Summary
This protocol entails a simple method for isolation, culturing, and in vitro differentiation of adult neural stem cells from the dentate gyrus in the hippocampus and the subventricular zone of adult mice. Cultured adult neural stem cells are an important in vitro model to investigate stem cell properties such as proliferation and differentiation and to expand the understanding of plasticity in the adult brain.
For complete details on the use and execution of this protocol, please refer to Isaksen et al. (2020).
Subject areas: Cell culture, Cell isolation, Neuroscience, Stem Cells, Cell Differentiation
Graphical abstract
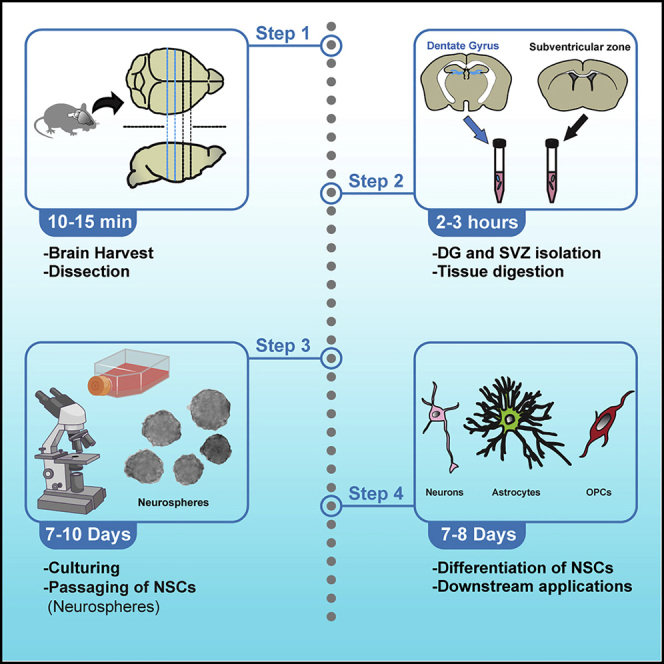
Highlights
-
•
A simple protocol for mouse adult neural stem cell isolation and culture
-
•
Targeted differentiation into various neural cells
-
•
Potential use of derived cells for studying brain plasticity
This protocol entails a simple method for isolation, culturing, and in vitro differentiation of adult neural stem cells from the dentate gyrus in the hippocampus and the subventricular zone of adult mice. Cultured adult neural stem cells are an important in vitro model to investigate stem cell properties such as proliferation and differentiation and to expand the understanding of plasticity in the adult brain.
Before you begin
Aliquot preparation
Timing: 1–2 h
-
1.
Dissolve Forskolin and Retinoic acid to 1mM in DMSO. Store as aliquots at −20°C.
Note: It is recommended to use Retinoic Acid aliquots within 1 month as it is unstable and very sensitive to light and air exposure, whereas Forskolin aliquots could be stored for up to 6 months.
-
2.
Use sterile H2O to reconstitute basic fibroblast growth factor (FGF2) and epidermal growth factor (EGF) to 100 μg/mL, apo-transferrin to 5 mg/mL, biotin and hydrocortisone to 1 μM, insulin to 5 mg/mL, PDGF-AA to 1 μg/mL and sodium selenite to 3 μM.
Store as aliquots at −20 to −80°C.
Note: The frozen aliquots could generally remain stable for up to 12 months. For PDFG-AA, storage for up to 6 months is recommended.
-
3.
Reconstitute bovine serum albumin (BSA) in PBS to 10% BSA aliquots and store at −20°C.
Note: BSA frozen aliquots could remain stable for up to 12–24 months.
Media preparation
Timing: 1 hour
-
4.
Prepare dissection buffer, wash media, and growth media (see materials and equipment for recipes).
