Abstract
The zoonotic pathogen Campylobacter jejuni is among the leading causes of foodborne diseases worldwide. While C. jejuni colonises many wild animals and livestock, persistence mechanisms enabling the bacterium to adapt to host species' guts are not fully understood. In order to identify putative determinants influencing host preferences of distinct lineages, bootstrapping based on stratified random sampling combined with a k-mer-based genome-wide association was conducted on 490 genomes from diverse origins in Germany and Canada. We show a strong association of both the core and the accessory genome characteristics with distinct host animal species, indicating multiple adaptive trajectories defining the evolution of C. jejuni lifestyle preferences in different ecosystems. Here, we demonstrate that adaptation towards a specific host niche ecology is most likely a long evolutionary and multifactorial process, expressed by gene absence or presence and allele variations of core genes. Several host-specific allelic variants from different phylogenetic backgrounds, including dnaE, rpoB, ftsX or pycB play important roles for genome maintenance and metabolic pathways. Thus, variants of genes important for C. jejuni to cope with specific ecological niches or hosts may be useful markers for both surveillance and future pathogen intervention strategies.
Subject terms: Computational biology and bioinformatics, Ecology, Microbiology
Introduction
Campylobacter jejuni is regarded as a common resident among the gut microbiota of many wild and agriculture-associated animals1, especially birds, poultry and cattle2,3. Contamination of (chicken) meat, water, raw-milk and other food products along the food production chain is therefore the most attributable factor of diarrheal disease caused by C. jejuni in humans3–6. As a result C. jejuni is a bacterium frequently isolated from human patients suffering from acute gastroenteritis6.
Previous research using multilocus sequence typing (MLST) of C. jejuni from different origins showed that specific sequence types (STs) were frequently associated with a particular host species7. While STs belonging to the clonal complexes (CC)-42 and CC-61 are common among C. jejuni of cattle and/or other ruminate origins, STs belonging to CC-257, CC-353 or CC-1034 are regarded as chicken-specific8–10. Isolates belonging to STs sharing a clonal complex such as CC-21, CC-45 or CC-48 commonly occur in samples of multiple host species, indicating the ability of these phylogenetic lineages to rapidly switch between different (intestinal) conditions, and, therefore, representing a typical host-generalist lifestyle11. Factors influencing adaptation of C. jejuni to certain host species, especially to poultry and cattle, were an important focus of Campylobacter research over the last decade12–14. In recent years, novel bioinformatic methods and tools such as genome-wide association studies (GWAS) proved their potential to identify genetic factors promoting host adaptation and/or pathogenicity in C. jejuni13–17. For instance, accessory genes encoding factors involved in the bacterial vitamin B5 biosynthesis pathway were found to be associated with cattle and its typical diet13, while proteins enhancing iron acquisition abilities of the bacteria during infection were harboured by isolates from human clinical samples 16. Previous studies employing GWAS often implemented a gene-by-gene approach for population scale analysis or focused on particular strains, such as CC-4513,15,16, a phylogenetic background known for its frequent association with cases of human diseases worldwide14,18–20 .
Most of these GWAS have been predominately focused on the variable set of genes commonly addressed as accessory genome. However, changes among (essential) core genes (i.e. basic cellular and regulatory functions) within the C. jejuni population may reflect adaptation towards a particular bacterial lifestyle as well.
Core genome alterations are thought to play an important role in overcoming specific host-associated intestinal stress conditions21,22, while other alterations may enable certain Campylobacter lineages to cope with colonisation inhibitors or even diets associated with gastrointestinal tracts of a much broader range of host species23. A recent GWAS study indicated that the worldwide intensified cattle farming for meat production was accompanied by a timeline of genomic events enhancing host adaptation of certain C. jejuni lineages to cattle24.
The aim of this study was to generate in-depth insights into the current population structure of C. jejuni by using high resolution of whole genome sequencing and a stratified random sampling approach combined with GWAS considering all nucleotide substrings of length k (k-mers) to investigate host adaptation, niche gene associations and outbreak potential associated with distinct C. jejuni lineages.
Results
C. jejuni core and accessory genome analysis
Here we report on 490 genomes of C. jejuni isolated from samples of animal, human and environmental origins from two distinct continents. The average size of the C. jejuni genomes was 1 690 635 bp. We identified 1 111 core genes that covered 60% of the average C. jejuni genome size, while a set of additional 7 250 genes was identified in at least one of the genomes under consideration and therefore assigned to the accessory gene content.
Core and accessory genome: phylogenetic structure and organisation of the C. jejuni population
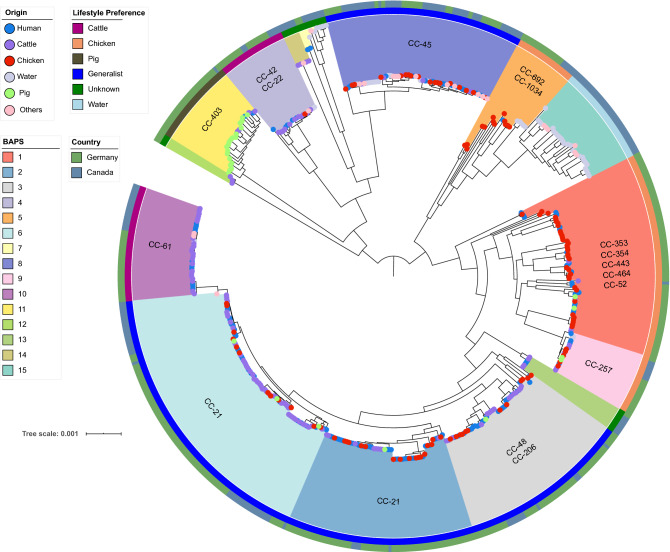
The phylogenetic representation of the 490 core genomes showed 15 distinct phylogenetic branches (1–15) that have been confirmed by BAPS clustering (Fig. 1). BAPS clusters identified here, which comprised of more than 15 C. jejuni genomes, were further evaluated according to their respective CCs, original sample source and lifestyle classification (Table S1).
Figure 1.
Population structure of C. jejuni based on the core genome alignment with BAPS clusters and clonal complexes colour-coded in the inner ring; Lifestyle preferences of the genomes coded in the second -ring; and country of genome origin described in the outer ring. The leaves are coloured by the origin of each sample.
For the original sample sources of the C. jejuni genomes investigated here, the relative proportion and absolute distribution for each of the BAPS clusters are visualised in Fig. 2a and supplementary Figure S1a. We identified a close phylogenetic relationship between genomes of BAPS cluster 5 representing the origin chicken with those of BAPS cluster 15 representing waterborne environmental C. jejuni (Fig. 1).
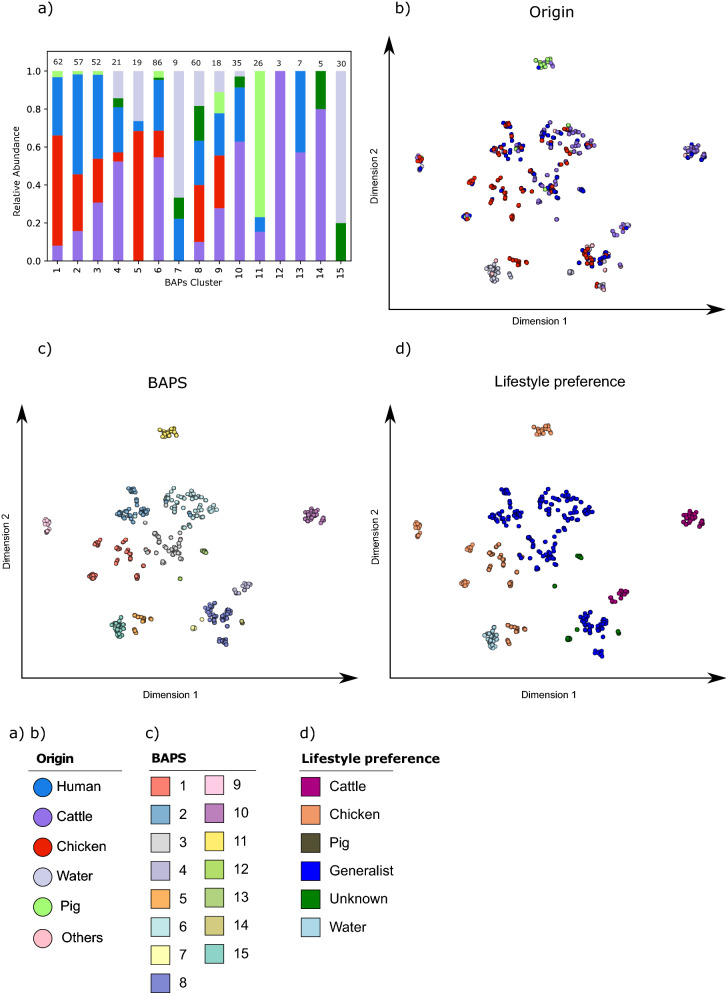
Figure 2.
