitary essay. They can then inhibit misdiagnosis and delays in treatment without rising costs or adding a new action to the procedure [98].
RT-PCR procedures might have repressive fragments and contamination problems; hence, different sample preparation, amplification, and analysis are required [99]. Additionally, nucleic acid-based methods are costly and lengthy and appear in many cases much less convenient than MS for regular laboratory recognition [100, 101]. For MALDI-TOF, there needs to be substantial development in closing the upfront of PCR processing to consist of DNA extraction and the PCR procedure itself. MALDI-TOF MS approaches likewise enable large-scale research studies for fresh samples and archival samples from numerous biological specimens [102].
One of the significant weaknesses of MALDI-TOF is that the database restricts the identification [50]. The mass profile is used as a mass spectrum to compare well-characterized microorganisms in a database [103]. The range generally consists of specific peaks, so that with an extensive collection of spectra, the recognition may be carried out by applying bioinformatics [104].
7. Future Perspectives
The application of MS to molecular diagnostics has become a highly active part of the study with considerable ramifications for medication and public health. One of the most sophisticated advancements has been created by precise matching of molecular methods with MALDI-TOF. MS as a discovery system is most matched for the recognition of complicated genetic markers without invoking sequences [105]. It can be anticipated that the future growth of MS-based molecular diagnostics will be connected to unique techniques of extracting scientifically and epidemiologically relevant info such as disorder intensity, medicine resistance, vaccination retreat, and transmission from the genetic markers applying mainly developed computational and mathematical versions [106]. An essential feature of the MS innovation will be the possibility of its application to the quick discovery of microbes triggering hospital infection [107]. If combined with proper fast and delicate modern technologies for the medical diagnosis of avoidable diseases, MS can also influence quality control of sterile blood items and food security [108]. Assimilation of molecular and computational strategies with MS needs to generate analysis assays for wide, regular public health and medical practice applications.
8. Conclusion
Rapid diagnosis of infection makes the control of it more straightforward, vital for protecting the community's health. There are various methods for rapid diagnosis of infections. One of them is MALDI-TOF MS, which is a fast, easy, and accurate method. Detecting bacterial proteinaceous agents helps us diagnose disorders quickly, which leads to more rapid treatment of the patient.
Acknowledgments
This study was supported by the National Institute for Medical Research Development (NIMAD) (957280) and Drug Applied Research Center and was approved by the local ethic committee. The authors thank all staff of DARC for their support.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Maryam Alizadeh, Leila Yousefi, and Farzaneh Pakdel equally contributed to this study.
References
- 1.Gholizadeh P., Pormohammad A., Eslami H., Shokouhi B., Fakhrzadeh V., Kafil H. S. Oral pathogenesis of Aggregatibacter actinomycetemcomitans. Microbial Pathogenesis. 2017;113:303–311. doi: 10.1016/j.micpath.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 2.McFall-Ngai M. J. Unseen forces: the influence of bacteria on animal development. Developmental Biology. 2002;242(1):1–14. doi: 10.1006/dbio.2001.0522. [DOI] [PubMed] [Google Scholar]
- 3.Glick B. R. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012;2012 doi: 10.6064/2012/963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kafil H. S., Mobarez A. M. Assessment of biofilm formation by enterococci isolates from urinary tract infections with different virulence profiles. Journal of King Saud University - Science. 2015;27(4):312–317. doi: 10.1016/j.jksus.2014.12.007. [DOI] [Google Scholar]
