Abstract
Purpose
In clinics, chest frontal view radiograph (CFR) is often taken for patients suspected of respiratory diseases and for assessing the heart and big vessels. CFR can be utilised to opportunistically detect osteoporotic vertebral fracture (OVF). However, for standard CFR, the site of highest OVF prevalence, i.e., the thoracolumbar junction, is usually ‘off-centre’ to the X-ray beam focus. This study tested the hypothesis that, if a CRF is taken with approximately two vertebrae lower than the standard X-ray beam positioning, the visualization of thoraco-lumbar junction can be much improved.
Materials
Four hospitals (A, B, C, D) provided 101 elderly women’s digital CFRs with standard filming (28, 20, 24, and 21 cases respectively). Eighty four elderly female patients were prospectively recruited from hospitals-A and B, who were consecutive patients referred for chest radiograph with indications other than spine disorders. For theses prospective CFRs, the focus of X-ray beam was adjusted from towards vertebra T6 to towards T8, and standard lateral radiographs were obtained for reference. Visibility of spine and detectability of OVF were assessed on the CFRs. OVF was diagnosed based on chest lateral radiograph (CLR) after excluding other potential causes both radiographically and clinically.
Results
For standardly filmed CFR, spine readability was similar among those from Hospitals-A, B, and C, while performed less well for those from Hospital-D. With the prospective cases from Hospitals-A and B, spines readable to vertebra L1 level or lower increased from 48.2% for standard filming to 80.7% for adjusted filming. Spines with ‘blurry’ labelling decreased from 35.7% for standard filming to 15.7% for adjusted filming. For the 84 prospective cases, 42.9% (36/84) of the patients had OVF, and 26 cases of CLR positive cases were detected as having vertebral deformity on CFR. For minimal OVF cases (<20% height loss), 38% (5/13) were detected on CFR. Among 22 cases with apparent OVF (≥20% height loss), two cases were missed on CFR. False positivity was labelled in five cases, among them four cases had ‘burry’ spines.
Conclusion
CFR can help opportunistically detect OVF, which can be further improved if X-ray beam is adjusted to towards vertebra T8 instead of towards vertebra T6.
The translational potential of this article
This study confirms that CFR can help detect OVF opportunistically, and the visibility of the mid/lower thoracic spine and thoracolumbar junction can be much improved after minor adjustment of X-ray beam positioning. This study also suggests high positive rate of OVF in elderly Chinese female patients indicated for chest radiograph. Radiologists should be trained and sensitized in vertebral deformity identification on CFR as the clinical management can be improved by opportunistic detection of OVF.
Keywords: Osteoporosis, Vertebral fracture, Radiograph, Chest, Vertebral deformity, Frontal view
Introduction
Fragility fractures may occur in almost all skeletal segments, but the preferential locations are the vertebral column, the proximal ends of the femur and humerus, and the distal end of the radius (Colles fracture). Trauma due to a fall is by far the most frequent cause of fractures affecting long bones, while it is more difficult to determine the cause and the exact time of fragility fractures of the vertebral body, which often go undiagnosed [[1], [2], [3], [4], [5]]. Osteoporotic vertebral fracture (OVF) has high prevalence in elderly women population. The detection of OVF in women suggests that the patient’s bone strength is compromised, and the risk of future fracture is substantially increased, both for further OVF and non-vertebral fragility fracture including the hip [[6], [7], [8], [9], [10], [11], [12]]. OVFs can be associated with decreases in trunk extension torque, spinal motion, functional reach, mobility skills and walking distance, and may also influence mortality because their association with chronic back pain, immobility and postural change [1]. Multiple and more severe grades of OVFs are associated with an even greater fracture risk [6,7,12].
An early detection of an OVF can lead to further investigation and appropriate therapy that decreases the risk of future fractures, critically to target pharmaceutical treatment to the patents aged 65 years or older with a hip or vertebral fracture [9]. Many fractures and associated complications, including secondary fractures and mortality, could be prevented by routine osteoporosis screening in older people and timely treatment initiation in at-risk individuals. Despite its importance, many patients with OVF and at high risk for further fracture remain undetected and untreated. Many guidelines suggest women with age ≥60 or ≥65 years should have osteoporosis screening [[8], [10], [13], [14], [15], [16], [17], [18]]. However, this measure is still not commonly taken by individuals. On the other hand, in daily clinics, chest frontal view radiograph (CFR) is often taken for patients suspected of respiratory diseases, pleural diseases, as well as to assess heart and big vessels, while chest lateral radiograph (CLR) is taken less often. FR (frontal view radiograph) of abdomen is taken in patients with abdominal pain, for assessing urinary stone, gastrointestinal gas etc. Based on the analysis of spine radiograph, we reported that moderate to severe vertebral deformities at mid-thoracic and lower thoracic spine as well as lumbar spine are mostly identifiable on FR, with a small proportion of ambiguous cases further clarified by additional lateral view imaging [19]. Moreover, some mild osteoporotic vertebral deformity (VD) and endplate and/or cortical fracture (ECF) are also visible on FR [19].
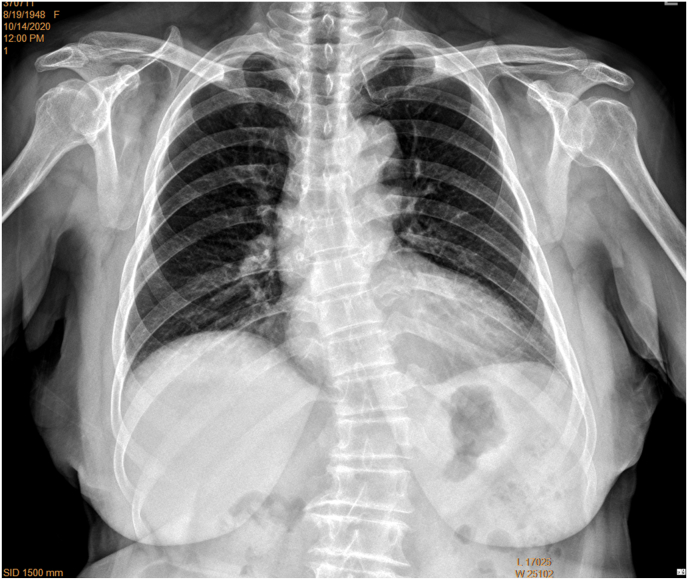
Following our study on spine FR evaluation of OVF, we did a further pilot study on chest and abdominal FR evaluation of OVF. We noticed that since FRs of abdomen are mostly commonly taken anterior-posteriorly (AP, mostly in a supine position), i.e., the spine is close to the X-ray detector, the vertebral borders on abdominal FR are usually sharply defined. For assessing OVF, abdominal FR is as good as lumbar FR (Fig. 1). On the other hand, CFRs are commonly taken posterior-anteriorly (PA), i.e., the anterior chest wall is close to the X-ray detector. Since the spine is at the back of the chest, compared with spine RFs which are taken with the spine close to the X-ray detector, the vertebrae are more magnified on CFR and the vertebral borders are less sharp. More importantly, while the most frequent site of OVF is the thoracolumbar junction (centred around L1, followed by T12 and then L2) [[20], [21], [22]], one of the difficulties of reading spine on CFR is that the thoracolumbar junction is usually ‘off-centre’ to the X-ray beam focus. Vertebrae T12, L1, and L2, when included in the field-of-view, may not be well displayed (Fig. 2A). We propose that if CRF is taken with approximately two vertebrae lower than the usual X-ray beam positioning, the visualization of thoraco-lumbar junction can be much improved (Fig. 2B). The aims of this study include to evaluate the visualization of thoraco-lumbar junction on CFR with adjusted X-ray beam positioning, and also the detectability of osteoporotic VD on CFR in two groups of random samples of clinical patients indicated for chest radiograph.
