Abstract
Glucose-6-phospate dehydrogenase (G6PD) deficiency is estimated to affect more than 400 million people world-wide. This X-linked genetic deficiency puts stress on red blood cells (RBC), which may be further augmented under certain pathophysiological conditions and drug treatments. These conditions can cause hemolytic anemia and eventually lead to multi-organ failure and mortality. G6PD is involved in the rate-limiting step of the pentose phosphate pathway, which generates reduced nicotinamide adenine dinucleotide phosphate (NADPH). In RBCs, the NADPH/G6PD pathway is the only source for recycling reduced glutathione and provides protection from oxidative stress.
Susceptibility of G6PD deficient populations to certain drug treatments and potential risks of hemolysis are important public health issues. A number of clinical trials are currently in progress investigating clinical factors associated with G6PD deficiency, validation of new diagnostic kits for G6PD deficiency, and evaluating drug safety, efficacy, and pathophysiology. More than 25 clinical studies in G6PD populations are currently in progress or have just been completed that have been examined for clinical pharmacology and potential therapeutic implications of G6PD deficiency. The information on clinical conditions, interventions, purpose, outcome, and status of these clinical trials has been studied. A critical review of ongoing clinical investigations on pharmacology and therapeutics of G6PD deficiency should be highly important for researchers, clinical pharmacologists, pharmaceutical companies, and global public health agencies. The information may be useful for developing strategies for treatment and control of hemolytic crisis and potential drug toxicities in G6PD deficient patients.
Keywords: Glucose-6-Phosphate Dehydrogenase, hemolytic anemia, malaria, G6PD deficiency
1. PATHOPHYSIOLOGY
Glucose-6-phospate dehydrogenase (G6PD) is an enzyme that protects all cells in the body, including red blood cells (RBC), from oxidative stress. This enzyme is the rate-limiting step of the pentose phosphate pathway (PPP) and supplies reducing energy to maintain levels of nicotinamide adenine dinucleotide phosphate (NADPH) (May, et al., 2019). The resultant NADPH is utilized in several metabolic functions, most importantly for generation of reduced glutathione (GSH), which is an antioxidant responsible for protecting cells from damaging reactive oxygen species (ROS). In some people this enzyme is defective due to genetic mutations/defects, leading to substantially decreased activity levels, diagnosed as G6PD deficiency. G6PD deficiency is estimated to affect more than 400 million people around the world especially those from African, Middle Eastern, and South Asian descent (May, et al., 2019). This enzyme deficiency results from a mutation on the X-chromosome so the disease can present differently in women versus men. Clinically, homozygous women present the same as men. Heterozygous women can show symptoms of the disease due to lyonization, inactivation of an X-chromosome, which leads to a mix of normal and G6PD deficient RBC. Mutation of the Xq28 band leads to different variations of G6PD deficiency. The most common variants are G6PD A(−) and G6PD Mediterranean (Cappellini & Fiorelli, 2008). The World Health Organization classification broadly divides G6PD deficiency into five classes based on enzyme activity with Class 1 being the most severe (<10% enzyme activity, chronic hemolytic anemia) to Class V being the least severe with no clinical manifestations.
Under normal conditions, G6PD deficiency does not have a noticeable clinical impact on patients. However, the G6PD pathway is the only source for NADPH and recycling GSH in RBC, so G6PD deficiency can lead to free radical damage under the pathophysiological and pharmacological conditions of high oxidative stress (Anantasomboon, et al., 2019; Hwang, et al., 2018). This results in a higher exposure risk to hemolytic anemia for G6PD deficient patients. There is evidence suggesting hemolytic crisis due to depleted GSH is both intravascular and extravascular evidenced by hemoglobinuria and hyperbilirubinemia (Luzzatto & Seneca, 2014; Rueangweerayut, et al., 2017). In G6PD deficient patients, hemolytic anemia develops following exposure to several pharmacological, physiological, and pathophysiological stressors such as ingestion of fava beans, mild viral infection, or exposure to certain drugs and can eventually lead to multi-organ failure and mortality (Dunyo, et al., 2011; Hygiene, Medicine, Council, & National Malaria Control Programme, 2004; Kiessling, et al., 2018; La Vieille, Lefebvre, Khalid, Decan, & Godefroy, 2019; Richardson & O'Malley, 2020; Sharma, et al., 2018). While there has been speculation that other foods and food colorings, such as blueberries, could contribute to hemolytic anemia in G6PD deficient patients, no conclusive data has shown a relationship between hemolysis and any food other than fava beans (Babu, Panachiyil, Sebastian, & Ravi, 2019; La Vieille, et al., 2019). Oxidative drugs like dapsone can cause hemolytic anemia in G6PD normal as well as deficient patients but the severity may be higher for G6PD deficient individuals than G6PD normal patients (Cappellini & Fiorelli, 2008; Lee & Geetha, 2015; Luzzatto, Nannelli, & Notaro, 2016; Luzzatto & Seneca, 2014). 8-aminoquinolines, a family of antimalarial drugs including Primaquine (PQ), have been shown to cause hemolytic anemia in G6PD deficient patients and even G6PD intermediate patients at high doses (Chu, Bancone, Nosten, White, & Luzzatto, 2018).
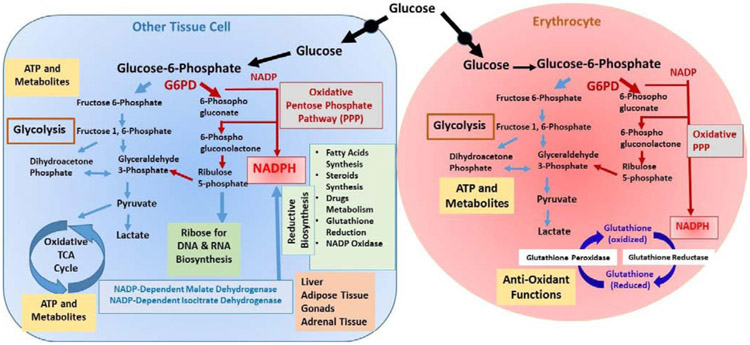
The NADPH/G6PD pathway in cells other than RBC is associated with a number of complex metabolic networks including glycolysis, the PPP, GSH homeostasis, and purine metabolism (Figure 1) (Luzzatto, et al., 2016). Besides its’ role in GSH mediated anti-oxidant defense functions, the NADPH cofactor provides high energy electrons for reductive biosynthesis pathways in other tissue cells. For example, biosynthesis of fatty acids, steroids, and proline especially in hepatic cells, adipose tissues, gonads and adrenal cortex (Figure 1). NADPH is also required for metabolic biotransformation of therapeutic drugs, xenobiotics, and environmental toxicants. In most of the nucleated cells the NADP recycling functions and lack of G6PD can be compensated by the cytosolic NADP-dependent malic enzyme 1 (ME1) and isocitrate dehydrogenase 1 (IDH1) with minimal pathophysiological consequences. The G6PD-mediated PPP is uniquely important for maintaining a normal physiological NADPH/NADP ratio in mammalian cells. The PPP is the largest source of cytosolic NADPH production in most mammalian cells. A recent study involving CRISPR deletion of G6PD, ME1, and IDH1 in HCT116 colon cancer cells suggested that loss of G6PD results in increased levels of cytosolic NADP. High levels of cytosolic NADP resulted in an increase in ME1 and IDH1 but inhibited dihydrofolate reductase and impaired folate-mediated functions in G6PD deletion mutants (L. Chen, et al., 2019). An increased expression of G6PD and translocation of the enzyme from the cytosol to the cell membrane was reported in adult cardiomyocytes experiencing oxidative stress (Jain et al. 2003). These studies suggest broader pathophysiological and clinical consequences of G6PD deficiency in other tissues besides erythrocytes.
Figure 1-. Pathways for pathophysiology of Glucose 6-phosphate dehydrogenase deficiency.
The metabolic pathways associated with glucose 6-phosphate dehydrogenase (G6PD) are illustrated in an erythrocyte (red) and a nucleated cell in tissue. The NADPH provides high energy electrons for antioxidant defense (in erythrocytes and other cells), reductive biosynthesis (in most nucleated cells), Cytochrome P450 mediated metabolism of drugs and xenobiotics (predominantly in hepatocytes), and NADPH oxidase function for generation of superoxides (phagocytic cells). In erythrocytes, the G6PD mediated oxidative Pentose phosphate pathway (PPP) is the sole source of NADPH. In other cells, cytosolic NADP-dependent malate dehydrogenase and isocitrate dehydrogenase can also recycle NADP to NADPH and may partly compensate G6PD function. However, the PPP is uniquely required in these cells to maintain a normal NADPH/NADP ratio so G6PD deficiency may have broad pathophysiological consequences (L. Chen, et al., 2019).
This review specifically aimed to compile and summarize recent reports and investigations on clinical pharmacology including clinical trials on individuals and populations with G6PD deficiency. Some recently completed clinical investigations on clinical pharmacology of G6PD deficiency reviewed here can be found in the supplemental table. A comprehensive up-to-date analysis has been made, especially regarding current tools under clinical evaluation for the diagnosis of G6PD deficiency, recent drug trials in G6PD deficient populations, and approaches for treatment of G6PD deficiency. Both recently completed and ongoing clinical trials are included here.
2. ASSOCIATION OF G6PD DEFICIENCY WITH CLINICAL CONDITIONS
Individuals with G6PD deficiency are mostly asymptomatic under normal circumstances. Primary clinical manifestations of G6PD deficiency are (a) neonatal jaundice, (b) acute hemolytic anemia (AHA) triggered by infections or treatment with certain drugs, exposure to environmental pollutants, and dietary consumption, and (c) chronic non-spherocytic hemolytic disease. Associations between G6PD deficiency and pathophysiology of other clinical conditions have also been reported.
Cardiovascular Disease
G6PD deficiency has been linked to pathophysiology of some cardiovascular conditions. Reduced levels of cytosolic GSH lead to impaired cardiomyocyte function and G6PD deficient mouse models experience cardiac dysfunction during reperfusion due to high levels of ROS (M. Jain, et al., 2003; M. Jain, et al., 2004). Reduced nitric oxide production and increased oxidant stress from lack of G6PD activity can lead to an influx of macrophages, cytokines, and other inflammatory response mechanisms causing atherosclerosis and cardiovascular disease (CVD) (Parsanathan & Jain, 2020; Pes, Parodi, & Dore, 2019; Thomas, et al., 2018). A U.S. military cross-sectional database study showed an almost 40% increased risk of developing CVD for G6PD deficient patients without accounting for sex, race, or other health conditions such as hypertension or CVD risk therapy (Thomas, et al., 2018). Additionally, a retrospective case-control study on a Northern Sardinian population, using samples from digestive endoscopy, found a 71% increased risk of CVD in patients with G6PD deficiency compared to G6PD normal patients after accounting for known risk factors. These findings contradicted a previous non-randomized study that showed a protective effect of qualitatively determined G6PD deficiency on CVD (Pes, Parodi, et al., 2019). It has been suggested that the association between increased risk of CVD and G6PD deficiency may be due to reduced function of protective pathways against oxidative stress, particularly in the early stages of atherogenesis (Parsanathan & Jain, 2020; Pes, Parodi, et al., 2019).
