Summary
Background
Despite recent trends in declining smoking rates, tobacco smoking remains the most preventable cause of cancer in Europe. We aimed to estimate numbers and proportions of future lung cancer cases that could be potentially prevented over a 20-year period if countries in Europe were to achieve a comprehensive implementation of tobacco control policies.
Methods
Historical data from population-based cancer incidence (or mortality) was used to predict sex-specific lung cancer incidence for 30 European countries up to 2037. Hypothetical country-specific smoking prevalence that would be expected if countries would have achieved the highest-level implementation of tobacco control policies (defined by the maximum total score of the Tobacco Control Scale, TCS) was estimated by combining national prevalence data on current smoking and data on the status of implementation of key tobacco control policies. Resulting numbers and proportions of potentially preventable lung cancer cases were estimated taking into account latency periods between changes in smoking prevalence and excess cancer risks.
Findings
In Europe, an estimated 1·65 million lung cancer cases (21·2%, 19·8% in men and 23·2% in women) could be prevented over a 20-year period with the highest-level implementation of tobacco control policies. Large variation was seen in European regions and countries reflecting the current level of tobacco control, with the largest potential for prevention in Western Europe (24·5%), Southern Europe (23·1%) and Eastern Europe (22·5%), and the lowest but still substantial potential for further prevention in Northern Europe (12·5%). In women, among whom lung cancer incidence is expected to increase, we estimated somewhat larger proportions of preventable lung cancer cases ranging from 9·9 to 33·9% as compared to men (8·6–28·5%). In the final year of study period (2037), these proportions even exceed 50% in women for some countries.
Interpretation
Improved and expanded implementation of evidence-based tobacco control policies at the most comprehensive level could reduce future lung cancer incidence considerably across Europe.
Funding
The study was funded by the German Cancer Aid (“Deutsche Krebshilfe”), grant number 70112097.
Key words: Lung cancer, Prevention, Tobacco control, Potential impact fraction
Abbreviations: APC, Age-period-cohort; ci5plus, Cancer incidence in five continents; FCTC, Framework Convention on Tobacco Control; ICD, International classification of diseases; PIF, Potential impact fraction; TCS, Tobacco Control Scale; WHO, World Health Organization
Research in context.
Evidence before this study
Tobacco smoking is a major preventable contributor to cancer morbidity and premature mortality. Tobacco control policies have shown significant impact to reducing the prevalence of smoking, in particular those embedded in the World Health Organization Framework Convention on Tobacco Control. Given the extended latency time to cancer occurrence, reductions in the number of cases are difficult to assign to specific prevention measures and tobacco control achievements therefore often remain unrecognized. To identify studies on predicted impact of uptake of comprehensive tobacco control policies on lung cancer in Europe, we searched PubMed for English articles between Jan 1 2010 and September 30, 2020 using the search terms (“Tobacco smoking policy” OR “Tobacco smoking control” OR “Tobacco control policy”) AND “Cancer”. Previous studies from different countries have modelled the benefits of implementing specific tobacco control policies on the national future cancer burden and suggest that up to 25% of future lung cancer cases could be avoided with a set of country-specific tobacco control policies. However, to our knowledge, no study has assessed the impact of a comprehensive tobacco control policies implementation on future lung cancer incidence across nations and regions in Europe.
Added value of this study
In this study, we estimated the impact of broadening the implementation of tobacco control polices on the future burden of lung cancer in Europe. We used long-term historical data from countries in Europe to provide a robust estimate of future cancer incidence if the current tobacco control practice and trends were to continue and compared this to a scenario where countries were to achieve the highest score of tobacco implementation. Our modeling study provides an assessment of tobacco control on the burden of lung cancer in Europe taking into account the time needed for cancer risk to decline after stopping smoking. Our findings provide a long-term view of the benefit of comprehensive implementation of tobacco control polices in Europe that could potentially prevent an estimated 1•65 million lung cancer cases over a 20-year period. Despite recent declines in smoking prevalence and expected future declines in lung cancer rates, our findings suggest that strengthening tobacco control could reduce 9•9 to 33•9% of lung cancer cases in women and 8•6 to 28•5% in men across European countries.
Implications of all the available evidence
Our estimates provide an assessment of future tobacco policy impact based on the relation of current implementation and tobacco smoking prevalence in countries and illustrate the great potential of comprehensive implementation of tobacco control policies for 30 European countries separately and combined. This information should be motivation for national and European-level policy makers to implement comprehensive tobacco control interventions including taxes, smoke-free legislation, public information campaigns, advertising bans, health warnings/standardised packaging and access to treatment. Our model could be extended to other cancer types and regions to highlight the wide-ranging benefits of tobacco control policies implementation.
With none of the European countries currently being covered by a comprehensive tobacco control, more rigorous efforts in increasing the uptake of tobacco control policies are needed in the face of substantial challenges that can be expected given the still high prevalence of smoking and the effects of population aging.
Alt-text: Unlabelled box
1. Introduction
Lung cancer is still the most frequently diagnosed cancer and the leading cause of cancer death worldwide [1]. Tobacco smoking is the largest preventable cause of lung cancer and contributes to greater than 80% to the occurrence of this disease [2]. Given the impact of population aging, the burden of lung cancer related to tobacco smoking is likely to increase in the coming decades [3,4]. Despite recent declines in smoking prevalence, Europe as a region remains to have the highest prevalence of tobacco smoking among adults (≥ 15 years) in the world with an estimated 36% of men and 20% of women smoking tobacco in 2020 [5,6].
As a global strategy to curb the global tobacco epidemic and to translate current knowledge of effective prevention strategies into practice, the WHO Framework Convention on Tobacco Control (WHO FCTC) was adopted in 2003. The WHO FCTC entered into force in 2005 and comprises evidence-based tobacco control policies to reduce the demand for and supply of tobacco [7]. To facilitate the implementation of these policies at country-level, the WHO introduced six cost-effective measures, the so-called MPOWER policy package, which correspond to one or more articles of the WHO FCTC: Monitoring tobacco use (article 20); Protect people from tobacco smoke (article 8); Offer help to quit tobacco use (article 14); Warn about the dangers of tobacco (articles 11 and 12); Enforce bans on tobacco advertising, promotion and sponsorship (article 13) and Raise taxes on tobacco (article 6).
Although there is promising evidence that the WHO FCTC has accelerated the implementation of tobacco control policies in different policy domains, considerable variation remains between countries in Europe [8,9]. This variation is also demonstrated by the Tobacco Control Scale (TCS), which was developed by Joossens and Raw [10] to systematically quantify the implementation of tobacco control policies at country-level across Europe. This scale is used to regularly assess the status of implementation of tobacco control policies and enables a comparison between countries. The TCS allocates points to six policy domains (price of cigarettes, smoke free public and workplaces, spending on public information campaigns, bans on advertising and promotion, health warning labels and standardised packaging, treatment to help smokers quit) which should be prioritised in a comprehensive tobacco control program with a maximum potential score of 100.
In order to quantify the potential of comprehensive tobacco control implementation, we estimated the numbers and proportions of future lung cancer cases that could have been prevented over a 20-year period. We modelled scenarios assuming that countries in Europe had smoking prevalences in 2018 that could be achieved through implementation of comprehensive tobacco control policies in line with the maximum achievable score of the Tobacco Control Scale. We compared these scenarios to a reference scenario in which we projected the current level of tobacco smoking and policy into the future.
2. Methods
To quantify the potential impact of tobacco control policies on future lung cancer incidence in 30 European countries, we used macro-simulation modeling estimating the potential impact fraction (PIF) [11]. This approach requires the following population-level information: 1) lung cancer incidence by age, sex, country and year; 2) country-specific implementation level of tobacco control policies; 3) estimates of the effect of tobacco control policies on smoking prevalence; 4) the prevalence of tobacco smoking by country, age and sex; and 5) risk estimates for the association between smoking and lung cancer.
