Abstract
Background
Androgenetic alopecia (AGA) is the most common type of hair loss in men. Its prevalence increases with advancing age. Characteristics of hair loss in male AGA reveal the possibility of different biophysical and physiological profiles between androgen-sensitive (vertex) and androgen-insensitive (occipital) scalps. However, these variations have not been well investigated.
Objective
We aimed to evaluate and compare scalp biophysical and physiological characteristics in male AGA patients and healthy controls.
Methods
Scalp biophysiological profiles were evaluated by non-invasive measuring techniques, including skin surface lipids (SSL), transepidermal water loss (TEWL), and stratum corneum hydration (SCH) on both vertex and occipital areas. Values were compared between scalp areas and study groups. Participants with AGA were further categorized based on disease severity (Hamilton–Norwood classification) for subgroup analyses. Correlation coefficients were evaluated to determine the effects of AGA severity and age on each functional parameter.
Results
Participants were 31 AGA subjects and 31 healthy controls. The vertex scalp of AGA patients had significantly higher SSL (p = 0.03) and lower SCH (p = 0.02) compared to the occipital scalp. TEWL was not significantly different (p = 0.31). AGA group SSL showed a positive correlation with severity of hair loss (r = 0.61, p = 0.03). When compared to controls, the AGA group vertex scalp had significantly higher SSL (p = 0.03) and lower TEWL (p < 0.001). The occipital area showed no statistically significant differences.
Conclusion
Male AGA presents with different biophysical and physiological characteristics in androgen-sensitive and androgen-insensitive areas, and with further differences from controls. These findings could direct further research and aid in the development of optimal hair and scalp treatments to improve scalp functional profiles in particular patients.
Keywords: alopecia, hair loss, hydration, sebum, transepidermal water loss
Introduction
Androgenetic alopecia (AGA) is the most common type of hair loss, it affects up to 80% of males with genetic predisposition.1 The prevalence of AGA increases steadily with advancing age.2 It is characterized by a progressive miniaturization of hair follicles limited to the frontotemporal and vertex areas, considered to be androgen-sensitive scalp regions.3–5 The pathogenesis of male AGA remains unclear; however, dihydrotestosterone (DHT), the main androgen converted from testosterone by 5-alpha reductase (5-AR), is thought to play an important role. DHT acts on androgen receptors (AR), causing terminal hairs to convert to miniaturized hairs, eventually resulting in AGA.2,6–9
The stratum corneum (SC) is a multilayered structure that acts as a barrier and regulates biological functions of the skin. Its specific biophysical and physiological properties vary between different body regions according to regional modifying factors.10,11 Scalp skin features specific characteristics compared to skin of other body areas; it has high proportions of hair follicles, sebaceous glands, and blood vessels.12 Increasingly, studies are finding that as well as hair follicles, androgen might also affect surrounding connective tissues and pilosebaceous units of balding scalp. Androgen-sensitive areas display significant enlargement of sebaceous glands, higher levels of DHT, and higher AR numbers compared to androgen-insensitive scalp areas.13,14 Moreover, hair serves to protect the scalp from environmental stressors such as ultraviolet (UV) radiation, chemical agents, and trauma.15 Therefore, scalps with alopecia have impaired physical defenses that might affect scalp biophysiological functioning. Several existing studies have already demonstrated abnormal barrier function in association with various skin conditions. We hypothesize that these factors might alter functional characteristics of AGA-affected scalps.
There is a paucity of information regarding biophysical and physiological profiles of scalp skin in AGA patients. One study has demonstrated that androgen-sensitive scalps have higher skin lipid levels and lower skin hydration compared to androgen-insensitive areas.16 However, to our knowledge, no comparative study with healthy controls has been conducted. The objective of this study was to evaluate and compare scalp skin barrier functions, including skin surface lipids (SSL), transepidermal water loss (TEWL), and SC hydration (SCH), in Thai males with AGA and healthy controls.
Materials and Methods
This study was approved by the Mahidol University Institutional Review Board for Ethics in Human Research (MURA2019/308) and was performed in accordance with the Helsinki Declaration. Written informed consent was received from each subject before enrolment. The sample size was calculated based on data from a previous study comparing skin functional properties between androgen-sensitive and androgen-insensitive scalps.16 A minimal sample size of 20 subjects per group was required to achieve a power of 80% with a confidence level of 95%.