Diabetes
Recently, a meta-analysis found an increased risk of diabetes associated with G6PD deficiency related to defective vasodilation and atherosclerosis (Lai, Lai, & Lee, 2017; Parsanathan & Jain, 2020). A number of mouse models have shown links between G6PD deficiency and diabetes (H. C. Yang, et al., 2019). The effects of diabetes in obese patients following gastric bypass surgery on G6PD deficiency have also been studied and these data show increased G6PD enzyme activity in type 2 diabetic patients compared to non-diabetic patients suggesting a correlation between G6PD activity and diabetes (Alabama, 2009). Based on a number of case studies, it has been suggested that increased risk of hemolysis in diabetic G6PD deficient patients is due to ketoacidosis however, a study on G6PD deficient patients with the Mediterranean mutant found no link between diabetic ketoacidosis and hemolysis (Mehta, Srivastav, Bhate, Gupta, & Nadkar, 2017; Shalev, Wollner, & Menczel, 1984). In obese mice, G6PD deficiency was associated with improved insulin resistance and an overall decrease in inflammatory signaling (H. C. Yang, et al., 2019). A recent study reported lower hemoglobin A1C (HbA1C), a glycemic indicator of diabetes, in Asian G6PD deficient males with the Canton and Kaiping variants. As this measurement is typically used to diagnose diabetes and pre-diabetes, it is suggested that fasting glucose measurements be used for accurate diagnosis in G6PD deficient patients. Additionally, management of diabetes using HbA1C measurements with certain G6PD deficient variants may not accurately reflect the disease state (Leong, et al., 2020).
Neonatal Jaundice/Sepsis
Hemolytic anemia from G6PD deficiency is believed to be associated with neonate jaundice, which is a sign of hyperbilirubinemia and can cause damage to the nervous system especially in neonates (Clinical Research Collaborative Group of Yinzhihuang Oral, 2011; InfaCare Pharmaceuticals Corporation & Mallinckrodt, 2013; Southern Medical University, 2015). A clinical study on 100 newborns with moderate to severe indirect hyperbilirubinemia and 50 normal neonates found an association between G6PD deficiency and neonatal hyperbilirubinemia (Eissa, Haji, & Al-Doski, 2019). A recent cross-sectional study on ethnic Egyptian neonates with indirect hyperbilirubinemia reported higher levels of serum bilirubin in neonates with G6PD deficiency (Kasemy, Bahbah, El Hefnawy, & Alkalash, 2020). However, another clinical trial looked at the prevalence of G6PD deficiency among other disease-causing gene mutations in neonates with severe hyperbilirubinemia but did not find a significant correlation between G6PD deficiency and hyperbilirubinemia despite additional disease contributors in the mutant population (Mednax Center for Research & Safety, 2006; Watchko, et al., 2009). A study in Iran did find G6PD deficiency was more prevalent in neonates admitted to the hospital for sepsis compared to the control group. The study had relatively small case and control populations, so while G6PD deficiency was significantly higher in the sepsis group, only 5% of patients had severe G6PD deficiency and 9% had moderate deficiency. The authors suggested that G6PD deficiency could be affecting white blood cell NADPH levels or increasing serum iron levels due to hemolysis, either of which could increase risk of sepsis (Zekavat, Makarem, Bahrami, Dastgheib, & Dehghani, 2019).
Cancer
A link between G6PD deficiency and cancer has also been suggested due to downregulation of the PPP. The PPP is normally preferred by cancer cells as it produces high levels of NADPH necessary for rapid growth. Abnormalities in redox signaling due to G6PD deficiency affect cell survival and apoptosis and have been implicated in the progression and outcomes of multiple types of cancers (Ghergurovich, et al., 2020; H. C. Yang, et al., 2019). Data from upper and lower endoscopy samples from Northern Sardinians was analyzed to determine the effect of G6PD deficiency on different forms of cancer (Pes, Errigo, Soro, Longo, & Dore, 2019). Upper endoscopy samples showed G6PD deficiency was inversely associated with hepatocellular carcinoma, decreasing risk by 55% regardless of age and gender (Dore, Vidili, Marras, Assy, & Pes, 2018). Upper and lower endoscopy samples showed a decreased risk for endodermal, gastric, and colorectal cancer in G6PD deficient patients compared to controls after adjusting for age, sex, smoking habits, diabetes, and socioeconomic status. However, G6PD deficiency did not appear to impact the risk of developing ectodermal/mesodermal related cancers such as breast, prostate, lung, and hematopoietic cancers (Pes, Errigo, et al., 2019). Additionally, a study involving retrospective analysis of the Taiwan National Health Insurance Research Database, from which 4,066 G6PD deficient patients and 16,264 controls were analyzed, suggested an increased risk of brain tumor development in G6PD deficient patients. Increased G6PD expression in grade II and III glioma was associated with poor patient survival but had no effect in glioblastoma (C. A. Yang, Huang, Lin, & Chang, 2018). A mouse model utilizing a p53 and K-Ras G12D deficiency for non-small-cell lung cancer found no effect from knockout of the G6PD enzyme on the formation or proliferation of tumors. There was only a moderate association between G6PD levels and cancer progression in regards to lung and breast cancers (Ghergurovich, et al., 2020).
Malaria
G6PD deficiency and other blood disorders are suggested to provide protection from some malarial infections. Lack of anti-oxidant defenses causes stress and damage to RBC allowing for destruction by phagocytosis. It has been suggested that parasite-infected RBC are included in this group and also destroyed by the body, breaking the chain of intra-erythrocytic growth of the malaria parasites (Hwang, et al., 2018). Removal of the infected along with the damaged RBC allows for enhanced P. falciparum protection potentially associated with some RBC disorders such as sickle cell, hemoglobin C, and G6PD deficiency (Agarwal, et al., 2000; Allergy, Diseases, & Center, 2005; Ayi, Turrini, Piga, & Arese, 2004). Hemoglobin C and sickle-cell trait in children from Mali provided protection from malaria but no effect on infection rates was seen in teenage sickle-cell patients (Allergy, Diseases, & Center, 2008; Lopera-Mesa, et al., 2015). A positive correlation between sickle-cell anemia and G6PD deficiency was found in an Indian population (Shivwanshi, Singh, & Kumar, 2019). The trial in Mali also found G6PD homozygous deficiency significantly reduced malaria risk for girls (Allergy, et al., 2008; Lopera-Mesa, et al., 2015). However, another study has suggested G6PD deficiency increases the risk for developing severe malarial anemia but is protective against cerebral malaria. Note though that those observations may have been a result of collider bias based on exclusion criteria due to G6PD deficient patient covariates (J. A. Watson, et al., 2019). A meta-analysis review of 28 studies did not find convincing evidence that G6PD deficiency provides protection from uncomplicated malaria, despite specific studies in certain countries presenting positively correlated data, and found no association with severe malaria risk (Mbanefo, et al., 2017). A recent study in Zambian children found similar results determining that G6PD normal children with a cytochrome b5 reductase 3 polymorphism were protected from severe malarial anemia but G6PD deficient A+/A− hemizygotes/homozygotes were not (Gordeuk, et al., 2020). A recent decreased risk of P. falciparum malaria infection in African populations suggests a positive selection towards the G6PD A- allele mutant in this area (Liang, et al., 2020). In general, there is great variation regarding risk of malarial infection in G6PD deficient individuals based on genetic and environmental factors (Mbanefo, et al.,2017).
Bacterial/Viral Infections and COVID-19
G6PD deficient cells have a compromised innate immune response due to changes in redox signaling associated with ROS generation. It has been suggested that increased oxidative stress could increase susceptibility to infection (Yen, et al., 2020; Zhao, et al., 2019). This was seen in reproductive age females in China without children where lower G6PD enzyme activity correlated with increased risk of hepatitis B viral infection (Zhao, et al., 2019). Increased risk of infection associated with G6PD deficiency is supported by in vitro data showing monocytes from G6PD deficient patients experienced increased risk of developing dengue virus (Chao, et al., 2008). However, a cross-sectional study on children ages 2-13 years from Myanmar with dengue infection did not show an association between G6PD deficiency and severity of infection, which correlates with other published data (May, et al., 2019). The additional strain on RBC from G6PD deficiency can cause severe hemolysis and multi-organ failure following acute hepatitis A infection despite the lack of complications associated with the infection normally (Sharma, et al., 2018). Infections such as hepatitis, typhoid fever, and pneumonia are also known to induce hemolytic response suggesting G6PD deficient patients with these infections could have a higher risk of multi-organ failure and mortality (Sharma, et al., 2018). Due to the known stress on RBC, one clinical trial looked at the effects of α-lipoic acid supplements given for 4 weeks to G6PD deficient patients on measures of blood redox status such as reduced GSH, uric acid, hemoglobin levels, etc. and found significantly higher levels of uric acid and decreased thiobarbituric acid and protein carbonyls in the G6PD deficient group. Following treatment, both G6PD deficient and normal patients had increased antioxidant capacity and G6PD deficient patients had increased GSH levels suggesting α-lipoic acid could affect redox status (Georgakouli, et al., 2013; Thessaly, 2016). Another trial by the same group aims to look at similar outcomes following N-acetyl Cysteine supplementation in G6PD deficient patients but has not yet begun recruiting (Thessaly, 2020).