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Alexa Fluor 488 – Secondary antibody (1:800) | Invitrogen | A-21042 |
| Alexa Fluor 568 – Secondary antibody (1:800) | Invitrogen | A-11011 |
| Anti-glial fibrillary acidic protein (GFAP) (1:2) | Dako | GA52461-2J |
| Anti-microtubule-associated protein 2 (Map2) (1:400) | Sigma-Aldrich | M4403 |
| Anti-nestin (1:400) | Merck Millipore | Mab353 |
| Anti-oligodendrocyte transcription factor (Olig2) (1:500) | Merck Millipore | Mabn50 |
| Anti-Sox2 (1:400) | Merck Millipore | Mab4343 |
| Chemicals, peptides, and recombinant proteins | ||
| 4′,6-diamidino-2-phenylindole (DAPI) | Thermo Fisher Scientific | D1306 |
| Antibiotic-Antimycotic (100×) | Thermo Fisher Scientific | 15240062 |
| apo-Transferrin | Sigma-Aldrich | T2036 |
| B27 without vitamin A (50×) | Thermo Fisher Scientific | 12587010 |
| Biotin | Sigma-Aldrich | B4501 |
| Bovine serum albumin | Sigma-Aldrich | A7906 |
| Bromodeoxyuridine/5-bromo-2'-deoxyuridine (BrdU) | Sigma-Aldrich | B5002 |
| DMEM/F-12 | Thermo Fisher Scientific | 11320033 |
| EGF | PeproTech | 315-09 |
| FGF2 | PeproTech | 100-18B |
| Forskolin | Sigma-Aldrich | F3917 |
| GlutaMAX (100×) | Thermo Fisher Scientific | 35050061 |
| Hank's balanced salt solution | Thermo Fisher Scientific | 14170070 |
| HEPES | Sigma-Aldrich | H3375 |
| Hydrocortisone | Sigma-Aldrich | H0888 |
| Insulin | Sigma-Aldrich | I6634 |
| Laminin | Sigma-Aldrich | L2020 |
| N2 supplement (×100) | Thermo Fisher Scientific | 175002048 |
| Neural Stem Cell Basal Medium | Merck Millipore | SCM003 |
| Platelet-Derived Growth Factor-AA (PDGF-AA) | PeproTech | 100-13a |
| Poly-L-ornithine | Sigma-Aldrich | P4957 |
| Retinoic acid | Sigma-Aldrich | R2625 |
| Sodium selenite | Sigma-Aldrich | S5261 |
| Trypsin-EDTA 0.5% (10×) | Sigma-Aldrich | T417 |
| Trypsin Inhibitor | Sigma-Aldrich | T6522 |
| Experimental models: organisms/strains | ||
| C57BL/6 mice | N/A | N/A |
| Other | ||
| 26G needle | Terumo | NN2613R |
| 27G needle | Terumo | NN2713R |
| Blade handle#3 | Feather | 2977#3 |
| Dumont #5 Forcepsa | Fine Science Tools | 11252-20 |
| Dumont #5/45 Forceps | Fine Science Tools | 11251-35 |
| Extra Fine Graefe Forceps | Fine Science Tools | 11150-10 |
| Friedman-Pearson Rongeurs | Fine Science Tools | 16221-14 |
| Hardened Fine Scissors | Fine Science Tools | 14091-09 |
| Micro scissors | Nadox | MB-50-10 |
| Surgical blade#11 | Feather | 2975#11 |
| Surgical scissors | Fine Science Tools | 14002-14 |
| Mouse Brain Slicer Matrix | Zivic Instruments | BSMAS001-1 |
| Stereomicroscope | Olympus | SZX7 |
| Ultra-Low Attachment 24-Well Plate | Sigma-Aldrich | CLS3473-24EA |
| Ultra-Low Attachment 6-Well Plate | Sigma-Aldrich | CLS3471-24EA |
Is used in both Surgery Kit sets A and B.
Materials and equipment
Surgery equipment
| Surgery Kit A |
|---|
| Dumont #5 Forceps |
| Dumont #5/45 Forceps |
| Extra Fine Graefe Forceps |
| Friedman-Pearson Rongeurs |
| Hardened Fine Scissors |
| Micro scissors |
| Surgical scissors |
| Surgery Kit B |
|---|
| 26G needle |
| 27G needle |
| Blade handle#3 |
| Dumont #5 Forceps |
| Surgical blade#11 |
Dissection buffer
| Components | Final concentration | Amount |
|---|---|---|
| Hank's balanced salt solution | n/a | ~50 mL |
| HEPES | 2 mM | 23.83 mg |
| Total | n/a | 50 mL |
Note: store the solution at 4 °C for up to 8 weeks.
Wash media
| Components | Final concentration | Amount |
|---|---|---|
| DMEM/F-12 | n/a | 48 mL |
| B27 without vitamin A | 1× | 1 mL |
| GlutaMAX | 1× | 500 μl |
| Antibiotic-Antimycotic | 1× | 500 μl |
| Total | n/a | 50 mL |
Note: store the medium at 4°C for up to 4 weeks.
Growth media
| Components | Final concentration | Amount |
|---|---|---|
| Neural Stem Cell Basal Medium | n/a | ~48 mL |
| B27 without vitamin A | 1× | 1 mL |
| GlutaMAX | 1× | 500 μl |
| Antibiotic-Antimycotic | 1× | 500 μl |
| FGF-2 | 20 ng/mL | 10 μl |
| EGF | 20 ng/mL | 10 μl |
| Total | n/a | 50 mL |
Note: Store the medium at 4°C for up to 2 weeks.
CRITICAL: To increase stability of growth factors, only preheat the necessary volume of media for each day.
Neuron/Astrocyte differentiation media:
| Components | Final concentration | Amount |
|---|---|---|
| Neural Stem Cell Basal Medium | n/a | ~47.9 mL |
| B27 without vitamin A | 1× | 1 mL |
| GlutaMAX | 1× | 500 μl |
| Antibiotic-Antimycotic | 1× | 500 μl |
| Forskolin | 1 μM | 50 μl |
| Retinoic acid | 1 μM | 50 μl |
| Total | n/a | 50 mL |
OPC media
| Components | Final concentration | Amount |
|---|---|---|
| DMEM/F12 | n/a | ~44.95 mL |
| GlutaMAX | 1× | 500 μl |
| B27 without vitamin A | 1× | 1 mL |
| N2 supplement | 1× | 500 μl |
| apo-Transferrin | 50 μg/mL | 500 μl |
| Biotin | 10 nM | 500 μl |
| Bovine serum albumin | 0.1% | 500 μl |
| Hydrocortisone | 10 nM | 500 μl |
| Insulin | 5 μg/mL | 50 μl |
| PDGF-AA | 10 ng/mL | 500 μl |
| Sodium selenite | 30 nM | 500 μl |
| Total | n/a | 50 mL |
CRITICAL:
- Retinoic acid is sensitive to UV light. Therefore, store the prepared differentiation media in the dark.
