Highlights
-
•
Ultrasonic/microwave-extracted green coffee oil showed the highest cafestol, kahweol and α-tocopherol contents.
-
•
The oil extracted from green coffee beans with the microwave technique presented a higher content of polyunsaturated fatty acids.
-
•
The highest content of γ-tocopherol and β-sitosterol was reported for green coffee oil derived by pressurized liquid extraction.
-
•
Data fusion combined with chemometrics was effective in evaluating green coffee oil obtained by different extraction techniques.
Keywords: Green coffee oil, Ultrasound, Physicochemical composition, Fatty acids, Chemometrics
Abstract
In this study, ultrasonic/microwave-assisted extraction (UMAE), microwave-assisted extraction (UAE), ultrasound-assisted extraction (UAE), and pressurized liquid extraction (PLE) were applied to extract green coffee oil (GCO), and the physicochemical indexes, fatty acids, tocopherols, diterpenes, and total phenols as well as antioxidant activity of GCO were investigated and compared. The results indicated that the extraction yield of UMAE was the highest (10.58 ± 0.32%), while that of PLE was the lowest (6.34 ± 0.65%), and linoleic acid and palmitic acid were the major fatty acids in the GCO, ranging from 40.67% to 43.77% and 36.57% to 38.71%, respectively. A large proportion of fatty acids and phytosterols were not significantly influenced by the four extraction techniques. However, tocopherols, diterpenes, total phenols, and the free radical scavenging activity were significantly different among these four GCOs. Moreover, structural changes in the coffee residues were explored by scanning electron microscopy and Fourier transform infrared spectroscopy. Overall, the high antioxidant activity of GCO demonstrated that it can be used as a highly economical natural product in the food and agricultural industries.
1. Introduction
Coffee is one of the most popular beverages in the world; it has played an important role in consumer culture since the mid-16th century and has undergone a transformation from a pure commodity to a specialty product over the past few decades [30]. Approximately 151.3 million 60 kg bags of coffee were consumed worldwide in 2015–2016 [20]. The United States (25 million bags) and Brazil (20 million bags) were the largest and second largest consumers, respectively. China has become the 12th largest coffee-producing country in the world, with total coffee production reaching 118,000 tons in 2014 [29], and Yunnan and Hainan Provinces contribute the most of this production. As a consumer product, coffee and its consumption are oriented to increase in the future [10] (Table 1).
Table 1.
Extraction yield and major physicochemical properties of green coffee oils extracted by different methods.
| Parameters | UMAE | UAE | MAE | PLE |
|---|---|---|---|---|
| Extraction yield (%) | 10.58 ± 0.32a | 9.06 ± 0.63b | 9.34 ± 0.21b | 6.34 ± 0.65c |
| Refractive index (25 °C) | 1.4689a | 1.4680a | 1.4673a | 1.4713a |
| Iodine value (g I2/100 g) | 60.21 ± 0.56a | 51.89 ± 2.96b | 59.78 ± 0.34a | 59.85 ± 0.42a |
| Peroxide value (meq O2/kg) | 6.95 ± 0.16ab | 1.07 ± 0.03c | 1.76 ± 0.17c | 8.14 ± 0.25a |
| Acid value (mg KOH/g) | 6.41 ± 0.24c | 7.65 ± 0.54b | 9.20 ± 0.54a | 9.17 ± 0.45a |
| Saponification value (mg KOH/g) | 158.48 ± 1.02b | 230.04 ± 10.14a | 131.18 ± 0.13c | 88.49 ± 1.48d |
| Free fatty acid (mg KOH/g oil) | 10.48 ± 0.12a | 5.85 ± 0.17c | 6.99 ± 0.32b | 3.57 ± 0.11d |
Results are expressed as the mean ± standard error. Different capital letters in the same row indicate significant differences at p < 0.05. Extraction yield, EY; refractive index, RI; iodine value, IV; peroxide value, PV; acid value, AV; saponification value, SV; free fatty acid, FFA.
Many nutrients, such as carbohydrates, lipids, vitamins, minerals and nitrogen compounds, are found in coffee [7]. Lipids are one of the most important components in coffee beans, which consist of wax, triglycerides and unsaponifiable matter, with an approximately 16.0% lipid content in Arabica coffee beans (C. arabica) and a 10.1% lipid content in Robusta coffee beans (C. Robusta). Green coffee oil (GCO) has been widely used in the cosmetics industry since it has the ability to maintain natural skin humidity. In addition, there is some evidence indicating that GCO has the ability to absorb UV radiation in the UVB range, which causes the greatest damage to human skin. Linoleic acid is the main fatty acid of GCO, which provides relief from eczema and has therapeutic properties in dermatitis. Moreover, some other important bioactive compounds, such as polyphenols, tocopherols, and phytosterols, also exist in GCO. The conventional techniques for extracting vegetable oils require a long extraction period, have environmental and health risks, consume a large amount of solvent, and possibly change the characteristics of the extracted oil. Therefore, alternative oil extraction methods need to be developed to provide high-quality products but do not need to use toxic chemicals [37], [22].
Some advanced extraction technologies have emerged in processes for plant component extraction. Mwaurah et al. [27] reviewed novel oil extraction technologies including processing conditions, quality parameters, and optimization. Ultrasonic/microwave-assisted extraction (UMAE), combining the advantages of microwaves and ultrasonication, presents many advantages [42], [28] and has been widely used for oil extraction. The UMAE procedure for polysaccharides from the fruit of Camptotheca acuminata (CAFP) was investigated and optimized by Sun et al. [34], and satisfactory yields of CAFP were achieved. Ultrasound-assisted extraction (UAE) is a modern method of extracting compounds from plants while maintaining their structural and molecular properties [32]. Microwave-assisted extraction (MAE) can rapidly heat the extract, accelerate the extraction process, and adsorb and desorb the target compound in the substrate [22]; MAE has been widely used to extract various biologically active components, such as green and roasted coffee extracts [26], GCO [38], roasted coffee carbohydrates, caffeine, chlorogenic acid and coloured compounds [25]. Pressurized liquid extraction (PLE) is known as accelerated solvent extraction and was first described in 1995 [9]. PLE was originally developed for laboratory analysis but is considered an innovation that could be used on a much larger scale.
Concerning GCO extraction, Chen et al. [5] integrated the UMAE technique with ethanol to extract GCO from Arabica coffee beans, which demonstrated that UMAE with ethanol is a rapid and efficient green technique for the extraction of GCO. Supercritical CO2 extraction of oil from green coffee beans was investigated by Cornelino-Sntiago et al. [8], and the solubility, triacylglycerol composition, thermophysical properties and thermodynamic modelling were systemically analysed. Tsukui et al. [38] carried out GCO extraction using microwave-assisted extraction and quantification of diterpenes by HPLC, while the space–time yield calculated on the diterpene content for sample AT1 (Arabica green coffee beans) showed a six times higher value compared to the traditional Soxhlet method. De Oliveira et al. [9] extracted GCO by PLE using ethanol as the solvent, with the greatest green coffee yield of 9.78%. The UAE method combined with response surface methodology was performed to extract kahweol and cafestol from roasted coffee, and the use of sonication allowed the extractive process to be simplified, reducing the number of stages (two steps compared to the conventional method), the amount of solvent and the analysis time. In addition, GCO obtained by cold pressing and hydroalcoholic extracts were utilized to develop carboxymethyl cellulose-based films [39]. Generally, different extraction methods may result in differences in the chemical composition of the obtained oils, including fatty acid content, tocopherols, sterols, diterpenes, and antioxidant activity. However, to date, there has been no literature on the application of different extraction techniques for comparative analysis of GCO originating from Yunnan Province.
Therefore, the objective of this study was to extract GCO by the UMAE, UAE, MAE, and PLE methods, after which the physicochemical properties, fatty acid profiles, tocopherols, diterpenes, total phenols, and free radical scavenging activity of GCO obtained by the various methods were comparatively evaluated. Additionally, the microstructural changes that occurred in the coffee powders were explored by scanning electron microscopy and Fourier transform infrared spectroscopy to assess the extraction features of the different techniques, which could produce oils with different characteristics that could define their use. Moreover, the fused dataset was subjected to principal component analysis (PCA), Circos map analysis (CAA), and hierarchical cluster analysis (HCA) to identify different characteristics of GCOs. This work could provide useful insights and a theoretical basis for the further development of the GCO industry.