- 5.Kafil H. S., Mobarez A. M., Moghadam M. F. Adhesion and virulence factor properties of Enterococci isolated from clinical samples in Iran. Indian Journal of Pathology and Microbiology. 2013;56(3):p. 238. doi: 10.4103/0377-4929.120375. [DOI] [PubMed] [Google Scholar]
- 6.McCutcheon J. P., Moran N. A. Extreme genome reduction in symbiotic bacteria. Nature Reviews Microbiology. 2012;10(1):13–26. doi: 10.1038/nrmicro2670. [DOI] [PubMed] [Google Scholar]
- 7.Bendtsen J. D., Kiemer L., Fausbøll A., Brunak S. Non-classical protein secretion in bacteria. BMC Microbiology. 2005;5(1):1–13. doi: 10.1186/1471-2180-5-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahroud B. D., Mokarram R. R., Khiabani M. S., et al. Low intensity ultrasound increases the fermentation efficiency of Lactobacillus casei subsp.casei ATTC 39392. International Journal of Biological Macromolecules. 2016;86:462–467. doi: 10.1016/j.ijbiomac.2016.01.103. [DOI] [PubMed] [Google Scholar]
- 9.Yudiati E., Subagiyo S., Djarod M. S. R. Preliminary study of polysaccharide and oligosaccharide alginate (AOS) as prebiotic of probiotic bacteria. Jurnal Kelautan Tropis. 2020;23(2):234–238. doi: 10.14710/jkt.v23i2.7674. [DOI] [Google Scholar]
- 10.Bogdanov M., Pyrshev K., Yesylevskyy S., et al. Phospholipid distribution in the cytoplasmic membrane of Gram-negative bacteria is highly asymmetric, dynamic, and cell shape-dependent. Science Advances. 2020;6(23):p. eaaz6333. doi: 10.1126/sciadv.aaz6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y., Sun J., Sun Y., et al. Genomic, transcriptomic, and proteomic insights into the symbiosis of deep-sea tubeworm holobionts. The ISME Journal. 2020;14(1):135–150. doi: 10.1038/s41396-019-0520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helena Duarte Sagawa C., Zaini P. A., de Assis R., et al. Deep learning neural network prediction method improves proteome profiling of vascular sap of grapevines during pierce’s disease development. Biology. 2020;9(9):p. 261. doi: 10.3390/biology9090261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lasch P., Schneider A., Blumenscheit C., Doellinger J. Identification of microorganisms by liquid chromatography-mass spectrometry (LC-MS1) and in silico peptide mass libraries. Molecular & Cellular Proteomics. 2020 doi: 10.1074/mcp.TIR120.002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vishwanath V., Sulyok M., Labuda R., Bicker W., Krska R. Simultaneous determination of 186 fungal and bacterial metabolites in indoor matrices by liquid chromatography/tandem mass spectrometry. Analytical and Bioanalytical Chemistry. 2009;395(5):1355–1372. doi: 10.1007/s00216-009-2995-2. [DOI] [PubMed] [Google Scholar]
- 15.Liang X., Zheng K., Qian M. G., Lubman D. M. Determination of bacterial protein profiles by matrix-assisted laser desorption/ionization mass spectrometry with high-performance liquid chromatography. Rapid Communications in Mass Spectrometry. 1996;10(10):1219–1226. doi: 10.1002/(sici)1097-0231(19960731)10:10<1219::aid-rcm660>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 16.Molloy M. P., Phadke N. D., Maddock J. R., Andrews P. C. Two-dimensional electrophoresis and peptide mass fingerprinting of bacterial outer membrane proteins. Electrophoresis. 2001;22(9):1686–1696. doi: 10.1002/1522-2683(200105)22:9<1686::aid-elps1686>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 17.Cash P. Characterisation of bacterial proteomes by two-dimensional electrophoresis. Analytica Chimica Acta. 1998;372(1-2):121–145. doi: 10.1016/s0003-2670(98)00346-8. [DOI] [Google Scholar]
- 18.Mitsutani A., Yamasaki I., Kitaguchi H., Kato J., Ueno S., Ishida Y. Analysis of algicidal proteins of a diatom-lytic marine bacterium Pseudoalteromonas sp. strain A25 by two-dimensional electrophoresis. Phycologia. 2001;40(3):286–291. doi: 10.2216/i0031-8884-40-3-286.1. [DOI] [Google Scholar]