Relative distribution of sample origin among BAPS clusters and t-SNE plots of the accessory genome profile. (a) Shows the relative proportion of sample origins within the BAPS cluster that are later used for the stratified random sampling approach. (b–d) Show t-SNE plots in the 2-dimensional space of the accessory genome profiles. The colours included in the legend represent the sampling source, the BAPS clusters and the lifestyle preference are included in the legend.
The genomes of BAPS cluster 15 and those of BAPS clusters with genomes from less than 15 isolates were not analysed with respect to their lifestyle preference and were therefore used as a control group in our study.
The lifestyle preference of each major BAPS cluster was determined and subjected to an internal assessment: As shown in Table S2, our assignments are generally concordant with lifestyle preferences reported for frequently occurring lineages such as CC-353, CC-354, CC-443, CC-464 and CC-52 (chicken), CC-42 and CC-61 (cattle) and CC-403 (pig). We also identified the probable lifestyle classification for the CC-22 lineage (cattle) and for isolates belonging to ST-2274 (chicken) (Table S1 and Table S2). Of note, the C. jejuni genomes associated with CC-21, CC-45 and CC-48 fulfilled the criteria for host-generalist lineages (Table S2).
Overall, the genomes assigned to individual BAPS clusters consisting of lineages considered as either host-specific for cattle (BAPS 4; including CC-42 and CC-22; BAPS 10, CC-61) or pigs (BAPS 11, CC-403) showed generally a less diverse population structure than those assigned to clusters associated with the host chicken (e.g. BAPS 5, including CC-1034 and CC-692). The distinct BAPS clusters comprising of host-generalist lineages (BAPS 8, including CC-45 and CC-283; BAPS 2, CC-21; BAPS 6, CC-21) showed a more diverse population structure (Fig. 1).
Our core genome-based phylogenetic analysis further revealed that cattle-related BAPS cluster 4 lineages (including CC-42 and CC-22) were more closely related to host-generalist lineages of BAPS cluster 6 with CC-21 than to other cattle-related lineages, for instance those of BAPS cluster 10 (Fig. 1). This also holds true for the chicken-related phylogenetic background (Fig. 1): While chicken-related BAPS cluster 1 was found being more closely related to BAPS cluster 6 of host-generalist lineage, BAPS cluster 5 showed less phylogenetic distance to BAPS cluster 8 (host-generalist lineage). These findings clearly reject the hypothesis of a common evolutionary background for host-specific lineages with respect to the host species represented here.
Minimum spanning trees based on MLST utilising BAPS cluster classification and lifestyle preferences are shown in the supplementary material (Figure S1). Finally, the accessory genome profiles of all genomes were visualised by t-SNE plots in Fig. 2b–d including sample origin, BAPS cluster and lifestyle preference. As expected, the overall population structure derived from the core genome is mirrored in the accessory genome content. Each BAPS cluster carries its unique set of accessory genes (Fig. 2c) confirming the population structure based on BAPS.
Also, C. jejuni genomes belonging to different BAPS clusters while sharing a particular lifestyle preference differ with respect to their accessory gene content (Fig. 2d). This observation is supported, for instance, by the accessory gene content identified for the cattle-specific BAPS clusters 4 and 10 (CC-42 and CC-61) and the chicken-specific BAPS clusters 1, 5 and 9 (CC-354, CC-692, CC-257, etc.) (Figs. 2c,d). Overall, BAPS clusters with a host-generalist lifestyle preference appear to have a broader gene pool within the accessory genome content than strains identified as host-specific.
Recombination events in Campylobacter jejuni lineages
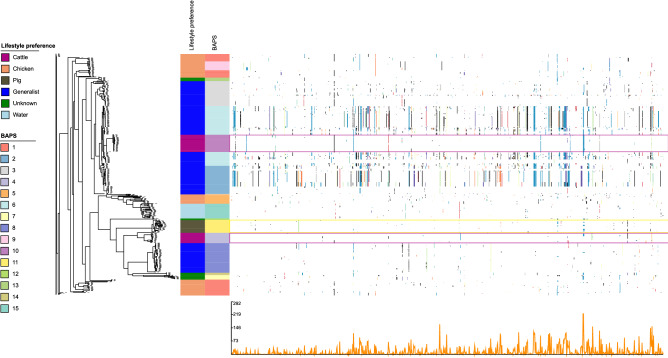
Recombination events that show more differences between taxa than expected by mutation-driven evolutionary processes alone were illustrated in Fig. 3. Overall, CCs assigned as cattle- or pig-associated as well as those belonging to the group of host-generalists showed recombination profiles most likely resulting from intra-lineage genomic events. The pig-associated lineages of BAPS cluster 11 and the cattle-associated lineages of BAPS cluster 4 shared limited recombination patterns with other lineages and yielded a low recombination rate compared with other clusters, indicating the possible presence of lineage-specific recombination barriers (Fig. 3). The cattle-associated genomes forming BAPS cluster 10 showed several recombination events which were also indicated in the host-generalist lineages assigned to BAPS clusters 2, 3 and 6 (Fig. 3). However, the cattle-associated BAPS clusters 4 and 10 shared a single recombination site only. The host-generalist BAPS clusters 2, 3, and 6 were found being associated with more recombination events and some of these were shared by host-specific lineages, i.e. BAPS cluster 10 (cattle) and BAPS clusters 1, 5 and 9 (chicken), indicating genomic exchanges between these lineages. In addition, the analysis revealed that chicken-associated lineages (BAPS clusters 1, 5 and 9) were prone to trade off genetic material with each other and with host-generalist lineages (Fig. 3).
Figure 3.
Recombination profile of the core genome alignment of 490 C. jejuni isolates calculated by BRATNextGen and visualized in Phandango. The left side shows the core genome phylogeny. The metadata provide information about lifestyle preferences (association) and BAPS clusters. Significant recombinations are marked by coloured dots and lines. Purple and yellow boxes highlight cattle- and pig-associated BAPS clusters, respectively. Presence of dot of the same colour across multiple isolates within a column represents acquisition of the same recombinant segment, otherwise colours are arbitrary. The line graph at the bottom presents recombination prevalence along the genome sequence.
In-silico identification of host-specific factors
After identifying significant k-mers using a consensus GWAS approach, the k-mers were mapped to an annotated reference genome in order to identify coding sequences (CDS) of the genome known to promote a particular lifestyle preference of C. jejuni25. A visualization of the resulting genes with corresponding p-values and frequencies for the matching k-mers are provided in supplementary Figure S2.
CDS identified by k-mers in the genomes of C. jejuni isolates with lifestyle preferences in pig and cattle showed a denser distribution around the expected allele frequency than the results obtained for the genomes representing chicken- or host-generalist lineages (Figure S2).
The genes identified by our analysis included accessory genes present in a limited number of genomic backgrounds and allelic variants of the core genome content. We identified several variants of core genes supporting specific lifestyle preferences in C. jejuni. To further evaluate the putative host-specific importance of the allelic variants identified, genes under consideration have been checked for non-synonymous base changes by comparing their predicted amino acid sequences. Several of these predicted aa sequences can be linked to particular lifestyle preferences of C. jejuni isolates. Details for all loci and aa sequence variants identified are provided in the Tables S3 (cattle), S4 (chicken), S5 (pig) and S6 (host-generalists).
Accessory genes and allelic variants of the core genome associated with C. jejuni lineages assigned as pig-specific
In the genomes belonging to BAPS cluster 11 (CC-403) we identified 21,681 k-mers which are significantly associated with the host pig. These k-mers mapped to 49 accessory genes and 78 allelic variants of the core genome (Table S5). Considering the accessory genes, 14 were exclusively found within C. jejuni genomes from pig hosts. (Table 1). Three accessory genes (A6J90_06670, A6J90_06675, A6J90_02350) belonged to transcription units encoding type II restriction modification systems (RM systems), while a further gene encodes the restriction subunit (R) of the host specificity determinant (hsdR; A6J90_08990) of a type I RM system. Additional 8/14 genes were annotated as hypothetical or putative proteins without any specific functional information available in NCBI GenBank (17.06.2020).
Table 1.