- Prepare fresh every time for each differentiation and store at 4°C for the duration of the differentiation protocol.
- Only preheat the necessary volume of Neuron/Astrocyte differentiation and OPC media for each day.
Step-by-step method details
Dissection and isolation of aNSCs
Timing: 2–3 h
This step describes the procedure for brain dissection followed by digestion and trituration of tissue into single cells. It is possible to use this protocol for isolation of adult neural stem cells (aNSCs) from the dentate gyrus of the hippocampus and the subventricular zone (SVZ).
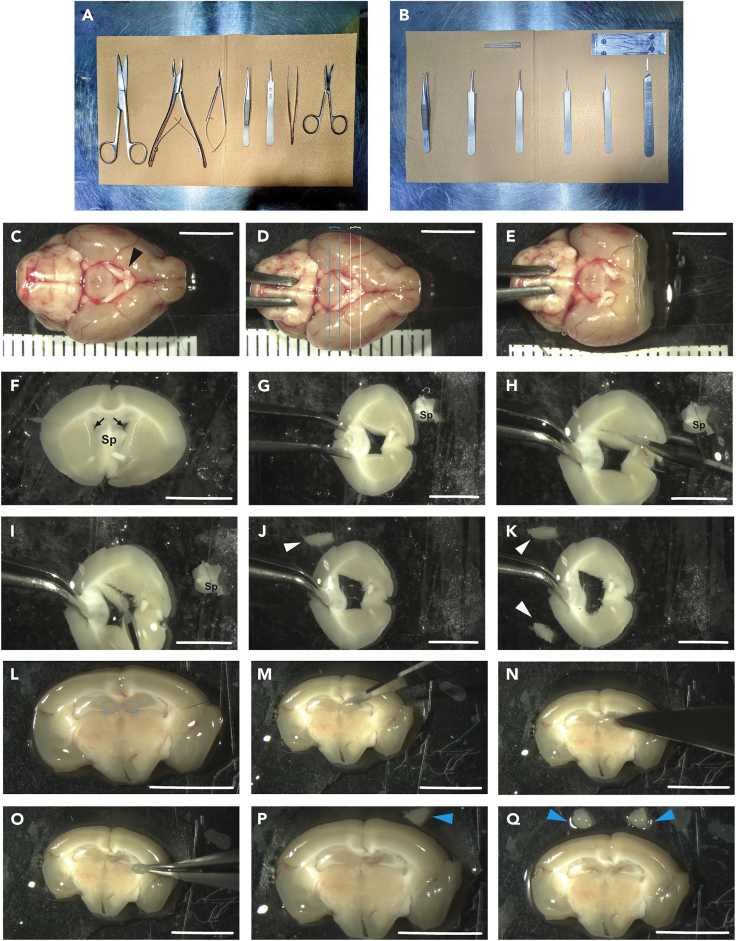
In the protocol, two separate sets of surgical kit are required to prevent contamination from mouse tissue, hair or other sources. Surgery Kit A: To harvest the brain tissue from the adult mouse (Figure 1A) and Surgery Kit B: for further dissection of the required brain regions (dentate gyrus of the hippocampus or the subventricular zone tissue) (Figure 1B).
CRITICAL: Good handling and quick transition between the steps of the procedure is desired.
-
1.
Euthanize one adult (age 6–24 weeks) C57BL/6 mouse.
-
2.
Sterilize the dissection area, surgery tools, and the head of the euthanized mouse with 70% ethanol.
-
3.
Surgically remove the brain and transfer it to a 50-mL tube with ice cold dissection buffer.
-
4.
Using the Optic Chiasm as an anatomical landmark (≈0 mm Bregma point) slice the brain into 1.0 mm coronal sections using a Mouse Brain Slicer Matrix at 0 -+1 mm Bregma and -1–-2 mm Bregma for the SVZ and the hippocampal DG respectively.
-
5.
Collect the slices containing the hippocampus or the SVZ into a 10 cm petri dish filled with ice cold dissection buffer.
-
6.
Under a microscope, quickly but carefully isolate the dentate gyrus or SVZ out of each relevant slice.Use a scalpel or a needle (26 or 27 G) to dissect the DG or SVZ while stabilizing the slice/section using angled forceps for easier and much accurate tissue isolation. Please refer to (troubleshooting 1 and 2)
- Then:
-
a.Immediately transfer the dissected tissue into the cap of a 15-mL tube or another petri dish and mince it with a fine scissor.
-
b.Once minced, attach the cap to its 15-mL tube with 10 mL ice cold dissection buffer and rotate slowly once to suspend the tissue chunks. Keep the tube on ice during further dissection.
-
c.Keep adding the dissected and minced tissue to the same 15-mL tube.
-
a.
-
7.
After finishing the dissection of all relevant brain sections, spin down the tissue at 200 g for 1 min at 18°C–25°C.
-
8.