Participants
A case–control study was conducted at the outpatient department, Division of Dermatology, Faculty of Medicine, Ramathibodi Hospital from May to December 2019. Thai males aged 18–70 years with a clinical diagnosis of AGA were recruited, and healthy subjects were enrolled as controls. Exclusion criteria included the presence of other hair and scalp disorders, and the use of topical/systemic drugs or systemic diseases that impaired skin barrier function and affect the hair growth cycle. Demographic data were collected from all subjects. Participants were instructed to avoid hair and scalp products 24 hours before examination.
Measurements
All measurements were carried out by a well-trained and experienced investigator under standardized conditions at a controlled temperature of 19–24°C and relative humidity of 50%–58%. All subjects were in a climate-controlled room for at least 15 minutes before examination. Measurement of SSL, TEWL, and SCH were performed using DermaLab® (Cortex Technology, Hadsund, Denmark) on two scalp areas for each subject. The hairs of the measured areas were trimmed at the level of the scalp surface immediately before the evaluation. Measurements were taken at the vertex (androgen-sensitive), located on the midline 24 cm from the frontal hairline, and the occipital region (androgen-insensitive), located on the midline 30 cm from the frontal hairline. Values were compared between scalp areas and study groups.
SSL was collected by placing microporous film on the scalp surface. The film’s translucency was modified by the amount of sebum collected, changing the amount of light that could pass through and reach a reader module. TEWL was assessed via a probe using an open chamber method. The sensor detected the humidity, and the evaporation rate was recorded in units of g/h/m2. SCH was measured by detecting any alteration of skin conduction properties when subjected to alternating voltage.
Statistics
Statistical analysis was performed using SPSS, version 18.0 (SPSS Inc., Chicago, IL, USA). Unpaired t-test and Mann–Whitney U-test were used to determine the differences between parameters in the AGA and control groups. Subgroup analyses based on disease severity were performed using the analysis of variance or the Kruskal–Wallis test. When the overall comparison p value was less than 0.05, pairwise comparisons of subgroups were conducted using the Turkey’s honest significance difference test or the Mann–Whitney U-test as appropriate. Correlations between each variable and AGA severity, and age were determined using Spearman’s and Pearson’s correlations, respectively. Statistical significance was determined as p < 0.05.
Results
Characterization of the Participants
There were 31 males with AGA and 31 healthy controls in this study. Mean age for the AGA and control groups was 51.25 ± 10.19 years and 47.81 ± 9.38 years, respectively. Regarding Hamilton–Norwood classifications, 10 (32%) AGA cases were grade III, 8 (26%) were grade IV, 7 (23%) were grade V, 3 (9.5%) were grade VI, and 3 (9.5%) were grade VII. There was no statistically significant difference in demographics between the groups. Subject characteristics are summarized in Table 1.
Table 1.
Characteristics of Included Participants
| AGA N = 31 | Control N = 31 | p value | |
|---|---|---|---|
| Age, y | |||
| Mean (SD) | 51.25 (10.19) | 47.81 (9.38) | 0.17 |
| Hamilton–Norwood type, n (%) | |||
| III | 10 (32) | – | – |
| IV | 8 (26) | – | |
| V | 7 (23) | – | |
| VI | 3 (9.5) | – | |
| VII | 3 (9.5) | – | |
| Frequency of hair washing, n (%) | |||
| < 1 time/day | 8 (25.8) | 14 (45.1) | 0.76 |
| 1 time/day | 21 (67.7) | 15 (48.4) | |
| ≥ 1 time/day | 2 (6.5) | 2 (6.5) |
Abbreviations: AGA, androgenetic alopecia; SD, standard deviation.
Scalp Biophysical Characteristics of Subjects with AGA
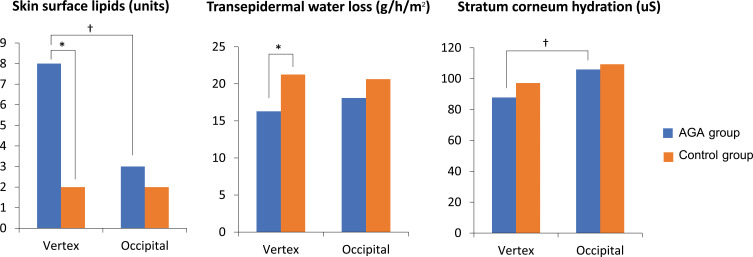
In the AGA group, the vertex had a statistically significant higher median SSL (8 [IQR 1–29] vs 3 [IQR 1–10], p = 0.03) and lower mean SCH (87.84 ± 18.75 vs 102.96 ± 29.29, p = 0.02) as compared to the occipital area. However, the vertex showed slightly lower mean TEWL compared to the occipital scalp, although this was not statistically significant (16.3 ± 5.15 vs 18.1 ± 4.2, p = 0.31) (Figure 1).