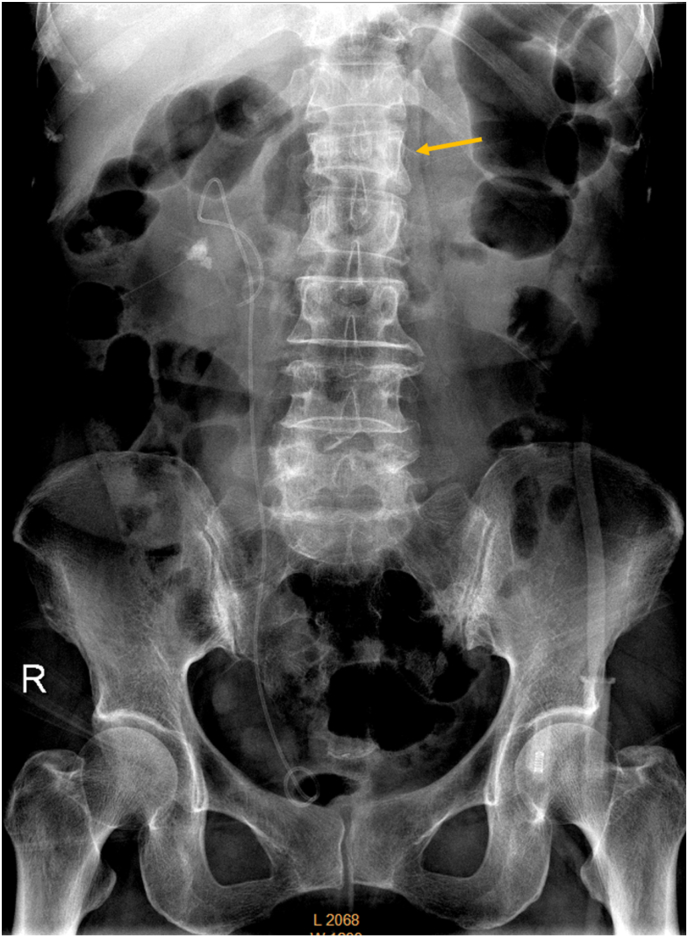
Fig. 1.
Abdominal radiograph of a 70-year-old female patient. A renal stone and urinary drainage catheter are noted on the right side. Arrow indicates L1 apparent vertebra deformity.
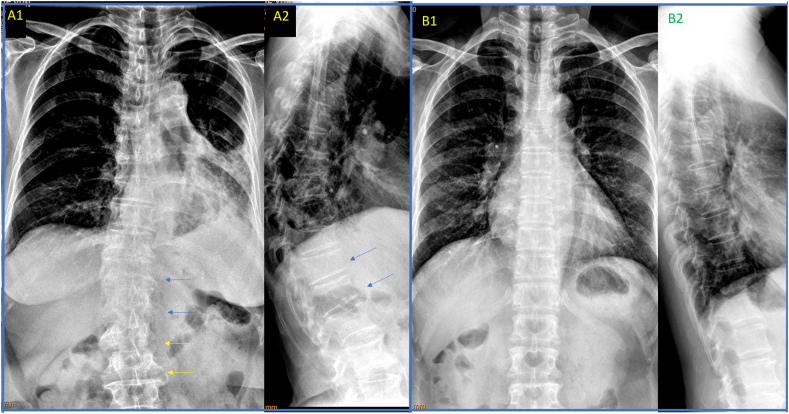
Fig. 2.
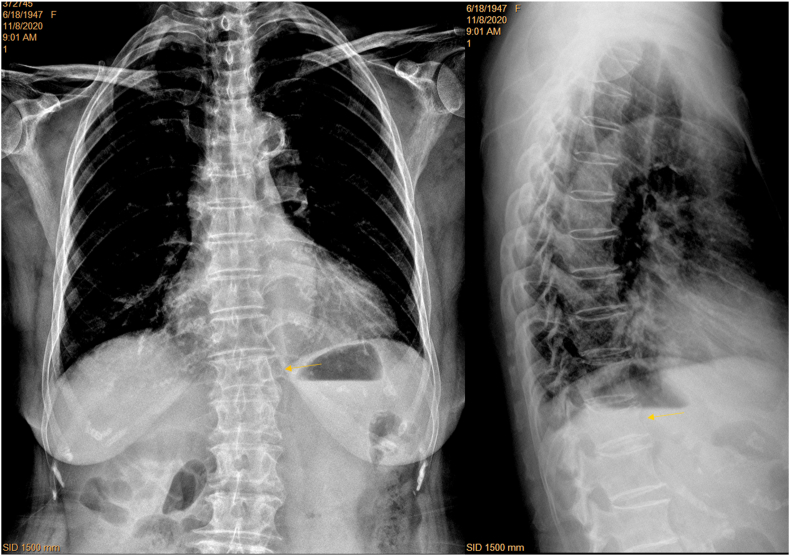
Chest frontal view radiographs taken with standard X-ray beam positioning may show blurry thoraco-lumbar junction, while radiographs taken with X-ray beam approximately two vertebrae lower than the standard positioning improve the visualization of thoraco-lumbar junction. A: chest frontal radiograph (brightness and contrast adjusted for viewing the spine) of a female patient of 72 years old taken according to standard positioning of X-ray beam, with the thoraco-lumbar junction poorly demonstrated for this case (A1). Lateral chest radiograph (A2) shows T12 and L1 minimal deformity (blue arrows), which cannot be evaluated on A1 (blue arrows). On frontal radiograph (A1), blurry appearance of thoraco-lumbar junction may be mis-labelled as with vertebral deformity. The shapes of L2 and L3 (yellow arrows) are distorted due to the X-ray projection. B: chest radiographs (B1: frontal view, brightness and contrast adjusted for viewing the spine; B2: lateral view) of another female patient of 72 years old taken according to the adjusted X-ray beam (two vertebrae lower than the standard positioning). The vertebrae at thoraco-lumbar junction are well demonstrated till L2 on (B1). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Materials and methods
This study has a retrospective part (part-1) and a prospective part (part-2). For part-1, four hospitals in China provided in total 101 randomly selected digital CFRs of elderly women examined during the first half of 2020 (Center-A, Dongguan Traditional Chinese Medicine Hospital, n = 28 CFRs; Center-B, General hospital of China Resources & Wuhan Iron and Steel Corporation, n = 28 CFRs; Center-C, Shenzhen People’s Hospital, n = 24 CFRs; and Center-D, the First Hospital of Jiaxing, n = 21 C FRs). Local ethics approval was obtained for retrospective analysis, while patient consent was waived. For these CFRs, after adjustment of image brightness and contrast for viewing the spine of vertebra T6 downward, radiographs were classified into 1) spine generally ‘blurry’, 2) lower thoracic spine and thoracolumbar conjunction ‘blurry’, 3) thoracolumbar conjunction ‘blurry’, 4) spine readable down to T12, 5) spine readable down to L1, 5) spine readable down to L2, 6) spine readable down to L3 or even lower. A spine segment being ‘blurry’ could be due to insufficient X-ray exposure, off-center to X-ray beam, or local kyphosis/scoliosis. A blurry spine did not necessarily mean it was useless for reading OVF. X-ray cone-beam may cause geometric distortion of vertebrae located at extremities of the field-of-view, and the accuracy of diagnosis of OVF can be reduced at the upper thoracic levels. Upper thoracic spine is often associated with physiological kyphosis; however, it has been known that OVF at levels above T6 is less common [[20], [21], [22]]. One local radiologist at each center evaluated the radiographs initially, and then the images in DICOM format were sent to Center-E (the Chinese University of Hong Kong) for additional evaluation, and final consensuses were reached.