2.1. Historical and future lung cancer incidence
For the prediction of country-specific numbers and rates of incident lung cancer cases up to 2037 by sex and age, numbers of past lung cancer cases (International Classification of Diseases (ICD-10: C33-C34) were obtained from Cancer Incidence in Five Continents (CI5plus), national cancer registries, and the World Health Organization (WHO) mortality database (if no incidence data were available). Observed and predicted (based on the UN medium-fertility variant) population sizes by country, year, sex and age were derived from the United Nation's World Population Prospects 2017 [12]. The different data sources and methods of incidence estimation are described in the supplementary material (Appendix A, Supplementary Table 1).
The prediction of country-specific lung cancer incidence in 5-year interval periods was conducted using the NORDPRED software package, developed by the cancer registry of Norway [13]. Data on observed cases were aggregated into the three or four most recent 5-year periods and were extrapolated using an age-period-cohort model. As it is assumed that current trends do not last forever, an attenuation effect was included using drift parameters of 25%, and 50% in the second and third prediction period, respectively, and 75% for the fourth and fifth prediction period.
Both the number of lung cancer cases and rates were interpolated, using the median year of each 5-year interval period as basis, in order to obtain number and rates by year of study period. We calculated age-standardised incidence rates (ASR) per 100,000 person-years for the population aged 35+ using the European population of 2013 as standard [14].
2.2. Implementation of tobacco control policy by country and effect of tobacco control policies on smoking prevalence
The Tobacco Control Scale (TCS) scores in 2010 and 2016 were used to assess the country-specific implementation level of tobacco control policies [15,16] (Supplementary Table 2). The TCS, introduced by Joossens and Raw, systematically quantifies the implementation of tobacco control policies at country level for different European countries using a scoring system comprising six evidence-based and cost-effective tobacco control interventions: tobacco taxes, public places smoking bans, public information campaigns, advertising bans, health warnings (including standardised packaging) and access to treatment [10].
To examine the potential impact of implementing tobacco control policies on smoking prevalence, we crossed the Tobacco Control Scale (TCS) score in 2010 with the relative change in tobacco smoking prevalence between 2009 and 2017. For both years, sex-specific and age-standardised smoking prevalence at country-level for the population aged 35 years and above were calculated using prevalence data on current tobacco smoking (including cigarettes, cigars, cigarillos, and pipe) from the Eurobarometer [17,18] (including 26 European countries). Prevalence for further four countries (France, Iceland, Norway, Switzerland) was extracted from national statistical institutes [19], [20], [21], [22].
We performed linear mixed effect models to examine the effect of total country-specific TCS score (independent variable) on the relative change in smoking prevalence (dependent variable). We included country as random effects in order to take into account the between-country variability and performed the regression models for men and women separately and for both sexes combined.
The TCS score regression coefficients of the sex-specific models were then utilised to calculate the expected relative change in tobacco smoking prevalence by country and specifically, if countries had implemented tobacco control policies at the highest level. For this purpose, the estimated relation between TCS score on sex-specific prevalence was multiplied by the difference between the TCS score for each country in 2016 and the maximum achievable score of 100 points.
2.3. Impact of tobacco control policies on future lung cancer incidence
To assess the number and the proportion of lung cancer incidence by country that could be reduced if the highest level of tobacco control policies were implemented, we estimated the population impact fraction (PIF) [23]. This was performed using data on the prevalence of current tobacco smoking and relative risk of lung cancer related to smoking which was superimposed with the effect estimate derived from the previous step.
2.3.1. Prevalence of tobacco smoking
For our simulation, we used most recent data on prevalence of current tobacco smoking (including cigarettes, cigars, cigarillos, and pipe) by sex and age group (35–44, 45–54, 55–64, 65+) in 2017 (Supplementary Table 3). By multiplying the age- and sex-specific prevalence with the sex-specific effect estimates, we derived a theoretical prevalence under a scenario of highest-level implementation of tobacco control policies in each country. The change in tobacco prevalence was assumed to occur in 2018, by applying the effect estimates derived from the regression coefficients calculated at step 2.2 (above).
2.3.2. Risk estimates for tobacco smoking and lung cancer
Sex- and age-specific relative risk (RR) estimates for the association between smoking and lung cancer describing the increased risk of dying from lung cancer among smokers as compared to never smokers were obtained from a published report of the US health authorities (Supplementary Table 4) [24].
To account for the time gap between the reduction of tobacco smoking and reduced risk of lung cancer, the decline in cancer risk was modelled using the concept of LAT and LAG times [25]. LAT is the time during which the cancer risk remains constant after changes in exposure to a cancer risk factor. LAG is the time during which the risk among previously exposed persons declines to the level of unexposed persons. For our simulation model, we defined the LAT time to be 5 years and the LAG time to be 15 years assuming an exponential decline in cancer risk. In our study we assumed that the risk for developing cancer among people who quit smoking will accordingly remain unchanged for the subsequent 5 years after smoking cessation, followed by a gradually 15 years decline in cancer risk until the level of never smokers (RR=1) is reached.
2.3.3. Statistical analysis
The PIF derives a proportional change in cancer risk from a change in risk factor exposure due to an intervention. In order to estimate the PIF we used the prevalence of tobacco smoking in combination with the relative risk of that risk factor related to cancer, using the following formula:
where p is the age- and sex-specific prevalence of tobacco smoking; RR is the corresponding time dependent relative risk of tobacco smoking and lung cancer, and p* is the prevalence of tobacco smoking in the counterfactual scenario [23].
Using the predicted lung cancer incidence as reference, we estimated for each country and year of study period the number of cancer cases that could be expected if tobacco control policies were to be implemented at the highest level in 2018 by multiplying the age- and sex-specific PIFs with the predicted number of lung cancers.
All analyses were performed using R 3.5.3 [26], including NORDPRED.
2.4. Sensitivity analyses and secondary results
To deal with uncertainty in the modeling assumptions, sensitivity analyses were conducted using different periods of latency time (LAT: 5 years and LAG: 10, 20, and 30 years) and modeling a linear decrease in lung cancer excess risk. Finally, to show-case impact of a medium-implementation scenario, we performed the analyses assuming tobacco smoking prevalence reduction by 30% (see Appendix E for more details).
2.5. Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. TG and IS had full access to all the data in the study and had final responsibility for the decision to submit to publication.
3. Results
In 2017, overall age-standardised prevalence of current tobacco smoking in the European population aged 35 year and older was 27·4% and 20·2% in men and women, respectively (Table 1). Looking at the different European regions, the proportion of current smokers varied considerably with the lowest proportion of smokers for both sexes found in Northern Europe (men: 16·3%, women: 15·6%) and highest in Eastern Europe (men: 34·4%, women: 22·3%). Large between-country and sex-specific variations can be observed with respect to lung cancer burden in 2017. While age-standardised lung cancer incidence rates ranged from 55·9 to 185·1 per 100,000 in Sweden and Hungary, among men, the range among women was between 24·2 and 136·5 per 100,000 in Portugal and Denmark, respectively. As for the implementation of tobacco control policies, the United Kingdom was leading with a TCS score of 81 (out of 100) in 2016, followed by Ireland (70), while Austria (36) and Germany (37) ranked bottom among the 30 European countries included in this study.
Table 1.