Figure 1.
Values of skin surface lipids, transepidermal water loss, and stratum corneum hydration measured at vertex and occipital areas of males with androgenetic alopecia (AGA) and healthy controls. †Statistically significant difference between vertex and occipital areas of males with AGA, *Statistically significant difference between AGA and control groups.
Subgroup Analyses of Subjects with AGA
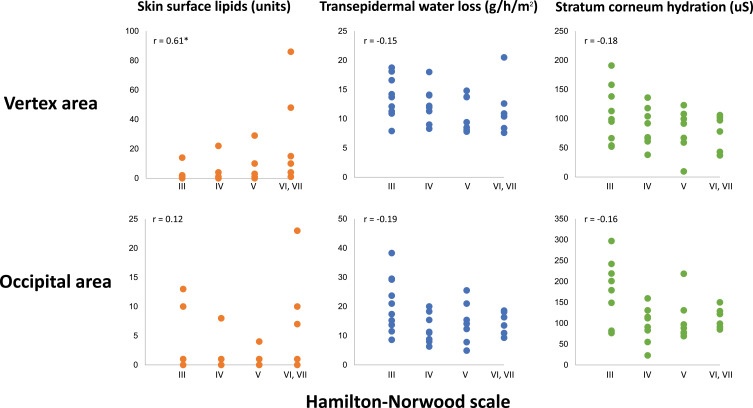
Participants with AGA were further categorized based on disease severity (Hamilton–Norwood classification). The overall comparisons among AGA subgroups and pairwise comparisons between each severity subgroup revealed no statistically significant differences in any of the measured values. Almost all parameters including TEWL, SCH, and occipital SSL revealed weak correlations with AGA severity. However, only vertex SSL showed a statistically significant positive correlation with AGA severity (r = 0.61, p = 0.03) (Figure 2). No correlation was found between the parameters and increasing age in both groups (all r < 0.1).
Figure 2.
Scatter plots of skin surface lipids, transepidermal water loss, and stratum corneum hydration measured at vertex and occipital areas of males with androgenetic alopecia, showing correlation coefficient (r). *Statistically significant.
Comparisons of Scalp Biophysical Characteristics Between AGA and Control Groups
Comparing measured values between AGA and control groups, median SSL on both vertex and occipital areas in the AGA group was higher compared to controls, but only vertex SSL was significantly different (p = 0.03). In contrast, mean TEWL was lower in the AGA group than in controls for both the vertex and occipital areas, with the vertex presenting a highly significant difference (p < 0.001). Mean SCH was lower in the AGA group than in controls for both areas; notably, mean vertex SCH tended to be lower than occipital SCH for both groups (Table 2).
Table 2.
Comparison of Measured Values Between Androgenetic Alopecia and Control Groups
| Biophysical Parameter | AGA N = 31 | Control N = 31 | p value |
|---|---|---|---|
| Skin surface lipids, median (IQR) | |||
| Vertex area | 8 (1–29) | 2 (1–9) | 0.03* |
| Occipital area | 3 (1–10) | 2 (1–8) | 0.32 |
| Transepidermal water loss, mean (SD) | |||
| Vertex area | 16.35 (5.15) | 21.26 (6.26) | <0.001* |
| Occipital area | 18.14 (4.20) | 20.64 (5.98) | 0.93 |
| Stratum corneum hydration, mean (SD) | |||
| Vertex area | 87.84 (18.75) | 97.17 (20.8) | 0.07 |
| Occipital area | 105.96 (29.29) | 109.32 (31.01) | 0.41 |
Note: *Statistically significant.
Abbreviations: AGA, androgenetic alopecia; IQR, interquartile range; SD, standard deviation.