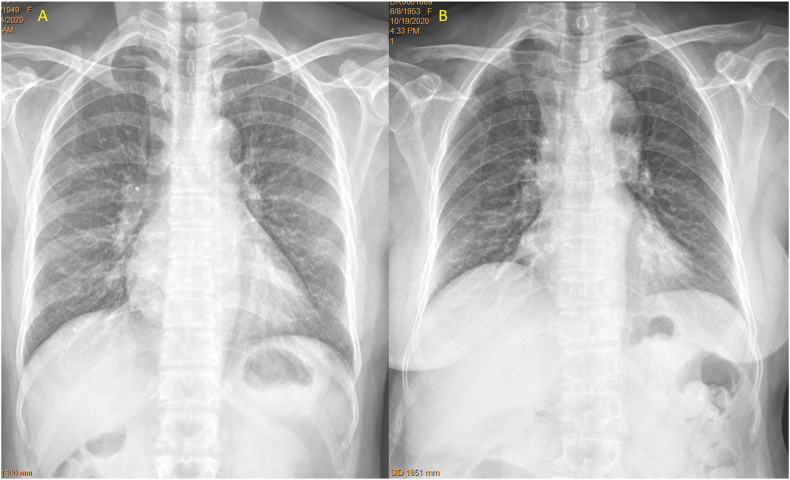
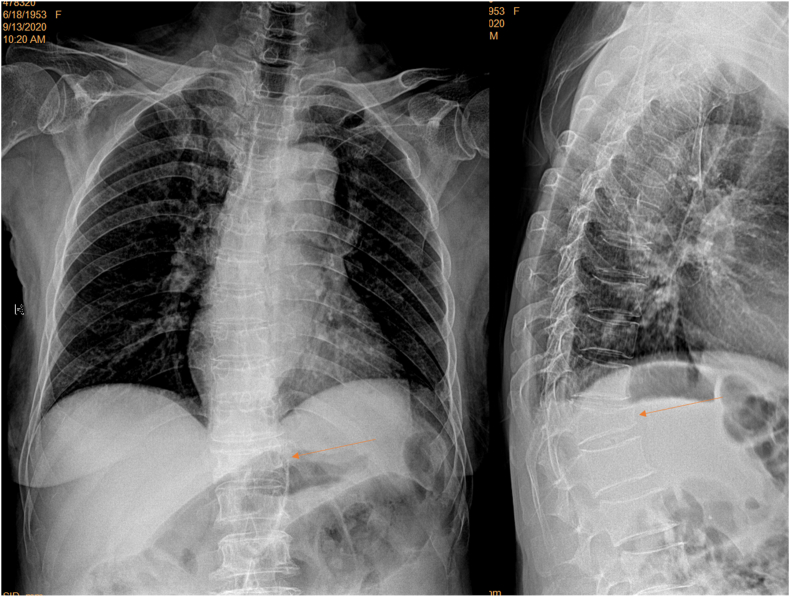
For part-2, this study prospectively recruited 87 patients (n = 61 from Center A, and n = 26 patients from Center B) during the period of September 23 to November 16, 2020. Local ethics approval was obtained, and all patients gave consent. The patients were consecutive elderly female patients referred for chest radiograph during this study period, with indications other than spine disorders. When CLR was not initially requested, it was added upon the consent of the patients and no patient refused this addition. For the CFR, the center of X-ray beam was adjusted two vertebrae lower (i.e., approximately 4–5 cm lower, and adjusted slightly even lower for those > 165 cm height) than the standard positioning (Fig. 3, Fig. 4). Thus, the center of X-ray beam was generally adjusted from towards vertebra T6 to towards vertebra T8. Field-of-view of X-ray beam was increased slightly for patients with big body size. Since the center of X-ray beam was adjusted lower and thus greater extent of sub-diaphragm solid organs were included, degree of X-ray exposure was increased. For all the X-ray equipment used in Center-A and Center-B, the degree of X-ray exposure was automatically adjusted. For apparent obese subjects, X-ray exposure was slightly further increased manually as evaluated necessary by the study radiographers (Fig. 5). After the radiographs were taken, two local radiologists (who were not an author of this paper) at center A or center B, respectively, read the CFR and both determined that the adjusted X-ray beam positioning did not negatively affect the interpretation of the intended indications (mostly for assessing the lungs) (Fig. 6). One local radiologist in center A or center B evaluated the visualization of spine using the same criteria as those in part-1, respectively, then the images in DICOM format were sent to Center-E for additional evaluation. Three patients from center-A were excluded from analysis due to that: one case had spine surgery history with metal implant; one case had injected cement in the thoracic spine after vertebroplasty; one case’s CLR was not taken properly with poor positioning.
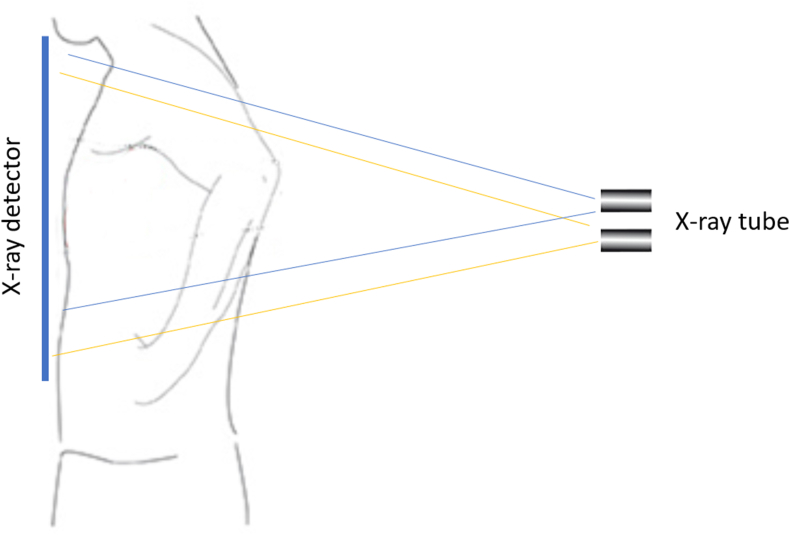
Fig. 3.
