Abstract
TACAN (Tmem120A), a mechanotransducing ion channel highly expressed in a subset of nociceptors, has recently been shown to contribute to detection of noxious mechanical stimulation. In the present study we evaluated its role in sensitization to mechanical stimuli associated with preclinical models of inflammatory and chemotherapy-induced neuropathic pain (CIPN). Intrathecal administration of an oligodeoxynucleotide antisense (AS-ODN) to TACAN mRNA attenuated TACAN protein expression in rat dorsal root ganglia (DRG). While TACAN AS-ODN produced only a modest increase in mechanical nociceptive threshold, it markedly reduced mechanical hyperalgesia produced by intradermal administration of prostaglandin E2 (PGE2), tumor necrosis factor alpha (TNFα) and low molecular weight hyaluronan (LMWH), and systemic administration of lipopolysaccharide (LPS), compatible with a prominent role of TACAN in mechanical hyperalgesia produced by inflammation. In contrast, TACAN AS-ODN had no effect on mechanical hyperalgesia associated with CIPN produced by oxaliplatin or paclitaxel. Our results provide evidence that TACAN plays a role in mechanical hyperalgesia induced by pronociceptive inflammatory mediators, but not CIPN, compatible with multiple mechanisms mediating mechanical nociception, and sensitization to mechanical stimuli in preclinical models of inflammatory versus CIPN.
Keywords: TACAN, Mechanical nociceptive threshold, Hyperalgesia, CIPN
INTRODUCTION
Most clinical pain syndromes are associated with sensitization of nociceptors to mechanical stimuli, especially those pain syndromes associated with inflammation33,42,44,46,54 and peripheral neuropathy12,22,31,53. Mechanotransduction in sensory neurons is a vital process underlying several physiological functions, including hearing, touch, pain, and proprioception14,18,41,61. The mechanisms underlying mechanotransduction in somatosensory neurons have only recently begun to be elucidated. Piezo2, a mechanotransducing ion channel is now well-established to contribute to the sensation of light touch34,62, and may also contribute to some forms of nociception37.
Identified in a screen for transmembrane proteins that underly stretch-activated currents in vascular smooth muscle cells55, transmembrane protein 120A (Tmem120A), also referred to as TACAN (movement in farsi) fulfills criteria for a mechanosensory transduction ion channel55. Like Piezos and Transmembrane Channel Like (TMC) proteins11,36, TACAN shares no sequence homology with other ion channels. TACAN is expressed on mouse dorsal root ganglion (DRG) neurons, predominately in small-diameter DRG neurons, colocalizing with isolectin-B4 (IB4) positivity, a marker of non-peptidergic nociceptors10,17.
Given that in the setting of inflammatory and neuropathic pain decreased mechanical threshold in nociceptors underlies mechanical hyperalgesia, we evaluated the role of TACAN in mechanical hyperalgesia in multiple preclinical models of inflammatory and neuropathic pain.
METHODS
Animals
Experiments were performed on 220–400 g male Sprague-Dawley rats (Charles River Laboratories, Hollister, CA). Experimental animals were housed three per cage, under a 12-hour light/dark cycle, in a temperature- and humidity-controlled room in the animal care facility at the University of California, San Francisco. Food and water were available ad libitum. Experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of California, San Francisco, and adhered to the National Institutes of Health guidelines for the care and use of laboratory animals.
Measuring nociceptive threshold
Mechanical nociceptive threshold was quantified using an Ugo Basile Analgesymeter (Stoelting, Wood Dale, IL), to perform the Randall-Selitto paw-withdrawal test. The analgesymeter applies a linearly increasing mechanical force to the dorsum of the rat’s hind paw52,59,60. Rats were placed in cylindrical acrylic restrainers, with lateral ports that allowed access to the hind paw for nociceptive threshold testing9. They were acclimatized to the testing procedure prior to experimental manipulations. Mechanical nociceptive threshold is defined as the force in grams at which a rat withdrew its paw; and baseline threshold was defined as the mean of three readings taken before injection of test agents. To minimize experimenter bias, individuals conducting the behavioral experiments (I.B and D.A) were blinded to experimental treatments; each experiment was performed on a different group of rats. Data are presented as mechanical nociceptive threshold (g).
Drugs
The following drugs were used in this study: low molecular weight hyaluronic acid (hyaluronan) sodium salt from Streptococcus equi (LMWH), prostaglandin E2 (PGE2), lipopolysaccharide (LPS), and the cancer chemotherapeutic agents paclitaxel and oxaliplatin, all from Sigma-Aldrich (St. Louis, MO); and, rat recombinant TNF-α from R&D Systems (Minneapolis, MN).
PGE2 was dissolved in absolute ethanol to a concentration of 1 μg/μL, and immediately before experiments diluted in saline. The final ethanol concentration of PGE2 was ~2%, a concentration previously shown to not affect mechanical nociceptive threshold after intradermal injection28. TNFα was first dissolved in phosphate buffered saline (PBS) containing 0.1% BSA, then further diluted in saline before administration. LMWH was dissolved in distilled water to a concentration of 1 μg/μL and further diluted in saline to the desired concentration, at the time of the experiment. PGE2 (100 ng), TNFα (100 ng) and LMWH (1 μg) were administered intradermally, in a volume of 5 μL, on the dorsum of the hind paw, using a 30-gauge hypodermic needle attached to a 50 μL Hamilton syringe (Hamilton, Reno, NV) by a segment of PE-10 polyethylene tubing (Becton Dickinson, Franklin Lakes, NJ). LPS was dissolved in saline to a concentration of 100 μg/kg and administered intraperitoneally (i.p.).
Oxaliplatin- and paclitaxel-induced neuropathy: We have previously described the preclinical models of oxaliplatin and paclitaxel painful chemotherapy-induced peripheral neuropathy (CIPN) used in these experiments23,26. Oxaliplatin was freshly dissolved in normal saline at a concentration of 2 mg/mL just prior to intravenous administration (1 mL/kg), via tail vein injection, in rats anesthetized with isoflurane (2.5% in O2). Paclitaxel was dissolved in absolute ethanol and polyethoxylated castor oil (Cremophor EL; 1:1; Sigma-Aldrich)1,8,16,20 and diluted in saline, to a concentration of 1 mg/mL, just before intraperitoneal (i.p.) injection15,32. Paclitaxel (1 mg/kg) was administered, every other day for a total of 4 doses, in rats anesthetized with isoflurane (2.5% in O2).
Oligodeoxynucleotides (ODNs) antisense to TACAN mRNA.
The antisense ODN sequence was directed against a unique region of the rat TACAN mRNA.
Antisense (AS) ODN sequence: 5’-CTT CTT CTG GCG TGT GAT AG-3’
Mismatch (MM) ODN sequence correspond to the antisense sequence with some bases mismatched (denoted by bold letters): 5’- CTC GTT CTT GCC AGT GAT AC- 3’.
ODNs, synthesized by Life Technologies (Life Technologies, Carlsbad, CA), were reconstituted in nuclease-free 0.9% NaCl and then administered intrathecally, at a dose of 6 μg/μL in a volume of 20 μL, for 3 consecutive days9. As described previously2, rats were anesthetized with isoflurane (2.5% in O2) and 120 μg of ODN in a volume of 20 μL injected intrathecally using a syringe (300 units/μL) attached to a 29-gauge needle inserted into the subarachnoid space between the L4 and L5 vertebrae. The intrathecal site of injection was confirmed by a sudden flick of the rat’s tail, a reflex that is evoked by subarachnoid space access and bolus intrathecal injection43. Animals regained consciousness approximately 2 minutes after the injection. The use of intrathecal AS-ODN, administered to attenuate the expression of proteins, essential for their role in nociceptor sensitization, is well supported by previous studies by others47,49,56–58, as well as our group6,9,13,28,29,48.