Discussion
Our study revealed differences in biophysiological characteristics between androgen-sensitive and androgen-insensitive scalp areas of individuals with AGA. The vertex had significantly higher SSL and lower SCH compared to the occipital scalp. Our results are consistent with a previous study, which also reported higher SSL and lower SCH for androgen-sensitive vs androgen-insensitive scalps.16 Furthermore, our study is the first to compare the scalp profiles of AGA patients and healthy controls. We demonstrated significant differences between the two groups in terms of higher vertex SSL and lower vertex TEWL in AGA patients, but comparable occipital area values. Our findings indicate alterations of skin barrier function in the scalps of AGA patients.
SSL comprises lipid components that originate in the epidermal lamellar bodies and sebaceous glands.17,18 It evenly overlays SC surface, providing specialized protective functions and regulating permeability barrier homeostasis.17,19 Our study demonstrated several SSL alterations on AGA-affected scalps: (1) significantly higher vertex SSL as compared to occipital SSL on AGA scalps; (2) positive correlation of SSL with AGA severity; and (3) significantly higher vertex SSL for the AGA group as compared to controls. We postulate that the role of androgens in AGA pathogenesis could explain our findings. Sawaya et al reported greater 5-AR enzymatic activity increasing conversion of androgens to DHT at AGA-affected areas.20 Moreover, sebaceous glands on balding scalps reveal higher binding affinity for DHT and express significantly higher ARs than healthy scalps.13,14 Apart from promoting follicular miniaturization, DHT also up-regulates sebocyte differentiation, leading to increased size of sebaceous glands in AGA-affected scalps.21 Kim et al confirmed these effects by demonstrating significant sebaceous gland enlargement and decreased hair follicle size in frontal and vertex areas compared to occipital scalp using serial cross-sectional histology and three-dimensional reconstruction software.22 Sebum production was found to be under hormonal control, and it increases with androgen activity.12 Therefore, AGA-affected areas show increased DHT activity and larger sebaceous glands producing more sebum, possibly resulting in increased SSL.
TEWL refers to the amount of water evaporating through the epidermis due to the water vapor pressure gradient across the skin. It varies across different body areas and can be affected by multiple factors. SC thickness influences skin TEWL and may explain our finding of lower vertex TEWL in AGA patients. Ya-Xian et al reported a negative correlation between number of corneocyte layers and TEWL, indicating that water barrier function of the skin related to SC thickness.10 Czekalla et al demonstrated the effect of UV radiation by showing that sun-exposed skin has a thicker epidermis and SC than sun-protected areas.23 In our study, AGA subjects showed lower vertex TEWL than occipital TEWL. Further, we found significantly lower vertex TEWL for AGA patients as compared with controls. Vertex scalp is more commonly exposed to UV than the occipital area due to perpendicularity of the scalp surface to light. In addition, AGA patients have reduced hair in this area. We theorize that vertex scalps with high UV exposure could result in SC thickening and eventually decreased TEWL. Furthermore, reduction of hair density and diameter on AGA-affected scalps may diminish TEWL since a meta-analysis reported a trend to decrease TEWL among body areas with a lower number of hair follicles.24
The quantity of water contained in SC, or SCH, is crucial for maintaining normal biophysiological functioning of the epidermis. Skin water content is altered by several factors, including an individual’s volume status, microcirculation, UV exposure, surrounding temperature, and humidity.25 Our study revealed significantly lower vertex compared to occipital SCH in AGA patients, but no significant difference in SCH between AGA and control groups. Scalp surface occlusion and UV exposure could explain our results. Kleesz et al reported that skin occlusion increased SCH approximately 30%, while SC thickening decreased SCH 10–20%.11 Compared to the occipital area, balding scalps have reduced hair coverage providing relatively less occlusive effects. As a result, there is more UV exposure and consequent increased SC thickness.23 This leads to significantly lower vertex as compared to occipital SCH in AGA patients. In the control group, SCH values echoed the AGA group, with vertex SCH lower than that of the occipital scalp, although this was not statistically significant. These findings may indicate scalp protective properties of hair fibers from the environment.