A diagram of shifting the beam of X-ray downward for approximately two vertebrae. Blue thin lines indicate the standard X-ray beam with focus towards T6 vertebra. Yellow thin lines indicate the adjusted X-ray beam with focus towards T8 vertebra. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
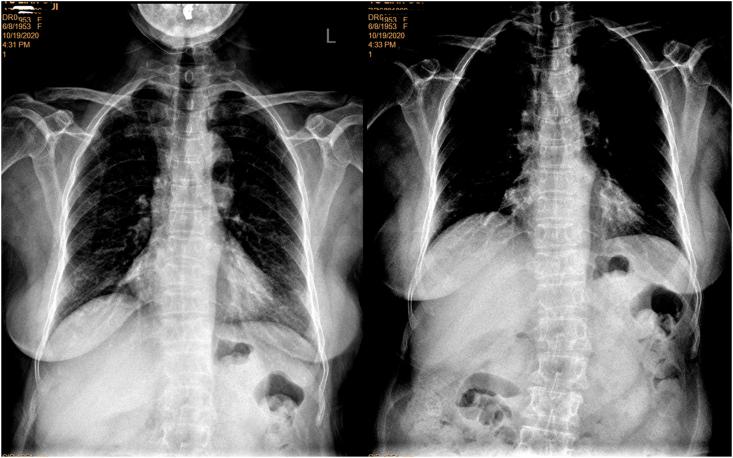
Fig. 4.
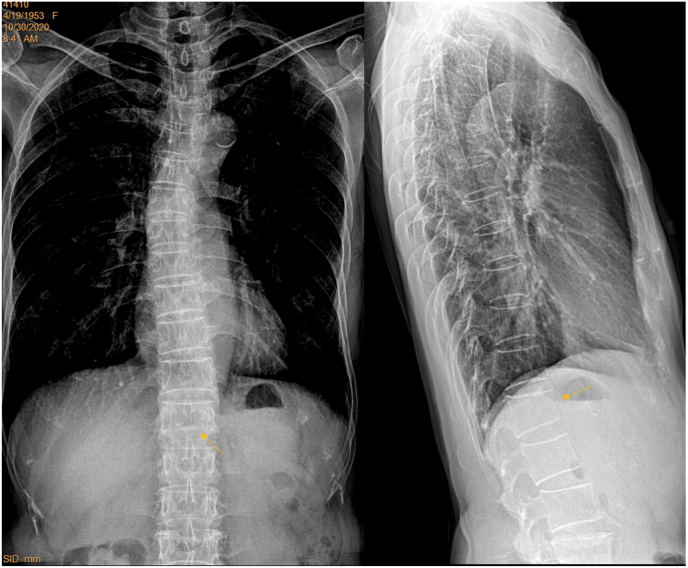
A female patient of 83 years old. Left: standard filming (brightness and contrast adjusted for viewing the spine) shows slight blurry of T12, L1 and L2. Right: filming with adjusted X-ray beam positioning better shows T12-L3 for the same patient.
Fig. 5.
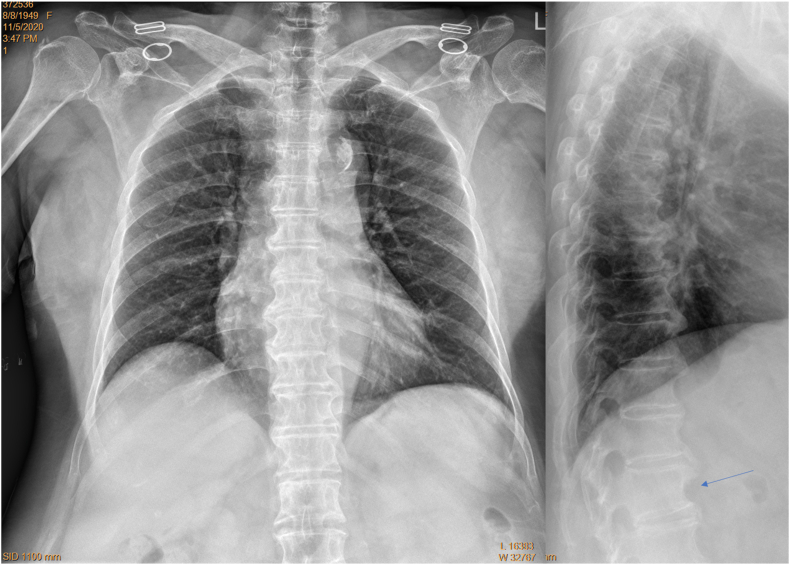
Frontal view radiograph of a female patient of 71 years old with adjusted filming (brightness and contrast adjusted for viewing the spine). Despite the apparent obesity of this subject, the spine is well demonstrated.
Fig. 6.
Frontal view radiographs of the patient of Fig. 2B (A) and the patient of Fig. 4 (B), with brightness and contrast of the radiographs adjusted for viewing the lungs.
Assessment of VD/OVF was initially performed by a reader in Center-E (YXJW). The focus of CFR reading was from T6 downward to the lowest readable lumbar vertebra, and image brightness and contrast were adjusted to better visualise the spinal structures. Signs suggesting VD on CFR included vertebral height loss, endplate depression, the loss of vertical continuity of vertebral morphology, alterations in the shape and configuration of the vertebrae relative to adjacent vertebrae and expected normal appearances, and regional increased density due to compression [19,23]. The results were then compared with the followed reading with CLR, which was considered as the reference. After the identification of VD, OVF was diagnosed based on CLR reading after excluding other potential causes both radiographically and clinically. OVF evaluation followed the principles of Genant SQ (semi-quantitative) criteria and ECF (endplate and/or cortex fracture) criteria [12,[24], [25], [26], [27]]. On CLR, OVFs were classified into 1) minimal OVF (radiological osteoporotic VD < 20% height loss) or 2) apparent OVF (radiological osteoporotic VD ≥ 20% height loss), by measurement of the height loss with the adjacent normal appearing vertebrae as reference [[28], [29], [30]]. VDs not detected on CFR was classified into 1) ‘false negative’ or 2) ‘missed’. ‘False negative’ reading referred to the results that after reading CLR the reader would still label this vertebra as negative. ‘Missed’ reading referred to the results that after reading CLR the reader retrospectively would label the vertebra as with VD on CFR (thought it was missed during initial reading of CFR). The readings at Center-E were then forwarded to Center-A and Center-B for consenting, and final consensuses were reached for all cases. During this study, the reader at Center-E was considered experienced in reading VD on FRs [19,23,31]. The readers at Center-A and B had past experience in reading OVF on CLRs but did not have prior specific training in reading VD on CFRs.
Results with consensus are presented descriptively. Categorical variables were compared by chi-square test or Fisher’s exact test (SAS, version 9.4, SAS Institute, Inc., Cary, NC, USA). A p value of less than 0.05 was considered statistically significant.
Results
Readabilities of mid-/lower thoracic spine and thoracolumbar junction for CFRs with standard filming and adjusted filming are shown in Table 1 and Table 2. The readability of standardly filmed spines from Centers-A, B, and C had similar performance, while that of Centers-D performed less well (supplementary Fig-1).
Table 1.