Selected accessory genes and allelic variants of the C. jejuni core genome content pig-associated.
| Locus taga | Gene | Predicted function | BfR- CA-14430b | COGc | COGc description | Lifestyle preferenced | Accessory/variante | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pig | Cattle | Chicken | Host generalists | Other | |||||||
| n | n | n | n | n | |||||||
| A6J90_00190 | – | Putative protein | – | – | – | 25 | 0 | 0 | 0 | 0 | A |
| A6J90_00195 | – | Hypothetical protein | – | S | Function unknown | 26 | 0 | 0 | 0 | 0 | A |
| A6J90_00200 | – | Hypothetical protein | – | – | – | 26 | 1 | 0 | 0 | 0 | A |
| A6J90_00270 | – | Putative protein | – | – | – | 26 | 0 | 0 | 0 | 0 | A |
| A6J90_00275 | dpnA | DNA methylase | – | L | Replication,recombination and repair | 26 | 0 | 0 | 0 | 0 | A |
| A6J90_01490 | – | Putative protein | – | – | – | 26 | 0 | 0 | 0 | 0 | A |
| A6J90_01500/A6J90_01505 | – | Hypothetical protein | – | V | Defense mechanisms | 25 | 0 | 0 | 0 | 0 | A |
| A6J90_02340 | – | Undecaprenyl-diphos-phooligosaccharide-protein glycotransferase | – | – | – | 25 | 0 | 0 | 0 | 0 | A |
| A6J90_02350 | – | R Pab1 restriction endonuclease | – | L | Replication, recombination and repair | 25 | 0 | 0 | 0 | 0 | A |
| A6J90_06670 | – | Type II restriction endonuclease | – | L | Replication, recombination and repair | 26 | 0 | 0 | 0 | 1 | A |
| A6J90_06675 | hhaIM | Cytosine-specificmethyl-transferase NlaX | – | H | Coenzyme transport and metabolism | 26 | 0 | 0 | 0 | 1 | A |
| A6J90_08990 | hsdR | Type I restriction enzyme EcoR124II R protein | – | V | Defense mechanisms | 26 | 0 | 1 | 0 | 0 | A |
| A6J90_01640 | – | Hypothetical protein | – | – | – | 26 | 0 | 0 | 0 | 0 | A |
| A6J90_02350 | (sua5) | Hypothetical protein | – | J | Translation, ribosomal structure and biogenesis | 26 | 0 | 0 | 0 | 0 | A |
| Cj0321 | dxs | l-Deoxy-d-xylulose-5-phosphate synthase | 298.748 | H | Coenzyme transport and metabolism | 26 | 56 | 90 | 255 | 63 | V |
| Cj1043c | tenI | Thiamine-phosphate Pyrophosphorylase | 991.366 | H | Coenzyme transport and metabolism | 26 | 56 | 90 | 255 | 63 | V |
| Cj1484c | - | Putative membraneprotein | 1.428.185 | – | – | 26 | 56 | 90 | 255 | 63 | V |
aLocus tag for accessory genes based on C. jejuni reference genome CP022076.1 (NCBI accession). Locus tags for allelic variants of the core genome refer to C. jejuni strain NCTC11168 (NCBI accession: AL111168.1).
bPosition of core genes in the reference strain BfR-CA-14430.
cClusters of orthologous groups (http://clovr.org/docs/clusters-of-orthologous-groups-cogs/).
dNumber of genomes assigned to a particular lifestyle carrying the gene or allelic variant (pig, cattle, chicken, host generalists, others).
eA indicates that a gene belongs to the accessory genome content of C. jejuni, while V indicates a specific allelic variant of the core genome content.
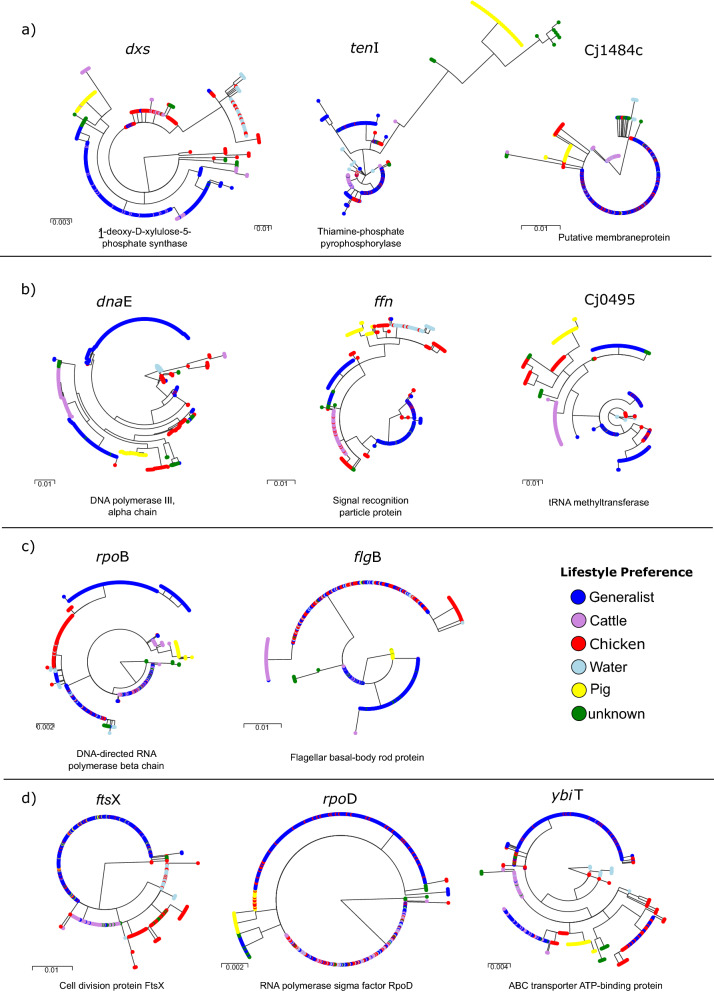
Considering the k-mer results for genes belonging to the core genome, nucleotide changes leading to actual effects with respect to host adaptation capabilities of certain lineages are difficult to pinpoint. Here, we noted alterations for the predicted aa sequences associated with the capability of C. jejuni to synthesize vitamins and enzyme co-factors such as TenI and Dxs (Fig. 4a). In addition, the predicted aa sequence for Cj1484 was found to be altered (Fig. 4a).
Figure 4.
Phylogenetic tree of predicted amino acid sequence variants encoded by dnaE, ffh, Cj0495, rpoB, flgB, ftsX, rpoD, ybiT, dxs, tenI and Cj484c (selected from Tables 2, 3 and 4) that show lifestyle associated variants (colour coded in legend) in different phylogenetic lineages originating from different genetic and geographic backgrounds (Fig. 1).
Accessory genes and allelic variants of the core genome associated with C. jejuni lineages assigned as cattle-specific
We further identified 66,491 k-mers for the cattle-associated genomes matching to 71 accessory genes and to 136 core gene variants (Table S3). According to our GWAS analysis, a particular accessory gene content which is representative for the lineages in both cattle-associated BAPS clusters (4 and 10) was not identified. However, 16 accessory genes were identified by k-mers significantly associated with CC-61 (BAPS cluster 10; Table S3). These genes belonged to a region of 9.9 kb size in C. jejuni (NCTC13261_01705 up to NCTC13261_01720). That particular locus contains 16 open reading frames encoding a HicA-HicB toxin/antitoxin system inhibiting the transfer of mRNA in case of nutrient limitation, a protein known to be involved in extracytoplasmatic stress response (YafQ) and regulatory protein RepA for plasmid DNA repair (Table S3).
Within the core genome we identified a 9.7 kb locus of 9 adjacent genes (Table 2) that encode for a ribosomal complex. While the allelic variants (non-synonymous substitutions) dnaE and ffh (Fig. 4b) were identified as cattle-specific, identical variants of arsC, aroF, uraH, rplS, trmD, rimM and rpsP were identified in host-generalist BAPS cluster 8, too. However, for the genes uraH, arsC, rplS and rpsP, detected SNPs lead to synonymous changes only, indicating their biological importance as conserved housekeeping genes within the C. jejuni lineages investigated here.
Table 2.