- 19.Folio P., Chavant P., Chafsey I., Belkorchia A., Chambon C., Hébraud M. Two-dimensional electrophoresis database ofListeria monocytogenes EGDe proteome and proteomic analysis of mid-log and stationary growth phase cells. Proteomics. 2004;4(10):3187–3201. doi: 10.1002/pmic.200300841. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh S.-Y., Tseng C.-L., Lee Y.-S., et al. Highly efficient classification and identification of human pathogenic bacteria by MALDI-TOF MS. Molecular & Cellular Proteomics. 2008;7(2):448–456. doi: 10.1074/mcp.m700339-mcp200. [DOI] [PubMed] [Google Scholar]
- 21.Biswas S., Rolain J.-M. Use of MALDI-TOF mass spectrometry for identification of bacteria that are difficult to culture. Journal of Microbiological Methods. 2013;92(1):14–24. doi: 10.1016/j.mimet.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 22.De Bruyne K., Slabbinck B., Waegeman W., Vauterin P., De Baets B., Vandamme P. Bacterial species identification from MALDI-TOF mass spectra through data analysis and machine learning. Systematic and Applied Microbiology. 2011;34(1):20–29. doi: 10.1016/j.syapm.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Aslam B., Basit M., Nisar M. A., Khurshid M., Rasool M. H. Proteomics: technologies and their applications. Journal of Chromatographic Science. 2017;55(2):182–196. doi: 10.1093/chromsci/bmw167. [DOI] [PubMed] [Google Scholar]
- 24.Wunschel S. C., Jarman K. H., Petersen C. E., et al. Bacterial analysis by MALDI-TOF mass spectrometry: an inter-laboratory comparison. Journal of the American Society for Mass Spectrometry. 2005;16(4):456–462. doi: 10.1016/j.jasms.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 25.La Scola B., Campocasso A., N’Dong R., et al. Tentative characterization of new environmental giant viruses by MALDI-TOF mass spectrometry. Intervirology. 2010;53(5):344–353. doi: 10.1159/000312919. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y. J., Freas A., Fenselau C. Analysis of viral glycoproteins by MALDI-TOF mass spectrometry. Analytical Chemistry. 2001;73(7):1544–1548. doi: 10.1021/ac001171p. [DOI] [PubMed] [Google Scholar]
- 27.Jurinke C., Zöllner B., Feucht H.-H., et al. Detection of hepatitis B virus DNA in serum samples via nested PCR and MALDI-TOF mass spectrometry. Genetic Analysis: Biomolecular Engineering. 1996;13(3):67–71. doi: 10.1016/1050-3862(95)00151-4. [DOI] [PubMed] [Google Scholar]
- 28.Chalupová J., Raus M., Sedlářová M., Šebela M. Identification of fungal microorganisms by MALDI-TOF mass spectrometry. Biotechnology Advances. 2014;32(1):230–241. doi: 10.1016/j.biotechadv.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Ranque S., Normand A.-C., Cassagne C., et al. MALDI-TOF mass spectrometry identification of filamentous fungi in the clinical laboratory. Mycoses. 2014;57(3):135–140. doi: 10.1111/myc.12115. [DOI] [PubMed] [Google Scholar]
- 30.Becker P. T., de Bel A., Martiny D., et al. Identification of filamentous fungi isolates by MALDI-TOF mass spectrometry: clinical evaluation of an extended reference spectra library. Medical Mycology. 2014;52(8):826–834. doi: 10.1093/mmy/myu064. [DOI] [PubMed] [Google Scholar]
- 31.Laroche M., Almeras L., Pecchi E., et al. MALDI-TOF MS as an innovative tool for detection of Plasmodium parasites in Anopheles mosquitoes. Malaria Journal. 2017;16(1):p. 5. doi: 10.1186/s12936-016-1657-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murugaiyan J., Roesler U. MALDI-TOF MS profiling-advances in species identification of pests, parasites, and vectors. Frontiers in Cellular and Infection Microbiology. 2017;7:p. 184. doi: 10.3389/fcimb.2017.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Millares P., LaCourse E. J., Perally S., et al. Proteomic profiling and protein identification by MALDI-TOF mass spectrometry in unsequenced parasitic nematodes. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0033590.e33590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Croxatto A., Prod’hom G., Greub G. Applications of MALDI-TOF mass spectrometry in clinical diagnostic microbiology. FEMS Microbiology Reviews. 2012;36(2):380–407. doi: 10.1111/j.1574-6976.2011.00298.x. [DOI] [PubMed] [Google Scholar]