Evidence suggests that altering the biophysiological profiles of AGA-affected scalps impacts disease severity. Lai et al reported that AGA patients frequently presented with oily scalps, and this was associated with moderate to severe AGA.26 Their findings correspond with ours: increased vertex SSL in the AGA group, and a positive correlation between SSL and AGA severity. In addition to genetic susceptibility and androgen involvement, scalp microinflammation might also contribute to AGA pathogenesis, accelerating disease onset and increasing its severity.27–31 Gatherwright et al conducted a study of 92 male identical twins who developed AGA to different stages; the presence of inflammatory scalp correlated with more severe AGA.32 Skin with low SCH exhibits increased levels of local and systemic pro-inflammatory cytokines.33–35 In contrast, SCH improvement appears to reduce these inflammatory substances.36 Therefore, prescribing optimal hair and scalp products that reduce SSL and enhance SCH alongside standard medical treatment could improve treatment outcomes.
The limitations of this study include the relatively small number of subjects, homogenous population (Asians), and the single center nature, as such, the results might not represent the general population. A multicenter study conducted with a larger subject group in different ethnicities would minimize these limitations. For more precise results, participants should be instructed to use the same shampoo/scalp cleanser for 1–2 weeks, with the last shampoo performed 24 hours before the measurement. Besides, variables such as occupations, UV exposure, diet, and daily activities should be collected since they may affect individuals’ scalp biophysical properties. Assessing skin thickness using scalp histopathology and/or ultrasonography could help better understanding scalp biophysical alterations. In addition, further research is required to elucidate whether biophysical parameters, especially SSL, are associated with AGA severity.
Conclusion
This study furthers our understanding of the biophysical and physiological characteristics of AGA-affected scalps and how they differ from non-AGA-affected scalps. The information reported herein could provide important reference values for further research of AGA in Asian populations. Moreover, our findings could guide development of optimal hair and scalp care products to improve treatment outcomes in patients.
Funding Statement
The authors received no financial support for this research.
Data Sharing Statement
All datasets are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Mahidol University Institutional Review Board for Ethics in Human Research (MURA2019/308) and was performed in accordance with the Helsinki Declaration. Written informed consent was received from each subject before enrolment.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ellis JA, Stebbing M, Harrap SB. Genetic analysis of male pattern baldness and the 5alpha-reductase genes. J Invest Dermatol. 1998;110(6):849–853. doi: 10.1046/j.1523-1747.1998.00224.x [DOI] [PubMed] [Google Scholar]
- 2.Dallob AL, Sadick NS, Unger W, et al. The effect of finasteride, a 5 alpha-reductase inhibitor, on scalp skin testosterone and dihydrotestosterone concentrations in patients with male pattern baldness. J Clin Endocrinol Metab. 1994;79(3):703–706. doi: 10.1210/jcem.79.3.8077349 [DOI] [PubMed] [Google Scholar]
- 3.Suchonwanit P, Iamsumang W, Leerunyakul K. Topical finasteride for the treatment of male androgenetic alopecia and female pattern hair loss: a review of the current literature. J Dermatolog Treat. 2020;1–6. doi: 10.1080/09546634.2020.1782324 [DOI] [PubMed] [Google Scholar]
- 4.Suchonwanit P, Srisuwanwattana P, Chalermroj N, Khunkhet S. A randomized, double-blind controlled study of the efficacy and safety of topical solution of 0.25% finasteride admixed with 3% minoxidil vs. 3% minoxidil solution in the treatment of male androgenetic alopecia. J Eur Acad Dermatol Venereol. 2018;32(12):2257–2263. doi: 10.1111/jdv.15171 [DOI] [PubMed] [Google Scholar]