In a laminar flow cabinet aspirate the dissection buffer and add 1 mL of 0.05% Trypsin-EDTA to the 15-mL tube containing tissue chunks.
-
9.Incubate at 37°C with slow mixing such as gentle rotation or light shaking in a water bath.
-
a.After the 15-20 min, confirm adequate digestion by pipetting slowly up and down a few times with a p1000 pipet.
-
b.If the digestion is inadequate, continue digestion at 37°C for 5-15 min. However, do not exceed 30 min of digestion as this can severely affect viability of the cells and result in increased debris in the culture afterward. Please refer to (troubleshooting 2)
-
a.
-
10.
Add 1 mL of Trypsin inhibitor solution to inhibit further digestion and incubate for 20 min at 37°C with slow mixing.
-
11.
Triturate the tissue by pipetting up and down 10–20 times until only few tissue clumps are visible (first using a p1000 then p200).
-
12.
Add 8 mL of wash media and pellet the triturate cells at 200 g for 5 min at 18°C–25°C.
-
13.
Wash the cells two times by resuspending the cells in 10 mL wash media and then spinning at 200 g for 5 min.
-
14.
Wash the cells once in 6 mL growth media and spin down at 200 g for 5 min.
-
15.
Resuspend the cells in 1 mL of growth media and plate them into a single well of a low-adherent 24-well culture plate.
-
16.
Incubate the cells in a 5% CO2 37°C incubator for 48 h.
-
17.
After 48 h, exchange half of the media with fresh growth media. For this, slowly aspirate media from a single periphery point as most neurospheres should be condensed towards the center of the well.
-
18.
Continue to replace half of the media with fresh growth media every second day.
-
19.
After 1–2 weeks neurospheres should form (Figure 2A), at this point proceed to next step for passage of aNSCs. Please refer to (troubleshooting 3)
CRITICAL: Avoid generating excess air bubbles during the triturating and wash steps as this can affect cell viability.
Optional: In the last wash step, it can be beneficial to pass the cells through a 100 μm cell strainer to remove undigested tissue.
Alternatives: Mice strains other than the C57BL/6 background can in principle be used. However, cell yield and viability might be affected. Thus, some method optimization for other mice strains might be required.
Figure 1.
Steps of DG and SVZ isolation from the adult mouse brain
(A) Photograph of the surgery equipment needed for harvest of the adult mouse brain.
(B) Photograph of the surgery equipment for the isolation of the SVZ and DG from harvested adult mouse brain. Note that two separate sets of surgical kit are used to prevent contamination from mouse tissue, hair or other sources.
(C–E) Dissection steps of the adult mouse brain. Through coronal sections the brain is dissected while being placed on its dorsal surface. The black arrowhead points to the Optic chiasm (C), the regions between the white lines and blue lines represent the SVZ and DG isolation sections respectively (D).
(F–K) Steps of SVZ isolation. The black arrows indicate the lateral ventricles (F). Firstly, the septal area (Sp) is removed, followed by dissecting the SVZs each at a time. The white arrowheads show the isolated SVZ tissue (J–F).
Note: the dashed white lines (F and G) represent the SVZ borders bilaterally.
(L–Q) Steps of DG isolation. The dashed blue lines represent the regions of the DG (L). A scalpel (or alternatively a needle 26–27G) is used to gently separate the DG from the surrounding tissue on both right and left sides (M–O). The blue arrowheads show the isolated DG tissue (P-Q).
Note: 1-mm thick sections were measured and used for isolation of both DG and SVZ. Moreover, the use of an angled forceps to fix/stabilize the brain sections during the isolation steps is preferred. Scale bars, 5 mm.
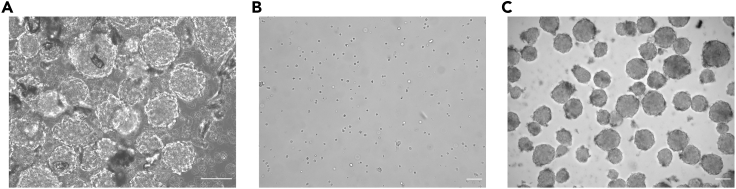
Figure 2.
Neurospheres derived from the adult mouse brain
Within 7–10 days post isolation of aNSCs, neurospheres were detected.
(A-C) Phase contrast images of SVZ derived neurospheres 7 days post isolation (A), after dissociation into single cells (B) and after the first passage (C) of aNSCs. Scale bars, 100 μm.
Passage of aNSCs
Timing: 30 min
This step outlines how to passage isolated aNSCs. We recommend to culture the aNSCs in low adherence 6 well plates or 35 mm petri dishes. Neurospheres should be passaged at around a size of 50–100 μm in diameter (Figure 2A) as described (Guo et al., 2011; Liu et al., 2010). Growing the neurospheres larger than 250 μm, could result in reduced growth rate (Mori et al., 2006) and inner core cell death due to lack of nutrients and oxygen.
-
20.
Carefully collect all neurospheres using a p1000 into a 15-mL tube and spin down at 200 g for 5 min at 18°C–25°C.
-
21.
Aspirate the media and add 1 mL of 0.05% Trypsin-EDTA for each well/dish.
-
22.
Incubate at 37°C with slow mixing for 1–2 min.