SDS-PAGE and Western blotting
To determine the efficacy of the antisense treatment Tmem120A expression in rat lumbar dorsal root ganglia (DRG) was analyzed. Rats were euthanized by exsanguination, while under isoflurane anesthesia, 24h after the last injection of antisense (or mismatch) ODN against Tmem120A mRNA. L4 and L5 DRG were then surgically removed and stored at −80°C until further use. DRGs were transferred into homogenization buffer (100mM NaCl, 1mM EDTA, 2% SDS, 50mM Tris-HCl, pH 7.4) that was supplemented with a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IL), and manually homogenized with a hand-held plastic pistil. Proteins were solubilized by incubating the homogenized DRG for 2 h at 37°C and 1400 rpm in an Eppendorf Thermomixer (Eppendorf AG, Hamburg, Germany). Solubilized proteins were extracted from insoluble cell and tissue components by centrifugation for 15 min at 14000 rpm in an Eppendorf tabletop centrifuge. Protein concentration of all samples was determined using the micro BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL) with bovine serum albumin (BSA) as the standard. Mixtures of 60 μg of protein per sample were denatured by boiling in sample buffer [3% SDS, 10% (v/v) Glycerol, 5% (v/v) β-Mercaptoethanol, 0.025 Bromphenol blue, 62.5mM Tris-HCl pH 6.8] for 10 min and electrophoresed on 4–15% pre-cast polyacrylamide gels (Biorad, Hercules, CA) in 25mM Tris containing 192mM glycine and 0.1% SDS. Proteins were electrophoretically transferred to a nitrocellulose membrane using the semidry method [transfer time 2h at 60mA/gel with 47.9mM Tris, 38.9mM Glycine, 0.038% SDS and 20% (v/v) Methanol]. Nitrocellulose membranes were saturated by shaking in antibody dilution buffer [5% BSA in Tris-buffered saline containing 0.1% Tween 20, pH 7.4 (TBST)] for 1h at room temperature, cut in half at ~64kDa and probed with either a rabbit polyclonal anti-Tmem120A antibody (MBS3223965, 1:500, MyBioSource, San Diego, CA) or a rabbit polyclonal anti-PKCε (sc-214, 1:500, Santa Cruz Biotechnology, Paso Robles, CA) antibody, in antibody dilution buffer at 4°C overnight. After rinsing with TBST (3 times at room temperature (RT), 15 min each) both blots were probed with a horseradish peroxidase conjugated anti-rabbit antibody (GE Healthcare Life Sciences, Pittsburgh, PA, 1:2500 in antibody dilution buffer) for 2h at RT. All blotting membranes were rinsed with TBST (3 times at RT, 15 min each) and the immunoreactivities visualized using the West femto chemiluminescence detection system (Pierce Biotechnology). Results were analyzed using computer-assisted densitometry and levels of Tmem120A immunoreactivity were normalized with respect to the PKCε control levels in each sample. The percentage decrease in Tmem120A expression was calculated as: [normalized density for AS/normalized density for MM × 100] −1003,5.
Statistical analysis
All data are presented as values for mechanical nociceptive threshold (grams) in individual rats. Prism 8.0 (GraphPad Software, San Diego, CA) was used for the graphics and to perform statistical analyses; P<0.05 was considered statistically significant. In all behavioral experiments, the dependent variable was mechanical nociceptive threshold (grams). We used 60 male rats in the behavioral tests. Repeated-measures 2-way ANOVA followed by Bonferroni’s multiple comparisons test or Student’s t-test was used to analyze data.
Our Western blot results are presented as arbitrary units (a.u.), normalized to the reference protein, PKCε. A total of 12 rats were used for this experiment (Fig. 1B). Differences between groups treated with AS- and MM-ODN for TACAN mRNA were analyzed using unpaired Student’s t-test..
Figure 1. Increase in mechanical nociceptive threshold in rats treated with ODN antisense to TACAN mRNA.
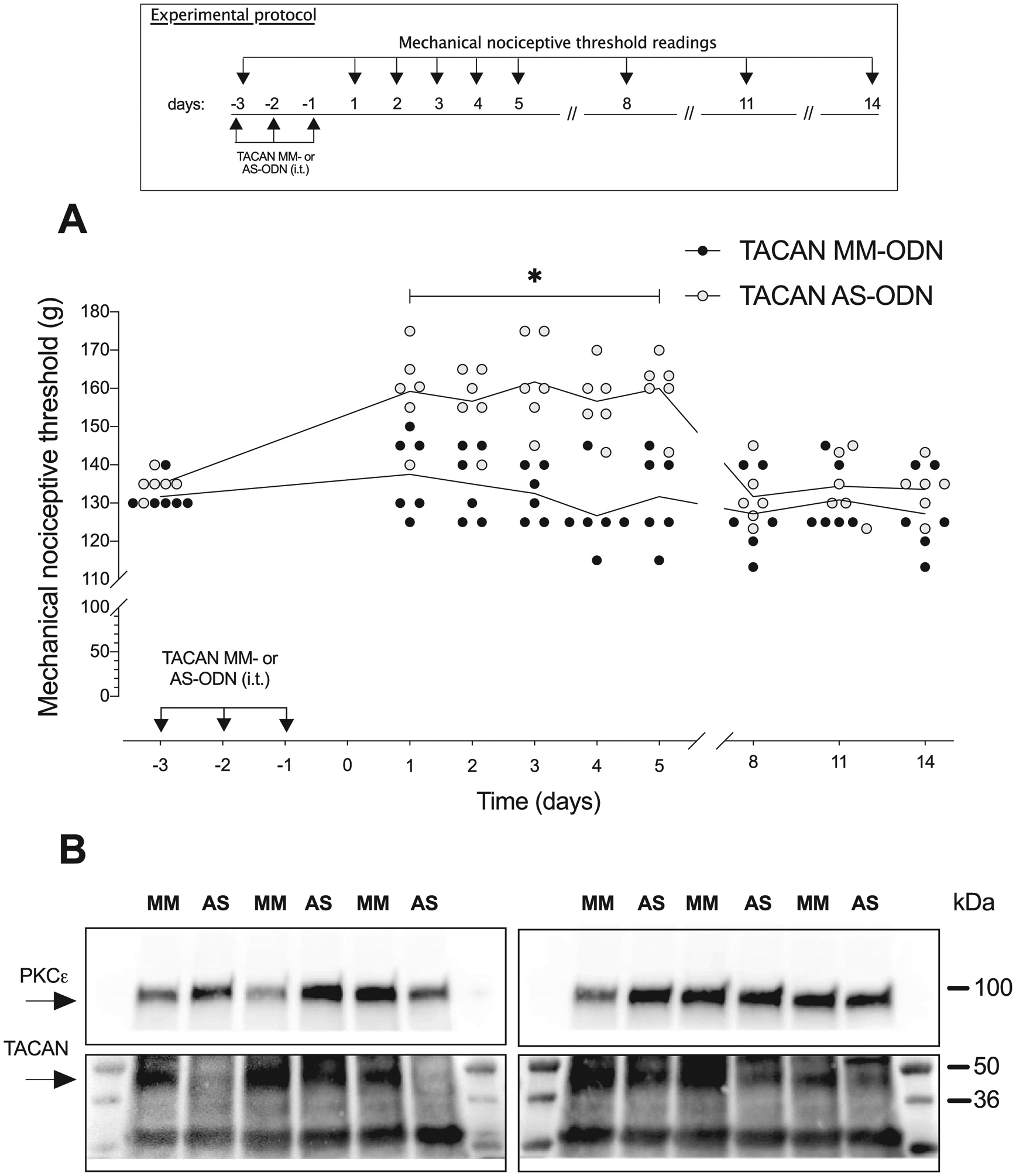
A. Rats were treated intrathecally with AS-ODN (120 μg/20 μL) or MM-ODN (120 μg/20 μL) for TACAN mRNA, once a day for three consecutive days. Mechanical nociceptive threshold was evaluated before the 1st intrathecal administration of AS- or MM-ODN and on days 1, 2, 3, 4, 5, 8, 11 and 14 after its last administration. The group that received TACAN AS-ODN showed an increase in mechanical nociceptive threshold (g) that persisted until 5 days after the last injection (F(8,80)=9.219, *p=0.0229 when the TACAN AS-ODN- is compared with the TACAN MM- ODN-treated group; two-way repeated-measures ANOVA followed by Bonferroni’s multiple comparison test). Data in all figures are presented as values for individual animals._n=6 per group.