- 5.Suchonwanit P, Rojhirunsakool S, Khunkhet S. A randomized, investigator-blinded, controlled, split-scalp study of the efficacy and safety of a 1550-nm fractional erbium-glass laser, used in combination with topical 5% minoxidil versus 5% minoxidil alone, for the treatment of androgenetic alopecia. Lasers Med Sci. 2019;34(9):1857–1864. doi: 10.1007/s10103-019-02783-8 [DOI] [PubMed] [Google Scholar]
- 6.Iamsumang W, Leerunyakul K, Suchonwanit P. Finasteride and its potential for the treatment of female pattern hair loss: evidence to date. Drug Des Devel Ther. 2020;14:951–959. doi: 10.2147/DDDT.S240615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suchonwanit P, Iamsumang W, Rojhirunsakool S. Efficacy of topical combination of 0.25% finasteride and 3% minoxidil versus 3% minoxidil solution in female pattern hair loss: a Randomized, Double-Blind, Controlled Study. Am J Clin Dermatol. 2019;20(1):147–153. doi: 10.1007/s40257-018-0387-0 [DOI] [PubMed] [Google Scholar]
- 8.Rojhirunsakool S, Suchonwanit P. Parietal scalp is another affected area in female pattern hair loss: an analysis of hair density and hair diameter. Clin Cosmet Investig Dermatol. 2018;11:7–12. doi: 10.2147/CCID.S153768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meephansan J, Thummakriengkrai J, Ponnikorn S, Yingmema W, Deenonpoe R, Suchonwanit P. Efficacy of topical tofacitinib in promoting hair growth in non-scarring alopecia: possible mechanism via VEGF induction. Arch Dermatol Res. 2017;309(9):729–738. doi: 10.1007/s00403-017-1777-5 [DOI] [PubMed] [Google Scholar]
- 10.Ya-Xian Z, Suetake T, Tagami H. Number of cell layers of the stratum corneum in normal skin - relationship to the anatomical location on the body, age, sex and physical parameters. Arch Dermatol Res. 1999;291(10):555–559. doi: 10.1007/s004030050453 [DOI] [PubMed] [Google Scholar]
- 11.Kleesz P, Darlenski R, Fluhr JW. Full-body skin mapping for six biophysical parameters: baseline values at 16 anatomical sites in 125 human subjects. Skin Pharmacol Physiol. 2012;25(1):25–33. doi: 10.1159/000330721 [DOI] [PubMed] [Google Scholar]
- 12.Suchonwanit P, Triyangkulsri K, Ploydaeng M, Leerunyakul K. Assessing biophysical and physiological profiles of scalp seborrheic dermatitis in the Thai population. Biomed Res Int. 2019;2019:5128376. doi: 10.1155/2019/5128376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Long D, Wang S. [Determination of scalp ER and AR of patients with male pattern baldness and the effects of hair transplantation on them]. Zhonghua Zheng Xing Wai Ke Za Zhi. 2000;16(2):105–107. Chinese. [PubMed] [Google Scholar]
- 14.Sawaya ME, Honig LS, Hsia SL. Increased androgen binding capacity in sebaceous glands in scalp of male-pattern baldness. J Invest Dermatol. 1989;92(1):91–95. doi: 10.1111/1523-1747.ep13071290 [DOI] [PubMed] [Google Scholar]
- 15.Leerunyakul K, Suchonwanit P. Asian hair: a review of structures, properties, and distinctive disorders. Clin Cosmet Investig Dermatol. 2020;13:309–318. doi: 10.2147/CCID.S247390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Goshi K, Iguchi M, Tagami H. Functional analysis of the stratum corneum of scalp skin: studies in patients with alopecia areata and androgenetic alopecia. Arch Dermatol Res. 2000;292(12):605–611. doi: 10.1007/s004030000185 [DOI] [PubMed] [Google Scholar]
- 17.Wertz PW. Lipids and the permeability and antimicrobial barriers of the skin. J Lipids. 2018;2018:5954034. doi: 10.1155/2018/5954034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harnchoowong S, Suchonwanit P. PPAR-γ agonists and their role in primary cicatricial alopecia. PPAR Res. 2017;2017:2501248. doi: 10.1155/2017/2501248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55(6):e338–343. doi: 10.1111/ijd.13061 [DOI] [PubMed] [Google Scholar]
- 20.Sawaya ME, Honig LS, Garland LD, Hsia SL. Delta 5-3 beta-hydroxysteroid dehydrogenase activity in sebaceous glands of scalp in male-pattern baldness. J Invest Dermatol. 1988;91(2):101–105. doi: 10.1111/1523-1747.ep12463393 [DOI] [PubMed] [Google Scholar]