-
23.
Add equal volume of Trypsin inhibitor and triturate into single cells (first using a p1000 then p200).
-
24.
Add equal volume of growth media and spin down at 200g 5 min.
-
25.
Aspirate and suspend the cells in growth media.
-
26.
Count cell number and viability.
-
27.
Seed cells at 1×10ˆ5 cells/mL (Figure 2B) into a low adherent 6-well plate (3 mL per plate) or low adherent 35mm petri dishes (2 mL per dish) and incubate the cells in a 5% CO2 37°C incubator.
-
28.
Passage the neurospheres every 2–4 days. Neurospheres should be around 50–100 μm in diameter when passaging (Figure 2C).
CRITICAL: Larger formation of spheres should be avoided as cell loss, mainly of inner/core cells due to reduced access to nutrients and oxygen.
-
29.
For general experimental usage, we recommend using the cells between passage 3 and 10.
Note: During the initial first passages, small tissue debris or clumps of dead cells can remain present. Thus, when collecting the neurospheres, try to avoid disrupting such debris on the bottom of the well. Furthermore, most of the neurospheres tend to accumulate in the center of the well, so collecting from the center only can also reduce the amount of debris.
Note: At times, instead of growing as neurospheres, some cultured aNSCs can start growing as adherent cells which can lead to differentiation and growth arrest. To avoid this, use low adherent cell plates for suspension culture.
Differentiation of aNSCs
Timing: 7 days
Here we outline a simple 5-day differentiation protocol for the aNSCs.
-
30.
Two days before seeding the cells for differentiation, coat flask/wells with 20 μg/mL of Poly-L-ornithine solution in phosphate-buffered saline (PBS) overnight at 37°C.
-
31.
Next day, wash 3×1 min with PBS and coat with 5mg/mL Laminin in PBS over night at 37°C.
-
32.
On the day of cell seeding, wash flask/wells 3×1 min with PBS. On the last wash, wait to aspirate PBS just before adding media/cells. Please refer to (troubleshooting 4)
-
33.
Collect neurospheres and triturate them into single cells as described for passaging.
-
34.
Seed aNSCs as appropriate for the cell flask/well (approximately 40000 cells per cm2, depending on downstream analysis) and keep the cells in a 5% CO2 37°C incubator.
CRITICAL: Please refer to the Limitations section under Seeding density part.
-
35.
The next day, exchange half the media with freshly prepared differentiation media. Repeat this every day for the neuron/astrocyte differentiation media and every other day when using the OPC medium to differentiate the aNSCs into neurons/astrocytes and OPCs respectively. This should be continued for a total of 5 days for differentiation.Please refer to (troubleshooting 5)
-
36.
After 5 days, differentiated cells can be analyzed by quantitative methods such as qPCR, immunofluorescence-based assays, and flow cytometry.
Confirming aNSCs identity
Timing: 4 days
In this final step, we suggest simple immunofluorescence-based assays to confirm aNSCs identity before using the cells in experimental assays.
Confirming presence of neural stem cell markers
-
37.
Seed aNSCs in Poly-L-ornithine/Laminin coated cell culture slides and incubate the cells for 12–16 hrs in a 5% CO2 37°C incubator.
-
38.
Next day, aspirate medium and wash 2×1 min with ice-cold PBS.
-
39.
Add ice-cold 4% paraformaldehyde in PBS pH 7.4 and incubate for 30 min at 18°C–25°C.
-
40.
Wash 3×5 min with PBS.
-
41.
Incubate fixed cells with 0.1% T×100 in PBS 15 min at 18°C–25°C , followed by 3% bovine serum albumin (BSA), 0.01% T×100, in PBS for 30 min.
-
42.
Incubate fixed cells with neural stem cell marker antibodies such as anti-nestin (1:400) and anti Sox2 (1:400) in 3% BSA, 0.01% T×100, PBS for 2 h at 18°C–25°C or at 4°C for 12-16 hrs.
-
43.
Wash 3×5 min in PBS.
-
44.
Incubate fixed cells with secondary fluorescence conjugated antibody (1:800) in 3% BSA, 0.01% T×100, PBS for 1 h at 18°C–25°C in the dark.
-
45.
Wash 1×5 min in PBS
-
46.
Incubate cells with DAPI (1:2000) for 5 min.
-
47.
Wash 2×5 min in PBS and mount cell slides with coverslip.
-
48.
Approximately >90% of all cells should be positive for nestin and Sox2 (Figure 3B and 3C respectively).
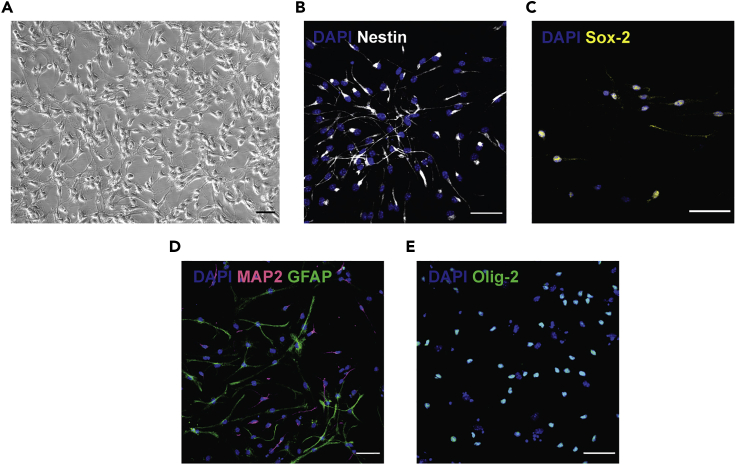
Figure 3.