- 35.Jazii F. R., Najafi Z., Malekzadeh R., et al. Identification of squamous cell carcinoma associated proteins by proteomics and loss of beta tropomyosin expression in esophageal cancer. World Journal of Gastroenterology. 2006;12(44):p. 7104. doi: 10.3748/wjg.v12.i44.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Connolly J. P., Comerci D., Alefantis T. G., et al. Proteomic analysis of Brucella abortus cell envelope and identification of immunogenic candidate proteins for vaccine development. Proteomics. 2006;6(13):3767–3780. doi: 10.1002/pmic.200500730. [DOI] [PubMed] [Google Scholar]
- 37.Qi Y.-J., He Q.-Y., Ma Y.-F., et al. Proteomic identification of malignant transformation-related proteins in esophageal squamous cell carcinoma. Journal of Cellular Biochemistry. 2008;104(5):1625–1635. doi: 10.1002/jcb.21727. [DOI] [PubMed] [Google Scholar]
- 38.Singhal N., Kumar M., Kanaujia P. K., Virdi J. S. MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Frontiers in Microbiology. 2015;6:p. 791. doi: 10.3389/fmicb.2015.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wieser A., Schneider L., Jung J., Schubert S. MALDI-TOF MS in microbiological diagnostics-identification of microorganisms and beyond (mini review) Applied Microbiology and Biotechnology. 2012;93(3):965–974. doi: 10.1007/s00253-011-3783-4. [DOI] [PubMed] [Google Scholar]
- 40.Gagnaire J., Dauwalder O., Boisset S., et al. Detection of Staphylococcus aureus delta-toxin production by whole-cell MALDI-TOF mass spectrometry. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0040660.e40660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griffin T. J., Smith L. M. Single-nucleotide polymorphism analysis by MALDI-TOF mass spectrometry. Trends in Biotechnology. 2000;18(2):77–84. doi: 10.1016/s0167-7799(99)01401-8. [DOI] [PubMed] [Google Scholar]
- 42.Kaltashov I. A., Eyles S. J. Studies of biomolecular conformations and conformational dynamics by mass spectrometry. Mass Spectrometry Reviews. 2002;21(1):37–71. doi: 10.1002/mas.10017. [DOI] [PubMed] [Google Scholar]
- 43.Hou T.-Y., Chiang-Ni C., Teng S.-H. Current status of MALDI-TOF mass spectrometry in clinical microbiology. Journal of Food and Drug Analysis. 2019;27(2):404–414. doi: 10.1016/j.jfda.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alam S. I., Kumar B., Kamboj D. V. Multiplex detection of protein toxins using MALDI-TOF-TOF tandem mass spectrometry: application in unambiguous toxin detection from bioaerosol. Analytical Chemistry. 2012;84(23):10500–10507. doi: 10.1021/ac3028678. [DOI] [PubMed] [Google Scholar]
- 45.Hrabák J., Chudáčková E., Walková R. Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry for detection of antibiotic resistance mechanisms: from research to routine diagnosis. Clinical Microbiology Reviews. 2013;26(1):103–114. doi: 10.1128/cmr.00058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Idelevich E. A., Sparbier K., Kostrzewa M., Becker K. Rapid detection of antibiotic resistance by MALDI-TOF mass spectrometry using a novel direct-on-target microdroplet growth assay. Clinical Microbiology and Infection. 2018;24(7):738–743. doi: 10.1016/j.cmi.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 47.Kostrzewa M., Sparbier K., Maier T., Schubert S. MALDI‐TOF MS: an upcoming tool for rapid detection of antibiotic resistance in microorganisms. PROTEOMICS–Clinical Applications. 2013;7(11-12):767–778. doi: 10.1002/prca.201300042. [DOI] [PubMed] [Google Scholar]
- 48.Calderaro A., Arcangeletti M.-C., Rodighiero I., et al. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry applied to virus identification. Scientific Reports. 2014;4(1):1–10. doi: 10.1038/srep06803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cobo F. Application of maldi-tof mass spectrometry in clinical virology: a review. The Open Virology Journal. 2013;7(1):84–90. doi: 10.2174/1874357920130927003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singhal N., Kumar M., Virdi J. S. MALDI-TOF MS in clinical parasitology: applications, constraints and prospects. Parasitology. 2016;143(12):1491–1500. doi: 10.1017/s0031182016001189. [DOI] [PubMed] [Google Scholar]