Chest fontal radiograph readability of mid-/lower thoracic spine and thoracolumbar junction with standard filming from four centers.
| Center-A | Center-B | Center-C | Center-D | ||
|---|---|---|---|---|---|
| 1# | Case number | 28 | 28 | 24 | 21 |
| Mean age (yrs) | 78.4 | 78.6 | 71.6 | 71.9 | |
| Age range (yrs) | 72–90 | 71–89 | 67–90 | 66–85 | |
| Readable to L3 ∗ | 2 (7.1%) | 4 (14.3%) | 1 (4.2%) | 0 | |
| 2# | Readable to L2 | 5 (17.9%) | 7 (25%) | 9 (37.5%) | 2 (9.5%) |
| 3# | Readable to L1 | 6 (21.4%) | 3 (10.7%) | 3 (12.5%) | 3 (14.2%) |
| 4# | Readable to T12 | 6 (21.4%) | 3 (10.7%) | 2 (8.3%) | 5 (23.8%) |
| 5# | Junction blurry | 6 (21.4%) | 5 (17.9%) | 3 (12.5%) | 2 (9.5%) |
| 6# | Lower T blurry | 1 (3.6%) | 2 (7.1%) | 2 (8.3%) | 0 |
| 7# | General blurry | 2 (7.1%) | 4 (14.3%) | 4 (16.7%) | 9 (42.8%) |
| Sum of 1#,2#,&3# | 46.4% | 50% | 54.2% | 23.8% | |
| Sum of 5#, 6#,&7# | 32.1% | 39.3% | 37.5% | 52.4% | |
∗ Readable to L3∗ or below. Junction blurry: thoracolumbar conjunction ‘blurry’; Lower T blurry: lower thoracic spine and thoracolumbar conjunction ‘blurry’; General blurry: spine generally ‘blurry’.
Table 2.
Chest fontal radiograph readability of mid-/lower thoracic spine and thoracolumbar junction with standard and adjusted filming: data from Center- A and Center-B.
| Adjusted filming |
Standard filming |
||||
|---|---|---|---|---|---|
| Center-A | Center-B | Mean of A &B | Mean of A &B | ||
| 1# | Case number | 57 | 26 | 83¶ | 56¶ |
| Mean age (yrs) | 75.1 | 73.6 | 74.7 | 78.5 | |
| Age range (yrs) | 67–89 | 64–88 | 64–89¶ | 71–90¶ | |
| Readable to L3∗ | 10 (17.5%) | 8 (30.8%) | 21.69% | 10.71% | |
| 2# | Readable to L2 | 27 (47.4%) | 9 (34.6%) | 43.47% | 21.42% |
| 3# | Readable to L1 | 10 (17.5%) | 3 (11.5%) | 15.66% | 16.07% |
| 4# | Readable to T12 | 1 (1.8%) | 2 (7.7%) | 3.61% | 16.07% |
| 5# | Junction blurry | 6 (10.5%) | 3 (11.5%) | 10.84% | 19.64% |
| 6# | Lower T blurry | 1 (1.8%) | 0 | 1.20% | 5.35% |
| 7# | General blurry | 2 (3.5%) | 1 (3.8%) | 3.61% | 10.71% |
| sum of 1#,2#,&3# | 82.5% | 76.9% | 80.72% | 48.21% | |
| sum of 5#, 6#,&7# | 15.8% | 15.4% | 15.67% | 35.71% | |
∗ Readable to L3∗ or below. ¶, total of A and B. Junction blurry: thoracolumbar conjunction ‘blurry’; Lower T blurry: lower thoracic spine and thoracolumbar conjunction ‘blurry’; General blurry: spine generally ‘blurry’.
For the combined results with cases from center-A and center-B, spines readable to L3 or lower increased from 10.7% for standard filming to 21.7% for adjusted filming (p = 0.09). Spines readable to L2 increased from 21.4% for standard filming to 43.5% for adjusted filming (p < 0.008). In total, spines readable to L1 or lower increased from 48.2% for standard filming to 80.7% for adjusted filming (p < 0.0001), and spines with ‘blurry’ labelling decreased from 35.7% for standard filming to 15.7% for adjusted filming (p < 0.006).
Based on CLR reading, in total 42.9% (36/84) of the patients had OVF (Table 3). 26 cases of CLR positive cases were detected as VD positive on CFR (26/36, 72.2%) (Fig. 7, Fig. 8), among them five minimal VD (one VD per patient) were detected (Fig. 9). Eight cases with minimal VDs were missed or labelled as false negative (Fig. 10, Fig. 11). This indicates 38% of the minimal VD cases were detected, while 62% of the minimal VD cases were not detected. Among 22 cases with apparent VD, two cases (one VD per patient) were missed on CFR (2/22, 9.1%, Fig. 12).
Table 3.
Reading results of adjusted filming chest fontal view radiograph with lateral view radiograph reading as reference.
| Center-A | Center-B | |
|---|---|---|
| Case number | 58 | 26 |
| Both CLR & CFR suggest negative | 31 | 12 |
| Singular apparent VD detected | 4 | 7 |
| Singular minimal VD detected | 4 | 1 |
| Multiple minimal VD detected | 1 | |
| Multiple app VD detected as Multiple app VD | 5 | 3 |
| Multiple app VD detected as single app VD | 1 | |
| Singular minimal VD false negative | 4 | 1 |
| Multiple minimal VD false negative | 2 | |
| Singular minimal VD missed | 1 | |
| Singular apparent VD missed ∗ | 1 | 1 |
| False positive in image quality ‘blurry’ spines | 4 | |
| False positive in image quality ‘readable’ spines | 1 | |
| Overall true VD rate based on CLR (42.9%) | 37.9% | 53.8% |
∗ Both in mid-thoracic regions. CFR: chest fontal view radiograph. CLR: chest lateral view radiograph. App VD: apparent vertebral deformity.
Fig. 7.
A female patient of 66 years old. L1 apparent vertebral deformity was detected on frontal radiograph (arrow, A; brightness and contrast adjusted for viewing the spine), which is confirmed on lateral radiograph (arrow, B).
Fig. 8.
A female patient of 77 years old. L1 apparent vertebral deformity was detected on frontal radiograph (arrow, A; brightness and contrast adjusted for viewing the spine), which is confirmed on lateral radiograph (arrow, B). C shows an image magnified around L1 which demonstrates apparent depression of upper endplate.
Fig. 9.
A female patient of 72 years old. Frontal view radiograph (brightness and contrast adjusted for viewing the spine) shows T12 deformity (arrow), which is confirmed on lateral view radiograph as apparent deformity (arrow).
Fig. 10.
A female patient of 66 years old. L1 minimal deformity can be identified on lateral view radiograph (arrow), which was missed during fontal view radiograph reading (brightness and contrast adjusted for viewing the spine, arrow indicating lower endplate blurry).
Fig. 11.
A female patient of 70 years old. L1 minimal deformity can be identified on lateral view radiograph (arrow), which was false negative on fontal view radiograph (brightness and contrast adjusted for viewing the spine).
Fig. 12.
A female patient of 66 years old. T7 vertebral deformity (arrow) was initially missed during frontal view radiograph reading (brightness and contrast adjusted for viewing the spine). Lateral view radiograph shows T7 apparent deformity (arrow).