2. Materials and methods
2.1. Materials and reagents
Fresh coffee fruit (Coffea arabica, Catimor 7963) was harvested in February 2019 from the coffee research farm of the Dehong Tropical Agriculture Research Institute of Yunnan (Yunnan, China). The wet processing method was applied to process the fresh coffee fruits, being continuous peeled and degummed, then dried until the average initial moisture content was about 11.0 ± 1.0 g/100 g dry weight. The green coffee beans were dried and peeled before oil extraction, milled in a pulverizer (model BJ-150, Baijie, China), passed through a 40 mesh screen, and then preserved in hermetically sealed bags at 25 °C. A thirty-seven fatty acid methyl ester (FAME) mix standard, methyl undecanoate standard, α-tocopherol, β-tocopherol, γ-tocopherol, δ-tocopherol standard, campesterol, β-sitosterol, stigmasterol, 5α-cholestane standard, bis (trimethylsilyl) trifluoroacetamide with 1% trimethylchlorosilane (BSTFA + TMCS; 99:1; v/v), kahweol standard, gallic acid, 2–2-diphenyl-1-picryihydrazyl (DPPH), and (+)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) were purchased from the Aladdin Company (Shanghai, China). 2,2-Azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS) and ferric reducing/antioxidant power assay (FRAP) kits were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Cafestol and β-tocopherol were purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). Other reagents were of analytical and chromatographic grade and obtained from Xilong Science (Guangdong, China) and the Aladdin Company (Shanghai, China).
2.2. Extraction of GCO by different methods
2.2.1. Ultrasound-assisted extraction (UAE)
Ultrasound equipment with a working frequency of 40 kHz and an electric power output of 50 W (SB-5200DT, Scient, Ningbo, China) was used for oil extraction from green coffee beans. Ten grams of green coffee powder was mixed with ethanol in an Erlenmeyer flask and placed into a water bath. The ratio of sample to ethanol was 1:30 (g/mL), and the extraction was performed at 35 °C for 50 min. The extraction conditions were based on our preliminary single-factor experiments. After UAE, the suspension was separated by centrifugation at 4500 rpm for 10 min, and the solvent was removed by a rotary evaporator at 40 °C. The oil yield was calculated using Eq. (1):
| (1) |
2.2.2. Microwave-assisted extraction (MAE)
Microwave-assisted extraction was performed using an ultrasound-microwave cooperative extractor (CW-2000, Xintuo, Shanghai, China) in which the ultrasound was always off. A 10.0 g powdered sample was loaded into the extractor with 100 mL ethanol and then extracted at 60 °C for 30 min, and the microwave power was fixed at 200 W. The experimental conditions were chosen according to the data from the pre-experiments. After MAE, the solvent was removed by vacuum evaporation at 40 °C, and the oil fraction was weighed and stored at −80 °C for further investigation.
2.2.3. Ultrasound-microwave assisted extraction (UMAE)
Ultrasound-microwave assisted extraction experiments were conducted as described in previous research by the research group [5]. The ground green coffee was weighed accurately and then transferred into the flask. A predetermined volume of ethanol (solid:liquid ratio: 1:28 g/mL) was added and sonicated in an ultrasonic-microwave extractor (CW-2000, Xintuo, Shanghai, China) at 60 °C for 10 min, with a microwave power of 350 W.
2.2.4. Pressurized liquid extraction (PLE)
Extraction was carried out in an accelerated solvent extractor (ASE, 350 system, Dionex, Sunnyvale, CA, USA) equipped with a controlled unit. To avoid any possible oxidation during the extraction process, the solvent was degassed for 10 min to remove the dissolved oxygen. A total of 20.0 g of green coffee powder was accurately weighed and loaded onto 66 mL stainless-steel extraction cells, and 40.0 mL of ethanol was added. Meanwhile, the furnace was heated to the test temperature, and ethanol was added until the pressure reached 100 bar. After pressurizing the system, the extractor was placed in the furnace with a fixed pressure and temperature, static extraction was conducted at 100 °C for 30 min, and the experimental conditions were adopted based on preliminary tests. The extract was dried under vacuum in a rotary evaporator (Buchi R 210, Switzerland) at 40 °C, and then the obtained GEO was stored at −80 °C for further analysis.
2.3. Physicochemical properties
The acid value (AV) [12], peroxide value (PV) [13], iodine value (IV) [14], and saponification value (SV) [15] were determined according to the Chinese National Standard Method. The refractive index (RI) of GCO was measured at 25 °C using a refractive index detector (RM50, Mettler Toledo, Switzerland). Each sample was determined in triplicate.
2.4. Determination of the fatty acid and free fatty acid (FFA) compositions
The fatty acid composition was determined according to the Chinese National Standard Method (GB 5009.168-2016). In brief, 8.0 mL of 2% sodium hydroxide methanol solution was added to the extracts, and circulation reflux continued in an 80 °C water bath until the oil droplets disappeared. Then, 7.0 mL of 15% boron trifluoride methanol solution was added, and reflux continued in an 80 °C water bath for 2 min and was then quickly cooled to room temperature. Then, 10.0 mL of n-heptane was added, and the mixture was shaken for 2 min. Saturated sodium chloride solution was added, and the solution was allowed to stand for 5 min. A series of supernatants was placed into 5.0 mL to 25.0 mL of test tubes. Then, 5.0 g of anhydrous sodium sulfate was added, and the mixture was shaken for 1 min. Then, the supernatant was used for determination. The contents of FAMEs were analysed with an Agilent 7890A gas chromatography with 5975C mass spectrometry system (Agilent Technologies, USA) coupled with a capillary column (DB-WAX, 30 m × 0.25 mm × 0.25 µm). The injection volume was 1.0 µL, the column temperature was held at 130 °C for 1 min after injection, and the temperature was programmed at 5 °C/min to 180 °C and then increased to 220 °C at 1 °C/min and held for 10 min. Helium was used as the carrier gas at a flow rate of 1.0 mL/min. The injector temperature and the detector temperature were both 250 °C, and the relative percentage (g/100 g oil) of fatty acids was calculated by comparing the retention time and peak area with the FAME standard. Free fatty acids were measured by a free fatty acids kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.5. Determination of the phytosterol contents
GCO (0.1 g) was saponified with 2.0 mL of 1.0 M methanolic potassium hydroxide at 80 °C for 1 h in a water bath. After cooling, 2.0 mL of distilled water and 5.0 mL of n-hexane were added, the mixture was shaken for 2 min, and the procedure was repeated three times. The combined n-hexane fractions were washed 3–5 times with distilled water (30.0 mL). Anhydrous sodium sulfate was added to the n-hexane layer to eliminate aqueous residues, the organic layer was then evaporated at 40 °C with a rotary evaporator, and the residue was redissolved in 5.0 mL of n-hexane. Finally, the extract was filtered through a 0.22 μm syringe filter and stored at −20 °C until analysis.
Phytosterol was simultaneously evaluated in a gas chromatograph equipped with a flame ionization detector using a capillary column (DB-5HT, 30 m × 0.25 mm × 0.1 μm). The extracted oils were derivatized using N,O-bis (trimethylsilyl) trifluoracetamide (BSTFA) for 30 min at 60 °C. The experimental conditions are based on the method described by Santos et al. [31] with minor modifications, the identification of phytosterol was achieved by comparing the peak time of the standard compounds, and the quantification analysis was carried out using 5α-cholestane as an internal standard.
2.6. Determination of tocopherol contents
Tocopherols were determined according to the Chinese National Standard Method (GB/T 26635-2011). Briefly, green coffee oil samples (0.5 g) were diluted in 2.0 mL of n-hexane and filtered through a 0.22 µm nylon filter before HPLC analysis. Chromatographic separation was carried out on a Waters e2695 HPLC system (Waters Corp., Milford, MA, USA) equipped with a 3300 evaporative light scattering detector (ELSD; Grace Inc., USA), and separation was carried out by a Lichrosorb Si 60 column (250 mm × 4.6 mm, 5 µm, Merck, Darmstadt, Germany). To detect the fluorescence of tocopherols, the excitation wavelength was set at 295 nm, the emission wavelength was set at 330 nm, the injection volume was 10 µL, the mobile phases were composed of methanol:water (v/v = 98:2), and an isocratic elution programme was used at a flow rate of 1.0 mL/min. Tocopherols were quantified by establishing a standard curve with α-tocopherol, δ-tocopherol, β-tocopherol, and γ-tocopherol standard solutions.
2.7. Determination of cafestol and kahweol contents
The contents of cafestol and kahweol in purified extract samples were determined by HPLC. HPLC analyses were carried out using an Agilent 1290 instrument (Agilent Technologies, Inc., Santa Clara, CA, USA). The system was equipped with a G4204A quaternary pump, a G4626A autosampler, a G1316C column oven, and a G4212A DAD detector. Chromatographic separation was achieved on an Agilent ZORBAX C18 column (4.6 mm × 100 mm, 3.5 μm) and a mobile phase methanol/water mixture (85/15, v/v) with a flow rate of 0.70 mL min−1, and the detection wavelength was set at 220 nm for maximal absorption [2]. The injection volume was 10.0 µL, and all the samples were filtered through a 0.22 µm syringe filter before analysis. Stock solutions of the cafestol and kahweol standards (1.0 mg/mL) were diluted in mobile phase at different concentrations (0.001–0.5 mg/mL), and 10 µL of standard solution was injected into the HPLC after filtration through a Millipore filter. The linearity was checked by regression analysis of at least six different concentrations.