Age-standardised smoking prevalence (35+ years), Tobacco Control Scale (TCS) scores in 2016, estimated number of cases and age-standardised incidence rates for lung cancer in 2017 in Europe by sex.
| Region/country | Smoking prevalence 1 |
TCS | Lung cancer cases |
Incidence rate per 100,000 |
|||
|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | ||
| Europe | 27·4 | 20·2 | 227,030 | 126,520 | 123·2 | 69·8 | |
| Eastern Europe | 34·4 | 22·3 | 40,170 | 17,460 | 140·8 | 51·7 | |
| Bulgaria | 45·4 | 26·7 | 47 | 3250 | 790 | 138·4 | 30·5 |
| Czech Republic | 32·3 | 19·6 | 40 | 4320 | 2410 | 123·2 | 65·7 |
| Hungary | 29·5 | 20·3 | 53 | 6480 | 4560 | 185·1 | 101·1 |
| Poland | 35·7 | 25·3 | 50 | 15,290 | 6110 | 138·1 | 47·6 |
| Romania | 33·8 | 18·8 | 56 | 8830 | 2710 | 136·4 | 35·8 |
| Slovakia | 26·3 | 17·8 | 41 | 2010 | 880 | 136·8 | 52·0 |
| Southern Europe | 32·3 | 21·1 | 60,600 | 25,940 | 119·0 | 51·4 | |
| Cyprus | 39·0 | 15·2 | 44 | 400 | 120 | 126·8 | 38·6 |
| Greece | 42·8 | 35·8 | 40 | 6660 | 1940 | 138·1 | 38·3 |
| Italy | 32·3 | 16·8 | 51 | 28,266 | 14,910 | 112·4 | 62·6 |
| Malta | 32·3 | 16·5 | 51 | 150 | 100 | 96·0 | 71·5 |
| Portugal | 29·8 | 17·3 | 50 | 3480 | 1240 | 86·2 | 24·2 |
| Slovenia | 30·9 | 21·8 | 43 | 790 | 460 | 110·1 | 63·0 |
| Spain | 30·4 | 23·7 | 55 | 20,860 | 7180 | 130·3 | 45·7 |
| Western Europe | 26·4 | 20·8 | 86,640 | 49,410 | 127·2 | 74·3 | |
| Austria | 34·1 | 22·7 | 36 | 3010 | 2100 | 100·7 | 70·8 |
| Belgium | 23·4 | 17·6 | 49 | 5680 | 2860 | 148·3 | 78·8 |
| France | 41·0 | 31·6 | 64 | 31,580 | 13,770 | 146·9 | 64·5 |
| Germany | 26·8 | 22·1 | 37 | 36,530 | 22,320 | 114·8 | 73·8 |
| Luxembourg | 27·0 | 12·3 | 37 | 210 | 120 | 86·4 | 54·7 |
| Netherlands | 21·7 | 16·5 | 53 | 7880 | 6150 | 129·3 | 112·2 |
| Switzerland | 28·9 | 20·8 | 46 | 2760 | 2100 | 93·9 | 76·8 |
| Northern Europe | 16·3 | 15·6 | 38,620 | 33,700 | 105·9 | 101·0 | |
| Denmark | 19·6 | 18·3 | 45 | 2500 | 2560 | 119·9 | 136·5 |
| Estonia | 31·7 | 14·9 | 46 | 550 | 210 | 146·4 | 40·7 |
| Finland | 24·6 | 15·1 | 60 | 1800 | 1060 | 86·0 | 51·8 |
| Iceland | 13·3 | 12·6 | 69 | 70 | 100 | 73·3 | 120·1 |
| Ireland | 18·1 | 15·9 | 70 | 1520 | 1280 | 113·7 | 107·3 |
| Latvia | 48·2 | 21·9 | 44 | 880 | 220 | 125·0 | 25·9 |
| Lithuania | 43·5 | 17·8 | 43 | 1090 | 310 | 134·1 | 27·0 |
| Norway | 22·0 | 17·4 | 63 | 1770 | 1560 | 101·7 | 102·0 |
| Sweden | 4·9 | 9·7 | 53 | 2090 | 2240 | 55·9 | 69·4 |
| United Kingdom | 14·0 | 15·9 | 81 | 26,350 | 24,160 | 111·5 | 113·2 |
Age-standardised smoking prevalence for Europe and the European regions were calculated by weighting according to population sizes.
The association between the country-specific TCS total score in 2010 and the relative change in smoking prevalence between 2009 and 2017 showed that the level of policy implementation was significantly associated with a decline in smoking prevalence in both sexes. A 10-unit increase in the TCS was associated with an average relative change in smoking prevalence by-7·9% (95%CI: −14·3 to −1·5) and −9·2% (95%:CI −17·2 to −1·2) among men and women, respectively. Detailed results of the regression models are presented in the appendix (Supplementary Table 5).
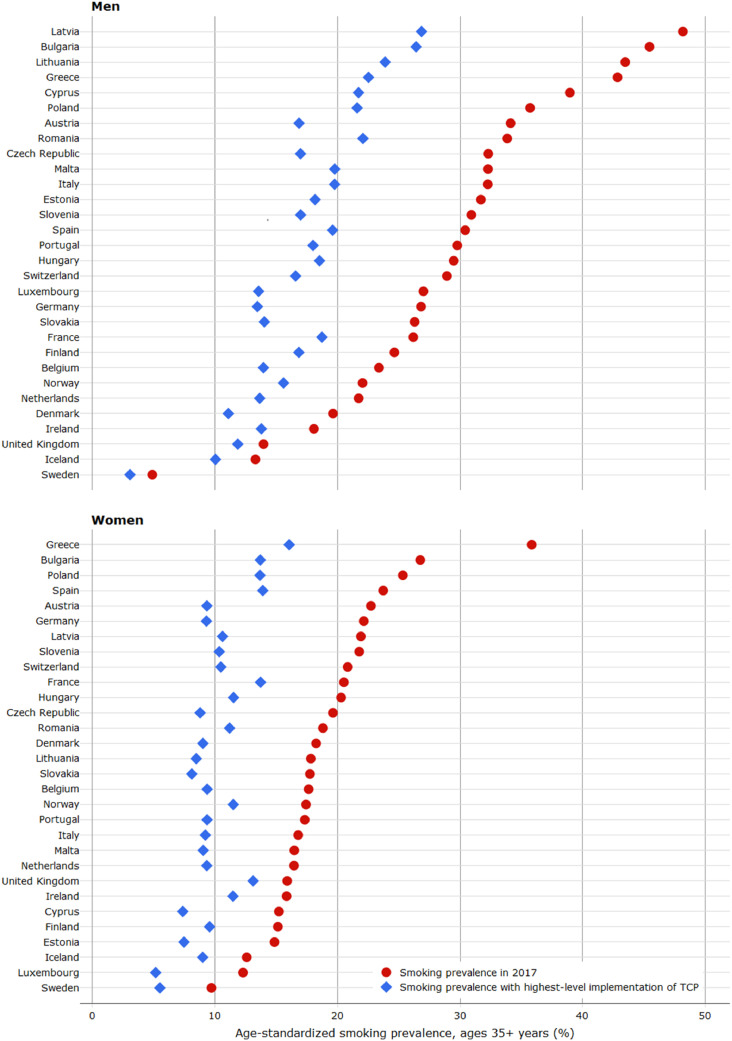
Fig. 1 shows the age-standardised smoking prevalence for the year 2017 and those estimated for a scenario of highest-level implementation of tobacco control policies. We observed large decline in smoking prevalence over the past decades in Sweden (men: -61·0%, women: −50·8%) and Ireland (men: −56·1%, women: −50·0%), while in Poland and Switzerland, smoking prevalence had only slightly declined. On the contrary, smoking prevalence among women increased in many countries, in particular in Lithuania, Latvia and Germany (Supplementary Table 6). If countries were to implement tobacco control policies at the highest level, the estimated proportional decreases in smoking prevalence ranged from 15·0% to 50·6% among men and from 17·5% to 58·9% among women, respectively. The largest impact was estimated for Austria, Germany and Luxembourg.
Fig. 1.
Age-standardised smoking prevalence in 2017 and estimated hypothetical prevalence with the highest-level of tobacco control policies (TCP) implementation (measured by the Tobacco Control Scale), by sex and country.
Overall, approximately 1·65 million lung cancer cases could have been prevented over the 20-year horizon if the 30 European countries were to implement tobacco control policies at highest level in 2018 (Table 2). This corresponds to an estimated 19·8% and 23·2% of all new lung cancer cases that are expected to occur among men and women, respectively. The proportion of potentially preventable lung cancer cases for both sexes was estimated to be largest in particular in Western Europe (24·5%), followed by Southern (23·1%) and Eastern Europe (22·5%), and lowest in Northern Europe (12·5%). Of all countries the largest proportion of prevented lung cancer cases over the 20-year study period was estimated in Austria (30·9%), followed by Germany (30·7%) and Luxembourg (30·0%). Overall, we also estimated a larger relative impact in future lung cancer cases among women; if the highest level of TCS was implemented we would expect 9·9 to 33·9% preventable lung cancers in women compared to 8·6 to 28·5% in men across European regions. Finally, we also see that the number of preventable cancers is largest at the end of the investigated time span (in 2037), with marked differences between European regions (and also across countries) ranging between 14·4 to 48·4% in men and 16·7 to 56·1% in women.