Selected accessory genes and allelic variants of the core genome content associated with the host cattle.
| Locus taga | Gene | Predicted function | BfR-CA-14430b | COGc | COGc description | Lifestyle preferenced | Accessory/variante | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pig | Cattle | Chicken | Host generalists | Other | |||||||
| n | n | n | n | n | |||||||
| Cj0718 | DnaE | DNA polymerase III, alpha chain | 679,065 | L | Replication, recombination and repair | 26 | 56 | 90 | 255 | 63 | V |
| Cj0717 | ArsC | Putative ArsC family protein | 678,288 | P | Inorganic ion transport and metabolism | 26 | 56 | 90 | 255 | 63 | V |
| Cj0716 | AroF | Putative phospho-2-dehydro-3-deoxyhep-tonate aldolase | 678,951 | E | Amino acid transport and metabolism | 26 | 56 | 90 | 255 | 63 | V |
| Cj0715 | uraH | Transthyretin-like periplasmic protein | 676,514 | S | Function unknown | 26 | 56 | 90 | 255 | 63 | V |
| Cj0714 | rplS | 50S ribosomal protein L19 | 676,024 | J | Translation, ribosomal structure and biogenesis | 26 | 56 | 90 | 255 | 63 | V |
| Cj0713 | trmD | tRNA (guanine-N1)-methyltransferase | 675,309 | J | Translation, ribosomal structure and biogenesis | 26 | 56 | 90 | 255 | 63 | V |
| Cj0712 | rimM | Putative 16S rRNA processing protein | 674,773 | J | Translation, ribosomal structure and biogenesis | 26 | 56 | 90 | 255 | 63 | V |
| Cj0710 | rpsP | 30S ribosomal protein S16 | 674,308 | J | Translation, ribosomal structure and biogenesis | 26 | 56 | 90 | 255 | 63 | V |
| Cj0709 | ffh | Signal recognition particle protein | 672,906 | U | Intracellular trafficking, secretion, and vesicular transport | 26 | 56 | 90 | 255 | 63 | V |
| Cj0495 | – | tRNA methyltransferase | 465,764 | J | Translation, ribosomal structure and biogenesis | 26 | 56 | 90 | 255 | 63 | V |
| Cj0017c | dsbI | Disulfid-deoxidoreductase | 825,673 | C | Energy production and conversion | 26 | 56 | 90 | 255 | 63 | V |
| Cj1233 | – | HAD-superfamily hydrolase | 1,175,101 | S | Function unknown | 26 | 56 | 90 | 255 | 63 | V |
| _01705 | Putative periplasmic protein | – | 35 | 38 | 0 | 193 | 43 | A | |||
| _01706 | – | RelE/ParE family plasmid Stabilization system | – | S | Function unknown | 35 | 20 | 0 | 0 | 4 | A |
| _01707 | – | Hypothetical protein | – | – | 35 | 0 | 0 | 0 | 0 | A | |
| _01708 | – | Hypothetical protein | – | – | 35 | 0 | 0 | 0 | 0 | A | |
| _01709 | – | Acyl carrier protein | – | K | Transcription | 34 | 0 | 0 | 0 | 0 | A |
| _01710 | – | Hypothetical protein | – | – | 35 | 0 | 0 | 0 | 0 | A | |
| _01711 | dnaG | DnaB-like protein helicase-like protein | – | L | Replication, recombination and repair | 30 | 19 | 0 | 0 | 4 | A |
| _01712 | – | Hypothetical protein | – | – | 34 | 7 | 0 | 1 | 4 | A | |
| _01713 | – | Hypothetical protein | – | – | 35 | 0 | 0 | 1 | 0 | A | |
| _01714 | – | Helix-turn-helix domain-containing | – | – | 35 | 19 | 0 | 1 | 4 | A | |
| _01716 | – | Putative protein | – | – | 35 | 0 | 0 | 0 | 0 | A | |
| _01717 | hicB | Antitoxin HicB | – | S | Function unknown | 34 | 14 | 0 | 1 | 4 | A |
| _01718 | Hypothetical protein | – | N | Cell motility | 35 | 20 | 0 | 0 | 4 | A | |
| _01719 | hicA | Probable mRNA interferase toxin HicA | – | – | 35 | 20 | 0 | 0 | 4 | A | |
| _01720 | – | Integrase | – | L | Replication, recombination and repair | 35 | 20 | 0 | 1 | 4 | A |
aLocus tags for accessory genes based on C. jejuni reference strain NCTC13261 genome LR134500.1 (NCBI accession) while locus tags for allelic variants of the core genome refer to C. jejuni strain NCTC11168 (NCBI accession: AL111168.1).
bPosition of core genes in the reference strain BfR-CA-14430.
cClusters of orthologous groups (http://clovr.org/docs/clusters-of-orthologous-groups-cogs/).
dNumber of genomes assigned to a particular lifestyle carrying the gene or allelic variant (pig, cattle, chicken, host generalists, others); e A indicates that a gene belongs to the accessory genome content of C. jejuni and V indicates a specific allelic variant of the core genome content.
Additional non-synonymous, cattle-specific allelic variants were also identified on independent positions within the genome, including the alleles Cj0495(Fig. 4b), dsbI and Cj1233 (Table 2).
Accessory genes and allelic variants of the core genome associated with C. jejuni lineages assigned as chicken-specific
In comparison to the lineages associated with cattle, pig or even the host-generalists, chicken-associated lineages showed the broadest phylogenetic diversification in our study, mirrored by multiple lineages and CCs (Fig. 1), including enhanced divergence within a specific CC (CC-353 or CC-1034). Accordingly, this particular heterogeneity resulted in less host-specific signatures. The 5 712 chicken-associated k-mers identified by our GWAS analysis cover 17 accessory genes and 25 core gene variants (Table S4). A gene for a TraG-like protein of the type IV secretion system26 was detected among the accessory genomes in 59/90 chicken-associated genomes (Table 3). TraG-like proteins are known to play a crucial role in the conjugative transfer of plasmids27. Additionally, two genes for putative proteins of unknown function are carried by 66 and 68 of the chicken associated strains, respectively (Table 3).
Table 3.
Selected accessory genes and allelic variants of the core genome content associated with the host chicken.
| Locus taga | Gene | Predicted function | BfR-CA-14430b | COGc | COGc description | Lifestyle preferenced | Accessory/variante | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pig | Cattle | Chicken | Host generalists | Other | |||||||
| n | n | n | n | n | |||||||
| Cj0933c | pycB | Putative pyruvate carboxylase B subunit | 882.094 | C | Energy production and conversion | 26 | 56 | 90 | 255 | 63 | V |
| Cj0478 | rpoB | DNA-directed RNA polymerase beta chain | 444.215 | K | Transcription | 26 | 56 | 90 | 255 | 63 | V |
| Cj0528c | flgB | Flagellar basal-body rod protein | 495.238 | N | Cell motility | 26 | 56 | 90 | 255 | 63 | V |
| _01618 | TraG | Conjugal transfer protein TraG | – | U | Intracellular trafficking, secretion, and vesicular transport | 1 | 1 | 59 | 1 | 13 | A |
| _01627 | – | Putative protein | – | – | – | 3 | 0 | 66 | 1 | 7 | A |
| _01633 | – | Putative protein | – | – | – | 3 | 0 | 68 | 0 | 0 | A |
aLocus tags for accessory genes based on C. jejuni reference strain NCTC13265 genome LR134498.1 (NCBI accession), while locus tags for allelic variants of the core genome refer to C. jejuni strain NCTC11168 (NCBI accession: AL111168.1).
bPosition of core genes in the reference strain BfR-CA-14430.
cClusters of orthologous groups (http://clovr.org/docs/clusters-of-orthologous-groups-cogs/).
dNumber of genomes assigned to a particular lifestyle carrying the gene or allelic variant (pig, cattle, chicken, host generalists, others).
eA indicates that a gene belongs to the accessory genome content of C. jejuni and V indicates a specific allelic variant of the core genome content.
Like the cattle-associated lineages, chicken-associated genomes carry host-adapted allelic variants (Table 3). The allele encoding a specific aa variant of rpoB was identified in most of the genomes in all three chicken-associated BAPS clusters (Table 3, Fig. 4c). The gene variant encoding FlgB (Fig. 4c) is identical in BAPS clusters 1 and 5 (chicken) and the host-generalist BAPS cluster 2 (CC-21). Furthermore, a very closely related aa variant was identified in BAPS cluster 9 (chicken) as well. Additionally, the same allelic variant of the pycB gene is carried by most genomes of BAPS clusters 1 and 9 (Table 3).
Independent adaptation of host-generalist lineages
Considering the core genome phylogeny of the C. jejuni strains presented here, the host-generalist lineages of BAPS cluster 8 appear to have evolved from independent genomic backgrounds, while other host-generalist lineages, for instance those of BAPS clusters 2, 3 and 6, appeared to be linked to each other (Fig. 1). In total, we have identified 37 339 k-mers which were mapped to 33 accessory genes and 87 core gene variants (Table S6). Accessory gene content exclusively associated with all host-generalist lineages was not identified by use of GWAS. A multitude of different allelic variants assigned to the core genome were identified for BAPS cluster 8 when compared with the genomes of the more closely related lineages of clusters 2, 3 and 6 (Table 4). Notably we also identified closely related variants for different core genes shared by all host-generalist lineages. These included ftsX, a gene involved in cell division, arsC, an arsenate reductase, further ribosomal genes (rplS and rpsP) and Cj0459c, known as a nicking endonuclease and purine-specific ribonuclease28 (Table 4). While the amino acid sequence encoded by ftsX shows a particular host-generalist-associated variant (Fig. 4d), the amino acid sequence determined by arsC, rplS, rpsP and Cj0459c are conserved in the C. jejuni population. Hence, k-mers identified for these CDS were associated with synonymous changes only. BAPS clusters 2, 3 and 6 harbour identical allelic variants for dnaE and ffh (Fig. 4b). The same is true for several other genes such as dxs, cysM and pckA (Table 4) that are broadly distributed across the C. jejuni genome and are involved in multiple metabolic pathways. Additionally, genes involved in transcriptional pathways such as rpoD and substrate transport functions like ybiT (Fig. 4d) were identified.