2.8. Determination of total phenolic content (TPC)
The TPC of GCO was analysed by the Folin-Ciocalteu method according to the protocol of Can-Cauich et al. [3] with slight modifications. In short, 1.0 mL polyphenolic extract was mixed with 5 mL of 10% (v/v) Folin-Ciocalteu reagent and left at room temperature for 5 min. Then, 4.0 mL of Na2CO3 (20%) was added and incubated at room temperature (25 ± 2 °C) for 90 min in the dark. The absorbance was determined at 760 nm using an ultraviolet spectrophotometer (Specord 250 plus, Analytik Jena AG, Germany). Gallic acid solutions (0.05–0.5 mg/mL) in methanol were used to build the calibration curve, and the TPC of GCO was expressed as gallic acid equivalents (mg GAE/100 g oil).
2.9. Antioxidant activity assays
The oils extracted from different methods were re-extracted by methanol three times. Then, they were further diluted to different concentrations and subjected to in vitro antioxidant activity assays. In particular, DPPH radical scavenging activity was measured according to the method described by Chouaibi et al. [6]. ABTS and FRAP antioxidant assays were performed using a total antioxidant capacity assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.10. Structural characterization of GCO
2.10.1. Scanning electron microscope (SEM) analysis
The morphological changes in coffee powders after extraction procedures were analysed by scanning electron microscopy (Hitachi, SU8020, Japan). The tested extracted samples were freeze-dried and positioned for SEM analysis. A small portion of each coffee sample was mounted onto the metal stub surface, coated with a thin layer of gold, and then photographed at 5000× magnification under high vacuum at an accelerating voltage of 10.0 kV [18].
2.10.2. Fourier transform infrared (FT-IR) spectroscopy
FT-IR spectra of the various GCOs were measured on Fourier transform infrared spectroscopy equipment (Bruker Optics, Ettlingen, Germany) equipped with a deuterated triglycine sulfate (DTGS) detector. The spectra of each sample were recorded in absorbance mode from 4000 to 400 cm−1 at a resolution of 4 cm−1. A small amount (5.0 μL) of the extracted oil sample was deposited between the two well-polished KBr pellets, and a Pasteur pipette was used to create a thin film. Pure spectroscopic grade KBr was used to correct for the background noise [24]. The spectra were baseline-corrected and deconvoluted using Omnic software. Determination was carried out in triplicate, and average spectra were used for further analysis.
2.11. Statistical analysis
The experimental data were evaluated by analysis of variance (ANOVA) using SPSS statistics 20.0 (SPSS Inc., Chicago, IL, USA), while significant differences in mean values were estimated at the probability level of p < 0.05. A Duncan's multiple range test was used to separate the means of the data when significant differences were observed. Moreover, the optimal extraction conditions were estimated through regression analysis and three-dimensional response surface plots of the independent variables and each dependent variable. The physicochemical indexes, compositions of fatty acid, tocopherol, diterpenes, and total phenol and the antioxidant activity of GCOs were normalized and merged into one matrix (12 rows × 24 columns) and then subjected to principal component analysis, Circos map analysis, and hierarchical clustering analysis to explore the marker compounds that could differentiate the GCO samples obtained by different extraction methods.
3. Results and discussion
3.1. Physicochemical properties
The influences of extraction methods on the oil yield and physicochemical indexes of GCOs are demonstrated in Fig. 1. UMAE achieved the highest oil extraction yield (10.58%), followed by MAE and UAE with extraction yields of 9.34% and 9.06%, respectively, while PLE showed obviously lower yields (6.34%) than the other three methods. This could be due to UMAE's combined advantages of MAE and UAE and to less contact between the solvent and raw material in PLE. The refractive index, acid value, iodine value, peroxide value, and saponification value are the main parameters for characterizing the quality of the oil [18]. The refractive index is the ratio of the speed at which light travels through the air to the speed at which it travels through the test sample. It can reflect the purity of the oil and indicate the unsaturation and chain length of fatty acids [36]. There was no significant difference between the different extraction methods, indicating that the extraction method has little influence on the unsaturation of GCO. The RI of the four oil samples ranged from 1.4680 to 1.4713, which was similar to that of kernel oil [17].
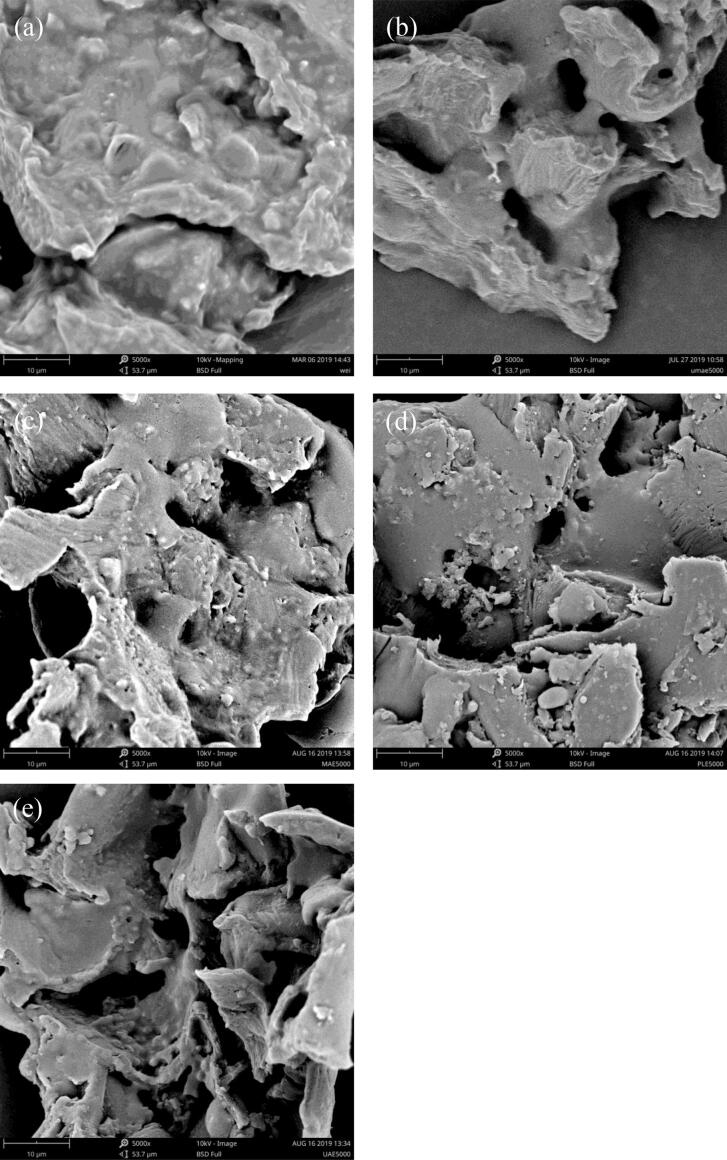
Fig. 1.
SEM of coffee powder at a magnification of 5000×: (a) raw green coffee powder before and after UMAE (b), UAE (c), MAE (d), and PLE (e) treatments.
The acid value is a measure of the number of free carboxylic acid groups in a compound (such as a fatty acid) or mixture and can be an indicator of the deterioration degree of oil. The result indicated that the lowest acid value was found with the UAE method and the highest value was found with the PLE method, demonstrating that GCO extracted by UAE has a better oil quality (Fig. 1) because a lower temperature was used in the UAE. The peroxide value showed a similar pattern, which was attributed to the higher temperature used in the extraction process leading to the decomposition and oxidation of triacylglycerol, which further leads to an increase in free fatty acids [33]. IV is an indicator of the degree of unsaturation in organic compounds, and the iodine value of GCO extracted by UAE was significantly lower than that of the other three methods, suggesting that GCO extracted by UAE has the lowest saturation and that temperature has an effect on oil saturation. The saponification value of GCO ranged from 88.49 ± 1.48 to 230.04 ± 10.14 mg KOH/g, indicating that different extraction methods have obvious effects on the fatty acid molecules of GCO, while the highest SV was found in the samples obtained by the UAE method, followed by the UMAE method (158.48 ± 1.02 mg KOH/g), with the PLE sample showing the lowest SV.
3.2. Fatty acid composition analysis
The fatty acid composition of GCO obtained by the different methods is shown in Table 2. It can be observed that the fatty acid composition of the extracted oils was very similar in percentage regardless of process, time, and extraction conditions. The main fatty acids in GCO were stearic acid (C18:0), oleic acid (C18:1), palmitic acid (C16:0), and linoleic acid (C18:2) in increasing order of abundance, with percentages of 6.70–7.11%, 8.54–9.94%, 36.57–38.71%, and 40.67–43.77%, respectively. In addition, palmitoleic acid, a minor monounsaturated fatty acid, was identified in GCO. The relative percentages of fatty acids in the GCO were slightly different by the different methods; the lowest content of linoleic acid and the highest content of palmitic acid were found in the UMAE method.