Table 2.
Predicted and preventable number (#) and proportions (%) of lung cancer cases by highest-level implementation of tobacco control policies over a 20-year period (2018–2037) and in 2037 in Europe by sex.
| Region/Country | Predicted lung cancer cases in 2018–2037 |
Preventable lung cancer cases in 2018–2037 |
Preventable lung cancer cases in 2037 |
|||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |
| # | # | # (%) | # (%) | # (%) | # (%) | |
| Europe | 4718,000 | 3095,000 | 935,000 (19·8) | 718,000 (23·2) | 83,000 (34·2) | 66,000 (38·9) |
| Eastern Europe | 742,000 | 400,000 | 154,000 (20·7) | 103,000 (25·7) | 13,000 (37·7) | 9000 (44·1) |
| Bulgaria | 58,400 | 17,600 | 12,600 (21·6) | 4700 (26·8) | 1000 (40·0) | 400 (46·4) |
| Czech Republic | 77,700 | 54,800 | 19,300 (24·8) | 16,800 (30·6) | 1600 (45·3) | 1500 (52·6) |
| Hungary | 116,400 | 102,900 | 22,500 (19·3) | 24,600 (23·9) | 1800 (35·4) | 2100 (41·2) |
| Poland | 282,700 | 141,100 | 59,000 (20·9) | 36,000 (25·5) | 5000 (37·7) | 3100 (43·9) |
| Romania | 165,300 | 62,100 | 30,000 (18·1) | 13,900 (22·4) | 2400 (33·1) | 1200 (38·4) |
| Slovakia | 41,600 | 21,900 | 10,600 (25·5) | 6700 (30·8) | 900 (44·4) | 600 (51·6) |
| Southern Europe | 1264,000 | 678,000 | 271,000 (21·5) | 177,000 (26·1) | 24,000 (37·0) | 17,000 (42·8) |
| Cyprus | 12,100 | 3400 | 3200 (26·8) | 1100 (30·6) | 300 (42·2) | 100 (48·9) |
| Greece | 142,500 | 52,800 | 37,700 (26·4) | 17,100 (32·4) | 3400 (45·3) | 1600 (52·6) |
| Italy | 551,600 | 365,700 | 116,300 (21·1) | 94,300 (25·8) | 10,400 (37·0) | 8800 (43·0) |
| Malta | 3100 | 3500 | 650 (21·0) | 1000 (27·6) | 50 (37·0) | 100 (42·6) |
| Portugal | 77,700 | 30,900 | 17,200 (22·1) | 8200 (26·4) | 1600 (37·7) | 800 (43·8) |
| Slovenia | 14,800 | 10,500 | 3600 (24·0) | 3100 (29·1) | 300 (43·1) | 300 (49·9) |
| Spain | 462,600 | 211,200 | 92,700 (20·0) | 52,100 (24·6) | 8430 (34·0) | 5100 (39·5) |
| Western Europe | 1851,000 | 1196,000 | 409,000 (22·1) | 333,000 (27·2) | 37,000 (38·3) | 30,000 (45·6) |
| Austria | 66,800 | 53,400 | 19,100 (28·5) | 18,100 (33·9) | 1800 (48·4) | 1700 (56·1) |
| Belgium | 119,800 | 72,500 | 26,700 (22·3) | 19,400 (26·7) | 2400 (38·5) | 1700 (44·7) |
| France | 672,600 | 359,200 | 105,000 (15·6) | 68,300 (19·0) | 9200 (27·1) | 6400 (31·5) |
| Germany | 749,900 | 543,400 | 205,200 (27·4) | 178,200 (32·8) | 18,500 (47·5) | 16,100 (55·2) |
| Luxembourg | 4300 | 2900 | 1200 (27·9) | 1000 (33·2) | 100 (47·4) | < 100 (55·0) |
| Netherlands | 178,900 | 143,400 | 37,600 (21·0) | 34,300 (23·9) | 3500 (35·5) | 3000 (41·2) |
| Switzerland | 58,200 | 50,300 | 13,800 (23·7) | 14,100 (28·0) | 1200 (40·8) | 1300 (47·3) |
| Northern Europe | 861,000 | 791,000 | 101,000 (11·8) | 106,000 (13·4) | 9000 (19·6) | 10,000 (22·4) |
| Denmark | 52,700 | 55,000 | 12,600 (23·9) | 15,200 (27·6) | 1100 (41·6) | 1300 (48·3) |
| Estonia | 10,000 | 4600 | 2200 (22·4) | 1300 (27·5) | 200 (40·7) | 100 (47·4) |
| Finland | 38,000 | 26,500 | 6600 (17·4) | 5600 (21·0) | 600 (30·2) | 500 (35·2) |
| Iceland | 1500 | 2400 | 200 (13·6) | 400 (16·1) | < 100 (23·4) | < 100 (27·2) |
| Ireland | 38,600 | 35,600 | 5400 (13·9) | 5800 (16·4) | 500 (22·6) | 600 (26·3) |
| Latvia | 14,500 | 4700 | 3200 (22·3) | 1300 (28·1) | 300 (42·2) | 100 (49·0) |
| Lithuania | 19,000 | 6900 | 4400 (23·3) | 2000 (29·5) | 400 (43·0) | 200 (50·0) |
| Norway | 40,600 | 35,900 | 6700 (16·5) | 6800 (18·9) | 600 (27·9) | 600 (32·5) |
| Sweden | 41,200 | 46,100 | 8200 (19·9) | 10,600 (23·0) | 700 (35·5) | 900 (41·2) |
| United Kingdom | 605,000 | 573,500 | 51,900 (8·6) | 56,800 (9·9) | 4900 (14·4) | 5200 (16·7) |
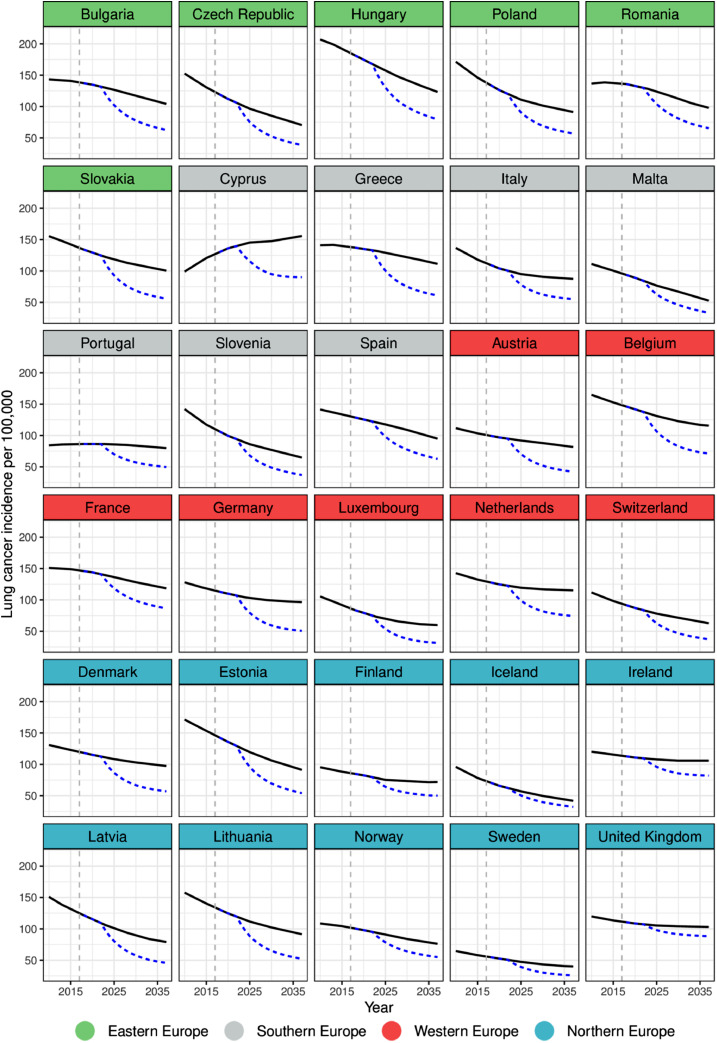
Fig. 2a, Fig. 2b show the observed and predicted trends in lung cancer rates under the status quo and under the counterfactual scenario. We observed large variation between predicted rates (with or without highest level of TCS) across regions and also within regions. Among men, decreases in lung cancer incidence rates are predicted in all countries with the exception of Cyprus (Fig. 2a). If tobacco control policies were implemented at highest-level, the lowest incidence rate in 2037 is expected in Sweden (25·8 per 100,000), while highest rates were estimated in the United Kingdom (88·2 per 100,000) and Cyprus (96·3 per 100,000).