Table 4.
Selected allelic variants of the core genome content associated with host-generalists.
| Locus taga | Gene | Predicted function | BfR-CA-14430b | COGc | COGc description | Lifestyle preferenced | Accessory/variante | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pig | Cattle | Chicken | Host generalists | Other | |||||||
| n | n | n | n | n | |||||||
| Cj1276c | ftsX | Cell division protein FtsX | 1.223.530 | D | Cell cycle control, cell division, chromosome partitioning | 26 | 56 | 90 | 255 | 63 | V |
| Cj0459c | - | Conserved hypothetical protein (32.5% identical to HP0268) | 428.984 | – | – | 26 | 56 | 90 | 255 | 63 | V |
| Cj0321 | dxs | 1-deoxy-d-xylulose-5-phosphate synthase | 296.904 | H | Coenzyme transport and metabolism | 26 | 56 | 90 | 255 | 63 | V |
| Cj0912c | cysM | Belongs to the cysteine synthase cystathionine beta-synthase family | 862.739 | E | Amino acid transport and metabolism | 26 | 56 | 90 | 255 | 63 | V |
| Cj1001 | rpoD | RNA polymerase sigma factor RpoD | 945.528 | K | Transcription | 26 | 56 | 90 | 255 | 63 | V |
| Cj0426 | ybiT | ABC transporter ATP-binding protein | 393.511 | S | Function unknown | 26 | 56 | 90 | 255 | 63 | V |
| Cj0932c | pckA | Phosphoenolpyruvate carboxykinase (ATP) | 880.507 | H | Coenzyme transport and metabolism | 26 | 56 | 90 | 255 | 63 | V |
aLocus tags for allelic variants of the core genome refer to C. jejuni strain NCTC11168 (NCBI accession: AL111168.1).
bPosition of core genes in the reference strain BfR-CA-14430.
cClusters of orthologous groups (http://clovr.org/docs/clusters-of-orthologous-groups-cogs/).
dNumber of genomes assigned to a particular lifestyle carrying the gene or allelic variant (pig, cattle, chicken, host generalists, others).
eA indicates that a gene belongs to the accessory genome content of C. jejuni. Variant (V) indicates a specific allelic variant of the core genome content.
Discussion
We show how the recently emerging research field of bacterial GWAS was able to identify genetic signatures that possibly play important roles for the host-specificity of Campylobacter. For each of the lifestyle preferences of C. jejuni investigated, we identified a broad set of allelic variants being associated with particular host-specific lineages from distantly related BAPS clusters, providing evidence for host-adaptive genetic signatures29.
We also extended the scheme of lifestyle preferences based on MLST to a whole genome level by applying BAPS and identified 15 distinct phylogenetic clusters. The efficiency of the proposed approach to identify lifestyle preferences by assigning host-specific or host-generalist C. jejuni lineages was verified by performing a comparison of the predicted lifestyles. For instance, CC-42 or CC-61 (cattle), CC-354 or CC-692 (chicken) and CC-403 (mammalian/pig) lifestyle assignments were verified with previously published reports on these C. jejuni lineages8,30,31. Additionally, putative novel lifestyle preferences of distinct lineages, i.e. CC-22 (cattle-specific) and ST-2274 (chicken-specific), were identified using the definition described above.
C. jejuni isolates assigned to either chicken or host-generalist lineages showed a diverse population structure, as reported before32. Contrarily, we found C. jejuni genomes identified as cattle-specific (CC-42 and CC-61) or pig-specific (CC-403) were less diverse and more clonal. Previous studies assumed that the tight clonal structure of the cattle-associated lineages CC-42 and CC-61 resulted from a more recent onset of the colonization of cattle by C. jejuni and therefore may reflect a bottleneck event in its evolution24,29. A similar host-adaptation process is possibly indicated by the limited diversity of CC-403 (pig-specific) assigned to BAPS cluster 11 in our study.
Genetic variation is known to be a pre-requisite to evolutionary change33. Since 2016, bacterial GWAS has advanced as a suitable method to identify genetic alterations associated with a phenotypical traits in large WGS datasets34,35, including studies on C. jejuni13–16. Acting like a “sieve”, genetic selection allows only a subset of mutations to persist and become an observable difference between genomes33. Allelic variants of C. jejuni core genes, independently acquired by different phylogenetic lineages leading to changes of known or predicted amino acid sequences, likely reflect adaptation to a particular ecological niche and/or host36,37. We have identified allelic variants of core genes which were clearly associated with the host species pig, cattle and chicken, even among distantly related BAPS clusters [BAPS 4 and 10 (cattle); BAPS 1, 5 and 9 (chicken)]. Further allelic variants (e.g. ftsX in CC-45 and CC-21) were identified as putative markers for host-generalist lineages. This observation is supported by the lack of notable recombination between CC-45 and CC-2130, indicating that these variants occurred independently of phylogenetic background and geographic origin. Therefore, mutant selection leading to homoplasy would be the most reasonable assumption. More research on the subject, including isolates covering a broader time span is needed to gain further insight into the bacterial evolution of C. jejuni.
For each of the CC-42, CC-22 and CC-61 cattle-associated lineages in BAPS cluster 4 and 10, a different set of specific accessory genes was identified. This may reflect independent colonisation events of that particular host in the evolutionary history of Campylobacter38. In BAPS cluster 10 we have identified genes associated with a HicA-HicB toxin/antitoxin system, which is suspected to inhibit the bacterial mRNA transfer in case of limited nutrient availability39–41.
Sharing the same host does not necessarily mean ample opportunities for DNA transfer with the host, since the preferred (sub-)niche of these CCs within the gut of cattle may differ, as it has been assumed for host-generalist lineages previously30. Furthermore, structure and composition of the gut microbiome may play a role, however little is known about the microbiome ecology and the putative lineage-specific differences among C. jejuni with respect to virulence-associated strategies such as attachment to host cell tissue42,43.
We identified a putative cattle-specific allelic variant of DNA polymerase III subunit alpha encoded by dnaE, in which mutations have been shown to increase the overall mutation rate of E. coli44,45. Since an increased mutation rate is well known as a factor influencing niche adaptation29, the dnaE variant may promote the host-specialization processes. In addition, we found cattle-specific changes of the gene encoding Ffh, a signal recognition particle protein (SRP). Ffh initiates the co-translational targeting of membrane and secretory proteins to the cytoplasmic bacterial membrane46, indicating adaptation of transport processes. In E. coli, the SRP system plays an important role in membrane protein biosynthesis, and previous research also indicated that Ffh is involved in the regulation of membrane protein translation47. Notably, a GTPase (FlhF) possessing an active domain most similar to Ffh, was found to be involved in flagellar gene regulation and biosynthesis in C. jejuni48. Again, the lack of corresponding recombination patterns indicated that niche-specific environmental pressure induced the predicted amino acid change of Ffh independently in distantly related lineages as we demonstrated in Fig. 3. Indeed, ffh has already been described as a homoplasic gene on a nucleotide level in cattle-associated C. jejuni genomes by a recent study24.
Most of the CC-403 and ST-1942 (pig-associated) C. jejuni in BAPS cluster 11 carry a unique set of genes encoding restriction modification (RM) systems (RM I and RM II) that may contribute to lineage-specific barriers shielding the bacteria from intrusion of foreign DNA, a phenomenon reported before49–51. As well, the frequency and pattern of intra-lineage recombination events was unique to CC-403 and its related STs, as noted before52. However, due to the limited number of pig-associated clades, particular differentiation between a lineage or host specific association is challenging.