- 51.Huguenin A., Depaquit J., Villena I., Ferté H. MALDI-TOF mass spectrometry: a new tool for rapid identification of cercariae (Trematoda, Digenea) Parasite. 2019;26 doi: 10.1051/parasite/2019011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cassagne C., Normand A.-C., L’Ollivier C., Ranque S., Piarroux R. Performance of MALDI-TOF MS platforms for fungal identification. Mycoses. 2016;59(11):678–690. doi: 10.1111/myc.12506. [DOI] [PubMed] [Google Scholar]
- 53.Patel R. A moldy application of MALDI: MALDI-ToF mass spectrometry for fungal identification. Journal of Fungi. 2019;5(1):p. 4. doi: 10.3390/jof5010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hegmans J. P. J. J., Bard M. P. L., Hemmes A., et al. Proteomic analysis of exosomes secreted by human mesothelioma cells. The American Journal of Pathology. 2004;164(5):1807–1815. doi: 10.1016/s0002-9440(10)63739-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baharvand H., Hajheidari M., Ashtiani S. K., Salekdeh G. H. Proteomic signature of human embryonic stem cells. Proteomics. 2006;6(12):3544–3549. doi: 10.1002/pmic.200500844. [DOI] [PubMed] [Google Scholar]
- 56.Ouedraogo R., Daumas A., Ghigo E., Capo C., Mege J.-L., Textoris J. Whole-cell MALDI-TOF MS: a new tool to assess the multifaceted activation of macrophages. Journal of Proteomics. 2012;75(18):5523–5532. doi: 10.1016/j.jprot.2012.07.046. [DOI] [PubMed] [Google Scholar]
- 57.Westphal Y., Schols H. A., Voragen A. G. J., Gruppen H. MALDI-TOF MS and CE-LIF fingerprinting of plant cell wall polysaccharide digests as a screening tool for Arabidopsis cell wall mutants. Journal of Agricultural and Food Chemistry. 2010;58(8):4644–4652. doi: 10.1021/jf100283b. [DOI] [PubMed] [Google Scholar]
- 58.Caruso G., Cavaliere C., Guarino C., Gubbiotti R., Foglia P., Laganà A. Identification of changes in Triticum durum L. leaf proteome in response to salt stress by two-dimensional electrophoresis and MALDI-TOF mass spectrometry. Analytical and Bioanalytical Chemistry. 2008;391(1):381–390. doi: 10.1007/s00216-008-2008-x. [DOI] [PubMed] [Google Scholar]
- 59.Majchrzykiewicz-Koehorst J. A., Heikens E., Trip H., et al. Rapid and generic identification of influenza A and other respiratory viruses with mass spectrometry. Journal of Virological Methods. 2015;213:75–83. doi: 10.1016/j.jviromet.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bader O. MALDI-TOF-MS-based species identification and typing approaches in medical mycology. Proteomics. 2013;13(5):788–799. doi: 10.1002/pmic.201200468. [DOI] [PubMed] [Google Scholar]
- 61.Vella A., De Carolis E., Mello E., et al. Potential use of MALDI-ToF mass spectrometry for rapid detection of antifungal resistance in the human pathogen Candida glabrata. Scientific Reports. 2017;7(1):1–9. doi: 10.1038/s41598-017-09329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Culha G., Akyar I., Zeyrek F. Y., et al. Leishmaniasis in Turkey: determination of Leishmania species by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) Iranian Journal of Parasitology. 2014;9(2):p. 239. [PMC free article] [PubMed] [Google Scholar]
- 63.Magnuson M. L., Owens J. H., Kelty C. A. Characterization of Cryptosporidium parvum by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Applied and Environmental Microbiology. 2000;66(11):4720–4724. doi: 10.1128/aem.66.11.4720-4724.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim J., Bae S.-S., Sung M.-H., Lee K.-H., Park S.-J. Comparative proteomic analysis of trophozoites versus cysts of Giardia lamblia. Parasitology Research. 2009;104(2):475–479. doi: 10.1007/s00436-008-1223-x. [DOI] [PubMed] [Google Scholar]