Oxidative burst and overproduction of free radical intermediates have been associated with pathogenesis of viral infections including the recently reported COVID-19 infections from SARS-CoV-2. In view of a strong association of G6PD deficiency with oxidative stress, researchers have suggested a potential association of G6PD deficiency with susceptibility to infection with SARS-CoV-2 and pathogenesis of COVID-19 (Wu, et al., 2015). An earlier study reported an enhanced susceptibility to infection with human coronavirus 229E (HCoV 229E) and higher viral load in G6PD deficient human fibroblast cells compared to cells with normal G6PD activity. Increased oxidative stress increased the risk of infection but, increasing G6PD enzyme function with the addition of the antioxidant α-lipoic acid decreased susceptibility to HCoV229E infection (Wu, et al., 2008). Another study reported that G6PD-dependent antiviral response was mediated through downregulation of the NADPH sensor HSCARG and upregulation of NF-κB signaling pathways in the host cells (Wu, et al., 2015). Increased oxidative stress resulting in reduced GSH levels has been shown to be associated with 25-hydroxyvitamin D deficiency. There is a strong belief that vitamin D deficiency may play a role in the severity of COVID-19 infection which disproportionally affects African Americans who have higher G6PD and vitamin D deficiency rates (S. K. Jain & Parsanathan, 2020). COVID-19 patients have increased ROS production and evidence of cytokine storm which would require high levels of antioxidants such as GSH, N-acetyl cysteine, and L-cysteine to combat (S. K. Jain, et al., 2020). Therefore, G6PD deficiency affects the cellular inflammatory response. The decreased antiviral response in G6PD-knockdown A549 cells mediated by tumor necrosis factor alpha (TNF-α) is a result of dysregulated NOX/MAPK/NF-κB/COX-2 signaling (Aydemir & Ulusu, 2020). These reports suggest potential association of G6PD deficiency with an enhanced risk of COVID-19 and other SARS coronavirus infections.
3. DIAGNOSIS OF G6PD DEFICIENCY
Management of G6PD deficiency to prevent hemolytic crisis requires simple, accurate enzyme testing. The gold standard test for G6PD deficiency utilizes spectrophotometry to detect the formation of NADPH from NADP by G6PD, indicated by the change in absorbance over a specific time interval (Alam, Kibria, Jahan, Price, & Ley, 2018). Spectrophotometric G6PD analysis is expensive and not accessible in many clinics, while inter-laboratory variability and lab-specific reference thresholds prove the need for universally accepted clinical enzyme cutoff values from a reliable testing platform (Pfeffer, et al., 2020). Historically, qualitative and point-of-care testing fails to identify intermediate deficiency indicated by 30-70% enzyme activity. However, more recently some point-of-care platforms have shown promise in ameliorating the vast number of issues seen with previously developed G6PD diagnostic tests (Djigo, et al., 2019; Ley, et al., 2019).
Mindray Medical International has developed a fast, large-scale UV enzyme assay using the BS-240 that can measure 40 samples of hemolysate in one hour. Results showed this assay was comparable to a spectrophotometric assay for some patients but still failed to detect intermediate deficiency (Anantasomboon, et al., 2019). A “sample-to-readout” point-of-care biosensor has been developed for direct detection of G6PD in whole blood by visualization using a simple color card. The color paper is stable for 6 weeks and works at a variety of temperatures and humidity levels. Initial diagnosis relies on the color of the sample and the time to colorization detecting deficient, intermediate, normal, and above 100% activity qualitatively at 80% accuracy. This novel G6PD colorimetric paper test could prove to be the best and easiest test for field diagnosis leading to quantitative clinical diagnosis, but the assay has not yet been tested in a clinical setting (White, Keramane, Capretta, & Brennan, 2020).
Two handheld biosensors, the CareStart™ G6PD Biosensor (CSG) by Accessbio and the STANDARD™ G6PD (STG) by SD Biosensor, have been developed for portable, minimally invasive G6PD enzyme level assessment in under five minutes. The CSG tested in the following studies should not be confused with the CareStart™ RDT colorization chamber for rapid, qualitative G6PD normal or deficient diagnosis. For both the CSG and STG, all samples are normalized to hemoglobin measures and there is no evidence that using venous versus capillary blood has an effect on measured G6PD activity (Bancone, et al., 2015; Ley, et al., 2017; Roca-Feltrer, et al., 2014). The CSG shows variable results in different regions due to differences in G6PD genotypes but overall is promising. Diagnostic accuracy of CSG was tested in a study cohort in China of 247 newborns admitted due to jaundice and was compared to qualitative analysis of G6PD enzyme levels and gene mutations. CSG effectively diagnosed all male newborns and 100% of female newborns with less than 60% enzyme activity but only 58.5% of heterozygous females with 60-100% enzyme activity (Yu, et al., 2020). Another study looked at G6PD activity measurements from CSG compared to the fluorescent spot test and a spectrophotometric reference test. CSG showed 97% accuracy at 30% enzyme activity and had good diagnostic ability with 88% accuracy for intermediate and deficient newborns (Pengboon, Thamwarokun, Changsri, Kaset, & Chomean, 2019). A meta-analysis for performance of CSG versus the gold standard on studies published between 2011 and 2019 found similar results. Out of the 8 studies with 5815 participants in total, CSG was determined to be suitable for diagnosis at the 30% threshold level. CSG performed significantly better in males versus females and it had a 99% Negative Predictive Value in samples with residual G6PD activity in the range of 5-30% (Ley, et al., 2019). However, a small trial in the Republic of Sudan tested the CSG against spectrophotometry and found only 11 of 21 patients with less than 60% activity levels were identified correctly as having either severe or intermediate deficiency (Hamid, et al., 2018; M. S. o. H. Research & Khartoum, 2015). A trial in Bangladesh found the sensitivity of the STG and CSG tests at the 70% cutoff of enzyme activity were similar but, the clinically relevant values correlating to the 30-70% enzyme activity window were narrower for the CSG compared to the STG (Alam, Kibria, Jahan, Thriemer, et al., 2018). The STG was tested on patients in the U.S. and Thailand and measurements highly correlated with values from the Pointe Scientific spectrophotometry kits with relatively high sensitivity and specificity values at 30%, 70%, and 80% thresholds across a wide range of temperatures and humidity levels (Pal, et al., 2019). Both tests could be used for field assessment of G6PD activity levels but it seems the STG from SDBiosensor gives more accurate enzyme readings across the entire enzyme level spectrum (Alam, Kibria, Jahan, Thriemer, et al., 2018; Pal, et al., 2019). Each of the G6PD enzyme testing methods discussed here are summarized in Table 1 below. The performance of individual methods of diagnosis of G6PD deficiency regarding sensitivity, specificity, and diagnostic accuracy as presented in Table 1 is subjective and may not be compared quantitatively.
Table 1-.
A comparative review of G6PD diagnostic tests
| Gold Standard |
Fluorescent Spot Test |
CareStart™ RDT |
CareStart™ G6PD Biosensor |
STANDARD™ G6PD by SD Biosensor |
“Sample -to- readout” |
BS-240 | |
|---|---|---|---|---|---|---|---|
| Method | Spectrophotometry | UV fluorescence visualization | Colorization chamber | Biosensor, test strip | Biosensor, test strip | Colorization, fabricated biosensor | Automated UV enzyme system |
| Volume | 10μL | 5μL | 2μL | 5μL | 10μL | 15μL | 20μL |
| Blood | Venous | Venous | Whole | Whole | Venous or capillary | Whole | Hemolys ate venous blood |
| Time | 5-10 min | 10 min + air dry time | 10 min | 4 min | 2 min | 10 min + air dry time | 2 min |
| Temperature | 30°C | Room temperature | Variable | Room temperature | Variable | Room temperature | 37°C |
| Intermediate Reading* | Yes | No | No | Yes | Yes | Yes | No |
| Measurement | Quantitative | Qualitative | Qualitative | Quantitative | Quantitative | Qualitative | Quantitative |
| Sensitivity # | Very High | Med-High | Med-High | High | High | Med-High | Med-High |
| Specificity # | Very High | Med-High | Med-High | High | High | Med-High | Med-High |
| Diagnostic Accuracy# | Very High | Low-Med | Medium | High | Very High | Med-High | Low-Med |
Intermediate readout for the G6PD activity is important for diagnosis on mild/intermediate G6PD deficiency. This provides the scope for determining threshold of G6PD deficiency for safety of therapeutics in G6PD deficient populations.
The performance of individual methods of diagnosis of G6PD deficiency regarding sensitivity, specificity, and diagnostic accuracy is subjective and may not be compared quantitatively.
Conventional classification of G6PD variants is based on residual G6PD activity. Individuals diagnosed with G6PD deficiency from field or laboratory methods are eventually genotyped for confirmation of genetic G6PD deficiency (Alina, et al., 2020). A group in Taiwan used a multiplex SNaPshot assay to detect G6PD activity in newborns from dried blood spot samples based on 21 known G6PD gene mutations. However, this method only detects known mutations (Chiu, et al., 2019). Recent studies in the Guangdong province in China and in Malaysia have used reverse dot blotting to identify variants for diagnosis and genotyping of G6PD deficiency. This method can uncover novel mutations. Identification of a genetic variant is the only definitive determination of carrier status in heterozygous females (Alina, et al., 2020; Lin, Lou, Xing, Zhang, & Yang, 2018).
Recent guidelines for laboratory diagnosis of G6PD deficiency published by the British Society for Haematology include recommendations for specimen collection/storage, measurements of G6PD activity, laboratory diagnosis of G6PD deficiency, factors affecting the clinical interpretation of G6PD deficiency, application of point-of-care tests for diagnosis, molecular diagnosis, and quality assurance for screening tests including the application of an accredited External Quality Assessment. These guidelines are all based on which test is being utilized and will vary among testing platforms (Roper, et al., 2020).
4. MODELS OF G6PD DEFICIENCY
Once G6PD deficiency has been diagnosed, hemolytic drugs need to be avoided by G6PD deficient patients. Assessing the hemolytic potential of newly developed compounds prior to human testing is crucial for creating safe alternatives for G6PD deficient populations. In general, simply incubating drugs known to cause hemolytic toxicity in vivo with human RBC in vitro does not initiate a response indicative of hemolysis. Bloom et al. determined that incubating human RBC with an NADPH generator and induced drug metabolizing microsomes taken from mouse livers resulted in correct identification of known hemolytic compounds 75% of the time (Bloom, Brewer, Magon, & Wetterstroem, 1983). Additional assays have since been developed using the framework of this method to characterize methemoglobin accumulation, ROS formation, and depletion of reduced GSH and total thiol levels by incubating human G6PD deficient RBC with pooled human liver microsomes or recombinant human cytochrome P450, an NADPH regeneration cocktail, and the testing compound (Ganesan, Chaurasiya, Sahu, Walker, & Tekwani, 2012; Ganesan, Sahu, Walker, & Tekwani, 2010; Ganesan, Tekwani, Sahu, Tripathi, & Walker, 2009). These assays, while relatively accurate at identifying hemolytic toxicity, do not account for all in vivo effects.