B. Western blot analysis of DRG extracts from rats injected with 120 μg of antisense ODN/day for three consecutive days revealed a significant decrease in anti-Tmem120A immunoreactivity when compared with the extracts of DRG from mismatch treated rats (−35.6 ± 14.6%, t(10)=2.438; *p=0.0175 unpaired student’s t-test). The calculated molecular weight of Tmem120A in rat tissue is ~41 kDa (according to UniProtKB database entry Q5HZE2). PKCε, which was used as loading control, has a calculated molecular weight of ~84 kDa (according to UniProtKB database entry P09216). n=6 per group.
RESULTS
TACAN antisense increases mechanical nociceptive threshold
To provide support for a role of TACAN in mechano-nociception, we measured mechanical nociceptive threshold before and after intrathecal administration of AS- or MM-ODN for TACAN mRNA. Both ODNs were administered once a day for three consecutive days. Mechanical nociceptive threshold (g) was increased when measured 24 h after the last dose of TACAN AS-ODN, and when measured 5 days later, but no longer elevated 8 days later (Fig. 1A). These observations provide support for TACAN as a mechanotransducing ion channel involved in mechanical nociception.
Antisense ODN decreases TACAN in DRG
Western blots of DRG from ODN treated rats demonstrated a 35.6 ± 14.6% (arbitrary units [a.u.] normalized to the reference protein) decrease in expression of TACAN in DRG from AS-ODN compared to MM-ODN treated rats (Fig. 1B). The magnitude of the decrease in TACAN protein is similar to that observed by us2,9,24,25,48, as well as by other investigators38,39, for diverse proteins in DRG neurons, following intrathecal administration of AS-ODN against their mRNAs.
TACAN AS-ODN attenuates mechanical hyperalgesia induced by pro-inflammatory mediators
To evaluate the contribution of TACAN to mechanical hyperalgesia induced by pronociceptive inflammatory mediators, groups of rats were treated with AS- or MM-ODN for TACAN mRNA, once a day for three consecutive days. On the fourth day (24 hours after the last injection of TACAN ODN), PGE2 (100 ng/ 5 μl) (Fig. 2A) or TNFα (100 ng/ 5 μl) (Fig. 2B) was injected intradermally, on the dorsum of the hind paw. Treatment with TACAN AS-ODN markedly attenuated mechanical hyperalgesia induced by both proinflammatory mediators. Hyperalgesia induced by intradermal LMWH (1 μg) was also markedly attenuated in the TACAN AS-ODN-treated group (Fig. 3). Additionally, we evaluated whether TACAN played a role in the hyperalgesia induced by systemically administered LPS (100 μg/kg, intraperitoneal), 24 hours after the third intrathecal administration of TACAN AS- or MM-ODN. Mechanical nociceptive threshold was evaluated 1 hour and 1, 2, 5 and 8 days after LPS. Hyperalgesia induced by LPS was markedly attenuated at 1 hour in the TACAN AS-ODN-treated group, and still attenuated 1, 2 and 5 days later (Fig. 4). These observations demonstrate a major contribution of TACAN to the mechanical hyperalgesia induced by diverse pronociceptive inflammatory mediators.
Figure 2. TACAN AS-ODN attenuates mechanical hyperalgesia induced by pronociceptive inflammatory mediators.
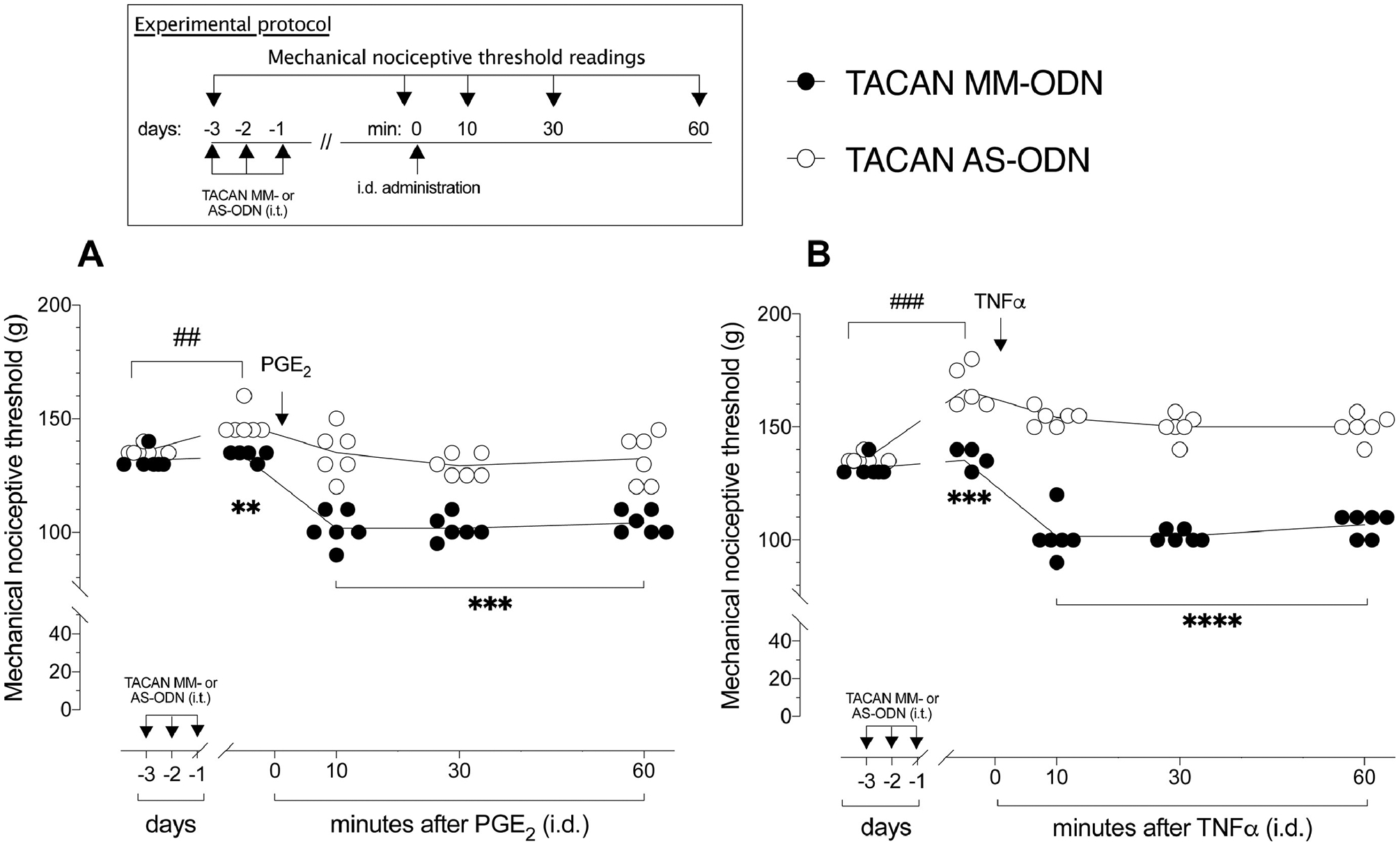
A. Rats were treated intrathecally with AS- (120 μg/20 μL) or MM- (120 μg/20 μL) ODN for TACAN mRNA, once a day for three consecutive days. On the fourth day, approximately 24 h after the last intrathecal administration of ODNs, when the mechanical nociceptive threshold was significantly elevated from pre-ODN baseline, in the TACAN AS-ODN-treated group (t(5)=1.000; p=0.3632, for the TACAN MM-ODN-treated group and, t(5)=7.000; ###p=0.0009, for the TACAN AS-ODN-treated group, when the mechanical nociceptive threshold is compared before and approximately 24 hours after the third intrathecal injection of ODNs; paired Student’s t-test), PGE2 (100 ng/5 μL) was injected intradermally on the dorsum of the hind paw. The mechanical nociceptive threshold was again evaluated 10, 30 and 60 min after PGE2. The group that received TACAN AS-ODN showed a decrease in PGE2-induced hyperalgesia when compared to the TACAN MM-ODN-treated group (F(4,40)=10.80, **p=0.0069, when the TACAN AS-ODN-treated group is compared with TACAN MM-ODN-treated group, before intradermal PGE2; **p=0.0033, when the TACAN AS-ODN- is compared with TACAN MM-ODN-treated group 10, 30 and 60 min after PGE2; two-way repeated-measures ANOVA followed by Bonferroni’s multiple comparison test). n=6 per group.