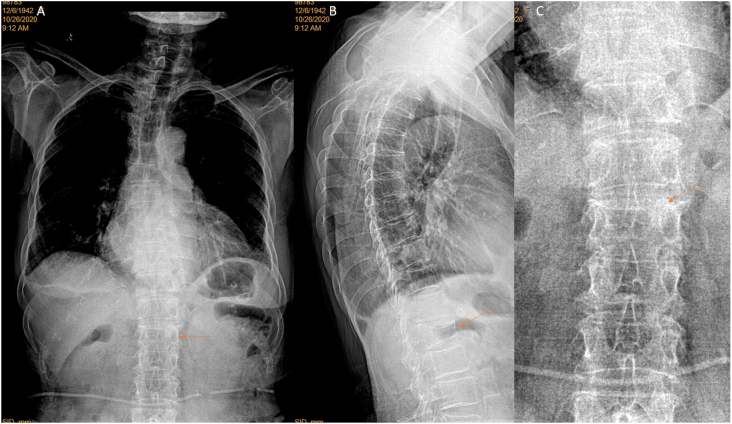
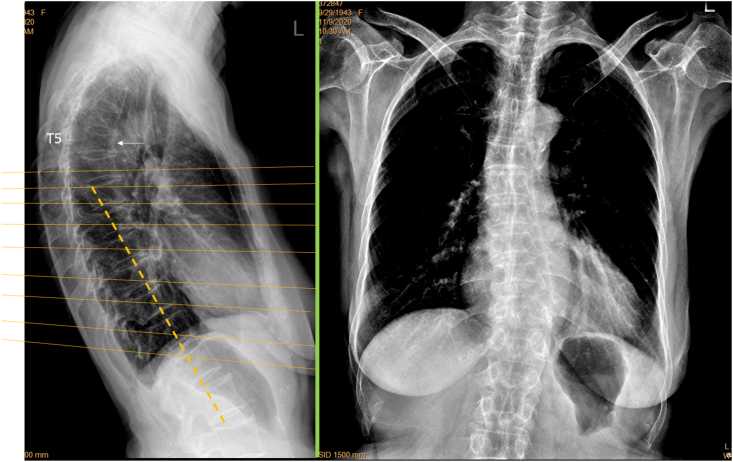
False positivity was labelled on CFR in five cases (5/84, 6.0%), among them four cases were with ‘burry’ spines (supplementary Fig-2). The remaining one CFR false positive case was noted on CLR as with poor positioning of spine relative to X-ray beam (Fig. 13).
Fig. 13.
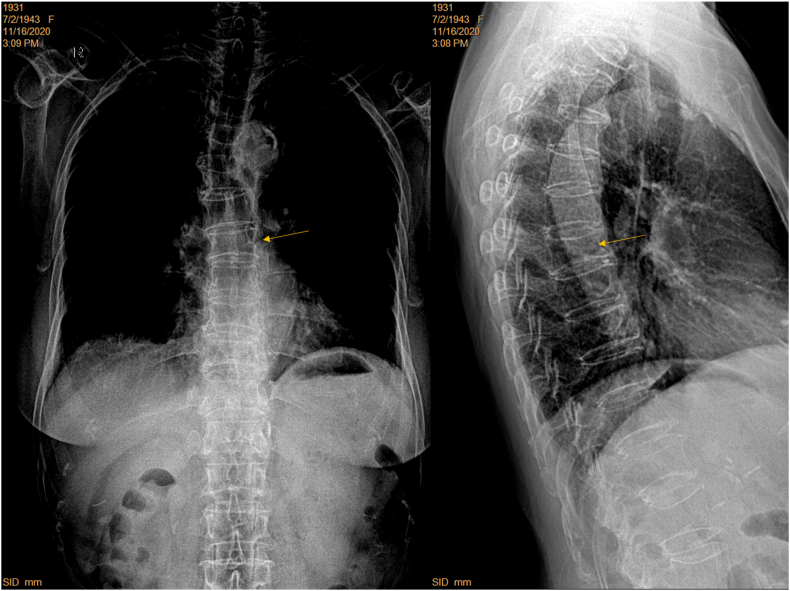
A female patient of 76 years old. T5 apparent deformity (arrow) is shown on lateral view radiograph but cannot be diagnosed on frontal view radiograph. Lower thoracic spine and thoracolumbar junction vertebrae are not well demonstrated on frontal view radiograph (brightness and contrast adjusted for viewing the spine), and in this case vertebrae at thoracolumbar junction can be miss-labelled as with deformity. For the thoracolumbar junction region, lateral view radiograph shows oblique projection of X-ray beam (yellow lines) relative to spine axis (dotted orange line). Green line: X-ray detector. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Discussion
Combining CFR data from Center-A and Center-B using standard filming and adjusted filming, this study showed that, by adjusted filming the proportion of CFR with spines readable down to vertebra L1 increased from 48.2% to 80.7%, and ‘blurry’ spines decreased from 35.7% to 15.7%. Most of the apparent OVFs were detected on CFR. Approximately 40% of the minimal OVFs were detected while 60% of the minimal OVFs were not detected on CFR. Thus, this study confirmed our two hypotheses that 1) CFR taken with approximately two vertebrae lower than the usual X-ray beam positioning improves the visualization of thoraco-lumbar junction; and 2) in two groups of random samples of clinical patients indicated for chest radiograph other than spine disorders, CFR with adjusted filming shows a good detection rate for apparent OVF. Center-C is a tertiary referral hospital, while Center-A and Center-B are both middle-sized general hospitals in China, their radiology department set-up and instruments would represent the general setup of radiology service. The CFRs from Center-E had less visibility for spine (while visible for lungs and heart/big vessels), estimated largely due to the less X-ray exposure during the filming and reflecting different practices in China. Even so, for some patients in Center-D their spines were still visible on CFR. According to CLR, the overall OVF rate was 42.9% in this study’s participants (mean age: 74.7 years). This OVF prevalence agrees with the expectation for elderly females of such an age range. As the study subjects were patients, their health status might be more compromised compared with general population of similar age.
VD is the appearance of OVF (though not every VD is an OVF). In assessing a vertebra, there are usually three stepwise questions: 1) does a VD exist? 2) is this VD likely a compressive VD? 3) can this compressive VD be called OVF? In the current study, OVF was diagnosed based on CLR assessment of VD morphology and after excluding other potential causes both radiographically and clinically. The most common differential diagnosis for OVF is degenerative changes (in the paper by Lentle et al. [32], a term of ‘morphometric vertebral deformity’ was used to describe these degenerative changes). Degenerative VDs often involve multiple adjacent vertebrae appearing similarly deformed, while fractural deformities tend more often to be singular appearing as a distinct loss of expected shape. Degenerative VDs and mild OVF can be mostly differentiated by experienced readers [4]. For the CLR based diagnosis, we chose to use the term OVF, instead of osteoporotic VD, as these deformities mostly have fracture under microscopy [33]. However, for the CFR readings, we used the term VD as we feel that we did not feel confident to differentiate osteoporotic deformity from other deformities, though for those of apparent grade VD, osteoporotic deformity would be the most common deformity in elderly females. Based on vertebral height loss on CLR, we separated OVFs into with <20% height loss and with ≥20% height loss [30]. Minimal OVFs would have included some of the Genant SQ grade-0.5 OVD as well as some of SQ grade-1 VD, as some radiological osteoporotic VDs without achieving 20% height loss threshold are classified as SQ mild VD by some experts [30,34]. It has been noted that, if VD grading based on measurement is taken as the reference, visual estimation has the tendency of slightly over-estimating the grades [28]. After excluding degenerative changes, SQ grade-1 OVFs can be clinically relevant. Johansson et al. [35] reported that grade 1 OVFs were associated with incident major osteoporotic fracture after adjustment for clinical risk factors and bone mineral density. In one recent study of ours [30], of the total five women with baseline minimal OVF, three had osteopenia and two had osteoporosis, and their OVF progression or new incident OVF rate at year-14 follow-up was similar to the women with baseline apparent OVF.