Total knockout of the G6PD enzyme is lethal but mouse models with reduced enzyme activity comparable to human levels have been developed (Luzzatto, et al., 2016). Originally, an offspring of mouse with an X-linked G6PD deficiency was recovered from a group of mice treated with 1-ethyl-1-nitrosourea that expressed 15-60% enzyme activity (Pretsch, Charles, & Merkle, 1988). Non-obese diabetic/severe combined immunodeficiency mice were engrafted with human normal or G6PD deficient RBC to test hemolytic potential in vivo. Oral ingestion of PQ caused dose-dependent hemolysis evidenced by formation of murine reticulocytes and depletion of human RBC as determined by flow cytometry. Other known hemolytic drugs, such as dapsone, had similar effects while non-hemolytic drugs, like Chloroquine (CQ), did not cause significant changes compared to vehicle controls (Rochford, et al., 2013). Genetic G6PD deficient mice with moderate deficiency similar to the African type A mutant were given PQ, pamaquine (a PQ analog), CQ, and Mefloquine (MQ) to determine hemolytic toxicity. These mice developed hemolytic anemia as anticipated following exposure to PQ and pamaquine evidenced by clinical measures including complete blood count, reticulocyte count, Heinz body counts, and haptoglobin levels. Reduced GSH levels were also decreased. CQ and MQ did not cause a hemolytic effect as expected based on previous clinical exposure in humans (Zhang, et al., 2013). Recently, Kitagawa et al. reported a G6PD deficient rat model with 20% enzyme activity and reduced function of the PPP (Kitagawa, et al., 2020). These animal models can aid in predicting the hemolytic toxicity of newly developed drugs in humans.
5. PHARMACOTHERAPY IN G6PD DEFICIENCY
Under normal circumstances, G6PD deficiency does not cause immediate harm to patients. However, when G6PD individuals are exposed to certain hemolytic drugs the results can range from mild hemolytic anemia to multi-organ failure and mortality (Sharma, et al., 2018). Some common markers of hemolysis namely bilirubin, lactate dehydrogenase, and reticulocytes are increased with decreased haptoglobin (Cappellini & Fiorelli, 2008; Zhang, et al., 2013). There are many published lists of known and suspected hemolytic drugs unsafe for G6PD deficient patients but definitive determination of drug-induced hemolytic anemia is difficult (Cappellini & Fiorelli, 2008; Luzzatto & Seneca, 2014; Renard & Rosselet, 2017). Potentially oxidative drugs causing hemolysis in G6PD deficient patients are prescribed for a range of disease states. However, generally the main focus is on hemolytic drugs used to treat malaria and therefore only those drugs will be reviewed here.
In 2018 alone, malaria was responsible for killing 405,000 people (Prevention, 2019). G6PD deficient populations are especially prevalent in areas with widespread malaria (Rueangweerayut, et al., 2017). Malaria is commonly treated with 8-aminoquinoline drugs alone or in combination usually with artemisinin or dihydroartemisinin-piperaquine (DHAP) therapy depending on the type of infection. The U.S. Centers for Disease Control and Prevention recommends drugs like CQ, MQ, and artemether-lumefantrine (AL) for treatment of the blood stage infection and 8-aminoquinolines like PQ to kill dormant liver stage parasites preventing relapse. 8-aminoquinonlines are the only drug class for radical cure of P. vivax and sterilization of P. falciparum malaria. However, PQ, the most commonly used 8-aminoquinoline, has a well-documented history of hemolytic response in G6PD deficient patients (Pal, et al., 2019; Recht, Ashley, & White, 2018; W. R. A. I. o. Research, 2003; Rueangweerayut, et al., 2017; von Seidlein, et al., 2013). Known toxicity of PQ in G6PD deficient patients forces many areas to forgo PQ use all together due to lack of reliable enzyme testing. This can lead to further spread of malaria and relapse (J. Watson, Taylor, Menard, Kheng, & White, 2017).
PQ toxicity was first discovered during World War II when patients began developing hemolytic anemia and jaundice. This toxicity was eventually linked to a lack of G6PD enzyme in RBC (Luzzatto, et al., 2016). PQ toxicity only occurs in vivo suggesting a metabolite rather than the drug itself is the cause of hemolysis (Luzzatto & Seneca, 2014). While the exact mechanism of parasite clearance is still unknown and many have been hypothesized, inactivation of CYP2D6 impairs PQ function suggesting both the efficacy and toxicity of PQ may result from cytochrome P450 mediated metabolite generation (Daher, et al., 2019). Additional pharmacokinetic (PK) studies on PQ are necessary to determine the mechanism of action so alternative drugs can be developed that are both efficacious and safe for all patients.
One clinical trial was recently completed at the University of Mississippi studying the PK profiles of individual enantiomers of PQ in G6PD normal patients with the goal of determining the safest enantiomers to then study in a G6PD deficient population (University of Mississippi, 2018). A different trial aimed to determine the safety of PQ treatment for P. vivax infection in G6PD normal breastfeeding mothers by studying the PK of drug distribution in the mothers’ blood, breast milk, and the infants’ blood to determine levels of the drug being passed to the child potentially causing hemolysis. This study found very low (<1% of the recommended dose) concentrations of PQ being passed to the infant during the 14 day treatment regimen suggesting PQ can be recommended for breastfeeding women with malaria regardless of the G6PD status of the child (Gilder, et al., 2018; Oxford, 2012). Currently there are two trials in Afghanistan and Brazil recruiting G6PD normal and deficient patients with P. vivax infection which will be treated with CQ in combination with different doses and timelines for PQ administration to study efficacy and PK profiles of drug treatment in regards to G6PD deficiency (Dourado, Foundation, & Tecnológico, 2018; Oxford, Unit, & University, 2016).
PQ is frequently used in combination with other antimalarials as it is highly effective at killing gametocytes but a poor eliminator of blood stage parasites. A single PQ dose is commonly used with schizonticides in artemisinin combination therapies (ACT) to prevent resistance of antimalarials and reduce spread. An in vitro study on P. falciparum gametocytes found a moderate synergistic relationship between PQ and piperaquine, CQ, and MQ. PQ had high synergism with naphthoquine while lumefantrine acted as an antagonist (Cabrera & Cui, 2015). This suggests these combinations may provide the necessary parasite clearance with a single dose of PQ making it safe for G6PD deficient patients. PQ has been studied extensively in combination therapy with other treatments in both G6PD normal and deficient patients with malaria. African G6PD deficient males with P. falciparum treated with a single low dose (SLD) of PQ in combination with either DHAP or AL did experience lower hemoglobin levels and some hemolysis but none was considered moderate or severe. It is not known whether the drop in hemoglobin and mild hemolysis is related to the PQ treatment or the infection. This trial found that the addition of PQ in certain cases substantially increased parasite clearance compared to each treatment (AL or DHAP) alone suggesting lower doses of PQ could be safe and effective for G6PD deficient patients (Bastiaens, et al., 2018; Eziefula, et al., 2014; Hygiene, Medicine, Centre National de Recherche et de Formation sur le Paludisme, & Nijmegen, 2014; Hygiene, Medicine, & Medical Research Council Unit, 2015). Another trial determined that a SLD of PQ tested in combination with DHAP is safe for P. falciparum-infected G6PD deficient patients to prevent the spread of malaria through Cambodia and a trial in Mali found a SLD of PQ between 0.4 and 0.5mg/kg was safe for G6PD deficient men and children without malaria (I. Chen, et al., 2018; Consortium, et al., 2014; Dysoley, et al., 2019; University of California, et al., 2015; Vantaux, et al., 2020). These studies suggest a SLD of PQ could be used in combination therapies even when G6PD testing is not available.
A promising new 8-aminoquinoline called Tafenoquine (TQ) was recently approved for the treatment of blood and liver stage parasites. TQ requires a single dose compared to the 14-day dose of PQ. Studies show this new drug, compared to PQ, does not significantly decrease incidence of hemolysis in G6PD non-deficient or intermediate patients with P. vivax. G6PD normal patients with P. vivax treated with PQ or TQ in combination with CQ experienced slight reductions in hemoglobin levels with both treatments but neither drug performed better than the other in terms of parasite clearance and prevention of relapse (GlaxoSmithKline & Venture, 2015; Llanos-Cuentas, et al., 2019). G6PD normal and intermediate (40-60% activity) females in Thailand experienced a dose dependent increase in hemolysis from TQ. At the therapeutic dose (300mg), the risk of hemolytic response was similar to the risk following a 14-day PQ dose (15mg/day) (Rueangweerayut, et al., 2017). A trial has just been completed monitoring pediatric patients in Colombia and Vietnam that tested positive for P. vivctx and were given an initial dose of CQ then treated with TQ tablets. The results will provide more information on PK profiles associated with TQ up to 120 days following treatment (GlaxoSmithKline & Venture, 2017). It is important to note that while a single dose of TQ may overall have higher parasite clearance rates than PQ treatment due to easier patient adherence to the dosing schedule, if severe hemolytic crisis occurs, PQ can be cleared from the body more quickly than TQ leading to better patient outcomes (Rueangweerayut, et al., 2017).
6. TREATMENT OF G6PD DEFICIENCY
G6PD deficiency causes a wide range of effects in patients and is associated with many different disease states. Hemolytic anemia is the most common life-threatening clinical manifestation of G6PD deficiency which is usually triggered by oxidative stress due to infections or consumption of certain foods and drugs, all of which should be avoided by G6PD deficient patients (Belfield & Tichy, 2018; Bubp, Jen, & Matuszewski, 2015). One of the many well-known factors that cause hemolytic anemia through oxidative stress in G6PD deficient individuals is ingestion of fava beans and as such, hemolytic crisis associated with G6PD deficiency has also been referred to as favism (Belfield & Tichy, 2018). β-Carotene, a precursor of vitamin A, has been used as an antioxidant to decrease oxidative stress and restore G6PD enzyme levels, serum glucose levels, and liver function in favism-induced rats compared to controls. Testicular and blood protein levels returned to normal following oral treatment with β-carotene in a dose-dependent manner suggesting antioxidant treatment for hemolysis (Koriem & Arbid, 2018). However, other studies have shown no effect from the use of antioxidants as treatment for hemolytic anemia from G6PD deficiency (Hwang, et al., 2018). Recently, the role of Laban, a fermented milk product from camels, was suggested as a source of lactic acid bacteria that could have an antioxidant effect. An ex vivo assay on human blood samples, from both normal and G6PD deficient children, exposed to fava beans showed a decrease in hemolysis with the addition of Laban. More studies would need to be done to determine the in vivo relationship between favism and Laban in regards to hemolytic crisis (Bin Masalam, et al., 2018; Zam & Belal, 2020).