B. Rats were treated intrathecally with TACAN AS- (120 μg/20 μL) or MM- (120 μg/20 μL) ODN, once a day, for three consecutive days. On the fourth day, approximately 24 h after the last intrathecal administration of ODN, when the mechanical nociceptive threshold was significantly elevated compared to pre-ODN baseline in the TACAN AS-ODN-treated group (t(5) = 2.072; p=0.0930, for the TACAN MM-ODN-treated group and, t(5)=10.07; ###p=0.0002, for the TACAN AS-ODN-treated group, when the mechanical nociceptive threshold is compared before and approximately 24 hours after the third intrathecal injection of ODNs; paired Student’s t-test), TNFα (100 ng/5 μL) was injected intradermally on the dorsum of the hind paw. The mechanical nociceptive threshold was again evaluated 10, 30 and 60 min after TNFα. In the group that received TACAN AS-ODN, TNFα-induced hyperalgesia was significantly inhibited compared to the TACAN MM-ODN-treated group, 30 and 60 min after TNFα (F(4,40)=38.61, ***p=0.0004, when the TACAN AS-ODN-treated group is compared with TACAN MM-ODN-treated group, before intradermal TNFα; ****p<0.0001 when the TACAN AS-ODN- is compared with TACAN MM-ODN-treated group 30 and 60 min after TNFα; two-way repeated-measures ANOVA followed by Bonferroni’s multiple comparison test). n=6 per group.
Figure 3. TACAN AS-ODN attenuates LMWH-induced mechanical hyperalgesia.
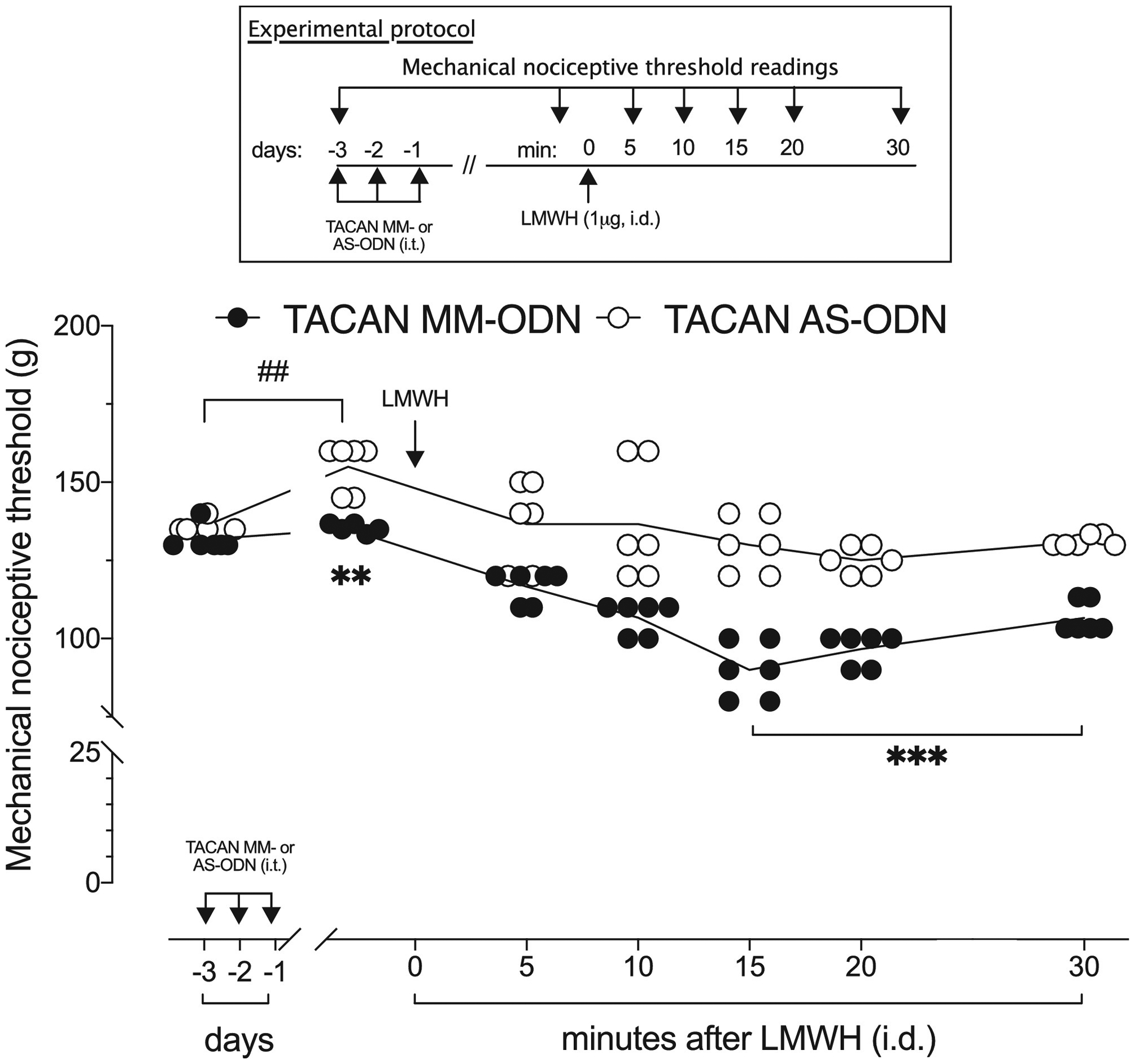
Rats were treated intrathecally with TACAN AS- (120 μg/20 μL) or MM- (120 μg/20 μL) ODN, once a day, for three consecutive days. On the fourth day, approximately 24 h after the last intrathecal administration of ODN, when the mechanical nociceptive threshold was significantly elevated from pre-ODN baseline in the TACAN AS-ODN-treated group (t(5) = 2.340; p=0.0664, for the TACAN MM-ODN-treated group and, t(5)=6.379; ##p=0.0014, for the TACAN AS-ODN-treated group, when the mechanical nociceptive threshold is compared before and approximately 24 hours after the third intrathecal injection of ODNs; paired Student’s t-test), LMWH (1 μg/5 μL) was injected intradermally on the dorsum of the hind paw. Mechanical nociceptive threshold was evaluated 5, 10, 15, 20 and 30 min after LMWH. In the group that received TACAN AS-ODN, hyperalgesia induced by LMWH was markedly attenuated 15, 20 and 30 min after LMWH, when compared with the TACAN MM-ODN- treated group (F(6,60)=5.828, **p<0.0085, when the TACAN AS-ODN- is compared with TACAN MM-ODN-treated group before intradermal LMWH; ***p=0.0002 when the TACAN AS-ODN- is compared with TACAN MM-ODN-treated group 15, 20 and 30 min after LMWH; two-way repeated-measures ANOVA followed by Bonferroni’s multiple comparison test). n=6 per group.
Figure 4. TACAN AS-ODN attenuates LPS-induced mechanical hyperalgesia.