Fig. 2a.
Observed and predicted age-standardised lung cancer incidence rates (per 100,000) among men by country in Europe. In Black (straight): Predicted rates if historical changes were to continue; In Blue (dashed): Predicted rates if the highest level of tobacco control policies were implemented. Dashed vertical gray line indicates baseline year 2017.
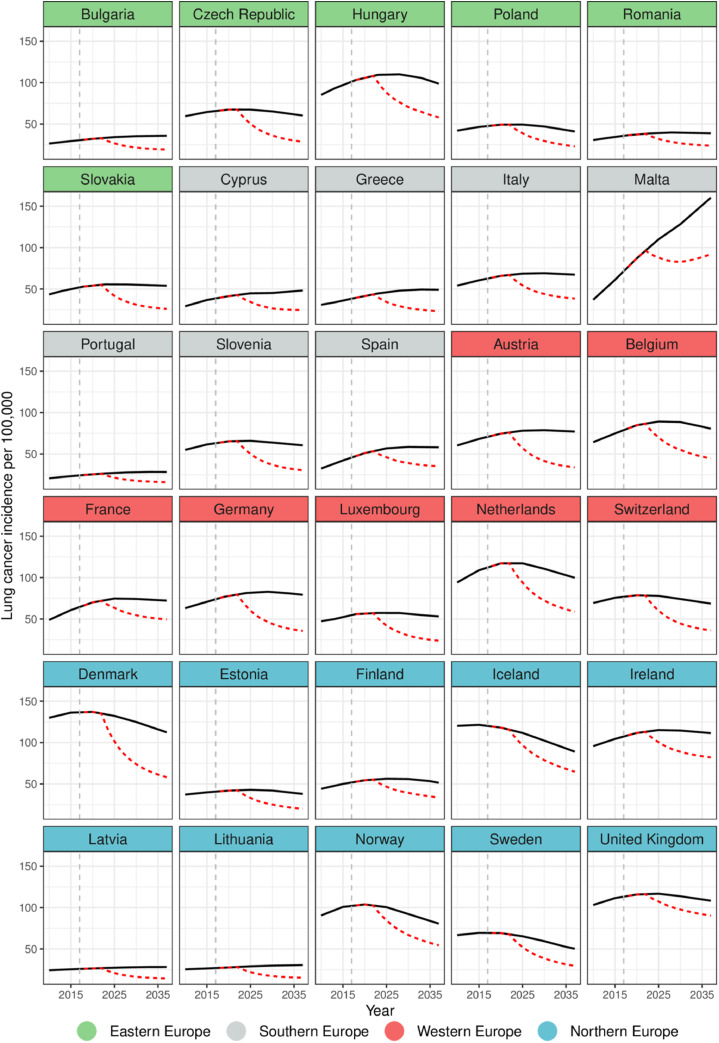
Fig. 2b.
Observed and predicted age-standardised lung cancer incidence rates (per 100,000) among women by country in Europe. In Black (straight): Predicted rates if historical changes were to continue; In Red (dashed): Predicted rates if the highest level of tobacco control policies were implemented. Dashed vertical gray line indicates baseline year 2017.
Predicted future lung cancer incidence rates among women are quite different as compared to those of men (Fig. 2b) in that they are expected to increase over few years, followed by a peak and a slight decline at the end. If the highest level of tobacco control policies was implemented, the lowest incidence rate in 2037 was estimated in Latvia (14·4 per 100,000), while the highest rate was expected in the United Kingdom and Malta (92·0 and 97·3 per 100,000).
The results from the sensitivity analyses using different periods of latency time (LAT and LAG) and modeling a linear decrease in lung cancer excess risk are presented in Appendix E.
4. Discussion
Given the current progress made in implementation of tobacco control policies in various countries in Europe, we sought to estimate the impact of an enforced implementation of tobacco control policies on the future lung cancer incidence in Europe. Our results suggest that of all lung cancer cases expected between 2018 and 2037 in the investigated 30 European countries, approximately 19·8% (~935,000 cases) among men and 23·2% (~718,000 cases) among women could be prevented by achieving the highest-level implementation of tobacco control policies by 2018. The impact varies across European regions, across countries, and between sex: at the end of our study period, in 2037, we could expect a reduction of lung cancer incidence by 14·4 to 48·4% in men and by 16·7 to 56·1% in women across European countries if tobacco control efforts were to be taken up to the highest level.
While lung cancer incidence rates are currently still lower in women than in men in almost all countries, they are projected to rise in the next decade reflecting the differential progression of the tobacco epidemic. Indeed, the tobacco smoking prevalence among women is declining much slower in many countries, such as France and Greece, or is even increasing in few countries such as Slovakia and the Czech Republic. Contributing factors to the gender disparity may be the participation of women in traditionally male-led socioeconomic activities as well as the tobacco companies’ specifically targeted marketing to women [5,27]. To address these differentiated trends in tobacco-use, gender-sensitive policy actions are needed to reverse the increasing trend in smoking among women in some countries and to further accelerate the downward trend among men.
Yet, we have observed a substantial decrease in smoking prevalence especially among the younger age groups [5]. Since the 1970s, smoking initiation rates in youth and young adults declined continuously across Europe, with the exception of South Europe where this decline leveled off after 1990 [28]. Although the reasons for this internationally observed trend have not yet been fully researched, alongside general social and cultural changes and an increased awareness of smoking as leading cause of preventable illness, the implementation of tobacco control policies over time may have played an important role [29]. In particular price increases on cigarettes [6], enforced legislation on smoke-free environments [30] as well as advertising bans [31] are measures that effectively address youth and young adults and may have been related to this decrease. In the Scandinavian countries, which stand at the forefront of efforts in implementing tobacco control policies, we can observe considerably lower rates of smoking initiation among youth and young adults than in other European regions [28]. As smoking behavior is typically established during adolescence, enhanced and targeted efforts are needed to protect young people and to ensure further prevalence declines in European countries.
Beside sex-specific differences in the stage of the epidemic, we also observed variation in lung cancer rates by region in Europe with higher current rates tending to be observed in Northern European countries among women and Eastern European countries among men. This pattern of the lung cancer burden corresponds well with the development of smoking epidemic in Europe where the uptake of smoking initially started in Northern Europe among men followed by Western Europe, Southern Europe and Eastern Europe [32].
The north-to-south gradient can partly be explained by differences in the socioeconomic development of countries with presently large socioeconomic differences in tobacco smoking and the tobacco related-cancer burden within all countries. For example, higher smoking prevalence was consistently reported among people with lower education in countries across Europe [5]. A review of socioeconomic difference in policy impact suggested that tobacco price increases have the greatest potential to reduce socioeconomic inequalities in smoking, and should therefore be considered when implementing national tobacco control policies [33].