While amino acid variants encoded by the tenl gene is thought to affect the thiamine metabolism and may serve as markers for cattle-specific niche adaption24, in this study we identified pig-specific variations as well. The amino acid changes associated with the allelic variant encoding final aromatase (TenI) needed in thiamine biosynthesis were extensive and may indicate functional alterations or even loss-of-function. Further research to characterise this gene would be useful for potential agrifood intervention strategies. Since industrial diets for pigs are generally supplemented with thiamine53, reduction or even shutting-off the metabolic pathway might conserve energy and seems therefore beneficial for pig-specialized C. jejuni lineages. In addition, we identified a pig-specific variant of the putative thiamine-dependent synthase encoded by dxs, again underlining the general importance of specific alterations of the thiamine pathway for host adaptation of C. jejuni lineages. The majority of the accessory genome assigned in this study as chicken-specific included, among others, genes for a putative conjugative transfer protein (TraG-like), which is commonly linked to a type IV secretion system essential for DNA transfer in bacterial conjugation54,55. These findings are in concordance with the recombination analysis for the chicken-specific lineages (e.g. CC-257 or CC-354), which indicated multiple horizontal gene transfer events. With respect to k-mers that indicate sequence alterations of the core genomes and lead to aa variants of the respective proteins, we noted significant k-mers mapping to the gene encoding PycB, the second subunit of the anaplerotic and glucogenic pyruvate carboxylase in C. jejuni56. This finding indicates a specific adaptation of a basal metabolic pathway in C. jejuni. In addition, we detected significant k-mers associated with a rpoB variant, a housekeeping gene used for investigating genetic relatedness within the Campylobacter genus57. Interestingly, several different mutations of rpoB enhance growth at 42.2 °C compared to the wildtype in E. coli58. Since the body temperature of poultry is commonly between 39 and 43 °C59, the rpoB variant might contribute to temperature–induced adaptive changes in C. jejuni.
The large host-generalist lineages belonging to either BAPS clusters 2, 3, 6 (CC-21/CC-48/CC-206) or BAPS cluster 8 (CC-45) showed clear differences concerning their accessory gene content, an observation confirmed by earlier results from Yahara et al., who tracked these lineages from the chicken flock through the meat production chain as well as in clinical samples of human origin14. Here, we have provided evidence that accessory gene patterns were mostly BAPS clusters-specific, irrespective of the sample origin (e.g. animal, human clinical or environment). Host-generalist BAPS clusters appear to possess a larger pool of accessory genes, possibly indicating a repertoire of genes promoting survival in different hosts and environments60,61. This idea is supported by our recombination analysis, showing that host-generalist lineages are prone to DNA exchange, thus, natural transformation and recombination between host-generalist lineages enhances adaptive possibilities needed to survive in different hosts.
Variation of predicted aa sequences possibly associated with a host–generalist lifestyle of specific C. jejuni lineages were, for instance, identified for the cell division protein encoded by ftsX. Recent work by Riedel et al. showed that ftsX transcription is downregulated in Campylobacter lari after exposure to heat stress62, possibly indicating certain allelic variants may differ with respect to their stress response. As mentioned earlier, allelic variants may have evolved individually in both lineages (CC-45 and CC-21/CC-48), since the recombination analysis suggests a limited number of recombination events between BAPS clusters 8 and 2, 3 and 6.
Distinct host-specific factors, such as body temperature, the structure and composition of the gut microbiota, mucosal structures and immune system shape the adaptation strategies of C. jejuni lineages. Focusing fundamental science research in these areas will enhance the opportunity to exploit this foodborne pathogen’s ability to thrive in niche environments with targeted intervention strategies in the future.
Material and methods
Strain selection and genome sequencing
A uniform stratified random collection comprising 324 C. jejuni isolates obtained from samples of four different species, including human (n = 96), chicken (n = 102), cattle (n = 98) and pig (n = 28). The original samples were collected in 16 different federal states in Germany, between 2010 and 2019. Isolates from healthy and diseased animals as well as human clinical isolates were included (Table S1). The animal-derived isolates were provided by the National Reference Laboratory for Campylobacter at the German Federal Institute for Risk Assessment (BFR) and the Institute of Microbiology and Epizootics (IMT) at Freie Universität Berlin, while the human-derived isolates were provided by the National Reference Centre for Salmonella and other Bacterial Enterics at the Robert Koch Institute (RKI). C. jejuni is rarely isolated from porcine, therefore porcine-derived isolates were limited. In order to limit spatial and temporal effects, the set of genomes investigated here was complemented by whole genome data of further 166 isolates from a Canadian study which included C. jejuni from cattle (n = 39), chicken (n = 12), human clinical cases (n = 40), environmental (n = 54) and other animal (n = 21) origins16. The original purpose of the Canadian study was to identify diagnostic markers which can be used for rapid screening approaches to detect C. jejuni subtypes16. The complete list of all 490 genomes, including available metadata such as sample origin/source and baseline typing data such as ST is provided in Table S1. Detailed protocols used for whole genome sequencing (WGS) are provided as supplementary material. Illumina raw read data sequenced for this study is available at the National Center for Biotechnology Information (NCBI) under Bioproject ID PRJNA648048. Furthermore we included the strain BfR-CA-14430, available at NCBI under the accessory numbers CP043763.1 and CP043764.1, already published as a representative C. jejuni genome by the zoonosis monitoring program of Germany63.
Assembly and annotation
The Illumina paired-end reads were adapter-trimmed by Flexbar v.3.0.364 and corrected using BayesHammer65. The de novo assembly was performed using SPAdes v3.11.166 with default settings. All genomes were annotated by Prokka v1.1367 employing a customized database which consist of 137 complete annotated reference genomes provided by NCBI as described before63.
Multilocus sequence type (MLST) analysis
In silico MLST was carried out on seven housekeeping genes (aspA, glnA, gltA, glyA, pgm, tkt, uncA) as described by Dingle et al.32. This was done with the BLAST-based tool “mlst” (https://github.com/tseemann/mlst) based on the Campylobacter jejuni/coli database of pubmlst68. Obtained MLST profiles were then used to calculate a minimum spanning tree by MSTree V2 that was visualized with GrapeTree69.
Pan-genome and phylogenetic analyses
Open reading frames (ORFs) predicted by Prokka were subsequently used as input for Roary v3.12.070 to calculate the pan-genome size and core genome alignment using default settings. The resulting alignment was used to calculate a maximum likelihood-based phylogeny with RAxML v.8.2.1071 with 100 bootstraps under the assumption of the gtr-gamma DNA substitution model72. ClonalFrameML v1.1173 was used to correct for recombination events and phylogenetic groups were identified with Bayesian Analysis of Population Structure (BAPS). Here, we used BAPS with hierarchical clustering that was implemented in the R packages RhierBAPS v1.0.174. Grouping of the accessory genome was further analysed by t-distributed stochastic neighbour embedding (t-SNE)75.
Recombination analysis
BratNextGen76 was used to reconstruct putative recombination events based on the analysis of the core genome alignment of our selection comprising 490 C. jejuni genomes. Parameter estimation was performed based on 20 iterations and significant recombinations (p-value 0.05) were obtained using permutation testing with 100 permutations executed in parallel.
Genome-wide association study (GWAS)
In order to perform an in-depth analysis of genomic alterations possibly associated with host specificity, pyseer v.1.1.225 was used for GWAS based on variable-length k-mer composition (9 to 100 base pairs) for all 490 genomes. To control the lineage-level associations reported for bacterial GWAS (Earle et al., 2016; PMID: 27572646) a linear mixed model (LMM) has been integrated (details are provided by the supplementary section on GWAS ). K-mers significantly representing distinct isolate origins (human, cattle, chicken or pig) were further mapped by bwa v0.7.1777 against selected reference genomes from this study set in order to identify putative origin-specific factors, genes and consecutive gene loci.
In order to reduce the false positive rate of the GWAS and account for highly unbalanced groups, we employed a bootstrapping approach. Further details can be found in the supplementary material.
The consequential set of genes was further analysed considering functional annotations and metabolic pathways using EggNog v.4.5.178,79.
C. jejuni lifestyle classification
In order to facilitate statistical comparison, we adapted a definition from Shepard et al.30 and defined a set of closely-related C. jejuni lineages as host-specific if ≥ 50% genomes building the respective BAPS cluster were associated with isolates from a specific animal origin (e.g. cattle, chicken) while each of the other isolate origins contributed less than 10% in the BAPS cluster. Potential host-generalist lineages were assumed when more than 25% of the clustering genomes represented in the corresponding BAPS cluster were from C. jejuni of human clinical cases while at least two further animal origins account for more than 10% of the remaining genomes, respectively.
Supplementary Information
Acknowledgements
We thank Petra Hahs and Corinna Fruth for their excellent assistance in the laboratory of the National Reference Centre for Salmonella and other Bacterial Enterics at the RKI.We also thank the Federal State Laboratories for isolating Campylobacter from food matrices and all members of the NRL for Campylobacter for technical support. This research was accomplished within the PAC-CAMPY research network, a part of the national Zoonotic Infectious Diseases Research Network which is funded by the Federal Ministry of Education and Research (BMBF) with grant 01KI1725F, 01KI2007F, and 01KI1725B. Additional funding was received from the BMBF-funded research network #1HealthPREVENT (grant 01KI1727F) and SFB project of BfR 1322-646.
Author contributions
T.S. designed and conceived the study; L.E. performed processing and downstream analyses of all sequencing data; B.W. assisted with biological interpretations; L.E., B.W., R.P. and N.J. wrote the original draft; M.K., A.F., A.F. and K.S. provided characterized isolates; M.K., A.T. and K.S. performed WGS; T.S., R.P. and L.W. supervised the work. All authors have approved the final manuscript draft.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-89683-6.