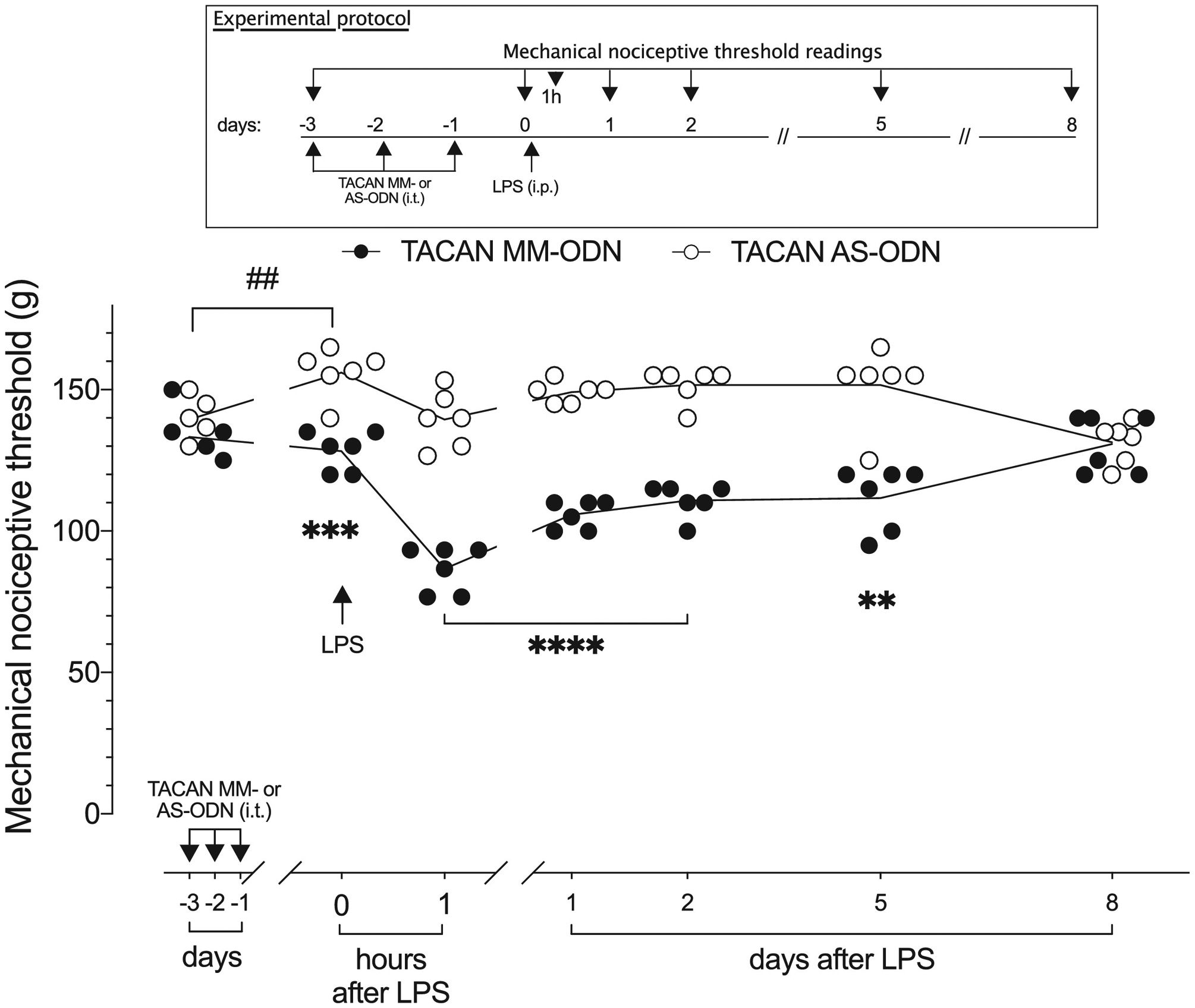
TACAN AS- (120 μg/20 μL) or MM- (120 μg/20 μL) ODN was administered intrathecally in rats, once a day, for three consecutive days. On the fourth day, approximately 24 h after the last intrathecal administration of AS-ODN, when the mechanical nociceptive threshold was significantly elevated from pre-ODN baseline in the TACAN AS-ODN-treated group (t(5)=1.291; p=0.2532, for the TACAN MM-ODN-treated group and, t(5)=5.534; ##p=0.0026, for the TACAN AS-ODN-treated group, when the mechanical nociceptive threshold is compared before and approximately 24 hours after the third intrathecal injection of ODNs; paired Student’s t-test), LPS (100 μg/kg) was injected intraperitoneally (i.p.). The mechanical nociceptive threshold was evaluated 1 h and 1, 2, 5 and 8 days after LPS. In the group that received TACAN AS-ODN, mechanical hyperalgesia induced by systemic LPS was robustly attenuated 1 hour after its i.p. administration, and still attenuated a 1, 2, and 5 days later, compared with the TACAN MM-ODN-treated group (F(6,60)=21.25; ***p<0.0009, when the TACAN AS-ODN- is compared with the TACAN MM-ODN-treated group before intraperitoneal LPS; ****p<0.0001 1 h and 1 and 2 day after intraperitoneal LPS: **p=0.0020; 5 days after intraperitoneal LPS; when the TACAN AS-ODN- is compared with TACAN MM-ODN-treated group; two-way repeated-measures ANOVA followed by Bonferroni’s multiple comparison test). Eight days after i.p. LPS there was no difference between the TACAN AS- and MM-ODN-treated groups. n=6 per group.
TACAN AS-ODN does not attenuate mechanical hyperalgesia in chemotherapy-induced peripheral neuropathy (CIPN)
To explore the role of TACAN in the mechanical hyperalgesia associated with the neuropathic pain in cancer patients, in two well-established models of chemotherapy-induced peripheral neuropathy (CIPN), TACAN AS- or MM-ODN mRNA was injected intrathecally, once a day for three consecutive days. Twenty-four hours after the last intrathecal ODN injection, oxaliplatin was administered intravenously (2 mg/kg)4,23, and mechanical nociceptive threshold evaluated 30 min and 1, 7, 14, 21 and 28 days later. Oxaliplatin-induced hyperalgesia was not attenuated in rats treated with TACAN AS-ODN (Fig. 5).
Figure 5. TACAN AS-ODN does not attenuate mechanical hyperalgesia associated with oxaliplatin chemotherapy-induced peripheral neuropathy (CIPN).

Rats were treated intrathecally with TACAN AS- (120 μg/20 μL) or MM- (120 μg/20 μL) ODN, once a day, for three consecutive days. On the fourth day, approximately 24 h after the last intrathecal administration of ODNs, (t(5)=0.5218; p=0.6241, for the TACAN MM- ODN-treated group and, t(5)=7.593; ###p=0.0006, for the TACAN AS-ODN-treated group, when the mechanical nociceptive threshold is compared before and approximately 24 hours after the third intrathecal injection of ODN; paired Student’s t-test), rats received an intravenous injection of oxaliplatin (2 mg/kg). The mechanical nociceptive threshold was evaluated before the 1st dose of ODNs, before oxaliplatin injection, and then 30min and 1, 7 14, 21 and 28 days after oxaliplatin. TACAN AS-ODN did not attenuate oxaliplatin-induced mechanical hyperalgesia at any time point evaluated (F(7,70)=5.874, *p<0.0136, when the TACAN AS-ODN- is compared with TACAN MM-ODN-treated group before intravenous oxaliplatin; p=0.5868 when the TACAN AS-ODN- is compared with TACAN MM-ODN-treated group after oxaliplatin; two-way repeated-measures ANOVA followed by Bonferroni’s multiple comparison test). n=6 per group.
We next evaluated the role of TACAN in mechanical hyperalgesia associated with paclitaxel CIPN26. TACAN AS- or MM-ODN was injected intrathecally, once a day, for three consecutive days. Paclitaxel was administered intraperitoneally (1 mg/kg), 24 hours after the third intrathecal injection of TACAN ODNs, and mechanical nociceptive threshold evaluated 1, 7, 14, 21 and 28 days later. Since paclitaxel was administered every other day, for a total of 4 doses, TACAN AS- and MM-ODNs were also administered every other day after the third consecutive intrathecal administration, until day 6 after paclitaxel (total of 6 doses of ODN). In rats treated with TACAN AS- and MM-ODN paclitaxel-induced hyperalgesia was actually slightly greater in the AS-ODN treated rats, although this difference was not statistically significant at any time point (Fig. 6). These findings support the suggestion that TACAN, does not play a role in chemotherapy-induced neuropathic pain, produced by either oxaliplatin or paclitaxel.
Figure 6. TACAN AS-ODN does not attenuate mechanical hyperalgesia associated with paclitaxel chemotherapy-induced peripheral neuropathy (CIPN).