Differentiation of aNSCs
aNSCs differentiated to different neural cells, namely neurons and astrocytes when incubated in neuron/astrocyte differentiation media and to oligodendrocyte progenitor cells when grown in OPC media containing the required factors.
(A) Phase-contrast image of SVZ derived aNSCs (Passage 7) 24 hrs post seeding into laminin coated wells.
(B and C) Proliferating aNSCs stained for the neural stem cell markers: Nestin (B) and Sox2 (C).
(D and E) represent differentiated aNSCs stained for the neuronal marker Map2 and the astrocytic marker GFAP (D) and OPCs stained for Olig2 (E).Scale bars, 50 μm.
Confirming differentiation potential
Timing: 8 days
-
49.
Induce differentiation of aNSCs seeded in cell culture slides as detailed in [differentiation of aNSCs].
-
50.
After 5 days of differentiation, aspirate the media and wash 2×1 min with ice-cold PBS.
-
51.
Add ice-cold 4% paraformaldehyde in PBS pH 7.4 and incubate for 30 min at 18°C–25°C.
-
52.
Wash 3×5 min with PBS.
-
53.
Incubate fixed cells with 0.1% T×100 in PBS 15 min at 18°C–25°C , followed by 3% BSA, 0.01% T×100, in PBS for 30 min.
-
54.
Incubate fixed cells with neural differentiation antibody markers, such as anti Map2 (1:400) as a neuronal marker, anti GFAP (1:2) as an astrocytic marker, and Olig2 as an oligodendrocyte progenitor cell marker (1:500), in 3% BSA, 0.01% T×100, PBS for 2 h at 18°C–25°C or at 4°C for 12–16 hrs.
-
55.
Wash 3×5 min in PBS.
-
56.
Incubate fixed cells with secondary fluorescence conjugated antibodies (1:800) in 3% BSA, 0.01% T×100, PBS for 1 h at 18°C–25°C in the dark.
-
57.
Wash 1×5 min in PBS
-
58.
Incubate cells with DAPI (1:2000) for 5 min.
-
59.
Wash 2×5 min in PBS and mount cell slides with coverslip.
-
60.
Using the Neuron/Astrocyte Differentiation media, usually the percentage of Map2 positive derived neurons and GFAP positive derived astrocytes are each roughly 10%–30% of total cells (Figure 3D). On the other hand, around 55%–65% of cells cultured in OPC media are positive for the oligodendrocyte progenitor cells marker Olig2 (Figure 3E).
Expected outcomes
Cultured aNSCs possess the ability to proliferate freely in growth media forming free floating neurospheres. These proliferating aNSCs are expected to be positive for neuronal stem cell markers such as nestin and Sox2 (>90% of cells) as previously described by (Isaksen et al., 2020).
Despite the fact that aNSCs will usually keep their stem cell potential for >15–20 passages, it is still strongly recommended to use the cultured aNSCs between passages 3 to 10 for experiments (Maslov et al., 2004) to prevent phenotypic changes associated with aged cultures.
Furthermore, the aNSCs can be induced to differentiate into neurons, astrocytes and oligodendrocyte progenitors. Investigating this differentiation potential can be done by analyzing differentiated cells with markers for each cell type. Standard markers used to test in vitro differentiation of aNSCs include Map2, neuron-specific class III beta-tubulin (Tuj1), and doublecortin (DCX) for neurons, GFAP for astrocytes, and Olig2 and platelet-derived growth factor alpha (PDGFRα) for oligodendrocyte progenitor cells.
Although several ways to differentiate NSCs have been shown, with spontaneous differentiation of aNSC by withdrawal of growth factors (EGF/b-FGF) being previously described (Wang et al., 2011), the use of Retinoic Acid/Forskolin to induce the NSCs, resulted in much efficient and faster differentiation (Jacobs et al., 2006; Janesick et al., 2015; Park et al., 2017) into neurons and astrocytes whereas the use of OPC media containing certain factors (e.g., PDGF-AA) direct towards more specific and reproducible differentiation of NSCs to OPCs (Biswas et al., 2019; Lee et al., 2018; Zhang et al., 2019).
Limitations
Mice age
Although this protocol is designed to be used for adult mice aged 6–24 weeks old of either sex, we noticed that the yield of NSCs was reduced with older age mice (Ahlenius et al., 2009; Encinas et al., 2011; Enwere et al., 2004) as and therefore we used 6 weeks old mice for most experiments.
Seeding density
Optimal NSCs seeding density could generally differ depending on the required assay.