- 65.Calderaro A., Piergianni M., Buttrini M., et al. MALDI-TOF mass spectrometry for the detection and differentiation of Entamoeba histolytica and Entamoeba dispar. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0122448.e0122448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang A.-Y., Wang H.-N., Tian G.-B., et al. Phenotypic and genotypic characterisation of antimicrobial resistance in faecal bacteria from 30 Giant pandas. International Journal of Antimicrobial Agents. 2009;33(5):456–460. doi: 10.1016/j.ijantimicag.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 67.Alizadeh N., Memar M. Y., Moaddab S. R., Kafil H. S. Aptamer-assisted novel technologies for detecting bacterial pathogens. Biomedicine & Pharmacotherapy. 2017;93:737–745. doi: 10.1016/j.biopha.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 68.Soro-Yao A. A., Schumann P., Thonart P., Djè K. M., Pukall R. The use of MALDI-TOF mass spectrometry, ribotyping and phenotypic tests to identify lactic acid bacteria from fermented cereal foods in abidjan (côte d’Ivoire) The Open Microbiology Journal. 2014;8(1):78–86. doi: 10.2174/1874285801408010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Domínguez-Medina C. C., Pérez-Toledo M., Schager A. E., et al. Outer membrane protein size and LPS O-antigen define protective antibody targeting to the Salmonella surface. Nature Communications. 2020;11(1):1–11. doi: 10.1038/s41467-020-14655-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Macnab R. M. Type III flagellar protein export and flagellar assembly. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2004;1694(1-3):207–217. doi: 10.1016/j.bbamcr.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 71.Abby S. S., Cury J., Guglielmini J., Néron B., Touchon M., Rocha E. P. Identification of protein secretion systems in bacterial genomes. Scientific Reports. 2016;6(1):1–14. doi: 10.1038/srep23080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Colodner R., Rock W., Chazan B., et al. Risk factors for the development of extended-spectrum beta-lactamase-producing bacteria in nonhospitalized patients. European Journal of Clinical Microbiology & Infectious Diseases. 2004;23(3):163–167. doi: 10.1007/s10096-003-1084-2. [DOI] [PubMed] [Google Scholar]
- 73.Rani S. A., Pitts B., Beyenal H., et al. Spatial patterns of DNA replication, protein synthesis, and oxygen concentration within bacterial biofilms reveal diverse physiological states. Journal of Bacteriology. 2007;189(11):4223–4233. doi: 10.1128/jb.00107-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kafil H. S., Mobarez A. M., Moghadam M. F., Hashemi Z. s., Yousefi M. Gentamicin induces efaA expression and biofilm formation in Enterococcus faecalis. Microbial Pathogenesis. 2016;92:30–35. doi: 10.1016/j.micpath.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 75.Böhme K., Fernández-No I. C., Barros-Velázquez J., Gallardo J. M., Cañas B., Calo-Mata P. Rapid species identification of seafood spoilage and pathogenic Gram-positive bacteria by MALDI-TOF mass fingerprinting. Electrophoresis. 2011;32(21):2951–2965. doi: 10.1002/elps.201100217. [DOI] [PubMed] [Google Scholar]
- 76.Fernández-Olmos A., García-Castillo M., Morosini M.-I., Lamas A., Máiz L., Cantón R. MALDI-TOF MS improves routine identification of non-fermenting Gram negative isolates from cystic fibrosis patients. Journal of Cystic Fibrosis. 2012;11(1):59–62. doi: 10.1016/j.jcf.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 77.Bille E., Dauphin B., Leto J., et al. MALDI-TOF MS Andromas strategy for the routine identification of bacteria, mycobacteria, yeasts, Aspergillus spp. and positive blood cultures. Clinical Microbiology and Infection. 2012;18(11):1117–1125. doi: 10.1111/j.1469-0691.2011.03688.x. [DOI] [PubMed] [Google Scholar]
- 78.Veloo A. C. M., Welling G. W., Degener J. E. The identification of anaerobic bacteria using MALDI-TOF MS. Anaerobe. 2011;17(4):211–212. doi: 10.1016/j.anaerobe.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 79.Sun L., Teramoto K., Sato H., Torimura M., Tao H., Shintani T. Characterization of ribosomal proteins as biomarkers for matrix-assisted laser desorption/ionization mass spectral identification ofLactobacillus plantarum. Rapid Communications in Mass Spectrometry. 2006;20(24):3789–3798. doi: 10.1002/rcm.2801. [DOI] [PubMed] [Google Scholar]