We reiterate our previous standpoint [19,23], for OVF high risk patients such as elderly females (>65 years) with suspected VDs or definite VDs on CFR, additional CLR or DXA imaging based VFA (vertebral fracture assessment, or other imaging modalities such as CT/magnetic resonance imaging when indicated) should be recommended. This can not only confirm (or refute) the suspected VFs on CFR, lateral imaging also helps to grade the OVF and detect possible additional OVF(s) missed on CFR. Multiple OVFs is a strong indication for initiation of anti-osteoporosis treatment. It should also be noted that to exclude VDs of non-osteoporotic causes is not always possible for every case, particularly for mild deformities caused by old trauma [4,36]. The age group with high prevalence of OVF is also the age group with high prevalence of spine metastatic tumors. Differentiating between OVF and metastatic deformity can be difficult in some cases by sole radiograph [27]. Further investigations such as MRI are required for ambiguous cases.
In this study, there were five cases of false positive labelling associated with CFR reading. Among them, four cases were with visually blurry spine, while in the remaining one case the spine was not recorded as ‘blurry’ on CRF but CLR showed mal-positioning of spine axis relative to X-ray beam. This mount of false positive libelling may be a minor concern for reading VD on CFR. A few strategies can be used to mitigate false positive labelling. One is to restrain from labelling VD when the spine looks blurry, though blurry spine may be caused by local kyphosis which can in turn be due to the existing local OVF [19,23]. The second is that, when the radiographer see these patients had substantial kyphosis/scoliosis or apparently ‘the patient could not stand straight’, the radiographer could mark down this point in the referral note so to alert the reporting radiologist. This approach was not done in this study but may be applied for the future. Lastly, increasing the skill of interpretating radiologists may also decrease false positive libelling. In this study, two cases with apparent VD were missed (2/22, 9.1%). Thought again this may be mitigated by gaining more experience, it shall be less a concern as the indications for chest radiograph were not spine disorder nor osteoporosis screening. For opportunistic detection of OVF, we may aim to maximize diagnosis specificity at the cost of sensitivity.
There are a number of limitations for this study. The first is that detecting VD on CFR is a relatively new concept, and experience should be further gained in the future. In this study, only one reader had prior experience in reading VD on CFR. For reading VD on CFR, the readers in Centers-A and B only agreed the readings from Center-E, i.e., they considered the readings from results from Center-E were reasonable. There was no golden standard for OVF detection and grading, and the CLR reading was used as the reference. It is still possible that a minority of the VDs recorded in this study may not be caused by osteoporosis. Currently, we still do not know how to exclude VD due to other causes based on CFR; we also did not offer grading on FR. We did not try to propose standards for filming, as we expect this will depend on individual hospital’s instrument and practice, and also general body size of the patients. Another limitation of this study is its limited sample size. However, we believe the current sample size is sufficient to prove the concept we wanted to emphasize. A multi-center study with much larger sample size is needed to validate the wider applicability of the approach described in this study.
In conclusion, this study suggests that CFR can help detect OVF opportunistically, and the visibility of the mid/lower thoracic spine and thoracolumbar junction can be much improved after minor adjustment of X-ray beam positioning. This study also confirms a high positive rate of OVF among elderly Chinese female patients indicated for chest radiograph other than spine disorders. Though CFR is not a perfect method to screen OVF, as a high proportion of minimal OVF and a small proportion of apparent OVF can be missed, and there is also a small risk of false positive labelling, radiologists should be trained and sensitized in OVF identification on CFR as the clinical management can be improved by opportunistic detection of OVF.
Declaration of competing interest
Authors Er-Zhu Du, Wei-Hong Liu, and Yì Xiáng Wáng all declare no conflict of interest.
Acknowledgement
We thank Dr Jingshan Gong, Department of Radiology, Shenzhen People’s Hospital, Shenzhen, Guangdong Province, China; and Dr Jianbing Ma, Department of Radiology, the First Hospital of Jiaxing, Jiaxing, Zhejiang Province, China, for providing the cases and assistance for this study. This study was partially supported by Innovation and technology fund (ITF) project of Hong Kong SAR (code: ITS/334/18).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2021.04.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kim D.H., Vaccaro A.R. Osteoporotic compression fractures of the spine; current options and considerations for treatment. Spine J. 2006;6:479–487. doi: 10.1016/j.spinee.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Delmas P.D., van de Langerijt L., Watts N.B., Eastell R., Genant H., Grauer A. IMPACT Study Group. Underdiagnosis of vertebral fractures is a worldwide problem: the IMPACT study. J Bone Miner Res. 2005;20:557–563. doi: 10.1359/JBMR.041214. [DOI] [PubMed] [Google Scholar]
- 3.Du M.M., Che-Nordin N., Ye P.P., Qiu S.W., Yan Z.H., Wang Y.X.J. Underreporting characteristics of osteoporotic vertebral fracture in back pain clinic patients of a tertiary hospital in China. J Orthop Translat. 2019;9(23):152–158. doi: 10.1016/j.jot.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y.X. Osteoporotic vertebral deformity: radiological appearances and their association with a history of trauma and the risk of further fragility fracture. Can Assoc Radiol J. 2020;Sep 16 doi: 10.1177/0846537120958471. [DOI] [PubMed] [Google Scholar]
- 5.Diacinti D., Vitali C., Gussoni G., Pisani D., Sinigaglia L., Bianchi G. Research Department of FADOI. Misdiagnosis of vertebral fractures on local radiographic readings of the multicentre POINT (prevalence of osteoporosis in INTernal medicine) study. Bone. 2017;101:230–235. doi: 10.1016/j.bone.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Weaver J., Sajjan S., Lewiecki E.M., Harris S.T., Marvos P. Prevalence and cost of subsequent fractures among U.S. Patients with an incident fracture. J Manag Care Spec Pharm. 2017;23:461–471. doi: 10.18553/jmcp.2017.23.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delmas P.D., Genant H.K., Crans G.G., Stock J.L., Wong M., Siris E. Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial. Bone. 2003;33:522–532. doi: 10.1016/s8756-3282(03)00241-2. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez-Rodriguez D., Bergmann P., Body J.J., Cavalier E., Gielen E., Goemaere S. The Belgian Bone Club 2020 guidelines for the management of osteoporosis in postmenopausal women. Maturitas. 2020;139:69–89. doi: 10.1016/j.maturitas.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Danila M.I., Saag K.G. Imminent fracture risk: a call to action for rheumatologists. Arthritis Care Res. 2020;72:741–743. doi: 10.1002/acr.24171. [DOI] [PubMed] [Google Scholar]
- 10.Nuti R., Brandi M.L., Checchia G., Di Munno O., Dominguez L., Falaschi P. Guidelines for the management of osteoporosis and fragility fractures. Intern Emerg Med. 2019;14:85–102. doi: 10.1007/s11739-018-1874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer A., Exuzides A., Spangler L., O’Malley C., Colby C., Johnston K. Burden of illness for osteoporotic fractures compared with other serious diseases among postmenopausal women in the United States. Mayo Clin Proc. 2015;90:53–62. doi: 10.1016/j.mayocp.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y.X., Che-Nordin N., Deng M., Leung J.C.S., Kwok A.W.L., He L.C. Osteoporotic vertebral deformity with endplate/cortex fracture is associated with higher further vertebral fracture risk: the Ms. OS (Hong Kong) study results. Osteoporos Int. 2019;30:897–905. doi: 10.1007/s00198-019-04856-4. [DOI] [PubMed] [Google Scholar]
- 13.Conley R.B., Adib G., Adler R.A., Åkesson K.E., Alexander I.M., Amenta K.C. Secondary fracture prevention: consensus clinical recommendations from a multistakeholder coalition. J Bone Miner Res. 2020;35:36–52. doi: 10.1002/jbmr.3877. [DOI] [PubMed] [Google Scholar]
- 14.Kwok T.C.Y., Law S.W., Leung E.M.F., Choy D.T.K., Lam P.M.S., Leung J.C.S. Hip fractures are preventable: a proposal for osteoporosis screening and fall prevention in older people. Hong Kong Med J. 2020;26:227–235. doi: 10.12809/hkmj198337. [DOI] [PubMed] [Google Scholar]
- 15.US Preventive Services Task Force. Curry S.J., Krist A.H., Owens D.K., Barry M.J., Caughey A.B., Davidson K.W. Screening for osteoporosis to prevent fractures: US preventive services task force recommendation statement. J Am Med Assoc. 2018;319:2521–2531. doi: 10.1001/jama.2018.7498. [DOI] [PubMed] [Google Scholar]
- 16.Brincat M., Calleja-Agius J., Erel C.T., Gambacciani M., Lambrinoudaki I., Moen M.H. EMAS. EMAS position statement: bone densitometry screening for osteoporosis. Maturitas. 2011;68:98–101. doi: 10.1016/j.maturitas.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Cheng X., Yuan H., Cheng J., Weng X., Xu H., Gao J. On behalf of bone and joint group of Chinese society of radiology, Chinese medical association (CMA), musculoskeletal radiology society of Chinese medical doctors association, osteoporosis group of Chinese orthopedic association, bone density group of Chinese society of imaging technology, CMA). Chinese expert consensus on the diagnosis of osteoporosis by imaging and bone mineral density. Quant Imag Med Surg. 2020;10:2066–2077. doi: 10.21037/qims-2020-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Society for Clinical Densitometry ISCD official positions–adult. International society for clinical densitometry (ISCD) 2019. https://www.iscd.org/official-positions/2019-iscd-official-positions-adult/ Available on-line: Accessed 17 November2020.