Table 2.
Fatty acid composition of green coffee oil obtained by different extraction methods (%).
| Fatty acids | UMAE | UAE | MAE | PLE |
|---|---|---|---|---|
| Myristic acid (C14:0) | 0.28 ± 0.01a | 0.29 ± 0.00a | 0.25 ± 0.01b | 0.27 ± 0.02ab |
| Ginkgolic acid (C15:0) | 0.12 ± 0.01a | 0.12 ± 0.01a | 0.10 ± 0.00b | 0.11 ± 0.00ab |
| Palmitic acid (C16:0) | 38.71 ± 1.12a | 37.38 ± 0.39ab | 36.57 ± 0.31b | 37.19 ± 0.54ab |
| Palmitoleic acid (C16:1) | 0.26 ± 0.00a | 0.24 ± 0.02ab | 0.22 ± 0.01b | 0.22 ± 0.01b |
| Heptadecanoic acid (C17:0) | 0.43 ± 0.07a | 0.32 ± 0.01a | 0.37 ± 0.08a | 0.36 ± 0.09a |
| Stearic acid (C18:0) | 7.02 ± 0.27a | 6.70 ± 0.24a | 6.93 ± 0.47a | 7.11 ± 0.39a |
| Oleic acid (C18:1) | 9.72 ± 0.49a | 9.89 ± 0.25a | 8.54 ± 0.62b | 9.94 ± 0.21a |
| Linoleic acid (C18:2) | 40.67 ± 1.36b | 42.06 ± 0.37ab | 43.77 ± 0.41a | 41.65 ± 0.58b |
| Arachidic acid (C20:0) | 2.85 ± 0.22a | 2.77 ± 0.18a | 2.90 ± 0.35a | 2.77 ± 0.01a |
| Methyl heneicosanoate (C21:0) | 0.11 ± 0.03a | 0.07 ± 0.01a | 0.08 ± 0.01a | 0.07 ± 0.00a |
| SFA | 48.85 ± 1.06a | 47.13 ± 0.46ab | 46.65 ± 0.70b | 47.58 ± 0.17ab |
| MUFA | 0.67 ± 0.10a | 0.51 ± 0.01a | 0.55 ± 0.07a | 0.48 ± 0.01a |
| PUFA | 50.48 ± 0.99b | 52.19 ± 0.41ab | 52.80 ± 0.65a | 51.81 ± 0.30ab |
| UFA/SFA | 1.05 ± 0.05a | 1.11 ± 0.03a | 1.14 ± 0.04a | 1.05 ± 0.08a |
Results are expressed as the mean ± standard error. Different capital letters in the same row indicate significant differences at p < 0.05. Saturated fatty acids, SFA; monounsaturated fatty acids, MUFA; polyunsaturated fatty acids, PUFA; UFA/SFA, unsaturated fatty acids/saturated fatty acids.
The saturated fatty acid (SFA) content of GCO ranged from 46.65 ± 0.70% to 48.85 ± 1.06%, much higher than that of other oils, such as red pepper seed oils [6] and echium seed oil [4]. While the highest SFA content of GCO was found in the UMAE method, this may be due to the high temperature resulting in the oxidation of unsaturated fatty acids, which were easily oxidized. The following conclusions were obtained by considering the composition of fatty acids: PUFA > SFA > MUFA. Differences in the fatty acid content among the GCOs extracted by the four techniques were not significant, thus proving that the extraction methods were not an important factor affecting the fatty acid composition of GCO. This result is consistent with previous research results [4]. It can be concluded that the green extraction technologies used to extract the components of GCO have no overall effect, but subtle differences can be found in some important fatty acids, such as linoleic acid and palmitoleic acid.
3.3. Tocopherol content of green coffee oil
Due to their observed thermal resistance and the protective effect against oxidative deterioration of the polyunsaturated fatty acids of vegetable oils [3], tocopherols are natural antioxidant compounds that can stabilize oils. The tocopherol content in the GCO extracted by the different methods is shown in Table 3. Four kinds of tocopherols were identified, which were α-tocopherol, δ-tocopherol, β-tocopherol, and γ-tocopherol. Regarding the tocopherols investigated in this work, γ-tocopherol was the major tocopherol in the GCOs, accounting for 64.82%–77.59% of the total tocopherols and ranging from 24.94 mg/100 g oil to 31.34 mg/100 g oil. PLE accounted for the highest tocopherol content in the GCO, while UMAE was accounted for the lowest. In addition, α-tocopherol in the GCO extracted by the different methods ranged from 5.63 to 9.67 mg/100 g, and the UMAE method led to the highest content, followed by UAE (7.87 mg/100 g oil) and MAE (6.54 mg/100 g oil). The δ-tocopherol content in GCO contributed approximately 5.30%–7.57% to the total tocopherols, lower than the contributions reported in red pepper seed oils [6]. As shown in Table 3, significant differences (p < 0.05) were found between the GCOs extracted by different methods, which suggested that the extraction method is an important factor affecting the tocopherol composition.
Table 3.
Tocopherols, phytosterols, diterpenes, total phenols, and antioxidant activity of green coffee oils extracted by different methods.
| Composition | UMAE | UAE | MAE | PLE |
|---|---|---|---|---|
| Tocopherol (mg/100 g oil) | ||||
| α-tocopherol | 9.67 ± 0.20a | 7.87 ± 0.46b | 6.54 ± 0.26a | 5.63 ± 0.11a |
| δ-tocopherol | 1.94 ± 0.04c | 2.89 ± 0.02a | 2.29 ± 0.12b | 3.06 ± 0.01a |
| β-tocopherol | 1.42 ± 0.20b | 2.05 ± 0.07a | 1.87 ± 0.05a | 2.07 ± 0.04a |
| γ-tocopherol | 24.94 ± 0.30c | 27.49 ± 0.64b | 30.52 ± 0.92a | 31.34 ± 0.24a |
| Total | 37.97 ± 0.36 | 40.03 ± 0.79 | 41.22 ± 0.97 | 42.10 ± 0.73 |
| Phytosterol (mg/g oil) | ||||
| Campesterol | 1.11 ± 0.03a | 0.92 ± 0.02b | 1.05 ± 0.03a | 1.11 ± 0.00a |
| Stigmasterol | 1.39 ± 0.04a | 1.18 ± 0.03b | 1.41 ± 0.04a | 1.45 ± 0.03a |
| β-sitosterol | 3.28 ± 0.02a | 2.99 ± 0.05b | 3.21 ± 0.09ab | 3.34 ± 0.06a |
| Total | 5.78 ± 0.06 | 5.10 ± 0.06 | 5.67 ± 0.11 | 5.90 ± 0.66 |
| Diterpenes (mg/100 g oil) | ||||
| Cafestol | 9.16 ± 0.63a | 6.57 ± 0.21c | 6.36 ± 0.16c | 7.82 ± 0.32b |
| Kahweol | 20.86 ± 1.51a | 14.84 ± 1.04bc | 13.76 ± 0.51c | 17.48 ± 0.77c |
| Total | 30.02 ± 1.64 | 21.41 ± 1.06 | 20.12 ± 0.53 | 25.30 ± 0.83 |
| Total phenols (mg GAE/100 g oil) | 14.81 ± 0.13bc | 14.40 ± 0.65c | 16.34 ± 1.31b | 26.33 ± 0.50a |
| Antioxidant activity (µmolTrolox/g oil) | ||||
| DPPH | 1.83 ± 0.11a | 1.41 ± 0.05c | 1.60 ± 0.02b | 1.58 ± 0.06b |
| ABTS | 1.00 ± 0.23a | 0.68 ± 0.24ab | 0.30 ± 0.05b | 0.93 ± 0.08a |
| FRAP (mmol Fe3SO4/g oil) | 2.16 ± 0.39b | 2.62 ± 0.14ab | 2.50 ± 0.18ab | 2.75 ± 0.12a |
Results are expressed as the mean ± standard error. Different capital letters in the same row indicate significant differences at p < 0.05.
3.4. Phytosterol contents of green coffee oil
Three kinds of phytosterols were identified in this study. The contents of three phytosterols can be found in Table 3, which demonstrates that the campesterol, stigmasterol, and β-sitosterol contents of GCOs increased in turn. The phytosterol content in GCO extracted by the UAE method (509.79 mg/100 g oil) was significantly lower than that in the oils extracted by the other three methods (p < 0.05), and there was no significant difference in phytosterol content among the other three methods. β-sitosterol, the principal plant sterol in many oil seeds, ranged from 2.99 ± 0.05 to 3.34 ± 0.06 mg/g oil in the GCO samples. The PLE samples showed the highest value, followed by the UMAE samples (3.28 ± 0.02 mg/g oil) and the MAE samples (3.21 ± 0.09 mg/g oil), while UAE samples showed the lowest value. Of these phytosterols, the highest content was found in the PLE samples, while UAE samples had the lowest contents of the three phytosterols. The sterol content of GCO was much higher than that of other vegetable oils, such as pumpkin oil [3] and kernel oils [17].
