Abstract
Intravascular large B-cell lymphoma (IVLBCL) is a very rare subtype of malignant lymphoma that is difficult to diagnose. Cases of myocardial infarction caused by IVLBCL are even rarer. Herein, we report a case presenting with heart failure and delayed enhancement in the hypokinetic cardiac septum on contrast-enhanced cardiac magnetic resonance imaging. Myocardial biopsy showed large B-cell lymphoma cells in the microvessels within the myocardium. To the best of our knowledge, this is the first report of imaging findings of cardiac involvement in IVLBCL.
Keywords: Intravascular lymphoma, Cardiac MRI, Cardiac involvement
Introduction
Intravascular large B-cell lymphoma (IVLBCL) is a rare subtype of malignant lymphoma [1]. IVLBCL is difficult to diagnose by imaging because the lymphoma cells remain in the blood vessels. Cases of myocardial infarction caused by IVLBCL are even rarer [2,3] and the imaging findings are unknown. Herein, we report the imaging findings of a case of IVLBCL confirmed by myocardial biopsy who developed heart failure.
Case report
A 65-year-old male was referred to our hospital with a rapid increase in body weight and abdominal bloating. He had a history of gout for 20 years and hypertension for 10 years, and was taking daily benzbromarone (50 mg), irbesartan (100 mg), and amlodipine (5 mg). He had a history of smoking 20 cigarettes per day for 40 years and drinking 113.6 g of alcohol equivalent per day.
Blood tests showed an elevated C-reactive protein (7.36 mg/dL), alkaline phosphatase (278 IU/L), gamma-glutamyl transpeptidase (356 IU/L), aspartate aminotransferase (42 IU/L), alanine aminotransferase (56 IU/L), lactic dehydrogenase (604 IU/L), total-bilirubin (1.9 mg/dL), and brain natriuretic peptide (89.8 pg/mL). Hepatitis B surface antigen and hepatitis C virus antibody were negative. Electrocardiography was in sinus rhythm.
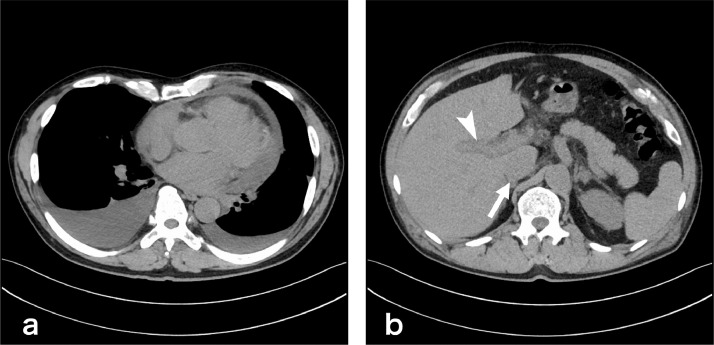
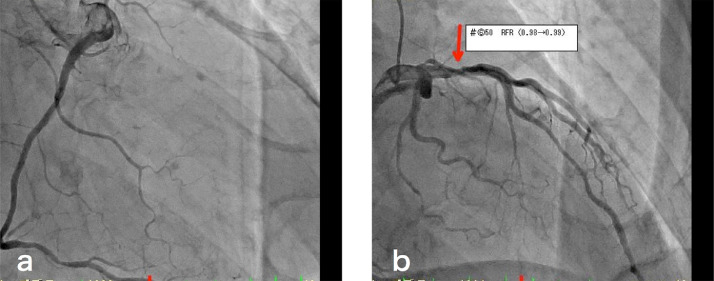
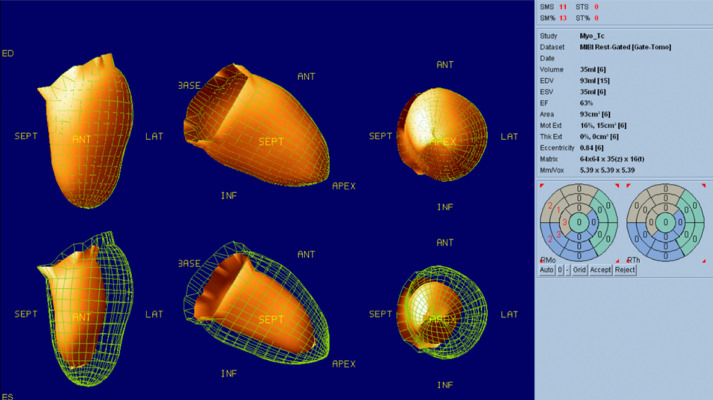
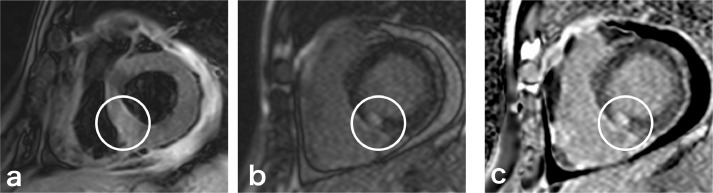
Chest to pelvis computed tomography (CT) demonstrated pericardial effusion and pleural effusion (Fig. 1a), periportal low attenuation, and a dilated inferior vena cava (Fig. 1b), which were suggestive of heart failure. Coronary angiography showed only a 50% stenosis at left anterior descending coronary artery #6. The stenosis was not significant, meaning it was not eligible for percutaneous coronary intervention. (Fig. 2). 99mTc-methoxy-isobutyl isonitrile (MIBI) scintigraphy showed hypokinesia of the ventricular septum and a decreased cardiac ejection fraction (63%) (Fig. 3). On contrast-enhanced magnetic resonance imaging (MRI), a nodular lesion was found on the ventricular septum. The lesion showed a mildly hyperintense signal at T2-weighted image (Fig. 4a) and a hyperintense signal at delayed phase T1-weighted image (Fig. 4b) and phase-sensitive inversion recovery image (Fig. 4c). The cause of heart failure could not be diagnosed at this time and the patient was discharged after his symptoms improved with furosemide treatment.