The declines in lung cancer incidence rates following decreases in smoking rates are evidence of the effectiveness of policies and highlight the potential benefit from reinforcing the prevention efforts. Due to the long latency period between tobacco smoking and the occurrence of cancer, the impact of tobacco control policies takes years to decades to become apparent in changes in cancer burden. Consequently, decreases in number of cancer cases are difficult to assign to specific prevention measures and tobacco control achievements therefore often remain unrecognized. Furthermore, recent progress made in the implementation of tobacco control policies in Europe are not yet evident in observed lung cancer cases and cannot be reflected in the predicted lung cancer incidence in our analysis. For example, the United Kingdom and Ireland had recently achieved substantial reductions in smoking prevalence by introducing smoking cessation services, smoke-free workplaces and standardised packaging [34]. As our predictions do not take into account those recent changes, the projected lung cancer rates for those countries are likely to be overestimated.
We expect a decline in future lung cancer rates which most likely relate to implementation of key tobacco control policies as per MPOWER policy package in some European countries [35], but considerable variation in the level of implementation remains between countries and policy domain: First, the implementation of health warnings has particularly progressed in recent years, and almost all countries in this study (excluding Iceland and Switzerland) are meeting the MPOWER recommendations by adopting large graphic warnings on both sides of the cigarette pack. Health warnings providing information about the risks associated with tobacco use can prompt smokers to reduce their consumption and to quit smoking [36]. More recently, France and the United Kingdom have implemented standardised tobacco packaging in 2017, followed by Norway, Ireland and Slovenia, which is expected to further reduce attractiveness of tobacco packaging [37].
Secondly, comprehensive bans on tobacco advertising, promotion and sponsorship (TAPS) activities which have been effective to particularly reduce youth tobacco use initiation and therefore smoking prevalence [31,38] have been comprehensively put in place only in few countries, namely in Finland, Iceland, Norway, Slovenia and Ireland [37].
Further, only a few countries (Hungary, Ireland, Romania, Spain, and the United Kingdom) have adopted comprehensive smoke-free laws covering all indoor public places and workplaces [37]. Comprehensive smoke-free laws are effective in decreasing exposure to second-hand smoke [39] and may additionally promote healthier behaviours such as the voluntary adoption of smoke-free homes [40].
Although all countries provide access to pharmacological therapy to support smoking cessation [35], only very few (Czech Republic, Denmark, Hungary, Ireland, Slovenia, and the United Kingdom) offer a national quit-line as well as full cost-coverage of their tobacco use cessation services [37]. Improved and expanded implementation of these domains across Europe could substantially reduce tobacco consumption.
Finally, raising taxes to increase the price of tobacco products has been shown to be the most effective population-level tobacco control policy: each 10% price increase is linked to a 4–5% reduction in tobacco smoking in high-income and low- and middle-income countries, respectively [41]. More than half of the observed countries in our study (Austria, Belgium, Bulgaria, Czech Republic, Estonia, Finland, France, Greece, Ireland, Italy, Malta, Poland, Slovakia, Slovenia, Spain, and the United Kingdom) have raised tobacco taxes to above 75% of the base price for most brands of cigarettes. However, tax rates vary considerably across Europe with a large proportion of countries still having a very high affordability of cigarettes [42]. Furthermore, real prices of cigarettes have often remained unchanged and tobacco control efforts are often undermined by the availability of cheap alternative tobacco products, such as roll-your-own tobacco [43].
Generally, evidence indicates that the most effective strategy to reduce smoking-related cancer burden is the implementation of comprehensive tobacco control policies. Although progress in tobacco control has been made in some policy domains since MPOWER was introduced in 2007, to date none of the 30 countries has fully implemented all six policy measures that were considered in this study. In this context, it is important to note that the FCTC comprises a number of further policy measures aiming at curbing the tobacco epidemic [35]. Further implementation of innovative effective measures is indeed needed if tobacco-related cancers were to be eliminated. Example to this is the new action are Australia [44] and New Zealand [45] using multifaceted tobacco control strategies (including ongoing annual tobacco tax increases, reduced tobacco supply and number of retail outlets) to finally eliminate smoking in their populations. In order to achieve a tobacco-free society in Europe, similar or even stronger tobacco control strategies [46] will be needed.
4.1. Strengths and limitations
This is the first modeling study to provide estimates of the impact of highest-level implementation of tobacco control policies on future cancer incidence in 30 European countries using recent smoking prevalence data and risk estimates. High quality population-based cancer registry data was used to predict the future cancer burden, using a well-established age-period-cohort model. For six countries lacking incidence data, incidence had to be estimated based on mortality data by applying a mortality to incidence ratio, a proxy for case fatality which though partly reflects health system effectiveness [47]. Overall, our results are in line with previous national or regional studies indicating that the future burden of lung cancer in Europe could be considerably reduced by implementing evidence-based tobacco control policies [11,[48], [49], [50], [51], [52]].
We examined the association between implementation of tobacco control polices and the percentage change in smoking prevalence over time. Our results indicate that, at the ecological level, countries with stronger tobacco control policies have a lower smoking prevalence and are more successful in reducing smoking rates [8,9,53]. However, given the low number of observations and tobacco control implementation as exclusive explanatory variable, there is large uncertainty in the final regression model which should be taken into account when looking at results pertaining to the impact of TCS implementation on tobacco prevalence by country. Yet compared to previous studies, similar impact size was found and used [54, 55].
Furthermore, we used the same sex-specific coefficients for all countries to calculate a hypothetical smoking prevalence under successful implementation of TCS. This is likely to overestimate the real effect of tobacco control policies for countries with already low smoking prevalence, e.g., Sweden, while the real effect is likely to be underestimated for countries with high smoking rates, such as Hungary. Some seemingly counterintuitive results might have emerged from the specific methodological approach taken in our study. For example, in France we saw smaller predicted decrease in smoking prevalence as compared to Austria. This is due to the high reported Tobacco Control Scale score in France and the ensuing smaller relative change in the smoking prevalence. As regards Sweden, it is also worth noting that by focusing on lung cancer we did not take into account other harmful effects of the Swedish Snus, which has widely substituted cigarettes especially in the male Swedish population [56] and which, albeit being less harmful than conventional cigarettes, is certainly not risk-free [57].
For the simulation in this study, we had to rely on the prevalence of current smoking reflecting both daily and occasional smoking. Nevertheless, even long-term low-intensity smoking has been shown to significantly increase lung cancer mortality [58]. While the stratification by age group at least partly reflects differences in smoking patterns over the life-course, differentiated modeling considering detailed smoking-related factors such as the intensity and duration of smoking over the life course, as well as the age at smoking cessation was however not possible due to limited availability of appropriate data, so we had to assume homogeneity of smoking patterns. It must be pointed out however, that this homogeneity assumption disregards substantial differences in the intensity of tobacco smoking across European countries, with for example daily smokers from Cyprus, Austria, Hungary or Greece smoking about 18 to 19 cigarettes per day and those from Sweden, Latvia, Spain, Lithuania or United Kingdom smoking around 12 cigarettes or less a day [18]. With respect to second-hand smoke exposure, direct and indirect effects of tobacco control accompanying the declines in smoking prevalence were likewise not considered in our study. We also could not incorporate diminishing or accelerating effects of policies over time in our projection model due to a limited availability of empirical evidence on the magnitude of such effects. Such factors may influence the impact of tobacco control policies on the smoking prevalence and may lead to either under- or overestimation of the potential of prevention. Further studies should be directed towards more differentiated modeling to enable detailed consideration of heterogeneity across countries and within populations.
In general, our simulation modeling framework is based on several assumptions, that inherently bring along some limitations and lead to a simplification of the complex reality. The purpose of this study was to highlight the difference in number of lung cancer cases by comparing a scenario of changing one factor, i.e., smoking prevalence, with a status quo scenario. Ultimately, our goal is to enable the quantification of the impact of tobacco control policies taking into account time trends in lung cancer incidence as well as latency periods between the implementation of tobacco control and change in cancer risk.