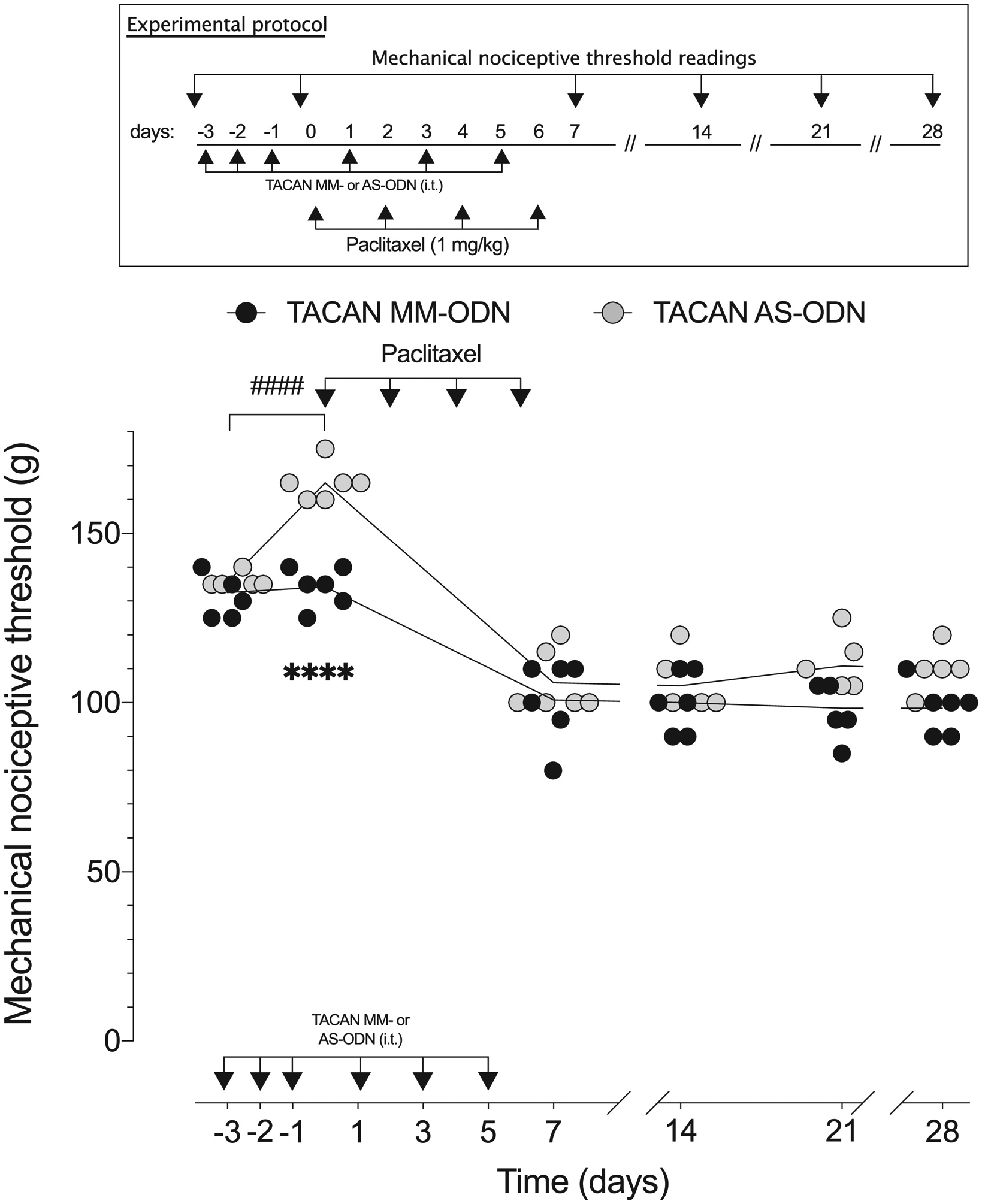
TACAN AS- (120 μg/20 μL) or MM- (120 μg/20 μL) ODN was administered to rats, once a day for three consecutive days. On the fourth day, approximately 24 h after the third intrathecal administration of ODN (t(5)= 0.7906; p=0. 4650, for the TACAN MM-ODN- treated group and, t(5)=18.49; ####p<0.0001, for the TACAN AS-ODN-treated group, when the mechanical nociceptive threshold is compared before and approximately 24 hours after the third intrathecal injection of ODNs; paired Student’s t-test), paclitaxel (1 mg/kg) was administered intraperitoneally, every other day for a total of 4 doses (days 0, 2, 4 and 6). TACAN AS- and MM-ODNs were also administered every other day, after the third consecutive daily intrathecal administration, until day 6 after paclitaxel (total of 6 doses). Mechanical nociceptive threshold was evaluated before the first dose of ODNs, before the first dose of paclitaxel, and then, on days 1, 7, 14, 21 and 28 after the first dose of paclitaxel. TACAN AS-ODN did not attenuate paclitaxel-induced mechanical hyperalgesia at any time point (F(5,50)=17.02, ****p<0.0001, when the TACAN AS-ODN- is compared with TACAN MM- ODN-treated group before intraperitoneal paclitaxel; p=0.6081 when the TACAN AS-ODN- is compared with TACAN MM-ODN-treated group; two-way repeated-measures ANOVA followed by Bonferroni’s multiple comparison test). n=6 per group.
DISCUSSION
TACAN, is a novel ion channel lacking sequence homology to other known classes of ion channels or other proteins7,21,40, that is blocked by mechanosensitive ion channel toxins10. Analysis of its tissue expression revealed that TACAN is expressed in dorsal root ganglion (DRG) neurons, being present in small- to medium-diameter cell bodies, especially in isolectin B4 (IB4)-positive neurons10, a population of nociceptors that have previously been implicated in mechanical nociception17, and in tyrosine hydroxylase (TH)-positive neurons. Inducible knockout of TACAN in nociceptors decreased, but did not eliminate, mechanosensitivity and nociceptive behaviors, measured as response frequency to mechanical stimuli10. These findings are in line with our results showing that the reversible knockdown of TACAN in nociceptors temporarily increases mechanical nociceptive threshold.
To evaluate the role of TACAN in the decrease in mechanical nociceptive threshold associated with inflammation and peripheral neuropathy, we administered an oligodeoxynucleotide antisense (AS-ODN) to TACAN mRNA, intrathecally2, in preclinical models of inflammatory and neuropathic pain. Intrathecal administration of AS-ODN decreased TACAN protein in DRG. This decrease in TACAN expression was associated with an increase in mechanical nociceptive threshold, providing support that TACAN is a nociceptor mechanotransducing ion channel10. Of note, while we did not completely eliminate TACAN protein in nociceptors, in knockout mice mechanical nociception was also only attenuated but not eliminated10.
In the present study we sought to determine if TACAN is involved in the decrease in mechanical nociceptive threshold associated with inflammation. In contrast to its modest effect on mechanical nociceptive threshold, TACAN AS-ODN markedly inhibited the hyperalgesia induced by four pronociceptive inflammatory mediators, PGE2, TNFα, LMWH and LPS. These findings support the hypothesis that TACAN plays an important role in the decrease of mechanical nociceptive threshold, mechanical hyperalgesia, produced by diverse inflammatory mediators.
While our present findings indicate that TACAN contributes to mechanotransduction and the development of mechanical hyperalgesia10, the magnitude of the attenuation of mechanical hyperalgesia appeared out of proportion to the more modest increase in nociceptive threshold. While this finding is compatible with the involvement of additional mechanotransduction mechanisms in nociceptors, it is not possible to rule out a contribution of other mechanisms to either mechanical nociceptive threshold or mechanical hyperalgesia (e.g., by ligand- or voltage-gated ion channels).
Since hypersensitivity to mechanical stimuli is also a prominent feature of neuropathic pain, we next evaluated whether TACAN also contributes to pain associated with chemotherapy-induced peripheral neuropathy (CIPN), produced by two commonly used clinical chemotherapy agents, oxaliplatin and paclitaxel26,27,30. Unexpectedly, treatment with TACAN AS-ODN did not attenuate the mechanical hyperalgesia induced by either oxaliplatin or paclitaxel, indicating that TACAN does not play a substantial role in the mechanical hyperalgesia associated with CIPN. However, given the heterogeneity in the mechanisms underlying the large number of types of neuropathic pain, widely recognized as amongst most difficult pain syndromes to manage, we cannot conclude that TACAN may have or not an effect in other models of neuropathic pain, including CIPN induced by other chemotherapeutic drugs.