Seeding densities ranging from (1–2 ×10 4 cells/cm2) described by (Cha et al., 2017; Krampert et al., 2010) reaching up to (105 cells/cm2) described by (Wang et al., 2020) have been shown. However, some previously mentioned using densities of (2.5 - 5 ×105 cells/cm2) for differentiation assays (Krampert et al., 2010; Pollard et al., 2006) and lower densities (1.5–2.5 ×105 cells/cm2) for proliferation assays (Cha et al., 2017; Krampert et al., 2010; Leone et al., 2005). Therefore, we recommend optimizing the required NSCs seeding density based on each assay or study purpose.
Therefore, in this protocol we recommend using 4×104 cells/cm2 as a starting seeding density followed by further optimizations by users.
Troubleshooting
Problem 1
Low viable cell yield after isolation (step 6 in dissection and isolation of aNSCs).
Potential solution
Optimizing time usage on each step during the isolation process is key to prevent cell loss. Practice of surgical steps is a good starting step for such optimization. Therefore, quick and smooth transition between tissue dissection and isolation steps is a crucial factor (Guo et al., 2012). Another important optimization during isolation is the trituration of tissue into single cells. Insufficient trituration results in few single cells release from the tissue, whereas excessive trituration results in high mechanical cell stress and cell death.
Problem 2
Excess debris in the culture (steps 6–14 in dissection and isolation of aNSCs).
Potential solution
Some debris is expected to be present and later on to be removed during the first few passages. However, excess debris can have an impact on cell viability and is undesired in analytical assays. Excess debris can also be due to a large tissue dissection, therefore good surgical skills to accurately obtain the DG and SVZ tissue as much as possible with minimal extra tissue is desired. If excess debris is observed, trying to use a cell strainer before seeding cells is often sufficient to remove these debris. Furthermore, performing additional wash steps of the cells following trypsin digestion and antitrypsin use, i.e., to resuspend cells in fresh media then spinning them down, can also help remove debris (Guo et al., 2012; Isaksen et al., 2020). However, prolonged handling of cells might also result in reduced viability.
Problem 3
Low proliferation rates (steps 20–29 in passage of aNSCs).
Potential solution
Avoid overgrowth of neurospheres in culture as it has been shown to reduce the growth rate of NSCs (Mori et al., 2006). Passage them when they reach 50–100 μm in diameter. Moreover, prepare fresh proliferation media using a new frozen stock of growth factors. The growth factors are relatively unstable, even at 4°C, and are critical for proper proliferation of the aNSCs.
Problem 4
Poor adherence after seed cells onto coated plates (steps 30–32 in differentiation of aNSCs).
Potential solution
This could probably be tackled by preparing fresh coating solutions and avoiding prolonged precoating of culture ware (Guo et al., 2012; Miyazaki et al., 2012).
Problem 5
Differentiation efficiency (step 35 in differentiation of aNSCs).
Potential solution
Low differentiation capacity can be the result of several factors. One of which is, the use of older cultures. It is generally preferred to use aNSCs between passage 3–10 (Maslov et al., 2004). Older cultures often exhibit loss of differentiation potential and impaired neurite growth as previously described by (Ferrón et al., 2009). Secondly, preparation of fresh differentiation media is also recommended since some of the factors used are relatively unstable and need to be used within short period. Finally, some cultures just exhibit lower differentiation rates. For such cultures, it is recommended to start all over.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, TY, yamashita@molneu.med.osaka-u.ac.jp.
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate any unique datasets or code.
Acknowledgments
T.J.I. was supported by a grant from the Independent Research Fund Denmark, Denmark (grant number: 7025-00042B). This work was furthermore supported by JSPS KAKENHI, Japan (grant number: JP17H06178) and AMED-CREST, Japan (grant number: 18gm1210005h0001) to T.Y.
Author contributions
All authors conceived and wrote the manuscript. A.K.M.A.A. performed and analyzed the experiments. A.K.M.A.A. and T.J.I. developed the methodology. T.J.I. and T.Y. acquired the funding and supervised the project.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Toke Jost Isaksen, Email: tokejost@gmail.com.
Toshihide Yamashita, Email: yamashita@molneu.med.osaka-u.ac.jp.