3.5. Diterpene content of green coffee oil
Cafestol and kahweol are two major diterpenes observed in GCO that are associated with increasing cholesterol levels in the body. The diterpene contents of the GCO obtained by different methods are shown in Table 3. The concentration of cafestol ranged from 6.36 ± 0.16 to 9.16 ± 0.63 mg/100 g oil, while that of kahweol ranged from 13.76 ± 0.51 to 20.86 ± 1.51 mg/100 g oil. The highest contents of kahweol and cafestol were found in the samples of the UMAE method, with 9.16 ± 0.63 and 20.86 ± 1.51 mg/100 g oil, respectively. In this study, the concentrations of cafestol and kahweol were high when ethanol was used as the solvent. In a supercritical extraction process, a higher temperature also has a positive effect on the extraction of diterpenes [1]. A similar phenomenon was discovered between the two processes when employing the same solvent. The results of the extracts were in agreement with the previous literature (de [9], and the diterpene contents were significantly different with the various extraction methods.
3.6. Total phenolic compounds (TPCs)
Phenolic compounds are important biologically active components of plant oils. The results of the total phenolic compounds are shown in Table 3. The total phenol content in the four oils tested in this study ranged from 14.00 ± 0.65 to 26.33 ± 0.50 mg GAE/100 g oil, higher than that of red pepper seed oil (12.56 mg/100 g oil) [6] and sesame oil (11.94 mg GAE/100 g oil) [33]. In particular, the oil obtained by the PLE method contributed the highest amounts of total phenols, followed by the MAE, UMAE and UAE methods in decreasing order. It is worth mentioning that high temperature plays an important role in the oxidation process because some unknown substances produced by the Maillard reaction increased the content of polyphenols. The results showed that the total phenols were significantly different with the different extraction methods (p < 0.05). One-way ANOVA showed that the extraction method had a great influence on the content of phenol compounds.
3.7. Free radical scavenging capacity (DPPH, ABTS, and FRAP)
The free radical scavenging capacity of the test GCOs was assessed by three methods (DPPH, ABTS, and FRAP). As shown in Table 3, the FRAP value (2.75 ± 0.12 mmol Fe3SO4/g oil) of oil by PLE was significantly higher than that of the other methods. In general, the PLE method showed a better performance than the other three methods (p < 0.05), indicating that the extraction method is a key factor affecting the free radical scavenging ability of GCO. The DPPH free radical scavenging ability of the GCO extracted by UMAE was the highest (1.81 ± 0.11 µmol Trolox/g oil), followed by the MAE (1.60 ± 0.02 µmol Trolox/g oil), PLE (1.58 ± 0.06 µmol Trolox/g oil), and UAE (1.41 ± 0.05 µmol Trolox/g oil) methods. In terms of ABTS total antioxidant capacity of the GCO studied in this work, its distribution in oils derived by UMAE and PLE was significantly higher than that in the MAE sample, while the UAE sample was in between these (p < 0.05).
3.8. Structural characterization
3.8.1. Scanning electron microscope of the coffee powder residue
Fig. 1a shows that the external structure of the coffee powder sample before extraction was intact and had a smooth cellular structure. After extraction by the four extraction techniques, dramatic changes in surface morphology could be observed in the samples. In the UMAE samples, the external structure was severely damaged, the surface of the coffee powder became rough, and large holes appeared (Fig. 1b). In general, extraction is achieved by penetration and solubility between the organic solvent and the oil; therefore, the microstructure of the sample is partially damaged. In the UMAE-treated samples, most of the microstructure was completely destroyed, with many irregular pores [19]. The ultrasound-treated sample (Fig. 1c) showed deep holes, which were due to violent shockwaves and high-speed jets striking the surface of the raw materials, leading to many pores. After extraction by MAE (Fig. 1d), most cells were ruptured, and many irregular pores and holes appeared. This phenomenon occurred because the microwave treatment obviously affected the external and internal structure of the samples, and heat was transferred by radiation, conduction, and convection during MAE. Zhou et al. [43] reported that an explosion takes place at the cell level due to the quick rise in temperature caused by microwave radiation. In addition, the high temperature and high pressure of PLE also led to cell damage of the coffee power (Fig. 1f). In summary, ultrasound, microwave, and pressurized treatments led to structural rupture of the raw samples and promoted the quick transfer of oil into the solvent.
3.8.2. Fourier transform infrared (FT-IR) spectral analysis
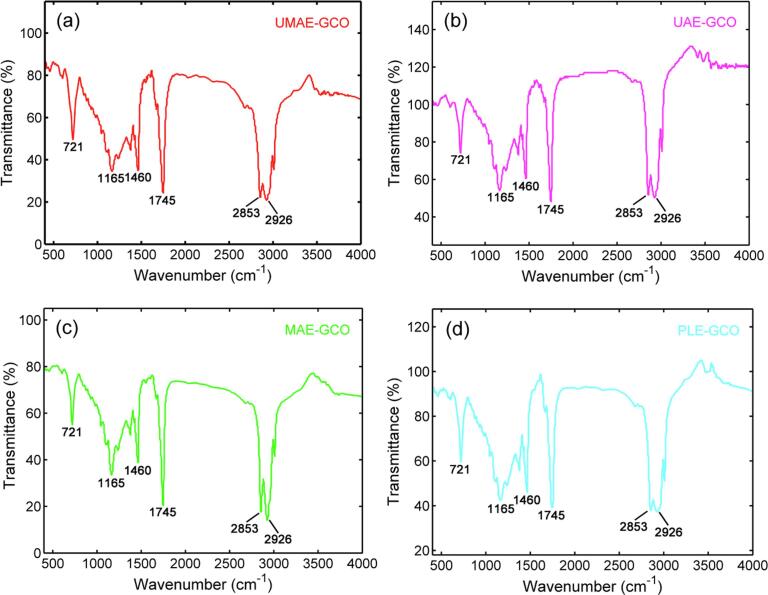
The spectra of GCOs were collected in the mid-infrared region from 4000 to 400 cm−1, and a representative FT-IR spectrum displaying the average of three replicates of GCO in the frequency region of 4000 to 400 cm−1 is shown in Fig. 2a–d. The FT-IR spectra of the GCOs obtained by the different extraction methods were quite similar because of the similar chemical composition of the oil samples. The absorption peak-to-peak position and types in the infrared spectra of oil samples were basically the same, indicating that the main components of GCOs extracted by the different methods were basically the same. As shown in Fig. 2a–d, the strong triplet peaks at 2980–2800 cm−1 are assigned to the C–H stretching of the methyl and methylene backbones of the oils [36]. The absorption peaks at 2926 cm−1 and 2853 cm−1 were attributed to the C–H stretching vibration peaks of the saturated carbon chain. The strong absorption peak at 1745 cm−1 was attributed to the C O carbonyl stretching of lipid and fatty acid ester groups. The overlapping peaks at 1460–1370 cm−1 corresponded to the combination of the methyl and methylene group deformation modes [16]. The peak at 1165 cm−1 was assigned to diacyl glycerol ethers in the oils. In addition, all spectra exhibited a peak related to cis C C out-of-plane bending at 721 cm−1.
Fig. 2.
FT-IR spectra of green coffee oils extracted through different methods (a: UMAE-GCO; b: UAE-GCO; c: MAE-GCO; d: PLE-GCO). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.9. Principal component analysis
PCA was used to explore the relationship between extraction methods and the analytical parameters determined for each GCO [3]. Before chemometric processing, all parameters were normalized to provide an equal contribution of variables in the prediction results. PCA was performed on the fused matrix composed of 12 rows corresponding to the GCO samples and 24 columns that were composed of the physicochemical indexes, compositions of fatty acids, tocopherols, diterpenes, and total phenol, and the antioxidant activity of GCOs. As shown in Fig. 2a, the first two principal components (PCs) explained 70.7% of the total variance (PC1: 35.9%; PC2: 34.8%), and the score plot shows a clear distribution of the oils according to the chemical composition. The PLE oil showed a positive value for PC1 and partly overlapped the x-axis, and the UMAE oil had negative and positive contributions to PC1 and PC2, respectively, which were located in the second quadrant of the plane. The UAE oil obtained negative PC1 and PC2 values, which were located in the third quadrant of the score plot. For the MAE samples, it can be observed that the MAE oil was closest to the origin and partially overlapped on the y-axis. Fig. 2b showed the loading plot in the PC1-PC2 plane. PC1 was positively related to ABTS, campesterol, iodine value, β-sitosterol, peroxide value, stigmasterol, total phenols, FRAP, γ-tocopherol, acid value, β-tocopherol, δ-tocopherol, and refractive index but was negatively related to the other parameters. The total phenols exhibited the highest positive loadings on PC1, from which we can affirm that the PLE sample was characterized by its high level of total phenols. The UMAE oil was mainly characterized by its content of α-tocopherol, free fatty acids, and extraction yield, and UAE oil showed a higher saponification value. All the information obtained confirms that the extraction methods can produce GCOs with different characteristics, which could define their special use in the food industry or for cosmetic purposes.