- 80.Friedrichs C., Rodloff A. C., Chhatwal G. S., Schellenberger W., Eschrich K. Rapid identification of viridans streptococci by mass spectrometric discrimination. Journal of Clinical Microbiology. 2007;45(8):2392–2397. doi: 10.1128/jcm.00556-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hettick J. M., Kashon M. L., Slaven J. E., et al. Discrimination of intact mycobacteria at the strain level: a combined MALDI-TOF MS and biostatistical analysis. Proteomics. 2006;6(24):6416–6425. doi: 10.1002/pmic.200600335. [DOI] [PubMed] [Google Scholar]
- 82.Deng C., Lin M., Hu C., et al. Exploring serological classification tree model of active pulmonary tuberculosis by magnetic beads pretreatment and MALDI-TOF MS analysis. Scandinavian Journal of Immunology. 2011;74(4):397–405. doi: 10.1111/j.1365-3083.2011.02590.x. [DOI] [PubMed] [Google Scholar]
- 83.Nagy E., Becker S., Kostrzewa M., Barta N., Urbán E. The value of MALDI-TOF MS for the identification of clinically relevant anaerobic bacteria in routine laboratories. Journal of Medical Microbiology. 2012;61(10):1393–1400. doi: 10.1099/jmm.0.043927-0. [DOI] [PubMed] [Google Scholar]
- 84.Eigner U., Holfelder M., Oberdorfer K., Betz-Wild U., Bertsch D., Fahr A.-M. Performance of a matrix-assisted laser desorption ionization-time-of-flight mass spectrometry system for the identification of bacterial isolates in the clinical routine laboratory. Clinical Laboratory Journal For Clinical Laboratories And Laboratories Related. 2009;55(7):p. 289. [PubMed] [Google Scholar]
- 85.Kuehne S. A., Cartman S. T., Heap J. T., Kelly M. L., Cockayne A., Minton N. P. The role of toxin A and toxin B in Clostridium difficile infection. Nature. 2010;467(7316):711–713. doi: 10.1038/nature09397. [DOI] [PubMed] [Google Scholar]
- 86.Rauchhaus M., Coats A. J., Anker S. D. The endotoxin-lipoprotein hypothesis. The Lancet. 2000;356(9233):930–933. doi: 10.1016/s0140-6736(00)02690-8. [DOI] [PubMed] [Google Scholar]
- 87.Kim B. S. Spatiotemporal regulation of Vibrio exotoxins by HlyU and other transcriptional regulators. Toxins. 2020;12(9):p. 544. doi: 10.3390/toxins12090544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsilia V., Devreese B., De Baenst I., et al. Application of MALDI-TOF mass spectrometry for the detection of enterotoxins produced by pathogenic strains of the Bacillus cereus group. Analytical and Bioanalytical Chemistry. 2012;404(6-7):1691–1702. doi: 10.1007/s00216-012-6254-6. [DOI] [PubMed] [Google Scholar]
- 89.Labandeira-Rey M., Couzon F., Boisset S., et al. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science. 2007;315(5815):1130–1133. doi: 10.1126/science.1137165. [DOI] [PubMed] [Google Scholar]
- 90.Bittar F., Ouchenane Z., Smati F., Raoult D., Rolain J.-M. MALDI-TOF-MS for rapid detection of staphylococcal Panton-Valentine leukocidin. International Journal of Antimicrobial Agents. 2009;34(5):467–470. doi: 10.1016/j.ijantimicag.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 91.Ranasinghe C., Akhurst R. J. Matrix assisted laser desorption ionisation time of flight mass spectrometry (MALDI-TOF MS) for detecting novel Bt toxins. Journal of Invertebrate Pathology. 2002;79(1):51–58. doi: 10.1016/s0022-2011(02)00004-6. [DOI] [PubMed] [Google Scholar]
- 92.Davies J., Davies D. Origins and evolution of antibiotic resistance. Microbiology and Molecular Biology Reviews. 2010;74(3):417–433. doi: 10.1128/mmbr.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wilke M. S., Lovering A. L., Strynadka N. C. β-Lactam antibiotic resistance: a current structural perspective. Current Opinion in Microbiology. 2005;8(5):525–533. doi: 10.1016/j.mib.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 94.Lu W.-J., Lin H.-J., Hsu P.-H., Lin H.-T. V. Determination of drug efflux pump efficiency in drug-resistant bacteria using MALDI-TOF MS. Antibiotics. 2020;9(10):p. 639. doi: 10.3390/antibiotics9100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Edwards-Jones V., Claydon M. A., Evason D. J., Walker J., Fox A. J., Gordon D. B. Rapid discrimination between methicillin-sensitive and methicillin-resistant Staphylococcus aureus by intact cell mass spectrometry. Journal of Medical Microbiology. 2000;49(3):295–300. doi: 10.1099/0022-1317-49-3-295. [DOI] [PubMed] [Google Scholar]