Fig. 1.
Chest to pelvis plain computed tomography (CT). (a) At the chest level, pericardial effusion and pleural effusion were found. (b) At the abdominal level, periportal low attenuation (arrow head) and a dilated inferior vena cava (arrow) were found.
Fig. 2.
Coronary angiography. (a) In the right coronary artery, there was no obstruction or stenosis. (b) In the left coronary artery, there was a 50% stenosis at left anterior descending coronary artery #6 (red arrow).
Fig. 3.
99mTc-methoxy-isobutyl isonitrile scintigraphy. Hypokinesia of the ventricular septum was shown. No significant decrease in myocardial perfusion was observed.
Fig. 4.
Contrast-enhanced cardiac magnetic resonance imaging (MRI). The image (c) is a phase-sensitive inversion recovery (PSIR) image, that is a corrected delayed contrast image. (a) T2-weighted image showed a nodular, mildly hyperintense signal area in the penetrating wall (circle). The nodular area showed hyperintense signals at T1-weighted image (b) and PSIR image (c).
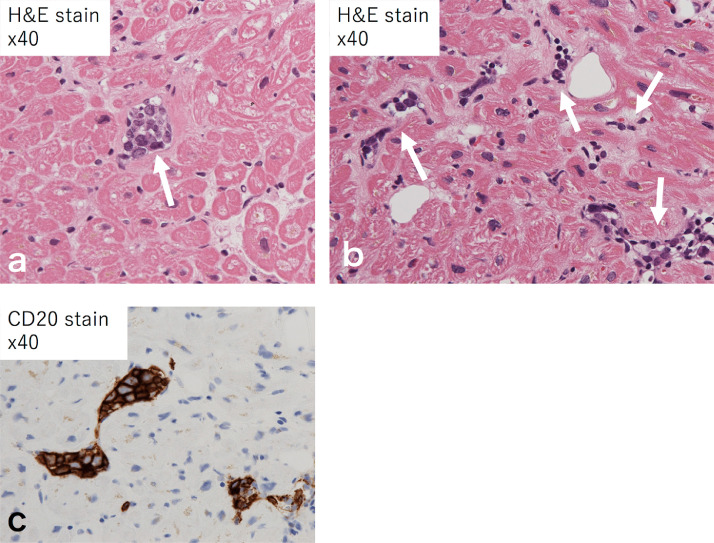
Three months later the patient returned to our hospital with a complaint of shortness of breath. CT of the trunk showed pleural effusion, pericardial effusion, and nodular pericardial thickening. There were no other masses or enlarged lymph nodes (Fig. 5). Blood tests showed a mild increase in free light chain kappa (51.2 mg/mL) suggestive of amyloidosis. As such, we performed biopsies from the skin, gastric mucosa, and bone marrow, although no abnormalities were found. We then performed transvascular myocardial biopsy of the septum, and, although there was no evidence of amyloidosis in the tissue, the lumen of the blood vessel was filled with atypical cells with a high nucleocytoplasmic ratio (Fig. 6a, b). The atypical cells immunostained positive for CD20 (Fig. 6c) and negative for CD3 and CD5, confirming a pathological diagnosis of IVLBCL.
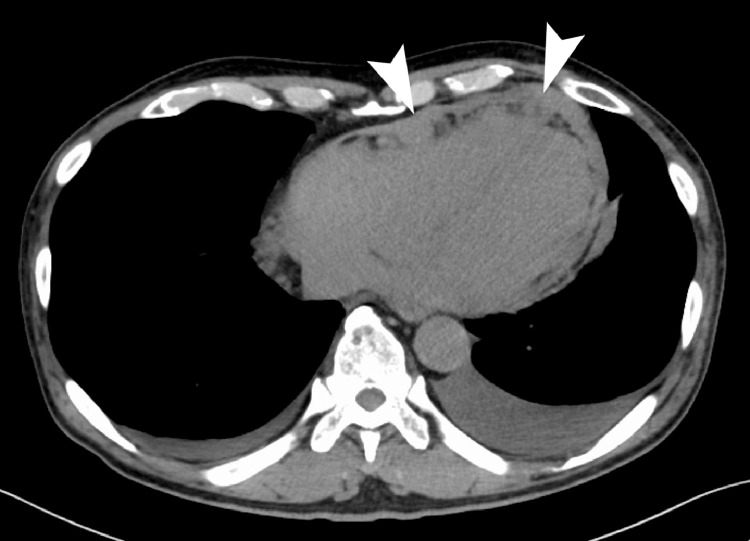
Fig. 5.