3.10. Circos map analysis
Circos maps have been widely used in comparative genetics and foods and are useful for visualizing the relationships among multidimensional data [23], [40]. The oils were clustered into four groups according to the different extraction methods (Fig. 4a). The UMAE and UAE samples were adjacent and distributed in the right region, and the MAE and PLE samples were distributed closely and located on the left bottom. The polyunsaturated fatty acids, tocopherols, and sterols had relatively longer intervals, indicating that the GCOs contained a higher level of these bioactive components, especially β-sitosterol and γ-tocopherol, which also exist widely in other vegetable oils [41], [11]. For β-sitosterol, it can be observed from the linkage-line colour that the β-sitosterol content was highest in the PLE samples, followed by the PLE and MAE samples, with a lower level in the MAE samples.
Fig. 4.
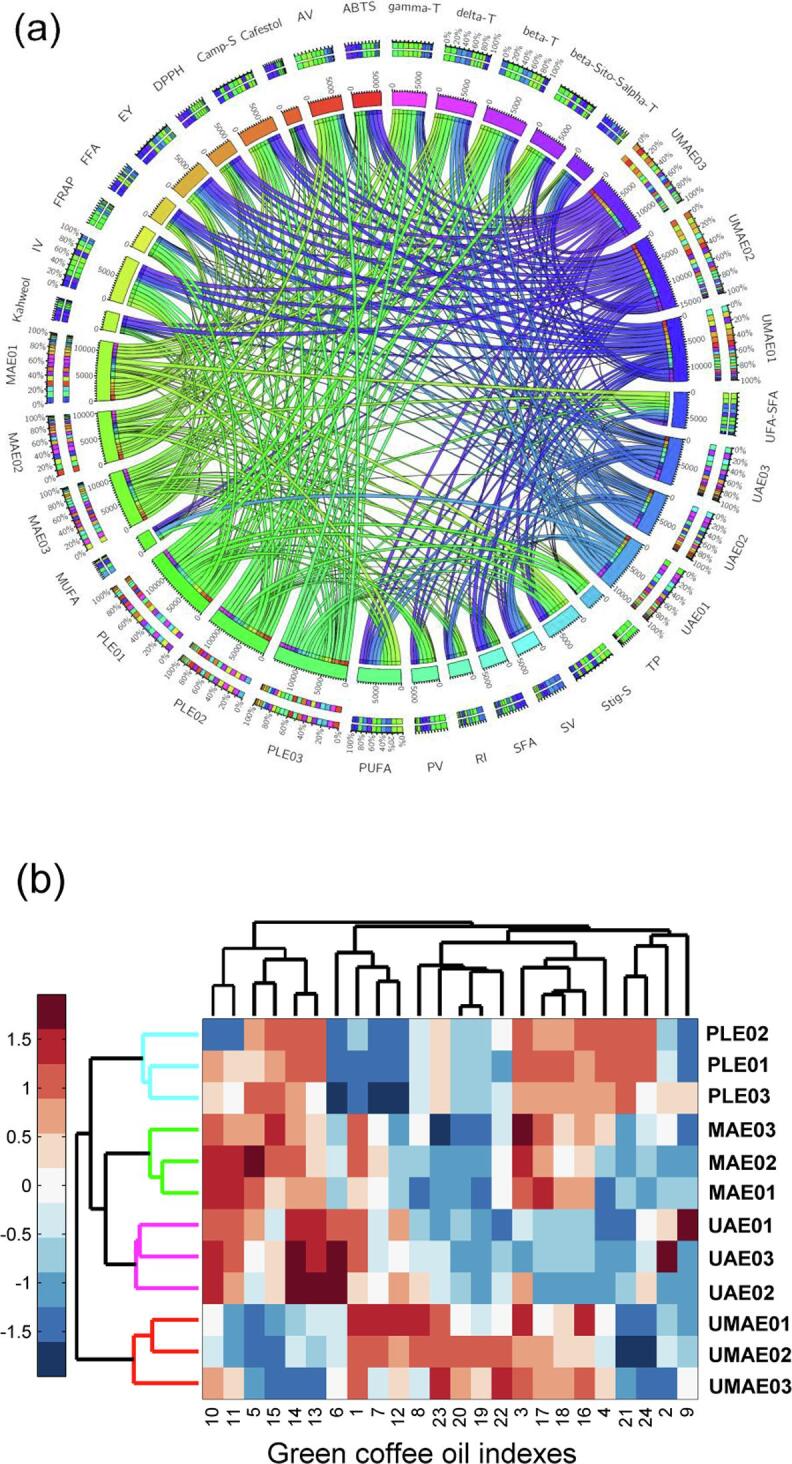
Circos map displaying the relationships among the oils, phytochemicals and oxidative indexes (a), cluster heat map hierarchically using the fused dataset matrix (b). Extraction yield, EY; refractive index, RI; iodine value, IV; peroxide value, PV; acid value, AV; saponification value, SV; free fatty acid, FFA; saturated fatty acids, SFA; monounsaturated fatty acids, MUFA; polyunsaturated fatty acids, PUFA; UFA/SFA, unsaturated fatty acids/saturated fatty acids; ɑ-tocopherol, Alpha-T; δ-tocopherol, Delta-T; β-tocopherol, Beta-T; γ-tocopherol, Gamma-T; campesterol, Camp-S; stigmasterol, Stig-S; β-sitosterol, beta-Sito-S; total phenols, TP.
3.11. Hierarchical cluster analysis
Heat maps have become an efficient tool for the evaluation of agricultural product quality and can reflect the chemical composition differences of samples prepared by different preprocessing methods [35], [21]. In this study, to investigate the differentiating power of the indexes used, HCA was performed with 24 analytical indexes, and a heat map was utilized to exhibit alterations of chemical indexes in the four kinds of samples using Euclidean distance as a similarity measure. Red indicates a high content, and blue indicates a low content. The oil samples from the four extraction methods were well distinguished through HCA (Fig. 4b), and four clusters were grouped. In addition, the PLE and MAE samples were always grouped as closely, and those clustering trends were in accordance with those in the PCA bioplot (Fig. 3a-b) and Circos map (Fig. 4a). Clustering demonstrated that the UMAE samples had high contents of ɑ-tocopherol. The UAE samples were separated from others in the heat map since they were characterized by saponification value, polyunsaturated fatty acids, δ-tocopherol, and β-tocopherol. In particular, the PLE samples clustered in one group that had the highest total phenol content.
Fig. 3.