- 96.Pomastowski P., Złoch M., Rodzik A., Ligor M., Kostrzewa M., Buszewski B. Analysis of bacteria associated with honeys of different geographical and botanical origin using two different identification approaches: MALDI-TOF MS and 16S rDNA PCR technique. PloS One. 2019;14(5) doi: 10.1371/journal.pone.0217078.e0217078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pinto T. C., Costa N. S., Castro L. F., et al. Potential of MALDI-TOF MS as an alternative approach for capsular typing Streptococcus pneumoniae isolates. Scientific Reports. 2017;7(1):1–5. doi: 10.1038/srep45572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khodadadi E., Zeinalzadeh E., Taghizadeh S., et al. Proteomic applications in antimicrobial resistance and clinical microbiology studies. Infection and Drug Resistance. 2020;Volume 13:1785–1806. doi: 10.2147/idr.s238446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kajiwara H., Murakami R. Application of RT-PCR and MALDI-TOF MS for the detection of RNA luteovirus. Analytical Biochemistry. 2017;539:45–47. doi: 10.1016/j.ab.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 100.Khodadadi E., Fahmideh L., Khodadadi E., et al. Current advances in DNA methylation analysis methods. BioMed Research International. 2021;2021:9. doi: 10.1155/2021/8827516.8827516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ruiz-Gaitán A. C., Fernández-Pereira J., Valentin E., et al. Molecular identification of Candida auris by PCR amplification of species-specific GPI protein-encoding genes. International Journal of Medical Microbiology. 2018;308(7):812–818. doi: 10.1016/j.ijmm.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 102.Zhao Y., Tsang C.-C., Xiao M., et al. Yeast identification by sequencing, biochemical kits, MALDI-TOF MS and rep-PCR DNA fingerprinting. Medical Mycology. 2018;56(7):816–827. doi: 10.1093/mmy/myx118. [DOI] [PubMed] [Google Scholar]
- 103.Oros D., Ceprnja M., Zucko J., et al. Identification of pathogens from native urine samples by MALDI-TOF/TOF tandem mass spectrometry. Clinical Proteomics. 2020;17(1):1–9. doi: 10.1186/s12014-020-09289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liébana-Martos C. The Use of Mass Spectrometry Technology (MALDI-TOF) in Clinical Microbiology. Amsterdam, Netherland: Elsevier; 2018. Indications, interpretation of results, advantages, disadvantages, and limitations of MALDI-TOF; pp. 75–86. [DOI] [Google Scholar]
- 105.Vrioni G., Tsiamis C., Oikonomidis G., Theodoridou K., Kapsimali V., Tsakris A. MALDI-TOF mass spectrometry technology for detecting biomarkers of antimicrobial resistance: current achievements and future perspectives. Annals of Translational Medicine. 2018;6(12) doi: 10.21037/atm.2018.06.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Florio W., Tavanti A., Ghelardi E., Lupetti A. MALDI-TOF MS applications to the detection of antifungal resistance: state of the art and future perspectives. Frontiers in Microbiology. 2018;9:p. 2577. doi: 10.3389/fmicb.2018.02577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Patel R. MALDI-TOF MS for the diagnosis of infectious diseases. Clinical Chemistry. 2015;61(1):100–111. doi: 10.1373/clinchem.2014.221770. [DOI] [PubMed] [Google Scholar]
- 108.Psaroulaki A., Chochlakis D. Use of MALDI-TOF mass spectrometry in the battle against bacterial infectious diseases: recent achievements and future perspectives. Expert Review of Proteomics. 2018;15(7):537–539. doi: 10.1080/14789450.2018.1499469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