References
- 1.Burnham PM, Hendrixson DR. Campylobacter jejuni: Collective components promoting a successful enteric lifestyle. Nat. Rev. Microbiol. 2018;16:551–565. doi: 10.1038/s41579-018-0037-9. [DOI] [PubMed] [Google Scholar]
- 2.Humphrey T, O’Brien S, Madsen M. Campylobacters as zoonotic pathogens: A food production perspective. Int. J. Food Microbiol. 2007;117:237–257. doi: 10.1016/j.ijfoodmicro.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Hale CR, et al. Estimates of enteric illness attributable to contact with animals and their environments in the United States. Clin. Infect. Dis. 2012;54:S472–S479. doi: 10.1093/cid/cis051. [DOI] [PubMed] [Google Scholar]
- 4.Friedman CR, et al. Risk factors for sporadic Campylobacter infection in the United States: A case-control study in FoodNet sites. Clin. Infect. Dis. 2004;38(3):S285–S296. doi: 10.1086/381598. [DOI] [PubMed] [Google Scholar]
- 5.Marder EP, et al. Incidence and trends of infections with pathogens transmitted commonly through food and the effect of increasing use of culture-independent diagnostic tests on surveillance—Foodborne diseases active surveillance network, 10 U.S. Sites, 2013–2016. MMWR. Morb. Mortal. Wkly. Rep. 2017;66:397–403. doi: 10.15585/mmwr.mm6615a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 2015;28:687–720. doi: 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Didelot X, Falush D. Inference of bacterial microevolution using multilocus sequence data. Genetics. 2007;175:1251–1266. doi: 10.1534/genetics.106.063305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheppard SK, et al. Niche segregation and genetic structure of Campylobacter jejuni populations from wild and agricultural host species. Mol. Ecol. 2011;20:3484–3490. doi: 10.1111/j.1365-294X.2011.05179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griekspoor P, et al. Marked host specificity and lack of phylogeographic population structure of Campylobacter jejuni in wild birds. Mol. Ecol. 2013;22:1463–1472. doi: 10.1111/mec.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogden ID, et al. Campylobacter excreted into the environment by animal sources: Prevalence, concentration shed, and host association. Foodborne Pathog. Dis. 2009;6:1161–1170. doi: 10.1089/fpd.2009.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dearlove BL, et al. Rapid host switching in generalist Campylobacter strains erodes the signal for tracing human infections. ISME J. 2016;10:721–729. doi: 10.1038/ismej.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermans D, et al. Colonization factors of Campylobacter jejuni in the chicken gut. Vet. Res. 2011;42:82. doi: 10.1186/1297-9716-42-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheppard SK, et al. Genome-wide association study identifies vitamin B5 biosynthesis as a host specificity factor in Campylobacter. Proc. Natl. Acad. Sci. U. S. A. 2013;110:11923–11927. doi: 10.1073/pnas.1305559110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yahara K, et al. Genome-wide association of functional traits linked with Campylobacter jejuni survival from farm to fork. Environ. Microbiol. 2017;19:361–380. doi: 10.1111/1462-2920.13628. [DOI] [PubMed] [Google Scholar]
- 15.Thépault A, et al. Genome-wide identification of host-segregating epidemiological markers for source attribution in Campylobacter jejuni. Appl. Environ. Microbiol. 2017;83:e03085–e3116. doi: 10.1128/AEM.03085-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchanan CJ, et al. A genome-wide association study to identify diagnostic markers for human pathogenic Campylobacter jejuni strains. Front. Microbiol. 2017;8:1224. doi: 10.3389/fmicb.2017.01224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Vries SPW, et al. Genome-wide fitness analyses of the foodborne pathogen Campylobacter jejuni in in vitro and in vivo models. Sci. Rep. 2017;7:1251. doi: 10.1038/s41598-017-01133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gormley FJ, et al. Has retail chicken played a role in the decline of human Campylobacteriosis? Appl. Environ. Microbiol. 2008 doi: 10.1128/AEM.01455-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korczak BM, Zurfluh M, Emler S, Kuhn-Oertli J, Kuhnert P. Multiplex strategy for multilocus sequence typing, fla typing, and genetic determination of antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolates collected in Switzerland. J. Clin. Microbiol. 2009 doi: 10.1128/JCM.00237-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lévesque S, Frost E, Arbeit RD, Michaud S. Multilocus sequence typing of Campylobacter jejuni isolates from humans, chickens, raw milk, and environmental water in Quebec, Canada. J. Clin. Microbiol. 2008 doi: 10.1128/JCM.00042-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Habib I, Uyttendaele M, De Zutter L. Survival of poultry-derived Campylobacter jejuni of multilocus sequence type clonal complexes 21 and 45 under freeze, chill, oxidative, acid and heat stresses. Food Microbiol. 2010;27:829–834. doi: 10.1016/j.fm.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Alter T, Scherer K. Stress response of Campylobacter spp. and its role in food processing. J. Vet. Med. Ser. B. 2010;53:351–357. doi: 10.1111/j.1439-0450.2006.00983.x. [DOI] [PubMed] [Google Scholar]
- 23.Murphy C, Carroll C, Jordan KN. Environmental survival mechanisms of the foodborne pathogen Campylobacter jejuni. J. Appl. Microbiol. 2006;100:623–632. doi: 10.1111/j.1365-2672.2006.02903.x. [DOI] [PubMed] [Google Scholar]
- 24.Mourkas E, et al. Agricultural intensification and the evolution of host specialism in the enteric pathogen Campylobacter jejuni. Proc. Natl. Acad. Sci. 2020;117:11018–11028. doi: 10.1073/pnas.1917168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lees JA, Galardini M, Bentley SD, Weiser JN, Corander J. pyseer: A comprehensive tool for microbial pangenome-wide association studies. Bioinformatics. 2018;34:4310–4312. doi: 10.1093/bioinformatics/bty539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schröder G, Lanka E. TraG-like proteins of type IV secretion systems: Functional dissection of the multiple activities of TraG (RP4) and TrwB (R388) J. Bacteriol. 2003;185:4371–4381. doi: 10.1128/JB.185.15.4371-4381.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poly F, Threadgill D, Stintzi A. Genomic diversity in Campylobacter jejuni: Identification of C. jejuni 81–176-specific genes. J. Clin. Microbiol. 2005;43:2330–2338. doi: 10.1128/JCM.43.5.2330-2338.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee K-Y, et al. Structure-based functional identification of Helicobacter pylori HP0268 as a nuclease with both DNA nicking and RNase activities. Nucleic Acids Res. 2015;43:5194–5207. doi: 10.1093/nar/gkv348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheppard SK, Guttman DS, Fitzgerald JR. Population genomics of bacterial host adaptation. Nat. Rev. Genet. 2018;19:549–565. doi: 10.1038/s41576-018-0032-z. [DOI] [PubMed] [Google Scholar]
- 30.Sheppard SK, et al. Cryptic ecology among host generalist Campylobacter jejuni in domestic animals. Mol. Ecol. 2014;23:2442–2451. doi: 10.1111/mec.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohan V, et al. Campylobacter jejuni colonization and population structure in urban populations of ducks and starlings in New Zealand. Microbiologyopen. 2013;2:659–673. doi: 10.1002/mbo3.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dingle KE, et al. Multilocus sequence typing system for Campylobacter jejuni. J. Clin. Microbiol. 2001;39:14–23. doi: 10.1128/JCM.39.1.14-23.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hershberg R. Mutation—The engine of evolution: Studying mutation and its role in the evolution of bacteria: Figure 1. Cold Spring Harb. Perspect. Biol. 2015;7:a018077. doi: 10.1101/cshperspect.a018077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falush D. Bacterial genomics: Microbial GWAS coming of age. Nat. Microbiol. 2016;1:16059. doi: 10.1038/nmicrobiol.2016.59. [DOI] [PubMed] [Google Scholar]
- 35.Power RA, Parkhill J, de Oliveira T. Microbial genome-wide association studies: Lessons from human GWAS. Nat. Rev. Genet. 2017;18:41–50. doi: 10.1038/nrg.2016.132. [DOI] [PubMed] [Google Scholar]
- 36.Brandley MC, Warren DL, Leaché AD, McGuire JA. Homoplasy and clade support. Syst. Biol. 2009;58:184–198. doi: 10.1093/sysbio/syp019. [DOI] [PubMed] [Google Scholar]
- 37.Hassanin A, Lecointre G, Tillier S. The ‘evolutionary signal’ of homoplasy in proteincoding gene sequences and its consequences for a priori weighting in phylogeny. C. R. l’Acad. Sci. Ser. III Sci. Vie. 1998;321:611–620. doi: 10.1016/S0764-4469(98)80464-2. [DOI] [PubMed] [Google Scholar]