We have previously demonstrated that CIPN induced by oxaliplatin is mediated by the IB4-positive population of nociceptors, as it is eliminated by IB4-saporin, a neurotoxin for this class of nociceptors35. Since TACAN is strongly expressed in IB4-positive nociceptors, this lack of effect of AS-ODN on the hyperalgesia associated with oxaliplatin CIPN is difficult to explain without invoking an additional class of mechanotransducer in IB4-positive nociceptors. Potential candidate mechanotransducers include degenerin/epithelial sodium channels (DEG/ENaC), transient receptor potential NOMPC-like (TRPN), and Piezo ion channels50. With regard to Piezo2, another mechanotransduction ion channel expressed in a subset of DRG neurons that innervate the skin (in low threshold mechanoreceptors)51, we recently demonstrated that intradermal AS-ODN for Piezo2 mRNA reversed oxaliplatin CIPN30.
While TRPN channels are implicated in proprioception19, DEG/ENaC, which regulates turning behavior, may also contribute to mechanical nociception45. As Piezo has also been implicated in mechanotransduction required for mechanical nociception45, different combinations of ion channels may serve different mechanosensory functions in the same neuron. Additionally, TACAN could co-activate or co-deactivate other ion channels in the cell membrane, leading to hyperpolarization or depolarization of its membrane potential.
TACAN is a TMEM family member. The co-expression of another member of this family, Tmem150C, was found to significantly decrease the apparent activation threshold of Piezo27. The same study also demonstrated that Tmem150C is a regulator of mechano-gated ion channels rather than itself a nonselective ion channel7. Thus, we cannot exclude the possibility that TACAN also produces a small decrease in the activation threshold in receptors and may act as a regulator of other mechano-gated ion channels.
In this study we provide support for the recent demonstration that TACAN is a mechanically-gated ion channel that plays a role in mechanical threshold in primary afferent nociceptors 10, and implicate it in mechanical hyperalgesia induced by pronociceptive inflammatory mediators, without contributing to neuropathic mechanical hyperalgesia induced by cancer chemotherapy. While further studies are needed to more precisely define the role of TACAN in nociceptor sensitization and mechanical hyperalgesia, the development of TACAN inhibitors could be a therapeutic target to treat pain, especially in inflammatory conditions.
Highlights.
Knocking down TACAN in DRG cells increases mechanical nociceptive threshold.
TACAN plays a crucial role in inflammatory mechanical hyperalgesia.
Chemotherapy-induced peripheral neuropathy is not dependent on TACAN.
Perspective.
We evaluated the role of TACAN, a mechanotransducing ion channel in nociceptors, in preclinical models of inflammatory and chemotherapy-induced neuropathic pain. Attenuation of TACAN expression reduced hyperalgesia produced by inflammatory mediators but had not chemotherapeutic agents. Our findings support the presence of multiple mechanotransducers in nociceptors.
Acknowledgements:
The authors would like to thank Samantha Stevens for technical assistance.
Disclosures: All authors report no financial interests or potential conflicts of interest. This study was funded by National Institutes of Health (NIH) grants CA250017 and AR075334.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Adams JD, Flora KP, Goldspiel BR, Wilson JW, Arbuck SG & Finley R Taxol: a history of pharmaceutical development and current pharmaceutical concerns. J Natl Cancer Inst Monogr 141–147 (1993). [PubMed] [Google Scholar]
- 2.Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB & Levine JD Hypotonicity induces TRPV4-mediated nociception in rat. Neuron 39, 497–511 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Alvarez P, Bogen O & Levine JD Nociceptor interleukin 33 receptor/ST2 signaling in vibration-induced muscle oain in the rat. J Pain 21, 506–512 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez P, Ferrari LF & Levine JD Muscle pain in models of chemotherapy-induced and alcohol-induced peripheral neuropathy. Ann Neurol 70, 101–109 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez P, Bogen O & Levine JD Interleukin 6 decreases nociceptor expression of the potassium channel KV1.4 in a rat model of hand–arm vibration syndrome. Pain 160, 1876–1882 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvarez P, Green PG & Levine JD Role for monocyte chemoattractant protein-1 in the induction of chronic muscle pain in the rat. Pain 155, 1161–1167 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson EO, Schneider ER, Matson JD, Gracheva EO & Bagriantsev SN TMEM150C/Tentonin3 is a regulator of mechano-gated Ion channels. Cell Rep 23, 701–708 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apfel SC, Lipton RB, Arezzo JC & Kessler JA Nerve growth factor prevents toxic neuropathy in mice. Ann Neurol 29, 87–90 (1991). [DOI] [PubMed] [Google Scholar]
- 9.Araldi D, Bogen O, Green PG & Levine JD Role of nociceptor toll-like receptor 4 (TLR4) in opioid-induced hyperalgesia and hyperalgesic priming. J Neurosci 39, 6414–6424 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beaulieu-Laroche L, Christin M, Donoghue A, Agosti F, Yousefpour N, Petitjean H, Davidova A, Stanton C, Khan U, Dietz C, Faure E, Fatima T, MacPherson A, Mouchbahani-Constance S, Bisson DG, Haglund L, Ouellet JA, Stone LS, Samson J, Smith MJ, Ask K, Ribeiro-da-Silva A, Blunck R, Poole K, Bourinet E & Sharif-Naeini R TACAN Is an ion channel involved in sensing mechanical pain. Cell 180, 956–967 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Beurg M, Kim KX & Fettiplace R Conductance and block of hair-cell mechanotransducer channels in transmembrane channel-like protein mutants. J Gen Physiol 144, 55–69 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ & Samad TA Nociceptors are interleukin-1beta sensors. J Neurosci 28, 14062–14073 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogen O, Alessandri-Haber N, Chu C, Gear RW & Levine JD Generation of a pain memory in the primary afferent nociceptor triggered by PKCε activation of CPEB. Journal of Neuroscience 32, 2018–2026 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyer AE, Quinn CP, Beesley CA, Gallegos-Candela M, Marston CK, Cronin LX, Lins RC, Stoddard RA, Li H, Schiffer J, Hossain MJ, Chakraborty A, Rahman M, Luby SP, Shieh WJ, Zaki S, Barr JR & Hoffmaster AR Lethal factor toxemia and anti-protective antigen antibody activity in naturally acquired cutaneous anthrax. J Infect Dis 204, 1321–1327 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavaletti G, Bogliun G, Crespi V, Marzorati L, Zincone A, Marzola M, Rota S, Galli A, Tredici P & Tredici G Neurotoxicity and ototoxicity of cisplatin plus paclitaxel in comparison to cisplatin plus cyclophosphamide in patients with epithelial ovarian cancer. J Clin Oncol 15, 199–206 (1997). [DOI] [PubMed] [Google Scholar]
- 16.Cavaletti G, Tredici G, Braga M & Tazzari S Experimental peripheral neuropathy induced in adult rats by repeated intraperitoneal administration of taxol. Exp Neurol 133, 64–72 (1995). [DOI] [PubMed] [Google Scholar]
- 17.Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI & Anderson DJ Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A 106, 9075–9080 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chalfie M Neurosensory mechanotransduction. Nat Rev Mol Cell Biol 10, 44–52 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Cheng W, Sun C & Zheng J Heteromerization of TRP channel subunits: extending functional diversity. Protein Cell 1, 802–810 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cliffer KD, Siuciak JA, Carson SR, Radley HE, Park JS, Lewis DR, Zlotchenko E, Nguyen T, Garcia K, Tonra JR, Stambler N, Cedarbaum JM, Bodine SC, Lindsay RM & DiStefano PS Physiological characterization of Taxol-induced large-fiber sensory neuropathy in the rat. Ann Neurol 43, 46–55 (1998). [DOI] [PubMed] [Google Scholar]
- 21.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE & Patapoutian A Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costigan M, Scholz J & Woolf CJ Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 32, 1–32 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dina OA, Chen X, Reichling D & Levine JD Role of protein kinase Cε and protein kinase A in a model of paclitaxel-induced painful peripheral neuropathy in the rat. Neuroscience (2001). [DOI] [PubMed] [Google Scholar]