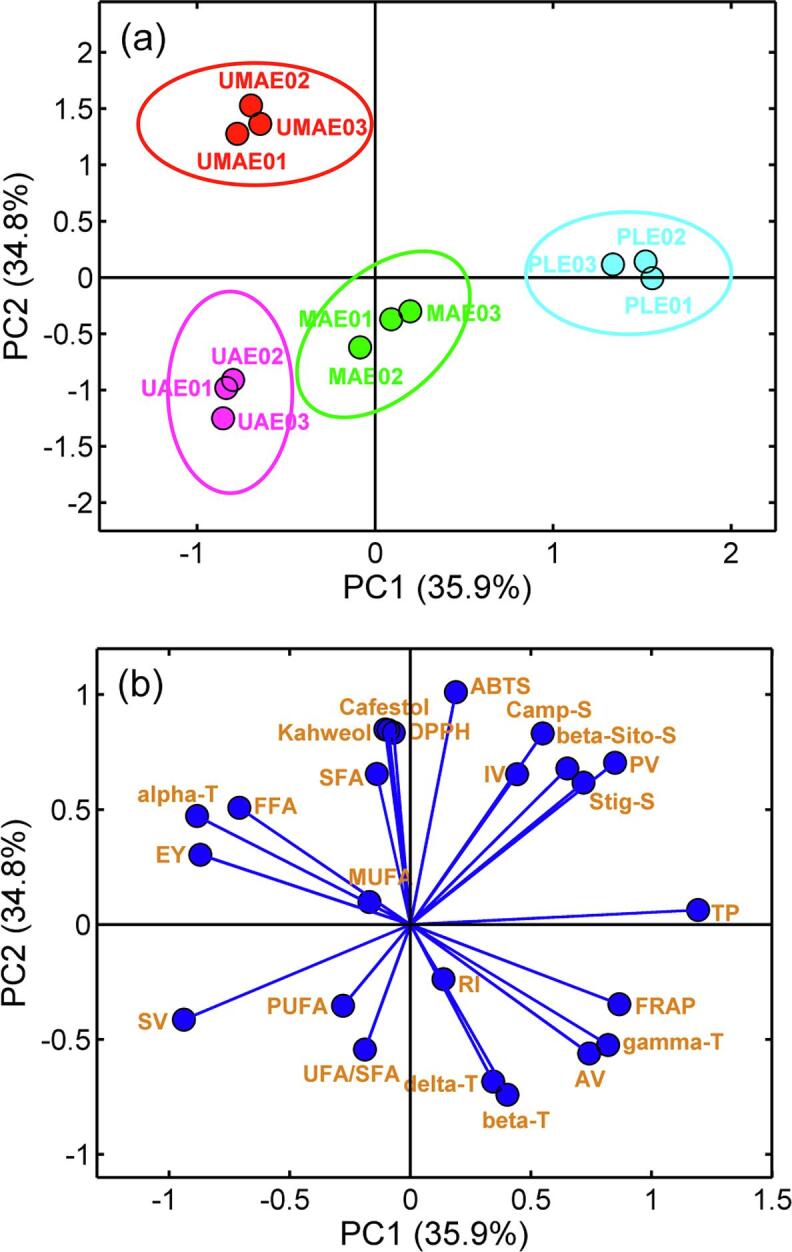
PCA bioplot obtained from the fused dataset matrix of green coffee oil from the different extraction methods (a: score biplot, b: loading biplot). Extraction yield, EY; refractive index, RI; iodine value, IV; peroxide value, PV; acid value, AV; saponification value, SV; free fatty acid, FFA; saturated fatty acids, SFA; monounsaturated fatty acids, MUFA; polyunsaturated fatty acids, PUFA; UFA/SFA, unsaturated fatty acids/saturated fatty acids; ɑ-tocopherol, Alpha-T; δ-tocopherol, Delta-T; β-tocopherol, Beta-T; γ-tocopherol, Gamma-T; campesterol, Camp-S; stigmasterol, Stig-S; β-sitosterol, beta-Sito-S; total phenols, TP. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Conclusions
In this study, four different extraction techniques were used and compared to extract green coffee oil from Arabica coffee beans using ethanol as a green solvent. It was demonstrated that extraction conditions exerted great effects on the physicochemical and antioxidant activities of green coffee beans. Significant differences existed in the extraction yield and the free fatty acid, γ-tocopherol, δ-tocopherol, cafestol, kahweol, and total phenol contents among the four extraction methods. However, other parameters, such as refractive index, fatty acid composition, and phytosterol content, were affected by the extraction methods and were small. All oils contained high proportions of β-sitosterol and γ-tocopherol. Chemometric analysis illustrated that PCA, Circos maps, and heat maps were useful and provided visualization of the evaluation of green coffee oils obtained by the various methods. The UMAE treatment is suggested for the extraction of GCO to obtain a product with a high content of SFA, α-tocopherol and kahweol. If green coffee oils rich in β-tocopherol are needed, the UAE or PLE methods would be more appropriate. In addition, if the purpose is to produce GCO with higher contents of PUFAs and UFAs/SFAs, the MAE method is suggested. The PLE method is ideal for preparing oils rich in δ-tocopherol, β-sitosterol, and total phenols. Overall, considering the extraction efficiency, good quality retention and production cost, the UMAE method, combined with ethanol, has the broadest market prospect for application. This method is proposed to obtain green coffee oil with a high quality and for industrial applications. Furthermore, more in-depth research should be carried out to confirm this efficient and eco-friendly extraction technique.
CRediT authorship contribution statement
Wenjiang Dong: Methodology, Software, Formal analysis, Investigation, Writing - original draft, Writing - review & editing, Visualization, Funding acquisition. Qiyu Chen: Methodology, Validation, Writing - original draft. Changqing Wei: Conceptualization, Supervision. : . Rongsuo Hu: Software, Investigation, Supervision. Yuzhou Long: Software, Supervision. Ying Zong: Supervision, Project administration. Zhong Chu: Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the Hainan Provincial Natural Science Foundation of China (2019CXTD416), National Natural Science Foundation of China (31872888), and the China Central Public-Interest Scientific Institution Basal Research Fund (1630142017005) for their financial support.
Contributor Information
Wenjiang Dong, Email: dongwenjiang.123@163.com.
Changqing Wei, Email: changqing_wei@126.com.
References
- 1.Barbosa H.M.A., de Melo M.M.R., Coimbra M.A., Passos C.P., Silva C.M. Optimization of the supercritical fluid coextraction of oil and diterpenes from spent coffee grounds using experimental design and response surface methodology. J. Supercrit. Fluids. 2014;85:165–172. [Google Scholar]
- 2.Belandria V., Aparecida de Oliveira P.M., Chartier A., Rabi J.A., de Oliveira A.L., Bostyn S. Pressurized-fluid extraction of cafestol and kahweol diterpenes from green coffee. Innov. Food Sci. Emerg. Technol. 2016;37:145–152. [Google Scholar]
- 3.Can-Cauich C.A., Sauri-Duch E., Moo-Huchin V.M., Betancur-Ancona D., Cuevas-Glory L.F. Effect of extraction method and specie on the content of bioactive compounds and antioxidant activity of pumpkin oil from Yucatan, Mexico. Food Chem. 2019;285:186–193. doi: 10.1016/j.foodchem.2019.01.153. [DOI] [PubMed] [Google Scholar]
- 4.Castejón N., Luna P., Señoráns F.J. Alternative oil extraction methods from Echium plantagineum L. seeds using advanced techniques and green solvents. Food Chem. 2018;244:75–82. doi: 10.1016/j.foodchem.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Chen Q.Y., Dong W.J., Wei C.Q., Hu R.S., Long Y.Z. Combing integrated ultrasonic-microwave technique with ethanol to maximize extraction of green coffee oil from Arabica coffee beans. Ind. Crops Prod. 2020;151 [Google Scholar]
- 6.Chouaibi M., Rezig L., Hamdi S., Ferrari G. Chemical characteristics and compositions of red pepper seed oils extracted by different methods. Ind. Crops Prod. 2019;128:363–370. [Google Scholar]
- 7.Cordoba N., Fernandez-Alduenda M., Moreno F.L., Ruiz Y. Coffee extraction: a review of parameters and their influence on the physicochemical characteristics and flavour of coffee brews. Trends Food Sci. Technol. 2020;96:45–60. [Google Scholar]
- 8.Cornelio-Santiago H.P., Goncalves C.B., De Oliveira N.A., De Oliveira A.L. Supercritical CO2 extraction of oil from green coffee beans: solubility, triacylglycerol composition, thermophysical properties and thermodymatic modeling. J. Supercrit. Fluids. 2017;128:386–394. [Google Scholar]
- 9.Oliveira N.A.d., Cornelio-Santiago H.P., Fukumasu H., Oliveira A.L.d. Green coffee extracts rich in diterpenes – process optimization of pressurized liquid extraction using ethanol as solvent. J. Food Eng. 2018;224:148–155. [Google Scholar]
- 10.Euromonitor International. Coffee in 2018: The new era of coffee everywhere (2018).
- 11.D. Firestone, Physical and chemical characteristics of oils, fats and waxes, 3rd ed., 2013, Softbound.
- 12.GB 5009.229-2016. The national standard test method for acid value. China Standard Publication House: Beijing (in Chinese) (2016).
- 13.GB/T 26635-2011. The national standard test method for tocopherol contents. China Standard Publication House: Beijing (in Chinese) (2011).
- 14.GB/T 5532-2008. The national standard test method for iodine value. China Standard Publication House: Beijing (in Chinese) (2008).
- 15.GB/T 5534-2008. The national standard test method for saponification value (in Chinese). China Standard Publication House: Beijing (in Chinese) (2008).
- 16.Gu Ling-Biao, Zhang Guang-Jie, Du Lei, Du Juan, Qi Kun, Zhu Xin-Liang, Zhang Xiao-Ying, Jiang Zhi-Hui. Comparative study on the extraction of Xanthoceras sorbifolia Bunge (yellow horn) seed oil using subcritical n-butane, supercritical CO2, and the Soxhlet method. LWT – Food Sci. Technol. 2019;111:548–554. [Google Scholar]
- 17.He Zhiyong, Zhu Haidong, Li Wangling, Zeng Maomao, Wu Shengfang, Chen Shangwei, Qin Fang, Chen Jie. Chemical components of cold pressed kernel oils from different Torreya grandis cultivars. Food Chem. 2016;209:196–202. doi: 10.1016/j.foodchem.2016.04.053. [DOI] [PubMed] [Google Scholar]
- 18.Hu Bin, Xi Xiaohui, Li Haochen, Qin Yvxian, Li Cheng, Zhang Zhiqing, Liu Yuntao, Zhang Qing, Liu Aiping, Liu Shuxiang, Luo Qingying. A comparison of extraction yield, quality and thermal properties from Sapindus mukorossi seed oil between microwave assisted extraction and Soxhlet extraction. Ind. Crops Prod. 2021;161:113185. doi: 10.1016/j.indcrop.2020.113185. [DOI] [Google Scholar]
- 19.Hu Bin, Wang Haoyuan, He Linfeng, Li Yi, Li Cheng, Zhang Zhiqing, Liu Yuntao, Zhou Kang, Zhang Qing, Liu Aiping, Liu Shuxiang, Zhu Yadong, Luo Qingying. A method for extracting oil from cherry seed by ultrasonic-microwave assisted aqueous enzymatic process and evaluation of its quality. J. Chromatogr. A. 2019;1587:50–60. doi: 10.1016/j.chroma.2018.12.027. [DOI] [PubMed] [Google Scholar]
- 20.International Coffee Organization (ICO). Coffee Market Report. March 2018. London (2018).