- 38.Sheppard SK, Maiden MCJ. The evolution of Campylobacter jejuni and Campylobacter coli. Cold Spring Harb. Perspect. Biol. 2015;7:a018119. doi: 10.1101/cshperspect.a018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Motiejūnaitė R, Armalytė J, Markuckas A, Sužiedėlienė E. Escherichia coli dinJ-yafQ genes act as a toxin-antitoxin module. FEMS Microbiol. Lett. 2007;268:112–119. doi: 10.1111/j.1574-6968.2006.00563.x. [DOI] [PubMed] [Google Scholar]
- 40.Buts L, Lah J, Dao-Thi M-H, Wyns L, Loris R. Toxin–antitoxin modules as bacterial metabolic stress managers. Trends Biochem. Sci. 2005;30:672–679. doi: 10.1016/j.tibs.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Gerdes K, Christensen SK, Løbner-Olesen A. Prokaryotic toxin–antitoxin stress response loci. Nat. Rev. Microbiol. 2005;3:371–382. doi: 10.1038/nrmicro1147. [DOI] [PubMed] [Google Scholar]
- 42.Han Z, et al. Influence of the gut microbiota composition on Campylobacter jejuni colonization in chickens. Infect. Immun. 2005 doi: 10.1128/IAI.00380-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Indikova I, Humphrey TJ, Hilbert F. Survival with a helping hand: Campylobacter and Microbiota. Front. Microbiol. 2015 doi: 10.3389/fmicb.2015.01266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fijalkowska IJ, Schaaper RM, Jonczyk P. DNA replication fidelity in Escherichia coli : A multi-DNA polymerase affair. FEMS Microbiol. Rev. 2012;36:1105–1121. doi: 10.1111/j.1574-6976.2012.00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vandewiele D, Fernández de Henestrosa AR, Timms AR, Bridges BA, Woodgate R. Sequence analysis and phenotypes of five temperature sensitive mutator alleles of dnaE, encoding modified α-catalytic subunits of Escherichia coli DNA polymerase III holoenzyme. Mutat. Res. Mol. Mech. Mutagen. 2002;499:85–95. doi: 10.1016/S0027-5107(01)00268-8. [DOI] [PubMed] [Google Scholar]
- 46.Shan S, Stroud RM, Walter P. Mechanism of association and reciprocal activation of two GTPases. PLoS Biol. 2004;2:e320. doi: 10.1371/journal.pbio.0020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yosef I, Bochkareva ES, Bibi E. Escherichia coli SRP, its protein subunit Ffh, and the Ffh M domain are able to selectively limit membrane protein expression when overexpressed. MBio. 2010 doi: 10.1128/mBio.00020-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balaban M, Joslin SN, Hendrixson DR. FlhF and its GTPase activity are required for distinct processes in flagellar gene regulation and biosynthesis in Campylobacter jejuni. J. Bacteriol. 2009;191:6602–6611. doi: 10.1128/JB.00884-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Budroni S, et al. Neisseria meningitidis is structured in clades associated with restriction modification systems that modulate homologous recombination. Proc. Natl. Acad. Sci. 2011;108:4494–4499. doi: 10.1073/pnas.1019751108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCarthy ND, et al. Host-associated genetic import in Campylobacter jejuni. Emerg. Infect. Dis. 2007;13:267–272. doi: 10.3201/eid1302.060620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asakura H, et al. Molecular evidence for the thriving of Campylobacter jejuni ST-4526 in Japan. PLoS ONE. 2012;7:e48394. doi: 10.1371/journal.pone.0048394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morley L, et al. Gene loss and lineage-specific restriction-modification systems associated with niche differentiation in the Campylobacter jejuni sequence type 403 clonal complex. Appl. Environ. Microbiol. 2015;81:3641–3647. doi: 10.1128/AEM.00546-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.National Research Council . Nutrient Requirements of Swine. National Academies Press; 2012. Nutrient Requirements of Swine. [Google Scholar]
- 54.Schröder G, et al. TraG-like proteins of DNA transfer systems and of the Helicobacter pylori type IV secretion system: Inner membrane gate for exported substrates? J. Bacteriol. 2002;184:2767–2779. doi: 10.1128/JB.184.10.2767-2779.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kienesberger S, et al. Interbacterial macromolecular transfer by the Campylobacter fetus subsp venerealis type IV secretion system. J. Bacteriol. 2011;193:744–758. doi: 10.1128/JB.00798-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Velayudhan J, Kelly DJ. Analysis of gluconeogenic and anaplerotic enzymes in Campylobacter jejuni: An essential role for phosphoenolpyruvate carboxykinase. Microbiology. 2002;148:685–694. doi: 10.1099/00221287-148-3-685. [DOI] [PubMed] [Google Scholar]
- 57.Korczak BM, et al. Genetic relatedness within the genus Campylobacter inferred from rpoB sequences. Int. J. Syst. Evol. Microbiol. 2006;56:937–945. doi: 10.1099/ijs.0.64109-0. [DOI] [PubMed] [Google Scholar]
- 58.González-González A, Hug SM, Rodríguez-Verdugo A, Patel JS, Gaut BS. Adaptive mutations in RNA polymerase and the transcriptional terminator rho have similar effects on Escherichia coli gene expression. Mol. Biol. Evol. 2017;34:2839–2855. doi: 10.1093/molbev/msx216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richards SA. The significance of changes in the temperature of the skin and body core of the chicken in the regulation of heat loss. J. Physiol. 1971;216:1–10. doi: 10.1113/jphysiol.1971.sp009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hottes AK, et al. Bacterial adaptation through loss of function. PLoS Genet. 2013;9:e1003617. doi: 10.1371/journal.pgen.1003617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iranzo J, Wolf YI, Koonin EV, Sela I. Gene gain and loss push prokaryotes beyond the homologous recombination barrier and accelerate genome sequence divergence. Nat. Commun. 2019;10:5376. doi: 10.1038/s41467-019-13429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Riedel C, et al. Differences in the transcriptomic response of Campylobacter coli and Campylobacter lari to heat stress. Front. Microbiol. 2019 doi: 10.3389/fmicb.2020.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Epping L, et al. Comparison of different technologies for the decipherment of the whole genome sequence of Campylobacter jejuni BfR-CA-14430. Gut Pathog. 2019;11:59. doi: 10.1186/s13099-019-0340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roehr JT, Dieterich C, Reinert K. Flexbar 3.0—SIMD and multicore parallelization. Bioinformatics. 2017;33:2941–2942. doi: 10.1093/bioinformatics/btx330. [DOI] [PubMed] [Google Scholar]
- 65.Nikolenko SI, Korobeynikov AI, Alekseyev MA. BayesHammer: Bayesian clustering for error correction in single-cell sequencing. BMC Genomics. 2013;14(1):S7. doi: 10.1186/1471-2164-14-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bankevich A, et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seemann T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 68.Jolley KA, Maiden MCJ. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinform. 2010;11:595. doi: 10.1186/1471-2105-11-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou Z, et al. GrapeTree: Visualization of core genomic relationships among 100,000 bacterial pathogens. Genome Res. 2018;28:1395–1404. doi: 10.1101/gr.232397.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Page AJ, et al. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015;31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tavaré S. Some probabilistic and statistical problems in the analysis of DNA sequences. Am. Math. Soc. Lect. Math. Life Sci. 1986;17:57–86. [Google Scholar]
- 73.Didelot X, Wilson DJ. ClonalFrameML: Efficient inference of recombination in whole bacterial genomes. PLOS Comput. Biol. 2015;11:e1004041. doi: 10.1371/journal.pcbi.1004041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tonkin-Hill G, Lees JA, Bentley SD, Frost SDWW, Corander J. RhierBAPs: An R implementation of the population clustering algorithm hierbaps [version 1; referees: 2 approved] Wellcome Open Res. 2018;3:93. doi: 10.12688/wellcomeopenres.14694.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van der Maaten L, Hinton G. Visualizing data using t-SNE. J. Mach. Learn. Res. 2008;9:2579–2605. [Google Scholar]
- 76.Marttinen P, et al. Detection of recombination events in bacterial genomes from large population samples. Nucleic Acids Res. 2012;40:e6. doi: 10.1093/nar/gkr928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huerta-Cepas J, et al. eggNOG 4.5: a hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences. Bioinformatics. 2009;44:D286–D293. doi: 10.1093/nar/gkv1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huerta-Cepas J, et al. Fast genome-wide functional annotation through orthology assignment by eggNOG-mapper. Mol. Biol. Evol. 2017;34:2115–2122. doi: 10.1093/molbev/msx148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.