- 19.Wang Y.X., Du M.M., Che-Nordin N., Ye P.P., Qiu S.W., Griffith J.F. Recognizing osteoporotic vertebral deformity on frontal view radiograph: a cohort analysis and a pictorial review. Arch Osteoporos. 2020;15:41. doi: 10.1007/s11657-020-00716-5. [DOI] [PubMed] [Google Scholar]
- 20.Deng M., Zeng X.J., He L.C., Leung J.C.S., Kwok A.W.L., Griffith J.F. Osteoporotic vertebral fracture prevalence in elderly Chinese men and women: a comparison of endplate/cortex fracture-based and morphometrical deformity-based methods. J Clin Densitom. 2019;22:409–419. doi: 10.1016/j.jocd.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Freitas S.S., Barrett-Connor E., Ensrud K.E., Fink H.A., Bauer D.C., Cawthon P.M. Osteoporotic Fractures in Men (MrOS) Research Group. Rate and circumstances of clinical vertebral fractures in older men. Osteoporos Int. 2008;19:615–623. doi: 10.1007/s00198-007-0510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wáng Y.X.J., Wang X.R., Che-Nordin N., Xu F.R., Huang Q.L. On the possibility of over-diagnosis of osteoporotic vertebral fracture at mid-thoracic level. J Thorac Dis. 2019;11:5708–5711. doi: 10.21037/jtd.2019.11.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y.X., Du E.Z., Gong J., Cheng X. Interpretation of osteoporotic vertebral deformity on frontal view radiographs of the chest and abdomen: a pictorial review. Quant Imag Med Surg. 2021;11:423–442. doi: 10.21037/qims-2020-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genant H.K., Jergas M. Assessment of prevalent and incident vertebral fractures in osteoporosis research. Osteoporos Int. 2003;14:S43–S55. doi: 10.1007/s00198-002-1348-1. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz E.N., Steinberg D. Detection of vertebral fractures. Curr Osteoporos Rep. 2005;3:126–135. doi: 10.1007/s11914-996-0015-4. [DOI] [PubMed] [Google Scholar]
- 26.Lentle B., Trollip J., Lian K. The radiology of osteoporotic vertebral fractures redux. J Clin Densitom. 2016;19:40–47. doi: 10.1016/j.jocd.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y.X.J., Santiago F.R., Deng M., Nogueira-Barbosa M.H. Identifying osteoporotic vertebral endplate and cortex fractures. Quant Imag Med Surg. 2017;7:555–591. doi: 10.21037/qims.2017.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y.X.J., Diacinti D., Yu W., Cheng X.G., Nogueira-Barbosa M.H., Che-Nordin N. Semi-quantitative grading and extended semi-quantitative grading for osteoporotic vertebral deformity: a radiographic image database for education and calibration. Ann Transl Med. 2020;8:398. doi: 10.21037/atm.2020.02.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y.X.J., Liu W.H., Diacinti D., Yang D.W., Iannacone A., Wang X.R. Diagnosis and grading of radiographic osteoporotic vertebral deformity by general radiologists after a brief self-learning period. J Thorac Dis. 2020;12:4702–4710. doi: 10.21037/jtd-20-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y.X., Che-Nordin N., Leung J.C.S., Man Yu B.W., Griffith J.F., Kwok T.C.Y. Elderly men have much lower vertebral fracture risk than elderly women even at advanced age: the MrOS and MsOS (Hong Kong) year 14 follow-up radiology results. Arch Osteoporos. 2020;15:176. doi: 10.1007/s11657-020-00845-x. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz Santiago F., Láinez Ramos-Bossini A.J., Wang Y.X., López Zúñiga D. The role of radiography in the study of spinal disorders. Quant Imag Med Surg. 2020;10:2322–2355. doi: 10.21037/qims-20-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lentle B.C., Berger C., Brown J.P., Probyn L., Langsetmo L., Hammond I. Vertebral fractures: which radiological criteria are better associated with the clinical course of osteoporosis? Can Assoc Radiol J. 2021;72:150–158. doi: 10.1177/0846537120943529. [DOI] [PubMed] [Google Scholar]
- 33.Antonacci M.D., Mody D.R., Rutz K., Weilbaecher D., Heggeness M.H. A histologic study of fractured human vertebral bodies. J Spinal Disord Tech. 2002;15:118–126. doi: 10.1097/00024720-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 34.https://www.iofbonehealth.org/what-we-do/training-andeducation/educational-slide-its/vertebral-fracture-teachingprogram
- 35.Johansson L., Sundh D., Magnusson P., Rukmangatharajan K., Mellström D., Nilsson A.G. Grade 1 vertebral fractures identified by densitometric lateral spine imaging predict incident major osteoporotic fracture independently of clinical risk factors and bone mineral density in older women. J Bone Miner Res. 2020;35:1942–1951. doi: 10.1002/jbmr.4108. [DOI] [PubMed] [Google Scholar]
- 36.Wang X.R., Xu F.R., Huang Q.L., Wang Y.X. Radiological features of traumatic vertebral endplate fracture: an analysis of 194 cases with 263 vertebral fractures. Chin Med J. 2020;133:2696–2702. doi: 10.1097/CM9.0000000000000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.