Since there are currently no approved treatments for G6PD deficiency, one group aimed to identify a small molecule that increases enzyme activity by screening agonists for the Canton mutant of the G6PD enzyme. They identified 2,2’-disulfanediylbis(N-(2-(1H-indol-3-yl)ethyl)ethan-1-amine) referred to as AG1. AG1 showed increased activation of the G6PD enzyme and promoted formation of dimers. AG1 increased activation and created an equilibrium dimeric state in multiple G6PD mutant variants from different regions of the world acting specifically on the G6PD enzyme without interacting with related enzymes such as 6-phosphogluconate dehydrogenase (6PGD), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and others. Treatment with AG1 in vivo significantly reduced ROS levels and increased NADPH levels following CQ exposure. This resulted in less pericardial edema in G6PD-deficient zebrafish embryos. Additionally, AG1 reduced hemolysis and ROS levels and increased GSH in both healthy RBC exposed to CQ and in RBC stored for 28 days that are usually subject to storage lesion (Hwang, et al., 2018). The mechanism of action of AG1 is believed to involve bridging two NADP+ molecules promoting G6PD dimerization and activation. G6PD cannot activate as a monomer (Raub, et al., 2019). This small molecule could serve as the lead compound for development of drugs for the treatment of G6PD deficiency eventually allowing safe antimalarial use in developing countries and possibly affecting heart disease, diabetes, and other pathologies shown to be related to G6PD deficiency (Hwang, et al., 2018; Raub, et al., 2019).
7. CONCLUSIONS
Deficient G6PD enzyme levels affect a number of metabolic systems in RBC and other cells leading to depleted NADPH, oxidative stress, hemolysis, and potentially multi-organ failure and mortality (Anantasomboon, et al., 2019; Hwang, et al., 2018; Kiessling, et al., 2018; Luzzatto, et al., 2016; May, et al., 2019). The effects of G6PD deficiency in both RBC and nucleated cells have been linked to a variety of disease states all over the body (L. Chen, et al., 2019). G6PD deficiency and some other blood diseases such as sickle cell trait have been shown to protect against development of severe malaria (Allergy, et al., 2005, 2008; Shivwanshi, et al., 2019; J. A. Watson, et al., 2019). G6PD deficiency has also been shown to decrease risk of hepatocellular, gastric, and colorectal cancers (Dore, et al., 2018; Pes, Errigo, et al., 2019). On the other hand, G6PD deficiency may increase risk of developing CVD and potentially increase the risk of infection and further development of serious complications (Pes, Parodi, et al., 2019; Sharma, et al., 2018; Thomas, et al., 2018). Additionally, there are convincing evidence to suggest that the G6PD enzyme is involved in regulating vascular function, insulin levels, and sepsis in neonates (Dore, et al., 2018; Parsanathan & Jain, 2020).
One of the greatest risks for G6PD deficient patients is developing drug-induced hemolytic anemia (Cappellini & Fiorelli, 2008; Luzzatto, et al., 2016). Accurately measuring levels of enzyme activity for diagnosis of G6PD deficiency is crucial to providing safe care and preventing hemolysis. Some of the assays described here show promise in correctly determining G6PD deficiency, including moderate deficiency, at the point-of-care in a cost effective manner. Hemolysis commonly occurs in G6PD deficient patients being treated for malaria. Currently, malaria treatment relies heavily on PQ and other 8-aminoquinoline drugs in combination as they eliminate blood and liver stages, but administration of these drugs can cause severe side effects in G6PD deficient populations (Rueangweerayut, et al., 2017; von Seidlein, et al., 2013). Two mouse models discussed above have been suggested as a safe way to determine the hemolytic potential of newly developed antimalarials for G6PD deficient patients (Rochford, et al., 2013; Zhang, et al., 2013). Additionally, SLD PQ combination therapies have been shown to be effective and safe regardless of G6PD status and wouldn’t require enzyme testing (Consortium, et al., 2014; Hygiene, et al., 2014; Hygiene, et al., 2015; University of California, et al., 2015). All of these methods can help avoid hemolytic crisis in G6PD deficient populations when treating for malaria.
Currently there is no cure for G6PD deficiency. Once thought to reverse the effects of this disease, antioxidant therapy has shown little effect on combating oxidative damage caused by the lack of effective G6PD enzyme (Bin Masalam, et al., 2018; Koriem & Arbid, 2018; Zam & Belal, 2020). However, a small molecule has been identified that increases enzyme levels in both in vitro and in vivo models suggesting this treatment could reverse the effects of G6PD deficiency potentially including other clinical states associated with the deficiency (Hwang, et al., 2018; Raub, et al., 2019). More work will need to be done to optimize this compound and determine the efficacy and safety profiles in patient populations.
Overall, the G6PD enzyme appears to play a role in many different mechanisms throughout the body, which manifest in many different disease states. While these studies have elucidated numerous facets of this enzymopathy, much is still unknown. Safer use of currently available drugs would be assisted by broader epidemiological surveys for G6PD deficiency and more accurate point-of-care quantitative tests for G6PD enzyme activity. In vitro and in vivo models can assist in the development of new drugs. Diagnosis of G6PD deficiency during the neonatal state and the development of a small molecule treatment would help to prevent hemolytic crisis in G6PD deficient patients and could affect the risk of cancers, CVD, and more. However, further investigation needs to be done to fully understand the most common enzyme deficiency in the world (La Vieille, et al., 2019).
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Mark Suto, Ph.D, Vice President of Drug Discovery Division at Southern Research for support. BLT is supported by the National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Health USA (R01AI132579).
LIST OF ABBREVIATIONS
- AL
Artemether-lumefantrine
- CQ
Chloroquine
- CSG
CareStart™ G6PD Biosensor
- CVD
Cardiovascular disease
- DHAP
Dihydroartemisinin-piperaquine
- G6PD
Glucose-6-phosphate dehydrogenase
- GSH
Glutathione
- HbA1c
Hemoglobin A1c
- IDH1
Isocitrate dehydrogenase 1
- ME1
Malic enzyme 1
- MQ
Mefloquine
- NADPH
Nicotinamide adenine dinucleotide phosphate
- PK
Pharmacokinetics
- PPP
Pentose phosphate pathway
- PQ
Primaquine
- RBC
Red blood cells
- ROS
Reactive oxygen species
- STG
STANDARD™ G6PD
- SLD
Single low dose
- TQ
Tafenoquine
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
This manuscript has not been published and is not under consideration for publication elsewhere.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agarwal A, Guindo A, Cissoko Y, Taylor JG, Coulibaly D, Kone A, Kayentao K, Djimde A, Plowe CV, Doumbo O, Wellems TE, & Diallo D (2000). Hemoglobin C associated with protection from severe malaria in the Dogon of Mali, a West African population with a low prevalence of hemoglobin S. Blood, 96, 2358–2363. [PubMed] [Google Scholar]
- Alabama, U. o. S. (2009). Erythrocyte and Adipocyte G6PD Activity Levels in Obesity. In: https://ClinicalTrials.gov/show/NCT01322035.
- Alam MS, Kibria MG, Jahan N, Price RN, & Ley B (2018). Spectrophotometry assays to determine G6PD activity from Trinity Biotech and Pointe Scientific G6PD show good correlation. BMC Res Notes, 11, 855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MS, Kibria MG, Jahan N, Thriemer K, Hossain MS, Douglas NM, Phru CS, Khan WA, Price RN, & Ley B (2018). Field evaluation of quantitative point of care diagnostics to measure glucose-6-phosphate dehydrogenase activity. PLoS One, 13, e0206331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alina MF, Azma RZ, Norunaluwar J, Azlin I, Darnina AJ, Cheah FC, Noor-Farisah AR, Siti-Hawa AA, Danny XRK, Zulkifli NF, & Ainoon O (2020). Genotyping of Malaysian G6PD-deficient neonates by reverse dot blot flow-through hybridisation. J Hum Genet, 65, 263–270. [DOI] [PubMed] [Google Scholar]
- Allergy, N. I. o., Diseases, I., & Center, N. I. o. H. C. (2005). Examination of Protective Factors Against Severe Malaria. In: https://ClinicalTrials.gov/show/NCT00342043.
- Allergy, N. I. o., Diseases, I., & Center, N. I. o. H. C. (2008). Innate and Acquired Resistance to Plasmodium Falciparum Malaria in Mali. In: https://ClinicalTrials.gov/show/NCT00669084.