Taken together, our results suggest that a substantial number of lung cancer cases in the next decades in Europe could be reduced by a comprehensive implementation of tobacco control policies. Further assessment of the impact of expanding the implementation of WHO/FCTC taking into account local context is needed. In view of the tremendous expected future burden of lung cancer there is a great need to reinforce prevention efforts by implementing evidence-based tobacco control policies at the most comprehensive level.
Contributors
TG, UM, HB, and IS conceived and designed the study. TG and IS had access to all the data and contributed to data analysis. TG, UM and IS contributed to manuscript writing. TG and TN designed the model and TG conducted the analyses. All authors evaluated the findings, commented on manuscript drafts and approved the final version of the manuscript.
Data sharing statement
All data used in this reseach are partly provided in the Supplemenatary Appendix or are publicly available and can be obtained following the corresponding reference.
Declaration of Interests
Mr. Gredner has nothing to disclose.
Dr. Mons reports grants from German Cancer Aid (Deutsche Krebshilfe), during the conduct of the study; and Dr. Mons headed the WHO Collaborating centre for Tobacco Control at the German Cancer Research Center (DKFZ) in Heidelberg from September 2016 till June 2020. In this role, Dr. Mons advised the German Government and other national and international policymaking bodies on topics related to tobacco control.
Dr. Niedermaier has nothing to disclose.
Dr. Brenner has nothing to disclose.
Dr. Soerjomataram has nothing to disclose.
Acknowledgements
This work was undertaken during the tenure of a doctoral research stay at the International Agency for Research on Cancer. The analyses for this project were supported by a grant from the German Cancer Aid (No. 70112097) to the German Cancer Research Center (Ute Mons, Hermann Brenner). The authors gratefully acknowledge all cancer registries and their staff for providing the data needed for this study.
Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanepe.2021.100074.
Appendix. Supplementary materials
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Kulhanova I., Forman D., Vignat J., Espina C., Brenner H., Storm H.H. Tobacco-related cancers in Europe: the scale of the epidemic in 2018. Eur J Cancer. 2020;139:27–36. doi: 10.1016/j.ejca.2020.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Forman D., Bauld L., Bonanni B., Brenner H., Brown K., Dillner J. Time for a European initiative for research to prevent cancer: a manifesto for Cancer Prevention Europe (CPE) J Cancer Policy. 2018;17:15–23. [Google Scholar]
- 4.Mons U., Brenner H. Demographic ageing and the evolution of smoking-attributable mortality: the example of Germany. Tob Control. 2017;26(4):455–457. doi: 10.1136/tobaccocontrol-2016-053008. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . 2019. Regional office for Europe. European tobacco use. trend.http://www.euro.who.int/en/health-topics/disease-prevention/tobacco/publications/2019/european-tobacco-use-trends-report-2019-2019 Available from: [Google Scholar]
- 6.2018. WHO global report on trends in prevalence of tobacco smoking 2000–2025.https://www.who.int/tobacco/publications/surveillance/trends-tobacco-smoking-second-edition/en/ Available from: [Google Scholar]
- 7.World Health Organization . 2003. WHO framework convention on tobacco control.https://www.who.int/fctc/text_download/en/ Available from: [Google Scholar]
- 8.Gravely S., Giovino G.A., Craig L., Commar A., D'Espaignet E.T., Schotte K. Implementation of key demand-reduction measures of the WHO Framework Convention on Tobacco Control and change in smoking prevalence in 126 countries: an association study. Lancet Public Health. 2017;2(4):e166–ee74. doi: 10.1016/S2468-2667(17)30045-2. [DOI] [PubMed] [Google Scholar]
- 9.Feliu A., Filippidis F.T., Joossens L., Fong G.T., Vardavas C.I., Baena A. Impact of tobacco control policies on smoking prevalence and quit ratios in 27 European Union countries from 2006 to 2014. Tob Control. 2019;28(1):101–109. doi: 10.1136/tobaccocontrol-2017-054119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joossens L., Raw M. The Tobacco Control Scale: a new scale to measure country activity. Tob Control. 2006;15(3):247–253. doi: 10.1136/tc.2005.015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soerjomataram I., de Vries E., Engholm G., Paludan-Müller G., Brønnum-Hansen H., Storm H.H. Impact of a smoking and alcohol intervention programme on lung and breast cancer incidence in Denmark: an example of dynamic modelling with Prevent. Eur J Cancer. 2010;46(14):2617–2624. doi: 10.1016/j.ejca.2010.07.051. [DOI] [PubMed] [Google Scholar]
- 12.United Nations, Department of Economics and Social Affairs, Population Division. World population prospects. the 2017 revision.
- 13.Moller B., Fekjaer H., Hakulinen T., Sigvaldason H., Storm H.H., Talback M. Prediction of cancer incidence in the Nordic countries: empirical comparison of different approaches. Stat Med. 2003;22(17):2751–2766. doi: 10.1002/sim.1481. [DOI] [PubMed] [Google Scholar]
- 14.Eurostat. European Comission . 213 edition. Publications Office of the European Union; Luxembourg: 2013. Revision of the European standard population. Report of Eurostat's task force. [Google Scholar]
- 15.Joossens L.R., Martin. The tobacco control scale 2016 in Europe. A report of the association of European cancer leagues. Available from: https://www.tobaccocontrolscale.org/
- 16.Joossens L.R., Martin. The tobacco control scale 2010 in Europe. A report of the association of European cancer leagues. Available from: https://www.tobaccocontrolscale.org/
- 17.European Commission . 2009. Eurobarometer 72.3. tobacco. Special Eurobarometer 332. [Google Scholar]
- 18.European Commission Attitudes of Europeans towards tobacco and electronic cigarettes. Spec Eurobarometer. 2017;458 [Google Scholar]
- 19.Statistics Iceland. Smoking habits by sex and age 1989-2017. directorate of health. Available from: https://statice.is/.
- 20.Statistics Norway. Tobacco, alcohol and other drugs. Available from: https://www.ssb.no/en/statbank/table/05307/.
- 21.Federal Statistical Office Switzerland. Swiss health survey 2017. Available from: https://www.bfs.admin.ch/bfs/en/home/statistics/health/determinants.html
- 22.Pasquereau A., Andler R., Guignard R., Richard J., Arwidson P., Nguyen-Thanh V. La consommation de tabac en France: premiers résultats du baromètre santé 2017. Bull épidémiol hebd. 2018;14:265–273. [Google Scholar]
- 23.Barendregt J.J., Veerman J.L. Categorical versus continuous risk factors and the calculation of potential impact fractions. J Epidemiol Commun Health. 2010;64(3):209–212. doi: 10.1136/jech.2009.090274. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Department of Health and Human Services . 2014. The health consequences of smoking: a report of the surgeon general report. [Google Scholar]
- 25.International Agency for Research on Cancer . Vol. 11. IARC Handbooks of Cancer Prevention; 2007. (Tobacco control: reversal of risk after quitting smoking). [Google Scholar]
- 26.R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2008. R: a language and environment for statistical computing.http://www.R-project.org [Google Scholar]
- 27.Samet J.M., Yoon S.-.Y. 2010. World health organization. Gender, women, and the tobacco epidemic: world health organization. [Google Scholar]
- 28.Marcon A., Pesce G., Calciano L., Bellisario V., Dharmage S.C., Garcia-Aymerich J. Trends in smoking initiation in Europe over 40 years: a retrospective cohort study. PLoS ONE. 2018;13(8) doi: 10.1371/journal.pone.0201881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ball J., Sim D., Edwards R. Why has adolescent smoking declined dramatically? Trend analysis using repeat cross-sectional data from New Zealand 2002–2015. BMJ Open. 2018;8(10) doi: 10.1136/bmjopen-2017-020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song A.V., Dutra L.M., Neilands T.B., Glantz S.A. Association of smoke-free laws with lower percentages of new and current smokers among adolescents and young adults: an 11-year longitudinal study. JAMA Pediatr. 2015;169(9) doi: 10.1001/jamapediatrics.2015.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lovato C., Watts A., Stead L.F. Cochrane Database Systematic Rev. 2011. Impact of tobacco advertising and promotion on increasing adolescent smoking behaviours. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lortet-Tieulent J., Renteria E., Sharp L., Weiderpass E., Comber H., Baas P. Convergence of decreasing male and increasing female incidence rates in major tobacco-related cancers in Europe in 1988-2010. Eur J Cancer. 2015;51(9):1144–1163. doi: 10.1016/j.ejca.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 33.Hill S., Amos A., Clifford D., Platt S. Impact of tobacco control interventions on socioeconomic inequalities in smoking: review of the evidence. Tob Control. 2014;23(e2):e89–e97. doi: 10.1136/tobaccocontrol-2013-051110. [DOI] [PubMed] [Google Scholar]
- 34.Hunt D., Knuchel-Takano A., Jaccard A., Bhimjiyani A., Retat L., Selvarajah C. Modelling the implications of reducing smoking prevalence: the public health and economic benefits of achieving a ‘tobacco-free'UK. Tob Control. 2018;27(2):129–135. doi: 10.1136/tobaccocontrol-2016-053507. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization . 2019. WHO report on the global tobacco epidemic, 2019: monitoring tobacco use and prevention policies.https://apps.who.int/iris/bitstream/handle/10665/326043/9789241516204-eng.pdf?ua=1 Available from: [Google Scholar]
- 36.Ngo A., Cheng K.-.W., Shang C., Huang J., Chaloupka F. Global evidence on the association between cigarette graphic warning labels and cigarette smoking prevalence and consumption. Int J Environ Res Public Health. 2018;15(3):421. doi: 10.3390/ijerph15030421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joossens L.R., Martin. The tobacco control scale 2019 in Europe. a report of the association of European cancer leagues. Available from: https://www.tobaccocontrolscale.org/2020.