- 24.Dina OA, Parada CA, Yeh J, Chen X, McCarter GC & Levine JD Integrin signaling in inflammatory and neuropathic pain in the rat. Eur J Neurosci 19, 634–642 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Ferrari LF, Araldi D, Bogen O, Green PG & Levine JD Systemic morphine produces dose-dependent nociceptor-mediated biphasic changes in nociceptive threshold and neuroplasticity. Neuroscience 398, 64–75 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrari LF, Araldi D, Green PG & Levine JD Marked sexual dimorphism in neuroendocrine mechanisms for the exacerbation of paclitaxel-induced painful peripheral neuropathy by stress. Pain 161, 865–874 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrari LF, Chum A, Bogen O, Reichling DB & Levine JD Role of Drp1, a key mitochondrial fission protein, in neuropathic pain. J Neurosci 31, 11404–11410 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrari LF, Khomula EV, Araldi D & Levine JD Marked sexual dimorphism in the role of the ryanodine receptor in a model of pain chronification in the rat. Sci Rep 6, 31221 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrari LF, Khomula EV, Araldi D & Levine JD CD44 signaling mediates high molecular weight hyaluronan-induced antihyperalgesia. J Neurosci 38, 308–321 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrari LF, Bogen O, Green P & Levine JD Contribution of Piezo2 to endothelium-dependent pain. Molecular Pain 11, 1–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gold MS & Gebhart GF Nociceptor sensitization in pain pathogenesis. Nat Med 16, 1248–1257 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamers FP, Pette C, Neijt JP & Gispen WH The ACTH-(4–9) analog, ORG 2766, prevents taxol-induced neuropathy in rats. Eur J Pharmacol 233, 177–178 (1993). [DOI] [PubMed] [Google Scholar]
- 33.He BH, Christin M, Mouchbahani-Constance S, Davidova A & Sharif-Naeini R Mechanosensitive ion channels in articular nociceptors drive mechanical allodynia in osteoarthritis. Osteoarthritis Cartilage 25, 2091–2099 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Ikeda R, Cha M, Ling J, Jia Z, Coyle D & Gu JG Merkel cells transduce and encode tactile stimuli to drive Aβ-afferent impulses. Cell 157, 664–675 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joseph EK, Chen X, Bogen O & Levine JD Oxaliplatin acts on IB4-positive nociceptors to induce an oxidative stress-dependent acute painful peripheral neuropathy. J Pain 9, 463–472 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Kawashima Y, Kurima K, Pan B, Griffith AJ & Holt JR Transmembrane channel-like (TMC) genes are required for auditory and vestibular mechanosensation. Pflugers Arch 467, 85–94 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SE, Coste B, Chadha A, Cook B & Patapoutian A The role of Drosophila Piezo in mechanical nociception. Nature 483, 209–212 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lai D, Weng S, Wang C, Qi L, Yu C, Fu L & Chen W Small antisense RNA to cyclin D1 generated by pre-tRNA splicing inhibits growth of human hepatoma cells. FEBS Lett 576, 481–486 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Lai J, Gold MS, Kim CS, Bian D, Ossipov MH, Hunter JC & Porreca F Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, NaV1.8. Pain 95, 143–152 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Li Fraine S, Patel A, Duprat F & Sharif-Naeini R Dynamic regulation of TREK1 gating by Polycystin 2 via a Filamin A-mediated cytoskeletal Mechanism. Sci Rep 7, 17403 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li MF, Hsiao CH, Chen YL, Huang WY, Lee YH, Huang HN & Lien HC Human herpesvirus 8-associated lymphoma mimicking cutaneous anaplastic large T-cell lymphoma in a patient with human immunodeficiency virus infection. J Cutan Pathol 39, 274–278 (2012). [DOI] [PubMed] [Google Scholar]
- 42.McQueen DS, Iggo A, Birrell GJ & Grubb BD Effects of paracetamol and aspirin on neural activity of joint mechanonociceptors in adjuvant arthritis. Br J Pharmacol 104, 178–182 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mestre C, Pélissier T, Fialip J, Wilcox G & Eschalier A A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods 32, 197–200 (1994). [DOI] [PubMed] [Google Scholar]
- 44.Neogi T, Guermazi A, Roemer F, Nevitt MC, Scholz J, Arendt-Nielsen L, Woolf C, Niu J, Bradley LA, Quinn E & Law LF Association of Joint Inflammation With Pain Sensitization in Knee Osteoarthritis: The Multicenter Osteoarthritis Study. Arthritis Rheumatol 68, 654–661 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nilius B & Owsianik G The transient receptor potential family of ion channels. Genome Biol 12, 218 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okun A, Liu P, Davis P, Ren J, Remeniuk B, Brion T, Ossipov MH, Xie J, Dussor GO, King T & Porreca F Afferent drive elicits ongoing pain in a model of advanced osteoarthritis. Pain 153, 924–933 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliveira-Fusaro MCG, Zanoni CIS, Dos Santos GG, Manzo LP, Araldi D, Bonet IJM, Tambeli CH, Dias EV & Parada CA Antihyperalgesic effect of CB1 receptor activation involves the modulation of P2X3 receptor in the primary afferent neuron. Eur J Pharmacol 798, 113–121 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Parada CA, Yeh JJ, Reichling DB & Levine JD Transient attenuation of protein kinase Cε can terminate a chronic hyperalgesic state in the rat. Neuroscience 120, 219–226 (2003). [DOI] [PubMed] [Google Scholar]
- 49.Quanhong Z, Ying X, Moxi C, Tao X, Jing W, Xin Z, Li W, Derong C, Xiaoli Z & Wei J Intrathecal PLC(β3) oligodeoxynucleotides antisense potentiates acute morphine efficacy and attenuates chronic morphine tolerance. Brain Res 1472, 38–44 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Ranade SS, Syeda R & Patapoutian A Mechanically activated ion channels. Neuron 87, 1162–1179 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Bégay V, Coste B, Mainquist J, Wilson AJ, Francisco AG, Reddy K, Qiu Z, Wood JN, Lewin GR & Patapoutian A Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516, 121–125 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Randall LO & Selitto JJ A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther 111, 409–419 (1957). [PubMed] [Google Scholar]
- 53.Schäfers M, Geis C, Svensson CI, Luo ZD & Sommer C Selective increase of tumour necrosis factor-alpha in injured and spared myelinated primary afferents after chronic constrictive injury of rat sciatic nerve. Eur J Neurosci 17, 791–804 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Schaible HG Nociceptive neurons detect cytokines in arthritis. Arthritis Res Ther 16, 470 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Lauritzen I, Arhatte M, Jodar M, Dedman A, Chatelain FC, Schulte U, Retailleau K, Loufrani L, Patel A, Sachs F, Delmas P, Peters DJ & Honoré E Polycystin-1 and −2 dosage regulates pressure sensing. Cell 139, 587–596 (2009). [DOI] [PubMed] [Google Scholar]
- 56.Song MJ, Wang YQ & Wu GC Additive anti-hyperalgesia of electroacupuncture and intrathecal antisense oligodeoxynucleotide to interleukin-1 receptor type I on carrageenan-induced inflammatory pain in rats. Brain Res Bull 78, 335–341 (2009). [DOI] [PubMed] [Google Scholar]
- 57.Su L, Wang C, Yu YH, Ren YY, Xie KL & Wang GL Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain. BMC Neurosci 12, 120 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun JL, Xiao C, Lu B, Zhang J, Yuan XZ, Chen W, Yu LN, Zhang FJ, Chen G & Yan M CX3CL1/CX3CR1 regulates nerve injury-induced pain hypersensitivity through the ERK5 signaling pathway. J Neurosci Res 91, 545–553 (2013). [DOI] [PubMed] [Google Scholar]
- 59.Taiwo YO, Coderre TJ & Levine JD The contribution of training to sensitivity in the nociceptive paw-withdrawal test. Brain Res 487, 148–151 (1989). [DOI] [PubMed] [Google Scholar]
- 60.Taiwo YO & Levine JD Prostaglandin effects after elimination of indirect hyperalgesic mechanisms in the skin of the rat. Brain Res 492, 397–399 (1989). [DOI] [PubMed] [Google Scholar]
- 61.Woo SH, Lukacs V, de Nooij JC, Zaytseva D, Criddle CR, Francisco A, Jessell TM, Wilkinson KA & Patapoutian A Piezo2 is the principal mechanotransduction channel for proprioception. Nat Neurosci 18, 1756–1762 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL & Patapoutian A Piezo2 is required for Merkel-cell mechanotransduction. Nature 509, 622–626 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