References
- Ahlenius H., Visan V., Kokaia M., Lindvall O., Kokaia Z. Neural stem and progenitor cells retain their potential for proliferation and differentiation into functional neurons despite lower number in aged brain. J. Neurosci. 2009;29:4408–4419. doi: 10.1523/JNEUROSCI.6003-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S., Chung S.H., Jiang P., Dehghan S., Deng W. Development of glial restricted human neural stem cells for oligodendrocyte differentiation in vitro and in vivo. Sci. Rep. 2019;9:1–14. doi: 10.1038/s41598-019-45247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha K.J., Kong S.Y., Lee J.S., Kim H.W., Shin J.Y., La M., Han B.W., Kim D.S., Kim H.J. Cell density-dependent differential proliferation of neural stem cells on omnidirectional nanopore-arrayed surface. Sci. Rep. 2017;7:1–14. doi: 10.1038/s41598-017-13372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas J.M., Michurina T.V., Peunova N., Park J.H., Tordo J., Peterson D.A., Fishell G., Koulakov A., Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwere E., Shingo T., Gregg C., Fujikawa H., Ohta S., Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J. Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrón S.R., Marqués-Torrejón M.Á., Mira H., Flores I., Taylor K., Blasco M.A., Fariñas I. Telomere shortening in neural stem cells disrupts neuronal differentiation and neuritogenesis. J. Neurosci. 2009;29:14394–14407. doi: 10.1523/JNEUROSCI.3836-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Allan A.M., Zong R., Zhang L., Johnson E.B., Schaller E.G., Murthy A.C., Goggin S.L., Eisch A.J., Oostra B.A. Ablation of Fmrp in adult neural stem cells disrupts hippocampus-dependent learning. Nat. Med. 2011;17:559–565. doi: 10.1038/nm.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Patzlaff N.E., Jobe E.M., Zhao X. Isolation of multipotent neural stem or progenitor cells from both the dentate gyrus and subventricular zone of a single adult mouse. Nat. Protoc. 2012;7:2005–2012. doi: 10.1038/nprot.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksen T.J., Fujita Y., Yamashita T. Stem Cell Reports. Stem Cell Rep. 2020;14:677–691. doi: 10.1016/j.stemcr.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Lie D.C., DeCicco K.L., Shi Y., DeLuca L.M., Gage F.H., Evans R.M. Retinoic acid is required early during adult neurogenesis in the dentate gyrus. Proc. Natl. Acad. Sci. U S A. 2006;103:3902–3907. doi: 10.1073/pnas.0511294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janesick A., Wu S.C., Blumberg B. Retinoic acid signaling and neuronal differentiation. Cell. Mol. Life Sci. 2015;72:1559–1576. doi: 10.1007/s00018-014-1815-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krampert M., Chirasani S.R., Wachs F.-P., Aigner R., Bogdahn U., Yingling J.M., Heldin C.-H., Aigner L., Heuchel R. Smad7 Regulates the Adult Neural Stem/Progenitor Cell Pool in a Transforming Growth Factor β- and Bone Morphogenetic Protein-Independent Manner. Mol. Cell. Biol. 2010;30:3685–3694. doi: 10.1128/mcb.00434-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Kim C.Y., Lee H.J., Kim J.G., Han D.W., Ko K., Walter J., Chung H.M., Schöler H.R., Bae Y.M. Two-step generation of oligodendrocyte progenitor cells from mouse fibroblasts for spinal cord injury. Front. Cell. Neurosci. 2018;12:1–8. doi: 10.3389/fncel.2018.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone D.P., Relvas J.B., Campos L.S., Hemmi S., Brakebusch C., Fässler R., Ffrench-Constant C., Suter U. Regulation of neural progenitor proliferation and survival by β1 integrins. J. Cell Sci. 2005;118:2589–2599. doi: 10.1242/jcs.02396. [DOI] [PubMed] [Google Scholar]
- Liu C., Teng Z.Q., Santistevan N.J., Szulwach K.E., Guo W., Jin P., Zhao X. Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell Stem Cell. 2010;6:433–444. doi: 10.1016/j.stem.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov A.Y., Barone T.A., Plunkett R.J., Pruitt S.C. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J. Neurosci. 2004;24:1726–1733. doi: 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T., Futaki S., Suemori H., Taniguchi Y., Yamada M., Kawasaki M., Hayashi M., Kumagai H., Nakatsuji N., Sekiguchi K. Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nat. Commun. 2012;3 doi: 10.1038/ncomms2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Ninomiya K., Kino-Oka M., Shofuda T., Islam M.O., Yamasaki M., Okano H., Taya M., Kanemura Y. Effect of neurosphere size on the growth rate of human neural stem/progenitor cells. J. Neurosci. Res. 2006;84:1682–1691. doi: 10.1002/jnr.21082. [DOI] [PubMed] [Google Scholar]
- Park S.J., Kim S., Kim S.Y., Jeon N.L., Song J.M., Won C., Min D.H. Highly efficient and rapid neural differentiation of mouse embryonic stem cells based on retinoic acid encapsulated porous nanoparticle. ACS Appl. Mater. Interfaces. 2017;9:34634–34640. doi: 10.1021/acsami.7b09760. [DOI] [PubMed] [Google Scholar]
- Pollard S.M., Conti L., Sun Y., Goffredo D., Smith A. Adherent neural stem (NS) cells from fetal and adult forebrain. Cereb. Cortex. 2006;16 doi: 10.1093/cercor/bhj167. [DOI] [PubMed] [Google Scholar]
- Wang M., Wei P.C., Lim C.K., Gallina I.S., Marshall S., Marchetto M.C., Alt F.W., Gage F.H. Increased neural progenitor proliferation in a hiPSC model of autism induces replication stress-associated genome instability. Cell Stem Cell. 2020;26:221–233.e6. doi: 10.1016/j.stem.2019.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Esbensen Y., Kunke D., Suganthan R., Rachek L., Bjørås M., Eide L. Mitochondrial DNA damage level determines neural stem cell differentiation fate. J. Neurosci. 2011;31:9746–9751. doi: 10.1523/JNEUROSCI.0852-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lu X.Y., Casella G., Tian J., Ye Z.Q., Yang T., Han J.J., Jia L.Y., Rostami A., Li X. Generation of oligodendrocyte progenitor cells from mouse bone marrow cells. Front. Cell. Neurosci. 2019;13:1–10. doi: 10.3389/fncel.2019.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate any unique datasets or code.