- 38.Blecher E. The impact of tobacco advertising bans on consumption in developing countries. J Health Econ. 2008;27(4):930–942. doi: 10.1016/j.jhealeco.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 39.U.S. Department of Health and Human Services . U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta: 2006. The health consequences of involuntary exposure to tobacco smoke: a report of the surgeon general. [Google Scholar]
- 40.Mons U., Nagelhout G.E., Allwright S., Guignard R., van den Putte B., Willemsen M.C. Impact of national smoke-free legislation on home smoking bans: findings from the International Tobacco Control Policy Evaluation Project Europe Surveys. Tob Control. 2013;22(e1):e2–e9. doi: 10.1136/tobaccocontrol-2011-050131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The economics of tobacco and tobacco control. 2017 https://www.who.int/tobacco/publications/economics/nci-monographseries-21/en/ and Geneva, Switzerland: World Health OrganizationAvailable from: [Google Scholar]
- 42.World Health Organization . 2019. WHO report on the global tobacco epidemic 2019: offer help to quit tobacco use.https://www.who.int/publications/i/item/who-report-on-the-global-tobacco-epidemic-2019-offer-help-to-quit-tobacco-use Available from: [Google Scholar]
- 43.Partos T.R., Gilmore A.B., Hitchman S.C., Hiscock R., Branston J.R., McNeill A. Availability and use of cheap tobacco in the United Kingdom 2002–2014: findings from the International Tobacco Control Project. Nicotine Tob Res. 2017;20(6):714–724. doi: 10.1093/ntr/ntx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirby T. Australia tax increases to price cigarettes out of reach. Lancet Oncol. 2016;17(6):e228. doi: 10.1016/S1470-2045(16)30136-X. [DOI] [PubMed] [Google Scholar]
- 45.van der Deen F.S., Wilson N., Cleghorn C.L., Kvizhinadze G., Cobiac L.J., Nghiem N. Impact of five tobacco endgame strategies on future smoking prevalence, population health and health system costs: two modelling studies to inform the tobacco endgame. Tob Control. 2018;27(3):278–286. doi: 10.1136/tobaccocontrol-2016-053585. [DOI] [PubMed] [Google Scholar]
- 46.Beaglehole R., Bonita R., Yach D., Mackay J., Reddy K.S. A tobacco-free world: a call to action to phase out the sale of tobacco products by 2040. Lancet. 2015;385(9972):1011–1018. doi: 10.1016/S0140-6736(15)60133-7. [DOI] [PubMed] [Google Scholar]
- 47.Asadzadeh Vostakolaei F., Karim-Kos H.E., Janssen-Heijnen M.L., Visser O., Verbeek A.L., Kiemeney L.A. The validity of the mortality to incidence ratio as a proxy for site-specific cancer survival. Eur J Public Health. 2011;21(5):573–577. doi: 10.1093/eurpub/ckq120. [DOI] [PubMed] [Google Scholar]
- 48.Poirier A.E., Ruan Y., Grevers X., Walter S.D., Villeneuve P.J., Friedenreich C.M. Estimates of the current and future burden of cancer attributable to active and passive tobacco smoking in Canada. Prev Med. 2019;122:9–19. doi: 10.1016/j.ypmed.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 49.Andersson T.M., Engholm G., Brink A.L., Pukkala E., Stenbeck M., Tryggvadottir L. Tackling the tobacco epidemic in the Nordic countries and lower cancer incidence by 1/5 in a 30-year period-The effect of envisaged scenarios changing smoking prevalence. Eur J Cancer. 2018;103:288–298. doi: 10.1016/j.ejca.2018.02.031. [DOI] [PubMed] [Google Scholar]
- 50.Didkowska J., Wojciechowska U., Koskinen H.L., Tavilla A., Dyba T., Hakulinen T. Future lung cancer incidence in Poland and Finland based on forecasts on hypothetical changes in smoking habits. Acta Oncol. 2011;50(1):81–87. doi: 10.3109/0284186X.2010.488247. [DOI] [PubMed] [Google Scholar]
- 51.Mulder I., Hoogenveen R.T., van Genugten M.L., Lankisch P.G., Lowenfels A.B., de Hollander A.E. Smoking cessation would substantially reduce the future incidence of pancreatic cancer in the European Union. Eur J Gastroenterol Hepatol. 2002;14(12):1343–1353. doi: 10.1097/00042737-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 52.Gredner T., Niedermaier T., Brenner H., Mons U. Impact of tobacco control policies on smoking-related cancer incidence in Germany 2020 to 2050-a simulation study. Cancer Epidemiol Biomarkers Prev. 2020;29(7):1413–1422. doi: 10.1158/1055-9965.EPI-19-1301. [DOI] [PubMed] [Google Scholar]
- 53.Ngo A., Cheng K.W., Chaloupka F.J., Shang C. The effect of MPOWER scores on cigarette smoking prevalence and consumption. Prev Med. 2017;105S doi: 10.1016/j.ypmed.2017.05.006. S10-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levy D.T., Huang A.T., Currie L.M., Clancy L. The benefits from complying with the framework convention on tobacco control: a SimSmoke analysis of 15 European nations. Health Policy Plan. 2014;29(8):1031–1042. doi: 10.1093/heapol/czt085. [DOI] [PubMed] [Google Scholar]
- 55.Levy D.T., Tam J., Kuo C., Fong G.T., Chaloupka F. The impact of implementing tobacco control policies: the 2017 tobacco control policy scorecard. J Public Health Manag Pract. 2018;24(5):448–457. doi: 10.1097/PHH.0000000000000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramström L., Borland R., Wikmans T. Patterns of smoking and snus use in Sweden: implications for public health. Int J Environ Res Public Health. 2016;13(11):1110. doi: 10.3390/ijerph13111110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Byhamre M.L., Araghi M., Alfredsson L., Bellocco R., Engström G., Eriksson M. Swedish snus use is associated with mortality: a pooled analysis of eight prospective studies. Int J Epidemiol. 2021;49(6):2041–2050. doi: 10.1093/ije/dyaa197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Inoue-Choi M., Liao L.M., Reyes-Guzman C., Hartge P., Caporaso N., Freedman N.D. Association of long-term, low-intensity smoking with all-cause and cause-specific mortality in the national institutes of health-AARP diet and health study. JAMA Intern Med. 2017;177(1):87–95. doi: 10.1001/jamainternmed.2016.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.