- Anantasomboon P, Chanda M, Jugnam-Ang W, Witoonpanich P, Cheepsunthom P, Nuchprayoon I, Fucharoen S, & Cheepsunthom CL (2019). Evaluating the performance of automated UV enzymatic assay for screening of glucose 6-phosphate dehydrogenase deficiency. Int J Lab Hematol, 41, 192–199. [DOI] [PubMed] [Google Scholar]
- Aydemir D, & Ulusu NN (2020). Is glucose-6-phosphate dehydrogenase enzyme deficiency a factor in Coronavirus-19 (COVID-19) infections and deaths? Pathog Glob Health, 114, 109–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayi K, Turrini F, Piga A, & Arese P (2004). Enhanced phagocytosis of ring-parasitized mutant erythrocytes: a common mechanism that may explain protection against falciparum malaria in sickle trait and beta-thalassemia trait. Blood, 104, 3364–3371. [DOI] [PubMed] [Google Scholar]
- Babu T, Panachiyil GM, Sebastian J, & Ravi MD (2019). Probable blueberry-induced haemolysis in a G6PD deficient child: A case report. Nutr Health, 25, 303–305. [DOI] [PubMed] [Google Scholar]
- Bancone G, Chu CS, Chowwiwat N, Somsakchaicharoen R, Wilaisrisak P, Charunwatthana P, Bansil P, McGray S, Domingo GJ, & Nosten FH (2015). Suitability of capillary blood for quantitative assessment of G6PD activity and performances of G6PD point-of-care tests. Am J Trop Med Hyg, 92, 818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaens GJH, Tiono AB, Okebe J, Pett HE, Coulibaly SA, Goncalves BP, Affara M, Ouedraogo A, Bougouma EC, Sanou GS, Nebie I, Bradley J, Lanke KHW, Niemi M, Sirima SB, d'Alessandro U, Bousema T, & Drakeley C (2018). Safety of single low-dose primaquine in glucose-6-phosphate dehydrogenase deficient falciparum-infected African males: Two open-label, randomized, safety trials. PLoS One, 13, e0190272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfield KD, & Tichy EM (2018). Review and drug therapy implications of glucose-6-phosphate dehydrogenase deficiency. Am J Health Syst Pharm, 75, 97–104. [DOI] [PubMed] [Google Scholar]
- Bin Masalam MS, Bahieldin A, Alharbi MG, Al-Masaudi S, Al-Jaouni SK, Harakeh SM, & Al-Hindi RR (2018). Isolation, Molecular Characterization and Probiotic Potential of Lactic Acid Bacteria in Saudi Raw and Fermented Milk. Evid Based Complement Alternat Med, 2018, 7970463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom KE, Brewer GJ, Magon AM, & Wetterstroem N (1983). Microsomal incubation test of potentially hemolytic drugs for glucose-6-phosphate dehydrogenase deficiency. Clin Pharmacol Ther, 33, 403–409. [DOI] [PubMed] [Google Scholar]
- Bubp J, Jen M, & Matuszewski K (2015). Caring for Glucose-6-Phosphate Dehydrogenase (G6PD)-Deficient Patients: Implications for Pharmacy. P T, 40, 572–574. [PMC free article] [PubMed] [Google Scholar]
- Cabrera M, & Cui L (2015). In Vitro Activities of Primaquine-Schizonticide Combinations on Asexual Blood Stages and Gametocytes of Plasmodium falciparum. Antimicrob Agents Chemother, 59, 7650–7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellini MD, & Fiorelli G (2008). Glucose-6-phosphate dehydrogenase deficiency. Lancet, 371, 64–74. [DOI] [PubMed] [Google Scholar]
- Chao YC, Huang CS, Lee CN, Chang SY, King CC, & Kao CL (2008). Higher infection of dengue virus serotype 2 in human monocytes of patients with G6PD deficiency. PLoS One, 3, e1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I, Diawara H, Mahamar A, Sanogo K, Keita S, Kone D, Diarra K, Djimde M, Keita M, Brown J, Roh ME, Hwang J, Pett H, Murphy M, Niemi M, Greenhouse B, Bousema T, Gosling R, & Dicko A (2018). Safety of Single-Dose Primaquine in G6PD-Deficient and G6PD-Normal Males in Mali Without Malaria: An Open-Label, Phase 1, Dose-Adjustment Trial. J Infect Dis, 217, 1298–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhang Z, Hoshino A, Zheng HD, Morley M, Arany Z, & Rabinowitz JD (2019). NADPH production by the oxidative pentose-phosphate pathway supports folate metabolism. Nat Metab, 1, 404–415. [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Chen HJ, Chang YC, Liu YN, Kao SM, Liu MY, Weng YY, Hsiao KJ, & Liu TT (2019). Applying a multiplexed primer extension method on dried blood spots increased the detection of carriers at risk of glucose-6-phosphate dehydrogenase deficiency in newborn screening program. Clin Chim Acta, 495, 271–277. [DOI] [PubMed] [Google Scholar]
- Chu CS, Bancone G, Nosten F, White NJ, & Luzzatto L (2018). Primaquine-induced haemolysis in females heterozygous for G6PD deficiency. Malar J, 17, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical Research Collaborative Group of Yinzhihuang Oral, S. (2011). [A multicenter randomized controlled study on the efficacy and safety of Yinzhihuang oral solution for the treatment of neonatal indirect hyperbilirubinemia in term newborn infants]. Zhonghua Er Ke Za Zhi, 49, 663–668. [PubMed] [Google Scholar]
- Consortium, M., National Centre for Parasitology, E., Malaria Control, C., Institute Pasteur, C., Organization, W. H., Control, C. f. D., & Prevention. (2014). Safety and Tolerability of Low Dose Primaquine. In: https://ClinicalTrials.gov/show/NCT02434952.
- Daher A, Aljayyoussi G, Pereira D, Lacerda MVG, Alexandre MAA, Nascimento CT, Alves JC, da Fonseca LB, da Silva DMD, Pinto DP, Rodrigues DF, Silvino ACR, de Sousa TN, de Brito CFA, Ter Kuile FO, & Lalloo DG (2019). Pharmacokinetics/pharmacodynamics of chloroquine and artemisinin-based combination therapy with primaquine. Malar J, 18, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djigo OKM, Bollahi MA, Hasni Ebou M, Ould Ahmedou Salem MS, Tahar R, Bogreau H, Basco L, & Ould Mohamed Salem Boukhary A (2019). Assessment of glucose-6-phosphate dehydrogenase activity using CareStart G6PD rapid diagnostic test and associated genetic variants in Plasmodium vivax malaria endemic setting in Mauritania. PLoS One, 14, e0220977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore MP, Vidili G, Marras G, Assy S, & Pes GM (2018). Inverse Association between Glucose6Phosphate Dehydrogenase Deficiency and Hepatocellular Carcinoma. Asian Pac J Cancer Prev, 19, 1069–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourado, F. d. M. T. D. H. V., Foundation, O. C., & Tecnológico, C. N. d. D. C. e. (2018). Safety and Efficacy of Different Regimens of Primaquine on Vivax Malaria Treatment in G6PD Deficient Patients. In: https://ClinicalTrials.gov/show/NCT03529396.
- Dunyo S, Sirugo G, Sesay S, Bisseye C, Njie F, Adiamoh M, Nwakanma D, Diatta M, Janha R, Sisay Joof F, Temple B, Snell P, Conway D, Walton R, Cheung YB, & Milligan P (2011). Randomized trial of safety and effectiveness of chlorproguanil-dapsone and lumefantrine-artemether for uncomplicated malaria in children in the Gambia. PLoS One, 6, e17371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dysoley L, Kim S, Lopes S, Khim N, Bjorges S, Top S, Huch C, Rekol H, Westercamp N, Fukuda MM, Hwang J, Roca-Feltrer A, Mukaka M, Menard D, & Taylor WR (2019). The tolerability of single low dose primaquine in glucose-6-phosphate deficient and normal falciparum-infected Cambodians. BMC Infect Dis, 19, 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissa AA, Haji BA, & Al-Doski AA (2019). G6PD Deficiency Prevalence as a Cause of Neonatal Jaundice in a Neonatal Ward in Dohuk, Iraq. Am JPerinatol. [DOI] [PubMed] [Google Scholar]
- Eziefula AC, Pett H, Grignard L, Opus S, Kiggundu M, Kamya MR, Yeung S, Staedke SG, Bousema T, & Drakeley C (2014). Glucose-6-phosphate dehydrogenase status and risk of hemolysis in Plasmodium falciparum-infected African children receiving single-dose primaquine. Antimicrob Agents Chemother, 58, 4971–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan S, Chaurasiya ND, Sahu R, Walker LA, & Tekwani BL (2012). Understanding the mechanisms for metabolism-linked hemolytic toxicity of primaquine against glucose 6-phosphate dehydrogenase deficient human erythrocytes: evaluation of eryptotic pathway. Toxicology, 294, 54–60. [DOI] [PubMed] [Google Scholar]
- Ganesan S, Sahu R, Walker LA, & Tekwani BL (2010). Cytochrome P450-dependent toxicity of dapsone in human erythrocytes. J Appl Toxicol, 30, 271–275. [DOI] [PubMed] [Google Scholar]
- Ganesan S, Tekwani BL, Sahu R, Tripathi LM, & Walker LA (2009). Cytochrome P(450)-dependent toxic effects of primaquine on human erythrocytes. Toxicol Appl Pharmacol, 241, 14–22. [DOI] [PubMed] [Google Scholar]
- Georgakouli K, Deli CK, Zalavras A, Fatouros IG, Kouretas D, Koutedakis Y, & Jamurtas AZ (2013). Alpha-lipoic acid supplementation up-regulates antioxidant capacity in adults with G6PD deficiency. Food Chem Toxicol, 61, 69–73. [DOI] [PubMed] [Google Scholar]
- Ghergurovich JM, Esposito M, Chen Z, Wang JZ, Bhatt V, Lan T, White E, Kang Y, Guo JY, & Rabinowitz JD (2020). Glucose-6-phosphate dehydrogenase is not essential for K-Ras-driven tumor growth or metastasis. Cancer Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilder ME, Hanpithakphong W, Hoglund RM, Tarning J, Win HH, Hilda N, Chu CS, Bancone G, Carrara VI, Singhasivanon P, White NJ, Nosten F, & McGready R (2018). Primaquine Pharmacokinetics in Lactating Women and Breastfed Infant Exposures. Clin Infect Dis, 67, 1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GlaxoSmithKline, & Venture, M. f. M. (2015). Study to Assess the Incidence of Hemolysis, Safety, and Efficacy of Tafenoquine (SB-252263, WR238605) Versus Primaquine in Subjects With Plasmodium Vivax Malaria. In: https://ClinicalTrials.gov/show/NCT02216123.
- GlaxoSmithKline, & Venture, M. f. M. (2017). A Pharmacokinetics, Safety and Efficacy Study of Tafenoquine (TQ) in Pediatric Subjects With Plasmodium Vivax (P. Vivax) Malaria. In: https://ClinicalTrials.gov/show/NCT02563496.
- Gordeuk VR, Shah BN, Zhang X, Thuma PE, Zulu S, Moono R, Reading NS, Song J, Zhang Y, Nouraie M, Campbell A, Minniti CP, Rana SR, Darbari DS, Kato GJ, Niu M, Castro OL, Machado R, Gladwin MT, & Prchal JT (2020). CYB5R3(c) (.350C>G) and G6PD A Alleles Modify Severity of Anemia in Malaria and Sickle Cell Disease. Am J Hematol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid MMA, Thriemer K, Elobied ME, Mahgoub NS, Boshara SA, Elsafi HMH, Gumaa SA, Hamid T, Abdelbagi H, Basheir HM, Marfurt J, Chen I, Gosling R, Price RN, & Ley B (2018). Low risk of recurrence following artesunate-Sulphadoxine-pyrimethamine plus primaquine for uncomplicated Plasmodium falciparum and Plasmodium vivax infections in the Republic of the Sudan. Malar J, 17, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S, Mruk K, Rahighi S, Raub AG, Chen CH, Dorn LE, Horikoshi N, Wakatsuki S, Chen JK, & Mochly-Rosen D (2018). Correcting glucose-6-phosphate dehydrogenase deficiency with a small-molecule activator. Nat Commun, 9, 4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hygiene, L. S. o., Medicine, T., Centre National de Recherche et de Formation sur le Paludisme, B. F., & Nijmegen, U. M. C. (2014). Safety of Primaquine + Artemether-lumefantrine in G6PD Deficient Males With an Asymptomatic Malaria Infection (SAFEPRIM). In: https://ClinicalTrials.gov/show/NCT02174900.
- Hygiene, L. S. o., Medicine, T., Council, M. R., & National Malaria Control Programme, T. G. (2004). Lapdap and Coartemether for Uncomplicated Malaria. In: https://ClinicalTrials.gov/show/NCT00118794.