- 21.Jiang Fan, Yuan Liyang, Shu Nanxi, Wang Wuliang, Liu Yuanfa, Xu Yong-Jiang. Foodomics revealed the effects of extract methods on the composition and nutrition of peanut oil. J. Agric. Food. Chem. 2020;68(4):1147–1156. doi: 10.1021/acs.jafc.9b06819. [DOI] [PubMed] [Google Scholar]
- 22.Koubaa Mohamed, Mhemdi Houcine, Barba Francisco J., Roohinejad Shahin, Greiner Ralf, Vorobiev Eugène. Oilseed treatment by ultrasounds and microwaves to improve oil yield and quality: an overview. Food Res. Int. 2016;85:59–66. doi: 10.1016/j.foodres.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Krzywinski M., Schein J., Birol I., Connors J., Gascoyne R., Horsman D., Jones S.J., Marra M.A. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19(9):1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Xue-Xia, Liu Hua-Min, Li Jin, Yan Yuan-Yuan, Wang Xue-De, Ma Yu-Xiang, Qin Guang-Yong. Effects of various oil extraction methods on the structural and functional properties of starches isolated from tigernut (Cyperus esculentus) tuber meals. Food Hydrocolloids. 2019;95:262–272. [Google Scholar]
- 25.Lopes Guido R., Passos Cláudia P., Rodrigues Carla, Teixeira José A., Coimbra Manuel A. Impact of microwave-assisted extraction on roasted coffee carbohydrates, caffeine, chlorogenic acids and coloured compounds. Food Res. Int. 2020;129:108864. doi: 10.1016/j.foodres.2019.108864. [DOI] [PubMed] [Google Scholar]
- 26.Montenegro Júlia, dos Santos Lauriza Silva, de Souza Rodrigo Gonçalves Gusmão, Lima Larissa Gabrielly Barbosa, Mattos Daniella Santos, Viana Bruna Prunes Pena Baroni, da Fonseca Bastos Ana Clara Santos, Muzzi Leda, Conte-Júnior Carlos Adam, Gimba Etel Rodrigues Pereira, Freitas-Silva Otniel, Teodoro Anderson Junger. Bioactive compounds, antioxidant activity and antiproliferative effects in prostate cancer cells of green and roasted coffee extracts obtained by microwave-assisted extraction (MAE) Food Res. Int. 2021;140:110014. doi: 10.1016/j.foodres.2020.110014. [DOI] [PubMed] [Google Scholar]
- 27.Mwaurah P.W., Kumar S., Kumar N., Attkan A.K., Panghal A., Singh V.K., Garg M.K. Novel oil extraction technologies: process conditions, quality parameters, and optimization. Compr. Rev. Food Sci. Food Saf. 2020;19:3–20. doi: 10.1111/1541-4337.12507. [DOI] [PubMed] [Google Scholar]
- 28.Patel Alok, Arora Neha, Pruthi Vikas, Pruthi Parul A. A novel rapid ultrasonication-microwave treatment for total lipid extraction from wet oleaginous yeast biomass for sustainable biodiesel production. Ultrason. Sonochem. 2019;51:504–516. doi: 10.1016/j.ultsonch.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Rigal Clément, Vaast Philippe, Xu Jianchu, Zang RunGuo. Using farmers' local knowledge of tree provision of ecosystem services to strengthen the emergence of coffee-agroforestry landscapes in southwest China. PLoS ONE. 2018;13(9):e0204046. doi: 10.1371/journal.pone.0204046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samoggia Antonella, Riedel Bettina. Coffee consumption and purchasing behavior review: Insights for further research. Appetite. 2018;129:70–81. doi: 10.1016/j.appet.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Santos Kátia Andressa, Bariccatti Reinaldo Aparecido, Cardozo-Filho Lúcio, Schneider Ricardo, Palú Fernando, Silva Camila da, Silva Edson Antônio da. Extraction of crambe seed oil using subcritical propane: kinetics, characterization and modeling. J. Supercrit. Fluids. 2015;104:54–61. [Google Scholar]
- 32.Sengar Animesh Singh, Rawson Ashish, Muthiah Manimekalai, Kalakandan Suresh Kumar. Comparison of different ultrasound assisted extraction techniques for pectin from tomato processing waste. Ultrason. Sonochem. 2020;61:104812. doi: 10.1016/j.ultsonch.2019.104812. [DOI] [PubMed] [Google Scholar]
- 33.Shi Long-Kai, Zheng Li, Liu Rui-Jie, Chang Ming, Jin Qing-Zhe, Wang Xing-Guo. Chemical characterization, oxidative stability, and in vitro antioxidant capacity of sesame oils extracted by supercritical and subcritical techniques and conventional methods: a comparative study using chemometrics. Eur. J. Lipid Sci. Technol. 2018;120(2):1700326. doi: 10.1002/ejlt.v120.210.1002/ejlt.201700326. [DOI] [Google Scholar]
- 34.Sun Haiyao, Li Chunying, Ni Yujiao, Yao Liping, Jiang Hongwei, Ren Xueting, Fu Yujie, Zhao Chunjian. Ultrasonic/microwave-assisted extraction of polysaccharides from Camptotheca acuminata fruits and its antitumor activity. Carbohydr. Polym. 2019;206:557–564. doi: 10.1016/j.carbpol.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Tian P., Zhan P., Tian H.L., Wang P., Lu C., Zhao Y., Ni R.J., Zhang Y.Y. Analysis of volatile compound changes in fried shallot (Allium cepa L. var. aggregatum) oil at different frying temperatures by GC-MS, OAV, and multivariate analysis. Food Chem. 2021;345 doi: 10.1016/j.foodchem.2020.128748. [DOI] [PubMed] [Google Scholar]
- 36.Timiksna Y.P., Vongsvivut J., Adhikari R., Adhikari B. Phsicochemical and thermal characteristics of Australian chia seed oil. Food Chem. 2017;228:384–402. doi: 10.1016/j.foodchem.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 37.Tiwari Brijesh K. Ultrasound: a clean, green extraction technology. Trends Anal. Chem. 2015;71:100–109. [Google Scholar]
- 38.Tsukui A., Santos Júnior H.M., Oigman S.S., de Souza R.O.M.A., Bizzo H.R., Rezende C.M. Microwave-assisted extraction of GCO and quantification of diterpenes by HPLC. Food Chem. 2014;164:266–271. doi: 10.1016/j.foodchem.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 39.Lombo Vidal Oscar, Tsukui Anna, Garrett Rafael, Miguez Rocha-Leão Maria Helena, Piler Carvalho Carlos Wanderlei, Pereira Freitas Suely, Moraes de Rezende Claudia, Simões Larraz Ferreira Mariana. Production of bioactive films of carboxymethyl cellulose enriched with green coffee oil and its residues. Int. J. Biol. Macromol. 2020;146:730–738. doi: 10.1016/j.ijbiomac.2019.10.123. [DOI] [PubMed] [Google Scholar]
- 40.Yu L., Wang Y.J., Wu G.C., Jin J., Jin Q.Z., Wang X.G. Chemical and volatile characteristics of olive oils extracted from four varieties grown in southeast of China. Food Res. Int. 2021;140 doi: 10.1016/j.foodres.2020.109987. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Tao, Wang Tao, Liu Ruijie, Chang Ming, Jin Qingzhe, Wang Xingguo. Chemical characterization of fourteen kinds of novel edible oils: a comparative study using chemometrics. LWT – Food Sci. Technol. 2020;118:108725. doi: 10.1016/j.lwt.2019.108725. [DOI] [Google Scholar]
- 42.Zhao C.J., Li Z., Li C.Y., Yang L., Yao L.P., Fu Y.J., He X., Shi K.M., Lu Z.C. Optimized extraction of polysaccharides from Taxus chinensis var. mairei fruits and its antitumor activity. Int. J. Biol. Macromol. 2015;75:192–198. doi: 10.1016/j.ijbiomac.2015.01.043. [DOI] [PubMed] [Google Scholar]
- 43.Zhou C., Sun D., Sun X., Zhu C., Wang Q. Combing ultrasound and microwave to improve the yield and quality of single-cell oil from Mortierella isabellina NTG-121. J. Am. Oil Chemists’ Soc. 2018;95:1535–1547. [Google Scholar]