- 21.Barrault C, Garnier J, Pedretti N, et al. Androgens induce sebaceous differentiation in sebocyte cells expressing a stable functional androgen receptor. J Steroid Biochem Mol Biol. 2015;152:34–44. doi: 10.1016/j.jsbmb.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 22.Kim JN, Lee JY, Shin KJ, Gil YC, Koh KS, Song WC. Morphological and morphometric study of the androgenetic alopecic scalp using two- and three-dimensional analysis comparing regional differences. Br J Dermatol. 2014;170(6):1313–1318. doi: 10.1111/bjd.12842 [DOI] [PubMed] [Google Scholar]
- 23.Czekalla C, Schonborn KH, Lademann J, Meinke MC. Noninvasive determination of epidermal and stratum corneum thickness in vivo using two-photon microscopy and optical coherence tomography: impact of body area, age, and gender. Skin Pharmacol Physiol. 2019;32(3):142–150. doi: 10.1159/000497475 [DOI] [PubMed] [Google Scholar]
- 24.Akdeniz M, Gabriel S, Lichterfeld-Kottner A, Blume-Peytavi U, Kottner J. Transepidermal water loss in healthy adults: a systematic review and meta-analysis update. Br J Dermatol. 2018;179(5):1049–1055. doi: 10.1111/bjd.17025 [DOI] [PubMed] [Google Scholar]
- 25.Verdier-Sevrain S, Bonte F. Skin hydration: a review on its molecular mechanisms. J Cosmet Dermatol. 2007;6(2):75–82. doi: 10.1111/j.1473-2165.2007.00300.x [DOI] [PubMed] [Google Scholar]
- 26.Lai CH, Chu NF, Chang CW, et al. Androgenic alopecia is associated with less dietary soy, lower [corrected] blood vanadium and rs1160312 1 polymorphism in Taiwanese communities. PLoS One. 2013;8(12):e79789. doi: 10.1371/journal.pone.0079789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahé YF, Michelet JF, Billoni N, et al. Androgenetic alopecia and microinflammation. Int J Dermatol. 2000;39(8):576–584. doi: 10.1046/j.1365-4362.2000.00612.x [DOI] [PubMed] [Google Scholar]
- 28.Katzer T, Leite Junior A, Beck R, da Silva C. Physiopathology and current treatments of androgenetic alopecia: going beyond androgens and anti-androgens. Dermatol Ther. 2019;32(5):e13059. doi: 10.1111/dth.13059 [DOI] [PubMed] [Google Scholar]
- 29.Chanasumon N, Sriphojanart T, Suchonwanit P. Therapeutic potential of bimatoprost for the treatment of eyebrow hypotrichosis. Drug Des Devel Ther. 2018;12:365–372. doi: 10.2147/DDDT.S156467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sriphojanart T, Khunkhet S, Suchonwanit P. A retrospective comparative study of the efficacy and safety of two regimens of diphenylcyclopropenone in the treatment of recalcitrant alopecia areata. Dermatol Rep. 2017;9(2):7399. doi: 10.4081/dr.2017.7399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Triyangkulsri K, Suchonwanit P. Role of janus kinase inhibitors in the treatment of alopecia areata. Drug Des Devel Ther. 2018;12:2323–2335. doi: 10.2147/DDDT.S172638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gatherwright J, Liu MT, Amirlak B, Gliniak C, Totonchi A, Guyuron B. The contribution of endogenous and exogenous factors to male alopecia: a study of identical twins. Plast Reconstr Surg. 2013;131(5):794e–801e. doi: 10.1097/PRS.0b013e3182865ca9 [DOI] [PubMed] [Google Scholar]
- 33.Kikuchi K, Kobayashi H, Hirao T, Ito A, Takahashi H, Tagami H. Improvement of mild inflammatory changes of the facial skin induced by winter environment with daily applications of a moisturizing cream. A half-side test of biophysical skin parameters, cytokine expression pattern and the formation of cornified envelope. Dermatology. 2003;207(3):269–275. doi: 10.1159/000073089 [DOI] [PubMed] [Google Scholar]
- 34.Yalçin B, Tamer E, Toy GG, Oztaş P, Hayran M, Alli N. The prevalence of skin diseases in the elderly: analysis of 4099 geriatric patients. Int J Dermatol. 2006;45(6):672–676. doi: 10.1111/j.1365-4632.2005.02607.x [DOI] [PubMed] [Google Scholar]
- 35.Hu L, Mauro TM, Dang E, et al. Epidermal dysfunction leads to an age-associated increase in levels of serum inflammatory cytokines. J Invest Dermatol. 2017;137(6):1277–1285. doi: 10.1016/j.jid.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye L, Mauro TM, Dang E, et al. Topical applications of an emollient reduce circulating pro-inflammatory cytokine levels in chronically aged humans: a pilot clinical study. J Eur Acad Dermatol Venereol. 2019;33(11):2197–2201. doi: 10.1111/jdv.15540 [DOI] [PMC free article] [PubMed] [Google Scholar]