- Hygiene, L. S. o., Medicine, T., & Medical Research Council Unit, T. G. (2015). Evaluation of the Safety of Primaquine in Combination With Dihydroartemisinin-piperaquine in G6PD Deficient Males in The Gambia. In: https://ClinicalTrials.gov/show/NCT02654730.
- InfaCare Pharmaceuticals Corporation, a. M. C., & Mallinckrodt. (2013). Stannsoporfin With Light Therapy for Newborn Babies With Jaundice. In: https://ClinicalTrials.gov/show/NCT01887327.
- Jain M, Brenner DA, Cui L, Lim CC, Wang B, Pimentel DR, Koh S, Sawyer DB, Leopold JA, Handy DE, Loscalzo J, Apstein CS, & Liao R (2003). Glucose-6-phosphate dehydrogenase modulates cytosolic redox status and contractile phenotype in adult cardiomyocytes. Circ Res, 93, e9–16. [DOI] [PubMed] [Google Scholar]
- Jain M, Cui L, Brenner DA, Wang B, Handy DE, Leopold JA, Loscalzo J, Apstein CS, & Liao R (2004). Increased myocardial dysfunction after ischemia-reperfusion in mice lacking glucose-6-phosphate dehydrogenase. Circulation, 109, 898–903. [DOI] [PubMed] [Google Scholar]
- Jain SK, & Parsanathan R (2020). Can Vitamin D and L-Cysteine Co-Supplementation Reduce 25(OH)-Vitamin D Deficiency and the Mortality Associated with COVID-19 in African Americans? J Am Coll Nutr, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain SK, Parsanathan R, Levine SN, Bocchini JA, Holick MF, & Vanchiere JA (2020). The potential link between inherited G6PD deficiency, oxidative stress, and vitamin D deficiency and the racial inequities in mortality associated with COVID-19. Free Radic Biol Med, 161, 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasemy ZA, Bahbah WA, El Hefnawy SM, & Alkalash SH (2020). Prevalence of and mothers' knowledge, attitude and practice towards glucose-6-phosphate dehydrogenase deficiency among neonates with jaundice: a cross-sectional study. BMJ Open, 10, e034079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling N, Brintrup J, Zeynudin A, Abduselam N, Gotz S, Mack M, Pritsch M, Wieser A, Kohne E, & Berens-Riha N (2018). Glucose-6-phosphate dehydrogenase activity measured by spectrophotometry and associated genetic variants from the Oromiya zone, Ethiopia. Malar J, 17, 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa A, Kizub I, Jacob C, Michael K, D'Alessandro A, Reisz JA, Grzybowski M, Geurts AM, Rocic P, Gupte R, Miano JM, & Gupte SA (2020). CRISPR-Mediated Single Nucleotide Polymorphism Modeling in Rats Reveals Insight Into Reduced Cardiovascular Risk Associated With Mediterranean G6PD Variant. Hypertension, 76, 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koriem KMM, & Arbid MS (2018). Evaluating of beta-carotene role in ameliorating of favism-induced disturbances in blood and testis. J Complement Integr Med, 15. [DOI] [PubMed] [Google Scholar]
- La Vieille S, Lefebvre DE, Khalid AF, Decan MR, & Godefroy S (2019). Dietary restrictions for people with glucose-6-phosphate dehydrogenase deficiency. Nutr Rev, 77, 96–106. [DOI] [PubMed] [Google Scholar]
- Lai YK, Lai NM, & Lee SW (2017). Glucose-6-phosphate dehydrogenase deficiency and risk of diabetes: a systematic review and meta-analysis. Ann Hematol, 96, 839–845. [DOI] [PubMed] [Google Scholar]
- Lee SM, & Geetha D (2015). Dapsone induced hemolysis in a patient with ANCA associated glomerulonephritis and normal G6PD level and implications for clinical practice: case report and review of the literature. Springerplus, 4, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong A, Lim VJY, Wang C, Chai JF, Dorajoo R, Heng CK, van Dam RM, Koh WP, Yuan JM, Jonas JB, Wang YX, Wei WB, Liu J, Reilly DF, Wong TY, Cheng CY, & Sim X (2020). Association of G6PD variants with hemoglobin A1c and impact on diabetes diagnosis in East Asian individuals. BMJ Open Diabetes Res Care, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley B, Bancone G, von Seidlein L, Thriemer K, Richards JS, Domingo GJ, & Price RN (2017). Methods for the field evaluation of quantitative G6PD diagnostics: a review. Malar J, 16, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley B, Winasti Satyagraha A, Rahmat H, von Fricken ME, Douglas NM, Pfeffer DA, Espino F, von Seidlein L, Henriques G, Oo NN, Menard D, Parikh S, Bancone G, Karahalios A, & Price RN (2019). Performance of the Access Bio/CareStart rapid diagnostic test for the detection of glucose-6-phosphate dehydrogenase deficiency: A systematic review and meta-analysis. PLoS Med, 16, e1002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang XY, Chen JT, Ma YB, Huang HY, Xie DD, Monte-Nguba SM, Ehapo CS, Eyi UM, Zheng YZ, Liu XZ, Zha GC, Lin LY, Chen WZ, Zhou X, & Lin M (2020). Evidence of positively selected G6PD A- allele reduces risk of Plasmodium falciparum infection in African population on Bioko Island. Mol Genet Genomic Med, 8, e1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Lou ZY, Xing SY, Zhang L, & Yang LY (2018). The gene spectrum of glucose-6-phosphate dehydrogenase (G6PD) deficiency in Guangdong province, China. Gene, 678, 312–317. [DOI] [PubMed] [Google Scholar]
- Llanos-Cuentas A, Lacerda MVG, Hien TT, Velez ID, Namaik-Larp C, Chu CS, Villegas MF, Val F, Monteiro WM, Brito MAM, Costa MRF, Chuquiyauri R, Casapia M, Nguyen CH, Aruachan S, Papwijitsil R, Nosten FH, Bancone G, Angus B, Duparc S, Craig G, Rousell VM, Jones SW, Hardaker E, Clover DD, Kendall L, Mohamed K, Koh G, Wilches VM, Breton JJ, & Green JA (2019). Tafenoquine versus Primaquine to Prevent Relapse of Plasmodium vivax Malaria. N Engl J Med, 380, 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopera-Mesa TM, Doumbia S, Konate D, Anderson JM, Doumbouya M, Keita AS, Diakite SA, Traore K, Krause MA, Diouf A, Moretz SE, Tullo GS, Miura K, Gu W, Fay MP, Taylor SM, Long CA, Diakite M, & Fairhurst RM (2015). Effect of red blood cell variants on childhood malaria in Mali: a prospective cohort study. Lancet Haematol, 2, e140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzatto L, Nannelli C, & Notaro R (2016). Glucose-6-Phosphate Dehydrogenase Deficiency. Hematol Oncol Clin North Am, 30, 373–393. [DOI] [PubMed] [Google Scholar]
- Luzzatto L, & Seneca E (2014). G6PD deficiency: a classic example of pharmacogenetics with on-going clinical implications. Br J Haematol, 164, 469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May WL, Kyaw MP, Blacksell SD, Pukrittayakamee S, Chotivanich K, Hanboonkunupakarn B, Thein KN, Lim CS, Thaipadungpanit J, Althaus T, & Jittamala P (2019). Impact of glucose-6-phosphate dehydrogenase deficiency on dengue infection in Myanmar children. PLoS One, 14, e0209204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbanefo EC, Ahmed AM, Titouna A, Elmaraezy A, Trang NT, Phuoc Long N, Hoang Anh N, Diem Nghi T, The Hung B, Van Hieu M, Ky Anh N, Huy NT, & Hirayama K (2017). Association of glucose-6-phosphate dehydrogenase deficiency and malaria: a systematic review and meta-analysis. Sci Rep, 7, 45963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednax Center for Research, E., Quality, & Safety. (2006). Demographic, Metabolic, and Genomic Description of Neonates With Severe Hyperbilirubinemia. In: https://ClinicalTrials.gov/show/NCT00383318.
- Mehta P, Srivastav V, Bhate P, Gupta V, & Nadkar MY (2017). Glucose-6-Phosphate Dehydrogenase Deficiency Unveiled by Diabetic Ketoacidosis: A Dual Dilemma. J Assoc Physicians India, 65, 98–102. [PubMed] [Google Scholar]
- Oxford, U. o. (2012). Primaquine Pharmacokinetics in Lactating Women and Their Infants. In: https://ClinicalTrials.gov/show/NCT01780753.
- Oxford, U. o., Unit, M. O. T. M. R., & University, N. (2016). G6PD Assessment Before Primaquine for Radical Treatment of Vivax Malaria. In: https://ClinicalTrials.gov/show/NCT02876549.
- Pal S, Bansil P, Bancone G, Hrutkay S, Kahn M, Gornsawun G, Penpitchaporn P, Chu CS, Nosten F, & Domingo GJ (2019). Evaluation of a Novel Quantitative Test for Glucose-6-Phosphate Dehydrogenase Deficiency: Bringing Quantitative Testing for Glucose-6-Phosphate Dehydrogenase Deficiency Closer to the Patient. Am J Trop Med Hyg, 100, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsanathan R, & Jain SK (2020). Glucose-6-phosphate dehydrogenase (G6PD) deficiency is linked with cardiovascular disease. Hypertens Res. [DOI] [PubMed] [Google Scholar]
- Pengboon P, Thamwarokun A, Changsri K, Kaset C, & Chomean S (2019). Evaluation of quantitative biosensor for glucose-6-phosphate dehydrogenase activity detection. PLoS One, 14, e0226927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pes GM, Errigo A, Soro S, Longo NP, & Dore MP (2019). Glucose-6-phosphate dehydrogenase deficiency reduces susceptibility to cancer of endodermal origin. Acta Oncol, 58, 1205–1211. [DOI] [PubMed] [Google Scholar]
- Pes GM, Parodi G, & Dore MP (2019). Glucose-6-phosphate dehydrogenase deficiency and risk of cardiovascular disease: A propensity score-matched study. Atherosclerosis, 282, 148–153. [DOI] [PubMed] [Google Scholar]
- Pfeffer DA, Ley B, Howes RE, Adu P, Alam MS, Bansil P, Boum Y 2nd, Brito M, Charoenkwan P, Clements A, Cui L, Deng Z, Egesie OJ, Espino FE, von Fricken ME, Hamid MMA, He Y, Henriques G, Khan WA, Khim N, Kim S, Lacerda M, Lon C, Mekuria AH, Menard D, Monteiro W, Nosten F, Oo NN, Pal S, Palasuwan D, Parikh S, Pitaloka Pasaribu A, Poespoprodjo JR, Price DJ, Roca-Feltrer A, Roh ME, Saunders DL, Spring MD, Sutanto I, Ley-Thriemer K, Weppelmann TA, von Seidlein L, Satyagraha AW, Bancone G, Domingo GJ, & Price RN (2020). Quantification of glucose-6-phosphate dehydrogenase activity by spectrophotometry: A systematic review and meta-analysis. PLoS Med, 17, e1003084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretsch W, Charles DJ, & Merkle S (1988). X-linked glucose-6-phosphate dehydrogenase deficiency in Mus musculus. Biochem Genet, 26, 89–103. [DOI] [PubMed] [Google Scholar]
- Prevention, C. f. D. C. a. (2019). Malaria. Centers for Disease Control and Prevention. [Google Scholar]
- Raub AG, Hwang S, Horikoshi N, Cunningham AD, Rahighi S, Wakatsuki S, & Mochly-Rosen D (2019). Small-Molecule Activators of Glucose-6-phosphate Dehydrogenase (G6PD) Bridging the Dimer Interface. Chem Med Chem, 14, 1321–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recht J, Ashley EA, & White NJ (2018). Use of primaquine and glucose-6-phosphate dehydrogenase deficiency testing: Divergent policies and practices in malaria endemic countries. PLoS Negl Trop Dis, 12, e0006230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard D, & Rosselet A (2017). Drug-induced hemolytic anemia: Pharmacological aspects. Transfus Clin Biol, 24, 110–114. [DOI] [PubMed] [Google Scholar]
- Research, M. S. o. H., & Khartoum, U. o. (2015). A Study to Assess Current Standard Malaria Treatment Guidelines in the Republic of the Sudan. In: https://ClinicalTrials.gov/show/NCT02592408.