Computed tomography (CT) at the return visit. Nodular pericardial thickening was found (arrow heads). Pleural effusion and pericardial effusion were also observed.
Fig. 6.
Histopathological specimen of the myocardial biopsy. Images (a) and (b) were stained with hematoxylin and eosin. The lumens of the blood vessels were filled with atypical cells with a high nucleocytoplasmic ratio (arrows). (c) The atypical cells were immunostained positive for CD20.
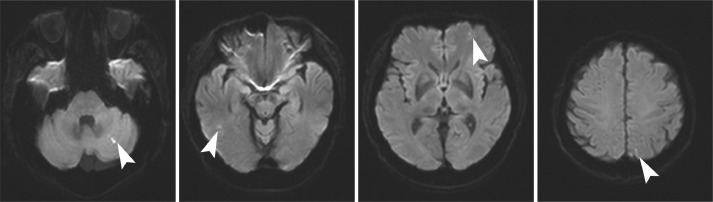
After diagnosis, elevated levels of interleukin-2 receptors (2154 U/mL) were found in the blood, while brain MRI showed multiple small diffusion-restricted areas in the cerebrum and cerebellum (Fig. 7). After steroid pulse therapy was administered, R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) therapy was initiated, resulting in decreased pericardial effusion and pleural effusion and reduced pericardial thickening on CT.
Fig. 7.
Brain diffusion-weighted magnetic resonance imaging (MRI).Multiple small diffusion restricted areas (arrow heads) were shown in the cerebrum and cerebellum.
Discussion
IVLBCL is a rare variant of non-Hodgkin's lymphoma. IVLBCL is characterized by its location in the small vessels of infiltrated organs and is symptomatic because of vascular occlusion and blockage of blood flow. The most common type of IVLBCL is multiple cerebral infarctions [4], while cases with cardiac involvement are rare [3]. In the present case, heart failure without obstruction of the larger coronary artery occurred prior to the multiple cerebral infarctions. This may result from lymphoma cells causing heart failure with a small myocardial infarction, as with the cerebral infarctions.
In a similar case to ours, Bauer et al. [2] reported evidence of lymphoma cell infiltration into the myocardial microvessels other than the main coronary artery at autopsy after death from myocardial infarction. However, there were no imaging findings in that case. In our case, the delayed enhancement of the penetrating wall on cardiac MRI was similar to that for myocardial infarction [5], but did not correspond to the dominant coronary artery. These signal changes were part of the myocardial infarction and may reflect edema and fibrosis of the injured myocardium. The pericardial thickening may also relate to the IVLBCL because it was reduced with R-CHOP therapy.
The lack of imaging findings and the difficulty of its detection may contribute to the poor prognosis of IVLBCL. Many of the reported cases are difficult to diagnose and the diagnosis is delayed [1]. Indeed, in our case the diagnosis was not made at the first visit and there was an exacerbation of symptoms at the second visit. Even though, the imaging findings we found in this case may contribute to the early detection and improved prognosis of IVLBCL.
In conclusion, we report a case of cardiac involvement of IVLBCL with imaging findings, which may provide clues for early diagnosis.
Patient consent
This study was approved by the Ethics Committee of our institution. Informed consent was obtained for the case report to be published.
Footnotes
Acknowledgments: We thank Edanz Group (https://en-author-services.edanz.com/ac) for editing a draft of this manuscript.
References
- 1.Ponzoni M, Campo E, Nakamura. S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks. Blood. 2018;132(15):1561–1567. doi: 10.1182/blood-2017-04-737445. [DOI] [PubMed] [Google Scholar]
- 2.Bauer A, Perras B, Sufke S, Horny HP, Kreft. B. Myocardial infarction as an uncommon clinical manifestation of intravascular large cell lymphoma. Acta cardiologica. 2005;60(5):551–555. doi: 10.2143/AC.60.5.2004979. [DOI] [PubMed] [Google Scholar]
- 3.Ferreri AJ, Campo E, Seymour JF, Willemze R, Ilariucci F, Ambrosetti. A. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant’1. Br J Haematol. 2004;127(2):173–183. doi: 10.1111/j.1365-2141.2004.05177.x. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto A, Kikuchi Y, Homma K, O'uchi T, Furui. S. Characteristics of intravascular large B-cell lymphoma on cerebral MR imaging. Am J Neuroradiol. 2012;33(2):292–296. doi: 10.3174/ajnr.A2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen. EL, Simonetti. O. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100(19):1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]