- Research, W. R. A. I. o. (2003). A Test to Predict the Hemolytic Potential of Drugs in G6PD Deficiency. In: https://ClinicalTrials.gov/show/NCT00076323.
- Richardson SR, & O'Malley GF (2020). Glucose 6 Phosphate Dehydrogenase (G6PD) Deficiency. In StatPearls. Treasure Island (FL). [Google Scholar]
- Roca-Feltrer A, Khim N, Kim S, Chy S, Canier L, Kerleguer A, Tor P, Chuor CM, Kheng S, Siv S, Kachur PS, Taylor WR, Hwang J, & Menard D (2014). Field trial evaluation of the performances of point-of-care tests for screening G6PD deficiency in Cambodia. PLoS One, 9, e116143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochford R, Ohrt C, Baresel PC, Campo B, Sampath A, Magill AJ, Tekwani BL, & Walker LA (2013). Humanized mouse model of glucose 6-phosphate dehydrogenase deficiency for in vivo assessment of hemolytic toxicity. Proc Natl Acad Sci U S A, 110, 17486–17491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper D, Layton M, Rees D, Lambert C, Vulliamy T, De la Salle B, D'Souza C, & British Society for, H. (2020). Laboratory diagnosis of G6PD deficiency. A British Society for Haematology Guideline. Br J Haematol. [DOI] [PubMed] [Google Scholar]
- Rueangweerayut R, Bancone G, Harrell EJ, Beelen AP, Kongpatanakul S, Mohrle JJ, Rousell V, Mohamed K, Qureshi A, Narayan S, Yubon N, Miller A, Nosten FH, Luzzatto L, Duparc S, Kleim JP, & Green JA (2017). Hemolytic Potential of Tafenoquine in Female Volunteers Heterozygous for Glucose-6-Phosphate Dehydrogenase (G6PD) Deficiency (G6PD Mahidol Variant) versus G6PD-Normal Volunteers. Am J Trop Med Hyg, 97, 702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev O, Wollner A, & Menczel J (1984). Diabetic ketoacidosis does not precipitate haemolysis in patients with the Mediterranean variant of glucose-6-phosphate dehydrogenase deficiency. Br Med J (Clin Res Ed), 288, 179–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, Singh O, Juneja D, Goel A, Garg SK, & Shekhar S (2018). Hepatitis A Virus-induced Severe Hemolysis Complicated by Severe Glucose-6-Phosphate Dehydrogenase Deficiency. Indian J Crit Care Med, 22, 670–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivwanshi LR, Singh E, & Kumar A (2019). A positive correlation between sickle cell anemia and g6pd deficiency from population of Chhattisgarh, India. Gene, 707, 143–150. [DOI] [PubMed] [Google Scholar]
- Southern Medical University, C. (2015). Efficacy of Yinzhihuang Oral Liquid on Indirect Bilirubin of Neonates With Glucose-6-phosphate Dehydrogenase Deficiency. In: https://ClinicalTrials.gov/show/NCT02594904.
- Thessaly, U. o. (2016). Effects of Alpha Lipoic Acid Supplementation in G6PD Deficient Individuals After Acute Exercise. In: https://ClinicalTrials.gov/show/NCT02937363.
- Thessaly, U. o. (2020). Effects of N-acetyl Cystein (NAC) Supplementation in G6PD Deficient Individuals After Acute Exercise. In: https://ClinicalTrials.gov/show/NCT02937376.
- Thomas JE, Kang S, Wyatt CJ, Kim FS, Mangelsdorff AD, & Weigel FK (2018). Glucose-6-Phosphate Dehydrogenase Deficiency is Associated with Cardiovascular Disease in U.S. Military Centers. Tex Heart Inst J, 45, 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of California, S. F., Research, M., Training Center, B., Mali, University, R., Center, U. o. M. M., Bill, & Foundation, M. G. (2015). Determining a Tolerable Dose of Primaquine in G6PD-deficient Persons Without Malaria in Mali. In: https://ClinicalTrials.gov/show/NCT02535767.
- University of Mississippi, O. (2018). Metabolism and Pharmacokinetics of Primaquine Enantiomers in Human Volunteers Receiving a Seven Day Dose Regimen. In: https://ClinicalTrials.gov/show/NCT03934450.
- Vantaux A, Kim S, Piv E, Chy S, Berne L, Khim N, Lek D, Siv S, Mukaka M, Taylor WR, & Menard D (2020). Significant Efficacy of a Single Low Dose of Primaquine Compared to Stand-Alone Artemisinin Combination Therapy in Reducing Gametocyte Carriage in Cambodian Patients with Uncomplicated Multidrug-Resistant Plasmodium falciparum Malaria. Antimicrob Agents Chemother, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Seidlein L, Auburn S, Espino F, Shanks D, Cheng Q, McCarthy J, Baird K, Moyes C, Howes R, Menard D, Bancone G, Winasti-Satyahraha A, Vestergaard LS, Green J, Domingo G, Yeung S, & Price R (2013). Review of key knowledge gaps in glucose-6-phosphate dehydrogenase deficiency detection with regard to the safe clinical deployment of 8-aminoquinoline treatment regimens: a workshop report. Malar J, 12, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watchko JF, Lin Z, Clark RH, Kelleher AS, Walker MW, Spitzer AR, & Pediatrix Hyperbilirubinemia Study, G. (2009). Complex multifactorial nature of significant hyperbilirubinemia in neonates. Pediatrics, 124, e868–877. [DOI] [PubMed] [Google Scholar]
- Watson J, Taylor WR, Menard D, Kheng S, & White NJ (2017). Modelling primaquine-induced haemolysis in G6PD deficiency. Elife, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JA, Leopold SJ, Simpson JA, Day NP, Dondorp AM, & White NJ (2019). Collider bias and the apparent protective effect of glucose-6-phosphate dehydrogenase deficiency on cerebral malaria. Elife, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D, Keramane M, Capretta A, & Brennan JD (2020). A paper-based biosensor for visual detection of glucose-6-phosphate dehydrogenase from whole blood. Analyst. [DOI] [PubMed] [Google Scholar]
- Wu YH, Chiu DT, Lin HR, Tang HY, Cheng ML, & Ho HY (2015). Glucose-6-Phosphate Dehydrogenase Enhances Antiviral Response through Downregulation of NADPH Sensor HSCARG and Upregulation of NF-kappaB Signaling. Viruses, 7, 6689–6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YH, Tseng CP, Cheng ML, Ho HY, Shih SR, & Chiu DT (2008). Glucose-6-phosphate dehydrogenase deficiency enhances human coronavirus 229E infection. J Infect Dis, 197, 812–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CA, Huang HY, Lin CL, & Chang JG (2018). G6PD as a predictive marker for glioma risk, prognosis and chemosensitivity. J Neurooncol, 139, 661–670. [DOI] [PubMed] [Google Scholar]
- Yang HC, Wu YH, Yen WC, Liu HY, Hwang TL, Stern A, & Chiu DT (2019). The Redox Role of G6PD in Cell Growth, Cell Death, and Cancer. Cells, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen WC, Wu YH, Wu CC, Lin HR, Stern A, Chen SH, Shu JC, & Tsun-Yee Chiu D (2020). Impaired inflammasome activation and bacterial clearance in G6PD deficiency due to defective NOX/p38 MAPK/AP-1 redox signaling. Redox Biol, 28, 101363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Zhang S, Chen B, Zhou Y, Ma C, Yang S, Tang Y, Huang D, Xie X, Xiao Q, & Wang L (2020). Evaluation of the Diagnostic Accuracy of the CareStart Glucose-6-Phosphate Dehydrogenase Deficiency Rapid Diagnostic Test among Chinese Newborns. J Trop Pediatr. [DOI] [PubMed] [Google Scholar]
- Zam W, & Belal L (2020). Ex Vivo Study of Laban's Role in Decreasing Hemolysis Crisis in G6PD-Deficient Patients. J Nutr Metab, 2020, 8034672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zekavat OR, Makarem A, Bahrami R, Dastgheib N, & Dehghani SJ (2019). Relationship of glucose-6-phosphate dehydrogenase deficiency and neonatal sepsis: a single-center investigation on the major cause of neonatal morbidity and mortality. Pediatric Health Med Ther, 10,33–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Gao X, Ishida H, Amnuaysirikul J, Weina PJ, Grogl M, O'Neil MT, Li Q, Caridha D, Ohrt C, Hickman M, Magill AJ, & Ray P (2013). An in vivo drug screening model using glucose-6-phosphate dehydrogenase deficient mice to predict the hemolytic toxicity of 8-aminoquinolines. Am J Trop MedHyg, 88, 1138–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhang X, Guan T, Dai Q, He W, Zhang H, Wang Y, Wang B, Peng Z, Hu X, Qi D, Yang X, Zhang Y, & Ma X (2019). The association between low glucose-6-phosphate dehydrogenase activity level and hepatitis B virus infection among pre-pregnant reproductive-age Chinese females. Sci Rep, 9, 3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.